User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Global melanoma incidence high and on the rise
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.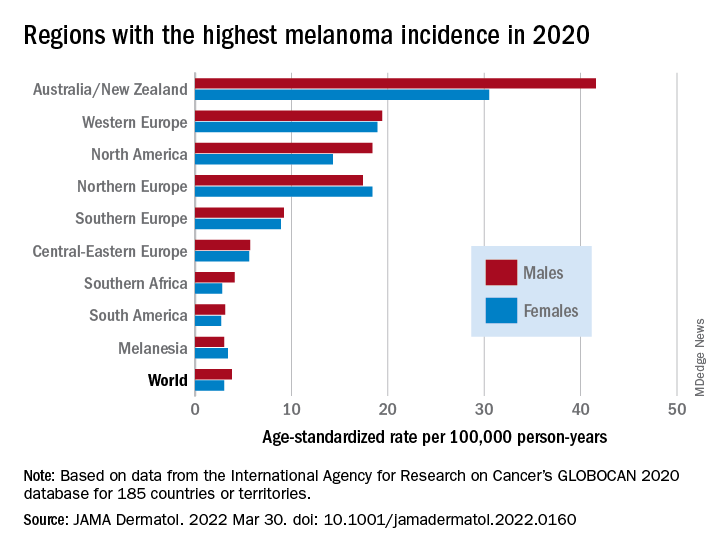
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
FROM JAMA DERMATOLOGY
‘Eye-opening’ experience on the other side of the hospital bed
The 5 days that she spent at her mother’s bedside were eye-opening for an oncologist used to being on the other side of the clinician–patient relationship.
“As a physician, I thought I had a unique perspective of things that were done well – and things that were not,” commented Pamela Kunz, MD.
Dr. Kunz, who was named the 2021 Woman Oncologist of the Year, is director of the Center for Gastrointestinal Cancers at Smilow Cancer Hospital and of the Yale Cancer Center, New Haven, Conn.
But she was propelled into quite a different role when her mother was admitted to the hospital.
Her mom, who has trouble hearing, was easily confused by jargon and by “all of the people coming in and out with no introductions,” she explained.
“She needed someone to translate what was going on because she didn’t feel well,” she added.
Seeing inpatient care through her mother’s eyes was enlightening, and at times it was “shocking to be on the other side.”
Physicians get used to “checking boxes, getting through the day,” she said. “It’s easy to forget the human side.”
“Seeing a loved one sick, [struggling] through this – I just wished I had seen things done differently,” added Dr. Kunz.
Her thread has since garnered thousands of “likes” and scores of comments and retweets.
She began the Twitter thread explaining what prompted her comments:
“I spent many hours last week observing the practice of medicine while sitting at my mom’s hospital bedside and was reminded of some important communication pearls. Some musings ...”
“1. Introduce yourself by full name, role, and team and have ID badges visible. It can get very confusing for [patients] and family members with the number of people in and out of rooms. E.g. ‘My name is Dr. X. I’m the intern on the primary internal medicine team.’
2. End your patient visit with a summary of the plan for the day.
3. Avoid medical jargon & speak slowly, clearly, and logically. Remember you are a teacher for your [patients] and their family.
4. Masks make it harder to hear, especially for [patients] with hearing loss (and they no longer have the aid of lip reading).
5. Many older [patients] get confused in the hospital. Repetition is a good thing.
6. Speak to a family member at least once per day to relay the plan.
7. Try to avoid last minute or surprise discharges – they make [patients] and family members anxious. Talk about discharge planning from day 1 and what milestones must occur prior to a safe discharge. ‘In order for you to leave the hospital, X, Y, X must happen.’
8. Talk with your [patients] about something other than what brought them to the hospital (a tip I once learned from a wise mentor).
9. When possible, sit at eye level with your patient (I love these stools from @YNHH).
10. Take time to listen.”
Dr. Kunz closed with her golden rule: “Lastly, treat your patients how you would want your own family member treated.”
Twitter user @BrunaPellini replied: “I love this, especially ‘Treat your patients how you would want your own family member treated.’ My mom and grandma always said that to me since I was a med student, and this is definitely one of my core values.”
Other clinicians shared similar experiences, and some added to Dr. Kunz’s list.
“Agree entirely, love the list – and while none of us can always practice perfectly, my experiences with my own mother’s illness taught me an enormous amount about communication,” @hoperugo responded.
Twitter user @mariejacork added: “Everyone in health care please read ... if you are lucky enough to not have had a loved one unwell in hospital, these may get forgotten. Having sat with my dad for a few days before he died a few years ago, I felt a lot of these, and it changed my practice forever.”
@bjcohenmd provided additional advice: “And use the dry erase board that should be in every room. Never start a medication without explaining it. Many docs will see the patient and then go to the computer, decide to order a med, but never go back to explain it.”
Patients also shared experiences and offered suggestions.
“As a chronic pain patient I’d add – we know it’s frustrating you can’t cure us but PLEASE do not SIGH if we say something didn’t work or [tell] us to be more positive. Just say ‘I know this is very hard, I’m here to listen.’ We don’t expect a cure, we do expect to be believed,” said @ppenguinsmt. “It makes me feel like I’m causing distress to you if I say the pain has been unrelenting. I leave feeling worse. ...You may have heard 10 [people] in pain before me but this is MY only [appointment].”
Twitter user @KatieCahoots added: “These are perfect. I wish doctors would do this not only in the hospital but in the doctor’s office, as well. I would add one caveat: When you try not to use medical jargon, don’t dumb it down as though I don’t know anything about science or haven’t done any of my own research.”
Dr. Kunz said she was taken aback but pleased by the response to her Tweet.
“It’s an example of the human side of medicine, so it resonates with physicians and with patients,” she commented. Seeing through her mom’s eyes how care was provided made her realize that medical training should include more emphasis on communication, including “real-time feedback to interns, residents, fellows, and students.”
Yes, it takes time, and “we don’t all have a lot of extra time,” she acknowledged.
“But some of these elements don’t take that much more time to do. They can help build trust and can, in the long run, actually save time if patients understand and family members feel engaged and like they are participants,” she said. “I think a little time investment will go a long way.”
In her case, she very much appreciated the one trainee who tried to call her and update her about her mother’s care each afternoon. “I really valued that,” she said.
A version of this article first appeared on Medscape.com.
The 5 days that she spent at her mother’s bedside were eye-opening for an oncologist used to being on the other side of the clinician–patient relationship.
“As a physician, I thought I had a unique perspective of things that were done well – and things that were not,” commented Pamela Kunz, MD.
Dr. Kunz, who was named the 2021 Woman Oncologist of the Year, is director of the Center for Gastrointestinal Cancers at Smilow Cancer Hospital and of the Yale Cancer Center, New Haven, Conn.
But she was propelled into quite a different role when her mother was admitted to the hospital.
Her mom, who has trouble hearing, was easily confused by jargon and by “all of the people coming in and out with no introductions,” she explained.
“She needed someone to translate what was going on because she didn’t feel well,” she added.
Seeing inpatient care through her mother’s eyes was enlightening, and at times it was “shocking to be on the other side.”
Physicians get used to “checking boxes, getting through the day,” she said. “It’s easy to forget the human side.”
“Seeing a loved one sick, [struggling] through this – I just wished I had seen things done differently,” added Dr. Kunz.
Her thread has since garnered thousands of “likes” and scores of comments and retweets.
She began the Twitter thread explaining what prompted her comments:
“I spent many hours last week observing the practice of medicine while sitting at my mom’s hospital bedside and was reminded of some important communication pearls. Some musings ...”
“1. Introduce yourself by full name, role, and team and have ID badges visible. It can get very confusing for [patients] and family members with the number of people in and out of rooms. E.g. ‘My name is Dr. X. I’m the intern on the primary internal medicine team.’
2. End your patient visit with a summary of the plan for the day.
3. Avoid medical jargon & speak slowly, clearly, and logically. Remember you are a teacher for your [patients] and their family.
4. Masks make it harder to hear, especially for [patients] with hearing loss (and they no longer have the aid of lip reading).
5. Many older [patients] get confused in the hospital. Repetition is a good thing.
6. Speak to a family member at least once per day to relay the plan.
7. Try to avoid last minute or surprise discharges – they make [patients] and family members anxious. Talk about discharge planning from day 1 and what milestones must occur prior to a safe discharge. ‘In order for you to leave the hospital, X, Y, X must happen.’
8. Talk with your [patients] about something other than what brought them to the hospital (a tip I once learned from a wise mentor).
9. When possible, sit at eye level with your patient (I love these stools from @YNHH).
10. Take time to listen.”
Dr. Kunz closed with her golden rule: “Lastly, treat your patients how you would want your own family member treated.”
Twitter user @BrunaPellini replied: “I love this, especially ‘Treat your patients how you would want your own family member treated.’ My mom and grandma always said that to me since I was a med student, and this is definitely one of my core values.”
Other clinicians shared similar experiences, and some added to Dr. Kunz’s list.
“Agree entirely, love the list – and while none of us can always practice perfectly, my experiences with my own mother’s illness taught me an enormous amount about communication,” @hoperugo responded.
Twitter user @mariejacork added: “Everyone in health care please read ... if you are lucky enough to not have had a loved one unwell in hospital, these may get forgotten. Having sat with my dad for a few days before he died a few years ago, I felt a lot of these, and it changed my practice forever.”
@bjcohenmd provided additional advice: “And use the dry erase board that should be in every room. Never start a medication without explaining it. Many docs will see the patient and then go to the computer, decide to order a med, but never go back to explain it.”
Patients also shared experiences and offered suggestions.
“As a chronic pain patient I’d add – we know it’s frustrating you can’t cure us but PLEASE do not SIGH if we say something didn’t work or [tell] us to be more positive. Just say ‘I know this is very hard, I’m here to listen.’ We don’t expect a cure, we do expect to be believed,” said @ppenguinsmt. “It makes me feel like I’m causing distress to you if I say the pain has been unrelenting. I leave feeling worse. ...You may have heard 10 [people] in pain before me but this is MY only [appointment].”
Twitter user @KatieCahoots added: “These are perfect. I wish doctors would do this not only in the hospital but in the doctor’s office, as well. I would add one caveat: When you try not to use medical jargon, don’t dumb it down as though I don’t know anything about science or haven’t done any of my own research.”
Dr. Kunz said she was taken aback but pleased by the response to her Tweet.
“It’s an example of the human side of medicine, so it resonates with physicians and with patients,” she commented. Seeing through her mom’s eyes how care was provided made her realize that medical training should include more emphasis on communication, including “real-time feedback to interns, residents, fellows, and students.”
Yes, it takes time, and “we don’t all have a lot of extra time,” she acknowledged.
“But some of these elements don’t take that much more time to do. They can help build trust and can, in the long run, actually save time if patients understand and family members feel engaged and like they are participants,” she said. “I think a little time investment will go a long way.”
In her case, she very much appreciated the one trainee who tried to call her and update her about her mother’s care each afternoon. “I really valued that,” she said.
A version of this article first appeared on Medscape.com.
The 5 days that she spent at her mother’s bedside were eye-opening for an oncologist used to being on the other side of the clinician–patient relationship.
“As a physician, I thought I had a unique perspective of things that were done well – and things that were not,” commented Pamela Kunz, MD.
Dr. Kunz, who was named the 2021 Woman Oncologist of the Year, is director of the Center for Gastrointestinal Cancers at Smilow Cancer Hospital and of the Yale Cancer Center, New Haven, Conn.
But she was propelled into quite a different role when her mother was admitted to the hospital.
Her mom, who has trouble hearing, was easily confused by jargon and by “all of the people coming in and out with no introductions,” she explained.
“She needed someone to translate what was going on because she didn’t feel well,” she added.
Seeing inpatient care through her mother’s eyes was enlightening, and at times it was “shocking to be on the other side.”
Physicians get used to “checking boxes, getting through the day,” she said. “It’s easy to forget the human side.”
“Seeing a loved one sick, [struggling] through this – I just wished I had seen things done differently,” added Dr. Kunz.
Her thread has since garnered thousands of “likes” and scores of comments and retweets.
She began the Twitter thread explaining what prompted her comments:
“I spent many hours last week observing the practice of medicine while sitting at my mom’s hospital bedside and was reminded of some important communication pearls. Some musings ...”
“1. Introduce yourself by full name, role, and team and have ID badges visible. It can get very confusing for [patients] and family members with the number of people in and out of rooms. E.g. ‘My name is Dr. X. I’m the intern on the primary internal medicine team.’
2. End your patient visit with a summary of the plan for the day.
3. Avoid medical jargon & speak slowly, clearly, and logically. Remember you are a teacher for your [patients] and their family.
4. Masks make it harder to hear, especially for [patients] with hearing loss (and they no longer have the aid of lip reading).
5. Many older [patients] get confused in the hospital. Repetition is a good thing.
6. Speak to a family member at least once per day to relay the plan.
7. Try to avoid last minute or surprise discharges – they make [patients] and family members anxious. Talk about discharge planning from day 1 and what milestones must occur prior to a safe discharge. ‘In order for you to leave the hospital, X, Y, X must happen.’
8. Talk with your [patients] about something other than what brought them to the hospital (a tip I once learned from a wise mentor).
9. When possible, sit at eye level with your patient (I love these stools from @YNHH).
10. Take time to listen.”
Dr. Kunz closed with her golden rule: “Lastly, treat your patients how you would want your own family member treated.”
Twitter user @BrunaPellini replied: “I love this, especially ‘Treat your patients how you would want your own family member treated.’ My mom and grandma always said that to me since I was a med student, and this is definitely one of my core values.”
Other clinicians shared similar experiences, and some added to Dr. Kunz’s list.
“Agree entirely, love the list – and while none of us can always practice perfectly, my experiences with my own mother’s illness taught me an enormous amount about communication,” @hoperugo responded.
Twitter user @mariejacork added: “Everyone in health care please read ... if you are lucky enough to not have had a loved one unwell in hospital, these may get forgotten. Having sat with my dad for a few days before he died a few years ago, I felt a lot of these, and it changed my practice forever.”
@bjcohenmd provided additional advice: “And use the dry erase board that should be in every room. Never start a medication without explaining it. Many docs will see the patient and then go to the computer, decide to order a med, but never go back to explain it.”
Patients also shared experiences and offered suggestions.
“As a chronic pain patient I’d add – we know it’s frustrating you can’t cure us but PLEASE do not SIGH if we say something didn’t work or [tell] us to be more positive. Just say ‘I know this is very hard, I’m here to listen.’ We don’t expect a cure, we do expect to be believed,” said @ppenguinsmt. “It makes me feel like I’m causing distress to you if I say the pain has been unrelenting. I leave feeling worse. ...You may have heard 10 [people] in pain before me but this is MY only [appointment].”
Twitter user @KatieCahoots added: “These are perfect. I wish doctors would do this not only in the hospital but in the doctor’s office, as well. I would add one caveat: When you try not to use medical jargon, don’t dumb it down as though I don’t know anything about science or haven’t done any of my own research.”
Dr. Kunz said she was taken aback but pleased by the response to her Tweet.
“It’s an example of the human side of medicine, so it resonates with physicians and with patients,” she commented. Seeing through her mom’s eyes how care was provided made her realize that medical training should include more emphasis on communication, including “real-time feedback to interns, residents, fellows, and students.”
Yes, it takes time, and “we don’t all have a lot of extra time,” she acknowledged.
“But some of these elements don’t take that much more time to do. They can help build trust and can, in the long run, actually save time if patients understand and family members feel engaged and like they are participants,” she said. “I think a little time investment will go a long way.”
In her case, she very much appreciated the one trainee who tried to call her and update her about her mother’s care each afternoon. “I really valued that,” she said.
A version of this article first appeared on Medscape.com.
Adding immunotherapy to chemo in lung cancer improves patient outcomes, new data show
according to an analysis presented at the annual European Lung Cancer Congress (ELCC) on March 30.
“Overall, it is very clear that chemotherapy plus immunotherapy prolongs the time to symptom deterioration and actually improves symptoms” in this patient population, said study discussant Luis Paz-Ares, MD, PhD, chair of medical oncology at the Hospital Universitario 12 de Octubre, Madrid, who was not involved in the research.
Last September, investigators reported efficacy outcomes from the phase 3 POSEIDON trial, which randomized 1,013 patients with EGFR/ALK wild-type mNSCLC to one of three first-line options: chemotherapy alone, chemotherapy plus the checkpoint inhibitor durvalumab, or chemotherapy plus two check-point inhibitors, durvalumab and tremelimumab. The analysis showed improved progression-free survival in both immunotherapy arms as well as a significant 2.3-month overall survival advantage with dual immunotherapy and a nonsignificant 1.6-month advantage with single agent durvalumab.
At the ELCC meeting, study presenter and lead investigator Edward Garon, MD, reported the latest data on the trial’s secondary endpoints: patient-reported outcomes. Global health status, functioning, and symptom scores were assessed using two questionnaires, the EORTC QLQ-C30 and EORTC QLQ-LC13.
Overall, Dr. Garon and colleagues reported a longer time to deterioration in all three areas – global health status, functioning, and symptoms – for patients who received immunotherapy versus chemotherapy alone, with similar results in both immunotherapy arms.
Time to deterioration in global health status, for instance, was a median of about 8 months on both immunotherapy regimens versus 5.6 months with chemotherapy alone. The positive findings held for many patient-reported treatment side effects, including dyspnea, hemoptysis, nausea/vomiting, and insomnia, but the benefits of adding immunotherapy weren’t always statistically significant.
Adding one or both checkpoint inhibitors to chemotherapy “improved efficacy while delaying deterioration in symptoms, functioning, and [health-related quality of life] versus chemotherapy alone in patients with mNSCLC,” concluded Dr. Garon, a thoracic medical oncologist at the University of California, Los Angeles. Plus, he added, “the pattern was observed across nearly all prespecified symptoms and domains of interest.”
According to study discussant Dr. Paz-Ares, “the data seem to be very consistent with all the trials asking similar questions.” The important thing here is figuring out the ideal candidates for dual inhibitor therapy, he said.
With positive efficacy and patient-reported outcomes for single and dual immunotherapy in this trial, it’s a “relatively straightforward” decision to add immunotherapy to chemotherapy for patients with mNSCLC, Massimo Di Maio, a medical oncologist at the University of Turin, Italy, said in an editorial on the ELCC’s news site.
However, that’s not always the case for every cancer type, which makes patient-reported outcomes “crucial” for determining the right treatment for each patient. Some might opt for a modest survival benefit regardless of the side effects, while others might favor a less toxic approach, even it means not living quite as long, he said.
The problem, he stressed, is that trials often release efficacy data well before patient-reported outcomes, which makes weighing the benefits and risks of a treat-ment option more difficult. The delay between efficacy and patient-reported outcome data was about 6 months in the POSEIDON trial.
“Timing is key when it comes to using [patient reported outcomes] for decision-making in oncology,” Dr. Di Maio said. “In fact, to enable a full assessment of a treatment, results should be published concurrently with the efficacy and safety data. Unfortunately, this is generally not the case.”
POSEIDON was funded by AstraZeneca, which markets durvalumab and is developing tremelimumab. Dr. Garon reported grants from the company. Dr. Paz-Ares reported honoraria and institutional research grants from AstraZeneca. Dr. Di Maio is a consultant for AstraZeneca and reported receiving honoraria and personal fees from the company.
according to an analysis presented at the annual European Lung Cancer Congress (ELCC) on March 30.
“Overall, it is very clear that chemotherapy plus immunotherapy prolongs the time to symptom deterioration and actually improves symptoms” in this patient population, said study discussant Luis Paz-Ares, MD, PhD, chair of medical oncology at the Hospital Universitario 12 de Octubre, Madrid, who was not involved in the research.
Last September, investigators reported efficacy outcomes from the phase 3 POSEIDON trial, which randomized 1,013 patients with EGFR/ALK wild-type mNSCLC to one of three first-line options: chemotherapy alone, chemotherapy plus the checkpoint inhibitor durvalumab, or chemotherapy plus two check-point inhibitors, durvalumab and tremelimumab. The analysis showed improved progression-free survival in both immunotherapy arms as well as a significant 2.3-month overall survival advantage with dual immunotherapy and a nonsignificant 1.6-month advantage with single agent durvalumab.
At the ELCC meeting, study presenter and lead investigator Edward Garon, MD, reported the latest data on the trial’s secondary endpoints: patient-reported outcomes. Global health status, functioning, and symptom scores were assessed using two questionnaires, the EORTC QLQ-C30 and EORTC QLQ-LC13.
Overall, Dr. Garon and colleagues reported a longer time to deterioration in all three areas – global health status, functioning, and symptoms – for patients who received immunotherapy versus chemotherapy alone, with similar results in both immunotherapy arms.
Time to deterioration in global health status, for instance, was a median of about 8 months on both immunotherapy regimens versus 5.6 months with chemotherapy alone. The positive findings held for many patient-reported treatment side effects, including dyspnea, hemoptysis, nausea/vomiting, and insomnia, but the benefits of adding immunotherapy weren’t always statistically significant.
Adding one or both checkpoint inhibitors to chemotherapy “improved efficacy while delaying deterioration in symptoms, functioning, and [health-related quality of life] versus chemotherapy alone in patients with mNSCLC,” concluded Dr. Garon, a thoracic medical oncologist at the University of California, Los Angeles. Plus, he added, “the pattern was observed across nearly all prespecified symptoms and domains of interest.”
According to study discussant Dr. Paz-Ares, “the data seem to be very consistent with all the trials asking similar questions.” The important thing here is figuring out the ideal candidates for dual inhibitor therapy, he said.
With positive efficacy and patient-reported outcomes for single and dual immunotherapy in this trial, it’s a “relatively straightforward” decision to add immunotherapy to chemotherapy for patients with mNSCLC, Massimo Di Maio, a medical oncologist at the University of Turin, Italy, said in an editorial on the ELCC’s news site.
However, that’s not always the case for every cancer type, which makes patient-reported outcomes “crucial” for determining the right treatment for each patient. Some might opt for a modest survival benefit regardless of the side effects, while others might favor a less toxic approach, even it means not living quite as long, he said.
The problem, he stressed, is that trials often release efficacy data well before patient-reported outcomes, which makes weighing the benefits and risks of a treat-ment option more difficult. The delay between efficacy and patient-reported outcome data was about 6 months in the POSEIDON trial.
“Timing is key when it comes to using [patient reported outcomes] for decision-making in oncology,” Dr. Di Maio said. “In fact, to enable a full assessment of a treatment, results should be published concurrently with the efficacy and safety data. Unfortunately, this is generally not the case.”
POSEIDON was funded by AstraZeneca, which markets durvalumab and is developing tremelimumab. Dr. Garon reported grants from the company. Dr. Paz-Ares reported honoraria and institutional research grants from AstraZeneca. Dr. Di Maio is a consultant for AstraZeneca and reported receiving honoraria and personal fees from the company.
according to an analysis presented at the annual European Lung Cancer Congress (ELCC) on March 30.
“Overall, it is very clear that chemotherapy plus immunotherapy prolongs the time to symptom deterioration and actually improves symptoms” in this patient population, said study discussant Luis Paz-Ares, MD, PhD, chair of medical oncology at the Hospital Universitario 12 de Octubre, Madrid, who was not involved in the research.
Last September, investigators reported efficacy outcomes from the phase 3 POSEIDON trial, which randomized 1,013 patients with EGFR/ALK wild-type mNSCLC to one of three first-line options: chemotherapy alone, chemotherapy plus the checkpoint inhibitor durvalumab, or chemotherapy plus two check-point inhibitors, durvalumab and tremelimumab. The analysis showed improved progression-free survival in both immunotherapy arms as well as a significant 2.3-month overall survival advantage with dual immunotherapy and a nonsignificant 1.6-month advantage with single agent durvalumab.
At the ELCC meeting, study presenter and lead investigator Edward Garon, MD, reported the latest data on the trial’s secondary endpoints: patient-reported outcomes. Global health status, functioning, and symptom scores were assessed using two questionnaires, the EORTC QLQ-C30 and EORTC QLQ-LC13.
Overall, Dr. Garon and colleagues reported a longer time to deterioration in all three areas – global health status, functioning, and symptoms – for patients who received immunotherapy versus chemotherapy alone, with similar results in both immunotherapy arms.
Time to deterioration in global health status, for instance, was a median of about 8 months on both immunotherapy regimens versus 5.6 months with chemotherapy alone. The positive findings held for many patient-reported treatment side effects, including dyspnea, hemoptysis, nausea/vomiting, and insomnia, but the benefits of adding immunotherapy weren’t always statistically significant.
Adding one or both checkpoint inhibitors to chemotherapy “improved efficacy while delaying deterioration in symptoms, functioning, and [health-related quality of life] versus chemotherapy alone in patients with mNSCLC,” concluded Dr. Garon, a thoracic medical oncologist at the University of California, Los Angeles. Plus, he added, “the pattern was observed across nearly all prespecified symptoms and domains of interest.”
According to study discussant Dr. Paz-Ares, “the data seem to be very consistent with all the trials asking similar questions.” The important thing here is figuring out the ideal candidates for dual inhibitor therapy, he said.
With positive efficacy and patient-reported outcomes for single and dual immunotherapy in this trial, it’s a “relatively straightforward” decision to add immunotherapy to chemotherapy for patients with mNSCLC, Massimo Di Maio, a medical oncologist at the University of Turin, Italy, said in an editorial on the ELCC’s news site.
However, that’s not always the case for every cancer type, which makes patient-reported outcomes “crucial” for determining the right treatment for each patient. Some might opt for a modest survival benefit regardless of the side effects, while others might favor a less toxic approach, even it means not living quite as long, he said.
The problem, he stressed, is that trials often release efficacy data well before patient-reported outcomes, which makes weighing the benefits and risks of a treat-ment option more difficult. The delay between efficacy and patient-reported outcome data was about 6 months in the POSEIDON trial.
“Timing is key when it comes to using [patient reported outcomes] for decision-making in oncology,” Dr. Di Maio said. “In fact, to enable a full assessment of a treatment, results should be published concurrently with the efficacy and safety data. Unfortunately, this is generally not the case.”
POSEIDON was funded by AstraZeneca, which markets durvalumab and is developing tremelimumab. Dr. Garon reported grants from the company. Dr. Paz-Ares reported honoraria and institutional research grants from AstraZeneca. Dr. Di Maio is a consultant for AstraZeneca and reported receiving honoraria and personal fees from the company.
FROM ELCC 2022
Anticoagulation not routinely needed after TAVR: ADAPT-TAVR
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
Breast cancer therapy toxicities: Education and communication
This transcript of a video roundtable, which is available on Medscape.com, has been edited for clarity.
Hope S. Rugo, MD: Hello. I’m Hope Rugo, a breast medical oncologist from the University of California, San Francisco. I’m joined here by three of my friends and colleagues to discuss the toxicity of new agents in the treatment of breast cancer. Fatima, do you want to start by introducing yourself?
Fatima F. Cardoso, MD: Sure. Hello, everyone. I’m Fatima Cardoso, a breast medical oncologist in Lisbon, Portugal.
Dr. Rugo: Sheila.
Sheila Pettiford: Hi, I’m Sheila Pettiford. I am a metastatic [breast cancer] patient and have been for almost 8 years in April. I used to live in Philadelphia, Pennsylvania, but moved to Delaware in the last couple of years during the pandemic. I’m happy to be here.
Dr. Rugo: Julia.
Julia Maués: Hi, everyone. I also am a person living with metastatic breast cancer. I was diagnosed in 2013, so it’s going to be 9 years, also in April.
Effective monitoring and management of side effects: A team effort
Dr. Rugo: We have an amazing group and an international representation, which is also really nice because we get different perspectives. What we’re going to talk about is important to providers and patients across the board. With the host of new agents for the treatment of breast cancer – most of which have really moved us forward in terms of having effective treatment options – we’ve also been faced with a lot of new toxicities or side effects that we haven’t seen before or that we might not have expected from the specific agent.
Those toxicities across the board include side effects that are quite familiar to us, like low blood counts, but we may not advise people well enough about other side effects such as mouth sores, inflammation of the lungs, immune toxicities, and skin toxicities.
Fatima, do you want to start and talk about how we can think about these toxicities and address them?
Dr. Cardoso: Sure. Thank you. From the health care provider point of view, what I would highlight is to educate. Educate before we start the treatment. It’s very important to inform the patient but in a balanced way, so we don’t overexaggerate certain types of side effects or underestimate certain types of side effects.
It’s very important because an informed patient will be attentive to the types of side effects that can happen. Also, teach the patient when it is a [cause for] alarm or something for which they might need to contact their health care team and when that’s not the case. I think this is one crucial topic.
The other one is to monitor. Find ways how to best communicate between the patient and the health care team but in a way that you can monitor, so you can act very early on. Most of these new side effects, if you act early on, will not become severe. It is very important to know about them and to act early on.
I believe there is something important that we don’t think about all the time, and that is prophylaxis. Do not be shy about using prophylactic measures, be it for the mouth sores, nausea and vomiting, diarrhea, and other things that really impact the quality of life of patients. Those, to start, are my three major points of attention for health care professionals.
Dr. Rugo: I think that’s so incredibly important – the comments that you’ve made – and also that prevention and prophylaxis are so important. You don’t want to have a patient have diarrhea in the middle of the night and not have any antipropulsive agents at home. Just as a very straightforward example, it’s really important.
Also, the ability to know what you should be looking for and how you can manage it [is important]. There are many examples of times when, even with some education, providers may not have communicated well to the patient. Then the patient is surprised and unhappy with the situation and unable to manage it.
The importance of education
Sheila, your comments on this from the patient perspective are so important. How important is the education piece, and how do you manage the fear of side effects vs actually managing the side effects that might be caused by the treatment you’re taking?
Ms. Pettiford: Thank you for that question. I really think it’s a dance. It’s a dance between the patient and the health care team. Yes, education is absolutely important. However, the health care professionals have to establish a relationship of trust with the patient. My own circumstances were that – and I was very fortunate in that my oncologist, who I chose just by looking and not by a recommendation – I did find an oncologist who listened to me.
When it came time for me to deal with a new medication, the education she provided me was sufficient because of the fact that there was a lot of listening that had gone on prior to the new medicine being given to me. I trusted what I was hearing, and it felt like there was a balanced situation that came about from what I was being told. I could look it up, too.
There still is that part of the patient who will be participating in the process, as well. They can still look up things, and that’s one of the downfalls of the information age we are in. It is a dance. I just want to go back to that. There’s a dance between the patient and the health care providers.
Dr. Rugo: Julia, from the patient’s side, how do you balance the benefits you might get from a treatment versus the side effects and how best to manage them?
Ms. Maués: I think it’s interesting that when we talk with our doctors, and especially when we read about a certain treatment, the attention is focused on the very severe and unlikely side effects that a drug has. We don’t talk as much about the side effects that are most likely to happen and will affect us but may not be life threatening.
Especially for those of us with metastatic cancer who are going to ideally be on a drug for a very long time, we’re then faced with low-grade nausea for the rest of our lives. That’s not okay either, right? I think it’s important to talk about all of the levels of toxicities and everything that can be done to avoid this.
Communication is key
Ms. Pettiford: I just want to add something that Dr. Cardoso said about monitoring that is absolutely important. We’re in a day and time when it’s very difficult to get someone on the telephone, but we do have digital charts and other ways that monitoring can take place. I was at a large teaching university, and I had to go monthly for my treatments. Every month, there were questions that were asked about my life and my condition. I could always get in contact with somebody through the digital chart.
Dr. Rugo: That’s an incredibly important comment, Sheila, about communication and how patients can feel like they have someone to go to in real time who can help manage things. Fatima, I’m interested in your comment on that.
Also, just to go to the next step, which is that when we see data reported on clinical trials and how the agent we’ve added or substituted is better than the standard, the toxicity tables are side effects that occur in at least 10% or more patients and sometimes even 20%. Then they’re graded, where often the division is grade 3 or greater. That may not actually reflect much about what the individual patient experience is. How do we interpret these data? Communication and interpretation?
Dr. Cardoso: Absolutely. I always call attention that perhaps, since we focus so much on grade 3 and 4 [side effects], that is the reason why we don’t see in the usual reporting differences quality of life between treatments. Quality of life is affected also significantly by grade 2 side effects. Or, like Julia was mentioning, even grade 1, if they are persistent, will eventually affect your quality of life.
Sometimes, like I was saying, don’t underestimate – it’s a little bit like that. We focus on explaining, “Look, this new immunotherapy can give you all these different side effects.” But then we forget to say: “Oh, by the way, it may also give you some nausea.” Actually, the nausea will affect the patient’s quality of life. I think that’s why it is so important to balance the way we provide the information.
I would like also to take on what Sheila said that sometimes too much information is not very helpful. That’s why sometimes we have to go stepwise. The first time you’re about to start the treatment, advise [the patient] on the most frequent side effects. Later on, you have time to say: “Okay, by the way, this can also give a rare side effect. This is what you should look for. If you have it, please contact your health care team.”
I think the most difficult part, at least from my experience, is for patients to understand what is really a sign of a severe side effect and what is normal for that type of treatment. Some of the new ways of communicating, like using some patient-reported outcome (PRO) apps, actually help the patient by saying, “This that you are feeling is normal. It can wait for your next appointment. This that you are feeling, it’s better if you try to reach your health care team right away. Or, this is an urgent thing and go to the emergency room near you.”
For this kind of triage, there are now new apps that can help. I think this is the most difficult part because when you are a patient, you don’t know if what you are feeling is actually a sign of something very severe or if it’s normal for the type of treatment you are receiving.
Dr. Rugo: I think that’s so important, and these new PRO apps may help with this. Of course, nothing substitutes for talking in the end if you’re confused or it doesn’t fit into whatever’s in that paradigm. I think it’s important.
Best practices in focusing on the individual patient
Julia, what do you think the best way of educating the patient is when you’re going to start a new treatment? You might be newly diagnosed with cancer or you might have had cancer for a number of years. You’re going to start a new treatment. What’s the best way to know what to look for and how to manage it?
Ms. Maués: I think the key here is that everyone’s different, so have that conversation, the doctor and the patient, about what the best way [of education] is for that specific person. Do they want a flyer listing all of the side effects? Do they want a link to a video they can watch and understand? Do they want someone to come in and give an extra explanation about things? Everyone learns so differently, and I think it’s really hard to assume there’s one way that all patients will understand.
I think the PRO apps are great, and also another benefit is that you keep track of your side effects. Sometimes we don’t even remember well. When did you have nausea? Was it in the morning? Was it in the evening? Is it every day? If you track it with these apps, then you will have the data stored there in the form to answer those questions.
Dr. Cardoso: There was recently a publication – I found it quite interesting – from Lesley Fallowfield’s group saying that the majority of patients would better absorb the information if it is not just text, but if it somehow has a video component, an image, or an infographic that would help them memorize a little bit more information.
Dr. Rugo: There’s been a move toward trying to make videos because the amount of education that’s needed on the providers’ side from our nurses and advanced practice providers may be overwhelming, so things might get missed. The idea of having videos to get everybody on the same page is very popular right now for this reason, and Lesley’s work is really groundbreaking.
Sheila, what do you think is the best way to communicate information?
Ms. Pettiford: Well, I definitely think it’s important for the doctors to recognize, as Julia said, that everyone is different, and all their patients are different. They could come with the same exact subtype of whatever cancer they have – in this situation, breast cancer – and still have so many different reactions. It’s so important for everybody on the health care team to listen to what the patient says because the patient is the one who is living with the illness and knows their body, hopefully.
It’s just one of those things. It’s not a one-size-fits-all situation. You give the standards, but I think it’s important to offer various ways of communicating to a patient because some people are visual. Some people want an overwhelming amount of information so they can sort through it. Then, you have some people who just want the bullet points. Again, it is important not to try to do it as a one-size-fits-all type thing.
Dr. Rugo: Yes, that’s such a good point. I’m always struck by the fact that some patients are totally on top of it and listen to it all, and then other people, we just can’t get them to even call in regarding their side effects. In some ways, it’s frightening for people to call in with issues. Maybe they’re afraid they won’t get the treatment, or that it is related to their cancer progressing, too. Trying to meet people on their own level is a real challenge and an important one.
We talked about education for providers. Fatima, how should we be best educating for these new drugs and new side effects? So many different manifestations can occur, and as we talked about, they might be quite uncommon. We just want people to keep their ears up for any kind of unusual toxicity we see. We all know that the presentation of efficacy data is not adequate for education.
Dr. Cardoso: When we present a new treatment, we focus usually on efficacy, right? Then we say a few things about safety, particularly if there is a new or a severe side effect, but we don’t go through details on how to best manage this in clinical practice.
Anecdotally, I remember that I contacted you because I was going to start using a new treatment and you had some experience. I asked, “What about nausea and vomiting? What do you do for prophylaxis?” I couldn’t find it anywhere in the manuscripts or the presentations. I think we need to focus a little bit more on practical tips. If you are about to start this new treatment, what you should think about and not just the very severe and rare side effects?
Of course, as health care professionals, we need to keep this in our minds. For example, with immunotherapy, side effects can often occur even after stopping the treatment. For other types of new treatments, we need to gain knowledge about endocrinology, for example, which is something that oncologists wouldn’t have to deal with that often in the past. Now, new skills are needed.
It’s also what makes our profession so exciting. There’s always something new to learn, and I like to look at it from that perspective. It’s not boring at all. We are always learning new things.
Dr. Rugo: Indeed. Certainly, you and I have worked together on trying to encourage our pharmaceutical colleagues to publish these papers alongside their urgency of distributing the efficacy data and publishing the papers on efficacy, and also to do a nitty-gritty review of safety and talk about management strategies. I’m really pleased that there seems to be a little more focus on that earlier now in the drug process – although still not early enough – but it’s getting there. That’s a good thing.
Ms. Pettiford: Julia, you mentioned earlier how important it is for the individual patient’s quality of life to understand how these side effects can affect them. It really is one of those things in which we have to make personal decisions. What might be good for one person in terms of what happens with side effects, and their ability to function might not work with someone else.
If you are a person who’s dealing with metastatic disease who has children, a household, a dog, and a cat to take care of, what I can handle being that I’m a single person is not what they can handle. That’s all a part of the education piece. That’s all part of the teamwork. That’s all part of the communication process. It all comes into play.
Dr. Rugo: That’s such an incredibly important point. As we’re wrapping up, it would be great if everybody had some points to make that pulled together some of our conversation. Julia, do you want to start?
Ms. Maués: Yes, I was going to add specifically about the topic you were just discussing, with all that an oncologist’s team has to know and all the different areas of our health being affected by these new treatments. One tip for patients and their teams is that the other providers around the patients may not be as informed about the disease and the treatments they are on. Sometimes we patients end up getting information that isn’t up to date with the latest drugs and things like that.
When we do talk with someone about our issues, make sure they are informed about the new drugs. For example, we often have skin issues. There are dermatologists that work with cancer patients often, and they’re very informed about the side effects that come with these drugs. There are others who never see these sorts of issues and may assume it’s something completely different.
I usually just go to doctors that my oncologist’s team collaborates with and gets referrals from because they send their patients to these doctors often. These are doctors that see cancer patients. We’re a very unique group.
Dr. Rugo: That’s a really good point. I have the same thing. We all have a little stable of people we refer to for various issues that we can reach on speed dial.
The importance of diversity in clinical trials to obtain the most useful outcomes
Fatima, there’s recently been, appropriately so, more of a push to try and evaluate side effects by racial and ethnic subgroups. I think we’re still pretty crummy at it, but we are making some progress. How important is that to you when you think about patients and managing them?
Dr. Cardoso: I think this is quite important. One area of research that is underused, really, is all the new genomics and sequencing technologies to understand why people react differently to the same treatment. Why is it that for some people, either for ethnic or other reasons, you have a different metabolism or something else that justifies a very high rate of side effects from a certain treatment, whereas in other regions of the world this doesn’t happen?
Not to go into these new drugs, but when using a very old drug like a taxane, I found a difference in reaction between the Portuguese patients and the Belgian patients, the two countries where I’ve worked. I even found that the cause might be genetic because the Portuguese living in Belgium reacted differently than the Belgians themselves.
Maybe there is something in the genetics that justifies the type of side effects that you have. I make a plea also for us to dedicate research to understanding why certain side effects are related to race and others are related to maybe some other types of genetic alterations that will lead to an increased side effect.
Dr. Rugo: Sheila, comments?
Ms. Pettiford: That is just excellent. It’s excellent to even consider it because it is so obvious. To me, it’s an obvious situation because there are things that are underneath the skin that we don’t understand. We have to take that into consideration when we are dealing with all these wonderful – I call them miracle – drugs that have come about in the last 20 years.
There still is much more to be done, and I try to participate in any type of organization that’s encouraging diversity in clinical trials because you need to have people of all different ethnicities in order for us to get to these answers. It’s fascinating that you found this out, doctor.
The patient-centered dosing initiative
Ms. Maués: I have the pleasure of being a member of a patient-led initiative called the Patient-Centered Dosing Initiative (PCDI). We are highlighting the discussion around dosages of drugs, especially in the metastatic setting. Metastatic breast cancer is what we’re focusing on, although it could apply to any type of cancer. We are advised by a number of wonderful, world-renowned physicians, Dr. Rugo being one of them. Anne Loeser, the leader of our group, has spoken at ASCO about this topic of dosage. What we’re seeing is that the dosage determination for oncology drugs is still done in the same way it used to be done decades ago and mostly with the curative intent of early-stage disease — metastatic cancer patients back then really didn’t live long at all. What we’re seeing right now is people with metastatic breast cancer that are able to, in some cases, live a long life managing their disease.
Patients are put on doses that are too high for them to be able to manage the side effects, and then they end up having to go off the drug, which means they have lost one of the tools in their toolbox. So, what we like to say about dosing is that, for metastatic cancer patients, it’s a marathon and not a sprint. If we throw all the poison at the patient from the very beginning, they won’t be able to take this for a very long time. And in the metastatic setting, the goal is to stay on each therapy for as long as possible. If we burn one of the cards early on, you have to move on to the next one. This is finite because at some point, there are not enough drugs that can help a particular patient. The PCDI is really getting a lot of visibility with the FDA and experts. People are talking more about dosages, and the FDA is now providing guidance for pharmaceutical companies to study different dosages in the clinical trials from the very beginning. This initiative is almost 3 years old, and we have made a tremendous impact since then.
Dr. Rugo: I think this is an incredibly important area moving forward, and thankfully, there’s so much interest now in not only promoting diversity, enrollment in trials, and education to promote diversity but also in looking at differences in efficacy and side effects.
I’ll just thank everybody for your contributions and amazing perspectives in this incredibly important area. As we move forward with better agents, we need to also make sure we’re understanding what the side effects are, managing them, and hearing the voices of our patients. Thanks very much.
Ms. Pettiford: Thank you so much.
Dr. Cardoso: Thank you.
Ms. Maués: Thank you.
Editor’s note: Our panelists would like to highlight these points:
- The patient and the health care team must build trust with each other.
- African Americans have historical reasons for not trusting the health care industry. Much outreach is still needed.
- Inform and educate before the start of treatment and during the treatment.
- Be balanced and do not underestimate common side effects or overestimate rare ones. Adapt the amount and the detail of the information to the wishes of the individual patient. Offer various methods of delivery (e.g., videos, pamphlets, fact sheets).
- Patients will research their condition and treatments online. Instead of trying to stop this, help them find the best sources.
- Patients will connect with others in the patient community and learn from each other’s experiences. Keep in mind that everyone is different, and decisions should always be made together with the medical team.
- Monitor patients regularly, especially during the first few treatment cycles.
- Use different forms of communication between the patient and health care providers (e.g., apps, digital charts, oncology nurses/nurse navigators, responsive oncologists, different forms of telemedicine), but don’t forget to speak directly with the patient.
- The use of new PRO apps can be very useful to help patients differentiate between urgent and nonurgent signs and symptoms.
- As much as possible, use preventive/prophylactic measures, namely for nausea, vomiting, diarrhea/constipation, and mucositis.
- Be aware of late side effects, especially with immunotherapy.
- Don’t forget that grade 1-2 side effects can substantially impact quality of life, particularly if they are persistent.
- Consider quality-of-life issues for each patient. What is acceptable for one patient may not be for another.
- Learn how to manage new and specific side effects (e.g., endocrine, skin related, pneumonitis).
- Keep an open dialogue about treatment and side effects. Things can change, and there are different ways to address issues such as medications for side effects and dosing changes.
- Listen to your patient and respond in a timely fashion.
- Ethnicity and genetics should be studied as a factor for individual side effects. Standard industry dosages of a new anticancer medication might not be as effective in one ethnic group as another due to the lack of diversity in clinical trials.
- Medications with hard-to-manage or dangerous side effects may be counterproductive regardless of effectiveness.
- Cancer treatment varies vastly depending on region and type of treatment facility. There are many unmet needs in rural areas because of lack of oncology personnel, finances, transportation, etc.
Dr. Rugo is a professor in the department of medicine, University of California San Francisco Comprehensive Cancer Center; director, Breast Oncology and Clinical Trials Education, Cancer Infusion Services, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco. Dr. Cardoso is director, breast unit, Champalimaud Clinical Centre, Lisbon. Financial disclosures for both Dr. Rugo and Dr. Cardoso are available on Medscape.com, where this article first appeared. Julia Maués is a patient in Washington. She has disclosed no relevant financial relationships. Sheila Pettiford is a patient in Middletown, Del. She has disclosed no relevant financial relationships.
This transcript of a video roundtable, which is available on Medscape.com, has been edited for clarity.
Hope S. Rugo, MD: Hello. I’m Hope Rugo, a breast medical oncologist from the University of California, San Francisco. I’m joined here by three of my friends and colleagues to discuss the toxicity of new agents in the treatment of breast cancer. Fatima, do you want to start by introducing yourself?
Fatima F. Cardoso, MD: Sure. Hello, everyone. I’m Fatima Cardoso, a breast medical oncologist in Lisbon, Portugal.
Dr. Rugo: Sheila.
Sheila Pettiford: Hi, I’m Sheila Pettiford. I am a metastatic [breast cancer] patient and have been for almost 8 years in April. I used to live in Philadelphia, Pennsylvania, but moved to Delaware in the last couple of years during the pandemic. I’m happy to be here.
Dr. Rugo: Julia.
Julia Maués: Hi, everyone. I also am a person living with metastatic breast cancer. I was diagnosed in 2013, so it’s going to be 9 years, also in April.
Effective monitoring and management of side effects: A team effort
Dr. Rugo: We have an amazing group and an international representation, which is also really nice because we get different perspectives. What we’re going to talk about is important to providers and patients across the board. With the host of new agents for the treatment of breast cancer – most of which have really moved us forward in terms of having effective treatment options – we’ve also been faced with a lot of new toxicities or side effects that we haven’t seen before or that we might not have expected from the specific agent.
Those toxicities across the board include side effects that are quite familiar to us, like low blood counts, but we may not advise people well enough about other side effects such as mouth sores, inflammation of the lungs, immune toxicities, and skin toxicities.
Fatima, do you want to start and talk about how we can think about these toxicities and address them?
Dr. Cardoso: Sure. Thank you. From the health care provider point of view, what I would highlight is to educate. Educate before we start the treatment. It’s very important to inform the patient but in a balanced way, so we don’t overexaggerate certain types of side effects or underestimate certain types of side effects.
It’s very important because an informed patient will be attentive to the types of side effects that can happen. Also, teach the patient when it is a [cause for] alarm or something for which they might need to contact their health care team and when that’s not the case. I think this is one crucial topic.
The other one is to monitor. Find ways how to best communicate between the patient and the health care team but in a way that you can monitor, so you can act very early on. Most of these new side effects, if you act early on, will not become severe. It is very important to know about them and to act early on.
I believe there is something important that we don’t think about all the time, and that is prophylaxis. Do not be shy about using prophylactic measures, be it for the mouth sores, nausea and vomiting, diarrhea, and other things that really impact the quality of life of patients. Those, to start, are my three major points of attention for health care professionals.
Dr. Rugo: I think that’s so incredibly important – the comments that you’ve made – and also that prevention and prophylaxis are so important. You don’t want to have a patient have diarrhea in the middle of the night and not have any antipropulsive agents at home. Just as a very straightforward example, it’s really important.
Also, the ability to know what you should be looking for and how you can manage it [is important]. There are many examples of times when, even with some education, providers may not have communicated well to the patient. Then the patient is surprised and unhappy with the situation and unable to manage it.
The importance of education
Sheila, your comments on this from the patient perspective are so important. How important is the education piece, and how do you manage the fear of side effects vs actually managing the side effects that might be caused by the treatment you’re taking?
Ms. Pettiford: Thank you for that question. I really think it’s a dance. It’s a dance between the patient and the health care team. Yes, education is absolutely important. However, the health care professionals have to establish a relationship of trust with the patient. My own circumstances were that – and I was very fortunate in that my oncologist, who I chose just by looking and not by a recommendation – I did find an oncologist who listened to me.
When it came time for me to deal with a new medication, the education she provided me was sufficient because of the fact that there was a lot of listening that had gone on prior to the new medicine being given to me. I trusted what I was hearing, and it felt like there was a balanced situation that came about from what I was being told. I could look it up, too.
There still is that part of the patient who will be participating in the process, as well. They can still look up things, and that’s one of the downfalls of the information age we are in. It is a dance. I just want to go back to that. There’s a dance between the patient and the health care providers.
Dr. Rugo: Julia, from the patient’s side, how do you balance the benefits you might get from a treatment versus the side effects and how best to manage them?
Ms. Maués: I think it’s interesting that when we talk with our doctors, and especially when we read about a certain treatment, the attention is focused on the very severe and unlikely side effects that a drug has. We don’t talk as much about the side effects that are most likely to happen and will affect us but may not be life threatening.
Especially for those of us with metastatic cancer who are going to ideally be on a drug for a very long time, we’re then faced with low-grade nausea for the rest of our lives. That’s not okay either, right? I think it’s important to talk about all of the levels of toxicities and everything that can be done to avoid this.
Communication is key
Ms. Pettiford: I just want to add something that Dr. Cardoso said about monitoring that is absolutely important. We’re in a day and time when it’s very difficult to get someone on the telephone, but we do have digital charts and other ways that monitoring can take place. I was at a large teaching university, and I had to go monthly for my treatments. Every month, there were questions that were asked about my life and my condition. I could always get in contact with somebody through the digital chart.
Dr. Rugo: That’s an incredibly important comment, Sheila, about communication and how patients can feel like they have someone to go to in real time who can help manage things. Fatima, I’m interested in your comment on that.
Also, just to go to the next step, which is that when we see data reported on clinical trials and how the agent we’ve added or substituted is better than the standard, the toxicity tables are side effects that occur in at least 10% or more patients and sometimes even 20%. Then they’re graded, where often the division is grade 3 or greater. That may not actually reflect much about what the individual patient experience is. How do we interpret these data? Communication and interpretation?
Dr. Cardoso: Absolutely. I always call attention that perhaps, since we focus so much on grade 3 and 4 [side effects], that is the reason why we don’t see in the usual reporting differences quality of life between treatments. Quality of life is affected also significantly by grade 2 side effects. Or, like Julia was mentioning, even grade 1, if they are persistent, will eventually affect your quality of life.
Sometimes, like I was saying, don’t underestimate – it’s a little bit like that. We focus on explaining, “Look, this new immunotherapy can give you all these different side effects.” But then we forget to say: “Oh, by the way, it may also give you some nausea.” Actually, the nausea will affect the patient’s quality of life. I think that’s why it is so important to balance the way we provide the information.
I would like also to take on what Sheila said that sometimes too much information is not very helpful. That’s why sometimes we have to go stepwise. The first time you’re about to start the treatment, advise [the patient] on the most frequent side effects. Later on, you have time to say: “Okay, by the way, this can also give a rare side effect. This is what you should look for. If you have it, please contact your health care team.”
I think the most difficult part, at least from my experience, is for patients to understand what is really a sign of a severe side effect and what is normal for that type of treatment. Some of the new ways of communicating, like using some patient-reported outcome (PRO) apps, actually help the patient by saying, “This that you are feeling is normal. It can wait for your next appointment. This that you are feeling, it’s better if you try to reach your health care team right away. Or, this is an urgent thing and go to the emergency room near you.”
For this kind of triage, there are now new apps that can help. I think this is the most difficult part because when you are a patient, you don’t know if what you are feeling is actually a sign of something very severe or if it’s normal for the type of treatment you are receiving.
Dr. Rugo: I think that’s so important, and these new PRO apps may help with this. Of course, nothing substitutes for talking in the end if you’re confused or it doesn’t fit into whatever’s in that paradigm. I think it’s important.
Best practices in focusing on the individual patient
Julia, what do you think the best way of educating the patient is when you’re going to start a new treatment? You might be newly diagnosed with cancer or you might have had cancer for a number of years. You’re going to start a new treatment. What’s the best way to know what to look for and how to manage it?
Ms. Maués: I think the key here is that everyone’s different, so have that conversation, the doctor and the patient, about what the best way [of education] is for that specific person. Do they want a flyer listing all of the side effects? Do they want a link to a video they can watch and understand? Do they want someone to come in and give an extra explanation about things? Everyone learns so differently, and I think it’s really hard to assume there’s one way that all patients will understand.
I think the PRO apps are great, and also another benefit is that you keep track of your side effects. Sometimes we don’t even remember well. When did you have nausea? Was it in the morning? Was it in the evening? Is it every day? If you track it with these apps, then you will have the data stored there in the form to answer those questions.
Dr. Cardoso: There was recently a publication – I found it quite interesting – from Lesley Fallowfield’s group saying that the majority of patients would better absorb the information if it is not just text, but if it somehow has a video component, an image, or an infographic that would help them memorize a little bit more information.
Dr. Rugo: There’s been a move toward trying to make videos because the amount of education that’s needed on the providers’ side from our nurses and advanced practice providers may be overwhelming, so things might get missed. The idea of having videos to get everybody on the same page is very popular right now for this reason, and Lesley’s work is really groundbreaking.
Sheila, what do you think is the best way to communicate information?
Ms. Pettiford: Well, I definitely think it’s important for the doctors to recognize, as Julia said, that everyone is different, and all their patients are different. They could come with the same exact subtype of whatever cancer they have – in this situation, breast cancer – and still have so many different reactions. It’s so important for everybody on the health care team to listen to what the patient says because the patient is the one who is living with the illness and knows their body, hopefully.
It’s just one of those things. It’s not a one-size-fits-all situation. You give the standards, but I think it’s important to offer various ways of communicating to a patient because some people are visual. Some people want an overwhelming amount of information so they can sort through it. Then, you have some people who just want the bullet points. Again, it is important not to try to do it as a one-size-fits-all type thing.
Dr. Rugo: Yes, that’s such a good point. I’m always struck by the fact that some patients are totally on top of it and listen to it all, and then other people, we just can’t get them to even call in regarding their side effects. In some ways, it’s frightening for people to call in with issues. Maybe they’re afraid they won’t get the treatment, or that it is related to their cancer progressing, too. Trying to meet people on their own level is a real challenge and an important one.
We talked about education for providers. Fatima, how should we be best educating for these new drugs and new side effects? So many different manifestations can occur, and as we talked about, they might be quite uncommon. We just want people to keep their ears up for any kind of unusual toxicity we see. We all know that the presentation of efficacy data is not adequate for education.
Dr. Cardoso: When we present a new treatment, we focus usually on efficacy, right? Then we say a few things about safety, particularly if there is a new or a severe side effect, but we don’t go through details on how to best manage this in clinical practice.
Anecdotally, I remember that I contacted you because I was going to start using a new treatment and you had some experience. I asked, “What about nausea and vomiting? What do you do for prophylaxis?” I couldn’t find it anywhere in the manuscripts or the presentations. I think we need to focus a little bit more on practical tips. If you are about to start this new treatment, what you should think about and not just the very severe and rare side effects?
Of course, as health care professionals, we need to keep this in our minds. For example, with immunotherapy, side effects can often occur even after stopping the treatment. For other types of new treatments, we need to gain knowledge about endocrinology, for example, which is something that oncologists wouldn’t have to deal with that often in the past. Now, new skills are needed.
It’s also what makes our profession so exciting. There’s always something new to learn, and I like to look at it from that perspective. It’s not boring at all. We are always learning new things.
Dr. Rugo: Indeed. Certainly, you and I have worked together on trying to encourage our pharmaceutical colleagues to publish these papers alongside their urgency of distributing the efficacy data and publishing the papers on efficacy, and also to do a nitty-gritty review of safety and talk about management strategies. I’m really pleased that there seems to be a little more focus on that earlier now in the drug process – although still not early enough – but it’s getting there. That’s a good thing.
Ms. Pettiford: Julia, you mentioned earlier how important it is for the individual patient’s quality of life to understand how these side effects can affect them. It really is one of those things in which we have to make personal decisions. What might be good for one person in terms of what happens with side effects, and their ability to function might not work with someone else.
If you are a person who’s dealing with metastatic disease who has children, a household, a dog, and a cat to take care of, what I can handle being that I’m a single person is not what they can handle. That’s all a part of the education piece. That’s all part of the teamwork. That’s all part of the communication process. It all comes into play.
Dr. Rugo: That’s such an incredibly important point. As we’re wrapping up, it would be great if everybody had some points to make that pulled together some of our conversation. Julia, do you want to start?
Ms. Maués: Yes, I was going to add specifically about the topic you were just discussing, with all that an oncologist’s team has to know and all the different areas of our health being affected by these new treatments. One tip for patients and their teams is that the other providers around the patients may not be as informed about the disease and the treatments they are on. Sometimes we patients end up getting information that isn’t up to date with the latest drugs and things like that.
When we do talk with someone about our issues, make sure they are informed about the new drugs. For example, we often have skin issues. There are dermatologists that work with cancer patients often, and they’re very informed about the side effects that come with these drugs. There are others who never see these sorts of issues and may assume it’s something completely different.
I usually just go to doctors that my oncologist’s team collaborates with and gets referrals from because they send their patients to these doctors often. These are doctors that see cancer patients. We’re a very unique group.
Dr. Rugo: That’s a really good point. I have the same thing. We all have a little stable of people we refer to for various issues that we can reach on speed dial.
The importance of diversity in clinical trials to obtain the most useful outcomes
Fatima, there’s recently been, appropriately so, more of a push to try and evaluate side effects by racial and ethnic subgroups. I think we’re still pretty crummy at it, but we are making some progress. How important is that to you when you think about patients and managing them?
Dr. Cardoso: I think this is quite important. One area of research that is underused, really, is all the new genomics and sequencing technologies to understand why people react differently to the same treatment. Why is it that for some people, either for ethnic or other reasons, you have a different metabolism or something else that justifies a very high rate of side effects from a certain treatment, whereas in other regions of the world this doesn’t happen?
Not to go into these new drugs, but when using a very old drug like a taxane, I found a difference in reaction between the Portuguese patients and the Belgian patients, the two countries where I’ve worked. I even found that the cause might be genetic because the Portuguese living in Belgium reacted differently than the Belgians themselves.
Maybe there is something in the genetics that justifies the type of side effects that you have. I make a plea also for us to dedicate research to understanding why certain side effects are related to race and others are related to maybe some other types of genetic alterations that will lead to an increased side effect.
Dr. Rugo: Sheila, comments?
Ms. Pettiford: That is just excellent. It’s excellent to even consider it because it is so obvious. To me, it’s an obvious situation because there are things that are underneath the skin that we don’t understand. We have to take that into consideration when we are dealing with all these wonderful – I call them miracle – drugs that have come about in the last 20 years.
There still is much more to be done, and I try to participate in any type of organization that’s encouraging diversity in clinical trials because you need to have people of all different ethnicities in order for us to get to these answers. It’s fascinating that you found this out, doctor.
The patient-centered dosing initiative
Ms. Maués: I have the pleasure of being a member of a patient-led initiative called the Patient-Centered Dosing Initiative (PCDI). We are highlighting the discussion around dosages of drugs, especially in the metastatic setting. Metastatic breast cancer is what we’re focusing on, although it could apply to any type of cancer. We are advised by a number of wonderful, world-renowned physicians, Dr. Rugo being one of them. Anne Loeser, the leader of our group, has spoken at ASCO about this topic of dosage. What we’re seeing is that the dosage determination for oncology drugs is still done in the same way it used to be done decades ago and mostly with the curative intent of early-stage disease — metastatic cancer patients back then really didn’t live long at all. What we’re seeing right now is people with metastatic breast cancer that are able to, in some cases, live a long life managing their disease.
Patients are put on doses that are too high for them to be able to manage the side effects, and then they end up having to go off the drug, which means they have lost one of the tools in their toolbox. So, what we like to say about dosing is that, for metastatic cancer patients, it’s a marathon and not a sprint. If we throw all the poison at the patient from the very beginning, they won’t be able to take this for a very long time. And in the metastatic setting, the goal is to stay on each therapy for as long as possible. If we burn one of the cards early on, you have to move on to the next one. This is finite because at some point, there are not enough drugs that can help a particular patient. The PCDI is really getting a lot of visibility with the FDA and experts. People are talking more about dosages, and the FDA is now providing guidance for pharmaceutical companies to study different dosages in the clinical trials from the very beginning. This initiative is almost 3 years old, and we have made a tremendous impact since then.
Dr. Rugo: I think this is an incredibly important area moving forward, and thankfully, there’s so much interest now in not only promoting diversity, enrollment in trials, and education to promote diversity but also in looking at differences in efficacy and side effects.
I’ll just thank everybody for your contributions and amazing perspectives in this incredibly important area. As we move forward with better agents, we need to also make sure we’re understanding what the side effects are, managing them, and hearing the voices of our patients. Thanks very much.
Ms. Pettiford: Thank you so much.
Dr. Cardoso: Thank you.
Ms. Maués: Thank you.
Editor’s note: Our panelists would like to highlight these points:
- The patient and the health care team must build trust with each other.
- African Americans have historical reasons for not trusting the health care industry. Much outreach is still needed.
- Inform and educate before the start of treatment and during the treatment.
- Be balanced and do not underestimate common side effects or overestimate rare ones. Adapt the amount and the detail of the information to the wishes of the individual patient. Offer various methods of delivery (e.g., videos, pamphlets, fact sheets).
- Patients will research their condition and treatments online. Instead of trying to stop this, help them find the best sources.
- Patients will connect with others in the patient community and learn from each other’s experiences. Keep in mind that everyone is different, and decisions should always be made together with the medical team.
- Monitor patients regularly, especially during the first few treatment cycles.
- Use different forms of communication between the patient and health care providers (e.g., apps, digital charts, oncology nurses/nurse navigators, responsive oncologists, different forms of telemedicine), but don’t forget to speak directly with the patient.
- The use of new PRO apps can be very useful to help patients differentiate between urgent and nonurgent signs and symptoms.
- As much as possible, use preventive/prophylactic measures, namely for nausea, vomiting, diarrhea/constipation, and mucositis.
- Be aware of late side effects, especially with immunotherapy.
- Don’t forget that grade 1-2 side effects can substantially impact quality of life, particularly if they are persistent.
- Consider quality-of-life issues for each patient. What is acceptable for one patient may not be for another.
- Learn how to manage new and specific side effects (e.g., endocrine, skin related, pneumonitis).
- Keep an open dialogue about treatment and side effects. Things can change, and there are different ways to address issues such as medications for side effects and dosing changes.
- Listen to your patient and respond in a timely fashion.
- Ethnicity and genetics should be studied as a factor for individual side effects. Standard industry dosages of a new anticancer medication might not be as effective in one ethnic group as another due to the lack of diversity in clinical trials.
- Medications with hard-to-manage or dangerous side effects may be counterproductive regardless of effectiveness.
- Cancer treatment varies vastly depending on region and type of treatment facility. There are many unmet needs in rural areas because of lack of oncology personnel, finances, transportation, etc.
Dr. Rugo is a professor in the department of medicine, University of California San Francisco Comprehensive Cancer Center; director, Breast Oncology and Clinical Trials Education, Cancer Infusion Services, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco. Dr. Cardoso is director, breast unit, Champalimaud Clinical Centre, Lisbon. Financial disclosures for both Dr. Rugo and Dr. Cardoso are available on Medscape.com, where this article first appeared. Julia Maués is a patient in Washington. She has disclosed no relevant financial relationships. Sheila Pettiford is a patient in Middletown, Del. She has disclosed no relevant financial relationships.
This transcript of a video roundtable, which is available on Medscape.com, has been edited for clarity.
Hope S. Rugo, MD: Hello. I’m Hope Rugo, a breast medical oncologist from the University of California, San Francisco. I’m joined here by three of my friends and colleagues to discuss the toxicity of new agents in the treatment of breast cancer. Fatima, do you want to start by introducing yourself?
Fatima F. Cardoso, MD: Sure. Hello, everyone. I’m Fatima Cardoso, a breast medical oncologist in Lisbon, Portugal.
Dr. Rugo: Sheila.
Sheila Pettiford: Hi, I’m Sheila Pettiford. I am a metastatic [breast cancer] patient and have been for almost 8 years in April. I used to live in Philadelphia, Pennsylvania, but moved to Delaware in the last couple of years during the pandemic. I’m happy to be here.
Dr. Rugo: Julia.
Julia Maués: Hi, everyone. I also am a person living with metastatic breast cancer. I was diagnosed in 2013, so it’s going to be 9 years, also in April.
Effective monitoring and management of side effects: A team effort
Dr. Rugo: We have an amazing group and an international representation, which is also really nice because we get different perspectives. What we’re going to talk about is important to providers and patients across the board. With the host of new agents for the treatment of breast cancer – most of which have really moved us forward in terms of having effective treatment options – we’ve also been faced with a lot of new toxicities or side effects that we haven’t seen before or that we might not have expected from the specific agent.
Those toxicities across the board include side effects that are quite familiar to us, like low blood counts, but we may not advise people well enough about other side effects such as mouth sores, inflammation of the lungs, immune toxicities, and skin toxicities.
Fatima, do you want to start and talk about how we can think about these toxicities and address them?
Dr. Cardoso: Sure. Thank you. From the health care provider point of view, what I would highlight is to educate. Educate before we start the treatment. It’s very important to inform the patient but in a balanced way, so we don’t overexaggerate certain types of side effects or underestimate certain types of side effects.
It’s very important because an informed patient will be attentive to the types of side effects that can happen. Also, teach the patient when it is a [cause for] alarm or something for which they might need to contact their health care team and when that’s not the case. I think this is one crucial topic.
The other one is to monitor. Find ways how to best communicate between the patient and the health care team but in a way that you can monitor, so you can act very early on. Most of these new side effects, if you act early on, will not become severe. It is very important to know about them and to act early on.
I believe there is something important that we don’t think about all the time, and that is prophylaxis. Do not be shy about using prophylactic measures, be it for the mouth sores, nausea and vomiting, diarrhea, and other things that really impact the quality of life of patients. Those, to start, are my three major points of attention for health care professionals.
Dr. Rugo: I think that’s so incredibly important – the comments that you’ve made – and also that prevention and prophylaxis are so important. You don’t want to have a patient have diarrhea in the middle of the night and not have any antipropulsive agents at home. Just as a very straightforward example, it’s really important.
Also, the ability to know what you should be looking for and how you can manage it [is important]. There are many examples of times when, even with some education, providers may not have communicated well to the patient. Then the patient is surprised and unhappy with the situation and unable to manage it.
The importance of education
Sheila, your comments on this from the patient perspective are so important. How important is the education piece, and how do you manage the fear of side effects vs actually managing the side effects that might be caused by the treatment you’re taking?
Ms. Pettiford: Thank you for that question. I really think it’s a dance. It’s a dance between the patient and the health care team. Yes, education is absolutely important. However, the health care professionals have to establish a relationship of trust with the patient. My own circumstances were that – and I was very fortunate in that my oncologist, who I chose just by looking and not by a recommendation – I did find an oncologist who listened to me.
When it came time for me to deal with a new medication, the education she provided me was sufficient because of the fact that there was a lot of listening that had gone on prior to the new medicine being given to me. I trusted what I was hearing, and it felt like there was a balanced situation that came about from what I was being told. I could look it up, too.
There still is that part of the patient who will be participating in the process, as well. They can still look up things, and that’s one of the downfalls of the information age we are in. It is a dance. I just want to go back to that. There’s a dance between the patient and the health care providers.
Dr. Rugo: Julia, from the patient’s side, how do you balance the benefits you might get from a treatment versus the side effects and how best to manage them?
Ms. Maués: I think it’s interesting that when we talk with our doctors, and especially when we read about a certain treatment, the attention is focused on the very severe and unlikely side effects that a drug has. We don’t talk as much about the side effects that are most likely to happen and will affect us but may not be life threatening.
Especially for those of us with metastatic cancer who are going to ideally be on a drug for a very long time, we’re then faced with low-grade nausea for the rest of our lives. That’s not okay either, right? I think it’s important to talk about all of the levels of toxicities and everything that can be done to avoid this.
Communication is key
Ms. Pettiford: I just want to add something that Dr. Cardoso said about monitoring that is absolutely important. We’re in a day and time when it’s very difficult to get someone on the telephone, but we do have digital charts and other ways that monitoring can take place. I was at a large teaching university, and I had to go monthly for my treatments. Every month, there were questions that were asked about my life and my condition. I could always get in contact with somebody through the digital chart.
Dr. Rugo: That’s an incredibly important comment, Sheila, about communication and how patients can feel like they have someone to go to in real time who can help manage things. Fatima, I’m interested in your comment on that.
Also, just to go to the next step, which is that when we see data reported on clinical trials and how the agent we’ve added or substituted is better than the standard, the toxicity tables are side effects that occur in at least 10% or more patients and sometimes even 20%. Then they’re graded, where often the division is grade 3 or greater. That may not actually reflect much about what the individual patient experience is. How do we interpret these data? Communication and interpretation?
Dr. Cardoso: Absolutely. I always call attention that perhaps, since we focus so much on grade 3 and 4 [side effects], that is the reason why we don’t see in the usual reporting differences quality of life between treatments. Quality of life is affected also significantly by grade 2 side effects. Or, like Julia was mentioning, even grade 1, if they are persistent, will eventually affect your quality of life.
Sometimes, like I was saying, don’t underestimate – it’s a little bit like that. We focus on explaining, “Look, this new immunotherapy can give you all these different side effects.” But then we forget to say: “Oh, by the way, it may also give you some nausea.” Actually, the nausea will affect the patient’s quality of life. I think that’s why it is so important to balance the way we provide the information.
I would like also to take on what Sheila said that sometimes too much information is not very helpful. That’s why sometimes we have to go stepwise. The first time you’re about to start the treatment, advise [the patient] on the most frequent side effects. Later on, you have time to say: “Okay, by the way, this can also give a rare side effect. This is what you should look for. If you have it, please contact your health care team.”
I think the most difficult part, at least from my experience, is for patients to understand what is really a sign of a severe side effect and what is normal for that type of treatment. Some of the new ways of communicating, like using some patient-reported outcome (PRO) apps, actually help the patient by saying, “This that you are feeling is normal. It can wait for your next appointment. This that you are feeling, it’s better if you try to reach your health care team right away. Or, this is an urgent thing and go to the emergency room near you.”
For this kind of triage, there are now new apps that can help. I think this is the most difficult part because when you are a patient, you don’t know if what you are feeling is actually a sign of something very severe or if it’s normal for the type of treatment you are receiving.
Dr. Rugo: I think that’s so important, and these new PRO apps may help with this. Of course, nothing substitutes for talking in the end if you’re confused or it doesn’t fit into whatever’s in that paradigm. I think it’s important.
Best practices in focusing on the individual patient
Julia, what do you think the best way of educating the patient is when you’re going to start a new treatment? You might be newly diagnosed with cancer or you might have had cancer for a number of years. You’re going to start a new treatment. What’s the best way to know what to look for and how to manage it?
Ms. Maués: I think the key here is that everyone’s different, so have that conversation, the doctor and the patient, about what the best way [of education] is for that specific person. Do they want a flyer listing all of the side effects? Do they want a link to a video they can watch and understand? Do they want someone to come in and give an extra explanation about things? Everyone learns so differently, and I think it’s really hard to assume there’s one way that all patients will understand.
I think the PRO apps are great, and also another benefit is that you keep track of your side effects. Sometimes we don’t even remember well. When did you have nausea? Was it in the morning? Was it in the evening? Is it every day? If you track it with these apps, then you will have the data stored there in the form to answer those questions.
Dr. Cardoso: There was recently a publication – I found it quite interesting – from Lesley Fallowfield’s group saying that the majority of patients would better absorb the information if it is not just text, but if it somehow has a video component, an image, or an infographic that would help them memorize a little bit more information.
Dr. Rugo: There’s been a move toward trying to make videos because the amount of education that’s needed on the providers’ side from our nurses and advanced practice providers may be overwhelming, so things might get missed. The idea of having videos to get everybody on the same page is very popular right now for this reason, and Lesley’s work is really groundbreaking.
Sheila, what do you think is the best way to communicate information?
Ms. Pettiford: Well, I definitely think it’s important for the doctors to recognize, as Julia said, that everyone is different, and all their patients are different. They could come with the same exact subtype of whatever cancer they have – in this situation, breast cancer – and still have so many different reactions. It’s so important for everybody on the health care team to listen to what the patient says because the patient is the one who is living with the illness and knows their body, hopefully.
It’s just one of those things. It’s not a one-size-fits-all situation. You give the standards, but I think it’s important to offer various ways of communicating to a patient because some people are visual. Some people want an overwhelming amount of information so they can sort through it. Then, you have some people who just want the bullet points. Again, it is important not to try to do it as a one-size-fits-all type thing.
Dr. Rugo: Yes, that’s such a good point. I’m always struck by the fact that some patients are totally on top of it and listen to it all, and then other people, we just can’t get them to even call in regarding their side effects. In some ways, it’s frightening for people to call in with issues. Maybe they’re afraid they won’t get the treatment, or that it is related to their cancer progressing, too. Trying to meet people on their own level is a real challenge and an important one.
We talked about education for providers. Fatima, how should we be best educating for these new drugs and new side effects? So many different manifestations can occur, and as we talked about, they might be quite uncommon. We just want people to keep their ears up for any kind of unusual toxicity we see. We all know that the presentation of efficacy data is not adequate for education.
Dr. Cardoso: When we present a new treatment, we focus usually on efficacy, right? Then we say a few things about safety, particularly if there is a new or a severe side effect, but we don’t go through details on how to best manage this in clinical practice.
Anecdotally, I remember that I contacted you because I was going to start using a new treatment and you had some experience. I asked, “What about nausea and vomiting? What do you do for prophylaxis?” I couldn’t find it anywhere in the manuscripts or the presentations. I think we need to focus a little bit more on practical tips. If you are about to start this new treatment, what you should think about and not just the very severe and rare side effects?
Of course, as health care professionals, we need to keep this in our minds. For example, with immunotherapy, side effects can often occur even after stopping the treatment. For other types of new treatments, we need to gain knowledge about endocrinology, for example, which is something that oncologists wouldn’t have to deal with that often in the past. Now, new skills are needed.
It’s also what makes our profession so exciting. There’s always something new to learn, and I like to look at it from that perspective. It’s not boring at all. We are always learning new things.
Dr. Rugo: Indeed. Certainly, you and I have worked together on trying to encourage our pharmaceutical colleagues to publish these papers alongside their urgency of distributing the efficacy data and publishing the papers on efficacy, and also to do a nitty-gritty review of safety and talk about management strategies. I’m really pleased that there seems to be a little more focus on that earlier now in the drug process – although still not early enough – but it’s getting there. That’s a good thing.
Ms. Pettiford: Julia, you mentioned earlier how important it is for the individual patient’s quality of life to understand how these side effects can affect them. It really is one of those things in which we have to make personal decisions. What might be good for one person in terms of what happens with side effects, and their ability to function might not work with someone else.
If you are a person who’s dealing with metastatic disease who has children, a household, a dog, and a cat to take care of, what I can handle being that I’m a single person is not what they can handle. That’s all a part of the education piece. That’s all part of the teamwork. That’s all part of the communication process. It all comes into play.
Dr. Rugo: That’s such an incredibly important point. As we’re wrapping up, it would be great if everybody had some points to make that pulled together some of our conversation. Julia, do you want to start?
Ms. Maués: Yes, I was going to add specifically about the topic you were just discussing, with all that an oncologist’s team has to know and all the different areas of our health being affected by these new treatments. One tip for patients and their teams is that the other providers around the patients may not be as informed about the disease and the treatments they are on. Sometimes we patients end up getting information that isn’t up to date with the latest drugs and things like that.
When we do talk with someone about our issues, make sure they are informed about the new drugs. For example, we often have skin issues. There are dermatologists that work with cancer patients often, and they’re very informed about the side effects that come with these drugs. There are others who never see these sorts of issues and may assume it’s something completely different.
I usually just go to doctors that my oncologist’s team collaborates with and gets referrals from because they send their patients to these doctors often. These are doctors that see cancer patients. We’re a very unique group.
Dr. Rugo: That’s a really good point. I have the same thing. We all have a little stable of people we refer to for various issues that we can reach on speed dial.
The importance of diversity in clinical trials to obtain the most useful outcomes
Fatima, there’s recently been, appropriately so, more of a push to try and evaluate side effects by racial and ethnic subgroups. I think we’re still pretty crummy at it, but we are making some progress. How important is that to you when you think about patients and managing them?
Dr. Cardoso: I think this is quite important. One area of research that is underused, really, is all the new genomics and sequencing technologies to understand why people react differently to the same treatment. Why is it that for some people, either for ethnic or other reasons, you have a different metabolism or something else that justifies a very high rate of side effects from a certain treatment, whereas in other regions of the world this doesn’t happen?
Not to go into these new drugs, but when using a very old drug like a taxane, I found a difference in reaction between the Portuguese patients and the Belgian patients, the two countries where I’ve worked. I even found that the cause might be genetic because the Portuguese living in Belgium reacted differently than the Belgians themselves.
Maybe there is something in the genetics that justifies the type of side effects that you have. I make a plea also for us to dedicate research to understanding why certain side effects are related to race and others are related to maybe some other types of genetic alterations that will lead to an increased side effect.
Dr. Rugo: Sheila, comments?
Ms. Pettiford: That is just excellent. It’s excellent to even consider it because it is so obvious. To me, it’s an obvious situation because there are things that are underneath the skin that we don’t understand. We have to take that into consideration when we are dealing with all these wonderful – I call them miracle – drugs that have come about in the last 20 years.
There still is much more to be done, and I try to participate in any type of organization that’s encouraging diversity in clinical trials because you need to have people of all different ethnicities in order for us to get to these answers. It’s fascinating that you found this out, doctor.
The patient-centered dosing initiative
Ms. Maués: I have the pleasure of being a member of a patient-led initiative called the Patient-Centered Dosing Initiative (PCDI). We are highlighting the discussion around dosages of drugs, especially in the metastatic setting. Metastatic breast cancer is what we’re focusing on, although it could apply to any type of cancer. We are advised by a number of wonderful, world-renowned physicians, Dr. Rugo being one of them. Anne Loeser, the leader of our group, has spoken at ASCO about this topic of dosage. What we’re seeing is that the dosage determination for oncology drugs is still done in the same way it used to be done decades ago and mostly with the curative intent of early-stage disease — metastatic cancer patients back then really didn’t live long at all. What we’re seeing right now is people with metastatic breast cancer that are able to, in some cases, live a long life managing their disease.
Patients are put on doses that are too high for them to be able to manage the side effects, and then they end up having to go off the drug, which means they have lost one of the tools in their toolbox. So, what we like to say about dosing is that, for metastatic cancer patients, it’s a marathon and not a sprint. If we throw all the poison at the patient from the very beginning, they won’t be able to take this for a very long time. And in the metastatic setting, the goal is to stay on each therapy for as long as possible. If we burn one of the cards early on, you have to move on to the next one. This is finite because at some point, there are not enough drugs that can help a particular patient. The PCDI is really getting a lot of visibility with the FDA and experts. People are talking more about dosages, and the FDA is now providing guidance for pharmaceutical companies to study different dosages in the clinical trials from the very beginning. This initiative is almost 3 years old, and we have made a tremendous impact since then.
Dr. Rugo: I think this is an incredibly important area moving forward, and thankfully, there’s so much interest now in not only promoting diversity, enrollment in trials, and education to promote diversity but also in looking at differences in efficacy and side effects.
I’ll just thank everybody for your contributions and amazing perspectives in this incredibly important area. As we move forward with better agents, we need to also make sure we’re understanding what the side effects are, managing them, and hearing the voices of our patients. Thanks very much.
Ms. Pettiford: Thank you so much.
Dr. Cardoso: Thank you.
Ms. Maués: Thank you.
Editor’s note: Our panelists would like to highlight these points:
- The patient and the health care team must build trust with each other.
- African Americans have historical reasons for not trusting the health care industry. Much outreach is still needed.
- Inform and educate before the start of treatment and during the treatment.
- Be balanced and do not underestimate common side effects or overestimate rare ones. Adapt the amount and the detail of the information to the wishes of the individual patient. Offer various methods of delivery (e.g., videos, pamphlets, fact sheets).
- Patients will research their condition and treatments online. Instead of trying to stop this, help them find the best sources.
- Patients will connect with others in the patient community and learn from each other’s experiences. Keep in mind that everyone is different, and decisions should always be made together with the medical team.
- Monitor patients regularly, especially during the first few treatment cycles.
- Use different forms of communication between the patient and health care providers (e.g., apps, digital charts, oncology nurses/nurse navigators, responsive oncologists, different forms of telemedicine), but don’t forget to speak directly with the patient.
- The use of new PRO apps can be very useful to help patients differentiate between urgent and nonurgent signs and symptoms.
- As much as possible, use preventive/prophylactic measures, namely for nausea, vomiting, diarrhea/constipation, and mucositis.
- Be aware of late side effects, especially with immunotherapy.
- Don’t forget that grade 1-2 side effects can substantially impact quality of life, particularly if they are persistent.
- Consider quality-of-life issues for each patient. What is acceptable for one patient may not be for another.
- Learn how to manage new and specific side effects (e.g., endocrine, skin related, pneumonitis).
- Keep an open dialogue about treatment and side effects. Things can change, and there are different ways to address issues such as medications for side effects and dosing changes.
- Listen to your patient and respond in a timely fashion.
- Ethnicity and genetics should be studied as a factor for individual side effects. Standard industry dosages of a new anticancer medication might not be as effective in one ethnic group as another due to the lack of diversity in clinical trials.
- Medications with hard-to-manage or dangerous side effects may be counterproductive regardless of effectiveness.
- Cancer treatment varies vastly depending on region and type of treatment facility. There are many unmet needs in rural areas because of lack of oncology personnel, finances, transportation, etc.
Dr. Rugo is a professor in the department of medicine, University of California San Francisco Comprehensive Cancer Center; director, Breast Oncology and Clinical Trials Education, Cancer Infusion Services, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco. Dr. Cardoso is director, breast unit, Champalimaud Clinical Centre, Lisbon. Financial disclosures for both Dr. Rugo and Dr. Cardoso are available on Medscape.com, where this article first appeared. Julia Maués is a patient in Washington. She has disclosed no relevant financial relationships. Sheila Pettiford is a patient in Middletown, Del. She has disclosed no relevant financial relationships.
How and why the language of medicine must change
The United States has never achieved a single high standard of medical care equity for all of its people, and the trend line does not appear favorable. The closest we have reached is basic Medicare (Parts A and B), military medicine, the Veterans Health Administration, and large nonprofit groups like Kaiser Permanente. It seems that the nature of we individualistic Americans is to always try to seek an advantage.
But even achieving equity in medical care would not ensure equity in health. The social determinants of health (income level, education, politics, government, geography, neighborhood, country of origin, language spoken, literacy, gender, and yes – race and ethnicity) have far more influence on health equity than does medical care.
Narratives can both reflect and influence culture. Considering the harmful effects of the current political divisiveness in the United States, the timing is ideal for our three leading medical and health education organizations – the American Medical Association, the Association of American Medical Colleges (AAMC), and the Centers for Disease Control and Prevention – to publish a definitive position paper called “Advancing Health Equity: A Guide to Language, Narrative and Concepts.”
What’s in a word?
According to William Shakespeare, “A rose by any other name would smell as sweet” (Romeo and Juliet). Maybe. But if the word used were “thorn” or “thistle,” it just would not be the same.
Words comprise language and wield enormous power with human beings. Wars are fought over geographic boundaries often defined by the language spoken by the people: think 2022, Russian-speaking Ukrainians. Think Winston Churchill’s massive 1,500-page “A History of the English-Speaking Peoples.” Think about the political power of French in Quebec, Canada.
Thus, it should be no surprise that words, acronyms, and abbreviations become rallying cries for political activists of all stripes: PC, January 6, Woke, 1619, BLM, Critical Race Theory, 1776, Remember Pearl Harbor, Remember the Alamo, the Civil War or the War Between the States, the War for Southern Independence, the War of Northern Aggression, the War of the Rebellion, or simply “The Lost Cause.” How about Realpolitik?
Is “medical language” the language of the people or of the profession? Physicians must understand each other, and physicians also must communicate clearly with patients using words that convey neutral meanings and don’t interfere with objective understanding. Medical editors prefer the brevity of one or a few words to clearly convey meaning.
I consider this document from the AMA and AAMC to be both profound and profoundly important for the healing professions. The contributors frequently use words like “humility” as they describe their efforts and products, knowing full well that they (and their organizations) stand to be figuratively torn limb from limb by a host of critics – or worse, ignored and marginalized.
Part 1 of the Health Equity Guide is titled “Language for promoting health equity.”(the reader is referred to the Health Equity Guide for the reasoning and explanations for all).
Part 2 of the Health Equity Guide is called “Why narratives matter.” It includes features of dominant narratives; a substantial section on the narrative of race and the narrative of individualism; the purpose of a health equity–based narrative; how to change the narrative; and how to see and think critically through dialogue.
Part 3 of the Health Equity Guide is a glossary of 138 key terms such as “class,” “discrimination,” “gender dysphoria,” “non-White,” “racial capitalism,” and “structural competency.”
The CDC also has a toolkit for inclusive communication, the “Health Equity Guiding Principles for Inclusive Communication.”
The substantive message of the Health Equity Guide could affect what you say, write, and do (even how you think) every day as well as how those with whom you interact view you. It can affect the entire communication milieu in which you live, whether or not you like it. Read it seriously, as though your professional life depended on it. It may.
Dr. Lundberg is consulting professor of health research policy and pathology at Stanford (Calif.) University. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The United States has never achieved a single high standard of medical care equity for all of its people, and the trend line does not appear favorable. The closest we have reached is basic Medicare (Parts A and B), military medicine, the Veterans Health Administration, and large nonprofit groups like Kaiser Permanente. It seems that the nature of we individualistic Americans is to always try to seek an advantage.
But even achieving equity in medical care would not ensure equity in health. The social determinants of health (income level, education, politics, government, geography, neighborhood, country of origin, language spoken, literacy, gender, and yes – race and ethnicity) have far more influence on health equity than does medical care.
Narratives can both reflect and influence culture. Considering the harmful effects of the current political divisiveness in the United States, the timing is ideal for our three leading medical and health education organizations – the American Medical Association, the Association of American Medical Colleges (AAMC), and the Centers for Disease Control and Prevention – to publish a definitive position paper called “Advancing Health Equity: A Guide to Language, Narrative and Concepts.”
What’s in a word?
According to William Shakespeare, “A rose by any other name would smell as sweet” (Romeo and Juliet). Maybe. But if the word used were “thorn” or “thistle,” it just would not be the same.
Words comprise language and wield enormous power with human beings. Wars are fought over geographic boundaries often defined by the language spoken by the people: think 2022, Russian-speaking Ukrainians. Think Winston Churchill’s massive 1,500-page “A History of the English-Speaking Peoples.” Think about the political power of French in Quebec, Canada.
Thus, it should be no surprise that words, acronyms, and abbreviations become rallying cries for political activists of all stripes: PC, January 6, Woke, 1619, BLM, Critical Race Theory, 1776, Remember Pearl Harbor, Remember the Alamo, the Civil War or the War Between the States, the War for Southern Independence, the War of Northern Aggression, the War of the Rebellion, or simply “The Lost Cause.” How about Realpolitik?
Is “medical language” the language of the people or of the profession? Physicians must understand each other, and physicians also must communicate clearly with patients using words that convey neutral meanings and don’t interfere with objective understanding. Medical editors prefer the brevity of one or a few words to clearly convey meaning.
I consider this document from the AMA and AAMC to be both profound and profoundly important for the healing professions. The contributors frequently use words like “humility” as they describe their efforts and products, knowing full well that they (and their organizations) stand to be figuratively torn limb from limb by a host of critics – or worse, ignored and marginalized.
Part 1 of the Health Equity Guide is titled “Language for promoting health equity.”(the reader is referred to the Health Equity Guide for the reasoning and explanations for all).

Part 2 of the Health Equity Guide is called “Why narratives matter.” It includes features of dominant narratives; a substantial section on the narrative of race and the narrative of individualism; the purpose of a health equity–based narrative; how to change the narrative; and how to see and think critically through dialogue.
Part 3 of the Health Equity Guide is a glossary of 138 key terms such as “class,” “discrimination,” “gender dysphoria,” “non-White,” “racial capitalism,” and “structural competency.”
The CDC also has a toolkit for inclusive communication, the “Health Equity Guiding Principles for Inclusive Communication.”
The substantive message of the Health Equity Guide could affect what you say, write, and do (even how you think) every day as well as how those with whom you interact view you. It can affect the entire communication milieu in which you live, whether or not you like it. Read it seriously, as though your professional life depended on it. It may.
Dr. Lundberg is consulting professor of health research policy and pathology at Stanford (Calif.) University. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The United States has never achieved a single high standard of medical care equity for all of its people, and the trend line does not appear favorable. The closest we have reached is basic Medicare (Parts A and B), military medicine, the Veterans Health Administration, and large nonprofit groups like Kaiser Permanente. It seems that the nature of we individualistic Americans is to always try to seek an advantage.
But even achieving equity in medical care would not ensure equity in health. The social determinants of health (income level, education, politics, government, geography, neighborhood, country of origin, language spoken, literacy, gender, and yes – race and ethnicity) have far more influence on health equity than does medical care.
Narratives can both reflect and influence culture. Considering the harmful effects of the current political divisiveness in the United States, the timing is ideal for our three leading medical and health education organizations – the American Medical Association, the Association of American Medical Colleges (AAMC), and the Centers for Disease Control and Prevention – to publish a definitive position paper called “Advancing Health Equity: A Guide to Language, Narrative and Concepts.”
What’s in a word?
According to William Shakespeare, “A rose by any other name would smell as sweet” (Romeo and Juliet). Maybe. But if the word used were “thorn” or “thistle,” it just would not be the same.
Words comprise language and wield enormous power with human beings. Wars are fought over geographic boundaries often defined by the language spoken by the people: think 2022, Russian-speaking Ukrainians. Think Winston Churchill’s massive 1,500-page “A History of the English-Speaking Peoples.” Think about the political power of French in Quebec, Canada.
Thus, it should be no surprise that words, acronyms, and abbreviations become rallying cries for political activists of all stripes: PC, January 6, Woke, 1619, BLM, Critical Race Theory, 1776, Remember Pearl Harbor, Remember the Alamo, the Civil War or the War Between the States, the War for Southern Independence, the War of Northern Aggression, the War of the Rebellion, or simply “The Lost Cause.” How about Realpolitik?
Is “medical language” the language of the people or of the profession? Physicians must understand each other, and physicians also must communicate clearly with patients using words that convey neutral meanings and don’t interfere with objective understanding. Medical editors prefer the brevity of one or a few words to clearly convey meaning.
I consider this document from the AMA and AAMC to be both profound and profoundly important for the healing professions. The contributors frequently use words like “humility” as they describe their efforts and products, knowing full well that they (and their organizations) stand to be figuratively torn limb from limb by a host of critics – or worse, ignored and marginalized.
Part 1 of the Health Equity Guide is titled “Language for promoting health equity.”(the reader is referred to the Health Equity Guide for the reasoning and explanations for all).

Part 2 of the Health Equity Guide is called “Why narratives matter.” It includes features of dominant narratives; a substantial section on the narrative of race and the narrative of individualism; the purpose of a health equity–based narrative; how to change the narrative; and how to see and think critically through dialogue.
Part 3 of the Health Equity Guide is a glossary of 138 key terms such as “class,” “discrimination,” “gender dysphoria,” “non-White,” “racial capitalism,” and “structural competency.”
The CDC also has a toolkit for inclusive communication, the “Health Equity Guiding Principles for Inclusive Communication.”
The substantive message of the Health Equity Guide could affect what you say, write, and do (even how you think) every day as well as how those with whom you interact view you. It can affect the entire communication milieu in which you live, whether or not you like it. Read it seriously, as though your professional life depended on it. It may.
Dr. Lundberg is consulting professor of health research policy and pathology at Stanford (Calif.) University. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
No link between cell phones and brain tumors in large U.K. study
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
“These results support the accumulating evidence that mobile phone use under usual conditions does not increase brain tumor risk,” study author Kirstin Pirie, MSc, from the cancer epidemiology unit at Oxford (England) Population Health, said in a statement.
However, an important limitation of the study is that it involved only women who were middle-aged and older; these people generally use cell phones less than younger women or men, the authors noted. In this study’s cohort, mobile phone use was low, with only 18% of users talking on the phone for 30 minutes or more each week.
The results were published in the Journal of the National Cancer Institute.
This study is a “welcome addition to the body of knowledge looking at the risk from mobile phones, and specifically in relation to certain types of tumor genesis. It is a well-designed, prospective study that identifies no causal link,” commented Malcolm Sperrin from Oxford University Hospitals, who was not involved in the research.
“There is always a need for further research work, especially as phones, wireless, etc., become ubiquitous, but this study should allay many existing concerns,” he commented on the UK Science Media Centre.
Concerns about a cancer risk, particularly brain tumors, have been circulating for decades, and to date, there have been some 30 epidemiologic studies on this issue.
In 2011, the International Agency for Research on Cancer announced that cell phones are “possibly carcinogenic.” That conclusion was based largely on the results of the large INTERPHONE international case-control study and a series of Swedish studies led by Hardell Lennart, MD.
In the latest article, the U.K. researchers suggest that a “likely explanation for the previous positive results is that for a very slow growing tumor, there may be detection bias if cellular telephone users seek medical advice because of awareness of typical symptoms of acoustic neuroma, such as unilateral hearing problems, earlier than nonusers.
“The totality of human evidence, from observational studies, time trends, and bioassays, suggests little or no increase in the risk of cellular telephone users developing a brain tumor,” the U.K. researchers concluded.
Commenting on the U.K. study, Joachim Schüz, PhD, branch head of the section of environment and radiation at the IARC, noted that “mobile technologies are improving all the time, so that the more recent generations emit substantially lower output power.
“Nevertheless, given the lack of evidence for heavy users, advising mobile phone users to reduce unnecessary exposures remains a good precautionary approach,” Dr. Schuz said in a statement.
Details of U.K. study
The U.K. study was conducted by researchers from Oxford Population Health and IARC, who used data from the ongoing UK Million Women Study. This study began in 1996 and has recruited 1.3 million women born from 1935 to 1950 (which amounts to 1 in every 4 women) through the U.K. National Health Service Breast Screening Programme. These women complete regular postal questionnaires about sociodemographic, medical, and lifestyle factors.
Questions about cell phone use were completed by about 776,000 women in 2001 (when they were 50-65 years old). About half of these women also answered these questions about mobile phone use 10 years later, in 2011 (when they were aged 60-75).
The answers indicated that by 2011, the majority of women (75%) aged between 60 and 64 years used a mobile phone, while just under half of those aged between 75 and 79 years used one.
These women were then followed for an average of 14 years through linkage to their NHS records.
The researchers looked for any mention of brain tumors, including glioma, acoustic neuroma, meningioma, and pituitary gland tumors, as well as eye tumors.
During the 14 year follow-up period, 3,268 (0.42%) of the participants developed a brain tumor, but there was no significant difference in that risk between individuals who had never used a mobile phone and those who were mobile phone users. These included tumors in the temporal and parietal lobes, which are the most exposed areas of the brain.
There was also no difference in the risk of developing tumors between women who reported using a mobile phone daily, those who used them for at least 20 minutes a week, and those who had used a mobile phone for over 10 years.
In addition, among the individuals who did develop a tumor, the incidence of right- and left-sided tumors was similar among mobile phone users, even though mobile phone use tends to involve the right side considerably more than the left side, the researchers noted.
The study was funded by the UK Medical Research Council and Cancer Research UK.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Obesity increasing the risk for cancer: It’s complicated
The link between obesity and cancer has increasingly been emphasized in public health messages, but is the current message correct?
“Being overweight or having obesity increases your risk of getting cancer,” warns the U.S. Centers for Disease Control and Prevention. It warns that overweight/obesity is “linked with a higher risk of getting 13 types of cancer ... [which] make up 40% of all cancers diagnosed in the United States each year.”
But that message, which is also promulgated by many cancer organizations, is based on data from observational studies, which have many limitations.
In addition, it found an inverse relationship for breast cancer, in which early-life obesity was associated with a reduced risk of breast cancer, and the relationship with obesity was “complicated” for lung and prostate cancer.
The study, headed by Zhe Fang, MBBS, Harvard T. H. Chan School of Public Health, Boston, Mass., was published in the Journal of the National Cancer Institute
“For a seemingly straightforward question of whether excessive body fatness causes cancer, the answer may not be straightforward after all,” writes Song Yao, PhD, professor of oncology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., in an accompanying editorial
“How to craft a simple public health message to convey the complexity and nuances of the relationships may be a challenge to be grappled with going forward,” he added.
In an interview, Dr. Yao said that it “really depends on what kind of message you want to get out.”
“If you want to talk about cancer overall, as one disease, we all know that a clear association with obesity does not exist,” he said. “It’s not that simple.”
“You really cannot say that obesity increases cancer risk overall,” he said.
For some cancers included in the study, Dr. Yao continued, it was “very clear that obesity increased the risk ... but for some other cancer types, we either don’t have enough data yet or the association is not as consistent.”
This, he said, is especially the case for prostate and lung cancer.
All of this indicates that there is a complex relationship between obesity and cancer risk, he maintains.
“We always think obesity is bad, not only for cancer but also for more common conditions, like hypertension, diabetes, and cardiovascular disease,” Dr. Yao noted. This points to the link between obesity and chronic inflammation, he added.
However, there are also other hypotheses, including synthesis of estrogen in adipose tissue, which may explain the link between obesity and breast cancer risk in older women.
However, in younger women, obesity protects against breast cancer, and “we really don’t know why,” Dr. Yao said.
The new study used Mendelian randomization to examine these relationships. This is a “new tool that we have developed over the past 20 years or so, largely because there is so much data coming from genome-wide association studies,” Dr. Yao explained.
It has “advantages” over other methods, including observational studies. One of its strengths is that it is “not impacted by reverse causality,” because genetic risk does not change over time.
However, he said, it is “quite straightforward to think that the genetics do not change, but at the same time, the environment we live in throughout our life course changes,” and the impact of genetic variants may be “washed out.”
How genetics influences cancer risk may therefore change over time, and it is a “dynamic process,” Dr. Yao commented.
In addition, this approach has its own limitations, he said, because it depends on how much of the variation in a given measure can be attributed to genetic factors.
New conclusions
In their study, Dr. Fang and colleagues reviewed 204 meta-analyses of 2,179 individual estimates from 507 cohort or case-control studies. They found “strong evidence” that supports the association between obesity and 11 cancers.
These are esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney.
They note, however, that the associations “may be causal for some malignancies” but that the co-occurrence of obesity with various cancer risk factors means that others may be “susceptible to potential confounding bias.”
To overcome some of these limitations, the team looked to Mendelian randomization studies that examined the association between genetic variants linked to body mass index (BMI), indicating lifetime risk of high BMI, and cancer risk for a range of cancer types.
These Mendelian randomization studies were then compared with the results of large-scale conventional observational studies, as well as with evidence in reports from the International Agency for Research on Cancer and the World Cancer Research Fund–American Institute of Cancer Research, which also include experimental studies.
The researchers say that, overall, the Mendelian randomization studies “further establish the causality of obesity” with six cancer types: colorectal, endometrial, ovarian, kidney, and pancreatic cancer, and esophageal adenocarcinoma.
In addition, these studies further establish the inverse relationship of early-life obesity with breast cancer.
However, the approach could not confirm a positive association between obesity and gallbladder and gastric cardia cancer, as well as multiple myeloma.
“This could be due to low power,” the team suggests, “and larger studies are required.”
With respect to lung cancer, the Mendelian randomization identified a positive association with obesity that supports the inverse association identified in observational studies, that is, that obesity may reduce the risk for lung cancer.
The researchers suggest this may reflect reverse causality related to the loss of lean body mass before diagnosis, as well as confounding by smoking.
For prostate cancer, the evidence was “conflicting” and “implies a complicated role of obesity,” Dr. Zhang and colleagues comment.
The link between obesity and lower prostate-specific antigen levels, they suggest, may result in a detection bias by masking the presence of prostate cancer, or it “could be biological” in origin, owing to reduced androgen levels.
For six cancer types for which a causal relationship with obesity could be established, the effect estimates from the Mendelian randomization studies were stronger than those seen in conventional studies, with the magnitude of risk ranging from 1.14-fold for early-life obesity and breast cancer to 1.37-fold for adult obesity and esophageal adenocarcinoma.
In another editorial accompanying the new study, Graham A. Colditz, MD, DrPH, from Washington University School of Medicine, St. Louis, underlined that childhood and adolescent obesity and their contribution to cancer risk need further attention.
“To reap the reward from past research, we must act to implement effective strategies to reduce childhood and adolescent adiposity, reduce excess weight gain in adult years, and maintain a healthy weight,” he writes.
“This will require us to change the way we live, but COVID-19 has shown we can make changes to how we live and work. Let us keep the changes we have already made, or take on new ones, that will cut our collective cancer toll,” he implores.
No funding for the study was described. Dr. Colditz is supported by the Breast Cancer Research Foundation. No other relevant financial relationships were described.
A version of this article first appeared on Medscape.com.
The link between obesity and cancer has increasingly been emphasized in public health messages, but is the current message correct?
“Being overweight or having obesity increases your risk of getting cancer,” warns the U.S. Centers for Disease Control and Prevention. It warns that overweight/obesity is “linked with a higher risk of getting 13 types of cancer ... [which] make up 40% of all cancers diagnosed in the United States each year.”
But that message, which is also promulgated by many cancer organizations, is based on data from observational studies, which have many limitations.
In addition, it found an inverse relationship for breast cancer, in which early-life obesity was associated with a reduced risk of breast cancer, and the relationship with obesity was “complicated” for lung and prostate cancer.
The study, headed by Zhe Fang, MBBS, Harvard T. H. Chan School of Public Health, Boston, Mass., was published in the Journal of the National Cancer Institute
“For a seemingly straightforward question of whether excessive body fatness causes cancer, the answer may not be straightforward after all,” writes Song Yao, PhD, professor of oncology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., in an accompanying editorial
“How to craft a simple public health message to convey the complexity and nuances of the relationships may be a challenge to be grappled with going forward,” he added.
In an interview, Dr. Yao said that it “really depends on what kind of message you want to get out.”
“If you want to talk about cancer overall, as one disease, we all know that a clear association with obesity does not exist,” he said. “It’s not that simple.”
“You really cannot say that obesity increases cancer risk overall,” he said.
For some cancers included in the study, Dr. Yao continued, it was “very clear that obesity increased the risk ... but for some other cancer types, we either don’t have enough data yet or the association is not as consistent.”
This, he said, is especially the case for prostate and lung cancer.
All of this indicates that there is a complex relationship between obesity and cancer risk, he maintains.
“We always think obesity is bad, not only for cancer but also for more common conditions, like hypertension, diabetes, and cardiovascular disease,” Dr. Yao noted. This points to the link between obesity and chronic inflammation, he added.
However, there are also other hypotheses, including synthesis of estrogen in adipose tissue, which may explain the link between obesity and breast cancer risk in older women.
However, in younger women, obesity protects against breast cancer, and “we really don’t know why,” Dr. Yao said.
The new study used Mendelian randomization to examine these relationships. This is a “new tool that we have developed over the past 20 years or so, largely because there is so much data coming from genome-wide association studies,” Dr. Yao explained.
It has “advantages” over other methods, including observational studies. One of its strengths is that it is “not impacted by reverse causality,” because genetic risk does not change over time.
However, he said, it is “quite straightforward to think that the genetics do not change, but at the same time, the environment we live in throughout our life course changes,” and the impact of genetic variants may be “washed out.”
How genetics influences cancer risk may therefore change over time, and it is a “dynamic process,” Dr. Yao commented.
In addition, this approach has its own limitations, he said, because it depends on how much of the variation in a given measure can be attributed to genetic factors.
New conclusions
In their study, Dr. Fang and colleagues reviewed 204 meta-analyses of 2,179 individual estimates from 507 cohort or case-control studies. They found “strong evidence” that supports the association between obesity and 11 cancers.
These are esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney.
They note, however, that the associations “may be causal for some malignancies” but that the co-occurrence of obesity with various cancer risk factors means that others may be “susceptible to potential confounding bias.”
To overcome some of these limitations, the team looked to Mendelian randomization studies that examined the association between genetic variants linked to body mass index (BMI), indicating lifetime risk of high BMI, and cancer risk for a range of cancer types.
These Mendelian randomization studies were then compared with the results of large-scale conventional observational studies, as well as with evidence in reports from the International Agency for Research on Cancer and the World Cancer Research Fund–American Institute of Cancer Research, which also include experimental studies.
The researchers say that, overall, the Mendelian randomization studies “further establish the causality of obesity” with six cancer types: colorectal, endometrial, ovarian, kidney, and pancreatic cancer, and esophageal adenocarcinoma.
In addition, these studies further establish the inverse relationship of early-life obesity with breast cancer.
However, the approach could not confirm a positive association between obesity and gallbladder and gastric cardia cancer, as well as multiple myeloma.
“This could be due to low power,” the team suggests, “and larger studies are required.”
With respect to lung cancer, the Mendelian randomization identified a positive association with obesity that supports the inverse association identified in observational studies, that is, that obesity may reduce the risk for lung cancer.
The researchers suggest this may reflect reverse causality related to the loss of lean body mass before diagnosis, as well as confounding by smoking.
For prostate cancer, the evidence was “conflicting” and “implies a complicated role of obesity,” Dr. Zhang and colleagues comment.
The link between obesity and lower prostate-specific antigen levels, they suggest, may result in a detection bias by masking the presence of prostate cancer, or it “could be biological” in origin, owing to reduced androgen levels.
For six cancer types for which a causal relationship with obesity could be established, the effect estimates from the Mendelian randomization studies were stronger than those seen in conventional studies, with the magnitude of risk ranging from 1.14-fold for early-life obesity and breast cancer to 1.37-fold for adult obesity and esophageal adenocarcinoma.
In another editorial accompanying the new study, Graham A. Colditz, MD, DrPH, from Washington University School of Medicine, St. Louis, underlined that childhood and adolescent obesity and their contribution to cancer risk need further attention.
“To reap the reward from past research, we must act to implement effective strategies to reduce childhood and adolescent adiposity, reduce excess weight gain in adult years, and maintain a healthy weight,” he writes.
“This will require us to change the way we live, but COVID-19 has shown we can make changes to how we live and work. Let us keep the changes we have already made, or take on new ones, that will cut our collective cancer toll,” he implores.
No funding for the study was described. Dr. Colditz is supported by the Breast Cancer Research Foundation. No other relevant financial relationships were described.
A version of this article first appeared on Medscape.com.
The link between obesity and cancer has increasingly been emphasized in public health messages, but is the current message correct?
“Being overweight or having obesity increases your risk of getting cancer,” warns the U.S. Centers for Disease Control and Prevention. It warns that overweight/obesity is “linked with a higher risk of getting 13 types of cancer ... [which] make up 40% of all cancers diagnosed in the United States each year.”
But that message, which is also promulgated by many cancer organizations, is based on data from observational studies, which have many limitations.
In addition, it found an inverse relationship for breast cancer, in which early-life obesity was associated with a reduced risk of breast cancer, and the relationship with obesity was “complicated” for lung and prostate cancer.
The study, headed by Zhe Fang, MBBS, Harvard T. H. Chan School of Public Health, Boston, Mass., was published in the Journal of the National Cancer Institute
“For a seemingly straightforward question of whether excessive body fatness causes cancer, the answer may not be straightforward after all,” writes Song Yao, PhD, professor of oncology, Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., in an accompanying editorial
“How to craft a simple public health message to convey the complexity and nuances of the relationships may be a challenge to be grappled with going forward,” he added.
In an interview, Dr. Yao said that it “really depends on what kind of message you want to get out.”
“If you want to talk about cancer overall, as one disease, we all know that a clear association with obesity does not exist,” he said. “It’s not that simple.”
“You really cannot say that obesity increases cancer risk overall,” he said.
For some cancers included in the study, Dr. Yao continued, it was “very clear that obesity increased the risk ... but for some other cancer types, we either don’t have enough data yet or the association is not as consistent.”
This, he said, is especially the case for prostate and lung cancer.
All of this indicates that there is a complex relationship between obesity and cancer risk, he maintains.
“We always think obesity is bad, not only for cancer but also for more common conditions, like hypertension, diabetes, and cardiovascular disease,” Dr. Yao noted. This points to the link between obesity and chronic inflammation, he added.
However, there are also other hypotheses, including synthesis of estrogen in adipose tissue, which may explain the link between obesity and breast cancer risk in older women.
However, in younger women, obesity protects against breast cancer, and “we really don’t know why,” Dr. Yao said.
The new study used Mendelian randomization to examine these relationships. This is a “new tool that we have developed over the past 20 years or so, largely because there is so much data coming from genome-wide association studies,” Dr. Yao explained.
It has “advantages” over other methods, including observational studies. One of its strengths is that it is “not impacted by reverse causality,” because genetic risk does not change over time.
However, he said, it is “quite straightforward to think that the genetics do not change, but at the same time, the environment we live in throughout our life course changes,” and the impact of genetic variants may be “washed out.”
How genetics influences cancer risk may therefore change over time, and it is a “dynamic process,” Dr. Yao commented.
In addition, this approach has its own limitations, he said, because it depends on how much of the variation in a given measure can be attributed to genetic factors.
New conclusions
In their study, Dr. Fang and colleagues reviewed 204 meta-analyses of 2,179 individual estimates from 507 cohort or case-control studies. They found “strong evidence” that supports the association between obesity and 11 cancers.
