User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Novel antiplatelet drug: Hope for efficacy without bleeding?
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
A new antiplatelet drug with a completely novel mechanism of action may hold the promise of delivering the holy grail – reducing cardiac events without increasing bleeding. That is the hope behind the new class of drugs directed against the platelet collagen glycoprotein VI (GPVI) receptor.
A phase 2 trial with the first agent in this class, known as revacept (advanceCOR), showed no increase in bleeding with the product when added to standard dual-antiplatelet therapy for patients with stable ischemic heart disease undergoing elective percutaneous coronary intervention (PCI), despite the drug’s being used at a dose that has been shown to increase platelet inhibition.
Unfortunately, there was no reduction in the primary clinical efficacy endpoint, a myocardial injury surrogate, but the authors pointed out that the overall event rate was low, and they were hopeful that future trials in a higher-risk population will show efficacy.
The ISAR PLASTER study was published online on March 31 in JAMA Cardiology.
“This new drug is targeting the collagen in the extracellular matrix of atherosclerotic plaque rather than the platelets themselves. So, in theory, this agent should not cause an increase in bleeding,” study author Steffen Massberg, DrMed, said in an interview.
Dr. Massberg explained that revacept targets the binding site for platelets on collagen that is exposed on rupture of atherosclerotic plaques and is a major trigger of platelet activation.
“In contrast to aspirin and P2Y12 inhibitors, which target all platelets, revacept only binds to sites where there is ruptured plaque. But the platelets themselves otherwise have normal function, so regular coagulation processes should be unaffected,” he commented.
“While collagen also has a role in the coagulation process, it is more involved in atherosclerotic plaque rupture, and in animal studies, revacept was effective in preventing clot formation in large arteries but only had a small effect on bleeding,” Dr. Massberg added.
In the JAMA Cardiology article, the authors further elaborated that, when collagen is exposed during atherosclerotic plaque rupture, it binds platelet GPVI, the major platelet collagen receptor.
“Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation. Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis,” they wrote.
The researchers describe revacept as a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.
In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation, they reported.
“As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion,” the authors said.
In animal studies and a phase 1 clinical trial, the drug was shown to inhibit atherothrombosis but to have little effect on systemic hemostasis or bleeding.
The current ISAR-PLASTER trial is the first study of the use of the agent for patients with coronary heart disease.
For the study, 334 patients with stable ischemic heart disease undergoing elective PCI were randomly assigned to receive a single intravenous infusion of revacept 160 mg, revacept 80 mg, or placebo prior to the start of PCI in addition to standard antithrombotic therapy.
The safety endpoint was bleeding of type 2-5, per Bleeding Academic Research Consortium (BARC) criteria, at 30 days.
Results showed no significant differences in the primary efficacy endpoint (the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin T [hsTnT] to at least five times the upper limit of normal within 48 hours from randomization) between the revacept and placebo groups. The primary efficacy endpoint occurred in 24.4% of the revacept 160-mg group, 25.0% of the revacept 80-mg group, and 23.3% of the placebo group.
The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, but adenosine 5-diphosphate–induced aggregation was not affected.
Revacept did not increase bleeding. Bleeding of BARC type 2 or higher at 30 days occurred in 5.0% of the 160-mg group, 5.9% of the 80-mg group, and 8.6% of the placebo group.
Dr. Massberg pointed out that one possible explanation for the lack of difference in the efficacy outcome was that the patients enrolled in the study were at low risk.
“The rate of major adverse cardiovascular events was very low (2.5% at 30 days), and this was a low-risk population undergoing elective PCI,” he commented.
The authors also pointed out that the five-times increase in hsTnT endpoint used in the current study has little prognostic impact.
In addition, Dr. Massberg noted that, in the stable situation, myocardial injury is mostly triggered by cholesterol embolism during PCI and side-branch occlusion due to distal plaque embolization, problems that are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
He suggested that better results may be achieved in patients with acute coronary syndrome (ACS). “In ACS patients, the myocardial injury is caused by ongoing thrombotic cascades, where the collagen-platelet interaction plays a much larger role, so in theory, this drug should show a greater effect in an ACS population.”
The researchers are now planning a larger phase 3 study in that group.
“I am still optimistic. I still believe it could work,” Dr. Massberg said. “The major aim for this study was safety and dosing. There was no difference in bleeding, so safety was supported,” he added.
The ISAR-PLASTER study was funded by the German Center for Cardiovascular Research, Deutsches Herzzentrum Munchen, the Federal Ministry of Education and Research, and advanceCOR (the manufacturer of revacept). One of the coauthors of the study is a cofounder of advanceCor.
A version of this article first appeared on Medscape.com.
About one in five clinicians considers quitting because of pandemic
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Squamous Cell Carcinoma in Hidradenitis Suppurativa Lesions Following Tumor Necrosis Factor α Inhibitors
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
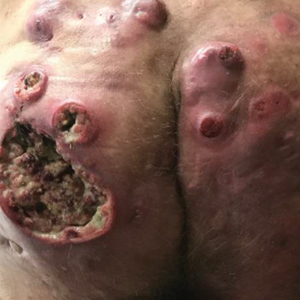
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.
