User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


How often should we check EKGs in patients starting antipsychotic medications?
Determining relative risk with available data
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 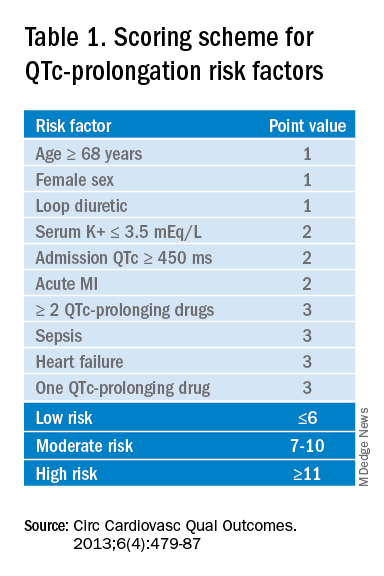
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.
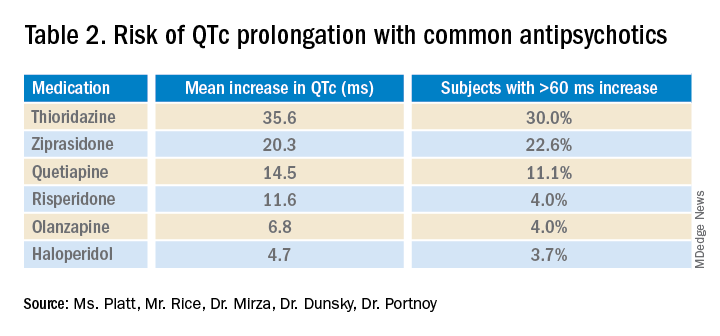
Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.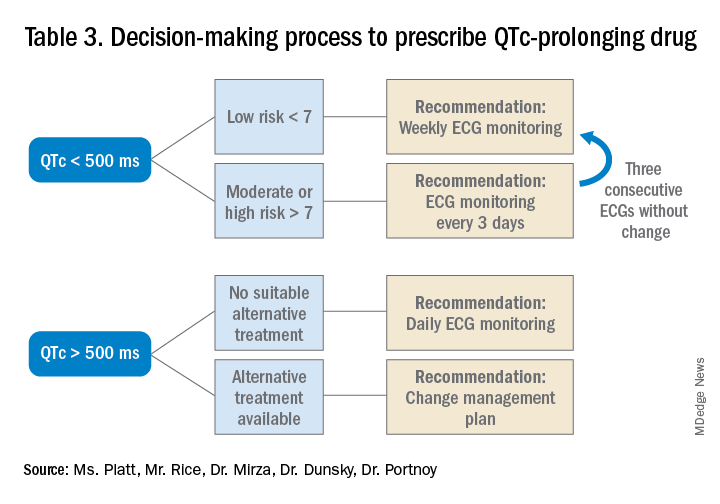
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Determining relative risk with available data
Determining relative risk with available data
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.

Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Case
An 88-year-old woman with history of osteoporosis, hyperlipidemia, and a remote myocardial infarction presents to the ED with altered mental status and agitation. The patient is admitted to the medicine service for further management. Her current medications include a thiazide and a statin. Psychiatry is consulted and recommends administering intravenous haloperidol. A baseline EKG shows a corrected QT interval (QTc) of 486 milliseconds (ms). How often should subsequent EKGs be ordered?
Overview of issue
A prolonged QT interval can predispose a patient to dangerous arrhythmias such as Torsades de pointes (TdP), which results in sudden cardiac death in about 10% of cases.1,2 A prolonged QTc interval can be caused by cardiac, renal, or hepatic dysfunction; congenital Long QT Syndrome (LQTS)2; electrolyte abnormalities; or as a result of many drugs, including most antipsychotic medications such as quetiapine, olanzapine, risperidone and haloperidol.
To diminish risk of TdP while taking these medications, it is necessary to monitor the QTc interval. Before commencing a QT-prolonging medication, it is recommended to get a baseline EKG, then perform EKG monitoring after administering the medication.
According to American Heart Association guidelines, a prolonged QT interval is considered more than 460 ms in women or above 450 ms in men.3 If an abnormal rhythm and/or prolonged QTc is detected via EKG monitoring, then the drug dosage can be changed or an alternative therapy selected.4 However, there are no current guidelines recommending how often EKG monitoring should be performed after a QT-prolonging antipsychotic medication is administered on an inpatient medicine unit. Without guidelines, there is potential for health care providers to under- or over-order EKG monitoring, possibly putting patients at risk of TdP or wasting hospital resources, respectively.
Overview of the data
There are currently no universally accepted guidelines regarding inpatient EKG monitoring for patients started on QTc prolonging antipsychotic medications. A 2018 review of the literature surrounding assessment and management of patients on QTc prolonging medications was performed to analyze the available data and make recommendations; notably the evidence was limited as none of the studies were randomized controlled trials.
The authors recommend assessing the drug for QTc prolonging potential, and if possible, choosing alternative treatment in patients with baseline prolonged QTc. If the QT prolonging medication is the best or only option, then the next step is assessing the patient’s risk for QTc prolongation based on that person’s current condition and medical history.5 They recommend using the QTc prolonging risk point system developed by Tisdale and colleagues, which identified patient risk factors for elevated QTc intervals based on EKG findings in cardiac care units at a large tertiary hospital center.6
Based on the patient’s demographics, current condition, and medication list, the score can be used to stratify patients into low-, medium-, and high-risk categories (see Table 1). 
Risk factors include age over 68 years, female sex, prior MI, concurrent use of other QTc prolonging medications, and sepsis, all of which have differing ability to cause QTc prolongation and thus are weighted differentially. This scoring system is helpful in identifying high risk patients; however, the review does not include recommendations for management of these patients beyond removing the offending drug or monitoring EKGs more aggressively in higher risk patients once identified.6
Low-risk patients can be managed expectantly. If the baseline QTc is < 500 ms, then the provider may administer the medication, but should obtain follow-up EKG monitoring to ensure the QTc does not rise above 500 ms; if it does, a management change is necessitated. For moderate- to high-risk patients with a baseline QTc > 500 ms, they recommend not administering the medication and consulting a cardiologist. The review does not provide a recommendation on how often EKG monitoring should be performed after prescribing an antipsychotic medication in an inpatient setting.5
A 2018 review article explored patient risk factors for a prolonged QTc in the setting of prescribing potentially QTc prolonging antipsychotics.7 The authors reiterate that QTc prolonging risk factors are important considerations when prescribing antipsychotics that can lead to adverse events, though they note that much of the literature associating antipsychotics with negative outcomes consists of case reports in which patients had independent risk factors for development of TdP, such as preexisting ventricular arrhythmias.
In addition, the data regarding the risk of each individual antipsychotic agent are not comprehensive. Some medications that have been deemed “QTc prolonging” were identified as such in only a handful of cases where patients had confounding comorbid risk factors. This raises concern that some medications are being unduly stigmatized in situations where there is little chance of TdP. If there is no equivalent or alternate treatment available, this may lead to an antipsychotic medication being held unnecessarily, which may exacerbate the psychiatric illness.
The authors note that the trend toward ordering baseline EKGs in the inpatient setting following administration of a new antipsychotic may be partly attributed to the ready availability of EKG testing in hospitals. They recommend a baseline EKG to assess the patient’s risk. For most agents, they recommend no further EKG monitoring unless there is a change in patient risk factors. Follow-up EKGs should be done in patients with multiple or significant risk factors to assess their QTc stability. In patients with a QTc > 500 ms on a follow-up EKG, daily monitoring is encouraged alongside reassessment of the current treatment regimen.7
Overall, the current literature suggests that providers should know which antipsychotics carry a risk for QTc prolongation and what other treatment options are available. The risk of QTc prolongation for common antipsychotic agents is provided in Table 2.

Providers should assess their patients’ risk factors for QTc prolongation and order a baseline EKG to help quantify the cardiac risk associated with prescribing the drug. In patients with many risk factors or complicated medication regimens, a follow-up EKG should be performed to assess the new QTc baseline. If the subsequent QTc is > 500 ms, then an alternative medication should be strongly considered. The majority of patients, however, will not have a clinically significant increase in their QTc, in which case there is no need for a change in medication and monitoring frequency can be deescalated.
Application of data to the case
Our 88-year-old patient has multiple risk factors for a prolonged QTc, and according to the Tisdale scoring system is at moderate risk (7-10 points). Her risk of developing TdP increases with the addition of IV haloperidol to her regimen.
Because of her increased risk, it is reasonable to consider alternative management. If she can cooperate with PO medications, then olanzapine could be given, which has a lesser effect on the QTc interval. If unable to take oral medications, she could be given haloperidol intramuscularly, which causes less QTc prolongation than the IV formulation. If an antipsychotic is administered, she should receive EKG monitoring.
Given the lack of evidence on the optimal monitoring strategy, a protocol should be utilized that balances the ability to capture a clinically meaningful increase in the QTc with appropriate stewardship of resources. Our practice is to initially monitor the EKG every 3 days in moderate- to high-risk patients with baseline QTc < 500 ms. If the QTc remains below 500 ms over three EKGs, then treatment may continue with EKG monitoring weekly while the patient is hospitalized. If the QTc rises above 500 ms, then a management change would be indicated (either dose reduction or a change of agents). If antipsychotic medications are continued, we check the EKG daily while the QTc is >500 ms until there are three unchanged EKGS, and then consider deescalating monitoring to every 3 days.
Bottom line
Prior to prescribing, perform a baseline EKG and assess the patient’s risk of QTc prolongation. If the patient is at increased risk, avoid prescribing QTc prolonging medications where alternatives exist. If a QTc prolonging medication is used in a patient with a moderate- to high-risk score, check an EKG every 3 days or daily if the QTc increases to > 500 ms.
Ms. Platt is a medical student at the Icahn School of Medicine at Mount Sinai in New York. Mr. Rice is a medical student at the Icahn School of Medicine. Dr. Mirza is assistant clinical professor of psychiatry at the Icahn School of Medicine. Dr. Dunsky is a cardiologist and assistant professor at the Icahn School of Medicine. Dr. Portnoy is a hospitalist and assistant professor at the Icahn School of Medicine.
References
1. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Supplements. 2001;3(suppl_K):K70-K80. doi: 10.1016/S1520-765X(01)90009-4.
2. Schwartz PJ, Woosley RL. Predicting the unpredictable: Drug-induced QT prolongation and torsades de pointes. J Am Coll Cardiol. 2016;67(13):1639-50. doi: 10.1016/j.jacc.2015.12.063.
3. Rautaharju PM et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009 Mar 17;53(11):982-91. doi: 10.1016/j.jacc.2008.12.014.
4. Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
5. Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
6. Tisdale JE et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479-87. doi: 10.1161/CIRCOUTCOMES.113.000152.
7. Beach SR et al. QT prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Key points
- An increased QTc interval can lead to TdP, ventricular fibrillation and cardiac death.
- The relative risk of each antipsychotic medication should be determined based on available data and the Tisdale scoring system can provide a system to assess a patient’s risk of QTc prolongation.
- Low-risk patients with a baseline QTc <500 ms should receive a baseline EKG and inpatient EKG monitoring weekly while moderate- to high-risk patients should receive EKG monitoring every 3 days.
- A QTc > 500 ms suggests the need for a management change (drug discontinuation, dose reduction, or a switch to another agent). If the antipsychotic is absolutely necessary, perform daily EKG monitoring until there are three unchanged EKGs, and then consider deescalating monitoring to every 3 days.
Additional reading
Beach SR et al. QT Prolongation, torsades de pointes, and psychotropic medications: A 5-year update. Psychosomatics. 2018;59(2):105-22. doi: 10.1016/j.psym.2017.10.009.
Drew BJ et al. Prevention of torsades de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;121(8):1047-60. doi: 10.1161/CIRCULATIONAHA.109.192704.
Zolezzi M, Cheung L. A literature-based algorithm for the assessment, management, and monitoring of drug-induced QTc prolongation in the psychiatric population. Neuropsychiatr Dis Treat. 2019;15:105-14. doi: 10.2147/NDT.S186474.
Quiz
A 70-year-old male inpatient on furosemide with last known potassium level of 3.3 is going to be started on olanzapine. His baseline EKG has a QTc of 470 ms.
How often should he receive EKG monitoring?
A. Daily
B. Every 3 days
C. Weekly
D. Monthly
Answer (C): He is a low risk patient (6 points: over 70 yrs, loop diuretic, K+< 3.5, QTc > 450 ms), so he should receive weekly EKG monitoring.
Navigating challenges in COVID-19 care
Early strategies for adapting to a moving target
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
Early strategies for adapting to a moving target
Early strategies for adapting to a moving target
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
During the early months of the COVID-19 pandemic, hospital groups and systems scrambled to create protocols and models to respond to the novel coronavirus. In the pre-pandemic world, hospital groups have traditionally focused on standardizing clinical protocols and care models that rely on evidence-based medical practices or extended experience.
During COVID-19, however, our team at Dell Medical School needed to rapidly and iteratively standardize care based on evolving science, effectively communicate that approach across rotating hospital medicine physicians and residents, and update care models, workflows, and technology every few days. In this article, we review our initial experiences, describe the strategies we employed to respond to these challenges, and reflect on the lessons learned and our proposed strategy moving forward.
Early pandemic challenges
Our initial inpatient strategies focused on containment, infection prevention, and bracing ourselves rather than creating a COVID Center of Excellence (COE). In fact, our hospital network’s initial strategy was to have COVID-19 patients transferred to a different hospital within our network. However, as March progressed, we became the designated COVID-19 hospital in our area’s network because of the increasing volume of patients we saw.
Patients from the surrounding regional hospitals were transferring their COVID-19 patients to us and we quickly saw the wide spectrum of illness, ranging from mild pneumonia to severe disease requiring mechanical ventilation upon admission. All frontline providers felt the stress of needing to find treatment options quickly for our sickest patients. We realized that to provide safe, effective, and high-quality care to COVID-19 patients, we needed to create a sustainable and standardized interdisciplinary approach.
COVID-19 testing was a major challenge when the pandemic hit as testing kits and personal protective equipment were in limited supply. How would we choose who to test or empirically place in COVID-19 isolation? In addition, we faced questions surrounding safe discharge practices, especially for patients who could not self-isolate in their home (if they even had one).
In March, emergency use authorization (EUA) for hydroxychloroquine was granted by the U.S. FDA despite limited data. This resulted in pressure from the public to use this drug in our patients. At the same time, we saw that some patients quickly got better on their own with supportive care. As clinicians striving to practice evidence-based medicine, we certainly did not want to give patients an unproven therapy that could do more harm than good. We also felt the need to respond with statements about what we could do that worked – rather than negotiate about withholding certain treatments featured in the news. Clearly, a “one-size-fits-all” approach to therapeutics was not going to work in treating patients with COVID-19.
We realized we were going to have to learn and adapt together – quickly. It became apparent that we needed to create structures to rapidly adjudicate and integrate emerging science into standardized clinical care delivery.
Solutions in the form of better structures
In response to these challenges, we created early morning meetings or “huddles” among COVID-19 care teams and hospital administration. A designated “COVID ID” physician from Infectious Diseases would meet with hospitalist and critical care teams each morning in our daily huddles to review all newly admitted patients, current hospitalized patients, and patients with pending COVID-19 tests or suspected initial false-negative tests.
Together, and via the newly developed Therapeutics and Informatics Committee, we created early treatment recommendations based upon available evidence, treatment availability, and the patient’s severity of illness. Within the first ten days of admitting our first patient, it had become standard practice to review eligible patients soon after admission for therapies such as convalescent plasma, and, later, remdesivir and steroids.
We codified these consensus recommendations and processes in our Dell Med COVID Manual, a living document that was frequently updated and disseminated to our group. It created a single ‘true north’ of standardized workflows for triage, diagnosis, management, discharge coordination, and end-of-life care. The document allowed for continuous and asynchronous multi-person collaboration and extremely rapid cycles of improvement. Between March and December 2020, this 100-page handbook went through more than 130 iterations.
Strategy for the future
This approach – communicating frequently, adapting on a daily to weekly basis, and continuously scanning the science for opportunities to improve our care delivery – became the foundation of our approach and the Therapeutics and Informatics Committee. Just as importantly, this created a culture of engagement, collaboration, and shared problem-solving that helped us stay organized, keep up to date with the latest science, and innovate rather than panic when faced with ongoing unpredictability and chaos in the early days of the pandemic.
As the pandemic enters into its 13th month, we carry this foundation and our strategies forward. The infrastructure and systems of communication that we have set in place will allow us to be nimble in our response as COVID-19 numbers surge in our region.
Dr. Gandhi is an assistant professor in the department of internal medicine at Dell Medical School, University of Texas, Austin. Dr. Mondy is chief of the division of infectious disease and associate professor in the department of internal medicine at Dell Medical School. Dr. Busch and Dr. Brode are assistant professors in the department of internal medicine at Dell Medical School. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
Blacks and Hispanics have higher inpatient use for mycosis fungoides
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
according to an analysis of the 2012-2017 National Inpatient Sample (NIS).
The findings are consistent with prior studies implicating earlier and more severe disease in Black and Hispanic patients, and reinforce the importance of accurate diagnosis and early treatment.
Dermatologists should maintain “a higher index of suspicion for MF in patients with skin of color, as early diagnosis may help mitigate the downstream costs of management,” Justin Choi, BA, a medical student at the University of Illinois at Chicago, said at the annual Skin of Color Society symposium.
Mr. Choi and coinvestigators, led by Shawn Kwatra, MD, of Johns Hopkins University, Baltimore, identified hospital admissions for MF in the NIS for 10,790 White patients, 4,020 Black patients, and 1,615 Hispanic patients over the 5-year period. The inpatient prevalence of MF – the most common variant of primary cutaneous T-cell lymphoma – was highest in these groups.
Black and Hispanic patients who were hospitalized for MF were significantly younger than White patients, with a mean age of 51.7 years and 48.5 years, respectively, compared with 59.9 years (P < .001 in each case). They also had longer lengths of stay: 8.34 days on average for Black patients and 8.88 for Hispanic patients, compared with 6.66 days for White patients (P < .001 and P = .001, respectively).
Hispanic patients accrued the highest costs of care (a mean of $107,242 vs. $64,049, P =.003) and underwent more procedures (a mean of 2.43 vs. 1.93, P = .004) than White patients. Black patients similarly had higher costs associated with their hospital stay (a mean of $75,053 vs. $64,049, P =.042).
In a multivariate linear regression adjusted for age, sex and insurance type, Black race remained significantly associated with a longer LOS than White race, and Hispanic ethnicity with a longer LOS, increased costs, and more procedures than White race.
The NIS is a publicly available, all-payer inpatient care database developed for the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.
Mr. Choi is a dermatology research fellow working under the guidance of Dr. Kwatra.
FROM SOC SOCIETY 2021
CDC panel: Pause of J&J COVID-19 vaccine to remain for now
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
The Advisory Committee on Immunization Practices decided there was not adequate information to change again recommend use of the Johnson & Johnson vaccine.
The committee’s decision comes the day after the CDC and Food and Drug Administration recommended that J&J injections be paused after reports of rare, but serious types of blood clots in six patients among the 6.8 million people who had received the J&J vaccine in the United States.
A member of the committee, Beth Bell, MD, said: “I do not want to be sending a message that there is some huge concern here on a different order of magnitude than any other vaccine safety signals that we evaluate. And I don’t want to send a message that there is something fundamentally wrong with the vaccine because that also I don’t agree with.”
At the end of the 4-hour meeting, ACIP members decided to call a meeting in 1 or 2 weeks and evaluate more safety data, specifically reports of people who have received the J&J vaccine in the past 2 weeks.
Some, however, pointed out that delaying a decision could have substantial consequences as well in terms of unused vaccine doses and public confidence.
Committee member Camiile Kotton, MD, described the pause as “devastating.”
“Putting this vaccine on pause for those of us that are frontline health care workers has really been devastating,” she said. “I agree in general that we don’t have enough data to make a decision at this time but we were planning on using this vaccine in the state of Massachusetts for people who were homebound and otherwise not able to get a vaccine. We were planning on using it for our vulnerable inpatient population often with many comorbidities and at high risk for disease but haven’t been able to get vaccinated otherwise.”
Pausing the one-and-done vaccine that doesn’t have the significant refrigeration requirements of the others “is a significant loss,” she said.
What is known, not known
Sara Oliver, MD, who leads the COVID-19 Vaccines ACIP Work Group, summarized what is known and unknown about the blood clots.
Among the six cases of cerebral venous sinus thrombosis reported to the Vaccine Adverse Event Reporting System after the J&J shot, all were women aged 18-48 years and all developed the clots 6-13 days after receiving the vaccine.
No cases of these clots have been reported from either the Pfizer or Moderna shots, she noted.
In the United States, the two mRNA vaccine alternatives – the Moderna and Pfizer vaccines – are available “and based on current projections supply of both vaccines are expected to be relatively stable in the near future,” she said.
She said 14 million doses of Pfizer and Moderna are expected each week in the United States and J&J vaccines makes up less than 5% of vaccines administered in the country.
Approximately 13 million J&J doses are available to order or are already at administration sites, she said.
But much more is unknown, she said.
“There may be more cases identified in the coming days to weeks,” Dr. Oliver said, referring back to the average time from vaccination to symptom onset.
Scott Ratzan, MD, editor-in-chief of the Journal of Health Communication: International Perspectives and executive director of Business Partners to CONVINCE (BP2C), a global network of employers that promotes COVID-19 vaccination among employees, suppliers, and customers, applauded ACIP’s delay on making a decision.
Dr. Ratzan, who watched the deliberations online, said in an interview the decision “shows an admirable abundance of caution in the distribution of COVID-19 vaccines.”
“Unfortunately,” he said, “the pause also worsens the existing and pervasive vaccine hesitancy issue.
“We need a rational strategy regarding who should or should not get the J&J/Janssen vaccine since these rare adverse events appear to affect a particular group of people, females aged 18-48. It is essential that we build vaccine confidence and retain the option of using this vaccine for people who are not in this risk group.”
He pointed out there are safety red flags with the Pfizer and Moderna COVID-19 vaccines.
“We should feel reassured about the process of ensuring vaccine safety as the FDA and CDC have quickly addressed risk and shared the data transparently of the J&J vaccine and taken appropriate action,” he said.
ACIP’s executive secretary, Amanda Cohn, MD, said the date for the next meeting would be set by April 16.
A version of this article first appeared on WebMD.com.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
How to counsel worried patients about the J&J vaccine news
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
On April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration issued a joint statement recommending a pause in Johnson & Johnson vaccine administration, pending review of six reported U.S. cases of a rare and severe type of blood clot occurring after receiving the Johnson & Johnson vaccine. To date, more than 6.8 million doses of that vaccine have been given in the United States, so at this point the rate of detected cases of this problem is less than one in a million.
The six cases occurred in women aged 18-48 years, and symptoms occurred 6-13 days after vaccination. In these cases, cerebral venous sinus thrombosis was seen in addition to thrombocytopenia.
Physicians may receive calls from concerned patients who have received a COVID vaccine. However, more than 95% of the vaccine administrations in the United States to date have been the Pfizer and Moderna messenger RNA vaccines. No association between these vaccines and blood clots has been detected. Also, these six cases occurred within 2 weeks of Johnson & Johnson vaccination, so even among those receiving the Johnson & Johnson vaccine, those who are more than 3 weeks out from their vaccination have no need for concern regarding this rare complication.
Physicians should counsel those who have received the Johnson & Johnson vaccine less than 3 weeks ago to watch for easy bruising, gum bleeding, nose bleeds, leg or arm pain or swelling, severe headache or abdominal pain, shortness of breath, or chest pain. If they notice one or more of those symptoms, they should seek medical attention.
The Centers for Disease Control and Prevention will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the six U.S. cases of the Johnson & Johnson vaccine and determine their significance.
Several cases of unusual thromboses and thrombocytopenia have been detected after the Oxford AstraZeneca vaccine, which uses the same adenovirus vector technology as the Johnson & Johnson vaccine, but which is not authorized for use in the United States. The Oxford AstraZeneca vaccine uses a recombinant deficient chimpanzee adenovirus to deliver the message to cells to produce antibody against the SARS-CoV-2 spike protein. The Johnson & Johnson vaccine uses a recombinant deficient human adenovirus to deliver this same message.
Two recent reports in the New England Journal of Medicine have reported on thrombosis and thrombocytopenia after the Oxford AstraZeneca vaccine in Europe. Both of these reports identified high levels of IgG antibodies to platelet factor 4–polyanion complexes, similar to the mechanism of heparin-induced thrombocytopenia. The term vaccine-induced immune thrombocytopenia was proposed for this phenomenon. Treatment of this condition involves administration of intravenous immunoglobulin and nonheparin anticoagulants. Recent updates from the World Health Organization report that 169 cases of cerebral venous sinus thrombosis and 53 of splanchnic venous thrombosis occurred after 34 million doses of the Oxford AstraZeneca vaccine was administered in the European Union and United Kingdom.
While this pause in Johnson & Johnson vaccination is disappointing news amid increased cases in parts of the country, the Johnson & Johnson vaccines make up less than 5% of the U.S. vaccine doses administered to date. According to the CDC, more than 122 million Americans have received at least one dose and more than 75 million are fully vaccinated.
Dr. Patterson has received an honorarium from Pfizer for an antifungal symposium and is a subinvestigator for the Novavax vaccine. Her spouse served as a consultant for SCYNEXIS, as a speaker for Gilead Sciences and Basilea, and has received a research grant from the National Institutes of Health for the ACTT remdesivir trial.
A version of this article first appeared on Medscape.com.
Data about COVID-19-related skin manifestations in children continue to emerge
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Two and stratifying children at risk for serious, systemic illness due to the virus.
In a single-center descriptive study carried out over a 9-month period, researchers in Madrid found that of 50 hospitalized children infected with COVID-19, 21 (42%) had mucocutaneous symptoms, most commonly exanthem, followed by conjunctival hyperemia without secretion and red cracked lips or strawberry tongue. In addition, 18 (36%) fulfilled criteria for Multisystem Inflammatory Syndrome in Children (MIS-C).
“Based on findings in adult patients, the skin manifestations of COVID-19 have been classified under five categories: acral pseudo-chilblain, vesicular eruptions, urticarial lesions, maculopapular eruptions, and livedo or necrosis,” David Andina-Martinez, MD, of Hospital Infantil Universitario Niño Jesús, Madrid, and colleagues wrote in the study, which was published online on April 2 in the Journal of the American Academy of Dermatology.
“Chilblain lesions in healthy children and adolescents have received much attention; these lesions resolve without complications after a few weeks,” they added. “Besides, other cutaneous manifestations of COVID-19 in children have been the matter of case reports or small case series. Nevertheless, the mucocutaneous manifestations in hospitalized children infected with SARS-CoV-2 and their implications on the clinical course have not yet been extensively described.”
In an effort to describe the mucocutaneous manifestations in children hospitalized for COVID-19, the researchers evaluated 50 children up to 18 years of age who were admitted between March 1 and Nov. 30, 2020, to Hospital Infantil Universitario Niño Jesús, which was designated as a pediatric reference center during the peak of the pandemic. The main reasons for admission were respiratory illness (40%) and MIS-C (40%).
Of the 50 patients, 44 (88%) had a positive RT-PCR for SARS-CoV-2 and 6 (12%) met clinical suspicion criteria and had a negative RT-PCR with a positive IgG serology. In 34 patients (68%), a close contact with a suspected or confirmed case of COVID-19 was referred, while the source of the infection remained unknown in the remaining 16 patients (32%).
