User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Delta variant among the most infectious respiratory viruses, CDC says
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
.
“Today, I want to speak about our need to come together against a common enemy. SARS-CoV-2 and the Delta variant is spreading with incredible efficiency, and now represents more than 83% of the virus circulating in the U.S.,” Dr. Walensky said at a news briefing July 22. “It is one of the most infectious respiratory viruses we know of and that I have seen in my 20-year career.”
Dr. Walensky said there were 46,318 cases of COVID-19 reported July 21, with a 7-day average of 37,700 cases per day -- up 53% from the previous week. Hospital admissions average about 3,500 per day, an increase of 32%. The 7-day average of deaths is 237 -- a 19% increase from the previous week.
Meanwhile, there are now 162 million Americans who are fully vaccinated against COVID-19.
Areas with low vaccination coverage continue to have the highest case numbers, she reported, with unvaccinated people accounting for 97% of hospitalizations and deaths.
But there may be early signs of progress. The four states with the highest case rates -- Arkansas, Florida, Louisiana, and Nevada -- had a higher rate of new vaccinations, compared with the national average over the past week, White House COVID-19 Response Coordinator Jeff Zients said.
He also announced that the administration will send $100 million to nearly 2,000 rural health clinics to support vaccine education and outreach efforts.
Dr. Walensky said despite the rising numbers, the CDC mask guidance remains the same, but she encouraged vaccinated people to wear masks if they choose.
“Whether you are vaccinated or not, please know we together are not out of the woods yet,” she said. “We are yet at another pivotal moment in this pandemic, with cases rising again and hospitals reaching their capacity in some areas.”
A version of this article first appeared on WebMD.com.
Acid suppression therapy increases intestinal colonization of MDROs
Background: Acid suppressants inhibit gastric acid secretion and disrupt the intestinal microbiome, but whether that facilitates colonization and infection with MDROs is unclear.
Study design: Systematic review and meta-analysis.
Setting: Observational studies searched from database through July 2019.
Synopsis: A total of 26 observational studies published during 1996-2019 with 29,382 patients were included in this meta-analysis. Of those, 24 studies directly measured intestinal MDRO carriage and 2 used urinary tract infections (UTIs) as the outcome measure, since most UTIs are caused by bacteria that colonize the intestinal tract. Target MDROs included multidrug-resistant Enterobacteriaceae (MRD-E) and vancomycin-resistant enterococci (VRE). Meta-analysis demonstrated that acid suppression is associated with increased odds of intestinal MDRO colonization (MDR-E: odds ratio, 1.60; 95% confidence interval, 1.33-1.92; VRE: OR, 1.97; 95% CI, 1.49-2.60), in both community and health care settings. The risk was similar for colonization with MDR-E and VRE. Regarding the effect of acid suppression by drug class, results were mixed with some studies demonstrating increased risk of MDRO in PPI users only while others reported increased risk only with H2-receptor antagonists.
Bottom line: Acid suppression therapy is associated with increased odds of MDRO colonization. While observational studies cannot prove causation, it is wise to avoid excessive use of acid suppressants.
Citation: Willems RPJ et al. Evaluation of the association between gastric acid suppression and risk of intestinal colonization with multidrug-resistant microorganisms: A systematic review and meta-analysis. JAMA Intern Med. 2020 Feb 24;180(4):561-71.
Dr. Li is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Acid suppressants inhibit gastric acid secretion and disrupt the intestinal microbiome, but whether that facilitates colonization and infection with MDROs is unclear.
Study design: Systematic review and meta-analysis.
Setting: Observational studies searched from database through July 2019.
Synopsis: A total of 26 observational studies published during 1996-2019 with 29,382 patients were included in this meta-analysis. Of those, 24 studies directly measured intestinal MDRO carriage and 2 used urinary tract infections (UTIs) as the outcome measure, since most UTIs are caused by bacteria that colonize the intestinal tract. Target MDROs included multidrug-resistant Enterobacteriaceae (MRD-E) and vancomycin-resistant enterococci (VRE). Meta-analysis demonstrated that acid suppression is associated with increased odds of intestinal MDRO colonization (MDR-E: odds ratio, 1.60; 95% confidence interval, 1.33-1.92; VRE: OR, 1.97; 95% CI, 1.49-2.60), in both community and health care settings. The risk was similar for colonization with MDR-E and VRE. Regarding the effect of acid suppression by drug class, results were mixed with some studies demonstrating increased risk of MDRO in PPI users only while others reported increased risk only with H2-receptor antagonists.
Bottom line: Acid suppression therapy is associated with increased odds of MDRO colonization. While observational studies cannot prove causation, it is wise to avoid excessive use of acid suppressants.
Citation: Willems RPJ et al. Evaluation of the association between gastric acid suppression and risk of intestinal colonization with multidrug-resistant microorganisms: A systematic review and meta-analysis. JAMA Intern Med. 2020 Feb 24;180(4):561-71.
Dr. Li is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Acid suppressants inhibit gastric acid secretion and disrupt the intestinal microbiome, but whether that facilitates colonization and infection with MDROs is unclear.
Study design: Systematic review and meta-analysis.
Setting: Observational studies searched from database through July 2019.
Synopsis: A total of 26 observational studies published during 1996-2019 with 29,382 patients were included in this meta-analysis. Of those, 24 studies directly measured intestinal MDRO carriage and 2 used urinary tract infections (UTIs) as the outcome measure, since most UTIs are caused by bacteria that colonize the intestinal tract. Target MDROs included multidrug-resistant Enterobacteriaceae (MRD-E) and vancomycin-resistant enterococci (VRE). Meta-analysis demonstrated that acid suppression is associated with increased odds of intestinal MDRO colonization (MDR-E: odds ratio, 1.60; 95% confidence interval, 1.33-1.92; VRE: OR, 1.97; 95% CI, 1.49-2.60), in both community and health care settings. The risk was similar for colonization with MDR-E and VRE. Regarding the effect of acid suppression by drug class, results were mixed with some studies demonstrating increased risk of MDRO in PPI users only while others reported increased risk only with H2-receptor antagonists.
Bottom line: Acid suppression therapy is associated with increased odds of MDRO colonization. While observational studies cannot prove causation, it is wise to avoid excessive use of acid suppressants.
Citation: Willems RPJ et al. Evaluation of the association between gastric acid suppression and risk of intestinal colonization with multidrug-resistant microorganisms: A systematic review and meta-analysis. JAMA Intern Med. 2020 Feb 24;180(4):561-71.
Dr. Li is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Sickle cell disease, trait may up risk for poor COVID outcomes
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Sickle cell disease (SCD) was associated with a greater than fourfold excess risk for COVID-19–related hospitalization and a greater than twofold risk for COVID-19–related death, according to a big-data analysis from the United Kingdom.
SCD was associated with an adjusted hazard ratio (HR) of 4.11 (95% confidence interval, 2.98-5.66) for admission to hospital and an HR of 2.55 (95% CI, 1.36-4.75) for death, report Ashley K. Clift, MBBS, a clinical research fellow at the University of Oxford, and colleagues. The results were published online July 20 in Annals of Internal Medicine.
Even those who carry just one copy of the sickle cell gene – the carrier status for sickle cell disease – appeared to be at heightened risk for these outcomes (HR for hospitalization, 1.38; 95% CI, 1.12-1.70; HR for death, 1.51; 95% CI, 1.13-2.00).
“Given the well-known ethnic patterning of sickle cell disorders, the predisposition they pose to other infections, and early evidence from smaller registries, we thought this would be an important analysis to run at the population level,” Dr. Clift said in an interview.
in terms of vaccination strategies and advice on nonpharmacological interventions,” he said.
“The best course of action for managing risk in this group is vaccination,” said Enrico M. Novelli, MD, director of the adult sickle cell program at the University of Pittsburgh Medical Center. Dr. Novelli, who is also section chief of benign hematology in the university’s School of Medicine, was not involved in the study. “To date, there are no specific studies of the effect of COVID-19 vaccination in patients with SCD, but there is no reason to believe it would be less effective or more risky in this patient population,” he said.
In addition, common-sense measures, such as masking and physical distancing, particularly at large, indoor gatherings, should be encouraged, Dr. Novelli added. Keeping SCD under good control with available treatments is also important. “Any patient with SCD who contracts COVID-19 should undergo close, outpatient monitoring with pulse oxygen measurements. If sick, they should be hospitalized in a center familiar with the care of SCD patients.”
The U.K. results are in line with and expand on earlier evidence from specialist centers and registries, but the association with sickle cell trait has been unclear and is notable in these findings, Dr. Clift said.
“The finding of the association with sickle cell trait is somewhat unexpected,” pediatric hematologist/oncologist Rabi Hanna, MD, director of pediatric bone marrow transplantation at Cleveland Clinic Children’s, told this news organization. “But I would question the accuracy of the numbers, since not all people with the trait realize they have it. In other respects, the study confirms earlier hypotheses and data from single-center studies.” Dr. Hanna did not participate in the U.K. study.
Study details
The SCD cohort consisted of 5,059 persons with SCD and 25,682 carriers, those with just one copy of the trait. Data were drawn from the United Kingdom’s large primary-care QResearch database. Follow-up for hospitalizations was conducted from Jan. 24, 2020 to Sept. 30, 2020; follow-up for deaths was conducted from Jan. 24, 2020 to Jan. 18, 2021. Among adults with SCD, there were 40 hospitalizations and 10 deaths. Among those with sickle cell trait, there were 98 hospitalizations and 50 deaths. No children died, and only a few (<5) required hospitalization.
Previous registry research showed similarly elevated risks for severe disease and fatality among patients with SCD who were infected with SARS-CoV-2.
Because SCD affects 8 to 12 million people globally – 100,000 in the United States – the authors say their results are important for policymakers and for prioritizing vaccination. They also note that trait carriers may be underdiagnosed.
“While SCD is part of newborn screening, there may be undiagnosed older people with the trait in the general population, but it’s difficult to quantify how much this is undiagnosed,” Dr. Clift said. “But now we have these results, it’s not that surprising that sickle cell trait is also associated with increased risk, albeit to a lower extent. This could suggest an almost dose-like effect of the sickle mutations on COVID hospitalization risk.”
Neonatal screening for the most common form of SCD is currently mandatory in the United States, but the Centers for Disease Control and Prevention has no clear data on how many people are aware they are carriers, Dr. Hanna said. “The states didn’t all begin screening at the same time – some started in the 1990s, others started in the 2000s – so many young adults may be unaware they have the trait,” he said.
Dr. Clift said the multiorgan complications of SCD, such as cardiac and immune problems, may be contributing to the heightened risk in individuals infected with SARS-CoV-2. “For example, we know that people with sickle cell disease are more susceptible to other viral infections. There is also some pathophysiological overlap between SCD disease and severe COVID, such as clotting dysfunction, so that may be worth further exploration,” he said.
The overlapping clotting problems associated with both COVID-19 and SCD could increase the risk for severe venous thromboembolism. In addition, experts noted that patients with SCD often have pre-COVID endothelial damage and baseline inflammation and are very sensitive to hypoxia; as well, a sizable proportion have lung disease.
The message to patients and physicians counseling patients is twofold, said Dr. Hanna: “SCD patients are at higher risk of COVID complications, and these are preventable with vaccination.”
The study was supported by the UK Medical Research Council. Dr. Clift is supported by Cancer Research UK. Coauthor Dr. Hippisley-Cox has received fees from ClinRisk and nonfinancial support from QResearch outside of the submitted work. Dr. Hanna has disclosed no relevant financial relationships. Dr. Novelli is a consultant for Novartis.
A version of this article first appeared on Medscape.com.
Statins again linked to lower COVID-19 mortality
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
Among patients hospitalized for COVID-19, those who had been taking statins had a substantially lower risk of death in a new large observational study.
Results showed that use of statins prior to admission was linked to a greater than 40% reduction in mortality and a greater than 25% reduction in risk of developing a severe outcome.
The findings come an analysis of data from the American Heart Association’s COVID-19 Cardiovascular Disease Registry on more than 10,000 patients hospitalized with COVID-19 at 104 hospitals across the United States published in PLoS One.
While several other studies have suggested benefits of statins in COVID-19, this is by far the largest study so far on this topic.
“I would say this is the most reliable study on statins in COVID-19 to date, with the results adjusted for many confounders, including socioeconomic factors and insurance type,” lead author Lori B. Daniels, MD, told this news organization. “However, it still an observational study and therefore falls short of a randomized study. But I would think a randomized study of statins in COVID-19 is probably not feasible, so this study provides excellent data at an observational level.”
After propensity matching for cardiovascular disease, results showed that most of the benefit of statins occurred in patients with known cardiovascular disease.
“While most patients taking statins will have cardiovascular disease, there are also many patients who take these drugs who don’t have heart disease but do have cardiovascular risk factors, such as those with raised cholesterol, or a family history of cardiovascular disease. For [such patients], the effect of statins was also in the same direction but it was not significant. This doesn’t exclude an effect,” noted Dr. Daniels, who is professor of medicine and director of cardiovascular intensive care at the University of California, San Diego.
“We are not saying that everyone should rush out and take a statin if they do not have risk factors for cardiovascular in order to lower their risk of dying from COVID. But if individuals do have an indication for a statin and are not taking one of these dugs this is another good reason to start taking them now,” she added.
The investigators embarked on the study because, although previous observational studies have found that statins may reduce the severity of COVID-19 infection, these studies have been limited in size with mostly single-center or regional studies, and some results have been conflicting. They therefore conducted the current, much larger analysis, in the AHA COVID-19 CVD Registry which systematically collected hospitalized patient–level data in a broad and diverse hospital and patient population across the United States.
For the analysis, the researchers analyzed data from 10,541 patients hospitalized with COVID-19 through September 2020 at 104 U.S. hospitals enrolled in the AHA registry to evaluate the associations between statin use and outcomes.
Most patients (71%) had either cardiovascular disease, hypertension, or both. Prior to admission, 42% of subjects used statins, with 7% being on statins alone and 35% on statins plus antihypertensives. Death (or discharge to hospice) occurred in 2,212 subjects (21%).
Results showed that outpatient use of statins, either alone or with antihypertensives, was associated with a 41% reduced risk of death (odds ratio, 0.59; 95% confidence interval, 0.50-0.69), after adjusting for demographic characteristics, underlying conditions, insurance status, hospital site, and concurrent medications. Statin use was also associated with a roughly 25% lower adjusted odds of developing severe disease.
Noting that patients on statins are also likely to be on antihypertensive medication, the researchers found that the statin benefit on mortality was seen in both patients taking a statin alone (OR, 0.54) and in those taking statins with an antihypertensive medication (OR, 0.60).
Use of antihypertensive drugs was associated with a smaller, albeit still substantial, 27% lower odds of death (OR, 0.73; 95% CI, 0.62-0.87).
In propensity-matched analyses, use of statins and/or antihypertensives was tied to a 32% reduced risk of death among those with a history of CVD and/or hypertension (OR, 0.68; 95% CI, 0.58-0.81). An observed 16% reduction in odds of death with statins and/or antihypertensive drugs among those without cardiovascular disease and/or hypertension was not statistically significant (OR, 0.84; 95% CI, 0.58-1.22).
Stabilizing the underlying disease
The researchers pointed out that the results of the propensity matching analysis are consistent with the hypothesis that the major benefit of these medications accrues from treating and/or stabilizing underlying disease.
“Although it is well known that statins improve long-term outcomes among patients with or at elevated risk for cardiovascular disease, the association with a large short-term benefit which accrues in the setting of hospitalization for COVID-19 is a new and intriguing finding,” they said.
They cited several “plausible mechanisms whereby statins could directly mitigate outcomes in COVID-19 beyond treating underlying disease conditions,” including anti-inflammatory effects and a direct inhibitory effect on the SARS-CoV-2 virus.
Dr. Daniels elaborated more on the potential mechanism at play in an interview: “I think what is happening is that the statin is stabilizing the coronary disease so patients are less likely to die from MI or stroke, and this gives them more time and strength to recover from COVID-19.”
She added: “Statins may also have some direct anti-COVID effects such as an anti-inflammatory actions, but I would guess that this is probably not the primary effect behind what we’re seeing here.”
‘Important clinical implications’
The authors say their findings have “important clinical implications.”
They noted that early in the pandemic there was speculation that certain medications, including statins, and the ACE inhibitor/angiotensin receptor blocker (ARB) classes of antihypertensives may confer an increased susceptibility to COVID-19 positivity and/or severity.
“Our study reinforces the AHA and others’ recommendations that not only is it safe to remain on these medications, but they may substantially reduce risk of severe COVID-19 and especially death from COVID-19, particularly statins, and particularly among those with associated underlying conditions,” the authors stressed.
Dr. Daniels added that, although statins are very safe drugs, there are always some patients who prefer not to take medication even if indicated, and others who may have borderline indications and decide not to take a statin at present.
“This study may persuade these patients that taking a statin is the right thing to do. It may give those patients on the cusp of thinking about taking one of these drugs a reason to go ahead,” she said.
‘Provocative but not definitive’
Commenting on the study, Robert Harrington, MD, professor of medicine and chair of the department of medicine at Stanford (Calif.) University, said: “These are interesting observational data but as such have all the limitations of nonrandomized comparisons despite the best attempts to adjust for a variety of potential confounders. For example, is this an effect of statins (perhaps through some anti-inflammatory mechanism) or is it more an effect that can be attributed to the patients who are prescribed and taking a statin, compared with those who are not?”
He added: “The primary clinical benefit of statins, based on many large randomized clinical trials, seems to be derived from their LDL lowering effect. Observational studies have suggested potential benefits from anti-inflammatory effects of statins, but the randomized trials have not confirmed these observations. So, the current data are interesting, even provocative, but ultimately hypothesis generating rather than definitive.”
Also commenting on the study, Steven Nissen, MD, professor of medicine at the Cleveland Clinic, said: “While statins have many established benefits, their role in preventing COVID-19 complications is very speculative. Like all observational studies, the current study must be viewed as hypothesis generating, not definitive evidence of benefit. There are many potential confounders. I’m skeptical.”
The authors of this study received no specific funding for this work and report no competing interests. Dr. Harrington was AHA president when the COVID registry was created and he is still a member of the AHA board, which has oversight over the project.
FROM PLOS ONE
‘Dealing with a different beast’: Why Delta has doctors worried
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Catherine O’Neal, MD, an infectious disease physician, took to the podium of the Louisiana governor’s press conference recently and did not mince words.
“The Delta variant is not last year’s virus, and it’s become incredibly apparent to healthcare workers that we are dealing with a different beast,” she said.
Louisiana is one of the least vaccinated states in the country. In the United States as a whole, 48.6% of the population is fully vaccinated. In Louisiana, it’s just 36%, and Delta is bearing down.
Dr. O’Neal spoke about the pressure that rising COVID cases were already putting on her hospital, Our Lady of the Lake Regional Medical Center in Baton Rouge. She talked about watching her peers, 30- and 40-year-olds, become severely ill with the latest iteration of the new coronavirus — the Delta variant — which is sweeping through the United States with astonishing speed, causing new cases, hospitalizations, and deaths to rise again.
Dr. O’Neal talked about parents who might not be alive to see their children go off to college in a few weeks. She talked about increasing hospital admissions for infected kids and pregnant women on ventilators.
“I want to be clear after seeing what we’ve seen the last two weeks. We only have two choices: We are either going to get vaccinated and end the pandemic, or we’re going to accept death and a lot of it,” Dr. O’Neal said, her voice choked by emotion.
Where Delta goes, death follows
Delta was first identified in India, where it caused a devastating surge in the spring. In a population that was largely unvaccinated, researchers think it may have caused as many as three million deaths. In just a few months’ time, it has sped across the globe.
, which was first identified in the United Kingdom).
Where a single infected person might have spread older versions of the virus to two or three others, mathematician and epidemiologist Adam Kucharski, PhD, an associate professor at the London School of Hygiene and Tropical Medicine, thinks that number — called the basic reproduction number — might be around six for Delta, meaning that, on average, each infected person spreads the virus to six others.
“The Delta variant is the most able and fastest and fittest of those viruses,” said Mike Ryan, executive director of the World Health Organization’s Health Emergencies Programme, in a recent press briefing.
Early evidence suggests it may also cause more severe disease in people who are not vaccinated.
“There’s clearly increased risk of ICU admission, hospitalization, and death,” said Ashleigh Tuite, PhD, MPH, an infectious disease epidemiologist at the University of Toronto in Ontario.
In a study published ahead of peer review, Dr. Tuite and her coauthor, David Fisman, MD, MPH, reviewed the health outcomes for more than 200,000 people who tested positive for SARS-CoV-2 in Ontario between February and June of 2021. Starting in February, Ontario began screening all positive COVID tests for mutations in the N501Y region for signs of mutation.
Compared with versions of the coronavirus that circulated in 2020, having an Alpha, Beta, or Gamma variant modestly increased the odds that an infected person would become sicker. The Delta variant raised the risk even higher, more than doubling the odds that an infected person would need to be hospitalized or could die from their infection.
Emerging evidence from England and Scotland, analyzed by Public Health England, also shows an increased risk for hospitalization with Delta. The increases are in line with the Canadian data. Experts caution that the picture may change over time as more evidence is gathered.
“What is causing that? We don’t know,” Dr. Tuite said.
Enhanced virus
The Delta variants (there’s actually more than one in the same viral family) have about 15 different mutations compared with the original virus. Two of these, L452R and E484Q, are mutations to the spike protein that were first flagged as problematic in other variants because they appear to help the virus escape the antibodies we make to fight it.
It has another mutation away from its binding site that’s also getting researchers’ attention — P681R.
This mutation appears to enhance the “springiness” of the parts of the virus that dock onto our cells, said Alexander Greninger, MD, PhD, assistant director of the UW Medicine Clinical Virology Laboratory at the University of Washington in Seattle. So it’s more likely to be in the right position to infect our cells if we come into contact with it.
Another theory is that P681R may also enhance the virus’s ability to fuse cells together into clumps that have several different nuclei. These balls of fused cells are called syncytia.
“So it turns into a big factory for making viruses,” said Kamran Kadkhoda, PhD, medical director of immunopathology at the Cleveland Clinic in Ohio.
This capability is not unique to Delta or even to the new coronavirus. Earlier versions and other viruses can do the same thing, but according to a recent paper in Nature, the syncytia that Delta creates are larger than the ones created by previous variants.
Scientists aren’t sure what these supersized syncytia mean, exactly, but they have some theories. They may help the virus copy itself more quickly, so a person’s viral load builds up quickly. That may enhance the ability of the virus to transmit from person to person.
And at least one recent study from China supports this idea. That study, which was posted ahead of peer review on the website Virological.org, tracked 167 people infected with Delta back to a single index case.
China has used extensive contact tracing to identify people that may have been exposed to the virus and sequester them quickly to tamp down its spread. Once a person is isolated or quarantined, they are tested daily with gold-standard PCR testing to determine whether or not they were infected.
Researchers compared the characteristics of Delta cases with those of people infected in 2020 with previous versions of the virus.
This study found that people infected by Delta tested positive more quickly than their predecessors did. In 2020, it took an average of 6 days for someone to test positive after an exposure. With Delta, it took an average of about 4 days.
When people tested positive, they had more than 1,000 times more virus in their bodies, suggesting that the Delta variant has a higher growth rate in the body.
This gives Delta a big advantage. According to Angie Rasmussen, PhD, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan in Canada, who posted a thread about the study on Twitter, if people are shedding 1,000 times more virus, it is much more likely that close contacts will be exposed to enough of it to become infected themselves.
And if they’re shedding earlier in the course of their infections, the virus has more opportunity to spread.
This may help explain why Delta is so much more contagious.
Beyond transmission, Delta’s ability to form syncytia may have two other important consequences. It may help the virus hide from our immune system, and it may make the virus more damaging to the body.
Commonly, when a virus infects a cell, it will corrupt the cell’s protein-making machinery to crank out more copies of itself. When the cell dies, these new copies are released into the plasma outside the cell where they can float over and infect new cells. It’s in this extracellular space where a virus can also be attacked by the neutralizing antibodies our immune system makes to fight it off.
“Antibodies don’t penetrate inside the cell. If these viruses are going from one cell to another by just fusing to each other, antibodies become less useful,” Dr. Kadkhoda said.
Escape artist
Recent studies show that Delta is also able to escape antibodies made in response to vaccination more effectively than the Alpha, or B.1.1.7 strain. The effect was more pronounced in older adults, who tend to have weaker responses to vaccines in general.
This evasion of the immune system is particularly problematic for people who are only partially vaccinated. Data from the United Kingdom show that a single dose of vaccine is only about 31% effective at preventing illness with Delta, and 75% effective at preventing hospitalization.
After two doses, the vaccines are still highly effective — even against Delta — reaching 80% protection for illness, and 94% for hospitalization, which is why U.S. officials are begging people to get both doses of their shots, and do it as quickly as possible.
Finally, the virus’s ability to form syncytia may leave greater damage behind in the body’s tissues and organs.
“Especially in the lungs,” Dr. Kadkhoda said. The lungs are very fragile tissues. Their tiny air sacs — the alveoli — are only a single-cell thick. They have to be very thin to exchange oxygen in the blood.
“Any damage like that can severely affect any oxygen exchange and the normal housekeeping activities of that tissue,” he said. “In those vital organs, it may be very problematic.”
The research is still early, but studies in animals and cell lines are backing up what doctors say they are seeing in hospitalized patients.
A recent preprint study from researchers in Japan found that hamsters infected with Delta lost more weight — a proxy for how sick they were — compared with hamsters infected with an older version of the virus. The researchers attribute this to the viruses› ability to fuse cells together to form syncytia.
Another investigation, from researchers in India, infected two groups of hamsters — one with the original “wild type” strain of the virus, the other with the Delta variant of the new coronavirus.
As in the Japanese study, the hamsters infected with Delta lost more weight. When the researchers performed necropsies on the animals, they found more lung damage and bleeding in hamsters infected with Delta. This study was also posted as a preprint ahead of peer review.
German researchers working with pseudotyped versions of the new coronavirus — viruses that have been genetically changed to make them safer to work with — watched what happened after they used these pseudoviruses to infect lung, colon, and kidney cells in the lab.
They, too, found that cells infected with the Delta variant formed more and larger syncytia compared with cells infected with the wild type strain of the virus. The authors write that their findings suggest Delta could “cause more tissue damage, and thus be more pathogenic, than previous variants.”Researchers say it’s important to remember that, while interesting, this research isn’t conclusive. Hamsters and cells aren’t humans. More studies are needed to prove these theories.
Scientists say that what we already know about Delta makes vaccination more important than ever.
“The net effect is really that, you know, this is worrisome in people who are unvaccinated and then people who have breakthrough infections, but it’s not…a reason to panic or to throw up our hands and say you know, this pandemic is never going to end,” Dr. Tuite said, “[b]ecause what we do see is that the vaccines continue to be highly protective.”
A version of this article first appeared on Medscape.com.
Empirical anti-MRSA therapy does not improve mortality in patients with pneumonia
Background: Empirical broad-spectrum antibiotics including anti-MRSA therapy are often selected because of concerns for resistant organisms. However, the outcomes of empirical anti-MRSA therapy among patients with pneumonia are unknown.
Study design: A national retrospective multicenter cohort study of hospitalizations for pneumonia.
Setting: This cohort study included 88,605 hospitalizations for pneumonia in the Veterans Health Administration health care system during 2008-2013, in which patients received either anti-MRSA or standard therapy for community-onset pneumonia.
Synopsis: Among 88,605 hospitalizations for pneumonia, 38% of the patients received empirical anti-MRSA therapy within the first day of hospitalization and vancomycin accounted for 98% of the therapy. The primary outcome was 30-day all-cause mortality after adjustment for patient comorbidities, vital signs, and laboratory results. Three treatment groups were studied: patients receiving anti-MRSA therapy (vancomycin hydrochloride or linezolid) plus guideline-recommended standard antibiotics (beta-lactam and macrolide or tetracycline hydrochloride, or fluoroquinolone); patients receiving anti-MRSA therapy without standard antibiotics; and patients receiving standard therapy alone. There was no mortality benefit of empirical anti-MRSA therapy versus standard antibiotics, even in those with risk factors for MRSA or in those whose clinical severity warranted admission to the ICU. Empirical anti-MRSA treatment was associated with greater 30-day mortality compared with standard therapy alone, with an adjusted risk ratio of 1.4 (95% confidence interval, 1.3-1.5) versus empirical anti-MRSA treatment plus standard therapy and 1.5 (1.4-1.6) versus empirical anti-MRSA treatment without standard therapy.
Bottom line: Empirical anti-MRSA therapy does not improve mortality and should not be routinely used in patients hospitalized for community-onset pneumonia, even in those with MRSA risk factors.
Citation: Jones BE et al. Empirical anti-MRSA vs. standard antibiotic therapy and risk of 30-day mortality in patients hospitalized for pneumonia. JAMA Intern Med. 2020 Feb 17;180(4):552-60.
Dr. Li is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Empirical broad-spectrum antibiotics including anti-MRSA therapy are often selected because of concerns for resistant organisms. However, the outcomes of empirical anti-MRSA therapy among patients with pneumonia are unknown.
Study design: A national retrospective multicenter cohort study of hospitalizations for pneumonia.
Setting: This cohort study included 88,605 hospitalizations for pneumonia in the Veterans Health Administration health care system during 2008-2013, in which patients received either anti-MRSA or standard therapy for community-onset pneumonia.
Synopsis: Among 88,605 hospitalizations for pneumonia, 38% of the patients received empirical anti-MRSA therapy within the first day of hospitalization and vancomycin accounted for 98% of the therapy. The primary outcome was 30-day all-cause mortality after adjustment for patient comorbidities, vital signs, and laboratory results. Three treatment groups were studied: patients receiving anti-MRSA therapy (vancomycin hydrochloride or linezolid) plus guideline-recommended standard antibiotics (beta-lactam and macrolide or tetracycline hydrochloride, or fluoroquinolone); patients receiving anti-MRSA therapy without standard antibiotics; and patients receiving standard therapy alone. There was no mortality benefit of empirical anti-MRSA therapy versus standard antibiotics, even in those with risk factors for MRSA or in those whose clinical severity warranted admission to the ICU. Empirical anti-MRSA treatment was associated with greater 30-day mortality compared with standard therapy alone, with an adjusted risk ratio of 1.4 (95% confidence interval, 1.3-1.5) versus empirical anti-MRSA treatment plus standard therapy and 1.5 (1.4-1.6) versus empirical anti-MRSA treatment without standard therapy.
Bottom line: Empirical anti-MRSA therapy does not improve mortality and should not be routinely used in patients hospitalized for community-onset pneumonia, even in those with MRSA risk factors.
Citation: Jones BE et al. Empirical anti-MRSA vs. standard antibiotic therapy and risk of 30-day mortality in patients hospitalized for pneumonia. JAMA Intern Med. 2020 Feb 17;180(4):552-60.
Dr. Li is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Empirical broad-spectrum antibiotics including anti-MRSA therapy are often selected because of concerns for resistant organisms. However, the outcomes of empirical anti-MRSA therapy among patients with pneumonia are unknown.
Study design: A national retrospective multicenter cohort study of hospitalizations for pneumonia.
Setting: This cohort study included 88,605 hospitalizations for pneumonia in the Veterans Health Administration health care system during 2008-2013, in which patients received either anti-MRSA or standard therapy for community-onset pneumonia.
Synopsis: Among 88,605 hospitalizations for pneumonia, 38% of the patients received empirical anti-MRSA therapy within the first day of hospitalization and vancomycin accounted for 98% of the therapy. The primary outcome was 30-day all-cause mortality after adjustment for patient comorbidities, vital signs, and laboratory results. Three treatment groups were studied: patients receiving anti-MRSA therapy (vancomycin hydrochloride or linezolid) plus guideline-recommended standard antibiotics (beta-lactam and macrolide or tetracycline hydrochloride, or fluoroquinolone); patients receiving anti-MRSA therapy without standard antibiotics; and patients receiving standard therapy alone. There was no mortality benefit of empirical anti-MRSA therapy versus standard antibiotics, even in those with risk factors for MRSA or in those whose clinical severity warranted admission to the ICU. Empirical anti-MRSA treatment was associated with greater 30-day mortality compared with standard therapy alone, with an adjusted risk ratio of 1.4 (95% confidence interval, 1.3-1.5) versus empirical anti-MRSA treatment plus standard therapy and 1.5 (1.4-1.6) versus empirical anti-MRSA treatment without standard therapy.
Bottom line: Empirical anti-MRSA therapy does not improve mortality and should not be routinely used in patients hospitalized for community-onset pneumonia, even in those with MRSA risk factors.
Citation: Jones BE et al. Empirical anti-MRSA vs. standard antibiotic therapy and risk of 30-day mortality in patients hospitalized for pneumonia. JAMA Intern Med. 2020 Feb 17;180(4):552-60.
Dr. Li is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Children and COVID: New vaccinations increase as cases continue to climb
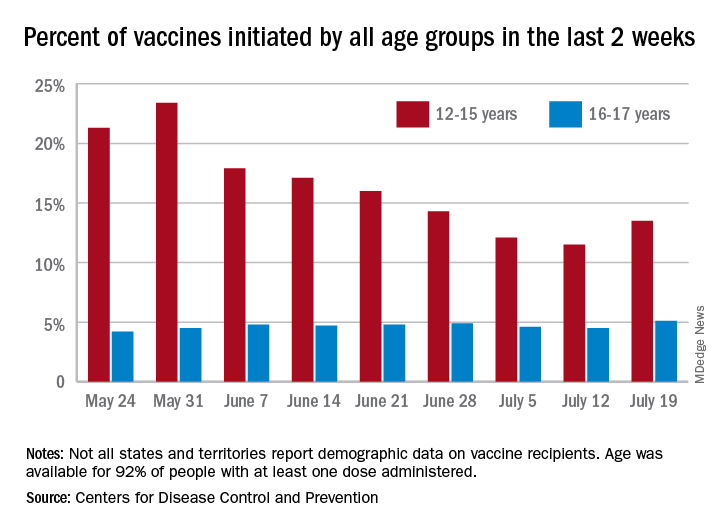
Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”

Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”

Children aged 12-15 years represented 13.5% of all first vaccinations received during the 2 weeks ending July 19, compared with 11.5% for the 2 weeks ending July 12, marking the first increase since the end of May. First vaccinations in 16- and 17-year-olds, who make up a much smaller share of the U.S. population, also went up, topping 5%, the Centers for Disease Control and Prevention said in its COVID Data Tracker.
The total number of vaccine initiations was almost 250,000 for the week ending July 19, after dropping to a low of 201,000 the previous week. Before that, first vaccinations had fallen in 5 of the previous 6 weeks, going from 1.4 million on May 24 to 307,000 on July 5, the CDC said.
New cases of COVID-19, unfortunately, continued to follow the trend among the larger population: As of July 15, weekly cases in children were up by 179% since dropping to 8,400 on June 24, the American Academy of Pediatrics and the Children’s Hospital Association said in a joint report. The 23,551 new cases in children for the week ending July 15 were 15.9% of all cases reported.
With those new cases, the total number of children infected with COVID-19 comes to almost 4.1 million since the start of the pandemic, the AAP and CHA said. The CDC data indicate that just over 5.35 million children aged 12-15 years and 3.53 million 16- and 17-year-olds have received at least one dose of the COVID-19 vaccine and that 6.8 million children aged 12-17 are fully vaccinated.
Fully vaccinated children represent 26.4% of all 12- to 15-year-olds and 38.3% of the 16- 17-year-olds as of July 19. The corresponding numbers for those who have received at least one dose are 35.2% (ages 12-15) and 46.8% (16-17), the CDC said.
The AAP recently recommended in-person learning with universal masking in schools this fall “because a significant portion of the student population is not yet eligible for vaccines. ... Many schools will not have a system to monitor vaccine status of students, teachers and staff, and some communities overall have low vaccination uptake where the virus may be circulating more prominently.”
HIV increases risk for severe COVID-19
according to a report from the World Health Organization on COVID-19 outcomes among people living with HIV. The study primarily included people from South Africa but also some data from other parts of the world, including the United States.
However, the report, presented at the 11th IAS Conference on HIV Science (IAS 2021), couldn’t answer some crucial questions clinicians have been wondering about since the COVID-19 pandemic began. For example, was the increase in COVID risk a result of the presence of HIV or because of the immune compromise caused by untreated HIV?
The report didn’t include data on viral load or CD counts, both used to evaluate the health of a person’s immune system. On effective treatment, people living with HIV have a lifespan close to their HIV-negative peers. And effective treatment causes undetectable viral loads which, when maintained for 6 months or more, eliminates transmission of HIV to sexual partners.
What’s clear is that in people with HIV, as in people without HIV, older people, men, and people with diabetes, hypertension, or obesity had the worst outcomes and were most likely to die from COVID-19.
For David Malebranche, MD, MPH, an internal medicine doctor who provides primary care for people in Atlanta, and who was not involved in the study, the WHO study didn’t add anything new. He already recommends the COVID-19 vaccine for all of his patients, HIV-positive or not.
“We don’t have any information from this about the T-cell counts [or] the rates of viral suppression, which I think is tremendously important,” he told this news organization. “To bypass that and not include that in any of the discussion puts the results in a questionable place for me.”
The results come from the WHO Clinical Platform, which culls data from WHO member country surveillance as well as manual case reports from all over the world. By April 29, data on 268,412 people hospitalized with COVID-19 from 37 countries were reported to the platform. Of those, 22,640 people are from the U.S.
A total of 15,522 participants worldwide were living with HIV, 664 in the United States. All U.S. cases were reported from the New York City Health and Hospitals system, Henry Ford Hospital in Detroit, and BronxCare Health System in New York City. Almost all of the remaining participants lived in South Africa – 14,682 of the 15,522, or 94.5%.
Of the 15,522 people living with HIV in the overall group, 37.1% of participants were male, and their median age was 45 years. More than 1 in 3 (36.2%) were admitted with severe or critical COVID-19, and nearly one quarter – 23.1% – with a known outcome died. More than half had one or more chronic conditions, including those that themselves are associated with worse COVID-19 outcomes, such as hypertension (in 33.2% of the participants), diabetes (22.7%), and BMIs above 30 (16.9%). In addition, 8.9% were smokers, 6.6% had chronic pulmonary disease, and 4.3% had chronic heart disease.
After adjusting for those chronic conditions, age, and sex, people living with HIV had a 6% higher rate of severe or critical COVID-19 illness. When investigators adjusted the analysis additionally to differentiate outcomes based on not just the presence of comorbid conditions but the number of them a person had, that increased risk rose to 13%. HIV itself is a comorbid condition, though it wasn’t counted as one in this adjusted analysis.
It didn’t matter whether researchers looked at risk for severe outcomes or deaths after removing the significant co-occurring conditions or if they looked at number of chronic illnesses (aside from HIV), said Silvia Bertagnolio, MD, medical officer at the World Health Organization and co-author of the analysis.
“Both models show almost identical [adjusted odds ratios], meaning that HIV was independently significantly associated with severe/critical presentation,” she told this news organization.
As for death, the analysis showed that, overall, people living with HIV were 30% more likely to die of COVID-19 compared with those not living with HIV. And while this held true even when they adjusted the data for comorbidities, people with HIV were more likely to die if they were over age 65 (risk increased by 82%), male (risk increased by 21%), had diabetes (risk increased by 50%), or had hypertension (risk increased by 26%).
When they broke down the data by WHO region – Africa, Europe, the Americas – investigators found that the increased risk for death held true in Africa. But there were not enough data from the other regions to model mortality risk. What’s more, when they broke the data down by country and excluded South Africa, they found that the elevated risk for death in people living with HIV did not reach statistical significance. Dr. Bertagnolio said she suspects that the small sample sizes from other regions made it impossible to detect a difference, but one could still be present.
One thing conspicuously absent from the analysis was information on viral load, CD4 T-cell count, progression of HIV to AIDS, and whether individuals were in HIV care. The first three factors were not reported in the platform, and the fourth was available for 60% of participants but was not included in the analysis. Dr. Bertagnolio pointed out that, for those 60% of participants, 91.8% were on antiretroviral treatment (ART).
“The majority of patients come from South Africa, and we know that in South Africa, over 90% of people receiving ART are virologically suppressed,” she told this news organization. “So we could speculate that this effect persists despite the use of ART, in a population likely to be virally suppressed, although we cannot assess this with certainty through the data set we had.”
A much smaller study of 749 people living with HIV and diagnosed with SARS-CoV-2, also presented at the conference, found that detectable HIV viral load was significantly associated with a slightly higher risk of severe outcomes (P < .039), but CD4 counts less than 200 cells/mm3 was not (P = .15).
And although both Dr. Bertagnolio and conference organizers presented this data as proof that HIV increases the risk for poor COVID-19 outcomes, Dr. Malebranche isn’t so sure. He estimates that only about half his patients have received the COVID-19 vaccine. But this study is unlikely to make him forcefully recommend a COVID-19 vaccination with young, otherwise healthy, and undetectable people in his care who express particular concern about long-term effects of the vaccine. He also manages a lot of people with HIV who have undetectable viral loads and CD4 counts of up to 1,200 but are older, with diabetes, obesity, and high blood pressure. Those are the people he will target with stronger messages regarding the vaccine.
“The young patients who are healthy, virally suppressed, and doing well may very much argue with me, ‘I’m not going to push it,’ but I will bring it up on the next visit,” he said. The analysis “just helps reinforce in me that I need to have these conversations and be a little bit more persuasive to my older patients with comorbid conditions.”
Dr. Bertagnolio has disclosed no relevant financial relationships. Dr. Malebranche serves on the pre-exposure prophylaxis (PrEP) speakers bureau for Gilead Sciences and has consulted and advised for ViiV Healthcare. This study was funded by the World Health Organization.
A version of this article first appeared on Medscape.com.
according to a report from the World Health Organization on COVID-19 outcomes among people living with HIV. The study primarily included people from South Africa but also some data from other parts of the world, including the United States.
However, the report, presented at the 11th IAS Conference on HIV Science (IAS 2021), couldn’t answer some crucial questions clinicians have been wondering about since the COVID-19 pandemic began. For example, was the increase in COVID risk a result of the presence of HIV or because of the immune compromise caused by untreated HIV?
The report didn’t include data on viral load or CD counts, both used to evaluate the health of a person’s immune system. On effective treatment, people living with HIV have a lifespan close to their HIV-negative peers. And effective treatment causes undetectable viral loads which, when maintained for 6 months or more, eliminates transmission of HIV to sexual partners.
What’s clear is that in people with HIV, as in people without HIV, older people, men, and people with diabetes, hypertension, or obesity had the worst outcomes and were most likely to die from COVID-19.
For David Malebranche, MD, MPH, an internal medicine doctor who provides primary care for people in Atlanta, and who was not involved in the study, the WHO study didn’t add anything new. He already recommends the COVID-19 vaccine for all of his patients, HIV-positive or not.
“We don’t have any information from this about the T-cell counts [or] the rates of viral suppression, which I think is tremendously important,” he told this news organization. “To bypass that and not include that in any of the discussion puts the results in a questionable place for me.”
The results come from the WHO Clinical Platform, which culls data from WHO member country surveillance as well as manual case reports from all over the world. By April 29, data on 268,412 people hospitalized with COVID-19 from 37 countries were reported to the platform. Of those, 22,640 people are from the U.S.
A total of 15,522 participants worldwide were living with HIV, 664 in the United States. All U.S. cases were reported from the New York City Health and Hospitals system, Henry Ford Hospital in Detroit, and BronxCare Health System in New York City. Almost all of the remaining participants lived in South Africa – 14,682 of the 15,522, or 94.5%.
Of the 15,522 people living with HIV in the overall group, 37.1% of participants were male, and their median age was 45 years. More than 1 in 3 (36.2%) were admitted with severe or critical COVID-19, and nearly one quarter – 23.1% – with a known outcome died. More than half had one or more chronic conditions, including those that themselves are associated with worse COVID-19 outcomes, such as hypertension (in 33.2% of the participants), diabetes (22.7%), and BMIs above 30 (16.9%). In addition, 8.9% were smokers, 6.6% had chronic pulmonary disease, and 4.3% had chronic heart disease.
After adjusting for those chronic conditions, age, and sex, people living with HIV had a 6% higher rate of severe or critical COVID-19 illness. When investigators adjusted the analysis additionally to differentiate outcomes based on not just the presence of comorbid conditions but the number of them a person had, that increased risk rose to 13%. HIV itself is a comorbid condition, though it wasn’t counted as one in this adjusted analysis.
It didn’t matter whether researchers looked at risk for severe outcomes or deaths after removing the significant co-occurring conditions or if they looked at number of chronic illnesses (aside from HIV), said Silvia Bertagnolio, MD, medical officer at the World Health Organization and co-author of the analysis.
“Both models show almost identical [adjusted odds ratios], meaning that HIV was independently significantly associated with severe/critical presentation,” she told this news organization.
As for death, the analysis showed that, overall, people living with HIV were 30% more likely to die of COVID-19 compared with those not living with HIV. And while this held true even when they adjusted the data for comorbidities, people with HIV were more likely to die if they were over age 65 (risk increased by 82%), male (risk increased by 21%), had diabetes (risk increased by 50%), or had hypertension (risk increased by 26%).
When they broke down the data by WHO region – Africa, Europe, the Americas – investigators found that the increased risk for death held true in Africa. But there were not enough data from the other regions to model mortality risk. What’s more, when they broke the data down by country and excluded South Africa, they found that the elevated risk for death in people living with HIV did not reach statistical significance. Dr. Bertagnolio said she suspects that the small sample sizes from other regions made it impossible to detect a difference, but one could still be present.
One thing conspicuously absent from the analysis was information on viral load, CD4 T-cell count, progression of HIV to AIDS, and whether individuals were in HIV care. The first three factors were not reported in the platform, and the fourth was available for 60% of participants but was not included in the analysis. Dr. Bertagnolio pointed out that, for those 60% of participants, 91.8% were on antiretroviral treatment (ART).
“The majority of patients come from South Africa, and we know that in South Africa, over 90% of people receiving ART are virologically suppressed,” she told this news organization. “So we could speculate that this effect persists despite the use of ART, in a population likely to be virally suppressed, although we cannot assess this with certainty through the data set we had.”
A much smaller study of 749 people living with HIV and diagnosed with SARS-CoV-2, also presented at the conference, found that detectable HIV viral load was significantly associated with a slightly higher risk of severe outcomes (P < .039), but CD4 counts less than 200 cells/mm3 was not (P = .15).
And although both Dr. Bertagnolio and conference organizers presented this data as proof that HIV increases the risk for poor COVID-19 outcomes, Dr. Malebranche isn’t so sure. He estimates that only about half his patients have received the COVID-19 vaccine. But this study is unlikely to make him forcefully recommend a COVID-19 vaccination with young, otherwise healthy, and undetectable people in his care who express particular concern about long-term effects of the vaccine. He also manages a lot of people with HIV who have undetectable viral loads and CD4 counts of up to 1,200 but are older, with diabetes, obesity, and high blood pressure. Those are the people he will target with stronger messages regarding the vaccine.
“The young patients who are healthy, virally suppressed, and doing well may very much argue with me, ‘I’m not going to push it,’ but I will bring it up on the next visit,” he said. The analysis “just helps reinforce in me that I need to have these conversations and be a little bit more persuasive to my older patients with comorbid conditions.”
Dr. Bertagnolio has disclosed no relevant financial relationships. Dr. Malebranche serves on the pre-exposure prophylaxis (PrEP) speakers bureau for Gilead Sciences and has consulted and advised for ViiV Healthcare. This study was funded by the World Health Organization.
A version of this article first appeared on Medscape.com.
according to a report from the World Health Organization on COVID-19 outcomes among people living with HIV. The study primarily included people from South Africa but also some data from other parts of the world, including the United States.
However, the report, presented at the 11th IAS Conference on HIV Science (IAS 2021), couldn’t answer some crucial questions clinicians have been wondering about since the COVID-19 pandemic began. For example, was the increase in COVID risk a result of the presence of HIV or because of the immune compromise caused by untreated HIV?
The report didn’t include data on viral load or CD counts, both used to evaluate the health of a person’s immune system. On effective treatment, people living with HIV have a lifespan close to their HIV-negative peers. And effective treatment causes undetectable viral loads which, when maintained for 6 months or more, eliminates transmission of HIV to sexual partners.
What’s clear is that in people with HIV, as in people without HIV, older people, men, and people with diabetes, hypertension, or obesity had the worst outcomes and were most likely to die from COVID-19.
For David Malebranche, MD, MPH, an internal medicine doctor who provides primary care for people in Atlanta, and who was not involved in the study, the WHO study didn’t add anything new. He already recommends the COVID-19 vaccine for all of his patients, HIV-positive or not.
“We don’t have any information from this about the T-cell counts [or] the rates of viral suppression, which I think is tremendously important,” he told this news organization. “To bypass that and not include that in any of the discussion puts the results in a questionable place for me.”
The results come from the WHO Clinical Platform, which culls data from WHO member country surveillance as well as manual case reports from all over the world. By April 29, data on 268,412 people hospitalized with COVID-19 from 37 countries were reported to the platform. Of those, 22,640 people are from the U.S.
A total of 15,522 participants worldwide were living with HIV, 664 in the United States. All U.S. cases were reported from the New York City Health and Hospitals system, Henry Ford Hospital in Detroit, and BronxCare Health System in New York City. Almost all of the remaining participants lived in South Africa – 14,682 of the 15,522, or 94.5%.
Of the 15,522 people living with HIV in the overall group, 37.1% of participants were male, and their median age was 45 years. More than 1 in 3 (36.2%) were admitted with severe or critical COVID-19, and nearly one quarter – 23.1% – with a known outcome died. More than half had one or more chronic conditions, including those that themselves are associated with worse COVID-19 outcomes, such as hypertension (in 33.2% of the participants), diabetes (22.7%), and BMIs above 30 (16.9%). In addition, 8.9% were smokers, 6.6% had chronic pulmonary disease, and 4.3% had chronic heart disease.
After adjusting for those chronic conditions, age, and sex, people living with HIV had a 6% higher rate of severe or critical COVID-19 illness. When investigators adjusted the analysis additionally to differentiate outcomes based on not just the presence of comorbid conditions but the number of them a person had, that increased risk rose to 13%. HIV itself is a comorbid condition, though it wasn’t counted as one in this adjusted analysis.
It didn’t matter whether researchers looked at risk for severe outcomes or deaths after removing the significant co-occurring conditions or if they looked at number of chronic illnesses (aside from HIV), said Silvia Bertagnolio, MD, medical officer at the World Health Organization and co-author of the analysis.
“Both models show almost identical [adjusted odds ratios], meaning that HIV was independently significantly associated with severe/critical presentation,” she told this news organization.
As for death, the analysis showed that, overall, people living with HIV were 30% more likely to die of COVID-19 compared with those not living with HIV. And while this held true even when they adjusted the data for comorbidities, people with HIV were more likely to die if they were over age 65 (risk increased by 82%), male (risk increased by 21%), had diabetes (risk increased by 50%), or had hypertension (risk increased by 26%).
When they broke down the data by WHO region – Africa, Europe, the Americas – investigators found that the increased risk for death held true in Africa. But there were not enough data from the other regions to model mortality risk. What’s more, when they broke the data down by country and excluded South Africa, they found that the elevated risk for death in people living with HIV did not reach statistical significance. Dr. Bertagnolio said she suspects that the small sample sizes from other regions made it impossible to detect a difference, but one could still be present.
One thing conspicuously absent from the analysis was information on viral load, CD4 T-cell count, progression of HIV to AIDS, and whether individuals were in HIV care. The first three factors were not reported in the platform, and the fourth was available for 60% of participants but was not included in the analysis. Dr. Bertagnolio pointed out that, for those 60% of participants, 91.8% were on antiretroviral treatment (ART).
“The majority of patients come from South Africa, and we know that in South Africa, over 90% of people receiving ART are virologically suppressed,” she told this news organization. “So we could speculate that this effect persists despite the use of ART, in a population likely to be virally suppressed, although we cannot assess this with certainty through the data set we had.”
A much smaller study of 749 people living with HIV and diagnosed with SARS-CoV-2, also presented at the conference, found that detectable HIV viral load was significantly associated with a slightly higher risk of severe outcomes (P < .039), but CD4 counts less than 200 cells/mm3 was not (P = .15).
And although both Dr. Bertagnolio and conference organizers presented this data as proof that HIV increases the risk for poor COVID-19 outcomes, Dr. Malebranche isn’t so sure. He estimates that only about half his patients have received the COVID-19 vaccine. But this study is unlikely to make him forcefully recommend a COVID-19 vaccination with young, otherwise healthy, and undetectable people in his care who express particular concern about long-term effects of the vaccine. He also manages a lot of people with HIV who have undetectable viral loads and CD4 counts of up to 1,200 but are older, with diabetes, obesity, and high blood pressure. Those are the people he will target with stronger messages regarding the vaccine.
“The young patients who are healthy, virally suppressed, and doing well may very much argue with me, ‘I’m not going to push it,’ but I will bring it up on the next visit,” he said. The analysis “just helps reinforce in me that I need to have these conversations and be a little bit more persuasive to my older patients with comorbid conditions.”
Dr. Bertagnolio has disclosed no relevant financial relationships. Dr. Malebranche serves on the pre-exposure prophylaxis (PrEP) speakers bureau for Gilead Sciences and has consulted and advised for ViiV Healthcare. This study was funded by the World Health Organization.
A version of this article first appeared on Medscape.com.
Pharmacologic and electrical cardioversion of acute Afib reduces hospital admissions
Background: Atrial fibrillation (Afib) is the most common arrhythmia requiring treatment in the ED. There is a paucity of literature regarding the management of acute (onset < 48 h) atrial fibrillation in this setting and no conclusive evidence exists regarding the superiority of pharmacologic vs. electrical cardioversion.
Study design: Multicenter, single-blind, randomized, placebo-controlled trial.
Setting: 11 Canadian academic medical centers.
Synopsis: In this trial of 396 patients with acute Afib, half were randomly assigned to pharmacologic cardioversion with procainamide infusion (followed by DC cardioversion, if unsuccessful), while half were given a placebo infusion then DC cardioversion. The primary outcome was conversion to sinus rhythm, with maintenance of sinus rhythm at 30 minutes. A secondary protocol evaluated the difference in efficacy between anterolateral (AL) and anteroposterior (AP) pad placement
The “drug-shock” group achieved and maintained sinus rhythm in 96% of cases, compared to 92% in the “placebo-shock” group (statistically insignificant difference). The procainamide infusion alone achieved and maintained sinus rhythm in 52% of recipients, who thereby avoided the need for procedural sedation and monitoring. Notably, only 2% of patients in the study required admission to the hospital. Pad placement was equally efficacious in the AL or AP positions. The most common adverse event observed was transient hypotension during infusion of procainamide. No strokes were observed in either arm. Follow-up ECGs obtained 14 days later showed that 95% of patients remained in sinus rhythm.
Bottom line: Pharmacologic cardioversion with procainamide infusion and/or electrical cardioversion is a safe and efficacious initial management strategy for acute atrial fibrillation, and all but eliminates the need for hospital admission.
Citation: Stiell IG et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomized trial. Lancet. 2020 Feb 1;395(10221):339-49.
Dr. Lawson is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Atrial fibrillation (Afib) is the most common arrhythmia requiring treatment in the ED. There is a paucity of literature regarding the management of acute (onset < 48 h) atrial fibrillation in this setting and no conclusive evidence exists regarding the superiority of pharmacologic vs. electrical cardioversion.
Study design: Multicenter, single-blind, randomized, placebo-controlled trial.
Setting: 11 Canadian academic medical centers.
Synopsis: In this trial of 396 patients with acute Afib, half were randomly assigned to pharmacologic cardioversion with procainamide infusion (followed by DC cardioversion, if unsuccessful), while half were given a placebo infusion then DC cardioversion. The primary outcome was conversion to sinus rhythm, with maintenance of sinus rhythm at 30 minutes. A secondary protocol evaluated the difference in efficacy between anterolateral (AL) and anteroposterior (AP) pad placement
The “drug-shock” group achieved and maintained sinus rhythm in 96% of cases, compared to 92% in the “placebo-shock” group (statistically insignificant difference). The procainamide infusion alone achieved and maintained sinus rhythm in 52% of recipients, who thereby avoided the need for procedural sedation and monitoring. Notably, only 2% of patients in the study required admission to the hospital. Pad placement was equally efficacious in the AL or AP positions. The most common adverse event observed was transient hypotension during infusion of procainamide. No strokes were observed in either arm. Follow-up ECGs obtained 14 days later showed that 95% of patients remained in sinus rhythm.
Bottom line: Pharmacologic cardioversion with procainamide infusion and/or electrical cardioversion is a safe and efficacious initial management strategy for acute atrial fibrillation, and all but eliminates the need for hospital admission.
Citation: Stiell IG et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomized trial. Lancet. 2020 Feb 1;395(10221):339-49.
Dr. Lawson is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Atrial fibrillation (Afib) is the most common arrhythmia requiring treatment in the ED. There is a paucity of literature regarding the management of acute (onset < 48 h) atrial fibrillation in this setting and no conclusive evidence exists regarding the superiority of pharmacologic vs. electrical cardioversion.
Study design: Multicenter, single-blind, randomized, placebo-controlled trial.
Setting: 11 Canadian academic medical centers.
Synopsis: In this trial of 396 patients with acute Afib, half were randomly assigned to pharmacologic cardioversion with procainamide infusion (followed by DC cardioversion, if unsuccessful), while half were given a placebo infusion then DC cardioversion. The primary outcome was conversion to sinus rhythm, with maintenance of sinus rhythm at 30 minutes. A secondary protocol evaluated the difference in efficacy between anterolateral (AL) and anteroposterior (AP) pad placement
The “drug-shock” group achieved and maintained sinus rhythm in 96% of cases, compared to 92% in the “placebo-shock” group (statistically insignificant difference). The procainamide infusion alone achieved and maintained sinus rhythm in 52% of recipients, who thereby avoided the need for procedural sedation and monitoring. Notably, only 2% of patients in the study required admission to the hospital. Pad placement was equally efficacious in the AL or AP positions. The most common adverse event observed was transient hypotension during infusion of procainamide. No strokes were observed in either arm. Follow-up ECGs obtained 14 days later showed that 95% of patients remained in sinus rhythm.
Bottom line: Pharmacologic cardioversion with procainamide infusion and/or electrical cardioversion is a safe and efficacious initial management strategy for acute atrial fibrillation, and all but eliminates the need for hospital admission.
Citation: Stiell IG et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomized trial. Lancet. 2020 Feb 1;395(10221):339-49.
Dr. Lawson is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Long COVID seen in patients with severe and mild disease
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.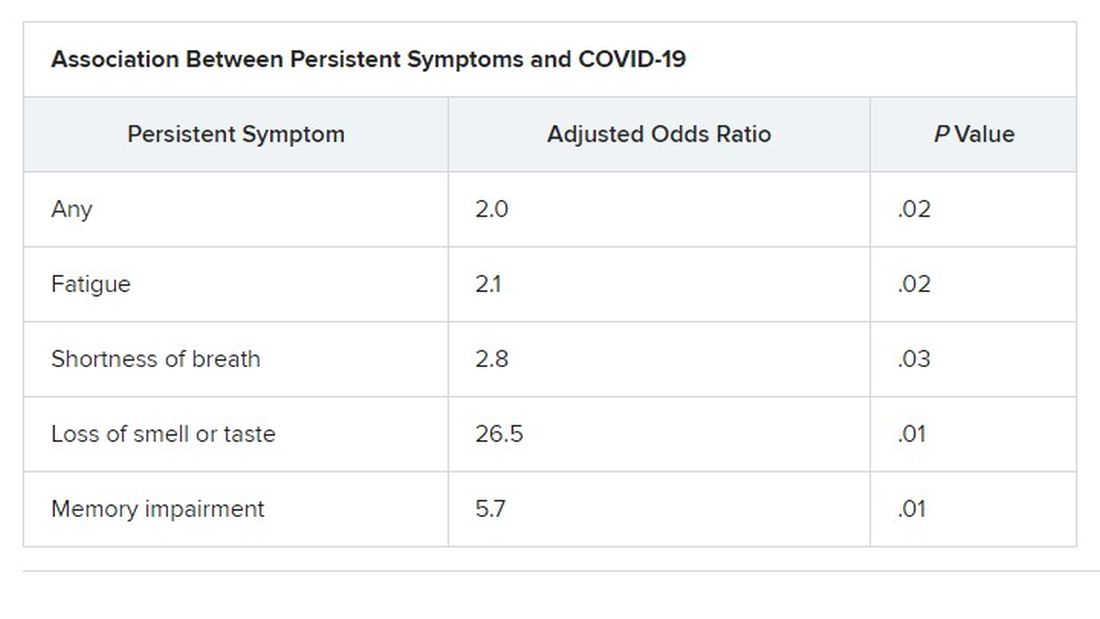
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN CONGRESS OF CLINICAL MICROBIOLOGY & INFECTIOUS DISEASES








