User login
-
Pandemic effect: All other health care visits can wait
according to survey conducted at the end of April.
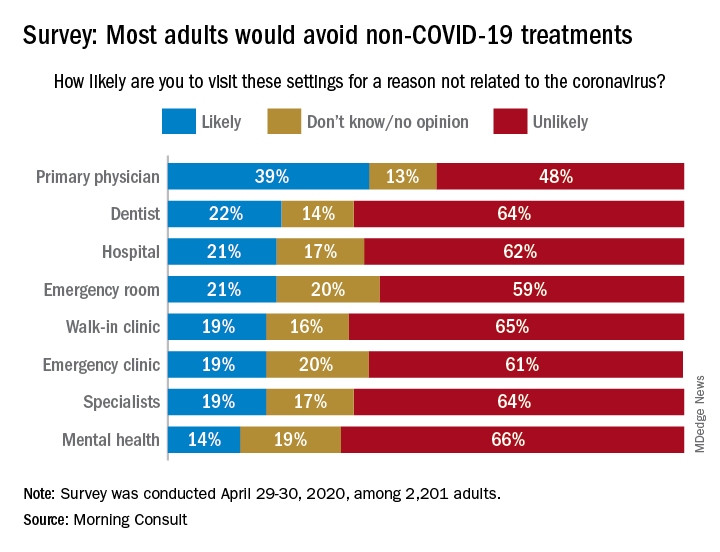
When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
according to survey conducted at the end of April.

When asked how likely they were to visit a variety of health care settings for treatment not related to the coronavirus, 62% of respondents said it was unlikely that they would go to a hospital, 64% wouldn’t go to a specialist, and 65% would avoid walk-in clinics, digital media company Morning Consult reported May 4.
The only setting with less than a majority on the unlikely-to-visit side was primary physicians, who managed to combine a 39% likely vote with a 13% undecided/no-opinion tally, Morning Consult said after surveying 2,201 adults on April 29-30 (margin of error, ±2 percentage points).
As to when they might feel comfortable making such an in-person visit with their primary physician, 24% of respondents said they would willing to go in the next month, 14% said 2 months, 18% said 3 months, 13% said 6 months, and 10% said more than 6 months, the Morning Consult data show.
“Hospitals, despite being overburdened in recent weeks in coronavirus hot spots such as New York City, have reported dips in revenue as a result of potential patients opting against receiving elective surgeries out of fear of contracting COVID-19,” Morning Consult wrote, and these poll results suggest that “health care companies could continue to feel the pinch as long as the coronavirus lingers.”
FDA grants EUA to muscle stimulator to reduce mechanical ventilator usage
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
The Food and Drug Administration has issued an Emergency Use Authorization (EUA) for the VentFree Respiratory Muscle Stimulator in order to potentially reduce the number of days adult patients, including those with COVID-19, require mechanical ventilation, according to a press release from Liberate Medical.
In comparison with mechanical ventilation, which is invasive and commonly weakens the breathing muscles, the VentFree system uses noninvasive neuromuscular electrical stimulation to contract the abdominal wall muscles in synchrony with exhalation during mechanical ventilation, according to the press release. This allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until they are weaned off of ventilation.
A pair of pilot randomized, controlled studies, completed in Europe and Australia, showed that VentFree helped to reduce ventilation duration and ICU length of stay, compared with placebo stimulation. The FDA granted VentFree Breakthrough Device status in 2019.
“We are grateful to the FDA for recognizing the potential of VentFree and feel privileged to have the opportunity to help patients on mechanical ventilation during the COVID-19 pandemic,” Angus McLachlan PhD, cofounder and CEO of Liberate Medical, said in the press release.
VentFree has been authorized for use only for the duration of the current COVID-19 emergency, as it has not yet been approved or cleared for usage by primary care providers.
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY
Fountains of Wayne, and a hospitalist’s first day, remembered
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Like many in the health care field, I have found it hard to watch the news over these past couple of months when it seems that almost every story is about COVID-19 or its repercussions. Luckily, I have two young daughters who “encourage” me to listen to the Frozen 2 soundtrack instead of putting on the evening news when I get home from work. Still, news manages to seep through my defenses. As I scrolled through some headlines recently, I learned of the death of musician Adam Schlesinger from COVID-19. He wasn’t a household name, but his death still hit me in unexpected ways.
I started internship in late June 2005, in a city (Portland, Ore.) about as different from my previous home (Dallas) as any two places can possibly be. I think the day before internship started still ranks as the most nervous of my life. I’m not sure how I slept at all that night, but somehow I did and arrived at the Portland Veterans Affairs Hospital the following morning to start my new career.
And then … nothing happened. Early on that first day, the electronic medical records crashed, and no patients were admitted during our time on “short call.” My upper level resident took care of the one or two established patients on the team (both discharged), so I ended the day with records that would not be broken during the remainder of my residency: 0 notes written, 0 patients seen. Perhaps the most successful first day that any intern, anywhere has ever had, although it prepared me quite poorly for all the subsequent days.
Since I had some time on my hands, I made the 20-minute walk to one of my new hometown’s record stores where Fountains of Wayne (FOW) was playing an acoustic in-store set. Their album from a few years prior, “Welcome Interstate Managers,” was in heavy rotation when I made the drive from Dallas to Portland. It was (and is) a great album for long drives – melodic, catchy, and (mostly) up-tempo. Adam and the band’s singer, Chris Collingwood, played several songs that night on the store’s stage. Then they headed out to the next city, and I headed back home and on to many far-busier days of residency.
We would cross paths again a decade later. I moved back to Texas and became a hospitalist. It turns out that, if you have enough hospitalists of a certain age and if enough of those hospitalists have unearned confidence in their musical ability, then a covers band will undoubtedly be formed. And so, it happened here in San Antonio. We were not selective in our song choices – we played songs from every decade of the last 50 years, bands as popular as the Beatles and as indie as the Rentals. And we played some FOW.
Our band (which will go nameless here so that our YouTube recordings are more difficult to find) played a grand total of one gig during our years of intermittent practicing. That one gig was my wedding rehearsal dinner and the penultimate song we played was “Stacy’s Mom,” which is notable for being both FOW’s biggest hit and a completely inappropriate song to play at a wedding rehearsal dinner. The crowd was probably around the same size as the one that had seen Adam and Chris play in Portland 10 years prior. I don’t think the applause we received was quite as genuine or deserved, though.
After Adam and Chris played their gig, there was an autograph session and I took home a signed poster. Last year, I decided to take it out of storage and hang it in my office. The date of the show and the first day of my physician career, a date now nearly 15 years ago, is written in psychedelic typography at the bottom. The store that I went to that day is no longer there, a victim of progress like so many other record stores across the country. Another location of the same store is still open in Portland. I hope that it and all the other small book and music stores across the country can survive this current crisis, but I know that many will not.
So, here’s to you Adam, and to all the others who have lost their lives to this terrible illness. As a small token of remembrance, I’ll be playing some Fountains of Wayne on the drive home tonight. It’s not quite the same as playing it on a cross-country drive, but hopefully, we will all be able to do that again soon.
Dr. Sehgal is a clinical associate professor of medicine in the division of general and hospital medicine at the South Texas Veterans Health Care System and UT-Health San Antonio. He is a member of the editorial advisory board for The Hospitalist.
Doctor with a mask: Enhancing communication and empathy
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Delivering a goodbye monologue to an elderly patient, I said: “Tomorrow, my colleague Dr. XYZ, who is an excellent physician, will be here in my place, and I will leave a detailed sign out for them.” I was on the last day of a 7-day-long block on hospital medicine service. Typically, when I say goodbye, some patients respond “thank you, enjoy your time,” some don’t care, and some show disappointment at the transition. This patient became uneasy, choking back tears, and said: “But, I don’t want a new doctor. You know me well. ... They don’t even allow my family in the hospital.”
That expression of anxiety, of having to build rapport with a new provider, concerns about continuity of care, and missing support of family members were not alien to me. As I instinctively took a step toward him to offer a comforting hug, an unsolicited voice in my head said, “social distancing.” I steered back, handing him a box of tissues. I continued: “You have come a long way, and things are looking good from here,” providing more details before I left the room. There was a change in my practice that week. I didn’t shake hands with my patients; I didn’t sit on any unassigned chair; I had no family members in the room asking me questions or supporting my patients. I was trying to show empathy or a smile behind a mask and protective eyewear. The business card with photograph had become more critical than ever for patients to “see” their doctor.
Moving from room to room and examining patients, it felt like the coronavirus was changing the practice of medicine beyond concerns of virus transmission, losing a patient, or putting in extra hours. I realized I was missing so-called “nonverbal communication” amid social distancing: facial expressions, social touch, and the support of family or friends to motivate or destress patients. With no visitors and curbed health care staff entries into patient’s rooms, social distancing was amounting to social isolation. My protective gear and social distancing seemed to be reducing my perceived empathy with patients, and the ability to build a good patient-physician relationship.
Amid alarms, beeps, and buzzes, patients were not only missing their families but also the familiar faces of their physicians. I needed to raise my game while embracing the “new normal” of health care. Cut to the next 13 patients: I paid more attention to voice, tone, and posture. I called patient families from the bedside instead of the office. I translated my emotions with words, loud and clear, replacing “your renal function looks better” (said without a smile) with “I am happy to see your renal function better.”
Through years of practice, I felt prepared to deal with feelings of denial, grief, anxiety, and much more, but the emotions arising as a result of this pandemic were unique. “I knew my mother was old, and this day would come,” said one of the inconsolable family members of a critically ill patient. “However, I wished to be at her side that day, not like this.” I spend my days listening to patient and family concerns about unemployment with quarantine, fears of spreading the disease to loved ones, and the possibility of medications not working.
After a long day, I went back to that first elderly patient to see if he was comfortable with the transition of care. I did a video conference with his daughter, and repeated my goodbyes. The patient smiled and said: “Doc, you deserve a break.” That day I learned about the challenges of good clinical rounding in coronavirus times, and how to overcome them. For “millennial” physicians, it is our first pandemic, and we are learning from it every day.
Driving home through empty streets, I concluded that my answers to the clinical questions asked by patients and families lean heavily on ever-changing data, and the treatments offered have yet to prove their mettle. As a result, I will continue to focus as much on the time-tested fundamentals of clinical practice: communication and empathy. I cannot allow the social distancing and the mask to hide my compassion, or take away from patient satisfaction. Shifting gears, I turned on my car radio, using music to reset my mind before attending to my now-homeschooling kids.
Dr. Saigal is a hospitalist and clinical assistant professor of medicine in the division of hospital medicine at the Ohio State University Wexner Medical Center, Columbus.
References
1. Wong CK et al. Effect of facemasks on empathy and relational continuity: A randomised controlled trial in primary care. BMC Fam Pract. 2013;14:200.
2. Little P et al. Randomised controlled trial of a brief intervention targeting predominantly nonverbal communication in general practice consultations. Br J Gen Pract. 2015;65(635):e351-6.
3. Varghese A. A doctor’s touch. TEDGlobal 2011. 2011 Jul. https://www.ted.com/talks/abraham_verghese_a_doctor_s_touch?language=en
Case reports illustrate heterogeneity of skin manifestations in COVID patients
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Two infection.
It is not yet clear from these or other case reports which, if any, skin eruptions accompanying COVID-19 infections are caused by the virus, but the authors of the editorial, led by Lauren M. Madigan, MD, of the department of dermatology at the University of Utah, Salt Lake City, urged dermatologists to lead efforts to find out.
“To fully characterize skin manifestations, it may be necessary for dermatologists to evaluate these patients directly; comprehensive evaluation could reveal important morphologic clues, such as the subtle purpuric nature of skin lesions or the characteristic mucosal or ophthalmologic features of COVID-19,” the authors of the editorial stated.
So far, the patterns of skin symptoms, which have been identified in up to 20% of COVID-19–infected patients in some series, have been heterogeneous as demonstrated in the two published case reports.
In one case, a papulosquamous and erythematous periumbilical patch that appeared on the trunk in an elderly patient 1 day after hospital admission for acute respiratory distress rapidly evolved into a digitate papulosquamous eruption involving the upper arms, shoulder, and back. It was described as “clinically reminiscent” of pityriasis rosea by the authors, from the divisions of dermatology and venereology, pathology, intensive care, and the virology laboratory, of the Hôpital Cochin, Paris.
In the other, pruritic erythematous macules, papules, and petechiae affecting the buttocks, popliteal fossae, anterior thighs, and lower abdomen appeared 3 days after the onset of fever in a 48-year-old man hospitalized in Madrid. A biopsy demonstrated a superficial perivascular lymphocytic infiltrate with red cell extravasation and focal papillary edema, “along with focal parakeratosis and isolated dyskeratotic cells,” according to the authors of this report, from the department of dermatology at Ramon y Cajal University, Madrid.
It was unclear whether COVID-19 directly caused either skin eruption. In the patient with the digitate papulosquamous eruption, no virus could be isolated from the skin. Based on high levels of proinflammatory cytokines, it was hypothesized that the rash might have been secondary to an immune response. The rash resolved within a week, but the patient subsequently died of the infection.
In the second case, the petechial lesions, which developed before any treatment was initiated, were said to resemble those associated with other viruses, such as parvovirus B19. This led the investigators to speculate that SARS-CoV-2 “could affect the skin in a similar way,” even though other potential etiologies could not be excluded. Treated with a topical steroid and an oral antihistamine, the skin lesions resolved after 5 days. This patient was discharged after recovering from the respiratory illness after 12 days.
Like previously reported cutaneous eruptions associated with COVID-19 infection, these cases “raise more questions than they provide answers,” wrote the authors of the editorial, but the limited information currently available was the basis for encouraging dermatologists to get involved.
To participate, dermatologists need not necessarily be affiliated with an academic center, according to one of the editorial coauthors, Kanade Shinkai, MD, PhD, professor of dermatology at the University of California, San Francisco. She noted that any health professional is invited to submit cases of COVID-19–associated dermatoses to a registry set up by the American Academy of Dermatology.
It is hoped that cases captured in this registry will create sufficient data to allow clinically relevant patterns and etiologies to be characterized.
The need for data is clear to those on the front lines. Kirsten Lo Sicco, MD, associate director of the skin and cancer unit at New York University, reported that her center is already set up to collect data systematically. “At NYU, we are currently working on standardizing laboratory and histopathology work up for COVID-19 patients who present with various skin eruptions.”
The goal, she added, is “to better determine COVID-19 pathophysiology, systemic associations, patient outcomes, and potential therapeutics.”
“Presumably, many of the eruptions seen in the setting of COVID-19 infection are related,” Dr. Lo Sicco explained in an interview. However, skin complications of infection “may overlap with or be a result of other etiologies as well.”
While better testing for COVID-19 and more lesion biopsies will play a critical role in differentiating etiologies, “we must not overcall COVID-19–related skin eruptions and potentially overlook other diagnoses,” Dr. Lo Sicco said.
In recounting some challenges from the NYU experience so far, Dr. Lo Sicco described the difficulty of differentiating COVID-19–related skin eruptions from skin eruptions caused by treatments, such as antibiotics and antivirals, when the presentation is delayed.
