User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Hematologic manifestations of COVID-19
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
While SARS-CoV-2 causes frequent and potentially severe pulmonary disease, extrapulmonary manifestations may be a prominent part of the clinical spectrum, according to a review published in Nature Medicine.
In this comprehensive literature review, Aakriti Gupta, MD, of New York-Presbyterian/Columbia University Irving Medical Center and colleagues detailed the epidemiologic and clinical multisystem effects of COVID-19. The authors explained what is known and/or suspected about the pathophysiology of those effects and outlined the resultant management considerations.
Key mechanisms for multiorgan injury include direct viral toxicity, endothelial cell damage with inflammatory mediation of thrombosis, aberrant immune response, and dysregulation of the renin-angiotensin-aldosterone system.
The relative importance of each pathway in the clinical presentation of COVID-19 and the mechanism for extrapulmonary spread of SARS-CoV-2 infection are imperfectly understood, Dr. Gupta and colleagues noted.
As for the hematologic effects of COVID-19, patients may present with several laboratory abnormalities, but the most clinically relevant complications are thromboembolic.
COVID-19-associated coagulopathy
Dr. Gupta and colleagues noted that COVID-19–associated coagulopathy (CAC) is accompanied by elevated levels of D-dimer and fibrinogen, with minor abnormalities in prothrombin time, activated partial thromboplastin time, and platelet counts in the initial stage of infection.
Elevated D-dimer levels have been reported in up to 46% of hospitalized patients, and a longitudinal increase while hospitalized is associated with higher mortality.
In initial reports from China and the Netherlands, thrombotic complications were seen in up to 30% of COVID-19 patients in ICUs. Thromboembolic events have been reported in 17%-22% of critically ill COVID-19 patients in studies from Italy and France.
Globally, in severely affected COVID-19 patients, there have been reports of thromboses in intravenous catheters and extracorporeal circuits as well as arterial vascular occlusive events, including myocardial infarction, acute limb ischemia, and stroke.
There have been multiple small studies in which critically ill COVID-19 patients were routinely screened for thrombotic disease. In these studies, rates of thrombotic complications ranged from 69% to 85%, despite thromboprophylaxis. Variability in prophylactic and screening protocols explain discrepancies in event rates.
Pathophysiology
The abnormally high blood levels of D-dimer and fibrinogen during the early stages of SARS-CoV-2 infection are reflective of excessive inflammation rather than overt disseminated intravascular coagulation (DIC), which may develop in later stages of illness, according to Dr. Gupta and colleagues. The authors theorized that uninhibited inflammation, along with hypoxia and direct viral-mediated cellular injury, contribute to thrombotic complications in COVID-19 patients.
“The increased expression of ACE2 in endothelial cells after infection with SARS-CoV-2 may perpetuate a vicious cycle of endothelialitis that promotes thromboinflammation,” the authors wrote. “Collectively, hemostatic and inflammatory changes, which reflect endothelial damage and activation as well as critical illness, constitute a prothrombotic milieu.”
The authors noted that small autopsy series have shown high rates of microvascular and macrovascular thromboses, particularly in the pulmonary circulation, in COVID-19 patients.
Management considerations
Dr. Gupta and colleagues referenced interim guidelines from the International Society of Thrombosis and Haemostasis that recommend serial complete blood counts, with white blood cell differential and assessment of D-dimer, prothrombin time, and fibrinogen for hospitalized patients with COVID-19. The authors also cited guidelines published in the Journal of the American College of Cardiology that recommend routine risk assessment for venous thromboembolism in all hospitalized patients with COVID-19 and the consideration of standard-dose pharmaco-prophylaxis in patients who lack absolute contraindications.
Empiric use of higher-than-routine prophylactic-dose or therapeutic-dose anticoagulation in ICU patients in the absence of proven thromboses has been implemented in some institutions, Dr. Gupta and colleagues noted. Parenteral anticoagulants (such as low-molecular-weight or unfractionated heparin) are preferred to oral anticoagulants because of short half-life, available reversal agents, and the potential for drug interactions between oral agents and antiviral and/or antibacterial treatment, according to the authors.
They wrote that randomized clinical trials “will be crucial to establishing effective and safe strategies” for anticoagulation in COVID-19 patients. To this point, few randomized trials have been published to guide management of COVID-19–associated extrapulmonary manifestations, including CAC.
Research priorities
A more complete understanding of the organ-specific pathophysiology of this multisystem disease is vital, according to Dr. Gupta and colleagues.
“Regional, national, and international collaborations of clinicians and scientists focused on high-quality, transparent, ethical, and evidence-based research practices would help propel the global community toward achieving success against this pandemic,” the authors wrote.
They noted that common definitions and data standards for research are key for cross-institutional and international collaborations.
Initial attention to high-quality prospective scientific documentation standards would have been valuable and will be required for dedicated trials to address the multisystem effects of COVID-19.
Community of learners
As much as at any prior time in their careers, during the COVID-19 pandemic, health care providers have been enveloped in a community of learners – a group of people who share values and beliefs and who actively engage in learning from one another.
Through a patchwork of sources – news media, social media, traditional medical journals, general and COVID-focused meetings, and, most importantly, patients – we have been living in a learning-centered environment. Academicians, clinicians, practicing physicians, researchers, patients, family members, and caregivers have been actively and intentionally building a knowledge base together.
Through their published review, Dr. Gupta and colleagues have contributed meaningfully to the understanding our learning community has of the various extrapulmonary manifestations of COVID-19. The authors have provided a nice template for further research and clinical advances.
Dr. Gupta and colleagues disclosed financial relationships with a range of pharmaceutical companies and other organizations.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Source: Gupta A et al. Nat Med. 2020 Jul;26(7):1017-32.
FROM NATURE MEDICINE
FDA okays new CAR T therapy, first for mantle cell lymphoma
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
Americans getting more sunburns
, for reasons that are unclear, Nicole L. Bolick, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
On the plus side, utilization of indoor tanning plunged in the United States during the same period, a statistic worth celebrating as a public health and legislative success, noted Dr. Bolick, who was at the Harvard T.H. Chan School of Public Health, Boston, when she conducted her study and is now at East Carolina University, Greenville, N.C.
More good news: Her analysis of data from 67,471 nationally representative participants in the Centers for Disease Control and Prevention’s National Health Information Survey for the years 2005, 2010, and 2015 also demonstrated that the public’s adoption of several key skin cancer prevention behaviors is on the rise, although she added that rates clearly remain suboptimal.
For example, the proportion of Americans who practice sun avoidance climbed from 31.7% in 2005 to 35.5% in 2010, and 36.8% in 2015 in a multivariate logistic regression analysis adjusted for demographics, alcohol use, location, smoking status, education level, health insurance, and family and personal history of skin cancer.
Similarly, the use of sunscreen always or most of the time when outdoors for more than 1 hour on a warm, sunny day rose from an adjusted 31.5% in 2005 to 33.1% in 2010 and to 34.3% in 2015.
Also, sun protective clothing – long pants, hats, and/or long-sleeved shirts – was utilized always or most of the time by 35.9% of respondents in 2005, 38.4% in 2010, and 37.2% in 2015.
In 2005, 19% of Americans reported having a lifetime history of a physician-performed full body skin examination. The prevalence of this secondary skin cancer prevention measure rose to 22.4% in 2010 and remained the same in 2015.
In the 2005 national survey, 14.1% of respondents reported engaging in indoor tanning within the past year. This figure dropped to 6.2% in 2010 and fell further to 4.1% in 2015.
