User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Dermatologic Management of Hidradenitis Suppurativa and Impact on Pregnancy and Breastfeeding
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
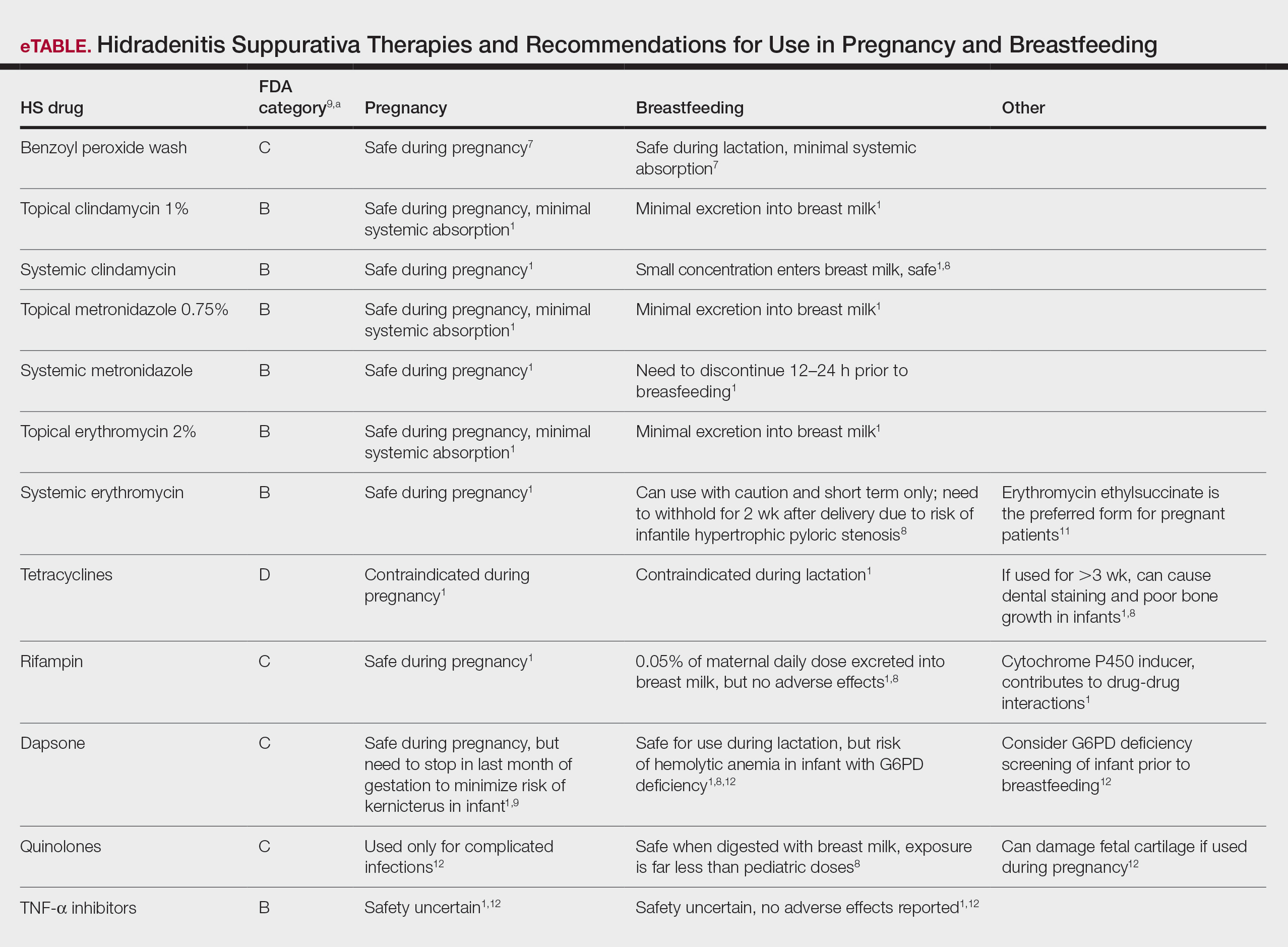
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9
Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5
CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with hyperandrogenism and is caused by occlusion or rupture of follicular units and inflammation of the apocrine glands.1-3 The disease most commonly affects women (female to male ratio of 3:1) of childbearing age.1,2,4,5 Body areas affected include the axillae and groin, and less commonly the perineum; perianal region; and skin folds, such as gluteal, inframammary, and infraumbilical folds.1,2 Symptoms manifest as painful subcutaneous nodules with possible accompanying purulent drainage, sinus tracts, and/or dermal contractures. Although the pathophysiology is unclear, androgens affect the course of HS during pregnancy by stimulating the affected glands and altering cytokines.1,2,6
During pregnancy, maternal immune function switches from cell-mediated T helper cell (TH1) to humoral TH2 cytokine production. The activity of sebaceous and eccrine glands increases while the activity of apocrine glands decreases, thus changing the inflammatory course of HS during pregnancy.3 Approximately 20% of women with HS experience improvement of symptoms during pregnancy, while the remainder either experience no relief or deterioration of symptoms.1 Improvement in symptoms during pregnancy was found to occur more frequently in those who had worsening symptoms during menses owing to the possible hormonal effect estrogen has on inhibiting TH1 and TH17 proinflammatory cytokines, which promotes an immunosuppressive environment.4
Lactation and breastfeeding abilities may be hindered if a woman has HS affecting the apocrine glands of breast tissue and a symptom flare in the postpartum period. If HS causes notable inflammation in the nipple-areolar complex during pregnancy, the patient may experience difficulties with lactation and milk fistula formation, leading to inability to breastfeed.2 Another reason why mothers with HS may not be able to breastfeed is that the medications required to treat the disease are unsafe if passed to the infant via breast milk. In addition, the teratogenic effects of HS medications may necessitate therapy adjustments in pregnancy.1 Here, we provide a brief overview of the medical management considerations of HS in the setting of pregnancy and the impact on breastfeeding.
MEDICAL MANAGEMENT AND DRUG SAFETY
Dermatologists prescribe a myriad of topical and systemic medications to ameliorate symptoms of HS. Therapy regimens often are multimodal and include antibiotics, biologics, and immunosuppressants.1,3
Antibiotics
First-line antibiotics include clindamycin, metronidazole, tetracyclines, erythromycin, rifampin, dapsone, and fluoroquinolones. Topical clindamycin 1%, metronidazole 0.75%, and erythromycin 2% are used for open or active HS lesions and are all safe to use in pregnancy since there is minimal systemic absorption and minimal excretion into breast milk.1 Topical antimicrobial washes such as benzoyl peroxide and chlorhexidine often are used in combination with systemic medications to treat HS. These washes are safe during pregnancy and lactation, as they have minimal systemic absorption.7
Of these first-line antibiotics, only tetracyclines are contraindicated during pregnancy and lactation, as they are deemed to be in category D by the US Food and Drug Administration (FDA).1 Aside from tetracyclines, these antibiotics do not cause birth defects and are safe for nursing infants.1,8 Systemic clindamycin is safe during pregnancy and breastfeeding. Systemic metronidazole also is safe for use in pregnant patients but needs to be discontinued 12 to 24 hours prior to breastfeeding, which often prohibits appropriate dosing.1
Systemic Erythromycin—There are several forms of systemic erythromycin, including erythromycin base, erythromycin estolate, erythromycin ethylsuccinate (EES), and erythromycin stearate. Erythromycin estolate is contraindicated in pregnancy because it is associated with reversible maternal hepatoxicity and jaundice.9-11 Erythromycin ethylsuccinate is the preferred form for pregnant patients. Providers should exercise caution when prescribing EES to lactating mothers, as small amounts are still secreted through breast milk.11 Some studies have shown an increased risk for development of infantile hypertrophic pyloric stenosis with systemic erythromycin use, especially if a neonate is exposed in the first 14 days of life. Thus, we recommend withholding EES for 2 weeks after delivery if the patient is breastfeeding. A follow-up study did not find any association between erythromycin and infantile hypertrophic pyloric stenosis; however, the American Academy of Pediatrics still recommends short-term use only of erythromycin if it is to be used in the systemic form.8
Rifampin—Rifampin is excreted into breast milk but without adverse effects to the infant. Rifampin also is safe in pregnancy but should be used on a case-by-case basis in pregnant or nursing women because it is a cytochrome P450 inducer.
Dapsone—Dapsone has no increased risk for congenital anomalies. However, it is associated with hemolytic anemia and neonatal hyperbilirubinemia, especially in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.12 Newborns exposed to dapsone are at an increased risk for methemoglobinemia owing to increased sensitivity of fetal erythrocytes to oxidizing agents.13 If dapsone use is necessary, stopping dapsone treatment in the last month of gestation is recommended to minimize risk for kernicterus.9 Dapsone can be found in high concentrations in breast milk at 14.3% of the maternal dose. It is still safe to use during breastfeeding, but there is a risk of the infant developing hyperbilirubinemia/G6PD deficiency.1,8 Thus, physicians may consider performing a G6PD screen on infants to determine if breastfeeding is safe.12
Fluoroquinolones—Quinolones are not contraindicated during pregnancy, but they can damage fetal cartilage and thus should be reserved for use in complicated infections when the benefits outweigh the risks.12 Quinolones are believed to increase risk for arthropathy but are safe for use in lactation. When quinolones are digested with milk, exposure decreases below pediatric doses because of the ionized property of calcium in milk.8
Tumor Necrosis Factor α Inhibitors—The safety of anti–tumor necrosis factor (TNF) α biologics in pregnancy is less certain when compared with antibiotics.1 Anti–TNF-α inhibitors such as etanercept, adalimumab, and infliximab are all labeled as FDA category B, meaning there are no well-controlled human studies of the drugs.9 There are limited data that support safe use of TNF-α inhibitors prior to the third trimester before maternal IgG antibodies are transferred to the fetus via the placenta.1,13 Anti–TNF-α inhibitors may be safe when breastfeeding because the drugs have large molecular weights that prevent them from entering breast milk in large amounts. Absorption also is limited due to the infant’s digestive acids and enzymes breaking down the protein structure of the medication.8 Overall, TNF-α inhibitor use is still controversial and only used if the benefits outweigh the risks during pregnancy or if there is no alternative treatment.1,3,9
Ustekinumab and Anakinra—Ustekinumab (an IL-12/IL-23 inhibitor) and anakinra (an IL-1α and IL-1β inhibitor) also are FDA category B drugs and have limited data supporting their use as HS treatment in pregnancy. Anakinra may have evidence of compatibility with breastfeeding, as endogenous IL-1α inhibitor is found in colostrum and mature breast milk.1
Immunosuppressants
Immunosuppressants that are used to treat HS include corticosteroids and cyclosporine.
