User login
EMERGENCY MEDICINE is a practical, peer-reviewed monthly publication and Web site that meets the educational needs of emergency clinicians and urgent care clinicians for their practice.
Findings of most heart failure trials reported late or not at all
A large proportion of results from heart failure trials registered with clinicaltrials.gov are published a year or more after completion or not at all, which violates the U.S. FDA Amendments Act (FDAAA), according to a detailed analysis of the interventional and observational trials in this database.
Of the 1,429 heart failure trials identified, 75% of which were randomized interventional studies and the remainder of which were observational, fewer than 20% met the FDAAA 1-year reporting requirement, and 44% have yet to be published at all, reported a team of collaborative investigators led by cardiologists from the Inova Heart and Vascular Institute (IHVI), Falls Church, Va.
“I believe the critical issue is that the FDAAA has thus far never been enforced,” reported Christopher M. O’Connor, MD, a cardiologist and president of IHVI. He was the senior author of the study, reported in the Journal of the American College of Cardiology.
To improve systematic reporting of clinical trials, including negative results, clinicaltrials.gov was created in 2000. In 2007, the FDAAA enacted rules to broaden the requirements for reporting and to make timely reporting of results mandatory.
Ten years later, the FDA was finally authorized to issue a penalty of $10,000 for failure to release results in a timely fashion, a provision of the 2007 amendment but not confirmed at that time, the investigators reported. In the majority of cases, timely reporting was defined as within 12 months of completion of the trial.
The new study shows that reporting of completed trials, timely or otherwise, remains low. Of the 1,243 trials completed after 2007, the proportion meeting the 1-year reporting requirement was just 20%. Although a significant improvement over the 13% reporting in this time frame before 2007, more than 80% of findings are not being released in a timely manner more than 10 years after this was made mandatory.
There are a number of reasons to consider this to be a serious issue, according to Mandeep R. Mehra, MD, of Brigham and Women’s Hospital, Boston. One of the authors of an accompanying editorial regarding this analysis, Dr. Mehra called underreporting “a public health matter because it is an impediment to medical discovery and poses plausible threats to patient safety.”
Among studies registered after 2007, publication rates were higher for trials funded by the National Institutes of Health (71%) relative to industry (49%) or the U.S. Veterans Affairs (45%).
Publication rates were also higher among interventional relative to observational trials (59% vs. 46%) and trials that enrolled more than 1,000 patients relative to those enrolling fewer than 150 (77% vs. 51%), although trial size was not a significant predictor of publication on multivariate analysis. Clinical endpoints, such as death or hospitalization, were also associated with a greater likelihood of publication relative to nonclinical endpoints.
Of the 251 trials terminated before completion, findings were published within 1 year in only 6%. Two years after completion, only 20% were published at all.
Results consistent with the primary hypothesis did not predict timely publication, but only 39% of the studies listed a primary hypothesis. Since 2017, this is another violation of the FDAAA, according to Dr. O’Connor.
The problem is not unique to heart failure trials, according to the authors who cited numerous studies showing low rates of timely publication in other therapeutic areas. Heart failure was selected for evaluation in this study mainly to keep the analysis feasible, although the authors contend this is an area with an urgent need for better treatments.
The problem needs to be fixed, according to Dr. Mehra. In his editorial, he called for rules to be “transitioned to regulations and action taken for underreporting.” Dr. O’Connor agreed.
“A combination of carrots and sticks might be needed to achieve sufficient result sharing,” Dr. O’Connor said. He suggested that stakeholders, such as investigators, sponsors, regulators, and journal editors, should collaborate to address the problem.
So far, the FDA has never levied a fine for lack of reporting or for failure to report in a timely manner. Routine imposition of large fines might not be viable, given the complex reasons that delay or inhibit publication of trial findings, but it would be a large source of revenue.
“According to the FDAAA TrialsTracker, a live tool that tracks FDAAA compliance and promotes trial transparency, the U.S. government could already have imposed more than $2.8 billion in fines for trials due after January 2018,” Dr. O’Connor reported.
The first and senior authors are among those who report financial relationships with pharmaceutical companies.
SOURCE: Psotka MA et al. J Am Coll Cardiol. 2020;75:3151-61.
A large proportion of results from heart failure trials registered with clinicaltrials.gov are published a year or more after completion or not at all, which violates the U.S. FDA Amendments Act (FDAAA), according to a detailed analysis of the interventional and observational trials in this database.
Of the 1,429 heart failure trials identified, 75% of which were randomized interventional studies and the remainder of which were observational, fewer than 20% met the FDAAA 1-year reporting requirement, and 44% have yet to be published at all, reported a team of collaborative investigators led by cardiologists from the Inova Heart and Vascular Institute (IHVI), Falls Church, Va.
“I believe the critical issue is that the FDAAA has thus far never been enforced,” reported Christopher M. O’Connor, MD, a cardiologist and president of IHVI. He was the senior author of the study, reported in the Journal of the American College of Cardiology.
To improve systematic reporting of clinical trials, including negative results, clinicaltrials.gov was created in 2000. In 2007, the FDAAA enacted rules to broaden the requirements for reporting and to make timely reporting of results mandatory.
Ten years later, the FDA was finally authorized to issue a penalty of $10,000 for failure to release results in a timely fashion, a provision of the 2007 amendment but not confirmed at that time, the investigators reported. In the majority of cases, timely reporting was defined as within 12 months of completion of the trial.
The new study shows that reporting of completed trials, timely or otherwise, remains low. Of the 1,243 trials completed after 2007, the proportion meeting the 1-year reporting requirement was just 20%. Although a significant improvement over the 13% reporting in this time frame before 2007, more than 80% of findings are not being released in a timely manner more than 10 years after this was made mandatory.
There are a number of reasons to consider this to be a serious issue, according to Mandeep R. Mehra, MD, of Brigham and Women’s Hospital, Boston. One of the authors of an accompanying editorial regarding this analysis, Dr. Mehra called underreporting “a public health matter because it is an impediment to medical discovery and poses plausible threats to patient safety.”
Among studies registered after 2007, publication rates were higher for trials funded by the National Institutes of Health (71%) relative to industry (49%) or the U.S. Veterans Affairs (45%).
Publication rates were also higher among interventional relative to observational trials (59% vs. 46%) and trials that enrolled more than 1,000 patients relative to those enrolling fewer than 150 (77% vs. 51%), although trial size was not a significant predictor of publication on multivariate analysis. Clinical endpoints, such as death or hospitalization, were also associated with a greater likelihood of publication relative to nonclinical endpoints.
Of the 251 trials terminated before completion, findings were published within 1 year in only 6%. Two years after completion, only 20% were published at all.
Results consistent with the primary hypothesis did not predict timely publication, but only 39% of the studies listed a primary hypothesis. Since 2017, this is another violation of the FDAAA, according to Dr. O’Connor.
The problem is not unique to heart failure trials, according to the authors who cited numerous studies showing low rates of timely publication in other therapeutic areas. Heart failure was selected for evaluation in this study mainly to keep the analysis feasible, although the authors contend this is an area with an urgent need for better treatments.
The problem needs to be fixed, according to Dr. Mehra. In his editorial, he called for rules to be “transitioned to regulations and action taken for underreporting.” Dr. O’Connor agreed.
“A combination of carrots and sticks might be needed to achieve sufficient result sharing,” Dr. O’Connor said. He suggested that stakeholders, such as investigators, sponsors, regulators, and journal editors, should collaborate to address the problem.
So far, the FDA has never levied a fine for lack of reporting or for failure to report in a timely manner. Routine imposition of large fines might not be viable, given the complex reasons that delay or inhibit publication of trial findings, but it would be a large source of revenue.
“According to the FDAAA TrialsTracker, a live tool that tracks FDAAA compliance and promotes trial transparency, the U.S. government could already have imposed more than $2.8 billion in fines for trials due after January 2018,” Dr. O’Connor reported.
The first and senior authors are among those who report financial relationships with pharmaceutical companies.
SOURCE: Psotka MA et al. J Am Coll Cardiol. 2020;75:3151-61.
A large proportion of results from heart failure trials registered with clinicaltrials.gov are published a year or more after completion or not at all, which violates the U.S. FDA Amendments Act (FDAAA), according to a detailed analysis of the interventional and observational trials in this database.
Of the 1,429 heart failure trials identified, 75% of which were randomized interventional studies and the remainder of which were observational, fewer than 20% met the FDAAA 1-year reporting requirement, and 44% have yet to be published at all, reported a team of collaborative investigators led by cardiologists from the Inova Heart and Vascular Institute (IHVI), Falls Church, Va.
“I believe the critical issue is that the FDAAA has thus far never been enforced,” reported Christopher M. O’Connor, MD, a cardiologist and president of IHVI. He was the senior author of the study, reported in the Journal of the American College of Cardiology.
To improve systematic reporting of clinical trials, including negative results, clinicaltrials.gov was created in 2000. In 2007, the FDAAA enacted rules to broaden the requirements for reporting and to make timely reporting of results mandatory.
Ten years later, the FDA was finally authorized to issue a penalty of $10,000 for failure to release results in a timely fashion, a provision of the 2007 amendment but not confirmed at that time, the investigators reported. In the majority of cases, timely reporting was defined as within 12 months of completion of the trial.
The new study shows that reporting of completed trials, timely or otherwise, remains low. Of the 1,243 trials completed after 2007, the proportion meeting the 1-year reporting requirement was just 20%. Although a significant improvement over the 13% reporting in this time frame before 2007, more than 80% of findings are not being released in a timely manner more than 10 years after this was made mandatory.
There are a number of reasons to consider this to be a serious issue, according to Mandeep R. Mehra, MD, of Brigham and Women’s Hospital, Boston. One of the authors of an accompanying editorial regarding this analysis, Dr. Mehra called underreporting “a public health matter because it is an impediment to medical discovery and poses plausible threats to patient safety.”
Among studies registered after 2007, publication rates were higher for trials funded by the National Institutes of Health (71%) relative to industry (49%) or the U.S. Veterans Affairs (45%).
Publication rates were also higher among interventional relative to observational trials (59% vs. 46%) and trials that enrolled more than 1,000 patients relative to those enrolling fewer than 150 (77% vs. 51%), although trial size was not a significant predictor of publication on multivariate analysis. Clinical endpoints, such as death or hospitalization, were also associated with a greater likelihood of publication relative to nonclinical endpoints.
Of the 251 trials terminated before completion, findings were published within 1 year in only 6%. Two years after completion, only 20% were published at all.
Results consistent with the primary hypothesis did not predict timely publication, but only 39% of the studies listed a primary hypothesis. Since 2017, this is another violation of the FDAAA, according to Dr. O’Connor.
The problem is not unique to heart failure trials, according to the authors who cited numerous studies showing low rates of timely publication in other therapeutic areas. Heart failure was selected for evaluation in this study mainly to keep the analysis feasible, although the authors contend this is an area with an urgent need for better treatments.
The problem needs to be fixed, according to Dr. Mehra. In his editorial, he called for rules to be “transitioned to regulations and action taken for underreporting.” Dr. O’Connor agreed.
“A combination of carrots and sticks might be needed to achieve sufficient result sharing,” Dr. O’Connor said. He suggested that stakeholders, such as investigators, sponsors, regulators, and journal editors, should collaborate to address the problem.
So far, the FDA has never levied a fine for lack of reporting or for failure to report in a timely manner. Routine imposition of large fines might not be viable, given the complex reasons that delay or inhibit publication of trial findings, but it would be a large source of revenue.
“According to the FDAAA TrialsTracker, a live tool that tracks FDAAA compliance and promotes trial transparency, the U.S. government could already have imposed more than $2.8 billion in fines for trials due after January 2018,” Dr. O’Connor reported.
The first and senior authors are among those who report financial relationships with pharmaceutical companies.
SOURCE: Psotka MA et al. J Am Coll Cardiol. 2020;75:3151-61.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
How to reboot elective CV procedures after COVID-19 lockdown
With the COVID-19 pandemic winding down in some parts of the United States, attention has turned to figuring out how to safely reboot elective cardiovascular (CV) services, which, for the most part, shut down in order to combat the virus and flatten the curve.
To aid in this effort, top cardiology societies have published a series of guidance documents. One, entitled Multimodality Cardiovascular Imaging in the Midst of the COVID-19 Pandemic: Ramping Up Safely to a New Normal, was initiated by the editors of JACC Cardiovascular Imaging and was developed in collaboration with the ACC Cardiovascular Imaging Council.
“As we enter a deceleration or indolent phase of the disease and a return to a ‘new normal’ for the foreseeable future, cardiovascular imaging laboratories will adjust to a different work flow and safety precautions for patients and staff alike,” write William Zoghbi, MD, of the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, and colleagues.
Minimize risk, maximize clinical benefit
The group outlined strategies and considerations on how to safely ramp up multimodality CV imaging laboratories in an environment of an abating but continuing pandemic.
The authors provide detailed advice on reestablishing echocardiography, transthoracic echocardiography, transesophageal echocardiography, stress testing modalities, treadmill testing, nuclear cardiology, cardiac CT, and cardiac MRI.
The advice is designed to “minimize risk, reduce resource utilization and maximize clinical benefit,” the authors wrote. They address patient and societal health; safety of healthcare professionals; choice of CV testing; and scheduling considerations.
Dr. Zoghbi and colleagues said that integrated communication among patients, referring physicians, the imaging teams, and administrative staff are key to reestablishing a more normal clinical operation.
“Recognizing that practice patterns and policies vary depending on institution and locale, the recommendations are not meant to be restrictive but rather to serve as a general framework during the COVID-19 pandemic and its recovery phase,” the writing group said.
Ultimately, the goal is to offer the necessary CV tests and information for the clinical team to provide the best care for patients, they added.
“To be successful in this new safety-driven modus operandi, innovation, coordination and adaptation among clinicians, staff and patients is necessary till herd immunity or control of COVID-19 is achieved,” they concluded.
Rebooting electrophysiology services
Uncertainty as to how to resume electrophysiology (EP) services for arrhythmia patients prompted representatives from the Heart Rhythm Society, the American Heart Association, and the ACC to develop a series of “guiding suggestions and principles” to help safely reestablish electrophysiological care.
The 28-page document is published in Circulation: Arrhythmia and Electrophysiology and the Journal of the American College of Cardiology Electrophysiology.
“Rebooting” EP services at many institutions may be more challenging than shutting down, wrote Dhanunjaya R. Lakkireddy, MD, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan., and colleagues.
Topics addressed by the writing group include the role of viral screening and serologic testing, return-to-work considerations for exposed or infected health care workers, risk stratification and management strategies based on COVID-19 disease burden, institutional preparedness for resumption of elective procedures, patient preparation and communication; prioritization of procedures, and development of outpatient and periprocedural care pathways.
They suggest creating an EP COVID-19 “reboot team” made up of stakeholders involved in the EP care continuum pathway that would coordinate with institutional or hospital-level COVID-19 leadership.
The reboot team may include an electrophysiologist, an EP laboratory manager, an outpatient clinic manager, an EP nurse, advanced practice providers, a device technician, an anesthesiologist, and an imaging team to provide insights into various aspects of the work flow.
“This team can clarify, interpret, iterate and disseminate policies, and also provide the necessary operational support to plan and successfully execute the reboot process as the efforts to contain COVID-19 continue,” the writing group said.
A mandatory component of the reboot plan should be planning for a second wave of the virus.
“We will have to learn to create relatively COVID-19 safe zones within the hospitals to help isolate patients from second waves and yet be able to provide regular care for non–COVID-19 patients,” the writing group said.
“Our main goal as health care professionals, whether we serve in a clinical, teaching, research, or administrative role, is to do everything we can to create a safe environment for our patients so that they receive the excellent care they deserve,” they concluded.
Defining moment for remote arrhythmia monitoring
In a separate report, an international team of heart rhythm specialists from the Latin American Heart Rhythm Society, the HRS, the European Heart Rhythm Association, the Asia Pacific Heart Rhythm Society, the AHA, and the ACC discussed how the pandemic has fueled adoption of telehealth and remote patient management across medicine, including heart rhythm monitoring.
Their report was simultaneously published in Circulation: Arrhythmia and Electrophysiology, EP Europace, the Journal of the American College of Cardiology, the Journal of Arrhythmia, and Heart Rhythm.
The COVID-19 pandemic has “catalyzed the use of wearables and digital medical tools,” and this will likely define medicine going forward, first author Niraj Varma, MD, PhD, of the Cleveland Clinic, said in an interview.
He noted that the technology has been available for some time, but the pandemic has forced people to use it. “Necessity is the mother of invention, and this has become necessary during the pandemic when we can’t see our patients,” said Dr. Varma.
He also noted that hospitals and physicians are now realizing that telehealth and remote arrhythmia monitoring “actually work, and regulatory agencies have moved very swiftly to dissolve traditional barriers and will now reimburse for it. So it’s a win-win.”
Dr. Varma and colleagues said that the time is right to “embed and grow remote services in everyday medical practice worldwide.” In their report, they offered a list of commonly used platforms for telehealth and examples of remote electrocardiogram and heart rate monitoring devices.
Development of the three reports had no commercial funding. Complete lists of disclosures for the writing groups are available in the original articles.
A version of this article originally appeared on Medscape.com.
With the COVID-19 pandemic winding down in some parts of the United States, attention has turned to figuring out how to safely reboot elective cardiovascular (CV) services, which, for the most part, shut down in order to combat the virus and flatten the curve.
To aid in this effort, top cardiology societies have published a series of guidance documents. One, entitled Multimodality Cardiovascular Imaging in the Midst of the COVID-19 Pandemic: Ramping Up Safely to a New Normal, was initiated by the editors of JACC Cardiovascular Imaging and was developed in collaboration with the ACC Cardiovascular Imaging Council.
“As we enter a deceleration or indolent phase of the disease and a return to a ‘new normal’ for the foreseeable future, cardiovascular imaging laboratories will adjust to a different work flow and safety precautions for patients and staff alike,” write William Zoghbi, MD, of the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, and colleagues.
Minimize risk, maximize clinical benefit
The group outlined strategies and considerations on how to safely ramp up multimodality CV imaging laboratories in an environment of an abating but continuing pandemic.
The authors provide detailed advice on reestablishing echocardiography, transthoracic echocardiography, transesophageal echocardiography, stress testing modalities, treadmill testing, nuclear cardiology, cardiac CT, and cardiac MRI.
The advice is designed to “minimize risk, reduce resource utilization and maximize clinical benefit,” the authors wrote. They address patient and societal health; safety of healthcare professionals; choice of CV testing; and scheduling considerations.
Dr. Zoghbi and colleagues said that integrated communication among patients, referring physicians, the imaging teams, and administrative staff are key to reestablishing a more normal clinical operation.
“Recognizing that practice patterns and policies vary depending on institution and locale, the recommendations are not meant to be restrictive but rather to serve as a general framework during the COVID-19 pandemic and its recovery phase,” the writing group said.
Ultimately, the goal is to offer the necessary CV tests and information for the clinical team to provide the best care for patients, they added.
“To be successful in this new safety-driven modus operandi, innovation, coordination and adaptation among clinicians, staff and patients is necessary till herd immunity or control of COVID-19 is achieved,” they concluded.
Rebooting electrophysiology services
Uncertainty as to how to resume electrophysiology (EP) services for arrhythmia patients prompted representatives from the Heart Rhythm Society, the American Heart Association, and the ACC to develop a series of “guiding suggestions and principles” to help safely reestablish electrophysiological care.
The 28-page document is published in Circulation: Arrhythmia and Electrophysiology and the Journal of the American College of Cardiology Electrophysiology.
“Rebooting” EP services at many institutions may be more challenging than shutting down, wrote Dhanunjaya R. Lakkireddy, MD, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan., and colleagues.
Topics addressed by the writing group include the role of viral screening and serologic testing, return-to-work considerations for exposed or infected health care workers, risk stratification and management strategies based on COVID-19 disease burden, institutional preparedness for resumption of elective procedures, patient preparation and communication; prioritization of procedures, and development of outpatient and periprocedural care pathways.
They suggest creating an EP COVID-19 “reboot team” made up of stakeholders involved in the EP care continuum pathway that would coordinate with institutional or hospital-level COVID-19 leadership.
The reboot team may include an electrophysiologist, an EP laboratory manager, an outpatient clinic manager, an EP nurse, advanced practice providers, a device technician, an anesthesiologist, and an imaging team to provide insights into various aspects of the work flow.
“This team can clarify, interpret, iterate and disseminate policies, and also provide the necessary operational support to plan and successfully execute the reboot process as the efforts to contain COVID-19 continue,” the writing group said.
A mandatory component of the reboot plan should be planning for a second wave of the virus.
“We will have to learn to create relatively COVID-19 safe zones within the hospitals to help isolate patients from second waves and yet be able to provide regular care for non–COVID-19 patients,” the writing group said.
“Our main goal as health care professionals, whether we serve in a clinical, teaching, research, or administrative role, is to do everything we can to create a safe environment for our patients so that they receive the excellent care they deserve,” they concluded.
Defining moment for remote arrhythmia monitoring
In a separate report, an international team of heart rhythm specialists from the Latin American Heart Rhythm Society, the HRS, the European Heart Rhythm Association, the Asia Pacific Heart Rhythm Society, the AHA, and the ACC discussed how the pandemic has fueled adoption of telehealth and remote patient management across medicine, including heart rhythm monitoring.
Their report was simultaneously published in Circulation: Arrhythmia and Electrophysiology, EP Europace, the Journal of the American College of Cardiology, the Journal of Arrhythmia, and Heart Rhythm.
The COVID-19 pandemic has “catalyzed the use of wearables and digital medical tools,” and this will likely define medicine going forward, first author Niraj Varma, MD, PhD, of the Cleveland Clinic, said in an interview.
He noted that the technology has been available for some time, but the pandemic has forced people to use it. “Necessity is the mother of invention, and this has become necessary during the pandemic when we can’t see our patients,” said Dr. Varma.
He also noted that hospitals and physicians are now realizing that telehealth and remote arrhythmia monitoring “actually work, and regulatory agencies have moved very swiftly to dissolve traditional barriers and will now reimburse for it. So it’s a win-win.”
Dr. Varma and colleagues said that the time is right to “embed and grow remote services in everyday medical practice worldwide.” In their report, they offered a list of commonly used platforms for telehealth and examples of remote electrocardiogram and heart rate monitoring devices.
Development of the three reports had no commercial funding. Complete lists of disclosures for the writing groups are available in the original articles.
A version of this article originally appeared on Medscape.com.
With the COVID-19 pandemic winding down in some parts of the United States, attention has turned to figuring out how to safely reboot elective cardiovascular (CV) services, which, for the most part, shut down in order to combat the virus and flatten the curve.
To aid in this effort, top cardiology societies have published a series of guidance documents. One, entitled Multimodality Cardiovascular Imaging in the Midst of the COVID-19 Pandemic: Ramping Up Safely to a New Normal, was initiated by the editors of JACC Cardiovascular Imaging and was developed in collaboration with the ACC Cardiovascular Imaging Council.
“As we enter a deceleration or indolent phase of the disease and a return to a ‘new normal’ for the foreseeable future, cardiovascular imaging laboratories will adjust to a different work flow and safety precautions for patients and staff alike,” write William Zoghbi, MD, of the department of cardiology at Houston Methodist DeBakey Heart and Vascular Center, and colleagues.
Minimize risk, maximize clinical benefit
The group outlined strategies and considerations on how to safely ramp up multimodality CV imaging laboratories in an environment of an abating but continuing pandemic.
The authors provide detailed advice on reestablishing echocardiography, transthoracic echocardiography, transesophageal echocardiography, stress testing modalities, treadmill testing, nuclear cardiology, cardiac CT, and cardiac MRI.
The advice is designed to “minimize risk, reduce resource utilization and maximize clinical benefit,” the authors wrote. They address patient and societal health; safety of healthcare professionals; choice of CV testing; and scheduling considerations.
Dr. Zoghbi and colleagues said that integrated communication among patients, referring physicians, the imaging teams, and administrative staff are key to reestablishing a more normal clinical operation.
“Recognizing that practice patterns and policies vary depending on institution and locale, the recommendations are not meant to be restrictive but rather to serve as a general framework during the COVID-19 pandemic and its recovery phase,” the writing group said.
Ultimately, the goal is to offer the necessary CV tests and information for the clinical team to provide the best care for patients, they added.
“To be successful in this new safety-driven modus operandi, innovation, coordination and adaptation among clinicians, staff and patients is necessary till herd immunity or control of COVID-19 is achieved,” they concluded.
Rebooting electrophysiology services
Uncertainty as to how to resume electrophysiology (EP) services for arrhythmia patients prompted representatives from the Heart Rhythm Society, the American Heart Association, and the ACC to develop a series of “guiding suggestions and principles” to help safely reestablish electrophysiological care.
The 28-page document is published in Circulation: Arrhythmia and Electrophysiology and the Journal of the American College of Cardiology Electrophysiology.
“Rebooting” EP services at many institutions may be more challenging than shutting down, wrote Dhanunjaya R. Lakkireddy, MD, Kansas City Heart Rhythm Institute and Research Foundation, Overland Park, Kan., and colleagues.
Topics addressed by the writing group include the role of viral screening and serologic testing, return-to-work considerations for exposed or infected health care workers, risk stratification and management strategies based on COVID-19 disease burden, institutional preparedness for resumption of elective procedures, patient preparation and communication; prioritization of procedures, and development of outpatient and periprocedural care pathways.
They suggest creating an EP COVID-19 “reboot team” made up of stakeholders involved in the EP care continuum pathway that would coordinate with institutional or hospital-level COVID-19 leadership.
The reboot team may include an electrophysiologist, an EP laboratory manager, an outpatient clinic manager, an EP nurse, advanced practice providers, a device technician, an anesthesiologist, and an imaging team to provide insights into various aspects of the work flow.
“This team can clarify, interpret, iterate and disseminate policies, and also provide the necessary operational support to plan and successfully execute the reboot process as the efforts to contain COVID-19 continue,” the writing group said.
A mandatory component of the reboot plan should be planning for a second wave of the virus.
“We will have to learn to create relatively COVID-19 safe zones within the hospitals to help isolate patients from second waves and yet be able to provide regular care for non–COVID-19 patients,” the writing group said.
“Our main goal as health care professionals, whether we serve in a clinical, teaching, research, or administrative role, is to do everything we can to create a safe environment for our patients so that they receive the excellent care they deserve,” they concluded.
Defining moment for remote arrhythmia monitoring
In a separate report, an international team of heart rhythm specialists from the Latin American Heart Rhythm Society, the HRS, the European Heart Rhythm Association, the Asia Pacific Heart Rhythm Society, the AHA, and the ACC discussed how the pandemic has fueled adoption of telehealth and remote patient management across medicine, including heart rhythm monitoring.
Their report was simultaneously published in Circulation: Arrhythmia and Electrophysiology, EP Europace, the Journal of the American College of Cardiology, the Journal of Arrhythmia, and Heart Rhythm.
The COVID-19 pandemic has “catalyzed the use of wearables and digital medical tools,” and this will likely define medicine going forward, first author Niraj Varma, MD, PhD, of the Cleveland Clinic, said in an interview.
He noted that the technology has been available for some time, but the pandemic has forced people to use it. “Necessity is the mother of invention, and this has become necessary during the pandemic when we can’t see our patients,” said Dr. Varma.
He also noted that hospitals and physicians are now realizing that telehealth and remote arrhythmia monitoring “actually work, and regulatory agencies have moved very swiftly to dissolve traditional barriers and will now reimburse for it. So it’s a win-win.”
Dr. Varma and colleagues said that the time is right to “embed and grow remote services in everyday medical practice worldwide.” In their report, they offered a list of commonly used platforms for telehealth and examples of remote electrocardiogram and heart rate monitoring devices.
Development of the three reports had no commercial funding. Complete lists of disclosures for the writing groups are available in the original articles.
A version of this article originally appeared on Medscape.com.
COVID-19: ‘dramatic’ surge in out-of-hospital cardiac arrests in NYC
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests (OHCAs) that placed a huge burden on first responders, a new analysis shows.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
New York City has the largest and busiest EMS system in the United States, serving a population of more than 8.4 million people and responding to more than 1.5 million calls every year.
To gauge the impact of COVID-19 on first responders, Dr. Prezant and colleagues analyzed data for adults with OHCA who received EMS resuscitation from March 1, when the first case of COVID-19 was diagnosed in the city, through April 25, when EMS call volume had receded to pre-COVID-19 levels.
Compared with the same period in 2019, the COVID-19 period had an excess of 2,653 patients with OHCA who underwent EMS resuscitation attempts (3,989 in 2020 vs. 1,336 in 2019, P < .001), an incidence rate triple that of 2019 (47.5 vs. 15.9 per 100,000).
On the worst day – Monday, April 6 – OHCAs peaked at 305 cases, an increase of nearly 10-fold compared with the same day in 2019.
Despite the surge in cases, the median response time of available EMS units to OHCAs increased by about 1 minute over 2019, a nonsignificant difference. Although the average time varied, median response time during the COVID-19 period was less than 3 minutes.
A more vulnerable group
Compared with 2019, patients suffering OHCA during the pandemic period were older (mean age 72 vs. 68 years), less likely to be white (20% white vs. 33%) and more likely to have hypertension (54% vs. 46%), diabetes (36% vs. 26%), physical limitations (57% vs. 48%) and cardiac rhythms that don’t respond to defibrillator shocks (92% vs. 81%).
Compared with 2019, the COVID-19 period had substantial reductions in return of spontaneous circulation (ROSC) (18% vs. 35%; P < .001) and sustained ROSC (11% vs. 25%; P < .001). The case fatality rate was 90% in the COVID-19 period vs. 75% a year earlier.
“The tragedy of the COVID-19 pandemic is not just the number of patients infected, but the large increase in OHCAs and deaths,” Dr. Prezant and colleagues said.
Identifying patients with the greatest risk for OHCA and death during the COVID-19 pandemic “should allow for early, targeted interventions in the outpatient setting that could lead to reductions in out-of-hospital deaths,” they noted.
“Vulnerable patient populations need outreach, telephonic medicine, televideo medicine, home visits, not just temperature monitoring but home O2 saturation monitoring,” Dr. Prezant said in an interview. “Barriers need to be removed, not just for this pandemic but for the future – no matter what the trigger is.”
Unsung heroes
In an Editor’s Note in JAMA Cardiology, Robert O. Bonow, MD, Northwestern University, Chicago, and colleagues said the American people owe a debt of gratitude to first responders for their “heroic work” triaging, resuscitating, and transporting thousands of people affected by COVID-19.
“Although the typically bustling NYC streets remained eerily deserted, the characteristic cacophony of sounds of the ‘City that Never Sleeps’ was replaced by sirens wailing all hours of the night,” they wrote.
First responders to OHCAs in the COVID-19 era place themselves at extremely high risk, in some cases without optimal personal protective equipment, they pointed out. “Sadly,” many first responders have fallen ill to COVID-19 infection, they added.
As of June 1, 29 EMS workers and volunteers across the United States had died of COVID-19.
They are James Villecco, Gregory Hodge, Tony Thomas, Mike Field, John Redd, Idris Bey, Richard Seaberry, and Sal Mancuso of New York; Israel Tolentino, Reuven Maroth, Liana Sá, Kevin Leiva, Frank Molinari, Robert Weber, Robert Tarrant, Solomon Donald, Scott Geiger, John Farrarella, John Careccia, Bill Nauta, and David Pinto of New Jersey; Kevin Bundy, Robert Zerman, and Jeremy Emerich of Pennsylvania; Paul Cary of Colorado; Paul Novicki of Michigan; David Martin of Mississippi; Billy Birmingham of Missouri; and John “JP” Granger of South Carolina.
“We offer their families, friends, and colleagues our sincerest condolences and honor their memory with our highest respect and gratitude,” Dr. Bonow and colleagues wrote.
This study was supported by the City of New York and the Fire Department of the City of New York. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests (OHCAs) that placed a huge burden on first responders, a new analysis shows.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
New York City has the largest and busiest EMS system in the United States, serving a population of more than 8.4 million people and responding to more than 1.5 million calls every year.
To gauge the impact of COVID-19 on first responders, Dr. Prezant and colleagues analyzed data for adults with OHCA who received EMS resuscitation from March 1, when the first case of COVID-19 was diagnosed in the city, through April 25, when EMS call volume had receded to pre-COVID-19 levels.
Compared with the same period in 2019, the COVID-19 period had an excess of 2,653 patients with OHCA who underwent EMS resuscitation attempts (3,989 in 2020 vs. 1,336 in 2019, P < .001), an incidence rate triple that of 2019 (47.5 vs. 15.9 per 100,000).
On the worst day – Monday, April 6 – OHCAs peaked at 305 cases, an increase of nearly 10-fold compared with the same day in 2019.
Despite the surge in cases, the median response time of available EMS units to OHCAs increased by about 1 minute over 2019, a nonsignificant difference. Although the average time varied, median response time during the COVID-19 period was less than 3 minutes.
A more vulnerable group
Compared with 2019, patients suffering OHCA during the pandemic period were older (mean age 72 vs. 68 years), less likely to be white (20% white vs. 33%) and more likely to have hypertension (54% vs. 46%), diabetes (36% vs. 26%), physical limitations (57% vs. 48%) and cardiac rhythms that don’t respond to defibrillator shocks (92% vs. 81%).
Compared with 2019, the COVID-19 period had substantial reductions in return of spontaneous circulation (ROSC) (18% vs. 35%; P < .001) and sustained ROSC (11% vs. 25%; P < .001). The case fatality rate was 90% in the COVID-19 period vs. 75% a year earlier.
“The tragedy of the COVID-19 pandemic is not just the number of patients infected, but the large increase in OHCAs and deaths,” Dr. Prezant and colleagues said.
Identifying patients with the greatest risk for OHCA and death during the COVID-19 pandemic “should allow for early, targeted interventions in the outpatient setting that could lead to reductions in out-of-hospital deaths,” they noted.
“Vulnerable patient populations need outreach, telephonic medicine, televideo medicine, home visits, not just temperature monitoring but home O2 saturation monitoring,” Dr. Prezant said in an interview. “Barriers need to be removed, not just for this pandemic but for the future – no matter what the trigger is.”
Unsung heroes
In an Editor’s Note in JAMA Cardiology, Robert O. Bonow, MD, Northwestern University, Chicago, and colleagues said the American people owe a debt of gratitude to first responders for their “heroic work” triaging, resuscitating, and transporting thousands of people affected by COVID-19.
“Although the typically bustling NYC streets remained eerily deserted, the characteristic cacophony of sounds of the ‘City that Never Sleeps’ was replaced by sirens wailing all hours of the night,” they wrote.
First responders to OHCAs in the COVID-19 era place themselves at extremely high risk, in some cases without optimal personal protective equipment, they pointed out. “Sadly,” many first responders have fallen ill to COVID-19 infection, they added.
As of June 1, 29 EMS workers and volunteers across the United States had died of COVID-19.
They are James Villecco, Gregory Hodge, Tony Thomas, Mike Field, John Redd, Idris Bey, Richard Seaberry, and Sal Mancuso of New York; Israel Tolentino, Reuven Maroth, Liana Sá, Kevin Leiva, Frank Molinari, Robert Weber, Robert Tarrant, Solomon Donald, Scott Geiger, John Farrarella, John Careccia, Bill Nauta, and David Pinto of New Jersey; Kevin Bundy, Robert Zerman, and Jeremy Emerich of Pennsylvania; Paul Cary of Colorado; Paul Novicki of Michigan; David Martin of Mississippi; Billy Birmingham of Missouri; and John “JP” Granger of South Carolina.
“We offer their families, friends, and colleagues our sincerest condolences and honor their memory with our highest respect and gratitude,” Dr. Bonow and colleagues wrote.
This study was supported by the City of New York and the Fire Department of the City of New York. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests (OHCAs) that placed a huge burden on first responders, a new analysis shows.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
New York City has the largest and busiest EMS system in the United States, serving a population of more than 8.4 million people and responding to more than 1.5 million calls every year.
To gauge the impact of COVID-19 on first responders, Dr. Prezant and colleagues analyzed data for adults with OHCA who received EMS resuscitation from March 1, when the first case of COVID-19 was diagnosed in the city, through April 25, when EMS call volume had receded to pre-COVID-19 levels.
Compared with the same period in 2019, the COVID-19 period had an excess of 2,653 patients with OHCA who underwent EMS resuscitation attempts (3,989 in 2020 vs. 1,336 in 2019, P < .001), an incidence rate triple that of 2019 (47.5 vs. 15.9 per 100,000).
On the worst day – Monday, April 6 – OHCAs peaked at 305 cases, an increase of nearly 10-fold compared with the same day in 2019.
Despite the surge in cases, the median response time of available EMS units to OHCAs increased by about 1 minute over 2019, a nonsignificant difference. Although the average time varied, median response time during the COVID-19 period was less than 3 minutes.
A more vulnerable group
Compared with 2019, patients suffering OHCA during the pandemic period were older (mean age 72 vs. 68 years), less likely to be white (20% white vs. 33%) and more likely to have hypertension (54% vs. 46%), diabetes (36% vs. 26%), physical limitations (57% vs. 48%) and cardiac rhythms that don’t respond to defibrillator shocks (92% vs. 81%).
Compared with 2019, the COVID-19 period had substantial reductions in return of spontaneous circulation (ROSC) (18% vs. 35%; P < .001) and sustained ROSC (11% vs. 25%; P < .001). The case fatality rate was 90% in the COVID-19 period vs. 75% a year earlier.
“The tragedy of the COVID-19 pandemic is not just the number of patients infected, but the large increase in OHCAs and deaths,” Dr. Prezant and colleagues said.
Identifying patients with the greatest risk for OHCA and death during the COVID-19 pandemic “should allow for early, targeted interventions in the outpatient setting that could lead to reductions in out-of-hospital deaths,” they noted.
“Vulnerable patient populations need outreach, telephonic medicine, televideo medicine, home visits, not just temperature monitoring but home O2 saturation monitoring,” Dr. Prezant said in an interview. “Barriers need to be removed, not just for this pandemic but for the future – no matter what the trigger is.”
Unsung heroes
In an Editor’s Note in JAMA Cardiology, Robert O. Bonow, MD, Northwestern University, Chicago, and colleagues said the American people owe a debt of gratitude to first responders for their “heroic work” triaging, resuscitating, and transporting thousands of people affected by COVID-19.
“Although the typically bustling NYC streets remained eerily deserted, the characteristic cacophony of sounds of the ‘City that Never Sleeps’ was replaced by sirens wailing all hours of the night,” they wrote.
First responders to OHCAs in the COVID-19 era place themselves at extremely high risk, in some cases without optimal personal protective equipment, they pointed out. “Sadly,” many first responders have fallen ill to COVID-19 infection, they added.
As of June 1, 29 EMS workers and volunteers across the United States had died of COVID-19.
They are James Villecco, Gregory Hodge, Tony Thomas, Mike Field, John Redd, Idris Bey, Richard Seaberry, and Sal Mancuso of New York; Israel Tolentino, Reuven Maroth, Liana Sá, Kevin Leiva, Frank Molinari, Robert Weber, Robert Tarrant, Solomon Donald, Scott Geiger, John Farrarella, John Careccia, Bill Nauta, and David Pinto of New Jersey; Kevin Bundy, Robert Zerman, and Jeremy Emerich of Pennsylvania; Paul Cary of Colorado; Paul Novicki of Michigan; David Martin of Mississippi; Billy Birmingham of Missouri; and John “JP” Granger of South Carolina.
“We offer their families, friends, and colleagues our sincerest condolences and honor their memory with our highest respect and gratitude,” Dr. Bonow and colleagues wrote.
This study was supported by the City of New York and the Fire Department of the City of New York. The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Dapagliflozin benefits low-EF heart failure regardless of diuretic dose: DAPA-HF
The DAPA-HF trial has already changed cardiology in opening up a new class of drugs to patients with heart failure (HF), whether or not they have diabetes. Now the trial is yielding clues as to how it benefits them. For now, it’s doing so by process of elimination.
A new analysis suggests that dapagliflozin (Farxiga, AstraZeneca) didn’t need help from loop diuretics to cut the risk for clinical events in patients with HF with reduced ejection fraction (HFrEF), a benefit seen across the spectrum of glycosylated hemoglobin levels and without compromising renal function, said DAPA-HF investigators. Also, use of dapagliflozin and its clinical effects were not associated with changes in loop diuretic dosage. Those findings and others suggest the drug helps in HFrEF at least partly by some other mechanism than its own diuretic effect, the researchers say.
Such insights will likely be important to case-by-case decisions on whether to use the drug, a sodium-glucose cotransporter 2 (SGLT2) inhibitor once reserved for patients with diabetes, given the recently broader landscape of HF treatment options.
As previously reported from DAPA-HF, with more than 4,700 patients, those who received dapagliflozin showed significant reductions in the primary end point, a composite of cardiovascular (CV) death, HF hospitalization, and urgent HF visit requiring IV therapy over about 18 months. The 45% of patients with and 55% without type 2 diabetes enjoyed about equal benefit in the placebo-controlled trial for that end point, as well as for all-cause mortality.
SGLT2 inhibitors work in diabetes by promoting urinary glucose excretion. That had led some to speculate that its benefit in HFrEF comes primarily from a diuretic effect; the current findings largely put that question to rest.
“Our findings show that treatment with dapagliflozin was effective regardless of diuretic use or diuretic dose. They also show that dapagliflozin did not lead to an increase in renal adverse events or discontinuation of therapy in patients treated with a diuretic,” trialist Alice M. Jackson, MB, ChB, said in an interview.
“In fact, renal adverse events were generally less common in patients treated with dapagliflozin, across the diuretic categories,” said Dr. Jackson, from the University of Glasgow.
Dr. Jackson presented the new analysis at a Late-Breaking Science Session during the European Society of Cardiology Heart Failure Discoveries virtual meeting. The HFA sessions were conducted virtually this year due to the COVID-19 pandemic.
At baseline, 84% of patients were on conventional diuretics. The post hoc analysis broke out all patients by loop-diuretic dosage level: none; less than 40 mg furosemide equivalents (FE); 40 mg FE; or more than 40 mg FE. Clinical outcomes were similar across the four groups.
Clinicians in the trial “were not given specific advice about adjusting diuretic doses, but were encouraged to assess volume status and make changes to medical therapy based on this, if necessary,” Dr. Jackson said. “This suggests that, for most patients, starting dapagliflozin will not necessitate a change in diuretic dose.”
With the caveat that the event rate was low in the relatively few patients not prescribed loop diuretics, she said, “the magnitude of the benefit from dapagliflozin appeared to be larger in patients not treated with a diuretic.”
There was no suggestion of a diuretic dose–response effect or statistical interaction between diuretic use and clinical outcomes on dapagliflozin, Dr. Jackson observed in the interview.
Of note in the analysis, hematocrit levels shot up soon after patients started active therapy, but they didn’t rise much in the placebo group. The sustained hematocrit elevation on dapagliflozin, seen at all diuretic dosage levels, persisted even after dosage reductions at 6 months, she said.
“Dapagliflozin is effective in HFrEF irrespective of background diuretic therapy; therefore, it is almost certainly not purely acting as a diuretic,” Andrew J. Coats, MD, DSc, MBA, said in an interview.
The findings also “lessen the concern that dapagliflozin’s beneficial effects are only seen only in patients without effective diuretic dosing,” said Dr. Coats, from University of Warwick, Coventry, England.
“Altogether, these data give further reassurance that dapagliflozin can safely be used in heart failure, and has a beneficial effect independent of the use of diuretic drugs,” invited discussant Wolfram Doehner, MD, PhD, Charité-Universitätsmedizin Berlin, said after Dr. Jackson’s presentation of the analysis.
He made special mention of the sustained hematocrit elevation on dapagliflozin. “While this effect may likely relate to the mild reduction in plasma volume secondary to dapagliflozin therapy, it is noted that the increase in hematocrit was independent of any change of the diuretic dose,” Doehner said. “If additional mechanisms have a role for this observed increase in hematocrit, it may be of interest in further investigations.”
Dr. Jackson pointed to several observations that suggest the hematocrit finding isn’t explained by hemoconcentration from reduced plasma volume, at least not entirely.
For example, hematocrit levels rose “without any suggestion of a relationship between diuretic dose and degree of hematocrit elevation with dapagliflozin,” she said.
The elevations persisted even with diuretic dose reductions at 6 and 12 months, “which should have led to a decrease in hemoconcentration if it was caused by volume contraction.”
Also, she said, “among patients not taking a diuretic, volume depletion occurred less frequently in the dapagliflozin group than in the placebo group, but there was still a similar rise in hematocrit with dapagliflozin.”
Both Dr. Jackson and Dr. Coats said the sustained elevation in hematocrit on the drug is unlikely to pose a major hazard.
Dr. Coats said that, theoretically, “increased hematocrit could reduce peripheral vessel blood flow, making ischemia and thrombosis more likely. But the size of the effect is small and unlikely to be clinically important.”
A diuretic dose could not be determined for 128 of the trial’s 4,744 randomized patients with HFrEF, so the post hoc analysis was limited to the remaining 4,616. Of those, 746 were not on diuretics at baseline, 1,311 were on loop diuretics at less than 40 mg FE or on non-loop diuretics only, 1,365 were taking 40 mg FE, and 1,204 were on higher doses of loop diuretics.
The mean baseline dosage was 60 mg FE, which rose slightly throughout the trial. But the baseline dosage and the increases were both similar in the placebo and dapagliflozin groups. Dr. Jackson said 84% and 83% of patients on dapagliflozin and placebo, respectively, maintained their baseline dose at 6 months and about 77% in both groups at 12 months.
The overall trial’s significant primary endpoint reduction for dapagliflozin versus placebo applied similarly to patients not on a diuretics and to those on any dose of diuretic, with an interaction P value of .23 for the effect of diuretic use. The hazard ratios (95% confidence interval) were 0.57 (0.36-0.92) for patients not on diuretics, 0.78 (0.68-0.90) for patients on any diuretic dosage, and 0.74 (0.65-0.85) overall
Dr. Jackson said during her formal online presentation that patients on diuretics showed a “tendency toward slightly more volume depletion in those on dapagliflozin than in those on placebo, but the excess was small and not greater than approximately 3% in those taking 40 mg furosemide equivalent diuretic. And fortunately, this did not result in an increase in frequency in renal adverse events nor of discontinuation of study drug.”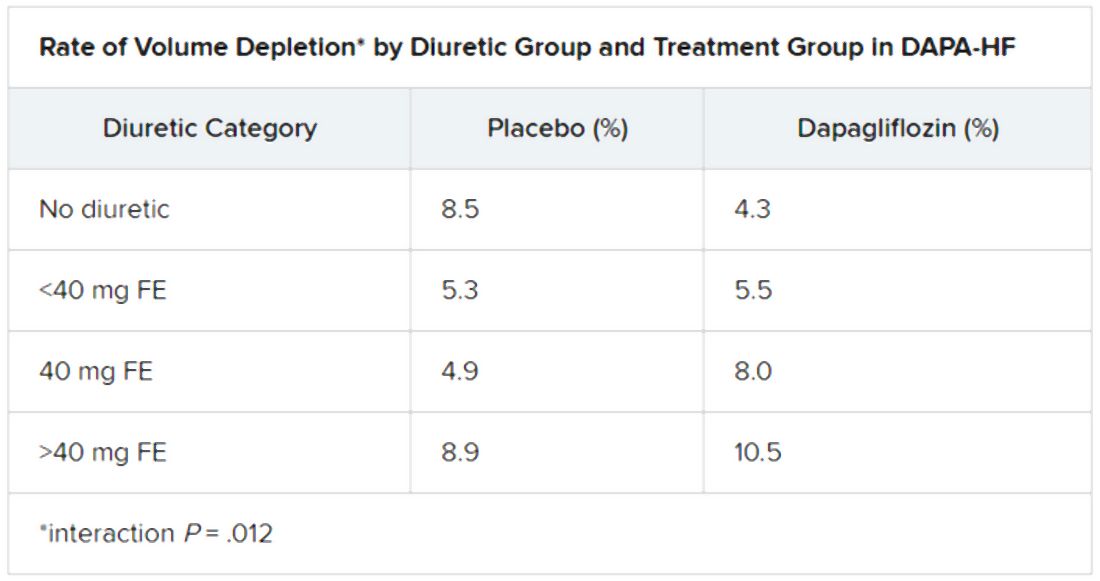
Renal adverse events were similarly prevalent in the two treatment groups, as were such events leading to treatment discontinuation. But serious renal events were less common in the dapagliflozin group (1.6% vs 2.7%; P = .009), as was investigator-reported serious acute kidney injury (1.0% vs 1.9%; P = .007).
“Overall, renal events were infrequent,” Dr. Jackson said, and “because of the small number of events, it is very difficult to draw conclusions about the impact of dapagliflozin on renal function according to diuretic-dose subgroups.”
Still, she said, worsening renal function was less common on dapagliflozin in three of the four groups by diuretic dosage; the exception was the less than 40 mg FE group, “but the absolute difference in this group was only two events.”
There seem to be dapagliflozin mechanisms “underneath the surface that need to be unraveled,” Dr. Doehner said as discussant, processes that are favorable for the treatment of HFrEF in which “diuretics play no big role.”
Dr. Jackson has no disclosures. Dr. Coats has disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor. Dr. Doener has recently disclosed receiving grants and personal fees from Vifor, Pfizer, Boehringer Ingelheim, Sphingotec, ZS Pharma, Bayer, and Medtronic.
A version of this article originally appeared on Medscape.com.
The DAPA-HF trial has already changed cardiology in opening up a new class of drugs to patients with heart failure (HF), whether or not they have diabetes. Now the trial is yielding clues as to how it benefits them. For now, it’s doing so by process of elimination.
A new analysis suggests that dapagliflozin (Farxiga, AstraZeneca) didn’t need help from loop diuretics to cut the risk for clinical events in patients with HF with reduced ejection fraction (HFrEF), a benefit seen across the spectrum of glycosylated hemoglobin levels and without compromising renal function, said DAPA-HF investigators. Also, use of dapagliflozin and its clinical effects were not associated with changes in loop diuretic dosage. Those findings and others suggest the drug helps in HFrEF at least partly by some other mechanism than its own diuretic effect, the researchers say.
Such insights will likely be important to case-by-case decisions on whether to use the drug, a sodium-glucose cotransporter 2 (SGLT2) inhibitor once reserved for patients with diabetes, given the recently broader landscape of HF treatment options.
As previously reported from DAPA-HF, with more than 4,700 patients, those who received dapagliflozin showed significant reductions in the primary end point, a composite of cardiovascular (CV) death, HF hospitalization, and urgent HF visit requiring IV therapy over about 18 months. The 45% of patients with and 55% without type 2 diabetes enjoyed about equal benefit in the placebo-controlled trial for that end point, as well as for all-cause mortality.
SGLT2 inhibitors work in diabetes by promoting urinary glucose excretion. That had led some to speculate that its benefit in HFrEF comes primarily from a diuretic effect; the current findings largely put that question to rest.
“Our findings show that treatment with dapagliflozin was effective regardless of diuretic use or diuretic dose. They also show that dapagliflozin did not lead to an increase in renal adverse events or discontinuation of therapy in patients treated with a diuretic,” trialist Alice M. Jackson, MB, ChB, said in an interview.
“In fact, renal adverse events were generally less common in patients treated with dapagliflozin, across the diuretic categories,” said Dr. Jackson, from the University of Glasgow.
Dr. Jackson presented the new analysis at a Late-Breaking Science Session during the European Society of Cardiology Heart Failure Discoveries virtual meeting. The HFA sessions were conducted virtually this year due to the COVID-19 pandemic.
At baseline, 84% of patients were on conventional diuretics. The post hoc analysis broke out all patients by loop-diuretic dosage level: none; less than 40 mg furosemide equivalents (FE); 40 mg FE; or more than 40 mg FE. Clinical outcomes were similar across the four groups.
Clinicians in the trial “were not given specific advice about adjusting diuretic doses, but were encouraged to assess volume status and make changes to medical therapy based on this, if necessary,” Dr. Jackson said. “This suggests that, for most patients, starting dapagliflozin will not necessitate a change in diuretic dose.”
With the caveat that the event rate was low in the relatively few patients not prescribed loop diuretics, she said, “the magnitude of the benefit from dapagliflozin appeared to be larger in patients not treated with a diuretic.”
There was no suggestion of a diuretic dose–response effect or statistical interaction between diuretic use and clinical outcomes on dapagliflozin, Dr. Jackson observed in the interview.
Of note in the analysis, hematocrit levels shot up soon after patients started active therapy, but they didn’t rise much in the placebo group. The sustained hematocrit elevation on dapagliflozin, seen at all diuretic dosage levels, persisted even after dosage reductions at 6 months, she said.
“Dapagliflozin is effective in HFrEF irrespective of background diuretic therapy; therefore, it is almost certainly not purely acting as a diuretic,” Andrew J. Coats, MD, DSc, MBA, said in an interview.
The findings also “lessen the concern that dapagliflozin’s beneficial effects are only seen only in patients without effective diuretic dosing,” said Dr. Coats, from University of Warwick, Coventry, England.
“Altogether, these data give further reassurance that dapagliflozin can safely be used in heart failure, and has a beneficial effect independent of the use of diuretic drugs,” invited discussant Wolfram Doehner, MD, PhD, Charité-Universitätsmedizin Berlin, said after Dr. Jackson’s presentation of the analysis.
He made special mention of the sustained hematocrit elevation on dapagliflozin. “While this effect may likely relate to the mild reduction in plasma volume secondary to dapagliflozin therapy, it is noted that the increase in hematocrit was independent of any change of the diuretic dose,” Doehner said. “If additional mechanisms have a role for this observed increase in hematocrit, it may be of interest in further investigations.”
Dr. Jackson pointed to several observations that suggest the hematocrit finding isn’t explained by hemoconcentration from reduced plasma volume, at least not entirely.
For example, hematocrit levels rose “without any suggestion of a relationship between diuretic dose and degree of hematocrit elevation with dapagliflozin,” she said.
The elevations persisted even with diuretic dose reductions at 6 and 12 months, “which should have led to a decrease in hemoconcentration if it was caused by volume contraction.”
Also, she said, “among patients not taking a diuretic, volume depletion occurred less frequently in the dapagliflozin group than in the placebo group, but there was still a similar rise in hematocrit with dapagliflozin.”
Both Dr. Jackson and Dr. Coats said the sustained elevation in hematocrit on the drug is unlikely to pose a major hazard.
Dr. Coats said that, theoretically, “increased hematocrit could reduce peripheral vessel blood flow, making ischemia and thrombosis more likely. But the size of the effect is small and unlikely to be clinically important.”
A diuretic dose could not be determined for 128 of the trial’s 4,744 randomized patients with HFrEF, so the post hoc analysis was limited to the remaining 4,616. Of those, 746 were not on diuretics at baseline, 1,311 were on loop diuretics at less than 40 mg FE or on non-loop diuretics only, 1,365 were taking 40 mg FE, and 1,204 were on higher doses of loop diuretics.
The mean baseline dosage was 60 mg FE, which rose slightly throughout the trial. But the baseline dosage and the increases were both similar in the placebo and dapagliflozin groups. Dr. Jackson said 84% and 83% of patients on dapagliflozin and placebo, respectively, maintained their baseline dose at 6 months and about 77% in both groups at 12 months.
The overall trial’s significant primary endpoint reduction for dapagliflozin versus placebo applied similarly to patients not on a diuretics and to those on any dose of diuretic, with an interaction P value of .23 for the effect of diuretic use. The hazard ratios (95% confidence interval) were 0.57 (0.36-0.92) for patients not on diuretics, 0.78 (0.68-0.90) for patients on any diuretic dosage, and 0.74 (0.65-0.85) overall
Dr. Jackson said during her formal online presentation that patients on diuretics showed a “tendency toward slightly more volume depletion in those on dapagliflozin than in those on placebo, but the excess was small and not greater than approximately 3% in those taking 40 mg furosemide equivalent diuretic. And fortunately, this did not result in an increase in frequency in renal adverse events nor of discontinuation of study drug.”
Renal adverse events were similarly prevalent in the two treatment groups, as were such events leading to treatment discontinuation. But serious renal events were less common in the dapagliflozin group (1.6% vs 2.7%; P = .009), as was investigator-reported serious acute kidney injury (1.0% vs 1.9%; P = .007).
“Overall, renal events were infrequent,” Dr. Jackson said, and “because of the small number of events, it is very difficult to draw conclusions about the impact of dapagliflozin on renal function according to diuretic-dose subgroups.”
Still, she said, worsening renal function was less common on dapagliflozin in three of the four groups by diuretic dosage; the exception was the less than 40 mg FE group, “but the absolute difference in this group was only two events.”
There seem to be dapagliflozin mechanisms “underneath the surface that need to be unraveled,” Dr. Doehner said as discussant, processes that are favorable for the treatment of HFrEF in which “diuretics play no big role.”
Dr. Jackson has no disclosures. Dr. Coats has disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor. Dr. Doener has recently disclosed receiving grants and personal fees from Vifor, Pfizer, Boehringer Ingelheim, Sphingotec, ZS Pharma, Bayer, and Medtronic.
A version of this article originally appeared on Medscape.com.
The DAPA-HF trial has already changed cardiology in opening up a new class of drugs to patients with heart failure (HF), whether or not they have diabetes. Now the trial is yielding clues as to how it benefits them. For now, it’s doing so by process of elimination.
A new analysis suggests that dapagliflozin (Farxiga, AstraZeneca) didn’t need help from loop diuretics to cut the risk for clinical events in patients with HF with reduced ejection fraction (HFrEF), a benefit seen across the spectrum of glycosylated hemoglobin levels and without compromising renal function, said DAPA-HF investigators. Also, use of dapagliflozin and its clinical effects were not associated with changes in loop diuretic dosage. Those findings and others suggest the drug helps in HFrEF at least partly by some other mechanism than its own diuretic effect, the researchers say.
Such insights will likely be important to case-by-case decisions on whether to use the drug, a sodium-glucose cotransporter 2 (SGLT2) inhibitor once reserved for patients with diabetes, given the recently broader landscape of HF treatment options.
As previously reported from DAPA-HF, with more than 4,700 patients, those who received dapagliflozin showed significant reductions in the primary end point, a composite of cardiovascular (CV) death, HF hospitalization, and urgent HF visit requiring IV therapy over about 18 months. The 45% of patients with and 55% without type 2 diabetes enjoyed about equal benefit in the placebo-controlled trial for that end point, as well as for all-cause mortality.
SGLT2 inhibitors work in diabetes by promoting urinary glucose excretion. That had led some to speculate that its benefit in HFrEF comes primarily from a diuretic effect; the current findings largely put that question to rest.
“Our findings show that treatment with dapagliflozin was effective regardless of diuretic use or diuretic dose. They also show that dapagliflozin did not lead to an increase in renal adverse events or discontinuation of therapy in patients treated with a diuretic,” trialist Alice M. Jackson, MB, ChB, said in an interview.
“In fact, renal adverse events were generally less common in patients treated with dapagliflozin, across the diuretic categories,” said Dr. Jackson, from the University of Glasgow.
Dr. Jackson presented the new analysis at a Late-Breaking Science Session during the European Society of Cardiology Heart Failure Discoveries virtual meeting. The HFA sessions were conducted virtually this year due to the COVID-19 pandemic.
At baseline, 84% of patients were on conventional diuretics. The post hoc analysis broke out all patients by loop-diuretic dosage level: none; less than 40 mg furosemide equivalents (FE); 40 mg FE; or more than 40 mg FE. Clinical outcomes were similar across the four groups.
Clinicians in the trial “were not given specific advice about adjusting diuretic doses, but were encouraged to assess volume status and make changes to medical therapy based on this, if necessary,” Dr. Jackson said. “This suggests that, for most patients, starting dapagliflozin will not necessitate a change in diuretic dose.”
With the caveat that the event rate was low in the relatively few patients not prescribed loop diuretics, she said, “the magnitude of the benefit from dapagliflozin appeared to be larger in patients not treated with a diuretic.”
There was no suggestion of a diuretic dose–response effect or statistical interaction between diuretic use and clinical outcomes on dapagliflozin, Dr. Jackson observed in the interview.
Of note in the analysis, hematocrit levels shot up soon after patients started active therapy, but they didn’t rise much in the placebo group. The sustained hematocrit elevation on dapagliflozin, seen at all diuretic dosage levels, persisted even after dosage reductions at 6 months, she said.
“Dapagliflozin is effective in HFrEF irrespective of background diuretic therapy; therefore, it is almost certainly not purely acting as a diuretic,” Andrew J. Coats, MD, DSc, MBA, said in an interview.
The findings also “lessen the concern that dapagliflozin’s beneficial effects are only seen only in patients without effective diuretic dosing,” said Dr. Coats, from University of Warwick, Coventry, England.
“Altogether, these data give further reassurance that dapagliflozin can safely be used in heart failure, and has a beneficial effect independent of the use of diuretic drugs,” invited discussant Wolfram Doehner, MD, PhD, Charité-Universitätsmedizin Berlin, said after Dr. Jackson’s presentation of the analysis.
He made special mention of the sustained hematocrit elevation on dapagliflozin. “While this effect may likely relate to the mild reduction in plasma volume secondary to dapagliflozin therapy, it is noted that the increase in hematocrit was independent of any change of the diuretic dose,” Doehner said. “If additional mechanisms have a role for this observed increase in hematocrit, it may be of interest in further investigations.”
Dr. Jackson pointed to several observations that suggest the hematocrit finding isn’t explained by hemoconcentration from reduced plasma volume, at least not entirely.
For example, hematocrit levels rose “without any suggestion of a relationship between diuretic dose and degree of hematocrit elevation with dapagliflozin,” she said.
The elevations persisted even with diuretic dose reductions at 6 and 12 months, “which should have led to a decrease in hemoconcentration if it was caused by volume contraction.”
Also, she said, “among patients not taking a diuretic, volume depletion occurred less frequently in the dapagliflozin group than in the placebo group, but there was still a similar rise in hematocrit with dapagliflozin.”
Both Dr. Jackson and Dr. Coats said the sustained elevation in hematocrit on the drug is unlikely to pose a major hazard.
Dr. Coats said that, theoretically, “increased hematocrit could reduce peripheral vessel blood flow, making ischemia and thrombosis more likely. But the size of the effect is small and unlikely to be clinically important.”
A diuretic dose could not be determined for 128 of the trial’s 4,744 randomized patients with HFrEF, so the post hoc analysis was limited to the remaining 4,616. Of those, 746 were not on diuretics at baseline, 1,311 were on loop diuretics at less than 40 mg FE or on non-loop diuretics only, 1,365 were taking 40 mg FE, and 1,204 were on higher doses of loop diuretics.
The mean baseline dosage was 60 mg FE, which rose slightly throughout the trial. But the baseline dosage and the increases were both similar in the placebo and dapagliflozin groups. Dr. Jackson said 84% and 83% of patients on dapagliflozin and placebo, respectively, maintained their baseline dose at 6 months and about 77% in both groups at 12 months.
The overall trial’s significant primary endpoint reduction for dapagliflozin versus placebo applied similarly to patients not on a diuretics and to those on any dose of diuretic, with an interaction P value of .23 for the effect of diuretic use. The hazard ratios (95% confidence interval) were 0.57 (0.36-0.92) for patients not on diuretics, 0.78 (0.68-0.90) for patients on any diuretic dosage, and 0.74 (0.65-0.85) overall
Dr. Jackson said during her formal online presentation that patients on diuretics showed a “tendency toward slightly more volume depletion in those on dapagliflozin than in those on placebo, but the excess was small and not greater than approximately 3% in those taking 40 mg furosemide equivalent diuretic. And fortunately, this did not result in an increase in frequency in renal adverse events nor of discontinuation of study drug.”
Renal adverse events were similarly prevalent in the two treatment groups, as were such events leading to treatment discontinuation. But serious renal events were less common in the dapagliflozin group (1.6% vs 2.7%; P = .009), as was investigator-reported serious acute kidney injury (1.0% vs 1.9%; P = .007).
“Overall, renal events were infrequent,” Dr. Jackson said, and “because of the small number of events, it is very difficult to draw conclusions about the impact of dapagliflozin on renal function according to diuretic-dose subgroups.”
Still, she said, worsening renal function was less common on dapagliflozin in three of the four groups by diuretic dosage; the exception was the less than 40 mg FE group, “but the absolute difference in this group was only two events.”
There seem to be dapagliflozin mechanisms “underneath the surface that need to be unraveled,” Dr. Doehner said as discussant, processes that are favorable for the treatment of HFrEF in which “diuretics play no big role.”
Dr. Jackson has no disclosures. Dr. Coats has disclosed receiving personal fees from Actimed, AstraZeneca, Faraday, WL Gore, Menarini, Novartis, Nutricia, Respicardia, Servier, Stealth Peptides, Verona, and Vifor. Dr. Doener has recently disclosed receiving grants and personal fees from Vifor, Pfizer, Boehringer Ingelheim, Sphingotec, ZS Pharma, Bayer, and Medtronic.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2020
VA readmissions program not linked to increased death
with no concurrent increase in 30-day mortality, a large cohort study suggests.
Unlike the Center for Medicare & Medicaid’s Hospital Readmissions Reduction Program (HRRP), whose primary objective is reducing payments to hospitals with excess readmissions, the VA’s efforts to reduce readmissions across their system did not include any financial penalties.
“The intervention focused on encouraging participation in transitions of care programs, such as the American College of Cardiology’s Hospital to Home Initiative and the creation of a heart failure provider network that included more than 900 heart failure providers throughout the VA system,” said the study’s lead author Justin T. Parizo, MD, of Stanford (Calif.) University.
The only measuring sticks the VA used were the public reporting of 30-day readmission rates (starting in 2012) and inclusion of those rates into hospitals’ overall star ratings (starting in 2014).
“The readmissions reductions we saw were similar in magnitude to those seen in patients in CMS fee-for-service categories in the HRRP,” said Dr. Parizo. “And while we had no ability to evaluate causality here, our best guess from what we can see is that there’s been no impact of the readmissions program on mortality,” he added.
Their results were published online June 17 in JAMA Cardiology.
Dr. Parizo and colleagues conducted a cohort study of 304,374 heart failure hospital admissions in 164,566 patients from January 2007 to September 2017. Importantly, he stressed, the researchers were able to do sophisticated risk adjustment for illness trends, something that has been a sticking point in some of the HRRP studies to date.
“We leveraged the robust dataset that the VA provides to adjust for illness severity. Accounting for clinical factors, like blood pressure, weight, creatinine, BNP [B-type natriuretic peptide], and other markers of heart failure severity, but also for changes in coding,” said Dr. Parizo.
Stratification according to left ventricular ejection fraction (LVEF) showed similar results both in terms of 30-day readmission and 30-day mortality for those with LVEF of 40% or greater and those with LVEF less than 40%.
In an interview, Dr. Parizo noted that they actually saw a small but significant uptick in mortality in the 2011-2012 period (compared with 2007-2008) that remains unexplained. “By the 2015-2017 period, 30-day death had returned to baseline levels,” he said.
In contrast, the HRRP, which was rolled out in 2012, has also been shown to reduce readmissions but, in most studies, 30-day mortality had gone up.
“The VA has a very robust quality infrastructure and a robust mechanism for prioritizing certain quality-improvement goals and getting them accomplished that I think they are underrecognized for,” said Leora Horwitz, MD, MHS, the director of the Center for Healthcare Innovation and Delivery Science at NYU Langone Medical Center, New York.
In an interview, she also noted some concern with the uptick seen in the 2011-2012 period, noting that the increase might be the same signal seen with the HRRP intervention.
“This is around the same time period where other people were writing the HRRP papers that showed an increase in mortality, so that’s something to consider,” she said.
Dr. Horwitz coauthored a study published in 2017 indicating that, on a hospital level (compared with a patient level, the approach most other studies took), reductions in readmissions were only weakly correlated with 30-day mortality rates after discharge.
“So, if you think that a hospital that’s behaving badly and keeping people out of the hospital inappropriately to cut down their readmissions, you’d expect to see increased mortality in that hospital, and in our study there was no correlation whatsoever. So there is still debate as to what is behind the increase in mortality on a patient level with heart failure that we’ve seen in some studies,” she said.
Dr. Horwitz doubts an intervention such as the one undertaken in the VA system – even with its fairly soft-touch “name and shame” component – would work in the non-VA hospital world.
“Those who have been in favor of financial penalties have pointed to the fact that, in general, it’s hard to get health systems to respond without financial alignment, even if it’s not an overt financial incentive,” she said.
“The VA is a unique environment,” she noted. “They have a very strong top-down command control focus where people are kind of used to being told, ‘OK, here are the measures we have to address this year.’ It’s good to see that the system that has worked for them for other outcomes also worked for them for heart failure readmissions too.”
Dr. Parizo has disclosed no relevant financial relationships. Dr. Horwitz has worked under contract to Medicare to develop readmission measures.
A version of this article originally appeared on Medscape.com.
with no concurrent increase in 30-day mortality, a large cohort study suggests.
Unlike the Center for Medicare & Medicaid’s Hospital Readmissions Reduction Program (HRRP), whose primary objective is reducing payments to hospitals with excess readmissions, the VA’s efforts to reduce readmissions across their system did not include any financial penalties.
“The intervention focused on encouraging participation in transitions of care programs, such as the American College of Cardiology’s Hospital to Home Initiative and the creation of a heart failure provider network that included more than 900 heart failure providers throughout the VA system,” said the study’s lead author Justin T. Parizo, MD, of Stanford (Calif.) University.
The only measuring sticks the VA used were the public reporting of 30-day readmission rates (starting in 2012) and inclusion of those rates into hospitals’ overall star ratings (starting in 2014).
“The readmissions reductions we saw were similar in magnitude to those seen in patients in CMS fee-for-service categories in the HRRP,” said Dr. Parizo. “And while we had no ability to evaluate causality here, our best guess from what we can see is that there’s been no impact of the readmissions program on mortality,” he added.
Their results were published online June 17 in JAMA Cardiology.
Dr. Parizo and colleagues conducted a cohort study of 304,374 heart failure hospital admissions in 164,566 patients from January 2007 to September 2017. Importantly, he stressed, the researchers were able to do sophisticated risk adjustment for illness trends, something that has been a sticking point in some of the HRRP studies to date.
“We leveraged the robust dataset that the VA provides to adjust for illness severity. Accounting for clinical factors, like blood pressure, weight, creatinine, BNP [B-type natriuretic peptide], and other markers of heart failure severity, but also for changes in coding,” said Dr. Parizo.
Stratification according to left ventricular ejection fraction (LVEF) showed similar results both in terms of 30-day readmission and 30-day mortality for those with LVEF of 40% or greater and those with LVEF less than 40%.
In an interview, Dr. Parizo noted that they actually saw a small but significant uptick in mortality in the 2011-2012 period (compared with 2007-2008) that remains unexplained. “By the 2015-2017 period, 30-day death had returned to baseline levels,” he said.
In contrast, the HRRP, which was rolled out in 2012, has also been shown to reduce readmissions but, in most studies, 30-day mortality had gone up.
“The VA has a very robust quality infrastructure and a robust mechanism for prioritizing certain quality-improvement goals and getting them accomplished that I think they are underrecognized for,” said Leora Horwitz, MD, MHS, the director of the Center for Healthcare Innovation and Delivery Science at NYU Langone Medical Center, New York.
In an interview, she also noted some concern with the uptick seen in the 2011-2012 period, noting that the increase might be the same signal seen with the HRRP intervention.
“This is around the same time period where other people were writing the HRRP papers that showed an increase in mortality, so that’s something to consider,” she said.
Dr. Horwitz coauthored a study published in 2017 indicating that, on a hospital level (compared with a patient level, the approach most other studies took), reductions in readmissions were only weakly correlated with 30-day mortality rates after discharge.
“So, if you think that a hospital that’s behaving badly and keeping people out of the hospital inappropriately to cut down their readmissions, you’d expect to see increased mortality in that hospital, and in our study there was no correlation whatsoever. So there is still debate as to what is behind the increase in mortality on a patient level with heart failure that we’ve seen in some studies,” she said.
Dr. Horwitz doubts an intervention such as the one undertaken in the VA system – even with its fairly soft-touch “name and shame” component – would work in the non-VA hospital world.
“Those who have been in favor of financial penalties have pointed to the fact that, in general, it’s hard to get health systems to respond without financial alignment, even if it’s not an overt financial incentive,” she said.
“The VA is a unique environment,” she noted. “They have a very strong top-down command control focus where people are kind of used to being told, ‘OK, here are the measures we have to address this year.’ It’s good to see that the system that has worked for them for other outcomes also worked for them for heart failure readmissions too.”
Dr. Parizo has disclosed no relevant financial relationships. Dr. Horwitz has worked under contract to Medicare to develop readmission measures.
A version of this article originally appeared on Medscape.com.
with no concurrent increase in 30-day mortality, a large cohort study suggests.
Unlike the Center for Medicare & Medicaid’s Hospital Readmissions Reduction Program (HRRP), whose primary objective is reducing payments to hospitals with excess readmissions, the VA’s efforts to reduce readmissions across their system did not include any financial penalties.
“The intervention focused on encouraging participation in transitions of care programs, such as the American College of Cardiology’s Hospital to Home Initiative and the creation of a heart failure provider network that included more than 900 heart failure providers throughout the VA system,” said the study’s lead author Justin T. Parizo, MD, of Stanford (Calif.) University.
The only measuring sticks the VA used were the public reporting of 30-day readmission rates (starting in 2012) and inclusion of those rates into hospitals’ overall star ratings (starting in 2014).
“The readmissions reductions we saw were similar in magnitude to those seen in patients in CMS fee-for-service categories in the HRRP,” said Dr. Parizo. “And while we had no ability to evaluate causality here, our best guess from what we can see is that there’s been no impact of the readmissions program on mortality,” he added.
Their results were published online June 17 in JAMA Cardiology.
Dr. Parizo and colleagues conducted a cohort study of 304,374 heart failure hospital admissions in 164,566 patients from January 2007 to September 2017. Importantly, he stressed, the researchers were able to do sophisticated risk adjustment for illness trends, something that has been a sticking point in some of the HRRP studies to date.
“We leveraged the robust dataset that the VA provides to adjust for illness severity. Accounting for clinical factors, like blood pressure, weight, creatinine, BNP [B-type natriuretic peptide], and other markers of heart failure severity, but also for changes in coding,” said Dr. Parizo.
Stratification according to left ventricular ejection fraction (LVEF) showed similar results both in terms of 30-day readmission and 30-day mortality for those with LVEF of 40% or greater and those with LVEF less than 40%.
In an interview, Dr. Parizo noted that they actually saw a small but significant uptick in mortality in the 2011-2012 period (compared with 2007-2008) that remains unexplained. “By the 2015-2017 period, 30-day death had returned to baseline levels,” he said.
In contrast, the HRRP, which was rolled out in 2012, has also been shown to reduce readmissions but, in most studies, 30-day mortality had gone up.
“The VA has a very robust quality infrastructure and a robust mechanism for prioritizing certain quality-improvement goals and getting them accomplished that I think they are underrecognized for,” said Leora Horwitz, MD, MHS, the director of the Center for Healthcare Innovation and Delivery Science at NYU Langone Medical Center, New York.
In an interview, she also noted some concern with the uptick seen in the 2011-2012 period, noting that the increase might be the same signal seen with the HRRP intervention.
“This is around the same time period where other people were writing the HRRP papers that showed an increase in mortality, so that’s something to consider,” she said.
Dr. Horwitz coauthored a study published in 2017 indicating that, on a hospital level (compared with a patient level, the approach most other studies took), reductions in readmissions were only weakly correlated with 30-day mortality rates after discharge.
“So, if you think that a hospital that’s behaving badly and keeping people out of the hospital inappropriately to cut down their readmissions, you’d expect to see increased mortality in that hospital, and in our study there was no correlation whatsoever. So there is still debate as to what is behind the increase in mortality on a patient level with heart failure that we’ve seen in some studies,” she said.
Dr. Horwitz doubts an intervention such as the one undertaken in the VA system – even with its fairly soft-touch “name and shame” component – would work in the non-VA hospital world.
“Those who have been in favor of financial penalties have pointed to the fact that, in general, it’s hard to get health systems to respond without financial alignment, even if it’s not an overt financial incentive,” she said.
“The VA is a unique environment,” she noted. “They have a very strong top-down command control focus where people are kind of used to being told, ‘OK, here are the measures we have to address this year.’ It’s good to see that the system that has worked for them for other outcomes also worked for them for heart failure readmissions too.”
Dr. Parizo has disclosed no relevant financial relationships. Dr. Horwitz has worked under contract to Medicare to develop readmission measures.
A version of this article originally appeared on Medscape.com.
Cortisol levels on COVID-19 admission may be a marker of severity
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to hospital have a substantially increased risk of dying, U.K. researchers have discovered.
Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London, and colleagues studied 535 patients admitted to major London hospitals. Their article was published online June 18 in Lancet Diabetes & Endocrinology.
“Our analyses show for the first time that patients with COVID-19 mount a marked and appropriate acute cortisol stress response,” said Dr. Dhillo and colleagues.
Moreover, “high cortisol concentrations were associated with increased mortality and a reduced median survival, probably because this is a marker of the severity of illness.”
So measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” Dr. Dhillo noted in a statement from his institution.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said.
However, it’s important to note that this means – particularly in the wake of the RECOVERY trial reported last week – that “in the early part of the disease you don’t need steroids,” he said.
In contrast to SARS, no adrenal insufficiency with COVID-19
Cortisol levels when healthy and resting are 100-200 nmol/L and nearly zero when sleeping, the researchers explained.
They decided to examine cortisol levels because, although physiological stress from critical illness normally increases levels of the hormone, the prior coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV), had the opposite effect and induced cortisol insufficiency in some patients.
“We would have said we’re not quite sure” what effect SARS-CoV-2 is having on cortisol levels, “so that’s why we collected the data,” Dr. Dhillo said in an interview.
The researchers studied patients admitted to three large London teaching hospitals between March 9 and April 22 with a clinical suspicion of SARS-CoV-2 infection. All patients had a standard set of blood tests, including full blood count, creatinine, C-reactive protein, D-dimer, and serum cortisol.
After exclusions, the team assessed 535 patients admitted over the study period who had baseline cortisol measured within 48 hours of admission.
Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing (88%) or a strong clinical and radiological suspicion, despite a negative test (12%).
In total, 132 (25%) individuals were not diagnosed with COVID-19.
Patients with COVID-19 were a mean age of 66.3 years, and 59.6% were men.
Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus (619 vs 519 nmol/L; P < .0001).
And by May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%; P < .0001).
Doubling of cortisol levels associated with 40% higher mortality
Multivariate analysis taking into account age, presence of comorbidities, and laboratory tests revealed that a doubling of cortisol concentrations among those with COVID-19 was associated with a significant increase in mortality, at a hazard ratio of 1.42 (P = .014).
And patients with COVID-19 whose baseline cortisol level was >744 nmol/L had a median survival of just 15 days, compared with those with a level ≤744 nmol/L, who had a median survival of 36 days (P < .0001).
The team notes that the cortisol stress responses in their patients with COVID-19 ranged up to 3,241 nmol/L, which is “a marked cortisol stress response, perhaps higher than is observed in patients undergoing major surgery.”
Of interest, there was no interaction between cortisol levels and ethnicity in their study; a subsequent analysis of the data stratified by black, Asian, and other minority ethnicities revealed no significant differences.
The team note that their results will need to be reproduced in other populations.
“Any potential role for cortisol measurement at baseline and later during an inpatient stay with COVID-19 as a prognostic biomarker, either by itself or in combination with other biomarkers, will require validation in a prospective study.”
Implications for treatment: Reserve dexamethasone for critically ill
Dr. Dhillo explained that, because their findings indicate that people initially infected with COVID-19 do mount an appropriate stress (cortisol) response, it is important that people properly understand this in the wake of the RECOVERY trial, reported last week.
The trial showed that the widely available steroid dexamethasone significantly reduced mortality among severely ill COVID-19 patients in the intensive care unit when given at a supraphysiologic dose of 6 mg.
But it would be hazardous for anyone to self-medicate with steroids at an early stage of COVID-19 because that would further increase cortisol levels and could suppress the immune system.
“For the average person on the street with COVID-19,” excess steroids will make their symptoms worse, Dr. Dhillo explained, adding this is important to emphasize because dexamethasone, and similar steroids, “are cheap and likely available on the Internet, and so misunderstanding of the RECOVERY trial could have serious implications.”
But once patients are very sick, with “inflammation in their lungs” and are in the intensive care unit, and often on ventilators – which is a very small subgroup of those with COVID-19 – it becomes a very different story, he stressed.
“RECOVERY shows clearly there seems to be a benefit once you need oxygen or are on a ventilator, and that makes sense because [dexamethasone] is going to be an anti-inflammatory,” in this instance when the “lungs are full of water.”
“But in the early days you definitely don’t need it and it could be harmful,” he reiterated.
The study is funded by the U.K. National Institute for Health Research and Medical Research Council. The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
T2D plus heart failure packs a deadly punch
It’s bad news for patients with newly diagnosed type 2 diabetes when they then develop heart failure during the next few years.
Patients with incident type 2 diabetes (T2D) who soon after also had heart failure appear faced a dramatically elevated mortality risk, higher than the incremental risk from any other cardiovascular or renal comorbidity that appeared following diabetes onset, in an analysis of more than 150,000 Danish patients with incident type 2 diabetes during 1998-2015.
The 5-year risk of death in patients who developed heart failure during the first 5 years following an initial diagnosis of T2D was about 48%, about threefold higher than in patients with newly diagnosed T2D who remained free of heart failure or any of the other studied comorbidities, Bochra Zareini, MD, and associates reported in a study published in Circulation: Cardiovascular Quality and Outcomes. The studied patients had no known cardiovascular or renal disease at the time of their first T2D diagnosis.
“Our study reports not only on the absolute 5-year risk” of mortality, “but also takes into consideration when patients developed” a comorbidity. “What is surprising and worrying is the very high risk of death following heart failure and the potential life years lost when compared to T2D patients who do not develop heart failure,” said Dr. Zareini, a cardiologist at Herlev and Gentofte University Hospital in Copenhagen. “The implications of our study are to create awareness and highlight the importance of early detection of heart failure development in patients with T2D.” The results also showed that “heart failure is a common cardiovascular disease” in patients with newly diagnosed T2D, she added in an interview.
The data she and her associates reported came from a retrospective analysis of 153,403 Danish citizens in national health records who received a prescription for an antidiabetes drug for the first time during 1998-2015, excluding patients with a prior diagnosis of heart failure, ischemic heart disease (IHD), stroke, peripheral artery disease (PAD), chronic kidney disease (CKD), or gestational diabetes. They followed these patients for a median of just under 10 years, during which time 45% of the cohort had an incident diagnosis of at least one of these cardiovascular and renal conditions, based on medical-record entries from hospitalization discharges or ambulatory contacts.
Nearly two-thirds of the T2D patients with an incident comorbidity during follow-up had a single new diagnosis, a quarter had two new comorbidities appear during follow-up, and 13% developed at least three new comorbidities.
Heart failure, least common but deadliest comorbidity
The most common of the tracked comorbidities was IHD, which appeared in 8% of the T2D patients within 5 years and in 13% after 10 years. Next most common was stroke, affecting 3% of patients after 5 years and 5% after 10 years. CKD occurred in 2.2% after 5 years and in 4.0% after 10 years, PAD occurred in 2.1% after 5 years and in 3.0% at 10 years, and heart failure occurred in 1.6% at 5 years and in 2.2% after 10 years.
But despite being the least common of the studied comorbidities, heart failure was by far the most deadly, roughly tripling the 5-year mortality rate, compared with T2D patients with no comorbidities, regardless of exactly when it first appeared during the first 5 years after the initial T2D diagnosis. The next most deadly comorbidities were stroke and PAD, which each roughly doubled mortality, compared with the patients who remained free of any studied comorbidity. CKD boosted mortality by 70%-110%, depending on exactly when it appeared during the first 5 years of follow-up, and IHD, while the most frequent comorbidity was also the most benign, increasing mortality by about 30%.
The most deadly combinations of two comorbidities were when heart failure appeared with either CKD or with PAD; each of these combinations boosted mortality by 300%-400% when it occurred during the first few years after a T2D diagnosis.
The findings came from “a very big and unselected patient group of patients, making our results highly generalizable in terms of assessing the prognostic consequences of heart failure,” Dr. Zareini stressed.
Management implications
The dangerous combination of T2D and heart failure has been documented for several years, and prompted a focused statement in 2019 about best practices for managing these patients (Circulation. 2019 Aug 3;140[7]:e294-324). “Heart failure has been known for some time to predict poorer outcomes in patients with T2D. Not much surprising” in the new findings reported by Dr. Zareini and associates, commented Robert H. Eckel, MD, a cardiovascular endocrinologist at the University of Colorado at Denver, Aurora. Heart failure “rarely acts alone, but in combination with other forms of heart or renal disease,” he noted in an interview.
Earlier studies may have “overlooked” heart failure’s importance compared with other comorbidities because they often “only investigated one cardiovascular disease in patients with T2D,” Dr. Zareini noted. In recent years the importance of heart failure occurring in patients with T2D also gained heightened significance because of the growing role of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drug class in treating patients with T2D and the documented ability of these drugs to significantly reduce hospitalizations for heart failure (J Am Coll Cardiol. 2020 Apr 28;75[16]:1956-74). Dr. Zareini and associates put it this way in their report: “Heart failure has in recent years been recognized as an important clinical endpoint ... in patients with T2D, in particular, after the results from randomized, controlled trials of SGLT2 inhibitors showed benefit on cardiovascular death and heart failure hospitalizations.”
Despite this, the new findings “do not address treatment with SGLT2 inhibitors in patients with T2D, nor can we use our data to address which patients should not be treated,” with this drug class, which instead should rely on “current evidence and expert consensus,” she said.
“Guidelines favor SGLT2 inhibitors or [glucagonlike peptide–1] receptor agonists in patients with a history of or high risk for major adverse coronary events,” and SGLT2 inhibitors are also “preferable in patients with renal disease,” Dr. Eckel noted.
Other avenues also exist for minimizing the onset of heart failure and other cardiovascular diseases in patients with T2D, Dr. Zareini said, citing modifiable risks that lead to heart failure that include hypertension, “diabetic cardiomyopathy,” and ISD. “Clinicians must treat all modifiable risk factors in patients with T2D in order to improve prognosis and limit development of cardiovascular and renal disease.”
The study received no commercial funding. Dr. Zareini and Dr. Eckel had no disclosures.
SOURCE: Zareini B et al. Circ Cardiovasc Qual Outcomes. 2020 Jun 23. doi: 10.1161/CIRCOUTCOMES.119.006260.
It’s bad news for patients with newly diagnosed type 2 diabetes when they then develop heart failure during the next few years.
Patients with incident type 2 diabetes (T2D) who soon after also had heart failure appear faced a dramatically elevated mortality risk, higher than the incremental risk from any other cardiovascular or renal comorbidity that appeared following diabetes onset, in an analysis of more than 150,000 Danish patients with incident type 2 diabetes during 1998-2015.
The 5-year risk of death in patients who developed heart failure during the first 5 years following an initial diagnosis of T2D was about 48%, about threefold higher than in patients with newly diagnosed T2D who remained free of heart failure or any of the other studied comorbidities, Bochra Zareini, MD, and associates reported in a study published in Circulation: Cardiovascular Quality and Outcomes. The studied patients had no known cardiovascular or renal disease at the time of their first T2D diagnosis.
“Our study reports not only on the absolute 5-year risk” of mortality, “but also takes into consideration when patients developed” a comorbidity. “What is surprising and worrying is the very high risk of death following heart failure and the potential life years lost when compared to T2D patients who do not develop heart failure,” said Dr. Zareini, a cardiologist at Herlev and Gentofte University Hospital in Copenhagen. “The implications of our study are to create awareness and highlight the importance of early detection of heart failure development in patients with T2D.” The results also showed that “heart failure is a common cardiovascular disease” in patients with newly diagnosed T2D, she added in an interview.
The data she and her associates reported came from a retrospective analysis of 153,403 Danish citizens in national health records who received a prescription for an antidiabetes drug for the first time during 1998-2015, excluding patients with a prior diagnosis of heart failure, ischemic heart disease (IHD), stroke, peripheral artery disease (PAD), chronic kidney disease (CKD), or gestational diabetes. They followed these patients for a median of just under 10 years, during which time 45% of the cohort had an incident diagnosis of at least one of these cardiovascular and renal conditions, based on medical-record entries from hospitalization discharges or ambulatory contacts.
Nearly two-thirds of the T2D patients with an incident comorbidity during follow-up had a single new diagnosis, a quarter had two new comorbidities appear during follow-up, and 13% developed at least three new comorbidities.
Heart failure, least common but deadliest comorbidity
The most common of the tracked comorbidities was IHD, which appeared in 8% of the T2D patients within 5 years and in 13% after 10 years. Next most common was stroke, affecting 3% of patients after 5 years and 5% after 10 years. CKD occurred in 2.2% after 5 years and in 4.0% after 10 years, PAD occurred in 2.1% after 5 years and in 3.0% at 10 years, and heart failure occurred in 1.6% at 5 years and in 2.2% after 10 years.
But despite being the least common of the studied comorbidities, heart failure was by far the most deadly, roughly tripling the 5-year mortality rate, compared with T2D patients with no comorbidities, regardless of exactly when it first appeared during the first 5 years after the initial T2D diagnosis. The next most deadly comorbidities were stroke and PAD, which each roughly doubled mortality, compared with the patients who remained free of any studied comorbidity. CKD boosted mortality by 70%-110%, depending on exactly when it appeared during the first 5 years of follow-up, and IHD, while the most frequent comorbidity was also the most benign, increasing mortality by about 30%.
The most deadly combinations of two comorbidities were when heart failure appeared with either CKD or with PAD; each of these combinations boosted mortality by 300%-400% when it occurred during the first few years after a T2D diagnosis.
The findings came from “a very big and unselected patient group of patients, making our results highly generalizable in terms of assessing the prognostic consequences of heart failure,” Dr. Zareini stressed.
Management implications
The dangerous combination of T2D and heart failure has been documented for several years, and prompted a focused statement in 2019 about best practices for managing these patients (Circulation. 2019 Aug 3;140[7]:e294-324). “Heart failure has been known for some time to predict poorer outcomes in patients with T2D. Not much surprising” in the new findings reported by Dr. Zareini and associates, commented Robert H. Eckel, MD, a cardiovascular endocrinologist at the University of Colorado at Denver, Aurora. Heart failure “rarely acts alone, but in combination with other forms of heart or renal disease,” he noted in an interview.
Earlier studies may have “overlooked” heart failure’s importance compared with other comorbidities because they often “only investigated one cardiovascular disease in patients with T2D,” Dr. Zareini noted. In recent years the importance of heart failure occurring in patients with T2D also gained heightened significance because of the growing role of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drug class in treating patients with T2D and the documented ability of these drugs to significantly reduce hospitalizations for heart failure (J Am Coll Cardiol. 2020 Apr 28;75[16]:1956-74). Dr. Zareini and associates put it this way in their report: “Heart failure has in recent years been recognized as an important clinical endpoint ... in patients with T2D, in particular, after the results from randomized, controlled trials of SGLT2 inhibitors showed benefit on cardiovascular death and heart failure hospitalizations.”
Despite this, the new findings “do not address treatment with SGLT2 inhibitors in patients with T2D, nor can we use our data to address which patients should not be treated,” with this drug class, which instead should rely on “current evidence and expert consensus,” she said.
“Guidelines favor SGLT2 inhibitors or [glucagonlike peptide–1] receptor agonists in patients with a history of or high risk for major adverse coronary events,” and SGLT2 inhibitors are also “preferable in patients with renal disease,” Dr. Eckel noted.
Other avenues also exist for minimizing the onset of heart failure and other cardiovascular diseases in patients with T2D, Dr. Zareini said, citing modifiable risks that lead to heart failure that include hypertension, “diabetic cardiomyopathy,” and ISD. “Clinicians must treat all modifiable risk factors in patients with T2D in order to improve prognosis and limit development of cardiovascular and renal disease.”
The study received no commercial funding. Dr. Zareini and Dr. Eckel had no disclosures.
SOURCE: Zareini B et al. Circ Cardiovasc Qual Outcomes. 2020 Jun 23. doi: 10.1161/CIRCOUTCOMES.119.006260.
It’s bad news for patients with newly diagnosed type 2 diabetes when they then develop heart failure during the next few years.
Patients with incident type 2 diabetes (T2D) who soon after also had heart failure appear faced a dramatically elevated mortality risk, higher than the incremental risk from any other cardiovascular or renal comorbidity that appeared following diabetes onset, in an analysis of more than 150,000 Danish patients with incident type 2 diabetes during 1998-2015.
The 5-year risk of death in patients who developed heart failure during the first 5 years following an initial diagnosis of T2D was about 48%, about threefold higher than in patients with newly diagnosed T2D who remained free of heart failure or any of the other studied comorbidities, Bochra Zareini, MD, and associates reported in a study published in Circulation: Cardiovascular Quality and Outcomes. The studied patients had no known cardiovascular or renal disease at the time of their first T2D diagnosis.
“Our study reports not only on the absolute 5-year risk” of mortality, “but also takes into consideration when patients developed” a comorbidity. “What is surprising and worrying is the very high risk of death following heart failure and the potential life years lost when compared to T2D patients who do not develop heart failure,” said Dr. Zareini, a cardiologist at Herlev and Gentofte University Hospital in Copenhagen. “The implications of our study are to create awareness and highlight the importance of early detection of heart failure development in patients with T2D.” The results also showed that “heart failure is a common cardiovascular disease” in patients with newly diagnosed T2D, she added in an interview.
The data she and her associates reported came from a retrospective analysis of 153,403 Danish citizens in national health records who received a prescription for an antidiabetes drug for the first time during 1998-2015, excluding patients with a prior diagnosis of heart failure, ischemic heart disease (IHD), stroke, peripheral artery disease (PAD), chronic kidney disease (CKD), or gestational diabetes. They followed these patients for a median of just under 10 years, during which time 45% of the cohort had an incident diagnosis of at least one of these cardiovascular and renal conditions, based on medical-record entries from hospitalization discharges or ambulatory contacts.
Nearly two-thirds of the T2D patients with an incident comorbidity during follow-up had a single new diagnosis, a quarter had two new comorbidities appear during follow-up, and 13% developed at least three new comorbidities.
Heart failure, least common but deadliest comorbidity
The most common of the tracked comorbidities was IHD, which appeared in 8% of the T2D patients within 5 years and in 13% after 10 years. Next most common was stroke, affecting 3% of patients after 5 years and 5% after 10 years. CKD occurred in 2.2% after 5 years and in 4.0% after 10 years, PAD occurred in 2.1% after 5 years and in 3.0% at 10 years, and heart failure occurred in 1.6% at 5 years and in 2.2% after 10 years.
But despite being the least common of the studied comorbidities, heart failure was by far the most deadly, roughly tripling the 5-year mortality rate, compared with T2D patients with no comorbidities, regardless of exactly when it first appeared during the first 5 years after the initial T2D diagnosis. The next most deadly comorbidities were stroke and PAD, which each roughly doubled mortality, compared with the patients who remained free of any studied comorbidity. CKD boosted mortality by 70%-110%, depending on exactly when it appeared during the first 5 years of follow-up, and IHD, while the most frequent comorbidity was also the most benign, increasing mortality by about 30%.
The most deadly combinations of two comorbidities were when heart failure appeared with either CKD or with PAD; each of these combinations boosted mortality by 300%-400% when it occurred during the first few years after a T2D diagnosis.
The findings came from “a very big and unselected patient group of patients, making our results highly generalizable in terms of assessing the prognostic consequences of heart failure,” Dr. Zareini stressed.
Management implications
The dangerous combination of T2D and heart failure has been documented for several years, and prompted a focused statement in 2019 about best practices for managing these patients (Circulation. 2019 Aug 3;140[7]:e294-324). “Heart failure has been known for some time to predict poorer outcomes in patients with T2D. Not much surprising” in the new findings reported by Dr. Zareini and associates, commented Robert H. Eckel, MD, a cardiovascular endocrinologist at the University of Colorado at Denver, Aurora. Heart failure “rarely acts alone, but in combination with other forms of heart or renal disease,” he noted in an interview.
Earlier studies may have “overlooked” heart failure’s importance compared with other comorbidities because they often “only investigated one cardiovascular disease in patients with T2D,” Dr. Zareini noted. In recent years the importance of heart failure occurring in patients with T2D also gained heightened significance because of the growing role of the sodium-glucose cotransporter 2 (SGLT2) inhibitor drug class in treating patients with T2D and the documented ability of these drugs to significantly reduce hospitalizations for heart failure (J Am Coll Cardiol. 2020 Apr 28;75[16]:1956-74). Dr. Zareini and associates put it this way in their report: “Heart failure has in recent years been recognized as an important clinical endpoint ... in patients with T2D, in particular, after the results from randomized, controlled trials of SGLT2 inhibitors showed benefit on cardiovascular death and heart failure hospitalizations.”
Despite this, the new findings “do not address treatment with SGLT2 inhibitors in patients with T2D, nor can we use our data to address which patients should not be treated,” with this drug class, which instead should rely on “current evidence and expert consensus,” she said.
“Guidelines favor SGLT2 inhibitors or [glucagonlike peptide–1] receptor agonists in patients with a history of or high risk for major adverse coronary events,” and SGLT2 inhibitors are also “preferable in patients with renal disease,” Dr. Eckel noted.
Other avenues also exist for minimizing the onset of heart failure and other cardiovascular diseases in patients with T2D, Dr. Zareini said, citing modifiable risks that lead to heart failure that include hypertension, “diabetic cardiomyopathy,” and ISD. “Clinicians must treat all modifiable risk factors in patients with T2D in order to improve prognosis and limit development of cardiovascular and renal disease.”
The study received no commercial funding. Dr. Zareini and Dr. Eckel had no disclosures.
SOURCE: Zareini B et al. Circ Cardiovasc Qual Outcomes. 2020 Jun 23. doi: 10.1161/CIRCOUTCOMES.119.006260.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES
Where does dexamethasone fit in with diabetic ketoacidosis in COVID-19?
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
A new article in the Journal of Clinical Endocrinology & Metabolism (JCEM) addresses unique concerns and considerations regarding diabetic ketoacidosis (DKA) in the setting of COVID-19.
Corresponding author Marie E. McDonnell, MD, director of the diabetes program at Brigham and Women’s Hospital, Boston, Massachusetts, discussed the recommendations with Medscape Medical News and also spoke about the news this week that the corticosteroid dexamethasone reduced death rates in severely ill patients with COVID-19.
The full JCEM article, by lead author Nadine E. Palermo, DO, Division of Endocrinology, Diabetes, and Hypertension, also at Brigham and Women’s Hospital, covers DKA diagnosis and triage, and emphasizes that usual hospital protocols for DKA management may need to be adjusted during COVID-19 to help preserve personal protective equipment and ICU beds.
“Hospitals and clinicians need to be able to quickly identify and manage DKA in COVID patients to save lives. This involves determining the options for management, including when less intensive subcutaneous insulin is indicated, and understanding how to guide patients on avoiding this serious complication,” McDonnell said in an Endocrine Society statement.
What about dexamethasone for severe COVID-19 in diabetes?
The new article briefly touches on the fact that upward adjustments to intensive intravenous insulin therapy for DKA may be necessary in patients with COVID-19 who are receiving concomitant corticosteroids or vasopressors.
But it was written prior to the June 16 announcement of the “RECOVERY” trial results with dexamethasone. The UK National Health Service immediately approved the drug’s use in the COVID-19 setting, despite the fact that there has been no published article on the findings yet.
McDonnell told Medscape Medical News that she would need to see formal results to better understand exactly which patients were studied and which ones benefited.
“The peer review will be critical. It looks as if it only benefits people who need respiratory support, but I want to understand that in much more detail,” she said. “If they all had acute respiratory distress syndrome [ARDS],” that’s different.
“There are already some data supporting steroid use in ARDS,” she noted, but added that not all of it suggests benefit.
She pointed to one of several studies now showing that diabetes, and hyperglycemia among people without a prior diabetes diagnosis, are both strong predictors of mortality in hospitalized patients with COVID-19.
“There was a very clear relationship between hyperglycemia and outcomes. We really shouldn’t put people at risk until we have clear data,” she said.
If, once the data are reviewed and appropriate dexamethasone becomes an established treatment for severe COVID-19, hyperglycemia would be a concern among all patients, not just those with previously diagnosed diabetes, she noted.
“We know a good number of people with prediabetes develop hyperglycemia when put on steroids. They can push people over the edge. We’re not going to miss anybody, but treating steroid-induced hyperglycemia is really hard,” McDonnell explained.
She also recommended 2014 guidance from Diabetes UK and the Association of British Clinical Diabetologists, which addresses management of inpatient steroid-induced DKA in patients with and without pre-existing diabetes.
Another major concern, she said, is “patients trying to get dexamethasone when they start to get sick” because this is not the right population to use this agent.
“We worry about people who do not need this drug. If they have diabetes, they put themselves at risk of hyperglycemia, which then increases the risk of severe COVID-19. And then they’re also putting themselves at risk of DKA. It would just be bad medicine,” she said.
Managing DKA in the face of COVID-19: Flexibility is key
In the JCEM article, Palermo and colleagues emphasize that the usual hospital protocols for DKA management may need to be adjusted during COVID-19 in the interest of reducing transmission risk and preserving scare resources.
They provide evidence for alternative treatment strategies, such as the use of subcutaneous rather than intravenous insulin when appropriate.
“We wanted to outline when exactly you should consider nonintensive management strategies for DKA,” McDonnell further explained to Medscape Medical News.
“That would include those with mild or some with moderate DKA. ... The idea is to remind our colleagues about that because hospitals tend to operate on a protocol-driven algorithmic methodology, they can forget to step off the usual care pathway even if evidence supports that,” she said.
But on the other hand, she also said that, in some very complex or severely ill patients with COVID-19, classical intravenous insulin therapy makes the most sense even if their DKA is mild.
The outpatient setting: Prevention and preparation
The new article also addresses several concerns regarding DKA prevention in the outpatient setting.
As with other guidelines, it includes a reminder that patients with diabetes should be advised to discontinue sodium-glucose cotransporter 2 (SGLT2) inhibitors if they become ill with COVID-19, especially if they’re not eating or drinking normally, because they raise the risk for DKA.
Also, for patients with type 1 diabetes, particularly those with a history of repeated DKA, “this is the time to make sure we reach out to patients to refill their insulin prescriptions and address issues related to cost and other access difficulties,” McDonnell said.
The authors also emphasize that insulin starts and education should not be postponed during the pandemic. “Patients identified as meeting criteria to start insulin should be referred for urgent education, either in person or, whenever possible and practical, via video teleconferencing,” they urge.
McDonnell has reported receiving research funding from Novo Nordisk. The other two authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Smart phones boosted compliance for cardiac device data transmission
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
A phone, an app, and the next generation of implanted cardiac device data signaling produced an unprecedented level of data transmission compliance in a single-arm, multicenter, pilot study with 245 patients, adding momentum to the expanding penetration of personal smart devices into cardiac electrophysiology.
During 12-month follow-up, the 245 patients who received either a medically indicated pacemaker or cardiac resynchronization therapy (CRT)–pacemaker equipped with Bluetooth remote transmission capability had successful data transfer to their clinicians for 95% of their scheduled data uploads while using a personal phone or tablet as the link between their heart implant and the Internet. This rate significantly surpassed the transmission-success rates tallied by traditional, bedside transmitters in historical control groups, Khaldoun G. Tarakji, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19.
A related analysis by Dr. Tarakji and colleagues of 811 patients from real-world practice who received similar implanted cardiac devices with the same remote-transmission capability showed a 93% rate of successful data transfers via smart devices.
In contrast, historical performance showed a 77% success rate in matched patients drawn from a pool of more than 69,000 people in routine care who had received a pacemaker or CRT-pacemaker that automatically transmitted to a bedside monitor. Historical transmission success among matched patients from a pool of more than 128,000 routine-care patients with similar implants who used a wand to interrogate their implants before the attached monitor transmitted their data had a 56% rate of successful transmissions.
Cardiac device signals that flow directly into a patient’s phone or pad and then relay automatically via an app to the clinic “are clearly much easier,” than the methods now used, observed Dr. Tarakji, a cardiac electrophysiologist at the Cleveland Clinic. “It is truly as seamless as possible. Patients don’t really need to do anything,” he said during a press briefing. The key is that most patients tend to keep their smart devices, especially their phones, near them all the time, which minimizes the chance that the implanted cardiac device might try to file a report when the patient is not positioned near the device that’s facilitating transmission. When patients use conventional, bedside transmitters they can forget to bring them on trips, while many fewer fail to take their phone. Another advantage is that the link between a phone and a cardiac implant can be started in the clinic once the patient downloads an app. Bedside units need home setup, and “some patients never even get theirs out of the box,” Dr. Tarakji lamented.
Another feature of handheld device transmissions that run off an app is that the app can display clinical metrics, activity, device performance, and transmission history, as well as educational information. All of these features can enhance patient engagement with their implanted device, their arrhythmia, and their health status. Bedside units often give patients little feedback, and they don’t display clinical data. “The real challenge for clinicians is what data you let patients see. That’s complicated,” Dr. Tarakji said.
“This study was designed to see whether the technology works. The next step is to study how it affects risk-factor modification” or other outcomes. “There are many opportunities” to explore with this new data transmission and processing capability, he concluded.
The BlueSync Field Evaluation study enrolled patients at 20 centers in the United States, France, Italy, and the United Kingdom during 2018, and the 245 patients who received a BlueSync device and were included in the analysis sent at least one of their scheduled data transmissions during their 12 months of follow-up. Participants were eligible if they were willing to use their own smart phone or pad that could interact with their cardiac implant, and included both first-time implant recipients as well as some patients who received replacement units.
Personal device–based data transmission from cardiac implants “will no doubt change the way we manage patients,” commented Nassir F. Marrouche, MD, a cardiac electrophysiologist and professor of medicine at Tulane University in New Orleans, and a designated discussant for the report. “Every implanted cardiac device should be able to connect with a phone, which can improve adoption and adherence,” he said.
But the study has several limitations for interpreting the implications of the findings, starting with its limited size and single-arm design, noted a second discussant, Roderick Tung, MD, director of cardiac electrophysiology at the University of Chicago. Another issue is the generalizability of the findings, which are likely biased by involving only patients who own a smart phone or tablet and may be more likely to transmit their data regardless of the means. And comparing transmission success in a prospective study with rates that occurred during real-world, routine practice could have a Hawthorne effect bias, where people under study behave differently than they do in everyday life. But that effect may be mitigated by confirmatory findings from a real-world group that also used smart-device transmission included in the report. Despite these caveats, it’s valuable to develop new ways of improving data collection from cardiac devices, Dr. Tung said.
The BlueSync Field Evaluation study was sponsored by Medtronic, the company that markets Bluetooth-enabled cardiac devices. Dr. Tarakji has been a consultant to Medtronic, and also to AliveCor, Boston Scientific, and Johnson & Johnson. Dr. Marrouche has been a consultant to Medtronic as well as to Biosense Webster, Biotronik, Cardiac Design, and Preventice, and he has received research funding from Abbott, Biosense Webster, Boston Scientific, and GE Healthcare. Dr. Tung has been a speaker on behalf of Abbott, Boston Scientific, and Biosense Webster.
SOURCE: Tarakji KG. Heart Rhythm 2020, Abstract D-LBCT04-01.
FROM HEART RHYTHM 2020
For COVID-19 plus diabetes, glycemic control tops treatment list
Optimizing glycemic control “is the key to overall treatment in people with diabetes and COVID-19,” said Antonio Ceriello, MD, during a June 5 webinar sponsored by Harvard Medical School, Boston.
Dr. Ceriello, a research consultant with the Italian Ministry of Health, IRCCS Multi-Medica, Milan, highlighted a recent study that examined the association of blood glucose control and outcomes in COVID-19 patients with preexisting type 2 diabetes.
Among 7,000 cases of COVID-19, type 2 diabetes correlated with a higher death rate. However, those with well-controlled blood glucose (upper limit ≤10 mmol/L) had a survival rate of 98.9%, compared with just 11% among those with poorly controlled blood glucose (upper limit >10 mmol/L), a reduction in risk of 86% (adjusted hazard ratio, 0.14; Cell Metab. 2020 May 1. doi: 10.1016/j.cmet.2020.04.021).
Clinicians should also consider the possible side effects of hypoglycemic agents in the evolution of this disease. This is true of all patients, not just diabetes patients, Dr. Ceriello said. “We have data showing that ... hyperglycemia contributes directly to worsening the prognosis of COVID-19 independent of the presence of diabetes.”
One study found that the glycosylation of ACE-2 played an important role in allowing cellular entry of the virus (Am J Physiol Endocrinol Metab. 2020 Mar 31;318:E736-41). “This is something that could be related to hyperglycemia,” he added.
Another risk factor is thrombosis, a clear contributor to death rates in COVID-19. Research on thrombosis incidence in COVID-19 patients with diabetes reported higher levels of D-dimer levels in people with diabetes, especially among those who couldn’t manage their disease.
Tying all of these factors together, Dr. Ceriello discussed how ACE-2 glycosylation, in combination with other factors in SARS-CoV-2 infection, could lead to hyperglycemia, thrombosis, and subsequently multiorgan damage in diabetes patients.
Other research has associated higher HbA1c levels (mean HbA1c, 7.5%) with higher mortality risk in COVID-19 patients, said another speaker, Linong Ji, MD, director for endocrinology and metabolism at Peking University People’s Hospital, Beijing, and director of Peking University’s Diabetes Center. Proper guidance is key to ensuring early detection of hyperglycemic crisis in people with diabetes, advised Dr. Ji.
Global management of diabetes in SARS-CoV-2 patients is “quite challenging,” given that most patients don’t have their diabetes under control, said host and moderator A. Enrique Caballero, MD, an endocrinologist/investigator in the division of endocrinology, diabetes, and hypertension and division of global health equity at Brigham and Women’s Hospital, Boston. “They are not meeting treatment targets for cholesterol or glucose control. So we’re not managing optimal care. And now on top of this, we have COVID-19.”
Optimizing glycemic control “is the key to overall treatment in people with diabetes and COVID-19,” said Antonio Ceriello, MD, during a June 5 webinar sponsored by Harvard Medical School, Boston.
Dr. Ceriello, a research consultant with the Italian Ministry of Health, IRCCS Multi-Medica, Milan, highlighted a recent study that examined the association of blood glucose control and outcomes in COVID-19 patients with preexisting type 2 diabetes.
Among 7,000 cases of COVID-19, type 2 diabetes correlated with a higher death rate. However, those with well-controlled blood glucose (upper limit ≤10 mmol/L) had a survival rate of 98.9%, compared with just 11% among those with poorly controlled blood glucose (upper limit >10 mmol/L), a reduction in risk of 86% (adjusted hazard ratio, 0.14; Cell Metab. 2020 May 1. doi: 10.1016/j.cmet.2020.04.021).
Clinicians should also consider the possible side effects of hypoglycemic agents in the evolution of this disease. This is true of all patients, not just diabetes patients, Dr. Ceriello said. “We have data showing that ... hyperglycemia contributes directly to worsening the prognosis of COVID-19 independent of the presence of diabetes.”
One study found that the glycosylation of ACE-2 played an important role in allowing cellular entry of the virus (Am J Physiol Endocrinol Metab. 2020 Mar 31;318:E736-41). “This is something that could be related to hyperglycemia,” he added.
Another risk factor is thrombosis, a clear contributor to death rates in COVID-19. Research on thrombosis incidence in COVID-19 patients with diabetes reported higher levels of D-dimer levels in people with diabetes, especially among those who couldn’t manage their disease.
Tying all of these factors together, Dr. Ceriello discussed how ACE-2 glycosylation, in combination with other factors in SARS-CoV-2 infection, could lead to hyperglycemia, thrombosis, and subsequently multiorgan damage in diabetes patients.
Other research has associated higher HbA1c levels (mean HbA1c, 7.5%) with higher mortality risk in COVID-19 patients, said another speaker, Linong Ji, MD, director for endocrinology and metabolism at Peking University People’s Hospital, Beijing, and director of Peking University’s Diabetes Center. Proper guidance is key to ensuring early detection of hyperglycemic crisis in people with diabetes, advised Dr. Ji.
Global management of diabetes in SARS-CoV-2 patients is “quite challenging,” given that most patients don’t have their diabetes under control, said host and moderator A. Enrique Caballero, MD, an endocrinologist/investigator in the division of endocrinology, diabetes, and hypertension and division of global health equity at Brigham and Women’s Hospital, Boston. “They are not meeting treatment targets for cholesterol or glucose control. So we’re not managing optimal care. And now on top of this, we have COVID-19.”
Optimizing glycemic control “is the key to overall treatment in people with diabetes and COVID-19,” said Antonio Ceriello, MD, during a June 5 webinar sponsored by Harvard Medical School, Boston.
Dr. Ceriello, a research consultant with the Italian Ministry of Health, IRCCS Multi-Medica, Milan, highlighted a recent study that examined the association of blood glucose control and outcomes in COVID-19 patients with preexisting type 2 diabetes.
Among 7,000 cases of COVID-19, type 2 diabetes correlated with a higher death rate. However, those with well-controlled blood glucose (upper limit ≤10 mmol/L) had a survival rate of 98.9%, compared with just 11% among those with poorly controlled blood glucose (upper limit >10 mmol/L), a reduction in risk of 86% (adjusted hazard ratio, 0.14; Cell Metab. 2020 May 1. doi: 10.1016/j.cmet.2020.04.021).
Clinicians should also consider the possible side effects of hypoglycemic agents in the evolution of this disease. This is true of all patients, not just diabetes patients, Dr. Ceriello said. “We have data showing that ... hyperglycemia contributes directly to worsening the prognosis of COVID-19 independent of the presence of diabetes.”
One study found that the glycosylation of ACE-2 played an important role in allowing cellular entry of the virus (Am J Physiol Endocrinol Metab. 2020 Mar 31;318:E736-41). “This is something that could be related to hyperglycemia,” he added.
Another risk factor is thrombosis, a clear contributor to death rates in COVID-19. Research on thrombosis incidence in COVID-19 patients with diabetes reported higher levels of D-dimer levels in people with diabetes, especially among those who couldn’t manage their disease.
Tying all of these factors together, Dr. Ceriello discussed how ACE-2 glycosylation, in combination with other factors in SARS-CoV-2 infection, could lead to hyperglycemia, thrombosis, and subsequently multiorgan damage in diabetes patients.
Other research has associated higher HbA1c levels (mean HbA1c, 7.5%) with higher mortality risk in COVID-19 patients, said another speaker, Linong Ji, MD, director for endocrinology and metabolism at Peking University People’s Hospital, Beijing, and director of Peking University’s Diabetes Center. Proper guidance is key to ensuring early detection of hyperglycemic crisis in people with diabetes, advised Dr. Ji.
Global management of diabetes in SARS-CoV-2 patients is “quite challenging,” given that most patients don’t have their diabetes under control, said host and moderator A. Enrique Caballero, MD, an endocrinologist/investigator in the division of endocrinology, diabetes, and hypertension and division of global health equity at Brigham and Women’s Hospital, Boston. “They are not meeting treatment targets for cholesterol or glucose control. So we’re not managing optimal care. And now on top of this, we have COVID-19.”
