“This is where collaboration with our dermatopathologists becomes important. Drug eruptions, viral exanthems, urticarial eruptions, vasculopathy, and vasculitis can all be differentiated on dermpath,” she said.
One early obstacle to the skin biopsies essential for these types of studies was the limited supply of personal protective equipment at many centers, including hospitals in New York. Biopsies could not be safely performed if supplies of masks and gowns were limited.
Recent evidence suggests that some of the more common morphologies, such as purpuric eruptions, livedo reticularis, and retiform purpura, are linked to the vasculopathy associated with COVID-19 infection, according to Dr. Lo Sicco, but this invites a new set of questions.
One is whether vasculopathies can be prevented with prophylactic anticoagulation. Many hospitalized COVID-19 patients are already receiving therapeutic anticoagulation, but Dr. Lo Sicco questioned whether prophylactic anticoagulation might improve prognosis for outpatients, such as those discharged or those never hospitalized. This is a strategy now being investigated.
Ultimately, she agreed with the thrust of the JAMA Dermatology editorial.
“Dermatologists are vital to determine if various morphologies, such as urticarial, vesicular, purpuric, or papulosquamous lesions, have any specific systemic implications or relate to differences in patient outcomes,” she said.
These are exactly the types of issues being actively investigated at her center.
Neither the authors of the case reports nor of the editorial reported any conflicts of interest.
SOURCEs: Madigan LM et al. JAMA Dermatol. 2020 Apr 30. doi:10.1001/jamadermatol.2020.1438; Diaz-Guimaraens B et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1741; Sanchez A et al. JAMA Dermatol. 2020 Apr 30. doi: 10.1001/jamadermatol.2020.1704.
Reporting for duty at the front lines of COVID-19: From your editor in chief
I am writing this in mid-April, in time for our May issue. These are unusual times. During unusual times, people rise up and do unusual and exemplary things. I firmly believe in the ability of humans to rise to the occasion and step out of their boundaries and boxes when needed. And the current COVID-19 pandemic is no exception. Our patients need us. The medical community needs us, and hematologists around the world have stepped up to help in any ways they can.
Since the beginning of the year with rumblings of the emergence of a novel SARs-CoV-2 virus in patients with influenza-like illness, the hematology community has banded together to figure out what this will mean for our patients battling malignant and nonmalignant blood disorders.
With very little published literature to go on, we have had to glean from our experience with the H1N1 influenza pandemic to develop a strategy to support hematology patients who may develop COVID-19 infection. With more questions than answers, institutions around the country and globally began to collaborate and communicate furiously with each other to learn from those who had experienced the effects of the virus before we had. We have been learning to anticipate blood supply changes, treatment modifications, and therapeutic needs for those who will inevitably get the virus.
Concern rose not just for the patients but also for the providers and clinical team who care for the hematology patients. How do we preserve and protect our workforce? A pandemic does not prevent new-diagnosis leukemia or myelodysplastic syndrome blast crisis from presenting as usual at 3 p.m. on a Friday afternoon. Who is at highest risk among the staff? If we practice social distancing what does that look like in a hematology clinic, in an infusion room? Or on the stem cell transplant in-patient unit? So many questions with minimal scientific evidence to guide our decisions.
As a sickle cell disease (SCD) specialist, I had some unique concerns. Roughly 10%-15% of the sickle cell population in the United States are supported by monthly red blood cell exchange transfusions, a lifesaving therapy to prevent recurrent stroke and to manage severe end organ damage. The vast majority of patients are on some disease-modifying therapy that requires ongoing lifelong monitoring of hematologic parameters, as well as renal and hepatic function. Most SCD patients also are members of racial minorities, live in densely populated parts of the city, and have significant social determinants of health that make adherence to social distancing mandates near impossible.
Frequent exposure to acute care for painful exacerbations of their disease, preexisting comorbidities involving the lung, heart, and kidney, and their underlying cellular and humoral immune dysfunction also put our patients at heightened risk of contracting COVID-19 infection.
So how have we handled the COVID-19 pandemic thus far? We have engaged various partners, collaborators, and colleagues to figure things out. Our institutions have established incident command operations to supervise and guide bed management, staff deployment/redeployment, and the supply chain, particularly as regards personal protective equipment; and to support physician and staff wellness. Our administrative leaders have partnered seamlessly with clinical leaders to rapidly roll out robust telemedicine strategies so that we can continue to provide ongoing medical care as best we can.
We have worked tirelessly across disciplines to develop guidance documents that are specialty specific with ways to support disease populations working with the hospitalist and acute care units to define testing, treatment, and admission and discharge criteria. We have engaged communications teams that have developed health-literate public messaging for the patients and the community about coronavirus as well as the rapidly changing public health guidelines to help #flattenthecurve.
As providers, we have reviewed our patient panels to determine who can tolerate appointment delays and who has to come in to be seen with minimal impact to health outcomes. We have read more articles in the past month than perhaps the past year; listened to more podcasts, webinars, and virtual lectures on COVID-19 and strategies to halt spread of the virus – just trying to learn more. We have engaged in social media – following COVID-19 public and private groups – to get and to offer support, as well as keep a finger on the pulse of the health community around this pandemic.
For my SCD population, I have had to decide who can tolerate simple rather than exchange transfusions for the next 3-6 months and what is the minimum number of red cell units we can safely use per red cell exchange procedure as we prepare for a possible blood supply shortage. The hematology community has worked tirelessly with national societies and numerous stakeholder groups to develop a comprehensive toolkit with regularly updated information about COVID-19 relevant to the hematology community (hematology.org/covid-19).
At a practice level, we are proactively reaching out to our hematology patients and their families to reassure them and connect them with resources and support while ensuring that they have adequate supply of their daily hematology medications with tips like using the pharmacy drive-through or home medication delivery options. The past 2 weeks in Charlotte, N.C., have been hectic with preparation. My days are long; a mixture of telemedicine visits, strategic meetings, and meetings to cascade the newest plan to the staff so that they know and are comfortable with it for the patients they take care of.
When the adrenaline from each day begins to wane, we think of our individual families; we worry about relatives far and near. We mourn the loss of loved ones or other hematologists or providers who have succumbed to the COVID-19 virus. We take a minute to think about ourselves and how this pandemic affects us individually and personally. I think about my older sister who runs a smaller hospital in the Bronx, N.Y. She is at the epicenter of the pandemic and is short-staffed in the ICU and medicine floors. Because she is an ob.gyn, she has called me for guidance on a pregnant woman with anemia and sickle cell trait. I hadn’t heard from her in 24 hours and I began to wonder – is she okay? Why didn’t she answer my call this morning? Is she sick? Did she get the information I sent to her linking her with our virtual ICU experts so she can implement a similar program for her hospital?
Next, I think of my younger sister in Long Island, N.Y., who was covering shifts as a hospitalist. She had asked me to send her some hematology tips on managing disseminated intravascular coagulopathy in COVID-19 patients as she has limited access to consultants. She sees an average of seven to nine COVID-19–positive patients and several persons under investigation per shift.
I also think of my 76-year-old mom who is upset that she cannot go to the adult center because of social distancing. So, even though I am weary, I do a FaceTime call with mom. I try to explain why it’s important for her to stay indoors. It’s only temporary, I reassure her; but I cannot say how long “temporary” is.
I pack up my bags, change out of my scrubs to head to my car thinking of my daughter who just turned 21 years old and was so excited about her college graduation in May. She had a meltdown yesterday because her university announced there will be no in-person gradation. I wonder how I can help her see the big picture and yet, why should she? She’s only 21.