Corticosteroids—Topical corticosteroids can be used safely in lactation if they are not applied directly to the nipple or any area that makes direct contact with the infant’s mouth. Intralesional corticosteroid injections are safe for use during both pregnancy and breastfeeding to decrease inflammation of acutely flaring lesions and can be considered first-line treatment.1 Oral glucocorticoids also can be safely used for acute flares during pregnancy; however, prolonged use is associated with pregnancy complications such as preeclampsia, eclampsia, premature delivery, and gestational diabetes.12 There also is a small risk of oral cleft deformity in the infant; thus, potent corticosteroids are recommended in short durations during pregnancy, and there are no adverse effects if the maternal dose is less than 10 mg daily.8,12 Systemic steroids are safe to use with breastfeeding, but patients should be advised to wait 4 hours after ingesting medication before breastfeeding.1,8
Cyclosporine—Topical and oral calcineurin inhibitors such as cyclosporine have low risk for transmission into breast milk; however, potential effects of exposure through breast milk are unknown. For that reason, manufacturers state that cyclosporine use is contraindicated during lactation.8 If cyclosporine is to be used by a breastfeeding woman, monitoring cyclosporine concentrations in the infant is suggested to ensure that the exposure is less than 5% to 10% of the therapeutic dose.13 The use of cyclosporine has been extensively studied in pregnant transplant patients and is considered relatively safe for use in pregnancy.14 Cyclosporine is lipid soluble and thus is quickly metabolized and spread throughout the body; it can easily cross the placenta.9,13 Blood concentration in the fetus is 30% to 64% that of the maternal circulation. However, cyclosporine is only toxic to the fetus at maternally toxic doses, which can result in low birth weight and increased prenatal and postnatal mortality.13
Isotretinoin, Oral Contraceptive Pills, and Spironolactone
Isotretinoin and hormonal treatments such as oral contraceptive pills and spironolactone (an androgen receptor blocker) commonly are used to treat HS, but all are contraindicated in pregnancy and lactation. Isotretinoin is a well-established teratogen, but adverse effects on nursing babies have not been described. However, the manufacturer of isotretinoin advises against its use in lactation. Oral contraceptive pill use in early pregnancy is associated with increased risk for Down syndrome. Oral contraceptive pill use also is contraindicated in lactation for 2 reasons: decreased milk production and risk for fetal feminization. Antiandrogenic agents such as spironolactone have been shown to be associated with hypospadias and feminization of the male fetus.7
COMMENT
Women with HS usually require ongoing medical treatment during pregnancy and immediately postpartum; thus, it is important that treatments are proven to be safe for use in this specific population. Current management guidelines are not entirely suitable for pregnant and breastfeeding women given that many HS drugs have teratogenic effects and/or can be excreted into breast milk.1 Several treatments have uncertain safety profiles in pregnancy and breastfeeding, which calls for dermatologists to change or create new regimens for their patients. Close management also is necessary to prevent excess inflammation of breast tissue and milk fistula formation, which would hinder normal breastfeeding.
The eTable lists medications used to treat HS. The FDA category is listed next to each drug. However, it should be noted that these FDA letter categories were replaced with the Pregnancy and Lactation Labeling Rule in 2015. The letter ratings were deemed overly simplistic and replaced with narrative-based labeling that provides more detailed adverse effects and clinical considerations.9

Risk Factors of HS—Predisposing risk factors for HS flares that are controllable include obesity and smoking.2 Pregnancy weight gain may cause increased skin maceration at intertriginous sites, which can contribute to worsening HS symptoms.1,5 Adipocytes play a role in HS exacerbation by promoting secretion of TNF-α, leading to increased inflammation.5 Dermatologists can help prevent postpartum HS flares by monitoring weight gain during pregnancy, encouraging smoking cessation, and promoting weight and nutrition goals as set by an obstetrician.1 In addition to medications, management of HS should include emotional support and education on wearing loose-fitting clothing to avoid irritation of the affected areas.3 An emphasis on dermatologist counseling for all patients with HS, even for those with milder disease, can reduce exacerbations during pregnancy.5

CONCLUSION
The selection of dermatologic drugs for the treatment of HS in the setting of pregnancy involves complex decision-making. Dermatologists need more guidelines and proven safety data in human trials, especially regarding use of biologics and immunosuppressants to better treat HS in pregnancy. With more data, they can create more evidence-based treatment regimens to help prevent postpartum exacerbations of HS. Thus, patients can breastfeed their infants comfortably and without any risks of impaired child development. In the meantime, dermatologists can continue to work together with obstetricians and psychiatrists to decrease disease flares through counseling patients on nutrition and weight gain and providing emotional support.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989. doi:10.1016/j.jaad.2016.10.032
- Samuel S, Tremelling A, Murray M. Presentation and surgical management of hidradenitis suppurativa of the breast during pregnancy: a case report. Int J Surg Case Rep. 2018;51:21-24. doi:10.1016/j.ijscr.2018.08.013
- Yang CS, Teeple M, Muglia J, et al. Inflammatory and glandular skin disease in pregnancy. Clin Dermatol. 2016;34:335-343. doi:10.1016/j.clindermatol.2016.02.005
- Vossen AR, van Straalen KR, Prens EP, et al. Menses and pregnancy affect symptoms in hidradenitis suppurativa: a cross-sectional study. J Am Acad Dermatol. 2017;76:155-156. doi:10.1016/j.jaad.2016.07.024
- Lyons AB, Peacock A, McKenzie SA, et al. Evaluation of hidradenitis suppurativa disease course during pregnancy and postpartum. JAMA Dermatol. 2020;156:681-685. doi:10.1001/jamadermatol.2020.0777
- Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa—a systematic review. Acta Dermatovenerol Croat. 2016;24:239-249.
- Kong YL, Tey HL. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787. doi:10.1007/s40265-013-0060-0
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. lactation. J Am Acad Dermatol. 2014;70:417:E1-E10. doi:10.1016/j.jaad.2013.09.009
- Wilmer E, Chai S, Kroumpouzos G. Drug safety: pregnancy rating classifications and controversies. Clin Dermatol. 2016;34:401-409. doi:10.1016/j.clindermatol.2016.02.013
- Inman WH, Rawson NS. Erythromycin estolate and jaundice. Br Med J (Clin Res Ed). 1983;286:1954-1955. doi:10.1136/bmj.286.6382.1954
- Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. pregnancy. J Am Acad Dermatol. 2014;70:401.e1-14; quiz 415. doi:10.1016/j.jaad.2013.09.010
- Brown SM, Aljefri K, Waas R, et al. Systemic medications used in treatment of common dermatological conditions: safety profile with respect to pregnancy, breast feeding and content in seminal fluid. J Dermatolog Treat. 2019;30:2-18. doi:10.1080/09546634.2016.1202402
- Kamarajah SK, Arntdz K, Bundred J, et al. Outcomes of pregnancy in recipients of liver transplants. Clin Gastroenterol Hepatol. 2019;17:1398-1404.e1. doi:10.1016/j.cgh.2018.11.055
Practice Points
- Some medications used to treat hidradenitis suppurativa (HS) may have teratogenic effects and be contraindicated during breastfeeding.
- We summarize what treatments are proven to be safe in pregnancy and breastfeeding and highlight the need for more guidelines and safety data for dermatologists to manage their pregnant patients with HS.
Reactivation of a BCG Vaccination Scar Following the First Dose of the Moderna COVID-19 Vaccine
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
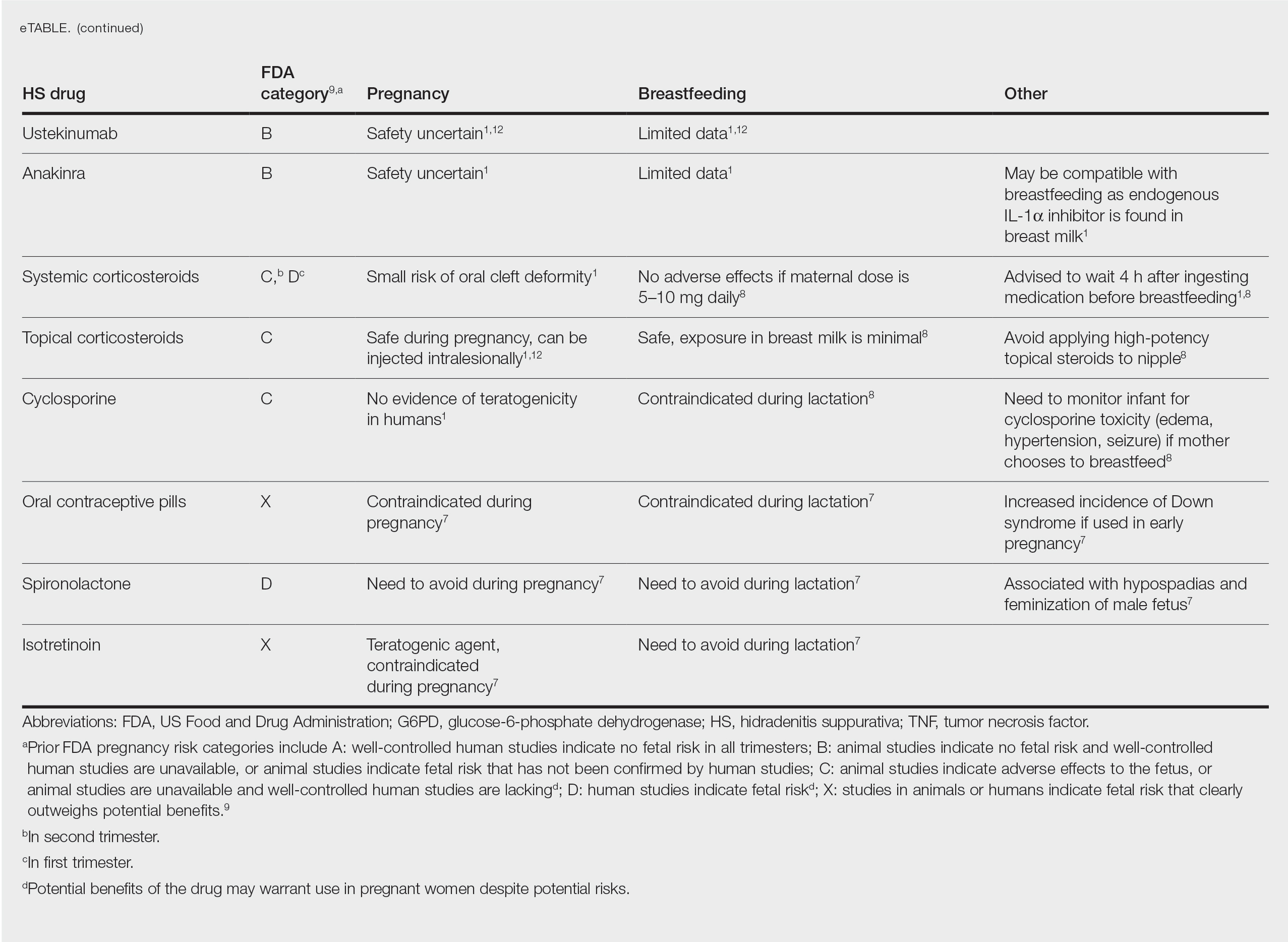
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
Practice Points
- BCG vaccination scar reactivation is a potential benign, self-limited reaction in patients who receive the Moderna COVID-19 Vaccine.
- Symptoms of BCG vaccination scar reactivation, which is seen more commonly in children with Kawasaki disease, include redness, swelling, and ulceration.
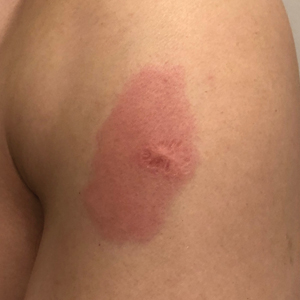
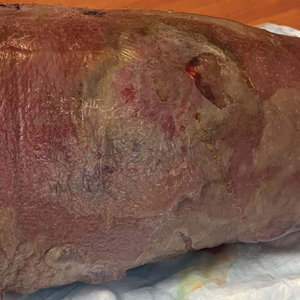
Rapidly Enlarging Bullous Plaque
The Diagnosis: Bullous Pyoderma Gangrenosum
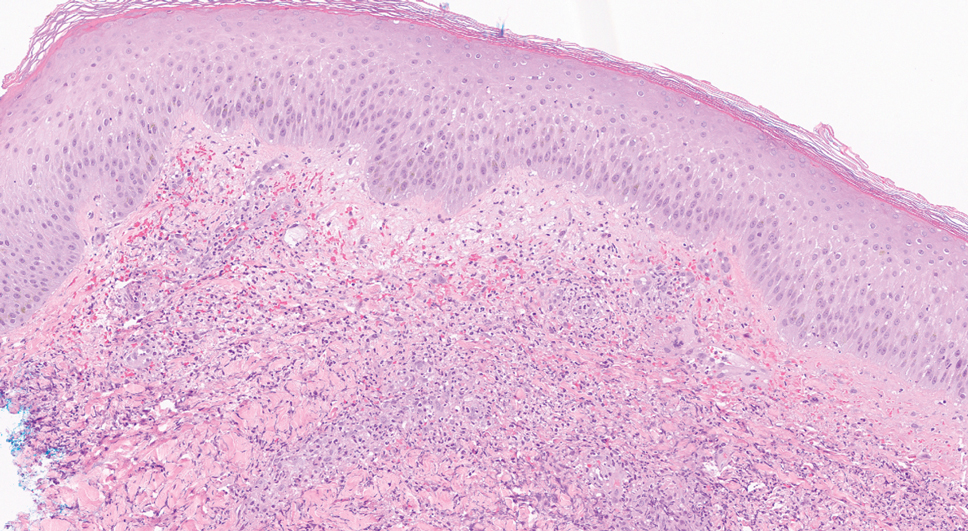
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.

First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.

First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
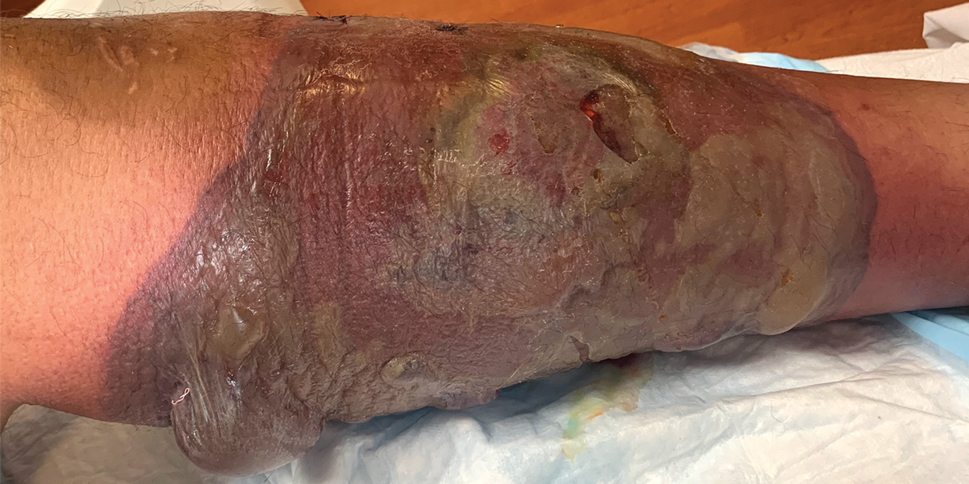
A 26-year-old previously healthy man presented to the emergency department with a new asymptomatic enlarging lesion on the lower leg that had appeared 4 days prior as a self-described “pimple” and rapidly evolved. The patient also reported chills, fatigue, and decreased appetite during that time. Physical examination revealed a red to violaceous, well-demarcated, bullous plaque involving much of the left lower leg. Laboratory studies demonstrated a hemoglobin level of 8.1 g/dL (reference range, 14.0–17.5 g/dL), hematocrit level of 23.7% (reference range, 41%–50%), platelet count of 26×103 /μL (reference range, 150–350×103 /μL), and a population of circulating blast cells and metamyelocytes.
Iododerma Following Exposure to Iodine: A Case of Explosive Acneform Eruption Overnight
To the Editor:
Iododerma is a rare dermatologic condition caused by exposure to iodinated contrast media, oral iodine suspensions, or topical povidone-iodine that can manifest as eruptive acneform lesions.1-3
A 27-year-old woman in septic shock presented for worsening facial lesions that showed no improvement on broad-spectrum antibiotics, antifungals, and antivirals. She initially presented to an outside hospital with abdominal pain and underwent computed tomography (CT) with intravenous (IV) iodinated contrast; 24 hours after this imaging study, the family reported the appearance of “explosive acne overnight.” The lesions first appeared as vegetative and acneform ulcerations on the face. A second abdominal CT scan with IV contrast was performed 4 days after the initial scan, given the concern for spontaneous bacterial peritonitis. Hours after the second study, the lesions progressed to involve the buccal mucosae, tongue, mucosal airway, and distal arms and legs. She became progressively disoriented and developed an altered mentation over the course of the following week. Due to progressive facial edema, she required intubation 5 days after the second CT scan.
The patient had a medical history of end-stage renal disease secondary to crescenteric glomerulonephritis on peritoneal dialysis. Physical examination revealed numerous beefy-red, heaped-up, weepy, crusted nodules clustered on the face (Figure 1) and a few newer bullous-appearing lesions on the hands and feet. She had similar lesions involving the buccal mucosae and tongue with substantial facial edema. Infectious workup was notable for a positive skin culture growing methicillin-susceptible Staphylococcus aureus. All blood and tissue cultures as well as serologies for fungal and viral etiologies were negative. A tissue biopsy revealed necrosis with a neutrophilic infiltrate with mixed cell inflammation (Figure 2), and direct immunofluorescence was negative.
The patient initially was thought to be septic due to viral or bacterial infection. She was transferred from an outside hospital 7 days after the initial appearance of the acneform lesions, having already received IV contrast on 2 occasions within the first 48 hours of illness. Infectious disease was consulted and initiated broad-spectrum antiviral, antimicrobial, and antifungal therapy with acyclovir, linezolid, meropenem, and later micafungin without improvement. The diagnosis of iododerma ultimately was established based on the patient’s elevated urinary iodine levels with preceding iodine exposure in the context of renal failure. The preferential involvement of sebaceous areas and pathology findings were supportive of this diagnosis. Aggressive supportive measures including respiratory support, IV fluids, and dialysis were initiated. Topical iodine solutions, iodine-containing medications, and additional contrast subsequently were avoided. Despite these supportive measures, the patient died within 48 hours of admission from acute respiratory failure. Her autopsy attributed “septic complications of multifocal ulcerative cutaneous disease” as the anatomic cause of death.
Iododerma is an extremely rare neutrophilic dermatosis. The proposed mechanism of action involves a cell-mediated hypersensitivity reaction to iodine with induction of neutrophil degranulation.2 There have been documented cases with exposure to oral potassium iodide supplements, amiodarone, topical povidone-iodine, and IV iodinated contrast material.1-3 Iododerma typically presents 1 to 3 days after exposure to iodine. The most common source is IV radiocontrast. Diagnosis is based on the clinical presentation including acneform to vegetative nodular or bullous eruptions involving sebaceous areas in the context of recent iodine exposure. Elevated urinary iodine levels and histologic findings of neutrophilic infiltrate of the dermis support the diagnosis.3,4
Although there have been reported cases of iododerma in patients with normal renal function, patients with renal failure are much more susceptible due to the decreased clearance of iodine.5 The plasma half-life of radiocontrast is 23 hours in patients with end-stage renal disease vs 2 hours in patients with normal kidney function.3 Dosage adjustments for renal impairment have not been well studied, and no specific guidelines exist for the prevention of iododerma in patients with renal failure.
The first step in treating iododerma is to remove the offending iodine-containing agent. In most cases, cutaneous lesions resolve in 4 to 6 weeks after discontinuation of the source of iodine; however, there have been reported fatalities in the literature secondary to pulmonary edema in patients with iododerma.6,7 Despite the rarity and diagnostically challenging nature of iododerma, early recognition of this disease is crucial. Although our patient showed symptoms of iododerma after 1 dose of radiocontrast, she was not diagnosed at that time and received a second imaging study with contrast less than 48 hours later. These 2 consecutive exposures to iodine as well as the delayed diagnosis unfortunately resulted in rapid clinical deterioration.
The mainstay of therapy for iododerma includes avoidance of iodine-containing materials as soon as the diagnosis is suspected as well as supportive care. Patients have been successfully treated with systemic corticosteroids, with the addition of cyclosporine and hemodialysis in severe cases.3 Patients with a history of iododerma are advised to avoid iodine in their diet, in topical preparations, and in future imaging studies.8
- Aliagaoglu C, Turan H, Uslu E, et al. Iododerma following topical povidone-iodine application. Cutan Ocul Toxicol. 2013;32:339-340.
- Torkamani, N, Sinclair R. Iododerma in pregnancy secondary to iodinated multivitamins. Australas J Dermatol. 2015;56:235-236.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast material. Br J Dermatol. 2014;170:1377-1379.
- Stavert R, Bunick CG, Modi B, et al. Vegetative plaques and hemorrhagic pustules. JAMA Dermatol. 2013;149:1231-1232.
- Rothman LR, Levender MM, Scharf MD, et al. Iododerma following serial computed tomography scans in a lung cancer patient. J Drugs Dermatol. 2013;12:574-576.
- Miranda-Romero A, Sánchez-Sambucety P, Gómez JE, et al. Vegetating iododerma with fatal outcome. Dermatology. 1999;198:295-297.
- Vailant L, Pengloan J, Blanchier D, et al. Iododerma and acute respiratory distress with leucocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232-233.
- Massé M, Flanaga V, Zhou LH. Use of topical povidone iodine resulting in an iododerma-like eruption. J Dermatol. 2008;35:744-747.
