User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Widespread carboplatin, cisplatin shortages: NCCN survey
The survey, which included responses from 27 NCCN member institutions, revealed that 93% are experiencing a shortage of carboplatin and that 70% have reported a shortage of cisplatin.
“This is an unacceptable situation,” Robert W. Carlson, MD, NCCN’s chief executive offer, said in the statement released by the network.
“We are hearing from oncologists and pharmacists across the country who have to scramble to find appropriate alternatives for treating their patients with cancer right now,” Dr. Carlson said. And while the survey results show patients are still able to get lifesaving care, “it comes at a burden to our overtaxed medical facilities.”
The NCCN called on the federal government, the pharmaceutical industry, providers, and payers to take steps to “help mitigate any impacts” from this cancer drug shortage.
“We need to work together to improve the current situation and prevent it from happening again in the future,” Dr. Carlson stressed.
Carboplatin and cisplatin, which are frequently used together for systemic treatment, are highly effective therapies prescribed to treat many cancer types, including lung, breast, and prostate cancers, as well as leukemias and lymphomas. An estimated 500,000 new patients with cancer receive these agents each year.
The current survey, conducted over the last week of May, found that 100% of responding centers are able to continue to treat patients who need cisplatin without delays.
The same cannot be said for carboplatin: only 64% of centers said they are still able to continue treating all current patients receiving the platinum-based therapy. Among 19 responding centers, 20% reported that they were continuing carboplatin regimens for some but not all patients. And 16% reported treatment delays from having to obtain prior authorization for modified treatment plans, though none reported denials.
“Carboplatin has been in short supply for months but in the last 4 weeks has reached a critical stage,” according to one survey comment. “Without additional inventory many of our sites will be out of drug by early next week.”
In response to the survey question, “Is your center experiencing a shortage of carboplatin,” others made similar comments:
- “Current shipments from established manufacturers have been paused.”
- “The supply of carboplatin available is not meeting our demands.”
- “Without additional supply in early June, we will have to implement several shortage mitigation strategies.”
Survey respondents also addressed whether manufacturers or suppliers have provided any indication of when these drugs will become readily available again. For both drugs, about 60% of respondents said no. And for those who do receive updates, many noted that the “information is tentative and variable.”
Respondents indicated that other cancer agents, including methotrexate (67%) and 5FU (26%), are also in short supply at their centers.
The shortage and the uncertainty as to when it will end are forcing some centers to develop conservation and mitigation strategies.
The NCCN has broadly outlined how the federal government, the pharmaceutical industry, providers, and payers can help with prevention and mitigation. The NCCN has called on the federal government and the pharmaceutical industry to work to secure a steady supply of core anticancer drugs and has asked payers to “put patients first and provide flexible and efficient systems of providing coverage for alternative therapies replacing anti-cancer drugs that are unavailable or in shortage.”
Overall, the survey results “demonstrate the widespread impact of the chemotherapy shortage,” said Alyssa Schatz, MSW, senior director of policy and advocacy for NCCN. “We hope that by sharing this survey and calling for united action across the oncology community, we can come together to prevent future drug shortages and ensure quality, effective, equitable, and accessible cancer care for all.”
A version of this article first appeared on Medscape.com.
The survey, which included responses from 27 NCCN member institutions, revealed that 93% are experiencing a shortage of carboplatin and that 70% have reported a shortage of cisplatin.
“This is an unacceptable situation,” Robert W. Carlson, MD, NCCN’s chief executive offer, said in the statement released by the network.
“We are hearing from oncologists and pharmacists across the country who have to scramble to find appropriate alternatives for treating their patients with cancer right now,” Dr. Carlson said. And while the survey results show patients are still able to get lifesaving care, “it comes at a burden to our overtaxed medical facilities.”
The NCCN called on the federal government, the pharmaceutical industry, providers, and payers to take steps to “help mitigate any impacts” from this cancer drug shortage.
“We need to work together to improve the current situation and prevent it from happening again in the future,” Dr. Carlson stressed.
Carboplatin and cisplatin, which are frequently used together for systemic treatment, are highly effective therapies prescribed to treat many cancer types, including lung, breast, and prostate cancers, as well as leukemias and lymphomas. An estimated 500,000 new patients with cancer receive these agents each year.
The current survey, conducted over the last week of May, found that 100% of responding centers are able to continue to treat patients who need cisplatin without delays.
The same cannot be said for carboplatin: only 64% of centers said they are still able to continue treating all current patients receiving the platinum-based therapy. Among 19 responding centers, 20% reported that they were continuing carboplatin regimens for some but not all patients. And 16% reported treatment delays from having to obtain prior authorization for modified treatment plans, though none reported denials.
“Carboplatin has been in short supply for months but in the last 4 weeks has reached a critical stage,” according to one survey comment. “Without additional inventory many of our sites will be out of drug by early next week.”
In response to the survey question, “Is your center experiencing a shortage of carboplatin,” others made similar comments:
- “Current shipments from established manufacturers have been paused.”
- “The supply of carboplatin available is not meeting our demands.”
- “Without additional supply in early June, we will have to implement several shortage mitigation strategies.”
Survey respondents also addressed whether manufacturers or suppliers have provided any indication of when these drugs will become readily available again. For both drugs, about 60% of respondents said no. And for those who do receive updates, many noted that the “information is tentative and variable.”
Respondents indicated that other cancer agents, including methotrexate (67%) and 5FU (26%), are also in short supply at their centers.
The shortage and the uncertainty as to when it will end are forcing some centers to develop conservation and mitigation strategies.
The NCCN has broadly outlined how the federal government, the pharmaceutical industry, providers, and payers can help with prevention and mitigation. The NCCN has called on the federal government and the pharmaceutical industry to work to secure a steady supply of core anticancer drugs and has asked payers to “put patients first and provide flexible and efficient systems of providing coverage for alternative therapies replacing anti-cancer drugs that are unavailable or in shortage.”
Overall, the survey results “demonstrate the widespread impact of the chemotherapy shortage,” said Alyssa Schatz, MSW, senior director of policy and advocacy for NCCN. “We hope that by sharing this survey and calling for united action across the oncology community, we can come together to prevent future drug shortages and ensure quality, effective, equitable, and accessible cancer care for all.”
A version of this article first appeared on Medscape.com.
The survey, which included responses from 27 NCCN member institutions, revealed that 93% are experiencing a shortage of carboplatin and that 70% have reported a shortage of cisplatin.
“This is an unacceptable situation,” Robert W. Carlson, MD, NCCN’s chief executive offer, said in the statement released by the network.
“We are hearing from oncologists and pharmacists across the country who have to scramble to find appropriate alternatives for treating their patients with cancer right now,” Dr. Carlson said. And while the survey results show patients are still able to get lifesaving care, “it comes at a burden to our overtaxed medical facilities.”
The NCCN called on the federal government, the pharmaceutical industry, providers, and payers to take steps to “help mitigate any impacts” from this cancer drug shortage.
“We need to work together to improve the current situation and prevent it from happening again in the future,” Dr. Carlson stressed.
Carboplatin and cisplatin, which are frequently used together for systemic treatment, are highly effective therapies prescribed to treat many cancer types, including lung, breast, and prostate cancers, as well as leukemias and lymphomas. An estimated 500,000 new patients with cancer receive these agents each year.
The current survey, conducted over the last week of May, found that 100% of responding centers are able to continue to treat patients who need cisplatin without delays.
The same cannot be said for carboplatin: only 64% of centers said they are still able to continue treating all current patients receiving the platinum-based therapy. Among 19 responding centers, 20% reported that they were continuing carboplatin regimens for some but not all patients. And 16% reported treatment delays from having to obtain prior authorization for modified treatment plans, though none reported denials.
“Carboplatin has been in short supply for months but in the last 4 weeks has reached a critical stage,” according to one survey comment. “Without additional inventory many of our sites will be out of drug by early next week.”
In response to the survey question, “Is your center experiencing a shortage of carboplatin,” others made similar comments:
- “Current shipments from established manufacturers have been paused.”
- “The supply of carboplatin available is not meeting our demands.”
- “Without additional supply in early June, we will have to implement several shortage mitigation strategies.”
Survey respondents also addressed whether manufacturers or suppliers have provided any indication of when these drugs will become readily available again. For both drugs, about 60% of respondents said no. And for those who do receive updates, many noted that the “information is tentative and variable.”
Respondents indicated that other cancer agents, including methotrexate (67%) and 5FU (26%), are also in short supply at their centers.
The shortage and the uncertainty as to when it will end are forcing some centers to develop conservation and mitigation strategies.
The NCCN has broadly outlined how the federal government, the pharmaceutical industry, providers, and payers can help with prevention and mitigation. The NCCN has called on the federal government and the pharmaceutical industry to work to secure a steady supply of core anticancer drugs and has asked payers to “put patients first and provide flexible and efficient systems of providing coverage for alternative therapies replacing anti-cancer drugs that are unavailable or in shortage.”
Overall, the survey results “demonstrate the widespread impact of the chemotherapy shortage,” said Alyssa Schatz, MSW, senior director of policy and advocacy for NCCN. “We hope that by sharing this survey and calling for united action across the oncology community, we can come together to prevent future drug shortages and ensure quality, effective, equitable, and accessible cancer care for all.”
A version of this article first appeared on Medscape.com.
After backlash, publisher to retract article that surveyed parents of children with gender dysphoria, says coauthor
The move is “due to concerns about lack of informed consent,” according to tweets by one of the paper’s authors.
The article, “Rapid Onset Gender Dysphoria: Parent Reports on 1655 Possible Cases,” was published in March in the Archives of Sexual Behavior. It has not been cited in the scientific literature, according to Clarivate’s Web of Science, but Altmetric, which tracks the online attention papers receive, ranks the article in the top 1% of all articles of a similar age.
Rapid Onset Gender Dysphoria (ROGD) is, the article stated, a “controversial theory” that “common cultural beliefs, values, and preoccupations cause some adolescents (especially female adolescents) to attribute their social problems, feelings, and mental health issues to gender dysphoria,” and that “youth with ROGD falsely believe that they are transgender,” in part due to social influences.
Michael Bailey, a psychology professor at Northwestern University in Evanston, Ill., and the paper’s corresponding author, tweeted: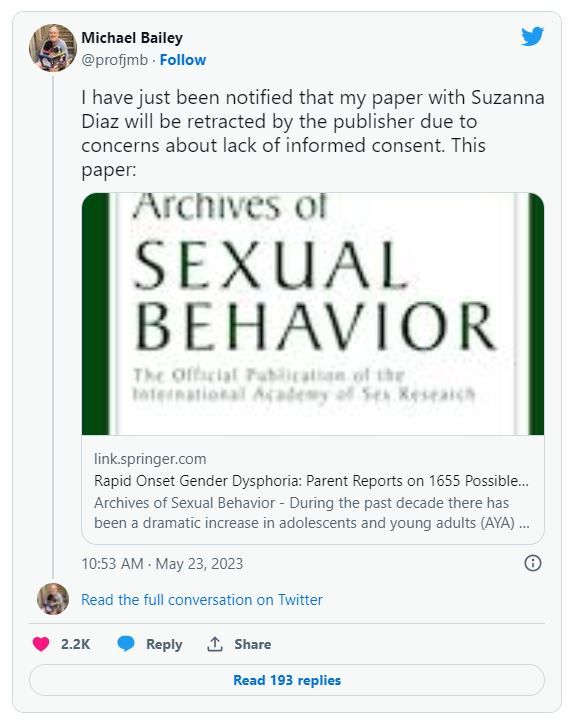
Bailey told Retraction Watch that he would “respond when [he] can” to our request for comment, following “new developments on our end.” Neither Springer Nature nor Kenneth Zucker, editor in chief of Archives of Sexual Behavior, has responded to similar requests.
The paper reported the results of a survey of parents who contacted the website ParentsofROGDKids.com, with which the first author is affiliated. According to the abstract, the authors found:
“Pre-existing mental health issues were common, and youths with these issues were more likely than those without them to have socially and medically transitioned. Parents reported that they had often felt pressured by clinicians to affirm their AYA [adolescent and young adult] child’s new gender and support their transition. According to the parents, AYA children’s mental health deteriorated considerably after social transition.”
Soon after publication, the paper attracted criticism that its method of gathering study participants was biased, and that the authors ignored information that didn’t support the theory of ROGD.
Archives of Sexual Behavior is the official publication of the International Academy of Sex Research, which tweeted on April 19: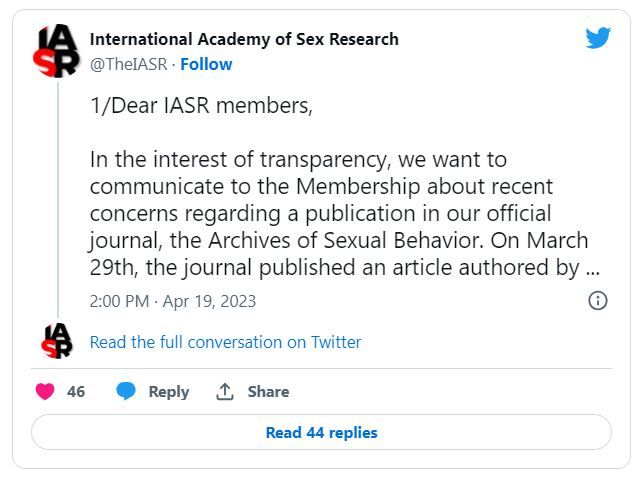
The episode prompted a May 5 “Open Letter in Support of Dr. Kenneth Zucker and the Need to Promote Robust Scientific Debate” from the Foundation Against Intolerance and Racism that has now been signed by nearly 2000 people.
On May 10, the following publisher’s note was added to the article:
“readers are alerted that concerns have been raised regarding methodology as described in this article. The publisher is currently investigating this matter and a further response will follow the conclusion of this investigation.
Six days later, the publisher removed the article’s supplementary information “due to a lack of documented consent by study participants.”
The story may feel familiar to readers who recall what happened to another paper in 2018. In that paper, Brown University’s Lisa Littman coined the term ROGD. Following a backlash, Brown took down a press release touting the results, and the paper was eventually republished with corrections.
Bailey has been accused of mistreating transgender research participants, but an investigation by bioethicist Alice Dreger found that of the many accusations, “almost none appear to have been legitimate.”
In a post on UnHerd earlier this month, Bailey responded to the reported concerns about the study lacking approval by an Institutional Review Board (IRB), and that the way the participants were recruited biased the results.
IRB approval was not necessary, Bailey wrote, because Suzanna Diaz, the first author who collected the data, was not affiliated with an institution that required it. “Suzanna Diaz” is a pseudonym for “the mother of a gender dysphoric child she believes has ROGD” who wishes to remain anonymous for the sake of her family, Bailey wrote.
The paper included the following statement about its ethical approval:
“The first author and creator of the survey is not affiliated with any university or hospital. Thus, she did not seek approval from an IRB. After seeing a presentation of preliminary survey results by the first author, the second author suggested the data to be analyzed and submitted as an academic article (he was not involved in collecting the data). The second author consulted with his university’s IRB, who declined to certify the study because data were already collected. However, they advised that publishing the results was likely ethical provided data were deidentified. Editor’s note: After I reviewed the manuscript, I concluded that its publication is ethically appropriate, consistent with Springer policy.”
In his UnHerd post, Bailey quoted from the journal’s submission guidelines:
“If a study has not been granted ethics committee approval prior to commencing, retrospective ethics approval usually cannot be obtained and it may not be possible to consider the manuscript for peer review. The decision on whether to proceed to peer review in such cases is at the Editor’s discretion.”
“Regarding the methodological limitations of the study, these were addressed forthrightly and thoroughly in our article,” Bailey wrote.
Adam Marcus, a cofounder of Retraction Watch, is an editor at this news organization.
A version of this article first appeared on RetractionWatch.com.
The move is “due to concerns about lack of informed consent,” according to tweets by one of the paper’s authors.
The article, “Rapid Onset Gender Dysphoria: Parent Reports on 1655 Possible Cases,” was published in March in the Archives of Sexual Behavior. It has not been cited in the scientific literature, according to Clarivate’s Web of Science, but Altmetric, which tracks the online attention papers receive, ranks the article in the top 1% of all articles of a similar age.
