User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Analysis of early onset cancers suggests need for genetic testing
according to a presentation at the AACR virtual meeting II.
Investigators analyzed blood samples from 1,201 patients who were aged 18-39 years when diagnosed with a solid tumor malignancy.
In this group, there were 877 patients with early onset cancers, defined as cancers for which 39 years of age is greater than 1 standard deviation below the mean age of diagnosis for the cancer type.
The remaining 324 patients had young adult cancers, defined as cancers for which 39 years of age is less than 1 standard deviation below the mean age of diagnosis.
The most common early onset cancers were breast, colorectal, kidney, pancreas, and ovarian cancer.
The most common young adult cancers were sarcoma, brain cancer, and testicular cancer, as expected, said investigator Zsofia K. Stadler, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Stadler and colleagues performed next-generation sequencing of the patient samples using a panel of up to 88 genes previously implicated in cancer predisposition. This revealed a significantly higher prevalence of germline mutations in patients with early onset cancers than in those with young adult cancers – 21% and 13%, respectively (P = .002).
In patients with only high- and moderate-risk cancer susceptibility genes, the prevalence was 15% in the early onset group and 10% in the young adult group (P = .01). “Among the early onset cancer group, pancreas, breast, and kidney cancer patients harbored the highest rates of germline mutations,” Dr. Stadler said, noting that the spectrum of mutated genes differed in early onset and young adult cancer patients.
“In early onset patients, the most commonly mutated genes were BRCA1 and BRCA2 [4.9%], Lynch syndrome genes [2.2%], ATM [1.6%], and CHECK2 [1.7%],” Dr. Stadler said. “On the other hand, in young adults, TP53 mutations [2.2%], and SDHA and SDHB mutations dominated [1.9%], with the majority of mutations occurring in sarcoma patients.”
These findings suggest the prevalence of inherited cancer susceptibility syndromes in young adults with cancer is not uniform.
“We found a very high prevalence of germline mutations in young patients with cancer types that typically present at later ages,” Dr. Stadler said, referring to the early onset patients.
Conversely, the young adult cancer patients had a prevalence and spectrum of mutations more similar to what is seen in pediatric cancer populations, she noted.
The findings are surprising, according to AACR past president Elaine R. Mardis, PhD, of The Ohio State University in Columbus.
Dr. Mardis said the results show that, in young adults with early onset cancers, “the germline prevalence of these mutations is significantly higher than we had previously thought.”
“Although representing only about 4% of all cancers, young adults with cancer ... face unique challenges,” Dr. Stadler said. “Identifying whether a young patient’s cancer occurred in the setting of an inherited cancer predisposition syndrome is especially important in this patient population.”
Such knowledge “can significantly impact the risk of second primary cancers and the need for increased surveillance measures or even risk-reducing surgeries,” Dr. Stadler explained. She added that it can also have implications for identifying at-risk family members, such as younger siblings or children who should pursue genetic testing and appropriate prevention measures.
“Our results suggest that, among patients with early onset cancer, the increased prevalence of germline mutations supports a role for genetic testing, irrespective of tumor type,” Dr. Stadler said.
This study was partially funded by the Precision, Interception and Prevention Program, the Robert and Katie Niehaus Center for Inherited Cancer Genomics, the Marie-Josee and Henry R. Kravis Center for Molecular Oncology, and a National Cancer Institute Cancer Center Core Grant. Dr. Stadler reported that an immediate family member serves as a consultant in ophthalmology for Allergan, Adverum Biotechnologies, Alimera Sciences, BioMarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Mardis disclosed relationships with Qiagen NV, Pact Pharma LLC, Moderna Inc., and Interpreta LLC.
SOURCE: Stadler Z et al. AACR 2020, Abstract 1122.
according to a presentation at the AACR virtual meeting II.
Investigators analyzed blood samples from 1,201 patients who were aged 18-39 years when diagnosed with a solid tumor malignancy.
In this group, there were 877 patients with early onset cancers, defined as cancers for which 39 years of age is greater than 1 standard deviation below the mean age of diagnosis for the cancer type.
The remaining 324 patients had young adult cancers, defined as cancers for which 39 years of age is less than 1 standard deviation below the mean age of diagnosis.
The most common early onset cancers were breast, colorectal, kidney, pancreas, and ovarian cancer.
The most common young adult cancers were sarcoma, brain cancer, and testicular cancer, as expected, said investigator Zsofia K. Stadler, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Stadler and colleagues performed next-generation sequencing of the patient samples using a panel of up to 88 genes previously implicated in cancer predisposition. This revealed a significantly higher prevalence of germline mutations in patients with early onset cancers than in those with young adult cancers – 21% and 13%, respectively (P = .002).
In patients with only high- and moderate-risk cancer susceptibility genes, the prevalence was 15% in the early onset group and 10% in the young adult group (P = .01). “Among the early onset cancer group, pancreas, breast, and kidney cancer patients harbored the highest rates of germline mutations,” Dr. Stadler said, noting that the spectrum of mutated genes differed in early onset and young adult cancer patients.
“In early onset patients, the most commonly mutated genes were BRCA1 and BRCA2 [4.9%], Lynch syndrome genes [2.2%], ATM [1.6%], and CHECK2 [1.7%],” Dr. Stadler said. “On the other hand, in young adults, TP53 mutations [2.2%], and SDHA and SDHB mutations dominated [1.9%], with the majority of mutations occurring in sarcoma patients.”
These findings suggest the prevalence of inherited cancer susceptibility syndromes in young adults with cancer is not uniform.
“We found a very high prevalence of germline mutations in young patients with cancer types that typically present at later ages,” Dr. Stadler said, referring to the early onset patients.
Conversely, the young adult cancer patients had a prevalence and spectrum of mutations more similar to what is seen in pediatric cancer populations, she noted.
The findings are surprising, according to AACR past president Elaine R. Mardis, PhD, of The Ohio State University in Columbus.
Dr. Mardis said the results show that, in young adults with early onset cancers, “the germline prevalence of these mutations is significantly higher than we had previously thought.”
“Although representing only about 4% of all cancers, young adults with cancer ... face unique challenges,” Dr. Stadler said. “Identifying whether a young patient’s cancer occurred in the setting of an inherited cancer predisposition syndrome is especially important in this patient population.”
Such knowledge “can significantly impact the risk of second primary cancers and the need for increased surveillance measures or even risk-reducing surgeries,” Dr. Stadler explained. She added that it can also have implications for identifying at-risk family members, such as younger siblings or children who should pursue genetic testing and appropriate prevention measures.
“Our results suggest that, among patients with early onset cancer, the increased prevalence of germline mutations supports a role for genetic testing, irrespective of tumor type,” Dr. Stadler said.
This study was partially funded by the Precision, Interception and Prevention Program, the Robert and Katie Niehaus Center for Inherited Cancer Genomics, the Marie-Josee and Henry R. Kravis Center for Molecular Oncology, and a National Cancer Institute Cancer Center Core Grant. Dr. Stadler reported that an immediate family member serves as a consultant in ophthalmology for Allergan, Adverum Biotechnologies, Alimera Sciences, BioMarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Mardis disclosed relationships with Qiagen NV, Pact Pharma LLC, Moderna Inc., and Interpreta LLC.
SOURCE: Stadler Z et al. AACR 2020, Abstract 1122.
according to a presentation at the AACR virtual meeting II.
Investigators analyzed blood samples from 1,201 patients who were aged 18-39 years when diagnosed with a solid tumor malignancy.
In this group, there were 877 patients with early onset cancers, defined as cancers for which 39 years of age is greater than 1 standard deviation below the mean age of diagnosis for the cancer type.
The remaining 324 patients had young adult cancers, defined as cancers for which 39 years of age is less than 1 standard deviation below the mean age of diagnosis.
The most common early onset cancers were breast, colorectal, kidney, pancreas, and ovarian cancer.
The most common young adult cancers were sarcoma, brain cancer, and testicular cancer, as expected, said investigator Zsofia K. Stadler, MD, of Memorial Sloan Kettering Cancer Center in New York.
Dr. Stadler and colleagues performed next-generation sequencing of the patient samples using a panel of up to 88 genes previously implicated in cancer predisposition. This revealed a significantly higher prevalence of germline mutations in patients with early onset cancers than in those with young adult cancers – 21% and 13%, respectively (P = .002).
In patients with only high- and moderate-risk cancer susceptibility genes, the prevalence was 15% in the early onset group and 10% in the young adult group (P = .01). “Among the early onset cancer group, pancreas, breast, and kidney cancer patients harbored the highest rates of germline mutations,” Dr. Stadler said, noting that the spectrum of mutated genes differed in early onset and young adult cancer patients.
“In early onset patients, the most commonly mutated genes were BRCA1 and BRCA2 [4.9%], Lynch syndrome genes [2.2%], ATM [1.6%], and CHECK2 [1.7%],” Dr. Stadler said. “On the other hand, in young adults, TP53 mutations [2.2%], and SDHA and SDHB mutations dominated [1.9%], with the majority of mutations occurring in sarcoma patients.”
These findings suggest the prevalence of inherited cancer susceptibility syndromes in young adults with cancer is not uniform.
“We found a very high prevalence of germline mutations in young patients with cancer types that typically present at later ages,” Dr. Stadler said, referring to the early onset patients.
Conversely, the young adult cancer patients had a prevalence and spectrum of mutations more similar to what is seen in pediatric cancer populations, she noted.
The findings are surprising, according to AACR past president Elaine R. Mardis, PhD, of The Ohio State University in Columbus.
Dr. Mardis said the results show that, in young adults with early onset cancers, “the germline prevalence of these mutations is significantly higher than we had previously thought.”
“Although representing only about 4% of all cancers, young adults with cancer ... face unique challenges,” Dr. Stadler said. “Identifying whether a young patient’s cancer occurred in the setting of an inherited cancer predisposition syndrome is especially important in this patient population.”
Such knowledge “can significantly impact the risk of second primary cancers and the need for increased surveillance measures or even risk-reducing surgeries,” Dr. Stadler explained. She added that it can also have implications for identifying at-risk family members, such as younger siblings or children who should pursue genetic testing and appropriate prevention measures.
“Our results suggest that, among patients with early onset cancer, the increased prevalence of germline mutations supports a role for genetic testing, irrespective of tumor type,” Dr. Stadler said.
This study was partially funded by the Precision, Interception and Prevention Program, the Robert and Katie Niehaus Center for Inherited Cancer Genomics, the Marie-Josee and Henry R. Kravis Center for Molecular Oncology, and a National Cancer Institute Cancer Center Core Grant. Dr. Stadler reported that an immediate family member serves as a consultant in ophthalmology for Allergan, Adverum Biotechnologies, Alimera Sciences, BioMarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Mardis disclosed relationships with Qiagen NV, Pact Pharma LLC, Moderna Inc., and Interpreta LLC.
SOURCE: Stadler Z et al. AACR 2020, Abstract 1122.
FROM AACR 2020
ctDNA clearance tracks with PFS in NSCLC subtype
The median PFS was 9.1 months for patients with ctDNA clearance and 3.9 months for those without ctDNA clearance three to four cycles after starting treatment with osimertinib, an EGFR tyrosine kinase inhibitor (TKI), and savolitinib, a MET TKI (P = 0.0146).
“[O]ur findings indicate that EGFR-mutant ctDNA clearance may be predictive of longer PFS for patients with EGFR-mutant, MET-amplified non–small cell lung cancer and detectable ctDNA at baseline,” said investigator Ryan Hartmaier, PhD, of AstraZeneca in Boston, Mass.
Dr. Hartmaier presented these findings at the AACR virtual meeting II.
Prior results of TATTON
Interim results of the TATTON study were published earlier this year (Lancet Oncol. 2020 Mar;21[3]:373-386). The trial enrolled patients with locally advanced or metastatic EGFR-mutant, MET-amplified NSCLC who had progressed on a prior EGFR TKI. Results included patients enrolled in parts B and D.
Part B consisted of patients who had previously received a third-generation EGFR TKI and patients who had not received a third-generation EGFR TKI and were either Thr790Met negative or Thr790Met positive. There were 144 patients in part B. All received oral osimertinib at 80 mg, 138 received savolitinib at 600 mg, and 8 received savolitinib at 300 mg daily. Part D included 42 patients who had not received a third-generation EGFR TKI and were Thr790Met negative. In this cohort, patients received osimertinib at 80 mg and savolitinib at 300 mg daily.
The objective response rate (all partial responses) was 48% in part B and 64% in part D. The median PFS was 7.6 months and 9.1 months, respectively.
Alexander E. Drilon, MD, of Memorial Sloan Kettering Cancer Center in New York said results of the TATTON study demonstrate that MET dependence is an actionable EGFR TKI resistance mechanism in EGFR-mutant lung cancers.
“We all would welcome the approval of an EGFR and MET TKI combination in the future,” Dr. Drilon said in a discussion of the study at the AACR meeting.
According to Dr. Hartmaier, MET-based resistance mechanisms are seen in up to 10% of patients with EGFR-mutated NSCLC following progression on first- and second-generation EGFR TKIs, and up to 25% of those progressing on osimertinib, a third-generation EGFR TKI.
“Nonclinical and clinical evidence suggests that combined treatment of a MET inhibitor and an EGFR TKI could overcome acquired MET-mediated resistance,” he said.
ctDNA analysis
Patients in the TATTON study had ctDNA samples collected at various time points from baseline through cycle five of treatment and until disease progression or treatment discontinuation.
Dr. Hartmaier’s analysis focused on ctDNA changes from baseline to day 1 of the third or fourth treatment cycle, time points at which the bulk of ctDNA could be observed, he said.
Among 34 evaluable patients in part B who received savolitinib at 600 mg, 22 had ctDNA clearance, and 12 had not. Among 16 evaluable patients in part D who received savolitinib at 300 mg, 13 had ctDNA clearance, and 3 had not.
Rates of ctDNA clearance were “remarkably similar” among the dosing groups, Dr. Hartmaier said.
In part B, the median PFS was 9.1 months for patients with ctDNA clearance and 3.9 months for patients without clearance (hazard ratio, 0.34; 95% confidence interval, 0.14-0.81; P = 0.0146).
Dr. Hartmaier did not present PFS results according to ctDNA clearance for patients in part D.
Dr. Drilon said serial ctDNA analyses can provide information on mechanisms of primary or acquired resistance, intra- and inter-tumoral heterogeneity, and the potential durability of benefit that can be achieved with combination targeted therapy. He acknowledged, however, that more work needs to be done in the field of MET-targeted therapy development.
“We need to work on standardizing diagnostic definitions of MET dependence, recognizing that loose definitions and poly-assay use make data challenging to interpret,” he said.
The TATTON study was supported by AstraZeneca. Dr. Hartmaier is an AstraZeneca employee and shareholder. Dr. Drilon disclosed relationships with AstraZeneca, Pfizer, Helsinn, Beigene, and other companies.
SOURCE: Hartmaier R, et al. AACR 2020, Abstract CT303.
The median PFS was 9.1 months for patients with ctDNA clearance and 3.9 months for those without ctDNA clearance three to four cycles after starting treatment with osimertinib, an EGFR tyrosine kinase inhibitor (TKI), and savolitinib, a MET TKI (P = 0.0146).
“[O]ur findings indicate that EGFR-mutant ctDNA clearance may be predictive of longer PFS for patients with EGFR-mutant, MET-amplified non–small cell lung cancer and detectable ctDNA at baseline,” said investigator Ryan Hartmaier, PhD, of AstraZeneca in Boston, Mass.
Dr. Hartmaier presented these findings at the AACR virtual meeting II.
Prior results of TATTON
Interim results of the TATTON study were published earlier this year (Lancet Oncol. 2020 Mar;21[3]:373-386). The trial enrolled patients with locally advanced or metastatic EGFR-mutant, MET-amplified NSCLC who had progressed on a prior EGFR TKI. Results included patients enrolled in parts B and D.
Part B consisted of patients who had previously received a third-generation EGFR TKI and patients who had not received a third-generation EGFR TKI and were either Thr790Met negative or Thr790Met positive. There were 144 patients in part B. All received oral osimertinib at 80 mg, 138 received savolitinib at 600 mg, and 8 received savolitinib at 300 mg daily. Part D included 42 patients who had not received a third-generation EGFR TKI and were Thr790Met negative. In this cohort, patients received osimertinib at 80 mg and savolitinib at 300 mg daily.
The objective response rate (all partial responses) was 48% in part B and 64% in part D. The median PFS was 7.6 months and 9.1 months, respectively.
Alexander E. Drilon, MD, of Memorial Sloan Kettering Cancer Center in New York said results of the TATTON study demonstrate that MET dependence is an actionable EGFR TKI resistance mechanism in EGFR-mutant lung cancers.
“We all would welcome the approval of an EGFR and MET TKI combination in the future,” Dr. Drilon said in a discussion of the study at the AACR meeting.
According to Dr. Hartmaier, MET-based resistance mechanisms are seen in up to 10% of patients with EGFR-mutated NSCLC following progression on first- and second-generation EGFR TKIs, and up to 25% of those progressing on osimertinib, a third-generation EGFR TKI.
“Nonclinical and clinical evidence suggests that combined treatment of a MET inhibitor and an EGFR TKI could overcome acquired MET-mediated resistance,” he said.
ctDNA analysis
Patients in the TATTON study had ctDNA samples collected at various time points from baseline through cycle five of treatment and until disease progression or treatment discontinuation.
Dr. Hartmaier’s analysis focused on ctDNA changes from baseline to day 1 of the third or fourth treatment cycle, time points at which the bulk of ctDNA could be observed, he said.
Among 34 evaluable patients in part B who received savolitinib at 600 mg, 22 had ctDNA clearance, and 12 had not. Among 16 evaluable patients in part D who received savolitinib at 300 mg, 13 had ctDNA clearance, and 3 had not.
Rates of ctDNA clearance were “remarkably similar” among the dosing groups, Dr. Hartmaier said.
In part B, the median PFS was 9.1 months for patients with ctDNA clearance and 3.9 months for patients without clearance (hazard ratio, 0.34; 95% confidence interval, 0.14-0.81; P = 0.0146).
Dr. Hartmaier did not present PFS results according to ctDNA clearance for patients in part D.
Dr. Drilon said serial ctDNA analyses can provide information on mechanisms of primary or acquired resistance, intra- and inter-tumoral heterogeneity, and the potential durability of benefit that can be achieved with combination targeted therapy. He acknowledged, however, that more work needs to be done in the field of MET-targeted therapy development.
“We need to work on standardizing diagnostic definitions of MET dependence, recognizing that loose definitions and poly-assay use make data challenging to interpret,” he said.
The TATTON study was supported by AstraZeneca. Dr. Hartmaier is an AstraZeneca employee and shareholder. Dr. Drilon disclosed relationships with AstraZeneca, Pfizer, Helsinn, Beigene, and other companies.
SOURCE: Hartmaier R, et al. AACR 2020, Abstract CT303.
The median PFS was 9.1 months for patients with ctDNA clearance and 3.9 months for those without ctDNA clearance three to four cycles after starting treatment with osimertinib, an EGFR tyrosine kinase inhibitor (TKI), and savolitinib, a MET TKI (P = 0.0146).
“[O]ur findings indicate that EGFR-mutant ctDNA clearance may be predictive of longer PFS for patients with EGFR-mutant, MET-amplified non–small cell lung cancer and detectable ctDNA at baseline,” said investigator Ryan Hartmaier, PhD, of AstraZeneca in Boston, Mass.
Dr. Hartmaier presented these findings at the AACR virtual meeting II.
Prior results of TATTON
Interim results of the TATTON study were published earlier this year (Lancet Oncol. 2020 Mar;21[3]:373-386). The trial enrolled patients with locally advanced or metastatic EGFR-mutant, MET-amplified NSCLC who had progressed on a prior EGFR TKI. Results included patients enrolled in parts B and D.
Part B consisted of patients who had previously received a third-generation EGFR TKI and patients who had not received a third-generation EGFR TKI and were either Thr790Met negative or Thr790Met positive. There were 144 patients in part B. All received oral osimertinib at 80 mg, 138 received savolitinib at 600 mg, and 8 received savolitinib at 300 mg daily. Part D included 42 patients who had not received a third-generation EGFR TKI and were Thr790Met negative. In this cohort, patients received osimertinib at 80 mg and savolitinib at 300 mg daily.
The objective response rate (all partial responses) was 48% in part B and 64% in part D. The median PFS was 7.6 months and 9.1 months, respectively.
Alexander E. Drilon, MD, of Memorial Sloan Kettering Cancer Center in New York said results of the TATTON study demonstrate that MET dependence is an actionable EGFR TKI resistance mechanism in EGFR-mutant lung cancers.
“We all would welcome the approval of an EGFR and MET TKI combination in the future,” Dr. Drilon said in a discussion of the study at the AACR meeting.
According to Dr. Hartmaier, MET-based resistance mechanisms are seen in up to 10% of patients with EGFR-mutated NSCLC following progression on first- and second-generation EGFR TKIs, and up to 25% of those progressing on osimertinib, a third-generation EGFR TKI.
“Nonclinical and clinical evidence suggests that combined treatment of a MET inhibitor and an EGFR TKI could overcome acquired MET-mediated resistance,” he said.
ctDNA analysis
Patients in the TATTON study had ctDNA samples collected at various time points from baseline through cycle five of treatment and until disease progression or treatment discontinuation.
Dr. Hartmaier’s analysis focused on ctDNA changes from baseline to day 1 of the third or fourth treatment cycle, time points at which the bulk of ctDNA could be observed, he said.
Among 34 evaluable patients in part B who received savolitinib at 600 mg, 22 had ctDNA clearance, and 12 had not. Among 16 evaluable patients in part D who received savolitinib at 300 mg, 13 had ctDNA clearance, and 3 had not.
Rates of ctDNA clearance were “remarkably similar” among the dosing groups, Dr. Hartmaier said.
In part B, the median PFS was 9.1 months for patients with ctDNA clearance and 3.9 months for patients without clearance (hazard ratio, 0.34; 95% confidence interval, 0.14-0.81; P = 0.0146).
Dr. Hartmaier did not present PFS results according to ctDNA clearance for patients in part D.
Dr. Drilon said serial ctDNA analyses can provide information on mechanisms of primary or acquired resistance, intra- and inter-tumoral heterogeneity, and the potential durability of benefit that can be achieved with combination targeted therapy. He acknowledged, however, that more work needs to be done in the field of MET-targeted therapy development.
“We need to work on standardizing diagnostic definitions of MET dependence, recognizing that loose definitions and poly-assay use make data challenging to interpret,” he said.
The TATTON study was supported by AstraZeneca. Dr. Hartmaier is an AstraZeneca employee and shareholder. Dr. Drilon disclosed relationships with AstraZeneca, Pfizer, Helsinn, Beigene, and other companies.
SOURCE: Hartmaier R, et al. AACR 2020, Abstract CT303.
FROM AACR 2020
AGA releases BRCA risk guidance
BRCA carrier status alone should not influence screening recommendations for colorectal cancer or pancreatic ductal adenocarcinoma, according to an American Gastroenterological Association clinical practice update.
Relationships between BRCA carrier status and risks of pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC) remain unclear, reported lead author Sonia S. Kupfer, MD, AGAF, of the University of Chicago, and colleagues.
“Pathogenic variants in BRCA1 and BRCA2 have ... been associated with variable risk of GI cancer, including CRC, PDAC, biliary, and gastric cancers,” the investigators wrote in Gastroenterology. “However, the magnitude of GI cancer risks is not well established and there is minimal evidence or guidance on screening for GI cancers among BRCA1 and BRCA2 carriers.”
According to the investigators, personalized screening for CRC is well supported by evidence, as higher-risk individuals, such as those with a family history of CRC, have been shown to benefit from earlier and more frequent colonoscopies. Although the value of risk-based screening is less clear for other types of GI cancer, the investigators cited a growing body of evidence that supports screening individuals at high risk of PDAC.
Still, data illuminating the role of BRCA carrier status are relatively scarce, which has led to variability in clinical practice.
“Lack of accurate CRC and PDAC risk estimates in BRCA1 and BRCA2 leave physicians and patients without guidance, and result in a range of screening recommendations and practices in this population,” wrote Dr. Kupfer and colleagues.
To offer some clarity, they drafted the present clinical practice update on behalf of the AGA. The recommendations are framed within a discussion of relevant publications.
Data from multiple studies, for instance, suggest that BRCA pathogenic variants are found in 1.3% of patients with early-onset CRC, 0.2% of those with high-risk CRC, and 1.0% of those with any type of CRC, all of which are higher rates “than would be expected by chance.
“However,” the investigators added, “this association is not proof that the observed BRCA1 and BRCA2 pathogenic variants play a causative role in CRC.”
The investigators went on to discuss a 2018 meta-analysis by Oho et al., which included 14 studies evaluating risk of CRC among BRCA carriers. The analysis found that BRCA carriers had a 24% increased risk of CRC, which Dr. Kupfer and colleagues described as “small but statistically significant.” Subgroup analysis suggested that BRCA1 carriers drove this association, with a 49% increased risk of CRC, whereas no significant link was found with BRCA2.
Dr. Kupfer and colleagues described the 49% increase as “very modest,” and therefore insufficient to warrant more intensive screening, particularly when considered in the context of other risk factors, such as Lynch syndrome, which may entail a 1,600% increased risk of CRC. For PDAC, no such meta-analysis has been conducted; however, multiple studies have pointed to associations between BRCA and risk of PDAC.
For example, a 2018 case-control study by Hu et al. showed that BRCA1 and BRCA2 had relative prevalence rates of 0.59% and 1.95% among patients with PDAC. These rates translated to a 158% increased risk of PDAC for BRCA1, and a 520% increase risk for BRCA2; but Dr. Kupfer and colleagues noted that the BRCA2 carriers were from high-risk families, so the findings may not extend to the general population.
In light of these findings, the update recommends PDAC screening for BRCA carriers only if they have a family history of PDAC, with the caveat that the association between risk and degree of family involvement remains unknown.
Ultimately, for both CRC and PDAC, the investigators called for further BRCA research, based on the conclusion that “results from published studies provide inconsistent levels of evidence.”
The investigators reported no conflicts of interest.
SOURCE: Kupfer SS et al. Gastroenterology. 2020 Apr 23. doi: 10.1053/j.gastro.2020.03.086.
BRCA carrier status alone should not influence screening recommendations for colorectal cancer or pancreatic ductal adenocarcinoma, according to an American Gastroenterological Association clinical practice update.
Relationships between BRCA carrier status and risks of pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC) remain unclear, reported lead author Sonia S. Kupfer, MD, AGAF, of the University of Chicago, and colleagues.
“Pathogenic variants in BRCA1 and BRCA2 have ... been associated with variable risk of GI cancer, including CRC, PDAC, biliary, and gastric cancers,” the investigators wrote in Gastroenterology. “However, the magnitude of GI cancer risks is not well established and there is minimal evidence or guidance on screening for GI cancers among BRCA1 and BRCA2 carriers.”
According to the investigators, personalized screening for CRC is well supported by evidence, as higher-risk individuals, such as those with a family history of CRC, have been shown to benefit from earlier and more frequent colonoscopies. Although the value of risk-based screening is less clear for other types of GI cancer, the investigators cited a growing body of evidence that supports screening individuals at high risk of PDAC.
Still, data illuminating the role of BRCA carrier status are relatively scarce, which has led to variability in clinical practice.
“Lack of accurate CRC and PDAC risk estimates in BRCA1 and BRCA2 leave physicians and patients without guidance, and result in a range of screening recommendations and practices in this population,” wrote Dr. Kupfer and colleagues.
To offer some clarity, they drafted the present clinical practice update on behalf of the AGA. The recommendations are framed within a discussion of relevant publications.
Data from multiple studies, for instance, suggest that BRCA pathogenic variants are found in 1.3% of patients with early-onset CRC, 0.2% of those with high-risk CRC, and 1.0% of those with any type of CRC, all of which are higher rates “than would be expected by chance.
“However,” the investigators added, “this association is not proof that the observed BRCA1 and BRCA2 pathogenic variants play a causative role in CRC.”
The investigators went on to discuss a 2018 meta-analysis by Oho et al., which included 14 studies evaluating risk of CRC among BRCA carriers. The analysis found that BRCA carriers had a 24% increased risk of CRC, which Dr. Kupfer and colleagues described as “small but statistically significant.” Subgroup analysis suggested that BRCA1 carriers drove this association, with a 49% increased risk of CRC, whereas no significant link was found with BRCA2.
Dr. Kupfer and colleagues described the 49% increase as “very modest,” and therefore insufficient to warrant more intensive screening, particularly when considered in the context of other risk factors, such as Lynch syndrome, which may entail a 1,600% increased risk of CRC. For PDAC, no such meta-analysis has been conducted; however, multiple studies have pointed to associations between BRCA and risk of PDAC.
For example, a 2018 case-control study by Hu et al. showed that BRCA1 and BRCA2 had relative prevalence rates of 0.59% and 1.95% among patients with PDAC. These rates translated to a 158% increased risk of PDAC for BRCA1, and a 520% increase risk for BRCA2; but Dr. Kupfer and colleagues noted that the BRCA2 carriers were from high-risk families, so the findings may not extend to the general population.
In light of these findings, the update recommends PDAC screening for BRCA carriers only if they have a family history of PDAC, with the caveat that the association between risk and degree of family involvement remains unknown.
Ultimately, for both CRC and PDAC, the investigators called for further BRCA research, based on the conclusion that “results from published studies provide inconsistent levels of evidence.”
The investigators reported no conflicts of interest.
SOURCE: Kupfer SS et al. Gastroenterology. 2020 Apr 23. doi: 10.1053/j.gastro.2020.03.086.
BRCA carrier status alone should not influence screening recommendations for colorectal cancer or pancreatic ductal adenocarcinoma, according to an American Gastroenterological Association clinical practice update.
Relationships between BRCA carrier status and risks of pancreatic ductal adenocarcinoma (PDAC) and colorectal cancer (CRC) remain unclear, reported lead author Sonia S. Kupfer, MD, AGAF, of the University of Chicago, and colleagues.
“Pathogenic variants in BRCA1 and BRCA2 have ... been associated with variable risk of GI cancer, including CRC, PDAC, biliary, and gastric cancers,” the investigators wrote in Gastroenterology. “However, the magnitude of GI cancer risks is not well established and there is minimal evidence or guidance on screening for GI cancers among BRCA1 and BRCA2 carriers.”
According to the investigators, personalized screening for CRC is well supported by evidence, as higher-risk individuals, such as those with a family history of CRC, have been shown to benefit from earlier and more frequent colonoscopies. Although the value of risk-based screening is less clear for other types of GI cancer, the investigators cited a growing body of evidence that supports screening individuals at high risk of PDAC.
Still, data illuminating the role of BRCA carrier status are relatively scarce, which has led to variability in clinical practice.
“Lack of accurate CRC and PDAC risk estimates in BRCA1 and BRCA2 leave physicians and patients without guidance, and result in a range of screening recommendations and practices in this population,” wrote Dr. Kupfer and colleagues.
To offer some clarity, they drafted the present clinical practice update on behalf of the AGA. The recommendations are framed within a discussion of relevant publications.
Data from multiple studies, for instance, suggest that BRCA pathogenic variants are found in 1.3% of patients with early-onset CRC, 0.2% of those with high-risk CRC, and 1.0% of those with any type of CRC, all of which are higher rates “than would be expected by chance.
“However,” the investigators added, “this association is not proof that the observed BRCA1 and BRCA2 pathogenic variants play a causative role in CRC.”
The investigators went on to discuss a 2018 meta-analysis by Oho et al., which included 14 studies evaluating risk of CRC among BRCA carriers. The analysis found that BRCA carriers had a 24% increased risk of CRC, which Dr. Kupfer and colleagues described as “small but statistically significant.” Subgroup analysis suggested that BRCA1 carriers drove this association, with a 49% increased risk of CRC, whereas no significant link was found with BRCA2.
Dr. Kupfer and colleagues described the 49% increase as “very modest,” and therefore insufficient to warrant more intensive screening, particularly when considered in the context of other risk factors, such as Lynch syndrome, which may entail a 1,600% increased risk of CRC. For PDAC, no such meta-analysis has been conducted; however, multiple studies have pointed to associations between BRCA and risk of PDAC.
For example, a 2018 case-control study by Hu et al. showed that BRCA1 and BRCA2 had relative prevalence rates of 0.59% and 1.95% among patients with PDAC. These rates translated to a 158% increased risk of PDAC for BRCA1, and a 520% increase risk for BRCA2; but Dr. Kupfer and colleagues noted that the BRCA2 carriers were from high-risk families, so the findings may not extend to the general population.
In light of these findings, the update recommends PDAC screening for BRCA carriers only if they have a family history of PDAC, with the caveat that the association between risk and degree of family involvement remains unknown.
Ultimately, for both CRC and PDAC, the investigators called for further BRCA research, based on the conclusion that “results from published studies provide inconsistent levels of evidence.”
The investigators reported no conflicts of interest.
SOURCE: Kupfer SS et al. Gastroenterology. 2020 Apr 23. doi: 10.1053/j.gastro.2020.03.086.
FROM GASTROENTEROLOGY
Hypercalcemia Is of Uncertain Significance in Patients With Advanced Adenocarcinoma of the Prostate
Hypercalcemia is found when the corrected serum calcium level is > 10.5 mg/dL.1 Its symptoms are not specific and may include polyuria, dehydration, polydipsia, anorexia, nausea and/or vomiting, constipation, and other central nervous system manifestations, including confusion, delirium, cognitive impairment, muscle weakness, psychotic symptoms, and even coma.1,2
Hypercalcemia has varied etiologies; however, malignancy-induced hypercalcemia is one of the most common causes. In the US, the most common causes of malignancy-induced hypercalcemia are primary tumors of the lung or breast, multiple myeloma (MM), squamous cell carcinoma of the head or neck, renal cancer, and ovarian cancer.1
Men with prostate cancer and bone metastasis have relatively worse prognosis than do patient with no metastasis.3 In a recent meta-analysis of patients with bone-involved castration-resistant prostate cancer, the median survival was 21 months.3
Hypercalcemia is a rare manifestation of prostate cancer. In a retrospective study conducted between 2009 and 2013 using the Oncology Services Comprehensive Electronic Records (OSCER) warehouse of electronic health records (EHR), the rates of malignancy-induced hypercalcemia were the lowest among patients with prostate cancer, ranging from 1.4 to 2.1%.1
We present this case to discuss different pathophysiologic mechanisms leading to hypercalcemia in a patient with prostate cancer with bone metastasis and to study the role of humoral and growth factors in the pathogenesis of the disease.
Case Presentation
An African American man aged 69 years presented to the emergency department (ED) with generalized weakness, fatigue, and lower extremities muscle weakness. He reported a 40-lb weight loss over the past 3 months, intermittent lower back pain, and a 50 pack-year smoking history. A physical examination suggested clinical signs of dehydration.
Laboratory test results indicated hypercalcemia, macrocytic anemia, and thrombocytopenia: calcium 15.8 mg/dL, serum albumin 4.1 mg/dL, alkaline phosphatase 139 μ/L, blood urea nitrogen 55 mg/dL, creatinine 3.4 mg/dL (baseline 1.4-1.5 mg/dL), hemoglobin 8 g/dL, mean corpuscular volume 99.6 fL, and platelets 100,000/μL. The patient was admitted for hypercalcemia. His intact parathyroid hormone (iPTH) was suppressed at 16 pg/mL, phosphorous was 3.8 mg/dL, parathyroid hormone-related peptide (PTHrP) was < 0.74 pmol/L, vitamin D (25 hydroxy cholecalciferol) was mildly decreased at 17.2 ng/mL, and 1,25 dihydroxy cholecalciferol (calcitriol) was < 5.0 (normal range 20-79.3 pg/mL).
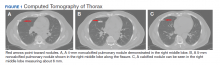
A computed tomography (CT) scan of the chest and abdomen was taken due to the patient’s heavy smoking history, an incidentally detected right lung base nodule on chest X-ray, and hypercalcemia. The CT scan showed multiple right middle lobe lung nodules with and without calcifications and calcified right hilar lymph nodes (Figure 1).
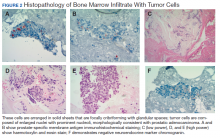
To evaluate the pancytopenia, a bone marrow biopsy was done, which showed that 80 to 90% of the marrow space was replaced by fibrosis and metastatic malignancy. Trilinear hematopoiesis was not seen (Figure 2). The tumor cells were positive for prostate- specific membrane antigen (PSMA) and negative for cytokeratin 7 and 20 (CK7 and CK20).4 The former is a membrane protein expressed on prostate tissues, including cancer; the latter is a form of protein used to identify adenocarcinoma of unknown primary origin (CK7 usually found in primary/ metastatic lung adenocarcinoma and CK20 usually in primary and some metastatic diseases of colon adenocarcinoma).5 A prostatic specific antigen (PSA) test was markedly elevated: 335.94 ng/mL (1.46 ng/mL on a previous 2011 test).
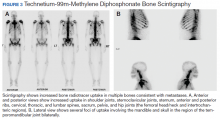
Metastatic adenocarcinoma of the prostate was diagnosed without a prostate biopsy. To determine the extent of bone metastases, a technetium-99m-methylene diphosphonate (MDP) bone scintigraphy demonstrated a superscan with intense foci of increased radiotracer uptake involving the bilateral shoulders, sternoclavicular joints, and sternum with heterogeneous uptake involving bilateral anterior and posterior ribs; cervical, thoracic, and lumbar spines; sacrum, pelvis, and bilateral hips, including the femoral head/neck and intertrochanteric regions. Also noted were several foci of radiotracer uptake involving the mandible and bilateral skull in the region of the temporomandibular joints (Figure 3).
The patient was initially treated with IV isotonic saline, followed by calcitonin and then pamidronate after kidney function improved. His calcium level responded to the therapy, and a plan was made by medical oncology to start androgen deprivation therapy (ADT) prior to discharge.
He was initially treated with bicalutamide, while a luteinizing hormone-releasing hormone agonist (leuprolide) was added 1 week later. Bicalutamide was then discontinued and a combined androgen blockade consisting of leuprolide, ketoconazole, and hydrocortisone was started. This therapy resulted in remission, and PSA declined to 1.73 ng/ mL 3 months later. At that time the patient enrolled in a clinical trial with leuprolide and bicalutamide combined therapy. About 6 months after his diagnosis, patient’s cancer progressed and became hormone refractory disease. At that time, bicalutamide was discontinued, and his therapy was switched to combined leuprolide and enzalutamide. After 6 months of therapy with enzalutamide, the patient’s cancer progressed again. He was later treated with docetaxel chemotherapy but died 16 months after diagnosis.
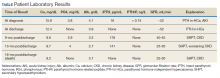
showed improvement of hypercalcemia at the time of discharge, but 9 months later and toward the time of expiration, our patient developed secondary hyperparathyroidism, with calcium maintained in the normal range, while iPTH was significantly elevated, a finding likely explained by a decline in kidney function and a fall in glomerular filtration rate (Table).
Discussion
Hypercalcemia in the setting of prostate cancer is a rare complication with an uncertain pathophysiology.6 Several mechanisms have been proposed for hypercalcemia of malignancy, these comprise humoral hypercalcemia of malignancy mediated by increased PTHrP; local osteolytic hypercalcemia with secretion of other humoral factors; excess extrarenal activation of vitamin D (1,25[OH]2D); PTH secretion, ectopic or primary; and multiple concurrent etiologies.7
PTHrP is the predominant mediator for hypercalcemia of malignancy and is estimated to account for 80% of hypercalcemia in patients with cancer. This protein shares a substantial sequence homology with PTH; in fact, 8 of the first 13 amino acids at the N-terminal portion of PTH were identical.8 PTHrP has multiple isoforms (PTHrP 141, PTHrP 139, and PTHrP 173). Like PTH, it enhances renal tubular reabsorption of calcium while increasing urinary phosphorus excretion.7 The result is both hypercalcemia and hypophosphatemia. However, unlike PTH, PTHrP does not increase 1,25(OH)2D and thus does not increase intestinal absorption of calcium and phosphorus. PTHrP acts on osteoblasts, leading to enhanced synthesis of receptor activator of nuclear factor-κB ligand (RANKL).7
In one study, PTHrP was detected immunohistochemically in prostate cancer cells. Iwamura and colleagues used 33 radical prostatectomy specimens from patients with clinically localized carcinoma of the prostate.9 None of these patients demonstrated hypercalcemia prior to the surgery. Using a mouse monoclonal antibody to an amino acid fragment, all cases demonstrated some degree of immunoreactivity throughout the cytoplasm of the tumor cells, but immunostaining was absent from inflammatory and stromal cells.9Furthermore, the intensity of the staining appeared to directly correlate with increasing tumor grade.9
Another study by Iwamura and colleagues suggested that PTHrP may play a significant role in the growth of prostate cancer by acting locally in an autocrine fashion.10 In this study, all prostate cancer cell lines from different sources expressed PTHrP immunoreactivity as well as evidence of DNA synthesis, the latter being measured by thymidine incorporation assay. Moreover, when these cells were incubated with various concentrations of mouse monoclonal antibody directed to PTHrP fragment, PTHrP-induced DNA synthesis was inhibited in a dose-dependent manner and almost completely neutralized at a specific concentration. Interestingly, the study demonstrated that cancer cell line derived from bone metastatic lesions secreted significantly greater amounts of PTHrP than did the cell line derived from the metastasis in the brain or in the lymph node. These findings suggest that PTHrP production may confer some advantage on the ability of prostate cancer cells to grow in bone.10
Ando and colleagues reported that neuroendocrine dedifferentiated prostate cancer can develop as a result of long-term ADT even after several years of therapy and has the potential to worsen and develop severe hypercalcemia.8 Neuron-specific enolase was used as the specific marker for the neuroendocrine cell, which suggested that the prostate cancer cell derived from the neuroendocrine cell might synthesize PTHrP and be responsible for the observed hypercalcemia.8
Other mechanisms cited for hypercalcemia of malignancy include other humoral factors associated with increased remodeling and comprise interleukin 1, 3, 6 (IL-1, IL-3, IL-6); tumor necrosis factor α; transforming growth factor A and B observed in metastatic bone lesions in breast cancer; lymphotoxin; E series prostaglandins; and macrophage inflammatory protein 1α seen in MM.
Local osteolytic hypercalcemia accounts for about 20% of cases and is usually associated with extensive bone metastases. It is most commonly seen in MM and metastatic breast cancer and less commonly in leukemia. The proposed mechanism is thought to be because of the release of local cytokines from the tumor, resulting in excess osteoclast activation and enhanced bone resorption often through RANK/RANKL interaction.
Extrarenal production of 1,25(OH)2D by the tumor accounts for about 1% of cases of hypercalcemia in malignancy. 1,25(OH)2D causes increased intestinal absorption of calcium and enhances osteolytic bone resorption, resulting in increased serum calcium. This mechanism is most commonly seen with Hodgkin and non-Hodgkin lymphoma and had been reported in ovarian dysgerminoma.7
In our patient, bone imaging showed osteoblastic lesions, a finding that likely contrasts the local osteolytic bone destruction theory. PTHrP was not significantly elevated in the serum, and PTH levels ruled out any form of primary hyperparathyroidism. In addition, histopathology showed no evidence of mosaicism or neuroendocrine dedifferentiation.
Findings in aggregate tell us that an exact pathophysiologic mechanism leading to hypercalcemia in prostate cancer is still unclear and may involve an interplay between growth factors and possible osteolytic materials, yet it must be studied thoroughly.
Conclusions
Hypercalcemia in pure metastatic adenocarcinoma of prostate is a rare finding and is of uncertain significance. Some studies suggested a search for unusual histopathologies, including neuroendocrine cancer and neuroendocrine dedifferentiation.8,11 However, in adenocarcinoma alone, it has an uncertain pathophysiology that needs to be further studied. Studies needed to investigate the role of PTHrP as a growth factor for both prostate cancer cells and development of hypercalcemia and possibly target-directed monoclonal antibody therapies may need to be extensively researched.
1. Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5(8):2091‐2100. doi:10.1002/cam4.749
2. Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000;1(4):253‐263. doi:10.1023/a:1026597816193
3. Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652‐1659. doi:10.1200/JCO.2015.65.7270
4. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(suppl 10):S13‐S18.
5. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86(12):1884‐1887. doi:10.1038/sj.bjc.6600326
6. Avashia JH, Walsh TD, Thomas AJ Jr, Kaye M, Licata A. Metastatic carcinoma of the prostate with hypercalcemia [published correction appears in Cleve Clin J Med. 1991;58(3):284]. Cleve Clin J Med. 1990;57(7):636‐638. doi:10.3949/ccjm.57.7.636.
7. Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12(5):426‐432. doi:10.1200/JOP.2016.011155.
8. Ando T, Watanabe K, Mizusawa T, Katagiri A. Hypercalcemia due to parathyroid hormone-related peptide secreted by neuroendocrine dedifferentiated prostate cancer. Urol Case Rep. 2018;22:67‐69. doi:10.1016/j.eucr.2018.11.001
9. Iwamura M, di Sant’Agnese PA, Wu G, et al. Immunohistochemical localization of parathyroid hormonerelated protein in human prostate cancer. Cancer Res. 1993;53(8):1724‐1726.
10. Iwamura M, Abrahamsson PA, Foss KA, Wu G, Cockett AT, Deftos LJ. Parathyroid hormone-related protein: a potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43(5):675‐679. doi:10.1016/0090-4295(94)90183-x
11. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol. 1992;10(3):499‐505. doi:10.1200/JCO.1992.10.3.499.
Hypercalcemia is found when the corrected serum calcium level is > 10.5 mg/dL.1 Its symptoms are not specific and may include polyuria, dehydration, polydipsia, anorexia, nausea and/or vomiting, constipation, and other central nervous system manifestations, including confusion, delirium, cognitive impairment, muscle weakness, psychotic symptoms, and even coma.1,2
Hypercalcemia has varied etiologies; however, malignancy-induced hypercalcemia is one of the most common causes. In the US, the most common causes of malignancy-induced hypercalcemia are primary tumors of the lung or breast, multiple myeloma (MM), squamous cell carcinoma of the head or neck, renal cancer, and ovarian cancer.1
Men with prostate cancer and bone metastasis have relatively worse prognosis than do patient with no metastasis.3 In a recent meta-analysis of patients with bone-involved castration-resistant prostate cancer, the median survival was 21 months.3
Hypercalcemia is a rare manifestation of prostate cancer. In a retrospective study conducted between 2009 and 2013 using the Oncology Services Comprehensive Electronic Records (OSCER) warehouse of electronic health records (EHR), the rates of malignancy-induced hypercalcemia were the lowest among patients with prostate cancer, ranging from 1.4 to 2.1%.1
We present this case to discuss different pathophysiologic mechanisms leading to hypercalcemia in a patient with prostate cancer with bone metastasis and to study the role of humoral and growth factors in the pathogenesis of the disease.
Case Presentation
An African American man aged 69 years presented to the emergency department (ED) with generalized weakness, fatigue, and lower extremities muscle weakness. He reported a 40-lb weight loss over the past 3 months, intermittent lower back pain, and a 50 pack-year smoking history. A physical examination suggested clinical signs of dehydration.
Laboratory test results indicated hypercalcemia, macrocytic anemia, and thrombocytopenia: calcium 15.8 mg/dL, serum albumin 4.1 mg/dL, alkaline phosphatase 139 μ/L, blood urea nitrogen 55 mg/dL, creatinine 3.4 mg/dL (baseline 1.4-1.5 mg/dL), hemoglobin 8 g/dL, mean corpuscular volume 99.6 fL, and platelets 100,000/μL. The patient was admitted for hypercalcemia. His intact parathyroid hormone (iPTH) was suppressed at 16 pg/mL, phosphorous was 3.8 mg/dL, parathyroid hormone-related peptide (PTHrP) was < 0.74 pmol/L, vitamin D (25 hydroxy cholecalciferol) was mildly decreased at 17.2 ng/mL, and 1,25 dihydroxy cholecalciferol (calcitriol) was < 5.0 (normal range 20-79.3 pg/mL).
A computed tomography (CT) scan of the chest and abdomen was taken due to the patient’s heavy smoking history, an incidentally detected right lung base nodule on chest X-ray, and hypercalcemia. The CT scan showed multiple right middle lobe lung nodules with and without calcifications and calcified right hilar lymph nodes (Figure 1).
To evaluate the pancytopenia, a bone marrow biopsy was done, which showed that 80 to 90% of the marrow space was replaced by fibrosis and metastatic malignancy. Trilinear hematopoiesis was not seen (Figure 2). The tumor cells were positive for prostate- specific membrane antigen (PSMA) and negative for cytokeratin 7 and 20 (CK7 and CK20).4 The former is a membrane protein expressed on prostate tissues, including cancer; the latter is a form of protein used to identify adenocarcinoma of unknown primary origin (CK7 usually found in primary/ metastatic lung adenocarcinoma and CK20 usually in primary and some metastatic diseases of colon adenocarcinoma).5 A prostatic specific antigen (PSA) test was markedly elevated: 335.94 ng/mL (1.46 ng/mL on a previous 2011 test).
Metastatic adenocarcinoma of the prostate was diagnosed without a prostate biopsy. To determine the extent of bone metastases, a technetium-99m-methylene diphosphonate (MDP) bone scintigraphy demonstrated a superscan with intense foci of increased radiotracer uptake involving the bilateral shoulders, sternoclavicular joints, and sternum with heterogeneous uptake involving bilateral anterior and posterior ribs; cervical, thoracic, and lumbar spines; sacrum, pelvis, and bilateral hips, including the femoral head/neck and intertrochanteric regions. Also noted were several foci of radiotracer uptake involving the mandible and bilateral skull in the region of the temporomandibular joints (Figure 3).
The patient was initially treated with IV isotonic saline, followed by calcitonin and then pamidronate after kidney function improved. His calcium level responded to the therapy, and a plan was made by medical oncology to start androgen deprivation therapy (ADT) prior to discharge.
He was initially treated with bicalutamide, while a luteinizing hormone-releasing hormone agonist (leuprolide) was added 1 week later. Bicalutamide was then discontinued and a combined androgen blockade consisting of leuprolide, ketoconazole, and hydrocortisone was started. This therapy resulted in remission, and PSA declined to 1.73 ng/ mL 3 months later. At that time the patient enrolled in a clinical trial with leuprolide and bicalutamide combined therapy. About 6 months after his diagnosis, patient’s cancer progressed and became hormone refractory disease. At that time, bicalutamide was discontinued, and his therapy was switched to combined leuprolide and enzalutamide. After 6 months of therapy with enzalutamide, the patient’s cancer progressed again. He was later treated with docetaxel chemotherapy but died 16 months after diagnosis.
showed improvement of hypercalcemia at the time of discharge, but 9 months later and toward the time of expiration, our patient developed secondary hyperparathyroidism, with calcium maintained in the normal range, while iPTH was significantly elevated, a finding likely explained by a decline in kidney function and a fall in glomerular filtration rate (Table).
Discussion
Hypercalcemia in the setting of prostate cancer is a rare complication with an uncertain pathophysiology.6 Several mechanisms have been proposed for hypercalcemia of malignancy, these comprise humoral hypercalcemia of malignancy mediated by increased PTHrP; local osteolytic hypercalcemia with secretion of other humoral factors; excess extrarenal activation of vitamin D (1,25[OH]2D); PTH secretion, ectopic or primary; and multiple concurrent etiologies.7
PTHrP is the predominant mediator for hypercalcemia of malignancy and is estimated to account for 80% of hypercalcemia in patients with cancer. This protein shares a substantial sequence homology with PTH; in fact, 8 of the first 13 amino acids at the N-terminal portion of PTH were identical.8 PTHrP has multiple isoforms (PTHrP 141, PTHrP 139, and PTHrP 173). Like PTH, it enhances renal tubular reabsorption of calcium while increasing urinary phosphorus excretion.7 The result is both hypercalcemia and hypophosphatemia. However, unlike PTH, PTHrP does not increase 1,25(OH)2D and thus does not increase intestinal absorption of calcium and phosphorus. PTHrP acts on osteoblasts, leading to enhanced synthesis of receptor activator of nuclear factor-κB ligand (RANKL).7
In one study, PTHrP was detected immunohistochemically in prostate cancer cells. Iwamura and colleagues used 33 radical prostatectomy specimens from patients with clinically localized carcinoma of the prostate.9 None of these patients demonstrated hypercalcemia prior to the surgery. Using a mouse monoclonal antibody to an amino acid fragment, all cases demonstrated some degree of immunoreactivity throughout the cytoplasm of the tumor cells, but immunostaining was absent from inflammatory and stromal cells.9Furthermore, the intensity of the staining appeared to directly correlate with increasing tumor grade.9
Another study by Iwamura and colleagues suggested that PTHrP may play a significant role in the growth of prostate cancer by acting locally in an autocrine fashion.10 In this study, all prostate cancer cell lines from different sources expressed PTHrP immunoreactivity as well as evidence of DNA synthesis, the latter being measured by thymidine incorporation assay. Moreover, when these cells were incubated with various concentrations of mouse monoclonal antibody directed to PTHrP fragment, PTHrP-induced DNA synthesis was inhibited in a dose-dependent manner and almost completely neutralized at a specific concentration. Interestingly, the study demonstrated that cancer cell line derived from bone metastatic lesions secreted significantly greater amounts of PTHrP than did the cell line derived from the metastasis in the brain or in the lymph node. These findings suggest that PTHrP production may confer some advantage on the ability of prostate cancer cells to grow in bone.10
Ando and colleagues reported that neuroendocrine dedifferentiated prostate cancer can develop as a result of long-term ADT even after several years of therapy and has the potential to worsen and develop severe hypercalcemia.8 Neuron-specific enolase was used as the specific marker for the neuroendocrine cell, which suggested that the prostate cancer cell derived from the neuroendocrine cell might synthesize PTHrP and be responsible for the observed hypercalcemia.8
Other mechanisms cited for hypercalcemia of malignancy include other humoral factors associated with increased remodeling and comprise interleukin 1, 3, 6 (IL-1, IL-3, IL-6); tumor necrosis factor α; transforming growth factor A and B observed in metastatic bone lesions in breast cancer; lymphotoxin; E series prostaglandins; and macrophage inflammatory protein 1α seen in MM.
Local osteolytic hypercalcemia accounts for about 20% of cases and is usually associated with extensive bone metastases. It is most commonly seen in MM and metastatic breast cancer and less commonly in leukemia. The proposed mechanism is thought to be because of the release of local cytokines from the tumor, resulting in excess osteoclast activation and enhanced bone resorption often through RANK/RANKL interaction.
Extrarenal production of 1,25(OH)2D by the tumor accounts for about 1% of cases of hypercalcemia in malignancy. 1,25(OH)2D causes increased intestinal absorption of calcium and enhances osteolytic bone resorption, resulting in increased serum calcium. This mechanism is most commonly seen with Hodgkin and non-Hodgkin lymphoma and had been reported in ovarian dysgerminoma.7
In our patient, bone imaging showed osteoblastic lesions, a finding that likely contrasts the local osteolytic bone destruction theory. PTHrP was not significantly elevated in the serum, and PTH levels ruled out any form of primary hyperparathyroidism. In addition, histopathology showed no evidence of mosaicism or neuroendocrine dedifferentiation.
Findings in aggregate tell us that an exact pathophysiologic mechanism leading to hypercalcemia in prostate cancer is still unclear and may involve an interplay between growth factors and possible osteolytic materials, yet it must be studied thoroughly.
Conclusions
Hypercalcemia in pure metastatic adenocarcinoma of prostate is a rare finding and is of uncertain significance. Some studies suggested a search for unusual histopathologies, including neuroendocrine cancer and neuroendocrine dedifferentiation.8,11 However, in adenocarcinoma alone, it has an uncertain pathophysiology that needs to be further studied. Studies needed to investigate the role of PTHrP as a growth factor for both prostate cancer cells and development of hypercalcemia and possibly target-directed monoclonal antibody therapies may need to be extensively researched.
Hypercalcemia is found when the corrected serum calcium level is > 10.5 mg/dL.1 Its symptoms are not specific and may include polyuria, dehydration, polydipsia, anorexia, nausea and/or vomiting, constipation, and other central nervous system manifestations, including confusion, delirium, cognitive impairment, muscle weakness, psychotic symptoms, and even coma.1,2
Hypercalcemia has varied etiologies; however, malignancy-induced hypercalcemia is one of the most common causes. In the US, the most common causes of malignancy-induced hypercalcemia are primary tumors of the lung or breast, multiple myeloma (MM), squamous cell carcinoma of the head or neck, renal cancer, and ovarian cancer.1
Men with prostate cancer and bone metastasis have relatively worse prognosis than do patient with no metastasis.3 In a recent meta-analysis of patients with bone-involved castration-resistant prostate cancer, the median survival was 21 months.3
Hypercalcemia is a rare manifestation of prostate cancer. In a retrospective study conducted between 2009 and 2013 using the Oncology Services Comprehensive Electronic Records (OSCER) warehouse of electronic health records (EHR), the rates of malignancy-induced hypercalcemia were the lowest among patients with prostate cancer, ranging from 1.4 to 2.1%.1
We present this case to discuss different pathophysiologic mechanisms leading to hypercalcemia in a patient with prostate cancer with bone metastasis and to study the role of humoral and growth factors in the pathogenesis of the disease.
Case Presentation
An African American man aged 69 years presented to the emergency department (ED) with generalized weakness, fatigue, and lower extremities muscle weakness. He reported a 40-lb weight loss over the past 3 months, intermittent lower back pain, and a 50 pack-year smoking history. A physical examination suggested clinical signs of dehydration.
Laboratory test results indicated hypercalcemia, macrocytic anemia, and thrombocytopenia: calcium 15.8 mg/dL, serum albumin 4.1 mg/dL, alkaline phosphatase 139 μ/L, blood urea nitrogen 55 mg/dL, creatinine 3.4 mg/dL (baseline 1.4-1.5 mg/dL), hemoglobin 8 g/dL, mean corpuscular volume 99.6 fL, and platelets 100,000/μL. The patient was admitted for hypercalcemia. His intact parathyroid hormone (iPTH) was suppressed at 16 pg/mL, phosphorous was 3.8 mg/dL, parathyroid hormone-related peptide (PTHrP) was < 0.74 pmol/L, vitamin D (25 hydroxy cholecalciferol) was mildly decreased at 17.2 ng/mL, and 1,25 dihydroxy cholecalciferol (calcitriol) was < 5.0 (normal range 20-79.3 pg/mL).
A computed tomography (CT) scan of the chest and abdomen was taken due to the patient’s heavy smoking history, an incidentally detected right lung base nodule on chest X-ray, and hypercalcemia. The CT scan showed multiple right middle lobe lung nodules with and without calcifications and calcified right hilar lymph nodes (Figure 1).
To evaluate the pancytopenia, a bone marrow biopsy was done, which showed that 80 to 90% of the marrow space was replaced by fibrosis and metastatic malignancy. Trilinear hematopoiesis was not seen (Figure 2). The tumor cells were positive for prostate- specific membrane antigen (PSMA) and negative for cytokeratin 7 and 20 (CK7 and CK20).4 The former is a membrane protein expressed on prostate tissues, including cancer; the latter is a form of protein used to identify adenocarcinoma of unknown primary origin (CK7 usually found in primary/ metastatic lung adenocarcinoma and CK20 usually in primary and some metastatic diseases of colon adenocarcinoma).5 A prostatic specific antigen (PSA) test was markedly elevated: 335.94 ng/mL (1.46 ng/mL on a previous 2011 test).
Metastatic adenocarcinoma of the prostate was diagnosed without a prostate biopsy. To determine the extent of bone metastases, a technetium-99m-methylene diphosphonate (MDP) bone scintigraphy demonstrated a superscan with intense foci of increased radiotracer uptake involving the bilateral shoulders, sternoclavicular joints, and sternum with heterogeneous uptake involving bilateral anterior and posterior ribs; cervical, thoracic, and lumbar spines; sacrum, pelvis, and bilateral hips, including the femoral head/neck and intertrochanteric regions. Also noted were several foci of radiotracer uptake involving the mandible and bilateral skull in the region of the temporomandibular joints (Figure 3).
The patient was initially treated with IV isotonic saline, followed by calcitonin and then pamidronate after kidney function improved. His calcium level responded to the therapy, and a plan was made by medical oncology to start androgen deprivation therapy (ADT) prior to discharge.
He was initially treated with bicalutamide, while a luteinizing hormone-releasing hormone agonist (leuprolide) was added 1 week later. Bicalutamide was then discontinued and a combined androgen blockade consisting of leuprolide, ketoconazole, and hydrocortisone was started. This therapy resulted in remission, and PSA declined to 1.73 ng/ mL 3 months later. At that time the patient enrolled in a clinical trial with leuprolide and bicalutamide combined therapy. About 6 months after his diagnosis, patient’s cancer progressed and became hormone refractory disease. At that time, bicalutamide was discontinued, and his therapy was switched to combined leuprolide and enzalutamide. After 6 months of therapy with enzalutamide, the patient’s cancer progressed again. He was later treated with docetaxel chemotherapy but died 16 months after diagnosis.
showed improvement of hypercalcemia at the time of discharge, but 9 months later and toward the time of expiration, our patient developed secondary hyperparathyroidism, with calcium maintained in the normal range, while iPTH was significantly elevated, a finding likely explained by a decline in kidney function and a fall in glomerular filtration rate (Table).
Discussion
Hypercalcemia in the setting of prostate cancer is a rare complication with an uncertain pathophysiology.6 Several mechanisms have been proposed for hypercalcemia of malignancy, these comprise humoral hypercalcemia of malignancy mediated by increased PTHrP; local osteolytic hypercalcemia with secretion of other humoral factors; excess extrarenal activation of vitamin D (1,25[OH]2D); PTH secretion, ectopic or primary; and multiple concurrent etiologies.7
PTHrP is the predominant mediator for hypercalcemia of malignancy and is estimated to account for 80% of hypercalcemia in patients with cancer. This protein shares a substantial sequence homology with PTH; in fact, 8 of the first 13 amino acids at the N-terminal portion of PTH were identical.8 PTHrP has multiple isoforms (PTHrP 141, PTHrP 139, and PTHrP 173). Like PTH, it enhances renal tubular reabsorption of calcium while increasing urinary phosphorus excretion.7 The result is both hypercalcemia and hypophosphatemia. However, unlike PTH, PTHrP does not increase 1,25(OH)2D and thus does not increase intestinal absorption of calcium and phosphorus. PTHrP acts on osteoblasts, leading to enhanced synthesis of receptor activator of nuclear factor-κB ligand (RANKL).7
In one study, PTHrP was detected immunohistochemically in prostate cancer cells. Iwamura and colleagues used 33 radical prostatectomy specimens from patients with clinically localized carcinoma of the prostate.9 None of these patients demonstrated hypercalcemia prior to the surgery. Using a mouse monoclonal antibody to an amino acid fragment, all cases demonstrated some degree of immunoreactivity throughout the cytoplasm of the tumor cells, but immunostaining was absent from inflammatory and stromal cells.9Furthermore, the intensity of the staining appeared to directly correlate with increasing tumor grade.9
Another study by Iwamura and colleagues suggested that PTHrP may play a significant role in the growth of prostate cancer by acting locally in an autocrine fashion.10 In this study, all prostate cancer cell lines from different sources expressed PTHrP immunoreactivity as well as evidence of DNA synthesis, the latter being measured by thymidine incorporation assay. Moreover, when these cells were incubated with various concentrations of mouse monoclonal antibody directed to PTHrP fragment, PTHrP-induced DNA synthesis was inhibited in a dose-dependent manner and almost completely neutralized at a specific concentration. Interestingly, the study demonstrated that cancer cell line derived from bone metastatic lesions secreted significantly greater amounts of PTHrP than did the cell line derived from the metastasis in the brain or in the lymph node. These findings suggest that PTHrP production may confer some advantage on the ability of prostate cancer cells to grow in bone.10
Ando and colleagues reported that neuroendocrine dedifferentiated prostate cancer can develop as a result of long-term ADT even after several years of therapy and has the potential to worsen and develop severe hypercalcemia.8 Neuron-specific enolase was used as the specific marker for the neuroendocrine cell, which suggested that the prostate cancer cell derived from the neuroendocrine cell might synthesize PTHrP and be responsible for the observed hypercalcemia.8
Other mechanisms cited for hypercalcemia of malignancy include other humoral factors associated with increased remodeling and comprise interleukin 1, 3, 6 (IL-1, IL-3, IL-6); tumor necrosis factor α; transforming growth factor A and B observed in metastatic bone lesions in breast cancer; lymphotoxin; E series prostaglandins; and macrophage inflammatory protein 1α seen in MM.
Local osteolytic hypercalcemia accounts for about 20% of cases and is usually associated with extensive bone metastases. It is most commonly seen in MM and metastatic breast cancer and less commonly in leukemia. The proposed mechanism is thought to be because of the release of local cytokines from the tumor, resulting in excess osteoclast activation and enhanced bone resorption often through RANK/RANKL interaction.
Extrarenal production of 1,25(OH)2D by the tumor accounts for about 1% of cases of hypercalcemia in malignancy. 1,25(OH)2D causes increased intestinal absorption of calcium and enhances osteolytic bone resorption, resulting in increased serum calcium. This mechanism is most commonly seen with Hodgkin and non-Hodgkin lymphoma and had been reported in ovarian dysgerminoma.7
In our patient, bone imaging showed osteoblastic lesions, a finding that likely contrasts the local osteolytic bone destruction theory. PTHrP was not significantly elevated in the serum, and PTH levels ruled out any form of primary hyperparathyroidism. In addition, histopathology showed no evidence of mosaicism or neuroendocrine dedifferentiation.
Findings in aggregate tell us that an exact pathophysiologic mechanism leading to hypercalcemia in prostate cancer is still unclear and may involve an interplay between growth factors and possible osteolytic materials, yet it must be studied thoroughly.
Conclusions
Hypercalcemia in pure metastatic adenocarcinoma of prostate is a rare finding and is of uncertain significance. Some studies suggested a search for unusual histopathologies, including neuroendocrine cancer and neuroendocrine dedifferentiation.8,11 However, in adenocarcinoma alone, it has an uncertain pathophysiology that needs to be further studied. Studies needed to investigate the role of PTHrP as a growth factor for both prostate cancer cells and development of hypercalcemia and possibly target-directed monoclonal antibody therapies may need to be extensively researched.
1. Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5(8):2091‐2100. doi:10.1002/cam4.749
2. Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000;1(4):253‐263. doi:10.1023/a:1026597816193
3. Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652‐1659. doi:10.1200/JCO.2015.65.7270
4. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(suppl 10):S13‐S18.
5. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86(12):1884‐1887. doi:10.1038/sj.bjc.6600326
6. Avashia JH, Walsh TD, Thomas AJ Jr, Kaye M, Licata A. Metastatic carcinoma of the prostate with hypercalcemia [published correction appears in Cleve Clin J Med. 1991;58(3):284]. Cleve Clin J Med. 1990;57(7):636‐638. doi:10.3949/ccjm.57.7.636.
7. Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12(5):426‐432. doi:10.1200/JOP.2016.011155.
8. Ando T, Watanabe K, Mizusawa T, Katagiri A. Hypercalcemia due to parathyroid hormone-related peptide secreted by neuroendocrine dedifferentiated prostate cancer. Urol Case Rep. 2018;22:67‐69. doi:10.1016/j.eucr.2018.11.001
9. Iwamura M, di Sant’Agnese PA, Wu G, et al. Immunohistochemical localization of parathyroid hormonerelated protein in human prostate cancer. Cancer Res. 1993;53(8):1724‐1726.
10. Iwamura M, Abrahamsson PA, Foss KA, Wu G, Cockett AT, Deftos LJ. Parathyroid hormone-related protein: a potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43(5):675‐679. doi:10.1016/0090-4295(94)90183-x
11. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol. 1992;10(3):499‐505. doi:10.1200/JCO.1992.10.3.499.
1. Gastanaga VM, Schwartzberg LS, Jain RK, et al. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5(8):2091‐2100. doi:10.1002/cam4.749
2. Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000;1(4):253‐263. doi:10.1023/a:1026597816193
3. Halabi S, Kelly WK, Ma H, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34(14):1652‐1659. doi:10.1200/JCO.2015.65.7270
4. Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(suppl 10):S13‐S18.
5. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002;86(12):1884‐1887. doi:10.1038/sj.bjc.6600326
6. Avashia JH, Walsh TD, Thomas AJ Jr, Kaye M, Licata A. Metastatic carcinoma of the prostate with hypercalcemia [published correction appears in Cleve Clin J Med. 1991;58(3):284]. Cleve Clin J Med. 1990;57(7):636‐638. doi:10.3949/ccjm.57.7.636.
7. Goldner W. Cancer-related hypercalcemia. J Oncol Pract. 2016;12(5):426‐432. doi:10.1200/JOP.2016.011155.
8. Ando T, Watanabe K, Mizusawa T, Katagiri A. Hypercalcemia due to parathyroid hormone-related peptide secreted by neuroendocrine dedifferentiated prostate cancer. Urol Case Rep. 2018;22:67‐69. doi:10.1016/j.eucr.2018.11.001
9. Iwamura M, di Sant’Agnese PA, Wu G, et al. Immunohistochemical localization of parathyroid hormonerelated protein in human prostate cancer. Cancer Res. 1993;53(8):1724‐1726.
10. Iwamura M, Abrahamsson PA, Foss KA, Wu G, Cockett AT, Deftos LJ. Parathyroid hormone-related protein: a potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43(5):675‐679. doi:10.1016/0090-4295(94)90183-x
11. Smith DC, Tucker JA, Trump DL. Hypercalcemia and neuroendocrine carcinoma of the prostate: a report of three cases and a review of the literature. J Clin Oncol. 1992;10(3):499‐505. doi:10.1200/JCO.1992.10.3.499.
Transitioning regimen may prolong proteasome inhibitor–based therapy for MM
Transitioning from parenteral bortezomib-based induction to all-oral ixazomib-lenalidomide-dexamethasone therapy increased proteasome inhibitor (PI)–based treatment adherence and duration, according to early results from a clinical trial designed to include patients representing the real-world U.S. multiple myeloma population.
The US MM-6 study was designed to evaluate a novel in-class therapy (iCT) transitioning approach from intravenous to oral treatment in the community-based setting with the aims of increasing PI-based treatment duration and adherence, maintaining health-related quality of life (HRQoL), and improving outcomes in a representative, real-world, community population of multiple myeloma patients, according to Sudhir Manda, MD, of Arizona Oncology/U.S. Oncology Research, Tucson, and colleagues.
Dr. Manda and colleagues reported on the early results of the US MM-6 trial (NCT03173092), which is a community-based, real-world, open-label, single-arm, phase 4 study of adult multiple myeloma patients who do not meet transplant-eligibility criteria, or for whom transplant would be delayed for 2 years or more, and who are receiving first-line bortezomib-based induction. All patients in the study had no evidence of progressive disease after three treatment cycles.
By the data cutoff for the reported analysis, 84 patients had been treated. The patients had a median age of 73 years; 49% were men; 15% black/African American; 10% Hispanic/Latino. A total of 62% of the patients remain on therapy, with a mean duration of total PI therapy of 10.1 months and of ixazomib-lenalidomide-dexamethasone (ixazomib-Rd) of 7.3 months.
The overall response rate was 62% (complete response, 4%; very good partial response, 25%; partial response, 33%) after bortezomib-based induction and 70% (complete response, 26%; very good partial response, 29%; partial response, 15%) after induction to all-oral ixazomib-Rd.
“The use of this novel iCT approach from parenteral bortezomib-based to oral ixazomib-based therapy facilitates long-term PI-based treatment that is well tolerated in real-world, nontransplant [newly diagnosed multiple myeloma] patients,” according to Dr. Manda and colleagues. In addition, “preliminary findings indicate that the iCT approach results in promising efficacy and high medication adherence, with no adverse impact on patients’ HRQoL or treatment satisfaction.”
The study was sponsored by Millennium Pharmaceuticals. Four of the authors are employees of Millennium Pharmaceuticals and several authors disclosed relationships with various pharmaceutical companies, including Millennium Pharmaceuticals.
SOURCE: Manda S et al. Clin Lymphoma Myeloma Leuk. 2020 Jun 30. doi: 10.1016/j.clml.2020.06.024.
Transitioning from parenteral bortezomib-based induction to all-oral ixazomib-lenalidomide-dexamethasone therapy increased proteasome inhibitor (PI)–based treatment adherence and duration, according to early results from a clinical trial designed to include patients representing the real-world U.S. multiple myeloma population.
The US MM-6 study was designed to evaluate a novel in-class therapy (iCT) transitioning approach from intravenous to oral treatment in the community-based setting with the aims of increasing PI-based treatment duration and adherence, maintaining health-related quality of life (HRQoL), and improving outcomes in a representative, real-world, community population of multiple myeloma patients, according to Sudhir Manda, MD, of Arizona Oncology/U.S. Oncology Research, Tucson, and colleagues.
Dr. Manda and colleagues reported on the early results of the US MM-6 trial (NCT03173092), which is a community-based, real-world, open-label, single-arm, phase 4 study of adult multiple myeloma patients who do not meet transplant-eligibility criteria, or for whom transplant would be delayed for 2 years or more, and who are receiving first-line bortezomib-based induction. All patients in the study had no evidence of progressive disease after three treatment cycles.
By the data cutoff for the reported analysis, 84 patients had been treated. The patients had a median age of 73 years; 49% were men; 15% black/African American; 10% Hispanic/Latino. A total of 62% of the patients remain on therapy, with a mean duration of total PI therapy of 10.1 months and of ixazomib-lenalidomide-dexamethasone (ixazomib-Rd) of 7.3 months.
The overall response rate was 62% (complete response, 4%; very good partial response, 25%; partial response, 33%) after bortezomib-based induction and 70% (complete response, 26%; very good partial response, 29%; partial response, 15%) after induction to all-oral ixazomib-Rd.
“The use of this novel iCT approach from parenteral bortezomib-based to oral ixazomib-based therapy facilitates long-term PI-based treatment that is well tolerated in real-world, nontransplant [newly diagnosed multiple myeloma] patients,” according to Dr. Manda and colleagues. In addition, “preliminary findings indicate that the iCT approach results in promising efficacy and high medication adherence, with no adverse impact on patients’ HRQoL or treatment satisfaction.”
The study was sponsored by Millennium Pharmaceuticals. Four of the authors are employees of Millennium Pharmaceuticals and several authors disclosed relationships with various pharmaceutical companies, including Millennium Pharmaceuticals.
SOURCE: Manda S et al. Clin Lymphoma Myeloma Leuk. 2020 Jun 30. doi: 10.1016/j.clml.2020.06.024.
Transitioning from parenteral bortezomib-based induction to all-oral ixazomib-lenalidomide-dexamethasone therapy increased proteasome inhibitor (PI)–based treatment adherence and duration, according to early results from a clinical trial designed to include patients representing the real-world U.S. multiple myeloma population.
The US MM-6 study was designed to evaluate a novel in-class therapy (iCT) transitioning approach from intravenous to oral treatment in the community-based setting with the aims of increasing PI-based treatment duration and adherence, maintaining health-related quality of life (HRQoL), and improving outcomes in a representative, real-world, community population of multiple myeloma patients, according to Sudhir Manda, MD, of Arizona Oncology/U.S. Oncology Research, Tucson, and colleagues.
Dr. Manda and colleagues reported on the early results of the US MM-6 trial (NCT03173092), which is a community-based, real-world, open-label, single-arm, phase 4 study of adult multiple myeloma patients who do not meet transplant-eligibility criteria, or for whom transplant would be delayed for 2 years or more, and who are receiving first-line bortezomib-based induction. All patients in the study had no evidence of progressive disease after three treatment cycles.
By the data cutoff for the reported analysis, 84 patients had been treated. The patients had a median age of 73 years; 49% were men; 15% black/African American; 10% Hispanic/Latino. A total of 62% of the patients remain on therapy, with a mean duration of total PI therapy of 10.1 months and of ixazomib-lenalidomide-dexamethasone (ixazomib-Rd) of 7.3 months.
The overall response rate was 62% (complete response, 4%; very good partial response, 25%; partial response, 33%) after bortezomib-based induction and 70% (complete response, 26%; very good partial response, 29%; partial response, 15%) after induction to all-oral ixazomib-Rd.
“The use of this novel iCT approach from parenteral bortezomib-based to oral ixazomib-based therapy facilitates long-term PI-based treatment that is well tolerated in real-world, nontransplant [newly diagnosed multiple myeloma] patients,” according to Dr. Manda and colleagues. In addition, “preliminary findings indicate that the iCT approach results in promising efficacy and high medication adherence, with no adverse impact on patients’ HRQoL or treatment satisfaction.”
The study was sponsored by Millennium Pharmaceuticals. Four of the authors are employees of Millennium Pharmaceuticals and several authors disclosed relationships with various pharmaceutical companies, including Millennium Pharmaceuticals.
SOURCE: Manda S et al. Clin Lymphoma Myeloma Leuk. 2020 Jun 30. doi: 10.1016/j.clml.2020.06.024.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
Geographical hot spots for early-onset colon cancer
The incidence of colorectal cancer (CRC) in adults younger than 55 years has been increasing in recent years ― a “dramatic increase” was noted in the United States in 2017, and an increase in incidence has subsequently been seen in many other countries across Europe, as well as Australia, New Zealand, and Canada.
A new study has identified geographic hot spots across the United States, characterized by distinct patterns of early-onset CRC with worse survival among men. The hot spots primarily include counties in the lower Mississippi Delta, west-central Appalachia, and eastern Virginia/North Carolina.
The study was published online on May 15 in the American Journal of Cancer Research.
These data can help to identify some of the risk factors associated with early-onset CRC/mortality, commented lead author Charles Rogers, PhD, MPH, a researcher at the Huntsman Cancer Institute and assistant professor of public health at the University of Utah, Salt Lake City.
“We noted potential explanations for the hot spots,” he told Medscape Medical News. “These include an enduring history of unique challenges, such as inadequate access to care, poor health literacy, and low educational attainment.”
Within hot-spot counties there were also higher rates of poverty, a lack of health insurance, and fewer primary care physicians.
“The disproportionate burden of early-onset colorectal cancer among non-Hispanic black men may result from distinctive stressors coupled with cultural and social expectations that impact screening and care behaviors,” said Rogers. “And while it’s estimated that approximately 14% of all US adults are current smokers, we observed that 24% of the adult population residing in hot-spot counties reported currently smoking and having smoked at least 100 cigarettes in their lifetime.”
Lifestyle and screening
Elements relating to the increase in early-onset CRC include environmental and geographical factors, as well as lifestyle factors, such as diet, obesity, and sedentary behaviors, Rogers commented.
“I think lifestyle factors are huge,” he said. “Consumption of high-fructose corn syrup and charred meat, for example, are worth considering and deserve more attention.”
He emphasized the importance of screening. Most health organizations in the United States recommend that screening start at age 50 years, but the American Cancer Society lowered this to 45 years, and the issue has been hotly debated. Rogers said that adults younger than 50 should be having conversations with their clinicians about screening for CRC. He noted that this is particularly important if they have any symptoms of CRC, have a family history of the disease, or reside in one of the hot spots that were identified in their study.
An expert who was approached for outside comment agreed. Chyke Doubeni, MBBS, MPH, director of the Center for Health Equity and Community Engagement Research at the Mayo Clinic in Rochester, Minnesota, said that anyone with health concerns should discuss preventive measures with their primary care physician.
“Screening for people younger than the age of 50 is currently controversial, as it is not recommended by some guidelines,” he said. “Recommendations for screening are different for people with a family history or certain genetic conditions.”
Such people include those younger than 50 years who have a family history of CRC or advanced adenomas. These patients should share that history with their primary care physician in order to determine when to begin screening and how often to be screened.
“People under the age of 50 who have symptoms such as unexplained rectal bleeding or iron deficiency anemia that may suggest the presence of colorectal cancer should be promptly evaluated for that possibility,” Doubeni added.
Hot spots versus other counties
The goal of the study was to identify mortality hot spots specific to men with early-onset CRC and to evaluate disparities while controlling for sex-specific differences. Rogers and colleagues identified counties with high early-onset CRC mortality rates using data from the Centers for Disease Control and Prevention (1999–2017) and linked them to data from the Surveillance, Epidemiology, and End Results (SEER) for men aged 15 to 49 years.
The team identified 232 US counties (7% of the total) as hot spots. The majority (214 of 232, 92%) were located in the South, and the remainder (18 of 232, 8%) were in the Midwest P < .01).
As compared to men living in other counties, those residing in hot-spot counties were more likely to be non-Hispanic blacks (30.82% vs 13.06%), less likely to be Hispanic (1.68% vs 16.65%; P < .01), and more likely to be diagnosed with metastatic disease (stage IV CRC) (2.58% vs 1.94%; P < .01).
Among men who lived in hot spots, CRC survival was poorer than was seen elsewhere (113.76 vs 129.04 months, respectively; P < .001). Among those with early-onset CRC, the risk for CRC-specific death was 24% higher (hazard ratio [HR], 1.24) than for men living outside of the hot-spot counties. However, that figure dropped to 12% after adjustment for county-level smoking (HR, 1.12).
With respect to racial/ethnic differences, non-Hispanic black (HR, 1.31) and Hispanic (HR, 1.12) patients had a 31% and 12% increased risk for CRC-specific death as compared to non-Hispanic white men (HR, 1.01) after adjusting for smoking status.
The authors note that among all determinants, “clinical stage explained the largest proportion of the variance” in early-onset CRC survival for men living in hot spots and other locations combined.
In the hot-spot counties, severe tumor grade was associated with greater CRC-specific mortality risk. Among patients with poorly differentiated tumors (HR, 1.87) and undifferentiated tumors (HR, 2.60), the mortality risk was nearly 2 times and 2.6 times greater, respectively, than those with well-differentiated tumors.
Compared to other counties, hot-spot counties were characterized by demographics that have been linked to poorer health outcomes, such as higher poverty rates (26.57% vs 16.77%), greater prevalence of adult obesity (34.94% vs 25.89%), higher adult smoking rates (23.97% vs 15.44%), higher uninsured rates (20.06% vs 17.91%), and fewer primary care physicians (58.28 vs 75.45 per 100,000 population).
Geographic distribution of CRC
Commenting to Medscape Medical News, Doubeni pointed out that the identified hot spots are similar to previously reported overall CRC hot spots.
“It shows the same patterns of geographic distribution of colorectal cancer in the United States,” he said. “These patterns tend to be associated with areas with high levels of poverty, as is the case with other chronic diseases, and may be related to clustering of risk factors and limited access to care in those areas.”
The research was supported by the National Cancer Institute of the National Institutes of Health, the Huntsman Cancer Foundation, and the Health Studies Fund of the Department of Family and Preventative Medicine at the University of Utah. The authors and Doubeni have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The incidence of colorectal cancer (CRC) in adults younger than 55 years has been increasing in recent years ― a “dramatic increase” was noted in the United States in 2017, and an increase in incidence has subsequently been seen in many other countries across Europe, as well as Australia, New Zealand, and Canada.
A new study has identified geographic hot spots across the United States, characterized by distinct patterns of early-onset CRC with worse survival among men. The hot spots primarily include counties in the lower Mississippi Delta, west-central Appalachia, and eastern Virginia/North Carolina.
The study was published online on May 15 in the American Journal of Cancer Research.
These data can help to identify some of the risk factors associated with early-onset CRC/mortality, commented lead author Charles Rogers, PhD, MPH, a researcher at the Huntsman Cancer Institute and assistant professor of public health at the University of Utah, Salt Lake City.
“We noted potential explanations for the hot spots,” he told Medscape Medical News. “These include an enduring history of unique challenges, such as inadequate access to care, poor health literacy, and low educational attainment.”
Within hot-spot counties there were also higher rates of poverty, a lack of health insurance, and fewer primary care physicians.
“The disproportionate burden of early-onset colorectal cancer among non-Hispanic black men may result from distinctive stressors coupled with cultural and social expectations that impact screening and care behaviors,” said Rogers. “And while it’s estimated that approximately 14% of all US adults are current smokers, we observed that 24% of the adult population residing in hot-spot counties reported currently smoking and having smoked at least 100 cigarettes in their lifetime.”
Lifestyle and screening
Elements relating to the increase in early-onset CRC include environmental and geographical factors, as well as lifestyle factors, such as diet, obesity, and sedentary behaviors, Rogers commented.
“I think lifestyle factors are huge,” he said. “Consumption of high-fructose corn syrup and charred meat, for example, are worth considering and deserve more attention.”
He emphasized the importance of screening. Most health organizations in the United States recommend that screening start at age 50 years, but the American Cancer Society lowered this to 45 years, and the issue has been hotly debated. Rogers said that adults younger than 50 should be having conversations with their clinicians about screening for CRC. He noted that this is particularly important if they have any symptoms of CRC, have a family history of the disease, or reside in one of the hot spots that were identified in their study.
An expert who was approached for outside comment agreed. Chyke Doubeni, MBBS, MPH, director of the Center for Health Equity and Community Engagement Research at the Mayo Clinic in Rochester, Minnesota, said that anyone with health concerns should discuss preventive measures with their primary care physician.
“Screening for people younger than the age of 50 is currently controversial, as it is not recommended by some guidelines,” he said. “Recommendations for screening are different for people with a family history or certain genetic conditions.”
Such people include those younger than 50 years who have a family history of CRC or advanced adenomas. These patients should share that history with their primary care physician in order to determine when to begin screening and how often to be screened.
“People under the age of 50 who have symptoms such as unexplained rectal bleeding or iron deficiency anemia that may suggest the presence of colorectal cancer should be promptly evaluated for that possibility,” Doubeni added.
Hot spots versus other counties
The goal of the study was to identify mortality hot spots specific to men with early-onset CRC and to evaluate disparities while controlling for sex-specific differences. Rogers and colleagues identified counties with high early-onset CRC mortality rates using data from the Centers for Disease Control and Prevention (1999–2017) and linked them to data from the Surveillance, Epidemiology, and End Results (SEER) for men aged 15 to 49 years.
The team identified 232 US counties (7% of the total) as hot spots. The majority (214 of 232, 92%) were located in the South, and the remainder (18 of 232, 8%) were in the Midwest P < .01).
As compared to men living in other counties, those residing in hot-spot counties were more likely to be non-Hispanic blacks (30.82% vs 13.06%), less likely to be Hispanic (1.68% vs 16.65%; P < .01), and more likely to be diagnosed with metastatic disease (stage IV CRC) (2.58% vs 1.94%; P < .01).
Among men who lived in hot spots, CRC survival was poorer than was seen elsewhere (113.76 vs 129.04 months, respectively; P < .001). Among those with early-onset CRC, the risk for CRC-specific death was 24% higher (hazard ratio [HR], 1.24) than for men living outside of the hot-spot counties. However, that figure dropped to 12% after adjustment for county-level smoking (HR, 1.12).
With respect to racial/ethnic differences, non-Hispanic black (HR, 1.31) and Hispanic (HR, 1.12) patients had a 31% and 12% increased risk for CRC-specific death as compared to non-Hispanic white men (HR, 1.01) after adjusting for smoking status.
The authors note that among all determinants, “clinical stage explained the largest proportion of the variance” in early-onset CRC survival for men living in hot spots and other locations combined.
In the hot-spot counties, severe tumor grade was associated with greater CRC-specific mortality risk. Among patients with poorly differentiated tumors (HR, 1.87) and undifferentiated tumors (HR, 2.60), the mortality risk was nearly 2 times and 2.6 times greater, respectively, than those with well-differentiated tumors.
Compared to other counties, hot-spot counties were characterized by demographics that have been linked to poorer health outcomes, such as higher poverty rates (26.57% vs 16.77%), greater prevalence of adult obesity (34.94% vs 25.89%), higher adult smoking rates (23.97% vs 15.44%), higher uninsured rates (20.06% vs 17.91%), and fewer primary care physicians (58.28 vs 75.45 per 100,000 population).
Geographic distribution of CRC
Commenting to Medscape Medical News, Doubeni pointed out that the identified hot spots are similar to previously reported overall CRC hot spots.
“It shows the same patterns of geographic distribution of colorectal cancer in the United States,” he said. “These patterns tend to be associated with areas with high levels of poverty, as is the case with other chronic diseases, and may be related to clustering of risk factors and limited access to care in those areas.”
The research was supported by the National Cancer Institute of the National Institutes of Health, the Huntsman Cancer Foundation, and the Health Studies Fund of the Department of Family and Preventative Medicine at the University of Utah. The authors and Doubeni have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The incidence of colorectal cancer (CRC) in adults younger than 55 years has been increasing in recent years ― a “dramatic increase” was noted in the United States in 2017, and an increase in incidence has subsequently been seen in many other countries across Europe, as well as Australia, New Zealand, and Canada.
A new study has identified geographic hot spots across the United States, characterized by distinct patterns of early-onset CRC with worse survival among men. The hot spots primarily include counties in the lower Mississippi Delta, west-central Appalachia, and eastern Virginia/North Carolina.
The study was published online on May 15 in the American Journal of Cancer Research.
These data can help to identify some of the risk factors associated with early-onset CRC/mortality, commented lead author Charles Rogers, PhD, MPH, a researcher at the Huntsman Cancer Institute and assistant professor of public health at the University of Utah, Salt Lake City.
“We noted potential explanations for the hot spots,” he told Medscape Medical News. “These include an enduring history of unique challenges, such as inadequate access to care, poor health literacy, and low educational attainment.”
Within hot-spot counties there were also higher rates of poverty, a lack of health insurance, and fewer primary care physicians.
“The disproportionate burden of early-onset colorectal cancer among non-Hispanic black men may result from distinctive stressors coupled with cultural and social expectations that impact screening and care behaviors,” said Rogers. “And while it’s estimated that approximately 14% of all US adults are current smokers, we observed that 24% of the adult population residing in hot-spot counties reported currently smoking and having smoked at least 100 cigarettes in their lifetime.”
Lifestyle and screening
Elements relating to the increase in early-onset CRC include environmental and geographical factors, as well as lifestyle factors, such as diet, obesity, and sedentary behaviors, Rogers commented.
“I think lifestyle factors are huge,” he said. “Consumption of high-fructose corn syrup and charred meat, for example, are worth considering and deserve more attention.”
He emphasized the importance of screening. Most health organizations in the United States recommend that screening start at age 50 years, but the American Cancer Society lowered this to 45 years, and the issue has been hotly debated. Rogers said that adults younger than 50 should be having conversations with their clinicians about screening for CRC. He noted that this is particularly important if they have any symptoms of CRC, have a family history of the disease, or reside in one of the hot spots that were identified in their study.
An expert who was approached for outside comment agreed. Chyke Doubeni, MBBS, MPH, director of the Center for Health Equity and Community Engagement Research at the Mayo Clinic in Rochester, Minnesota, said that anyone with health concerns should discuss preventive measures with their primary care physician.
“Screening for people younger than the age of 50 is currently controversial, as it is not recommended by some guidelines,” he said. “Recommendations for screening are different for people with a family history or certain genetic conditions.”
Such people include those younger than 50 years who have a family history of CRC or advanced adenomas. These patients should share that history with their primary care physician in order to determine when to begin screening and how often to be screened.
“People under the age of 50 who have symptoms such as unexplained rectal bleeding or iron deficiency anemia that may suggest the presence of colorectal cancer should be promptly evaluated for that possibility,” Doubeni added.
Hot spots versus other counties
The goal of the study was to identify mortality hot spots specific to men with early-onset CRC and to evaluate disparities while controlling for sex-specific differences. Rogers and colleagues identified counties with high early-onset CRC mortality rates using data from the Centers for Disease Control and Prevention (1999–2017) and linked them to data from the Surveillance, Epidemiology, and End Results (SEER) for men aged 15 to 49 years.
The team identified 232 US counties (7% of the total) as hot spots. The majority (214 of 232, 92%) were located in the South, and the remainder (18 of 232, 8%) were in the Midwest P < .01).
As compared to men living in other counties, those residing in hot-spot counties were more likely to be non-Hispanic blacks (30.82% vs 13.06%), less likely to be Hispanic (1.68% vs 16.65%; P < .01), and more likely to be diagnosed with metastatic disease (stage IV CRC) (2.58% vs 1.94%; P < .01).
Among men who lived in hot spots, CRC survival was poorer than was seen elsewhere (113.76 vs 129.04 months, respectively; P < .001). Among those with early-onset CRC, the risk for CRC-specific death was 24% higher (hazard ratio [HR], 1.24) than for men living outside of the hot-spot counties. However, that figure dropped to 12% after adjustment for county-level smoking (HR, 1.12).