To the Editor:
Iododerma is a rare dermatologic condition caused by exposure to iodinated contrast media, oral iodine suspensions, or topical povidone-iodine that can manifest as eruptive acneform lesions.1-3
A 27-year-old woman in septic shock presented for worsening facial lesions that showed no improvement on broad-spectrum antibiotics, antifungals, and antivirals. She initially presented to an outside hospital with abdominal pain and underwent computed tomography (CT) with intravenous (IV) iodinated contrast; 24 hours after this imaging study, the family reported the appearance of “explosive acne overnight.” The lesions first appeared as vegetative and acneform ulcerations on the face. A second abdominal CT scan with IV contrast was performed 4 days after the initial scan, given the concern for spontaneous bacterial peritonitis. Hours after the second study, the lesions progressed to involve the buccal mucosae, tongue, mucosal airway, and distal arms and legs. She became progressively disoriented and developed an altered mentation over the course of the following week. Due to progressive facial edema, she required intubation 5 days after the second CT scan.

The patient had a medical history of end-stage renal disease secondary to crescenteric glomerulonephritis on peritoneal dialysis. Physical examination revealed numerous beefy-red, heaped-up, weepy, crusted nodules clustered on the face (Figure 1) and a few newer bullous-appearing lesions on the hands and feet. She had similar lesions involving the buccal mucosae and tongue with substantial facial edema. Infectious workup was notable for a positive skin culture growing methicillin-susceptible Staphylococcus aureus. All blood and tissue cultures as well as serologies for fungal and viral etiologies were negative. A tissue biopsy revealed necrosis with a neutrophilic infiltrate with mixed cell inflammation (Figure 2), and direct immunofluorescence was negative.

The patient initially was thought to be septic due to viral or bacterial infection. She was transferred from an outside hospital 7 days after the initial appearance of the acneform lesions, having already received IV contrast on 2 occasions within the first 48 hours of illness. Infectious disease was consulted and initiated broad-spectrum antiviral, antimicrobial, and antifungal therapy with acyclovir, linezolid, meropenem, and later micafungin without improvement. The diagnosis of iododerma ultimately was established based on the patient’s elevated urinary iodine levels with preceding iodine exposure in the context of renal failure. The preferential involvement of sebaceous areas and pathology findings were supportive of this diagnosis. Aggressive supportive measures including respiratory support, IV fluids, and dialysis were initiated. Topical iodine solutions, iodine-containing medications, and additional contrast subsequently were avoided. Despite these supportive measures, the patient died within 48 hours of admission from acute respiratory failure. Her autopsy attributed “septic complications of multifocal ulcerative cutaneous disease” as the anatomic cause of death.
Iododerma is an extremely rare neutrophilic dermatosis. The proposed mechanism of action involves a cell-mediated hypersensitivity reaction to iodine with induction of neutrophil degranulation.2 There have been documented cases with exposure to oral potassium iodide supplements, amiodarone, topical povidone-iodine, and IV iodinated contrast material.1-3 Iododerma typically presents 1 to 3 days after exposure to iodine. The most common source is IV radiocontrast. Diagnosis is based on the clinical presentation including acneform to vegetative nodular or bullous eruptions involving sebaceous areas in the context of recent iodine exposure. Elevated urinary iodine levels and histologic findings of neutrophilic infiltrate of the dermis support the diagnosis.3,4
Although there have been reported cases of iododerma in patients with normal renal function, patients with renal failure are much more susceptible due to the decreased clearance of iodine.5 The plasma half-life of radiocontrast is 23 hours in patients with end-stage renal disease vs 2 hours in patients with normal kidney function.3 Dosage adjustments for renal impairment have not been well studied, and no specific guidelines exist for the prevention of iododerma in patients with renal failure.
The first step in treating iododerma is to remove the offending iodine-containing agent. In most cases, cutaneous lesions resolve in 4 to 6 weeks after discontinuation of the source of iodine; however, there have been reported fatalities in the literature secondary to pulmonary edema in patients with iododerma.6,7 Despite the rarity and diagnostically challenging nature of iododerma, early recognition of this disease is crucial. Although our patient showed symptoms of iododerma after 1 dose of radiocontrast, she was not diagnosed at that time and received a second imaging study with contrast less than 48 hours later. These 2 consecutive exposures to iodine as well as the delayed diagnosis unfortunately resulted in rapid clinical deterioration.
The mainstay of therapy for iododerma includes avoidance of iodine-containing materials as soon as the diagnosis is suspected as well as supportive care. Patients have been successfully treated with systemic corticosteroids, with the addition of cyclosporine and hemodialysis in severe cases.3 Patients with a history of iododerma are advised to avoid iodine in their diet, in topical preparations, and in future imaging studies.8
To the Editor:
Iododerma is a rare dermatologic condition caused by exposure to iodinated contrast media, oral iodine suspensions, or topical povidone-iodine that can manifest as eruptive acneform lesions.1-3
A 27-year-old woman in septic shock presented for worsening facial lesions that showed no improvement on broad-spectrum antibiotics, antifungals, and antivirals. She initially presented to an outside hospital with abdominal pain and underwent computed tomography (CT) with intravenous (IV) iodinated contrast; 24 hours after this imaging study, the family reported the appearance of “explosive acne overnight.” The lesions first appeared as vegetative and acneform ulcerations on the face. A second abdominal CT scan with IV contrast was performed 4 days after the initial scan, given the concern for spontaneous bacterial peritonitis. Hours after the second study, the lesions progressed to involve the buccal mucosae, tongue, mucosal airway, and distal arms and legs. She became progressively disoriented and developed an altered mentation over the course of the following week. Due to progressive facial edema, she required intubation 5 days after the second CT scan.

The patient had a medical history of end-stage renal disease secondary to crescenteric glomerulonephritis on peritoneal dialysis. Physical examination revealed numerous beefy-red, heaped-up, weepy, crusted nodules clustered on the face (Figure 1) and a few newer bullous-appearing lesions on the hands and feet. She had similar lesions involving the buccal mucosae and tongue with substantial facial edema. Infectious workup was notable for a positive skin culture growing methicillin-susceptible Staphylococcus aureus. All blood and tissue cultures as well as serologies for fungal and viral etiologies were negative. A tissue biopsy revealed necrosis with a neutrophilic infiltrate with mixed cell inflammation (Figure 2), and direct immunofluorescence was negative.

The patient initially was thought to be septic due to viral or bacterial infection. She was transferred from an outside hospital 7 days after the initial appearance of the acneform lesions, having already received IV contrast on 2 occasions within the first 48 hours of illness. Infectious disease was consulted and initiated broad-spectrum antiviral, antimicrobial, and antifungal therapy with acyclovir, linezolid, meropenem, and later micafungin without improvement. The diagnosis of iododerma ultimately was established based on the patient’s elevated urinary iodine levels with preceding iodine exposure in the context of renal failure. The preferential involvement of sebaceous areas and pathology findings were supportive of this diagnosis. Aggressive supportive measures including respiratory support, IV fluids, and dialysis were initiated. Topical iodine solutions, iodine-containing medications, and additional contrast subsequently were avoided. Despite these supportive measures, the patient died within 48 hours of admission from acute respiratory failure. Her autopsy attributed “septic complications of multifocal ulcerative cutaneous disease” as the anatomic cause of death.
Iododerma is an extremely rare neutrophilic dermatosis. The proposed mechanism of action involves a cell-mediated hypersensitivity reaction to iodine with induction of neutrophil degranulation.2 There have been documented cases with exposure to oral potassium iodide supplements, amiodarone, topical povidone-iodine, and IV iodinated contrast material.1-3 Iododerma typically presents 1 to 3 days after exposure to iodine. The most common source is IV radiocontrast. Diagnosis is based on the clinical presentation including acneform to vegetative nodular or bullous eruptions involving sebaceous areas in the context of recent iodine exposure. Elevated urinary iodine levels and histologic findings of neutrophilic infiltrate of the dermis support the diagnosis.3,4
Although there have been reported cases of iododerma in patients with normal renal function, patients with renal failure are much more susceptible due to the decreased clearance of iodine.5 The plasma half-life of radiocontrast is 23 hours in patients with end-stage renal disease vs 2 hours in patients with normal kidney function.3 Dosage adjustments for renal impairment have not been well studied, and no specific guidelines exist for the prevention of iododerma in patients with renal failure.
The first step in treating iododerma is to remove the offending iodine-containing agent. In most cases, cutaneous lesions resolve in 4 to 6 weeks after discontinuation of the source of iodine; however, there have been reported fatalities in the literature secondary to pulmonary edema in patients with iododerma.6,7 Despite the rarity and diagnostically challenging nature of iododerma, early recognition of this disease is crucial. Although our patient showed symptoms of iododerma after 1 dose of radiocontrast, she was not diagnosed at that time and received a second imaging study with contrast less than 48 hours later. These 2 consecutive exposures to iodine as well as the delayed diagnosis unfortunately resulted in rapid clinical deterioration.
The mainstay of therapy for iododerma includes avoidance of iodine-containing materials as soon as the diagnosis is suspected as well as supportive care. Patients have been successfully treated with systemic corticosteroids, with the addition of cyclosporine and hemodialysis in severe cases.3 Patients with a history of iododerma are advised to avoid iodine in their diet, in topical preparations, and in future imaging studies.8
- Aliagaoglu C, Turan H, Uslu E, et al. Iododerma following topical povidone-iodine application. Cutan Ocul Toxicol. 2013;32:339-340.
- Torkamani, N, Sinclair R. Iododerma in pregnancy secondary to iodinated multivitamins. Australas J Dermatol. 2015;56:235-236.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast material. Br J Dermatol. 2014;170:1377-1379.
- Stavert R, Bunick CG, Modi B, et al. Vegetative plaques and hemorrhagic pustules. JAMA Dermatol. 2013;149:1231-1232.
- Rothman LR, Levender MM, Scharf MD, et al. Iododerma following serial computed tomography scans in a lung cancer patient. J Drugs Dermatol. 2013;12:574-576.
- Miranda-Romero A, Sánchez-Sambucety P, Gómez JE, et al. Vegetating iododerma with fatal outcome. Dermatology. 1999;198:295-297.
- Vailant L, Pengloan J, Blanchier D, et al. Iododerma and acute respiratory distress with leucocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232-233.
- Massé M, Flanaga V, Zhou LH. Use of topical povidone iodine resulting in an iododerma-like eruption. J Dermatol. 2008;35:744-747.
- Aliagaoglu C, Turan H, Uslu E, et al. Iododerma following topical povidone-iodine application. Cutan Ocul Toxicol. 2013;32:339-340.
- Torkamani, N, Sinclair R. Iododerma in pregnancy secondary to iodinated multivitamins. Australas J Dermatol. 2015;56:235-236.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast material. Br J Dermatol. 2014;170:1377-1379.
- Stavert R, Bunick CG, Modi B, et al. Vegetative plaques and hemorrhagic pustules. JAMA Dermatol. 2013;149:1231-1232.
- Rothman LR, Levender MM, Scharf MD, et al. Iododerma following serial computed tomography scans in a lung cancer patient. J Drugs Dermatol. 2013;12:574-576.
- Miranda-Romero A, Sánchez-Sambucety P, Gómez JE, et al. Vegetating iododerma with fatal outcome. Dermatology. 1999;198:295-297.
- Vailant L, Pengloan J, Blanchier D, et al. Iododerma and acute respiratory distress with leucocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232-233.
- Massé M, Flanaga V, Zhou LH. Use of topical povidone iodine resulting in an iododerma-like eruption. J Dermatol. 2008;35:744-747.
Practice Points
- Iododerma should be considered for patients who develop rapidly progressive, vegetative lesions, especially in those with renal failure. A thorough history should be obtained in these cases, focusing on medications and recent studies involving iodinated contrast.
- The most important first step in treating iododerma is to remove the iodine-containing agent to avoid continued exposure.
- Therapies for iododerma include supportive care, cyclosporine, systemic corticosteroids, and hemodialysis in severe cases.
Necrotic Ulcerations After the Use of an Over-the-counter Mole and Skin Tag Removal Product
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.
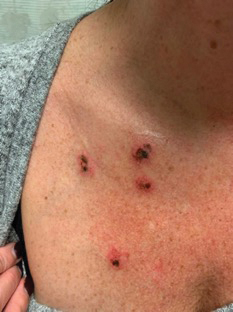
The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
Practice Point
- Self-administered mole and skin tag removal products are rising in popularity, but unregulated ingredients in over-the-counter products that are not approved by the US Food and Drug Administration may mask underlying transformation of atypical nevi.
At-Home Treatment of Pigmented Lesions With a Zinc Chloride Preparation
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
Practice Points
- Zinc chloride preparations are readily available over the counter and unregulated.
- Patients may attempt to self-treat pigmented lesions based on claims they see online.
- When asking patients about prior treatments, it may be prudent to specifically ask about over-the-counter products and their ingredients.
Patch Testing on Dupilumab: Reliable or Not?
In patients with persistent atopic dermatitis (AD) who are taking dupilumab, is there benefit of patch testing to determine if allergic contact dermatitis (ACD) also is contributing to their disease? Results of patch testing are likely be influenced by the immunomodulatory effects of dupilumab. Similar to the recommendation for patients to refrain from using topical or systemic corticosteroids for 1 week or more prior to patch testing to eliminate false negatives, we reviewed the literature to create practice guidelines for dermatologists regarding patch testing while a patient is taking dupilumab.
Pathophysiology and Pathomechanism
Dupilumab functions through the blockade of T helper 2 (TH2) cells; ACD is propagated through the T helper 1 (TH1) cellular pathway. However, patients with ACD that is unresponsive to allergen avoidance and traditional therapies, such as topical and oral corticosteroids, have responded to dupilumab. The more common reports of this responsiveness are with fragrances; multiple case series described patients with ACD to fragrance mix I1 and balsam of Peru1,2 who improved on dupilumab when other treatments failed. There also are reports of response when ACD was secondary to nickel,2,3p-phenylenediamine,1 Compositae,4 and non–formaldehyde-releasing preservatives (non-FRPs).5 Therefore, not all ACD is propagated through the TH1 cellular pathway.
As noted in these cases, ACD can be a response to an allergen whose pathogenesis involves the TH2 pathway or when patient characteristics favor a TH2 response. It has been suggested that AD patients are more susceptible to TH2-mediated contact sensitization to less-potent allergens, such as fragrances.6
Patch Test Results
Positive patch test results for allergens have been reported while patients are on dupilumab therapy, including a few studies in which results prior to starting dupilumab were compared with those while patients were on dupilumab therapy. In a retrospective chart review of 48 patients on dupilumab for AD with persistent disease, 23 patients were patch tested before and during dupilumab therapy. In these patients, the majority of contact allergies were persistent and only 10% (13/125) of patch test–positive results resolved on dupilumab therapy.7 Contact allergies that resolved included those to emulsifiers (propylene glycol, Amerchol L101 [lanolin-containing products found in cosmetics and other goods], dimethylaminopropylamine), fragrances (fragrance mix I, balsam of Peru), sunscreens (sulisobenzone, phenylbenzimidazole-5-sulfonic acid), and metals (vanadium chloride, phenylmercuric acetate).7 The following results observed in individual cases demonstrated conflicting findings: persistence of allergy to non-FRPs (methylisothiazolinone [MI]) but resolution of allergy to formaldehyde8; persistence of allergy to corticosteroids (budesonide and alclometasone)9; persistence of allergy to an antibiotic (neomycin sulfate) but resolution of allergies to a different antibiotic (bacitracin), glues (ethyl acrylate), bleach, and glutaraldehyde9; persistence of nickel allergy but resolution of allergies to fragrances (cinnamic aldehyde, balsam of Peru) and non-FRPs (methylchloroisothiazolinone or MI)10; and persistence of allergies to non-FRPs (MI) and FRPs (bronopol) but resolution of allergies to nickel, fragrances (hydroperoxides of linalool), and Compositae.11 Additional case reports of positive patch test results while on dupilumab but with no pretreatment results for comparison include allergies to rubber additives,12-14 nickel,14 textile dyes,14 cosmetic and hair care additives,12,14,15 corticosteroids,15 FRPs,15 fragrances,15,16 emulsifiers,16 and non-FRPs.17
An evident theme in the dupilumab patch-testing literature has been that results are variable and case specific: a given patient with ACD to an allergen will respond to dupilumab treatment and have subsequent negative patch testing, while another patient will not respond to dupilumab treatment and have persistent positive patch testing. This is likely because, in certain individuals, the allergen-immune system combination shifts ACD pathogenesis from a purely TH1 response to at least a partial TH2 response, thus allowing for benefit from dupilumab therapy. T helper 1 cell–mediated ACD should not be affected by dupilumab; therefore, reliable results can be elucidated from patch testing despite the drug.
Final Thoughts
We propose that AD patients with residual disease after taking dupilumab undergo patch testing. Positive results indicate allergens that are not inhibited by the drug. Patients will need to follow strict allergen avoidance to resolve this component of their disease; failure to improve might suggest the result was a nonrelevant positive.
If patch testing is negative, an alternative cause for residual disease must be sought. We do not recommend stopping dupilumab prior to patch testing to avoid a disease flare from AD or possible TH2-mediated ACD.
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020:83;137-139. doi:10.1111/cod.13545
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701. doi:10.1111/dth.12701
- Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317. doi:10.1016/j.jaad.2016.03.010
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Suresh R, Murase JE. The role of expanded series patch testing in identifying causality of residual facial dermatitis following initiation of dupilumab therapy. JAAD Case Rep. 2018;4:899-904. doi:10.1016/j.jdcr.2018.08.027
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162. doi:10.1016/j.jaad.2019.03.020
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Hoot JW, Douglas JD, Falo LD Jr. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164. doi:10.1097/DER.0000000000000357
- Crepy M-N, Nosbaum A, Bensefa-Colas L. Blocking type 2 inflammation by dupilumab does not control classic (type 1-driven) allergic contact dermatitis in chronic hand eczema. Contact Dermatitis. 2019;81:145-147. doi:10.1111/cod.13266
- Raffi J, Chen R, Botto N. Wide dye reactors. JAAD Case Rep. 2019;5:877-879. doi:10.1016/j.jdcr.2019.08.005
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Raffi J, Suresh R, Fishman H, et al. Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD). Int J Womens Dermatol. 2019;5:308-313. doi:10.1016/j.ijwd.2019.10.001
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
In patients with persistent atopic dermatitis (AD) who are taking dupilumab, is there benefit of patch testing to determine if allergic contact dermatitis (ACD) also is contributing to their disease? Results of patch testing are likely be influenced by the immunomodulatory effects of dupilumab. Similar to the recommendation for patients to refrain from using topical or systemic corticosteroids for 1 week or more prior to patch testing to eliminate false negatives, we reviewed the literature to create practice guidelines for dermatologists regarding patch testing while a patient is taking dupilumab.
Pathophysiology and Pathomechanism
Dupilumab functions through the blockade of T helper 2 (TH2) cells; ACD is propagated through the T helper 1 (TH1) cellular pathway. However, patients with ACD that is unresponsive to allergen avoidance and traditional therapies, such as topical and oral corticosteroids, have responded to dupilumab. The more common reports of this responsiveness are with fragrances; multiple case series described patients with ACD to fragrance mix I1 and balsam of Peru1,2 who improved on dupilumab when other treatments failed. There also are reports of response when ACD was secondary to nickel,2,3p-phenylenediamine,1 Compositae,4 and non–formaldehyde-releasing preservatives (non-FRPs).5 Therefore, not all ACD is propagated through the TH1 cellular pathway.
As noted in these cases, ACD can be a response to an allergen whose pathogenesis involves the TH2 pathway or when patient characteristics favor a TH2 response. It has been suggested that AD patients are more susceptible to TH2-mediated contact sensitization to less-potent allergens, such as fragrances.6
Patch Test Results
Positive patch test results for allergens have been reported while patients are on dupilumab therapy, including a few studies in which results prior to starting dupilumab were compared with those while patients were on dupilumab therapy. In a retrospective chart review of 48 patients on dupilumab for AD with persistent disease, 23 patients were patch tested before and during dupilumab therapy. In these patients, the majority of contact allergies were persistent and only 10% (13/125) of patch test–positive results resolved on dupilumab therapy.7 Contact allergies that resolved included those to emulsifiers (propylene glycol, Amerchol L101 [lanolin-containing products found in cosmetics and other goods], dimethylaminopropylamine), fragrances (fragrance mix I, balsam of Peru), sunscreens (sulisobenzone, phenylbenzimidazole-5-sulfonic acid), and metals (vanadium chloride, phenylmercuric acetate).7 The following results observed in individual cases demonstrated conflicting findings: persistence of allergy to non-FRPs (methylisothiazolinone [MI]) but resolution of allergy to formaldehyde8; persistence of allergy to corticosteroids (budesonide and alclometasone)9; persistence of allergy to an antibiotic (neomycin sulfate) but resolution of allergies to a different antibiotic (bacitracin), glues (ethyl acrylate), bleach, and glutaraldehyde9; persistence of nickel allergy but resolution of allergies to fragrances (cinnamic aldehyde, balsam of Peru) and non-FRPs (methylchloroisothiazolinone or MI)10; and persistence of allergies to non-FRPs (MI) and FRPs (bronopol) but resolution of allergies to nickel, fragrances (hydroperoxides of linalool), and Compositae.11 Additional case reports of positive patch test results while on dupilumab but with no pretreatment results for comparison include allergies to rubber additives,12-14 nickel,14 textile dyes,14 cosmetic and hair care additives,12,14,15 corticosteroids,15 FRPs,15 fragrances,15,16 emulsifiers,16 and non-FRPs.17
An evident theme in the dupilumab patch-testing literature has been that results are variable and case specific: a given patient with ACD to an allergen will respond to dupilumab treatment and have subsequent negative patch testing, while another patient will not respond to dupilumab treatment and have persistent positive patch testing. This is likely because, in certain individuals, the allergen-immune system combination shifts ACD pathogenesis from a purely TH1 response to at least a partial TH2 response, thus allowing for benefit from dupilumab therapy. T helper 1 cell–mediated ACD should not be affected by dupilumab; therefore, reliable results can be elucidated from patch testing despite the drug.
Final Thoughts
We propose that AD patients with residual disease after taking dupilumab undergo patch testing. Positive results indicate allergens that are not inhibited by the drug. Patients will need to follow strict allergen avoidance to resolve this component of their disease; failure to improve might suggest the result was a nonrelevant positive.
