User login
In Case You Missed It: COVID
Rationale for baricitinib’s use in COVID-19 patients demonstrated
It should not be surprising that the RA drug baricitinib (Olumiant), a Janus kinase (JAK) 1/2 inhibitor, might be beneficial in controlling the cytokine storm of hyperinflammation that can follow severe SARS-CoV-2 infections and lead to lung damage and acute respiratory distress syndrome – the leading cause of death from the virus.
But to demonstrate within a matter of months, at least preliminarily, that baricitinib reduces mortality and morbidity in hospitalized patients with COVID-19 pneumonia required a widely cross-disciplinary international team of researchers from 10 countries working at breakneck speed, said Justin Stebbing, PhD, the principal investigator of a new baricitinib study published Nov. 13 in Science Advances. “We went from modeling and mechanistic investigations to clinical tests in a number of settings and laboratory analysis in record time.”
The international team of 50 researchers included medical specialists in rheumatology, virology, geriatrics, oncology, and general medicine, along with experts in molecular and cellular biology, bioinformatics, statistics and trial design, computer modeling, pathology, genetics, and super-resolution microscopy, Dr. Stebbing, professor of cancer medicine and medical oncology at Imperial College, London, said in an interview.
Artificial intelligence, provided by the London-based firm BenevolentAI, was used to sift through a huge repository of structured medical information to identify drugs that might block the SARS-CoV-2 infection process. It predicted that baricitinib would be a promising candidate to inhibit inflammation and reduce viral load in COVID-19. Previous reports by Dr. Stebbing and colleagues (here and here) describe this AI-mediated testing, which was validated by the new study.
The researchers also used three-dimensional miniature human liver organoids in vitro and super-resolution microscopy to perform further lab investigations, which showed that baricitinib reversed expression of the SARS-CoV-2 receptor ACE2 triggered by type I interferons. Baricitinib inhibited the significant increase in ACE2 expression caused by interferon alpha-2, and thus cytokine-mediated inflammation, and also reduced infectivity, Dr. Stebbing said. “Our study of baricitinib shows that it has both antiviral and anticytokine effects and appears to be safe.”
71% mortality reduction
The team found a 71% reduction in mortality for a group of 83 hospitalized patients with COVID-19 pneumonia in Italy and Spain – early epicenters of the pandemic – who received baricitinib along with standard care, compared with propensity-matched groups that received only standard care. At that time, between mid-March and mid-April, standard COVID-19 care included antibiotics, glucocorticoids, hydroxychloroquine, low-molecular-weight heparin, and the antiretroviral combination lopinavir/ritonavir.
In the Spanish and Italian cohorts, baricitinib was generally well tolerated, although not without side effects, including bacterial infections and increases in liver enzyme levels, which may not have been related to baricitinib. Patients showed reductions in inflammation within days of starting treatment. “We did not observe thrombotic or vascular events in our cohorts, but most of the patients were receiving low molecular weight heparin,” he said.
The fact that baricitinib is approved by the Food and Drug Administration, is already well studied for safety, can be taken conveniently as a once-daily oral tablet, and is less expensive than many other antiviral treatments all make it an good target for further study, including randomized, controlled trials that are already underway, Dr. Stebbing noted. His study cohort also included elderly patients (median age, 81 years) who are the most likely to experience severe disease or death from COVID-19.
The National Library of Medicine’s clinicaltrials.gov registry of federally funded clinical studies lists 15 current research initiatives involving baricitinib and COVID-19. Dr. Stebbing suggested that data generated so far are helping to guide ongoing studies on dose and duration of treatment – in other words, who it works for, when to give it, and at what dose it should be taken and for how long.
Manufacturer Eli Lilly, which markets baricitinib in 2-mg or 4-mg tablets, announced in October that initial data are starting to emerge from 1,000-plus patients enrolled in ACTT-2 (the Adaptive COVID-19 Treatment Trial 2). ACTT-2 compared patients on the broad-spectrum intravenous antiviral drug remdesivir (Veklury) with those receiving remdesivir in combination with baricitinib. Based on ACTT-2 results that suggested a reduced time to recovery and improved clinical outcomes for the combination group, the FDA issued an emergency-use authorization on Nov. 19 for the combination of baricitinib and remdesivir for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients aged 2 years or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Interrupting the cytokine outbreak
Baricitinib has the potential to reduce or interrupt the passage of SARS-CoV-2 into cells, and thus to inhibit the JAK1- and JAK2-mediated cytokine outbreak, researcher Heinz-Josef Lenz, MD, professor of medicine and preventive medicine at the University of Southern California’s Norris Comprehensive Cancer Center in Los Angeles, said in a comment. Baricitinib was also identified, using BenevolentAI’s proprietary, artificial intelligence-derived knowledge graph, as a numb-associated kinase inhibitor, with high affinity for AP2-associated protein kinase 1, an important endocytosis regulator.
Early clinical data suggest a potent biologic effect of baricitinib 2 mg or 4 mg daily on circulating interleukin-6 levels and other inflammatory markers, including C-reactive protein. Dr. Lenz said the evidence for advantageous action of baricitinib on viral endocytosis and excessive cytokine release constitutes the rationale for using it in combination with other antivirals such as remdesivir in patients with moderate to severe COVID-19 illness.
“Although baricitinib may display antiviral activity on its own, its anti-inflammatory effects could hypothetically delay viral clearance,” Dr. Lenz added. “The data from Stebbing et al. confirm the dual actions of baricitinib, demonstrating its ability to inhibit viral entry into primary human hepatocyte spheroids and the reduction in inflammatory markers in COVID-19 patients.”
Other JAK inhibitors were not advanced as promising candidates for the research team’s attention by its artificial intelligence search, Dr. Stebbing noted. “The history of the pandemic has taught us the importance of well-designed observational studies as well as randomized, controlled trials. When it comes to COVID, pyrite looks much like gold, as failed studies of four antivirals have shown.”
Although the current translational research study did not use a placebo group, it is an important next step toward future randomized, controlled trials. “What’s great about this study is its high degree of collaboration, done with real urgency,” he added. “It’s harder to produce a paper that crosses multiple boundaries, like this one does, than a single-focused piece of work. But we wanted to link all of these threads together.”
The study was supported by the Imperial Biomedical Research Centre and Experimental Cancer Medicine Centre, the National Institute for Health Research, and the U.K. National Health Service’s Accelerated Access Collaborative. Dr. Stebbing has served on scientific advisory boards for Eli Lilly and other companies. Dr. Lenz had no relevant disclosures to report.
SOURCE: Stebbing J et al. Sci Adv. 2020 Nov 13. doi: 10.1126/sciadv.abe4724.
It should not be surprising that the RA drug baricitinib (Olumiant), a Janus kinase (JAK) 1/2 inhibitor, might be beneficial in controlling the cytokine storm of hyperinflammation that can follow severe SARS-CoV-2 infections and lead to lung damage and acute respiratory distress syndrome – the leading cause of death from the virus.
But to demonstrate within a matter of months, at least preliminarily, that baricitinib reduces mortality and morbidity in hospitalized patients with COVID-19 pneumonia required a widely cross-disciplinary international team of researchers from 10 countries working at breakneck speed, said Justin Stebbing, PhD, the principal investigator of a new baricitinib study published Nov. 13 in Science Advances. “We went from modeling and mechanistic investigations to clinical tests in a number of settings and laboratory analysis in record time.”
The international team of 50 researchers included medical specialists in rheumatology, virology, geriatrics, oncology, and general medicine, along with experts in molecular and cellular biology, bioinformatics, statistics and trial design, computer modeling, pathology, genetics, and super-resolution microscopy, Dr. Stebbing, professor of cancer medicine and medical oncology at Imperial College, London, said in an interview.
Artificial intelligence, provided by the London-based firm BenevolentAI, was used to sift through a huge repository of structured medical information to identify drugs that might block the SARS-CoV-2 infection process. It predicted that baricitinib would be a promising candidate to inhibit inflammation and reduce viral load in COVID-19. Previous reports by Dr. Stebbing and colleagues (here and here) describe this AI-mediated testing, which was validated by the new study.
The researchers also used three-dimensional miniature human liver organoids in vitro and super-resolution microscopy to perform further lab investigations, which showed that baricitinib reversed expression of the SARS-CoV-2 receptor ACE2 triggered by type I interferons. Baricitinib inhibited the significant increase in ACE2 expression caused by interferon alpha-2, and thus cytokine-mediated inflammation, and also reduced infectivity, Dr. Stebbing said. “Our study of baricitinib shows that it has both antiviral and anticytokine effects and appears to be safe.”
71% mortality reduction
The team found a 71% reduction in mortality for a group of 83 hospitalized patients with COVID-19 pneumonia in Italy and Spain – early epicenters of the pandemic – who received baricitinib along with standard care, compared with propensity-matched groups that received only standard care. At that time, between mid-March and mid-April, standard COVID-19 care included antibiotics, glucocorticoids, hydroxychloroquine, low-molecular-weight heparin, and the antiretroviral combination lopinavir/ritonavir.
In the Spanish and Italian cohorts, baricitinib was generally well tolerated, although not without side effects, including bacterial infections and increases in liver enzyme levels, which may not have been related to baricitinib. Patients showed reductions in inflammation within days of starting treatment. “We did not observe thrombotic or vascular events in our cohorts, but most of the patients were receiving low molecular weight heparin,” he said.
The fact that baricitinib is approved by the Food and Drug Administration, is already well studied for safety, can be taken conveniently as a once-daily oral tablet, and is less expensive than many other antiviral treatments all make it an good target for further study, including randomized, controlled trials that are already underway, Dr. Stebbing noted. His study cohort also included elderly patients (median age, 81 years) who are the most likely to experience severe disease or death from COVID-19.
The National Library of Medicine’s clinicaltrials.gov registry of federally funded clinical studies lists 15 current research initiatives involving baricitinib and COVID-19. Dr. Stebbing suggested that data generated so far are helping to guide ongoing studies on dose and duration of treatment – in other words, who it works for, when to give it, and at what dose it should be taken and for how long.
Manufacturer Eli Lilly, which markets baricitinib in 2-mg or 4-mg tablets, announced in October that initial data are starting to emerge from 1,000-plus patients enrolled in ACTT-2 (the Adaptive COVID-19 Treatment Trial 2). ACTT-2 compared patients on the broad-spectrum intravenous antiviral drug remdesivir (Veklury) with those receiving remdesivir in combination with baricitinib. Based on ACTT-2 results that suggested a reduced time to recovery and improved clinical outcomes for the combination group, the FDA issued an emergency-use authorization on Nov. 19 for the combination of baricitinib and remdesivir for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients aged 2 years or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Interrupting the cytokine outbreak
Baricitinib has the potential to reduce or interrupt the passage of SARS-CoV-2 into cells, and thus to inhibit the JAK1- and JAK2-mediated cytokine outbreak, researcher Heinz-Josef Lenz, MD, professor of medicine and preventive medicine at the University of Southern California’s Norris Comprehensive Cancer Center in Los Angeles, said in a comment. Baricitinib was also identified, using BenevolentAI’s proprietary, artificial intelligence-derived knowledge graph, as a numb-associated kinase inhibitor, with high affinity for AP2-associated protein kinase 1, an important endocytosis regulator.
Early clinical data suggest a potent biologic effect of baricitinib 2 mg or 4 mg daily on circulating interleukin-6 levels and other inflammatory markers, including C-reactive protein. Dr. Lenz said the evidence for advantageous action of baricitinib on viral endocytosis and excessive cytokine release constitutes the rationale for using it in combination with other antivirals such as remdesivir in patients with moderate to severe COVID-19 illness.
“Although baricitinib may display antiviral activity on its own, its anti-inflammatory effects could hypothetically delay viral clearance,” Dr. Lenz added. “The data from Stebbing et al. confirm the dual actions of baricitinib, demonstrating its ability to inhibit viral entry into primary human hepatocyte spheroids and the reduction in inflammatory markers in COVID-19 patients.”
Other JAK inhibitors were not advanced as promising candidates for the research team’s attention by its artificial intelligence search, Dr. Stebbing noted. “The history of the pandemic has taught us the importance of well-designed observational studies as well as randomized, controlled trials. When it comes to COVID, pyrite looks much like gold, as failed studies of four antivirals have shown.”
Although the current translational research study did not use a placebo group, it is an important next step toward future randomized, controlled trials. “What’s great about this study is its high degree of collaboration, done with real urgency,” he added. “It’s harder to produce a paper that crosses multiple boundaries, like this one does, than a single-focused piece of work. But we wanted to link all of these threads together.”
The study was supported by the Imperial Biomedical Research Centre and Experimental Cancer Medicine Centre, the National Institute for Health Research, and the U.K. National Health Service’s Accelerated Access Collaborative. Dr. Stebbing has served on scientific advisory boards for Eli Lilly and other companies. Dr. Lenz had no relevant disclosures to report.
SOURCE: Stebbing J et al. Sci Adv. 2020 Nov 13. doi: 10.1126/sciadv.abe4724.
It should not be surprising that the RA drug baricitinib (Olumiant), a Janus kinase (JAK) 1/2 inhibitor, might be beneficial in controlling the cytokine storm of hyperinflammation that can follow severe SARS-CoV-2 infections and lead to lung damage and acute respiratory distress syndrome – the leading cause of death from the virus.
But to demonstrate within a matter of months, at least preliminarily, that baricitinib reduces mortality and morbidity in hospitalized patients with COVID-19 pneumonia required a widely cross-disciplinary international team of researchers from 10 countries working at breakneck speed, said Justin Stebbing, PhD, the principal investigator of a new baricitinib study published Nov. 13 in Science Advances. “We went from modeling and mechanistic investigations to clinical tests in a number of settings and laboratory analysis in record time.”