A history of two or more sunburns within the past year was reported by 18.2% of subjects in 2005, by 21.1% in 2010, and by 19.9% in 2015. It’s unclear whether this unwelcome phenomenon is due to inadequate use of sun protection or increased awareness of the link between sun exposure and skin cancer, with a resultant increase in reporting of sunburns. The influence of climate change is another possible explanation worthy of further study, according to Dr. Bolick.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
, for reasons that are unclear, Nicole L. Bolick, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
On the plus side, utilization of indoor tanning plunged in the United States during the same period, a statistic worth celebrating as a public health and legislative success, noted Dr. Bolick, who was at the Harvard T.H. Chan School of Public Health, Boston, when she conducted her study and is now at East Carolina University, Greenville, N.C.
More good news: Her analysis of data from 67,471 nationally representative participants in the Centers for Disease Control and Prevention’s National Health Information Survey for the years 2005, 2010, and 2015 also demonstrated that the public’s adoption of several key skin cancer prevention behaviors is on the rise, although she added that rates clearly remain suboptimal.
For example, the proportion of Americans who practice sun avoidance climbed from 31.7% in 2005 to 35.5% in 2010, and 36.8% in 2015 in a multivariate logistic regression analysis adjusted for demographics, alcohol use, location, smoking status, education level, health insurance, and family and personal history of skin cancer.
Similarly, the use of sunscreen always or most of the time when outdoors for more than 1 hour on a warm, sunny day rose from an adjusted 31.5% in 2005 to 33.1% in 2010 and to 34.3% in 2015.
Also, sun protective clothing – long pants, hats, and/or long-sleeved shirts – was utilized always or most of the time by 35.9% of respondents in 2005, 38.4% in 2010, and 37.2% in 2015.
In 2005, 19% of Americans reported having a lifetime history of a physician-performed full body skin examination. The prevalence of this secondary skin cancer prevention measure rose to 22.4% in 2010 and remained the same in 2015.
In the 2005 national survey, 14.1% of respondents reported engaging in indoor tanning within the past year. This figure dropped to 6.2% in 2010 and fell further to 4.1% in 2015.
A history of two or more sunburns within the past year was reported by 18.2% of subjects in 2005, by 21.1% in 2010, and by 19.9% in 2015. It’s unclear whether this unwelcome phenomenon is due to inadequate use of sun protection or increased awareness of the link between sun exposure and skin cancer, with a resultant increase in reporting of sunburns. The influence of climate change is another possible explanation worthy of further study, according to Dr. Bolick.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
, for reasons that are unclear, Nicole L. Bolick, MD, reported at the virtual annual meeting of the American Academy of Dermatology.
On the plus side, utilization of indoor tanning plunged in the United States during the same period, a statistic worth celebrating as a public health and legislative success, noted Dr. Bolick, who was at the Harvard T.H. Chan School of Public Health, Boston, when she conducted her study and is now at East Carolina University, Greenville, N.C.
More good news: Her analysis of data from 67,471 nationally representative participants in the Centers for Disease Control and Prevention’s National Health Information Survey for the years 2005, 2010, and 2015 also demonstrated that the public’s adoption of several key skin cancer prevention behaviors is on the rise, although she added that rates clearly remain suboptimal.
For example, the proportion of Americans who practice sun avoidance climbed from 31.7% in 2005 to 35.5% in 2010, and 36.8% in 2015 in a multivariate logistic regression analysis adjusted for demographics, alcohol use, location, smoking status, education level, health insurance, and family and personal history of skin cancer.
Similarly, the use of sunscreen always or most of the time when outdoors for more than 1 hour on a warm, sunny day rose from an adjusted 31.5% in 2005 to 33.1% in 2010 and to 34.3% in 2015.
Also, sun protective clothing – long pants, hats, and/or long-sleeved shirts – was utilized always or most of the time by 35.9% of respondents in 2005, 38.4% in 2010, and 37.2% in 2015.
In 2005, 19% of Americans reported having a lifetime history of a physician-performed full body skin examination. The prevalence of this secondary skin cancer prevention measure rose to 22.4% in 2010 and remained the same in 2015.
In the 2005 national survey, 14.1% of respondents reported engaging in indoor tanning within the past year. This figure dropped to 6.2% in 2010 and fell further to 4.1% in 2015.
A history of two or more sunburns within the past year was reported by 18.2% of subjects in 2005, by 21.1% in 2010, and by 19.9% in 2015. It’s unclear whether this unwelcome phenomenon is due to inadequate use of sun protection or increased awareness of the link between sun exposure and skin cancer, with a resultant increase in reporting of sunburns. The influence of climate change is another possible explanation worthy of further study, according to Dr. Bolick.
She reported having no financial conflicts regarding her study, conducted free of commercial support.
FROM AAD 20
CCC19, other registries help define COVID/cancer landscape
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
FROM AACR: COVID-19 and CANCER
Genetic differences by ancestry shouldn’t impact efficacy of prostate cancer therapies
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
, according to an analysis published in Clinical Cancer Research.
“[N]o significant differences were seen in clinically actionable DNA repair genes, MSI-high [microsatellite instability–high] status, and tumor mutation burden, suggesting that current therapeutic strategies may be equally beneficial in both populations,” wrote study author Yusuke Koga, of the Boston University, and colleagues.
“Since these findings suggest that the frequency of targetable genetic alterations is similar in patients of predominantly African versus European ancestry, offering comprehensive genomic profiling and biomarker-based therapies to all patients, including African American patients, is a critical component of promoting equity in the management of metastatic prostate cancer,” said Atish D. Choudhury, MD, PhD, of the Dana-Farber Cancer Institute in Boston, who was not involved in this study.
Mr. Koga and colleagues noted that, when compared with European-American men, African American men have a higher incidence of prostate cancer, present with more advanced disease at an earlier age, and have increased mortality. These differences persist even after adjustment for socioeconomic covariates. That raises the question of the role of genetics.
“There is emerging evidence that, across some clinical trials and equal-access health systems, outcomes between AFR [African-American] men and European-American men with prostate cancer are similar,” the investigators wrote. “Although these data suggest that disparities can be ameliorated, there is limited knowledge of the genomic alterations that differ between groups and that could impact clinical outcomes.”
Study details and results
To get a handle on the issue, the investigators performed a meta-analysis of tumors from 250 African American men and 611 European-American men to compare the frequencies of somatic alterations across datasets from the Cancer Genome Atlas, the African Ancestry prostate cancer cohort, and the Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets panel.
The team also compared prostate cancer sequencing data from a commercial platform, the Foundation Medicine assay, from 436 African-American men and 3,018 European-American men.
In the meta-analysis, mutations in ZFHX3 and focal deletions in ETV3 were more common in tumors from African American men than in tumors from European-American men. Both genes are putative prostate cancer tumor suppressors, the investigators noted.
TP53 mutations, meanwhile, were associated with increasing Gleason scores in both groups, suggesting “that if TP53 mutations are found in low-grade disease, they may potentially indicate a more aggressive clinical trajectory,” the investigators wrote.
In the analysis with the commercial assay, MYC amplifications were more frequent in African American men with metastatic disease, raising “the possibility that MYC amplifications may also contribute to high-risk disease in this population,” the team wrote.
Deletions in PTEN and rearrangements in TMPRSS2-ERG were less frequent in tumors from African American men, but KMT2D truncations and CCND1 amplifications were more frequent.
“Higher expression of CCND1 has been implicated with perineural invasion in prostate cancer, an aggressive histological feature in prostate cancer. Truncating mutations in KMT2D have been reported in both localized and metastatic prostate cancer patients with unclear clinical significance,” the investigators noted.
“The genomic differences seen in genes such as MYC, ZFHX3, PTEN, and TMPRSS2-ERG suggest that different pathways of carcinogenesis may be active in AFR [African American] men, which could lead to further disparities if targeted therapies for some of these alterations become available,” the team wrote.
They noted that the meta-analysis was limited by the fact that some cohorts lacked matched tumors from European-American men, which limited the investigators’ ability to control for differences in region, clinical setting, or sequencing assay. Furthermore, age, tumor stage, and Gleason grade were unavailable in the cohort analyzed with the commercial assay.
This research was funded by the Department of Defense, the National Cancer Institute, and the Prostate Cancer Foundation. Two authors are employees of Foundation Medicine.
SOURCE: Koga Y et al. Clin Cancer Res. 2020 Jul 10. doi: 10.1158/1078-0432.CCR-19-4112.
FROM CLINICAL CANCER RESEARCH
Survey: U.S. oncologists have high net worth, live within their means
The average annual income for oncologists surveyed was $377,000, which was 5% higher than the $359,000 reported for 2018. This put oncologists in eleventh place among 29 specialties.
However, this information was obtained prior to February 11, 2020, before the COVID-19 pandemic took hold in the United States, and the financial situation has changed for many physicians.
For example, primary care physicians have reported a 55% decrease in revenue along with a 20% to 30% reduction in patient volume. The decline has even led some to shutter their physical offices, according to the larger survey of all physicians, the Medscape Physician Debt and Net Worth Report 2020. This full survey included 17, 461 physicians and represented 30 specialties.
Physicians in specialty practices may be facing even greater reductions. “Specialists are currently having more troubles than PCPs because they’re largely dependent on elective cases, which can’t be directly addressed by telemedicine,” commented Joel Greenwald, MD, CEO of Greenwald Wealth Management, St. Louis Park, Minnesota, in the survey.
Community oncology clinics and practices have reported a substantial decline in office visits and new patients because of the COVID-19 pandemic. Even before the pandemic, clinics had been closing in recent years as a result of being acquired, merging, or because of financial struggles, although that trend has been plateauing, according to the latest report from the Community Oncology Alliance.
Oncologists’ net worth
With regard to net worth, 42% of the oncologists surveyed reported having assets totaling from $1 million to $5 million, which is about the same for physicians in general. Only 15% reported a net worth of $5 million or higher; a quarter reported a net worth of less than $500,000.
Wealth is more evenly divided when it comes to gender in comparison with other specialties. For all physicians, 56% of men and 39% of women reported a net worth of more than $1 million. For oncologists, that ratio is 59% of men and 54% of women.
Not surprisingly, net worth also increased by age. Only about a quarter (27%) of oncologists younger than age 45 reported a net worth of $1 million to $5 million, compared to 48% aged 45-54 and 56% of physicians aged 55-64. This makes sense, inasmuch as earnings generally increase over time and early-career debt is paid down. However, net worth does appear to decline somewhat after the age of 65, presumably because of a decrease in income on retirement.
Debts and expenses
For debts and expenses that are currently being paid off, mortgage on a primary residence (59%) topped the list. More than half of oncologists reported living in a home that is 3,000 sq ft or larger, and nearly half (49%) have a mortgage of $300,000 or higher. About a third of the oncologists surveyed have no mortgage or one that has been paid off.
Car loan payments (35%) and college education/medical school loans (25%) were the second and third most common sources of debt. As compared with other specialties, oncologists land right in the middle of those still paying off school loans. Only 15% reported that they had no debts or expenses to be paid off.
Savings and living within one’s means
The average American has four credit cards. About half of oncologists surveyed reported having four or fewer, although about a fifth (22%) have seven or more. But the vast majority reported living within their means (49%) or below their means (46%). Only 6% reported living above their means.
Surveyed oncologists also reported putting money aside in a tax-deferred retirement account or college savings account. Almost half (48%) are putting aside more than $2000 every month, and 28% save from $1000 to $2000. A small percentage (8%) reported not doing this on a regular basis.
A smaller percentage (40%) responded that they put more than $2000 a month into a taxable retirement or college savings account; 18% reported not doing this on a regular basis. More than two thirds also reported either having a written budget or a mental one for their personal expenses.
In 2019, most oncologists (77%) did not experience a financial loss. For those who did, bad investments on the stock market (14%) were the main cause. A smaller number reported real estate losses, problems with their practice, or job loss.
Nearly half (49%) reported that they currently work with a financial planner or have done so in the past.
This article first appeared on Medscape.com.
The average annual income for oncologists surveyed was $377,000, which was 5% higher than the $359,000 reported for 2018. This put oncologists in eleventh place among 29 specialties.
However, this information was obtained prior to February 11, 2020, before the COVID-19 pandemic took hold in the United States, and the financial situation has changed for many physicians.
For example, primary care physicians have reported a 55% decrease in revenue along with a 20% to 30% reduction in patient volume. The decline has even led some to shutter their physical offices, according to the larger survey of all physicians, the Medscape Physician Debt and Net Worth Report 2020. This full survey included 17, 461 physicians and represented 30 specialties.
Physicians in specialty practices may be facing even greater reductions. “Specialists are currently having more troubles than PCPs because they’re largely dependent on elective cases, which can’t be directly addressed by telemedicine,” commented Joel Greenwald, MD, CEO of Greenwald Wealth Management, St. Louis Park, Minnesota, in the survey.
Community oncology clinics and practices have reported a substantial decline in office visits and new patients because of the COVID-19 pandemic. Even before the pandemic, clinics had been closing in recent years as a result of being acquired, merging, or because of financial struggles, although that trend has been plateauing, according to the latest report from the Community Oncology Alliance.
Oncologists’ net worth
With regard to net worth, 42% of the oncologists surveyed reported having assets totaling from $1 million to $5 million, which is about the same for physicians in general. Only 15% reported a net worth of $5 million or higher; a quarter reported a net worth of less than $500,000.
Wealth is more evenly divided when it comes to gender in comparison with other specialties. For all physicians, 56% of men and 39% of women reported a net worth of more than $1 million. For oncologists, that ratio is 59% of men and 54% of women.
Not surprisingly, net worth also increased by age. Only about a quarter (27%) of oncologists younger than age 45 reported a net worth of $1 million to $5 million, compared to 48% aged 45-54 and 56% of physicians aged 55-64. This makes sense, inasmuch as earnings generally increase over time and early-career debt is paid down. However, net worth does appear to decline somewhat after the age of 65, presumably because of a decrease in income on retirement.
Debts and expenses
For debts and expenses that are currently being paid off, mortgage on a primary residence (59%) topped the list. More than half of oncologists reported living in a home that is 3,000 sq ft or larger, and nearly half (49%) have a mortgage of $300,000 or higher. About a third of the oncologists surveyed have no mortgage or one that has been paid off.
Car loan payments (35%) and college education/medical school loans (25%) were the second and third most common sources of debt. As compared with other specialties, oncologists land right in the middle of those still paying off school loans. Only 15% reported that they had no debts or expenses to be paid off.
Savings and living within one’s means
The average American has four credit cards. About half of oncologists surveyed reported having four or fewer, although about a fifth (22%) have seven or more. But the vast majority reported living within their means (49%) or below their means (46%). Only 6% reported living above their means.
Surveyed oncologists also reported putting money aside in a tax-deferred retirement account or college savings account. Almost half (48%) are putting aside more than $2000 every month, and 28% save from $1000 to $2000. A small percentage (8%) reported not doing this on a regular basis.
A smaller percentage (40%) responded that they put more than $2000 a month into a taxable retirement or college savings account; 18% reported not doing this on a regular basis. More than two thirds also reported either having a written budget or a mental one for their personal expenses.
In 2019, most oncologists (77%) did not experience a financial loss. For those who did, bad investments on the stock market (14%) were the main cause. A smaller number reported real estate losses, problems with their practice, or job loss.
Nearly half (49%) reported that they currently work with a financial planner or have done so in the past.
This article first appeared on Medscape.com.
The average annual income for oncologists surveyed was $377,000, which was 5% higher than the $359,000 reported for 2018. This put oncologists in eleventh place among 29 specialties.
However, this information was obtained prior to February 11, 2020, before the COVID-19 pandemic took hold in the United States, and the financial situation has changed for many physicians.
For example, primary care physicians have reported a 55% decrease in revenue along with a 20% to 30% reduction in patient volume. The decline has even led some to shutter their physical offices, according to the larger survey of all physicians, the Medscape Physician Debt and Net Worth Report 2020. This full survey included 17, 461 physicians and represented 30 specialties.
Physicians in specialty practices may be facing even greater reductions. “Specialists are currently having more troubles than PCPs because they’re largely dependent on elective cases, which can’t be directly addressed by telemedicine,” commented Joel Greenwald, MD, CEO of Greenwald Wealth Management, St. Louis Park, Minnesota, in the survey.
Community oncology clinics and practices have reported a substantial decline in office visits and new patients because of the COVID-19 pandemic. Even before the pandemic, clinics had been closing in recent years as a result of being acquired, merging, or because of financial struggles, although that trend has been plateauing, according to the latest report from the Community Oncology Alliance.
Oncologists’ net worth
With regard to net worth, 42% of the oncologists surveyed reported having assets totaling from $1 million to $5 million, which is about the same for physicians in general. Only 15% reported a net worth of $5 million or higher; a quarter reported a net worth of less than $500,000.
Wealth is more evenly divided when it comes to gender in comparison with other specialties. For all physicians, 56% of men and 39% of women reported a net worth of more than $1 million. For oncologists, that ratio is 59% of men and 54% of women.
Not surprisingly, net worth also increased by age. Only about a quarter (27%) of oncologists younger than age 45 reported a net worth of $1 million to $5 million, compared to 48% aged 45-54 and 56% of physicians aged 55-64. This makes sense, inasmuch as earnings generally increase over time and early-career debt is paid down. However, net worth does appear to decline somewhat after the age of 65, presumably because of a decrease in income on retirement.
Debts and expenses
For debts and expenses that are currently being paid off, mortgage on a primary residence (59%) topped the list. More than half of oncologists reported living in a home that is 3,000 sq ft or larger, and nearly half (49%) have a mortgage of $300,000 or higher. About a third of the oncologists surveyed have no mortgage or one that has been paid off.
Car loan payments (35%) and college education/medical school loans (25%) were the second and third most common sources of debt. As compared with other specialties, oncologists land right in the middle of those still paying off school loans. Only 15% reported that they had no debts or expenses to be paid off.
Savings and living within one’s means
The average American has four credit cards. About half of oncologists surveyed reported having four or fewer, although about a fifth (22%) have seven or more. But the vast majority reported living within their means (49%) or below their means (46%). Only 6% reported living above their means.
Surveyed oncologists also reported putting money aside in a tax-deferred retirement account or college savings account. Almost half (48%) are putting aside more than $2000 every month, and 28% save from $1000 to $2000. A small percentage (8%) reported not doing this on a regular basis.
A smaller percentage (40%) responded that they put more than $2000 a month into a taxable retirement or college savings account; 18% reported not doing this on a regular basis. More than two thirds also reported either having a written budget or a mental one for their personal expenses.
In 2019, most oncologists (77%) did not experience a financial loss. For those who did, bad investments on the stock market (14%) were the main cause. A smaller number reported real estate losses, problems with their practice, or job loss.
Nearly half (49%) reported that they currently work with a financial planner or have done so in the past.
This article first appeared on Medscape.com.
Heavy toll from ongoing cancer referral delays
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Early screening may halve breast cancer mortality in childhood cancer survivors
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
Two strategies – annual mammography with MRI and annual MRI alone – at least halved breast cancer mortality when started at the ages of 25 or 30 years.
Jennifer M. Yeh, PhD, of Harvard Medical School in Boston and colleagues reported these results in the Annals of Internal Medicine.
When cost was also considered, 30 years emerged as the preferred starting age, dropping the incremental cost-effectiveness ratio (ICER) below the generally accepted threshold of $100,000 per quality-adjusted life-year gained.
“Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with these patients,” Dr. Yeh and colleagues wrote.
“Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors,” the authors recommended. “Our findings also highlight the importance of ensuring that survivors have access to health insurance coverage for MRI screening.”
Implications for awareness, coverage
“My hope is that, by showing the significantly decreased risk of death associated with early breast cancer screening, with harm-benefit ratios considerably lower than benchmarks for average-risk women, this study will help health insurance companies see the benefit in covering early screening for at-risk survivors,” commented Karen E. Effinger, MD, of Emory University, Atlanta, and the Aflac Cancer & Blood Disorders Center at Children’s Healthcare of Atlanta.
“In many survivors, the cost of current screening [as recommended by] guidelines is prohibitive,” added Dr. Effinger, who was not involved in the current study.
The main concern regarding the study’s findings is generalizability to the contemporary era, given the use of a cohort diagnosed and treated decades ago and changes in radiation techniques and dosing since then, she noted in an interview. This limitation was addressed in a sensitivity analysis that halved the women’s base-case lifetime risk of breast cancer and still netted similar results.
“However, it will take many years to determine the true risk reduction of our current treatment strategies,” Dr. Effinger acknowledged.
“It is crucial that we improve our education of both survivors and our colleagues who care for these survivors, especially in regard to risk of subsequent malignancies and the benefits of screening,” Dr. Effinger maintained. “While many people are aware of the risk of breast cancer associated with BRCA mutations, the increased risk in survivors of childhood cancer is not as recognized by nononcologists. This study reinforces that increasing this awareness can save lives.”
In educating their patients about preventive care, health care providers must strike “a fine balance between discussing the risks and benefits of screening without provoking significant anxiety,” she concluded. “It is important for survivors to establish care with a primary care provider in order to develop trust and receive the guidance they need to decrease the risk of early mortality.”
Study details
Dr. Yeh and colleagues developed models to compare outcomes with various screening strategies among women aged 20 years who had received chest radiotherapy for childhood cancer during 1970-1986. The women had been diagnosed with Hodgkin lymphoma (55%), Wilms tumor (12%), non-Hodgkin lymphoma (8%), and other cancers.
The investigators conducted their analysis using data from the Childhood Cancer Survivor Study and other published sources, a lifetime time horizon, and a payer perspective.
The team assessed three strategies: no screening; digital mammography with MRI screening starting at 25 years of age (the current Children’s Oncology Group recommendation), 30 years, or 35 years and continuing to 74 years of age; and MRI only starting at age 25, 30, or 35 years and continuing to age 74 years.
The main study results showed that, without screening, women who had received chest radiation for childhood cancer had a 10%-11% lifetime risk of breast cancer mortality across models.
Relative to no screening, starting at age 25 years, the largest share of deaths was averted with the strategy of annual mammography with MRI – 56.3%-71.2% – or with the strategy of annual MRI alone – 55.7%-62.0%.
These two strategies also yielded the most screening tests, as well as the most false-positive test results and benign biopsy results.
For women who started screening at age 25, there were 4,188-4,879 false-positive test results per 1,000 women for mammography plus MRI and 3,283-3,764 false-positive results per 1,000 women for MRI alone.
For women who started screening at age 25, there were 1,340-1,561 benign biopsy results per 1,000 women for mammography plus MRI and 1,248-1,430 benign results per 1,000 women for MRI alone.
After cost was factored in, beginning screening at age 30 emerged as the preferred strategy to achieve an ICER threshold of less than $100,000 per quality-adjusted life-year gained.
When started at 30 years of age, annual mammography with MRI averted 54.7%-68.8% of breast cancer deaths, with an ICER of $25,400-$113,200 per quality-adjusted life-year gained. Annual MRI alone averted 54.0%-60.0% of breast cancer deaths, with an ICER of $21,800-$50,580 per quality-adjusted life-year gained.
This research was supported by grants from the National Cancer Institute, American Cancer Society, and American Lebanese Syrian Associated Charities. The authors disclosed relationships with GE Healthcare and Biovector. Dr. Effinger disclosed no relevant conflicts of interest.
SOURCE: Yeh JM et al. Ann Intern Med. 2020 Jul 7. doi: 10.7326/M19-3481.
FROM ANNALS OF INTERNAL MEDICINE
Don’t overlook treating older patients with acute promyelocytic leukemia, expert says
The estimated one third of patients with acute promyelocytic leukemia (APL) who are older than 60 years now enjoy a notably better prognosis than in years past, thanks to the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). However, such patients still require special management considerations, and can only benefit from treatment advantages if properly identified.
In a recently published set of recommendations, the International Society of Geriatric Oncology Task Force outlined the latest information on the treatment of APL in older patients. Medscape spoke with the lead author of the article, Heidi Klepin, MD, MS, professor in the section on hematology and oncology at Wake Forest School of Medicine in Winston Salem, N.C., who highlighted the key points that clinicians need to know about this often highly treatable subtype of acute myeloid leukemia (AML). This interview has been edited for length and clarity.
Medscape: How do the potential benefits of therapy for APL compare with other AML subtypes in older persons?
Dr. Klepin: Potential benefits of therapy are dramatically better for APL, compared with other AML subtypes. The use of non–chemotherapy based regimens with ATRA and ATO has substantially changed options for APL management. ATRA+ATO are associated with high remission and cure rates. The chance of cure with less toxicity extends the clinical benefit to adults of advanced age and, to some extent, with comorbidities.
How has the management strategy for this subgroup of patients with APL changed in recent years?
Management options have changed dramatically with the advent of non–chemotherapy-based regimens. The majority of treated older adults could be expected to achieve remissions that are durable, with less risk of major side effects during treatment. Adults with comorbid conditions, at advanced age, and with some functional limitations could also still benefit from treatment.
Does that management strategy change based on whether patients are considered low-risk or high-risk?
Clinical trials are lacking to provide best evidence for the optimal treatment for adults over age 70 years. However, based on available data and experience, the expert consensus provided in this report recommends that older adults regardless of age with low-risk disease should be offered ATRA+ATO–based therapy if available.
The optimal approach for patients with high-risk disease is less clear based on available studies. For fit older adults without cardiac disease, the use of single-drug anthracycline chemotherapy with ATRA plus/minus ATO is appropriate. However, treatment with ATRA+ATO may also provide a good response with less side-effect risk. For older patients with high-risk disease and comorbidity or poor functional status, the use of non-chemotherapy regimen ATRA+ATO is preferred.
What role does frailty have in making treatment decisions in this population?
Although frail older adults have not been specifically studied in clinical trials, it is reasonable to offer treatment with a non–chemotherapy based regimen for many of these patients, particularly if frailty may in part be related to disease burden. Frailty is a dynamic state. Rapid initiation of therapy can improve function and symptoms, potentially reversing the phenotype of frailty if driven largely by disease burden.
What is the role of consolidation and maintenance therapy in older patients with APL?
Consolidation therapy is recommended with ATRA+ATO as a standard consideration for most patients when available, although protocol-based treatments may vary. For those older adults treated with chemotherapy+ATRA for high-risk disease, decreased anthracycline [chemotherapy] exposure during consolidation results in less mortality risk. Maintenance therapy is not needed when ATRA+ATO are used for induction and consolidation and after achieving a molecular remission.
What other patient factors should influence treatment decisions?
In practice, older age, concurrent comorbid conditions [particularly cardiac disease], and physical function may all influence treatment decisions. Regarding the disease itself, a high white blood cell count at diagnosis, which is classified as higher-risk disease, directs choice of therapy, particularly for fit older adults. Cardiac disease can limit certain treatment options because of risk of side effects. In particular, the use of anthracycline chemotherapy is contraindicated for people with heart failure, and the use of ATO can increase risk of arrhythmia and is not used with certain EKG findings.
Special considerations in older patients with APL
How would you characterize older individuals’ involvement in clinical trials?
Older adults are underrepresented on clinical trials, with very limited inclusion of those over age 75 years. Some APL trials have had upper age exclusions, which is something we have advocated to remove.
Are there unique challenges in diagnosing older adults with APL?
The presentation of APL with low blood counts can look similar to other types of AML or myelodysplastic syndrome when reviewing routine lab results. If additional testing is not done quickly, the diagnosis will be missed, as well as the opportunity for effective treatment. Rapid diagnosis is essential in this disease.
Are there age-related differences in the presentation of APL?
There are no available data to support more-aggressive APL biology in older adults.
How does age impact the outcomes of patients with APL?
Although the outcomes in APL have improved, the survival difference between age groups has not decreased in recent years and the magnitude of improvement in survival in older patients still lags behind younger patients. Older age is also associated with worse outcomes driven largely by increased early death, with greater rates of infection and multiorgan failure leading to a decreased overall survival.
How important is a geriatric assessment for older patients with APL? What role does it play in management?
There are no data on the use of a geriatric assessment specifically in APL, although a geriatric assessment is recommended for older adults starting new chemotherapy in general. A geriatric assessment may help determine who is fit enough to be treated like a younger patient, which has the greatest implications for those with high-risk disease where chemotherapy would be added.
A geriatric assessment can also play an important role in management by identifying vulnerabilities that could be addressed to minimize complications during treatment regardless of the type of treatment given. An example would be identifying and addressing polypharmacy (commonly defined as ≥5 medications). One challenge faced when treating older patients is the use of multiple concomitant medications. Polypharmacy is common among older patients with cancer. Among older adults, each new drug increases the risk of adverse drug events by 10%. Drugs commonly used for the treatment of APL, such as ATRA and ATO, have many potential drug interactions, which must be carefully assessed by a pharmacist prior to and during treatment. Active deprescribing of medications that are not critical during treatment for APL should be done to minimize risks.
What is differentiation syndrome? What role does age appear to play in the risk of developing it and in strategies for managing it?
Differentiation syndrome is a serious side effect that may occur in patients with APL who have been treated with certain anticancer drugs. Differentiation syndrome usually occurs within a week or 2 of starting treatment. It is caused by a large, rapid release of cytokines [immune substances] from leukemia cells. The most common symptoms include fever; cough; shortness of breath; weight gain; swelling of the arms, legs, and neck; build-up of excess fluid around the heart and lungs; low blood pressure; and kidney failure. Differentiation syndrome can be life-threatening if not recognized and treated early.
Some evidence suggests older adults may be at a higher risk for developing differentiation syndrome and may be less likely to tolerate it. A risk factor is kidney dysfunction, which is more common in older adults.
It is not clear that management should differ by age, but vigilance is critical. The use of prophylactic steroids is considered for high-risk patients [high white cell count or kidney disease]. The treatment for differentiation syndrome involves rapid use of steroids.
Does the management of infections differ in older people with APL?
There is no clear data to support a different management of infection prevention for older adults, although preventive antibiotics can be considered as older adults are at a higher risk for infectious complications. However, drug interactions need to be carefully considered in this context.
Guiding clinicians toward better treatment of APL
Why did you decide to formulate these recommendations now?
It is particularly important to draw attention to the management of older adults with APL given the availability of effective non–chemotherapy based therapies and the large distinction between expected outcomes with APL vs. other types of acute leukemia in this population. This diagnosis should not be missed. Further, we highlight the importance of ensuring that older adults are included in trials to provide best evidence for both treatment choice and supportive care management.
How do you see these recommendations affecting clinical practice?
We want to emphasize that advanced age should not preclude treatment, which can have meaningful benefit with expectation of remission and quality time gained.
We hope that these recommendations provide a useful blueprint for guiding the management of older adults, particularly consolidating information to help inform treatment for those patients older than 75 years that can provide best estimates of side effects and benefits when making a decision with patients. We also hope that these recommendations will be used to educate providers on the importance of looking for this diagnosis in our older patients.
From a practical standpoint, it will be important that this information gets to those providers who are making the referrals to oncologists, which can include primary care physicians and emergency room providers, to ensure prompt diagnostic workup. Treatment decisions can only be made once a diagnosis has been recognized, and time is critical with this disease.
Dr. Klepin disclosed a consultancy for Genentech and Pfizer and is a contributor to UpToDate.
A version of this article originally appeared on Medscape.com.
The estimated one third of patients with acute promyelocytic leukemia (APL) who are older than 60 years now enjoy a notably better prognosis than in years past, thanks to the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). However, such patients still require special management considerations, and can only benefit from treatment advantages if properly identified.
In a recently published set of recommendations, the International Society of Geriatric Oncology Task Force outlined the latest information on the treatment of APL in older patients. Medscape spoke with the lead author of the article, Heidi Klepin, MD, MS, professor in the section on hematology and oncology at Wake Forest School of Medicine in Winston Salem, N.C., who highlighted the key points that clinicians need to know about this often highly treatable subtype of acute myeloid leukemia (AML). This interview has been edited for length and clarity.
Medscape: How do the potential benefits of therapy for APL compare with other AML subtypes in older persons?
Dr. Klepin: Potential benefits of therapy are dramatically better for APL, compared with other AML subtypes. The use of non–chemotherapy based regimens with ATRA and ATO has substantially changed options for APL management. ATRA+ATO are associated with high remission and cure rates. The chance of cure with less toxicity extends the clinical benefit to adults of advanced age and, to some extent, with comorbidities.
How has the management strategy for this subgroup of patients with APL changed in recent years?
Management options have changed dramatically with the advent of non–chemotherapy-based regimens. The majority of treated older adults could be expected to achieve remissions that are durable, with less risk of major side effects during treatment. Adults with comorbid conditions, at advanced age, and with some functional limitations could also still benefit from treatment.
Does that management strategy change based on whether patients are considered low-risk or high-risk?
Clinical trials are lacking to provide best evidence for the optimal treatment for adults over age 70 years. However, based on available data and experience, the expert consensus provided in this report recommends that older adults regardless of age with low-risk disease should be offered ATRA+ATO–based therapy if available.
The optimal approach for patients with high-risk disease is less clear based on available studies. For fit older adults without cardiac disease, the use of single-drug anthracycline chemotherapy with ATRA plus/minus ATO is appropriate. However, treatment with ATRA+ATO may also provide a good response with less side-effect risk. For older patients with high-risk disease and comorbidity or poor functional status, the use of non-chemotherapy regimen ATRA+ATO is preferred.
What role does frailty have in making treatment decisions in this population?
Although frail older adults have not been specifically studied in clinical trials, it is reasonable to offer treatment with a non–chemotherapy based regimen for many of these patients, particularly if frailty may in part be related to disease burden. Frailty is a dynamic state. Rapid initiation of therapy can improve function and symptoms, potentially reversing the phenotype of frailty if driven largely by disease burden.
What is the role of consolidation and maintenance therapy in older patients with APL?
Consolidation therapy is recommended with ATRA+ATO as a standard consideration for most patients when available, although protocol-based treatments may vary. For those older adults treated with chemotherapy+ATRA for high-risk disease, decreased anthracycline [chemotherapy] exposure during consolidation results in less mortality risk. Maintenance therapy is not needed when ATRA+ATO are used for induction and consolidation and after achieving a molecular remission.
What other patient factors should influence treatment decisions?
In practice, older age, concurrent comorbid conditions [particularly cardiac disease], and physical function may all influence treatment decisions. Regarding the disease itself, a high white blood cell count at diagnosis, which is classified as higher-risk disease, directs choice of therapy, particularly for fit older adults. Cardiac disease can limit certain treatment options because of risk of side effects. In particular, the use of anthracycline chemotherapy is contraindicated for people with heart failure, and the use of ATO can increase risk of arrhythmia and is not used with certain EKG findings.
Special considerations in older patients with APL
How would you characterize older individuals’ involvement in clinical trials?
Older adults are underrepresented on clinical trials, with very limited inclusion of those over age 75 years. Some APL trials have had upper age exclusions, which is something we have advocated to remove.
Are there unique challenges in diagnosing older adults with APL?
The presentation of APL with low blood counts can look similar to other types of AML or myelodysplastic syndrome when reviewing routine lab results. If additional testing is not done quickly, the diagnosis will be missed, as well as the opportunity for effective treatment. Rapid diagnosis is essential in this disease.
Are there age-related differences in the presentation of APL?
There are no available data to support more-aggressive APL biology in older adults.
How does age impact the outcomes of patients with APL?
Although the outcomes in APL have improved, the survival difference between age groups has not decreased in recent years and the magnitude of improvement in survival in older patients still lags behind younger patients. Older age is also associated with worse outcomes driven largely by increased early death, with greater rates of infection and multiorgan failure leading to a decreased overall survival.
How important is a geriatric assessment for older patients with APL? What role does it play in management?
There are no data on the use of a geriatric assessment specifically in APL, although a geriatric assessment is recommended for older adults starting new chemotherapy in general. A geriatric assessment may help determine who is fit enough to be treated like a younger patient, which has the greatest implications for those with high-risk disease where chemotherapy would be added.
A geriatric assessment can also play an important role in management by identifying vulnerabilities that could be addressed to minimize complications during treatment regardless of the type of treatment given. An example would be identifying and addressing polypharmacy (commonly defined as ≥5 medications). One challenge faced when treating older patients is the use of multiple concomitant medications. Polypharmacy is common among older patients with cancer. Among older adults, each new drug increases the risk of adverse drug events by 10%. Drugs commonly used for the treatment of APL, such as ATRA and ATO, have many potential drug interactions, which must be carefully assessed by a pharmacist prior to and during treatment. Active deprescribing of medications that are not critical during treatment for APL should be done to minimize risks.