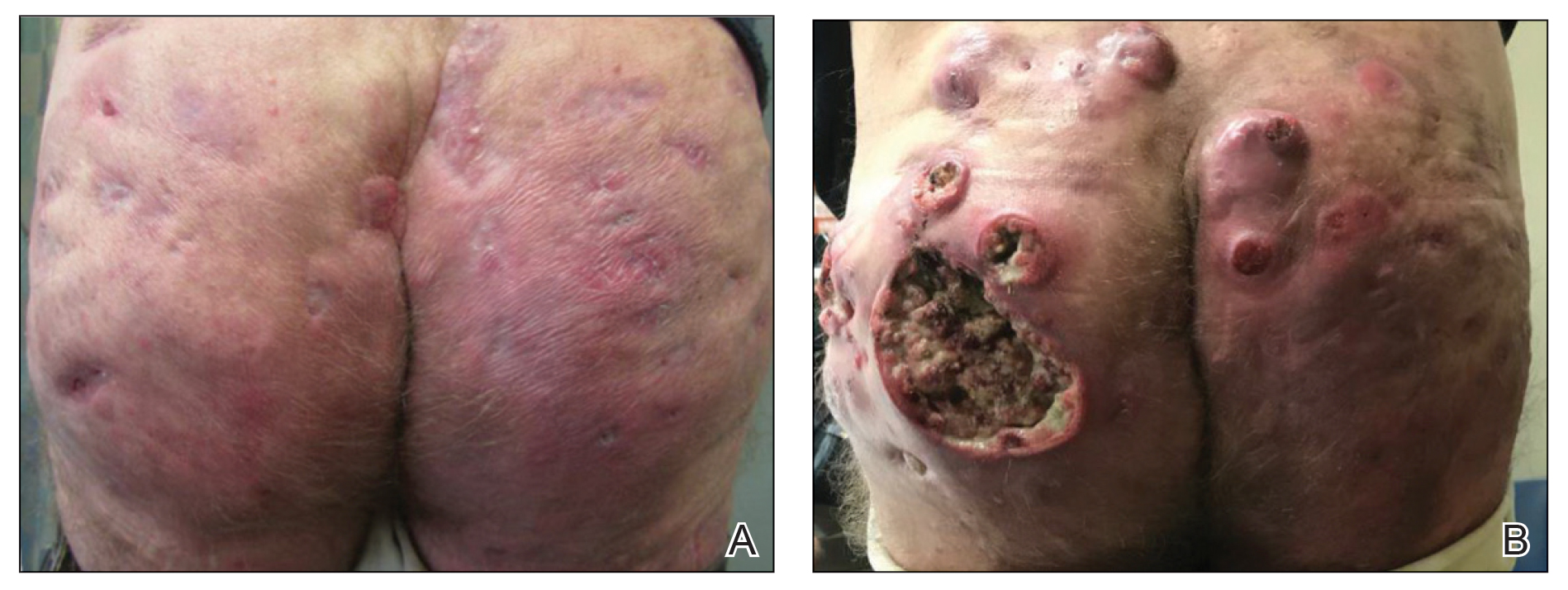
The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.
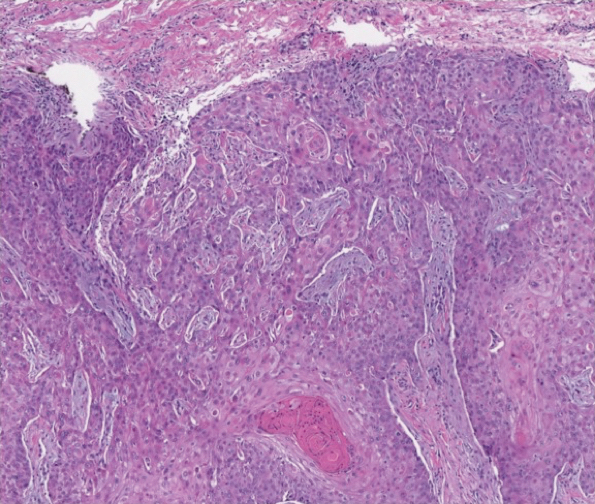
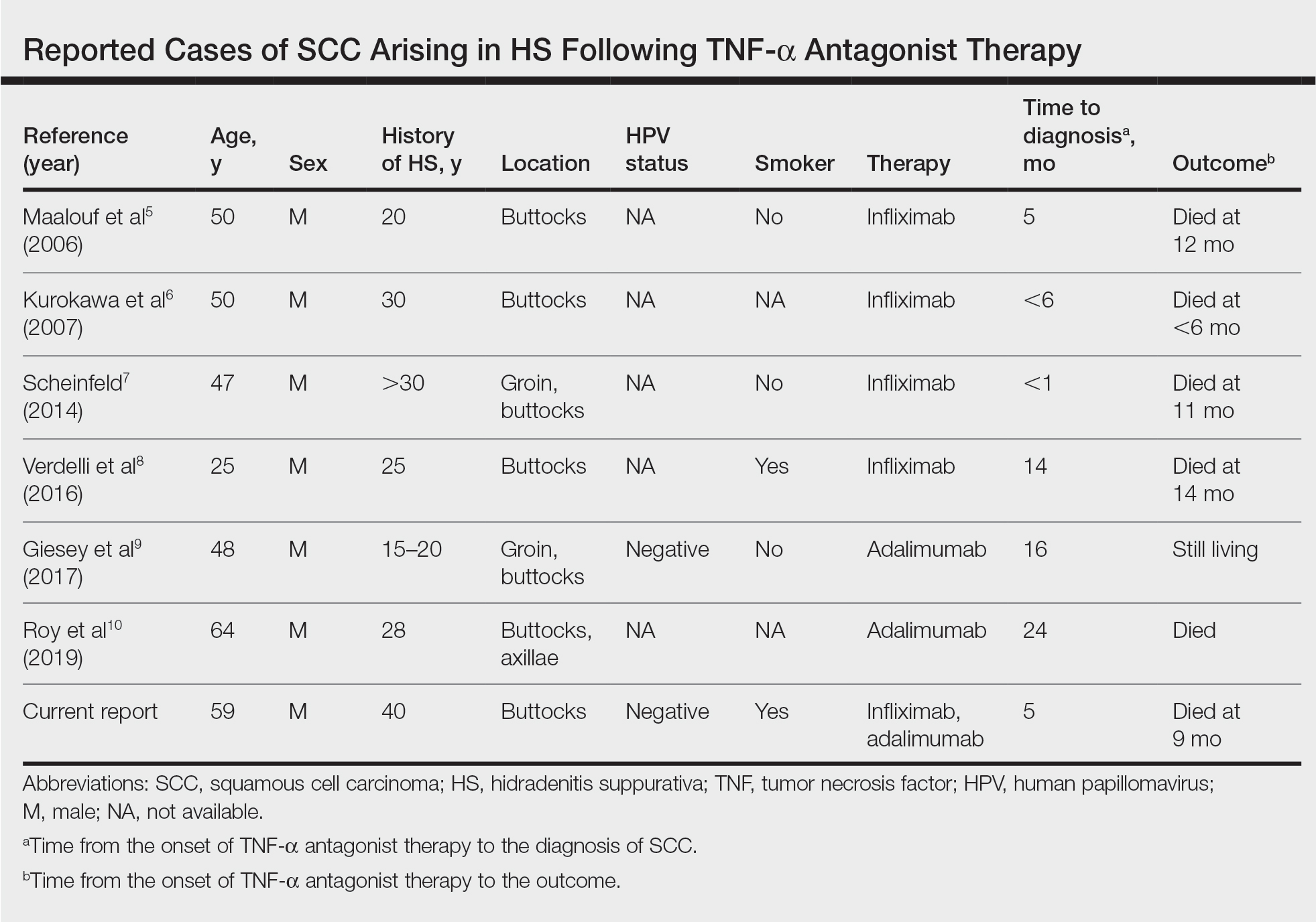
Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.
Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
Practice Points
- Consider biopsy of representative lesions in men older than 20 years with moderate to severe disease of the groin and/or buttocks prior to initiation of tumor necrosis factor inhibitors.
- Consider more frequent clinical monitoring with a decrease in threshold to perform biopsy of any new or ulcerating lesions.
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
Cardiovascular disease remains leading cause of type 2 diabetes mortality
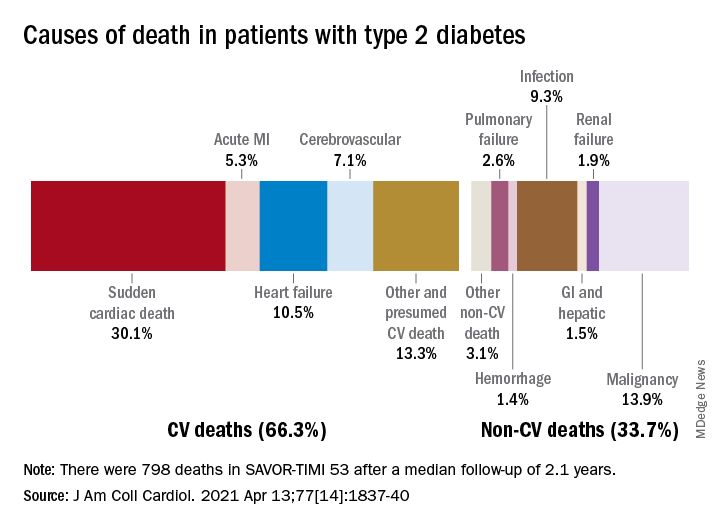
Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.

Two-thirds (66.3%) of all 798 deaths after a median 2.1 years of follow-up were caused by one of five cardiovascular (CV) conditions, with sudden cardiac death accounting for the largest share (30.1%) of the total, Ilaria Cavallari, MD, PhD, and associates said in the Journal of the American College of Cardiology.
Most common among the non-CV causes was malignancy at 13.9% of all deaths in a T2DM population at high/very high risk for CV disease (n = 16,492), followed by infection (9.3%), the members of the TIMI Study Group noted.
After variables independently associated with overall mortality were identified, a subdistribution of competing risks was constructed using a competing-risk analysis based on the proportional hazards model, they explained.
Prior heart failure was the clinical variable most associated with CV death and could, along with older age, worse glycemic control, prior CV events, peripheral artery disease, and kidney complications, “identify a subgroup of T2DM patients at high risk of mortality who are likely to achieve the greatest benefit from aggressive management of modifiable risk factors and newer glucose-lowering agents,” the investigators wrote.
It was a pair of laboratory measurements, however, that had the largest subdistribution hazard ratios. “Interestingly, the magnitude of associations of abnormal N-terminal pro–B-type natriuretic peptide [sHR, 2.82] and high-sensitivity troponin T [sHR, 2.46] measured in a stable population were greater than clinical variables in the prediction of all causes of death,” Dr. Cavallari and associates said.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Excess deaths jump 23% in U.S. in 2020, mostly because of COVID-19
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
Is there a need for tPA before thrombectomy in patients with stroke?
In a new randomized trial that investigated the question of whether thrombolysis can be omitted for patients with stroke who are undergoing endovascular thrombectomy for a large-vessel occlusion, results were similar for both approaches.
and functional outcomes were not significantly different. In addition, hemorrhage rates with or without intravenous alteplase administration before endovascular treatment were similar.
“From the MR CLEAN NO IV results, we cannot change standard practice, as we failed to show superiority of the direct endovascular approach, and we also didn’t meet the noninferiority criteria. So, the standard practice of giving tPA to those eligible still holds,” said co–lead investigator Yvo Roos, MD.
“But I think we can say that these results suggest that there may also not be such a need for tPA in patients who can go straight for endovascular therapy,” said Dr. Roos, who is professor of neurology at Amsterdam Medical Center.
“If we are not sure whether a patient is suitable for tPA because they have a higher bleeding risk, I think we can be reassured about missing the tPA out and going straight to endovascular treatment. So, if in doubt, leave it out,” he added.
Results of the MR CLEAN NO IV trial were presented at the International Stroke Conference sponsored by the American Heart Association.
“If in doubt, leave it out”
Dr. Roos noted that three trials have investigated the question regarding dropping thrombolysis for patients who can receive thrombectomy quickly. These are the DIRECT MT, SKIP, and DEVT studies. All of these trials were conducted in Asian countries, and none found differences in functional outcomes between the two approaches.
The largest of these studies – the DIRECT-MT trial, from China, which was a sister study to MR CLEAN NO IV – did show noninferiority of the direct endovascular approach to tPA plus endovascular treatment.
But because of differences in health care logistics and trial populations, the benefits and risks of dropping thrombolysis in Western countries are not known, explained Charles Majoie, MD, who is co–lead investigator of the current trial and is chair of neuroradiology at Amsterdam Medical Center.