The researchers reported that 21 patients (42%) had mucocutaneous symptoms, most commonly maculopapular exanthem (86%), conjunctival hyperemia (81%), and red cracked lips or strawberry tongue (43%). In addition, 18 of the 21 patients (86%) fulfilled criteria for MIS-C.
“A tricky thing about MIS-C is that it often manifests 4-5 weeks after a child had COVID-19,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study. “MIS-C is associated with characteristic bright red lips and a red tongue that might resemble a strawberry. Such oral findings should prompt rapid evaluation for other signs and symptoms. There can be redness of the eyes or other more nonspecific skin findings (large or small areas of redness on the trunk or limbs, sometimes with surface change), but more importantly, fever, a rapid heartbeat, diarrhea, or breathing issues. The risk with MIS-C is a rapid decline in a child’s health, with admission to an intensive care unit.”
Dr. Andina-Martinez and his colleagues also contrast the skin findings of MIS-C, which are not generally on the hands or feet, with the so-called “COVID toe” or finger phenomenon, which has also been associated with SARS-CoV-2, particularly in children. “Only one of the patients in this series had skin involvement of a finger, and it only appeared after recovery from MIS-C,” Dr. Ko noted. “Distinguishing COVID toes from MIS-C is important, as COVID toes has a very good outcome, while MIS-C can have severe consequences, including protracted heart disease.”
In other findings, patients who presented with mucocutaneous signs tended to be older than those without skin signs and they presented at the emergency department with poor general status and extreme tachycardia. They also had higher C-reactive protein and D-dimer levels and lower lymphocyte counts and faced a more than a 10-fold increased risk of being admitted to the PICU, compared with patients who did not have skin signs (OR, 10.24; P = .003).
In a separate study published online on April 7 in JAMA Dermatology, Zachary E. Holcomb, MD, of the combined dermatology residency program at Massachusetts General Hospital, Boston, and colleagues presented what is believed to be the first case report of reactive infectious mucocutaneous eruption (RIME) triggered by SARS-CoV-2. RIME is the preferred term for pediatric patients who present with mucositis and rash (often a scant or even absent skin eruption) triggered by various infectious agents.
The patient, a 17-year-old male, presented to the emergency department with 3 days of mouth pain and nonpainful penile erosions. “One week prior, he experienced transient anosmia and ageusia that had since spontaneously resolved,” the researchers wrote. “At that time, he was tested for SARS-CoV-2 infection via nasopharyngeal polymerase chain reaction (PCR), the results of which were positive.”
At presentation, the patient had no fever, his vital signs were normal, and the physical exam revealed shallow erosions of the vermilion lips and hard palate, circumferential erythematous erosions of the periurethral glans penis, and five small vesicles on the trunk and upper extremities. Serum analysis revealed a normal white blood cell count with mild absolute lymphopenia, slightly elevated creatinine level, normal liver function, slightly elevated C-reactive protein level, and normal ferritin level.
Dr. Holcomb and colleagues made a diagnosis of SARS-CoV-2–associated RIME based on microbiological results, which revealed positive repeated SARS-CoV-2 nasopharyngeal PCR and negative nasopharyngeal PCR testing for Mycoplasma pneumoniae, adenovirus, Chlamydophila pneumoniae, human metapneumovirus, influenza A/B, parainfluenza 1 to 4, rhinovirus, and respiratory syncytial virus. In addition, titers of Mycoplasma pneumoniae IgM levels were negative, but Mycoplasma pneumoniae IgG levels were elevated.
The lesions resolved with 60 mg of oral prednisone taken daily for 4 days. A recurrence of oral mucositis 3 months later responded to 80 mg oral prednisone taken daily for 6 days.
“It’s not surprising that SARS-CoV-2 is yet another trigger for RIME,” said Anna Yasmine Kirkorian, MD, chief of the division of dermatology at Children’s National Hospital, Washington, who was asked to comment about the case report.
“The take-home message is for clinicians to be aware of this association and distinguish these patients from those with MIS-C, because patients with MIS-C require monitoring and urgent systemic treatment. RIME and MIS-C may potentially be distinguished clinically based on the nature of the mucositis (hemorrhagic and erosive in RIME, dry, cracked lips with ‘strawberry tongue’ in MIS-C) but more importantly patients with RIME lack laboratory evidence of severe systemic inflammation,” such as ESR, CRP, or ferritin, she said.
“A final interesting point in this article was the recurrence of mucositis in this patient, which could mean that recurrent mucositis/recurrent RIME might be yet another manifestation of ‘long-COVID’ (now called post-Acute Sequelae of SARS-CoV-2 infection) in some patients,” Dr. Kirkorian added. She noted that the American Academy of Dermatology–International League of Dermatologic Societies COVID-19 Dermatology Registry and articles like these “provide invaluable ‘hot off the presses’ information for clinicians who are facing the protean manifestations of a novel viral epidemic.”
The researchers reported having no financial disclosures.
Making a difference
Hospitalists engaging in advocacy efforts
Hospitalists around the country are devoting large portions of their spare time to a wide range of advocacy efforts. From health policy to caring for the unhoused population to diversity and equity to advocating for fellow hospitalists, these physicians are passionate about their causes and determined to make a difference.
Championing the unhoused
Sarah Stella, MD, FHM, a hospitalist at Denver Health, was initially drawn there because of the population the hospital serves, which includes a high concentration of people experiencing homelessness. As she cared for her patients, Dr. Stella, who is also associate professor of hospital medicine at the University of Colorado, increasingly felt the desire to help prevent the negative downstream outcomes the hospital sees.
To understand the experiences of the unhoused outside the hospital, Dr. Stella started talking to her patients and people in community-based organizations that serve this population. “I learned a ton,” she said. “Homelessness feels like such an intractable, hopeless thing, but the more I talked to people, the more opportunities I saw to work toward something better.”
This led to a pilot grant to work with the Colorado Coalition for the Homeless to set up a community advisory panel. “My goal was to better understand their experiences and to develop a shared vision for how we collectively can do better,” said Dr. Stella. Eventually, she also received a grant from the University of Colorado, and multiple opportunities have sprung up ever since.
For the past several years, Dr. Stella has worked with Denver Health leadership to improve care for the homeless. “Right now, I’m working with a community team on developing an idea to provide peer support from people with a shared lived experience for people who are experiencing homelessness when they’re hospitalized. That’s really where my passion has been in working on the partnership,” she said.
Her advocacy role has been beneficial in her work as a hospitalist, particularly when COVID began. Dr. Stella again partnered with the Colorado Coalition for the Homeless to start a joint task force. “Everyone on our task force is motivated by this powerful desire to improve the health and lives of this community and that’s one of the silver linings in this pandemic for me,” said Dr. Stella.
Advocacy work has also increased Dr. Stella’s knowledge of what community support options are available for the unhoused. This allows her to educate her patients about their options and how to access them.
While she has colleagues who are able to compartmentalize their work, “I absolutely could not be a hospitalist without being an advocate,” Dr. Stella said. “For me, it has been a protective strategy in terms of burnout because I have to feel like I’m working to advocate for better policies and more appropriate resources to address the gaps that I’m seeing.”
Dr. Stella believes that physicians have a special credibility to advocate, tell stories, and use data to back their stories up. “We have to realize that we have this power, and we have it so we can empower others,” she said. “The people I’ve seen in my community who are working so hard to help people who are experiencing homelessness are the heroes. Understanding that and giving power to those people through our voice and our well-respected place in society drives me.”
Strengthening diversity, equity, and inclusion
In September 2020, Michael Bryant, MD, became the inaugural vice chair of Diversity, Equity, and Inclusion for the department of pediatrics at Children’s Hospital Los Angeles, where he is also the division head of pediatric hospital medicine. “I was motivated to apply for this position because I wanted to be an agent for change to eliminate the institutional racism, social injustice, and marginalization that continues to threaten the lives and well-beings of so many Americans,” Dr. Bryant said.
Between the pandemic, the economic decline it has created, and the divisive political landscape, people of color have been especially affected. “These are poignant examples of the ever-widening divide and disenfranchisement many Americans feel,” said Dr. Bryant. “Gandhi said, ‘Be the change that you want to see,’ and that is what I want to model.”
At work, advocacy for diversity, equality, and inclusion is an innate part of everything he does. From the new physicians he recruits to the candidates he considers for leadership positions, Dr. Bryant strives “to have a workforce that mirrors the diversity of the patients we humbly care for and serve.”
Advocacy is intrinsic to Dr. Bryant’s worldview, in his quest to understand and accept each individual’s uniqueness, his desire “to embrace cultural humility,” his recognition that “our differences enhance us instead of diminishing us,” and his willingness to engage in difficult conversations.
“Advocacy means that I acknowledge that intent does not equal impact and that I must accept that what I do and what I say may have unintended consequences,” he said. “When that happens, I must resist becoming defensive and instead be willing to listen and learn.”
Dr. Bryant is proud of his accomplishments and enjoys his advocacy work. In his workplace, there are few African Americans in leadership roles. This means that he is in high demand when it comes to making sure there’s representation during various processes such as hiring and vetting, a disparity known as the “minority tax.”
“I am thankful for the opportunities, but it does take a toll at times,” Dr. Bryant said, which is yet another reason why he is a proponent of increasing diversity and inclusion. “This allows us to build the resource pool as these needs arise and minimizes the toll of the ‘minority tax’ on any single person or small group of individuals.”
This summer, physicians from Dr. Bryant’s hospital participated in the national “White Coats for Black Lives” effort. He found it to be “an incredibly moving event” that hundreds of his colleagues participated in.
Dr. Bryant’s advice for hospitalists who want to get involved in advocacy efforts is to check out the movie “John Lewis: Good Trouble.” “He was a champion of human rights and fought for these rights until his death,” Dr. Bryant said. “He is a true American hero and a wonderful example.”
Bolstering health care change
Since his residency, Joshua Lenchus, DO, FACP, SFHM, has developed an ever-increasing interest in legislative advocacy, particularly health policy. Getting involved in this arena requires an understanding of civics and government that goes beyond just the basics. “My desire to affect change in my own profession really served as the catalyst to get involved,” said Dr. Lenchus, the regional chief medical officer at Broward Health Medical Center in Fort Lauderdale, Fla. “What better way to do that than by combining what we do on a daily basis in the practice of medicine with this new understanding of how laws are passed and promulgated?”
Dr. Lenchus has been involved with both state and national medical organizations and has served on public policy committees as a member and as a chair. “The charge of these committees is to monitor and navigate position statements and policies that will drive the entire organization,” he said. This means becoming knowledgeable enough about a topic to be able to talk about it eloquently and adding supporting personal or professional illustrations that reinforce the position to lawmakers.
He finds his advocacy efforts “incredibly rewarding” because they contribute to his endeavors “to help my colleagues practice medicine in a safe, efficient, and productive manner.” For instance, some of the organizations Dr. Lenchus was involved with helped make changes to the Affordable Care Act that ended up in its final version, as well as changes after it passed. “There are tangible things that advocacy enables us to do in our daily practice,” he said.
When something his organizations have advocated for does not pass, they know they need to try a different outlet. “You can’t win every fight,” he said. “Every time you go and comment on an issue, you have to understand that you’re there to do your best, and to the extent that the people you’re talking to are willing to listen to what you have to say, that’s where I think you can make the most impact.” When changes he has helped fight for do pass, “it really is amazing that you can tell your colleagues about your role in achieving meaningful change in the profession.”
Dr. Lenchus acknowledges that advocacy “can be all-consuming at times. We have to understand our limits.” That said, he thinks not engaging in advocacy could increase stress and potential burnout. “I think being involved in advocacy efforts really helps people conduct meaningful work and educates them about what it means not just to them, but to the rest of the medical profession and the patients that we serve,” he said.
For hospitalists who are interested in health policy advocacy, there are many ways to get involved, Dr. Lenchus said. You could join an organization (many organized medical societies have public policy committees), participate in advocacy activities, work on a political campaign, or even run for office yourself. “Ultimately, education and some level of involvement really will make the difference in who navigates our future as hospitalists,” he said.
Questioning co-management practices
Though he says he’s in the minority, Hardik Vora, MD, SFHM, medical director for hospital medicine at Riverside Regional Medical Center in Newport News, Va., believes that co-management is going to “make or break hospital medicine. It’s going to have a huge impact on our specialty.”
In the roughly 25-year history of hospital medicine, it has evolved from admitting and caring for patients of primary care physicians to patients of specialists and, more recently, surgical patients. “Now there are (hospital medicine) programs across the country that are pretty much admitting everything,” said Dr. Vora.
As a recruiter for the Riverside Health System for the past eight years, “I have not met a single resident who is trained to do what we’re doing in hospital medicine, because you’re admitting surgical patients all the time and you have primary attending responsibility,” Dr. Vora said. “I see that as a cause of a significant amount of stress because now you’re responsible for something that you don’t have adequate training for.”
In the co-management discussion, Dr. Vora notes that people often bring up the research that shows that the practice has improved surgeon satisfaction. “What bothers me is that…you need to add one more question – how does it affect your hospitalists? And I bet the answer to that question is ‘it has a terrible effect.’”
The expectations surrounding hospitalists these days is a big concern in terms of burnout, Dr. Vora said. “We talk a lot about the drivers of burnout, whether it’s schedule or COVID,” he said. The biggest issue when it comes to burnout, as he sees it, is not COVID; it’s when hospitalists are performing tasks that make them feel they aren’t adding value. “I think that’s a huge topic in hospital medicine right now.”
Dr. Vora believes there should be more discussion and awareness of the potential pitfalls. “Hospitalists should get involved in co-management where they are adding value and certainly not take up the attending responsibility where they’re not adding value and it’s out of the scope of their training and expertise,” he said. “Preventing scope creep and burnout from co-management are some of the key issues I’m really passionate about.”
Dr. Vora said it is important to set realistic goals and remember that it takes time to make change when it comes to advocacy. “You still have to operate within whatever environment is given to you and then you can make change from within,” he said.
His enthusiasm for co-management awareness has led to creating a co-management forum through SHM in his local Hampton Roads chapter. He was also a panelist for an SHM webinar in February 2021 in which the panelists debated co-management.
“I think we really need to look at this as a specialty. Are we going in the right direction?” Dr. Vora asked. “We need to come together as a specialty and make a decision, which is going to be hard because there are competing financial interests and various practice models.”
Improving patient care
Working as a hospitalist at University Medical Center, a safety net hospital in New Orleans, Celeste Newby, MD, PhD, sees plenty of patients who are underinsured or not insured at all. “A lot of my interest in health policy stems from that,” she said.
During her residency, which she finished in 2015, Louisiana became a Medicaid expansion state. This impressed upon Dr. Newby how much Medicaid improved the lives of patients who had previously been uninsured. “We saw procedures getting done that had been put on hold because of financial concerns or medicines that were now affordable that weren’t before,” she said. “It really did make a difference.”
When repeated attempts to repeal the Affordable Care Act began, “it was a call to do health policy work for me personally that just hadn’t come up in the past,” said Dr. Newby, who is also assistant professor of medicine at Tulane University in New Orleans. “I personally found that the best way to do (advocacy work) was to go through medical societies because there is a much stronger voice when you have more people saying the same thing,” she said.
Dr. Newby sits on the Council of Legislation for the Louisiana State Medical Society and participates in the Leadership and Health Policy (LEAHP) Program through the Society of General Internal Medicine.
The LEAHP Program has been instrumental in expanding Dr. Newby’s knowledge of how health policy is made and the mechanisms behind it. It has also taught her “how we can either advise, guide, leverage, or advocate for things that we think would be important for change and moving the country in the right direction in terms of health care.”
Another reason involvement in medical societies is helpful is because, as a busy clinician, it is impossible to keep up with everything. “Working with medical societies, you have people who are more directly involved in the legislature and can give you quicker notice about things that are coming up that are going to be important to you or your co-workers or your patients,” Dr. Newby said.
Dr. Newby feels her advocacy work is an outlet for stress and “a way to work at more of a macro level on problems that I see with my individual patients. It’s a nice compliment.” At the hospital, she can only help one person at a time, but with her advocacy efforts, there’s potential to make changes for many.
“Advocacy now is such a large umbrella that encompasses so many different projects at all kinds of levels,” Dr. Newby said. She suggests looking around your community to see where the needs lie. If you’re passionate about a certain topic or population, see what you can do to help advocate for change there.
Hospitalists engaging in advocacy efforts
Hospitalists engaging in advocacy efforts
Hospitalists around the country are devoting large portions of their spare time to a wide range of advocacy efforts. From health policy to caring for the unhoused population to diversity and equity to advocating for fellow hospitalists, these physicians are passionate about their causes and determined to make a difference.
Championing the unhoused
Sarah Stella, MD, FHM, a hospitalist at Denver Health, was initially drawn there because of the population the hospital serves, which includes a high concentration of people experiencing homelessness. As she cared for her patients, Dr. Stella, who is also associate professor of hospital medicine at the University of Colorado, increasingly felt the desire to help prevent the negative downstream outcomes the hospital sees.
To understand the experiences of the unhoused outside the hospital, Dr. Stella started talking to her patients and people in community-based organizations that serve this population. “I learned a ton,” she said. “Homelessness feels like such an intractable, hopeless thing, but the more I talked to people, the more opportunities I saw to work toward something better.”
This led to a pilot grant to work with the Colorado Coalition for the Homeless to set up a community advisory panel. “My goal was to better understand their experiences and to develop a shared vision for how we collectively can do better,” said Dr. Stella. Eventually, she also received a grant from the University of Colorado, and multiple opportunities have sprung up ever since.
For the past several years, Dr. Stella has worked with Denver Health leadership to improve care for the homeless. “Right now, I’m working with a community team on developing an idea to provide peer support from people with a shared lived experience for people who are experiencing homelessness when they’re hospitalized. That’s really where my passion has been in working on the partnership,” she said.
Her advocacy role has been beneficial in her work as a hospitalist, particularly when COVID began. Dr. Stella again partnered with the Colorado Coalition for the Homeless to start a joint task force. “Everyone on our task force is motivated by this powerful desire to improve the health and lives of this community and that’s one of the silver linings in this pandemic for me,” said Dr. Stella.
Advocacy work has also increased Dr. Stella’s knowledge of what community support options are available for the unhoused. This allows her to educate her patients about their options and how to access them.
While she has colleagues who are able to compartmentalize their work, “I absolutely could not be a hospitalist without being an advocate,” Dr. Stella said. “For me, it has been a protective strategy in terms of burnout because I have to feel like I’m working to advocate for better policies and more appropriate resources to address the gaps that I’m seeing.”