Rapid Onset Gender Dysphoria (ROGD) is, the article stated, a “controversial theory” that “common cultural beliefs, values, and preoccupations cause some adolescents (especially female adolescents) to attribute their social problems, feelings, and mental health issues to gender dysphoria,” and that “youth with ROGD falsely believe that they are transgender,” in part due to social influences.
Michael Bailey, a psychology professor at Northwestern University in Evanston, Ill., and the paper’s corresponding author, tweeted:
Bailey told Retraction Watch that he would “respond when [he] can” to our request for comment, following “new developments on our end.” Neither Springer Nature nor Kenneth Zucker, editor in chief of Archives of Sexual Behavior, has responded to similar requests.
The paper reported the results of a survey of parents who contacted the website ParentsofROGDKids.com, with which the first author is affiliated. According to the abstract, the authors found:
“Pre-existing mental health issues were common, and youths with these issues were more likely than those without them to have socially and medically transitioned. Parents reported that they had often felt pressured by clinicians to affirm their AYA [adolescent and young adult] child’s new gender and support their transition. According to the parents, AYA children’s mental health deteriorated considerably after social transition.”
Soon after publication, the paper attracted criticism that its method of gathering study participants was biased, and that the authors ignored information that didn’t support the theory of ROGD.
Archives of Sexual Behavior is the official publication of the International Academy of Sex Research, which tweeted on April 19:
The episode prompted a May 5 “Open Letter in Support of Dr. Kenneth Zucker and the Need to Promote Robust Scientific Debate” from the Foundation Against Intolerance and Racism that has now been signed by nearly 2000 people.
On May 10, the following publisher’s note was added to the article:
“readers are alerted that concerns have been raised regarding methodology as described in this article. The publisher is currently investigating this matter and a further response will follow the conclusion of this investigation.
Six days later, the publisher removed the article’s supplementary information “due to a lack of documented consent by study participants.”
The story may feel familiar to readers who recall what happened to another paper in 2018. In that paper, Brown University’s Lisa Littman coined the term ROGD. Following a backlash, Brown took down a press release touting the results, and the paper was eventually republished with corrections.
Bailey has been accused of mistreating transgender research participants, but an investigation by bioethicist Alice Dreger found that of the many accusations, “almost none appear to have been legitimate.”
In a post on UnHerd earlier this month, Bailey responded to the reported concerns about the study lacking approval by an Institutional Review Board (IRB), and that the way the participants were recruited biased the results.
IRB approval was not necessary, Bailey wrote, because Suzanna Diaz, the first author who collected the data, was not affiliated with an institution that required it. “Suzanna Diaz” is a pseudonym for “the mother of a gender dysphoric child she believes has ROGD” who wishes to remain anonymous for the sake of her family, Bailey wrote.
The paper included the following statement about its ethical approval:
“The first author and creator of the survey is not affiliated with any university or hospital. Thus, she did not seek approval from an IRB. After seeing a presentation of preliminary survey results by the first author, the second author suggested the data to be analyzed and submitted as an academic article (he was not involved in collecting the data). The second author consulted with his university’s IRB, who declined to certify the study because data were already collected. However, they advised that publishing the results was likely ethical provided data were deidentified. Editor’s note: After I reviewed the manuscript, I concluded that its publication is ethically appropriate, consistent with Springer policy.”
In his UnHerd post, Bailey quoted from the journal’s submission guidelines:
“If a study has not been granted ethics committee approval prior to commencing, retrospective ethics approval usually cannot be obtained and it may not be possible to consider the manuscript for peer review. The decision on whether to proceed to peer review in such cases is at the Editor’s discretion.”
“Regarding the methodological limitations of the study, these were addressed forthrightly and thoroughly in our article,” Bailey wrote.
Adam Marcus, a cofounder of Retraction Watch, is an editor at this news organization.
A version of this article first appeared on RetractionWatch.com.
The move is “due to concerns about lack of informed consent,” according to tweets by one of the paper’s authors.
The article, “Rapid Onset Gender Dysphoria: Parent Reports on 1655 Possible Cases,” was published in March in the Archives of Sexual Behavior. It has not been cited in the scientific literature, according to Clarivate’s Web of Science, but Altmetric, which tracks the online attention papers receive, ranks the article in the top 1% of all articles of a similar age.
Rapid Onset Gender Dysphoria (ROGD) is, the article stated, a “controversial theory” that “common cultural beliefs, values, and preoccupations cause some adolescents (especially female adolescents) to attribute their social problems, feelings, and mental health issues to gender dysphoria,” and that “youth with ROGD falsely believe that they are transgender,” in part due to social influences.
Michael Bailey, a psychology professor at Northwestern University in Evanston, Ill., and the paper’s corresponding author, tweeted:
Bailey told Retraction Watch that he would “respond when [he] can” to our request for comment, following “new developments on our end.” Neither Springer Nature nor Kenneth Zucker, editor in chief of Archives of Sexual Behavior, has responded to similar requests.
The paper reported the results of a survey of parents who contacted the website ParentsofROGDKids.com, with which the first author is affiliated. According to the abstract, the authors found:
“Pre-existing mental health issues were common, and youths with these issues were more likely than those without them to have socially and medically transitioned. Parents reported that they had often felt pressured by clinicians to affirm their AYA [adolescent and young adult] child’s new gender and support their transition. According to the parents, AYA children’s mental health deteriorated considerably after social transition.”
Soon after publication, the paper attracted criticism that its method of gathering study participants was biased, and that the authors ignored information that didn’t support the theory of ROGD.
Archives of Sexual Behavior is the official publication of the International Academy of Sex Research, which tweeted on April 19:
The episode prompted a May 5 “Open Letter in Support of Dr. Kenneth Zucker and the Need to Promote Robust Scientific Debate” from the Foundation Against Intolerance and Racism that has now been signed by nearly 2000 people.
On May 10, the following publisher’s note was added to the article:
“readers are alerted that concerns have been raised regarding methodology as described in this article. The publisher is currently investigating this matter and a further response will follow the conclusion of this investigation.
Six days later, the publisher removed the article’s supplementary information “due to a lack of documented consent by study participants.”
The story may feel familiar to readers who recall what happened to another paper in 2018. In that paper, Brown University’s Lisa Littman coined the term ROGD. Following a backlash, Brown took down a press release touting the results, and the paper was eventually republished with corrections.
Bailey has been accused of mistreating transgender research participants, but an investigation by bioethicist Alice Dreger found that of the many accusations, “almost none appear to have been legitimate.”
In a post on UnHerd earlier this month, Bailey responded to the reported concerns about the study lacking approval by an Institutional Review Board (IRB), and that the way the participants were recruited biased the results.
IRB approval was not necessary, Bailey wrote, because Suzanna Diaz, the first author who collected the data, was not affiliated with an institution that required it. “Suzanna Diaz” is a pseudonym for “the mother of a gender dysphoric child she believes has ROGD” who wishes to remain anonymous for the sake of her family, Bailey wrote.
The paper included the following statement about its ethical approval:
“The first author and creator of the survey is not affiliated with any university or hospital. Thus, she did not seek approval from an IRB. After seeing a presentation of preliminary survey results by the first author, the second author suggested the data to be analyzed and submitted as an academic article (he was not involved in collecting the data). The second author consulted with his university’s IRB, who declined to certify the study because data were already collected. However, they advised that publishing the results was likely ethical provided data were deidentified. Editor’s note: After I reviewed the manuscript, I concluded that its publication is ethically appropriate, consistent with Springer policy.”
In his UnHerd post, Bailey quoted from the journal’s submission guidelines:
“If a study has not been granted ethics committee approval prior to commencing, retrospective ethics approval usually cannot be obtained and it may not be possible to consider the manuscript for peer review. The decision on whether to proceed to peer review in such cases is at the Editor’s discretion.”
“Regarding the methodological limitations of the study, these were addressed forthrightly and thoroughly in our article,” Bailey wrote.
Adam Marcus, a cofounder of Retraction Watch, is an editor at this news organization.
A version of this article first appeared on RetractionWatch.com.
Wildfire smoke and air quality: How long could health effects last?
People with moderate to severe asthma, chronic obstructive pulmonary disease, and other risk factors are used to checking air quality warnings before heading outside. But this situation is anything but typical.
Even people not normally at risk can have burning eyes, a runny nose, and a hard time breathing. These are among the symptoms to watch for as health effects of wildfire smoke. Special considerations should be made for people with heart disease, lung disease, and other conditions that put them at increased risk. Those affected can also have trouble sleeping, anxiety, and ongoing mental health issues.
The smoke will stick around the next few days, possibly clearing out early next week when the winds change direction, Weather Channel meteorologist Ari Sarsalari predicted June 8. But that doesn’t mean any physical or mental health effects will clear up as quickly.
“We are seeing dramatic increases in air pollution, and we are seeing increases in patients coming to the ED and the hospital. We expect that this will increase in the days ahead,” said Meredith McCormack, MD, MHS, a volunteer medical spokesperson for the American Lung Association.
“The air quality in our area – Baltimore – and other surrounding areas is not healthy for anyone,” said Dr. McCormack, who specializes in pulmonary and critical care medicine at Johns Hopkins University, Baltimore.
How serious are the health warnings?
Residents of California might be more familiar with the hazards of wildfire smoke, but this is a novel experience for many people along the East Coast. Air quality advisories are popping up on cellphones for people living in Boston, New York, and as far south as Northern Virginia. What should the estimated 75 million to 128 million affected Americans do?
We asked experts to weigh in on when it’s safe or not safe to spend time outside, when to seek medical help, and the best ways for people to protect themselves.
“It’s important to stay indoors and close all windows to reduce exposure to smoke from wildfires. It’s also essential to stay away from any windows that may not have a good seal, in order to minimize any potential exposure to smoke,” said Robert Glatter, MD, editor at large for Medscape Emergency Medicine and an emergency medicine doctor at Lenox Hill Hospital/Northwell Health in New York.
Dr. Glatter noted that placing moist towels under doors and sealing leaking windows can help.
Monitor your symptoms, and contact your doctor or go to urgent care, Dr. McCormack advised, if you see any increase in concerning symptoms. These include shortness of breath, coughing, chest tightness, or wheezing. Also make sure you take recommended medications and have enough on hand, she said.
Fine particles, big concerns
The weather is warming in many parts of the country, and that can mean air conditioning. Adding a MERV 13 filter to a central air conditioning system could reduce exposure to wildfire smoke. Using a portable indoor air purifier with a HEPA filter also can help people without central air conditioning. The filter can help remove small particles in the air but must be replaced regularly.
Smoke from wildfires contains multiple toxins, including heavy metals, carcinogens, and fine particulate matter (PM) under 2.5 microns. Dr. Glatter explained that these particles are about 100 times thinner than a human hair. Because of their size, they can embed deeper into the airways in the lungs and trigger chronic inflammation.
“This has also been linked to increased rates of lung cancer and brain tumors,” he said, based on a 2022 study in Canada.
The effects of smoke from wildfires can continue for many years. After the 2014 Hazelwood coal mine fire, emergency department visits for respiratory conditions and cardiovascular complaints remained higher for up to 2-5 years later, Dr. Glatter said. Again, large quantities of fine particulate matter in the smoke, less than 2.5 microns (PM 2.5), was to blame.
Exposure to smoke from wildfires during pregnancy has also been linked to abnormal fetal growth, preterm birth, as well as low birth weight, a January 2023 preprint on MedRxiv suggested.
Time to wear a mask again?
A properly fitted N95 mask will be the best approach to lessen exposure to smoke from wildfires, “but by itself cannot eliminate all of the risk,” Dr. Glatter said. Surgical masks can add minimal protection, and cloth masks will not provide any significant protection against the damaging effects of smoke from wildfires.
KN95 masks tend to be more comfortable to wear than N95s. But leakage often occurs that can make this type of protection less effective, Dr. Glatter said.
“Masks are important if you need to go outdoors,” Dr. McCormack said. Also, if you’re traveling by car, set the air conditioning system to recirculate to filter the air inside the vehicle, she recommended.
What does that number mean?
The federal government monitors air quality nationwide. In case you’re unfamiliar, the U.S. Air Quality Index includes a color-coded scale for ozone levels and particle pollution, the main concern from wildfire smoke. The lowest risk is the Green or satisfactory air quality category, where air pollution poses little or no risk, with an Index number from 0 to 50.
The index gets progressively more serious, from Yellow for moderate risk (51-100) up to a Maroon category, a hazardous range of 300 or higher on the index. When a Maroon advisory is issued, it means an emergency health warning where “everyone is more likely to be affected.”
How do you know if your outside air is polluted? Your local Air Quality Index (AQI) from the EPA can help. It’s a scale of 0 to 500, and the greater the number, the more harmful pollution in the air. It has six levels: good, moderate, unhealthy for sensitive groups, unhealthy, very unhealthy, and hazardous. You can find it at AirNow.gov.
New York is under an air quality alert until midnight Friday with a current “unhealthy” Index report of 200. The city recorded its worst-ever air quality on Wednesday. The New York State Department of Environmental Conservation warns that fine particulate levels – small particles that can enter a person’s lungs – are the biggest concern.
AirNow.gov warns that western New England down to Washington has air quality in the three worst categories – ranging from unhealthy to very unhealthy and hazardous. The ten worst locations on the U.S. Air Quality Index as of 10 a.m. ET on June 8 include the Wilmington, Del., area with an Index of 241, or “very unhealthy.”
Other “very unhealthy” locations have the following Index readings:
- 244: Suburban Washington/Maryland.
- 252: Southern coastal New Jersey.
- 252: Kent County, Del.
- 270: Philadelphia.
- 291: Greater New Castle County, Del.
- 293: Northern Virginia.
- 293: Metropolitan Washington.
These two locations are in the “hazardous” or health emergency warning category:
- 309: Lehigh Valley, Pa.
- 399: Susquehanna Valley, Pa.
To check an air quality advisory in your area, enter your ZIP code at AirNow.gov.
A version of this article first appeared on WebMD.com.
People with moderate to severe asthma, chronic obstructive pulmonary disease, and other risk factors are used to checking air quality warnings before heading outside. But this situation is anything but typical.
Even people not normally at risk can have burning eyes, a runny nose, and a hard time breathing. These are among the symptoms to watch for as health effects of wildfire smoke. Special considerations should be made for people with heart disease, lung disease, and other conditions that put them at increased risk. Those affected can also have trouble sleeping, anxiety, and ongoing mental health issues.
The smoke will stick around the next few days, possibly clearing out early next week when the winds change direction, Weather Channel meteorologist Ari Sarsalari predicted June 8. But that doesn’t mean any physical or mental health effects will clear up as quickly.
“We are seeing dramatic increases in air pollution, and we are seeing increases in patients coming to the ED and the hospital. We expect that this will increase in the days ahead,” said Meredith McCormack, MD, MHS, a volunteer medical spokesperson for the American Lung Association.
“The air quality in our area – Baltimore – and other surrounding areas is not healthy for anyone,” said Dr. McCormack, who specializes in pulmonary and critical care medicine at Johns Hopkins University, Baltimore.
How serious are the health warnings?
Residents of California might be more familiar with the hazards of wildfire smoke, but this is a novel experience for many people along the East Coast. Air quality advisories are popping up on cellphones for people living in Boston, New York, and as far south as Northern Virginia. What should the estimated 75 million to 128 million affected Americans do?
We asked experts to weigh in on when it’s safe or not safe to spend time outside, when to seek medical help, and the best ways for people to protect themselves.
“It’s important to stay indoors and close all windows to reduce exposure to smoke from wildfires. It’s also essential to stay away from any windows that may not have a good seal, in order to minimize any potential exposure to smoke,” said Robert Glatter, MD, editor at large for Medscape Emergency Medicine and an emergency medicine doctor at Lenox Hill Hospital/Northwell Health in New York.
Dr. Glatter noted that placing moist towels under doors and sealing leaking windows can help.
Monitor your symptoms, and contact your doctor or go to urgent care, Dr. McCormack advised, if you see any increase in concerning symptoms. These include shortness of breath, coughing, chest tightness, or wheezing. Also make sure you take recommended medications and have enough on hand, she said.
Fine particles, big concerns
The weather is warming in many parts of the country, and that can mean air conditioning. Adding a MERV 13 filter to a central air conditioning system could reduce exposure to wildfire smoke. Using a portable indoor air purifier with a HEPA filter also can help people without central air conditioning. The filter can help remove small particles in the air but must be replaced regularly.
Smoke from wildfires contains multiple toxins, including heavy metals, carcinogens, and fine particulate matter (PM) under 2.5 microns. Dr. Glatter explained that these particles are about 100 times thinner than a human hair. Because of their size, they can embed deeper into the airways in the lungs and trigger chronic inflammation.
“This has also been linked to increased rates of lung cancer and brain tumors,” he said, based on a 2022 study in Canada.
The effects of smoke from wildfires can continue for many years. After the 2014 Hazelwood coal mine fire, emergency department visits for respiratory conditions and cardiovascular complaints remained higher for up to 2-5 years later, Dr. Glatter said. Again, large quantities of fine particulate matter in the smoke, less than 2.5 microns (PM 2.5), was to blame.
Exposure to smoke from wildfires during pregnancy has also been linked to abnormal fetal growth, preterm birth, as well as low birth weight, a January 2023 preprint on MedRxiv suggested.
Time to wear a mask again?
A properly fitted N95 mask will be the best approach to lessen exposure to smoke from wildfires, “but by itself cannot eliminate all of the risk,” Dr. Glatter said. Surgical masks can add minimal protection, and cloth masks will not provide any significant protection against the damaging effects of smoke from wildfires.
KN95 masks tend to be more comfortable to wear than N95s. But leakage often occurs that can make this type of protection less effective, Dr. Glatter said.
“Masks are important if you need to go outdoors,” Dr. McCormack said. Also, if you’re traveling by car, set the air conditioning system to recirculate to filter the air inside the vehicle, she recommended.
What does that number mean?
The federal government monitors air quality nationwide. In case you’re unfamiliar, the U.S. Air Quality Index includes a color-coded scale for ozone levels and particle pollution, the main concern from wildfire smoke. The lowest risk is the Green or satisfactory air quality category, where air pollution poses little or no risk, with an Index number from 0 to 50.
The index gets progressively more serious, from Yellow for moderate risk (51-100) up to a Maroon category, a hazardous range of 300 or higher on the index. When a Maroon advisory is issued, it means an emergency health warning where “everyone is more likely to be affected.”
How do you know if your outside air is polluted? Your local Air Quality Index (AQI) from the EPA can help. It’s a scale of 0 to 500, and the greater the number, the more harmful pollution in the air. It has six levels: good, moderate, unhealthy for sensitive groups, unhealthy, very unhealthy, and hazardous. You can find it at AirNow.gov.
New York is under an air quality alert until midnight Friday with a current “unhealthy” Index report of 200. The city recorded its worst-ever air quality on Wednesday. The New York State Department of Environmental Conservation warns that fine particulate levels – small particles that can enter a person’s lungs – are the biggest concern.
AirNow.gov warns that western New England down to Washington has air quality in the three worst categories – ranging from unhealthy to very unhealthy and hazardous. The ten worst locations on the U.S. Air Quality Index as of 10 a.m. ET on June 8 include the Wilmington, Del., area with an Index of 241, or “very unhealthy.”
Other “very unhealthy” locations have the following Index readings:
- 244: Suburban Washington/Maryland.
- 252: Southern coastal New Jersey.
- 252: Kent County, Del.
- 270: Philadelphia.
- 291: Greater New Castle County, Del.
- 293: Northern Virginia.
- 293: Metropolitan Washington.
These two locations are in the “hazardous” or health emergency warning category:
- 309: Lehigh Valley, Pa.
- 399: Susquehanna Valley, Pa.
To check an air quality advisory in your area, enter your ZIP code at AirNow.gov.
A version of this article first appeared on WebMD.com.
People with moderate to severe asthma, chronic obstructive pulmonary disease, and other risk factors are used to checking air quality warnings before heading outside. But this situation is anything but typical.
Even people not normally at risk can have burning eyes, a runny nose, and a hard time breathing. These are among the symptoms to watch for as health effects of wildfire smoke. Special considerations should be made for people with heart disease, lung disease, and other conditions that put them at increased risk. Those affected can also have trouble sleeping, anxiety, and ongoing mental health issues.
The smoke will stick around the next few days, possibly clearing out early next week when the winds change direction, Weather Channel meteorologist Ari Sarsalari predicted June 8. But that doesn’t mean any physical or mental health effects will clear up as quickly.
“We are seeing dramatic increases in air pollution, and we are seeing increases in patients coming to the ED and the hospital. We expect that this will increase in the days ahead,” said Meredith McCormack, MD, MHS, a volunteer medical spokesperson for the American Lung Association.
“The air quality in our area – Baltimore – and other surrounding areas is not healthy for anyone,” said Dr. McCormack, who specializes in pulmonary and critical care medicine at Johns Hopkins University, Baltimore.
How serious are the health warnings?
Residents of California might be more familiar with the hazards of wildfire smoke, but this is a novel experience for many people along the East Coast. Air quality advisories are popping up on cellphones for people living in Boston, New York, and as far south as Northern Virginia. What should the estimated 75 million to 128 million affected Americans do?
We asked experts to weigh in on when it’s safe or not safe to spend time outside, when to seek medical help, and the best ways for people to protect themselves.
“It’s important to stay indoors and close all windows to reduce exposure to smoke from wildfires. It’s also essential to stay away from any windows that may not have a good seal, in order to minimize any potential exposure to smoke,” said Robert Glatter, MD, editor at large for Medscape Emergency Medicine and an emergency medicine doctor at Lenox Hill Hospital/Northwell Health in New York.
Dr. Glatter noted that placing moist towels under doors and sealing leaking windows can help.
Monitor your symptoms, and contact your doctor or go to urgent care, Dr. McCormack advised, if you see any increase in concerning symptoms. These include shortness of breath, coughing, chest tightness, or wheezing. Also make sure you take recommended medications and have enough on hand, she said.
Fine particles, big concerns
The weather is warming in many parts of the country, and that can mean air conditioning. Adding a MERV 13 filter to a central air conditioning system could reduce exposure to wildfire smoke. Using a portable indoor air purifier with a HEPA filter also can help people without central air conditioning. The filter can help remove small particles in the air but must be replaced regularly.
Smoke from wildfires contains multiple toxins, including heavy metals, carcinogens, and fine particulate matter (PM) under 2.5 microns. Dr. Glatter explained that these particles are about 100 times thinner than a human hair. Because of their size, they can embed deeper into the airways in the lungs and trigger chronic inflammation.
“This has also been linked to increased rates of lung cancer and brain tumors,” he said, based on a 2022 study in Canada.
The effects of smoke from wildfires can continue for many years. After the 2014 Hazelwood coal mine fire, emergency department visits for respiratory conditions and cardiovascular complaints remained higher for up to 2-5 years later, Dr. Glatter said. Again, large quantities of fine particulate matter in the smoke, less than 2.5 microns (PM 2.5), was to blame.
Exposure to smoke from wildfires during pregnancy has also been linked to abnormal fetal growth, preterm birth, as well as low birth weight, a January 2023 preprint on MedRxiv suggested.
Time to wear a mask again?
A properly fitted N95 mask will be the best approach to lessen exposure to smoke from wildfires, “but by itself cannot eliminate all of the risk,” Dr. Glatter said. Surgical masks can add minimal protection, and cloth masks will not provide any significant protection against the damaging effects of smoke from wildfires.
KN95 masks tend to be more comfortable to wear than N95s. But leakage often occurs that can make this type of protection less effective, Dr. Glatter said.
“Masks are important if you need to go outdoors,” Dr. McCormack said. Also, if you’re traveling by car, set the air conditioning system to recirculate to filter the air inside the vehicle, she recommended.
What does that number mean?
The federal government monitors air quality nationwide. In case you’re unfamiliar, the U.S. Air Quality Index includes a color-coded scale for ozone levels and particle pollution, the main concern from wildfire smoke. The lowest risk is the Green or satisfactory air quality category, where air pollution poses little or no risk, with an Index number from 0 to 50.
The index gets progressively more serious, from Yellow for moderate risk (51-100) up to a Maroon category, a hazardous range of 300 or higher on the index. When a Maroon advisory is issued, it means an emergency health warning where “everyone is more likely to be affected.”
How do you know if your outside air is polluted? Your local Air Quality Index (AQI) from the EPA can help. It’s a scale of 0 to 500, and the greater the number, the more harmful pollution in the air. It has six levels: good, moderate, unhealthy for sensitive groups, unhealthy, very unhealthy, and hazardous. You can find it at AirNow.gov.
New York is under an air quality alert until midnight Friday with a current “unhealthy” Index report of 200. The city recorded its worst-ever air quality on Wednesday. The New York State Department of Environmental Conservation warns that fine particulate levels – small particles that can enter a person’s lungs – are the biggest concern.
AirNow.gov warns that western New England down to Washington has air quality in the three worst categories – ranging from unhealthy to very unhealthy and hazardous. The ten worst locations on the U.S. Air Quality Index as of 10 a.m. ET on June 8 include the Wilmington, Del., area with an Index of 241, or “very unhealthy.”
Other “very unhealthy” locations have the following Index readings:
- 244: Suburban Washington/Maryland.
- 252: Southern coastal New Jersey.
- 252: Kent County, Del.
- 270: Philadelphia.
- 291: Greater New Castle County, Del.
- 293: Northern Virginia.
- 293: Metropolitan Washington.
These two locations are in the “hazardous” or health emergency warning category:
- 309: Lehigh Valley, Pa.
- 399: Susquehanna Valley, Pa.
To check an air quality advisory in your area, enter your ZIP code at AirNow.gov.
A version of this article first appeared on WebMD.com.
Is ChatGPT a friend or foe of medical publishing?
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
. These tools should not be listed as authors, and researchers must denote how AI-assisted technologies were used, the committee said.
These new guidelines are the latest effort for medical journals to define policies for using these large-scale language models (LLMs) in scientific publication. While these AI-assisted tools can help with tasks such as writing, analyzing data, catching mistakes, and much more, they are also prone to errors, noted Casey Greene, PhD, a professor of biomedical informatics at the University of Colorado at Denver, Aurora. It is also not totally clear how information is stored and processed in these kinds of tools, and who has access to that information, he noted.
At the same time, experts argue that these AI tools could have a positive impact on the field by limiting some of the linguistic disparities in scientific publishing as well as alleviating the burden of some monotonous or mechanical tasks that come along with manuscript writing.
What experts can agree on, though, is that the use of AI tools is here to stay. “This is going to become a common tool,” Dr. Greene said. “I don’t think there’s a way out of that at this point.”
A change in medical publishing
OpenAI released ChatGPT in November 2022. In its own words, ChatGPT is “a deep learning model that has been trained on a massive amount of text data to understand and generate humanlike text.” Enter a question or a prompt, and it will respond. For example, when asked how the AI tool can be used in scientific publishing, ChatGPT responded:
“ChatGPT can aid scientific publishing by generating ideas, clarifying concepts, conducting preliminary literature reviews, providing proofreading and editing suggestions, and assisting with outlining and organizing scientific papers. However, it should be used as a supportive tool alongside domain expertise and validation from trusted scientific sources.”
Just a few months after ChatGPT became available, researchers began using this tool in their own work. One individual, Som Biswas, MD, a radiologist at the University of Tennessee Health Science Center in Memphis, reportedly used ChatGPT to author 16 scientific articles in just 4 months, according to the Daily Beast. Five of these articles have been published in four different journals. Dr. Biswas declined to be interviewed for this article.
There were also reports of papers with ChatGPT as one of the listed authors, which sparked backlash. In response, JAMA, Nature, and Science all published editorials in January outlining their policies for using ChatGPT and other large language models in the scientific authoring process. Editors from the journals of the American College of Cardiology and the American College of Rheumatology also updated their policies to reflect the influence of AI authoring tools.
The consensus is that AI has no place on the author byline.
“We think that’s not appropriate, because coauthorship means that you are taking responsibility for the analysis and the generation of data that are included in a manuscript. A machine that is dictated by AI can’t take responsibility,” said Daniel Solomon, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, and the editor in chief of the ACR journal Arthritis & Rheumatology.
Issues with AI
One of the big concerns around using AI in writing is that it can generate text that seems plausible but is untrue or not supported by data. For example, Dr. Greene and colleague Milton Pividori, PhD, also of the University of Colorado, were writing a journal article about new software they developed that uses a large language model to revise scientific manuscripts.
“We used the same software to revise that article and at one point, it added a line that noted that the large language model had been fine-tuned on a data set of manuscripts from within the same field. This makes a lot of sense, and is absolutely something you could do, but was not something that we did,” Dr. Greene said. “Without a really careful review of the content, it becomes possible to invent things that were not actually done.”
In another case, ChatGPT falsely stated that a prominent law professor had been accused of sexual assault, citing a Washington Post article that did not exist.
“We live in a society where we are extremely concerned about fake news,” Dr. Pividori added, “and [these kinds of errors] could certainly exacerbate that in the scientific community, which is very concerning because science informs public policy.”
Another issue is the lack of transparency around how large language models like ChatGPT process and store data used to make queries.
“We have no idea how they are recording all the prompts and things that we input into ChatGPT and their systems,” Dr. Pividori said.
OpenAI recently addressed some privacy concerns by allowing users to turn off their chat history with the AI chatbot, so conversations cannot be used to train or improve the company’s models. But Dr. Greene noted that the terms of service “still remain pretty nebulous.”
Dr. Solomon is also concerned with researchers using these AI tools in authoring without knowing how they work. “The thing we are really concerned about is that fact that [LLMs] are a bit of a black box – people don’t really understand the methodologies,” he said.
A positive tool?
But despite these concerns, many think that these types of AI-assisted tools could have a positive impact on medical publishing, particularly for researchers for whom English is not their first language, noted Catherine Gao, MD, a pulmonary and critical care instructor at Northwestern University, Chicago. She recently led research comparing scientific abstracts written by ChatGPT and real abstracts and discovered that reviewers found it “surprisingly difficult” to differentiate the two.
“The majority of research is published in English,” she said in an email. “Responsible use of LLMs can potentially reduce the burden of writing for busy scientists and improve equity for those who are not native English speakers.”
Dr. Pividori agreed, adding that as a non-native English speaker, he spends much more time working on the structure and grammar of sentences when authoring a manuscript, compared with people who speak English as a first language. He noted that these tools can also be used to automate some of the more monotonous tasks that come along with writing manuscripts and allow researchers to focus on the more creative aspects.
In the future, “I want to focus more on the things that only a human can do and let these tools do all the rest of it,” he said.
New rules
But despite how individual researchers feel about LLMs, they agree that these AI tools are here to stay.
“I think that we should anticipate that they will become part of the medical research establishment over time, when we figure out how to use them appropriately,” Dr. Solomon said.
While the debate of how to best use AI in medical publications will continue, journal editors agree that all authors of a manuscript are solely responsible for content in articles that used AI-assisted technology.
“Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased,” the ICMJE guidelines state. “Authors should be able to assert that there is no plagiarism in their paper, including in text and images produced by the AI.” This includes appropriate attribution of all cited materials.
The committee also recommends that authors write in both the cover letter and submitted work how AI was used in the manuscript writing process. Recently updated guidelines from the World Association of Medical Editors recommend that all prompts used to generate new text or analytical work should be provided in submitted work. Dr. Greene also noted that if authors used an AI tool to revise their work, they can include a version of the manuscript untouched by LLMs.
It is similar to a preprint, he said, but rather than publishing a version of a paper prior to peer review, someone is showing a version of a manuscript before it was reviewed and revised by AI. “This type of practice could be a path that lets us benefit from these models,” he said, “without having the drawbacks that many are concerned about.”
Dr. Solomon has financial relationships with AbbVie, Amgen, Janssen, CorEvitas, and Moderna. Both Dr. Greene and Dr. Pividori are inventors in the U.S. Provisional Patent Application No. 63/486,706 that the University of Colorado has filed for the “Publishing Infrastructure For AI-Assisted Academic Authoring” invention with the U.S. Patent and Trademark Office. Dr. Greene and Dr. Pividori also received a grant from the Alfred P. Sloan Foundation to improve their AI-based manuscript revision tool. Dr. Gao reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Risk of falls seen with newer antiandrogens for prostate cancer
Second-generation antiandrogens (AAs) – abiraterone, apalutamide, darolutamide, and enzalutamide – are a cornerstone of modern prostate cancer treatment, improving outcomes and survival.
However, they carry a significant caveat, according to a new meta-analysis of 12 clinical trials with over 13,000 patients.
the authors reported.
These findings carry “important public health indications” because use of second-generation AAs, currently first-line treatment for advanced and castration-resistant prostate cancer, is expanding with new indications, meaning that the pool of men at risk for such problems is large and growing, the team wrote.
The take-home message is that the findings give men – and the physicians who counsel them – a fuller idea of what to expect when considering using the agents, the researchers comment. This information is key at a time when so much of prostate cancer treatment involves carefully weighing the risks and benefits, they added.
The study was published in JAMA Oncology. It was conducted by a team of researchers from the University of Texas MD Anderson Cancer Center, Houston, and was led by Malgorzata Nowakowska, a medical student at Baylor College of Medicine, Houston.
Two prostate cancer specialists agreed and gave an example to bring the point home in an accompanying editorial.
The risk-benefit ratio of adding a second-generation AA to treatment may be different for a patient who wants to stay alert and sharp to keep a complex job “versus someone whose primary goal is to see their young children graduate high school,” Alexandra Sokolova, MD, of the Oregon Health and Science University, Portland, and Julie Graff, MD, of the VA Portland (Ore.) Health Care System, wrote in their editorial.
The study fills a “critical gap” when it comes to counseling men about the drugs and will help guide discussions, they said.
The investigators said their study also highlights the need for additional research to identify who is most at risk for the side effects and the best way to prevent and treat them. “Interventions currently under investigation include donepezil, methylphenidate, low-fat diet, acupuncture, martial arts, and high-intensity exercise, among many others,” Ms. Nowakowska and colleagues noted.
Study details
The 12 trials in the meta-analysis, which compared second-generation AAs with placebo, were conducted from 2008 to 2021. These trials were multinational investigations that included patients with metastatic disease as well as those with nonmetastatic disease. The median age across the studies ranged from 67 to 74 years, and trial follow-up ranged from 3.9 to 48 months.
The rates of adverse cognitive effects and attention disorders and disturbances ranged from 2% to 8% among patients who received second-generation AAs versus 2%-3% among those who received placebo, a more than doubling of the risk of cognitive toxic effects (P = .002).
Fatigue of any grade was reported in 5%-45% of participants taking second-generation AAs versus 2%-42% of patients taking placebos, which translates to a 34% higher risk (P < .001).
The use of AAs was associated with an 87% increase in the risk of falls in comparison with placebo, regardless of severity. For falls of grade 3 or higher that required hospitalization or invasive treatment, the increase in risk with second-generation AAs was 72% (P = .05).
The findings were consistent for cognitive toxicity and fatigue in studies that included traditional hormone therapy in both the treatment and control arms. Increased age was associated with a greater risk of fatigue.
Study limits include the fact that it was not known how long patients were taking the drugs before they encountered problems. In addition, the findings were not broken down with respect to medication, so it’s unknown whether such problems are worse with some second-generation AAs than with others.
The editorialists noted that real-world patients tend to be older and sicker than patients in trials, so the risk of falls, fatigue, and cognition problems might be higher among everyday patients.
The study was funded by the National Institutes of Health and others. The investigators disclosed no relevant financial relationships. Dr. Sokolova has received personal fees from Lantheus and travel grants from AstraZeneca. Dr. Graff has received nonfinancial support from Janssen, Pfizer/Astellas, and Sanofi.
A version of this article first appeared on Medscape.com.
Second-generation antiandrogens (AAs) – abiraterone, apalutamide, darolutamide, and enzalutamide – are a cornerstone of modern prostate cancer treatment, improving outcomes and survival.
However, they carry a significant caveat, according to a new meta-analysis of 12 clinical trials with over 13,000 patients.
the authors reported.
These findings carry “important public health indications” because use of second-generation AAs, currently first-line treatment for advanced and castration-resistant prostate cancer, is expanding with new indications, meaning that the pool of men at risk for such problems is large and growing, the team wrote.
The take-home message is that the findings give men – and the physicians who counsel them – a fuller idea of what to expect when considering using the agents, the researchers comment. This information is key at a time when so much of prostate cancer treatment involves carefully weighing the risks and benefits, they added.
The study was published in JAMA Oncology. It was conducted by a team of researchers from the University of Texas MD Anderson Cancer Center, Houston, and was led by Malgorzata Nowakowska, a medical student at Baylor College of Medicine, Houston.
Two prostate cancer specialists agreed and gave an example to bring the point home in an accompanying editorial.
The risk-benefit ratio of adding a second-generation AA to treatment may be different for a patient who wants to stay alert and sharp to keep a complex job “versus someone whose primary goal is to see their young children graduate high school,” Alexandra Sokolova, MD, of the Oregon Health and Science University, Portland, and Julie Graff, MD, of the VA Portland (Ore.) Health Care System, wrote in their editorial.
The study fills a “critical gap” when it comes to counseling men about the drugs and will help guide discussions, they said.
The investigators said their study also highlights the need for additional research to identify who is most at risk for the side effects and the best way to prevent and treat them. “Interventions currently under investigation include donepezil, methylphenidate, low-fat diet, acupuncture, martial arts, and high-intensity exercise, among many others,” Ms. Nowakowska and colleagues noted.
Study details
The 12 trials in the meta-analysis, which compared second-generation AAs with placebo, were conducted from 2008 to 2021. These trials were multinational investigations that included patients with metastatic disease as well as those with nonmetastatic disease. The median age across the studies ranged from 67 to 74 years, and trial follow-up ranged from 3.9 to 48 months.
The rates of adverse cognitive effects and attention disorders and disturbances ranged from 2% to 8% among patients who received second-generation AAs versus 2%-3% among those who received placebo, a more than doubling of the risk of cognitive toxic effects (P = .002).
Fatigue of any grade was reported in 5%-45% of participants taking second-generation AAs versus 2%-42% of patients taking placebos, which translates to a 34% higher risk (P < .001).
The use of AAs was associated with an 87% increase in the risk of falls in comparison with placebo, regardless of severity. For falls of grade 3 or higher that required hospitalization or invasive treatment, the increase in risk with second-generation AAs was 72% (P = .05).
The findings were consistent for cognitive toxicity and fatigue in studies that included traditional hormone therapy in both the treatment and control arms. Increased age was associated with a greater risk of fatigue.
Study limits include the fact that it was not known how long patients were taking the drugs before they encountered problems. In addition, the findings were not broken down with respect to medication, so it’s unknown whether such problems are worse with some second-generation AAs than with others.
The editorialists noted that real-world patients tend to be older and sicker than patients in trials, so the risk of falls, fatigue, and cognition problems might be higher among everyday patients.
The study was funded by the National Institutes of Health and others. The investigators disclosed no relevant financial relationships. Dr. Sokolova has received personal fees from Lantheus and travel grants from AstraZeneca. Dr. Graff has received nonfinancial support from Janssen, Pfizer/Astellas, and Sanofi.
A version of this article first appeared on Medscape.com.
Second-generation antiandrogens (AAs) – abiraterone, apalutamide, darolutamide, and enzalutamide – are a cornerstone of modern prostate cancer treatment, improving outcomes and survival.
However, they carry a significant caveat, according to a new meta-analysis of 12 clinical trials with over 13,000 patients.
the authors reported.
These findings carry “important public health indications” because use of second-generation AAs, currently first-line treatment for advanced and castration-resistant prostate cancer, is expanding with new indications, meaning that the pool of men at risk for such problems is large and growing, the team wrote.
The take-home message is that the findings give men – and the physicians who counsel them – a fuller idea of what to expect when considering using the agents, the researchers comment. This information is key at a time when so much of prostate cancer treatment involves carefully weighing the risks and benefits, they added.
The study was published in JAMA Oncology. It was conducted by a team of researchers from the University of Texas MD Anderson Cancer Center, Houston, and was led by Malgorzata Nowakowska, a medical student at Baylor College of Medicine, Houston.
Two prostate cancer specialists agreed and gave an example to bring the point home in an accompanying editorial.
The risk-benefit ratio of adding a second-generation AA to treatment may be different for a patient who wants to stay alert and sharp to keep a complex job “versus someone whose primary goal is to see their young children graduate high school,” Alexandra Sokolova, MD, of the Oregon Health and Science University, Portland, and Julie Graff, MD, of the VA Portland (Ore.) Health Care System, wrote in their editorial.
The study fills a “critical gap” when it comes to counseling men about the drugs and will help guide discussions, they said.
The investigators said their study also highlights the need for additional research to identify who is most at risk for the side effects and the best way to prevent and treat them. “Interventions currently under investigation include donepezil, methylphenidate, low-fat diet, acupuncture, martial arts, and high-intensity exercise, among many others,” Ms. Nowakowska and colleagues noted.
Study details
The 12 trials in the meta-analysis, which compared second-generation AAs with placebo, were conducted from 2008 to 2021. These trials were multinational investigations that included patients with metastatic disease as well as those with nonmetastatic disease. The median age across the studies ranged from 67 to 74 years, and trial follow-up ranged from 3.9 to 48 months.
The rates of adverse cognitive effects and attention disorders and disturbances ranged from 2% to 8% among patients who received second-generation AAs versus 2%-3% among those who received placebo, a more than doubling of the risk of cognitive toxic effects (P = .002).
Fatigue of any grade was reported in 5%-45% of participants taking second-generation AAs versus 2%-42% of patients taking placebos, which translates to a 34% higher risk (P < .001).
The use of AAs was associated with an 87% increase in the risk of falls in comparison with placebo, regardless of severity. For falls of grade 3 or higher that required hospitalization or invasive treatment, the increase in risk with second-generation AAs was 72% (P = .05).
The findings were consistent for cognitive toxicity and fatigue in studies that included traditional hormone therapy in both the treatment and control arms. Increased age was associated with a greater risk of fatigue.
Study limits include the fact that it was not known how long patients were taking the drugs before they encountered problems. In addition, the findings were not broken down with respect to medication, so it’s unknown whether such problems are worse with some second-generation AAs than with others.
The editorialists noted that real-world patients tend to be older and sicker than patients in trials, so the risk of falls, fatigue, and cognition problems might be higher among everyday patients.
The study was funded by the National Institutes of Health and others. The investigators disclosed no relevant financial relationships. Dr. Sokolova has received personal fees from Lantheus and travel grants from AstraZeneca. Dr. Graff has received nonfinancial support from Janssen, Pfizer/Astellas, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Muscle fat: A new risk factor for cognitive decline?
Investigators assessed muscle fat in more than 1,600 adults in their 70s and evaluated their cognitive function over a 10-year period. They found that increases in muscle adiposity from year 1 to year 6 were associated with greater cognitive decline over time, independent of total weight, other fat deposits, muscle characteristics, and traditional dementia risk factors.
The findings were similar between Black and White people and between men and women.
“Increasing adiposity – or fat deposition – in skeletal muscles predicted faster cognitive decline, irrespective of demographics or other disease, and this effect was distinct from that of other types of fat or other muscle characteristics, such as strength or mass,” study investigator Caterina Rosano MD, MPH, professor of epidemiology at the University of Pittsburgh, said in an interview.
The study was published in the Journal of the American Geriatrics Society.
Biologically plausible
“There has been a growing recognition that overall adiposity and muscle measures, such as strength and mass, are individual indicators of future dementia risk and both strengthen the algorithms to predict cognitive decline,” said Dr. Rosano, associate director for clinical translation at the University of Pittsburgh’s Aging Institute. “However, adiposity in the muscle has not been examined.”
Some evidence supports a “biologically plausible link” between muscle adiposity and dementia risk. For example, muscle adiposity increases the risk for type 2 diabetes and hypertension, both of which are dementia risk factors.
Skeletal muscle adiposity increases with older age, even in older adults who lose weight, and is “highly prevalent” among older adults of African ancestry.
The researchers examined a large, biracial sample of older adults participating in the Health, Aging and Body Composition study, which enrolled men and women aged between 70 and 79 years. Participants were followed for an average of 9.0 ± 1.8 years.
During years 1 and 6, participants’ body composition was analyzed, including intermuscular adipose tissue (IMAT), visceral and subcutaneous adiposity, total fat mass, and muscle area.
In years 1, 3, 5, 8, and 10, participants’ cognition was measured using the modified Mini-Mental State (3MS) exam.
The main independent variable was 5-year change in thigh IMAT (year 6 minus year 1), and the main dependent variable was 3MS decline (from year 5 to year 10).
The researchers adjusted all the models for traditional dementia risk factors at baseline including 3MS, education, apo E4 allele, diabetes, hypertension, and physical activity and also calculated interactions between IMAT change by race or sex.
These models also accounted for change in muscle strength, muscle area, body weight, abdominal subcutaneous and visceral adiposity, and total body fat mass as well as cytokines related to adiposity.
‘Rich and engaging crosstalk’
The final sample included 1634 participants (mean age, 73.38 years at baseline; 48% female; 35% Black; mean baseline 3MS score, 91.6).
Thigh IMAT increased by 39.0% in all participants from year 1 to year 6, which corresponded to an increase of 4.85 cm2 or 0.97 cm2/year. During the same time period, muscle strength decreased by 14.0% (P < .05), although thigh muscle area remained stable, decreasing less than 0.5%.
There were decreases in both abdominal subcutaneous and visceral adiposity of 3.92% and 6.43%, respectively (P < .05). There was a decrease of 3.3% in 3MS from year 5 to year 10.
Several variables were associated with 3MS decline, independent of any change in thigh IMAT: older age, less education, and having at least one copy of the APOe4 allele. These variables were included in the model of IMAT change predicting 3MS change.
A statistically significant association of IMAT increase with 3MS decline was found. The IMAT increase of 4.85 cm2 corresponded to a 3MS decline of an additional 3.6 points (P < .0001) from year 5 to year 10, “indicating a clinically important change.”
The association between increasing thigh IMAT with declining 3MS “remained statistically significant” after adjusting for race, age, education, and apo E4 (P < .0001) and was independent of changes in thigh muscle area, muscle strength, and other adiposity measures.
In participants with increased IMAT in years 1-6, the mean 3MS score fell to approximately 87 points at year 10, compared with those without increased IMAT, with a 3MS score that dropped to approximately 89 points.
Interactions by race and sex were not statistically significant (P > .08).
“Our results suggest that adiposity in muscles can predict cognitive decline, in addition to (not instead of) other traditional dementia risk factors,” said Dr. Rosano.
There is “a rich and engaging crosstalk between muscle, adipose tissue, and the brain all throughout our lives, happening through factors released in the bloodstream that can reach the brain, however, the specific identity of the factors responsible for the crosstalk of muscle adiposity and brain in older adults has not yet been discovered,” she noted.
Although muscle adiposity is “not yet routinely measured in clinical settings, it is being measured opportunistically on clinical CT scans obtained as part of routine patient care,” she added. “These CT measurements have already been validated in many studies of older adults; thus, clinicians could have access to this novel information without additional cost, time, or radiation exposure.”
Causality not proven
In a comment, Bruce Albala, PhD, professor, department of environmental and occupational health, University of California, Irvine, noted that the 3MS assessment is scored on a 100-point scale, with a score less than 78 “generally regarded as indicating cognitive impairment or approaching a dementia condition.” In the current study, the mean 3MS score of participants with increased IMAT was still “well above the dementia cut-off.”
Moreover, “even if there is a relationship or correlation between IMAT and cognition, this does not prove or even suggest causality, especially from a biological mechanistic approach,” said Dr. Albaba, an adjunct professor of neurology, who was not involved in the study. “Clearly, more research is needed even to understand the relationship between these two factors.”
The study was supported by the National Institute on Aging. Dr. Rosano and coauthors and Dr. Albala declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators assessed muscle fat in more than 1,600 adults in their 70s and evaluated their cognitive function over a 10-year period. They found that increases in muscle adiposity from year 1 to year 6 were associated with greater cognitive decline over time, independent of total weight, other fat deposits, muscle characteristics, and traditional dementia risk factors.
The findings were similar between Black and White people and between men and women.
“Increasing adiposity – or fat deposition – in skeletal muscles predicted faster cognitive decline, irrespective of demographics or other disease, and this effect was distinct from that of other types of fat or other muscle characteristics, such as strength or mass,” study investigator Caterina Rosano MD, MPH, professor of epidemiology at the University of Pittsburgh, said in an interview.
The study was published in the Journal of the American Geriatrics Society.
Biologically plausible
“There has been a growing recognition that overall adiposity and muscle measures, such as strength and mass, are individual indicators of future dementia risk and both strengthen the algorithms to predict cognitive decline,” said Dr. Rosano, associate director for clinical translation at the University of Pittsburgh’s Aging Institute. “However, adiposity in the muscle has not been examined.”
Some evidence supports a “biologically plausible link” between muscle adiposity and dementia risk. For example, muscle adiposity increases the risk for type 2 diabetes and hypertension, both of which are dementia risk factors.
Skeletal muscle adiposity increases with older age, even in older adults who lose weight, and is “highly prevalent” among older adults of African ancestry.
The researchers examined a large, biracial sample of older adults participating in the Health, Aging and Body Composition study, which enrolled men and women aged between 70 and 79 years. Participants were followed for an average of 9.0 ± 1.8 years.
During years 1 and 6, participants’ body composition was analyzed, including intermuscular adipose tissue (IMAT), visceral and subcutaneous adiposity, total fat mass, and muscle area.
In years 1, 3, 5, 8, and 10, participants’ cognition was measured using the modified Mini-Mental State (3MS) exam.
The main independent variable was 5-year change in thigh IMAT (year 6 minus year 1), and the main dependent variable was 3MS decline (from year 5 to year 10).
The researchers adjusted all the models for traditional dementia risk factors at baseline including 3MS, education, apo E4 allele, diabetes, hypertension, and physical activity and also calculated interactions between IMAT change by race or sex.
These models also accounted for change in muscle strength, muscle area, body weight, abdominal subcutaneous and visceral adiposity, and total body fat mass as well as cytokines related to adiposity.
‘Rich and engaging crosstalk’
The final sample included 1634 participants (mean age, 73.38 years at baseline; 48% female; 35% Black; mean baseline 3MS score, 91.6).
Thigh IMAT increased by 39.0% in all participants from year 1 to year 6, which corresponded to an increase of 4.85 cm2 or 0.97 cm2/year. During the same time period, muscle strength decreased by 14.0% (P < .05), although thigh muscle area remained stable, decreasing less than 0.5%.
There were decreases in both abdominal subcutaneous and visceral adiposity of 3.92% and 6.43%, respectively (P < .05). There was a decrease of 3.3% in 3MS from year 5 to year 10.
Several variables were associated with 3MS decline, independent of any change in thigh IMAT: older age, less education, and having at least one copy of the APOe4 allele. These variables were included in the model of IMAT change predicting 3MS change.
A statistically significant association of IMAT increase with 3MS decline was found. The IMAT increase of 4.85 cm2 corresponded to a 3MS decline of an additional 3.6 points (P < .0001) from year 5 to year 10, “indicating a clinically important change.”
The association between increasing thigh IMAT with declining 3MS “remained statistically significant” after adjusting for race, age, education, and apo E4 (P < .0001) and was independent of changes in thigh muscle area, muscle strength, and other adiposity measures.
In participants with increased IMAT in years 1-6, the mean 3MS score fell to approximately 87 points at year 10, compared with those without increased IMAT, with a 3MS score that dropped to approximately 89 points.
Interactions by race and sex were not statistically significant (P > .08).
“Our results suggest that adiposity in muscles can predict cognitive decline, in addition to (not instead of) other traditional dementia risk factors,” said Dr. Rosano.
There is “a rich and engaging crosstalk between muscle, adipose tissue, and the brain all throughout our lives, happening through factors released in the bloodstream that can reach the brain, however, the specific identity of the factors responsible for the crosstalk of muscle adiposity and brain in older adults has not yet been discovered,” she noted.
Although muscle adiposity is “not yet routinely measured in clinical settings, it is being measured opportunistically on clinical CT scans obtained as part of routine patient care,” she added. “These CT measurements have already been validated in many studies of older adults; thus, clinicians could have access to this novel information without additional cost, time, or radiation exposure.”
Causality not proven
In a comment, Bruce Albala, PhD, professor, department of environmental and occupational health, University of California, Irvine, noted that the 3MS assessment is scored on a 100-point scale, with a score less than 78 “generally regarded as indicating cognitive impairment or approaching a dementia condition.” In the current study, the mean 3MS score of participants with increased IMAT was still “well above the dementia cut-off.”
Moreover, “even if there is a relationship or correlation between IMAT and cognition, this does not prove or even suggest causality, especially from a biological mechanistic approach,” said Dr. Albaba, an adjunct professor of neurology, who was not involved in the study. “Clearly, more research is needed even to understand the relationship between these two factors.”
The study was supported by the National Institute on Aging. Dr. Rosano and coauthors and Dr. Albala declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Investigators assessed muscle fat in more than 1,600 adults in their 70s and evaluated their cognitive function over a 10-year period. They found that increases in muscle adiposity from year 1 to year 6 were associated with greater cognitive decline over time, independent of total weight, other fat deposits, muscle characteristics, and traditional dementia risk factors.
The findings were similar between Black and White people and between men and women.
“Increasing adiposity – or fat deposition – in skeletal muscles predicted faster cognitive decline, irrespective of demographics or other disease, and this effect was distinct from that of other types of fat or other muscle characteristics, such as strength or mass,” study investigator Caterina Rosano MD, MPH, professor of epidemiology at the University of Pittsburgh, said in an interview.
The study was published in the Journal of the American Geriatrics Society.
Biologically plausible
“There has been a growing recognition that overall adiposity and muscle measures, such as strength and mass, are individual indicators of future dementia risk and both strengthen the algorithms to predict cognitive decline,” said Dr. Rosano, associate director for clinical translation at the University of Pittsburgh’s Aging Institute. “However, adiposity in the muscle has not been examined.”
Some evidence supports a “biologically plausible link” between muscle adiposity and dementia risk. For example, muscle adiposity increases the risk for type 2 diabetes and hypertension, both of which are dementia risk factors.
Skeletal muscle adiposity increases with older age, even in older adults who lose weight, and is “highly prevalent” among older adults of African ancestry.
The researchers examined a large, biracial sample of older adults participating in the Health, Aging and Body Composition study, which enrolled men and women aged between 70 and 79 years. Participants were followed for an average of 9.0 ± 1.8 years.
During years 1 and 6, participants’ body composition was analyzed, including intermuscular adipose tissue (IMAT), visceral and subcutaneous adiposity, total fat mass, and muscle area.
In years 1, 3, 5, 8, and 10, participants’ cognition was measured using the modified Mini-Mental State (3MS) exam.
The main independent variable was 5-year change in thigh IMAT (year 6 minus year 1), and the main dependent variable was 3MS decline (from year 5 to year 10).
The researchers adjusted all the models for traditional dementia risk factors at baseline including 3MS, education, apo E4 allele, diabetes, hypertension, and physical activity and also calculated interactions between IMAT change by race or sex.
These models also accounted for change in muscle strength, muscle area, body weight, abdominal subcutaneous and visceral adiposity, and total body fat mass as well as cytokines related to adiposity.
‘Rich and engaging crosstalk’
The final sample included 1634 participants (mean age, 73.38 years at baseline; 48% female; 35% Black; mean baseline 3MS score, 91.6).
Thigh IMAT increased by 39.0% in all participants from year 1 to year 6, which corresponded to an increase of 4.85 cm2 or 0.97 cm2/year. During the same time period, muscle strength decreased by 14.0% (P < .05), although thigh muscle area remained stable, decreasing less than 0.5%.
There were decreases in both abdominal subcutaneous and visceral adiposity of 3.92% and 6.43%, respectively (P < .05). There was a decrease of 3.3% in 3MS from year 5 to year 10.
Several variables were associated with 3MS decline, independent of any change in thigh IMAT: older age, less education, and having at least one copy of the APOe4 allele. These variables were included in the model of IMAT change predicting 3MS change.
A statistically significant association of IMAT increase with 3MS decline was found. The IMAT increase of 4.85 cm2 corresponded to a 3MS decline of an additional 3.6 points (P < .0001) from year 5 to year 10, “indicating a clinically important change.”
The association between increasing thigh IMAT with declining 3MS “remained statistically significant” after adjusting for race, age, education, and apo E4 (P < .0001) and was independent of changes in thigh muscle area, muscle strength, and other adiposity measures.
In participants with increased IMAT in years 1-6, the mean 3MS score fell to approximately 87 points at year 10, compared with those without increased IMAT, with a 3MS score that dropped to approximately 89 points.
Interactions by race and sex were not statistically significant (P > .08).
“Our results suggest that adiposity in muscles can predict cognitive decline, in addition to (not instead of) other traditional dementia risk factors,” said Dr. Rosano.
There is “a rich and engaging crosstalk between muscle, adipose tissue, and the brain all throughout our lives, happening through factors released in the bloodstream that can reach the brain, however, the specific identity of the factors responsible for the crosstalk of muscle adiposity and brain in older adults has not yet been discovered,” she noted.
Although muscle adiposity is “not yet routinely measured in clinical settings, it is being measured opportunistically on clinical CT scans obtained as part of routine patient care,” she added. “These CT measurements have already been validated in many studies of older adults; thus, clinicians could have access to this novel information without additional cost, time, or radiation exposure.”
Causality not proven
In a comment, Bruce Albala, PhD, professor, department of environmental and occupational health, University of California, Irvine, noted that the 3MS assessment is scored on a 100-point scale, with a score less than 78 “generally regarded as indicating cognitive impairment or approaching a dementia condition.” In the current study, the mean 3MS score of participants with increased IMAT was still “well above the dementia cut-off.”
Moreover, “even if there is a relationship or correlation between IMAT and cognition, this does not prove or even suggest causality, especially from a biological mechanistic approach,” said Dr. Albaba, an adjunct professor of neurology, who was not involved in the study. “Clearly, more research is needed even to understand the relationship between these two factors.”
The study was supported by the National Institute on Aging. Dr. Rosano and coauthors and Dr. Albala declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
Guide explains nonsurgical management of major hemorrhage
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new guide offers recommendations for the nonsurgical management of major hemorrhage, which is a challenging clinical problem.
Major hemorrhage is a significant cause of death and can occur in a myriad of clinical settings.
“In Ontario, we’ve been collecting quality metrics on major hemorrhages to try and make sure that a higher percentage of patients gets the best possible care when they are experiencing significant bleeding,” author Jeannie Callum, MD, professor and director of transfusion medicine at Kingston (Ont.) Health Sciences Centre and Queen’s University, also in Kingston, said in an interview. “There were some gaps, so this is our effort to get open, clear information out to the emergency doctors, intensive care unit doctors, the surgeons, and everyone else involved in managing major hemorrhage, to help close these gaps.”
The guide was published in the Canadian Medical Association Journal.
Fast care essential
The guide aims to provide answers, based on the latest research, to questions such as when to activate a massive hemorrhage protocol (MHP), which patients should receive tranexamic acid (TXA), which blood products should be transfused before laboratory results are available, how to monitor the effects of blood transfusion, and when fibrinogen concentrate or prothrombin complex concentrate should be given.
Not all recommendations will be followed, Dr. Callum said, especially in rural hospitals with limited resources. But the guide is adaptable, and rural hospitals can create protocols that are customized to their unique circumstances.
Care must be “perfect and fast” in the first hour of major injury, said Dr. Callum. “You need to get a proclotting drug in that first hour if you have a traumatic or postpartum bleed. You have to make sure your clotting factors never fail you throughout your resuscitation. You have to be fast with the transfusion. You have to monitor for the complications of the transfusion, electrolyte disturbances, and the patient’s temperature dropping. It’s a complicated situation that needs a multidisciplinary team.”
Bleeding affects everybody in medicine, from family doctors in smaller institutions who work in emergency departments to obstetricians and surgeons, she added.
“For people under the age of 45, trauma is the most common cause of death. When people die of trauma, they die of bleeding. So many people experience these extreme bleeds. We believe that some of them might be preventable with faster, more standardized, more aggressive care. That’s why we wrote this review,” said Dr. Callum.
Administer TXA quickly
The first recommendation is to ensure that every hospital has a massive hemorrhage protocol. Such a protocol is vital for the emergency department, operating room, and obstetric unit. “Making sure you’ve got a protocol that is updated every 3 years and adjusted to the local hospital context is essential,” said Dr. Callum.
Smaller hospitals will have to adjust their protocols according to the capabilities of their sites. “Some smaller hospitals do not have platelets in stock and get their platelets from another hospital, so you need to adjust your protocol to what you are able to do. Not every hospital can control bleeding in a trauma patient, so your protocol would be to stabilize and call a helicopter. Make sure all of this is detailed so that implementing it becomes automatic,” said Dr. Callum.
An MHP should be activated for patients with uncontrolled hemorrhage who meet the clinical criteria of the local hospital and are expected to need blood product support and red blood cells.
“Lots of people bleed, but not everybody is bleeding enough that they need a code transfusion,” said Dr. Callum. Most patients with gastrointestinal bleeds caused by NSAID use can be managed with uncrossed matched blood from the local blood bank. “But in patients who need the full code transfusion because they are going to need plasma, clotting factor replacement, and many other drugs, that is when the MHP should be activated. Don’t activate it when you don’t need it, because doing so activates the whole hospital and diverts care away from other patients.”
TXA should be administered as soon as possible after onset of hemorrhage in most patients, with the exception of gastrointestinal hemorrhage, where a benefit has not been shown.
TXA has been a major advance in treating massive bleeding, Dr. Callum said. “TXA was invented by a Japanese husband-and-wife research team. We know that it reduces the death rate in trauma and in postpartum hemorrhage, and it reduces the chance of major bleeding with major surgical procedures. We give it routinely in surgical procedures. If a patient gets TXA within 60 minutes of injury, it dramatically reduces the death rate. And it costs $10 per patient. It’s cheap, it’s easy, it has no side effects. It’s just amazing.”
Future research must address several unanswered questions, said Dr. Callum. These questions include whether prehospital transfusion improves patient outcomes, whether whole blood has a role in the early management of major hemorrhage, and what role factor concentrates play in patients with major bleeding.
‘Optimal recommendations’
Commenting on the document, Bourke Tillmann, MD, PhD, trauma team leader at Sunnybrook Health Sciences Centre and the Ross Tilley Burn Center in Toronto, said: “Overall, I think it is a good overview of MHPs as an approach to major hemorrhage.”
The review also is timely, since Ontario released its MHP guidelines in 2021, he added. “I would have liked to see more about the treatment aspects than just an overview of an MHP. But if you are the person overseeing the emergency department or running the blood bank, these protocols are incredibly useful and incredibly important.”
“This report is a nice and thoughtful overview of best practices in many areas, especially trauma, and makes recommendations that are optimal, although they are not necessarily practical in all centers,” Eric L. Legome, MD, professor and chair of emergency medicine at Mount Sinai West and Mount Sinai Morningside, New York, said in an interview.
“If you’re in a small rural hospital with one lab technician, trying to do all of these things, it will not be possible. These are optimal recommendations that people can use to the best of their ability, but they are not standard of care, because some places will not be able to provide this level of care,” he added. “This paper provides practical, reasonable advice that should be looked at as you are trying to implement transfusion policies and processes, with the understanding that it is not necessarily applicable or practical for very small hospitals in very rural centers that might not have access to these types of products and tools, but it’s a reasonable and nicely written paper.”
No outside funding for the guideline was reported. Dr. Callum has received research funding from Canadian Blood Services and Octapharma. She sits on the nominating committee with the Association for the Advancement of Blood & Biotherapies and on the data safety monitoring boards for the Tranexamic Acid for Subdural Hematoma trial and the Fibrinogen Replacement in Trauma trial. Dr. Tillmann and Dr. Legome reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Unraveling the mechanisms behind FMT efficacy needed to expand its use
A deeper understanding of the mechanisms underlying the success of fecal microbiota transplantation (FMT) is needed to further improve its effectiveness, according to two recent reviews published in Cell Host and Microbe.
how closely the donor’s microbial composition matches the patient’s existing microbiome, and the presence of nonbacterial gut inhabitants like viruses and fungi – affect FMT success, according to a press release.
FMT is most often used to treat recurrent Clostridioides difficile infections, which don’t always respond to antibiotics. Success rates range from 60% to 90%, depending on the administration route and study design, notes an international research team led by Abbas Yadegar, PhD, a medical bacteriologist at the Shahid Beheshti University of Medical Sciences in Tehran, Iran.
The understanding of how FMT works is incomplete, however, and the reasons some patients fail to benefit is unclear, note Dr. Yadegar and colleagues. Little attention has been paid to the role that other components of the patient’s microbiome, along with outside factors, play in the treatment’s success, they add.
“We wanted other researchers to look beyond changes in stool microbial composition and function, which have been the focus of research in the past few years,” Dr. Yadegar’s team said in a statement provided to this news organization.
Dr. Yadegar and colleagues’ review of more than 130 studies summarizes recent evidence on the mechanisms contributing to FMT success against recurrent C. difficile infection, highlights knowledge gaps, and proposes future research directions in the field.
Factors that influence FMT’s effectiveness and the potential the procedure holds for treatment of other diseases associated with gut dysbiosis are the subject of a review of 149 studies by a team of researchers led by Serena Porcari, MD, a gastroenterologist at the Fondazione Policlinico Universitario Gemelli and Università Cattolica del Sacro Cuore, in Rome.
“Our main goal was not only to unravel the different mechanisms of FMT efficacy but also to introduce some mindset shifts that are needed to bring FMT forward, mainly covering the gap that exists between basic scientists and clinicians,” Gianluca Ianiro, MD, PhD, a senior researcher in digestive diseases who works with Dr. Porcari and is the review’s lead author, told this news organization.
Engraftment may influence success
Engraftment of donor microbial strains in recipients appears to be key to the therapeutic success of FMT, both reviews note.
Three factors influence engraftment: the donor’s bacteria fitness relative to the recipient, the bacteria already present in the recipient, and whether antibiotics are used prior to FMT to open a niche for the incoming donor microbes, according to Dr. Yadegar and colleagues.
How to calculate strain engraftment has not yet been standardized in the field, and the number of strains detected in the recipient’s fecal sample is dependent on the depth of sequencing techniques, Dr. Porcari and colleagues note.
The use of whole-genome sequencing has enabled more precise evaluation of engraftment, they add.
“With this approach, microbial engraftment has been associated with clinical success, regardless of the disease, in a large metagenomic metanalysis of 24 FMT trials and almost 1,400 fecal samples,” Dr. Porcari and colleagues write. However, these results have not been replicated, likely because of differences between the studies.
More study on the topic is needed, both articles note.
“Because the recent metagenomics studies compared pre- and post-FMT only in cases with successful treatment outcomes, it is not possible to link engraftment to clinical outcomes,” Dr. Yadegar and colleagues write in their statement to this news organization.
A closer look at donor-recipient pairings
Clinicians usually enlist healthy, carefully screened individuals as FMT donors.
However, both research groups conclude that fine-scale taxonomic and metabolic analyses of donor and recipient microbiomes would better inform clinical decisions, especially when treating diseases other than C. difficile.
This may call for a more personalized approach to choosing donor-recipient pairings. Investigators should assess the patient’s diet and genetic background and how closely the donor’s microbiome matches that of the patient.
“Most studies focused on profiling stool samples before and after FMT without also including functional analyses; therefore, there are still a lot of aspects of host microbial interactions that remain unknown,” write Dr. Yadegar and colleagues in their statement.
Ecologic factors, including diet and host genetics, are often not included in clinical studies of C. difficile, but they “may potentially be the missing links” to treatment failure in the small portion of patients whose condition doesn’t respond to FMT, they write.
Pairing donor-recipient combinations on the basis of dietary patterns and preferences could improve FMT efficacy because the donor microbiota would be preadapted to the recipient’s diet, Dr. Yadegar and colleagues write. The team is examining how donor and recipient diet may affect outcomes.
Dr. Porcari and colleagues add that while some studies support the existence of shared characteristics that make up super-donors, others found that the optimal donor is more patient specific. They call for personalized selection strategies that employ microbiome sequencing tools rather than a “one stool fits all” approach.
Currently, many clinicians aren’t familiar with microbiome sequencing and analysis, but they’ll need to be in the near future, note Dr. Porcari and colleagues.
“Identifying microbiome characteristics that maximize strain engraftment in the FMT will allow clinicians to select the best donor for each single patient,” they write.
The possible role of viruses and fungi
In FMT research, investigators tend to focus on the bacteria in the human microbiome. However, viruses and fungi also appear to play a role, both articles note.
“Other microbial kingdoms that inhabit the intestine should be taken into account when considering predictors of post-FMT microbial transfer,” write Dr. Porcari and colleagues.
Although few studies have examined the gut virome’s impact on FMT effectiveness against C. difficile, the existing research, although limited, indicates that bacteriophage viruses could play a role, Dr. Yadegar and colleagues note. For example, high levels of donor-derived Caudoviralesbacteriophages in recipients were associated with FMT efficacy in one preliminary study, they write.
In a small human study, fecal filtrate from healthy donors who had bacteriophages but no live bacteria successfully treated five patients with recurrent C. difficile infection, Dr. Yadegar and colleagues write.
“Therefore, the idea that viruses may play a role is very provocative,” write Dr. Yadegar’s team in their statement.
It’s important to note that these studies are associative, which means they can’t definitively answer the question of how or whether viruses play a role, Dr. Yadegar’s team added.
Researchers “know even less about how fungi may or may not play a role,” write Dr. Yadegar and colleagues. However, in early research that involved patients who had successfully undergone FMT for C. difficile, there was higher relative abundance of Saccharomyces and Aspergillus, whereas Candida, if prominent, may impede response, they write in their article.
Additionally, to explore whether live bacteria are necessary for FMT to work, Dr. Yadegar and colleagues informed this news organization that they are conducting a study “comparing traditional FMT to a fecal filtrate that contains no live bacteria, but has all other components, to see if we can achieve similar success rates in recurrent C. difficile infection.”
Repeat treatment for sustained response
Dr. Yadegar’s team offered another important takeaway: A single FMT treatment will not sustain a positive response, especially when treating chronic noncommunicable conditions in which intestinal dysbiosis may play a role. Repeat treatment will be needed, as with other chronic conditions. This has been shown even in C. difficile infection.
“Recent studies have documented a significant advantage of repeated FMT over single FMT on the cure rates of recurrent C. difficile,” especially for patients with inflammatory bowel disorder, Dr. Yadegar’s team told this news organization.
“What we don’t know is which patient is likely to respond to microbial-based therapy, or what the dose or frequency should be, or which bacteria are responsible for the effects,” Dr. Yadegar and team said.
Dr. Porcari and colleagues are examining whether FMT could be refined to improve its success against other diseases. This may involve selecting specific donors, monitoring the gut microbiome of both donors and recipients, or using a specific means of delivery, such as lyophilized capsules, Dr. Ianiro said.
A response to FMT for chronic, noncommunicable disorders typically is not sustained long term, note Dr. Porcari and colleagues. However, they add that “sequential transplants have been applied in this setting with promising results, suggesting that chronic modulation of the patient microbiome may be beneficial in noncommunicable chronic disorders.” Dr. Porcari and colleagues point to the success of repeated, long-term FMT in studies of patients with ulcerative colitis and irritable bowel syndrome.
The use of cutting-edge technologies for microbiome assessment and a change in the view of FMT as only an acute, single-use therapy could improve FMT protocols and outcomes for noncommunicable conditions, they write.
Expanding FMT beyond C. difficile
Dr. Yadegar and colleagues’ article “really breaks down what is known about the mechanisms of FMT in C. difficile infection, which is important as other live biotherapeutic products are developed,” Colleen Kelly, MD, an associate professor of medicine at Brown University in Providence, R.I., who was not involved with the reviews, said in an interview.
Dr. Yadegar and colleagues concur. They note in a press release that as the mechanisms behind FMT success are understood, that information should be used to design new standardized therapies.
“Although highly effective, there are substantial drawbacks with [FMT], including infectious risks and sparse long-term safety data,” they write. “Better treatment options for recurrent C. difficile infections that are targeted, safe, and donor-independent are thus desired.”
In December 2022, the U.S. Food and Drug Administration approved the first fecal microbiota product, Rebyota, to prevent recurrence of C. difficile. More recently, in April 2023, the FDA approved Vowst, a pill for treating recurrent C. difficile infections.
Dr. Kelly also noted that the article by Dr. Yadegar and colleagues “may help us understand why a small percentage of patients fail to achieve cure after FMT.”
Regarding Dr. Porcari and colleagues’ article, Dr. Kelly said, “There is a lot of hope that FMT or other gut microbiome therapies will be beneficial for conditions outside of C. difficile.
“They do a good job reviewing the state of the science of FMT and highlight the many unknowns around the use of FMT in conditions outside of C. difficile,” added Dr. Kelly, who has been using FMT to treat C. difficile for more than 15 years.
Data supporting FMT for conditions such as ulcerative colitis and autism are compelling, Dr. Kelly acknowledged. But in her view, FMT isn’t ready for “prime time” outside of C. difficile – at least not yet.
“Academic investigators and those in industry are actively conducting research in many non–C. difficile indications, and I predict we will see the emergence of gut microbiome–based therapies for other indications within the next 5-10 years,” Dr. Kelly said.
Dr. Yadegar reports no relevant financial relationships. One coauthor of the Yadegar study has served on the adjudication board for Finch Therapeutics and has received consulting fees and a speaking honorarium from Rebiotix/Ferring Pharmaceuticals. Dr. Ianiro reports no relevant financial relationships. Dr. Kelly has consulted for Sebela Pharmaceuticals and is one of the principal investigators for the FMT National Patient Registry funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
A version of this article originally appeared on Medscape.com.
A deeper understanding of the mechanisms underlying the success of fecal microbiota transplantation (FMT) is needed to further improve its effectiveness, according to two recent reviews published in Cell Host and Microbe.
how closely the donor’s microbial composition matches the patient’s existing microbiome, and the presence of nonbacterial gut inhabitants like viruses and fungi – affect FMT success, according to a press release.
FMT is most often used to treat recurrent Clostridioides difficile infections, which don’t always respond to antibiotics. Success rates range from 60% to 90%, depending on the administration route and study design, notes an international research team led by Abbas Yadegar, PhD, a medical bacteriologist at the Shahid Beheshti University of Medical Sciences in Tehran, Iran.
The understanding of how FMT works is incomplete, however, and the reasons some patients fail to benefit is unclear, note Dr. Yadegar and colleagues. Little attention has been paid to the role that other components of the patient’s microbiome, along with outside factors, play in the treatment’s success, they add.
“We wanted other researchers to look beyond changes in stool microbial composition and function, which have been the focus of research in the past few years,” Dr. Yadegar’s team said in a statement provided to this news organization.
Dr. Yadegar and colleagues’ review of more than 130 studies summarizes recent evidence on the mechanisms contributing to FMT success against recurrent C. difficile infection, highlights knowledge gaps, and proposes future research directions in the field.
Factors that influence FMT’s effectiveness and the potential the procedure holds for treatment of other diseases associated with gut dysbiosis are the subject of a review of 149 studies by a team of researchers led by Serena Porcari, MD, a gastroenterologist at the Fondazione Policlinico Universitario Gemelli and Università Cattolica del Sacro Cuore, in Rome.
“Our main goal was not only to unravel the different mechanisms of FMT efficacy but also to introduce some mindset shifts that are needed to bring FMT forward, mainly covering the gap that exists between basic scientists and clinicians,” Gianluca Ianiro, MD, PhD, a senior researcher in digestive diseases who works with Dr. Porcari and is the review’s lead author, told this news organization.
Engraftment may influence success
Engraftment of donor microbial strains in recipients appears to be key to the therapeutic success of FMT, both reviews note.
Three factors influence engraftment: the donor’s bacteria fitness relative to the recipient, the bacteria already present in the recipient, and whether antibiotics are used prior to FMT to open a niche for the incoming donor microbes, according to Dr. Yadegar and colleagues.
How to calculate strain engraftment has not yet been standardized in the field, and the number of strains detected in the recipient’s fecal sample is dependent on the depth of sequencing techniques, Dr. Porcari and colleagues note.
The use of whole-genome sequencing has enabled more precise evaluation of engraftment, they add.
“With this approach, microbial engraftment has been associated with clinical success, regardless of the disease, in a large metagenomic metanalysis of 24 FMT trials and almost 1,400 fecal samples,” Dr. Porcari and colleagues write. However, these results have not been replicated, likely because of differences between the studies.
More study on the topic is needed, both articles note.
“Because the recent metagenomics studies compared pre- and post-FMT only in cases with successful treatment outcomes, it is not possible to link engraftment to clinical outcomes,” Dr. Yadegar and colleagues write in their statement to this news organization.
A closer look at donor-recipient pairings
Clinicians usually enlist healthy, carefully screened individuals as FMT donors.
However, both research groups conclude that fine-scale taxonomic and metabolic analyses of donor and recipient microbiomes would better inform clinical decisions, especially when treating diseases other than C. difficile.
This may call for a more personalized approach to choosing donor-recipient pairings. Investigators should assess the patient’s diet and genetic background and how closely the donor’s microbiome matches that of the patient.
“Most studies focused on profiling stool samples before and after FMT without also including functional analyses; therefore, there are still a lot of aspects of host microbial interactions that remain unknown,” write Dr. Yadegar and colleagues in their statement.
Ecologic factors, including diet and host genetics, are often not included in clinical studies of C. difficile, but they “may potentially be the missing links” to treatment failure in the small portion of patients whose condition doesn’t respond to FMT, they write.
Pairing donor-recipient combinations on the basis of dietary patterns and preferences could improve FMT efficacy because the donor microbiota would be preadapted to the recipient’s diet, Dr. Yadegar and colleagues write. The team is examining how donor and recipient diet may affect outcomes.
Dr. Porcari and colleagues add that while some studies support the existence of shared characteristics that make up super-donors, others found that the optimal donor is more patient specific. They call for personalized selection strategies that employ microbiome sequencing tools rather than a “one stool fits all” approach.
Currently, many clinicians aren’t familiar with microbiome sequencing and analysis, but they’ll need to be in the near future, note Dr. Porcari and colleagues.
“Identifying microbiome characteristics that maximize strain engraftment in the FMT will allow clinicians to select the best donor for each single patient,” they write.
The possible role of viruses and fungi
In FMT research, investigators tend to focus on the bacteria in the human microbiome. However, viruses and fungi also appear to play a role, both articles note.
“Other microbial kingdoms that inhabit the intestine should be taken into account when considering predictors of post-FMT microbial transfer,” write Dr. Porcari and colleagues.
Although few studies have examined the gut virome’s impact on FMT effectiveness against C. difficile, the existing research, although limited, indicates that bacteriophage viruses could play a role, Dr. Yadegar and colleagues note. For example, high levels of donor-derived Caudoviralesbacteriophages in recipients were associated with FMT efficacy in one preliminary study, they write.
In a small human study, fecal filtrate from healthy donors who had bacteriophages but no live bacteria successfully treated five patients with recurrent C. difficile infection, Dr. Yadegar and colleagues write.
“Therefore, the idea that viruses may play a role is very provocative,” write Dr. Yadegar’s team in their statement.
It’s important to note that these studies are associative, which means they can’t definitively answer the question of how or whether viruses play a role, Dr. Yadegar’s team added.