These are esophageal adenocarcinoma, multiple myeloma, and cancers of the gastric cardia, colon, rectum, biliary tract system, pancreas, breast, endometrium, ovary, and kidney.
They note, however, that the associations “may be causal for some malignancies” but that the co-occurrence of obesity with various cancer risk factors means that others may be “susceptible to potential confounding bias.”
To overcome some of these limitations, the team looked to Mendelian randomization studies that examined the association between genetic variants linked to body mass index (BMI), indicating lifetime risk of high BMI, and cancer risk for a range of cancer types.
These Mendelian randomization studies were then compared with the results of large-scale conventional observational studies, as well as with evidence in reports from the International Agency for Research on Cancer and the World Cancer Research Fund–American Institute of Cancer Research, which also include experimental studies.
The researchers say that, overall, the Mendelian randomization studies “further establish the causality of obesity” with six cancer types: colorectal, endometrial, ovarian, kidney, and pancreatic cancer, and esophageal adenocarcinoma.
In addition, these studies further establish the inverse relationship of early-life obesity with breast cancer.
However, the approach could not confirm a positive association between obesity and gallbladder and gastric cardia cancer, as well as multiple myeloma.
“This could be due to low power,” the team suggests, “and larger studies are required.”
With respect to lung cancer, the Mendelian randomization identified a positive association with obesity that supports the inverse association identified in observational studies, that is, that obesity may reduce the risk for lung cancer.
The researchers suggest this may reflect reverse causality related to the loss of lean body mass before diagnosis, as well as confounding by smoking.
For prostate cancer, the evidence was “conflicting” and “implies a complicated role of obesity,” Dr. Zhang and colleagues comment.
The link between obesity and lower prostate-specific antigen levels, they suggest, may result in a detection bias by masking the presence of prostate cancer, or it “could be biological” in origin, owing to reduced androgen levels.
For six cancer types for which a causal relationship with obesity could be established, the effect estimates from the Mendelian randomization studies were stronger than those seen in conventional studies, with the magnitude of risk ranging from 1.14-fold for early-life obesity and breast cancer to 1.37-fold for adult obesity and esophageal adenocarcinoma.
In another editorial accompanying the new study, Graham A. Colditz, MD, DrPH, from Washington University School of Medicine, St. Louis, underlined that childhood and adolescent obesity and their contribution to cancer risk need further attention.
“To reap the reward from past research, we must act to implement effective strategies to reduce childhood and adolescent adiposity, reduce excess weight gain in adult years, and maintain a healthy weight,” he writes.
“This will require us to change the way we live, but COVID-19 has shown we can make changes to how we live and work. Let us keep the changes we have already made, or take on new ones, that will cut our collective cancer toll,” he implores.
No funding for the study was described. Dr. Colditz is supported by the Breast Cancer Research Foundation. No other relevant financial relationships were described.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Cancer Data Trends 2022
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Is this the most controversial issue in early breast cancer treatment?
Is this the most controversial topic in breast oncology? Quite likely: the results of a recent online poll show split votes and no consensus.
The topic is the use of chemotherapy for premenopausal women with early-stage hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2-) breast cancer.
, as the other expert countered?
The debate was held during the recent San Antonio Breast Cancer Symposium (SABCS), at which new results were presented that increased the controversy.
The controversy had arisen the previous year over results from the RxPONDER trial.
Five-year follow-up data from RxPONDER showed that adding chemotherapy to endocrine therapy did not improve outcomes over endocrine therapy alone for postmenopausal women with low-risk, node-positive HR+, HER2- breast cancer. This suggests that older women with early-stage breast cancer may safely forgo chemotherapy.
However, the same trial included premenopausal women with the same disease profile, and the results in this subgroup showed that there was benefit from chemotherapy, with a 5-year invasive disease-free survival (IDFS) rate of 94.2%, versus 89.0% for endocrine therapy alone (P = .0004).
The results were immediately controversial.
Some experts suggested the effect was due to the chemotherapy incidentally causing ovarian suppression, not the cytotoxic effect of the drugs on cancer cells. These experts were skeptical about the suggestion that chemotherapy works differently in premenopausal women than it does in postmenopausal women.
Some clinicians feel the lack of clarity creates an opportunity for greater discussion with women when making the treatment decision.
“When I have this conversation with patients, it’s really nuanced,” Stephanie L. Graff, MD, director of breast oncology, Lifespan Cancer Institute, Providence, R.I., told this news organization.
“I would choose chemotherapy for myself, but I’m a chemotherapy doctor, so I’m very comfortable with these medications and their side effects, and I am also very familiar with the slow burn of the side effects of endocrine therapy,” she said.
But for patients who are hearing their options for the first time, the idea of chemotherapy “feels scary,” and there is “a lot of stigma” associated with it, she commented.
Ultimately, she believes in offering patients as much information as possible, inasmuch as “knowledge is power.”
For Dr. Graff, the message from RxPONDER was that, in premenopausal patients with lymph node positive, HR+ breast cancer, “all comers benefited from chemotherapy.”
“And so if the goal is to be maximally aggressive and optimally lower your risk of distant recurrence, which is a life-threatening event, chemotherapy should offered.”
But chemotherapy comes with side effects, so it’s an important conversation to have with patients; RxPONDER showed that the absolute difference in the rate of distant recurrence with chemotherapy was relatively minor, she added.
Debate rages on
The debate at SABCS was moderated by Harold J. Burstein, MD, PhD, from the Dana-Farber Cancer Institute, Boston, who commented that if this was “a compelling question last week in clinic, it has now become red hot.”
At the meeting, held in December 2021, new longer-term data from the SOFT and TEXT clinical trials were presented, showing that ovarian suppression with tamoxifen plus an aromatase inhibitor provides a greater reduction in long-term risk of recurrence than tamoxifen alone.
Moreover, updated results from RxPONDER presented at the same session revealed that chemoendocrine therapy was associated with longer IDFS and distant relapse-free survival than endocrine therapy alone for women with one to three positive lymph nodes and a recurrence score of 25 or lower on the Oncotype DX (Genomic Health) 21-gene breast cancer assay.
Dr. Burstein said the debate over the use of chemotherapy in premenopausal women “is the most interesting question right now in early-stage breast cancer.”
The debate focused on the effect of chemotherapy in these patients – was it all down to ovarian function suppression?
Yes, argued Michael Gnant, MD, from the Medical University of Vienna.
Data from “modern adjuvant chemotherapy trials” suggest that chemo offers a 2%-3% benefit in distant disease-free survival at 5 years for premenopausal women, he noted. But the effect is much larger with ovarian function suppression via endocrine therapy, which provides 5-year disease-free and overall survival benefits of 9%-13%.
Older studies have shown that the benefit with chemotherapy is seen only in women who experience amenorrhea with the cytotoxic drugs, Dr. Gnant noted.
“In short, if you give adjuvant chemotherapy and you induce amenorrhea, then there is going to be a survival difference,” he said. “But if you give adjuvant chemotherapy and there is no amenorrhea, there won’t be an outcome difference.”
The ABSCG-05 trial, which compared endocrine therapy with chemotherapy, showed that “in the presence of optimal endocrine adjuvant treatment, adjuvant chemotherapy doesn’t add anything, because you have already achieved the effect of treatment-induced amenorrhea.”
So Dr. Gnant argued that the effect of chemotherapy in RxPONDER was due to ovarian function suppression.
But the real question is: “What does it mean for clinical practice?”
Dr. Gnant asserted that for the “large group of lower-risk premenopausal patients, tamoxifen will be good enough,” while those at moderate or intermediate risk should receive ovarian function suppression with either tamoxifen or an aromatase inhibitor, with the choice dictated by their adverse effects.
Chemotherapy “is just a graceless method of ovarian function suppression and should only be given to high-risk patients and to patients with endocrine nonresponsive disease,” he argued.
On the other side of the debate, Sibylle Loibl, MD, PhD, from the Centre of Hematology and Oncology, Bethanien, Frankfurt, argued that the effect is not all due to ovarian function suppression and that chemotherapy also has a cytotoxic effect in these patients.
“We need chemotherapy” because “cancer in young women is biologically different,” she asserted.
Dr. Loibl pointed to data currently awaiting publication in the Journal of the National Cancer Institute that suggest that younger women have “higher immune gene expression” that may make them more chemotherapy sensitive, and lower expression of hormone receptor genes, which “could make them less endocrine sensitive.”
She also cited data from a study from her own group that showed that pathologic complete response rates to neoadjuvant chemotherapy were higher in younger women with HR+, HER2- breast cancer, indicating a direct effect of chemotherapy on the disease and that age was an important prognostic factor.
The data on the induction of amenorrhea by chemotherapy is also not as clearcut as it seems, she commented. Chemotherapy does not achieve 100% amenorrhea, and gonadotropin-releasing hormone analogues are unable to suppress ovarian function in 20% of women.
Dr. Loibl concluded that the “chemotherapy effect is there, it is higher in young women with HR+, HER2- breast cancer,” and that the effect has two components.
“There is a direct cytotoxic effect which cannot be neglected, and there is an endocrine effect on the ovarian function suppression,” she argued.
“I think both are needed in young premenopausal patients,” she added.
Audience responses
After the debate, the audience was polled on what effect they thought chemotherapy was having in lower-risk HR+, HER2- breast cancer patients. About two-thirds responded that it was all or mostly due to ovarian function suppression.
However, the next question split the audience. They were presented with a clinical scenario: a 43-year-old woman with a mammographically detected 1.4-cm, intermediate grade, HR+, HER2- breast cancer who also had metastatic disease in one of three sentinel lymph nodes and whose recurrence score was 13.
When asked about the treatment plan they would choose for this patient, the audience was split over whether to opt for chemoendocrine therapy or endocrine therapy alone.
A similar clinical question was posited recently on Twitter, when Angela Toss, MD, PhD, from the University of Modena and Reggio Emilia, Italy, asked respondents which they would chose from among three options.
From the 815 votes that were cast, 46% chose Oncotype DX testing to determine the likely benefit of chemotherapy, 48% chose chemotherapy, and 6% picked ovarian function suppression and an aromatase inhibitor.
In response, Paolo Tarantino, MD, from the Dana-Farber Cancer Institute, commented: “If you had any doubt of which is the most controversial topic in breast oncology, doubt no more. 815 votes, no consensus.”
Approached for comment, Eric Winer, MD, director of the Yale Cancer Center, New Haven, Conn., said that the data from RxPONDER “in many ways was helpful, but ... it created about as many questions as it answered, if not more.”
Because the results showed a benefit from chemotherapy for premenopausal women but not for postmenopausal women with breast cancer, Dr. Winer told this news organization that one of the outstanding questions is “whether premenopausal women are fundamentally different from postmenopausal women ... and my answer to that is that is very unlikely.”
Dr. Winer added that the “real tragedy” of this trial was that it did not include women with more than three positive nodes, particularly those who have a low recurrence score, he said.
Clinicians are therefore left either “extrapolating” data from those with fewer nodes or “marching down a path that we’ve taken for years of just giving those people chemotherapy routinely,” even though there may be no benefit, Dr. Winer commented.
Another expert who was approached for comment had a different take on the data. Matteo Lambertini, MD, IRCCS Ospedale Policlinico San Martino, Genoa, Italy, agreed with Loibl’s argument that chemotherapy has a cytotoxic effect in premenopausal women with HR+, HER2- breast cancer in addition to its effect on ovarian function suppression.
He did not agree, however, that there is a question mark over what to do for patients with more than three positive lymph nodes.
Dr. Lambertini said in an interview that he thinks “too much” trust is placed in genomic testing and that there is a “risk of forgetting about all the other factors that we normally use to make our treatment choices.”
A patient with five positive nodes will benefit from chemotherapy, “even if she had a very low recurrence score,” he said, “because there is a very high clinical risk of disease recurrence,” and chemotherapy “is of benefit” in these situations, he asserted.
Dr. Lambertini said that the RxPONDER results – and also studies such as TAILORx, which demonstrated the ability of Oncotype DX to identify which patients with early breast cancer could skip chemotherapy – show that “chemotherapy has a role to play” and that most patients should receive it.
He suggested, however, that “probably the benefit of chemotherapy is smaller” in real life than was seen in these trials, because in the trials, they did not use optimal adjuvant endocrine therapy.
Treating individual patients
When it comes to making treatment decisions for individual patients, Dr. Winer said he has a “conversation with people about what the results of the study showed and what [he believes] that they need.”
For patients whose Oncotype DX score is in the “very low range, I do not recommend chemotherapy,” he said, preferring instead to use endocrine therapy for ovarian function suppression.
For women with a more intermediate score, “I explain that I don’t think we have an answer and that, if they would want to take the most traditional and conservative path, it would be to get chemotherapy.
“But I’m certainly not rigid about my recommendations, and I’m particularly open” to ovarian function suppression for a premenopausal woman with an Onctyope DX score of 20 and two positive nodes who does not have “other adverse features.”
“Ultimately, what pushes me in one direction or another,” Dr. Winer said, aside from number of positive nodes or the size of the tumor, “is the patient’s preferences.”
This was a theme taken up by Kim Sabelko, PhD, vice-president of scientific strategy and programs at Susan G. Komen, Dallas.
The results from RxPONDER and similar studies are “really interesting,” as researchers are “working out how to individualize treatment,” and that it is not a matter of “one size fits all.”
“We need to understand when to use chemotherapy and other drugs, and more importantly, when not to, because we don’t want to overtreat people who don’t necessarily need these drugs,” she commented.
Dr. Sabelko emphasized that treatment decisions “should be shared” between the patient and their doctor, and she noted that there “will be some people who are going to refuse chemotherapy for different reasons.”
These clinical trial results help clinicians to explain the risks and benefits of treatment options, but the treatment decision should be taken “together” with the patient, she emphasized.
Dr. Gnant has relationships with Sandoz, Amge, Daiichi Sankyo, AstraZeneca, Eli Lilly, Nanostring, Novartis, Pierre Fabre, TLC Pharmaceuticals, and Life Brain. Dr. Loibl has relationships with AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Meyers Squibb, Celgene, Daiichi Sankyo, Eirgenix, GSK, Gilead, Lilly, Merck, Novartis, Pfizer, Pierre Fabre, Medscape, Puma, Roche, Samsung, Seagen, VM Scope, and GBG Forschungs.
A version of this article first appeared on Medscape.com.
Is this the most controversial topic in breast oncology? Quite likely: the results of a recent online poll show split votes and no consensus.
The topic is the use of chemotherapy for premenopausal women with early-stage hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2-) breast cancer.
, as the other expert countered?
The debate was held during the recent San Antonio Breast Cancer Symposium (SABCS), at which new results were presented that increased the controversy.
The controversy had arisen the previous year over results from the RxPONDER trial.
Five-year follow-up data from RxPONDER showed that adding chemotherapy to endocrine therapy did not improve outcomes over endocrine therapy alone for postmenopausal women with low-risk, node-positive HR+, HER2- breast cancer. This suggests that older women with early-stage breast cancer may safely forgo chemotherapy.
However, the same trial included premenopausal women with the same disease profile, and the results in this subgroup showed that there was benefit from chemotherapy, with a 5-year invasive disease-free survival (IDFS) rate of 94.2%, versus 89.0% for endocrine therapy alone (P = .0004).
The results were immediately controversial.
Some experts suggested the effect was due to the chemotherapy incidentally causing ovarian suppression, not the cytotoxic effect of the drugs on cancer cells. These experts were skeptical about the suggestion that chemotherapy works differently in premenopausal women than it does in postmenopausal women.
Some clinicians feel the lack of clarity creates an opportunity for greater discussion with women when making the treatment decision.
“When I have this conversation with patients, it’s really nuanced,” Stephanie L. Graff, MD, director of breast oncology, Lifespan Cancer Institute, Providence, R.I., told this news organization.
“I would choose chemotherapy for myself, but I’m a chemotherapy doctor, so I’m very comfortable with these medications and their side effects, and I am also very familiar with the slow burn of the side effects of endocrine therapy,” she said.
But for patients who are hearing their options for the first time, the idea of chemotherapy “feels scary,” and there is “a lot of stigma” associated with it, she commented.
Ultimately, she believes in offering patients as much information as possible, inasmuch as “knowledge is power.”
For Dr. Graff, the message from RxPONDER was that, in premenopausal patients with lymph node positive, HR+ breast cancer, “all comers benefited from chemotherapy.”
“And so if the goal is to be maximally aggressive and optimally lower your risk of distant recurrence, which is a life-threatening event, chemotherapy should offered.”
But chemotherapy comes with side effects, so it’s an important conversation to have with patients; RxPONDER showed that the absolute difference in the rate of distant recurrence with chemotherapy was relatively minor, she added.
Debate rages on
The debate at SABCS was moderated by Harold J. Burstein, MD, PhD, from the Dana-Farber Cancer Institute, Boston, who commented that if this was “a compelling question last week in clinic, it has now become red hot.”
At the meeting, held in December 2021, new longer-term data from the SOFT and TEXT clinical trials were presented, showing that ovarian suppression with tamoxifen plus an aromatase inhibitor provides a greater reduction in long-term risk of recurrence than tamoxifen alone.
Moreover, updated results from RxPONDER presented at the same session revealed that chemoendocrine therapy was associated with longer IDFS and distant relapse-free survival than endocrine therapy alone for women with one to three positive lymph nodes and a recurrence score of 25 or lower on the Oncotype DX (Genomic Health) 21-gene breast cancer assay.
Dr. Burstein said the debate over the use of chemotherapy in premenopausal women “is the most interesting question right now in early-stage breast cancer.”
The debate focused on the effect of chemotherapy in these patients – was it all down to ovarian function suppression?
Yes, argued Michael Gnant, MD, from the Medical University of Vienna.
Data from “modern adjuvant chemotherapy trials” suggest that chemo offers a 2%-3% benefit in distant disease-free survival at 5 years for premenopausal women, he noted. But the effect is much larger with ovarian function suppression via endocrine therapy, which provides 5-year disease-free and overall survival benefits of 9%-13%.
Older studies have shown that the benefit with chemotherapy is seen only in women who experience amenorrhea with the cytotoxic drugs, Dr. Gnant noted.
“In short, if you give adjuvant chemotherapy and you induce amenorrhea, then there is going to be a survival difference,” he said. “But if you give adjuvant chemotherapy and there is no amenorrhea, there won’t be an outcome difference.”
The ABSCG-05 trial, which compared endocrine therapy with chemotherapy, showed that “in the presence of optimal endocrine adjuvant treatment, adjuvant chemotherapy doesn’t add anything, because you have already achieved the effect of treatment-induced amenorrhea.”
So Dr. Gnant argued that the effect of chemotherapy in RxPONDER was due to ovarian function suppression.
But the real question is: “What does it mean for clinical practice?”
Dr. Gnant asserted that for the “large group of lower-risk premenopausal patients, tamoxifen will be good enough,” while those at moderate or intermediate risk should receive ovarian function suppression with either tamoxifen or an aromatase inhibitor, with the choice dictated by their adverse effects.
Chemotherapy “is just a graceless method of ovarian function suppression and should only be given to high-risk patients and to patients with endocrine nonresponsive disease,” he argued.
On the other side of the debate, Sibylle Loibl, MD, PhD, from the Centre of Hematology and Oncology, Bethanien, Frankfurt, argued that the effect is not all due to ovarian function suppression and that chemotherapy also has a cytotoxic effect in these patients.
“We need chemotherapy” because “cancer in young women is biologically different,” she asserted.
Dr. Loibl pointed to data currently awaiting publication in the Journal of the National Cancer Institute that suggest that younger women have “higher immune gene expression” that may make them more chemotherapy sensitive, and lower expression of hormone receptor genes, which “could make them less endocrine sensitive.”
She also cited data from a study from her own group that showed that pathologic complete response rates to neoadjuvant chemotherapy were higher in younger women with HR+, HER2- breast cancer, indicating a direct effect of chemotherapy on the disease and that age was an important prognostic factor.
The data on the induction of amenorrhea by chemotherapy is also not as clearcut as it seems, she commented. Chemotherapy does not achieve 100% amenorrhea, and gonadotropin-releasing hormone analogues are unable to suppress ovarian function in 20% of women.
Dr. Loibl concluded that the “chemotherapy effect is there, it is higher in young women with HR+, HER2- breast cancer,” and that the effect has two components.
“There is a direct cytotoxic effect which cannot be neglected, and there is an endocrine effect on the ovarian function suppression,” she argued.
“I think both are needed in young premenopausal patients,” she added.
Audience responses
After the debate, the audience was polled on what effect they thought chemotherapy was having in lower-risk HR+, HER2- breast cancer patients. About two-thirds responded that it was all or mostly due to ovarian function suppression.
However, the next question split the audience. They were presented with a clinical scenario: a 43-year-old woman with a mammographically detected 1.4-cm, intermediate grade, HR+, HER2- breast cancer who also had metastatic disease in one of three sentinel lymph nodes and whose recurrence score was 13.
When asked about the treatment plan they would choose for this patient, the audience was split over whether to opt for chemoendocrine therapy or endocrine therapy alone.
A similar clinical question was posited recently on Twitter, when Angela Toss, MD, PhD, from the University of Modena and Reggio Emilia, Italy, asked respondents which they would chose from among three options.
From the 815 votes that were cast, 46% chose Oncotype DX testing to determine the likely benefit of chemotherapy, 48% chose chemotherapy, and 6% picked ovarian function suppression and an aromatase inhibitor.
In response, Paolo Tarantino, MD, from the Dana-Farber Cancer Institute, commented: “If you had any doubt of which is the most controversial topic in breast oncology, doubt no more. 815 votes, no consensus.”
Approached for comment, Eric Winer, MD, director of the Yale Cancer Center, New Haven, Conn., said that the data from RxPONDER “in many ways was helpful, but ... it created about as many questions as it answered, if not more.”
Because the results showed a benefit from chemotherapy for premenopausal women but not for postmenopausal women with breast cancer, Dr. Winer told this news organization that one of the outstanding questions is “whether premenopausal women are fundamentally different from postmenopausal women ... and my answer to that is that is very unlikely.”
Dr. Winer added that the “real tragedy” of this trial was that it did not include women with more than three positive nodes, particularly those who have a low recurrence score, he said.
Clinicians are therefore left either “extrapolating” data from those with fewer nodes or “marching down a path that we’ve taken for years of just giving those people chemotherapy routinely,” even though there may be no benefit, Dr. Winer commented.
Another expert who was approached for comment had a different take on the data. Matteo Lambertini, MD, IRCCS Ospedale Policlinico San Martino, Genoa, Italy, agreed with Loibl’s argument that chemotherapy has a cytotoxic effect in premenopausal women with HR+, HER2- breast cancer in addition to its effect on ovarian function suppression.
He did not agree, however, that there is a question mark over what to do for patients with more than three positive lymph nodes.
Dr. Lambertini said in an interview that he thinks “too much” trust is placed in genomic testing and that there is a “risk of forgetting about all the other factors that we normally use to make our treatment choices.”
A patient with five positive nodes will benefit from chemotherapy, “even if she had a very low recurrence score,” he said, “because there is a very high clinical risk of disease recurrence,” and chemotherapy “is of benefit” in these situations, he asserted.
Dr. Lambertini said that the RxPONDER results – and also studies such as TAILORx, which demonstrated the ability of Oncotype DX to identify which patients with early breast cancer could skip chemotherapy – show that “chemotherapy has a role to play” and that most patients should receive it.
He suggested, however, that “probably the benefit of chemotherapy is smaller” in real life than was seen in these trials, because in the trials, they did not use optimal adjuvant endocrine therapy.
Treating individual patients
When it comes to making treatment decisions for individual patients, Dr. Winer said he has a “conversation with people about what the results of the study showed and what [he believes] that they need.”
For patients whose Oncotype DX score is in the “very low range, I do not recommend chemotherapy,” he said, preferring instead to use endocrine therapy for ovarian function suppression.
For women with a more intermediate score, “I explain that I don’t think we have an answer and that, if they would want to take the most traditional and conservative path, it would be to get chemotherapy.
“But I’m certainly not rigid about my recommendations, and I’m particularly open” to ovarian function suppression for a premenopausal woman with an Onctyope DX score of 20 and two positive nodes who does not have “other adverse features.”
“Ultimately, what pushes me in one direction or another,” Dr. Winer said, aside from number of positive nodes or the size of the tumor, “is the patient’s preferences.”
This was a theme taken up by Kim Sabelko, PhD, vice-president of scientific strategy and programs at Susan G. Komen, Dallas.
The results from RxPONDER and similar studies are “really interesting,” as researchers are “working out how to individualize treatment,” and that it is not a matter of “one size fits all.”
“We need to understand when to use chemotherapy and other drugs, and more importantly, when not to, because we don’t want to overtreat people who don’t necessarily need these drugs,” she commented.
Dr. Sabelko emphasized that treatment decisions “should be shared” between the patient and their doctor, and she noted that there “will be some people who are going to refuse chemotherapy for different reasons.”
These clinical trial results help clinicians to explain the risks and benefits of treatment options, but the treatment decision should be taken “together” with the patient, she emphasized.
Dr. Gnant has relationships with Sandoz, Amge, Daiichi Sankyo, AstraZeneca, Eli Lilly, Nanostring, Novartis, Pierre Fabre, TLC Pharmaceuticals, and Life Brain. Dr. Loibl has relationships with AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Meyers Squibb, Celgene, Daiichi Sankyo, Eirgenix, GSK, Gilead, Lilly, Merck, Novartis, Pfizer, Pierre Fabre, Medscape, Puma, Roche, Samsung, Seagen, VM Scope, and GBG Forschungs.
A version of this article first appeared on Medscape.com.
Is this the most controversial topic in breast oncology? Quite likely: the results of a recent online poll show split votes and no consensus.
The topic is the use of chemotherapy for premenopausal women with early-stage hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2-) breast cancer.
, as the other expert countered?
The debate was held during the recent San Antonio Breast Cancer Symposium (SABCS), at which new results were presented that increased the controversy.
The controversy had arisen the previous year over results from the RxPONDER trial.
Five-year follow-up data from RxPONDER showed that adding chemotherapy to endocrine therapy did not improve outcomes over endocrine therapy alone for postmenopausal women with low-risk, node-positive HR+, HER2- breast cancer. This suggests that older women with early-stage breast cancer may safely forgo chemotherapy.
However, the same trial included premenopausal women with the same disease profile, and the results in this subgroup showed that there was benefit from chemotherapy, with a 5-year invasive disease-free survival (IDFS) rate of 94.2%, versus 89.0% for endocrine therapy alone (P = .0004).
The results were immediately controversial.
Some experts suggested the effect was due to the chemotherapy incidentally causing ovarian suppression, not the cytotoxic effect of the drugs on cancer cells. These experts were skeptical about the suggestion that chemotherapy works differently in premenopausal women than it does in postmenopausal women.
Some clinicians feel the lack of clarity creates an opportunity for greater discussion with women when making the treatment decision.
“When I have this conversation with patients, it’s really nuanced,” Stephanie L. Graff, MD, director of breast oncology, Lifespan Cancer Institute, Providence, R.I., told this news organization.
“I would choose chemotherapy for myself, but I’m a chemotherapy doctor, so I’m very comfortable with these medications and their side effects, and I am also very familiar with the slow burn of the side effects of endocrine therapy,” she said.
But for patients who are hearing their options for the first time, the idea of chemotherapy “feels scary,” and there is “a lot of stigma” associated with it, she commented.
Ultimately, she believes in offering patients as much information as possible, inasmuch as “knowledge is power.”
For Dr. Graff, the message from RxPONDER was that, in premenopausal patients with lymph node positive, HR+ breast cancer, “all comers benefited from chemotherapy.”
“And so if the goal is to be maximally aggressive and optimally lower your risk of distant recurrence, which is a life-threatening event, chemotherapy should offered.”
But chemotherapy comes with side effects, so it’s an important conversation to have with patients; RxPONDER showed that the absolute difference in the rate of distant recurrence with chemotherapy was relatively minor, she added.
Debate rages on
The debate at SABCS was moderated by Harold J. Burstein, MD, PhD, from the Dana-Farber Cancer Institute, Boston, who commented that if this was “a compelling question last week in clinic, it has now become red hot.”
At the meeting, held in December 2021, new longer-term data from the SOFT and TEXT clinical trials were presented, showing that ovarian suppression with tamoxifen plus an aromatase inhibitor provides a greater reduction in long-term risk of recurrence than tamoxifen alone.
Moreover, updated results from RxPONDER presented at the same session revealed that chemoendocrine therapy was associated with longer IDFS and distant relapse-free survival than endocrine therapy alone for women with one to three positive lymph nodes and a recurrence score of 25 or lower on the Oncotype DX (Genomic Health) 21-gene breast cancer assay.
Dr. Burstein said the debate over the use of chemotherapy in premenopausal women “is the most interesting question right now in early-stage breast cancer.”
The debate focused on the effect of chemotherapy in these patients – was it all down to ovarian function suppression?
Yes, argued Michael Gnant, MD, from the Medical University of Vienna.
Data from “modern adjuvant chemotherapy trials” suggest that chemo offers a 2%-3% benefit in distant disease-free survival at 5 years for premenopausal women, he noted. But the effect is much larger with ovarian function suppression via endocrine therapy, which provides 5-year disease-free and overall survival benefits of 9%-13%.
Older studies have shown that the benefit with chemotherapy is seen only in women who experience amenorrhea with the cytotoxic drugs, Dr. Gnant noted.
“In short, if you give adjuvant chemotherapy and you induce amenorrhea, then there is going to be a survival difference,” he said. “But if you give adjuvant chemotherapy and there is no amenorrhea, there won’t be an outcome difference.”
The ABSCG-05 trial, which compared endocrine therapy with chemotherapy, showed that “in the presence of optimal endocrine adjuvant treatment, adjuvant chemotherapy doesn’t add anything, because you have already achieved the effect of treatment-induced amenorrhea.”
So Dr. Gnant argued that the effect of chemotherapy in RxPONDER was due to ovarian function suppression.
But the real question is: “What does it mean for clinical practice?”
Dr. Gnant asserted that for the “large group of lower-risk premenopausal patients, tamoxifen will be good enough,” while those at moderate or intermediate risk should receive ovarian function suppression with either tamoxifen or an aromatase inhibitor, with the choice dictated by their adverse effects.
Chemotherapy “is just a graceless method of ovarian function suppression and should only be given to high-risk patients and to patients with endocrine nonresponsive disease,” he argued.
On the other side of the debate, Sibylle Loibl, MD, PhD, from the Centre of Hematology and Oncology, Bethanien, Frankfurt, argued that the effect is not all due to ovarian function suppression and that chemotherapy also has a cytotoxic effect in these patients.
“We need chemotherapy” because “cancer in young women is biologically different,” she asserted.
Dr. Loibl pointed to data currently awaiting publication in the Journal of the National Cancer Institute that suggest that younger women have “higher immune gene expression” that may make them more chemotherapy sensitive, and lower expression of hormone receptor genes, which “could make them less endocrine sensitive.”
She also cited data from a study from her own group that showed that pathologic complete response rates to neoadjuvant chemotherapy were higher in younger women with HR+, HER2- breast cancer, indicating a direct effect of chemotherapy on the disease and that age was an important prognostic factor.
The data on the induction of amenorrhea by chemotherapy is also not as clearcut as it seems, she commented. Chemotherapy does not achieve 100% amenorrhea, and gonadotropin-releasing hormone analogues are unable to suppress ovarian function in 20% of women.
Dr. Loibl concluded that the “chemotherapy effect is there, it is higher in young women with HR+, HER2- breast cancer,” and that the effect has two components.
“There is a direct cytotoxic effect which cannot be neglected, and there is an endocrine effect on the ovarian function suppression,” she argued.
“I think both are needed in young premenopausal patients,” she added.
Audience responses
After the debate, the audience was polled on what effect they thought chemotherapy was having in lower-risk HR+, HER2- breast cancer patients. About two-thirds responded that it was all or mostly due to ovarian function suppression.
However, the next question split the audience. They were presented with a clinical scenario: a 43-year-old woman with a mammographically detected 1.4-cm, intermediate grade, HR+, HER2- breast cancer who also had metastatic disease in one of three sentinel lymph nodes and whose recurrence score was 13.
When asked about the treatment plan they would choose for this patient, the audience was split over whether to opt for chemoendocrine therapy or endocrine therapy alone.
A similar clinical question was posited recently on Twitter, when Angela Toss, MD, PhD, from the University of Modena and Reggio Emilia, Italy, asked respondents which they would chose from among three options.
From the 815 votes that were cast, 46% chose Oncotype DX testing to determine the likely benefit of chemotherapy, 48% chose chemotherapy, and 6% picked ovarian function suppression and an aromatase inhibitor.
In response, Paolo Tarantino, MD, from the Dana-Farber Cancer Institute, commented: “If you had any doubt of which is the most controversial topic in breast oncology, doubt no more. 815 votes, no consensus.”
Approached for comment, Eric Winer, MD, director of the Yale Cancer Center, New Haven, Conn., said that the data from RxPONDER “in many ways was helpful, but ... it created about as many questions as it answered, if not more.”
Because the results showed a benefit from chemotherapy for premenopausal women but not for postmenopausal women with breast cancer, Dr. Winer told this news organization that one of the outstanding questions is “whether premenopausal women are fundamentally different from postmenopausal women ... and my answer to that is that is very unlikely.”
Dr. Winer added that the “real tragedy” of this trial was that it did not include women with more than three positive nodes, particularly those who have a low recurrence score, he said.
Clinicians are therefore left either “extrapolating” data from those with fewer nodes or “marching down a path that we’ve taken for years of just giving those people chemotherapy routinely,” even though there may be no benefit, Dr. Winer commented.
Another expert who was approached for comment had a different take on the data. Matteo Lambertini, MD, IRCCS Ospedale Policlinico San Martino, Genoa, Italy, agreed with Loibl’s argument that chemotherapy has a cytotoxic effect in premenopausal women with HR+, HER2- breast cancer in addition to its effect on ovarian function suppression.
He did not agree, however, that there is a question mark over what to do for patients with more than three positive lymph nodes.
Dr. Lambertini said in an interview that he thinks “too much” trust is placed in genomic testing and that there is a “risk of forgetting about all the other factors that we normally use to make our treatment choices.”
A patient with five positive nodes will benefit from chemotherapy, “even if she had a very low recurrence score,” he said, “because there is a very high clinical risk of disease recurrence,” and chemotherapy “is of benefit” in these situations, he asserted.
Dr. Lambertini said that the RxPONDER results – and also studies such as TAILORx, which demonstrated the ability of Oncotype DX to identify which patients with early breast cancer could skip chemotherapy – show that “chemotherapy has a role to play” and that most patients should receive it.
He suggested, however, that “probably the benefit of chemotherapy is smaller” in real life than was seen in these trials, because in the trials, they did not use optimal adjuvant endocrine therapy.
Treating individual patients
When it comes to making treatment decisions for individual patients, Dr. Winer said he has a “conversation with people about what the results of the study showed and what [he believes] that they need.”
For patients whose Oncotype DX score is in the “very low range, I do not recommend chemotherapy,” he said, preferring instead to use endocrine therapy for ovarian function suppression.
For women with a more intermediate score, “I explain that I don’t think we have an answer and that, if they would want to take the most traditional and conservative path, it would be to get chemotherapy.
“But I’m certainly not rigid about my recommendations, and I’m particularly open” to ovarian function suppression for a premenopausal woman with an Onctyope DX score of 20 and two positive nodes who does not have “other adverse features.”
“Ultimately, what pushes me in one direction or another,” Dr. Winer said, aside from number of positive nodes or the size of the tumor, “is the patient’s preferences.”
This was a theme taken up by Kim Sabelko, PhD, vice-president of scientific strategy and programs at Susan G. Komen, Dallas.
The results from RxPONDER and similar studies are “really interesting,” as researchers are “working out how to individualize treatment,” and that it is not a matter of “one size fits all.”
“We need to understand when to use chemotherapy and other drugs, and more importantly, when not to, because we don’t want to overtreat people who don’t necessarily need these drugs,” she commented.
Dr. Sabelko emphasized that treatment decisions “should be shared” between the patient and their doctor, and she noted that there “will be some people who are going to refuse chemotherapy for different reasons.”
These clinical trial results help clinicians to explain the risks and benefits of treatment options, but the treatment decision should be taken “together” with the patient, she emphasized.
Dr. Gnant has relationships with Sandoz, Amge, Daiichi Sankyo, AstraZeneca, Eli Lilly, Nanostring, Novartis, Pierre Fabre, TLC Pharmaceuticals, and Life Brain. Dr. Loibl has relationships with AbbVie, Amgen, AstraZeneca, Bayer, Bristol-Meyers Squibb, Celgene, Daiichi Sankyo, Eirgenix, GSK, Gilead, Lilly, Merck, Novartis, Pfizer, Pierre Fabre, Medscape, Puma, Roche, Samsung, Seagen, VM Scope, and GBG Forschungs.
A version of this article first appeared on Medscape.com.



