User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
COVID vaccine preprint study prompts Twitter outrage
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
Three ‘bad news’ payment changes coming soon for physicians
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Physicians are bracing for upcoming changes in reimbursement that may start within a few months. As doctors gear up for another wave of COVID, payment trends may not be the top priority, but some “uh oh” announcements in the fall of 2021 could have far-reaching implications that could affect your future.
The Centers for Medicare & Medicaid Services issued a proposed rule in the summer covering key aspects of physician payment. Although the rule contained some small bright lights, the most important changes proposed were far from welcome.
Here’s what could be in store:
1. The highly anticipated Medicare Physician Fee Schedule ruling confirmed a sweeping payment cut. The drive to maintain budget neutrality forced the federal agency to reduce Medicare payments, on average, by nearly 4%. Many physicians are outraged at the proposed cut.
2. More bad news for 2022: Sequestration will be back. Sequestration is the mandatory, pesky, negative 2% adjustment on all Medicare payments. It had been put on hold and is set to return at the beginning of 2022.
Essentially, sequestration reduces what Medicare pays its providers for health services, but Medicare beneficiaries bear no responsibility for the cost difference. To prevent further debt, CMS imposes financially on hospitals, physicians, and other health care providers.
The Health Resources and Services Administration has funds remaining to reimburse for all COVID-related testing, treatment, and vaccines provided to uninsured individuals. You can apply and be reimbursed at Medicare rates for these services when COVID is the primary diagnosis (or secondary in the case of pregnancy). Patients need not be American citizens for you to get paid.
3. Down to a nail-biter: The final ruling is expected in early November. The situation smacks of earlier days when physicians clung to a precipice, waiting in anticipation for a legislative body to save them from the dreaded income plunge. Indeed, we are slipping back to the decade-long period when Congress kept coming to the rescue simply to maintain the status quo.
Many anticipate a last-minute Congressional intervention to save the day, particularly in the midst of another COVID spike. The promises of a stable reimbursement system made possible by the Medicare Access and CHIP Reauthorization Act have been far from realized, and there are signs that the payment landscape is in the midst of a fundamental transformation.
Other changes proposed in the 1,747-page ruling include:
Positive:
- More telehealth services will be covered by Medicare, including home visits.
- Tele–mental health services got a big boost; many restrictions were removed so that now the patient’s home is considered a permissible originating site. It also allows for audio-only (no visual required) encounters; the audio-only allowance will extend to opioid use disorder treatment services. Phone treatment is covered.
- Permanent adoption of G2252: The 11- to 20-minute virtual check-in code wasn’t just a one-time payment but will be reimbursed in perpetuity.
- Boosts in reimbursement for chronic care and principal care management codes, which range on the basis of service but indicate a commitment to pay for care coordination.
- Clarification of roles and billing opportunities for split/shared visits, which occur if a physician and advanced practice provider see the same patient on a particular day. Prepare for new coding rules to include a modifier. Previously, the rules for billing were muddled, so transparency helps guide payment opportunities.
- Delay of the appropriate use criteria for advanced imaging for 1 (more) year, a welcome postponement of the ruling that carries a significant administrative burden.
- Physician assistants will be able to bill Medicare directly, and referrals to be made to medical nutrition therapy by a nontreating physician.
- A new approach to patient cost-sharing for colorectal cancer screenings will be phased in. This area has caused problems in the past when the physician identifies a need for additional services (for example, polyp removal by a gastroenterologist during routine colonoscopy).
Not positive:
- Which specialties benefit and which get zapped? The anticipated impact by specialty ranges from hits to interventional radiologists (–9%) and vascular surgeons (–8%), to increases for family practitioners, hand surgeons, endocrinologists, and geriatricians, each estimated to gain a modest 2%. (The exception is portable x-ray supplier, with an estimated increase of 10%.) All other specialties fall in between.
- The proposed conversion factor for 2022 is $33.58, a 3.75% drop from the 2021 conversion factor of $34.89.
The proposed ruling also covered the Quality Payment Program, the overarching program of which the Merit-based Incentive Payment System (MIPS) is the main track for participation. The proposal incorporates additional episode-based cost measures as well as updates to quality indicators and improvement activities.
MIPS penalties. The stakes are higher now, with 9% penalties on the table for nonparticipants. The government offers physicians the ability to officially get out of the program in 2021 because of the COVID-19 pandemic, thereby staving off the steep penalty. The option, which is available through the end of the year, requires a simple application that can be completed on behalf of the entire practice. If you want out, now is the time to find and fill out that application.
Exempt from technology requirements. If the proposal is accepted, small practices – defined by CMS as 15 eligible clinicians or fewer – won’t have to file an annual application to reweight the “promoting interoperability” portion of the program. If acknowledged, small practices will automatically be exempt from the program’s technology section. That’s a big plus, as one of the many chief complaints from small practices is the onus of meeting the technology requirements, which include a security risk analysis, bi-directional health information exchange, public health reporting, and patient access to health information. Meeting the requirements is no small feat. That will only affect future years, so be sure to apply in 2021 if applicable for your practice.
Changes in MIPS. MIPS Value Pathways (MVPs) are anticipated for 2023, with the government releasing details about proposed models for heart disease, rheumatology, joint repair, and more. The MVPs are slated to take over the traditional MIPS by 2027.
The program will shift to 30% of your score coming from the “cost” category, which is based on the government’s analysis of a physician’s claims – and, if attributed, the claims of the patients for whom you care. This area is tricky to manage, but recognize that the costs under scrutiny are the expenses paid by Medicare on behalf of its patients.
In essence, Medicare is measuring the cost of your patients as compared with your colleagues’ costs (in the form of specialty-based benchmarks). Therefore, if you’re referring, or ordering, a more costly set of diagnostic tests, assessments, or interventions than your peers, you’ll be dinged.
However, physicians are more likely this year to flat out reject participation in the federal payment program. Payouts have been paltry and dismal to date, and the buzz is that physicians just don’t consider it worth the effort. Of course, clearing the threshold (which is proposed at 70 points next year) is a must to avoid the penalty, but don’t go crazy to get a perfect score as it won’t count for much. 2022 is the final year that there are any monies for exceptional performance.
Considering that the payouts for exceptional performance have been less than 2% for several years now, it’s hard to justify dedicating resources to achieve perfection. Experts believe that even exceptional performance will only be worth pennies in bonus payments.
The fear of the stick, therefore, may be the only motivation. And that is subjective, as physicians weigh the effort required versus just taking the hit on the penalty. But the penalty is substantial, and so even without the incentive, it’s important to participate at least at the threshold.
Fewer cost-sharing waivers. While the federal government’s payment policies have a major impact on reimbursement, other forces may have broader implications. Commercial payers have rolled back cost-sharing waivers, bringing to light the significant financial responsibility that patients have for their health care in the form of deductibles, coinsurance, and so forth.
More than a third of Americans had trouble paying their health care bills before the pandemic; as patients catch up with services that were postponed or delayed because of the pandemic, this may expose challenges for you. Patients with unpaid bills translate into your financial burden.
Virtual-first health plans. Patients may be seeking alternatives to avoid the frustrating cycle of unpaid medical bills. This may be a factor propelling another trend: Lower-cost virtual-first health plans such as Alignment Health have taken hold in the market. As the name implies, insurance coverage features telehealth that extends to in-person services if necessary.
These disruptors may have their hands at least somewhat tied, however. The market may not be able to fully embrace telemedicine until state licensure is addressed. Despite the federal regulatory relaxations, states still control the distribution of medical care through licensure requirements. Many are rolling back their pandemic-based emergency orders and only allowing licensed physicians to see patients in their state, even over telemedicine.
While seemingly frustrating for physicians who want to see patients over state lines, the delays imposed by states may actually have a welcome effect. If licensure migrates to the federal level, there are many implications. For the purposes of this article, the competitive landscape will become incredibly aggressive. You will need to compete with Amazon Care, Walmart, Cigna, and many other well-funded national players that would love nothing more than to launch a campaign to target the entire nation. Investors are eager to capture part of the nearly quarter-trillion-dollar market, with telemedicine at 38 times prepandemic levels and no signs of abating.
Increased competition for insurers. While the proposed drop in Medicare reimbursement is frustrating, keep a pulse on the fact that your patients may soon be lured by vendors like Amazon and others eager to gain access to physician payments. Instead of analyzing Federal Registers in the future, we may be assessing stock prices.
Consider, therefore, how to ensure that your digital front door is at least available, if not wide open, in the meantime. The nature of physician payments is surely changing.
Ms. Woodcock is president of Woodcock & Associates, Atlanta. She has disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
COVID wars, part nine: The rise of iodine
Onions and iodine and COVID, oh my!
As surely as the sun rises, anti-vaxxers will come up with some wacky and dangerous new idea to prevent COVID. While perhaps nothing will top horse medication, gargling iodine (or spraying it into the nose) is also not a great idea.
Multiple social media posts have extolled the virtues of gargling Betadine (povidone iodine), which is a TOPICAL disinfectant commonly used in EDs and operating rooms. One post cited a paper by a Bangladeshi plastic surgeon who hypothesized on the subject, and if that’s not a peer-reviewed, rigorously researched source, we don’t know what is.
Perhaps unsurprisingly, actual medical experts do not recommend using Betadine to prevent COVID. Ingesting it can cause iodine poisoning and plenty of nasty GI side effects; while Betadine does make a diluted product safe for gargling use (used for the treatment of sore throats), it has not shown any effectiveness against viruses or COVID in particular.
A New York ED doctor summed it up best in the Rolling Stone article when he was told anti-vaxxers were gargling iodine: He offered a choice four-letter expletive, then said, “Of course they are.”
But wait! We’ve got a two-for-one deal on dubious COVID cures this week. Health experts in Myanmar (Burma to all the “Seinfeld” fans) and Thailand have been combating social media posts claiming that onion fumes will cure COVID. All you need to do is slice an onion in half, sniff it for a while, then chew on a second onion, and your COVID will be cured!
In what is surely the most radical understatement of the year, a professor in the department of preventive and social medicine at Chulalongkorn University, Bangkok, said in the AFP article that there is “no solid evidence” to support onion sniffing from “any clinical research.”
We’re just going to assume the expletives that surely followed were kept off the record.
Pro-Trump state governor encourages vaccination
Clearly, the politics of COVID-19 have been working against the science of COVID-19. Politicians can’t, or won’t, agree on what to do about it, and many prominent Republicans have been actively resisting vaccine and mask mandates.
There is at least one Republican governor who has wholeheartedly encouraged vaccination in his pro-Trump state. We’re talking about Gov. Jim Justice of West Virginia, and not for the first time.
The Washington Post has detailed his efforts to promote the COVID vaccine, and we would like to share a couple of examples.
In June he suggested that people who didn’t get vaccinated were “entering the death drawing.” He followed that by saying, “If I knew for certain that there was going to be eight or nine people die by next Tuesday, and I could be one of them if I don’t take the vaccine ... What in the world do you think I would do? I mean, I would run over top of somebody.”
More recently, Gov. Justice took on vaccine conspiracy theories.
“For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the very same people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Nuff said.
Jet lag may be a gut feeling
After a week-long vacation halfway around the world, it’s time to go back to your usual routine and time zone. But don’t forget about that free souvenir, jet lag. A disrupted circadian rhythm can be a real bummer, but researchers may have found the fix in your belly.
In a study funded by the U.S. Navy, researchers at the University of Colorado, Boulder, looked into how the presence of a prebiotic in one’s diet can have on the disrupted biological clocks. They’re not the same as probiotics, which help you stay regular in another way. Prebiotics work as food to help the good gut bacteria you already have. An earlier study had suggested that prebiotics may have a positive effect on the brain.
To test the theory, the researchers gave one group of rats their regular food while another group received food with two different prebiotics. After manipulating the rats’ light-dark cycle for 8 weeks to give the illusion of traveling to a time zone 12 hours ahead every week, they found that the rats who ate the prebiotics were able to bounce back faster.
The possibility of ingesting something to keep your body clock regular sounds like a dream, but the researchers don’t really advise you to snatch all the supplements you can at your local pharmacy just yet.
“If you know you are going to come into a challenge, you could take a look at some of the prebiotics that are available. Just realize that they are not customized yet, so it might work for you but it won’t work for your neighbor,” said senior author Monika Fleshner.
Until there’s more conclusive research, just be good to your bacteria.
How to make stuff up and influence people
You’ve probably heard that we use only 10% of our brain. It’s right up there with “the Earth is flat” and “an apple a day keeps the doctor away.”
The idea that we use only 10% of our brains can probably be traced back to the early 1900s, suggests Discover magazine, when psychologist William James wrote, “Compared with what we ought to be, we are only half awake. Our fires are damped, our drafts are checked. We are making use of only a small part of our possible mental and physical resources.”
There are many different takes on it, but it is indeed a myth that we use only 10% of our brains. Dale Carnegie, the public speaking teacher, seems to be the one who put the specific number of 10% on James’ idea in his 1936 book, “How to Win Friends and Influence People.”
“We think that people are excited by this pseudo fact because it’s very optimistic,” neuroscientist Sandra Aamodt told Discover. “Wouldn’t we all love to think our brains had some giant pool of untapped potential that we’re not using?”
The reality is, we do use our whole brain. Functional MRI shows that different parts of the brain are used for different things such as language and memories. “Not all at the same time, of course. But every part of the brain has a job to do,” the Discover article explained.
There are many things we don’t know about how the brain works, but at least you know you use more than 10%. After all, a brain just told you so.
Onions and iodine and COVID, oh my!
As surely as the sun rises, anti-vaxxers will come up with some wacky and dangerous new idea to prevent COVID. While perhaps nothing will top horse medication, gargling iodine (or spraying it into the nose) is also not a great idea.
Multiple social media posts have extolled the virtues of gargling Betadine (povidone iodine), which is a TOPICAL disinfectant commonly used in EDs and operating rooms. One post cited a paper by a Bangladeshi plastic surgeon who hypothesized on the subject, and if that’s not a peer-reviewed, rigorously researched source, we don’t know what is.
Perhaps unsurprisingly, actual medical experts do not recommend using Betadine to prevent COVID. Ingesting it can cause iodine poisoning and plenty of nasty GI side effects; while Betadine does make a diluted product safe for gargling use (used for the treatment of sore throats), it has not shown any effectiveness against viruses or COVID in particular.
A New York ED doctor summed it up best in the Rolling Stone article when he was told anti-vaxxers were gargling iodine: He offered a choice four-letter expletive, then said, “Of course they are.”
But wait! We’ve got a two-for-one deal on dubious COVID cures this week. Health experts in Myanmar (Burma to all the “Seinfeld” fans) and Thailand have been combating social media posts claiming that onion fumes will cure COVID. All you need to do is slice an onion in half, sniff it for a while, then chew on a second onion, and your COVID will be cured!
In what is surely the most radical understatement of the year, a professor in the department of preventive and social medicine at Chulalongkorn University, Bangkok, said in the AFP article that there is “no solid evidence” to support onion sniffing from “any clinical research.”
We’re just going to assume the expletives that surely followed were kept off the record.
Pro-Trump state governor encourages vaccination
Clearly, the politics of COVID-19 have been working against the science of COVID-19. Politicians can’t, or won’t, agree on what to do about it, and many prominent Republicans have been actively resisting vaccine and mask mandates.
There is at least one Republican governor who has wholeheartedly encouraged vaccination in his pro-Trump state. We’re talking about Gov. Jim Justice of West Virginia, and not for the first time.
The Washington Post has detailed his efforts to promote the COVID vaccine, and we would like to share a couple of examples.
In June he suggested that people who didn’t get vaccinated were “entering the death drawing.” He followed that by saying, “If I knew for certain that there was going to be eight or nine people die by next Tuesday, and I could be one of them if I don’t take the vaccine ... What in the world do you think I would do? I mean, I would run over top of somebody.”
More recently, Gov. Justice took on vaccine conspiracy theories.
“For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the very same people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Nuff said.
Jet lag may be a gut feeling
After a week-long vacation halfway around the world, it’s time to go back to your usual routine and time zone. But don’t forget about that free souvenir, jet lag. A disrupted circadian rhythm can be a real bummer, but researchers may have found the fix in your belly.
In a study funded by the U.S. Navy, researchers at the University of Colorado, Boulder, looked into how the presence of a prebiotic in one’s diet can have on the disrupted biological clocks. They’re not the same as probiotics, which help you stay regular in another way. Prebiotics work as food to help the good gut bacteria you already have. An earlier study had suggested that prebiotics may have a positive effect on the brain.
To test the theory, the researchers gave one group of rats their regular food while another group received food with two different prebiotics. After manipulating the rats’ light-dark cycle for 8 weeks to give the illusion of traveling to a time zone 12 hours ahead every week, they found that the rats who ate the prebiotics were able to bounce back faster.
The possibility of ingesting something to keep your body clock regular sounds like a dream, but the researchers don’t really advise you to snatch all the supplements you can at your local pharmacy just yet.
“If you know you are going to come into a challenge, you could take a look at some of the prebiotics that are available. Just realize that they are not customized yet, so it might work for you but it won’t work for your neighbor,” said senior author Monika Fleshner.
Until there’s more conclusive research, just be good to your bacteria.
How to make stuff up and influence people
You’ve probably heard that we use only 10% of our brain. It’s right up there with “the Earth is flat” and “an apple a day keeps the doctor away.”
The idea that we use only 10% of our brains can probably be traced back to the early 1900s, suggests Discover magazine, when psychologist William James wrote, “Compared with what we ought to be, we are only half awake. Our fires are damped, our drafts are checked. We are making use of only a small part of our possible mental and physical resources.”
There are many different takes on it, but it is indeed a myth that we use only 10% of our brains. Dale Carnegie, the public speaking teacher, seems to be the one who put the specific number of 10% on James’ idea in his 1936 book, “How to Win Friends and Influence People.”
“We think that people are excited by this pseudo fact because it’s very optimistic,” neuroscientist Sandra Aamodt told Discover. “Wouldn’t we all love to think our brains had some giant pool of untapped potential that we’re not using?”
The reality is, we do use our whole brain. Functional MRI shows that different parts of the brain are used for different things such as language and memories. “Not all at the same time, of course. But every part of the brain has a job to do,” the Discover article explained.
There are many things we don’t know about how the brain works, but at least you know you use more than 10%. After all, a brain just told you so.
Onions and iodine and COVID, oh my!
As surely as the sun rises, anti-vaxxers will come up with some wacky and dangerous new idea to prevent COVID. While perhaps nothing will top horse medication, gargling iodine (or spraying it into the nose) is also not a great idea.
Multiple social media posts have extolled the virtues of gargling Betadine (povidone iodine), which is a TOPICAL disinfectant commonly used in EDs and operating rooms. One post cited a paper by a Bangladeshi plastic surgeon who hypothesized on the subject, and if that’s not a peer-reviewed, rigorously researched source, we don’t know what is.
Perhaps unsurprisingly, actual medical experts do not recommend using Betadine to prevent COVID. Ingesting it can cause iodine poisoning and plenty of nasty GI side effects; while Betadine does make a diluted product safe for gargling use (used for the treatment of sore throats), it has not shown any effectiveness against viruses or COVID in particular.
A New York ED doctor summed it up best in the Rolling Stone article when he was told anti-vaxxers were gargling iodine: He offered a choice four-letter expletive, then said, “Of course they are.”
But wait! We’ve got a two-for-one deal on dubious COVID cures this week. Health experts in Myanmar (Burma to all the “Seinfeld” fans) and Thailand have been combating social media posts claiming that onion fumes will cure COVID. All you need to do is slice an onion in half, sniff it for a while, then chew on a second onion, and your COVID will be cured!
In what is surely the most radical understatement of the year, a professor in the department of preventive and social medicine at Chulalongkorn University, Bangkok, said in the AFP article that there is “no solid evidence” to support onion sniffing from “any clinical research.”
We’re just going to assume the expletives that surely followed were kept off the record.
Pro-Trump state governor encourages vaccination
Clearly, the politics of COVID-19 have been working against the science of COVID-19. Politicians can’t, or won’t, agree on what to do about it, and many prominent Republicans have been actively resisting vaccine and mask mandates.
There is at least one Republican governor who has wholeheartedly encouraged vaccination in his pro-Trump state. We’re talking about Gov. Jim Justice of West Virginia, and not for the first time.
The Washington Post has detailed his efforts to promote the COVID vaccine, and we would like to share a couple of examples.
In June he suggested that people who didn’t get vaccinated were “entering the death drawing.” He followed that by saying, “If I knew for certain that there was going to be eight or nine people die by next Tuesday, and I could be one of them if I don’t take the vaccine ... What in the world do you think I would do? I mean, I would run over top of somebody.”
More recently, Gov. Justice took on vaccine conspiracy theories.
“For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the very same people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Nuff said.
Jet lag may be a gut feeling
After a week-long vacation halfway around the world, it’s time to go back to your usual routine and time zone. But don’t forget about that free souvenir, jet lag. A disrupted circadian rhythm can be a real bummer, but researchers may have found the fix in your belly.
In a study funded by the U.S. Navy, researchers at the University of Colorado, Boulder, looked into how the presence of a prebiotic in one’s diet can have on the disrupted biological clocks. They’re not the same as probiotics, which help you stay regular in another way. Prebiotics work as food to help the good gut bacteria you already have. An earlier study had suggested that prebiotics may have a positive effect on the brain.
To test the theory, the researchers gave one group of rats their regular food while another group received food with two different prebiotics. After manipulating the rats’ light-dark cycle for 8 weeks to give the illusion of traveling to a time zone 12 hours ahead every week, they found that the rats who ate the prebiotics were able to bounce back faster.
The possibility of ingesting something to keep your body clock regular sounds like a dream, but the researchers don’t really advise you to snatch all the supplements you can at your local pharmacy just yet.
“If you know you are going to come into a challenge, you could take a look at some of the prebiotics that are available. Just realize that they are not customized yet, so it might work for you but it won’t work for your neighbor,” said senior author Monika Fleshner.
Until there’s more conclusive research, just be good to your bacteria.
How to make stuff up and influence people
You’ve probably heard that we use only 10% of our brain. It’s right up there with “the Earth is flat” and “an apple a day keeps the doctor away.”
The idea that we use only 10% of our brains can probably be traced back to the early 1900s, suggests Discover magazine, when psychologist William James wrote, “Compared with what we ought to be, we are only half awake. Our fires are damped, our drafts are checked. We are making use of only a small part of our possible mental and physical resources.”
There are many different takes on it, but it is indeed a myth that we use only 10% of our brains. Dale Carnegie, the public speaking teacher, seems to be the one who put the specific number of 10% on James’ idea in his 1936 book, “How to Win Friends and Influence People.”
“We think that people are excited by this pseudo fact because it’s very optimistic,” neuroscientist Sandra Aamodt told Discover. “Wouldn’t we all love to think our brains had some giant pool of untapped potential that we’re not using?”
The reality is, we do use our whole brain. Functional MRI shows that different parts of the brain are used for different things such as language and memories. “Not all at the same time, of course. But every part of the brain has a job to do,” the Discover article explained.
There are many things we don’t know about how the brain works, but at least you know you use more than 10%. After all, a brain just told you so.
Texts boost activity, quality of life in patients with heart failure and diabetes
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
FROM HFSA 2021
Weight-loss surgery linked to fewer cardiovascular events, more so with RYGB
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
FROM DIABETES CARE
Man dies after 43 full ICUs turn him away
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
PRESERVED-HF: Dapagliflozin improves physical limitations in patients with HFpEF
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
FROM HFSA 2021
Cavernous gender gap in Medicare payments to cardiologists
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Women cardiologists receive dramatically smaller payments from the U.S. Centers for Medicare & Medicaid Services (CMS) than their male counterparts, new research suggests.
An analysis of 2016 claims data revealed male cardiologists received on average 45% more reimbursement than women in the inpatient setting, with the median payment 39% higher ($62,897 vs. $45,288).
In the outpatient setting, men received on average 62% more annual CMS payments, with the median payment 75% higher ($91,053 vs. $51,975; P < .001 for both).
The difference remained significant after the exclusion of the top and bottom 2.5% of earning physicians and cardiology subspecialties, like electrophysiology and interventional cardiology, with high procedural volumes and greater gender imbalances.
“This is one study among others which demonstrates a wage gap between men and women in medicine in cardiology,” lead author Inbar Raber, MD, Beth Israel Deaconess Medical Center, Boston, said in an interview. “I hope by increasing awareness [and] understanding of possible etiologies, it will enable some sustainable solutions, and those include access to additional support staff and equitable models surrounding parental leave and childcare support.”
The study, published online September 8 in JAMA Cardiology, comes on the heels of a recent cross-sectional analysis that put cardiology at the bottom of 13 internal medicine subspecialties with just 21% female faculty representation and one of only three specialties in which women’s median salaries did not reach 90% of men’s.
The new findings build on a 2017 report that showed Medicare payments to women physicians in 2013 were 55% of those to male physicians across all specialties.
“It can be disheartening, especially as an early career woman cardiologist, seeing these differences, but I think the responsibility on all of us is to take these observations and really try to understand more deeply why they exist,” Nosheen Reza, MD, from the University of Pennsylvania, Philadelphia, and coauthor of the cross-sectional analysis, told this news organization.
Several factors could be contributing to the disparity, but “it’s not gender discrimination from Medicare,” Dr. Raber said. “The gap in reimbursement is really driven by the types and the volume of charges submitted.”
Indeed, a direct comparison of the three most common inpatient and outpatient billing codes showed no difference in payments between the sexes.
Men, however, submitted 24% more median inpatient charges to CMS than women (1,190 vs. 959), and 94% more outpatient charges (1,685 vs. 870).
Men also submitted slightly more unique billing codes (median inpatient, 10 vs. 9; median outpatient, 11 vs. 8).
Notably, women made up just 13% of the 17,524 cardiologists who received CMS payments in the inpatient setting in 2016 and 13% of the 16,929 cardiologists who did so in the outpatient setting.
Louisiana had the dubious distinction of having the largest gender gap in mean CMS payments, with male cardiologists earning $145,323 (235%) more than women, whereas women cardiologists in Vermont out-earned men by $31,483 (38%).
Overall, male cardiologists had more years in practice than women cardiologists and cared for slightly older Medicare beneficiaries.
Differences in CMS payments persisted, however, after adjustment for years since graduation, physician subspecialty, number of charges, number of unique billing codes, and patient complexity. The resulting β coefficient was -0.06, which translates into women receiving an average of 94% of the CMS payments received by men.
“The first takeaway, if you were really crass and focused on the bottom line, might be: ‘Hey, let me get a few more male cardiologists because they’re going to bring more into the organization.’ But we shouldn’t do that because, unless you link these data with quality outcomes, they’re an interesting observation and hypothesis-generating,” said Sharonne Hayes, MD, coauthor of the 2017 report and professor of cardiovascular medicine at Mayo Clinic in Rochester, Minn., where she has served as director of diversity and inclusion for a decade.
She noted that there are multiple examples that the style of medicine women practice, on average, may be more effective, may be more outcomes based, and may save lives, as suggested by a recent analysis of hospitalized Medicare beneficiaries.
“The gap was not much different, like within 1% or so, but when you take that over the literally millions of Medicare patients cared for each year by hospitalists, that’s a substantial number of people,” Dr. Hayes said. “So, I think we need to take a step back, and we have to include these observations on studies like this and better understand the compensation gaps.”
She pointed out that the present study lacks data on full-time-equivalent status but that female physicians are more likely to work part-time, thus reducing the volume of claims.
Women might also care for different patient populations. “I practice in a women’s heart clinic and take care of [spontaneous coronary artery dissection] SCAD patients where the average age of SCAD is 42. So, the vast majority of patients I see on a day-to-day basis aren’t going to be Medicare age,” observed Dr. Hayes.
The differences in charges might also reflect the increased obligations in nonreimbursed work that women can have, Dr. Raber said. These can be things like mentoring, teaching roles, and serving on committees, which is a hypothesis supported by a 2021 study that showed women physicians spend more time on these “citizenship tasks” than men.
Finally, there could be organizational barriers that affect women’s clinical volumes, including less access to support from health care personnel. Added support is especially important, though, amid a 100-year pandemic, the women agreed.
“Within the first year of the pandemic, we saw women leaving the workforce in droves across all sectors, including medicine, including academic medicine. And, as the pandemic goes on without any signs of abatement, those threats continue to exist and continue to be amplified,” Dr. Reza said.
The groundswell of support surrounding the importance of diversity, equity, and inclusion initiatives across the board has helped bring attention to the issue, she said. Some institutions, including the National Institutes of Health, are making efforts to extend relief to women with young families, caregivers, or those in academic medicine who, for example, need extensions on grants or bridge funding.
“There’s certainly a lot left to do, but I do think within the last year, there’s been an acceleration of literature that has come out, not only pointing out the disparities, but pointing out that perhaps women physicians do have better outcomes and are better liked by their patients and that losing women in the workforce would be a huge detriment to the field overall,” Dr. Reza said.
Dr. Raber, Dr. Reza, and Dr. Hayes reports no relevant financial relationships. Coauthor conflict of interest disclosures are listed in the paper.
A version of this article first appeared on Medscape.com.
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”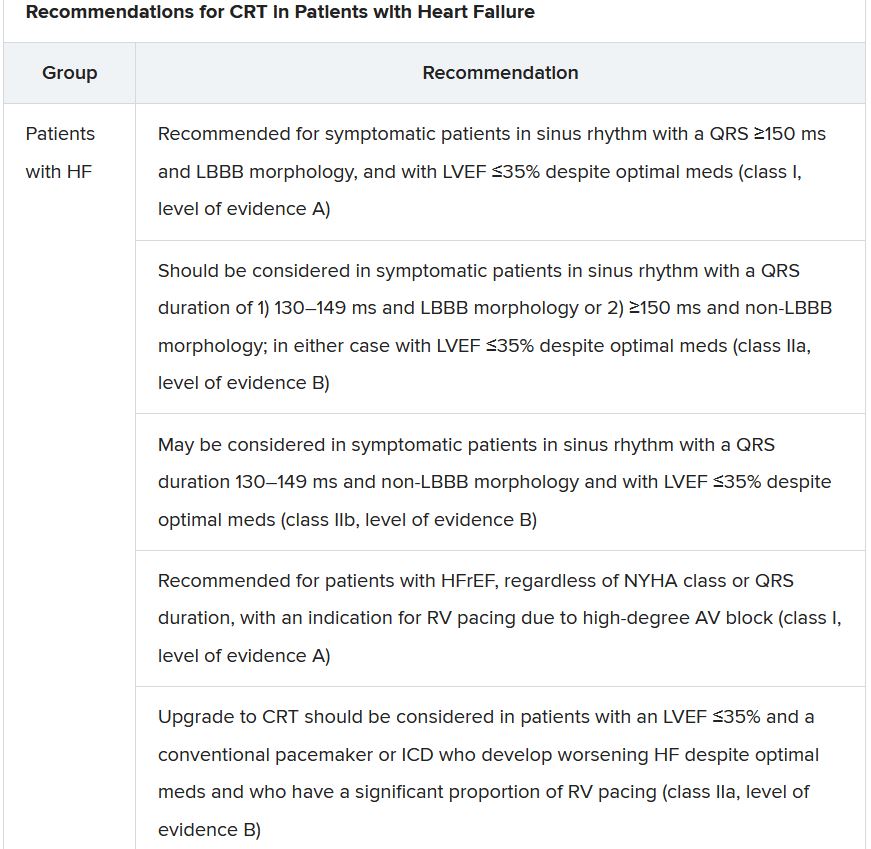
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.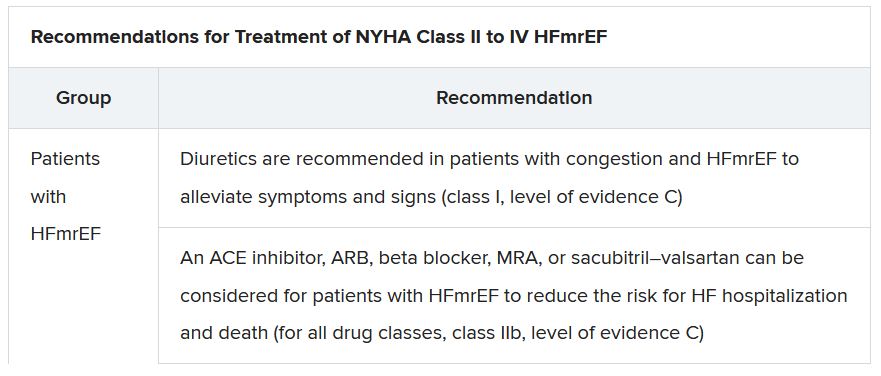
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
Study gives bleeding risk estimates for VTE patients on anticoagulants
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
The meta-analysis of data from 27 studies with 17,202 patients was published in the Annals of Internal Medicine. According to two of the paper’s coauthors, Faizan Khan, MSc, and Marc A. Rodger, MD, it “provides best available estimates of long-term bleeding risk with different anticoagulants in patients with unprovoked VTE,” including subgroups at increased risk.
Patients at increased risk for major bleeding include those who are older; those using antiplatelet therapy; and patients with kidney disease, a history of bleeding, or anemia, noted the coauthors, who work for the Ottawa Hospital Research Institute.
The researchers focused on randomized controlled trials (RCTs) and prospective cohort studies that reported major bleeding among patients with a first unprovoked or weakly provoked VTE who received oral anticoagulation for at least 6 months beyond an initial anticoagulant treatment course of at least 3 months.
The investigators analyzed data from 14 RCTs and 13 cohort studies. In all, 9,982 patients received a vitamin K antagonist (VKA), and 7,220 received a direct oral anticoagulant (DOAC).
The incidence of major bleeding per 100 person-years was 1.7 events with VKAs, compared with 1.1 events with DOACs. The researchers estimated that the 5-year cumulative incidence of major bleeding with VKAs was 6.3%. The available data for DOACs were insufficient to estimate the incidence of major bleeding beyond 1 year.
“This information can help clinicians counsel patients and inform shared decision-making about extended therapy,” the researchers said.
Risks of serious bleeding ‘not trivial’
Margaret Fang, MD, with the University of California, San Francisco, agreed that the study can help clinicians and patients weigh the risks of extended anticoagulation for common types of VTE.
The study also “highlights that the risks of serious bleeding are not trivial” and points out gaps in the literature regarding the long-term use of DOACs for extended VTE therapy, Dr. Fang said.
Better ways to predict which patients will develop bleeding on anticoagulants are needed, Dr. Fang added. “It will also be important to establish which of the various therapies for preventing recurrent VTE – full dose versus lowered dose, or even aspirin – has the best balance of safety and efficacy,” she said.
‘Standardized approach’ for identifying high-risk patients lacking
Clinical practice guidelines recommend indefinite anticoagulation for an unprovoked VTE, except when patients are at high risk of bleeding, the authors noted. But clinicians lack a “standardized approach to identify patients at high risk of bleeding,” Mr. Khan and Dr. Rodger said. “Evidence from randomized trials on net long-term benefit of extended therapy is limited, and current guideline recommendations are largely based on expert consensus opinion. Major bleeding events are two to three times more likely to be fatal than recurrent VTE events, so extended therapy is not always associated with a net mortality benefit, particularly in patients at low risk of recurrent VTE or high risk of bleeding.”
The analysis indicates that there is “a clinically meaningful difference in long-term risk for anticoagulant-related major bleeding among patients with a first unprovoked VTE stratified according to presence or absence of the following risk factors: age older than 65 years, creatinine clearance less than 50 mL/min, history of bleeding, concomitant use of antiplatelet therapy, and hemoglobin level less than 100 g/L,” the authors said.
For example, the researchers found that the incidence of major bleeding was higher among those older than 65 years, compared with younger patients (incidence rate ratio, 1.84 with VKAs and 2.92 with DOACs), and among those with creatinine clearance less than 50 mL/min (IRR, 2.83 with VKAs and 3.71 with DOACs).
The case-fatality rate of major bleeding was 8.3% with VKAs and 9.7% with DOACs.
The study received funding from the Canadian Institutes of Health Research. Some of the coauthors are employees of or have financial ties to pharmaceutical companies. Mr. Khan, Dr. Rodger, and Dr. Fang had no relevant disclosures.
FROM ANNALS OF INTERNAL MEDICINE














