User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID-19 mortality in hospitalized HF patients: Nearly 1 in 4
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine rollout faces delays
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
If the current pace of vaccination continues, “it’s going to take years, not months, to vaccinate the American people,” President-elect Joe Biden said during a briefing Dec. 29.
In fact, at the current rate, it would take nearly 10 years to vaccinate enough Americans to bring the pandemic under control, according to NBC News. To reach 80% of the country by late June, 3 million people would need to receive a COVID-19 vaccine each day.
“As I long feared and warned, the effort to distribute and administer the vaccine is not progressing as it should,” Mr. Biden said, reemphasizing his pledge to get 100 million doses to Americans during his first 100 days as president.
So far, 11.4 million doses have been distributed and 2.1 million people have received a vaccine, according to the Centers for Disease Control and Prevention. Most states have administered a fraction of the doses they’ve received, according to data compiled by The New York Times.
Federal officials have said there’s an “expected lag” between delivery of doses, shots going into arms, and the data being reported to the CDC, according to CNN. The Food and Drug Administration must assess each shipment for quality control, which has slowed down distribution, and the CDC data are just now beginning to include the Moderna vaccine, which the FDA authorized for emergency use on Dec. 18.
The 2.1 million number is “an underestimate,” Brett Giroir, MD, the assistant secretary of the U.S. Department of Health & Human Services, told NBC News Dec. 29. At the same time, the U.S. won’t meet the goal of vaccinating 20 million people in the next few days, he said.
Another 30 million doses will go out in January, Dr. Giroir said, followed by 50 million in February.
Some vaccine experts have said they’re not surprised by the speed of vaccine distribution.
“It had to go this way,” Paul Offit, MD, a professor of pediatrics at Children’s Hospital of Philadelphia, told STAT. “We had to trip and fall and stumble and figure this out.”
To speed up distribution in 2021, the federal government will need to help states, Mr. Biden said Dec. 29. He plans to use the Defense Authorization Act to ramp up production of vaccine supplies. Even still, the process will take months, he said.
A version of this article first appeared on WebMD.com .
CDC issues COVID-19 vaccine guidance for underlying conditions
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has issued updated guidance for people with underlying medical conditions who are considering getting the coronavirus vaccine.
“Adults of any age with certain underlying medical conditions are at increased risk for severe illness from the virus that causes COVID-19,” the CDC said in the guidance, posted on Dec. 26. “mRNA COVID-19 vaccines may be administered to people with underlying medical conditions provided they have not had a severe allergic reaction to any of the ingredients in the vaccine.”
Both the Pfizer and Moderna vaccines use mRNA, or messenger RNA.
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome (GBS) and Bell’s palsy who are thinking of getting the vaccine.
People with HIV and weakened immune systems “may receive a COVID-19 vaccine. However, they should be aware of the limited safety data,” the CDC said.
There’s no information available yet about the safety of the vaccines for people with weakened immune systems. People with HIV were included in clinical trials, but “safety data specific to this group are not yet available at this time,” the CDC said.
Cases of Bell’s palsy, a temporary facial paralysis, were reported in people receiving the Pfizer and Moderna vaccines in clinical trials, the Food and Drug Administration said Dec. 17.
But the new CDC guidance said that the FDA “does not consider these to be above the rate expected in the general population. They have not concluded these cases were caused by vaccination. Therefore, persons who have previously had Bell’s palsy may receive an mRNA COVID-19 vaccine.”
Researchers have determined the vaccines are safe for people with GBS, a rare autoimmune disorder in which the body’s immune system attacks nerves just as they leave the spinal cord, the CDC said.
“To date, no cases of GBS have been reported following vaccination among participants in the mRNA COVID-19 vaccine clinical trials,” the CDC guidance said. “With few exceptions, the independent Advisory Committee on Immunization Practices general best practice guidelines for immunization do not include a history of GBS as a precaution to vaccination with other vaccines.”
For months, the CDC and other health authorities have said that people with certain medical conditions are at an increased risk of developing severe cases of COVID-19.
A version of this article first appeared on Medscape.com.
Elite soccer players have big hearts and that’s okay
Elite American soccer players have, on average, larger, thicker, and heavier hearts than the general population, according to a new study that provides clinicians with normative echocardiogram and electrocardiogram (ECG) cutoffs to use when assessing the heart health of competitive athletes.
To provide these age- and sex-specific reference values, a team from Massachusetts General Hospital, Boston, led by Timothy W. Churchill, MD, and Aaron L. Baggish, MD, analyzed data from 122 female and 116 male soccer players from the American national teams preparing for World Cup play and undergoing FIFA-mandated preparticipation screening.
The athletes frequently exceeded normal echocardiographic ranges for left ventricular (LV) mass, volume, and wall thickness – structural cardiac parameters responsive to exercise-induced remodeling – but with none showing pathologic findings that might indicate the need to restrict their participation in the sport.
Almost one-third (30%) of female athletes and 41% of male athletes exceeded the American Society of Echocardiography’s upper limit of normal for LV wall thickness, with a measure greater than 12 mm seen in 12% of men and 1% of women.
The majority (51% of females and 59% of males) exceeded normal ranges for body surface area–indexed LV mass, with 77% and 68%, respectively, having LV volumes above the normal range.
Dr. Baggish stressed in an interview, however, that these data tell a story about healthy hearts, not at-risk hearts.
“These are the healthiest, highest-performing elite soccer players that we have in the United States, and this is really a look at how adaptive the heart can be, how much it can grow and change in size, shape, structure, and function in response to sport,” said Dr. Baggish.
The mean age of screened athletes was 20 years (range, 15-40 years). The majority of the female players were White (71%), whereas the male players were more evenly divided between Black (34%), Hispanic (33%), and White (32%).
Screening was performed at U.S. Soccer training sites by experienced clinicians affiliated with the Massachusetts General Hospital cardiovascular performance program.
Interestingly, the study debunks the idea that women, on average, have smaller chamber sizes. “When we did body-size correction, the men and women actually looked pretty similar with respect to their ability to adapt to strenuous exercise,” noted Dr. Baggish.
They did see, however, that women were more likely than men to have abnormal ECG findings. Male athletes showed a higher prevalence of “normal” training-related ECG findings, whereas female athletes were more likely to have abnormal ECG patterns (11.5% vs. 0.0% in the male cohort), most often pathologic T-wave inversions (TWI) confined to the anterior precordial lead distribution.
“This is important because ECGs are the most common screening tool used and we wanted to alert people to the fact that these women who showed some abnormalities on ECG went on to have a total healthy-looking echo, so a false-positive ECG is something to consider,” said Dr. Baggish.
This excess in anterior TWIs has been seen in previous studies and is thought to be benign, although the mechanism remains unclear. Four of the nine female athletes with abnormal ECG findings on initial evaluation had normalized on repeat testing 2-4 years later. Serial data were available in only a subset of athletes.
Clarity needed after COVID
The data, published recently in JAMA Cardiology, are particularly valuable these days given concern over the effects of COVID-19 on the heart and return-to-play recommendations.
“Athletes who have had COVID are being sent for echocardiograms before they can return to play to check for COVID-induced heart disease – which is real – but what we’re seeing is that there’s confusion out there in terms of what is a COVID-related abnormality and what is a normal, adapted athletic heart,” said Dr. Baggish.
“In this paper, we provide a dataset of normal values – generated before COVID was on anyone’s radar – to let cardiologists know what’s ‘big good’ and not ‘big bad.’ ”
More sport-specific data needed
“Although these numbers are still small, this dataset is an important step forward in our understanding of athletic adaptations,” said Matthew Martinez, MD, in an interview. “Many factors impact physiologic athletic changes, and the study aids in our understanding of gender- and sport-specific changes in athletes.”
Dr. Martinez, who is the director of sports cardiology at Atlantic Health–Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, also in Morristown, and the chair of Sports and Exercise Cardiology Section Leadership Council for the American College of Cardiology, noted the relatively young mean age of screened athletes.
“The data represent collegiate-age athletes with some older groups mixed in, but it does not represent older established elite athlete changes,” he said.
Mean age was 21 years in the female players but only 18 years in the males because the men’s senior national team failed to qualify for the World Cup during the study period and was therefore not screened. The authors acknowledged the “dearth of older men in the cohort.”
There is, overall, little age-, sport-, and sex-specific normative data for differentiating training-related cardiovascular adaptations from potentially pathologic phenotypes, wrote the authors.
It exists for men playing in the National Football League and for both sexes participating in the National Basketball Association, but most other studies have mixed the sports and focused mainly on men. That said, Dr. Baggish does not consider these data to be applicable to all elite athletes.
“Soccer is kind of in a league of its own with respect to the mixed amount of explosive or resistant and aerobic work that these athletes have to do, and also it’s the most popular sport in the world, so we really wanted to focus on them,” said Dr. Baggish.
Although the findings are perhaps applicable to athletes from other team sports characterized by explosive spurts of high-intensity activity – like hockey, lacrosse, and field hockey – he would not suggest they be applied to, say, long-distance runners, cyclists, or other sports that require a similar type of aerobic output.
Dr. Baggish reported no relevant conflict of interest. Dr. Martinez is league cardiologist for Major League Soccer.
A version of this article first appeared on Medscape.com.
Elite American soccer players have, on average, larger, thicker, and heavier hearts than the general population, according to a new study that provides clinicians with normative echocardiogram and electrocardiogram (ECG) cutoffs to use when assessing the heart health of competitive athletes.
To provide these age- and sex-specific reference values, a team from Massachusetts General Hospital, Boston, led by Timothy W. Churchill, MD, and Aaron L. Baggish, MD, analyzed data from 122 female and 116 male soccer players from the American national teams preparing for World Cup play and undergoing FIFA-mandated preparticipation screening.
The athletes frequently exceeded normal echocardiographic ranges for left ventricular (LV) mass, volume, and wall thickness – structural cardiac parameters responsive to exercise-induced remodeling – but with none showing pathologic findings that might indicate the need to restrict their participation in the sport.
Almost one-third (30%) of female athletes and 41% of male athletes exceeded the American Society of Echocardiography’s upper limit of normal for LV wall thickness, with a measure greater than 12 mm seen in 12% of men and 1% of women.
The majority (51% of females and 59% of males) exceeded normal ranges for body surface area–indexed LV mass, with 77% and 68%, respectively, having LV volumes above the normal range.
Dr. Baggish stressed in an interview, however, that these data tell a story about healthy hearts, not at-risk hearts.
“These are the healthiest, highest-performing elite soccer players that we have in the United States, and this is really a look at how adaptive the heart can be, how much it can grow and change in size, shape, structure, and function in response to sport,” said Dr. Baggish.
The mean age of screened athletes was 20 years (range, 15-40 years). The majority of the female players were White (71%), whereas the male players were more evenly divided between Black (34%), Hispanic (33%), and White (32%).
Screening was performed at U.S. Soccer training sites by experienced clinicians affiliated with the Massachusetts General Hospital cardiovascular performance program.
Interestingly, the study debunks the idea that women, on average, have smaller chamber sizes. “When we did body-size correction, the men and women actually looked pretty similar with respect to their ability to adapt to strenuous exercise,” noted Dr. Baggish.
They did see, however, that women were more likely than men to have abnormal ECG findings. Male athletes showed a higher prevalence of “normal” training-related ECG findings, whereas female athletes were more likely to have abnormal ECG patterns (11.5% vs. 0.0% in the male cohort), most often pathologic T-wave inversions (TWI) confined to the anterior precordial lead distribution.
“This is important because ECGs are the most common screening tool used and we wanted to alert people to the fact that these women who showed some abnormalities on ECG went on to have a total healthy-looking echo, so a false-positive ECG is something to consider,” said Dr. Baggish.
This excess in anterior TWIs has been seen in previous studies and is thought to be benign, although the mechanism remains unclear. Four of the nine female athletes with abnormal ECG findings on initial evaluation had normalized on repeat testing 2-4 years later. Serial data were available in only a subset of athletes.
Clarity needed after COVID
The data, published recently in JAMA Cardiology, are particularly valuable these days given concern over the effects of COVID-19 on the heart and return-to-play recommendations.
“Athletes who have had COVID are being sent for echocardiograms before they can return to play to check for COVID-induced heart disease – which is real – but what we’re seeing is that there’s confusion out there in terms of what is a COVID-related abnormality and what is a normal, adapted athletic heart,” said Dr. Baggish.
“In this paper, we provide a dataset of normal values – generated before COVID was on anyone’s radar – to let cardiologists know what’s ‘big good’ and not ‘big bad.’ ”
More sport-specific data needed
“Although these numbers are still small, this dataset is an important step forward in our understanding of athletic adaptations,” said Matthew Martinez, MD, in an interview. “Many factors impact physiologic athletic changes, and the study aids in our understanding of gender- and sport-specific changes in athletes.”
Dr. Martinez, who is the director of sports cardiology at Atlantic Health–Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, also in Morristown, and the chair of Sports and Exercise Cardiology Section Leadership Council for the American College of Cardiology, noted the relatively young mean age of screened athletes.
“The data represent collegiate-age athletes with some older groups mixed in, but it does not represent older established elite athlete changes,” he said.
Mean age was 21 years in the female players but only 18 years in the males because the men’s senior national team failed to qualify for the World Cup during the study period and was therefore not screened. The authors acknowledged the “dearth of older men in the cohort.”
There is, overall, little age-, sport-, and sex-specific normative data for differentiating training-related cardiovascular adaptations from potentially pathologic phenotypes, wrote the authors.
It exists for men playing in the National Football League and for both sexes participating in the National Basketball Association, but most other studies have mixed the sports and focused mainly on men. That said, Dr. Baggish does not consider these data to be applicable to all elite athletes.
“Soccer is kind of in a league of its own with respect to the mixed amount of explosive or resistant and aerobic work that these athletes have to do, and also it’s the most popular sport in the world, so we really wanted to focus on them,” said Dr. Baggish.
Although the findings are perhaps applicable to athletes from other team sports characterized by explosive spurts of high-intensity activity – like hockey, lacrosse, and field hockey – he would not suggest they be applied to, say, long-distance runners, cyclists, or other sports that require a similar type of aerobic output.
Dr. Baggish reported no relevant conflict of interest. Dr. Martinez is league cardiologist for Major League Soccer.
A version of this article first appeared on Medscape.com.
Elite American soccer players have, on average, larger, thicker, and heavier hearts than the general population, according to a new study that provides clinicians with normative echocardiogram and electrocardiogram (ECG) cutoffs to use when assessing the heart health of competitive athletes.
To provide these age- and sex-specific reference values, a team from Massachusetts General Hospital, Boston, led by Timothy W. Churchill, MD, and Aaron L. Baggish, MD, analyzed data from 122 female and 116 male soccer players from the American national teams preparing for World Cup play and undergoing FIFA-mandated preparticipation screening.
The athletes frequently exceeded normal echocardiographic ranges for left ventricular (LV) mass, volume, and wall thickness – structural cardiac parameters responsive to exercise-induced remodeling – but with none showing pathologic findings that might indicate the need to restrict their participation in the sport.
Almost one-third (30%) of female athletes and 41% of male athletes exceeded the American Society of Echocardiography’s upper limit of normal for LV wall thickness, with a measure greater than 12 mm seen in 12% of men and 1% of women.
The majority (51% of females and 59% of males) exceeded normal ranges for body surface area–indexed LV mass, with 77% and 68%, respectively, having LV volumes above the normal range.
Dr. Baggish stressed in an interview, however, that these data tell a story about healthy hearts, not at-risk hearts.
“These are the healthiest, highest-performing elite soccer players that we have in the United States, and this is really a look at how adaptive the heart can be, how much it can grow and change in size, shape, structure, and function in response to sport,” said Dr. Baggish.
The mean age of screened athletes was 20 years (range, 15-40 years). The majority of the female players were White (71%), whereas the male players were more evenly divided between Black (34%), Hispanic (33%), and White (32%).
Screening was performed at U.S. Soccer training sites by experienced clinicians affiliated with the Massachusetts General Hospital cardiovascular performance program.
Interestingly, the study debunks the idea that women, on average, have smaller chamber sizes. “When we did body-size correction, the men and women actually looked pretty similar with respect to their ability to adapt to strenuous exercise,” noted Dr. Baggish.
They did see, however, that women were more likely than men to have abnormal ECG findings. Male athletes showed a higher prevalence of “normal” training-related ECG findings, whereas female athletes were more likely to have abnormal ECG patterns (11.5% vs. 0.0% in the male cohort), most often pathologic T-wave inversions (TWI) confined to the anterior precordial lead distribution.
“This is important because ECGs are the most common screening tool used and we wanted to alert people to the fact that these women who showed some abnormalities on ECG went on to have a total healthy-looking echo, so a false-positive ECG is something to consider,” said Dr. Baggish.
This excess in anterior TWIs has been seen in previous studies and is thought to be benign, although the mechanism remains unclear. Four of the nine female athletes with abnormal ECG findings on initial evaluation had normalized on repeat testing 2-4 years later. Serial data were available in only a subset of athletes.
Clarity needed after COVID
The data, published recently in JAMA Cardiology, are particularly valuable these days given concern over the effects of COVID-19 on the heart and return-to-play recommendations.
“Athletes who have had COVID are being sent for echocardiograms before they can return to play to check for COVID-induced heart disease – which is real – but what we’re seeing is that there’s confusion out there in terms of what is a COVID-related abnormality and what is a normal, adapted athletic heart,” said Dr. Baggish.
“In this paper, we provide a dataset of normal values – generated before COVID was on anyone’s radar – to let cardiologists know what’s ‘big good’ and not ‘big bad.’ ”
More sport-specific data needed
“Although these numbers are still small, this dataset is an important step forward in our understanding of athletic adaptations,” said Matthew Martinez, MD, in an interview. “Many factors impact physiologic athletic changes, and the study aids in our understanding of gender- and sport-specific changes in athletes.”
Dr. Martinez, who is the director of sports cardiology at Atlantic Health–Morristown (N.J.) Medical Center and the Gagnon Cardiovascular Institute, also in Morristown, and the chair of Sports and Exercise Cardiology Section Leadership Council for the American College of Cardiology, noted the relatively young mean age of screened athletes.
“The data represent collegiate-age athletes with some older groups mixed in, but it does not represent older established elite athlete changes,” he said.
Mean age was 21 years in the female players but only 18 years in the males because the men’s senior national team failed to qualify for the World Cup during the study period and was therefore not screened. The authors acknowledged the “dearth of older men in the cohort.”
There is, overall, little age-, sport-, and sex-specific normative data for differentiating training-related cardiovascular adaptations from potentially pathologic phenotypes, wrote the authors.
It exists for men playing in the National Football League and for both sexes participating in the National Basketball Association, but most other studies have mixed the sports and focused mainly on men. That said, Dr. Baggish does not consider these data to be applicable to all elite athletes.
“Soccer is kind of in a league of its own with respect to the mixed amount of explosive or resistant and aerobic work that these athletes have to do, and also it’s the most popular sport in the world, so we really wanted to focus on them,” said Dr. Baggish.
Although the findings are perhaps applicable to athletes from other team sports characterized by explosive spurts of high-intensity activity – like hockey, lacrosse, and field hockey – he would not suggest they be applied to, say, long-distance runners, cyclists, or other sports that require a similar type of aerobic output.
Dr. Baggish reported no relevant conflict of interest. Dr. Martinez is league cardiologist for Major League Soccer.
A version of this article first appeared on Medscape.com.
Temper enthusiasm for long-term treatment with bisphosphonates?
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women treated with oral bisphosphonate drugs for osteoporosis for 5 years get no additional benefit – in terms of hip fracture risk – if the treatment is extended for another 5 years, new research shows.
“We found that hip fracture risk in women did not differ if women stopped bisphosphonate use after 5 years or stayed on the medication for 10 years,” coauthor Joan C. Lo, MD, Kaiser Permanente Northern California, Oakland, said in an interview.
The new study, published Dec. 7 in JAMA Network Open, did show a small benefit in continuing the treatment through 7 years vs. 5 years, but it wasn’t clear if this was significant.
“Whether there is a benefit to staying on the drug for 7 years needs to be further studied in randomized trials,” Dr. Lo stressed.
It is well established that oral bisphosphonates are effective in reducing the risk for fracture within the first 3-5 years of treatment; however, evidence on the effects of treatment beyond 5 years is lacking.
The most recent guidance from the American Society of Bone and Mineral Research (ASBMR) on the issue, which were released in 2015, recommends continuation of bisphosphonates beyond 5 years for high-risk patients, but it recommends a “drug holiday” for low-risk patients.
Study adds important new evidence
However, that guidance acknowledges that data are limited regarding long-term use. This large new study adds important new evidence to the discussion, Robert A. Adler, MD, who was a member of the ASBMR Task Force for the recent guidance, said in an interview.
“[With the lack of recent research,] this new study from Kaiser Permanente is of great interest,” said Dr. Adler, chief of endocrinology and metabolism at Central Virginia Veterans Affairs Health Care System and professor of internal medicine and of epidemiology at Virginia Commonwealth University, Richmond.
“It is new data and suggests we might temper our enthusiasm for long-term treatment with bisphosphonates,” he said.
“Importantly, it is the first large observational trial and is closer to a real-world setting than a randomized controlled trial,” he said.
But, Dr. Adler emphasized: “The take-home message is that while this suggests that patients can probably be given a drug holiday for a couple of years ... they should be retested, and if they appear to be at an increased risk of fracture, they probably should restart again.
“Osteoporosis is a chronic disorder,” he emphasized. “It isn’t cured by any of our treatments, and as people get older, they are at a higher fracture risk.
“So we really need to follow our patients for a lifetime and reassess their fracture risk every couple of years – whether they are still on therapy or on a drug holiday.”
Possible that 7 years is better than 5 but remains to be proven
The new study involved data from Kaiser Permanente Northern and Southern California on 29,685 women who had completed 5 years of treatment with oral bisphosphonates, including alendronate, risedronate, or ibandronate, between 2002 and 2014.
Among the women, 11,105 (37%) continued taking the drugs beyond 5 years to 7 years, and 2,725 (9.2%) completed a total of 10 years of treatment.
Their median age was 71. Among those for whom bone mineral density data were available, 37% had osteoporosis after the first 5 years of treatment.
During these 5 years of treatment, 507 hip fractures occurred.
The cumulative incidence of hip fracture among for those who discontinued study therapy at entry, i.e., those who underwent treatment for 5 years, was 23.0 per 1,000 individuals.
After 7 years of treatment, the rate was 20.8 per 1000. For those who continued therapy for 10 years, the rate was 26.8 per 1000 individuals.
The rate in the 7-year treatment group was based on patients taking a 6-month drug holiday after the initial 5 years, but the results are hard to interpret, Dr. Lo said.
“It’s possible that 7 years is better than 5, but this is not a randomized trial, and some of the data analyses done in the study suggest more research should be done to look at a benefit after 7 years.
“At the end of the day, doctors and women need to decide at 5 years what an individual woman’s risk fracture risk is and determine if she should stay on the drug longer,” Dr. Lo emphasized.
Limitations: Subgroups not identified, adherence hard to assess
The uncertainty of any benefit of treatment with bisphosphonates beyond 5 years is further reflected in U.S. recommendations – the Food and Drug Administration has concluded on the basis of pooled data from the extension phase of major clinical trials that any advantages of treatment beyond 3-5 years are unclear.
Key limitations of the current study include the fact that the incidence of hip fracture was not evaluated in low-risk vs. high-risk subgroups; therefore, “these findings may not be applicable to older women at higher risk of osteoporotic fracture,” the authors wrote.
Furthermore, the study did not assess outcomes of fractures other than hip fractures, such as vertebral fractures, they noted.
Dr. Adler pointed out that another limitation is that adherence in the trial was defined as taking 60% of prescribed pills.
“I think this is the biggest weakness with the study,” he said. “Particularly with medications like oral bisphosphonates that don’t really make patients feel any different, it’s a real challenge to make sure patients continue to take these drugs properly.”
The findings should give some reassurance for patients who take a break from the drugs after 5 years. However, reassessment of their risk is critical, Dr. Adler reiterated.
The study was supported by a grant from the National Institute on Aging and the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health. The authors and Adler have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 case fatality doubled in heart transplant patients
Heart transplant recipients infected with SARS-CoV-2 are about twice as likely to die from COVID-19 and should be immediately referred to a transplant center for care, according to transplant experts from Northern Italy.
In a COVID Rapid Report published Dec. 9 in JACC Heart Failure, a group led by Tomaso Bottio, MD, PhD, from the University of Padua, Italy, presented findings on 47 heart transplant recipients who tested positive for SARS-Cov-2 between Feb. 21 and June 30.
The investigators found a case fatality rate of 29.7%, compared with 15.4% in the general population. Prevalence of infection was also much higher at 18 cases (vs. 7) per 1,000 population.
“In our opinion, prompt referral to a heart transplant center is crucial for immunosuppressive therapy optimization and cardiologic follow-up,” Dr. Bottio said in an interview.
Beyond the need for careful adjustment of immunosuppression, graft function should be assessed to “avoid acute rejection or decompensation,” he added.
Dr. Bottio and colleagues tracked COVID-19 cases from among the 2,676 heart transplant recipients alive before the onset of the pandemic at seven heart transplant centers in Northern Italy.
Of the 47 recipients who contracted SARS-CoV-2, 38 required hospitalization while 9 remained at home and 14 died. Mean length of stay in hospital was 17.8 days, much longer in survivors than nonsurvivors (23.2 days vs. 8.5 days; P < .001).
Nonsurvivors were significantly older than survivors (72 vs. 58 years; P = .002). Nonsurvivors were also more likely to present with diabetes (P = .04), extra-cardiac arteriopathy (P = .04), previous percutaneous coronary intervention (P = .04), more allograft vasculopathy (P = .04), and more symptoms of heart failure (P = .02).
Although the authors said the high case fatality rate was, unfortunately, expected, they did not expect so many patients to do well at home.
“What most surprised us was the proportion of a- or pauci-symptomatic heart transplanted patients who did well being treated at home without any therapy modifications,” Dr. Bottio shared. They were also surprised to see there were no cases of graft failure caused by infection-related myocarditis.
These findings from Northern Italy are not dissimilar from the 25% case fatality rate seen in a cohort of heart transplant recipients who caught COVID-19 in New York City early in the pandemic.
In another study, this time looking at a wider group of solid organ transplant recipients with SARS-CoV-2 infection at two centers during the first 3 weeks of the outbreak in New York City, 16 of 90 patients (18%) died.
Treatment recommendations?
Recognizing that there is no randomized trial data informing the treatment of this vulnerable patient population, Dr. Bottio and colleagues suggested that, based on their experience, no change in immunosuppression is needed in those who are “pauci-symptomatic” (mildly symptomatic).
“On the other hand, in hospitalized patients a partial reduction in immunosuppressive therapy avoiding full discontinuation and risk of graft rejection seems to be a common strategy in facing the viral infection,” he said. “In addition, the introduction of corticosteroids could help to suspend the onset of the inflammatory cascade responsible for severe forms of the disease.”
Antibiotic prophylaxis appears to be “fundamental,” he added, particularly in hospitalized patients, but “the role of specific antiviral therapies is still not fully understood in our population.”
Since July 1, they’ve seen an additional six patients with a positive test for SARS-CoV-2. Five were asymptomatic and quarantined at home without changing their immunosuppressive therapy. One patient was hospitalized for pneumonia and had immunosuppressive therapy reduced.
Dr. Bottio and the study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heart transplant recipients infected with SARS-CoV-2 are about twice as likely to die from COVID-19 and should be immediately referred to a transplant center for care, according to transplant experts from Northern Italy.
In a COVID Rapid Report published Dec. 9 in JACC Heart Failure, a group led by Tomaso Bottio, MD, PhD, from the University of Padua, Italy, presented findings on 47 heart transplant recipients who tested positive for SARS-Cov-2 between Feb. 21 and June 30.
The investigators found a case fatality rate of 29.7%, compared with 15.4% in the general population. Prevalence of infection was also much higher at 18 cases (vs. 7) per 1,000 population.
“In our opinion, prompt referral to a heart transplant center is crucial for immunosuppressive therapy optimization and cardiologic follow-up,” Dr. Bottio said in an interview.
Beyond the need for careful adjustment of immunosuppression, graft function should be assessed to “avoid acute rejection or decompensation,” he added.
Dr. Bottio and colleagues tracked COVID-19 cases from among the 2,676 heart transplant recipients alive before the onset of the pandemic at seven heart transplant centers in Northern Italy.
Of the 47 recipients who contracted SARS-CoV-2, 38 required hospitalization while 9 remained at home and 14 died. Mean length of stay in hospital was 17.8 days, much longer in survivors than nonsurvivors (23.2 days vs. 8.5 days; P < .001).
Nonsurvivors were significantly older than survivors (72 vs. 58 years; P = .002). Nonsurvivors were also more likely to present with diabetes (P = .04), extra-cardiac arteriopathy (P = .04), previous percutaneous coronary intervention (P = .04), more allograft vasculopathy (P = .04), and more symptoms of heart failure (P = .02).
Although the authors said the high case fatality rate was, unfortunately, expected, they did not expect so many patients to do well at home.
“What most surprised us was the proportion of a- or pauci-symptomatic heart transplanted patients who did well being treated at home without any therapy modifications,” Dr. Bottio shared. They were also surprised to see there were no cases of graft failure caused by infection-related myocarditis.
These findings from Northern Italy are not dissimilar from the 25% case fatality rate seen in a cohort of heart transplant recipients who caught COVID-19 in New York City early in the pandemic.
In another study, this time looking at a wider group of solid organ transplant recipients with SARS-CoV-2 infection at two centers during the first 3 weeks of the outbreak in New York City, 16 of 90 patients (18%) died.
Treatment recommendations?
Recognizing that there is no randomized trial data informing the treatment of this vulnerable patient population, Dr. Bottio and colleagues suggested that, based on their experience, no change in immunosuppression is needed in those who are “pauci-symptomatic” (mildly symptomatic).
“On the other hand, in hospitalized patients a partial reduction in immunosuppressive therapy avoiding full discontinuation and risk of graft rejection seems to be a common strategy in facing the viral infection,” he said. “In addition, the introduction of corticosteroids could help to suspend the onset of the inflammatory cascade responsible for severe forms of the disease.”
Antibiotic prophylaxis appears to be “fundamental,” he added, particularly in hospitalized patients, but “the role of specific antiviral therapies is still not fully understood in our population.”
Since July 1, they’ve seen an additional six patients with a positive test for SARS-CoV-2. Five were asymptomatic and quarantined at home without changing their immunosuppressive therapy. One patient was hospitalized for pneumonia and had immunosuppressive therapy reduced.
Dr. Bottio and the study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heart transplant recipients infected with SARS-CoV-2 are about twice as likely to die from COVID-19 and should be immediately referred to a transplant center for care, according to transplant experts from Northern Italy.
In a COVID Rapid Report published Dec. 9 in JACC Heart Failure, a group led by Tomaso Bottio, MD, PhD, from the University of Padua, Italy, presented findings on 47 heart transplant recipients who tested positive for SARS-Cov-2 between Feb. 21 and June 30.
The investigators found a case fatality rate of 29.7%, compared with 15.4% in the general population. Prevalence of infection was also much higher at 18 cases (vs. 7) per 1,000 population.
“In our opinion, prompt referral to a heart transplant center is crucial for immunosuppressive therapy optimization and cardiologic follow-up,” Dr. Bottio said in an interview.
Beyond the need for careful adjustment of immunosuppression, graft function should be assessed to “avoid acute rejection or decompensation,” he added.
Dr. Bottio and colleagues tracked COVID-19 cases from among the 2,676 heart transplant recipients alive before the onset of the pandemic at seven heart transplant centers in Northern Italy.
Of the 47 recipients who contracted SARS-CoV-2, 38 required hospitalization while 9 remained at home and 14 died. Mean length of stay in hospital was 17.8 days, much longer in survivors than nonsurvivors (23.2 days vs. 8.5 days; P < .001).
Nonsurvivors were significantly older than survivors (72 vs. 58 years; P = .002). Nonsurvivors were also more likely to present with diabetes (P = .04), extra-cardiac arteriopathy (P = .04), previous percutaneous coronary intervention (P = .04), more allograft vasculopathy (P = .04), and more symptoms of heart failure (P = .02).
Although the authors said the high case fatality rate was, unfortunately, expected, they did not expect so many patients to do well at home.
“What most surprised us was the proportion of a- or pauci-symptomatic heart transplanted patients who did well being treated at home without any therapy modifications,” Dr. Bottio shared. They were also surprised to see there were no cases of graft failure caused by infection-related myocarditis.
These findings from Northern Italy are not dissimilar from the 25% case fatality rate seen in a cohort of heart transplant recipients who caught COVID-19 in New York City early in the pandemic.
In another study, this time looking at a wider group of solid organ transplant recipients with SARS-CoV-2 infection at two centers during the first 3 weeks of the outbreak in New York City, 16 of 90 patients (18%) died.
Treatment recommendations?
Recognizing that there is no randomized trial data informing the treatment of this vulnerable patient population, Dr. Bottio and colleagues suggested that, based on their experience, no change in immunosuppression is needed in those who are “pauci-symptomatic” (mildly symptomatic).
“On the other hand, in hospitalized patients a partial reduction in immunosuppressive therapy avoiding full discontinuation and risk of graft rejection seems to be a common strategy in facing the viral infection,” he said. “In addition, the introduction of corticosteroids could help to suspend the onset of the inflammatory cascade responsible for severe forms of the disease.”
Antibiotic prophylaxis appears to be “fundamental,” he added, particularly in hospitalized patients, but “the role of specific antiviral therapies is still not fully understood in our population.”
Since July 1, they’ve seen an additional six patients with a positive test for SARS-CoV-2. Five were asymptomatic and quarantined at home without changing their immunosuppressive therapy. One patient was hospitalized for pneumonia and had immunosuppressive therapy reduced.
Dr. Bottio and the study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Does daily inhaler monitoring improve asthma outcomes in children?
Among children with moderate or severe persistent asthma, a randomized trial suggests.
But the intervention also may lead to more ED visits and increased hospitalization rates.
“We improved asthma symptom control but did not reduce health care use,” Ruchi S. Gupta, MD, MPH, and colleagues, wrote in a study published in Pediatrics.
The monitoring system alerted clinicians when a patient used a short-acting beta-agonist more than four times in a day. It could be that the “alerts enabled providers to detect asthma exacerbation virtually and refer for clinically appropriate care that included directing children to the ED,” the authors suggested. It also is possible that the intervention led caregivers to be more vigilant about symptoms and more empowered to seek care.
Adherence to preventive regimens
Many patients with asthma need to use preventive medications such as daily inhaled corticosteroids to control symptoms. Researchers have developed sensor-based inhaler monitoring interventions to improve treatment adherence, but the effectiveness of these interventions in improving asthma outcomes in urban and minority populations is unclear.
To assess the effectiveness of a clinically integrated, sensor-based inhaler monitoring intervention on improving asthma symptom control and related outcomes in children, Dr. Gupta, of Northwestern University and Ann & Robert H. Lurie Children’s Hospital of Chicago, and colleagues conducted a randomized, unblinded study, known as the Improving Technology-Assisted Recording of Asthma Control in Children (iTRACC) trial. They included 252 children: 127 in the control group and 125 in the intervention group.
Patients in the intervention group received Propeller Health’s Food and Drug Administration–cleared inhaler sensors for inhaled corticosteroids and short-acting beta-agonists. Caregivers could use a mobile application and clinicians could use a Web portal to track patients’ medication use. The app featured personalized insights, educational content, encouragement, surveys, and care team services.
Researchers recruited caregivers and children from five Chicago clinics for the study, which was conducted between 2016 and 2018. They included children aged 4-17 years who had a prescription for daily inhaled corticosteroids for at least 1 year before enrollment. In addition, participants had at least 1 exacerbation requiring oral corticosteroids in the previous year. They excluded children with other respiratory conditions. They also excluded participants who did not speak English because the app was available only in English.
“Sensors monitored inhaled medication use, capturing the date, time, and number of uses, and transmitted this information via Bluetooth to a paired smartphone and the provider portal in real-time,” the authors said.
Clinicians were alerted to call participants if a patient missed inhaled corticosteroid doses for 4 continuous days or used more than 4 short-acting beta-agonist doses per day. Clinicians could help guide asthma management, schedule an appointment, refill medications, and address technical difficulties with the sensors.
The intervention and control groups had similar baseline characteristics. About one-third of the patients were female, and the mean age was 9.3 years. In the control group, 28% identified as Hispanic, and 33% identified as non-Hispanic Black. In the intervention group, 40% identified as Hispanic, and 23% identified as non-Hispanic Black. About 59% reported Medicaid insurance. The intervention and control arms completed electronic surveys at 1, 3, 6, 9, and 12 months.
Average Asthma Control Test score increased from 19 to 22 in the intervention group, compared with an increase from 19 to 20 in the control group. Adjusted rates of emergency department visits and hospitalizations were greater in the intervention group (incidence rate ratios, 2.2 and 3.4, respectively). A measure of caregiver quality of life was greater in the intervention group, although the difference was not significant.
During the trial, more caregivers in the intervention group reported asthma attacks for which steroids were prescribed by a medical office (73% vs. 35%).
Some participants had to manually enter the number of daily puffs into the app because their inhalers were incompatible with the sensors. In addition, some data were missing because of incomplete or missing survey responses and sensor failure over time. “The number of intervention participants with actively transmitting sensors decreased from 102 at baseline to 56 at 12 months,” Dr. Gupta and associates noted.
Important area of research
“One interesting finding of this study is the increase in health care use in the intervention group to nearly twice as many emergency department (ED) visits and three times as many hospitalizations as the control group over 12 months,” Rachelle R. Ramsey, PhD, and Theresa W. Guilbert, MD, MS, of the University of Cincinnati, wrote in a related commentary. “Although it is plausible that, as the authors suggest, greater asthma knowledge and monitoring may have led to increased vigilance of asthma symptoms, it seems that this would have only led to an increase in ED visits but not hospitalizations.”
The mixture of objective electronic monitoring and subjective self-reported adherence may complicate interpretation of the results, they added.
“Overall, this article underscores the feasibility and importance of sensor-based electronic monitoring of adherence in pediatric asthma and encourages future research in this area,” Dr. Ramsey and Dr. Guilbert said.
The trial was supported by the UnitedHealth Group. Dr. Gupta has received grants from the National Institutes of Health, Rho, and other organizations, and has served as a medical consultant and adviser for a variety of companies. Dr. Ramsey is supported by the NIH. Dr. Guilbert reported fees from the American Board of Pediatrics, the Pediatric Pulmonary Subboard, and some pharmaceutical companies, plus grants from the NIH, grants and personal fees from Sanofi, Regeneron, and AstraZeneca, and royalties from UpToDate.
SOURCE: Gupta RS et al. Pediatrics. 2020 Dec 22. doi: 10.1542/peds.2020-1330.
Among children with moderate or severe persistent asthma, a randomized trial suggests.
But the intervention also may lead to more ED visits and increased hospitalization rates.
“We improved asthma symptom control but did not reduce health care use,” Ruchi S. Gupta, MD, MPH, and colleagues, wrote in a study published in Pediatrics.
The monitoring system alerted clinicians when a patient used a short-acting beta-agonist more than four times in a day. It could be that the “alerts enabled providers to detect asthma exacerbation virtually and refer for clinically appropriate care that included directing children to the ED,” the authors suggested. It also is possible that the intervention led caregivers to be more vigilant about symptoms and more empowered to seek care.
Adherence to preventive regimens
Many patients with asthma need to use preventive medications such as daily inhaled corticosteroids to control symptoms. Researchers have developed sensor-based inhaler monitoring interventions to improve treatment adherence, but the effectiveness of these interventions in improving asthma outcomes in urban and minority populations is unclear.
To assess the effectiveness of a clinically integrated, sensor-based inhaler monitoring intervention on improving asthma symptom control and related outcomes in children, Dr. Gupta, of Northwestern University and Ann & Robert H. Lurie Children’s Hospital of Chicago, and colleagues conducted a randomized, unblinded study, known as the Improving Technology-Assisted Recording of Asthma Control in Children (iTRACC) trial. They included 252 children: 127 in the control group and 125 in the intervention group.
Patients in the intervention group received Propeller Health’s Food and Drug Administration–cleared inhaler sensors for inhaled corticosteroids and short-acting beta-agonists. Caregivers could use a mobile application and clinicians could use a Web portal to track patients’ medication use. The app featured personalized insights, educational content, encouragement, surveys, and care team services.
Researchers recruited caregivers and children from five Chicago clinics for the study, which was conducted between 2016 and 2018. They included children aged 4-17 years who had a prescription for daily inhaled corticosteroids for at least 1 year before enrollment. In addition, participants had at least 1 exacerbation requiring oral corticosteroids in the previous year. They excluded children with other respiratory conditions. They also excluded participants who did not speak English because the app was available only in English.
“Sensors monitored inhaled medication use, capturing the date, time, and number of uses, and transmitted this information via Bluetooth to a paired smartphone and the provider portal in real-time,” the authors said.
Clinicians were alerted to call participants if a patient missed inhaled corticosteroid doses for 4 continuous days or used more than 4 short-acting beta-agonist doses per day. Clinicians could help guide asthma management, schedule an appointment, refill medications, and address technical difficulties with the sensors.
The intervention and control groups had similar baseline characteristics. About one-third of the patients were female, and the mean age was 9.3 years. In the control group, 28% identified as Hispanic, and 33% identified as non-Hispanic Black. In the intervention group, 40% identified as Hispanic, and 23% identified as non-Hispanic Black. About 59% reported Medicaid insurance. The intervention and control arms completed electronic surveys at 1, 3, 6, 9, and 12 months.
Average Asthma Control Test score increased from 19 to 22 in the intervention group, compared with an increase from 19 to 20 in the control group. Adjusted rates of emergency department visits and hospitalizations were greater in the intervention group (incidence rate ratios, 2.2 and 3.4, respectively). A measure of caregiver quality of life was greater in the intervention group, although the difference was not significant.
During the trial, more caregivers in the intervention group reported asthma attacks for which steroids were prescribed by a medical office (73% vs. 35%).
Some participants had to manually enter the number of daily puffs into the app because their inhalers were incompatible with the sensors. In addition, some data were missing because of incomplete or missing survey responses and sensor failure over time. “The number of intervention participants with actively transmitting sensors decreased from 102 at baseline to 56 at 12 months,” Dr. Gupta and associates noted.
Important area of research
“One interesting finding of this study is the increase in health care use in the intervention group to nearly twice as many emergency department (ED) visits and three times as many hospitalizations as the control group over 12 months,” Rachelle R. Ramsey, PhD, and Theresa W. Guilbert, MD, MS, of the University of Cincinnati, wrote in a related commentary. “Although it is plausible that, as the authors suggest, greater asthma knowledge and monitoring may have led to increased vigilance of asthma symptoms, it seems that this would have only led to an increase in ED visits but not hospitalizations.”
The mixture of objective electronic monitoring and subjective self-reported adherence may complicate interpretation of the results, they added.
“Overall, this article underscores the feasibility and importance of sensor-based electronic monitoring of adherence in pediatric asthma and encourages future research in this area,” Dr. Ramsey and Dr. Guilbert said.
The trial was supported by the UnitedHealth Group. Dr. Gupta has received grants from the National Institutes of Health, Rho, and other organizations, and has served as a medical consultant and adviser for a variety of companies. Dr. Ramsey is supported by the NIH. Dr. Guilbert reported fees from the American Board of Pediatrics, the Pediatric Pulmonary Subboard, and some pharmaceutical companies, plus grants from the NIH, grants and personal fees from Sanofi, Regeneron, and AstraZeneca, and royalties from UpToDate.
SOURCE: Gupta RS et al. Pediatrics. 2020 Dec 22. doi: 10.1542/peds.2020-1330.
Among children with moderate or severe persistent asthma, a randomized trial suggests.
But the intervention also may lead to more ED visits and increased hospitalization rates.
“We improved asthma symptom control but did not reduce health care use,” Ruchi S. Gupta, MD, MPH, and colleagues, wrote in a study published in Pediatrics.
The monitoring system alerted clinicians when a patient used a short-acting beta-agonist more than four times in a day. It could be that the “alerts enabled providers to detect asthma exacerbation virtually and refer for clinically appropriate care that included directing children to the ED,” the authors suggested. It also is possible that the intervention led caregivers to be more vigilant about symptoms and more empowered to seek care.
Adherence to preventive regimens
Many patients with asthma need to use preventive medications such as daily inhaled corticosteroids to control symptoms. Researchers have developed sensor-based inhaler monitoring interventions to improve treatment adherence, but the effectiveness of these interventions in improving asthma outcomes in urban and minority populations is unclear.
To assess the effectiveness of a clinically integrated, sensor-based inhaler monitoring intervention on improving asthma symptom control and related outcomes in children, Dr. Gupta, of Northwestern University and Ann & Robert H. Lurie Children’s Hospital of Chicago, and colleagues conducted a randomized, unblinded study, known as the Improving Technology-Assisted Recording of Asthma Control in Children (iTRACC) trial. They included 252 children: 127 in the control group and 125 in the intervention group.
Patients in the intervention group received Propeller Health’s Food and Drug Administration–cleared inhaler sensors for inhaled corticosteroids and short-acting beta-agonists. Caregivers could use a mobile application and clinicians could use a Web portal to track patients’ medication use. The app featured personalized insights, educational content, encouragement, surveys, and care team services.
Researchers recruited caregivers and children from five Chicago clinics for the study, which was conducted between 2016 and 2018. They included children aged 4-17 years who had a prescription for daily inhaled corticosteroids for at least 1 year before enrollment. In addition, participants had at least 1 exacerbation requiring oral corticosteroids in the previous year. They excluded children with other respiratory conditions. They also excluded participants who did not speak English because the app was available only in English.
“Sensors monitored inhaled medication use, capturing the date, time, and number of uses, and transmitted this information via Bluetooth to a paired smartphone and the provider portal in real-time,” the authors said.
Clinicians were alerted to call participants if a patient missed inhaled corticosteroid doses for 4 continuous days or used more than 4 short-acting beta-agonist doses per day. Clinicians could help guide asthma management, schedule an appointment, refill medications, and address technical difficulties with the sensors.
The intervention and control groups had similar baseline characteristics. About one-third of the patients were female, and the mean age was 9.3 years. In the control group, 28% identified as Hispanic, and 33% identified as non-Hispanic Black. In the intervention group, 40% identified as Hispanic, and 23% identified as non-Hispanic Black. About 59% reported Medicaid insurance. The intervention and control arms completed electronic surveys at 1, 3, 6, 9, and 12 months.
Average Asthma Control Test score increased from 19 to 22 in the intervention group, compared with an increase from 19 to 20 in the control group. Adjusted rates of emergency department visits and hospitalizations were greater in the intervention group (incidence rate ratios, 2.2 and 3.4, respectively). A measure of caregiver quality of life was greater in the intervention group, although the difference was not significant.
During the trial, more caregivers in the intervention group reported asthma attacks for which steroids were prescribed by a medical office (73% vs. 35%).
Some participants had to manually enter the number of daily puffs into the app because their inhalers were incompatible with the sensors. In addition, some data were missing because of incomplete or missing survey responses and sensor failure over time. “The number of intervention participants with actively transmitting sensors decreased from 102 at baseline to 56 at 12 months,” Dr. Gupta and associates noted.
Important area of research
“One interesting finding of this study is the increase in health care use in the intervention group to nearly twice as many emergency department (ED) visits and three times as many hospitalizations as the control group over 12 months,” Rachelle R. Ramsey, PhD, and Theresa W. Guilbert, MD, MS, of the University of Cincinnati, wrote in a related commentary. “Although it is plausible that, as the authors suggest, greater asthma knowledge and monitoring may have led to increased vigilance of asthma symptoms, it seems that this would have only led to an increase in ED visits but not hospitalizations.”
