User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Lawmakers question mental health disclosure rules
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
State medical licensing queries criticized
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
FROM A HOUSE ENERGY AND COMMERCE’S HEALTH SUBCOMMITTEE HEARING
Chewed prasugrel for primary PCI? Forget it!
And cangrelor, in turn, is superior to oral prasugrel, according to the randomized FABOLUS FASTER trial, Marco Valgimigli, MD, PhD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, contrary to conventional wisdom, chewed prasugrel (Effient) proved no better than swallowing the tablets whole for platelet inhibition, said Dr. Valgimigli, an interventional cardiologist at the University of Bern (Switzerland).
He explained that standard administration of the newer oral P2Y12 inhibitors prasugrel and ticagrelor (Brilinta) in patients undergoing percutaneous coronary intervention (PCI) for ST-elevation MI (STEMI) does not provide optimal early inhibition of platelet aggregation. The parenteral antiplatelet drugs tirofiban and cangrelor have been shown to provide faster and more prolonged inhibition of platelet aggregation than the oral P2Y12 inhibitors.
But there has been no head-to-head comparative data for the glycoprotein IIb/IIIA inhibitor tirofiban (Aggrastat) and the P2Y12 inhibitor cangrelor (Kengreal) in the setting of primary PCI for STEMI. This was the impetus for FABOLUS FASTER, the first study to compare the pharmacodynamic effects of the two parenteral antiplatelet agents. The trial also looked at how these potent parenteral drugs, compared with chewed prasugrel, another previously unexamined yet highly practical issue.
The three-center, multinational, open-label FABOLUS FASTER trial randomized 122 patients undergoing primary PCI for STEMI to one of three arms: a standard intravenous bolus and 2-hour infusion of either the P2Y12 inhibitor cangrelor (Kengreal) or the glycoprotein IIb/IIIA inhibitor tirofiban (Aggrastat), followed in either case by 60 mg of oral prasugrel, or a third arm in which patients didn’t receive either drug but were instead randomized to a 60-mg loading dose of chewed or whole prasugrel tablets.
The primary study endpoint was inhibition of platelet aggregation at 30 minutes as measured by light transmittance aggregometry in response to 20 mcmol/L of adenosine diphosphate (ADP).
Tirofiban was the unequivocal winner with 95% inhibition, as compared with 34.1% with cangrelor, 10.5% with chewed prasugrel, and 6.3% with prasugrel swallowed whole, even though the concentration of prasugrel’s active metabolite was far greater at 62.3 ng/mL after prasugrel was chewed, compared with 17.1 ng/mL when swallowed in integral tablet form.
The rate of nonresponsiveness to tirofiban as defined by greater than 59% platelet aggregation was zero for tirofiban during its 2-hour infusion, then a scant 8% thereafter during repeated testing at 3 and 4-6 hours. In contrast, the cangrelor nonresponsiveness rate was 50%-58% during the 2-hour infusion, rising to 82% at 3 hours.
FABOLUS FASTER, while not powered for clinical endpoints, might nevertheless have important clinical implications, according to Dr. Valgimigli. First, the superiority of the intravenous drugs tirofiban and cangrelor over prasugrel for early, strong platelet inhibition underscores the importance of giving parenteral antiplatelet drugs over oral therapy during the acute phase of STEMI therapy. Moreover, tirofiban’s outstanding performance – and the high residual platelet reactivity associated with cangrelor – makes a strong case for large comparative, randomized trials of the two drugs, with hard clinical endpoints.
Discussant Christoph K. Naber, MD, PhD, opined that he personally doesn’t consider the FABOLUS FASTER results practice changing, for a couple of reasons.
“Platelet inhibition measured by ADP in vitro is not necessarily related to true effects in vivo. We know that platelets are activated by multiple mechanisms, and the ADP pathway is just one of them,” said Dr. Naber, an interventional cardiologist at the Wilhemshaven (Germany) Clinic.
Also, there’s a good reason why no glycoprotein IIb/IIIA inhibitors are approved for treatment of STEMI, and why tirofiban, despite its impressive antiplatelet effects, is currently largely reserved for bailout situations, such as complex lesions with large thrombus burden. It’s because tirofiban’s potent antiplatelet activity is accompanied by a high risk of bleeding, he added.
However, Dr. Valgimigli noted that this conviction about excessive bleeding risk is mainly based on older studies in which glycoprotein IIb/IIIA inhibitors were administered for prolonged duration through femoral access sites. He argued that it’s time for large clinical trials examining the risk/benefit ratio of short infusion of these agents in the contemporary practice of primary PCI for STEMI.
Simultaneously with Dr. Valgimigli’s presentation, the FABOLUS FASTER results were published online (Circulation. 2020 Jun 27; doi: 10.1161/CIRCULATIONAHA.120.046928).
Dr. Valgimigli reported that Medicure, the sponsor of the FABOLUS FASTER trial, provided an institutional research grant to conduct the study. He also disclosed receiving research grants and personal fees outside the scope of this study from a dozen pharmaceutical and medical device companies. Dr. Naber reported having no financial conflicts.
And cangrelor, in turn, is superior to oral prasugrel, according to the randomized FABOLUS FASTER trial, Marco Valgimigli, MD, PhD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, contrary to conventional wisdom, chewed prasugrel (Effient) proved no better than swallowing the tablets whole for platelet inhibition, said Dr. Valgimigli, an interventional cardiologist at the University of Bern (Switzerland).
He explained that standard administration of the newer oral P2Y12 inhibitors prasugrel and ticagrelor (Brilinta) in patients undergoing percutaneous coronary intervention (PCI) for ST-elevation MI (STEMI) does not provide optimal early inhibition of platelet aggregation. The parenteral antiplatelet drugs tirofiban and cangrelor have been shown to provide faster and more prolonged inhibition of platelet aggregation than the oral P2Y12 inhibitors.
But there has been no head-to-head comparative data for the glycoprotein IIb/IIIA inhibitor tirofiban (Aggrastat) and the P2Y12 inhibitor cangrelor (Kengreal) in the setting of primary PCI for STEMI. This was the impetus for FABOLUS FASTER, the first study to compare the pharmacodynamic effects of the two parenteral antiplatelet agents. The trial also looked at how these potent parenteral drugs, compared with chewed prasugrel, another previously unexamined yet highly practical issue.
The three-center, multinational, open-label FABOLUS FASTER trial randomized 122 patients undergoing primary PCI for STEMI to one of three arms: a standard intravenous bolus and 2-hour infusion of either the P2Y12 inhibitor cangrelor (Kengreal) or the glycoprotein IIb/IIIA inhibitor tirofiban (Aggrastat), followed in either case by 60 mg of oral prasugrel, or a third arm in which patients didn’t receive either drug but were instead randomized to a 60-mg loading dose of chewed or whole prasugrel tablets.
The primary study endpoint was inhibition of platelet aggregation at 30 minutes as measured by light transmittance aggregometry in response to 20 mcmol/L of adenosine diphosphate (ADP).
Tirofiban was the unequivocal winner with 95% inhibition, as compared with 34.1% with cangrelor, 10.5% with chewed prasugrel, and 6.3% with prasugrel swallowed whole, even though the concentration of prasugrel’s active metabolite was far greater at 62.3 ng/mL after prasugrel was chewed, compared with 17.1 ng/mL when swallowed in integral tablet form.
The rate of nonresponsiveness to tirofiban as defined by greater than 59% platelet aggregation was zero for tirofiban during its 2-hour infusion, then a scant 8% thereafter during repeated testing at 3 and 4-6 hours. In contrast, the cangrelor nonresponsiveness rate was 50%-58% during the 2-hour infusion, rising to 82% at 3 hours.
FABOLUS FASTER, while not powered for clinical endpoints, might nevertheless have important clinical implications, according to Dr. Valgimigli. First, the superiority of the intravenous drugs tirofiban and cangrelor over prasugrel for early, strong platelet inhibition underscores the importance of giving parenteral antiplatelet drugs over oral therapy during the acute phase of STEMI therapy. Moreover, tirofiban’s outstanding performance – and the high residual platelet reactivity associated with cangrelor – makes a strong case for large comparative, randomized trials of the two drugs, with hard clinical endpoints.
Discussant Christoph K. Naber, MD, PhD, opined that he personally doesn’t consider the FABOLUS FASTER results practice changing, for a couple of reasons.
“Platelet inhibition measured by ADP in vitro is not necessarily related to true effects in vivo. We know that platelets are activated by multiple mechanisms, and the ADP pathway is just one of them,” said Dr. Naber, an interventional cardiologist at the Wilhemshaven (Germany) Clinic.
Also, there’s a good reason why no glycoprotein IIb/IIIA inhibitors are approved for treatment of STEMI, and why tirofiban, despite its impressive antiplatelet effects, is currently largely reserved for bailout situations, such as complex lesions with large thrombus burden. It’s because tirofiban’s potent antiplatelet activity is accompanied by a high risk of bleeding, he added.
However, Dr. Valgimigli noted that this conviction about excessive bleeding risk is mainly based on older studies in which glycoprotein IIb/IIIA inhibitors were administered for prolonged duration through femoral access sites. He argued that it’s time for large clinical trials examining the risk/benefit ratio of short infusion of these agents in the contemporary practice of primary PCI for STEMI.
Simultaneously with Dr. Valgimigli’s presentation, the FABOLUS FASTER results were published online (Circulation. 2020 Jun 27; doi: 10.1161/CIRCULATIONAHA.120.046928).
Dr. Valgimigli reported that Medicure, the sponsor of the FABOLUS FASTER trial, provided an institutional research grant to conduct the study. He also disclosed receiving research grants and personal fees outside the scope of this study from a dozen pharmaceutical and medical device companies. Dr. Naber reported having no financial conflicts.
And cangrelor, in turn, is superior to oral prasugrel, according to the randomized FABOLUS FASTER trial, Marco Valgimigli, MD, PhD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Moreover, contrary to conventional wisdom, chewed prasugrel (Effient) proved no better than swallowing the tablets whole for platelet inhibition, said Dr. Valgimigli, an interventional cardiologist at the University of Bern (Switzerland).
He explained that standard administration of the newer oral P2Y12 inhibitors prasugrel and ticagrelor (Brilinta) in patients undergoing percutaneous coronary intervention (PCI) for ST-elevation MI (STEMI) does not provide optimal early inhibition of platelet aggregation. The parenteral antiplatelet drugs tirofiban and cangrelor have been shown to provide faster and more prolonged inhibition of platelet aggregation than the oral P2Y12 inhibitors.
But there has been no head-to-head comparative data for the glycoprotein IIb/IIIA inhibitor tirofiban (Aggrastat) and the P2Y12 inhibitor cangrelor (Kengreal) in the setting of primary PCI for STEMI. This was the impetus for FABOLUS FASTER, the first study to compare the pharmacodynamic effects of the two parenteral antiplatelet agents. The trial also looked at how these potent parenteral drugs, compared with chewed prasugrel, another previously unexamined yet highly practical issue.
The three-center, multinational, open-label FABOLUS FASTER trial randomized 122 patients undergoing primary PCI for STEMI to one of three arms: a standard intravenous bolus and 2-hour infusion of either the P2Y12 inhibitor cangrelor (Kengreal) or the glycoprotein IIb/IIIA inhibitor tirofiban (Aggrastat), followed in either case by 60 mg of oral prasugrel, or a third arm in which patients didn’t receive either drug but were instead randomized to a 60-mg loading dose of chewed or whole prasugrel tablets.
The primary study endpoint was inhibition of platelet aggregation at 30 minutes as measured by light transmittance aggregometry in response to 20 mcmol/L of adenosine diphosphate (ADP).
Tirofiban was the unequivocal winner with 95% inhibition, as compared with 34.1% with cangrelor, 10.5% with chewed prasugrel, and 6.3% with prasugrel swallowed whole, even though the concentration of prasugrel’s active metabolite was far greater at 62.3 ng/mL after prasugrel was chewed, compared with 17.1 ng/mL when swallowed in integral tablet form.
The rate of nonresponsiveness to tirofiban as defined by greater than 59% platelet aggregation was zero for tirofiban during its 2-hour infusion, then a scant 8% thereafter during repeated testing at 3 and 4-6 hours. In contrast, the cangrelor nonresponsiveness rate was 50%-58% during the 2-hour infusion, rising to 82% at 3 hours.
FABOLUS FASTER, while not powered for clinical endpoints, might nevertheless have important clinical implications, according to Dr. Valgimigli. First, the superiority of the intravenous drugs tirofiban and cangrelor over prasugrel for early, strong platelet inhibition underscores the importance of giving parenteral antiplatelet drugs over oral therapy during the acute phase of STEMI therapy. Moreover, tirofiban’s outstanding performance – and the high residual platelet reactivity associated with cangrelor – makes a strong case for large comparative, randomized trials of the two drugs, with hard clinical endpoints.
Discussant Christoph K. Naber, MD, PhD, opined that he personally doesn’t consider the FABOLUS FASTER results practice changing, for a couple of reasons.
“Platelet inhibition measured by ADP in vitro is not necessarily related to true effects in vivo. We know that platelets are activated by multiple mechanisms, and the ADP pathway is just one of them,” said Dr. Naber, an interventional cardiologist at the Wilhemshaven (Germany) Clinic.
Also, there’s a good reason why no glycoprotein IIb/IIIA inhibitors are approved for treatment of STEMI, and why tirofiban, despite its impressive antiplatelet effects, is currently largely reserved for bailout situations, such as complex lesions with large thrombus burden. It’s because tirofiban’s potent antiplatelet activity is accompanied by a high risk of bleeding, he added.
However, Dr. Valgimigli noted that this conviction about excessive bleeding risk is mainly based on older studies in which glycoprotein IIb/IIIA inhibitors were administered for prolonged duration through femoral access sites. He argued that it’s time for large clinical trials examining the risk/benefit ratio of short infusion of these agents in the contemporary practice of primary PCI for STEMI.
Simultaneously with Dr. Valgimigli’s presentation, the FABOLUS FASTER results were published online (Circulation. 2020 Jun 27; doi: 10.1161/CIRCULATIONAHA.120.046928).
Dr. Valgimigli reported that Medicure, the sponsor of the FABOLUS FASTER trial, provided an institutional research grant to conduct the study. He also disclosed receiving research grants and personal fees outside the scope of this study from a dozen pharmaceutical and medical device companies. Dr. Naber reported having no financial conflicts.
REPORTING FROM EUROPCR 2020
Cushing’s and COVID-19: Nontraditional symptoms keys to assessment, treatments
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
Lifestyle changes may explain skin lesions in pandemic-era patients
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
Daily Recap: Hospitalized COVID patients need MRIs; Americans vote for face masks
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
COVID-19: Haiti is vulnerable, but the international community can help
Doctors Without Borders, other groups urged to mobilize
Do you want to know what keeps us up at night? As 4th-year medical students born, raised, and living in Haiti, we worry about the impact of COVID-19 on our patients.
The pandemic has shaken the world, and Haiti is no exception.
It has taken several months for the disease to spread, and it began with two confirmed cases, one from France and the other from Belgium, on March 19.1 Much of the spread of COVID-19 in Haiti has been tied to workers returning from the Dominican Republic. As of June 29, Haiti had 5,975 confirmed cases and 105 deaths.2 Of course, those numbers sound minuscule, compared with those in the United States, where the number of deaths from COVID-19 surpassed 100,000 several weeks ago. But the population of Haiti is 30 times smaller than that of the United States, and Haiti is the poorest country in the Western Hemisphere. We have watched in horror as the virus has ravaged marginalized groups in the United States and worry that it will do the same in our own country.
Just as the Haitian Ministry of Health worked with various groups to reach the 1-year free of cholera mark in Haiti, groups such as Doctors Without Borders must mobilize to rein in COVID-19.
Community transmission rapid
After the first two cases were confirmed, a state of health emergency was immediately declared. Haitian President Jovenel Moïse and other government officials called for the implementation of several measures aimed at limiting the spread of COVID-19.
Schools, universities, clinical training programs, vocational centers, factories, airports, and ports, except for the transport of goods, were all ordered to close until further notice. Gatherings of larger than 10 people were banned. A curfew from 8 p.m. EST time to 5 a.m. EST was imposed. Measures such as those encouraged by U.S. Centers for Disease Control and Prevention, such as hand washing, physical distancing, and staying at home were also encouraged by the Haitian Ministry of Health. Mask wearing in public places was deemed mandatory.
The latest testing data show that community spread has been occurring among the Haitian population at a rapid rate. According to Jean William Pape, MD, Haiti’s top infectious diseases expert and founder of GHESKIO, an iconic infectious disease center that cares for people with HIV-AIDS and tuberculosis, a COVID-19 simulation from Cornell University in New York shows that about 35% of the Haitian population will be infected by the end of August 2020. A simulation by the University of Oxford (England) paints an even more dire picture. That simulation shows that 86% of the population could be infected, More than 9,000 additional hospital beds would be needed, and 20,000 people would be likely to die from COVID-19, Dr. Pape said in an interview with Haiti’s Nouvelliste newspaper.3
Medical response
We know that there is a global shortage of health care workers,4 and Haiti is no exception. According to a 2018 report from the Haitian Ministry of Health, the country has 11,775 health care professionals, including about 3,354 medical doctors, to care for more than 11 million people. That translates to about 23.4 physicians per 100,000.5
The pandemic has led some members of this already anemic health care workforce to stay home because of a lack of personal protective equipment. Others, because of reduced hospital or clinic budgets, have been furloughed, making the COVID-19 national health emergency even harder to manage.
But a severe health care shortage is not the only challenge facing Haiti. It spends about $131 U.S. per capita, which makes Haiti one of most vulnerable among low- and middle-income countries in the world. As a poor country,7 its health care infrastructure is among the most inadequate and weakest. Prior to COVID-19, medical advocacy groups already had started movements and strikes demanding that the government improve the health care system. The country’s precarious health care infrastructure includes a lack of hospital beds, and basic medical supplies and equipment, such as oxygen and ventilators.8 The emergence of COVID-19 has only exacerbated the situation.
Clinical training programs have been suspended, many doctors and nurses are on quarantine, and some hospitals and clinics are closing. We have witnessed makeshift voodoo clinics built by Haitian voodoo leaders to receive, hospitalize, and treat COVID-19 patients through rituals and herbal remedies. In some areas of the country, residents have protested against the opening of several COVID-19 treatment and management centers.
Unique cultural challenges
Public health officials around the world are facing challenges persuading citizens to engage in behaviors that could protect them from the virus.
Just as in America, where many people opt to not wear face coverings9,10 despite the public health risks, deep distrust of the Haitian government has undermined the messages of President Moïse and public health officials about the role of masks in limiting the spread of COVID. We see large numbers of unmasked people on the streets in the informal markets every day. Crammed tap-taps and overloaded motorcycles are moving everywhere. This also could be tied to cultural attitudes about COVID that persist among some Haitians. For example, many people with signs and symptoms of COVID-19 are afraid of going to the hospital to get tested and receive care, and resort to going to the voodoo clinics. Along with rituals, voodoo priests have been serving up teas with ingredients, including moringa, eucalyptus, ginger, and honey to those seeking COVID-19 care in the centers. The voodoo priests claim that the teas they serve strengthen the immune system.
In addition, it is difficult for poor people who live in small quarters with several other people to adhere to physical distancing.11
Stigma and violence
Other barriers in the fight against COVID-19 in Haiti are stigma and violence. If widespread testing were available, some Haitians would opt not to do so – despite clear signs and symptoms of the infection. Some people who would get tested if they could are afraid to do so because of fears tied to being attacked by neighbors.
When Haitian University professor Bellamy Nelson and his girlfriend returned to Haiti from the United States in March and began experiencing some pain and fever, he experienced attacks from neighbors, he said in an interview. He said neighbors threatened to burn down his house. When an ambulance arrived at his house to transport him to a hospital, it had to drive through back roads to avoid people armed with rocks, fire, and machetes, he told us. No hospital wanted to admit him. Eventually, Professor Nelson self-quarantined at home, he said.
In another incident, a national ambulance center in Gonaïves, a town toward the northern region of Haiti, reportedly was vandalized, because COVID-19 equipment and supplies used to treat people had been stored there. Hospital Bernard Mevs, along with many other hospitals, was forced by the area’s residents to suspend the plan to open a center for COVID-19 management. Threats to burn down the hospitals caused the leaders of the hospitals to back down and give up a plan to build a 20-bed COVID-19 response center.
Maternal health
Another concern we have about the pandemic is the risk it could be to pregnant women. On average, 94,000 deaths occur annually in Haiti. Out of this number, maternal mortality accounts for 1,000. In 2017, for every 100,000 live births for women of reproductive age from 15 to 49 years old, 480 women died. In contrast, in the Dominican Republic, 95 women died per 100,000 that same year. In the United States, 19 died, and in Norway, no more than 2 died that year.12
Some of the primary factors contributing to the crisis are limited accessibility, inadequate health care facilities, and an inadequate number of trained health care practitioners; low percentages of skilled attendants at deliveries and of prenatal and postnatal visits; and high numbers of high-risk deliveries in nonqualified health facilities.
During the COVID-19 national health emergency, with most hospitals reducing their health care personnel either because of budget-related reasons or because they are on quarantine, this maternal-fetal health crisis has escalated.
One of the biggest hospitals in Jacmel, a town in the southern region of Haiti, has stopped its prenatal care program. In Delmas, the city with the highest incidence and prevalence of COVID-19, Hôpital Universitaire de la Paix has reduced this program to 50% of its capacity and gynecologic care has been completely suspended. Hôpital St. Luc, one of the first hospitals in the western region of Haiti to open its doors to care for COVID-19 patients, has recently shut down the entire maternal-fetal department.
So, access to prenatal and postnatal care, including the ability to deliver babies in health care institutions, is significantly reduced because of COVID-19. This leaves thousands of already vulnerable pregnant women at risk and having to deliver domestically with little to no health care professional assistance. We worry that, in light of the data, more women and babies will die because of the COVID-19 pandemic.
A call to action
Despite these conditions, there are reasons for hope. Various groups, both from the international community and locally have mobilized to respond to the pandemic.
International health care organizations such as Doctors Without Borders and Partners in Health, and local groups such as GHESKIO, the St. Luke Foundation for Haiti, and others have been collaborating with the Haitian Ministry of Health to devise and strategic plans and deploy valuable resources with the common goal of saving lives from COVID-19.
GHESKIO, for example, under Dr. Pape’s leadership, currently has one of the three COVID-19 testing centers in the country. It also has two COVID-19 treatment centers in full operation, in Port-au-Prince, the capital city, managing and treating 520 patients with confirmed COVID-19. GHESKIO, which has been in the front lines of previous major infectious disease outbreaks,13 has trained about 200 clinicians from both public and private health care institutions to care for COVID-19 patients.
Doctors Without Borders has been investing in efforts to support the Ministry of Health by converting and renovating its Burn Center in Drouillard, a small section of the city of Cité Soleil, one of the country’s biggest slums. In May, as part of its COVID-19 response, it launched a 20-bed capacity center that can accommodate up to 45 beds to care for patients who have tested positive for COVID-19.
Partners in Health, the Boston-based nonprofit health care organization cofounded in 1987 by American anthropologist and infectious disease specialist, Paul Farmer, MD, and the largest nonprofit health care provider in Haiti, also joined the Ministry of Health through its national and public health efforts to tackle COVID-19 in Haiti. Partners in Health, through its sister organization, Zanmi Lasante, has pioneered the movement of diagnosing and treating people with HIV-AIDS and TB. Since the late 1990s, its efforts against both infectious diseases have helped 15,000 HIV-positive patients begin and remain on treatment. And every year, 1,500 TB patients have started treatment on the path to a cure.
Early in the pandemic in Haiti, Partners in Health, through its state-of-the-art 300-bed university hospital (Hôpital Universitaire de Mirebalais de Mirebalais), was the first to open a COVID-19 center with a 20-bed capacity and has been caring for COVID-19 patients since then. In June, Partners in Health supported and inaugurated the renovation of the internal medicine department at one of its affiliated community hospitals, Hôpital Saint-Nicolas de Saint Marc. That department will have a 24-bed capacity that can extend up to 36 beds to manage and treat COVID-19 patients.
In total, currently, 26 COVID-19 centers with a capacity of 1,011 beds are available to serve, manage, and treat Haitian patients affected with COVID-19. But are those efforts enough? No.
Haiti, as a weak state even before COVID-19, continues to need funding from the international community so it can strengthen its health care infrastructure to be effective and strong in fighting against COVID-19.
In addition, we would like to see preventive initiatives implemented on the local level. Our family has taken on a role that, we think, could help conquer COVID-19 if others followed suit on a large scale.
As part of our contribution in tackling COVID-19, the two of us have launched a small-scale community experiment. We have educated our family in Delmas about COVID-19 and subsequently launched an awareness campaign in the community. We dispatched small groups that go door to door in the community to educate neighbors about the disease in an effort to help them understand that COVID-19 is real and it is normal for people that feel they may have the disease to seek medical care. This approach helps suppress the transmission of the virus. This pilot project could be reproduced in several other communities. It is easy to operate, rapid, effective, and cost-free. The community has been very receptive to and grateful for our efforts.
Like other countries across the world, Haiti was not ready for COVID-19. But we are confident that, with help from the international community, organizations such as GHESKIO,14 and with due diligence on the local level, we are strong and resilient enough to beat COVID. We must act together – quickly.
References
1. Sénat JD. Coronavirus: 2 cas confirmés en Haïti, Jovenel Moïse décrète l’état d’ur-gence sanitaire. 2020 Le Nouvelliste.
2. Haitian Ministry of Health.
3. “Entre appel a la solidarite et de sombres previsions, le Dr William Pape fait le point.” Le Nouvelliste.
4. Darzi A and Evans T. Lancet. 2016 Nov-Dec 26. 388;10060:2576-7.
5. Rapport Statistique 2018. 2019 Republic of Haiti.
6. Sentlinger K. “Water Crisis in Haiti.” The Water Project.
7. The World Bank in Haiti. worldbank.org.
8. Cenat JM. Travel Med Infect Dis. 2020 Mar 28. doi: 10.1016/jtmaid.2020.101684.
9. Block D. “Why some Americans resist wearing face masks.” voanews.com. 2020 May 31.
10. Panceski B and Douglas J. “Masks could help stop coronavirus. So why are they still controversial?” wsj.com. Updated 29 Jun 2020.
11. Bojarski S. “Social distancing: A luxury Haiti’s poor cannot afford. The Haitian Times. 2020 Apr.
12. World Health Organization, UNICEF, World Bank Group, and the U.N. Population Division. Maternal mortality ratio, Haiti.
13. Feliciano I and Kargbo C. “As COVID cases surge, Haiti’s Dr. Pape is on the front line again.” PBS NewsHour Weekend. 2020 Jun 13.
14. Liautaud B and Deschamps MM. New Engl J Med. 2020 Jun 16.
Mr. Dorcela is a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince, Haiti. He also is a medical intern at Unité de Médecine Familiale Hôpital Saint Nicolas in Saint-Marc. Mr. Dorcela has no disclosures. Mr. St. Jean, who is Mr. Dorcela’s brother, is also a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince. He has no disclosures.
Doctors Without Borders, other groups urged to mobilize
Doctors Without Borders, other groups urged to mobilize
Do you want to know what keeps us up at night? As 4th-year medical students born, raised, and living in Haiti, we worry about the impact of COVID-19 on our patients.
The pandemic has shaken the world, and Haiti is no exception.
It has taken several months for the disease to spread, and it began with two confirmed cases, one from France and the other from Belgium, on March 19.1 Much of the spread of COVID-19 in Haiti has been tied to workers returning from the Dominican Republic. As of June 29, Haiti had 5,975 confirmed cases and 105 deaths.2 Of course, those numbers sound minuscule, compared with those in the United States, where the number of deaths from COVID-19 surpassed 100,000 several weeks ago. But the population of Haiti is 30 times smaller than that of the United States, and Haiti is the poorest country in the Western Hemisphere. We have watched in horror as the virus has ravaged marginalized groups in the United States and worry that it will do the same in our own country.
Just as the Haitian Ministry of Health worked with various groups to reach the 1-year free of cholera mark in Haiti, groups such as Doctors Without Borders must mobilize to rein in COVID-19.
Community transmission rapid
After the first two cases were confirmed, a state of health emergency was immediately declared. Haitian President Jovenel Moïse and other government officials called for the implementation of several measures aimed at limiting the spread of COVID-19.
Schools, universities, clinical training programs, vocational centers, factories, airports, and ports, except for the transport of goods, were all ordered to close until further notice. Gatherings of larger than 10 people were banned. A curfew from 8 p.m. EST time to 5 a.m. EST was imposed. Measures such as those encouraged by U.S. Centers for Disease Control and Prevention, such as hand washing, physical distancing, and staying at home were also encouraged by the Haitian Ministry of Health. Mask wearing in public places was deemed mandatory.
The latest testing data show that community spread has been occurring among the Haitian population at a rapid rate. According to Jean William Pape, MD, Haiti’s top infectious diseases expert and founder of GHESKIO, an iconic infectious disease center that cares for people with HIV-AIDS and tuberculosis, a COVID-19 simulation from Cornell University in New York shows that about 35% of the Haitian population will be infected by the end of August 2020. A simulation by the University of Oxford (England) paints an even more dire picture. That simulation shows that 86% of the population could be infected, More than 9,000 additional hospital beds would be needed, and 20,000 people would be likely to die from COVID-19, Dr. Pape said in an interview with Haiti’s Nouvelliste newspaper.3
Medical response
We know that there is a global shortage of health care workers,4 and Haiti is no exception. According to a 2018 report from the Haitian Ministry of Health, the country has 11,775 health care professionals, including about 3,354 medical doctors, to care for more than 11 million people. That translates to about 23.4 physicians per 100,000.5
The pandemic has led some members of this already anemic health care workforce to stay home because of a lack of personal protective equipment. Others, because of reduced hospital or clinic budgets, have been furloughed, making the COVID-19 national health emergency even harder to manage.
But a severe health care shortage is not the only challenge facing Haiti. It spends about $131 U.S. per capita, which makes Haiti one of most vulnerable among low- and middle-income countries in the world. As a poor country,7 its health care infrastructure is among the most inadequate and weakest. Prior to COVID-19, medical advocacy groups already had started movements and strikes demanding that the government improve the health care system. The country’s precarious health care infrastructure includes a lack of hospital beds, and basic medical supplies and equipment, such as oxygen and ventilators.8 The emergence of COVID-19 has only exacerbated the situation.
Clinical training programs have been suspended, many doctors and nurses are on quarantine, and some hospitals and clinics are closing. We have witnessed makeshift voodoo clinics built by Haitian voodoo leaders to receive, hospitalize, and treat COVID-19 patients through rituals and herbal remedies. In some areas of the country, residents have protested against the opening of several COVID-19 treatment and management centers.
Unique cultural challenges
Public health officials around the world are facing challenges persuading citizens to engage in behaviors that could protect them from the virus.
Just as in America, where many people opt to not wear face coverings9,10 despite the public health risks, deep distrust of the Haitian government has undermined the messages of President Moïse and public health officials about the role of masks in limiting the spread of COVID. We see large numbers of unmasked people on the streets in the informal markets every day. Crammed tap-taps and overloaded motorcycles are moving everywhere. This also could be tied to cultural attitudes about COVID that persist among some Haitians. For example, many people with signs and symptoms of COVID-19 are afraid of going to the hospital to get tested and receive care, and resort to going to the voodoo clinics. Along with rituals, voodoo priests have been serving up teas with ingredients, including moringa, eucalyptus, ginger, and honey to those seeking COVID-19 care in the centers. The voodoo priests claim that the teas they serve strengthen the immune system.
In addition, it is difficult for poor people who live in small quarters with several other people to adhere to physical distancing.11
Stigma and violence
Other barriers in the fight against COVID-19 in Haiti are stigma and violence. If widespread testing were available, some Haitians would opt not to do so – despite clear signs and symptoms of the infection. Some people who would get tested if they could are afraid to do so because of fears tied to being attacked by neighbors.
When Haitian University professor Bellamy Nelson and his girlfriend returned to Haiti from the United States in March and began experiencing some pain and fever, he experienced attacks from neighbors, he said in an interview. He said neighbors threatened to burn down his house. When an ambulance arrived at his house to transport him to a hospital, it had to drive through back roads to avoid people armed with rocks, fire, and machetes, he told us. No hospital wanted to admit him. Eventually, Professor Nelson self-quarantined at home, he said.
In another incident, a national ambulance center in Gonaïves, a town toward the northern region of Haiti, reportedly was vandalized, because COVID-19 equipment and supplies used to treat people had been stored there. Hospital Bernard Mevs, along with many other hospitals, was forced by the area’s residents to suspend the plan to open a center for COVID-19 management. Threats to burn down the hospitals caused the leaders of the hospitals to back down and give up a plan to build a 20-bed COVID-19 response center.
Maternal health
Another concern we have about the pandemic is the risk it could be to pregnant women. On average, 94,000 deaths occur annually in Haiti. Out of this number, maternal mortality accounts for 1,000. In 2017, for every 100,000 live births for women of reproductive age from 15 to 49 years old, 480 women died. In contrast, in the Dominican Republic, 95 women died per 100,000 that same year. In the United States, 19 died, and in Norway, no more than 2 died that year.12
Some of the primary factors contributing to the crisis are limited accessibility, inadequate health care facilities, and an inadequate number of trained health care practitioners; low percentages of skilled attendants at deliveries and of prenatal and postnatal visits; and high numbers of high-risk deliveries in nonqualified health facilities.
During the COVID-19 national health emergency, with most hospitals reducing their health care personnel either because of budget-related reasons or because they are on quarantine, this maternal-fetal health crisis has escalated.
One of the biggest hospitals in Jacmel, a town in the southern region of Haiti, has stopped its prenatal care program. In Delmas, the city with the highest incidence and prevalence of COVID-19, Hôpital Universitaire de la Paix has reduced this program to 50% of its capacity and gynecologic care has been completely suspended. Hôpital St. Luc, one of the first hospitals in the western region of Haiti to open its doors to care for COVID-19 patients, has recently shut down the entire maternal-fetal department.
So, access to prenatal and postnatal care, including the ability to deliver babies in health care institutions, is significantly reduced because of COVID-19. This leaves thousands of already vulnerable pregnant women at risk and having to deliver domestically with little to no health care professional assistance. We worry that, in light of the data, more women and babies will die because of the COVID-19 pandemic.
A call to action
Despite these conditions, there are reasons for hope. Various groups, both from the international community and locally have mobilized to respond to the pandemic.
International health care organizations such as Doctors Without Borders and Partners in Health, and local groups such as GHESKIO, the St. Luke Foundation for Haiti, and others have been collaborating with the Haitian Ministry of Health to devise and strategic plans and deploy valuable resources with the common goal of saving lives from COVID-19.
GHESKIO, for example, under Dr. Pape’s leadership, currently has one of the three COVID-19 testing centers in the country. It also has two COVID-19 treatment centers in full operation, in Port-au-Prince, the capital city, managing and treating 520 patients with confirmed COVID-19. GHESKIO, which has been in the front lines of previous major infectious disease outbreaks,13 has trained about 200 clinicians from both public and private health care institutions to care for COVID-19 patients.
Doctors Without Borders has been investing in efforts to support the Ministry of Health by converting and renovating its Burn Center in Drouillard, a small section of the city of Cité Soleil, one of the country’s biggest slums. In May, as part of its COVID-19 response, it launched a 20-bed capacity center that can accommodate up to 45 beds to care for patients who have tested positive for COVID-19.
Partners in Health, the Boston-based nonprofit health care organization cofounded in 1987 by American anthropologist and infectious disease specialist, Paul Farmer, MD, and the largest nonprofit health care provider in Haiti, also joined the Ministry of Health through its national and public health efforts to tackle COVID-19 in Haiti. Partners in Health, through its sister organization, Zanmi Lasante, has pioneered the movement of diagnosing and treating people with HIV-AIDS and TB. Since the late 1990s, its efforts against both infectious diseases have helped 15,000 HIV-positive patients begin and remain on treatment. And every year, 1,500 TB patients have started treatment on the path to a cure.
Early in the pandemic in Haiti, Partners in Health, through its state-of-the-art 300-bed university hospital (Hôpital Universitaire de Mirebalais de Mirebalais), was the first to open a COVID-19 center with a 20-bed capacity and has been caring for COVID-19 patients since then. In June, Partners in Health supported and inaugurated the renovation of the internal medicine department at one of its affiliated community hospitals, Hôpital Saint-Nicolas de Saint Marc. That department will have a 24-bed capacity that can extend up to 36 beds to manage and treat COVID-19 patients.
In total, currently, 26 COVID-19 centers with a capacity of 1,011 beds are available to serve, manage, and treat Haitian patients affected with COVID-19. But are those efforts enough? No.
Haiti, as a weak state even before COVID-19, continues to need funding from the international community so it can strengthen its health care infrastructure to be effective and strong in fighting against COVID-19.
In addition, we would like to see preventive initiatives implemented on the local level. Our family has taken on a role that, we think, could help conquer COVID-19 if others followed suit on a large scale.
As part of our contribution in tackling COVID-19, the two of us have launched a small-scale community experiment. We have educated our family in Delmas about COVID-19 and subsequently launched an awareness campaign in the community. We dispatched small groups that go door to door in the community to educate neighbors about the disease in an effort to help them understand that COVID-19 is real and it is normal for people that feel they may have the disease to seek medical care. This approach helps suppress the transmission of the virus. This pilot project could be reproduced in several other communities. It is easy to operate, rapid, effective, and cost-free. The community has been very receptive to and grateful for our efforts.
Like other countries across the world, Haiti was not ready for COVID-19. But we are confident that, with help from the international community, organizations such as GHESKIO,14 and with due diligence on the local level, we are strong and resilient enough to beat COVID. We must act together – quickly.
References
1. Sénat JD. Coronavirus: 2 cas confirmés en Haïti, Jovenel Moïse décrète l’état d’ur-gence sanitaire. 2020 Le Nouvelliste.
2. Haitian Ministry of Health.
3. “Entre appel a la solidarite et de sombres previsions, le Dr William Pape fait le point.” Le Nouvelliste.
4. Darzi A and Evans T. Lancet. 2016 Nov-Dec 26. 388;10060:2576-7.
5. Rapport Statistique 2018. 2019 Republic of Haiti.
6. Sentlinger K. “Water Crisis in Haiti.” The Water Project.
7. The World Bank in Haiti. worldbank.org.
8. Cenat JM. Travel Med Infect Dis. 2020 Mar 28. doi: 10.1016/jtmaid.2020.101684.
9. Block D. “Why some Americans resist wearing face masks.” voanews.com. 2020 May 31.
10. Panceski B and Douglas J. “Masks could help stop coronavirus. So why are they still controversial?” wsj.com. Updated 29 Jun 2020.
11. Bojarski S. “Social distancing: A luxury Haiti’s poor cannot afford. The Haitian Times. 2020 Apr.
12. World Health Organization, UNICEF, World Bank Group, and the U.N. Population Division. Maternal mortality ratio, Haiti.
13. Feliciano I and Kargbo C. “As COVID cases surge, Haiti’s Dr. Pape is on the front line again.” PBS NewsHour Weekend. 2020 Jun 13.
14. Liautaud B and Deschamps MM. New Engl J Med. 2020 Jun 16.
Mr. Dorcela is a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince, Haiti. He also is a medical intern at Unité de Médecine Familiale Hôpital Saint Nicolas in Saint-Marc. Mr. Dorcela has no disclosures. Mr. St. Jean, who is Mr. Dorcela’s brother, is also a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince. He has no disclosures.
Do you want to know what keeps us up at night? As 4th-year medical students born, raised, and living in Haiti, we worry about the impact of COVID-19 on our patients.
The pandemic has shaken the world, and Haiti is no exception.
It has taken several months for the disease to spread, and it began with two confirmed cases, one from France and the other from Belgium, on March 19.1 Much of the spread of COVID-19 in Haiti has been tied to workers returning from the Dominican Republic. As of June 29, Haiti had 5,975 confirmed cases and 105 deaths.2 Of course, those numbers sound minuscule, compared with those in the United States, where the number of deaths from COVID-19 surpassed 100,000 several weeks ago. But the population of Haiti is 30 times smaller than that of the United States, and Haiti is the poorest country in the Western Hemisphere. We have watched in horror as the virus has ravaged marginalized groups in the United States and worry that it will do the same in our own country.
Just as the Haitian Ministry of Health worked with various groups to reach the 1-year free of cholera mark in Haiti, groups such as Doctors Without Borders must mobilize to rein in COVID-19.
Community transmission rapid
After the first two cases were confirmed, a state of health emergency was immediately declared. Haitian President Jovenel Moïse and other government officials called for the implementation of several measures aimed at limiting the spread of COVID-19.
Schools, universities, clinical training programs, vocational centers, factories, airports, and ports, except for the transport of goods, were all ordered to close until further notice. Gatherings of larger than 10 people were banned. A curfew from 8 p.m. EST time to 5 a.m. EST was imposed. Measures such as those encouraged by U.S. Centers for Disease Control and Prevention, such as hand washing, physical distancing, and staying at home were also encouraged by the Haitian Ministry of Health. Mask wearing in public places was deemed mandatory.
The latest testing data show that community spread has been occurring among the Haitian population at a rapid rate. According to Jean William Pape, MD, Haiti’s top infectious diseases expert and founder of GHESKIO, an iconic infectious disease center that cares for people with HIV-AIDS and tuberculosis, a COVID-19 simulation from Cornell University in New York shows that about 35% of the Haitian population will be infected by the end of August 2020. A simulation by the University of Oxford (England) paints an even more dire picture. That simulation shows that 86% of the population could be infected, More than 9,000 additional hospital beds would be needed, and 20,000 people would be likely to die from COVID-19, Dr. Pape said in an interview with Haiti’s Nouvelliste newspaper.3
Medical response
We know that there is a global shortage of health care workers,4 and Haiti is no exception. According to a 2018 report from the Haitian Ministry of Health, the country has 11,775 health care professionals, including about 3,354 medical doctors, to care for more than 11 million people. That translates to about 23.4 physicians per 100,000.5
The pandemic has led some members of this already anemic health care workforce to stay home because of a lack of personal protective equipment. Others, because of reduced hospital or clinic budgets, have been furloughed, making the COVID-19 national health emergency even harder to manage.
But a severe health care shortage is not the only challenge facing Haiti. It spends about $131 U.S. per capita, which makes Haiti one of most vulnerable among low- and middle-income countries in the world. As a poor country,7 its health care infrastructure is among the most inadequate and weakest. Prior to COVID-19, medical advocacy groups already had started movements and strikes demanding that the government improve the health care system. The country’s precarious health care infrastructure includes a lack of hospital beds, and basic medical supplies and equipment, such as oxygen and ventilators.8 The emergence of COVID-19 has only exacerbated the situation.
Clinical training programs have been suspended, many doctors and nurses are on quarantine, and some hospitals and clinics are closing. We have witnessed makeshift voodoo clinics built by Haitian voodoo leaders to receive, hospitalize, and treat COVID-19 patients through rituals and herbal remedies. In some areas of the country, residents have protested against the opening of several COVID-19 treatment and management centers.
Unique cultural challenges
Public health officials around the world are facing challenges persuading citizens to engage in behaviors that could protect them from the virus.
Just as in America, where many people opt to not wear face coverings9,10 despite the public health risks, deep distrust of the Haitian government has undermined the messages of President Moïse and public health officials about the role of masks in limiting the spread of COVID. We see large numbers of unmasked people on the streets in the informal markets every day. Crammed tap-taps and overloaded motorcycles are moving everywhere. This also could be tied to cultural attitudes about COVID that persist among some Haitians. For example, many people with signs and symptoms of COVID-19 are afraid of going to the hospital to get tested and receive care, and resort to going to the voodoo clinics. Along with rituals, voodoo priests have been serving up teas with ingredients, including moringa, eucalyptus, ginger, and honey to those seeking COVID-19 care in the centers. The voodoo priests claim that the teas they serve strengthen the immune system.
In addition, it is difficult for poor people who live in small quarters with several other people to adhere to physical distancing.11
Stigma and violence
Other barriers in the fight against COVID-19 in Haiti are stigma and violence. If widespread testing were available, some Haitians would opt not to do so – despite clear signs and symptoms of the infection. Some people who would get tested if they could are afraid to do so because of fears tied to being attacked by neighbors.
When Haitian University professor Bellamy Nelson and his girlfriend returned to Haiti from the United States in March and began experiencing some pain and fever, he experienced attacks from neighbors, he said in an interview. He said neighbors threatened to burn down his house. When an ambulance arrived at his house to transport him to a hospital, it had to drive through back roads to avoid people armed with rocks, fire, and machetes, he told us. No hospital wanted to admit him. Eventually, Professor Nelson self-quarantined at home, he said.
In another incident, a national ambulance center in Gonaïves, a town toward the northern region of Haiti, reportedly was vandalized, because COVID-19 equipment and supplies used to treat people had been stored there. Hospital Bernard Mevs, along with many other hospitals, was forced by the area’s residents to suspend the plan to open a center for COVID-19 management. Threats to burn down the hospitals caused the leaders of the hospitals to back down and give up a plan to build a 20-bed COVID-19 response center.
Maternal health
Another concern we have about the pandemic is the risk it could be to pregnant women. On average, 94,000 deaths occur annually in Haiti. Out of this number, maternal mortality accounts for 1,000. In 2017, for every 100,000 live births for women of reproductive age from 15 to 49 years old, 480 women died. In contrast, in the Dominican Republic, 95 women died per 100,000 that same year. In the United States, 19 died, and in Norway, no more than 2 died that year.12
Some of the primary factors contributing to the crisis are limited accessibility, inadequate health care facilities, and an inadequate number of trained health care practitioners; low percentages of skilled attendants at deliveries and of prenatal and postnatal visits; and high numbers of high-risk deliveries in nonqualified health facilities.
During the COVID-19 national health emergency, with most hospitals reducing their health care personnel either because of budget-related reasons or because they are on quarantine, this maternal-fetal health crisis has escalated.
One of the biggest hospitals in Jacmel, a town in the southern region of Haiti, has stopped its prenatal care program. In Delmas, the city with the highest incidence and prevalence of COVID-19, Hôpital Universitaire de la Paix has reduced this program to 50% of its capacity and gynecologic care has been completely suspended. Hôpital St. Luc, one of the first hospitals in the western region of Haiti to open its doors to care for COVID-19 patients, has recently shut down the entire maternal-fetal department.
So, access to prenatal and postnatal care, including the ability to deliver babies in health care institutions, is significantly reduced because of COVID-19. This leaves thousands of already vulnerable pregnant women at risk and having to deliver domestically with little to no health care professional assistance. We worry that, in light of the data, more women and babies will die because of the COVID-19 pandemic.
A call to action
Despite these conditions, there are reasons for hope. Various groups, both from the international community and locally have mobilized to respond to the pandemic.
International health care organizations such as Doctors Without Borders and Partners in Health, and local groups such as GHESKIO, the St. Luke Foundation for Haiti, and others have been collaborating with the Haitian Ministry of Health to devise and strategic plans and deploy valuable resources with the common goal of saving lives from COVID-19.
GHESKIO, for example, under Dr. Pape’s leadership, currently has one of the three COVID-19 testing centers in the country. It also has two COVID-19 treatment centers in full operation, in Port-au-Prince, the capital city, managing and treating 520 patients with confirmed COVID-19. GHESKIO, which has been in the front lines of previous major infectious disease outbreaks,13 has trained about 200 clinicians from both public and private health care institutions to care for COVID-19 patients.
Doctors Without Borders has been investing in efforts to support the Ministry of Health by converting and renovating its Burn Center in Drouillard, a small section of the city of Cité Soleil, one of the country’s biggest slums. In May, as part of its COVID-19 response, it launched a 20-bed capacity center that can accommodate up to 45 beds to care for patients who have tested positive for COVID-19.
Partners in Health, the Boston-based nonprofit health care organization cofounded in 1987 by American anthropologist and infectious disease specialist, Paul Farmer, MD, and the largest nonprofit health care provider in Haiti, also joined the Ministry of Health through its national and public health efforts to tackle COVID-19 in Haiti. Partners in Health, through its sister organization, Zanmi Lasante, has pioneered the movement of diagnosing and treating people with HIV-AIDS and TB. Since the late 1990s, its efforts against both infectious diseases have helped 15,000 HIV-positive patients begin and remain on treatment. And every year, 1,500 TB patients have started treatment on the path to a cure.
Early in the pandemic in Haiti, Partners in Health, through its state-of-the-art 300-bed university hospital (Hôpital Universitaire de Mirebalais de Mirebalais), was the first to open a COVID-19 center with a 20-bed capacity and has been caring for COVID-19 patients since then. In June, Partners in Health supported and inaugurated the renovation of the internal medicine department at one of its affiliated community hospitals, Hôpital Saint-Nicolas de Saint Marc. That department will have a 24-bed capacity that can extend up to 36 beds to manage and treat COVID-19 patients.
In total, currently, 26 COVID-19 centers with a capacity of 1,011 beds are available to serve, manage, and treat Haitian patients affected with COVID-19. But are those efforts enough? No.
Haiti, as a weak state even before COVID-19, continues to need funding from the international community so it can strengthen its health care infrastructure to be effective and strong in fighting against COVID-19.
In addition, we would like to see preventive initiatives implemented on the local level. Our family has taken on a role that, we think, could help conquer COVID-19 if others followed suit on a large scale.
As part of our contribution in tackling COVID-19, the two of us have launched a small-scale community experiment. We have educated our family in Delmas about COVID-19 and subsequently launched an awareness campaign in the community. We dispatched small groups that go door to door in the community to educate neighbors about the disease in an effort to help them understand that COVID-19 is real and it is normal for people that feel they may have the disease to seek medical care. This approach helps suppress the transmission of the virus. This pilot project could be reproduced in several other communities. It is easy to operate, rapid, effective, and cost-free. The community has been very receptive to and grateful for our efforts.
Like other countries across the world, Haiti was not ready for COVID-19. But we are confident that, with help from the international community, organizations such as GHESKIO,14 and with due diligence on the local level, we are strong and resilient enough to beat COVID. We must act together – quickly.
References
1. Sénat JD. Coronavirus: 2 cas confirmés en Haïti, Jovenel Moïse décrète l’état d’ur-gence sanitaire. 2020 Le Nouvelliste.
2. Haitian Ministry of Health.
3. “Entre appel a la solidarite et de sombres previsions, le Dr William Pape fait le point.” Le Nouvelliste.
4. Darzi A and Evans T. Lancet. 2016 Nov-Dec 26. 388;10060:2576-7.
5. Rapport Statistique 2018. 2019 Republic of Haiti.
6. Sentlinger K. “Water Crisis in Haiti.” The Water Project.
7. The World Bank in Haiti. worldbank.org.
8. Cenat JM. Travel Med Infect Dis. 2020 Mar 28. doi: 10.1016/jtmaid.2020.101684.
9. Block D. “Why some Americans resist wearing face masks.” voanews.com. 2020 May 31.
10. Panceski B and Douglas J. “Masks could help stop coronavirus. So why are they still controversial?” wsj.com. Updated 29 Jun 2020.
11. Bojarski S. “Social distancing: A luxury Haiti’s poor cannot afford. The Haitian Times. 2020 Apr.
12. World Health Organization, UNICEF, World Bank Group, and the U.N. Population Division. Maternal mortality ratio, Haiti.
13. Feliciano I and Kargbo C. “As COVID cases surge, Haiti’s Dr. Pape is on the front line again.” PBS NewsHour Weekend. 2020 Jun 13.
14. Liautaud B and Deschamps MM. New Engl J Med. 2020 Jun 16.
Mr. Dorcela is a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince, Haiti. He also is a medical intern at Unité de Médecine Familiale Hôpital Saint Nicolas in Saint-Marc. Mr. Dorcela has no disclosures. Mr. St. Jean, who is Mr. Dorcela’s brother, is also a senior medical student at Faculté des Sciences de la Santé Université Quisqueya in Port-au-Prince. He has no disclosures.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
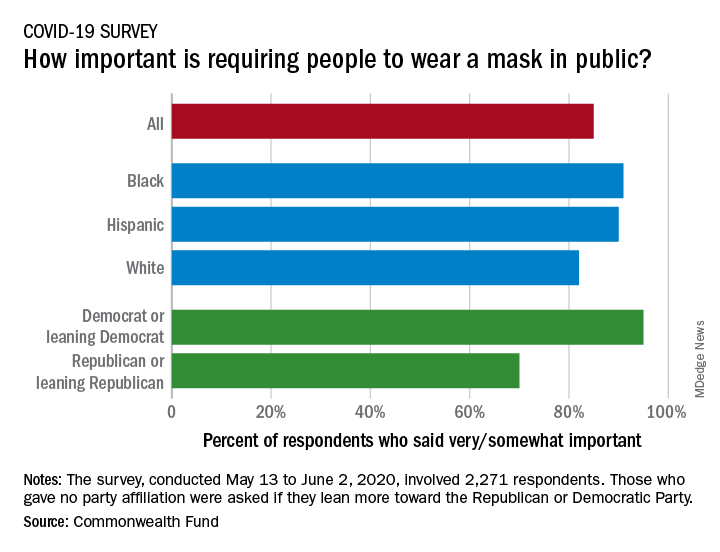
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Three stages to COVID-19 brain damage, new review suggests
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Daily Recap: Docs are good at saving money; SARS-CoV-2 vaccine trials advance
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Phase 3 COVID-19 vaccine trials launching in July, expert says
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
FROM PEDIATRIC DERMATOLOGY 2020