The MR CLEAN NO IV trial was designed to show superiority of the direct endovascular approach with noninferiority for hemorrhage. It enrolled 540 European patients who were eligible for both thrombolysis and thrombectomy and who presented to a thrombectomy-capable center. They were randomly assigned to receive thrombolysis plus endovascular therapy or direct endovascular therapy alone.
The mean time from stroke onset to groin puncture (the start of endovascular therapy) was very fast in both groups – 130 minutes in the direct group, and 135 minutes in the tPA group.
The primary outcome was a shift analysis of the Modified Rankin Scale (mRS). On that outcome, the trial failed to show significant superiority of the direct approach (odds ratio, 0.88; 95% confidence interval, 0.65-1.19).
A good functional outcome (mRS, 0-2) was achieved in 49% of the direct thrombectomy group and in 51% of the tPA group (OR, 0.95; 95% CI, 0.65-1.40).
Safety results showed no difference in any of the hemorrhage endpoints between the two groups. The rate of symptomatic intracranial hemorrhage was actually numerically higher in the direct thrombectomy group (5.9% vs. 5.3%).
“One of the most intriguing results of this study is that there was no increase in hemorrhage in the tPA group,” Dr. Roos commented. “This is very surprising, as we have always thought thrombolysis causes an increased bleeding risk. But after these results, we may have to rethink that idea – perhaps it is not the tPA itself that causes bleeding risk but rather the opening up of the vessel.”
On the failure to show noninferiority of the direct approach, Dr. Roos suggested that the trial may have been underpowered in this respect.
“Our sister trial, DIRECT-MT, was a noninferiority study. They had 650 patients, and they just reached noninferiority,” he said. “In MR CLEAN NO IV, we were aiming for superiority, and we had fewer patients – 540. We didn’t show superiority, and we didn’t have quite enough patients to show noninferiority.”
He added that, considering all the four studies together, the results look very similar and suggest no difference between the two approaches.
Individualized approach probable
Dr. Majoie suggested that different patients may be suitable for the different approaches.
“I think we are heading for individualized treatment. If we have a young patient and the angiography suite is ready, we could probably skip tPA, but it would be for the neurologist/neuroradiologist to make individualized decisions on this,” he said. “We need to look at subgroups for more information.”
Another large trial that investigated this issue, SWIFT-DIRECT, is expected to be presented later this year. An Australian trial, DIRECT-SAFE, is ongoing and is at an early stage of recruitment.
Dr. Roos said that the data from all the trials will be combined for a more comprehensive analysis of the benefits and risks of the two approaches in various subgroups.
Commenting on the study was cochair of the ISC session at which it was presented, Tudor Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, N.J.
“Putting these results together with the previous Asian studies, I think we can say that direct thrombectomy without tPA is clearly not superior to the combined approach of tPA plus thrombectomy,” he said.
Dr. Jovin explained that, in theory, direct thrombectomy could be faster than the combined approach and that the risk for symptomatic intracerebral hemorrhage could be lower. But neither of these two possible benefits were seen in this study.
He agreed with Dr. Roos that MR CLEAN NO IV could have failed to show noninferiority of the direct strategy because the sample was not large enough.
“The results of the two approaches are very similar in this study and in the Asian studies, so it doesn’t appear that tPA adds very much, and it is associated with a significant increase in costs,” he said.
“The answer will probably be that there is not a ‘one-size-fits-all’ strategy, and we may end up using different approaches for different patient groups,” Dr. Jovin added. “Information on this will come from subgroups analyses from these trials.”
MR CLEAN NO-IV trial was part of the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative (an initiative of the Dutch Heart Foundation), the Brain Foundation Netherlands, Medtronic, Health-Holland, and Top Sector Life Sciences. The study received additional unrestricted funding from Stryker European Operations. Dr. Roos and Dr. Majoie are shareholders of Nico Lab.
A version of this article first appeared on Medscape.com.
In a new randomized trial that investigated the question of whether thrombolysis can be omitted for patients with stroke who are undergoing endovascular thrombectomy for a large-vessel occlusion, results were similar for both approaches.
and functional outcomes were not significantly different. In addition, hemorrhage rates with or without intravenous alteplase administration before endovascular treatment were similar.
“From the MR CLEAN NO IV results, we cannot change standard practice, as we failed to show superiority of the direct endovascular approach, and we also didn’t meet the noninferiority criteria. So, the standard practice of giving tPA to those eligible still holds,” said co–lead investigator Yvo Roos, MD.
“But I think we can say that these results suggest that there may also not be such a need for tPA in patients who can go straight for endovascular therapy,” said Dr. Roos, who is professor of neurology at Amsterdam Medical Center.
“If we are not sure whether a patient is suitable for tPA because they have a higher bleeding risk, I think we can be reassured about missing the tPA out and going straight to endovascular treatment. So, if in doubt, leave it out,” he added.
Results of the MR CLEAN NO IV trial were presented at the International Stroke Conference sponsored by the American Heart Association.
“If in doubt, leave it out”
Dr. Roos noted that three trials have investigated the question regarding dropping thrombolysis for patients who can receive thrombectomy quickly. These are the DIRECT MT, SKIP, and DEVT studies. All of these trials were conducted in Asian countries, and none found differences in functional outcomes between the two approaches.
The largest of these studies – the DIRECT-MT trial, from China, which was a sister study to MR CLEAN NO IV – did show noninferiority of the direct endovascular approach to tPA plus endovascular treatment.
But because of differences in health care logistics and trial populations, the benefits and risks of dropping thrombolysis in Western countries are not known, explained Charles Majoie, MD, who is co–lead investigator of the current trial and is chair of neuroradiology at Amsterdam Medical Center.
The MR CLEAN NO IV trial was designed to show superiority of the direct endovascular approach with noninferiority for hemorrhage. It enrolled 540 European patients who were eligible for both thrombolysis and thrombectomy and who presented to a thrombectomy-capable center. They were randomly assigned to receive thrombolysis plus endovascular therapy or direct endovascular therapy alone.