If patch testing is negative, an alternative cause for residual disease must be sought. We do not recommend stopping dupilumab prior to patch testing to avoid a disease flare from AD or possible TH2-mediated ACD.
In patients with persistent atopic dermatitis (AD) who are taking dupilumab, is there benefit of patch testing to determine if allergic contact dermatitis (ACD) also is contributing to their disease? Results of patch testing are likely be influenced by the immunomodulatory effects of dupilumab. Similar to the recommendation for patients to refrain from using topical or systemic corticosteroids for 1 week or more prior to patch testing to eliminate false negatives, we reviewed the literature to create practice guidelines for dermatologists regarding patch testing while a patient is taking dupilumab.
Pathophysiology and Pathomechanism
Dupilumab functions through the blockade of T helper 2 (TH2) cells; ACD is propagated through the T helper 1 (TH1) cellular pathway. However, patients with ACD that is unresponsive to allergen avoidance and traditional therapies, such as topical and oral corticosteroids, have responded to dupilumab. The more common reports of this responsiveness are with fragrances; multiple case series described patients with ACD to fragrance mix I1 and balsam of Peru1,2 who improved on dupilumab when other treatments failed. There also are reports of response when ACD was secondary to nickel,2,3p-phenylenediamine,1 Compositae,4 and non–formaldehyde-releasing preservatives (non-FRPs).5 Therefore, not all ACD is propagated through the TH1 cellular pathway.
As noted in these cases, ACD can be a response to an allergen whose pathogenesis involves the TH2 pathway or when patient characteristics favor a TH2 response. It has been suggested that AD patients are more susceptible to TH2-mediated contact sensitization to less-potent allergens, such as fragrances.6
Patch Test Results
Positive patch test results for allergens have been reported while patients are on dupilumab therapy, including a few studies in which results prior to starting dupilumab were compared with those while patients were on dupilumab therapy. In a retrospective chart review of 48 patients on dupilumab for AD with persistent disease, 23 patients were patch tested before and during dupilumab therapy. In these patients, the majority of contact allergies were persistent and only 10% (13/125) of patch test–positive results resolved on dupilumab therapy.7 Contact allergies that resolved included those to emulsifiers (propylene glycol, Amerchol L101 [lanolin-containing products found in cosmetics and other goods], dimethylaminopropylamine), fragrances (fragrance mix I, balsam of Peru), sunscreens (sulisobenzone, phenylbenzimidazole-5-sulfonic acid), and metals (vanadium chloride, phenylmercuric acetate).7 The following results observed in individual cases demonstrated conflicting findings: persistence of allergy to non-FRPs (methylisothiazolinone [MI]) but resolution of allergy to formaldehyde8; persistence of allergy to corticosteroids (budesonide and alclometasone)9; persistence of allergy to an antibiotic (neomycin sulfate) but resolution of allergies to a different antibiotic (bacitracin), glues (ethyl acrylate), bleach, and glutaraldehyde9; persistence of nickel allergy but resolution of allergies to fragrances (cinnamic aldehyde, balsam of Peru) and non-FRPs (methylchloroisothiazolinone or MI)10; and persistence of allergies to non-FRPs (MI) and FRPs (bronopol) but resolution of allergies to nickel, fragrances (hydroperoxides of linalool), and Compositae.11 Additional case reports of positive patch test results while on dupilumab but with no pretreatment results for comparison include allergies to rubber additives,12-14 nickel,14 textile dyes,14 cosmetic and hair care additives,12,14,15 corticosteroids,15 FRPs,15 fragrances,15,16 emulsifiers,16 and non-FRPs.17
An evident theme in the dupilumab patch-testing literature has been that results are variable and case specific: a given patient with ACD to an allergen will respond to dupilumab treatment and have subsequent negative patch testing, while another patient will not respond to dupilumab treatment and have persistent positive patch testing. This is likely because, in certain individuals, the allergen-immune system combination shifts ACD pathogenesis from a purely TH1 response to at least a partial TH2 response, thus allowing for benefit from dupilumab therapy. T helper 1 cell–mediated ACD should not be affected by dupilumab; therefore, reliable results can be elucidated from patch testing despite the drug.
Final Thoughts
We propose that AD patients with residual disease after taking dupilumab undergo patch testing. Positive results indicate allergens that are not inhibited by the drug. Patients will need to follow strict allergen avoidance to resolve this component of their disease; failure to improve might suggest the result was a nonrelevant positive.
If patch testing is negative, an alternative cause for residual disease must be sought. We do not recommend stopping dupilumab prior to patch testing to avoid a disease flare from AD or possible TH2-mediated ACD.
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020:83;137-139. doi:10.1111/cod.13545
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701. doi:10.1111/dth.12701
- Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317. doi:10.1016/j.jaad.2016.03.010
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Suresh R, Murase JE. The role of expanded series patch testing in identifying causality of residual facial dermatitis following initiation of dupilumab therapy. JAAD Case Rep. 2018;4:899-904. doi:10.1016/j.jdcr.2018.08.027
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162. doi:10.1016/j.jaad.2019.03.020
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Hoot JW, Douglas JD, Falo LD Jr. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164. doi:10.1097/DER.0000000000000357
- Crepy M-N, Nosbaum A, Bensefa-Colas L. Blocking type 2 inflammation by dupilumab does not control classic (type 1-driven) allergic contact dermatitis in chronic hand eczema. Contact Dermatitis. 2019;81:145-147. doi:10.1111/cod.13266
- Raffi J, Chen R, Botto N. Wide dye reactors. JAAD Case Rep. 2019;5:877-879. doi:10.1016/j.jdcr.2019.08.005
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Raffi J, Suresh R, Fishman H, et al. Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD). Int J Womens Dermatol. 2019;5:308-313. doi:10.1016/j.ijwd.2019.10.001
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
- Chipalkatti N, Lee N, Zancanaro P, et al. Dupilumab as a treatment for allergic contact dermatitis. Dermatitis. 2018;29:347-348. doi:10.1097/DER.0000000000000414
- Jacob SE, Sung CT, Machler BC. Dupilumab for systemic allergy syndrome with dermatitis. Dermatitis. 2019;30:164-167. doi:10.1097/DER.0000000000000446
- Joshi SR, Khan DA. Effective use of dupilumab in managing systemic allergic contact dermatitis. Dermatitis. 2018;29:282-284. doi:10.1097/DER.0000000000000409
- Ruge IF, Skov L, Zachariae C, et al. Dupilumab treatment in two patients with severe allergic contact dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2020:83;137-139. doi:10.1111/cod.13545
- Goldminz AM, Scheinman PL. A case series of dupilumab-treated allergic contact dermatitis patients. Dermatol Ther. 2018;31:e12701. doi:10.1111/dth.12701
- Kohli N, Nedorost S. Inflamed skin predisposes to sensitization to less potent allergens. J Am Acad Dermatol. 2016;75:312-317. doi:10.1016/j.jaad.2016.03.010
- Raffi J, Suresh R, Botto N, et al. The impact of dupilumab on patch testing and the prevalence of comorbid allergic contact dermatitis in recalcitrant atopic dermatitis: a retrospective chart review. J Am Acad Dermatol. 2020;82:132-138. doi:10.1016/j.jaad.2019.09.028
- Puza CJ, Atwater AR. Positive patch test reaction in a patient taking dupilumab. Dermatitis. 2018;29:89. doi:10.1097/DER.0000000000000346
- Suresh R, Murase JE. The role of expanded series patch testing in identifying causality of residual facial dermatitis following initiation of dupilumab therapy. JAAD Case Rep. 2018;4:899-904. doi:10.1016/j.jdcr.2018.08.027
- Stout M, Silverberg JI. Variable impact of dupilumab on patch testing results and allergic contact dermatitis in adults with atopic dermatitis. J Am Acad Dermatol. 2019;81:157-162. doi:10.1016/j.jaad.2019.03.020
- Raffi J, Botto N. Patch testing and allergen-specific inhibition in a patient taking dupilumab. JAMA Dermatol. 2019;155:120-121. doi:10.1001/jamadermatol.2018.4098
- Hoot JW, Douglas JD, Falo LD Jr. Patch testing in a patient on dupilumab. Dermatitis. 2018;29:164. doi:10.1097/DER.0000000000000357
- Crepy M-N, Nosbaum A, Bensefa-Colas L. Blocking type 2 inflammation by dupilumab does not control classic (type 1-driven) allergic contact dermatitis in chronic hand eczema. Contact Dermatitis. 2019;81:145-147. doi:10.1111/cod.13266
- Raffi J, Chen R, Botto N. Wide dye reactors. JAAD Case Rep. 2019;5:877-879. doi:10.1016/j.jdcr.2019.08.005
- Koblinski JE, Hamann D. Mixed occupational and iatrogenic allergic contact dermatitis in a hairdresser. Occup Med (Lond). 2020;70:523-526. doi:10.1093/occmed/kqaa152
- Raffi J, Suresh R, Fishman H, et al. Investigating the role of allergic contact dermatitis in residual ocular surface disease on dupilumab (ROSDD). Int J Womens Dermatol. 2019;5:308-313. doi:10.1016/j.ijwd.2019.10.001
- Zhu GA, Chen JK, Chiou A, et al. Repeat patch testing in a patient with allergic contact dermatitis improved on dupilumab. JAAD Case Rep. 2019;5:336-338. doi:10.1016/j.jdcr.2019.01.023
Practice Points
- Allergic contact dermatitis is an important diagnostic consideration in patients with refractory or persistent dermatitis.
- Patch testing is important to help determine a possible allergic contactant, but there is confusion about its accuracy in patients taking dupilumab.
- Patients with residual dermatitis while on dupilumab are likely to benefit from patch testing.
Wet Your Whistles: Alcohol-Induced Flushing With Use of Topical Calcineurin Inhibitors
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.
