User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Remibrutinib safe for Sjögren’s in phase 2
PHILADELPHIA – Oral remibrutinib was well tolerated and had a good safety profile over 24 weeks among patients with moderate to severe Sjögren syndrome (SS), according to new phase 2 data presented at the annual meeting of the American College of Rheumatology.
Thomas Dörner, MD, with the department of rheumatology and clinical immunology at Charité Universitätsmedizin Berlin, presented the data from the double-blind, randomized, placebo-controlled, proof-of-concept study.
The authors said the results of the study suggest that remibrutinib, a highly specific inhibitor of Bruton tyrosine kinase, has the potential to become the first effective oral disease-modifying therapy for SS.
The 73 participants in the study had moderate to severe SS. The baseline EULAR Sjögren Syndrome Disease Activity Index (ESSDAI) score was at least 5, EULAR Sjögren Syndrome Patient Reported Index (ESSPRI) was at least 5, and anti-Ro/SSA antibody positivity was 3 months or less before screening. The patients’ unstimulated whole salivary flow rate was > 0 mL/min.
Overall, 73 patients (71 women) were randomly assigned to receive either remibrutinib 100 mg twice a day (n = 24), remibrutinib 100 mg four times a day (n = 25), or placebo (n = 24) between August 2019 and May 2021.
Remibrutinib met the primary endpoint and resulted in a statistically significant improvement in ESSDAI score for both regimens combined compared with placebo at week 24 (ESSDAI, –2.86).
Patient-reported outcomes similar to placebo
Patient-reported outcomes, including scores on ESSPRI, Functional Assessment of Chronic Illness Therapy–Fatigue, and EuroQol-5 Dimension, were similar in the treatment groups and the placebo group.
“All of the patients, including the placebo patients, improved over the time of the study,” Dr. Dörner said.
The average age of the patients was 51.8 years (range, 18-75 years). Groups were generally balanced with regard to demographic qualities and disease severity at baseline, and the patients represented the SS population well, Dr. Dörner said.
No severe adverse events were reported. Infections were the most frequently reported adverse events, and the rates were similar with the study drug and placebo. No notable liver abnormalities were reported in any of the groups.
Chrisanna Dobrowolski, MD, assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York, told this news organization, “Preliminary results are promising, but they failed to show improvements in patient-reported quality-of-life measures.
“Having statistical improvements in disease activity measures without clinically meaningful improvement in patient quality of life may limit the value of this treatment,” she said.
Dr. Dobrowolski added that the follow-up period of 6 months is short, and larger studies over a longer period are needed to better assess the effect on patients’ quality of life.
“Regardless, this is the first oral medication which has shown disease-modifying potential for the glandular symptoms of SS and is an exciting new avenue of investigation to be further explored,” she said.
Patients with SS 15 to 20 times more likely to develop B-cell lymphoma as a life-threatening complication. SS is a systemic autoimmune disease characterized by B-cell hyperactivation, lymphoid infiltration, progressive destruction of exocrine glands, and various complications outside the glands, the study authors wrote in the abstract.
Nearly 4 million in U.S. live with the disease
Nearly 4 million people in the United States live with the disease. Common symptoms include light sensitivity, dry eye, dry mouth, fatigue, and joint pain.
SS can be difficult to diagnose because the symptoms vary from person to person and can be confused with those caused by other diseases.
Ardy Fenando, MD, a rheumatology fellow with the University of Kansas Medical Center, said in an interview, “We need more therapies for Sjögren’s. Heterogeneity complicates the way we set the primary endpoints. Therefore, we haven’t had a proven treatment for Sjögren’s. This is supported by previous RCTs [randomized controlled trials] that failed to meet the primary end points.”
Dr. Dörner has relationships with AbbVie, Eli Lilly, Roche/Genentech, Janssen, Novartis, Bristol-Myers Squibb), and UCB. Other authors have various relationships with industry. Dr. Fenando and Dr. Dobrowolski have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Oral remibrutinib was well tolerated and had a good safety profile over 24 weeks among patients with moderate to severe Sjögren syndrome (SS), according to new phase 2 data presented at the annual meeting of the American College of Rheumatology.
Thomas Dörner, MD, with the department of rheumatology and clinical immunology at Charité Universitätsmedizin Berlin, presented the data from the double-blind, randomized, placebo-controlled, proof-of-concept study.
The authors said the results of the study suggest that remibrutinib, a highly specific inhibitor of Bruton tyrosine kinase, has the potential to become the first effective oral disease-modifying therapy for SS.
The 73 participants in the study had moderate to severe SS. The baseline EULAR Sjögren Syndrome Disease Activity Index (ESSDAI) score was at least 5, EULAR Sjögren Syndrome Patient Reported Index (ESSPRI) was at least 5, and anti-Ro/SSA antibody positivity was 3 months or less before screening. The patients’ unstimulated whole salivary flow rate was > 0 mL/min.
Overall, 73 patients (71 women) were randomly assigned to receive either remibrutinib 100 mg twice a day (n = 24), remibrutinib 100 mg four times a day (n = 25), or placebo (n = 24) between August 2019 and May 2021.
Remibrutinib met the primary endpoint and resulted in a statistically significant improvement in ESSDAI score for both regimens combined compared with placebo at week 24 (ESSDAI, –2.86).
Patient-reported outcomes similar to placebo
Patient-reported outcomes, including scores on ESSPRI, Functional Assessment of Chronic Illness Therapy–Fatigue, and EuroQol-5 Dimension, were similar in the treatment groups and the placebo group.
“All of the patients, including the placebo patients, improved over the time of the study,” Dr. Dörner said.
The average age of the patients was 51.8 years (range, 18-75 years). Groups were generally balanced with regard to demographic qualities and disease severity at baseline, and the patients represented the SS population well, Dr. Dörner said.
No severe adverse events were reported. Infections were the most frequently reported adverse events, and the rates were similar with the study drug and placebo. No notable liver abnormalities were reported in any of the groups.
Chrisanna Dobrowolski, MD, assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York, told this news organization, “Preliminary results are promising, but they failed to show improvements in patient-reported quality-of-life measures.
“Having statistical improvements in disease activity measures without clinically meaningful improvement in patient quality of life may limit the value of this treatment,” she said.
Dr. Dobrowolski added that the follow-up period of 6 months is short, and larger studies over a longer period are needed to better assess the effect on patients’ quality of life.
“Regardless, this is the first oral medication which has shown disease-modifying potential for the glandular symptoms of SS and is an exciting new avenue of investigation to be further explored,” she said.
Patients with SS 15 to 20 times more likely to develop B-cell lymphoma as a life-threatening complication. SS is a systemic autoimmune disease characterized by B-cell hyperactivation, lymphoid infiltration, progressive destruction of exocrine glands, and various complications outside the glands, the study authors wrote in the abstract.
Nearly 4 million in U.S. live with the disease
Nearly 4 million people in the United States live with the disease. Common symptoms include light sensitivity, dry eye, dry mouth, fatigue, and joint pain.
SS can be difficult to diagnose because the symptoms vary from person to person and can be confused with those caused by other diseases.
Ardy Fenando, MD, a rheumatology fellow with the University of Kansas Medical Center, said in an interview, “We need more therapies for Sjögren’s. Heterogeneity complicates the way we set the primary endpoints. Therefore, we haven’t had a proven treatment for Sjögren’s. This is supported by previous RCTs [randomized controlled trials] that failed to meet the primary end points.”
Dr. Dörner has relationships with AbbVie, Eli Lilly, Roche/Genentech, Janssen, Novartis, Bristol-Myers Squibb), and UCB. Other authors have various relationships with industry. Dr. Fenando and Dr. Dobrowolski have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Oral remibrutinib was well tolerated and had a good safety profile over 24 weeks among patients with moderate to severe Sjögren syndrome (SS), according to new phase 2 data presented at the annual meeting of the American College of Rheumatology.
Thomas Dörner, MD, with the department of rheumatology and clinical immunology at Charité Universitätsmedizin Berlin, presented the data from the double-blind, randomized, placebo-controlled, proof-of-concept study.
The authors said the results of the study suggest that remibrutinib, a highly specific inhibitor of Bruton tyrosine kinase, has the potential to become the first effective oral disease-modifying therapy for SS.
The 73 participants in the study had moderate to severe SS. The baseline EULAR Sjögren Syndrome Disease Activity Index (ESSDAI) score was at least 5, EULAR Sjögren Syndrome Patient Reported Index (ESSPRI) was at least 5, and anti-Ro/SSA antibody positivity was 3 months or less before screening. The patients’ unstimulated whole salivary flow rate was > 0 mL/min.
Overall, 73 patients (71 women) were randomly assigned to receive either remibrutinib 100 mg twice a day (n = 24), remibrutinib 100 mg four times a day (n = 25), or placebo (n = 24) between August 2019 and May 2021.
Remibrutinib met the primary endpoint and resulted in a statistically significant improvement in ESSDAI score for both regimens combined compared with placebo at week 24 (ESSDAI, –2.86).
Patient-reported outcomes similar to placebo
Patient-reported outcomes, including scores on ESSPRI, Functional Assessment of Chronic Illness Therapy–Fatigue, and EuroQol-5 Dimension, were similar in the treatment groups and the placebo group.
“All of the patients, including the placebo patients, improved over the time of the study,” Dr. Dörner said.
The average age of the patients was 51.8 years (range, 18-75 years). Groups were generally balanced with regard to demographic qualities and disease severity at baseline, and the patients represented the SS population well, Dr. Dörner said.
No severe adverse events were reported. Infections were the most frequently reported adverse events, and the rates were similar with the study drug and placebo. No notable liver abnormalities were reported in any of the groups.
Chrisanna Dobrowolski, MD, assistant professor of medicine at Icahn School of Medicine at Mount Sinai in New York, told this news organization, “Preliminary results are promising, but they failed to show improvements in patient-reported quality-of-life measures.
“Having statistical improvements in disease activity measures without clinically meaningful improvement in patient quality of life may limit the value of this treatment,” she said.
Dr. Dobrowolski added that the follow-up period of 6 months is short, and larger studies over a longer period are needed to better assess the effect on patients’ quality of life.
“Regardless, this is the first oral medication which has shown disease-modifying potential for the glandular symptoms of SS and is an exciting new avenue of investigation to be further explored,” she said.
Patients with SS 15 to 20 times more likely to develop B-cell lymphoma as a life-threatening complication. SS is a systemic autoimmune disease characterized by B-cell hyperactivation, lymphoid infiltration, progressive destruction of exocrine glands, and various complications outside the glands, the study authors wrote in the abstract.
Nearly 4 million in U.S. live with the disease
Nearly 4 million people in the United States live with the disease. Common symptoms include light sensitivity, dry eye, dry mouth, fatigue, and joint pain.
SS can be difficult to diagnose because the symptoms vary from person to person and can be confused with those caused by other diseases.
Ardy Fenando, MD, a rheumatology fellow with the University of Kansas Medical Center, said in an interview, “We need more therapies for Sjögren’s. Heterogeneity complicates the way we set the primary endpoints. Therefore, we haven’t had a proven treatment for Sjögren’s. This is supported by previous RCTs [randomized controlled trials] that failed to meet the primary end points.”
Dr. Dörner has relationships with AbbVie, Eli Lilly, Roche/Genentech, Janssen, Novartis, Bristol-Myers Squibb), and UCB. Other authors have various relationships with industry. Dr. Fenando and Dr. Dobrowolski have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2022
First recommendations for cancer screening in myositis issued
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
AT ACR 2022
PHILADELPHIA – The first consensus screening guidelines for patients with idiopathic inflammatory myopathy (IIM) provide recommendations on risk stratification for individuals, basic and enhanced screening protocols, and screening frequency.
The recommendations, issued by the International Myositis Assessment and Clinical Studies Group (IMACS), stratify cancer risk for individual patients into low, intermediate, or high categories based on the IIM disease subtype, autoantibody status, and clinical features, reported Alexander Oldroyd, PhD, MSc, MBChB of the University of Manchester, England.
“There’s a big unmet need for cancer screening. One in four adults with myositis has cancer, either 3 years before or after a diagnosis of myositis. It’s one of the leading causes of death in these patients, and they’re overwhelmingly diagnosed at a late stage, so we need standardized approaches to get early diagnosis,” he said in an interview at the annual meeting of the American College of Rheumatology.
Sharon Kolasinski, MD, of the University of Pennsylvania in Philadelphia, said in an interview that the guideline is a welcome development for rheumatologists. Dr. Kolasinski moderated the session where Dr. Oldroyd described the guideline, but she was not involved in its formulation.
“I think that we all have wondered for a very long time: What is the optimal cancer screening for myositis patients? We all worry that the onset of their diseases is associated with a coincident cancer, or that they will develop it soon,” she said.
Dr. Oldroyd emphasized that all patients with myositis have elevated risk for cancer compared with the general population and that the guideline categories of low, intermediate, and high are relative only to patients with IIM.
International consensus
The data on which the recommendations are based come from a systematic review and meta-analysis by Dr. Oldroyd and colleagues of 69 studies on cancer risk factors and 9 on IIM-specific cancer screening.
The authors of that paper found that the dermatomyositis subtype, older age, male sex, dysphagia, cutaneous ulceration and antitranscriptional intermediary factor-1 gamma (anti-TIF1-gamma) positivity were associated with significantly increased risk of cancer.
In contrast, polymyositis and clinically amyopathic dermatomyositis subtypes, Raynaud’s phenomenon, interstitial lung disease, very high serum creatine kinase or lactate dehydrogenase levels, and positivity for anti-Jo1 or anti-EJ antibodies were associated with significantly reduced risk of cancer.
The consensus recommendations were developed with anonymous contributions from 75 expert participants in 22 countries, with additional input from 3 patient partners.
Do this
The guideline lists 18 recommendations, of which 13 are strong and 5 are conditional.
An example of a strong recommendation is number 3, based on a moderate level of evidences:
“All adult IIM patients, irrespective of cancer risk, should continue to participate in country/region-specific age and sex appropriate cancer screening programs,” the guideline recommends.
Patients with verified inclusion body myositis or juvenile-onset IIM do not, however, require routine screening for myositis-associated cancer, the guideline says (recommendations 1 and 2).
There are also recommendations that all adults with new-onset IIM be tested for myositis-specific and myositis-associated autoantibodies to assist in stratifying patients by risk category.
The guideline divides screening recommendations into basic and enhanced. The basic screening should include a comprehensive history and physical exam, complete blood count, liver functions tests, erythrocyte sedimentation rates/plasma viscosity, serum protein electrophoresis, urinalysis, and chest x-ray.
Adults with IIM who are determined to be at low risk for IIM-related cancer should have basic cancer screening at the time of IIM diagnosis. Adults with intermediate risk should undergo both basic and enhanced screening at the time of IIM diagnosis, and those with high risk should undergo enhanced screening at the time of myositis diagnosis, with basic screening annually for 3 years, the recommendations say.
Consider doing this
Conditional recommendations (“clinicians should consider ...”) include the use of PET/CT for adults at high risk for cancer when an underlying cancer has not been detected at the time of IIM diagnosis. They also include a single screening test for anti-TIF1-gamma positive dermatomyositis patients whose disease onset was after age 40 and who have at least one additional risk factor.
Also conditionally recommended are upper and lower gastrointestinal endoscopy for patients at high risk when an underlying cancer is not found at the time of IIM diagnosis, nasoendoscopy in geographical regions with elevated risk for nasopharyngeal cancers, and screening for all IIM patients with red-flag symptoms or clinical features of cancer, including unexplained weight loss, family history of cancer, smoking, unexplained fever, or night sweats.
Guided steps
“I think clinicians have a lot of questions such as, ‘well, what should I do, when should I do it?’ These are important clinical questions, and we need guidance about this. We need to balance comprehensiveness with cost-effectiveness, and we need expert opinion about what steps we should take now and which should we take later,” Dr. Kolasinski said.
The guideline development process was supported by the University of Manchester, IMACS, National Institute for Health Research (United Kingdom), National Institutes of Health, National Health Service Northern Care Alliance, The Myositis Association, Myositis UK, University of Pittsburgh, Versus Arthritis, and the Center for Musculoskeletal Research. Dr. Oldroyd and Dr. Kolasinski reported having no relevant conflicts of interest.
Serum dupilumab levels do not predict clinical response
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
The finding that serum levels, according to a study published in JAMA Dermatology.
The study results mean that researchers should continue exploring potential AD drugs with novel mechanisms to help patients who fail type 2 inflammatory inhibition, experts told this news organization. The search for accurate augurs of clinical performance also must continue.
Addressing inadequate response
Quantifying nonresponse and incomplete response levels with dupilumab is difficult, said Jonathan I. Silverberg, MD, PhD, MPH, offering perspective on the study. “True nonresponse is probably less than 20%, but many other patients are inadequate responders even if they are having partial response.” Dr. Silverberg, professor of dermatology and director of clinical research, at George Washington University, Washington, was not an investigator.
Robert Sidbury, MD, MPH, added, “When a patient doesn’t respond to a medication that you expect they should, we always ask ourselves why.” Dermatologists have long assumed that, as with biologics for psoriasis, low blood levels were to blame for dupilumab nonresponse, said Dr. Sidbury, who is division chief of dermatology at Seattle Children’s Hospital and was not involved with the study. “This study showed that there was no correlation between response and blood levels.”
In the study, Lotte S. Spekhorst, MD, of National Expertise Center for Atopic Dermatitis, department of dermatology and allergology, University Medical Center Utrecht (the Netherlands) and coinvestigators prospectively followed 295 consecutive adult patients with moderate AD who were treated with dupilumab for 1 year. All patients received the same loading (600 mg) and biweekly (300 mg) doses.
The median dupilumab level at 16 weeks was 86.6 mcg/mL, which is higher than serum levels observed with other monoclonal antibodies used for other indications, such as psoriasis and inflammatory bowel disease, the authors noted. More importantly, researchers found no significant relationship between median week 16 dupilumab levels and 1-year clinical responses measured either discretely (Eczema Area and Severity Index [EASI] < 50, 50, 75, or 90; P = .18) or as quartiles (P = .06).
“It may be that response is dependent on target availability of the IL-4R-alpha, with an interpatient variability producing heterogeneity in response,” the authors wrote. But because serum dupilumab levels were relatively high, they said, all patients’ IL-4R-alpha “was likely fully saturated” at 16 weeks.
“This would explain why serum dupilumab levels were not related to effectiveness,” they noted, “although we cannot rule out differential effects in the tissue associated with heterogeneity in serum dupilumab levels.”
The study helps explain why some patients do not fully respond to dupilumab, said Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, who was not involved with the study.
“One hypothesis would be that drug serum levels differ due to metabolism or absorption reasons,” Dr. Simpson said in an interview. Results also suggest that heterogeneity in disease biology, such as other uninhibited cytokine pathways, might explain differences in clinical results. “Thus, more therapeutics that target different inflammatory pathways are needed to capture responses in patients not adequately responding to type 2 inflammatory blockade,” he said.
Assessing AEs
As with response levels, serum dupilumab levels at week 16 did not predict AEs including dupilumab-associated ocular surface disease (DAOSD), which impacted 46.4% of 216 patients who reported AEs. These findings also contradict what happens with biologics in other diseases such as psoriasis and inflammatory bowel disease, said Dr. Sidbury, wherein serum drug levels may predict both clinical response and side-effect risks.
A previous study showed that lowering dupilumab levels led to improvement in DAOSD. Authors of the current study therefore surmised that DAOSD development might be more associated with interpatient variability in IL-4R-alpha expression than with serum drug levels. “More research is necessary to confirm the hypothesis of interpatient variability of the IL-4Ra and the pharmacokinetics of dupilumab,” they concluded.
For now, said Dr. Sidbury, the study helps clinicians look beyond serum drug levels when patients respond inadequately to dupilumab. Moreover, added Dr. Silverberg, study results mean that physicians must find other ways to predict dupilumab response levels. “We need better predictors of clinical response – theranostic markers that we could test the patient to and understand how well they’re going to do,” he said.
Be it dupilumab or any other medication, he said, physicians lack even confirmatory biomarkers to reflect when a drug is working well. “Right now, we go with clinical assessments. But if it’s not drug levels, we have to figure out why some patients do markedly better than others.”
It was not unreasonable, Dr. Silverberg said, for the investigators to seek a biomarker in blood rather than tissue. “But in this disease, we believe that the more important place to look for biomarkers and drug levels would be in the skin itself. So we are still left with the issue” that drug levels in tissue might reflect response when serum levels do not.
The study was supported by grants from AbbVie, Eli Lilly, Leo Pharma, Pfizer, and Sanofi. Study patients participated in the BioDay Registry, which is sponsored by Sanofi, Regeneron, AbbVie, Eli Lilly, LEO Pharma, and Pfizer; the sponsors had no role in the study design and conduct. Dr. Spekhorst discloses receiving speaking fees from Abbvie outside the work; disclosures of other authors included receiving advisory, speaking consulting, and/or investigator fees from Sanofi Genzyme during the study. Several authors had no disclosures.
Dr. Simpson has been an investigator and consultant for Regeneron and Sanofi, makers of dupilumab. Dr. Silverberg has been an investigator, consultant, and speaker for Regeneron and Sanofi. Dr. Sidbury has been a clinical investigator for all dupilumab pediatric trials. (His institution has a contract with Regeneron and Sanofi, but he receives no money from the arrangement.)
FROM JAMA DERMATOLOGY
Monkeypox in children appears rare and relatively mild
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox virus infections in children and adolescents in the United States are rare, and young patients with known infections have all recovered, according to a study from the Centers for Disease Control and Prevention.
In addition, evidence suggests that secondary transmission in schools or childcare facilities may be unlikely.
The study was the first comprehensive study on the impact of monkeypox on children during the 2022 outbreak, according to a statement emailed to this news organization from the California Department of Public Health, one of the state health departments that partnered with the CDC to share information.
News of low infection rates and relatively mild disease was welcome to clinicians, who had braced for severe findings on the basis of sparse prior data, according to Peter Chin-Hong, MD, a professor of medicine and an infectious diseases physician at the University of California, San Francisco.
“We were on heightened alert that kids may do poorly,” said Dr. Chin-Hong, who was not involved in the study but who cared for monkeypox patients during the outbreak. “I think this study is reassuring.
“The other silver lining about it is that most of the kids got infected in the household setting from ways that you would expect them to get [infected],” Dr. Chin-Hong said in an interview.
However, Black and Hispanic children were more likely to contract the disease, underscoring troubling inequities.
“Early on, individuals of color were much less likely to be able to successfully access vaccination,” said first author Ian Hennessee, PhD, MPH, an epidemic intelligence service officer with the CDC and a member of the Special Case Investigation Unit of the Multinational Monkeypox Response Team at the CDC. “We think those kinds of structural inequities really trickled down towards the children and adolescents that have been affected by this outbreak.”
The study was published in Morbidity and Mortality Weekly Report.
A nationwide look at the data
The researchers discussed 83 children and adolescents with monkeypox who came to the CDC’s attention between May 17 and Sept. 24, 2022.
The 83 cases represent 0.3% of the 25,038 reported monkeypox cases in the United States over that period. Of the 28 children aged 12 years or younger, 18 (64%) were boys. Sixteen children were younger than 4 years.
Exposure data were available for 20 (71%) of those aged 0-12. In that group, 19 were exposed at home; 17 cases were due to routine skin-to-skin contact with a household caregiver; and one case was suspected to be caused by fomites (such as a shared towel). Exposure information was unavailable for the remaining case.
Most of the children experienced lesions on the trunk. No lesions were anogenital. Two patients in the youngest age group were hospitalized because of widespread rash that involved the eyelids, and a patient in the 5- to 12-year-old group was hospitalized because of periorbital cellulitis and conjunctivitis.
Among those aged 13-17, there were 55 cases. Of these patients, 89% were boys. Exposure data were available for 35 (64%). In 32 of these patients, the infection occurred from presumed sexual contact. Twenty-three of those adolescents reported male-to-male sexual contact. No case was found to be connected with sexual abuse.
Lesions in the adolescents were mostly truncal or anogenital. Six in this group were hospitalized, and all of them recovered. One adolescent was found to be HIV positive.
Black and Hispanic children accounted for 47% and 35% of all cases, respectively.
Eleven percent of all the children and adolescents were hospitalized, and none received intensive care.
Treatments, when given, included the antiviral drug tecovirimat, intravenous vaccinia immune globulin, and topical trifluridine. There were no deaths.
Ten symptomatic patients attended school or daycare. Among these patients, no secondary transmissions were found to have occurred. Some contacts were offered the JYNNEOS monkeypox vaccine as postexposure prophylaxis.
Limitations of the study included potentially overlooked cases. Data were collected through routine surveillance, children frequently experience rashes, and access to testing has been a challenge, Dr. Hennessee explained.
In addition, data on exposure characteristics were missing for some children.
Inequities and the risks of being judged
The outbreak in the United States has eased in recent months. However, though uncommon in children, monkeypox has affected some racial groups disproportionately.
“Especially in the later course of the outbreak, the majority of cases were among Black and Hispanic individuals,” said co-author Rachel E. Harold, MD, an infectious diseases specialist and supervisory medical officer with the District of Columbia Department of Health’s HIV/AIDS, Hepatitis, STDs, and TB Administration.
“Unfortunately, the pediatric cases do reflect the outbreak overall,” she told this news organization.
Dr. Harold noted there have been efforts in D.C. and other jurisdictions, as well as by the White House monkeypox response team, to reach populations at greatest risk and that they were “really trying to make vaccine available to people of color.”
Vaccination clinics often popped up in unexpected locations at short notice, and that made it hard for some people to get to them, Dr. Chin-Hong pointed out.
Another factor was “the public aspect of accessing diagnostics and vaccines and the way that that’s linked to potential judgment or sexual risk,” he added.
“Not everybody’s out,” Dr. Chin-Hong said, referring to members of the LGBTQ community. “In many communities of color, going to get a test or going to get a vaccine essentially means that you’re out.”
For clinicians who suspect monkeypox in a child, Dr. Harold suggests keeping a broad differential diagnosis, looking for an epidemiologic link, and contacting the CDC for assistance. Infected children should be encouraged to avoid touching their own eyes or mucous membranes, she added.
In addition, she said, tecovirimat is a reasonable treatment and is well tolerated by pediatric monkeypox patients with eczema, an underlying condition that could lead to severe disease.
For infected caregivers, Dr. Hennessee said, measures to prevent infecting children at home include isolation, contact precautions, and in some cases, postexposure prophylaxis via vaccination.
For sexually active adolescents, he advised that clinicians offer vaccination, education on sexual health, and testing for HIV and other sexually transmitted infections.
“It’s important to remember that adolescents may be sexually active, and clinicians should do a thorough and nonjudgmental sexual history,” Dr. Harold added. “That is always true, but especially if there is concern for [monkeypox].”
Dr. Hennessee, Dr. Chin-Hong, and Dr. Harold have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
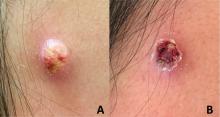
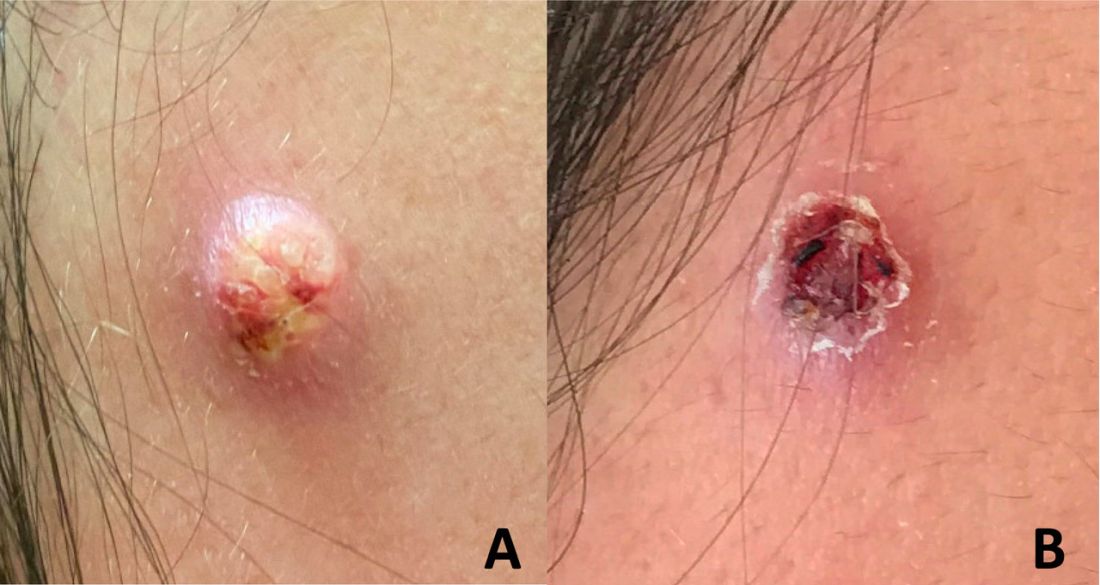
An adolescent male presents with an eroded bump on the temple
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
The correct answer is (D), molluscum contagiosum. Upon surgical excision, the pathology indicated the lesion was consistent with molluscum contagiosum.
Molluscum contagiosum is a benign skin disorder caused by a pox virus and is frequently seen in children. This disease is transmitted primarily through direct skin contact with an infected individual.1 Contaminated fomites have been suggested as another source of infection.2 The typical lesion appears dome-shaped, round, and pinkish-purple in color.1 The incubation period ranges from 2 weeks to 6 months and is typically self-limited in immunocompetent hosts; however, in immunocompromised persons, molluscum contagiosum lesions may present atypically such that they are larger in size and/or resemble malignancies, such as basal cell carcinoma or keratoacanthoma (for single lesions), or other infectious diseases, such as cryptococcosis and histoplasmosis (for more numerous lesions).3,4 A giant atypical molluscum contagiosum is rarely seen in healthy individuals.
What’s on the differential?
The recent episode of bleeding raises concern for other neoplastic processes of the skin including squamous cell carcinoma or basal cell carcinoma as well as cutaneous metastatic rhabdoid tumor, given the patient’s history.
Eruptive keratoacanthomas are also reported in patients taking nivolumab, an anti-PD-1 immunotherapy, which the patient has received for treatment of his recurrent metastatic rhabdoid tumor.5 More common entities such as a pyogenic granuloma or verruca are also included on the differential. The initial presentation of the lesion, however, is more consistent with the pearly umbilicated papules associated with molluscum contagiosum.
Comments from Dr. Eichenfield
This is a very hard diagnosis to make with the clinical findings and history.
Molluscum contagiosum infections are common, but with this patient’s medical history, biopsy and excision with pathologic examination was an appropriate approach to make a certain diagnosis.
Ms. Moyal is a research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
References
1. Brown J et al. Int J Dermatol. 2006 Feb;45(2):93-9.
2. Hanson D and Diven DG. Dermatol Online J. 2003 Mar;9(2).
3. Badri T and Gandhi GR. Molluscum contagiosum. 2022. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing.
4. Schwartz JJ and Myskowski PL. J Am Acad Dermatol. 1992 Oct 1;27(4):583-8.
5. Antonov NK et al. JAAD Case Rep. 2019 Apr 5;5(4):342-5.
Breaking the itch-scratch cycle with mindfulness
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
FROM IDS 2022
ICD-10 code can identify patients with melasma for future study
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
FROM JAMA DERMATOLOGY
In patients with untreated AIDS, monkeypox can be life-threatening
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Monkeypox, though often mild, may be severe and even fatal in immunocompromised individuals, particularly those with untreated AIDS, according to a Centers for Disease Control and Prevention study in Morbidity and Mortality Weekly Report.
The study described a group of patients recently treated for severe monkeypox. The majority were Black, HIV positive, and not receiving treatment. Many were also facing homelessness.
The authors urged HIV testing for all sexually active individuals with suspected monkeypox. Early or prolonged monkeypox treatment may be necessary, they concluded.
Coauthor John T. Brooks, MD, called the study “a real call to action.”
“If we want to reduce cases of severe monkeypox, we need to reduce the number of persons with HIV who are undiagnosed and not treated,” said Dr. Brooks, a medical epidemiologist who is chief medical officer of CDC›s multinational monkeypox response. Dr. Brooks also leads the epidemiology research team in CDC’s division of HIV/AIDS prevention.
noted Richard Silvera, MD, MPH, CPH, who is associate program director of the infectious diseases fellowship and assistant professor of medicine (infectious diseases) at the Icahn School of Medicine at Mount Sinai, New York. He was not involved with the study.
“These patients really have not been served by the health care system,” Dr. Silvera said. “Monkeypox is just really taking advantage of that.”
How severe monkeypox can manifest
The authors reported on 57 adults hospitalized with severe monkeypox between Aug. 10 and Sept. 10, 2022, for whose care the providers sought CDC consultation.
The vast majority (95%) were men, their median age was 34 years, and 68% were Black. Nearly one in four were homeless (23%).
Overall, 47 (82%) were HIV positive, of whom just 4 had been receiving antiretroviral therapy (ART). Of 43 for whom CD4 counts were known, 71% had fewer than 50 CD4 cells/mm3.
Clinical signs included severe skin lesions in all patients and severe mucosal lesions in 68%. Other affected organ systems included lungs (21%), eyes (21%), and central nervous system (7%).
Treatments included oral or intravenous tecovirimat (93% and 65%, respectively), vaccinia immune globulin intravenous (VIGIV, 51%), and cidofovir (23%).
Nearly 1 in 3 patients (30%) received care in an ICU; 12 died (21%). Monkeypox was considered the cause or a contributing factor in five of the deaths and not a factor in one death; the remaining six deaths are under investigation.
Case studies
The report included details of three representative cases of the CDC consultations.
One was a Hispanic man in his 20s with a fever of 102.8° F, a rash including eschars, oral lesions, neck mass, and cervical lymphadenopathy. He had tested positive for monkeypox as an outpatient and upon admission was found to be HIV positive, with a CD4 count of 79 cells/mm3. He experienced a severe and ultimately fatal clinical course that included intubation, refractory hypotension, seizures, renal failure, and cardiac arrest. An autopsy revealed diffuse organ necrosis plus orthopoxvirus and cytomegalovirus.
The second was a Black man in his 30s with untreated AIDS and diffuse rash. He was tested and treated for gonorrhea, chlamydia, and syphilis before phimosis and urinary retention led to admission and a monkeypox diagnosis 4 weeks after his rash began. He was discharged with oral tecovirimat, but his skin lesions developed necrosis and he was readmitted twice, each time with new lesions. His clinical course included methicillin-resistant Staphylococcus aureus bacteremia, atrial fibrillation, eye and ear involvement, a suprapubic catheter, and progressive necrosis of his lesions. As of the CDC report, he was receiving ART and intravenous tecovirimat.
The third patient, a White man in his 40s with untreated AIDS, presented with diffuse rash. He was promptly diagnosed with monkeypox and admitted for pain control. He was discharged with oral tecovirimat and ART, but homelessness and food insecurity jeopardized the absorption of his tecovirimat (which depends on a full fatty meal), and the lesions worsened. Despite readmission and aggressive medical treatment, the patient required finger debridement and a toe amputation. After discharge, he was again readmitted for lesions and pain and, at report publication, remained hospitalized, taking oral tecovirimat and ART.
The patients in the study may not be typical of severe monkeypox cases, wrote the authors reported. Deaths after the study period were not counted.
Fewer cases, some severe
As of Nov. 7, the CDC has confirmed 28,709 monkeypox cases. These have trended downward since August. Most people with recent diagnoses are men who are gay, bisexual, same gender loving, or who have sex with men, and most are Black, according to Brooks.
Dr. Brooks urges clinicians to report suspected monkeypox cases – especially severe ones – to their health departments.
“We don’t have a good bead on exactly how many severe cases there are in the States because of complexities in our surveillance systems,” Dr. Brooks said.
For patients with suspected or confirmed monkeypox, Brooks recommends testing for sexually transmitted infections, including HIV if status is unknown. Patients with HIV should receive prompt ART. For those at risk for severe disease, the authors recommend early treatment for suspected monkeypox, even before results are back. Some patients may benefit from tecovirimat courses lasting beyond 14 days, plus additional antivirals (cidofovir or brincidofovir) and/or VIGIV.
“With severe cases, clinicians may want to consider the value of more than one drug to attack the virus at different stages of its replication cycle,” Dr. Brooks said.
Inequities matter
The authors called on providers to engage communities burdened by HIV and to ensure access to not only monkeypox vaccination, diagnosis, and treatment but also sustained HIV care.
Dr. Silvera added that providers need to tailor care plans to patients’ social determinants of health. For example, he explained, inpatient care for monkeypox could be appropriate for some patients facing homelessness and food insecurity – even if they are able to take tecovirimat orally.
He recommends tapping others’ expertise: “Our social work colleagues are well versed in this.”
“I don’t think these clinicians failed these patients. ... I think everyone made all the right choices medically,” Dr. Silvera added. “I think that the system failed these patients – and we as clinicians are part of those systems. So we also have the power to change those systems. And I think we just need to start opening our eyes to that and [start] to work together towards that goal to take better care of our patients.”
Dr. Brooks reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Study finds high rate of psychiatric burden in cosmetic dermatology patients
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
results from a large retrospective analysis showed.
“As the rate of cosmetic procedures continues to increase, it is crucial that physicians understand that many patients with a psychiatric disorder require clear communication and appropriate consultation visits,” lead study author Patricia Richey, MD, told this news organization.
While studies have displayed links between the desire for a cosmetic procedure and psychiatric stressors and disorders – most commonly mood disorders, personality disorders, body dysmorphic disorder, and addiction-like behavior – the scarce literature on the subject mostly comes from the realm of plastic surgery.
“The relationship between psychiatric disease and the motivation for dermatologic cosmetic procedures has never been fully elucidated,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “A possible association between psychiatric disorder and the motivation for cosmetic procedures is critical to understand given increasing procedure rates and the need for clear communication and appropriate consultation visits with these patients.”
For the retrospective cohort study, which was published online in the Journal of the American Academy of Dermatology, Dr. Richey; Mathew Avram, MD, JD, director of the Dermatology Laser and Cosmetic Center at MGH; and Ryan W. Chapin, PharmD, of Beth Israel Deaconess Medical Center, Boston, reviewed the medical records of 1,000 patients from a cosmetic dermatology clinic and 1,000 patients from a medical dermatology clinic, both at MGH. Those who crossed over between the two clinics were excluded from the analysis.
Patients in the cosmetic group were significantly younger than those in the medical group (a mean of 48 vs. 56 years, respectively; P < .0001), and there was a higher percentage of women than men in both groups (78.5% vs. 21.5% in the cosmetic group and 61.4% vs. 38.6% in the medical group; P < .00001).
The researchers found that 49% of patients in the cosmetic group had been diagnosed with at least one psychiatric disorder, compared with 33% in the medical group (P < .00001), most commonly anxiety, depression, ADHD, and insomnia. In addition, 39 patients in the cosmetic group had 2 or more psychiatric disorders, compared with 22 of those in the medical group.
Similarly, 44% of patients in the cosmetic group were on a psychiatric medication, compared with 28% in the medical group (P < .00001). The average number of medications among those on more than one psychiatric medication was 1.67 among those in the cosmetic dermatology group versus 1.48 among those in the medical dermatology group (P = .020).
By drug class, a higher percentage of patients in the cosmetic group, compared with those in the medical group, were taking antidepressants (33% vs. 21%, respectively; P < .00001), anxiolytics (26% vs. 13%; P < .00001), mood stabilizers (2.80% vs. 1.10%; P = .006), and stimulants (15.2% vs. 7.20%; P < .00001). The proportion of those taking antipsychotics was essentially even in the two groups (2.50% vs. 2.70%; P = .779).
Dr. Richey and colleagues also observed that patients in the cosmetic group had significantly higher rates of obsessive-compulsive disorder (OCD) and ADHD than those in the medical group. “This finding did not particularly surprise me,” she said, since she and her colleagues recently published a study on the association of stimulant use with psychocutaneous disease.
“Stimulants are used to treat ADHD and are also known to trigger OCD-like symptoms,” she said. “I was surprised that no patients had been diagnosed with body dysmorphic disorder, but we know that with increased patient access to medical records, physicians are often cautious in their documentation.”
She added that the overall results of the new study underscore the importance of consultation visits with cosmetic patients, including obtaining a full medication list and accurate medical history, if possible. “One could also consider well-studied screening tools mostly from the mood disorder realm, such as the Patient Health Questionnaire–2,” Dr. Richey said. “Much can be gained from simply talking to the patient and trying to understand him/her and underlying motivations prior to performing a procedure.”
Evan Rieder, MD, a New York City–based dermatologist and psychiatrist who was asked to comment on the study, characterized the analysis as demonstrating what medical and cosmetic dermatologists have been seeing in their practices for years. “While this study is limited by its single-center retrospective nature in an academic center that may not be representative of the general population, it does demonstrate a high burden of psychopathology and psychopharmacologic treatments in aesthetic patients,” Dr. Rieder said in an interview.
“While psychiatric illness is not a contraindication to cosmetic treatment, a high percentage of patients with ADHD, OCD, and likely [body dysmorphic disorder] in cosmetic dermatology practices should give us pause.” The nature of these diseases may indicate that some people are seeking aesthetic treatments for reasons yet to be elucidated, he added.
“It certainly indicates that dermatologists should be equipped to screen for, identify, and provide such patients with the appropriate resources for psychological treatment, regardless if they are deemed appropriate candidates for cosmetic intervention,” he said.
In an interview, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, noted that previous studies have demonstrated the interplay between mood disorders and dermatologic conditions for years, namely in acne, atopic dermatitis, psoriasis, and immune mediated disorders.
“In these conditions, the psychiatric stressors can worsen the skin condition and impede treatment,” Dr. Sodha said. “This study is an important segue into further elucidating our cosmetic patient population, and we should try to ask the next important question: how do we as physicians build a better rapport with these patients, understand their motivations for care, and effectively guide the patient through the consultation process to realistically address their concerns? It might help us both.”
Neither the researchers nor Dr. Sodha reported having financial disclosures. Dr. Rieder disclosed that he is a consultant for Allergan, Almirall, Bristol-Myers Squibb, Dr. Brandt, L’Oreal, Procter & Gamble, and Unilever.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Have you heard the one about the emergency dept. that called 911?
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.
Who watches the ED staff?
We heard a really great joke recently, one we simply have to share.
A man in Seattle went to a therapist. “I’m depressed,” he says. “Depressed, overworked, and lonely.”
“Oh dear, that sounds quite serious,” the therapist replies. “Tell me all about it.”
“Life just seems so harsh and cruel,” the man explains. “The pandemic has caused 300,000 health care workers across the country to leave the industry.”
“Such as the doctor typically filling this role in the joke,” the therapist, who is not licensed to prescribe medicine, nods.
“Exactly! And with so many respiratory viruses circulating and COVID still hanging around, emergency departments all over the country are facing massive backups. People are waiting outside the hospital for hours, hoping a bed will open up. Things got so bad at a hospital near Seattle in October that a nurse called 911 on her own ED. Told the 911 operator to send the fire department to help out, since they were ‘drowning’ and ‘in dire straits.’ They had 45 patients waiting and only five nurses to take care of them.”
“That is quite serious,” the therapist says, scribbling down unseen notes.
“The fire chief did send a crew out, and they cleaned rooms, changed beds, and took vitals for 90 minutes until the crisis passed,” the man says. “But it’s only a matter of time before it happens again. The hospital president said they have 300 open positions, and literally no one has applied to work in the emergency department. Not one person.”
“And how does all this make you feel?” the therapist asks.
“I feel all alone,” the man says. “This world feels so threatening, like no one cares, and I have no idea what will come next. It’s so vague and uncertain.”
“Ah, I think I have a solution for you,” the therapist says. “Go to the emergency department at St. Michael Medical Center in Silverdale, near Seattle. They’ll get your bad mood all settled, and they’ll prescribe you the medicine you need to relax.”
The man bursts into tears. “You don’t understand,” he says. “I am the emergency department at St. Michael Medical Center.”
Good joke. Everybody laugh. Roll on snare drum. Curtains.
Myth buster: Supplements for cholesterol lowering
When it comes to that nasty low-density lipoprotein cholesterol, some people swear by supplements over statins as a holistic approach. Well, we’re busting the myth that those heart-healthy supplements are even effective in comparison.
Which supplements are we talking about? These six are always on sale at the pharmacy: fish oil, cinnamon, garlic, turmeric, plant sterols, and red yeast rice.
In a study presented at the recent American Heart Association scientific sessions, researchers compared these supplements’ effectiveness in lowering LDL cholesterol with low-dose rosuvastatin or placebo among 199 adults aged 40-75 years who didn’t have a personal history of cardiovascular disease.
Participants who took the statin for 28 days had an average of 24% decrease in total cholesterol and a 38% reduction in LDL cholesterol, while 28 days’ worth of the supplements did no better than the placebo in either measure. Compared with placebo, the plant sterols supplement notably lowered HDL cholesterol and the garlic supplement notably increased LDL cholesterol.
Even though there are other studies showing the validity of plant sterols and red yeast rice to lower LDL cholesterol, author Luke J. Laffin, MD, of the Cleveland Clinic noted that this study shows how supplement results can vary and that more research is needed to see the effect they truly have on cholesterol over time.
So, should you stop taking or recommending supplements for heart health or healthy cholesterol levels? Well, we’re not going to come to your house and raid your medicine cabinet, but the authors of this study are definitely not saying that you should rely on them.
Consider this myth mostly busted.
COVID dept. of unintended consequences, part 2
The surveillance testing programs conducted in the first year of the pandemic were, in theory, meant to keep everyone safer. Someone, apparently, forgot to explain that to the students of the University of Wyoming and the University of Idaho.
We’re all familiar with the drill: Students at the two schools had to undergo frequent COVID screening to keep the virus from spreading, thereby making everyone safer. Duck your head now, because here comes the unintended consequence.
The students who didn’t get COVID eventually, and perhaps not so surprisingly, “perceived that the mandatory testing policy decreased their risk of contracting COVID-19, and … this perception led to higher participation in COVID-risky events,” Chian Jones Ritten, PhD, and associates said in PNAS Nexus.
They surveyed 757 students from the Univ. of Washington and 517 from the Univ. of Idaho and found that those who were tested more frequently perceived that they were less likely to contract the virus. Those respondents also more frequently attended indoor gatherings, both small and large, and spent more time in restaurants and bars.
The investigators did not mince words: “From a public health standpoint, such behavior is problematic.”
Current parents/participants in the workforce might have other ideas about an appropriate response to COVID.
At this point, we probably should mention that appropriation is the second-most sincere form of flattery.