User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
FFR-guided PCI falls short vs. surgery in multivessel disease: FAME 3
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”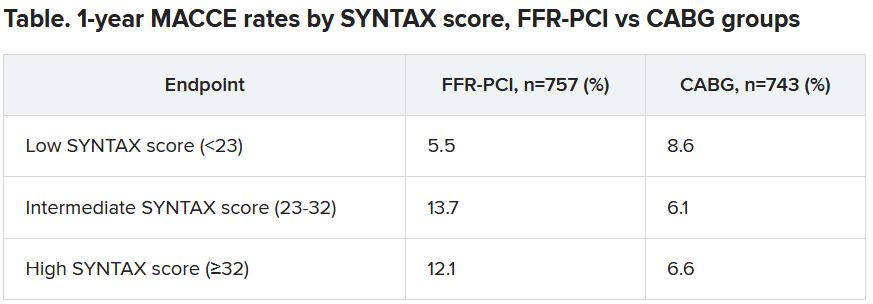
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
Coronary stenting guided by fractional flow reserve (FFR) readings, considered to reflect the targeted lesion’s functional impact, was no match for coronary bypass surgery (CABG) in patients with multivessel disease (MVD) in a major international randomized trial.
Indeed, FFR-guided percutaneous coronary intervention (PCI) using one of the latest drug-eluting stents (DES) seemed to perform poorly in the trial, compared with surgery, apparently upping the risk for clinical events by 50% over 1 year.
Designed statistically for noninferiority, the third Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME 3) trial, with 1,500 randomized patients, showed that FFR-guided PCI was “not noninferior” to CABG. Of those randomized to PCI, 10.6% met the 1-year primary endpoint of major adverse cardiac or cerebrovascular events (MACCE), compared with only 6.9% of patients assigned to CABG.
The trial enrolled only patients with three-vessel coronary disease with no left-main coronary artery involvement, who were declared by their institution’s multidisciplinary heart team to be appropriate for either form of revascularization.
One of the roles of FFR for PCI guidance is to identify significant lesions “that are underrecognized by the angiogram,” which is less likely to happen in patients with very complex coronary anatomy, study chair William F. Fearon, MD, Stanford (Calif.) University, said in an interview.
“That’s what we saw in a subgroup analysis based on SYNTAX score,” an index of lesion complexity. “In patients with very high SYNTAX scores, CABG outperformed FFR-guided PCI. But if you look at patients with low SYNTAX scores, actually, FFR-guided PCI outperformed CABG for 1-year MACCE.”
Dr. Fearon is lead author on the study’s Nov. 4, 2021, publication in the New England Journal of Medicine, its release timed to coincide with his presentation of the trial at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
He noted that FAME-3 “wasn’t designed or powered to test for superiority,” so its results do not imply CABG is superior to FFR-PCI in patients with MVD, and remains “inconclusive” on that question.
“I think what this study does is provide both the physician and patients more contemporary data and information on options and expected outcomes in multivessel disease. So if you are a patient who has less complex disease, I think you can feel comfortable that you will get an equivalent result with FFR-guided PCI.” But, at least based on FAME-3, Dr. Fearon said, CABG provides better outcomes in patients with more complex disease.
“I think there are still patients that look at trade-offs. Some patients will accept a higher event rate in order to avoid a long recovery, and vice versa.” So the trial may allow patients and physicians to make more informed decisions, he said.
A main message of FAME-3 “is that we’re getting very good results with three-vessel PCI, but better results with surgery,” Ran Kornowski, MD, Rabin Medical Center, Petah Tikva, Israel, and Tel Aviv University, said as a discussant following Dr. Fearon’s presentation of the trial. The subanalysis by SYNTAX score, he agreed, probably could be used as part of shared decision-making with patients.
Not all that surprising
“It’s a well-designed study, with a lot of patients,” said surgeon Frank W. Sellke, MD, of Rhode Island Hospital, Miriam Hospital, and Brown University, all in Providence.
“I don’t think it’s all that surprising,” he said in an interview. “It’s very consistent with what other studies have shown, that for three-vessel disease, surgery tends to have the edge,” even when pitted against FFR-guided PCI.
Indeed, pressure-wire FFR-PCI has a spotty history, even as an alternative to standard angiography-based PCI. For example, it has performed well in registry and other cohort studies but showed no advantage in the all-comers RIPCORD-2 trial or in the setting of complete revascularization PCI for acute MI in FLOWER-MI. And it emitted an increased-mortality signal in the prematurely halted FUTURE trial.
In FAME-3, “the 1-year follow-up was the best chance for FFR-PCI to be noninferior to CABG. The CABG advantage is only going to get better with time if prior experience and pathobiology is true,” Sanjay Kaul, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
Overall, “the quality and quantity of evidence is insufficient to support FFR-guided PCI” in patients with complex coronary artery disease (CAD), he said. “I would also argue that the evidence for FFR-guided PCI for simple CAD is also not high quality.”
Dr. Kaul also blasted the claim that FFR-PCI was seen to perform better against CABG in patients with low SYNTAX scores. “In general, one cannot use a positive subgroup in a null or negative trial, as is the case with FAME-3, to ‘rescue’ the treatment intervention.” Such a positive subgroup finding, he said, “would at best be deemed hypothesis-generating and not hypothesis validating.”
Dr. Fearon agreed that the subgroup analysis by SYNTAX score, though prespecified, was only hypothesis generating. “But I think that other studies have shown the same thing – that in less complex disease, the two strategies appear to perform in a similar fashion.”
The FAME-3 trial’s 1,500 patients were randomly assigned at 48 centers to undergo standard CABG or FFR-guided PCI with Resolute Integrity (Medtronic) zotarolimus-eluting DES. Lesions with a pressure-wire FFR of 0.80 or less were stented and those with higher FFR readings were deferred.
The 1-year hazard ratio for the primary endpoint—a composite of death from any cause, MI, stroke, or repeat revascularization – was 1.5 (95% confidence interval, 1.1-2.2) with a noninferiority P value of .35 for the comparison of FFR-PCI versus CABG.
FFR-guided PCI fared significantly better than CABG for some safety endpoints, including major bleeding (1.6% vs 3.8%, P < .01), arrhythmia including atrial fibrillation (2.4% vs. 14.1%, P < .001), acute kidney injury (0.1% vs 0.9%, P < .04), and 30-day rehospitalization (5.5% vs 10.2%, P < .001).
Did the primary endpoint favor CABG?
At a media briefing prior to Dr. Fearon’s TCT 2021 presentation of the trail, Roxana Mehran, MD, Icahn School of Medicine at Mount Sinai, New York, proposed that the inclusion of repeat revascularization in the trial’s composite primary endpoint tilted the outcome in favor of CABG. “To me, the FAME-3 results are predictable because repeat revascularization is in the equation.”
It’s well recognized that the endpoint is less likely after CABG than PCI. The latter treats focal lesions that are a limited part of a coronary artery in which CAD is still likely progressing. CABG, on the other hand, can bypass longer segments of diseased artery.
Indeed, as Dr. Fearon reported, the rates of death, MI, or stroke excluding repeat revascularization were 7.3% with FFR-PCI and 5.2% for CABG, for an HR of 1.4 (95% CI, 0.9-2.1).
Dr. Mehran also proposed that intravascular-ultrasound (IVUS) guidance, had it been part of the trial, could potentially have boosted the performance of FFR-PCI.
Repeat revascularization, Dr. Kaul agreed, “should not have been included” in the trial’s primary endpoint. It had been added “to amplify events and to minimize sample size. Not including revascularization would render the sample size prohibitive. There is always give and take in designing clinical trials.”
And he agreed that “IVUS-based PCI optimization would have further improved PCI outcomes.” However, “IVUS plus FFR adds to the procedural burden and limited resources available.” Dr. Fearon said when interviewed that the trial’s definition of procedural MI, a component of the primary endpoint, might potentially be seen as controversial. Procedural MIs in both the PCI and CABG groups were required to meet the standards of CABG-related type-5 MI according to the third and fourth Universal Definitions. The had also had to be accompanied by “a significant finding like new Q waves or a new wall-motion abnormality on echocardiography,” he said.
“That’s fairly strict. Because of that, we had a low rate of periprocedural MI and it was similar between the two groups, around 1.5% in both arms.”
FAME-3 was funded by Medtronic and Abbott Vascular. Dr. Kaul disclosed no relevant financial relationships. Dr. Kornowsky receives royalties from or holds intellectual property rights with CathWorks. Dr. Mehran disclosed financial ties to numerous pharmaceutical and device companies, and that she, her spouse, or her institution hold equity in Elixir Medical, Applied Therapeutics, and ControlRad.
A version of this article first appeared on Medscape.com.
SUGAR trial finds superior stent for those with diabetes and CAD
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Superiority shown on TLF endpoint
Superiority shown on TLF endpoint
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Designed to show noninferiority for treatment of coronary artery disease (CAD) in patients with diabetes, a head-to-head comparison of contemporary stents ended up showing that one was superior to the for the primary endpoint of target lesion failure (TLF).
In the superiority analysis, the 35% relative reduction in the risk of TLF at 1 year for the Cre8 EVO (Alvimedica) stent relative to the Resolute Onyx (Medtronic) device reached significance, according to Rafael Romaguera, MD, PhD, an interventional cardiologist at the Bellvitge University Hospital, Barcelona.
At 1 year, the rates of TLF were 7.2% and 10.5% for the Cre8 EVO and Resolute Onyx stents, respectively. On the basis of noninferiority, the 3.73% reduction in TLF at 1 year among those receiving the Cre8 EVO device provided a highly significant confirmation of noninferiority (P < .001) and triggered the preplanned superiority analysis.
When the significant advantage on the TLF endpoint (P = .03) was broken down into its components, the Cre8 EVO stent was linked to numerically lower rates of cardiac death (2.1% vs. 2.7%), target vessel MI (5.3% vs. 7.2%), and target lesion revascularization (2.4% vs. 3.9%), according to the SUGAR (Second-Generation Drug-Eluting Stents in Diabetes) trial results presented at the Transcatheter Cardiovascular Therapeutics annual meeting, held virtually and live in Orlando and sponsored by the Cardiovascular Research Foundation.
In a previous study comparing these devices, called the ReCre8 trial, the rates of TLF in an all-comer CAD population were similar at 1 year. When an updated 3-year analysis was presented earlier in 2021 at the Cardiovascular Research Technologies meeting, they remained similar.
Diabetes-centered trial was unmet need
The rationale for conducting a new trial limited to patients with diabetes was based on the greater risk in this population, according to Dr. Romaguera. He cited data that indicate the risk of major adverse cardiac events are about two times higher 2 years after stent implantation in patients with diabetes relative to those without, even when contemporary drug-eluting stents are used.
Both the Cre8 EVO and Resolute Onyx stent are drug eluting and employ contemporary architecture that provides the basis for marketing claims that they are suitable for complex patients; but they have differences.
“There are three features that I think differentiate the Cre8 EVO stent,” Dr. Romaguera reported at the meeting, sponsored by the Cardiovascular Research Foundation.
One is the absence of polymer, which contrasts with the permanent polymer of the Resolute device. This feature affects the dissolution of the anti-inflammatory drug and might be one explanation for the greater protection from ischemic events, according to Dr. Romaguera.
Another is the thickness of the struts, which range from 70 to 80 mm for the Cre8 EVO device and from 92 to 102 mm for the Resolute Onyx device. In experimental studies, strut thickness has been associated with greater risk of thrombus formation, although it is unclear if this modest difference is clinically significant.
Also important, the Cre8 EVO device employs sirolimus for an anti-inflammatory effect, while the Resolute Onyx elutes zotarolimus. Again, experimental evidence suggests a greater anti-inflammatory effect reduces the need for dual-antiplatelet therapy (DAPT); that might offer a relative advantage in patients with an elevated risk of bleeding.
It is not clear whether all of these features contribute to the better results observed in this trial in diabetes patients, but Dr. Romaguera indicated that the lower risk of TLF with Cre8 EVO is not just statistically significant but also clinically meaningful.
In SUGAR, which included 23 centers in Spain, 1,175 patients with confirmed diabetes scheduled for percutaneous intervention (PCI) were randomized to one of the two stents. The study was purposely designed with very few exclusion criteria.
SUGAR trial employed all-comer design
“This was an all-comer design and there was no limitation in regard to clinical presentation, complexity, number of lesions, or other disease features,” said Dr. Romaguera. The major exclusions were a life expectancy of less than 2 years and a contraindication to taking DAPT for at least 1 month,
The patients were almost equally divided between those who had a non–ST-segment elevation MI) and those with chronic coronary artery disease, but patients with a STEMI, representing about 12% of the population, were included. Almost all of the patients (about 95%) had type 2 diabetes; nearly one-third were on insulin at the time of randomization.
According to Dr. Romaguera, “SUGAR is the first powered trial to compare new-generation drug-eluting stents in patients with diabetes,” and he emphasized the all-comer design in supporting its clinical relevance.
Several of those participating in discussion of the trial during the late-breaker session agreed. Although the moderator, Gregg Stone, MD, of the Icahn School of Medicine at Mount Sinai, New York, expressed surprise that the trial “actually demonstrated superiority” given the difficulty of showing a difference between modern stents, he called the findings “remarkable.”
Others seemed to suggest that it would alter their practice.
“This study is sweet like sugar for us, because now we have a stent that is dedicated and fitted for the diabetic population,” said Gennaro Sardella, MD, of Sapienza University of Rome.
For Marc Etienne Jolicoeur, MD, an interventional cardiologist associated with Duke University, Durham, N.C., one of the impressive findings was the early separation of the curves in favor of Cre8 EVO. Calling SUGAR a “fantastic trial,” he indicated that the progressive advantage over time reinforced his impression that the difference is real.
However, David Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta, was more circumspect. He did not express any criticisms of the trial, but he called for “a larger evidence base” before declaring the Cre8 EVO device a standard of care for patients with diabetes undergoing PCI.
The SUGAR results were published in the European Heart Journal at the time of presentation at the meeting.
The trial was funded by the Spanish Society of Cardiology. Dr. Romaguera reported financial relationships with Biotronik and Boston Scientific. Dr. Stone, has financial relationships with more than 10 pharmaceutical companies, including those developing devices used in PCI. Dr. Sardella and Dr. Jolicoeur reported no financial relationships relevant to this topic. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
COVID-19 has brought more complex, longer office visits
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Ivermectin–COVID-19 study retracted; authors blame file mix-up
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
AHA dietary guidance cites structural challenges to heart-healthy patterns
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
In a new scientific statement on diet and lifestyle recommendations, the American Heart Association is highlighting, for the first time, structural challenges that impede the adoption of heart-healthy dietary patterns.
This is in addition to stressing aspects of diet that improve cardiovascular health and reduce cardiovascular risk, with an emphasis on dietary patterns and food-based guidance beyond naming individual foods or nutrients.
The 2021 Dietary Guidance to Improve Cardiovascular Health scientific statement, developed under Alice H. Lichtenstein, DSc, chair of the AHA writing group, provides 10 evidence-based guidance recommendations to promote cardiometabolic health.
“The way to make heart-healthy choices every day,” said Dr. Lichtenstein, of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University in Boston, in a statement, “is to step back, look at the environment in which you eat, whether it be at home, at work, during social interaction, and then identify what the best choices are. And if there are no good choices, then think about how you can modify your environment so that there are good choices.”
The statement, published in Circulation, underscores growing evidence that nutrition-related chronic diseases have maternal-nutritional origins, and that prevention of pediatric obesity is a key to preserving and prolonging ideal cardiovascular health.
The features are as follows:
- Adjust energy intake and expenditure to achieve and maintain a healthy body weight. To counter the shift toward higher energy intake and more sedentary lifestyles over the past 3 decades, the statement recommends at least 150 minutes of moderate physical activity per week, adjusted for individual’s age, activity level, sex, and size.
- Eat plenty of fruits and vegetables; choose a wide variety. Observational and intervention studies document that dietary patterns rich in varied fruits and vegetables, with the exception of white potatoes, are linked to a lower risk of cardiovascular disease (CVD). Also, whole fruits and vegetables, which more readily provide fiber and satiety, are preferred over juices.
- Choose whole grain foods and products made mostly with whole grains rather than refined grains. Evidence from observational, interventional, and clinical studies confirm the benefits of frequent consumption of whole grains over infrequent consumption or over refined grains in terms of CVD risk, coronary heart disease (CHD), stroke, metabolic syndrome, cardiometabolic risk factors, laxation, and gut microbiota.
- Choose healthy sources of protein, mostly from plants (legumes and nuts).
- Higher intake of legumes, which are rich in protein and fiber, is associated with lower CVD risk, while higher nut intake is associated with lower risks of CVD, CHD, and stroke mortality/incidence. Replacing animal-source foods with plant-source whole foods, beyond health benefits, lowers the diet’s carbon footprint. Meat alternatives are often ultraprocessed and evidence on their short- and long-term health effects is limited. Unsaturated fats are preferred, as are lean, nonprocessed meats.
- Use liquid plant oils rather than tropical oils (coconut, palm, and palm kernel), animal fats (butter and lard), and partially hydrogenated fats. Saturated and trans fats (animal and dairy fats, and partially hydrogenated fat) should be replaced with nontropical liquid plant oils. Evidence supports cardiovascular benefits of dietary unsaturated fats, especially polyunsaturated fats primarily from plant oils (e.g. soybean, corn, safflower and sunflower oils, walnuts, and flax seeds).
- Choose minimally processed foods instead of ultraprocessed foods. Because of their proven association with adverse health outcomes, including overweight and obesity, cardiometabolic disorders (type 2 diabetes, CVD), and all-cause mortality, the consumption of many ultraprocessed foods is of concern. Ultraprocessed foods include artificial colors and flavors and preservatives that promote shelf stability, preserve texture, and increase palatability. A general principle is to emphasize unprocessed or minimally processed foods.
- Minimize intake of beverages and foods with added sugars. Added sugars (commonly glucose, dextrose, sucrose, corn syrup, honey, maple syrup, and concentrated fruit juice) are tied to elevated risk for type 2 diabetes, high cholesterol, and excess body weight. Findings from meta-analyses on body weight and metabolic outcomes for replacing added sugars with low-energy sweeteners are mixed, and the possibility of reverse causality has been raised.
- Choose and prepare foods with little or no salt. In general, the effects of sodium reduction on blood pressure tend to be higher in Black people, middle-aged and older people, and those with hypertension. In the United States, the main combined sources of sodium intake are processed foods, those prepared outside the home, packaged foods, and restaurant foods. Potassium-enriched salts are a promising alternative.
- If you don’t drink alcohol, don’t start; if you choose to drink, limit intake.
- While relationships between alcohol intake and cardiovascular outcomes are complex, the 2020 Dietary Guidelines Advisory Committee recently concluded that those who do drink should consume no more than one drink per day and should not drink alcohol in binges; the 2020 Dietary Guidelines for Americans continues to recommend no more than one drink per day for women and two drinks per day for men.
- Adhere to the guidance regardless in all settings. Food-based dietary guidance applies to all foods and beverages, regardless of where prepared, procured, and consumed. Policies should be enacted that encourage healthier default options (for example, whole grains, minimized sodium and sugar content).
Recognizing impediments
The AHA/ASA scientific statement closes with the declaration: “Creating an environment that facilitates, rather than impedes, adherence to heart-healthy dietary patterns among all individuals is a public health imperative.” It points to the National Institutes of Health’s 2020-2030 Strategic Plan for National Institutes of Health Nutrition Research, which focuses on precision nutrition as a means “to determine the impact on health of not only what individuals eat, but also of why, when, and how they eat throughout the life course.”
Ultimately, precision nutrition may provide personalized diets for CVD prevention. But the “food environment,” often conditioned by “rampant nutrition misinformation” through local, state, and federal practices and policies, may impede the adoption of heart-healthy dietary patterns. Factors such as targeted food marketing (for example, of processed food and beverages in minority neighborhoods), structural racism, neighborhood segregation, unhealthy built environments, and food insecurity create environments in which unhealthy foods are the default option.”
These factors compound adverse dietary and health effects, and underscore a need to “directly combat nutrition misinformation among the public and health care professionals.” They also explain why, despite widespread knowledge of heart-healthy dietary pattern components, little progress has been made in achieving dietary goals in the United States.
Dr. Lichtenstein’s office, in response to a request regarding AHA advocacy and consumer programs, provided the following links: Voices for Healthy Kids initiative site and choosing healthier processed foods and one on fresh, frozen, and canned fruits and vegetables.
Dr. Lichtenstein had no disclosures. Disclosures for the writing group members are included in the statement.
FROM CIRCULATION
Feds launch COVID-19 worker vaccine mandates
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
Renal denervation remains only promising, per latest meta-analysis
Questions remain despite efficacy
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Questions remain despite efficacy
Questions remain despite efficacy
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
James Bond taken down by an epidemiologist
No, Mr. Bond, I expect you to die
Movie watching usually requires a certain suspension of disbelief, and it’s safe to say James Bond movies require this more than most. Between the impossible gadgets and ludicrous doomsday plans, very few have ever stopped to consider the health risks of the James Bond universe.
Now, however, Bond, James Bond, has met his most formidable opponent: Wouter Graumans, a graduate student in epidemiology from the Netherlands. During a foray to Burkina Faso to study infectious diseases, Mr. Graumans came down with a case of food poisoning, which led him to wonder how 007 is able to trot across this big world of ours without contracting so much as a sinus infection.
Because Mr. Graumans is a man of science and conviction, mere speculation wasn’t enough. He and a group of coauthors wrote an entire paper on the health risks of the James Bond universe.
Doing so required watching over 3,000 minutes of numerous movies and analyzing Bond’s 86 total trips to 46 different countries based on current Centers for Disease Control and Prevention advice for travel to those countries. Time which, the authors state in the abstract, “could easily have been spent on more pressing societal issues or forms of relaxation that are more acceptable in academic circles.”
Naturally, Mr. Bond’s line of work entails exposure to unpleasant things, such as poison, dehydration, heatstroke, and dangerous wildlife (everything from ticks to crocodiles), though oddly enough he never succumbs to any of it. He’s also curiously immune to hangovers, despite rarely drinking anything nonalcoholic. There are also less obvious risks: For one, 007 rarely washes his hands. During one movie, he handles raw chicken to lure away a pack of crocodiles but fails to wash his hands afterward, leaving him at risk for multiple food-borne illnesses.
Of course, we must address the elephant in the bedroom: Mr. Bond’s numerous, er, encounters with women. One would imagine the biggest risk to those women would be from the various STDs that likely course through Bond’s body, but of the 27% who died shortly after … encountering … him, all involved violence, with disease playing no obvious role. Who knows, maybe he’s clean? Stranger things have happened.
The timing of this article may seem a bit suspicious. Was it a PR stunt by the studio? Rest assured, the authors addressed this, noting that they received no funding for the study, and that, “given the futility of its academic value, this is deemed entirely appropriate by all authors.” We love when a punchline writes itself.
How to see Atlanta on $688.35 a day
The world is always changing, so we have to change with it. This week, LOTME becomes a travel guide, and our first stop is the Big A, the Big Peach, Dogwood City, Empire City of the South, Wakanda.
There’s lots to do in Atlanta: Celebrate a World Series win, visit the College Football Hall of Fame or the World of Coca Cola, or take the Stranger Things/Upside Down film locations tour. Serious adventurers, however, get out of the city and go to Emory Decatur Hospital in – you guessed it – Decatur (unofficial motto: “Everything is Greater in Decatur”).
Find the emergency room and ask for Taylor Davis, who will be your personal guide. She’ll show you how to check in at the desk, sit in the waiting room for 7 hours, and then leave without seeing any medical personnel or receiving any sort of attention whatsoever. All the things she did when she went there in July for a head injury.
Ms. Davis told Fox5 Atlanta: “I didn’t get my vitals taken, nobody called my name. I wasn’t seen at all.”
But wait! There’s more! By booking your trip through LOTMEgo* and using the code “Decatur,” you’ll get the Taylor Davis special, which includes a bill/cover charge for $688.35 from the hospital. An Emory Healthcare patient financial services employee told Ms. Davis that “you get charged before you are seen. Not for being seen.”
If all this has you ready to hop in your car (really?), then check out LOTMEgo* on Twittbook and InstaTok. You’ll also find trick-or-treating tips and discounts on haunted hospital tours.
*Does not actually exist
Breaking down the hot flash
Do you ever wonder why we scramble for cold things when we’re feeling nauseous? Whether it’s the cool air that needs to hit your face in the car or a cold, damp towel on the back of your neck, scientists think it could possibly be an evolutionary mechanism at the cellular level.
Motion sickness it’s actually a battle of body temperature, according to an article from LiveScience. Capillaries in the skin dilate, allowing for more blood flow near the skin’s surface and causing core temperature to fall. Once body temperature drops, the hypothalamus, which regulates temperature, tries to do its job by raising body temperature. Thus the hot flash!
The cold compress and cool air help fight the battle by counteracting the hypothalamus, but why the drop in body temperature to begin with?
There are a few theories. Dr. Robert Glatter, an emergency physician at Lenox Hill Hospital in New York, told LiveScience that the lack of oxygen needed in body tissue to survive at lower temperatures could be making it difficult to get oxygen to the body when a person is ill, and is “more likely an adaptive response influenced by poorly understood mechanisms at the cellular level.”
Another theory is that the nausea and body temperature shift is the body’s natural response to help people vomit.
Then there’s the theory of “defensive hypothermia,” which suggests that cold sweats are a possible mechanism to conserve energy so the body can fight off an intruder, which was supported by a 2014 study and a 2016 review.
It’s another one of the body’s many survival tricks.
Teachers were right: Pupils can do the math
Teachers liked to preach that we wouldn’t have calculators with us all the time, but that wound up not being true. Our phones have calculators at the press of a button. But maybe even calculators aren’t always needed because our pupils do more math than you think.
The pupil light reflex – constrict in light and dilate in darkness – is well known, but recent work shows that pupil size is also regulated by cognitive and perceptual factors. By presenting subjects with images of various numbers of dots and measuring pupil size, the investigators were able to show “that numerical information is intrinsically related to perception,” lead author Dr. Elisa Castaldi of Florence University noted in a written statement.
The researchers found that pupils are responsible for important survival techniques. Coauthor David Burr of the University of Sydney and the University of Florence gave an evolutionary perspective: “When we look around, we spontaneously perceive the form, size, movement and colour of a scene. Equally spontaneously, we perceive the number of items before us. This ability, shared with most other animals, is an evolutionary fundamental: It reveals immediately important quantities, such as how many apples there are on the tree, or how many enemies are attacking.”
Useful information, indeed, but our pupils seem to be more interested in the quantity of beers in the refrigerator.
No, Mr. Bond, I expect you to die
Movie watching usually requires a certain suspension of disbelief, and it’s safe to say James Bond movies require this more than most. Between the impossible gadgets and ludicrous doomsday plans, very few have ever stopped to consider the health risks of the James Bond universe.
Now, however, Bond, James Bond, has met his most formidable opponent: Wouter Graumans, a graduate student in epidemiology from the Netherlands. During a foray to Burkina Faso to study infectious diseases, Mr. Graumans came down with a case of food poisoning, which led him to wonder how 007 is able to trot across this big world of ours without contracting so much as a sinus infection.
Because Mr. Graumans is a man of science and conviction, mere speculation wasn’t enough. He and a group of coauthors wrote an entire paper on the health risks of the James Bond universe.
Doing so required watching over 3,000 minutes of numerous movies and analyzing Bond’s 86 total trips to 46 different countries based on current Centers for Disease Control and Prevention advice for travel to those countries. Time which, the authors state in the abstract, “could easily have been spent on more pressing societal issues or forms of relaxation that are more acceptable in academic circles.”
Naturally, Mr. Bond’s line of work entails exposure to unpleasant things, such as poison, dehydration, heatstroke, and dangerous wildlife (everything from ticks to crocodiles), though oddly enough he never succumbs to any of it. He’s also curiously immune to hangovers, despite rarely drinking anything nonalcoholic. There are also less obvious risks: For one, 007 rarely washes his hands. During one movie, he handles raw chicken to lure away a pack of crocodiles but fails to wash his hands afterward, leaving him at risk for multiple food-borne illnesses.
Of course, we must address the elephant in the bedroom: Mr. Bond’s numerous, er, encounters with women. One would imagine the biggest risk to those women would be from the various STDs that likely course through Bond’s body, but of the 27% who died shortly after … encountering … him, all involved violence, with disease playing no obvious role. Who knows, maybe he’s clean? Stranger things have happened.
The timing of this article may seem a bit suspicious. Was it a PR stunt by the studio? Rest assured, the authors addressed this, noting that they received no funding for the study, and that, “given the futility of its academic value, this is deemed entirely appropriate by all authors.” We love when a punchline writes itself.
How to see Atlanta on $688.35 a day
The world is always changing, so we have to change with it. This week, LOTME becomes a travel guide, and our first stop is the Big A, the Big Peach, Dogwood City, Empire City of the South, Wakanda.
There’s lots to do in Atlanta: Celebrate a World Series win, visit the College Football Hall of Fame or the World of Coca Cola, or take the Stranger Things/Upside Down film locations tour. Serious adventurers, however, get out of the city and go to Emory Decatur Hospital in – you guessed it – Decatur (unofficial motto: “Everything is Greater in Decatur”).
Find the emergency room and ask for Taylor Davis, who will be your personal guide. She’ll show you how to check in at the desk, sit in the waiting room for 7 hours, and then leave without seeing any medical personnel or receiving any sort of attention whatsoever. All the things she did when she went there in July for a head injury.
Ms. Davis told Fox5 Atlanta: “I didn’t get my vitals taken, nobody called my name. I wasn’t seen at all.”
But wait! There’s more! By booking your trip through LOTMEgo* and using the code “Decatur,” you’ll get the Taylor Davis special, which includes a bill/cover charge for $688.35 from the hospital. An Emory Healthcare patient financial services employee told Ms. Davis that “you get charged before you are seen. Not for being seen.”
If all this has you ready to hop in your car (really?), then check out LOTMEgo* on Twittbook and InstaTok. You’ll also find trick-or-treating tips and discounts on haunted hospital tours.
*Does not actually exist
Breaking down the hot flash
Do you ever wonder why we scramble for cold things when we’re feeling nauseous? Whether it’s the cool air that needs to hit your face in the car or a cold, damp towel on the back of your neck, scientists think it could possibly be an evolutionary mechanism at the cellular level.
Motion sickness it’s actually a battle of body temperature, according to an article from LiveScience. Capillaries in the skin dilate, allowing for more blood flow near the skin’s surface and causing core temperature to fall. Once body temperature drops, the hypothalamus, which regulates temperature, tries to do its job by raising body temperature. Thus the hot flash!
The cold compress and cool air help fight the battle by counteracting the hypothalamus, but why the drop in body temperature to begin with?
There are a few theories. Dr. Robert Glatter, an emergency physician at Lenox Hill Hospital in New York, told LiveScience that the lack of oxygen needed in body tissue to survive at lower temperatures could be making it difficult to get oxygen to the body when a person is ill, and is “more likely an adaptive response influenced by poorly understood mechanisms at the cellular level.”
Another theory is that the nausea and body temperature shift is the body’s natural response to help people vomit.
Then there’s the theory of “defensive hypothermia,” which suggests that cold sweats are a possible mechanism to conserve energy so the body can fight off an intruder, which was supported by a 2014 study and a 2016 review.
It’s another one of the body’s many survival tricks.
Teachers were right: Pupils can do the math
Teachers liked to preach that we wouldn’t have calculators with us all the time, but that wound up not being true. Our phones have calculators at the press of a button. But maybe even calculators aren’t always needed because our pupils do more math than you think.
The pupil light reflex – constrict in light and dilate in darkness – is well known, but recent work shows that pupil size is also regulated by cognitive and perceptual factors. By presenting subjects with images of various numbers of dots and measuring pupil size, the investigators were able to show “that numerical information is intrinsically related to perception,” lead author Dr. Elisa Castaldi of Florence University noted in a written statement.
The researchers found that pupils are responsible for important survival techniques. Coauthor David Burr of the University of Sydney and the University of Florence gave an evolutionary perspective: “When we look around, we spontaneously perceive the form, size, movement and colour of a scene. Equally spontaneously, we perceive the number of items before us. This ability, shared with most other animals, is an evolutionary fundamental: It reveals immediately important quantities, such as how many apples there are on the tree, or how many enemies are attacking.”
Useful information, indeed, but our pupils seem to be more interested in the quantity of beers in the refrigerator.
No, Mr. Bond, I expect you to die
Movie watching usually requires a certain suspension of disbelief, and it’s safe to say James Bond movies require this more than most. Between the impossible gadgets and ludicrous doomsday plans, very few have ever stopped to consider the health risks of the James Bond universe.
Now, however, Bond, James Bond, has met his most formidable opponent: Wouter Graumans, a graduate student in epidemiology from the Netherlands. During a foray to Burkina Faso to study infectious diseases, Mr. Graumans came down with a case of food poisoning, which led him to wonder how 007 is able to trot across this big world of ours without contracting so much as a sinus infection.
Because Mr. Graumans is a man of science and conviction, mere speculation wasn’t enough. He and a group of coauthors wrote an entire paper on the health risks of the James Bond universe.
Doing so required watching over 3,000 minutes of numerous movies and analyzing Bond’s 86 total trips to 46 different countries based on current Centers for Disease Control and Prevention advice for travel to those countries. Time which, the authors state in the abstract, “could easily have been spent on more pressing societal issues or forms of relaxation that are more acceptable in academic circles.”
Naturally, Mr. Bond’s line of work entails exposure to unpleasant things, such as poison, dehydration, heatstroke, and dangerous wildlife (everything from ticks to crocodiles), though oddly enough he never succumbs to any of it. He’s also curiously immune to hangovers, despite rarely drinking anything nonalcoholic. There are also less obvious risks: For one, 007 rarely washes his hands. During one movie, he handles raw chicken to lure away a pack of crocodiles but fails to wash his hands afterward, leaving him at risk for multiple food-borne illnesses.
Of course, we must address the elephant in the bedroom: Mr. Bond’s numerous, er, encounters with women. One would imagine the biggest risk to those women would be from the various STDs that likely course through Bond’s body, but of the 27% who died shortly after … encountering … him, all involved violence, with disease playing no obvious role. Who knows, maybe he’s clean? Stranger things have happened.
The timing of this article may seem a bit suspicious. Was it a PR stunt by the studio? Rest assured, the authors addressed this, noting that they received no funding for the study, and that, “given the futility of its academic value, this is deemed entirely appropriate by all authors.” We love when a punchline writes itself.
How to see Atlanta on $688.35 a day
The world is always changing, so we have to change with it. This week, LOTME becomes a travel guide, and our first stop is the Big A, the Big Peach, Dogwood City, Empire City of the South, Wakanda.
There’s lots to do in Atlanta: Celebrate a World Series win, visit the College Football Hall of Fame or the World of Coca Cola, or take the Stranger Things/Upside Down film locations tour. Serious adventurers, however, get out of the city and go to Emory Decatur Hospital in – you guessed it – Decatur (unofficial motto: “Everything is Greater in Decatur”).
Find the emergency room and ask for Taylor Davis, who will be your personal guide. She’ll show you how to check in at the desk, sit in the waiting room for 7 hours, and then leave without seeing any medical personnel or receiving any sort of attention whatsoever. All the things she did when she went there in July for a head injury.
Ms. Davis told Fox5 Atlanta: “I didn’t get my vitals taken, nobody called my name. I wasn’t seen at all.”
But wait! There’s more! By booking your trip through LOTMEgo* and using the code “Decatur,” you’ll get the Taylor Davis special, which includes a bill/cover charge for $688.35 from the hospital. An Emory Healthcare patient financial services employee told Ms. Davis that “you get charged before you are seen. Not for being seen.”
If all this has you ready to hop in your car (really?), then check out LOTMEgo* on Twittbook and InstaTok. You’ll also find trick-or-treating tips and discounts on haunted hospital tours.
*Does not actually exist
Breaking down the hot flash
Do you ever wonder why we scramble for cold things when we’re feeling nauseous? Whether it’s the cool air that needs to hit your face in the car or a cold, damp towel on the back of your neck, scientists think it could possibly be an evolutionary mechanism at the cellular level.
Motion sickness it’s actually a battle of body temperature, according to an article from LiveScience. Capillaries in the skin dilate, allowing for more blood flow near the skin’s surface and causing core temperature to fall. Once body temperature drops, the hypothalamus, which regulates temperature, tries to do its job by raising body temperature. Thus the hot flash!
The cold compress and cool air help fight the battle by counteracting the hypothalamus, but why the drop in body temperature to begin with?
There are a few theories. Dr. Robert Glatter, an emergency physician at Lenox Hill Hospital in New York, told LiveScience that the lack of oxygen needed in body tissue to survive at lower temperatures could be making it difficult to get oxygen to the body when a person is ill, and is “more likely an adaptive response influenced by poorly understood mechanisms at the cellular level.”
Another theory is that the nausea and body temperature shift is the body’s natural response to help people vomit.
Then there’s the theory of “defensive hypothermia,” which suggests that cold sweats are a possible mechanism to conserve energy so the body can fight off an intruder, which was supported by a 2014 study and a 2016 review.
It’s another one of the body’s many survival tricks.
Teachers were right: Pupils can do the math
Teachers liked to preach that we wouldn’t have calculators with us all the time, but that wound up not being true. Our phones have calculators at the press of a button. But maybe even calculators aren’t always needed because our pupils do more math than you think.
The pupil light reflex – constrict in light and dilate in darkness – is well known, but recent work shows that pupil size is also regulated by cognitive and perceptual factors. By presenting subjects with images of various numbers of dots and measuring pupil size, the investigators were able to show “that numerical information is intrinsically related to perception,” lead author Dr. Elisa Castaldi of Florence University noted in a written statement.
The researchers found that pupils are responsible for important survival techniques. Coauthor David Burr of the University of Sydney and the University of Florence gave an evolutionary perspective: “When we look around, we spontaneously perceive the form, size, movement and colour of a scene. Equally spontaneously, we perceive the number of items before us. This ability, shared with most other animals, is an evolutionary fundamental: It reveals immediately important quantities, such as how many apples there are on the tree, or how many enemies are attacking.”
Useful information, indeed, but our pupils seem to be more interested in the quantity of beers in the refrigerator.
CDC endorses Pfizer’s COVID-19 vaccine for young kids
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
FDA class I recall of CardioSave hybrid/rescue IABPs
Datascope/Getinge/Maquet is recalling CardioSave Hybrid and Rescue intra-aortic balloon pumps (IABPs) because some battery packs may have a shortened run time and fail unexpectedly, according to a medical device recall notice posted on the U.S. Food and Drug Administration website.
The FDA has identified this as a class I recall, the most serious type of recall, because of the risk for serious injury or death.
The recalled IABPs have substandard batteries that do not meet performance specifications and were mistakenly released to a limited number of customers.
If a patient requires life-supporting therapy with an IABP and the device does not work or stops working during use because of battery failure, the patient will be at risk for serious injury, including death, the FDA cautions.
Both IABP monitors display battery life and have low battery alarms when alternative power sources are needed.
Datascope/Getting/Maquet has received six complaints but no reports of injury or death related to this issue.
“However, there is a potential for underreporting since the end user reporting a failed battery or short battery run time cannot be aware that they originally received a substandard battery,” the FDA said.
The recall involves 137 battery packs distributed in the United States between Sept. 23, 2017, and Aug. 17, 2021. Product codes and lot numbers are available in the recall notice.
The company sent an urgent medical device removal letter to customers requesting that they check inventory to determine if there are any CardioSave LiIon battery packs with part number/reference number 0146-00-0097 and with serial numbers listed in the letter.
Customers are asked to replace any affected battery with an unaffected battery and remove the affected product from areas of use.
The company will issue credit or a replacement battery at no cost to the facility upon receipt of the response form attached to the letter.
Distributors who shipped any affected product to customers are asked to forward the device removal letter to customers.
All customers, regardless of whether or not they have defective batteries, are asked to complete and sign the response form to acknowledge that they received the notification and disposed of the affected batteries.
Completed forms can be scanned and emailed to Datascope/Getinge/Maquet at [email protected] or by FAX to 1-877-446-3360.
Customers who have questions about this recall should contact their Datascope/Getinge/Maquet sales representative or, for technical questions, customer service (1-888-943-8872, option 2), Monday through Friday, 8:00 a.m. to 6:00 p.m. ET.
Any adverse events or suspected adverse events related to the recalled CardioSave Hybrid/Rescue IABPs should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article first appeared on Medscape.com.
Datascope/Getinge/Maquet is recalling CardioSave Hybrid and Rescue intra-aortic balloon pumps (IABPs) because some battery packs may have a shortened run time and fail unexpectedly, according to a medical device recall notice posted on the U.S. Food and Drug Administration website.
The FDA has identified this as a class I recall, the most serious type of recall, because of the risk for serious injury or death.
The recalled IABPs have substandard batteries that do not meet performance specifications and were mistakenly released to a limited number of customers.
If a patient requires life-supporting therapy with an IABP and the device does not work or stops working during use because of battery failure, the patient will be at risk for serious injury, including death, the FDA cautions.
Both IABP monitors display battery life and have low battery alarms when alternative power sources are needed.
Datascope/Getting/Maquet has received six complaints but no reports of injury or death related to this issue.
“However, there is a potential for underreporting since the end user reporting a failed battery or short battery run time cannot be aware that they originally received a substandard battery,” the FDA said.
The recall involves 137 battery packs distributed in the United States between Sept. 23, 2017, and Aug. 17, 2021. Product codes and lot numbers are available in the recall notice.
The company sent an urgent medical device removal letter to customers requesting that they check inventory to determine if there are any CardioSave LiIon battery packs with part number/reference number 0146-00-0097 and with serial numbers listed in the letter.
Customers are asked to replace any affected battery with an unaffected battery and remove the affected product from areas of use.
The company will issue credit or a replacement battery at no cost to the facility upon receipt of the response form attached to the letter.
Distributors who shipped any affected product to customers are asked to forward the device removal letter to customers.
All customers, regardless of whether or not they have defective batteries, are asked to complete and sign the response form to acknowledge that they received the notification and disposed of the affected batteries.
Completed forms can be scanned and emailed to Datascope/Getinge/Maquet at [email protected] or by FAX to 1-877-446-3360.
Customers who have questions about this recall should contact their Datascope/Getinge/Maquet sales representative or, for technical questions, customer service (1-888-943-8872, option 2), Monday through Friday, 8:00 a.m. to 6:00 p.m. ET.
Any adverse events or suspected adverse events related to the recalled CardioSave Hybrid/Rescue IABPs should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article first appeared on Medscape.com.
Datascope/Getinge/Maquet is recalling CardioSave Hybrid and Rescue intra-aortic balloon pumps (IABPs) because some battery packs may have a shortened run time and fail unexpectedly, according to a medical device recall notice posted on the U.S. Food and Drug Administration website.
The FDA has identified this as a class I recall, the most serious type of recall, because of the risk for serious injury or death.
The recalled IABPs have substandard batteries that do not meet performance specifications and were mistakenly released to a limited number of customers.
If a patient requires life-supporting therapy with an IABP and the device does not work or stops working during use because of battery failure, the patient will be at risk for serious injury, including death, the FDA cautions.
Both IABP monitors display battery life and have low battery alarms when alternative power sources are needed.
Datascope/Getting/Maquet has received six complaints but no reports of injury or death related to this issue.
“However, there is a potential for underreporting since the end user reporting a failed battery or short battery run time cannot be aware that they originally received a substandard battery,” the FDA said.
The recall involves 137 battery packs distributed in the United States between Sept. 23, 2017, and Aug. 17, 2021. Product codes and lot numbers are available in the recall notice.
The company sent an urgent medical device removal letter to customers requesting that they check inventory to determine if there are any CardioSave LiIon battery packs with part number/reference number 0146-00-0097 and with serial numbers listed in the letter.
Customers are asked to replace any affected battery with an unaffected battery and remove the affected product from areas of use.
The company will issue credit or a replacement battery at no cost to the facility upon receipt of the response form attached to the letter.
Distributors who shipped any affected product to customers are asked to forward the device removal letter to customers.
All customers, regardless of whether or not they have defective batteries, are asked to complete and sign the response form to acknowledge that they received the notification and disposed of the affected batteries.
Completed forms can be scanned and emailed to Datascope/Getinge/Maquet at [email protected] or by FAX to 1-877-446-3360.
Customers who have questions about this recall should contact their Datascope/Getinge/Maquet sales representative or, for technical questions, customer service (1-888-943-8872, option 2), Monday through Friday, 8:00 a.m. to 6:00 p.m. ET.
Any adverse events or suspected adverse events related to the recalled CardioSave Hybrid/Rescue IABPs should be reported to the FDA through MedWatch, its adverse event reporting program.
A version of this article first appeared on Medscape.com.