With respect to racial/ethnic differences, non-Hispanic black (HR, 1.31) and Hispanic (HR, 1.12) patients had a 31% and 12% increased risk for CRC-specific death as compared to non-Hispanic white men (HR, 1.01) after adjusting for smoking status.
The authors note that among all determinants, “clinical stage explained the largest proportion of the variance” in early-onset CRC survival for men living in hot spots and other locations combined.
In the hot-spot counties, severe tumor grade was associated with greater CRC-specific mortality risk. Among patients with poorly differentiated tumors (HR, 1.87) and undifferentiated tumors (HR, 2.60), the mortality risk was nearly 2 times and 2.6 times greater, respectively, than those with well-differentiated tumors.
Compared to other counties, hot-spot counties were characterized by demographics that have been linked to poorer health outcomes, such as higher poverty rates (26.57% vs 16.77%), greater prevalence of adult obesity (34.94% vs 25.89%), higher adult smoking rates (23.97% vs 15.44%), higher uninsured rates (20.06% vs 17.91%), and fewer primary care physicians (58.28 vs 75.45 per 100,000 population).
Geographic distribution of CRC
Commenting to Medscape Medical News, Doubeni pointed out that the identified hot spots are similar to previously reported overall CRC hot spots.
“It shows the same patterns of geographic distribution of colorectal cancer in the United States,” he said. “These patterns tend to be associated with areas with high levels of poverty, as is the case with other chronic diseases, and may be related to clustering of risk factors and limited access to care in those areas.”
The research was supported by the National Cancer Institute of the National Institutes of Health, the Huntsman Cancer Foundation, and the Health Studies Fund of the Department of Family and Preventative Medicine at the University of Utah. The authors and Doubeni have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Use of nonopioid pain meds is on the rise
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Opioid and nonopioid prescription pain medications have taken different journeys since 2009, but they ended up in the same place in 2018, according to a recent report from the National Center for Health Statistics.

At least by one measure, anyway. Survey data from 2009 to 2010 show that 6.2% of adults aged 20 years and older had taken at least one prescription opioid in the last 30 days and 4.3% had used a prescription nonopioid without an opioid. By 2017-2018, past 30-day use of both drug groups was 5.7%, Craig M. Hales, MD, and associates said in an NCHS data brief.
“Opioids may be prescribed together with nonopioid pain medications, [but] nonpharmacologic and nonopioid-containing pharmacologic therapies are preferred for management of chronic pain,” the NCHS researchers noted.
as did the short-term increase in nonopioids from 2015-2016 to 2017-2018, but the 10-year trend for opioids was not significant, based on data from the National Health and Nutrition Examination Survey.
Much of the analysis focused on 2015-2018, when 30-day use of any prescription pain medication was reported by 10.7% of adults aged 20 years and older, with use of opioids at 5.7% and nonopioids at 5.0%. For women, use of any pain drug was 12.6% (6.4% opioid, 6.2% nonopioid) from 2015 to 2018, compared with 8.7% for men (4.9%, 3.8%), Dr. Hales and associates reported.
Past 30-day use of both opioids and nonopioids over those 4 years was highest for non-Hispanic whites and lowest, by a significant margin for both drug groups, among non-Hispanic Asian adults, a pattern that held for both men and women, they said.
Antihypertensives linked to reduced risk of colorectal cancer
Treating hypertension with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) was associated with a reduced risk for colorectal cancer, according to findings from a large retrospective study.
However, another study reported just over a year ago suggested that ACE inhibitors, but not ARBs, are associated with an increased risk for lung cancer. An expert approached for comment emphasized that both studies are observational, and, as such, they only show an association, not causation.
In this latest study, published online July 6 in the journal Hypertension, the use of ACE inhibitors/ARBs was associated with a 22% lower risk for colorectal cancer developing within 3 years after a negative baseline colonoscopy.
This is the largest study to date, with a cohort of more than 185,000 patients, to suggest a significant protective effect for these two common antihypertensive medications, the authors note. The risk of developing colorectal cancer decreased with longer duration of ACE inhibitor/ARB use, with a 5% reduction in adjusted hazard ratio risk for each year of use. However, this effect was limited to patients who had negative colonoscopies within a 3-year period and did not extend beyond that point.
Lead author Wai K. Leung, MD, clinical professor of medicine at the University of Hong Kong, explained that they are not advising patients to take ACE inhibitors simply to prevent cancer. “Unlike aspirin and statins, the potential chemopreventive role of ACE inhibitors on cancer has never been established,” he said in an interview. “The study findings may favor the use of ACE inhibitors in the treatment of hypertension, over many other antihypertensives, in some patients for preventing colorectal cancer.”
Increased or reduced risk?
There has been considerable debate about the potential carcinogenic effects of ACE inhibitors and ARBs, and the relationship with “various solid organ cancer risks have been unsettled,” the authors note. Studies have produced conflicting results – showing no overall cancer risk and a modestly increased overall cancer risk – associated with these agents.
A recent study reported that ACE inhibitors, as compared with ARBs, increased risk for lung cancer by 14%. The risk for lung cancer increased by 22% among those using ACE inhibitors for 5 years, and the risk peaked at 31% for patients who took ACE inhibitors for 10 years or longer.
The lead author of that lung cancer study, Laurent Azoulay, PhD, of McGill University in Montreal, offered some thoughts on the seemingly conflicting data now being reported showing a reduction in the risk of colorectal cancer.
“In a nutshell, this study has important methodologic issues that can explain the observed findings,” he said in an interview.
Dr. Azoulay pointed out that, in the univariate model, the use of ACE inhibitors/ARBs was associated with a 26% increased risk of colorectal cancer. “It is only after propensity score adjustment that the effect estimate reversed in the protective direction,” he pointed out. “However, the variables included in the propensity score model were measured in the same time window as the exposure, which can lead to an overadjustment bias and generate spurious findings.”
Another issue is that the study period did not begin at the time of the exposure, but rather at a distant point after treatment initiation – in this case, colorectal cancer screening. “As such, the authors excluded patients who were previously diagnosed with colorectal cancer prior to that point, which likely included patients exposed to ACE inhibitors/ARBs,” he said. “This approach can lead to the inclusion of the ‘survivors’ for whom the risk of developing colorectal cancer is lower.
“But certainly,” Dr. Azoulay added, “this possible association should be investigated using methodologically sound approaches.”
Take-home message for physicians
Another expert emphasized the observational nature of both studies. Raymond Townsend, MD, director of the Hypertension Program and a professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia, said: “First and foremost, these are observational studies and cannot make inference about causality; they can only show associations.”
He pointed out that, sometimes, associations are truly present, whereas at other times, there is bias or confounding that cannot be controlled for statistically because it is “unknown.” That said, the size of this latest study is a plus, and there is a reasonable follow-up period.
“The take-home [message] for practitioners is that there may be a benefit in keeping older people on ACE inhibitors on the likelihood of developing colorectal cancer if your last colonoscopy was negative,” Dr. Townsend, who was not involved in the study, said in an interview.
But there are some questions that remain unanswered regarding characteristics of the cohort, Dr. Townsend noted. “Who were the people having the colonoscopy in the first place? Were they a group at higher risk? Why were some on an ACE inhibitors/ARBs and many others not?”
There are other conclusions that clinicians can glean from this. “Make a choice of treatment for a patient based on your best estimate of what will lower their blood pressure and prevent hypertension-mediated organ damage,” said Dr. Townsend, who is also an American Heart Association volunteer expert. “Keep in mind that patients hear about these studies and read unreviewed blogs on the web and so have questions.”
He emphasized that it always comes back to two things. “One is that every treatment decision is inherently a risk-benefit scenario,” he said. “And second is that most of our patients are adults, and if they choose to not be treated for their hypertension despite our best advice and reasoning with them, relinquish control and let them proceed as they wish, offering to renegotiate in the future when and if they reconsider.”
Study details
In the latest study, Dr. Leung and colleagues conducted a retrospective cohort study and used data from an electronic health care database of the Hong Kong Hospital Authority. A total of 187,897 individuals aged 40 years and older had undergone colonoscopy between 2005 and 2013 with a negative result and were included in the analysis.
The study’s primary outcome was colorectal cancer that was diagnosed between 6 and 36 months after undergoing colonoscopy, and the median age at colonoscopy was 60.6 years. Within this population, 30,856 patients (16.4%) used ACE inhibitors/ARBs.
Between 6 months and 3 years after undergoing colonoscopy, 854 cases of colorectal cancer were diagnosed, with an incidence rate of 15.2 per 10,000 person-years. The median time between colonoscopy and diagnosis was 1.2 years.
ACE inhibitor/ARB users had a median duration of 3.3 years of use within the 5-year period before their colonoscopy. Within this group, there were 169 (0.55%) cases of colorectal cancer. On univariate analysis, the crude hazard ratio (HR) of colorectal cancer and ACE inhibitor/ARB use was 1.26 (P = .008), but on propensity score regression adjustment, the adjusted HR became 0.78.
The propensity score absolute reduction in risk for users was 3.2 per 10,000 person-years versus nonusers, and stratification by subsite showed an HR of 0.77 for distal cancers and 0.83 for proximal cancers.
In a subgroup analysis, the benefits of ACE inhibitors and ARBs were seen in patients aged 55 years or older (adjusted HR, 0.79) and in those with a history of colonic polyps (adjusted HR, 0.71).
The authors also assessed if there was an association between these medications and other types of cancer. On univariate analysis, usage was associated with an increased risk of lung and prostate cancer but lower risk of breast cancer. But after propensity score regression adjustment, the associations were no longer there.
The study was funded by the Health and Medical Research Fund of the Hong Kong SAR Government. Dr. Leung has received honorarium for attending advisory board meetings of AbbVie, Takeda, and Abbott Laboratories; coauthor Esther W. Chan has received funding support from Pfizer, Bristol-Myers Squibb, Bayer, Takeda, Janssen (a division of Johnson & Johnson); Research Grants Council of Hong Kong; Narcotics Division, Security Bureau; and the National Natural Science Foundation of China, all for work unrelated to the current study. None of the other authors have disclosed relevant financial relationships. Dr. Azoulay has disclosed no relevant financial relationships. Dr. Townsend is employed by Penn Medicine.
A version of this article originally appeared on Medscape.com.
Treating hypertension with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) was associated with a reduced risk for colorectal cancer, according to findings from a large retrospective study.
However, another study reported just over a year ago suggested that ACE inhibitors, but not ARBs, are associated with an increased risk for lung cancer. An expert approached for comment emphasized that both studies are observational, and, as such, they only show an association, not causation.
In this latest study, published online July 6 in the journal Hypertension, the use of ACE inhibitors/ARBs was associated with a 22% lower risk for colorectal cancer developing within 3 years after a negative baseline colonoscopy.
This is the largest study to date, with a cohort of more than 185,000 patients, to suggest a significant protective effect for these two common antihypertensive medications, the authors note. The risk of developing colorectal cancer decreased with longer duration of ACE inhibitor/ARB use, with a 5% reduction in adjusted hazard ratio risk for each year of use. However, this effect was limited to patients who had negative colonoscopies within a 3-year period and did not extend beyond that point.
Lead author Wai K. Leung, MD, clinical professor of medicine at the University of Hong Kong, explained that they are not advising patients to take ACE inhibitors simply to prevent cancer. “Unlike aspirin and statins, the potential chemopreventive role of ACE inhibitors on cancer has never been established,” he said in an interview. “The study findings may favor the use of ACE inhibitors in the treatment of hypertension, over many other antihypertensives, in some patients for preventing colorectal cancer.”
Increased or reduced risk?
There has been considerable debate about the potential carcinogenic effects of ACE inhibitors and ARBs, and the relationship with “various solid organ cancer risks have been unsettled,” the authors note. Studies have produced conflicting results – showing no overall cancer risk and a modestly increased overall cancer risk – associated with these agents.
A recent study reported that ACE inhibitors, as compared with ARBs, increased risk for lung cancer by 14%. The risk for lung cancer increased by 22% among those using ACE inhibitors for 5 years, and the risk peaked at 31% for patients who took ACE inhibitors for 10 years or longer.
The lead author of that lung cancer study, Laurent Azoulay, PhD, of McGill University in Montreal, offered some thoughts on the seemingly conflicting data now being reported showing a reduction in the risk of colorectal cancer.
“In a nutshell, this study has important methodologic issues that can explain the observed findings,” he said in an interview.
Dr. Azoulay pointed out that, in the univariate model, the use of ACE inhibitors/ARBs was associated with a 26% increased risk of colorectal cancer. “It is only after propensity score adjustment that the effect estimate reversed in the protective direction,” he pointed out. “However, the variables included in the propensity score model were measured in the same time window as the exposure, which can lead to an overadjustment bias and generate spurious findings.”
Another issue is that the study period did not begin at the time of the exposure, but rather at a distant point after treatment initiation – in this case, colorectal cancer screening. “As such, the authors excluded patients who were previously diagnosed with colorectal cancer prior to that point, which likely included patients exposed to ACE inhibitors/ARBs,” he said. “This approach can lead to the inclusion of the ‘survivors’ for whom the risk of developing colorectal cancer is lower.
“But certainly,” Dr. Azoulay added, “this possible association should be investigated using methodologically sound approaches.”
Take-home message for physicians
Another expert emphasized the observational nature of both studies. Raymond Townsend, MD, director of the Hypertension Program and a professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia, said: “First and foremost, these are observational studies and cannot make inference about causality; they can only show associations.”
He pointed out that, sometimes, associations are truly present, whereas at other times, there is bias or confounding that cannot be controlled for statistically because it is “unknown.” That said, the size of this latest study is a plus, and there is a reasonable follow-up period.
“The take-home [message] for practitioners is that there may be a benefit in keeping older people on ACE inhibitors on the likelihood of developing colorectal cancer if your last colonoscopy was negative,” Dr. Townsend, who was not involved in the study, said in an interview.
But there are some questions that remain unanswered regarding characteristics of the cohort, Dr. Townsend noted. “Who were the people having the colonoscopy in the first place? Were they a group at higher risk? Why were some on an ACE inhibitors/ARBs and many others not?”
There are other conclusions that clinicians can glean from this. “Make a choice of treatment for a patient based on your best estimate of what will lower their blood pressure and prevent hypertension-mediated organ damage,” said Dr. Townsend, who is also an American Heart Association volunteer expert. “Keep in mind that patients hear about these studies and read unreviewed blogs on the web and so have questions.”
He emphasized that it always comes back to two things. “One is that every treatment decision is inherently a risk-benefit scenario,” he said. “And second is that most of our patients are adults, and if they choose to not be treated for their hypertension despite our best advice and reasoning with them, relinquish control and let them proceed as they wish, offering to renegotiate in the future when and if they reconsider.”
Study details
In the latest study, Dr. Leung and colleagues conducted a retrospective cohort study and used data from an electronic health care database of the Hong Kong Hospital Authority. A total of 187,897 individuals aged 40 years and older had undergone colonoscopy between 2005 and 2013 with a negative result and were included in the analysis.
The study’s primary outcome was colorectal cancer that was diagnosed between 6 and 36 months after undergoing colonoscopy, and the median age at colonoscopy was 60.6 years. Within this population, 30,856 patients (16.4%) used ACE inhibitors/ARBs.
Between 6 months and 3 years after undergoing colonoscopy, 854 cases of colorectal cancer were diagnosed, with an incidence rate of 15.2 per 10,000 person-years. The median time between colonoscopy and diagnosis was 1.2 years.