Then I get a page – it’s a patient with sickle cell disease – my first COVID-19–positive patient. As I take the history and turn my computer back on to do this consult, I realize that this is what all the preparation was for. The sickle cell guidance document we had worked on over the past weeks to outline a step-by-step approach to managing a SCD patient with COVID-19 that is intentionally aligned with our institutions COVID-19 treatment protocol with specific nuances relevant to patients with red blood cell disorders, was now being put to use. I felt glad for my patient that we were prepared and had a semblance of a plan on how to approach his care.
The battle is far from over. Actually, as of my writing this, it’s just starting in my part of the country. The days will continue to be long. I continue to appreciate the beauty of the human spirit among the people we work with, the hematology community we belong to, and the patients that we serve. I am committed (as are all of you) to staying “on-duty” for as long as I can, and I’d like to take this opportunity to say to all the hematologists out there – “Thank you for your service and for reporting for duty to the front lines.”
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
I am writing this in mid-April, in time for our May issue. These are unusual times. During unusual times, people rise up and do unusual and exemplary things. I firmly believe in the ability of humans to rise to the occasion and step out of their boundaries and boxes when needed. And the current COVID-19 pandemic is no exception. Our patients need us. The medical community needs us, and hematologists around the world have stepped up to help in any ways they can.
Since the beginning of the year with rumblings of the emergence of a novel SARs-CoV-2 virus in patients with influenza-like illness, the hematology community has banded together to figure out what this will mean for our patients battling malignant and nonmalignant blood disorders.
With very little published literature to go on, we have had to glean from our experience with the H1N1 influenza pandemic to develop a strategy to support hematology patients who may develop COVID-19 infection. With more questions than answers, institutions around the country and globally began to collaborate and communicate furiously with each other to learn from those who had experienced the effects of the virus before we had. We have been learning to anticipate blood supply changes, treatment modifications, and therapeutic needs for those who will inevitably get the virus.
Concern rose not just for the patients but also for the providers and clinical team who care for the hematology patients. How do we preserve and protect our workforce? A pandemic does not prevent new-diagnosis leukemia or myelodysplastic syndrome blast crisis from presenting as usual at 3 p.m. on a Friday afternoon. Who is at highest risk among the staff? If we practice social distancing what does that look like in a hematology clinic, in an infusion room? Or on the stem cell transplant in-patient unit? So many questions with minimal scientific evidence to guide our decisions.
As a sickle cell disease (SCD) specialist, I had some unique concerns. Roughly 10%-15% of the sickle cell population in the United States are supported by monthly red blood cell exchange transfusions, a lifesaving therapy to prevent recurrent stroke and to manage severe end organ damage. The vast majority of patients are on some disease-modifying therapy that requires ongoing lifelong monitoring of hematologic parameters, as well as renal and hepatic function. Most SCD patients also are members of racial minorities, live in densely populated parts of the city, and have significant social determinants of health that make adherence to social distancing mandates near impossible.
Frequent exposure to acute care for painful exacerbations of their disease, preexisting comorbidities involving the lung, heart, and kidney, and their underlying cellular and humoral immune dysfunction also put our patients at heightened risk of contracting COVID-19 infection.
So how have we handled the COVID-19 pandemic thus far? We have engaged various partners, collaborators, and colleagues to figure things out. Our institutions have established incident command operations to supervise and guide bed management, staff deployment/redeployment, and the supply chain, particularly as regards personal protective equipment; and to support physician and staff wellness. Our administrative leaders have partnered seamlessly with clinical leaders to rapidly roll out robust telemedicine strategies so that we can continue to provide ongoing medical care as best we can.
We have worked tirelessly across disciplines to develop guidance documents that are specialty specific with ways to support disease populations working with the hospitalist and acute care units to define testing, treatment, and admission and discharge criteria. We have engaged communications teams that have developed health-literate public messaging for the patients and the community about coronavirus as well as the rapidly changing public health guidelines to help #flattenthecurve.
As providers, we have reviewed our patient panels to determine who can tolerate appointment delays and who has to come in to be seen with minimal impact to health outcomes. We have read more articles in the past month than perhaps the past year; listened to more podcasts, webinars, and virtual lectures on COVID-19 and strategies to halt spread of the virus – just trying to learn more. We have engaged in social media – following COVID-19 public and private groups – to get and to offer support, as well as keep a finger on the pulse of the health community around this pandemic.
For my SCD population, I have had to decide who can tolerate simple rather than exchange transfusions for the next 3-6 months and what is the minimum number of red cell units we can safely use per red cell exchange procedure as we prepare for a possible blood supply shortage. The hematology community has worked tirelessly with national societies and numerous stakeholder groups to develop a comprehensive toolkit with regularly updated information about COVID-19 relevant to the hematology community (hematology.org/covid-19).
At a practice level, we are proactively reaching out to our hematology patients and their families to reassure them and connect them with resources and support while ensuring that they have adequate supply of their daily hematology medications with tips like using the pharmacy drive-through or home medication delivery options. The past 2 weeks in Charlotte, N.C., have been hectic with preparation. My days are long; a mixture of telemedicine visits, strategic meetings, and meetings to cascade the newest plan to the staff so that they know and are comfortable with it for the patients they take care of.
When the adrenaline from each day begins to wane, we think of our individual families; we worry about relatives far and near. We mourn the loss of loved ones or other hematologists or providers who have succumbed to the COVID-19 virus. We take a minute to think about ourselves and how this pandemic affects us individually and personally. I think about my older sister who runs a smaller hospital in the Bronx, N.Y. She is at the epicenter of the pandemic and is short-staffed in the ICU and medicine floors. Because she is an ob.gyn, she has called me for guidance on a pregnant woman with anemia and sickle cell trait. I hadn’t heard from her in 24 hours and I began to wonder – is she okay? Why didn’t she answer my call this morning? Is she sick? Did she get the information I sent to her linking her with our virtual ICU experts so she can implement a similar program for her hospital?
Next, I think of my younger sister in Long Island, N.Y., who was covering shifts as a hospitalist. She had asked me to send her some hematology tips on managing disseminated intravascular coagulopathy in COVID-19 patients as she has limited access to consultants. She sees an average of seven to nine COVID-19–positive patients and several persons under investigation per shift.
I also think of my 76-year-old mom who is upset that she cannot go to the adult center because of social distancing. So, even though I am weary, I do a FaceTime call with mom. I try to explain why it’s important for her to stay indoors. It’s only temporary, I reassure her; but I cannot say how long “temporary” is.
I pack up my bags, change out of my scrubs to head to my car thinking of my daughter who just turned 21 years old and was so excited about her college graduation in May. She had a meltdown yesterday because her university announced there will be no in-person gradation. I wonder how I can help her see the big picture and yet, why should she? She’s only 21.
Then I get a page – it’s a patient with sickle cell disease – my first COVID-19–positive patient. As I take the history and turn my computer back on to do this consult, I realize that this is what all the preparation was for. The sickle cell guidance document we had worked on over the past weeks to outline a step-by-step approach to managing a SCD patient with COVID-19 that is intentionally aligned with our institutions COVID-19 treatment protocol with specific nuances relevant to patients with red blood cell disorders, was now being put to use. I felt glad for my patient that we were prepared and had a semblance of a plan on how to approach his care.
The battle is far from over. Actually, as of my writing this, it’s just starting in my part of the country. The days will continue to be long. I continue to appreciate the beauty of the human spirit among the people we work with, the hematology community we belong to, and the patients that we serve. I am committed (as are all of you) to staying “on-duty” for as long as I can, and I’d like to take this opportunity to say to all the hematologists out there – “Thank you for your service and for reporting for duty to the front lines.”
