User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


3D Printing for the Development of Palatal Defect Prosthetics
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
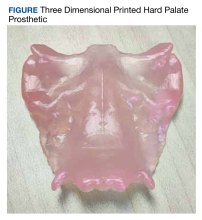
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
Improving Fecal Immunochemical Test Collection for Colorectal Cancer Screening During the COVID-19 Pandemic
Colorectal cancer (CRC) is the third-most common cancer worldwide and accounts for almost 11% of all cancer diagnoses, with > 1.9 million cases reported globally.1,2 CRC is the second-most deadly cancer, responsible for about 935,000 deaths.1 Over the past several decades, a steady decline in CRC incidence and mortality has been reported in developed countries, including the US.3,4 From 2008 through 2017, an annual reduction of 3% in CRC death rates was reported in individuals aged ≥ 65 years.5 This decline can mainly be attributed to improvements made in health systems and advancements in CRC screening programs.3,5
US Preventive Services Task Force (USPSTF) recommends CRC screening in individuals aged 45 to 75 years. USPSTF recommends direct visualization tests, such as colonoscopy and flexible sigmoidoscopy for CRC screening.6 Although colonoscopy is commonly used for CRC screening, it is an invasive procedure that requires bowel preparation and sedation, and has the potential risk of colonic perforation, bleeding, and infection. Additionally, social determinants—such as health care costs, missed work, and geographic location (eg, rural communities)—may limit colonoscopy utilization.7 As a result, other cost-effective, noninvasive tests such as high-sensitivity guaiac-based fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are also used for CRC screening. These tests detect occult blood in the stool of individuals who may be at risk for CRC, helping direct them to colonoscopy if they screen positive.8
The gFOBT relies on simple oxidation and requires a stool sample to detect the presence of the heme component of blood.9 If heme is present in the stool sample, it will enable the oxidation of guaiac to form a blue-colored dye when added to hydrogen peroxide. It is important to note that the oxidation component of this test may lead to false-positive results, as it may detect dietary hemoglobin present in red meat. Medications or foods that have peroxidase properties may also result in a false-positive gFOBT result. Additionally, false-negative results may be caused by antioxidants, which may interfere with the oxidation of guaiac.
FIT uses antibodies, which bind to the intact globin component of human hemoglobin.9 The quantity of bound antibody-hemoglobin complex is detected and measured by a variety of automated quantitative techniques. This testing strategy eliminates the need for food or medication restrictions and the subjective visual assessment of change in color, as required for the gFOBT.9 A 2016 meta-analysis found that FIT performed better compared with gFOBT in terms of specificity, positivity rate, number needed to scope, and number needed to screen.8 The FIT screening method has also been found to have greater adherence rates, which is likely due to fewer stool sampling requirements and the lack of medication or dietary restrictions, compared with gFOBT.7,8
The COVID-19 pandemic had a drastic impact on CRC preventive care services. In March 2020, elective colonoscopies were temporarily ceased across the country and the US Department of Veterans Affairs (VA) deferred all elective surgeries and medical procedures, including screening and surveillance colonoscopies. In line with these recommendations, elective colonoscopies were temporarily ceased across the country.10 The National Cancer Institute’s Population-Based Research to Optimize the Screening Process consortium reported that CRC screening rates decreased by 82% across the US in 2020.11 Public health measures are likely the main reason for this decline, but other factors may include a lack of resource availability in outpatient settings and public fear of the pandemic.10
The James A. Haley Veterans Affairs Hospital (JAHVAH) in Tampa, Florida, encouraged the use of FIT in place of colonoscopies to avoid delaying preventive services. The initiative to continue CRC screening methods via FIT was scrutinized when laboratory personnel reported that in fiscal year (FY) 2020, 62% of the FIT kits that patients returned to the laboratory were missing information or had other errors (Figure 1). These improperly returned FIT kits led to delayed processing, canceled orders, increased staff workload, and more costs for FIT repetition.
Research shows many patients often fail to adhere to the instructions for proper FIT sample collection and return. Wang and colleagues reported that of 4916 FIT samples returned to the laboratory, 971 (20%) had collection errors, and 910 (94%) of those samples were missing a sample collection date.12 The sample collection date is important because hemoglobin degradation occurs over time, which may create false-negative FIT results. Although studies have found that sample return times of ≤ 10 days are not associated with a decrease in FIT positive rates, it is recommended to mail completed FITs within 24 hours of sample collection.13
Because remote screening methods like FIT were preferred during the COVID-19 pandemic, we conducted a quality improvement (QI) project to address FIT inefficiency. The aim of this initiative was to determine the root cause behind incorrectly returned FIT kits and to increase correctly collected and testable FIT kits upon initial laboratory arrival by at least 20% by the second quarter of FY 2021.
Quality Improvement Project
This QI project was conducted from July 2020 to June 2021 at the JAHVAH, which provides primary care and specialty health services to veterans in central and south Florida. The QI was designed based on the Plan-Do-Study-Act (PDSA) model of health care improvement. The QI team consisted of physicians, nurses, administrative staff, and laboratory personnel. A SIPOC (Suppliers, Input, Process, Output, Customers) map was initially designed to help clarify the different groups involved in the process of FIT kit distribution and return. This map helped the team decide who should be involved in the solution process.
The QI team performed a root cause analysis using a fishbone diagram and identified the reasons FIT kits were returned to the laboratory with errors that prevented processing. The team brainstormed potential change ideas and created an impact vs effort chart to increase the number of correctly returned and testable FIT kits upon initial arrival at the laboratory by at least 20% by the second quarter of FY 2021. We identified strengths and prioritized change ideas to improve the number of testable and correctly returned FIT kits to the hospital laboratory. These ideas included centralizing FIT kit dispersal to a new administrative group, building redundant patient reminders on kit completion and giving patients more accessible places for kit return.
Patients included in the study were adults aged 50 to 75 years seen at the JAHVAH outpatient clinic who were asked to undergo FIT CRC screening. FIT orders for other facilities were excluded. The primary endpoint of this project was to improve the number of correctly returned FITs. The number of correct and incorrect returned FITs were measured from July 2020 to June 2021. FITs returned with errors were categorized by the type of error, including: no order on file in the electronic health record (EHR), canceled test, expired test, unable to identify test, missing information, and missing collection date.
We attempted to calculate costs of FITs that were returned to the laboratory but could not be analyzed and were discarded. In FY 2020, 1568 FITs were discarded. Each FIT cost about $7.80 to process for an annualized expense of $12,230 for discarded FITs.
Root Cause Analysis
Root causes were obtained by making a fishbone diagram. From this diagram, an impact vs effort chart was created to form and prioritize ideas for our PDSA cycles. Data about correctly and incorrectly returned kits were collected monthly from laboratory personnel, then analyzed by the QI team using run charts to look for change in frequency and patterns.
To improve this process, a swim lane chart for FIT processing was assembled and later used to make a comprehensive fishbone diagram to establish the 6 main root cause errors: missing FIT EHR order, cancelled FIT EHR order, expired stool specimen, partial patient identifiers, no patient identifiers, and no stool collection date. Pareto and run charts were superimposed with the laboratory data. The most common cause of incorrectly returned FITs was no collection date.
PDSA Cycles
Beginning in January 2021, PDSA cycles from the ideas in the impact vs effort chart were used. Organization and implementation of the project occurred from July 2020 to April 2021. The team reassessed the data in April 2021 to evaluate progress after PDSA initiation. The mean rate of missing collection date dropped from 24% in FY 2020 prior to PDSA cycles to 14% in April 2021; however, the number of incorrectly returned kits was similar to the baseline level. When reviewing this discrepancy, the QI team found that although the missing collection date rate had improved, the rate of FITs with not enough information had increased from 5% in FY 2020 to 67% in April 2021 (Figure 2). After discussing with laboratory personnel, it was determined that the EHR order was missing when the process pathway changed. Our PDSA initiative changed the process pathway and different individuals were responsible for FIT dispersal. The error was quickly addressed with the help of clinical and administrative staff; a 30-day follow-up on June 21, 2021, revealed that only 9% of the patients had sent back kits with not enough information.
After troubleshooting, the team achieved a sustainable increase in the number of correctly returned FIT kits from an average of 38% before the project to 72% after 30-day follow-up.
Discussion
Proper collection and return of FIT samples are vital for process efficiency for both physicians and patients. This initiative aimed to improve the rate of correctly returned FIT kits by 20%, but its final numbers showed an improvement of 33.6%. Operational benefits from this project included early detection of CRC, improved laboratory workflow, decreased FIT kit waste, and increased patient satisfaction.
The multipronged PDSA cycle attempted to increase the rate of correctly returned FIT kits. We improved kit comprehension and laboratory accessibility, and instituted redundant return reminders for patients. We also centralized a new process pathway for FIT distribution and educated physicians and support staff. Sampling and FIT return may seem like a simple procedure, but the FIT can be cumbersome for patients and directions can be confusing. Therefore, to maximize screening participation, it is essential to minimize confusion in the collection and return of a FIT sample.14,15
This QI initiative was presented at Grand Rounds at the University of South Florida in June 2021 and has since been shared with other VA hospitals. It was also presented at the American College of Gastroenterology Conference in 2021.
Limitations
This study was a single-center QI project and focused mostly on FIT kit return rates. To fully address CRC screening, it is important to ensure that individuals with a positive screen are appropriately followed up with a colonoscopy. Although follow-up was not in the scope of this project, it is key to CRC screening in general and should be the subject of future research.
Conclusions
FIT is a useful method for CRC screening that can be particularly helpful when in-person visits are limited, as seen during the COVID-19 pandemic. This increase in demand for FITs during the pandemic revealed process deficiencies and gave JAHVAH an opportunity to improve workflow. Through the aid of a multidisciplinary team, the process to complete and return FITs improved and surpassed the goal of 20% improvement. Our goal is to continue to fine-tune the workflow and troubleshoot the system as needed.
1. Sawicki T, Ruszkowska M, Danielewicz A, Niedz′wiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. Published 2021 Apr 22. doi:10.3390/cancers13092025
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103. doi:10.5114/pg.2018.81072
3. Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120(18):2893-2901. doi:10.1002/cncr.28794
4. Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1296-1302. doi:10.1158/1055-9965.EPI-11-0250
5. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. doi:10.3322/caac.21601
6. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive
Colorectal cancer (CRC) is the third-most common cancer worldwide and accounts for almost 11% of all cancer diagnoses, with > 1.9 million cases reported globally.1,2 CRC is the second-most deadly cancer, responsible for about 935,000 deaths.1 Over the past several decades, a steady decline in CRC incidence and mortality has been reported in developed countries, including the US.3,4 From 2008 through 2017, an annual reduction of 3% in CRC death rates was reported in individuals aged ≥ 65 years.5 This decline can mainly be attributed to improvements made in health systems and advancements in CRC screening programs.3,5
US Preventive Services Task Force (USPSTF) recommends CRC screening in individuals aged 45 to 75 years. USPSTF recommends direct visualization tests, such as colonoscopy and flexible sigmoidoscopy for CRC screening.6 Although colonoscopy is commonly used for CRC screening, it is an invasive procedure that requires bowel preparation and sedation, and has the potential risk of colonic perforation, bleeding, and infection. Additionally, social determinants—such as health care costs, missed work, and geographic location (eg, rural communities)—may limit colonoscopy utilization.7 As a result, other cost-effective, noninvasive tests such as high-sensitivity guaiac-based fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are also used for CRC screening. These tests detect occult blood in the stool of individuals who may be at risk for CRC, helping direct them to colonoscopy if they screen positive.8
The gFOBT relies on simple oxidation and requires a stool sample to detect the presence of the heme component of blood.9 If heme is present in the stool sample, it will enable the oxidation of guaiac to form a blue-colored dye when added to hydrogen peroxide. It is important to note that the oxidation component of this test may lead to false-positive results, as it may detect dietary hemoglobin present in red meat. Medications or foods that have peroxidase properties may also result in a false-positive gFOBT result. Additionally, false-negative results may be caused by antioxidants, which may interfere with the oxidation of guaiac.
FIT uses antibodies, which bind to the intact globin component of human hemoglobin.9 The quantity of bound antibody-hemoglobin complex is detected and measured by a variety of automated quantitative techniques. This testing strategy eliminates the need for food or medication restrictions and the subjective visual assessment of change in color, as required for the gFOBT.9 A 2016 meta-analysis found that FIT performed better compared with gFOBT in terms of specificity, positivity rate, number needed to scope, and number needed to screen.8 The FIT screening method has also been found to have greater adherence rates, which is likely due to fewer stool sampling requirements and the lack of medication or dietary restrictions, compared with gFOBT.7,8
The COVID-19 pandemic had a drastic impact on CRC preventive care services. In March 2020, elective colonoscopies were temporarily ceased across the country and the US Department of Veterans Affairs (VA) deferred all elective surgeries and medical procedures, including screening and surveillance colonoscopies. In line with these recommendations, elective colonoscopies were temporarily ceased across the country.10 The National Cancer Institute’s Population-Based Research to Optimize the Screening Process consortium reported that CRC screening rates decreased by 82% across the US in 2020.11 Public health measures are likely the main reason for this decline, but other factors may include a lack of resource availability in outpatient settings and public fear of the pandemic.10
The James A. Haley Veterans Affairs Hospital (JAHVAH) in Tampa, Florida, encouraged the use of FIT in place of colonoscopies to avoid delaying preventive services. The initiative to continue CRC screening methods via FIT was scrutinized when laboratory personnel reported that in fiscal year (FY) 2020, 62% of the FIT kits that patients returned to the laboratory were missing information or had other errors (Figure 1). These improperly returned FIT kits led to delayed processing, canceled orders, increased staff workload, and more costs for FIT repetition.
Research shows many patients often fail to adhere to the instructions for proper FIT sample collection and return. Wang and colleagues reported that of 4916 FIT samples returned to the laboratory, 971 (20%) had collection errors, and 910 (94%) of those samples were missing a sample collection date.12 The sample collection date is important because hemoglobin degradation occurs over time, which may create false-negative FIT results. Although studies have found that sample return times of ≤ 10 days are not associated with a decrease in FIT positive rates, it is recommended to mail completed FITs within 24 hours of sample collection.13
Because remote screening methods like FIT were preferred during the COVID-19 pandemic, we conducted a quality improvement (QI) project to address FIT inefficiency. The aim of this initiative was to determine the root cause behind incorrectly returned FIT kits and to increase correctly collected and testable FIT kits upon initial laboratory arrival by at least 20% by the second quarter of FY 2021.
Quality Improvement Project
This QI project was conducted from July 2020 to June 2021 at the JAHVAH, which provides primary care and specialty health services to veterans in central and south Florida. The QI was designed based on the Plan-Do-Study-Act (PDSA) model of health care improvement. The QI team consisted of physicians, nurses, administrative staff, and laboratory personnel. A SIPOC (Suppliers, Input, Process, Output, Customers) map was initially designed to help clarify the different groups involved in the process of FIT kit distribution and return. This map helped the team decide who should be involved in the solution process.
The QI team performed a root cause analysis using a fishbone diagram and identified the reasons FIT kits were returned to the laboratory with errors that prevented processing. The team brainstormed potential change ideas and created an impact vs effort chart to increase the number of correctly returned and testable FIT kits upon initial arrival at the laboratory by at least 20% by the second quarter of FY 2021. We identified strengths and prioritized change ideas to improve the number of testable and correctly returned FIT kits to the hospital laboratory. These ideas included centralizing FIT kit dispersal to a new administrative group, building redundant patient reminders on kit completion and giving patients more accessible places for kit return.
Patients included in the study were adults aged 50 to 75 years seen at the JAHVAH outpatient clinic who were asked to undergo FIT CRC screening. FIT orders for other facilities were excluded. The primary endpoint of this project was to improve the number of correctly returned FITs. The number of correct and incorrect returned FITs were measured from July 2020 to June 2021. FITs returned with errors were categorized by the type of error, including: no order on file in the electronic health record (EHR), canceled test, expired test, unable to identify test, missing information, and missing collection date.
We attempted to calculate costs of FITs that were returned to the laboratory but could not be analyzed and were discarded. In FY 2020, 1568 FITs were discarded. Each FIT cost about $7.80 to process for an annualized expense of $12,230 for discarded FITs.
Root Cause Analysis
Root causes were obtained by making a fishbone diagram. From this diagram, an impact vs effort chart was created to form and prioritize ideas for our PDSA cycles. Data about correctly and incorrectly returned kits were collected monthly from laboratory personnel, then analyzed by the QI team using run charts to look for change in frequency and patterns.
To improve this process, a swim lane chart for FIT processing was assembled and later used to make a comprehensive fishbone diagram to establish the 6 main root cause errors: missing FIT EHR order, cancelled FIT EHR order, expired stool specimen, partial patient identifiers, no patient identifiers, and no stool collection date. Pareto and run charts were superimposed with the laboratory data. The most common cause of incorrectly returned FITs was no collection date.
PDSA Cycles
Beginning in January 2021, PDSA cycles from the ideas in the impact vs effort chart were used. Organization and implementation of the project occurred from July 2020 to April 2021. The team reassessed the data in April 2021 to evaluate progress after PDSA initiation. The mean rate of missing collection date dropped from 24% in FY 2020 prior to PDSA cycles to 14% in April 2021; however, the number of incorrectly returned kits was similar to the baseline level. When reviewing this discrepancy, the QI team found that although the missing collection date rate had improved, the rate of FITs with not enough information had increased from 5% in FY 2020 to 67% in April 2021 (Figure 2). After discussing with laboratory personnel, it was determined that the EHR order was missing when the process pathway changed. Our PDSA initiative changed the process pathway and different individuals were responsible for FIT dispersal. The error was quickly addressed with the help of clinical and administrative staff; a 30-day follow-up on June 21, 2021, revealed that only 9% of the patients had sent back kits with not enough information.
After troubleshooting, the team achieved a sustainable increase in the number of correctly returned FIT kits from an average of 38% before the project to 72% after 30-day follow-up.
Discussion
Proper collection and return of FIT samples are vital for process efficiency for both physicians and patients. This initiative aimed to improve the rate of correctly returned FIT kits by 20%, but its final numbers showed an improvement of 33.6%. Operational benefits from this project included early detection of CRC, improved laboratory workflow, decreased FIT kit waste, and increased patient satisfaction.
The multipronged PDSA cycle attempted to increase the rate of correctly returned FIT kits. We improved kit comprehension and laboratory accessibility, and instituted redundant return reminders for patients. We also centralized a new process pathway for FIT distribution and educated physicians and support staff. Sampling and FIT return may seem like a simple procedure, but the FIT can be cumbersome for patients and directions can be confusing. Therefore, to maximize screening participation, it is essential to minimize confusion in the collection and return of a FIT sample.14,15
This QI initiative was presented at Grand Rounds at the University of South Florida in June 2021 and has since been shared with other VA hospitals. It was also presented at the American College of Gastroenterology Conference in 2021.
Limitations
This study was a single-center QI project and focused mostly on FIT kit return rates. To fully address CRC screening, it is important to ensure that individuals with a positive screen are appropriately followed up with a colonoscopy. Although follow-up was not in the scope of this project, it is key to CRC screening in general and should be the subject of future research.
Conclusions
FIT is a useful method for CRC screening that can be particularly helpful when in-person visits are limited, as seen during the COVID-19 pandemic. This increase in demand for FITs during the pandemic revealed process deficiencies and gave JAHVAH an opportunity to improve workflow. Through the aid of a multidisciplinary team, the process to complete and return FITs improved and surpassed the goal of 20% improvement. Our goal is to continue to fine-tune the workflow and troubleshoot the system as needed.
Colorectal cancer (CRC) is the third-most common cancer worldwide and accounts for almost 11% of all cancer diagnoses, with > 1.9 million cases reported globally.1,2 CRC is the second-most deadly cancer, responsible for about 935,000 deaths.1 Over the past several decades, a steady decline in CRC incidence and mortality has been reported in developed countries, including the US.3,4 From 2008 through 2017, an annual reduction of 3% in CRC death rates was reported in individuals aged ≥ 65 years.5 This decline can mainly be attributed to improvements made in health systems and advancements in CRC screening programs.3,5
US Preventive Services Task Force (USPSTF) recommends CRC screening in individuals aged 45 to 75 years. USPSTF recommends direct visualization tests, such as colonoscopy and flexible sigmoidoscopy for CRC screening.6 Although colonoscopy is commonly used for CRC screening, it is an invasive procedure that requires bowel preparation and sedation, and has the potential risk of colonic perforation, bleeding, and infection. Additionally, social determinants—such as health care costs, missed work, and geographic location (eg, rural communities)—may limit colonoscopy utilization.7 As a result, other cost-effective, noninvasive tests such as high-sensitivity guaiac-based fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are also used for CRC screening. These tests detect occult blood in the stool of individuals who may be at risk for CRC, helping direct them to colonoscopy if they screen positive.8
The gFOBT relies on simple oxidation and requires a stool sample to detect the presence of the heme component of blood.9 If heme is present in the stool sample, it will enable the oxidation of guaiac to form a blue-colored dye when added to hydrogen peroxide. It is important to note that the oxidation component of this test may lead to false-positive results, as it may detect dietary hemoglobin present in red meat. Medications or foods that have peroxidase properties may also result in a false-positive gFOBT result. Additionally, false-negative results may be caused by antioxidants, which may interfere with the oxidation of guaiac.
FIT uses antibodies, which bind to the intact globin component of human hemoglobin.9 The quantity of bound antibody-hemoglobin complex is detected and measured by a variety of automated quantitative techniques. This testing strategy eliminates the need for food or medication restrictions and the subjective visual assessment of change in color, as required for the gFOBT.9 A 2016 meta-analysis found that FIT performed better compared with gFOBT in terms of specificity, positivity rate, number needed to scope, and number needed to screen.8 The FIT screening method has also been found to have greater adherence rates, which is likely due to fewer stool sampling requirements and the lack of medication or dietary restrictions, compared with gFOBT.7,8
The COVID-19 pandemic had a drastic impact on CRC preventive care services. In March 2020, elective colonoscopies were temporarily ceased across the country and the US Department of Veterans Affairs (VA) deferred all elective surgeries and medical procedures, including screening and surveillance colonoscopies. In line with these recommendations, elective colonoscopies were temporarily ceased across the country.10 The National Cancer Institute’s Population-Based Research to Optimize the Screening Process consortium reported that CRC screening rates decreased by 82% across the US in 2020.11 Public health measures are likely the main reason for this decline, but other factors may include a lack of resource availability in outpatient settings and public fear of the pandemic.10
The James A. Haley Veterans Affairs Hospital (JAHVAH) in Tampa, Florida, encouraged the use of FIT in place of colonoscopies to avoid delaying preventive services. The initiative to continue CRC screening methods via FIT was scrutinized when laboratory personnel reported that in fiscal year (FY) 2020, 62% of the FIT kits that patients returned to the laboratory were missing information or had other errors (Figure 1). These improperly returned FIT kits led to delayed processing, canceled orders, increased staff workload, and more costs for FIT repetition.
Research shows many patients often fail to adhere to the instructions for proper FIT sample collection and return. Wang and colleagues reported that of 4916 FIT samples returned to the laboratory, 971 (20%) had collection errors, and 910 (94%) of those samples were missing a sample collection date.12 The sample collection date is important because hemoglobin degradation occurs over time, which may create false-negative FIT results. Although studies have found that sample return times of ≤ 10 days are not associated with a decrease in FIT positive rates, it is recommended to mail completed FITs within 24 hours of sample collection.13
Because remote screening methods like FIT were preferred during the COVID-19 pandemic, we conducted a quality improvement (QI) project to address FIT inefficiency. The aim of this initiative was to determine the root cause behind incorrectly returned FIT kits and to increase correctly collected and testable FIT kits upon initial laboratory arrival by at least 20% by the second quarter of FY 2021.
Quality Improvement Project
This QI project was conducted from July 2020 to June 2021 at the JAHVAH, which provides primary care and specialty health services to veterans in central and south Florida. The QI was designed based on the Plan-Do-Study-Act (PDSA) model of health care improvement. The QI team consisted of physicians, nurses, administrative staff, and laboratory personnel. A SIPOC (Suppliers, Input, Process, Output, Customers) map was initially designed to help clarify the different groups involved in the process of FIT kit distribution and return. This map helped the team decide who should be involved in the solution process.
The QI team performed a root cause analysis using a fishbone diagram and identified the reasons FIT kits were returned to the laboratory with errors that prevented processing. The team brainstormed potential change ideas and created an impact vs effort chart to increase the number of correctly returned and testable FIT kits upon initial arrival at the laboratory by at least 20% by the second quarter of FY 2021. We identified strengths and prioritized change ideas to improve the number of testable and correctly returned FIT kits to the hospital laboratory. These ideas included centralizing FIT kit dispersal to a new administrative group, building redundant patient reminders on kit completion and giving patients more accessible places for kit return.
Patients included in the study were adults aged 50 to 75 years seen at the JAHVAH outpatient clinic who were asked to undergo FIT CRC screening. FIT orders for other facilities were excluded. The primary endpoint of this project was to improve the number of correctly returned FITs. The number of correct and incorrect returned FITs were measured from July 2020 to June 2021. FITs returned with errors were categorized by the type of error, including: no order on file in the electronic health record (EHR), canceled test, expired test, unable to identify test, missing information, and missing collection date.
We attempted to calculate costs of FITs that were returned to the laboratory but could not be analyzed and were discarded. In FY 2020, 1568 FITs were discarded. Each FIT cost about $7.80 to process for an annualized expense of $12,230 for discarded FITs.
Root Cause Analysis
Root causes were obtained by making a fishbone diagram. From this diagram, an impact vs effort chart was created to form and prioritize ideas for our PDSA cycles. Data about correctly and incorrectly returned kits were collected monthly from laboratory personnel, then analyzed by the QI team using run charts to look for change in frequency and patterns.
To improve this process, a swim lane chart for FIT processing was assembled and later used to make a comprehensive fishbone diagram to establish the 6 main root cause errors: missing FIT EHR order, cancelled FIT EHR order, expired stool specimen, partial patient identifiers, no patient identifiers, and no stool collection date. Pareto and run charts were superimposed with the laboratory data. The most common cause of incorrectly returned FITs was no collection date.
PDSA Cycles
Beginning in January 2021, PDSA cycles from the ideas in the impact vs effort chart were used. Organization and implementation of the project occurred from July 2020 to April 2021. The team reassessed the data in April 2021 to evaluate progress after PDSA initiation. The mean rate of missing collection date dropped from 24% in FY 2020 prior to PDSA cycles to 14% in April 2021; however, the number of incorrectly returned kits was similar to the baseline level. When reviewing this discrepancy, the QI team found that although the missing collection date rate had improved, the rate of FITs with not enough information had increased from 5% in FY 2020 to 67% in April 2021 (Figure 2). After discussing with laboratory personnel, it was determined that the EHR order was missing when the process pathway changed. Our PDSA initiative changed the process pathway and different individuals were responsible for FIT dispersal. The error was quickly addressed with the help of clinical and administrative staff; a 30-day follow-up on June 21, 2021, revealed that only 9% of the patients had sent back kits with not enough information.
After troubleshooting, the team achieved a sustainable increase in the number of correctly returned FIT kits from an average of 38% before the project to 72% after 30-day follow-up.
Discussion
Proper collection and return of FIT samples are vital for process efficiency for both physicians and patients. This initiative aimed to improve the rate of correctly returned FIT kits by 20%, but its final numbers showed an improvement of 33.6%. Operational benefits from this project included early detection of CRC, improved laboratory workflow, decreased FIT kit waste, and increased patient satisfaction.
The multipronged PDSA cycle attempted to increase the rate of correctly returned FIT kits. We improved kit comprehension and laboratory accessibility, and instituted redundant return reminders for patients. We also centralized a new process pathway for FIT distribution and educated physicians and support staff. Sampling and FIT return may seem like a simple procedure, but the FIT can be cumbersome for patients and directions can be confusing. Therefore, to maximize screening participation, it is essential to minimize confusion in the collection and return of a FIT sample.14,15
This QI initiative was presented at Grand Rounds at the University of South Florida in June 2021 and has since been shared with other VA hospitals. It was also presented at the American College of Gastroenterology Conference in 2021.
Limitations
This study was a single-center QI project and focused mostly on FIT kit return rates. To fully address CRC screening, it is important to ensure that individuals with a positive screen are appropriately followed up with a colonoscopy. Although follow-up was not in the scope of this project, it is key to CRC screening in general and should be the subject of future research.
Conclusions
FIT is a useful method for CRC screening that can be particularly helpful when in-person visits are limited, as seen during the COVID-19 pandemic. This increase in demand for FITs during the pandemic revealed process deficiencies and gave JAHVAH an opportunity to improve workflow. Through the aid of a multidisciplinary team, the process to complete and return FITs improved and surpassed the goal of 20% improvement. Our goal is to continue to fine-tune the workflow and troubleshoot the system as needed.
1. Sawicki T, Ruszkowska M, Danielewicz A, Niedz′wiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. Published 2021 Apr 22. doi:10.3390/cancers13092025
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103. doi:10.5114/pg.2018.81072
3. Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120(18):2893-2901. doi:10.1002/cncr.28794
4. Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1296-1302. doi:10.1158/1055-9965.EPI-11-0250
5. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. doi:10.3322/caac.21601
6. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive
1. Sawicki T, Ruszkowska M, Danielewicz A, Niedz′wiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. Published 2021 Apr 22. doi:10.3390/cancers13092025
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103. doi:10.5114/pg.2018.81072
3. Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120(18):2893-2901. doi:10.1002/cncr.28794
4. Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1296-1302. doi:10.1158/1055-9965.EPI-11-0250
5. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. doi:10.3322/caac.21601
6. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive
Potential Impact of USPS Mail Delivery Delays on Colorectal Cancer Screening Programs
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
Few Cancer Survivors Meet ACS Nutrition, Exercise Guidelines
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The ACS has published nutrition and exercise guidelines for cancer survivors, which include recommendations to maintain a healthy weight and diet, cut out alcohol, and participate in regular physical activities. Engaging in these behaviors is associated with longer survival among cancer survivors, but whether survivors follow these nutrition and activity recommendations has not been systematically tracked.
- Researchers evaluated data on 10,020 individuals (mean age, 64.2 years) who had completed cancer treatment. Data came from the Behavioral Risk Factor Surveillance System telephone-based survey administered in 2017, 2019, and 2021, which represents 2.7 million cancer survivors.
- The researchers estimated survivors’ adherence to guidelines across four domains: Weight, physical activity, fruit and vegetable consumption, and alcohol intake. Factors associated with adherence were also evaluated.
- Overall, 9,121 survivors (91%) completed questionnaires for all four domains.
TAKEAWAY:
Only 4% of patients (365 of 9121) followed ACS guidelines in all four categories.
When assessing adherence to each category, the researchers found that 72% of cancer survivors reported engaging in recommended levels of physical activity, 68% maintained a nonobese weight, 50% said they did not consume alcohol, and 12% said they consumed recommended quantities of fruits and vegetables.
Compared with people in the general population, cancer survivors generally engaged in fewer healthy behaviors than those who had never been diagnosed with cancer.
The authors identified certain factors associated with greater guideline adherence, including female sex, older age, Black (vs White) race, and higher education level (college graduate).
IN PRACTICE:
This study highlights a potential “gap between published guidelines regarding behavioral modifications for cancer survivors and uptake of these behaviors,” the authors wrote, adding that “it is essential for oncologists and general internists to improve widespread and systematic counseling on these guidelines to improve uptake of healthy behaviors in this vulnerable patient population.”
SOURCE:
This work, led by Carter Baughman, MD, from the Division of Internal Medicine at Beth Israel Deaconess Medical Center, Boston, Massachusetts, was published online in JAMA Oncology.
LIMITATIONS:
The authors reported several study limitations, most notably that self-reported data may introduce biases.
DISCLOSURES:
The study funding source was not reported. One author received grants from the US Highbush Blueberry Council outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
FDA Approves Second Gene Therapy for Hemophilia B
Patients are eligible for a one-time infusion of Pfizer’s gene therapy if they are currently using clotting factor IX prophylaxis therapy; have current or historical life-threatening hemorrhages; or have repeated, serious spontaneous bleeding episodes.
Beqvez is the second gene therapy the agency has approved for hemophilia B, a deficiency in clotting factor IX because of a faulty gene that occurs mostly in males. The FDA approved the first gene therapy, etranacogene dezaparvovec (Hemgenix), in November 2022.
Both therapies deliver a functional copy of the factor IX gene to liver cells via a viral vector.
Pfizer said the list price of Beqvez will be $3.5 million — the same price as Hemgenix. The argument for this hefty price tag is that these gene therapies offer the possibility of a cure whereas ongoing factor IX infusions can cost more than $20 million over a patient’s lifetime. Uptake of Hemgenix, however, has been slow, given the cost and concerns about the therapy’s durability and safety.
Beqvez was approved on the basis of the phase 3 BENEGENE-2 trial in 45 men with moderate to severe hemophilia B. These men had been on factor IX prophylaxis for at least 6 months and had tested negative for antibodies against the viral delivery vector.
The annualized bleeding rate fell from a mean of 4.5 events during the pretreatment period of at least 6 months to a mean of 2.5 events between week 12 and data cutoff (median, 1.8 years of follow-up), according to Pfizer’s press release. Overall, bleeding events were eliminated in 60% of patients who received the one-time infusion vs 29% of patients on factor IX prophylaxis therapy.
Overall, Pfizer reported that the gene therapy was “generally well-tolerated,” with an increase in transaminases reported as the most common adverse event. No deaths, serious infusion reactions, thrombotic events, or development of factor IX antibodies occurred.
Pfizer has said it will continue to monitor patients to assess the therapy’s long-term durability and safety over a 15-year period.
A version of this article appeared on Medscape.com.
Patients are eligible for a one-time infusion of Pfizer’s gene therapy if they are currently using clotting factor IX prophylaxis therapy; have current or historical life-threatening hemorrhages; or have repeated, serious spontaneous bleeding episodes.
Beqvez is the second gene therapy the agency has approved for hemophilia B, a deficiency in clotting factor IX because of a faulty gene that occurs mostly in males. The FDA approved the first gene therapy, etranacogene dezaparvovec (Hemgenix), in November 2022.
Both therapies deliver a functional copy of the factor IX gene to liver cells via a viral vector.
Pfizer said the list price of Beqvez will be $3.5 million — the same price as Hemgenix. The argument for this hefty price tag is that these gene therapies offer the possibility of a cure whereas ongoing factor IX infusions can cost more than $20 million over a patient’s lifetime. Uptake of Hemgenix, however, has been slow, given the cost and concerns about the therapy’s durability and safety.
Beqvez was approved on the basis of the phase 3 BENEGENE-2 trial in 45 men with moderate to severe hemophilia B. These men had been on factor IX prophylaxis for at least 6 months and had tested negative for antibodies against the viral delivery vector.
The annualized bleeding rate fell from a mean of 4.5 events during the pretreatment period of at least 6 months to a mean of 2.5 events between week 12 and data cutoff (median, 1.8 years of follow-up), according to Pfizer’s press release. Overall, bleeding events were eliminated in 60% of patients who received the one-time infusion vs 29% of patients on factor IX prophylaxis therapy.
Overall, Pfizer reported that the gene therapy was “generally well-tolerated,” with an increase in transaminases reported as the most common adverse event. No deaths, serious infusion reactions, thrombotic events, or development of factor IX antibodies occurred.
Pfizer has said it will continue to monitor patients to assess the therapy’s long-term durability and safety over a 15-year period.
A version of this article appeared on Medscape.com.
Patients are eligible for a one-time infusion of Pfizer’s gene therapy if they are currently using clotting factor IX prophylaxis therapy; have current or historical life-threatening hemorrhages; or have repeated, serious spontaneous bleeding episodes.
Beqvez is the second gene therapy the agency has approved for hemophilia B, a deficiency in clotting factor IX because of a faulty gene that occurs mostly in males. The FDA approved the first gene therapy, etranacogene dezaparvovec (Hemgenix), in November 2022.
Both therapies deliver a functional copy of the factor IX gene to liver cells via a viral vector.
Pfizer said the list price of Beqvez will be $3.5 million — the same price as Hemgenix. The argument for this hefty price tag is that these gene therapies offer the possibility of a cure whereas ongoing factor IX infusions can cost more than $20 million over a patient’s lifetime. Uptake of Hemgenix, however, has been slow, given the cost and concerns about the therapy’s durability and safety.
Beqvez was approved on the basis of the phase 3 BENEGENE-2 trial in 45 men with moderate to severe hemophilia B. These men had been on factor IX prophylaxis for at least 6 months and had tested negative for antibodies against the viral delivery vector.
The annualized bleeding rate fell from a mean of 4.5 events during the pretreatment period of at least 6 months to a mean of 2.5 events between week 12 and data cutoff (median, 1.8 years of follow-up), according to Pfizer’s press release. Overall, bleeding events were eliminated in 60% of patients who received the one-time infusion vs 29% of patients on factor IX prophylaxis therapy.
Overall, Pfizer reported that the gene therapy was “generally well-tolerated,” with an increase in transaminases reported as the most common adverse event. No deaths, serious infusion reactions, thrombotic events, or development of factor IX antibodies occurred.
Pfizer has said it will continue to monitor patients to assess the therapy’s long-term durability and safety over a 15-year period.
A version of this article appeared on Medscape.com.
Oregon Physician Assistants Get Name Change
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
‘We Need to Rethink Our Options’: Lung Cancer Recurrence
This transcript has been edited for clarity.
Hello. It’s Mark Kris reporting back after attending the New York Lung Cancer Foundation Summit here in New York. A large amount of discussion went on, but as usual, I was most interested in the perioperative space.
In previous videos, I’ve talked about this ongoing discussion of whether you should operate and give adjuvant therapy or give neoadjuvant therapy, and I’ve addressed that already. One thing I want to bring up – and as we move off of that argument, which frankly doesn’t have an answer today, with neoadjuvant therapy, having all the data to support it – is
I was taught early on by my surgical mentors that the issue here was systemic control. While they could do very successful surgery to get high levels of local control, they could not control systemic disease. Sadly, the tools we had early on with chemotherapy were just not good enough. Suddenly, we have better tools to control systemic spread. In the past, the vast majority of occurrences were systemic; they’re now local.
What I think we need to do as a group of practitioners trying to deal with the problems getting in the way of curing our patients is look at what the issue is now. Frankly, the big issue now, as systemic therapy has controlled metastatic disease, is recurrence in the chest.
We give adjuvant osimertinib. Please remember what the numbers are. In the osimertinib arm, of the 11 recurrences reported in the European Society for Medical Oncology presentation a few years back, nine of them were in the chest or mediastinal nodes. In the arm that got no osimertinib afterward, there were 46 recurrences, and 32 of those 46 recurrences were in the chest, either the lung or mediastinal nodes. Therefore, 74% of the recurrences are suddenly in the chest. What’s the issue here?
The issue is we need to find strategies to give better disease control in the chest, as we have made inroads in controlling systemic disease with the targeted therapies in the endothelial growth factor receptor space, and very likely the checkpoint inhibitors, too, as that data kind of filters out. We need to think about how better to get local control.
I think rather than continue to get into this argument of neoadjuvant vs adjuvant, we should move to what’s really hurting our patients. Again, the data I quoted you was from the ADAURA trial, which was adjuvant therapy, and I’m sure the neoadjuvant is going to show the same thing. It’s better systemic therapy but now, more trouble in the chest.
How are we going to deal with that? I’d like to throw out one strategy, and that is to rethink the role of radiation in these patients. Again, if the problem is local in the chest, lung, and lymph nodes, we have to think about local therapy. Yes, we’re not recommending it routinely for everybody, but now that we have better systemic control, we need to rethink our options. The obvious one to rethink is about giving radiotherapy.
We should also use what we learned in the earlier trials, which is that there is harm in giving excessive radiation to the heart. If you avoid the heart, you avoid the harm. We have better planning strategies for stereotactic body radiotherapy and more traditional radiation, and of course, we have proton therapy as well.
As we continue to struggle with the idea of that patient with stage II or III disease, whether to give adjuvant vs neoadjuvant therapy, please remember to consider their risk in 2024. Their risk for first recurrence is in the chest.
What are we going to do to better control disease in the chest? We have a challenge. I’m sure we can meet it if we put our heads together.
Dr. Kris is professor of medicine at Weill Cornell Medical College, and attending physician, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. It’s Mark Kris reporting back after attending the New York Lung Cancer Foundation Summit here in New York. A large amount of discussion went on, but as usual, I was most interested in the perioperative space.
In previous videos, I’ve talked about this ongoing discussion of whether you should operate and give adjuvant therapy or give neoadjuvant therapy, and I’ve addressed that already. One thing I want to bring up – and as we move off of that argument, which frankly doesn’t have an answer today, with neoadjuvant therapy, having all the data to support it – is
I was taught early on by my surgical mentors that the issue here was systemic control. While they could do very successful surgery to get high levels of local control, they could not control systemic disease. Sadly, the tools we had early on with chemotherapy were just not good enough. Suddenly, we have better tools to control systemic spread. In the past, the vast majority of occurrences were systemic; they’re now local.
What I think we need to do as a group of practitioners trying to deal with the problems getting in the way of curing our patients is look at what the issue is now. Frankly, the big issue now, as systemic therapy has controlled metastatic disease, is recurrence in the chest.
We give adjuvant osimertinib. Please remember what the numbers are. In the osimertinib arm, of the 11 recurrences reported in the European Society for Medical Oncology presentation a few years back, nine of them were in the chest or mediastinal nodes. In the arm that got no osimertinib afterward, there were 46 recurrences, and 32 of those 46 recurrences were in the chest, either the lung or mediastinal nodes. Therefore, 74% of the recurrences are suddenly in the chest. What’s the issue here?
The issue is we need to find strategies to give better disease control in the chest, as we have made inroads in controlling systemic disease with the targeted therapies in the endothelial growth factor receptor space, and very likely the checkpoint inhibitors, too, as that data kind of filters out. We need to think about how better to get local control.
I think rather than continue to get into this argument of neoadjuvant vs adjuvant, we should move to what’s really hurting our patients. Again, the data I quoted you was from the ADAURA trial, which was adjuvant therapy, and I’m sure the neoadjuvant is going to show the same thing. It’s better systemic therapy but now, more trouble in the chest.
How are we going to deal with that? I’d like to throw out one strategy, and that is to rethink the role of radiation in these patients. Again, if the problem is local in the chest, lung, and lymph nodes, we have to think about local therapy. Yes, we’re not recommending it routinely for everybody, but now that we have better systemic control, we need to rethink our options. The obvious one to rethink is about giving radiotherapy.
We should also use what we learned in the earlier trials, which is that there is harm in giving excessive radiation to the heart. If you avoid the heart, you avoid the harm. We have better planning strategies for stereotactic body radiotherapy and more traditional radiation, and of course, we have proton therapy as well.
As we continue to struggle with the idea of that patient with stage II or III disease, whether to give adjuvant vs neoadjuvant therapy, please remember to consider their risk in 2024. Their risk for first recurrence is in the chest.
What are we going to do to better control disease in the chest? We have a challenge. I’m sure we can meet it if we put our heads together.
Dr. Kris is professor of medicine at Weill Cornell Medical College, and attending physician, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. It’s Mark Kris reporting back after attending the New York Lung Cancer Foundation Summit here in New York. A large amount of discussion went on, but as usual, I was most interested in the perioperative space.
In previous videos, I’ve talked about this ongoing discussion of whether you should operate and give adjuvant therapy or give neoadjuvant therapy, and I’ve addressed that already. One thing I want to bring up – and as we move off of that argument, which frankly doesn’t have an answer today, with neoadjuvant therapy, having all the data to support it – is
I was taught early on by my surgical mentors that the issue here was systemic control. While they could do very successful surgery to get high levels of local control, they could not control systemic disease. Sadly, the tools we had early on with chemotherapy were just not good enough. Suddenly, we have better tools to control systemic spread. In the past, the vast majority of occurrences were systemic; they’re now local.
What I think we need to do as a group of practitioners trying to deal with the problems getting in the way of curing our patients is look at what the issue is now. Frankly, the big issue now, as systemic therapy has controlled metastatic disease, is recurrence in the chest.
We give adjuvant osimertinib. Please remember what the numbers are. In the osimertinib arm, of the 11 recurrences reported in the European Society for Medical Oncology presentation a few years back, nine of them were in the chest or mediastinal nodes. In the arm that got no osimertinib afterward, there were 46 recurrences, and 32 of those 46 recurrences were in the chest, either the lung or mediastinal nodes. Therefore, 74% of the recurrences are suddenly in the chest. What’s the issue here?
The issue is we need to find strategies to give better disease control in the chest, as we have made inroads in controlling systemic disease with the targeted therapies in the endothelial growth factor receptor space, and very likely the checkpoint inhibitors, too, as that data kind of filters out. We need to think about how better to get local control.
I think rather than continue to get into this argument of neoadjuvant vs adjuvant, we should move to what’s really hurting our patients. Again, the data I quoted you was from the ADAURA trial, which was adjuvant therapy, and I’m sure the neoadjuvant is going to show the same thing. It’s better systemic therapy but now, more trouble in the chest.
How are we going to deal with that? I’d like to throw out one strategy, and that is to rethink the role of radiation in these patients. Again, if the problem is local in the chest, lung, and lymph nodes, we have to think about local therapy. Yes, we’re not recommending it routinely for everybody, but now that we have better systemic control, we need to rethink our options. The obvious one to rethink is about giving radiotherapy.
We should also use what we learned in the earlier trials, which is that there is harm in giving excessive radiation to the heart. If you avoid the heart, you avoid the harm. We have better planning strategies for stereotactic body radiotherapy and more traditional radiation, and of course, we have proton therapy as well.
As we continue to struggle with the idea of that patient with stage II or III disease, whether to give adjuvant vs neoadjuvant therapy, please remember to consider their risk in 2024. Their risk for first recurrence is in the chest.
What are we going to do to better control disease in the chest? We have a challenge. I’m sure we can meet it if we put our heads together.
Dr. Kris is professor of medicine at Weill Cornell Medical College, and attending physician, Thoracic Oncology Service, Memorial Sloan Kettering Cancer Center, New York. He disclosed ties with AstraZeneca, Roche/Genentech, Ariad Pharmaceuticals, Pfizer, and PUMA.
A version of this article appeared on Medscape.com.
Can Rectal Cancer Patients Benefit from Deintensification of Treatment?
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
New and evolving research in locally advanced rectal cancer suggests that selective use of treatments in some patients can achieve outcomes similar to those of standard regimens, according to the chair of the Department of Radiation Oncology at Duke University School of Medicine, Durham, North Carolina.
Total neoadjuvant therapy (TNT) is the standard treatment that involves systemic chemotherapy and radiation therapy before surgery for patients with locally advanced rectal cancer, Christopher G. Willett, MD, explained, in an interview. However, recent clinical trials support several strategies for “deintensification” of TNT for patients with locally advanced rectal cancer, he said.
Some patients may not require surgery or radiation therapy, or they may not require any treatment modalities including radiation therapy, chemotherapy, and surgery, Dr. Willett continued.
However, “these patients require close surveillance post treatment to identify any recurrence that may require salvage treatment,” he added.
During a presentation at the 2024 National Comprehensive Cancer Network Annual Conference, Dr. Willett primarily discussed the following three strategies for deintensifying overall therapy for locally advanced rectal cancer:
- Selective surgical omission for patients with rectal cancer having a complete clinical response after TNT with close surveillance following treatment.
- Selective omission of radiation therapy for patients with surgery such as sphincter-sparing surgery.
- Selective omission of all treatment modalities (radiation therapy, chemotherapy and surgery).
Does Watch and Wait Work?
Selective surgical omission, also known as a “watch and wait” or nonoperative management (NOM), involves treating patients with chemotherapy or a combination of chemo and radiation therapy but without surgery, Dr. Willett said during his presentation at the meeting.
Data from the OPRA trial published in the Journal of Clinical Oncology showed that 36% of patients who started on NOM developed tumor regrowth, most of which occurred in the first 2-3 years. Five-year disease-free survival rates were similar in patients who had total mesorectal excision (TME) upfront and those who had salvage TME procedures after tumor regrowth (61% and 62%, respectively). An update to the OPRA trial showed that the clinical outcomes persisted, and the results suggest no significant differences in disease-free survival between upfront surgery vs. watch and wait, Dr. Willett said.
Does Selective Omission of Radiotherapy Work?
Selective omission of radiotherapy is another option for reducing the overall treatment burden in patients with locally advanced rectal cancer, Dr. Willett. For these patients, who are at relatively low risk for recurrence, radiation along with surgery may not be needed.
Data from the FOWARC trial, published in the Journal of Clinical Oncology in 2016 and 2019, included 495 patients from 15 centers in China. In the randomized trial, the researchers found no significant difference in the primary outcome of disease-free survival between patients assigned in a 1:1:1 ratio to three arms:
- FOLFOX chemotherapy alone (a combination of chemotherapy drugs including folinic acid, fluorouracil, and oxaliplatin).
- FOLFOX plus chemoradiation.
- FU (fluorouracil)/LV (leucovorin calcium) plus chemoradiation.
Although the data were ultimately inconclusive because of potential staging bias, the findings were “promising for recommending radiation omission in these patients,” Dr. Willett said.
The larger PROSPECT study published in The New England Journal of Medicine in 2023 was similarly encouraging, he said. In this trial, 1194 patients with locally advanced rectal cancer were randomized to FOLFOX or chemoradiation prior to sphincter-sparing surgery. The two groups showed similar 5-year estimated overall survival, complete resection (R0), and pathological complete response.
“These further data support the idea that we don’t need radiotherapy anymore,” Dr. Willett said.
PROSPECT was “a very well-done trial” that also showed important patient reported outcomes, he said. At 12 months after surgery, patients in the chemoradiation group had higher scores on fatigue and neuropathy measures, but less than 15% were severe. Sexual function scores for men and women were worse in the chemoradiation group, although overall health-related quality-of-life scores were not significantly different between the groups, he noted.
Does Dropping Everything But Immunotherapy Work?
Research is very preliminary, but a small study of 12 patients with mismatch repair-deficit (MMRd) locally advanced rectal cancer published in The New England Journal of Medicine “lends optimism” to a personalized treatment approach via a programmed death 1 (PD-1) blockade, Dr. Willett said. The “small, but impressive numbers” showed that all 12 patients treated with dostarlimab only (an anti-PD-1 monoclonal antibody) had durable disease control at a follow-up of 6-24 months.
This option is feasible for patients with MMRd locally advanced rectal cancer, Dr. Willett said in an interview. “Patients treated with only dostarlimab (a PD-1 inhibitor) had excellent outcomes and did not require radiation therapy, chemotherapy, and surgery. This is potentially a new paradigm of treatment for MMRd rectal cancer.”
What are the Clinical Implications and Next Steps?
Patients should be carefully evaluated and selected for treatment approaches by experienced multidisciplinary teams with vigilant posttreatment surveillance, including history and physical exam, endoscopy, computed tomography (CT) of the chest, and abdomen and pelvic magnetic resonance imaging (MRI), Dr. Willett said in the interview.
Data on the treatment of patients with MMRd rectal cancer using dostarlimab and other immune checkpoint inhibitors are preliminary; more patients and further follow-up are required, he said. This treatment is applicable to only 5%-10% of patients with rectal cancer, he continued.
“There is a need for biomarkers such as circulating tumor DNA to further aid in selection and monitoring of patients with rectal cancer,” Dr. Willett said.
Other preliminary research is examining circulating tumor DNA analysis to guide adjuvant treatment for patients with resected stage II colon cancer, he noted in his presentation. Currently, ctDNA-driven therapy is not recommended by the NCCN, but more research is needed to determine whether this strategy might be applied to decision-making in rectal cancer patients, especially with watch and wait/nonoperative strategies, he said.
What Are the Takeaways for Deintensifying Treatment of Rectal Cancer?
The global continuum of rectal cancer clinical trials has provided significant evidence that, for select patients, the deintensification of treatment strategies may result in the avoidance of radiation and even avoidance of surgery, which can profoundly improve long-term quality of life, Al B. Benson III, MD, said in an interview.
“A critical takeaway message for clinicians who are determining which individual patient might benefit from a less intensive regimen to treat locally advanced rectal cancer is to first have a multidisciplinary consensus which should encompass review of a rectal MRI, pathology, chest and abdominal imaging, colonoscopy, as well as the patient’s clinical status including comorbidities,” said Dr. Benson, who served as chair of the NCCN Guidelines Panel for Colon/Rectal/Anal Cancers and Small Intestine Adenocarcinoma.
“The location of the rectal tumor (distal versus proximal) and clinical TNM stage also will inform the discussion as to which of the potential total neoadjuvant therapy regimens would be most optimal to reduce the risk of local recurrence and maintain long-term quality of life for the individual patient,” explained Dr. Benson, professor of medicine at Robert H. Lurie Comprehensive Cancer Center of Northwestern University in Chicago.
The effectiveness of less intense treatment for rectal cancer remains a work in progress, Dr. Benson said in an interview. “There is much we still do not know, such as the optimal selection of patients and the durability of this approach over time.”
Patients who undergo watch and wait require intensive follow-up, including sigmoidoscopy, digital rectal exam, and rectal MRI, to detect any evidence of local recurrence that would warrant further intervention, including possible radiation and surgery, he said. A highly skilled multidisciplinary team is a must for individuals who are potential candidates for a less intense treatment regimen, he emphasized.
The treatment of locally advanced rectal cancer continues to evolve, but there is no question that TNT has transformed patient outcomes, including the ability to deintensify treatment for select patients, Dr. Benson said.
However, many research gaps remain, Dr. Benson said in an interview. “For the MSI/dMMR patient who has achieved a complete response from immunotherapy we will need more long-term data to determine the durability of a complete clinical response and long-term avoidance of other interventions including radiation, chemotherapy and surgery.
“The wait and watch strategy for the much more common MSS patient also will require much longer follow-up to determine which patients are destined to recur and which are not,” he added.
“The introduction of monitoring with ctDNA determination over time offers an opportunity to streamline surveillance of patients who have completed combination therapy and for those undergoing watch and wait; however, much more information is required to determine which of the various ctDNA assays are most optimal, and the frequency and duration of ctDNA determination that will lend this approach as a standard of care,” Dr. Benson said.
Dr. Willett and Dr. Benson had no financial conflicts to disclose.
FROM NCCN 2024
FDA Approves New Bladder Cancer Drug
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
Specifically, the agent is approved to treat patients with BCG-unresponsive non–muscle-invasive bladder cancer carcinoma in situ with or without Ta or T1 papillary disease.
The FDA declined an initial approval for the combination in May 2023 because of deficiencies the agency observed during its prelicense inspection of third-party manufacturing organizations. In October 2023, ImmunityBio resubmitted the Biologics License Application, which was accepted.
The new therapy represents addresses “an unmet need” in this high-risk bladder cancer population, the company stated in a press release announcing the initial study findings. Typically, patients with intermediate or high-risk disease undergo bladder tumor resection followed by treatment with BCG, but the cancer recurs in up to 50% of patients, including those who experience a complete response, explained ImmunityBio, which acquired Altor BioScience.
Approval was based on findings from the single arm, phase 2/3 open-label QUILT-3.032 study, which included 77 patients with BCG-unresponsive, high-risk disease following transurethral resection. All had Eastern Cooperative Oncology Group performance status of 0-2.
Patients received nogapendekin alfa inbakicept-pmln induction via intravesical instillation with BCG followed by maintenance therapy for up to 37 months.
According to the FDA’s press release, 62% of patients had a complete response, defined as a negative cystoscopy and urine cytology; 58% of those with a complete response had a duration of response lasting at least 12 months and 40% had a duration of response lasting 24 months or longer.
The safety of the combination was evaluated in a cohort of 88 patients. Serious adverse reactions occurred in 16% of patients. The most common treatment-emergent adverse effects included dysuria, pollakiuria, and hematuria, which are associated with intravesical BCG; 86% of these events were grade 1 or 2. Overall, 7% of patients discontinued the combination owing to adverse reactions.
The recommended dose is 400 mcg administered intravesically with BCG once a week for 6 weeks as induction therapy, with an option for a second induction course if patients don’t achieve a complete response at 3 months. The recommended maintenance therapy dose is 400 mcg with BCG once a week for 3 weeks at months 4, 7, 10, 13, and 19. Patients who achieve a complete response at 25 months and beyond may receive maintenance instillations with BCG once a week for 3 weeks at months 25, 31, and 37. The maximum treatment duration is 37 months.
The FDA recommends discontinuing treatment if disease persists after second induction or owing to disease recurrence, progression, or unacceptable toxicity.
A version of this article appeared on Medscape.com.
New Screening Protocol May Improve Prostate Cancer Detection
TOPLINE:
according to preliminary findings from the Finnish ProScreen randomized clinical trial.
METHODOLOGY:
- Prostate-specific antigen (PSA) screening is currently recommended for men in the United States starting at age 55. However, the test is controversial, in large part because it often detects prostate cancer that is not clinically relevant and may lead to overtreatment of men with low-grade disease.
- The current ProScreen trial assessed a screening intervention that aims to reduce unnecessary diagnoses of prostate cancer but still catch relevant cancers and reduce prostate cancer mortality.
- The researchers randomized 60,745 eligible men aged 50-63 years to be invited to a three-phase screening intervention (n = 15,201) or to be part of a control group that was not invited to screen (n = 45,544).
- The screening group who agreed to participate (n = 7744) first underwent a PSA test. Those with a PSA of ≥ 3.0 ng/mL then underwent a four-kallikrein panel to identify high-grade prostate cancer. Those with a kallikrein panel risk score of 7.5% or higher underwent an MRI of the prostate gland.
- Targeted biopsies were performed in those with abnormal prostate gland findings on MRI. Most patients with a negative MRI were not recommended for systematic biopsy unless they had a PSA density of ≥ 0.15 ng/mL.
TAKEAWAY:
- Among the 7744 invited men who agreed to the three-phase screening protocol (51%), ultimately 209 (2.7% of all screened participants) had a targeted transrectal prostate biopsy. Overall, 136 of the biopsies (65%) detected cancer — 32 low-grade and 128 high-grade prostate cancers, for cumulative incidence rates of 0.41% and 1.65%, respectively.
- Over a 3.2-year median follow-up among the 7457 invited men who refused screening, seven low-grade and 44 high-grade prostate cancers were detected (cumulative incidence rates, 0.1% and 0.6%, respectively).
- Among the entire invited screening group, 39 low-grade (cumulative incidence, 0.26%) and 172 high-grade prostate cancers (cumulative incidence, 1.13%) were detected.
- Among men in the control group, 65 low-grade prostate cancers were ultimately identified and 282 high-grade. The risk difference between the invited screening group and control group was 0.11% for low-grade disease and 0.51% for high-grade disease. Compared with the control group, the intervention led to the detection of one additional low-grade prostate cancer per 909 men invited to screen and one additional high-grade prostate cancer per 196 men invited.
IN PRACTICE:
The three-phase screening approach used in this study detected additional cancers, compared with a control group not invited for screening, but “these results are descriptive and should be interpreted provisionally pending results from the trial on the primary outcomes of prostate cancer mortality,” the investigators said.
SOURCE:
This study was conducted by the ProScreen Trial Investigators, including first author Anssi Auvinen, MD, PhD, of Tampere University, Tampere, Finland, and was published online in JAMAalongside an accompanying editorial.
LIMITATIONS:
Absolute differences between the two randomized groups in this study were small and had unclear clinical importance. Prior screening was reported by several participants and may have reduced cancer detection. The results are based on a single invitation for screening, meaning some high-grade cancers were likely missed; subsequent screening invitations may identify missed cancers. No data were available on cancers missed at screening, and interval cancer incidence is needed to assess sensitivity of the screening protocol used in the study.
DISCLOSURES:
The ProScreen trial is funded by grants from the Academy of Finland, the Finnish Cancer Foundation, the Jane and Aatos Erkko Foundation, the Finland State Research Funding, Helsinki University Hospital, the Sigrid Jusélius Foundation, and the Päivikki and Sakari Sohlberg Foundation. Dr. Auvinen reported having no disclosures. Multiple co-authors reported associations outside the submitted work. The full list of author disclosures is included with the full text of the article.
A version of this article appeared on Medscape.com.
TOPLINE:
according to preliminary findings from the Finnish ProScreen randomized clinical trial.
METHODOLOGY:
- Prostate-specific antigen (PSA) screening is currently recommended for men in the United States starting at age 55. However, the test is controversial, in large part because it often detects prostate cancer that is not clinically relevant and may lead to overtreatment of men with low-grade disease.
- The current ProScreen trial assessed a screening intervention that aims to reduce unnecessary diagnoses of prostate cancer but still catch relevant cancers and reduce prostate cancer mortality.
- The researchers randomized 60,745 eligible men aged 50-63 years to be invited to a three-phase screening intervention (n = 15,201) or to be part of a control group that was not invited to screen (n = 45,544).
- The screening group who agreed to participate (n = 7744) first underwent a PSA test. Those with a PSA of ≥ 3.0 ng/mL then underwent a four-kallikrein panel to identify high-grade prostate cancer. Those with a kallikrein panel risk score of 7.5% or higher underwent an MRI of the prostate gland.
- Targeted biopsies were performed in those with abnormal prostate gland findings on MRI. Most patients with a negative MRI were not recommended for systematic biopsy unless they had a PSA density of ≥ 0.15 ng/mL.
TAKEAWAY:
- Among the 7744 invited men who agreed to the three-phase screening protocol (51%), ultimately 209 (2.7% of all screened participants) had a targeted transrectal prostate biopsy. Overall, 136 of the biopsies (65%) detected cancer — 32 low-grade and 128 high-grade prostate cancers, for cumulative incidence rates of 0.41% and 1.65%, respectively.
- Over a 3.2-year median follow-up among the 7457 invited men who refused screening, seven low-grade and 44 high-grade prostate cancers were detected (cumulative incidence rates, 0.1% and 0.6%, respectively).
- Among the entire invited screening group, 39 low-grade (cumulative incidence, 0.26%) and 172 high-grade prostate cancers (cumulative incidence, 1.13%) were detected.
- Among men in the control group, 65 low-grade prostate cancers were ultimately identified and 282 high-grade. The risk difference between the invited screening group and control group was 0.11% for low-grade disease and 0.51% for high-grade disease. Compared with the control group, the intervention led to the detection of one additional low-grade prostate cancer per 909 men invited to screen and one additional high-grade prostate cancer per 196 men invited.
IN PRACTICE:
The three-phase screening approach used in this study detected additional cancers, compared with a control group not invited for screening, but “these results are descriptive and should be interpreted provisionally pending results from the trial on the primary outcomes of prostate cancer mortality,” the investigators said.
SOURCE:
This study was conducted by the ProScreen Trial Investigators, including first author Anssi Auvinen, MD, PhD, of Tampere University, Tampere, Finland, and was published online in JAMAalongside an accompanying editorial.
LIMITATIONS:
Absolute differences between the two randomized groups in this study were small and had unclear clinical importance. Prior screening was reported by several participants and may have reduced cancer detection. The results are based on a single invitation for screening, meaning some high-grade cancers were likely missed; subsequent screening invitations may identify missed cancers. No data were available on cancers missed at screening, and interval cancer incidence is needed to assess sensitivity of the screening protocol used in the study.
DISCLOSURES:
The ProScreen trial is funded by grants from the Academy of Finland, the Finnish Cancer Foundation, the Jane and Aatos Erkko Foundation, the Finland State Research Funding, Helsinki University Hospital, the Sigrid Jusélius Foundation, and the Päivikki and Sakari Sohlberg Foundation. Dr. Auvinen reported having no disclosures. Multiple co-authors reported associations outside the submitted work. The full list of author disclosures is included with the full text of the article.
A version of this article appeared on Medscape.com.
TOPLINE:
according to preliminary findings from the Finnish ProScreen randomized clinical trial.
METHODOLOGY:
- Prostate-specific antigen (PSA) screening is currently recommended for men in the United States starting at age 55. However, the test is controversial, in large part because it often detects prostate cancer that is not clinically relevant and may lead to overtreatment of men with low-grade disease.
- The current ProScreen trial assessed a screening intervention that aims to reduce unnecessary diagnoses of prostate cancer but still catch relevant cancers and reduce prostate cancer mortality.
- The researchers randomized 60,745 eligible men aged 50-63 years to be invited to a three-phase screening intervention (n = 15,201) or to be part of a control group that was not invited to screen (n = 45,544).
- The screening group who agreed to participate (n = 7744) first underwent a PSA test. Those with a PSA of ≥ 3.0 ng/mL then underwent a four-kallikrein panel to identify high-grade prostate cancer. Those with a kallikrein panel risk score of 7.5% or higher underwent an MRI of the prostate gland.
- Targeted biopsies were performed in those with abnormal prostate gland findings on MRI. Most patients with a negative MRI were not recommended for systematic biopsy unless they had a PSA density of ≥ 0.15 ng/mL.
TAKEAWAY:
- Among the 7744 invited men who agreed to the three-phase screening protocol (51%), ultimately 209 (2.7% of all screened participants) had a targeted transrectal prostate biopsy. Overall, 136 of the biopsies (65%) detected cancer — 32 low-grade and 128 high-grade prostate cancers, for cumulative incidence rates of 0.41% and 1.65%, respectively.
- Over a 3.2-year median follow-up among the 7457 invited men who refused screening, seven low-grade and 44 high-grade prostate cancers were detected (cumulative incidence rates, 0.1% and 0.6%, respectively).
- Among the entire invited screening group, 39 low-grade (cumulative incidence, 0.26%) and 172 high-grade prostate cancers (cumulative incidence, 1.13%) were detected.
- Among men in the control group, 65 low-grade prostate cancers were ultimately identified and 282 high-grade. The risk difference between the invited screening group and control group was 0.11% for low-grade disease and 0.51% for high-grade disease. Compared with the control group, the intervention led to the detection of one additional low-grade prostate cancer per 909 men invited to screen and one additional high-grade prostate cancer per 196 men invited.
IN PRACTICE:
The three-phase screening approach used in this study detected additional cancers, compared with a control group not invited for screening, but “these results are descriptive and should be interpreted provisionally pending results from the trial on the primary outcomes of prostate cancer mortality,” the investigators said.
SOURCE:
This study was conducted by the ProScreen Trial Investigators, including first author Anssi Auvinen, MD, PhD, of Tampere University, Tampere, Finland, and was published online in JAMAalongside an accompanying editorial.
LIMITATIONS:
Absolute differences between the two randomized groups in this study were small and had unclear clinical importance. Prior screening was reported by several participants and may have reduced cancer detection. The results are based on a single invitation for screening, meaning some high-grade cancers were likely missed; subsequent screening invitations may identify missed cancers. No data were available on cancers missed at screening, and interval cancer incidence is needed to assess sensitivity of the screening protocol used in the study.
DISCLOSURES:
The ProScreen trial is funded by grants from the Academy of Finland, the Finnish Cancer Foundation, the Jane and Aatos Erkko Foundation, the Finland State Research Funding, Helsinki University Hospital, the Sigrid Jusélius Foundation, and the Päivikki and Sakari Sohlberg Foundation. Dr. Auvinen reported having no disclosures. Multiple co-authors reported associations outside the submitted work. The full list of author disclosures is included with the full text of the article.
A version of this article appeared on Medscape.com.