User login
-
Physicians’ trust in health care leadership drops in pandemic
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.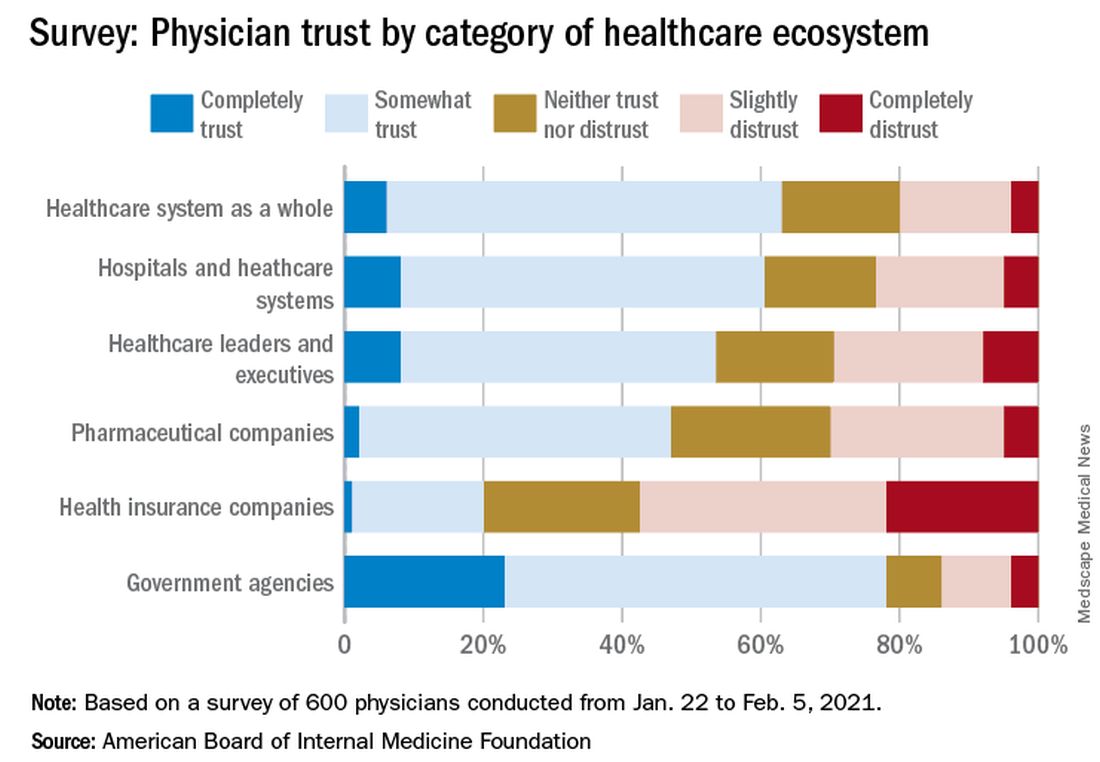
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a survey conducted by NORC at the University of Chicago on behalf of the American Board of Internal Medicine Foundation.
Survey results, released May 21, indicate that 30% of physicians say their trust in the U.S. health care system and health care leadership has decreased during the pandemic. Only 18% reported an increase in trust.
Physicians, however, have great trust in their fellow clinicians.
In the survey of 600 physicians, 94% said they trust doctors within their practice; 85% trusted doctors outside of their practice; and 89% trusted nurses. That trust increased during the pandemic, with 41% saying their trust in fellow physicians rose and 37% saying their trust in nurses did.
In a separate survey, NORC asked patients about their trust in various aspects of health care. Among 2,069 respondents, a wide majority reported that they trust doctors (84%) and nurses (85%), but only 64% trusted the health care system as a whole. One in three consumers (32%) said their trust in the health care system decreased during the pandemic, compared with 11% who said their trust increased.
The ABIM Foundation released the research findings on May 21 as part of Building Trust, a national campaign that aims to boost trust among patients, clinicians, system leaders, researchers, and others.
Richard J. Baron, MD, president and chief executive officer of the ABIM Foundation, said in an interview, “Clearly there’s lower trust in health care organization leaders and executives, and that’s troubling.
“Science by itself is not enough,” he said. “Becoming trustworthy has to be a core project of everybody in health care.”
Deterioration in physicians’ trust during the pandemic comes in part from failed promises of adequate personal protective equipment and some physicians’ loss of income as a result of the crisis, Dr. Baron said.
He added that the vaccine rollout was very uneven and that policies as to which elective procedures could be performed were handled differently in different parts of the country.
He also noted that, early on, transparency was lacking as to how many COVID patients hospitals were treating, which may have contributed to the decrease in trust in the system.
Fear of being known as ‘the COVID hospital’
Hospitals were afraid of being known as “the COVID hospital” and losing patients who were afraid to come there, Dr. Baron said.
He said the COVID-19 epidemic exacerbated problems regarding trust, but that trust has been declining for some time. The Building Trust campaign will focus on solutions in breaches of trust as physicians move increasingly toward being employees of huge systems, according to Dr. Baron.
However, trust works both ways, Dr. Baron notes. Physicians can be champions for their health care system or “throw the system under the bus,” he said.
For example, if a patient complains about the appointment system, clinicians who trust their institutions may say the system usually works and that they will try to make sure the patient has a better experience next time. Clinicians without trust may say they agree that the health care system doesn’t know what it is doing, and patients may further lose confidence when physicians validate their complaint, and patients may then go elsewhere.
78% of patients trust primary care doctor
When asked whether they trust their primary care physician, 78% of patients said yes. However, trust in doctors was higher among people who were older (90%), White (82%), or had high income (89%). Among people reporting lower trust, 25% said their physician spends too little time with them, and 14% said their doctor does not know or listen to them.
The survey shows that government agencies have work to do to earn trust. Responses indicate that 43% of physicians said they have “complete trust” in government health care agencies, such as the U.S. Food and Drug Administration and the Centers for Disease Control and Prevention, which is substantially higher than other parts of the health care system. However, trust in agencies declined for 43% of physician respondents and increased for 21%.
Dhruv Khullar, MD, MPP, of the department of health policy and economics at Weill Cornell Medical College in New York, told this news organization the survey results match what he sees anecdotally in medicine – that physicians have been losing trust in the system but not in their colleagues.
He said the sample size of 600 is enough to be influential, though he said he would like to know the response rate, which was not calculated for this survey.
He added that, in large part, physicians’ lack of trust in their systems may come from generally being asked to see more patients and to meet more metrics during the same or shorter periods.
Physicians’ lack of trust in the system can have significant consequences, he said. It can lead to burnout, which has been linked with poorer quality of care and physician turnover, he noted.
COVID-19 led some physicians to wonder whether their system had their best interests at heart, insofar as access to adequate medicines and supplies as well as emotional support were inconsistent, Dr. Khullar said.
He said that to regain trust health care systems need to ask themselves questions in three areas. The first is whether their goals are focused on the best interest of the organization or the best interest of the patient.
“Next is competency,” Dr. Khullar said. “Maybe your motives are right, but are you able to deliver? Are you delivering a good product, whether clinical services or something else?”
The third area is transparency, he said. “Are you going to be honest and forthright in what we’re doing and where we’re going?”
Caroline Pearson, senior vice president of health care strategy for NORC, said the emailed survey was conducted between Dec. 29, 2020, and Feb. 5, 2021, with a health care survey partner that maintains a nationwide panel of physicians across specialties.
She said this report is fairly novel insofar as surveys are more typically conducted regarding patients’ trust of their doctors or of the health care system.
Ms. Pearson said because health care is delivered in teams, understanding the level of trust among the entities helps ensure that care will be delivered effectively and seamlessly with high quality.
“We want our patients to trust our doctors, but we really want doctors to trust each other and trust the hospitals and systems in which they’re working,” she said.
Dr. Baron, Ms. Pearson, and Dr. Khullar report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ID experts dole out practical advice to help with mask confusion
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s latest guidance on what fully vaccinated people can do safely – including not socially distancing and not wearing a mask indoors or outdoors unless other regulations require it – has been widely misinterpreted and caused confusion, two infectious disease experts said at a briefing on May 20 hosted by the Infectious Diseases Society of America (IDSA).
The CDC did not ‘’lift” the mask mandate, but rather supplied guidance for those who are fully vaccinated. However, many questions and gray areas remain, and the experts addressed those. ‘’The CDC guidance is really directed at people who are fully vaccinated and who we know are likely to have a really solid response to the vaccine,” said Jeanne Marrazzo, MD, MPH, director of infectious diseases at the University of Alabama at Birmingham and an IDSA board member.
That message was largely lost, said Dr. Marrazzo and Jeffrey Duchin, MD, health officer of public health for Seattle and King County, Washington, and also an IDSA board member. Dr. Duchin said many people mistakenly regarded the new guidance as a message that the pandemic is over.
Among their practical tips on how to interpret the guidance:
To mask or not?
To make the decision, people need to think about not only the numbers of vaccinated versus unvaccinated individuals in their community but the local rates of disease, the experts said. And they need to know that the CDC guidance doesn’t apply if regulations by federal or state authorities or businesses and workplace are in conflict.
Deciding on mask use sometimes depends on where you are going. What about going into grocery stores or large bin stores without a mask? “If you are fully vaccinated and have no other conditions that compromise your immune system, and the rates of COVID are relatively low where you live, and the vaccination rates are high, I would be 100% fine” without a mask, Dr. Marrazzo said. But it’s important to think of all these factors in calculating your risk.
“I’m still wearing a mask when I go anywhere in public,” she said, citing vaccination rates that have not yet reached 50% in her area.
If that rate reached 80%, the typical percentage talked about for herd immunity, and new cases were low, Dr. Marrazzo said she might shed the mask.
The CDC also continues to recommend masks on mass transit for all.
One population that also must be considered, and who must evaluate their risk, even if vaccinated, are the immunocompromised, Dr. Marrazzo said. While people think of the immunocompromised as those with HIV or organ transplants, the numbers are actually much larger.
“A study a couple of years ago indicated up to 3% of Americans may actually have been told by their physician they have some of level of being immunocompromised,” she said. Among the examples are those who are on dialysis, on chemotherapy, or those taking any of the medications that modify the immune system.
“Millions of people fit this bill, and we have [very] little data on whether the vaccine works in them. We think it does,” Dr. Marrazzo said.
Still, she said, it’s a reason for these people to be cautious. For some other vaccines, the dose is modified for those who are immunocompromised. What’s not known yet is whether additional doses of the COVID vaccines might boost protection for those who are immunocompromised.
Many people, even after vaccination, may choose to keep wearing a mask especially in indoor, crowded settings, Dr. Duchin said. “We need to expect, accept, and respect continued mask wearing by anyone at any time.”
In most outdoor settings, he said, “I think masks are probably not necessary, vaccinated or not, regardless of age.” One exception: close face-to-face contact, such as in certain sports.
How to protect toddlers and infants
With masks not practical or recommended for infants and toddlers under 2 years old, Dr. Marrazzo said adults should remember that ‘’those very little kids don’t do poorly at all [even if infected], although there is not a ton of data.”
Adults should still treat young children as vulnerable, especially newborns. Adults not yet vaccinated should wear a mask when around them, she said.
J & J vaccine recipients
With less ‘’real world” data on the Johnson & Johnson vaccine, should those who got it think of themselves in a different risk group than those who got Moderna or Pfizer and adjust their behavior accordingly?
“The J&J vaccine, based on everything we know, does provide a great deal of protection,” Dr. Marrazzo said. ‘’We don’t know as much about prevention of transmission in the asymptomatic cases in the J&J.”
Most of that data, she said, is from the mRNA vaccines Pfizer and Moderna. “I think it’s an important area to study and learn about.” But all three vaccines, overall, provide a high level of protection, she said.
A version of this article first appeared on Medscape.com.
Photobiomodulation reduced acute radiodermatitis severity in head and neck cancer patients
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
The delivery of , according to results from the first randomized study of its kind.
“The use of light therapy-based applications for cancer therapy-related adverse events has steadily increased in the past 40 years,” lead study author Jolien Robijns, MSc, PhD, told this news organization during the annual conference of the American Society for Laser Medicine and Surgery. “The most well-known and studied indication of photobiomodulation therapy in supportive cancer care is oral mucositis,” she said, referring to a recent systematic review, which found that based on the available evidence, PBMT is an effective therapy for the prevention of oral mucositis, using well-defined PBM parameters in specific patient populations. “Various internationally well-recognized health organizations in oncology recommend PBMT to prevent and manage oral mucositis,” she added.
Based on the wound-healing and anti-inflammatory properties of PBMT, several studies have investigated its use for the prevention and management of acute radiodermatitis (ARD) since the 1990s, said Dr. Robijns, a postdoctoral researcher at Limburg Clinical Research Center in Hasselt, Belgium. Under the supervision of Jeroen Mebis, MD, PhD, at the Limburg Oncologic Laser Institute, she and her colleagues have been conducting clinical research on PBMT and ARD since 2014, with successful results. In 2020 they published a narrative review, which showed that based on nine clinical trials, PBMT could effectively reduce the incidence of severe ARD, decrease accompanying pain, and improve patients’ quality of life.
For the current study, known as the DERMISHEAD trial and published online March 9, 2021, in Radiotherapy and Oncology, investigators at Limburg Oncology Center at Jessa Hospital in Hasselt, and Hasselt University, recruited head and neck cancer patients who underwent bilateral radiotherapy with or without chemotherapy, for a total dose of 30-35 x 2 Gy . All patients received standard skin care combined with two PBMT or sham sessions twice per week during the complete course of RT, which resulted in 14 total sessions.
As described in the Radiotherapy and Oncology study, the commercially available device used for PBMT “consists of two laser diodes with different wavelengths (808-905 nm), peak powers (1.1-25 W), and emission modes (continuous and pulsed). Both diodes work simultaneously and synchronously with coincident propagation axes (average radiant power 3.3 W). The energy density (fluence) was set at 4 J/cm2 based on earlier recommendations and on our clinical experience.” A blinded study nurse used Radiation Therapy Oncology Group criteria to evaluate the skin reactions.
After 303 patients were initially assessed for eligibility, 46 patients were enrolled in DERMISHEAD (18 in the placebo group and 28 in the PBMT group). At the end of radiotherapy, 77.8% of patients in the placebo group had a grade 2 or 3 skin reaction, compared with 28.6% of patients in the PBMT group (P = .001).
“The DERMISHEAD trial proved that PBMT significantly reduces the severity of ARD,” Dr. Robijns said. “Thereby, it improves the patients’ quality of life during their radiotherapy course. The trial supports the further implementation of PBM in the supportive care of cancer patients undergoing radiotherapy.”
The results are similar to those in the TRANSDERMIS trial, in which Dr. Robijns and her colleagues used PMBT to treat breast cancer patients.
“However, an interesting difference is that the percentage decrease in severe ARD was higher in the DERMISHEAD trial than in the TRANSDERMIS trial: 49% vs. 23%, respectively,” she noted. “This difference can be rationalized because in total, more control head and neck cancer patients developed grade 3 ARD than did control breast cancer patients (17% vs. 5%). A possible explanation of this finding can be related to the difference in treatment regimens and radiotherapy parameters between the two trials.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn., who was asked to comment on the study, said that acute radiation dermatitis “can be very painful and distressing to patients, and over time, the skin changes can create long-term problems. Prevention of acute and chronic radiation dermatitis is worthwhile, particularly for patients at risk.”
This study, she added, “shows a benefit of photobiomodulation therapy as a potential preventative treatment. Notably, patients did not always follow up appropriately for the therapy, and the authors said that it is yet another thing that patients need to keep track of, in addition to their cancer therapy visits. Thus, optimally, it would be useful to have a biomarker of which patients would most benefit from treatments that prevent/potentiate radiation dermatitis.”
Dr. Robijns acknowledged certain limitations of the trial, including its small sample size and the scarcity of clinical trials on PBM and acute radiation dermatitis. “More studies are needed,” she said. “Future studies should focus on randomized controlled study designs with well-described and complete PBMT parameters in a larger and more diverse patient population. This would enable the implementation of PBM in the field of ARD and supportive cancer care, which would enhance wound care management and improve the patient’s quality of life.”
This work won a “best of clinical applications” abstract award from the ASLMS.
The research is part of the Limburg Clinical Research Center UHasselt-ZOL-Jessa, financially supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish Government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital. The research is also funded by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society, Limburgs Kankerfonds, and ASA Srl. Dr. Robijns reported having no financial disclosures.
FROM ASLMS 2021
Medical homes a boon to patients with bleeding disorders
As bleeding disorders are increasingly recognized as a national health priority, hematologists are focusing on how the patient-centered medical home – a widely accepted concept in primary care and in some specialties – can improve outcomes and quality life for their patients.
The patient-centered medical home is a model of health care delivery in which patients receive comprehensive, accessible care that is fully integrated across all providers and elements of a healthcare system.1 The concept emerged in the 1960s among pediatricians seeking to better coordinate care for children with complex medical needs. Since then, the patient-centered medical home has become a globally recognized standard – not only in primary care, but also in specialties such as endocrinology, oncology, and geriatric medicine. The movement to establish medical homes for patients with bleeding disorders is more recent and is receiving national attention.
Why a medical home?
The advent of prophylactic therapies for bleeding disorders has vastly improved the outlook for many patients compared to just a few decades ago. However, treatment options remain limited, and patients who have severe disease or complications – such as an inadequate treatment response or the development of inhibitory antibodies to replacement clotting factors – are at risk for recurrent breakthrough bleeding that can lead to synovitis and ultimately culminate in progressive, irreversible joint damage. The resulting pain and limitation of motion greatly compromises patients’ quality of life across physical, psychological, and social domains, undermines their ability to live and work independently, and greatly increases treatment costs.2-4 Family members, too, face high stress and lower quality of life when they struggle to obtain and manage treatment while caring for loved ones with bleeding disorders.5
For patients with bleeding disorders, a patient-centered medical home can help address or surmount these challenges, said Amy Shapiro, MD, medical director of the Indiana Hemophilia and Thrombosis Center in Indianapolis, Ind., which was the first hemophilia treatment center in the country to be formally certified as a medical home.
Dr. Shapiro explained that a patient-centered medical home leverages the care of an integrated multidisciplinary team to help optimize therapies and patient outcomes across all domains of life. She sees the medical home concept as a natural fit for patients with bleeding disorders, given the complexity of their needs and the number of specialties involved. “When you have hemophilia, you don’t just need a hematologist to manage your care. You need nurses, physical therapists, and social workers. You need coordinated care for genetic counseling. You also need to coordinate dental hygiene and surgical interventions, if these are required. Patients need nutrition counseling, and they may need assistance with education or career options if too many days are missed from work or school. Patients or their families may need counseling on choosing the right insurance program so they don’t choose a plan that may create more hardships for them because of their chronic disorder.”
