User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Feds slap UPMC, lead cardiothoracic surgeon with fraud lawsuit
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Following a 2-year investigation, the U.S. government has filed suit against the University of Pittsburgh Medical Center (UPMC), University of Pittsburgh Physicians (UPP), and James Luketich, MD, for billing related to concurrent surgeries performed by the long-time chair of cardiothoracic surgery.
The lawsuit alleges that UPMC “knowingly allowed” Dr. Luketich to “book and perform three surgeries at the same time, to miss the surgical time outs at the outset of those procedures, to go back-and-forth between operating rooms and even hospital facilities while his surgical patients remain under general anesthesia...”
UPMC, the lawsuit claims, also allowed Dr. Luketich to falsely attest that “he was with his patients throughout the entirety of their surgical procedures or during all ‘key and critical’ portions of those procedures and to unlawfully bill Government Health Benefit Programs for those procedures, all in order to increase surgical volume, maximize UPMC and UPP’s revenue, and/or appease Dr. Luketich.”
These practices violate the statutes and regulations governing the defendants, including those that prohibit “teaching physicians” like Dr. Luketich from performing and billing the U.S. for concurrent surgeries, the Department of Justice said in news release.
The Justice Department contends the defendants “knowingly submitted hundreds of materially false claims for payment” to Medicare, Medicaid, and other government programs over the past 6 years.
“The laws prohibiting ‘concurrent surgeries’ are in place for a reason: To protect patients and ensure they receive appropriate and focused medical care,” Stephen R. Kaufman, Acting U.S. Attorney for the Western District of Pennsylvania, said in the release.
According to the lawsuit, “some of Dr. Luketich’s patients were forced to endure additional surgical procedures and/or extended hospital stays as a result of his unlawful conduct. Numerous patients developed painful pressure ulcers. A few were diagnosed with compartment syndrome. And at least two had to undergo amputations.”
The allegations were originally brought forward under the federal False Claims Act’s whistleblower provisions by Jonathan D’Cunha, MD, PhD, who worked closely with Dr. Luketich from 2012 to 2019 and now chairs the department of cardiothoracic surgery at the Mayo Clinic, Phoenix.
The charges cited in the lawsuit include three counts of violating the False Claims Act, one count of unjust enrichment, and one count of payment by mistake.
The 56-page lawsuit includes numerous case examples and cites an October 2015 Boston Globe Spotlight Team report on the safety of running concurrent operations, which reportedly prompted UPMC to reevaluate its policies and identify physicians or departments in potential violation.
Hospital officials met with Dr. Luketich in March 2016 and devised a “plan” to ensure his availability and “compliance with concurrency rules,” it alleges, but also highlights an email that notes “continued problems” with Dr. Luketich’s schedule.
“UPMC has persistently ignored or minimized complaints by employees and staff regarding Dr. Luketich, his hyper-busy schedule, his refusal to delegate surgeries and surgical tasks” and “protected him from meaningful sanction; refused to curtail his surgical practice; and continued to allow Dr. Luketich to skirt the rules and endanger his patients,” according to the lawsuit.
The suit notes that Dr. Luketich is one of UPMC and UPP’s highest sources of revenue and that UPMC advertises him as a “life-saving pioneer” who routinely performs dramatic, last-ditch procedures on patients who are otherwise hopeless.
In response to an interview request from this news organization, a UPMC spokesperson wrote: “As the government itself concedes in its complaint, many of Dr. Luketich’s surgical patients are elderly, frail, and/or very ill. They include the ‘hopeless’ patients ... who suffer from chronic illness or metastatic cancer, and/or have extensive surgical histories and choose UPMC and Dr. Luketich when other physicians and health care providers have turned them down.”
“Dr. Luketich always performs the most critical portions of every operation he undertakes,” the spokesperson said, adding that no law or regulation prohibits overlapping surgeries or billing for those surgeries, “let alone surgeries conducted by teams of surgeons like those led by Dr. Luketich.”
“The government’s claims are, rather, based on a misapplication or misinterpretation of UPMC’s internal policies and [Centers for Medicare & Medicaid Services] guidance, neither of which can support a claim for fraudulent billing. UPMC and Dr. Luketich plan to vigorously defend against the government’s claims,” the spokesperson concluded.
The claims asserted against the defendants are allegations only; there has been no determination of liability. The government is seeking three times the amount of actual damages suffered as a result of the alleged false claims and/or fraud; a sum of $23,331 (or the maximum penalty, whichever is greater) for each false claim submitted by UPMC, UPP, and/or Dr. Luketich; and costs and expenses associated with the civil suit.
A version of this article first appeared on Medscape.com.
Novel diabetic foot ulcer cream shows promise in phase 3 trial
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months, much still unknown about diabetes and COVID-19
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA could authorize COVID-19 vaccine for ages 5-11 in October
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
Biden vaccine mandate rule could be ready within weeks
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
Case: Patient with statin-associated muscle symptoms
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
A 66-year-old woman is discharged from the hospital after an MI. Her discharge medications include atorvastatin 40 mg, lisinopril 20 mg, acetylsalicylic acid 81 mg, and clopidogrel 75 mg. At this patient’s follow-up appointment, she mentions that she has muscle pain and stiffness in both legs and her back. Her labs include thyroid-stimulating hormone of 2.0 and vitamin D of 40. She stops the atorvastatin for 2 weeks with resolution of her symptoms.
Which treatment recommendation would you make for this patient?
A. Restart atorvastatin
B. Start rosuvastatin twice a week
C. Start ezetimibe
D. Start a PCSK9 inhibitor
We often see high-risk cardiovascular disease patients who are concerned about muscle side effects brought on by statins. I think we all can agree that this patient needs aggressive medical therapy for prevention of secondary cardiovascular events. I would restart her atorvastatin.
Neilsen and Nordestgaard found that early statin discontinuation rates increased from 6% in 1995 to 18% in 2010.1
Early statin discontinuation correlated with negative statin-related news stories, their paper states. This suggests either an increased awareness of side effects or a possible nocebo effect.
Statin rechallenge results
Joy and colleagues reported the results on eight patients who had developed myalgias within 3 weeks of starting a statin. These patients, who received placebo or statin, completed an N-of-1 trial with three double-blind, crossover comparisons separated by 3-week washout periods.
Patients were evaluated pain on a visual analog scale (VAS). For each N-of-1 trial there was no statistically significant difference in pain or myalgia score between those who took statin and placebo. Five of the eight patients chose to continue on statins at the end of the trial.
Herrett and colleagues performed a more extensive series of N-of-1 trials involving 200 patients who had stopped or were considering stopping statins because of muscle symptoms.3 Participants either received 2 months of atorvastatin 20 mg or placebo for 2-month blocks six times. They rated their muscle symptoms on a VAS at the end of each block. There was no difference in muscle symptom scores between the statin and placebo periods.
Wood and colleagues took it a step further, when they studied an N-of-1 trial that included statin, placebo, and no treatment.4 Each participant received four bottles of atorvastatin 20 mg, four bottles of placebo, and four empty bottles. Each month they used treatment from the bottles based on random sequence and reported daily symptom scores. The mean symptom intensity was 8.0 during no-tablet months, 15.4 during placebo months (P < .001, compared with no-tablet months), and 16.3 during statin months (P < .001, compared with no-tablet months; P = .39, compared with placebo).
Taylor and colleagues studied 120 patients who had prior statin-associated muscle complaints.5 Each patient received either simvastatin 20 mg or placebo for 4 weeks, and then were switched for an additional 4 weeks. A total of 43 patients (36%) had pain on simvastatin but not placebo, 21 (17%) had no pain with either treatment, 21 (17%) reported pain with both treatments, and 35 (29%) had pain with placebo but not simvastatin. These studies support the concept of nocebo effect in patients who have muscle symptoms on statins.
So what should be done? Brennan and Roy did a retrospective study of 118 patients referred to a lipid clinic as being statin intolerant to two or more statins.6 Most of the patients were able to tolerate a statin: 71% tolerated same statin rechallenge, 53% tolerated statin switch, and 57% tolerated a nonstatin therapy.
In the Prosisa study, only 27% of patients who reported statin-associated muscle symptoms had reappearance of muscle symptoms after rechallenge with a statin.7
Research implications
Rechallenge with the same statin seems to be a reasonable first step, followed by switching to a different statin. I also share the concept of nocebo effect with my patients, and tell them I believe they have an excellent chance of tolerating the statin.
Pearl: The majority of patients with muscle symptoms while taking a statin likely have a nocebo effect, and are likely to tolerate rechallenge with the same statin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Nielsen SF and Nordestgaard BG. Eur Heart J. 2016;37:908-16.
2. Joy TR et al. Ann Intern Med. 2014;160:301-10.
3. Herrett E et al. BMJ. 2021 Feb 24;372:n135.
4. Wood FA et al. N Engl J Med 2020;383:2182-4.
5. Taylor BA et al. Atherosclerosis. 2017;256:100-4.
6. Brennen ET and Roy TR. Can J Card. 2017;33(5):666-73.
7. Bonaiti Fet al. Atherosclerosis. 2020;315:E13-4.
‘Quadpill’ bests monotherapy for initial BP lowering: QUARTET
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
A “quadpill” containing quarter doses of four blood pressure (BP)–lowering medications was more effective than monotherapy for initial treatment of hypertension, with similar tolerability, in the 1-year, phase 3 QUARTET randomized, active-control trial.
Clara Chow, MD, PhD, academic director of the Westmead Applied Research Centre, University of Sydney, presented the findings in a late-breaking trial session at the annual congress of the European Society of Cardiology. The study was simultaneously published in The Lancet.
The primary outcome, mean unattended office BP at 12 weeks, dropped from 142/86 mm Hg to 120/71 mm Hg in patients who received the daily quadpill – a capsule containing irbesartan, amlodipine, indapamide, and bisoprolol – and fell from 140/83 mm Hg to 127/79 mm Hg in patients who received a daily full dose of irbesartan.
This 6.9 mm Hg greater drop in systolic BP at 12 months is clinically meaningful, Dr. Chow told this news organization. “If maintained, it would be expected to confer about a 15%-20% reduction” in heart disease, stroke, and heart failure.
In the SPRINT study, she noted, the final systolic BP was 120 mm Hg in the intervention group and 134 mm Hg in the control group, and the difference was associated with a 27% reduction in the composite cardiovascular (CV) outcome.
The results of QUARTET suggest that, “even in those with stage 1 hypertension, we can safely reduce BP to a significant degree by this simple approach, compared to usual care,” Salim Yusuf, MD, DPhil, a long-time advocate of a polypill approach, said in an email.
Importantly, Dr. Chow pointed out, at 12 months, 81% of patients treated with the quadpill versus 62% of patients treated with monotherapy had BP control (<140/90 mm Hg). Patients who received monotherapy did not “catch up,” even though a higher percentage received stepped-up therapy.
The quadpill dosing strategy aligns with the latest 2018 ESC/European Society of Hypertension guidelines, which recommend starting antihypertensive treatment with more than one drug, session cochair Thomas Kahan, MD, PhD, Karolinska Institute, Danderyd Hospital, department of clinical sciences, Stockholm, commented.
“How many drugs should be in the initial step?” he asked. “Is four better than three or two, or should we have even more drugs at low doses?”
The trial was not designed to answer these questions, Dr. Chow replied. “We were really comparing [the quadpill] against what the majority of people around the planet are still doing, which is starting on one drug and slowly but surely stepping it up,” she said.
The quadpill was actually a capsule, she clarified, that contained four generic BP medications available in half doses in Australia. The half doses were cut in half and the medications were encapsulated. The control drug was prepared in an identical-looking capsule.
It is important to note that “the time to BP control was shorter in patients who received the quadpill versus monotherapy,” session cochair Felix Mahfoud, MD, internal medicine and cardiology, Saarland University Hospital, Hamburg, Germany, pointed out, because “in clinical practice we aim to get patients to BP control as quickly as possible.”
“What is new here is the use of four drugs, each given at quarter doses,” Dr. Yusuf, director of the Population Health Research Institute, McMaster University, Hamilton, Ont., said. Although a few questions remain, “this study emphasizes the importance and potential benefits and simplicity of using combination BP-lowering drugs at low doses.”
For guidelines to be changed, he observed, the findings would have to be replicated in independent studies, and the quadpill would likely have to be shown to be superior to the dual pill.
“It took about 20 years [to change guidelines] after the first evidence that combinations of two pills were preferable to single-drug combinations,” he noted.
“I hope that in most people with elevated BP, at least a two-drug combination plus a statin plus aspirin will be prescribed,” Dr. Yusuf said. “This can reduce the risk of CVD events by 50% or more – a big impact both for the individual and for populations. The quad pill may have a role in this approach.”
Four-in-one pill
Worldwide, hypertension control is poor, Dr. Chow said, because of the need for multiple medications, treatment inertia, and concerns about adverse events.
The researchers hypothesized that initial antihypertensive treatment with a four-in-one pill with quarter doses of each medication would minimize side effects, maximize BP lowering, and overcome these treatment barriers and concerns. A pilot study of this strategy published by the group in 2017 showed promise.
QUARTET randomized 591 adults with hypertension, seen at clinics in four states in Australia from June 2017 through August 2020.
Patients were either receiving no antihypertensive medication and had an unattended standard office BP of 140/90 to 179/109 mm Hg or daytime ambulatory BP greater than 135/95 mm Hg, or they were on BP-lowering monotherapy and had a BP of 130/85 to 179/109 mm Hg or daytime ambulatory BP greater than 125/80 mm Hg. Patients who were taking antihypertensive therapy entered a washout period before the trial.
The researchers randomized 291 participants to receive 150 mg irbesartan daily (usual care or control group).
The other 300 participants received a daily quadpill containing 37.5 mg irbesartan, 1.25 mg amlodipine, 0.625 mg indapamide, and 2.5 mg bisoprolol. The first three drugs are the most commonly prescribed angiotensin II receptor blocker, calcium channel blocker, and thiazide or thiazidelike diuretic in Australia, and the last drug, a beta-blocker, has a long duration of action, the study protocol explains.
Patients in both groups had similar baseline characteristics. They were a mean age of 58 years, 40% were women, and 82% were White. They also had a mean body mass index of 31 kg/m2. About 8% were current smokers, and about 54% were not taking a BP-lowering drug.
Participants had clinic visits at baseline, 6 weeks, and 12 weeks, and if they continued the study, at 26 weeks and 52 weeks.
If a patient’s blood pressure was higher than 140/90 mm Hg, clinicians could add another medication, starting with amlodipine 5 mg.
At 12 weeks, 15% of patients in the intervention group and 40% in the control group had stepped up treatment.
Despite greater up-titration in the usual care group, BP control remained higher in the quadpill group, Dr. Chow pointed out. That is, patients in the quadpill group were more likely than patients in the usual care group to have a BP less than 140/90 mm Hg (76% vs. 58% respectively; P < .0001).
Patients in the quadpill group also had lower daytime and nighttime ambulatory systolic BP.
At 12 months, among the 417 patients who continued treatment, patients in the quadpill group had a 7.7 mm Hg greater drop in systolic BP, compared with patients in the control group, (P < .001).
There were no significant differences in adverse events, which were most commonly dizziness (31% and 25%) or muscle cramps, gastrointestinal complaints, headache, or musculoskeletal complaints.
At 12 weeks, there were seven serious adverse events in the intervention group versus three in the control group. There were 12 treatment withdrawals in the intervention group versus seven in the control group (P = .27).
Remaining questions, upcoming phase 3 U.S. study
“While the [QUARTET] results are impressive, we are left with a number of questions,” Dr. Yusuf said.
Would the results be the same with a three-drug combo or even a two-drug combo at half doses? In the HOPE 3 trial, a two-drug combo at half doses provided similar results to the current study, over a much longer mean follow-up of 5.6 years, he noted.
Also, is the quadpill associated with higher rates of diabetes or higher creatinine levels in the long term? “Given that we do not have any data on long-term clinical outcomes from a four-drug combination,” Dr. Yusuf said, “caution should be utilized.”
Would the reduced risk of CVD be greater with a combination of low doses of two BP-lowering drugs plus a statin plus aspirin? That may be superior, he said, “based on recent information published on the polypill indicating a 50% relative risk reduction in CVD events.”
The related phase 2 QUARTET US trial should shed further light on a quadpill strategy. Patients with hypertension are being randomized to a daily quadpill containing 2 mg candesartan, 1.25 mg amlodipine besylate, 0.625 mg indapamide, and 2.5 mg bisoprolol, or to usual care, 8 mg candesartan daily.
Investigators plan to enroll 87 participants in the Chicago area, with estimated study completion by March 31, 2023.
The study was supported by an Australian National Health and Medical Research Council grant. The George Institute for Global Health has submitted patent applications for low–fixed-dose combination products to treat CV or cardiometabolic disease. Dr. Chow and coauthor Kris Rogers, PhD, senior biostatistician at The George Institute for Global Health, Newtown, Australia, are listed as inventors, but they do not have direct financial interests in these patent applications.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2021
New guidance on preventing cutaneous SCC in solid organ transplant patients
An expert panel of 48 dermatologists from 13 countries has developed recommendations to guide efforts aimed at preventing cutaneous squamous cell carcinoma (CSCC) in solid organ transplant recipients.
The recommendations were published online on Sept. 1 in JAMA Dermatology.
Because of lifelong immunosuppression, solid organ transplant recipients (SOTRs) have a risk of CSCC that is 20-200 times higher than in the general population and despite a growing literature on prevention of CSCC in these patients, uncertainty remains regarding best practices for various patient scenarios.
Paul Massey, MD, MPH, of the department of dermatology, Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to identify consensus-based medical management recommendations for prevention of CSCC in SOTRs.
The survey design was guided by a novel actinic damage and skin cancer index (AD-SCI) made up of six ordinal stages corresponding to an increasing burden of actinic damage and CSCC.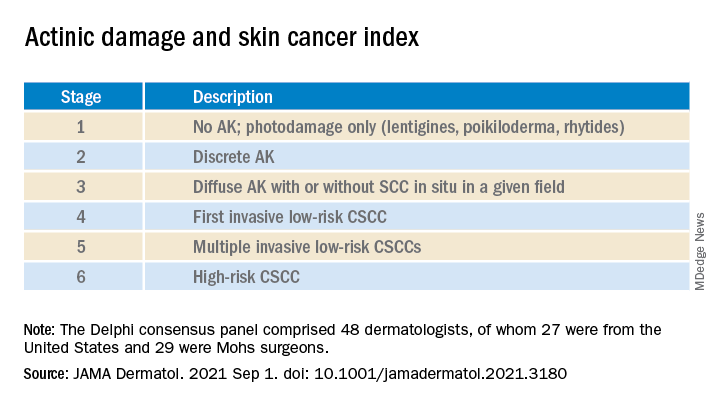
The AD-SCI stage-based recommendations were established when consensus was reached (80% or higher concordance) or near consensus was reached (70%-80% concordance) among panel members.
For five of the six AD-SCI stages, the panel was able to make recommendations. Key recommendations include:
- Cryotherapy for scattered AK.
- Field therapy for AK when grouped in one site, unless AKs are thick, in which case field therapy and cryotherapy are recommended.
- Combination lesion-directed and field therapy with fluorouracil for field cancerized skin.
- Initiation of acitretin therapy and discussion of immunosuppression reduction or modification for patients who develop multiple CSCCs at a high rate (10 per year) or develop high-risk CSCC (defined by a tumor with roughly ≥20% risk of nodal metastasis). The panel did not make a recommendation as to the best immunosuppression modification strategy to pursue.
Lingering questions
The panel was unable to reach consensus on a recommendation for SOTRs with a first low-risk CSCC, reflecting “clinical equipoise” in this situation and the need for further study in this clinical scenario, they say.
The panel did not make a recommendation for use of nicotinamide or capecitabine in any of the six stages, which is “notable,” they acknowledge, given results of a double-blind randomized controlled trial in immunocompetent patients demonstrating benefit in preventing AKs and CSCCs, as reported previously.
Nearly three-quarters of the panel felt that a lack of efficacy data specifically for the SOTR population limited their use of nicotinamide. “Given the low cost, high safety, and demonstration of CSCC reduction in non-SOTRs, nicotinamide administration may be an area for further consideration and expanded study,” the panel wrote.
As for capecitabine, the panel notes that case series in SOTRs have found efficacy for chemoprevention, but randomized controlled studies are lacking. More than half of the panel noted that they did not have routine access to capecitabine in their practice.
The panel recommended routine skin surveillance and sunscreen use for all patients.
“These recommendations reflect consensus among expert transplant dermatologists and the incorporation of limited and sometimes contradictory evidence into real-world clinical experience across a range of CSCC disease severity,” the panel said.
“Areas of consensus may aid physicians in establishing best practices regarding prevention of CSCC in SOTRs in the setting of limited high level of evidence data in this population,” they added.
This research had no specific funding. Author disclosures included serving as a consultant to Regeneron, Sanofi, and receiving research funding from Castle Biosciences, Regeneron, Novartis, and Genentech. A complete list of disclosures for panel members is available with the original article.
An expert panel of 48 dermatologists from 13 countries has developed recommendations to guide efforts aimed at preventing cutaneous squamous cell carcinoma (CSCC) in solid organ transplant recipients.
The recommendations were published online on Sept. 1 in JAMA Dermatology.
Because of lifelong immunosuppression, solid organ transplant recipients (SOTRs) have a risk of CSCC that is 20-200 times higher than in the general population and despite a growing literature on prevention of CSCC in these patients, uncertainty remains regarding best practices for various patient scenarios.
Paul Massey, MD, MPH, of the department of dermatology, Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to identify consensus-based medical management recommendations for prevention of CSCC in SOTRs.
The survey design was guided by a novel actinic damage and skin cancer index (AD-SCI) made up of six ordinal stages corresponding to an increasing burden of actinic damage and CSCC.
The AD-SCI stage-based recommendations were established when consensus was reached (80% or higher concordance) or near consensus was reached (70%-80% concordance) among panel members.
For five of the six AD-SCI stages, the panel was able to make recommendations. Key recommendations include:
- Cryotherapy for scattered AK.
- Field therapy for AK when grouped in one site, unless AKs are thick, in which case field therapy and cryotherapy are recommended.
- Combination lesion-directed and field therapy with fluorouracil for field cancerized skin.
- Initiation of acitretin therapy and discussion of immunosuppression reduction or modification for patients who develop multiple CSCCs at a high rate (10 per year) or develop high-risk CSCC (defined by a tumor with roughly ≥20% risk of nodal metastasis). The panel did not make a recommendation as to the best immunosuppression modification strategy to pursue.
Lingering questions
The panel was unable to reach consensus on a recommendation for SOTRs with a first low-risk CSCC, reflecting “clinical equipoise” in this situation and the need for further study in this clinical scenario, they say.
The panel did not make a recommendation for use of nicotinamide or capecitabine in any of the six stages, which is “notable,” they acknowledge, given results of a double-blind randomized controlled trial in immunocompetent patients demonstrating benefit in preventing AKs and CSCCs, as reported previously.
Nearly three-quarters of the panel felt that a lack of efficacy data specifically for the SOTR population limited their use of nicotinamide. “Given the low cost, high safety, and demonstration of CSCC reduction in non-SOTRs, nicotinamide administration may be an area for further consideration and expanded study,” the panel wrote.
As for capecitabine, the panel notes that case series in SOTRs have found efficacy for chemoprevention, but randomized controlled studies are lacking. More than half of the panel noted that they did not have routine access to capecitabine in their practice.
The panel recommended routine skin surveillance and sunscreen use for all patients.
“These recommendations reflect consensus among expert transplant dermatologists and the incorporation of limited and sometimes contradictory evidence into real-world clinical experience across a range of CSCC disease severity,” the panel said.
“Areas of consensus may aid physicians in establishing best practices regarding prevention of CSCC in SOTRs in the setting of limited high level of evidence data in this population,” they added.
This research had no specific funding. Author disclosures included serving as a consultant to Regeneron, Sanofi, and receiving research funding from Castle Biosciences, Regeneron, Novartis, and Genentech. A complete list of disclosures for panel members is available with the original article.
An expert panel of 48 dermatologists from 13 countries has developed recommendations to guide efforts aimed at preventing cutaneous squamous cell carcinoma (CSCC) in solid organ transplant recipients.
The recommendations were published online on Sept. 1 in JAMA Dermatology.
Because of lifelong immunosuppression, solid organ transplant recipients (SOTRs) have a risk of CSCC that is 20-200 times higher than in the general population and despite a growing literature on prevention of CSCC in these patients, uncertainty remains regarding best practices for various patient scenarios.
Paul Massey, MD, MPH, of the department of dermatology, Brigham and Women’s Hospital, Boston, and colleagues used a Delphi process to identify consensus-based medical management recommendations for prevention of CSCC in SOTRs.
The survey design was guided by a novel actinic damage and skin cancer index (AD-SCI) made up of six ordinal stages corresponding to an increasing burden of actinic damage and CSCC.
The AD-SCI stage-based recommendations were established when consensus was reached (80% or higher concordance) or near consensus was reached (70%-80% concordance) among panel members.
For five of the six AD-SCI stages, the panel was able to make recommendations. Key recommendations include:
- Cryotherapy for scattered AK.
- Field therapy for AK when grouped in one site, unless AKs are thick, in which case field therapy and cryotherapy are recommended.
- Combination lesion-directed and field therapy with fluorouracil for field cancerized skin.
- Initiation of acitretin therapy and discussion of immunosuppression reduction or modification for patients who develop multiple CSCCs at a high rate (10 per year) or develop high-risk CSCC (defined by a tumor with roughly ≥20% risk of nodal metastasis). The panel did not make a recommendation as to the best immunosuppression modification strategy to pursue.
Lingering questions
The panel was unable to reach consensus on a recommendation for SOTRs with a first low-risk CSCC, reflecting “clinical equipoise” in this situation and the need for further study in this clinical scenario, they say.
The panel did not make a recommendation for use of nicotinamide or capecitabine in any of the six stages, which is “notable,” they acknowledge, given results of a double-blind randomized controlled trial in immunocompetent patients demonstrating benefit in preventing AKs and CSCCs, as reported previously.
Nearly three-quarters of the panel felt that a lack of efficacy data specifically for the SOTR population limited their use of nicotinamide. “Given the low cost, high safety, and demonstration of CSCC reduction in non-SOTRs, nicotinamide administration may be an area for further consideration and expanded study,” the panel wrote.
As for capecitabine, the panel notes that case series in SOTRs have found efficacy for chemoprevention, but randomized controlled studies are lacking. More than half of the panel noted that they did not have routine access to capecitabine in their practice.
The panel recommended routine skin surveillance and sunscreen use for all patients.
“These recommendations reflect consensus among expert transplant dermatologists and the incorporation of limited and sometimes contradictory evidence into real-world clinical experience across a range of CSCC disease severity,” the panel said.
“Areas of consensus may aid physicians in establishing best practices regarding prevention of CSCC in SOTRs in the setting of limited high level of evidence data in this population,” they added.
This research had no specific funding. Author disclosures included serving as a consultant to Regeneron, Sanofi, and receiving research funding from Castle Biosciences, Regeneron, Novartis, and Genentech. A complete list of disclosures for panel members is available with the original article.
Even those who just test positive at more risk for long COVID: CDC
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021