Dr. Stella believes that physicians have a special credibility to advocate, tell stories, and use data to back their stories up. “We have to realize that we have this power, and we have it so we can empower others,” she said. “The people I’ve seen in my community who are working so hard to help people who are experiencing homelessness are the heroes. Understanding that and giving power to those people through our voice and our well-respected place in society drives me.”
Strengthening diversity, equity, and inclusion
In September 2020, Michael Bryant, MD, became the inaugural vice chair of Diversity, Equity, and Inclusion for the department of pediatrics at Children’s Hospital Los Angeles, where he is also the division head of pediatric hospital medicine. “I was motivated to apply for this position because I wanted to be an agent for change to eliminate the institutional racism, social injustice, and marginalization that continues to threaten the lives and well-beings of so many Americans,” Dr. Bryant said.
Between the pandemic, the economic decline it has created, and the divisive political landscape, people of color have been especially affected. “These are poignant examples of the ever-widening divide and disenfranchisement many Americans feel,” said Dr. Bryant. “Gandhi said, ‘Be the change that you want to see,’ and that is what I want to model.”
At work, advocacy for diversity, equality, and inclusion is an innate part of everything he does. From the new physicians he recruits to the candidates he considers for leadership positions, Dr. Bryant strives “to have a workforce that mirrors the diversity of the patients we humbly care for and serve.”
Advocacy is intrinsic to Dr. Bryant’s worldview, in his quest to understand and accept each individual’s uniqueness, his desire “to embrace cultural humility,” his recognition that “our differences enhance us instead of diminishing us,” and his willingness to engage in difficult conversations.
“Advocacy means that I acknowledge that intent does not equal impact and that I must accept that what I do and what I say may have unintended consequences,” he said. “When that happens, I must resist becoming defensive and instead be willing to listen and learn.”
Dr. Bryant is proud of his accomplishments and enjoys his advocacy work. In his workplace, there are few African Americans in leadership roles. This means that he is in high demand when it comes to making sure there’s representation during various processes such as hiring and vetting, a disparity known as the “minority tax.”
“I am thankful for the opportunities, but it does take a toll at times,” Dr. Bryant said, which is yet another reason why he is a proponent of increasing diversity and inclusion. “This allows us to build the resource pool as these needs arise and minimizes the toll of the ‘minority tax’ on any single person or small group of individuals.”
This summer, physicians from Dr. Bryant’s hospital participated in the national “White Coats for Black Lives” effort. He found it to be “an incredibly moving event” that hundreds of his colleagues participated in.
Dr. Bryant’s advice for hospitalists who want to get involved in advocacy efforts is to check out the movie “John Lewis: Good Trouble.” “He was a champion of human rights and fought for these rights until his death,” Dr. Bryant said. “He is a true American hero and a wonderful example.”
Bolstering health care change
Since his residency, Joshua Lenchus, DO, FACP, SFHM, has developed an ever-increasing interest in legislative advocacy, particularly health policy. Getting involved in this arena requires an understanding of civics and government that goes beyond just the basics. “My desire to affect change in my own profession really served as the catalyst to get involved,” said Dr. Lenchus, the regional chief medical officer at Broward Health Medical Center in Fort Lauderdale, Fla. “What better way to do that than by combining what we do on a daily basis in the practice of medicine with this new understanding of how laws are passed and promulgated?”
Dr. Lenchus has been involved with both state and national medical organizations and has served on public policy committees as a member and as a chair. “The charge of these committees is to monitor and navigate position statements and policies that will drive the entire organization,” he said. This means becoming knowledgeable enough about a topic to be able to talk about it eloquently and adding supporting personal or professional illustrations that reinforce the position to lawmakers.
He finds his advocacy efforts “incredibly rewarding” because they contribute to his endeavors “to help my colleagues practice medicine in a safe, efficient, and productive manner.” For instance, some of the organizations Dr. Lenchus was involved with helped make changes to the Affordable Care Act that ended up in its final version, as well as changes after it passed. “There are tangible things that advocacy enables us to do in our daily practice,” he said.
When something his organizations have advocated for does not pass, they know they need to try a different outlet. “You can’t win every fight,” he said. “Every time you go and comment on an issue, you have to understand that you’re there to do your best, and to the extent that the people you’re talking to are willing to listen to what you have to say, that’s where I think you can make the most impact.” When changes he has helped fight for do pass, “it really is amazing that you can tell your colleagues about your role in achieving meaningful change in the profession.”
Dr. Lenchus acknowledges that advocacy “can be all-consuming at times. We have to understand our limits.” That said, he thinks not engaging in advocacy could increase stress and potential burnout. “I think being involved in advocacy efforts really helps people conduct meaningful work and educates them about what it means not just to them, but to the rest of the medical profession and the patients that we serve,” he said.
For hospitalists who are interested in health policy advocacy, there are many ways to get involved, Dr. Lenchus said. You could join an organization (many organized medical societies have public policy committees), participate in advocacy activities, work on a political campaign, or even run for office yourself. “Ultimately, education and some level of involvement really will make the difference in who navigates our future as hospitalists,” he said.
Questioning co-management practices
Though he says he’s in the minority, Hardik Vora, MD, SFHM, medical director for hospital medicine at Riverside Regional Medical Center in Newport News, Va., believes that co-management is going to “make or break hospital medicine. It’s going to have a huge impact on our specialty.”
In the roughly 25-year history of hospital medicine, it has evolved from admitting and caring for patients of primary care physicians to patients of specialists and, more recently, surgical patients. “Now there are (hospital medicine) programs across the country that are pretty much admitting everything,” said Dr. Vora.
As a recruiter for the Riverside Health System for the past eight years, “I have not met a single resident who is trained to do what we’re doing in hospital medicine, because you’re admitting surgical patients all the time and you have primary attending responsibility,” Dr. Vora said. “I see that as a cause of a significant amount of stress because now you’re responsible for something that you don’t have adequate training for.”
In the co-management discussion, Dr. Vora notes that people often bring up the research that shows that the practice has improved surgeon satisfaction. “What bothers me is that…you need to add one more question – how does it affect your hospitalists? And I bet the answer to that question is ‘it has a terrible effect.’”
The expectations surrounding hospitalists these days is a big concern in terms of burnout, Dr. Vora said. “We talk a lot about the drivers of burnout, whether it’s schedule or COVID,” he said. The biggest issue when it comes to burnout, as he sees it, is not COVID; it’s when hospitalists are performing tasks that make them feel they aren’t adding value. “I think that’s a huge topic in hospital medicine right now.”
Dr. Vora believes there should be more discussion and awareness of the potential pitfalls. “Hospitalists should get involved in co-management where they are adding value and certainly not take up the attending responsibility where they’re not adding value and it’s out of the scope of their training and expertise,” he said. “Preventing scope creep and burnout from co-management are some of the key issues I’m really passionate about.”
Dr. Vora said it is important to set realistic goals and remember that it takes time to make change when it comes to advocacy. “You still have to operate within whatever environment is given to you and then you can make change from within,” he said.
His enthusiasm for co-management awareness has led to creating a co-management forum through SHM in his local Hampton Roads chapter. He was also a panelist for an SHM webinar in February 2021 in which the panelists debated co-management.
“I think we really need to look at this as a specialty. Are we going in the right direction?” Dr. Vora asked. “We need to come together as a specialty and make a decision, which is going to be hard because there are competing financial interests and various practice models.”
Improving patient care
Working as a hospitalist at University Medical Center, a safety net hospital in New Orleans, Celeste Newby, MD, PhD, sees plenty of patients who are underinsured or not insured at all. “A lot of my interest in health policy stems from that,” she said.
During her residency, which she finished in 2015, Louisiana became a Medicaid expansion state. This impressed upon Dr. Newby how much Medicaid improved the lives of patients who had previously been uninsured. “We saw procedures getting done that had been put on hold because of financial concerns or medicines that were now affordable that weren’t before,” she said. “It really did make a difference.”
When repeated attempts to repeal the Affordable Care Act began, “it was a call to do health policy work for me personally that just hadn’t come up in the past,” said Dr. Newby, who is also assistant professor of medicine at Tulane University in New Orleans. “I personally found that the best way to do (advocacy work) was to go through medical societies because there is a much stronger voice when you have more people saying the same thing,” she said.
Dr. Newby sits on the Council of Legislation for the Louisiana State Medical Society and participates in the Leadership and Health Policy (LEAHP) Program through the Society of General Internal Medicine.
The LEAHP Program has been instrumental in expanding Dr. Newby’s knowledge of how health policy is made and the mechanisms behind it. It has also taught her “how we can either advise, guide, leverage, or advocate for things that we think would be important for change and moving the country in the right direction in terms of health care.”
Another reason involvement in medical societies is helpful is because, as a busy clinician, it is impossible to keep up with everything. “Working with medical societies, you have people who are more directly involved in the legislature and can give you quicker notice about things that are coming up that are going to be important to you or your co-workers or your patients,” Dr. Newby said.
Dr. Newby feels her advocacy work is an outlet for stress and “a way to work at more of a macro level on problems that I see with my individual patients. It’s a nice compliment.” At the hospital, she can only help one person at a time, but with her advocacy efforts, there’s potential to make changes for many.
