User login
-
Older age, r/r disease in lymphoma patients tied to increased COVID-19 death rate
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
Patients with B-cell lymphoma are immunocompromised because of the disease and its treatments. This presents the question of their outcomes upon infection with SARS-CoV-2. Researchers assessed the characteristics of patients with lymphoma hospitalized for COVID-19 and analyzed determinants of mortality in a retrospective database study. The investigators looked at data from adult patients with lymphoma who were hospitalized for COVID-19 in March and April 2020 in three French regions.
Older age and relapsed/refractory (r/r) disease in B-cell lymphoma patients were both found to be independent risk factors of increased death rate from COVID-19, according to the online report in EClinicalMedicine, published by The Lancet.
These results encourage “the application of standard Covid-19 treatment, including intubation, for lymphoma patients with Covid-19 lymphoma diagnosis, under first- or second-line chemotherapy, or in remission,” according to Sylvain Lamure, MD, of Montellier (France) University, and colleagues.
The study examined a series of 89 consecutive patients from three French regions who had lymphoma and were hospitalized for COVID-19 in March and April 2020. The population was homogeneous; most patients were diagnosed with B-cell non-Hodgkin lymphoma (NHL) and had been treated for their lymphoma within 1 year.
Promising results for many
There were a significant associations between 30-day mortality and increasing age (over age 70 years) and r/r lymphoma. However, in the absence of those factors, mortality of the lymphoma patients with COVID-19 was comparable with that of the reference French COVID-19 population. In addition, there was no significant impact of active lymphoma treatment that had been given within 1 year, except for those patients who received bendamustine, which was associated with greater mortality, according to the researchers.
With a median follow-up of 33 days from admission, the Kaplan-Meier estimate of 30-day overall survival was 71% (95% confidence interval, 62%-81%). According to histological type of the lymphoma, 30-day overall survival rates were 80% (95% CI, 45%-100%) for Hodgkin lymphoma, 71% (95% CI, 61%-82%) for B-cell non-Hodgkin Lymphoma, and 71% (95% CI, 38%-100%) for T-cell non-Hodgkin Lymphoma.
The main factors associated with mortality were age 70 years and older (hazard ratio, 3.78; 95% CI, 1.73-8.25; P = .0009), hypertension (HR, 2.20; 95% CI, 1.06-4.59; P = .03), previous cancer (HR, 2.11; 95% CI, 0.90-4.92; P = .08), use of bendamustine within 12 months before admission to hospital (HR, 3.05; 95% CI, 1.31-7.11; P = .01), and r/r lymphoma (HR, 2.62; 95% CI, 1.20-5.72; P = .02).
Overall, the Kaplan-Meier estimates of 30-day overall survival were 61% for patients with r/r lymphoma, 52% in patients age 70 years with non–r/r lymphoma, and 88% for patients younger than 70 years with non–r/r, which was comparable with general population survival data among French populations, according to the researchers.
“Longer term clinical follow-up and biological monitoring of immune responses is warranted to explore the impact of lymphoma and its treatment on the immunity and prolonged outcome of Covid-19 patients,” they concluded.
The study was unsponsored. Several of the authors reported financial relationships with a number of biotechnology and pharmaceutical companies.
SOURCE: Lamure S et al. EClinicalMedicine. 2020 Oct 12. doi: 10.1016/j.eclinm.2020.100549.
FROM ECLINICALMEDICINE
Link between vitamin D and ICU outcomes unclear
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
We can “stop putting money on vitamin D” to help patients who require critical care, said Todd Rice, MD, FCCP.
“Results from vitamin D trials have not been uniformly one way, but they have been pretty uniformly disappointing,” Dr. Rice, from Vanderbilt University Medical Center, Nashville, Tenn., reported at the annual meeting of the American College of Chest Physicians.
Low levels of vitamin D in critically ill COVID-19 patients have been reported in numerous recent studies, and researchers are looking for ways to boost those levels and improve outcomes.
We are seeing “the exact same story” in the critically ill COVID-19 population as we see in the general ICU population, said Dr. Rice. “The whole scenario is repeating itself. I’m pessimistic.”
Still, vitamin D levels can be elevated so, in theory, “the concept makes sense,” he said. There is evidence that, “when given enterally, the levels rise nicely” and vitamin D is absorbed reasonably well.” But is that enough?
When patients are admitted to the ICU, some biomarkers in the body are too high and others are too low. Vitamin D is often too low. So far, though, “supplementing vitamin D in the ICU has not significantly improved outcomes,” said Dr. Rice.
In the Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) trial, Dr. Rice and colleagues found no statistical benefit when a 540,000 IU boost of vitamin D was administered to 2,624 critically ill patients, as reported by Medscape Medical News.
“Early administration of high-dose enteral vitamin D3 did not provide an advantage over placebo with respect to 90-day mortality or other nonfatal outcomes among critically ill, vitamin D–deficient patients,” the researchers write in their recent report.
In fact, VIOLET ended before enrollment had reached the planned 3,000-patient cohort because the statistical analysis clearly did not show benefit. Those enrolled were in the ICU because of, among other things, pneumonia, sepsis, the need for mechanical ventilation or vasopressors, and risk for acute respiratory distress syndrome.
“It doesn’t look like vitamin D is going to be the answer to our critical care problems,” Dr. Rice said in an interview.
Maintenance dose needed?
One theory suggests that VIOLET might have failed because a maintenance dose is needed after the initial boost of vitamin D.
In the ongoing VITDALIZE trial, critically ill patients with severe vitamin D deficiency (12 ng/mL or less at admission) receive an initial 540,000-IU dose followed by 4,000 IU per day.
The highly anticipated VITDALIZE results are expected in the middle of next year, Dr. Rice reported, so “let’s wait to see.”
“Vitamin D may not have an acute effect,” he theorized. “We can raise your levels, but that doesn’t give you all the benefits of having a sufficient level for a long period of time.”
Another theory suggests that a low level of vitamin D is simply a signal of the severity of disease, not a direct influence on disease pathology.
Some observational data have shown an association between low levels of vitamin D and outcomes in COVID-19 patients (Nutrients. 2020 May 9;12[5]:1359; medRxiv 2020 Apr 24. doi: 10.1101/2020.04.24.20075838; JAMA Netw Open. 2020;3[9]:e2019722; FEBS J. 2020 Jul 23;10.1111/febs.15495; Clin Endocrinol [Oxf]. 2020 Jul 3;10.1111/cen.14276), but some have shown no association (medRxiv. 2020 Jun 26. doi: 10.1101/2020.06.26.20140921; J Public Health [Oxf]. 2020 Aug 18;42[3]:451-60).
Dr. Rice conducted a search of Clinicaltrials.gov immediately before his presentation on Sunday, and found 41 ongoing interventional studies – “not observational studies” – looking at COVID-19 and vitamin D.
“They’re recruiting, they’re enrolling; hopefully we’ll have data soon,” he said.
Researchers have checked a lot of boxes with a resounding yes on the vitamin D question, so there’s reason to think an association does exist for ICU patients, whether or not they have COVID-19.
“Is there a theoretical benefit of vitamin D in the ICU?” Dr. Rice asked. “Yes. Is vitamin D deficient in patients in the ICU? Yes. Is that deficiency associated with poor outcomes? Yes. Can it be replaced safely? Yes.”
However, “we’re not really sure that it improves outcomes,” he said.
A chronic issue?
“Do you think it’s really an issue of the patients being critically ill with vitamin D,” or is it “a chronic issue of having low vitamin D?” asked session moderator Antine Stenbit, MD, PhD, from the University of California, San Diego.
“We don’t know for sure,” Dr. Rice said. Vitamin D might not have a lot of acute effects; it might have effects that are chronic, that work with levels over a period of time, he explained.
“It’s not clear we can correct that with a single dose or with a few days of giving a level that is adequate,” he acknowledged.
Dr. Rice is an investigator in the PETAL network. Dr. Stenbit disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM CHEST 2020
COVID-19 transforms medical education: No ‘back to normal’
The COVID-19 pandemic has thrown a monkey wrench into the medical education landscape across the entire health care spectrum, disrupting the plans of medical students, residents, fellows, and program directors.
As cases of COVID-19 spread across the United States in early 2020, it became clear to training program directors that immediate action was required to meet the needs of medical learners. The challenges were unlike those surrounding the Ebola virus in 2014, “where we could more easily prevent students and trainees from exposure due to the fact that there were simply not significant numbers of cases in the United States,” Tiffany Murano, MD, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. Dr. Murano is professor of emergency medicine at Rutgers New Jersey Medical School, Newark, and president-elect of the Council of Residency Directors in Emergency Medicine. “COVID was a completely different scenario. We quickly realized that not only was personal protective equipment in short supply, but we also lacked the testing and tracking capabilities for potential exposures. Medical students and other supportive workers who were considered nonessential were removed from the clinical setting. This was after a trial of limiting who the students saw, essentially dampening the risk of exposure. But this proved to be flawed as COVID patients presented with symptoms that were unexpected.”
To complicate matters, she continued, many medical clinics either shut down, had limited access, or converted to telemedicine. Elective surgeries were canceled. This led to an overall pause in clinical medical student rotations and no direct patient care activities. As social distancing mandates were instituted, licensing examination testing centers were closed, and exams and on-campus activities were postponed.
Limiting trainee exposure
On the graduate medical education front, some training programs attempted to limit exposure of their trainees to persons under investigation for COVID-19. “As the number of COVID cases grew and encompassed most of what we were seeing in the hospital, it was obvious that residents had to play a vital part in the care of these patients,” said Dr. Murano, who is also a member of the American Council of Graduate Medical Education’s emergency review and recognition committee. “However, there was a consensus among all of the specialties that the procedures that posed the highest risk of exposure would be limited to the most senior or experienced trainees or professionals, and closely supervised by the faculty.”
ACGME activities such as accreditation site visits, clinical environment learning reviews, self-study, and resident and faculty surveys were suspended, postponed, or modified in some way, she said. The ACGME created stages of COVID status to guide sponsoring institutions to suspend learning curricula in order for patients to be cared for. Stage 1 was business as usual, “so there was no significant impact on patient care,” Dr. Murano said. “Stage 2 was increased but manageable clinical demand, while stage 3 was pandemic emergency status, where there were extraordinary circumstances where the clinical demand was so high and strenuous that the routine patient care and education really needed to be reconfigured in order to care for the patients.”
New requirements to manage training
The ACGME also implemented four requirements to manage training that were consistent among institutions, regardless of their COVID stage status. These included making sure that trainees continued to be held to work-hour limit requirements, ensuring adequate resources for training, ensuring that all residents had the appropriate level of supervision at all times, and allowing fellows to function in the core specialty in which they completed their residency training. “This was only possible if the fellows were ABMS [American Board of Medical Specialties] or AOA [American Osteopathic Association] board-eligible, or certified in their core specialty,” Dr. Murano said. “The fellows had to be appointed to the medical staff at the sponsoring institution, and their time spent on the core specialty service would be limited to 20% of their annual education time in any academic year.”
Mindful that there may have been trainees who required a 2-week quarantine period following exposure or potential exposure to COVID-19, some specialty boards showed leniency in residency time required to sit for the written exam. “Testing centers were being forced to close to observe social distancing requirements and heed sanitation recommendations, so exams were either canceled or postponed,” Dr. Murano said. “This posed a special concern for the board certification process, and those specialties with oral examinations had to make a heavy decision regarding whether or not they would allow these exams to take place. Naturally, travel among institutions was suspended or limited, or had quarantine requirements upon returning home from endemic areas. Conferences were either being canceled or converted to virtual formats.”
Subani Chandra, MD, FCCP, of the division of pulmonary, allergy, and critical care medicine at Columbia University, New York, is the internal medicine residency program director and the associate vice-chair of education for the department of medicine, and she recognized the problem created for medical trainees by the changes necessitated by the pandemic.
“The variability in caseloads and clinical exposure has given thrust to the move toward competency-based assessments rather than number- or time-based criteria for determining proficiency and graduation,” she wrote in an email interview. In addition, she noted the impact on medical meetings and the need to adapt. “Early on, before large regional and national conferences adapted to a virtual format, many were canceled altogether. Students, residents, and fellows expecting to have the opportunity to present their scholarly work were suddenly no longer able to do so. Understanding the importance of scholarly interaction, the virtual format of CHEST 2020 is designed with opportunities to present, interact with experts in the field, ask questions, network, and meet mentors.”
No return to ‘normal’
By April 2020, cases in the northeast continued to rise, particularly in the New York, New Jersey, and Connecticut region. “These states were essentially shut down in order to contain spread of the virus,” she said. “This was a real turning point because we realized that things were not going to return to ‘normal’ in the foreseeable future.” With the clinical experience essentially halted for medical students during this time, some medical schools allowed their senior students who met requirements to graduate early. “There were a lot of mixed feelings about this, recognizing that PPE [personal protective equipment] was still in short supply in many areas,” Dr. Murano said. “So, institutions took on these early graduates into roles in which they were not learners in particular, but rather medical workers. They were helping with informatics and technology, telehealth, virtual or telephone call follow-ups, and other tasks like this. There was a movement to virtual learning for the preclinical undergraduate learners, so classes were now online, recorded, or livestreamed.”
Early graduation, matching, and residencies
On April 3, the ACGME released a statement regarding graduating students early and appointing them early to the clinical learning environment. “They pointed out that institutions that were in emergency pandemic status lacked the ability to offer the comprehensive orientation and training in PPE and direct supervision required for new residents at the start of their residency,” Dr. Murano said. “Their opinion maintained that graduating medical students matriculate in their previously matched program, the National Resident Match Program start date, or other date that would be nationally determined to be the beginning of the 2020-2021 academic year.”
As May 2020 rolled around, the overriding feeling was uncertainty regarding when, if, and how medical schools were going to open in the early summer and fall. “There was also uncertainty about how graduating medical students were going to function in their new role as residents,” she said. “Same for the graduating residents. There were some who had signed contracts for jobs months before, and had them rescinded, and physicians were being furloughed due to financial hardships that institutions faced. There was also postponement of board certification exams, so people were uncertain about when they would become board certified.”
July 2020 ushered in what Dr. Murano characterized as “a whole new level of stress.” For medical students in particular, “we were entering the application season for residency positions,” she said. “Due to travel restrictions placed by various states and institutions, away rotations were limited or nonexistent. Application release dates through the Electronic Residency Application Service were moved to later in the year. The United States Medical Licensing Examination clinical skills exam was suspended, and there were modifications made for Education Commission for Foreign Medical Graduates requirements. Letters of recommendation were also going to be limited, so there had to be some degree of leniency within specialties to take a more holistic approach to review of applications for residencies.”
On the graduate medical education front, the ACGME sunsetted the initial stages and created two categories: nonemergency, which was formerly stages 1 and 2, and emergency, which was formerly stage 3. “All emergency stages are applied for and granted at 1-month intervals,” Dr. Murano said. Board certification exams were modified to accommodate either later exams or online formats, and specialties with oral examinations faced the task of potentially creating virtual oral exams.
Despite the challenges, Dr. Chandra has seen medical training programs respond with new ideas. “The flexibility and agile adaptability of the entire educational enterprise has been remarkable. The inherent uncertainty in a very dynamic and changing learning environment can be challenging. Recognizing this, many programs are creating additional ways to support the mental, emotional, physical, and financial health of students, residents, and fellows and all health care workers. The importance of this innovative response cannot be overstated.”
New learning formats
The pandemic forced Dr. Murano and other medical educators to consider unorthodox learning formats, and virtual learning took center stage. “Residency programs had shared national livestream conferences and grand rounds, and there were virtual curricula made for medical students as well as virtual simulation,” she said. “Telemedicine and telehealth really became important parts of education as well, as this may have been the only face-to-face contact that students and residents had with patients who had non–COVID-related complaints.”
To level the playing field for medical residents during this unprecedented time, a work group of the Coalition for Physician Accountability developed a set of recommendations that include limiting the number of letters of recommendation accepted, limiting the number of away rotations, and allowing alternative or less conventional letters of recommendation. “Keeping an open mind and taking a more holistic approach to applicants has really been needed during this time,” Dr. Murano said. “Virtual interview days have been agreed upon for all specialties. They’re safer, and they allow for students to virtually meet faculty and residents from distant programs that in the past would have been a deterrent due to distance and travel costs. This is not without its own downside, as it’s difficult to determine how well a student will fit into a program without [him or her] actually visiting the institution.”
Dr. Chandra agreed that virtual interviews are necessary but have inherent limitations. However, “we will all learn a lot, and very likely the future process will blend the benefits of both virtual and in-person interviews.”
‘We need to keep moving forward’
Dr. Murano concluded her presentation by noting that the COVID-19 pandemic has created opportunities for growth and innovation in medical education, “so we need to keep moving forward. I’ve heard many say that they can’t wait for things to go back to normal. But I think it’s important to go ahead to new and better ways of learning. We’re now thinking outside of the typical education model and are embracing technology and alternative means of education. We don’t know yet if this education is better, worse, or equivalent to traditional methods, but that will be determined and studied in months and years to come, so we’re certainly looking to the future.”
Dr. Murano and Dr. Chandra reported having no financial disclosures.
The COVID-19 pandemic has thrown a monkey wrench into the medical education landscape across the entire health care spectrum, disrupting the plans of medical students, residents, fellows, and program directors.
As cases of COVID-19 spread across the United States in early 2020, it became clear to training program directors that immediate action was required to meet the needs of medical learners. The challenges were unlike those surrounding the Ebola virus in 2014, “where we could more easily prevent students and trainees from exposure due to the fact that there were simply not significant numbers of cases in the United States,” Tiffany Murano, MD, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. Dr. Murano is professor of emergency medicine at Rutgers New Jersey Medical School, Newark, and president-elect of the Council of Residency Directors in Emergency Medicine. “COVID was a completely different scenario. We quickly realized that not only was personal protective equipment in short supply, but we also lacked the testing and tracking capabilities for potential exposures. Medical students and other supportive workers who were considered nonessential were removed from the clinical setting. This was after a trial of limiting who the students saw, essentially dampening the risk of exposure. But this proved to be flawed as COVID patients presented with symptoms that were unexpected.”
To complicate matters, she continued, many medical clinics either shut down, had limited access, or converted to telemedicine. Elective surgeries were canceled. This led to an overall pause in clinical medical student rotations and no direct patient care activities. As social distancing mandates were instituted, licensing examination testing centers were closed, and exams and on-campus activities were postponed.
Limiting trainee exposure
On the graduate medical education front, some training programs attempted to limit exposure of their trainees to persons under investigation for COVID-19. “As the number of COVID cases grew and encompassed most of what we were seeing in the hospital, it was obvious that residents had to play a vital part in the care of these patients,” said Dr. Murano, who is also a member of the American Council of Graduate Medical Education’s emergency review and recognition committee. “However, there was a consensus among all of the specialties that the procedures that posed the highest risk of exposure would be limited to the most senior or experienced trainees or professionals, and closely supervised by the faculty.”
ACGME activities such as accreditation site visits, clinical environment learning reviews, self-study, and resident and faculty surveys were suspended, postponed, or modified in some way, she said. The ACGME created stages of COVID status to guide sponsoring institutions to suspend learning curricula in order for patients to be cared for. Stage 1 was business as usual, “so there was no significant impact on patient care,” Dr. Murano said. “Stage 2 was increased but manageable clinical demand, while stage 3 was pandemic emergency status, where there were extraordinary circumstances where the clinical demand was so high and strenuous that the routine patient care and education really needed to be reconfigured in order to care for the patients.”
New requirements to manage training
The ACGME also implemented four requirements to manage training that were consistent among institutions, regardless of their COVID stage status. These included making sure that trainees continued to be held to work-hour limit requirements, ensuring adequate resources for training, ensuring that all residents had the appropriate level of supervision at all times, and allowing fellows to function in the core specialty in which they completed their residency training. “This was only possible if the fellows were ABMS [American Board of Medical Specialties] or AOA [American Osteopathic Association] board-eligible, or certified in their core specialty,” Dr. Murano said. “The fellows had to be appointed to the medical staff at the sponsoring institution, and their time spent on the core specialty service would be limited to 20% of their annual education time in any academic year.”
Mindful that there may have been trainees who required a 2-week quarantine period following exposure or potential exposure to COVID-19, some specialty boards showed leniency in residency time required to sit for the written exam. “Testing centers were being forced to close to observe social distancing requirements and heed sanitation recommendations, so exams were either canceled or postponed,” Dr. Murano said. “This posed a special concern for the board certification process, and those specialties with oral examinations had to make a heavy decision regarding whether or not they would allow these exams to take place. Naturally, travel among institutions was suspended or limited, or had quarantine requirements upon returning home from endemic areas. Conferences were either being canceled or converted to virtual formats.”
Subani Chandra, MD, FCCP, of the division of pulmonary, allergy, and critical care medicine at Columbia University, New York, is the internal medicine residency program director and the associate vice-chair of education for the department of medicine, and she recognized the problem created for medical trainees by the changes necessitated by the pandemic.
“The variability in caseloads and clinical exposure has given thrust to the move toward competency-based assessments rather than number- or time-based criteria for determining proficiency and graduation,” she wrote in an email interview. In addition, she noted the impact on medical meetings and the need to adapt. “Early on, before large regional and national conferences adapted to a virtual format, many were canceled altogether. Students, residents, and fellows expecting to have the opportunity to present their scholarly work were suddenly no longer able to do so. Understanding the importance of scholarly interaction, the virtual format of CHEST 2020 is designed with opportunities to present, interact with experts in the field, ask questions, network, and meet mentors.”
No return to ‘normal’
By April 2020, cases in the northeast continued to rise, particularly in the New York, New Jersey, and Connecticut region. “These states were essentially shut down in order to contain spread of the virus,” she said. “This was a real turning point because we realized that things were not going to return to ‘normal’ in the foreseeable future.” With the clinical experience essentially halted for medical students during this time, some medical schools allowed their senior students who met requirements to graduate early. “There were a lot of mixed feelings about this, recognizing that PPE [personal protective equipment] was still in short supply in many areas,” Dr. Murano said. “So, institutions took on these early graduates into roles in which they were not learners in particular, but rather medical workers. They were helping with informatics and technology, telehealth, virtual or telephone call follow-ups, and other tasks like this. There was a movement to virtual learning for the preclinical undergraduate learners, so classes were now online, recorded, or livestreamed.”
Early graduation, matching, and residencies
On April 3, the ACGME released a statement regarding graduating students early and appointing them early to the clinical learning environment. “They pointed out that institutions that were in emergency pandemic status lacked the ability to offer the comprehensive orientation and training in PPE and direct supervision required for new residents at the start of their residency,” Dr. Murano said. “Their opinion maintained that graduating medical students matriculate in their previously matched program, the National Resident Match Program start date, or other date that would be nationally determined to be the beginning of the 2020-2021 academic year.”
As May 2020 rolled around, the overriding feeling was uncertainty regarding when, if, and how medical schools were going to open in the early summer and fall. “There was also uncertainty about how graduating medical students were going to function in their new role as residents,” she said. “Same for the graduating residents. There were some who had signed contracts for jobs months before, and had them rescinded, and physicians were being furloughed due to financial hardships that institutions faced. There was also postponement of board certification exams, so people were uncertain about when they would become board certified.”
July 2020 ushered in what Dr. Murano characterized as “a whole new level of stress.” For medical students in particular, “we were entering the application season for residency positions,” she said. “Due to travel restrictions placed by various states and institutions, away rotations were limited or nonexistent. Application release dates through the Electronic Residency Application Service were moved to later in the year. The United States Medical Licensing Examination clinical skills exam was suspended, and there were modifications made for Education Commission for Foreign Medical Graduates requirements. Letters of recommendation were also going to be limited, so there had to be some degree of leniency within specialties to take a more holistic approach to review of applications for residencies.”
On the graduate medical education front, the ACGME sunsetted the initial stages and created two categories: nonemergency, which was formerly stages 1 and 2, and emergency, which was formerly stage 3. “All emergency stages are applied for and granted at 1-month intervals,” Dr. Murano said. Board certification exams were modified to accommodate either later exams or online formats, and specialties with oral examinations faced the task of potentially creating virtual oral exams.
Despite the challenges, Dr. Chandra has seen medical training programs respond with new ideas. “The flexibility and agile adaptability of the entire educational enterprise has been remarkable. The inherent uncertainty in a very dynamic and changing learning environment can be challenging. Recognizing this, many programs are creating additional ways to support the mental, emotional, physical, and financial health of students, residents, and fellows and all health care workers. The importance of this innovative response cannot be overstated.”
New learning formats
The pandemic forced Dr. Murano and other medical educators to consider unorthodox learning formats, and virtual learning took center stage. “Residency programs had shared national livestream conferences and grand rounds, and there were virtual curricula made for medical students as well as virtual simulation,” she said. “Telemedicine and telehealth really became important parts of education as well, as this may have been the only face-to-face contact that students and residents had with patients who had non–COVID-related complaints.”
To level the playing field for medical residents during this unprecedented time, a work group of the Coalition for Physician Accountability developed a set of recommendations that include limiting the number of letters of recommendation accepted, limiting the number of away rotations, and allowing alternative or less conventional letters of recommendation. “Keeping an open mind and taking a more holistic approach to applicants has really been needed during this time,” Dr. Murano said. “Virtual interview days have been agreed upon for all specialties. They’re safer, and they allow for students to virtually meet faculty and residents from distant programs that in the past would have been a deterrent due to distance and travel costs. This is not without its own downside, as it’s difficult to determine how well a student will fit into a program without [him or her] actually visiting the institution.”
Dr. Chandra agreed that virtual interviews are necessary but have inherent limitations. However, “we will all learn a lot, and very likely the future process will blend the benefits of both virtual and in-person interviews.”
‘We need to keep moving forward’
Dr. Murano concluded her presentation by noting that the COVID-19 pandemic has created opportunities for growth and innovation in medical education, “so we need to keep moving forward. I’ve heard many say that they can’t wait for things to go back to normal. But I think it’s important to go ahead to new and better ways of learning. We’re now thinking outside of the typical education model and are embracing technology and alternative means of education. We don’t know yet if this education is better, worse, or equivalent to traditional methods, but that will be determined and studied in months and years to come, so we’re certainly looking to the future.”
Dr. Murano and Dr. Chandra reported having no financial disclosures.
The COVID-19 pandemic has thrown a monkey wrench into the medical education landscape across the entire health care spectrum, disrupting the plans of medical students, residents, fellows, and program directors.
As cases of COVID-19 spread across the United States in early 2020, it became clear to training program directors that immediate action was required to meet the needs of medical learners. The challenges were unlike those surrounding the Ebola virus in 2014, “where we could more easily prevent students and trainees from exposure due to the fact that there were simply not significant numbers of cases in the United States,” Tiffany Murano, MD, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. Dr. Murano is professor of emergency medicine at Rutgers New Jersey Medical School, Newark, and president-elect of the Council of Residency Directors in Emergency Medicine. “COVID was a completely different scenario. We quickly realized that not only was personal protective equipment in short supply, but we also lacked the testing and tracking capabilities for potential exposures. Medical students and other supportive workers who were considered nonessential were removed from the clinical setting. This was after a trial of limiting who the students saw, essentially dampening the risk of exposure. But this proved to be flawed as COVID patients presented with symptoms that were unexpected.”
To complicate matters, she continued, many medical clinics either shut down, had limited access, or converted to telemedicine. Elective surgeries were canceled. This led to an overall pause in clinical medical student rotations and no direct patient care activities. As social distancing mandates were instituted, licensing examination testing centers were closed, and exams and on-campus activities were postponed.
Limiting trainee exposure
On the graduate medical education front, some training programs attempted to limit exposure of their trainees to persons under investigation for COVID-19. “As the number of COVID cases grew and encompassed most of what we were seeing in the hospital, it was obvious that residents had to play a vital part in the care of these patients,” said Dr. Murano, who is also a member of the American Council of Graduate Medical Education’s emergency review and recognition committee. “However, there was a consensus among all of the specialties that the procedures that posed the highest risk of exposure would be limited to the most senior or experienced trainees or professionals, and closely supervised by the faculty.”
ACGME activities such as accreditation site visits, clinical environment learning reviews, self-study, and resident and faculty surveys were suspended, postponed, or modified in some way, she said. The ACGME created stages of COVID status to guide sponsoring institutions to suspend learning curricula in order for patients to be cared for. Stage 1 was business as usual, “so there was no significant impact on patient care,” Dr. Murano said. “Stage 2 was increased but manageable clinical demand, while stage 3 was pandemic emergency status, where there were extraordinary circumstances where the clinical demand was so high and strenuous that the routine patient care and education really needed to be reconfigured in order to care for the patients.”
New requirements to manage training
The ACGME also implemented four requirements to manage training that were consistent among institutions, regardless of their COVID stage status. These included making sure that trainees continued to be held to work-hour limit requirements, ensuring adequate resources for training, ensuring that all residents had the appropriate level of supervision at all times, and allowing fellows to function in the core specialty in which they completed their residency training. “This was only possible if the fellows were ABMS [American Board of Medical Specialties] or AOA [American Osteopathic Association] board-eligible, or certified in their core specialty,” Dr. Murano said. “The fellows had to be appointed to the medical staff at the sponsoring institution, and their time spent on the core specialty service would be limited to 20% of their annual education time in any academic year.”
Mindful that there may have been trainees who required a 2-week quarantine period following exposure or potential exposure to COVID-19, some specialty boards showed leniency in residency time required to sit for the written exam. “Testing centers were being forced to close to observe social distancing requirements and heed sanitation recommendations, so exams were either canceled or postponed,” Dr. Murano said. “This posed a special concern for the board certification process, and those specialties with oral examinations had to make a heavy decision regarding whether or not they would allow these exams to take place. Naturally, travel among institutions was suspended or limited, or had quarantine requirements upon returning home from endemic areas. Conferences were either being canceled or converted to virtual formats.”
Subani Chandra, MD, FCCP, of the division of pulmonary, allergy, and critical care medicine at Columbia University, New York, is the internal medicine residency program director and the associate vice-chair of education for the department of medicine, and she recognized the problem created for medical trainees by the changes necessitated by the pandemic.
“The variability in caseloads and clinical exposure has given thrust to the move toward competency-based assessments rather than number- or time-based criteria for determining proficiency and graduation,” she wrote in an email interview. In addition, she noted the impact on medical meetings and the need to adapt. “Early on, before large regional and national conferences adapted to a virtual format, many were canceled altogether. Students, residents, and fellows expecting to have the opportunity to present their scholarly work were suddenly no longer able to do so. Understanding the importance of scholarly interaction, the virtual format of CHEST 2020 is designed with opportunities to present, interact with experts in the field, ask questions, network, and meet mentors.”
No return to ‘normal’
By April 2020, cases in the northeast continued to rise, particularly in the New York, New Jersey, and Connecticut region. “These states were essentially shut down in order to contain spread of the virus,” she said. “This was a real turning point because we realized that things were not going to return to ‘normal’ in the foreseeable future.” With the clinical experience essentially halted for medical students during this time, some medical schools allowed their senior students who met requirements to graduate early. “There were a lot of mixed feelings about this, recognizing that PPE [personal protective equipment] was still in short supply in many areas,” Dr. Murano said. “So, institutions took on these early graduates into roles in which they were not learners in particular, but rather medical workers. They were helping with informatics and technology, telehealth, virtual or telephone call follow-ups, and other tasks like this. There was a movement to virtual learning for the preclinical undergraduate learners, so classes were now online, recorded, or livestreamed.”
Early graduation, matching, and residencies
On April 3, the ACGME released a statement regarding graduating students early and appointing them early to the clinical learning environment. “They pointed out that institutions that were in emergency pandemic status lacked the ability to offer the comprehensive orientation and training in PPE and direct supervision required for new residents at the start of their residency,” Dr. Murano said. “Their opinion maintained that graduating medical students matriculate in their previously matched program, the National Resident Match Program start date, or other date that would be nationally determined to be the beginning of the 2020-2021 academic year.”
As May 2020 rolled around, the overriding feeling was uncertainty regarding when, if, and how medical schools were going to open in the early summer and fall. “There was also uncertainty about how graduating medical students were going to function in their new role as residents,” she said. “Same for the graduating residents. There were some who had signed contracts for jobs months before, and had them rescinded, and physicians were being furloughed due to financial hardships that institutions faced. There was also postponement of board certification exams, so people were uncertain about when they would become board certified.”
July 2020 ushered in what Dr. Murano characterized as “a whole new level of stress.” For medical students in particular, “we were entering the application season for residency positions,” she said. “Due to travel restrictions placed by various states and institutions, away rotations were limited or nonexistent. Application release dates through the Electronic Residency Application Service were moved to later in the year. The United States Medical Licensing Examination clinical skills exam was suspended, and there were modifications made for Education Commission for Foreign Medical Graduates requirements. Letters of recommendation were also going to be limited, so there had to be some degree of leniency within specialties to take a more holistic approach to review of applications for residencies.”
On the graduate medical education front, the ACGME sunsetted the initial stages and created two categories: nonemergency, which was formerly stages 1 and 2, and emergency, which was formerly stage 3. “All emergency stages are applied for and granted at 1-month intervals,” Dr. Murano said. Board certification exams were modified to accommodate either later exams or online formats, and specialties with oral examinations faced the task of potentially creating virtual oral exams.
Despite the challenges, Dr. Chandra has seen medical training programs respond with new ideas. “The flexibility and agile adaptability of the entire educational enterprise has been remarkable. The inherent uncertainty in a very dynamic and changing learning environment can be challenging. Recognizing this, many programs are creating additional ways to support the mental, emotional, physical, and financial health of students, residents, and fellows and all health care workers. The importance of this innovative response cannot be overstated.”
New learning formats
The pandemic forced Dr. Murano and other medical educators to consider unorthodox learning formats, and virtual learning took center stage. “Residency programs had shared national livestream conferences and grand rounds, and there were virtual curricula made for medical students as well as virtual simulation,” she said. “Telemedicine and telehealth really became important parts of education as well, as this may have been the only face-to-face contact that students and residents had with patients who had non–COVID-related complaints.”
To level the playing field for medical residents during this unprecedented time, a work group of the Coalition for Physician Accountability developed a set of recommendations that include limiting the number of letters of recommendation accepted, limiting the number of away rotations, and allowing alternative or less conventional letters of recommendation. “Keeping an open mind and taking a more holistic approach to applicants has really been needed during this time,” Dr. Murano said. “Virtual interview days have been agreed upon for all specialties. They’re safer, and they allow for students to virtually meet faculty and residents from distant programs that in the past would have been a deterrent due to distance and travel costs. This is not without its own downside, as it’s difficult to determine how well a student will fit into a program without [him or her] actually visiting the institution.”
Dr. Chandra agreed that virtual interviews are necessary but have inherent limitations. However, “we will all learn a lot, and very likely the future process will blend the benefits of both virtual and in-person interviews.”
‘We need to keep moving forward’
Dr. Murano concluded her presentation by noting that the COVID-19 pandemic has created opportunities for growth and innovation in medical education, “so we need to keep moving forward. I’ve heard many say that they can’t wait for things to go back to normal. But I think it’s important to go ahead to new and better ways of learning. We’re now thinking outside of the typical education model and are embracing technology and alternative means of education. We don’t know yet if this education is better, worse, or equivalent to traditional methods, but that will be determined and studied in months and years to come, so we’re certainly looking to the future.”
Dr. Murano and Dr. Chandra reported having no financial disclosures.
FROM AN SCCM VIRTUAL MEETING
Efforts to close the ‘AYA gap’ in lymphoma
In the 1970s, cancer survival was poor for young children and older adults in the United States, as shown by data published in the Journal of the National Cancer Institute.
Great progress has been made since the 1970s, but improvements in outcome have been less impressive for cancer patients aged 15-39 years, as shown by research published in Cancer.
Patients aged 15-39 years have been designated by the National Institutes of Health (NIH) as “adolescents and young adults (AYAs),” and the lag in survival benefit has been termed “the AYA gap.”
The AYA gap persists in lymphoma patients, and an expert panel recently outlined differences between lymphoma in AYAs and lymphoma in other age groups.
The experts spoke at a special session of the AACR Virtual Meeting: Advances in Malignant Lymphoma moderated by Somali M. Smith, MD, of the University of Chicago.
Factors that contribute to the AYA gap
About 89,000 AYAs are diagnosed with cancer each year in the United States, according to data from the National Cancer Institute (NCI). Lymphomas and thyroid cancer are the most common cancers among younger AYAs, aged 15-24 years.
In a report commissioned by the NIH in 2006, many factors contributing to the AYA gap were identified. Chief among them were:
- Limitations in access to care.
- Delayed diagnosis.
- Inconsistency in treatment and follow-up.
- Long-term toxicity (fertility, second malignancies, and cardiovascular disease).
These factors compromise health-related survival, even when cancer-specific survival is improved.
Panelist Kara Kelly, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., noted that there are additional unique challenges for AYAs with cancer. These include:
- Pubertal changes.
- Developmental transition to independence.
- Societal impediments such as insurance coverage and disparities in access to specialized centers.
- Psychosocial factors such as health literacy and adherence to treatment and follow-up.
Focusing on lymphoma specifically, Dr. Kelly noted that lymphoma biology differs across the age spectrum and by race and ethnicity. Both tumor and host factors require further study, she said.
Clinical trial access for AYAs
Dr. Kelly emphasized that, unfortunately, clinical research participation is low among AYAs. A major impediment is that adult clinical trials historically required participants to be at least 18 years old.
In addition, there has not been a focused effort to educate AYAs about regulatory safeguards to ensure safety and the promise of enhanced benefit to them in NCI Cancer Trials Network (NCTN) trials. As a result, the refusal rate is high.
A multi-stakeholder workshop, convened in May 2016 by the American Society of Clinical Oncology and Friends of Cancer Research, outlined opportunities for expanding trial eligibility to include children younger than 18 years in first-in-human and other adult cancer clinical trials, enhancing their access to new agents, without compromising safety.
Recently, collaborative efforts between the adult and children’s NCTN research groups have included AYAs in studies addressing cancers that span the age spectrum, including lymphoma.
However, as Dr. Kelly noted, there are differences in AYA lymphoid malignancy types with a transition from more pediatric to more adult types.
Hodgkin lymphoma and primary mediastinal B-cell lymphoma
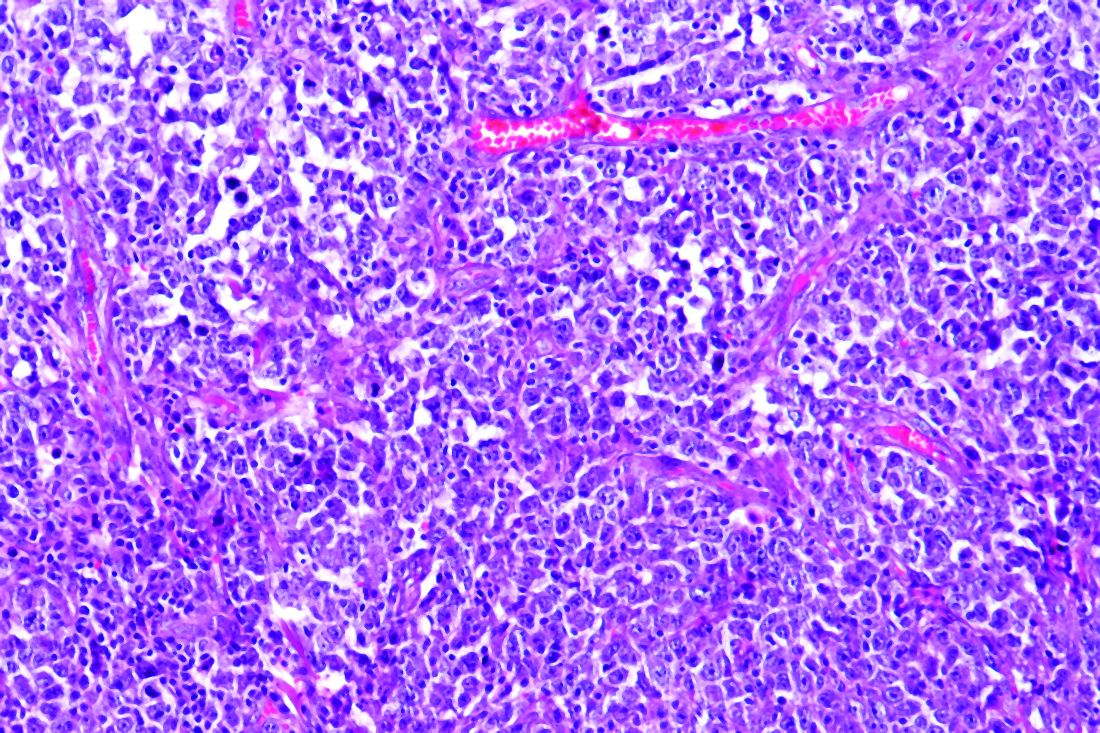
Panelist Lisa G. Roth, MD, of Weill Cornell Medicine, New York, reviewed the genomic landscape of Hodgkin lymphoma (HL) and primary mediastinal B-cell lymphoma (PMBCL).
Dr. Roth explained that both HL and PMBCL are derived from thymic B cells, predominantly affect the mediastinum, and are CD30-positive lymphomas. Both are characterized by upregulation of JAK/STAT and NF-kappaB as well as overexpression of PD-L1.
Dr. Roth noted that HL is challenging to sequence by standard methods because Reed Sternberg (HRS) cells represent less than 1% of the cellular infiltrate. Recurrently mutated genes in HL cluster by histologic subtype.
Whole-exome sequencing of HRS cells show loss of beta-2 microglobulin and MHC-1 expression, HLA-B, NF-kappaB signaling, and JAK-STAT signaling, according to data published in Blood Advances in 2019.
Dr. Roth’s lab performed immunohistochemistry on tissue microarrays in 145 cases of HL (unpublished data). Results showed that loss of beta-2 microglobulin is more common in younger HL patients. For other alterations, there were too few cases to know.
Dr. Roth’s lab is a member of a pediatric/AYA HL sequencing multi-institutional consortium that has been able to extract DNA and RNA from samples submitted for whole-exome sequencing. The consortium’s goal is to shed light on implications of other genomic alterations that may differ by age in HL patients.
Dr. Roth cited research showing that PMBCL shares molecular alterations similar to those of HL. Alterations in PMBCL suggest dysregulated cellular signaling and immune evasion mechanisms (e.g., deletions in MHC type 1 and 2, beta-2 microglobulin, JAK-STAT, and NF-kappaB mutations) that provide opportunities to study novel agents, according to data published in Blood in 2019.
By early 2021, the S1826 and ANHL1931 studies, which have no age restriction, will be available to AYA lymphoma patients with HL and PMBCL, respectively, Dr. Roth said.
Follicular lymphoma: Clinical features by age
Panelist Abner Louissaint Jr, MD, PhD, of Massachusetts General Hospital in Boston, discussed age-related differences in follicular lymphoma (FL).
He noted that FL typically presents at an advanced stage, with low- or high-grade histology. It is increasingly common in adults in their 50s and 60s, representing 20% of all lymphomas. FL is rare in children and AYAs.
Dr. Louissaint explained that the typical flow cytometric findings in FL are BCL2 translocations, occurring in up to 85%-90% of low-grade and 50% of high-grade cases. The t(14;18)(q32;q21) translocation juxtaposes BCL2 on 18q21 to regulatory sequences and enhances the expression of elements of the Ig heavy chain.
Malignant cells in FL patients express CD20, CD10, CD21, and BCL2 (in contrast to normal germinal centers) and overexpress BCL6 (in contrast to normal follicles), Dr. Louissaint noted. He said the Ki-67 proliferative index of the malignant cells is typically low.
Pediatric-type FL is rare, but case series show clinical, pathologic, and molecular features that are distinctive from adult FL, Dr. Louissaint explained.
He then discussed the features of pediatric-type FL in multiple domains. In the clinical domain, there is a male predilection, and stage tends to be low. There is frequent involvement of nodes of the head and neck region and rare involvement of internal lymph node chains.
Pathologically, the malignant cells appear high grade, with architectural effacement, expansile follicular pattern, large lymphocyte size, and an elevated proliferation index. In contrast to adult FL, malignant cells in pediatric-type FL lack aberrant BCL2 expression.
Most importantly, for pediatric-type FL, the prognosis is excellent with durable remissions after surgical excision, Dr. Louissaint said.
Follicular lymphoma: Molecular features by age
Because of the excellent prognosis in pediatric-type FL, it is important to assess whether young adults with FL have adult-type or pediatric-type lesions, Dr. Louissaint said.
He cited many studies showing differences in adult and pediatric-type FL. In adult FL, the mutational landscape is characterized by frequent chromatin-modifying mutations in genes such as CREBBP, KM22D, and EP300.
In contrast, in pediatric-type FL, there are frequent activating MAPK pathway mutations, including mutations in the negative regulatory domain of MAP2K1. These mutations are not seen in adult FL.
Dr. Louissaint noted that there may be mutations in epigenetic modifiers (CREBBP, TNFRSF14) in both adult and pediatric-type FL. However, CREBBP is very unusual in pediatric-type FL and common in adult FL. This suggests the alterations in pediatric-type FL do not simply represent an early stage of the same disease as adult FL.
Despite a high proliferating fraction and absence of BCL2/BCL6/IRF4 rearrangements in pediatric-type FL, the presence of these features was associated with dramatic difference in progression-free survival, according to research published in Blood in 2012.
A distinct entity
In 2016, the World Health Organization recognized pediatric-type FL as a distinct entity, with the following diagnostic criteria (published in Blood):
- At least partial effacement of nodal architecture, expansile follicles, intermediate-size blastoid cells, and no component of diffuse large B-cell lymphoma.
- Immunohistochemistry showing BCL6 positivity, BCL2 negativity or weak positivity, and a high proliferative fraction.
- Genomic studies showing no BCL2 amplification.
- Clinical features of nodal disease in the head and neck region, early clinical stage, age younger than 40 years, typically in a male with no internal nodes involved.
When FL occurs in AYAs, the diagnostic findings of pediatric-type FL suggest the patient will do well with conservative management (e.g., excision alone), Dr. Louissaint noted.
Two sizes do not fit all
The strategies that have improved cancer outcomes since the 1970s for children and older adults have been much less successful for AYAs with cancer.
As an oncologic community, we should not allow the AYA gap to persist. As always, the solutions are likely to involve focused clinical research, education, and communication. Effort will need to be targeted specifically to the AYA population.
Since health-related mortality is high even when cancer-specific outcomes improve, adopting and maintaining a healthy lifestyle must be a key part of the discussion with these young patients.
The biologic differences associated with AYA lymphomas demand participation in clinical trials.
Oncologists should vigorously support removing impediments to the participation of AYAs in prospective clinical trials, stratified (but unrestricted) by age, with careful analysis of patient-reported outcomes, late adverse effects, and biospecimen collection.
As Dr. Kelly noted in the question-and-answer period, the Children’s Oncology Group has an existing biobank of paraffin-embedded tumor samples, DNA from lymphoma specimens, plasma, and sera with clinically annotated data that can be given to investigators upon request and justification.
Going beyond eligibility for clinical trials
Unfortunately, we will likely find that broadening eligibility criteria is the “low-hanging fruit.” There are protocol-, patient-, and physician-related obstacles, according to a review published in Cancer in 2019.
Patient-related obstacles include fear of toxicity, uncertainty about placebos, a steep learning curve for health literacy, insurance-related impediments, and other access-related issues.
Discussions will need to be tailored to the AYA population. Frank, early conversations about fertility, sexuality, financial hardship, career advancement, work-life balance, and cognitive risks may not only facilitate treatment planning but also encourage the trust that is essential for patients to enroll in trials.
The investment in time, multidisciplinary staff and physician involvement, and potential delays in treatment initiation may be painful and inconvenient, but the benefits for long-term health outcomes and personal-professional relationships will be gratifying beyond measure.
Dr. Smith disclosed relationships with Genentech/Roche, Celgene, TGTX, Karyopharm, Janssen, and Bantem. Dr. Roth disclosed relationships with Janssen, ADC Therapeutics, and Celgene. Dr. Kelly and Dr. Louissaint had no financial relationships to disclose.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
In the 1970s, cancer survival was poor for young children and older adults in the United States, as shown by data published in the Journal of the National Cancer Institute.
Great progress has been made since the 1970s, but improvements in outcome have been less impressive for cancer patients aged 15-39 years, as shown by research published in Cancer.
Patients aged 15-39 years have been designated by the National Institutes of Health (NIH) as “adolescents and young adults (AYAs),” and the lag in survival benefit has been termed “the AYA gap.”
The AYA gap persists in lymphoma patients, and an expert panel recently outlined differences between lymphoma in AYAs and lymphoma in other age groups.
The experts spoke at a special session of the AACR Virtual Meeting: Advances in Malignant Lymphoma moderated by Somali M. Smith, MD, of the University of Chicago.
Factors that contribute to the AYA gap
About 89,000 AYAs are diagnosed with cancer each year in the United States, according to data from the National Cancer Institute (NCI). Lymphomas and thyroid cancer are the most common cancers among younger AYAs, aged 15-24 years.
In a report commissioned by the NIH in 2006, many factors contributing to the AYA gap were identified. Chief among them were:
- Limitations in access to care.
- Delayed diagnosis.
- Inconsistency in treatment and follow-up.
- Long-term toxicity (fertility, second malignancies, and cardiovascular disease).
These factors compromise health-related survival, even when cancer-specific survival is improved.
Panelist Kara Kelly, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., noted that there are additional unique challenges for AYAs with cancer. These include:
- Pubertal changes.
- Developmental transition to independence.
- Societal impediments such as insurance coverage and disparities in access to specialized centers.
- Psychosocial factors such as health literacy and adherence to treatment and follow-up.
Focusing on lymphoma specifically, Dr. Kelly noted that lymphoma biology differs across the age spectrum and by race and ethnicity. Both tumor and host factors require further study, she said.
Clinical trial access for AYAs
Dr. Kelly emphasized that, unfortunately, clinical research participation is low among AYAs. A major impediment is that adult clinical trials historically required participants to be at least 18 years old.
In addition, there has not been a focused effort to educate AYAs about regulatory safeguards to ensure safety and the promise of enhanced benefit to them in NCI Cancer Trials Network (NCTN) trials. As a result, the refusal rate is high.
A multi-stakeholder workshop, convened in May 2016 by the American Society of Clinical Oncology and Friends of Cancer Research, outlined opportunities for expanding trial eligibility to include children younger than 18 years in first-in-human and other adult cancer clinical trials, enhancing their access to new agents, without compromising safety.
Recently, collaborative efforts between the adult and children’s NCTN research groups have included AYAs in studies addressing cancers that span the age spectrum, including lymphoma.
However, as Dr. Kelly noted, there are differences in AYA lymphoid malignancy types with a transition from more pediatric to more adult types.
Hodgkin lymphoma and primary mediastinal B-cell lymphoma
Panelist Lisa G. Roth, MD, of Weill Cornell Medicine, New York, reviewed the genomic landscape of Hodgkin lymphoma (HL) and primary mediastinal B-cell lymphoma (PMBCL).
Dr. Roth explained that both HL and PMBCL are derived from thymic B cells, predominantly affect the mediastinum, and are CD30-positive lymphomas. Both are characterized by upregulation of JAK/STAT and NF-kappaB as well as overexpression of PD-L1.
Dr. Roth noted that HL is challenging to sequence by standard methods because Reed Sternberg (HRS) cells represent less than 1% of the cellular infiltrate. Recurrently mutated genes in HL cluster by histologic subtype.
Whole-exome sequencing of HRS cells show loss of beta-2 microglobulin and MHC-1 expression, HLA-B, NF-kappaB signaling, and JAK-STAT signaling, according to data published in Blood Advances in 2019.
Dr. Roth’s lab performed immunohistochemistry on tissue microarrays in 145 cases of HL (unpublished data). Results showed that loss of beta-2 microglobulin is more common in younger HL patients. For other alterations, there were too few cases to know.
Dr. Roth’s lab is a member of a pediatric/AYA HL sequencing multi-institutional consortium that has been able to extract DNA and RNA from samples submitted for whole-exome sequencing. The consortium’s goal is to shed light on implications of other genomic alterations that may differ by age in HL patients.
Dr. Roth cited research showing that PMBCL shares molecular alterations similar to those of HL. Alterations in PMBCL suggest dysregulated cellular signaling and immune evasion mechanisms (e.g., deletions in MHC type 1 and 2, beta-2 microglobulin, JAK-STAT, and NF-kappaB mutations) that provide opportunities to study novel agents, according to data published in Blood in 2019.
By early 2021, the S1826 and ANHL1931 studies, which have no age restriction, will be available to AYA lymphoma patients with HL and PMBCL, respectively, Dr. Roth said.
Follicular lymphoma: Clinical features by age
Panelist Abner Louissaint Jr, MD, PhD, of Massachusetts General Hospital in Boston, discussed age-related differences in follicular lymphoma (FL).
He noted that FL typically presents at an advanced stage, with low- or high-grade histology. It is increasingly common in adults in their 50s and 60s, representing 20% of all lymphomas. FL is rare in children and AYAs.
Dr. Louissaint explained that the typical flow cytometric findings in FL are BCL2 translocations, occurring in up to 85%-90% of low-grade and 50% of high-grade cases. The t(14;18)(q32;q21) translocation juxtaposes BCL2 on 18q21 to regulatory sequences and enhances the expression of elements of the Ig heavy chain.
Malignant cells in FL patients express CD20, CD10, CD21, and BCL2 (in contrast to normal germinal centers) and overexpress BCL6 (in contrast to normal follicles), Dr. Louissaint noted. He said the Ki-67 proliferative index of the malignant cells is typically low.
Pediatric-type FL is rare, but case series show clinical, pathologic, and molecular features that are distinctive from adult FL, Dr. Louissaint explained.
He then discussed the features of pediatric-type FL in multiple domains. In the clinical domain, there is a male predilection, and stage tends to be low. There is frequent involvement of nodes of the head and neck region and rare involvement of internal lymph node chains.
Pathologically, the malignant cells appear high grade, with architectural effacement, expansile follicular pattern, large lymphocyte size, and an elevated proliferation index. In contrast to adult FL, malignant cells in pediatric-type FL lack aberrant BCL2 expression.
Most importantly, for pediatric-type FL, the prognosis is excellent with durable remissions after surgical excision, Dr. Louissaint said.
Follicular lymphoma: Molecular features by age
Because of the excellent prognosis in pediatric-type FL, it is important to assess whether young adults with FL have adult-type or pediatric-type lesions, Dr. Louissaint said.
He cited many studies showing differences in adult and pediatric-type FL. In adult FL, the mutational landscape is characterized by frequent chromatin-modifying mutations in genes such as CREBBP, KM22D, and EP300.
In contrast, in pediatric-type FL, there are frequent activating MAPK pathway mutations, including mutations in the negative regulatory domain of MAP2K1. These mutations are not seen in adult FL.
Dr. Louissaint noted that there may be mutations in epigenetic modifiers (CREBBP, TNFRSF14) in both adult and pediatric-type FL. However, CREBBP is very unusual in pediatric-type FL and common in adult FL. This suggests the alterations in pediatric-type FL do not simply represent an early stage of the same disease as adult FL.
Despite a high proliferating fraction and absence of BCL2/BCL6/IRF4 rearrangements in pediatric-type FL, the presence of these features was associated with dramatic difference in progression-free survival, according to research published in Blood in 2012.
A distinct entity
In 2016, the World Health Organization recognized pediatric-type FL as a distinct entity, with the following diagnostic criteria (published in Blood):
- At least partial effacement of nodal architecture, expansile follicles, intermediate-size blastoid cells, and no component of diffuse large B-cell lymphoma.
- Immunohistochemistry showing BCL6 positivity, BCL2 negativity or weak positivity, and a high proliferative fraction.
- Genomic studies showing no BCL2 amplification.
- Clinical features of nodal disease in the head and neck region, early clinical stage, age younger than 40 years, typically in a male with no internal nodes involved.
When FL occurs in AYAs, the diagnostic findings of pediatric-type FL suggest the patient will do well with conservative management (e.g., excision alone), Dr. Louissaint noted.
Two sizes do not fit all
The strategies that have improved cancer outcomes since the 1970s for children and older adults have been much less successful for AYAs with cancer.
As an oncologic community, we should not allow the AYA gap to persist. As always, the solutions are likely to involve focused clinical research, education, and communication. Effort will need to be targeted specifically to the AYA population.
Since health-related mortality is high even when cancer-specific outcomes improve, adopting and maintaining a healthy lifestyle must be a key part of the discussion with these young patients.
The biologic differences associated with AYA lymphomas demand participation in clinical trials.
Oncologists should vigorously support removing impediments to the participation of AYAs in prospective clinical trials, stratified (but unrestricted) by age, with careful analysis of patient-reported outcomes, late adverse effects, and biospecimen collection.
As Dr. Kelly noted in the question-and-answer period, the Children’s Oncology Group has an existing biobank of paraffin-embedded tumor samples, DNA from lymphoma specimens, plasma, and sera with clinically annotated data that can be given to investigators upon request and justification.
Going beyond eligibility for clinical trials
Unfortunately, we will likely find that broadening eligibility criteria is the “low-hanging fruit.” There are protocol-, patient-, and physician-related obstacles, according to a review published in Cancer in 2019.
Patient-related obstacles include fear of toxicity, uncertainty about placebos, a steep learning curve for health literacy, insurance-related impediments, and other access-related issues.
Discussions will need to be tailored to the AYA population. Frank, early conversations about fertility, sexuality, financial hardship, career advancement, work-life balance, and cognitive risks may not only facilitate treatment planning but also encourage the trust that is essential for patients to enroll in trials.
The investment in time, multidisciplinary staff and physician involvement, and potential delays in treatment initiation may be painful and inconvenient, but the benefits for long-term health outcomes and personal-professional relationships will be gratifying beyond measure.
Dr. Smith disclosed relationships with Genentech/Roche, Celgene, TGTX, Karyopharm, Janssen, and Bantem. Dr. Roth disclosed relationships with Janssen, ADC Therapeutics, and Celgene. Dr. Kelly and Dr. Louissaint had no financial relationships to disclose.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
In the 1970s, cancer survival was poor for young children and older adults in the United States, as shown by data published in the Journal of the National Cancer Institute.
Great progress has been made since the 1970s, but improvements in outcome have been less impressive for cancer patients aged 15-39 years, as shown by research published in Cancer.
Patients aged 15-39 years have been designated by the National Institutes of Health (NIH) as “adolescents and young adults (AYAs),” and the lag in survival benefit has been termed “the AYA gap.”
The AYA gap persists in lymphoma patients, and an expert panel recently outlined differences between lymphoma in AYAs and lymphoma in other age groups.
The experts spoke at a special session of the AACR Virtual Meeting: Advances in Malignant Lymphoma moderated by Somali M. Smith, MD, of the University of Chicago.
Factors that contribute to the AYA gap
About 89,000 AYAs are diagnosed with cancer each year in the United States, according to data from the National Cancer Institute (NCI). Lymphomas and thyroid cancer are the most common cancers among younger AYAs, aged 15-24 years.
In a report commissioned by the NIH in 2006, many factors contributing to the AYA gap were identified. Chief among them were:
- Limitations in access to care.
- Delayed diagnosis.
- Inconsistency in treatment and follow-up.
- Long-term toxicity (fertility, second malignancies, and cardiovascular disease).
These factors compromise health-related survival, even when cancer-specific survival is improved.
Panelist Kara Kelly, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., noted that there are additional unique challenges for AYAs with cancer. These include:
- Pubertal changes.
- Developmental transition to independence.
- Societal impediments such as insurance coverage and disparities in access to specialized centers.
- Psychosocial factors such as health literacy and adherence to treatment and follow-up.
Focusing on lymphoma specifically, Dr. Kelly noted that lymphoma biology differs across the age spectrum and by race and ethnicity. Both tumor and host factors require further study, she said.
Clinical trial access for AYAs
Dr. Kelly emphasized that, unfortunately, clinical research participation is low among AYAs. A major impediment is that adult clinical trials historically required participants to be at least 18 years old.
In addition, there has not been a focused effort to educate AYAs about regulatory safeguards to ensure safety and the promise of enhanced benefit to them in NCI Cancer Trials Network (NCTN) trials. As a result, the refusal rate is high.
A multi-stakeholder workshop, convened in May 2016 by the American Society of Clinical Oncology and Friends of Cancer Research, outlined opportunities for expanding trial eligibility to include children younger than 18 years in first-in-human and other adult cancer clinical trials, enhancing their access to new agents, without compromising safety.
Recently, collaborative efforts between the adult and children’s NCTN research groups have included AYAs in studies addressing cancers that span the age spectrum, including lymphoma.
However, as Dr. Kelly noted, there are differences in AYA lymphoid malignancy types with a transition from more pediatric to more adult types.
Hodgkin lymphoma and primary mediastinal B-cell lymphoma
Panelist Lisa G. Roth, MD, of Weill Cornell Medicine, New York, reviewed the genomic landscape of Hodgkin lymphoma (HL) and primary mediastinal B-cell lymphoma (PMBCL).
Dr. Roth explained that both HL and PMBCL are derived from thymic B cells, predominantly affect the mediastinum, and are CD30-positive lymphomas. Both are characterized by upregulation of JAK/STAT and NF-kappaB as well as overexpression of PD-L1.
Dr. Roth noted that HL is challenging to sequence by standard methods because Reed Sternberg (HRS) cells represent less than 1% of the cellular infiltrate. Recurrently mutated genes in HL cluster by histologic subtype.
Whole-exome sequencing of HRS cells show loss of beta-2 microglobulin and MHC-1 expression, HLA-B, NF-kappaB signaling, and JAK-STAT signaling, according to data published in Blood Advances in 2019.
Dr. Roth’s lab performed immunohistochemistry on tissue microarrays in 145 cases of HL (unpublished data). Results showed that loss of beta-2 microglobulin is more common in younger HL patients. For other alterations, there were too few cases to know.
Dr. Roth’s lab is a member of a pediatric/AYA HL sequencing multi-institutional consortium that has been able to extract DNA and RNA from samples submitted for whole-exome sequencing. The consortium’s goal is to shed light on implications of other genomic alterations that may differ by age in HL patients.
Dr. Roth cited research showing that PMBCL shares molecular alterations similar to those of HL. Alterations in PMBCL suggest dysregulated cellular signaling and immune evasion mechanisms (e.g., deletions in MHC type 1 and 2, beta-2 microglobulin, JAK-STAT, and NF-kappaB mutations) that provide opportunities to study novel agents, according to data published in Blood in 2019.
By early 2021, the S1826 and ANHL1931 studies, which have no age restriction, will be available to AYA lymphoma patients with HL and PMBCL, respectively, Dr. Roth said.
Follicular lymphoma: Clinical features by age
Panelist Abner Louissaint Jr, MD, PhD, of Massachusetts General Hospital in Boston, discussed age-related differences in follicular lymphoma (FL).
He noted that FL typically presents at an advanced stage, with low- or high-grade histology. It is increasingly common in adults in their 50s and 60s, representing 20% of all lymphomas. FL is rare in children and AYAs.
Dr. Louissaint explained that the typical flow cytometric findings in FL are BCL2 translocations, occurring in up to 85%-90% of low-grade and 50% of high-grade cases. The t(14;18)(q32;q21) translocation juxtaposes BCL2 on 18q21 to regulatory sequences and enhances the expression of elements of the Ig heavy chain.
Malignant cells in FL patients express CD20, CD10, CD21, and BCL2 (in contrast to normal germinal centers) and overexpress BCL6 (in contrast to normal follicles), Dr. Louissaint noted. He said the Ki-67 proliferative index of the malignant cells is typically low.
Pediatric-type FL is rare, but case series show clinical, pathologic, and molecular features that are distinctive from adult FL, Dr. Louissaint explained.
He then discussed the features of pediatric-type FL in multiple domains. In the clinical domain, there is a male predilection, and stage tends to be low. There is frequent involvement of nodes of the head and neck region and rare involvement of internal lymph node chains.
Pathologically, the malignant cells appear high grade, with architectural effacement, expansile follicular pattern, large lymphocyte size, and an elevated proliferation index. In contrast to adult FL, malignant cells in pediatric-type FL lack aberrant BCL2 expression.
Most importantly, for pediatric-type FL, the prognosis is excellent with durable remissions after surgical excision, Dr. Louissaint said.
Follicular lymphoma: Molecular features by age
Because of the excellent prognosis in pediatric-type FL, it is important to assess whether young adults with FL have adult-type or pediatric-type lesions, Dr. Louissaint said.
He cited many studies showing differences in adult and pediatric-type FL. In adult FL, the mutational landscape is characterized by frequent chromatin-modifying mutations in genes such as CREBBP, KM22D, and EP300.
In contrast, in pediatric-type FL, there are frequent activating MAPK pathway mutations, including mutations in the negative regulatory domain of MAP2K1. These mutations are not seen in adult FL.
Dr. Louissaint noted that there may be mutations in epigenetic modifiers (CREBBP, TNFRSF14) in both adult and pediatric-type FL. However, CREBBP is very unusual in pediatric-type FL and common in adult FL. This suggests the alterations in pediatric-type FL do not simply represent an early stage of the same disease as adult FL.
Despite a high proliferating fraction and absence of BCL2/BCL6/IRF4 rearrangements in pediatric-type FL, the presence of these features was associated with dramatic difference in progression-free survival, according to research published in Blood in 2012.
A distinct entity
In 2016, the World Health Organization recognized pediatric-type FL as a distinct entity, with the following diagnostic criteria (published in Blood):
- At least partial effacement of nodal architecture, expansile follicles, intermediate-size blastoid cells, and no component of diffuse large B-cell lymphoma.
- Immunohistochemistry showing BCL6 positivity, BCL2 negativity or weak positivity, and a high proliferative fraction.
- Genomic studies showing no BCL2 amplification.
- Clinical features of nodal disease in the head and neck region, early clinical stage, age younger than 40 years, typically in a male with no internal nodes involved.
When FL occurs in AYAs, the diagnostic findings of pediatric-type FL suggest the patient will do well with conservative management (e.g., excision alone), Dr. Louissaint noted.
Two sizes do not fit all
The strategies that have improved cancer outcomes since the 1970s for children and older adults have been much less successful for AYAs with cancer.
As an oncologic community, we should not allow the AYA gap to persist. As always, the solutions are likely to involve focused clinical research, education, and communication. Effort will need to be targeted specifically to the AYA population.
Since health-related mortality is high even when cancer-specific outcomes improve, adopting and maintaining a healthy lifestyle must be a key part of the discussion with these young patients.
The biologic differences associated with AYA lymphomas demand participation in clinical trials.
Oncologists should vigorously support removing impediments to the participation of AYAs in prospective clinical trials, stratified (but unrestricted) by age, with careful analysis of patient-reported outcomes, late adverse effects, and biospecimen collection.
As Dr. Kelly noted in the question-and-answer period, the Children’s Oncology Group has an existing biobank of paraffin-embedded tumor samples, DNA from lymphoma specimens, plasma, and sera with clinically annotated data that can be given to investigators upon request and justification.
Going beyond eligibility for clinical trials
Unfortunately, we will likely find that broadening eligibility criteria is the “low-hanging fruit.” There are protocol-, patient-, and physician-related obstacles, according to a review published in Cancer in 2019.
Patient-related obstacles include fear of toxicity, uncertainty about placebos, a steep learning curve for health literacy, insurance-related impediments, and other access-related issues.
Discussions will need to be tailored to the AYA population. Frank, early conversations about fertility, sexuality, financial hardship, career advancement, work-life balance, and cognitive risks may not only facilitate treatment planning but also encourage the trust that is essential for patients to enroll in trials.
The investment in time, multidisciplinary staff and physician involvement, and potential delays in treatment initiation may be painful and inconvenient, but the benefits for long-term health outcomes and personal-professional relationships will be gratifying beyond measure.
Dr. Smith disclosed relationships with Genentech/Roche, Celgene, TGTX, Karyopharm, Janssen, and Bantem. Dr. Roth disclosed relationships with Janssen, ADC Therapeutics, and Celgene. Dr. Kelly and Dr. Louissaint had no financial relationships to disclose.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FROM AACR ADVANCES IN MALIGNANT LYMPHOMA 2020
Cancer researchers cross over to COVID-19 clinical trials
When the first reports emerged of “cytokine storm” in patients with severe COVID-19, all eyes turned to cancer research. Oncologists have years of experience reigning in “cytokine release syndrome” (CRS) in patients treated with chimeric antigen receptor (CAR) therapies for advanced blood cancers.
There was hope that drugs used to quell CRS in patients with cancer would be effective in patients with severe COVID. But the promise of a quick fix with oncology medications has yet to be fully realized.
Part of the problem is that the two conditions, while analogous, are “not the same,” said Nirali Shah, MD, head of the hematologic malignancies section in the pediatric oncology branch at the National Cancer Institute.
“You have to understand the underlying pathophysiology, what triggers the inflammation,” Dr. Shah said.
CAR T–related CRS is caused by activated T cells in patients with cancer who often do not have an infection, she explained. In contrast, cytokine storm in COVID-19 is triggered by a viral pathogen that can drive “out of control” inflammation. These differences may explain why drugs work in the first instance, but not in the second, she added. Drugs that inhibit interleukin-6 (such as tocilizumab, sarilumab, and siltuximab) are used with great success to dampen down the CRS in patients receiving CAR therapy for blood cancers. And although trials of these agents in patients with COVID are still ongoing, initial results are disappointing.
The first global, phase 3 randomized controlled trial of tocilizumab in severe COVID-19 failed to meet its primary endpoint of improved clinical status, and it did not meet its secondary endpoint of improved mortality at week 4.
In its recent recommendations, the National Institutes of Health noted a lack of data to support the efficacy of IL-6 inhibitors in COVID-19, and recommended against their use, except as part of a clinical trial.
Trimming the tree vs. cutting it down
As researchers have begun to decode the immune process underlying severe COVID-19, they have turned to other cancer drugs to tame cytokine storm.
Louis Staudt, MD, PhD, and Wyndham Wilson, MD, PhD, both at the NCI, think that cytokine storm in COVID-19 is driven by macrophages, which trigger release of multiple cytokines.
For years, the pair have been studying lymphoid tumors. Dr. Staudt is chief of the lymphoid malignancies branch at the NCI, and Wilson is head of the lymphoma therapeutics section. In past work, Dr. Staudt discovered that inhibiting an enzyme called bruton tyrosine kinase (BTK) dampens macrophage function.
When the pandemic began, Dr. Staudt and Dr. Wilson realized that singling out just one cytokine like IL-6 may not be enough. They thought that a more effective approach may be to target macrophages with a BTK inhibitor called acalabrutinib (Calquence), which would inhibit multiple cytokines at the same time.
Dr. Staudt likens the immune response to a tree, with the macrophages composing the tree trunk and the limbs made up of individual cytokines.
“Targeting macrophages is getting at the trunk of the problem,” he said. “You’re only cutting off the limbs with tocilizumab.”
In just 3 days, Dr. Staudt and Dr. Wilson went from concept to approval to launching a prospective, observational study. The study took place at five centers in the US, and included 19 patients hospitalized with COVID-19; the results were published in Science Immunology. Over a treatment course of 14 days, the majority of patients treated off-label with acalabrutinib improved, some within 24 hours. Eight of 11 patients on supplemental oxygen were discharged on room air. Four of eight patients on ventilators were extubated, with two of these discharged on room air. Two patients on ventilators died. No discernible toxicity was noted.
Analyses also showed increased BTK activity and elevated IL-6 levels in monocytes – precursors of macrophages – in patients with severe COVID-19, compared with healthy volunteers.
“We showed that the target of acalabrutinib was active in the immune cells of patients with severe COVID-19,” Dr. Staudt said. “So we have the target. We have the drug to hit the target. And we have an apparent clinical benefit.”
Those three things were compelling enough to launch the CALAVI phase 2 trial, an open-label, randomized, controlled trial, sponsored by AstraZeneca and the NCI, that is being conducted in the United States and internationally. It is testing acalabrutinib with best supportive care versus BSC alone in people hospitalized with COVID-19. The trial is scheduled to be completed on Nov. 26.
Preliminary insights from this trial are expected soon. “These are not insights that we will likely publish, but they are important insights that will lead to the launch of a definitive double-blind, randomized, phase 3 trial, which we hope to launch in the next month or so,” Dr. Wilson said.
Targeting inflammation and infection simultaneously
Other scientists are investigating inhibitors of Janus kinase (JAK), a family of enzymes that play a key role in orchestrating immune responses, particularly cytokines. Interest in JAK inhibition to control hyperinflammation in cancer goes back at least 15 years, and drugs that act as JAK inhibitors are already approved for use in the treatment of myelofibrosis (ruxolitinib [Jakafi], fedratinib [Inrebic]) and also for rheumatoid arthritis (upadacitinib [Rinvoq], baricitinib [Olumiant]).
“It wasn’t a huge leap for those of us with a lot of understanding of JAK inhibitors to propose taking them into the clinic to treat patients with COVID-19,” commented John Mascarenhas, MD, the leader of clinical investigation in the myeloproliferative disorders program at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Mascarenhas is also principal investigator of the PRE-VENT trial, which is comparing the investigational JAK2 inhibitor pacritinib plus standard of care to standard of care alone in patients hospitalized with severe COVID-19, with and without cancer. The trial is sponsored by CTI BioPharma (manufacturer of pacritinib), and is taking place at 10 sites in the United States.
In a move that may raise eyebrows, PRE-VENT skipped phase 1 and 2 and went straight to phase 3. Pacritinib has yet to receive FDA approval and has mostly been studied in myelofibrosis, an intensely inflammatory disease.
The decision was based on trials of pacritinib in hematologic malignancies and also on results from a phase 2 study in China that found possible clinical benefit for the JAK 1/2 inhibitor ruxolitinib in 43 patients hospitalized with severe COVID-19, although results were not statistically significant, Dr. Mascarenhas explained.
Recent results from Lilly’s ACTT-2 study have provided further support for the role of JAK inhibitors in treating cytokine storm. ACTT-2 is a phase 3, double-blind, placebo-controlled, randomized, controlled trial sponsored by the NIH and NIAID comparing the JAK 1/2 inhibitor baricitinib plus the antiviral remdesivir with remdesivir alone in patients hospitalized with COVID-19. In September, Lilly announced that the trial met its primary endpoint of decreased time to recovery in patients who received baricitinib in combination with remdesivir.
But pacritinib’s mechanism of action may take things a step further. The drug selectively inhibits JAK2 and spares JAK1, which is important for antiviral activity in the immune system. Also, in vitro data suggests pacritinib may simultaneously reduce inflammation and fight off the virus by selectively inhibiting two additional enzymes and two other receptors.
“The rationale to me is very strong for using pacritinib,” Dr. Mascarenhas said. “I think this approach was bold but appropriate.”
The main safety concern with pacritinib could be bleeding, especially among patients on anticoagulants, Dr. Mascarenhas said. Because some patients with severe COVID-19 tend to develop blood clots, anticoagulation has become the standard of care at many institutions.
Because the trial is just beginning – only a minority of the total planned population of 358 patients has been enrolled – no interim results are available.
Right drug, wrong time?
IL-6 inhibition could still have a role to play in COVID-19, but the trick could be in the timing. Most of the trials so far have studied tocilizumab in patients with severe COVID-19, many of whom were already on ventilators. At that point, it may be too late to reverse the damage that has already taken place.
One of the main reasons tocilizumab works so well in CRS after CAR T therapy is that oncologists have learned how to use it early, often within 24 hours of fever onset. Oncologists use the American Society for Transplantation and Cellular Therapy consensus grading system, which helps them identify CRS when it is easier to control.
But applying the ASTCT grading system to COVID-19 is problematic. “Almost by definition, patients hospitalized with COVID-19 have low oxygen levels, which throws off the scale,” said Joshua Hill, MD, an infectious disease specialist at Fred Hutchinson Cancer Research Center in Seattle, who has research expertise in infectious complications after CAR T therapy.
“The key is to intervene earlier to prevent damage to the lungs and other end organs. We don’t have anything magical that will reverse that damage,” Dr. Hill said.
Results from the phase 3 trial EMPACTA trial (sponsored by Genentech) seem to bear this out. EMPACTA is evaluating use of tocilizumab in hospitalized patients with less severe COVID-19 who do not yet require mechanical ventilation. The trial is notable for being the first global phase 3 trial to demonstrate efficacy for tocilizumab vs placebo in hospitalized patients with COVID-19 pneumonia, and for including a high percentage of racial/ethnic minorities (85% of 389 participants), who have been hard hit by the pandemic and have historically been underrepresented in drug trials.
Last month, Roche announced that EMPACTA met its primary endpoint. Results showed that patients hospitalized with COVID-19 pneumonia who received tocilizumab plus standard of care were 44% less likely to go on mechanical ventilation or die, compared with those who received placebo plus standard of care (P = .0348), although there were no statistically significant differences in death by day 28 between tocilizumab and placebo (10.4% vs. 8.6%, P = .5146).
However, earlier administration of tocilizumab raises another issue. IL-6 and its pathway are important for clearing viral infections. Using tocilizumab in the context of an ongoing infection could raise safety issues.
Also, tocilizumab sticks around in the body for a relatively long time. In the treatment of rheumatoid arthritis, it is dosed once a month, and it carries a black box warning for reactivation of tuberculosis.
Whereas results from EMPACTA showed similar rates of infection associated with tocilizumab and placebo (10% vs. 11%), at least one other study has found increased rates of superinfection in patients with severe COVID-19 who received tocilizumab. Overall, though, the drug was associated with decreased risk of death in the latter study.
A phase 2 trial called COVIDOSE is tackling the safety issue. COVIDOSE is evaluating whether low-dose tocilizumab is effective in noncritical COVID-19 patients, with the idea that lower doses could be safer. Early results published as a preprint before peer review indicated that low-dose tocilizumab (ranging from 40 mg to 200 mg) was associated with clinical improvement in 32 noncritical patients hospitalized with COVID-19.
Five patients (15.6%) developed bacterial superinfections, and five (15.6%) died by 28-day follow-up, although there wasn’t a perfect “overlap” between these groups of patients. Bacterial superinfection was not the cause of death in all five patients who died, and not all patients who died developed bacterial superinfections, according to senior author Pankti Reid, MD, MPH, an assistant professor of medicine at the University of Chicago.
Results from COVIDOSE also showed that treatment with tocilizumab did not seem to affect the ability of patients to develop antibodies against COVID-19. The results set the stage for a larger randomized, controlled trial (still ongoing) to determine the optimal dose of tocilizumab.
Still, Dr. Hill urges caution.
Many of these immunomodulators have been used only in the context of a clinical trial, or only for patients with terminal cancer and no other treatment options. In patients with cancer, these drugs have been studied and have shown an “acceptable safety profile,” according to Dr. Shah.
But this is a different situation, and when it comes to repurposing them to relatively healthy patients with COVID-19, Dr. Hill emphasized the need for careful research.
“We’re always very concerned about giving drugs that suppress the immune response if people have active infections,” Dr. Hill said. “Often times we think it makes things worse, and it typically does.”
Dr. Mascarenhas reported institutional research funding from CTI Biopharma. Dr. Hill, Dr. Staudt, Dr. Wilson, and Dr. Shah disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
When the first reports emerged of “cytokine storm” in patients with severe COVID-19, all eyes turned to cancer research. Oncologists have years of experience reigning in “cytokine release syndrome” (CRS) in patients treated with chimeric antigen receptor (CAR) therapies for advanced blood cancers.
There was hope that drugs used to quell CRS in patients with cancer would be effective in patients with severe COVID. But the promise of a quick fix with oncology medications has yet to be fully realized.
Part of the problem is that the two conditions, while analogous, are “not the same,” said Nirali Shah, MD, head of the hematologic malignancies section in the pediatric oncology branch at the National Cancer Institute.
“You have to understand the underlying pathophysiology, what triggers the inflammation,” Dr. Shah said.
CAR T–related CRS is caused by activated T cells in patients with cancer who often do not have an infection, she explained. In contrast, cytokine storm in COVID-19 is triggered by a viral pathogen that can drive “out of control” inflammation. These differences may explain why drugs work in the first instance, but not in the second, she added. Drugs that inhibit interleukin-6 (such as tocilizumab, sarilumab, and siltuximab) are used with great success to dampen down the CRS in patients receiving CAR therapy for blood cancers. And although trials of these agents in patients with COVID are still ongoing, initial results are disappointing.
The first global, phase 3 randomized controlled trial of tocilizumab in severe COVID-19 failed to meet its primary endpoint of improved clinical status, and it did not meet its secondary endpoint of improved mortality at week 4.
In its recent recommendations, the National Institutes of Health noted a lack of data to support the efficacy of IL-6 inhibitors in COVID-19, and recommended against their use, except as part of a clinical trial.
Trimming the tree vs. cutting it down
As researchers have begun to decode the immune process underlying severe COVID-19, they have turned to other cancer drugs to tame cytokine storm.
Louis Staudt, MD, PhD, and Wyndham Wilson, MD, PhD, both at the NCI, think that cytokine storm in COVID-19 is driven by macrophages, which trigger release of multiple cytokines.
For years, the pair have been studying lymphoid tumors. Dr. Staudt is chief of the lymphoid malignancies branch at the NCI, and Wilson is head of the lymphoma therapeutics section. In past work, Dr. Staudt discovered that inhibiting an enzyme called bruton tyrosine kinase (BTK) dampens macrophage function.
When the pandemic began, Dr. Staudt and Dr. Wilson realized that singling out just one cytokine like IL-6 may not be enough. They thought that a more effective approach may be to target macrophages with a BTK inhibitor called acalabrutinib (Calquence), which would inhibit multiple cytokines at the same time.
Dr. Staudt likens the immune response to a tree, with the macrophages composing the tree trunk and the limbs made up of individual cytokines.
“Targeting macrophages is getting at the trunk of the problem,” he said. “You’re only cutting off the limbs with tocilizumab.”
In just 3 days, Dr. Staudt and Dr. Wilson went from concept to approval to launching a prospective, observational study. The study took place at five centers in the US, and included 19 patients hospitalized with COVID-19; the results were published in Science Immunology. Over a treatment course of 14 days, the majority of patients treated off-label with acalabrutinib improved, some within 24 hours. Eight of 11 patients on supplemental oxygen were discharged on room air. Four of eight patients on ventilators were extubated, with two of these discharged on room air. Two patients on ventilators died. No discernible toxicity was noted.
Analyses also showed increased BTK activity and elevated IL-6 levels in monocytes – precursors of macrophages – in patients with severe COVID-19, compared with healthy volunteers.
“We showed that the target of acalabrutinib was active in the immune cells of patients with severe COVID-19,” Dr. Staudt said. “So we have the target. We have the drug to hit the target. And we have an apparent clinical benefit.”
Those three things were compelling enough to launch the CALAVI phase 2 trial, an open-label, randomized, controlled trial, sponsored by AstraZeneca and the NCI, that is being conducted in the United States and internationally. It is testing acalabrutinib with best supportive care versus BSC alone in people hospitalized with COVID-19. The trial is scheduled to be completed on Nov. 26.
Preliminary insights from this trial are expected soon. “These are not insights that we will likely publish, but they are important insights that will lead to the launch of a definitive double-blind, randomized, phase 3 trial, which we hope to launch in the next month or so,” Dr. Wilson said.
Targeting inflammation and infection simultaneously
Other scientists are investigating inhibitors of Janus kinase (JAK), a family of enzymes that play a key role in orchestrating immune responses, particularly cytokines. Interest in JAK inhibition to control hyperinflammation in cancer goes back at least 15 years, and drugs that act as JAK inhibitors are already approved for use in the treatment of myelofibrosis (ruxolitinib [Jakafi], fedratinib [Inrebic]) and also for rheumatoid arthritis (upadacitinib [Rinvoq], baricitinib [Olumiant]).
“It wasn’t a huge leap for those of us with a lot of understanding of JAK inhibitors to propose taking them into the clinic to treat patients with COVID-19,” commented John Mascarenhas, MD, the leader of clinical investigation in the myeloproliferative disorders program at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Mascarenhas is also principal investigator of the PRE-VENT trial, which is comparing the investigational JAK2 inhibitor pacritinib plus standard of care to standard of care alone in patients hospitalized with severe COVID-19, with and without cancer. The trial is sponsored by CTI BioPharma (manufacturer of pacritinib), and is taking place at 10 sites in the United States.
In a move that may raise eyebrows, PRE-VENT skipped phase 1 and 2 and went straight to phase 3. Pacritinib has yet to receive FDA approval and has mostly been studied in myelofibrosis, an intensely inflammatory disease.
The decision was based on trials of pacritinib in hematologic malignancies and also on results from a phase 2 study in China that found possible clinical benefit for the JAK 1/2 inhibitor ruxolitinib in 43 patients hospitalized with severe COVID-19, although results were not statistically significant, Dr. Mascarenhas explained.
Recent results from Lilly’s ACTT-2 study have provided further support for the role of JAK inhibitors in treating cytokine storm. ACTT-2 is a phase 3, double-blind, placebo-controlled, randomized, controlled trial sponsored by the NIH and NIAID comparing the JAK 1/2 inhibitor baricitinib plus the antiviral remdesivir with remdesivir alone in patients hospitalized with COVID-19. In September, Lilly announced that the trial met its primary endpoint of decreased time to recovery in patients who received baricitinib in combination with remdesivir.
But pacritinib’s mechanism of action may take things a step further. The drug selectively inhibits JAK2 and spares JAK1, which is important for antiviral activity in the immune system. Also, in vitro data suggests pacritinib may simultaneously reduce inflammation and fight off the virus by selectively inhibiting two additional enzymes and two other receptors.
“The rationale to me is very strong for using pacritinib,” Dr. Mascarenhas said. “I think this approach was bold but appropriate.”
The main safety concern with pacritinib could be bleeding, especially among patients on anticoagulants, Dr. Mascarenhas said. Because some patients with severe COVID-19 tend to develop blood clots, anticoagulation has become the standard of care at many institutions.
Because the trial is just beginning – only a minority of the total planned population of 358 patients has been enrolled – no interim results are available.
Right drug, wrong time?
IL-6 inhibition could still have a role to play in COVID-19, but the trick could be in the timing. Most of the trials so far have studied tocilizumab in patients with severe COVID-19, many of whom were already on ventilators. At that point, it may be too late to reverse the damage that has already taken place.
One of the main reasons tocilizumab works so well in CRS after CAR T therapy is that oncologists have learned how to use it early, often within 24 hours of fever onset. Oncologists use the American Society for Transplantation and Cellular Therapy consensus grading system, which helps them identify CRS when it is easier to control.
But applying the ASTCT grading system to COVID-19 is problematic. “Almost by definition, patients hospitalized with COVID-19 have low oxygen levels, which throws off the scale,” said Joshua Hill, MD, an infectious disease specialist at Fred Hutchinson Cancer Research Center in Seattle, who has research expertise in infectious complications after CAR T therapy.
“The key is to intervene earlier to prevent damage to the lungs and other end organs. We don’t have anything magical that will reverse that damage,” Dr. Hill said.
Results from the phase 3 trial EMPACTA trial (sponsored by Genentech) seem to bear this out. EMPACTA is evaluating use of tocilizumab in hospitalized patients with less severe COVID-19 who do not yet require mechanical ventilation. The trial is notable for being the first global phase 3 trial to demonstrate efficacy for tocilizumab vs placebo in hospitalized patients with COVID-19 pneumonia, and for including a high percentage of racial/ethnic minorities (85% of 389 participants), who have been hard hit by the pandemic and have historically been underrepresented in drug trials.
Last month, Roche announced that EMPACTA met its primary endpoint. Results showed that patients hospitalized with COVID-19 pneumonia who received tocilizumab plus standard of care were 44% less likely to go on mechanical ventilation or die, compared with those who received placebo plus standard of care (P = .0348), although there were no statistically significant differences in death by day 28 between tocilizumab and placebo (10.4% vs. 8.6%, P = .5146).
However, earlier administration of tocilizumab raises another issue. IL-6 and its pathway are important for clearing viral infections. Using tocilizumab in the context of an ongoing infection could raise safety issues.
Also, tocilizumab sticks around in the body for a relatively long time. In the treatment of rheumatoid arthritis, it is dosed once a month, and it carries a black box warning for reactivation of tuberculosis.
Whereas results from EMPACTA showed similar rates of infection associated with tocilizumab and placebo (10% vs. 11%), at least one other study has found increased rates of superinfection in patients with severe COVID-19 who received tocilizumab. Overall, though, the drug was associated with decreased risk of death in the latter study.
A phase 2 trial called COVIDOSE is tackling the safety issue. COVIDOSE is evaluating whether low-dose tocilizumab is effective in noncritical COVID-19 patients, with the idea that lower doses could be safer. Early results published as a preprint before peer review indicated that low-dose tocilizumab (ranging from 40 mg to 200 mg) was associated with clinical improvement in 32 noncritical patients hospitalized with COVID-19.
Five patients (15.6%) developed bacterial superinfections, and five (15.6%) died by 28-day follow-up, although there wasn’t a perfect “overlap” between these groups of patients. Bacterial superinfection was not the cause of death in all five patients who died, and not all patients who died developed bacterial superinfections, according to senior author Pankti Reid, MD, MPH, an assistant professor of medicine at the University of Chicago.
Results from COVIDOSE also showed that treatment with tocilizumab did not seem to affect the ability of patients to develop antibodies against COVID-19. The results set the stage for a larger randomized, controlled trial (still ongoing) to determine the optimal dose of tocilizumab.
Still, Dr. Hill urges caution.
Many of these immunomodulators have been used only in the context of a clinical trial, or only for patients with terminal cancer and no other treatment options. In patients with cancer, these drugs have been studied and have shown an “acceptable safety profile,” according to Dr. Shah.
But this is a different situation, and when it comes to repurposing them to relatively healthy patients with COVID-19, Dr. Hill emphasized the need for careful research.
“We’re always very concerned about giving drugs that suppress the immune response if people have active infections,” Dr. Hill said. “Often times we think it makes things worse, and it typically does.”
Dr. Mascarenhas reported institutional research funding from CTI Biopharma. Dr. Hill, Dr. Staudt, Dr. Wilson, and Dr. Shah disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
When the first reports emerged of “cytokine storm” in patients with severe COVID-19, all eyes turned to cancer research. Oncologists have years of experience reigning in “cytokine release syndrome” (CRS) in patients treated with chimeric antigen receptor (CAR) therapies for advanced blood cancers.
There was hope that drugs used to quell CRS in patients with cancer would be effective in patients with severe COVID. But the promise of a quick fix with oncology medications has yet to be fully realized.
Part of the problem is that the two conditions, while analogous, are “not the same,” said Nirali Shah, MD, head of the hematologic malignancies section in the pediatric oncology branch at the National Cancer Institute.
“You have to understand the underlying pathophysiology, what triggers the inflammation,” Dr. Shah said.
CAR T–related CRS is caused by activated T cells in patients with cancer who often do not have an infection, she explained. In contrast, cytokine storm in COVID-19 is triggered by a viral pathogen that can drive “out of control” inflammation. These differences may explain why drugs work in the first instance, but not in the second, she added. Drugs that inhibit interleukin-6 (such as tocilizumab, sarilumab, and siltuximab) are used with great success to dampen down the CRS in patients receiving CAR therapy for blood cancers. And although trials of these agents in patients with COVID are still ongoing, initial results are disappointing.
The first global, phase 3 randomized controlled trial of tocilizumab in severe COVID-19 failed to meet its primary endpoint of improved clinical status, and it did not meet its secondary endpoint of improved mortality at week 4.
In its recent recommendations, the National Institutes of Health noted a lack of data to support the efficacy of IL-6 inhibitors in COVID-19, and recommended against their use, except as part of a clinical trial.
Trimming the tree vs. cutting it down
As researchers have begun to decode the immune process underlying severe COVID-19, they have turned to other cancer drugs to tame cytokine storm.
Louis Staudt, MD, PhD, and Wyndham Wilson, MD, PhD, both at the NCI, think that cytokine storm in COVID-19 is driven by macrophages, which trigger release of multiple cytokines.
For years, the pair have been studying lymphoid tumors. Dr. Staudt is chief of the lymphoid malignancies branch at the NCI, and Wilson is head of the lymphoma therapeutics section. In past work, Dr. Staudt discovered that inhibiting an enzyme called bruton tyrosine kinase (BTK) dampens macrophage function.
When the pandemic began, Dr. Staudt and Dr. Wilson realized that singling out just one cytokine like IL-6 may not be enough. They thought that a more effective approach may be to target macrophages with a BTK inhibitor called acalabrutinib (Calquence), which would inhibit multiple cytokines at the same time.
Dr. Staudt likens the immune response to a tree, with the macrophages composing the tree trunk and the limbs made up of individual cytokines.
“Targeting macrophages is getting at the trunk of the problem,” he said. “You’re only cutting off the limbs with tocilizumab.”
In just 3 days, Dr. Staudt and Dr. Wilson went from concept to approval to launching a prospective, observational study. The study took place at five centers in the US, and included 19 patients hospitalized with COVID-19; the results were published in Science Immunology. Over a treatment course of 14 days, the majority of patients treated off-label with acalabrutinib improved, some within 24 hours. Eight of 11 patients on supplemental oxygen were discharged on room air. Four of eight patients on ventilators were extubated, with two of these discharged on room air. Two patients on ventilators died. No discernible toxicity was noted.
Analyses also showed increased BTK activity and elevated IL-6 levels in monocytes – precursors of macrophages – in patients with severe COVID-19, compared with healthy volunteers.
“We showed that the target of acalabrutinib was active in the immune cells of patients with severe COVID-19,” Dr. Staudt said. “So we have the target. We have the drug to hit the target. And we have an apparent clinical benefit.”
Those three things were compelling enough to launch the CALAVI phase 2 trial, an open-label, randomized, controlled trial, sponsored by AstraZeneca and the NCI, that is being conducted in the United States and internationally. It is testing acalabrutinib with best supportive care versus BSC alone in people hospitalized with COVID-19. The trial is scheduled to be completed on Nov. 26.
Preliminary insights from this trial are expected soon. “These are not insights that we will likely publish, but they are important insights that will lead to the launch of a definitive double-blind, randomized, phase 3 trial, which we hope to launch in the next month or so,” Dr. Wilson said.
Targeting inflammation and infection simultaneously
Other scientists are investigating inhibitors of Janus kinase (JAK), a family of enzymes that play a key role in orchestrating immune responses, particularly cytokines. Interest in JAK inhibition to control hyperinflammation in cancer goes back at least 15 years, and drugs that act as JAK inhibitors are already approved for use in the treatment of myelofibrosis (ruxolitinib [Jakafi], fedratinib [Inrebic]) and also for rheumatoid arthritis (upadacitinib [Rinvoq], baricitinib [Olumiant]).
“It wasn’t a huge leap for those of us with a lot of understanding of JAK inhibitors to propose taking them into the clinic to treat patients with COVID-19,” commented John Mascarenhas, MD, the leader of clinical investigation in the myeloproliferative disorders program at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Mascarenhas is also principal investigator of the PRE-VENT trial, which is comparing the investigational JAK2 inhibitor pacritinib plus standard of care to standard of care alone in patients hospitalized with severe COVID-19, with and without cancer. The trial is sponsored by CTI BioPharma (manufacturer of pacritinib), and is taking place at 10 sites in the United States.
In a move that may raise eyebrows, PRE-VENT skipped phase 1 and 2 and went straight to phase 3. Pacritinib has yet to receive FDA approval and has mostly been studied in myelofibrosis, an intensely inflammatory disease.
The decision was based on trials of pacritinib in hematologic malignancies and also on results from a phase 2 study in China that found possible clinical benefit for the JAK 1/2 inhibitor ruxolitinib in 43 patients hospitalized with severe COVID-19, although results were not statistically significant, Dr. Mascarenhas explained.
Recent results from Lilly’s ACTT-2 study have provided further support for the role of JAK inhibitors in treating cytokine storm. ACTT-2 is a phase 3, double-blind, placebo-controlled, randomized, controlled trial sponsored by the NIH and NIAID comparing the JAK 1/2 inhibitor baricitinib plus the antiviral remdesivir with remdesivir alone in patients hospitalized with COVID-19. In September, Lilly announced that the trial met its primary endpoint of decreased time to recovery in patients who received baricitinib in combination with remdesivir.
But pacritinib’s mechanism of action may take things a step further. The drug selectively inhibits JAK2 and spares JAK1, which is important for antiviral activity in the immune system. Also, in vitro data suggests pacritinib may simultaneously reduce inflammation and fight off the virus by selectively inhibiting two additional enzymes and two other receptors.
“The rationale to me is very strong for using pacritinib,” Dr. Mascarenhas said. “I think this approach was bold but appropriate.”
The main safety concern with pacritinib could be bleeding, especially among patients on anticoagulants, Dr. Mascarenhas said. Because some patients with severe COVID-19 tend to develop blood clots, anticoagulation has become the standard of care at many institutions.
Because the trial is just beginning – only a minority of the total planned population of 358 patients has been enrolled – no interim results are available.
Right drug, wrong time?
IL-6 inhibition could still have a role to play in COVID-19, but the trick could be in the timing. Most of the trials so far have studied tocilizumab in patients with severe COVID-19, many of whom were already on ventilators. At that point, it may be too late to reverse the damage that has already taken place.
One of the main reasons tocilizumab works so well in CRS after CAR T therapy is that oncologists have learned how to use it early, often within 24 hours of fever onset. Oncologists use the American Society for Transplantation and Cellular Therapy consensus grading system, which helps them identify CRS when it is easier to control.
But applying the ASTCT grading system to COVID-19 is problematic. “Almost by definition, patients hospitalized with COVID-19 have low oxygen levels, which throws off the scale,” said Joshua Hill, MD, an infectious disease specialist at Fred Hutchinson Cancer Research Center in Seattle, who has research expertise in infectious complications after CAR T therapy.
“The key is to intervene earlier to prevent damage to the lungs and other end organs. We don’t have anything magical that will reverse that damage,” Dr. Hill said.
Results from the phase 3 trial EMPACTA trial (sponsored by Genentech) seem to bear this out. EMPACTA is evaluating use of tocilizumab in hospitalized patients with less severe COVID-19 who do not yet require mechanical ventilation. The trial is notable for being the first global phase 3 trial to demonstrate efficacy for tocilizumab vs placebo in hospitalized patients with COVID-19 pneumonia, and for including a high percentage of racial/ethnic minorities (85% of 389 participants), who have been hard hit by the pandemic and have historically been underrepresented in drug trials.
Last month, Roche announced that EMPACTA met its primary endpoint. Results showed that patients hospitalized with COVID-19 pneumonia who received tocilizumab plus standard of care were 44% less likely to go on mechanical ventilation or die, compared with those who received placebo plus standard of care (P = .0348), although there were no statistically significant differences in death by day 28 between tocilizumab and placebo (10.4% vs. 8.6%, P = .5146).
However, earlier administration of tocilizumab raises another issue. IL-6 and its pathway are important for clearing viral infections. Using tocilizumab in the context of an ongoing infection could raise safety issues.
Also, tocilizumab sticks around in the body for a relatively long time. In the treatment of rheumatoid arthritis, it is dosed once a month, and it carries a black box warning for reactivation of tuberculosis.
Whereas results from EMPACTA showed similar rates of infection associated with tocilizumab and placebo (10% vs. 11%), at least one other study has found increased rates of superinfection in patients with severe COVID-19 who received tocilizumab. Overall, though, the drug was associated with decreased risk of death in the latter study.
A phase 2 trial called COVIDOSE is tackling the safety issue. COVIDOSE is evaluating whether low-dose tocilizumab is effective in noncritical COVID-19 patients, with the idea that lower doses could be safer. Early results published as a preprint before peer review indicated that low-dose tocilizumab (ranging from 40 mg to 200 mg) was associated with clinical improvement in 32 noncritical patients hospitalized with COVID-19.
Five patients (15.6%) developed bacterial superinfections, and five (15.6%) died by 28-day follow-up, although there wasn’t a perfect “overlap” between these groups of patients. Bacterial superinfection was not the cause of death in all five patients who died, and not all patients who died developed bacterial superinfections, according to senior author Pankti Reid, MD, MPH, an assistant professor of medicine at the University of Chicago.
Results from COVIDOSE also showed that treatment with tocilizumab did not seem to affect the ability of patients to develop antibodies against COVID-19. The results set the stage for a larger randomized, controlled trial (still ongoing) to determine the optimal dose of tocilizumab.
Still, Dr. Hill urges caution.
Many of these immunomodulators have been used only in the context of a clinical trial, or only for patients with terminal cancer and no other treatment options. In patients with cancer, these drugs have been studied and have shown an “acceptable safety profile,” according to Dr. Shah.
But this is a different situation, and when it comes to repurposing them to relatively healthy patients with COVID-19, Dr. Hill emphasized the need for careful research.
“We’re always very concerned about giving drugs that suppress the immune response if people have active infections,” Dr. Hill said. “Often times we think it makes things worse, and it typically does.”
Dr. Mascarenhas reported institutional research funding from CTI Biopharma. Dr. Hill, Dr. Staudt, Dr. Wilson, and Dr. Shah disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Sleepless nights, hair loss, and cracked teeth: Pandemic stress takes its toll
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Survey: Doctors lonely, burned out in COVID-19
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Fauci: Cautious optimism for COVID-19 vaccine by end of 2020
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
FROM CHEST 2020
The unsteady state
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Mastering mask communicating
. For those specialties not accustomed to wearing a mask all day, it’s frustrating: How many times have you had to repeat yourself today? Or ask your patient to say something again? (Ain’t no one got time to repeat a third time how to do that prednisone taper). Worse, we’re losing important nonverbal cues that help us connect with our patients. How can we be understood when our faces are covered and 6 feet away?
Masks muffle both verbal and nonverbal communication. For soft-spoken or high-pitched speakers, the verbal effect is significant. In particular, masks make hearing consonants more difficult. They can make the “sh,” “th,” “f,” and “s” sounds difficult to distinguish. Typically, we’d use context and lip reading to boost the signal, but this fix is blocked (and the clear mouth-window masks are kinda creepy).
Masks also prevent us from seeing facial microexpressions, critical information when you are trying to connect with someone or to build trust. A randomized controlled trial published in 2013 indeed showed that doctors wearing a mask were perceived as less empathetic and had diminished relational continuity with patients as compared to doctors not wearing a mask. There are a few things we can do to help.
Speak more loudly is obvious advice. Loud talking has limitations though, as it can feel rude, and it blunts inflections, which add richness and emotion. (Shouting “THIS WILL ONLY HURT A LITTLE” seems a mixed message). More important than the volume is your choice of words. Try to use simple terms and short sentences. Pause between points. Hit your consonants harder.
It’s also important that you have their full attention and are giving yours. As much as possible, try to align squared up with patients. Facing your computer exacerbates the problem. Look them in their eyes and be sure they are connected with you before any complex or difficult conversations. Hearing-impaired patients are now sometimes leaving out their aids because it’s too uncomfortable to wear them with their mask. You might ask them to put them back in. Check in with patients and repeat back what you heard them say. This can help with clarity and with connecting. Use your face more: if you’ve ever acted on stage, this would be your on-stage face. Exaggerate your expressions so it’s a little easier for them to read you.
Lastly, there are apps such as Ava or Google Live Translator, which can transcribe your speech real time. You could then share your screen with the patient so they can read exactly what you’ve said.
Some of us are natural communicators. Even if you are not, you can mitigate some of our current challenges. I’ll admit, it’s been a bit easier for me than for others. Between my prominent eyebrows and Italian-American upbringing, I can express my way through pretty much any face covering. If you’d like to learn how to use your hands better, then just watch this little girl: https://youtu.be/Z5wAWyqDrnc.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
. For those specialties not accustomed to wearing a mask all day, it’s frustrating: How many times have you had to repeat yourself today? Or ask your patient to say something again? (Ain’t no one got time to repeat a third time how to do that prednisone taper). Worse, we’re losing important nonverbal cues that help us connect with our patients. How can we be understood when our faces are covered and 6 feet away?
Masks muffle both verbal and nonverbal communication. For soft-spoken or high-pitched speakers, the verbal effect is significant. In particular, masks make hearing consonants more difficult. They can make the “sh,” “th,” “f,” and “s” sounds difficult to distinguish. Typically, we’d use context and lip reading to boost the signal, but this fix is blocked (and the clear mouth-window masks are kinda creepy).
Masks also prevent us from seeing facial microexpressions, critical information when you are trying to connect with someone or to build trust. A randomized controlled trial published in 2013 indeed showed that doctors wearing a mask were perceived as less empathetic and had diminished relational continuity with patients as compared to doctors not wearing a mask. There are a few things we can do to help.
Speak more loudly is obvious advice. Loud talking has limitations though, as it can feel rude, and it blunts inflections, which add richness and emotion. (Shouting “THIS WILL ONLY HURT A LITTLE” seems a mixed message). More important than the volume is your choice of words. Try to use simple terms and short sentences. Pause between points. Hit your consonants harder.
It’s also important that you have their full attention and are giving yours. As much as possible, try to align squared up with patients. Facing your computer exacerbates the problem. Look them in their eyes and be sure they are connected with you before any complex or difficult conversations. Hearing-impaired patients are now sometimes leaving out their aids because it’s too uncomfortable to wear them with their mask. You might ask them to put them back in. Check in with patients and repeat back what you heard them say. This can help with clarity and with connecting. Use your face more: if you’ve ever acted on stage, this would be your on-stage face. Exaggerate your expressions so it’s a little easier for them to read you.
Lastly, there are apps such as Ava or Google Live Translator, which can transcribe your speech real time. You could then share your screen with the patient so they can read exactly what you’ve said.
Some of us are natural communicators. Even if you are not, you can mitigate some of our current challenges. I’ll admit, it’s been a bit easier for me than for others. Between my prominent eyebrows and Italian-American upbringing, I can express my way through pretty much any face covering. If you’d like to learn how to use your hands better, then just watch this little girl: https://youtu.be/Z5wAWyqDrnc.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
. For those specialties not accustomed to wearing a mask all day, it’s frustrating: How many times have you had to repeat yourself today? Or ask your patient to say something again? (Ain’t no one got time to repeat a third time how to do that prednisone taper). Worse, we’re losing important nonverbal cues that help us connect with our patients. How can we be understood when our faces are covered and 6 feet away?
Masks muffle both verbal and nonverbal communication. For soft-spoken or high-pitched speakers, the verbal effect is significant. In particular, masks make hearing consonants more difficult. They can make the “sh,” “th,” “f,” and “s” sounds difficult to distinguish. Typically, we’d use context and lip reading to boost the signal, but this fix is blocked (and the clear mouth-window masks are kinda creepy).
Masks also prevent us from seeing facial microexpressions, critical information when you are trying to connect with someone or to build trust. A randomized controlled trial published in 2013 indeed showed that doctors wearing a mask were perceived as less empathetic and had diminished relational continuity with patients as compared to doctors not wearing a mask. There are a few things we can do to help.
Speak more loudly is obvious advice. Loud talking has limitations though, as it can feel rude, and it blunts inflections, which add richness and emotion. (Shouting “THIS WILL ONLY HURT A LITTLE” seems a mixed message). More important than the volume is your choice of words. Try to use simple terms and short sentences. Pause between points. Hit your consonants harder.
It’s also important that you have their full attention and are giving yours. As much as possible, try to align squared up with patients. Facing your computer exacerbates the problem. Look them in their eyes and be sure they are connected with you before any complex or difficult conversations. Hearing-impaired patients are now sometimes leaving out their aids because it’s too uncomfortable to wear them with their mask. You might ask them to put them back in. Check in with patients and repeat back what you heard them say. This can help with clarity and with connecting. Use your face more: if you’ve ever acted on stage, this would be your on-stage face. Exaggerate your expressions so it’s a little easier for them to read you.
Lastly, there are apps such as Ava or Google Live Translator, which can transcribe your speech real time. You could then share your screen with the patient so they can read exactly what you’ve said.
Some of us are natural communicators. Even if you are not, you can mitigate some of our current challenges. I’ll admit, it’s been a bit easier for me than for others. Between my prominent eyebrows and Italian-American upbringing, I can express my way through pretty much any face covering. If you’d like to learn how to use your hands better, then just watch this little girl: https://youtu.be/Z5wAWyqDrnc.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].