The mixture of objective electronic monitoring and subjective self-reported adherence may complicate interpretation of the results, they added.
“Overall, this article underscores the feasibility and importance of sensor-based electronic monitoring of adherence in pediatric asthma and encourages future research in this area,” Dr. Ramsey and Dr. Guilbert said.
The trial was supported by the UnitedHealth Group. Dr. Gupta has received grants from the National Institutes of Health, Rho, and other organizations, and has served as a medical consultant and adviser for a variety of companies. Dr. Ramsey is supported by the NIH. Dr. Guilbert reported fees from the American Board of Pediatrics, the Pediatric Pulmonary Subboard, and some pharmaceutical companies, plus grants from the NIH, grants and personal fees from Sanofi, Regeneron, and AstraZeneca, and royalties from UpToDate.
SOURCE: Gupta RS et al. Pediatrics. 2020 Dec 22. doi: 10.1542/peds.2020-1330.
FROM PEDIATRICS
Disparities in child abuse evaluation arise from implicit bias
according to research discussed by Tiffani J. Johnson, MD, an assistant professor of emergency medicine at the University of California, Davis.
“These disparities in child abuse evaluation and reporting are bidirectional,” she said. “We also recognize that abuse is more likely to be unrecognized in White children.”
Dr. Johnson presented data on the health disparities in child abuse reporting in a session at the annual meeting of the American Academy of Pediatrics, held virtually this year. Health care disparities, as defined by the National Academy of Sciences, refers to differences in the quality of care between minority and nonminority populations that are not caused by clinical appropriateness, access, need, or patient preference, she explained. Instead, they result from discrimination, bias, stereotyping, and uncertainty.
Disparities lead to harm in all children
For example, a 2018 systematic review found that Black and other non-White children were significantly more likely than White children to be evaluated with a skeletal survey. In one of the studies included, at a large urban academic center, Black and Latinx children with accidental fractures were 8.75 times more likely to undergo a skeletal survey than White children and 4.3 times more likely to be reported to child protective services.
“And let me emphasize that these are children who were ultimately found to have accidental fractures,” Dr. Johnson said.
Meanwhile, in an analysis of known cases of head trauma, researchers found that abuse was missed in 37% of White children, compared with 19% of non-White children.
“These only represent the tip of the iceberg as a true number of cases of abuse may never really be detected because some cases are still unknown,” Dr. Johnson told attendees. And the harm of these disparities runs in both directions.
“Failing to diagnose abuse in White children clearly puts them at increased risk for return visits and return evaluations for repeated abuse by the perpetrators,” she said. But harm also can result from overreporting and investigation, including psychological trauma and a waste of limited resources. Overinvestigation also can erode family-physician relationships and perpetuate distrust of medical care in communities of color.
Yet at the same time, it’s clear that Black children, adolescents, and young adults are not protected from harm in society more generally, when at home, where they learn, or where they play, Dr. Johnson said, referencing the deaths of Breonna Taylor and Tamir Rice as examples.
“And now we’re seeing increased evidence that children are not protected in the health care center when we think about the many disparities that have been identified in the care and outcomes of children, including the disproportionality in terms of child abuse evaluation and referrals,” Dr. Johnson said.
Racism is a root cause of that harm to Black children, she said, as the systemic structure of opportunities unfairly disadvantages some individuals and communities while unfairly advantaging others, thereby “sapping the strength of the whole society through the waste of human resources.”
Tonya Chaffee, MD, MPH, a clinical professor of health sciences at the University of California, San Francisco, who attended the session, said she particularly appreciated “seeing data on which racial/ethnic populations have child abuse reports made and the disparities that exist that are similar to what we are noticing in our own institution.”
Role of individual-level implicit bias and racism
While institutional and structural racism play a substantial role in health care disparities, Dr. Johnson focused primarily on the impact of personal racism when it comes to child abuse evaluations, through overt discrimination, explicit bias, implicit bias, and stigmatization. The most challenging of these to identify and acknowledge is often implicit bias, a tendency to believe, even unconsciously, that some people or ideas are better than others, which results in unfair treatment.
For example, a 2016 study found that half of medical students and residents held at least one biological belief about differences between Black and White individuals that was actually false, such as Black people having more pain tolerance or stronger bones than White people, which then affected treatment recommendations.
“Implicit bias refers to our attitudes that lie below the surface, but they can still influence our behaviors,” Dr. Johnson explained. She encouraged providers to take the implicit bias test online to learn about their own unrecognized implicit biases. These biases have a hand in influencing decisions particularly in fast-paced environments where cognitive load is high – such as EDs, where many child abuse evaluations occur.
For example, in one study Dr. Johnson led, the researchers measured implicit bias in participants before and after an ED shift to assess how cognitive load affected bias. They found that participants who care for more than 10 patients, the average score for implicit bias increased.
Similarly, “when the ED was more overcrowded, there was also increased bias at the end of the shift, compared to the beginning of the shift,” Dr. Johnson said. She asked clinicians to take into consideration that at the start of the shift, they may feel well rested and freshly caffeinated, able to suppress or overcome the biases that they know they have.
“But our biases [are] more likely to come into play with every subsequent decision that we make throughout the day when we’re engaged in clinical encounters,” such as who does and does not receive a skeletal survey or get referred to child protective services, she said.
In another study where she hypothesized that resident physicians would have less bias on the child race implicit bias test than on the adult race one, Dr. Johnson reported that 85% of 91 residents working in an ED had an implicit pro-White/anti-Black bias in the test on adult race, but an even higher bias score – 91% – with child race.
Research has found that even children’s names can conjure implicit bias when it comes to stereotypically “White-sounding” names versus stereotypically “Black-sounding names.”
The implicit bias among clinicians extends beyond care of different children. Research has also identified association between higher implicit bias scores and less interpersonal treatment, less supportive communication, less patient-centered communication, poorer patient ratings of satisfaction, and greater patient-reported difficulty with following recommendations, Dr. Johnson said.
“I want you to think about that because I know that when we’re engaging with parents and making decisions about whether or not we’re going to do a skeletal survey or report someone for it, there is a lot of subjectivity that comes into play with how you’re interacting with families,” Dr. Johnson said. Those verbal and nonverbal cues may be triggering to parents, which then affects your interaction with them. Further, research shows that these biases may impact treatment decisions as well.
Personal-mediated racism also shows up in the use of stigmatizing language, Dr. Johnson said.
“When providers read stigmatizing language in the patient’s medical records, it was associated with them having more negative attitudes about that patient,” which then influenced their clinical decision-making, she said. “So when providers got primed with stigmatizing language, they subsequently had less aggressive pain management for those patients.”
Clinical implications for patient care
Dr. Johnson encouraged attendees to be careful about the language and tone they use in communicating with other health care providers and during documentation in medical records. Disparities in child abuse evaluation and reporting tend to be greater in EDs with more subjective conditions, whereas disparities are lower in departments with more established protocols.
She recommended several changes to practice that can reduce the impact of implicit bias. Universal screening for child abuse can increase how many injuries are found, but usually at the cost of increased resources and radiation. Another option is use of validated clinical decision support rules to identify who is at high or low risk for maltreatment, something Dr. Johnson is working on in her research.
But it’s also important for individual providers to confront their personal biases. Evidence-based strategies for reducing bias include perspective taking, focusing on common identities with patients, using counter-stereotypical imaging, seeking increased opportunity for cross-cultural contact, and mindfulness meditation.
“When you interact with people of different backgrounds, it helps to reduce the impact of stereotypes in society about those individuals,” Dr. Johnson told attendees. It’s also important to recognize how diversity in your clinical team can reduce bias.
“We need to work with our institutions to confront racial biases in child abuse reporting and develop quality improvement projects to ensure reporting is done objectively,” Dr. Chaffee said in an interview after attending the session. “This will require training and likely policy changes, including how reports are made to child welfare and/or the police moving forward.”
according to research discussed by Tiffani J. Johnson, MD, an assistant professor of emergency medicine at the University of California, Davis.
“These disparities in child abuse evaluation and reporting are bidirectional,” she said. “We also recognize that abuse is more likely to be unrecognized in White children.”
Dr. Johnson presented data on the health disparities in child abuse reporting in a session at the annual meeting of the American Academy of Pediatrics, held virtually this year. Health care disparities, as defined by the National Academy of Sciences, refers to differences in the quality of care between minority and nonminority populations that are not caused by clinical appropriateness, access, need, or patient preference, she explained. Instead, they result from discrimination, bias, stereotyping, and uncertainty.
Disparities lead to harm in all children
For example, a 2018 systematic review found that Black and other non-White children were significantly more likely than White children to be evaluated with a skeletal survey. In one of the studies included, at a large urban academic center, Black and Latinx children with accidental fractures were 8.75 times more likely to undergo a skeletal survey than White children and 4.3 times more likely to be reported to child protective services.
“And let me emphasize that these are children who were ultimately found to have accidental fractures,” Dr. Johnson said.
Meanwhile, in an analysis of known cases of head trauma, researchers found that abuse was missed in 37% of White children, compared with 19% of non-White children.
“These only represent the tip of the iceberg as a true number of cases of abuse may never really be detected because some cases are still unknown,” Dr. Johnson told attendees. And the harm of these disparities runs in both directions.
“Failing to diagnose abuse in White children clearly puts them at increased risk for return visits and return evaluations for repeated abuse by the perpetrators,” she said. But harm also can result from overreporting and investigation, including psychological trauma and a waste of limited resources. Overinvestigation also can erode family-physician relationships and perpetuate distrust of medical care in communities of color.
Yet at the same time, it’s clear that Black children, adolescents, and young adults are not protected from harm in society more generally, when at home, where they learn, or where they play, Dr. Johnson said, referencing the deaths of Breonna Taylor and Tamir Rice as examples.
“And now we’re seeing increased evidence that children are not protected in the health care center when we think about the many disparities that have been identified in the care and outcomes of children, including the disproportionality in terms of child abuse evaluation and referrals,” Dr. Johnson said.
Racism is a root cause of that harm to Black children, she said, as the systemic structure of opportunities unfairly disadvantages some individuals and communities while unfairly advantaging others, thereby “sapping the strength of the whole society through the waste of human resources.”
Tonya Chaffee, MD, MPH, a clinical professor of health sciences at the University of California, San Francisco, who attended the session, said she particularly appreciated “seeing data on which racial/ethnic populations have child abuse reports made and the disparities that exist that are similar to what we are noticing in our own institution.”
Role of individual-level implicit bias and racism
While institutional and structural racism play a substantial role in health care disparities, Dr. Johnson focused primarily on the impact of personal racism when it comes to child abuse evaluations, through overt discrimination, explicit bias, implicit bias, and stigmatization. The most challenging of these to identify and acknowledge is often implicit bias, a tendency to believe, even unconsciously, that some people or ideas are better than others, which results in unfair treatment.
For example, a 2016 study found that half of medical students and residents held at least one biological belief about differences between Black and White individuals that was actually false, such as Black people having more pain tolerance or stronger bones than White people, which then affected treatment recommendations.
“Implicit bias refers to our attitudes that lie below the surface, but they can still influence our behaviors,” Dr. Johnson explained. She encouraged providers to take the implicit bias test online to learn about their own unrecognized implicit biases. These biases have a hand in influencing decisions particularly in fast-paced environments where cognitive load is high – such as EDs, where many child abuse evaluations occur.
For example, in one study Dr. Johnson led, the researchers measured implicit bias in participants before and after an ED shift to assess how cognitive load affected bias. They found that participants who care for more than 10 patients, the average score for implicit bias increased.
Similarly, “when the ED was more overcrowded, there was also increased bias at the end of the shift, compared to the beginning of the shift,” Dr. Johnson said. She asked clinicians to take into consideration that at the start of the shift, they may feel well rested and freshly caffeinated, able to suppress or overcome the biases that they know they have.
“But our biases [are] more likely to come into play with every subsequent decision that we make throughout the day when we’re engaged in clinical encounters,” such as who does and does not receive a skeletal survey or get referred to child protective services, she said.
In another study where she hypothesized that resident physicians would have less bias on the child race implicit bias test than on the adult race one, Dr. Johnson reported that 85% of 91 residents working in an ED had an implicit pro-White/anti-Black bias in the test on adult race, but an even higher bias score – 91% – with child race.
Research has found that even children’s names can conjure implicit bias when it comes to stereotypically “White-sounding” names versus stereotypically “Black-sounding names.”
The implicit bias among clinicians extends beyond care of different children. Research has also identified association between higher implicit bias scores and less interpersonal treatment, less supportive communication, less patient-centered communication, poorer patient ratings of satisfaction, and greater patient-reported difficulty with following recommendations, Dr. Johnson said.
“I want you to think about that because I know that when we’re engaging with parents and making decisions about whether or not we’re going to do a skeletal survey or report someone for it, there is a lot of subjectivity that comes into play with how you’re interacting with families,” Dr. Johnson said. Those verbal and nonverbal cues may be triggering to parents, which then affects your interaction with them. Further, research shows that these biases may impact treatment decisions as well.
Personal-mediated racism also shows up in the use of stigmatizing language, Dr. Johnson said.
“When providers read stigmatizing language in the patient’s medical records, it was associated with them having more negative attitudes about that patient,” which then influenced their clinical decision-making, she said. “So when providers got primed with stigmatizing language, they subsequently had less aggressive pain management for those patients.”
Clinical implications for patient care
Dr. Johnson encouraged attendees to be careful about the language and tone they use in communicating with other health care providers and during documentation in medical records. Disparities in child abuse evaluation and reporting tend to be greater in EDs with more subjective conditions, whereas disparities are lower in departments with more established protocols.
She recommended several changes to practice that can reduce the impact of implicit bias. Universal screening for child abuse can increase how many injuries are found, but usually at the cost of increased resources and radiation. Another option is use of validated clinical decision support rules to identify who is at high or low risk for maltreatment, something Dr. Johnson is working on in her research.
But it’s also important for individual providers to confront their personal biases. Evidence-based strategies for reducing bias include perspective taking, focusing on common identities with patients, using counter-stereotypical imaging, seeking increased opportunity for cross-cultural contact, and mindfulness meditation.
“When you interact with people of different backgrounds, it helps to reduce the impact of stereotypes in society about those individuals,” Dr. Johnson told attendees. It’s also important to recognize how diversity in your clinical team can reduce bias.
“We need to work with our institutions to confront racial biases in child abuse reporting and develop quality improvement projects to ensure reporting is done objectively,” Dr. Chaffee said in an interview after attending the session. “This will require training and likely policy changes, including how reports are made to child welfare and/or the police moving forward.”
according to research discussed by Tiffani J. Johnson, MD, an assistant professor of emergency medicine at the University of California, Davis.
“These disparities in child abuse evaluation and reporting are bidirectional,” she said. “We also recognize that abuse is more likely to be unrecognized in White children.”
Dr. Johnson presented data on the health disparities in child abuse reporting in a session at the annual meeting of the American Academy of Pediatrics, held virtually this year. Health care disparities, as defined by the National Academy of Sciences, refers to differences in the quality of care between minority and nonminority populations that are not caused by clinical appropriateness, access, need, or patient preference, she explained. Instead, they result from discrimination, bias, stereotyping, and uncertainty.
Disparities lead to harm in all children
For example, a 2018 systematic review found that Black and other non-White children were significantly more likely than White children to be evaluated with a skeletal survey. In one of the studies included, at a large urban academic center, Black and Latinx children with accidental fractures were 8.75 times more likely to undergo a skeletal survey than White children and 4.3 times more likely to be reported to child protective services.
“And let me emphasize that these are children who were ultimately found to have accidental fractures,” Dr. Johnson said.
Meanwhile, in an analysis of known cases of head trauma, researchers found that abuse was missed in 37% of White children, compared with 19% of non-White children.
“These only represent the tip of the iceberg as a true number of cases of abuse may never really be detected because some cases are still unknown,” Dr. Johnson told attendees. And the harm of these disparities runs in both directions.
“Failing to diagnose abuse in White children clearly puts them at increased risk for return visits and return evaluations for repeated abuse by the perpetrators,” she said. But harm also can result from overreporting and investigation, including psychological trauma and a waste of limited resources. Overinvestigation also can erode family-physician relationships and perpetuate distrust of medical care in communities of color.
Yet at the same time, it’s clear that Black children, adolescents, and young adults are not protected from harm in society more generally, when at home, where they learn, or where they play, Dr. Johnson said, referencing the deaths of Breonna Taylor and Tamir Rice as examples.
“And now we’re seeing increased evidence that children are not protected in the health care center when we think about the many disparities that have been identified in the care and outcomes of children, including the disproportionality in terms of child abuse evaluation and referrals,” Dr. Johnson said.
Racism is a root cause of that harm to Black children, she said, as the systemic structure of opportunities unfairly disadvantages some individuals and communities while unfairly advantaging others, thereby “sapping the strength of the whole society through the waste of human resources.”
Tonya Chaffee, MD, MPH, a clinical professor of health sciences at the University of California, San Francisco, who attended the session, said she particularly appreciated “seeing data on which racial/ethnic populations have child abuse reports made and the disparities that exist that are similar to what we are noticing in our own institution.”
Role of individual-level implicit bias and racism
While institutional and structural racism play a substantial role in health care disparities, Dr. Johnson focused primarily on the impact of personal racism when it comes to child abuse evaluations, through overt discrimination, explicit bias, implicit bias, and stigmatization. The most challenging of these to identify and acknowledge is often implicit bias, a tendency to believe, even unconsciously, that some people or ideas are better than others, which results in unfair treatment.
For example, a 2016 study found that half of medical students and residents held at least one biological belief about differences between Black and White individuals that was actually false, such as Black people having more pain tolerance or stronger bones than White people, which then affected treatment recommendations.
“Implicit bias refers to our attitudes that lie below the surface, but they can still influence our behaviors,” Dr. Johnson explained. She encouraged providers to take the implicit bias test online to learn about their own unrecognized implicit biases. These biases have a hand in influencing decisions particularly in fast-paced environments where cognitive load is high – such as EDs, where many child abuse evaluations occur.
For example, in one study Dr. Johnson led, the researchers measured implicit bias in participants before and after an ED shift to assess how cognitive load affected bias. They found that participants who care for more than 10 patients, the average score for implicit bias increased.
Similarly, “when the ED was more overcrowded, there was also increased bias at the end of the shift, compared to the beginning of the shift,” Dr. Johnson said. She asked clinicians to take into consideration that at the start of the shift, they may feel well rested and freshly caffeinated, able to suppress or overcome the biases that they know they have.
“But our biases [are] more likely to come into play with every subsequent decision that we make throughout the day when we’re engaged in clinical encounters,” such as who does and does not receive a skeletal survey or get referred to child protective services, she said.
In another study where she hypothesized that resident physicians would have less bias on the child race implicit bias test than on the adult race one, Dr. Johnson reported that 85% of 91 residents working in an ED had an implicit pro-White/anti-Black bias in the test on adult race, but an even higher bias score – 91% – with child race.
Research has found that even children’s names can conjure implicit bias when it comes to stereotypically “White-sounding” names versus stereotypically “Black-sounding names.”
The implicit bias among clinicians extends beyond care of different children. Research has also identified association between higher implicit bias scores and less interpersonal treatment, less supportive communication, less patient-centered communication, poorer patient ratings of satisfaction, and greater patient-reported difficulty with following recommendations, Dr. Johnson said.
“I want you to think about that because I know that when we’re engaging with parents and making decisions about whether or not we’re going to do a skeletal survey or report someone for it, there is a lot of subjectivity that comes into play with how you’re interacting with families,” Dr. Johnson said. Those verbal and nonverbal cues may be triggering to parents, which then affects your interaction with them. Further, research shows that these biases may impact treatment decisions as well.
Personal-mediated racism also shows up in the use of stigmatizing language, Dr. Johnson said.
“When providers read stigmatizing language in the patient’s medical records, it was associated with them having more negative attitudes about that patient,” which then influenced their clinical decision-making, she said. “So when providers got primed with stigmatizing language, they subsequently had less aggressive pain management for those patients.”
Clinical implications for patient care
Dr. Johnson encouraged attendees to be careful about the language and tone they use in communicating with other health care providers and during documentation in medical records. Disparities in child abuse evaluation and reporting tend to be greater in EDs with more subjective conditions, whereas disparities are lower in departments with more established protocols.
She recommended several changes to practice that can reduce the impact of implicit bias. Universal screening for child abuse can increase how many injuries are found, but usually at the cost of increased resources and radiation. Another option is use of validated clinical decision support rules to identify who is at high or low risk for maltreatment, something Dr. Johnson is working on in her research.
But it’s also important for individual providers to confront their personal biases. Evidence-based strategies for reducing bias include perspective taking, focusing on common identities with patients, using counter-stereotypical imaging, seeking increased opportunity for cross-cultural contact, and mindfulness meditation.
“When you interact with people of different backgrounds, it helps to reduce the impact of stereotypes in society about those individuals,” Dr. Johnson told attendees. It’s also important to recognize how diversity in your clinical team can reduce bias.
“We need to work with our institutions to confront racial biases in child abuse reporting and develop quality improvement projects to ensure reporting is done objectively,” Dr. Chaffee said in an interview after attending the session. “This will require training and likely policy changes, including how reports are made to child welfare and/or the police moving forward.”
FROM AAP 2020
Child abuse visits to EDs declined in 2020, but not admissions
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.
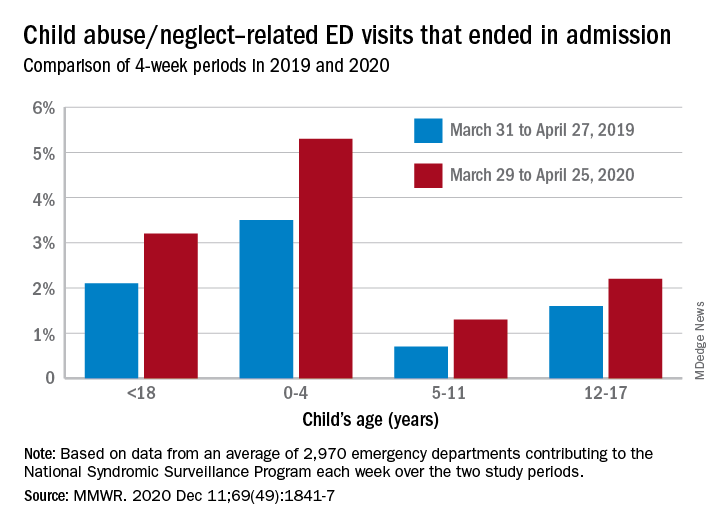
The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
but the visits in 2020 were significantly more likely to result in hospitalization, based on analysis of a national ED database.

The number of ED visits involving child abuse and neglect was down by 53% during the 4-week period from March 29 to April 25, 2020, compared with the 4 weeks from March 31 to April 27, 2019. The proportion of those ED visits that ended in hospitalizations, however, increased from 2.1% in 2019 to 3.2% in 2020, Elizabeth Swedo, MD, and associates at the Centers for Disease Control and Prevention said in the Morbidity and Mortality Weekly Report.
“ED visits related to suspected or confirmed child abuse and neglect decreased beginning the week of March 15, 2020, coinciding with the declaration of a national emergency related to COVID-19 and implementation of community mitigation measures,” they wrote.
An earlier study involving the same database (the National Syndromic Surveillance Program) showed that, over the two same 4-week periods, the volume of all ED visits in 2020 was down 72% for children aged 10 years and younger and 71% for those aged 11-14 years.
In the current study, however, all age subgroups had significant increases in hospital admissions. The proportion of ED visits related to child abuse and neglect that resulted in hospitalization rose from 3.5% in 2019 to 5.3% in 2020 among ages 0-4 years, 0.7% to 1.3% for ages 5-11 years, and 1.6% to 2.2% for adolescents aged 12-17, Dr. Swedo and associates reported.
The absence of a corresponding drop in hospitalizations may be tied to risk factors related to the pandemic, “such as loss of income, increased stress related to parental child care and schooling responsibilities, and increased substance use and mental health conditions among adults,” the investigators added.
The National Syndromic Surveillance Program receives daily data from 3,310 EDs in 47 states, but the number of facilities meeting the investigators’ criteria averaged 2,970 a week for the 8 weeks of the study period.
SOURCE: Swedo E et al. MMWR. 2020 Dec. 11;69(49):1841-7.
FROM MMWR
Tier 4 lockdown in England as virus variant spreading fast
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.
Mr. Johnson told a Downing Street briefing: “The new variant could increase the R by 0.4 or more, and although there’s considerable uncertainty, it may be up to 70% more transmissible than the old variant, the original version of the disease.”
England’s Tier 4 is the equivalent of the old national lockdown restrictions and began Dec. 20.
It prevents Christmas relaxation for gatherings in Tier 4, aside from support bubbles.
Non-essential shops, gyms, and hairdressers will also close. People shouldn’t enter or leave Tier 4.
In the rest of England, special Christmas measures are reduced to 1 day down from the previous 5.
Canceling Christmas
Mr. Johnson had previously said it would be “inhuman” to cancel Christmas.
“When the science changes, we must change our response,” Mr. Johnson said. “When the virus changes its method of attack, we must change our method of defence. And as your Prime Minister I sincerely believe there is no alternative open to me.”
He added: “We’re sacrificing the chance to see our loved ones this Christmas so we have a better chance of protecting their lives so that we can see them at future Christmases.”
He denied he’d been slow to react to rising cases and evidence around the virus variant.
Rest of the UK
The PM’s announcement for England followed calls with the cabinet, and with the leaders of Scotland, Wales, and Northern Ireland.
Wales has brought forward its planned national lockdown to start at midnight with rules eased on Christmas Day. First Minister Mark Drakeford said: “The situation is incredibly serious. I cannot overstate this.”
Seventeen new variant cases have already been identified in Scotland. First Minister Nicola Sturgeon said: “We do now face a very serious situation. It is, in fact, probably the most serious and potentially dangerous juncture we have faced since the start of the COVID pandemic in February, and March.”
Although she said the situation in Scotland is not as severe as other parts of the UK, preventative measures were needed.
Restrictions will now only be lifted on Christmas day itself, and there’s a ban on non-essential travel to and from the rest of the UK.
Level 4 measures will be applied to all of mainland Scotland for 3 weeks from Boxing Day.
Ms. Sturgeon said making the announcement about Christmas made her want to cry.
New variant
The variant was identified through Public Health England genomic surveillance. Chief Medical Adviser Professor Chris Whitty issued a statement saying: “As a result of the rapid spread of the new variant, preliminary modelling data and rapidly rising incidence rates in the South East, the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) now consider that the new strain can spread more quickly.
“We have alerted the World Health Organisation and are continuing to analyse the available data to improve our understanding.
“There is no current evidence to suggest the new strain causes a higher mortality rate or that it affects vaccines and treatments although urgent work is underway to confirm this.”
He told the news briefing: “In the South East, 43% of the virus is now this new variant, in Eastern England it’s 59%, and in London 62%.”
Rates of hospitalisation were higher where the new variant was more prevalent.
‘Cause for concern’
Chief Scientific Adviser Sir Patrick Vallance said: “The new variant contains 23 different changes, many of them associated with changes in the protein that the virus makes. This is an unusually large number of variants. It’s also got variants in areas of the virus that are known to be associated with how the virus binds to cells and enters cells. So there are some changes, which cause concern in terms of how the virus looks.”
He added: “This virus transmits and spreads fast.”
The variant may have originated in the UK, Sir Patrick said: “There’s a large outbreak in the UK, it may have started here, we don’t know for sure.”
Earlier, SAGE member, and Director of the Wellcome Trust, Sir Jeremy Farrar tweeted: “The new strain of COVID-19 is worrying & real cause for concern & extra caution. Research is ongoing to understand more, but acting urgently now is critical. There is no part of the UK & globally that should not be concerned. As in many countries, the situation is fragile.”
Dr. Samantha Batt-Rawden, president of Doctors’ Association UK and a senior intensive care registrar in the South East of England commented: “We realise how disappointing the new restrictions will be for many today, especially those in Tier 4 areas. However, doctors across the UK, but especially those in the South East are telling us that the surge in cases is already putting hospitals and critical care units under enormous strain.”
Vaccines
Mr. Johnson said 350,000 people across the UK have now had the first dose of the Pfizer/BioNTech vaccine.
On Dec. 18, the US FDA granted emergency use of Moderna’s messenger RNA COVID-19 vaccine, the country’s second after the Pfizer/BioNTech product.
The Moderna vaccine, and the Oxford/AstraZeneca jab, are still being assessed by the UK’s MHRA.
Daily data
In Dec. 19’s daily data another 27,052 UK positive tests were reported and 534 deaths.
The total number of deaths within 28 days of a positive test now stands at 67,075.
There are 18,771 COVID-19 patients in hospital and 1,364 ventilator beds are in use.
A version of this article first appeared on Medscape.com.