User login
AVAHO
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]


Skull Base Regeneration During Treatment With Chemoradiation for Nasopharyngeal Carcinoma: A Case Report
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
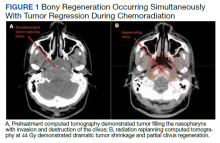
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
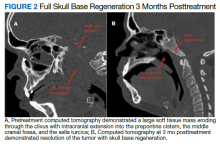
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
Nasopharyngeal carcinoma (NPC) differs from other head and neck (H&N) cancers in its epidemiology and treatment. Unlike other H&N cancers, NPC has a distinct geographical distribution with a much higher incidence in endemic areas, such as southern China, than in areas where it is relatively uncommon, such as the United States.1 The etiology of NPC varies based on the geographical distribution, with Epstein-Barr virus (EBV) thought to be the primary etiologic agent in endemic areas. On the other hand, in North America 2 additional subsets of NPC have been identified: human papillomavirus (HPV)–positive/EBV-negative and HPV-negative/EBV-negative.2,3 NPC arises from the epithelial lining of the nasopharynx, often in the fossa of Rosenmuller, and is the most seen tumor in the nasopharynx.4 NPC is less surgically accessible than other H&N cancers, and surgery to the nasopharynx poses more risks given the proximity of critical surrounding structures. NPC is radiosensitive, and therefore radiotherapy (RT), in combination with chemotherapy for locally advanced tumors, has become the mainstay of treatment for nonmetastatic NPC.4
NPC often presents with an asymptomatic neck mass or with symptoms of epistaxis, nasal obstruction, and otitis media.5 Advanced cases of NPC can present with direct extension into the skull base, paranasal sinuses, and orbit, as well as involvement of cranial nerves. Radiation planning for tumors of the nasopharynx is complicated by the need to deliver an adequate dose to the tumor while limiting dose and toxicity to nearby critical structures such as the brainstem, optic chiasm, eyes, spinal cord (SC), temporal lobes, and cochleae. Achieving an adequate dose to nasopharyngeal primary tumors is especially complicated for T4 tumors invading the skull base with intracranial extension, in direct contact with these critical structures (Table 1).
Skull base invasion is a poor prognostic factor, predicting for an increased risk of locoregional recurrence and worse overall survival. Furthermore, the extent of skull base invasion in NPC affects overall prognosis, with cranial nerve involvement and intracranial extension predictive for worse outcomes.5 Depending on the extent of destruction, a bony defect along the skull base could develop with tumor shrinkage during RT, resulting in complications such as cerebrospinal fluid leaks, herniation, and atlantoaxial instability.6
There is a paucity of literature on the ability of bone to regenerate during or after RT for cases of NPC with skull base destruction. To our knowledge, nothing has been published detailing the extent of bony regeneration that can occur during treatment itself, as the tumor regresses and poses a threat of a skull base defect. Here we present a case of T4 HPV-positive/EBV-negative NPC with intracranial extension and describe the RT planning methods leading to prolonged local control, limited toxicities, and bony regeneration of the skull base during treatment.
Case Presentation
A 34-year-old male patient with no previous medical history presented to the emergency department with worsening diplopia, nasal obstruction, facial pain, and neck stiffness. The patient reported a 3 pack-year smoking history with recent smoking cessation. His physical examination was notable for a right abducens nerve palsy and an ulcerated nasopharyngeal mass on endoscopy.
Computed tomography (CT) scan revealed a 7-cm mass in the nasopharynx, eroding through the skull base with destruction and replacement of the clivus by tumor. Also noted was erosion of the petrous apices, carotid canals, sella turcica, dens, and the bilateral occipital condyles. There was intracranial extension with replacement of portions of the cavernous sinuses as well as mass effect on the prepontine cistern. Additional brain imaging studies, including magnetic resonance imaging (MRI) and positron emission tomography (PET) scans, were obtained for completion of the staging workup. The MRI correlated with the findings noted on CT and demonstrated involvement of Meckel cave, foramen ovale, foramen rotundum, Dorello canal, and the hypoglossal canals. No cervical lymphadenopathy or distant metastases were noted on imaging. Pathology from biopsy revealed poorly differentiated squamous cell carcinoma, EBV-negative, strongly p16-positive, HPV-16 positive, and P53-negative.
The H&N multidisciplinary tumor board recommended concurrent chemoradiation for this stage IVA (T4N0M0) EBV-negative, HPV-positive, Word Health Organization type I NPC (Table 2). The patient underwent CT simulation for RT planning, and both tumor volumes and critical normal structures were contoured. The goal was to deliver 70 Gy to the gross tumor. However, given the inability to deliver this dose while meeting the SC dose tolerance of < 45 Gy, a 2-Gy fraction was removed. Therefore, 34 fractions of 2 Gy were delivered to the tumor volume for a total dose of 68 Gy. Weekly cisplatin, at a dose of 40 mg/m2, was administered concurrently with RT.
RT planning was complicated by the tumor’s contact with the brainstem and upper cervical SC, as well as proximity of the tumor to the optic apparatus. The patient underwent 2 replanning CT scans at 26 Gy and 44 Gy to evaluate for tumor shrinkage. These CT scans demonstrated shrinkage of the tumor away from critical neural structures, allowing the treatment volume to be reduced away from these structures in order to achieve required dose tolerances (brainstem < 54 Gy, optic nerves and chiasm < 50 Gy, SC < 45 Gy for this case). The replanning CT scan at 44 Gy, 5 weeks after treatment initiation, demonstrated that dramatic tumor shrinkage had occurred early in treatment, with separation of the remaining tumor from the area of the SC and brainstem with which it was initially in contact (Figure 1). This improvement allowed for shrinkage of the high-dose radiation field away from these critical neural structures.
Baseline destruction of the skull base by tumor raised concern for craniospinal instability with tumor response. The patient was evaluated by neurosurgery before the start of RT, and the recommendation was for reimaging during treatment and close follow-up of the patient’s symptoms to determine whether surgical fixation would be indicated during or after treatment. The patient underwent a replanning CT scan at 44 Gy, 5 weeks after treatment initiation, that demonstrated impressive bony regeneration occurring during chemoradiation. New bone formation was noted in the region of the clivus and bilateral occipital condyles, which had been absent on CT prior to treatment initiation. Another CT at 54 Gy demonstrated further ossification of the clivus and bilateral occipital condyles, and bony regeneration occurring rapidly during chemoradiation. The posttreatment CT 3 months after completion of chemoradiation demonstrated complete skull base regeneration, maintaining stability of this area and precluding the need for neurosurgical intervention (Figure 2).
During RT,
The patient had no evidence of disease at 5 years posttreatment. After completing treatment, the patient experienced ongoing intermittent nasal congestion and occasional aural fullness. He experienced an early decay of several teeth starting 1 year after completion of RT, and he continues to visit his dentist for management. He experienced no other treatment-related toxicities. In particular, he has exhibited no signs of neurologic toxicity to date.
Discussion
RT for NPC is complicated by the proximity of these tumors to critical surrounding neural structures. It is challenging to achieve the required dose constraints to surrounding neural tissues while delivering the usual 70-Gy dose to the gross tumor, especially when the tumor comes into direct contact with these structures.
This case provides an example of response-adapted RT using imaging during treatment to shrink the high-dose target as the tumor shrinks away from critical surrounding structures.7 This strategy permits delivery of the maximum dose to the tumor while minimizing radiation dose, and therefore risk of toxicity, to normal surrounding structures. While it is typical to deliver 70 Gy to the full extent of tumor involvement for H&N tumors, this was not possible in this case as the tumor was in contact with the brainstem and upper cervical SC. Delivering the full 70 Gy to these areas of tumor would have placed this patient at substantial risk of brainstem and/or SC toxicity. This report demonstrates that response-adapted RT with shrinking fields can allow for tumor control while avoiding toxicity to critical neural structures for cases of locally advanced NPC in which tumor is abutting these structures.
Bony regeneration of the skull base following RT has been reported in the literature, but in limited reviews. Early reports used plain radiography to follow changes. Unger and colleagues demonstrated the regeneration of bone using skull radiographs 4 to 6 months after completion of RT for NPC.8 More recent literature details the ability of bone to regenerate after RT based on CT findings. Fang and colleagues reported on 90 cases of NPC with skull base destruction, with 63% having bony regeneration on posttreatment CT.9 Most of the patients in Fang’s report had bony regeneration within 1 year of treatment, and in general, bony regeneration became more evident on imaging with longer follow-up. Of note, local control was significantly greater in patients with regeneration vs persistent destruction (77% vs 21%, P < .001). On multivariate analysis, complete tumor response was significantly associated with bony regeneration; other factors such as age, sex, radiation dose, and chemotherapy were not significantly associated with the likelihood of bony regeneration.
Our report details a nasopharyngeal tumor that destroyed the skull base with no intact bony barrier. In such cases, concern arises regarding craniospinal instability with tumor regression if there is not simultaneous bone regeneration. Tumor invasion of the skull base and C1-2 vertebral bodies and complications from treatment of such tumor extent can lead to symptoms of craniospinal instability, including pain, difficulty with neck range of motion, and loss of strength and sensation in the upper and lower extremities.10 A case report of a woman treated with chemoradiation for a plasmacytoma of the skull base detailed her posttreatment presentation with quadriparesis resulting from craniospinal instability after tumor regression.11 Such instability is generally treated surgically, and during this woman’s surgery, there was an injury to the right vertebral artery, although this did not cause any additional neurologic deficits.
RT leads to hypocellularity, hypovascularity, and hypoxia of treated tissues, resulting in a reduced ability for growth and healing. Studies demonstrate that irradiated bone contains fewer osteoblast cells and osteocytes than unirradiated bone, resulting in reduced regenerative capacity.12,13 Furthermore, the reconstruction of bony defects resulting after cancer treatment has been shown to be difficult and associated with a high risk of complications.14 Given the impaired ability of irradiated bone to regenerate, studies have evaluated the use of growth factors and gene therapy to promote bone formation after treatment.15 Bone marrow stem cells have been shown to reverse radiation-induced cellular depletion and to increase osteocyte counts in animal studies.12 Further, overexpression of miR-34a, a tumor suppressor involved in tissue development, has been shown to improve osteoblastic differentiation of irradiated bone marrow stem cells and promote bone regeneration in vitro and in animal studies.13 While several techniques are being studied in vitro and in animal studies to promote bony regeneration after RT, there is a lack of data on use of these techniques in humans with cancer.
With our case, there was great uncertainty related to the ability of bone to regenerate during treatment and concern regarding consequences of formation of a skull base defect during treatment. CT imaging revealed bony regeneration of the central skull base and clivus, as well as occipital condyles, that occurred throughout the RT course. There was clear evidence of bone regeneration on the replanning CT obtained 5 weeks after treatment initiation. To our knowledge, this is the first report to demonstrate rapid bony regeneration during RT, thereby maintaining the integrity of the skull base and precluding the need for neurosurgical intervention. Moving forward, imaging should be considered during treatment for patients with tumor-related destruction of the skull base and upper cervical spine to evaluate the extent of bony regeneration during treatment and estimate the potential risk of craniocervical instability. Further studies with imaging during treatment are needed for more information on the likelihood of bony regeneration and factors that correlate with bony regeneration during treatment. As in other reports, our case demonstrates that bony regeneration may predict complete response to RT.9
Our patient’s tumor was HPV-positive and EBV-negative. In the US, the rate of HPV-positive NPC is 35%.16 However, HPV-positive NPC is much less common in endemic areas. A recent study from China of 1,328 patients with NPC revealed a 6.4% rate of HPV-positive/EBV-negative cases.17 In that study, patients with HPV-positive/EBV-negative tumors had improved survival compared to patients whose tumors were HPV-negative/EBV-positive. Another study suggests that the impact of HPV in NPC varies according to race, with HPV-positivity predicting for improved outcomes in East Asian patients and worse outcomes in White patients.17 A study from the University of Michigan suggests that both HPV-positive/EBV-negative and HPV-negative/EBV-negative NPC are associated with worse overall survival and locoregional control than EBV-positive NPC.2 Overall, the prognostic role of HPV in NPC remains unclear given conflicting information in the literature and the lack of large population studies.18
Conclusions
There is a paucity of literature on bony regeneration in patients with skull base destruction from advanced NPC, and in particular, the ability of skull base regeneration to occur during treatment simultaneous with tumor regression. Our patient had HPV-positive/EBV-negative NPC, but it is unclear how this subtype affected his prognosis. Factors such as tumor histology, radiosensitivity with rapid tumor regression, and young age may have all contributed to the rapidity of bone regeneration in our patient. This case report demonstrates that an impressive tumor response to chemoradiation with simultaneous bony regeneration is possible among patients presenting with tumor destruction of the skull base, precluding the need for neurosurgical intervention.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
1. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777. doi:10.1158/1055-9965.EPI-06-0353
2. Stenmark MH, McHugh JB, Schipper M, et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys. 2014;88(3):580-588. doi:10.1016/j.ijrobp.2013.11.246
3. Maxwell JH, Kumar B, Feng FY, et al. HPV-positive/p16-positive/EBV-negative nasopharyngeal carcinoma in white North Americans. Head Neck. 2010;32(5):562-567. doi:10.1002/hed.21216
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64-80. doi:10.1016/S0140-6736(19)30956-0
5. Roh JL, Sung MW, Kim KH, et al.. Nasopharyngeal carcinoma with skull base invasion: a necessity of staging subdivision. Am J Otolaryngol. 2004;25(1):26-32. doi:10.1016/j.amjoto.2003.09.011
6. Orr RD, Salo PT. Atlantoaxial instability complicating radiation therapy for recurrent nasopharyngeal carcinoma. A case report. Spine. 1998;23(11):1280-1282. doi:10.1097/00007632-199806010-00021
7. Morgan HE, Sher DJ. Adaptive radiotherapy for head and neck cancer. Cancers Head Neck. 2020;5:1. doi:10.1186/s41199-019-0046-z
8. Unger JD, Chiang LC, Unger GF. Apparent reformation of the base of the skull following radiotherapy for nasopharyngeal carcinoma. Radiology. 1978;126(3):779-782. doi:10.1148/126.3.779
9. Fang FM, Leung SW, Wang CJ, et al. Computed tomography findings of bony regeneration after radiotherapy for nasopharyngeal carcinoma with skull base destruction: implications for local control. Int J Radiat Oncol Biol Phys. 1999;44(2):305-309. doi:10.1016/s0360-3016(99)00004-8
10. Tiruchelvarayan R, Lee KA, Ng I. Surgery for atlanto-axial (C1-2) involvement or instability in nasopharyngeal carcinoma patients. Singapore Med J. 2012;53(6):416-421.
11. Samprón N, Arrazola M, Urculo E. Skull-base plasmacytoma with craniocervical instability [in Spanish]. Neurocirugia (Astur). 2009;20(5):478-483.
12. Zheutlin AR, Deshpande SS, Nelson NS, et al. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: a histomorphometric evaluation. Cytotherapy. 2016;18(5):664-672. doi:10.1016/j.jcyt.2016.01.013
13. Liu H, Dong Y, Feng X, et al. miR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblast differentiation of mesenchymal stromal cells in rats. Stem Cell Res Ther. 2019;10(1):180. doi:10.1186/s13287-019-1285-y
14. Holzapfel BM, Wagner F, Martine LC, et al. Tissue engineering and regenerative medicine in musculoskeletal oncology. Cancer Metastasis Rev. 2016;35(3):475-487. doi:10.1007/s10555-016-9635-z
15. Hu WW, Ward BB, Wang Z, Krebsbach PH. Bone regeneration in defects compromised by radiotherapy. J Dent Res. 2010;89(1):77-81. doi:10.1177/0022034509352151
16. Wotman M, Oh EJ, Ahn S, Kraus D, Constantino P, Tham T. HPV status in patients with nasopharyngeal carcinoma in the United States: a SEER database study. Am J Otolaryngol. 2019;40(5):705-710. doi:10.1016/j.amjoto.2019.06.00717. Huang WB, Chan JYW, Liu DL. Human papillomavirus and World Health Organization type III nasopharyngeal carcinoma: multicenter study from an endemic area in Southern China. Cancer. 2018;124(3):530-536. doi:10.1002/cncr.31031.
18. Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck. 2018;40(4):696-706. doi:10.1002/hed.24978
19. Lee AWM, Lydiatt WM, Colevas AD, et al. Nasopharynx. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th ed. Springer; 2017:103.
20. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. Pathology and genetics of head and neck tumors. In: World Health Organization Classification of Tumours. IARC Press; 2005.
Early-onset colon cancer projected to double by 2030
from 7.9 to 12.9 cases in 2015 per 100,000 people. The reason for the increase isn’t well understood.
The findings were highlighted in a recent review article published online in the New England Journal of Medicine. “It’s a national phenomenon and it’s also occurring in other parts of the developed world. We’re used to seeing mostly older people who have this diagnosis. Now we’re seeing a lot of younger people with this disease. It’s rather alarming,” said author Frank Sinicrope, MD, a medical oncologist with Mayo Clinic, Rochester, Minn.
The trend contrasts with a decline in later-onset CRC likely attributable to increases in screening. As a result of the two trends, but especially the increased number of early-onset cases, the median age of diagnosis dropped from 72 in the early 2000s to 66 today.
“Although patients with early-onset colorectal cancer are more likely to have a hereditary syndrome than those who have later-onset disease, most cases are sporadic, with no identifiable cause. Furthermore, somatic mutational profiling of early-onset colorectal cancers has not revealed previously unidentified or actionable alterations to inform our understanding of the pathogenesis of these cancers or to guide treatment,” he wrote in the review.
“Early-onset colorectal cancers are most commonly detected in the rectum, followed by the distal colon; more than 70% of early-onset colorectal cancers are in the left colon at presentation,” he wrote in the review. Younger patients tend to be unfamiliar with CRC symptoms, which are often mistaken for benign conditions.
“We’ve moved the screening age down to 45, but that still is not going to capture a lot of these patients,” Dr. Sinicrope said. He estimates that 25% of rectal cancers and 10%-12% of colon cancers diagnosed in the next 10 years will be early onset.
Although the direct cause of the increased incidence isn’t clear, Dr. Sinicrope suggested it may reflect changing dietary habits and rising obesity among adolescents. “The sugar-containing beverages, the processed sugar and a lot of red meat in the diet and refined grains … reflect changes in the diet over the last 50 years. We may now be seeing the end result of many of these dietary changes that have occurred,” he said, calling for a greater emphasis on plant-based diets, which promote a healthier gut microbiome that may reduce CRC risk. Western-style diets can change the gut microbiome leading to inflammation which increases the risk of CRC.
Most patients with early CRC present with advanced disease in the left colon. And, pathogenic germline variants are present in one in six patients – half of which are associated with Lynch syndrome which increases the risk for CRC.
Dr. Sinicrope highlighted the need for more risk-based intervention, which in turn requires a better knowledge of family history.
“We need to do better job to risk stratify, and that will help us figure out who’s best to target our screening efforts toward,” Dr. Sinicrope said. He pointed out guidelines from the U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society that can help physicians identify patients who might benefit from earlier screening. The American Cancer Society recommends that CRC screening be conducted at 45 years for average-risk individuals.
“The best screening test is the one that the patient will do,” Dr. Sinicrope said.
from 7.9 to 12.9 cases in 2015 per 100,000 people. The reason for the increase isn’t well understood.
The findings were highlighted in a recent review article published online in the New England Journal of Medicine. “It’s a national phenomenon and it’s also occurring in other parts of the developed world. We’re used to seeing mostly older people who have this diagnosis. Now we’re seeing a lot of younger people with this disease. It’s rather alarming,” said author Frank Sinicrope, MD, a medical oncologist with Mayo Clinic, Rochester, Minn.
The trend contrasts with a decline in later-onset CRC likely attributable to increases in screening. As a result of the two trends, but especially the increased number of early-onset cases, the median age of diagnosis dropped from 72 in the early 2000s to 66 today.
“Although patients with early-onset colorectal cancer are more likely to have a hereditary syndrome than those who have later-onset disease, most cases are sporadic, with no identifiable cause. Furthermore, somatic mutational profiling of early-onset colorectal cancers has not revealed previously unidentified or actionable alterations to inform our understanding of the pathogenesis of these cancers or to guide treatment,” he wrote in the review.
“Early-onset colorectal cancers are most commonly detected in the rectum, followed by the distal colon; more than 70% of early-onset colorectal cancers are in the left colon at presentation,” he wrote in the review. Younger patients tend to be unfamiliar with CRC symptoms, which are often mistaken for benign conditions.
“We’ve moved the screening age down to 45, but that still is not going to capture a lot of these patients,” Dr. Sinicrope said. He estimates that 25% of rectal cancers and 10%-12% of colon cancers diagnosed in the next 10 years will be early onset.
Although the direct cause of the increased incidence isn’t clear, Dr. Sinicrope suggested it may reflect changing dietary habits and rising obesity among adolescents. “The sugar-containing beverages, the processed sugar and a lot of red meat in the diet and refined grains … reflect changes in the diet over the last 50 years. We may now be seeing the end result of many of these dietary changes that have occurred,” he said, calling for a greater emphasis on plant-based diets, which promote a healthier gut microbiome that may reduce CRC risk. Western-style diets can change the gut microbiome leading to inflammation which increases the risk of CRC.
Most patients with early CRC present with advanced disease in the left colon. And, pathogenic germline variants are present in one in six patients – half of which are associated with Lynch syndrome which increases the risk for CRC.
Dr. Sinicrope highlighted the need for more risk-based intervention, which in turn requires a better knowledge of family history.
“We need to do better job to risk stratify, and that will help us figure out who’s best to target our screening efforts toward,” Dr. Sinicrope said. He pointed out guidelines from the U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society that can help physicians identify patients who might benefit from earlier screening. The American Cancer Society recommends that CRC screening be conducted at 45 years for average-risk individuals.
“The best screening test is the one that the patient will do,” Dr. Sinicrope said.
from 7.9 to 12.9 cases in 2015 per 100,000 people. The reason for the increase isn’t well understood.
The findings were highlighted in a recent review article published online in the New England Journal of Medicine. “It’s a national phenomenon and it’s also occurring in other parts of the developed world. We’re used to seeing mostly older people who have this diagnosis. Now we’re seeing a lot of younger people with this disease. It’s rather alarming,” said author Frank Sinicrope, MD, a medical oncologist with Mayo Clinic, Rochester, Minn.
The trend contrasts with a decline in later-onset CRC likely attributable to increases in screening. As a result of the two trends, but especially the increased number of early-onset cases, the median age of diagnosis dropped from 72 in the early 2000s to 66 today.
“Although patients with early-onset colorectal cancer are more likely to have a hereditary syndrome than those who have later-onset disease, most cases are sporadic, with no identifiable cause. Furthermore, somatic mutational profiling of early-onset colorectal cancers has not revealed previously unidentified or actionable alterations to inform our understanding of the pathogenesis of these cancers or to guide treatment,” he wrote in the review.
“Early-onset colorectal cancers are most commonly detected in the rectum, followed by the distal colon; more than 70% of early-onset colorectal cancers are in the left colon at presentation,” he wrote in the review. Younger patients tend to be unfamiliar with CRC symptoms, which are often mistaken for benign conditions.
“We’ve moved the screening age down to 45, but that still is not going to capture a lot of these patients,” Dr. Sinicrope said. He estimates that 25% of rectal cancers and 10%-12% of colon cancers diagnosed in the next 10 years will be early onset.
Although the direct cause of the increased incidence isn’t clear, Dr. Sinicrope suggested it may reflect changing dietary habits and rising obesity among adolescents. “The sugar-containing beverages, the processed sugar and a lot of red meat in the diet and refined grains … reflect changes in the diet over the last 50 years. We may now be seeing the end result of many of these dietary changes that have occurred,” he said, calling for a greater emphasis on plant-based diets, which promote a healthier gut microbiome that may reduce CRC risk. Western-style diets can change the gut microbiome leading to inflammation which increases the risk of CRC.
Most patients with early CRC present with advanced disease in the left colon. And, pathogenic germline variants are present in one in six patients – half of which are associated with Lynch syndrome which increases the risk for CRC.
Dr. Sinicrope highlighted the need for more risk-based intervention, which in turn requires a better knowledge of family history.
“We need to do better job to risk stratify, and that will help us figure out who’s best to target our screening efforts toward,” Dr. Sinicrope said. He pointed out guidelines from the U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society that can help physicians identify patients who might benefit from earlier screening. The American Cancer Society recommends that CRC screening be conducted at 45 years for average-risk individuals.
“The best screening test is the one that the patient will do,” Dr. Sinicrope said.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Head and neck cancer patients recommend 11 needed improvements in health care
HNC has a high burden of treatment-related adverse events, along with frequent trouble with speech, swallowing, facial disfigurement, and psychological distress.
Among cancer patients, “they have the highest rates of emergency department use and hospitalization during treatment. They also have the highest rates of psychological distress. We have some Ontario data that shows they’ve got the highest rates of suicide and self-harm. So I think this is a really special population that we need to support,” Christopher Noel, MD, PhD, said in an interview. Dr. Noel was the lead author of the study, which was published in JAMA Otolaryngology – Head & Neck Surgery.
These issues can strongly affect quality of life, and even patient outcomes. “Even a 1-day interruption in treatment has been shown to impact oncologic outcomes. This is a very big issue whether you’re a surgeon, a medical oncologist, or a radiation oncologist,” said Dr. Noel, who is a resident physician at the University of Toronto.
He advocates that physicians interview patients and review the results in a structured way and then act on it. “If we just rely on patient [provided] communication, we’re going to miss about 50% of patient symptoms,” he said.
The researchers aimed for the patient’s perspective on treatment. “What is the patient’s perception of going through head neck cancer and their treatment, and managing their symptoms at home? And where do they think that we could do better?” Dr. Noel asked.
The most pressing issue was that patients felt their emotional and informational needs often were not met. That challenge is even harder for patients who have trouble communicating, which in turn makes them more prone to isolation and loneliness. Many felt that they had to get the information on their own. “They wanted it to be a more effortless process,” said Dr. Noel.
He described one patient with oropharynx cancer who was able to talk to people about her grief over her diagnosis, but treatment led to her throat becoming swollen and she lost the ability to communicate. “She felt very isolated and lonely. She really highlighted the emotional and psychosocial barriers in cancer care. Her treatment inherently leaves her feeling very isolated and lonely, and she had such a hard time connecting with a psychotherapist,” Dr. Noel said.
Another common issue revolved around efforts to communicate about symptoms and adverse effects of treatment. Resources often aren’t available on evenings or weekends, and it can take time for a nurse to call them back. Patients wanted to see more modern approaches, such as use of email or apps.
The patients in the study recommended 11 health care improvements.
- 1. Nurse navigator teams should have hours extended to evenings and weekends.
- 2. Patient communication methods should be expanded, using methods like email or apps.
- 3. HNC resources should be more broadly disseminated.
- 4. Education and information approaches should be individualized to the patient.
- 5. All HNC patients should be offered psychological resources.
- 6. Mental health needs should be assessed repeatedly throughout treatment and extended care.
- 7. Physicians should recognize the added symptom burden often faced by patients who travel extensively for treatment.
- 8. Partners and caregivers should be included as part of the treatment team.
- 9. Share symptom data with patients, which can improve engagement.
- 10. Review symptom scores and act on them regularly.
- 11. A member of the care team should be identified to oversee symptom management.
Dr. Noel had no relevant financial disclosures.
HNC has a high burden of treatment-related adverse events, along with frequent trouble with speech, swallowing, facial disfigurement, and psychological distress.
Among cancer patients, “they have the highest rates of emergency department use and hospitalization during treatment. They also have the highest rates of psychological distress. We have some Ontario data that shows they’ve got the highest rates of suicide and self-harm. So I think this is a really special population that we need to support,” Christopher Noel, MD, PhD, said in an interview. Dr. Noel was the lead author of the study, which was published in JAMA Otolaryngology – Head & Neck Surgery.
These issues can strongly affect quality of life, and even patient outcomes. “Even a 1-day interruption in treatment has been shown to impact oncologic outcomes. This is a very big issue whether you’re a surgeon, a medical oncologist, or a radiation oncologist,” said Dr. Noel, who is a resident physician at the University of Toronto.
He advocates that physicians interview patients and review the results in a structured way and then act on it. “If we just rely on patient [provided] communication, we’re going to miss about 50% of patient symptoms,” he said.
The researchers aimed for the patient’s perspective on treatment. “What is the patient’s perception of going through head neck cancer and their treatment, and managing their symptoms at home? And where do they think that we could do better?” Dr. Noel asked.
The most pressing issue was that patients felt their emotional and informational needs often were not met. That challenge is even harder for patients who have trouble communicating, which in turn makes them more prone to isolation and loneliness. Many felt that they had to get the information on their own. “They wanted it to be a more effortless process,” said Dr. Noel.
He described one patient with oropharynx cancer who was able to talk to people about her grief over her diagnosis, but treatment led to her throat becoming swollen and she lost the ability to communicate. “She felt very isolated and lonely. She really highlighted the emotional and psychosocial barriers in cancer care. Her treatment inherently leaves her feeling very isolated and lonely, and she had such a hard time connecting with a psychotherapist,” Dr. Noel said.
Another common issue revolved around efforts to communicate about symptoms and adverse effects of treatment. Resources often aren’t available on evenings or weekends, and it can take time for a nurse to call them back. Patients wanted to see more modern approaches, such as use of email or apps.
The patients in the study recommended 11 health care improvements.
- 1. Nurse navigator teams should have hours extended to evenings and weekends.
- 2. Patient communication methods should be expanded, using methods like email or apps.
- 3. HNC resources should be more broadly disseminated.
- 4. Education and information approaches should be individualized to the patient.
- 5. All HNC patients should be offered psychological resources.
- 6. Mental health needs should be assessed repeatedly throughout treatment and extended care.
- 7. Physicians should recognize the added symptom burden often faced by patients who travel extensively for treatment.
- 8. Partners and caregivers should be included as part of the treatment team.
- 9. Share symptom data with patients, which can improve engagement.
- 10. Review symptom scores and act on them regularly.
- 11. A member of the care team should be identified to oversee symptom management.
Dr. Noel had no relevant financial disclosures.
HNC has a high burden of treatment-related adverse events, along with frequent trouble with speech, swallowing, facial disfigurement, and psychological distress.
Among cancer patients, “they have the highest rates of emergency department use and hospitalization during treatment. They also have the highest rates of psychological distress. We have some Ontario data that shows they’ve got the highest rates of suicide and self-harm. So I think this is a really special population that we need to support,” Christopher Noel, MD, PhD, said in an interview. Dr. Noel was the lead author of the study, which was published in JAMA Otolaryngology – Head & Neck Surgery.
These issues can strongly affect quality of life, and even patient outcomes. “Even a 1-day interruption in treatment has been shown to impact oncologic outcomes. This is a very big issue whether you’re a surgeon, a medical oncologist, or a radiation oncologist,” said Dr. Noel, who is a resident physician at the University of Toronto.
He advocates that physicians interview patients and review the results in a structured way and then act on it. “If we just rely on patient [provided] communication, we’re going to miss about 50% of patient symptoms,” he said.
The researchers aimed for the patient’s perspective on treatment. “What is the patient’s perception of going through head neck cancer and their treatment, and managing their symptoms at home? And where do they think that we could do better?” Dr. Noel asked.
The most pressing issue was that patients felt their emotional and informational needs often were not met. That challenge is even harder for patients who have trouble communicating, which in turn makes them more prone to isolation and loneliness. Many felt that they had to get the information on their own. “They wanted it to be a more effortless process,” said Dr. Noel.
