User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


The febrile infant: New AAP guidance for the first 2 months of life
Sixteen years in the making, the American Academy of Pediatrics just released a new clinical practice guideline (CPG), “Evaluation and Management of Well-Appearing Febrile Infants 8-60 Days Old”. The recommendations were derived from interpretations of sequential studies in young, febrile, but well-appearing infants that covered invasive bacterial infection (IBI) incidence, diagnostic modalities, and treatment during the first 2 months of life, further refining approaches to evaluation and empirical treatment.
Pediatricians have long had solid information to help assess the risk for IBI among febrile infants aged 0-3 months, but there has been an ongoing desire to further refine the suggested evaluation of these very young infants. A study of febrile infants from the Pediatric Research in Office Settings network along with subsequent evidence has identified the first 3 weeks of life as the period of highest risk for IBI, with risk declining in a graded fashion aged between 22 and 56 days.
Critical caveats
First, some caveats. Infants 0-7 days are not addressed in the CPG, and all should be treated as high risk and receive full IBI evaluation according to newborn protocols. Second, the recommendations apply only to “well-appearing” infants. Any ill-appearing infant should be treated as high risk and receive full IBI evaluation and begun on empirical antimicrobials. Third, even though the CPG deals with infants as young as 8-21 days old, the recommendations are to treat all infants in this age group as high risk, even if well-appearing, and complete full IBI evaluation and empirical therapy while awaiting results. Fourth, these guidelines apply only to infants born at 37 weeks’ gestation or more. Finally, the new CPG action statements are meant to be recommendations rather than a standard of medical care, leaving some leeway for clinician interpretation of individual patient scenarios. Where appropriate, parents’ values and preferences should be incorporated as part of shared decision-making.
The CPG divides young, febrile infants into three cohorts based on age:
- 8-21 days old
- 22-28 days old
- 29-60 days old
Age 8-21 days
For well-appearing febrile infants 8-21 days old, the CPG recommends a complete IBI evaluation that includes urine, blood, and cerebrospinal fluid (CSF) for culture, approaching all infants in this cohort as high risk. Inflammatory markers may be obtained, but the evidence is not comprehensive enough to evaluate their role in decision-making for this age group. A two-step urine evaluation method (urine analysis followed by culture if the urine analysis looks concerning) is not recommended for infants aged 8-21 days. Urine samples for culture from these young infants should be obtained by catheterization or suprapubic aspiration.
The CPG recommends drawing blood cultures and CSF by lumbar puncture from this cohort. These infants should be admitted to the hospital, treated empirically with antimicrobials, and actively monitored. However, if the cultures are negative at 24-36 hours, the clinician should discontinue antimicrobials and discharge the infant if there is no other reason for continued hospitalization.
Age 22-28 days
Well-appearing, febrile infants 22-28 days old are in an intermediate-risk zone. The recommendation for infants in this cohort is to obtain a urine specimen by catheterization or suprapubic aspiration for both urine analysis and culture. Clinicians may consider obtaining urine samples for analysis noninvasively (e.g., urine bag) in this cohort, but this is not the preferred method.
Blood culture should be obtained from all infants in this group. Inflammatory markers can help clinicians identify infants at greater risk for IBI, including meningitis. Previous data suggested that inflammatory markers such as serum white blood cell counts greater than 11,000/mcL, a serum absolute neutrophil count of greater than 4,000/mcL, and elevated C-reactive protein and procalcitonin levels could help providers identify febrile infants with true IBI. A 2008 study demonstrated that procalcitonin had the best receiver operating characteristic curve in regard to predicting IBI in young febrile infants. Other research backed up that finding and identified cutoff values for procalcitonin levels greater than 1.0 ng/mL. The CPG recommends considering a procalcitonin value of 0.5 ng/mL or higher as positive, indicating that the infant is at greater risk for IBI and potentially should undergo an expanded IBI workup. Therefore, in infants aged 22-28 days, inflammatory markers can play a role in deciding whether to perform a lumbar puncture.
Many more nuanced recommendations for whether to and how to empirically treat with antimicrobials in this cohort can be found in the CPG, including whether to manage in the hospital or at home. Treatment recommendations vary greatly for this cohort on the basis of the tests obtained and whether tests were positive or negative at the initial evaluation.
Age 29-60 days
The CPG will be most helpful when clinicians are faced with well-appearing, febrile infants in the 29- to 60-day age group. As with the other groups, a urine evaluation is recommended; however, the CPG suggests that the two-step approach – obtaining a urine analysis by a noninvasive method and only obtaining culture if the urine analysis is positive – is reasonable. This means that a bag or free-flowing urine specimen would be appropriate for urinalysis, followed by catheterization/suprapubic aspiration if a culture is necessary. This would save approximately 90% of infants from invasive urine collection. Regardless, only catheter or suprapubic specimens are appropriate for urine culture.
The CPG also recommends that clinicians obtain blood culture on all of these infants. Inflammatory markers should be assessed in this cohort because avoiding lumbar puncture for CSF culture would be appropriate in this cohort if the inflammatory markers are negative. If CSF is obtained in this age cohort, enterovirus testing should be added to the testing regimen. Again, for any infant considered at higher risk for IBI on the basis of screening tests, the CPG recommends a 24- to 36-hour rule-out period with empirical antimicrobial treatment and active monitoring in the hospital.
Summary
The recommended approach for febrile infants 8-21 days old is relatively aggressive, with urine, blood, and CSF evaluation for IBI. Clinicians gain some leeway for infants age 22-28 days, but the guidelines recommend a more flexible approach to evaluating well-appearing, febrile infants age 29-60 days, when a two-step urine evaluation and inflammatory marker assessment can help clinicians and parents have a better discussion about the risk-benefit trade-offs of more aggressive testing and empirical treatment.
The author would like to thank Ken Roberts, MD, for his review and helpful comments on this summary of the CPG highlights. Summary points of the CPG were presented by the writing group at the 2021 Pediatric Academic Societies meeting.
William T. Basco, Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Sixteen years in the making, the American Academy of Pediatrics just released a new clinical practice guideline (CPG), “Evaluation and Management of Well-Appearing Febrile Infants 8-60 Days Old”. The recommendations were derived from interpretations of sequential studies in young, febrile, but well-appearing infants that covered invasive bacterial infection (IBI) incidence, diagnostic modalities, and treatment during the first 2 months of life, further refining approaches to evaluation and empirical treatment.
Pediatricians have long had solid information to help assess the risk for IBI among febrile infants aged 0-3 months, but there has been an ongoing desire to further refine the suggested evaluation of these very young infants. A study of febrile infants from the Pediatric Research in Office Settings network along with subsequent evidence has identified the first 3 weeks of life as the period of highest risk for IBI, with risk declining in a graded fashion aged between 22 and 56 days.
Critical caveats
First, some caveats. Infants 0-7 days are not addressed in the CPG, and all should be treated as high risk and receive full IBI evaluation according to newborn protocols. Second, the recommendations apply only to “well-appearing” infants. Any ill-appearing infant should be treated as high risk and receive full IBI evaluation and begun on empirical antimicrobials. Third, even though the CPG deals with infants as young as 8-21 days old, the recommendations are to treat all infants in this age group as high risk, even if well-appearing, and complete full IBI evaluation and empirical therapy while awaiting results. Fourth, these guidelines apply only to infants born at 37 weeks’ gestation or more. Finally, the new CPG action statements are meant to be recommendations rather than a standard of medical care, leaving some leeway for clinician interpretation of individual patient scenarios. Where appropriate, parents’ values and preferences should be incorporated as part of shared decision-making.
The CPG divides young, febrile infants into three cohorts based on age:
- 8-21 days old
- 22-28 days old
- 29-60 days old
Age 8-21 days
For well-appearing febrile infants 8-21 days old, the CPG recommends a complete IBI evaluation that includes urine, blood, and cerebrospinal fluid (CSF) for culture, approaching all infants in this cohort as high risk. Inflammatory markers may be obtained, but the evidence is not comprehensive enough to evaluate their role in decision-making for this age group. A two-step urine evaluation method (urine analysis followed by culture if the urine analysis looks concerning) is not recommended for infants aged 8-21 days. Urine samples for culture from these young infants should be obtained by catheterization or suprapubic aspiration.
The CPG recommends drawing blood cultures and CSF by lumbar puncture from this cohort. These infants should be admitted to the hospital, treated empirically with antimicrobials, and actively monitored. However, if the cultures are negative at 24-36 hours, the clinician should discontinue antimicrobials and discharge the infant if there is no other reason for continued hospitalization.
Age 22-28 days
Well-appearing, febrile infants 22-28 days old are in an intermediate-risk zone. The recommendation for infants in this cohort is to obtain a urine specimen by catheterization or suprapubic aspiration for both urine analysis and culture. Clinicians may consider obtaining urine samples for analysis noninvasively (e.g., urine bag) in this cohort, but this is not the preferred method.
Blood culture should be obtained from all infants in this group. Inflammatory markers can help clinicians identify infants at greater risk for IBI, including meningitis. Previous data suggested that inflammatory markers such as serum white blood cell counts greater than 11,000/mcL, a serum absolute neutrophil count of greater than 4,000/mcL, and elevated C-reactive protein and procalcitonin levels could help providers identify febrile infants with true IBI. A 2008 study demonstrated that procalcitonin had the best receiver operating characteristic curve in regard to predicting IBI in young febrile infants. Other research backed up that finding and identified cutoff values for procalcitonin levels greater than 1.0 ng/mL. The CPG recommends considering a procalcitonin value of 0.5 ng/mL or higher as positive, indicating that the infant is at greater risk for IBI and potentially should undergo an expanded IBI workup. Therefore, in infants aged 22-28 days, inflammatory markers can play a role in deciding whether to perform a lumbar puncture.
Many more nuanced recommendations for whether to and how to empirically treat with antimicrobials in this cohort can be found in the CPG, including whether to manage in the hospital or at home. Treatment recommendations vary greatly for this cohort on the basis of the tests obtained and whether tests were positive or negative at the initial evaluation.
Age 29-60 days
The CPG will be most helpful when clinicians are faced with well-appearing, febrile infants in the 29- to 60-day age group. As with the other groups, a urine evaluation is recommended; however, the CPG suggests that the two-step approach – obtaining a urine analysis by a noninvasive method and only obtaining culture if the urine analysis is positive – is reasonable. This means that a bag or free-flowing urine specimen would be appropriate for urinalysis, followed by catheterization/suprapubic aspiration if a culture is necessary. This would save approximately 90% of infants from invasive urine collection. Regardless, only catheter or suprapubic specimens are appropriate for urine culture.
The CPG also recommends that clinicians obtain blood culture on all of these infants. Inflammatory markers should be assessed in this cohort because avoiding lumbar puncture for CSF culture would be appropriate in this cohort if the inflammatory markers are negative. If CSF is obtained in this age cohort, enterovirus testing should be added to the testing regimen. Again, for any infant considered at higher risk for IBI on the basis of screening tests, the CPG recommends a 24- to 36-hour rule-out period with empirical antimicrobial treatment and active monitoring in the hospital.
Summary
The recommended approach for febrile infants 8-21 days old is relatively aggressive, with urine, blood, and CSF evaluation for IBI. Clinicians gain some leeway for infants age 22-28 days, but the guidelines recommend a more flexible approach to evaluating well-appearing, febrile infants age 29-60 days, when a two-step urine evaluation and inflammatory marker assessment can help clinicians and parents have a better discussion about the risk-benefit trade-offs of more aggressive testing and empirical treatment.
The author would like to thank Ken Roberts, MD, for his review and helpful comments on this summary of the CPG highlights. Summary points of the CPG were presented by the writing group at the 2021 Pediatric Academic Societies meeting.
William T. Basco, Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Sixteen years in the making, the American Academy of Pediatrics just released a new clinical practice guideline (CPG), “Evaluation and Management of Well-Appearing Febrile Infants 8-60 Days Old”. The recommendations were derived from interpretations of sequential studies in young, febrile, but well-appearing infants that covered invasive bacterial infection (IBI) incidence, diagnostic modalities, and treatment during the first 2 months of life, further refining approaches to evaluation and empirical treatment.
Pediatricians have long had solid information to help assess the risk for IBI among febrile infants aged 0-3 months, but there has been an ongoing desire to further refine the suggested evaluation of these very young infants. A study of febrile infants from the Pediatric Research in Office Settings network along with subsequent evidence has identified the first 3 weeks of life as the period of highest risk for IBI, with risk declining in a graded fashion aged between 22 and 56 days.
Critical caveats
First, some caveats. Infants 0-7 days are not addressed in the CPG, and all should be treated as high risk and receive full IBI evaluation according to newborn protocols. Second, the recommendations apply only to “well-appearing” infants. Any ill-appearing infant should be treated as high risk and receive full IBI evaluation and begun on empirical antimicrobials. Third, even though the CPG deals with infants as young as 8-21 days old, the recommendations are to treat all infants in this age group as high risk, even if well-appearing, and complete full IBI evaluation and empirical therapy while awaiting results. Fourth, these guidelines apply only to infants born at 37 weeks’ gestation or more. Finally, the new CPG action statements are meant to be recommendations rather than a standard of medical care, leaving some leeway for clinician interpretation of individual patient scenarios. Where appropriate, parents’ values and preferences should be incorporated as part of shared decision-making.
The CPG divides young, febrile infants into three cohorts based on age:
- 8-21 days old
- 22-28 days old
- 29-60 days old
Age 8-21 days
For well-appearing febrile infants 8-21 days old, the CPG recommends a complete IBI evaluation that includes urine, blood, and cerebrospinal fluid (CSF) for culture, approaching all infants in this cohort as high risk. Inflammatory markers may be obtained, but the evidence is not comprehensive enough to evaluate their role in decision-making for this age group. A two-step urine evaluation method (urine analysis followed by culture if the urine analysis looks concerning) is not recommended for infants aged 8-21 days. Urine samples for culture from these young infants should be obtained by catheterization or suprapubic aspiration.
The CPG recommends drawing blood cultures and CSF by lumbar puncture from this cohort. These infants should be admitted to the hospital, treated empirically with antimicrobials, and actively monitored. However, if the cultures are negative at 24-36 hours, the clinician should discontinue antimicrobials and discharge the infant if there is no other reason for continued hospitalization.
Age 22-28 days
Well-appearing, febrile infants 22-28 days old are in an intermediate-risk zone. The recommendation for infants in this cohort is to obtain a urine specimen by catheterization or suprapubic aspiration for both urine analysis and culture. Clinicians may consider obtaining urine samples for analysis noninvasively (e.g., urine bag) in this cohort, but this is not the preferred method.
Blood culture should be obtained from all infants in this group. Inflammatory markers can help clinicians identify infants at greater risk for IBI, including meningitis. Previous data suggested that inflammatory markers such as serum white blood cell counts greater than 11,000/mcL, a serum absolute neutrophil count of greater than 4,000/mcL, and elevated C-reactive protein and procalcitonin levels could help providers identify febrile infants with true IBI. A 2008 study demonstrated that procalcitonin had the best receiver operating characteristic curve in regard to predicting IBI in young febrile infants. Other research backed up that finding and identified cutoff values for procalcitonin levels greater than 1.0 ng/mL. The CPG recommends considering a procalcitonin value of 0.5 ng/mL or higher as positive, indicating that the infant is at greater risk for IBI and potentially should undergo an expanded IBI workup. Therefore, in infants aged 22-28 days, inflammatory markers can play a role in deciding whether to perform a lumbar puncture.