ACE inhibitor/ARB users had a median duration of 3.3 years of use within the 5-year period before their colonoscopy. Within this group, there were 169 (0.55%) cases of colorectal cancer. On univariate analysis, the crude hazard ratio (HR) of colorectal cancer and ACE inhibitor/ARB use was 1.26 (P = .008), but on propensity score regression adjustment, the adjusted HR became 0.78.
The propensity score absolute reduction in risk for users was 3.2 per 10,000 person-years versus nonusers, and stratification by subsite showed an HR of 0.77 for distal cancers and 0.83 for proximal cancers.
In a subgroup analysis, the benefits of ACE inhibitors and ARBs were seen in patients aged 55 years or older (adjusted HR, 0.79) and in those with a history of colonic polyps (adjusted HR, 0.71).
The authors also assessed if there was an association between these medications and other types of cancer. On univariate analysis, usage was associated with an increased risk of lung and prostate cancer but lower risk of breast cancer. But after propensity score regression adjustment, the associations were no longer there.
The study was funded by the Health and Medical Research Fund of the Hong Kong SAR Government. Dr. Leung has received honorarium for attending advisory board meetings of AbbVie, Takeda, and Abbott Laboratories; coauthor Esther W. Chan has received funding support from Pfizer, Bristol-Myers Squibb, Bayer, Takeda, Janssen (a division of Johnson & Johnson); Research Grants Council of Hong Kong; Narcotics Division, Security Bureau; and the National Natural Science Foundation of China, all for work unrelated to the current study. None of the other authors have disclosed relevant financial relationships. Dr. Azoulay has disclosed no relevant financial relationships. Dr. Townsend is employed by Penn Medicine.
A version of this article originally appeared on Medscape.com.
Treating hypertension with angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) was associated with a reduced risk for colorectal cancer, according to findings from a large retrospective study.
However, another study reported just over a year ago suggested that ACE inhibitors, but not ARBs, are associated with an increased risk for lung cancer. An expert approached for comment emphasized that both studies are observational, and, as such, they only show an association, not causation.
In this latest study, published online July 6 in the journal Hypertension, the use of ACE inhibitors/ARBs was associated with a 22% lower risk for colorectal cancer developing within 3 years after a negative baseline colonoscopy.
This is the largest study to date, with a cohort of more than 185,000 patients, to suggest a significant protective effect for these two common antihypertensive medications, the authors note. The risk of developing colorectal cancer decreased with longer duration of ACE inhibitor/ARB use, with a 5% reduction in adjusted hazard ratio risk for each year of use. However, this effect was limited to patients who had negative colonoscopies within a 3-year period and did not extend beyond that point.
Lead author Wai K. Leung, MD, clinical professor of medicine at the University of Hong Kong, explained that they are not advising patients to take ACE inhibitors simply to prevent cancer. “Unlike aspirin and statins, the potential chemopreventive role of ACE inhibitors on cancer has never been established,” he said in an interview. “The study findings may favor the use of ACE inhibitors in the treatment of hypertension, over many other antihypertensives, in some patients for preventing colorectal cancer.”
Increased or reduced risk?
There has been considerable debate about the potential carcinogenic effects of ACE inhibitors and ARBs, and the relationship with “various solid organ cancer risks have been unsettled,” the authors note. Studies have produced conflicting results – showing no overall cancer risk and a modestly increased overall cancer risk – associated with these agents.
A recent study reported that ACE inhibitors, as compared with ARBs, increased risk for lung cancer by 14%. The risk for lung cancer increased by 22% among those using ACE inhibitors for 5 years, and the risk peaked at 31% for patients who took ACE inhibitors for 10 years or longer.
The lead author of that lung cancer study, Laurent Azoulay, PhD, of McGill University in Montreal, offered some thoughts on the seemingly conflicting data now being reported showing a reduction in the risk of colorectal cancer.
“In a nutshell, this study has important methodologic issues that can explain the observed findings,” he said in an interview.
Dr. Azoulay pointed out that, in the univariate model, the use of ACE inhibitors/ARBs was associated with a 26% increased risk of colorectal cancer. “It is only after propensity score adjustment that the effect estimate reversed in the protective direction,” he pointed out. “However, the variables included in the propensity score model were measured in the same time window as the exposure, which can lead to an overadjustment bias and generate spurious findings.”
Another issue is that the study period did not begin at the time of the exposure, but rather at a distant point after treatment initiation – in this case, colorectal cancer screening. “As such, the authors excluded patients who were previously diagnosed with colorectal cancer prior to that point, which likely included patients exposed to ACE inhibitors/ARBs,” he said. “This approach can lead to the inclusion of the ‘survivors’ for whom the risk of developing colorectal cancer is lower.
“But certainly,” Dr. Azoulay added, “this possible association should be investigated using methodologically sound approaches.”
Take-home message for physicians
Another expert emphasized the observational nature of both studies. Raymond Townsend, MD, director of the Hypertension Program and a professor of medicine at the Hospital of the University of Pennsylvania, Philadelphia, said: “First and foremost, these are observational studies and cannot make inference about causality; they can only show associations.”
He pointed out that, sometimes, associations are truly present, whereas at other times, there is bias or confounding that cannot be controlled for statistically because it is “unknown.” That said, the size of this latest study is a plus, and there is a reasonable follow-up period.
“The take-home [message] for practitioners is that there may be a benefit in keeping older people on ACE inhibitors on the likelihood of developing colorectal cancer if your last colonoscopy was negative,” Dr. Townsend, who was not involved in the study, said in an interview.
But there are some questions that remain unanswered regarding characteristics of the cohort, Dr. Townsend noted. “Who were the people having the colonoscopy in the first place? Were they a group at higher risk? Why were some on an ACE inhibitors/ARBs and many others not?”
There are other conclusions that clinicians can glean from this. “Make a choice of treatment for a patient based on your best estimate of what will lower their blood pressure and prevent hypertension-mediated organ damage,” said Dr. Townsend, who is also an American Heart Association volunteer expert. “Keep in mind that patients hear about these studies and read unreviewed blogs on the web and so have questions.”
He emphasized that it always comes back to two things. “One is that every treatment decision is inherently a risk-benefit scenario,” he said. “And second is that most of our patients are adults, and if they choose to not be treated for their hypertension despite our best advice and reasoning with them, relinquish control and let them proceed as they wish, offering to renegotiate in the future when and if they reconsider.”
Study details
In the latest study, Dr. Leung and colleagues conducted a retrospective cohort study and used data from an electronic health care database of the Hong Kong Hospital Authority. A total of 187,897 individuals aged 40 years and older had undergone colonoscopy between 2005 and 2013 with a negative result and were included in the analysis.
The study’s primary outcome was colorectal cancer that was diagnosed between 6 and 36 months after undergoing colonoscopy, and the median age at colonoscopy was 60.6 years. Within this population, 30,856 patients (16.4%) used ACE inhibitors/ARBs.
Between 6 months and 3 years after undergoing colonoscopy, 854 cases of colorectal cancer were diagnosed, with an incidence rate of 15.2 per 10,000 person-years. The median time between colonoscopy and diagnosis was 1.2 years.
ACE inhibitor/ARB users had a median duration of 3.3 years of use within the 5-year period before their colonoscopy. Within this group, there were 169 (0.55%) cases of colorectal cancer. On univariate analysis, the crude hazard ratio (HR) of colorectal cancer and ACE inhibitor/ARB use was 1.26 (P = .008), but on propensity score regression adjustment, the adjusted HR became 0.78.
The propensity score absolute reduction in risk for users was 3.2 per 10,000 person-years versus nonusers, and stratification by subsite showed an HR of 0.77 for distal cancers and 0.83 for proximal cancers.
In a subgroup analysis, the benefits of ACE inhibitors and ARBs were seen in patients aged 55 years or older (adjusted HR, 0.79) and in those with a history of colonic polyps (adjusted HR, 0.71).
The authors also assessed if there was an association between these medications and other types of cancer. On univariate analysis, usage was associated with an increased risk of lung and prostate cancer but lower risk of breast cancer. But after propensity score regression adjustment, the associations were no longer there.
The study was funded by the Health and Medical Research Fund of the Hong Kong SAR Government. Dr. Leung has received honorarium for attending advisory board meetings of AbbVie, Takeda, and Abbott Laboratories; coauthor Esther W. Chan has received funding support from Pfizer, Bristol-Myers Squibb, Bayer, Takeda, Janssen (a division of Johnson & Johnson); Research Grants Council of Hong Kong; Narcotics Division, Security Bureau; and the National Natural Science Foundation of China, all for work unrelated to the current study. None of the other authors have disclosed relevant financial relationships. Dr. Azoulay has disclosed no relevant financial relationships. Dr. Townsend is employed by Penn Medicine.
A version of this article originally appeared on Medscape.com.
Lipophilic statins linked to lower mortality in ovarian cancer
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
, findings from a large observational study suggest.
The study included 10,062 patients with epithelial ovarian cancer enrolled in the Finnish national cancer registry. There were 2,621 patients who were prescribed statins between 1995 and 2015, and 80% of them used lipophilic statins.
When compared with no statin use, any statin use was associated with a 40% reduction in ovarian cancer mortality (weighted hazard ratio, 0.60), and any use of lipophilic statins was associated with a 43% reduction in ovarian cancer mortality (wHR, 0.57).
Kala Visvanathan, MD, of Johns Hopkins University in Baltimore, and colleagues reported these findings in a poster at the AACR virtual meeting II.
Reductions in ovarian cancer mortality were observed in women who took simvastatin or atorvastatin (wHRs 0.24 and 0.20, respectively), the researchers found.
Lipophilic statin use also was associated with a reduction in ovarian cancer mortality across disease subtypes, although the magnitude of reduction varied. The hazard ratios were 0.60 for high-grade serous ovarian cancer, 0.50 for endometrioid ovarian cancer, 0.20 for clear cell ovarian cancer, 0.30 for mucinous ovarian cancer, and 0.27 for borderline disease.
Survival benefits were evident both in patients who started statins prior to their ovarian cancer diagnosis and in those who started statins after diagnosis.
Never-statin users had a median age of 62 years at baseline, and ever-statin users had a median age of 67 years. The median follow-up was 3.6 years and 5.5 years, respectively.
Data from the registry were linked to prescription claims, and a series of analyses were conducted to examine the association between pre- and postdiagnostic statin use and mortality. The findings were adjusted for age at diagnosis, stage, ovarian cancer subtype, treatments, year of diagnosis, and chronic disease medications. Adherence to statins was greater than 90%.
Implications and next steps
The idea of using statins for the treatment of ovarian cancer is appealing because of the promising survival data as well as the broad access, low cost, and tolerability of statins, Dr. Visvanathan said in a statement. About 28% of U.S. adults over age 40 routinely take statins for cholesterol control, and statins are widely used in other countries, she said.
“Our results support research to evaluate the repurposing of therapies that are well tolerated and inexpensive in order to help reduce the global cancer burden,” Dr. Visvanathan and colleagues wrote in their poster.
“Our results provide evidence in support of the evaluation of lipophilic statins, particularly atorvastatin and/or simvastatin, for the treatment of [epithelial ovarian cancer] in conjunction with existing therapies,” the researchers wrote. They added that these statins should be “evaluated in randomized clinical trials that include correlative endpoints.”
Further, the researchers argued that “the results are biologically plausible based on known mechanisms associated with statin use and highlight the fact that statins may be effective to treat more than one disease/outcome (i.e., high cholesterol, EOC [epithelial ovarian cancer], breast cancer).”
The results of this study are intriguing, according to James Yarmolinsky, MSc, of the University of Bristol, England. Mr. Yarmolinsky is the lead author of a case-control study that showed an association between genetically proxied 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibition and lower odds of developing epithelial ovarian cancer (JAMA. 2020;323[7]:646-655).
Mr. Yarmolinsky and colleagues found that HMG-CoA reductase inhibition equivalent to a 38.7-mg/dL reduction in low-density lipoprotein cholesterol was significantly associated with lower odds of epithelial ovarian cancer in the general population (odds ratio, 0.60) and among BRCA1/2 mutation carriers (hazard ratio, 0.69). The findings raised questions about whether a similar association would be seen with medications such as statins that inhibit HMG-CoA reductase.
“These findings linking statin use to lower ovarian cancer mortality are really interesting given our own research suggesting that these drugs may also lower women’s risk of developing this disease in the first place,” Mr. Yarmolinsky said.
“The survival rate for ovarian cancer remains the lowest among all gynecological cancers in the United States, so use of these medications in either a preventive or therapeutic context could offer an important approach for reducing disease burden,” he added. “If the findings reported by Visvanathan and colleagues can be shown to replicate in other large population-based studies, testing the efficacy of statins in a randomized clinical trial could provide definitive evidence of whether these medications lower ovarian cancer mortality.”
The Department of Defense and the Breast Cancer Research Foundation funded the current study. Dr. Visvanathan and Mr. Yarmolinsky reported no disclosures.
SOURCE: Visvanathan K et al. AACR 2020, Abstract 5782.
FROM AACR 2020
Study supports changing classification of renal cell carcinoma
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
, according to a population-level cohort study published in Cancer.
While patients with lymph node–negative stage III disease had superior overall survival at 5 years, survival rates were similar between patients with node–positive stage III disease and stage IV disease. This supports reclassifying stage III node-positive RCC to stage IV, according to researchers.
“Prior institutional studies have indicated that, among patients with stage III disease, those with lymph node disease have worse oncologic outcomes and experience survival that is similar to that of patients with American Joint Committee on Cancer (AJCC) stage IV disease,” wrote Arnav Srivastava, MD, of Rutgers Cancer Institute of New Jersey, New Brunswick, and colleagues.
The researchers used data from the National Cancer Database to identify patients with AJCC stage III or stage IV RCC who had undergone nephrectomy and lymph node dissection.
The cohort included 8,988 patients, 6,587 of whom had node–negative stage III disease, 2,218 of whom had node–positive stage III disease, and 183 of whom had stage IV metastatic disease. The researchers compared relative survival between staging groups.
The 5-year overall survival rate was 61.9% in patients with node–negative stage III RCC (95% confidence interval, 60.3%-63.4%), 22.7% in patients with node-positive stage III RCC (95% CI, 20.6%-24.9%), and 15.6% in patients with stage IV RCC (95% CI, 11.1%-23.8%).
“Patients with lymph node–positive stage III disease and those with stage IV disease were found to have overlapping 95% CIs when measuring 5-year survival; both demonstrated similar mortality,” the researchers reported. They further noted that these findings remained unchanged when patients were stratified by clear cell and non–clear cell histology.
In an accompanying editorial, Daniel D. Shapiro, MD, of the University of Texas MD Anderson Cancer Center, Houston, and E. Jason Abel, MD, of the University of Wisconsin–Madison, said the study results suggest the clinical phenotype of patients with isolated lymph node metastases is different from other stage III RCCs.
“Future editions of the AJCC staging system [should] recognize the increased risk with [lymph node–positive stage III] tumors and consider reclassification of [these] tumors as stage IV tumors so that baseline risks are more accurately measured in these rare populations,” they recommended.
Dr. Srivastava and colleagues acknowledged that two key limitations of the study were the retrospective design and the absence of data on other survival measures, such as metastasis-free and cancer-specific survival.
“Despite these limitations, we believe the current study was able to significantly build on prior work recommending the reclassification of lymph node–positive RCC as stage IV cancer,” they concluded.
The National Cancer Institute supported the study. Some study authors disclosed relationships with pharmaceutical companies and other organizations for work performed outside of the current study. The editorial authors disclosed no conflicts of interest.
SOURCE: Srivastava A et al. Cancer. 2020 Jul 1;126(13):2991-3001.
FROM CANCER