User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Facial lipoatrophy with semaglutide-related weight loss
Ozempic and Wegovy are two prescription drugs that have transformed the management of type 2 diabetes and obesity. Both are a form of semaglutide; the Food and Drug Administration approved Ozempic for treating type 2 diabetes in 2017, followed by Wegovy in 2021 for weight loss in adults with obesity or those who are overweight and have least one weight-related health condition, such as hypertension or hypercholesterolemia. Ozempic is not approved for weight loss, but it has been prescribed off label for that purpose.
An effective treatment, participants with overweight or obesity in one study experienced almost a mean 15% drop in body weight with subcutaneous semaglutide administered once a week versus about 2% with placebo after 68 weeks.
In 2022, high demand and global supply constraints gave rise to shortages of both medications. The FDA reported a Wegovy shortage in March 2022, followed by an Ozempic shortage in August. Social media attention and increased off-label prescribing, with some patients purporting to have had significant improvements with weight loss and their quality of life, including having their clothing fit better and being able to bend over and tie their shoes, increased attention on these medications to the point that off-label prescribing of both drugs for weight loss resulted in some patients with type 2 diabetes unable to receive their medication on time. In late January 2023, NBC reported that Ozempic prescriptions had “tripled from 2021 to 2022,” based on data from the prescription drug discount company SingleCare.
Semaglutide is designed to mimic a hormone that signals to the brain when a person is full and promotes the release of insulin. In turn, the medications can result in lower blood glucose levels, appetite suppression, and reduced caloric intake. Injected once weekly, the medication, a glucagonlike peptide–1 receptor agonist, specifically, activates GLP-1 receptors in the brain, increasing insulin secretion, decreasing glucagon secretion, and delaying gastric emptying (acting as an incretin mimetic).
‘Ozempic face’
Common adverse events with semaglutide can include nausea, vomiting, diarrhea, abdominal pain, constipation, and injection-site reactions. Rare, but more severe adverse events may include thyroid C-cell tumor (in animal studies), medullary thyroid cancer risk, hypersensitivity reaction, anaphylaxis, acute renal injury, chronic renal failure exacerbation, pancreatitis, and cholelithiasis.
A less severe but noticeable side effect that has gained attention is facial wasting and aging, reportedly coined “Ozempic face” by a dermatologist interviewed for an article published in January in The New York Times.
As of Feb. 9, TikTok videos from individuals describing their personal experiences, health care professionals, and others with the tag #ozempicface had 4.8 million views.
Theories as to why noticeable facial changes occur with these medications include: accelerated loss of facial pads that already tend to diminish or shift with normal aging, as well as the inability of skin elasticity to keep up with the loss of volume (fat), resulting in more prominent hanging skin and the appearance of “jowls.” Wan and colleagues have described the fat pad distribution in the face and the facial aging that occurs as a result of the loss and shifting of these fat pads over time.
In the same way that we use facial fillers to help treat and correct volume/fat loss associated with photoaging, facial fillers may be used to help restore volume where it’s been lost after weight loss. The sagging skin or loss of elasticity often associated with Ozempic-related weight loss or with rapid or noticeable weight loss in general, may or may not also require other interventions that include treatment with tissue tightening devices – such as radiofrequency energy, high-focused ultrasound energy, threads, and/or surgery – such as a face lift. The potential high cost of both off-label prescribing of these medications (especially without use of prescription health insurance) as well as treatment to correct any facial wasting has also received attention in news media and social media discussions of this topic.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at [email protected]. She has no relevant disclosures.
*Correction 1/28/23: An earlier version of this story misstated the approval date of Wegovy. It was in 2021.
Ozempic and Wegovy are two prescription drugs that have transformed the management of type 2 diabetes and obesity. Both are a form of semaglutide; the Food and Drug Administration approved Ozempic for treating type 2 diabetes in 2017, followed by Wegovy in 2021 for weight loss in adults with obesity or those who are overweight and have least one weight-related health condition, such as hypertension or hypercholesterolemia. Ozempic is not approved for weight loss, but it has been prescribed off label for that purpose.
An effective treatment, participants with overweight or obesity in one study experienced almost a mean 15% drop in body weight with subcutaneous semaglutide administered once a week versus about 2% with placebo after 68 weeks.
In 2022, high demand and global supply constraints gave rise to shortages of both medications. The FDA reported a Wegovy shortage in March 2022, followed by an Ozempic shortage in August. Social media attention and increased off-label prescribing, with some patients purporting to have had significant improvements with weight loss and their quality of life, including having their clothing fit better and being able to bend over and tie their shoes, increased attention on these medications to the point that off-label prescribing of both drugs for weight loss resulted in some patients with type 2 diabetes unable to receive their medication on time. In late January 2023, NBC reported that Ozempic prescriptions had “tripled from 2021 to 2022,” based on data from the prescription drug discount company SingleCare.
Semaglutide is designed to mimic a hormone that signals to the brain when a person is full and promotes the release of insulin. In turn, the medications can result in lower blood glucose levels, appetite suppression, and reduced caloric intake. Injected once weekly, the medication, a glucagonlike peptide–1 receptor agonist, specifically, activates GLP-1 receptors in the brain, increasing insulin secretion, decreasing glucagon secretion, and delaying gastric emptying (acting as an incretin mimetic).
‘Ozempic face’
Common adverse events with semaglutide can include nausea, vomiting, diarrhea, abdominal pain, constipation, and injection-site reactions. Rare, but more severe adverse events may include thyroid C-cell tumor (in animal studies), medullary thyroid cancer risk, hypersensitivity reaction, anaphylaxis, acute renal injury, chronic renal failure exacerbation, pancreatitis, and cholelithiasis.
A less severe but noticeable side effect that has gained attention is facial wasting and aging, reportedly coined “Ozempic face” by a dermatologist interviewed for an article published in January in The New York Times.
As of Feb. 9, TikTok videos from individuals describing their personal experiences, health care professionals, and others with the tag #ozempicface had 4.8 million views.
Theories as to why noticeable facial changes occur with these medications include: accelerated loss of facial pads that already tend to diminish or shift with normal aging, as well as the inability of skin elasticity to keep up with the loss of volume (fat), resulting in more prominent hanging skin and the appearance of “jowls.” Wan and colleagues have described the fat pad distribution in the face and the facial aging that occurs as a result of the loss and shifting of these fat pads over time.
In the same way that we use facial fillers to help treat and correct volume/fat loss associated with photoaging, facial fillers may be used to help restore volume where it’s been lost after weight loss. The sagging skin or loss of elasticity often associated with Ozempic-related weight loss or with rapid or noticeable weight loss in general, may or may not also require other interventions that include treatment with tissue tightening devices – such as radiofrequency energy, high-focused ultrasound energy, threads, and/or surgery – such as a face lift. The potential high cost of both off-label prescribing of these medications (especially without use of prescription health insurance) as well as treatment to correct any facial wasting has also received attention in news media and social media discussions of this topic.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at [email protected]. She has no relevant disclosures.
*Correction 1/28/23: An earlier version of this story misstated the approval date of Wegovy. It was in 2021.
Ozempic and Wegovy are two prescription drugs that have transformed the management of type 2 diabetes and obesity. Both are a form of semaglutide; the Food and Drug Administration approved Ozempic for treating type 2 diabetes in 2017, followed by Wegovy in 2021 for weight loss in adults with obesity or those who are overweight and have least one weight-related health condition, such as hypertension or hypercholesterolemia. Ozempic is not approved for weight loss, but it has been prescribed off label for that purpose.
An effective treatment, participants with overweight or obesity in one study experienced almost a mean 15% drop in body weight with subcutaneous semaglutide administered once a week versus about 2% with placebo after 68 weeks.
In 2022, high demand and global supply constraints gave rise to shortages of both medications. The FDA reported a Wegovy shortage in March 2022, followed by an Ozempic shortage in August. Social media attention and increased off-label prescribing, with some patients purporting to have had significant improvements with weight loss and their quality of life, including having their clothing fit better and being able to bend over and tie their shoes, increased attention on these medications to the point that off-label prescribing of both drugs for weight loss resulted in some patients with type 2 diabetes unable to receive their medication on time. In late January 2023, NBC reported that Ozempic prescriptions had “tripled from 2021 to 2022,” based on data from the prescription drug discount company SingleCare.
Semaglutide is designed to mimic a hormone that signals to the brain when a person is full and promotes the release of insulin. In turn, the medications can result in lower blood glucose levels, appetite suppression, and reduced caloric intake. Injected once weekly, the medication, a glucagonlike peptide–1 receptor agonist, specifically, activates GLP-1 receptors in the brain, increasing insulin secretion, decreasing glucagon secretion, and delaying gastric emptying (acting as an incretin mimetic).
‘Ozempic face’
Common adverse events with semaglutide can include nausea, vomiting, diarrhea, abdominal pain, constipation, and injection-site reactions. Rare, but more severe adverse events may include thyroid C-cell tumor (in animal studies), medullary thyroid cancer risk, hypersensitivity reaction, anaphylaxis, acute renal injury, chronic renal failure exacerbation, pancreatitis, and cholelithiasis.
A less severe but noticeable side effect that has gained attention is facial wasting and aging, reportedly coined “Ozempic face” by a dermatologist interviewed for an article published in January in The New York Times.
As of Feb. 9, TikTok videos from individuals describing their personal experiences, health care professionals, and others with the tag #ozempicface had 4.8 million views.
Theories as to why noticeable facial changes occur with these medications include: accelerated loss of facial pads that already tend to diminish or shift with normal aging, as well as the inability of skin elasticity to keep up with the loss of volume (fat), resulting in more prominent hanging skin and the appearance of “jowls.” Wan and colleagues have described the fat pad distribution in the face and the facial aging that occurs as a result of the loss and shifting of these fat pads over time.
In the same way that we use facial fillers to help treat and correct volume/fat loss associated with photoaging, facial fillers may be used to help restore volume where it’s been lost after weight loss. The sagging skin or loss of elasticity often associated with Ozempic-related weight loss or with rapid or noticeable weight loss in general, may or may not also require other interventions that include treatment with tissue tightening devices – such as radiofrequency energy, high-focused ultrasound energy, threads, and/or surgery – such as a face lift. The potential high cost of both off-label prescribing of these medications (especially without use of prescription health insurance) as well as treatment to correct any facial wasting has also received attention in news media and social media discussions of this topic.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at [email protected]. She has no relevant disclosures.
*Correction 1/28/23: An earlier version of this story misstated the approval date of Wegovy. It was in 2021.
Three wild technologies about to change health care
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
When I was a child, I watched syndicated episodes of the original “Star Trek.” I was dazzled by the space travel, sure, but also the medical technology.
A handheld “tricorder” detected diseases, while an intramuscular injector (“hypospray”) could treat them. Sickbay “biobeds” came with real-time health monitors that looked futuristic at the time but seem primitive today.
Such visions inspired a lot of us kids to pursue science. Little did we know the real-life advances many of us would see in our lifetimes.
Artificial intelligence helping to spot disease, robots performing surgery, even video calls between doctor and patient – all these once sounded fantastical but now happen in clinical care.
Now, in the 23rd year of the 21st century, you might not believe wht we’ll be capable of next. Three especially wild examples are moving closer to clinical reality.
Human hibernation
Captain America, Han Solo, and “Star Trek” villain Khan – all were preserved at low temperatures and then revived, waking up alive and well months, decades, or centuries later. These are fictional examples, to be sure, but the science they’re rooted in is real.
(In one extreme case, a climber survived after almost 9 hours of efforts to revive him.)
Useful for a space traveler? Maybe not. But it’s potentially huge for someone with life-threatening injuries from a car accident or a gunshot wound.
That’s the thinking behind a breakthrough procedure that came after decades of research on pigs and dogs, now in a clinical trial. The idea: A person with massive blood loss whose heart has stopped is injected with an ice-cold fluid, cooling them from the inside, down to about 50° F.
Doctors already induce more modest hypothermia to protect the brain and other organs after cardiac arrest and during surgery on the aortic arch (the main artery carrying blood from the heart).
But this experimental procedure – called emergency preservation and resuscitation (EPR) – goes far beyond that, dramatically “decreasing the body’s need for oxygen and blood flow,” says Samuel Tisherman, MD, a trauma surgeon at the University of Maryland Medical Center and the trial’s lead researcher. This puts the patient in a state of suspended animation that “could buy time for surgeons to stop the bleeding and save more of these patients.”
The technique has been done on at least six patients, though none were reported to survive. The trial is expected to include 20 people by the time it wraps up in December, according to the listing on the U.S. clinical trials database. Though given the strict requirements for candidates (emergency trauma victims who are not likely to survive), one can’t exactly rely on a set schedule.
Still, the technology is promising. Someday we may even use it to keep patients in suspended animation for months or years, experts predict, helping astronauts through decades-long spaceflights, or stalling death in sick patients awaiting a cure.
Artificial womb
Another sci-fi classic: growing human babies outside the womb. Think the fetus fields from “The Matrix,” or the frozen embryos in “Alien: Covenant.”
In 1923, British biologist J.B.S. Haldane coined a term for that – ectogenesis. He predicted that 70% of pregnancies would take place, from fertilization to birth, in artificial wombs by 2074. That many seems unlikely, but the timeline is on track.
Developing an embryo outside the womb is already routine in in vitro fertilization. And technology enables preterm babies to survive through much of the second half of gestation. Normal human pregnancy is 40 weeks, and the youngest preterm baby ever to survive was 21 weeks and 1 day old, just a few days younger than a smattering of others who lived.
The biggest obstacle for babies younger than that is lung viability. Mechanical ventilation can damage the lungs and lead to a chronic (sometimes fatal) lung disease known as bronchopulmonary dysplasia. Avoiding this would mean figuring out a way to maintain fetal circulation – the intricate system that delivers oxygenated blood from the placenta to the fetus via the umbilical cord. Researchers at Children’s Hospital of Philadelphia have done this using a fetal lamb.
The key to their invention is a substitute placenta: an oxygenator connected to the lamb’s umbilical cord. Tubes inserted through the umbilical vein and arteries carry oxygenated blood from the “placenta” to the fetus, and deoxygenated blood back out. The lamb resides in an artificial, fluid-filled amniotic sac until its lungs and other organs are developed.
Fertility treatment could benefit, too. “An artificial womb may substitute in situations in which a gestational carrier – surrogate – is indicated,” says Paula Amato, MD, a professor of obstetrics and gynecology at Oregon Health and Science University, Portland. (Dr. Amato is not involved in the CHOP research.) For example: when the mother is missing a uterus or can’t carry a pregnancy safely.
No date is set for clinical trials yet. But according to the research, the main difference between human and lamb may come down to size. A lamb’s umbilical vessels are larger, so feeding in a tube is easier. With today’s advances in miniaturizing surgical methods, that seems like a challenge scientists can overcome.
Messenger RNA therapeutics
Back to “Star Trek.” The hypospray injector’s contents could cure just about any disease, even one newly discovered on a strange planet. That’s not unlike messenger RNA (mRNA) technology, a breakthrough that enabled scientists to quickly develop some of the first COVID-19 vaccines.
But vaccines are just the beginning of what this technology can do.
A whole field of immunotherapy is emerging that uses mRNA to deliver instructions to produce chimeric antigen receptor–modified immune cells (CAR-modified immune cells). These cells are engineered to target diseased cells and tissues, like cancer cells and harmful fibroblasts (scar tissue) that promote fibrosis in, for example, the heart and lungs.
The field is bursting with rodent research, and clinical trials have started for treating some advanced-stage malignancies.
Actual clinical use may be years away, but if all goes well, these medicines could help treat or even cure the core medical problems facing humanity. We’re talking cancer, heart disease, neurodegenerative disease – transforming one therapy into another by simply changing the mRNA’s “nucleotide sequence,” the blueprint containing instructions telling it what to do, and what disease to attack.
As this technology matures, we may start to feel as if we’re really on “Star Trek,” where Dr. Leonard “Bones” McCoy pulls out the same device to treat just about every disease or injury.
A version of this article first appeared on WebMD.com.
Pound of flesh buys less prison time
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Pound of flesh buys less prison time
We should all have more Shakespeare in our lives. Yeah, yeah, Shakespeare is meant to be played, not read, and it can be a struggle to herd teenagers through the Bard’s interesting and bloody tragedies, but even a perfunctory reading of “The Merchant of Venice” would hopefully have prevented the dystopian nightmare Massachusetts has presented us with today.
The United States has a massive shortage of donor organs. This is an unfortunate truth. So, to combat this issue, a pair of Massachusetts congresspeople have proposed HD 3822, which would allow prisoners to donate organs and/or bone marrow (a pound of flesh, so to speak) in exchange for up to a year in reduced prison time. Yes, that’s right. Give up pieces of yourself and the state of Massachusetts will deign to reduce your long prison sentence.
Oh, and before you dismiss this as typical Republican antics, the bill was sponsored by two Democrats, and in a statement one of them hoped to address racial disparities in organ donation, as people of color are much less likely to receive organs. Never mind that Black people are imprisoned at a much higher rate than Whites.
Yeah, this whole thing is what people in the business like to call an ethical disaster.
Fortunately, the bill will likely never be passed and it’s probably illegal anyway. A federal law from 1984 (how’s that for a coincidence) prevents people from donating organs for use in human transplantation in exchange for “valuable consideration.” In other words, you can’t sell your organs for profit, and in this case, reducing prison time would probably count as valuable consideration in the eyes of the courts.
Oh, and in case you’ve never read Merchant of Venice, Shylock, the character looking for the pound of flesh as payment for a debt? He’s the villain. In fact, it’s pretty safe to say that anyone looking to extract payment from human dismemberment is probably the bad guy of the story. Apparently that wasn’t clear.
How do you stop a fungi? With a deadly guy
Thanks to the new HBO series “The Last of Us,” there’s been a lot of talk about the upcoming fungi-pocalypse, as the show depicts the real-life “zombie fungus” Cordyceps turning humans into, you know, zombies.
No need to worry, ladies and gentleman, because science has discovered a way to turn back the fungal horde. A heroic, and environmentally friendly, alternative to chemical pesticides “in the fight against resistant fungi [that] are now resistant to antimycotics – partly because they are used in large quantities in agricultural fields,” investigators at the Leibniz Institute for Natural Product Research and Infection Biology in Jena, Germany, said in a written statement.
We are, of course, talking about Keanu Reeves. Wait a second. He’s not even in “The Last of Us.” Sorry folks, we are being told that it really is Keanu Reeves. Our champion in the inevitable fungal pandemic is movie star Keanu Reeves. Sort of. It’s actually keanumycin, a substance produced by bacteria of the genus Pseudomonas.
Really? Keanumycin? “The lipopeptides kill so efficiently that we named them after Keanu Reeves because he, too, is extremely deadly in his roles,” lead author Sebastian Götze, PhD, explained.
Dr. Götze and his associates had been working with pseudomonads for quite a while before they were able to isolate the toxins responsible for their ability to kill amoebae, which resemble fungi in some characteristics. When then finally tried the keanumycin against gray mold rot on hydrangea leaves, the intensely contemplative star of “The Matrix” and “John Wick” – sorry, wrong Keanu – the bacterial derivative significantly inhibited growth of the fungus, they said.
Additional testing has shown that keanumycin is not highly toxic to human cells and is effective against fungi such as Candida albicans in very low concentrations, which makes it a good candidate for future pharmaceutical development.
To that news there can be only one response from the substance’s namesake.
High fat, bye parasites
Fat. Fat. Fat. Seems like everyone is trying to avoid it these days, but fat may be good thing when it comes to weaseling out a parasite.
The parasite in this case is the whipworm, aka Trichuris trichiura. You can find this guy in the intestines of millions of people, where it causes long-lasting infections. Yikes … Researchers have found that the plan of attack to get rid of this invasive species is to boost the immune system, but instead of vitamin C and zinc it’s fat they’re pumping in. Yes, fat.
The developing countries with poor sewage that are at the highest risk for contracting parasites such as this also are among those where people ingest cheaper diets that are generally higher in fat. The investigators were interested to see how a high-fat diet would affect immune responses to the whipworms.
And, as with almost everything else, the researchers turned to mice, which were introduced to a closely related species, Trichuris muris.
A high-fat diet, rather than obesity itself, increases a molecule on T-helper cells called ST2, and this allows an increased T-helper 2 response, effectively giving eviction notices to the parasites in the intestinal lining.
To say the least, the researchers were surprised since “high-fat diets are mostly associated with increased pathology during disease,” said senior author Richard Grencis, PhD, of the University of Manchester (England), who noted that ST2 is not normally triggered with a standard diet in mice but the high-fat diet gave it a boost and an “alternate pathway” out.
Now before you start ordering extra-large fries at the drive-through to keep the whipworms away, the researchers added that they “have previously published that weight loss can aid the expulsion of a different gut parasite worm.” Figures.
Once again, though, signs are pointing to the gut for improved health.
Study documents link between preadolescent acne and elevated BMI
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
FROM PEDIATRIC DERMATOLOGY
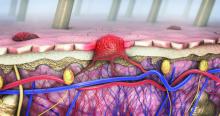
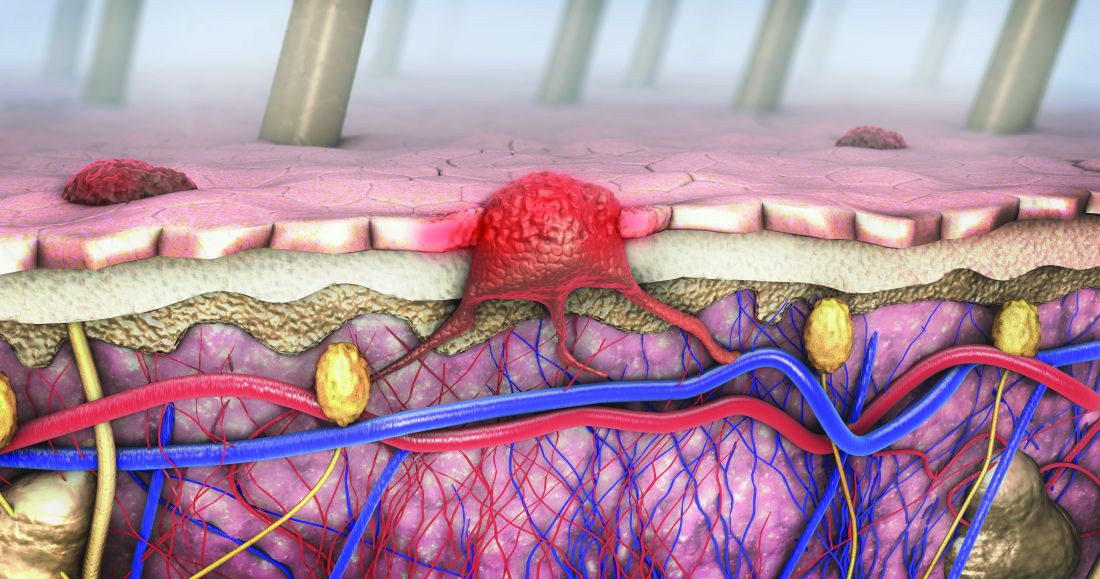
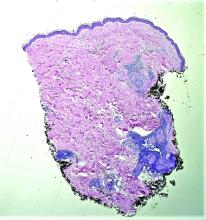
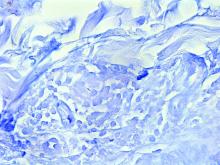
Dermoscopy, other modalities for improving melanoma diagnoses reviewed
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
San Diego – .
“I don’t think that’s going to change in the short term,” Travis W. Blalock, MD, director of dermatologic surgery, Mohs micrographic surgery, and cutaneous oncology at Emory University, Atlanta, said at the annual Cutaneous Malignancy Update. “But I do think we can supplement that with other modalities that will improve the clinical examination and help dermatopathologists as they assess and evaluate these lesions,” he said, adding: “The reality is, histopathology, while it may be the gold standard, is not necessarily a consistently reproducible evaluation. That raises the question: What can we do better?”
According to Dr. Blalock, the future may include more routine use of noninvasive genetic molecular assays to assist with the diagnostics challenges linked to the visual image and pattern recognition approach of detecting cutaneous melanoma. For example, a two-gene classification method based on LINC00518 and preferentially expressed antigen in melanoma (PRAME) gene expression was evaluated and validated in 555 pigmented lesions obtained noninvasively via adhesive patch biopsy.
“Today, you can pick up a kit from your local pharmacy that can tell you a bit about broad genetic susceptibilities,” he said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. He predicted that using adhesive patch biopsies to assess suspicious melanocytic lesions “is likely the wave of the future.” This may increase patient understanding “as to the types of risks they have, the different lesions they have, and minimize invasive disease, but it also will pose different challenges for us when it comes to deploying patient-centered health care. For example, in a patient with multiple different lesions, how are you going to keep track of them all?”
Dermoscopy
In Dr. Blalock’s clinical opinion, dermoscopy improves the sensitivity of human visual detection of melanoma and may allow detection before a lesion displays classical features described with the “ABCDE rule.” However, the learning curve for dermoscopy is steep, he added, and whether the technique should be considered a first-line tool or as a supplement to other methods of examining cutaneous lesions remains a matter of debate.
“Dermoscopy is our version of the stethoscope,” he said. “We need to figure out when we’re going to use it. Should we be using it all of the time or only some of the time? Based on the clinical setting, maybe it’s a personal choice, but this can be a helpful skill and art in your practice if you’re willing to take the time to learn.”
In 2007, the International Dermoscopy Society (IDS) established a proposal for the standardization and recommended criteria necessary to effectively convey dermoscopic findings to consulting physicians and colleagues. The document includes 10 points categorized as either recommended or optional for a standardized dermoscopy report.
“The first step is to assess the lesion to determine whether or not it’s melanocytic in the first place,” said Dr. Blalock. “There are many different features – the mile-high [global features] evaluation of the lesions – then more specific local features that may clue you in to specific diagnoses,” he noted. “Once we get past that first step of determining that a lesion is melanocytic, it’s not enough to stop there, because we don’t want to biopsy every single lesion that’s melanocytic,” so there is a need to determine which ones require intervention, which is where dermoscopy “gets trickier and a little more challenging.”
According to the IDS, a standard dermoscopy report should include the patient’s age, relevant history pertaining to the lesion, pertinent personal and family history (recommended); clinical description of the lesion (recommended); the two-step method of dermoscopy differentiating melanocytic from nonmelanocytic tumors (recommended); and the use of standardized terms to describe structures as defined by the Dermoscopy Consensus Report published in 2003.
For new terms, the document states, “it would be helpful” for the physician to provide a working definition (recommended); the dermoscopic algorithm used should be mentioned (optional); information on the imaging equipment and magnification (recommended); clinical and dermoscopic images of the tumor (recommended); a diagnosis or differential diagnosis (recommended); decision concerning management (recommended), and specific comments for the pathologist when excision and histopathologic examination are recommended (optional).
The 2007 IDS document also includes a proposed seven-point checklist to differentiate between benign and melanocytic lesions on dermoscopy. Three major criteria are worth two points each: The presence of an atypical pigment network, gray-blue areas (commonly known as the veil), and an atypical vascular pattern. Four minor criteria are worth one point each: Irregular streaks, irregular dots/globules, irregular pigmentation, and regression structures. A minimum total score of 3 is required to establish a diagnosis of melanoma.
Another diagnostic technique, digital mole mapping, involves the use of photography to detect new or changing lesions. Dr. Blalock described this approach as rife with limitations, including variations in quality, challenges of storing and maintaining records, cost, time required to evaluate them, and determining which patients are appropriate candidates.
Other techniques being evaluated include computer algorithms to help dermatologists determine the diagnosis of melanoma from dermoscopic images, electrical impedance spectroscopy for noninvasive evaluation of atypical pigmented lesions, and ultrasound for staging of cutaneous malignant tumors.
Ultimately, “I think we’ll have multiple tools in our belt,” Dr. Blalock said, adding, “How do we pull them out at the right time to improve the lives of our patients? Are we going to use ultrasound? Dermoscopy? Integrate them with some of the genetic findings?”
Dr. Blalock disclosed that he has served as a principal investigator for Castle Biosciences.
AT MELANOMA 2023
Spectrum of dermatologic adverse events associated with amivantamab use
associated with EGFR inhibitors and atypical presentations. Toxic effects, however, were mitigated by dose interruptions, dAE management, and amivantamab dose reductions, allowing for cancer therapy continuation in all cases. Amivantamab doses were reduced in 5 out of 6 cases, according to a research letter published in JAMA Dermatology.
The EGFR exon 20 insertion–mutation portends insensitivity to EGFR tyrosine kinase inhibitors and poor prognosis. Amivantamab, a bispecific monoclonal antibody targeting EGFR and mesenchymal epithelial transition factor (MET) is Food and Drug Administration approved for this population. Acneiform eruptions and pruritus are the most common dAEs associated with EGFR inhibitors, with xerosis, fissures, and nail and hair changes occurring additionally. While no FDA-approved monoclonal antibody targets MET exclusively, capmatinib and tepotinib (both tyrosine kinase inhibitors) inhibit MET. They have been associated with photosensitivity, acneiform rash, paronychia, xerosis, pruritus, and mucositis.
The Belzer et al. letter reviewed six consecutive cases (mean age, 58) of dAEs associated with amivantamab at two academic health centers (treated June 2021 to August 2022) in order to describe dAEs associated with amivantamab use. “I suspect the rate of dAEs with amivantamab is similar to the rate of dAEs associated with first- and second-generation EGFR inhibitors, where the majority of patients, actually 75%-90%, develop cutaneous toxicity,” said Jonathan Leventhal, MD, Yale University, New Haven, Conn., corresponding author for the Belzer et al. letter.
Time from treatment initiation with amivantamab to dAE ranged from less than 1 month to 4 months. All dAEs were grade 2 or 3 and all included acneiform eruptions. These were widespread in four cases and in another case complicated by impetiginization (culture results positive for methicillin-susceptible Staphylococcus aureus), and a further case was limited to the scalp, face, upper back, and upper chest. Others with widespread acneiform eruption included the face with hyperkeratotic crust of the scalp and dermatitis of the posterior neck. Fissuring of the palms and soles was noted in two cases with widespread acneiform eruptions. Paronychia with pyogenic granulomas was reported in four cases. Another case included onycholysis with suppurative paronychia.
In five cases amivantamab was stopped but successfully reinitiated at 67%-75% of the original dose. In one case amivantamab was continued at the original dose.
Doxycycline at 100 mg twice daily was included among all of the treatments for cutaneous dAEs. Silver nitrate cautery was applied for pyogenic granulomas in clinic. The case of grade 3 acneiform eruption of the scalp and face was treated with hydrogen peroxide soaks with debridement in clinic, doxycycline, aluminum acetate soaks, and triamcinolone ointment. All dermatologic cases resolved fully without scarring.
“It is very likely that this series highlights the more severe and unusual presentations of dAEs which were referred to oncodermatology. I suspect milder presentations were likely managed by oncologists,” Dr. Leventhal said in the interview.
“It is important for dermatologists and oncologists to be aware of the more severe and atypical dAEs associated with this novel FDA-approved targeted therapy.” Dr. Belzer said. “As amivantamab use increases, oncologists and dermatologists need to collaborate to ensure swift diagnosis and management of dAEs.”
One trial, the authors stated, revealed more than half of patients receiving EGFR inhibitors taking preemptive treatment with moisturizers, sunscreen, topical corticosteroids, and an oral tetracycline to have more than a 50% reduction in grade 2 or higher dAEs. Belzer et al. concluded that prophylactic treatment, including sun protection, should be considered before initiating treatment with amivantamab.
A limitation of the study, Belzer et al. acknowledged, was the small sample size.
Dr. Leventhal reported receiving personal fees from the advisory boards of Sanofi, Regeneron, and La Roche-Posay as well as clinical trial funding from Azitra and OnQuality Pharmaceuticals outside the submitted work.
associated with EGFR inhibitors and atypical presentations. Toxic effects, however, were mitigated by dose interruptions, dAE management, and amivantamab dose reductions, allowing for cancer therapy continuation in all cases. Amivantamab doses were reduced in 5 out of 6 cases, according to a research letter published in JAMA Dermatology.
The EGFR exon 20 insertion–mutation portends insensitivity to EGFR tyrosine kinase inhibitors and poor prognosis. Amivantamab, a bispecific monoclonal antibody targeting EGFR and mesenchymal epithelial transition factor (MET) is Food and Drug Administration approved for this population. Acneiform eruptions and pruritus are the most common dAEs associated with EGFR inhibitors, with xerosis, fissures, and nail and hair changes occurring additionally. While no FDA-approved monoclonal antibody targets MET exclusively, capmatinib and tepotinib (both tyrosine kinase inhibitors) inhibit MET. They have been associated with photosensitivity, acneiform rash, paronychia, xerosis, pruritus, and mucositis.
The Belzer et al. letter reviewed six consecutive cases (mean age, 58) of dAEs associated with amivantamab at two academic health centers (treated June 2021 to August 2022) in order to describe dAEs associated with amivantamab use. “I suspect the rate of dAEs with amivantamab is similar to the rate of dAEs associated with first- and second-generation EGFR inhibitors, where the majority of patients, actually 75%-90%, develop cutaneous toxicity,” said Jonathan Leventhal, MD, Yale University, New Haven, Conn., corresponding author for the Belzer et al. letter.
Time from treatment initiation with amivantamab to dAE ranged from less than 1 month to 4 months. All dAEs were grade 2 or 3 and all included acneiform eruptions. These were widespread in four cases and in another case complicated by impetiginization (culture results positive for methicillin-susceptible Staphylococcus aureus), and a further case was limited to the scalp, face, upper back, and upper chest. Others with widespread acneiform eruption included the face with hyperkeratotic crust of the scalp and dermatitis of the posterior neck. Fissuring of the palms and soles was noted in two cases with widespread acneiform eruptions. Paronychia with pyogenic granulomas was reported in four cases. Another case included onycholysis with suppurative paronychia.
In five cases amivantamab was stopped but successfully reinitiated at 67%-75% of the original dose. In one case amivantamab was continued at the original dose.
Doxycycline at 100 mg twice daily was included among all of the treatments for cutaneous dAEs. Silver nitrate cautery was applied for pyogenic granulomas in clinic. The case of grade 3 acneiform eruption of the scalp and face was treated with hydrogen peroxide soaks with debridement in clinic, doxycycline, aluminum acetate soaks, and triamcinolone ointment. All dermatologic cases resolved fully without scarring.
“It is very likely that this series highlights the more severe and unusual presentations of dAEs which were referred to oncodermatology. I suspect milder presentations were likely managed by oncologists,” Dr. Leventhal said in the interview.
“It is important for dermatologists and oncologists to be aware of the more severe and atypical dAEs associated with this novel FDA-approved targeted therapy.” Dr. Belzer said. “As amivantamab use increases, oncologists and dermatologists need to collaborate to ensure swift diagnosis and management of dAEs.”
One trial, the authors stated, revealed more than half of patients receiving EGFR inhibitors taking preemptive treatment with moisturizers, sunscreen, topical corticosteroids, and an oral tetracycline to have more than a 50% reduction in grade 2 or higher dAEs. Belzer et al. concluded that prophylactic treatment, including sun protection, should be considered before initiating treatment with amivantamab.
A limitation of the study, Belzer et al. acknowledged, was the small sample size.
Dr. Leventhal reported receiving personal fees from the advisory boards of Sanofi, Regeneron, and La Roche-Posay as well as clinical trial funding from Azitra and OnQuality Pharmaceuticals outside the submitted work.
associated with EGFR inhibitors and atypical presentations. Toxic effects, however, were mitigated by dose interruptions, dAE management, and amivantamab dose reductions, allowing for cancer therapy continuation in all cases. Amivantamab doses were reduced in 5 out of 6 cases, according to a research letter published in JAMA Dermatology.
The EGFR exon 20 insertion–mutation portends insensitivity to EGFR tyrosine kinase inhibitors and poor prognosis. Amivantamab, a bispecific monoclonal antibody targeting EGFR and mesenchymal epithelial transition factor (MET) is Food and Drug Administration approved for this population. Acneiform eruptions and pruritus are the most common dAEs associated with EGFR inhibitors, with xerosis, fissures, and nail and hair changes occurring additionally. While no FDA-approved monoclonal antibody targets MET exclusively, capmatinib and tepotinib (both tyrosine kinase inhibitors) inhibit MET. They have been associated with photosensitivity, acneiform rash, paronychia, xerosis, pruritus, and mucositis.
The Belzer et al. letter reviewed six consecutive cases (mean age, 58) of dAEs associated with amivantamab at two academic health centers (treated June 2021 to August 2022) in order to describe dAEs associated with amivantamab use. “I suspect the rate of dAEs with amivantamab is similar to the rate of dAEs associated with first- and second-generation EGFR inhibitors, where the majority of patients, actually 75%-90%, develop cutaneous toxicity,” said Jonathan Leventhal, MD, Yale University, New Haven, Conn., corresponding author for the Belzer et al. letter.
Time from treatment initiation with amivantamab to dAE ranged from less than 1 month to 4 months. All dAEs were grade 2 or 3 and all included acneiform eruptions. These were widespread in four cases and in another case complicated by impetiginization (culture results positive for methicillin-susceptible Staphylococcus aureus), and a further case was limited to the scalp, face, upper back, and upper chest. Others with widespread acneiform eruption included the face with hyperkeratotic crust of the scalp and dermatitis of the posterior neck. Fissuring of the palms and soles was noted in two cases with widespread acneiform eruptions. Paronychia with pyogenic granulomas was reported in four cases. Another case included onycholysis with suppurative paronychia.
In five cases amivantamab was stopped but successfully reinitiated at 67%-75% of the original dose. In one case amivantamab was continued at the original dose.
Doxycycline at 100 mg twice daily was included among all of the treatments for cutaneous dAEs. Silver nitrate cautery was applied for pyogenic granulomas in clinic. The case of grade 3 acneiform eruption of the scalp and face was treated with hydrogen peroxide soaks with debridement in clinic, doxycycline, aluminum acetate soaks, and triamcinolone ointment. All dermatologic cases resolved fully without scarring.
“It is very likely that this series highlights the more severe and unusual presentations of dAEs which were referred to oncodermatology. I suspect milder presentations were likely managed by oncologists,” Dr. Leventhal said in the interview.
“It is important for dermatologists and oncologists to be aware of the more severe and atypical dAEs associated with this novel FDA-approved targeted therapy.” Dr. Belzer said. “As amivantamab use increases, oncologists and dermatologists need to collaborate to ensure swift diagnosis and management of dAEs.”
One trial, the authors stated, revealed more than half of patients receiving EGFR inhibitors taking preemptive treatment with moisturizers, sunscreen, topical corticosteroids, and an oral tetracycline to have more than a 50% reduction in grade 2 or higher dAEs. Belzer et al. concluded that prophylactic treatment, including sun protection, should be considered before initiating treatment with amivantamab.
A limitation of the study, Belzer et al. acknowledged, was the small sample size.
Dr. Leventhal reported receiving personal fees from the advisory boards of Sanofi, Regeneron, and La Roche-Posay as well as clinical trial funding from Azitra and OnQuality Pharmaceuticals outside the submitted work.
FROM JAMA DERMATOLOGY
Little evidence to support lasers for ‘vaginal rejuvenation’
Laser devices licensed in Canada to treat genitourinary syndrome of menopause (GSM) are often marketed for vaginal rejuvenation with claims that they will tighten the vagina and improve sexual function, despite lack of evidence, a new commentary reveals.
Vaginal lasers heat the vaginal epithelium and cause thermal necrosis. This intervention induces collagen remodeling and synthesis, neovascularization, and elastin formation and may result in improved vaginal elasticity and restoration of premenopausal epithelial function, according to coauthors Blayne Welk, MD, MSc, an associate professor of urologic surgery at Western University, London, Ont., and Erin Kelly, MD, a lecturer in obstetrics and gynecology at the University of Alberta, Edmonton.
Their patients’ questions and experiences with the laser devices prompted the commentary, they told this news organization.
“A large part of my practice involves addressing GSM and urinary incontinence,” said Dr. Kelly. “Many women present to the clinic having heard of vaginal laser procedures, having had vaginal laser procedures, or having been told they need vaginal laser procedures. My impression has been that these procedures are being marketed to women … without rigorous study.”
“Many women are reluctant to have mesh slings for stress incontinence due to some of the potential risks,” and they are looking for less invasive options, said Dr. Welk. Over the past few years, he has had increasing questions from patients about the use of lasers to improve this condition.
The commentary was published online in the Canadian Medical Association Journal.
Transparency needed
The first vaginal energy device was licensed by Health Canada in 2015 to treat GSM. That meant the device was deemed to have met basic safety, effectiveness, and quality criteria. But no controlled studies are required for regulatory approval of such devices, and after licensing, some providers rebranded the device indication from GSM to vaginal rejuvenation, said Dr. Kelly and Dr. Welk.
Vaginal laser therapies are offered throughout Canada, with at least one provider of vaginal rejuvenation procedures in the 10 most populous cities. Under the current system, the number of patients who pay for these procedures and the amount that they pay cannot be tracked. Nor can the number of vaginal laser systems active in Canada be tracked. Patients can refer themselves for the service, and providers’ publicly quoted costs (on websites, for example) are thousands of dollars for treatment.
The rebranding for vaginal rejuvenation “represents a difference between the licensing of a medical device by Health Canada and the way that these devices are used and marketed,” according to the commentary. “A procedure with limited high-quality evidence supporting its efficacy and a potential financial conflict of interest for providers may not be serving the best interests of people in Canada, even if the risk of adverse events is low.”
Updates to Canada’s medical devices action plan, including mandatory reporting of serious incidents and the ability to compel manufacturers to provide information on safety and effectiveness, “represent important progress,” according to Dr. Kelly and Dr. Welk. However, problems persist, including lack of a requirement for peer-reviewed, controlled studies.
Furthermore, women who undergo laser treatment for GSM, urinary incontinence, or vaginal rejuvenation may not receive a proper medical evaluation and standard treatments, the authors noted.
“I would like to see more transparency and public-facing information available on approved medical devices,” said Dr. Welk. “Health Canada has an online database of approved devices, but no information around the evidence submitted during the approval process is available, nor are the indications for the various devices.”
In addition, he said, many devices in the registry are listed by a serial number rather than the name that would be familiar to the public, “making it hard to match up information.”
Dr. Kelly added the “encouraging” news that the Canadian Society for Pelvic Medicine is working with Health Canada to “improve knowledge translation when it comes to transparency regarding medical devices.”
Medicine before marketing
“The commentary provides an accurate and evidence-based assessment of the use of vaginal laser treatments,” Jason Abbott, B Med (Hons), PhD, professor of gynecology at the University of New South Wales, Sydney, told this news organization. “The marketing of this device is a case of putting the cart before the horse. It is essential that strong, scientific, and reproducible studies be available on efficacy and safety before there is a direct-to-consumer marketing approach.”
Clinicians should advise patients when the treatment effect is likely to be minimal or risky, especially when there is a financial incentive to the clinician, he said. “Governments, regulators, and medical societies have a duty of care to the public to make sure that the medicine comes before the marketing. Otherwise, we are no better than snake oil sellers.
“Given the size of studies to date, the improvement in symptoms following treatment may be less than a few percent,” he noted. “That may be acceptable to some women. We don’t know.”
Dr. Abbott’s team is conducting research to define what women would want as a minimal level of improvement, the maximum cost, and the maximum risk from the laser procedure.
“In cancer … the benefit of a new treatment may only be a few percent for survival,” he said. “That may be completely acceptable for some or even many patients. What we cannot do, however, is extrapolate those same expectations to a treatment for a benign condition where quality of life is compromised.”
Echoing Dr. Kelly and Dr. Welk, Dr. Abbott said, “It is important that there be transparency in the clinical communication. Patients should be told that the best scientific studies that are judged based on their quality show there is no benefit to laser treatment for GSM or urinary incontinence.”
Although the medical risks may be low, he added, “financial risk also needs to be discussed. Patients should be encouraged to participate in clinical trials where there is no cost to them to gain the information first, before wholesale uptake of the treatment. … Should patients still wish to undergo the procedure once the risks and an honest account of the evidence is given to them, that of course is their choice.” Dr. Kelly, Dr. Welk, and Dr. Abbott had no commercial funding or relevant financial relationships to report.
A version of this article first appeared on Medscape.com.
Laser devices licensed in Canada to treat genitourinary syndrome of menopause (GSM) are often marketed for vaginal rejuvenation with claims that they will tighten the vagina and improve sexual function, despite lack of evidence, a new commentary reveals.
Vaginal lasers heat the vaginal epithelium and cause thermal necrosis. This intervention induces collagen remodeling and synthesis, neovascularization, and elastin formation and may result in improved vaginal elasticity and restoration of premenopausal epithelial function, according to coauthors Blayne Welk, MD, MSc, an associate professor of urologic surgery at Western University, London, Ont., and Erin Kelly, MD, a lecturer in obstetrics and gynecology at the University of Alberta, Edmonton.
Their patients’ questions and experiences with the laser devices prompted the commentary, they told this news organization.
“A large part of my practice involves addressing GSM and urinary incontinence,” said Dr. Kelly. “Many women present to the clinic having heard of vaginal laser procedures, having had vaginal laser procedures, or having been told they need vaginal laser procedures. My impression has been that these procedures are being marketed to women … without rigorous study.”
“Many women are reluctant to have mesh slings for stress incontinence due to some of the potential risks,” and they are looking for less invasive options, said Dr. Welk. Over the past few years, he has had increasing questions from patients about the use of lasers to improve this condition.
The commentary was published online in the Canadian Medical Association Journal.
Transparency needed
The first vaginal energy device was licensed by Health Canada in 2015 to treat GSM. That meant the device was deemed to have met basic safety, effectiveness, and quality criteria. But no controlled studies are required for regulatory approval of such devices, and after licensing, some providers rebranded the device indication from GSM to vaginal rejuvenation, said Dr. Kelly and Dr. Welk.
Vaginal laser therapies are offered throughout Canada, with at least one provider of vaginal rejuvenation procedures in the 10 most populous cities. Under the current system, the number of patients who pay for these procedures and the amount that they pay cannot be tracked. Nor can the number of vaginal laser systems active in Canada be tracked. Patients can refer themselves for the service, and providers’ publicly quoted costs (on websites, for example) are thousands of dollars for treatment.
The rebranding for vaginal rejuvenation “represents a difference between the licensing of a medical device by Health Canada and the way that these devices are used and marketed,” according to the commentary. “A procedure with limited high-quality evidence supporting its efficacy and a potential financial conflict of interest for providers may not be serving the best interests of people in Canada, even if the risk of adverse events is low.”
Updates to Canada’s medical devices action plan, including mandatory reporting of serious incidents and the ability to compel manufacturers to provide information on safety and effectiveness, “represent important progress,” according to Dr. Kelly and Dr. Welk. However, problems persist, including lack of a requirement for peer-reviewed, controlled studies.
Furthermore, women who undergo laser treatment for GSM, urinary incontinence, or vaginal rejuvenation may not receive a proper medical evaluation and standard treatments, the authors noted.
“I would like to see more transparency and public-facing information available on approved medical devices,” said Dr. Welk. “Health Canada has an online database of approved devices, but no information around the evidence submitted during the approval process is available, nor are the indications for the various devices.”
In addition, he said, many devices in the registry are listed by a serial number rather than the name that would be familiar to the public, “making it hard to match up information.”
Dr. Kelly added the “encouraging” news that the Canadian Society for Pelvic Medicine is working with Health Canada to “improve knowledge translation when it comes to transparency regarding medical devices.”
Medicine before marketing
“The commentary provides an accurate and evidence-based assessment of the use of vaginal laser treatments,” Jason Abbott, B Med (Hons), PhD, professor of gynecology at the University of New South Wales, Sydney, told this news organization. “The marketing of this device is a case of putting the cart before the horse. It is essential that strong, scientific, and reproducible studies be available on efficacy and safety before there is a direct-to-consumer marketing approach.”
Clinicians should advise patients when the treatment effect is likely to be minimal or risky, especially when there is a financial incentive to the clinician, he said. “Governments, regulators, and medical societies have a duty of care to the public to make sure that the medicine comes before the marketing. Otherwise, we are no better than snake oil sellers.
“Given the size of studies to date, the improvement in symptoms following treatment may be less than a few percent,” he noted. “That may be acceptable to some women. We don’t know.”
Dr. Abbott’s team is conducting research to define what women would want as a minimal level of improvement, the maximum cost, and the maximum risk from the laser procedure.
“In cancer … the benefit of a new treatment may only be a few percent for survival,” he said. “That may be completely acceptable for some or even many patients. What we cannot do, however, is extrapolate those same expectations to a treatment for a benign condition where quality of life is compromised.”
Echoing Dr. Kelly and Dr. Welk, Dr. Abbott said, “It is important that there be transparency in the clinical communication. Patients should be told that the best scientific studies that are judged based on their quality show there is no benefit to laser treatment for GSM or urinary incontinence.”
Although the medical risks may be low, he added, “financial risk also needs to be discussed. Patients should be encouraged to participate in clinical trials where there is no cost to them to gain the information first, before wholesale uptake of the treatment. … Should patients still wish to undergo the procedure once the risks and an honest account of the evidence is given to them, that of course is their choice.” Dr. Kelly, Dr. Welk, and Dr. Abbott had no commercial funding or relevant financial relationships to report.
A version of this article first appeared on Medscape.com.
Laser devices licensed in Canada to treat genitourinary syndrome of menopause (GSM) are often marketed for vaginal rejuvenation with claims that they will tighten the vagina and improve sexual function, despite lack of evidence, a new commentary reveals.
Vaginal lasers heat the vaginal epithelium and cause thermal necrosis. This intervention induces collagen remodeling and synthesis, neovascularization, and elastin formation and may result in improved vaginal elasticity and restoration of premenopausal epithelial function, according to coauthors Blayne Welk, MD, MSc, an associate professor of urologic surgery at Western University, London, Ont., and Erin Kelly, MD, a lecturer in obstetrics and gynecology at the University of Alberta, Edmonton.
Their patients’ questions and experiences with the laser devices prompted the commentary, they told this news organization.
“A large part of my practice involves addressing GSM and urinary incontinence,” said Dr. Kelly. “Many women present to the clinic having heard of vaginal laser procedures, having had vaginal laser procedures, or having been told they need vaginal laser procedures. My impression has been that these procedures are being marketed to women … without rigorous study.”
“Many women are reluctant to have mesh slings for stress incontinence due to some of the potential risks,” and they are looking for less invasive options, said Dr. Welk. Over the past few years, he has had increasing questions from patients about the use of lasers to improve this condition.
The commentary was published online in the Canadian Medical Association Journal.
Transparency needed
The first vaginal energy device was licensed by Health Canada in 2015 to treat GSM. That meant the device was deemed to have met basic safety, effectiveness, and quality criteria. But no controlled studies are required for regulatory approval of such devices, and after licensing, some providers rebranded the device indication from GSM to vaginal rejuvenation, said Dr. Kelly and Dr. Welk.
Vaginal laser therapies are offered throughout Canada, with at least one provider of vaginal rejuvenation procedures in the 10 most populous cities. Under the current system, the number of patients who pay for these procedures and the amount that they pay cannot be tracked. Nor can the number of vaginal laser systems active in Canada be tracked. Patients can refer themselves for the service, and providers’ publicly quoted costs (on websites, for example) are thousands of dollars for treatment.
The rebranding for vaginal rejuvenation “represents a difference between the licensing of a medical device by Health Canada and the way that these devices are used and marketed,” according to the commentary. “A procedure with limited high-quality evidence supporting its efficacy and a potential financial conflict of interest for providers may not be serving the best interests of people in Canada, even if the risk of adverse events is low.”
Updates to Canada’s medical devices action plan, including mandatory reporting of serious incidents and the ability to compel manufacturers to provide information on safety and effectiveness, “represent important progress,” according to Dr. Kelly and Dr. Welk. However, problems persist, including lack of a requirement for peer-reviewed, controlled studies.
Furthermore, women who undergo laser treatment for GSM, urinary incontinence, or vaginal rejuvenation may not receive a proper medical evaluation and standard treatments, the authors noted.
“I would like to see more transparency and public-facing information available on approved medical devices,” said Dr. Welk. “Health Canada has an online database of approved devices, but no information around the evidence submitted during the approval process is available, nor are the indications for the various devices.”
In addition, he said, many devices in the registry are listed by a serial number rather than the name that would be familiar to the public, “making it hard to match up information.”
Dr. Kelly added the “encouraging” news that the Canadian Society for Pelvic Medicine is working with Health Canada to “improve knowledge translation when it comes to transparency regarding medical devices.”
Medicine before marketing
“The commentary provides an accurate and evidence-based assessment of the use of vaginal laser treatments,” Jason Abbott, B Med (Hons), PhD, professor of gynecology at the University of New South Wales, Sydney, told this news organization. “The marketing of this device is a case of putting the cart before the horse. It is essential that strong, scientific, and reproducible studies be available on efficacy and safety before there is a direct-to-consumer marketing approach.”
Clinicians should advise patients when the treatment effect is likely to be minimal or risky, especially when there is a financial incentive to the clinician, he said. “Governments, regulators, and medical societies have a duty of care to the public to make sure that the medicine comes before the marketing. Otherwise, we are no better than snake oil sellers.
“Given the size of studies to date, the improvement in symptoms following treatment may be less than a few percent,” he noted. “That may be acceptable to some women. We don’t know.”
Dr. Abbott’s team is conducting research to define what women would want as a minimal level of improvement, the maximum cost, and the maximum risk from the laser procedure.
“In cancer … the benefit of a new treatment may only be a few percent for survival,” he said. “That may be completely acceptable for some or even many patients. What we cannot do, however, is extrapolate those same expectations to a treatment for a benign condition where quality of life is compromised.”
Echoing Dr. Kelly and Dr. Welk, Dr. Abbott said, “It is important that there be transparency in the clinical communication. Patients should be told that the best scientific studies that are judged based on their quality show there is no benefit to laser treatment for GSM or urinary incontinence.”
Although the medical risks may be low, he added, “financial risk also needs to be discussed. Patients should be encouraged to participate in clinical trials where there is no cost to them to gain the information first, before wholesale uptake of the treatment. … Should patients still wish to undergo the procedure once the risks and an honest account of the evidence is given to them, that of course is their choice.” Dr. Kelly, Dr. Welk, and Dr. Abbott had no commercial funding or relevant financial relationships to report.
A version of this article first appeared on Medscape.com.
Systemic sclerosis antibodies show link to interstitial lung disease in RA
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF TRANSLATIONAL AUTOIMMUNITY
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
A White male presented with a 1½-year history of a progressive hypoesthetic annular, hyperpigmented plaque on the upper arm
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
A 44-year-old White male presented with a 1½-year history of a progressive hypoesthetic annular, mildly hyperpigmented plaque on the left posterior upper arm.
He denied pruritus, pain, or systemic symptoms including weight loss, visual changes, cough, dyspnea, and abdominal pain. He also denied any paresthesia or weakness. On physical examination, there is a subtle, solitary 4-cm annular skin-colored thin plaque on the patient's left posterior upper arm (Figure 1).
Punch biopsy of the lesion was performed, and the histopathological findings are illustrated in Figure 2.