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
I am writing this in mid-April, in time for our May issue. These are unusual times. During unusual times, people rise up and do unusual and exemplary things. I firmly believe in the ability of humans to rise to the occasion and step out of their boundaries and boxes when needed. And the current COVID-19 pandemic is no exception. Our patients need us. The medical community needs us, and hematologists around the world have stepped up to help in any ways they can.
Since the beginning of the year with rumblings of the emergence of a novel SARs-CoV-2 virus in patients with influenza-like illness, the hematology community has banded together to figure out what this will mean for our patients battling malignant and nonmalignant blood disorders.
With very little published literature to go on, we have had to glean from our experience with the H1N1 influenza pandemic to develop a strategy to support hematology patients who may develop COVID-19 infection. With more questions than answers, institutions around the country and globally began to collaborate and communicate furiously with each other to learn from those who had experienced the effects of the virus before we had. We have been learning to anticipate blood supply changes, treatment modifications, and therapeutic needs for those who will inevitably get the virus.
Concern rose not just for the patients but also for the providers and clinical team who care for the hematology patients. How do we preserve and protect our workforce? A pandemic does not prevent new-diagnosis leukemia or myelodysplastic syndrome blast crisis from presenting as usual at 3 p.m. on a Friday afternoon. Who is at highest risk among the staff? If we practice social distancing what does that look like in a hematology clinic, in an infusion room? Or on the stem cell transplant in-patient unit? So many questions with minimal scientific evidence to guide our decisions.
As a sickle cell disease (SCD) specialist, I had some unique concerns. Roughly 10%-15% of the sickle cell population in the United States are supported by monthly red blood cell exchange transfusions, a lifesaving therapy to prevent recurrent stroke and to manage severe end organ damage. The vast majority of patients are on some disease-modifying therapy that requires ongoing lifelong monitoring of hematologic parameters, as well as renal and hepatic function. Most SCD patients also are members of racial minorities, live in densely populated parts of the city, and have significant social determinants of health that make adherence to social distancing mandates near impossible.
Frequent exposure to acute care for painful exacerbations of their disease, preexisting comorbidities involving the lung, heart, and kidney, and their underlying cellular and humoral immune dysfunction also put our patients at heightened risk of contracting COVID-19 infection.
So how have we handled the COVID-19 pandemic thus far? We have engaged various partners, collaborators, and colleagues to figure things out. Our institutions have established incident command operations to supervise and guide bed management, staff deployment/redeployment, and the supply chain, particularly as regards personal protective equipment; and to support physician and staff wellness. Our administrative leaders have partnered seamlessly with clinical leaders to rapidly roll out robust telemedicine strategies so that we can continue to provide ongoing medical care as best we can.
We have worked tirelessly across disciplines to develop guidance documents that are specialty specific with ways to support disease populations working with the hospitalist and acute care units to define testing, treatment, and admission and discharge criteria. We have engaged communications teams that have developed health-literate public messaging for the patients and the community about coronavirus as well as the rapidly changing public health guidelines to help #flattenthecurve.
As providers, we have reviewed our patient panels to determine who can tolerate appointment delays and who has to come in to be seen with minimal impact to health outcomes. We have read more articles in the past month than perhaps the past year; listened to more podcasts, webinars, and virtual lectures on COVID-19 and strategies to halt spread of the virus – just trying to learn more. We have engaged in social media – following COVID-19 public and private groups – to get and to offer support, as well as keep a finger on the pulse of the health community around this pandemic.
For my SCD population, I have had to decide who can tolerate simple rather than exchange transfusions for the next 3-6 months and what is the minimum number of red cell units we can safely use per red cell exchange procedure as we prepare for a possible blood supply shortage. The hematology community has worked tirelessly with national societies and numerous stakeholder groups to develop a comprehensive toolkit with regularly updated information about COVID-19 relevant to the hematology community (hematology.org/covid-19).
At a practice level, we are proactively reaching out to our hematology patients and their families to reassure them and connect them with resources and support while ensuring that they have adequate supply of their daily hematology medications with tips like using the pharmacy drive-through or home medication delivery options. The past 2 weeks in Charlotte, N.C., have been hectic with preparation. My days are long; a mixture of telemedicine visits, strategic meetings, and meetings to cascade the newest plan to the staff so that they know and are comfortable with it for the patients they take care of.
When the adrenaline from each day begins to wane, we think of our individual families; we worry about relatives far and near. We mourn the loss of loved ones or other hematologists or providers who have succumbed to the COVID-19 virus. We take a minute to think about ourselves and how this pandemic affects us individually and personally. I think about my older sister who runs a smaller hospital in the Bronx, N.Y. She is at the epicenter of the pandemic and is short-staffed in the ICU and medicine floors. Because she is an ob.gyn, she has called me for guidance on a pregnant woman with anemia and sickle cell trait. I hadn’t heard from her in 24 hours and I began to wonder – is she okay? Why didn’t she answer my call this morning? Is she sick? Did she get the information I sent to her linking her with our virtual ICU experts so she can implement a similar program for her hospital?
Next, I think of my younger sister in Long Island, N.Y., who was covering shifts as a hospitalist. She had asked me to send her some hematology tips on managing disseminated intravascular coagulopathy in COVID-19 patients as she has limited access to consultants. She sees an average of seven to nine COVID-19–positive patients and several persons under investigation per shift.
I also think of my 76-year-old mom who is upset that she cannot go to the adult center because of social distancing. So, even though I am weary, I do a FaceTime call with mom. I try to explain why it’s important for her to stay indoors. It’s only temporary, I reassure her; but I cannot say how long “temporary” is.
I pack up my bags, change out of my scrubs to head to my car thinking of my daughter who just turned 21 years old and was so excited about her college graduation in May. She had a meltdown yesterday because her university announced there will be no in-person gradation. I wonder how I can help her see the big picture and yet, why should she? She’s only 21.
Then I get a page – it’s a patient with sickle cell disease – my first COVID-19–positive patient. As I take the history and turn my computer back on to do this consult, I realize that this is what all the preparation was for. The sickle cell guidance document we had worked on over the past weeks to outline a step-by-step approach to managing a SCD patient with COVID-19 that is intentionally aligned with our institutions COVID-19 treatment protocol with specific nuances relevant to patients with red blood cell disorders, was now being put to use. I felt glad for my patient that we were prepared and had a semblance of a plan on how to approach his care.
The battle is far from over. Actually, as of my writing this, it’s just starting in my part of the country. The days will continue to be long. I continue to appreciate the beauty of the human spirit among the people we work with, the hematology community we belong to, and the patients that we serve. I am committed (as are all of you) to staying “on-duty” for as long as I can, and I’d like to take this opportunity to say to all the hematologists out there – “Thank you for your service and for reporting for duty to the front lines.”
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
FDA tightens requirements for COVID-19 antibody tests
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
Hydroxychloroquine-triggered QTc-interval prolongations mount in COVID-19 patients
The potential for serious arrhythmias from hydroxychloroquine treatment of COVID-19 patients received further documentation from a pair of studies released on May 1, casting further doubt on whether the uncertain benefit from this or related drugs to infected patients is worth the clear risks the agents pose.
A report from 90 confirmed COVID-19 patients treated with hydroxychloroquine at one Boston hospital during March-April 2020 identified a significantly prolonged, corrected QT (QTc) interval of at least 500 msec in 18 patients (20%), which included 10 patients whose QTc rose by at least 60 msec above baseline, and a total of 21 patients (23%) having a notable prolongation (JAMA Cardiol. 2020 May 4. doi: 10.1001/jamacardio.2020.1834). This series included one patient who developed torsades de pointes following treatment with hydroxychloroquine and azithromycin, “which to our knowledge has yet to be reported elsewhere in the literature,” the report said.
The second report, from a single center in Lyon, France, included 40 confirmed COVID-19 patients treated with hydroxychloroquine during 2 weeks in late March, and found that 37 (93%) had some increase in the QTc interval, including 14 patients (36%) with an increase of at least 60 msec, and 7 patients (18%) whose QTc rose to at least 500 msec (JAMA Cardiol. 2020 May. doi: 10.1001/jamacardio.2020.1787). However, none of the 40 patients in this series developed an identified ventricular arrhythmia. All patients in both studies received hydroxychloroquine for at least 1 day, and roughly half the patients in each series also received concurrent azithromycin, another drug that can prolong the QTc interval and that has been frequently used in combination with hydroxychloroquine as an unproven COVID-19 treatment cocktail.
These two reports, as well as prior report from Brazil on COVID-19 patients treated with chloroquine diphosphate (JAMA Netw Open. 2020;3[4]:e208857), “underscore the potential risk associated with widespread use of hydroxychloroquine and the combination of hydroxychloroquine and azithromycin in ambulatory patients with known or suspected COVID-19. Understanding whether this risk is worth taking in the absence of evidence of therapeutic efficacy creates a knowledge gap that needs to be addressed,” wrote Robert O. Bonow, MD, a professor of medicine at Northwestern University in Chicago, and coauthors in an editorial that accompanied the two reports (JAMA Cardiol. 2020 May 4;doi: 10.1001/jamacardio.2020.1782). The editorial cited two recently-begun prospective trials, ORCHID and RECOVERY, that are more systematically assessing the safety and efficacy of hydroxychloroquine treatment in COVID-19 patients.
The findings lend further support to a Safety Communication from the U.S. Food and Drug Administration on April 24 that reminded clinicians that the Emergency Use Authorization for hydroxychloroquine and chloroquine in COVID-19 patients that the FDA issued on March 28 applied to only certain hospitalized patients or those enrolled in clinical trials. The Safety Communication also said that agency was aware of reports of adverse arrhythmia events when COVID-19 patients received these drugs outside a hospital setting as well as uninfected people who had received one of these drugs for preventing infection.
In addition, leaders of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society on April 10 issued a summary of considerations when using hydroxychloroquine and azithromycin to treat COVID-19 patients, and noted that a way to minimized the risk from these drugs is to withhold them from patients with a QTc interval of 500 msec or greater at baseline (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). The summary also highlighted the need for regular ECG monitoring of COVID-19 patients who receive drugs that can prolong the QTc interval, and recommended withdrawing treatment from patients when their QTc exceeds the 500 msec threshold.
None of the authors of the two reports and editorial had relevant commercial disclosures.
The potential for serious arrhythmias from hydroxychloroquine treatment of COVID-19 patients received further documentation from a pair of studies released on May 1, casting further doubt on whether the uncertain benefit from this or related drugs to infected patients is worth the clear risks the agents pose.
A report from 90 confirmed COVID-19 patients treated with hydroxychloroquine at one Boston hospital during March-April 2020 identified a significantly prolonged, corrected QT (QTc) interval of at least 500 msec in 18 patients (20%), which included 10 patients whose QTc rose by at least 60 msec above baseline, and a total of 21 patients (23%) having a notable prolongation (JAMA Cardiol. 2020 May 4. doi: 10.1001/jamacardio.2020.1834). This series included one patient who developed torsades de pointes following treatment with hydroxychloroquine and azithromycin, “which to our knowledge has yet to be reported elsewhere in the literature,” the report said.
The second report, from a single center in Lyon, France, included 40 confirmed COVID-19 patients treated with hydroxychloroquine during 2 weeks in late March, and found that 37 (93%) had some increase in the QTc interval, including 14 patients (36%) with an increase of at least 60 msec, and 7 patients (18%) whose QTc rose to at least 500 msec (JAMA Cardiol. 2020 May. doi: 10.1001/jamacardio.2020.1787). However, none of the 40 patients in this series developed an identified ventricular arrhythmia. All patients in both studies received hydroxychloroquine for at least 1 day, and roughly half the patients in each series also received concurrent azithromycin, another drug that can prolong the QTc interval and that has been frequently used in combination with hydroxychloroquine as an unproven COVID-19 treatment cocktail.
These two reports, as well as prior report from Brazil on COVID-19 patients treated with chloroquine diphosphate (JAMA Netw Open. 2020;3[4]:e208857), “underscore the potential risk associated with widespread use of hydroxychloroquine and the combination of hydroxychloroquine and azithromycin in ambulatory patients with known or suspected COVID-19. Understanding whether this risk is worth taking in the absence of evidence of therapeutic efficacy creates a knowledge gap that needs to be addressed,” wrote Robert O. Bonow, MD, a professor of medicine at Northwestern University in Chicago, and coauthors in an editorial that accompanied the two reports (JAMA Cardiol. 2020 May 4;doi: 10.1001/jamacardio.2020.1782). The editorial cited two recently-begun prospective trials, ORCHID and RECOVERY, that are more systematically assessing the safety and efficacy of hydroxychloroquine treatment in COVID-19 patients.
The findings lend further support to a Safety Communication from the U.S. Food and Drug Administration on April 24 that reminded clinicians that the Emergency Use Authorization for hydroxychloroquine and chloroquine in COVID-19 patients that the FDA issued on March 28 applied to only certain hospitalized patients or those enrolled in clinical trials. The Safety Communication also said that agency was aware of reports of adverse arrhythmia events when COVID-19 patients received these drugs outside a hospital setting as well as uninfected people who had received one of these drugs for preventing infection.
In addition, leaders of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society on April 10 issued a summary of considerations when using hydroxychloroquine and azithromycin to treat COVID-19 patients, and noted that a way to minimized the risk from these drugs is to withhold them from patients with a QTc interval of 500 msec or greater at baseline (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). The summary also highlighted the need for regular ECG monitoring of COVID-19 patients who receive drugs that can prolong the QTc interval, and recommended withdrawing treatment from patients when their QTc exceeds the 500 msec threshold.
None of the authors of the two reports and editorial had relevant commercial disclosures.
The potential for serious arrhythmias from hydroxychloroquine treatment of COVID-19 patients received further documentation from a pair of studies released on May 1, casting further doubt on whether the uncertain benefit from this or related drugs to infected patients is worth the clear risks the agents pose.
A report from 90 confirmed COVID-19 patients treated with hydroxychloroquine at one Boston hospital during March-April 2020 identified a significantly prolonged, corrected QT (QTc) interval of at least 500 msec in 18 patients (20%), which included 10 patients whose QTc rose by at least 60 msec above baseline, and a total of 21 patients (23%) having a notable prolongation (JAMA Cardiol. 2020 May 4. doi: 10.1001/jamacardio.2020.1834). This series included one patient who developed torsades de pointes following treatment with hydroxychloroquine and azithromycin, “which to our knowledge has yet to be reported elsewhere in the literature,” the report said.
The second report, from a single center in Lyon, France, included 40 confirmed COVID-19 patients treated with hydroxychloroquine during 2 weeks in late March, and found that 37 (93%) had some increase in the QTc interval, including 14 patients (36%) with an increase of at least 60 msec, and 7 patients (18%) whose QTc rose to at least 500 msec (JAMA Cardiol. 2020 May. doi: 10.1001/jamacardio.2020.1787). However, none of the 40 patients in this series developed an identified ventricular arrhythmia. All patients in both studies received hydroxychloroquine for at least 1 day, and roughly half the patients in each series also received concurrent azithromycin, another drug that can prolong the QTc interval and that has been frequently used in combination with hydroxychloroquine as an unproven COVID-19 treatment cocktail.
These two reports, as well as prior report from Brazil on COVID-19 patients treated with chloroquine diphosphate (JAMA Netw Open. 2020;3[4]:e208857), “underscore the potential risk associated with widespread use of hydroxychloroquine and the combination of hydroxychloroquine and azithromycin in ambulatory patients with known or suspected COVID-19. Understanding whether this risk is worth taking in the absence of evidence of therapeutic efficacy creates a knowledge gap that needs to be addressed,” wrote Robert O. Bonow, MD, a professor of medicine at Northwestern University in Chicago, and coauthors in an editorial that accompanied the two reports (JAMA Cardiol. 2020 May 4;doi: 10.1001/jamacardio.2020.1782). The editorial cited two recently-begun prospective trials, ORCHID and RECOVERY, that are more systematically assessing the safety and efficacy of hydroxychloroquine treatment in COVID-19 patients.
The findings lend further support to a Safety Communication from the U.S. Food and Drug Administration on April 24 that reminded clinicians that the Emergency Use Authorization for hydroxychloroquine and chloroquine in COVID-19 patients that the FDA issued on March 28 applied to only certain hospitalized patients or those enrolled in clinical trials. The Safety Communication also said that agency was aware of reports of adverse arrhythmia events when COVID-19 patients received these drugs outside a hospital setting as well as uninfected people who had received one of these drugs for preventing infection.
In addition, leaders of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society on April 10 issued a summary of considerations when using hydroxychloroquine and azithromycin to treat COVID-19 patients, and noted that a way to minimized the risk from these drugs is to withhold them from patients with a QTc interval of 500 msec or greater at baseline (J Am Coll Cardiol. 2020 Apr 10. doi: 10.1016/j.jacc.2020.04.016). The summary also highlighted the need for regular ECG monitoring of COVID-19 patients who receive drugs that can prolong the QTc interval, and recommended withdrawing treatment from patients when their QTc exceeds the 500 msec threshold.
None of the authors of the two reports and editorial had relevant commercial disclosures.
FROM JAMA CARDIOLOGY
COVID-19: Social distancing with young children
Emma just celebrated her second birthday, and she has been working on the usual things that children start to master at this age: potty training, making friends, exerting her will through both actions and words, and generally enjoying life as the center of attention for both her parents and grandparents. Like everyone else in Maryland, Emma’s life changed suddenly with the coronavirus stay-at-home order that was issued on March 30. There is no more day care and her parents work from home while caring for her. Her grandparents visit, but only outside and only from a distance – there are no more hugs and there is no more sitting in her grandfather’s lap while he reads stories.
One afternoon a few weeks ago, Emma was looking out the window when she saw her friend, Max, walk by with his parents. Before her parents could stop her, Emma bolted out the door, and she and little Max wrapped each other in a tight embrace. Their parents snapped a photo of the smiling toddlers hugging before they separated the children. The photo is adorable, but as all struggle with social distancing, the poignance of two innocent toddlers in a forbidden embrace is a bit heartbreaking.
Everyone who has ever observed children knows that social distancing is not in their nature. Children play, they hug, they wrestle and tackle and poke, and sometimes even bite. And every student of social psychology has been taught about Harry Harlow’s experiments with rhesus macaques who were separated from their mothers and given access to an inanimate object to serve as a surrogate mother. The Harlow studies, while controversial, were revolutionary in demonstrating that early interactions with both a mother and with playmates were essential in the development of normal social relationships.
Regine Galanti, PhD, is a clinical psychologist at Long Island Behavioral Psychology, Cedarhurst, N.Y., who specializes in the treatment of anxiety and behavior problems. With young children she uses parent-child interaction therapy (PCIT) to help build relationships and discipline. Dr. Galanti said: “I don’t think we’re well prepared as a field to answer questions about the long-term effects of social distancing. If you need young children to socially distance, the responsibility has to fall on the adults. It’s important to explain to children what’s going on and to be honest in a developmentally appropriate way.”
Dr. Galanti has noticed that the issues that people had before COVID-19 are exacerbated by the stress of the current situation. What we do know is that young children thrive on structure.”
Tovah P. Klein, PhD, is the author of “How Toddlers Thrive” (Touchstone, 2015) and is the director of the Barnard College Center for Toddler Development in Manhattan. “When this started, we thought we would be closed for a few weeks,” Dr. Klein said. “We wanted to maintain a connection to the children, so we made videos for the parents to show to the kids, just to say ‘We’re still here.’ But as time went on and we realized it was going to be a while, we felt it was important to provide connection, so we launched a virtual program.”
Dr. Klein said that the teachers meet with their classes of 13 2-year-olds over Zoom, and when they first started, she asked the teachers to try to meet for 10 minutes. They are now meeting for 40 minutes twice a week. The children like seeing their teachers in their homes and they like seeing each other. In addition, the teachers make videos to send home and they are currently working on one to demystify masks. “We’re working on normalizing masks and showing children that when you put the mask on, you’re still there underneath.”
The center has existed for 48 years. There have been struggles for some of the children who attend; some of the parents have been hospitalized with the virus, and some work on the front line and so parents may be living away from a child.
“We’ve seen more challenging behaviors during this time, more tantrums, toileting issues, night awakenings, and more fragility. But as the new normal takes hold, things are settling in. Parents have been good about getting new routines and it helps if parents can handle their own stress,” Dr. Klein said. She also pointed out that for parents working at home while caring for their children, this can be particularly difficult on a young child. “The child knows the parent is home, but isn’t spending time with him, and he sees it as a rejection.”
Margaret Adams, MD, is a child psychiatrist in Maryland who works with very young children and their parents. She says that some of the children are thriving with the extra attention from their parents. “I often have seen difficulties with readjustment to the routine of separations to day care after a family vacation of a week, or sometimes even a weekend, even for those young ones who seem to love the social aspects of day care. I think it is likely a big impact will come upon return, depending on the developmental stage of the child,” Dr. Adams noted.
Despite the hardships of the moment, all three experts expressed hopefulness about the future for these children.
“Young children are super-resilient and that’s the blessing of this,” Dr. Galanti said. “I think they will be okay.”
Emma is home for now with her parents, who are expecting another child soon. Her mother notes: “The days are long and balancing work is an impossible challenge, but being with Emma has been a total blessing, and when would I ever have this much time to spend with my kid? She’s at such a fun age – so curious and adventurous – it’s amazing to watch her language and skills progress. I wish we weren’t in the midst of a pandemic, but Emma is definitely the bright spot.”
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Emma just celebrated her second birthday, and she has been working on the usual things that children start to master at this age: potty training, making friends, exerting her will through both actions and words, and generally enjoying life as the center of attention for both her parents and grandparents. Like everyone else in Maryland, Emma’s life changed suddenly with the coronavirus stay-at-home order that was issued on March 30. There is no more day care and her parents work from home while caring for her. Her grandparents visit, but only outside and only from a distance – there are no more hugs and there is no more sitting in her grandfather’s lap while he reads stories.
One afternoon a few weeks ago, Emma was looking out the window when she saw her friend, Max, walk by with his parents. Before her parents could stop her, Emma bolted out the door, and she and little Max wrapped each other in a tight embrace. Their parents snapped a photo of the smiling toddlers hugging before they separated the children. The photo is adorable, but as all struggle with social distancing, the poignance of two innocent toddlers in a forbidden embrace is a bit heartbreaking.
Everyone who has ever observed children knows that social distancing is not in their nature. Children play, they hug, they wrestle and tackle and poke, and sometimes even bite. And every student of social psychology has been taught about Harry Harlow’s experiments with rhesus macaques who were separated from their mothers and given access to an inanimate object to serve as a surrogate mother. The Harlow studies, while controversial, were revolutionary in demonstrating that early interactions with both a mother and with playmates were essential in the development of normal social relationships.
Regine Galanti, PhD, is a clinical psychologist at Long Island Behavioral Psychology, Cedarhurst, N.Y., who specializes in the treatment of anxiety and behavior problems. With young children she uses parent-child interaction therapy (PCIT) to help build relationships and discipline. Dr. Galanti said: “I don’t think we’re well prepared as a field to answer questions about the long-term effects of social distancing. If you need young children to socially distance, the responsibility has to fall on the adults. It’s important to explain to children what’s going on and to be honest in a developmentally appropriate way.”
Dr. Galanti has noticed that the issues that people had before COVID-19 are exacerbated by the stress of the current situation. What we do know is that young children thrive on structure.”
Tovah P. Klein, PhD, is the author of “How Toddlers Thrive” (Touchstone, 2015) and is the director of the Barnard College Center for Toddler Development in Manhattan. “When this started, we thought we would be closed for a few weeks,” Dr. Klein said. “We wanted to maintain a connection to the children, so we made videos for the parents to show to the kids, just to say ‘We’re still here.’ But as time went on and we realized it was going to be a while, we felt it was important to provide connection, so we launched a virtual program.”
Dr. Klein said that the teachers meet with their classes of 13 2-year-olds over Zoom, and when they first started, she asked the teachers to try to meet for 10 minutes. They are now meeting for 40 minutes twice a week. The children like seeing their teachers in their homes and they like seeing each other. In addition, the teachers make videos to send home and they are currently working on one to demystify masks. “We’re working on normalizing masks and showing children that when you put the mask on, you’re still there underneath.”
The center has existed for 48 years. There have been struggles for some of the children who attend; some of the parents have been hospitalized with the virus, and some work on the front line and so parents may be living away from a child.
“We’ve seen more challenging behaviors during this time, more tantrums, toileting issues, night awakenings, and more fragility. But as the new normal takes hold, things are settling in. Parents have been good about getting new routines and it helps if parents can handle their own stress,” Dr. Klein said. She also pointed out that for parents working at home while caring for their children, this can be particularly difficult on a young child. “The child knows the parent is home, but isn’t spending time with him, and he sees it as a rejection.”
Margaret Adams, MD, is a child psychiatrist in Maryland who works with very young children and their parents. She says that some of the children are thriving with the extra attention from their parents. “I often have seen difficulties with readjustment to the routine of separations to day care after a family vacation of a week, or sometimes even a weekend, even for those young ones who seem to love the social aspects of day care. I think it is likely a big impact will come upon return, depending on the developmental stage of the child,” Dr. Adams noted.
Despite the hardships of the moment, all three experts expressed hopefulness about the future for these children.
“Young children are super-resilient and that’s the blessing of this,” Dr. Galanti said. “I think they will be okay.”
Emma is home for now with her parents, who are expecting another child soon. Her mother notes: “The days are long and balancing work is an impossible challenge, but being with Emma has been a total blessing, and when would I ever have this much time to spend with my kid? She’s at such a fun age – so curious and adventurous – it’s amazing to watch her language and skills progress. I wish we weren’t in the midst of a pandemic, but Emma is definitely the bright spot.”
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.
Emma just celebrated her second birthday, and she has been working on the usual things that children start to master at this age: potty training, making friends, exerting her will through both actions and words, and generally enjoying life as the center of attention for both her parents and grandparents. Like everyone else in Maryland, Emma’s life changed suddenly with the coronavirus stay-at-home order that was issued on March 30. There is no more day care and her parents work from home while caring for her. Her grandparents visit, but only outside and only from a distance – there are no more hugs and there is no more sitting in her grandfather’s lap while he reads stories.
One afternoon a few weeks ago, Emma was looking out the window when she saw her friend, Max, walk by with his parents. Before her parents could stop her, Emma bolted out the door, and she and little Max wrapped each other in a tight embrace. Their parents snapped a photo of the smiling toddlers hugging before they separated the children. The photo is adorable, but as all struggle with social distancing, the poignance of two innocent toddlers in a forbidden embrace is a bit heartbreaking.
Everyone who has ever observed children knows that social distancing is not in their nature. Children play, they hug, they wrestle and tackle and poke, and sometimes even bite. And every student of social psychology has been taught about Harry Harlow’s experiments with rhesus macaques who were separated from their mothers and given access to an inanimate object to serve as a surrogate mother. The Harlow studies, while controversial, were revolutionary in demonstrating that early interactions with both a mother and with playmates were essential in the development of normal social relationships.
Regine Galanti, PhD, is a clinical psychologist at Long Island Behavioral Psychology, Cedarhurst, N.Y., who specializes in the treatment of anxiety and behavior problems. With young children she uses parent-child interaction therapy (PCIT) to help build relationships and discipline. Dr. Galanti said: “I don’t think we’re well prepared as a field to answer questions about the long-term effects of social distancing. If you need young children to socially distance, the responsibility has to fall on the adults. It’s important to explain to children what’s going on and to be honest in a developmentally appropriate way.”
Dr. Galanti has noticed that the issues that people had before COVID-19 are exacerbated by the stress of the current situation. What we do know is that young children thrive on structure.”
Tovah P. Klein, PhD, is the author of “How Toddlers Thrive” (Touchstone, 2015) and is the director of the Barnard College Center for Toddler Development in Manhattan. “When this started, we thought we would be closed for a few weeks,” Dr. Klein said. “We wanted to maintain a connection to the children, so we made videos for the parents to show to the kids, just to say ‘We’re still here.’ But as time went on and we realized it was going to be a while, we felt it was important to provide connection, so we launched a virtual program.”
Dr. Klein said that the teachers meet with their classes of 13 2-year-olds over Zoom, and when they first started, she asked the teachers to try to meet for 10 minutes. They are now meeting for 40 minutes twice a week. The children like seeing their teachers in their homes and they like seeing each other. In addition, the teachers make videos to send home and they are currently working on one to demystify masks. “We’re working on normalizing masks and showing children that when you put the mask on, you’re still there underneath.”
The center has existed for 48 years. There have been struggles for some of the children who attend; some of the parents have been hospitalized with the virus, and some work on the front line and so parents may be living away from a child.
“We’ve seen more challenging behaviors during this time, more tantrums, toileting issues, night awakenings, and more fragility. But as the new normal takes hold, things are settling in. Parents have been good about getting new routines and it helps if parents can handle their own stress,” Dr. Klein said. She also pointed out that for parents working at home while caring for their children, this can be particularly difficult on a young child. “The child knows the parent is home, but isn’t spending time with him, and he sees it as a rejection.”
Margaret Adams, MD, is a child psychiatrist in Maryland who works with very young children and their parents. She says that some of the children are thriving with the extra attention from their parents. “I often have seen difficulties with readjustment to the routine of separations to day care after a family vacation of a week, or sometimes even a weekend, even for those young ones who seem to love the social aspects of day care. I think it is likely a big impact will come upon return, depending on the developmental stage of the child,” Dr. Adams noted.
Despite the hardships of the moment, all three experts expressed hopefulness about the future for these children.
“Young children are super-resilient and that’s the blessing of this,” Dr. Galanti said. “I think they will be okay.”
Emma is home for now with her parents, who are expecting another child soon. Her mother notes: “The days are long and balancing work is an impossible challenge, but being with Emma has been a total blessing, and when would I ever have this much time to spend with my kid? She’s at such a fun age – so curious and adventurous – it’s amazing to watch her language and skills progress. I wish we weren’t in the midst of a pandemic, but Emma is definitely the bright spot.”
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. Dr. Miller has no disclosures.