Meeting these needs requires the help of an integrated care team, which many individuals with bleeding disorders lack. “If you are just out there in the community and you have medical issues that need to be dealt with, often the individuals themselves have to coordinate their own care, including their medications and their appointments with different specialists,” said Dr. Shapiro. “For example, a care provider may tell a patient that they need a physical therapist and give them some names, and then the patient has to take it from there and not only find the provider, but also determine if their insurance provides coverage.”
A medical home takes a completely different approach, she explained. “At my center, when we say you need a physical therapist, we have a physical therapist on staff. Our therapist provides an assessment and determines the need for ongoing PT and whether that can be done at home with a plan and intermittent oversight, or whether the patient needs a referral, and whether the person the patient is referred to needs education on how to provide PT for someone with hemophilia. A medical home provides all this in one place. It is a place where patients know they will receive either direct services, or support to shepherd their care and outcomes, and oversight of that support as well.”
Few studies have directly assessed the medical home model in the setting of bleeding disorders, but a number have evaluated the impact of integrated care, a more general term for the practice of coordinating multidisciplinary care to improve access and outcomes while eliminating redundancies and unnecessary costs. In a recent systematic review and meta-analysis of 27 nonrandomized studies of patients with hemophilia, integrated care was linked to lower mortality, fewer emergency room visits and hospitalizations, shorter lengths of stay in the hospital, and fewer missed days of school and work.6 Such findings, combined with promising outcomes data from studies of patient-centered medical homes in other disease settings, suggest that the patient-centered medical home can significantly benefit patients with bleeding disorders and their families and caregivers.
Creating a medical home
Establishing a patient-centered medical home can be challenging, involving a plethora of stakeholders and a considerable investment of time, energy, and resources. Organizations such as the National Committee for Quality Assurance and the Accreditation Association for Ambulatory Health Care have formal certification programs to help ensure that an inpatient or outpatient center that calls itself a medical home truly is one.7-8
The certification process requires centers to document activities in areas such as patients’ rights and responsibilities, administration and governance, patient and care team relationships, clinical records and other health information, and quality, comprehensiveness, continuity, and accessibility.7 Achieving certification is rigorous, often requiring centers to document compliance with more than 100 policies, procedures, and standards.
For the Indiana Hemophilia and Thrombosis Center, becoming certified as a medical home “was a multiyear process and an ongoing process,” said Dr. Shapiro. “It involves documentation of quality improvement initiatives, obtaining input from patients to document their satisfaction, and looking at all types of systems within our center and how we integrate care so that all those systems function together. It’s a difficult process, but treatment centers are a medical home for patients with bleeding disorders, and this is an effort to provide some documentation on a national level of how we’re doing everything that we are doing.”
She noted that the process of obtaining medical home certification may require an even higher level of commitment if a bleeding disorder (hemophilia) treatment center is embedded in a university or academic medical center. In this case, more stakeholders are involved, and more hoops may need to be jumped through to implement processes that meet medical home standards while still adhering to any requirements at the organizational level.
Certification programs for patient-centered medical homes are not designed around specific disorders or diseases, but a closer look at their compliance metrics underscores how medical homes can benefit patients with bleeding disorders. For example, to receive medical home certification from the Accreditation Association for Ambulatory Health Care, a center needs to be able to document that patients’ care is not transferred without first making arrangements with a receiving health care provider, that the quality improvement programs are peer-led, and that these programs assess and address diverse measures of clinical performance, cost-effectiveness, and administrative functioning.7-9
Medical homes, the NHPCC, and Healthy People 2030
Creating patient-centered medical homes for patients with bleeding disorders is now a quality improvement objective of the National Hemophilia Program Coordinating Center, or NHPCC. Established in 2012 and funded by the federal Health Resources and Services Administration, the NHPCC partners with the eight regional hemophilia networks and more than 140 federally funded hemophilia treatment centers across the United States to identify gaps, standardize and improve access to care, and share and promote best practices for the treatment and management of blood disorders.10
In the United States, receiving care in a hemophilia treatment center (which, despite its name, typically offers care for other disorders such as von Willebrand disease) has been linked to lower mortality and fewer hospitalizations related to bleeding complications.11 To continue to improve on these outcomes, the NHPCC, regional networks, and hemophilia treatment centers are prioritizing medical homes and ranking their establishments alongside core objectives such as bettering patient and family engagement and improving the transition from pediatric to adult care.12
As part of this quality improvement work, the NHPCC, regional leadership, and hemophilia treatment centers meet regularly to identify needs and priorities, plan programs, and ensure that each center is meeting the goals and objectives set out by its federal grant.13 Such partnerships help improve and integrate care within a coordinated national framework, Dr. Shapiro said. “We all are charged with this same mission,” she added. “That doesn’t mean that every treatment center looks exactly the same, has the same number of staff, or does everything the same way, but we all have the same mission, and we know what that is. That is the work of the NHPCC, to determine and document that and help level and improve care throughout the country.”
The NHPCC also engages other stakeholders, including consumer agencies and professional organizations. Recent achievements have included a first-ever national patient needs assessment, a tandem technical needs assessment of hemophilia treatment centers, an educational outreach program for genetic counselors, a webinar on transitioning care for adolescents, a national survey of the federal 340B Drug Pricing Program, and a survey of minority patients to identify and characterize problems such as language and insurance barriers, the lack of culturally appropriate educational materials on blood disorders, and difficulties getting transportation to treatment centers or educational programs.14
In part because of this advocacy work, the U.S. Department of Health and Human Services recently included hemophilia for the first time in Healthy People, its evidence-based set of decade-long objectives aimed at improving the health of all Americans. In Healthy People 2030, the specific objective for hemophilia is to reduce the proportion of patients with severe disease who experience more than four joint bleeds per year to 13.3% (the current estimate is 16.9%).15
For Healthy People to prioritize hemophilia for the first time alongside much more common conditions such as diabetes and heart disease reflects the challenges of managing bleeding disorders and the efforts by the NHPCC and other stakeholders to raise awareness about current needs. To track progress in meeting the Healthy People 2030 objective, the NHPCC will work with federal partners to analyze patient-level data gathered through the Centers for Disease Control’s Community Counts Registry for Bleeding Disorders Surveillance program, which collects data from hemophilia treatment centers across the United States and includes patients with all levels of disease severity.
“The inclusion of bleeding disorders in Healthy People 2030 is really very significant,” said Dr. Shapiro. “These are disorders that affect less than 200,000 Americans, which is the definition of a rare disease in this context. To have hemophilia considered as a national priority is very important, not only for hemophilia, but also for other rare diseases that may in the future also be considered as being as of national importance in this way.”
References
1. Rodriguez-Saldana J. 2019. The Patient-Centered Medical Home, Primary Care, and Diabetes. In: Rodriguez-Saldana J. (eds) The Diabetes Textbook. Springer, Cham.
2. J Comorb. 2011;1:51-59.
3. Eur J Haematol. 2018 Apr;100 Suppl 1:5-13.
4. Blood. 2003;102(7):2358-63.
5. Haemophilia. 2014 Jul;20(4):541-9.
6. Haemophilia. 2016;22(Suppl 3):31-40.
7. AAAHC. Medical Home.
8. NCQA. Patient-centered medical home (PCMH).
9. AAAHC, 2013. Medical Home On-Site Certification Handbook.
10. Centers for Disease Control and Prevention. HTC Population Profile.
11. Blood Transfus. 2014;12 Suppl 3(Suppl 3):e542-e548.
12. American Thrombosis and Hemostasis Network.
13. The Great Lakes Regional Hemophilia Network.
14. American Thrombosis and Hemostasis Network. What the NHPCC does.
15. U.S. Department of Health and Human Services. Healthy People 2030: Blood Disorders.
As bleeding disorders are increasingly recognized as a national health priority, hematologists are focusing on how the patient-centered medical home – a widely accepted concept in primary care and in some specialties – can improve outcomes and quality life for their patients.
The patient-centered medical home is a model of health care delivery in which patients receive comprehensive, accessible care that is fully integrated across all providers and elements of a healthcare system.1 The concept emerged in the 1960s among pediatricians seeking to better coordinate care for children with complex medical needs. Since then, the patient-centered medical home has become a globally recognized standard – not only in primary care, but also in specialties such as endocrinology, oncology, and geriatric medicine. The movement to establish medical homes for patients with bleeding disorders is more recent and is receiving national attention.
Why a medical home?
The advent of prophylactic therapies for bleeding disorders has vastly improved the outlook for many patients compared to just a few decades ago. However, treatment options remain limited, and patients who have severe disease or complications – such as an inadequate treatment response or the development of inhibitory antibodies to replacement clotting factors – are at risk for recurrent breakthrough bleeding that can lead to synovitis and ultimately culminate in progressive, irreversible joint damage. The resulting pain and limitation of motion greatly compromises patients’ quality of life across physical, psychological, and social domains, undermines their ability to live and work independently, and greatly increases treatment costs.2-4 Family members, too, face high stress and lower quality of life when they struggle to obtain and manage treatment while caring for loved ones with bleeding disorders.5
For patients with bleeding disorders, a patient-centered medical home can help address or surmount these challenges, said Amy Shapiro, MD, medical director of the Indiana Hemophilia and Thrombosis Center in Indianapolis, Ind., which was the first hemophilia treatment center in the country to be formally certified as a medical home.