Researchers “know even less about how fungi may or may not play a role,” write Dr. Yadegar and colleagues. However, in early research that involved patients who had successfully undergone FMT for C. difficile, there was higher relative abundance of Saccharomyces and Aspergillus, whereas Candida, if prominent, may impede response, they write in their article.
Additionally, to explore whether live bacteria are necessary for FMT to work, Dr. Yadegar and colleagues informed this news organization that they are conducting a study “comparing traditional FMT to a fecal filtrate that contains no live bacteria, but has all other components, to see if we can achieve similar success rates in recurrent C. difficile infection.”
Repeat treatment for sustained response
Dr. Yadegar’s team offered another important takeaway: A single FMT treatment will not sustain a positive response, especially when treating chronic noncommunicable conditions in which intestinal dysbiosis may play a role. Repeat treatment will be needed, as with other chronic conditions. This has been shown even in C. difficile infection.
“Recent studies have documented a significant advantage of repeated FMT over single FMT on the cure rates of recurrent C. difficile,” especially for patients with inflammatory bowel disorder, Dr. Yadegar’s team told this news organization.
“What we don’t know is which patient is likely to respond to microbial-based therapy, or what the dose or frequency should be, or which bacteria are responsible for the effects,” Dr. Yadegar and team said.
Dr. Porcari and colleagues are examining whether FMT could be refined to improve its success against other diseases. This may involve selecting specific donors, monitoring the gut microbiome of both donors and recipients, or using a specific means of delivery, such as lyophilized capsules, Dr. Ianiro said.
A response to FMT for chronic, noncommunicable disorders typically is not sustained long term, note Dr. Porcari and colleagues. However, they add that “sequential transplants have been applied in this setting with promising results, suggesting that chronic modulation of the patient microbiome may be beneficial in noncommunicable chronic disorders.” Dr. Porcari and colleagues point to the success of repeated, long-term FMT in studies of patients with ulcerative colitis and irritable bowel syndrome.
The use of cutting-edge technologies for microbiome assessment and a change in the view of FMT as only an acute, single-use therapy could improve FMT protocols and outcomes for noncommunicable conditions, they write.
Expanding FMT beyond C. difficile
Dr. Yadegar and colleagues’ article “really breaks down what is known about the mechanisms of FMT in C. difficile infection, which is important as other live biotherapeutic products are developed,” Colleen Kelly, MD, an associate professor of medicine at Brown University in Providence, R.I., who was not involved with the reviews, said in an interview.
Dr. Yadegar and colleagues concur. They note in a press release that as the mechanisms behind FMT success are understood, that information should be used to design new standardized therapies.
“Although highly effective, there are substantial drawbacks with [FMT], including infectious risks and sparse long-term safety data,” they write. “Better treatment options for recurrent C. difficile infections that are targeted, safe, and donor-independent are thus desired.”
In December 2022, the U.S. Food and Drug Administration approved the first fecal microbiota product, Rebyota, to prevent recurrence of C. difficile. More recently, in April 2023, the FDA approved Vowst, a pill for treating recurrent C. difficile infections.
Dr. Kelly also noted that the article by Dr. Yadegar and colleagues “may help us understand why a small percentage of patients fail to achieve cure after FMT.”
Regarding Dr. Porcari and colleagues’ article, Dr. Kelly said, “There is a lot of hope that FMT or other gut microbiome therapies will be beneficial for conditions outside of C. difficile.
“They do a good job reviewing the state of the science of FMT and highlight the many unknowns around the use of FMT in conditions outside of C. difficile,” added Dr. Kelly, who has been using FMT to treat C. difficile for more than 15 years.
Data supporting FMT for conditions such as ulcerative colitis and autism are compelling, Dr. Kelly acknowledged. But in her view, FMT isn’t ready for “prime time” outside of C. difficile – at least not yet.
“Academic investigators and those in industry are actively conducting research in many non–C. difficile indications, and I predict we will see the emergence of gut microbiome–based therapies for other indications within the next 5-10 years,” Dr. Kelly said.
Dr. Yadegar reports no relevant financial relationships. One coauthor of the Yadegar study has served on the adjudication board for Finch Therapeutics and has received consulting fees and a speaking honorarium from Rebiotix/Ferring Pharmaceuticals. Dr. Ianiro reports no relevant financial relationships. Dr. Kelly has consulted for Sebela Pharmaceuticals and is one of the principal investigators for the FMT National Patient Registry funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
A version of this article originally appeared on Medscape.com.
A deeper understanding of the mechanisms underlying the success of fecal microbiota transplantation (FMT) is needed to further improve its effectiveness, according to two recent reviews published in Cell Host and Microbe.
how closely the donor’s microbial composition matches the patient’s existing microbiome, and the presence of nonbacterial gut inhabitants like viruses and fungi – affect FMT success, according to a press release.
FMT is most often used to treat recurrent Clostridioides difficile infections, which don’t always respond to antibiotics. Success rates range from 60% to 90%, depending on the administration route and study design, notes an international research team led by Abbas Yadegar, PhD, a medical bacteriologist at the Shahid Beheshti University of Medical Sciences in Tehran, Iran.
The understanding of how FMT works is incomplete, however, and the reasons some patients fail to benefit is unclear, note Dr. Yadegar and colleagues. Little attention has been paid to the role that other components of the patient’s microbiome, along with outside factors, play in the treatment’s success, they add.
“We wanted other researchers to look beyond changes in stool microbial composition and function, which have been the focus of research in the past few years,” Dr. Yadegar’s team said in a statement provided to this news organization.
Dr. Yadegar and colleagues’ review of more than 130 studies summarizes recent evidence on the mechanisms contributing to FMT success against recurrent C. difficile infection, highlights knowledge gaps, and proposes future research directions in the field.
Factors that influence FMT’s effectiveness and the potential the procedure holds for treatment of other diseases associated with gut dysbiosis are the subject of a review of 149 studies by a team of researchers led by Serena Porcari, MD, a gastroenterologist at the Fondazione Policlinico Universitario Gemelli and Università Cattolica del Sacro Cuore, in Rome.
“Our main goal was not only to unravel the different mechanisms of FMT efficacy but also to introduce some mindset shifts that are needed to bring FMT forward, mainly covering the gap that exists between basic scientists and clinicians,” Gianluca Ianiro, MD, PhD, a senior researcher in digestive diseases who works with Dr. Porcari and is the review’s lead author, told this news organization.
Engraftment may influence success
Engraftment of donor microbial strains in recipients appears to be key to the therapeutic success of FMT, both reviews note.
Three factors influence engraftment: the donor’s bacteria fitness relative to the recipient, the bacteria already present in the recipient, and whether antibiotics are used prior to FMT to open a niche for the incoming donor microbes, according to Dr. Yadegar and colleagues.
How to calculate strain engraftment has not yet been standardized in the field, and the number of strains detected in the recipient’s fecal sample is dependent on the depth of sequencing techniques, Dr. Porcari and colleagues note.
The use of whole-genome sequencing has enabled more precise evaluation of engraftment, they add.
“With this approach, microbial engraftment has been associated with clinical success, regardless of the disease, in a large metagenomic metanalysis of 24 FMT trials and almost 1,400 fecal samples,” Dr. Porcari and colleagues write. However, these results have not been replicated, likely because of differences between the studies.
More study on the topic is needed, both articles note.
“Because the recent metagenomics studies compared pre- and post-FMT only in cases with successful treatment outcomes, it is not possible to link engraftment to clinical outcomes,” Dr. Yadegar and colleagues write in their statement to this news organization.
A closer look at donor-recipient pairings
Clinicians usually enlist healthy, carefully screened individuals as FMT donors.
However, both research groups conclude that fine-scale taxonomic and metabolic analyses of donor and recipient microbiomes would better inform clinical decisions, especially when treating diseases other than C. difficile.
This may call for a more personalized approach to choosing donor-recipient pairings. Investigators should assess the patient’s diet and genetic background and how closely the donor’s microbiome matches that of the patient.
“Most studies focused on profiling stool samples before and after FMT without also including functional analyses; therefore, there are still a lot of aspects of host microbial interactions that remain unknown,” write Dr. Yadegar and colleagues in their statement.
Ecologic factors, including diet and host genetics, are often not included in clinical studies of C. difficile, but they “may potentially be the missing links” to treatment failure in the small portion of patients whose condition doesn’t respond to FMT, they write.
Pairing donor-recipient combinations on the basis of dietary patterns and preferences could improve FMT efficacy because the donor microbiota would be preadapted to the recipient’s diet, Dr. Yadegar and colleagues write. The team is examining how donor and recipient diet may affect outcomes.
Dr. Porcari and colleagues add that while some studies support the existence of shared characteristics that make up super-donors, others found that the optimal donor is more patient specific. They call for personalized selection strategies that employ microbiome sequencing tools rather than a “one stool fits all” approach.
Currently, many clinicians aren’t familiar with microbiome sequencing and analysis, but they’ll need to be in the near future, note Dr. Porcari and colleagues.
“Identifying microbiome characteristics that maximize strain engraftment in the FMT will allow clinicians to select the best donor for each single patient,” they write.
The possible role of viruses and fungi
In FMT research, investigators tend to focus on the bacteria in the human microbiome. However, viruses and fungi also appear to play a role, both articles note.
“Other microbial kingdoms that inhabit the intestine should be taken into account when considering predictors of post-FMT microbial transfer,” write Dr. Porcari and colleagues.
Although few studies have examined the gut virome’s impact on FMT effectiveness against C. difficile, the existing research, although limited, indicates that bacteriophage viruses could play a role, Dr. Yadegar and colleagues note. For example, high levels of donor-derived Caudoviralesbacteriophages in recipients were associated with FMT efficacy in one preliminary study, they write.
In a small human study, fecal filtrate from healthy donors who had bacteriophages but no live bacteria successfully treated five patients with recurrent C. difficile infection, Dr. Yadegar and colleagues write.
“Therefore, the idea that viruses may play a role is very provocative,” write Dr. Yadegar’s team in their statement.
It’s important to note that these studies are associative, which means they can’t definitively answer the question of how or whether viruses play a role, Dr. Yadegar’s team added.
Researchers “know even less about how fungi may or may not play a role,” write Dr. Yadegar and colleagues. However, in early research that involved patients who had successfully undergone FMT for C. difficile, there was higher relative abundance of Saccharomyces and Aspergillus, whereas Candida, if prominent, may impede response, they write in their article.
Additionally, to explore whether live bacteria are necessary for FMT to work, Dr. Yadegar and colleagues informed this news organization that they are conducting a study “comparing traditional FMT to a fecal filtrate that contains no live bacteria, but has all other components, to see if we can achieve similar success rates in recurrent C. difficile infection.”
Repeat treatment for sustained response
Dr. Yadegar’s team offered another important takeaway: A single FMT treatment will not sustain a positive response, especially when treating chronic noncommunicable conditions in which intestinal dysbiosis may play a role. Repeat treatment will be needed, as with other chronic conditions. This has been shown even in C. difficile infection.
“Recent studies have documented a significant advantage of repeated FMT over single FMT on the cure rates of recurrent C. difficile,” especially for patients with inflammatory bowel disorder, Dr. Yadegar’s team told this news organization.
“What we don’t know is which patient is likely to respond to microbial-based therapy, or what the dose or frequency should be, or which bacteria are responsible for the effects,” Dr. Yadegar and team said.
Dr. Porcari and colleagues are examining whether FMT could be refined to improve its success against other diseases. This may involve selecting specific donors, monitoring the gut microbiome of both donors and recipients, or using a specific means of delivery, such as lyophilized capsules, Dr. Ianiro said.
A response to FMT for chronic, noncommunicable disorders typically is not sustained long term, note Dr. Porcari and colleagues. However, they add that “sequential transplants have been applied in this setting with promising results, suggesting that chronic modulation of the patient microbiome may be beneficial in noncommunicable chronic disorders.” Dr. Porcari and colleagues point to the success of repeated, long-term FMT in studies of patients with ulcerative colitis and irritable bowel syndrome.
The use of cutting-edge technologies for microbiome assessment and a change in the view of FMT as only an acute, single-use therapy could improve FMT protocols and outcomes for noncommunicable conditions, they write.
Expanding FMT beyond C. difficile
Dr. Yadegar and colleagues’ article “really breaks down what is known about the mechanisms of FMT in C. difficile infection, which is important as other live biotherapeutic products are developed,” Colleen Kelly, MD, an associate professor of medicine at Brown University in Providence, R.I., who was not involved with the reviews, said in an interview.
Dr. Yadegar and colleagues concur. They note in a press release that as the mechanisms behind FMT success are understood, that information should be used to design new standardized therapies.
“Although highly effective, there are substantial drawbacks with [FMT], including infectious risks and sparse long-term safety data,” they write. “Better treatment options for recurrent C. difficile infections that are targeted, safe, and donor-independent are thus desired.”
In December 2022, the U.S. Food and Drug Administration approved the first fecal microbiota product, Rebyota, to prevent recurrence of C. difficile. More recently, in April 2023, the FDA approved Vowst, a pill for treating recurrent C. difficile infections.
Dr. Kelly also noted that the article by Dr. Yadegar and colleagues “may help us understand why a small percentage of patients fail to achieve cure after FMT.”
Regarding Dr. Porcari and colleagues’ article, Dr. Kelly said, “There is a lot of hope that FMT or other gut microbiome therapies will be beneficial for conditions outside of C. difficile.
“They do a good job reviewing the state of the science of FMT and highlight the many unknowns around the use of FMT in conditions outside of C. difficile,” added Dr. Kelly, who has been using FMT to treat C. difficile for more than 15 years.
Data supporting FMT for conditions such as ulcerative colitis and autism are compelling, Dr. Kelly acknowledged. But in her view, FMT isn’t ready for “prime time” outside of C. difficile – at least not yet.
“Academic investigators and those in industry are actively conducting research in many non–C. difficile indications, and I predict we will see the emergence of gut microbiome–based therapies for other indications within the next 5-10 years,” Dr. Kelly said.
Dr. Yadegar reports no relevant financial relationships. One coauthor of the Yadegar study has served on the adjudication board for Finch Therapeutics and has received consulting fees and a speaking honorarium from Rebiotix/Ferring Pharmaceuticals. Dr. Ianiro reports no relevant financial relationships. Dr. Kelly has consulted for Sebela Pharmaceuticals and is one of the principal investigators for the FMT National Patient Registry funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
A version of this article originally appeared on Medscape.com.
Should antibiotic treatment be used toward the end of life?
Diagnosing an infection is complex because of the presence of symptoms that are often nonspecific and that are common in patients in decline toward the end of life. Use of antibiotic therapy in this patient population is still controversial, because the clinical benefits are not clear and the risk of pointless overmedicalization is very high.
Etiology
For patients who are receiving palliative care, the following factors predispose to an infection:
- Increasing fragility.
- Bedbound status and anorexia/cachexia syndrome.
- Weakened immune defenses owing to disease or treatments.
- Changes to skin integrity, related to venous access sites and/or bladder catheterization.
Four-week cutoff
For patients who are expected to live for fewer than 4 weeks, evidence from the literature shows that antimicrobial therapy does not resolve a potential infection or improve the prognosis. Antibiotics should therefore be used only for improving symptom management.
In practice, the most common infections in patients receiving end-of-life care are in the urinary and respiratory tracts. Antibiotics are beneficial in the short term in managing symptoms associated with urinary tract infections (effective in 60%-92% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death.
Antibiotics are also beneficial in managing symptoms associated with respiratory tract infections (effective in up to 53% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death. However, the risk of futility is high. As an alternative, opioids and antitussives could provide greater benefit for patients with dyspnea and cough.
No benefit has been observed with the use of antibiotics to treat symptoms associated with sepsis, abscesses, and deep and complicated infections. Antibiotics are therefore deemed futile in these cases.
In unclear cases, the “2-day rule” is useful. This involves waiting for 2 days, and if the patient remains clinically stable, prescribing antibiotics. If the patient’s condition deteriorates rapidly and progressively, antibiotics should not be prescribed.
Alternatively, one can prescribe antibiotics immediately. If no clinical improvement is observed after 2 days, the antibiotics should be stopped, especially if deterioration of the patient’s condition is rapid and progressive.
