User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
WHO tracking new Omicron subvariant in India
The subvariant, a sublineage of BA.2 being called BA.2.75, has been reported in eight countries and hasn’t yet been declared a variant of concern.
“There’s been an emergence of a ‘could be’ subvariant. It’s been not yet officially called, but some people are referring to it as BA.2.75,” Soumya Swaminathan, MD, the WHO’s chief scientist, said in a video posted on Twitter.
The subvariant appears to have mutations similar to other contagious strains, she said, though there are a limited number of sequences available to analyze. How transmissible and severe it is, and how well it can evade our immunity, aren’t yet known.
“We have to wait and see, and of course, we are tracking it,” Dr. Swaminathan said.
The WHO committee responsible for analyzing global coronavirus data will label the subvariant officially and release more information as the situation warrants it, she said.
Public health experts around the world are also talking about the subvariant, which has been nicknamed Centaurus. BA.2.75 was first found in India in May and is now competing with BA.5, which has become dominant in the United States.
BA.2.75 has eight mutations beyond those seen in BA.5, which “could make immune escape worse than what we’re seeing now,” Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief at Medscape, wrote in a Twitter post.
Individually, the extra mutations aren’t too concerning, “but all appearing together at once is another matter,” Tom Peacock, PhD, a virologist at Imperial College London, wrote in a Twitter post.
The “apparent rapid growth and wide geographical spread” are “worth keeping a close eye on,” he said.
BA.2.75 has been found in a handful of cases in the United States, Australia, Canada, Germany, Japan, New Zealand, and the United Kingdom. In India, the sequence accounts for about 23% of recent samples.
“It is really too early to know if BA.2.75 will take over relative to BA.2 or even relative to BA.5,” Ulrich Elling, PhD, a researcher at Australia’s Institute of Molecular Biotechnology, wrote in a Twitter post.
“Just to emphasize it again: While the distribution across Indian regions as well as internationally and the very rapid appearance makes it likely we are dealing with a variant spreading fast and spread widely already, the absolute data points are few,” he said.
Globally, coronavirus cases have increased nearly 30% during the past 2 weeks, the WHO said July 6. Four out of six of the WHO subregions reported an increase in the last week, with BA.4 and BA.5 driving waves in the United States and Europe.
A version of this article first appeared on WebMD.com.
The subvariant, a sublineage of BA.2 being called BA.2.75, has been reported in eight countries and hasn’t yet been declared a variant of concern.
“There’s been an emergence of a ‘could be’ subvariant. It’s been not yet officially called, but some people are referring to it as BA.2.75,” Soumya Swaminathan, MD, the WHO’s chief scientist, said in a video posted on Twitter.
The subvariant appears to have mutations similar to other contagious strains, she said, though there are a limited number of sequences available to analyze. How transmissible and severe it is, and how well it can evade our immunity, aren’t yet known.
“We have to wait and see, and of course, we are tracking it,” Dr. Swaminathan said.
The WHO committee responsible for analyzing global coronavirus data will label the subvariant officially and release more information as the situation warrants it, she said.
Public health experts around the world are also talking about the subvariant, which has been nicknamed Centaurus. BA.2.75 was first found in India in May and is now competing with BA.5, which has become dominant in the United States.
BA.2.75 has eight mutations beyond those seen in BA.5, which “could make immune escape worse than what we’re seeing now,” Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief at Medscape, wrote in a Twitter post.
Individually, the extra mutations aren’t too concerning, “but all appearing together at once is another matter,” Tom Peacock, PhD, a virologist at Imperial College London, wrote in a Twitter post.
The “apparent rapid growth and wide geographical spread” are “worth keeping a close eye on,” he said.
BA.2.75 has been found in a handful of cases in the United States, Australia, Canada, Germany, Japan, New Zealand, and the United Kingdom. In India, the sequence accounts for about 23% of recent samples.
“It is really too early to know if BA.2.75 will take over relative to BA.2 or even relative to BA.5,” Ulrich Elling, PhD, a researcher at Australia’s Institute of Molecular Biotechnology, wrote in a Twitter post.
“Just to emphasize it again: While the distribution across Indian regions as well as internationally and the very rapid appearance makes it likely we are dealing with a variant spreading fast and spread widely already, the absolute data points are few,” he said.
Globally, coronavirus cases have increased nearly 30% during the past 2 weeks, the WHO said July 6. Four out of six of the WHO subregions reported an increase in the last week, with BA.4 and BA.5 driving waves in the United States and Europe.
A version of this article first appeared on WebMD.com.
The subvariant, a sublineage of BA.2 being called BA.2.75, has been reported in eight countries and hasn’t yet been declared a variant of concern.
“There’s been an emergence of a ‘could be’ subvariant. It’s been not yet officially called, but some people are referring to it as BA.2.75,” Soumya Swaminathan, MD, the WHO’s chief scientist, said in a video posted on Twitter.
The subvariant appears to have mutations similar to other contagious strains, she said, though there are a limited number of sequences available to analyze. How transmissible and severe it is, and how well it can evade our immunity, aren’t yet known.
“We have to wait and see, and of course, we are tracking it,” Dr. Swaminathan said.
The WHO committee responsible for analyzing global coronavirus data will label the subvariant officially and release more information as the situation warrants it, she said.
Public health experts around the world are also talking about the subvariant, which has been nicknamed Centaurus. BA.2.75 was first found in India in May and is now competing with BA.5, which has become dominant in the United States.
BA.2.75 has eight mutations beyond those seen in BA.5, which “could make immune escape worse than what we’re seeing now,” Eric Topol, MD, founder and director of the Scripps Research Translational Institute and editor-in-chief at Medscape, wrote in a Twitter post.
Individually, the extra mutations aren’t too concerning, “but all appearing together at once is another matter,” Tom Peacock, PhD, a virologist at Imperial College London, wrote in a Twitter post.
The “apparent rapid growth and wide geographical spread” are “worth keeping a close eye on,” he said.
BA.2.75 has been found in a handful of cases in the United States, Australia, Canada, Germany, Japan, New Zealand, and the United Kingdom. In India, the sequence accounts for about 23% of recent samples.
“It is really too early to know if BA.2.75 will take over relative to BA.2 or even relative to BA.5,” Ulrich Elling, PhD, a researcher at Australia’s Institute of Molecular Biotechnology, wrote in a Twitter post.
“Just to emphasize it again: While the distribution across Indian regions as well as internationally and the very rapid appearance makes it likely we are dealing with a variant spreading fast and spread widely already, the absolute data points are few,” he said.
Globally, coronavirus cases have increased nearly 30% during the past 2 weeks, the WHO said July 6. Four out of six of the WHO subregions reported an increase in the last week, with BA.4 and BA.5 driving waves in the United States and Europe.
A version of this article first appeared on WebMD.com.
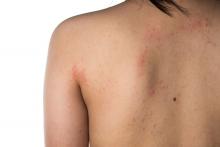
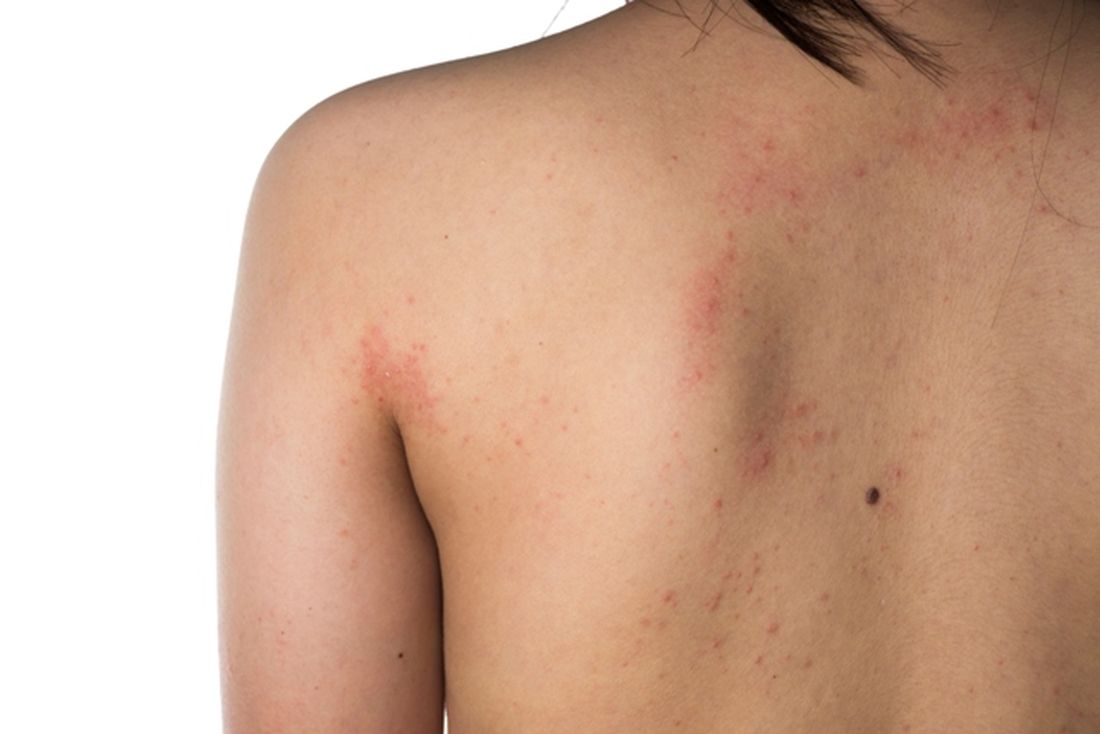
Eczema severity, time spent on management strongly associated with overall disease burden
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
. However, AD severity and spending 11 hours or more per week managing the condition did correlate with higher overall disease burden.
“Research has documented the disease burden of AD, including its visible nature and the effect on itch and sleep, but knowledge gaps remain,” Aaron M. Drucker, MD, of the division of dermatology at the University of Toronto, and colleagues wrote in the study published online in JAMA Dermatology. “Gaps include a poor understanding of symptoms other than itch, patients’ treatment experience, and how different elements of burden of disease interact.”
Dr. Drucker and colleagues collected data from an externally led patient-focused drug development survey on AD, a 32-item questionnaire that was administered electronically between Aug. 1, 2019, and Oct. 11, 2019. Respondents were asked to rate the overall impact of their AD in the past months and the specific elements of disease burden on a 1-5 scale, with 1 meaning no impact, and 5 meaning a significant impact. They were also asked to rate current mood changes and mood changes at the worst point of AD on a 4-point scale that ranged from “not present” to “severe.” The researchers used multivariable ordinal regression to examine associations between demographic and clinical variables and patient-reported overall AD impact scores.
Survey results
Of the 1,065 respondents, 33% were aged 18-34 years, 50% were aged 35-50 years, 17% were aged 65 years or older, and 83% were female. Nearly half (45%) reported having moderate AD, while 28% had severe AD. When asked about the overall disease burden of AD symptoms in the past month, 30% reported a significant impact on life, 28% reported a moderate impact score, 21% reported a high impact score, 18% reported a low impact score, and 3% of respondents reported no impact.
In the multivariable proportional odds analysis, moderate AD (odds ratio [OR], 4.13) and severe AD (OR, 13.63) were both associated with greater disease burden compared with mild AD. Also, spending 11 or more hours per week managing AD symptoms was associated with greater disease burden compared with 0 to 4 hours (an OR of 2.67 for 11-20 hours per week spent managing AD and OR of 5.34 for 21 or more hours per week spent managing AD).
Correlations between specific impact domains such as sleep, cognitive thinking, and physical activity and overall AD impact scores ranged from weak to moderate, and no individual aspect of disease burden correlated strongly with overall impact scores. The researchers observed similar results after they stratified the analysis by age, current severity, and time spent managing AD.
In other findings, 40% of study participants reported mild changes in mood related to their AD, 30% reported moderate changes, 9% reported severe changes, while the remainder reported no changes in mood. The variable most strongly associated with current mood changes was having severe AD at the time of the survey (OR 5.29).
Understanding of disease burden ‘limited’
“Atopic dermatitis is associated with an immense clinical burden,” said Raj Chovatiya, MD, PhD, assistant professor in the department of dermatology at Northwestern University, Chicago, who was asked to comment on the study. “However, our understanding of disease burden from the patient perspective is limited,” he added.
“Interestingly, no single specific element of disease burden was strongly correlated with overall burden, further supporting the complex, multidimensional nature” of the impact of AD, he said, noting that the study “highlights the need for clinicians to look beyond the skin when it comes to AD and underscores the need for additional research to better understand the patient and caregiver perspective.”
Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was also asked to comment on the study, noted that aside from the well discussed impact and burden of itch and its impact on sleep loss, much remains to be learned about the full impact of AD, particularly among adults.
“For example, it is commonly accepted and expected that patients with more severe AD likely experience higher disease burden, but are there other factors that can influence this risk?” she asked. “Can we explain the high impact of AD disease aside from the level of disease severity, particularly among adults with AD?”
The study, she added, “is important because it provides additional insights into those possible factors, including ‘time spent managing their disease’ and ‘associated depression.’ In particular, understanding the association between ‘time spent managing their disease’ and higher disease burden is critical because, in my opinion, it emphasizes the need to develop better strategies for improving the care of patients with AD including the development of more efficacious and safer treatment strategies.”
Dr. Drucker and colleagues acknowledged certain limitations of the analysis, including its cross-sectional design, the potential for selection bias, and the fact that it did not use the patient-oriented outcome measure or the dermatology life quality index. “Further work to address the complex burden of AD, including strategies to reduce time spent managing AD, and understanding the fullness of the patient experience is needed,” they concluded.
The work was supported in part by a grant from the National Eczema Association (NEA). Dr. Drucker reported that he receives compensation from the British Journal of Dermatology (as reviewer and section editor), American Academy of Dermatology (guidelines writer), and NEA (grant reviewer). Coauthors representing the NEA and other patient organizations including the Allergy & Asthma Network, Asthma and Allergy Foundation of America, Global Parents for Eczema Research, and International Topical Steroid Awareness Network received organizational grants (Pfizer) and sponsorship funding for these analyses from AbbVie, Eli Lilly, Incyte, LEO Pharma, Regeneron Pharmaceuticals, and Sanofi Genzyme.
Dr. Chovatiya disclosed that he has served as an advisory board member, consultant, speaker, and/or investigator for AbbVie, Arcutis, Arena, Beiersdorf, Bristol Myers Squibb, Dermavant, Eli Lilly and Company, EPI Health, Incyte, L’Oréal, the NEA, Pfizer, Regeneron, Sanofi, and UCB.
Dr. Chiesa Fuxench disclosed that she has received research grants from Lilly, LEO Pharma, Regeneron, Sanofi, Tioga, and Vanda for work related to AD She has served as consultant for the Asthma and Allergy Foundation of America, NEA, AbbVie, Incyte Corporation, and Pfizer; and received honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi and Pfizer.
FROM JAMA DERMATOLOGY
Study explores gender differences in pediatric melanoma
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Ruxolitinib found to benefit adolescents with vitiligo up to one year
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Doc releases song after racist massacre in Buffalo
Physician-musician Cleveland Francis, MD, responded to the recent mass shooting in Buffalo, New York, which left 10 dead, in the only way he knew how. He wrote and recorded a song to honor the victims as “a plea to the other side to recognize us as people,” the Black cardiologist told this news organization.
He couldn’t sleep after the shooting, and “this song was just in my head.” In the 1990s, Dr. Francis took a 3-year sabbatical from medicine to perform and tour as a country singer. He leveraged his Nashville connections to get “Buffalo” produced and recorded.
Acclaimed artist James Threalkill created the accompanying art, titled “The Heavenly Escort of the Buffalo 10,” after listening to a scratch demo.
Dr. Francis doesn’t want people to overlook the massacre as just another gun violence incident because this was “overt hate-crime racism,” he said.
According to the affidavit submitted by FBI agent Christopher J. Dlugokinski, the suspect’s “motive for the mass shooting was to prevent Black people from replacing White people and eliminating the White race, and to inspire others to commit similar attacks.”
Dr. Francis views the Buffalo shooting as distinct from cases like the murder of George Floyd that involved crime or police. It immediately made him think of the Mother Emanuel Church shooting in Charleston, South Carolina. “Having a black skin is now a death warrant,” he said.
The song is also an appeal for White people to fight racism. Dr. Francis is concerned about young men caught up in white supremacy and suggests that we be more alert to children or grandchildren who disconnect from their families, spend time on the dark web, and access guns. The lyrics deliberately don’t mention guns because Dr. Francis wanted to stay out of that debate. “I just sang: ‘What else do I have to do to prove to you that I’m human too?’ ”
Despite his country credentials, Dr. Francis wrote “Buffalo” as a Gospel song because that genre “connects with Black people more and because that civil rights movement was through the church with Dr. Martin Luther King,” he explained. Although he sings all styles of music, the song is performed by Nashville-based singer Michael Lusk so that it’s not a “Cleve Francis thing,” he said, referring to his stage name.
Songwriter Norman Kerner collaborated on the song. The music was produced and recorded by David Thein and mixed by Bob Bullock of Nashville, who Dr. Francis had worked with when he was an artist on Capitol Records.
They sent the video and artwork to the Mayor of Buffalo, Byron Brown, but have yet to hear back. Dr. Francis hopes it could be part of their healing, noting that some people used the song in their Juneteenth celebrations.
The Louisiana native grew up during segregation and was one of two Black students in the Medical College of Virginia class of 1973. After completing his cardiology fellowship, no one would hire him, so Dr. Francis set up his own practice in Northern Virginia. He now works at Inova Heart and Vascular Institute in Alexandria, Va. He remains optimistic about race relations in America and would love a Black pop or Gospel star to record “Buffalo” and bring it to a wider audience.
Dr. Francis is a regular blogger for Medscape. His contribution to country music is recognized in the National Museum of African American History and Culture in Washington, DC. You can find more of his music on YouTube.
A version of this article first appeared on Medscape.com.
Physician-musician Cleveland Francis, MD, responded to the recent mass shooting in Buffalo, New York, which left 10 dead, in the only way he knew how. He wrote and recorded a song to honor the victims as “a plea to the other side to recognize us as people,” the Black cardiologist told this news organization.
He couldn’t sleep after the shooting, and “this song was just in my head.” In the 1990s, Dr. Francis took a 3-year sabbatical from medicine to perform and tour as a country singer. He leveraged his Nashville connections to get “Buffalo” produced and recorded.
Acclaimed artist James Threalkill created the accompanying art, titled “The Heavenly Escort of the Buffalo 10,” after listening to a scratch demo.
Dr. Francis doesn’t want people to overlook the massacre as just another gun violence incident because this was “overt hate-crime racism,” he said.
According to the affidavit submitted by FBI agent Christopher J. Dlugokinski, the suspect’s “motive for the mass shooting was to prevent Black people from replacing White people and eliminating the White race, and to inspire others to commit similar attacks.”
Dr. Francis views the Buffalo shooting as distinct from cases like the murder of George Floyd that involved crime or police. It immediately made him think of the Mother Emanuel Church shooting in Charleston, South Carolina. “Having a black skin is now a death warrant,” he said.
The song is also an appeal for White people to fight racism. Dr. Francis is concerned about young men caught up in white supremacy and suggests that we be more alert to children or grandchildren who disconnect from their families, spend time on the dark web, and access guns. The lyrics deliberately don’t mention guns because Dr. Francis wanted to stay out of that debate. “I just sang: ‘What else do I have to do to prove to you that I’m human too?’ ”
Despite his country credentials, Dr. Francis wrote “Buffalo” as a Gospel song because that genre “connects with Black people more and because that civil rights movement was through the church with Dr. Martin Luther King,” he explained. Although he sings all styles of music, the song is performed by Nashville-based singer Michael Lusk so that it’s not a “Cleve Francis thing,” he said, referring to his stage name.
Songwriter Norman Kerner collaborated on the song. The music was produced and recorded by David Thein and mixed by Bob Bullock of Nashville, who Dr. Francis had worked with when he was an artist on Capitol Records.
They sent the video and artwork to the Mayor of Buffalo, Byron Brown, but have yet to hear back. Dr. Francis hopes it could be part of their healing, noting that some people used the song in their Juneteenth celebrations.
The Louisiana native grew up during segregation and was one of two Black students in the Medical College of Virginia class of 1973. After completing his cardiology fellowship, no one would hire him, so Dr. Francis set up his own practice in Northern Virginia. He now works at Inova Heart and Vascular Institute in Alexandria, Va. He remains optimistic about race relations in America and would love a Black pop or Gospel star to record “Buffalo” and bring it to a wider audience.
Dr. Francis is a regular blogger for Medscape. His contribution to country music is recognized in the National Museum of African American History and Culture in Washington, DC. You can find more of his music on YouTube.
A version of this article first appeared on Medscape.com.
Physician-musician Cleveland Francis, MD, responded to the recent mass shooting in Buffalo, New York, which left 10 dead, in the only way he knew how. He wrote and recorded a song to honor the victims as “a plea to the other side to recognize us as people,” the Black cardiologist told this news organization.
He couldn’t sleep after the shooting, and “this song was just in my head.” In the 1990s, Dr. Francis took a 3-year sabbatical from medicine to perform and tour as a country singer. He leveraged his Nashville connections to get “Buffalo” produced and recorded.
Acclaimed artist James Threalkill created the accompanying art, titled “The Heavenly Escort of the Buffalo 10,” after listening to a scratch demo.
Dr. Francis doesn’t want people to overlook the massacre as just another gun violence incident because this was “overt hate-crime racism,” he said.
According to the affidavit submitted by FBI agent Christopher J. Dlugokinski, the suspect’s “motive for the mass shooting was to prevent Black people from replacing White people and eliminating the White race, and to inspire others to commit similar attacks.”
Dr. Francis views the Buffalo shooting as distinct from cases like the murder of George Floyd that involved crime or police. It immediately made him think of the Mother Emanuel Church shooting in Charleston, South Carolina. “Having a black skin is now a death warrant,” he said.
The song is also an appeal for White people to fight racism. Dr. Francis is concerned about young men caught up in white supremacy and suggests that we be more alert to children or grandchildren who disconnect from their families, spend time on the dark web, and access guns. The lyrics deliberately don’t mention guns because Dr. Francis wanted to stay out of that debate. “I just sang: ‘What else do I have to do to prove to you that I’m human too?’ ”
Despite his country credentials, Dr. Francis wrote “Buffalo” as a Gospel song because that genre “connects with Black people more and because that civil rights movement was through the church with Dr. Martin Luther King,” he explained. Although he sings all styles of music, the song is performed by Nashville-based singer Michael Lusk so that it’s not a “Cleve Francis thing,” he said, referring to his stage name.
Songwriter Norman Kerner collaborated on the song. The music was produced and recorded by David Thein and mixed by Bob Bullock of Nashville, who Dr. Francis had worked with when he was an artist on Capitol Records.
They sent the video and artwork to the Mayor of Buffalo, Byron Brown, but have yet to hear back. Dr. Francis hopes it could be part of their healing, noting that some people used the song in their Juneteenth celebrations.
The Louisiana native grew up during segregation and was one of two Black students in the Medical College of Virginia class of 1973. After completing his cardiology fellowship, no one would hire him, so Dr. Francis set up his own practice in Northern Virginia. He now works at Inova Heart and Vascular Institute in Alexandria, Va. He remains optimistic about race relations in America and would love a Black pop or Gospel star to record “Buffalo” and bring it to a wider audience.
Dr. Francis is a regular blogger for Medscape. His contribution to country music is recognized in the National Museum of African American History and Culture in Washington, DC. You can find more of his music on YouTube.
A version of this article first appeared on Medscape.com.
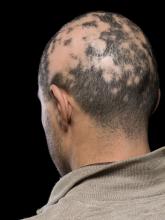
Baricitinib’s approval for alopecia areata: Considerations for starting patients on treatment
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
On June 13, the FDA approved baricitinib, a Janus kinase inhibitor (Olumiant, Lilly), for severe AA, and two other options may not be far behind. Pfizer and Concert Pharmaceuticals have JAK inhibitors in late-stage development for AA. JAK inhibitors, including baricitinib, are already on the market for treating rheumatoid arthritis and other autoimmune diseases.
Meanwhile, dermatologists have been fielding calls from hopeful patients and sorting out who should get the treatment, how to advise patients on risks and benefits, and what tests should be used before and after starting treatment.
Uptake for new systemic drugs, such as biologics, can be slow in dermatology, noted Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, as some doctors like to stick with what they know.
He told this news organization that he hopes that uptake for baricitinib is quicker, as it is the only approved oral systemic treatment for patients with severe alopecia areata, which affects about 300,000 people a year in the United States. Other treatments, including steroid injections in the scalp, have lacked efficacy and convenience.
Beyond the physical effects, the mental toll of patchy hair clumps and missing brows and lashes can be devastating for patients with alopecia areata.
Fielding patient inquiries
Word of the FDA approval spread fast, and calls and emails are coming into dermatologists’ offices and clinics from interested patients.
Physicians should be ready for patients with any kind of hair loss, not just severe alopecia areata, to ask about the drug, Dr. Friedman said. Some patients contacting him don’t fit the indication, which “highlights how disabling hair loss” is for people, considering that, in general, “people see this and think it is for them.”
Baricitinib is not a new drug, but a drug with a new indication. It had already been approved for treating moderate to severe RA in patients who have had an inadequate response to one or more tumor necrosis factor blockers, and for treating COVID-19 in certain hospitalized adults.
Boxed warning
Patients may ask about the boxed warning in the baricitinib label about the increased risk for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Natasha A. Mesinkovska, MD, PhD, an investigator in the clinical trials that led to FDA approval of baricitinib and the chief scientific officer at the National Alopecia Areata Foundation, told this news organization that several aspects of the label are important to point out.
One is that the warning is for all the JAK inhibitors used to treat RA and other inflammatory conditions, not just baricitinib. Also, the warning is based mostly on data on patients with RA who, she noted, have substantial comorbidities and have been taking toxic immunosuppressive medications. The RA population is also typically many years older than the alopecia areata population.
“Whether the warnings apply to the alopecia areata patients is as yet unclear,” said Dr. Mesinkovska, who is also an associate professor of dermatology at the University of California, Irvine.
Patients are also asking about how well it works.
In one of the two trials that led up to the FDA approval, which enrolled patients with at least 50% scalp hair loss for over 6 months, 22% of the patients who received 2 mg of baricitinib and 35% of those who received 4 mg saw adequate hair coverage (at least 80%) at week 36, compared with 5% on placebo. In the second trial, 17% of those who received 2 mg and 32% who received 4 mg saw adequate hair coverage, compared with 3% on placebo.
Common side effects associated with baricitinib, according to the FDA, are lower respiratory tract infections, headache, acne, high cholesterol, increased creatinine phosphokinase, urinary tract infection, liver enzyme elevations, folliculitis, fatigue, nausea, genital yeast infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain.
The risk-benefit discussions with patients should also include potential benefits beyond hair regrowth on the scalp. Loss of hair in the ears and nose can affect hearing and allergies, Dr. Mesinkovska said.
“About 30%-50% with alopecia areata, depending on age group or part of the world, will have allergies,” she said.
Patients should also know that baricitinib will need to be taken “for a very long time,” Dr. Mesinkovska noted. It’s possible that could be forever and that stopping the medication at any point may result in hair falling out again, she says, but duration will vary from case to case.
The good news is that it has been well tolerated. “We give a lot of medications for acne like doxycycline and other antibiotics and people have more stomach problems and angst with those than with [baricitinib],” she said.
Regrowth takes time
Benjamin Ungar, MD, a dermatologist at the Alopecia Center of Excellence at Mount Sinai, New York, told this news organization that an important message for patients is that hair regrowth takes time. For some other skin conditions, patients start treatment and see almost instant improvement.
“That is not the case for alopecia areata,” he said. “The expectation is that it will take months for regrowth in general.”
He said he hasn’t started prescribing baricitinib yet, but plans to do so soon.
“Obviously, I’ll have conversations with patients about it, but it’s a medication I’m going to be using, definitely. I have no reservations,” Dr. Ungar said.
After initial testing, physicians may find that some patients might not be ideal candidates, he added. People with liver disease, a history of blood clots, abnormal blood counts, or low neutrophils are among those who may not be the best candidates for baricitinib.
For most with severe alopecia areata, though, baricitinib provides hope.
“Treatment options have been not readily available, often inaccessible, ineffective, often dangerous,” he said. “There’s a treatment now that can be accessed, generally is safe and is effective for many people.”
Be up front with patients about the unknown
Additionally, it’s important to tell patients what is not yet known, the experts interviewed say.
“Alopecia areata is a chronic disease. We don’t have long-term data on the patient population yet,” Dr. Friedman said.
Also unknown is how easy it will be for physicians to get insurance to reimburse for baricitinib, which, at the end of June, was priced at about $5,000 a month for the 4-mg dose. FDA approval was important in that regard. Previously, some claims had been rejected for drugs used off label for AA.
“We dermatologists know how much it affects patients,” Dr. Mesinkovska said. “As long as we stick by what we know and convey to insurers how much it affects people’s lives, they should cover it.”
Another unknown is what other drugs can be taken with baricitinib. In clinical trials, it was used alone, she said. Currently, concomitant use of other immune suppressants – such as methotrexate or prednisone – is not recommended. But it remains to be seen what other medications will be safe to use at the same time as more long-term data are available.
Lynne J. Goldberg, MD, professor of dermatology, pathology, and laboratory medicine, Boston University, and director of the Hair Clinic at Boston Medical Center, said that she received a slew of emails from patients asking about baricitinib, but most of them did not have alopecia areata and were not candidates for this treatment.
She said that nurses in her clinic have been instructed on what to tell patients about which patients the drug is meant to treat, side effects, and benefits.
Access won’t be immediate
Dr. Goldberg said the drug’s approval does not mean immediate access. The patient has to come in, discuss the treatment, and get lab tests first. “It’s not a casual drug. This is a potent immunosuppressant drug. You need lab tests and once you start it you need blood tests every 3 months to stay on it.”
Those tests may vary by physician, but people will generally need a standard blood count and a comprehensive metabolic panel and lipid panel. “There’s nothing esoteric,” she said.
She added that physicians will need to check for presence of infections including tuberculosis and hepatitis B and C before prescribing, just as they would before they start prescribing a biologic.
“You don’t want to reactivate something,” she noted.
But, Dr. Goldberg added, the benefits for all who have been either living with only patches of hair or no hair or who put on a wig or hat every day are “life changing.”
Dr. Mesinkovska is on the advisory boards and runs trials for Eli Lilly, Pfizer, and Concert Pharmaceuticals. Dr. Friedman, Dr. Goldberg, and Dr. Ungar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mosquitoes and the vicious circle that’s gone viral
These viruses want mosquitoes with good taste
Taste can be a pretty subjective sense. Not everyone agrees on what tastes good and what tastes bad. Most people would agree that freshly baked cookies taste good, but what about lima beans? And what about mosquitoes? What tastes good to a mosquito?
The answer? Blood. Blood tastes good to a mosquito. That really wasn’t a very hard question, was it? You did know the answer, didn’t you? They don’t care about cookies, and they certainly don’t care about lima beans. It’s blood that they love.
That brings us back to subjectivity, because it is possible for blood to taste even better. The secret ingredient is dengue … and Zika.
A study just published in Cell demonstrates that mice infected with dengue and Zika viruses release a volatile compound called acetophenone. “We found that flavivirus [like dengue and Zika] can utilize the increased release of acetophenone to help itself achieve its lifecycles more effectively by making their hosts more attractive to mosquito vectors,” senior author Gong Cheng of Tsinghua University, Beijing, said in a written statement.
How do they do it? The viruses, he explained, promote the proliferation of acetophenone-producing skin bacteria. “As a result, some bacteria overreplicate and produce more acetophenone. Suddenly, these sick individuals smell as delicious to mosquitoes as a tray of freshly baked cookies to a group of five-year-old children,” the statement said.
And how do you stop a group of tiny, flying 5-year-olds? That’s right, with acne medication. Really? You knew that one but not the blood one before? The investigators fed isotretinoin to the infected mice, which led to reduced acetophenone release from skin bacteria and made the animals no more attractive to the mosquitoes than their uninfected counterparts.
The investigators are planning to take the next step – feeding isotretinoin to people with dengue and Zika – having gotten the official fictional taste-test approval of celebrity chef Gordon Ramsay, who said, “You’re going to feed this #$^% to sick people? ARE YOU &%*$@#& KIDDING ME?”
Okay, so maybe approval isn’t quite the right word.
Welcome to bladders of the rich and famous!
Don’t you hate it when you’re driving out to your multimillion-dollar second home in the Hamptons and traffic is so bad you absolutely have to find a place to “rest” along the way? But wouldn’t you know it, there just isn’t anywhere to stop! Geez, how do we live?
That’s where David Shusterman, MD, a urologist in New York City and a true American hero, comes in. He’s identified a market and positioned himself as the king of both bladder surgery and “bladder Botox” for the wealthy New Yorkers who regularly make long journeys from the city out to their second homes in the Hamptons. Traffic has increased dramatically on Long Island roads in recent years, and the journey can now taking upward of 4 hours. Some people just can’t make it that long without a bathroom break, and there are very few places to stop along the way.
Dr. Shusterman understands the plight of the Hamptons vacationer, as he told Insider.com: “I can’t tell you how many arguments I personally get into – I’ve lost three friends because I’m the driver and refuse to stop for them.” A tragedy worthy of Shakespeare himself.
During the summer season, Dr. Shusterman performs about 10 prostate artery embolizations a week, an hour-long procedure that shrinks the prostate, which is great for 50- to 60-year-old men with enlarged prostates that cause more frequent bathroom trips. He also performs Botox injections into the bladder once or twice a week for women, which reduces the need to urinate for roughly 6 months. The perfect amount of time to get them through the summer season.
These procedures are sometimes covered by insurance but can cost as much as $20,000 if paid out of pocket. That’s a lot of money to us, but if you’re the sort of person who has a second home in the Hamptons, $20,000 is chump change, especially if it means you won’t have to go 2 entire minutes out of your way to use a gas-station bathroom. Then again, having seen a more than a few gas-station bathrooms in our time, maybe they have a point.
Ditch the apples. Go for the avocados
We’ve all heard about “an apple a day,” but instead of apples you might want to go with avocados.
Avocados are generally thought to be a healthy fat. A study just published in the Journal of the American Heart Association proves that they actually don’t do anything for your waistline but will work wonders on your cholesterol level. The study involved 923 participants who were considered overweight/obese split into two groups: One was asked to consume an avocado a day, and the other continued their usual diets and were asked to consume fewer than two avocados a month.
At the end of the 6 months, the researchers found total cholesterol decreased by an additional 2.9 mg/dL and LDL cholesterol by 2.5 mg/dL in those who ate one avocado every day, compared with the usual-diet group. And even though avocados have a lot of calories, there was no clinical evidence that it impacted weight gain or any cardiometabolic risk factors, according to a statement from Penn State University.
Avocados, then, can be considered a guilt-free food. The findings from this study suggest it can give a substantial boost to your overall quality of diet, in turn lessening your risk of developing type 2 diabetes and some cancers, Kristina Peterson, PhD, assistant professor of nutritional sciences at Texas Tech University, said in the statement.
So get creative with your avocado recipes. You can only eat so much guacamole.
Your nose knows a good friend for you
You’ve probably noticed how dogs sniff other dogs and people before becoming friends. It would be pretty comical if people did the same thing, right? Just walked up to strangers and started sniffing them like dogs?
Well, apparently humans do go by smell when it comes to making friends, and they prefer people who smell like them. Maybe you’ve noticed that your friends look like you, share your values, and think the same way as you. You’re probably right, seeing as previous research has pointed to this.
For the current study, done to show how smell affects human behavior, researchers recruited people who befriended each other quickly, before knowing much about each other. They assumed that the relationships between these same-sex, nonromantic “click friends” relied more on physiological traits, including smell. After collecting samples from the click friends, researchers used an eNose to scan chemical signatures. In another experiment, human volunteers sniffed samples to determine if any were similar. Both experiments showed that click friends had more similar smells than pairs of random people.
“This is not to say that we act like goats or shrews – humans likely rely on other, far more dominant cues in their social decision-making. Nevertheless, our study’s results do suggest that our nose plays a bigger role than previously thought in our choice of friends,” said senior author Noam Sobel, PhD, of the Weizmann Institute of Science in Rehovot, Israel.
Lead author Inbal Ravreby, a graduate student at the institute, put it this way: “These results imply that, as the saying goes, there is chemistry in social chemistry.”
These viruses want mosquitoes with good taste
Taste can be a pretty subjective sense. Not everyone agrees on what tastes good and what tastes bad. Most people would agree that freshly baked cookies taste good, but what about lima beans? And what about mosquitoes? What tastes good to a mosquito?
The answer? Blood. Blood tastes good to a mosquito. That really wasn’t a very hard question, was it? You did know the answer, didn’t you? They don’t care about cookies, and they certainly don’t care about lima beans. It’s blood that they love.
That brings us back to subjectivity, because it is possible for blood to taste even better. The secret ingredient is dengue … and Zika.
A study just published in Cell demonstrates that mice infected with dengue and Zika viruses release a volatile compound called acetophenone. “We found that flavivirus [like dengue and Zika] can utilize the increased release of acetophenone to help itself achieve its lifecycles more effectively by making their hosts more attractive to mosquito vectors,” senior author Gong Cheng of Tsinghua University, Beijing, said in a written statement.
How do they do it? The viruses, he explained, promote the proliferation of acetophenone-producing skin bacteria. “As a result, some bacteria overreplicate and produce more acetophenone. Suddenly, these sick individuals smell as delicious to mosquitoes as a tray of freshly baked cookies to a group of five-year-old children,” the statement said.
And how do you stop a group of tiny, flying 5-year-olds? That’s right, with acne medication. Really? You knew that one but not the blood one before? The investigators fed isotretinoin to the infected mice, which led to reduced acetophenone release from skin bacteria and made the animals no more attractive to the mosquitoes than their uninfected counterparts.
The investigators are planning to take the next step – feeding isotretinoin to people with dengue and Zika – having gotten the official fictional taste-test approval of celebrity chef Gordon Ramsay, who said, “You’re going to feed this #$^% to sick people? ARE YOU &%*$@#& KIDDING ME?”
Okay, so maybe approval isn’t quite the right word.
Welcome to bladders of the rich and famous!
Don’t you hate it when you’re driving out to your multimillion-dollar second home in the Hamptons and traffic is so bad you absolutely have to find a place to “rest” along the way? But wouldn’t you know it, there just isn’t anywhere to stop! Geez, how do we live?
That’s where David Shusterman, MD, a urologist in New York City and a true American hero, comes in. He’s identified a market and positioned himself as the king of both bladder surgery and “bladder Botox” for the wealthy New Yorkers who regularly make long journeys from the city out to their second homes in the Hamptons. Traffic has increased dramatically on Long Island roads in recent years, and the journey can now taking upward of 4 hours. Some people just can’t make it that long without a bathroom break, and there are very few places to stop along the way.
Dr. Shusterman understands the plight of the Hamptons vacationer, as he told Insider.com: “I can’t tell you how many arguments I personally get into – I’ve lost three friends because I’m the driver and refuse to stop for them.” A tragedy worthy of Shakespeare himself.
During the summer season, Dr. Shusterman performs about 10 prostate artery embolizations a week, an hour-long procedure that shrinks the prostate, which is great for 50- to 60-year-old men with enlarged prostates that cause more frequent bathroom trips. He also performs Botox injections into the bladder once or twice a week for women, which reduces the need to urinate for roughly 6 months. The perfect amount of time to get them through the summer season.
These procedures are sometimes covered by insurance but can cost as much as $20,000 if paid out of pocket. That’s a lot of money to us, but if you’re the sort of person who has a second home in the Hamptons, $20,000 is chump change, especially if it means you won’t have to go 2 entire minutes out of your way to use a gas-station bathroom. Then again, having seen a more than a few gas-station bathrooms in our time, maybe they have a point.
Ditch the apples. Go for the avocados
We’ve all heard about “an apple a day,” but instead of apples you might want to go with avocados.
Avocados are generally thought to be a healthy fat. A study just published in the Journal of the American Heart Association proves that they actually don’t do anything for your waistline but will work wonders on your cholesterol level. The study involved 923 participants who were considered overweight/obese split into two groups: One was asked to consume an avocado a day, and the other continued their usual diets and were asked to consume fewer than two avocados a month.
At the end of the 6 months, the researchers found total cholesterol decreased by an additional 2.9 mg/dL and LDL cholesterol by 2.5 mg/dL in those who ate one avocado every day, compared with the usual-diet group. And even though avocados have a lot of calories, there was no clinical evidence that it impacted weight gain or any cardiometabolic risk factors, according to a statement from Penn State University.
Avocados, then, can be considered a guilt-free food. The findings from this study suggest it can give a substantial boost to your overall quality of diet, in turn lessening your risk of developing type 2 diabetes and some cancers, Kristina Peterson, PhD, assistant professor of nutritional sciences at Texas Tech University, said in the statement.
So get creative with your avocado recipes. You can only eat so much guacamole.
Your nose knows a good friend for you
You’ve probably noticed how dogs sniff other dogs and people before becoming friends. It would be pretty comical if people did the same thing, right? Just walked up to strangers and started sniffing them like dogs?
Well, apparently humans do go by smell when it comes to making friends, and they prefer people who smell like them. Maybe you’ve noticed that your friends look like you, share your values, and think the same way as you. You’re probably right, seeing as previous research has pointed to this.
For the current study, done to show how smell affects human behavior, researchers recruited people who befriended each other quickly, before knowing much about each other. They assumed that the relationships between these same-sex, nonromantic “click friends” relied more on physiological traits, including smell. After collecting samples from the click friends, researchers used an eNose to scan chemical signatures. In another experiment, human volunteers sniffed samples to determine if any were similar. Both experiments showed that click friends had more similar smells than pairs of random people.
“This is not to say that we act like goats or shrews – humans likely rely on other, far more dominant cues in their social decision-making. Nevertheless, our study’s results do suggest that our nose plays a bigger role than previously thought in our choice of friends,” said senior author Noam Sobel, PhD, of the Weizmann Institute of Science in Rehovot, Israel.
Lead author Inbal Ravreby, a graduate student at the institute, put it this way: “These results imply that, as the saying goes, there is chemistry in social chemistry.”
These viruses want mosquitoes with good taste
Taste can be a pretty subjective sense. Not everyone agrees on what tastes good and what tastes bad. Most people would agree that freshly baked cookies taste good, but what about lima beans? And what about mosquitoes? What tastes good to a mosquito?
The answer? Blood. Blood tastes good to a mosquito. That really wasn’t a very hard question, was it? You did know the answer, didn’t you? They don’t care about cookies, and they certainly don’t care about lima beans. It’s blood that they love.
That brings us back to subjectivity, because it is possible for blood to taste even better. The secret ingredient is dengue … and Zika.
A study just published in Cell demonstrates that mice infected with dengue and Zika viruses release a volatile compound called acetophenone. “We found that flavivirus [like dengue and Zika] can utilize the increased release of acetophenone to help itself achieve its lifecycles more effectively by making their hosts more attractive to mosquito vectors,” senior author Gong Cheng of Tsinghua University, Beijing, said in a written statement.
How do they do it? The viruses, he explained, promote the proliferation of acetophenone-producing skin bacteria. “As a result, some bacteria overreplicate and produce more acetophenone. Suddenly, these sick individuals smell as delicious to mosquitoes as a tray of freshly baked cookies to a group of five-year-old children,” the statement said.
And how do you stop a group of tiny, flying 5-year-olds? That’s right, with acne medication. Really? You knew that one but not the blood one before? The investigators fed isotretinoin to the infected mice, which led to reduced acetophenone release from skin bacteria and made the animals no more attractive to the mosquitoes than their uninfected counterparts.
The investigators are planning to take the next step – feeding isotretinoin to people with dengue and Zika – having gotten the official fictional taste-test approval of celebrity chef Gordon Ramsay, who said, “You’re going to feed this #$^% to sick people? ARE YOU &%*$@#& KIDDING ME?”
Okay, so maybe approval isn’t quite the right word.
Welcome to bladders of the rich and famous!
Don’t you hate it when you’re driving out to your multimillion-dollar second home in the Hamptons and traffic is so bad you absolutely have to find a place to “rest” along the way? But wouldn’t you know it, there just isn’t anywhere to stop! Geez, how do we live?
That’s where David Shusterman, MD, a urologist in New York City and a true American hero, comes in. He’s identified a market and positioned himself as the king of both bladder surgery and “bladder Botox” for the wealthy New Yorkers who regularly make long journeys from the city out to their second homes in the Hamptons. Traffic has increased dramatically on Long Island roads in recent years, and the journey can now taking upward of 4 hours. Some people just can’t make it that long without a bathroom break, and there are very few places to stop along the way.
Dr. Shusterman understands the plight of the Hamptons vacationer, as he told Insider.com: “I can’t tell you how many arguments I personally get into – I’ve lost three friends because I’m the driver and refuse to stop for them.” A tragedy worthy of Shakespeare himself.
During the summer season, Dr. Shusterman performs about 10 prostate artery embolizations a week, an hour-long procedure that shrinks the prostate, which is great for 50- to 60-year-old men with enlarged prostates that cause more frequent bathroom trips. He also performs Botox injections into the bladder once or twice a week for women, which reduces the need to urinate for roughly 6 months. The perfect amount of time to get them through the summer season.
These procedures are sometimes covered by insurance but can cost as much as $20,000 if paid out of pocket. That’s a lot of money to us, but if you’re the sort of person who has a second home in the Hamptons, $20,000 is chump change, especially if it means you won’t have to go 2 entire minutes out of your way to use a gas-station bathroom. Then again, having seen a more than a few gas-station bathrooms in our time, maybe they have a point.
Ditch the apples. Go for the avocados
We’ve all heard about “an apple a day,” but instead of apples you might want to go with avocados.
Avocados are generally thought to be a healthy fat. A study just published in the Journal of the American Heart Association proves that they actually don’t do anything for your waistline but will work wonders on your cholesterol level. The study involved 923 participants who were considered overweight/obese split into two groups: One was asked to consume an avocado a day, and the other continued their usual diets and were asked to consume fewer than two avocados a month.
At the end of the 6 months, the researchers found total cholesterol decreased by an additional 2.9 mg/dL and LDL cholesterol by 2.5 mg/dL in those who ate one avocado every day, compared with the usual-diet group. And even though avocados have a lot of calories, there was no clinical evidence that it impacted weight gain or any cardiometabolic risk factors, according to a statement from Penn State University.
Avocados, then, can be considered a guilt-free food. The findings from this study suggest it can give a substantial boost to your overall quality of diet, in turn lessening your risk of developing type 2 diabetes and some cancers, Kristina Peterson, PhD, assistant professor of nutritional sciences at Texas Tech University, said in the statement.
So get creative with your avocado recipes. You can only eat so much guacamole.
Your nose knows a good friend for you
You’ve probably noticed how dogs sniff other dogs and people before becoming friends. It would be pretty comical if people did the same thing, right? Just walked up to strangers and started sniffing them like dogs?
Well, apparently humans do go by smell when it comes to making friends, and they prefer people who smell like them. Maybe you’ve noticed that your friends look like you, share your values, and think the same way as you. You’re probably right, seeing as previous research has pointed to this.
For the current study, done to show how smell affects human behavior, researchers recruited people who befriended each other quickly, before knowing much about each other. They assumed that the relationships between these same-sex, nonromantic “click friends” relied more on physiological traits, including smell. After collecting samples from the click friends, researchers used an eNose to scan chemical signatures. In another experiment, human volunteers sniffed samples to determine if any were similar. Both experiments showed that click friends had more similar smells than pairs of random people.
“This is not to say that we act like goats or shrews – humans likely rely on other, far more dominant cues in their social decision-making. Nevertheless, our study’s results do suggest that our nose plays a bigger role than previously thought in our choice of friends,” said senior author Noam Sobel, PhD, of the Weizmann Institute of Science in Rehovot, Israel.
Lead author Inbal Ravreby, a graduate student at the institute, put it this way: “These results imply that, as the saying goes, there is chemistry in social chemistry.”
Skin reactions after COVID-19 vaccination have six patterns
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Skin manifestations of COVID-19 were among the topics presented in several sessions at the 49th Congress of the Spanish Academy of Dermatology and Venereology. Specialists agreed that fewer skin changes associated with this virus have been seen with the latest variants of SARS-CoV-2. They highlighted the results of the most remarkable research on this topic that were presented in this forum.
In the study, which was carried out by Spanish dermatologists with the support of the AEDV, researchers analyzed skin reactions associated with the COVID-19 vaccine.
Study author Cristina Galván, MD, a dermatologist at the University Hospital of Móstoles, Madrid, said, of the dermatological manifestations caused as a reaction to these vaccines.”
The study was carried out during the first months of COVID-19 vaccination, Dr. Galván told this news organization. It was proposed as a continuation of a COVID skin study that was published in the British Journal of Dermatology. That study documented the first classification of skin lesions associated with COVID-19. Dr. Galván is the lead author of the latter study.
“The objectives of this study were to characterize and classify skin reactions after vaccination, identify their chronology, and analyze the associations with a series of antecedents: dermatological and allergic diseases, previous SARS-CoV-2 infection, and skin reactions associated with COVID-19,” said Dr. Galván. The study was a team effort, she added.
“It was conducted between Feb. 15 and May 12, 2021, and information was gathered on 405 reactions that appeared during the 21 days after any dose of the COVID-19 vaccines approved at that time in Spain: the Pfizer/BioNTech, Moderna, and University of Oxford/AstraZeneca vaccines,” she added.
Dr. Galván explained that the study shows very clear patterns and investigators reached conclusions that match those of other groups that have investigated this topic. “Six reaction patterns were described according to their frequency. The first is the ‘COVID-19 arm,’ which consists of a local reaction at the injection site and occurs almost exclusively in women and in 70% of cases after inoculation with the Moderna serum. It is a manifestation that resolves well and does not always recur in subsequent doses. More than half are of delayed onset: biopsied patients show signs of a delayed hypersensitivity reaction. In line with all the publications in this regard, it was found that this reaction is not a reason to skip or delay a dose.”
Herpes zoster reactivation
The second pattern is urticarial, which, according to the specialist, occurs with equal frequency after the administration of all vaccines and is well controlled with antihistamines. “This is a very nonspecific pattern, which does not prevent it from still being frequent. It was not associated with drug intake.
“The morbilliform pattern is more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. It affects the trunk and extremities, and up to a quarter of the cases required systemic corticosteroids. The papulovesicular and pityriasis rosea–like patterns are equally frequent in all vaccines. The latter is found in a younger age group. Finally, there is the purpuric pattern, more localized in the extremities and more frequent after the Pfizer/BioNTech and AstraZeneca vaccines. On biopsy, this pattern showed small-vessel vasculitis.”
Less frequently, reactivations or de novo onset of different dermatologic diseases were found. “Varicella-zoster virus reactivations were observed with a frequency of 13.8%, being more common after the Pfizer/BioNTech vaccine,” said Dr. Galván. “Other studies have corroborated this increase in herpes zoster, although it has been seen that the absolute number is low, so the benefits of the vaccine outweigh this eventual complication. At the same time and along the same lines, vaccination against herpes zoster is recommended for those over 50 years of age.”
Another fact revealed by the study is that these reactions were not significantly more severe in people with dermatologic diseases, those with previous infection, or those with skin manifestation associated with COVID-19.
Dr. Galván highlighted that, except for the COVID-19 arm, these patterns were among those associated with the disease, “which supports [the idea] that it does not demonstrate that the host’s immune reaction to the infection was playing a role.”
Women and young people
“As for pseudoperniosis, it is poorly represented in our series: 0.7% compared to 2% in the American registry. Although neither the SARS-CoV-2–pseudoperniosis association nor its pathophysiology is clear, the idea is that if this manifestation is related to the host’s immune response during infection, pseudoperniosis after vaccination could also be linked to the immune response to the vaccine,” said Dr. Galván.
Many of these reactions are more intense in women. “Before starting to use these vaccines, we already knew that messenger RNA vaccines (a powerful activator of innate immunity) induce frequent reactions, that adjuvants and excipients (polyethylene glycol and polysorbate) also generate them, and that other factors influence reactogenicity, among those of us of the same age and sex, reactions being more frequent in younger people and in women,” said Dr. Galván. “This may be one of the reasons why the COVID-19 arm is so much more prevalent in the female population and that 80% of all reactions that were collected were in women.”
In relation to the fact that manifestations differed, depending on the type of inoculated serum, Dr. Galván said, “Some reactions are just as common after any of the vaccines. However, others are not, as is the case with the COVID-19 arm for the Moderna vaccine or reactivations of the herpes virus, more frequent after the Pfizer/BioNTech vaccine.
“Undoubtedly, behind these differences are particularities in the immune reaction caused by each of the vaccines and their composition, including the excipients,” she said.
Regarding the fact that these reactions were the same throughout the vaccine regimen or that they varied in intensity, depending on the dose, Dr. Galván said, “In our study, as in those carried out by other groups, there were no significant differences in terms of frequency after the first and second doses. One thing to keep in mind is that, due to the temporary design of our study and the time at which it was conducted, it was not possible to collect reactions after second doses of AstraZeneca.
“Manifestations have generally been mild and well controlled. Many of them did not recur after the second dose, and the vast majority did not prevent completion of the vaccination scheme, but we must not lose sight of the fact that 20% of these manifestations were assessed by the dermatologist as serious or very serious,” Dr. Galván added.
Regarding the next steps planned for this line of research, Dr. Galván commented, “We are awaiting the evolution of the reported cases and the reactions that may arise, although for now, our group does not have any open studies. The most important thing now is to be alert and report the data observed in the pharmacovigilance systems, in open registries, and in scientific literature to generate evidence.”
Dr. Galván has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox mutating faster than expected
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The monkeypox virus is evolving 6-12 times faster than would be expected, according to a new study.
The virus is thought to have a single origin, the genetic data suggests, and is a likely descendant of the strain involved in the 2017-2018 monkeypox outbreak in Nigeria. It’s not clear if these mutations have aided the transmissibility of the virus among people or have any other clinical implications, João Paulo Gomes, PhD, from Portugal’s National Institute of Health, Lisbon, said in an email.
Since the monkeypox outbreak began in May, nearly 7,000 cases of monkeypox have been reported across 52 countries and territories.
Orthopoxviruses – the genus to which monkeypox belongs – are large DNA viruses that usually only gain one or two mutations every year. (For comparison, SARS-CoV-2 gains around two mutations every month.) One would expect 5 to 10 mutations in the 2022 monkeypox virus, compared with the 2017 strain, Dr. Gomes said.
In the study, Dr. Gomes and colleagues analyzed 15 monkeypox DNA sequences made available by Portugal and the National Center for Biotechnology Information, Bethesda, Md., between May 20 and May 27, 2022. The analysis revealed that this most recent strain differed by 50 single-nucleotide polymorphisms, compared with previous strains of the virus in 2017-2018.
“This is far beyond what we would expect, specifically for orthopoxvirus,” Andrew Lover, PhD, an epidemiologist at the University of Massachusetts Amherst School of Public Health & Health Sciences, told this news organization. He was not involved with the research. “That suggests [the virus] is trying to figure out the best way to deal with a new host species,” he added.
Rodents are thought to be the natural hosts of the monkeypox virus, he explained, and, in 2022, the infection transferred to humans. “Moving into a new species can ‘turbocharge’ mutations as the virus adapts to a new biological environment,” he explained, though it is not clear if the new mutations Dr. Gomes’s team detected help the 2022 virus spread more easily among people.
Researchers also found that the 2022 virus belonged in clade 3 of the virus, which is part of the less-lethal West-African clade. While the West-African clade has a fatality rate of less than 1%, the Central African clade has a fatality rate of over 10%.
The rapid changes in the viral genome could be driven by a family of proteins thought to play a role in antiviral immunity: apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3 (APOBEC3). These enzymes can make changes to a viral genome, Dr. Gomes explained, “but sometimes the system is not ‘well regulated,’ and the changes in the genome are not detrimental to the virus.” These APOBEC3-driven mutations have a signature pattern, he said, which was also detected in most of the 50 new mutations Dr. Gomes’s team identified.
However, it is not known if these mutations have clinical implications, Dr. Lover said.
The 2022 monkeypox virus does appear to behave differently than previous strains of the virus, he noted. In the current outbreak, sexual transmission appears to be very common, which is not the case for previous outbreaks, he said. Also, while monkeypox traditionally presents with a rash that can spread to all parts of the body, there have been several instances of patients presenting with just a few “very innocuous lesions,” he added.
Dr. Gomes hopes that specialized lab groups will now be able to tease out whether there is a connection between these identified mutations and changes in the behavior of the virus, including transmissibility.
While none of the findings in this analysis raises any serious concerns, the study “suggests there [are] definitely gaps in our knowledge about monkeypox,” Dr. Lover said. As for the global health response, he said, “We probably should err on the side of caution. ... There are clearly things that we absolutely don’t understand here, in terms of how quickly mutations are popping up.”
Dr. Gomes and Dr. Lover report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is a single dose of HPV vaccine enough?
In an April press release, the Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization (WHO) reported the findings of their review concerning the efficacy of various dose schedules for human papillomavirus (HPV). “A single-dose HPV vaccine delivers solid protection against HPV, the virus that causes cervical cancer, that is comparable to 2-dose schedules,” according to SAGE.
This statement comes on the heels of an article published in the November 2021 issue of Lancet Oncology about a study in India. It found that a single dose of the vaccine provides protection against persistent infection from HPV 16 and 18 similar to that provided by two or three doses.
Will this new information lead French authorities to change their recommendations? What do French specialists think? At the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases (SFCPCV), Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, shared his thoughts.
With respect to the Indian study, Dr. Canlorbe pointed out that while its findings would need “to be confirmed by other studies,” they were, nonetheless,
India and France
During the congress press conference, he went on to say that, at this stage, the findings “cannot be extrapolated” to France. This is because the country’s situation is different. HPV vaccination coverage is low; estimates put it at 23.7%, placing the country 28th out of 31 in Europe.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
In addition, he drew attention to several limitations of the Indian study. Initially, the team had planned to enroll 20,000 participants. In the end, there were around 17,000, and these were allocated to three cohorts: single-dose, two-dose, and three-dose. Furthermore, the primary objective, which had initially been focused on precancerous and cancerous lesions, was revised. The new aim was to compare vaccine efficacy of single dose to that of three and two doses in protecting against persistent HPV 16 and 18 infection at 10 years postvaccination. In about 90% of cases, the HPV infection went away spontaneously in 2 years without inducing lesions. Finally, the participants were women in India; therefore, the results cannot necessarily be generalized to the French population.
“This information has to be confirmed. However, as far as I know, there are no new studies going on at the moment. The Indian study, on the other hand, is still in progress,” said Dr. Canlorbe.
“In France, I think that for the time being we should stick to the studies that are currently available, which have demonstrated the efficacy and safety of two or three doses,” he concluded. In support of this approach, he cited a study on the effects of the national HPV vaccination program in England; there, the vaccination coverage is 80%.
This program was associated with a 95% risk reduction for precancerous lesions and an 87% reduction in the number of cancers, confirming the good results already achieved by Sweden and Australia.
In his comments on the WHO’s stance (which differs from that of the French experts), Jean-Luc Mergui, MD, gynecologist in the department of colposcopy and hysteroscopy at Pitié-Salpêtrière, and former president of the SFCPCV, offered an eloquent comparison: “The WHO also recommends 6 months of breastfeeding as a method of contraception, but this isn’t what’s recommended in France, for the risk of getting pregnant nevertheless remains.”
Indian study highlights
Partha Basu, MD, PhD, of the International Agency for Research on Cancer (IARC) in Lyon, France, and colleagues compared vaccine efficacy of a single dose of Gardasil (HPV 9-valent vaccine, recombinant) to that of two and three doses in protecting against persistent HPV 16 and HPV 18 infection at 10 years postvaccination.
According to the protocol, the plan was to recruit 20,000 unmarried girls, aged 10-18 years, from across India. Recruitment was initiated in September 2009. However, in response to seven unexplained deaths reported in another ongoing HPV vaccination demonstration program in the country, the Indian government issued a notification in April 2010 to stop further recruitment and HPV vaccination in all clinical trials. At this point, Dr. Basu and his team had recruited 17,729 eligible girls.
After suspension of recruitment and vaccination, their randomized trial was converted to a longitudinal, prospective, cohort study by default.
Vaccinated participants were followed up over a median duration of 9 years. In all, 4,348 participants had three doses, 4,980 had two doses (at 0 and 6 months), and 4,949 had a single dose. Cervical specimens were collected from participants 18 months after marriage or 6 months after first childbirth, whichever was earlier, to assess incident and persistent HPV infections. Participants were invited to an annual cervical cancer screening once they reached age 25 years and were married.
A single dose of HPV vaccine provides similar protection against persistent infection from HPV 16 and HPV 18, the genotypes responsible for nearly 70% of cervical cancers, compared with that provided by two or three doses. Vaccine efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% (95% confidence interval [CI], 85.0-99.9) in the single-dose default cohort (2,135 women assessed), 93.1% (95% CI, 77.3-99.8) in the two-dose cohort (1,452 women assessed), and 93.3% (95% CI, 77.5-99.7) in three-dose recipients (1,460 women assessed).
Dr. Canlorbe reported no relevant financial relationships regarding the content of this article.
This article was translated from the Medscape French edition. An English version appeared on Medscape.com.
In an April press release, the Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization (WHO) reported the findings of their review concerning the efficacy of various dose schedules for human papillomavirus (HPV). “A single-dose HPV vaccine delivers solid protection against HPV, the virus that causes cervical cancer, that is comparable to 2-dose schedules,” according to SAGE.
This statement comes on the heels of an article published in the November 2021 issue of Lancet Oncology about a study in India. It found that a single dose of the vaccine provides protection against persistent infection from HPV 16 and 18 similar to that provided by two or three doses.
Will this new information lead French authorities to change their recommendations? What do French specialists think? At the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases (SFCPCV), Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, shared his thoughts.
With respect to the Indian study, Dr. Canlorbe pointed out that while its findings would need “to be confirmed by other studies,” they were, nonetheless,
India and France
During the congress press conference, he went on to say that, at this stage, the findings “cannot be extrapolated” to France. This is because the country’s situation is different. HPV vaccination coverage is low; estimates put it at 23.7%, placing the country 28th out of 31 in Europe.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
In addition, he drew attention to several limitations of the Indian study. Initially, the team had planned to enroll 20,000 participants. In the end, there were around 17,000, and these were allocated to three cohorts: single-dose, two-dose, and three-dose. Furthermore, the primary objective, which had initially been focused on precancerous and cancerous lesions, was revised. The new aim was to compare vaccine efficacy of single dose to that of three and two doses in protecting against persistent HPV 16 and 18 infection at 10 years postvaccination. In about 90% of cases, the HPV infection went away spontaneously in 2 years without inducing lesions. Finally, the participants were women in India; therefore, the results cannot necessarily be generalized to the French population.
“This information has to be confirmed. However, as far as I know, there are no new studies going on at the moment. The Indian study, on the other hand, is still in progress,” said Dr. Canlorbe.
“In France, I think that for the time being we should stick to the studies that are currently available, which have demonstrated the efficacy and safety of two or three doses,” he concluded. In support of this approach, he cited a study on the effects of the national HPV vaccination program in England; there, the vaccination coverage is 80%.
This program was associated with a 95% risk reduction for precancerous lesions and an 87% reduction in the number of cancers, confirming the good results already achieved by Sweden and Australia.
In his comments on the WHO’s stance (which differs from that of the French experts), Jean-Luc Mergui, MD, gynecologist in the department of colposcopy and hysteroscopy at Pitié-Salpêtrière, and former president of the SFCPCV, offered an eloquent comparison: “The WHO also recommends 6 months of breastfeeding as a method of contraception, but this isn’t what’s recommended in France, for the risk of getting pregnant nevertheless remains.”
Indian study highlights
Partha Basu, MD, PhD, of the International Agency for Research on Cancer (IARC) in Lyon, France, and colleagues compared vaccine efficacy of a single dose of Gardasil (HPV 9-valent vaccine, recombinant) to that of two and three doses in protecting against persistent HPV 16 and HPV 18 infection at 10 years postvaccination.
According to the protocol, the plan was to recruit 20,000 unmarried girls, aged 10-18 years, from across India. Recruitment was initiated in September 2009. However, in response to seven unexplained deaths reported in another ongoing HPV vaccination demonstration program in the country, the Indian government issued a notification in April 2010 to stop further recruitment and HPV vaccination in all clinical trials. At this point, Dr. Basu and his team had recruited 17,729 eligible girls.
After suspension of recruitment and vaccination, their randomized trial was converted to a longitudinal, prospective, cohort study by default.
Vaccinated participants were followed up over a median duration of 9 years. In all, 4,348 participants had three doses, 4,980 had two doses (at 0 and 6 months), and 4,949 had a single dose. Cervical specimens were collected from participants 18 months after marriage or 6 months after first childbirth, whichever was earlier, to assess incident and persistent HPV infections. Participants were invited to an annual cervical cancer screening once they reached age 25 years and were married.
A single dose of HPV vaccine provides similar protection against persistent infection from HPV 16 and HPV 18, the genotypes responsible for nearly 70% of cervical cancers, compared with that provided by two or three doses. Vaccine efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% (95% confidence interval [CI], 85.0-99.9) in the single-dose default cohort (2,135 women assessed), 93.1% (95% CI, 77.3-99.8) in the two-dose cohort (1,452 women assessed), and 93.3% (95% CI, 77.5-99.7) in three-dose recipients (1,460 women assessed).
Dr. Canlorbe reported no relevant financial relationships regarding the content of this article.
This article was translated from the Medscape French edition. An English version appeared on Medscape.com.
In an April press release, the Strategic Advisory Group of Experts on Immunization (SAGE) of the World Health Organization (WHO) reported the findings of their review concerning the efficacy of various dose schedules for human papillomavirus (HPV). “A single-dose HPV vaccine delivers solid protection against HPV, the virus that causes cervical cancer, that is comparable to 2-dose schedules,” according to SAGE.
This statement comes on the heels of an article published in the November 2021 issue of Lancet Oncology about a study in India. It found that a single dose of the vaccine provides protection against persistent infection from HPV 16 and 18 similar to that provided by two or three doses.
Will this new information lead French authorities to change their recommendations? What do French specialists think? At the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases (SFCPCV), Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, shared his thoughts.
With respect to the Indian study, Dr. Canlorbe pointed out that while its findings would need “to be confirmed by other studies,” they were, nonetheless,
India and France
During the congress press conference, he went on to say that, at this stage, the findings “cannot be extrapolated” to France. This is because the country’s situation is different. HPV vaccination coverage is low; estimates put it at 23.7%, placing the country 28th out of 31 in Europe.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
In addition, he drew attention to several limitations of the Indian study. Initially, the team had planned to enroll 20,000 participants. In the end, there were around 17,000, and these were allocated to three cohorts: single-dose, two-dose, and three-dose. Furthermore, the primary objective, which had initially been focused on precancerous and cancerous lesions, was revised. The new aim was to compare vaccine efficacy of single dose to that of three and two doses in protecting against persistent HPV 16 and 18 infection at 10 years postvaccination. In about 90% of cases, the HPV infection went away spontaneously in 2 years without inducing lesions. Finally, the participants were women in India; therefore, the results cannot necessarily be generalized to the French population.
“This information has to be confirmed. However, as far as I know, there are no new studies going on at the moment. The Indian study, on the other hand, is still in progress,” said Dr. Canlorbe.
“In France, I think that for the time being we should stick to the studies that are currently available, which have demonstrated the efficacy and safety of two or three doses,” he concluded. In support of this approach, he cited a study on the effects of the national HPV vaccination program in England; there, the vaccination coverage is 80%.
This program was associated with a 95% risk reduction for precancerous lesions and an 87% reduction in the number of cancers, confirming the good results already achieved by Sweden and Australia.
In his comments on the WHO’s stance (which differs from that of the French experts), Jean-Luc Mergui, MD, gynecologist in the department of colposcopy and hysteroscopy at Pitié-Salpêtrière, and former president of the SFCPCV, offered an eloquent comparison: “The WHO also recommends 6 months of breastfeeding as a method of contraception, but this isn’t what’s recommended in France, for the risk of getting pregnant nevertheless remains.”
Indian study highlights
Partha Basu, MD, PhD, of the International Agency for Research on Cancer (IARC) in Lyon, France, and colleagues compared vaccine efficacy of a single dose of Gardasil (HPV 9-valent vaccine, recombinant) to that of two and three doses in protecting against persistent HPV 16 and HPV 18 infection at 10 years postvaccination.
According to the protocol, the plan was to recruit 20,000 unmarried girls, aged 10-18 years, from across India. Recruitment was initiated in September 2009. However, in response to seven unexplained deaths reported in another ongoing HPV vaccination demonstration program in the country, the Indian government issued a notification in April 2010 to stop further recruitment and HPV vaccination in all clinical trials. At this point, Dr. Basu and his team had recruited 17,729 eligible girls.
After suspension of recruitment and vaccination, their randomized trial was converted to a longitudinal, prospective, cohort study by default.
Vaccinated participants were followed up over a median duration of 9 years. In all, 4,348 participants had three doses, 4,980 had two doses (at 0 and 6 months), and 4,949 had a single dose. Cervical specimens were collected from participants 18 months after marriage or 6 months after first childbirth, whichever was earlier, to assess incident and persistent HPV infections. Participants were invited to an annual cervical cancer screening once they reached age 25 years and were married.
A single dose of HPV vaccine provides similar protection against persistent infection from HPV 16 and HPV 18, the genotypes responsible for nearly 70% of cervical cancers, compared with that provided by two or three doses. Vaccine efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% (95% confidence interval [CI], 85.0-99.9) in the single-dose default cohort (2,135 women assessed), 93.1% (95% CI, 77.3-99.8) in the two-dose cohort (1,452 women assessed), and 93.3% (95% CI, 77.5-99.7) in three-dose recipients (1,460 women assessed).
Dr. Canlorbe reported no relevant financial relationships regarding the content of this article.
This article was translated from the Medscape French edition. An English version appeared on Medscape.com.