User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


U.S. Surgeon General Vivek Murthy to Speak at SHM Converge
U.S. Surgeon General, Vice Admiral Vivek H. Murthy, MD, MBA, will be a keynote speaker at SHM Converge, the Society of Hospital Meidicine’s annual conference.
Dr. Murthy will discuss burnout and well-being in health care, key themes of the conference, during a fireside chat with Danielle Scheurer, MD, MSCR, SFHM, president of SHM’s board of directors. The conversation will be held on Thursday, May 6, 2021 from 1:30 to 2:00 p.m. ET.
“It is an honor to welcome Dr. Murthy back to SHM’s annual conference during a time when his leadership is more important than ever,” said Eric E. Howell, MD, MHM, chief executive officer of SHM. “Dr. Murthy is dedicated to the health of the American people and to the well-being of those who care for them. I know his message will resonate with hospitalists who have been on the front lines of the pandemic since day one. They are key to navigating the future of our nation’s health care system.”
Dr. Murthy has devoted himself to improving public health through service, clinical care, research, education, and entrepreneurship. In addition to clinical practice, Dr. Murthy has two decades of experience in improving health in communities around the world and served as the 19th U.S. Surgeon General from 2014 to 2017, prior to being reappointed with an expanded role earlier in 2021. As “America’s Doctor,” the Surgeon General’s role is to provide clear, consistent guidance and healthcare resources for the public, ensuring to reach the nation’s most vulnerable communities.
“As we guide our nation out of the pandemic, hospitalists can look to Dr. Murthy as a leader and innovator who will do what is best for our hospitalist community and the patients they serve,” Dr. Scheurer said. “Dr. Murthy will provide a unique perspective to help hospitalists care for themselves during a time when they have been hyper-focused on caring for others.”
In addition to Dr. Murthy, SHM Converge will feature three keynote speakers who will address pressing topics in hospital medicine:
- Mark Hertling, DBA: Coming Out of Combat: Post-Pandemic Recovery
- Vineet Arora, MD, MAPP, MHM: Hospitalists Healing: Surviving, Salvaging, Sustaining, and Succeeding for a Pandemic World
- Larry Wellikson, MD, MHM: Out of COVID and Into the Light: Hospitalists More Essential Than Ever
SHM Converge is the premier educational experience for hospital medicine professionals. This year’s virtual conference will be held from May 3-7, 2021 and offers 21 educational tracks, more than 250 speakers, advanced learning courses, networking opportunities, a scientific abstract competition, and more. SHM Converge On Demand is also available until 2024, including the opportunity to earn additional continuing medical education credit.
To register for SHM Converge, visit shmconverge.org. If you are a member of the media who would like to obtain a press pass, email [email protected].
U.S. Surgeon General, Vice Admiral Vivek H. Murthy, MD, MBA, will be a keynote speaker at SHM Converge, the Society of Hospital Meidicine’s annual conference.
Dr. Murthy will discuss burnout and well-being in health care, key themes of the conference, during a fireside chat with Danielle Scheurer, MD, MSCR, SFHM, president of SHM’s board of directors. The conversation will be held on Thursday, May 6, 2021 from 1:30 to 2:00 p.m. ET.
“It is an honor to welcome Dr. Murthy back to SHM’s annual conference during a time when his leadership is more important than ever,” said Eric E. Howell, MD, MHM, chief executive officer of SHM. “Dr. Murthy is dedicated to the health of the American people and to the well-being of those who care for them. I know his message will resonate with hospitalists who have been on the front lines of the pandemic since day one. They are key to navigating the future of our nation’s health care system.”
Dr. Murthy has devoted himself to improving public health through service, clinical care, research, education, and entrepreneurship. In addition to clinical practice, Dr. Murthy has two decades of experience in improving health in communities around the world and served as the 19th U.S. Surgeon General from 2014 to 2017, prior to being reappointed with an expanded role earlier in 2021. As “America’s Doctor,” the Surgeon General’s role is to provide clear, consistent guidance and healthcare resources for the public, ensuring to reach the nation’s most vulnerable communities.
“As we guide our nation out of the pandemic, hospitalists can look to Dr. Murthy as a leader and innovator who will do what is best for our hospitalist community and the patients they serve,” Dr. Scheurer said. “Dr. Murthy will provide a unique perspective to help hospitalists care for themselves during a time when they have been hyper-focused on caring for others.”
In addition to Dr. Murthy, SHM Converge will feature three keynote speakers who will address pressing topics in hospital medicine:
- Mark Hertling, DBA: Coming Out of Combat: Post-Pandemic Recovery
- Vineet Arora, MD, MAPP, MHM: Hospitalists Healing: Surviving, Salvaging, Sustaining, and Succeeding for a Pandemic World
- Larry Wellikson, MD, MHM: Out of COVID and Into the Light: Hospitalists More Essential Than Ever
SHM Converge is the premier educational experience for hospital medicine professionals. This year’s virtual conference will be held from May 3-7, 2021 and offers 21 educational tracks, more than 250 speakers, advanced learning courses, networking opportunities, a scientific abstract competition, and more. SHM Converge On Demand is also available until 2024, including the opportunity to earn additional continuing medical education credit.
To register for SHM Converge, visit shmconverge.org. If you are a member of the media who would like to obtain a press pass, email [email protected].
U.S. Surgeon General, Vice Admiral Vivek H. Murthy, MD, MBA, will be a keynote speaker at SHM Converge, the Society of Hospital Meidicine’s annual conference.
Dr. Murthy will discuss burnout and well-being in health care, key themes of the conference, during a fireside chat with Danielle Scheurer, MD, MSCR, SFHM, president of SHM’s board of directors. The conversation will be held on Thursday, May 6, 2021 from 1:30 to 2:00 p.m. ET.
“It is an honor to welcome Dr. Murthy back to SHM’s annual conference during a time when his leadership is more important than ever,” said Eric E. Howell, MD, MHM, chief executive officer of SHM. “Dr. Murthy is dedicated to the health of the American people and to the well-being of those who care for them. I know his message will resonate with hospitalists who have been on the front lines of the pandemic since day one. They are key to navigating the future of our nation’s health care system.”
Dr. Murthy has devoted himself to improving public health through service, clinical care, research, education, and entrepreneurship. In addition to clinical practice, Dr. Murthy has two decades of experience in improving health in communities around the world and served as the 19th U.S. Surgeon General from 2014 to 2017, prior to being reappointed with an expanded role earlier in 2021. As “America’s Doctor,” the Surgeon General’s role is to provide clear, consistent guidance and healthcare resources for the public, ensuring to reach the nation’s most vulnerable communities.
“As we guide our nation out of the pandemic, hospitalists can look to Dr. Murthy as a leader and innovator who will do what is best for our hospitalist community and the patients they serve,” Dr. Scheurer said. “Dr. Murthy will provide a unique perspective to help hospitalists care for themselves during a time when they have been hyper-focused on caring for others.”
In addition to Dr. Murthy, SHM Converge will feature three keynote speakers who will address pressing topics in hospital medicine:
- Mark Hertling, DBA: Coming Out of Combat: Post-Pandemic Recovery
- Vineet Arora, MD, MAPP, MHM: Hospitalists Healing: Surviving, Salvaging, Sustaining, and Succeeding for a Pandemic World
- Larry Wellikson, MD, MHM: Out of COVID and Into the Light: Hospitalists More Essential Than Ever
SHM Converge is the premier educational experience for hospital medicine professionals. This year’s virtual conference will be held from May 3-7, 2021 and offers 21 educational tracks, more than 250 speakers, advanced learning courses, networking opportunities, a scientific abstract competition, and more. SHM Converge On Demand is also available until 2024, including the opportunity to earn additional continuing medical education credit.
To register for SHM Converge, visit shmconverge.org. If you are a member of the media who would like to obtain a press pass, email [email protected].
COVID plus MI confers poor prognosis; 1 in 3 die in hospital
COVID-19 patients with ST-segment elevation MI (STEMI) represent a population with unique demographic and clinical features resulting in a high risk for mortality, according to initial findings from the North American Cardiovascular COVID-19 Myocardial Infarction (NACMI) Registry.

“This is the largest registry of COVID-positive patients presenting with STEMI [and] the results clearly illustrate the challenges and uniqueness of this patient population that deserves prompt and special attention,” study cochair Timothy Henry, MD, president-elect of the Society for Cardiovascular Angiography & Interventions, said in a news release.
The NACMI registry is a collaborative effort between the SCAI, the American College of Cardiology Interventional Council, and the Canadian Association of Interventional Cardiology.
“The rapid development of this ongoing, critically important prospective registry reflects the strong and unique collaboration of all three societies. It was gratifying to be part of this process and hopefully the results will improve the care of our patients and stimulate further research,” Dr. Henry said in the news release.
The registry has enrolled 1,185 patients presenting with STEMI at 64 sites across the United States and Canada. Participants include 230 COVID-positive STEMI patients; 495 STEMI patients suspected but ultimately confirmed not to have COVID-19; and 460 age-and sex-matched control STEMI patients treated prior to the pandemic who are part of the Midwest STEMI Consortium.
The initial findings from the registry were published online in the Journal of the American College of Cardiology.
Atypical symptoms may explain high death rate
The primary outcome – a composite of in-hospital death, stroke, recurrent MI, or repeat unplanned revascularization – occurred in 36% of COVID-positive patients, compared with 13% of COVID-negative patients and 5% of control patients (P < .001 relative to controls).
This difference was driven largely by a “very high” in-hospital death rate in COVID-positive patients, lead author Santiago Garcia, MD, Minneapolis Heart Institute Foundation, said in an interview.
The in-hospital death rate was 33% in COVID-positive patients, compared with 11% in COVID-negative patients and 4% in controls. Stroke also occurred more often in COVID-positive patients at 3% versus 2% in COVID-negative and 0% in controls.
These initial findings suggest that the combination of STEMI and COVID-19 infection “confers a poor prognosis, with one in three patients succumbing to the disease, even among patients selected for invasive angiography (28% mortality),” the investigators wrote.
The data also show that STEMI in COVID-positive patients disproportionately affects ethnic minorities (23% Hispanic and 24% Black) with diabetes, which was present in 46% of COVID-positive patients.
COVID-positive patients with STEMI are more likely to present with atypical symptoms such as dyspnea (54%), pulmonary infiltrates on chest x-ray (46%), and high-risk conditions such as cardiogenic shock (18%), “which may explain the high fatality rate,” Dr. Garcia said.
Despite these high-risk features, COVID-positive patients are less apt to undergo invasive angiography when compared with COVID-negative and control STEMI patients (78% vs. 96% vs. 100%).
The majority of patients (71%) who did under angiography received primary percutaneous coronary intervention (PPCI) with very small treatment delays (at 15 minutes) during the pandemic.
Another notable finding is that “many patients (23%) have ‘no culprit’ vessel and may represent different etiologies of ST-segment elevation including microemboli, myocarditis, Takotsubo cardiomyopathy,” Dr. Garcia said in an interview.
“In line with current guidelines, patients with suspected STEMI should be managed with PPCI, without delay while the safety of health care providers is ensured,” Ran Kornowski, MD, and Katia Orvin, MD, both with Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, wrote in a linked editorial.
“In this case, PPCI should be performed routinely, even if the patient is presumed to have COVID-19, because PPCI should not be postponed. Confirmation of SARS-CoV-2 infection should not delay urgent decision management concerning reperfusion strategy,” they advised.
Looking ahead, Garcia said plans for the registry include determining predictors of in-hospital mortality and studying demographic and treatment trends as the pandemic continues with more virulent strains of the virus.
Various subgroup analyses are also planned as well as an independent angiographic and electrocardiographic core lab analysis. A comparative analysis of data from the US and Canada is also planned.
This work was supported by an ACC Accreditation Grant, Saskatchewan Health Research Foundation, and grants from Medtronic and Abbott Vascular to SCAI. Dr. Garcia has received institutional research grants from Edwards Lifesciences, BSCI, Medtronic, and Abbott Vascular; has served as a consultant for Medtronic and BSCI; and has served as a proctor for Edwards Lifesciences. Dr. Kornowski and Dr. Orvin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients with ST-segment elevation MI (STEMI) represent a population with unique demographic and clinical features resulting in a high risk for mortality, according to initial findings from the North American Cardiovascular COVID-19 Myocardial Infarction (NACMI) Registry.

“This is the largest registry of COVID-positive patients presenting with STEMI [and] the results clearly illustrate the challenges and uniqueness of this patient population that deserves prompt and special attention,” study cochair Timothy Henry, MD, president-elect of the Society for Cardiovascular Angiography & Interventions, said in a news release.
The NACMI registry is a collaborative effort between the SCAI, the American College of Cardiology Interventional Council, and the Canadian Association of Interventional Cardiology.
“The rapid development of this ongoing, critically important prospective registry reflects the strong and unique collaboration of all three societies. It was gratifying to be part of this process and hopefully the results will improve the care of our patients and stimulate further research,” Dr. Henry said in the news release.
The registry has enrolled 1,185 patients presenting with STEMI at 64 sites across the United States and Canada. Participants include 230 COVID-positive STEMI patients; 495 STEMI patients suspected but ultimately confirmed not to have COVID-19; and 460 age-and sex-matched control STEMI patients treated prior to the pandemic who are part of the Midwest STEMI Consortium.
The initial findings from the registry were published online in the Journal of the American College of Cardiology.
Atypical symptoms may explain high death rate
The primary outcome – a composite of in-hospital death, stroke, recurrent MI, or repeat unplanned revascularization – occurred in 36% of COVID-positive patients, compared with 13% of COVID-negative patients and 5% of control patients (P < .001 relative to controls).
This difference was driven largely by a “very high” in-hospital death rate in COVID-positive patients, lead author Santiago Garcia, MD, Minneapolis Heart Institute Foundation, said in an interview.
The in-hospital death rate was 33% in COVID-positive patients, compared with 11% in COVID-negative patients and 4% in controls. Stroke also occurred more often in COVID-positive patients at 3% versus 2% in COVID-negative and 0% in controls.
These initial findings suggest that the combination of STEMI and COVID-19 infection “confers a poor prognosis, with one in three patients succumbing to the disease, even among patients selected for invasive angiography (28% mortality),” the investigators wrote.
The data also show that STEMI in COVID-positive patients disproportionately affects ethnic minorities (23% Hispanic and 24% Black) with diabetes, which was present in 46% of COVID-positive patients.
COVID-positive patients with STEMI are more likely to present with atypical symptoms such as dyspnea (54%), pulmonary infiltrates on chest x-ray (46%), and high-risk conditions such as cardiogenic shock (18%), “which may explain the high fatality rate,” Dr. Garcia said.
Despite these high-risk features, COVID-positive patients are less apt to undergo invasive angiography when compared with COVID-negative and control STEMI patients (78% vs. 96% vs. 100%).
The majority of patients (71%) who did under angiography received primary percutaneous coronary intervention (PPCI) with very small treatment delays (at 15 minutes) during the pandemic.
Another notable finding is that “many patients (23%) have ‘no culprit’ vessel and may represent different etiologies of ST-segment elevation including microemboli, myocarditis, Takotsubo cardiomyopathy,” Dr. Garcia said in an interview.
“In line with current guidelines, patients with suspected STEMI should be managed with PPCI, without delay while the safety of health care providers is ensured,” Ran Kornowski, MD, and Katia Orvin, MD, both with Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, wrote in a linked editorial.
“In this case, PPCI should be performed routinely, even if the patient is presumed to have COVID-19, because PPCI should not be postponed. Confirmation of SARS-CoV-2 infection should not delay urgent decision management concerning reperfusion strategy,” they advised.
Looking ahead, Garcia said plans for the registry include determining predictors of in-hospital mortality and studying demographic and treatment trends as the pandemic continues with more virulent strains of the virus.
Various subgroup analyses are also planned as well as an independent angiographic and electrocardiographic core lab analysis. A comparative analysis of data from the US and Canada is also planned.
This work was supported by an ACC Accreditation Grant, Saskatchewan Health Research Foundation, and grants from Medtronic and Abbott Vascular to SCAI. Dr. Garcia has received institutional research grants from Edwards Lifesciences, BSCI, Medtronic, and Abbott Vascular; has served as a consultant for Medtronic and BSCI; and has served as a proctor for Edwards Lifesciences. Dr. Kornowski and Dr. Orvin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 patients with ST-segment elevation MI (STEMI) represent a population with unique demographic and clinical features resulting in a high risk for mortality, according to initial findings from the North American Cardiovascular COVID-19 Myocardial Infarction (NACMI) Registry.

“This is the largest registry of COVID-positive patients presenting with STEMI [and] the results clearly illustrate the challenges and uniqueness of this patient population that deserves prompt and special attention,” study cochair Timothy Henry, MD, president-elect of the Society for Cardiovascular Angiography & Interventions, said in a news release.
The NACMI registry is a collaborative effort between the SCAI, the American College of Cardiology Interventional Council, and the Canadian Association of Interventional Cardiology.
“The rapid development of this ongoing, critically important prospective registry reflects the strong and unique collaboration of all three societies. It was gratifying to be part of this process and hopefully the results will improve the care of our patients and stimulate further research,” Dr. Henry said in the news release.
The registry has enrolled 1,185 patients presenting with STEMI at 64 sites across the United States and Canada. Participants include 230 COVID-positive STEMI patients; 495 STEMI patients suspected but ultimately confirmed not to have COVID-19; and 460 age-and sex-matched control STEMI patients treated prior to the pandemic who are part of the Midwest STEMI Consortium.
The initial findings from the registry were published online in the Journal of the American College of Cardiology.
Atypical symptoms may explain high death rate
The primary outcome – a composite of in-hospital death, stroke, recurrent MI, or repeat unplanned revascularization – occurred in 36% of COVID-positive patients, compared with 13% of COVID-negative patients and 5% of control patients (P < .001 relative to controls).
This difference was driven largely by a “very high” in-hospital death rate in COVID-positive patients, lead author Santiago Garcia, MD, Minneapolis Heart Institute Foundation, said in an interview.
The in-hospital death rate was 33% in COVID-positive patients, compared with 11% in COVID-negative patients and 4% in controls. Stroke also occurred more often in COVID-positive patients at 3% versus 2% in COVID-negative and 0% in controls.
These initial findings suggest that the combination of STEMI and COVID-19 infection “confers a poor prognosis, with one in three patients succumbing to the disease, even among patients selected for invasive angiography (28% mortality),” the investigators wrote.
The data also show that STEMI in COVID-positive patients disproportionately affects ethnic minorities (23% Hispanic and 24% Black) with diabetes, which was present in 46% of COVID-positive patients.
COVID-positive patients with STEMI are more likely to present with atypical symptoms such as dyspnea (54%), pulmonary infiltrates on chest x-ray (46%), and high-risk conditions such as cardiogenic shock (18%), “which may explain the high fatality rate,” Dr. Garcia said.
Despite these high-risk features, COVID-positive patients are less apt to undergo invasive angiography when compared with COVID-negative and control STEMI patients (78% vs. 96% vs. 100%).
The majority of patients (71%) who did under angiography received primary percutaneous coronary intervention (PPCI) with very small treatment delays (at 15 minutes) during the pandemic.
Another notable finding is that “many patients (23%) have ‘no culprit’ vessel and may represent different etiologies of ST-segment elevation including microemboli, myocarditis, Takotsubo cardiomyopathy,” Dr. Garcia said in an interview.
“In line with current guidelines, patients with suspected STEMI should be managed with PPCI, without delay while the safety of health care providers is ensured,” Ran Kornowski, MD, and Katia Orvin, MD, both with Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, wrote in a linked editorial.
“In this case, PPCI should be performed routinely, even if the patient is presumed to have COVID-19, because PPCI should not be postponed. Confirmation of SARS-CoV-2 infection should not delay urgent decision management concerning reperfusion strategy,” they advised.
Looking ahead, Garcia said plans for the registry include determining predictors of in-hospital mortality and studying demographic and treatment trends as the pandemic continues with more virulent strains of the virus.
Various subgroup analyses are also planned as well as an independent angiographic and electrocardiographic core lab analysis. A comparative analysis of data from the US and Canada is also planned.
This work was supported by an ACC Accreditation Grant, Saskatchewan Health Research Foundation, and grants from Medtronic and Abbott Vascular to SCAI. Dr. Garcia has received institutional research grants from Edwards Lifesciences, BSCI, Medtronic, and Abbott Vascular; has served as a consultant for Medtronic and BSCI; and has served as a proctor for Edwards Lifesciences. Dr. Kornowski and Dr. Orvin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Assessing the efficacy and safety of dapagliflozin in patients with HFrEF
Background: Guideline-directed medical therapy (use of beta-blockers, ACE inhibitor/angiotensin receptor blockers, and mineralocorticoid antagonists) provides clear benefits on mortality and morbidity in patients with HFrEF. Dapagliflozin (Farxiga) belongs to a class of sodium-glucose transporter 2 (SGLT2) inhibitors that inhibits reabsorption of sodium and glucose in the kidney and treats type 2 diabetes. This new class of drugs is emerging as an effective tool in the management of HFrEF based on the recent publication of the primary results of the DAPA-HF trial (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure). It demonstrated substantial benefits in terms of heart failure symptoms, hospitalizations, and mortality when added to triple therapy for patients with chronic HFrEF regardless of the presence of diabetes.
Study design: Randomized, controlled double-blind trials.
Setting: 410 participating institutions in 20 countries.
Synopsis: Men and women aged 18 years and older with HFrEF who had New York Heart Association (NYHA) functional class II or higher, and optimally treated with pharmacologic and device therapy for HF were randomized to receive dapagliflozin or placebo. A total of 4,744 patients, aged 22-94 years were enrolled in the study.
- Dapagliflozin showed a clinically significant benefit on health status (symptoms, physical function, and quality of life). Improved health-related quality of life (as measured by the well-validated Kansas City Cardiomyopathy Questionnaire score) with dapagliflozin in comparison with placebo was sustained for more than 8 months.
- Dapagliflozin reduced the risk of death and worsening heart failure and improved symptoms across the broad spectrum of ages studied in DAPA-HF. There was no significant imbalance in tolerability or safety events between dapagliflozin and placebo, even in elderly individuals.
Bottom line: Follow-up DAPA-HF studies further support the role of SGLT2 inhibitor dapagliflozin in improving mortality, reducing hospitalization, and improving the quality of life in patients with HFrEF and is considered a safe option across all age groups.
Citations: Kosiborod MN et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF trial. Circulation. 2020 Jan 14;141(2):90-9. Martinez FA et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age. Insights from DAPA-HF. Circulation. 2020 Jan 14;141:100-11.
Dr. Garg is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Guideline-directed medical therapy (use of beta-blockers, ACE inhibitor/angiotensin receptor blockers, and mineralocorticoid antagonists) provides clear benefits on mortality and morbidity in patients with HFrEF. Dapagliflozin (Farxiga) belongs to a class of sodium-glucose transporter 2 (SGLT2) inhibitors that inhibits reabsorption of sodium and glucose in the kidney and treats type 2 diabetes. This new class of drugs is emerging as an effective tool in the management of HFrEF based on the recent publication of the primary results of the DAPA-HF trial (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure). It demonstrated substantial benefits in terms of heart failure symptoms, hospitalizations, and mortality when added to triple therapy for patients with chronic HFrEF regardless of the presence of diabetes.
Study design: Randomized, controlled double-blind trials.
Setting: 410 participating institutions in 20 countries.
Synopsis: Men and women aged 18 years and older with HFrEF who had New York Heart Association (NYHA) functional class II or higher, and optimally treated with pharmacologic and device therapy for HF were randomized to receive dapagliflozin or placebo. A total of 4,744 patients, aged 22-94 years were enrolled in the study.
- Dapagliflozin showed a clinically significant benefit on health status (symptoms, physical function, and quality of life). Improved health-related quality of life (as measured by the well-validated Kansas City Cardiomyopathy Questionnaire score) with dapagliflozin in comparison with placebo was sustained for more than 8 months.
- Dapagliflozin reduced the risk of death and worsening heart failure and improved symptoms across the broad spectrum of ages studied in DAPA-HF. There was no significant imbalance in tolerability or safety events between dapagliflozin and placebo, even in elderly individuals.
Bottom line: Follow-up DAPA-HF studies further support the role of SGLT2 inhibitor dapagliflozin in improving mortality, reducing hospitalization, and improving the quality of life in patients with HFrEF and is considered a safe option across all age groups.
Citations: Kosiborod MN et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF trial. Circulation. 2020 Jan 14;141(2):90-9. Martinez FA et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age. Insights from DAPA-HF. Circulation. 2020 Jan 14;141:100-11.
Dr. Garg is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Guideline-directed medical therapy (use of beta-blockers, ACE inhibitor/angiotensin receptor blockers, and mineralocorticoid antagonists) provides clear benefits on mortality and morbidity in patients with HFrEF. Dapagliflozin (Farxiga) belongs to a class of sodium-glucose transporter 2 (SGLT2) inhibitors that inhibits reabsorption of sodium and glucose in the kidney and treats type 2 diabetes. This new class of drugs is emerging as an effective tool in the management of HFrEF based on the recent publication of the primary results of the DAPA-HF trial (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with Chronic Heart Failure). It demonstrated substantial benefits in terms of heart failure symptoms, hospitalizations, and mortality when added to triple therapy for patients with chronic HFrEF regardless of the presence of diabetes.
Study design: Randomized, controlled double-blind trials.
Setting: 410 participating institutions in 20 countries.
Synopsis: Men and women aged 18 years and older with HFrEF who had New York Heart Association (NYHA) functional class II or higher, and optimally treated with pharmacologic and device therapy for HF were randomized to receive dapagliflozin or placebo. A total of 4,744 patients, aged 22-94 years were enrolled in the study.
- Dapagliflozin showed a clinically significant benefit on health status (symptoms, physical function, and quality of life). Improved health-related quality of life (as measured by the well-validated Kansas City Cardiomyopathy Questionnaire score) with dapagliflozin in comparison with placebo was sustained for more than 8 months.
- Dapagliflozin reduced the risk of death and worsening heart failure and improved symptoms across the broad spectrum of ages studied in DAPA-HF. There was no significant imbalance in tolerability or safety events between dapagliflozin and placebo, even in elderly individuals.
Bottom line: Follow-up DAPA-HF studies further support the role of SGLT2 inhibitor dapagliflozin in improving mortality, reducing hospitalization, and improving the quality of life in patients with HFrEF and is considered a safe option across all age groups.
Citations: Kosiborod MN et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF trial. Circulation. 2020 Jan 14;141(2):90-9. Martinez FA et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age. Insights from DAPA-HF. Circulation. 2020 Jan 14;141:100-11.
Dr. Garg is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Early palliative care consultation in the medical ICU
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Mortality rates in critically ill patients remain in excess of 20% in many institutions. In the last 2 decades, palliative care has become a core component of ICU care. Current literature recommends a palliative care consult in the ICU setting; however, implementing this recommendation in a meaningful way has been challenging. The purpose of this study is to evaluate whether consulting palliative care in ICU earlier improves patient outcomes.
Study design: Single-center cluster randomized crossover trial.
Setting: Two medical ICUs at Barnes Jewish Hospital, St. Louis.
Synopsis: 199 patients were enrolled using palliative care criteria to identify patients at high risk for morbidity and mortality. In the intervention arm patients received a palliative care consultation from an inter-professional team led by board-certified palliative care providers within 48 hours of ICU admission.
The primary outcome of this study was a change in code status to Do Not Resuscitate/Do Not Intubate (DNR/DNI), which was significantly higher in the intervention group (50.5% vs. 23.4%; P less than .0001). The intervention group also had more hospice discharges, fewer ventilated days, a lower rate of tracheostomy, and fewer hospital readmissions. However, mortality and ICU/hospital length of stay were not significantly different between the two arms. Limitations of this study include using a single academic center and the fact that establishing a DNR/DNI may not measure quality of life or patient/family satisfaction. Further studies are needed to focus on clinical outcomes as well as patient and family satisfaction.
Bottom line: Early goal-directed palliative care consults with experienced clinicians board certified in palliative care influences goals of care, code status, and discharge plans for the critically ill and can improve medical resource utilization.
Citation: Ma J et al. Early palliative care consultation in the medical ICU: A cluster randomized crossover trial. Crit Care Med. 2019 Dec;47: 1707-15.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Stroke is ‘not a common complication’ in COVID-19
One study showed a stroke rate of 2.2% among patients with COVID-19 admitted to intensive care in 52 different countries. Another found a stroke rate of 1.48% in patients hospitalized with COVID-19 from 70 different countries. These researchers also found a reduction in stroke presentations and stroke care during the pandemic.
Both studies will be presented at the American Academy of Neurology’s 2021 annual meeting.
“Stroke has been a known serious complication of COVID-19, with some studies reporting a higher-than-expected occurrence, especially in young people,” said coauthor of the intensive care study, Jonathon Fanning, MBBS, PhD, University of Queensland, Brisbane, Australia.
“However, among the sickest of COVID patients – those admitted to an ICU – our research found that stroke was not a common complication and that ischemic stroke did not increase the risk of death,” he added.
Hemorrhagic stroke more common?
In this study, researchers analyzed a database of 2,699 patients who were admitted to the intensive care unit with COVID-19 in 52 countries and found that 59 of these patients (2.2%) subsequently sustained a stroke.
Most of the strokes identified in this cohort were hemorrhagic (46%), with 32% being ischemic and 22% unspecified. Hemorrhagic stroke was associated with a fivefold increased risk for death compared with patients who did not have a stroke. Of those with a hemorrhagic stroke, 72% died, but only 15% died of the stroke. Rather, multiorgan failure was the leading cause of death.
There was no association between ischemic stroke and mortality.
“There is scarce research on new-onset stroke complicating ICU admissions, and many of the limitations of assessing stroke in ICU populations confound the true values and result in variability in reported incidence anywhere from a 1%-4% incidence,” Dr. Fanning said.
He noted that a large Korean study had shown a 1.2% rate of stroke in patients without COVID admitted to non-neurologic ICUs. “In light of this, I think this 2% is higher than we would expect in a general ICU population, but in the context of earlier reports of COVID-19–associated risk for stroke, this figure is actually somewhat reassuring,” Dr. Fanning said.
Asked how this study compared with the large American Heart Association study recently reported that showed an overall rate of ischemic stroke of 0.75%, Dr. Fanning said the two studies reported on different populations, which makes them difficult to compare.
“Our study specifically reports on new-onset stroke complicating ICU admission,” he noted. “The AHA study is a large study of all patients admitted to hospital, but both studies identified less than previous estimates of COVID-related stroke.”
Largest sample to date
The other study, which includes 119,967 COVID-19 hospitalizations and represents the largest sample reporting the concomitant diagnoses of stroke and SARS-CoV-2 infection to date, was presented at the AAN meeting by Thanh N. Nguyen, MD, a professor at Boston University.
This study has also been published online in Neurology, with first author Raul G. Nogueira, MD, Emory University, Atlanta.
In this international observational, retrospective study across 6 continents, 70 countries, and 457 stroke centers, there was a 1.48% stroke rate across 119,967 COVID-19 hospitalizations. SARS-CoV-2 infection was noted in 3.3% (1,722) of all stroke admissions, which numbered 52,026.
The researchers identified stroke diagnoses by the International Classification of Diseases, 10th revision, codes and/or classifications in stroke center databases, and rates of stroke hospitalizations and numbers of patients receiving thrombolysis were compared between the first 4 months of the pandemic (March to June 2020) compared with two control 4-month periods.
Global decline in stroke care during pandemic
Results showed a global decline in the number of stroke patients admitted to the hospital as well as acute stroke treatments, such as thrombolysis, during the first wave of the COVID-19 pandemic. The researchers found that there were 91,373 stroke admissions in the 4 months immediately before the pandemic, compared with 80,894 admissions during the first 4 pandemic months, representing an 11.5% decline.
They also report that 13,334 stroke patients received intravenous thrombolysis in the 4 months preceding the pandemic, compared with 11,570 during the first 4 pandemic months, representing a 13.2% drop.
Interhospital transfers after thrombolysis for a higher level of stroke care decreased from 1,337 before the pandemic to 1,178 during the pandemic, a reduction of 11.9%.
There were greater declines in primary compared with comprehensive stroke centers for stroke hospitalizations (change, –17.3% vs. –10.3%) and for the number of patients receiving thrombolysis (change, –15.5% vs. –12.6%).