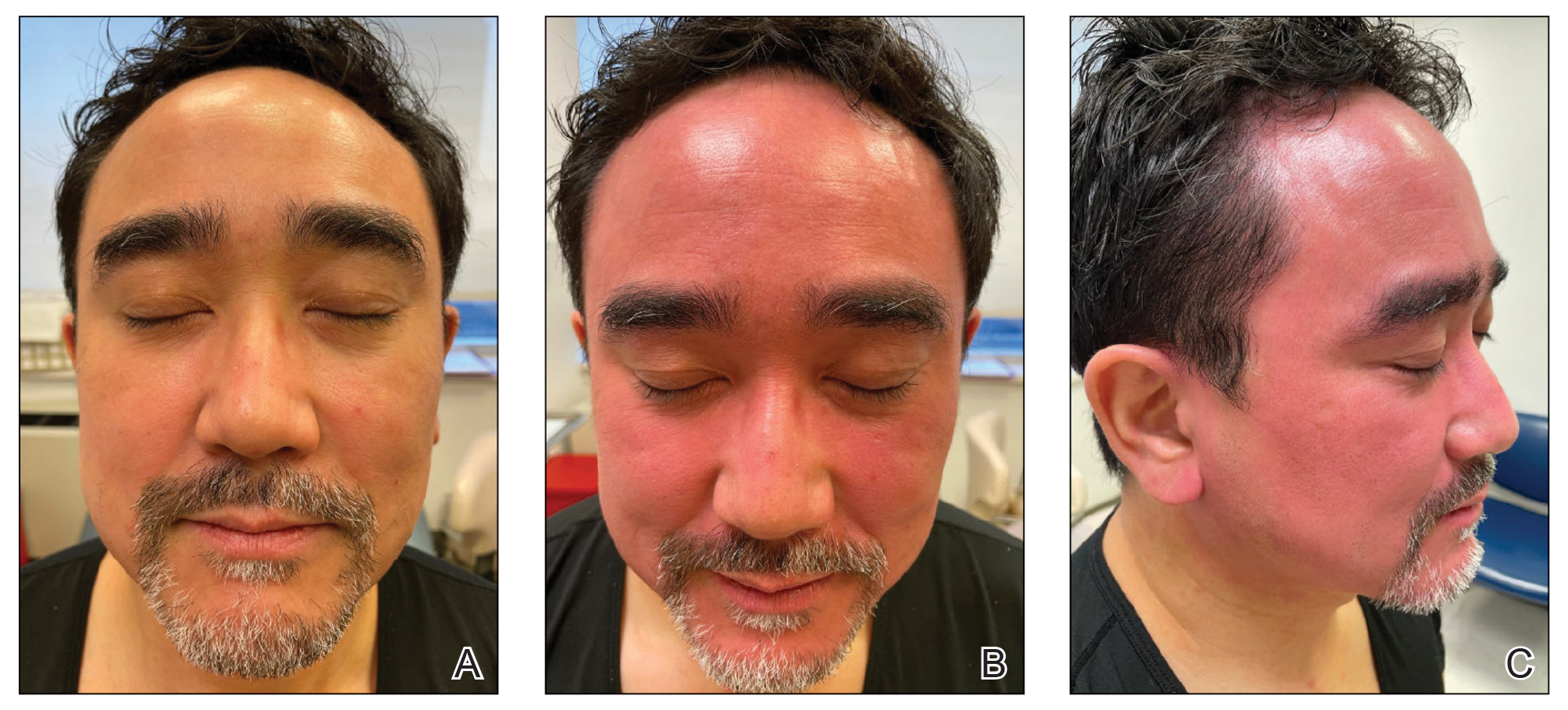
Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.
Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.

Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.

Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
Practice Gap
The topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are US Food and Drug Administration approved for the treatment of atopic dermatitis.1 In addition, these 2 drugs are utilized off label for many other dermatologic conditions, including vitiligo, psoriasis, and periorificial dermatitis. They can be used safely for prolonged periods and on sensitive areas, including the face.
Treatment with a TCI provides advantages over topical steroids, which can cause atrophy, telangiectasia, dyspigmentation, ocular hypertension, cataracts, and tachyphylaxis after prolonged use. Adverse events resulting from use of a TCI most commonly include transient burning, warmth, and erythema in areas of application. Patients typically acclimate to these effects after a few consecutive days of use.
Localized flushing after alcohol ingestion is a known potential side effect of TCIs1; however, this association may be underappreciated and underreported to patients.
Counseling Patients Taking TCIs
Topical calcineurin inhibitors cause alcohol-induced flushing on areas of application (Figures 1 and 2) in approximately 3.4% to 6.9% of patients.1 The reaction has been reported with both topical TCIs but more often is noted with tacrolimus.2,3 Typically, flushing begins 2 to 4 weeks after treatment is initiated and within 5 to 20 minutes after alcohol intake.4 The phenomenon is self-limited; erythema typically resolves in 20 to 60 minutes.

Topical calcineurin inhibitors are hypothesized to cause alcohol-induced flushing by locally inhibiting acetaldehyde dehydrogenase, an enzyme necessary for alcohol metabolism. This leads to accumulation of acetaldehyde, a by-product of alcohol metabolism, which indirectly causes concentrated vasodilation by means of prostaglandins, histamines, and other vasodilatory mediators. The combination of ethanol and a TCI also might induce release of neuropeptides, which could cause vasodilation.4
Alcohol-related flushing commonly is seen among individuals who are aldehyde dehydrogenase 2 (ALDH2) deficient; it is sometimes accompanied by nausea, headache, and tachycardia. The same pathway is implicated in disulfiram reactions, to a more intense and systemic degree, to discourage alcohol intake.
Oral calcineurin inhibitors are not reported to cause generalized flushing, perhaps because of differences in the relative dose. For example, topical tacrolimus 0.1% is 1 mg/g that is applied to a relatively small body surface area; oral calcineurin inhibitors are dosed at a range of 1 to 15 mg for an entire person.
Notably, erythema that develops after alcohol intake in a patient taking a topical TCI can mimic the dermatosis being treated—similar to one of our patients (Figure 2) whose flushing was mistaken for a flare of periorificial dermatitis—contact dermatitis or another flushing disorder such as rosacea. Uninformed patients might mistakenly self-diagnose the flushing as an allergic or anaphylactic reaction to foods, drugs, or other exposures contemporaneous with alcohol ingestion. The side effect can be frustrating owing to its appearance and discomfort, which often coincide with social interactions involving alcohol.

Techniques to Avoid Flushing
Discontinuing a TCI altogether leads to resolution of associated adverse effects, including flushing, typically within weeks to 1 month.5 Alternatively, oral aspirin (81 mg) might eliminate or attenuate symptoms, as documented in a double-blind, controlled trial in which relief of TCI-induced flushing after consuming wine was investigated.6
Another approach (albeit nontraditional) is for patients who experience this phenomenon to “wet their whistles” with an alcoholic drink before a social engagement. After flushing resolves in 20 to 60 minutes, subsequent drinks do not appear to elicit symptoms again in most patients. That said, we stop short of calling this tip “doctor’s orders.”
Practical Implication
Counseling patients who will be using a TCI—tacrolimus or pimecrolimus—about the potential for these drugs to produce localized flushing after alcohol ingestion as well as techniques for lessening or eliminating this adverse effect are important facets of their dermatologic care.
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
- Soter NA, Fleischer AB Jr, Webster GF, et al. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol. 2001;44(suppl 1):S39-S46. doi:10.1067/mjd.2001.109817
- Milingou M, Antille C, Sorg O, et al. Alcohol intolerance and facial flushing in patients treated with topical tacrolimus. Arch Dermatol. 2004;140:1542-1544. doi:10.1001/archderm.140.12.1542-b
- Sabater-Abad J, Matellanes-Palacios M, Millán Parrilla F. Image gallery: interaction between alcohol and topical tacrolimus as a cause of facial flushing. Br J Dermatol. 2019;180:E144. doi:10.1111/bjd.17611
- Stinco G, Piccirillo F, Sallustio M, et al. Facial flush reaction after alcohol ingestion during topical pimecrolimus and tacrolimus treatment. Dermatology. 2009;218:71-72. doi:10.1159/000161123
- Lübbe J, Milingou M. Images in clinical medicine. tacrolimus ointment, alcohol, and facial flushing. N Engl J Med. 2004;351:2740. doi:10.1056/NEJMicm040139
- Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140:1014-1015. doi:10.1001/archderm.140.8.1014
Oral Lichen Planus Treated With Plasma Rich in Growth Factors
Lichen planus is a chronic inflammatory mucocutaneous disease that usually affects the skin and/or the genital and oral mucosae.1,2 This disease classically presents with clinical relapses or outbreaks that alternate with periods of remission or latency. Oral lichen planus (OLP) can present with or without extraoral manifestation. It sometimes is difficult to differentiate OLP from oral lichenoid reactions, which can be related to dental materials, some drugs, and systemic conditions or can be idiopathic.1,2
Oral lichen planus is one of the most common noninfectious diseases of the oral cavity, with a reported prevalence of 1% worldwide and marked geographical differences. In Europe, the prevalence of OLP ranges from 1% to 2%.3,4 It is more frequent in women (1.5:1 to 2:1) and usually appears in the fourth and fifth decades of life.1-4
The causes of OLP have not been entirely elucidated, but it is broadly accepted that there is a deregulation on different T lymphocytes that in turn causes effects on CD8 lymphocytes in response to an external noxa. This unknown “trigger” or starting factor also produces an impact on basal keratinocytes. Therefore, the pathogenesis of lichen planus is influenced by a series of cellular events mediated by different cytokines.2,5,6 Among these, tumor necrosis factor α and IL-1 are known to have important roles in the disease. More recently, other cytokines, such as IL-4, secreted by type 2 helper T cells, also have been related to the development and progression of the oral lesions.5,6 In addition to the factors that generate the onset of the disease, there are others that may precipitate clinical outbreaks. Different factors have been related to the progression of the disease, influencing the initiation, perpetuation, and/or worsening of OLP lesions.1,2 Exactly how these factors affect disease progression is another challenging question. The list of possible or potential factors related to disease progression is long; nonetheless, in the vast majority, a clear explanation at a molecular level has not been clearly demonstrated.2,5
Conventionally, 6 clinical presentations of OLP lesions divided into 2 main groups have been described in the oral cavity: white forms (reticular, papular, and plaquelike) and red forms (erythematous, atrophic-erosive, and bullous).1,7-9
Oral lichen planus mainly is treated with topically or systemically administered steroids based on the presence of symptoms such as pain and inability to perform daily activities (eg, eating, talking).5,10 The treatment of choice often is based on the professional’s experience, as there are no broadly accepted national or international clinical practice guidelines on steroid type, administration route, dose, vehicle for administration, or maintenance.11 Despite this lack of unified criteria, different topical and systemic steroid administration protocols allow a reduction in the symptoms or even the disappearance of the red lesions to be achieved in many cases. Unfortunately, there are many patients with lesions refractory to standard treatments for OLP.12 Several alternatives for these patients have been described in the literature, though on many occasions these alternatives present substantial side effects for the patient.13 The search for an effective treatment without side effects is still challenging. One of the treatments tested under this premise has been the application of plasma rich in growth factors (PRGF) by means of infiltration or topical application, in both cases obtaining good results without side effects.14
We sought to analyze the information from a case series of patients treated at the Eduardo Anitua Clinic (Vitoria-Gasteiz, Spain) and describe the results and follow-up of patients with erosive OLP refractory to standard therapy who have been successfully treated by local infiltration of PRGF as the only treatment.
Material and Methods
Patients—We included data from the database of the clinical center with de-identified information of patients with erosive OLP diagnosed clinically and histopathologically who did not respond to conventional treatment (ie, topical and/or systemic corticosteroids [depending on the case]) as well as patients who presented with extensive erosive OLP with systemic involvement and whose systemic treatment was not effective in resolving oral manifestations.
Therapies Administered and Evaluations—Lesions refractory to conventional corticosteroid protocols had been previously treated for 30 days with 0.5% triamcinolone acetonide mouth rinse followed by a cycle of 1% triamcinolone acetonide mouth rinse. Subsequently, a cycle of oral corticosteroids (prednisone for 30 days: 1 mg/kg/d in a single morning dose with staged reduction after the first week) had been administered. One dayafter the corticosteroid treatment was suspended, the patients were treated by PRGF-Endoret (BTI Biotechnology Institute) infiltration following the protocol described by Anitua et al.15,16
Before starting the infiltrations with PRGF, the patient had been asked to rate the pain level on a visual analog scale (VAS) of 1 to 10, with 10 being the most intense imaginable pain. Pain score was subsequently rated and registered during every visit. An initial photograph of the lesion also was obtained to establish a starting point for further comparisons of clinical evolution of the lesions.
Prior to each infiltration, the plasma was separated into 2 fractions. The second fraction was the one that corresponded to the highest number of platelets and included the 2 mL of plasma just above the white series (or buffy coat). This fraction of plasma was the one used to infiltrate the lesions.
Plasma rich in growth factors was activated just before infiltration. The activation was done by adding 10% calcium chloride. Once activated, it was infiltrated into the active lesion using a 31-G × 1/6-in hypodermic needle and a 2-mL Luer-lock syringe. Infiltrations were performed without anesthesia. Four punctures were made for each ulcerative lesion, dividing the lesion into 4 points: upper, lower, right, and left. Plasma rich in growth factors was infiltrated until a slight blanching was observed in the surrounding tissue. At that moment, the infiltration was stopped and was carried out in the next infiltration site.
One treatment session was performed per week, with follow-up 1 week after treatment. In the control visit, the state of the lesions was re-evaluated, and it was decided whether new infiltrations were needed. The treatment was finished when complete epithelialization of the lesion was visualized or the associated symptoms disappeared. At each visit, photographs were taken, and the patient assessed the severity of pain on the VAS.
Statistical Analysis—A Shapiro-Wilk test was carried out with the obtained data to check the normal distribution of the sample. The evolution of pain during the study was compared by paired t test. The qualitative variables were described by means of a frequency analysis. Quantitative variables were described by the mean and the SD. The data were analyzed with SPSS V15.0 for Windows (SPSS Inc). P<.05 showed statistical significance.
Results
A total of 15 patients were included in the study, all with atrophic-erosive lichen planus. Two patients were male, and 13 were female. The mean age (SD) of the patients included in the study was 55.27 (14.19) years. The mean number of outbreaks per year (SD) was 3.2 (1.7), with a range of 1 to 8 outbreaks.
Healing of OLP Lesions—The number of treatment sessions to achieve complete healing varied among the patients (Figures 1 and 2). Ten patients (66.7%) required a single session, 2 patients (13.3%) required 2 sessions, and 3 patients (20%) required 3 sessions. The mean time (SD) without lesions for the patients who required a single session was 10.9 (5.2) months (range, 6–24 months).
Pain Assessment—The mean (SD) score obtained on the VAS before treatment with PRGF was 8.27 (1.16); this score dropped to 1.27 (1.53) after the first treatment session and was a statistically significant difference (P=.006).
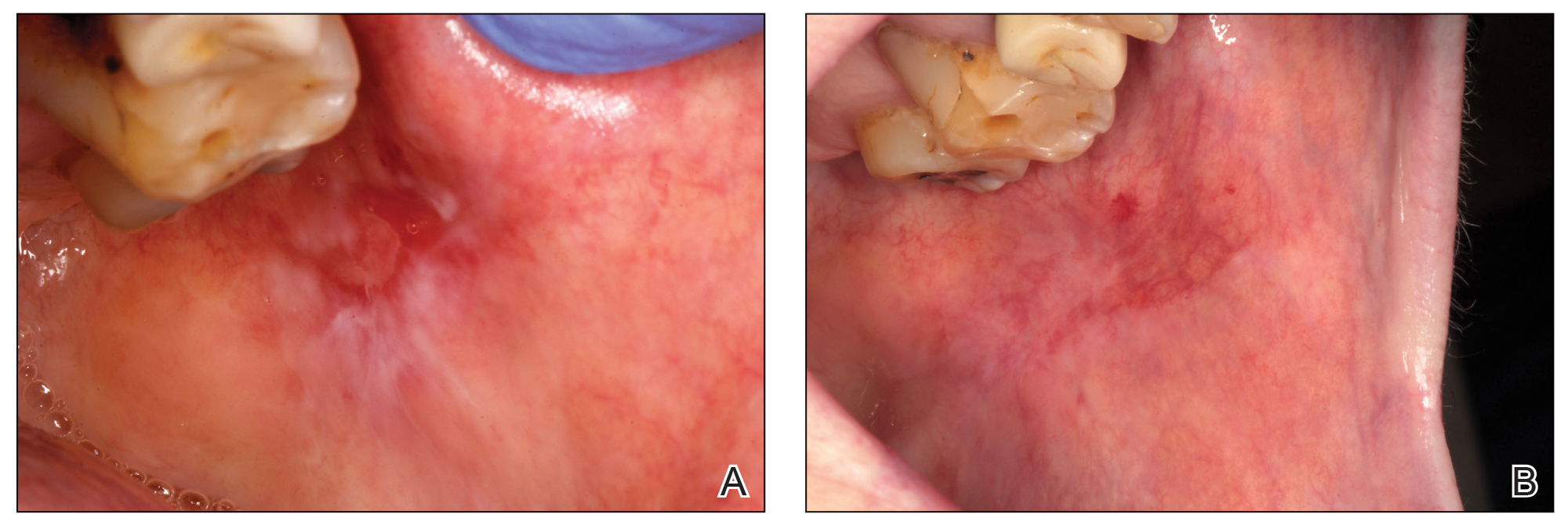
For those patients requiring more than 1 session, the mean (SD) pain scores decreased by 0.75 (0.97) points and 0 points after the first and second sessions of treatment, respectively. The mean (SD) amount of PRGF infiltrated in each patient in the first session was 2.60 (0.63) mL. In the second session, the mean (SD) amount was 1.2 (0.33) mL; these differences were statistically significant (P=.008). In the last session, the mean (SD) amount was 1.1 (0.22) mL.
Follow-up and Adverse Effects—The mean (SD) follow-up time was 47.16 (15.78) months. The patients were free of symptoms, and there were no adverse effects derived from the treatment during follow-up.
Comment
The primary goal of OLP treatment is to stop the outbreaks.1,9,13 The lack of potency of corticosteroids in some patients with OLP could be due in part to the inadequate selection of the vehicle (ointment/oral rinse) for the extension and characteristics of the lesion or because of an inappropriate prescription dose, time, and/or frequency, as described by González-Moles.17 However, even when using an appropriate protocol, some lesions are resistant to topical treatment and require other therapeutic modalities.1,9,13 Previously proposed topical treatments include different immunosuppressants, such as the mammalian target of rapamycin, tacrolimus ointment 0.1%, pimecrolimus cream 1%, or cyclosporine A (50–100 mg/mL) formulations.18 Nevertheless, these drugs seem to have a greater number of side effects than topical steroids, and tacrolimus has been associated with cases of oral malignancy after continuing treatment.15
Severe and/or recalcitrant lesions and extraoral involvement have been successfully treated with systemic prednisone (40–80 mg/d).1,9,13 Nevertheless, systemic corticosteroid toxicity requires that these treatments should be used only when necessary at the lowest possible dose and for the shortest possible duration.19 Other nonpharmacologic options for treatment are photodynamic, UV, and low-level laser therapy.20,21 They have been accepted as supplementary modalities in different inflammatory skin conditions but present important technical requirements. Their effectiveness in corticosteroid-resistant cases have not been definitively assessed. Interestingly, promising results recently have been reported by Bennardo et al22 when comparing the efficacy of autologous platelet concentrates with triamcinolone injection.
In our study, the use of PRGF stopped the lesions’ evolution since the first treatment session, reducing them by 6.5-fold. The positive effects observed may have been promoted by the activity of different proteins present in PRGF (eg, platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor, epidermal growth factor, fibroblast growth factor, fibronectin). These molecules contribute to collagen synthesis; angiogenesis; endothelial cell migration and proliferation; or keratinocyte cell migration, proliferation, differentiation, growth, and migration—phenomena that are essential for healing and re-epithelialization.23-25
Different studies also have supported an anti-inflammatory effect of PRGF mediated by an inhibition of the transcription of nuclear factor–κB and the expression of cyclooxygenase-2 and chemokine receptor type 4 produced by its high content of hepatocyte growth factor or the reduction of inflammatory marker expression, such as intercellular adhesion molecule 1. The development of an efficient 3-dimensional fibrin scaffold formation that occurs after PRGF administration also could facilitate healing, helping some cell populations to guide their position and function.23-25
Limitations of our study include the small number of patients and the absence of a control group. The higher number of female patients in the study did not seem to affect the results, as differences related to gender have not been reported when treating patients with OLP with autologous platelet concentrates or other modalities of treatment.
Conclusion
Results from our study indicate that the use of PRGF could be a new treatment option for OLP cases refractory to conventional therapy. No complications were observed during the treatment procedure or during the complete follow-up period. Nonetheless, new prospective studies with a greater number of patients and longer follow-up periods are needed to confirm these preliminary results.
- Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:1-12.
- Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72-80.
- McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447-453.
- González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27:813-828.
- Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19:1161-1167.
- Mehrbani SP, Motahari P, Azar FP, et al. Role of interleukin-4 in pathogenesis of oral lichen planus: a systematic review. Med Oral Patol Oral Cir Bucal. 2020;25:E410-E415.
- Edwards PC, Kelsch R. Oral lichen planus: clinical presentation and management. J Can Dent Assoc. 2002;68:494-499.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826.
- Babu A, Chellaswamy S, Muthukumar S, et al. Bullous lichen planus: case report and review. J Pharm Bioallied Sci. 2019;11(suppl 2):S499-S506.
- Thongprasom K, Carrozzo M, Furness S, et al. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011;7:CD001168.
- López-Jornet P, Martínez-Beneyto Y, Nicolás AV, et al. Professional attitudes toward oral lichen planus: need for national and international guidelines. J Eval Clin Pract. 2009;15:541-542.
- Yang H, Wu Y, Jiang L, et al. Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:496-509.
- Ribero S, Borradori L. Re: risk of malignancy and systemic absorption after application of topical tacrolimus in oral lichen planus. J Eur Acad Dermatol Venereol. 2017;31:E85-E86.
- Piñas L, Alkhraisat MH, Fernández RS, et al. Biological therapy of refractory ulcerative oral lichen planus with plasma rich in growth factors. Am J Clin Dermatol. 2017;18:429-433.
- Anitua E, Zalduendo MM, Prado R, et al. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103:1011-1020.
- Anitua E, Prado R, Sánchez M, et al. Platelet-rich plasma: preparation and formulation. Oper Tech Orthop. 2012;22:25-32.
- González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Pathol Oral Cir Bucal. 2010;15:E827-E831.
- Siponen M, Huuskonen L, Kallio-Pulkkinen S, et al. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: a pilot randomized controlled trial. Oral Dis. 2017;23:660-668.
- Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388-393.
- Lavaee F, Shadmanpour M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019;25:1954-1963.
- Derikvand N, Ghasemi SS, