User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Radial arteries show CABG survival advantage over saphenous veins
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
FROM ACC 2020
COVID-19 less severe in children, yet questions for pediatricians remain
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
COVID-19 is less severe in children, compared with adults, early data suggest. “Yet many questions remain, especially regarding the effects on children with special health care needs,” according to a viewpoint recently published in JAMA Pediatrics.
The COVID-19 pandemic also raises questions about clinic visits for healthy children in communities with widespread transmission and about the unintended effects of school closures and other measures aimed at slowing the spread of the disease, wrote Sonja A. Rasmussen, MD, and Lindsay A. Thompson, MD, both of the University of Florida, Gainesville.
In communities with widespread outbreaks, telephone triage and expanded use of telehealth may be needed to limit nonurgent clinic visits, they suggested.
“Community mitigation interventions, such as school closures, cancellation of mass gatherings, and closure of public places are appropriate” in places with widespread transmission, Dr. Rasmussen and Dr. Thompson wrote. “If these measures are required, pediatricians need to advocate to alleviate unintended consequences or inadvertent expansion of health disparities on children, such as by finding ways to maintain nutrition for those who depend on school lunches and provide online mental health services for stress management for families whose routines might be severely interrupted for an extended period of time.”
Continued preventive care for infants and vaccinations for younger children may be warranted, they wrote.
Clinical course
Overall, children have experienced lower-than-expected rates of COVID-19 disease, and deaths in this population appear to be rare, Dr. Rasmussen and Dr. Thompson wrote.
Common symptoms of COVID-19 in adults include fever, cough, myalgia, shortness of breath, headache, and diarrhea, and children have similar manifestations. In adults, older age and underlying illness increase the risk of severe disease. There has not been convincing evidence of intrauterine transmission of COVID-19, and whether breastfeeding can transmit the virus is unknown, they noted.
An analysis of more than 72,000 cases from China found that 1.2% were in patients aged 10-19 years, and 0.9% were in patients younger than 10 years. One death occurred in the adolescent age range. A separate analysis of 2,143 confirmed and suspected pediatric cases in China indicated that infants were at higher risk of severe disease (11%), compared with older children – 4% for those aged 11-15 years, and 3% in those 16 years and older.
There is less data available about the clinical course of COVID-19 in children in the United States, the authors noted. But among more than 4,000 patients with COVID-19 in the United States through March 16, no ICU admissions or deaths were reported for patients aged younger than 19 years (MMWR Morb Mortal Wkly Rep. 2020 Mar 26;69[12]:343-6).
Still, researchers have suggested that children with underlying illness may be at greater risk of COVID-19. In a study of 20 children with COVID-19 in China, 7 of the patients had a history of congenital or acquired disease, potentially indicating that they were more susceptible to the virus (Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718). Chest CT consolidations with surrounding halo sign was evident in half of the patients, and procalcitonin elevation was seen in 80% of the children; these were signs common in children, but not in adults with COVID-19.
“About 10% of children in the U.S. have asthma; many children live with other pulmonary, cardiac, neuromuscular, or genetic diseases that affect their ability to handle respiratory disease, and other children are immunosuppressed because of illness or its treatment,” Dr. Rasmussen and Dr. Thompson wrote. “It is possible that these children will experience COVID-19 differently than counterparts of the same ages who are healthy.”
The authors reported that they had no financial disclosures.
SOURCE: Rasmussen SA, Thompson LA. JAMA Pediatr. 2020 Apr 3. doi: 10.1001/jamapediatrics.2020.1224.
FROM JAMA PEDIATRICS
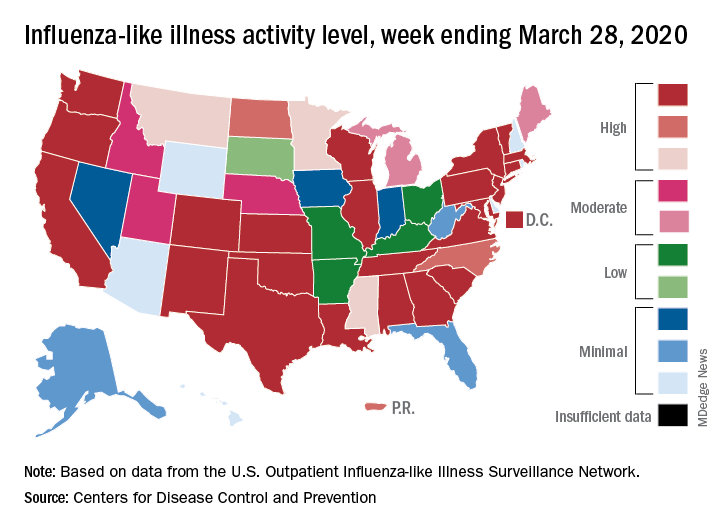
Flu activity down from its third peak of the season, COVID-19 still a factor
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
First report of MM patient successfully treated for COVID-19 with tocilizumab
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
Recent research has shown that severe cases of COVID-19 show an excessive immune response and a strong cytokine storm, which may include high levels of granulocyte-macrophage colony-stimulating factor (GSF) and interleukin-6 (IL-6). Following up on that research, investigators from China reported the first case of COVID-19 in a patient with multiple myeloma (MM) who was successfully treated with the humanized anti–IL-6 receptor antibody tocilizumab (an off-label use in the United States). The exceptional case report was published online in Blood Advances, an American Society of Hematology journal.
A 60-year-old man working in Wuhan, China, developed chest tightness without fever and cough on Feb. 1, 2020, and was admitted immediately after computed tomography (CT) imaging of his chest showed multiple ground-glass opacities and pneumatocele located in both subpleural spaces. He received 400 mg of moxifloxacin IV daily for 3 days while swab specimens were collected and tested by real-time reverse transcriptase–polymerase chain reaction. A positive result for SARS-CoV-2 infection was received 3 days later. The patient was subsequently given 200-mg umifenovir (Arbidol) tablets orally, three times daily, for antiviral treatment.
The patient had a history of symptomatic MM, which was diagnosed in 2015. The patient received two cycles of induction chemotherapy consisting of bortezomib, thalidomide, and dexamethasone, and his symptoms completely disappeared. After that, he received thalidomide for maintenance.
Chest CT imaging on hospital day 8 showed that the bilateral, multiple ground-glass opacities from the first scan remained, and laboratory investigations revealed a high level of serum IL-6. On hospital day 9, the patient was given a single, one-time dose of 8 mg/kg tocilizumab, administered by IV. On hospital day 12, his chest tightness disappeared. “After tocilizumab administration, the IL-6 level decreased gradually over the following 10 days (from 121.59 to 20.81 pg/mL), then increased rapidly to the peak (317.38 pg/mL), and then decreased to a low level (117.10 pg/mL). The transient rebounding of the IL-6 level to the peak does not mean COVID-19 relapse: Instead, this might be attributed to the recovery of the normal T cells,” the authors wrote.
On hospital day 19, the patient’s chest CT scan showed that the range of ground-glass opacities had obviously decreased, and he was declared cured and discharged from the hospital. The patient had no symptoms of MM, and related laboratory findings were all in normal ranges, according to the researchers.
“This case is the first to prove that tocilizumab is effective in the treatment of COVID-19 in MM with obvious clinical recovery; however, randomized controlled trials are needed to determine the safety and efficacy of tocilizumab,” the researchers concluded.
The authors declared that they had no conflicts of interest.
SOURCE: Zhang X et al. Blood Adv. 2020;4(7):1307-10.
FROM BLOOD ADVANCES
FDA grants emergency authorization for first rapid antibody test for COVID-19
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
The U.S. Food and Drug Administration has granted Cellex an emergency use authorization to market a rapid antibody test for COVID-19, the first antibody test released amidst the pandemic.
“It is reasonable to believe that your product may be effective in diagnosing COVID-19,” and “there is no adequate, approved, and available alternative,” the agency said in a letter to Cellex.
A drop of serum, plasma, or whole blood is placed into a well on a small cartridge, and the results are read 15-20 minutes later; lines indicate the presence of IgM, IgG, or both antibodies against the SARS-CoV-2 virus.
Of 128 samples confirmed positive by reverse transcription polymerase chain reaction in premarket testing, 120 tested positive by IgG, IgM, or both. Of 250 confirmed negative, 239 were negative by the rapid test.
The numbers translated to a positive percent agreement with RT-PCR of 93.8% (95% CI: 88.06-97.26%) and a negative percent agreement of 96.4% (95% CI: 92.26-97.78%), according to labeling.
“Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection,” the labeling states.
Negative results do not rule out infection; antibodies might not have had enough time to form or the virus could have had a minor amino acid mutation in the epitope recognized by the antibodies screened for in the test. False positives can occur due to cross-reactivity with antibodies from previous infections, such as from other coronaviruses.
Labeling suggests that people who test negative should be checked again in a few days, and positive results should be confirmed by other methods. Also, the intensity of the test lines do not necessarily correlate with SARS-CoV-2 antibody titers.
As part of its authorization, the FDA waived good manufacturing practice requirements, but stipulated that advertising must state that the test has not been formally approved by the agency.
Testing is limited to Clinical Laboratory Improvement Amendments-certified labs. Positive results are required to be reported to public health authorities. The test can be ordered through Cellex distributors or directly from the company.
IgM antibodies are generally detectable several days after the initial infection, while IgG antibodies take longer. It’s not known how long COVID-19 antibodies persist after the infection has cleared, the agency said.
Survey: COVID-19 is getting in our heads
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
As the COVID-19 pandemic sweeps across the United States, it is increasingly affecting those who are not infected. Social bonds are being broken, businesses are closing, jobs are being lost, and the stress is mounting.

In a poll conducted March 25-30, 45% of Americans said that stress resulting from the pandemic is having a negative impact on their mental health, compared with 32% expressing that view just 2 weeks earlier, the Kaiser Family Foundation reported April 2.
In the later survey, the effect looked like this: 19% of all respondents said that the pandemic has had a major negative impact and 26% said it has been minor so far. Women were more likely than men (24% vs. 15%) to report a major impact, as were blacks and Hispanic adults (both at 24%) compared with whites (17%), the KFF investigators said.
More Hispanic (44%) and black (42%) respondents also said that they had already lost their job, lost income, or had their hours reduced without pay as a result of the pandemic, compared with whites (36%). Among all respondents, 26% had lost income from a job or business and 28% had lost their job, been laid off, or had their hours reduced without pay, according to KFF.
A majority of respondents (57%) reported “being worried they will put themselves at risk of exposure to coronavirus because they can’t afford to stay home and miss work,” the researchers said. That figure is up from 35% in the earlier survey.
Anxiety about work-related exposure was even higher among hourly workers or those who get paid by the job (61%) and among employed adults who earn less than $40,000 annually (72%), they reported.
Overall, 72% of respondents said that their lives have been disrupted “a lot” or “some” by the coronavirus outbreak, and that is a jump of 32 percentage points over the previous poll, the investigators noted.
The disruption is expected to continue, it seems, as 74% believe that the worst is yet to come “in spite of the health, social and economic upheaval that Americans are already experiencing,” they wrote.
First presumptive case of encephalitis linked to COVID-19 reported
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
“As the number of patients with COVID-19 increases worldwide, clinicians and radiologists should be watching for this presentation among patients presenting with COVID-19 and altered mental status,” the clinicians advise in a report published online March 31 in Radiology.
“This is significant for all providers to be aware of and looking out for in [COVID-19] patients who present with an altered level of consciousness. This complication is as devastating as severe lung disease,” Elissa Fory, MD, a neurologist with Henry Ford who was part of the team of medical experts that made the diagnosis, said in a statement.
“We need to be thinking of how we’re going to incorporate patients with severe neurological disease into our treatment paradigm,” Fory added.
Brent Griffith, MD, radiologist with Henry Ford and senior author of the case report, said the case shows “the important role that imaging can play in COVID-19 cases.”
Diagnosed via neuroimaging
The 58-year-old woman presented with a 3-day history of fever, cough, and muscle aches ― symptoms consistent with COVID-19. She was transported by ambulance to the emergency department and showed signs of confusion, lethargy, and disorientation.
The woman tested negative for influenza, but a rapid COVID-19 test confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was later diagnosed with acute hemorrhagic necrotizing encephalopathy.
“The team had suspected encephalitis at the outset, but then back-to-back CT and MRI scans made the diagnosis,” Fory said in the statement.
Noncontrast head CT revealed “symmetric hypoattenuation within the bilateral medial thalami with a normal CT angiogram and CT venogram,” the team reports in their article. Brain MRI showed “hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.”
The patient was started on intravenous immunoglobulin but not high-dose steroids, because of concern for respiratory compromise. As of April 1, the patient was hospitalized in serious condition. Henry Ford Hospital has not provided an update.
Acute necrotizing encephalopathy (ANE) is a rare complication of viral infections, but until now, it has not been known to have occurred as a result of COVID-19 infection. ANE has been associated with intracranial “cytokine storms,” and a recent report in the Lancet suggested that a subgroup of patients with severe COVID-19 might develop a cytokine storm syndrome.
Commenting for Medscape Medical News, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University in St. Louis, Missouri, said, “Since this is just one report of one patient, the findings are the most preliminary we can conceive, and more research is needed to determine the extent to which COVID-19 may affect the central nervous system.”
Fory, Griffith, and Raji have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Survey shows just how dire PPE shortages are at many hospitals
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
As the COVID-19 pandemic spreads over the country, nearly half (48%) of US healthcare facilities — of various types and sizes — are already or almost out of respirators for treating patients, according to the results of a national online survey of infection prevention professionals.
Conducted during March 23-25 by the Association for Professionals in Infection Control and Epidemiology (APIC), the survey asked APIC’s 11,922 US-based infection preventionist members to rank their facilities’ supply of personal protective equipment (PPE) and key items, such as hand sanitizer and cleaning products, on a 5-point scale from having “plenty” to “none.”
Overall, 1,140 (9.6%) infection preventionists responded. Almost 70% of respondents represented a healthcare system rather than a single facility, and facilities ranged from hospitals (42.7%) to ambulatory care (17.4%) and dialysis (2.7%). The centers, from all 50 states and Washington, D.C., ranged in size from those with 1 to 50 beds to those with more than 300 beds.
and 317 (27.8%) said they were almost out of the devices, which are needed to protect healthcare workers managing patients with COVID-19 and different infectious diseases.
The survey was posted Friday on the APIC website.
Other findings from the survey include:
- Nearly half of respondents (49.2%) said their centers lack sufficient enough face shields, with 36.5% reporting being almost out and 12.6% reporting being completely out.
- Approximately one third (31.7%) of respondents reported being completely or nearly out of face masks.
- Even simple hand sanitizer is in short supply at more than 1 in 4 facilities surveyed; 25.6% of respondents said they are almost out and 2.6% are completely out.
- Nearly 30% of respondents reported accessing supplemental PPE through state or local resources, while 24.6% said they accepted private donations of supplies.
- Fewer than one-third (31.5%) said they had sufficient gowns.
- About 28% said they were almost out of protective respirators, while 20.5% said they have none.
- Only 12.3% said they have received supplies from federal resources, including the Strategic National Stockpile, which is controlled by the Department of Health and Human Services.
- 17.2% of respondents reported resorting to DIY measures such as sewing their own masks.
In terms of staffing resources, 67% of respondents said their center has only one (or fewer) full-time–equivalent infection preventionist on staff to develop protocols for managing COVID-19. That is not surprising given the general underresourcing of infection control programs, the survey compilers said.
“Hospitals and health facilities with fewer than one full-time person on staff to direct infection prevention activities may have been disadvantaged even before the COVID-19 pandemic,” said APIC president Connie Steed, MSN, RN, in a related news release.
On a more positive note, about two thirds of facilities said they have sufficient supplies of gloves (63.4%) and hand washing soap (67.1%).
“I am concerned that many facilities will not be able to protect healthcare workers and patients from not only COVID-19, but also MRSA, C diff., and other antibiotic-resistant infections,” Steed said.
At some centers, however, the situation is not so grim — yet. The large Harris Health System in Houston has enough PPE on hand to support all infection prevention protocols in place, according to Bryan McLeod, director of corporate communications. “The PPE inventory varies from a few weeks to well over a month depending on the specific item,” McLeod told Medscape Medical News. “But everything is dependent on the utilization rate, which can vary with patient volume. Our concern is long-term resupply while demand is peaking around the world, and we continue to pursue all avenues to secure resupply.”
Above all, Steed emphasizes healthcare workers’ need for clarity. “They need to know when exactly they can expect desperately needed supplies to arrive so they don’t have to turn to unproven crisis methods for PPE,” she said. “There have been grim reports from health officials about the supply shortage for weeks and we’re not getting any answers. This is unacceptable.”
APIC is urging the federal government for immediate activation of the Cold War–era Defense Production Act and any other available means to quickly manufacture vital supplies to protect healthcare workers treating the escalating numbers of COVID-19 patients.
In the meantime, frontline healthcare workers are scouring the Internet for suppliers and begging online for donations of masks.
APIC notes that the COVID-19 pandemic is compounded by this year’s particularly severe influenza season, which had already led overcrowded healthcare facilities.
This article first appeared on Medscape.com.
iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
Case study shows CLL may mask COVID-19 infection
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
Characteristics of patients with chronic lymphocytic leukemia can mask COVID-19 infection, creating a risk for patients, practitioners, and the community, according to a case study published in the Lancet Haematology.
A 39-year-old man with a history of non-Hodgkin lymphoma and chronic lymphocytic leukemia (CLL) presented at a clinic in Wenzhou, China, with symptoms of fever, sore throat, productive cough, and dyspnea, according to the authors. COVID-19 infection was not initially suspected, as his whole blood cell and lymphocyte counts were high, the CLL masked a potential infection, and the patient claimed he had no suspect recent travel history.
However, a CT chest scan showed bilateral ground-glass opacities and a small amount of fluid in the patient’s left pleural cavity, leading the attending physician to suspect COVID-19. Testing was ordered and the real-time reverse-transcription polymerase chain reaction assay result was positive. The patient was immediately transferred to the isolation ward for management and confirmed COVID-19 infection.
Subsequently, the patient admitted travel to the COVID-19 epicenter in Wuhan province, although it was 25 days prior, indicating a longer period of incubation than generally believed, according to the authors. The patient survived treatment and was eventually discharged.
“Clinical and biochemical data of COVID-19 might be partly masked by coexisting chronic lymphocytic leukemia; better diagnostic strategies (i.e., superior CT differential techniques such as radiomics) could be used for diagnosis,” the researchers concluded, speculating that the apparently longer-than-normal COVID-19 incubation period might be the result of the patient’s compromised immune system.
The authors reported that they had no conflicts of interest.
SOURCE: Jin X-H et al. Lancet Haematol. 2020;7(4):E351-2.
FROM THE LANCET HAEMATOLOGY