User login
-
Omics analysis links blood type to COVID-19
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with CLL have significantly reduced response to COVID-19 vaccine
Patients with chronic lymphocytic leukemia (CLL) have increased risk for severe COVID-19 disease as well as mortality.
Such patients are likely to have compromised immune systems, making them respond poorly to vaccines, as has been seen in studies involving pneumococcal, hepatitis B, and influenza A and B vaccination.
In order to determine if vaccination against COVID-19 disease will be effective among these patients, researchers performed a study to determine the efficacy of a single COVID-19 vaccine in patients with CLL. They found that the response rate of patients with CLL to vaccination was significantly lower than that of healthy controls, according to the study published in Blood Advances.
Study details
The study (NCT04746092) assessed the humoral immune responses to BNT162b2 mRNA COVID-19 (Pfizer) vaccination in adult patients with CLL and compared responses with those obtained in age-matched healthy controls. Patients received two vaccine doses, 21 days apart, and antibody titers were measured 2-3 weeks after administration of the second dose, according to Yair Herishanu, MD, of the Tel-Aviv Sourasky Medical Center, Tel Aviv University, and colleagues.
Troubling results
The researchers found an antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in only 66 of 167 (39.5%) of all patients with CLL. The response rate of 52 of these responding patients with CLL to the vaccine was significantly lower than that occurring in 52 age- and sex-matched healthy controls (52% vs. 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001).
Among the patients with CLL, the response rate was highest in those who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients, and it was only 16% in patients under treatment at the time of vaccination.
In patients treated with either BTK inhibitors or venetoclax with and without anti-CD20 antibody, response rates were low (16.0% and 13.6%, respectively). In particular, none of the patients exposed to anti-CD20 antibodies less than 12 months prior to vaccination responded, according to the researchers.
Multivariate analysis showed that the independent predictors of a vaccine response were age (65 years or younger; odds ratio, 3.17; P = .025), sex (women; OR, 3.66; P = .006), lack of active therapy (including treatment naive and previously treated patients; OR 6.59; P < .001), IgG levels 550 mg/dL or greater (OR, 3.70; P = .037), and IgM levels 40mg/dL or greater (OR, 2.92; P = .017).
Within a median follow-up period of 75 days since the first vaccine dose, none of the CLL patients developed COVID-19 infection, the researchers reported.
“Vaccinated patients with CLL should continue to adhere to masking, social distancing, and vaccination of their close contacts should be strongly recommended. Serological tests after the second injection of the COVID-19 vaccine can provide valuable information to the individual patient and perhaps, may be integrated in future clinical decisions,” the researchers concluded.
The study was sponsored by the Tel-Aviv Sourasky Medical Center. The authors reported that they had no conflicts of interest.
Patients with chronic lymphocytic leukemia (CLL) have increased risk for severe COVID-19 disease as well as mortality.
Such patients are likely to have compromised immune systems, making them respond poorly to vaccines, as has been seen in studies involving pneumococcal, hepatitis B, and influenza A and B vaccination.
In order to determine if vaccination against COVID-19 disease will be effective among these patients, researchers performed a study to determine the efficacy of a single COVID-19 vaccine in patients with CLL. They found that the response rate of patients with CLL to vaccination was significantly lower than that of healthy controls, according to the study published in Blood Advances.
Study details
The study (NCT04746092) assessed the humoral immune responses to BNT162b2 mRNA COVID-19 (Pfizer) vaccination in adult patients with CLL and compared responses with those obtained in age-matched healthy controls. Patients received two vaccine doses, 21 days apart, and antibody titers were measured 2-3 weeks after administration of the second dose, according to Yair Herishanu, MD, of the Tel-Aviv Sourasky Medical Center, Tel Aviv University, and colleagues.
Troubling results
The researchers found an antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in only 66 of 167 (39.5%) of all patients with CLL. The response rate of 52 of these responding patients with CLL to the vaccine was significantly lower than that occurring in 52 age- and sex-matched healthy controls (52% vs. 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001).
Among the patients with CLL, the response rate was highest in those who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients, and it was only 16% in patients under treatment at the time of vaccination.
In patients treated with either BTK inhibitors or venetoclax with and without anti-CD20 antibody, response rates were low (16.0% and 13.6%, respectively). In particular, none of the patients exposed to anti-CD20 antibodies less than 12 months prior to vaccination responded, according to the researchers.
Multivariate analysis showed that the independent predictors of a vaccine response were age (65 years or younger; odds ratio, 3.17; P = .025), sex (women; OR, 3.66; P = .006), lack of active therapy (including treatment naive and previously treated patients; OR 6.59; P < .001), IgG levels 550 mg/dL or greater (OR, 3.70; P = .037), and IgM levels 40mg/dL or greater (OR, 2.92; P = .017).
Within a median follow-up period of 75 days since the first vaccine dose, none of the CLL patients developed COVID-19 infection, the researchers reported.
“Vaccinated patients with CLL should continue to adhere to masking, social distancing, and vaccination of their close contacts should be strongly recommended. Serological tests after the second injection of the COVID-19 vaccine can provide valuable information to the individual patient and perhaps, may be integrated in future clinical decisions,” the researchers concluded.
The study was sponsored by the Tel-Aviv Sourasky Medical Center. The authors reported that they had no conflicts of interest.
Patients with chronic lymphocytic leukemia (CLL) have increased risk for severe COVID-19 disease as well as mortality.
Such patients are likely to have compromised immune systems, making them respond poorly to vaccines, as has been seen in studies involving pneumococcal, hepatitis B, and influenza A and B vaccination.
In order to determine if vaccination against COVID-19 disease will be effective among these patients, researchers performed a study to determine the efficacy of a single COVID-19 vaccine in patients with CLL. They found that the response rate of patients with CLL to vaccination was significantly lower than that of healthy controls, according to the study published in Blood Advances.
Study details
The study (NCT04746092) assessed the humoral immune responses to BNT162b2 mRNA COVID-19 (Pfizer) vaccination in adult patients with CLL and compared responses with those obtained in age-matched healthy controls. Patients received two vaccine doses, 21 days apart, and antibody titers were measured 2-3 weeks after administration of the second dose, according to Yair Herishanu, MD, of the Tel-Aviv Sourasky Medical Center, Tel Aviv University, and colleagues.
Troubling results
The researchers found an antibody-mediated response to the BNT162b2 mRNA COVID-19 vaccine in only 66 of 167 (39.5%) of all patients with CLL. The response rate of 52 of these responding patients with CLL to the vaccine was significantly lower than that occurring in 52 age- and sex-matched healthy controls (52% vs. 100%, respectively; adjusted odds ratio, 0.010; 95% confidence interval, 0.001-0.162; P < .001).
Among the patients with CLL, the response rate was highest in those who obtained clinical remission after treatment (79.2%), followed by 55.2% in treatment-naive patients, and it was only 16% in patients under treatment at the time of vaccination.
In patients treated with either BTK inhibitors or venetoclax with and without anti-CD20 antibody, response rates were low (16.0% and 13.6%, respectively). In particular, none of the patients exposed to anti-CD20 antibodies less than 12 months prior to vaccination responded, according to the researchers.
Multivariate analysis showed that the independent predictors of a vaccine response were age (65 years or younger; odds ratio, 3.17; P = .025), sex (women; OR, 3.66; P = .006), lack of active therapy (including treatment naive and previously treated patients; OR 6.59; P < .001), IgG levels 550 mg/dL or greater (OR, 3.70; P = .037), and IgM levels 40mg/dL or greater (OR, 2.92; P = .017).
Within a median follow-up period of 75 days since the first vaccine dose, none of the CLL patients developed COVID-19 infection, the researchers reported.
“Vaccinated patients with CLL should continue to adhere to masking, social distancing, and vaccination of their close contacts should be strongly recommended. Serological tests after the second injection of the COVID-19 vaccine can provide valuable information to the individual patient and perhaps, may be integrated in future clinical decisions,” the researchers concluded.
The study was sponsored by the Tel-Aviv Sourasky Medical Center. The authors reported that they had no conflicts of interest.
FROM BLOOD ADVANCES
ADAPTABLE: Low-dose aspirin as good as high-dose in CHD?
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
CDC: Vaccinated? You don’t need a mask indoors
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
CDC recommends use of Pfizer’s COVID vaccine in 12- to 15-year-olds
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
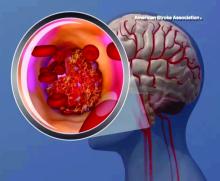
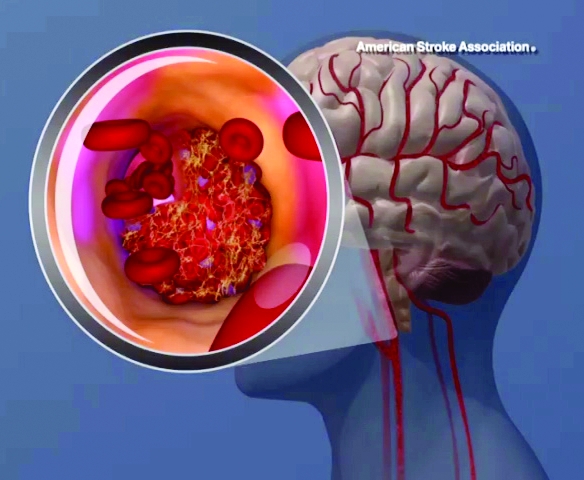
High teen BMI linked to stroke risk in young adulthood
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
FROM STROKE
Reassuring data on impact of mild COVID-19 on the heart
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What to know about COVID-19 vaccines and skin reactions
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
FROM AAD VMX 2021
Dr. Fauci: Feds may ease indoor mask mandates soon
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.