User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Cushing’s and COVID-19: Nontraditional symptoms keys to assessment, treatments
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
Do not rely on more traditional signs and symptoms of COVID-19 like fever and dyspnea when assessing patients with Cushing’s syndrome for the novel coronavirus, Rosario Pivonello, MD, PhD, and colleagues urged.
Physicians evaluating patients with Cushing’s syndrome for COVID-19 “should be suspicious of any change in health status of their patients with Cushing’s syndrome, rather than relying on fever and [dyspnea] as typical features,” Dr. Pivonello, an endocrinologist with the University of Naples (Italy) Federico II, and colleagues wrote in a commentary published in The Lancet Diabetes & Endocrinology.
COVID-19 symptoms are a unique concern among patients with Cushing’s syndrome because many of the cardiometabolic and immune impairments that place someone at higher risk of more severe disease or mortality for the novel coronavirus – such as obesity, hypertension, diabetes, and immunodeficiency syndromes – are also shared with Cushing’s syndrome.
Increased cardiovascular risk factors and susceptibility to severe infection are “two leading causes of death” for patients with Cushing’s syndrome, Dr. Pivonello and colleagues noted.
The immunocompromised state of patients with Cushing’s syndrome may make detection of COVID-19 infection difficult, the authors say. For example, fever is a common symptom of patients with COVID-19, but in patients with active Cushing’s syndrome, “low-grade chronic inflammation and the poor immune response might limit febrile response in the early phase of infection,” Dr. Pivonello and colleagues wrote.
In other cases, because Cushing’s syndrome and COVID-19 have overlapping symptoms, it may be difficult to attribute a particular symptom to either disease. Dyspnea is a common symptom of COVID-19, but may present in Cushing’s syndrome because of “cardiac insufficiency or weakness of respiratory muscles,” the authors wrote. Instead, physicians should look to other COVID-19 symptoms, such as cough, dysgeusia, anosmia, and diarrhea, for signs of the disease.
Patients with Cushing’s syndrome may also be predisposed to a more severe course of COVID-19 because of the prevalence of obesity, hypertension, or diabetes in these patients, which have been identified as comorbidities that increase the likelihood of severe COVID-19 and progression to acute respiratory distress syndrome (ARDS). “However, a key element in the development of ARDS during COVID-19 is the exaggerated cellular response induced by the cytokine increase, leading to massive alveolar–capillary wall damage and a decline in gas exchange,” Dr. Pivonello and colleagues wrote. “Because patients with Cushing’s syndrome might not mount a normal cytokine response, these patients might [paradoxically] be less prone to develop severe ARDS with COVID-19.”
As both Cushing’s syndrome and COVID-19 are associated with hypercoagulability, the authors “strongly advise” using low-molecular-weight heparin in hospitalized patients with active Cushing’s syndrome who develop COVID-19. In both diseases, there is also a risk of longer duration of viral infections and opportunistic infections such as atypical bacterial and invasive fungal infections. For this reason, the authors also recommended patients with Cushing’s syndrome who have COVID-19 be placed on prolonged antiviral and broad-spectrum antibiotic treatment as a prophylactic measure.
During the pandemic, avoiding surgery for Cushing’s syndrome should be considered to reduce the likelihood of acquiring COVID-19 in a hospital setting, the authors wrote. Medical therapy can be temporarily used where appropriate, such as using ketoconazole, metyrapone, osilodrostat, and etomidate to lower cortisol levels. They acknowledge that some cases of malignant Cushing’s syndrome may require “expeditious definitive diagnosis and proper surgical resolution.”
After remission, while infection risk should be significantly lowered, other comorbidities like obesity, hypertension, diabetes, and thromboembolic diathesis may remain. “Because these are features associated with an increased death risk in patients with COVID-19, patients with Cushing’s syndrome in remission should be considered a high-risk population and consequently adopt adequate self-protection strategies to [minimize] contagion risk,” the authors wrote.
Dr. Pivonello reported relationships with Novartis, Strongbridge Biopharma, HRA Pharma, Ipsen, Shire, and Pfizer, Corcept Therapeutics, IBSA Farmaceutici, Ferring, and Italfarmaco in the form of receiving grants and/or personal fees. One coauthor reported receiving grants and/or nonfinancial support from Takeda, Ipsen, Shire, Pfizer, and Corcept Therapeutics. One coauthor reported receiving grants and personal fees from Novartis and Strongbridge, and grants from Millendo Therapeutics. Another coauthor reported receiving grants and/or personal fees from Novartis, Ipsen, Shire, Pfizer, Italfarmaco, Lilly, Merck, and Novo Nordisk. The other authors reported no relevant conflicts of interest.
SOURCE: Pivonello R et al. Lancet Diabetes Endocrinol. 2020 Jun 9. doi: 10.1016/S2213-8587(20)30215-1.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
Lifestyle changes may explain skin lesions in pandemic-era patients
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
Once again, no survival benefit with PCI, surgery in stable CAD
Coronary revascularization does not confer a survival advantage over initial medical therapy in patients with stable ischemic heart disease (SIHD) but reduces unstable angina, according to a new study-level meta-analysis.
Routine upfront revascularization is also associated with less spontaneous myocardial infarction but this is at the cost of increased procedural infarctions, reported lead investigator Sripal Bangalore, MD, of New York University.
“These relationships should be taken into consideration for shared decision-making for the management of patients with stable ischemic heart disease,” he said in a late-breaking trial session at PCR e-Course 2020, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR).
The results, simultaneously published in Circulation, are consistent with last year’s ISCHEMIA trial and other contemporary trials, such as COURAGE, FAME 2, and BARI 2D, that have failed to show a reduction in mortality with revascularization alone in SIHD. Guidelines continue, however, to recommend revascularization to improve survival in SIHD based on trials performed in the 1980s when medical therapy was limited, Dr. Bangalore observed.
The updated meta-analysis included 14 randomized controlled trials, including the aforementioned, and 14,877 patients followed for a weighted mean of 4.5 years. Most trials enrolled patients who had preserved left ventricular function and low symptom burden (Canadian Cardiovascular Society Class I/II).
In the revascularization group, 87.5% of patients underwent any revascularization. Percutaneous coronary intervention (PCI) was the first procedure in 71.3% and bypass surgery the first choice in 16.2%. In eight trials, stents were used in at least 50% of PCI patients; drug-eluting stents were mainly used in FAME 2, ISCHEMIA, and ISCHEMIA-CKD.
In eight trials, statins were used in at least 50% of patients. Nearly 1 in 3 patients (31.9%) treated initially with medical therapy underwent revascularization during follow-up.
Results show no reduction in mortality risk with routine revascularization in the overall analysis (relative risk, 0.99; 95% confidence interval, 0.90-1.09) or when analyzed by whether studies did or did not use stents (P for interaction = .85).
Trial sequential analysis also showed that the cumulative z-curve crossed the futility boundary, “suggesting we have great data to show that there is lack of even a 10% reduction in death with revascularization,” Dr. Bangalore said.
Results were very similar for cardiovascular death (RR, 0.92; 95% CI, 0.80-1.06), including when analyzed by study stent status (P for interaction = .60).
There was no significant reduction in overall MI risk with revascularization, although a borderline significant 11% decrease in MIs was found in the contemporary stent era trials (RR, 0.89; 95% CI, 0.80-0.998).
Revascularization was associated with a 148% increase in the risk of procedural MI (RR, 2.48; 95% CI, 1.86-3.31) but reduced risk of spontaneous MI (RR, 0.76; 95% CI, 0.67-0.85).