Dr. Shapiro explained that a patient-centered medical home leverages the care of an integrated multidisciplinary team to help optimize therapies and patient outcomes across all domains of life. She sees the medical home concept as a natural fit for patients with bleeding disorders, given the complexity of their needs and the number of specialties involved. “When you have hemophilia, you don’t just need a hematologist to manage your care. You need nurses, physical therapists, and social workers. You need coordinated care for genetic counseling. You also need to coordinate dental hygiene and surgical interventions, if these are required. Patients need nutrition counseling, and they may need assistance with education or career options if too many days are missed from work or school. Patients or their families may need counseling on choosing the right insurance program so they don’t choose a plan that may create more hardships for them because of their chronic disorder.”
Meeting these needs requires the help of an integrated care team, which many individuals with bleeding disorders lack. “If you are just out there in the community and you have medical issues that need to be dealt with, often the individuals themselves have to coordinate their own care, including their medications and their appointments with different specialists,” said Dr. Shapiro. “For example, a care provider may tell a patient that they need a physical therapist and give them some names, and then the patient has to take it from there and not only find the provider, but also determine if their insurance provides coverage.”
A medical home takes a completely different approach, she explained. “At my center, when we say you need a physical therapist, we have a physical therapist on staff. Our therapist provides an assessment and determines the need for ongoing PT and whether that can be done at home with a plan and intermittent oversight, or whether the patient needs a referral, and whether the person the patient is referred to needs education on how to provide PT for someone with hemophilia. A medical home provides all this in one place. It is a place where patients know they will receive either direct services, or support to shepherd their care and outcomes, and oversight of that support as well.”
Few studies have directly assessed the medical home model in the setting of bleeding disorders, but a number have evaluated the impact of integrated care, a more general term for the practice of coordinating multidisciplinary care to improve access and outcomes while eliminating redundancies and unnecessary costs. In a recent systematic review and meta-analysis of 27 nonrandomized studies of patients with hemophilia, integrated care was linked to lower mortality, fewer emergency room visits and hospitalizations, shorter lengths of stay in the hospital, and fewer missed days of school and work.6 Such findings, combined with promising outcomes data from studies of patient-centered medical homes in other disease settings, suggest that the patient-centered medical home can significantly benefit patients with bleeding disorders and their families and caregivers.
Creating a medical home
Establishing a patient-centered medical home can be challenging, involving a plethora of stakeholders and a considerable investment of time, energy, and resources. Organizations such as the National Committee for Quality Assurance and the Accreditation Association for Ambulatory Health Care have formal certification programs to help ensure that an inpatient or outpatient center that calls itself a medical home truly is one.7-8
The certification process requires centers to document activities in areas such as patients’ rights and responsibilities, administration and governance, patient and care team relationships, clinical records and other health information, and quality, comprehensiveness, continuity, and accessibility.7 Achieving certification is rigorous, often requiring centers to document compliance with more than 100 policies, procedures, and standards.
For the Indiana Hemophilia and Thrombosis Center, becoming certified as a medical home “was a multiyear process and an ongoing process,” said Dr. Shapiro. “It involves documentation of quality improvement initiatives, obtaining input from patients to document their satisfaction, and looking at all types of systems within our center and how we integrate care so that all those systems function together. It’s a difficult process, but treatment centers are a medical home for patients with bleeding disorders, and this is an effort to provide some documentation on a national level of how we’re doing everything that we are doing.”
She noted that the process of obtaining medical home certification may require an even higher level of commitment if a bleeding disorder (hemophilia) treatment center is embedded in a university or academic medical center. In this case, more stakeholders are involved, and more hoops may need to be jumped through to implement processes that meet medical home standards while still adhering to any requirements at the organizational level.
Certification programs for patient-centered medical homes are not designed around specific disorders or diseases, but a closer look at their compliance metrics underscores how medical homes can benefit patients with bleeding disorders. For example, to receive medical home certification from the Accreditation Association for Ambulatory Health Care, a center needs to be able to document that patients’ care is not transferred without first making arrangements with a receiving health care provider, that the quality improvement programs are peer-led, and that these programs assess and address diverse measures of clinical performance, cost-effectiveness, and administrative functioning.7-9
Medical homes, the NHPCC, and Healthy People 2030
Creating patient-centered medical homes for patients with bleeding disorders is now a quality improvement objective of the National Hemophilia Program Coordinating Center, or NHPCC. Established in 2012 and funded by the federal Health Resources and Services Administration, the NHPCC partners with the eight regional hemophilia networks and more than 140 federally funded hemophilia treatment centers across the United States to identify gaps, standardize and improve access to care, and share and promote best practices for the treatment and management of blood disorders.10
In the United States, receiving care in a hemophilia treatment center (which, despite its name, typically offers care for other disorders such as von Willebrand disease) has been linked to lower mortality and fewer hospitalizations related to bleeding complications.11 To continue to improve on these outcomes, the NHPCC, regional networks, and hemophilia treatment centers are prioritizing medical homes and ranking their establishments alongside core objectives such as bettering patient and family engagement and improving the transition from pediatric to adult care.12
As part of this quality improvement work, the NHPCC, regional leadership, and hemophilia treatment centers meet regularly to identify needs and priorities, plan programs, and ensure that each center is meeting the goals and objectives set out by its federal grant.13 Such partnerships help improve and integrate care within a coordinated national framework, Dr. Shapiro said. “We all are charged with this same mission,” she added. “That doesn’t mean that every treatment center looks exactly the same, has the same number of staff, or does everything the same way, but we all have the same mission, and we know what that is. That is the work of the NHPCC, to determine and document that and help level and improve care throughout the country.”
The NHPCC also engages other stakeholders, including consumer agencies and professional organizations. Recent achievements have included a first-ever national patient needs assessment, a tandem technical needs assessment of hemophilia treatment centers, an educational outreach program for genetic counselors, a webinar on transitioning care for adolescents, a national survey of the federal 340B Drug Pricing Program, and a survey of minority patients to identify and characterize problems such as language and insurance barriers, the lack of culturally appropriate educational materials on blood disorders, and difficulties getting transportation to treatment centers or educational programs.14
In part because of this advocacy work, the U.S. Department of Health and Human Services recently included hemophilia for the first time in Healthy People, its evidence-based set of decade-long objectives aimed at improving the health of all Americans. In Healthy People 2030, the specific objective for hemophilia is to reduce the proportion of patients with severe disease who experience more than four joint bleeds per year to 13.3% (the current estimate is 16.9%).15
For Healthy People to prioritize hemophilia for the first time alongside much more common conditions such as diabetes and heart disease reflects the challenges of managing bleeding disorders and the efforts by the NHPCC and other stakeholders to raise awareness about current needs. To track progress in meeting the Healthy People 2030 objective, the NHPCC will work with federal partners to analyze patient-level data gathered through the Centers for Disease Control’s Community Counts Registry for Bleeding Disorders Surveillance program, which collects data from hemophilia treatment centers across the United States and includes patients with all levels of disease severity.
“The inclusion of bleeding disorders in Healthy People 2030 is really very significant,” said Dr. Shapiro. “These are disorders that affect less than 200,000 Americans, which is the definition of a rare disease in this context. To have hemophilia considered as a national priority is very important, not only for hemophilia, but also for other rare diseases that may in the future also be considered as being as of national importance in this way.”
References
1. Rodriguez-Saldana J. 2019. The Patient-Centered Medical Home, Primary Care, and Diabetes. In: Rodriguez-Saldana J. (eds) The Diabetes Textbook. Springer, Cham.
2. J Comorb. 2011;1:51-59.
3. Eur J Haematol. 2018 Apr;100 Suppl 1:5-13.
4. Blood. 2003;102(7):2358-63.
5. Haemophilia. 2014 Jul;20(4):541-9.
6. Haemophilia. 2016;22(Suppl 3):31-40.
7. AAAHC. Medical Home.
8. NCQA. Patient-centered medical home (PCMH).
9. AAAHC, 2013. Medical Home On-Site Certification Handbook.
10. Centers for Disease Control and Prevention. HTC Population Profile.
11. Blood Transfus. 2014;12 Suppl 3(Suppl 3):e542-e548.
12. American Thrombosis and Hemostasis Network.
13. The Great Lakes Regional Hemophilia Network.
14. American Thrombosis and Hemostasis Network. What the NHPCC does.
15. U.S. Department of Health and Human Services. Healthy People 2030: Blood Disorders.
As bleeding disorders are increasingly recognized as a national health priority, hematologists are focusing on how the patient-centered medical home – a widely accepted concept in primary care and in some specialties – can improve outcomes and quality life for their patients.
The patient-centered medical home is a model of health care delivery in which patients receive comprehensive, accessible care that is fully integrated across all providers and elements of a healthcare system.1 The concept emerged in the 1960s among pediatricians seeking to better coordinate care for children with complex medical needs. Since then, the patient-centered medical home has become a globally recognized standard – not only in primary care, but also in specialties such as endocrinology, oncology, and geriatric medicine. The movement to establish medical homes for patients with bleeding disorders is more recent and is receiving national attention.
Why a medical home?
The advent of prophylactic therapies for bleeding disorders has vastly improved the outlook for many patients compared to just a few decades ago. However, treatment options remain limited, and patients who have severe disease or complications – such as an inadequate treatment response or the development of inhibitory antibodies to replacement clotting factors – are at risk for recurrent breakthrough bleeding that can lead to synovitis and ultimately culminate in progressive, irreversible joint damage. The resulting pain and limitation of motion greatly compromises patients’ quality of life across physical, psychological, and social domains, undermines their ability to live and work independently, and greatly increases treatment costs.2-4 Family members, too, face high stress and lower quality of life when they struggle to obtain and manage treatment while caring for loved ones with bleeding disorders.5
For patients with bleeding disorders, a patient-centered medical home can help address or surmount these challenges, said Amy Shapiro, MD, medical director of the Indiana Hemophilia and Thrombosis Center in Indianapolis, Ind., which was the first hemophilia treatment center in the country to be formally certified as a medical home.
Dr. Shapiro explained that a patient-centered medical home leverages the care of an integrated multidisciplinary team to help optimize therapies and patient outcomes across all domains of life. She sees the medical home concept as a natural fit for patients with bleeding disorders, given the complexity of their needs and the number of specialties involved. “When you have hemophilia, you don’t just need a hematologist to manage your care. You need nurses, physical therapists, and social workers. You need coordinated care for genetic counseling. You also need to coordinate dental hygiene and surgical interventions, if these are required. Patients need nutrition counseling, and they may need assistance with education or career options if too many days are missed from work or school. Patients or their families may need counseling on choosing the right insurance program so they don’t choose a plan that may create more hardships for them because of their chronic disorder.”
Meeting these needs requires the help of an integrated care team, which many individuals with bleeding disorders lack. “If you are just out there in the community and you have medical issues that need to be dealt with, often the individuals themselves have to coordinate their own care, including their medications and their appointments with different specialists,” said Dr. Shapiro. “For example, a care provider may tell a patient that they need a physical therapist and give them some names, and then the patient has to take it from there and not only find the provider, but also determine if their insurance provides coverage.”
A medical home takes a completely different approach, she explained. “At my center, when we say you need a physical therapist, we have a physical therapist on staff. Our therapist provides an assessment and determines the need for ongoing PT and whether that can be done at home with a plan and intermittent oversight, or whether the patient needs a referral, and whether the person the patient is referred to needs education on how to provide PT for someone with hemophilia. A medical home provides all this in one place. It is a place where patients know they will receive either direct services, or support to shepherd their care and outcomes, and oversight of that support as well.”
Few studies have directly assessed the medical home model in the setting of bleeding disorders, but a number have evaluated the impact of integrated care, a more general term for the practice of coordinating multidisciplinary care to improve access and outcomes while eliminating redundancies and unnecessary costs. In a recent systematic review and meta-analysis of 27 nonrandomized studies of patients with hemophilia, integrated care was linked to lower mortality, fewer emergency room visits and hospitalizations, shorter lengths of stay in the hospital, and fewer missed days of school and work.6 Such findings, combined with promising outcomes data from studies of patient-centered medical homes in other disease settings, suggest that the patient-centered medical home can significantly benefit patients with bleeding disorders and their families and caregivers.
Creating a medical home
Establishing a patient-centered medical home can be challenging, involving a plethora of stakeholders and a considerable investment of time, energy, and resources. Organizations such as the National Committee for Quality Assurance and the Accreditation Association for Ambulatory Health Care have formal certification programs to help ensure that an inpatient or outpatient center that calls itself a medical home truly is one.7-8
The certification process requires centers to document activities in areas such as patients’ rights and responsibilities, administration and governance, patient and care team relationships, clinical records and other health information, and quality, comprehensiveness, continuity, and accessibility.7 Achieving certification is rigorous, often requiring centers to document compliance with more than 100 policies, procedures, and standards.
For the Indiana Hemophilia and Thrombosis Center, becoming certified as a medical home “was a multiyear process and an ongoing process,” said Dr. Shapiro. “It involves documentation of quality improvement initiatives, obtaining input from patients to document their satisfaction, and looking at all types of systems within our center and how we integrate care so that all those systems function together. It’s a difficult process, but treatment centers are a medical home for patients with bleeding disorders, and this is an effort to provide some documentation on a national level of how we’re doing everything that we are doing.”
She noted that the process of obtaining medical home certification may require an even higher level of commitment if a bleeding disorder (hemophilia) treatment center is embedded in a university or academic medical center. In this case, more stakeholders are involved, and more hoops may need to be jumped through to implement processes that meet medical home standards while still adhering to any requirements at the organizational level.
Certification programs for patient-centered medical homes are not designed around specific disorders or diseases, but a closer look at their compliance metrics underscores how medical homes can benefit patients with bleeding disorders. For example, to receive medical home certification from the Accreditation Association for Ambulatory Health Care, a center needs to be able to document that patients’ care is not transferred without first making arrangements with a receiving health care provider, that the quality improvement programs are peer-led, and that these programs assess and address diverse measures of clinical performance, cost-effectiveness, and administrative functioning.7-9
Medical homes, the NHPCC, and Healthy People 2030
Creating patient-centered medical homes for patients with bleeding disorders is now a quality improvement objective of the National Hemophilia Program Coordinating Center, or NHPCC. Established in 2012 and funded by the federal Health Resources and Services Administration, the NHPCC partners with the eight regional hemophilia networks and more than 140 federally funded hemophilia treatment centers across the United States to identify gaps, standardize and improve access to care, and share and promote best practices for the treatment and management of blood disorders.10
In the United States, receiving care in a hemophilia treatment center (which, despite its name, typically offers care for other disorders such as von Willebrand disease) has been linked to lower mortality and fewer hospitalizations related to bleeding complications.11 To continue to improve on these outcomes, the NHPCC, regional networks, and hemophilia treatment centers are prioritizing medical homes and ranking their establishments alongside core objectives such as bettering patient and family engagement and improving the transition from pediatric to adult care.12
As part of this quality improvement work, the NHPCC, regional leadership, and hemophilia treatment centers meet regularly to identify needs and priorities, plan programs, and ensure that each center is meeting the goals and objectives set out by its federal grant.13 Such partnerships help improve and integrate care within a coordinated national framework, Dr. Shapiro said. “We all are charged with this same mission,” she added. “That doesn’t mean that every treatment center looks exactly the same, has the same number of staff, or does everything the same way, but we all have the same mission, and we know what that is. That is the work of the NHPCC, to determine and document that and help level and improve care throughout the country.”
The NHPCC also engages other stakeholders, including consumer agencies and professional organizations. Recent achievements have included a first-ever national patient needs assessment, a tandem technical needs assessment of hemophilia treatment centers, an educational outreach program for genetic counselors, a webinar on transitioning care for adolescents, a national survey of the federal 340B Drug Pricing Program, and a survey of minority patients to identify and characterize problems such as language and insurance barriers, the lack of culturally appropriate educational materials on blood disorders, and difficulties getting transportation to treatment centers or educational programs.14
In part because of this advocacy work, the U.S. Department of Health and Human Services recently included hemophilia for the first time in Healthy People, its evidence-based set of decade-long objectives aimed at improving the health of all Americans. In Healthy People 2030, the specific objective for hemophilia is to reduce the proportion of patients with severe disease who experience more than four joint bleeds per year to 13.3% (the current estimate is 16.9%).15
For Healthy People to prioritize hemophilia for the first time alongside much more common conditions such as diabetes and heart disease reflects the challenges of managing bleeding disorders and the efforts by the NHPCC and other stakeholders to raise awareness about current needs. To track progress in meeting the Healthy People 2030 objective, the NHPCC will work with federal partners to analyze patient-level data gathered through the Centers for Disease Control’s Community Counts Registry for Bleeding Disorders Surveillance program, which collects data from hemophilia treatment centers across the United States and includes patients with all levels of disease severity.
“The inclusion of bleeding disorders in Healthy People 2030 is really very significant,” said Dr. Shapiro. “These are disorders that affect less than 200,000 Americans, which is the definition of a rare disease in this context. To have hemophilia considered as a national priority is very important, not only for hemophilia, but also for other rare diseases that may in the future also be considered as being as of national importance in this way.”
References
1. Rodriguez-Saldana J. 2019. The Patient-Centered Medical Home, Primary Care, and Diabetes. In: Rodriguez-Saldana J. (eds) The Diabetes Textbook. Springer, Cham.
2. J Comorb. 2011;1:51-59.
3. Eur J Haematol. 2018 Apr;100 Suppl 1:5-13.
4. Blood. 2003;102(7):2358-63.
5. Haemophilia. 2014 Jul;20(4):541-9.
6. Haemophilia. 2016;22(Suppl 3):31-40.
7. AAAHC. Medical Home.
8. NCQA. Patient-centered medical home (PCMH).
9. AAAHC, 2013. Medical Home On-Site Certification Handbook.
10. Centers for Disease Control and Prevention. HTC Population Profile.
11. Blood Transfus. 2014;12 Suppl 3(Suppl 3):e542-e548.
12. American Thrombosis and Hemostasis Network.
13. The Great Lakes Regional Hemophilia Network.
14. American Thrombosis and Hemostasis Network. What the NHPCC does.
15. U.S. Department of Health and Human Services. Healthy People 2030: Blood Disorders.
Sex differences in pediatric B-ALL outcomes persist
Even in the age of intensive therapy and extensive risk stratification, there are small but significant differences in outcomes between boys and girls with B-lineage acute lymphoblastic leukemia (B-ALL).
This finding comes from a review of 10 years of clinical trials by the Children’s Oncology Group (COG), which showed that, among patients with B-ALL, 5-year event-free survival (EFS) and overall survival (OS) were inferior with boys, compared with girls, even when adjusted for prognostic factors, reported Sumit Gupta, MD, PhD, FRCPC, from the Hospital for Sick Children in Toronto.
“Inferior outcomes, although small in absolute terms, continue to exist among boys versus girls despite modern therapy and after adjusting for other risk factors. These persist also despite the longer duration of therapy among boys,” he said in an oral abstract presentation during the annual meeting of the American Society of Pediatric Hematology/Oncology. (Abstract 2025).
Among pediatric patients with T-cell lineage ALL (T-ALL), however, there were no significant sex-based differences in either EFS or OS, he said.
Although survival for children with ALL has continued to improve, previous studies found inferior survival outcomes in boys, and suggested that the difference might be explained by imbalances in risk factors.
To see whether sex-based disparities persist with modern intensive therapy protocols after adjustment for risk factors, and to determine whether there are sex-based differences in toxicities or patterns of treatment failure, Dr. Gupta and colleagues created a cohort of all patients age 1-30 years enrolled in frontline COG trial for B-ALL and T-ALL from 2004 to 2014.
During this period, boys received an extra year of maintenance. Cranial radiation was limited to B-ALL patients with slow treatment responses and central nervous system status 3, signifying definite CNS involvement. Among patients with T-ALL, cranial radiation was given to all intermediate- and high-risk patients.
Sex differences small, but significant
The investigators identified a total of 8,202 patients (4,463 males and 3,739 females) with B-ALL, and 1,562 (1,161 males and 401 females) with T-ALL. Boys were likely to be older (P < .0001), and to have a small but significantly greater likelihood of having unfavorable B-ALL cytogenetics, compared with girls (P = .05).
Boys with B-ALL were less likely to be negative for minimal residual disease (76.1% vs. 78.1%, P = .04), but the opposite was true for those with T-ALL (59% vs. 56.8%, P = .01).
As noted before, among pediatric patients with B-ALL, EFS and OS were both inferior for males, with a hazard ratio for higher EFS rates in girls of 1.19 (P = .001) and a HR for OS of 1.17 (P = .046).
Both EFS and OS were similar between the sexes among patients with T-ALL.
The differences in EFS in patients with B-ALL was attributable to higher CNS relapses among boys (4.2% vs. 2.5%, P < .0001). The CNS relapses occurred at a median of 2.5 years in boys versus 2.1 years in girls, although most relapses occurred during therapy.
There were no differences in cumulative isolated bone marrow relapses, however.
Treatment-related mortality rates were the same, but osteonecrosis rates were significantly lower for boys, with a 5-year cumulative incidence of 5.2% versus 6.7% for girls (P = .001).
Possible explanations
Dr. Gupta noted that the inferior outcomes among boys may be attributable to extramedullary relapses among patients with B-ALL.
In addition, the lack of sex-based differences in T-ALL may be caused in part by the increased use of CNS radiation in this population. Previous studies in which CNS radiation was omitted showed an increase in CNS relapsed rates among boys but not girls, he pointed out.
“This does imply that in the more recent generation of T-lineage ALL treatment trials that we’ll need to monitor sex-based differences in outcome, as fewer and fewer patients with T-ALL disease received cranial radiation in these more recent trials and in contemporary therapy,” he said.
One possible mechanism for sex-based outcome differences might be differences in steroid metabolism, as suggested by the higher osteonecrosis rate among girls, he added.
In the question-and-answer following the presentation, William G. Woods, MD, from Emory University, Atlanta, asked what role testicular relapse played in outcomes.
Dr. Gupta replied that the investigators had considered that the excess risk for extramedullary relapse in boys might be accounted for by testicular relapse, but “when you take away testicular relapse from those numbers and really just concentrate on CNS, it’s still that substantial difference when you’re talking about B-lineage disease.”
In patients with T-ALL as well, CNS relapse was more common in boys after controlling for testicular relapse, he said.
Another audience member asked whether the data suggest a benefit to treating boys with CNS-penetrating drugs such as dexamethasone or high-dose methotrexate,
Dr. Gupta said that it’s still uncertain whether it is clinically sound to subject a boy with otherwise–standard-risk disease to more intensive high-risk therapy, given the relatively small absolute differences in outcomes between the sexes.
The study was supported by grants from the National Cancer Institute and the St. Baldrick’s Foundation. Dr. Gupta, Dr. Woods, and Dr. Meret had no relevant conflicts of interest to report.
Even in the age of intensive therapy and extensive risk stratification, there are small but significant differences in outcomes between boys and girls with B-lineage acute lymphoblastic leukemia (B-ALL).
This finding comes from a review of 10 years of clinical trials by the Children’s Oncology Group (COG), which showed that, among patients with B-ALL, 5-year event-free survival (EFS) and overall survival (OS) were inferior with boys, compared with girls, even when adjusted for prognostic factors, reported Sumit Gupta, MD, PhD, FRCPC, from the Hospital for Sick Children in Toronto.
“Inferior outcomes, although small in absolute terms, continue to exist among boys versus girls despite modern therapy and after adjusting for other risk factors. These persist also despite the longer duration of therapy among boys,” he said in an oral abstract presentation during the annual meeting of the American Society of Pediatric Hematology/Oncology. (Abstract 2025).
Among pediatric patients with T-cell lineage ALL (T-ALL), however, there were no significant sex-based differences in either EFS or OS, he said.
Although survival for children with ALL has continued to improve, previous studies found inferior survival outcomes in boys, and suggested that the difference might be explained by imbalances in risk factors.
To see whether sex-based disparities persist with modern intensive therapy protocols after adjustment for risk factors, and to determine whether there are sex-based differences in toxicities or patterns of treatment failure, Dr. Gupta and colleagues created a cohort of all patients age 1-30 years enrolled in frontline COG trial for B-ALL and T-ALL from 2004 to 2014.
During this period, boys received an extra year of maintenance. Cranial radiation was limited to B-ALL patients with slow treatment responses and central nervous system status 3, signifying definite CNS involvement. Among patients with T-ALL, cranial radiation was given to all intermediate- and high-risk patients.
Sex differences small, but significant
The investigators identified a total of 8,202 patients (4,463 males and 3,739 females) with B-ALL, and 1,562 (1,161 males and 401 females) with T-ALL. Boys were likely to be older (P < .0001), and to have a small but significantly greater likelihood of having unfavorable B-ALL cytogenetics, compared with girls (P = .05).
Boys with B-ALL were less likely to be negative for minimal residual disease (76.1% vs. 78.1%, P = .04), but the opposite was true for those with T-ALL (59% vs. 56.8%, P = .01).
As noted before, among pediatric patients with B-ALL, EFS and OS were both inferior for males, with a hazard ratio for higher EFS rates in girls of 1.19 (P = .001) and a HR for OS of 1.17 (P = .046).
Both EFS and OS were similar between the sexes among patients with T-ALL.
The differences in EFS in patients with B-ALL was attributable to higher CNS relapses among boys (4.2% vs. 2.5%, P < .0001). The CNS relapses occurred at a median of 2.5 years in boys versus 2.1 years in girls, although most relapses occurred during therapy.
There were no differences in cumulative isolated bone marrow relapses, however.
Treatment-related mortality rates were the same, but osteonecrosis rates were significantly lower for boys, with a 5-year cumulative incidence of 5.2% versus 6.7% for girls (P = .001).
Possible explanations
Dr. Gupta noted that the inferior outcomes among boys may be attributable to extramedullary relapses among patients with B-ALL.
In addition, the lack of sex-based differences in T-ALL may be caused in part by the increased use of CNS radiation in this population. Previous studies in which CNS radiation was omitted showed an increase in CNS relapsed rates among boys but not girls, he pointed out.
“This does imply that in the more recent generation of T-lineage ALL treatment trials that we’ll need to monitor sex-based differences in outcome, as fewer and fewer patients with T-ALL disease received cranial radiation in these more recent trials and in contemporary therapy,” he said.
One possible mechanism for sex-based outcome differences might be differences in steroid metabolism, as suggested by the higher osteonecrosis rate among girls, he added.
In the question-and-answer following the presentation, William G. Woods, MD, from Emory University, Atlanta, asked what role testicular relapse played in outcomes.
Dr. Gupta replied that the investigators had considered that the excess risk for extramedullary relapse in boys might be accounted for by testicular relapse, but “when you take away testicular relapse from those numbers and really just concentrate on CNS, it’s still that substantial difference when you’re talking about B-lineage disease.”
In patients with T-ALL as well, CNS relapse was more common in boys after controlling for testicular relapse, he said.
Another audience member asked whether the data suggest a benefit to treating boys with CNS-penetrating drugs such as dexamethasone or high-dose methotrexate,
Dr. Gupta said that it’s still uncertain whether it is clinically sound to subject a boy with otherwise–standard-risk disease to more intensive high-risk therapy, given the relatively small absolute differences in outcomes between the sexes.
The study was supported by grants from the National Cancer Institute and the St. Baldrick’s Foundation. Dr. Gupta, Dr. Woods, and Dr. Meret had no relevant conflicts of interest to report.
Even in the age of intensive therapy and extensive risk stratification, there are small but significant differences in outcomes between boys and girls with B-lineage acute lymphoblastic leukemia (B-ALL).
This finding comes from a review of 10 years of clinical trials by the Children’s Oncology Group (COG), which showed that, among patients with B-ALL, 5-year event-free survival (EFS) and overall survival (OS) were inferior with boys, compared with girls, even when adjusted for prognostic factors, reported Sumit Gupta, MD, PhD, FRCPC, from the Hospital for Sick Children in Toronto.
“Inferior outcomes, although small in absolute terms, continue to exist among boys versus girls despite modern therapy and after adjusting for other risk factors. These persist also despite the longer duration of therapy among boys,” he said in an oral abstract presentation during the annual meeting of the American Society of Pediatric Hematology/Oncology. (Abstract 2025).
Among pediatric patients with T-cell lineage ALL (T-ALL), however, there were no significant sex-based differences in either EFS or OS, he said.
Although survival for children with ALL has continued to improve, previous studies found inferior survival outcomes in boys, and suggested that the difference might be explained by imbalances in risk factors.
To see whether sex-based disparities persist with modern intensive therapy protocols after adjustment for risk factors, and to determine whether there are sex-based differences in toxicities or patterns of treatment failure, Dr. Gupta and colleagues created a cohort of all patients age 1-30 years enrolled in frontline COG trial for B-ALL and T-ALL from 2004 to 2014.
During this period, boys received an extra year of maintenance. Cranial radiation was limited to B-ALL patients with slow treatment responses and central nervous system status 3, signifying definite CNS involvement. Among patients with T-ALL, cranial radiation was given to all intermediate- and high-risk patients.
Sex differences small, but significant
The investigators identified a total of 8,202 patients (4,463 males and 3,739 females) with B-ALL, and 1,562 (1,161 males and 401 females) with T-ALL. Boys were likely to be older (P < .0001), and to have a small but significantly greater likelihood of having unfavorable B-ALL cytogenetics, compared with girls (P = .05).
Boys with B-ALL were less likely to be negative for minimal residual disease (76.1% vs. 78.1%, P = .04), but the opposite was true for those with T-ALL (59% vs. 56.8%, P = .01).
As noted before, among pediatric patients with B-ALL, EFS and OS were both inferior for males, with a hazard ratio for higher EFS rates in girls of 1.19 (P = .001) and a HR for OS of 1.17 (P = .046).
Both EFS and OS were similar between the sexes among patients with T-ALL.
The differences in EFS in patients with B-ALL was attributable to higher CNS relapses among boys (4.2% vs. 2.5%, P < .0001). The CNS relapses occurred at a median of 2.5 years in boys versus 2.1 years in girls, although most relapses occurred during therapy.
There were no differences in cumulative isolated bone marrow relapses, however.
Treatment-related mortality rates were the same, but osteonecrosis rates were significantly lower for boys, with a 5-year cumulative incidence of 5.2% versus 6.7% for girls (P = .001).
Possible explanations
Dr. Gupta noted that the inferior outcomes among boys may be attributable to extramedullary relapses among patients with B-ALL.
In addition, the lack of sex-based differences in T-ALL may be caused in part by the increased use of CNS radiation in this population. Previous studies in which CNS radiation was omitted showed an increase in CNS relapsed rates among boys but not girls, he pointed out.
“This does imply that in the more recent generation of T-lineage ALL treatment trials that we’ll need to monitor sex-based differences in outcome, as fewer and fewer patients with T-ALL disease received cranial radiation in these more recent trials and in contemporary therapy,” he said.
One possible mechanism for sex-based outcome differences might be differences in steroid metabolism, as suggested by the higher osteonecrosis rate among girls, he added.
In the question-and-answer following the presentation, William G. Woods, MD, from Emory University, Atlanta, asked what role testicular relapse played in outcomes.
Dr. Gupta replied that the investigators had considered that the excess risk for extramedullary relapse in boys might be accounted for by testicular relapse, but “when you take away testicular relapse from those numbers and really just concentrate on CNS, it’s still that substantial difference when you’re talking about B-lineage disease.”
In patients with T-ALL as well, CNS relapse was more common in boys after controlling for testicular relapse, he said.
Another audience member asked whether the data suggest a benefit to treating boys with CNS-penetrating drugs such as dexamethasone or high-dose methotrexate,
Dr. Gupta said that it’s still uncertain whether it is clinically sound to subject a boy with otherwise–standard-risk disease to more intensive high-risk therapy, given the relatively small absolute differences in outcomes between the sexes.
The study was supported by grants from the National Cancer Institute and the St. Baldrick’s Foundation. Dr. Gupta, Dr. Woods, and Dr. Meret had no relevant conflicts of interest to report.
FROM ASPHO 2021
High-dose methotrexate of no CNS benefit for patients with high-risk DLBCL
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
Patients with high-risk diffuse large B-cell lymphoma (DLBCL) have a greater than 10% risk of central nervous system (CNS) relapse, and the use of prophylactic high-dose methotrexate (HD-MTX) has been proposed as a preventative measure.
However, the use of prophylactic HD-MTX did not improve CNS or survival outcomes of patients with high-risk DLBCL, but instead was associated with increased toxicities, according to the results of a retrospective study by Hyehyun Jeong, MD, of University of Ulsan College of Medicine, Seoul, Republic of Korea, and colleagues.
The researchers evaluated the effects of prophylactic HD-MTX on CNS relapse and survival outcomes in newly diagnosed R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–treated patients with high-risk DLBCL. The assessment was based on the initial treatment intent (ITT) of the physician on the use of prophylactic HD-MTX.
A total of 5,130 patients were classified into an ITT HD-MTX group and an equal number into a non-ITT HD-MTX group, according to the report, published online in Blood Advances.
Equivalent results
The study showed that the CNS relapse rate was not significantly different between the two groups, with 2-year CNS relapse rates of 12.4% and 13.9%, respectively (P = .96). Three-year progression-free survival and overall survival rates in the ITT HD-MTX and non-ITT HD-MTX groups were 62.4% vs. 64.5% (P = .94) and 71.7% vs. 71.4% (P = .7), respectively. In addition, the propensity score–matched analyses showed no significant differences in the time-to-CNS relapse, progression-free survival, or overall survival, according to the researchers.
One key concern, however, was the increase in toxicity seen in the HD-MTX group. In this study, the ITT HD-MTX group had a statistically higher incidence of grade 3/4 oral mucositis and elevated alanine aminotransferase (ALT) levels, a marker for liver damage. In addition, the ITT HD-MTX group tended to have a higher incidence of elevated creatinine levels during treatment compared with the non-ITT HD-MTX group.
The HD-MTX group also showed a more common treatment delay or a dose reduction in R-CHOP, which might be attributable to toxicities related to intercalated HD-MTX treatments between R-CHOP cycles, the researchers suggested, potentially resulting in a reduced dose intensity of R-CHOP that could play a role in the lack of an observed survival benefit with additional HD-MTX.
“Another vital issue to consider is that HD-MTX treatment requires hospitalization because intensive hydration and leucovorin rescue is needed, which increases the medical costs,” the authors added.
“This real-world experience, which is unique in its scope and analytical methods, should provide insightful information on the role of HD-MTX prophylaxis to help guide current practice, given the lack of prospective clinical evidence in this patient population,” the researchers concluded.
The authors reported that they had no competing financial interests.
FROM BLOOD ADVANCES
New guidance for those fully vaccinated against COVID-19
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
As has been dominating the headlines, the Centers for Disease Control and Prevention recently released updated public health guidance for those who are fully vaccinated against COVID-19.
This new guidance applies to those who are fully vaccinated as indicated by 2 weeks after the second dose in a 2-dose series or 2 weeks after a single-dose vaccine. Those who meet these criteria no longer need to wear a mask or physically distance themselves from others in both indoor and outdoor settings. For those not fully vaccinated, masking and social distancing should continue to be practiced.
The new guidance indicates that quarantine after a known exposure is no longer necessary.
Unless required by local, state, or territorial health authorities, testing is no longer required following domestic travel for fully vaccinated individuals. A negative test is still required prior to boarding an international flight to the United States and testing 3-5 days after arrival is still recommended. Self-quarantine is no longer required after international travel for fully vaccinated individuals.
The new guidance recommends that individuals who are fully vaccinated not participate in routine screening programs when feasible. Finally, if an individual has tested positive for COVID-19, regardless of vaccination status, that person should isolate and not visit public or private settings for a minimum of ten days.1
Updated guidance for health care facilities
In addition to changes for the general public in all settings, the CDC updated guidance for health care facilities on April 27, 2021. These updated guidelines allow for communal dining and visitation for fully vaccinated patients and their visitors. The guidelines indicate that fully vaccinated health care personnel (HCP) do not require quarantine after exposure to patients who have tested positive for COVID-19 as long as the HCP remains asymptomatic. They should, however, continue to utilize personal protective equipment as previously recommended. HCPs are able to be in break and meeting rooms unmasked if all HCPs are vaccinated.2
There are some important caveats to these updated guidelines. They do not apply to those who have immunocompromising conditions, including those using immunosuppressant agents. They also do not apply to locations subject to federal, state, local, tribal, or territorial laws, rules, and regulations, including local business and workplace guidance.
Those who work or reside in correction or detention facilities and homeless shelters are also still required to test after known exposures. Masking is still required by all travelers on all forms of public transportation into and within the United States.
Most importantly, the guidelines apply only to those who are fully vaccinated. Finally, no vaccine is perfect. As such, anyone who experiences symptoms indicative of COVID-19, regardless of vaccination status, should obtain viral testing and isolate themselves from others.1,2
Pros and cons to new guidance
Both sets of updated guidelines are a great example of public health guidance that is changing as the evidence is gathered and changes. This guidance is also a welcome encouragement that the vaccines are effective at decreasing transmission of this virus that has upended our world.
These guidelines leave room for change as evidence is gathered on emerging novel variants. There are, however, a few remaining concerns.
My first concern is for those who are not yet able to be vaccinated, including children under the age of 12. For families with members who are not fully vaccinated, they may have first heard the headlines of “you do not have to mask” to then read the fine print that remains. When truly following these guidelines, many social situations in both the public and private setting should still include both masking and social distancing.
There is no clarity on how these guidelines are enforced. Within the guidance, it is clear that individuals’ privacy is of utmost importance. In the absence of knowledge, that means that the assumption should be that all are not yet vaccinated. Unless there is a way to reliably demonstrate vaccination status, it would likely still be safer to assume that there are individuals who are not fully vaccinated within the setting.
Finally, although this is great news surrounding the efficacy of the vaccine, some are concerned that local mask mandates that have already started to be lifted will be completely removed. As there is still a large portion of the population not yet fully vaccinated, it seems premature for local, state, and territorial authorities to lift these mandates.
How to continue exercising caution
With the outstanding concerns, I will continue to mask in settings, particularly indoors, where I do not definitely know that everyone is vaccinated. I will continue to do this to protect my children and my patients who are not yet vaccinated, and my patients who are immunosuppressed for whom we do not yet have enough information.
I will continue to advise my patients to be thoughtful about the risk for themselves and their families as well.
There has been more benefit to these public health measures then just decreased transmission of COVID-19. I hope that this year has reinforced within us the benefits of masking and self-isolation in the cases of any contagious illnesses.
Although I am looking forward to the opportunities to interact in person with more colleagues and friends, I think we should continue to do this with caution and thoughtfulness. We must be prepared for the possibility of vaccines having decreased efficacy against novel variants as well as eventually the possibility of waning immunity. If these should occur, we need to be prepared for additional recommendation changes and tightening of restrictions.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. Centers for Disease Control and Prevention. Interim Public Health Recommendations for Fully Vaccinated People. U.S. Department of Health & Human Services, May 13, 2021.
2. Centers for Disease Control and Prevention. Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. U.S. Department of Health and Human Services, April 27, 2021.
Use your court awareness to go faster in practice
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Have you ever had a nightmare you’re running late? Recently I dreamt I was seeing patients on a ship, a little cruiser like the ones that give you tours of Boston Harbor, with low ceilings and narrow iron stairs. My nurse stood where what would have been the coffee and danish window. My first patient was a newborn (this was a nightmare, in case you forgot) who was enormous. She had a big belly and spindly legs that hung off the table. Uniform, umbilicated papules and pustules covered her body. At the sight of her, terror ripped through me – no clue. I rushed to the doctor lounge (nice the ship had one) and flipped channels on a little TV mounted on the ceiling. Suddenly, my nurse burst in, she was frantic because dozens of angry adults and crying children were crammed in the hallway. Apparently, I had been watching TV for hours and my whole clinic was now backed up.
Running-late dreams are common and usually relate to real life. For us, the clinic has been busy lately. Vaccinated patients are returning after a year with their skin cancers that have flourished and psoriasis covering them like kudzu. In particular, they “see the floor” better than other docs and therefore make continual adjustments to stay on pace. At its essence, they are using super-powers of observation to make decisions. It reminded me of a podcast about court awareness and great passers in basketball like the Charlotte Hornets’ LaMelo Ball and NBA great, Bill Bradley.
Bradley had an extraordinary ability to know where all the players were, and where they would be, at any given moment. He spent years honing this skill, noticing details in store windows as he stared straight ahead walking down a street. It’s reported his peripheral vision extended 5%-15% wider than average and he used it to gather more information and to process it more quickly. As a result he made outstanding decisions and fast, ultimately earning a spot in the Hall of Fame in Springfield.
Hall of Fame clinicians similarly take in a wider view than others and process that information quickly. They know how much time they have spent in the room, sense the emotional needs of the patient and anticipate the complexity of the problem. They quickly get to the critical questions and examinations that will make the diagnosis. They know the experience and skill of their medical assistant. They know the level of difficulty and even the temperament of patients who lie ahead on the schedule. All this is processed and used in moment-to-moment decision making. Do I sit down or stand up now? Can I excise this today, or reschedule? Do I ask another question? Do I step out of this room and see another in parallel while this biopsy is set up? And always, do I dare ask about grandkids or do I politely move on?
By broadening out their vision, they optimize their clinic, providing the best possible service, whether the day is busy or slow. I found their economy of motion also means they are less exhausted at the end of the day. I bet if when they dream of being on a ship, they’re sipping a Mai Tai, lounging on the deck.
For more on Bill Bradley and becoming more observant about your surroundings, you might appreciate the following:
www.newyorker.com/magazine/1965/01/23/a-sense-of-where-you-are and freakonomics.com/podcast/nsq-mindfulness/
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Dr. Fauci: Extraordinary challenges, scientific triumphs with COVID-19
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
FDA approves new treatment option for rare anemia
A rare, life-threatening anemia now has a new treatment option. The Food and Drug Administration announced the approval of pegcetacoplan (Empaveli) injection to treat adults with paroxysmal nocturnal hemoglobinuria (PNH). Pegcetacoplan is the first PNH treatment that binds to complement protein C3, according to the FDA announcement. Complement protein C3 is a key component of host immunity and defense.
Special concern
Because of the risk of severe side effects, the drug is available only through a restricted program under a risk evaluation and mitigation strategy (REMS). Serious infections can occur in patients taking pegcetacoplan that can become life-threatening or fatal if not treated early. According to the FDA, REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication, and only a few drugs require a REMS.
The most common other side effects are injection site reactions, diarrhea, abdominal pain, and fatigue.
Pegcetacoplan was approved based upon a study of 80 patients with PNH and anemia who had been taking eculizumab, a previously approved treatment. During 16 weeks of treatment, patients in the pegcetacoplan group had an average increase in their hemoglobin of 2.4 g/dL, while patients in the eculizumab group had an average decrease in their hemoglobin of 1.5 g/dL.
About the disease
PNH is caused by gene mutations that affect red blood cells, causing them to be defective and susceptible to destruction by a patient’s own immune system. Red blood cells in people with these mutations are defective and can be destroyed by the immune system, causing anemia.
Other symptoms include blood clots and destruction of bone marrow. The disease affects 1-1.5 people per million, with diagnosis typically occurring around ages 35-40, and a median survival of only 10 years after diagnosis, according to the FDA.
A rare, life-threatening anemia now has a new treatment option. The Food and Drug Administration announced the approval of pegcetacoplan (Empaveli) injection to treat adults with paroxysmal nocturnal hemoglobinuria (PNH). Pegcetacoplan is the first PNH treatment that binds to complement protein C3, according to the FDA announcement. Complement protein C3 is a key component of host immunity and defense.
Special concern
Because of the risk of severe side effects, the drug is available only through a restricted program under a risk evaluation and mitigation strategy (REMS). Serious infections can occur in patients taking pegcetacoplan that can become life-threatening or fatal if not treated early. According to the FDA, REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication, and only a few drugs require a REMS.
The most common other side effects are injection site reactions, diarrhea, abdominal pain, and fatigue.
Pegcetacoplan was approved based upon a study of 80 patients with PNH and anemia who had been taking eculizumab, a previously approved treatment. During 16 weeks of treatment, patients in the pegcetacoplan group had an average increase in their hemoglobin of 2.4 g/dL, while patients in the eculizumab group had an average decrease in their hemoglobin of 1.5 g/dL.
About the disease
PNH is caused by gene mutations that affect red blood cells, causing them to be defective and susceptible to destruction by a patient’s own immune system. Red blood cells in people with these mutations are defective and can be destroyed by the immune system, causing anemia.
Other symptoms include blood clots and destruction of bone marrow. The disease affects 1-1.5 people per million, with diagnosis typically occurring around ages 35-40, and a median survival of only 10 years after diagnosis, according to the FDA.
A rare, life-threatening anemia now has a new treatment option. The Food and Drug Administration announced the approval of pegcetacoplan (Empaveli) injection to treat adults with paroxysmal nocturnal hemoglobinuria (PNH). Pegcetacoplan is the first PNH treatment that binds to complement protein C3, according to the FDA announcement. Complement protein C3 is a key component of host immunity and defense.
Special concern
Because of the risk of severe side effects, the drug is available only through a restricted program under a risk evaluation and mitigation strategy (REMS). Serious infections can occur in patients taking pegcetacoplan that can become life-threatening or fatal if not treated early. According to the FDA, REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication, and only a few drugs require a REMS.
The most common other side effects are injection site reactions, diarrhea, abdominal pain, and fatigue.
Pegcetacoplan was approved based upon a study of 80 patients with PNH and anemia who had been taking eculizumab, a previously approved treatment. During 16 weeks of treatment, patients in the pegcetacoplan group had an average increase in their hemoglobin of 2.4 g/dL, while patients in the eculizumab group had an average decrease in their hemoglobin of 1.5 g/dL.
About the disease
PNH is caused by gene mutations that affect red blood cells, causing them to be defective and susceptible to destruction by a patient’s own immune system. Red blood cells in people with these mutations are defective and can be destroyed by the immune system, causing anemia.
Other symptoms include blood clots and destruction of bone marrow. The disease affects 1-1.5 people per million, with diagnosis typically occurring around ages 35-40, and a median survival of only 10 years after diagnosis, according to the FDA.









