User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
Diabetes hospitalizations halved with FreeStyle Libre glucose monitor
among patients with diabetes, data from two new studies indicate.
The results were presented June 13 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
One large database analysis, from France, revealed that use of the Libre system halved hospitalization rates for DKA among people with type 1 or type 2 diabetes.
In the other study, a retrospective analysis of data from over 1200 insulin-treated individuals with type 2 diabetes in the United States, use of the Libre was associated with significant reductions in both hospitalizations for acute diabetes-related emergency events and all-cause hospitalizations.
The Libre system reads glucose levels through a sensor worn on the back of the upper arm for up to 14 days. Users wave a scanner over the device to obtain a reading.
Asked to comment, Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, told Medscape Medical News: “One of the biggest problems with access to continuous glucose monitoring is cost. Payers need to see that there’s some cost-saving to offset the cost of paying for these devices. I think both of these studies are important for that reason.”
However, Argento also said he recommends that people with type 1 diabetes use the Dexcom continuous glucose monitor (CGM) if possible rather than the Libre, despite the former’s higher cost, because it has an alarm feature that the Libre doesn’t and is more accurate in the hypoglycemic range.
Large French study: Libre cuts DKA hospitalizations by 50%
The FreeStyle Libre system has been reimbursed in France since June 1, 2017 for patients over 4 years of age with type 1 or type 2 diabetes who take at least 3 insulin injections per day or use an insulin pump.
The new results were presented by Ronan Roussel, MD, PhD, chief of the endocrinology, diabetes, and nutrition department at Hôpital Bichat, Fédération de Diabétologie, AP-HP, Paris, France.
The DKA hospitalization data Roussel reported were part of a larger longitudinal retrospective cohort study looking at overall prescribing and use of the Libre system, and its impact on healthcare outcomes and associated costs in standard practice in France. The data came from a large nationwide claims database containing all healthcare expenses for over 66 million people.
The current study participants were 74,076 individuals with at least a full year of follow-up beginning in 2017 with the date of first reimbursement for the FreeStyle Libre system. Of those, 44.8% (33,203) had type 1 diabetes and 55.2% (40,955) had type 2 diabetes.
Prior to initiation of Libre use, about a quarter of each group was using 0 fingerstick test strips per day, about 19% of the type 1 diabetes group and 28% of the type 2 diabetes group were using 1-3 strips per day, and about half of both groups were using 4 or more strips per day.
Compared with the year prior to the date of first reimbursement for the Libre, hospitalization rates for DKA during the first year of Libre use fell by 52% in the type 1 diabetes group, from 5.46 to 2.59 per 100 patient-years, and by 47% in the type 2 diabetes group, from 1.70 to 0.90 per 100 patient-years.
The impact of Libre on DKA hospitalizations was most dramatic among those not using any test strips prior to Libre use, with a 60% reduction for the type 1 diabetes group (8.31 to 3.31 per 100 patient-years) and a 51% reduction in the type 2 diabetes group (2.51 to 1.23 per 100 patient-years).
But interestingly, the next-biggest impact was among those who had been using more than 5 test strips per day, with drops of 59% among those with type 1 diabetes (5.55 to 2.26 per 100 patient-years) and 52% in the type 2 diabetes group (1.88 to 0.90 per 100 patient-years).
This finding is important for the United States, Argento said, because some insurers, including Medicare, require that the patient performs at least 4 fingerstick glucose measurements per day to qualify for reimbursement for the Libre or any CGM system.
“I think that speaks to the importance of not requiring that patients first show they’re frequently doing self-blood glucose monitoring before they can get these devices,” he observed.
The large benefit in the high strip use group is interesting too, Argento said. “It’s a different group of people. They’re more engaged in their care...This U-shaped curve they showed is fascinating.”
Reductions in DKA hospitalizations were also similar between patients using insulin pumps and those using multiple daily injections of insulin, Roussel reported.
“It is plausible that use of the FreeStyle Libre system allowed people to detect and limit persistent hyperglycemia, and subsequently ketoacidosis,” Roussel said.
“This analysis has significant implications for patient-centered clinical care in diabetes and also for long-term health economic outcomes in the treatment of diabetes at a national level.”
All-cause hospitalizations drop 30% with Libre in type 2 diabetes
Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, presented the US results, obtained from the IBM Watson Health MarketScan, a database of commercial and Medicare supplemental insurance claims for over 30 million Americans.
The study population included 2463 patients with type 2 diabetes using basal-bolus daily insulin injections but who had not previously used Libre or any other CGM, and for whom data were available 6 months prior to and after Libre initiation.
Compared with 6 months prior to Libre use, the number of acute diabetes-related events — including hyperglycemia, hypoglycemia, DKA, hypoglycemic coma, and hyperosmolarity — in the subsequent 6 months dropped by 60%, from 0.180 to 0.072 events per patient-year (P < .001).
Similarly significant reductions were seen between males and females, and among those aged ≥ 50 years or < 50 years.
All-cause hospitalizations also significantly dropped by 33% (P < 0.001), from 0.420 to 0.283 events per patient-year. Among diagnostic codes for the hospitalizations, circulatory system causes remained number one during both time periods, with little change from pre-Libre to during Libre use.
However, “endocrine, nutritional, and metabolism system” codes dropped from the second position pre-Libre (6.4 events/100 patient-years) down to the fifth position (2.6 events/100 patient-years).
And, Bergenstal noted, other major diagnostic categories that also dropped included respiratory (3.5 to 2.1 events/100 patient-years), kidney and urinary tract (3.3 to 1.7 events/100 patient-years), and hepatobiliary system and pancreas (2.4 to 1.4 events/100 patient-years).
“We’re seeing a resurgence of certain types of complications, but all of these were reduced in the 6 months after Libre,” Bergenstal pointed out.
And, pertinent to the current COVID-19 situation, “infectious and parasitic disease and disorders” dropped as well, from 4.8 to 2.8 per 100 patient-years.
Argento commented: “The fact that infections went down speaks to something that is important right now. Hyperglycemia impairs immune function chronically, but also acutely...so patients who become ill and their blood glucose deteriorates rapidly are much more likely to have a poor outcome regardless of infection. There are data for COVID-19 now.”
“These findings provide compelling support for use of [Libre] to improve both clinical outcomes and potentially reduce costs in this patient population,” Bergenstal concluded.
Roussel has reported being on advisory panels for Abbott, AstraZeneca, Diabnext, Eli Lilly, Merck, Mundipharma International, Novo Nordisk, and Sanofi-Aventis. Bergenstal has reported being a consultant for Ascensia Diabetes Care, Johnson & Johnson, and has other relationships with Abbott, Dexcom, Hygieia, Lilly Diabetes, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Sanofi, and UnitedHealth Group. Argento has reported consulting and being on speaker bureaus for Omnipod, Eli Lilly, Novo Nordisk, Dexcom, and Boehringer Ingelheim.
This article first appeared on Medscape.com.
among patients with diabetes, data from two new studies indicate.
The results were presented June 13 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
One large database analysis, from France, revealed that use of the Libre system halved hospitalization rates for DKA among people with type 1 or type 2 diabetes.
In the other study, a retrospective analysis of data from over 1200 insulin-treated individuals with type 2 diabetes in the United States, use of the Libre was associated with significant reductions in both hospitalizations for acute diabetes-related emergency events and all-cause hospitalizations.
The Libre system reads glucose levels through a sensor worn on the back of the upper arm for up to 14 days. Users wave a scanner over the device to obtain a reading.
Asked to comment, Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, told Medscape Medical News: “One of the biggest problems with access to continuous glucose monitoring is cost. Payers need to see that there’s some cost-saving to offset the cost of paying for these devices. I think both of these studies are important for that reason.”
However, Argento also said he recommends that people with type 1 diabetes use the Dexcom continuous glucose monitor (CGM) if possible rather than the Libre, despite the former’s higher cost, because it has an alarm feature that the Libre doesn’t and is more accurate in the hypoglycemic range.
Large French study: Libre cuts DKA hospitalizations by 50%
The FreeStyle Libre system has been reimbursed in France since June 1, 2017 for patients over 4 years of age with type 1 or type 2 diabetes who take at least 3 insulin injections per day or use an insulin pump.
The new results were presented by Ronan Roussel, MD, PhD, chief of the endocrinology, diabetes, and nutrition department at Hôpital Bichat, Fédération de Diabétologie, AP-HP, Paris, France.
The DKA hospitalization data Roussel reported were part of a larger longitudinal retrospective cohort study looking at overall prescribing and use of the Libre system, and its impact on healthcare outcomes and associated costs in standard practice in France. The data came from a large nationwide claims database containing all healthcare expenses for over 66 million people.
The current study participants were 74,076 individuals with at least a full year of follow-up beginning in 2017 with the date of first reimbursement for the FreeStyle Libre system. Of those, 44.8% (33,203) had type 1 diabetes and 55.2% (40,955) had type 2 diabetes.
Prior to initiation of Libre use, about a quarter of each group was using 0 fingerstick test strips per day, about 19% of the type 1 diabetes group and 28% of the type 2 diabetes group were using 1-3 strips per day, and about half of both groups were using 4 or more strips per day.
Compared with the year prior to the date of first reimbursement for the Libre, hospitalization rates for DKA during the first year of Libre use fell by 52% in the type 1 diabetes group, from 5.46 to 2.59 per 100 patient-years, and by 47% in the type 2 diabetes group, from 1.70 to 0.90 per 100 patient-years.
The impact of Libre on DKA hospitalizations was most dramatic among those not using any test strips prior to Libre use, with a 60% reduction for the type 1 diabetes group (8.31 to 3.31 per 100 patient-years) and a 51% reduction in the type 2 diabetes group (2.51 to 1.23 per 100 patient-years).
But interestingly, the next-biggest impact was among those who had been using more than 5 test strips per day, with drops of 59% among those with type 1 diabetes (5.55 to 2.26 per 100 patient-years) and 52% in the type 2 diabetes group (1.88 to 0.90 per 100 patient-years).
This finding is important for the United States, Argento said, because some insurers, including Medicare, require that the patient performs at least 4 fingerstick glucose measurements per day to qualify for reimbursement for the Libre or any CGM system.
“I think that speaks to the importance of not requiring that patients first show they’re frequently doing self-blood glucose monitoring before they can get these devices,” he observed.
The large benefit in the high strip use group is interesting too, Argento said. “It’s a different group of people. They’re more engaged in their care...This U-shaped curve they showed is fascinating.”
Reductions in DKA hospitalizations were also similar between patients using insulin pumps and those using multiple daily injections of insulin, Roussel reported.
“It is plausible that use of the FreeStyle Libre system allowed people to detect and limit persistent hyperglycemia, and subsequently ketoacidosis,” Roussel said.
“This analysis has significant implications for patient-centered clinical care in diabetes and also for long-term health economic outcomes in the treatment of diabetes at a national level.”
All-cause hospitalizations drop 30% with Libre in type 2 diabetes
Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, presented the US results, obtained from the IBM Watson Health MarketScan, a database of commercial and Medicare supplemental insurance claims for over 30 million Americans.
The study population included 2463 patients with type 2 diabetes using basal-bolus daily insulin injections but who had not previously used Libre or any other CGM, and for whom data were available 6 months prior to and after Libre initiation.
Compared with 6 months prior to Libre use, the number of acute diabetes-related events — including hyperglycemia, hypoglycemia, DKA, hypoglycemic coma, and hyperosmolarity — in the subsequent 6 months dropped by 60%, from 0.180 to 0.072 events per patient-year (P < .001).
Similarly significant reductions were seen between males and females, and among those aged ≥ 50 years or < 50 years.
All-cause hospitalizations also significantly dropped by 33% (P < 0.001), from 0.420 to 0.283 events per patient-year. Among diagnostic codes for the hospitalizations, circulatory system causes remained number one during both time periods, with little change from pre-Libre to during Libre use.
However, “endocrine, nutritional, and metabolism system” codes dropped from the second position pre-Libre (6.4 events/100 patient-years) down to the fifth position (2.6 events/100 patient-years).
And, Bergenstal noted, other major diagnostic categories that also dropped included respiratory (3.5 to 2.1 events/100 patient-years), kidney and urinary tract (3.3 to 1.7 events/100 patient-years), and hepatobiliary system and pancreas (2.4 to 1.4 events/100 patient-years).
“We’re seeing a resurgence of certain types of complications, but all of these were reduced in the 6 months after Libre,” Bergenstal pointed out.
And, pertinent to the current COVID-19 situation, “infectious and parasitic disease and disorders” dropped as well, from 4.8 to 2.8 per 100 patient-years.
Argento commented: “The fact that infections went down speaks to something that is important right now. Hyperglycemia impairs immune function chronically, but also acutely...so patients who become ill and their blood glucose deteriorates rapidly are much more likely to have a poor outcome regardless of infection. There are data for COVID-19 now.”
“These findings provide compelling support for use of [Libre] to improve both clinical outcomes and potentially reduce costs in this patient population,” Bergenstal concluded.
Roussel has reported being on advisory panels for Abbott, AstraZeneca, Diabnext, Eli Lilly, Merck, Mundipharma International, Novo Nordisk, and Sanofi-Aventis. Bergenstal has reported being a consultant for Ascensia Diabetes Care, Johnson & Johnson, and has other relationships with Abbott, Dexcom, Hygieia, Lilly Diabetes, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Sanofi, and UnitedHealth Group. Argento has reported consulting and being on speaker bureaus for Omnipod, Eli Lilly, Novo Nordisk, Dexcom, and Boehringer Ingelheim.
This article first appeared on Medscape.com.
among patients with diabetes, data from two new studies indicate.
The results were presented June 13 during the virtual American Diabetes Association (ADA) 80th Scientific Sessions.
One large database analysis, from France, revealed that use of the Libre system halved hospitalization rates for DKA among people with type 1 or type 2 diabetes.
In the other study, a retrospective analysis of data from over 1200 insulin-treated individuals with type 2 diabetes in the United States, use of the Libre was associated with significant reductions in both hospitalizations for acute diabetes-related emergency events and all-cause hospitalizations.
The Libre system reads glucose levels through a sensor worn on the back of the upper arm for up to 14 days. Users wave a scanner over the device to obtain a reading.
Asked to comment, Nicholas Argento, MD, diabetes technology director at Maryland Endocrine and Diabetes, Columbia, told Medscape Medical News: “One of the biggest problems with access to continuous glucose monitoring is cost. Payers need to see that there’s some cost-saving to offset the cost of paying for these devices. I think both of these studies are important for that reason.”
However, Argento also said he recommends that people with type 1 diabetes use the Dexcom continuous glucose monitor (CGM) if possible rather than the Libre, despite the former’s higher cost, because it has an alarm feature that the Libre doesn’t and is more accurate in the hypoglycemic range.
Large French study: Libre cuts DKA hospitalizations by 50%
The FreeStyle Libre system has been reimbursed in France since June 1, 2017 for patients over 4 years of age with type 1 or type 2 diabetes who take at least 3 insulin injections per day or use an insulin pump.
The new results were presented by Ronan Roussel, MD, PhD, chief of the endocrinology, diabetes, and nutrition department at Hôpital Bichat, Fédération de Diabétologie, AP-HP, Paris, France.
The DKA hospitalization data Roussel reported were part of a larger longitudinal retrospective cohort study looking at overall prescribing and use of the Libre system, and its impact on healthcare outcomes and associated costs in standard practice in France. The data came from a large nationwide claims database containing all healthcare expenses for over 66 million people.
The current study participants were 74,076 individuals with at least a full year of follow-up beginning in 2017 with the date of first reimbursement for the FreeStyle Libre system. Of those, 44.8% (33,203) had type 1 diabetes and 55.2% (40,955) had type 2 diabetes.
Prior to initiation of Libre use, about a quarter of each group was using 0 fingerstick test strips per day, about 19% of the type 1 diabetes group and 28% of the type 2 diabetes group were using 1-3 strips per day, and about half of both groups were using 4 or more strips per day.
Compared with the year prior to the date of first reimbursement for the Libre, hospitalization rates for DKA during the first year of Libre use fell by 52% in the type 1 diabetes group, from 5.46 to 2.59 per 100 patient-years, and by 47% in the type 2 diabetes group, from 1.70 to 0.90 per 100 patient-years.
The impact of Libre on DKA hospitalizations was most dramatic among those not using any test strips prior to Libre use, with a 60% reduction for the type 1 diabetes group (8.31 to 3.31 per 100 patient-years) and a 51% reduction in the type 2 diabetes group (2.51 to 1.23 per 100 patient-years).
But interestingly, the next-biggest impact was among those who had been using more than 5 test strips per day, with drops of 59% among those with type 1 diabetes (5.55 to 2.26 per 100 patient-years) and 52% in the type 2 diabetes group (1.88 to 0.90 per 100 patient-years).
This finding is important for the United States, Argento said, because some insurers, including Medicare, require that the patient performs at least 4 fingerstick glucose measurements per day to qualify for reimbursement for the Libre or any CGM system.
“I think that speaks to the importance of not requiring that patients first show they’re frequently doing self-blood glucose monitoring before they can get these devices,” he observed.
The large benefit in the high strip use group is interesting too, Argento said. “It’s a different group of people. They’re more engaged in their care...This U-shaped curve they showed is fascinating.”
Reductions in DKA hospitalizations were also similar between patients using insulin pumps and those using multiple daily injections of insulin, Roussel reported.
“It is plausible that use of the FreeStyle Libre system allowed people to detect and limit persistent hyperglycemia, and subsequently ketoacidosis,” Roussel said.
“This analysis has significant implications for patient-centered clinical care in diabetes and also for long-term health economic outcomes in the treatment of diabetes at a national level.”
All-cause hospitalizations drop 30% with Libre in type 2 diabetes
Richard M. Bergenstal, MD, executive director of the International Diabetes Center at Park Nicollet, Minneapolis, Minnesota, presented the US results, obtained from the IBM Watson Health MarketScan, a database of commercial and Medicare supplemental insurance claims for over 30 million Americans.
The study population included 2463 patients with type 2 diabetes using basal-bolus daily insulin injections but who had not previously used Libre or any other CGM, and for whom data were available 6 months prior to and after Libre initiation.
Compared with 6 months prior to Libre use, the number of acute diabetes-related events — including hyperglycemia, hypoglycemia, DKA, hypoglycemic coma, and hyperosmolarity — in the subsequent 6 months dropped by 60%, from 0.180 to 0.072 events per patient-year (P < .001).
Similarly significant reductions were seen between males and females, and among those aged ≥ 50 years or < 50 years.
All-cause hospitalizations also significantly dropped by 33% (P < 0.001), from 0.420 to 0.283 events per patient-year. Among diagnostic codes for the hospitalizations, circulatory system causes remained number one during both time periods, with little change from pre-Libre to during Libre use.
However, “endocrine, nutritional, and metabolism system” codes dropped from the second position pre-Libre (6.4 events/100 patient-years) down to the fifth position (2.6 events/100 patient-years).
And, Bergenstal noted, other major diagnostic categories that also dropped included respiratory (3.5 to 2.1 events/100 patient-years), kidney and urinary tract (3.3 to 1.7 events/100 patient-years), and hepatobiliary system and pancreas (2.4 to 1.4 events/100 patient-years).
“We’re seeing a resurgence of certain types of complications, but all of these were reduced in the 6 months after Libre,” Bergenstal pointed out.
And, pertinent to the current COVID-19 situation, “infectious and parasitic disease and disorders” dropped as well, from 4.8 to 2.8 per 100 patient-years.
Argento commented: “The fact that infections went down speaks to something that is important right now. Hyperglycemia impairs immune function chronically, but also acutely...so patients who become ill and their blood glucose deteriorates rapidly are much more likely to have a poor outcome regardless of infection. There are data for COVID-19 now.”
“These findings provide compelling support for use of [Libre] to improve both clinical outcomes and potentially reduce costs in this patient population,” Bergenstal concluded.
Roussel has reported being on advisory panels for Abbott, AstraZeneca, Diabnext, Eli Lilly, Merck, Mundipharma International, Novo Nordisk, and Sanofi-Aventis. Bergenstal has reported being a consultant for Ascensia Diabetes Care, Johnson & Johnson, and has other relationships with Abbott, Dexcom, Hygieia, Lilly Diabetes, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Sanofi, and UnitedHealth Group. Argento has reported consulting and being on speaker bureaus for Omnipod, Eli Lilly, Novo Nordisk, Dexcom, and Boehringer Ingelheim.
This article first appeared on Medscape.com.
FROM ADA 2020
Half of young adults with diabetes have diastolic dysfunction
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
Roughly half of adolescents and young adults with either type 1 or type 2 diabetes for about a decade had diastolic dysfunction, a direct precursor to heart failure, in a multicenter echocardiography survey of 479 American patients.
Using tissue Doppler echocardiography findings from 258 adolescents and young adults with type 1 diabetes, and 221 with type 2 diabetes, the study found at least one imaging marker of ventricular stiffness – diastolic dysfunction – in 58% of the patients with type 2 diabetes and in 47% of those with type 1 diabetes. The type 1 patients averaged 21 years of age with a median 12 years of diagnosed disease, while the type 2 patients had an average age of 25 years and a median 11 years disease duration.
The analysis also identified several measures that significantly linked with the presence of diastolic dysfunction: older age, female sex, nonwhite race, type 2 diabetes, higher heart rate, higher body mass index, higher systolic blood pressure, and higher hemoglobin A1c.
“Our data suggest targeting modifiable risk factors” in these patients in an effort to slow the process causing the diastolic dysfunction, Amy S. Shah, MD, said at the virtual annual scientific sessions of the American Diabetes Association. She particularly cited interventions aimed at reducing body mass index, lowering blood pressure, and improving glycemic control, as well as preventing type 2 diabetes in the first place.
Prevention of type 2 diabetes, as well as prevention of diastolic dysfunction development and progression, are key steps because of the substantial clinical consequences of diastolic dysfunction, triggered by stiffening of the left ventricle. Diastolic dysfunction leads to increased left ventricular diastolic pressure, left atrial dysfunction, and ultimately heart failure with preserved ejection fraction, a common diabetes complication that currently has no treatment with proven efficacy, said Dr. Shah, a pediatric endocrinologist and director of the Adolescent Type 2 Diabetes Program at Cincinnati Children’s Hospital Medical Center.
“It’s very concerning that diastolic dysfunction is so prevalent in this age group,” commented Robert A. Gabbay, MD, Chief Science & Medical Officer of the American Diabetes Association. “An important question is whether you can see an improvement by reversing risk factors.” He noted the importance of confirming the finding in additional cohorts as well as running prospective studies looking at the impact of risk factor modification.
Dr. Shah and her associates used data collected at four U.S. centers from patients enrolled in the SEARCH for Diabetes in Youth study who underwent a tissue Doppler examination during 2016-2019, and used three measures derived from the scans to identify diastolic dysfunction:
- The E/A ratio, which compares the early flow wave across the mitral valve (E) with the atrial flow wave (A) that occurs after atrial contraction. Lower values reflect worse pathology.
- The E/e’ ratio, which compares the early flow wave across the mitral valve (E) with the rate of cardiac wall relaxation in early diastole (e’). Higher values reflect worse pathology.
- The e’/a’ ratio, which compares the rate of cardiac wall relaxation in early diastole (e’) with the rate of cardiac wall relaxation in late diastole (a’). Lower values reflect worse pathology.
The most common abnormality involved the e’/a’ measure, which occurred in roughly 38% of the patients with type 2 diabetes and in about 23% of those with type 1 diabetes. Next most common was an abnormally high E/e’ ratio, and fewer than 10% of patients had an abnormally low E/A ratio. Both the E/A and E/e’ values were significantly worse among patients with type 2 diabetes compared with type 1 patients, while no statistically significant difference separated the two subgroups for prevalence of an e’/a’ abnormality after adjustment for body mass index, blood pressure, and HbA1c values.
Average body mass index among the 221 studied patients with type 2 diabetes was 38 kg/m2, 74% were girls or women, and 57% were non-Hispanic black and 24% non-Hispanic white. Mean blood pressure among the patients with type 2 diabetes was 123/80 mm Hg, while it was 110/72 mm Hg among the 258 patients with type 1 diabetes.
SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
SOURCE: Shah AS et al. ADA 2020 abstract 58-OR.
FROM ADA 2020
Key clinical point: .
Major finding: Tissue Doppler echocardiography detected diastolic dysfunction in 58% of patients with type 2 diabetes and 47% of type 1 patients.
Study details: SEARCH for Diabetes in Youth study, with 479 American adolescents and young adults with diabetes.
Disclosures: SEARCH for Diabetes in Youth receives no commercial funding. Dr. Shah had no disclosures.
Source: Shah AS et al. ADA 2020, Abstract 58-OR.
EMPA-REG OUTCOME: Empagliflozin cut insulin need in type 2 diabetes
Patients with type 2 diabetes treated with the SGLT2 inhibitor empagliflozin during the landmark EMPA-REG OUTCOME trial had a solidly reduced need to either start insulin treatment or intensify existing insulin treatment, compared with those given placebo, in a post-hoc analysis of the study’s findings.
“Empagliflozin markedly and durably delayed the need for insulin initiation, and reduced the need for large dose increases in patients already using insulin,” Muthiah Vaduganathan, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The patients in the empagliflozin (Jardiance) arm of EMPA-REG OUTCOME had a 9% rate of initiating insulin treatment after 4 years in the study, compared with a 20% rate among patients who received placebo, a statistically significant 60% relative risk reduction. All patients in the trial continued on their background oral glucose-lowering medications.
Among the 48% of study patients who entered the study already using insulin as part of their usual regimen, 18% of those receiving empagliflozin required a significant increase in their insulin dosage (an increase of at least 20% from baseline) after 4 years. But among the control patients, 35% needed this level of insulin-dosage intensification, again a statistically significant difference that computed to a 58% relative reduction in the need for boosting the insulin dosage.
For both of these endpoints, the divergence between the empagliflozin and control arms became apparent within the first 6 months on treatment, and the between-group differences steadily increased during further follow-up. The analyses pooled the patients who received empagliflozin in the trial, which studied two different dosages of the drug.
Results add to the ‘risk and benefit conversation’
“This is one of the first studies to look at this question in a more granular fashion” in patients with type 2 diabetes receiving a drug from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class, said Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston. It provides “compelling” information to include when discussing oral diabetes-drug options with patients, he said in an interview.
Patients newly diagnosed with type 2 diabetes “often think about insulin” and their potential need to eventually start taking it, with the requirements it brings for training, monitoring, and drug delivery, along with the costs for insulin and glucose monitoring. “Patients are very attuned to potentially needing insulin and often ask about it. A reduced need for insulin will be an important part of the risk and benefit conversation” with patients about potential use of an SGLT2 inhibitor, he said.
Dr. Vaduganathan hypothesized that three factors could contribute to the impact of empagliflozin on insulin initiation and dosage level: a direct glycemic-control effect of the drug, the drug’s positive impact on overall well-being and function that could enhance patient movement, and the documented ability of treatment with empagliflozin and other drugs in its class to cut the rate of heart failure hospitalizations. This last feature is potentially relevant because insulin treatment often starts in patients with type 2 diabetes during a hospitalization, he noted.
Handelsman: Analysis shows no ‘spectacular effect’
The association of empagliflozin treatment with a reduced need for insulin seen in these data is consistent with expectations for patients with type 2 diabetes who receive an additional oral drug, commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of American in Tarzana, Calif. “In large part it has to do with patients on placebo having to get more insulin” because their additional oral-drug options were limited. Dr. Handelsman pointed out that during the period when the EMPA-REG OUTCOME trial ran, from 2010-2015, fewer oral drugs were available than today, and clinicians in the study were encouraged to treat patients to their goal glycemia level according to local guidelines. In addition to a modest but useful glycemic control effect from SGLT2 inhibitors that, on average, cut hemoglobin A1c levels by about 0.5%, they may also give a small boost to insulin sensitivity that can also defer the need to add or increase insulin. The level of insulin-treatment deference reported in the new analysis was “not a spectacular effect” he said in an interview.
The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study followed 7,020 patients at 590 sites in 42 countries for a median of 3.1 years. The study’s primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction (excluding silent MI), or nonfatal stroke, and the results showed a statistically significant 14% relative risk reduction with empagliflozin treatment (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ). The results also showed that 12 weeks into the study, before patients could receive any additional drugs, HbA1c levels averaged 0.54%-0.6% lower among the empagliflozin-treated patients than those in the placebo arm, with smaller between-group differences maintained through the balance of the study. At entry, more than half the enrolled patients were routinely treated with metformin, and close to half were receiving a sulfonyurea agent.
The EMPA-REG OUTCOME results were also notable as showing for the first time that treatment with an SGLT2 inhibitor drug produced a substantial decrease in heart failure hospitalizations, incident heart failure, and progression of renal dysfunction, effects subsequently confirmed and also found for other agents in this drug class.
EMPA-REG OUTCOME was funded in part by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin (Jardiance). Dr. Vaduganathan has been an advisor to Boehringer Ingelheim and to Amgen, AstraZeneca, Baxter, Bayer, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including Boehringer Ingelheim and Eli Lilly.
SOURCE: Vaduganathan M et al. ADA 2020, Abstract 30-OR.
Patients with type 2 diabetes treated with the SGLT2 inhibitor empagliflozin during the landmark EMPA-REG OUTCOME trial had a solidly reduced need to either start insulin treatment or intensify existing insulin treatment, compared with those given placebo, in a post-hoc analysis of the study’s findings.
“Empagliflozin markedly and durably delayed the need for insulin initiation, and reduced the need for large dose increases in patients already using insulin,” Muthiah Vaduganathan, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The patients in the empagliflozin (Jardiance) arm of EMPA-REG OUTCOME had a 9% rate of initiating insulin treatment after 4 years in the study, compared with a 20% rate among patients who received placebo, a statistically significant 60% relative risk reduction. All patients in the trial continued on their background oral glucose-lowering medications.
Among the 48% of study patients who entered the study already using insulin as part of their usual regimen, 18% of those receiving empagliflozin required a significant increase in their insulin dosage (an increase of at least 20% from baseline) after 4 years. But among the control patients, 35% needed this level of insulin-dosage intensification, again a statistically significant difference that computed to a 58% relative reduction in the need for boosting the insulin dosage.
For both of these endpoints, the divergence between the empagliflozin and control arms became apparent within the first 6 months on treatment, and the between-group differences steadily increased during further follow-up. The analyses pooled the patients who received empagliflozin in the trial, which studied two different dosages of the drug.
Results add to the ‘risk and benefit conversation’
“This is one of the first studies to look at this question in a more granular fashion” in patients with type 2 diabetes receiving a drug from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class, said Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston. It provides “compelling” information to include when discussing oral diabetes-drug options with patients, he said in an interview.
Patients newly diagnosed with type 2 diabetes “often think about insulin” and their potential need to eventually start taking it, with the requirements it brings for training, monitoring, and drug delivery, along with the costs for insulin and glucose monitoring. “Patients are very attuned to potentially needing insulin and often ask about it. A reduced need for insulin will be an important part of the risk and benefit conversation” with patients about potential use of an SGLT2 inhibitor, he said.
Dr. Vaduganathan hypothesized that three factors could contribute to the impact of empagliflozin on insulin initiation and dosage level: a direct glycemic-control effect of the drug, the drug’s positive impact on overall well-being and function that could enhance patient movement, and the documented ability of treatment with empagliflozin and other drugs in its class to cut the rate of heart failure hospitalizations. This last feature is potentially relevant because insulin treatment often starts in patients with type 2 diabetes during a hospitalization, he noted.
Handelsman: Analysis shows no ‘spectacular effect’
The association of empagliflozin treatment with a reduced need for insulin seen in these data is consistent with expectations for patients with type 2 diabetes who receive an additional oral drug, commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of American in Tarzana, Calif. “In large part it has to do with patients on placebo having to get more insulin” because their additional oral-drug options were limited. Dr. Handelsman pointed out that during the period when the EMPA-REG OUTCOME trial ran, from 2010-2015, fewer oral drugs were available than today, and clinicians in the study were encouraged to treat patients to their goal glycemia level according to local guidelines. In addition to a modest but useful glycemic control effect from SGLT2 inhibitors that, on average, cut hemoglobin A1c levels by about 0.5%, they may also give a small boost to insulin sensitivity that can also defer the need to add or increase insulin. The level of insulin-treatment deference reported in the new analysis was “not a spectacular effect” he said in an interview.
The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study followed 7,020 patients at 590 sites in 42 countries for a median of 3.1 years. The study’s primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction (excluding silent MI), or nonfatal stroke, and the results showed a statistically significant 14% relative risk reduction with empagliflozin treatment (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ). The results also showed that 12 weeks into the study, before patients could receive any additional drugs, HbA1c levels averaged 0.54%-0.6% lower among the empagliflozin-treated patients than those in the placebo arm, with smaller between-group differences maintained through the balance of the study. At entry, more than half the enrolled patients were routinely treated with metformin, and close to half were receiving a sulfonyurea agent.
The EMPA-REG OUTCOME results were also notable as showing for the first time that treatment with an SGLT2 inhibitor drug produced a substantial decrease in heart failure hospitalizations, incident heart failure, and progression of renal dysfunction, effects subsequently confirmed and also found for other agents in this drug class.
EMPA-REG OUTCOME was funded in part by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin (Jardiance). Dr. Vaduganathan has been an advisor to Boehringer Ingelheim and to Amgen, AstraZeneca, Baxter, Bayer, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including Boehringer Ingelheim and Eli Lilly.
SOURCE: Vaduganathan M et al. ADA 2020, Abstract 30-OR.
Patients with type 2 diabetes treated with the SGLT2 inhibitor empagliflozin during the landmark EMPA-REG OUTCOME trial had a solidly reduced need to either start insulin treatment or intensify existing insulin treatment, compared with those given placebo, in a post-hoc analysis of the study’s findings.
“Empagliflozin markedly and durably delayed the need for insulin initiation, and reduced the need for large dose increases in patients already using insulin,” Muthiah Vaduganathan, MD, said at the virtual annual scientific sessions of the American Diabetes Association.
The patients in the empagliflozin (Jardiance) arm of EMPA-REG OUTCOME had a 9% rate of initiating insulin treatment after 4 years in the study, compared with a 20% rate among patients who received placebo, a statistically significant 60% relative risk reduction. All patients in the trial continued on their background oral glucose-lowering medications.
Among the 48% of study patients who entered the study already using insulin as part of their usual regimen, 18% of those receiving empagliflozin required a significant increase in their insulin dosage (an increase of at least 20% from baseline) after 4 years. But among the control patients, 35% needed this level of insulin-dosage intensification, again a statistically significant difference that computed to a 58% relative reduction in the need for boosting the insulin dosage.
For both of these endpoints, the divergence between the empagliflozin and control arms became apparent within the first 6 months on treatment, and the between-group differences steadily increased during further follow-up. The analyses pooled the patients who received empagliflozin in the trial, which studied two different dosages of the drug.
Results add to the ‘risk and benefit conversation’
“This is one of the first studies to look at this question in a more granular fashion” in patients with type 2 diabetes receiving a drug from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class, said Dr. Vaduganathan, a cardiologist at Brigham and Women’s Hospital in Boston. It provides “compelling” information to include when discussing oral diabetes-drug options with patients, he said in an interview.
Patients newly diagnosed with type 2 diabetes “often think about insulin” and their potential need to eventually start taking it, with the requirements it brings for training, monitoring, and drug delivery, along with the costs for insulin and glucose monitoring. “Patients are very attuned to potentially needing insulin and often ask about it. A reduced need for insulin will be an important part of the risk and benefit conversation” with patients about potential use of an SGLT2 inhibitor, he said.
Dr. Vaduganathan hypothesized that three factors could contribute to the impact of empagliflozin on insulin initiation and dosage level: a direct glycemic-control effect of the drug, the drug’s positive impact on overall well-being and function that could enhance patient movement, and the documented ability of treatment with empagliflozin and other drugs in its class to cut the rate of heart failure hospitalizations. This last feature is potentially relevant because insulin treatment often starts in patients with type 2 diabetes during a hospitalization, he noted.
Handelsman: Analysis shows no ‘spectacular effect’
The association of empagliflozin treatment with a reduced need for insulin seen in these data is consistent with expectations for patients with type 2 diabetes who receive an additional oral drug, commented Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of The Metabolic Institute of American in Tarzana, Calif. “In large part it has to do with patients on placebo having to get more insulin” because their additional oral-drug options were limited. Dr. Handelsman pointed out that during the period when the EMPA-REG OUTCOME trial ran, from 2010-2015, fewer oral drugs were available than today, and clinicians in the study were encouraged to treat patients to their goal glycemia level according to local guidelines. In addition to a modest but useful glycemic control effect from SGLT2 inhibitors that, on average, cut hemoglobin A1c levels by about 0.5%, they may also give a small boost to insulin sensitivity that can also defer the need to add or increase insulin. The level of insulin-treatment deference reported in the new analysis was “not a spectacular effect” he said in an interview.
The EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) study followed 7,020 patients at 590 sites in 42 countries for a median of 3.1 years. The study’s primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction (excluding silent MI), or nonfatal stroke, and the results showed a statistically significant 14% relative risk reduction with empagliflozin treatment (N Engl J Med. 2015 Nov 26;373[22]:2117-28 ). The results also showed that 12 weeks into the study, before patients could receive any additional drugs, HbA1c levels averaged 0.54%-0.6% lower among the empagliflozin-treated patients than those in the placebo arm, with smaller between-group differences maintained through the balance of the study. At entry, more than half the enrolled patients were routinely treated with metformin, and close to half were receiving a sulfonyurea agent.
The EMPA-REG OUTCOME results were also notable as showing for the first time that treatment with an SGLT2 inhibitor drug produced a substantial decrease in heart failure hospitalizations, incident heart failure, and progression of renal dysfunction, effects subsequently confirmed and also found for other agents in this drug class.
EMPA-REG OUTCOME was funded in part by Boehringer Ingelheim and Eli Lilly, the companies that market empagliflozin (Jardiance). Dr. Vaduganathan has been an advisor to Boehringer Ingelheim and to Amgen, AstraZeneca, Baxter, Bayer, Cytokinetics, and Relypsa. Dr. Handelsman has been a consultant to several drug companies including Boehringer Ingelheim and Eli Lilly.
SOURCE: Vaduganathan M et al. ADA 2020, Abstract 30-OR.
FROM ADA 2020
Half of type 1 diabetes patients with COVID-19 manage at home
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing [email protected].
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing [email protected].
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
New preliminary data from the T1D Exchange suggest that, although hyperglycemia and diabetic ketoacidosis (DKA) are common in people with type 1 diabetes who develop COVID-19, many are still able to manage the illness at home and overall mortality is relatively low.
The new findings – the first US data on individuals with type 1 diabetes and COVID-19 – were published online June 5 in Diabetes Care by Osagie A. Ebekozien, MD, vice president, quality improvement and population health at the T1D Exchange, and colleagues.
Two UK studies are the only prior ones to previously examine the topic.
The newly published study includes data as of May 5 on 64 individuals from a total of 64 US sites, including 15 T1D Exchange member clinics and an additional 49 endocrinology clinics from around the country. Since the paper was submitted, there are now 220 patients from 68 sites. Another publication with a more detailed analysis of risk factors and adjustment for confounders is planned for later this year.
Some of the findings from the preliminary data have shifted, but many aspects remain consistent, Ebekozien told Medscape Medical News.
“One thing still very true, even with the unpublished findings, is the influence of A1c and glycemic management. ...With higher A1c levels, we’re seeing more COVID-19 hospitalizations and worse outcomes,” he said.
And as has been generally reported for COVID-19, high body mass index was a major risk factor in the preliminary dataset – and remains so.
There were two deaths in the preliminary report, both individuals with comorbidities in addition to type 1 diabetes, Ebekozien said. There have been a few more deaths in the larger dataset, but the mortality rate remains relatively low.
Interestingly, females predominate in both cohorts. That may be a reporting phenomenon, another factor that is being analyzed.
Hyperglycemia Remains a Major Risk Factor
The study is specifically being conducted by the T1D Exchange’s Quality Improvement Collaborative, which Ebekozien heads.
Data were obtained for 33 patients with type 1 diabetes who tested positive for COVID-19, and another 31 who were classified as “COVID-19–like” because they had symptoms consistent with COVID-19, as identified by the Centers for Disease Control and Prevention, but hadn’t been tested for the virus.
For all 64 patients, the mean age was 20.9 years and two thirds (65.6%) were aged 18 or younger. A higher proportion of the COVID-19–like patients were pediatric than the confirmed cases. The larger dataset includes more adult patients, Ebekozien told Medscape Medical News.
Overall, 60.9% of patients were female. Nearly half were white, a quarter Hispanic, and 18.8% black. More confirmed COVID-19 cases were black compared with suspected cases (30.3% vs 6.5%).
Median A1c for the overall group (including suspected COVID-19 cases) was 8.0%, but it was 8.5% among confirmed cases. Overall, six patients (9.8%) presented with new-onset type 1 diabetes after they developed COVID-19.
Hyperglycemia was present in half (32) of patients overall. DKA occurred in 19 people (30.2%): 15 of the confirmed COVID-19 cases (45.5%) versus just 4 (13.3%) of the COVID-19–like cases. Nausea was reported in 30.2% of patients overall.
Other symptoms were typical of COVID-19, including fever (41.3%), dry cough (38.1%), and shortness of breath (27.0%). Loss of taste and smell was less common, at just 9.5% overall.
Obesity was present in 39.7% of patients overall, with similar proportions in the confirmed and suspected COVID-19 groups. Hypertension and/or cardiovascular disease were present in 14.3% of patients overall, and the rate was similar between the two subgroups.
One of the two patients who died was a 79-year-old man who had hypertension and a prior stroke in addition to type 1 diabetes. The other was a 19-year-old woman with a history of asthma who developed a pulmonary embolism during the onset of COVID-19. Neither had DKA.
Even in Type 1 Diabetes, COVID-19 Can Be Managed at Home
Overall, 34.9% of patients were able to manage COVID-19 entirely at home, with 27.3% of the confirmed and 43.3% of the suspected cases able to do so.
At the other extreme, 22.2% of patients overall were admitted to the intensive care unit; 30.3% of the confirmed versus 13.3% of suspected cases.
Including the small proportion of patients sent home after being seen in emergency or urgent care, overall roughly half were not admitted to hospital.
“Interestingly, even in this preliminary study, half were managed at home via telemedicine with an endocrinologist and infectious disease specialist. ... I think it continues to be a case-by-case clinical decision between the patient and their provider,” Ebekozien said.
“But, we’re seeing a good number of patients who are managed at home and the symptoms resolve in a week or two, and the illness runs its course, and they don’t have to even be seen,” he added.
The research team is also collecting data on barriers to remote care, including challenges with telemedicine and how frontline providers are navigating them.
“Those are all things that our future paper will be able to shed more light on,” he explained.
Endocrinologists around the country are invited to report cases of COVID-19 in patients with type 1 diabetes to the T1D Exchange by emailing [email protected].
And in fact, Ebekozien also requested that clinicians with a large type 1 diabetes population also report if they’ve had no COVID-19 cases.
“Even if they haven’t had a case, that’s very useful information for us to know. One of the things we want to calculate down the line is the incidence ratio. Not all participating sites have had a case.”
Endocrinologists from all the participating sites have formed a dedicated community that meets regularly via webinars to share information, he noted. “It’s been a very selfless effort to work collaboratively as a community to quickly answer critical questions.”
The Helmsley Charitable Trust funds the T1D Exchange Quality Improvement Collaborative. The T1D Exchange received financial support for this study from Abbott Diabetes, Dexcom, JDRF, Insulet Corporation, Lilly, Medtronic, and Tandem Diabetes Care. No other relevant financial relationships were reported.
This article first appeared on Medscape.com.
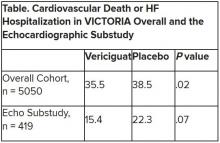
Mortality differs by LVEF between women and men
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
, Simon Stewart, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
This analysis from the ongoing National Echocardiography Database of Australia (NEDA) included 499,153 men and women who underwent echocardiography in routine clinical practice for a variety of indications, with more than 3 million person-years of follow-up.
This study broke new ground. There is surprisingly little information from routine clinical practice to describe the spectrum and prognostic importance of left ventricular ejection fraction (LVEF). Indeed, most data have come from clinical trials in patients with heart failure with reduced ejection fraction (HFrEF), in which women are traditionally underrepresented. By comparison, the NEDA analysis included 237,046 women in routine care, noted Dr. Stewart, a National Health and Medical Research Council of Australia Senior Principal Research Fellow at Torrens University in Adelaide.
Among the novel findings in the new NEDA analysis: an LVEF below 50% was more than twice as common in men than women, occurring in 17.6% and 8.3%, respectively. Also, women had a higher average LVEF: 64.2%, compared with 59.5% in men. The overall 1- and 5-year all-cause mortality rates in the half-million participants were 5.8% and 18.4%.
Cardiovascular-related mortality occurred in 7.1% of women in median of 5.6 years of follow-up and in 8.1% of men with 5.5 years of follow-up.
All-cause and cardiovascular mortality rates followed a J-shaped curve, with the clear nadir occurring at an LVEF of 65%-69.9% in both women and men. But for LVEF values outside the nadir, a striking sex-based difference was present. Cardiovascular mortality, when adjusted for body mass index, age, heart rate, valvular heart disease, E-wave velocity, and other potential confounders, wasn’t significantly different between men whose LVEF was 65%-69.9% and those with an LVEF of 45%-64.9%. It started climbing in earnest only at an LVEF below 45%. In contrast, women with an LVEF of 45%-54.9% had a statistically significant twofold increased cardiovascular mortality rate compared to those in the nadir. Moreover, women with an LVEF of 55%-59.9% showed a trend in the same unwanted direction.
High LVEF, higher mortality in women
Dr. Stewart drew attention to an inflection point in the mortality curve for women whereby mortality began climbing at LVEF values of 70% or more. Values in that high range were documented in 72,379 women and 51,317 men.
He noted that the NEDA finding of an increasing mortality risk at LVEFs of at least 70%, especially in women, is similar to a recent report from another big data study, this one involving more than 200,000 patients who underwent echocardiography in routine clinical practice in the Geisinger health system in Pennsylvania. The investigators found in this retrospective study that during a median of 4 years of follow-up after echocardiography, the adjusted risk for all-cause mortality followed a U-shaped curve. The nadir of risk occurred in patients with an LVEF of 60%-65%, with a 1.71-fold increased risk at an LVEF at 70% or more and a near-identical 1.73-fold increased risk at an LVEF of 35%-40%. In this study, however, which was less than half the size of the NEDA analysis, the U-shaped LVEF/mortality curve applied to both men and women. Similar findings were seen in a validation cohort of nearly 36,000 patients from New Zealand (Eur Heart J. 2020 Mar 21;41[12]:1249-57).
The investigators predicted that in addition to the existing categories of HFrEF, heart failure with preserved ejection fraction (HFpEF), and the more recently proposed heart failure with midrange ejection fraction (HFmrEF), their results “may herald the recognition of a new phenotype characterized by supranormal LVEF,” with a moniker of HFsnEF.
New treatment opportunity for women?
Discussant Lars Lund, MD, PhD, professor of cardiology at the Karolinska Institute, Stockholm, said that it’s not possible to make any statements about what constitutes a “normal” LVEF in men or women based on the NEDA study, since all participants underwent medically indicated echocardiography. He added that what he found most interesting about the NEDA analysis was the observation that women with mid-range or mildly reduced LVEF had increased mortality, while men didn’t. That’s a finding that helps explain the suggestion of possible benefit for sacubitril-valsartan in patients with lower ejection fraction and in women in the PARAGON-HF trial of angiotensin-neprilysin inhibition in patients with heart failure with preserved ejection fraction (N Engl J Med. 2019 Oct 24;381[17]:1609-20).
Dr. Lund expressed the hope that the NEDA investigators will do an analysis of the relationship between echocardiographic left atrial size and mortality. Dr. Stewart replied that, as a matter of fact,such a study is planned. The enormous and continuously growing NEDA database has already been used to provide new insights into aortic stenosis and pulmonary hypertension, he noted.
Session moderator Andrew Coats, MD, incoming president of the ESC Heart Failure Association, said that there are many different methods used for echocardiographic measurement of LVEF. He wondered about the validity of pooling them in a single analysis.
Dr. Stewart replied that NEDA software applies a hierarchical weighting of the various methods used to quantify LVEF. And the submitted data come from the top echocardiography laboratories throughout Australia.
“We’ve done some sensitivity analyses around the different methods of quantifying LVEF and we get the same patterns,” he said. “We’re comfortable with the validity of what we’ve done. The big data allows us to do that.”
Dr. Stewart reported receiving speakers fees and travel support from Novartis, a partial funder of NEDA.
SOURCE: Stewart S. ESC Heart Failure 2020.
FROM ESC HEART FAILURE 2020
VICTORIA results deepen mystery of vericiguat in low-EF heart failure
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
Although clinical outcomes improved for patients with high-risk heart failure (HF) who received vericiguat (Merck/Bayer) on top of standard therapy in a major randomized trial, a subgroup study failed to show any corresponding gains in ventricular function.
The discordant results from the 5,050-patient VICTORIA trial and its echocardiographic substudy highlight something of a mystery as to the mechanism of the investigational oral soluble guanylate cyclase stimulator’s clinical effects. In the overall trial, they included a drop in risk of cardiovascular (CV) death or first HF hospitalization, the primary endpoint.
In the echo substudy, which assessed patients with evaluable echocardiograms at both baseline and 8 months, vericiguat, compared with placebo, had no significant effect on two measures of left ventricular (LV) function. Patients in the prospectively conducted substudy made up less than 10% of the total trial population.
Both LV ejection fraction (LVEF) and LV end-systolic volume index (LVESVI) significantly improved in the vericiguat and control groups, but vericiguat “had no additional significant effect,” said Burkert Pieske, MD, of Charité University Medicine Berlin.
Still, he said, there was “evidence of a lower risk of events, evidence of a clinical benefit,” for those who received vericiguat, although it fell slightly short of significance in the substudy cohort of fewer than 500 patients.
Dr. Pieske reported the VICTORIA echo substudy results June 5 in a Late-Breaking Science Session during HFA Discoveries, the online backup for the Heart Failure Association of the European Society of Cardiology annual scientific meeting.
The traditional live HFA meeting had been scheduled for Barcelona but was canceled this year as a result of the COVID-19 pandemic.
Pointing to the significant echo improvements in both treatment groups, invited discussant Rudolf A. de Boer, MD, PhD, University of Groningen (the Netherlands), said the substudy shows that HF in high-risk patients “is associated with a transient deterioration of LV function and geometry, which can to a certain extent be reversed over time.”
That the effect apparently wasn’t influenced by vericiguat “may be explained by the fact that, in randomized controlled trials, patients – including those on placebo – tend to be treated very well.” In clinical practice, he said, “less complete reverse remodeling may be expected.”
Dr. de Boer also pointed to likely survivor bias in the study, in that only patients who survived to at least 8 months were included. That meant, among other things, that they were likely at lower overall risk than the total VICTORIA population, leaving less room for any treatment effect.
“Further, likely because of the play of chance in this substudy, the LV volumes were smaller in the vericiguat group at baseline, creating less of an opportunity for vericiguat to make a difference,” he said. “It could be speculated that, with larger volumes, the window of opportunity for vericiguat would have been wider.”
But “most strikingly,” the lack of vericiguat effect on echo parameters contrasts with the clinical benefits associated with the drug in the main trial, and possibly in the echo substudy, Dr. de Boer said, “creating a dissociation between the surrogate echo parameters and the clinical hard endpoints. And it could be imagined that the rather crude echo measures presented here, LVEF and LV volume, miss a more subtle effect of vericiguat.”
For example, it’s possible that the drug’s clinical effect in heart failure does not depend on any improvements in ventricular function, Dr. de Boer said, adding that vericiguat “may potentially also have important effects on pulmonary and peripheral vasculature,” so he recommended future studies look for any changes in arterial and right ventricular function from the drug.
VICTORIA enrolled only patients with HF and reduced ejection fraction who had previously experienced a decompensation event, usually only within the last 3 months, as it turned out. Those assigned to vericiguat on top of standard drug and device therapies showed a modest 10% decline in adjusted relative risk (P = .019) for the trial’s primary endpoint, CV death or first HF hospitalization.
But when the results were unveiled at a meeting, trialists and observers were more enthused about the drug’s effect in absolute terms, which by one measure was 4.2 fewer events on vericiguat per 100 patient-years. That translated to a number to treat of 24 to prevent one event, said to be impressive, given that the study’s patients were so high risk.
The echo substudy included 419 prospectively selected patients, 208 on vericiguat and 211 assigned to placebo, who had evaluable echocardiograms at both baseline and 8 months, as assessed at the VICTORIA echo core lab. They averaged 64.5 years in age with a mean baseline LVEF of 29%; about 27% were women.
Their clinical outcomes paralleled the overall study, with lower event rates overall and a difference between treatment groups that fell short of significance.
Neither of the study’s primary endpoints, the two echo parameters, responded differently to vericiguat, compared with placebo.
The overall VICTORIA trial “showed a modest but useful benefit in the combined endpoint of hospitalizations and mortality, but all due to fewer hospitalizations,” Andrew J. Coats, MD, DSc, MBA, told this news organization.
“The echo substudy was smaller, and many drugs that reduce hospitalization do not do it through effects on LV function,” said Dr. Coats of the University of Warwick, Coventry, England, who wasn’t a part of VICTORIA. “Other mechanisms may be via improved peripheral vascular or renal effects.”
VICTORIA and the echocardiographic substudy were supported by Merck Sharp & Dohme and Bayer AG. Dr. Pieske disclosed serving on a speakers bureau, advisory board, or committee for Bayer Healthcare, Merck, Novartis, AstraZeneca, Stealth, Servier, Daiichi-Sankyo, Biotronic, Abbott Vascular, and Bristol-Myers Squibb. Dr. de Boer disclosed receiving speaker fees from Abbott, AstraZeneca, Novartis, and Roche. Dr. Coats disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2020
Cardiology societies unite to denounce racist violence
The death of George Floyd and other African Americans spurred the Association of Black Cardiologists, the American Heart Association, and the American College of Cardiology to join forces and issue an urgent letter denouncing recent and ongoing events.
Starting off by acknowledging that these are “difficult and disturbing times,” the presidents of the three societies tied the violence into the bigger public health picture. “Like cardiovascular disease, acts of violence and racism are core causes of psychosocial stress that promote poor well-being and cardiovascular health, especially for communities of color.”
“It’s not just one quick solution, one quick letter. It’s more of an ongoing project to raise awareness and have really defined projects. We want to have goals, tactics, and measurable outcomes. We want to make sure it’s not just a banner on the wall,” Athena Poppas, MD, president of the American College of Cardiology and one of three physicians signing the letter, said in an interview.
The Association of Black Cardiologists drafted the statement and asked the AHA and ACC if they wanted to sign on. “It felt important to join them and follow their lead,” she said. “There is a clear link between psychosocial stress and discrimination and health equity in the communities.”
Interestingly, the ABC and ACC have an existing partnership, one that included creating a “Campaign for the Future” a little more than a year ago. One of the focuses is on reducing health disparities and starting a diversity and inclusion task force that later became a committee. The groups held a joint board of trustees meeting at Morehouse University, Atlanta, in January 2020. Thinking about that time, Dr. Poppas added, “who knew what was about to transpire over the next few months?”
The letter is only one component of an ongoing effort to “find concrete ways to make change, both within the college and within our profession,” added Dr. Poppas, chief of cardiology and professor of medicine at Brown University, Providence, R.I., and director of the Lifespan Cardiovascular Institute of Rhode Island, Miriam Hospitals, and Newport Hospitals. “Thereby, there is good data that you affect health equity in the population as well.”
“We DENOUNCE incidents of racism and violence that continue to ravage our communities,” the society leaders wrote in the letter. “Given that heart disease and stroke are the leading causes of death for communities of color, particularly African Americans who have the lowest life expectancy of all racial/ethnic groups living in the United States, we are extremely disturbed by violent acts that cut to the core of the lives of our community.”
Other societies released similar statements. For example, the American College of Physicians expressed “grave concern” about recent events and the American Medical Association released a statement entitled “Police brutality must stop.”
A cardiologist speaks out
“Thank you to my organizations, the Association of Black Cardiologists and the American College of Cardiology, for taking a stand,” Travis C. Batts, MD, said in a video statement posted to YouTube on June 2, 2020.
“As an African American male who has sons, brothers, and friends who are also African American, I oftentimes have angst, particularly with my sons. Despite what I do to create an environment that cultivates education and puts them in the right position, there are some people who would stop just at how they look when they approach them,” Dr. Batts said.
“I always have that fear as a father that at some point they may engage with law enforcement – and it may not turn out the way we want it to,” said Dr. Batts, chairman of medical sub-specialties and medical director of the cardiology clinic at Wilford Hall Ambulatory Surgical Center at Lackland Air Force Base, Tex. He also is an associate professor of cardiovascular medicine for the Uniformed Services University of the Health Sciences, Bethesda, Md., and is an adjunct assistant professor at Texas A&M University. He went on in the video to describe how a personal encounter with police years ago changed his life.
The urgent letter from the cardiology societies speaks to health care disparities, Dr. Batts said, “but it doesn’t stop there. It talks about their goals to balance these issues that we see as a pervasive problem in our community.”
The societies point out that George Floyd’s death is not an isolated incident. “Mr. Floyd’s death comes on the heels of other recent incidents caught on camera. In another 2020 incident, Ahmaud Arbery was shot and killed while jogging in his hometown of Brunswick, Ga. Christian Cooper is fortunately alive and well to speak to the Memorial Day incident in New York’s Central Park where he was accused of threatening the life of a woman while bird watching.” They added that “another senseless death involves officers entering the Louisville, Kent., home of emergency medical technician Breonna Taylor.”
Dr. Batts said this portion of the statement was particularly poignant: “We stand and link arms in solidarity with efforts to dismantle systems that maintain excess morbidity and mortality, especially among vulnerable populations and those historically oppressed. Indeed, our collective vast membership, many of whom are at the front lines of clinical health care, has taken an oath to decisively and with kindness, compassion and grace act to relieve suffering related to ‘I can’t breathe’ in order to preserve life.”
A Positive Response
The response to the urgent letter has been “overwhelmingly positive,” Dr. Poppas said. “This isn’t political, per se. This is really about justice, about health equity, and about being moral and conscious human beings. People I hadn’t heard from in years said, ‘thank you for doing this.’ ” The comments on social media were “almost uniformly positive,” she added. “There is always one or two people who feel this isn’t what cardiology is about.”
“Although making a statement is important, so is doing the hard work to make change,” Dr. Poppas said. The goal involves “rolling up our sleeves and spending the time, the money and the energy to make changes – so 5-10 years from now, it looks different.”
In addition to Dr. Poppas, Michelle A. Albert, MD, MPH, president of the Association of Black Cardiologists and Robert A. Harrington, MD, president of the American Heart Association, signed the letter. Dr. Pappas and Dr. Batts had no relevant disclosures.
The death of George Floyd and other African Americans spurred the Association of Black Cardiologists, the American Heart Association, and the American College of Cardiology to join forces and issue an urgent letter denouncing recent and ongoing events.
Starting off by acknowledging that these are “difficult and disturbing times,” the presidents of the three societies tied the violence into the bigger public health picture. “Like cardiovascular disease, acts of violence and racism are core causes of psychosocial stress that promote poor well-being and cardiovascular health, especially for communities of color.”
“It’s not just one quick solution, one quick letter. It’s more of an ongoing project to raise awareness and have really defined projects. We want to have goals, tactics, and measurable outcomes. We want to make sure it’s not just a banner on the wall,” Athena Poppas, MD, president of the American College of Cardiology and one of three physicians signing the letter, said in an interview.
The Association of Black Cardiologists drafted the statement and asked the AHA and ACC if they wanted to sign on. “It felt important to join them and follow their lead,” she said. “There is a clear link between psychosocial stress and discrimination and health equity in the communities.”
Interestingly, the ABC and ACC have an existing partnership, one that included creating a “Campaign for the Future” a little more than a year ago. One of the focuses is on reducing health disparities and starting a diversity and inclusion task force that later became a committee. The groups held a joint board of trustees meeting at Morehouse University, Atlanta, in January 2020. Thinking about that time, Dr. Poppas added, “who knew what was about to transpire over the next few months?”
The letter is only one component of an ongoing effort to “find concrete ways to make change, both within the college and within our profession,” added Dr. Poppas, chief of cardiology and professor of medicine at Brown University, Providence, R.I., and director of the Lifespan Cardiovascular Institute of Rhode Island, Miriam Hospitals, and Newport Hospitals. “Thereby, there is good data that you affect health equity in the population as well.”
“We DENOUNCE incidents of racism and violence that continue to ravage our communities,” the society leaders wrote in the letter. “Given that heart disease and stroke are the leading causes of death for communities of color, particularly African Americans who have the lowest life expectancy of all racial/ethnic groups living in the United States, we are extremely disturbed by violent acts that cut to the core of the lives of our community.”
Other societies released similar statements. For example, the American College of Physicians expressed “grave concern” about recent events and the American Medical Association released a statement entitled “Police brutality must stop.”
A cardiologist speaks out
“Thank you to my organizations, the Association of Black Cardiologists and the American College of Cardiology, for taking a stand,” Travis C. Batts, MD, said in a video statement posted to YouTube on June 2, 2020.
“As an African American male who has sons, brothers, and friends who are also African American, I oftentimes have angst, particularly with my sons. Despite what I do to create an environment that cultivates education and puts them in the right position, there are some people who would stop just at how they look when they approach them,” Dr. Batts said.
“I always have that fear as a father that at some point they may engage with law enforcement – and it may not turn out the way we want it to,” said Dr. Batts, chairman of medical sub-specialties and medical director of the cardiology clinic at Wilford Hall Ambulatory Surgical Center at Lackland Air Force Base, Tex. He also is an associate professor of cardiovascular medicine for the Uniformed Services University of the Health Sciences, Bethesda, Md., and is an adjunct assistant professor at Texas A&M University. He went on in the video to describe how a personal encounter with police years ago changed his life.
The urgent letter from the cardiology societies speaks to health care disparities, Dr. Batts said, “but it doesn’t stop there. It talks about their goals to balance these issues that we see as a pervasive problem in our community.”
The societies point out that George Floyd’s death is not an isolated incident. “Mr. Floyd’s death comes on the heels of other recent incidents caught on camera. In another 2020 incident, Ahmaud Arbery was shot and killed while jogging in his hometown of Brunswick, Ga. Christian Cooper is fortunately alive and well to speak to the Memorial Day incident in New York’s Central Park where he was accused of threatening the life of a woman while bird watching.” They added that “another senseless death involves officers entering the Louisville, Kent., home of emergency medical technician Breonna Taylor.”
Dr. Batts said this portion of the statement was particularly poignant: “We stand and link arms in solidarity with efforts to dismantle systems that maintain excess morbidity and mortality, especially among vulnerable populations and those historically oppressed. Indeed, our collective vast membership, many of whom are at the front lines of clinical health care, has taken an oath to decisively and with kindness, compassion and grace act to relieve suffering related to ‘I can’t breathe’ in order to preserve life.”
A Positive Response
The response to the urgent letter has been “overwhelmingly positive,” Dr. Poppas said. “This isn’t political, per se. This is really about justice, about health equity, and about being moral and conscious human beings. People I hadn’t heard from in years said, ‘thank you for doing this.’ ” The comments on social media were “almost uniformly positive,” she added. “There is always one or two people who feel this isn’t what cardiology is about.”
“Although making a statement is important, so is doing the hard work to make change,” Dr. Poppas said. The goal involves “rolling up our sleeves and spending the time, the money and the energy to make changes – so 5-10 years from now, it looks different.”
In addition to Dr. Poppas, Michelle A. Albert, MD, MPH, president of the Association of Black Cardiologists and Robert A. Harrington, MD, president of the American Heart Association, signed the letter. Dr. Pappas and Dr. Batts had no relevant disclosures.
The death of George Floyd and other African Americans spurred the Association of Black Cardiologists, the American Heart Association, and the American College of Cardiology to join forces and issue an urgent letter denouncing recent and ongoing events.
Starting off by acknowledging that these are “difficult and disturbing times,” the presidents of the three societies tied the violence into the bigger public health picture. “Like cardiovascular disease, acts of violence and racism are core causes of psychosocial stress that promote poor well-being and cardiovascular health, especially for communities of color.”
“It’s not just one quick solution, one quick letter. It’s more of an ongoing project to raise awareness and have really defined projects. We want to have goals, tactics, and measurable outcomes. We want to make sure it’s not just a banner on the wall,” Athena Poppas, MD, president of the American College of Cardiology and one of three physicians signing the letter, said in an interview.
The Association of Black Cardiologists drafted the statement and asked the AHA and ACC if they wanted to sign on. “It felt important to join them and follow their lead,” she said. “There is a clear link between psychosocial stress and discrimination and health equity in the communities.”
Interestingly, the ABC and ACC have an existing partnership, one that included creating a “Campaign for the Future” a little more than a year ago. One of the focuses is on reducing health disparities and starting a diversity and inclusion task force that later became a committee. The groups held a joint board of trustees meeting at Morehouse University, Atlanta, in January 2020. Thinking about that time, Dr. Poppas added, “who knew what was about to transpire over the next few months?”
The letter is only one component of an ongoing effort to “find concrete ways to make change, both within the college and within our profession,” added Dr. Poppas, chief of cardiology and professor of medicine at Brown University, Providence, R.I., and director of the Lifespan Cardiovascular Institute of Rhode Island, Miriam Hospitals, and Newport Hospitals. “Thereby, there is good data that you affect health equity in the population as well.”
“We DENOUNCE incidents of racism and violence that continue to ravage our communities,” the society leaders wrote in the letter. “Given that heart disease and stroke are the leading causes of death for communities of color, particularly African Americans who have the lowest life expectancy of all racial/ethnic groups living in the United States, we are extremely disturbed by violent acts that cut to the core of the lives of our community.”
Other societies released similar statements. For example, the American College of Physicians expressed “grave concern” about recent events and the American Medical Association released a statement entitled “Police brutality must stop.”
A cardiologist speaks out
“Thank you to my organizations, the Association of Black Cardiologists and the American College of Cardiology, for taking a stand,” Travis C. Batts, MD, said in a video statement posted to YouTube on June 2, 2020.
“As an African American male who has sons, brothers, and friends who are also African American, I oftentimes have angst, particularly with my sons. Despite what I do to create an environment that cultivates education and puts them in the right position, there are some people who would stop just at how they look when they approach them,” Dr. Batts said.
“I always have that fear as a father that at some point they may engage with law enforcement – and it may not turn out the way we want it to,” said Dr. Batts, chairman of medical sub-specialties and medical director of the cardiology clinic at Wilford Hall Ambulatory Surgical Center at Lackland Air Force Base, Tex. He also is an associate professor of cardiovascular medicine for the Uniformed Services University of the Health Sciences, Bethesda, Md., and is an adjunct assistant professor at Texas A&M University. He went on in the video to describe how a personal encounter with police years ago changed his life.
The urgent letter from the cardiology societies speaks to health care disparities, Dr. Batts said, “but it doesn’t stop there. It talks about their goals to balance these issues that we see as a pervasive problem in our community.”
The societies point out that George Floyd’s death is not an isolated incident. “Mr. Floyd’s death comes on the heels of other recent incidents caught on camera. In another 2020 incident, Ahmaud Arbery was shot and killed while jogging in his hometown of Brunswick, Ga. Christian Cooper is fortunately alive and well to speak to the Memorial Day incident in New York’s Central Park where he was accused of threatening the life of a woman while bird watching.” They added that “another senseless death involves officers entering the Louisville, Kent., home of emergency medical technician Breonna Taylor.”
Dr. Batts said this portion of the statement was particularly poignant: “We stand and link arms in solidarity with efforts to dismantle systems that maintain excess morbidity and mortality, especially among vulnerable populations and those historically oppressed. Indeed, our collective vast membership, many of whom are at the front lines of clinical health care, has taken an oath to decisively and with kindness, compassion and grace act to relieve suffering related to ‘I can’t breathe’ in order to preserve life.”
A Positive Response
The response to the urgent letter has been “overwhelmingly positive,” Dr. Poppas said. “This isn’t political, per se. This is really about justice, about health equity, and about being moral and conscious human beings. People I hadn’t heard from in years said, ‘thank you for doing this.’ ” The comments on social media were “almost uniformly positive,” she added. “There is always one or two people who feel this isn’t what cardiology is about.”
“Although making a statement is important, so is doing the hard work to make change,” Dr. Poppas said. The goal involves “rolling up our sleeves and spending the time, the money and the energy to make changes – so 5-10 years from now, it looks different.”
In addition to Dr. Poppas, Michelle A. Albert, MD, MPH, president of the Association of Black Cardiologists and Robert A. Harrington, MD, president of the American Heart Association, signed the letter. Dr. Pappas and Dr. Batts had no relevant disclosures.
High-dose tafamidis boosts survival in transthyretin amyloidosis cardiomyopathy
Treatment with oral tafamidis at 80 mg/day provided a significantly greater survival benefit than dosing at 20 mg/day in patients with transthyretin amyloid cardiomyopathy in the long-term extension of the landmark ATTR-ACT trial, Thibaud Damy, MD, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
Moreover, the superior survival benefit achieved by taking four 20-mg capsules of tafamidis (Vyndaqel) once daily – or its more convenient once-daily, single-capsule, 61-mg bioequivalent formulation marketed as Vyndamax – came at no cost in terms of side effects and toxicity, compared with low-dose therapy for this progressive multisystem disease, according to Dr. Damy, professor of cardiology at the University of Paris and head of the French National Referral Center for Cardiac Amyloidosis at Henri Mondor University Hospital, Créteil, France.
“There are no side effects with tafamidis,” he said. “It doesn’t act on any receptors, it just acts on the formation of amyloid fibrils, so there are no side effects at whatever dosage is used. And in ATTR-ACT there was actually a trend towards increased side effects in the placebo group because the amyloidosis is everywhere, so by decreasing the amyloidosis process you improve not only the heart but all the organs, and the patient has a better quality of life.”
ATTR-ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) was a phase 3, double-blind study in which 441 patients with transthyretin amyloidosis cardiomyopathy (TAC) in 13 countries were randomized to tafamidis at either 80 mg or 20 mg per day or placebo and followed prospectively for 30 months. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a statistically significant and clinically important 30% relative risk reduction, establishing tafamidis as the first disease-modifying therapy for this disease (N Engl J Med. 2018 Sep 13;379[11]:1007-16).
Patients in the 80-mg group had a 20% reduction in the risk of death, compared with the 20-mg group, at 30 months in an analysis adjusted for baseline age, 6-minute walk distance, and N-terminal pro-B-type natriuretic peptide, all of which are known to impact survival in TAC. This between-group survival difference wasn’t statistically significant, providing one impetus for the subsequent long-term extension study, in which patients remained on their original dose of tafamidis, and the controls who’d been on placebo for 30 months were randomized 2:1 to tafamidis at 80 mg or 20 mg per day.
The primary endpoint in the long-term extension was a composite of all-cause mortality, heart transplantation, or implantation of a ventricular assist device. At a median follow-up of 39 months since ATTR-ACT began, the high-dose tafamidis group had an adjusted 33% reduction in the risk of this endpoint, compared with patients on 20 mg per day, a difference that barely missed statistical significance. At that point, everyone in the long-term extension was switched to the once-daily 61-mg formulation of tafamidis free acid, which is bioequivalent to four 20-mg capsules of tafamidis.
Dr. Damy’s key message: At a median of 51 months of follow-up, the group originally on 80 mg of tafamidis displayed a highly significant adjusted 43% reduction in risk of the composite endpoint, compared with those who had been on 20 mg per day.
Session chair Petar M. Seferovic, MD, PhD, pronounced the ATTR-ACT trial and its long-term extension “a breakthrough advancement.”
“This is the first time in human medical history that we have a drug which improves the long-term outcome, including survival, in patients with this form of hypertrophic cardiomyopathy. So this is extremely important. It’s one of the major steps forward in the treatment of patients with myocardial disease,” said Dr. Seferovic, president of the European Society of Cardiology Heart Failure Association and professor of internal medicine at the University of Belgrade, Serbia.
Discussant Loreena Hill, PhD, of Queen’s University in Belfast, Northern Ireland, observed that TAC is a devastating disease with a formidable symptom burden and an average survival of just 2-5 years after diagnosis.
“It is often underdiagnosed, and yet it is estimated to account for up to 13% of patients with heart failure and preserved ejection fraction,” she said, adding that she considers the long-term extension results “extremely positive.”
Nailing down the prevalence of hereditary TAC: the DISCOVERY study
TAC occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium and elsewhere. In the heart, the result is progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive nonischemic heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild-type protein.
Dr. Damy was a coinvestigator in the recently published multicenter DISCOVERY study, in which 1,001 patients with clinically suspected cardiac amyloidosis, the great majority of them from the United States, were screened for pathogenic transthyretin genetic mutations. The overall prevalence of such mutations was 8% in the American patients, with the Val122Ile mutation being identified in 11% of African Americans (Amyloid. 2020 May 26;1-8).
The prevalence of wild-type amyloidosis causing TAC hasn’t yet been studied with anything approaching the rigor of DISCOVERY, but the available evidence suggests the wild-type version is roughly as common as the hereditary forms.
Although DISCOVERY and other studies indicate that TAC is far more common than generally realized, Pfizer has priced Vyndaqel and Vyndamax as though TAC is a rare disease, with a U.S. list price of around $225,000 per year.
“Obviously, the cost will go down over time,” Dr. Seferovic predicted.
Diagnosing TAC
Audience members mostly wanted to know how to identify individuals with TAC who are buried within the huge population of patients with heart failure with preserved ejection fraction. Dr. Damy said it’s actually a simple matter using a screening framework developed by an 11-member TAC expert panel on which he served. A definitive diagnosis can usually be achieved noninvasively at a low cost using bone scintigraphy, he added.
The panel recommended screening via bone scintigraphy in patients with an increased left ventricular wall thickness of 14 mm or more in men over age 65 and women older than 70 who either have heart failure or red flag symptoms.
These red flags for TAC include an echocardiographic finding of reduced longitudinal strain with relative apical sparing, a discrepancy between left ventricular wall thickness on imaging and normal or low-normal voltages on a standard 12-lead ECG, diffuse gadolinium enhancement or marked extracellular volume expansion on cardiac magnetic resonance imaging, a history of bilateral carpal tunnel syndrome, symptoms of polyneuropathy, and mildly increased serum troponin levels on multiple occasions (JACC Heart Fail. 2019 Aug;7[8]:709-16).
Dr. Damy reported receiving institutional research grant support from Pfizer, the study sponsor, and serving on a scientific advisory board for the company.
Treatment with oral tafamidis at 80 mg/day provided a significantly greater survival benefit than dosing at 20 mg/day in patients with transthyretin amyloid cardiomyopathy in the long-term extension of the landmark ATTR-ACT trial, Thibaud Damy, MD, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
Moreover, the superior survival benefit achieved by taking four 20-mg capsules of tafamidis (Vyndaqel) once daily – or its more convenient once-daily, single-capsule, 61-mg bioequivalent formulation marketed as Vyndamax – came at no cost in terms of side effects and toxicity, compared with low-dose therapy for this progressive multisystem disease, according to Dr. Damy, professor of cardiology at the University of Paris and head of the French National Referral Center for Cardiac Amyloidosis at Henri Mondor University Hospital, Créteil, France.
“There are no side effects with tafamidis,” he said. “It doesn’t act on any receptors, it just acts on the formation of amyloid fibrils, so there are no side effects at whatever dosage is used. And in ATTR-ACT there was actually a trend towards increased side effects in the placebo group because the amyloidosis is everywhere, so by decreasing the amyloidosis process you improve not only the heart but all the organs, and the patient has a better quality of life.”
ATTR-ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) was a phase 3, double-blind study in which 441 patients with transthyretin amyloidosis cardiomyopathy (TAC) in 13 countries were randomized to tafamidis at either 80 mg or 20 mg per day or placebo and followed prospectively for 30 months. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a statistically significant and clinically important 30% relative risk reduction, establishing tafamidis as the first disease-modifying therapy for this disease (N Engl J Med. 2018 Sep 13;379[11]:1007-16).
Patients in the 80-mg group had a 20% reduction in the risk of death, compared with the 20-mg group, at 30 months in an analysis adjusted for baseline age, 6-minute walk distance, and N-terminal pro-B-type natriuretic peptide, all of which are known to impact survival in TAC. This between-group survival difference wasn’t statistically significant, providing one impetus for the subsequent long-term extension study, in which patients remained on their original dose of tafamidis, and the controls who’d been on placebo for 30 months were randomized 2:1 to tafamidis at 80 mg or 20 mg per day.
The primary endpoint in the long-term extension was a composite of all-cause mortality, heart transplantation, or implantation of a ventricular assist device. At a median follow-up of 39 months since ATTR-ACT began, the high-dose tafamidis group had an adjusted 33% reduction in the risk of this endpoint, compared with patients on 20 mg per day, a difference that barely missed statistical significance. At that point, everyone in the long-term extension was switched to the once-daily 61-mg formulation of tafamidis free acid, which is bioequivalent to four 20-mg capsules of tafamidis.
Dr. Damy’s key message: At a median of 51 months of follow-up, the group originally on 80 mg of tafamidis displayed a highly significant adjusted 43% reduction in risk of the composite endpoint, compared with those who had been on 20 mg per day.
Session chair Petar M. Seferovic, MD, PhD, pronounced the ATTR-ACT trial and its long-term extension “a breakthrough advancement.”
“This is the first time in human medical history that we have a drug which improves the long-term outcome, including survival, in patients with this form of hypertrophic cardiomyopathy. So this is extremely important. It’s one of the major steps forward in the treatment of patients with myocardial disease,” said Dr. Seferovic, president of the European Society of Cardiology Heart Failure Association and professor of internal medicine at the University of Belgrade, Serbia.
Discussant Loreena Hill, PhD, of Queen’s University in Belfast, Northern Ireland, observed that TAC is a devastating disease with a formidable symptom burden and an average survival of just 2-5 years after diagnosis.
“It is often underdiagnosed, and yet it is estimated to account for up to 13% of patients with heart failure and preserved ejection fraction,” she said, adding that she considers the long-term extension results “extremely positive.”
Nailing down the prevalence of hereditary TAC: the DISCOVERY study
TAC occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium and elsewhere. In the heart, the result is progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive nonischemic heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild-type protein.
Dr. Damy was a coinvestigator in the recently published multicenter DISCOVERY study, in which 1,001 patients with clinically suspected cardiac amyloidosis, the great majority of them from the United States, were screened for pathogenic transthyretin genetic mutations. The overall prevalence of such mutations was 8% in the American patients, with the Val122Ile mutation being identified in 11% of African Americans (Amyloid. 2020 May 26;1-8).
The prevalence of wild-type amyloidosis causing TAC hasn’t yet been studied with anything approaching the rigor of DISCOVERY, but the available evidence suggests the wild-type version is roughly as common as the hereditary forms.
Although DISCOVERY and other studies indicate that TAC is far more common than generally realized, Pfizer has priced Vyndaqel and Vyndamax as though TAC is a rare disease, with a U.S. list price of around $225,000 per year.
“Obviously, the cost will go down over time,” Dr. Seferovic predicted.
Diagnosing TAC
Audience members mostly wanted to know how to identify individuals with TAC who are buried within the huge population of patients with heart failure with preserved ejection fraction. Dr. Damy said it’s actually a simple matter using a screening framework developed by an 11-member TAC expert panel on which he served. A definitive diagnosis can usually be achieved noninvasively at a low cost using bone scintigraphy, he added.
The panel recommended screening via bone scintigraphy in patients with an increased left ventricular wall thickness of 14 mm or more in men over age 65 and women older than 70 who either have heart failure or red flag symptoms.
These red flags for TAC include an echocardiographic finding of reduced longitudinal strain with relative apical sparing, a discrepancy between left ventricular wall thickness on imaging and normal or low-normal voltages on a standard 12-lead ECG, diffuse gadolinium enhancement or marked extracellular volume expansion on cardiac magnetic resonance imaging, a history of bilateral carpal tunnel syndrome, symptoms of polyneuropathy, and mildly increased serum troponin levels on multiple occasions (JACC Heart Fail. 2019 Aug;7[8]:709-16).
Dr. Damy reported receiving institutional research grant support from Pfizer, the study sponsor, and serving on a scientific advisory board for the company.
Treatment with oral tafamidis at 80 mg/day provided a significantly greater survival benefit than dosing at 20 mg/day in patients with transthyretin amyloid cardiomyopathy in the long-term extension of the landmark ATTR-ACT trial, Thibaud Damy, MD, PhD, reported at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
Moreover, the superior survival benefit achieved by taking four 20-mg capsules of tafamidis (Vyndaqel) once daily – or its more convenient once-daily, single-capsule, 61-mg bioequivalent formulation marketed as Vyndamax – came at no cost in terms of side effects and toxicity, compared with low-dose therapy for this progressive multisystem disease, according to Dr. Damy, professor of cardiology at the University of Paris and head of the French National Referral Center for Cardiac Amyloidosis at Henri Mondor University Hospital, Créteil, France.
“There are no side effects with tafamidis,” he said. “It doesn’t act on any receptors, it just acts on the formation of amyloid fibrils, so there are no side effects at whatever dosage is used. And in ATTR-ACT there was actually a trend towards increased side effects in the placebo group because the amyloidosis is everywhere, so by decreasing the amyloidosis process you improve not only the heart but all the organs, and the patient has a better quality of life.”
ATTR-ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) was a phase 3, double-blind study in which 441 patients with transthyretin amyloidosis cardiomyopathy (TAC) in 13 countries were randomized to tafamidis at either 80 mg or 20 mg per day or placebo and followed prospectively for 30 months. At 30 months, all-cause mortality was 29.5% in patients who received tafamidis, compared with 42.9% in controls, for a statistically significant and clinically important 30% relative risk reduction, establishing tafamidis as the first disease-modifying therapy for this disease (N Engl J Med. 2018 Sep 13;379[11]:1007-16).
Patients in the 80-mg group had a 20% reduction in the risk of death, compared with the 20-mg group, at 30 months in an analysis adjusted for baseline age, 6-minute walk distance, and N-terminal pro-B-type natriuretic peptide, all of which are known to impact survival in TAC. This between-group survival difference wasn’t statistically significant, providing one impetus for the subsequent long-term extension study, in which patients remained on their original dose of tafamidis, and the controls who’d been on placebo for 30 months were randomized 2:1 to tafamidis at 80 mg or 20 mg per day.
The primary endpoint in the long-term extension was a composite of all-cause mortality, heart transplantation, or implantation of a ventricular assist device. At a median follow-up of 39 months since ATTR-ACT began, the high-dose tafamidis group had an adjusted 33% reduction in the risk of this endpoint, compared with patients on 20 mg per day, a difference that barely missed statistical significance. At that point, everyone in the long-term extension was switched to the once-daily 61-mg formulation of tafamidis free acid, which is bioequivalent to four 20-mg capsules of tafamidis.
Dr. Damy’s key message: At a median of 51 months of follow-up, the group originally on 80 mg of tafamidis displayed a highly significant adjusted 43% reduction in risk of the composite endpoint, compared with those who had been on 20 mg per day.
Session chair Petar M. Seferovic, MD, PhD, pronounced the ATTR-ACT trial and its long-term extension “a breakthrough advancement.”
“This is the first time in human medical history that we have a drug which improves the long-term outcome, including survival, in patients with this form of hypertrophic cardiomyopathy. So this is extremely important. It’s one of the major steps forward in the treatment of patients with myocardial disease,” said Dr. Seferovic, president of the European Society of Cardiology Heart Failure Association and professor of internal medicine at the University of Belgrade, Serbia.
Discussant Loreena Hill, PhD, of Queen’s University in Belfast, Northern Ireland, observed that TAC is a devastating disease with a formidable symptom burden and an average survival of just 2-5 years after diagnosis.
“It is often underdiagnosed, and yet it is estimated to account for up to 13% of patients with heart failure and preserved ejection fraction,” she said, adding that she considers the long-term extension results “extremely positive.”
Nailing down the prevalence of hereditary TAC: the DISCOVERY study
TAC occurs when transthyretin, a transport protein, becomes destabilized and misfolds, promoting deposition of amyloid fibrils in the myocardium and elsewhere. In the heart, the result is progressive ventricular wall thickening and stiffness, manifest as restrictive cardiomyopathy and progressive nonischemic heart failure. The cause of transthyretin destabilization can be either autosomal dominant inheritance of any of more than 100 pathogenic mutations in the transthyretin gene identified to date or a spontaneous wild-type protein.
Dr. Damy was a coinvestigator in the recently published multicenter DISCOVERY study, in which 1,001 patients with clinically suspected cardiac amyloidosis, the great majority of them from the United States, were screened for pathogenic transthyretin genetic mutations. The overall prevalence of such mutations was 8% in the American patients, with the Val122Ile mutation being identified in 11% of African Americans (Amyloid. 2020 May 26;1-8).
The prevalence of wild-type amyloidosis causing TAC hasn’t yet been studied with anything approaching the rigor of DISCOVERY, but the available evidence suggests the wild-type version is roughly as common as the hereditary forms.
Although DISCOVERY and other studies indicate that TAC is far more common than generally realized, Pfizer has priced Vyndaqel and Vyndamax as though TAC is a rare disease, with a U.S. list price of around $225,000 per year.
“Obviously, the cost will go down over time,” Dr. Seferovic predicted.
Diagnosing TAC
Audience members mostly wanted to know how to identify individuals with TAC who are buried within the huge population of patients with heart failure with preserved ejection fraction. Dr. Damy said it’s actually a simple matter using a screening framework developed by an 11-member TAC expert panel on which he served. A definitive diagnosis can usually be achieved noninvasively at a low cost using bone scintigraphy, he added.
The panel recommended screening via bone scintigraphy in patients with an increased left ventricular wall thickness of 14 mm or more in men over age 65 and women older than 70 who either have heart failure or red flag symptoms.
These red flags for TAC include an echocardiographic finding of reduced longitudinal strain with relative apical sparing, a discrepancy between left ventricular wall thickness on imaging and normal or low-normal voltages on a standard 12-lead ECG, diffuse gadolinium enhancement or marked extracellular volume expansion on cardiac magnetic resonance imaging, a history of bilateral carpal tunnel syndrome, symptoms of polyneuropathy, and mildly increased serum troponin levels on multiple occasions (JACC Heart Fail. 2019 Aug;7[8]:709-16).
Dr. Damy reported receiving institutional research grant support from Pfizer, the study sponsor, and serving on a scientific advisory board for the company.
FROM ESC HEART FAILURE 2020
Spinning of results common in industry-sponsored interventional cardiovascular trials
As the rigor of COVID-19 research comes under increasing scrutiny, a deep dive into contemporary trials of invasive cardiovascular interventions finds intricate ties with industry and the art of spin on full display.
After examining 216 randomized, controlled trials published in the past decade, researchers found that more than half (53.2%) were commercially funded. In 18.3% of these trials, the sponsor was involved with the trial conduct and reporting.
Commercially sponsored trials were significantly more likely to report results that favored the experimental therapy than trials without commercial sponsorship (64.3% vs. 48.5%; P = .02).
The association remained statistically significant after adjustment for differences in trial characteristics (exponent of regression coefficient beta, 2.80; 95% confidence interval, 1.09-7.18; P = .03), the authors reported in JAMA Internal Medicine.
“To make this clear, this is not an attack on industry-sponsored trials,” study author and cardiac surgeon Mario Gaudino, MD, of New York–Presbyterian and Weill Cornell Medical Center, New York, said in an interview. “Because industry has more money, they have the best trialists, the best research organization. So they generally do a pretty good trial; they’re larger, they have a higher Fragility Index, which means they’re more solid.
“And, most importantly, more than half of the trials were sponsored by industry,” he said. “So without industry, there wouldn’t be half the research in that 10-year period we explored.”
Previous research in cardiology and in other fields has shown that trials supported by for-profit organizations are more likely to report positive findings. The explanations often focus on bias and differential quality in how the trials were designed and reported.
In the present analysis, however, the authors found no difference between trials with and without industry funding in terms of estimated treatment effect, length of follow-up, use of composite or clinically significant outcomes, or outcome modification, compared with the published protocol.
Part of the explanation may be that industry-sponsored trials more often used a noninferiority design (26.1% vs. 14.9%) and had a higher loss of patients to follow-up (median of sample, 1.0% vs. 0.1%), Dr. Gaudino said. “But I think more, in general, it’s not so much a difference in the measurable characteristics of the trial. It’s the selection of the sites that participate, the patient population that is targeted that makes the trial very likely to get the result that industry would like to see.”
“Just think of the differences in the transcatheter MitraClip results between MITRA-FR and COAPT – basically they were related to the fact they enrolled different patients,” he said.
Significant spin
The analysis included 216 coronary, vascular, and structural interventional cardiology and vascular and cardiac surgical randomized, controlled trials published from January 2008 to May 31, 2019. Most were multicenter trials (78.7%); 58% originated from Europe, 12% from North America, and 10.6% from Asia.
One in six trials (16.2%) were not prospectively registered before the start of enrollment, and at least one major discrepancy existed between the registered and published primary outcome in 38% of registered trials.
“If you don’t register the trial then you can make all the changes you want to the protocol up until the moment you publish,” Dr. Gaudino observed. “There really is no rational justification for not registering a trial.”
Overall, the trials were not particularly robust, he noted. In 62 trials in which the Fragility Index was measured, only a median of five patients experiencing a different outcome in a commercially sponsored trial would change statistically significant results to nonsignificant. For noncommercially sponsored trials, that number was 4.5 and in four trials; the change in condition of only one patient was needed to switch the statistical significance.
“This finding is concerning given the substantial role that [randomized, controlled trials] results play in federal device approvals, payer criteria, and clinical consensus guidelines,” the authors wrote.
The authors also looked for interpretation bias in the trials. In the 84 trials with nonsignificant differences in the primary outcomes, 65.5% contained spin, such as focusing on statistically significant secondary outcomes or interpreting nonsignificant primary outcomes as showing treatment equivalence or comparable effectiveness. Spin was present in 80.6% of the trials with commercial sponsorship and in 54.2% without (P = .02) – a finding that remained significant after trial differences were controlled for (beta, 4.64; 95% CI, 1.05-20.54; P = .04).
A pivot point
“It’s just another paper showing there are issues with conflicts of interest in industry trials. I’m not particularly surprised,” said David Moher, PhD, MSc, director of the Centre for Journalology, based at the Ottawa Hospital Research Institute.
“It’s sort of high time people from all sides sat down together and tried to resolve how to actually move forward with industry wanting to do trials,” he said. “They are hugely important in drug development. How can these trials be done where the impact of industry and, for that matter, academia is minimized?”
Dr. Gaudino suggested the “ideal situation” would be to have industry put its funding into an existing funding organization, such as the National Institutes of Health or a newly created independent organization – a concept that has been floated before without much forward movement.
“We may be at a pivot point,” Dr. Moher said. “It’s quite clear that COVID has indicated some serious problems with how trials are done, how they’re disseminated, the notion of open science. I think this could be an opportunity. Whether there is so much noise, whether anybody will be able to take any of these initiative forward, I don’t know.”
No matter how trial funding is revised, patients must be brought to the table, he said.
“What frustrates me quite a bit is this almost parental view of all of this – the scientists know best, industry knows best,” Dr. Moher said. “We actually need the most important groups: patients and the public. They need to have an enormous amount of say in how this actually is formed.”
Commenting further, Dr. Moher said that “industry and academia can only do trials when they have patients willing to participate, and yet in the discussions you and I are having, what do patients think about spin in trials? I would imagine they would be horrified that they are going into studies – in a sense in many cases risking their lives – and yet people are spinning the results.”
Dr. Gaudino and Dr. Moher reported having no relevant conflicts of interest.
A version of this story originally appeared on Medscape.com.
As the rigor of COVID-19 research comes under increasing scrutiny, a deep dive into contemporary trials of invasive cardiovascular interventions finds intricate ties with industry and the art of spin on full display.
After examining 216 randomized, controlled trials published in the past decade, researchers found that more than half (53.2%) were commercially funded. In 18.3% of these trials, the sponsor was involved with the trial conduct and reporting.
Commercially sponsored trials were significantly more likely to report results that favored the experimental therapy than trials without commercial sponsorship (64.3% vs. 48.5%; P = .02).
The association remained statistically significant after adjustment for differences in trial characteristics (exponent of regression coefficient beta, 2.80; 95% confidence interval, 1.09-7.18; P = .03), the authors reported in JAMA Internal Medicine.
“To make this clear, this is not an attack on industry-sponsored trials,” study author and cardiac surgeon Mario Gaudino, MD, of New York–Presbyterian and Weill Cornell Medical Center, New York, said in an interview. “Because industry has more money, they have the best trialists, the best research organization. So they generally do a pretty good trial; they’re larger, they have a higher Fragility Index, which means they’re more solid.
“And, most importantly, more than half of the trials were sponsored by industry,” he said. “So without industry, there wouldn’t be half the research in that 10-year period we explored.”
Previous research in cardiology and in other fields has shown that trials supported by for-profit organizations are more likely to report positive findings. The explanations often focus on bias and differential quality in how the trials were designed and reported.
In the present analysis, however, the authors found no difference between trials with and without industry funding in terms of estimated treatment effect, length of follow-up, use of composite or clinically significant outcomes, or outcome modification, compared with the published protocol.
Part of the explanation may be that industry-sponsored trials more often used a noninferiority design (26.1% vs. 14.9%) and had a higher loss of patients to follow-up (median of sample, 1.0% vs. 0.1%), Dr. Gaudino said. “But I think more, in general, it’s not so much a difference in the measurable characteristics of the trial. It’s the selection of the sites that participate, the patient population that is targeted that makes the trial very likely to get the result that industry would like to see.”
“Just think of the differences in the transcatheter MitraClip results between MITRA-FR and COAPT – basically they were related to the fact they enrolled different patients,” he said.
Significant spin
The analysis included 216 coronary, vascular, and structural interventional cardiology and vascular and cardiac surgical randomized, controlled trials published from January 2008 to May 31, 2019. Most were multicenter trials (78.7%); 58% originated from Europe, 12% from North America, and 10.6% from Asia.
One in six trials (16.2%) were not prospectively registered before the start of enrollment, and at least one major discrepancy existed between the registered and published primary outcome in 38% of registered trials.
“If you don’t register the trial then you can make all the changes you want to the protocol up until the moment you publish,” Dr. Gaudino observed. “There really is no rational justification for not registering a trial.”
Overall, the trials were not particularly robust, he noted. In 62 trials in which the Fragility Index was measured, only a median of five patients experiencing a different outcome in a commercially sponsored trial would change statistically significant results to nonsignificant. For noncommercially sponsored trials, that number was 4.5 and in four trials; the change in condition of only one patient was needed to switch the statistical significance.
“This finding is concerning given the substantial role that [randomized, controlled trials] results play in federal device approvals, payer criteria, and clinical consensus guidelines,” the authors wrote.
The authors also looked for interpretation bias in the trials. In the 84 trials with nonsignificant differences in the primary outcomes, 65.5% contained spin, such as focusing on statistically significant secondary outcomes or interpreting nonsignificant primary outcomes as showing treatment equivalence or comparable effectiveness. Spin was present in 80.6% of the trials with commercial sponsorship and in 54.2% without (P = .02) – a finding that remained significant after trial differences were controlled for (beta, 4.64; 95% CI, 1.05-20.54; P = .04).
A pivot point
“It’s just another paper showing there are issues with conflicts of interest in industry trials. I’m not particularly surprised,” said David Moher, PhD, MSc, director of the Centre for Journalology, based at the Ottawa Hospital Research Institute.
“It’s sort of high time people from all sides sat down together and tried to resolve how to actually move forward with industry wanting to do trials,” he said. “They are hugely important in drug development. How can these trials be done where the impact of industry and, for that matter, academia is minimized?”
Dr. Gaudino suggested the “ideal situation” would be to have industry put its funding into an existing funding organization, such as the National Institutes of Health or a newly created independent organization – a concept that has been floated before without much forward movement.
“We may be at a pivot point,” Dr. Moher said. “It’s quite clear that COVID has indicated some serious problems with how trials are done, how they’re disseminated, the notion of open science. I think this could be an opportunity. Whether there is so much noise, whether anybody will be able to take any of these initiative forward, I don’t know.”
No matter how trial funding is revised, patients must be brought to the table, he said.
“What frustrates me quite a bit is this almost parental view of all of this – the scientists know best, industry knows best,” Dr. Moher said. “We actually need the most important groups: patients and the public. They need to have an enormous amount of say in how this actually is formed.”
Commenting further, Dr. Moher said that “industry and academia can only do trials when they have patients willing to participate, and yet in the discussions you and I are having, what do patients think about spin in trials? I would imagine they would be horrified that they are going into studies – in a sense in many cases risking their lives – and yet people are spinning the results.”
Dr. Gaudino and Dr. Moher reported having no relevant conflicts of interest.
A version of this story originally appeared on Medscape.com.
As the rigor of COVID-19 research comes under increasing scrutiny, a deep dive into contemporary trials of invasive cardiovascular interventions finds intricate ties with industry and the art of spin on full display.
After examining 216 randomized, controlled trials published in the past decade, researchers found that more than half (53.2%) were commercially funded. In 18.3% of these trials, the sponsor was involved with the trial conduct and reporting.
Commercially sponsored trials were significantly more likely to report results that favored the experimental therapy than trials without commercial sponsorship (64.3% vs. 48.5%; P = .02).
The association remained statistically significant after adjustment for differences in trial characteristics (exponent of regression coefficient beta, 2.80; 95% confidence interval, 1.09-7.18; P = .03), the authors reported in JAMA Internal Medicine.
“To make this clear, this is not an attack on industry-sponsored trials,” study author and cardiac surgeon Mario Gaudino, MD, of New York–Presbyterian and Weill Cornell Medical Center, New York, said in an interview. “Because industry has more money, they have the best trialists, the best research organization. So they generally do a pretty good trial; they’re larger, they have a higher Fragility Index, which means they’re more solid.
“And, most importantly, more than half of the trials were sponsored by industry,” he said. “So without industry, there wouldn’t be half the research in that 10-year period we explored.”
Previous research in cardiology and in other fields has shown that trials supported by for-profit organizations are more likely to report positive findings. The explanations often focus on bias and differential quality in how the trials were designed and reported.
In the present analysis, however, the authors found no difference between trials with and without industry funding in terms of estimated treatment effect, length of follow-up, use of composite or clinically significant outcomes, or outcome modification, compared with the published protocol.
Part of the explanation may be that industry-sponsored trials more often used a noninferiority design (26.1% vs. 14.9%) and had a higher loss of patients to follow-up (median of sample, 1.0% vs. 0.1%), Dr. Gaudino said. “But I think more, in general, it’s not so much a difference in the measurable characteristics of the trial. It’s the selection of the sites that participate, the patient population that is targeted that makes the trial very likely to get the result that industry would like to see.”
“Just think of the differences in the transcatheter MitraClip results between MITRA-FR and COAPT – basically they were related to the fact they enrolled different patients,” he said.
Significant spin
The analysis included 216 coronary, vascular, and structural interventional cardiology and vascular and cardiac surgical randomized, controlled trials published from January 2008 to May 31, 2019. Most were multicenter trials (78.7%); 58% originated from Europe, 12% from North America, and 10.6% from Asia.
One in six trials (16.2%) were not prospectively registered before the start of enrollment, and at least one major discrepancy existed between the registered and published primary outcome in 38% of registered trials.
“If you don’t register the trial then you can make all the changes you want to the protocol up until the moment you publish,” Dr. Gaudino observed. “There really is no rational justification for not registering a trial.”
Overall, the trials were not particularly robust, he noted. In 62 trials in which the Fragility Index was measured, only a median of five patients experiencing a different outcome in a commercially sponsored trial would change statistically significant results to nonsignificant. For noncommercially sponsored trials, that number was 4.5 and in four trials; the change in condition of only one patient was needed to switch the statistical significance.
“This finding is concerning given the substantial role that [randomized, controlled trials] results play in federal device approvals, payer criteria, and clinical consensus guidelines,” the authors wrote.
The authors also looked for interpretation bias in the trials. In the 84 trials with nonsignificant differences in the primary outcomes, 65.5% contained spin, such as focusing on statistically significant secondary outcomes or interpreting nonsignificant primary outcomes as showing treatment equivalence or comparable effectiveness. Spin was present in 80.6% of the trials with commercial sponsorship and in 54.2% without (P = .02) – a finding that remained significant after trial differences were controlled for (beta, 4.64; 95% CI, 1.05-20.54; P = .04).
A pivot point
“It’s just another paper showing there are issues with conflicts of interest in industry trials. I’m not particularly surprised,” said David Moher, PhD, MSc, director of the Centre for Journalology, based at the Ottawa Hospital Research Institute.
“It’s sort of high time people from all sides sat down together and tried to resolve how to actually move forward with industry wanting to do trials,” he said. “They are hugely important in drug development. How can these trials be done where the impact of industry and, for that matter, academia is minimized?”
Dr. Gaudino suggested the “ideal situation” would be to have industry put its funding into an existing funding organization, such as the National Institutes of Health or a newly created independent organization – a concept that has been floated before without much forward movement.
“We may be at a pivot point,” Dr. Moher said. “It’s quite clear that COVID has indicated some serious problems with how trials are done, how they’re disseminated, the notion of open science. I think this could be an opportunity. Whether there is so much noise, whether anybody will be able to take any of these initiative forward, I don’t know.”
No matter how trial funding is revised, patients must be brought to the table, he said.
“What frustrates me quite a bit is this almost parental view of all of this – the scientists know best, industry knows best,” Dr. Moher said. “We actually need the most important groups: patients and the public. They need to have an enormous amount of say in how this actually is formed.”
Commenting further, Dr. Moher said that “industry and academia can only do trials when they have patients willing to participate, and yet in the discussions you and I are having, what do patients think about spin in trials? I would imagine they would be horrified that they are going into studies – in a sense in many cases risking their lives – and yet people are spinning the results.”
Dr. Gaudino and Dr. Moher reported having no relevant conflicts of interest.
A version of this story originally appeared on Medscape.com.
Lancet, NEJM retract studies on hydroxychloroquine for COVID-19
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
The Lancet article, titled “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis” was originally published online May 22. The NEJM article, “Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19” was initially published May 1.
Three authors of the Lancet article, Mandeep R. Mehra, MD, Frank Ruschitzka, MD, and Amit N. Patel, MD, wrote in a letter that the action came after concerns were raised about the integrity of the data, and about how the analysis was conducted by Chicago-based Surgisphere Corp and study coauthor Sapan Desai, MD, Surgisphere’s founder and CEO.
The authors asked for an independent third-party review of Surgisphere to evaluate the integrity of the trial elements and to replicate the analyses in the article.
“Our independent peer reviewers informed us that Surgisphere would not transfer the full dataset, client contracts, and the full ISO audit report to their servers for analysis, as such transfer would violate client agreements and confidentiality requirements,” the authors wrote.
Therefore, reviewers were not able to conduct the review and notified the authors they would withdraw from the peer-review process.
The Lancet said in a statement: “The Lancet takes issues of scientific integrity extremely seriously, and there are many outstanding questions about Surgisphere and the data that were allegedly included in this study. Following guidelines from the Committee on Publication Ethics and International Committee of Medical Journal Editors, institutional reviews of Surgisphere’s research collaborations are urgently needed.”
The authors wrote, “We can never forget the responsibility we have as researchers to scrupulously ensure that we rely on data sources that adhere to our high standards. Based on this development, we can no longer vouch for the veracity of the primary data sources. Due to this unfortunate development, the authors request that the paper be retracted.
“We all entered this collaboration to contribute in good faith and at a time of great need during the COVID-19 pandemic. We deeply apologize to you, the editors, and the journal readership for any embarrassment or inconvenience that this may have caused.”
In a similar, if briefer, note, the authors requested that the New England Journal of Medicine retract the earlier article as well. The retraction notice on the website reads: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article, ‘Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19.’ We therefore request that the article be retracted. We apologize to the editors and to readers of the Journal for the difficulties that this has caused.”
Both journals had already published “Expression of Concern” notices about the articles. The expression of concern followed an open letter, endorsed by more than 200 scientists, ethicists, and clinicians and posted on May 28, questioning the data and ethics of the study.
A version of this article originally appeared on Medscape.com.