Unstable angina was reduced in patients undergoing revascularization (RR, 0.64; 95% CI, 0.45-0.92), driven by a 55% reduction in the contemporary stent era trials. Freedom from angina was also greater with routine revascularization but the difference was modest, Dr. Bangalore said. There was no difference between the two strategies in heart failure or stroke.
“This meta-analysis is well done but really doesn’t change what we already know,” Rasha Al-Lamee, MBBS, of Imperial College, London, said in an interview. “The most important message is that intervention in stable CAD does not change survival. We don’t need to rush to intervene: We have time to plan the best strategy for each patient and to modify our plans based on their response.”
The analysis addresses some of the issues with previous meta-analyses that have included trials that were not strictly stable CAD trials such as SWISSI-2, COMPARE-ACUTE, and DANAMI-3-PRIMULTI, she noted. “However a study like this is only as good as the trials that are included. We must remember that unblinded trials really cannot be used to accurately assess endpoints that are prone to bias such as unstable angina and freedom from angina.”
Following the presentation, dedicated discussant Davide Capodanno, MD, PhD, of the University of Catania (Italy) said, “We have seen beyond any doubt that there is no difference in mortality. For cardiovascular death, it’s pretty much the same. It’s a little bit more mixed and nuanced, the story of myocardial infarction.”
“Additional science is needed to understand the prognostic implications,” he said. “Of course we know that spontaneous myocardial infarction is bad, but I’m not so sure about periprocedural MI. Is this something that is as important as spontaneous myocardial infarction?”
The meta-analysis is the largest ever performed, but there was clinical heterogeneity in the individual studies, especially in the definition of MI, Dr. Capodanno observed. Because of the use of trial-level data rather than patient-level data, the analysis also could not account for adherence to treatment or the effect of stent type or medication dosage.
The MI issue really depends on the trial definition of MI, Dr. Al-Lamee said. “We need long-term follow-up from ISCHEMIA to understand what it means for our patients. While revascularization clearly increases procedural MI rates, it also results in lower spontaneous MI rates with no impact on overall MI or death,” she said. “We will only know if these MIs are important if we see what impact they have in the long term.”
Although the meta-analysis combined data from several decades, it’s likely that the outdated revascularization techniques in the older trials are balanced out by the outdated medical therapy in the same trials, Dr. Al-Lamee observed.
The new findings can certainly be used in patient-physician discussions, with more follow-up from ISCHEMIA to provide additional insights, she said.
“We will of course hear more about the placebo-controlled efficacy of PCI in the blinded ORBITA-2 trial. And I would really like to see some of the older studies of patients and perceptions of the effect of PCI repeated,” Dr. Al-Lamee said. “Now we have more data, are we informing our patients and referrers correctly of the impact of our procedures, and do they truly choose revascularization with a true awareness of what it does and does not do?”
Dr. Bangalore reported grants from the National Heart, Lung, and Blood Institute and Abbott Vascular; and serving on the advisory boards of Abbott Vascular, Biotronik, Meril, SMT, Pfizer, Amgen, and Reata. Dr. Al-Lamee reported speaker’s honorarium from Philips Volcano and Menarini Pharmaceuticals. Dr. Capodanno has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Coronary revascularization does not confer a survival advantage over initial medical therapy in patients with stable ischemic heart disease (SIHD) but reduces unstable angina, according to a new study-level meta-analysis.
Routine upfront revascularization is also associated with less spontaneous myocardial infarction but this is at the cost of increased procedural infarctions, reported lead investigator Sripal Bangalore, MD, of New York University.
“These relationships should be taken into consideration for shared decision-making for the management of patients with stable ischemic heart disease,” he said in a late-breaking trial session at PCR e-Course 2020, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR).
The results, simultaneously published in Circulation, are consistent with last year’s ISCHEMIA trial and other contemporary trials, such as COURAGE, FAME 2, and BARI 2D, that have failed to show a reduction in mortality with revascularization alone in SIHD. Guidelines continue, however, to recommend revascularization to improve survival in SIHD based on trials performed in the 1980s when medical therapy was limited, Dr. Bangalore observed.
The updated meta-analysis included 14 randomized controlled trials, including the aforementioned, and 14,877 patients followed for a weighted mean of 4.5 years. Most trials enrolled patients who had preserved left ventricular function and low symptom burden (Canadian Cardiovascular Society Class I/II).
In the revascularization group, 87.5% of patients underwent any revascularization. Percutaneous coronary intervention (PCI) was the first procedure in 71.3% and bypass surgery the first choice in 16.2%. In eight trials, stents were used in at least 50% of PCI patients; drug-eluting stents were mainly used in FAME 2, ISCHEMIA, and ISCHEMIA-CKD.
In eight trials, statins were used in at least 50% of patients. Nearly 1 in 3 patients (31.9%) treated initially with medical therapy underwent revascularization during follow-up.
Results show no reduction in mortality risk with routine revascularization in the overall analysis (relative risk, 0.99; 95% confidence interval, 0.90-1.09) or when analyzed by whether studies did or did not use stents (P for interaction = .85).
Trial sequential analysis also showed that the cumulative z-curve crossed the futility boundary, “suggesting we have great data to show that there is lack of even a 10% reduction in death with revascularization,” Dr. Bangalore said.
Results were very similar for cardiovascular death (RR, 0.92; 95% CI, 0.80-1.06), including when analyzed by study stent status (P for interaction = .60).
There was no significant reduction in overall MI risk with revascularization, although a borderline significant 11% decrease in MIs was found in the contemporary stent era trials (RR, 0.89; 95% CI, 0.80-0.998).
Revascularization was associated with a 148% increase in the risk of procedural MI (RR, 2.48; 95% CI, 1.86-3.31) but reduced risk of spontaneous MI (RR, 0.76; 95% CI, 0.67-0.85).
Unstable angina was reduced in patients undergoing revascularization (RR, 0.64; 95% CI, 0.45-0.92), driven by a 55% reduction in the contemporary stent era trials. Freedom from angina was also greater with routine revascularization but the difference was modest, Dr. Bangalore said. There was no difference between the two strategies in heart failure or stroke.
“This meta-analysis is well done but really doesn’t change what we already know,” Rasha Al-Lamee, MBBS, of Imperial College, London, said in an interview. “The most important message is that intervention in stable CAD does not change survival. We don’t need to rush to intervene: We have time to plan the best strategy for each patient and to modify our plans based on their response.”
The analysis addresses some of the issues with previous meta-analyses that have included trials that were not strictly stable CAD trials such as SWISSI-2, COMPARE-ACUTE, and DANAMI-3-PRIMULTI, she noted. “However a study like this is only as good as the trials that are included. We must remember that unblinded trials really cannot be used to accurately assess endpoints that are prone to bias such as unstable angina and freedom from angina.”
Following the presentation, dedicated discussant Davide Capodanno, MD, PhD, of the University of Catania (Italy) said, “We have seen beyond any doubt that there is no difference in mortality. For cardiovascular death, it’s pretty much the same. It’s a little bit more mixed and nuanced, the story of myocardial infarction.”
“Additional science is needed to understand the prognostic implications,” he said. “Of course we know that spontaneous myocardial infarction is bad, but I’m not so sure about periprocedural MI. Is this something that is as important as spontaneous myocardial infarction?”
The meta-analysis is the largest ever performed, but there was clinical heterogeneity in the individual studies, especially in the definition of MI, Dr. Capodanno observed. Because of the use of trial-level data rather than patient-level data, the analysis also could not account for adherence to treatment or the effect of stent type or medication dosage.
The MI issue really depends on the trial definition of MI, Dr. Al-Lamee said. “We need long-term follow-up from ISCHEMIA to understand what it means for our patients. While revascularization clearly increases procedural MI rates, it also results in lower spontaneous MI rates with no impact on overall MI or death,” she said. “We will only know if these MIs are important if we see what impact they have in the long term.”
Although the meta-analysis combined data from several decades, it’s likely that the outdated revascularization techniques in the older trials are balanced out by the outdated medical therapy in the same trials, Dr. Al-Lamee observed.
The new findings can certainly be used in patient-physician discussions, with more follow-up from ISCHEMIA to provide additional insights, she said.
“We will of course hear more about the placebo-controlled efficacy of PCI in the blinded ORBITA-2 trial. And I would really like to see some of the older studies of patients and perceptions of the effect of PCI repeated,” Dr. Al-Lamee said. “Now we have more data, are we informing our patients and referrers correctly of the impact of our procedures, and do they truly choose revascularization with a true awareness of what it does and does not do?”
Dr. Bangalore reported grants from the National Heart, Lung, and Blood Institute and Abbott Vascular; and serving on the advisory boards of Abbott Vascular, Biotronik, Meril, SMT, Pfizer, Amgen, and Reata. Dr. Al-Lamee reported speaker’s honorarium from Philips Volcano and Menarini Pharmaceuticals. Dr. Capodanno has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Coronary revascularization does not confer a survival advantage over initial medical therapy in patients with stable ischemic heart disease (SIHD) but reduces unstable angina, according to a new study-level meta-analysis.
Routine upfront revascularization is also associated with less spontaneous myocardial infarction but this is at the cost of increased procedural infarctions, reported lead investigator Sripal Bangalore, MD, of New York University.
“These relationships should be taken into consideration for shared decision-making for the management of patients with stable ischemic heart disease,” he said in a late-breaking trial session at PCR e-Course 2020, the virtual meeting of the Congress of European Association of Percutaneous Cardiovascular Interventions (EuroPCR).
The results, simultaneously published in Circulation, are consistent with last year’s ISCHEMIA trial and other contemporary trials, such as COURAGE, FAME 2, and BARI 2D, that have failed to show a reduction in mortality with revascularization alone in SIHD. Guidelines continue, however, to recommend revascularization to improve survival in SIHD based on trials performed in the 1980s when medical therapy was limited, Dr. Bangalore observed.
The updated meta-analysis included 14 randomized controlled trials, including the aforementioned, and 14,877 patients followed for a weighted mean of 4.5 years. Most trials enrolled patients who had preserved left ventricular function and low symptom burden (Canadian Cardiovascular Society Class I/II).
In the revascularization group, 87.5% of patients underwent any revascularization. Percutaneous coronary intervention (PCI) was the first procedure in 71.3% and bypass surgery the first choice in 16.2%. In eight trials, stents were used in at least 50% of PCI patients; drug-eluting stents were mainly used in FAME 2, ISCHEMIA, and ISCHEMIA-CKD.
In eight trials, statins were used in at least 50% of patients. Nearly 1 in 3 patients (31.9%) treated initially with medical therapy underwent revascularization during follow-up.
Results show no reduction in mortality risk with routine revascularization in the overall analysis (relative risk, 0.99; 95% confidence interval, 0.90-1.09) or when analyzed by whether studies did or did not use stents (P for interaction = .85).
Trial sequential analysis also showed that the cumulative z-curve crossed the futility boundary, “suggesting we have great data to show that there is lack of even a 10% reduction in death with revascularization,” Dr. Bangalore said.
Results were very similar for cardiovascular death (RR, 0.92; 95% CI, 0.80-1.06), including when analyzed by study stent status (P for interaction = .60).
There was no significant reduction in overall MI risk with revascularization, although a borderline significant 11% decrease in MIs was found in the contemporary stent era trials (RR, 0.89; 95% CI, 0.80-0.998).
Revascularization was associated with a 148% increase in the risk of procedural MI (RR, 2.48; 95% CI, 1.86-3.31) but reduced risk of spontaneous MI (RR, 0.76; 95% CI, 0.67-0.85).
Unstable angina was reduced in patients undergoing revascularization (RR, 0.64; 95% CI, 0.45-0.92), driven by a 55% reduction in the contemporary stent era trials. Freedom from angina was also greater with routine revascularization but the difference was modest, Dr. Bangalore said. There was no difference between the two strategies in heart failure or stroke.
“This meta-analysis is well done but really doesn’t change what we already know,” Rasha Al-Lamee, MBBS, of Imperial College, London, said in an interview. “The most important message is that intervention in stable CAD does not change survival. We don’t need to rush to intervene: We have time to plan the best strategy for each patient and to modify our plans based on their response.”
The analysis addresses some of the issues with previous meta-analyses that have included trials that were not strictly stable CAD trials such as SWISSI-2, COMPARE-ACUTE, and DANAMI-3-PRIMULTI, she noted. “However a study like this is only as good as the trials that are included. We must remember that unblinded trials really cannot be used to accurately assess endpoints that are prone to bias such as unstable angina and freedom from angina.”
Following the presentation, dedicated discussant Davide Capodanno, MD, PhD, of the University of Catania (Italy) said, “We have seen beyond any doubt that there is no difference in mortality. For cardiovascular death, it’s pretty much the same. It’s a little bit more mixed and nuanced, the story of myocardial infarction.”
“Additional science is needed to understand the prognostic implications,” he said. “Of course we know that spontaneous myocardial infarction is bad, but I’m not so sure about periprocedural MI. Is this something that is as important as spontaneous myocardial infarction?”
The meta-analysis is the largest ever performed, but there was clinical heterogeneity in the individual studies, especially in the definition of MI, Dr. Capodanno observed. Because of the use of trial-level data rather than patient-level data, the analysis also could not account for adherence to treatment or the effect of stent type or medication dosage.
The MI issue really depends on the trial definition of MI, Dr. Al-Lamee said. “We need long-term follow-up from ISCHEMIA to understand what it means for our patients. While revascularization clearly increases procedural MI rates, it also results in lower spontaneous MI rates with no impact on overall MI or death,” she said. “We will only know if these MIs are important if we see what impact they have in the long term.”
Although the meta-analysis combined data from several decades, it’s likely that the outdated revascularization techniques in the older trials are balanced out by the outdated medical therapy in the same trials, Dr. Al-Lamee observed.
The new findings can certainly be used in patient-physician discussions, with more follow-up from ISCHEMIA to provide additional insights, she said.
“We will of course hear more about the placebo-controlled efficacy of PCI in the blinded ORBITA-2 trial. And I would really like to see some of the older studies of patients and perceptions of the effect of PCI repeated,” Dr. Al-Lamee said. “Now we have more data, are we informing our patients and referrers correctly of the impact of our procedures, and do they truly choose revascularization with a true awareness of what it does and does not do?”
Dr. Bangalore reported grants from the National Heart, Lung, and Blood Institute and Abbott Vascular; and serving on the advisory boards of Abbott Vascular, Biotronik, Meril, SMT, Pfizer, Amgen, and Reata. Dr. Al-Lamee reported speaker’s honorarium from Philips Volcano and Menarini Pharmaceuticals. Dr. Capodanno has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Daily Recap: Hospitalized COVID patients need MRIs; Americans vote for face masks
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Three stages to COVID-19 brain damage, new review suggests
A new review outlined a three-stage classification of the impact of COVID-19 on the central nervous system and recommended all hospitalized patients with the virus undergo MRI to flag potential neurologic damage and inform postdischarge monitoring.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” said lead author Majid Fotuhi, MD, PhD. The review was published online in the Journal of Alzheimer’s Disease. Read more.
Topline results for novel intranasal med to treat opioid overdose
Topline results show positive results for the experimental intranasal nalmefene product OX125 for opioid overdose reversal, Orexo, the drug’s manufacturer, announced.
A crossover, comparative bioavailability study was conducted in healthy volunteers to assess nalmefene absorption of three development formulations of OX125. Preliminary results showed “extensive and rapid absorption” across all three formulations versus an intramuscular injection of nalmefene, Orexo reported.
“As the U.S. heroin crisis has developed to a fentanyl crisis, the medical need for novel and more powerful opioid rescue medications is vast,” Nikolaj Sørensen, president and CEO of Orexo, said in a press release. Read more.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
Regarding regular testing, 66% of Republicans and those leaning Republican said that such testing was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Read more.
Weight loss failures drive bariatric surgery regrets
Not all weight loss surgery patients “live happily ever after,” according to Daniel B. Jones, MD.
A 2014 study of 22 women who underwent weight loss surgery reported lower energy, worse quality of life, and persistent eating disorders.
Of gastric band patients, “almost 20% did not think they made the right decision,” he said. As for RYGP patients, 13% of patients at 1 year and 4 years reported that weight loss surgery caused “some” or “a lot” of negative effects. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Two-stent technique shown superior for complex coronary bifurcations
A systematic two-stent approach to complex coronary bifurcation lesions led to significantly improved clinical outcomes at 1 year, compared with the long-popular provisional stenting technique, in the first randomized trial to prospectively validate a standardized definition of what constitutes a complex bifurcation.
Since the double-kissing (DK) crush technique was employed in 78% of the systematic two-stent procedures, and the two-stent approach provided superior outcomes, it’s reasonable to infer that the DK crush is the preferred technique in patients with truly complex coronary bifurcation lesions (CBLs), Shao-Liang Chen, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
He presented the results of the DEFINITION II trial, a multinational trial in which 653 patients at 49 medical centers who fulfilled the criteria for complex CBLs were randomized to a systematic two-stent approach or provisional stenting, with a second stent deployed by interventionalists as needed. Dr. Chen, director of the cardiology department and deputy president of Nanjing (China) Medical University, and coworkers had previously published their standardized criteria for CBLs (JACC Cardiovasc Interv. 2014 Nov;7[11]:1266-76), which they developed by analysis of a large bifurcation cohort; however, until the DEFINITION II trial, the criteria had never been used in a prospective randomized trial.
According to the standardized definition developed by Dr. Chen and associates, complex coronary bifurcation lesions must meet one major and two minor criteria.
Major criteria:
- A side branch lesion length of at least 10 mm with a diameter stenosis of 70% or more for distal left main bifurcation lesions.
- For non–left main bifurcation lesions, a side branch diameter stenosis of at least 90% along with a side branch lesion length of at least 10 mm.
Minor criteria:
- Moderate to severe calcification multiple lesions
- Bifurcation angle of <45 degrees or >70 degrees
- Thrombus-containing lesions
- Main vessel residual diameter <2.5 mm
- Main vessel lesion length of at least 25 mm
Interventionalists were strongly encouraged to utilize the DK crush or culotte stenting techniques in patients randomized to the systematic two-stent approach. In contrast, in the provisional stenting group, where 23% of patients received a second stent, that stent was placed using the T and small protrusion technique 64% of the time.
The primary endpoint was the target lesion failure rate at 1-year of follow-up. Target lesion failure was a composite comprising cardiac death, target vessel MI, and clinically driven target vessel revascularization. The rate was 6.1% in the systematic two-stent group and 11.4% with provisional stenting, for a highly significant 48% relative risk reduction. The difference was driven largely by the systematic two-stent group’s lower rates of target vessel MI – 3.0% versus 7.1% with provisional stenting – and target lesion revascularization, with rates of 2.4% and 5.5%, respectively.
“The underlying mechanisms for the increased target vessel MI rate after the provisional stenting technique are unclear, and further study is urgently warranted,” Dr. Chen said.
There were no significant between-group differences in all-cause mortality or cardiac death, although both endpoints were numerically less frequent in the two-stent group.
The primary safety outcome was the 12-month rate of definite or probable stent thrombosis. This occurred in 1.2% of the systematic two-stent group and 2.5% of the provisional stent patients, a nonsignificant difference.
Discussant Davide Capodanno, MD, PhD, declared the DEFINITE II trial to be “another success for this DK crush technique everyone is talking about recently.”
He noted that, in a recent meta-analysis of 21 randomized, controlled trials including 5,711 patients with bifurcation lesions treated using five different percutaneous coronary intervention techniques, DK crush stood out from the pack. Particularly impressive was the finding that the target lesion revascularization rate in patients treated using the DK crush technique was 64% lower than with provisional stenting (JACC Cardiovasc Interv. 2020 Jun 22;13[12]:1432-44).
Dr. Capodanno said that, although the DEFINITE II results were strongly positive in favor of the systematic two-stent approach and DK crush technique, he’s not convinced of the generalizability of the study results.
“These investigators are very expert in this technique. They invented it. They’ve been using it for 10 years. So of course you may expect excellent results when you have masters of this technique,” observed Dr. Capodanno, a cardiologist at the University of Catania (Italy).
Independent replication of the DEFINITE II findings is needed. Fortunately, two ongoing randomized trials are addressing the issue of how to best treat bifurcation lesions. The EBC-MAIN trial is comparing the provisional approach with the systematic two-stent strategy in patients with left main bifurcation lesions; the study will include the DK crush as well as culotte and TAP PCI techniques, with a primary endpoint consisting of the 12-month rate of death, MI, and target lesion revascularization. And the BBK-3 trial will compare systematic two-stent strategies pitting the culotte against the DK crush, with the primary endpoint being the 9-month rate of angiographic restenosis by quantitative coronary angiography.
“After these trials are complete, we’ll probably know much more about the tailoring of bifurcation techniques for particular patients,” according to Dr. Capodanno.
Simultaneous with Dr. Chen’s presentation, the results of the DEFINITION II trial were published online (Eur Heart J. 2020 Jun 26.doi: 10.1093/eurheartj/ehaa543).
Dr. Chen and Dr. Capodanno reported having no financial conflicts of interest regarding the study, which was funded mainly by the National Science Foundation of China.
A systematic two-stent approach to complex coronary bifurcation lesions led to significantly improved clinical outcomes at 1 year, compared with the long-popular provisional stenting technique, in the first randomized trial to prospectively validate a standardized definition of what constitutes a complex bifurcation.
Since the double-kissing (DK) crush technique was employed in 78% of the systematic two-stent procedures, and the two-stent approach provided superior outcomes, it’s reasonable to infer that the DK crush is the preferred technique in patients with truly complex coronary bifurcation lesions (CBLs), Shao-Liang Chen, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
He presented the results of the DEFINITION II trial, a multinational trial in which 653 patients at 49 medical centers who fulfilled the criteria for complex CBLs were randomized to a systematic two-stent approach or provisional stenting, with a second stent deployed by interventionalists as needed. Dr. Chen, director of the cardiology department and deputy president of Nanjing (China) Medical University, and coworkers had previously published their standardized criteria for CBLs (JACC Cardiovasc Interv. 2014 Nov;7[11]:1266-76), which they developed by analysis of a large bifurcation cohort; however, until the DEFINITION II trial, the criteria had never been used in a prospective randomized trial.
According to the standardized definition developed by Dr. Chen and associates, complex coronary bifurcation lesions must meet one major and two minor criteria.
Major criteria:
- A side branch lesion length of at least 10 mm with a diameter stenosis of 70% or more for distal left main bifurcation lesions.
- For non–left main bifurcation lesions, a side branch diameter stenosis of at least 90% along with a side branch lesion length of at least 10 mm.
Minor criteria:
- Moderate to severe calcification multiple lesions
- Bifurcation angle of <45 degrees or >70 degrees
- Thrombus-containing lesions
- Main vessel residual diameter <2.5 mm
- Main vessel lesion length of at least 25 mm
Interventionalists were strongly encouraged to utilize the DK crush or culotte stenting techniques in patients randomized to the systematic two-stent approach. In contrast, in the provisional stenting group, where 23% of patients received a second stent, that stent was placed using the T and small protrusion technique 64% of the time.
The primary endpoint was the target lesion failure rate at 1-year of follow-up. Target lesion failure was a composite comprising cardiac death, target vessel MI, and clinically driven target vessel revascularization. The rate was 6.1% in the systematic two-stent group and 11.4% with provisional stenting, for a highly significant 48% relative risk reduction. The difference was driven largely by the systematic two-stent group’s lower rates of target vessel MI – 3.0% versus 7.1% with provisional stenting – and target lesion revascularization, with rates of 2.4% and 5.5%, respectively.
“The underlying mechanisms for the increased target vessel MI rate after the provisional stenting technique are unclear, and further study is urgently warranted,” Dr. Chen said.
There were no significant between-group differences in all-cause mortality or cardiac death, although both endpoints were numerically less frequent in the two-stent group.
The primary safety outcome was the 12-month rate of definite or probable stent thrombosis. This occurred in 1.2% of the systematic two-stent group and 2.5% of the provisional stent patients, a nonsignificant difference.
Discussant Davide Capodanno, MD, PhD, declared the DEFINITE II trial to be “another success for this DK crush technique everyone is talking about recently.”
He noted that, in a recent meta-analysis of 21 randomized, controlled trials including 5,711 patients with bifurcation lesions treated using five different percutaneous coronary intervention techniques, DK crush stood out from the pack. Particularly impressive was the finding that the target lesion revascularization rate in patients treated using the DK crush technique was 64% lower than with provisional stenting (JACC Cardiovasc Interv. 2020 Jun 22;13[12]:1432-44).
Dr. Capodanno said that, although the DEFINITE II results were strongly positive in favor of the systematic two-stent approach and DK crush technique, he’s not convinced of the generalizability of the study results.
“These investigators are very expert in this technique. They invented it. They’ve been using it for 10 years. So of course you may expect excellent results when you have masters of this technique,” observed Dr. Capodanno, a cardiologist at the University of Catania (Italy).
Independent replication of the DEFINITE II findings is needed. Fortunately, two ongoing randomized trials are addressing the issue of how to best treat bifurcation lesions. The EBC-MAIN trial is comparing the provisional approach with the systematic two-stent strategy in patients with left main bifurcation lesions; the study will include the DK crush as well as culotte and TAP PCI techniques, with a primary endpoint consisting of the 12-month rate of death, MI, and target lesion revascularization. And the BBK-3 trial will compare systematic two-stent strategies pitting the culotte against the DK crush, with the primary endpoint being the 9-month rate of angiographic restenosis by quantitative coronary angiography.
“After these trials are complete, we’ll probably know much more about the tailoring of bifurcation techniques for particular patients,” according to Dr. Capodanno.
Simultaneous with Dr. Chen’s presentation, the results of the DEFINITION II trial were published online (Eur Heart J. 2020 Jun 26.doi: 10.1093/eurheartj/ehaa543).
Dr. Chen and Dr. Capodanno reported having no financial conflicts of interest regarding the study, which was funded mainly by the National Science Foundation of China.
A systematic two-stent approach to complex coronary bifurcation lesions led to significantly improved clinical outcomes at 1 year, compared with the long-popular provisional stenting technique, in the first randomized trial to prospectively validate a standardized definition of what constitutes a complex bifurcation.
Since the double-kissing (DK) crush technique was employed in 78% of the systematic two-stent procedures, and the two-stent approach provided superior outcomes, it’s reasonable to infer that the DK crush is the preferred technique in patients with truly complex coronary bifurcation lesions (CBLs), Shao-Liang Chen, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
He presented the results of the DEFINITION II trial, a multinational trial in which 653 patients at 49 medical centers who fulfilled the criteria for complex CBLs were randomized to a systematic two-stent approach or provisional stenting, with a second stent deployed by interventionalists as needed. Dr. Chen, director of the cardiology department and deputy president of Nanjing (China) Medical University, and coworkers had previously published their standardized criteria for CBLs (JACC Cardiovasc Interv. 2014 Nov;7[11]:1266-76), which they developed by analysis of a large bifurcation cohort; however, until the DEFINITION II trial, the criteria had never been used in a prospective randomized trial.
According to the standardized definition developed by Dr. Chen and associates, complex coronary bifurcation lesions must meet one major and two minor criteria.
Major criteria:
- A side branch lesion length of at least 10 mm with a diameter stenosis of 70% or more for distal left main bifurcation lesions.
- For non–left main bifurcation lesions, a side branch diameter stenosis of at least 90% along with a side branch lesion length of at least 10 mm.
Minor criteria:
- Moderate to severe calcification multiple lesions
- Bifurcation angle of <45 degrees or >70 degrees
- Thrombus-containing lesions
- Main vessel residual diameter <2.5 mm
- Main vessel lesion length of at least 25 mm
Interventionalists were strongly encouraged to utilize the DK crush or culotte stenting techniques in patients randomized to the systematic two-stent approach. In contrast, in the provisional stenting group, where 23% of patients received a second stent, that stent was placed using the T and small protrusion technique 64% of the time.
The primary endpoint was the target lesion failure rate at 1-year of follow-up. Target lesion failure was a composite comprising cardiac death, target vessel MI, and clinically driven target vessel revascularization. The rate was 6.1% in the systematic two-stent group and 11.4% with provisional stenting, for a highly significant 48% relative risk reduction. The difference was driven largely by the systematic two-stent group’s lower rates of target vessel MI – 3.0% versus 7.1% with provisional stenting – and target lesion revascularization, with rates of 2.4% and 5.5%, respectively.
“The underlying mechanisms for the increased target vessel MI rate after the provisional stenting technique are unclear, and further study is urgently warranted,” Dr. Chen said.
There were no significant between-group differences in all-cause mortality or cardiac death, although both endpoints were numerically less frequent in the two-stent group.
The primary safety outcome was the 12-month rate of definite or probable stent thrombosis. This occurred in 1.2% of the systematic two-stent group and 2.5% of the provisional stent patients, a nonsignificant difference.
Discussant Davide Capodanno, MD, PhD, declared the DEFINITE II trial to be “another success for this DK crush technique everyone is talking about recently.”
He noted that, in a recent meta-analysis of 21 randomized, controlled trials including 5,711 patients with bifurcation lesions treated using five different percutaneous coronary intervention techniques, DK crush stood out from the pack. Particularly impressive was the finding that the target lesion revascularization rate in patients treated using the DK crush technique was 64% lower than with provisional stenting (JACC Cardiovasc Interv. 2020 Jun 22;13[12]:1432-44).
Dr. Capodanno said that, although the DEFINITE II results were strongly positive in favor of the systematic two-stent approach and DK crush technique, he’s not convinced of the generalizability of the study results.
“These investigators are very expert in this technique. They invented it. They’ve been using it for 10 years. So of course you may expect excellent results when you have masters of this technique,” observed Dr. Capodanno, a cardiologist at the University of Catania (Italy).
Independent replication of the DEFINITE II findings is needed. Fortunately, two ongoing randomized trials are addressing the issue of how to best treat bifurcation lesions. The EBC-MAIN trial is comparing the provisional approach with the systematic two-stent strategy in patients with left main bifurcation lesions; the study will include the DK crush as well as culotte and TAP PCI techniques, with a primary endpoint consisting of the 12-month rate of death, MI, and target lesion revascularization. And the BBK-3 trial will compare systematic two-stent strategies pitting the culotte against the DK crush, with the primary endpoint being the 9-month rate of angiographic restenosis by quantitative coronary angiography.
“After these trials are complete, we’ll probably know much more about the tailoring of bifurcation techniques for particular patients,” according to Dr. Capodanno.
Simultaneous with Dr. Chen’s presentation, the results of the DEFINITION II trial were published online (Eur Heart J. 2020 Jun 26.doi: 10.1093/eurheartj/ehaa543).
Dr. Chen and Dr. Capodanno reported having no financial conflicts of interest regarding the study, which was funded mainly by the National Science Foundation of China.
FROM EUROPCR 2020
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
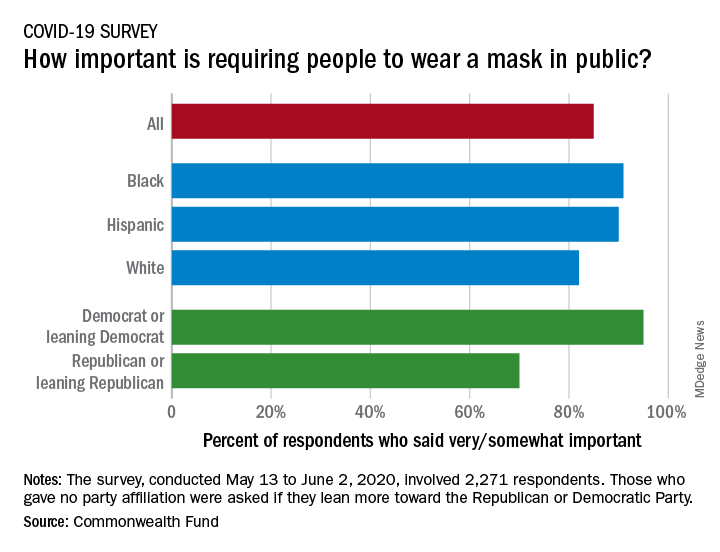
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Zoledronic acid fails to impact abdominal aortic calcification
A single yearly dose of zoledronic acid had no impact on the progression of abdominal aortic calcification in postmenopausal women with osteoporosis, based on data from 502 women.
Although bisphosphonates have been shown to reduce the formation and progression of vascular calcification in animal studies, the impact on aortic calcification in humans has not been studied, wrote Guoqi Cai, PhD, of the University of Tasmania, Australia, and colleagues.
In a post hoc analysis published in Osteoporosis International, the researchers reviewed data from the HORIZON Pivotal Fracture trial of women with osteoporosis.
The study population included 234 postmenopausal women with osteoporosis who received an annual infusion of 5 mg zoledronic acid (ZA) and 268 who received a placebo. The mean age of the women was 72.5 years. Overall, abdominal aortic calcification (AAC) was present in 292 women (58%) at baseline, defined as an AAC score greater than 0, and AAC scores were similar between the intervention and placebo groups.
Over 3 years, AAC progressed similarly between the ZA and placebo groups (29% and 31%, respectively). Progression was defined as an increase in AAC score, which was measured by comparing spinal x-rays at baseline and after 3 years. In a subgroup analysis, progression of AAC was similar between the ZA and placebo groups with and without baseline AAC.
“The lack of effect on the progression of vascular calcification with zoledronic acid treatment in this study does not rule out a potential role of bisphosphonates in reducing cardiovascular mortality mediated through other mechanisms,” the researchers noted.
No correlation appeared between change in AAC score and change in bone mineral density at the total hip and femoral neck during the study period in any of the groups.
The study findings were limited by several factors including the post hoc analysis, potential lack of sensitivity of the AAC-8 scale in measuring small AAC changes, and homogenous study population, the researchers noted.
However, the study is the first to examine the impact of zoledronic acid on aortic calcification in humans, and was strengthened by the randomized design, the researchers said. Although other studies on the impact of bisphosphonates on vascular calcification have been inconsistent, the “finding that zoledronic acid was not protective against vascular calcification agrees with previous trials of nitrogen-containing bisphosphonates conducted in postmenopausal women with osteoporosis,” as well as chronic kidney disease patients and renal transplant patients, they said.
“Thus, our findings do not support the use of zoledronic acid for the treatment of vascular calcification,” they concluded.
The study was supported by Novartis. Dr. Cai had no financial conflicts to disclose.
SOURCE: Cai G. et al. Osteoporosis Int. 2020 May 2. doi: 10.1007/s00198-020-05430-z.
A single yearly dose of zoledronic acid had no impact on the progression of abdominal aortic calcification in postmenopausal women with osteoporosis, based on data from 502 women.
Although bisphosphonates have been shown to reduce the formation and progression of vascular calcification in animal studies, the impact on aortic calcification in humans has not been studied, wrote Guoqi Cai, PhD, of the University of Tasmania, Australia, and colleagues.
In a post hoc analysis published in Osteoporosis International, the researchers reviewed data from the HORIZON Pivotal Fracture trial of women with osteoporosis.
The study population included 234 postmenopausal women with osteoporosis who received an annual infusion of 5 mg zoledronic acid (ZA) and 268 who received a placebo. The mean age of the women was 72.5 years. Overall, abdominal aortic calcification (AAC) was present in 292 women (58%) at baseline, defined as an AAC score greater than 0, and AAC scores were similar between the intervention and placebo groups.
Over 3 years, AAC progressed similarly between the ZA and placebo groups (29% and 31%, respectively). Progression was defined as an increase in AAC score, which was measured by comparing spinal x-rays at baseline and after 3 years. In a subgroup analysis, progression of AAC was similar between the ZA and placebo groups with and without baseline AAC.
“The lack of effect on the progression of vascular calcification with zoledronic acid treatment in this study does not rule out a potential role of bisphosphonates in reducing cardiovascular mortality mediated through other mechanisms,” the researchers noted.
No correlation appeared between change in AAC score and change in bone mineral density at the total hip and femoral neck during the study period in any of the groups.
The study findings were limited by several factors including the post hoc analysis, potential lack of sensitivity of the AAC-8 scale in measuring small AAC changes, and homogenous study population, the researchers noted.
However, the study is the first to examine the impact of zoledronic acid on aortic calcification in humans, and was strengthened by the randomized design, the researchers said. Although other studies on the impact of bisphosphonates on vascular calcification have been inconsistent, the “finding that zoledronic acid was not protective against vascular calcification agrees with previous trials of nitrogen-containing bisphosphonates conducted in postmenopausal women with osteoporosis,” as well as chronic kidney disease patients and renal transplant patients, they said.
“Thus, our findings do not support the use of zoledronic acid for the treatment of vascular calcification,” they concluded.
The study was supported by Novartis. Dr. Cai had no financial conflicts to disclose.
SOURCE: Cai G. et al. Osteoporosis Int. 2020 May 2. doi: 10.1007/s00198-020-05430-z.
A single yearly dose of zoledronic acid had no impact on the progression of abdominal aortic calcification in postmenopausal women with osteoporosis, based on data from 502 women.
Although bisphosphonates have been shown to reduce the formation and progression of vascular calcification in animal studies, the impact on aortic calcification in humans has not been studied, wrote Guoqi Cai, PhD, of the University of Tasmania, Australia, and colleagues.
In a post hoc analysis published in Osteoporosis International, the researchers reviewed data from the HORIZON Pivotal Fracture trial of women with osteoporosis.
The study population included 234 postmenopausal women with osteoporosis who received an annual infusion of 5 mg zoledronic acid (ZA) and 268 who received a placebo. The mean age of the women was 72.5 years. Overall, abdominal aortic calcification (AAC) was present in 292 women (58%) at baseline, defined as an AAC score greater than 0, and AAC scores were similar between the intervention and placebo groups.
Over 3 years, AAC progressed similarly between the ZA and placebo groups (29% and 31%, respectively). Progression was defined as an increase in AAC score, which was measured by comparing spinal x-rays at baseline and after 3 years. In a subgroup analysis, progression of AAC was similar between the ZA and placebo groups with and without baseline AAC.
“The lack of effect on the progression of vascular calcification with zoledronic acid treatment in this study does not rule out a potential role of bisphosphonates in reducing cardiovascular mortality mediated through other mechanisms,” the researchers noted.
No correlation appeared between change in AAC score and change in bone mineral density at the total hip and femoral neck during the study period in any of the groups.
The study findings were limited by several factors including the post hoc analysis, potential lack of sensitivity of the AAC-8 scale in measuring small AAC changes, and homogenous study population, the researchers noted.
However, the study is the first to examine the impact of zoledronic acid on aortic calcification in humans, and was strengthened by the randomized design, the researchers said. Although other studies on the impact of bisphosphonates on vascular calcification have been inconsistent, the “finding that zoledronic acid was not protective against vascular calcification agrees with previous trials of nitrogen-containing bisphosphonates conducted in postmenopausal women with osteoporosis,” as well as chronic kidney disease patients and renal transplant patients, they said.
“Thus, our findings do not support the use of zoledronic acid for the treatment of vascular calcification,” they concluded.
The study was supported by Novartis. Dr. Cai had no financial conflicts to disclose.
SOURCE: Cai G. et al. Osteoporosis Int. 2020 May 2. doi: 10.1007/s00198-020-05430-z.
FROM OSTEOPOROSIS INTERNATIONAL
Three stages to COVID-19 brain damage, new review suggests
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In stage 1, viral damage is limited to epithelial cells of the nose and mouth, and in stage 2 blood clots that form in the lungs may travel to the brain, leading to stroke. In stage 3, the virus crosses the blood-brain barrier and invades the brain.
“Our major take-home points are that patients with COVID-19 symptoms, such as shortness of breath, headache, or dizziness, may have neurological symptoms that, at the time of hospitalization, might not be noticed or prioritized, or whose neurological symptoms may become apparent only after they leave the hospital,” lead author Majid Fotuhi, MD, PhD, medical director of NeuroGrow Brain Fitness Center in McLean, Va., said.
“Hospitalized patients with COVID-19 should have a neurological evaluation and ideally a brain MRI before leaving the hospital; and, if there are abnormalities, they should follow up with a neurologist in 3-4 months,” said Dr. Fotuhi, who is also affiliate staff at Johns Hopkins Medicine, Baltimore.
The review was published online June 8 in the Journal of Alzheimer’s Disease.
Wreaks CNS havoc
It has become “increasingly evident” that SARS-CoV-2 can cause neurologic manifestations, including anosmia, seizures, stroke, confusion, encephalopathy, and total paralysis, the authors wrote.
They noted that SARS-CoV-2 binds to ACE2, which facilitates the conversion of angiotensin II to angiotensin. After ACE2 has bound to respiratory epithelial cells and then to epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a “cytokine storm.”
These cytokines, in turn, increase vascular permeability, edema, and widespread inflammation, as well as triggering “hypercoagulation cascades,” which cause small and large blood clots that affect multiple organs.
If SARS-CoV-2 crosses the blood-brain barrier, directly entering the brain, it can contribute to demyelination or neurodegeneration.
“We very thoroughly reviewed the literature published between Jan. 1 and May 1, 2020, about neurological issues [in COVID-19] and what I found interesting is that so many neurological things can happen due to a virus which is so small,” said Dr. Fotuhi.
“This virus’ DNA has such limited information, and yet it can wreak havoc on our nervous system because it kicks off such a potent defense system in our body that damages our nervous system,” he said.
Three-stage classification
- Stage 1: The extent of SARS-CoV-2 binding to the ACE2 receptors is limited to the nasal and gustatory epithelial cells, with the cytokine storm remaining “low and controlled.” During this stage, patients may experience smell or taste impairments, but often recover without any interventions.
- Stage 2: A “robust immune response” is activated by the virus, leading to inflammation in the blood vessels, increased hypercoagulability factors, and the formation of blood clots in cerebral arteries and veins. The patient may therefore experience either large or small strokes. Additional stage 2 symptoms include fatigue, hemiplegia, sensory loss, , tetraplegia, , or ataxia.
- Stage 3: The cytokine storm in the blood vessels is so severe that it causes an “explosive inflammatory response” and penetrates the blood-brain barrier, leading to the entry of cytokines, blood components, and viral particles into the brain parenchyma and causing neuronal cell death and encephalitis. This stage can be characterized by seizures, confusion, , coma, loss of consciousness, or death.
“Patients in stage 3 are more likely to have long-term consequences, because there is evidence that the virus particles have actually penetrated the brain, and we know that SARS-CoV-2 can remain dormant in neurons for many years,” said Dr. Fotuhi.
“Studies of coronaviruses have shown a link between the viruses and the risk of multiple sclerosis or Parkinson’s disease even decades later,” he added.
“Based on several reports in recent months, between 36% to 55% of patients with COVID-19 that are hospitalized have some neurological symptoms, but if you don’t look for them, you won’t see them,” Dr. Fotuhi noted.
As a result, patients should be monitored over time after discharge, as they may develop cognitive dysfunction down the road.
Additionally, “it is imperative for patients [hospitalized with COVID-19] to get a baseline MRI before leaving the hospital so that we have a starting point for future evaluation and treatment,” said Dr. Fotuhi.
“The good news is that neurological manifestations of COVID-19 are treatable,” and “can improve with intensive training,” including lifestyle changes – such as a heart-healthy diet, regular physical activity, stress reduction, improved sleep, biofeedback, and brain rehabilitation, Dr. Fotuhi added.
Routine MRI not necessary
Kenneth Tyler, MD, chair of the department of neurology at the University of Colorado at Denver, Aurora, disagreed that all hospitalized patients with COVID-19 should routinely receive an MRI.
“Whenever you are using a piece of equipment on patients who are COVID-19 infected, you risk introducing the infection to uninfected patients,” he said. Instead, “the indication is in patients who develop unexplained neurological manifestations – altered mental status or focal seizures, for example – because in those cases, you do need to understand whether there are underlying structural abnormalities,” said Dr. Tyler, who was not involved in the review.
Also commenting on the review, Vanja Douglas, MD, associate professor of clinical neurology, University of California, San Francisco, described the review as “thorough” and suggested it may “help us understand how to design observational studies to test whether the associations are due to severe respiratory illness or are specific to SARS-CoV-2 infection.”
Dr. Douglas, who was not involved in the review, added that it is “helpful in giving us a sense of which neurologic syndromes have been observed in COVID-19 patients, and therefore which patients neurologists may want to screen more carefully during the pandemic.”
The study had no specific funding. Dr. Fotuhi disclosed no relevant financial relationships. One coauthor reported receiving consulting fees as a member of the scientific advisory board for Brainreader and reports royalties for expert witness consultation in conjunction with Neurevolution. Dr. Tyler and Dr. Douglas disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Daily Recap: Docs are good at saving money; SARS-CoV-2 vaccine trials advance
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Many physicians live within their means and save
Although about two of five physicians report a net worth of between $1 million and $5 million, about half report that they are living at or below their means, according to the latest Medscape Physician Debt and Net Worth Report 2020.
Net worth figures varied greatly by specialty. Among specialists, orthopedists were most likely (at 19%) to top the $5 million level, followed by plastic surgeons and gastroenterologists (both at 16%). Conversely, 46% of family physicians and 44% of pediatricians reported that their net worth was under $500,000. Gender gaps were also apparent in the data, especially at the highest levels. Twice as many male physicians (10%) as their female counterparts (5%) had a net worth of more than $5 million.
Asked about saving habits, 43% of physicians reported they live below their means. Just 7% said they live above their means. How do they save money? Survey respondents reported putting bonus money into an investment account, putting extra money toward paying down the mortgage, and bringing lunch to work everyday.
The survey responses on salary, debt, and net worth from more than 17,000 physicians spanning 30 specialties were collected prior to Feb. 11, before COVID-19 was declared a pandemic. Read more.
Phase 3 COVID-19 vaccine trials launching in July
There are now 120 Investigational New Drug applications to the Food and Drug Administration for a SARS-CoV-2 vaccine, and researchers at more than 70 companies across the globe are interested in making a vaccine, according to Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.” Read more.
FDA approves in-home breast cancer treatment
The Food and Drug Administration has approved a combination of subcutaneous breast cancer treatments that could be administered at home, following completion of chemotherapy.
The agency gave the green light to pertuzumab (Perjeta, Genentech/Roche), trastuzumab (Herceptin, Genentech/Roche) and hyaluronidase (Phesgo, Genentech/Roche), administered subcutaneously rather than intravenously, for the treatment of early and metastatic HER2-positive breast cancers.
Phesgo is initially used in combination with chemotherapy at an infusion center but could continue to be administered in a patient’s home by a qualified health care professional once chemotherapy is complete. Read more.
Could a visual tool aid migraine management?
A new visual tool aims to streamline patient-clinician communication about risk factors for progression from episodic to chronic migraines.
The tool is still just a prototype, but it could eventually synthesize patient responses to an integrated questionnaire and produce a chart illustrating where the patient stands with respect to a range of modifiable risk factors from depression to insomnia.
Physicians must see patients in short appointment periods, making it difficult to communicate all of the risk factors and behavioral characteristics that can contribute to risk of progression. “If you have a patient and you’re able to look at a visualization tool quickly and say: ‘Okay, my patient really is having insomnia and sleep issues,’ you can focus the session talking about sleep, cognitive-behavioral therapy for insomnia, and all the things we can help patients with,” lead researcher Ami Cuneo, MD, who is a headache fellow at the University of Washington, Seattle, said in an interview.
Dr. Cuneo presented a poster describing the concept at the virtual annual meeting of the American Headache Society. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Phase 3 COVID-19 vaccine trials launching in July, expert says
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
The race to develop a SARS-CoV-2 vaccine is unlike any other global research and development effort in modern medicine.
According to Paul A. Offit, MD, there are now 120 Investigational New Drug applications to the Food and Drug Administration for these vaccines, and researchers at more than 70 companies across the globe are interested in making a vaccine. The Biomedical Advanced Research and Development Authority (BARDA) has awarded $2.5 billion to five different pharmaceutical companies to make a vaccine.
“The good news is that the new coronavirus is relatively stable,” Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, said during the virtual Pediatric Dermatology 2020: Best Practices and Innovations Conference. “Although it is a single-stranded RNA virus, it does mutate to some extent, but it doesn’t look like it’s going to mutate away from the vaccine. So, this is not going to be like influenza virus, where you must give a vaccine every year. I think we can make a vaccine that will last for several years. And we know the protein we’re interested in. We’re interested in antibodies directed against the spike glycoprotein, which is abundantly present on the surface of the virus. We know that if we make an antibody response to that protein, we can therefore prevent infection.”
Some research groups are interested in developing a whole, killed virus like those used in the inactivated polio vaccine, and vaccines for hepatitis A virus and rabies, said Dr. Offit, who is a member of Accelerating COVID-19 Technical Innovations And Vaccines, a public-private partnership formed by the National Institutes of Health. Other groups are interested in making a live-attenuated vaccine like those for measles, mumps, and rubella. “Some are interested in using a vectored vaccine, where you take a virus that is relatively weak and doesn’t cause disease in people, like vesicular stomatitis virus, and then clone into that the gene that codes for this coronavirus spike protein, which is the way that we made the Ebola virus vaccine,” Dr. Offit said. “Those approaches have all been used before, with success.”
Novel approaches are also being employed to make this vaccine, including using a replication-defective adenovirus. “That means that the virus can’t reproduce itself, but it can make proteins,” he explained. “There are some proteins that are made, but most aren’t. Therefore, the virus can’t reproduce itself. We’ll see whether or not that [approach] works, but it’s never been used before.”
Another approach is to inject messenger RNA that codes for the coronavirus spike protein, where that genetic material is translated into the spike protein. The other platform being evaluated is a DNA vaccine, in which “you give DNA which is coded for that spike protein, which is transcribed to messenger RNA and then is translated to other proteins.”
Typical vaccine development involves animal models to prove the concept, dose-ranging studies in humans, and progressively larger safety and immunogenicity studies in hundreds of thousands of people. Next come phase 3 studies, “where the proof is in the pudding,” he said. “These are large, prospective placebo-controlled trials to prove that the vaccine is safe. This is the only way whether you can prove or not a vaccine is effective.”
“Some companies may branch out on their own and do smaller studies than that,” he said. “We’ll see how this plays out. Keep your eyes open for that, because you really want to make sure you have a fairly large phase 3 trial. That’s the best way to show whether something works and whether it’s safe.”
The tried and true vaccines that emerge from the effort will not be FDA-licensed products. Rather, they will be approved products under the Emergency Use Authorization program. “Ever since the 1950s, every vaccine that has been used in the U.S. has been under the auspices of FDA licensure,” said Dr. Offit, who is also professor of pediatrics and the Maurice R. Hilleman professor of vaccinology at the University of Pennsylvania, Philadelphia. “That’s not going to be true here. The FDA is involved every step of the way but here they have a somewhat lighter touch.”
A few candidate vaccines are being mass-produced at risk, “meaning they’re being produced not knowing whether these vaccines are safe and effective yet or not,” he said. “But when they’re shown in a phase 3 trial to be safe and effective, you will have already produced it, and then it’s much easier to roll it out to the general public the minute you’ve shown that it works. This is what we did for the polio vaccine back in the 1950s. We mass-produced that vaccine at risk.”
Dr. Offit emphasized the importance of managing expectations once a COVID-19 vaccine gets approved for use. “Regarding safety, these vaccines will be tested in tens of thousands of people, not tens of millions of people, so although you can disprove a relatively uncommon side effect preapproval, you’re not going to disprove a rare side effect preapproval. You’re only going to know that post approval. I think we need to make people aware of that and to let them know that through groups like the Vaccine Safety Datalink, we’re going to be monitoring these vaccines once they’re approved.”
Regarding efficacy, he continued, “we’re not going know about the rates of immunity initially; we’re only going to know about that after the vaccine [has been administered]. My guess is the protection is going to be short lived and incomplete. By short lived, I mean that protection would last for years but not decades. By incomplete, I mean that protection will be against moderate to severe disease, which is fine. You don’t need protection against all of the disease; it’s hard to do that with respiratory viruses. That means you can keep people out of the hospital, and you can keep them from dying. That’s the main goal.”
Dr. Offit closed his remarks by noting that much is at stake in this effort to develop a vaccine so quickly and that it “could go one of two ways. We could find that the vaccine is a lifesaver, and [that] we can finally end this awful pandemic. Or, if we cut corners and don’t prove that the vaccines are safe and effective as we should before they’re released, we could shake what is a fragile vaccine confidence in this country. Hopefully, it doesn’t play out that way.”
FROM PEDIATRIC DERMATOLOGY 2020