The volume of stroke hospitalizations increased by 9.5% in the two later pandemic months (May, June) versus the two earlier months (March, April), with greater recovery in hospitals with lower COVID-19 hospitalization volume, high-volume stroke centers, and comprehensive stroke centers.
Dr. Nguyen suggested that reasons for the reductions in these stroke numbers at the beginning of the pandemic could include a reduction in stroke risk due to a reduction of exposure to other viral infections or patients not presenting to the hospital for fear of contracting the coronavirus.
The higher recovery of stroke volume in high-volume stroke centers and comprehensive stroke centers may represent patients with higher needs – those having more severe strokes – seeking care more frequently than those with milder symptoms, she noted.
“Preserving access to stroke care and emergency stroke care amidst a pandemic is as important as educating patients on the importance of presenting to the hospital in the event of stroke-like symptoms,” Dr. Nguyen concluded.
“We continue to advocate that if a patient has stroke-like symptoms, such as loss of speech, strength, vision, or balance, it is important for the patient to seek medical care as an emergency, as there are treatments that can improve a patient’s ability to recover from disabling stroke in earlier rather than later time windows,” she added.
In the publication, the authors wrote, “Our results concur with other recent reports on the collateral effects of the COVID-19 pandemic on stroke systems of care,” but added that “this is among the first descriptions of the change at a global level, including primary and comprehensive stroke centers.”
They said that hospital access related to high COVID-19 burden was unlikely a factor because the decline was seen in centers with a few or no patients with COVID-19. They suggested that patient fear of contracting coronavirus may have played a role, along with a decrease in presentation of transient ischemic attacks, mild strokes, or moderate strokes, and physical distancing measures may have prevented the timely witnessing of a stroke.
A version of this article first appeared on Medscape.com.
One study showed a stroke rate of 2.2% among patients with COVID-19 admitted to intensive care in 52 different countries. Another found a stroke rate of 1.48% in patients hospitalized with COVID-19 from 70 different countries. These researchers also found a reduction in stroke presentations and stroke care during the pandemic.
Both studies will be presented at the American Academy of Neurology’s 2021 annual meeting.
“Stroke has been a known serious complication of COVID-19, with some studies reporting a higher-than-expected occurrence, especially in young people,” said coauthor of the intensive care study, Jonathon Fanning, MBBS, PhD, University of Queensland, Brisbane, Australia.
“However, among the sickest of COVID patients – those admitted to an ICU – our research found that stroke was not a common complication and that ischemic stroke did not increase the risk of death,” he added.
Hemorrhagic stroke more common?
In this study, researchers analyzed a database of 2,699 patients who were admitted to the intensive care unit with COVID-19 in 52 countries and found that 59 of these patients (2.2%) subsequently sustained a stroke.
Most of the strokes identified in this cohort were hemorrhagic (46%), with 32% being ischemic and 22% unspecified. Hemorrhagic stroke was associated with a fivefold increased risk for death compared with patients who did not have a stroke. Of those with a hemorrhagic stroke, 72% died, but only 15% died of the stroke. Rather, multiorgan failure was the leading cause of death.
There was no association between ischemic stroke and mortality.
“There is scarce research on new-onset stroke complicating ICU admissions, and many of the limitations of assessing stroke in ICU populations confound the true values and result in variability in reported incidence anywhere from a 1%-4% incidence,” Dr. Fanning said.
He noted that a large Korean study had shown a 1.2% rate of stroke in patients without COVID admitted to non-neurologic ICUs. “In light of this, I think this 2% is higher than we would expect in a general ICU population, but in the context of earlier reports of COVID-19–associated risk for stroke, this figure is actually somewhat reassuring,” Dr. Fanning said.
Asked how this study compared with the large American Heart Association study recently reported that showed an overall rate of ischemic stroke of 0.75%, Dr. Fanning said the two studies reported on different populations, which makes them difficult to compare.
“Our study specifically reports on new-onset stroke complicating ICU admission,” he noted. “The AHA study is a large study of all patients admitted to hospital, but both studies identified less than previous estimates of COVID-related stroke.”
Largest sample to date
The other study, which includes 119,967 COVID-19 hospitalizations and represents the largest sample reporting the concomitant diagnoses of stroke and SARS-CoV-2 infection to date, was presented at the AAN meeting by Thanh N. Nguyen, MD, a professor at Boston University.
This study has also been published online in Neurology, with first author Raul G. Nogueira, MD, Emory University, Atlanta.
In this international observational, retrospective study across 6 continents, 70 countries, and 457 stroke centers, there was a 1.48% stroke rate across 119,967 COVID-19 hospitalizations. SARS-CoV-2 infection was noted in 3.3% (1,722) of all stroke admissions, which numbered 52,026.
The researchers identified stroke diagnoses by the International Classification of Diseases, 10th revision, codes and/or classifications in stroke center databases, and rates of stroke hospitalizations and numbers of patients receiving thrombolysis were compared between the first 4 months of the pandemic (March to June 2020) compared with two control 4-month periods.
Global decline in stroke care during pandemic
Results showed a global decline in the number of stroke patients admitted to the hospital as well as acute stroke treatments, such as thrombolysis, during the first wave of the COVID-19 pandemic. The researchers found that there were 91,373 stroke admissions in the 4 months immediately before the pandemic, compared with 80,894 admissions during the first 4 pandemic months, representing an 11.5% decline.
They also report that 13,334 stroke patients received intravenous thrombolysis in the 4 months preceding the pandemic, compared with 11,570 during the first 4 pandemic months, representing a 13.2% drop.
Interhospital transfers after thrombolysis for a higher level of stroke care decreased from 1,337 before the pandemic to 1,178 during the pandemic, a reduction of 11.9%.
There were greater declines in primary compared with comprehensive stroke centers for stroke hospitalizations (change, –17.3% vs. –10.3%) and for the number of patients receiving thrombolysis (change, –15.5% vs. –12.6%).
The volume of stroke hospitalizations increased by 9.5% in the two later pandemic months (May, June) versus the two earlier months (March, April), with greater recovery in hospitals with lower COVID-19 hospitalization volume, high-volume stroke centers, and comprehensive stroke centers.
Dr. Nguyen suggested that reasons for the reductions in these stroke numbers at the beginning of the pandemic could include a reduction in stroke risk due to a reduction of exposure to other viral infections or patients not presenting to the hospital for fear of contracting the coronavirus.
The higher recovery of stroke volume in high-volume stroke centers and comprehensive stroke centers may represent patients with higher needs – those having more severe strokes – seeking care more frequently than those with milder symptoms, she noted.
“Preserving access to stroke care and emergency stroke care amidst a pandemic is as important as educating patients on the importance of presenting to the hospital in the event of stroke-like symptoms,” Dr. Nguyen concluded.
“We continue to advocate that if a patient has stroke-like symptoms, such as loss of speech, strength, vision, or balance, it is important for the patient to seek medical care as an emergency, as there are treatments that can improve a patient’s ability to recover from disabling stroke in earlier rather than later time windows,” she added.
In the publication, the authors wrote, “Our results concur with other recent reports on the collateral effects of the COVID-19 pandemic on stroke systems of care,” but added that “this is among the first descriptions of the change at a global level, including primary and comprehensive stroke centers.”
They said that hospital access related to high COVID-19 burden was unlikely a factor because the decline was seen in centers with a few or no patients with COVID-19. They suggested that patient fear of contracting coronavirus may have played a role, along with a decrease in presentation of transient ischemic attacks, mild strokes, or moderate strokes, and physical distancing measures may have prevented the timely witnessing of a stroke.
A version of this article first appeared on Medscape.com.
One study showed a stroke rate of 2.2% among patients with COVID-19 admitted to intensive care in 52 different countries. Another found a stroke rate of 1.48% in patients hospitalized with COVID-19 from 70 different countries. These researchers also found a reduction in stroke presentations and stroke care during the pandemic.
Both studies will be presented at the American Academy of Neurology’s 2021 annual meeting.
“Stroke has been a known serious complication of COVID-19, with some studies reporting a higher-than-expected occurrence, especially in young people,” said coauthor of the intensive care study, Jonathon Fanning, MBBS, PhD, University of Queensland, Brisbane, Australia.
“However, among the sickest of COVID patients – those admitted to an ICU – our research found that stroke was not a common complication and that ischemic stroke did not increase the risk of death,” he added.
Hemorrhagic stroke more common?
In this study, researchers analyzed a database of 2,699 patients who were admitted to the intensive care unit with COVID-19 in 52 countries and found that 59 of these patients (2.2%) subsequently sustained a stroke.
Most of the strokes identified in this cohort were hemorrhagic (46%), with 32% being ischemic and 22% unspecified. Hemorrhagic stroke was associated with a fivefold increased risk for death compared with patients who did not have a stroke. Of those with a hemorrhagic stroke, 72% died, but only 15% died of the stroke. Rather, multiorgan failure was the leading cause of death.
There was no association between ischemic stroke and mortality.
“There is scarce research on new-onset stroke complicating ICU admissions, and many of the limitations of assessing stroke in ICU populations confound the true values and result in variability in reported incidence anywhere from a 1%-4% incidence,” Dr. Fanning said.
He noted that a large Korean study had shown a 1.2% rate of stroke in patients without COVID admitted to non-neurologic ICUs. “In light of this, I think this 2% is higher than we would expect in a general ICU population, but in the context of earlier reports of COVID-19–associated risk for stroke, this figure is actually somewhat reassuring,” Dr. Fanning said.
Asked how this study compared with the large American Heart Association study recently reported that showed an overall rate of ischemic stroke of 0.75%, Dr. Fanning said the two studies reported on different populations, which makes them difficult to compare.
“Our study specifically reports on new-onset stroke complicating ICU admission,” he noted. “The AHA study is a large study of all patients admitted to hospital, but both studies identified less than previous estimates of COVID-related stroke.”
Largest sample to date
The other study, which includes 119,967 COVID-19 hospitalizations and represents the largest sample reporting the concomitant diagnoses of stroke and SARS-CoV-2 infection to date, was presented at the AAN meeting by Thanh N. Nguyen, MD, a professor at Boston University.
This study has also been published online in Neurology, with first author Raul G. Nogueira, MD, Emory University, Atlanta.
In this international observational, retrospective study across 6 continents, 70 countries, and 457 stroke centers, there was a 1.48% stroke rate across 119,967 COVID-19 hospitalizations. SARS-CoV-2 infection was noted in 3.3% (1,722) of all stroke admissions, which numbered 52,026.
The researchers identified stroke diagnoses by the International Classification of Diseases, 10th revision, codes and/or classifications in stroke center databases, and rates of stroke hospitalizations and numbers of patients receiving thrombolysis were compared between the first 4 months of the pandemic (March to June 2020) compared with two control 4-month periods.
Global decline in stroke care during pandemic
Results showed a global decline in the number of stroke patients admitted to the hospital as well as acute stroke treatments, such as thrombolysis, during the first wave of the COVID-19 pandemic. The researchers found that there were 91,373 stroke admissions in the 4 months immediately before the pandemic, compared with 80,894 admissions during the first 4 pandemic months, representing an 11.5% decline.
They also report that 13,334 stroke patients received intravenous thrombolysis in the 4 months preceding the pandemic, compared with 11,570 during the first 4 pandemic months, representing a 13.2% drop.
Interhospital transfers after thrombolysis for a higher level of stroke care decreased from 1,337 before the pandemic to 1,178 during the pandemic, a reduction of 11.9%.
There were greater declines in primary compared with comprehensive stroke centers for stroke hospitalizations (change, –17.3% vs. –10.3%) and for the number of patients receiving thrombolysis (change, –15.5% vs. –12.6%).
The volume of stroke hospitalizations increased by 9.5% in the two later pandemic months (May, June) versus the two earlier months (March, April), with greater recovery in hospitals with lower COVID-19 hospitalization volume, high-volume stroke centers, and comprehensive stroke centers.
Dr. Nguyen suggested that reasons for the reductions in these stroke numbers at the beginning of the pandemic could include a reduction in stroke risk due to a reduction of exposure to other viral infections or patients not presenting to the hospital for fear of contracting the coronavirus.
The higher recovery of stroke volume in high-volume stroke centers and comprehensive stroke centers may represent patients with higher needs – those having more severe strokes – seeking care more frequently than those with milder symptoms, she noted.
“Preserving access to stroke care and emergency stroke care amidst a pandemic is as important as educating patients on the importance of presenting to the hospital in the event of stroke-like symptoms,” Dr. Nguyen concluded.
“We continue to advocate that if a patient has stroke-like symptoms, such as loss of speech, strength, vision, or balance, it is important for the patient to seek medical care as an emergency, as there are treatments that can improve a patient’s ability to recover from disabling stroke in earlier rather than later time windows,” she added.
In the publication, the authors wrote, “Our results concur with other recent reports on the collateral effects of the COVID-19 pandemic on stroke systems of care,” but added that “this is among the first descriptions of the change at a global level, including primary and comprehensive stroke centers.”
They said that hospital access related to high COVID-19 burden was unlikely a factor because the decline was seen in centers with a few or no patients with COVID-19. They suggested that patient fear of contracting coronavirus may have played a role, along with a decrease in presentation of transient ischemic attacks, mild strokes, or moderate strokes, and physical distancing measures may have prevented the timely witnessing of a stroke.
A version of this article first appeared on Medscape.com.
From AAN 2021
Ten reasons airborne transmission of SARS-CoV-2 appears airtight
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
The scientific evidence for airborne transmission of the SARS-CoV-2 virus from different researchers all point in the same direction – that infectious aerosols are the principal means of person-to-person transmission, according to experts.
Not that it’s without controversy.
The science backing aerosol transmission “is clear-cut, but it is not accepted in many circles,” Trisha Greenhalgh, PhD, said in an interview.
“In particular, some in the evidence-based medicine movement and some infectious diseases clinicians are remarkably resistant to the evidence,” added Dr. Greenhalgh, professor of primary care health sciences at the University of Oxford (England).
“It’s very hard to see why, since the evidence all stacks up,” Dr. Greenhalgh said.
“The scientific evidence on spread from both near-field and far-field aerosols has been clear since early on in the pandemic, but there was resistance to acknowledging this in some circles, including the medical journals,” Joseph G. Allen, DSc, MPH, told this news organization when asked to comment.
“This is the week the dam broke. Three new commentaries came out … in top medical journals – BMJ, The Lancet, JAMA – all making the same point that aerosols are the dominant mode of transmission,” added Dr. Allen, associate professor of exposure assessment science at the Harvard T.H. Chan School of Public Health in Boston.
Dr. Greenhalgh and colleagues point to an increase in COVID-19 cases in the aftermath of so-called “super-spreader” events, spread of SARS-CoV-2 to people across different hotel rooms, and the relatively lower transmission detected after outdoor events.
Top 10 reasons
They outlined 10 scientific reasons backing airborne transmission in a commentary published online April 15 in The Lancet:
- The dominance of airborne transmission is supported by long-range transmission observed at super-spreader events.
- Long-range transmission has been reported among rooms at COVID-19 quarantine hotels, settings where infected people never spent time in the same room.
- Asymptomatic individuals account for an estimated 33%-59% of SARS-CoV-2 transmission, and could be spreading the virus through speaking, which produces thousands of aerosol particles and few large droplets.
- Transmission outdoors and in well-ventilated indoor spaces is lower than in enclosed spaces.
- Nosocomial infections are reported in health care settings where protective measures address large droplets but not aerosols.
- Viable SARS-CoV-2 has been detected in the air of hospital rooms and in the car of an infected person.
- Investigators found SARS-CoV-2 in hospital air filters and building ducts.
- It’s not just humans – infected animals can infect animals in other cages connected only through an air duct.
- No strong evidence refutes airborne transmission, and contact tracing supports secondary transmission in crowded, poorly ventilated indoor spaces.
- Only limited evidence supports other means of SARS-CoV-2 transmission, including through fomites or large droplets.
“We thought we’d summarize [the evidence] to clarify the arguments for and against. We looked hard for evidence against but found none,” Dr. Greenhalgh said.
“Although other routes can contribute, we believe that the airborne route is likely to be dominant,” the authors note.
The evidence on airborne transmission was there very early on but the Centers for Disease Control and Prevention, World Health Organization, and others repeated the message that the primary concern was droplets and fomites.