Many more nuanced recommendations for whether to and how to empirically treat with antimicrobials in this cohort can be found in the CPG, including whether to manage in the hospital or at home. Treatment recommendations vary greatly for this cohort on the basis of the tests obtained and whether tests were positive or negative at the initial evaluation.
Age 29-60 days
The CPG will be most helpful when clinicians are faced with well-appearing, febrile infants in the 29- to 60-day age group. As with the other groups, a urine evaluation is recommended; however, the CPG suggests that the two-step approach – obtaining a urine analysis by a noninvasive method and only obtaining culture if the urine analysis is positive – is reasonable. This means that a bag or free-flowing urine specimen would be appropriate for urinalysis, followed by catheterization/suprapubic aspiration if a culture is necessary. This would save approximately 90% of infants from invasive urine collection. Regardless, only catheter or suprapubic specimens are appropriate for urine culture.
The CPG also recommends that clinicians obtain blood culture on all of these infants. Inflammatory markers should be assessed in this cohort because avoiding lumbar puncture for CSF culture would be appropriate in this cohort if the inflammatory markers are negative. If CSF is obtained in this age cohort, enterovirus testing should be added to the testing regimen. Again, for any infant considered at higher risk for IBI on the basis of screening tests, the CPG recommends a 24- to 36-hour rule-out period with empirical antimicrobial treatment and active monitoring in the hospital.
Summary
The recommended approach for febrile infants 8-21 days old is relatively aggressive, with urine, blood, and CSF evaluation for IBI. Clinicians gain some leeway for infants age 22-28 days, but the guidelines recommend a more flexible approach to evaluating well-appearing, febrile infants age 29-60 days, when a two-step urine evaluation and inflammatory marker assessment can help clinicians and parents have a better discussion about the risk-benefit trade-offs of more aggressive testing and empirical treatment.
The author would like to thank Ken Roberts, MD, for his review and helpful comments on this summary of the CPG highlights. Summary points of the CPG were presented by the writing group at the 2021 Pediatric Academic Societies meeting.
William T. Basco, Jr, MD, MS, is a professor of pediatrics at the Medical University of South Carolina, Charleston, and director of the division of general pediatrics. He is an active health services researcher and has published more than 60 manuscripts in the peer-reviewed literature.
A version of this article first appeared on Medscape.com.
Treatment of opioid use disorder with buprenorphine and methadone effective but underutilized
Background: Opioid use disorder (OUD) is a chronic disease with a high health care and societal burden from overdose and complications requiring hospitalization. Though clinical trials demonstrate effectiveness of methadone and buprenorphine, most patients do not have access to these medications.
Study design: Retrospective comparative effectiveness study.
Setting: Nationwide claims database of commercial and Medicare Advantage Enrollees.
Synopsis: A total of 40,885 individuals aged 16 years or older with OUD were studied in an intent-to-treat analysis of six unique treatment pathways. Though used in just 12.5% of patients, only treatment with buprenorphine or methadone was protective against overdose at 3 and 12 months, compared with no treatment. Additionally, these medications and nonintensive behavioral health counseling were associated with lower incidence of acute care episodes from complications of opioid use. Notably, those treated with buprenorphine or methadone for more than 6 months received the greatest benefit. With use of only health care encounters, the results may underestimate incidence of complications of ongoing opioid misuse.
Bottom line: Buprenorphine and methadone for OUD were associated with reduced overdose and opioid-related morbidity, compared with opioid antagonist therapy, inpatient treatment, or intensive outpatient behavioral interventions and should be considered a first-line treatment.
Citation: Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
Dr. Inofuentes is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Opioid use disorder (OUD) is a chronic disease with a high health care and societal burden from overdose and complications requiring hospitalization. Though clinical trials demonstrate effectiveness of methadone and buprenorphine, most patients do not have access to these medications.
Study design: Retrospective comparative effectiveness study.
Setting: Nationwide claims database of commercial and Medicare Advantage Enrollees.
Synopsis: A total of 40,885 individuals aged 16 years or older with OUD were studied in an intent-to-treat analysis of six unique treatment pathways. Though used in just 12.5% of patients, only treatment with buprenorphine or methadone was protective against overdose at 3 and 12 months, compared with no treatment. Additionally, these medications and nonintensive behavioral health counseling were associated with lower incidence of acute care episodes from complications of opioid use. Notably, those treated with buprenorphine or methadone for more than 6 months received the greatest benefit. With use of only health care encounters, the results may underestimate incidence of complications of ongoing opioid misuse.
Bottom line: Buprenorphine and methadone for OUD were associated with reduced overdose and opioid-related morbidity, compared with opioid antagonist therapy, inpatient treatment, or intensive outpatient behavioral interventions and should be considered a first-line treatment.
Citation: Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
Dr. Inofuentes is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Opioid use disorder (OUD) is a chronic disease with a high health care and societal burden from overdose and complications requiring hospitalization. Though clinical trials demonstrate effectiveness of methadone and buprenorphine, most patients do not have access to these medications.
Study design: Retrospective comparative effectiveness study.
Setting: Nationwide claims database of commercial and Medicare Advantage Enrollees.
Synopsis: A total of 40,885 individuals aged 16 years or older with OUD were studied in an intent-to-treat analysis of six unique treatment pathways. Though used in just 12.5% of patients, only treatment with buprenorphine or methadone was protective against overdose at 3 and 12 months, compared with no treatment. Additionally, these medications and nonintensive behavioral health counseling were associated with lower incidence of acute care episodes from complications of opioid use. Notably, those treated with buprenorphine or methadone for more than 6 months received the greatest benefit. With use of only health care encounters, the results may underestimate incidence of complications of ongoing opioid misuse.
Bottom line: Buprenorphine and methadone for OUD were associated with reduced overdose and opioid-related morbidity, compared with opioid antagonist therapy, inpatient treatment, or intensive outpatient behavioral interventions and should be considered a first-line treatment.
Citation: Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
Dr. Inofuentes is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Large remdesivir study finds no COVID-19 survival benefit
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A lack of consensus in the evidence regarding the antiviral remdesivir (Veklury) to treat people with COVID-19 continues, leaving clinicians without clear direction on one of the few treatments for the illness approved under U.S. Food and Drug Administration emergency use authorization.
The latest research comes from Michael Ohl, MD, MSPH, and colleagues, who studied a large group of VA patients hospitalized with COVID-19. Compared with a matched group of veterans who did not receive the antiviral, remdesivir did not significantly improve survival.
The percentages were close: 12.2% of patients in the remdesivir group died within 30 days compared with 10.6% of those in the control group.
At the same time, the retrospective cohort study showed remdesivir was associated with more days in the hospital.
“There is still uncertainty about the role of remdesivir in treatment for people hospitalized with COVID-19,” Dr. Ohl told this news organization.
“It is reasonable to follow the CDC and Infectious Diseases Society of America guidelines for remdesivir use, “but clinicians should avoid admitting people or keeping people in the hospital solely to receive remdesivir if they do not meet other criteria for hospitalization,” said Dr. Ohl, lead author and an infectious disease specialist at the Center for Access & Delivery Research and Evaluation, Iowa City Veterans Affairs (VA) Health Care System.
The study was published online July 15 in JAMA Network Open.
Sticking with the official protocol?
The longer a hospital stays associated with remdesivir, a median 6 days versus 3 days, could be a result of treating people for 5 or 10 days with the antiviral agent. In other words, it is “possible that clinicians were not discharging patients who otherwise met the criteria for hospital discharge until the remdesivir course was completed,” Dr. Ohl and colleagues note.
Not doing so, they add, could have resulted in “increased used of scarce hospital beds during the pandemic.”
“The recommended remdesivir treatment course is a somewhat arbitrary 5 or 10 days depending on illness severity, and remdesivir is currently available only as an intravenous formulation for use in health care settings,” they add.
This is the “most likely explanation,” notes Gio J. Baracco, MD, in an invited commentary accompanying the study.
At the time of the study, use of remdesivir also required patient consent, close adverse event monitoring, and ongoing testing, Dr. Baracco notes.
He added that an option to discharge patients earlier if they responded to treatment might have been lost in translation from clinical trial protocol to real-world use in the VA system.
While a large clinical trial protocol called for the remdesivir infusions to be stopped early if the patient met the primary outcome and was ready to be discharged, “this detail was not adequately translated to the clinicians treating these patients,” added Dr. Baracco, who’s with the Division of Infectious Diseases at the University of Miami Miller School of Medicine and the Miami VA Healthcare System.
Conflicting evidence
Another large study, the World Health Organization Solidarity Trial, found remdesivir was not associated with shorter hospital stays or improved survival compared with standard of care. For this reason, the WHO recommends against use of remdesivir.
In contrast, the double-blind, randomized Adaptive COVID-19 Treatment Trial (ACTT-1) linked remdesivir treatment to shorter stays in the hospital, a median 10 days versus 15 days in a placebo group.
The FDA included the 2020 ACTT-1 in its consideration for remdesivir emergency use authorization. The FDA issued the EUA in May 2020, followed by full approval as the first treatment indicated for COVID-19 in October.
ACTT-1 lead author John H. Beigel, MD, and colleagues also looked at the death rates for remdesivir versus placebo.
By day 15, the proportion of people who died was 6.7% in the remdesivir group versus 11%. By day 29, the rate was 11.4% among those who received the antiviral versus 15.2% among those who did not.
When asked why the VA and ACTT-1 studies yielded different results, Dr. Beigel cited two reasons. The timing was different, with the VA study starting after the remdesivir EUA was issued, and ACTT-1 findings were announced.
“So at that point, clinicians understood those populations most likely to benefit from remdesivir. The use of remdesivir likely did not occur at random; it was likely to be more commonly used in those who were sicker or at higher risk for poor outcomes,” said Dr. Beigel, associate director for clinical research in the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases (NIAID).
In addition, the studies evaluated very different populations, he said. The differences in median duration of hospitalization between the trials reflects this, Dr. Beigel added.
Furthermore, when asked if he thinks the new evidence should affect clinical use of remdesivir, Dr. Beigel replied, “No. Observational studies, even with adjustments such as propensity score matching, are not equivalent levels of proof compared to randomized trials.”
Study details
Dr. Ohl and colleagues identified patients admitted to one of 123 VA hospitals for the first time for COVID-19 from May 1 to Oct. 8, 2020. Each had a PCR-confirmed SARS-CoV-2 infection. The researchers then compared 1,172 patients receiving remdesivir to another 1,172 patients not receiving the agent.
Those receiving remdesivir were more likely to be older, White, have chronic obstructive pulmonary disease and have more severe COVID-19. A total 94% of the remdesivir group were men.
“Over 90% of the people included in VA study were men, mostly over the age of 60,” Dr. Ohl said when asked how generalizable the findings would be to a non-VA population.
“There is no obvious biological reason that remdesivir should have different effects in men and women, but we should be cautious about extrapolating study findings to women and younger individuals,” he added.
Limitations of the study include its observational design, which makes unadjusted confounding based on illness severity a possibility. In addition, the investigators were unable to identify specific subgroups that might benefit from remdesivir treatment.
The data did suggest that remdesivir was more effective earlier in the course of disease when patients required supplemental oxygen and before need for mechanical ventilation.
Dr. Baracco pointed out the contradictory findings in his commentary: “The real-life application of a drug promising to hasten discharge from the hospital as its primary beneficial outcome must include an assessment of how easy it is to do so and make it clear that once a patient reaches that point, they can discontinue the drug.”
“The paradoxical findings in the study by Dr. Ohl et al. compared with the study used for its authorization illustrate this point very clearly,” he adds.
Dr. Ohl reported receiving grants from Veterans Affairs Health Services Research and Development during the conduct of the study and consulting for Gilead Pharmaceuticals outside the submitted work. Dr. Baracco reported receiving salary support from the U.S. Department of Veterans Affairs. Dr. Beigel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DOACs linked to lower mortality than vitamin K antagonist: 3-year TAVR registry
Following a transcatheter aortic valve replacement (TAVR), direct oral anticoagulants (DOACs) are preferable to vitamin K antagonists (VKAs) in patients who are candidates for oral anticoagulants, according to data drawn from a large multicenter French TAVR registry.
When oral anticoagulation is appropriate following TAVR, such as in patients with atrial fibrillation, “DOACs are associated with improved survival and lower incidence of bleeding, compared to VKA,” reported a team of investigators led by Martine Gilard, MD, PhD, director of interventional cardiology, Brest (France) University Hospital Center.
The comparison, using propensity score matching, is not definitive, but it might be the best data currently available to support DOACs over VKA until a randomized trial is completed, according to Dr. Gilard, senior author of the newly published study.
Asked in an interview if DOACs should now be used preferentially after TAVR when patients are indicated for oral anticoagulation, Dr. Gilard replied, “My answer is yes.”
Of more than 24,000 TAVR patients in the French TAVI and FRANCE2 multicenter registries, which are linked to the French single-payer claims database (SNDS), 8,962 (36.4%) received an oral anticoagulant following their procedure. Of these, 2,180 (24.3%) received a DOAC and the remaining received VKA.
By linking data from the registries to the SNDS, outcomes were tracked. Propensity matching was employed to control for differences in baseline characteristics, including age, body mass index, functional class, diabetes, comorbidities, and past medical history.
On the primary endpoint of mortality at the end of 3 years, the rates were 35.6% and 31.2% for VKA and DOACs, respectively. This translated in a 37% greater hazard ratio for death among those treated with VKA (P < .005).
The rate of major bleeding, a secondary endpoint, was also higher (12.3% vs. 8.4%) and significantly different (HR, 1.65; P < .005) for VKA versus DOACs. The rates of ischemic stroke, acute coronary syndrome, and hemorrhagic stroke were all numerically higher in patients treated with VKA than DOACs, although none of these differences reached statistical significance.
Residual confounding cannot be discounted
“The large number of events allowed for taking into account a higher number of potential confounders with appropriate statistical power,” according to the authors. However, they acknowledged that residual confounding cannot be eliminated by propensity matching and conceded that prospective data are needed for a definitive comparison.
In an accompanying editorial, Daniele Giacoppo, MD, a cardiologist at Alto Vicentino Hospital, Santorso, Italy, enlarged on this point . In addition to the inherent limitations of retrospective data, he also noted that data from other studies addressing the same question have been inconsistent.
Of these studies, he pointed to the ATLANTIS trial, presented 2 months ago at the annual meeting of the American College of Cardiology. This study failed to show an advantage for the DOAC apixaban over VKA in TAVR patients for the primary composite outcome of time to death, myocardial infarction, systemic emboli, valve thrombosis, or major bleeding. Although this study was not limited to patients with an indication for oral anticoagulants, Dr. Giacoppo pointed out that there was no advantage, even among the subgroup of patients who did have an indication.
Data are supportive in absence of trial results
In general, Dr. Giacoppo agreed that the French registry are generally supportive of DOACs over VKA in TAVR patients with an indication for oral anticoagulation, but he cautioned that blanket statements are difficult. He anticipates better information from a randomized trial called ENVISAGE-TAVI AF, which is comparing edoxaban with VKA following TAVR in atrial fibrillation patients who have an indication for oral anticoagulation, but he indicated that some individualization of choice will be needed among those high or low relative risks of thrombotic events or bleeding.
“The concerns related to DOACs after TAVR are most confined to patients without an indication for oral anticoagulation,” Dr. Giacoppo said in an interview. In patients with an indication, “oral anticoagulation alone without antithrombotic therapy significantly reduced the risk of bleeding” in several studies, he added, citing in particular the POPular TAVI trial.
Issues about when to employ – or not employ – both oral anticoagulation and antithrombotic therapy based on such factors as bleeding risk remain unresolved, but “in aggregate, waiting for additional high-quality data, the use of a DOAC in patients with an indication for oral anticoagulation who underwent TAVR seems to be safe,” Dr. Giacoppo said. He thinks that the “higher predictability of DOACS compared to vitamin K antagonists might translate into lower bleeding rates over time in a real-world, unselected population.”
Benefit-to-risk ratio requires attention
A similar concern about balancing risks and benefits of oral anticoagulation in TAVR patients with an indication for oral anticoagulation was emphasized by Ron Waksman, MD, associate director, division of cardiology, MedStar Washington (D.C.) Hospital Center.
“The TAVR population is elderly in general and so are at high risk for bleeding with any additional anticoagulation,” Dr. Waksman said. He cited data that bring into question the utility of using a DOAC in TAVR patients without an additional indication for anticoagulation, but he believes DOACs do make sense in those who were on and had an indication for a DOAC even before TAVR.
Patients who had atrial fibrillation or another indication “should continue to take the DOAC after TAVR. This population can be assumed to have less bleeding risk as they are vetted as safe for DOACs before their TAVR procedure,” he said.
Although mortality was the primary endpoint of the French registry evaluation, it is the bleeding risk that is a dominant concern, according to Romain Didier, MD, PhD, the first author of this study who performed this work in collaboration with Dr. Gilard.
“We really believe that VKA use in real life after TAVR, even with INR monitoring, is associated with a higher risk of bleeding as compared to DOACs,” he said. It is for this reason that “we currently use DOACs as a first choice in patients who require anticoagulant after TAVR.”
Dr. Gilard, Dr. Didier, and Dr. Giacoppo reported no potential conflicts of interest. Dr. Waksman reported financial relationships with Amgen, AstraZeneca, Boston Scientific, Cardioset, Cardiovascular Systems, Chiesi, MedAlliance, Medtronic, and Pi-Cardia.
Following a transcatheter aortic valve replacement (TAVR), direct oral anticoagulants (DOACs) are preferable to vitamin K antagonists (VKAs) in patients who are candidates for oral anticoagulants, according to data drawn from a large multicenter French TAVR registry.
When oral anticoagulation is appropriate following TAVR, such as in patients with atrial fibrillation, “DOACs are associated with improved survival and lower incidence of bleeding, compared to VKA,” reported a team of investigators led by Martine Gilard, MD, PhD, director of interventional cardiology, Brest (France) University Hospital Center.
The comparison, using propensity score matching, is not definitive, but it might be the best data currently available to support DOACs over VKA until a randomized trial is completed, according to Dr. Gilard, senior author of the newly published study.
Asked in an interview if DOACs should now be used preferentially after TAVR when patients are indicated for oral anticoagulation, Dr. Gilard replied, “My answer is yes.”
Of more than 24,000 TAVR patients in the French TAVI and FRANCE2 multicenter registries, which are linked to the French single-payer claims database (SNDS), 8,962 (36.4%) received an oral anticoagulant following their procedure. Of these, 2,180 (24.3%) received a DOAC and the remaining received VKA.
By linking data from the registries to the SNDS, outcomes were tracked. Propensity matching was employed to control for differences in baseline characteristics, including age, body mass index, functional class, diabetes, comorbidities, and past medical history.
On the primary endpoint of mortality at the end of 3 years, the rates were 35.6% and 31.2% for VKA and DOACs, respectively. This translated in a 37% greater hazard ratio for death among those treated with VKA (P < .005).
The rate of major bleeding, a secondary endpoint, was also higher (12.3% vs. 8.4%) and significantly different (HR, 1.65; P < .005) for VKA versus DOACs. The rates of ischemic stroke, acute coronary syndrome, and hemorrhagic stroke were all numerically higher in patients treated with VKA than DOACs, although none of these differences reached statistical significance.
Residual confounding cannot be discounted
“The large number of events allowed for taking into account a higher number of potential confounders with appropriate statistical power,” according to the authors. However, they acknowledged that residual confounding cannot be eliminated by propensity matching and conceded that prospective data are needed for a definitive comparison.
In an accompanying editorial, Daniele Giacoppo, MD, a cardiologist at Alto Vicentino Hospital, Santorso, Italy, enlarged on this point . In addition to the inherent limitations of retrospective data, he also noted that data from other studies addressing the same question have been inconsistent.
Of these studies, he pointed to the ATLANTIS trial, presented 2 months ago at the annual meeting of the American College of Cardiology. This study failed to show an advantage for the DOAC apixaban over VKA in TAVR patients for the primary composite outcome of time to death, myocardial infarction, systemic emboli, valve thrombosis, or major bleeding. Although this study was not limited to patients with an indication for oral anticoagulants, Dr. Giacoppo pointed out that there was no advantage, even among the subgroup of patients who did have an indication.
Data are supportive in absence of trial results
In general, Dr. Giacoppo agreed that the French registry are generally supportive of DOACs over VKA in TAVR patients with an indication for oral anticoagulation, but he cautioned that blanket statements are difficult. He anticipates better information from a randomized trial called ENVISAGE-TAVI AF, which is comparing edoxaban with VKA following TAVR in atrial fibrillation patients who have an indication for oral anticoagulation, but he indicated that some individualization of choice will be needed among those high or low relative risks of thrombotic events or bleeding.
“The concerns related to DOACs after TAVR are most confined to patients without an indication for oral anticoagulation,” Dr. Giacoppo said in an interview. In patients with an indication, “oral anticoagulation alone without antithrombotic therapy significantly reduced the risk of bleeding” in several studies, he added, citing in particular the POPular TAVI trial.
Issues about when to employ – or not employ – both oral anticoagulation and antithrombotic therapy based on such factors as bleeding risk remain unresolved, but “in aggregate, waiting for additional high-quality data, the use of a DOAC in patients with an indication for oral anticoagulation who underwent TAVR seems to be safe,” Dr. Giacoppo said. He thinks that the “higher predictability of DOACS compared to vitamin K antagonists might translate into lower bleeding rates over time in a real-world, unselected population.”
Benefit-to-risk ratio requires attention
A similar concern about balancing risks and benefits of oral anticoagulation in TAVR patients with an indication for oral anticoagulation was emphasized by Ron Waksman, MD, associate director, division of cardiology, MedStar Washington (D.C.) Hospital Center.
“The TAVR population is elderly in general and so are at high risk for bleeding with any additional anticoagulation,” Dr. Waksman said. He cited data that bring into question the utility of using a DOAC in TAVR patients without an additional indication for anticoagulation, but he believes DOACs do make sense in those who were on and had an indication for a DOAC even before TAVR.
Patients who had atrial fibrillation or another indication “should continue to take the DOAC after TAVR. This population can be assumed to have less bleeding risk as they are vetted as safe for DOACs before their TAVR procedure,” he said.
Although mortality was the primary endpoint of the French registry evaluation, it is the bleeding risk that is a dominant concern, according to Romain Didier, MD, PhD, the first author of this study who performed this work in collaboration with Dr. Gilard.
“We really believe that VKA use in real life after TAVR, even with INR monitoring, is associated with a higher risk of bleeding as compared to DOACs,” he said. It is for this reason that “we currently use DOACs as a first choice in patients who require anticoagulant after TAVR.”
Dr. Gilard, Dr. Didier, and Dr. Giacoppo reported no potential conflicts of interest. Dr. Waksman reported financial relationships with Amgen, AstraZeneca, Boston Scientific, Cardioset, Cardiovascular Systems, Chiesi, MedAlliance, Medtronic, and Pi-Cardia.
Following a transcatheter aortic valve replacement (TAVR), direct oral anticoagulants (DOACs) are preferable to vitamin K antagonists (VKAs) in patients who are candidates for oral anticoagulants, according to data drawn from a large multicenter French TAVR registry.
When oral anticoagulation is appropriate following TAVR, such as in patients with atrial fibrillation, “DOACs are associated with improved survival and lower incidence of bleeding, compared to VKA,” reported a team of investigators led by Martine Gilard, MD, PhD, director of interventional cardiology, Brest (France) University Hospital Center.
The comparison, using propensity score matching, is not definitive, but it might be the best data currently available to support DOACs over VKA until a randomized trial is completed, according to Dr. Gilard, senior author of the newly published study.
Asked in an interview if DOACs should now be used preferentially after TAVR when patients are indicated for oral anticoagulation, Dr. Gilard replied, “My answer is yes.”
Of more than 24,000 TAVR patients in the French TAVI and FRANCE2 multicenter registries, which are linked to the French single-payer claims database (SNDS), 8,962 (36.4%) received an oral anticoagulant following their procedure. Of these, 2,180 (24.3%) received a DOAC and the remaining received VKA.
By linking data from the registries to the SNDS, outcomes were tracked. Propensity matching was employed to control for differences in baseline characteristics, including age, body mass index, functional class, diabetes, comorbidities, and past medical history.
On the primary endpoint of mortality at the end of 3 years, the rates were 35.6% and 31.2% for VKA and DOACs, respectively. This translated in a 37% greater hazard ratio for death among those treated with VKA (P < .005).
The rate of major bleeding, a secondary endpoint, was also higher (12.3% vs. 8.4%) and significantly different (HR, 1.65; P < .005) for VKA versus DOACs. The rates of ischemic stroke, acute coronary syndrome, and hemorrhagic stroke were all numerically higher in patients treated with VKA than DOACs, although none of these differences reached statistical significance.
Residual confounding cannot be discounted
“The large number of events allowed for taking into account a higher number of potential confounders with appropriate statistical power,” according to the authors. However, they acknowledged that residual confounding cannot be eliminated by propensity matching and conceded that prospective data are needed for a definitive comparison.
In an accompanying editorial, Daniele Giacoppo, MD, a cardiologist at Alto Vicentino Hospital, Santorso, Italy, enlarged on this point . In addition to the inherent limitations of retrospective data, he also noted that data from other studies addressing the same question have been inconsistent.
Of these studies, he pointed to the ATLANTIS trial, presented 2 months ago at the annual meeting of the American College of Cardiology. This study failed to show an advantage for the DOAC apixaban over VKA in TAVR patients for the primary composite outcome of time to death, myocardial infarction, systemic emboli, valve thrombosis, or major bleeding. Although this study was not limited to patients with an indication for oral anticoagulants, Dr. Giacoppo pointed out that there was no advantage, even among the subgroup of patients who did have an indication.
Data are supportive in absence of trial results
In general, Dr. Giacoppo agreed that the French registry are generally supportive of DOACs over VKA in TAVR patients with an indication for oral anticoagulation, but he cautioned that blanket statements are difficult. He anticipates better information from a randomized trial called ENVISAGE-TAVI AF, which is comparing edoxaban with VKA following TAVR in atrial fibrillation patients who have an indication for oral anticoagulation, but he indicated that some individualization of choice will be needed among those high or low relative risks of thrombotic events or bleeding.
“The concerns related to DOACs after TAVR are most confined to patients without an indication for oral anticoagulation,” Dr. Giacoppo said in an interview. In patients with an indication, “oral anticoagulation alone without antithrombotic therapy significantly reduced the risk of bleeding” in several studies, he added, citing in particular the POPular TAVI trial.
Issues about when to employ – or not employ – both oral anticoagulation and antithrombotic therapy based on such factors as bleeding risk remain unresolved, but “in aggregate, waiting for additional high-quality data, the use of a DOAC in patients with an indication for oral anticoagulation who underwent TAVR seems to be safe,” Dr. Giacoppo said. He thinks that the “higher predictability of DOACS compared to vitamin K antagonists might translate into lower bleeding rates over time in a real-world, unselected population.”
Benefit-to-risk ratio requires attention
A similar concern about balancing risks and benefits of oral anticoagulation in TAVR patients with an indication for oral anticoagulation was emphasized by Ron Waksman, MD, associate director, division of cardiology, MedStar Washington (D.C.) Hospital Center.
“The TAVR population is elderly in general and so are at high risk for bleeding with any additional anticoagulation,” Dr. Waksman said. He cited data that bring into question the utility of using a DOAC in TAVR patients without an additional indication for anticoagulation, but he believes DOACs do make sense in those who were on and had an indication for a DOAC even before TAVR.
Patients who had atrial fibrillation or another indication “should continue to take the DOAC after TAVR. This population can be assumed to have less bleeding risk as they are vetted as safe for DOACs before their TAVR procedure,” he said.
Although mortality was the primary endpoint of the French registry evaluation, it is the bleeding risk that is a dominant concern, according to Romain Didier, MD, PhD, the first author of this study who performed this work in collaboration with Dr. Gilard.
“We really believe that VKA use in real life after TAVR, even with INR monitoring, is associated with a higher risk of bleeding as compared to DOACs,” he said. It is for this reason that “we currently use DOACs as a first choice in patients who require anticoagulant after TAVR.”
Dr. Gilard, Dr. Didier, and Dr. Giacoppo reported no potential conflicts of interest. Dr. Waksman reported financial relationships with Amgen, AstraZeneca, Boston Scientific, Cardioset, Cardiovascular Systems, Chiesi, MedAlliance, Medtronic, and Pi-Cardia.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Legalization of cannabis tied to drop in opioid-related ED visits
State laws permitting recreational marijuana use have not led to an increase in opioid-related emergency department visits, as many had feared.
On the contrary, states that legalize recreational marijuana may see a short-term decrease in opioid-related ED visits in the first 6 months, after which rates may return to prelegalization levels, new research suggests.
Previous research suggests that individuals may reduce the use of opioids when they have an alternative and that cannabis can provide pain relief.
“At the same time, we often hear claims from politicians that we should not legalize cannabis because it may act as a ‘gateway drug’ that leads to use of other drugs,” lead researcher Coleman Drake, PhD, Department of Health Policy and Management, University of Pittsburgh Graduate School of Public Health, told this news organization.
“Our findings indicate that cannabis legalization does not effect any increase in opioid-related ED visits, contradicting the gateway drug explanation,” Dr. Drake said.
The study was published online July 12 in Health Economics.
Significant reduction
So far, 19 states have legalized recreational cannabis, meaning that nearly half of the U.S. population lives in a state that allows recreational cannabis use.
The investigators analyzed data on opioid-related ED visits from 29 states between 2011 and 2017. Four states – California, Maine, Massachusetts, and Nevada – legalized recreational marijuana during the study period; the remaining 25 states did not.
The four states with recreational cannabis laws experienced a 7.6% reduction in opioid-related ED visits for 6 months after the law went into effect in comparison with the states that did not legalize recreational marijuana.
“This isn’t trivial – a decline in opioid-related emergency department visits, even if only for 6 months, is a welcome public health development,” Dr. Drake said in a statement.
Not surprisingly, these effects are driven by men and adults aged 25 to 44 years. “These are populations that are more likely to use cannabis, and the reduction in opioid-related ED visits that we find is concentrated among them,” Dr. Drake told this news organization.
However, the downturn in opioid-related ED visits after making marijuana legal was only temporary.
“
Encouragingly, he said, the data show that opioid-related ED visits don’t increase above baseline after recreational marijuana laws are adopted.
“We conclude that cannabis legalization likely is not a panacea for the opioid epidemic, but there are some helpful effects,” Dr. Drake said in an interview.
The study was supported by the National Institute on Drug Abuse. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws permitting recreational marijuana use have not led to an increase in opioid-related emergency department visits, as many had feared.
On the contrary, states that legalize recreational marijuana may see a short-term decrease in opioid-related ED visits in the first 6 months, after which rates may return to prelegalization levels, new research suggests.
Previous research suggests that individuals may reduce the use of opioids when they have an alternative and that cannabis can provide pain relief.
“At the same time, we often hear claims from politicians that we should not legalize cannabis because it may act as a ‘gateway drug’ that leads to use of other drugs,” lead researcher Coleman Drake, PhD, Department of Health Policy and Management, University of Pittsburgh Graduate School of Public Health, told this news organization.
“Our findings indicate that cannabis legalization does not effect any increase in opioid-related ED visits, contradicting the gateway drug explanation,” Dr. Drake said.
The study was published online July 12 in Health Economics.
Significant reduction
So far, 19 states have legalized recreational cannabis, meaning that nearly half of the U.S. population lives in a state that allows recreational cannabis use.
The investigators analyzed data on opioid-related ED visits from 29 states between 2011 and 2017. Four states – California, Maine, Massachusetts, and Nevada – legalized recreational marijuana during the study period; the remaining 25 states did not.
The four states with recreational cannabis laws experienced a 7.6% reduction in opioid-related ED visits for 6 months after the law went into effect in comparison with the states that did not legalize recreational marijuana.
“This isn’t trivial – a decline in opioid-related emergency department visits, even if only for 6 months, is a welcome public health development,” Dr. Drake said in a statement.
Not surprisingly, these effects are driven by men and adults aged 25 to 44 years. “These are populations that are more likely to use cannabis, and the reduction in opioid-related ED visits that we find is concentrated among them,” Dr. Drake told this news organization.
However, the downturn in opioid-related ED visits after making marijuana legal was only temporary.
“
Encouragingly, he said, the data show that opioid-related ED visits don’t increase above baseline after recreational marijuana laws are adopted.
“We conclude that cannabis legalization likely is not a panacea for the opioid epidemic, but there are some helpful effects,” Dr. Drake said in an interview.
The study was supported by the National Institute on Drug Abuse. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
State laws permitting recreational marijuana use have not led to an increase in opioid-related emergency department visits, as many had feared.
On the contrary, states that legalize recreational marijuana may see a short-term decrease in opioid-related ED visits in the first 6 months, after which rates may return to prelegalization levels, new research suggests.
Previous research suggests that individuals may reduce the use of opioids when they have an alternative and that cannabis can provide pain relief.
“At the same time, we often hear claims from politicians that we should not legalize cannabis because it may act as a ‘gateway drug’ that leads to use of other drugs,” lead researcher Coleman Drake, PhD, Department of Health Policy and Management, University of Pittsburgh Graduate School of Public Health, told this news organization.
“Our findings indicate that cannabis legalization does not effect any increase in opioid-related ED visits, contradicting the gateway drug explanation,” Dr. Drake said.
The study was published online July 12 in Health Economics.
Significant reduction
So far, 19 states have legalized recreational cannabis, meaning that nearly half of the U.S. population lives in a state that allows recreational cannabis use.
The investigators analyzed data on opioid-related ED visits from 29 states between 2011 and 2017. Four states – California, Maine, Massachusetts, and Nevada – legalized recreational marijuana during the study period; the remaining 25 states did not.
The four states with recreational cannabis laws experienced a 7.6% reduction in opioid-related ED visits for 6 months after the law went into effect in comparison with the states that did not legalize recreational marijuana.
“This isn’t trivial – a decline in opioid-related emergency department visits, even if only for 6 months, is a welcome public health development,” Dr. Drake said in a statement.
Not surprisingly, these effects are driven by men and adults aged 25 to 44 years. “These are populations that are more likely to use cannabis, and the reduction in opioid-related ED visits that we find is concentrated among them,” Dr. Drake told this news organization.
However, the downturn in opioid-related ED visits after making marijuana legal was only temporary.
“
Encouragingly, he said, the data show that opioid-related ED visits don’t increase above baseline after recreational marijuana laws are adopted.
“We conclude that cannabis legalization likely is not a panacea for the opioid epidemic, but there are some helpful effects,” Dr. Drake said in an interview.
The study was supported by the National Institute on Drug Abuse. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term outcome data suggest optimism for MIS-C patients
Only 1 child from a cohort of 45 children hospitalized with multisystem inflammatory syndrome following COVID-19 infection had persistent mild cardiac dysfunction after 9 months, according to data from patients younger than 21 years seen at a single center in 2020.
In a study published in Pediatrics, Kanwal M. Farooqi, MD, of Columbia University, New York, and colleagues provided the first report on longitudinal cardiac and immunologic outcomes in North American children hospitalized with multisystem inflammatory syndrome (MIS-C). In response to the COVID-19 pandemic, clinicians at New York–Presbyterian Hospital consolidated pediatric admissions and developed an interdisciplinary inpatient and outpatient MIS-C follow-up program to monitor cardiac and immunologic outcomes in their patients.
The study included all children younger than 21 years admitted to Columbia University Irving Medical Center/New York–Presbyterian Morgan Stanley Children’s Hospital for MIS-C in 2020. The median age of the patients was 9 years, and the median length of hospital stay was 5 days. Follow-up visits occurred at 1-4 weeks (average 2 weeks), 1-4 months (average 2 months), and 4-9 months (average 6 months) after hospital discharge. Follow-up visits included echocardiograms and measures of inflammatory markers.
Most of the children (84%) had no underlying medical conditions, but 24% presented with some level of respiratory distress or oxygen requirement, and 64% had vasodilatory shock. In addition, 80% had at least mild cardiac abnormalities and 66% had significant lymphopenia on admission.
Inflammatory profiles on admission showed elevation of C-reactive protein, ferritin, and D-dimer in 87%-98% of the patients. Consistent with cardiac involvement, 64% of the patients also had elevated troponin levels, and 91% had elevated N-terminal pro-brain natriuretic peptide (NT-proBNP) levels.
“These parameters peaked at or shortly after admission and then gradually normalized,” the researchers said. “By the first follow-up, [C-reactive protein], troponin, and NT-proBNP had normalized in nearly all tested patients (97%-100%),” they noted.
By the first follow-up period at 1-4 weeks, all patients had normal coronary arteries, and 18% (seven patients) had mild echocardiographic findings. However, approximately one-third (32%) of the patients had persistent lymphocytosis at 1-4 weeks, and 23 of the 24 patients assessed had elevated double-negative T cells, which persisted in 96% of the patients at 1-4 months’ follow-up. However, during the last follow-up of 4-9 months, only one patient had persistent mild biventricular dysfunction and a second patient had mild mitral and tricuspid valve regurgitation.
All patients were treated with steroids and immunoglobulins (2 g/kg), as well as enoxaparin prophylaxis or low-dose aspirin and GI prophylaxis. Treatment with methylprednisolone varied based on disease severity; patients with mild presentation received 2 mg/kg per day; those with moderate presentation received a methylprednisolone pulse of 10 mg/kg per day, followed by 2 mg/kg per day; those with severe disease received methylprednisolone at 20-30 mg/kg per day for 1-3 days, followed by 2 mg/kg per day.
“Aggressive use of steroids may also explain the lower incidence of coronary artery abnormalities in our cohort,” the researchers noted.
The study findings were limited by the observational design and inability to make definitive conclusions about treatment and outcomes, as well as the evolving case definitions for MIS-C, the researchers said.
The persistence of double-negative T cells was surprising, and “likely represent a prolonged postinflammatory recovery cell population, but further study is ongoing to better define this observation,” they noted.
“Our study reveals generally encouraging medium-term outcomes, including rapid normalization of inflammatory markers and significant cardiac abnormalities in the majority of patients with MIS-C,” the researchers said. “The exact nature and potential for long-term cardiac fibrosis, exercise intolerance, or other changes remain unknown,” and long-term caution and follow-up are recommended, they concluded.
Cautious optimism, long-term monitoring
The study is important to provide guidance for clinicians on how to manage their patients who have been hospitalized with MIS-C, said Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H.
“It was both surprising and reassuring to see that so many of the patients had positive outcomes in terms of cardiac function and that during the acute stage there were no deaths,” said Dr. Boulter. “Hospitalizations were brief, averaging just 5 days. The patients had many symptoms, but unlike adults, there was not a preponderance of underlying risk factors in this cohort of patients,” she said.
The results suggest optimism for MIS-C patients in that they generally recover, but the take-home message for clinicians is that these patients will require careful monitoring for long-term issues, Dr. Boulter said.
“These patients should be followed for years to assess long-term effects on morbidity and mortality,” Dr. Boulter emphasized.
The study was funded by Genentech. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Only 1 child from a cohort of 45 children hospitalized with multisystem inflammatory syndrome following COVID-19 infection had persistent mild cardiac dysfunction after 9 months, according to data from patients younger than 21 years seen at a single center in 2020.
In a study published in Pediatrics, Kanwal M. Farooqi, MD, of Columbia University, New York, and colleagues provided the first report on longitudinal cardiac and immunologic outcomes in North American children hospitalized with multisystem inflammatory syndrome (MIS-C). In response to the COVID-19 pandemic, clinicians at New York–Presbyterian Hospital consolidated pediatric admissions and developed an interdisciplinary inpatient and outpatient MIS-C follow-up program to monitor cardiac and immunologic outcomes in their patients.
The study included all children younger than 21 years admitted to Columbia University Irving Medical Center/New York–Presbyterian Morgan Stanley Children’s Hospital for MIS-C in 2020. The median age of the patients was 9 years, and the median length of hospital stay was 5 days. Follow-up visits occurred at 1-4 weeks (average 2 weeks), 1-4 months (average 2 months), and 4-9 months (average 6 months) after hospital discharge. Follow-up visits included echocardiograms and measures of inflammatory markers.
Most of the children (84%) had no underlying medical conditions, but 24% presented with some level of respiratory distress or oxygen requirement, and 64% had vasodilatory shock. In addition, 80% had at least mild cardiac abnormalities and 66% had significant lymphopenia on admission.
Inflammatory profiles on admission showed elevation of C-reactive protein, ferritin, and D-dimer in 87%-98% of the patients. Consistent with cardiac involvement, 64% of the patients also had elevated troponin levels, and 91% had elevated N-terminal pro-brain natriuretic peptide (NT-proBNP) levels.
“These parameters peaked at or shortly after admission and then gradually normalized,” the researchers said. “By the first follow-up, [C-reactive protein], troponin, and NT-proBNP had normalized in nearly all tested patients (97%-100%),” they noted.
By the first follow-up period at 1-4 weeks, all patients had normal coronary arteries, and 18% (seven patients) had mild echocardiographic findings. However, approximately one-third (32%) of the patients had persistent lymphocytosis at 1-4 weeks, and 23 of the 24 patients assessed had elevated double-negative T cells, which persisted in 96% of the patients at 1-4 months’ follow-up. However, during the last follow-up of 4-9 months, only one patient had persistent mild biventricular dysfunction and a second patient had mild mitral and tricuspid valve regurgitation.
All patients were treated with steroids and immunoglobulins (2 g/kg), as well as enoxaparin prophylaxis or low-dose aspirin and GI prophylaxis. Treatment with methylprednisolone varied based on disease severity; patients with mild presentation received 2 mg/kg per day; those with moderate presentation received a methylprednisolone pulse of 10 mg/kg per day, followed by 2 mg/kg per day; those with severe disease received methylprednisolone at 20-30 mg/kg per day for 1-3 days, followed by 2 mg/kg per day.
“Aggressive use of steroids may also explain the lower incidence of coronary artery abnormalities in our cohort,” the researchers noted.
The study findings were limited by the observational design and inability to make definitive conclusions about treatment and outcomes, as well as the evolving case definitions for MIS-C, the researchers said.
The persistence of double-negative T cells was surprising, and “likely represent a prolonged postinflammatory recovery cell population, but further study is ongoing to better define this observation,” they noted.
“Our study reveals generally encouraging medium-term outcomes, including rapid normalization of inflammatory markers and significant cardiac abnormalities in the majority of patients with MIS-C,” the researchers said. “The exact nature and potential for long-term cardiac fibrosis, exercise intolerance, or other changes remain unknown,” and long-term caution and follow-up are recommended, they concluded.
Cautious optimism, long-term monitoring
The study is important to provide guidance for clinicians on how to manage their patients who have been hospitalized with MIS-C, said Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H.
“It was both surprising and reassuring to see that so many of the patients had positive outcomes in terms of cardiac function and that during the acute stage there were no deaths,” said Dr. Boulter. “Hospitalizations were brief, averaging just 5 days. The patients had many symptoms, but unlike adults, there was not a preponderance of underlying risk factors in this cohort of patients,” she said.
The results suggest optimism for MIS-C patients in that they generally recover, but the take-home message for clinicians is that these patients will require careful monitoring for long-term issues, Dr. Boulter said.
“These patients should be followed for years to assess long-term effects on morbidity and mortality,” Dr. Boulter emphasized.
The study was funded by Genentech. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
Only 1 child from a cohort of 45 children hospitalized with multisystem inflammatory syndrome following COVID-19 infection had persistent mild cardiac dysfunction after 9 months, according to data from patients younger than 21 years seen at a single center in 2020.
In a study published in Pediatrics, Kanwal M. Farooqi, MD, of Columbia University, New York, and colleagues provided the first report on longitudinal cardiac and immunologic outcomes in North American children hospitalized with multisystem inflammatory syndrome (MIS-C). In response to the COVID-19 pandemic, clinicians at New York–Presbyterian Hospital consolidated pediatric admissions and developed an interdisciplinary inpatient and outpatient MIS-C follow-up program to monitor cardiac and immunologic outcomes in their patients.
The study included all children younger than 21 years admitted to Columbia University Irving Medical Center/New York–Presbyterian Morgan Stanley Children’s Hospital for MIS-C in 2020. The median age of the patients was 9 years, and the median length of hospital stay was 5 days. Follow-up visits occurred at 1-4 weeks (average 2 weeks), 1-4 months (average 2 months), and 4-9 months (average 6 months) after hospital discharge. Follow-up visits included echocardiograms and measures of inflammatory markers.
Most of the children (84%) had no underlying medical conditions, but 24% presented with some level of respiratory distress or oxygen requirement, and 64% had vasodilatory shock. In addition, 80% had at least mild cardiac abnormalities and 66% had significant lymphopenia on admission.
Inflammatory profiles on admission showed elevation of C-reactive protein, ferritin, and D-dimer in 87%-98% of the patients. Consistent with cardiac involvement, 64% of the patients also had elevated troponin levels, and 91% had elevated N-terminal pro-brain natriuretic peptide (NT-proBNP) levels.
“These parameters peaked at or shortly after admission and then gradually normalized,” the researchers said. “By the first follow-up, [C-reactive protein], troponin, and NT-proBNP had normalized in nearly all tested patients (97%-100%),” they noted.
By the first follow-up period at 1-4 weeks, all patients had normal coronary arteries, and 18% (seven patients) had mild echocardiographic findings. However, approximately one-third (32%) of the patients had persistent lymphocytosis at 1-4 weeks, and 23 of the 24 patients assessed had elevated double-negative T cells, which persisted in 96% of the patients at 1-4 months’ follow-up. However, during the last follow-up of 4-9 months, only one patient had persistent mild biventricular dysfunction and a second patient had mild mitral and tricuspid valve regurgitation.
All patients were treated with steroids and immunoglobulins (2 g/kg), as well as enoxaparin prophylaxis or low-dose aspirin and GI prophylaxis. Treatment with methylprednisolone varied based on disease severity; patients with mild presentation received 2 mg/kg per day; those with moderate presentation received a methylprednisolone pulse of 10 mg/kg per day, followed by 2 mg/kg per day; those with severe disease received methylprednisolone at 20-30 mg/kg per day for 1-3 days, followed by 2 mg/kg per day.
“Aggressive use of steroids may also explain the lower incidence of coronary artery abnormalities in our cohort,” the researchers noted.
The study findings were limited by the observational design and inability to make definitive conclusions about treatment and outcomes, as well as the evolving case definitions for MIS-C, the researchers said.
The persistence of double-negative T cells was surprising, and “likely represent a prolonged postinflammatory recovery cell population, but further study is ongoing to better define this observation,” they noted.
“Our study reveals generally encouraging medium-term outcomes, including rapid normalization of inflammatory markers and significant cardiac abnormalities in the majority of patients with MIS-C,” the researchers said. “The exact nature and potential for long-term cardiac fibrosis, exercise intolerance, or other changes remain unknown,” and long-term caution and follow-up are recommended, they concluded.
Cautious optimism, long-term monitoring
The study is important to provide guidance for clinicians on how to manage their patients who have been hospitalized with MIS-C, said Susan Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H.
“It was both surprising and reassuring to see that so many of the patients had positive outcomes in terms of cardiac function and that during the acute stage there were no deaths,” said Dr. Boulter. “Hospitalizations were brief, averaging just 5 days. The patients had many symptoms, but unlike adults, there was not a preponderance of underlying risk factors in this cohort of patients,” she said.
The results suggest optimism for MIS-C patients in that they generally recover, but the take-home message for clinicians is that these patients will require careful monitoring for long-term issues, Dr. Boulter said.
“These patients should be followed for years to assess long-term effects on morbidity and mortality,” Dr. Boulter emphasized.
The study was funded by Genentech. The researchers had no financial conflicts to disclose. Dr. Boulter had no financial conflicts to disclose, but serves on the Pediatric News Editorial Advisory Board.
FROM PEDIATRICS
Disconnect between POLST orders and end-of-life care
Background: In order to reduce the mismatch between patients’ desired and actual end-of-life care, the Physician Orders for Life-Sustaining Treatment (POLST) was created. POLST is a portable document delineating medical orders for emergency care treatment at the end of life including whether to attempt resuscitation and general level of medical interventions. For nursing home residents, an association between POLST creation and reduction of unwanted CPR has been substantiated. Outside of this population, the association is unknown.
Study design: Retrospective cohort study.
Setting: Two academic hospitals in Washington.
Synopsis: Patients older than age 18 years who had one of nine chronic health conditions associated with 90% of deaths among Medicare beneficiaries were identified using Washington state death certificates. Additional inclusion criteria included hospital admission in the last 6 months of life and creation of a POLST prior to this admission. This led to identification of 1,818 patients. Patients with full-treatment POLST orders were significantly more likely to be admitted to the ICU as well as receive life-sustaining treatments such as mechanical ventilation, vasoactive infusions, or CPR, compared with patients with limited interventions or comfort-only POLST orders (P < .001 for both). 38% of patients with treatment-limiting POLSTs received aggressive end-of-life care that was discordant with their previously documented wishes.
Bottom line: Completion of POLST was associated with a greater likelihood of receiving end-of-life care that was in line with patients’ previously documented wishes regarding admission to ICU and life-sustaining treatment. Washington was one of the first states to adopt POLST in 2005 and therefore these results may not be broadly applicable.
Citation: Lee RY et al. Association of physician orders for life-sustaining treatment with ICU admission among patients hospitalized hear the end of life. JAMA. 2020 Feb 16;323(10):950-60.
Dr. Dreicer is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: In order to reduce the mismatch between patients’ desired and actual end-of-life care, the Physician Orders for Life-Sustaining Treatment (POLST) was created. POLST is a portable document delineating medical orders for emergency care treatment at the end of life including whether to attempt resuscitation and general level of medical interventions. For nursing home residents, an association between POLST creation and reduction of unwanted CPR has been substantiated. Outside of this population, the association is unknown.
Study design: Retrospective cohort study.
Setting: Two academic hospitals in Washington.
Synopsis: Patients older than age 18 years who had one of nine chronic health conditions associated with 90% of deaths among Medicare beneficiaries were identified using Washington state death certificates. Additional inclusion criteria included hospital admission in the last 6 months of life and creation of a POLST prior to this admission. This led to identification of 1,818 patients. Patients with full-treatment POLST orders were significantly more likely to be admitted to the ICU as well as receive life-sustaining treatments such as mechanical ventilation, vasoactive infusions, or CPR, compared with patients with limited interventions or comfort-only POLST orders (P < .001 for both). 38% of patients with treatment-limiting POLSTs received aggressive end-of-life care that was discordant with their previously documented wishes.
Bottom line: Completion of POLST was associated with a greater likelihood of receiving end-of-life care that was in line with patients’ previously documented wishes regarding admission to ICU and life-sustaining treatment. Washington was one of the first states to adopt POLST in 2005 and therefore these results may not be broadly applicable.
Citation: Lee RY et al. Association of physician orders for life-sustaining treatment with ICU admission among patients hospitalized hear the end of life. JAMA. 2020 Feb 16;323(10):950-60.
Dr. Dreicer is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: In order to reduce the mismatch between patients’ desired and actual end-of-life care, the Physician Orders for Life-Sustaining Treatment (POLST) was created. POLST is a portable document delineating medical orders for emergency care treatment at the end of life including whether to attempt resuscitation and general level of medical interventions. For nursing home residents, an association between POLST creation and reduction of unwanted CPR has been substantiated. Outside of this population, the association is unknown.
Study design: Retrospective cohort study.
Setting: Two academic hospitals in Washington.
Synopsis: Patients older than age 18 years who had one of nine chronic health conditions associated with 90% of deaths among Medicare beneficiaries were identified using Washington state death certificates. Additional inclusion criteria included hospital admission in the last 6 months of life and creation of a POLST prior to this admission. This led to identification of 1,818 patients. Patients with full-treatment POLST orders were significantly more likely to be admitted to the ICU as well as receive life-sustaining treatments such as mechanical ventilation, vasoactive infusions, or CPR, compared with patients with limited interventions or comfort-only POLST orders (P < .001 for both). 38% of patients with treatment-limiting POLSTs received aggressive end-of-life care that was discordant with their previously documented wishes.
Bottom line: Completion of POLST was associated with a greater likelihood of receiving end-of-life care that was in line with patients’ previously documented wishes regarding admission to ICU and life-sustaining treatment. Washington was one of the first states to adopt POLST in 2005 and therefore these results may not be broadly applicable.
Citation: Lee RY et al. Association of physician orders for life-sustaining treatment with ICU admission among patients hospitalized hear the end of life. JAMA. 2020 Feb 16;323(10):950-60.
Dr. Dreicer is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Early heparin treatment linked to lower COVID-19 mortality
Early treatment with low-molecular-weight heparin (LMWH) reduces the risk for death in patients with COVID-19, a retrospective cohort study shows.
Heparin could reduce the risk for blood clots, Andrea De Vito, MD, of the unit of infectious diseases at the University of Sassari, Italy, said during his online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
“Several studies try to describe the role played by coagulopathies in COVID-19 death,” but the mechanism causing them is still unclear, Dr. De Vito explained.
Some guidelines have suggested heparin as a treatment for hospitalized COVID-19 patients, but few have looked at nonhospitalized patients. In fact, the National Institutes of Health discourages the use of heparin in nonhospitalized COVID-19 patients, and guidance for the home care of COVID-19 patients from the World Health Organization doesn’t mention heparin treatment at all, he said.
To examine the benefits of early heparin – whether administered at home or in the hospital – Dr. De Vito and colleagues looked at a cohort of older adults with COVID-19 who were evaluated or treated at an Italian university hospital.
“Some patients were hospitalized immediately after symptoms onset; other people preferred to call their general practitioner and started the treatment at home,” Dr. De Vito said in an interview. “Other people were hospitalized for worsening of symptoms later in the course of the disease.”
Of the 734 patients, 296 received heparin within 5 days of the onset of symptoms or a positive COVID-19 test. Of the remaining 438 patients, 196 received LMWH treatment later during the disease course, and the rest never received LMWH.
All patients who received early heparin were treated with LMWH 4,000 IU, or 6,000 IU if their body mass index was above 30 kg/m2. This was reduced to 2,000 IU if estimated glomerular filtration rate (eGFR) dropped below 30 mL/min. None of the patients had previously received heparin.
Median age was slightly younger for patients who received early heparin than for those who did not (76.8 vs. 78.5 years).
Other demographic characteristics, such as sex and BMI, were similar in the two groups, as were rates of comorbidities, such as hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, kidney disease, and neurologic conditions. Also similar were the frequency of symptoms (such as fever, cough, and shortness of breath) and rates of treatment with remdesivir or steroids.
Rates of hospital admission were not significantly different between patients who received early heparin and those who did not (65% vs. 61%). There was also no significant difference in use of a venturi mask (35% vs. 28%), noninvasive ventilation (13% vs. 14%), or intubation (5% vs. 8%).
However, rates of death were significantly lower in patients who received early heparin than in those who did not (13% vs. 25%; P < .0001).
There was a trend toward shorter hospital stays for patients treated with early heparin, but the difference was not significant (median, 10 vs. 13 days; P = .08).
Researchers also conducted a separate analysis of 219 COVID-19 patients who received LMWH at home, regardless of when during their disease course they received it. These patients were significantly less likely to be hospitalized than were patients who did not receive LMWH at home (odds ratio, 0.2; P < .0001).
Comparatively, early heparin treatment had a greater effect on the risk for death and the risk for hospitalization than did other factors.
“Thromboemboli are a major complication of COVID. There is good consensus that hospitalized patients with COVID should receive anticoagulants prophylactically, although the best dose is being studied,” said Judy Stone, MD, an infectious disease physician and journalist who was not involved in the study.
“This study extends those findings of benefit from anticoagulants to nonhospitalized patients, with fewer deaths in those treated with low-molecular-weight heparin,” Dr. Stone told this news organization. “The major limitation is that the study is retrospective and observational. The next step would be to confirm these findings prospectively, randomizing a similar group to LMWH or no anticoagulation.”
Another limitation of the study is that some of the patients lived in nursing homes and might have received care from nurses that eliminated the need for hospitalization, Dr. De Vito added.
The study did not note any external funding. The authors have disclosed no relevant financial relationships. Dr. Stone is a member of the advisory committee for the C-Path CURE Drug Repurposing Collaboratory (CDRC) program and has written for Medscape.
A version of this article first appeared on Medscape.com.
Early treatment with low-molecular-weight heparin (LMWH) reduces the risk for death in patients with COVID-19, a retrospective cohort study shows.
Heparin could reduce the risk for blood clots, Andrea De Vito, MD, of the unit of infectious diseases at the University of Sassari, Italy, said during his online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
“Several studies try to describe the role played by coagulopathies in COVID-19 death,” but the mechanism causing them is still unclear, Dr. De Vito explained.
Some guidelines have suggested heparin as a treatment for hospitalized COVID-19 patients, but few have looked at nonhospitalized patients. In fact, the National Institutes of Health discourages the use of heparin in nonhospitalized COVID-19 patients, and guidance for the home care of COVID-19 patients from the World Health Organization doesn’t mention heparin treatment at all, he said.
To examine the benefits of early heparin – whether administered at home or in the hospital – Dr. De Vito and colleagues looked at a cohort of older adults with COVID-19 who were evaluated or treated at an Italian university hospital.
“Some patients were hospitalized immediately after symptoms onset; other people preferred to call their general practitioner and started the treatment at home,” Dr. De Vito said in an interview. “Other people were hospitalized for worsening of symptoms later in the course of the disease.”
Of the 734 patients, 296 received heparin within 5 days of the onset of symptoms or a positive COVID-19 test. Of the remaining 438 patients, 196 received LMWH treatment later during the disease course, and the rest never received LMWH.
All patients who received early heparin were treated with LMWH 4,000 IU, or 6,000 IU if their body mass index was above 30 kg/m2. This was reduced to 2,000 IU if estimated glomerular filtration rate (eGFR) dropped below 30 mL/min. None of the patients had previously received heparin.
Median age was slightly younger for patients who received early heparin than for those who did not (76.8 vs. 78.5 years).
Other demographic characteristics, such as sex and BMI, were similar in the two groups, as were rates of comorbidities, such as hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, kidney disease, and neurologic conditions. Also similar were the frequency of symptoms (such as fever, cough, and shortness of breath) and rates of treatment with remdesivir or steroids.
Rates of hospital admission were not significantly different between patients who received early heparin and those who did not (65% vs. 61%). There was also no significant difference in use of a venturi mask (35% vs. 28%), noninvasive ventilation (13% vs. 14%), or intubation (5% vs. 8%).
However, rates of death were significantly lower in patients who received early heparin than in those who did not (13% vs. 25%; P < .0001).
There was a trend toward shorter hospital stays for patients treated with early heparin, but the difference was not significant (median, 10 vs. 13 days; P = .08).
Researchers also conducted a separate analysis of 219 COVID-19 patients who received LMWH at home, regardless of when during their disease course they received it. These patients were significantly less likely to be hospitalized than were patients who did not receive LMWH at home (odds ratio, 0.2; P < .0001).
Comparatively, early heparin treatment had a greater effect on the risk for death and the risk for hospitalization than did other factors.
“Thromboemboli are a major complication of COVID. There is good consensus that hospitalized patients with COVID should receive anticoagulants prophylactically, although the best dose is being studied,” said Judy Stone, MD, an infectious disease physician and journalist who was not involved in the study.
“This study extends those findings of benefit from anticoagulants to nonhospitalized patients, with fewer deaths in those treated with low-molecular-weight heparin,” Dr. Stone told this news organization. “The major limitation is that the study is retrospective and observational. The next step would be to confirm these findings prospectively, randomizing a similar group to LMWH or no anticoagulation.”
Another limitation of the study is that some of the patients lived in nursing homes and might have received care from nurses that eliminated the need for hospitalization, Dr. De Vito added.
The study did not note any external funding. The authors have disclosed no relevant financial relationships. Dr. Stone is a member of the advisory committee for the C-Path CURE Drug Repurposing Collaboratory (CDRC) program and has written for Medscape.
A version of this article first appeared on Medscape.com.
Early treatment with low-molecular-weight heparin (LMWH) reduces the risk for death in patients with COVID-19, a retrospective cohort study shows.
Heparin could reduce the risk for blood clots, Andrea De Vito, MD, of the unit of infectious diseases at the University of Sassari, Italy, said during his online presentation of the findings at the 31st European Congress of Clinical Microbiology & Infectious Diseases.
“Several studies try to describe the role played by coagulopathies in COVID-19 death,” but the mechanism causing them is still unclear, Dr. De Vito explained.
Some guidelines have suggested heparin as a treatment for hospitalized COVID-19 patients, but few have looked at nonhospitalized patients. In fact, the National Institutes of Health discourages the use of heparin in nonhospitalized COVID-19 patients, and guidance for the home care of COVID-19 patients from the World Health Organization doesn’t mention heparin treatment at all, he said.
To examine the benefits of early heparin – whether administered at home or in the hospital – Dr. De Vito and colleagues looked at a cohort of older adults with COVID-19 who were evaluated or treated at an Italian university hospital.
“Some patients were hospitalized immediately after symptoms onset; other people preferred to call their general practitioner and started the treatment at home,” Dr. De Vito said in an interview. “Other people were hospitalized for worsening of symptoms later in the course of the disease.”
Of the 734 patients, 296 received heparin within 5 days of the onset of symptoms or a positive COVID-19 test. Of the remaining 438 patients, 196 received LMWH treatment later during the disease course, and the rest never received LMWH.
All patients who received early heparin were treated with LMWH 4,000 IU, or 6,000 IU if their body mass index was above 30 kg/m2. This was reduced to 2,000 IU if estimated glomerular filtration rate (eGFR) dropped below 30 mL/min. None of the patients had previously received heparin.
Median age was slightly younger for patients who received early heparin than for those who did not (76.8 vs. 78.5 years).
Other demographic characteristics, such as sex and BMI, were similar in the two groups, as were rates of comorbidities, such as hypertension, cardiovascular disease, diabetes, chronic obstructive pulmonary disease, kidney disease, and neurologic conditions. Also similar were the frequency of symptoms (such as fever, cough, and shortness of breath) and rates of treatment with remdesivir or steroids.
Rates of hospital admission were not significantly different between patients who received early heparin and those who did not (65% vs. 61%). There was also no significant difference in use of a venturi mask (35% vs. 28%), noninvasive ventilation (13% vs. 14%), or intubation (5% vs. 8%).
However, rates of death were significantly lower in patients who received early heparin than in those who did not (13% vs. 25%; P < .0001).
There was a trend toward shorter hospital stays for patients treated with early heparin, but the difference was not significant (median, 10 vs. 13 days; P = .08).
Researchers also conducted a separate analysis of 219 COVID-19 patients who received LMWH at home, regardless of when during their disease course they received it. These patients were significantly less likely to be hospitalized than were patients who did not receive LMWH at home (odds ratio, 0.2; P < .0001).
Comparatively, early heparin treatment had a greater effect on the risk for death and the risk for hospitalization than did other factors.
“Thromboemboli are a major complication of COVID. There is good consensus that hospitalized patients with COVID should receive anticoagulants prophylactically, although the best dose is being studied,” said Judy Stone, MD, an infectious disease physician and journalist who was not involved in the study.
“This study extends those findings of benefit from anticoagulants to nonhospitalized patients, with fewer deaths in those treated with low-molecular-weight heparin,” Dr. Stone told this news organization. “The major limitation is that the study is retrospective and observational. The next step would be to confirm these findings prospectively, randomizing a similar group to LMWH or no anticoagulation.”
Another limitation of the study is that some of the patients lived in nursing homes and might have received care from nurses that eliminated the need for hospitalization, Dr. De Vito added.
The study did not note any external funding. The authors have disclosed no relevant financial relationships. Dr. Stone is a member of the advisory committee for the C-Path CURE Drug Repurposing Collaboratory (CDRC) program and has written for Medscape.
A version of this article first appeared on Medscape.com.
Hospital medicine and the future of smart care
People often overestimate what will happen in the next two years and underestimate what will happen in ten. – Bill Gates
The COVID-19 pandemic set in motion a series of innovations catalyzing the digital transformation of the health care landscape.
Telemedicine use exploded over the last 12 months to the point that it has almost become ubiquitous. With that, we saw a rapid proliferation of wearables and remote patient monitoring devices. Thanks to virtual care, care delivery is no longer strictly dependent on having onsite specialists, and care itself is not confined to the boundaries of hospitals or doctors’ offices anymore.
We saw the formation of the digital front door and the emergence of new virtual care sites like virtual urgent care, virtual home health, virtual office visits, virtual hospital at home that allowed clinical care to be delivered safely outside the boundaries of hospitals. Nonclinical public places like gyms, schools, and community centers were being transformed into virtual health care portals that brought care closer to the people.
Inside the hospital, we saw a fusion of traditional inpatient care and virtual care. Onsite hospital teams embraced telemedicine during the pandemic for various reasons; to conserve personal protective equipment (PPE), limit exposure, boost care capacity, improve access to specialists at distant sites, and bring family memberse to “webside” who cannot be at a patient’s bedside.
In clinical trials as well, virtual care is a welcome change. According to one survey1, most trial participants favored the use of telehealth services for clinical trials, as these helped them stay engaged, compliant, monitored, and on track while remaining at home. Furthermore, we are seeing the integration of artificial intelligence (AI) into telehealth, whether it is to aid physicians in clinical decision-making or to generate reminders to help patients with chronic disease management. However, this integration is only beginning to scratch the surface of the combination of two technologies’ real potential.
What’s next?
Based on these trends, it should be no surprise that digital health will become a vital sign for health care organizations.
The next 12 to 24 months will set new standards for digital health and play a significant role in defining the next generation of virtual care. There are projections that global health care industry revenues will exceed $2.6 trillion by 2025, with AI and telehealth playing a prominent role in this growth.2 According to estimates, telehealth itself will be a $175 billion market by 2026 and approximately one in three patient encounters will go virtual.3,4 Moreover, virtual care will continue to make exciting transformations, helping to make quality care accessible to everyone in innovative ways. For example, the University of Cincinnati has recently developed a pilot project using a drone equipped with video technology, artificial intelligence, sensors, and first aid kits to go to hard-to-reach areas to deliver care via telemedicine.5
Smart hospitals
In coming years, we can expect the integration of AI, augmented reality (AR), and virtual reality (VR) into telemedicine at lightning speed – and at a much larger scale – that will enable surgeons from different parts of the globe to perform procedures remotely and more precisely.
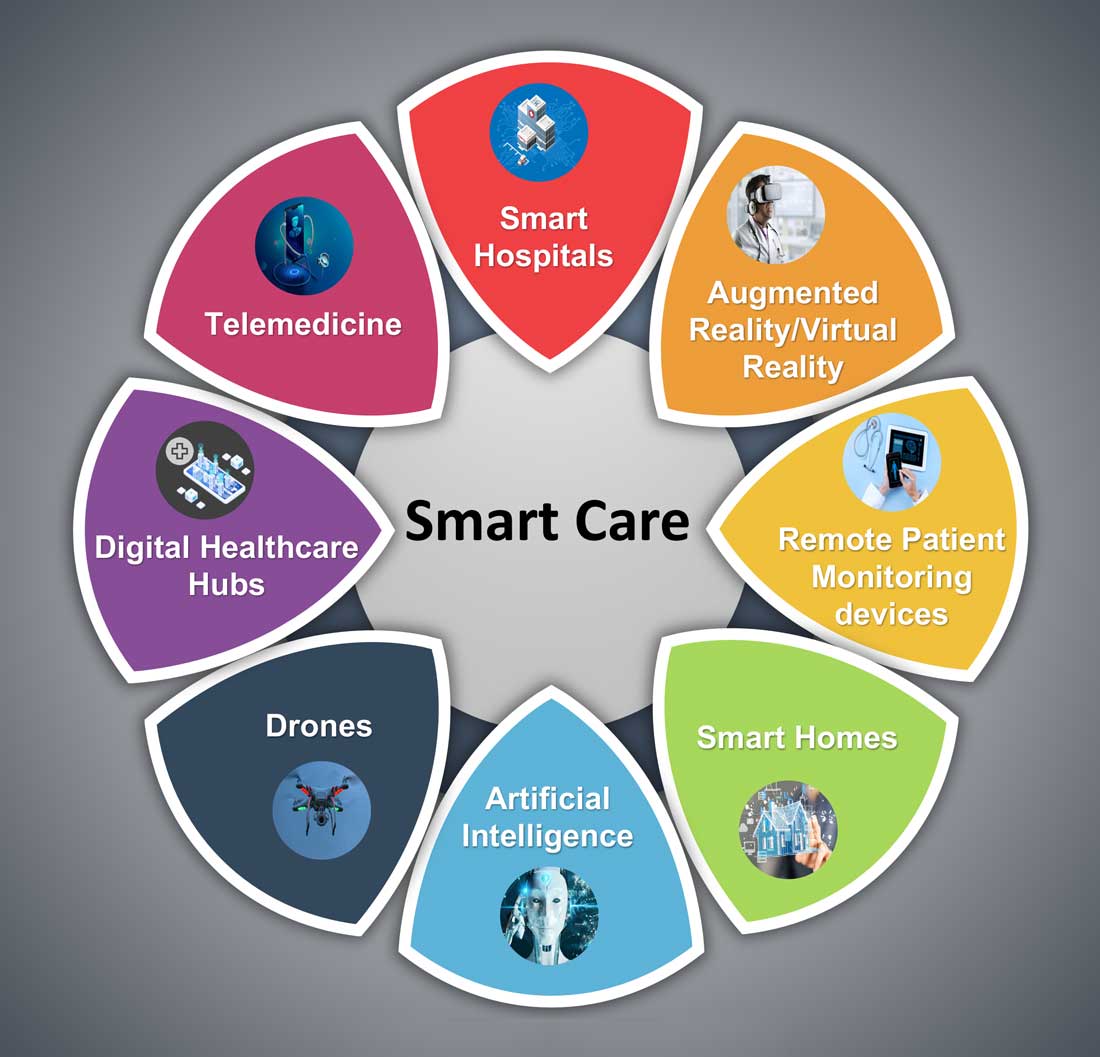
AI is already gaining traction in different fields within health care – whether it’s predicting length of stay in the ICU, or assisting in triage decisions, or reading radiological images, to name just a few. The Mayo Clinic is using AI and computer-aided decision-making tools to predict the risk of surgery and potential post-op complications, which could allow even better collaboration between medical and surgical teams. We hear about the “X-ray” vision offered to proceduralists using HoloLens – mixed reality smartglasses – a technology that enables them to perform procedures more precisely. Others project that there will be more sensors and voice recognition tools in the OR that will be used to gather data to develop intelligent algorithms, and to build a safety net for interventionalists that can notify them of potential hazards or accidental sterile field breaches. The insights gained will be used to create best practices and even allow some procedures to be performed outside the traditional OR setting.
Additionally, we are seeing the development of “smart” patient rooms. For example, one health system in Florida is working on deploying Amazon Alexa in 2,500 patient rooms to allow patients to connect more easily to their care team members. In the not-so-distant future, smart hospitals with smart patient rooms and smart ORs equipped with telemedicine, AI, AR, mixed reality, and computer-aided decision-making tools will no longer be an exception.
Smart homes for smart care
Smart homes with technologies like gas detectors, movement sensors, and sleep sensors will continue to evolve. According to one estimate, the global smart home health care market was $8.7 billion in 2019, and is expected to be $96.2 billion by 2030.6
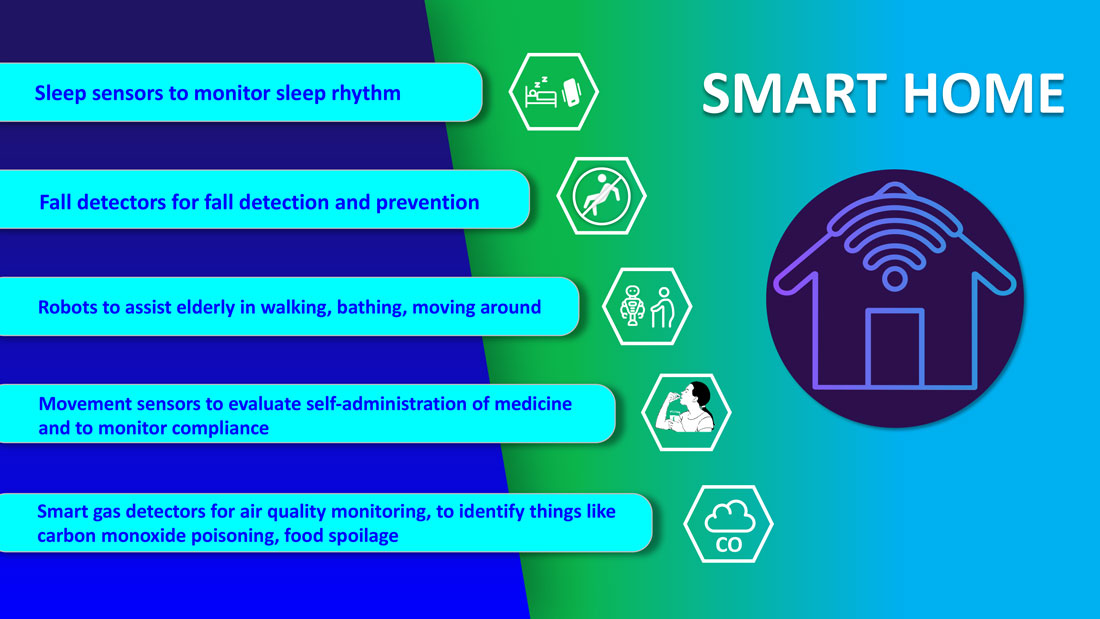
Smart technologies will have applications in fall detection and prevention, evaluation of self-administration of medicine, sleep rhythm monitoring, air quality monitoring for the detection of abnormal gas levels, and identification of things like carbon monoxide poisoning or food spoilage. In coming years, expect to see more virtual medical homes and digital health care complexes. Patients, from the convenience of their homes, might be able to connect to a suite of caregivers, all working collaboratively to provide more coordinated, effective care. The “hospital at home” model that started with six hospitals has already grown to over 100 hospitals across 29 states. The shift from onsite specialists to onscreen specialists will continue, providing greater access to specialized services.
With these emerging trends, it can be anticipated that much acute care will be provided to patients outside the hospital – either under the hospital at home model, via drone technology using telemedicine, through smart devices in smart homes, or via wearables and artificial intelligence. Hence, hospitals’ configuration in the future will be much different and more compact than currently, and many hospitals will be reserved for trauma patients, casualties of natural disasters, higher acuity diseases requiring complex procedures, and other emergencies.
The role of hospitalists has evolved over the years and is still evolving. It should be no surprise if, in the future, we work alongside a digital hospitalist twin to provide better and more personalized care to our patients. Change is uncomfortable but it is inevitable. When COVID hit, we were forced to find innovative ways to deliver care to our patients. One thing is for certain: post-pandemic (AD, or After Disease) we are not going back to a Before COVID (BC) state in terms of virtual care. With the new dawn of digital era, the crucial questions to address will be: What will the future role of a hospitalist look like? How can we leverage technology and embrace our flexibility to adapt to these trends? How can we apply the lessons learned during the pandemic to propel hospital medicine into the future? And is it time to rethink our role and even reclassify ourselves – from hospitalists to Acute Care Experts (ACE) or Primary Acute Care Physicians?
Dr. Zia is a hospitalist, physician advisor, and founder of Virtual Hospitalist - a telemedicine company with a 360-degree care model for hospital patients.
References
1. www.subjectwell.com/news/data-shows-a-majority-of-patients-remain-interested-in-clinical-trials-during-the-coronavirus-pandemic/
2. ww2.frost.com/news/press-releases/technology-innovations-and-virtual-consultations-drive-healthcare-2025/
3. www.gminsights.com/industry-analysis/telemedicine-market
4. www.healthcareitnews.com/blog/frost-sullivans-top-10-predictions-healthcare-2021
5. www.uc.edu/news/articles/2021/03/virtual-medicine--new-uc-telehealth-drone-makes-house-calls.html
6. www.psmarketresearch.com/market-analysis/smart-home-healthcare-market
People often overestimate what will happen in the next two years and underestimate what will happen in ten. – Bill Gates
The COVID-19 pandemic set in motion a series of innovations catalyzing the digital transformation of the health care landscape.
Telemedicine use exploded over the last 12 months to the point that it has almost become ubiquitous. With that, we saw a rapid proliferation of wearables and remote patient monitoring devices. Thanks to virtual care, care delivery is no longer strictly dependent on having onsite specialists, and care itself is not confined to the boundaries of hospitals or doctors’ offices anymore.
We saw the formation of the digital front door and the emergence of new virtual care sites like virtual urgent care, virtual home health, virtual office visits, virtual hospital at home that allowed clinical care to be delivered safely outside the boundaries of hospitals. Nonclinical public places like gyms, schools, and community centers were being transformed into virtual health care portals that brought care closer to the people.
Inside the hospital, we saw a fusion of traditional inpatient care and virtual care. Onsite hospital teams embraced telemedicine during the pandemic for various reasons; to conserve personal protective equipment (PPE), limit exposure, boost care capacity, improve access to specialists at distant sites, and bring family memberse to “webside” who cannot be at a patient’s bedside.
In clinical trials as well, virtual care is a welcome change. According to one survey1, most trial participants favored the use of telehealth services for clinical trials, as these helped them stay engaged, compliant, monitored, and on track while remaining at home. Furthermore, we are seeing the integration of artificial intelligence (AI) into telehealth, whether it is to aid physicians in clinical decision-making or to generate reminders to help patients with chronic disease management. However, this integration is only beginning to scratch the surface of the combination of two technologies’ real potential.
What’s next?
Based on these trends, it should be no surprise that digital health will become a vital sign for health care organizations.
The next 12 to 24 months will set new standards for digital health and play a significant role in defining the next generation of virtual care. There are projections that global health care industry revenues will exceed $2.6 trillion by 2025, with AI and telehealth playing a prominent role in this growth.2 According to estimates, telehealth itself will be a $175 billion market by 2026 and approximately one in three patient encounters will go virtual.3,4 Moreover, virtual care will continue to make exciting transformations, helping to make quality care accessible to everyone in innovative ways. For example, the University of Cincinnati has recently developed a pilot project using a drone equipped with video technology, artificial intelligence, sensors, and first aid kits to go to hard-to-reach areas to deliver care via telemedicine.5
Smart hospitals
In coming years, we can expect the integration of AI, augmented reality (AR), and virtual reality (VR) into telemedicine at lightning speed – and at a much larger scale – that will enable surgeons from different parts of the globe to perform procedures remotely and more precisely.

AI is already gaining traction in different fields within health care – whether it’s predicting length of stay in the ICU, or assisting in triage decisions, or reading radiological images, to name just a few. The Mayo Clinic is using AI and computer-aided decision-making tools to predict the risk of surgery and potential post-op complications, which could allow even better collaboration between medical and surgical teams. We hear about the “X-ray” vision offered to proceduralists using HoloLens – mixed reality smartglasses – a technology that enables them to perform procedures more precisely. Others project that there will be more sensors and voice recognition tools in the OR that will be used to gather data to develop intelligent algorithms, and to build a safety net for interventionalists that can notify them of potential hazards or accidental sterile field breaches. The insights gained will be used to create best practices and even allow some procedures to be performed outside the traditional OR setting.
Additionally, we are seeing the development of “smart” patient rooms. For example, one health system in Florida is working on deploying Amazon Alexa in 2,500 patient rooms to allow patients to connect more easily to their care team members. In the not-so-distant future, smart hospitals with smart patient rooms and smart ORs equipped with telemedicine, AI, AR, mixed reality, and computer-aided decision-making tools will no longer be an exception.
Smart homes for smart care
Smart homes with technologies like gas detectors, movement sensors, and sleep sensors will continue to evolve. According to one estimate, the global smart home health care market was $8.7 billion in 2019, and is expected to be $96.2 billion by 2030.6

Smart technologies will have applications in fall detection and prevention, evaluation of self-administration of medicine, sleep rhythm monitoring, air quality monitoring for the detection of abnormal gas levels, and identification of things like carbon monoxide poisoning or food spoilage. In coming years, expect to see more virtual medical homes and digital health care complexes. Patients, from the convenience of their homes, might be able to connect to a suite of caregivers, all working collaboratively to provide more coordinated, effective care. The “hospital at home” model that started with six hospitals has already grown to over 100 hospitals across 29 states. The shift from onsite specialists to onscreen specialists will continue, providing greater access to specialized services.
With these emerging trends, it can be anticipated that much acute care will be provided to patients outside the hospital – either under the hospital at home model, via drone technology using telemedicine, through smart devices in smart homes, or via wearables and artificial intelligence. Hence, hospitals’ configuration in the future will be much different and more compact than currently, and many hospitals will be reserved for trauma patients, casualties of natural disasters, higher acuity diseases requiring complex procedures, and other emergencies.
The role of hospitalists has evolved over the years and is still evolving. It should be no surprise if, in the future, we work alongside a digital hospitalist twin to provide better and more personalized care to our patients. Change is uncomfortable but it is inevitable. When COVID hit, we were forced to find innovative ways to deliver care to our patients. One thing is for certain: post-pandemic (AD, or After Disease) we are not going back to a Before COVID (BC) state in terms of virtual care. With the new dawn of digital era, the crucial questions to address will be: What will the future role of a hospitalist look like? How can we leverage technology and embrace our flexibility to adapt to these trends? How can we apply the lessons learned during the pandemic to propel hospital medicine into the future? And is it time to rethink our role and even reclassify ourselves – from hospitalists to Acute Care Experts (ACE) or Primary Acute Care Physicians?
Dr. Zia is a hospitalist, physician advisor, and founder of Virtual Hospitalist - a telemedicine company with a 360-degree care model for hospital patients.
References
1. www.subjectwell.com/news/data-shows-a-majority-of-patients-remain-interested-in-clinical-trials-during-the-coronavirus-pandemic/
2. ww2.frost.com/news/press-releases/technology-innovations-and-virtual-consultations-drive-healthcare-2025/
3. www.gminsights.com/industry-analysis/telemedicine-market
4. www.healthcareitnews.com/blog/frost-sullivans-top-10-predictions-healthcare-2021
5. www.uc.edu/news/articles/2021/03/virtual-medicine--new-uc-telehealth-drone-makes-house-calls.html
6. www.psmarketresearch.com/market-analysis/smart-home-healthcare-market
People often overestimate what will happen in the next two years and underestimate what will happen in ten. – Bill Gates
The COVID-19 pandemic set in motion a series of innovations catalyzing the digital transformation of the health care landscape.
Telemedicine use exploded over the last 12 months to the point that it has almost become ubiquitous. With that, we saw a rapid proliferation of wearables and remote patient monitoring devices. Thanks to virtual care, care delivery is no longer strictly dependent on having onsite specialists, and care itself is not confined to the boundaries of hospitals or doctors’ offices anymore.
We saw the formation of the digital front door and the emergence of new virtual care sites like virtual urgent care, virtual home health, virtual office visits, virtual hospital at home that allowed clinical care to be delivered safely outside the boundaries of hospitals. Nonclinical public places like gyms, schools, and community centers were being transformed into virtual health care portals that brought care closer to the people.
Inside the hospital, we saw a fusion of traditional inpatient care and virtual care. Onsite hospital teams embraced telemedicine during the pandemic for various reasons; to conserve personal protective equipment (PPE), limit exposure, boost care capacity, improve access to specialists at distant sites, and bring family memberse to “webside” who cannot be at a patient’s bedside.
In clinical trials as well, virtual care is a welcome change. According to one survey1, most trial participants favored the use of telehealth services for clinical trials, as these helped them stay engaged, compliant, monitored, and on track while remaining at home. Furthermore, we are seeing the integration of artificial intelligence (AI) into telehealth, whether it is to aid physicians in clinical decision-making or to generate reminders to help patients with chronic disease management. However, this integration is only beginning to scratch the surface of the combination of two technologies’ real potential.
What’s next?
Based on these trends, it should be no surprise that digital health will become a vital sign for health care organizations.
The next 12 to 24 months will set new standards for digital health and play a significant role in defining the next generation of virtual care. There are projections that global health care industry revenues will exceed $2.6 trillion by 2025, with AI and telehealth playing a prominent role in this growth.2 According to estimates, telehealth itself will be a $175 billion market by 2026 and approximately one in three patient encounters will go virtual.3,4 Moreover, virtual care will continue to make exciting transformations, helping to make quality care accessible to everyone in innovative ways. For example, the University of Cincinnati has recently developed a pilot project using a drone equipped with video technology, artificial intelligence, sensors, and first aid kits to go to hard-to-reach areas to deliver care via telemedicine.5
Smart hospitals
In coming years, we can expect the integration of AI, augmented reality (AR), and virtual reality (VR) into telemedicine at lightning speed – and at a much larger scale – that will enable surgeons from different parts of the globe to perform procedures remotely and more precisely.

AI is already gaining traction in different fields within health care – whether it’s predicting length of stay in the ICU, or assisting in triage decisions, or reading radiological images, to name just a few. The Mayo Clinic is using AI and computer-aided decision-making tools to predict the risk of surgery and potential post-op complications, which could allow even better collaboration between medical and surgical teams. We hear about the “X-ray” vision offered to proceduralists using HoloLens – mixed reality smartglasses – a technology that enables them to perform procedures more precisely. Others project that there will be more sensors and voice recognition tools in the OR that will be used to gather data to develop intelligent algorithms, and to build a safety net for interventionalists that can notify them of potential hazards or accidental sterile field breaches. The insights gained will be used to create best practices and even allow some procedures to be performed outside the traditional OR setting.
Additionally, we are seeing the development of “smart” patient rooms. For example, one health system in Florida is working on deploying Amazon Alexa in 2,500 patient rooms to allow patients to connect more easily to their care team members. In the not-so-distant future, smart hospitals with smart patient rooms and smart ORs equipped with telemedicine, AI, AR, mixed reality, and computer-aided decision-making tools will no longer be an exception.
Smart homes for smart care
Smart homes with technologies like gas detectors, movement sensors, and sleep sensors will continue to evolve. According to one estimate, the global smart home health care market was $8.7 billion in 2019, and is expected to be $96.2 billion by 2030.6

Smart technologies will have applications in fall detection and prevention, evaluation of self-administration of medicine, sleep rhythm monitoring, air quality monitoring for the detection of abnormal gas levels, and identification of things like carbon monoxide poisoning or food spoilage. In coming years, expect to see more virtual medical homes and digital health care complexes. Patients, from the convenience of their homes, might be able to connect to a suite of caregivers, all working collaboratively to provide more coordinated, effective care. The “hospital at home” model that started with six hospitals has already grown to over 100 hospitals across 29 states. The shift from onsite specialists to onscreen specialists will continue, providing greater access to specialized services.
With these emerging trends, it can be anticipated that much acute care will be provided to patients outside the hospital – either under the hospital at home model, via drone technology using telemedicine, through smart devices in smart homes, or via wearables and artificial intelligence. Hence, hospitals’ configuration in the future will be much different and more compact than currently, and many hospitals will be reserved for trauma patients, casualties of natural disasters, higher acuity diseases requiring complex procedures, and other emergencies.
The role of hospitalists has evolved over the years and is still evolving. It should be no surprise if, in the future, we work alongside a digital hospitalist twin to provide better and more personalized care to our patients. Change is uncomfortable but it is inevitable. When COVID hit, we were forced to find innovative ways to deliver care to our patients. One thing is for certain: post-pandemic (AD, or After Disease) we are not going back to a Before COVID (BC) state in terms of virtual care. With the new dawn of digital era, the crucial questions to address will be: What will the future role of a hospitalist look like? How can we leverage technology and embrace our flexibility to adapt to these trends? How can we apply the lessons learned during the pandemic to propel hospital medicine into the future? And is it time to rethink our role and even reclassify ourselves – from hospitalists to Acute Care Experts (ACE) or Primary Acute Care Physicians?
Dr. Zia is a hospitalist, physician advisor, and founder of Virtual Hospitalist - a telemedicine company with a 360-degree care model for hospital patients.
References
1. www.subjectwell.com/news/data-shows-a-majority-of-patients-remain-interested-in-clinical-trials-during-the-coronavirus-pandemic/
2. ww2.frost.com/news/press-releases/technology-innovations-and-virtual-consultations-drive-healthcare-2025/
3. www.gminsights.com/industry-analysis/telemedicine-market
4. www.healthcareitnews.com/blog/frost-sullivans-top-10-predictions-healthcare-2021
5. www.uc.edu/news/articles/2021/03/virtual-medicine--new-uc-telehealth-drone-makes-house-calls.html
6. www.psmarketresearch.com/market-analysis/smart-home-healthcare-market
Bullying in academic medicine rife, underreported
Bullying in academic medicine, especially among women, is rife, underreported, and remains largely unaddressed, new research suggests.
Investigators reviewed close to 70 studies, encompassing over 82,000 medical consultants or trainees in academic medical settings, and found that men were identified as the most common perpetrators – close to 70% of respondents – whereas women were the most common victims (56%).
Collectively, respondents in all of the studies identified the most common bullies to be consultants (54%), followed by residents (22%), and nurses (15%).
Disturbingly, less than one-third of victims overall reported that they were bullied, and close to 60% who formally reported the abuse said they did not have a positive outcome.
“We found that bullies are commonly men and senior consultants, while more than half of their victims are women,” senior author Harriette G.C. Van Spall, MD, MPH, associate professor of medicine and director of e-health and virtual care, Division of Cardiology, McMaster University, Hamilton, Ont., said in an interview.
“The greatest barriers to addressing academic bullying are the fear of reprisal, lack of impact of reporting, and non-enforcement of anti-bullying policies,” she added.
The study was published online July 12 in BMJ Open.
Personal experience
“Some behaviors were excruciating to deal with, protesting against them would bring more on, and every day was filled with dread. It took sheer will to show up at work to care for patients, to complete research I was leading, and to have hope, and my academic output, income, and personal well-being dropped during those years,” she added.
Dr. Van Spall thought the subject “merited research because our performance as clinicians, researchers, and educators relies on our work environment.”
To investigate, the researchers reviewed 68 studies (n = 82,349 respondents) conducted between 1999 and 2021 in academic medical settings, in which victims were either consultants or trainees. Many of the studies (31) were conducted in the U.S.
Other countries included the United Kingdom, Canada, Australia, Pakistan, Egypt, Iran, Turkey, New Zealand, Lithuania, Greece, India, Germany, Nigeria, Oman, and Finland.
Studies were required to describe the method and impact of bullying; characteristics of the perpetrators and victims; or interventions that were used to address the bullying.
“Bullying” was defined as “the abuse of authority by a perpetrator who targets the victim in an academic setting through punishing behaviors that include overwork, destabilization, and isolation in order to impede the education or career of the target.”
Systemic sexism
Bullying behaviors, reported in 28 studies (n = 35,779 respondents), were grouped into destabilization, threats to professional status, overwork, and isolation, with overwork found to be the most common form of bullying.
The most common impact of being bullied was psychological distress, reported by 39.1% of respondents in 14 studies, followed by considerations of quitting (35.9%; 7 studies), and worsening of clinical performance (34.6%, 8 studies).
“Among demographic groups, men were identified as the most common perpetrators (67.2% of 4,722 respondents in 5 studies) and women the most common victims (56.2% of 15,246 respondents in 27 studies),” the authors report.
“Academic medicine in many institutions is encumbered by systemic sexism that is evident in processes around remuneration, recognition, opportunities for advancement, and leadership positions,” said Dr. Van Spall.
“There are fewer women at decision-making tables in academic medicine, the climb is uphill at the best of times, and women are likely easier targets for bullies, as their voices are easier to drown out,” she added.
She noted that many men do “exhibit wonderful attributes of professionalism and decency,” but “some in positions of power are given impunity by virtue of other accomplishments.”
Multiple deterrents
Thirty-one studies (n = 15,868) described characteristics of the bullies and showed the most common to be consultants (53.6% [30 studies]), residents (22% [22 studies]), and nurses (14.9% [21 studies]).
Only a minority of victims (28.9% of 9,410 victims [10 studies]) formally reported the bullying. The researchers identified multiple deterrents to reporting.
When a formal complaint was submitted (n = 1,139 respondents), it most frequently had no perceived effect (35.6%); more than one-fifth (21.9%) experienced worsening of the bullying, and only 13.7% reported improvement.
The common institutional facilitators of bullying, described in 25 studies, included lack of enforcement of anti-bullying policies (13 studies), the hierarchical structure of medicine (7 studies), and normalization of bullying (10 studies).
Forty-nine studies looked at strategies to address academic bullying, including anti-bullying policies, mandatory workshops on mistreatment, establishing an anti-bullying oversight committee, and institutional support for victims. However, the studies testing the effectiveness of these interventions “had a high risk of bias.”
Support available
Commenting on the research for this news organization, Roberta Gebhard, DO, past president of the American Medical Women’s Association (AMWA) and a member of the advisory board for Physician Just Equity, called it a “good study, large, international, and well-written.”
Dr. Gebhard, a member of the Governing Council for the American Medical Association Women Physician Section, was not associated with this study but said she is currently researching women who left medical school and residency.
“A common reason for leaving is being bullied. Bullying is often not reported and if reported, often not addressed. Or, if addressed, the person who reports it is often retaliated against, which is a common experience, especially in women.”
She advised female physicians who are bullied to get support from other female physicians – for example, by joining the AMWA, which has an online women’s leadership group.
“Having other women physicians throughout the country you can call for advice and support can be helpful,” said Dr. Gebhard, a family practice physician based in Grand Island, New York.
Dr. Van Spall receives support from the Canadian Institutes of Health Research, the Heart and Stroke Foundation, the Women As One Escalator Award, and McMaster Department of Medicine. The study authors and Dr. Gebhard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bullying in academic medicine, especially among women, is rife, underreported, and remains largely unaddressed, new research suggests.
Investigators reviewed close to 70 studies, encompassing over 82,000 medical consultants or trainees in academic medical settings, and found that men were identified as the most common perpetrators – close to 70% of respondents – whereas women were the most common victims (56%).
Collectively, respondents in all of the studies identified the most common bullies to be consultants (54%), followed by residents (22%), and nurses (15%).
Disturbingly, less than one-third of victims overall reported that they were bullied, and close to 60% who formally reported the abuse said they did not have a positive outcome.
“We found that bullies are commonly men and senior consultants, while more than half of their victims are women,” senior author Harriette G.C. Van Spall, MD, MPH, associate professor of medicine and director of e-health and virtual care, Division of Cardiology, McMaster University, Hamilton, Ont., said in an interview.
“The greatest barriers to addressing academic bullying are the fear of reprisal, lack of impact of reporting, and non-enforcement of anti-bullying policies,” she added.
The study was published online July 12 in BMJ Open.
Personal experience
“Some behaviors were excruciating to deal with, protesting against them would bring more on, and every day was filled with dread. It took sheer will to show up at work to care for patients, to complete research I was leading, and to have hope, and my academic output, income, and personal well-being dropped during those years,” she added.
Dr. Van Spall thought the subject “merited research because our performance as clinicians, researchers, and educators relies on our work environment.”
To investigate, the researchers reviewed 68 studies (n = 82,349 respondents) conducted between 1999 and 2021 in academic medical settings, in which victims were either consultants or trainees. Many of the studies (31) were conducted in the U.S.
Other countries included the United Kingdom, Canada, Australia, Pakistan, Egypt, Iran, Turkey, New Zealand, Lithuania, Greece, India, Germany, Nigeria, Oman, and Finland.
Studies were required to describe the method and impact of bullying; characteristics of the perpetrators and victims; or interventions that were used to address the bullying.
“Bullying” was defined as “the abuse of authority by a perpetrator who targets the victim in an academic setting through punishing behaviors that include overwork, destabilization, and isolation in order to impede the education or career of the target.”
Systemic sexism
Bullying behaviors, reported in 28 studies (n = 35,779 respondents), were grouped into destabilization, threats to professional status, overwork, and isolation, with overwork found to be the most common form of bullying.
The most common impact of being bullied was psychological distress, reported by 39.1% of respondents in 14 studies, followed by considerations of quitting (35.9%; 7 studies), and worsening of clinical performance (34.6%, 8 studies).
“Among demographic groups, men were identified as the most common perpetrators (67.2% of 4,722 respondents in 5 studies) and women the most common victims (56.2% of 15,246 respondents in 27 studies),” the authors report.
“Academic medicine in many institutions is encumbered by systemic sexism that is evident in processes around remuneration, recognition, opportunities for advancement, and leadership positions,” said Dr. Van Spall.
“There are fewer women at decision-making tables in academic medicine, the climb is uphill at the best of times, and women are likely easier targets for bullies, as their voices are easier to drown out,” she added.
She noted that many men do “exhibit wonderful attributes of professionalism and decency,” but “some in positions of power are given impunity by virtue of other accomplishments.”
Multiple deterrents
Thirty-one studies (n = 15,868) described characteristics of the bullies and showed the most common to be consultants (53.6% [30 studies]), residents (22% [22 studies]), and nurses (14.9% [21 studies]).
Only a minority of victims (28.9% of 9,410 victims [10 studies]) formally reported the bullying. The researchers identified multiple deterrents to reporting.
When a formal complaint was submitted (n = 1,139 respondents), it most frequently had no perceived effect (35.6%); more than one-fifth (21.9%) experienced worsening of the bullying, and only 13.7% reported improvement.
The common institutional facilitators of bullying, described in 25 studies, included lack of enforcement of anti-bullying policies (13 studies), the hierarchical structure of medicine (7 studies), and normalization of bullying (10 studies).
Forty-nine studies looked at strategies to address academic bullying, including anti-bullying policies, mandatory workshops on mistreatment, establishing an anti-bullying oversight committee, and institutional support for victims. However, the studies testing the effectiveness of these interventions “had a high risk of bias.”
Support available
Commenting on the research for this news organization, Roberta Gebhard, DO, past president of the American Medical Women’s Association (AMWA) and a member of the advisory board for Physician Just Equity, called it a “good study, large, international, and well-written.”
Dr. Gebhard, a member of the Governing Council for the American Medical Association Women Physician Section, was not associated with this study but said she is currently researching women who left medical school and residency.
“A common reason for leaving is being bullied. Bullying is often not reported and if reported, often not addressed. Or, if addressed, the person who reports it is often retaliated against, which is a common experience, especially in women.”
She advised female physicians who are bullied to get support from other female physicians – for example, by joining the AMWA, which has an online women’s leadership group.
“Having other women physicians throughout the country you can call for advice and support can be helpful,” said Dr. Gebhard, a family practice physician based in Grand Island, New York.
Dr. Van Spall receives support from the Canadian Institutes of Health Research, the Heart and Stroke Foundation, the Women As One Escalator Award, and McMaster Department of Medicine. The study authors and Dr. Gebhard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Bullying in academic medicine, especially among women, is rife, underreported, and remains largely unaddressed, new research suggests.
Investigators reviewed close to 70 studies, encompassing over 82,000 medical consultants or trainees in academic medical settings, and found that men were identified as the most common perpetrators – close to 70% of respondents – whereas women were the most common victims (56%).
Collectively, respondents in all of the studies identified the most common bullies to be consultants (54%), followed by residents (22%), and nurses (15%).
Disturbingly, less than one-third of victims overall reported that they were bullied, and close to 60% who formally reported the abuse said they did not have a positive outcome.
“We found that bullies are commonly men and senior consultants, while more than half of their victims are women,” senior author Harriette G.C. Van Spall, MD, MPH, associate professor of medicine and director of e-health and virtual care, Division of Cardiology, McMaster University, Hamilton, Ont., said in an interview.
“The greatest barriers to addressing academic bullying are the fear of reprisal, lack of impact of reporting, and non-enforcement of anti-bullying policies,” she added.
The study was published online July 12 in BMJ Open.
Personal experience
“Some behaviors were excruciating to deal with, protesting against them would bring more on, and every day was filled with dread. It took sheer will to show up at work to care for patients, to complete research I was leading, and to have hope, and my academic output, income, and personal well-being dropped during those years,” she added.
Dr. Van Spall thought the subject “merited research because our performance as clinicians, researchers, and educators relies on our work environment.”
To investigate, the researchers reviewed 68 studies (n = 82,349 respondents) conducted between 1999 and 2021 in academic medical settings, in which victims were either consultants or trainees. Many of the studies (31) were conducted in the U.S.
Other countries included the United Kingdom, Canada, Australia, Pakistan, Egypt, Iran, Turkey, New Zealand, Lithuania, Greece, India, Germany, Nigeria, Oman, and Finland.
Studies were required to describe the method and impact of bullying; characteristics of the perpetrators and victims; or interventions that were used to address the bullying.
“Bullying” was defined as “the abuse of authority by a perpetrator who targets the victim in an academic setting through punishing behaviors that include overwork, destabilization, and isolation in order to impede the education or career of the target.”
Systemic sexism
Bullying behaviors, reported in 28 studies (n = 35,779 respondents), were grouped into destabilization, threats to professional status, overwork, and isolation, with overwork found to be the most common form of bullying.
The most common impact of being bullied was psychological distress, reported by 39.1% of respondents in 14 studies, followed by considerations of quitting (35.9%; 7 studies), and worsening of clinical performance (34.6%, 8 studies).
“Among demographic groups, men were identified as the most common perpetrators (67.2% of 4,722 respondents in 5 studies) and women the most common victims (56.2% of 15,246 respondents in 27 studies),” the authors report.
“Academic medicine in many institutions is encumbered by systemic sexism that is evident in processes around remuneration, recognition, opportunities for advancement, and leadership positions,” said Dr. Van Spall.
“There are fewer women at decision-making tables in academic medicine, the climb is uphill at the best of times, and women are likely easier targets for bullies, as their voices are easier to drown out,” she added.
She noted that many men do “exhibit wonderful attributes of professionalism and decency,” but “some in positions of power are given impunity by virtue of other accomplishments.”
Multiple deterrents
Thirty-one studies (n = 15,868) described characteristics of the bullies and showed the most common to be consultants (53.6% [30 studies]), residents (22% [22 studies]), and nurses (14.9% [21 studies]).
Only a minority of victims (28.9% of 9,410 victims [10 studies]) formally reported the bullying. The researchers identified multiple deterrents to reporting.
When a formal complaint was submitted (n = 1,139 respondents), it most frequently had no perceived effect (35.6%); more than one-fifth (21.9%) experienced worsening of the bullying, and only 13.7% reported improvement.
The common institutional facilitators of bullying, described in 25 studies, included lack of enforcement of anti-bullying policies (13 studies), the hierarchical structure of medicine (7 studies), and normalization of bullying (10 studies).
Forty-nine studies looked at strategies to address academic bullying, including anti-bullying policies, mandatory workshops on mistreatment, establishing an anti-bullying oversight committee, and institutional support for victims. However, the studies testing the effectiveness of these interventions “had a high risk of bias.”
Support available
Commenting on the research for this news organization, Roberta Gebhard, DO, past president of the American Medical Women’s Association (AMWA) and a member of the advisory board for Physician Just Equity, called it a “good study, large, international, and well-written.”
Dr. Gebhard, a member of the Governing Council for the American Medical Association Women Physician Section, was not associated with this study but said she is currently researching women who left medical school and residency.
“A common reason for leaving is being bullied. Bullying is often not reported and if reported, often not addressed. Or, if addressed, the person who reports it is often retaliated against, which is a common experience, especially in women.”
She advised female physicians who are bullied to get support from other female physicians – for example, by joining the AMWA, which has an online women’s leadership group.
“Having other women physicians throughout the country you can call for advice and support can be helpful,” said Dr. Gebhard, a family practice physician based in Grand Island, New York.
Dr. Van Spall receives support from the Canadian Institutes of Health Research, the Heart and Stroke Foundation, the Women As One Escalator Award, and McMaster Department of Medicine. The study authors and Dr. Gebhard have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.





