User login
-
Isa-Kd improves PFS in relapsed/refractory multiple myeloma
The risk of progression or death for patients with relapsed or refractory multiple myeloma was nearly halved with the addition of isatuximab to carfilzomib and dexamethasone, according to an interim analysis of the phase 3 IKEMA trial (NCT03275285).
After a median follow-up of 20.7 months, the median progression-free survival had not been reached for 179 patients treated with isatuximab (Sarclisa), carfilzomib (Kyprolis), and dexamethasone (together, known as Isa-Kd), compared with 19.5 months for 123 patients treated with carfilzomib and dexamethasone alone (Kd). The hazard ratio for progression free survival with the triple combination was 0.531 (P = .0007), reported coprincipal investigator Phillipe Moreau, MD, from the University Hospital Hôtel-Dieu in Nantes, France.
“The benefit of the triplet combination was observed across subgroups, including patients difficult to treat, such as [those with] high-risk cytogenetics or elderly patients,” he said in a late-breaking abstract presentation during the virtual annual congress of the European Hematology Association.
Isatuximab is an immunoglobulin G1 monoclonal antibody targeting a CD38 transmembrane glycoprotein on multiple myeloma cells, with a mechanism of action similar to that of another anti-CD38 antibody, daratumumab (Darzalex). Isatuximab is approved in the United States and Europe in combination with pomalidomide and dexamethasone for patients with relapsed/refractory multiple myeloma after at least two prior lines of therapy.
A ‘me too’ agent?
It’s unclear, however, whether isatuximab offers any additional benefit over daratumumab, an agent approved for use both in front line therapy combinations and for patients with relapsed/refractory disease, said Brea C. Lipe, MD, a multiple myeloma specialist at the University of Rochester (N.Y.) Wilmot Cancer Institute, who was not involved in the study.
“Every time we get a new drug it’s nice to have another option, but it doesn’t really add anything different from daratumumab at this point,” she said in an interview.
Dr. Lipe noted the IKEMA results are similar to those seen in the phase 3 CANDOR trial, comparing carfilzomib, dexamethasone, and daratumumab to carfilzomib/dexamethasone alone in patients with relapsed/refractory myeloma. In addition, it’s unknown whether patients with disease that is refractory to daratumumab could benefit from isatuximab, she said.
Although isatuximab has been touted as offering more rapid and more convenient dosing than daratumumab, the introduction of rapid infusion and subcutaneous administration of daratumumab has negated any theoretical advantage of the newcomer, Dr. Lipe added.
Study details
In the IKEMA trial, 302 patients with relapsed/refractory multiple myeloma who’d received one to three prior lines of therapy were stratified by the number of prior lines and by revised Multiple Myeloma International Staging System (R-ISS) and were then randomized on a 3:2 basis to treatment with carfilzomib 20 mg/m2 on days 1, 2, 8, 9, 15, and 16 of cycle 1 and 56 mg/m2 on the corresponding days of each subsequent cycle plus dexamethasone 20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of each cycle, with or without isatuximab. The antibody was dosed 10 mg/kg on days 1, 8, 15, and 22 in cycle 1 then every 2 weeks thereafter.
Treatments were continued until disease progression, unacceptable toxicity, or patient choice intervened.
At a prespecified interim analysis, the trial met its primary endpoint of a minimum 41% risk reduction in the hazard rate for progression free survival (PFS), with an actual risk reduction of 47%.
An analysis of PFS by subgroup showed significant benefits with the triple combination for patients aged 65 years and older, those with baseline estimated glomerular filtration rates below 60 mL/min per 1.73 m2, those with more than one prior line of therapy, those who had not previously received a proteasome inhibitor (e.g., bortezomib) or immunomodulatory agent (e.g., lenalidomide), those with high-risk cytogenetic status, and those with R-ISS stage II at study entry.
Overall response rates were similar between the study arms, at 86.6% with Isa-Kd and 82.9% with Kd, but the rate of very good partial responses or better was significantly higher with the triplet, at 72.6% versus 56.1% (P = .0011). The rate of minimal residual disease negativity was also significantly lower with Isa-Kd in the intent-to-treat population, at 29.6% versus 13%, respectively (P = .0004).
Overall survival data were not mature at the time of data cutoff and will be reported later, Dr. Moreau said.
Grade 3 or greater treatment-emergent adverse events (TEAEs) occurred in 76.8% of patients on the triplet and 67.2% of those on Kd. The incidences of death, serious TEAEs, or adverse events leading to discontinuation of therapy did not differ markedly between the treatment arms, however. Grade 3 or greater cardiac failure occurred in seven patients treated with the triplet (4%) and five treated with Kd (4.1%); respective rates of grade 3 or greater hematologic abnormalities included anemia in 22% and 19.7%, neutropenia in 19.2% and 7.4%, and thrombocytopenia in 29.9% and 23.8%.
The primary completion date for the trial is estimated to occur in November 2020, with final results in November 2023.
The study was sponsored by Sanofi. Dr. Moreau disclosed honoraria and a consulting or advisory role with several companies, not including Sanofi. Dr. Lipe disclosed impending advisory board activity for Janssen.
SOURCE: Moreau P et al. EHA Congress, Abstract LB2603.
The risk of progression or death for patients with relapsed or refractory multiple myeloma was nearly halved with the addition of isatuximab to carfilzomib and dexamethasone, according to an interim analysis of the phase 3 IKEMA trial (NCT03275285).
After a median follow-up of 20.7 months, the median progression-free survival had not been reached for 179 patients treated with isatuximab (Sarclisa), carfilzomib (Kyprolis), and dexamethasone (together, known as Isa-Kd), compared with 19.5 months for 123 patients treated with carfilzomib and dexamethasone alone (Kd). The hazard ratio for progression free survival with the triple combination was 0.531 (P = .0007), reported coprincipal investigator Phillipe Moreau, MD, from the University Hospital Hôtel-Dieu in Nantes, France.
“The benefit of the triplet combination was observed across subgroups, including patients difficult to treat, such as [those with] high-risk cytogenetics or elderly patients,” he said in a late-breaking abstract presentation during the virtual annual congress of the European Hematology Association.
Isatuximab is an immunoglobulin G1 monoclonal antibody targeting a CD38 transmembrane glycoprotein on multiple myeloma cells, with a mechanism of action similar to that of another anti-CD38 antibody, daratumumab (Darzalex). Isatuximab is approved in the United States and Europe in combination with pomalidomide and dexamethasone for patients with relapsed/refractory multiple myeloma after at least two prior lines of therapy.
A ‘me too’ agent?
It’s unclear, however, whether isatuximab offers any additional benefit over daratumumab, an agent approved for use both in front line therapy combinations and for patients with relapsed/refractory disease, said Brea C. Lipe, MD, a multiple myeloma specialist at the University of Rochester (N.Y.) Wilmot Cancer Institute, who was not involved in the study.
“Every time we get a new drug it’s nice to have another option, but it doesn’t really add anything different from daratumumab at this point,” she said in an interview.
Dr. Lipe noted the IKEMA results are similar to those seen in the phase 3 CANDOR trial, comparing carfilzomib, dexamethasone, and daratumumab to carfilzomib/dexamethasone alone in patients with relapsed/refractory myeloma. In addition, it’s unknown whether patients with disease that is refractory to daratumumab could benefit from isatuximab, she said.
Although isatuximab has been touted as offering more rapid and more convenient dosing than daratumumab, the introduction of rapid infusion and subcutaneous administration of daratumumab has negated any theoretical advantage of the newcomer, Dr. Lipe added.
Study details
In the IKEMA trial, 302 patients with relapsed/refractory multiple myeloma who’d received one to three prior lines of therapy were stratified by the number of prior lines and by revised Multiple Myeloma International Staging System (R-ISS) and were then randomized on a 3:2 basis to treatment with carfilzomib 20 mg/m2 on days 1, 2, 8, 9, 15, and 16 of cycle 1 and 56 mg/m2 on the corresponding days of each subsequent cycle plus dexamethasone 20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of each cycle, with or without isatuximab. The antibody was dosed 10 mg/kg on days 1, 8, 15, and 22 in cycle 1 then every 2 weeks thereafter.
Treatments were continued until disease progression, unacceptable toxicity, or patient choice intervened.
At a prespecified interim analysis, the trial met its primary endpoint of a minimum 41% risk reduction in the hazard rate for progression free survival (PFS), with an actual risk reduction of 47%.
An analysis of PFS by subgroup showed significant benefits with the triple combination for patients aged 65 years and older, those with baseline estimated glomerular filtration rates below 60 mL/min per 1.73 m2, those with more than one prior line of therapy, those who had not previously received a proteasome inhibitor (e.g., bortezomib) or immunomodulatory agent (e.g., lenalidomide), those with high-risk cytogenetic status, and those with R-ISS stage II at study entry.
Overall response rates were similar between the study arms, at 86.6% with Isa-Kd and 82.9% with Kd, but the rate of very good partial responses or better was significantly higher with the triplet, at 72.6% versus 56.1% (P = .0011). The rate of minimal residual disease negativity was also significantly lower with Isa-Kd in the intent-to-treat population, at 29.6% versus 13%, respectively (P = .0004).
Overall survival data were not mature at the time of data cutoff and will be reported later, Dr. Moreau said.
Grade 3 or greater treatment-emergent adverse events (TEAEs) occurred in 76.8% of patients on the triplet and 67.2% of those on Kd. The incidences of death, serious TEAEs, or adverse events leading to discontinuation of therapy did not differ markedly between the treatment arms, however. Grade 3 or greater cardiac failure occurred in seven patients treated with the triplet (4%) and five treated with Kd (4.1%); respective rates of grade 3 or greater hematologic abnormalities included anemia in 22% and 19.7%, neutropenia in 19.2% and 7.4%, and thrombocytopenia in 29.9% and 23.8%.
The primary completion date for the trial is estimated to occur in November 2020, with final results in November 2023.
The study was sponsored by Sanofi. Dr. Moreau disclosed honoraria and a consulting or advisory role with several companies, not including Sanofi. Dr. Lipe disclosed impending advisory board activity for Janssen.
SOURCE: Moreau P et al. EHA Congress, Abstract LB2603.
The risk of progression or death for patients with relapsed or refractory multiple myeloma was nearly halved with the addition of isatuximab to carfilzomib and dexamethasone, according to an interim analysis of the phase 3 IKEMA trial (NCT03275285).
After a median follow-up of 20.7 months, the median progression-free survival had not been reached for 179 patients treated with isatuximab (Sarclisa), carfilzomib (Kyprolis), and dexamethasone (together, known as Isa-Kd), compared with 19.5 months for 123 patients treated with carfilzomib and dexamethasone alone (Kd). The hazard ratio for progression free survival with the triple combination was 0.531 (P = .0007), reported coprincipal investigator Phillipe Moreau, MD, from the University Hospital Hôtel-Dieu in Nantes, France.
“The benefit of the triplet combination was observed across subgroups, including patients difficult to treat, such as [those with] high-risk cytogenetics or elderly patients,” he said in a late-breaking abstract presentation during the virtual annual congress of the European Hematology Association.
Isatuximab is an immunoglobulin G1 monoclonal antibody targeting a CD38 transmembrane glycoprotein on multiple myeloma cells, with a mechanism of action similar to that of another anti-CD38 antibody, daratumumab (Darzalex). Isatuximab is approved in the United States and Europe in combination with pomalidomide and dexamethasone for patients with relapsed/refractory multiple myeloma after at least two prior lines of therapy.
A ‘me too’ agent?
It’s unclear, however, whether isatuximab offers any additional benefit over daratumumab, an agent approved for use both in front line therapy combinations and for patients with relapsed/refractory disease, said Brea C. Lipe, MD, a multiple myeloma specialist at the University of Rochester (N.Y.) Wilmot Cancer Institute, who was not involved in the study.
“Every time we get a new drug it’s nice to have another option, but it doesn’t really add anything different from daratumumab at this point,” she said in an interview.
Dr. Lipe noted the IKEMA results are similar to those seen in the phase 3 CANDOR trial, comparing carfilzomib, dexamethasone, and daratumumab to carfilzomib/dexamethasone alone in patients with relapsed/refractory myeloma. In addition, it’s unknown whether patients with disease that is refractory to daratumumab could benefit from isatuximab, she said.
Although isatuximab has been touted as offering more rapid and more convenient dosing than daratumumab, the introduction of rapid infusion and subcutaneous administration of daratumumab has negated any theoretical advantage of the newcomer, Dr. Lipe added.
Study details
In the IKEMA trial, 302 patients with relapsed/refractory multiple myeloma who’d received one to three prior lines of therapy were stratified by the number of prior lines and by revised Multiple Myeloma International Staging System (R-ISS) and were then randomized on a 3:2 basis to treatment with carfilzomib 20 mg/m2 on days 1, 2, 8, 9, 15, and 16 of cycle 1 and 56 mg/m2 on the corresponding days of each subsequent cycle plus dexamethasone 20 mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of each cycle, with or without isatuximab. The antibody was dosed 10 mg/kg on days 1, 8, 15, and 22 in cycle 1 then every 2 weeks thereafter.
Treatments were continued until disease progression, unacceptable toxicity, or patient choice intervened.
At a prespecified interim analysis, the trial met its primary endpoint of a minimum 41% risk reduction in the hazard rate for progression free survival (PFS), with an actual risk reduction of 47%.
An analysis of PFS by subgroup showed significant benefits with the triple combination for patients aged 65 years and older, those with baseline estimated glomerular filtration rates below 60 mL/min per 1.73 m2, those with more than one prior line of therapy, those who had not previously received a proteasome inhibitor (e.g., bortezomib) or immunomodulatory agent (e.g., lenalidomide), those with high-risk cytogenetic status, and those with R-ISS stage II at study entry.
Overall response rates were similar between the study arms, at 86.6% with Isa-Kd and 82.9% with Kd, but the rate of very good partial responses or better was significantly higher with the triplet, at 72.6% versus 56.1% (P = .0011). The rate of minimal residual disease negativity was also significantly lower with Isa-Kd in the intent-to-treat population, at 29.6% versus 13%, respectively (P = .0004).
Overall survival data were not mature at the time of data cutoff and will be reported later, Dr. Moreau said.
Grade 3 or greater treatment-emergent adverse events (TEAEs) occurred in 76.8% of patients on the triplet and 67.2% of those on Kd. The incidences of death, serious TEAEs, or adverse events leading to discontinuation of therapy did not differ markedly between the treatment arms, however. Grade 3 or greater cardiac failure occurred in seven patients treated with the triplet (4%) and five treated with Kd (4.1%); respective rates of grade 3 or greater hematologic abnormalities included anemia in 22% and 19.7%, neutropenia in 19.2% and 7.4%, and thrombocytopenia in 29.9% and 23.8%.
The primary completion date for the trial is estimated to occur in November 2020, with final results in November 2023.
The study was sponsored by Sanofi. Dr. Moreau disclosed honoraria and a consulting or advisory role with several companies, not including Sanofi. Dr. Lipe disclosed impending advisory board activity for Janssen.
SOURCE: Moreau P et al. EHA Congress, Abstract LB2603.
FROM EHA CONGRESS
Telehealth and medical liability
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
COVID-19: Medicare data show long hospital stays, disparities
according to a new analysis by the Centers for Medicare & Medicaid Services.
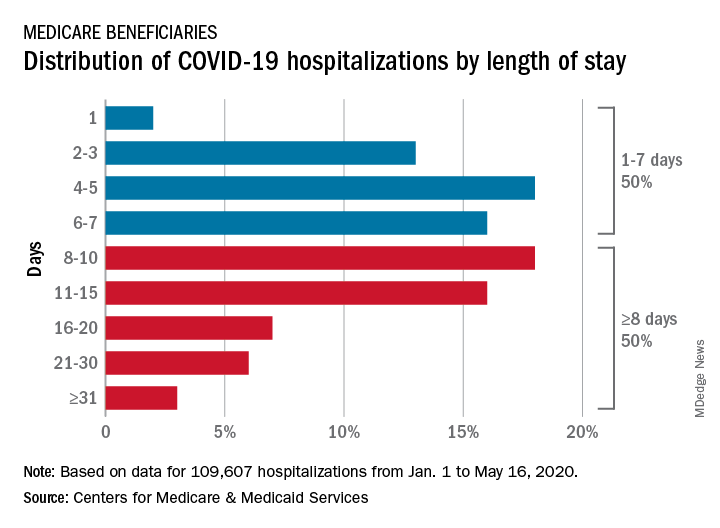
CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
Guidance on infection prevention for health care personnel
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
ACR issues guidances for MIS-C and pediatric rheumatic disease during pandemic
Two new clinical guidance documents from the American College of Rheumatology provide evidence-based recommendations for managing pediatric rheumatic disease during the COVID-19 pandemic as well as diagnostic and treatment recommendations for multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 infection.
Although several children’s hospitals have published their treatment protocols for MIS-C since the condition’s initial discovery, the ACR appears to be the first medical organization to review all the most current evidence to issue interim guidance with the expectations that it will change as more data become available.
“It is challenging having to make recommendations not having a lot of scientific evidence, but we still felt we had to use whatever’s out there to the best of our ability and use our experience to put together these recommendations,” Dawn M. Wahezi, MD, chief of pediatric rheumatology at Children’s Hospital at Montefiore and an associate professor of pediatrics at Albert Einstein College of Medicine, New York, said in an interview.
“We wanted to be mindful of the fact that there are things we know and things we don’t know, and we have to be careful about what we’re recommending,” said Dr. Wahezi, a member of the ACR working group that assembled the recommendations for pediatric rheumatic disease management during the pandemic. “We’re recommending the best we can at this moment, but if there are new studies that come out and suggest otherwise, we will definitely have to go back and amend the document.”
The foremost priority of the pediatric rheumatic disease guidance focuses on maintaining control of the disease and avoiding flares that may put children at greater risk of infection. Dr. Wahezi said the ACR has received many calls from patients and clinicians asking whether patients should continue their immunosuppressant medications. Fear of the coronavirus infection, medication shortages, difficulty getting to the pharmacy, uneasiness about going to the clinic or hospital for infusions, and other barriers may have led to gaps in medication.
“We didn’t want people to be too quick to hold patients’ medications just because they were scared of COVID,” Dr. Wahezi said. “If they did have medication stopped for one reason or another and their disease flared, having active disease, regardless of which disease it is, actually puts you at higher risk for infection. By controlling their disease, that would be the way to protect them the most.”
A key takeaway in the guidance on MIS-C, meanwhile, is an emphasis on its rarity lest physicians be too quick to diagnose it and miss another serious condition with overlapping symptoms, explained Lauren Henderson, MD, an attending rheumatologist at Boston Children’s Hospital and assistant professor of pediatrics at Harvard Medical School, Boston. Dr. Henderson participated in the ACR group that wrote the MIS-C guidance.
“The first thing we want to be thoughtful about clinically is to recognize that children in general with the acute infectious phase of SARS-CoV-2 have mild symptoms and generally do well,” Dr. Henderson said. “From what we can tell from all the data, MIS-C is rare. That really needs to be considered when clinicians on the ground are doing the diagnostic evaluation” because of concerns that clinicians “could rush to diagnose and treat patients with MIS-C and miss important diagnoses like malignancies and infections.”
Management of pediatric rheumatic disease during the pandemic
The COVID-19 clinical guidance for managing pediatric rheumatic disease grew from the work of the North American Pediatric Rheumatology Clinical Guidance Task Force, which included seven pediatric rheumatologists, two pediatric infectious disease physicians, one adult rheumatologist, and one pediatric nurse practitioner. The general guidance covers usual preventive measures for reducing risk for COVID-19 infection, the recommendation that children continue to receive recommended vaccines unless contraindicated by medication, and routine in-person visits for ophthalmologic surveillance of those with a history of uveitis or at high risk for chronic uveitis. The guidance also notes the risk of mental health concerns, such as depression and anxiety, related to quarantine and the pandemic.
The top recommendation is initiation or continuation of all medications necessary to control underlying disease, including NSAIDs, hydroxychloroquine, ACE inhibitors/angiotensin II receptor blockers, colchicine, conventional disease-modifying antirheumatic drugs (cDMARDs), biologic DMARDs, and targeted synthetic DMARDs. Even patients who may have had exposure to COVID-19 or who have an asymptomatic COVID-19 infection should continue to take these medications with the exception of ACEi/ARBs.
In those with pediatric rheumatic disease who have a symptomatic COVID-19 infection, “NSAIDs, HCQ, and colchicine may be continued, if necessary, to control underlying disease,” as can interleukin (IL)-1 and IL-6 inhibitors, but “cDMARDs, bDMARDs [except IL-1 and IL-6 inhibitors] and tsDMARDs should be temporarily delayed or withheld,” according to the guidance. Glucocorticoids can be continued at the lowest possible dose to control disease.
“There’s nothing in the literature that suggests people who have rheumatic disease, especially children, and people who are on these medications, really are at increased risk for COVID-19,” Dr. Wahezi said. “That’s why we didn’t want people to be overcautious in stopping medications when the main priority is to control their disease.”
She noted some experts’ speculations that these medications may actually benefit patients with rheumatic disease who develop a COVID-19 infection because the medications keep the immune response in check. “If you allow them to have this dysregulated immune response and have active disease, you’re potentially putting them at greater risk,” Dr. Wahezi said, although she stressed that inadequate evidence exists to support these speculations right now.
Lack of evidence has been the biggest challenge all around with developing this guidance, she said.
“Because this is such an unprecedented situation and because people are so desperate to find treatments both for the illness and to protect those at risk for it, there are lots of people trying to put evidence out there, but it may not be the best-quality evidence,” Dr. Wahezi said.
Insufficient evidence also drove the group’s determination that “SARS-CoV-2 antibody testing is not useful in informing on the history of infection or risk of reinfection,” as the guidance states. Too much variability in the assays exist, Dr. Wahezi said, and, further, it’s unclear what the clinical significance of a positive test would be.
“We didn’t want anyone to feel they had to make clinical decisions based on the results of that antibody testing,” she said. “Even if the test is accurate, we don’t know how to interpret it because it’s so new.”
The guidance also notes that patients with stable disease and previously stable lab markers on stable doses of their medication may be able to extend the interval for medication toxicity lab testing a few months if there is concern about exposure to COVID-19 to get the blood work.
“If you’re just starting a medicine or there’s someone who’s had abnormalities with the medicine in the past or you’re making medication adjustments, you wouldn’t do it in those scenarios, but if there’s someone who’s been on the drug for a long time and are nervous to get [blood] drawn, it’s probably okay to delay it,” Dr. Wahezi said. Lab work for disease activity measures, on the other hand, remain particularly important, especially since telemedicine visits may require clinicians to rely on lab results more than previously.
Management of MIS-C associated with COVID-19
The task force that developed guidance for the new inflammatory condition recently linked to SARS-CoV-2 infections in children included nine pediatric rheumatologists, two adult rheumatologists, two pediatric cardiologists, two pediatric infectious disease specialists, and one pediatric critical care physician.
The guidance includes a figure for the diagnostic pathway in evaluating children suspected of having MIS-C and extensive detail on diagnostic work-up, but the task force intentionally avoided providing a case definition for the condition. Existing case definitions from the Centers for Disease Control and Prevention, World Health Organization, and the United Kingdom’s Royal College of Paediatrics and Child Health differ from one another and are based on unclear evidence, Dr. Henderson noted. “We really don’t have enough data to know the sensitivity and specificity of each parameter, and until that’s available, we didn’t want to add to the confusion,” she said.
The guidance also stresses that MIS-C is a rare complication, so patients suspected of having the condition who do not have “life-threatening manifestations should undergo diagnostic evaluation for MIS-C as well as other possible infectious and noninfectious etiologies before immunomodulatory treatment is initiated,” the guidance states.
Unless a child is in shock or otherwise requires urgent care, physicians should take the time to complete the diagnostic work-up while monitoring the child, Dr. Henderson said. If the child does have MIS-C, the guidance currently recommends intravenous immunoglobulin (IVIG) and/or glucocorticoids to prevent coronary artery aneurysms, the same treatment other institutions have been recommending.
“We don’t have rigorous comparative studies looking at different types of treatments,” Dr. Henderson said, noting that the vast majority of children in the literature received IVIG and/or glucocorticoid treatment. “Often children really responded quite forcefully to those treatments, but we don’t have high-quality data yet to know that this treatment is better than supportive care or another medication.”
Dr. Henderson also stressed the importance of children receiving care at a facility with the necessary expertise to manage MIS-C and receiving long-term follow-up care from a multidisciplinary clinical team that includes a rheumatologist, an infectious disease doctor, a cardiologist, and possibly a hematologist.
“Making sure children are admitted to a hospital that has the resources and are followed by physicians with expertise or understanding of the intricacies of MIS-C is really important,” she said, particularly for children with cardiac involvement. “We don’t know if all the kids presenting with left ventricular dysfunction and shock are at risk for having myocardial fibrosis down the line,” she noted. “There is so much we do not understand and very little data to guide us on what to do, so these children really need to be under the care of a cardiologist and rheumatologist to make sure that their care is tailored to them.”
Although MIS-C shares overlapping symptoms with Kawasaki disease, it’s still unclear how similar or different the two conditions are, Dr. Henderson said.
“We can definitely say that when we look at MIS-C and compare it to historical groups of Kawasaki disease before the pandemic, there are definitely different features in the MIS-C group,” she said. Kawasaki disease generally only affects children under age 5, whereas MIS-C patients run the gamut from age 1-17. Racial demographics are also different, with a higher proportion of black children affected by MIS-C.
It’s possible that the pathophysiology of both conditions will turn out to be similar, particularly given the hypothesis that Kawasaki disease is triggered by infections in genetically predisposed people. However, the severity of symptoms and risk of aneurysms appear greater with MIS-C so far.
“The degree to which these patients are presenting with left ventricular dysfunction and shock is much higher than what we’ve seen previously,” Dr. Henderson said. “Children can have aneurysms even if they don’t meet all the Kawasaki disease features, which makes it feel that this is somehow clinically different from what we’ve seen before. It’s not just the kids who have the rash and the conjunctivitis and the extremity changes and oral changes who have the aneurysms.”
The reason for including both IVIG and glucocorticoids as possible first-line drugs to prevent aneurysms is that some evidence suggests children with MIS-C may have higher levels of IVIG resistance, she said.
Like Dr. Wahezi, Dr. Henderson emphasized the necessarily transient nature of these recommendations.
“These recommendations will almost certainly change based on evolving understanding of MIS-C and the data,” Dr. Henderson said, adding that this new, unique condition highlights the importance of including children in allocating funding for research and in clinical trials.
“Children are not always identical to adults, and it’s really important that we have high-quality data to inform our decisions about how to care for them,” she said.
Dr. Wahezi had no disclosures. Dr. Henderson has consulted for Sobi and Adaptive Technologies. The guidelines did not note other disclosures for members of the ACR groups.
SOURCES: COVID-19 Clinical Guidance for Pediatric Patients with Rheumatic Disease and Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19
Two new clinical guidance documents from the American College of Rheumatology provide evidence-based recommendations for managing pediatric rheumatic disease during the COVID-19 pandemic as well as diagnostic and treatment recommendations for multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 infection.
Although several children’s hospitals have published their treatment protocols for MIS-C since the condition’s initial discovery, the ACR appears to be the first medical organization to review all the most current evidence to issue interim guidance with the expectations that it will change as more data become available.
“It is challenging having to make recommendations not having a lot of scientific evidence, but we still felt we had to use whatever’s out there to the best of our ability and use our experience to put together these recommendations,” Dawn M. Wahezi, MD, chief of pediatric rheumatology at Children’s Hospital at Montefiore and an associate professor of pediatrics at Albert Einstein College of Medicine, New York, said in an interview.
“We wanted to be mindful of the fact that there are things we know and things we don’t know, and we have to be careful about what we’re recommending,” said Dr. Wahezi, a member of the ACR working group that assembled the recommendations for pediatric rheumatic disease management during the pandemic. “We’re recommending the best we can at this moment, but if there are new studies that come out and suggest otherwise, we will definitely have to go back and amend the document.”
The foremost priority of the pediatric rheumatic disease guidance focuses on maintaining control of the disease and avoiding flares that may put children at greater risk of infection. Dr. Wahezi said the ACR has received many calls from patients and clinicians asking whether patients should continue their immunosuppressant medications. Fear of the coronavirus infection, medication shortages, difficulty getting to the pharmacy, uneasiness about going to the clinic or hospital for infusions, and other barriers may have led to gaps in medication.
“We didn’t want people to be too quick to hold patients’ medications just because they were scared of COVID,” Dr. Wahezi said. “If they did have medication stopped for one reason or another and their disease flared, having active disease, regardless of which disease it is, actually puts you at higher risk for infection. By controlling their disease, that would be the way to protect them the most.”
A key takeaway in the guidance on MIS-C, meanwhile, is an emphasis on its rarity lest physicians be too quick to diagnose it and miss another serious condition with overlapping symptoms, explained Lauren Henderson, MD, an attending rheumatologist at Boston Children’s Hospital and assistant professor of pediatrics at Harvard Medical School, Boston. Dr. Henderson participated in the ACR group that wrote the MIS-C guidance.
“The first thing we want to be thoughtful about clinically is to recognize that children in general with the acute infectious phase of SARS-CoV-2 have mild symptoms and generally do well,” Dr. Henderson said. “From what we can tell from all the data, MIS-C is rare. That really needs to be considered when clinicians on the ground are doing the diagnostic evaluation” because of concerns that clinicians “could rush to diagnose and treat patients with MIS-C and miss important diagnoses like malignancies and infections.”
Management of pediatric rheumatic disease during the pandemic
The COVID-19 clinical guidance for managing pediatric rheumatic disease grew from the work of the North American Pediatric Rheumatology Clinical Guidance Task Force, which included seven pediatric rheumatologists, two pediatric infectious disease physicians, one adult rheumatologist, and one pediatric nurse practitioner. The general guidance covers usual preventive measures for reducing risk for COVID-19 infection, the recommendation that children continue to receive recommended vaccines unless contraindicated by medication, and routine in-person visits for ophthalmologic surveillance of those with a history of uveitis or at high risk for chronic uveitis. The guidance also notes the risk of mental health concerns, such as depression and anxiety, related to quarantine and the pandemic.
The top recommendation is initiation or continuation of all medications necessary to control underlying disease, including NSAIDs, hydroxychloroquine, ACE inhibitors/angiotensin II receptor blockers, colchicine, conventional disease-modifying antirheumatic drugs (cDMARDs), biologic DMARDs, and targeted synthetic DMARDs. Even patients who may have had exposure to COVID-19 or who have an asymptomatic COVID-19 infection should continue to take these medications with the exception of ACEi/ARBs.
In those with pediatric rheumatic disease who have a symptomatic COVID-19 infection, “NSAIDs, HCQ, and colchicine may be continued, if necessary, to control underlying disease,” as can interleukin (IL)-1 and IL-6 inhibitors, but “cDMARDs, bDMARDs [except IL-1 and IL-6 inhibitors] and tsDMARDs should be temporarily delayed or withheld,” according to the guidance. Glucocorticoids can be continued at the lowest possible dose to control disease.
“There’s nothing in the literature that suggests people who have rheumatic disease, especially children, and people who are on these medications, really are at increased risk for COVID-19,” Dr. Wahezi said. “That’s why we didn’t want people to be overcautious in stopping medications when the main priority is to control their disease.”
She noted some experts’ speculations that these medications may actually benefit patients with rheumatic disease who develop a COVID-19 infection because the medications keep the immune response in check. “If you allow them to have this dysregulated immune response and have active disease, you’re potentially putting them at greater risk,” Dr. Wahezi said, although she stressed that inadequate evidence exists to support these speculations right now.
Lack of evidence has been the biggest challenge all around with developing this guidance, she said.
“Because this is such an unprecedented situation and because people are so desperate to find treatments both for the illness and to protect those at risk for it, there are lots of people trying to put evidence out there, but it may not be the best-quality evidence,” Dr. Wahezi said.
Insufficient evidence also drove the group’s determination that “SARS-CoV-2 antibody testing is not useful in informing on the history of infection or risk of reinfection,” as the guidance states. Too much variability in the assays exist, Dr. Wahezi said, and, further, it’s unclear what the clinical significance of a positive test would be.
“We didn’t want anyone to feel they had to make clinical decisions based on the results of that antibody testing,” she said. “Even if the test is accurate, we don’t know how to interpret it because it’s so new.”
The guidance also notes that patients with stable disease and previously stable lab markers on stable doses of their medication may be able to extend the interval for medication toxicity lab testing a few months if there is concern about exposure to COVID-19 to get the blood work.
“If you’re just starting a medicine or there’s someone who’s had abnormalities with the medicine in the past or you’re making medication adjustments, you wouldn’t do it in those scenarios, but if there’s someone who’s been on the drug for a long time and are nervous to get [blood] drawn, it’s probably okay to delay it,” Dr. Wahezi said. Lab work for disease activity measures, on the other hand, remain particularly important, especially since telemedicine visits may require clinicians to rely on lab results more than previously.
Management of MIS-C associated with COVID-19
The task force that developed guidance for the new inflammatory condition recently linked to SARS-CoV-2 infections in children included nine pediatric rheumatologists, two adult rheumatologists, two pediatric cardiologists, two pediatric infectious disease specialists, and one pediatric critical care physician.
The guidance includes a figure for the diagnostic pathway in evaluating children suspected of having MIS-C and extensive detail on diagnostic work-up, but the task force intentionally avoided providing a case definition for the condition. Existing case definitions from the Centers for Disease Control and Prevention, World Health Organization, and the United Kingdom’s Royal College of Paediatrics and Child Health differ from one another and are based on unclear evidence, Dr. Henderson noted. “We really don’t have enough data to know the sensitivity and specificity of each parameter, and until that’s available, we didn’t want to add to the confusion,” she said.
The guidance also stresses that MIS-C is a rare complication, so patients suspected of having the condition who do not have “life-threatening manifestations should undergo diagnostic evaluation for MIS-C as well as other possible infectious and noninfectious etiologies before immunomodulatory treatment is initiated,” the guidance states.
Unless a child is in shock or otherwise requires urgent care, physicians should take the time to complete the diagnostic work-up while monitoring the child, Dr. Henderson said. If the child does have MIS-C, the guidance currently recommends intravenous immunoglobulin (IVIG) and/or glucocorticoids to prevent coronary artery aneurysms, the same treatment other institutions have been recommending.
“We don’t have rigorous comparative studies looking at different types of treatments,” Dr. Henderson said, noting that the vast majority of children in the literature received IVIG and/or glucocorticoid treatment. “Often children really responded quite forcefully to those treatments, but we don’t have high-quality data yet to know that this treatment is better than supportive care or another medication.”
Dr. Henderson also stressed the importance of children receiving care at a facility with the necessary expertise to manage MIS-C and receiving long-term follow-up care from a multidisciplinary clinical team that includes a rheumatologist, an infectious disease doctor, a cardiologist, and possibly a hematologist.
“Making sure children are admitted to a hospital that has the resources and are followed by physicians with expertise or understanding of the intricacies of MIS-C is really important,” she said, particularly for children with cardiac involvement. “We don’t know if all the kids presenting with left ventricular dysfunction and shock are at risk for having myocardial fibrosis down the line,” she noted. “There is so much we do not understand and very little data to guide us on what to do, so these children really need to be under the care of a cardiologist and rheumatologist to make sure that their care is tailored to them.”
Although MIS-C shares overlapping symptoms with Kawasaki disease, it’s still unclear how similar or different the two conditions are, Dr. Henderson said.
“We can definitely say that when we look at MIS-C and compare it to historical groups of Kawasaki disease before the pandemic, there are definitely different features in the MIS-C group,” she said. Kawasaki disease generally only affects children under age 5, whereas MIS-C patients run the gamut from age 1-17. Racial demographics are also different, with a higher proportion of black children affected by MIS-C.
It’s possible that the pathophysiology of both conditions will turn out to be similar, particularly given the hypothesis that Kawasaki disease is triggered by infections in genetically predisposed people. However, the severity of symptoms and risk of aneurysms appear greater with MIS-C so far.
“The degree to which these patients are presenting with left ventricular dysfunction and shock is much higher than what we’ve seen previously,” Dr. Henderson said. “Children can have aneurysms even if they don’t meet all the Kawasaki disease features, which makes it feel that this is somehow clinically different from what we’ve seen before. It’s not just the kids who have the rash and the conjunctivitis and the extremity changes and oral changes who have the aneurysms.”
The reason for including both IVIG and glucocorticoids as possible first-line drugs to prevent aneurysms is that some evidence suggests children with MIS-C may have higher levels of IVIG resistance, she said.
Like Dr. Wahezi, Dr. Henderson emphasized the necessarily transient nature of these recommendations.
“These recommendations will almost certainly change based on evolving understanding of MIS-C and the data,” Dr. Henderson said, adding that this new, unique condition highlights the importance of including children in allocating funding for research and in clinical trials.
“Children are not always identical to adults, and it’s really important that we have high-quality data to inform our decisions about how to care for them,” she said.
Dr. Wahezi had no disclosures. Dr. Henderson has consulted for Sobi and Adaptive Technologies. The guidelines did not note other disclosures for members of the ACR groups.
SOURCES: COVID-19 Clinical Guidance for Pediatric Patients with Rheumatic Disease and Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19
Two new clinical guidance documents from the American College of Rheumatology provide evidence-based recommendations for managing pediatric rheumatic disease during the COVID-19 pandemic as well as diagnostic and treatment recommendations for multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 infection.
Although several children’s hospitals have published their treatment protocols for MIS-C since the condition’s initial discovery, the ACR appears to be the first medical organization to review all the most current evidence to issue interim guidance with the expectations that it will change as more data become available.
“It is challenging having to make recommendations not having a lot of scientific evidence, but we still felt we had to use whatever’s out there to the best of our ability and use our experience to put together these recommendations,” Dawn M. Wahezi, MD, chief of pediatric rheumatology at Children’s Hospital at Montefiore and an associate professor of pediatrics at Albert Einstein College of Medicine, New York, said in an interview.
“We wanted to be mindful of the fact that there are things we know and things we don’t know, and we have to be careful about what we’re recommending,” said Dr. Wahezi, a member of the ACR working group that assembled the recommendations for pediatric rheumatic disease management during the pandemic. “We’re recommending the best we can at this moment, but if there are new studies that come out and suggest otherwise, we will definitely have to go back and amend the document.”
The foremost priority of the pediatric rheumatic disease guidance focuses on maintaining control of the disease and avoiding flares that may put children at greater risk of infection. Dr. Wahezi said the ACR has received many calls from patients and clinicians asking whether patients should continue their immunosuppressant medications. Fear of the coronavirus infection, medication shortages, difficulty getting to the pharmacy, uneasiness about going to the clinic or hospital for infusions, and other barriers may have led to gaps in medication.
“We didn’t want people to be too quick to hold patients’ medications just because they were scared of COVID,” Dr. Wahezi said. “If they did have medication stopped for one reason or another and their disease flared, having active disease, regardless of which disease it is, actually puts you at higher risk for infection. By controlling their disease, that would be the way to protect them the most.”
A key takeaway in the guidance on MIS-C, meanwhile, is an emphasis on its rarity lest physicians be too quick to diagnose it and miss another serious condition with overlapping symptoms, explained Lauren Henderson, MD, an attending rheumatologist at Boston Children’s Hospital and assistant professor of pediatrics at Harvard Medical School, Boston. Dr. Henderson participated in the ACR group that wrote the MIS-C guidance.
“The first thing we want to be thoughtful about clinically is to recognize that children in general with the acute infectious phase of SARS-CoV-2 have mild symptoms and generally do well,” Dr. Henderson said. “From what we can tell from all the data, MIS-C is rare. That really needs to be considered when clinicians on the ground are doing the diagnostic evaluation” because of concerns that clinicians “could rush to diagnose and treat patients with MIS-C and miss important diagnoses like malignancies and infections.”
Management of pediatric rheumatic disease during the pandemic
The COVID-19 clinical guidance for managing pediatric rheumatic disease grew from the work of the North American Pediatric Rheumatology Clinical Guidance Task Force, which included seven pediatric rheumatologists, two pediatric infectious disease physicians, one adult rheumatologist, and one pediatric nurse practitioner. The general guidance covers usual preventive measures for reducing risk for COVID-19 infection, the recommendation that children continue to receive recommended vaccines unless contraindicated by medication, and routine in-person visits for ophthalmologic surveillance of those with a history of uveitis or at high risk for chronic uveitis. The guidance also notes the risk of mental health concerns, such as depression and anxiety, related to quarantine and the pandemic.
The top recommendation is initiation or continuation of all medications necessary to control underlying disease, including NSAIDs, hydroxychloroquine, ACE inhibitors/angiotensin II receptor blockers, colchicine, conventional disease-modifying antirheumatic drugs (cDMARDs), biologic DMARDs, and targeted synthetic DMARDs. Even patients who may have had exposure to COVID-19 or who have an asymptomatic COVID-19 infection should continue to take these medications with the exception of ACEi/ARBs.
In those with pediatric rheumatic disease who have a symptomatic COVID-19 infection, “NSAIDs, HCQ, and colchicine may be continued, if necessary, to control underlying disease,” as can interleukin (IL)-1 and IL-6 inhibitors, but “cDMARDs, bDMARDs [except IL-1 and IL-6 inhibitors] and tsDMARDs should be temporarily delayed or withheld,” according to the guidance. Glucocorticoids can be continued at the lowest possible dose to control disease.
“There’s nothing in the literature that suggests people who have rheumatic disease, especially children, and people who are on these medications, really are at increased risk for COVID-19,” Dr. Wahezi said. “That’s why we didn’t want people to be overcautious in stopping medications when the main priority is to control their disease.”
She noted some experts’ speculations that these medications may actually benefit patients with rheumatic disease who develop a COVID-19 infection because the medications keep the immune response in check. “If you allow them to have this dysregulated immune response and have active disease, you’re potentially putting them at greater risk,” Dr. Wahezi said, although she stressed that inadequate evidence exists to support these speculations right now.
Lack of evidence has been the biggest challenge all around with developing this guidance, she said.
“Because this is such an unprecedented situation and because people are so desperate to find treatments both for the illness and to protect those at risk for it, there are lots of people trying to put evidence out there, but it may not be the best-quality evidence,” Dr. Wahezi said.
Insufficient evidence also drove the group’s determination that “SARS-CoV-2 antibody testing is not useful in informing on the history of infection or risk of reinfection,” as the guidance states. Too much variability in the assays exist, Dr. Wahezi said, and, further, it’s unclear what the clinical significance of a positive test would be.
“We didn’t want anyone to feel they had to make clinical decisions based on the results of that antibody testing,” she said. “Even if the test is accurate, we don’t know how to interpret it because it’s so new.”
The guidance also notes that patients with stable disease and previously stable lab markers on stable doses of their medication may be able to extend the interval for medication toxicity lab testing a few months if there is concern about exposure to COVID-19 to get the blood work.
“If you’re just starting a medicine or there’s someone who’s had abnormalities with the medicine in the past or you’re making medication adjustments, you wouldn’t do it in those scenarios, but if there’s someone who’s been on the drug for a long time and are nervous to get [blood] drawn, it’s probably okay to delay it,” Dr. Wahezi said. Lab work for disease activity measures, on the other hand, remain particularly important, especially since telemedicine visits may require clinicians to rely on lab results more than previously.
Management of MIS-C associated with COVID-19
The task force that developed guidance for the new inflammatory condition recently linked to SARS-CoV-2 infections in children included nine pediatric rheumatologists, two adult rheumatologists, two pediatric cardiologists, two pediatric infectious disease specialists, and one pediatric critical care physician.
The guidance includes a figure for the diagnostic pathway in evaluating children suspected of having MIS-C and extensive detail on diagnostic work-up, but the task force intentionally avoided providing a case definition for the condition. Existing case definitions from the Centers for Disease Control and Prevention, World Health Organization, and the United Kingdom’s Royal College of Paediatrics and Child Health differ from one another and are based on unclear evidence, Dr. Henderson noted. “We really don’t have enough data to know the sensitivity and specificity of each parameter, and until that’s available, we didn’t want to add to the confusion,” she said.
The guidance also stresses that MIS-C is a rare complication, so patients suspected of having the condition who do not have “life-threatening manifestations should undergo diagnostic evaluation for MIS-C as well as other possible infectious and noninfectious etiologies before immunomodulatory treatment is initiated,” the guidance states.
Unless a child is in shock or otherwise requires urgent care, physicians should take the time to complete the diagnostic work-up while monitoring the child, Dr. Henderson said. If the child does have MIS-C, the guidance currently recommends intravenous immunoglobulin (IVIG) and/or glucocorticoids to prevent coronary artery aneurysms, the same treatment other institutions have been recommending.
“We don’t have rigorous comparative studies looking at different types of treatments,” Dr. Henderson said, noting that the vast majority of children in the literature received IVIG and/or glucocorticoid treatment. “Often children really responded quite forcefully to those treatments, but we don’t have high-quality data yet to know that this treatment is better than supportive care or another medication.”
Dr. Henderson also stressed the importance of children receiving care at a facility with the necessary expertise to manage MIS-C and receiving long-term follow-up care from a multidisciplinary clinical team that includes a rheumatologist, an infectious disease doctor, a cardiologist, and possibly a hematologist.
“Making sure children are admitted to a hospital that has the resources and are followed by physicians with expertise or understanding of the intricacies of MIS-C is really important,” she said, particularly for children with cardiac involvement. “We don’t know if all the kids presenting with left ventricular dysfunction and shock are at risk for having myocardial fibrosis down the line,” she noted. “There is so much we do not understand and very little data to guide us on what to do, so these children really need to be under the care of a cardiologist and rheumatologist to make sure that their care is tailored to them.”
Although MIS-C shares overlapping symptoms with Kawasaki disease, it’s still unclear how similar or different the two conditions are, Dr. Henderson said.
“We can definitely say that when we look at MIS-C and compare it to historical groups of Kawasaki disease before the pandemic, there are definitely different features in the MIS-C group,” she said. Kawasaki disease generally only affects children under age 5, whereas MIS-C patients run the gamut from age 1-17. Racial demographics are also different, with a higher proportion of black children affected by MIS-C.
It’s possible that the pathophysiology of both conditions will turn out to be similar, particularly given the hypothesis that Kawasaki disease is triggered by infections in genetically predisposed people. However, the severity of symptoms and risk of aneurysms appear greater with MIS-C so far.
“The degree to which these patients are presenting with left ventricular dysfunction and shock is much higher than what we’ve seen previously,” Dr. Henderson said. “Children can have aneurysms even if they don’t meet all the Kawasaki disease features, which makes it feel that this is somehow clinically different from what we’ve seen before. It’s not just the kids who have the rash and the conjunctivitis and the extremity changes and oral changes who have the aneurysms.”
The reason for including both IVIG and glucocorticoids as possible first-line drugs to prevent aneurysms is that some evidence suggests children with MIS-C may have higher levels of IVIG resistance, she said.
Like Dr. Wahezi, Dr. Henderson emphasized the necessarily transient nature of these recommendations.
“These recommendations will almost certainly change based on evolving understanding of MIS-C and the data,” Dr. Henderson said, adding that this new, unique condition highlights the importance of including children in allocating funding for research and in clinical trials.
“Children are not always identical to adults, and it’s really important that we have high-quality data to inform our decisions about how to care for them,” she said.
Dr. Wahezi had no disclosures. Dr. Henderson has consulted for Sobi and Adaptive Technologies. The guidelines did not note other disclosures for members of the ACR groups.
SOURCES: COVID-19 Clinical Guidance for Pediatric Patients with Rheumatic Disease and Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 and Hyperinflammation in COVID-19
FDA approves selinexor for relapsed/refractory DLBCL
The Food and Drug Administration has granted accelerated approval of selinexor, a nuclear transport inhibitor, for the treatment of relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
Selinexor (marketed as XPOVIO by Karyopharm Therapeutics) is intended for adult patients with relapsed/refractory DLBCL, not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy, according to the FDA’s announcement.
The FDA granted selinexor accelerated approval for this indication based on the response rate seen in the SADAL trial. Continued approval for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
The SADAL trial (NCT02227251) was a phase 2, single-arm, open-label study of patients with DLBCL who had previously received two to five systemic regimens. The patients received selinexor at 60 mg orally on days 1 and 3 of each week.
Results in 134 patients showed an overall response rate of 29% (95% confidence interval: 22-38), with complete responses in 13% of patients. Of 39 patients who achieved a partial or complete response, 38% had a response duration of at least 6 months, and 15% had a response duration of at least 12 months, according to the FDA announcement.
The most common adverse reactions in this trial were fatigue, nausea, diarrhea, appetite decrease, weight decrease, constipation, vomiting, and pyrexia. Grade 3-4 laboratory abnormalities occurred in 15% or more of the patients, and comprised thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia.
Serious adverse reactions occurred in 46% of patients, most often from infection. Gastrointestinal toxicity occurred in 80% of patients, and any-grade hyponatremia occurred in 61%. Central neurological adverse reactions occurred in 25% of patients, including dizziness and mental status changes, according to the announcement.
Warnings and precautions for adverse events – including thrombocytopenia, neutropenia, gastrointestinal toxicity, hyponatremia, serious infection, neurological toxicity, and embryo-fetal toxicity – are provided in the prescribing information.
Selinexor acts through the inhibition of exportin-1 and leads to an accumulation of tumor suppressor proteins, a reduction in oncoproteins, and apoptosis of cancer cells. The drug was previously approved in 2019 for the treatment of relapsed/refractory multiple myeloma.
The SADAL trial was sponsored by Karyopharm Therapeutics.
SOURCE: U.S. Food and Drug Administration. 2020. Approval announcement.
The Food and Drug Administration has granted accelerated approval of selinexor, a nuclear transport inhibitor, for the treatment of relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
Selinexor (marketed as XPOVIO by Karyopharm Therapeutics) is intended for adult patients with relapsed/refractory DLBCL, not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy, according to the FDA’s announcement.
The FDA granted selinexor accelerated approval for this indication based on the response rate seen in the SADAL trial. Continued approval for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
The SADAL trial (NCT02227251) was a phase 2, single-arm, open-label study of patients with DLBCL who had previously received two to five systemic regimens. The patients received selinexor at 60 mg orally on days 1 and 3 of each week.
Results in 134 patients showed an overall response rate of 29% (95% confidence interval: 22-38), with complete responses in 13% of patients. Of 39 patients who achieved a partial or complete response, 38% had a response duration of at least 6 months, and 15% had a response duration of at least 12 months, according to the FDA announcement.
The most common adverse reactions in this trial were fatigue, nausea, diarrhea, appetite decrease, weight decrease, constipation, vomiting, and pyrexia. Grade 3-4 laboratory abnormalities occurred in 15% or more of the patients, and comprised thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia.
Serious adverse reactions occurred in 46% of patients, most often from infection. Gastrointestinal toxicity occurred in 80% of patients, and any-grade hyponatremia occurred in 61%. Central neurological adverse reactions occurred in 25% of patients, including dizziness and mental status changes, according to the announcement.
Warnings and precautions for adverse events – including thrombocytopenia, neutropenia, gastrointestinal toxicity, hyponatremia, serious infection, neurological toxicity, and embryo-fetal toxicity – are provided in the prescribing information.
Selinexor acts through the inhibition of exportin-1 and leads to an accumulation of tumor suppressor proteins, a reduction in oncoproteins, and apoptosis of cancer cells. The drug was previously approved in 2019 for the treatment of relapsed/refractory multiple myeloma.
The SADAL trial was sponsored by Karyopharm Therapeutics.
SOURCE: U.S. Food and Drug Administration. 2020. Approval announcement.
The Food and Drug Administration has granted accelerated approval of selinexor, a nuclear transport inhibitor, for the treatment of relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
Selinexor (marketed as XPOVIO by Karyopharm Therapeutics) is intended for adult patients with relapsed/refractory DLBCL, not otherwise specified, including DLBCL arising from follicular lymphoma, after at least two lines of systemic therapy, according to the FDA’s announcement.
The FDA granted selinexor accelerated approval for this indication based on the response rate seen in the SADAL trial. Continued approval for this indication “may be contingent upon verification and description of clinical benefit in confirmatory trials,” according to the FDA.
The SADAL trial (NCT02227251) was a phase 2, single-arm, open-label study of patients with DLBCL who had previously received two to five systemic regimens. The patients received selinexor at 60 mg orally on days 1 and 3 of each week.
Results in 134 patients showed an overall response rate of 29% (95% confidence interval: 22-38), with complete responses in 13% of patients. Of 39 patients who achieved a partial or complete response, 38% had a response duration of at least 6 months, and 15% had a response duration of at least 12 months, according to the FDA announcement.
The most common adverse reactions in this trial were fatigue, nausea, diarrhea, appetite decrease, weight decrease, constipation, vomiting, and pyrexia. Grade 3-4 laboratory abnormalities occurred in 15% or more of the patients, and comprised thrombocytopenia, lymphopenia, neutropenia, anemia, and hyponatremia.
Serious adverse reactions occurred in 46% of patients, most often from infection. Gastrointestinal toxicity occurred in 80% of patients, and any-grade hyponatremia occurred in 61%. Central neurological adverse reactions occurred in 25% of patients, including dizziness and mental status changes, according to the announcement.
Warnings and precautions for adverse events – including thrombocytopenia, neutropenia, gastrointestinal toxicity, hyponatremia, serious infection, neurological toxicity, and embryo-fetal toxicity – are provided in the prescribing information.
Selinexor acts through the inhibition of exportin-1 and leads to an accumulation of tumor suppressor proteins, a reduction in oncoproteins, and apoptosis of cancer cells. The drug was previously approved in 2019 for the treatment of relapsed/refractory multiple myeloma.
The SADAL trial was sponsored by Karyopharm Therapeutics.
SOURCE: U.S. Food and Drug Administration. 2020. Approval announcement.
FROM THE FDA
Oxidatative stress–related genetic variant tied to stroke risk in sickle cell patients
Oxidative stress-related genetic variants, in particular, the according to Igor F. Domingos of the Genetics Postgraduate Program, Federal University of Pernambuco, Recife, Brazil, and colleagues.
The researchers genotyped 499 unrelated adult patients with sickle cell anemia (SCA) for a variety of polymorphisms, along with alpha-thalassemia status and beta-globin gene haplotypes.
They found that SOD2 Val16Ala polymorphism was independently associated with risk of stroke (odds ratio, 1.98; 95% confidence interval, 1.18-3.32; P = .009) and with the long-term cumulative incidence of stroke (hazard ratio, 2.24; 95% CI, 1.3-3.9; P = .004).
A crucial limitation identified by the authors was the inability to replicate their results in a validation cohort. They suggested that there could have been genetic differences between the Brazilian population and the validation cohort of 231 patients followed at King’s College London for whom biological samples was available. They also suggested that patient treatment history between the two countries may be a factor.
“We believe that our study represents an alternative for understudied SCA populations with no access to TCD [transcranial Doppler ultrasound screening] and imaging exams, in which genetic modifiers may be a useful tool for predicting stroke in SCA,” the authors concluded.
The authors reported that they had no competing financial interests.
[email protected]
SOURCE: Domingos IF et al. J Neurol Sci. 2020 Apr 16; doi: 10.1016/j.jns.2020.116839.
Oxidative stress-related genetic variants, in particular, the according to Igor F. Domingos of the Genetics Postgraduate Program, Federal University of Pernambuco, Recife, Brazil, and colleagues.
The researchers genotyped 499 unrelated adult patients with sickle cell anemia (SCA) for a variety of polymorphisms, along with alpha-thalassemia status and beta-globin gene haplotypes.
They found that SOD2 Val16Ala polymorphism was independently associated with risk of stroke (odds ratio, 1.98; 95% confidence interval, 1.18-3.32; P = .009) and with the long-term cumulative incidence of stroke (hazard ratio, 2.24; 95% CI, 1.3-3.9; P = .004).
A crucial limitation identified by the authors was the inability to replicate their results in a validation cohort. They suggested that there could have been genetic differences between the Brazilian population and the validation cohort of 231 patients followed at King’s College London for whom biological samples was available. They also suggested that patient treatment history between the two countries may be a factor.
“We believe that our study represents an alternative for understudied SCA populations with no access to TCD [transcranial Doppler ultrasound screening] and imaging exams, in which genetic modifiers may be a useful tool for predicting stroke in SCA,” the authors concluded.
The authors reported that they had no competing financial interests.
[email protected]
SOURCE: Domingos IF et al. J Neurol Sci. 2020 Apr 16; doi: 10.1016/j.jns.2020.116839.
Oxidative stress-related genetic variants, in particular, the according to Igor F. Domingos of the Genetics Postgraduate Program, Federal University of Pernambuco, Recife, Brazil, and colleagues.
The researchers genotyped 499 unrelated adult patients with sickle cell anemia (SCA) for a variety of polymorphisms, along with alpha-thalassemia status and beta-globin gene haplotypes.
They found that SOD2 Val16Ala polymorphism was independently associated with risk of stroke (odds ratio, 1.98; 95% confidence interval, 1.18-3.32; P = .009) and with the long-term cumulative incidence of stroke (hazard ratio, 2.24; 95% CI, 1.3-3.9; P = .004).
A crucial limitation identified by the authors was the inability to replicate their results in a validation cohort. They suggested that there could have been genetic differences between the Brazilian population and the validation cohort of 231 patients followed at King’s College London for whom biological samples was available. They also suggested that patient treatment history between the two countries may be a factor.
“We believe that our study represents an alternative for understudied SCA populations with no access to TCD [transcranial Doppler ultrasound screening] and imaging exams, in which genetic modifiers may be a useful tool for predicting stroke in SCA,” the authors concluded.
The authors reported that they had no competing financial interests.
[email protected]
SOURCE: Domingos IF et al. J Neurol Sci. 2020 Apr 16; doi: 10.1016/j.jns.2020.116839.
FROM THE JOURNAL OF NEUROLOGICAL SCIENCES
Key clinical point: SOD2 Val16Ala polymorphism may represent a simple and inexpensive alternative for identifying sickle cell patients at risk of stroke.
Major finding: SOD2 Val16Ala polymorphism was independently associated with an increased risk of stroke (odds ratio, 1.98; P = .009).
Study details: A total of 499 unrelated adult patients with sickle cell disease were genotyped.
Disclosures: The authors reported that they had no competing financial interests.
Source: Domingos IF et al. J Neurol Sci. 2020 Apr 16. doi: org/10.1016/j.jns.2020.116839.
Cortisol levels on COVID-19 admission may be a marker of severity
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Experts publish imaging recommendations for pediatric COVID-19
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has devised recommendations for ordering imaging studies in suspected cases of the infection.
The review also included useful radiographic findings to help in the differential diagnosis of COVID-19 pneumonia from other respiratory infections. Alexandra M. Foust, DO, of Boston Children’s Hospital, and colleagues reported the summary of findings and recommendations in Pediatric Pulmonology.
“Pediatricians face numerous challenges created by increasing reports of severe COVID-19 related findings in affected children,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. “[The current review] represents a multinational collaboration to provide up to date information and key imaging findings to guide chest physicians caring for children with pneumonia symptoms during the COVID-19 pandemic.”
Clinical presentation in children
In general, pediatric patients infected with the virus show milder symptoms compared with adults, and based on the limited evidence reported to date, the most common clinical symptoms of COVID-19 in children are rhinorrhea and/or nasal congestion, fever and cough with sore throat, fatigue or dyspnea, and diarrhea.
As with other viral pneumonias in children, the laboratory parameters are usually nonspecific; however, while the complete blood count (CBC) is often normal, lymphopenia, thrombocytopenia, and neutropenia have been reported in some cases of pediatric COVID-19, the authors noted.
The current Centers for Disease Control and Prevention (CDC) recommendation for initial diagnosis of SARS-CoV-2 is obtaining a nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) testing, they explained.
Role of imaging in diagnosis
The researchers reported that current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as a upfront test to diagnose pediatric COVID-19, but they may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course.
The potential benefits of utilizing radiologic evaluation, such as establishing a baseline for monitoring disease progression, must be balanced with potential drawbacks, which include radiation exposure, and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Recommendations for ordering imaging studies
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia.
The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia.
“The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Dr. Cataletto. “By synthesizing the available imaging case series and guidelines, this primer provides a useful tool for the practicing pulmonologist,” she explained.
Key recommendations: CXR
“For pediatric patients with suspected or known COVID-19 infection with moderate to severe clinical symptoms requiring hospitalization (i.e., hypoxia, moderate or severe dyspnea, signs of sepsis, shock, cardiovascular compromise, altered mentation), CXR is usually indicated to establish an imaging baseline and to assess for an alternative diagnosis,” they recommended.
“Sequential CXRs may be helpful to assess pediatric patients with COVID-19 who demonstrate worsening clinical symptoms or to assess response to supportive therapy,” they wrote.
Key recommendations: CT
“Due to the increased radiation sensitivity of pediatric patients, chest CT is not recommended as an initial diagnostic test for pediatric patients with known or suspected COVID-19 pneumonia,” they explained.
The guide also included several considerations around the differential diagnosis of COVID-19 pneumonia from other pediatric lung disorders, including immune-related conditions, infectious etiologies, hematological dyscrasias, and inhalation-related lung injury.
As best practice recommendations for COVID-19 continue to evolve, the availability of practical clinical decision-making tools becomes essential to ensure optimal patient care.
No funding sources or financial disclosures were reported in the manuscript.
SOURCE: Foust AM et al. Pediatr Pulmonol. 2020 May 28. doi: 10.1002/ppul.24870.
FROM PEDIATRIC PULMONOLOGY
Ibrutinib-venetoclax produces high MRD-negative rates in CLL/SLL
In patients with previously untreated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), a once-daily oral regimen of ibrutinib and venetoclax was associated with deep molecular remissions in both bone marrow and peripheral blood, including in patients with high-risk disease, according to investigators in the phase 2 CAPTIVATE MRD trial.
An intention-to-treat analysis of 164 patients with CLL/SLL treated with the combination of ibrutinib (Imbruvica) and venetoclax (Venclexta) showed a 75% rate of minimal residual disease (MRD) negativity in peripheral blood, and a 68% rate of MRD negativity in bone marrow among patients who received up to 12 cycles of the combination, reported Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif., and colleagues.
“This phase 2 study supports synergistic antitumor activity of the combination with notable deep responses across multiple compartments,” she said in an oral presentation during the virtual annual congress of the European Hematology Association.
Not ready to change practice
A hematologist/oncologist who was not involved in the study said that the data from CAPTIVATE MRD look good, but it’s still not known whether concurrent or sequential administration of the agents is optimal, and whether other regimens may be more effective in the first line.
“I think this is promising, but the informative and practice-changing study would be to compare this combination to ibrutinib monotherapy or to venetoclax and obinutuzumab, and that’s actually the subject of the next large German cooperative group study, CLL17,” said Catherine C. Coombs, MD, assistant professor of medicine at the University of North Carolina, and the UNC Lineberger Cancer Center, Chapel Hill.
She noted that the combination of venetoclax and obinutuzumab (Gazyva) is also associated with high rates of MRD negativity in the first-line setting, and that use of this regimen allows clinicians to reserve ibrutinib or acalabrutinib (Calquence) for patients in the relapsed setting.
Prerandomization results
Dr. Siddiqi presented prerandomization results from the MRD cohort of the CAPTIVATE trial (NCT02910583), which is evaluating the combination of ibrutinib and venetoclax for depth of MRD response. Following 12 cycles of the combinations, patients in this cohort are then randomized based on confirmed MRD status, with patients who are MRD negative randomized to maintenance with either ibrutinib or placebo, and patients with residual disease (MRD positive) randomized to maintenance with either ibrutinib alone or with venetoclax.
A total of 164 patients with previously untreated CLL/SLL and active disease requiring treatment who were under age 70 and had good performance status were enrolled. Following an ibrutinib lead-in period with the drug given at 420 mg once daily for three cycles of 28 days, the patients were continued on ibrutinib, and were started on venetoclax with a ramp up to 400 mg once daily, for 12 additional cycles.
As planned, patients were assessed after 15 cycles for tumor lysis syndrome (TLS) risk assessment, MRD, and hematologic, clinical, imaging, and bone marrow exams for response.
The median patient age was 58, with poor-risk features such as deletion 17p seen in 16%, complex karyotype in 19%, and unmutated immunoglobulin heavy chain variable (IGHV) in 59%.
A total of 152 patients (90%) completed all 12 cycles of the combined agents, with a median treatment duration of 14.7 months on ibrutinib and 12 months on venetoclax. Eight patients had adverse events leading to discontinuation, but there were no treatment-related deaths.
A majority of patients had reductions in lymph node burden after the three-cycle ibrutinib lead in. TLS risk also decreased during the lead-in period, with 90% of patients who had a high baseline TLS risk shifting to medium or low-risk categories, and no patients moved into the high-risk category.
“Hospitalization because of this was no longer required in 66% of at-risk patients after three cycles of ibrutinib lead in, and 82% of patients initiated venetoclax ramp up without the need for hospitalization,” Dr. Siddiqi said.
The best response of undetectable MRD was seen in peripheral blood of 75% of 163 evaluable patients, and in bone marrow of 72% of 155 patients. As noted before, the respective rates of MRD negativity in the intention-to-treat population were 75% and 68%. The proportion of patients with undetectable MRD in peripheral blood increased over time, from 57% after six cycles of the combination, she said.
The overall response rate was 97%, including 51% complete responses (CR) or CR with incomplete bone marrow recovery (CRi), and 46% partial (PR) or nodular PR (nPR). Among patients with CR/CRi, 85% had undetectable MRD in peripheral blood and 80% were MRD negative in bone marrow. In patients with PR/nPR, the respective rates were 69% and 59%. The high rates of undetectable MRD were seen irrespective of baseline disease characteristics, including bulky disease, cytogenetic risk category, del(17p) or TP53 mutation, and complex karyotype.
The most common adverse events with the combination were grade 1 or 2 diarrhea, arthralgia, fatigue, headache, and nausea. Grade 3 neutropenia was seen in 17% of patients, and grade 4 neutropenia was seen in 16%. Grade 3 febrile neutropenia and laboratory confirmed TLS occurred in 2 patients each (1%), and there were no grade 4 instances of either adverse event.
Postrandomization follow-up and analyses are currently being conducted, and results will be reported at a future meeting, real or virtual. An analysis of data on a separate cohort of 159 patients treated with the ibrutinib-venetoclax combination for a fixed duration is currently ongoing.
Dr. Siddiqi disclosed research funding and speakers bureau activity for Pharmacyclics, which sponsored the study, and others, as well as consulting/advising for several companies. Dr. Coombs disclosed consulting for AbbVie.
SOURCE: Siddiqi T et al. EHA25. Abstract S158.
In patients with previously untreated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), a once-daily oral regimen of ibrutinib and venetoclax was associated with deep molecular remissions in both bone marrow and peripheral blood, including in patients with high-risk disease, according to investigators in the phase 2 CAPTIVATE MRD trial.
An intention-to-treat analysis of 164 patients with CLL/SLL treated with the combination of ibrutinib (Imbruvica) and venetoclax (Venclexta) showed a 75% rate of minimal residual disease (MRD) negativity in peripheral blood, and a 68% rate of MRD negativity in bone marrow among patients who received up to 12 cycles of the combination, reported Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif., and colleagues.