The mean time from stroke onset to groin puncture (the start of endovascular therapy) was very fast in both groups – 130 minutes in the direct group, and 135 minutes in the tPA group.
The primary outcome was a shift analysis of the Modified Rankin Scale (mRS). On that outcome, the trial failed to show significant superiority of the direct approach (odds ratio, 0.88; 95% confidence interval, 0.65-1.19).
A good functional outcome (mRS, 0-2) was achieved in 49% of the direct thrombectomy group and in 51% of the tPA group (OR, 0.95; 95% CI, 0.65-1.40).
Safety results showed no difference in any of the hemorrhage endpoints between the two groups. The rate of symptomatic intracranial hemorrhage was actually numerically higher in the direct thrombectomy group (5.9% vs. 5.3%).
“One of the most intriguing results of this study is that there was no increase in hemorrhage in the tPA group,” Dr. Roos commented. “This is very surprising, as we have always thought thrombolysis causes an increased bleeding risk. But after these results, we may have to rethink that idea – perhaps it is not the tPA itself that causes bleeding risk but rather the opening up of the vessel.”
On the failure to show noninferiority of the direct approach, Dr. Roos suggested that the trial may have been underpowered in this respect.
“Our sister trial, DIRECT-MT, was a noninferiority study. They had 650 patients, and they just reached noninferiority,” he said. “In MR CLEAN NO IV, we were aiming for superiority, and we had fewer patients – 540. We didn’t show superiority, and we didn’t have quite enough patients to show noninferiority.”
He added that, considering all the four studies together, the results look very similar and suggest no difference between the two approaches.
Individualized approach probable
Dr. Majoie suggested that different patients may be suitable for the different approaches.
“I think we are heading for individualized treatment. If we have a young patient and the angiography suite is ready, we could probably skip tPA, but it would be for the neurologist/neuroradiologist to make individualized decisions on this,” he said. “We need to look at subgroups for more information.”
Another large trial that investigated this issue, SWIFT-DIRECT, is expected to be presented later this year. An Australian trial, DIRECT-SAFE, is ongoing and is at an early stage of recruitment.
Dr. Roos said that the data from all the trials will be combined for a more comprehensive analysis of the benefits and risks of the two approaches in various subgroups.
Commenting on the study was cochair of the ISC session at which it was presented, Tudor Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, N.J.
“Putting these results together with the previous Asian studies, I think we can say that direct thrombectomy without tPA is clearly not superior to the combined approach of tPA plus thrombectomy,” he said.
Dr. Jovin explained that, in theory, direct thrombectomy could be faster than the combined approach and that the risk for symptomatic intracerebral hemorrhage could be lower. But neither of these two possible benefits were seen in this study.
He agreed with Dr. Roos that MR CLEAN NO IV could have failed to show noninferiority of the direct strategy because the sample was not large enough.
“The results of the two approaches are very similar in this study and in the Asian studies, so it doesn’t appear that tPA adds very much, and it is associated with a significant increase in costs,” he said.
“The answer will probably be that there is not a ‘one-size-fits-all’ strategy, and we may end up using different approaches for different patient groups,” Dr. Jovin added. “Information on this will come from subgroups analyses from these trials.”
MR CLEAN NO-IV trial was part of the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative (an initiative of the Dutch Heart Foundation), the Brain Foundation Netherlands, Medtronic, Health-Holland, and Top Sector Life Sciences. The study received additional unrestricted funding from Stryker European Operations. Dr. Roos and Dr. Majoie are shareholders of Nico Lab.
A version of this article first appeared on Medscape.com.
In a new randomized trial that investigated the question of whether thrombolysis can be omitted for patients with stroke who are undergoing endovascular thrombectomy for a large-vessel occlusion, results were similar for both approaches.
and functional outcomes were not significantly different. In addition, hemorrhage rates with or without intravenous alteplase administration before endovascular treatment were similar.
“From the MR CLEAN NO IV results, we cannot change standard practice, as we failed to show superiority of the direct endovascular approach, and we also didn’t meet the noninferiority criteria. So, the standard practice of giving tPA to those eligible still holds,” said co–lead investigator Yvo Roos, MD.
“But I think we can say that these results suggest that there may also not be such a need for tPA in patients who can go straight for endovascular therapy,” said Dr. Roos, who is professor of neurology at Amsterdam Medical Center.
“If we are not sure whether a patient is suitable for tPA because they have a higher bleeding risk, I think we can be reassured about missing the tPA out and going straight to endovascular treatment. So, if in doubt, leave it out,” he added.
Results of the MR CLEAN NO IV trial were presented at the International Stroke Conference sponsored by the American Heart Association.
“If in doubt, leave it out”
Dr. Roos noted that three trials have investigated the question regarding dropping thrombolysis for patients who can receive thrombectomy quickly. These are the DIRECT MT, SKIP, and DEVT studies. All of these trials were conducted in Asian countries, and none found differences in functional outcomes between the two approaches.
The largest of these studies – the DIRECT-MT trial, from China, which was a sister study to MR CLEAN NO IV – did show noninferiority of the direct endovascular approach to tPA plus endovascular treatment.
But because of differences in health care logistics and trial populations, the benefits and risks of dropping thrombolysis in Western countries are not known, explained Charles Majoie, MD, who is co–lead investigator of the current trial and is chair of neuroradiology at Amsterdam Medical Center.
The MR CLEAN NO IV trial was designed to show superiority of the direct endovascular approach with noninferiority for hemorrhage. It enrolled 540 European patients who were eligible for both thrombolysis and thrombectomy and who presented to a thrombectomy-capable center. They were randomly assigned to receive thrombolysis plus endovascular therapy or direct endovascular therapy alone.
The mean time from stroke onset to groin puncture (the start of endovascular therapy) was very fast in both groups – 130 minutes in the direct group, and 135 minutes in the tPA group.
The primary outcome was a shift analysis of the Modified Rankin Scale (mRS). On that outcome, the trial failed to show significant superiority of the direct approach (odds ratio, 0.88; 95% confidence interval, 0.65-1.19).
A good functional outcome (mRS, 0-2) was achieved in 49% of the direct thrombectomy group and in 51% of the tPA group (OR, 0.95; 95% CI, 0.65-1.40).
Safety results showed no difference in any of the hemorrhage endpoints between the two groups. The rate of symptomatic intracranial hemorrhage was actually numerically higher in the direct thrombectomy group (5.9% vs. 5.3%).
“One of the most intriguing results of this study is that there was no increase in hemorrhage in the tPA group,” Dr. Roos commented. “This is very surprising, as we have always thought thrombolysis causes an increased bleeding risk. But after these results, we may have to rethink that idea – perhaps it is not the tPA itself that causes bleeding risk but rather the opening up of the vessel.”
On the failure to show noninferiority of the direct approach, Dr. Roos suggested that the trial may have been underpowered in this respect.
“Our sister trial, DIRECT-MT, was a noninferiority study. They had 650 patients, and they just reached noninferiority,” he said. “In MR CLEAN NO IV, we were aiming for superiority, and we had fewer patients – 540. We didn’t show superiority, and we didn’t have quite enough patients to show noninferiority.”
He added that, considering all the four studies together, the results look very similar and suggest no difference between the two approaches.
Individualized approach probable
Dr. Majoie suggested that different patients may be suitable for the different approaches.
“I think we are heading for individualized treatment. If we have a young patient and the angiography suite is ready, we could probably skip tPA, but it would be for the neurologist/neuroradiologist to make individualized decisions on this,” he said. “We need to look at subgroups for more information.”
Another large trial that investigated this issue, SWIFT-DIRECT, is expected to be presented later this year. An Australian trial, DIRECT-SAFE, is ongoing and is at an early stage of recruitment.
Dr. Roos said that the data from all the trials will be combined for a more comprehensive analysis of the benefits and risks of the two approaches in various subgroups.
Commenting on the study was cochair of the ISC session at which it was presented, Tudor Jovin, MD, chair of neurology at Cooper University Hospital, Cherry Hill, N.J.
“Putting these results together with the previous Asian studies, I think we can say that direct thrombectomy without tPA is clearly not superior to the combined approach of tPA plus thrombectomy,” he said.
Dr. Jovin explained that, in theory, direct thrombectomy could be faster than the combined approach and that the risk for symptomatic intracerebral hemorrhage could be lower. But neither of these two possible benefits were seen in this study.
He agreed with Dr. Roos that MR CLEAN NO IV could have failed to show noninferiority of the direct strategy because the sample was not large enough.
“The results of the two approaches are very similar in this study and in the Asian studies, so it doesn’t appear that tPA adds very much, and it is associated with a significant increase in costs,” he said.
“The answer will probably be that there is not a ‘one-size-fits-all’ strategy, and we may end up using different approaches for different patient groups,” Dr. Jovin added. “Information on this will come from subgroups analyses from these trials.”
MR CLEAN NO-IV trial was part of the CONTRAST consortium, which is supported by the Netherlands Cardiovascular Research Initiative (an initiative of the Dutch Heart Foundation), the Brain Foundation Netherlands, Medtronic, Health-Holland, and Top Sector Life Sciences. The study received additional unrestricted funding from Stryker European Operations. Dr. Roos and Dr. Majoie are shareholders of Nico Lab.
A version of this article first appeared on Medscape.com.
FROM ISC 2021
Six pregnancy complications flag later heart disease risk
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
Six pregnancy-related complications increase a woman’s risk of developing risk factors for cardiovascular disease (CVD) and subsequently developing CVD, the American Heart Association says in a new scientific statement.
They are hypertensive disorders of pregnancy, preterm delivery, gestational diabetes, small-for-gestational-age (SGA) delivery, placental abruption (abruptio placentae), and pregnancy loss.
A history of any of these adverse pregnancy outcomes should prompt “more vigorous primordial prevention of CVD risk factors and primary prevention of CVD,” the writing group says.
“Adverse pregnancy outcomes are linked to women having hypertension, diabetes, abnormal cholesterol, and cardiovascular disease events, including heart attack and stroke, long after their pregnancies,” Nisha I. Parikh, MD, MPH, chair of the writing group, said in a news release.
Adverse pregnancy outcomes can be a “powerful window” into CVD prevention “if women and their health care professionals harness the knowledge and use it for health improvement,” said Dr. Parikh, associate professor of medicine in the cardiovascular division at the University of California, San Francisco.
The statement was published online March 29 in Circulation.
For the scientific statement, the writing group reviewed the latest scientific literature on adverse pregnancy outcomes and CVD risk.
The evidence in the literature linking adverse pregnancy outcomes to later CVD is “consistent over many years and confirmed in nearly every study we examined,” Dr. Parikh said. Among their key findings:
- Gestational hypertension is associated with an increased risk of CVD later in life by 67% and the odds of stroke by 83%. Moderate and severe is associated with a more than twofold increase in the risk for CVD.
- Gestational diabetes is associated with an increase in the risk for CVD by 68% and the risk of developing after pregnancy by 10-fold.
- Preterm delivery (before 37 weeks) is associated with double the risk of developing CVD and is strongly associated with later heart disease, stroke, and CVD.
- Placental abruption is associated with an 82% increased risk for CVD.
- Stillbirth is associated with about double the risk for CVD.
“This statement should inform future prevention guidelines in terms of the important factors to consider for determining women’s risk for heart diseases and stroke,” Dr. Parikh added.
The statement emphasizes the importance of recognizing these adverse pregnancy outcomes when evaluating CVD risk in women but notes that their value in reclassifying CVD risk may not be established.
It highlights the importance of adopting a heart-healthy diet and increasing physical activity among women with any of these pregnancy-related complications, starting right after childbirth and continuing across the life span to decrease CVD risk.
Lactation and breastfeeding may lower a woman’s later cardiometabolic risk, the writing group notes.
‘Golden year of opportunity’
The statement highlights several opportunities to improve transition of care for women with adverse pregnancy outcomes and to implement strategies to reduce their long-term CVD risk.
One strategy is longer postpartum follow-up care, sometimes referred to as the “fourth trimester,” to screen for CVD risk factors and provide CVD prevention counseling.
Another strategy involves improving the transfer of health information between ob/gyns and primary care physicians to eliminate inconsistencies in electronic health record documentation, which should improve patient care.
A third strategy is obtaining a short and targeted health history for each woman to confirm if she has any of the six pregnancy-related complications.
“If a woman has had any of these adverse pregnancy outcomes, consider close blood pressure monitoring, type 2 diabetes and lipid screening, and more aggressive risk factor modification and CVD prevention recommendations,” Dr. Parikh advised.
“Our data [lend] support to the prior AHA recommendation that these important adverse pregnancy outcomes should be ‘risk enhancers’ to guide consideration for statin therapy aimed at CVD prevention in women,” Dr. Parikh added.
In a commentary in Circulation, Eliza C. Miller, MD, assistant professor of neurology at Columbia University, New York, notes that pregnancy and the postpartum period are a critical time window in a woman’s life to identify CVD risk and improve a woman’s health trajectory.
“The so-called ‘Golden Hour’ for conditions such as sepsis and acute stroke refers to a critical time window for early recognition and treatment, when we can change a patient’s clinical trajectory and prevent severe morbidity and mortality,” writes Dr. Miller.
“Pregnancy and the postpartum period can be considered a ‘Golden Year’ in a woman’s life, offering a rare opportunity for clinicians to identify young women at risk and work with them to improve their cardiovascular health trajectories,” she notes.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Council on Epidemiology and Prevention; the Council on Arteriosclerosis, Thrombosis and Vascular Biology; the Council on Cardiovascular and Stroke Nursing; and the Stroke Council.
The authors of the scientific statement have disclosed no relevant financial relationships. Dr. Miller received personal compensation from Finch McCranie and Argionis & Associates for expert testimony regarding maternal stroke; and personal compensation from Elsevier for editorial work on Handbook of Clinical Neurology, Vol. 171 and 172 (Neurology of Pregnancy).
A version of this article first appeared on Medscape.com.
AstraZeneca COVID vaccine: Clotting disorder mechanism revealed?
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
The European Medicines Agency continues to reassure the public about the safety of the AstraZeneca COVID-19 vaccine, although several countries have imposed new restrictions on the product, owing to its link to a rare clotting disorder.
Use of the vaccine has been suspended for individuals younger than 55 or 60 years in several European countries and in Canada after reports of a prothrombotic disorder and thrombocytopenia, mainly in younger individuals.
Now, more information on the prothrombotic disorder has become available. The vaccine appears to be linked to a condition that clinically resembles heparin-induced thrombocytopenia (HIT) and that occurs mainly in younger women.
Researchers have described clinical and laboratory details of nine patients from Germany and Austria who developed this condition 4-16 days after receiving the AstraZeneca vaccine in a preprint article published March 28, 2021, on Research Square.
They found that serum from four patients who were tested showed platelet-activating antibodies directed against platelet factor 4 (PF4), similar to what is seen in HIT.
They are proposing naming the condition “vaccine-induced prothrombotic immune thrombocytopenia (VIPIT)” to avoid confusion with HIT.
At a press conference March 31, the EMA said its ongoing review of the situation “has not identified any specific risk factors, such as age, gender, or a previous medical history of clotting disorders, for these very rare events. A causal link with the vaccine is not proven but is possible, and further analysis is continuing.”
A statement from the agency noted: “EMA is of the view that the benefits of the AstraZeneca vaccine in preventing COVID-19, with its associated risk of hospitalization and death, outweigh the risks of side effects.”
But it added: “Vaccinated people should be aware of the remote possibility of these very rare types of blood clots occurring. If they have symptoms suggestive of clotting problems as described in the product information, they should seek immediate medical attention and inform health care professionals of their recent vaccination.”
VIPIT study
In the Research Square preprint article, a group led by Andreas Greinacher, MD, professor of transfusion medicine at the Greifswald (Germany) University Clinic, reported on clinical and laboratory features of nine patients (eight of whom were women) in Germany and Austria who developed thrombosis and thrombocytopenia after they received the AstraZeneca vaccine.
The researchers explained that they investigated whether these patients could have a prothrombotic disorder caused by platelet-activating antibodies directed against PF4, which is known to be caused by heparin and sometimes environmental triggers.
The nine patients were aged 22-49 years and presented with thrombosis beginning 4-16 days post vaccination. Seven patients had cerebral venous thrombosis (CVT), one had pulmonary embolism, and one had splanchnic vein thrombosis and CVT. Four patients died. None had received heparin prior to symptom onset.
Serum from four patients was tested for anti-PF4/heparin antibodies, and all four tested strongly positive. All four also tested strongly positive on platelet activation assay for the presence of PF4 independently of heparin.
The authors noted that it has been recognized that triggers other than heparin, including some infections, can rarely cause a disorder that strongly resembles HIT. These cases have been referred to as spontaneous HIT syndrome.
They said that their current findings have several important clinical implications.
“Clinicians should be aware that onset of (venous or arterial) thrombosis particularly at unusual sites such as in the brain or abdomen and thrombocytopenia beginning approximately 5-14 days after vaccination can represent a rare adverse effect of preceding COVID-19 vaccination,” they wrote. To date, this has only been reported with the AstraZeneca vaccine.
They pointed out that enzyme immunoassays for HIT are widely available and can be used to investigate for potential postvaccination anti-PF4 antibody–associated thrombocytopenia/thrombosis. For such patients, referral should be made to a laboratory that performs platelet-activation assays.
Although this syndrome differs from typical HIT, the researchers noted that at least one patient showed strong platelet activation in the presence of heparin. They thus recommended therapy with nonheparin anticoagulants, such as the direct oral anticoagulants.
They also wrote that high-dose intravenous immunoglobulin has been shown to be effective for treating severe HIT and could also be an important treatment adjunct for patients who develop life-threatening thrombotic events, such as cerebral vein sinus thrombosis (CVST), after being vaccinated.
EMA data to date
Updated data, reported at the EMA press briefing on March 31, indicate that 62 cases of CVST have been reported worldwide (44 from the European Union). These data may not yet include all the German cases.
Peter Arlett, MD, head of pharmacovigilance and epidemiology at the EMA, said there were more cases than expected in the 2-week window after vaccination among patients younger than 60 and that health care professionals should be alert to features of this condition, including headache and blurred vision.
He suggested that the higher rate of the condition among younger women may reflect the population that received this vaccine, because initially, the vaccine was not recommended for older people in many countries and was targeted toward younger health care workers, who were mainly women.
The German regulatory agency, the Paul Ehrlich Institute, reported this week that it has now registered 31 cases of CVST among nearly 2.7 million people who had received the vaccine in Germany. Of these patients, 19 also were found to have a deficiency of blood platelets or thrombocytopenia. Nine of the affected patients died. All but two of the cases occurred in women aged 20-63 years. The two men were aged 36 and 57 years.
These data have prompted the German authorities to limit use of the AstraZeneca vaccine to those aged 60 years and older. Even before this decision, senior clinicians in Germany had been urging a change in the vaccination recommendations.
For example, Bernd Salzberger, MD, head of infectious diseases, University Hospital Regensburg (Germany), told the Science Media Center: “In women, a complicated course of COVID disease is less common from the start and is so rare in younger women that the chance of avoiding a fatal course through vaccination in women without comorbidities is of the same order of magnitude as the risk of this rare side effect.”
Sandra Ciesek, MD, a virologist at Goethe University, Frankfurt, Germany, told the journal Science: “The argument I keep hearing is that the risk-benefit ratio is still positive. But we do not have just one vaccine, we have several. So, restricting the AstraZeneca vaccine to older people makes sense to me, and it does not waste any doses.”
Concerns put in perspective
Commenting of the latest developments, thrombosis expert Saskia Middeldorp, MD, head of internal medicine at Radboud University Medical Center, Nijmegen, the Netherlands, said it was vitally important that these concerns be put in perspective and that the vaccination program with the AstraZeneca product continue.
“There are some concerning reports about very rare blood clotting disorders and low platelet counts possibly associated with the AstraZeneca vaccine. Groups from Germany and Norway have identified a syndrome similar to HIT, which seems to explain the cause of this very rare side effect,” Dr. Middeldorp noted.
“But with such a high pressure from the virus and many countries now going into a third wave of infection, anything that might slow down vaccination rates will cause much more harm than good,” she warned.
Dr. Middeldorp believes the incidence of this HIT-type syndrome linked to the vaccine is about 1-2 per million. “These are estimates based on the number of reports of this side effect and denominators from the U.K. and EU populations,” she explained. However, Germany has restricted the vaccine on the basis of German data, which appear to show higher rates of the condition. It is not known why the rates are higher in Germany.
“The European Medicines Agency is looking at this very closely. Their statement is quite clear. There is no foundation for changing policy on vaccination,” Dr. Middeldorp stated.
She cautioned that these reports were reducing confidence in the AstraZeneca vaccine, particularly among young people, which she said was causing “a major setback” for the vaccination program.
Noting that everything must be viewed in the context of this severe pandemic, Dr. Middeldorp emphasized that the benefit of the vaccine outweighed any risk, even among young people.
“To those who may be hesitating to have the vaccine as they don’t think they are at high risk of severe COVID infection, I would say there are a lot of young people in the ICU at present with COVID, and your chance of a severe COVID illness is far higher than the 1 or 2 in a million risk of a severe reaction to the vaccine,” she stated.
Dr. Greinacher has received grants and nonfinancial support from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Paringenix, Bayer Healthcare, Gore, Rovi, Sagent, and Biomarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, Merck Sharp & Dohme, Macopharma, Bristol-Myers Squibb, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and GTH outside the submitted work.
A version of this article first appeared on Medscape.com.
FDA approves new ready-to-inject glucagon product
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved dasiglucagon (Zegalogue 0.6 mg/0.6 mL, Zealand Pharma) autoinjector and prefilled syringe for the treatment of severe hypoglycemia in people with diabetes aged 6 years and older.
The product has a shelf-life of 36 months at refrigerated temperatures and is stable for up to 12 months at room temperature.
“This approval will help enable appropriate children and adults with diabetes to be able to address sudden and severe hypoglycemia, which can quickly progress from a mild event to an emergency,” Jeremy Pettus, MD, assistant professor of medicine at the University of California, San Diego, said in a company statement.
The approval marks the latest step in the development of newer glucagon formulations that are easier to use in hypoglycemic emergencies than the traditional formulation that requires several steps for reconstitution.
The first intranasal glucagon (Baqsimi, Eli Lilly) was approved in the United States in July 2019 for people with diabetes age 4 years and older.
In September 2019, the FDA approved another prefilled glucagon rescue pen (Gvoke HypoPen, Xeris Pharmaceuticals) for the treatment of severe hypoglycemia in adult and pediatric patients age 2 years and older with diabetes.
Dasiglucagon is currently in phase 3 trials as a subcutaneous infusion for treating congenital hyperinsulinemia, and in phase 2 trials as part of a bihormonal artificial pancreas pump system.
The FDA approval was based on results from three randomized, double-blind, placebo-controlled, phase 3 studies of dasiglucagon in children age 6-17 years and adults with type 1 diabetes.
The primary endpoint was time to achieving an increase in blood glucose of 20 mg/dL or greater from time of administration without additional intervention within 45 minutes. That endpoint was achieved in all three studies, with a median time to blood glucose recovery of 10 minutes overall, with 99% of adults recovering within 15 minutes.
The most common adverse events reported in 2% or more of study participants were nausea, vomiting, headache, and injection-site pain in both children and adults. Diarrhea was also reported in adults.
Full launch is expected in late June 2021.
A version of this article first appeared on Medscape.com.