“Advocacy now is such a large umbrella that encompasses so many different projects at all kinds of levels,” Dr. Newby said. She suggests looking around your community to see where the needs lie. If you’re passionate about a certain topic or population, see what you can do to help advocate for change there.
Hospitalists around the country are devoting large portions of their spare time to a wide range of advocacy efforts. From health policy to caring for the unhoused population to diversity and equity to advocating for fellow hospitalists, these physicians are passionate about their causes and determined to make a difference.
Championing the unhoused
Sarah Stella, MD, FHM, a hospitalist at Denver Health, was initially drawn there because of the population the hospital serves, which includes a high concentration of people experiencing homelessness. As she cared for her patients, Dr. Stella, who is also associate professor of hospital medicine at the University of Colorado, increasingly felt the desire to help prevent the negative downstream outcomes the hospital sees.
To understand the experiences of the unhoused outside the hospital, Dr. Stella started talking to her patients and people in community-based organizations that serve this population. “I learned a ton,” she said. “Homelessness feels like such an intractable, hopeless thing, but the more I talked to people, the more opportunities I saw to work toward something better.”
This led to a pilot grant to work with the Colorado Coalition for the Homeless to set up a community advisory panel. “My goal was to better understand their experiences and to develop a shared vision for how we collectively can do better,” said Dr. Stella. Eventually, she also received a grant from the University of Colorado, and multiple opportunities have sprung up ever since.
For the past several years, Dr. Stella has worked with Denver Health leadership to improve care for the homeless. “Right now, I’m working with a community team on developing an idea to provide peer support from people with a shared lived experience for people who are experiencing homelessness when they’re hospitalized. That’s really where my passion has been in working on the partnership,” she said.
Her advocacy role has been beneficial in her work as a hospitalist, particularly when COVID began. Dr. Stella again partnered with the Colorado Coalition for the Homeless to start a joint task force. “Everyone on our task force is motivated by this powerful desire to improve the health and lives of this community and that’s one of the silver linings in this pandemic for me,” said Dr. Stella.
Advocacy work has also increased Dr. Stella’s knowledge of what community support options are available for the unhoused. This allows her to educate her patients about their options and how to access them.
While she has colleagues who are able to compartmentalize their work, “I absolutely could not be a hospitalist without being an advocate,” Dr. Stella said. “For me, it has been a protective strategy in terms of burnout because I have to feel like I’m working to advocate for better policies and more appropriate resources to address the gaps that I’m seeing.”
Dr. Stella believes that physicians have a special credibility to advocate, tell stories, and use data to back their stories up. “We have to realize that we have this power, and we have it so we can empower others,” she said. “The people I’ve seen in my community who are working so hard to help people who are experiencing homelessness are the heroes. Understanding that and giving power to those people through our voice and our well-respected place in society drives me.”
Strengthening diversity, equity, and inclusion
In September 2020, Michael Bryant, MD, became the inaugural vice chair of Diversity, Equity, and Inclusion for the department of pediatrics at Children’s Hospital Los Angeles, where he is also the division head of pediatric hospital medicine. “I was motivated to apply for this position because I wanted to be an agent for change to eliminate the institutional racism, social injustice, and marginalization that continues to threaten the lives and well-beings of so many Americans,” Dr. Bryant said.
Between the pandemic, the economic decline it has created, and the divisive political landscape, people of color have been especially affected. “These are poignant examples of the ever-widening divide and disenfranchisement many Americans feel,” said Dr. Bryant. “Gandhi said, ‘Be the change that you want to see,’ and that is what I want to model.”
At work, advocacy for diversity, equality, and inclusion is an innate part of everything he does. From the new physicians he recruits to the candidates he considers for leadership positions, Dr. Bryant strives “to have a workforce that mirrors the diversity of the patients we humbly care for and serve.”
Advocacy is intrinsic to Dr. Bryant’s worldview, in his quest to understand and accept each individual’s uniqueness, his desire “to embrace cultural humility,” his recognition that “our differences enhance us instead of diminishing us,” and his willingness to engage in difficult conversations.
“Advocacy means that I acknowledge that intent does not equal impact and that I must accept that what I do and what I say may have unintended consequences,” he said. “When that happens, I must resist becoming defensive and instead be willing to listen and learn.”
Dr. Bryant is proud of his accomplishments and enjoys his advocacy work. In his workplace, there are few African Americans in leadership roles. This means that he is in high demand when it comes to making sure there’s representation during various processes such as hiring and vetting, a disparity known as the “minority tax.”
“I am thankful for the opportunities, but it does take a toll at times,” Dr. Bryant said, which is yet another reason why he is a proponent of increasing diversity and inclusion. “This allows us to build the resource pool as these needs arise and minimizes the toll of the ‘minority tax’ on any single person or small group of individuals.”
This summer, physicians from Dr. Bryant’s hospital participated in the national “White Coats for Black Lives” effort. He found it to be “an incredibly moving event” that hundreds of his colleagues participated in.
Dr. Bryant’s advice for hospitalists who want to get involved in advocacy efforts is to check out the movie “John Lewis: Good Trouble.” “He was a champion of human rights and fought for these rights until his death,” Dr. Bryant said. “He is a true American hero and a wonderful example.”
Bolstering health care change
Since his residency, Joshua Lenchus, DO, FACP, SFHM, has developed an ever-increasing interest in legislative advocacy, particularly health policy. Getting involved in this arena requires an understanding of civics and government that goes beyond just the basics. “My desire to affect change in my own profession really served as the catalyst to get involved,” said Dr. Lenchus, the regional chief medical officer at Broward Health Medical Center in Fort Lauderdale, Fla. “What better way to do that than by combining what we do on a daily basis in the practice of medicine with this new understanding of how laws are passed and promulgated?”
Dr. Lenchus has been involved with both state and national medical organizations and has served on public policy committees as a member and as a chair. “The charge of these committees is to monitor and navigate position statements and policies that will drive the entire organization,” he said. This means becoming knowledgeable enough about a topic to be able to talk about it eloquently and adding supporting personal or professional illustrations that reinforce the position to lawmakers.
He finds his advocacy efforts “incredibly rewarding” because they contribute to his endeavors “to help my colleagues practice medicine in a safe, efficient, and productive manner.” For instance, some of the organizations Dr. Lenchus was involved with helped make changes to the Affordable Care Act that ended up in its final version, as well as changes after it passed. “There are tangible things that advocacy enables us to do in our daily practice,” he said.
When something his organizations have advocated for does not pass, they know they need to try a different outlet. “You can’t win every fight,” he said. “Every time you go and comment on an issue, you have to understand that you’re there to do your best, and to the extent that the people you’re talking to are willing to listen to what you have to say, that’s where I think you can make the most impact.” When changes he has helped fight for do pass, “it really is amazing that you can tell your colleagues about your role in achieving meaningful change in the profession.”
Dr. Lenchus acknowledges that advocacy “can be all-consuming at times. We have to understand our limits.” That said, he thinks not engaging in advocacy could increase stress and potential burnout. “I think being involved in advocacy efforts really helps people conduct meaningful work and educates them about what it means not just to them, but to the rest of the medical profession and the patients that we serve,” he said.
For hospitalists who are interested in health policy advocacy, there are many ways to get involved, Dr. Lenchus said. You could join an organization (many organized medical societies have public policy committees), participate in advocacy activities, work on a political campaign, or even run for office yourself. “Ultimately, education and some level of involvement really will make the difference in who navigates our future as hospitalists,” he said.
Questioning co-management practices
Though he says he’s in the minority, Hardik Vora, MD, SFHM, medical director for hospital medicine at Riverside Regional Medical Center in Newport News, Va., believes that co-management is going to “make or break hospital medicine. It’s going to have a huge impact on our specialty.”
In the roughly 25-year history of hospital medicine, it has evolved from admitting and caring for patients of primary care physicians to patients of specialists and, more recently, surgical patients. “Now there are (hospital medicine) programs across the country that are pretty much admitting everything,” said Dr. Vora.
As a recruiter for the Riverside Health System for the past eight years, “I have not met a single resident who is trained to do what we’re doing in hospital medicine, because you’re admitting surgical patients all the time and you have primary attending responsibility,” Dr. Vora said. “I see that as a cause of a significant amount of stress because now you’re responsible for something that you don’t have adequate training for.”
In the co-management discussion, Dr. Vora notes that people often bring up the research that shows that the practice has improved surgeon satisfaction. “What bothers me is that…you need to add one more question – how does it affect your hospitalists? And I bet the answer to that question is ‘it has a terrible effect.’”
The expectations surrounding hospitalists these days is a big concern in terms of burnout, Dr. Vora said. “We talk a lot about the drivers of burnout, whether it’s schedule or COVID,” he said. The biggest issue when it comes to burnout, as he sees it, is not COVID; it’s when hospitalists are performing tasks that make them feel they aren’t adding value. “I think that’s a huge topic in hospital medicine right now.”
Dr. Vora believes there should be more discussion and awareness of the potential pitfalls. “Hospitalists should get involved in co-management where they are adding value and certainly not take up the attending responsibility where they’re not adding value and it’s out of the scope of their training and expertise,” he said. “Preventing scope creep and burnout from co-management are some of the key issues I’m really passionate about.”
Dr. Vora said it is important to set realistic goals and remember that it takes time to make change when it comes to advocacy. “You still have to operate within whatever environment is given to you and then you can make change from within,” he said.
His enthusiasm for co-management awareness has led to creating a co-management forum through SHM in his local Hampton Roads chapter. He was also a panelist for an SHM webinar in February 2021 in which the panelists debated co-management.
“I think we really need to look at this as a specialty. Are we going in the right direction?” Dr. Vora asked. “We need to come together as a specialty and make a decision, which is going to be hard because there are competing financial interests and various practice models.”
Improving patient care
Working as a hospitalist at University Medical Center, a safety net hospital in New Orleans, Celeste Newby, MD, PhD, sees plenty of patients who are underinsured or not insured at all. “A lot of my interest in health policy stems from that,” she said.
During her residency, which she finished in 2015, Louisiana became a Medicaid expansion state. This impressed upon Dr. Newby how much Medicaid improved the lives of patients who had previously been uninsured. “We saw procedures getting done that had been put on hold because of financial concerns or medicines that were now affordable that weren’t before,” she said. “It really did make a difference.”
When repeated attempts to repeal the Affordable Care Act began, “it was a call to do health policy work for me personally that just hadn’t come up in the past,” said Dr. Newby, who is also assistant professor of medicine at Tulane University in New Orleans. “I personally found that the best way to do (advocacy work) was to go through medical societies because there is a much stronger voice when you have more people saying the same thing,” she said.
Dr. Newby sits on the Council of Legislation for the Louisiana State Medical Society and participates in the Leadership and Health Policy (LEAHP) Program through the Society of General Internal Medicine.
The LEAHP Program has been instrumental in expanding Dr. Newby’s knowledge of how health policy is made and the mechanisms behind it. It has also taught her “how we can either advise, guide, leverage, or advocate for things that we think would be important for change and moving the country in the right direction in terms of health care.”
Another reason involvement in medical societies is helpful is because, as a busy clinician, it is impossible to keep up with everything. “Working with medical societies, you have people who are more directly involved in the legislature and can give you quicker notice about things that are coming up that are going to be important to you or your co-workers or your patients,” Dr. Newby said.
Dr. Newby feels her advocacy work is an outlet for stress and “a way to work at more of a macro level on problems that I see with my individual patients. It’s a nice compliment.” At the hospital, she can only help one person at a time, but with her advocacy efforts, there’s potential to make changes for many.
“Advocacy now is such a large umbrella that encompasses so many different projects at all kinds of levels,” Dr. Newby said. She suggests looking around your community to see where the needs lie. If you’re passionate about a certain topic or population, see what you can do to help advocate for change there.
COVID-19 in children: New cases on the rise again
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
The number of new COVID-19 cases in children rose for the third time in the last 4 weeks, reaching the highest point since mid-February, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Just over 73,000 cases were reported during the week of April 2-8, up by 14.6% over the previous week. For the latest week, children represented 18.8% of all COVID-19 cases in the United States – also up from the week before and the second-highest proportion seen during the entire pandemic, based on data in the weekly AAP/CHA report.
The 3.54 million children who have been infected with SARS-CoV-2 make up 13.5% of all cases reported in the United States during the pandemic, a figure that climbed again after 2 weeks at 13.4%. The overall rate of infection was just over 4,700 cases per 100,000 children as of April 8, the AAP and CHA said.
State-level data show that Vermont, Michigan, and Maine have been the COVID-19 hotspots over the past 2 weeks. The total number of cases has jumped by almost 19% in Vermont since the week of March 19-25, by 18% in Michigan, and by 12% in Maine, according to the report.
Cumulative data also indicate that the children of Vermont are bearing a greater share of the COVID-19 burden – 21.5% of all cases – than in any other state. North Dakota, meanwhile, has the highest cumulative rate of infection at 9,057 cases per 100,000 children, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The number of COVID-19–related deaths in children increased by 8 during the week of April 2-8 and now stands at 292, just 0.06% of all deaths reported in the 43 states (along with New York City, Puerto Rico, and Guam) that provide age distributions for mortality data, the AAP and CHA said.
Arthritis drug may curb myocardial damage in acute STEMI
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.
Early use of tocilizumab (Actemra) does not reduce myocardial infarct size but modestly increases myocardial salvage in patients with acute ST-segment elevation MI (STEMI), results of the ASSAIL-MI trial suggest.
“We’re among the first to show that you can actually affect the reperfusion injury through anti-inflammatory treatment – it’s sort of a new attack point for treatments in STEMI,” lead author Kaspar Broch, MD, PhD, Oslo University Hospital Rikshospitalet, said in an interview. “What we do now is reperfuse as soon as we can and then add drugs in order to prevent new events, but we don’t really attack the reperfusion injury that occurs when you perform PCI [percutaneous coronary intervention], which has been shown to actually account for some 50% of the final injury.”
The phase 2, proof-of-concept study was prompted by the team’s earlier work in non-STEMI patients, in which a single dose of the interleukin-6 receptor antagonist cut C-reactive protein (CRP) levels by more than 50% during hospitalization and reduced troponin T release after PCI.
For ASSAIL-MI, Dr. Broch and colleagues randomly assigned 199 patients presenting with acute STEMI within 6 hours of symptom onset to a single intravenous injection of 280 mg tocilizumab or placebo during PCI. Patients, study personnel, and caretakers were blinded to treatment. Data were available for 195 patients for the primary endpoint of myocardial salvage index.
As reported in the Journal of the American College of Cardiology, tocilizumab was associated with a higher adjusted myocardial salvage index on cardiac MRI 3-7 days after PCI than placebo (69.3% vs. 63.6%; P = .04).
The extent of microvascular obstruction was less with tocilizumab (0% vs. 4%; P = .03), as was the area under the curve of CRP during hospitalization (1.9 vs. 8.6 mg/L per hour; P < .001).
The final infarct size at 6 months was 21% lower in the tocilizumab group but the difference did not reach statistical significance (7.2% vs. 9.1% of left ventricular mass; P = .08).
There were no between-group differences in troponin T area under the curve during hospitalization (1,614 vs. 2,357 ng/L per hour; P = .13), N-terminal of the prohormone brain natriuretic peptide concentrations at 6 months (79 vs. 63 ng/L; P = .25), or baseline-adjusted left ventricular end-diastolic volume at 6 months (157 vs. 160 mL; P = .54).
Subgroup analyses suggested the positive effect of tocilizumab on myocardial salvage index is limited to patients presenting at least 3 hours after symptom onset versus 3 hours or less (P = .034), with a trend for greater benefit among men versus women (P = .053).
Dr. Broch noted that the absolute effect of tocilizumab on myocardial necrosis was smaller than anticipated when the trial was designed, which may explain the lack of significant reduction in infarct size.
“We were aiming for patients with larger infarctions than we actually ended up with, which is partly due to the strict inclusion criteria and the fact that, with modern treatments, patients don’t end up with large myocardial infarctions,” he said. “But if they had been larger, I think that 20% absolute reduction would have meant a lot in terms of clinical events.”
The study also used a very modest dose of tocilizumab, compared with that used for inflammatory diseases, to minimize a potential negative effect on myocardial healing, for instance, myocardial ruptures, Dr. Broch said. “I’m not sure whether you gain anything by giving a larger dose.”
Serious adverse events were similar in the tocilizumab and placebo groups (19 vs. 15; P = .57). There were no myocardial ruptures, and no patient died or developed heart failure. LDL cholesterol, triglycerides, and liver enzymes increased in the tocilizumab group but were similar at 3 and 6 months.
“IL-6 is a central cytokine involved in all stages of plaque growth, progression, and rupture,” Paul Ridker, MD, MPH, of the Brigham and Women’s Hospital in Boston, and a long-standing investigator in inflammation and atherothrombosis, said in an interview. “These preliminary data in STEMI, like the authors’ prior data in non-STEMI, are consistent with the idea that inhibiting IL-6 could have clinical benefit, a concept that will be taken into a major cardiovascular outcomes trial later this year.”
The cardiovascular outcomes trial, known as ZEUS, will test the novel IL-6 inhibitor ziltivekimab among more than 6,000 very-high-risk atherosclerosis patients who have moderate to severe chronic kidney disease and high sensitivity CRP greater than 2 mg/L, he noted.
Moving beyond IL-1b blockade as done in CANTOS to direct downstream inhibition of IL-6 represents a “logical next scientific step” in the development of anti-inflammatory therapies for acute ischemia and chronic atherosclerosis, Dr. Ridker, who led the CANTOS trial, noted in an accompanying editorial.
“Preventive cardiologists, however, need not wait until outcome trials are complete to use this evolving biological knowledge to their patient’s advantage,” he wrote. “As recently confirmed in the pages of the Journal, exercise, smoking cessation, and a healthy diet reduce both C-reactive protein and IL-6, and clearly have lifelong benefits. Our immediate task is thus to incorporate inflammation inhibition through lifestyle management into our daily practice.”
The study was supported by the South-Eastern Norway Regional Health Authority, Central Norway Regional Health Authority, and Roche, which provided the medicinal products and an unrestricted grant. Dr. Broch has disclosed no relevant financial relationships. Dr. Ridker has received investigator-initiated research grant support from Kowa, Novartis, Amarin, Pfizer, and the National Heart, Lung, and Blood Institute; and has served as a consultant to Novartis, Janssen, Agepha, Flame, Civi Biopharma, Inflazome, Corvidia, Novo Nordisk, SOCAR, IQVIA, and AstraZeneca.
A version of this article first appeared on Medscape.com.