“This phase 2 study supports synergistic antitumor activity of the combination with notable deep responses across multiple compartments,” she said in an oral presentation during the virtual annual congress of the European Hematology Association.
Not ready to change practice
A hematologist/oncologist who was not involved in the study said that the data from CAPTIVATE MRD look good, but it’s still not known whether concurrent or sequential administration of the agents is optimal, and whether other regimens may be more effective in the first line.
“I think this is promising, but the informative and practice-changing study would be to compare this combination to ibrutinib monotherapy or to venetoclax and obinutuzumab, and that’s actually the subject of the next large German cooperative group study, CLL17,” said Catherine C. Coombs, MD, assistant professor of medicine at the University of North Carolina, and the UNC Lineberger Cancer Center, Chapel Hill.
She noted that the combination of venetoclax and obinutuzumab (Gazyva) is also associated with high rates of MRD negativity in the first-line setting, and that use of this regimen allows clinicians to reserve ibrutinib or acalabrutinib (Calquence) for patients in the relapsed setting.
Prerandomization results
Dr. Siddiqi presented prerandomization results from the MRD cohort of the CAPTIVATE trial (NCT02910583), which is evaluating the combination of ibrutinib and venetoclax for depth of MRD response. Following 12 cycles of the combinations, patients in this cohort are then randomized based on confirmed MRD status, with patients who are MRD negative randomized to maintenance with either ibrutinib or placebo, and patients with residual disease (MRD positive) randomized to maintenance with either ibrutinib alone or with venetoclax.
A total of 164 patients with previously untreated CLL/SLL and active disease requiring treatment who were under age 70 and had good performance status were enrolled. Following an ibrutinib lead-in period with the drug given at 420 mg once daily for three cycles of 28 days, the patients were continued on ibrutinib, and were started on venetoclax with a ramp up to 400 mg once daily, for 12 additional cycles.
As planned, patients were assessed after 15 cycles for tumor lysis syndrome (TLS) risk assessment, MRD, and hematologic, clinical, imaging, and bone marrow exams for response.
The median patient age was 58, with poor-risk features such as deletion 17p seen in 16%, complex karyotype in 19%, and unmutated immunoglobulin heavy chain variable (IGHV) in 59%.
A total of 152 patients (90%) completed all 12 cycles of the combined agents, with a median treatment duration of 14.7 months on ibrutinib and 12 months on venetoclax. Eight patients had adverse events leading to discontinuation, but there were no treatment-related deaths.
A majority of patients had reductions in lymph node burden after the three-cycle ibrutinib lead in. TLS risk also decreased during the lead-in period, with 90% of patients who had a high baseline TLS risk shifting to medium or low-risk categories, and no patients moved into the high-risk category.
“Hospitalization because of this was no longer required in 66% of at-risk patients after three cycles of ibrutinib lead in, and 82% of patients initiated venetoclax ramp up without the need for hospitalization,” Dr. Siddiqi said.
The best response of undetectable MRD was seen in peripheral blood of 75% of 163 evaluable patients, and in bone marrow of 72% of 155 patients. As noted before, the respective rates of MRD negativity in the intention-to-treat population were 75% and 68%. The proportion of patients with undetectable MRD in peripheral blood increased over time, from 57% after six cycles of the combination, she said.
The overall response rate was 97%, including 51% complete responses (CR) or CR with incomplete bone marrow recovery (CRi), and 46% partial (PR) or nodular PR (nPR). Among patients with CR/CRi, 85% had undetectable MRD in peripheral blood and 80% were MRD negative in bone marrow. In patients with PR/nPR, the respective rates were 69% and 59%. The high rates of undetectable MRD were seen irrespective of baseline disease characteristics, including bulky disease, cytogenetic risk category, del(17p) or TP53 mutation, and complex karyotype.
The most common adverse events with the combination were grade 1 or 2 diarrhea, arthralgia, fatigue, headache, and nausea. Grade 3 neutropenia was seen in 17% of patients, and grade 4 neutropenia was seen in 16%. Grade 3 febrile neutropenia and laboratory confirmed TLS occurred in 2 patients each (1%), and there were no grade 4 instances of either adverse event.
Postrandomization follow-up and analyses are currently being conducted, and results will be reported at a future meeting, real or virtual. An analysis of data on a separate cohort of 159 patients treated with the ibrutinib-venetoclax combination for a fixed duration is currently ongoing.
Dr. Siddiqi disclosed research funding and speakers bureau activity for Pharmacyclics, which sponsored the study, and others, as well as consulting/advising for several companies. Dr. Coombs disclosed consulting for AbbVie.
SOURCE: Siddiqi T et al. EHA25. Abstract S158.
In patients with previously untreated chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), a once-daily oral regimen of ibrutinib and venetoclax was associated with deep molecular remissions in both bone marrow and peripheral blood, including in patients with high-risk disease, according to investigators in the phase 2 CAPTIVATE MRD trial.
An intention-to-treat analysis of 164 patients with CLL/SLL treated with the combination of ibrutinib (Imbruvica) and venetoclax (Venclexta) showed a 75% rate of minimal residual disease (MRD) negativity in peripheral blood, and a 68% rate of MRD negativity in bone marrow among patients who received up to 12 cycles of the combination, reported Tanya Siddiqi, MD, of City of Hope National Medical Center, Duarte, Calif., and colleagues.
“This phase 2 study supports synergistic antitumor activity of the combination with notable deep responses across multiple compartments,” she said in an oral presentation during the virtual annual congress of the European Hematology Association.
Not ready to change practice
A hematologist/oncologist who was not involved in the study said that the data from CAPTIVATE MRD look good, but it’s still not known whether concurrent or sequential administration of the agents is optimal, and whether other regimens may be more effective in the first line.
“I think this is promising, but the informative and practice-changing study would be to compare this combination to ibrutinib monotherapy or to venetoclax and obinutuzumab, and that’s actually the subject of the next large German cooperative group study, CLL17,” said Catherine C. Coombs, MD, assistant professor of medicine at the University of North Carolina, and the UNC Lineberger Cancer Center, Chapel Hill.
She noted that the combination of venetoclax and obinutuzumab (Gazyva) is also associated with high rates of MRD negativity in the first-line setting, and that use of this regimen allows clinicians to reserve ibrutinib or acalabrutinib (Calquence) for patients in the relapsed setting.
Prerandomization results
Dr. Siddiqi presented prerandomization results from the MRD cohort of the CAPTIVATE trial (NCT02910583), which is evaluating the combination of ibrutinib and venetoclax for depth of MRD response. Following 12 cycles of the combinations, patients in this cohort are then randomized based on confirmed MRD status, with patients who are MRD negative randomized to maintenance with either ibrutinib or placebo, and patients with residual disease (MRD positive) randomized to maintenance with either ibrutinib alone or with venetoclax.
A total of 164 patients with previously untreated CLL/SLL and active disease requiring treatment who were under age 70 and had good performance status were enrolled. Following an ibrutinib lead-in period with the drug given at 420 mg once daily for three cycles of 28 days, the patients were continued on ibrutinib, and were started on venetoclax with a ramp up to 400 mg once daily, for 12 additional cycles.
As planned, patients were assessed after 15 cycles for tumor lysis syndrome (TLS) risk assessment, MRD, and hematologic, clinical, imaging, and bone marrow exams for response.
The median patient age was 58, with poor-risk features such as deletion 17p seen in 16%, complex karyotype in 19%, and unmutated immunoglobulin heavy chain variable (IGHV) in 59%.
A total of 152 patients (90%) completed all 12 cycles of the combined agents, with a median treatment duration of 14.7 months on ibrutinib and 12 months on venetoclax. Eight patients had adverse events leading to discontinuation, but there were no treatment-related deaths.
A majority of patients had reductions in lymph node burden after the three-cycle ibrutinib lead in. TLS risk also decreased during the lead-in period, with 90% of patients who had a high baseline TLS risk shifting to medium or low-risk categories, and no patients moved into the high-risk category.
“Hospitalization because of this was no longer required in 66% of at-risk patients after three cycles of ibrutinib lead in, and 82% of patients initiated venetoclax ramp up without the need for hospitalization,” Dr. Siddiqi said.
The best response of undetectable MRD was seen in peripheral blood of 75% of 163 evaluable patients, and in bone marrow of 72% of 155 patients. As noted before, the respective rates of MRD negativity in the intention-to-treat population were 75% and 68%. The proportion of patients with undetectable MRD in peripheral blood increased over time, from 57% after six cycles of the combination, she said.
The overall response rate was 97%, including 51% complete responses (CR) or CR with incomplete bone marrow recovery (CRi), and 46% partial (PR) or nodular PR (nPR). Among patients with CR/CRi, 85% had undetectable MRD in peripheral blood and 80% were MRD negative in bone marrow. In patients with PR/nPR, the respective rates were 69% and 59%. The high rates of undetectable MRD were seen irrespective of baseline disease characteristics, including bulky disease, cytogenetic risk category, del(17p) or TP53 mutation, and complex karyotype.
The most common adverse events with the combination were grade 1 or 2 diarrhea, arthralgia, fatigue, headache, and nausea. Grade 3 neutropenia was seen in 17% of patients, and grade 4 neutropenia was seen in 16%. Grade 3 febrile neutropenia and laboratory confirmed TLS occurred in 2 patients each (1%), and there were no grade 4 instances of either adverse event.
Postrandomization follow-up and analyses are currently being conducted, and results will be reported at a future meeting, real or virtual. An analysis of data on a separate cohort of 159 patients treated with the ibrutinib-venetoclax combination for a fixed duration is currently ongoing.
Dr. Siddiqi disclosed research funding and speakers bureau activity for Pharmacyclics, which sponsored the study, and others, as well as consulting/advising for several companies. Dr. Coombs disclosed consulting for AbbVie.
SOURCE: Siddiqi T et al. EHA25. Abstract S158.
FROM EHA 2020