He described one patient with oropharynx cancer who was able to talk to people about her grief over her diagnosis, but treatment led to her throat becoming swollen and she lost the ability to communicate. “She felt very isolated and lonely. She really highlighted the emotional and psychosocial barriers in cancer care. Her treatment inherently leaves her feeling very isolated and lonely, and she had such a hard time connecting with a psychotherapist,” Dr. Noel said.
Another common issue revolved around efforts to communicate about symptoms and adverse effects of treatment. Resources often aren’t available on evenings or weekends, and it can take time for a nurse to call them back. Patients wanted to see more modern approaches, such as use of email or apps.
The patients in the study recommended 11 health care improvements.
- 1. Nurse navigator teams should have hours extended to evenings and weekends.
- 2. Patient communication methods should be expanded, using methods like email or apps.
- 3. HNC resources should be more broadly disseminated.
- 4. Education and information approaches should be individualized to the patient.
- 5. All HNC patients should be offered psychological resources.
- 6. Mental health needs should be assessed repeatedly throughout treatment and extended care.
- 7. Physicians should recognize the added symptom burden often faced by patients who travel extensively for treatment.
- 8. Partners and caregivers should be included as part of the treatment team.
- 9. Share symptom data with patients, which can improve engagement.
- 10. Review symptom scores and act on them regularly.
- 11. A member of the care team should be identified to oversee symptom management.
Dr. Noel had no relevant financial disclosures.
FROM JAMA OTOLARYNGOLOGY – HEAD & NECK SURGERY
Dodging potholes from cancer care to hospice transitions
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
Misconceptions remain on gene signature use in breast cancer
BERLIN – , a European survey suggests.
The authors found, for instance, that while most specialists agreed that molecular intrinsic subtypes had clinical utility for understanding prognosis in early-stage hormone receptor (HR)–positive disease and for identifying patients for whom chemotherapy could be safely avoided, about 1 in 4 experts either disagreed or felt neutral about the use of signatures in these settings.
Similarly, almost 75% of respondents felt that these signatures were not useful in the triple-negative or metastatic setting, but a small percentage believed they were, and about 10% were neutral.
“Considering that breast cancer multigene signatures were developed in the post menopausal HR+/HER2- early breast cancer setting, the fact that some experts consider [them] useful in triple-negative, HER2+ breast cancer or in the metastatic setting corroborates a misunderstanding on how to interpret the results,” study author Giuseppe Curigliano, MD, PhD, associate professor of medical oncology at the University of Milan, and colleagues wrote.
Dr. Curigliano, who is also head of the Division of Early Drug Development at the European Institute of Oncology, presented the survey findings on May 4 at the European Society for Medical Oncology (ESMO BCC) Breast Cancer Congress.
Although several breast cancer multigene signatures are available to profile early breast cancer, little information exists on how these signatures should be used in clinical practice.
To investigate, Dr. Curigliano and colleagues convened a scientific committee of eight breast cancer experts to develop a Delphi questionnaire to examine respondents’ opinions and uses of these signatures.
The questionnaire asked about the clinical utility of multigene signatures in breast cancer and recommendations for their use in clinical practice.
In all, 133 breast cancer specialists from 11 European countries completed the questionnaire. Respondents were about 49 years old on average, and most (86.5%) worked in a teaching hospital. More than 72% were medical oncologists; 12% were pathologists.
Consensus was considered to be reached when 70% or more of the respondents were in agreement on a topic.
Participants had “extensive experience in the management of breast cancer patients and have been using breast cancer multigene signatures in clinical practice,” Dr. Curigliano said.
Almost all respondents (93.6%) reported using breast cancer multigene signatures routinely or in selected patients, and 73.4% had more than 5 years of experience with them.
Overall, more than 70% of respondents agreed that identifying tumor intrinsic subtype via gene expression profiling was important in making prognostic and treatment decisions; however, a consensus was not reached on the use of immunohistochemistry.
In addition, most respondents (76%) agreed that identifying breast cancer molecular intrinsic subtypes had clinical utility for prognosis in early-stage HR-positive disease and for identifying patients for whom chemotherapy can be safely avoided (75%). However, in both cases, about one-quarter of respondents either disagreed or felt neutral.
No consensus was reached on the clinical utility of these subtypes for selecting the most appropriate chemotherapy treatment – two-thirds disagreed, while 13% agreed and 17% felt neutral.
When deciding on the use of chemotherapy in the adjuvant setting in early node-negative breast cancer, 88% of respondents felt that breast cancer multigene signatures were important. Moreover, 75% considered such signatures important when deciding whether to use chemotherapy in the adjuvant setting for patients with one to three positive lymph nodes. However, no consensus was reached on the utility of signatures for deciding whether to extend endocrine therapy in either setting.
When examining the usefulness of signatures in more special settings, the authors found that the vast majority (90%) of respondents believed that multigene signatures had clinical utility for postmenopausal early breast cancer patients, and 82% did not consider signatures clinically useful in the early-stage HER2-overexpressed setting.
In addition, 74% thought that breast cancer multigene signatures were not useful in triple-negative disease or in the metastatic setting.
Respondents did not reach a consensus on the clinical utility of multigene signatures in the neoadjuvant setting – only 27% considered them useful, and almost half did not.
The “low percentage” of respondents using the signatures in the neoadjuvant setting and the “misconception regarding the predictive value of these tests on chemotherapy benefits suggest there is still room for training on results interpretation [for breast cancer multigene signatures],” the authors write.
The study was sponsored by Veracyte. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, and Veracyte. No other relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
This article was updated 5/9/22.
BERLIN – , a European survey suggests.
The authors found, for instance, that while most specialists agreed that molecular intrinsic subtypes had clinical utility for understanding prognosis in early-stage hormone receptor (HR)–positive disease and for identifying patients for whom chemotherapy could be safely avoided, about 1 in 4 experts either disagreed or felt neutral about the use of signatures in these settings.
Similarly, almost 75% of respondents felt that these signatures were not useful in the triple-negative or metastatic setting, but a small percentage believed they were, and about 10% were neutral.
“Considering that breast cancer multigene signatures were developed in the post menopausal HR+/HER2- early breast cancer setting, the fact that some experts consider [them] useful in triple-negative, HER2+ breast cancer or in the metastatic setting corroborates a misunderstanding on how to interpret the results,” study author Giuseppe Curigliano, MD, PhD, associate professor of medical oncology at the University of Milan, and colleagues wrote.
Dr. Curigliano, who is also head of the Division of Early Drug Development at the European Institute of Oncology, presented the survey findings on May 4 at the European Society for Medical Oncology (ESMO BCC) Breast Cancer Congress.
Although several breast cancer multigene signatures are available to profile early breast cancer, little information exists on how these signatures should be used in clinical practice.
To investigate, Dr. Curigliano and colleagues convened a scientific committee of eight breast cancer experts to develop a Delphi questionnaire to examine respondents’ opinions and uses of these signatures.
The questionnaire asked about the clinical utility of multigene signatures in breast cancer and recommendations for their use in clinical practice.
In all, 133 breast cancer specialists from 11 European countries completed the questionnaire. Respondents were about 49 years old on average, and most (86.5%) worked in a teaching hospital. More than 72% were medical oncologists; 12% were pathologists.
Consensus was considered to be reached when 70% or more of the respondents were in agreement on a topic.
Participants had “extensive experience in the management of breast cancer patients and have been using breast cancer multigene signatures in clinical practice,” Dr. Curigliano said.
Almost all respondents (93.6%) reported using breast cancer multigene signatures routinely or in selected patients, and 73.4% had more than 5 years of experience with them.
Overall, more than 70% of respondents agreed that identifying tumor intrinsic subtype via gene expression profiling was important in making prognostic and treatment decisions; however, a consensus was not reached on the use of immunohistochemistry.
In addition, most respondents (76%) agreed that identifying breast cancer molecular intrinsic subtypes had clinical utility for prognosis in early-stage HR-positive disease and for identifying patients for whom chemotherapy can be safely avoided (75%). However, in both cases, about one-quarter of respondents either disagreed or felt neutral.
No consensus was reached on the clinical utility of these subtypes for selecting the most appropriate chemotherapy treatment – two-thirds disagreed, while 13% agreed and 17% felt neutral.
When deciding on the use of chemotherapy in the adjuvant setting in early node-negative breast cancer, 88% of respondents felt that breast cancer multigene signatures were important. Moreover, 75% considered such signatures important when deciding whether to use chemotherapy in the adjuvant setting for patients with one to three positive lymph nodes. However, no consensus was reached on the utility of signatures for deciding whether to extend endocrine therapy in either setting.
When examining the usefulness of signatures in more special settings, the authors found that the vast majority (90%) of respondents believed that multigene signatures had clinical utility for postmenopausal early breast cancer patients, and 82% did not consider signatures clinically useful in the early-stage HER2-overexpressed setting.
In addition, 74% thought that breast cancer multigene signatures were not useful in triple-negative disease or in the metastatic setting.
Respondents did not reach a consensus on the clinical utility of multigene signatures in the neoadjuvant setting – only 27% considered them useful, and almost half did not.
The “low percentage” of respondents using the signatures in the neoadjuvant setting and the “misconception regarding the predictive value of these tests on chemotherapy benefits suggest there is still room for training on results interpretation [for breast cancer multigene signatures],” the authors write.
The study was sponsored by Veracyte. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, and Veracyte. No other relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
This article was updated 5/9/22.
BERLIN – , a European survey suggests.
The authors found, for instance, that while most specialists agreed that molecular intrinsic subtypes had clinical utility for understanding prognosis in early-stage hormone receptor (HR)–positive disease and for identifying patients for whom chemotherapy could be safely avoided, about 1 in 4 experts either disagreed or felt neutral about the use of signatures in these settings.
Similarly, almost 75% of respondents felt that these signatures were not useful in the triple-negative or metastatic setting, but a small percentage believed they were, and about 10% were neutral.
“Considering that breast cancer multigene signatures were developed in the post menopausal HR+/HER2- early breast cancer setting, the fact that some experts consider [them] useful in triple-negative, HER2+ breast cancer or in the metastatic setting corroborates a misunderstanding on how to interpret the results,” study author Giuseppe Curigliano, MD, PhD, associate professor of medical oncology at the University of Milan, and colleagues wrote.
Dr. Curigliano, who is also head of the Division of Early Drug Development at the European Institute of Oncology, presented the survey findings on May 4 at the European Society for Medical Oncology (ESMO BCC) Breast Cancer Congress.
Although several breast cancer multigene signatures are available to profile early breast cancer, little information exists on how these signatures should be used in clinical practice.
To investigate, Dr. Curigliano and colleagues convened a scientific committee of eight breast cancer experts to develop a Delphi questionnaire to examine respondents’ opinions and uses of these signatures.
The questionnaire asked about the clinical utility of multigene signatures in breast cancer and recommendations for their use in clinical practice.
In all, 133 breast cancer specialists from 11 European countries completed the questionnaire. Respondents were about 49 years old on average, and most (86.5%) worked in a teaching hospital. More than 72% were medical oncologists; 12% were pathologists.
Consensus was considered to be reached when 70% or more of the respondents were in agreement on a topic.
Participants had “extensive experience in the management of breast cancer patients and have been using breast cancer multigene signatures in clinical practice,” Dr. Curigliano said.
Almost all respondents (93.6%) reported using breast cancer multigene signatures routinely or in selected patients, and 73.4% had more than 5 years of experience with them.
Overall, more than 70% of respondents agreed that identifying tumor intrinsic subtype via gene expression profiling was important in making prognostic and treatment decisions; however, a consensus was not reached on the use of immunohistochemistry.
In addition, most respondents (76%) agreed that identifying breast cancer molecular intrinsic subtypes had clinical utility for prognosis in early-stage HR-positive disease and for identifying patients for whom chemotherapy can be safely avoided (75%). However, in both cases, about one-quarter of respondents either disagreed or felt neutral.
No consensus was reached on the clinical utility of these subtypes for selecting the most appropriate chemotherapy treatment – two-thirds disagreed, while 13% agreed and 17% felt neutral.
When deciding on the use of chemotherapy in the adjuvant setting in early node-negative breast cancer, 88% of respondents felt that breast cancer multigene signatures were important. Moreover, 75% considered such signatures important when deciding whether to use chemotherapy in the adjuvant setting for patients with one to three positive lymph nodes. However, no consensus was reached on the utility of signatures for deciding whether to extend endocrine therapy in either setting.
When examining the usefulness of signatures in more special settings, the authors found that the vast majority (90%) of respondents believed that multigene signatures had clinical utility for postmenopausal early breast cancer patients, and 82% did not consider signatures clinically useful in the early-stage HER2-overexpressed setting.
In addition, 74% thought that breast cancer multigene signatures were not useful in triple-negative disease or in the metastatic setting.
Respondents did not reach a consensus on the clinical utility of multigene signatures in the neoadjuvant setting – only 27% considered them useful, and almost half did not.
The “low percentage” of respondents using the signatures in the neoadjuvant setting and the “misconception regarding the predictive value of these tests on chemotherapy benefits suggest there is still room for training on results interpretation [for breast cancer multigene signatures],” the authors write.
The study was sponsored by Veracyte. Dr. Curigliano has relationships with Pfizer, Novartis, Lilly, Roche, Seattle Genetics, Celltrion, and Veracyte. No other relevant financial relationships were disclosed.
A version of this article first appeared on Medscape.com.
This article was updated 5/9/22.
AT ESMO BCC 2022
Is it time to remove ‘cancer’ label from low-risk prostate tumors?
Physicians often advise that men with low-risk prostate tumors wait to see if the disease worsens – an approach called “active surveillance” – rather than rushing to treat the condition. After all, low-grade tumors rarely cause harm, and therapies such as radiation and surgery can carry serious side effects, including impotence and urinary leakage.
Yet doctors still label these lesions “cancer,” and as a result, some experts say, many men in the United States opt for treatment they don’t need.
In a new paper likely to stoke debate, experts from a range of disciplines, as well as one patient, argue that overtreatment could be reduced by removing the word “cancer” from low-risk disease. Tumors that rate 6 on the Gleason score (GS) cannot invade other organs but nonetheless scare patients into undergoing risky treatments, they argue. Fewer than 1% of men with GS6 prostate tumors experience metastatic disease or die from cancer within 15 years of the initial diagnosis, they report.
“No matter how much time a physician may spend downplaying the significance of a GS6 diagnosis or emphasizing the phrase low-risk, the words ‘you have cancer’ have a potent psychological effect on most men and their families,” they wrote in a paper published in the Journal of Clinical Oncology.
Dropping the C word for low-risk tumors, which make up about half of 268,000 prostate cancer diagnoses annually in the United States, is not a new idea. An independent panel convened by the National Institutes of Health proposed just that in 2011.
However, clinician support for the shift appears to be growing, said Scott Eggener, MD, a urologic oncologist and professor of surgery at the University of Chicago, and a coauthor of the new article.
Dr. Eggener said active surveillance has been increasing dramatically in the United States, to about 60% of patients with GS6. “We feel like the landscape is right now to be talking about this issue,” Dr. Eggener told this news organization.
Reducing unnecessary treatment, he and his coauthors argue, could reduce the cost of health care — and boost the benefit of prostate-specific antigen testing for prostate cancer, which the U.S. Preventive Services Task Force at the moment deems small.
In addition, patients with prostate cancer diagnoses encounter increased risk of depression and suicide, disqualification or higher rates for life insurance, and questions from family and friends if they choose active surveillance over treatment – all of which might be ameliorated by a change in terminology.
The word “cancer” has been dropped from bladder, cervical, and thyroid conditions and prostate abnormalities that used to be classified as Gleason 2 through 5, they noted.
Keeping the status quo
But some physicians say GS6 doesn’t need a name change.
From a scientific standpoint, GS6 disease has molecular hallmarks of cancer, according to Jonathan Epstein, MD, professor of pathology, urology, and oncology at Johns Hopkins University, Baltimore. More important, Dr. Epstein told Medscape, the classification does not guarantee that more serious cancer is not present, only that it has not been found yet in tissue samples.
Dr. Eggener acknowledged that while GS6 does have molecular markers associated with cancer – a fact that’s “challenging to reconcile with” – giving it another name “would still require surveillance, and since the window of opportunity for curing localized [prostate cancer] is typically measured in years or decades, evidence of histologic progression to a higher-grade cancer would far precede the potential time of future metastasis in the majority of cases.”
Still, Dr. Epstein worries that dropping the cancer designation may lead some patients to forgo active surveillance, which involves repeated imaging and biopsies to check for worse disease. Without such monitoring, he said, “if they do have higher grade cancer that’s unsampled, it will pose a threat to their life.”
Gleason 6 tumors “may progress, some significantly, or be incompletely sampled at the time of diagnosis. Both clinicians and patients need to understand such risk,” Peter Carroll, MD, MPH, a urologist at the University of California, San Francisco, who is critical of the proposed name change, told this news organization.
Regardless of what it’s called, Gleason 6 disease warrants close monitoring, said Joe Gallo, a 77-year-old Pennsylvania man whose high-risk cancer was detected during active surveillance. “If I had taken a laid-back, or less, approach” to monitoring, Mr. Gallo said, “necessary treatment may have been delayed and my condition may have become more serious.”
Some advocates say patients and their families need to be educated that cancer exists on a spectrum of severity.
Mark Lichty, 73, chairman of a support group called Active Surveillance Patients International, received a Gleason 6 diagnosis 17 years ago. He resisted treatment against medical advice, and the cancer never progressed.
Mr. Lichty said active surveillance has been more widely adopted in Sweden, where physicians assure patients that treatment is unnecessary and support systems exist. “Yes, a diagnosis of cancer is frightening,” he said in an interview. But “we can do a lot better in how we communicate the diagnosis.”
Dr. Eggener reported consulting or advisory roles with Sophiris Bio, Francis Medical, Insightec, Profound Medical, and Candel Therapeutics; speakers bureau at Janssen; and fees for travel, accommodations, and expenses from Janssen Biotech and Insightec; as well as an uncompensated relationship with Steba Biotech. The remaining coauthors reported several financial relationships, which are listed in the paper. Dr. Epstein and Dr. Carroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians often advise that men with low-risk prostate tumors wait to see if the disease worsens – an approach called “active surveillance” – rather than rushing to treat the condition. After all, low-grade tumors rarely cause harm, and therapies such as radiation and surgery can carry serious side effects, including impotence and urinary leakage.
Yet doctors still label these lesions “cancer,” and as a result, some experts say, many men in the United States opt for treatment they don’t need.
In a new paper likely to stoke debate, experts from a range of disciplines, as well as one patient, argue that overtreatment could be reduced by removing the word “cancer” from low-risk disease. Tumors that rate 6 on the Gleason score (GS) cannot invade other organs but nonetheless scare patients into undergoing risky treatments, they argue. Fewer than 1% of men with GS6 prostate tumors experience metastatic disease or die from cancer within 15 years of the initial diagnosis, they report.
“No matter how much time a physician may spend downplaying the significance of a GS6 diagnosis or emphasizing the phrase low-risk, the words ‘you have cancer’ have a potent psychological effect on most men and their families,” they wrote in a paper published in the Journal of Clinical Oncology.
Dropping the C word for low-risk tumors, which make up about half of 268,000 prostate cancer diagnoses annually in the United States, is not a new idea. An independent panel convened by the National Institutes of Health proposed just that in 2011.
However, clinician support for the shift appears to be growing, said Scott Eggener, MD, a urologic oncologist and professor of surgery at the University of Chicago, and a coauthor of the new article.
Dr. Eggener said active surveillance has been increasing dramatically in the United States, to about 60% of patients with GS6. “We feel like the landscape is right now to be talking about this issue,” Dr. Eggener told this news organization.
Reducing unnecessary treatment, he and his coauthors argue, could reduce the cost of health care — and boost the benefit of prostate-specific antigen testing for prostate cancer, which the U.S. Preventive Services Task Force at the moment deems small.
In addition, patients with prostate cancer diagnoses encounter increased risk of depression and suicide, disqualification or higher rates for life insurance, and questions from family and friends if they choose active surveillance over treatment – all of which might be ameliorated by a change in terminology.
The word “cancer” has been dropped from bladder, cervical, and thyroid conditions and prostate abnormalities that used to be classified as Gleason 2 through 5, they noted.
Keeping the status quo
But some physicians say GS6 doesn’t need a name change.
From a scientific standpoint, GS6 disease has molecular hallmarks of cancer, according to Jonathan Epstein, MD, professor of pathology, urology, and oncology at Johns Hopkins University, Baltimore. More important, Dr. Epstein told Medscape, the classification does not guarantee that more serious cancer is not present, only that it has not been found yet in tissue samples.
Dr. Eggener acknowledged that while GS6 does have molecular markers associated with cancer – a fact that’s “challenging to reconcile with” – giving it another name “would still require surveillance, and since the window of opportunity for curing localized [prostate cancer] is typically measured in years or decades, evidence of histologic progression to a higher-grade cancer would far precede the potential time of future metastasis in the majority of cases.”
Still, Dr. Epstein worries that dropping the cancer designation may lead some patients to forgo active surveillance, which involves repeated imaging and biopsies to check for worse disease. Without such monitoring, he said, “if they do have higher grade cancer that’s unsampled, it will pose a threat to their life.”
Gleason 6 tumors “may progress, some significantly, or be incompletely sampled at the time of diagnosis. Both clinicians and patients need to understand such risk,” Peter Carroll, MD, MPH, a urologist at the University of California, San Francisco, who is critical of the proposed name change, told this news organization.
Regardless of what it’s called, Gleason 6 disease warrants close monitoring, said Joe Gallo, a 77-year-old Pennsylvania man whose high-risk cancer was detected during active surveillance. “If I had taken a laid-back, or less, approach” to monitoring, Mr. Gallo said, “necessary treatment may have been delayed and my condition may have become more serious.”
Some advocates say patients and their families need to be educated that cancer exists on a spectrum of severity.
Mark Lichty, 73, chairman of a support group called Active Surveillance Patients International, received a Gleason 6 diagnosis 17 years ago. He resisted treatment against medical advice, and the cancer never progressed.
Mr. Lichty said active surveillance has been more widely adopted in Sweden, where physicians assure patients that treatment is unnecessary and support systems exist. “Yes, a diagnosis of cancer is frightening,” he said in an interview. But “we can do a lot better in how we communicate the diagnosis.”
Dr. Eggener reported consulting or advisory roles with Sophiris Bio, Francis Medical, Insightec, Profound Medical, and Candel Therapeutics; speakers bureau at Janssen; and fees for travel, accommodations, and expenses from Janssen Biotech and Insightec; as well as an uncompensated relationship with Steba Biotech. The remaining coauthors reported several financial relationships, which are listed in the paper. Dr. Epstein and Dr. Carroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physicians often advise that men with low-risk prostate tumors wait to see if the disease worsens – an approach called “active surveillance” – rather than rushing to treat the condition. After all, low-grade tumors rarely cause harm, and therapies such as radiation and surgery can carry serious side effects, including impotence and urinary leakage.
Yet doctors still label these lesions “cancer,” and as a result, some experts say, many men in the United States opt for treatment they don’t need.
In a new paper likely to stoke debate, experts from a range of disciplines, as well as one patient, argue that overtreatment could be reduced by removing the word “cancer” from low-risk disease. Tumors that rate 6 on the Gleason score (GS) cannot invade other organs but nonetheless scare patients into undergoing risky treatments, they argue. Fewer than 1% of men with GS6 prostate tumors experience metastatic disease or die from cancer within 15 years of the initial diagnosis, they report.
“No matter how much time a physician may spend downplaying the significance of a GS6 diagnosis or emphasizing the phrase low-risk, the words ‘you have cancer’ have a potent psychological effect on most men and their families,” they wrote in a paper published in the Journal of Clinical Oncology.
Dropping the C word for low-risk tumors, which make up about half of 268,000 prostate cancer diagnoses annually in the United States, is not a new idea. An independent panel convened by the National Institutes of Health proposed just that in 2011.
However, clinician support for the shift appears to be growing, said Scott Eggener, MD, a urologic oncologist and professor of surgery at the University of Chicago, and a coauthor of the new article.
Dr. Eggener said active surveillance has been increasing dramatically in the United States, to about 60% of patients with GS6. “We feel like the landscape is right now to be talking about this issue,” Dr. Eggener told this news organization.
Reducing unnecessary treatment, he and his coauthors argue, could reduce the cost of health care — and boost the benefit of prostate-specific antigen testing for prostate cancer, which the U.S. Preventive Services Task Force at the moment deems small.
In addition, patients with prostate cancer diagnoses encounter increased risk of depression and suicide, disqualification or higher rates for life insurance, and questions from family and friends if they choose active surveillance over treatment – all of which might be ameliorated by a change in terminology.
The word “cancer” has been dropped from bladder, cervical, and thyroid conditions and prostate abnormalities that used to be classified as Gleason 2 through 5, they noted.
Keeping the status quo
But some physicians say GS6 doesn’t need a name change.
From a scientific standpoint, GS6 disease has molecular hallmarks of cancer, according to Jonathan Epstein, MD, professor of pathology, urology, and oncology at Johns Hopkins University, Baltimore. More important, Dr. Epstein told Medscape, the classification does not guarantee that more serious cancer is not present, only that it has not been found yet in tissue samples.
Dr. Eggener acknowledged that while GS6 does have molecular markers associated with cancer – a fact that’s “challenging to reconcile with” – giving it another name “would still require surveillance, and since the window of opportunity for curing localized [prostate cancer] is typically measured in years or decades, evidence of histologic progression to a higher-grade cancer would far precede the potential time of future metastasis in the majority of cases.”
Still, Dr. Epstein worries that dropping the cancer designation may lead some patients to forgo active surveillance, which involves repeated imaging and biopsies to check for worse disease. Without such monitoring, he said, “if they do have higher grade cancer that’s unsampled, it will pose a threat to their life.”
Gleason 6 tumors “may progress, some significantly, or be incompletely sampled at the time of diagnosis. Both clinicians and patients need to understand such risk,” Peter Carroll, MD, MPH, a urologist at the University of California, San Francisco, who is critical of the proposed name change, told this news organization.
Regardless of what it’s called, Gleason 6 disease warrants close monitoring, said Joe Gallo, a 77-year-old Pennsylvania man whose high-risk cancer was detected during active surveillance. “If I had taken a laid-back, or less, approach” to monitoring, Mr. Gallo said, “necessary treatment may have been delayed and my condition may have become more serious.”
Some advocates say patients and their families need to be educated that cancer exists on a spectrum of severity.
Mark Lichty, 73, chairman of a support group called Active Surveillance Patients International, received a Gleason 6 diagnosis 17 years ago. He resisted treatment against medical advice, and the cancer never progressed.
Mr. Lichty said active surveillance has been more widely adopted in Sweden, where physicians assure patients that treatment is unnecessary and support systems exist. “Yes, a diagnosis of cancer is frightening,” he said in an interview. But “we can do a lot better in how we communicate the diagnosis.”
Dr. Eggener reported consulting or advisory roles with Sophiris Bio, Francis Medical, Insightec, Profound Medical, and Candel Therapeutics; speakers bureau at Janssen; and fees for travel, accommodations, and expenses from Janssen Biotech and Insightec; as well as an uncompensated relationship with Steba Biotech. The remaining coauthors reported several financial relationships, which are listed in the paper. Dr. Epstein and Dr. Carroll have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Q&A with Hubert (Hugh) Greenway, MD
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
who was also recently selected as program director for cutaneous oncology at Scripps MD Anderson Cancer Center in San Diego. He is also a former president of the American College of Mohs Surgery.
After earning his medical degree from the Medical College of Georgia, Augusta, in 1974, Dr. Greenway was fellowship trained in Mohs skin cancer surgery by Frederic E. Mohs, MD, at the University of Wisconsin–Madison. He completed his dermatology residency at the Naval Medical Center San Diego and joined Scripps Clinic in 1983, where he launched the institution’s first Mohs surgery program, as well as a popular annual intensive course in superficial anatomy and cutaneous surgery that bears his name. He was also the first physician in the world to use interferon as a nonsurgical treatment of basal cell carcinoma.

To date, Dr. Greenway has performed more than 41,000 Mohs surgery cases and has trained 61 fellows who practice in academic and clinical settings. In 2017, he received the Frederic E. Mohs Award from the ACMS at the college’s annual meeting. He is also a past CEO of Scripps Clinic. In this Q&A, Dr. Greenway opens up about what it was like to train with Dr. Mohs, what makes a good Mohs surgeon, and why he’s excited about the future of dermatology.
I understand that you first became interested in a medical career after meeting Dr. Carl Jones, a friend of your father who was your Scoutmaster in the Boy Scouts in Georgia. What about Dr. Jones inspired you to pursue a career in medicine?
Dr. Jones was an internist/allergist in Atlanta, where I grew up. His three sons and I were friends. My dad had dealt with several medical problems being injured in World War II and subsequently undergoing a couple of kidney transplantations, so I developed an interest in medicine personally. Even though Dr. Jones was a specialist, he started out as a family doctor like I did, so he was interested in the whole person and all of his or her medical problems as opposed to those related to his specialty only. I traveled with the Boy Scouts to camp at places like Valley Forge in Pennsylvania, and Dr. Jones was involved with the medical set-ups of those large events. That also contributed to my interest in medicine.
As part of your 9-year service in the U.S. Navy, you spent 2 years as the flight surgeon at NAS Atlanta/Dobbins Air Force Base. What was your most memorable experience from that assignment?
Dobbins is a large facility with two Lockheed plants, and the Air Force had built the medical clinic, which was staffed by the Navy. Getting to know some of the active-duty members of the Air Force, the Navy, and the National Guard, and their commitment to our country, was memorable. Jimmy Carter was the president in those days. When he would fly in Dobbins, one of my jobs as the flight surgeon was to be on base when Air Force One landed or departed. One night, we had a DC-9 commercial aircraft coming from Huntsville, Ala., to Atlanta that got caught in a thunderstorm a little above 30,000 feet. Both engines went out and the aircraft essentially became a glider. The pilots tried to land on our runway but unfortunately, they ended up 4 miles short. We were heavily involved in responding to the crash, which was a tragic event. I also learned to fly (second seat) different types of aircraft during my assignment at NAS Atlanta/Dobbins Air Force Base, everything from the large C-5s to Navy fighter jets and helicopters. Coincidentally, Dr. Jones was involved with a couple of free health clinics in Atlanta when I was stationed there. Every Tuesday night, my wife (who is a nurse) and I would volunteer at a clinic in Cabbagetown, which was one of the poorer areas of Atlanta. It was a chance to give back to a group of people who didn’t have a whole lot.
In the middle your dermatology residency at Naval Medical Center San Diego, you were selected by Dr. Mohs for fellowship training in Mohs skin cancer surgery at the University of Wisconsin–Madison. What do you remember most about your training with Dr. Mohs?
Dr. Mohs was a kind, humble man who had this great idea about skin cancer. He was not a dermatologist; he was a general surgeon. The technique he developed was originally called chemosurgery because he put a chemical onto the skin. This was known as the fixed-tissue technique. Then we had a fresh-tissue technique, where we did not use the chemical, but we were able to use local anesthesia right away. That developed into the Mohs surgery we know today. Dr. Mohs did not name it that; he was very humble, but he was very proud of his technique. He was also a very hard worker. On the first day of my fellowship, I started at 7 in the morning and ended at 7 at night. It was the same for the last day of my fellowship. He also had an excellent office staff, many of whom had worked with him for many years. Patients with difficult skin cancers traveled to Madison from all over the world because there weren’t that many Mohs surgery clinics in those days. During the latter part of my fellowship, Michael McCall, MD, and I had the opportunity to remove a skin cancer from the nose of Dr. Mohs. We presented the case at a national conference, and I titled the talk “Mohs Surgery for Mohs’ Nose.”
Early in your career Dr. Mohs asked you to take over his practice, but you accepted an offer to establish the first Mohs surgery office at Scripps in San Diego instead. What convinced you to head West?
After my fellowship, I returned to San Diego to complete my residency with the Navy, where we opened a Mohs surgery clinic. Dr. Mohs came out for the ribbon cutting. During that time, I was taking care of several patients that he had treated in Wisconsin. Through that my wife and I ended up going to dinner with Cecil and Ida Green, philanthropists who made several financial gifts to Scripps Clinic – and for whom Scripps Green Hospital is named. Cecil cofounded Texas Instruments and was knighted by Queen Elizabeth. During dinner, he suggested that I stay in San Diego for a year and work at Scripps after my residency assignment with the Navy. I agreed and have been here ever since.
What do you find most interesting about Mohs surgery?
In Mohs surgery, you’re able to provide not only surgical care to eliminate the tumor, but also the pathology and the reconstruction. That was interesting to me. Dr. Mohs was not that interested in reconstruction. He was more focused on the tumor, in part because with the original fixed-tissue technique you could not do the reconstruction. You had to wait for an extra layer of tissue to separate. But with the fresh-tissue technique, you were able to provide the reconstruction that day. Mohs surgery deals with a subset of tumors that are challenging to treat. That also spiked my academic and clinical interest.
In your opinion, what’s been the most important advance in Mohs surgery to date?
In recent years, immunology has come into play, so now we have teams of clinicians in dermatology, medical oncology, surgery, and other subspecialties providing patients the best of care. In the arena of Mohs surgery itself, in the 1980s, the American College of Mohs Surgery developed a 1-year fellowship program, which enabled us to train many men and women to practice Mohs surgery. Most of them are dermatologists.
Please complete the sentence: “You can tell a good Mohs surgeon by the way he/she ...”
Treats patients, is willing to spend time with them, and shows an interest in them. One of the things we should strive for is to let patients know that they as a person are important; it’s not just the melanoma on their nose. We’re not only dealing with a skin cancer; we’re dealing with a patient who has skin cancer.
For the past 39 years, you have led Hugh Greenway’s Superficial Anatomy and Cutaneous Surgery course, which takes place every January in San Diego. What’s been key to sustaining this training course for nearly 4 decades?
There have been many people involved in its success, so it’s not just me. When I first started my practice, there really was not a focus on anatomy in the general dermatologic community. Dermatologic surgery textbooks contained very little content on surgical anatomy so I developed an interest a putting together a course that would cover some of this material. I met with Terence Davidson, MD, an otolaryngologist who was dean of continuing medical education at the University of California, San Diego. The course includes lectures from experts in many subspecialties and hands-on laboratories using cadavers to work on anatomy and surgical techniques. After about 16 years of doing the course Dr. Davidson told me: “When we started this course, as a group, the head and neck surgeons were the best to do the reconstructions on the face with skin flaps and grafts and layered closures. But now, as a group, the dermatologists are best at doing that.” That’s what we want to hear in medical education.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint?
I’m fortunate to practice at a place like Scripps, where there are many resources to look at what was happening with COVID-19. Clinically, we had to put a lot of things on hold, but we tried our best to keep our cancer patients in particular in the forefront of care. It has been a challenge, but fortunately we have been able to take care of patients after a brief timeout. Many of us remember the polio vaccine back in the 1950s. Having worked overseas and at missionary hospital where we had children die of measles because they were not vaccinated gave me a larger appreciation for the importance of vaccines. I recommend all young physicians who work with me to read, “The Great Influenza: The Story of the Deadliest Pandemic in History,” by John M. Barry, which recounts the 1918 flu epidemic.
Who inspires you most in your work today?
I don’t view what I do as work. Dr. Jones and Dr. Mohs continue to inspire me with what they accomplished during their careers. You have to love people and love patients. Every patient who comes to see me has a story, so I try to understand their story. One of the things I really enjoy is training the young fellows. We train three Mohs fellows per year at Scripps, and it’s a great challenge every day.
What development in dermatology are you most excited about in the next 5 years?
Dermatology will continue to evolve just like all other medical specialties. We’re going to see a large growth in telemedicine, and immunotherapy is playing a key role in dermatologic oncology. What excites me the most in medicine is the young people who enter the field willing to contribute their lives to helping others.
‘Unprecedented crisis’: Hodgkin drug shortage persists
The persistent shortage of dacarbazine has led to an “acute and unprecedented crisis” in the treatment of patients with advanced classical Hodgkin lymphoma, experts say.
In a recent review, oncologists scoured decades of data to find the best alternatives for a range of scenarios. For fit adults younger than 60, the group recommends the seven-drug regimen BEACOPP – bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone.
“Among all available regimens,” BEACOPP has “the most robust evidence” as a substitute for the four-drug standard ABVD, which includes doxorubicin, bleomycin, vinblastine, and dacarbazine, Pallawi Torka, MD, a hematologic oncologist at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues wrote in JCO Oncology Practice.
Last October, the Food and Drug Administration posted a notice about the dacarbazine shortage. According to the notice, the shortage occurred because of “manufacturing delays” and a “demand increase” affecting three companies supplying the U.S. market – Fresenius Kabi USA, Hikma Pharmaceuticals, and Teva. In an update issued May 4, the FDA said that 100-mg and 200-mg vials of the drug are now available from Fresenius. An update from April 8 said that 200-mg vials were available from Hikma.
Dacarbazine is hardly the only oncology drug to fall into short supply. Recent data show that shortages of oncology drugs have become more common in the United States in recent years, particularly generic drugs and those targeting hematologic malignancies.
In a recent national survey of oncology pharmacists, researchers found that almost two-thirds of institutions reported at least one drug shortage in the past month, representing a 34% increase between 2018 and 2019.
“This shortage of [dacarbazine] is not the first shortage of oncolytic drugs, and it certainly will not be the last,” Nicole Soriano, PharmD, hematology/oncology clinical pharmacist at Northwestern Memorial Hospital, Chicago, and colleagues wrote in a commentary accompanying the review.
According to Dr. Soriano and coauthors, “some studies have found that shortages are significant across many oncology disciplines and may lead to delays, changes in therapy, interference with clinical research, increased risk of medication errors, adverse outcomes, and increased costs.”
Finding a substitute
In the current analysis, Dr. Torka and her team conducted an exhaustive literature review in which they examined studies going back decades.
The authors highlight more than 10 alternative regimens for treating advanced classical Hodgkin lymphoma. They also provide a detailed treatment algorithm to help oncologists choose the best option for their individual patients as well as strategies for reintegrating ABVD into patient care should the supply of dacarbazine return to normal.
The first considerations: Can patients tolerate intensive chemotherapy, and are patients younger than 60?
For fit adults younger than 60, Dr. Torka and colleagues conclude that the BEACOPP regimen is the “preferred” option. In trials comparing ABVD to BEACOPP, both regimens demonstrated similar overall survival. And while BEACOPP may provide slightly “better disease control,” this approach may also come with greater toxicities in the short and long term, compared with ABVD, depending on the dosing strategy.
The authors also propose an alternative treatment strategy in case the supply of dacarbazine returns to normal mid-treatment. In this scenario, patients could receive an escalated BEACOPP regimen for two cycles and then undergo an interim positron-emission tomography scan. If the scan is negative and dacarbazine is available, the patient’s regimen could be deescalated to ABVD for four cycles without affecting disease control.
For pediatric patients, the authors recommend the ABVE-PC regimen, which includes six drugs – doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide. Data show that the 5-year overall survival among pediatric patients receiving ABVE-PC is 95%.
Stanford V-C – cyclophosphamide, doxorubicin hydrochloride, vinblastine, vincristine, bleomycin, etoposide, and prednisone – is another “acceptable approach” for pediatric patients, the authors noted.
For older patients with advanced disease or those unfit for intensive chemotherapy, the authors suggest evaluating them for fitness for anthracyclines to determine whether doxorubicin, in particular, is an option.
The researchers suggest one of the following three strategies for those who are doxorubicin-eligible: PVAG (prednisone, vinblastine, doxorubicin, and gemcitabine), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), or EVA (etoposide, vinblastine, and doxorubicin).
For those unfit for anthracyclines, the options include COPP (cyclophosphamide, vincristine, procarbazine, and prednisone) or ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisone).
For frail patients who are ineligible for chemotherapy, the team recommends brentuximab alone or in combination with nivolumab.
Given the limited availability of dacarbazine, the authors say that the “current supply should be triaged to prioritize patients whose therapy cannot be changed and those without alternative acceptable options.”
To stretch available dacarbazine supplies as much as possible, the researchers and editorialists advocate for rounding doses within 5%-10% of the prescribed dose.
For example, Dr. Torka and colleagues explained, rounding a dose from 750 mg down to 700 mg would save one vial of dacarbazine.
Vial sharing and using drugs beyond their use dates by compounding with closed-system transfer devices are other strategies to preserve the existing supply of dacarbazine.
The goal of this review “is to give as many patients as possible the most optimal and efficacious therapy even with the strain on supply,” the editorialists wrote.
No funding for the study was reported. Dr. Torka is an adviser for Genentech, ADC Therapeutics, and TG Therapeutics. Dr. Soriano has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The persistent shortage of dacarbazine has led to an “acute and unprecedented crisis” in the treatment of patients with advanced classical Hodgkin lymphoma, experts say.
In a recent review, oncologists scoured decades of data to find the best alternatives for a range of scenarios. For fit adults younger than 60, the group recommends the seven-drug regimen BEACOPP – bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone.
“Among all available regimens,” BEACOPP has “the most robust evidence” as a substitute for the four-drug standard ABVD, which includes doxorubicin, bleomycin, vinblastine, and dacarbazine, Pallawi Torka, MD, a hematologic oncologist at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues wrote in JCO Oncology Practice.
Last October, the Food and Drug Administration posted a notice about the dacarbazine shortage. According to the notice, the shortage occurred because of “manufacturing delays” and a “demand increase” affecting three companies supplying the U.S. market – Fresenius Kabi USA, Hikma Pharmaceuticals, and Teva. In an update issued May 4, the FDA said that 100-mg and 200-mg vials of the drug are now available from Fresenius. An update from April 8 said that 200-mg vials were available from Hikma.
Dacarbazine is hardly the only oncology drug to fall into short supply. Recent data show that shortages of oncology drugs have become more common in the United States in recent years, particularly generic drugs and those targeting hematologic malignancies.
In a recent national survey of oncology pharmacists, researchers found that almost two-thirds of institutions reported at least one drug shortage in the past month, representing a 34% increase between 2018 and 2019.
“This shortage of [dacarbazine] is not the first shortage of oncolytic drugs, and it certainly will not be the last,” Nicole Soriano, PharmD, hematology/oncology clinical pharmacist at Northwestern Memorial Hospital, Chicago, and colleagues wrote in a commentary accompanying the review.
According to Dr. Soriano and coauthors, “some studies have found that shortages are significant across many oncology disciplines and may lead to delays, changes in therapy, interference with clinical research, increased risk of medication errors, adverse outcomes, and increased costs.”
Finding a substitute
In the current analysis, Dr. Torka and her team conducted an exhaustive literature review in which they examined studies going back decades.
The authors highlight more than 10 alternative regimens for treating advanced classical Hodgkin lymphoma. They also provide a detailed treatment algorithm to help oncologists choose the best option for their individual patients as well as strategies for reintegrating ABVD into patient care should the supply of dacarbazine return to normal.
The first considerations: Can patients tolerate intensive chemotherapy, and are patients younger than 60?
For fit adults younger than 60, Dr. Torka and colleagues conclude that the BEACOPP regimen is the “preferred” option. In trials comparing ABVD to BEACOPP, both regimens demonstrated similar overall survival. And while BEACOPP may provide slightly “better disease control,” this approach may also come with greater toxicities in the short and long term, compared with ABVD, depending on the dosing strategy.
The authors also propose an alternative treatment strategy in case the supply of dacarbazine returns to normal mid-treatment. In this scenario, patients could receive an escalated BEACOPP regimen for two cycles and then undergo an interim positron-emission tomography scan. If the scan is negative and dacarbazine is available, the patient’s regimen could be deescalated to ABVD for four cycles without affecting disease control.
For pediatric patients, the authors recommend the ABVE-PC regimen, which includes six drugs – doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide. Data show that the 5-year overall survival among pediatric patients receiving ABVE-PC is 95%.
Stanford V-C – cyclophosphamide, doxorubicin hydrochloride, vinblastine, vincristine, bleomycin, etoposide, and prednisone – is another “acceptable approach” for pediatric patients, the authors noted.
For older patients with advanced disease or those unfit for intensive chemotherapy, the authors suggest evaluating them for fitness for anthracyclines to determine whether doxorubicin, in particular, is an option.
The researchers suggest one of the following three strategies for those who are doxorubicin-eligible: PVAG (prednisone, vinblastine, doxorubicin, and gemcitabine), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), or EVA (etoposide, vinblastine, and doxorubicin).
For those unfit for anthracyclines, the options include COPP (cyclophosphamide, vincristine, procarbazine, and prednisone) or ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisone).
For frail patients who are ineligible for chemotherapy, the team recommends brentuximab alone or in combination with nivolumab.
Given the limited availability of dacarbazine, the authors say that the “current supply should be triaged to prioritize patients whose therapy cannot be changed and those without alternative acceptable options.”
To stretch available dacarbazine supplies as much as possible, the researchers and editorialists advocate for rounding doses within 5%-10% of the prescribed dose.
For example, Dr. Torka and colleagues explained, rounding a dose from 750 mg down to 700 mg would save one vial of dacarbazine.
Vial sharing and using drugs beyond their use dates by compounding with closed-system transfer devices are other strategies to preserve the existing supply of dacarbazine.
The goal of this review “is to give as many patients as possible the most optimal and efficacious therapy even with the strain on supply,” the editorialists wrote.
No funding for the study was reported. Dr. Torka is an adviser for Genentech, ADC Therapeutics, and TG Therapeutics. Dr. Soriano has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The persistent shortage of dacarbazine has led to an “acute and unprecedented crisis” in the treatment of patients with advanced classical Hodgkin lymphoma, experts say.
In a recent review, oncologists scoured decades of data to find the best alternatives for a range of scenarios. For fit adults younger than 60, the group recommends the seven-drug regimen BEACOPP – bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone.
“Among all available regimens,” BEACOPP has “the most robust evidence” as a substitute for the four-drug standard ABVD, which includes doxorubicin, bleomycin, vinblastine, and dacarbazine, Pallawi Torka, MD, a hematologic oncologist at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., and colleagues wrote in JCO Oncology Practice.
Last October, the Food and Drug Administration posted a notice about the dacarbazine shortage. According to the notice, the shortage occurred because of “manufacturing delays” and a “demand increase” affecting three companies supplying the U.S. market – Fresenius Kabi USA, Hikma Pharmaceuticals, and Teva. In an update issued May 4, the FDA said that 100-mg and 200-mg vials of the drug are now available from Fresenius. An update from April 8 said that 200-mg vials were available from Hikma.
Dacarbazine is hardly the only oncology drug to fall into short supply. Recent data show that shortages of oncology drugs have become more common in the United States in recent years, particularly generic drugs and those targeting hematologic malignancies.
In a recent national survey of oncology pharmacists, researchers found that almost two-thirds of institutions reported at least one drug shortage in the past month, representing a 34% increase between 2018 and 2019.
“This shortage of [dacarbazine] is not the first shortage of oncolytic drugs, and it certainly will not be the last,” Nicole Soriano, PharmD, hematology/oncology clinical pharmacist at Northwestern Memorial Hospital, Chicago, and colleagues wrote in a commentary accompanying the review.
According to Dr. Soriano and coauthors, “some studies have found that shortages are significant across many oncology disciplines and may lead to delays, changes in therapy, interference with clinical research, increased risk of medication errors, adverse outcomes, and increased costs.”
Finding a substitute
In the current analysis, Dr. Torka and her team conducted an exhaustive literature review in which they examined studies going back decades.
The authors highlight more than 10 alternative regimens for treating advanced classical Hodgkin lymphoma. They also provide a detailed treatment algorithm to help oncologists choose the best option for their individual patients as well as strategies for reintegrating ABVD into patient care should the supply of dacarbazine return to normal.
The first considerations: Can patients tolerate intensive chemotherapy, and are patients younger than 60?
For fit adults younger than 60, Dr. Torka and colleagues conclude that the BEACOPP regimen is the “preferred” option. In trials comparing ABVD to BEACOPP, both regimens demonstrated similar overall survival. And while BEACOPP may provide slightly “better disease control,” this approach may also come with greater toxicities in the short and long term, compared with ABVD, depending on the dosing strategy.
The authors also propose an alternative treatment strategy in case the supply of dacarbazine returns to normal mid-treatment. In this scenario, patients could receive an escalated BEACOPP regimen for two cycles and then undergo an interim positron-emission tomography scan. If the scan is negative and dacarbazine is available, the patient’s regimen could be deescalated to ABVD for four cycles without affecting disease control.
For pediatric patients, the authors recommend the ABVE-PC regimen, which includes six drugs – doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide. Data show that the 5-year overall survival among pediatric patients receiving ABVE-PC is 95%.
Stanford V-C – cyclophosphamide, doxorubicin hydrochloride, vinblastine, vincristine, bleomycin, etoposide, and prednisone – is another “acceptable approach” for pediatric patients, the authors noted.
For older patients with advanced disease or those unfit for intensive chemotherapy, the authors suggest evaluating them for fitness for anthracyclines to determine whether doxorubicin, in particular, is an option.
The researchers suggest one of the following three strategies for those who are doxorubicin-eligible: PVAG (prednisone, vinblastine, doxorubicin, and gemcitabine), CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), or EVA (etoposide, vinblastine, and doxorubicin).
For those unfit for anthracyclines, the options include COPP (cyclophosphamide, vincristine, procarbazine, and prednisone) or ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisone).
For frail patients who are ineligible for chemotherapy, the team recommends brentuximab alone or in combination with nivolumab.
Given the limited availability of dacarbazine, the authors say that the “current supply should be triaged to prioritize patients whose therapy cannot be changed and those without alternative acceptable options.”
To stretch available dacarbazine supplies as much as possible, the researchers and editorialists advocate for rounding doses within 5%-10% of the prescribed dose.
For example, Dr. Torka and colleagues explained, rounding a dose from 750 mg down to 700 mg would save one vial of dacarbazine.
Vial sharing and using drugs beyond their use dates by compounding with closed-system transfer devices are other strategies to preserve the existing supply of dacarbazine.
The goal of this review “is to give as many patients as possible the most optimal and efficacious therapy even with the strain on supply,” the editorialists wrote.
No funding for the study was reported. Dr. Torka is an adviser for Genentech, ADC Therapeutics, and TG Therapeutics. Dr. Soriano has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many state cancer plans drift from USPSTF breast cancer recommendations
When it comes to mammography recommendations state comprehensive cancer control (CCC) plans vary considerably and don’t always closely match the U.S. Preventive Services Task Force (USPSTF) recommendations for mammography frequency in women at average risk for breast cancer, according to a new cross-sectional study of CCC plans in all 50 states and the District of Columbia. The recommended age for initiation varied widely among CCC plans, and nearly one in five bore little resemblance at all to the USPTF recommendations.
According to the authors of the study, the variation among suggested ages of initiation may indicate a lack of consensus among state agencies. “For a recommendation tied to service coverage, this is a serious gap in public health policy,” they wrote in the study published online in JAMA Network Open.
CCC plans include goals, measurable objectives, and evidence-based strategies to combat cancers that are both common and preventable. They include input from multiple groups, frequently take 4-6 years to create, and should be updated regularly. Funding from the Centers for Disease Control and Prevention requires that the plans include data cancer screening prevalence rates and specific objectives and strategies.
Breast cancer is the most common cancer in women in the United States, and the second highest cause of cancer death. Regular, high-quality screening reduces breast cancer mortality by 25%-31% among women aged 50-69. As a result, the American Cancer Society, the USPSTF, the American College of Physicians, and the American Academy of Family Physicians have produced guidelines for mammography screening in women at average risk of breast cancer.
Although the benefits of screening are widely accepted, there is disagreement about the ages it should be initiated and ended. These inconsistencies stem from different evidence used to support recommendations, as well as different standards for benefits and harms from screening. Common concerns include overdiagnosis, false-positive results, and radiation damage from mammography.
Because these benefits and harms can vary based on age and values, there is an emphasis on shared decision-making between clinicians and women, especially those aged 40-49.
The most recent USPTF recommendation, issued in 2016, states that women aged 50-74 with average risk should be screened with mammography every 2 years. The choice of mammography in average-risk women under 50 should be approached on an individual basis. USPTF defines average risk as having no signs, symptoms, or previous diagnosis of breast cancer, and no family history or genetic causes for concern.
In the new study, researchers conducted a point-in-time evaluation of CCC plans from 50 states and the District of Columbia, between Nov. 1, 2019, and June 30, 2021.
Thirty-one percent of the plans included the complete USPTF recommendations of biennial mammography between ages 50 and 74; 51% included some, but not all of the USPTF recommendations; and 18% were not consistent at all with USPTF recommendations.
Overall, 59% of plans recommended initiation at age 50 and 37% at age 40, which is consistent with the older 2009 USPSTF recommendation. Eight percent of plans recommended starting at both 40 and 50, and 20% of plans had no recommended age of initiation.
Among the plans that were partially consistent with USPTF, 73% recommended initiation of mammography at age 40 and 31% at age 50. Eighty-five percent did not include an age to stop mammography; 15% did not include a recommended frequency; and 15% had an initiation age other than 40 or 50. Eighty-five percent of plans partially consistent with USPSTF included a recommendation that mammography should be conducted biennially.
The authors state that CCC plans could be improved by a unified emphasis on biennial screening of the general population of women aged 50-74, as well as clear differentiation between women at average risk and those at high risk, who could be screened at ages younger than 50 in consultation with their physician.
The study is limited by the fact that plans were reviewed a single time, while state CCC plans are updated with varying periodicity. The authors agree that implementation of population-based screening should be tailored to individual states and health care systems.
When it comes to mammography recommendations state comprehensive cancer control (CCC) plans vary considerably and don’t always closely match the U.S. Preventive Services Task Force (USPSTF) recommendations for mammography frequency in women at average risk for breast cancer, according to a new cross-sectional study of CCC plans in all 50 states and the District of Columbia. The recommended age for initiation varied widely among CCC plans, and nearly one in five bore little resemblance at all to the USPTF recommendations.
According to the authors of the study, the variation among suggested ages of initiation may indicate a lack of consensus among state agencies. “For a recommendation tied to service coverage, this is a serious gap in public health policy,” they wrote in the study published online in JAMA Network Open.
CCC plans include goals, measurable objectives, and evidence-based strategies to combat cancers that are both common and preventable. They include input from multiple groups, frequently take 4-6 years to create, and should be updated regularly. Funding from the Centers for Disease Control and Prevention requires that the plans include data cancer screening prevalence rates and specific objectives and strategies.
Breast cancer is the most common cancer in women in the United States, and the second highest cause of cancer death. Regular, high-quality screening reduces breast cancer mortality by 25%-31% among women aged 50-69. As a result, the American Cancer Society, the USPSTF, the American College of Physicians, and the American Academy of Family Physicians have produced guidelines for mammography screening in women at average risk of breast cancer.
Although the benefits of screening are widely accepted, there is disagreement about the ages it should be initiated and ended. These inconsistencies stem from different evidence used to support recommendations, as well as different standards for benefits and harms from screening. Common concerns include overdiagnosis, false-positive results, and radiation damage from mammography.
Because these benefits and harms can vary based on age and values, there is an emphasis on shared decision-making between clinicians and women, especially those aged 40-49.
The most recent USPTF recommendation, issued in 2016, states that women aged 50-74 with average risk should be screened with mammography every 2 years. The choice of mammography in average-risk women under 50 should be approached on an individual basis. USPTF defines average risk as having no signs, symptoms, or previous diagnosis of breast cancer, and no family history or genetic causes for concern.
In the new study, researchers conducted a point-in-time evaluation of CCC plans from 50 states and the District of Columbia, between Nov. 1, 2019, and June 30, 2021.
Thirty-one percent of the plans included the complete USPTF recommendations of biennial mammography between ages 50 and 74; 51% included some, but not all of the USPTF recommendations; and 18% were not consistent at all with USPTF recommendations.
Overall, 59% of plans recommended initiation at age 50 and 37% at age 40, which is consistent with the older 2009 USPSTF recommendation. Eight percent of plans recommended starting at both 40 and 50, and 20% of plans had no recommended age of initiation.
Among the plans that were partially consistent with USPTF, 73% recommended initiation of mammography at age 40 and 31% at age 50. Eighty-five percent did not include an age to stop mammography; 15% did not include a recommended frequency; and 15% had an initiation age other than 40 or 50. Eighty-five percent of plans partially consistent with USPSTF included a recommendation that mammography should be conducted biennially.
The authors state that CCC plans could be improved by a unified emphasis on biennial screening of the general population of women aged 50-74, as well as clear differentiation between women at average risk and those at high risk, who could be screened at ages younger than 50 in consultation with their physician.
The study is limited by the fact that plans were reviewed a single time, while state CCC plans are updated with varying periodicity. The authors agree that implementation of population-based screening should be tailored to individual states and health care systems.
When it comes to mammography recommendations state comprehensive cancer control (CCC) plans vary considerably and don’t always closely match the U.S. Preventive Services Task Force (USPSTF) recommendations for mammography frequency in women at average risk for breast cancer, according to a new cross-sectional study of CCC plans in all 50 states and the District of Columbia. The recommended age for initiation varied widely among CCC plans, and nearly one in five bore little resemblance at all to the USPTF recommendations.
According to the authors of the study, the variation among suggested ages of initiation may indicate a lack of consensus among state agencies. “For a recommendation tied to service coverage, this is a serious gap in public health policy,” they wrote in the study published online in JAMA Network Open.
CCC plans include goals, measurable objectives, and evidence-based strategies to combat cancers that are both common and preventable. They include input from multiple groups, frequently take 4-6 years to create, and should be updated regularly. Funding from the Centers for Disease Control and Prevention requires that the plans include data cancer screening prevalence rates and specific objectives and strategies.
Breast cancer is the most common cancer in women in the United States, and the second highest cause of cancer death. Regular, high-quality screening reduces breast cancer mortality by 25%-31% among women aged 50-69. As a result, the American Cancer Society, the USPSTF, the American College of Physicians, and the American Academy of Family Physicians have produced guidelines for mammography screening in women at average risk of breast cancer.
Although the benefits of screening are widely accepted, there is disagreement about the ages it should be initiated and ended. These inconsistencies stem from different evidence used to support recommendations, as well as different standards for benefits and harms from screening. Common concerns include overdiagnosis, false-positive results, and radiation damage from mammography.
Because these benefits and harms can vary based on age and values, there is an emphasis on shared decision-making between clinicians and women, especially those aged 40-49.
The most recent USPTF recommendation, issued in 2016, states that women aged 50-74 with average risk should be screened with mammography every 2 years. The choice of mammography in average-risk women under 50 should be approached on an individual basis. USPTF defines average risk as having no signs, symptoms, or previous diagnosis of breast cancer, and no family history or genetic causes for concern.
In the new study, researchers conducted a point-in-time evaluation of CCC plans from 50 states and the District of Columbia, between Nov. 1, 2019, and June 30, 2021.
Thirty-one percent of the plans included the complete USPTF recommendations of biennial mammography between ages 50 and 74; 51% included some, but not all of the USPTF recommendations; and 18% were not consistent at all with USPTF recommendations.
Overall, 59% of plans recommended initiation at age 50 and 37% at age 40, which is consistent with the older 2009 USPSTF recommendation. Eight percent of plans recommended starting at both 40 and 50, and 20% of plans had no recommended age of initiation.
Among the plans that were partially consistent with USPTF, 73% recommended initiation of mammography at age 40 and 31% at age 50. Eighty-five percent did not include an age to stop mammography; 15% did not include a recommended frequency; and 15% had an initiation age other than 40 or 50. Eighty-five percent of plans partially consistent with USPSTF included a recommendation that mammography should be conducted biennially.
The authors state that CCC plans could be improved by a unified emphasis on biennial screening of the general population of women aged 50-74, as well as clear differentiation between women at average risk and those at high risk, who could be screened at ages younger than 50 in consultation with their physician.
The study is limited by the fact that plans were reviewed a single time, while state CCC plans are updated with varying periodicity. The authors agree that implementation of population-based screening should be tailored to individual states and health care systems.
FROM JAMA NETWORK OPEN
Reading Chekhov on the Cancer Ward
Burnout and other forms of psychosocial distress are common among health care professionals necessitating measures to promote well-being and reduce burnout.1 Studies have shown that nonmedical reading is associated with low burnout and that small group study sections can promote wellness.2,3 Narrative medicine, which proposes a model for humane and effective medical practice, advocates for the necessity of narrative competence.
Short Story Club
Narrative competence is the ability to acknowledge, interpret, and act on the stories of others. The narrative skill of close reading also encourages reflective practice, equipping practitioners to better weather the tides of illness.4 In our case, we formed a short story club intervention to closely read, or read and reflect, on literary fiction. We explored how reading and reflecting would result in profound changes in thinking and feeling and noted different ways by which they can cause such well-being. We describe here the 7 ways in which stories led us to increase bonding, improve empathy, and promote meaning in medicine.
Slowing Down
The short story club helped to bond us together and increase our sense of meaning in medicine by slowing us down. One member of the group likened the experience to increasing the pixels in a painting, thereby improving the resolution and seeing more clearly. Another member mentioned the experience as a form of meditation in slowing down the brain, breathing in the story, and breathing out impressions. One story by Anatole Broyard emphasized the importance of slowing down and “brooding” over a patient.5 The author describes his experience as a prostate cancer patient, in which his body was treated but his story was ignored. He begged his doctors to pay more attention to his story to listen and to brood over him. This story was enlightening to us; we saw how desperate our patients are to tell their stories, and for us to hear their stories.
Mirrors and Windows
Another way reading and reflecting on short stories helped was by reflecting our practices to ourselves, as though looking into a mirror to see ourselves and out of a window to see others. We found that stories mirrored our own world and allowed us to discuss issues close to us without the embarrassment or stigma of owning the story. In one session we read “The Doctor’s Visit” by Anton Chekhov.6 Some of the members resonated with the doctor of this story who awkwardly attended to his lady patient whose son was dying of a brain tumor. The doctor was nervous, insecure, and unable to express any empathy. He was also the father of the child who was dying and refused to admit any responsibility. One member of the group stated that he could relate to the doctor’s insecurities and mentioned that he too felt insecure and even sometimes felt like an imposter. This led to a discussion of insecurities, ways to bolster self-confidence, and ways to accept and respect limitations. This was a conversation that may not have taken place without the story as anchor to discuss insecurities that we individually may not have been willing to admit to the group.
In a different session, we discussed the story “Interpreter of Maladies” by Jhumpa Lahiri in which a settled Indian American family returns to India to tour and learn about their heritage from a guide (the interpreter of maladies) who interpreted the culture for them.7 The family professed to be interested in knowing about the culture but could not concentrate: the wife stayed busy flirting with the guide and revealing outrageous secrets to him, the children were engrossed in their squabbles, and the father was essentially absent taking photographs as souvenirs instead of seeing the sites firsthand. Some of the members of the group were Indian American and could relate to the alienation from their home and nostalgia for their country, while others could relate to the same alienation, albeit from other cultures and countries. This allowed us to talk about deeply personal topics, without having to own the topic or reveal personal issues. The discussion led to a deep understanding and empathy for us and our colleagues knowing the pain of alienation that some of them felt but could not discuss.
The stories also served as windows into the world of others which enabled us to see and become the other. For example, in one session we reflected on “Babylon Revisited” by F. Scott Fitzgerald.8 In this story, an American man returns to Paris after the Great Depression and recalls his life as a young artist in the American artist expatriate community of Paris in the 1920s and 1930s. During that time, he partied, drank in excess, lost his wife to pneumonia (for which he was at least partially responsible), lost custody of his daughter, and lost his fortune. As he returned to Paris to try to reclaim his daughter, we feel his pain as he tries but fails to overcome chronic alcoholism, sexual indiscretions, and losses. This gave rise to discussion of losses in general as we became one with the main character. This increased our empathy for others in a way that could not have been possible without this short story as anchor.
In another session we reflected on “Hills Like White Elephants” by Ernest Hemingway, in which a man is waiting for a train while proposing his girlfriend get an abortion.9 She agonizes over her choices and makes no decision in this story. Yet, we the reader could “become” the woman in the story faced with hard choices of having a baby but losing the man she loves, or having an abortion and maybe losing him anyway. In becoming this woman, we could experience the complex emotions and feel an experience of the other.
Exploring the Taboo
A third aspect of the club was enabling discussion of controversial topics. There were topics that arose in the group which never would have arisen in clinical practice discussions. These had to do with the taboo topics such as romantic attachments to patients. We read “The Caves of Lascaux” and reflected on the story of a young doctor who becomes enamored and obsessed with his beautiful but dying patient.10 He becomes so obsessed with her that he almost abandons his wife, family, and stable livelihood to descend with her into the caves. This story gave rise to discussions about romantic attachment to patients and how to handle and extricate one from the situation. The senior doctors explained some of their relevant experiences and how they either transferred care or sought counseling to extricate themselves from a potentially dangerous situation, especially when they too fell under the spell of forbidden romance.
Moral Grounding
These sessions also served to define the moral basis of our own practice. Much of health care psychosocial distress is related to moral injury in which health care professionals do the wrong thing or fail to do the right thing at the right time, due to external pressures related to financial or other gains. Reading and reflecting put us face-to-face with moral dilemmas and let us find our moral grounding. In reading “The Haircut” by Ring Lardner, we explored the disruptive town scoundrel who harassed and tortured his friends and neighbors but in such outrageous ways that he was considered a comedian rather than an abuser.11 Despite his hurtful acts, the townspeople (including the narrator) considered him a clown and laughed at his racist and sexist statements as well as his tricks.He faced no consequences such as confrontation, until the end when fate caught up. This story gave rise to a discussion of how we handle unkind, racist, sexist, or other comments which are disguised as humor, and to what extent we tolerate such controversial behavior. Do we go along with the scoundrel and laugh, or do we confront such people and insist that they respect and honor other people? The story sensitized the group to the ways in which prejudice and racism or sexism can be masked as humor, and to consider our moral responsibilities in society.
In another session we read and reflected on “Three Questions” by Leo Tolstoy.12 In this story, a king travels to another territory but gets distracted by helping a neighbor in need, and thereby inadvertently and fortunately avoids the trap that had been devised to kill him. The author gives us his moral basis by asking and answering 3 questions: Who is the most important person? What is the most important thing to do? What is the most important thing to do now? His answers provided his moral grounding. We discussed our answers and the basis of our moral grounding, whether it be the injunction do no harm, the more complex religious backgrounds of our childhood, or otherwise.
Symbols and Metaphors
The practice of reading and reflecting also taught us symbols and metaphors. Symbols and metaphors are the essence of storytelling, and they provide keys to understanding people. We sought out and studied the metaphors and symbols in each of the stories we read. In “I Stood There Ironing”, a woman is ironing as she is being questioned by a social worker on the upbringing of her first daughter, and its impact on her psychosocial distress.13 The woman remembers the hardships in raising her daughter and her neglect and abuse of the child due to circumstances beyond her control. She keeps ironing back and forth as she recounts the ways in which she neglected her child. The ironing provides a metaphor for attempting to straighten out her life and for recognizing finally at the end of the story that the daughter should not be the dress, under which her iron is pressing. This gave rise to a discussion of metaphors in our lives and the meanings they carry.
Problem-solving Guide
A sixth way the reflections helped was by serving as a guide to solving our problems. Some of the stories we read resonated deeply with members of the group and provided guides to solving problems. In one meeting we discussed “Those Are as Brothers” by Nancy Hale, a story in which a Nazi concentration camp survivor finds refuge in a country home and develops a friendship with a survivor of an abusive marriage.14 Reading and reflecting on this story enabled us to see the impact of trauma on ourselves, our life choices, professions, ways of being, philosophies, and even on our next generation. The story was personal for several members of the group, some of whom were second-generation Holocaust survivors, and for one who admitted to severe trauma as a child. Discussing our backgrounds together, we empathized with each other and helped each other heal. The story also provided a guide to healing from trauma, as its title indicates: sharing stories together can be a way to heal. The solidarity of standing together, as brothers, heals. The concentration camp survivor was mistreated in his job, but the abuse trauma victim rushes to his defense and vows her friendship and support. This soothed his soul and healed his mind. The guidance is clear: we can do the same, find friends, treat them like brothers, support each other and heal.
Bonding Through Shared Experience
The final and possibly most important way in which the club helped was by serving as an adventure to bond group members together through shared experience. We believe that literature can capture imagination in extraordinary ways and provide an opportunity to undertake remarkable journeys. As such, together we traveled to the ends of the earth from the beginning to the end of time and beyond. We traveled through the hills of Africa, meandered in the streets of Russia and Poland, watched the racetracks in Italy, toured the Taj Mahal in India, and descended into the caves of Lascaux, all while working in Little Rock, Arkansas. We shared a wide array of experiences together, which allowed us to know ourselves and others better, to share stories, and to develop a common vision, common ground, and common culture.
Conclusions
Through reading and reflecting on stories, we bonded as a group, increased our empathy for each other and others, and found meaning in medicine. Other studies have shown that participation in small study groups promote physician well-being, improve job satisfaction, and decrease burnout.3 We synergized this effort by reading nonmedical stories on a consistent basis, hoping to gain resilience to psychosocial distress.3 We chose short stories rather than novels to minimize any stress from excess reading. Combining these interventions, small group studies and nonmedical reading, into a single intervention as is typical in the practice of narrative medicine may provide a way to improve team functioning.
This pilot study showed that it is possible to form short story clubs even in a busy oncology program and that such programs benefit participants in a variety of ways with no apparent adverse effects. Further research is needed to study the impact of reading and reflecting on medical work in small study groups in larger numbers of subjects and to evaluate their impact on burnout. Further study is also needed to develop narrative medicine curricula that best address the needs of particular subspecialties and to determine the optimal conditions for implementation.
Acknowledgments
The authors acknowledge Dr. Erick Messias for inspiring and encouraging this project at the University of Arkansas for Medical Sciences where he was Associate Dean for Faculty Affairs. He is presently Chair of Psychiatry and Behavioral Neuroscience at the St. Louis University School of Medicine, St. Louis, Missouri.
1. Messias E, Gathright MM, Freeman ES, et al. Differences in burnout prevalence between clinical professionals and biomedical scientists in an academic medical centre: a cross-sectional survey. BMJ Open. 2019;9(2):e023506. doi:10.1136/bmjopen-2018-023506
2. Marchalik D, Rodriguez A, Namath A, et al. The impact of non-medical reading on clinical burnout: a national survey of palliative care providers. Ann Palliat Med. 2019;8(4):428-435. doi:10.21037/apm.2019.05.02
3. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533. doi:10.1001/jamainternmed.2013.14387
4. Charon R. Narrative medicine: a model for empathy, reflection, profession, and tust. JAMA. 2001;286(15):1897–1902. doi:10.1001/jama.286.15.1897
5. Broyard A. Doctor Talk to Me. August 26, 1990. Accessed September 2021. https://www.nytimes.com/1990/08/26/magazine/doctor-talk-to-me.html
6. Chekhov A. A Doctor’s Visit,. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:50-59.
7. Lahiri J. Interpreter of Maladies. In: Lahiri J. Interpreter of Maladies. Mariner Books;2019.
8. Fitzgerald FS. Babylon Revisited. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:62-81.
9. Hemingway E. Hills like White Elephants. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:108-111.
10. Karmel M. Caves of Lascaux. In: Ofri D, Staff of the Bellavue Literary Review, eds. The Best of the Bellevue Literary Review. Bellevue Literary Press;2008:168-174.
11. Lardner R. The Haircut. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:48-61.
12. Tolstoy L. The Three Questions. Accessed September 2021. https://www.plough.com/en/topics/culture/short-stories/the-three-questions
13. Olsen T. I Stand Here Ironing. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:173-180.
14. Hale N. Those Are as Brothers. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:132-141.
Burnout and other forms of psychosocial distress are common among health care professionals necessitating measures to promote well-being and reduce burnout.1 Studies have shown that nonmedical reading is associated with low burnout and that small group study sections can promote wellness.2,3 Narrative medicine, which proposes a model for humane and effective medical practice, advocates for the necessity of narrative competence.
Short Story Club
Narrative competence is the ability to acknowledge, interpret, and act on the stories of others. The narrative skill of close reading also encourages reflective practice, equipping practitioners to better weather the tides of illness.4 In our case, we formed a short story club intervention to closely read, or read and reflect, on literary fiction. We explored how reading and reflecting would result in profound changes in thinking and feeling and noted different ways by which they can cause such well-being. We describe here the 7 ways in which stories led us to increase bonding, improve empathy, and promote meaning in medicine.
Slowing Down
The short story club helped to bond us together and increase our sense of meaning in medicine by slowing us down. One member of the group likened the experience to increasing the pixels in a painting, thereby improving the resolution and seeing more clearly. Another member mentioned the experience as a form of meditation in slowing down the brain, breathing in the story, and breathing out impressions. One story by Anatole Broyard emphasized the importance of slowing down and “brooding” over a patient.5 The author describes his experience as a prostate cancer patient, in which his body was treated but his story was ignored. He begged his doctors to pay more attention to his story to listen and to brood over him. This story was enlightening to us; we saw how desperate our patients are to tell their stories, and for us to hear their stories.
Mirrors and Windows
Another way reading and reflecting on short stories helped was by reflecting our practices to ourselves, as though looking into a mirror to see ourselves and out of a window to see others. We found that stories mirrored our own world and allowed us to discuss issues close to us without the embarrassment or stigma of owning the story. In one session we read “The Doctor’s Visit” by Anton Chekhov.6 Some of the members resonated with the doctor of this story who awkwardly attended to his lady patient whose son was dying of a brain tumor. The doctor was nervous, insecure, and unable to express any empathy. He was also the father of the child who was dying and refused to admit any responsibility. One member of the group stated that he could relate to the doctor’s insecurities and mentioned that he too felt insecure and even sometimes felt like an imposter. This led to a discussion of insecurities, ways to bolster self-confidence, and ways to accept and respect limitations. This was a conversation that may not have taken place without the story as anchor to discuss insecurities that we individually may not have been willing to admit to the group.
In a different session, we discussed the story “Interpreter of Maladies” by Jhumpa Lahiri in which a settled Indian American family returns to India to tour and learn about their heritage from a guide (the interpreter of maladies) who interpreted the culture for them.7 The family professed to be interested in knowing about the culture but could not concentrate: the wife stayed busy flirting with the guide and revealing outrageous secrets to him, the children were engrossed in their squabbles, and the father was essentially absent taking photographs as souvenirs instead of seeing the sites firsthand. Some of the members of the group were Indian American and could relate to the alienation from their home and nostalgia for their country, while others could relate to the same alienation, albeit from other cultures and countries. This allowed us to talk about deeply personal topics, without having to own the topic or reveal personal issues. The discussion led to a deep understanding and empathy for us and our colleagues knowing the pain of alienation that some of them felt but could not discuss.
The stories also served as windows into the world of others which enabled us to see and become the other. For example, in one session we reflected on “Babylon Revisited” by F. Scott Fitzgerald.8 In this story, an American man returns to Paris after the Great Depression and recalls his life as a young artist in the American artist expatriate community of Paris in the 1920s and 1930s. During that time, he partied, drank in excess, lost his wife to pneumonia (for which he was at least partially responsible), lost custody of his daughter, and lost his fortune. As he returned to Paris to try to reclaim his daughter, we feel his pain as he tries but fails to overcome chronic alcoholism, sexual indiscretions, and losses. This gave rise to discussion of losses in general as we became one with the main character. This increased our empathy for others in a way that could not have been possible without this short story as anchor.
In another session we reflected on “Hills Like White Elephants” by Ernest Hemingway, in which a man is waiting for a train while proposing his girlfriend get an abortion.9 She agonizes over her choices and makes no decision in this story. Yet, we the reader could “become” the woman in the story faced with hard choices of having a baby but losing the man she loves, or having an abortion and maybe losing him anyway. In becoming this woman, we could experience the complex emotions and feel an experience of the other.
Exploring the Taboo
A third aspect of the club was enabling discussion of controversial topics. There were topics that arose in the group which never would have arisen in clinical practice discussions. These had to do with the taboo topics such as romantic attachments to patients. We read “The Caves of Lascaux” and reflected on the story of a young doctor who becomes enamored and obsessed with his beautiful but dying patient.10 He becomes so obsessed with her that he almost abandons his wife, family, and stable livelihood to descend with her into the caves. This story gave rise to discussions about romantic attachment to patients and how to handle and extricate one from the situation. The senior doctors explained some of their relevant experiences and how they either transferred care or sought counseling to extricate themselves from a potentially dangerous situation, especially when they too fell under the spell of forbidden romance.
Moral Grounding
These sessions also served to define the moral basis of our own practice. Much of health care psychosocial distress is related to moral injury in which health care professionals do the wrong thing or fail to do the right thing at the right time, due to external pressures related to financial or other gains. Reading and reflecting put us face-to-face with moral dilemmas and let us find our moral grounding. In reading “The Haircut” by Ring Lardner, we explored the disruptive town scoundrel who harassed and tortured his friends and neighbors but in such outrageous ways that he was considered a comedian rather than an abuser.11 Despite his hurtful acts, the townspeople (including the narrator) considered him a clown and laughed at his racist and sexist statements as well as his tricks.He faced no consequences such as confrontation, until the end when fate caught up. This story gave rise to a discussion of how we handle unkind, racist, sexist, or other comments which are disguised as humor, and to what extent we tolerate such controversial behavior. Do we go along with the scoundrel and laugh, or do we confront such people and insist that they respect and honor other people? The story sensitized the group to the ways in which prejudice and racism or sexism can be masked as humor, and to consider our moral responsibilities in society.
In another session we read and reflected on “Three Questions” by Leo Tolstoy.12 In this story, a king travels to another territory but gets distracted by helping a neighbor in need, and thereby inadvertently and fortunately avoids the trap that had been devised to kill him. The author gives us his moral basis by asking and answering 3 questions: Who is the most important person? What is the most important thing to do? What is the most important thing to do now? His answers provided his moral grounding. We discussed our answers and the basis of our moral grounding, whether it be the injunction do no harm, the more complex religious backgrounds of our childhood, or otherwise.
Symbols and Metaphors
The practice of reading and reflecting also taught us symbols and metaphors. Symbols and metaphors are the essence of storytelling, and they provide keys to understanding people. We sought out and studied the metaphors and symbols in each of the stories we read. In “I Stood There Ironing”, a woman is ironing as she is being questioned by a social worker on the upbringing of her first daughter, and its impact on her psychosocial distress.13 The woman remembers the hardships in raising her daughter and her neglect and abuse of the child due to circumstances beyond her control. She keeps ironing back and forth as she recounts the ways in which she neglected her child. The ironing provides a metaphor for attempting to straighten out her life and for recognizing finally at the end of the story that the daughter should not be the dress, under which her iron is pressing. This gave rise to a discussion of metaphors in our lives and the meanings they carry.
Problem-solving Guide
A sixth way the reflections helped was by serving as a guide to solving our problems. Some of the stories we read resonated deeply with members of the group and provided guides to solving problems. In one meeting we discussed “Those Are as Brothers” by Nancy Hale, a story in which a Nazi concentration camp survivor finds refuge in a country home and develops a friendship with a survivor of an abusive marriage.14 Reading and reflecting on this story enabled us to see the impact of trauma on ourselves, our life choices, professions, ways of being, philosophies, and even on our next generation. The story was personal for several members of the group, some of whom were second-generation Holocaust survivors, and for one who admitted to severe trauma as a child. Discussing our backgrounds together, we empathized with each other and helped each other heal. The story also provided a guide to healing from trauma, as its title indicates: sharing stories together can be a way to heal. The solidarity of standing together, as brothers, heals. The concentration camp survivor was mistreated in his job, but the abuse trauma victim rushes to his defense and vows her friendship and support. This soothed his soul and healed his mind. The guidance is clear: we can do the same, find friends, treat them like brothers, support each other and heal.
Bonding Through Shared Experience
The final and possibly most important way in which the club helped was by serving as an adventure to bond group members together through shared experience. We believe that literature can capture imagination in extraordinary ways and provide an opportunity to undertake remarkable journeys. As such, together we traveled to the ends of the earth from the beginning to the end of time and beyond. We traveled through the hills of Africa, meandered in the streets of Russia and Poland, watched the racetracks in Italy, toured the Taj Mahal in India, and descended into the caves of Lascaux, all while working in Little Rock, Arkansas. We shared a wide array of experiences together, which allowed us to know ourselves and others better, to share stories, and to develop a common vision, common ground, and common culture.
Conclusions
Through reading and reflecting on stories, we bonded as a group, increased our empathy for each other and others, and found meaning in medicine. Other studies have shown that participation in small study groups promote physician well-being, improve job satisfaction, and decrease burnout.3 We synergized this effort by reading nonmedical stories on a consistent basis, hoping to gain resilience to psychosocial distress.3 We chose short stories rather than novels to minimize any stress from excess reading. Combining these interventions, small group studies and nonmedical reading, into a single intervention as is typical in the practice of narrative medicine may provide a way to improve team functioning.
This pilot study showed that it is possible to form short story clubs even in a busy oncology program and that such programs benefit participants in a variety of ways with no apparent adverse effects. Further research is needed to study the impact of reading and reflecting on medical work in small study groups in larger numbers of subjects and to evaluate their impact on burnout. Further study is also needed to develop narrative medicine curricula that best address the needs of particular subspecialties and to determine the optimal conditions for implementation.
Acknowledgments
The authors acknowledge Dr. Erick Messias for inspiring and encouraging this project at the University of Arkansas for Medical Sciences where he was Associate Dean for Faculty Affairs. He is presently Chair of Psychiatry and Behavioral Neuroscience at the St. Louis University School of Medicine, St. Louis, Missouri.
Burnout and other forms of psychosocial distress are common among health care professionals necessitating measures to promote well-being and reduce burnout.1 Studies have shown that nonmedical reading is associated with low burnout and that small group study sections can promote wellness.2,3 Narrative medicine, which proposes a model for humane and effective medical practice, advocates for the necessity of narrative competence.
Short Story Club
Narrative competence is the ability to acknowledge, interpret, and act on the stories of others. The narrative skill of close reading also encourages reflective practice, equipping practitioners to better weather the tides of illness.4 In our case, we formed a short story club intervention to closely read, or read and reflect, on literary fiction. We explored how reading and reflecting would result in profound changes in thinking and feeling and noted different ways by which they can cause such well-being. We describe here the 7 ways in which stories led us to increase bonding, improve empathy, and promote meaning in medicine.
Slowing Down
The short story club helped to bond us together and increase our sense of meaning in medicine by slowing us down. One member of the group likened the experience to increasing the pixels in a painting, thereby improving the resolution and seeing more clearly. Another member mentioned the experience as a form of meditation in slowing down the brain, breathing in the story, and breathing out impressions. One story by Anatole Broyard emphasized the importance of slowing down and “brooding” over a patient.5 The author describes his experience as a prostate cancer patient, in which his body was treated but his story was ignored. He begged his doctors to pay more attention to his story to listen and to brood over him. This story was enlightening to us; we saw how desperate our patients are to tell their stories, and for us to hear their stories.
Mirrors and Windows
Another way reading and reflecting on short stories helped was by reflecting our practices to ourselves, as though looking into a mirror to see ourselves and out of a window to see others. We found that stories mirrored our own world and allowed us to discuss issues close to us without the embarrassment or stigma of owning the story. In one session we read “The Doctor’s Visit” by Anton Chekhov.6 Some of the members resonated with the doctor of this story who awkwardly attended to his lady patient whose son was dying of a brain tumor. The doctor was nervous, insecure, and unable to express any empathy. He was also the father of the child who was dying and refused to admit any responsibility. One member of the group stated that he could relate to the doctor’s insecurities and mentioned that he too felt insecure and even sometimes felt like an imposter. This led to a discussion of insecurities, ways to bolster self-confidence, and ways to accept and respect limitations. This was a conversation that may not have taken place without the story as anchor to discuss insecurities that we individually may not have been willing to admit to the group.
In a different session, we discussed the story “Interpreter of Maladies” by Jhumpa Lahiri in which a settled Indian American family returns to India to tour and learn about their heritage from a guide (the interpreter of maladies) who interpreted the culture for them.7 The family professed to be interested in knowing about the culture but could not concentrate: the wife stayed busy flirting with the guide and revealing outrageous secrets to him, the children were engrossed in their squabbles, and the father was essentially absent taking photographs as souvenirs instead of seeing the sites firsthand. Some of the members of the group were Indian American and could relate to the alienation from their home and nostalgia for their country, while others could relate to the same alienation, albeit from other cultures and countries. This allowed us to talk about deeply personal topics, without having to own the topic or reveal personal issues. The discussion led to a deep understanding and empathy for us and our colleagues knowing the pain of alienation that some of them felt but could not discuss.
The stories also served as windows into the world of others which enabled us to see and become the other. For example, in one session we reflected on “Babylon Revisited” by F. Scott Fitzgerald.8 In this story, an American man returns to Paris after the Great Depression and recalls his life as a young artist in the American artist expatriate community of Paris in the 1920s and 1930s. During that time, he partied, drank in excess, lost his wife to pneumonia (for which he was at least partially responsible), lost custody of his daughter, and lost his fortune. As he returned to Paris to try to reclaim his daughter, we feel his pain as he tries but fails to overcome chronic alcoholism, sexual indiscretions, and losses. This gave rise to discussion of losses in general as we became one with the main character. This increased our empathy for others in a way that could not have been possible without this short story as anchor.
In another session we reflected on “Hills Like White Elephants” by Ernest Hemingway, in which a man is waiting for a train while proposing his girlfriend get an abortion.9 She agonizes over her choices and makes no decision in this story. Yet, we the reader could “become” the woman in the story faced with hard choices of having a baby but losing the man she loves, or having an abortion and maybe losing him anyway. In becoming this woman, we could experience the complex emotions and feel an experience of the other.
Exploring the Taboo
A third aspect of the club was enabling discussion of controversial topics. There were topics that arose in the group which never would have arisen in clinical practice discussions. These had to do with the taboo topics such as romantic attachments to patients. We read “The Caves of Lascaux” and reflected on the story of a young doctor who becomes enamored and obsessed with his beautiful but dying patient.10 He becomes so obsessed with her that he almost abandons his wife, family, and stable livelihood to descend with her into the caves. This story gave rise to discussions about romantic attachment to patients and how to handle and extricate one from the situation. The senior doctors explained some of their relevant experiences and how they either transferred care or sought counseling to extricate themselves from a potentially dangerous situation, especially when they too fell under the spell of forbidden romance.
Moral Grounding
These sessions also served to define the moral basis of our own practice. Much of health care psychosocial distress is related to moral injury in which health care professionals do the wrong thing or fail to do the right thing at the right time, due to external pressures related to financial or other gains. Reading and reflecting put us face-to-face with moral dilemmas and let us find our moral grounding. In reading “The Haircut” by Ring Lardner, we explored the disruptive town scoundrel who harassed and tortured his friends and neighbors but in such outrageous ways that he was considered a comedian rather than an abuser.11 Despite his hurtful acts, the townspeople (including the narrator) considered him a clown and laughed at his racist and sexist statements as well as his tricks.He faced no consequences such as confrontation, until the end when fate caught up. This story gave rise to a discussion of how we handle unkind, racist, sexist, or other comments which are disguised as humor, and to what extent we tolerate such controversial behavior. Do we go along with the scoundrel and laugh, or do we confront such people and insist that they respect and honor other people? The story sensitized the group to the ways in which prejudice and racism or sexism can be masked as humor, and to consider our moral responsibilities in society.
In another session we read and reflected on “Three Questions” by Leo Tolstoy.12 In this story, a king travels to another territory but gets distracted by helping a neighbor in need, and thereby inadvertently and fortunately avoids the trap that had been devised to kill him. The author gives us his moral basis by asking and answering 3 questions: Who is the most important person? What is the most important thing to do? What is the most important thing to do now? His answers provided his moral grounding. We discussed our answers and the basis of our moral grounding, whether it be the injunction do no harm, the more complex religious backgrounds of our childhood, or otherwise.
Symbols and Metaphors
The practice of reading and reflecting also taught us symbols and metaphors. Symbols and metaphors are the essence of storytelling, and they provide keys to understanding people. We sought out and studied the metaphors and symbols in each of the stories we read. In “I Stood There Ironing”, a woman is ironing as she is being questioned by a social worker on the upbringing of her first daughter, and its impact on her psychosocial distress.13 The woman remembers the hardships in raising her daughter and her neglect and abuse of the child due to circumstances beyond her control. She keeps ironing back and forth as she recounts the ways in which she neglected her child. The ironing provides a metaphor for attempting to straighten out her life and for recognizing finally at the end of the story that the daughter should not be the dress, under which her iron is pressing. This gave rise to a discussion of metaphors in our lives and the meanings they carry.
Problem-solving Guide
A sixth way the reflections helped was by serving as a guide to solving our problems. Some of the stories we read resonated deeply with members of the group and provided guides to solving problems. In one meeting we discussed “Those Are as Brothers” by Nancy Hale, a story in which a Nazi concentration camp survivor finds refuge in a country home and develops a friendship with a survivor of an abusive marriage.14 Reading and reflecting on this story enabled us to see the impact of trauma on ourselves, our life choices, professions, ways of being, philosophies, and even on our next generation. The story was personal for several members of the group, some of whom were second-generation Holocaust survivors, and for one who admitted to severe trauma as a child. Discussing our backgrounds together, we empathized with each other and helped each other heal. The story also provided a guide to healing from trauma, as its title indicates: sharing stories together can be a way to heal. The solidarity of standing together, as brothers, heals. The concentration camp survivor was mistreated in his job, but the abuse trauma victim rushes to his defense and vows her friendship and support. This soothed his soul and healed his mind. The guidance is clear: we can do the same, find friends, treat them like brothers, support each other and heal.
Bonding Through Shared Experience
The final and possibly most important way in which the club helped was by serving as an adventure to bond group members together through shared experience. We believe that literature can capture imagination in extraordinary ways and provide an opportunity to undertake remarkable journeys. As such, together we traveled to the ends of the earth from the beginning to the end of time and beyond. We traveled through the hills of Africa, meandered in the streets of Russia and Poland, watched the racetracks in Italy, toured the Taj Mahal in India, and descended into the caves of Lascaux, all while working in Little Rock, Arkansas. We shared a wide array of experiences together, which allowed us to know ourselves and others better, to share stories, and to develop a common vision, common ground, and common culture.
Conclusions
Through reading and reflecting on stories, we bonded as a group, increased our empathy for each other and others, and found meaning in medicine. Other studies have shown that participation in small study groups promote physician well-being, improve job satisfaction, and decrease burnout.3 We synergized this effort by reading nonmedical stories on a consistent basis, hoping to gain resilience to psychosocial distress.3 We chose short stories rather than novels to minimize any stress from excess reading. Combining these interventions, small group studies and nonmedical reading, into a single intervention as is typical in the practice of narrative medicine may provide a way to improve team functioning.
This pilot study showed that it is possible to form short story clubs even in a busy oncology program and that such programs benefit participants in a variety of ways with no apparent adverse effects. Further research is needed to study the impact of reading and reflecting on medical work in small study groups in larger numbers of subjects and to evaluate their impact on burnout. Further study is also needed to develop narrative medicine curricula that best address the needs of particular subspecialties and to determine the optimal conditions for implementation.
Acknowledgments
The authors acknowledge Dr. Erick Messias for inspiring and encouraging this project at the University of Arkansas for Medical Sciences where he was Associate Dean for Faculty Affairs. He is presently Chair of Psychiatry and Behavioral Neuroscience at the St. Louis University School of Medicine, St. Louis, Missouri.
1. Messias E, Gathright MM, Freeman ES, et al. Differences in burnout prevalence between clinical professionals and biomedical scientists in an academic medical centre: a cross-sectional survey. BMJ Open. 2019;9(2):e023506. doi:10.1136/bmjopen-2018-023506
2. Marchalik D, Rodriguez A, Namath A, et al. The impact of non-medical reading on clinical burnout: a national survey of palliative care providers. Ann Palliat Med. 2019;8(4):428-435. doi:10.21037/apm.2019.05.02
3. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533. doi:10.1001/jamainternmed.2013.14387
4. Charon R. Narrative medicine: a model for empathy, reflection, profession, and tust. JAMA. 2001;286(15):1897–1902. doi:10.1001/jama.286.15.1897
5. Broyard A. Doctor Talk to Me. August 26, 1990. Accessed September 2021. https://www.nytimes.com/1990/08/26/magazine/doctor-talk-to-me.html
6. Chekhov A. A Doctor’s Visit,. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:50-59.
7. Lahiri J. Interpreter of Maladies. In: Lahiri J. Interpreter of Maladies. Mariner Books;2019.
8. Fitzgerald FS. Babylon Revisited. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:62-81.
9. Hemingway E. Hills like White Elephants. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:108-111.
10. Karmel M. Caves of Lascaux. In: Ofri D, Staff of the Bellavue Literary Review, eds. The Best of the Bellevue Literary Review. Bellevue Literary Press;2008:168-174.
11. Lardner R. The Haircut. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:48-61.
12. Tolstoy L. The Three Questions. Accessed September 2021. https://www.plough.com/en/topics/culture/short-stories/the-three-questions
13. Olsen T. I Stand Here Ironing. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:173-180.
14. Hale N. Those Are as Brothers. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:132-141.
1. Messias E, Gathright MM, Freeman ES, et al. Differences in burnout prevalence between clinical professionals and biomedical scientists in an academic medical centre: a cross-sectional survey. BMJ Open. 2019;9(2):e023506. doi:10.1136/bmjopen-2018-023506
2. Marchalik D, Rodriguez A, Namath A, et al. The impact of non-medical reading on clinical burnout: a national survey of palliative care providers. Ann Palliat Med. 2019;8(4):428-435. doi:10.21037/apm.2019.05.02
3. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533. doi:10.1001/jamainternmed.2013.14387
4. Charon R. Narrative medicine: a model for empathy, reflection, profession, and tust. JAMA. 2001;286(15):1897–1902. doi:10.1001/jama.286.15.1897
5. Broyard A. Doctor Talk to Me. August 26, 1990. Accessed September 2021. https://www.nytimes.com/1990/08/26/magazine/doctor-talk-to-me.html
6. Chekhov A. A Doctor’s Visit,. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:50-59.
7. Lahiri J. Interpreter of Maladies. In: Lahiri J. Interpreter of Maladies. Mariner Books;2019.
8. Fitzgerald FS. Babylon Revisited. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:62-81.
9. Hemingway E. Hills like White Elephants. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:108-111.
10. Karmel M. Caves of Lascaux. In: Ofri D, Staff of the Bellavue Literary Review, eds. The Best of the Bellevue Literary Review. Bellevue Literary Press;2008:168-174.
11. Lardner R. The Haircut. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:48-61.
12. Tolstoy L. The Three Questions. Accessed September 2021. https://www.plough.com/en/topics/culture/short-stories/the-three-questions
13. Olsen T. I Stand Here Ironing. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:173-180.
14. Hale N. Those Are as Brothers. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:132-141.