Increased body temperature is somewhat common in the last days and hours of life and is not generally associated with symptoms. Fever in these cases is not an indication for the use of antimicrobial therapy.
The most common laboratory markers of infection (C-reactive protein level, erythrocyte sedimentation rate, leukocyte level) are not particularly useful in this patient population, because they are affected by the baseline condition as well as by any treatments given and the state of systemic inflammation, which is associated with the decline in overall health in the last few weeks of life.
The choice should be individualized and shared with patients and family members so that the clinical appropriateness of the therapeutic strategy is evident and that decisions regarding antibiotic treatment are not regarded as a failure to treat the patient.
The longer term
In deciding to start antibiotic therapy, consideration must be given to the patient’s overall health, the treatment objectives, the possibility that the antibiotic will resolve the infection or improve the patient’s symptoms, and the estimated prognosis, which must be sufficiently long to allow the antibiotic time to take effect.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Diagnosing an infection is complex because of the presence of symptoms that are often nonspecific and that are common in patients in decline toward the end of life. Use of antibiotic therapy in this patient population is still controversial, because the clinical benefits are not clear and the risk of pointless overmedicalization is very high.
Etiology
For patients who are receiving palliative care, the following factors predispose to an infection:
- Increasing fragility.
- Bedbound status and anorexia/cachexia syndrome.
- Weakened immune defenses owing to disease or treatments.
- Changes to skin integrity, related to venous access sites and/or bladder catheterization.
Four-week cutoff
For patients who are expected to live for fewer than 4 weeks, evidence from the literature shows that antimicrobial therapy does not resolve a potential infection or improve the prognosis. Antibiotics should therefore be used only for improving symptom management.
In practice, the most common infections in patients receiving end-of-life care are in the urinary and respiratory tracts. Antibiotics are beneficial in the short term in managing symptoms associated with urinary tract infections (effective in 60%-92% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death.
Antibiotics are also beneficial in managing symptoms associated with respiratory tract infections (effective in up to 53% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death. However, the risk of futility is high. As an alternative, opioids and antitussives could provide greater benefit for patients with dyspnea and cough.
No benefit has been observed with the use of antibiotics to treat symptoms associated with sepsis, abscesses, and deep and complicated infections. Antibiotics are therefore deemed futile in these cases.
In unclear cases, the “2-day rule” is useful. This involves waiting for 2 days, and if the patient remains clinically stable, prescribing antibiotics. If the patient’s condition deteriorates rapidly and progressively, antibiotics should not be prescribed.
Alternatively, one can prescribe antibiotics immediately. If no clinical improvement is observed after 2 days, the antibiotics should be stopped, especially if deterioration of the patient’s condition is rapid and progressive.
Increased body temperature is somewhat common in the last days and hours of life and is not generally associated with symptoms. Fever in these cases is not an indication for the use of antimicrobial therapy.
The most common laboratory markers of infection (C-reactive protein level, erythrocyte sedimentation rate, leukocyte level) are not particularly useful in this patient population, because they are affected by the baseline condition as well as by any treatments given and the state of systemic inflammation, which is associated with the decline in overall health in the last few weeks of life.
The choice should be individualized and shared with patients and family members so that the clinical appropriateness of the therapeutic strategy is evident and that decisions regarding antibiotic treatment are not regarded as a failure to treat the patient.
The longer term
In deciding to start antibiotic therapy, consideration must be given to the patient’s overall health, the treatment objectives, the possibility that the antibiotic will resolve the infection or improve the patient’s symptoms, and the estimated prognosis, which must be sufficiently long to allow the antibiotic time to take effect.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Diagnosing an infection is complex because of the presence of symptoms that are often nonspecific and that are common in patients in decline toward the end of life. Use of antibiotic therapy in this patient population is still controversial, because the clinical benefits are not clear and the risk of pointless overmedicalization is very high.
Etiology
For patients who are receiving palliative care, the following factors predispose to an infection:
- Increasing fragility.
- Bedbound status and anorexia/cachexia syndrome.
- Weakened immune defenses owing to disease or treatments.
- Changes to skin integrity, related to venous access sites and/or bladder catheterization.
Four-week cutoff
For patients who are expected to live for fewer than 4 weeks, evidence from the literature shows that antimicrobial therapy does not resolve a potential infection or improve the prognosis. Antibiotics should therefore be used only for improving symptom management.
In practice, the most common infections in patients receiving end-of-life care are in the urinary and respiratory tracts. Antibiotics are beneficial in the short term in managing symptoms associated with urinary tract infections (effective in 60%-92% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death.
Antibiotics are also beneficial in managing symptoms associated with respiratory tract infections (effective in up to 53% of cases), so they should be considered if the patient is not in the agonal or pre-agonal phase of death. However, the risk of futility is high. As an alternative, opioids and antitussives could provide greater benefit for patients with dyspnea and cough.
No benefit has been observed with the use of antibiotics to treat symptoms associated with sepsis, abscesses, and deep and complicated infections. Antibiotics are therefore deemed futile in these cases.
In unclear cases, the “2-day rule” is useful. This involves waiting for 2 days, and if the patient remains clinically stable, prescribing antibiotics. If the patient’s condition deteriorates rapidly and progressively, antibiotics should not be prescribed.
Alternatively, one can prescribe antibiotics immediately. If no clinical improvement is observed after 2 days, the antibiotics should be stopped, especially if deterioration of the patient’s condition is rapid and progressive.
Increased body temperature is somewhat common in the last days and hours of life and is not generally associated with symptoms. Fever in these cases is not an indication for the use of antimicrobial therapy.
The most common laboratory markers of infection (C-reactive protein level, erythrocyte sedimentation rate, leukocyte level) are not particularly useful in this patient population, because they are affected by the baseline condition as well as by any treatments given and the state of systemic inflammation, which is associated with the decline in overall health in the last few weeks of life.
The choice should be individualized and shared with patients and family members so that the clinical appropriateness of the therapeutic strategy is evident and that decisions regarding antibiotic treatment are not regarded as a failure to treat the patient.
The longer term
In deciding to start antibiotic therapy, consideration must be given to the patient’s overall health, the treatment objectives, the possibility that the antibiotic will resolve the infection or improve the patient’s symptoms, and the estimated prognosis, which must be sufficiently long to allow the antibiotic time to take effect.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
Long COVID risk not higher with rheumatic diseases
MILAN – presented at the annual European Congress of Rheumatology.
Although more patients with inflammatory rheumatic diseases (iRD) report symptoms resembling long COVID, the data suggest that many of these symptoms can be attributed to the underlying rheumatic disease. “Overall, we find the data quite reassuring,” said Laura Boekel, Amsterdam Rheumatology and Immunology Center, Amsterdam University Medical Center.
The results were also published in The Lancet Rheumatology.
The risk of developing long COVID after infection with the Omicron variant appeared to be higher in patients with iRD, with 21% meeting the criteria set by the World Health Organization, compared with 13% of healthy individuals (odds ratio, 1.58; P = .037). Fatigue and loss of fitness were the most common long COVID symptoms reported by both iRD patients and controls. However, the difference in risk decreased after accounting for factors that are significantly associated with an increased risk for long COVID, such as body mass index and the severity of the acute COVID-19 infection (adjusted OR, 1.46; P = .081). The duration of symptoms did not show a statistically significant difference.
Kim Lauper, MD, University of Geneva, who chaired the session in which Ms. Boekel reported the study, said in an interview that the data should be interpreted with caution. “The data demonstrate that rheumatic disease itself is not a risk factor for long COVID. However, patients with rheumatic diseases are at a higher risk of severe disease, which in turn increases the likelihood of long COVID. Therefore, as a population, these patients are more susceptible to long COVID overall.”
Moreover, irrespective of their previous COVID-19 infection status, iRD patients often exhibit symptoms similar to those of long COVID even without a prior COVID-19 infection. (There was no history of COVID-19 in 21% of iRD patients vs. 11% of controls.) This suggests that some of the reported long COVID symptoms may actually be clinical manifestations of the underlying rheumatic disease, thereby complicating the diagnosis of long COVID in this population. The study employed the WHO definition of long COVID, which includes persistent symptoms lasting at least 8 weeks, beginning within 3 months of a confirmed SARS-CoV-2 infection, and that cannot be attributed to an alternative diagnosis. However, the data presented in Milan indicate that the WHO definition “is not well suited for patients with iRD due to significant overlap in symptoms and features,” Ms. Boekel concluded.
The cases of Omicron COVID-19 were identified during Jan. 1–April 25, 2022, among iRD patients recruited from the Amsterdam Rheumatology and Immunology Center. The population with confirmed SARS-CoV-2 Omicron infection during this period was monitored for long COVID. The total number of patients included in the study consisted of 77 iRD patients and 23 healthy controls. When asked about the potential risk of selection bias in the survey, Ms. Boekel stated that only approximately 8% of participants declined to respond, and the nonresponders were comparable with the respondents. She concluded that “the risk of selection bias is minimal.”
In an editorial published in The Lancet Rheumatology, Leonard H. Calabrese, DO, Cleveland Clinic, provided his insights on the findings. He emphasized that, “at present, long COVID remains an important reality that significantly impacts the lives of millions of individuals, yet it remains incompletely defined. ... These limitations in defining cases should not in any way undermine the experiences of those suffering from long COVID. Instead, they should serve as a reminder that, at this stage of the pandemic, we unfortunately still lack validated classification criteria for long COVID. It is crucial to include non–SARS-CoV-2–infected controls in all studies to further enhance our understanding.”
Ms. Boekel and coauthors, as well as Dr. Lauper and Dr. Calabrese, reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
MILAN – presented at the annual European Congress of Rheumatology.
Although more patients with inflammatory rheumatic diseases (iRD) report symptoms resembling long COVID, the data suggest that many of these symptoms can be attributed to the underlying rheumatic disease. “Overall, we find the data quite reassuring,” said Laura Boekel, Amsterdam Rheumatology and Immunology Center, Amsterdam University Medical Center.
The results were also published in The Lancet Rheumatology.
The risk of developing long COVID after infection with the Omicron variant appeared to be higher in patients with iRD, with 21% meeting the criteria set by the World Health Organization, compared with 13% of healthy individuals (odds ratio, 1.58; P = .037). Fatigue and loss of fitness were the most common long COVID symptoms reported by both iRD patients and controls. However, the difference in risk decreased after accounting for factors that are significantly associated with an increased risk for long COVID, such as body mass index and the severity of the acute COVID-19 infection (adjusted OR, 1.46; P = .081). The duration of symptoms did not show a statistically significant difference.
Kim Lauper, MD, University of Geneva, who chaired the session in which Ms. Boekel reported the study, said in an interview that the data should be interpreted with caution. “The data demonstrate that rheumatic disease itself is not a risk factor for long COVID. However, patients with rheumatic diseases are at a higher risk of severe disease, which in turn increases the likelihood of long COVID. Therefore, as a population, these patients are more susceptible to long COVID overall.”
Moreover, irrespective of their previous COVID-19 infection status, iRD patients often exhibit symptoms similar to those of long COVID even without a prior COVID-19 infection. (There was no history of COVID-19 in 21% of iRD patients vs. 11% of controls.) This suggests that some of the reported long COVID symptoms may actually be clinical manifestations of the underlying rheumatic disease, thereby complicating the diagnosis of long COVID in this population. The study employed the WHO definition of long COVID, which includes persistent symptoms lasting at least 8 weeks, beginning within 3 months of a confirmed SARS-CoV-2 infection, and that cannot be attributed to an alternative diagnosis. However, the data presented in Milan indicate that the WHO definition “is not well suited for patients with iRD due to significant overlap in symptoms and features,” Ms. Boekel concluded.
The cases of Omicron COVID-19 were identified during Jan. 1–April 25, 2022, among iRD patients recruited from the Amsterdam Rheumatology and Immunology Center. The population with confirmed SARS-CoV-2 Omicron infection during this period was monitored for long COVID. The total number of patients included in the study consisted of 77 iRD patients and 23 healthy controls. When asked about the potential risk of selection bias in the survey, Ms. Boekel stated that only approximately 8% of participants declined to respond, and the nonresponders were comparable with the respondents. She concluded that “the risk of selection bias is minimal.”
In an editorial published in The Lancet Rheumatology, Leonard H. Calabrese, DO, Cleveland Clinic, provided his insights on the findings. He emphasized that, “at present, long COVID remains an important reality that significantly impacts the lives of millions of individuals, yet it remains incompletely defined. ... These limitations in defining cases should not in any way undermine the experiences of those suffering from long COVID. Instead, they should serve as a reminder that, at this stage of the pandemic, we unfortunately still lack validated classification criteria for long COVID. It is crucial to include non–SARS-CoV-2–infected controls in all studies to further enhance our understanding.”
Ms. Boekel and coauthors, as well as Dr. Lauper and Dr. Calabrese, reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
MILAN – presented at the annual European Congress of Rheumatology.
Although more patients with inflammatory rheumatic diseases (iRD) report symptoms resembling long COVID, the data suggest that many of these symptoms can be attributed to the underlying rheumatic disease. “Overall, we find the data quite reassuring,” said Laura Boekel, Amsterdam Rheumatology and Immunology Center, Amsterdam University Medical Center.
The results were also published in The Lancet Rheumatology.
The risk of developing long COVID after infection with the Omicron variant appeared to be higher in patients with iRD, with 21% meeting the criteria set by the World Health Organization, compared with 13% of healthy individuals (odds ratio, 1.58; P = .037). Fatigue and loss of fitness were the most common long COVID symptoms reported by both iRD patients and controls. However, the difference in risk decreased after accounting for factors that are significantly associated with an increased risk for long COVID, such as body mass index and the severity of the acute COVID-19 infection (adjusted OR, 1.46; P = .081). The duration of symptoms did not show a statistically significant difference.
Kim Lauper, MD, University of Geneva, who chaired the session in which Ms. Boekel reported the study, said in an interview that the data should be interpreted with caution. “The data demonstrate that rheumatic disease itself is not a risk factor for long COVID. However, patients with rheumatic diseases are at a higher risk of severe disease, which in turn increases the likelihood of long COVID. Therefore, as a population, these patients are more susceptible to long COVID overall.”
Moreover, irrespective of their previous COVID-19 infection status, iRD patients often exhibit symptoms similar to those of long COVID even without a prior COVID-19 infection. (There was no history of COVID-19 in 21% of iRD patients vs. 11% of controls.) This suggests that some of the reported long COVID symptoms may actually be clinical manifestations of the underlying rheumatic disease, thereby complicating the diagnosis of long COVID in this population. The study employed the WHO definition of long COVID, which includes persistent symptoms lasting at least 8 weeks, beginning within 3 months of a confirmed SARS-CoV-2 infection, and that cannot be attributed to an alternative diagnosis. However, the data presented in Milan indicate that the WHO definition “is not well suited for patients with iRD due to significant overlap in symptoms and features,” Ms. Boekel concluded.
The cases of Omicron COVID-19 were identified during Jan. 1–April 25, 2022, among iRD patients recruited from the Amsterdam Rheumatology and Immunology Center. The population with confirmed SARS-CoV-2 Omicron infection during this period was monitored for long COVID. The total number of patients included in the study consisted of 77 iRD patients and 23 healthy controls. When asked about the potential risk of selection bias in the survey, Ms. Boekel stated that only approximately 8% of participants declined to respond, and the nonresponders were comparable with the respondents. She concluded that “the risk of selection bias is minimal.”
In an editorial published in The Lancet Rheumatology, Leonard H. Calabrese, DO, Cleveland Clinic, provided his insights on the findings. He emphasized that, “at present, long COVID remains an important reality that significantly impacts the lives of millions of individuals, yet it remains incompletely defined. ... These limitations in defining cases should not in any way undermine the experiences of those suffering from long COVID. Instead, they should serve as a reminder that, at this stage of the pandemic, we unfortunately still lack validated classification criteria for long COVID. It is crucial to include non–SARS-CoV-2–infected controls in all studies to further enhance our understanding.”
Ms. Boekel and coauthors, as well as Dr. Lauper and Dr. Calabrese, reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023