The international team of 50 researchers included medical specialists in rheumatology, virology, geriatrics, oncology, and general medicine, along with experts in molecular and cellular biology, bioinformatics, statistics and trial design, computer modeling, pathology, genetics, and super-resolution microscopy, Dr. Stebbing, professor of cancer medicine and medical oncology at Imperial College, London, said in an interview.
Artificial intelligence, provided by the London-based firm BenevolentAI, was used to sift through a huge repository of structured medical information to identify drugs that might block the SARS-CoV-2 infection process. It predicted that baricitinib would be a promising candidate to inhibit inflammation and reduce viral load in COVID-19. Previous reports by Dr. Stebbing and colleagues (here and here) describe this AI-mediated testing, which was validated by the new study.
The researchers also used three-dimensional miniature human liver organoids in vitro and super-resolution microscopy to perform further lab investigations, which showed that baricitinib reversed expression of the SARS-CoV-2 receptor ACE2 triggered by type I interferons. Baricitinib inhibited the significant increase in ACE2 expression caused by interferon alpha-2, and thus cytokine-mediated inflammation, and also reduced infectivity, Dr. Stebbing said. “Our study of baricitinib shows that it has both antiviral and anticytokine effects and appears to be safe.”
71% mortality reduction
The team found a 71% reduction in mortality for a group of 83 hospitalized patients with COVID-19 pneumonia in Italy and Spain – early epicenters of the pandemic – who received baricitinib along with standard care, compared with propensity-matched groups that received only standard care. At that time, between mid-March and mid-April, standard COVID-19 care included antibiotics, glucocorticoids, hydroxychloroquine, low-molecular-weight heparin, and the antiretroviral combination lopinavir/ritonavir.
In the Spanish and Italian cohorts, baricitinib was generally well tolerated, although not without side effects, including bacterial infections and increases in liver enzyme levels, which may not have been related to baricitinib. Patients showed reductions in inflammation within days of starting treatment. “We did not observe thrombotic or vascular events in our cohorts, but most of the patients were receiving low molecular weight heparin,” he said.
The fact that baricitinib is approved by the Food and Drug Administration, is already well studied for safety, can be taken conveniently as a once-daily oral tablet, and is less expensive than many other antiviral treatments all make it an good target for further study, including randomized, controlled trials that are already underway, Dr. Stebbing noted. His study cohort also included elderly patients (median age, 81 years) who are the most likely to experience severe disease or death from COVID-19.
The National Library of Medicine’s clinicaltrials.gov registry of federally funded clinical studies lists 15 current research initiatives involving baricitinib and COVID-19. Dr. Stebbing suggested that data generated so far are helping to guide ongoing studies on dose and duration of treatment – in other words, who it works for, when to give it, and at what dose it should be taken and for how long.
Manufacturer Eli Lilly, which markets baricitinib in 2-mg or 4-mg tablets, announced in October that initial data are starting to emerge from 1,000-plus patients enrolled in ACTT-2 (the Adaptive COVID-19 Treatment Trial 2). ACTT-2 compared patients on the broad-spectrum intravenous antiviral drug remdesivir (Veklury) with those receiving remdesivir in combination with baricitinib. Based on ACTT-2 results that suggested a reduced time to recovery and improved clinical outcomes for the combination group, the FDA issued an emergency-use authorization on Nov. 19 for the combination of baricitinib and remdesivir for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized adults and pediatric patients aged 2 years or older requiring supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Interrupting the cytokine outbreak
Baricitinib has the potential to reduce or interrupt the passage of SARS-CoV-2 into cells, and thus to inhibit the JAK1- and JAK2-mediated cytokine outbreak, researcher Heinz-Josef Lenz, MD, professor of medicine and preventive medicine at the University of Southern California’s Norris Comprehensive Cancer Center in Los Angeles, said in a comment. Baricitinib was also identified, using BenevolentAI’s proprietary, artificial intelligence-derived knowledge graph, as a numb-associated kinase inhibitor, with high affinity for AP2-associated protein kinase 1, an important endocytosis regulator.
Early clinical data suggest a potent biologic effect of baricitinib 2 mg or 4 mg daily on circulating interleukin-6 levels and other inflammatory markers, including C-reactive protein. Dr. Lenz said the evidence for advantageous action of baricitinib on viral endocytosis and excessive cytokine release constitutes the rationale for using it in combination with other antivirals such as remdesivir in patients with moderate to severe COVID-19 illness.
“Although baricitinib may display antiviral activity on its own, its anti-inflammatory effects could hypothetically delay viral clearance,” Dr. Lenz added. “The data from Stebbing et al. confirm the dual actions of baricitinib, demonstrating its ability to inhibit viral entry into primary human hepatocyte spheroids and the reduction in inflammatory markers in COVID-19 patients.”
Other JAK inhibitors were not advanced as promising candidates for the research team’s attention by its artificial intelligence search, Dr. Stebbing noted. “The history of the pandemic has taught us the importance of well-designed observational studies as well as randomized, controlled trials. When it comes to COVID, pyrite looks much like gold, as failed studies of four antivirals have shown.”
Although the current translational research study did not use a placebo group, it is an important next step toward future randomized, controlled trials. “What’s great about this study is its high degree of collaboration, done with real urgency,” he added. “It’s harder to produce a paper that crosses multiple boundaries, like this one does, than a single-focused piece of work. But we wanted to link all of these threads together.”
The study was supported by the Imperial Biomedical Research Centre and Experimental Cancer Medicine Centre, the National Institute for Health Research, and the U.K. National Health Service’s Accelerated Access Collaborative. Dr. Stebbing has served on scientific advisory boards for Eli Lilly and other companies. Dr. Lenz had no relevant disclosures to report.
SOURCE: Stebbing J et al. Sci Adv. 2020 Nov 13. doi: 10.1126/sciadv.abe4724.
FROM SCIENCE ADVANCES
IDSA updates COVID guidelines for antibodies, antivirals, other drugs
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
An infectious disease expert panel cautions against routine use of bamlanivimab (Eli Lilly) and notes that remdesivir (Veklury) can shorten the clinical course of COVID-19 – which could be critical “as hospitals fill up” across the United States.
The group also said the monoclonal antibodies approved for emergency use by the Food and Drug Administration and still in development hold promise, although more clinical trial data are needed.
These and other recommendations appear in updated guidelines from the Infectious Diseases Society of America, released Nov. 18 and Nov. 22.
A conditional ‘no’ on routine bamlanivimab
“The guideline panel gave a conditional recommendation against the routine use of bamlanivimab,” Adarsh Bhimraj, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel, said.
On Nov. 10, the FDA issued an emergency-use authorization (EUA) for bamlanivimab for use in ambulatory patients with mild to moderate COVID-19.
“We did have a remark that it may be used in patients who have increased risk of severe COVID-19, as it is outlined in the FDA emergency-use authorization issued last week,” he said. He added that use should follow an informed discussion between provider and patient, one in which “the patient puts a very high value on the uncertain benefits and a low value on uncertain adverse events.”
The panel’s rationale was based in part on interim analysis of the phase 2 BLAZE-1 trial, which found 1.6% of people randomly assigned to bamlanivimab had an emergency department visit or hospitalization compared with 6.3% of those receiving a placebo.
“We thought the estimate was too fragile because the number in each arm was very low. Even a small change in these numbers could make the difference nonsignificant,” said Bhimraj, head of the neurologic infectious diseases section in the department of infectious diseases at the Cleveland (Ohio) Clinic.
Awaiting more data on antibody combination
On November 21, the FDA granted an EUA to the casirivimab and imbdevimab monoclonal antibody combination (Regeneron), indicated to treated mild to moderate COVID-19.
“Surprisingly, the preliminary results released in the EUA look a lot like bamlanivimab,” Dr. Bhimraj said.
Unlike bamlanivimab, for which trial details were published, the panel does not yet have the totality of data on casirivimab and imbdevimab, and therefore is not yet making a recommendation. “We want to be cautious as a guideline panel. We are anxiously awaiting the full publication,” he added.
“I do think these monoclonal antibodies show potential for benefit, but as Dr. Bhimraj said, it’s very difficult with the relatively small numbers we’re talking about,” said Rajesh T. Gandhi, MD, cochair of the IDSA COVID-19 Treatment and Management Guidelines Expert Panel.
Remaining questions include the degree of efficacy these antibody therapies will have, as well as which patients are most likely to benefit, added Dr. Gandhi, who is also a professor of medicine at Harvard Medical School and director of HIV Clinical Services and Education at Massachusetts General Hospital, both in Boston.
Furthermore, although there appear to be adequate supplies of remdesivir and dexamethasone, for example, availability and distribution of monoclonal antibodies could present logistic challenges. Prioritizing which high-risk patients receive this therapy and ensuring equity and access to communities most affected by COVID-19, including minority and low socioeconomic populations, need to be addressed, Dr. Gandhi said.
Remdesivir recommended to shorten hospital stays
The panel’s recommendations regarding the use of remdesivir “has largely remained the same,” Dr. Gandhi said. Evidence indicates recovery is faster with remdesivir at 10 days vs 15 days in people taking a placebo.
In the ACTT-1 trial, for example, participants in the treatment group recovered in a median 10 days versus 15 days in the placebo group.
Therefore, the IDSA panel continues to recommend remdesivir treatment for hospitalized patients with COVID-19.
“As hospitals around the United States fill up, the IDSA panel believes the effect of remdesivir on speeding up recovery could be an important benefit, and that is why we continue to suggest its use,” Dr. Gandhi said.
When asked about the World Health Organization–sponsored trial that showed no benefit in terms of mortality, he replied, “Remdesivir is not a home run – we need better drugs.”
A recommendation against lopinavir and ritonavir
In contrast, the panel recommends against use of the lopinavir/ritonavir protease inhibitor combination therapy, based in part on data from a preprint of the Solidarity study.
The open-label Solidarity trial in 30 countries, sponsored by WHO, assessed hydroxychloroquine, interferon, lopinavir/ritonavir, and remdesivir in people hospitalized with COVID-19.
None of these drugs showed an effect on mortality, Dr. Gandhi said. “Better medicines that improve survival are clearly needed.”
Dexamethasone remains the only agent demonstrated to reduce mortality in people hospitalized with COVID-19, he added.
Tocilizumab not for routine use
After critical review of the studies that have emerged since the last IDSA recommendation regarding tocilizumab (Actemra) in September, “the panel still stood with the recommendation against routine use of tocilizumab in hospitalized patients with COVID-19,” Dr. Bhimraj said.
The guidance is based on trials including COVACTA and EMPACTA. Treatment with tocilizumab was not associated with significant differences in mortality. In these and other studies, “we did not really find a significant difference, and that was the reason for the conditional recommendation against routine use of tocilizumab in hospitalized patients,” Dr. Bhimraj said.
Also, although the trials were blinded, “we know treatment with tocilizumab can cause a reduction in C-reactive protein levels,” which could indicate to researchers which participants were receiving active treatment versus placebo, he said.
Jury still out on baricitinib, remdesivir combination
The FDA granted an EUA to the combination of remdesivir and baricitinib (Olumiant) on Nov. 19. However, the IDSA panel is reserving its recommendation on this therapeutic combination until more data emerge.
“We still don’t have complete results of the ACTT-2 study, and the information we do have is what is available in the EUA,” Dr. Bhimraj said. The panel expects to issue guidance once the totality of data become available.
Unanswered questions include why investigators chose a 4-mg dose of baricitinib – twice the 2-mg dose commonly used for treating rheumatoid arthritis – and how many patients in the trial also were treated with steroids.
Dr. Gandhi agreed that the proportion of patients taking a steroid is “really an important consideration.” He added that dexamethasone has become standard of care because it reduces mortality, as well as the number of people requiring oxygen. He said it will be important to know how the baricitinib/remdesivir combination compares with dexamethasone.
“You don’t want to give a drug with less certain benefit over a drug for which there is more certain benefit,” Dr. Gandhi said.
Future possibilities
“The monoclonal antibodies are important to continue studying, particularly in combinations,” Dr. Gandhi said. Researchers are investigating formulations other than IV infusion to make therapy more convenient. For example, a subcutaneous injection like insulin would make administration at home more of a possibility.
Investigators are also looking at oral antiviral therapy, inhaled antivirals, and the promise of using interferon therapy. Dr. Gandhi added there is also “a lot of work around medications to reduce the excess inflammation that drives very severe COVID-19.”
‘Exciting news’ on AstraZeneca vaccine
Although not part of the IDSA guidelines, “we saw the news from AstraZeneca this morning, which is exciting,” Dr. Gandhi said during a media briefing.
Unlike the Pfizer and Moderna messenger RNA vaccines, which use the genetic material of the virus to make the virus proteins that elicit an immune response, the AstraZeneca/Oxford University vaccine uses a viral vector to carry the SARS-CoV-2 protein, to which the body produces an immune response.
“I’m thrilled that several different vaccines are showing important effects at rates higher than the FDA benchmark of 50%, and these are well exceeding that,” Dr. Gandhi said.
“One interesting thing from the [AstraZeneca] press release is they show asymptomatic infection being reduced,” he added. “That is critical because we know a lot of transmission of SARS-CoV-2 comes from asymptomatic people.”
Reasons for optimism
In response to a question about whether the experts feel more optimistic about COVID-19, Dr. Bhimraj said he is cautiously optimistic. “We have made tremendous progress in therapeutic agents, and in how the world has come together in the middle of a catastrophe to collaborate, setting our differences apart, to do trials. That is commendable.”
Dr. Gandhi said he felt more optimistic than he did in the spring. He pointed out that physicians and researchers know a lot more about potential blood clotting complications, how to support patients through severe COVID-19 and keep them off a ventilator whenever possible, and how to provide dexamethasone to reduce the risk of death.
Those benefits are in hospitalized patients, however, and “we need ways to prevent people from getting into the hospital, and that is why we are looking at the monoclonal antibodies,” Dr. Gandhi said. “If borne out in larger trials, that will be a major advance.”
“We need to keep our focus on prevention and go back to our idea of flattening the curve. That is critical so our health care systems do not get overwhelmed during this massive surge we are in,” Dr. Gandhi said. “So masking and social distancing are just as important as they always have been.”
Dr. Bhimraj disclosed no relevant financial relationships. Dr. Gandhi has no disclosures for the past 12 months; in the past 3 years, he has served on scientific advisory boards for Gilead and Merck.
This article first appeared on Medscape.com.
Equitable Post-COVID-19 Care: A Practical Framework to Integrate Health Equity in Diabetes Management
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.
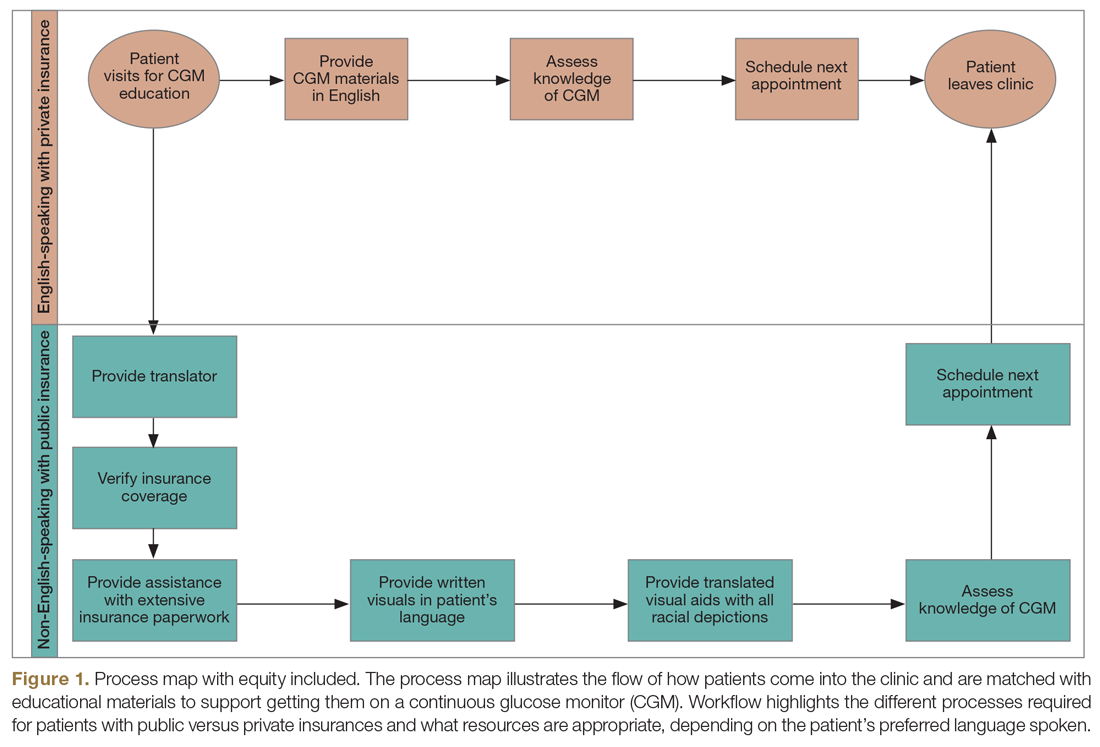
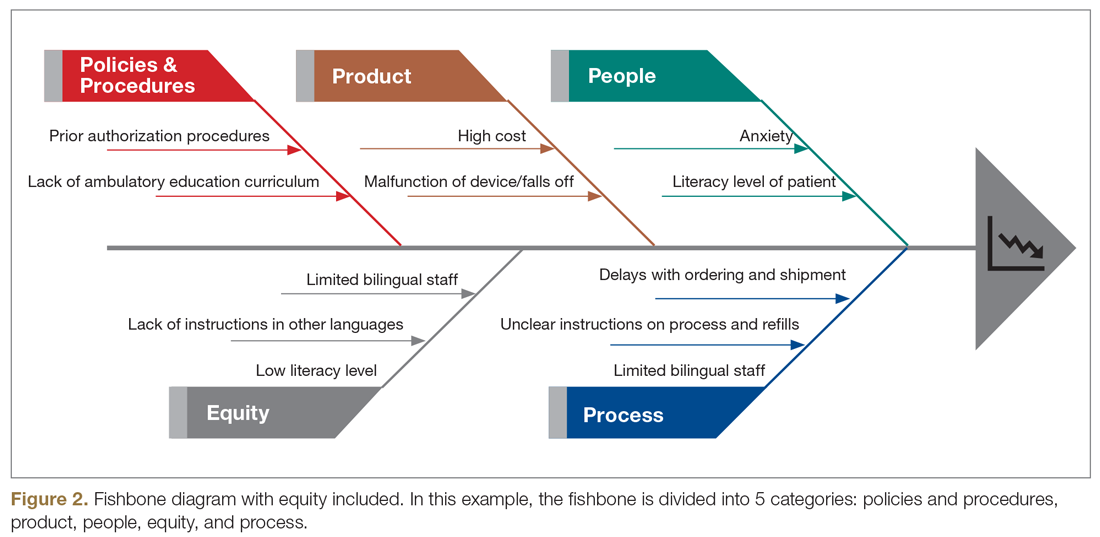
Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.
Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
From T1D Exchange, Boston, MA (Dr. Ebekozien, Dr. Odugbesan, and Nicole Rioles); Barbara Davis Center, University of Colorado, Boulder, CO (Dr. Majidi); Cincinnati Children’s Hospital Medical Center, Cincinnati, OH (Dr. Jones); and Nationwide Children’s Hospital, Columbus, OH (Dr. Kamboj)
Health equity has been described as the opportunity for all persons to obtain their highest level of health possible.1 Unfortunately, even with advances in technology and care practices, disparities persist in health care outcomes. Disparities in prevalence, prognosis, and outcomes still exist in diabetes management.2 Non-Hispanic Black and/or Hispanic populations are more likely to have worse glycemic control,3,4 to encounter more barriers in access to care,5 and to have higher levels of acute complications,4 and to use advanced technologies less frequently.4 Diabetes is one of the preexisting conditions that increase morbidity and mortality in COVID-19.6,7 Unfortunately, adverse outcomes from COVID-19 also disproportionately impact a specific vulnerable population.8,9 The urgent transition to managing diabetes remotely during the COVID-19 pandemic may exacerbate long-term inequities because some vulnerable patients might not have access to technology devices necessary for effective remote management.
Here, we describe how quality improvement (QI) tools and principles can be adapted into a framework for advancing health equity. Specifically, we describe a 10-step framework that may be applied in diabetes care management to achieve improvement, using a hypothetical example of increasing use of continuous glucose monitors (CGMs) among patients with type 1 diabetes mellitus.10 This framework was developed to address the literature gap on practical ways health care providers can address inequities using QI principles, and was implemented by 1 of the authors at a local public health department.11 The framework’s iterative and comprehensive design makes it ideal for addressing inequities in chronic diseases like diabetes, which have multiple root causes with no easy solutions. The improvement program pilot received a national model practice award.11,12
10-Step Framework
Step 1: Review program/project baseline data for existing disparities. Diabetes programs and routine QI processes encourage existing data review to determine how effective the current system is working and if the existing process has a predictable pattern.13,14 Our equity-revised framework proposes a more in-depth review to stratify baseline data based on factors that might contribute to inequities, including race, age, income levels, ethnicity, language, sexual orientation, insurance type, and zip code. This process will identify patients not served or unfairly impacted due to socioeconomic factors. For example, using the hypothetical example of improving CGM use, a team completes a preliminary data review and determines that baseline CGM use is 30% in the clinic population. However, in a review to assess for disparities, they also identify that patients on public insurance have a significantly lower CGM uptake of only 15%.
Step 2: Build an equitable project team, including patients with lived experiences. Routine projects typically have clinicians, administrative staff, and analytic staff as members of their team. In a post-COVID-19 world, every team needs to learn directly from people impacted and share decision-making power. The traditional approach to receiving feedback has generally been to collect responses using surveys or focus groups. We propose that individuals/families who are disproportionately impacted be included as active members on QI teams. For example, in the hypothetical example of the CGM project, team members would include patients with type 1 diabetes who are on public insurance and their families.
Step 3: Develop equity-focused goals. The traditional program involves the development of aims that are SMART (specific, measurable, achievable, realistic, time-bound).15 The proposed framework encourages the inclusion of equity-revised goals (SMARTer) using insights from Steps 1 and 2. For example, your typical smart goal might be to increase the percentage of patients using CGM by 20% in 6 months, while a SMARTer goal would be to increase the proportion of patients using CGM by 20% and reduce the disparities among public and private insurance patients by 30% in 6 months.
Step 4: Identify inequitable processes/pathways. Traditional QI programs might use a process map or flow diagram to depict the current state of a process visually.16 For example, in Figure 1, the process map diagram depicts some differences in the process for patients with public insurance as opposed to those with private insurance. The framework also advocates for using visual tools like process maps to depict how there might be inequitable pathways in a system. Visually identifying inequitable processes/pathways can help a team see barriers, address challenges, and pilot innovative solutions.

Step 5: Identify how socioeconomic factors are contributing to the current outcome. A good understanding of factors that contribute to the problem is an essential part of finding fundamental solutions. The fishbone diagram16 is a visualization tool used to identify contributing factors. When investigating contributing factors, it is commonplace to identify factors that fit into 1 of 5 categories: people, process, place, product, and policies (5 Ps). An equity-focused process will include equity as a new major factor category, and the socioeconomic impacts that contribute to inequities will be brainstormed and visually represented. For example, in the hypothetical CGM improvement example, an equity contributing factor is extensive CGM application paperwork for patients on public insurance as compared to those on private insurance. Figure 2 shows equity integrated into a fishbone diagram.

Step 6: Brainstorm possible improvements. Potential improvement ideas for the hypothetical CGM example might include redesigning the existing workflow, piloting CGM educational classes, and using a CGM barrier assessment tool to identify and address barriers to adoption.
Step 7: Use the decision matrix with equity as a criterion to prioritize improvement ideas. Decision matrix15 is a great tool that is frequently used to help teams prioritize potential ideas. Project team members must decide what criteria are important in prioritizing ideas to implement. Common criteria include implementation cost, time, and resources, but in addition to the common criteria, the team can specify ”impact on equity” as one of their criteria, alongside other standard criteria like impact.
Step 8: Test one small change at a time. This step is consistent with other traditional improvement models using the Plan, Do, Study, Act (PDSA) model for improvement.17 During this phase, the team should make predictions on the expected impact of the intervention on outcomes. For example, in the hypothetical example, the team predicts that testing and expanding CGM classes will reduce disparities among public versus private health insurance users by 5% and increase overall CGM uptake by 10%.
Step 9: Measure and compare results with predictions to identify inequitable practices or consequences. After each test of change, the team should review the results, including implementation cost considerations, and compare them to the predictions in the earlier step. The team should also document the potential reasons why their predictions were correct or inaccurate, and whether there were any unforeseen outcomes from the intervention.
Step 10: Celebrate small wins and repeat the process. Making fundamental and equitable changes takes time. This framework aimed at undoing inequities, particularly those inequities that have been amplified by the COVID-19 pandemic, is iterative and ongoing.18,19 Not every test of change will impact the outcome or reduce inequity, but over time, each change will impact the next, generating sustainable effects.
Conclusion
There are ongoing studies examining the adverse outcomes and potential health inequities for patients with diabetes impacted by COVID-19.20 Health care providers need to plan for post-COVID-19 care, keeping in mind that the pandemic might worsen already existing health disparities in diabetes management.3,4,21 This work will involve an intentional approach to address structural and systemic racism.22 Therefore, the work of building health equity solutions must be rooted in racial justice, economic equity, and equitable access to health care and education.
Initiatives like this are currently being funded through foundation grants as well as state and federal research or program grants. Regional and national payors, including the Centers for Medicare & Medicaid Services, are currently piloting long-term sustainable funding models through programs like accountable care organizations and the Accountable Health Communities Model.23
Health systems can successfully address health equity and racial justice, using a framework as described above, to identify determinants of health, develop policies to expand access to care for the most vulnerable patients, distribute decision-making power, and train staff by naming structural racism as a driver of health inequities.
Acknowledgment: The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1D Exchange Quality Improvement Collaborative, who continually seek to improve care and outcomes for people living with diabetes.
Corresponding author: Osagie Ebekozien, MD, 11 Avenue De La Fayette, Boston, MA 02115; [email protected].
Financial disclosures: None.
Funding: T1D Exchange QI Collaborative is funded by The Leona M. and Harry B. Helmsley Charitable Trust. No specific funding was received for this manuscript or the development of this framework.
Keywords: type 1 diabetes; quality improvement; QI framework; racial justice; health disparities.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
1. American Public Health Association Health Equity web site. https://www.apha.org/topics-and-issues/health-equity. Accessed June 4, 2020.
2. Lado J, Lipman T. Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinol Metab Clin North Am. 2016;45:453-461.
3. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1:e181851.
4. Willi SM, Miller KM, DiMeglio LA, et al; T1D Exchange Clinic Network. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics. 2015;135:424-434.
5. Valenzuela JM, Seid M, Waitzfelder B, et al. Prevalence of and disparities in barriers to care experienced by youth with type 1 diabetes. J Pediatr. 2014;164:1369-1375.
6. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
7. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012-2022.
8. Laurencin CT, McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398-402.
9. Shah M, Sachdeva M, Dodiuk-Gad RP. COVID-19 and racial disparities. J Am Acad Dermatol. 2020;83:e35.
10. Ebekozien O, Rioles N, DeSalvo D, et al. Improving continuous glucose monitoring (CGM) use across national centers: results from the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Diabetes. 2020;69(Supplement 1):145-LB.
11. Ebekozien O. QI methodology to address health equity. Presented at American Society of Quality BOSCON 2018; Boston, MA; March 19 and 20, 2018.
12. 2019 Model Practice Award, Building A Culture of Improvement. National Association of County and City Health Officials web site. www.naccho.org/membership/awards/model-practices. Accessed June 4, 2020.
13. Nuckols TK, Keeler E, Anderson LJ, et al. Economic evaluation of quality improvement interventions designed to improve glycemic control in diabetes: a systematic review and weighted regression analysis. Diabetes Care. 2018;41:985‐993.
14. Rossi MC, Nicolucci A, Arcangeli A, et al. Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care. 2008;31:2166‐2168.
15. McQuillan RF, Silver SA, Harel Z, et al. How to measure and interpret quality improvement data. Clin J Am Soc Nephrol. 2016;11:908-914.
16. Siddiqi FS. Quality improvement in diabetes care: time for us to step up? Can J Diabetes. 2019;43:233.
17. Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290‐298.
18. Ferdinand KC, Nasser SA. African American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746-2748..
19. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318:E736-E741.
20. Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43:e83-e85.
21. Majidi S, Ebekozien O, Noor N, et al. Inequities in health outcomes among patients in the T1D Exchange-QI Collaborative. Diabetes. 2020;69(Supplement 1):1220-P. https://doi.org/10.2337/ db20-1220.-P.
22. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20-47.
23. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. CMS.gov web site. https://innovation.cms.gov/innovation-models/ahcm. Accessed October 10, 2020.
Telepsychiatry poised to thrive after the pandemic
Once the fog lifts on a global pandemic that led to an explosion in telehealth visits in 2020, mental health experts expect virtual and in-person visits to merge to become a standard model of care in clinical psychiatry.
Hybrid care is the future, Peter Yellowlees, MBBS, MD, a professor of psychiatry and chief wellness officer at the University of California, Davis, said in an interview. “I’ve been working this way for several years – where all of my patients get to see me in person or online, or both.”
The model’s increasing popularity reflects a major shift toward virtual consults. Telemedicine offers safer, quicker, and less expensive alternatives, Steven Chan, MD, MBA, of Stanford (Calif.) University, said in an interview. “This continuity is essential to helping to reduce emergency room visits, reduce inpatient hospitalizations and readmissions, and improving adherence to treatment,” Dr. Chan said. State and federal regulators’ actions to lift certain licensing and prescribing restrictions and expand coverage made it easier for clinical psychiatrists to offer and get paid for these services.
The catch is that no one knows whether these easements will remain in place once COVID-19 recedes, ending the national public health emergency, Jay H. Shore, MD, MPH, chairperson of the American Psychiatric Association telepsychiatry committee, and professor and director of telemedicine programming at the University of Colorado at Denver, Aurora’s department of psychiatry, said in an interview. “These all temporarily changed during this time period, but we have no idea when they’re going to end or if they’re going to continue off of COVID. So now, there’s a lot of uncertainty.”
‘Suite of different technologies’
New freedoms to deliver telehealth care left some practices scrambling to adopt or refine technology. Once COVID-19 hit, “I can’t think of a psychiatrist or provider or institution that didn’t have to rapidly virtualize and do at least some video conferencing,” Dr. Shore said. This immediate shift signaled a key move toward hybrid patient-doctor relations. “It means you hold a relationship with your patients through multiple different mediums, email, portal, telephone, in person. It’s not just about in-person versus video, it’s about a suite of different technologies.”
Dr. Shore began practicing telehealth 20 years ago, long before the age of COVID. He’s since established rewarding relationships with patients he’s never met. “I’ve done everything from medical management to long-term psychotherapy, group psychotherapy, and I’ve been successful with different populations.”
Many nuances exist around matching the right patient with the right videoconferencing adaptation, Dr. Shore continued. However, “in general, the literature supports that you can get equal clinical outcomes with telehealth versus in-person treatment.”
‘I see their garden’
While some may eschew the idea of providing care over a virtual platform, other physicians see it as an insightful window into a person’s mental state. “I think some are actually quite surprised by how much good care you can give using video,” said Dr. Yellowlees. Unlike a phone conversation, video allows you to see a person’s home. “The beauty of a video is not just that you can see the person, you can also look around their home, and learn more about them.”
In his own visits, he asks patients to take him on a virtual “walk” through their house, provided there are no confidentiality issues.
“I get to meet their pets, the carers, the spouses; I get to see their garden. I get to see what their interests are from looking at the paintings on their walls. I learn more about my patients that way. If you use video to purely see people from the neck up, then that’s fine, but I think you can also use it to your advantage, seeing people at home.” He also encourages patients to do visits in their cars – as long as the windows are shut, they’re in a safe area, and most importantly, they’re not driving.
Nina Vasan, MD, MBA, who treats patients at Stanford and in a concierge private practice for executives, agrees that seeing patients in the home offers a more direct view of patients’ lives. “These little extra pieces of information are things that we didn’t get before COVID, when patients would come by themselves into the office week after week. I do feel like I know some patients much better by being able to see their surroundings and home interactions,” Dr. Vasan, founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation and chair of the APA’s committee on innovation, said in an interview.
She uses Zoom for her sessions, and regularly texts with her concierge patients. In both clinics, where most visits have gone virtual, “the no-show rate is near zero. Before COVID, it was around 10%-20%.”
Safety is a key advantage of virtual visits, noted Dr. Shore. “It’s certainly better than some of the risks of seeing people in person.”
Lack of integration causes frustration
Some challenges exist around access and adaptation of virtual technology. The pandemic’s sudden onslaught left many clinicians scrambling to adjust to a virtual format without any training or tools to support them. “This has caused some stress,” Dr. Shore said.
Electronic health record systems may or may not integrate video visits, noted Dr. Chan. “For instance, I work at a health system where mental health questionnaires, imaging systems, notes, and video visit scheduling are housed in separate systems.”
Without a seamless national, integrated system in place, physicians are often left to couple different IT formats together. “There are some systems that work pretty well, and there are some that are really clunky where you’re forcing the two together, so that’s a challenge,” Dr. Shore said. Providers may be using one system for their office and another system to provide care. “They may be using different EHRs and different teleconferencing platforms. That adds complexity.” Video conferencing platforms aren’t as challenging to use as EHRs. However, if some people are self-taught, there’s no way of knowing if they’re using best practices, he added.
Many health systems do offer video built into EMRs. Companies such as Epic have provided integrations with Vidyo, Amwell, and other platforms, noted Dr. Chan.
An integrated platform is useful if done well – but isn’t necessarily essential, according to Dr. Yellowlees. In his own setup, he signs a laptop into an EMR and uses an iPad to communicate with patients via video on the iPad and all video on the phone.
“The big advantage of telemedicine is you can type your notes in a socially appropriate way while talking to the patient on video. I do that, and it saves me a considerable amount of time. I don’t have to spend time after a consultation typing up notes.”
Still, others may struggle with bandwidth or connectivity. “Perhaps there’s a problem with privacy or the types of patients you’re dealing with,” Dr. Shore said. Older patients in a nursing home, for example, may require a team of people that works onsite. Suddenly, their care has to transition to a virtual system. “You may need to figure how to put the right team together to do straightforward and individual interactions,” he said.
Virtual care also suffers from the “digital divide,” an issue that predates COVID-19, said Dr. Shore. Not all patients have access to bandwidth and the technology to see clinicians. Other lack expertise and comfort with using the technology, or can’t afford the equipment necessary to bring them online. The pandemic has highlighted all of these disparities, he emphasized.
Dr. Chan offered that the digital divide is part of a larger socioeconomic divide, where barriers to any care – including transportation for in-person care – exist.
Technology and access issues aside, insidious “Zoom fatigue” is affecting everyone right now. “That’s clearly real,” Dr. Shore said. “And it’s not just about videoconferencing; it’s about being in quarantine. We’re all in this virtual lockdown, where people are using the term ‘videoconferencing fatigue.’ It’s a complicated problem.”
To assist with telehealth implementation, the APA has issued practice guidance and a toolkit that includes an extensive set of educational materials, including 40 videos on various topics. It’s also hosted webinars on telehealth policies and written up standard operating procedures on this topic, Dr. Yellowlees said. “The most important thing is APA, like other organizations, is advocating to have the relaxation of regulations during COVID made permanent,” he said.
Reimbursement post pandemic
As virtual visits rose in 2020, so did billing for such services. According to America’s Health Insurance Plans, claims to private insurers exploded by more than 4,000% in 2020. AHIP last July reported that mental health conditions made up one-third or more than 33% of telehealth claims to private insurers during the pandemic. Dr. Yellowlees said the health insurers he’s dealt with “have been good about paying for telehealth visits during the COVID-declared emergency.”
Whether this coverage will continue once the public health emergency ends, is unclear, some contend. “My sense is that there will be a rollback of coverage, but it won’t be back to what we had prepandemic,” said Ateev Mehrotra, MD, MPH, who studies telemedicine trends, in an interview. Right now, there’s still a great deal of uncertainty about reimbursement, “and that’s what’s giving providers pause,” said Dr. Mehrotra, of the department of health care policy and medicine at Harvard Medical School and a hospitalist at Beth Israel Deaconess Medical Center, both in Boston.
AHIP will continue to support federal and state policies to further promote telehealth access during the public health emergency, spokesman David Allen said in a statement. “Insurance providers have independently shifted their policies to increase access to care and services, ranging from acute care needs and triage services to chronic disease management and behavioral health. We are still awaiting information on what changes will remain in place beyond the public health emergency,” he said.
Even before COVID-19, Mr. Allen noted, nearly all large employers (96%) offered access to telehealth services as a covered benefit in 2019. In May, the Kaiser Family Foundation reported that many insurers were reducing or lifting cost sharing for telehealth for limited time periods.
Some of the larger payers said they’re continuing benefits, although it’s not clear how long some benefits will remain in place.
UnitedHealthcare offers no-cost coverage for COVID-19 testing–related telehealth visits, but that benefit is set to expire once the public health emergency presumably ends on Jan. 20, 2021. Through Dec. 31, 2020, the payer said it would offer coverage with no cost sharing for telehealth visits related to COVID-19 treatment and expanded access to telehealth visits not related to COVID-19 through its network. Similarly, Anthem is waiving cost sharing for COVID-19 treatments via telehealth or in-person visits, and for telehealth visits not related to COVID-19 for Medicare members through the end of the year.
Anthem will continue to cover telehealth and encourage members and clinicians to use telehealth for behavioral health, a spokesperson said. Anthem also has a telehealth provider, LiveHealth Online, “another safe and effective way for members to see a doctor to receive health guidance from their home via mobile device or a computer with a webcam,” said the spokesperson.
Dr. Vasan hopes that insurers will increase coverage for telehealth and at the same rate as in-person visits, especially for mental health. “I have not felt that the quality of the clinic has decreased, and in fact, in some ways it’s gotten better, and insurance coverage should reflect this.”
Outlook for the hybrid model
As long as there’s COVID-19, psychiatry practices must remain virtual, at least for now, Dr. Shore said. “We will emerge from this pandemic, I suspect in bits and starts.” When that happens, practices will need to have a transition plan in place. “Once we get away from COVID, I don’t think our mental health will ever be the same again. We’ll have much more virtual technology along the lines of a hybrid model, where we’ll see patients in person, but we’ll use more technology to work with patients, but it will be more of a blend.” Practices will also have to address the regulatory, reimbursement, and prescribing conditions the new world offers.
Some practices are already discovering the benefits of relying less on a brick-and-mortar office.
Dr. Chan said his colleagues are finding that they no longer have to deal with the expense and upkeep of renting and furnishing office space. “Many are taking their practice virtual-only because the monthly recurring costs are so cheap, and they can see patients in distant, underserved communities.” In-person visits are now inconvenient and risky, he continued. They require expensive personal protective equipment and cleaning protocols. “Plus, there’s the risk that services must shut down when stay-at-home orders return or when a staff member gets infected.”
Physicians prescribing certain controlled substances will likely continue to use office space, once the public health emergency expires and face-to-face visits resume. “In such cases, they can rent office space part-time,” Dr. Chan added.
Dr. Vasan, also of the department of psychiatry at Stanford and chief medical officer of Real, hopes that such a model prevails. “I do miss seeing patients in person and think a hybrid will be a good balance.”
Most patients want it, as do the influx of Generation Z physicians coming into the profession, Dr. Yellowlees noted. These are young, technologically savvy doctors who grew up in the age of the Internet. “I think the silver lining of COVID is it led telemedicine past the tipping point, where both patients and providers are learning that it’s an appropriate way to get care, as long as you’re careful, use professional guidelines – and don’t drop your standards of care.”
Dr. Yellowlees and Dr. Shore are coauthors of Telepsychiatry and Health Technologies: A Guide for Mental Health Professionals (Washington: American Psychiatric Association Publishing, 2018), and receive royalties from the book. Dr. Shore also reported working with AccessCare and receiving royalties from Springer Press. Dr. Chan reported consulting for Orbit Health. Dr. Vasan reported no conflicts of interest. Dr. Mehrotra has received research funding from several U.S. agencies, including the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke.
Once the fog lifts on a global pandemic that led to an explosion in telehealth visits in 2020, mental health experts expect virtual and in-person visits to merge to become a standard model of care in clinical psychiatry.
Hybrid care is the future, Peter Yellowlees, MBBS, MD, a professor of psychiatry and chief wellness officer at the University of California, Davis, said in an interview. “I’ve been working this way for several years – where all of my patients get to see me in person or online, or both.”
The model’s increasing popularity reflects a major shift toward virtual consults. Telemedicine offers safer, quicker, and less expensive alternatives, Steven Chan, MD, MBA, of Stanford (Calif.) University, said in an interview. “This continuity is essential to helping to reduce emergency room visits, reduce inpatient hospitalizations and readmissions, and improving adherence to treatment,” Dr. Chan said. State and federal regulators’ actions to lift certain licensing and prescribing restrictions and expand coverage made it easier for clinical psychiatrists to offer and get paid for these services.
The catch is that no one knows whether these easements will remain in place once COVID-19 recedes, ending the national public health emergency, Jay H. Shore, MD, MPH, chairperson of the American Psychiatric Association telepsychiatry committee, and professor and director of telemedicine programming at the University of Colorado at Denver, Aurora’s department of psychiatry, said in an interview. “These all temporarily changed during this time period, but we have no idea when they’re going to end or if they’re going to continue off of COVID. So now, there’s a lot of uncertainty.”
‘Suite of different technologies’
New freedoms to deliver telehealth care left some practices scrambling to adopt or refine technology. Once COVID-19 hit, “I can’t think of a psychiatrist or provider or institution that didn’t have to rapidly virtualize and do at least some video conferencing,” Dr. Shore said. This immediate shift signaled a key move toward hybrid patient-doctor relations. “It means you hold a relationship with your patients through multiple different mediums, email, portal, telephone, in person. It’s not just about in-person versus video, it’s about a suite of different technologies.”
Dr. Shore began practicing telehealth 20 years ago, long before the age of COVID. He’s since established rewarding relationships with patients he’s never met. “I’ve done everything from medical management to long-term psychotherapy, group psychotherapy, and I’ve been successful with different populations.”
Many nuances exist around matching the right patient with the right videoconferencing adaptation, Dr. Shore continued. However, “in general, the literature supports that you can get equal clinical outcomes with telehealth versus in-person treatment.”
‘I see their garden’
While some may eschew the idea of providing care over a virtual platform, other physicians see it as an insightful window into a person’s mental state. “I think some are actually quite surprised by how much good care you can give using video,” said Dr. Yellowlees. Unlike a phone conversation, video allows you to see a person’s home. “The beauty of a video is not just that you can see the person, you can also look around their home, and learn more about them.”
In his own visits, he asks patients to take him on a virtual “walk” through their house, provided there are no confidentiality issues.
“I get to meet their pets, the carers, the spouses; I get to see their garden. I get to see what their interests are from looking at the paintings on their walls. I learn more about my patients that way. If you use video to purely see people from the neck up, then that’s fine, but I think you can also use it to your advantage, seeing people at home.” He also encourages patients to do visits in their cars – as long as the windows are shut, they’re in a safe area, and most importantly, they’re not driving.
Nina Vasan, MD, MBA, who treats patients at Stanford and in a concierge private practice for executives, agrees that seeing patients in the home offers a more direct view of patients’ lives. “These little extra pieces of information are things that we didn’t get before COVID, when patients would come by themselves into the office week after week. I do feel like I know some patients much better by being able to see their surroundings and home interactions,” Dr. Vasan, founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation and chair of the APA’s committee on innovation, said in an interview.
She uses Zoom for her sessions, and regularly texts with her concierge patients. In both clinics, where most visits have gone virtual, “the no-show rate is near zero. Before COVID, it was around 10%-20%.”
Safety is a key advantage of virtual visits, noted Dr. Shore. “It’s certainly better than some of the risks of seeing people in person.”
Lack of integration causes frustration
Some challenges exist around access and adaptation of virtual technology. The pandemic’s sudden onslaught left many clinicians scrambling to adjust to a virtual format without any training or tools to support them. “This has caused some stress,” Dr. Shore said.
Electronic health record systems may or may not integrate video visits, noted Dr. Chan. “For instance, I work at a health system where mental health questionnaires, imaging systems, notes, and video visit scheduling are housed in separate systems.”
Without a seamless national, integrated system in place, physicians are often left to couple different IT formats together. “There are some systems that work pretty well, and there are some that are really clunky where you’re forcing the two together, so that’s a challenge,” Dr. Shore said. Providers may be using one system for their office and another system to provide care. “They may be using different EHRs and different teleconferencing platforms. That adds complexity.” Video conferencing platforms aren’t as challenging to use as EHRs. However, if some people are self-taught, there’s no way of knowing if they’re using best practices, he added.
Many health systems do offer video built into EMRs. Companies such as Epic have provided integrations with Vidyo, Amwell, and other platforms, noted Dr. Chan.
An integrated platform is useful if done well – but isn’t necessarily essential, according to Dr. Yellowlees. In his own setup, he signs a laptop into an EMR and uses an iPad to communicate with patients via video on the iPad and all video on the phone.
“The big advantage of telemedicine is you can type your notes in a socially appropriate way while talking to the patient on video. I do that, and it saves me a considerable amount of time. I don’t have to spend time after a consultation typing up notes.”
Still, others may struggle with bandwidth or connectivity. “Perhaps there’s a problem with privacy or the types of patients you’re dealing with,” Dr. Shore said. Older patients in a nursing home, for example, may require a team of people that works onsite. Suddenly, their care has to transition to a virtual system. “You may need to figure how to put the right team together to do straightforward and individual interactions,” he said.
Virtual care also suffers from the “digital divide,” an issue that predates COVID-19, said Dr. Shore. Not all patients have access to bandwidth and the technology to see clinicians. Other lack expertise and comfort with using the technology, or can’t afford the equipment necessary to bring them online. The pandemic has highlighted all of these disparities, he emphasized.
Dr. Chan offered that the digital divide is part of a larger socioeconomic divide, where barriers to any care – including transportation for in-person care – exist.
Technology and access issues aside, insidious “Zoom fatigue” is affecting everyone right now. “That’s clearly real,” Dr. Shore said. “And it’s not just about videoconferencing; it’s about being in quarantine. We’re all in this virtual lockdown, where people are using the term ‘videoconferencing fatigue.’ It’s a complicated problem.”
To assist with telehealth implementation, the APA has issued practice guidance and a toolkit that includes an extensive set of educational materials, including 40 videos on various topics. It’s also hosted webinars on telehealth policies and written up standard operating procedures on this topic, Dr. Yellowlees said. “The most important thing is APA, like other organizations, is advocating to have the relaxation of regulations during COVID made permanent,” he said.
Reimbursement post pandemic
As virtual visits rose in 2020, so did billing for such services. According to America’s Health Insurance Plans, claims to private insurers exploded by more than 4,000% in 2020. AHIP last July reported that mental health conditions made up one-third or more than 33% of telehealth claims to private insurers during the pandemic. Dr. Yellowlees said the health insurers he’s dealt with “have been good about paying for telehealth visits during the COVID-declared emergency.”
Whether this coverage will continue once the public health emergency ends, is unclear, some contend. “My sense is that there will be a rollback of coverage, but it won’t be back to what we had prepandemic,” said Ateev Mehrotra, MD, MPH, who studies telemedicine trends, in an interview. Right now, there’s still a great deal of uncertainty about reimbursement, “and that’s what’s giving providers pause,” said Dr. Mehrotra, of the department of health care policy and medicine at Harvard Medical School and a hospitalist at Beth Israel Deaconess Medical Center, both in Boston.
AHIP will continue to support federal and state policies to further promote telehealth access during the public health emergency, spokesman David Allen said in a statement. “Insurance providers have independently shifted their policies to increase access to care and services, ranging from acute care needs and triage services to chronic disease management and behavioral health. We are still awaiting information on what changes will remain in place beyond the public health emergency,” he said.
Even before COVID-19, Mr. Allen noted, nearly all large employers (96%) offered access to telehealth services as a covered benefit in 2019. In May, the Kaiser Family Foundation reported that many insurers were reducing or lifting cost sharing for telehealth for limited time periods.
Some of the larger payers said they’re continuing benefits, although it’s not clear how long some benefits will remain in place.
UnitedHealthcare offers no-cost coverage for COVID-19 testing–related telehealth visits, but that benefit is set to expire once the public health emergency presumably ends on Jan. 20, 2021. Through Dec. 31, 2020, the payer said it would offer coverage with no cost sharing for telehealth visits related to COVID-19 treatment and expanded access to telehealth visits not related to COVID-19 through its network. Similarly, Anthem is waiving cost sharing for COVID-19 treatments via telehealth or in-person visits, and for telehealth visits not related to COVID-19 for Medicare members through the end of the year.
Anthem will continue to cover telehealth and encourage members and clinicians to use telehealth for behavioral health, a spokesperson said. Anthem also has a telehealth provider, LiveHealth Online, “another safe and effective way for members to see a doctor to receive health guidance from their home via mobile device or a computer with a webcam,” said the spokesperson.
Dr. Vasan hopes that insurers will increase coverage for telehealth and at the same rate as in-person visits, especially for mental health. “I have not felt that the quality of the clinic has decreased, and in fact, in some ways it’s gotten better, and insurance coverage should reflect this.”
Outlook for the hybrid model
As long as there’s COVID-19, psychiatry practices must remain virtual, at least for now, Dr. Shore said. “We will emerge from this pandemic, I suspect in bits and starts.” When that happens, practices will need to have a transition plan in place. “Once we get away from COVID, I don’t think our mental health will ever be the same again. We’ll have much more virtual technology along the lines of a hybrid model, where we’ll see patients in person, but we’ll use more technology to work with patients, but it will be more of a blend.” Practices will also have to address the regulatory, reimbursement, and prescribing conditions the new world offers.
Some practices are already discovering the benefits of relying less on a brick-and-mortar office.
Dr. Chan said his colleagues are finding that they no longer have to deal with the expense and upkeep of renting and furnishing office space. “Many are taking their practice virtual-only because the monthly recurring costs are so cheap, and they can see patients in distant, underserved communities.” In-person visits are now inconvenient and risky, he continued. They require expensive personal protective equipment and cleaning protocols. “Plus, there’s the risk that services must shut down when stay-at-home orders return or when a staff member gets infected.”
Physicians prescribing certain controlled substances will likely continue to use office space, once the public health emergency expires and face-to-face visits resume. “In such cases, they can rent office space part-time,” Dr. Chan added.
Dr. Vasan, also of the department of psychiatry at Stanford and chief medical officer of Real, hopes that such a model prevails. “I do miss seeing patients in person and think a hybrid will be a good balance.”
Most patients want it, as do the influx of Generation Z physicians coming into the profession, Dr. Yellowlees noted. These are young, technologically savvy doctors who grew up in the age of the Internet. “I think the silver lining of COVID is it led telemedicine past the tipping point, where both patients and providers are learning that it’s an appropriate way to get care, as long as you’re careful, use professional guidelines – and don’t drop your standards of care.”
Dr. Yellowlees and Dr. Shore are coauthors of Telepsychiatry and Health Technologies: A Guide for Mental Health Professionals (Washington: American Psychiatric Association Publishing, 2018), and receive royalties from the book. Dr. Shore also reported working with AccessCare and receiving royalties from Springer Press. Dr. Chan reported consulting for Orbit Health. Dr. Vasan reported no conflicts of interest. Dr. Mehrotra has received research funding from several U.S. agencies, including the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke.
Once the fog lifts on a global pandemic that led to an explosion in telehealth visits in 2020, mental health experts expect virtual and in-person visits to merge to become a standard model of care in clinical psychiatry.
Hybrid care is the future, Peter Yellowlees, MBBS, MD, a professor of psychiatry and chief wellness officer at the University of California, Davis, said in an interview. “I’ve been working this way for several years – where all of my patients get to see me in person or online, or both.”
The model’s increasing popularity reflects a major shift toward virtual consults. Telemedicine offers safer, quicker, and less expensive alternatives, Steven Chan, MD, MBA, of Stanford (Calif.) University, said in an interview. “This continuity is essential to helping to reduce emergency room visits, reduce inpatient hospitalizations and readmissions, and improving adherence to treatment,” Dr. Chan said. State and federal regulators’ actions to lift certain licensing and prescribing restrictions and expand coverage made it easier for clinical psychiatrists to offer and get paid for these services.
The catch is that no one knows whether these easements will remain in place once COVID-19 recedes, ending the national public health emergency, Jay H. Shore, MD, MPH, chairperson of the American Psychiatric Association telepsychiatry committee, and professor and director of telemedicine programming at the University of Colorado at Denver, Aurora’s department of psychiatry, said in an interview. “These all temporarily changed during this time period, but we have no idea when they’re going to end or if they’re going to continue off of COVID. So now, there’s a lot of uncertainty.”
‘Suite of different technologies’
New freedoms to deliver telehealth care left some practices scrambling to adopt or refine technology. Once COVID-19 hit, “I can’t think of a psychiatrist or provider or institution that didn’t have to rapidly virtualize and do at least some video conferencing,” Dr. Shore said. This immediate shift signaled a key move toward hybrid patient-doctor relations. “It means you hold a relationship with your patients through multiple different mediums, email, portal, telephone, in person. It’s not just about in-person versus video, it’s about a suite of different technologies.”
Dr. Shore began practicing telehealth 20 years ago, long before the age of COVID. He’s since established rewarding relationships with patients he’s never met. “I’ve done everything from medical management to long-term psychotherapy, group psychotherapy, and I’ve been successful with different populations.”
Many nuances exist around matching the right patient with the right videoconferencing adaptation, Dr. Shore continued. However, “in general, the literature supports that you can get equal clinical outcomes with telehealth versus in-person treatment.”
‘I see their garden’
While some may eschew the idea of providing care over a virtual platform, other physicians see it as an insightful window into a person’s mental state. “I think some are actually quite surprised by how much good care you can give using video,” said Dr. Yellowlees. Unlike a phone conversation, video allows you to see a person’s home. “The beauty of a video is not just that you can see the person, you can also look around their home, and learn more about them.”
In his own visits, he asks patients to take him on a virtual “walk” through their house, provided there are no confidentiality issues.
“I get to meet their pets, the carers, the spouses; I get to see their garden. I get to see what their interests are from looking at the paintings on their walls. I learn more about my patients that way. If you use video to purely see people from the neck up, then that’s fine, but I think you can also use it to your advantage, seeing people at home.” He also encourages patients to do visits in their cars – as long as the windows are shut, they’re in a safe area, and most importantly, they’re not driving.
Nina Vasan, MD, MBA, who treats patients at Stanford and in a concierge private practice for executives, agrees that seeing patients in the home offers a more direct view of patients’ lives. “These little extra pieces of information are things that we didn’t get before COVID, when patients would come by themselves into the office week after week. I do feel like I know some patients much better by being able to see their surroundings and home interactions,” Dr. Vasan, founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation and chair of the APA’s committee on innovation, said in an interview.
She uses Zoom for her sessions, and regularly texts with her concierge patients. In both clinics, where most visits have gone virtual, “the no-show rate is near zero. Before COVID, it was around 10%-20%.”
Safety is a key advantage of virtual visits, noted Dr. Shore. “It’s certainly better than some of the risks of seeing people in person.”
Lack of integration causes frustration
Some challenges exist around access and adaptation of virtual technology. The pandemic’s sudden onslaught left many clinicians scrambling to adjust to a virtual format without any training or tools to support them. “This has caused some stress,” Dr. Shore said.
Electronic health record systems may or may not integrate video visits, noted Dr. Chan. “For instance, I work at a health system where mental health questionnaires, imaging systems, notes, and video visit scheduling are housed in separate systems.”
Without a seamless national, integrated system in place, physicians are often left to couple different IT formats together. “There are some systems that work pretty well, and there are some that are really clunky where you’re forcing the two together, so that’s a challenge,” Dr. Shore said. Providers may be using one system for their office and another system to provide care. “They may be using different EHRs and different teleconferencing platforms. That adds complexity.” Video conferencing platforms aren’t as challenging to use as EHRs. However, if some people are self-taught, there’s no way of knowing if they’re using best practices, he added.
Many health systems do offer video built into EMRs. Companies such as Epic have provided integrations with Vidyo, Amwell, and other platforms, noted Dr. Chan.
An integrated platform is useful if done well – but isn’t necessarily essential, according to Dr. Yellowlees. In his own setup, he signs a laptop into an EMR and uses an iPad to communicate with patients via video on the iPad and all video on the phone.
“The big advantage of telemedicine is you can type your notes in a socially appropriate way while talking to the patient on video. I do that, and it saves me a considerable amount of time. I don’t have to spend time after a consultation typing up notes.”
Still, others may struggle with bandwidth or connectivity. “Perhaps there’s a problem with privacy or the types of patients you’re dealing with,” Dr. Shore said. Older patients in a nursing home, for example, may require a team of people that works onsite. Suddenly, their care has to transition to a virtual system. “You may need to figure how to put the right team together to do straightforward and individual interactions,” he said.
Virtual care also suffers from the “digital divide,” an issue that predates COVID-19, said Dr. Shore. Not all patients have access to bandwidth and the technology to see clinicians. Other lack expertise and comfort with using the technology, or can’t afford the equipment necessary to bring them online. The pandemic has highlighted all of these disparities, he emphasized.
Dr. Chan offered that the digital divide is part of a larger socioeconomic divide, where barriers to any care – including transportation for in-person care – exist.
Technology and access issues aside, insidious “Zoom fatigue” is affecting everyone right now. “That’s clearly real,” Dr. Shore said. “And it’s not just about videoconferencing; it’s about being in quarantine. We’re all in this virtual lockdown, where people are using the term ‘videoconferencing fatigue.’ It’s a complicated problem.”
To assist with telehealth implementation, the APA has issued practice guidance and a toolkit that includes an extensive set of educational materials, including 40 videos on various topics. It’s also hosted webinars on telehealth policies and written up standard operating procedures on this topic, Dr. Yellowlees said. “The most important thing is APA, like other organizations, is advocating to have the relaxation of regulations during COVID made permanent,” he said.
Reimbursement post pandemic
As virtual visits rose in 2020, so did billing for such services. According to America’s Health Insurance Plans, claims to private insurers exploded by more than 4,000% in 2020. AHIP last July reported that mental health conditions made up one-third or more than 33% of telehealth claims to private insurers during the pandemic. Dr. Yellowlees said the health insurers he’s dealt with “have been good about paying for telehealth visits during the COVID-declared emergency.”
Whether this coverage will continue once the public health emergency ends, is unclear, some contend. “My sense is that there will be a rollback of coverage, but it won’t be back to what we had prepandemic,” said Ateev Mehrotra, MD, MPH, who studies telemedicine trends, in an interview. Right now, there’s still a great deal of uncertainty about reimbursement, “and that’s what’s giving providers pause,” said Dr. Mehrotra, of the department of health care policy and medicine at Harvard Medical School and a hospitalist at Beth Israel Deaconess Medical Center, both in Boston.
AHIP will continue to support federal and state policies to further promote telehealth access during the public health emergency, spokesman David Allen said in a statement. “Insurance providers have independently shifted their policies to increase access to care and services, ranging from acute care needs and triage services to chronic disease management and behavioral health. We are still awaiting information on what changes will remain in place beyond the public health emergency,” he said.
Even before COVID-19, Mr. Allen noted, nearly all large employers (96%) offered access to telehealth services as a covered benefit in 2019. In May, the Kaiser Family Foundation reported that many insurers were reducing or lifting cost sharing for telehealth for limited time periods.
Some of the larger payers said they’re continuing benefits, although it’s not clear how long some benefits will remain in place.
UnitedHealthcare offers no-cost coverage for COVID-19 testing–related telehealth visits, but that benefit is set to expire once the public health emergency presumably ends on Jan. 20, 2021. Through Dec. 31, 2020, the payer said it would offer coverage with no cost sharing for telehealth visits related to COVID-19 treatment and expanded access to telehealth visits not related to COVID-19 through its network. Similarly, Anthem is waiving cost sharing for COVID-19 treatments via telehealth or in-person visits, and for telehealth visits not related to COVID-19 for Medicare members through the end of the year.
Anthem will continue to cover telehealth and encourage members and clinicians to use telehealth for behavioral health, a spokesperson said. Anthem also has a telehealth provider, LiveHealth Online, “another safe and effective way for members to see a doctor to receive health guidance from their home via mobile device or a computer with a webcam,” said the spokesperson.
Dr. Vasan hopes that insurers will increase coverage for telehealth and at the same rate as in-person visits, especially for mental health. “I have not felt that the quality of the clinic has decreased, and in fact, in some ways it’s gotten better, and insurance coverage should reflect this.”
Outlook for the hybrid model
As long as there’s COVID-19, psychiatry practices must remain virtual, at least for now, Dr. Shore said. “We will emerge from this pandemic, I suspect in bits and starts.” When that happens, practices will need to have a transition plan in place. “Once we get away from COVID, I don’t think our mental health will ever be the same again. We’ll have much more virtual technology along the lines of a hybrid model, where we’ll see patients in person, but we’ll use more technology to work with patients, but it will be more of a blend.” Practices will also have to address the regulatory, reimbursement, and prescribing conditions the new world offers.
Some practices are already discovering the benefits of relying less on a brick-and-mortar office.
Dr. Chan said his colleagues are finding that they no longer have to deal with the expense and upkeep of renting and furnishing office space. “Many are taking their practice virtual-only because the monthly recurring costs are so cheap, and they can see patients in distant, underserved communities.” In-person visits are now inconvenient and risky, he continued. They require expensive personal protective equipment and cleaning protocols. “Plus, there’s the risk that services must shut down when stay-at-home orders return or when a staff member gets infected.”
Physicians prescribing certain controlled substances will likely continue to use office space, once the public health emergency expires and face-to-face visits resume. “In such cases, they can rent office space part-time,” Dr. Chan added.
Dr. Vasan, also of the department of psychiatry at Stanford and chief medical officer of Real, hopes that such a model prevails. “I do miss seeing patients in person and think a hybrid will be a good balance.”
Most patients want it, as do the influx of Generation Z physicians coming into the profession, Dr. Yellowlees noted. These are young, technologically savvy doctors who grew up in the age of the Internet. “I think the silver lining of COVID is it led telemedicine past the tipping point, where both patients and providers are learning that it’s an appropriate way to get care, as long as you’re careful, use professional guidelines – and don’t drop your standards of care.”
Dr. Yellowlees and Dr. Shore are coauthors of Telepsychiatry and Health Technologies: A Guide for Mental Health Professionals (Washington: American Psychiatric Association Publishing, 2018), and receive royalties from the book. Dr. Shore also reported working with AccessCare and receiving royalties from Springer Press. Dr. Chan reported consulting for Orbit Health. Dr. Vasan reported no conflicts of interest. Dr. Mehrotra has received research funding from several U.S. agencies, including the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke.
A call to make four telehealth provisions permanent
Lawmakers, physicians, and advocates alike have hailed a relaxation of telehealth rules under the COVID-19 emergency declaration, and they’d like things to stay this way.
Regulators previously restricted telemedicine use “by insisting that you could only see patients in the state you’re licensed in, by not reimbursing as widely for telehealth, and by not allowing us to prescribe controlled substances. They also didn’t allow us to see patients on the phone. So, there’s very good reasons to keep those regulations permanently relaxed,” said Peter Yellowlees, MBBS, MD, a professor of psychiatry and chief wellness officer at the University of California, Davis.
In his view, changes should take place in four key areas:
- Licensing. “Traditionally, state medical boards have been very insistent that clinical psychiatrists license in the state the patient resides in. This means physicians must have licenses in many different states. It’s very restrictive, because physicians can’t follow patients from one state to another. Under COVID, we can do this, but physicians want these licensing changes to be made permanent.”
- Reimbursement. “In the past, federal regulators have only allowed reimbursement for telemedicine in very specific, defined rural areas and specified clinical environments. This rule has since been relaxed, allowing us to see patients anywhere, especially in their homes. This is another area that should become permanent. Payers should continue to pay telehealth services on par with in-person visits.”
- Telephony. “Psychiatrists and other physicians haven’t been traditionally paid for telephone visits. But there’s no doubt that telephone follow-up visits can be very beneficial, so while I wouldn’t personally see a new patient on the phone, I now follow up with them on the phone once I have gotten to know them, and this works well.”
- Prescribing. The Ryan Haight Online Pharmacy Consumer Protection Act of 2008 was introduced to stop overseas pharmacies prescribing narcotics. “It was very successful, but as a side effect, it stopped most physicians from prescribing controlled substances on video. With COVID, we can now do this. For psychiatry, this is very important because it means we can use video to treat people for addictions with medications like buprenorphine and [prescribe] stimulants for children with ADHD. The U.S. Drug Enforcement Administration should finalize regulations for the Ryan Haight Act to allow for the prescribing of controlled substances via telehealth without a prior in-person exam.”
The American Psychiatric Association has called for an extension of the telehealth waiver authority under COVID-19 beyond the emergency declaration to study its impact. It will continue to advocate to allow for telephone-only telehealth to be reimbursed at the same rate as live audio-video, said a spokesperson. “We also will continue to advocate for the removal of geographic and originating site restrictions in Medicare, which prevent Medicare patients from being seen in the home,” with some exceptions, the spokesperson said.
The APA has also issued guidance to practitioners seeking clarity on telehealth coverage and COVID-19.
Lawmakers, physicians, and advocates alike have hailed a relaxation of telehealth rules under the COVID-19 emergency declaration, and they’d like things to stay this way.
Regulators previously restricted telemedicine use “by insisting that you could only see patients in the state you’re licensed in, by not reimbursing as widely for telehealth, and by not allowing us to prescribe controlled substances. They also didn’t allow us to see patients on the phone. So, there’s very good reasons to keep those regulations permanently relaxed,” said Peter Yellowlees, MBBS, MD, a professor of psychiatry and chief wellness officer at the University of California, Davis.
In his view, changes should take place in four key areas:
- Licensing. “Traditionally, state medical boards have been very insistent that clinical psychiatrists license in the state the patient resides in. This means physicians must have licenses in many different states. It’s very restrictive, because physicians can’t follow patients from one state to another. Under COVID, we can do this, but physicians want these licensing changes to be made permanent.”
- Reimbursement. “In the past, federal regulators have only allowed reimbursement for telemedicine in very specific, defined rural areas and specified clinical environments. This rule has since been relaxed, allowing us to see patients anywhere, especially in their homes. This is another area that should become permanent. Payers should continue to pay telehealth services on par with in-person visits.”
- Telephony. “Psychiatrists and other physicians haven’t been traditionally paid for telephone visits. But there’s no doubt that telephone follow-up visits can be very beneficial, so while I wouldn’t personally see a new patient on the phone, I now follow up with them on the phone once I have gotten to know them, and this works well.”
- Prescribing. The Ryan Haight Online Pharmacy Consumer Protection Act of 2008 was introduced to stop overseas pharmacies prescribing narcotics. “It was very successful, but as a side effect, it stopped most physicians from prescribing controlled substances on video. With COVID, we can now do this. For psychiatry, this is very important because it means we can use video to treat people for addictions with medications like buprenorphine and [prescribe] stimulants for children with ADHD. The U.S. Drug Enforcement Administration should finalize regulations for the Ryan Haight Act to allow for the prescribing of controlled substances via telehealth without a prior in-person exam.”
The American Psychiatric Association has called for an extension of the telehealth waiver authority under COVID-19 beyond the emergency declaration to study its impact. It will continue to advocate to allow for telephone-only telehealth to be reimbursed at the same rate as live audio-video, said a spokesperson. “We also will continue to advocate for the removal of geographic and originating site restrictions in Medicare, which prevent Medicare patients from being seen in the home,” with some exceptions, the spokesperson said.
The APA has also issued guidance to practitioners seeking clarity on telehealth coverage and COVID-19.
Lawmakers, physicians, and advocates alike have hailed a relaxation of telehealth rules under the COVID-19 emergency declaration, and they’d like things to stay this way.
Regulators previously restricted telemedicine use “by insisting that you could only see patients in the state you’re licensed in, by not reimbursing as widely for telehealth, and by not allowing us to prescribe controlled substances. They also didn’t allow us to see patients on the phone. So, there’s very good reasons to keep those regulations permanently relaxed,” said Peter Yellowlees, MBBS, MD, a professor of psychiatry and chief wellness officer at the University of California, Davis.
In his view, changes should take place in four key areas:
- Licensing. “Traditionally, state medical boards have been very insistent that clinical psychiatrists license in the state the patient resides in. This means physicians must have licenses in many different states. It’s very restrictive, because physicians can’t follow patients from one state to another. Under COVID, we can do this, but physicians want these licensing changes to be made permanent.”
- Reimbursement. “In the past, federal regulators have only allowed reimbursement for telemedicine in very specific, defined rural areas and specified clinical environments. This rule has since been relaxed, allowing us to see patients anywhere, especially in their homes. This is another area that should become permanent. Payers should continue to pay telehealth services on par with in-person visits.”
- Telephony. “Psychiatrists and other physicians haven’t been traditionally paid for telephone visits. But there’s no doubt that telephone follow-up visits can be very beneficial, so while I wouldn’t personally see a new patient on the phone, I now follow up with them on the phone once I have gotten to know them, and this works well.”
- Prescribing. The Ryan Haight Online Pharmacy Consumer Protection Act of 2008 was introduced to stop overseas pharmacies prescribing narcotics. “It was very successful, but as a side effect, it stopped most physicians from prescribing controlled substances on video. With COVID, we can now do this. For psychiatry, this is very important because it means we can use video to treat people for addictions with medications like buprenorphine and [prescribe] stimulants for children with ADHD. The U.S. Drug Enforcement Administration should finalize regulations for the Ryan Haight Act to allow for the prescribing of controlled substances via telehealth without a prior in-person exam.”
The American Psychiatric Association has called for an extension of the telehealth waiver authority under COVID-19 beyond the emergency declaration to study its impact. It will continue to advocate to allow for telephone-only telehealth to be reimbursed at the same rate as live audio-video, said a spokesperson. “We also will continue to advocate for the removal of geographic and originating site restrictions in Medicare, which prevent Medicare patients from being seen in the home,” with some exceptions, the spokesperson said.
The APA has also issued guidance to practitioners seeking clarity on telehealth coverage and COVID-19.
2020 and the telehealth boom
This year saw an unprecedented rise in medical consults over virtual platforms, as the COVID-19 pandemic raged on in the United States and worldwide.
Statistics from major health care groups and payers underscore this effect. Polling 1,004 U.S. adults this fall, the American Psychiatric Association found that 31% had used telehealth services – with 72% reporting they had ventured into this mode of care over the last 6 months.
The Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, was a major catalyst, waiving geographic and Medicare telehealth payment restrictions for mental health services during certain emergency periods. Medicare beneficiaries gained access to telehealth services – they could start seeing doctors via videoconferencing in their homes, regardless of location. The Centers for Medicare and Medicaid Services began paying doctors for telehealth services at the same rate as in-office visits for all diagnoses and issued a toolkit to promote adoption of telehealth coverage policies among state Medicaid agencies.
Most states responded, expanding telehealth in Medicaid programs and relaxing restrictions on provider licensing, online prescribing, and patient consent for telehealth, the Kaiser Family Foundation reported in May. Other federal agencies took actions during the public health emergency. The Drug Enforcement Administration allowed for the prescribing of controlled substances through telemedicine, and the U.S. Department of Health & Human Services’s Office for Civil Rights agreed not to impose penalties for noncompliance of HIPAA during video conferencing, provided that physicians were acting in the best interests of the patient.
“The benefits we’re seeing on both sides – for patients and for doctors – around convenience and access are wonderful,” Nina Vasan, MD, MBA, founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation and chair of APA’s Committee on Innovation, said in an interview. Before COVID began, only a handful of clinicians were seeing patients via televideo at Stanford, said Dr. Vasan. “Now, almost everyone is. The forced uptake and change of behavior was something we’ve needed for years, and now that it has happened, I don’t see it going away.”
This year saw an unprecedented rise in medical consults over virtual platforms, as the COVID-19 pandemic raged on in the United States and worldwide.
Statistics from major health care groups and payers underscore this effect. Polling 1,004 U.S. adults this fall, the American Psychiatric Association found that 31% had used telehealth services – with 72% reporting they had ventured into this mode of care over the last 6 months.
The Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, was a major catalyst, waiving geographic and Medicare telehealth payment restrictions for mental health services during certain emergency periods. Medicare beneficiaries gained access to telehealth services – they could start seeing doctors via videoconferencing in their homes, regardless of location. The Centers for Medicare and Medicaid Services began paying doctors for telehealth services at the same rate as in-office visits for all diagnoses and issued a toolkit to promote adoption of telehealth coverage policies among state Medicaid agencies.
Most states responded, expanding telehealth in Medicaid programs and relaxing restrictions on provider licensing, online prescribing, and patient consent for telehealth, the Kaiser Family Foundation reported in May. Other federal agencies took actions during the public health emergency. The Drug Enforcement Administration allowed for the prescribing of controlled substances through telemedicine, and the U.S. Department of Health & Human Services’s Office for Civil Rights agreed not to impose penalties for noncompliance of HIPAA during video conferencing, provided that physicians were acting in the best interests of the patient.
“The benefits we’re seeing on both sides – for patients and for doctors – around convenience and access are wonderful,” Nina Vasan, MD, MBA, founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation and chair of APA’s Committee on Innovation, said in an interview. Before COVID began, only a handful of clinicians were seeing patients via televideo at Stanford, said Dr. Vasan. “Now, almost everyone is. The forced uptake and change of behavior was something we’ve needed for years, and now that it has happened, I don’t see it going away.”
This year saw an unprecedented rise in medical consults over virtual platforms, as the COVID-19 pandemic raged on in the United States and worldwide.
Statistics from major health care groups and payers underscore this effect. Polling 1,004 U.S. adults this fall, the American Psychiatric Association found that 31% had used telehealth services – with 72% reporting they had ventured into this mode of care over the last 6 months.
The Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, was a major catalyst, waiving geographic and Medicare telehealth payment restrictions for mental health services during certain emergency periods. Medicare beneficiaries gained access to telehealth services – they could start seeing doctors via videoconferencing in their homes, regardless of location. The Centers for Medicare and Medicaid Services began paying doctors for telehealth services at the same rate as in-office visits for all diagnoses and issued a toolkit to promote adoption of telehealth coverage policies among state Medicaid agencies.
Most states responded, expanding telehealth in Medicaid programs and relaxing restrictions on provider licensing, online prescribing, and patient consent for telehealth, the Kaiser Family Foundation reported in May. Other federal agencies took actions during the public health emergency. The Drug Enforcement Administration allowed for the prescribing of controlled substances through telemedicine, and the U.S. Department of Health & Human Services’s Office for Civil Rights agreed not to impose penalties for noncompliance of HIPAA during video conferencing, provided that physicians were acting in the best interests of the patient.
“The benefits we’re seeing on both sides – for patients and for doctors – around convenience and access are wonderful,” Nina Vasan, MD, MBA, founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation and chair of APA’s Committee on Innovation, said in an interview. Before COVID began, only a handful of clinicians were seeing patients via televideo at Stanford, said Dr. Vasan. “Now, almost everyone is. The forced uptake and change of behavior was something we’ve needed for years, and now that it has happened, I don’t see it going away.”
AMA takes on vaccine misinformation, physician vaccines, racism
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
The American Medical Association House of Delegates has adopted a policy to educate physicians on how to speak with patients about COVID-19 vaccination to counteract widespread misinformation about the vaccine development process.
Other highlights of the AMA’s recent special meeting include a new policy on the ethics of physicians getting immunized against COVID-19 and a far-reaching statement about racism.
Under the organization’s new vaccination education policy, the AMA will provide physicians with “culturally appropriate patient education materials,” according to a news release.
This campaign will be conducted “bearing in mind the historical context of ‘experimentation’ with vaccines and other medication in communities of color,” the AMA said, apparently alluding to the infamous Tuskegee study of syphilis in Black men.
Educating the public about the safety and efficacy of the COVID-19 vaccine programs is an “urgent priority,” the AMA said. This is especially true among populations that have been disproportionately affected by the disease. Black and Latino people are being hospitalized for COVID-19 at far higher rates than White Americans.
“Under the new policy, the AMA will help address patient concerns, dispel misinformation, and build confidence in COVID-19 vaccination,” the release states. The AMA also plans to build a coalition of health care and public health organizations to develop and implement a joint public education program.
Polls have indicated that many people will not get vaccinated when supplies of the new COVID-19 vaccines are available, although public support is rising. A recent Gallup poll found that 58% of surveyed adults were willing to be inoculated, up from 50% in September.
A Kaiser Family Foundation survey in September found that a majority of Americans were skeptical of a rushed vaccine, because they were concerned that the Trump administration was pressuring the Food and Drug Administration to approve a vaccine before the election.
“Given the unprecedented situation with COVID-19 and with vaccine development moving at a rapid pace, many of our patients and the public have questions and concerns,” said AMA President Susan R. Bailey, MD, in the release. “It is essential that we speak together as a strong, unified voice across health care and public health, inclusive of organizations respected in communities of color; to use scientific, fact-based evidence to help allay public concerns; and build confidence in COVID-19 vaccine candidates that are determined to be safe and effective.”
Physician, immunize thyself
The AMA also adopted a new ethics policy about physician immunization. On Monday, the AMA House of Delegates stated that physicians who are not immunized from a vaccine-preventable disease have an ethical responsibility to take appropriate actions to protect patients and colleagues.
The AMA code of ethics has long maintained that physicians have a strong ethical duty to accept immunizations when a safe, effective vaccine is available. However, the organization said in a news release, “it is not ethically problematic to exempt individuals when a specific vaccine poses a risk due to underlying medical conditions.”
Ethical concerns arise when physicians are allowed to decline vaccinations for nonmedical reasons, according to a report presented to the House of Delegates by the AMA Council on Ethical and Judicial Affairs.
According to the newly amended AMA ethical guidance, “physicians who are not or cannot be immunized have a responsibility to voluntarily take appropriate actions to protect patients, fellow health care workers and others.” This includes refraining from direct patient contact.
The delegates also approved a guidance asserting that physician practices and health care institutions are responsible for developing policies and procedures for responding to pandemics and epidemics. These policies and procedures should outline appropriate protective equipment allocation, staff immunization programs, and infection control practices.
Combating systemic racism
In an effort to reduce racial disparities in healthcare, the AMA House of Delegates adopted new policies recognizing race as a social construct, rather than a biological construct.
“The policies aim to advance data-driven, antiracist concepts challenging the current clinical application of race and its effects on vulnerable patient populations,” an AMA statement said.
The new AMA policies “reflect an understanding of race as a socially constructed category different from ethnicity, genetic ancestry, or biology, and aim to end the misinterpretation of race as a biological category defined by genetic traits or biological differences,” the AMA said.
According to the AMA, the practice of accepting race as a biological construct “exacerbates health disparities and results in detrimental health outcomes for marginalized and minoritized communities.”
Specifically, the AMA said it supports ending the practice of using race as a proxy for biology in medical education, research, and clinical practice. It also encourages medical education programs to recognize the harmful effects of this approach. It recommends that clinicians and researchers focus on genetics and biology, the experience of racism, and social determinants of health when describing risk factors for disease.
“The AMA is dedicated to dismantling racist and discriminatory policies and practices across all of health care, and that includes the way we define race in medicine,” said AMA board member Michael Suk, MD, in its statement. “We believe it is not sufficient for medicine to be nonracist, which is why the AMA is committed to pushing for a shift in thinking from race as a biological risk factor to a deeper understanding of racism as a determinant of health.”
The AMA also plans to partner with physician organizations and other stakeholders “to identify any problematic aspects of medical education that may perpetuate institutional and structural racism.” For example, the AMA will work with other organizations to improve clinical algorithms that incorrectly adjust for race and lead to less-than-optimal care for minority patients.
A version of this article originally appeared on Medscape.com.
Antidepressant shows early promise for mild COVID-19
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
Early treatment with the antidepressant fluvoxamine (Luvox) may help prevent respiratory deterioration in patients with mild symptomatic COVID-19, results of a preliminary randomized controlled trial suggest.
In the trial, none of the patients who took fluvoxamine within 7 days of first symptoms developed serious breathing difficulties or required hospitalization for respiratory deterioration.
“Most investigational treatments for COVID-19 have been aimed at the very sickest patients, but it’s also important to find therapies that prevent patients from getting sick enough to require supplemental oxygen or to have to go to the hospital,” study investigator Eric J. Lenze, MD, professor of psychiatry and director of the Healthy Mind Lab at Washington University, St. Louis, said in a statement.
“Our study suggests fluvoxamine may help fill that niche,” Lenze added.
The study was published online Nov. 12 in the JAMA.
Antiviral effects?
The study included 152 nonhospitalized adults (mean age, 46 years; 72% women) with confirmed SARS-CoV-2 infection and mild COVID-19 symptoms starting within 7 days and oxygen saturation of 92% or greater.
Eighty were randomly assigned to 100 mg of fluvoxamine three times daily for 15 days and 72 to matching placebo.
The primary outcome was clinical deterioration within 15 days of randomization defined by meeting two criteria. These included shortness of breath or hospitalization for shortness of breath or pneumonia and oxygen saturation <92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Clinical deterioration occurred in none of the 80 patients taking fluvoxamine compared with 6 of 72 (8.3%) patients taking placebo, an absolute difference of 8.7% (95% confidence interval, 1.8%-16.4%).
Clinical deterioration in the placebo group happened from 1 to 7 days after randomization and from 3 to 12 days after the onset of COVID-19 symptoms. Four of the 6 patients with clinical deterioration were admitted to the hospital for 4-21 days. One patient required mechanical ventilation for 10 days. No patients died.
Hypothesis generating
The authors cautioned that the study was small and with short follow-up and that the findings “need to be interpreted as hypothesis generating rather than as a demonstration of efficacy.”
However, they noted, if the drug turns out to be effective for COVID-19, the potential advantages of fluvoxamine for outpatient use include its safety, widespread availability, low cost, and oral administration.
Carolyn Machamer, PhD, member of the COVID-19 Early Treatment Fund (CETF) scientific advisory board, which funded the study, noted that there are several reasons fluvoxamine might be helpful in COVID-19.
“The preliminary data suggest the mechanism involves activation of the sigma-1 receptor, which has a number of documented activities. One strong possibility is that activation dampens cytokine release and thus the inflammatory response,” she said in an interview.
“Other possible mechanisms can include inhibition of platelet activation and modulation of autophagy. Coronaviruses usurp some autophagy machinery to remodel membranes for replicating their genomes, so this last mechanism might actually be antiviral,” said Dr. Machamer.
She added that a much larger trial is “crucial to see if the initial striking results can be reproduced, and the Healthy Mind Lab and CETF are currently coordinating these next steps.”
The editors of JAMA published an “Editor’s Note” with the study. In it, they wrote the pilot study addresses a “critically important question during the pandemic of how to prevent individuals who acquire COVID-19 from deteriorating to serious illness. If an effective treatment is found for this key gap in treatment, it will affect the health of millions of people worldwide.”
However, the study has “important limitations, and the findings should be interpreted as only hypothesis generating; they should not be used as the basis for current treatment decisions,” cautioned authors Christopher Seymour, MD, Howard Bauchner, MD, and Robert Golub, MD.
This study was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the CETF. Additional support was provided by the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and the National Institutes of Health.
Dr. Lenze has received grants from the Patient-Centered Outcomes Research Institute, Takeda, Alkermes, Janssen, Acadia, and the Barnes Jewish Hospital Foundation and has received consulting fees from Janssen and Jazz Pharmaceuticals. Dr. Machamer has disclosed no relevant financial relationships. Dr. Seymour has received grants from the National Institutes of Health and personal fees from Beckman Coulter and Edwards Lifesciences.
A version of this article originally appeared on Medscape.com.
Pfizer files for FDA emergency use authorization of COVID vaccine
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Tips for physicians, patients to make the most of the holidays amid COVID
“We must accept finite disappointment, but never lose infinite hope,” Martin Luther King, Jr.
This holiday season will be like no other. We will remember it for the rest of our lives, and we will look back to see how we faced the holidays during a pandemic.
Like the rest of 2020, the holidays will need to be reimagined. Years and even decades of tradition will be broken as we place health above merriment.
Here are a few tips to help all of us and our patients make the most of this holiday season.
- Reprioritize: This holiday season will be about depth not breadth, quality not quantity, and less not more. Trips are canceled and gatherings have shrunk. We are not running from store to store or party to party. Instead, you will find yourself surrounded by fewer friends and family. Some will be alone to optimally protect their health and the health of others. Do your best to focus on the half-full portion.
- Embrace change: Don’t compare or try to make this year like previous years. Be creative and try to find ways to make a new format fun. Meeting during the day and limiting alcohol intake can assist in making sure everyone stays safe. It has been interesting to see how many of my patients have decreased their alcohol use during quarantine. I hope this pattern will continue over the next weeks and months.
- Practice self-care: As health care professionals, we must remember the old adage “physician, heal thyself.” This year has been so difficult for almost all of us. It was filled with unprecedented levels of personal and professional stress. Holidays are often about what we can do for others, but this year we may need to place self-care first. Do what brings you happiness.
Even though you aren’t traveling, you can still disconnect from work. Set up a schedule and stick to it making sure you take plenty of time to rest and enjoy. Many of us have been working extremely long hours and a break is so needed. Take it if you possibly can. Detox from your screen! Limit the news. Creativity and productivity will be enhanced in 2021 if we can come in recharged.
For those remaining on the front lines, be patient; the end is nearing. Take care of yourself when you are not working. We are all so grateful to those in our field who have sacrificed so much to care for others. Eat, drink, and rest well to keep your immune system strong.
- Acknowledge your negative emotions: As we all know, if you try to deny negative emotions, they continue to pop up. If we give them time and space to be felt, we will find they diminish in intensity. Long work hours may have prevented us from feeling our emotions, so don’t be surprised if they surface when we take a break.
Let yourself feel the sadness for what you have experienced this year. Be open about missing those who can’t be with you because of travel or other restrictions. Let yourself feel the disappointment about your holiday travel plans that you can’t embark upon.
You may elect to share these emotions with someone close to you or with a professional. To paraphrase Carl Jung, “what we resist, persists,” so don’t try to hide from your negative emotions. Most of us had lots of them in 2020, so don’t be shy about admitting it.
- Focus on growth: What have we learned from 2020 and how can we be better equipped in 2021 and beyond?
Trauma can bring growth not just disorder. This year has returned well-deserved prestige to our fields. We are being lauded as heroes as we have scarified our health and the health of our loved ones to serve others. Can we choose to celebrate our accomplishments?
We have become more resilient and learned to continue on in the face of great hardship. Many of us have gained confidence as we confronted this historic challenge. As we have been reminded of death daily, we learn to appreciate life more fully and not take any day for granted.
I am proud to be a physician during this pandemic, and I hope you are, too!
- Find joy: Often times, we find real happiness in smaller moments and experiences. For many, this time of year is filled with so much stress that it can be hard to carve out moments of joy. As we may be less busy socially this holiday season, might we find even more joy?
Joy can only be experienced in the present moment. Tap into all your senses. Eat slowly making sure to smell and taste every bite. Cherish those who can still gather at your table. If you find yourself alone, embrace that experience. Safety must continue to come first, and we can’t let down our guard now.
- Reflect: New Year’s Eve is always a time for reflection and hope for the future. Most of us will be glad to see 2020 in the rearview mirror. With multiple and very promising vaccines on the horizon, we can anticipate a brighter future. We must continue to work hard; remain patient; and be creative, resilient, and optimistic. Let’s try to fill our days with hope and purpose and work together to achieve a brighter future for all.
“Learn from yesterday, live for today, hope for tomorrow,” Albert Einstein
Wishing you health and happiness in this holiday season and beyond.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She also is founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world.
“We must accept finite disappointment, but never lose infinite hope,” Martin Luther King, Jr.
This holiday season will be like no other. We will remember it for the rest of our lives, and we will look back to see how we faced the holidays during a pandemic.
Like the rest of 2020, the holidays will need to be reimagined. Years and even decades of tradition will be broken as we place health above merriment.
Here are a few tips to help all of us and our patients make the most of this holiday season.
- Reprioritize: This holiday season will be about depth not breadth, quality not quantity, and less not more. Trips are canceled and gatherings have shrunk. We are not running from store to store or party to party. Instead, you will find yourself surrounded by fewer friends and family. Some will be alone to optimally protect their health and the health of others. Do your best to focus on the half-full portion.
- Embrace change: Don’t compare or try to make this year like previous years. Be creative and try to find ways to make a new format fun. Meeting during the day and limiting alcohol intake can assist in making sure everyone stays safe. It has been interesting to see how many of my patients have decreased their alcohol use during quarantine. I hope this pattern will continue over the next weeks and months.
- Practice self-care: As health care professionals, we must remember the old adage “physician, heal thyself.” This year has been so difficult for almost all of us. It was filled with unprecedented levels of personal and professional stress. Holidays are often about what we can do for others, but this year we may need to place self-care first. Do what brings you happiness.
Even though you aren’t traveling, you can still disconnect from work. Set up a schedule and stick to it making sure you take plenty of time to rest and enjoy. Many of us have been working extremely long hours and a break is so needed. Take it if you possibly can. Detox from your screen! Limit the news. Creativity and productivity will be enhanced in 2021 if we can come in recharged.
For those remaining on the front lines, be patient; the end is nearing. Take care of yourself when you are not working. We are all so grateful to those in our field who have sacrificed so much to care for others. Eat, drink, and rest well to keep your immune system strong.
- Acknowledge your negative emotions: As we all know, if you try to deny negative emotions, they continue to pop up. If we give them time and space to be felt, we will find they diminish in intensity. Long work hours may have prevented us from feeling our emotions, so don’t be surprised if they surface when we take a break.
Let yourself feel the sadness for what you have experienced this year. Be open about missing those who can’t be with you because of travel or other restrictions. Let yourself feel the disappointment about your holiday travel plans that you can’t embark upon.
You may elect to share these emotions with someone close to you or with a professional. To paraphrase Carl Jung, “what we resist, persists,” so don’t try to hide from your negative emotions. Most of us had lots of them in 2020, so don’t be shy about admitting it.
- Focus on growth: What have we learned from 2020 and how can we be better equipped in 2021 and beyond?
Trauma can bring growth not just disorder. This year has returned well-deserved prestige to our fields. We are being lauded as heroes as we have scarified our health and the health of our loved ones to serve others. Can we choose to celebrate our accomplishments?
We have become more resilient and learned to continue on in the face of great hardship. Many of us have gained confidence as we confronted this historic challenge. As we have been reminded of death daily, we learn to appreciate life more fully and not take any day for granted.
I am proud to be a physician during this pandemic, and I hope you are, too!
- Find joy: Often times, we find real happiness in smaller moments and experiences. For many, this time of year is filled with so much stress that it can be hard to carve out moments of joy. As we may be less busy socially this holiday season, might we find even more joy?
Joy can only be experienced in the present moment. Tap into all your senses. Eat slowly making sure to smell and taste every bite. Cherish those who can still gather at your table. If you find yourself alone, embrace that experience. Safety must continue to come first, and we can’t let down our guard now.
- Reflect: New Year’s Eve is always a time for reflection and hope for the future. Most of us will be glad to see 2020 in the rearview mirror. With multiple and very promising vaccines on the horizon, we can anticipate a brighter future. We must continue to work hard; remain patient; and be creative, resilient, and optimistic. Let’s try to fill our days with hope and purpose and work together to achieve a brighter future for all.
“Learn from yesterday, live for today, hope for tomorrow,” Albert Einstein
Wishing you health and happiness in this holiday season and beyond.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She also is founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world.
“We must accept finite disappointment, but never lose infinite hope,” Martin Luther King, Jr.
This holiday season will be like no other. We will remember it for the rest of our lives, and we will look back to see how we faced the holidays during a pandemic.
Like the rest of 2020, the holidays will need to be reimagined. Years and even decades of tradition will be broken as we place health above merriment.
Here are a few tips to help all of us and our patients make the most of this holiday season.
- Reprioritize: This holiday season will be about depth not breadth, quality not quantity, and less not more. Trips are canceled and gatherings have shrunk. We are not running from store to store or party to party. Instead, you will find yourself surrounded by fewer friends and family. Some will be alone to optimally protect their health and the health of others. Do your best to focus on the half-full portion.
- Embrace change: Don’t compare or try to make this year like previous years. Be creative and try to find ways to make a new format fun. Meeting during the day and limiting alcohol intake can assist in making sure everyone stays safe. It has been interesting to see how many of my patients have decreased their alcohol use during quarantine. I hope this pattern will continue over the next weeks and months.
- Practice self-care: As health care professionals, we must remember the old adage “physician, heal thyself.” This year has been so difficult for almost all of us. It was filled with unprecedented levels of personal and professional stress. Holidays are often about what we can do for others, but this year we may need to place self-care first. Do what brings you happiness.
Even though you aren’t traveling, you can still disconnect from work. Set up a schedule and stick to it making sure you take plenty of time to rest and enjoy. Many of us have been working extremely long hours and a break is so needed. Take it if you possibly can. Detox from your screen! Limit the news. Creativity and productivity will be enhanced in 2021 if we can come in recharged.
For those remaining on the front lines, be patient; the end is nearing. Take care of yourself when you are not working. We are all so grateful to those in our field who have sacrificed so much to care for others. Eat, drink, and rest well to keep your immune system strong.
- Acknowledge your negative emotions: As we all know, if you try to deny negative emotions, they continue to pop up. If we give them time and space to be felt, we will find they diminish in intensity. Long work hours may have prevented us from feeling our emotions, so don’t be surprised if they surface when we take a break.
Let yourself feel the sadness for what you have experienced this year. Be open about missing those who can’t be with you because of travel or other restrictions. Let yourself feel the disappointment about your holiday travel plans that you can’t embark upon.
You may elect to share these emotions with someone close to you or with a professional. To paraphrase Carl Jung, “what we resist, persists,” so don’t try to hide from your negative emotions. Most of us had lots of them in 2020, so don’t be shy about admitting it.
- Focus on growth: What have we learned from 2020 and how can we be better equipped in 2021 and beyond?
Trauma can bring growth not just disorder. This year has returned well-deserved prestige to our fields. We are being lauded as heroes as we have scarified our health and the health of our loved ones to serve others. Can we choose to celebrate our accomplishments?
We have become more resilient and learned to continue on in the face of great hardship. Many of us have gained confidence as we confronted this historic challenge. As we have been reminded of death daily, we learn to appreciate life more fully and not take any day for granted.
I am proud to be a physician during this pandemic, and I hope you are, too!
- Find joy: Often times, we find real happiness in smaller moments and experiences. For many, this time of year is filled with so much stress that it can be hard to carve out moments of joy. As we may be less busy socially this holiday season, might we find even more joy?
Joy can only be experienced in the present moment. Tap into all your senses. Eat slowly making sure to smell and taste every bite. Cherish those who can still gather at your table. If you find yourself alone, embrace that experience. Safety must continue to come first, and we can’t let down our guard now.
- Reflect: New Year’s Eve is always a time for reflection and hope for the future. Most of us will be glad to see 2020 in the rearview mirror. With multiple and very promising vaccines on the horizon, we can anticipate a brighter future. We must continue to work hard; remain patient; and be creative, resilient, and optimistic. Let’s try to fill our days with hope and purpose and work together to achieve a brighter future for all.
“Learn from yesterday, live for today, hope for tomorrow,” Albert Einstein
Wishing you health and happiness in this holiday season and beyond.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018). She also is founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world.
