What is differentiation syndrome? What role does age appear to play in the risk of developing it and in strategies for managing it?
Differentiation syndrome is a serious side effect that may occur in patients with APL who have been treated with certain anticancer drugs. Differentiation syndrome usually occurs within a week or 2 of starting treatment. It is caused by a large, rapid release of cytokines [immune substances] from leukemia cells. The most common symptoms include fever; cough; shortness of breath; weight gain; swelling of the arms, legs, and neck; build-up of excess fluid around the heart and lungs; low blood pressure; and kidney failure. Differentiation syndrome can be life-threatening if not recognized and treated early.
Some evidence suggests older adults may be at a higher risk for developing differentiation syndrome and may be less likely to tolerate it. A risk factor is kidney dysfunction, which is more common in older adults.
It is not clear that management should differ by age, but vigilance is critical. The use of prophylactic steroids is considered for high-risk patients [high white cell count or kidney disease]. The treatment for differentiation syndrome involves rapid use of steroids.
Does the management of infections differ in older people with APL?
There is no clear data to support a different management of infection prevention for older adults, although preventive antibiotics can be considered as older adults are at a higher risk for infectious complications. However, drug interactions need to be carefully considered in this context.
Guiding clinicians toward better treatment of APL
Why did you decide to formulate these recommendations now?
It is particularly important to draw attention to the management of older adults with APL given the availability of effective non–chemotherapy based therapies and the large distinction between expected outcomes with APL vs. other types of acute leukemia in this population. This diagnosis should not be missed. Further, we highlight the importance of ensuring that older adults are included in trials to provide best evidence for both treatment choice and supportive care management.
How do you see these recommendations affecting clinical practice?
We want to emphasize that advanced age should not preclude treatment, which can have meaningful benefit with expectation of remission and quality time gained.
We hope that these recommendations provide a useful blueprint for guiding the management of older adults, particularly consolidating information to help inform treatment for those patients older than 75 years that can provide best estimates of side effects and benefits when making a decision with patients. We also hope that these recommendations will be used to educate providers on the importance of looking for this diagnosis in our older patients.
From a practical standpoint, it will be important that this information gets to those providers who are making the referrals to oncologists, which can include primary care physicians and emergency room providers, to ensure prompt diagnostic workup. Treatment decisions can only be made once a diagnosis has been recognized, and time is critical with this disease.
Dr. Klepin disclosed a consultancy for Genentech and Pfizer and is a contributor to UpToDate.
A version of this article originally appeared on Medscape.com.
The estimated one third of patients with acute promyelocytic leukemia (APL) who are older than 60 years now enjoy a notably better prognosis than in years past, thanks to the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). However, such patients still require special management considerations, and can only benefit from treatment advantages if properly identified.
In a recently published set of recommendations, the International Society of Geriatric Oncology Task Force outlined the latest information on the treatment of APL in older patients. Medscape spoke with the lead author of the article, Heidi Klepin, MD, MS, professor in the section on hematology and oncology at Wake Forest School of Medicine in Winston Salem, N.C., who highlighted the key points that clinicians need to know about this often highly treatable subtype of acute myeloid leukemia (AML). This interview has been edited for length and clarity.
Medscape: How do the potential benefits of therapy for APL compare with other AML subtypes in older persons?
Dr. Klepin: Potential benefits of therapy are dramatically better for APL, compared with other AML subtypes. The use of non–chemotherapy based regimens with ATRA and ATO has substantially changed options for APL management. ATRA+ATO are associated with high remission and cure rates. The chance of cure with less toxicity extends the clinical benefit to adults of advanced age and, to some extent, with comorbidities.
How has the management strategy for this subgroup of patients with APL changed in recent years?
Management options have changed dramatically with the advent of non–chemotherapy-based regimens. The majority of treated older adults could be expected to achieve remissions that are durable, with less risk of major side effects during treatment. Adults with comorbid conditions, at advanced age, and with some functional limitations could also still benefit from treatment.
Does that management strategy change based on whether patients are considered low-risk or high-risk?
Clinical trials are lacking to provide best evidence for the optimal treatment for adults over age 70 years. However, based on available data and experience, the expert consensus provided in this report recommends that older adults regardless of age with low-risk disease should be offered ATRA+ATO–based therapy if available.
The optimal approach for patients with high-risk disease is less clear based on available studies. For fit older adults without cardiac disease, the use of single-drug anthracycline chemotherapy with ATRA plus/minus ATO is appropriate. However, treatment with ATRA+ATO may also provide a good response with less side-effect risk. For older patients with high-risk disease and comorbidity or poor functional status, the use of non-chemotherapy regimen ATRA+ATO is preferred.
What role does frailty have in making treatment decisions in this population?
Although frail older adults have not been specifically studied in clinical trials, it is reasonable to offer treatment with a non–chemotherapy based regimen for many of these patients, particularly if frailty may in part be related to disease burden. Frailty is a dynamic state. Rapid initiation of therapy can improve function and symptoms, potentially reversing the phenotype of frailty if driven largely by disease burden.
What is the role of consolidation and maintenance therapy in older patients with APL?
Consolidation therapy is recommended with ATRA+ATO as a standard consideration for most patients when available, although protocol-based treatments may vary. For those older adults treated with chemotherapy+ATRA for high-risk disease, decreased anthracycline [chemotherapy] exposure during consolidation results in less mortality risk. Maintenance therapy is not needed when ATRA+ATO are used for induction and consolidation and after achieving a molecular remission.
What other patient factors should influence treatment decisions?
In practice, older age, concurrent comorbid conditions [particularly cardiac disease], and physical function may all influence treatment decisions. Regarding the disease itself, a high white blood cell count at diagnosis, which is classified as higher-risk disease, directs choice of therapy, particularly for fit older adults. Cardiac disease can limit certain treatment options because of risk of side effects. In particular, the use of anthracycline chemotherapy is contraindicated for people with heart failure, and the use of ATO can increase risk of arrhythmia and is not used with certain EKG findings.
Special considerations in older patients with APL
How would you characterize older individuals’ involvement in clinical trials?
Older adults are underrepresented on clinical trials, with very limited inclusion of those over age 75 years. Some APL trials have had upper age exclusions, which is something we have advocated to remove.
Are there unique challenges in diagnosing older adults with APL?
The presentation of APL with low blood counts can look similar to other types of AML or myelodysplastic syndrome when reviewing routine lab results. If additional testing is not done quickly, the diagnosis will be missed, as well as the opportunity for effective treatment. Rapid diagnosis is essential in this disease.
Are there age-related differences in the presentation of APL?
There are no available data to support more-aggressive APL biology in older adults.
How does age impact the outcomes of patients with APL?
Although the outcomes in APL have improved, the survival difference between age groups has not decreased in recent years and the magnitude of improvement in survival in older patients still lags behind younger patients. Older age is also associated with worse outcomes driven largely by increased early death, with greater rates of infection and multiorgan failure leading to a decreased overall survival.
How important is a geriatric assessment for older patients with APL? What role does it play in management?
There are no data on the use of a geriatric assessment specifically in APL, although a geriatric assessment is recommended for older adults starting new chemotherapy in general. A geriatric assessment may help determine who is fit enough to be treated like a younger patient, which has the greatest implications for those with high-risk disease where chemotherapy would be added.
A geriatric assessment can also play an important role in management by identifying vulnerabilities that could be addressed to minimize complications during treatment regardless of the type of treatment given. An example would be identifying and addressing polypharmacy (commonly defined as ≥5 medications). One challenge faced when treating older patients is the use of multiple concomitant medications. Polypharmacy is common among older patients with cancer. Among older adults, each new drug increases the risk of adverse drug events by 10%. Drugs commonly used for the treatment of APL, such as ATRA and ATO, have many potential drug interactions, which must be carefully assessed by a pharmacist prior to and during treatment. Active deprescribing of medications that are not critical during treatment for APL should be done to minimize risks.
What is differentiation syndrome? What role does age appear to play in the risk of developing it and in strategies for managing it?
Differentiation syndrome is a serious side effect that may occur in patients with APL who have been treated with certain anticancer drugs. Differentiation syndrome usually occurs within a week or 2 of starting treatment. It is caused by a large, rapid release of cytokines [immune substances] from leukemia cells. The most common symptoms include fever; cough; shortness of breath; weight gain; swelling of the arms, legs, and neck; build-up of excess fluid around the heart and lungs; low blood pressure; and kidney failure. Differentiation syndrome can be life-threatening if not recognized and treated early.
Some evidence suggests older adults may be at a higher risk for developing differentiation syndrome and may be less likely to tolerate it. A risk factor is kidney dysfunction, which is more common in older adults.
It is not clear that management should differ by age, but vigilance is critical. The use of prophylactic steroids is considered for high-risk patients [high white cell count or kidney disease]. The treatment for differentiation syndrome involves rapid use of steroids.
Does the management of infections differ in older people with APL?
There is no clear data to support a different management of infection prevention for older adults, although preventive antibiotics can be considered as older adults are at a higher risk for infectious complications. However, drug interactions need to be carefully considered in this context.
Guiding clinicians toward better treatment of APL
Why did you decide to formulate these recommendations now?
It is particularly important to draw attention to the management of older adults with APL given the availability of effective non–chemotherapy based therapies and the large distinction between expected outcomes with APL vs. other types of acute leukemia in this population. This diagnosis should not be missed. Further, we highlight the importance of ensuring that older adults are included in trials to provide best evidence for both treatment choice and supportive care management.
How do you see these recommendations affecting clinical practice?
We want to emphasize that advanced age should not preclude treatment, which can have meaningful benefit with expectation of remission and quality time gained.
We hope that these recommendations provide a useful blueprint for guiding the management of older adults, particularly consolidating information to help inform treatment for those patients older than 75 years that can provide best estimates of side effects and benefits when making a decision with patients. We also hope that these recommendations will be used to educate providers on the importance of looking for this diagnosis in our older patients.
From a practical standpoint, it will be important that this information gets to those providers who are making the referrals to oncologists, which can include primary care physicians and emergency room providers, to ensure prompt diagnostic workup. Treatment decisions can only be made once a diagnosis has been recognized, and time is critical with this disease.
Dr. Klepin disclosed a consultancy for Genentech and Pfizer and is a contributor to UpToDate.
A version of this article originally appeared on Medscape.com.
Early data support further study of ivosidenib in mIDH1 glioma
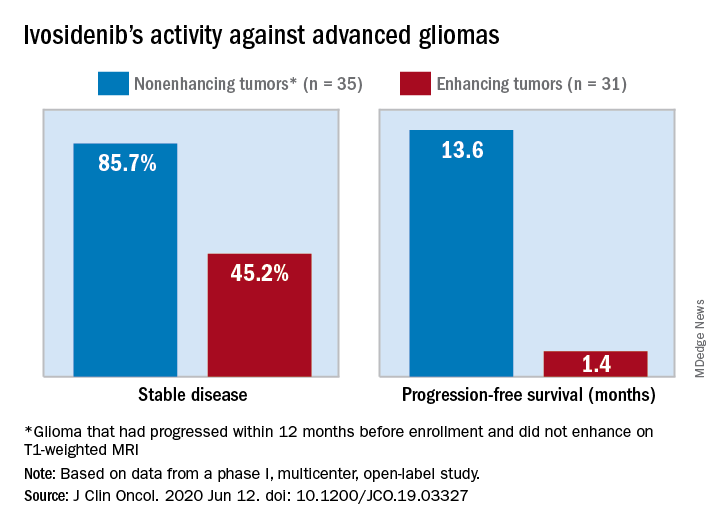
The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327

The median progression-free survival was 13.6 months for patients with nonenhancing tumors and 1.4 months for patients with enhancing tumors in a study of 66 adults with mIDH1 advanced glioma.
“On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted,” Ingo Mellinghoff, MD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues wrote in the Journal of Clinical Oncology.
“This is not a home run but is of interest to the community,” said Lawrence Recht, MD, of Stanford (Calif.) University, who was not involved in this study. “Other companies are also developing agents like this.”
Considering that the ivosidenib study “is uncontrolled, one cannot say for sure that this wasn’t just the natural history of the disease,” Dr. Recht continued. “This type of tumor can behave very indolently, and patients can survive years without treatment, so this is rather a short interval to make a long-time statement. I think the authors are a bit overenthusiastic.”
The authors tested ivosidenib in 66 adults with mIDH1 glioma – 35 with nonenhancing glioma and 31 with enhancing glioma. Tumors had recurred after, or did not respond to, initial surgery, radiation, or chemotherapy.
The patients’ median age was 41 years (range, 21-71 years), and 25 patients (37.9%) were women. The most common tumor type at screening was oligodendroglioma in 23 patients (34.8%).
Patients received ivosidenib at doses ranging from 100 mg twice a day to 900 mg once a day. A total of 50 patients received the phase 2 recommended dose – 500 mg once a day. There were no dose-limiting toxicities, and there was no maximum-tolerated dose.
Adverse events of grade 3 or higher occurred in 19.7% of patients and included headache, seizure, hyperglycemia, neutropenia, and hypophosphatemia. Grade 3 or higher treatment-related adverse events occurred in two patients.
A total of 30 patients with nonenhancing tumors (85.7%) and 14 with enhancing tumors (45.2%) had a best response of stable disease. There was one partial response in a nonenhancing patient on 500 mg/day. The rest of the subjects had a best response of progressive disease.
The median treatment duration was 18.4 months among patients with nonenhancing tumors and 1.9 months among those with enhancing tumors. Discontinuation was caused byo progression in all but one case.
Among patients with measurable disease, tumor measurements decreased from baseline in 22 nonenhancing tumors (66.7%) and in 9 enhancing tumors (33.3%).
“Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas,” the investigators wrote. “This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery.
“We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia,” the investigators wrote.
The team also noted that the median progression-free survival for patients with nonenhancing gliomas in the current study “compares favorably to that reported for temozolomide” in advanced mIDH1 low-grade glioma, which was approximately 7 months.
This research was funded by Agios Pharmaceuticals, the company developing ivosidenib. Dr. Mellinghoff receives travel compensation from and is an adviser to the company. Several other investigators are employees. Dr. Recht disclosed no conflicts of interest.
SOURCE: Mellinghoff I et al. J Clin Oncol. 2020 Jun 12. doi: 10.1200/JCO.19.03327
FROM THE JOURNAL OF CLINICAL ONCOLOGY





