User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Pan-coronavirus vaccines may be key to fighting future pandemics
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
As the COVID-19 pandemic winds down – for the time being at least – efforts are ramping up to develop next-generation vaccines that can protect against future novel coronaviruses and variants. Several projects are presenting clever combinations of viral parts to the immune system that evoke a robust and hopefully lasting response.
The coming generation of “pan” vaccines aims to tamp down SARS-CoV-2, its closest relatives, and whatever may come into tamer respiratory viruses like the common cold. Whatever the eventual components of this new generation of vaccines, experts agree on the goal: preventing severe disease and death. And a broader approach is critical.
“All the vaccines have been amazing. But we’re playing a whack-a-mole game with the variants. We need to take a step back and ask if a pan-variant vaccine is possible. That’s important because Omicron isn’t the last variant,” said Jacob Lemieux, MD, PhD, instructor in medicine and infectious disease specialist at Massachusetts General Hospital, Boston.
A broad spectrum vaccine
The drive to create a vaccine that would deter multiple coronaviruses arose early, among many researchers. An article published in Nature in May 2020 by National Institute of Allergy and Infectious Diseases researcher Luca T. Giurgea, MD, and colleagues said it all in the title: “Universal coronavirus vaccines: the time to start is now.”
Their concerns? The diversity of bat coronaviruses poised to jump into humans; the high mutability of the spike gene that the immune response recognizes; and the persistence of mutations in an RNA virus, which can’t repair errors.
Work on broader vaccines began in several labs as SARS-CoV-2 spawned variant after variant.
On Sept. 28, NIAID announced funding for developing ‘pan-coronavirus’ vaccines – the quotation marks theirs to indicate that a magic bullet against any new coronavirus is unrealistic. “These new awards are designed to look ahead and prepare for the next generation of coronaviruses with pandemic potential,” said NIAID director Anthony S. Fauci, MD. An initial three awards went to groups at the University of Wisconsin, Brigham and Women’s Hospital, and Duke University.
President Biden mentioned the NIAID funding in his State of the Union Address. He also talked about how the Biomedical Advanced Research and Development Authority, founded in 2006 to prepare for public health emergencies, is spearheading development of new vaccine platforms and vaccines that target a broader swath of pathogen parts.
Meanwhile, individual researchers from eclectic fields are finding new ways to prevent future pandemics.
Artem Babaian, PhD, a computational biologist at the University of Cambridge (England), had the idea to probe National Institutes of Health genome databases, going back more than a decade, for overlooked novel coronaviruses. He started the project while he was between jobs as the pandemic was unfurling, using a telltale enzyme unique to the RNA viruses to fish out COVID cousins. The work is published in Nature and the data freely available at serratus.io.
Among the nearly 132,000 novel RNA viruses Dr. Babaian’s team found, 9 were from previously unrecognized coronaviruses. The novel nine came from “ecologically diverse sources”: a seahorse, an axolotl, an eel, and several fishes. Deciphering the topographies of these coronaviruses may provide clues to developing vaccines that stay ahead of future pandemics.
But optics are important in keeping expectations reasonable. “‘Universal vaccine’ is a misnomer. I think about it as ‘broad spectrum vaccines.’ It’s critical to be up front that these vaccines can never guarantee immunity against all coronaviruses. There are no absolutes in biology, but they hopefully will work against the dangers that we do know exist. A vaccine that mimics exposure to many coronaviruses could protect against a currently unknown coronavirus, especially if slower-evolving antigens are included,” Dr. Babaian said in an interview.
Nikolai Petrovsky, MD, PhD, of Flinders University, Adelaide, and the biotechnology company Vaccine Pty, agrees, calling a literal pan-coronavirus vaccine a “pipe dream. What I do think is achievable is a broadly protective, pan–CoV-19 vaccine – I can say that because we have already developed and tested it, combining antigens rather than trying just one that can do everything.”
Immunity lures
The broader vaccines in development display viral antigens, such as spike proteins, to the immune system on diverse frameworks. Here are a few approaches.
Ferritin nanoparticles: A candidate vaccine from the emerging infectious diseases branch of Water Reed National Military Medical Center began phase 1 human trials in April 2021. Called SpFN, the vaccine consists of arrays of ferritin nanoparticles linked to spike proteins from various variants and species. Ferritin is a protein that binds and stores iron in the body.
“The repetitive and ordered display of the coronavirus spike protein on a multifaced nanoparticle may stimulate immunity in such a way as to translate into significantly broader protection,” said Walter Reed’s branch director and vaccine coinventor Kayvon Modjarrad, MD, PhD.
A second vaccine targets only the “bullseye” part of the spike that the virus uses to attach and gain access to human cells, called the receptor-binding domain (RBD), of SARS-CoV-2 variants and of the virus behind the original SARS. The preclinical data appeared in Science Translational Medicine.
Barton Haynes, MD and colleagues at the Duke Human Vaccine Institute are also using ferritin to design and develop a “pan-betacoronavirus vaccine,” referring to the genus to which SARS-CoV-2 belongs. They say their results in macaques, published in Nature, “demonstrate that current mRNA-based vaccines may provide some protection from future outbreaks of zoonotic betacoronaviruses.”
Mosaic nanoparticles: Graduate student Alexander Cohen is leading an effort at CalTech, in the lab of Pamela Bjorkman, PhD, that uses nanoparticles consisting of proteins from a bacterium (Strep pyogenes) to which RBDs from spike proteins of four or eight different betacoronaviruses are attached. The strategy demonstrates that the whole is greater than the sum of the parts.
“Alex’s results show that it is possible to raise diverse neutralizing antibody responses, even against coronavirus strains that were not represented on the injected nanoparticle. We are hopeful that this technology could be used to protect against future animal coronaviruses that cross into humans,” said Dr. Björkman. The work appeared in Science.
Candidate vaccines from Inovio Pharmaceuticals also use a mosaic spike strategy, but with DNA rings (plasmids) rather than nanoparticles. One version works against pre-Omicron variants and is being tested against Omicron, and another with “pan–COVID-19” coverage has tested well in animal models. Inovio’s vaccines are delivered into the skin using a special device that applies an electric pulse that increases the cells’ permeability.
Chimeric spikes: Yet another approach is to fashion vaccines from various parts of the betacoronaviruses that are most closely related to SARS-CoV-2 – the pathogens behind Middle East respiratory syndrome and severe acute respiratory syndrome as well as several bat viruses and a few pangolin ones. The abundance and ubiquity of these viruses provide a toolbox of sorts, with instructions written in the language of RNA, from which to select, dissect, recombine, and customize vaccines.
“SARS-like viruses can recombine and exhibit great genetic diversity in several parts of the genome. We designed chimeric spikes to improve coverage of a multiplexed vaccine,” said David Martinez, PhD.
His team at the University of North Carolina at Chapel Hill has developed mRNA vaccines that deliver “scrambled coronavirus spikes” representing various parts, not just the RBD, as described in Science.
In mice, the chimeric vaccines elicit robust T- and B-cell immune responses, which stimulate antibody production and control other facets of building immunity.
Beyond the spike bullseye
The challenge of developing pan-coronavirus vaccines is dual. “The very best vaccines are highly specific to each strain, and the universal vaccines have to sacrifice effectiveness to get broad coverage. Life is a trade-off.” Dr. Petrovsky told this news organization.
Efforts to broaden vaccine efficacy venture beyond targeting the RBD bullseyes of the spike triplets that festoon the virus. Some projects are focusing on less changeable spike parts that are more alike among less closely related coronaviruses than is the mutation-prone RBD. For example, the peptides that twist into the “stem-helix” portion of the part of the spike that adheres to host cells are the basis of some candidate vaccines now in preclinical studies.
Still other vaccines aren’t spike based at all. French company Osivax, for example, is working on a vaccine that targets the nucleocapsid protein that shields the viral RNA. The hope is that presenting various faces of the pathogen may spark immunity beyond an initial antibody rush and evoke more diverse and lasting T-cell responses.
With the myriad efforts to back up the first generation of COVID-19 vaccines with new ones offering broader protection, it appears that science may have finally learned from history.
“After the SARS outbreak, we lost interest and failed to complete development of a vaccine for use in case of a recurrent outbreak. We must not make the same mistake again,” Dr. Giurgea and colleagues wrote in their Nature article about universal coronavirus vaccines.
A version of this article first appeared on Medscape.com.
Antivaccine physician pleads guilty to role in Capitol riot
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
Concussion increases risk of mental health issues in children
Among children and adolescents aged 5-18 years, concussion was associated with a higher risk of mental health problems, compared with age- and sex-matched children and adolescents with an orthopedic injury, according to a cohort study published in JAMA Network Open.
While concussions are one of the most common head injuries in the pediatric population, the extent to which they increase the risk of new onset psychiatric disorders or subsequent psychopathology is unclear, lead author Andrée-Anne Ledoux, PhD, of the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, and colleagues explained.
The researchers conducted a population-based retrospective cohort study to evaluate associations between concussion and risk of subsequent mental health issues, psychiatric hospitalizations, self-harm, or suicides in children and adolescents, with follow-up ranging from 1 month to 10 years.
The data were obtained from province-wide health administrative databases. Participants with concussion were included in an exposed group, while those with an orthopedic injury were included in a 1:2 age- and sex-matched comparison group.
Results
The study cohort comprised 448,803 participants, including 152,321 and 296,482 children and adolescents with concussion and orthopedic injury, respectively.
The incidence rates of any mental health problem were 11,141 per 100,000 person-years in the exposed group and 7,960 per 100,000 person-years in the unexposed group (difference, 3,181; 95% confidence interval, 3,073-3,291 per 100,000 person-years).
After concussion, the exposed group had a greater risk of developing a mental health issue (adjusted hazard ratio, 1.39; 95% CI, 1.37-1.40), psychiatric hospitalization (aHR, 1.47; 95% CI, 1.41-1.53), and self-harm (aHR, 1.49; 95% CI, 1.42-1.56). In addition, there was no significant difference in death by suicide between the exposed and unexposed groups (HR, 1.54; 95% CI, 0.90-2.61).
“Our results suggest that clinicians should assess for preexisting and new mental health symptoms throughout concussion recovery and treat mental health conditions or symptoms or refer the patient to a specialist in pediatric mental health,” wrote Dr. Ledoux and colleagues. “[Clinicians should also] assess suicidal ideation and self-harm behaviors during evaluation and follow-up visits for concussion.”
The researchers acknowledged that a key limitation of the study was the retrospective observational design. In addition, the identification of exposures using diagnostic billing codes could have introduced exposure or outcome misclassification.
Expert-recommended resources
“For more information, I’d recommend ‘Pedsconcussion,’ which are evidence-based living guidelines for pediatric concussion care,” Dr. Ledoux said in an interview. “Within domain 8, there are specific guidelines related to the management of mental health issues post concussion.”
Neuropsychology expert Talin Babikian, PhD, of the University of California, Los Angeles, commented: “Studies have shown that even a single psychoeducational session early after a concussion can minimize prolonged recoveries. Ensuring all stakeholders (family, clinicians, school, coach, peers) are on the same page and providing the same information is important to build trust and a sense of safety and agency.
“We want to provide psychoeducation early in the process to avoid unnecessary fear and avoidance. We also want to curtail misattribution of everyday symptoms or symptoms related to an unrelated condition to a brain injury, which are easier to do when caught early,” Dr. Babikian added.
This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care. One author reported financial relationships with the University of Ottawa, the National Football League, Parachute Canada, and 360 Concussion Care, an interdisciplinary concussion clinic; no other conflicts of interest were reported.
Among children and adolescents aged 5-18 years, concussion was associated with a higher risk of mental health problems, compared with age- and sex-matched children and adolescents with an orthopedic injury, according to a cohort study published in JAMA Network Open.
While concussions are one of the most common head injuries in the pediatric population, the extent to which they increase the risk of new onset psychiatric disorders or subsequent psychopathology is unclear, lead author Andrée-Anne Ledoux, PhD, of the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, and colleagues explained.
The researchers conducted a population-based retrospective cohort study to evaluate associations between concussion and risk of subsequent mental health issues, psychiatric hospitalizations, self-harm, or suicides in children and adolescents, with follow-up ranging from 1 month to 10 years.
The data were obtained from province-wide health administrative databases. Participants with concussion were included in an exposed group, while those with an orthopedic injury were included in a 1:2 age- and sex-matched comparison group.
Results
The study cohort comprised 448,803 participants, including 152,321 and 296,482 children and adolescents with concussion and orthopedic injury, respectively.
The incidence rates of any mental health problem were 11,141 per 100,000 person-years in the exposed group and 7,960 per 100,000 person-years in the unexposed group (difference, 3,181; 95% confidence interval, 3,073-3,291 per 100,000 person-years).
After concussion, the exposed group had a greater risk of developing a mental health issue (adjusted hazard ratio, 1.39; 95% CI, 1.37-1.40), psychiatric hospitalization (aHR, 1.47; 95% CI, 1.41-1.53), and self-harm (aHR, 1.49; 95% CI, 1.42-1.56). In addition, there was no significant difference in death by suicide between the exposed and unexposed groups (HR, 1.54; 95% CI, 0.90-2.61).
“Our results suggest that clinicians should assess for preexisting and new mental health symptoms throughout concussion recovery and treat mental health conditions or symptoms or refer the patient to a specialist in pediatric mental health,” wrote Dr. Ledoux and colleagues. “[Clinicians should also] assess suicidal ideation and self-harm behaviors during evaluation and follow-up visits for concussion.”
The researchers acknowledged that a key limitation of the study was the retrospective observational design. In addition, the identification of exposures using diagnostic billing codes could have introduced exposure or outcome misclassification.
Expert-recommended resources
“For more information, I’d recommend ‘Pedsconcussion,’ which are evidence-based living guidelines for pediatric concussion care,” Dr. Ledoux said in an interview. “Within domain 8, there are specific guidelines related to the management of mental health issues post concussion.”
Neuropsychology expert Talin Babikian, PhD, of the University of California, Los Angeles, commented: “Studies have shown that even a single psychoeducational session early after a concussion can minimize prolonged recoveries. Ensuring all stakeholders (family, clinicians, school, coach, peers) are on the same page and providing the same information is important to build trust and a sense of safety and agency.
“We want to provide psychoeducation early in the process to avoid unnecessary fear and avoidance. We also want to curtail misattribution of everyday symptoms or symptoms related to an unrelated condition to a brain injury, which are easier to do when caught early,” Dr. Babikian added.
This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care. One author reported financial relationships with the University of Ottawa, the National Football League, Parachute Canada, and 360 Concussion Care, an interdisciplinary concussion clinic; no other conflicts of interest were reported.
Among children and adolescents aged 5-18 years, concussion was associated with a higher risk of mental health problems, compared with age- and sex-matched children and adolescents with an orthopedic injury, according to a cohort study published in JAMA Network Open.
While concussions are one of the most common head injuries in the pediatric population, the extent to which they increase the risk of new onset psychiatric disorders or subsequent psychopathology is unclear, lead author Andrée-Anne Ledoux, PhD, of the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, and colleagues explained.
The researchers conducted a population-based retrospective cohort study to evaluate associations between concussion and risk of subsequent mental health issues, psychiatric hospitalizations, self-harm, or suicides in children and adolescents, with follow-up ranging from 1 month to 10 years.
The data were obtained from province-wide health administrative databases. Participants with concussion were included in an exposed group, while those with an orthopedic injury were included in a 1:2 age- and sex-matched comparison group.
Results
The study cohort comprised 448,803 participants, including 152,321 and 296,482 children and adolescents with concussion and orthopedic injury, respectively.
The incidence rates of any mental health problem were 11,141 per 100,000 person-years in the exposed group and 7,960 per 100,000 person-years in the unexposed group (difference, 3,181; 95% confidence interval, 3,073-3,291 per 100,000 person-years).
After concussion, the exposed group had a greater risk of developing a mental health issue (adjusted hazard ratio, 1.39; 95% CI, 1.37-1.40), psychiatric hospitalization (aHR, 1.47; 95% CI, 1.41-1.53), and self-harm (aHR, 1.49; 95% CI, 1.42-1.56). In addition, there was no significant difference in death by suicide between the exposed and unexposed groups (HR, 1.54; 95% CI, 0.90-2.61).
“Our results suggest that clinicians should assess for preexisting and new mental health symptoms throughout concussion recovery and treat mental health conditions or symptoms or refer the patient to a specialist in pediatric mental health,” wrote Dr. Ledoux and colleagues. “[Clinicians should also] assess suicidal ideation and self-harm behaviors during evaluation and follow-up visits for concussion.”
The researchers acknowledged that a key limitation of the study was the retrospective observational design. In addition, the identification of exposures using diagnostic billing codes could have introduced exposure or outcome misclassification.
Expert-recommended resources
“For more information, I’d recommend ‘Pedsconcussion,’ which are evidence-based living guidelines for pediatric concussion care,” Dr. Ledoux said in an interview. “Within domain 8, there are specific guidelines related to the management of mental health issues post concussion.”
Neuropsychology expert Talin Babikian, PhD, of the University of California, Los Angeles, commented: “Studies have shown that even a single psychoeducational session early after a concussion can minimize prolonged recoveries. Ensuring all stakeholders (family, clinicians, school, coach, peers) are on the same page and providing the same information is important to build trust and a sense of safety and agency.
“We want to provide psychoeducation early in the process to avoid unnecessary fear and avoidance. We also want to curtail misattribution of everyday symptoms or symptoms related to an unrelated condition to a brain injury, which are easier to do when caught early,” Dr. Babikian added.
This study was supported by the Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Long-term Care. One author reported financial relationships with the University of Ottawa, the National Football League, Parachute Canada, and 360 Concussion Care, an interdisciplinary concussion clinic; no other conflicts of interest were reported.
FROM JAMA NETWORK OPEN
Depression, suicidal ideation continue to plague physicians: Survey
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
Now, as they bear the weight of a multiyear pandemic alongside the perpetual struggle to maintain some semblance of work-life balance, their resiliency has been stretched to the brink.
In 2022, the Medscape Physician Suicide Report surveyed more than 13,000 physicians in 29 specialties who were candid about their experiences with suicidal thoughts, how they support their besieged colleagues, and their go-to coping strategies.
Overall, 21% of physicians reported having feelings of depression. Of those, 24% had clinical depression and 64% had colloquial depression. Physicians who felt sad or blue decreased slightly, compared with the 2021 report, but the number of physicians experiencing severe depression rose 4%.
One in 10 physicians said they have thought about or attempted suicide. However, the number of physicians with suicidal thoughts dropped to 9%, down substantially from the 22% who reported similar feelings in 2020.
Still, there was a slight uptick in women physicians contemplating suicide, likely linked to their larger share of childcare and family responsibilities.
“They have needed to pull double duty even more than usual, and that may have increased their sense of burnout and vulnerability to suicidal thoughts,” said Andrea Giedinghagen, MD, assistant professor in the department of psychiatry at Washington University in St. Louis, and coauthor of “Physician Suicide: A Call to Action
Fighting the stigma of seeking mental health help
Although the number of physicians attempting, but not completing suicide, has remained steady at 1% for several years, the recent passage of the Dr. Lorna Breen Health Care Provider Protection Act by Congress aims to drive that figure even lower. Dr. Breen, an ED physician at New York–Presbyterian Hospital, died by suicide in April 2020. Overwhelmed by the onslaught of COVID patients, Dr. Breen was reluctant to seek mental health services for fear of being ostracized.
“Many physicians don’t seek mental health care due to fear of negative consequences in the workplace, including retribution, exclusion, loss of license, or even their job,” Gary Price, MD, president of The Physicians Foundation, told this news organization. “This was the experience of Dr. Lorna Breen. She was convinced that if she talked to a professional, she would lose her medical license. Perhaps if Dr. Breen was equipped with the accurate information – there is no mental health reporting requirement in her state’s medical license application – it might have saved her life.”
This same stigma was reflected in the survey, with one physician saying: “I’m afraid that if I spoke to a therapist, I’d have to report receiving psychiatric treatment to credentialing or licensing boards.” Roughly 40% of survey respondents, regardless of age, chose not to disclose their suicidal thoughts to anyone, not even a family member or suicide hotline. And just a tiny portion of physicians (10% of men and 13% of women) said that a colleague had discussed their suicidal thoughts with them.
“There is a longstanding culture of silence around physician mental health in the medical community,” said Dr. Price. “The strategies within the Act are critical to fixing this culture and making it acceptable and normalized for physicians to seek mental health care,” and for it to “become a fundamental and ongoing element of being a practicing physician.”
As part of the legislation, the Department of Health & Human Services must award grants to hospitals, medical associations, and other entities to facilitate mental health programs for providers. They must also establish policy recommendations and conduct campaigns to improve providers’ mental and behavioral health, encourage providers to seek mental health support and assistance, remove barriers to such treatment, and identify best practices to prevent suicide and promote resiliency
Addressing barriers to mental health
The new bill is a step in the right direction, but Dr. Price said health organizations must do more to address the six key structural barriers that are “discouraging physicians from seeking [mental health] help,” such as the inclusion of “intrusive mental health questions on medical board, hospital credentialing, and malpractice insurance applications.”
In addition, employers should allow physicians to seek out-of-network mental health services, if necessary, and not cause further humiliation by requiring them to be treated by colleagues within their hospital system. A similar proposal has recently been introduced and is gaining traction in Utah, following the suicide of ED physician Scott Jolley, MD, in 2021 after he was admitted for psychiatric care where he worked.
Diminishing the stigma surrounding physicians’ mental health encourages a more open dialogue, so if a colleague reaches out – listen. “Start by thanking the colleague for sharing the information: ‘I’m sure that wasn’t easy but I appreciate that you respect me enough to share this. Let’s talk more,’ ” said Michael F. Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn . “Then ask what you can do to help, which cuts down on the sense of isolation that colleague may feel.”
According to the survey, many physicians have developed strategies to support their happiness and mental health. Although fewer than 10% said reducing work hours or transitioning to a part-time schedule was most effective, the majority of physicians relied on spending time with family and friends (68%) – a choice that has considerable benefits.
“Close and intimate relationships are the single most protective factor for our mental health,” said Peter Yellowlees, MBBS, MD, chief wellness officer for UC Davis Health and professor of psychiatry at the University of California, Davis. “Isolation and loneliness are very important stressors, and we know that about 25% of the population reports being lonely.”
A version of this article first appeared on Medscape.com.
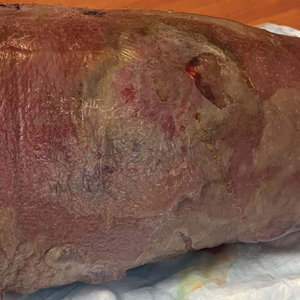
Rapidly Enlarging Bullous Plaque
The Diagnosis: Bullous Pyoderma Gangrenosum
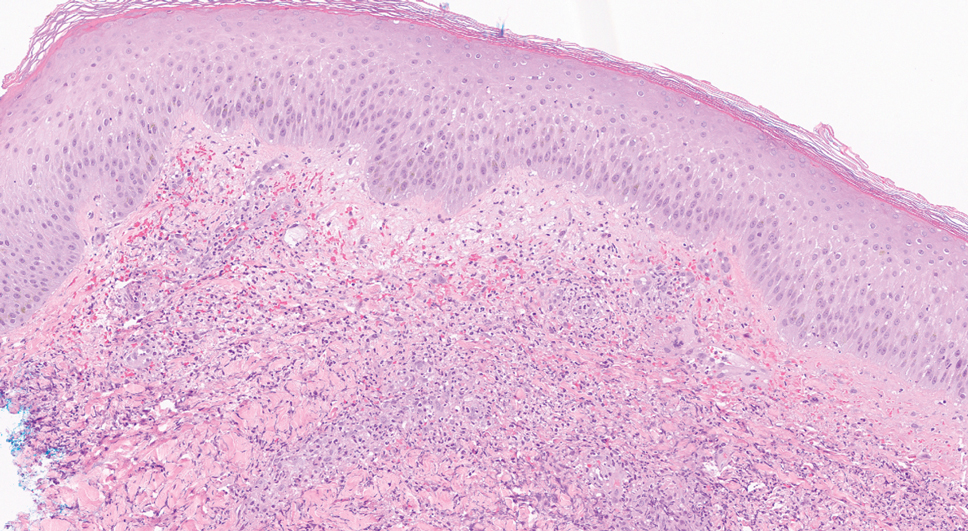
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.

First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.

First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
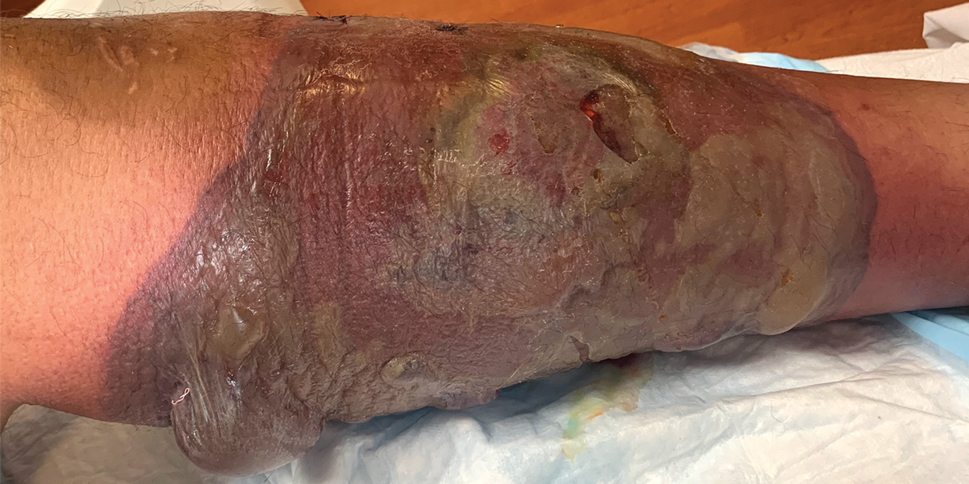
A 26-year-old previously healthy man presented to the emergency department with a new asymptomatic enlarging lesion on the lower leg that had appeared 4 days prior as a self-described “pimple” and rapidly evolved. The patient also reported chills, fatigue, and decreased appetite during that time. Physical examination revealed a red to violaceous, well-demarcated, bullous plaque involving much of the left lower leg. Laboratory studies demonstrated a hemoglobin level of 8.1 g/dL (reference range, 14.0–17.5 g/dL), hematocrit level of 23.7% (reference range, 41%–50%), platelet count of 26×103 /μL (reference range, 150–350×103 /μL), and a population of circulating blast cells and metamyelocytes.
Lawsuit: 18-inch sponge left in stomach for 5 years; migrates internally
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
ARBs and cancer risk: New meta-analysis raises questions again
The debate on whether the popular class of antihypertensive drugs, angiotensin receptor blockers (ARBs), may be associated with an increased risk for cancer has been reopened with the publication of a new meta-analysis.
The analysis found an increasing risk for cancer, and specifically lung cancer, with increasing cumulative exposure to these drugs.
The findings are reported in a study published online in PLOS ONE.
The author of this new meta-analysis is Ilke Sipahi, MD, a cardiologist from Acibadem University Medical School, Istanbul, who previously raised this issue in an initial meta-analysis published in 2010.
“The new meta-analysis is important because it is the first study to investigate whether there is a dose response in the association between ARBs and cancer,” Dr. Sipahi told this news organization.
“I found a clear signal of increased risk of cancer as exposure to ARBs increased, and the association started to become significant when the maximum dose was taken for 3 years,” he added.
Dr. Sipahi explained that in the first meta-analysis published in Lancet Oncology, he and his colleagues reported an increased cancer risk with ARBs based on observations from high-exposure trials – those that included higher doses of ARBs with a long duration of follow-up.
Following this publication, an investigation by the U.S. Food and Drug Administration refuted the risk, and a collaboration of ARB trial investigators also performed an analysis published in the Journal of Hypertension (2011. doi: 10.1097/HJH.0b013e328344a7de), which again did not show an increased risk for cancer with use of ARBs.
Dr. Sipahi claims that those analyses by the FDA and the ARB Trialists Collaboration, which were all trial-level meta-analyses, diluted the “high exposure” data (including higher doses taken for longer periods of time) with a large amount of other data on much lower exposures (lower doses and/or shorter time periods).
“The overall risk would then inevitably become nonsignificant. These analyses also did not look at different exposure levels,” he says.
“For cancer, the degree of exposure is obviously very important. The risk associated with smoking 2 or 3 cigarettes a day for a year is very different from that of smoking 2 packs a day for 40 years. The same principle applies to taking a medication,” Dr. Sipahi asserts.
From these latest data, he estimates that 120 patients needed to be treated with the maximal daily dose of an ARB for 4.7 years for one excess cancer diagnosis, and 464 patients needed to be treated for one excess lung cancer.
“Given that at least 200 million individuals are being treated with an ARB globally, approximately 1.7 million excess cancers (and 430,000 lung cancers) in 4.6 years could be potentially caused by this class of drugs,” he suggests.
For the current analysis, Dr. Sipahi used trial-level data taken from the paper by the ARB Trialists Collaboration and investigated the effect of exposure to ARBs – including both the dose taken and the length of treatment – on risk for cancer. He performed metaregression analyses that he says has not been done before.
“I mathematically quantitated the degree of exposure in each trial. And when the degree of exposure was correlated with risk of cancer, there was a significant association.”
The new meta-analysis includes 15 randomized controlled trials. The two coprimary outcomes were the relationship between cumulative exposure to ARBs and risk for all cancers combined and the relationship between cumulative exposure and risk for lung cancer.
In the trials, 74,021 patients were randomly assigned to an ARB, resulting in a total cumulative exposure of 172,389 person-years of exposure to daily high dose (or equivalent), and 61,197 patients were randomly assigned to control.
Results showed a highly significant correlation between the degree of cumulative exposure to ARBs and risk for all cancers combined (slope = 0.07; 95% confidence interval, 0.03-0.11; P < .001) and also lung cancer (slope = 0.16; 95% CI, 0.05-0.27; P = .003).
In trials where the cumulative exposure was greater than 3 years of exposure to daily high dose, there was a statistically significant increase in risk for all cancers combined (risk ratio, 1.11; 95% CI, 1.03-1.19; P = .006).
There was also a statistically significant increase in risk for lung cancers in trials where the cumulative exposure was greater than 2.5 years (RR, 1.21; 95% CI, 1.02-1.44; P = .03).
In trials with lower cumulative exposure to ARBs, there was no increased risk either for all cancers combined or lung cancer.
Dr. Sipahi reports that the cumulative exposure-risk relationship with ARBs was independent of background angiotensin-converting enzyme (ACE) inhibitor treatment or the type of control (placebo or nonplacebo control).
But he acknowledges that since this is a trial-level analysis, the effects of patient characteristics such as age and smoking status could not be examined because of lack of patient-level data.
Dr. Sipahi says he does not know the mechanism behind these findings, but he draws attention to the recent withdrawal of several thousand lots of ARB formulations because of the presence of potentially carcinogenic impurities that have been suggested to be a byproduct of ARB synthesis.
He also claims that unlike some other classes of antihypertensives, ARBs have not been shown to reduce the risk for MI, leading him to conclude that “other classes of antihypertensives with good safety and efficacy data (such as ACE-inhibitors, calcium-channel blockers or others) should become the preferred first-line agents in the treatment of hypertension.”
Dr. Sipahi wants the FDA to reinvestigate the issue of ARBs and cancer risk using individual patient data. “They already have the patient-level data from the trials. They should look at it more carefully and look at exposure levels and how they relate to cancer risk,” he said. “And the fact that there have been studies linking high ARB exposure levels to increased cancer risk should at least get a warning on the drug labels.”
A ‘clear increase’ in risk
Dr. Sipahi also points out that a link between ARBs and cancer has been found in another meta-analysis performed in 2013 by senior FDA analyst Thomas Marciniak, MD.
“Because he worked at the FDA, [Dr.] Marciniak had access to individual patent data. This is the best type of analysis and generally produces more accurate results than a trial-level meta-analysis,” Dr. Sipahi commented.
Dr. Marciniak’s analysis, which is available on the FDA website as part of another document, was not officially published elsewhere, and no further action has been taken on the issue.
Contacted by this news organization, Dr. Marciniak, who has now retired from the FDA, said he not only conducted a patient-level meta-analysis but also followed up adverse effects reported in the trials that could have been a symptom of cancer to establish further whether the patient was later diagnosed with cancer or not.
“I used every scrap of information sent in, including serious adverse event reports. I saw a clear increase in lung cancer risk with the ARBs,” Dr. Marciniak said. He did not, however, perform a dose-response relationship analysis.
Asked why his analysis and those from Dr. Sipahi reach different conclusions to those from the ARB Trialists Collaboration and the official FDA investigations, Dr. Marciniak said: “It may be that there were too many low-exposure trials that just washed out the difference. But trial data generally do not capture adverse events such as cancer, which takes a long time to develop, very well, and if you’re not really looking for it, you’re probably not going to find it.”
Dr. Marciniak said that Dr. Sipahi’s current findings are in line with his results. “Finding a dose response, to me, is extremely compelling, and I think the signal here is real,” he commented. “I think this new paper from Dr. Sipahi verifies what I found. I think the FDA should now release all individual patient data it has.”
Contacted for comment, an FDA spokesperson said, “Generally the FDA does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
They added: “The FDA has ongoing assessment, surveillance, compliance, and pharmaceutical quality efforts across every product area, and we will continue to work with drug manufacturers to ensure safe, effective, and high-quality drugs for the American public. When we identify new and previously unrecognized risks to safety and quality, we react swiftly to resolve the problem, as we have done in responding to the recent findings of nitrosamines in certain medicines.”
Analysis ‘should be taken seriously’
Commenting on this new study, Steve Nissen, MD, a key figure in analyzing such complex data and who has himself uncovered problems with high-profile drugs in the past, says the current analysis should be taken seriously.
Dr. Nissen, who was Dr. Sipahi’s senior during his post-doc position at the Cleveland Clinic, wrote an editorial accompanying Dr. Sipahi’s first paper and calling for urgent regulatory review of the evidence.
He says the new findings add to previous evidence suggesting a possible risk for cancer with ARBs.
“[Dr.] Sipahi is a capable researcher, and this analysis needs to be taken seriously, but it needs to be verified. It is not possible to draw a strong conclusion on this analysis, as it is not based on individual patient data, but I don’t think it should be ignored,” Dr. Nissen stated.
“I will say again what I said 12 years ago – that the regulatory agencies need to carefully review all their data in a very detailed way. The FDA and EMA have access to the individual patient data and are both very capable of doing the required analyses.”
Limitations of trial-level analysis
Asked to evaluate the statistics in the current paper, Andrew Althouse, PhD, an assistant professor of medicine at the University of Pittsburgh, and a clinical trial statistician, explained that the best way to do a thorough analysis of the relationship between ARB exposure and risk for incident cancer would involve the use of patient-level data.
“As such data were not available to Dr. Sipahi, I believe he is doing as well as he can. But without full access to individual patient-level data from the respective trials, it is difficult to support any firm conclusions,” Dr. Althouse said in an interview.
He suggested that the meta-regression analyses used in the paper were unable to properly estimate the relationship between ARB exposure and risk for incident cancer.
“Taken at face value, the current analysis suggests that [in] trials with longer follow-up duration (and therefore greater cumulative exposure to ARB for the treatment group), the risk of developing cancer for patients in the ARB group versus the non-ARB group was progressively higher. But this study doesn’t take into account the actual amount of follow-up time for individual patients or potential differences in the amount of follow-up time between the two groups in each trial,” he noted.
Dr. Althouse says this raises the possibility of “competing risks” or the idea that if ARBs reduce cardiovascular disease and cardiovascular death, then there would be more patients remaining in that arm who could go on to develop cancer. “So a crude count of the number of cancer cases may look as though patients receiving ARBs are ‘more likely’ to develop cancer, but this is a mirage.”
He added: “When there are some patients dying during the study, the only way to tell whether the intervention actually increased the risk of other health-related complications is to have an analysis that properly accounts for each patient’s time-at-risk of the outcome. Unfortunately, properly analyzing this requires the use of patient-level data.”
Cardiologists skeptical?
Cardiology experts asked for thoughts on the new meta-analysis were also cautious to read too much into the findings.
Franz Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “Perhaps one would simply ignore this rambling, cherrypicking-based condemnation of ARBs if it were not for the powerful negative connotation of the word cancer. Thus, the meta-analysis of Dr. Sipahi purporting that ARBs could be increasing the development of cancers in a cumulative way is of concern to both physicians and patients.”
But, raising a similar point to Dr. Althouse about competing risks, Dr. Messerli said: “We have to consider that as one gets older, the cardiovascular disease state and cancer state will compete with each other for the outcome of death. The better that therapies protect against cardiovascular death, the more they will increase life expectancy and thus the risk of cancer.”
He also added that “in head-to-head comparisons with ACE inhibitors, ARBs showed similar efficacy in terms of death, CV mortality, MI, stroke, and end-stage kidney disease, so can we agree that the attempt of Dr. Sipahi to disparage ARBs as a class is much ado about nothing?”
Dr. Nissen, however, said he views the idea of competing risk as “a bit of a stretch” in this case. “Although ARBs are effective antihypertensive drugs, I would say there is very little evidence that they would prolong survival versus other antihypertensives.”
Dr. Sipahi also claims that this argument is not relevant to the current analysis. “ARBs did not increase survival in any of the high-exposure trials that showed an excess in cancers. Therefore, competing outcomes, or ‘survival bias’ to be more specific, is not a possibility here,” he says.
George Bakris, MD, professor of medicine at the University of Chicago Medicine, noted that while the current study shows a slight increase in cancer incidence, especially lung cancer, among those taking ARBs for more than 3 years, it “totally ignores the overwhelming cardiovascular risk reduction seen in the trials.”
“Moreover,” he adds, “the author notes that the findings were independent of ACE-inhibitors, but he can’t rule out smoking and age as factors, two major risk factors for cancer and lung cancer, specifically. Thus, as typical of these types of analyses, the associations are probably true/true unrelated or, at best, partially related.”
Dr. Bakris referred to the potentially carcinogenic nitrosamine and azido compounds found in several ARB formulations that have resulted in recalls.
“At any stage of drug synthesis throughout each product’s lifetime, these impurities may evolve if an amine reacts with a nitrosating agent coexisting under appropriate conditions,” he said. “Drug regulatory authorities worldwide have established stringent guidelines on nitrosamine contamination for all drug products. The studies noted in the author’s analysis were done well before these guidelines were implemented. Hence, many of the issues raised by the authors using trials from 10-20 years ago are not of significant concern.”
Still, the cardiology experts all agreed on one thing – that patients should continue to take ARBs as prescribed.
Noting that worldwide authorities are now addressing the issue of possible carcinogen contamination, Dr. Bakris stressed that patients “should not panic and should not stop their meds.”
Dr. Nissen added: “What we don’t want is for patents who are taking ARBs to stop taking these medications – hypertension is a deadly disorder, and these drugs have proven cardiovascular benefits.”
Dr. Sipahi received no specific funding for this work. He reports receiving lecture honoraria from Novartis, Boehringer Ingelheim, Sanofi, Sandoz, Bristol-Myers Squibb, Bayer, Pfizer, Ranbaxy, Servier, and ARIS and served on advisory boards for Novartis, Sanofi, Servier, Bristol-Myers Squibb, Pfizer, Bayer and I.E. Ulagay. The other commenters do not report any relevant disclosures.
A version of this article first appeared on Medscape.com.
The debate on whether the popular class of antihypertensive drugs, angiotensin receptor blockers (ARBs), may be associated with an increased risk for cancer has been reopened with the publication of a new meta-analysis.
The analysis found an increasing risk for cancer, and specifically lung cancer, with increasing cumulative exposure to these drugs.
The findings are reported in a study published online in PLOS ONE.
The author of this new meta-analysis is Ilke Sipahi, MD, a cardiologist from Acibadem University Medical School, Istanbul, who previously raised this issue in an initial meta-analysis published in 2010.
“The new meta-analysis is important because it is the first study to investigate whether there is a dose response in the association between ARBs and cancer,” Dr. Sipahi told this news organization.
“I found a clear signal of increased risk of cancer as exposure to ARBs increased, and the association started to become significant when the maximum dose was taken for 3 years,” he added.
Dr. Sipahi explained that in the first meta-analysis published in Lancet Oncology, he and his colleagues reported an increased cancer risk with ARBs based on observations from high-exposure trials – those that included higher doses of ARBs with a long duration of follow-up.
Following this publication, an investigation by the U.S. Food and Drug Administration refuted the risk, and a collaboration of ARB trial investigators also performed an analysis published in the Journal of Hypertension (2011. doi: 10.1097/HJH.0b013e328344a7de), which again did not show an increased risk for cancer with use of ARBs.
Dr. Sipahi claims that those analyses by the FDA and the ARB Trialists Collaboration, which were all trial-level meta-analyses, diluted the “high exposure” data (including higher doses taken for longer periods of time) with a large amount of other data on much lower exposures (lower doses and/or shorter time periods).
“The overall risk would then inevitably become nonsignificant. These analyses also did not look at different exposure levels,” he says.
“For cancer, the degree of exposure is obviously very important. The risk associated with smoking 2 or 3 cigarettes a day for a year is very different from that of smoking 2 packs a day for 40 years. The same principle applies to taking a medication,” Dr. Sipahi asserts.
From these latest data, he estimates that 120 patients needed to be treated with the maximal daily dose of an ARB for 4.7 years for one excess cancer diagnosis, and 464 patients needed to be treated for one excess lung cancer.
“Given that at least 200 million individuals are being treated with an ARB globally, approximately 1.7 million excess cancers (and 430,000 lung cancers) in 4.6 years could be potentially caused by this class of drugs,” he suggests.
For the current analysis, Dr. Sipahi used trial-level data taken from the paper by the ARB Trialists Collaboration and investigated the effect of exposure to ARBs – including both the dose taken and the length of treatment – on risk for cancer. He performed metaregression analyses that he says has not been done before.
“I mathematically quantitated the degree of exposure in each trial. And when the degree of exposure was correlated with risk of cancer, there was a significant association.”
The new meta-analysis includes 15 randomized controlled trials. The two coprimary outcomes were the relationship between cumulative exposure to ARBs and risk for all cancers combined and the relationship between cumulative exposure and risk for lung cancer.
In the trials, 74,021 patients were randomly assigned to an ARB, resulting in a total cumulative exposure of 172,389 person-years of exposure to daily high dose (or equivalent), and 61,197 patients were randomly assigned to control.
Results showed a highly significant correlation between the degree of cumulative exposure to ARBs and risk for all cancers combined (slope = 0.07; 95% confidence interval, 0.03-0.11; P < .001) and also lung cancer (slope = 0.16; 95% CI, 0.05-0.27; P = .003).
In trials where the cumulative exposure was greater than 3 years of exposure to daily high dose, there was a statistically significant increase in risk for all cancers combined (risk ratio, 1.11; 95% CI, 1.03-1.19; P = .006).
There was also a statistically significant increase in risk for lung cancers in trials where the cumulative exposure was greater than 2.5 years (RR, 1.21; 95% CI, 1.02-1.44; P = .03).
In trials with lower cumulative exposure to ARBs, there was no increased risk either for all cancers combined or lung cancer.
Dr. Sipahi reports that the cumulative exposure-risk relationship with ARBs was independent of background angiotensin-converting enzyme (ACE) inhibitor treatment or the type of control (placebo or nonplacebo control).
But he acknowledges that since this is a trial-level analysis, the effects of patient characteristics such as age and smoking status could not be examined because of lack of patient-level data.
Dr. Sipahi says he does not know the mechanism behind these findings, but he draws attention to the recent withdrawal of several thousand lots of ARB formulations because of the presence of potentially carcinogenic impurities that have been suggested to be a byproduct of ARB synthesis.
He also claims that unlike some other classes of antihypertensives, ARBs have not been shown to reduce the risk for MI, leading him to conclude that “other classes of antihypertensives with good safety and efficacy data (such as ACE-inhibitors, calcium-channel blockers or others) should become the preferred first-line agents in the treatment of hypertension.”
Dr. Sipahi wants the FDA to reinvestigate the issue of ARBs and cancer risk using individual patient data. “They already have the patient-level data from the trials. They should look at it more carefully and look at exposure levels and how they relate to cancer risk,” he said. “And the fact that there have been studies linking high ARB exposure levels to increased cancer risk should at least get a warning on the drug labels.”
A ‘clear increase’ in risk
Dr. Sipahi also points out that a link between ARBs and cancer has been found in another meta-analysis performed in 2013 by senior FDA analyst Thomas Marciniak, MD.
“Because he worked at the FDA, [Dr.] Marciniak had access to individual patent data. This is the best type of analysis and generally produces more accurate results than a trial-level meta-analysis,” Dr. Sipahi commented.
Dr. Marciniak’s analysis, which is available on the FDA website as part of another document, was not officially published elsewhere, and no further action has been taken on the issue.
Contacted by this news organization, Dr. Marciniak, who has now retired from the FDA, said he not only conducted a patient-level meta-analysis but also followed up adverse effects reported in the trials that could have been a symptom of cancer to establish further whether the patient was later diagnosed with cancer or not.
“I used every scrap of information sent in, including serious adverse event reports. I saw a clear increase in lung cancer risk with the ARBs,” Dr. Marciniak said. He did not, however, perform a dose-response relationship analysis.
Asked why his analysis and those from Dr. Sipahi reach different conclusions to those from the ARB Trialists Collaboration and the official FDA investigations, Dr. Marciniak said: “It may be that there were too many low-exposure trials that just washed out the difference. But trial data generally do not capture adverse events such as cancer, which takes a long time to develop, very well, and if you’re not really looking for it, you’re probably not going to find it.”
Dr. Marciniak said that Dr. Sipahi’s current findings are in line with his results. “Finding a dose response, to me, is extremely compelling, and I think the signal here is real,” he commented. “I think this new paper from Dr. Sipahi verifies what I found. I think the FDA should now release all individual patient data it has.”
Contacted for comment, an FDA spokesperson said, “Generally the FDA does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
They added: “The FDA has ongoing assessment, surveillance, compliance, and pharmaceutical quality efforts across every product area, and we will continue to work with drug manufacturers to ensure safe, effective, and high-quality drugs for the American public. When we identify new and previously unrecognized risks to safety and quality, we react swiftly to resolve the problem, as we have done in responding to the recent findings of nitrosamines in certain medicines.”
Analysis ‘should be taken seriously’
Commenting on this new study, Steve Nissen, MD, a key figure in analyzing such complex data and who has himself uncovered problems with high-profile drugs in the past, says the current analysis should be taken seriously.
Dr. Nissen, who was Dr. Sipahi’s senior during his post-doc position at the Cleveland Clinic, wrote an editorial accompanying Dr. Sipahi’s first paper and calling for urgent regulatory review of the evidence.
He says the new findings add to previous evidence suggesting a possible risk for cancer with ARBs.
“[Dr.] Sipahi is a capable researcher, and this analysis needs to be taken seriously, but it needs to be verified. It is not possible to draw a strong conclusion on this analysis, as it is not based on individual patient data, but I don’t think it should be ignored,” Dr. Nissen stated.
“I will say again what I said 12 years ago – that the regulatory agencies need to carefully review all their data in a very detailed way. The FDA and EMA have access to the individual patient data and are both very capable of doing the required analyses.”
Limitations of trial-level analysis
Asked to evaluate the statistics in the current paper, Andrew Althouse, PhD, an assistant professor of medicine at the University of Pittsburgh, and a clinical trial statistician, explained that the best way to do a thorough analysis of the relationship between ARB exposure and risk for incident cancer would involve the use of patient-level data.
“As such data were not available to Dr. Sipahi, I believe he is doing as well as he can. But without full access to individual patient-level data from the respective trials, it is difficult to support any firm conclusions,” Dr. Althouse said in an interview.
He suggested that the meta-regression analyses used in the paper were unable to properly estimate the relationship between ARB exposure and risk for incident cancer.
“Taken at face value, the current analysis suggests that [in] trials with longer follow-up duration (and therefore greater cumulative exposure to ARB for the treatment group), the risk of developing cancer for patients in the ARB group versus the non-ARB group was progressively higher. But this study doesn’t take into account the actual amount of follow-up time for individual patients or potential differences in the amount of follow-up time between the two groups in each trial,” he noted.
Dr. Althouse says this raises the possibility of “competing risks” or the idea that if ARBs reduce cardiovascular disease and cardiovascular death, then there would be more patients remaining in that arm who could go on to develop cancer. “So a crude count of the number of cancer cases may look as though patients receiving ARBs are ‘more likely’ to develop cancer, but this is a mirage.”
He added: “When there are some patients dying during the study, the only way to tell whether the intervention actually increased the risk of other health-related complications is to have an analysis that properly accounts for each patient’s time-at-risk of the outcome. Unfortunately, properly analyzing this requires the use of patient-level data.”
Cardiologists skeptical?
Cardiology experts asked for thoughts on the new meta-analysis were also cautious to read too much into the findings.
Franz Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “Perhaps one would simply ignore this rambling, cherrypicking-based condemnation of ARBs if it were not for the powerful negative connotation of the word cancer. Thus, the meta-analysis of Dr. Sipahi purporting that ARBs could be increasing the development of cancers in a cumulative way is of concern to both physicians and patients.”
But, raising a similar point to Dr. Althouse about competing risks, Dr. Messerli said: “We have to consider that as one gets older, the cardiovascular disease state and cancer state will compete with each other for the outcome of death. The better that therapies protect against cardiovascular death, the more they will increase life expectancy and thus the risk of cancer.”
He also added that “in head-to-head comparisons with ACE inhibitors, ARBs showed similar efficacy in terms of death, CV mortality, MI, stroke, and end-stage kidney disease, so can we agree that the attempt of Dr. Sipahi to disparage ARBs as a class is much ado about nothing?”
Dr. Nissen, however, said he views the idea of competing risk as “a bit of a stretch” in this case. “Although ARBs are effective antihypertensive drugs, I would say there is very little evidence that they would prolong survival versus other antihypertensives.”
Dr. Sipahi also claims that this argument is not relevant to the current analysis. “ARBs did not increase survival in any of the high-exposure trials that showed an excess in cancers. Therefore, competing outcomes, or ‘survival bias’ to be more specific, is not a possibility here,” he says.
George Bakris, MD, professor of medicine at the University of Chicago Medicine, noted that while the current study shows a slight increase in cancer incidence, especially lung cancer, among those taking ARBs for more than 3 years, it “totally ignores the overwhelming cardiovascular risk reduction seen in the trials.”
“Moreover,” he adds, “the author notes that the findings were independent of ACE-inhibitors, but he can’t rule out smoking and age as factors, two major risk factors for cancer and lung cancer, specifically. Thus, as typical of these types of analyses, the associations are probably true/true unrelated or, at best, partially related.”
Dr. Bakris referred to the potentially carcinogenic nitrosamine and azido compounds found in several ARB formulations that have resulted in recalls.
“At any stage of drug synthesis throughout each product’s lifetime, these impurities may evolve if an amine reacts with a nitrosating agent coexisting under appropriate conditions,” he said. “Drug regulatory authorities worldwide have established stringent guidelines on nitrosamine contamination for all drug products. The studies noted in the author’s analysis were done well before these guidelines were implemented. Hence, many of the issues raised by the authors using trials from 10-20 years ago are not of significant concern.”
Still, the cardiology experts all agreed on one thing – that patients should continue to take ARBs as prescribed.
Noting that worldwide authorities are now addressing the issue of possible carcinogen contamination, Dr. Bakris stressed that patients “should not panic and should not stop their meds.”
Dr. Nissen added: “What we don’t want is for patents who are taking ARBs to stop taking these medications – hypertension is a deadly disorder, and these drugs have proven cardiovascular benefits.”
Dr. Sipahi received no specific funding for this work. He reports receiving lecture honoraria from Novartis, Boehringer Ingelheim, Sanofi, Sandoz, Bristol-Myers Squibb, Bayer, Pfizer, Ranbaxy, Servier, and ARIS and served on advisory boards for Novartis, Sanofi, Servier, Bristol-Myers Squibb, Pfizer, Bayer and I.E. Ulagay. The other commenters do not report any relevant disclosures.
A version of this article first appeared on Medscape.com.
The debate on whether the popular class of antihypertensive drugs, angiotensin receptor blockers (ARBs), may be associated with an increased risk for cancer has been reopened with the publication of a new meta-analysis.
The analysis found an increasing risk for cancer, and specifically lung cancer, with increasing cumulative exposure to these drugs.
The findings are reported in a study published online in PLOS ONE.
The author of this new meta-analysis is Ilke Sipahi, MD, a cardiologist from Acibadem University Medical School, Istanbul, who previously raised this issue in an initial meta-analysis published in 2010.
“The new meta-analysis is important because it is the first study to investigate whether there is a dose response in the association between ARBs and cancer,” Dr. Sipahi told this news organization.
“I found a clear signal of increased risk of cancer as exposure to ARBs increased, and the association started to become significant when the maximum dose was taken for 3 years,” he added.
Dr. Sipahi explained that in the first meta-analysis published in Lancet Oncology, he and his colleagues reported an increased cancer risk with ARBs based on observations from high-exposure trials – those that included higher doses of ARBs with a long duration of follow-up.
Following this publication, an investigation by the U.S. Food and Drug Administration refuted the risk, and a collaboration of ARB trial investigators also performed an analysis published in the Journal of Hypertension (2011. doi: 10.1097/HJH.0b013e328344a7de), which again did not show an increased risk for cancer with use of ARBs.
Dr. Sipahi claims that those analyses by the FDA and the ARB Trialists Collaboration, which were all trial-level meta-analyses, diluted the “high exposure” data (including higher doses taken for longer periods of time) with a large amount of other data on much lower exposures (lower doses and/or shorter time periods).
“The overall risk would then inevitably become nonsignificant. These analyses also did not look at different exposure levels,” he says.
“For cancer, the degree of exposure is obviously very important. The risk associated with smoking 2 or 3 cigarettes a day for a year is very different from that of smoking 2 packs a day for 40 years. The same principle applies to taking a medication,” Dr. Sipahi asserts.
From these latest data, he estimates that 120 patients needed to be treated with the maximal daily dose of an ARB for 4.7 years for one excess cancer diagnosis, and 464 patients needed to be treated for one excess lung cancer.
“Given that at least 200 million individuals are being treated with an ARB globally, approximately 1.7 million excess cancers (and 430,000 lung cancers) in 4.6 years could be potentially caused by this class of drugs,” he suggests.
For the current analysis, Dr. Sipahi used trial-level data taken from the paper by the ARB Trialists Collaboration and investigated the effect of exposure to ARBs – including both the dose taken and the length of treatment – on risk for cancer. He performed metaregression analyses that he says has not been done before.
“I mathematically quantitated the degree of exposure in each trial. And when the degree of exposure was correlated with risk of cancer, there was a significant association.”
The new meta-analysis includes 15 randomized controlled trials. The two coprimary outcomes were the relationship between cumulative exposure to ARBs and risk for all cancers combined and the relationship between cumulative exposure and risk for lung cancer.
In the trials, 74,021 patients were randomly assigned to an ARB, resulting in a total cumulative exposure of 172,389 person-years of exposure to daily high dose (or equivalent), and 61,197 patients were randomly assigned to control.
Results showed a highly significant correlation between the degree of cumulative exposure to ARBs and risk for all cancers combined (slope = 0.07; 95% confidence interval, 0.03-0.11; P < .001) and also lung cancer (slope = 0.16; 95% CI, 0.05-0.27; P = .003).
In trials where the cumulative exposure was greater than 3 years of exposure to daily high dose, there was a statistically significant increase in risk for all cancers combined (risk ratio, 1.11; 95% CI, 1.03-1.19; P = .006).
There was also a statistically significant increase in risk for lung cancers in trials where the cumulative exposure was greater than 2.5 years (RR, 1.21; 95% CI, 1.02-1.44; P = .03).
In trials with lower cumulative exposure to ARBs, there was no increased risk either for all cancers combined or lung cancer.
Dr. Sipahi reports that the cumulative exposure-risk relationship with ARBs was independent of background angiotensin-converting enzyme (ACE) inhibitor treatment or the type of control (placebo or nonplacebo control).
But he acknowledges that since this is a trial-level analysis, the effects of patient characteristics such as age and smoking status could not be examined because of lack of patient-level data.
Dr. Sipahi says he does not know the mechanism behind these findings, but he draws attention to the recent withdrawal of several thousand lots of ARB formulations because of the presence of potentially carcinogenic impurities that have been suggested to be a byproduct of ARB synthesis.
He also claims that unlike some other classes of antihypertensives, ARBs have not been shown to reduce the risk for MI, leading him to conclude that “other classes of antihypertensives with good safety and efficacy data (such as ACE-inhibitors, calcium-channel blockers or others) should become the preferred first-line agents in the treatment of hypertension.”
Dr. Sipahi wants the FDA to reinvestigate the issue of ARBs and cancer risk using individual patient data. “They already have the patient-level data from the trials. They should look at it more carefully and look at exposure levels and how they relate to cancer risk,” he said. “And the fact that there have been studies linking high ARB exposure levels to increased cancer risk should at least get a warning on the drug labels.”
A ‘clear increase’ in risk
Dr. Sipahi also points out that a link between ARBs and cancer has been found in another meta-analysis performed in 2013 by senior FDA analyst Thomas Marciniak, MD.
“Because he worked at the FDA, [Dr.] Marciniak had access to individual patent data. This is the best type of analysis and generally produces more accurate results than a trial-level meta-analysis,” Dr. Sipahi commented.
Dr. Marciniak’s analysis, which is available on the FDA website as part of another document, was not officially published elsewhere, and no further action has been taken on the issue.
Contacted by this news organization, Dr. Marciniak, who has now retired from the FDA, said he not only conducted a patient-level meta-analysis but also followed up adverse effects reported in the trials that could have been a symptom of cancer to establish further whether the patient was later diagnosed with cancer or not.
“I used every scrap of information sent in, including serious adverse event reports. I saw a clear increase in lung cancer risk with the ARBs,” Dr. Marciniak said. He did not, however, perform a dose-response relationship analysis.
Asked why his analysis and those from Dr. Sipahi reach different conclusions to those from the ARB Trialists Collaboration and the official FDA investigations, Dr. Marciniak said: “It may be that there were too many low-exposure trials that just washed out the difference. But trial data generally do not capture adverse events such as cancer, which takes a long time to develop, very well, and if you’re not really looking for it, you’re probably not going to find it.”
Dr. Marciniak said that Dr. Sipahi’s current findings are in line with his results. “Finding a dose response, to me, is extremely compelling, and I think the signal here is real,” he commented. “I think this new paper from Dr. Sipahi verifies what I found. I think the FDA should now release all individual patient data it has.”
Contacted for comment, an FDA spokesperson said, “Generally the FDA does not comment on specific studies but evaluates them as part of the body of evidence to further our understanding about a particular issue and assist in our mission to protect public health.”
They added: “The FDA has ongoing assessment, surveillance, compliance, and pharmaceutical quality efforts across every product area, and we will continue to work with drug manufacturers to ensure safe, effective, and high-quality drugs for the American public. When we identify new and previously unrecognized risks to safety and quality, we react swiftly to resolve the problem, as we have done in responding to the recent findings of nitrosamines in certain medicines.”
Analysis ‘should be taken seriously’
Commenting on this new study, Steve Nissen, MD, a key figure in analyzing such complex data and who has himself uncovered problems with high-profile drugs in the past, says the current analysis should be taken seriously.
Dr. Nissen, who was Dr. Sipahi’s senior during his post-doc position at the Cleveland Clinic, wrote an editorial accompanying Dr. Sipahi’s first paper and calling for urgent regulatory review of the evidence.
He says the new findings add to previous evidence suggesting a possible risk for cancer with ARBs.
“[Dr.] Sipahi is a capable researcher, and this analysis needs to be taken seriously, but it needs to be verified. It is not possible to draw a strong conclusion on this analysis, as it is not based on individual patient data, but I don’t think it should be ignored,” Dr. Nissen stated.
“I will say again what I said 12 years ago – that the regulatory agencies need to carefully review all their data in a very detailed way. The FDA and EMA have access to the individual patient data and are both very capable of doing the required analyses.”
Limitations of trial-level analysis
Asked to evaluate the statistics in the current paper, Andrew Althouse, PhD, an assistant professor of medicine at the University of Pittsburgh, and a clinical trial statistician, explained that the best way to do a thorough analysis of the relationship between ARB exposure and risk for incident cancer would involve the use of patient-level data.
“As such data were not available to Dr. Sipahi, I believe he is doing as well as he can. But without full access to individual patient-level data from the respective trials, it is difficult to support any firm conclusions,” Dr. Althouse said in an interview.
He suggested that the meta-regression analyses used in the paper were unable to properly estimate the relationship between ARB exposure and risk for incident cancer.
“Taken at face value, the current analysis suggests that [in] trials with longer follow-up duration (and therefore greater cumulative exposure to ARB for the treatment group), the risk of developing cancer for patients in the ARB group versus the non-ARB group was progressively higher. But this study doesn’t take into account the actual amount of follow-up time for individual patients or potential differences in the amount of follow-up time between the two groups in each trial,” he noted.
Dr. Althouse says this raises the possibility of “competing risks” or the idea that if ARBs reduce cardiovascular disease and cardiovascular death, then there would be more patients remaining in that arm who could go on to develop cancer. “So a crude count of the number of cancer cases may look as though patients receiving ARBs are ‘more likely’ to develop cancer, but this is a mirage.”
He added: “When there are some patients dying during the study, the only way to tell whether the intervention actually increased the risk of other health-related complications is to have an analysis that properly accounts for each patient’s time-at-risk of the outcome. Unfortunately, properly analyzing this requires the use of patient-level data.”
Cardiologists skeptical?
Cardiology experts asked for thoughts on the new meta-analysis were also cautious to read too much into the findings.
Franz Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “Perhaps one would simply ignore this rambling, cherrypicking-based condemnation of ARBs if it were not for the powerful negative connotation of the word cancer. Thus, the meta-analysis of Dr. Sipahi purporting that ARBs could be increasing the development of cancers in a cumulative way is of concern to both physicians and patients.”
But, raising a similar point to Dr. Althouse about competing risks, Dr. Messerli said: “We have to consider that as one gets older, the cardiovascular disease state and cancer state will compete with each other for the outcome of death. The better that therapies protect against cardiovascular death, the more they will increase life expectancy and thus the risk of cancer.”
He also added that “in head-to-head comparisons with ACE inhibitors, ARBs showed similar efficacy in terms of death, CV mortality, MI, stroke, and end-stage kidney disease, so can we agree that the attempt of Dr. Sipahi to disparage ARBs as a class is much ado about nothing?”
Dr. Nissen, however, said he views the idea of competing risk as “a bit of a stretch” in this case. “Although ARBs are effective antihypertensive drugs, I would say there is very little evidence that they would prolong survival versus other antihypertensives.”
Dr. Sipahi also claims that this argument is not relevant to the current analysis. “ARBs did not increase survival in any of the high-exposure trials that showed an excess in cancers. Therefore, competing outcomes, or ‘survival bias’ to be more specific, is not a possibility here,” he says.
George Bakris, MD, professor of medicine at the University of Chicago Medicine, noted that while the current study shows a slight increase in cancer incidence, especially lung cancer, among those taking ARBs for more than 3 years, it “totally ignores the overwhelming cardiovascular risk reduction seen in the trials.”
“Moreover,” he adds, “the author notes that the findings were independent of ACE-inhibitors, but he can’t rule out smoking and age as factors, two major risk factors for cancer and lung cancer, specifically. Thus, as typical of these types of analyses, the associations are probably true/true unrelated or, at best, partially related.”
Dr. Bakris referred to the potentially carcinogenic nitrosamine and azido compounds found in several ARB formulations that have resulted in recalls.
“At any stage of drug synthesis throughout each product’s lifetime, these impurities may evolve if an amine reacts with a nitrosating agent coexisting under appropriate conditions,” he said. “Drug regulatory authorities worldwide have established stringent guidelines on nitrosamine contamination for all drug products. The studies noted in the author’s analysis were done well before these guidelines were implemented. Hence, many of the issues raised by the authors using trials from 10-20 years ago are not of significant concern.”
Still, the cardiology experts all agreed on one thing – that patients should continue to take ARBs as prescribed.
Noting that worldwide authorities are now addressing the issue of possible carcinogen contamination, Dr. Bakris stressed that patients “should not panic and should not stop their meds.”
Dr. Nissen added: “What we don’t want is for patents who are taking ARBs to stop taking these medications – hypertension is a deadly disorder, and these drugs have proven cardiovascular benefits.”
Dr. Sipahi received no specific funding for this work. He reports receiving lecture honoraria from Novartis, Boehringer Ingelheim, Sanofi, Sandoz, Bristol-Myers Squibb, Bayer, Pfizer, Ranbaxy, Servier, and ARIS and served on advisory boards for Novartis, Sanofi, Servier, Bristol-Myers Squibb, Pfizer, Bayer and I.E. Ulagay. The other commenters do not report any relevant disclosures.
A version of this article first appeared on Medscape.com.
Geriatric guideline implementation remains unrealistic in most EDs
Many emergency departments are currently unable to provide care for geriatric patients that meets best practices and guidelines recommended by several major medical organizations, but a panel discussion in 2021 at the American Academy of Emergency Medicine’s Scientific Assembly identified three areas in which realistic improvements might be achieved.
In an article published online in the Journal of Emergency Medicine, Richard D. Shih, MD, of Florida Atlantic University, Boca Raton, and colleagues synthesized the presentation and discussion of an expert panel on the topic of the GED guidelines and the current realities of patient care.
The Geriatric Emergency Department (GED) Guidelines, published in 2014 in Annals of Emergency Medicine, were endorsed by the American College of Emergency Physicians, American Geriatrics Society, Emergency Nurses Association, and Society for Academic Emergency Medicine.
“With the substantial challenges in providing guideline-recommended care in EDs, this article will explore three high-impact GED clinical conditions to highlight guideline recommendations, challenges, and opportunities, and discuss realistically achievable expectations for non–GED-accredited institutions,” the authors wrote.
Geriatric patients and delirium
When delirium in older adults is not identified in the ED, the patient’s 6-month mortality rate significantly increases, but few EDs have delirium screening protocols, the authors said. Challenges included the time and money needed to educate staff, on top of multiple mandatory training requirements on other topics. Delirium screening in the clinical setting also requires personnel to conduct assessments, and time to document symptoms and screening results in medical records.
“Perhaps the highest priority challenge for delirium experts is to evaluate and publish effective delirium intervention strategies because current evidence is completely lacking for ED-based delirium prevention or treatment,” they said. In the meantime, developing outcome measures for quality improvement of delirium care will require institutional support as well as education.
Geriatric patients and falls
Approximately one third of community-dwelling adults older than 65 years suffer falls, but data suggest that fewer than half of these individuals report falls to their doctors. “Older adults who present to an ED after a fall have an approximately 30% greater risk of functional decline and depression at 6 months after the event,” the authors noted.
The GED guidelines call for a comprehensive approach to evaluating and managing falls in older adults, but many of these “are untested in the ED,” the authors said. The recommended protocol includes an initial assessment of fall risk, followed by, for those at low risk, tailored recommendations for education and the use of community resources. Additional recommendations for those at high risk of falls include multifactorial assessment of modifiable risk factors, including peripheral neuropathy, balance/gait assessment, and medication review.
However, this best practice workflow is beyond the resource capacity of most EDs, the authors noted. “When ED resources are insufficient to support best practices, the care should focus on educating patients and caregivers about the significance of a fall event, providing educational materials (e.g., [the Centers for Disease Control and Prevention’s] STEADI materials), and assessing safety with respect to mobility for immediate return to the home environment and follow-up with a PCP.”
Geriatric patients and polypharmacy
Polypharmacy is common among older adults by virtue of their greater number of illnesses and comorbid conditions, and polypharmacy also has been associated with more adverse drug reactions, the authors said. The AGS Beers Criteria identifies medications associated with adverse drug reactions, but it is not practical for use in a busy ED setting. Instead, the authors suggested a more practical approach of focusing on a smaller list of common medications that tend to cause the adverse events that may result in ED visits.
“Perhaps targeting patients on multiple (three or more) psychoactive medications, drugs that can cause hypotension, or hypoglycemics could not only be done quickly, but identify patients in whom deprescribing should be considered in the ED,” the authors wrote. Deprescribing is a complicated process, however, and may be more effective when done via the patient’s primary care provider or in a geriatric consultation.
The GED Guidelines highlighted the specific needs of the geriatric population in the ED, the authors said. Widespread implementation remains a challenge, but many organizations provide resources to help improve care of geriatric patients in the ED and beyond.
In particular, the Geriatric Emergency Care Applied Research Network and Geriatric Emergency Department Collaborative provide funding opportunities, updated and focused published reviews, and webinars (some including free continuing medical education) for the entire health care team, including hospital administrators, the authors said.
Article brings attention to clinical realities
“The reality is that the overwhelming majority of emergency departments in the United States, if not globally, are simply not equipped – operationally or financially – to meet the rigorous standards that are required to fulfill the goals of operating an accredited geriatric ED,” Robert D. Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, said in an interview.
“Drawing attention to this important gap in accreditation is critical to not only inform hospitals, health care providers and stakeholders, but the public, patients, and their families about the important work that needs to be done to better equip all EDs with the proper tools and educational approaches to more effectively care for the geriatric community,” Dr. Glatter emphasized.
“There are currently three tiers of accreditation, with level 1 being the highest,” he explained, but there are only 100 geriatric ED accreditation-certified hospitals across the United States.
“I am not surprised at all by the challenges of implementing current GED guidelines,” said Dr. Glatter. “It comes down to operational and budget considerations, which ultimately compete with many other departments and regulatory constraints in any given hospital.”
However, “the bottom line is that such guidelines are designed with patient safety in mind, making them important issues in the eyes of any hospital administrator looking to improve outcomes and reduce medicolegal risk or exposure impacting geriatric patients in the emergency department,” he noted.
Ultimately, guideline adherence “comes down to budget decisions, and where hospitals must invest their money to meet the bottom line,” said Dr. Glatter. “Making modifications to hospital infrastructure and architecture to accommodate geriatric patients may not be the top priority of hospital administrators when confronted with multiple competing interests. But, if it impacts patient safety, the decision to invest in structural and operational improvements may certainly have additional and important considerations.
“Until Medicare, or even the Joint Commission on Accreditation of Hospitals, adopts geriatric guidelines in emergency departments as a requirement for accreditation, there may not be adequate incentives in place currently to satisfy the intent of having a rigorous set of guidelines in the first place,” Dr. Glatter added.
Despite the limitations of applying the current guidelines, there are some steps hospitals can take, said Dr. Glatter. “They can institute new measures in a graded fashion, with the goal of taking the important steps to satisfy at least some components of the guidelines. Attention to details can go a long way, such as rails in bathrooms, better lighting, and treads on floors that may reduce the risk of falls in the ED itself.
“Attention to fall prevention by assessing contributors including polypharmacy, gait instability, and quality of footwear can impact risk of future ED visits. Having incentives in place by Medicare or JACO may force the hand of hospital administrators to comply with geriatric guidelines and place emphasis on compliance,” noted Dr. Glatter.
More research is needed that “looks at costs of implementing geriatric guidelines in typical community and academic EDs and how this impacts key metrics such as length of stay, effect on reimbursement per ICD-10 code, and savings, if any, realized in reduced malpractice claims related to missed diagnoses (such as delirium), injuries, (patient falls), or medical misadventures due to polypharmacy,” he said.
The article received no outside funding. The authors disclosed no relevant financial relationships. Dr. Glatter disclosed no relevant financial relationships, and serves on the advisory board of Medscape Emergency Medicine.
A version of this article first appeared on Medscape.com.
Many emergency departments are currently unable to provide care for geriatric patients that meets best practices and guidelines recommended by several major medical organizations, but a panel discussion in 2021 at the American Academy of Emergency Medicine’s Scientific Assembly identified three areas in which realistic improvements might be achieved.
In an article published online in the Journal of Emergency Medicine, Richard D. Shih, MD, of Florida Atlantic University, Boca Raton, and colleagues synthesized the presentation and discussion of an expert panel on the topic of the GED guidelines and the current realities of patient care.
The Geriatric Emergency Department (GED) Guidelines, published in 2014 in Annals of Emergency Medicine, were endorsed by the American College of Emergency Physicians, American Geriatrics Society, Emergency Nurses Association, and Society for Academic Emergency Medicine.
“With the substantial challenges in providing guideline-recommended care in EDs, this article will explore three high-impact GED clinical conditions to highlight guideline recommendations, challenges, and opportunities, and discuss realistically achievable expectations for non–GED-accredited institutions,” the authors wrote.
Geriatric patients and delirium
When delirium in older adults is not identified in the ED, the patient’s 6-month mortality rate significantly increases, but few EDs have delirium screening protocols, the authors said. Challenges included the time and money needed to educate staff, on top of multiple mandatory training requirements on other topics. Delirium screening in the clinical setting also requires personnel to conduct assessments, and time to document symptoms and screening results in medical records.
“Perhaps the highest priority challenge for delirium experts is to evaluate and publish effective delirium intervention strategies because current evidence is completely lacking for ED-based delirium prevention or treatment,” they said. In the meantime, developing outcome measures for quality improvement of delirium care will require institutional support as well as education.
Geriatric patients and falls
Approximately one third of community-dwelling adults older than 65 years suffer falls, but data suggest that fewer than half of these individuals report falls to their doctors. “Older adults who present to an ED after a fall have an approximately 30% greater risk of functional decline and depression at 6 months after the event,” the authors noted.
The GED guidelines call for a comprehensive approach to evaluating and managing falls in older adults, but many of these “are untested in the ED,” the authors said. The recommended protocol includes an initial assessment of fall risk, followed by, for those at low risk, tailored recommendations for education and the use of community resources. Additional recommendations for those at high risk of falls include multifactorial assessment of modifiable risk factors, including peripheral neuropathy, balance/gait assessment, and medication review.
However, this best practice workflow is beyond the resource capacity of most EDs, the authors noted. “When ED resources are insufficient to support best practices, the care should focus on educating patients and caregivers about the significance of a fall event, providing educational materials (e.g., [the Centers for Disease Control and Prevention’s] STEADI materials), and assessing safety with respect to mobility for immediate return to the home environment and follow-up with a PCP.”
Geriatric patients and polypharmacy
Polypharmacy is common among older adults by virtue of their greater number of illnesses and comorbid conditions, and polypharmacy also has been associated with more adverse drug reactions, the authors said. The AGS Beers Criteria identifies medications associated with adverse drug reactions, but it is not practical for use in a busy ED setting. Instead, the authors suggested a more practical approach of focusing on a smaller list of common medications that tend to cause the adverse events that may result in ED visits.
“Perhaps targeting patients on multiple (three or more) psychoactive medications, drugs that can cause hypotension, or hypoglycemics could not only be done quickly, but identify patients in whom deprescribing should be considered in the ED,” the authors wrote. Deprescribing is a complicated process, however, and may be more effective when done via the patient’s primary care provider or in a geriatric consultation.
The GED Guidelines highlighted the specific needs of the geriatric population in the ED, the authors said. Widespread implementation remains a challenge, but many organizations provide resources to help improve care of geriatric patients in the ED and beyond.
In particular, the Geriatric Emergency Care Applied Research Network and Geriatric Emergency Department Collaborative provide funding opportunities, updated and focused published reviews, and webinars (some including free continuing medical education) for the entire health care team, including hospital administrators, the authors said.
Article brings attention to clinical realities
“The reality is that the overwhelming majority of emergency departments in the United States, if not globally, are simply not equipped – operationally or financially – to meet the rigorous standards that are required to fulfill the goals of operating an accredited geriatric ED,” Robert D. Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, said in an interview.
“Drawing attention to this important gap in accreditation is critical to not only inform hospitals, health care providers and stakeholders, but the public, patients, and their families about the important work that needs to be done to better equip all EDs with the proper tools and educational approaches to more effectively care for the geriatric community,” Dr. Glatter emphasized.
“There are currently three tiers of accreditation, with level 1 being the highest,” he explained, but there are only 100 geriatric ED accreditation-certified hospitals across the United States.
“I am not surprised at all by the challenges of implementing current GED guidelines,” said Dr. Glatter. “It comes down to operational and budget considerations, which ultimately compete with many other departments and regulatory constraints in any given hospital.”
However, “the bottom line is that such guidelines are designed with patient safety in mind, making them important issues in the eyes of any hospital administrator looking to improve outcomes and reduce medicolegal risk or exposure impacting geriatric patients in the emergency department,” he noted.
Ultimately, guideline adherence “comes down to budget decisions, and where hospitals must invest their money to meet the bottom line,” said Dr. Glatter. “Making modifications to hospital infrastructure and architecture to accommodate geriatric patients may not be the top priority of hospital administrators when confronted with multiple competing interests. But, if it impacts patient safety, the decision to invest in structural and operational improvements may certainly have additional and important considerations.
“Until Medicare, or even the Joint Commission on Accreditation of Hospitals, adopts geriatric guidelines in emergency departments as a requirement for accreditation, there may not be adequate incentives in place currently to satisfy the intent of having a rigorous set of guidelines in the first place,” Dr. Glatter added.
Despite the limitations of applying the current guidelines, there are some steps hospitals can take, said Dr. Glatter. “They can institute new measures in a graded fashion, with the goal of taking the important steps to satisfy at least some components of the guidelines. Attention to details can go a long way, such as rails in bathrooms, better lighting, and treads on floors that may reduce the risk of falls in the ED itself.
“Attention to fall prevention by assessing contributors including polypharmacy, gait instability, and quality of footwear can impact risk of future ED visits. Having incentives in place by Medicare or JACO may force the hand of hospital administrators to comply with geriatric guidelines and place emphasis on compliance,” noted Dr. Glatter.
More research is needed that “looks at costs of implementing geriatric guidelines in typical community and academic EDs and how this impacts key metrics such as length of stay, effect on reimbursement per ICD-10 code, and savings, if any, realized in reduced malpractice claims related to missed diagnoses (such as delirium), injuries, (patient falls), or medical misadventures due to polypharmacy,” he said.
The article received no outside funding. The authors disclosed no relevant financial relationships. Dr. Glatter disclosed no relevant financial relationships, and serves on the advisory board of Medscape Emergency Medicine.
A version of this article first appeared on Medscape.com.
Many emergency departments are currently unable to provide care for geriatric patients that meets best practices and guidelines recommended by several major medical organizations, but a panel discussion in 2021 at the American Academy of Emergency Medicine’s Scientific Assembly identified three areas in which realistic improvements might be achieved.
In an article published online in the Journal of Emergency Medicine, Richard D. Shih, MD, of Florida Atlantic University, Boca Raton, and colleagues synthesized the presentation and discussion of an expert panel on the topic of the GED guidelines and the current realities of patient care.
The Geriatric Emergency Department (GED) Guidelines, published in 2014 in Annals of Emergency Medicine, were endorsed by the American College of Emergency Physicians, American Geriatrics Society, Emergency Nurses Association, and Society for Academic Emergency Medicine.
“With the substantial challenges in providing guideline-recommended care in EDs, this article will explore three high-impact GED clinical conditions to highlight guideline recommendations, challenges, and opportunities, and discuss realistically achievable expectations for non–GED-accredited institutions,” the authors wrote.
Geriatric patients and delirium
When delirium in older adults is not identified in the ED, the patient’s 6-month mortality rate significantly increases, but few EDs have delirium screening protocols, the authors said. Challenges included the time and money needed to educate staff, on top of multiple mandatory training requirements on other topics. Delirium screening in the clinical setting also requires personnel to conduct assessments, and time to document symptoms and screening results in medical records.
“Perhaps the highest priority challenge for delirium experts is to evaluate and publish effective delirium intervention strategies because current evidence is completely lacking for ED-based delirium prevention or treatment,” they said. In the meantime, developing outcome measures for quality improvement of delirium care will require institutional support as well as education.
Geriatric patients and falls
Approximately one third of community-dwelling adults older than 65 years suffer falls, but data suggest that fewer than half of these individuals report falls to their doctors. “Older adults who present to an ED after a fall have an approximately 30% greater risk of functional decline and depression at 6 months after the event,” the authors noted.
The GED guidelines call for a comprehensive approach to evaluating and managing falls in older adults, but many of these “are untested in the ED,” the authors said. The recommended protocol includes an initial assessment of fall risk, followed by, for those at low risk, tailored recommendations for education and the use of community resources. Additional recommendations for those at high risk of falls include multifactorial assessment of modifiable risk factors, including peripheral neuropathy, balance/gait assessment, and medication review.
However, this best practice workflow is beyond the resource capacity of most EDs, the authors noted. “When ED resources are insufficient to support best practices, the care should focus on educating patients and caregivers about the significance of a fall event, providing educational materials (e.g., [the Centers for Disease Control and Prevention’s] STEADI materials), and assessing safety with respect to mobility for immediate return to the home environment and follow-up with a PCP.”
Geriatric patients and polypharmacy
Polypharmacy is common among older adults by virtue of their greater number of illnesses and comorbid conditions, and polypharmacy also has been associated with more adverse drug reactions, the authors said. The AGS Beers Criteria identifies medications associated with adverse drug reactions, but it is not practical for use in a busy ED setting. Instead, the authors suggested a more practical approach of focusing on a smaller list of common medications that tend to cause the adverse events that may result in ED visits.
“Perhaps targeting patients on multiple (three or more) psychoactive medications, drugs that can cause hypotension, or hypoglycemics could not only be done quickly, but identify patients in whom deprescribing should be considered in the ED,” the authors wrote. Deprescribing is a complicated process, however, and may be more effective when done via the patient’s primary care provider or in a geriatric consultation.
The GED Guidelines highlighted the specific needs of the geriatric population in the ED, the authors said. Widespread implementation remains a challenge, but many organizations provide resources to help improve care of geriatric patients in the ED and beyond.
In particular, the Geriatric Emergency Care Applied Research Network and Geriatric Emergency Department Collaborative provide funding opportunities, updated and focused published reviews, and webinars (some including free continuing medical education) for the entire health care team, including hospital administrators, the authors said.
Article brings attention to clinical realities
“The reality is that the overwhelming majority of emergency departments in the United States, if not globally, are simply not equipped – operationally or financially – to meet the rigorous standards that are required to fulfill the goals of operating an accredited geriatric ED,” Robert D. Glatter, MD, an emergency medicine physician at Lenox Hill Hospital, New York, said in an interview.
“Drawing attention to this important gap in accreditation is critical to not only inform hospitals, health care providers and stakeholders, but the public, patients, and their families about the important work that needs to be done to better equip all EDs with the proper tools and educational approaches to more effectively care for the geriatric community,” Dr. Glatter emphasized.
“There are currently three tiers of accreditation, with level 1 being the highest,” he explained, but there are only 100 geriatric ED accreditation-certified hospitals across the United States.
“I am not surprised at all by the challenges of implementing current GED guidelines,” said Dr. Glatter. “It comes down to operational and budget considerations, which ultimately compete with many other departments and regulatory constraints in any given hospital.”
However, “the bottom line is that such guidelines are designed with patient safety in mind, making them important issues in the eyes of any hospital administrator looking to improve outcomes and reduce medicolegal risk or exposure impacting geriatric patients in the emergency department,” he noted.
Ultimately, guideline adherence “comes down to budget decisions, and where hospitals must invest their money to meet the bottom line,” said Dr. Glatter. “Making modifications to hospital infrastructure and architecture to accommodate geriatric patients may not be the top priority of hospital administrators when confronted with multiple competing interests. But, if it impacts patient safety, the decision to invest in structural and operational improvements may certainly have additional and important considerations.
“Until Medicare, or even the Joint Commission on Accreditation of Hospitals, adopts geriatric guidelines in emergency departments as a requirement for accreditation, there may not be adequate incentives in place currently to satisfy the intent of having a rigorous set of guidelines in the first place,” Dr. Glatter added.
Despite the limitations of applying the current guidelines, there are some steps hospitals can take, said Dr. Glatter. “They can institute new measures in a graded fashion, with the goal of taking the important steps to satisfy at least some components of the guidelines. Attention to details can go a long way, such as rails in bathrooms, better lighting, and treads on floors that may reduce the risk of falls in the ED itself.
“Attention to fall prevention by assessing contributors including polypharmacy, gait instability, and quality of footwear can impact risk of future ED visits. Having incentives in place by Medicare or JACO may force the hand of hospital administrators to comply with geriatric guidelines and place emphasis on compliance,” noted Dr. Glatter.
More research is needed that “looks at costs of implementing geriatric guidelines in typical community and academic EDs and how this impacts key metrics such as length of stay, effect on reimbursement per ICD-10 code, and savings, if any, realized in reduced malpractice claims related to missed diagnoses (such as delirium), injuries, (patient falls), or medical misadventures due to polypharmacy,” he said.
The article received no outside funding. The authors disclosed no relevant financial relationships. Dr. Glatter disclosed no relevant financial relationships, and serves on the advisory board of Medscape Emergency Medicine.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF EMERGENCY MEDICINE
Long COVID patients may develop nerve damage: Study
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
according to a new study published in the journal Neurology: Neuroimmunology & Neuroinflammation (doi: 10.1212/NXI.0000000000001146).
The nerve damage, which has been seen even among mild coronavirus cases, appears to be caused by immunity problems triggered by infection.
“This is one of the early papers looking into causes of long COVID, which will steadily increase in importance as acute COVID wanes,” Anne Louise Oaklander, MD, the lead study author and a neurologist at Massachusetts General Hospital, Boston, said in a statement.
“Our findings suggest that some long COVID patients had damage to their peripheral nerve fibers and that damage to the small-fiber type of nerve cell may be prominent,” she said.
The research team analyzed data from 17 COVID-19 survivors with lingering symptoms who had no history or risks of neuropathy, or nerve damage or disease. The patients were from 10 states and territories, and all but one had mild infections.
They found that 10 patients – or 59% – had at least one test that confirmed neuropathy. Two patients had rare neuropathies that affected muscle nerves, and 10 were diagnosed with small-fiber neuropathy, which is a cause of chronic pain. Common symptoms included fatigue, weakness, changes in their senses, and pain in their hands and feet.
For treatment, 11 patients were given immunotherapies such as corticosteroids or intravenous immunoglobulins, and the five patients who received repeated IgG treatments appeared to benefit. Over time, 52% of patients improved, though none had all of their symptoms go away.
“Research from our team and others is clarifying what the different types of post-COVID neuropathy are and how best to diagnose and treat them,” she said. “Most long COVID neuropathies described so far appear to reflect immune responses to the virus that went off course.”
Dr. Oaklander noted that researchers haven’t been able to do clinical trials to evaluate specific post-COVID neuropathy treatments. But some existing treatments may help.
“Some patients seem to improve from standard treatments for other immune-related neuropathies,” she said.
A version of this article first appeared on WebMD.com.
FROM NEUROLOGY: NEUROIMMUNOLOGY & NEUROINFLAMMATION
Cardiac arrest survival lower in COVID-19 inpatients
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
FROM JAMA NETWORK OPEN