User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Sick call
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
They call me and I go.
– William Carlos Williams
I never get sick. I’ve never had the flu. When everyone’s got a cold, I’m somehow immune. The last time I threw up was June 29th, 1980. You see, I work out almost daily, eat vegan, and sleep plenty. I drink gallons of pressed juice and throw down a few high-quality supplements. Yes, I’m that guy: The one who never gets sick. Well, I was anyway.
I am no longer that guy since our little girl became a supersocial little toddler. My undefeated welterweight “never-sick” title has been obliterated by multiple knockouts. One was a wicked adenovirus that broke the no-vomit streak. At one point, I lay on the luxury gray tile bathroom floor hoping to go unconscious to make the nausea stop. I actually called out sick that day. Then with a nasty COVID-despite-vaccine infection. I called out again. Later with a hacking lower respiratory – RSV?! – bug. Called out. All of which our 2-year-old blonde, curly-haired vector transmitted to me with remarkable efficiency.
In fact, That’s saying a lot. Our docs, like most, don’t call out sick.
We physicians have legendary stamina. Compared with other professionals, we are no less likely to become ill but a whopping 80% less likely to call out sick.
Presenteeism is our physician version of Omerta, a code of honor to never give in even at the expense of our, or our family’s, health and well-being. Every medical student is regaled with stories of physicians getting an IV before rounds or finishing clinic after their water broke. Why? In part it’s an indoctrination into this thing of ours we call Medicine: An elitist club that admits only those able to pass O-chem and hold diarrhea. But it is also because our medical system is so brittle that the slightest bend causes it to shatter. When I cancel a clinic, patients who have waited weeks for their spot have to be sent home. And for critical cases or those patients who don’t get the message, my already slammed colleagues have to cram the unlucky ones in between already-scheduled appointments. The guilt induced by inconveniencing our colleagues and our patients is more potent than dry heaves. And so we go. Suck it up. Sip ginger ale. Load up on acetaminophen. Carry on. This harms not only us, but also patients whom we put in the path of transmission. We become terrible 2-year-olds.
Of course, it’s not always easy to tell if you’re sick enough to stay home. But the stigma of calling out is so great that we often show up no matter what symptoms. A recent Medscape survey of physicians found that 85% said they had come to work sick in 2022.
We can do better. Perhaps creating sick-leave protocols could help? For example, if you have a fever above 100.4, have contact with someone positive for influenza, are unable to take POs, etc. then stay home. So might building rolling slack into schedules to accommodate the inevitable physician illness, parenting emergency, or death of an beloved uncle. And if there is one thing artificial intelligence could help us with, it would be smart scheduling. Can’t we build algorithms for anticipating and absorbing these predictable events? I’d take that over an AI skin cancer detector any day. Yet this year we’ll struggle through the cold and flu (and COVID) season again and nothing will have changed.
Our daughter hasn’t had hand, foot, and mouth disease yet. It’s not a question of if, but rather when she, and her mom and I, will get it. I hope it happens on a Friday so that my Monday clinic will be bearable when I show up.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Higher metal contact allergy rates found in metalworkers
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Give bacterial diversity a chance: The antibiotic dichotomy
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
What’s the opposite of an antibiotic?
Everyone knows that LOTME loves a good dichotomy: yin/yang, good/evil, heads/tails, particle/wave, peanut butter/jelly. They’re all great. We’re also big fans of microbiomes, particularly the gut microbiome. But what if we could combine the two? A healthy and nutritious story about the gut microbiome, with a dash of added dichotomy for flavor. Is such a thing even possible? Let’s find out.
First, we need an antibiotic, a drug designed to fight bacterial infections. If you’ve got strep throat, otitis media, or bubonic plague, there’s a good chance you will receive an antibiotic. That antibiotic will kill the bad bacteria that are making you sick, but it will also kill a lot of the good bacteria that inhabit your gut microbiome, which results in side effects like bloating and diarrhea.
It comes down to diversity, explained Elisa Marroquin, PhD, of Texas Christian University (Go Horned Frogs!): “In a human community, we need people that have different professions because we don’t all know how to do every single job. And so the same happens with bacteria. We need lots of different gut bacteria that know how to do different things.”
She and her colleagues reviewed 29 studies published over the last 7 years and found a way to preserve the diversity of a human gut microbiome that’s dealing with an antibiotic. Their solution? Prescribe a probiotic.
The way to fight the effects of stopping a bacterial infection is to provide food for what are, basically, other bacterial infections. Antibiotic/probiotic is a prescription for dichotomy, and it means we managed to combine gut microbiomes with a dichotomy. And you didn’t think we could do it.
The earphone of hearing aids
It’s estimated that up to 75% of people who need hearing aids don’t wear them. Why? Well, there’s the social stigma about not wanting to appear too old, and then there’s the cost factor.
Is there a cheaper, less stigmatizing option to amplify hearing? The answer, according to otolaryngologist Yen-fu Cheng, MD, of Taipei Veterans General Hospital and associates, is wireless earphones. AirPods, if you want to be brand specific.
Airpods can be on the more expensive side – running about $129 for AirPods 2 and $249 for AirPods Pro – but when compared with premium hearing aids ($10,000), or even basic aids (about $1,500), the Apple products come off inexpensive after all.
The team tested the premium and basic hearing aids against the AirPods 2 and the AirPod Pro using Apple’s Live Listen feature, which helps amplify sound through the company’s wireless earphones and iPhones and was initially designed to assist people with normal hearing in situations such as birdwatching.
The AirPods Pro worked just as well as the basic hearing aid but not quite as well as the premium hearing aid in a quiet setting, while the AirPods 2 performed the worst. When tested in a noisy setting, the AirPods Pro was pretty comparable to the premium hearing aid, as long as the noise came from a lateral direction. Neither of the AirPod models did as well as the hearing aids with head-on noises.
Wireless earbuds may not be the perfect solution from a clinical standpoint, but they’re a good start for people who don’t have access to hearing aids, Dr. Cheng noted.
So who says headphones damage your hearing? They might actually help.
Now I lay me down to sleep, I pray the computer my soul to keep
Radiation is the boring hazard of space travel. No one dies in a space horror movie because they’ve been slowly exposed to too much cosmic radiation. It’s always “thrown out the airlock” this and “eaten by a xenomorph” that.
Radiation, however, is not something that can be ignored, but it turns out that a potential solution is another science fiction staple: artificial hibernation. Generally in sci-fi, hibernation is a plot convenience to get people from point A to point B in a ship that doesn’t break the laws of physics. Here on Earth, though, it is well known that animals naturally entering a state of torpor during hibernation gain significant resistance to radiation.
The problem, of course, is that humans don’t hibernate, and no matter how hard people who work 100-hour weeks for Elon Musk try, sleeping for months on end is simply something we can’t do. However, a new study shows that it’s possible to induce this torpor state in animals that don’t naturally hibernate. By injecting rats with adenosine 5’-monophosphate monohydrate and keeping them in a room held at 16° C, an international team of scientists successfully induced a synthetic torpor state.
That’s not all they did: The scientists also exposed the hibernating rats to a large dose of radiation approximating that found in deep space. Which isn’t something we’d like to explain to our significant other when we got home from work. “So how was your day?” “Oh, I irradiated a bunch of sleeping rats. … Don’t worry they’re fine!” Which they were. Thanks to the hypoxic and hypothermic state, the tissue was spared damage from the high-energy ion radiation.
Obviously, there’s a big difference between a rat and a human and a lot of work to be done, but the study does show that artificial hibernation is possible. Perhaps one day we’ll be able to fall asleep and wake up light-years away under an alien sky, and we won’t be horrifically mutated or riddled with cancer. If, however, you find yourself in hibernation on your way to Jupiter (or Saturn) to investigate a mysterious black monolith, we suggest sleeping with one eye open and gripping your pillow tight.
Is there a doctor on the plane? Tips for providing in-flight assistance
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.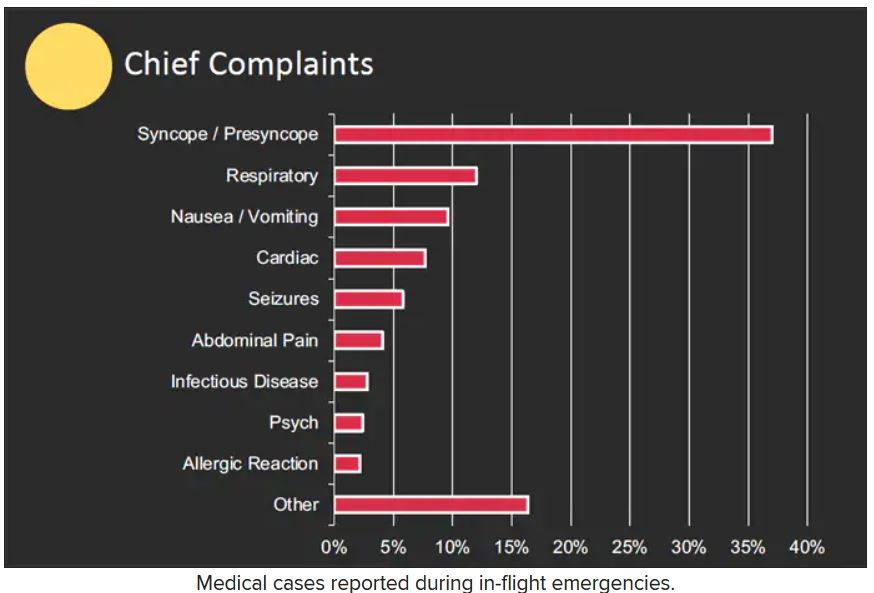
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In most cases, passengers on an airline flight are representative of the general population, which means that anyone could have an emergency at any time.
as determined on the basis of in-flight medical emergencies that resulted in calls to a physician-directed medical communications center, said Amy Faith Ho, MD, MPH of Integrative Emergency Services, Dallas–Fort Worth, in a presentation at the annual meeting of the American College of Emergency Physicians.
The study authors reviewed records of 11,920 in-flight medical emergencies between Jan. 1, 2008, and Oct. 31, 2010. The data showed that physician passengers provided medical assistance in nearly half of in-flight emergencies (48.1%) and that flights were diverted because of the emergency in 7.3% of cases.
The majority of the in-flight emergencies involved syncope or presyncope (37.4% of cases), followed by respiratory symptoms (12.1%) and nausea or vomiting (9.5%), according to the study.
When a physician is faced with an in-flight emergency, the medical team includes the physician himself, medical ground control, and the flight attendants, said Dr. Ho. Requirements may vary among airlines, but all flight attendants will be trained in cardiopulmonary resuscitation (CPR) or basic life support, as well as use of automated external defibrillators (AEDs).
Physician call centers (medical ground control) can provide additional assistance remotely, she said.
The in-flight medical bag
Tools in a physician’s in-flight toolbox start with the first-aid kit. Airplanes also have an emergency medical kit (EMK), an oxygen tank, and an AED.
The minimum EMK contents are mandated by the Federal Aviation Administration, said Dr. Ho. The standard equipment includes a stethoscope, a sphygmomanometer, and three sizes of oropharyngeal airways. Other items include self-inflating manual resuscitation devices and CPR masks in thee sizes, alcohol sponges, gloves, adhesive tape, scissors, a tourniquet, as well as saline solution, needles, syringes, and an intravenous administration set consisting of tubing and two Y connectors.
An EMK also should contain the following medications: nonnarcotic analgesic tablets, antihistamine tablets, an injectable antihistamine, atropine, aspirin tablets, a bronchodilator, and epinephrine (both 1:1000; 1 injectable cc and 1:10,000; two injectable cc). Nitroglycerin tablets and 5 cc of 20 mg/mL injectable cardiac lidocaine are part of the mandated kit as well, according to Dr. Ho.
Some airlines carry additional supplies on all their flights, said Dr. Ho. Notably, American Airlines and British Airways carry EpiPens for adults and children, as well as opioid reversal medication (naloxone) and glucose for managing low blood sugar. American Airlines and Delta stock antiemetics, and Delta also carries naloxone. British Airways is unique in stocking additional cardiac medications, both oral and injectable.
How to handle an in-flight emergency
Physicians should always carry a copy of their medical license when traveling for documentation by the airline if they assist in a medical emergency during a flight, Dr. Ho emphasized. “Staff” personnel should be used. These include the flight attendants, medical ground control, and other passengers who might have useful skills, such as nursing, the ability to perform CPR, or therapy/counseling to calm a frightened patient. If needed, “crowdsource additional supplies from passengers,” such as a glucometer or pulse oximeter.
Legal lessons
Physicians are not obligated to assist during an in-flight medical emergency, said Dr. Ho. Legal jurisdiction can vary. In the United States, a bystander who assists in an emergency is generally protected by Good Samaritan laws; for international airlines, the laws may vary; those where the airline is based usually apply.
The Aviation Medical Assistance Act, passed in 1998, protects individuals from being sued for negligence while providing medical assistance, “unless the individual, while rendering such assistance, is guilty of gross negligence of willful misconduct,” Dr. Ho noted. The Aviation Medical Assistance Act also protects the airline itself “if the carrier in good faith believes that the passenger is a medically qualified individual.”
Dr. Ho disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Microtox and Mesotox
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
The terms when they mention one of these terms.
Let’s settle the nomenclature confusion. In this column, I define and outline suggested terminology based on studies and my 15 years of experience using neuromodulators. If any readers or colleagues disagree, please write to me and we can discuss the alternatives in a subsequent article; if you agree, please also write to me so we can collaboratively correct the discrepancies in the literature accordingly.
The term mesotherapy, originating from the Greek “mesos” referring to the early embryonic mesoderm, was identified in the 1950’s by Dr. Michel Pistor, a French physician who administered drugs intradermally. The term was defined as a minimally invasive technique by which drugs or bioactive substances are given in small quantities through dermal micropunctures. Drugs administered intradermally diffuse very slowly and therefore, stay in the tissue longer than those administered intramuscularly.
Thus, Mesotox is defined not by the concentration of the neuromodulator or location, but by the depth of injection in the superficial dermis. It can be delivered through individual injections or through a microneedling pen.
Microtox refers to the dilution of the neuromodulator at concentrations below the proposed dilution guidelines of the manufacturer: Less than 2.5 U per 0.1 mL for onabotulinumtoxinA (OBA), incobotulinumtoxinA (IBA), and prabotulinumtoxinA (PBA); and less than 10 U per 0.1 mL for abobotulinumtoxinA (ABO), This method allows for the injection of superficial cutaneous muscles softening the dynamic rhytids without complete paralysis.
Mesotox is widely used off label for facial lifting, reduction in skin laxity or crepiness, flushing of rosacea, acne, hyperhidrosis of the face, keloids, seborrhea, neck rejuvenation, contouring of the mandibular border, and scalp oiliness. Based on a review of articles using this technique, dilution methods were less than 2.5 U per 1 mL (OBA, IBA) and less than 10 U per 0.1 mL (ABO) depth of injection was the superficial to mid-dermis with injection points 0.5 cm to 1 cm apart.
In a study by Atwa and colleagues, 25 patients with mild facial skin laxity received intradermal Botox-A on one side and saline on the other. This split face study showed a highly significant difference with facial lifting on the treated side. Mesotox injection points vary based on the clinical indication and area being treated.
The treatment of dynamic muscles using standard neuromodulator dosing protocols include the treatment of the glabella, crow’s feet, forehead lines, masseter hypertrophy, bunny lines, gummy smile, perioral lines, mentalis hypertonia, platysmal bands, and marionette lines.
However, hyperdilute neuromodulators or Microtox can effectively be used alone or in combination with standard dosing for the following off-label uses. Used in combination with standard dosing of the forehead lines, I use Microtox in the lateral brow to soften the frontalis muscle without dropping the brow in patients with a low-set brow or lid laxity. I also use it for the jelly roll of the eyes and to open the aperture of the eyes. Along the nose, Microtox can also be used to treat a sagging nasal tip, decrease the width of the ala, and treat overactive facial muscles adjacent to the nose resulting in an overactive nasolabial fold.
Similarly, Microtox can be used to treat lateral smile lines and downward extensions of the crow’s feet. In all of the aforementioned treatment areas, I recommend approximately 0.5-1 U of toxin in each area divided at 1-cm intervals.Mesotox and Microtox are both highly effective strategies to treat the aging face. However, the nomenclature is not interchangeable. I propose that the term Mesotox be used only to articulate or define the superficial injection of a neuromodulator for the improvement of the skin that does not involve the injection into or paralysis of a cutaneous muscle (“tox” being used generically for all neuromodulators). I also propose that the term Microtox should be used to define the dilution of a neuromodulator beyond the manufacturer-recommended dilution protocols – used for the paralysis of a cutaneous muscle. In addition, I recommend that the terms MicroBotox and MesoBotox no longer be used. These procedures all have risks, and adverse events associated with Microtox and Mesotox are similar to those of any neuromodulator injection at FDA-recommended maximum doses, and dilution and storage protocols and proper injection techniques need to be followed. Expertise and training is crucial and treatment by a board-certified dermatologist or plastic surgeon is imperative.
Dr. Talakoub and Naissan O. Wesley, MD, are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to her at [email protected]. Dr. Talakoub had no relevant disclosures.
References
Awaida CJ et al. Plast Reconstr Surg. 2018 Sep;142(3):640-9.
Calvani F et al. Plast Surg (Oakv). 2019 May;27(2):156-61.
Iranmanesh B et al. J Cosmet Dermatol. 2022 Oct;21(10):4160-70.
Kandhari R et al. J Cutan Aesthet Surg. 2022 Apr-Jun;15(2):101-7.
Lewandowski M et al. Molecules. 2022 May 13;27(10):3143.
Mammucari M et al. Eur Rev Med Pharmacol Sci. 2011 Jun;15(6):682-94.
Park KY et al. Ann Dermatol. 2018 Dec;30(6):688-93.
Pistor M. Chir Dent Fr. 1976;46:59-60.
Rho NK, Gil YC. Toxins (Basel). 2021 Nov 19;13(11):817.
Wu WTL. Plast Reconstr Surg. 2015 Nov;136(5 Suppl):92S-100S.
Zhang H et al. Clin Cosmet Investig Dermatol. 2021 Apr 30;14:407-17.
Ulmus davidiana root extract
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at [email protected].
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at [email protected].
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
Ulmus davidiana, commonly known as yugeunpi, has a long history of use in Korea in treating burns, eczema, frostbite, difficulties in urination, inflammation, and psoriasis,1 and has also been used in China for some of these indications, including skin inflammation.2,3 Currently, there are several areas in which the bioactivity of U. davidiana are under investigation, with numerous potential applications in dermatology. This column focuses briefly on the evidence supporting the traditional uses of the plant and potential new applications.
Anti-inflammatory activity
Eom and colleagues studied the potential of a polysaccharide extract from the root bark of U. davidiana to serve as a suitable cosmetic ingredient for conferring moisturizing, anti-inflammatory, and photoprotective activity. In this 2006 investigation, the composition of the polysaccharide extract was found to be primarily rhamnose, galactose, and glucose. The root extract exhibited a similar humectant moisturizing effect as hyaluronic acid, the researchers reported. The U. davidiana root extract was also found to dose-dependently suppress prostaglandin E2. The inhibition of the release of interleukin-6 and IL-8 was also reported to be significant. The use of the U. davidiana extract also stimulated the recovery of human fibroblasts (two times that of positive control) exposed to UVA irradiation. The researchers suggested that their overall results point to the viability of U. davidiana root extract as a cosmetic agent ingredient to protect skin from UV exposure and the inflammation that follows.2
In 2013, Choi and colleagues found that a methanol extract of the stem and root barks of U. davidiana revealed anti-inflammatory properties, with activity attributed to two trihydroxy acids [then-new trihydroxy fatty acid, 9,12,13-trihydroxyoctadeca-10(Z),15(Z)-dienoic acid, and pinellic acid], both of which blocked prostaglandin D₂ production.4
That same year, Lyu and colleagues studied the antiallergic and anti-inflammatory effects of U. davidiana using a 1-fluoro-2,4-dinitrofluorobenzene (DNFB)–induced contact dermatitis mouse model. They found that treatment at a dose of 10 mg/mL successfully prevented skin lesions caused by consistent DNFB application. Further, the researchers observed that topically applied U. davidiana suppressed spongiosis and reduced total serum immunoglobulin and IgG2a levels. Overall, they concluded that the botanical treatment improved contact dermatitis in mice.1
In 2019, So and colleagues studied the chemical components of U. davidiana root bark (isolating a chromane derivative and 22 known substances) and reported data supporting the traditional use of the root bark for gastroenteric and inflammatory indications.3
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, including U. davidiana, is used for its anti-inflammatory properties in traditional Korean medicine.5 Choi and colleagues determined that bakuchiol exhibited robust anti-inflammatory activity in a study of U. davidiana constituents, at least partially accounting for the anti-inflammatory functions of the plant.5
Antifungal activity
In 2021, Alishir and colleagues conducted a phytochemical analysis of the root bark extract of U. davidiana, resulting in the isolation of 10 substances including the novel coumarin glycoside derivative ulmusakidian. Some of the compounds exhibited antifungal activity against Cryptococcus neoformans, though none demonstrated antifungal activity against Candida albicans.6
Wound dressing
Park and colleagues demonstrated in 2020 that superabsorbing hydrogel wound dressings composed of U. davidiana root bark powders, which exhibit gelling activity, performed effectively in speeding up wound closure and cutaneous regeneration in skin-wound mice models. These dressings also displayed thermal stability and superior mechanical properties to pullulan-only gel films. The researchers concluded that gel films composed of U. davidiana have potential to surpass the effectiveness of current products.7
Anti–hair loss activity
Early in 2022, Kwon and colleagues investigated the anti–hair loss mechanism of U. davidiana and determined that supercritical extraction-residues of U. davidiana significantly hinder the secretion of transforming growth factor–beta but dose dependently salvage insulinlike growth factor 1, and substantially decrease dihydrotestosterone synthesis. They concluded that these U. davidiana supercritical fluid extract residues have the potential to halt the loss of human hair.8
Photoprotective potential
Late in 2020, Her and colleagues reported on their development and analysis of a new distillate derived from a fermented mixture of nine anti-inflammatory herbs including U. davidiana. The investigators assessed the effects of the topically applied distillate on UVB-induced skin damage in Institute of Cancer Research mice, finding significant improvements in the dorsal skin photodamage. Application of the distillate also ameliorated collagen production impairment and diminished proinflammatory cytokine levels of tumor necrosis factor (TNF)–alpha and IL-1B. The researchers concluded that this anti-inflammatory herbal distillate, which includes U. davidiana, displays the potential to serve as a photoprotective agent.9
Antiaging activity
In 2011, Yang and colleagues set out to identify constituent substances of the root bark of U. davidiana that have the capacity to suppress cellular senescence in human fibroblasts and human umbilical vein endothelial cells. They isolated 22 compounds, of which epifriedelanol, ssioriside, and catechin-7-O-beta-D-glucopyranoside impeded adriamycin-induced cellular senescence in human dermal fibroblasts and friedelin, epifriedelanol, and catechin-7-O-beta-apiofuranoside in the umbilical vein endothelial cells. Epifriedelanol was the most potent of the substances, leading the researchers to conclude that this U. davidiana component can diminish cellular senescence in human primary cells and has the potential as an oral and/or topical antiaging agent.10
Also that year, in a study on the protective effects of U. davidiana on UVB-irradiated hairless mice, the authors claimed that an ethanol extract of U. davidiana significantly suppressed wrinkle development in mice chronically exposed to UVB.11 This study showed that U. davidiana extract exerts antioxidant activity as evidenced by a decrease in MMP-1 activity. It also demonstrated antielastase activity. The treated mice showed a decrease in wrinkles as compared with water-treated mice.11 Although this is just one study in mice, it may demonstrate a protective effect on elastic fibers on skin exposed to UVB light.
Late in 2020, Lee and colleagues reported on their study of the possible antiaging effects on the skin of (-)-phenolic compounds isolated from the root bark of U. davidiana. The function of collagenase MMP-1 was found to be inhibited by the isolate (-)-catechin, which also halted collagen degradation caused by TNF-alpha in normal human dermal fibroblasts. Further, the investigators demonstrated that the U. davidiana isolate (-)-catechin reduced the expression of proinflammatory cytokines such as IL-1B and IL-6. They concluded that the U. davidiana isolate exhibits the potential to combat intrinsic as well as extrinsic cutaneous aging.12
These findings are particularly intriguing. There is much overlap between intrinsic and extrinsic aging. If U. davidiana can keep collagen intact and inhibit cellular senescence, it may serve as an early intervention toward slowing or preventing skin aging.
Summary
Of greatest interest now, perhaps, is its potential to impede cellular senescence. Senescent cells release a multitude of inflammatory and other factors that hasten intrinsic aging. Blocking cellular senescence is an important approach to the prevention and treatment of skin aging.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an ecommerce solution. Write to her at [email protected].
References
1. Lyu J et al. J Pharmacopuncture. 2013 Jun;16(2):41-5.
2. Eom SY et al. J Cosmet Sci. 2006 Sep-Oct;57(5):355-67.
3. So HM et al. Bioorg Chem. 2019 Oct;91:103145.
4. Choi HG et al. Phytother Res. 2013 Sep;27(9):1376-80.
5. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
6. Alishir A et al. Bioorg Med Chem Lett. 2021 Mar 15;36:127828.
7. Park TH et al. Saudi Pharm J. 2020 Jul;28(7):791-802.
8. Kwon YE et al. Molecules. 2022 Feb 19;27(4):1419.
9. Her Y et al. Molecules. 2020 Dec 29;26(1):124.
10. Yang HH et al. Planta Med. 2011 Mar;77(5):441-9.
11. Kim YO et al. Korean Journal of Medicinal Crop Science. 2011;19(6):508-13.
12. Lee S et al. Antioxidants (Basel). 2020 Oct 13;9(10):981.
Combination therapy shows mixed results for scleroderma-related lung disease
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
PHILADELPHIA – Combining the immunomodulatory agent mycophenolate with the antifibrotic pirfenidone led to more rapid improvement and showed a trend to be more effective than mycophenolate mofetil alone for treating the signs and symptoms of scleroderma-related interstitial lung disease, but the combination therapy came with an increase in side effects, according to results from the Scleroderma Lung Study III.
Dinesh Khanna, MBBS, MSc, of the University of Michigan, Ann Arbor, presented the results at the annual meeting of the American College of Rheumatology. He noted some problems with the study – namely its small size, enrolling only 51 patients, about one-third of its original goal. But he also said it showed a potential signal for efficacy and that the study itself could serve as a “template” for future studies of combination mycophenolate mofetil (MMF) plus pirfenidone therapy for scleroderma-related interstitial lung disease (SSc-ILD).
“The pirfenidone patients had quite a bit more GI side effects and photosensitivity, and those are known side effects,” Dr. Khanna said in an interview. “So the combination therapy had more side effects but trends to higher efficacy.”
The design of SLS-III, a phase 2 clinical trial, was a challenge, Dr. Khanna explained. The goal was to enroll 150 SSc-ILD patients who hadn’t had any previous treatment for their disease. Finding those patients proved difficult. “In fact, if you look at the recent history, 70% of the patients with early diffuse scleroderma are on MMF,” he said in his presentation. Compounding low study enrollment was the intervening COVID-19 pandemic, he added.
Testing a faster-acting combination
Nonetheless, the trial managed to enroll 27 patients in the combination therapy group and 24 in the MMF-plus-placebo group and compared their outcomes over 18 months. Study dosing was 1,500 mg MMF twice daily and pirfenidone 801 mg three times daily, titrated to the tolerable dose.
Despite the study’s being underpowered, Dr. Khanna said, it still reported some notable outcomes that merit further investigation. “I think what was intriguing in the study was the long-term benefit in the patient-reported outcomes and the structural changes,” he said in the interview.
Among those notable outcomes was a clinically significant change in forced vital capacity (FVC) percentage for the combination vs. the placebo groups: 2.24% vs. 2.09%. He also noted that the combination group saw a somewhat more robust improvement in FVC at six months: 2.59% (± 0.98%) vs. 0.92% (± 1.1%) in the placebo group.
The combination group showed greater improvements in high-resolution computed tomography-evaluated lung involvement and lung fibrosis and patient-reported outcomes, including a statistically significant 3.67-point greater improvement in PROMIS-29 physical function score (4.42 vs. 0.75).
The patients on combination therapy had higher rates of serious adverse events (SAEs), and seven discontinued one or both study drugs early, all in the combined arm. Four combination therapy patients had six SAEs, compared to two placebo patients with three SAEs. In the combination group, SAEs included chest pain, herpes zoster ophthalmicus, nodular basal cell cancer, marginal zone B cell lymphoma, renal crisis, and dyspnea. SAEs in the placebo group were colitis, COVID-19 and hypoxic respiratory failure.
Study design challenges
Nonetheless, Dr. Khanna said the SLS-III data are consistent with the SLS-II findings, with mean improvements in FVC of 2.24% and 2.1%, respectively.
“The next study may be able to replicate what we tried to do, keeping in mind that there are really no MMF-naive patients who are walking around,” Dr. Khanna said. “So the challenge is about the feasibility of recruiting within a trial vs. trying to show a statistical difference between the drug and placebo.”
This study could serve as a foundation for future studies of MMF in patients with SSc-ILD, Robert Spiera, MD, of the Hospital for Special Surgery in New York, said in an interview. “There are lessons to be learned both from the study but also from prior studies looking at MMF use in the background in patients treated with other drugs in clinical trials,” he said.
Dr. Spiera noted that the study had other challenges besides the difficulty in recruiting patients who hadn’t been on MMF therapy. “A great challenge is that the benefit with regard to the impact on the lungs from MMF seems most prominent in the first 6 months to a year to even 2 years that somebody is on the drug,” he said.
The other challenge with this study is that a large proportion of patients had limited systemic disease and relatively lower levels of skin disease compared with other studies of patients on MMF, Dr. Spiera said.
“The optimal treatment of scleroderma-associated lung disease remains a very important and not-adequately met need,” he said. “Particularly, we’re looking for drugs that are tolerable in a patient population that are very prone to GI side effects in general. This study and others have taught us a lot about trial design, and I think more globally this will allow us to move this field forward.”
Dr. Khanna disclosed relationships with Actelion, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Horizon Therapeutics USA, Janssen Global Services, Prometheus Biosciences, Mitsubishi Tanabe Pharma Corp., Genentech/Roche, Theraly, and Pfizer. Genentech provided funding for the study and pirfenidone and placebo drugs at no cost.
Dr. Spiera disclosed relationships with GlaxoSmithKline, Boehringer-Ingelheim, Corbus Pharmaceutical, InflaRx, AbbVie/Abbott, Sanofi, Novartis, Chemocentryx, Roche and Vera.
AT ACR 2022
Starting a podcast
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I discussed . At this writing (November 2022), more than 600 million blogs are online, compared with about 2 million podcasts, and relatively few of them are run by physicians. With podcasts, you have a better chance of standing out in a crowded online world.
Starting a podcast is not difficult, but there are several steps you need to go through before launching one.
As with blogging, start by outlining a long-range plan. Your general topic will probably be your specialty, but you will need to narrow your focus to a few specific subjects, such as the problems you see most often, or a subspecialty that you concentrate on. You can always expand your topic later, as you get more popular. Choose a name for your podcast, and purchase a domain name that accurately describes it.
You will also need to choose a hosting service. Numerous inexpensive hosting platforms are available, and a simple Google search will find them for you. Many of them provide free learning materials, helpful creative tools, and customer support to get you through the confusing technical aspects. They can also help you choose a music introduction (to add a bit of polish), and help you piece together your audio segments. Buzzsprout, RSS.com, and Podbean get good reviews on many sites. (As always, I have no financial interest in any company or service mentioned herein.)
Hosting services can assist you in creating a template – a framework that you can reuse each time you record an episode – containing your intro and exit music, tracks for your conversations, etc. This will make your podcasts instantly recognizable each time your listeners tune in.
Many podcasting experts recommend recruiting a co-host. This can be an associate within your practice, a friend who practices elsewhere, or perhaps a resident in an academic setting. You will be able to spread the workload of creating, editing, and promoting. Plus, it is much easier to generate interesting content when two people are having a conversation, rather than one person lecturing from a prepared script. You might also consider having multiple co-hosts, either to expand episodes into group discussions, or to take turns working with you in covering different subjects.
How long you make your podcast is entirely up to you. Some consultants recommend specific time frames, such as 5 minutes (because that’s an average attention span), or 28 minutes (because that’s the average driving commute time). There are short podcasts and long ones; whatever works for you is fine, as long as you don’t drift off the topic. Furthermore, no one says they must all be the same length; when you are finished talking, you are done. And no one says you must stick with one subject throughout. Combining several short segments might hold more listeners’ interest and will make it easier to share small clips on social media.
Content guidelines are similar to those for blogs. Give people content that will be of interest or benefit to them. Talk about subjects – medical and otherwise – that are relevant to your practice or are prominent in the news.
As with blogs, try to avoid polarizing political discussions, and while it’s fine to discuss treatments and procedures that you offer, aggressive solicitation tends to make viewers look elsewhere. Keep any medical advice in general terms; don’t portray any specific patients as examples.
When your podcast is ready, your hosting platform will show you how to submit it to iTunes, and how to submit your podcast RSS feed to other podcast directories. As you upload new episodes, your host will automatically update your RSS feed, so that any directory you are listed on will receive the new episode.
Once you are uploaded, you can use your host’s social sharing tools to spread the word. As with blogs, use social media, such as your practice’s Facebook page, to push podcast updates into patients’ feeds and track relevant Twitter hashtags to find online communities that might be interested in your subject matter. You should also find your episode embed code (which your host will have) and place it in a prominent place on your website so patients can listen directly from there.
Transcriptions are another excellent promotional tool. Search engines will “read” your podcasts and list them in searches. Some podcast hosts will do transcribing for a fee, but there are independent transcription services as well.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Infant anaphylaxis: Study characterizes symptoms, treatment
LOUISVILLE, KY. – , research findings indicate.
Given that early administration of epinephrine can be potentially lifesaving for infants with anaphylaxis, the study highlighted the real-world successes in increased uptake of treatment in this vulnerable patient population.
Most infants in the study who presented to the ED and received epinephrine were able to be discharged home after just a few hours, with only 1 out of 10 requiring hospitalization.
The study also reported that most symptoms were in the skin/mucosal, gastrointestinal, respiratory, and cardiovascular (CV) systems, providing improved characterization of anaphylaxis symptoms in the infant population.
Nearly “all episodes were triggered by food – especially egg, peanut, milk, and cashew,” commented Colleen Shannon, MD, a pediatrician at Children’s Hospital of Philadelphia, who presented the research findings at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Dr. Shannon noted that despite previous research demonstrating age-based differences in the presentation of anaphylaxis, the symptomatology of anaphylaxis in infants has not been robustly characterized. Better characterization of anaphylaxis in infants with allergies may help ensure earlier and more accurate diagnosis and management, she said.
For the study, the researchers performed a retrospective chart review of 169 patients between 0 and 24 months of age (mean age, 1.0 years) who presented to the emergency department of a pediatric tertiary referral center between 2019 and 2022.
All patients in the study met diagnostic criteria for anaphylaxis. The investigators used the medical records of patients to evaluate for demographics, as well as presenting symptoms and treatment.
More than half (56.2%) of infants in the study were 12 months of age or younger, and 64.5% were male.
Nearly all (96.5%) anaphylaxis episodes presenting to the ED were triggered by food. The most common foods triggering these episodes were egg (26.6%), peanut (25.4%), milk (13.6%), and cashew (10.1%).
Most symptoms involved the skin/mucosal (97.6%) and GI (74.6%) systems, followed by respiratory (56.8%) and CV (34.3%) systems. Isolated tachycardia was recorded in 84.5% of patients with CV-related symptoms.
Epinephrine was administered to 86.4% of infants who presented to the ED with anaphylaxis. Nearly a third (30.1%) of these infants received epinephrine before arriving to the ED, and 9.5% required more than 1 dose.
The researchers also found that 10.1% of patients required hospital admission, but none had symptoms severe enough to require intensive care.
Jennifer Hoffmann, MD, an emergency medicine physician at the Lurie Children’s Hospital of Chicago, told this news organization that while characterizing anaphylaxis symptoms is relevant for clinicians, it also remains vitally important “to teach parents of infants how to recognize the signs of anaphylaxis, particularly as they begin to introduce new foods,” to ensure timely treatment.
She added that since most infants in the study improved after a single dose of epinephrine, most infants presenting to the ED with anaphylaxis can therefore be safely discharged home after only a brief period of observation. “That is, age alone should not be a reason for admission,” explained Dr. Hoffmann, who wasn’t involved in the research study.
The study was independently supported. Dr. Shannon and Dr. Hoffmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LOUISVILLE, KY. – , research findings indicate.
Given that early administration of epinephrine can be potentially lifesaving for infants with anaphylaxis, the study highlighted the real-world successes in increased uptake of treatment in this vulnerable patient population.
Most infants in the study who presented to the ED and received epinephrine were able to be discharged home after just a few hours, with only 1 out of 10 requiring hospitalization.
The study also reported that most symptoms were in the skin/mucosal, gastrointestinal, respiratory, and cardiovascular (CV) systems, providing improved characterization of anaphylaxis symptoms in the infant population.
Nearly “all episodes were triggered by food – especially egg, peanut, milk, and cashew,” commented Colleen Shannon, MD, a pediatrician at Children’s Hospital of Philadelphia, who presented the research findings at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Dr. Shannon noted that despite previous research demonstrating age-based differences in the presentation of anaphylaxis, the symptomatology of anaphylaxis in infants has not been robustly characterized. Better characterization of anaphylaxis in infants with allergies may help ensure earlier and more accurate diagnosis and management, she said.
For the study, the researchers performed a retrospective chart review of 169 patients between 0 and 24 months of age (mean age, 1.0 years) who presented to the emergency department of a pediatric tertiary referral center between 2019 and 2022.
All patients in the study met diagnostic criteria for anaphylaxis. The investigators used the medical records of patients to evaluate for demographics, as well as presenting symptoms and treatment.
More than half (56.2%) of infants in the study were 12 months of age or younger, and 64.5% were male.
Nearly all (96.5%) anaphylaxis episodes presenting to the ED were triggered by food. The most common foods triggering these episodes were egg (26.6%), peanut (25.4%), milk (13.6%), and cashew (10.1%).
Most symptoms involved the skin/mucosal (97.6%) and GI (74.6%) systems, followed by respiratory (56.8%) and CV (34.3%) systems. Isolated tachycardia was recorded in 84.5% of patients with CV-related symptoms.
Epinephrine was administered to 86.4% of infants who presented to the ED with anaphylaxis. Nearly a third (30.1%) of these infants received epinephrine before arriving to the ED, and 9.5% required more than 1 dose.
The researchers also found that 10.1% of patients required hospital admission, but none had symptoms severe enough to require intensive care.
Jennifer Hoffmann, MD, an emergency medicine physician at the Lurie Children’s Hospital of Chicago, told this news organization that while characterizing anaphylaxis symptoms is relevant for clinicians, it also remains vitally important “to teach parents of infants how to recognize the signs of anaphylaxis, particularly as they begin to introduce new foods,” to ensure timely treatment.
She added that since most infants in the study improved after a single dose of epinephrine, most infants presenting to the ED with anaphylaxis can therefore be safely discharged home after only a brief period of observation. “That is, age alone should not be a reason for admission,” explained Dr. Hoffmann, who wasn’t involved in the research study.
The study was independently supported. Dr. Shannon and Dr. Hoffmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LOUISVILLE, KY. – , research findings indicate.
Given that early administration of epinephrine can be potentially lifesaving for infants with anaphylaxis, the study highlighted the real-world successes in increased uptake of treatment in this vulnerable patient population.
Most infants in the study who presented to the ED and received epinephrine were able to be discharged home after just a few hours, with only 1 out of 10 requiring hospitalization.
The study also reported that most symptoms were in the skin/mucosal, gastrointestinal, respiratory, and cardiovascular (CV) systems, providing improved characterization of anaphylaxis symptoms in the infant population.
Nearly “all episodes were triggered by food – especially egg, peanut, milk, and cashew,” commented Colleen Shannon, MD, a pediatrician at Children’s Hospital of Philadelphia, who presented the research findings at the annual meeting of the American College of Allergy, Asthma, and Immunology.
Dr. Shannon noted that despite previous research demonstrating age-based differences in the presentation of anaphylaxis, the symptomatology of anaphylaxis in infants has not been robustly characterized. Better characterization of anaphylaxis in infants with allergies may help ensure earlier and more accurate diagnosis and management, she said.
For the study, the researchers performed a retrospective chart review of 169 patients between 0 and 24 months of age (mean age, 1.0 years) who presented to the emergency department of a pediatric tertiary referral center between 2019 and 2022.
All patients in the study met diagnostic criteria for anaphylaxis. The investigators used the medical records of patients to evaluate for demographics, as well as presenting symptoms and treatment.
More than half (56.2%) of infants in the study were 12 months of age or younger, and 64.5% were male.
Nearly all (96.5%) anaphylaxis episodes presenting to the ED were triggered by food. The most common foods triggering these episodes were egg (26.6%), peanut (25.4%), milk (13.6%), and cashew (10.1%).
Most symptoms involved the skin/mucosal (97.6%) and GI (74.6%) systems, followed by respiratory (56.8%) and CV (34.3%) systems. Isolated tachycardia was recorded in 84.5% of patients with CV-related symptoms.
Epinephrine was administered to 86.4% of infants who presented to the ED with anaphylaxis. Nearly a third (30.1%) of these infants received epinephrine before arriving to the ED, and 9.5% required more than 1 dose.
The researchers also found that 10.1% of patients required hospital admission, but none had symptoms severe enough to require intensive care.
Jennifer Hoffmann, MD, an emergency medicine physician at the Lurie Children’s Hospital of Chicago, told this news organization that while characterizing anaphylaxis symptoms is relevant for clinicians, it also remains vitally important “to teach parents of infants how to recognize the signs of anaphylaxis, particularly as they begin to introduce new foods,” to ensure timely treatment.
She added that since most infants in the study improved after a single dose of epinephrine, most infants presenting to the ED with anaphylaxis can therefore be safely discharged home after only a brief period of observation. “That is, age alone should not be a reason for admission,” explained Dr. Hoffmann, who wasn’t involved in the research study.
The study was independently supported. Dr. Shannon and Dr. Hoffmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACAAI
Lower hydroxychloroquine dose for lupus tied to hospitalizations for flares
PHILADELPHIA – Patients with systemic lupus erythematosus treated with lower doses of hydroxychloroquine (HCQ) had an increased risk for hospitalization for flares, according to study results presented during a press conference at the annual meeting of the American College of Rheumatology.
HCQ is a cornerstone treatment for SLE as it has been shown to increase survival and decrease disease flares.
Doses decreased with changing guidelines
Guidelines over the years have recommended decreasing doses of HCQ. In 2011, ophthalmology guidelines recommended limiting HCQ dosing to 6.5 mg/kg per day or less of ideal body weight to reduce the chance of retinopathy. For many patients, this required a dose lower than 400 mg/day, an amount frequently used to treat lupus.
In 2016, updated guidelines further lowered the dosage of HCQ, recommending 5 mg/kg or less of patient’s actual body weight.
The effects that lower dosing has had on SLE-associated hospitalizations was unknown, which inspired Dr. Nestor’s research.
The team conducted a case-crossover study within the Mass General Brigham SLE cohort.
Hospitalizations studied over a decade
Dr. Nestor and colleagues identified patients with SLE (via electronic health records) who had at least one visit for SLE and were prescribed HCQ between January 2011 and December 2021, the period over which the recommendations were made.
They identified patients who had been hospitalized during that decade with SLE as the primary discharge diagnosis.
Patients were excluded if they had non-SLE indications, such as kidney transplant or infection without a concomitant SLE flare.
Of 2,971 patients with SLE who used HCQ, 576 had at least one hospitalization with primary discharge diagnosis of SLE.
Of these, 108 were hospitalized for an SLE flare and had used HCQ prior to that hospitalization and had at least one control period with HCQ use during the study period.
All of the patients in the study had to have a case period and a control period, Dr. Nestor explained. The case period was 6 months on HCQ ending in hospitalization for lupus and the control period was 6 months on HCQ that did not end in hospitalization for lupus.
Significantly increased hospitalizations
Low-dose HCQ by weight-based dose (≤ 5 vs. > 5 mg/kg per day) and by non–weight-based dose (< 400 vs. 400 mg per day) were both associated with significantly increased hospitalizations for SLE (adjusted odds ratio, 4.41; 95% confidence interval, 1.50-12.98; and AOR, 3.48; 95% CI, 1.33-9.13, respectively).
The average age of the hospitalized group was 36 years. Most patients (92%) were female, 43.5% were White, and 32.4% were Black.
In calling for reassessment of the dosing, Dr. Nestor said, “We are protecting our patients against a very long-term side effect of hydroxychloroquine retinopathy. [It] typically takes 10-20 years to develop in our patients. But by doing that, we’re missing many of the short-term benefits from hydroxychloroquine in our patients, leading to more lupus flares, which leads to more end-organ damage.”
She said patients taking HCQ for lupus are asked to see an ophthalmologist once a year to monitor for the side effect, adding that rheumatologists and ophthalmologists could work together to adjust the guidelines.
Dr. Nestor suggested it’s possible that patients need higher doses of HCQ earlier in their disease and lower doses later. “Perhaps it’s just the patients who are particularly active who need the higher doses,” she said.
“I don’t think this question is settled,” he told this news organization. “The 5 mg/kg dose recommendation was based on terms of safety but not of effectiveness. We don’t know what the effective dose of HCQ is, and this study shows that low dose is less effective.”
He agreed there needs to be a risk/benefit balance, but noted, “HCQ retinopathy is very rare and we have great tools to screen for it.”
Study limitations include incomplete information on whether patients adhered to treatment plans and reasons for using lower-dose HCQ.
The study authors and Dr. Duarte Garcia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with systemic lupus erythematosus treated with lower doses of hydroxychloroquine (HCQ) had an increased risk for hospitalization for flares, according to study results presented during a press conference at the annual meeting of the American College of Rheumatology.
HCQ is a cornerstone treatment for SLE as it has been shown to increase survival and decrease disease flares.
Doses decreased with changing guidelines
Guidelines over the years have recommended decreasing doses of HCQ. In 2011, ophthalmology guidelines recommended limiting HCQ dosing to 6.5 mg/kg per day or less of ideal body weight to reduce the chance of retinopathy. For many patients, this required a dose lower than 400 mg/day, an amount frequently used to treat lupus.
In 2016, updated guidelines further lowered the dosage of HCQ, recommending 5 mg/kg or less of patient’s actual body weight.
The effects that lower dosing has had on SLE-associated hospitalizations was unknown, which inspired Dr. Nestor’s research.
The team conducted a case-crossover study within the Mass General Brigham SLE cohort.
Hospitalizations studied over a decade
Dr. Nestor and colleagues identified patients with SLE (via electronic health records) who had at least one visit for SLE and were prescribed HCQ between January 2011 and December 2021, the period over which the recommendations were made.
They identified patients who had been hospitalized during that decade with SLE as the primary discharge diagnosis.
Patients were excluded if they had non-SLE indications, such as kidney transplant or infection without a concomitant SLE flare.
Of 2,971 patients with SLE who used HCQ, 576 had at least one hospitalization with primary discharge diagnosis of SLE.
Of these, 108 were hospitalized for an SLE flare and had used HCQ prior to that hospitalization and had at least one control period with HCQ use during the study period.
All of the patients in the study had to have a case period and a control period, Dr. Nestor explained. The case period was 6 months on HCQ ending in hospitalization for lupus and the control period was 6 months on HCQ that did not end in hospitalization for lupus.
Significantly increased hospitalizations
Low-dose HCQ by weight-based dose (≤ 5 vs. > 5 mg/kg per day) and by non–weight-based dose (< 400 vs. 400 mg per day) were both associated with significantly increased hospitalizations for SLE (adjusted odds ratio, 4.41; 95% confidence interval, 1.50-12.98; and AOR, 3.48; 95% CI, 1.33-9.13, respectively).
The average age of the hospitalized group was 36 years. Most patients (92%) were female, 43.5% were White, and 32.4% were Black.
In calling for reassessment of the dosing, Dr. Nestor said, “We are protecting our patients against a very long-term side effect of hydroxychloroquine retinopathy. [It] typically takes 10-20 years to develop in our patients. But by doing that, we’re missing many of the short-term benefits from hydroxychloroquine in our patients, leading to more lupus flares, which leads to more end-organ damage.”
She said patients taking HCQ for lupus are asked to see an ophthalmologist once a year to monitor for the side effect, adding that rheumatologists and ophthalmologists could work together to adjust the guidelines.
Dr. Nestor suggested it’s possible that patients need higher doses of HCQ earlier in their disease and lower doses later. “Perhaps it’s just the patients who are particularly active who need the higher doses,” she said.
“I don’t think this question is settled,” he told this news organization. “The 5 mg/kg dose recommendation was based on terms of safety but not of effectiveness. We don’t know what the effective dose of HCQ is, and this study shows that low dose is less effective.”
He agreed there needs to be a risk/benefit balance, but noted, “HCQ retinopathy is very rare and we have great tools to screen for it.”
Study limitations include incomplete information on whether patients adhered to treatment plans and reasons for using lower-dose HCQ.
The study authors and Dr. Duarte Garcia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Patients with systemic lupus erythematosus treated with lower doses of hydroxychloroquine (HCQ) had an increased risk for hospitalization for flares, according to study results presented during a press conference at the annual meeting of the American College of Rheumatology.
HCQ is a cornerstone treatment for SLE as it has been shown to increase survival and decrease disease flares.
Doses decreased with changing guidelines
Guidelines over the years have recommended decreasing doses of HCQ. In 2011, ophthalmology guidelines recommended limiting HCQ dosing to 6.5 mg/kg per day or less of ideal body weight to reduce the chance of retinopathy. For many patients, this required a dose lower than 400 mg/day, an amount frequently used to treat lupus.
In 2016, updated guidelines further lowered the dosage of HCQ, recommending 5 mg/kg or less of patient’s actual body weight.
The effects that lower dosing has had on SLE-associated hospitalizations was unknown, which inspired Dr. Nestor’s research.
The team conducted a case-crossover study within the Mass General Brigham SLE cohort.
Hospitalizations studied over a decade
Dr. Nestor and colleagues identified patients with SLE (via electronic health records) who had at least one visit for SLE and were prescribed HCQ between January 2011 and December 2021, the period over which the recommendations were made.
They identified patients who had been hospitalized during that decade with SLE as the primary discharge diagnosis.
Patients were excluded if they had non-SLE indications, such as kidney transplant or infection without a concomitant SLE flare.
Of 2,971 patients with SLE who used HCQ, 576 had at least one hospitalization with primary discharge diagnosis of SLE.
Of these, 108 were hospitalized for an SLE flare and had used HCQ prior to that hospitalization and had at least one control period with HCQ use during the study period.
All of the patients in the study had to have a case period and a control period, Dr. Nestor explained. The case period was 6 months on HCQ ending in hospitalization for lupus and the control period was 6 months on HCQ that did not end in hospitalization for lupus.
Significantly increased hospitalizations
Low-dose HCQ by weight-based dose (≤ 5 vs. > 5 mg/kg per day) and by non–weight-based dose (< 400 vs. 400 mg per day) were both associated with significantly increased hospitalizations for SLE (adjusted odds ratio, 4.41; 95% confidence interval, 1.50-12.98; and AOR, 3.48; 95% CI, 1.33-9.13, respectively).
The average age of the hospitalized group was 36 years. Most patients (92%) were female, 43.5% were White, and 32.4% were Black.
In calling for reassessment of the dosing, Dr. Nestor said, “We are protecting our patients against a very long-term side effect of hydroxychloroquine retinopathy. [It] typically takes 10-20 years to develop in our patients. But by doing that, we’re missing many of the short-term benefits from hydroxychloroquine in our patients, leading to more lupus flares, which leads to more end-organ damage.”
She said patients taking HCQ for lupus are asked to see an ophthalmologist once a year to monitor for the side effect, adding that rheumatologists and ophthalmologists could work together to adjust the guidelines.
Dr. Nestor suggested it’s possible that patients need higher doses of HCQ earlier in their disease and lower doses later. “Perhaps it’s just the patients who are particularly active who need the higher doses,” she said.
“I don’t think this question is settled,” he told this news organization. “The 5 mg/kg dose recommendation was based on terms of safety but not of effectiveness. We don’t know what the effective dose of HCQ is, and this study shows that low dose is less effective.”
He agreed there needs to be a risk/benefit balance, but noted, “HCQ retinopathy is very rare and we have great tools to screen for it.”
Study limitations include incomplete information on whether patients adhered to treatment plans and reasons for using lower-dose HCQ.
The study authors and Dr. Duarte Garcia report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2022

