Response to a review
The top 10 list is also part rebuttal of a systematic review funded by the WHO and published last month that points to inconclusive evidence for airborne transmission. The researchers involved with that review state that “the lack of recoverable viral culture samples of SARS-CoV-2 prevents firm conclusions to be drawn about airborne transmission.”
However, Dr. Greenhalgh and colleagues note that “this conclusion, and the wide circulation of the review’s findings, is concerning because of the public health implications.”
The current authors also argue that enough evidence already exists on airborne transmission. “Policy should change. We don’t need more research on this topic; we need different policy,” Dr. Greenhalgh said. “We need ventilation front and center, air filtration when necessary, and better-fitting masks worn whenever indoors.”
Dr. Allen agreed that guidance hasn’t always kept pace with the science. “With all of the new evidence accumulated on airborne transmission since last winter, there is still widespread confusion in the public about modes of transmission,” he said. Dr. Allen also serves as commissioner of The Lancet COVID-19 Commission and is chair of the commission’s Task Force on Safe Work, Safe Schools, and Safe Travel.
“It was only just last week that CDC pulled back on guidance on ‘deep cleaning’ and in its place correctly said that the risk from touching surfaces is low,” he added. “The science has been clear on this for over a year, but official guidance was only recently updated.”
As a result, many companies and organizations continued to focus on “hygiene theatre,” Dr. Allen said, “wasting resources on overcleaning surfaces. Unbelievably, many schools still close for an entire day each week for deep cleaning and some still quarantine library books. The message that shared air is the problem, not shared surfaces, is a message that still needs to be reinforced.”
The National Institute for Health Research, Economic and Social Research Council, and Wellcome support Dr. Greenhalgh’s research. Dr. Greenhalgh and Dr. Allen had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Correlating hospitalist work schedules with patient outcomes
Background: Studies show better outcomes, decreased length of stay, increased patient satisfaction, improved quality, and decreased readmission rates when hospitalist services are used. This study looks at how hospitalist schedules affect these outcomes.
Study design: Retrospective cohort study.
Setting: 229 hospitals in Texas.
Synopsis: This cohort study used 3 years of Medicare data from 229 hospitals in Texas. It included 114,777 medical admissions of patients with a 3- to 6-day length of stay. The study used the percentage of hospitalist working days that were blocks of 7 days or longer. ICU stays and patients requiring two or more E&M codes were excluded since they are associated with greater illness severity.
The primary outcome was mortality within 30 days of discharge and secondary outcomes were 30-day readmission rates, discharge destination, and 30-day postdischarge costs.
Patients receiving care from hospitalists working several days in a row had better outcomes. It is postulated that continuity of care by one hospitalist is important for several reasons. Most importantly, the development of rapport with patient and family is key to deciding the plan of care and destination post discharge as it is quite challenging to effectively transfer all important information during verbal or written handoffs.
Bottom line: Care provided by hospitalists working more days in a row improved patient outcomes. A variety of hospitalist schedules are being practiced currently; however, these findings must be taken into account as schedules are designed.
Citation: Goodwin JS et al. Association of the work schedules of hospitalists with patient outcomes of hospitalization. JAMA Intern Med. 2020;180(2):215-22. doi: 10.1001/jamainternmed.2019.5193.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Studies show better outcomes, decreased length of stay, increased patient satisfaction, improved quality, and decreased readmission rates when hospitalist services are used. This study looks at how hospitalist schedules affect these outcomes.
Study design: Retrospective cohort study.
Setting: 229 hospitals in Texas.
Synopsis: This cohort study used 3 years of Medicare data from 229 hospitals in Texas. It included 114,777 medical admissions of patients with a 3- to 6-day length of stay. The study used the percentage of hospitalist working days that were blocks of 7 days or longer. ICU stays and patients requiring two or more E&M codes were excluded since they are associated with greater illness severity.
The primary outcome was mortality within 30 days of discharge and secondary outcomes were 30-day readmission rates, discharge destination, and 30-day postdischarge costs.
Patients receiving care from hospitalists working several days in a row had better outcomes. It is postulated that continuity of care by one hospitalist is important for several reasons. Most importantly, the development of rapport with patient and family is key to deciding the plan of care and destination post discharge as it is quite challenging to effectively transfer all important information during verbal or written handoffs.
Bottom line: Care provided by hospitalists working more days in a row improved patient outcomes. A variety of hospitalist schedules are being practiced currently; however, these findings must be taken into account as schedules are designed.
Citation: Goodwin JS et al. Association of the work schedules of hospitalists with patient outcomes of hospitalization. JAMA Intern Med. 2020;180(2):215-22. doi: 10.1001/jamainternmed.2019.5193.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: Studies show better outcomes, decreased length of stay, increased patient satisfaction, improved quality, and decreased readmission rates when hospitalist services are used. This study looks at how hospitalist schedules affect these outcomes.
Study design: Retrospective cohort study.
Setting: 229 hospitals in Texas.
Synopsis: This cohort study used 3 years of Medicare data from 229 hospitals in Texas. It included 114,777 medical admissions of patients with a 3- to 6-day length of stay. The study used the percentage of hospitalist working days that were blocks of 7 days or longer. ICU stays and patients requiring two or more E&M codes were excluded since they are associated with greater illness severity.
The primary outcome was mortality within 30 days of discharge and secondary outcomes were 30-day readmission rates, discharge destination, and 30-day postdischarge costs.
Patients receiving care from hospitalists working several days in a row had better outcomes. It is postulated that continuity of care by one hospitalist is important for several reasons. Most importantly, the development of rapport with patient and family is key to deciding the plan of care and destination post discharge as it is quite challenging to effectively transfer all important information during verbal or written handoffs.
Bottom line: Care provided by hospitalists working more days in a row improved patient outcomes. A variety of hospitalist schedules are being practiced currently; however, these findings must be taken into account as schedules are designed.
Citation: Goodwin JS et al. Association of the work schedules of hospitalists with patient outcomes of hospitalization. JAMA Intern Med. 2020;180(2):215-22. doi: 10.1001/jamainternmed.2019.5193.
Dr. Ahmed is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Children’s share of COVID-19 burden has never been higher
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
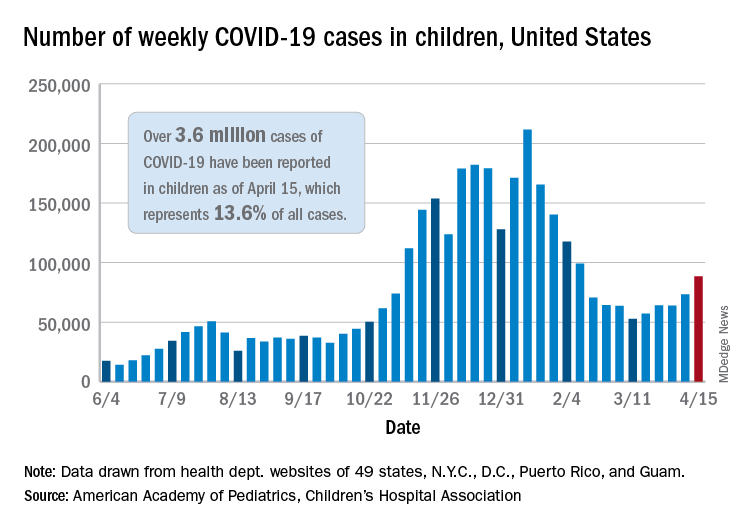
That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
For the first time since the pandemic began, children’s share of weekly COVID-19 cases topped 20% in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

That represented 20.6% of all new cases for the week, eclipsing the previous high of 19.1% recorded just 3 weeks ago, based on data collected by the AAP and CHA from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Cumulative cases of COVID-19 in children exceed 3.6 million in those jurisdictions, which is 13.6% of the total reported among all ages, and the overall rate of coronavirus infection is 4,824 cases per 100,000 children in the population, the AAP and CHA said in their weekly COVID-19 report.
Among the 53 reporting jurisdictions, North Dakota has the highest cumulative rate, 9,167 per 100,000 children, followed by Tennessee (8,580), South Carolina (7,948), South Dakota (7,938), and Connecticut (7,707). Children’s share of cumulative cases is highest in Vermont, at 21.9%, with Alaska next at 20.0% and Wyoming at 19.2%, the AAP and CHA said.
Since the beginning of April, the largest local increases in cases reported came in Michigan (21.6%), Vermont (15.9%), and Maine (15.6%). Nationally, the increase over those same 2 weeks is just under 5%, the two organizations noted.
There were 5 deaths among children during the week of April 9-15, bringing the total to 297, but the recent increases in cases have not affected the long-term trends for serious illness. The death rate for children with COVID-19 has been 0.01% since early November – 43 states, New York City, Puerto Rico, and Guam are reporting such data – and the hospitalization rate has been 0.8% since mid-January in 24 states and New York City, the AAP/CHA data show.
Watch for abnormal movements in hospitalized COVID-19 patients
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
Myoclonus was diagnosed in about half of hospitalized COVID-19 patients who were evaluated for movement disorders, data from 50 cases show.
Abnormal movements often occur as complications from critical illness, and neurologic consultation can determine whether patients have experienced a seizure or stroke. However, restriction of bedside assessment in the wake of the COVID-19 pandemic increases the risk that abnormal movements will be missed, Jeffrey R. Clark and Eric M. Liotta, MD, of Northwestern University, Chicago, and colleagues wrote.
“Given the limited reports of abnormal movements in hospitalized COVID-19 patients and increased recognition of neurologic manifestations of COVID-19, we sought to examine the frequency and etiology of this finding as an indication of neurologic consultation,” they said.
In a study published in the Journal of the Neurological Sciences, the researchers reviewed data from the first 50 consecutive patients with COVID-19 symptoms who were hospitalized at a single center and underwent neurologic consultation between March 17, 2020, and May 18, 2020.
Overall, 11 patients (22.0%) of patients experienced abnormal movement, and all were admitted to the ICU within 7 days of meeting criteria for severe COVID-19. These patients included nine men and two women with an age range of 36-78 years. The most common comorbidities were obesity, hypertension, diabetes, chronic kidney disease, and coronary artery disease.
Myoclonus (generalized and focal) was the most common abnormal movement, and present in 6 of the 11 patients. Three cases were attributed to high-intensity sedation, and three to toxic-metabolic disturbances. In two patients, abnormal movements were attributed to focal seizures in the setting of encephalopathy, with focal facial twitching. An additional two patients experienced tremors; one showed an acute subdural hemorrhage on CT imaging. The second patient showed no sign of stroke or other abnormality on MRI and the tremor improved during the hospital stay. One patient who experienced abnormal high-amplitude nonrhythmic movements of the lower extremities was diagnosed with serotonin syndrome that resolved after discontinuing high-dose fentanyl.
The study findings were limited by several factors, including the small study population and limited availability of MRI, the researchers noted. Assessing severe COVID-19 cases in the ICU setting presents a challenge because of limited patient participation and the potentially confounding effects of sedation and mechanical ventilation.
However, the researchers said.
“A heightened awareness of abnormal eye movements, or subtle facial tremoring, may be the first steps in recognizing potentially dangerous neurologic manifestations,” and clinicians caring for patients with severe COVID-19 should be able to recognize abnormal movements and seek neurologic consultation when indicated, they emphasized.
The study was supported in part by grants to coauthors Nicholas J. Reish, MD, and Dr. Liotta from the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF THE NEUROLOGICAL SCIENCES
Pneumonia risk soars in heart failure patients, especially HFpEF
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
Patients with heart failure get pneumonia at a rate almost three times greater than expected and, once they do get pneumonia, have about a fourfold greater risk of death, investigators for a retrospective analysis of 13,000 patients from two landmark randomized HF trials have found.
The investigators also found that HF patients with preserved ejection fraction (HFpEF) are at the highest risk of developing pneumonia. The findings underscore the importance of patients with HF getting a pneumonia vaccination, they found.
The analysis showed that 6.3% of patients in the PARADIGM-HF trial and 10.6% of those in the PARAGON-HF trial developed pneumonia, reported the study authors, led by John J.V. McMurray, MD, of the British Heart Foundation Cardiovascular Research Center at the University of Glasgow in Scotland (J Am Coll Cardiol. 2021;77:1961-73).
“The main reason for doing this study was the fact that many heart failure patients are not vaccinated, as they should be, against pneumonia – both pneumococcus and influenza vaccination,” Dr. McMurray said in an interview. “We wanted to document the frequency and consequences of pneumonia in patients with heart failure to help highlight this deficiency in care.”
Dr. McMurray said he believes this is the first study to document the incidence of pneumonia and pneumonia-related outcomes according to the two major ejection fraction phenotypes.
PARADIGM-HF and PARAGON-HF
The post hoc analysis consisted of 8,399 patients with HF with reduced ejection fraction (HFrEF) in PARADIGM-HF (Eur J Heart Fail. 2013 Sep;15[9]:1062-73) and 4,796 patients with HFpEF in PARAGON-HF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). The analysis focused on the 528 and 510 patients in each study, respectively, who developed pneumonia. Those rates translated to an incidence rate of 29 per 1,000 patient-years (95% confidence interval, 27-31) in PARADIGM-HF and 39 per 1,000 patient-years (95% CI, 36-42) in PARAGON-HF.
After pneumonia, the risk of death in patients increased substantially. In PARADIGM-HF, the adjusted hazard ratio for the risk of death from any cause after pneumonia was 4.34 (95% CI, 3.73-5.05). In PARAGON-HF, it was 3.76 (95% CI, 3.09-4.58). HF patients who contracted pneumonia also tended to have HF longer than their counterparts who didn’t develop pneumonia, but the frequency of previous hospitalization for HF didn’t vary between the pneumonia and no-pneumonia groups.
Patients who developed pneumonia tended to be older (average age of 66.9 years vs. 64.6 years, P < .001) and male (83.9% vs. 77.8%, P < .001). The mean age of patients in PARADIGM-HF was almost a decade younger than those in PARAGON-HF, 64 vs. 73 years.
Pneumonia patients also had worse Kansas City Cardiomyopathy Questionnaire scores (76 vs. 80 on average), but no difference in New York Heart Association functional class. “In general, patients who developed pneumonia had more symptoms and signs and HF than those who did not develop pneumonia,” Dr. McMurray and colleagues wrote.
Pneumonia patients also had higher rates of chronic obstructive pulmonary disease (26% vs. 12%), diabetes (43% vs. 34%), and atrial fibrillation (46% vs. 36%).
Another reason for conducting the study, Dr. McMurray said, “was the prior findings in patients with coronary disease and acute myocardial infarction that the risk associated with an episode of pneumonia [e.g., in subsequent vascular events and deaths] persisted long after the acute event. We wanted to see if this was also the case for heart failure, and indeed it was.”
For example, the adjusted HR for cardiovascular death or hospitalization in the first month following an episode of pneumonia was 9.48 (range of 6.85-13.12, P < .001), leveling off to 1.59 after 3 months or more.
Vaccination crucial in HF patients
Dr. McMurray noted that this study emphasizes the importance of pneumonia vaccination for patients with HF. “Given that we have so few treatments to offer patients with HFpEF, this makes the potential value of vaccination in these patients all the greater,” he said.
The COVID-19 pandemic, Dr. McMurray said, is a “good reminder of the dangers of a respiratory infection and the importance of vaccination in these patients. COVID-19 has interesting parallels in being a systemic disease and one with postacute, persisting effects.”
The persistent risk for adverse cardiovascular events 3 months and later after pneumonia is a novel finding of the study, wrote Donna Mancini, MD, and Gregory Gibson, MD, in an invited commentary (J Am Coll Cardiol. 2021;77:1974-6). Both are with the Icahn School of Medicine at Mt. Sinai in New York. The post hoc study also “serves as an important reminder” of pneumonia risk in patients with HF, especially during the pandemic, they wrote.
“Although vaccination alone appears unlikely to be a panacea, it is a readily accessible tool for mitigating disease severity and improving outcomes,” Dr. Mancini and Dr. Gibson wrote. “After all, an ounce of prevention is worth a pound of cure.”
Novartis provided funding for the PARADIGM-HF and PARAGON-HF trials, and Dr. McMurray and coauthors disclosed financial relationships with Novartis. Dr. Mancini and Dr. Gibson have no relevant financial relationships to disclose.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY







