User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Cardiac arrest survival lower in COVID-19 inpatients
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
FROM JAMA NETWORK OPEN
Analysis questions tocilizumab in ventilated COVID patients
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Proper steps for physicians to follow if they find themselves under investigation
Physician clients will find themselves in difficult legal situations from time to time. Sometimes it’s an investigation for Medicare fraud or other illegal conduct. Other times it’s a review related to Drug Enforcement Administration or licensure compliance. More commonly, physicians are involved in employer inquiries into workplace misconduct.
but how they choose to deal with the issue can have significant consequences.
In my opinion, physicians should have a relationship with a health care lawyer or firm in place before any investigation occurs. Whether they are being investigated for a license or medical staff issue, Medicare fraud, or contract issue, it’s important to know where to go for help quickly. Even if the physician does not retain a lawyer in advance, having the name of a qualified person who can be called for a variety of health care issues is already a step in the right direction.
More important than having a knowledgeable lawyer is actually contacting that lawyer. Some physicians will sit and chat with the Federal Bureau of Investigation or other investigators for hours, only to call me after the visitors leave. I have other clients who handle important medical staff hearings, discipline meetings, and license investigations on their own without consulting counsel first. In all of these situations, it can be too late to help a physician once their case has progressed too far down the road.
Employment issues arising in the workplace setting are the most common and troubling. Physicians will – without a second thought – attend a human resources–called or other meeting without thinking through the reason for the meeting, whether they are prepared or not, and without considering whether counsel could be helpful. Sometimes in the moment, there may be no choice, but most meetings are scheduled in advance with ample time for consultation and planning.
Many issues that arise in the workplace setting are troubling because they can be easily avoided. The No. 1 piece of advice which I offer to young physician clients as they enter the workplace is: Remember that nobody in the workplace is your friend. Every word that is said, text that is sent, gesture that is made, can put you at risk. You must assume that all conversations and messages will be shared with others. Joking around in the operating room about sexual escapades, sending texts with flirtatious comments, making comments that can be construed as racist or homophobic, or raising your voice in a moment of frustration are all real examples of situations where physicians ended up disciplined and terminated. Are these innocent comments or ones the doctor thought they could get away with among “friends?” From a human resources perspective, there is little tolerance for such conduct, regardless of the doctor’s intent.
There are also situations in the workplace that are more troubling. Many times a physician is accused of noncompliance with a contract or a policy, when in fact the accuser is retaliating or engaging in efforts to discredit a doctor. I have seen this happen where minority physicians complain about how they are treated and are suddenly investigated for a performance issue. I have had female physicians criticize a business decision at a committee meeting, only to receive a formal notice that their “negative attitude” violated a policy.
In these situations, talking with counsel before a meeting with the employer representative is recommended and can impact the trajectory of a physician’s career. Physicians cannot and should not handle such events on their own.
If a physician is forced or chooses to attend a meeting with an investigator or other party without counsel, there are some steps to consider (subject to the type of meeting and the specific circumstances).
- Listen more than you talk. Make sure you know the name of everyone who is present and their role within the organization.
- If you have previously provided any written or oral statements, or have written correspondence related to the issues at hand, review all materials in advance. If there is anything you think needs to be corrected or added, let the interviewer know that at the outset.
- Be familiar with your own employment agreement/policies and the terms that may be relevant to the discussion or meeting.
- Be calm, honest, and forthcoming in response to the questions, and don’t embellish or exaggerate.
- Avoid personal attacks on anyone. This generally serves to weaken an argument and credibility.
- Be prepared to explain your allegations or defense, and when you do so, keep in mind that the interviewer may not know the history, background, or details of any of the issues.
- If the reason for the situation relates to race or national origin, age, gender, sexual orientation, disability, or other protected category, don’t hesitate to say so.
- Answer the question you’re asked, but if you feel that the interviewer needs more information or is not understanding what you’ve said, feel free to explain. Be forthcoming, but don’t dominate the conversation.
- If they ask whether you have counsel, be honest, but decline to provide them any information about what you discussed with counsel, as those conversations are privileged.
- If the interviewer asks to record the conversation, you can agree, but ask to be provided a copy of the recording.
- Know your rights in advance. If the subject of the meeting is governed by bylaws or policies, for example, you may have the right to bring an attorney or adviser to the meeting, receive advance notice of who will be attending the meeting and the subject matter, and avail yourself of specific procedures or appeal rights of any discipline or decisions decided during the meeting.
There are many circumstances that can lead to a physician being under investigation or interrogation. In every single circumstance, it is ideal to seek legal counsel immediately. Whether the physician has actually engaged in wrongful conduct or not, without proper handling a physician’s career can be permanently, and sometimes irrevocably, affected.
Ms. Adler is a shareholder and health law practice group manager for Chicago-based law firm Roetzel, a member of the Illinois Association of Healthcare Attorneys, and a current advisory board member at DePaul College of Law Health Law Institute. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physician clients will find themselves in difficult legal situations from time to time. Sometimes it’s an investigation for Medicare fraud or other illegal conduct. Other times it’s a review related to Drug Enforcement Administration or licensure compliance. More commonly, physicians are involved in employer inquiries into workplace misconduct.
but how they choose to deal with the issue can have significant consequences.
In my opinion, physicians should have a relationship with a health care lawyer or firm in place before any investigation occurs. Whether they are being investigated for a license or medical staff issue, Medicare fraud, or contract issue, it’s important to know where to go for help quickly. Even if the physician does not retain a lawyer in advance, having the name of a qualified person who can be called for a variety of health care issues is already a step in the right direction.
More important than having a knowledgeable lawyer is actually contacting that lawyer. Some physicians will sit and chat with the Federal Bureau of Investigation or other investigators for hours, only to call me after the visitors leave. I have other clients who handle important medical staff hearings, discipline meetings, and license investigations on their own without consulting counsel first. In all of these situations, it can be too late to help a physician once their case has progressed too far down the road.
Employment issues arising in the workplace setting are the most common and troubling. Physicians will – without a second thought – attend a human resources–called or other meeting without thinking through the reason for the meeting, whether they are prepared or not, and without considering whether counsel could be helpful. Sometimes in the moment, there may be no choice, but most meetings are scheduled in advance with ample time for consultation and planning.
Many issues that arise in the workplace setting are troubling because they can be easily avoided. The No. 1 piece of advice which I offer to young physician clients as they enter the workplace is: Remember that nobody in the workplace is your friend. Every word that is said, text that is sent, gesture that is made, can put you at risk. You must assume that all conversations and messages will be shared with others. Joking around in the operating room about sexual escapades, sending texts with flirtatious comments, making comments that can be construed as racist or homophobic, or raising your voice in a moment of frustration are all real examples of situations where physicians ended up disciplined and terminated. Are these innocent comments or ones the doctor thought they could get away with among “friends?” From a human resources perspective, there is little tolerance for such conduct, regardless of the doctor’s intent.
There are also situations in the workplace that are more troubling. Many times a physician is accused of noncompliance with a contract or a policy, when in fact the accuser is retaliating or engaging in efforts to discredit a doctor. I have seen this happen where minority physicians complain about how they are treated and are suddenly investigated for a performance issue. I have had female physicians criticize a business decision at a committee meeting, only to receive a formal notice that their “negative attitude” violated a policy.
In these situations, talking with counsel before a meeting with the employer representative is recommended and can impact the trajectory of a physician’s career. Physicians cannot and should not handle such events on their own.
If a physician is forced or chooses to attend a meeting with an investigator or other party without counsel, there are some steps to consider (subject to the type of meeting and the specific circumstances).
- Listen more than you talk. Make sure you know the name of everyone who is present and their role within the organization.
- If you have previously provided any written or oral statements, or have written correspondence related to the issues at hand, review all materials in advance. If there is anything you think needs to be corrected or added, let the interviewer know that at the outset.
- Be familiar with your own employment agreement/policies and the terms that may be relevant to the discussion or meeting.
- Be calm, honest, and forthcoming in response to the questions, and don’t embellish or exaggerate.
- Avoid personal attacks on anyone. This generally serves to weaken an argument and credibility.
- Be prepared to explain your allegations or defense, and when you do so, keep in mind that the interviewer may not know the history, background, or details of any of the issues.
- If the reason for the situation relates to race or national origin, age, gender, sexual orientation, disability, or other protected category, don’t hesitate to say so.
- Answer the question you’re asked, but if you feel that the interviewer needs more information or is not understanding what you’ve said, feel free to explain. Be forthcoming, but don’t dominate the conversation.
- If they ask whether you have counsel, be honest, but decline to provide them any information about what you discussed with counsel, as those conversations are privileged.
- If the interviewer asks to record the conversation, you can agree, but ask to be provided a copy of the recording.
- Know your rights in advance. If the subject of the meeting is governed by bylaws or policies, for example, you may have the right to bring an attorney or adviser to the meeting, receive advance notice of who will be attending the meeting and the subject matter, and avail yourself of specific procedures or appeal rights of any discipline or decisions decided during the meeting.
There are many circumstances that can lead to a physician being under investigation or interrogation. In every single circumstance, it is ideal to seek legal counsel immediately. Whether the physician has actually engaged in wrongful conduct or not, without proper handling a physician’s career can be permanently, and sometimes irrevocably, affected.
Ms. Adler is a shareholder and health law practice group manager for Chicago-based law firm Roetzel, a member of the Illinois Association of Healthcare Attorneys, and a current advisory board member at DePaul College of Law Health Law Institute. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Physician clients will find themselves in difficult legal situations from time to time. Sometimes it’s an investigation for Medicare fraud or other illegal conduct. Other times it’s a review related to Drug Enforcement Administration or licensure compliance. More commonly, physicians are involved in employer inquiries into workplace misconduct.
but how they choose to deal with the issue can have significant consequences.
In my opinion, physicians should have a relationship with a health care lawyer or firm in place before any investigation occurs. Whether they are being investigated for a license or medical staff issue, Medicare fraud, or contract issue, it’s important to know where to go for help quickly. Even if the physician does not retain a lawyer in advance, having the name of a qualified person who can be called for a variety of health care issues is already a step in the right direction.
More important than having a knowledgeable lawyer is actually contacting that lawyer. Some physicians will sit and chat with the Federal Bureau of Investigation or other investigators for hours, only to call me after the visitors leave. I have other clients who handle important medical staff hearings, discipline meetings, and license investigations on their own without consulting counsel first. In all of these situations, it can be too late to help a physician once their case has progressed too far down the road.
Employment issues arising in the workplace setting are the most common and troubling. Physicians will – without a second thought – attend a human resources–called or other meeting without thinking through the reason for the meeting, whether they are prepared or not, and without considering whether counsel could be helpful. Sometimes in the moment, there may be no choice, but most meetings are scheduled in advance with ample time for consultation and planning.
Many issues that arise in the workplace setting are troubling because they can be easily avoided. The No. 1 piece of advice which I offer to young physician clients as they enter the workplace is: Remember that nobody in the workplace is your friend. Every word that is said, text that is sent, gesture that is made, can put you at risk. You must assume that all conversations and messages will be shared with others. Joking around in the operating room about sexual escapades, sending texts with flirtatious comments, making comments that can be construed as racist or homophobic, or raising your voice in a moment of frustration are all real examples of situations where physicians ended up disciplined and terminated. Are these innocent comments or ones the doctor thought they could get away with among “friends?” From a human resources perspective, there is little tolerance for such conduct, regardless of the doctor’s intent.
There are also situations in the workplace that are more troubling. Many times a physician is accused of noncompliance with a contract or a policy, when in fact the accuser is retaliating or engaging in efforts to discredit a doctor. I have seen this happen where minority physicians complain about how they are treated and are suddenly investigated for a performance issue. I have had female physicians criticize a business decision at a committee meeting, only to receive a formal notice that their “negative attitude” violated a policy.
In these situations, talking with counsel before a meeting with the employer representative is recommended and can impact the trajectory of a physician’s career. Physicians cannot and should not handle such events on their own.
If a physician is forced or chooses to attend a meeting with an investigator or other party without counsel, there are some steps to consider (subject to the type of meeting and the specific circumstances).
- Listen more than you talk. Make sure you know the name of everyone who is present and their role within the organization.
- If you have previously provided any written or oral statements, or have written correspondence related to the issues at hand, review all materials in advance. If there is anything you think needs to be corrected or added, let the interviewer know that at the outset.
- Be familiar with your own employment agreement/policies and the terms that may be relevant to the discussion or meeting.
- Be calm, honest, and forthcoming in response to the questions, and don’t embellish or exaggerate.
- Avoid personal attacks on anyone. This generally serves to weaken an argument and credibility.
- Be prepared to explain your allegations or defense, and when you do so, keep in mind that the interviewer may not know the history, background, or details of any of the issues.
- If the reason for the situation relates to race or national origin, age, gender, sexual orientation, disability, or other protected category, don’t hesitate to say so.
- Answer the question you’re asked, but if you feel that the interviewer needs more information or is not understanding what you’ve said, feel free to explain. Be forthcoming, but don’t dominate the conversation.
- If they ask whether you have counsel, be honest, but decline to provide them any information about what you discussed with counsel, as those conversations are privileged.
- If the interviewer asks to record the conversation, you can agree, but ask to be provided a copy of the recording.
- Know your rights in advance. If the subject of the meeting is governed by bylaws or policies, for example, you may have the right to bring an attorney or adviser to the meeting, receive advance notice of who will be attending the meeting and the subject matter, and avail yourself of specific procedures or appeal rights of any discipline or decisions decided during the meeting.
There are many circumstances that can lead to a physician being under investigation or interrogation. In every single circumstance, it is ideal to seek legal counsel immediately. Whether the physician has actually engaged in wrongful conduct or not, without proper handling a physician’s career can be permanently, and sometimes irrevocably, affected.
Ms. Adler is a shareholder and health law practice group manager for Chicago-based law firm Roetzel, a member of the Illinois Association of Healthcare Attorneys, and a current advisory board member at DePaul College of Law Health Law Institute. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Self-care tips for clinicians as COVID-19 lingers
LAS VEGAS – according to Jon A. Levenson, MD.
“There are those who will need mental health treatment, so creating an easy way to reach out for help and facilitate linkage with care is critically important,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The vast majority of our workforce will thrive with proper support. But what can each of us do to take care of ourselves?”
Step one is to recognize common stress reactions as well as signs of distress. He offered the oxygen mask metaphor, the idea that before we can take care of and support anyone else, we must first take care of ourselves. “When people are stressed, they don’t always think about the oxygen mask metaphor,” Dr. Levenson said. Step two is to practice and model self-care by adopting principles often discussed in acceptance and commitment therapy: to focus on what you can control, not on what you can’t control.
“We can’t control the amount of toilet paper at the grocery store, how long the pandemic will last, or how others have reacted,” Dr. Levenson said. “We also can’t control other people’s motives, predict what will happen, or the actions of others, including whether they will follow social distancing guidelines or not.”
How about what we can control? One is a positive attitude, “which can sustain people during times of intense stress,” he said. “Other things that we can do include turn off the news and find fun and enriching activities to do at home, whether it be playing a game with family or reaching out to friends through an iPad or a smartphone. You can also follow [Centers for Disease Control and Prevention] recommendations, control your own social distancing, and limit social media activity, which can be stressful. We can also control our kindness and grace.” He added that resilience does not mean “snapping back” to how you were before the pandemic, but rather “learning to integrate the adverse experiences into who you are and growing with them, which is sometimes known as posttraumatic growth.”
Dr. Levenson encouraged health care workers to use their coping resources, connect to others, and cultivate their values and purpose in life as they navigate these challenging times. “You also want to promote realistic optimism; find a way to stay positive,” he said. “We emphasize to our staff that while you won’t forget this time, focus on what you can control – your positive relationships – and remind yourself of your values and sources of gratitude. Figure out, and reflect on, what you care about, and then care about it. Remind yourself in a deliberate, purposeful way what anchors you to your job, which in the health care setting tends to be a desire to care for others, to assist those in need, and to work in teams. We also encourage staff to refrain from judgment. Guilt is a normal and near-universal response to this stressor, but there are many ways to contribute without a judgmental or guilty tone.”
Other tips for self-support are to remind yourself that it is not selfish to take breaks. “The needs of your patients are not more important than your own needs,” Dr. Levenson said. “Working nonstop can put you at higher risk for stress, exhaustion, and illness. You may need to give yourself more time to step back and recover from workplace challenges or extended coverage for peers; this is important. We remind our staff that your work may feel more emotionally draining than usual because everything is more intense overall during the COVID-19 pandemic. This reminder helps staff normalize what they already may be experiencing, and in turn, to further support each other.”
Soothing activities to relieve stress include meditation, prayer, deep and slow breathing, relaxation exercises, yoga, mindfulness, stretching, staying hydrated, eating healthfully, exercise, and getting sufficient sleep. Other stress management tips include avoiding excessive alcohol intake, reaching out to others, asking for assistance, and delegating when possible. “We want to promote psychological flexibility: the ability to stay in contact with the present moment,” he said. “We encourage our peers to be aware of unpleasant thoughts and feelings, and to try to redirect negative thought patterns to a proactive problem-solving approach; this includes choosing one’s behaviors based on the situation and personal values.”
Dr. Levenson reported having no disclosures related to his presentation.
LAS VEGAS – according to Jon A. Levenson, MD.
“There are those who will need mental health treatment, so creating an easy way to reach out for help and facilitate linkage with care is critically important,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The vast majority of our workforce will thrive with proper support. But what can each of us do to take care of ourselves?”
Step one is to recognize common stress reactions as well as signs of distress. He offered the oxygen mask metaphor, the idea that before we can take care of and support anyone else, we must first take care of ourselves. “When people are stressed, they don’t always think about the oxygen mask metaphor,” Dr. Levenson said. Step two is to practice and model self-care by adopting principles often discussed in acceptance and commitment therapy: to focus on what you can control, not on what you can’t control.
“We can’t control the amount of toilet paper at the grocery store, how long the pandemic will last, or how others have reacted,” Dr. Levenson said. “We also can’t control other people’s motives, predict what will happen, or the actions of others, including whether they will follow social distancing guidelines or not.”
How about what we can control? One is a positive attitude, “which can sustain people during times of intense stress,” he said. “Other things that we can do include turn off the news and find fun and enriching activities to do at home, whether it be playing a game with family or reaching out to friends through an iPad or a smartphone. You can also follow [Centers for Disease Control and Prevention] recommendations, control your own social distancing, and limit social media activity, which can be stressful. We can also control our kindness and grace.” He added that resilience does not mean “snapping back” to how you were before the pandemic, but rather “learning to integrate the adverse experiences into who you are and growing with them, which is sometimes known as posttraumatic growth.”
Dr. Levenson encouraged health care workers to use their coping resources, connect to others, and cultivate their values and purpose in life as they navigate these challenging times. “You also want to promote realistic optimism; find a way to stay positive,” he said. “We emphasize to our staff that while you won’t forget this time, focus on what you can control – your positive relationships – and remind yourself of your values and sources of gratitude. Figure out, and reflect on, what you care about, and then care about it. Remind yourself in a deliberate, purposeful way what anchors you to your job, which in the health care setting tends to be a desire to care for others, to assist those in need, and to work in teams. We also encourage staff to refrain from judgment. Guilt is a normal and near-universal response to this stressor, but there are many ways to contribute without a judgmental or guilty tone.”
Other tips for self-support are to remind yourself that it is not selfish to take breaks. “The needs of your patients are not more important than your own needs,” Dr. Levenson said. “Working nonstop can put you at higher risk for stress, exhaustion, and illness. You may need to give yourself more time to step back and recover from workplace challenges or extended coverage for peers; this is important. We remind our staff that your work may feel more emotionally draining than usual because everything is more intense overall during the COVID-19 pandemic. This reminder helps staff normalize what they already may be experiencing, and in turn, to further support each other.”
Soothing activities to relieve stress include meditation, prayer, deep and slow breathing, relaxation exercises, yoga, mindfulness, stretching, staying hydrated, eating healthfully, exercise, and getting sufficient sleep. Other stress management tips include avoiding excessive alcohol intake, reaching out to others, asking for assistance, and delegating when possible. “We want to promote psychological flexibility: the ability to stay in contact with the present moment,” he said. “We encourage our peers to be aware of unpleasant thoughts and feelings, and to try to redirect negative thought patterns to a proactive problem-solving approach; this includes choosing one’s behaviors based on the situation and personal values.”
Dr. Levenson reported having no disclosures related to his presentation.
LAS VEGAS – according to Jon A. Levenson, MD.
“There are those who will need mental health treatment, so creating an easy way to reach out for help and facilitate linkage with care is critically important,” Dr. Levenson, associate professor of psychiatry at Columbia University Irving Medical Center, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The vast majority of our workforce will thrive with proper support. But what can each of us do to take care of ourselves?”
Step one is to recognize common stress reactions as well as signs of distress. He offered the oxygen mask metaphor, the idea that before we can take care of and support anyone else, we must first take care of ourselves. “When people are stressed, they don’t always think about the oxygen mask metaphor,” Dr. Levenson said. Step two is to practice and model self-care by adopting principles often discussed in acceptance and commitment therapy: to focus on what you can control, not on what you can’t control.
“We can’t control the amount of toilet paper at the grocery store, how long the pandemic will last, or how others have reacted,” Dr. Levenson said. “We also can’t control other people’s motives, predict what will happen, or the actions of others, including whether they will follow social distancing guidelines or not.”
How about what we can control? One is a positive attitude, “which can sustain people during times of intense stress,” he said. “Other things that we can do include turn off the news and find fun and enriching activities to do at home, whether it be playing a game with family or reaching out to friends through an iPad or a smartphone. You can also follow [Centers for Disease Control and Prevention] recommendations, control your own social distancing, and limit social media activity, which can be stressful. We can also control our kindness and grace.” He added that resilience does not mean “snapping back” to how you were before the pandemic, but rather “learning to integrate the adverse experiences into who you are and growing with them, which is sometimes known as posttraumatic growth.”
Dr. Levenson encouraged health care workers to use their coping resources, connect to others, and cultivate their values and purpose in life as they navigate these challenging times. “You also want to promote realistic optimism; find a way to stay positive,” he said. “We emphasize to our staff that while you won’t forget this time, focus on what you can control – your positive relationships – and remind yourself of your values and sources of gratitude. Figure out, and reflect on, what you care about, and then care about it. Remind yourself in a deliberate, purposeful way what anchors you to your job, which in the health care setting tends to be a desire to care for others, to assist those in need, and to work in teams. We also encourage staff to refrain from judgment. Guilt is a normal and near-universal response to this stressor, but there are many ways to contribute without a judgmental or guilty tone.”
Other tips for self-support are to remind yourself that it is not selfish to take breaks. “The needs of your patients are not more important than your own needs,” Dr. Levenson said. “Working nonstop can put you at higher risk for stress, exhaustion, and illness. You may need to give yourself more time to step back and recover from workplace challenges or extended coverage for peers; this is important. We remind our staff that your work may feel more emotionally draining than usual because everything is more intense overall during the COVID-19 pandemic. This reminder helps staff normalize what they already may be experiencing, and in turn, to further support each other.”
Soothing activities to relieve stress include meditation, prayer, deep and slow breathing, relaxation exercises, yoga, mindfulness, stretching, staying hydrated, eating healthfully, exercise, and getting sufficient sleep. Other stress management tips include avoiding excessive alcohol intake, reaching out to others, asking for assistance, and delegating when possible. “We want to promote psychological flexibility: the ability to stay in contact with the present moment,” he said. “We encourage our peers to be aware of unpleasant thoughts and feelings, and to try to redirect negative thought patterns to a proactive problem-solving approach; this includes choosing one’s behaviors based on the situation and personal values.”
Dr. Levenson reported having no disclosures related to his presentation.
AT NPA 2022
Tastier chocolate may be healthier chocolate
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
Chocolate: Now part of a well-balanced diet
Asking if someone loves chocolate is like asking if they love breathing. It’s really not a question that needs to be asked. The thing with chocolate, however, is that most people who love chocolate actually love sugar, since your typical milk chocolate contains only about 30% cacao. The rest, of course, is sugar.
Now, dark chocolate is actually kind of good for you since it contains beneficial flavonoids and less sugar. But that healthiness comes at a cost: Dark chocolate is quite bitter, and gets more so as the cacao content rises, to the point where 100% cacao chocolate is very nearly inedible. That’s the chocolate conundrum, the healthier it is, the worse it tastes. But what if there’s another way? What if you can have tasty chocolate that’s good for you?
That’s the question a group of researchers from Penn State University dared to ask. The secret, they discovered, is to subject the cacao beans to extra-intense roasting. We’re not sure how screaming insults at a bunch of beans will help, but if science says so ... YOU USELESS LUMP OF BARELY EDIBLE FOOD! HOW DARE YOU EXIST!
Oh, not that kind of roasting. Oops.
For their study, the researchers made 27 unsweetened chocolates, prepared using various cacao bean roasting times and temperatures, and served them to volunteers. Those volunteers reported that chocolates made with cacao beans roasted more intensely (such as 20 minutes at 340° F, 80 min at 275° F, and 54 min at 304° F) were far more acceptable than were chocolates prepared with raw or lightly roasted cacao beans.
The implications of healthy yet tasty chocolate are obvious: Master the chocolate and you’ll make millions. Imagine a future where parents say to their kids: “Don’t forget to eat your chocolate.” So, we’re off to do some cooking. Don’t want Hershey to make all the money off of this revelation.
The villain hiding in dairy for some MS patients
For some of us, lactose can be a real heartbreaker when it comes to dairy consumption, but for people with multiple sclerosis (MS) there’s another villain they may also have to face that can make their symptoms worse.
Physicians at the Institute of Anatomy at University Hospital Bonn (Germany) were getting so many complaints from patients with MS about how much worse they felt about after having cheese, yogurt, and milk that they decided to get to the bottom of it. The culprit, it seems, is casein, a protein specifically found in cow’s milk.
The researchers injected mice with various proteins found in cow’s milk and found perforated myelin sheaths in those given casein. In MS, the patient’s own immune system destroys that sheath, which leads to paresthesia, vision problems, and movement disorders.
“The body’s defenses actually attack the casein, but in the process they also destroy proteins involved in the formation of myelin, “ said Rittika Chunder, a postdoctoral fellow at the University of Bonn. How? Apparently it’s all a big misunderstanding.
While looking at molecules needed for myelin production, the researchers came across MAG, which is very similar to casein, which is a problem when patients with MS are allergic to casein. After they have dairy products, the B-cell squad gets called in to clean up the evil twin, casein, but can’t differentiate it from the good twin, MAG, so it all gets a wash and the myelin sheath suffers.
Since this happens only to patients with MS who have a casein allergy, the researchers advise them to stay away from milk, yogurt, or cottage cheese while they work on a self-test to check if patients carry the antibodies.
A small price to pay, perhaps, to stop a villainous evil twin.
You would even say it glows
If you’re anything like us – and we think you are since you’re reading this – you’ve been asking yourself: Are there any common medications in my house that will make good radiation sensors?
Not that anyone needs to worry about excess radiation or anything. Far from it. We were just wondering.
It just so happens that Anna Mrozik and Paweł Bilski, both of the Institute of Nuclear Physics Polish Academy of Sciences (IFJ PAN) in Kraków, Poland, were wondering the same thing: “During an uncontrolled release of radiation, it is highly unlikely that members of the public will be equipped with personal radiation dose monitors.”
People would need to use something they had lying around the house. A smartphone would work, the investigators explained in a statement from the IFJ PAN, but the process of converting one to radiation-sensor duty, which involves dismantling it and breaking the display glass, “is laborious and time-consuming [and] the destruction of a valuable and useful device does not seem to be the optimal solution.”
Naturally, they turned to drugs. The key, in this case, is optically stimulated luminescence. They needed to find materials that would glow with greater intensity as the radiation dose increased. Turns out that ibuprofen- and paracetamol-based painkillers fit the bill quite nicely, although aspirin also works.
It’s not known exactly which substance is causing the luminescence, but rest assured, the “physicists from the IFJ PAN intend to identify it.”
This is why you don’t interrupt someone using headphones
There’s nothing like taking a nice relaxing walk with your headphones. Whether you’re listening to a podcast or a song or talking on the phone, it’s an escape from reality that makes you feel like you’re completely in tune with what you’re listening to.
According to a new study, headphones, as opposed to speakers, make people feel more connected to what they are listening to. Data collected from more than 4,000 people showed that listening with headphones makes more of an impact than listening to speakers.
“Headphones produce a phenomenon called in-head localization, which makes the speaker sound as if they’re inside your head,” study coauthor On Amir of the University of California, San Diego, said in a statement. Because of this, people feel like the speakers are close to them and there’s more of a sense of empathy for the speakers and the listener is more likely to be swayed toward the ideas of the speaker.
These findings could lead to more efficient training programs, online work, and advertising, the investigators suggested.
We now finally understand why people get so mad when they have to take out their headphones to answer or talk to us. We ruined a satisfying moment going on in their brains.
‘Striking’ differences in BP when wrong cuff size is used
Strong new evidence on the need to use an appropriately sized cuff in blood pressure measurement has come from the cross-sectional randomized trial Cuff(SZ).
The study found that in people in whom a small adult cuff was appropriate, systolic BP readings were on average 3.6 mm Hg lower when a regular adult size cuff was used.
However, systolic readings were on average 4.8 mm Hg higher when a regular cuff was used in people who required a large adult cuff and 19.5 mm Hg higher in those needing an extra-large cuff based on their mid-arm circumference.
The diastolic readings followed a similar pattern (-1.3 mm Hg, 1.8 mm Hg, and 7.4 mm Hg, respectively).
“We found that using the regular adult cuff in all individuals had striking differences in blood pressure,” lead author Tammy M. Brady, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization. “And that has a lot of clinical implications.”
She noted, for example, that people who required an extra-large cuff and were measured with a regular cuff had an average BP of 144/86.7 mm Hg, which is in the stage 2 hypertension range. But when the correct size cuff was used, the average BP was 124.5/79.3 mm Hg, or in the prehypertensive range.
Overall, the overestimation of BP due to using too small a cuff misclassified 39% of people as being hypertensive, while the underestimation of BP due to using a cuff that was too large missed 22% of people with hypertension.
“So, I think clinicians really need to have a renewed emphasis on cuff size, especially in populations where obesity is highly prevalent and many of their patients require extra-large cuffs, because those are the populations that are most impacted by mis-cuffing,” Dr. Brady said.
The findings were presented in an E-poster at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health (EPI/Lifestyle) 2022 conference sponsored by the American Heart Association.
Willie Lawrence, MD, chair of the AHA’s National Hypertension Control Initiative Advisory Committee, said in an interview that the magnitude of inaccuracy observed by the researchers “makes this a very, very important study.”
“Is it the first of its kind, no, but it’s incredibly important because it was so well done, and it comes at a time when people are once again dealing with issues around equity, and this study can have a significant impact on the state of hypertension in diverse communities,” said Dr. Lawrence, a cardiologist with Spectrum Health Lakeland, Benton Harbor, Michigan.
Previous studies examining the issue were older, had few participants, and used mercury sphygmomanometers instead of automated devices, which are typically recommended by professional societies for screening hypertension in adults, Dr. Brady explained.
For the Cuff Size Blood Pressure Measurement trial, 195 adults recruited from the community underwent 2 to 3 sets of 3 BP readings, 30 seconds apart, with an automated and validated device (Welch Allyn ProB 2000) using a BP cuff that was appropriated sized, one size lower, and one size higher. The order of cuff sizes was randomized. Before each set, patients walked for 2 minutes, followed by 5 minutes of rest to eliminate the potential effect of longer resting periods between tests on the results. The room was also kept quiet and participants were asked not to speak or use a smart phone.
Participants had a mean age of 54 years, 34% were male, 68% were Black, and 36% had a body mass index of at least 30 kg/m2, meeting the criteria for obesity.
Roughly one-half had a self-reported hypertension diagnosis, 31% had a systolic BP of 130 mm Hg or greater, and 26% had a diastolic BP of 80 mm Hg or greater.
Based on arm circumference (mean, 34 cm), the appropriate adult cuff size was small (20-25 cm) in 18%, regular (25.1-32 cm) in 28%, large (32.1-40 cm) in 34%, and extra-large (40.1-55 cm) in 21%.
Dr. Brady pointed out that the most recent hypertension guidelines detail sources of inaccuracy in BP measurement and say that if too small a cuff size is used, the blood pressure could be different by 2 to 11 mm Hg. “And what we show, is it can be anywhere from 5 to 20 mm Hg. So, I think that’s a significant difference from what studies have shown so far and is going to be very surprising to clinicians.”
A 2019 AHA scientific statement on the measurement of blood pressure stresses the importance of cuff size, and last year, the American Medical Association launched a new initiative to standardize training in BP measurement for future physicians and health care professionals.
Previous work also showed that children as young as 3 to 5 years of age often require an adult cuff size, and those in the 12- to 15-year age group may need an extra-large cuff, or what is often referred to as a thigh cuff, said Dr. Brady, who is also the medical director of the pediatric hypertension program at Johns Hopkins Children’s Center.
“Part of the problem is that many physicians aren’t often the one doing the measurement and that others may not be as in tune with some of these data and initiatives,” she said.
Other barriers are cost and availability. Offices and clinics don’t routinely stock multiple cuff sizes in exam rooms, and devices sold over the counter typically come with a regular adult cuff, Dr. Brady said. An extra cuff could add $25 to $50 on top of the $25 to $50 for the device for the growing number of patients measuring BP remotely.
“During the pandemic, I was trying to do telemedicine with my hypertensive patients, but the children who had significant obesity couldn’t afford or find blood pressure devices that had a cuff that was big enough for them,” she said. “It just wasn’t something that they could get. So I think people just don’t recognize how important this is.”
A version of this article first appeared on Medscape.com.
Strong new evidence on the need to use an appropriately sized cuff in blood pressure measurement has come from the cross-sectional randomized trial Cuff(SZ).
The study found that in people in whom a small adult cuff was appropriate, systolic BP readings were on average 3.6 mm Hg lower when a regular adult size cuff was used.
However, systolic readings were on average 4.8 mm Hg higher when a regular cuff was used in people who required a large adult cuff and 19.5 mm Hg higher in those needing an extra-large cuff based on their mid-arm circumference.
The diastolic readings followed a similar pattern (-1.3 mm Hg, 1.8 mm Hg, and 7.4 mm Hg, respectively).
“We found that using the regular adult cuff in all individuals had striking differences in blood pressure,” lead author Tammy M. Brady, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization. “And that has a lot of clinical implications.”
She noted, for example, that people who required an extra-large cuff and were measured with a regular cuff had an average BP of 144/86.7 mm Hg, which is in the stage 2 hypertension range. But when the correct size cuff was used, the average BP was 124.5/79.3 mm Hg, or in the prehypertensive range.
Overall, the overestimation of BP due to using too small a cuff misclassified 39% of people as being hypertensive, while the underestimation of BP due to using a cuff that was too large missed 22% of people with hypertension.
“So, I think clinicians really need to have a renewed emphasis on cuff size, especially in populations where obesity is highly prevalent and many of their patients require extra-large cuffs, because those are the populations that are most impacted by mis-cuffing,” Dr. Brady said.
The findings were presented in an E-poster at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health (EPI/Lifestyle) 2022 conference sponsored by the American Heart Association.
Willie Lawrence, MD, chair of the AHA’s National Hypertension Control Initiative Advisory Committee, said in an interview that the magnitude of inaccuracy observed by the researchers “makes this a very, very important study.”
“Is it the first of its kind, no, but it’s incredibly important because it was so well done, and it comes at a time when people are once again dealing with issues around equity, and this study can have a significant impact on the state of hypertension in diverse communities,” said Dr. Lawrence, a cardiologist with Spectrum Health Lakeland, Benton Harbor, Michigan.
Previous studies examining the issue were older, had few participants, and used mercury sphygmomanometers instead of automated devices, which are typically recommended by professional societies for screening hypertension in adults, Dr. Brady explained.
For the Cuff Size Blood Pressure Measurement trial, 195 adults recruited from the community underwent 2 to 3 sets of 3 BP readings, 30 seconds apart, with an automated and validated device (Welch Allyn ProB 2000) using a BP cuff that was appropriated sized, one size lower, and one size higher. The order of cuff sizes was randomized. Before each set, patients walked for 2 minutes, followed by 5 minutes of rest to eliminate the potential effect of longer resting periods between tests on the results. The room was also kept quiet and participants were asked not to speak or use a smart phone.
Participants had a mean age of 54 years, 34% were male, 68% were Black, and 36% had a body mass index of at least 30 kg/m2, meeting the criteria for obesity.
Roughly one-half had a self-reported hypertension diagnosis, 31% had a systolic BP of 130 mm Hg or greater, and 26% had a diastolic BP of 80 mm Hg or greater.
Based on arm circumference (mean, 34 cm), the appropriate adult cuff size was small (20-25 cm) in 18%, regular (25.1-32 cm) in 28%, large (32.1-40 cm) in 34%, and extra-large (40.1-55 cm) in 21%.
Dr. Brady pointed out that the most recent hypertension guidelines detail sources of inaccuracy in BP measurement and say that if too small a cuff size is used, the blood pressure could be different by 2 to 11 mm Hg. “And what we show, is it can be anywhere from 5 to 20 mm Hg. So, I think that’s a significant difference from what studies have shown so far and is going to be very surprising to clinicians.”
A 2019 AHA scientific statement on the measurement of blood pressure stresses the importance of cuff size, and last year, the American Medical Association launched a new initiative to standardize training in BP measurement for future physicians and health care professionals.
Previous work also showed that children as young as 3 to 5 years of age often require an adult cuff size, and those in the 12- to 15-year age group may need an extra-large cuff, or what is often referred to as a thigh cuff, said Dr. Brady, who is also the medical director of the pediatric hypertension program at Johns Hopkins Children’s Center.
“Part of the problem is that many physicians aren’t often the one doing the measurement and that others may not be as in tune with some of these data and initiatives,” she said.
Other barriers are cost and availability. Offices and clinics don’t routinely stock multiple cuff sizes in exam rooms, and devices sold over the counter typically come with a regular adult cuff, Dr. Brady said. An extra cuff could add $25 to $50 on top of the $25 to $50 for the device for the growing number of patients measuring BP remotely.
“During the pandemic, I was trying to do telemedicine with my hypertensive patients, but the children who had significant obesity couldn’t afford or find blood pressure devices that had a cuff that was big enough for them,” she said. “It just wasn’t something that they could get. So I think people just don’t recognize how important this is.”
A version of this article first appeared on Medscape.com.
Strong new evidence on the need to use an appropriately sized cuff in blood pressure measurement has come from the cross-sectional randomized trial Cuff(SZ).
The study found that in people in whom a small adult cuff was appropriate, systolic BP readings were on average 3.6 mm Hg lower when a regular adult size cuff was used.
However, systolic readings were on average 4.8 mm Hg higher when a regular cuff was used in people who required a large adult cuff and 19.5 mm Hg higher in those needing an extra-large cuff based on their mid-arm circumference.
The diastolic readings followed a similar pattern (-1.3 mm Hg, 1.8 mm Hg, and 7.4 mm Hg, respectively).
“We found that using the regular adult cuff in all individuals had striking differences in blood pressure,” lead author Tammy M. Brady, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, told this news organization. “And that has a lot of clinical implications.”
She noted, for example, that people who required an extra-large cuff and were measured with a regular cuff had an average BP of 144/86.7 mm Hg, which is in the stage 2 hypertension range. But when the correct size cuff was used, the average BP was 124.5/79.3 mm Hg, or in the prehypertensive range.
Overall, the overestimation of BP due to using too small a cuff misclassified 39% of people as being hypertensive, while the underestimation of BP due to using a cuff that was too large missed 22% of people with hypertension.
“So, I think clinicians really need to have a renewed emphasis on cuff size, especially in populations where obesity is highly prevalent and many of their patients require extra-large cuffs, because those are the populations that are most impacted by mis-cuffing,” Dr. Brady said.
The findings were presented in an E-poster at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health (EPI/Lifestyle) 2022 conference sponsored by the American Heart Association.
Willie Lawrence, MD, chair of the AHA’s National Hypertension Control Initiative Advisory Committee, said in an interview that the magnitude of inaccuracy observed by the researchers “makes this a very, very important study.”
“Is it the first of its kind, no, but it’s incredibly important because it was so well done, and it comes at a time when people are once again dealing with issues around equity, and this study can have a significant impact on the state of hypertension in diverse communities,” said Dr. Lawrence, a cardiologist with Spectrum Health Lakeland, Benton Harbor, Michigan.
Previous studies examining the issue were older, had few participants, and used mercury sphygmomanometers instead of automated devices, which are typically recommended by professional societies for screening hypertension in adults, Dr. Brady explained.
For the Cuff Size Blood Pressure Measurement trial, 195 adults recruited from the community underwent 2 to 3 sets of 3 BP readings, 30 seconds apart, with an automated and validated device (Welch Allyn ProB 2000) using a BP cuff that was appropriated sized, one size lower, and one size higher. The order of cuff sizes was randomized. Before each set, patients walked for 2 minutes, followed by 5 minutes of rest to eliminate the potential effect of longer resting periods between tests on the results. The room was also kept quiet and participants were asked not to speak or use a smart phone.
Participants had a mean age of 54 years, 34% were male, 68% were Black, and 36% had a body mass index of at least 30 kg/m2, meeting the criteria for obesity.
Roughly one-half had a self-reported hypertension diagnosis, 31% had a systolic BP of 130 mm Hg or greater, and 26% had a diastolic BP of 80 mm Hg or greater.
Based on arm circumference (mean, 34 cm), the appropriate adult cuff size was small (20-25 cm) in 18%, regular (25.1-32 cm) in 28%, large (32.1-40 cm) in 34%, and extra-large (40.1-55 cm) in 21%.
Dr. Brady pointed out that the most recent hypertension guidelines detail sources of inaccuracy in BP measurement and say that if too small a cuff size is used, the blood pressure could be different by 2 to 11 mm Hg. “And what we show, is it can be anywhere from 5 to 20 mm Hg. So, I think that’s a significant difference from what studies have shown so far and is going to be very surprising to clinicians.”
A 2019 AHA scientific statement on the measurement of blood pressure stresses the importance of cuff size, and last year, the American Medical Association launched a new initiative to standardize training in BP measurement for future physicians and health care professionals.
Previous work also showed that children as young as 3 to 5 years of age often require an adult cuff size, and those in the 12- to 15-year age group may need an extra-large cuff, or what is often referred to as a thigh cuff, said Dr. Brady, who is also the medical director of the pediatric hypertension program at Johns Hopkins Children’s Center.
“Part of the problem is that many physicians aren’t often the one doing the measurement and that others may not be as in tune with some of these data and initiatives,” she said.
Other barriers are cost and availability. Offices and clinics don’t routinely stock multiple cuff sizes in exam rooms, and devices sold over the counter typically come with a regular adult cuff, Dr. Brady said. An extra cuff could add $25 to $50 on top of the $25 to $50 for the device for the growing number of patients measuring BP remotely.
“During the pandemic, I was trying to do telemedicine with my hypertensive patients, but the children who had significant obesity couldn’t afford or find blood pressure devices that had a cuff that was big enough for them,” she said. “It just wasn’t something that they could get. So I think people just don’t recognize how important this is.”
A version of this article first appeared on Medscape.com.
Elective surgery should be delayed 7 weeks after COVID-19 infection for unvaccinated patients, statement recommends
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
.
For patients fully vaccinated against COVID-19 with breakthrough infections, there is no consensus on how vaccination affects the time between COVID-19 infection and elective surgery. Clinicians should use their clinical judgment to schedule procedures, said Randall M. Clark, MD, president of the American Society of Anesthesiologists (ASA). “We need all physicians, anesthesiologists, surgeons, and others to base their decision to go ahead with elective surgery on the patient’s symptoms, their need for the procedure, and whether delays could cause other problems with their health,” he said in an interview.
Prior to these updated recommendations, which were published Feb. 22, the ASA and the APSF recommended a 4-week gap between COVID-19 diagnosis and elective surgery for asymptomatic or mild cases, regardless of a patient’s vaccination status.
Extending the wait time from 4 to 7 weeks was based on a multination study conducted in October 2020 following more than 140,000 surgical patients. Patients with previous COVID-19 infection had an increased risk for complications and death in elective surgery for up to 6 weeks following their diagnosis, compared with patients without COVID-19. Additional research in the United States found that patients with a preoperative COVID diagnosis were at higher risk for postoperative complications of respiratory failure for up to 4 weeks after diagnosis and postoperative pneumonia complications for up to 8 weeks after diagnosis.
Because these studies were conducted in unvaccinated populations or those with low vaccination rates, and preliminary data suggest vaccinated patients with breakthrough infections may have a lower risk for complications and death postinfection, “we felt that it was prudent to just make recommendations specific to unvaccinated patients,” Dr. Clark added.
Although this guidance is “very helpful” in that it summarizes the currently available research to give evidence-based recommendations, the 7-week wait time is a “very conservative estimate,” Brent Matthews, MD, surgeon-in-chief of the surgery care division of Atrium Health, Charlotte, N.C., told this news organization. At Atrium Health, surgery is scheduled at least 21 days after a patient’s COVID-19 diagnosis, regardless of their vaccination status, Dr. Matthews said.
The studies currently available were conducted earlier in the pandemic, when a different variant was prevalent, Dr. Matthews explained. The Omicron variant is currently the most prevalent COVID-19 variant and is less virulent than earlier strains of the virus. The joint statement does note that there is currently “no robust data” on patients infected with the Delta or Omicron variants of COVID-19, and that “the Omicron variant causes less severe disease and is more likely to reside in the oro- and nasopharynx without infiltration and damage to the lungs.”
Still, the new recommendations are a reminder to re-evaluate the potential complications from surgery for previously infected patients and to consider what comorbidities might make them more vulnerable, Dr. Matthews said. “The real power of the joint statement is to get people to ensure that they make an assessment of every patient that comes in front of them who has had a recent positive COVID test.”
A version of this article first appeared on Medscape.com.
How Lp(a) can help improve ASCVD risk assessment
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).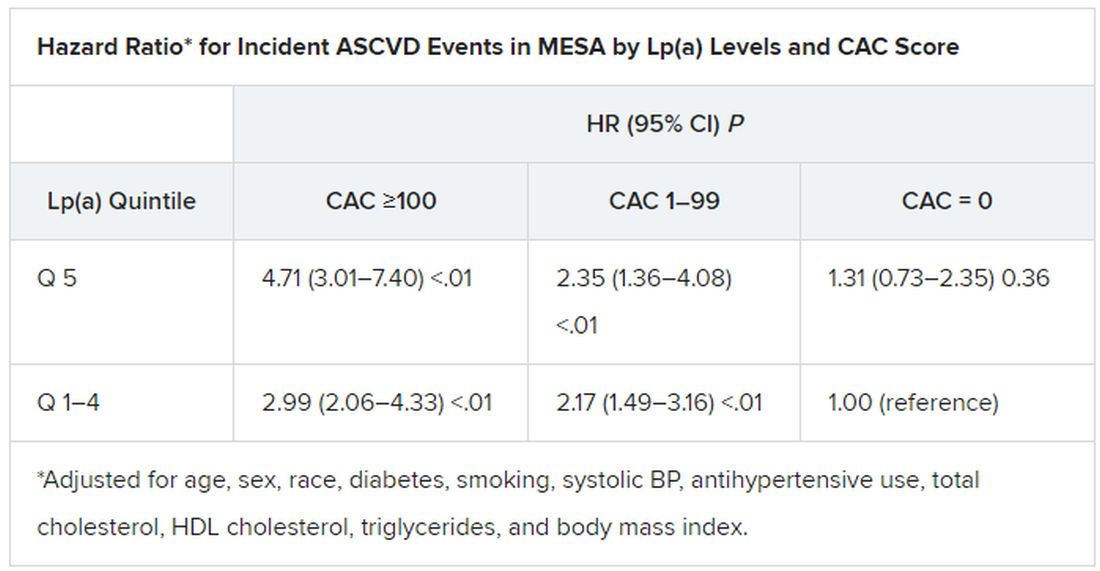
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).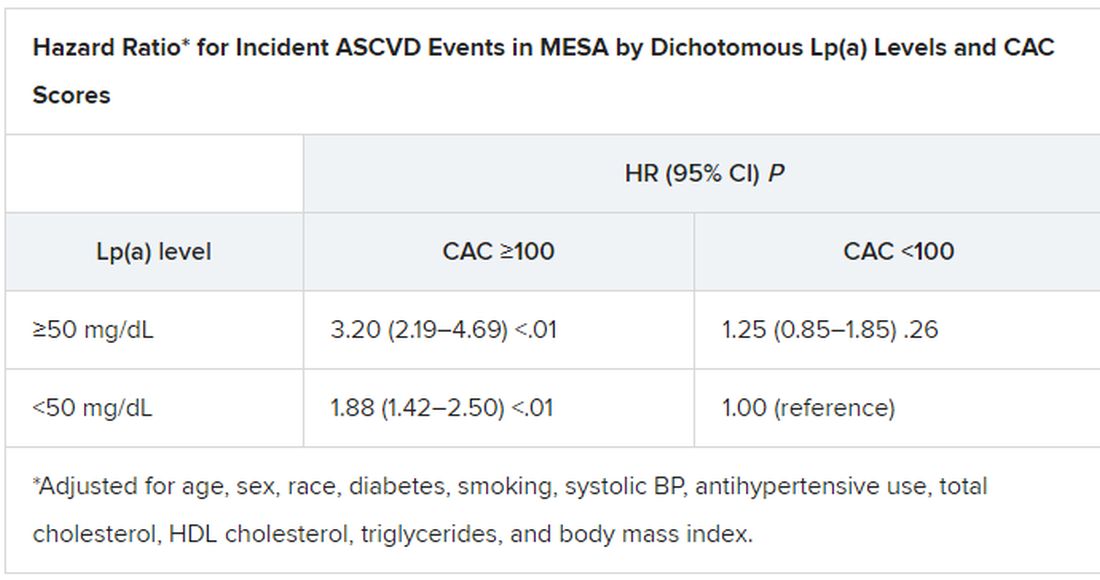
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
A look back at a pair of large cohort studies suggests a telling relation between two distinct predictors of atherosclerotic cardiovascular disease (ASCVD) risk and may offer guidance on how to interpret them together.
Elevated levels of lipoprotein(a), or Lp(a), and high coronary artery calcium (CAC) scores were both predictive of ASCVD risk over 10 years, but independent of each other and a host of more traditional cardiovascular risk factors, for example, in the analysis of data from the MESA (Multi-Ethnic Study of Atherosclerosis) and DHS (Dallas Heart Study) longitudinal cohorts.
Notably, the risk when both Lp(a) and CAC scores were high far exceeded that associated with either marker alone. But when CAC scores were less than 100 Agatston units, predicted ASCVD risk wasn’t influenced by levels of Lp(a). Indeed, a CAC score of 0 predicted the lowest levels of ASCVD risk, even with elevated Lp(a).
That is, the findings suggest, the addition of Lp(a) makes a difference to the risk assessment only when CAC scores are high, at least 100 units, and elevated Lp(a) doesn’t mean increased ASCVD risk in the absence of coronary calcium.
“Our novel findings indicate that elevated Lp(a) drives ASCVD risk independent of the subclinical coronary atherosclerosis burden captured by CAC score,” concluded a report on the analysis, published in the Journal of the American College of Cardiology, with lead author Anurag Mehta, MD, Emory University, Atlanta.
There are no formal recommendations on how to interpret Lp(a) and CAC scores together, but the current findings “provide impetus for measuring Lp(a) in more individuals as part of the shared decision-making process,” the authors contended.
“Really, the calcium score carries the majority of the information in terms of risk, except in the highest CAC score group. That is, if you have a high Lp(a) and a high burden of calcium, your risk is significantly higher than if you just have the high calcium score and the normal Lp(a),” senior author Parag H. Joshi, MD, MHS, said in an interview.
“We thought we would see that the group with higher Lp(a) would have more events over 10 years, even among those who didn’t have coronary calcium,” said Dr. Joshi, of the University of Texas Southwestern Medical Center, Dallas. “But we really don’t see that, at least in a statistically significant way.”
A CAC score of 0 would at least support a more conservative approach in a patient with elevated Lp(a) “who is hesitant to be on a statin or to be more aggressive managing their risk,” Dr. Joshi said.
“This study should be very reassuring for a patient like that,” Ron Blankstein, MD, director of cardiac computed tomography at Brigham and Women’s Hospital, Boston, said in an interview.
“If you have a high Lp(a) and you’re concerned, I think this study really supports the role of calcium scoring for further risk assessment,” said Dr. Blankstein, who is not associated with the new report. “We often check Lp(a) in individuals who perhaps have a family history or who come to see us in a preventive cardiology clinic. If it is high and there is concern, a calcium score can be very helpful. If it’s zero, that really means a very low risk of events. And if it’s elevated, I think we’re going to be more concerned about that patient.”
The current analysis suggests “that, when a patient without clinical cardiovascular disease is identified with either CAC ≥100 or Lp(a) >50 mg/dL, the next step in the risk evaluation should be to measure either Lp(a) or CAC, respectively – if not already performed – to identify the patients at highest risk,” Sotirios Tsimikas, MD, director of vascular medicine at University of California, San Diego, wrote in an accompanying editorial.
“Both Lp(a) and CAC should be more broadly applied in clinical care settings in patients without prior ASCVD to identify those that most likely will benefit from more aggressive therapy and, in the future, from Lp(a)-lowering therapies,” he wrote.
The analyses were conducted separately on data from 4,512 initially asymptomatic patients in MESA and 2,078 from the DHS cohort, who were followed for ASCVD events an average of 13 years and 11 years, respectively. Such events included coronary heart disease–related death, nonfatal MI, and fatal or nonfatal stroke.
In the MESA cohort – 52% women, 36.8% White, 29.3% Black, 22.2% Hispanic, and 11.7% Chinese – elevated Lp(a) (quintile 5 vs. quintiles 1-4) and CAC scores of 1-99 and above 100 (both compared with 0) were each independently associated with increased risk for ASCVD events. The hazard ratio was 1.29 (P = .02) for elevated Lp(a), 1.68 (P < .01) for a CAC score of 1-99, and 2.66 (P < .01) for a CAC score of at least 100.
The corresponding HRs in the DHS cohort were 1.54 (P = .07) for Lp(a), 3.32 (P < .01) for a CAC score of 1-99, and 5.21 (P < .01) for a CAC score of at least 100.
Of note, the authors wrote, ASCVD risk among MESA participants with a CAC score of 0 was not significantly different in those with normal and elevated Lp(a).
The findings were similar in the corresponding DHS analysis, the authors noted.
When both Lp(a) and CAC scores are considered as dichotomous variables, the highest 10-year ASCVD incidence in MESA was in participants with both elevated Lp(a) (≥50 mg/dL) and a high CAC score (≥100). The lowest risk was seen when Lp(a) was normal (<50 mg/dL) and the CAC score was no more than moderately high (<100).
The results in the corresponding DHS analysis, according to the report, again mirrored those from MESA.
“This study has important implications for our patients and also potentially for future clinical trial design,” Dr. Blankstein noted. “A big part of developing a trial in this space is identifying the patients who are at higher risk,” and the current analysis supports CAC scores for identifying the highest-risk patient among those with elevated Lp(a).
Current wisdom is that, for the most part, Lp(a) levels are genetically mediated and are mostly unaffected by interventions such as diet management or exercise. It’s unknown whether reducing elevated Lp(a) levels pharmacologically will cut ASCVD risk, but there are a number of clinical trial programs currently aimed at learning just that. They include the Novartis-sponsored phase 3 HORIZON trial of the antisense agent pelacarsen (TQJ230), with an estimated enrollment of almost 7,700; a randomized, controlled dose-finding study of the small interfering RNA agent olpasiran (AMG890), with 290 patients and funded by Amgen; and an 88-patient phase 1 study of another siRNA agent, SLN360, supported by Silence Therapeutics.
Dr. Mehta reported no relevant relationships. Dr. Joshi has received grant support from Novo Nordisk and consulting income from Bayer and Regeneron; holds equity in G3 Therapeutics; and has served as site investigator for GlaxoSmithKline, Sanofi, AstraZeneca, and Novartis. Dr. Blankstein reported serving as a consultant to Amgen, Novartis, and Silence Therapeutics.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
What is the healthiest salt for you?
When we refer to “regular table salt,” it is most commonly in the form of sodium chloride, which is also a major constituent of packaged and ultraprocessed foods.
The best approach to finding the “healthiest salt” – which really means the lowest in sodium – is to look for the amount on the label. “Sodium-free” usually indicates less than 5 mg of sodium per serving, and “low-sodium” usually means 140 mg or less per serving. In contrast, regular table salt can contain as much as 560 mg of sodium in one serving.
Other en vogue salts, such as pink Himalayan salt, sea salt, and kosher salt, are high in sodium content – like regular table salt – but because of their larger crystal size, less sodium is delivered per serving.
Most salt substitutes are reduced in sodium, with the addition of potassium chloride instead.
FDA issues guidance on reducing salt
Currently, the U.S. sodium dietary guidelines for persons older than 14 stipulate 2,300 mg/d, which is equivalent to 1 teaspoon a day. However it is estimated that the average person in the United States consumes more than this – around 3,400 mg of sodium daily.
In October 2021, the U.S. Food and Drug Administration published guidance on voluntary sodium limitations in commercially processed, packaged, and prepared food. The FDA’s short-term approach is to slowly reduce exposure to sodium in processed and restaurant food by 2025, on the basis that people will eventually get used to less salt, as has happened in the United Kingdom and other countries.
Such strategies to reduce salt intake are now being used in national programs in several countries. Many of these successful initiatives include active engagement with the food industry to reduce the amount of sodium added to processed food, as well as public awareness campaigns to alert consumers to the dangers of eating too much salt. This includes increasing potassium in manufactured foods, primarily to target hypertension and heart disease, as described by Clare Farrand, MSc, BSc, and colleagues, in the Journal of Clinical Hypertension. The authors also make several recommendations regarding salt reduction policies:
- Food manufacturers should gradually reduce sodium in food to the lowest possible levels and explore the use of potassium-based sodium replacers to reduce sodium levels even further.
- Governments should continue to monitor sodium and potassium levels in processed foods.
- Further consideration may need to be given to how best to label salt substitutes (namely potassium) in processed foods to ensure that people who may be adversely affected are aware.
- Governments should systematically monitor potassium intake at the population level, including for specific susceptible groups.
- Governments should continue to systematically monitor sodium (salt) intake and iodine intake at the population level to adjust salt iodization over time as necessary, depending on observed salt intake in specific targeted groups, to ensure that they have sufficient but not excessive iodine intakes as salt intakes are reduced.
- Governments should consider opportunities for promoting and subsidizing salt substitutes, particularly in countries where salt added during cooking or at the table is the major source of salt in the diet.
The new FDA document includes 163 subcategories of foods in its voluntary salt reduction strategy.
Salt substitutes, high blood pressure, and mortality
Lowering sodium intake is almost certainly beneficial for persons with high blood pressure. In 2020, a review in Hypertension highlighted the benefit of salt substitutes in reducing hypertension, reporting that they lower systolic blood pressure by 5.58 mm Hg and diastolic blood pressure by 2.88 mm Hg.
And changes to dietary sodium intake can potentially reduce or obviate the need for medications for essential hypertension in some individuals. Although there are only a few studies on this topic, a study by Bruce Neal, MB, ChB, PhD, and colleagues, revealed a reduction in stroke, cardiovascular events, and deaths with the use of potassium-based salt substitutes.
Salt substitutes and sodium and potassium handling in the kidneys
Many studies have shown that potassium-rich salt substitutes are safe in individuals with normal kidney function, but are they safe and beneficial for people with chronic kidney disease (CKD)?
For anyone who is on a renal diet, potassium and sodium intake goals are limited according to their absolute level of kidney function.
There have been case reports of life-threatening blood potassium levels (hyperkalemia) due to potassium-rich salt substitutes in people with CKD, but no larger published studies on this topic can be found.
A diet modeling study by Rebecca Morrison and colleagues evaluated varying degrees of potassium-enriched salt substituted bread products and their impact on dietary intake in persons with CKD. They used dietary data from the National Nutrition and Physical Activity Survey 2011-2012 in Australia for 12,152 participants, 154 of whom had CKD. Replacing the sodium in bread with varying amounts of potassium chloride (20%, 30%, and 40%) would result in one-third of people with CKD exceeding the safe limits for dietary potassium consumption (31.8%, 32.6%, and 33%, respectively), they found.
“Potassium chloride substitution in staple foods such as bread and bread products have serious and potentially fatal consequences for people who need to restrict dietary potassium. Improved food labelling is required for consumers to avoid excessive consumption,” Ms. Morrison and colleagues concluded. They added that more studies are needed to further understand the risks of potassium dietary intake and hyperkalemia in CKD from potassium-based salt substitutes.
The American Heart Association recommends no more than 1,500 mg of sodium intake daily for persons with CKD, diabetes, or high blood pressure; those older than 51; and African American persons of any age.
The recommended daily intake of potassium in persons with CKD can range from 2,000 mg to 4,000 mg, depending on the individual and their degree of CKD. The potassium content in some salt substitutes varies from 440 mg to 2,800 mg per teaspoon.
The best recommendation for individuals with CKD and a goal to reduce their sodium intake is to use herbs and lower-sodium seasonings as a substitute, but these should always be reviewed with their physician and renal nutritionist.
Dr. Brookins is a board-certified nephrologist and internist practicing in Georgia. She is the founder and owner of Remote Renal Care, a telehealth kidney practice. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When we refer to “regular table salt,” it is most commonly in the form of sodium chloride, which is also a major constituent of packaged and ultraprocessed foods.
The best approach to finding the “healthiest salt” – which really means the lowest in sodium – is to look for the amount on the label. “Sodium-free” usually indicates less than 5 mg of sodium per serving, and “low-sodium” usually means 140 mg or less per serving. In contrast, regular table salt can contain as much as 560 mg of sodium in one serving.
Other en vogue salts, such as pink Himalayan salt, sea salt, and kosher salt, are high in sodium content – like regular table salt – but because of their larger crystal size, less sodium is delivered per serving.
Most salt substitutes are reduced in sodium, with the addition of potassium chloride instead.
FDA issues guidance on reducing salt
Currently, the U.S. sodium dietary guidelines for persons older than 14 stipulate 2,300 mg/d, which is equivalent to 1 teaspoon a day. However it is estimated that the average person in the United States consumes more than this – around 3,400 mg of sodium daily.
In October 2021, the U.S. Food and Drug Administration published guidance on voluntary sodium limitations in commercially processed, packaged, and prepared food. The FDA’s short-term approach is to slowly reduce exposure to sodium in processed and restaurant food by 2025, on the basis that people will eventually get used to less salt, as has happened in the United Kingdom and other countries.
Such strategies to reduce salt intake are now being used in national programs in several countries. Many of these successful initiatives include active engagement with the food industry to reduce the amount of sodium added to processed food, as well as public awareness campaigns to alert consumers to the dangers of eating too much salt. This includes increasing potassium in manufactured foods, primarily to target hypertension and heart disease, as described by Clare Farrand, MSc, BSc, and colleagues, in the Journal of Clinical Hypertension. The authors also make several recommendations regarding salt reduction policies:
- Food manufacturers should gradually reduce sodium in food to the lowest possible levels and explore the use of potassium-based sodium replacers to reduce sodium levels even further.
- Governments should continue to monitor sodium and potassium levels in processed foods.
- Further consideration may need to be given to how best to label salt substitutes (namely potassium) in processed foods to ensure that people who may be adversely affected are aware.
- Governments should systematically monitor potassium intake at the population level, including for specific susceptible groups.
- Governments should continue to systematically monitor sodium (salt) intake and iodine intake at the population level to adjust salt iodization over time as necessary, depending on observed salt intake in specific targeted groups, to ensure that they have sufficient but not excessive iodine intakes as salt intakes are reduced.
- Governments should consider opportunities for promoting and subsidizing salt substitutes, particularly in countries where salt added during cooking or at the table is the major source of salt in the diet.
The new FDA document includes 163 subcategories of foods in its voluntary salt reduction strategy.
Salt substitutes, high blood pressure, and mortality
Lowering sodium intake is almost certainly beneficial for persons with high blood pressure. In 2020, a review in Hypertension highlighted the benefit of salt substitutes in reducing hypertension, reporting that they lower systolic blood pressure by 5.58 mm Hg and diastolic blood pressure by 2.88 mm Hg.
And changes to dietary sodium intake can potentially reduce or obviate the need for medications for essential hypertension in some individuals. Although there are only a few studies on this topic, a study by Bruce Neal, MB, ChB, PhD, and colleagues, revealed a reduction in stroke, cardiovascular events, and deaths with the use of potassium-based salt substitutes.
Salt substitutes and sodium and potassium handling in the kidneys
Many studies have shown that potassium-rich salt substitutes are safe in individuals with normal kidney function, but are they safe and beneficial for people with chronic kidney disease (CKD)?
For anyone who is on a renal diet, potassium and sodium intake goals are limited according to their absolute level of kidney function.
There have been case reports of life-threatening blood potassium levels (hyperkalemia) due to potassium-rich salt substitutes in people with CKD, but no larger published studies on this topic can be found.
A diet modeling study by Rebecca Morrison and colleagues evaluated varying degrees of potassium-enriched salt substituted bread products and their impact on dietary intake in persons with CKD. They used dietary data from the National Nutrition and Physical Activity Survey 2011-2012 in Australia for 12,152 participants, 154 of whom had CKD. Replacing the sodium in bread with varying amounts of potassium chloride (20%, 30%, and 40%) would result in one-third of people with CKD exceeding the safe limits for dietary potassium consumption (31.8%, 32.6%, and 33%, respectively), they found.
“Potassium chloride substitution in staple foods such as bread and bread products have serious and potentially fatal consequences for people who need to restrict dietary potassium. Improved food labelling is required for consumers to avoid excessive consumption,” Ms. Morrison and colleagues concluded. They added that more studies are needed to further understand the risks of potassium dietary intake and hyperkalemia in CKD from potassium-based salt substitutes.
The American Heart Association recommends no more than 1,500 mg of sodium intake daily for persons with CKD, diabetes, or high blood pressure; those older than 51; and African American persons of any age.
The recommended daily intake of potassium in persons with CKD can range from 2,000 mg to 4,000 mg, depending on the individual and their degree of CKD. The potassium content in some salt substitutes varies from 440 mg to 2,800 mg per teaspoon.
The best recommendation for individuals with CKD and a goal to reduce their sodium intake is to use herbs and lower-sodium seasonings as a substitute, but these should always be reviewed with their physician and renal nutritionist.
Dr. Brookins is a board-certified nephrologist and internist practicing in Georgia. She is the founder and owner of Remote Renal Care, a telehealth kidney practice. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
When we refer to “regular table salt,” it is most commonly in the form of sodium chloride, which is also a major constituent of packaged and ultraprocessed foods.
The best approach to finding the “healthiest salt” – which really means the lowest in sodium – is to look for the amount on the label. “Sodium-free” usually indicates less than 5 mg of sodium per serving, and “low-sodium” usually means 140 mg or less per serving. In contrast, regular table salt can contain as much as 560 mg of sodium in one serving.
Other en vogue salts, such as pink Himalayan salt, sea salt, and kosher salt, are high in sodium content – like regular table salt – but because of their larger crystal size, less sodium is delivered per serving.
Most salt substitutes are reduced in sodium, with the addition of potassium chloride instead.
FDA issues guidance on reducing salt
Currently, the U.S. sodium dietary guidelines for persons older than 14 stipulate 2,300 mg/d, which is equivalent to 1 teaspoon a day. However it is estimated that the average person in the United States consumes more than this – around 3,400 mg of sodium daily.
In October 2021, the U.S. Food and Drug Administration published guidance on voluntary sodium limitations in commercially processed, packaged, and prepared food. The FDA’s short-term approach is to slowly reduce exposure to sodium in processed and restaurant food by 2025, on the basis that people will eventually get used to less salt, as has happened in the United Kingdom and other countries.
Such strategies to reduce salt intake are now being used in national programs in several countries. Many of these successful initiatives include active engagement with the food industry to reduce the amount of sodium added to processed food, as well as public awareness campaigns to alert consumers to the dangers of eating too much salt. This includes increasing potassium in manufactured foods, primarily to target hypertension and heart disease, as described by Clare Farrand, MSc, BSc, and colleagues, in the Journal of Clinical Hypertension. The authors also make several recommendations regarding salt reduction policies:
- Food manufacturers should gradually reduce sodium in food to the lowest possible levels and explore the use of potassium-based sodium replacers to reduce sodium levels even further.
- Governments should continue to monitor sodium and potassium levels in processed foods.
- Further consideration may need to be given to how best to label salt substitutes (namely potassium) in processed foods to ensure that people who may be adversely affected are aware.
- Governments should systematically monitor potassium intake at the population level, including for specific susceptible groups.
- Governments should continue to systematically monitor sodium (salt) intake and iodine intake at the population level to adjust salt iodization over time as necessary, depending on observed salt intake in specific targeted groups, to ensure that they have sufficient but not excessive iodine intakes as salt intakes are reduced.
- Governments should consider opportunities for promoting and subsidizing salt substitutes, particularly in countries where salt added during cooking or at the table is the major source of salt in the diet.
The new FDA document includes 163 subcategories of foods in its voluntary salt reduction strategy.
Salt substitutes, high blood pressure, and mortality
Lowering sodium intake is almost certainly beneficial for persons with high blood pressure. In 2020, a review in Hypertension highlighted the benefit of salt substitutes in reducing hypertension, reporting that they lower systolic blood pressure by 5.58 mm Hg and diastolic blood pressure by 2.88 mm Hg.
And changes to dietary sodium intake can potentially reduce or obviate the need for medications for essential hypertension in some individuals. Although there are only a few studies on this topic, a study by Bruce Neal, MB, ChB, PhD, and colleagues, revealed a reduction in stroke, cardiovascular events, and deaths with the use of potassium-based salt substitutes.
Salt substitutes and sodium and potassium handling in the kidneys
Many studies have shown that potassium-rich salt substitutes are safe in individuals with normal kidney function, but are they safe and beneficial for people with chronic kidney disease (CKD)?
For anyone who is on a renal diet, potassium and sodium intake goals are limited according to their absolute level of kidney function.
There have been case reports of life-threatening blood potassium levels (hyperkalemia) due to potassium-rich salt substitutes in people with CKD, but no larger published studies on this topic can be found.
A diet modeling study by Rebecca Morrison and colleagues evaluated varying degrees of potassium-enriched salt substituted bread products and their impact on dietary intake in persons with CKD. They used dietary data from the National Nutrition and Physical Activity Survey 2011-2012 in Australia for 12,152 participants, 154 of whom had CKD. Replacing the sodium in bread with varying amounts of potassium chloride (20%, 30%, and 40%) would result in one-third of people with CKD exceeding the safe limits for dietary potassium consumption (31.8%, 32.6%, and 33%, respectively), they found.
“Potassium chloride substitution in staple foods such as bread and bread products have serious and potentially fatal consequences for people who need to restrict dietary potassium. Improved food labelling is required for consumers to avoid excessive consumption,” Ms. Morrison and colleagues concluded. They added that more studies are needed to further understand the risks of potassium dietary intake and hyperkalemia in CKD from potassium-based salt substitutes.
The American Heart Association recommends no more than 1,500 mg of sodium intake daily for persons with CKD, diabetes, or high blood pressure; those older than 51; and African American persons of any age.
The recommended daily intake of potassium in persons with CKD can range from 2,000 mg to 4,000 mg, depending on the individual and their degree of CKD. The potassium content in some salt substitutes varies from 440 mg to 2,800 mg per teaspoon.
The best recommendation for individuals with CKD and a goal to reduce their sodium intake is to use herbs and lower-sodium seasonings as a substitute, but these should always be reviewed with their physician and renal nutritionist.
Dr. Brookins is a board-certified nephrologist and internist practicing in Georgia. She is the founder and owner of Remote Renal Care, a telehealth kidney practice. She reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New data explore risk of magnetic interference with implantable devices
Building on several previous reports that the newest models of mobile telephones and other electronics that use magnets pose a threat to the function of defibrillators and other implantable cardiovascular devices, a new study implicates any device that emits a 10-gauss (G) magnetic field more than a couple of inches.
“Beside the devices described in our manuscript, this can be any portable consumer product [with magnets] like electric cigarettes or smart watches,” explained study author Sven Knecht, DSc, a research electrophysiologist associated with the department of cardiology, University Hospital Basel (Switzerland).
In the newly published article, the investigators evaluated earphones, earphone charging cases, and two electronic pens used to draw on electronic tablets. These particular devices are of interest because, like mobile phones, they are of a size and shape to fit in a breast pocket adjacent to where many cardiovascular devices are implanted.
The study joins several previous studies that have shown the same risk, but this study used three-dimensional (3D) mapping of the magnetic field rather than a one-axis sensor, which is a standard adopted by the U.S. Food and Drug Administration, according to the investigators.
3D mapping assessment used
Because of the 3D nature of magnetic fields, 3D mapping serves as a better tool to assess the risk of the magnetic force as the intensity gradient diminishes with distance from the source, the authors contended. The 3D maps used in this study have a resolution to 2 mm.
The ex vivo measurements of the magnetic field, which could be displayed in a configurable 3D volume in relation to the electronic products were performed on five different explanted cardioverter defibrillators from two manufacturers.
In the ex vivo setting, the ability of the earphones, earphone charging cases, and electronic pens to interfere with defibrillator function was compared to that of the Apple iPhone 12 Max, which was the subject of a small in vivo study published in 2021. When the iPhone 12 Max was placed on the skin over a cardiac implantable device in that study, clinically identifiable interference could be detected in all 3 patients evaluated.
Based on previous work, the International Organization for Standardization has established that a minimal field strength of 10 G is needed to interfere with an implantable device, but the actual risk from any specific device is determined by the distance at which this strength of magnetic field is projected.
In the 3D analysis, the 10-G intensity was found to project 20 mm from the surface of the ear phones, ear phone charging case, and one of the electronic pens and to project 29 mm from the other electronic pen. When tested against the five defibrillators, magnetic reversion mode was triggered by the portable electronics at distances ranging from 8 to 18 mm.
In an interview, Dr. Knecht explained that this study adds more devices to the list of those associated with potential for interfering with implantable cardiovascular devices, but added that the more important point is that any device that contains magnets emitting a force of 10 G or greater for more than a few inches can be expected to be associated with clinically meaningful interference. The devices tested in this study were produced by Apple and Microsoft, but a focus on specific devices obscures the main message.
“All portable electronics with an embedded permanent magnet creating a 10-G magnetic field have a theoretical capability of triggering implantable devices,” he said.
For pacemakers, the interference is likely to trigger constant pacing, which would not be expected to pose a significant health threat if detected with a reasonable period, according to Dr. Knecht. Interference is potentially more serious for defibrillators, which might fail during magnetic interference to provide the shock needed to terminate a serious arrhythmia.
The combination of events – interference at the time of an arrhythmia – make this risk “very low,” but Dr. Knecht said it is sufficient to mean that patients receiving an implantable cardiovascular device should be made aware of the risk and the need to avoid placing portable electronic products near the implanted device.
When in vivo evidence of a disturbance with the iPhone 12 was reported in 2021, it amplified existing concern. The American Heart Association maintains a list of electronic products with the potential to interfere with implantable devices on its website. But, again, understanding the potential for risk and the need to keep electronic products with magnets at a safe distance from cardiovascular implantable devices is more important than trying to memorize the ever-growing list of devices with this capability.
“Prudent education of patients receiving an implantable device is important,” said N.A. Mark Estes III, MD, professor of medicine in the division of cardiology at the University of Pittsburgh. However, in an interview, he warned that the growing list of implicated devices makes a complete survey impractical, and, even if achievable, likely to leave patients “feeling overwhelmed.”
In Dr. Estes’s practice, he does provide printed information about the risks of electronics to interfere with implantable devices as well as a list of dos and don’ts. He agreed that the absolute risk of interference from a device causing significant clinical complications is low, but the goal is to “bring it as close to zero as possible.”
“No clinical case of a meaningful interaction of an electronic product and dysfunction of an implantable device has ever been documented,” he said. Given the widespread use of the new generation of cellphones that contain magnets powerful enough to induce dysfunction in an implantable device, “this speaks to the fact that the risk continues to be very low.”
Dr. Knecht and coinvestigators, along with Dr. Estes, reported no potential conflicts of interest.
Building on several previous reports that the newest models of mobile telephones and other electronics that use magnets pose a threat to the function of defibrillators and other implantable cardiovascular devices, a new study implicates any device that emits a 10-gauss (G) magnetic field more than a couple of inches.
“Beside the devices described in our manuscript, this can be any portable consumer product [with magnets] like electric cigarettes or smart watches,” explained study author Sven Knecht, DSc, a research electrophysiologist associated with the department of cardiology, University Hospital Basel (Switzerland).
In the newly published article, the investigators evaluated earphones, earphone charging cases, and two electronic pens used to draw on electronic tablets. These particular devices are of interest because, like mobile phones, they are of a size and shape to fit in a breast pocket adjacent to where many cardiovascular devices are implanted.
The study joins several previous studies that have shown the same risk, but this study used three-dimensional (3D) mapping of the magnetic field rather than a one-axis sensor, which is a standard adopted by the U.S. Food and Drug Administration, according to the investigators.
3D mapping assessment used
Because of the 3D nature of magnetic fields, 3D mapping serves as a better tool to assess the risk of the magnetic force as the intensity gradient diminishes with distance from the source, the authors contended. The 3D maps used in this study have a resolution to 2 mm.
The ex vivo measurements of the magnetic field, which could be displayed in a configurable 3D volume in relation to the electronic products were performed on five different explanted cardioverter defibrillators from two manufacturers.
In the ex vivo setting, the ability of the earphones, earphone charging cases, and electronic pens to interfere with defibrillator function was compared to that of the Apple iPhone 12 Max, which was the subject of a small in vivo study published in 2021. When the iPhone 12 Max was placed on the skin over a cardiac implantable device in that study, clinically identifiable interference could be detected in all 3 patients evaluated.
Based on previous work, the International Organization for Standardization has established that a minimal field strength of 10 G is needed to interfere with an implantable device, but the actual risk from any specific device is determined by the distance at which this strength of magnetic field is projected.
In the 3D analysis, the 10-G intensity was found to project 20 mm from the surface of the ear phones, ear phone charging case, and one of the electronic pens and to project 29 mm from the other electronic pen. When tested against the five defibrillators, magnetic reversion mode was triggered by the portable electronics at distances ranging from 8 to 18 mm.
In an interview, Dr. Knecht explained that this study adds more devices to the list of those associated with potential for interfering with implantable cardiovascular devices, but added that the more important point is that any device that contains magnets emitting a force of 10 G or greater for more than a few inches can be expected to be associated with clinically meaningful interference. The devices tested in this study were produced by Apple and Microsoft, but a focus on specific devices obscures the main message.
“All portable electronics with an embedded permanent magnet creating a 10-G magnetic field have a theoretical capability of triggering implantable devices,” he said.
For pacemakers, the interference is likely to trigger constant pacing, which would not be expected to pose a significant health threat if detected with a reasonable period, according to Dr. Knecht. Interference is potentially more serious for defibrillators, which might fail during magnetic interference to provide the shock needed to terminate a serious arrhythmia.
The combination of events – interference at the time of an arrhythmia – make this risk “very low,” but Dr. Knecht said it is sufficient to mean that patients receiving an implantable cardiovascular device should be made aware of the risk and the need to avoid placing portable electronic products near the implanted device.
When in vivo evidence of a disturbance with the iPhone 12 was reported in 2021, it amplified existing concern. The American Heart Association maintains a list of electronic products with the potential to interfere with implantable devices on its website. But, again, understanding the potential for risk and the need to keep electronic products with magnets at a safe distance from cardiovascular implantable devices is more important than trying to memorize the ever-growing list of devices with this capability.
“Prudent education of patients receiving an implantable device is important,” said N.A. Mark Estes III, MD, professor of medicine in the division of cardiology at the University of Pittsburgh. However, in an interview, he warned that the growing list of implicated devices makes a complete survey impractical, and, even if achievable, likely to leave patients “feeling overwhelmed.”
In Dr. Estes’s practice, he does provide printed information about the risks of electronics to interfere with implantable devices as well as a list of dos and don’ts. He agreed that the absolute risk of interference from a device causing significant clinical complications is low, but the goal is to “bring it as close to zero as possible.”
“No clinical case of a meaningful interaction of an electronic product and dysfunction of an implantable device has ever been documented,” he said. Given the widespread use of the new generation of cellphones that contain magnets powerful enough to induce dysfunction in an implantable device, “this speaks to the fact that the risk continues to be very low.”
Dr. Knecht and coinvestigators, along with Dr. Estes, reported no potential conflicts of interest.
Building on several previous reports that the newest models of mobile telephones and other electronics that use magnets pose a threat to the function of defibrillators and other implantable cardiovascular devices, a new study implicates any device that emits a 10-gauss (G) magnetic field more than a couple of inches.
“Beside the devices described in our manuscript, this can be any portable consumer product [with magnets] like electric cigarettes or smart watches,” explained study author Sven Knecht, DSc, a research electrophysiologist associated with the department of cardiology, University Hospital Basel (Switzerland).
In the newly published article, the investigators evaluated earphones, earphone charging cases, and two electronic pens used to draw on electronic tablets. These particular devices are of interest because, like mobile phones, they are of a size and shape to fit in a breast pocket adjacent to where many cardiovascular devices are implanted.
The study joins several previous studies that have shown the same risk, but this study used three-dimensional (3D) mapping of the magnetic field rather than a one-axis sensor, which is a standard adopted by the U.S. Food and Drug Administration, according to the investigators.
3D mapping assessment used
Because of the 3D nature of magnetic fields, 3D mapping serves as a better tool to assess the risk of the magnetic force as the intensity gradient diminishes with distance from the source, the authors contended. The 3D maps used in this study have a resolution to 2 mm.
The ex vivo measurements of the magnetic field, which could be displayed in a configurable 3D volume in relation to the electronic products were performed on five different explanted cardioverter defibrillators from two manufacturers.
In the ex vivo setting, the ability of the earphones, earphone charging cases, and electronic pens to interfere with defibrillator function was compared to that of the Apple iPhone 12 Max, which was the subject of a small in vivo study published in 2021. When the iPhone 12 Max was placed on the skin over a cardiac implantable device in that study, clinically identifiable interference could be detected in all 3 patients evaluated.
Based on previous work, the International Organization for Standardization has established that a minimal field strength of 10 G is needed to interfere with an implantable device, but the actual risk from any specific device is determined by the distance at which this strength of magnetic field is projected.
In the 3D analysis, the 10-G intensity was found to project 20 mm from the surface of the ear phones, ear phone charging case, and one of the electronic pens and to project 29 mm from the other electronic pen. When tested against the five defibrillators, magnetic reversion mode was triggered by the portable electronics at distances ranging from 8 to 18 mm.
In an interview, Dr. Knecht explained that this study adds more devices to the list of those associated with potential for interfering with implantable cardiovascular devices, but added that the more important point is that any device that contains magnets emitting a force of 10 G or greater for more than a few inches can be expected to be associated with clinically meaningful interference. The devices tested in this study were produced by Apple and Microsoft, but a focus on specific devices obscures the main message.
“All portable electronics with an embedded permanent magnet creating a 10-G magnetic field have a theoretical capability of triggering implantable devices,” he said.
For pacemakers, the interference is likely to trigger constant pacing, which would not be expected to pose a significant health threat if detected with a reasonable period, according to Dr. Knecht. Interference is potentially more serious for defibrillators, which might fail during magnetic interference to provide the shock needed to terminate a serious arrhythmia.
The combination of events – interference at the time of an arrhythmia – make this risk “very low,” but Dr. Knecht said it is sufficient to mean that patients receiving an implantable cardiovascular device should be made aware of the risk and the need to avoid placing portable electronic products near the implanted device.
When in vivo evidence of a disturbance with the iPhone 12 was reported in 2021, it amplified existing concern. The American Heart Association maintains a list of electronic products with the potential to interfere with implantable devices on its website. But, again, understanding the potential for risk and the need to keep electronic products with magnets at a safe distance from cardiovascular implantable devices is more important than trying to memorize the ever-growing list of devices with this capability.
“Prudent education of patients receiving an implantable device is important,” said N.A. Mark Estes III, MD, professor of medicine in the division of cardiology at the University of Pittsburgh. However, in an interview, he warned that the growing list of implicated devices makes a complete survey impractical, and, even if achievable, likely to leave patients “feeling overwhelmed.”
In Dr. Estes’s practice, he does provide printed information about the risks of electronics to interfere with implantable devices as well as a list of dos and don’ts. He agreed that the absolute risk of interference from a device causing significant clinical complications is low, but the goal is to “bring it as close to zero as possible.”
“No clinical case of a meaningful interaction of an electronic product and dysfunction of an implantable device has ever been documented,” he said. Given the widespread use of the new generation of cellphones that contain magnets powerful enough to induce dysfunction in an implantable device, “this speaks to the fact that the risk continues to be very low.”
Dr. Knecht and coinvestigators, along with Dr. Estes, reported no potential conflicts of interest.
FROM CIRCULATION: ARRHYTHMIAS & ELECTROPHYSIOLOGY












