User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Tapinarof Cream Under FDA Review for Atopic Dermatitis Indication
On February 14, Dermavant Sciences announced that the company had submitted a supplemental New Drug Application (sNDA) to the Food and Drug Administration for tapinarof cream, 1%, for treating atopic dermatitis (AD) in adults and children 2 years of age and older.
Tapinarof cream, 1%, is an aryl hydrocarbon receptor agonist marketed under the brand name VTAMA that was approved in 2022 for treating plaque psoriasis in adults.
According to a Dermavant press release, the sNDA is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials and interim results from the phase 3 ADORING 3 open-label, long-term extension 48-week trial. In ADORING 1 and ADORING 2, tapinarof cream demonstrated statistically significant improvements in the primary endpoint of Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) treatment success, defined as a vIGA-AD score of 0 (clear) or 1 (almost clear) with at least a 2-grade improvement from baseline; demonstrated treatment success over vehicle at week 8; and met all key secondary endpoints with statistical significance, according to the company.
The most common adverse reactions in patients treated with VTAMA cream include folliculitis, nasopharyngitis, contact dermatitis, headache, and pruritus.
On February 14, Dermavant Sciences announced that the company had submitted a supplemental New Drug Application (sNDA) to the Food and Drug Administration for tapinarof cream, 1%, for treating atopic dermatitis (AD) in adults and children 2 years of age and older.
Tapinarof cream, 1%, is an aryl hydrocarbon receptor agonist marketed under the brand name VTAMA that was approved in 2022 for treating plaque psoriasis in adults.
According to a Dermavant press release, the sNDA is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials and interim results from the phase 3 ADORING 3 open-label, long-term extension 48-week trial. In ADORING 1 and ADORING 2, tapinarof cream demonstrated statistically significant improvements in the primary endpoint of Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) treatment success, defined as a vIGA-AD score of 0 (clear) or 1 (almost clear) with at least a 2-grade improvement from baseline; demonstrated treatment success over vehicle at week 8; and met all key secondary endpoints with statistical significance, according to the company.
The most common adverse reactions in patients treated with VTAMA cream include folliculitis, nasopharyngitis, contact dermatitis, headache, and pruritus.
On February 14, Dermavant Sciences announced that the company had submitted a supplemental New Drug Application (sNDA) to the Food and Drug Administration for tapinarof cream, 1%, for treating atopic dermatitis (AD) in adults and children 2 years of age and older.
Tapinarof cream, 1%, is an aryl hydrocarbon receptor agonist marketed under the brand name VTAMA that was approved in 2022 for treating plaque psoriasis in adults.
According to a Dermavant press release, the sNDA is based on positive data from the phase 3 ADORING 1 and ADORING 2 pivotal trials and interim results from the phase 3 ADORING 3 open-label, long-term extension 48-week trial. In ADORING 1 and ADORING 2, tapinarof cream demonstrated statistically significant improvements in the primary endpoint of Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) treatment success, defined as a vIGA-AD score of 0 (clear) or 1 (almost clear) with at least a 2-grade improvement from baseline; demonstrated treatment success over vehicle at week 8; and met all key secondary endpoints with statistical significance, according to the company.
The most common adverse reactions in patients treated with VTAMA cream include folliculitis, nasopharyngitis, contact dermatitis, headache, and pruritus.
Mixing Paxlovid With Specific Immunosuppressants Risks Serious Adverse Reactions
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has issued a reminder to healthcare professionals regarding the potential serious adverse reactions associated with Paxlovid when administered in combination with specific immunosuppressants.
These immunosuppressants, encompassing calcineurin inhibitors (tacrolimus and ciclosporin) and mTOR inhibitors (everolimus and sirolimus), possess a narrow safe dosage range. They are recognized for their role in diminishing the activity of the immune system and are typically prescribed for autoimmune conditions and organ transplant recipients.
The highlighted risk arises due to drug-drug interactions, which can compromise the body’s ability to eliminate these medicines effectively.
Paxlovid, also known as nirmatrelvir with ritonavir, is an antiviral medication used to treat COVID-19 in adults who do not require supplemental oxygen and who are at an increased risk of progressing to severe COVID-19. It should be administered as soon as possible after a diagnosis of COVID-19 has been made and within 5 days of symptom onset.
Conditional marketing authorization for Paxlovid was granted across the European Union (EU) on January 28, 2022, and subsequently transitioned to full marketing authorization on February 24, 2023.
Developed by Pfizer, Paxlovid exhibited an 89% reduction in the risk for hospitalization or death among unvaccinated individuals in a phase 2-3 clinical trial. This led the National Institutes of Health to prioritize Paxlovid over other COVID-19 treatments. Subsequent real-world studies have affirmed its effectiveness, even among the vaccinated.
When combining Paxlovid with tacrolimus, ciclosporin, everolimus, or sirolimus, healthcare professionals need to actively monitor their blood levels. This proactive approach is essential to mitigate the risk for drug-drug interactions and potential serious reactions. They should collaborate with a multidisciplinary team of specialists to navigate the complexities of administering these medications concurrently.
Further, Paxlovid must not be coadministered with medications highly reliant on CYP3A liver enzymes for elimination, such as the immunosuppressant voclosporin. When administered together, there is a risk for these drugs interfering with each other’s metabolism, potentially leading to altered blood levels, reduced effectiveness, or an increased risk for adverse reactions.
After a thorough review, PRAC has highlighted potential serious adverse reactions, including fatal cases, due to drug interactions between Paxlovid and specified immunosuppressants. Thus, it issued a direct healthcare professional communication (DHPC) to emphasize the recognized risk for these interactions, as previously outlined in Paxlovid’s product information.
The DHPC for Paxlovid will undergo further evaluation by EMA’s Committee for Medicinal Products for Human Use and, upon adoption, will be disseminated to healthcare professionals. The communication plan will include publication on the DHPCs page and in national registers across EU Member States.
A version of this article appeared on Medscape.com.
Healing From Trauma
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
Management of Tinea Capitis in Children Varies, Survey Finds
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The fungal scalp infection tinea capitis affects an estimated 3%-13% of children.
- While international guidelines exist for the treatment of tinea capitis in infants and children, no such document has been developed in the United States.
- Researchers distributed a survey by email to dermatologists through the and the Society for Pediatric Dermatology in the United States, asking about how they treated and managed pediatric patients with tinea capitis; 56 dermatologists participated.
TAKEAWAY:
- Most respondents (88.2%) said they felt comfortable prescribing oral medications prior to confirmation for those aged 2-18 years ( was the most common choice in 60.4% of cases), compared with 81.6% for those aged 2 months to 2 years ( was the most common treatment choice in 41.5% of cases), and 48.7% for those aged 0-2 months ( was the most common choice in 28.6% of cases).
- When asked what topical medication they would start prior to confirmation, most respondents said shampoo (62.3% for those aged 0-2 months and 75.5% each for those aged 2 months to 2 years and those aged 2-18 years), yet between 11.3% and 13% said they would use none.
- The most common form of confirmatory testing was , followed by potassium hydroxide preparation, trichoscopy, and Wood’s lamp.
- More than half of survey respondents would alter their choice of oral medication based on culture results, but most would not change their topical medication preference.
IN PRACTICE:
“The management of tinea capitis in the United States is currently variable, particularly with the introduction of newer antifungals,” the authors wrote. “Future steps involve establishing evidence-based clinical practice guidelines that consider drug efficacy, safety profiles, and costs.”
SOURCE:
Bernard Cohen, MD, of the Departments of Pediatrics and Dermatology at Johns Hopkins University, Baltimore, Maryland, led the research, which was published in Pediatric Dermatology.
LIMITATIONS:
Lower response rates associated with online surveys and predefined age groups restrict the granularity of responses.
DISCLOSURES:
The authors reported having no financial disclosures.
A version of this article appeared on Medscape.com.
Expert Hopes to Expand Ohio Model of Melanoma Case Reporting
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
SAN DIEGO – Soon after Brett M. Coldiron, MD, launched his Cincinnati-based dermatology and Mohs surgery practice more than 20 years ago, he reported his first three cases of thin melanomas to the Ohio Department of Health, as mandated by state law.
“I got sent reams of paperwork to fill out that I did not understand,” Dr. Coldiron, a past president of the American College of Mohs Surgery and the American Academy of Dermatology, recalled at the annual Cutaneous Malignancy Update. “Then, I got chewed out for not reporting sooner and threatened with thousands of dollars in fines if I did not promptly report the forms in the future. It was an obnoxious experience.”
About 15 years later, while testifying at the Ohio Legislature on medical reasons to restrict the use of tanning beds, a lobbyist for the tanning bed industry told him that the melanoma rates had been stable in Ohio for the previous 5 years. “It turns out they were cherry picking certain segments of data to fit their narrative,” Dr. Coldiron said. “I was stunned and it kind of deflated me. I thought about this for a long time, and thought, ‘how do we solve this issue of reporting melanoma cases without adding work to existing staff if you’re a small practice and without spending significant amounts of money? Let’s make this easier.’ ”
In addition to reducing the use of tanning beds, proper reporting of melanoma cases is important for reasons that include efforts to increase sunscreen use and to be counted in ongoing research efforts to obtain a realistic snapshot of melanoma prevalence and incidence, he said.
Quality of melanoma case reporting relies on the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), and the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which collects data on the incidence, treatment, staging, and survival for 28% of the US population. All 50 states and US territories require melanoma to be reported to the NPCR, but while most hospital systems have reporting protocols and dedicated data registrars, private practices may not.
Also, many dermatopathology practices operate independently and do not have dedicated registrars and may not report cases. “Melanoma is unique in that it is often completely managed in outpatient settings and these melanomas may never be reported,” said Dr. Coldiron, current president of the Ohio Dermatological Foundation. “That’s the practice gap.” One study published in 2018 found that only 49% of dermatologists knew that melanoma was a reportable disease and only 34% routinely reported newly diagnosed cases to their state’s cancer registry. He characterized melanoma reporting as an unfunded mandate.
“Hospitals are doing the most of them, because they have a registrar,” he said. “Small practices have to assign someone to do this, and it can be difficult to train that person. It’s time consuming. The first time we did it, it took an hour,” but, he said, taking a 2-hour tutorial from the Ohio Department of Health helped.
He noted that there is a lack of awareness and clinicians think it’s the dermatopathologist’s job to report cases, “while the dermatopathologist thinks it’s the clinician’s job,” and many of the entry fields are not applicable to thinner melanomas.
There is also a “patchwork” of ways that state departments of health accept the information, not all electronically, he continued. For example, those in Arizona, Montana, West Virginia, Delaware, Vermont, and Maine accept paper copies only, “meaning you have to download a PDF, fill it out, and fax it back to them,” Dr. Coldiron said at the meeting, which was hosted by Scripps Cancer Center.
“We have them sign a HIPAA form and take the two-hour online tutorial,” he said. They download data that Ohio dermatologists have faxed to a dedicated secure HIPAA-compliant cloud-based fax line that Dr. Coldiron has set up, and the cases are then sent to the Ohio Department of Health.
Dr. Coldiron and colleagues have also partnered with the University of Cincinnati Clermont, which offers a National Cancer Registries Association–accredited certificate program — one of several nationwide. Students in this program are trained to become cancer registrars. “The university staff are gung-ho about it because they are looking for easy cases to train the students on. Also, the Ohio Department of Health staff are keen to help train the students and even help them find jobs or hire them after they complete the degree. Staff from the department of health and college faculty are fully engaged and supervising. It’s a win-win for all.”
According to Dr. Coldiron, in 2023, 8 Ohio dermatology practices were sending their reports to the fax line he set up and 7 more have signed up in recent months, making 15 practices to date. “It’s self-perpetuating at this point,” he said. “The Ohio Department of Health and the University of Cincinnati are invested in this program long-term.” The fax service costs Dr. Coldiron $42 per month — a small price to pay, he said, for being a clearinghouse for private Ohio dermatology practices looking for a practical way to report their melanoma cases. The model has increased melanoma reporting in Ohio by 2.8% in the last 2 years, “which doesn’t seem like that many, but if there are 6500 cases of melanoma, and you can increase reporting by a couple hundred cases, that’s a lot,” he said.
His goal is to expand this model to more states. “Dermatologists, surgical oncologists, and cancer center administrators should embrace this opportunity to make their practices a clearinghouse for their state,” he said. “This is an opportunity to improve state health, quality improvement projects, help providers, and gain recognition as a center of excellence. The increase in incidence of melanoma will lend great clout to public and legislative requests for prevention, treatment, and research dollars.”
In an interview, Hugh Greenway, MD, the head of Mohs and dermatologic surgery at Scripps Clinic in San Diego, also noted that cutaneous melanoma is significantly underreported in spite of individual state requirements. “As Dr. Coldiron reminds us, the main reason is that in many cases the pathology diagnosis and report come from the dermatologist’s/dermatopathologist’s office,” Dr. Greenway said. “With no hospital or large multispecialty laboratory involved, the reporting may be incomplete or not done. This is not the case with almost all other cancers where a hospital laboratory is involved.”
If widespread adoption of Dr. Coldiron’s model can occur, he added, “then we will have much better melanoma reporting data on which to both help our patients and specialty. He is to be applauded for producing a workable solution to the problem of underreporting.”
Dr. Coldiron reported having no relevant disclosures. Dr. Greenway reported that he conducts research for Castle Biosciences. He is also course director of the annual Cutaneous Malignancy Update.
FROM MELANOMA 2024
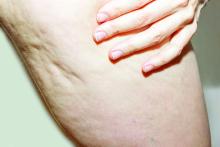
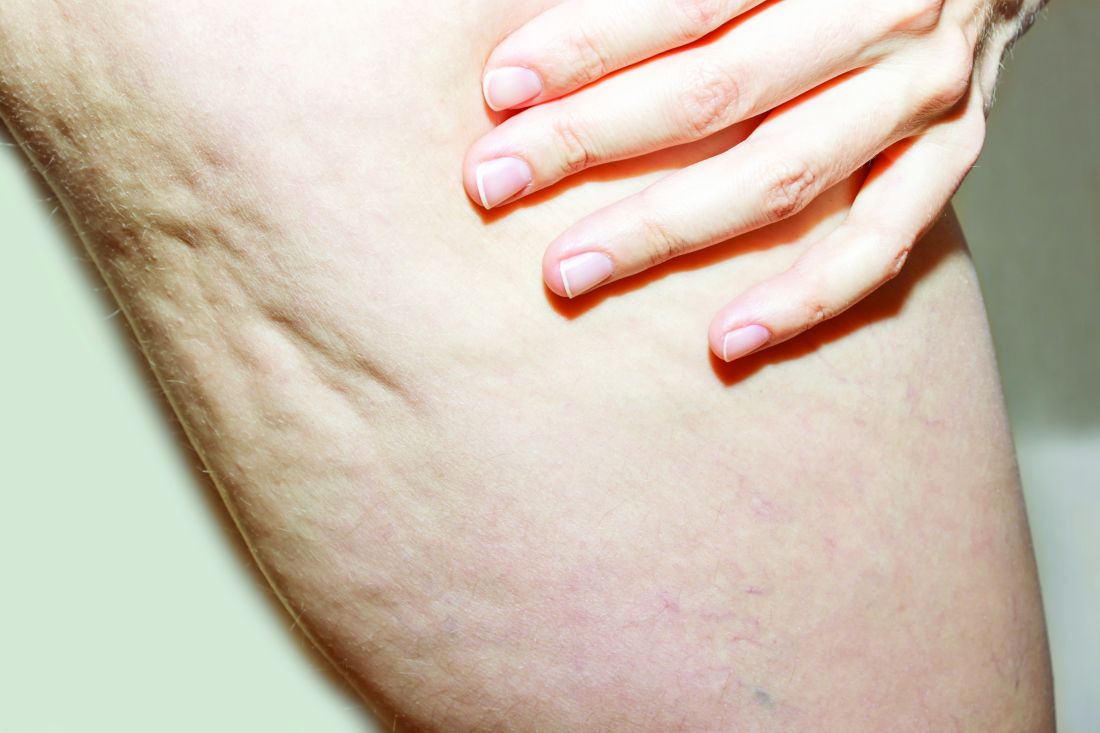
What Do Results from Acoustic Subcision for Cellulite Look Like at One Year?
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
FROM DERMATOLOGIC SURGERY
OTC Topical Scar Products May Contain Allergens, Study Finds
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- OTC topical scar treatments have the potential to cause an allergic reaction, but the prevalence of North American Contact Dermatitis Group (NACDG) core allergens in these products is unclear.
- Researchers used the word scar in a query of Amazon.com and four other retail websites to identify topical scar products for consumers and noted the list of ingredients.
- The investigators also surveyed the American Contact Dermatitis Society’s Contact Allergen Management Program (CAMP), a resource that helps patients with allergies find personal care products that are safe to use, for pertinent products.
TAKEAWAY:
- The search query identified 156 products. Of these, 119 (76.2%) were gels, creams, or oils and 37 (23.7%) were sheets, strips, or tape.
- Of the 125 products that had a list of ingredients, 69 (55.2%) contained at least one NACDG allergen and 45 (36%) contained more than one.
- The top six most common allergens listed in the ingredients were fragrance (16.8%), phenoxyethanol (16.8%), parabens (14.4%), panthenol (12.8%), sodium benzoate (9.60%), and ethylhexylglycerin (8%).
- Analysis of CAMP revealed that the program only had five unique scar products in its list, suggesting that CAMP might not be a reliable source of scar product information for patients with known allergies to pertinent NACDG allergens.
IN PRACTICE:
“Patients can consider trying a ‘use test’ on the inner forearm before applying to the surgical site,” the authors wrote. “It may reveal they are sensitive or sensitized by a product.
SOURCE:
First author Meera Kattapuram, MD, of the Department of Internal Medicine at Mount Sinai Hospital, New York, led the study, published in the February issue of Dermatologic Surgery.
LIMITATIONS:
Limitations include the selection of five retailers and the top 100 products from each website and the potential for ingredient list inaccuracies.
DISCLOSURES:
The authors reported having no financial conflicts of interest. The research was supported by a grant from the National Institutes of Health/National Cancer Institute.
A version of this article appeared on Medscape.com.
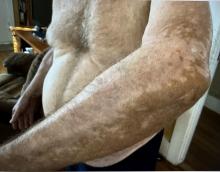
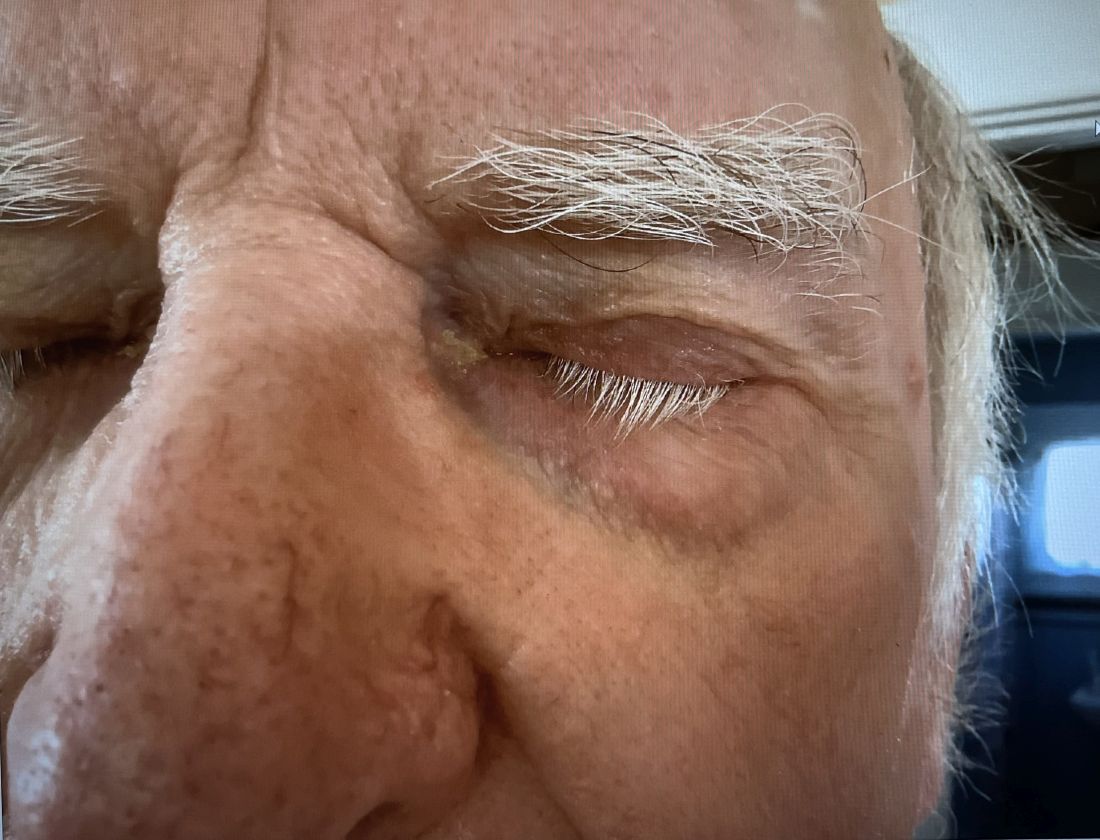
A 74-year-old White male presented with a 1-year history of depigmented patches on the hands, arms, and face, as well as white eyelashes and eyebrows
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
Biosimilars Have Driven Down Cost of Infliximab
LAS VEGAS — , according to two new retrospective analyses that separately looked at private insurance and Medicare patients.
“It is interesting to see that biosimilar use is increasing in patients with Medicare insurance in the U.S. since introduction into the market. This shows that clinicians are getting more comfortable with their use in clinical practice,” said Sara Horst, MD, associate professor of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center in Nashville, Tennessee. That “is a continued important question in use, especially in the older population,” Dr. Horst said at the poster session where the two studies were presented, at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
One study examined Medicare part D participants. The researchers included infliximab prescriptions between 2013 and 2021, with a break in 2017 when biosimilars were first introduced. Between 2013 and 2017, there was a 93.7% annual increase in infliximab cost, and a 29.0% increase in annual claims. Between 2017 and 2021, those numbers declined to 23.9% and 12.1%, respectively. The researchers also examined trends by individual states and found a wide range of results. “Nationwide, it seems to be doing exactly what you’d expect, and I’d say it’s encouraging. This is exactly what you want with the introduction of biosimilars, and this is what you want for the patient population. But there are some states that have not really kept up with the rest of the country, and may need to look further into what types of trends are there,” said Modan Goldman, who presented the study. He is a medical student at Carle Illinois College of Medicine in Urbana, Illinois.
The other study looked at use of infliximab versus a biosimilar between 2015 and 2021 in 42,009 patients 64 or younger, drawing data from Merative Marketscan Commercial Claims and Encounters Database. They excluded government-funded insurance. Between January 2015 and December 2017, the cost of a vial of infliximab increased by $6.31 per month, reaching a maxim cost of $1,491 per vial. In January 2018, the cost decreased by $62 per vial, with a downward trend after that of $12.93 per vial per month.
“[Getting] access to these drugs, especially for pediatric IBD, has been an uphill battle with insurance companies and getting the medication and the doses we want,” said Samantha Paglinco, DO, who presented the Marketscan study.
The findings demonstrated clear cost reductions. “At the beginning of our period, infliximab originator Remicade was a little over $1,200 per vial, and at the end of our period of 2021, we expected it to be around $1,800 per vial. However, with the introduction of multiple infliximab biosimilars, we did see it come down to $800 per vial, which generated a savings of $1,000, which is pretty significant. We’re hoping that by generating overall cost savings to insurance companies, this will allow for greater access, because we know infliximab is such a good medication for pediatric IBD and for many other autoimmune conditions,” said Dr. Paglinco, a pediatric GI fellow at Nationwide Children’s Hospital in Columbus, Ohio.
The decrease in the cost of medication per vial in both the originator and biosimilar is an important finding in light of cost containment strategies, although Dr. Horst pointed out that the study didn’t determine if there were any savings to patients. “That is what clinicians and patients care about the most. I worry that while these savings were seen at insurance and health care organizational levels, patients may not have experienced any cost savings, and this is something we need to consider in the future,” she said.
The FDA created the biosimilar regulatory pathway in 2010 in an effort to reduce costs. “And they have. The cost savings however, has not directly translated to the individual patient in their copays or necessarily in changes to access when prescribing. Nor has it allowed dose escalation more easily, which is frequently needed,” said David T. Rubin, MD, AGAF, who was asked for comment.
Although the changes in prescribing patterns may reflect cost savings to patients, there are some limits to this interpretation, according to Dr. Rubin, of the University of Chicago. Payers are often the ones who determine a switch to biosimilar. The study also does not account for disease activity or phenotype, which can be an important confounder. Newer biologics that have come on to the market in recent years may also have affected the prescribing patterns for infliximab.
Nevertheless, an association between introduction of biosimilars and declining cost in the originator product (Remicade) is welcome, according to Dr. Rubin. “This is nice to see and we have seen it in our practices,” he said.
Unfortunately, “it has also not been seen to translate into reduced costs for patients nor has it been associated with increased access or changes in payer policies to allow these drugs to be used, or dose escalations to occur,” Dr. Rubin added.
Dr. Paglinco and Mr. Goldman have no relevant financial disclosures. Dr. Horst has consulted for Janssen, Takeda, Bristol-Myers Squibb, and Abbvie. Dr. Rubin has received grant support from Takeda and has consulted for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
LAS VEGAS — , according to two new retrospective analyses that separately looked at private insurance and Medicare patients.
“It is interesting to see that biosimilar use is increasing in patients with Medicare insurance in the U.S. since introduction into the market. This shows that clinicians are getting more comfortable with their use in clinical practice,” said Sara Horst, MD, associate professor of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center in Nashville, Tennessee. That “is a continued important question in use, especially in the older population,” Dr. Horst said at the poster session where the two studies were presented, at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
One study examined Medicare part D participants. The researchers included infliximab prescriptions between 2013 and 2021, with a break in 2017 when biosimilars were first introduced. Between 2013 and 2017, there was a 93.7% annual increase in infliximab cost, and a 29.0% increase in annual claims. Between 2017 and 2021, those numbers declined to 23.9% and 12.1%, respectively. The researchers also examined trends by individual states and found a wide range of results. “Nationwide, it seems to be doing exactly what you’d expect, and I’d say it’s encouraging. This is exactly what you want with the introduction of biosimilars, and this is what you want for the patient population. But there are some states that have not really kept up with the rest of the country, and may need to look further into what types of trends are there,” said Modan Goldman, who presented the study. He is a medical student at Carle Illinois College of Medicine in Urbana, Illinois.
The other study looked at use of infliximab versus a biosimilar between 2015 and 2021 in 42,009 patients 64 or younger, drawing data from Merative Marketscan Commercial Claims and Encounters Database. They excluded government-funded insurance. Between January 2015 and December 2017, the cost of a vial of infliximab increased by $6.31 per month, reaching a maxim cost of $1,491 per vial. In January 2018, the cost decreased by $62 per vial, with a downward trend after that of $12.93 per vial per month.
“[Getting] access to these drugs, especially for pediatric IBD, has been an uphill battle with insurance companies and getting the medication and the doses we want,” said Samantha Paglinco, DO, who presented the Marketscan study.
The findings demonstrated clear cost reductions. “At the beginning of our period, infliximab originator Remicade was a little over $1,200 per vial, and at the end of our period of 2021, we expected it to be around $1,800 per vial. However, with the introduction of multiple infliximab biosimilars, we did see it come down to $800 per vial, which generated a savings of $1,000, which is pretty significant. We’re hoping that by generating overall cost savings to insurance companies, this will allow for greater access, because we know infliximab is such a good medication for pediatric IBD and for many other autoimmune conditions,” said Dr. Paglinco, a pediatric GI fellow at Nationwide Children’s Hospital in Columbus, Ohio.
The decrease in the cost of medication per vial in both the originator and biosimilar is an important finding in light of cost containment strategies, although Dr. Horst pointed out that the study didn’t determine if there were any savings to patients. “That is what clinicians and patients care about the most. I worry that while these savings were seen at insurance and health care organizational levels, patients may not have experienced any cost savings, and this is something we need to consider in the future,” she said.
The FDA created the biosimilar regulatory pathway in 2010 in an effort to reduce costs. “And they have. The cost savings however, has not directly translated to the individual patient in their copays or necessarily in changes to access when prescribing. Nor has it allowed dose escalation more easily, which is frequently needed,” said David T. Rubin, MD, AGAF, who was asked for comment.
Although the changes in prescribing patterns may reflect cost savings to patients, there are some limits to this interpretation, according to Dr. Rubin, of the University of Chicago. Payers are often the ones who determine a switch to biosimilar. The study also does not account for disease activity or phenotype, which can be an important confounder. Newer biologics that have come on to the market in recent years may also have affected the prescribing patterns for infliximab.
Nevertheless, an association between introduction of biosimilars and declining cost in the originator product (Remicade) is welcome, according to Dr. Rubin. “This is nice to see and we have seen it in our practices,” he said.
Unfortunately, “it has also not been seen to translate into reduced costs for patients nor has it been associated with increased access or changes in payer policies to allow these drugs to be used, or dose escalations to occur,” Dr. Rubin added.
Dr. Paglinco and Mr. Goldman have no relevant financial disclosures. Dr. Horst has consulted for Janssen, Takeda, Bristol-Myers Squibb, and Abbvie. Dr. Rubin has received grant support from Takeda and has consulted for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
LAS VEGAS — , according to two new retrospective analyses that separately looked at private insurance and Medicare patients.
“It is interesting to see that biosimilar use is increasing in patients with Medicare insurance in the U.S. since introduction into the market. This shows that clinicians are getting more comfortable with their use in clinical practice,” said Sara Horst, MD, associate professor of gastroenterology, hepatology, and nutrition at Vanderbilt University Medical Center in Nashville, Tennessee. That “is a continued important question in use, especially in the older population,” Dr. Horst said at the poster session where the two studies were presented, at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
One study examined Medicare part D participants. The researchers included infliximab prescriptions between 2013 and 2021, with a break in 2017 when biosimilars were first introduced. Between 2013 and 2017, there was a 93.7% annual increase in infliximab cost, and a 29.0% increase in annual claims. Between 2017 and 2021, those numbers declined to 23.9% and 12.1%, respectively. The researchers also examined trends by individual states and found a wide range of results. “Nationwide, it seems to be doing exactly what you’d expect, and I’d say it’s encouraging. This is exactly what you want with the introduction of biosimilars, and this is what you want for the patient population. But there are some states that have not really kept up with the rest of the country, and may need to look further into what types of trends are there,” said Modan Goldman, who presented the study. He is a medical student at Carle Illinois College of Medicine in Urbana, Illinois.
The other study looked at use of infliximab versus a biosimilar between 2015 and 2021 in 42,009 patients 64 or younger, drawing data from Merative Marketscan Commercial Claims and Encounters Database. They excluded government-funded insurance. Between January 2015 and December 2017, the cost of a vial of infliximab increased by $6.31 per month, reaching a maxim cost of $1,491 per vial. In January 2018, the cost decreased by $62 per vial, with a downward trend after that of $12.93 per vial per month.
“[Getting] access to these drugs, especially for pediatric IBD, has been an uphill battle with insurance companies and getting the medication and the doses we want,” said Samantha Paglinco, DO, who presented the Marketscan study.
The findings demonstrated clear cost reductions. “At the beginning of our period, infliximab originator Remicade was a little over $1,200 per vial, and at the end of our period of 2021, we expected it to be around $1,800 per vial. However, with the introduction of multiple infliximab biosimilars, we did see it come down to $800 per vial, which generated a savings of $1,000, which is pretty significant. We’re hoping that by generating overall cost savings to insurance companies, this will allow for greater access, because we know infliximab is such a good medication for pediatric IBD and for many other autoimmune conditions,” said Dr. Paglinco, a pediatric GI fellow at Nationwide Children’s Hospital in Columbus, Ohio.
The decrease in the cost of medication per vial in both the originator and biosimilar is an important finding in light of cost containment strategies, although Dr. Horst pointed out that the study didn’t determine if there were any savings to patients. “That is what clinicians and patients care about the most. I worry that while these savings were seen at insurance and health care organizational levels, patients may not have experienced any cost savings, and this is something we need to consider in the future,” she said.
The FDA created the biosimilar regulatory pathway in 2010 in an effort to reduce costs. “And they have. The cost savings however, has not directly translated to the individual patient in their copays or necessarily in changes to access when prescribing. Nor has it allowed dose escalation more easily, which is frequently needed,” said David T. Rubin, MD, AGAF, who was asked for comment.
Although the changes in prescribing patterns may reflect cost savings to patients, there are some limits to this interpretation, according to Dr. Rubin, of the University of Chicago. Payers are often the ones who determine a switch to biosimilar. The study also does not account for disease activity or phenotype, which can be an important confounder. Newer biologics that have come on to the market in recent years may also have affected the prescribing patterns for infliximab.
Nevertheless, an association between introduction of biosimilars and declining cost in the originator product (Remicade) is welcome, according to Dr. Rubin. “This is nice to see and we have seen it in our practices,” he said.
Unfortunately, “it has also not been seen to translate into reduced costs for patients nor has it been associated with increased access or changes in payer policies to allow these drugs to be used, or dose escalations to occur,” Dr. Rubin added.
Dr. Paglinco and Mr. Goldman have no relevant financial disclosures. Dr. Horst has consulted for Janssen, Takeda, Bristol-Myers Squibb, and Abbvie. Dr. Rubin has received grant support from Takeda and has consulted for Abbvie, Bristol-Myers Squibb, Janssen Pharmaceuticals, Lilly, Pfizer, and Takeda.
FROM CROHN’S AND COLITIS CONGRESS
Oral IL-23 Inhibitor Calms Moderate to Severe Psoriasis
A novel oral drug for plaque psoriasis that targets the same inflammatory pathway as currently available parenteral therapies showed promise for treating moderate to severe disease in a phase 2 dose-finding trial.
Among 255 at week 16 of at least 75% (PASI 75) compared with 9% of patients assigned to placebo, reported Robert Bissonnette, MD, from Innovaderm Research in Montreal, Quebec, Canada, and colleagues.
“The level of reduction of psoriasis that was observed with higher doses of JNJ-77242113 at week 16 was similar in magnitude to the responses seen with several of the injectable biologics that are currently approved for psoriasis,” investigators in the FRONTIER 1 trial wrote in The New England Journal of Medicine.
The investigators noted that among patients assigned to the 100-mg dose of the active drug, 60% had a PASI 90 response, which compares favorably with that seen in phase 3 trials of two other orally available therapies for psoriasis, deucravacitinib (Sotyktu) and apremilast (Otezla). They cautioned, however, against drawing any further inferences from these data, because these agents have not been tested head-to-head against JNJ-77242113 in comparison trials.
Targets IL-23 and IL-17
The investigational agent is an oral IL-23 receptor antagonist peptide that selectively blocks IL-23 proximal signaling as well as the production of downstream inflammatory cytokines such as IL-17, according to the authors.
“Modulation of the interleukin-23 pathway with the use of monoclonal antibodies has shown efficacy in the treatment of psoriasis and is considered to be associated with a more favorable safety profile than older oral therapies (eg, cyclosporine, acitretin, methotrexate, and dimethyl fumarate),” the investigators wrote.
Currently available biologic agents targeting IL-23 include guselkumab (Tremfya), risankizumab (Skyrizi) and tildrakizumab (Ilumya). These agents require intravenous or subcutaneous administration, whereas JNJ-77242113 is taken orally, giving it a theoretical advantage in terms of patient preference.
The novel drug must be taken twice daily on an empty stomach at least 2 hours before food or drink, and those who take it must wait an additional 30 minutes to eat or drink after taking the drug. (This news organization has learned that in planned phase 3 studies, patients will be instructed to take a double daily dose on awakening and then wait 30 minutes for eating or drinking.)
‘Profoundly Effective’
The results of this study have convinced at least one former skeptic of the efficacy of the novel agent.
“They asked me to do the trial, and I turned it down, because I didn’t believe it would work,” said Mark G. Lebwohl, MD, dean for Clinical Therapeutics at the Icahn School of Medicine at Mount Sinai and professor and chairman emeritus of the Department of Dermatology at Mount Sinai Medicine in New York, NY.
In an interview with this news organization, Dr. Lebwohl said that he was initially dubious that a peptide, a short chain of amino acids directed against a receptor, could be effective because it would likely be digested in the intestinal tract.
“Indeed, more than 99% of it is digested, but the data show that the tiny amount that gets through is profoundly effective,” he said.
“I would never have believed that this was going to work – and it did,” Dr. Lebwohl added.
He has signed on as an investigator in the currently recruiting phase 3 ICONIC-LEAD trial, in which JNJ-77242113 will be tested against placebo in adolescents and adults with moderate to severe plaque psoriasis.
In an editorial accompanying the study in the NEJM, Joel M. Gelfand, MD, MSCE, vice chair of clinical research and medical director of the Dermatology Clinical Studies Unit at the University of Pennsylvania in Philadelphia, noted that if confirmed in larger studies, the PASI 90 rate at the highest dose “would be similar to the most effective injectable biologics,” with no evidence of increased adverse events at higher doses.
“However, two occurrences of infection (COVID-19 and an infected cyst) and a suicide attempt were reported as serious adverse events; larger trials will be needed to determine whether such events are attributable to chance, psoriasis itself, or inhibition of interleukin-23 signaling,” cautioned Dr. Gelfand, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania.
In an interview, Dr. Lebwohl said that currently available IL-23 signaling inhibitors have an excellent safety profile and that the investigational oral agent also appears to be very safe. “It’s seeing a target whose effects are known, and the effects are all good and not bad,” he said.
FRONTIER-1 Details
The investigators enrolled eligible adults aged 18 years or older who had moderate to severe plaque psoriasis as defined by an Investigator’s Global Assessment score ≥ 3, a total body-surface area of psoriasis involvement of at least 10%, and a PASI score ≥ 12 who had received their diagnosis of plaque psoriasis at least 6 months before starting the trial. The participants had to be candidates for phototherapy or systemic psoriasis therapy.
Patients were randomly assigned to the active agent at doses of 25 mg once or twice daily, 50 mg once daily, or 100 mg once or twice daily for 16 weeks.
There was a clear dose response, with 37% of patients assigned to 25-mg once-daily dose meeting the primary endpoint of a PASI 75 response at week 16 compared with 51% of those assigned to the 25-mg twice-daily dose, 58% assigned to 50-mg once-daily dose, 65% assigned to 100-mg once-daily dose, and 79% assigned to 100-mg twice-daily dose (P for dose response < .001).
As noted previously, 9% of patients in the placebo group had a PASI 75 response at week 16.
After a mean duration of 15.9 weeks, adverse events after the first dose of JNJ-77242113 (all dose groups were pooled for the safety analysis) were reported in 47% of patients on the 25-mg once-daily dose, 49% on 25-mg twice-daily dose, 60% on 50-mg once-daily dose, 44% on 100-mg once-daily dose, and 62% on 100-mg twice-daily dose. Adverse events after the first dose occurred in 51% of patients assigned to placebo.
The incidence of adverse events did not increase significantly with successively higher dose levels.
As noted by Dr. Gelfand in his editorial, there were three serious adverse events, all occurring in patients on the active drug: a case of COVID-19 in one patient and a suicide attempt in one patient, both in the 100-mg once-daily dose group, and an infected cyst in the 50-mg once-daily group. All three events were determined by the principal investigator and the sponsor to be unrelated to JNJ-77242113.
There were no reports of deaths, major adverse cardiovascular events, or incident cancers during the trial.
The study was supported by Janssen Research and Development. Dr. Bissonnette disclosed institutional research funding and advisory board participation and honoraria with Janssen. Dr. Gelfand disclosed consulting for Janssen Biotech. Dr. Lebwohl disclosed institutional research funding from Janssen but no personal fees.
A version of this article first appeared on Medscape.com.
A novel oral drug for plaque psoriasis that targets the same inflammatory pathway as currently available parenteral therapies showed promise for treating moderate to severe disease in a phase 2 dose-finding trial.
Among 255 at week 16 of at least 75% (PASI 75) compared with 9% of patients assigned to placebo, reported Robert Bissonnette, MD, from Innovaderm Research in Montreal, Quebec, Canada, and colleagues.
“The level of reduction of psoriasis that was observed with higher doses of JNJ-77242113 at week 16 was similar in magnitude to the responses seen with several of the injectable biologics that are currently approved for psoriasis,” investigators in the FRONTIER 1 trial wrote in The New England Journal of Medicine.
The investigators noted that among patients assigned to the 100-mg dose of the active drug, 60% had a PASI 90 response, which compares favorably with that seen in phase 3 trials of two other orally available therapies for psoriasis, deucravacitinib (Sotyktu) and apremilast (Otezla). They cautioned, however, against drawing any further inferences from these data, because these agents have not been tested head-to-head against JNJ-77242113 in comparison trials.
Targets IL-23 and IL-17
The investigational agent is an oral IL-23 receptor antagonist peptide that selectively blocks IL-23 proximal signaling as well as the production of downstream inflammatory cytokines such as IL-17, according to the authors.
“Modulation of the interleukin-23 pathway with the use of monoclonal antibodies has shown efficacy in the treatment of psoriasis and is considered to be associated with a more favorable safety profile than older oral therapies (eg, cyclosporine, acitretin, methotrexate, and dimethyl fumarate),” the investigators wrote.
Currently available biologic agents targeting IL-23 include guselkumab (Tremfya), risankizumab (Skyrizi) and tildrakizumab (Ilumya). These agents require intravenous or subcutaneous administration, whereas JNJ-77242113 is taken orally, giving it a theoretical advantage in terms of patient preference.
The novel drug must be taken twice daily on an empty stomach at least 2 hours before food or drink, and those who take it must wait an additional 30 minutes to eat or drink after taking the drug. (This news organization has learned that in planned phase 3 studies, patients will be instructed to take a double daily dose on awakening and then wait 30 minutes for eating or drinking.)
‘Profoundly Effective’
The results of this study have convinced at least one former skeptic of the efficacy of the novel agent.
“They asked me to do the trial, and I turned it down, because I didn’t believe it would work,” said Mark G. Lebwohl, MD, dean for Clinical Therapeutics at the Icahn School of Medicine at Mount Sinai and professor and chairman emeritus of the Department of Dermatology at Mount Sinai Medicine in New York, NY.
In an interview with this news organization, Dr. Lebwohl said that he was initially dubious that a peptide, a short chain of amino acids directed against a receptor, could be effective because it would likely be digested in the intestinal tract.
“Indeed, more than 99% of it is digested, but the data show that the tiny amount that gets through is profoundly effective,” he said.
“I would never have believed that this was going to work – and it did,” Dr. Lebwohl added.
He has signed on as an investigator in the currently recruiting phase 3 ICONIC-LEAD trial, in which JNJ-77242113 will be tested against placebo in adolescents and adults with moderate to severe plaque psoriasis.
In an editorial accompanying the study in the NEJM, Joel M. Gelfand, MD, MSCE, vice chair of clinical research and medical director of the Dermatology Clinical Studies Unit at the University of Pennsylvania in Philadelphia, noted that if confirmed in larger studies, the PASI 90 rate at the highest dose “would be similar to the most effective injectable biologics,” with no evidence of increased adverse events at higher doses.
“However, two occurrences of infection (COVID-19 and an infected cyst) and a suicide attempt were reported as serious adverse events; larger trials will be needed to determine whether such events are attributable to chance, psoriasis itself, or inhibition of interleukin-23 signaling,” cautioned Dr. Gelfand, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania.
In an interview, Dr. Lebwohl said that currently available IL-23 signaling inhibitors have an excellent safety profile and that the investigational oral agent also appears to be very safe. “It’s seeing a target whose effects are known, and the effects are all good and not bad,” he said.
FRONTIER-1 Details
The investigators enrolled eligible adults aged 18 years or older who had moderate to severe plaque psoriasis as defined by an Investigator’s Global Assessment score ≥ 3, a total body-surface area of psoriasis involvement of at least 10%, and a PASI score ≥ 12 who had received their diagnosis of plaque psoriasis at least 6 months before starting the trial. The participants had to be candidates for phototherapy or systemic psoriasis therapy.
Patients were randomly assigned to the active agent at doses of 25 mg once or twice daily, 50 mg once daily, or 100 mg once or twice daily for 16 weeks.
There was a clear dose response, with 37% of patients assigned to 25-mg once-daily dose meeting the primary endpoint of a PASI 75 response at week 16 compared with 51% of those assigned to the 25-mg twice-daily dose, 58% assigned to 50-mg once-daily dose, 65% assigned to 100-mg once-daily dose, and 79% assigned to 100-mg twice-daily dose (P for dose response < .001).
As noted previously, 9% of patients in the placebo group had a PASI 75 response at week 16.
After a mean duration of 15.9 weeks, adverse events after the first dose of JNJ-77242113 (all dose groups were pooled for the safety analysis) were reported in 47% of patients on the 25-mg once-daily dose, 49% on 25-mg twice-daily dose, 60% on 50-mg once-daily dose, 44% on 100-mg once-daily dose, and 62% on 100-mg twice-daily dose. Adverse events after the first dose occurred in 51% of patients assigned to placebo.
The incidence of adverse events did not increase significantly with successively higher dose levels.
As noted by Dr. Gelfand in his editorial, there were three serious adverse events, all occurring in patients on the active drug: a case of COVID-19 in one patient and a suicide attempt in one patient, both in the 100-mg once-daily dose group, and an infected cyst in the 50-mg once-daily group. All three events were determined by the principal investigator and the sponsor to be unrelated to JNJ-77242113.
There were no reports of deaths, major adverse cardiovascular events, or incident cancers during the trial.
The study was supported by Janssen Research and Development. Dr. Bissonnette disclosed institutional research funding and advisory board participation and honoraria with Janssen. Dr. Gelfand disclosed consulting for Janssen Biotech. Dr. Lebwohl disclosed institutional research funding from Janssen but no personal fees.
A version of this article first appeared on Medscape.com.
A novel oral drug for plaque psoriasis that targets the same inflammatory pathway as currently available parenteral therapies showed promise for treating moderate to severe disease in a phase 2 dose-finding trial.
Among 255 at week 16 of at least 75% (PASI 75) compared with 9% of patients assigned to placebo, reported Robert Bissonnette, MD, from Innovaderm Research in Montreal, Quebec, Canada, and colleagues.
“The level of reduction of psoriasis that was observed with higher doses of JNJ-77242113 at week 16 was similar in magnitude to the responses seen with several of the injectable biologics that are currently approved for psoriasis,” investigators in the FRONTIER 1 trial wrote in The New England Journal of Medicine.
The investigators noted that among patients assigned to the 100-mg dose of the active drug, 60% had a PASI 90 response, which compares favorably with that seen in phase 3 trials of two other orally available therapies for psoriasis, deucravacitinib (Sotyktu) and apremilast (Otezla). They cautioned, however, against drawing any further inferences from these data, because these agents have not been tested head-to-head against JNJ-77242113 in comparison trials.
Targets IL-23 and IL-17
The investigational agent is an oral IL-23 receptor antagonist peptide that selectively blocks IL-23 proximal signaling as well as the production of downstream inflammatory cytokines such as IL-17, according to the authors.
“Modulation of the interleukin-23 pathway with the use of monoclonal antibodies has shown efficacy in the treatment of psoriasis and is considered to be associated with a more favorable safety profile than older oral therapies (eg, cyclosporine, acitretin, methotrexate, and dimethyl fumarate),” the investigators wrote.
Currently available biologic agents targeting IL-23 include guselkumab (Tremfya), risankizumab (Skyrizi) and tildrakizumab (Ilumya). These agents require intravenous or subcutaneous administration, whereas JNJ-77242113 is taken orally, giving it a theoretical advantage in terms of patient preference.
The novel drug must be taken twice daily on an empty stomach at least 2 hours before food or drink, and those who take it must wait an additional 30 minutes to eat or drink after taking the drug. (This news organization has learned that in planned phase 3 studies, patients will be instructed to take a double daily dose on awakening and then wait 30 minutes for eating or drinking.)
‘Profoundly Effective’
The results of this study have convinced at least one former skeptic of the efficacy of the novel agent.
“They asked me to do the trial, and I turned it down, because I didn’t believe it would work,” said Mark G. Lebwohl, MD, dean for Clinical Therapeutics at the Icahn School of Medicine at Mount Sinai and professor and chairman emeritus of the Department of Dermatology at Mount Sinai Medicine in New York, NY.
In an interview with this news organization, Dr. Lebwohl said that he was initially dubious that a peptide, a short chain of amino acids directed against a receptor, could be effective because it would likely be digested in the intestinal tract.
“Indeed, more than 99% of it is digested, but the data show that the tiny amount that gets through is profoundly effective,” he said.
“I would never have believed that this was going to work – and it did,” Dr. Lebwohl added.
He has signed on as an investigator in the currently recruiting phase 3 ICONIC-LEAD trial, in which JNJ-77242113 will be tested against placebo in adolescents and adults with moderate to severe plaque psoriasis.
In an editorial accompanying the study in the NEJM, Joel M. Gelfand, MD, MSCE, vice chair of clinical research and medical director of the Dermatology Clinical Studies Unit at the University of Pennsylvania in Philadelphia, noted that if confirmed in larger studies, the PASI 90 rate at the highest dose “would be similar to the most effective injectable biologics,” with no evidence of increased adverse events at higher doses.
“However, two occurrences of infection (COVID-19 and an infected cyst) and a suicide attempt were reported as serious adverse events; larger trials will be needed to determine whether such events are attributable to chance, psoriasis itself, or inhibition of interleukin-23 signaling,” cautioned Dr. Gelfand, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania.
In an interview, Dr. Lebwohl said that currently available IL-23 signaling inhibitors have an excellent safety profile and that the investigational oral agent also appears to be very safe. “It’s seeing a target whose effects are known, and the effects are all good and not bad,” he said.
FRONTIER-1 Details
The investigators enrolled eligible adults aged 18 years or older who had moderate to severe plaque psoriasis as defined by an Investigator’s Global Assessment score ≥ 3, a total body-surface area of psoriasis involvement of at least 10%, and a PASI score ≥ 12 who had received their diagnosis of plaque psoriasis at least 6 months before starting the trial. The participants had to be candidates for phototherapy or systemic psoriasis therapy.
Patients were randomly assigned to the active agent at doses of 25 mg once or twice daily, 50 mg once daily, or 100 mg once or twice daily for 16 weeks.
There was a clear dose response, with 37% of patients assigned to 25-mg once-daily dose meeting the primary endpoint of a PASI 75 response at week 16 compared with 51% of those assigned to the 25-mg twice-daily dose, 58% assigned to 50-mg once-daily dose, 65% assigned to 100-mg once-daily dose, and 79% assigned to 100-mg twice-daily dose (P for dose response < .001).
As noted previously, 9% of patients in the placebo group had a PASI 75 response at week 16.
After a mean duration of 15.9 weeks, adverse events after the first dose of JNJ-77242113 (all dose groups were pooled for the safety analysis) were reported in 47% of patients on the 25-mg once-daily dose, 49% on 25-mg twice-daily dose, 60% on 50-mg once-daily dose, 44% on 100-mg once-daily dose, and 62% on 100-mg twice-daily dose. Adverse events after the first dose occurred in 51% of patients assigned to placebo.
The incidence of adverse events did not increase significantly with successively higher dose levels.
As noted by Dr. Gelfand in his editorial, there were three serious adverse events, all occurring in patients on the active drug: a case of COVID-19 in one patient and a suicide attempt in one patient, both in the 100-mg once-daily dose group, and an infected cyst in the 50-mg once-daily group. All three events were determined by the principal investigator and the sponsor to be unrelated to JNJ-77242113.
There were no reports of deaths, major adverse cardiovascular events, or incident cancers during the trial.
The study was supported by Janssen Research and Development. Dr. Bissonnette disclosed institutional research funding and advisory board participation and honoraria with Janssen. Dr. Gelfand disclosed consulting for Janssen Biotech. Dr. Lebwohl disclosed institutional research funding from Janssen but no personal fees.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE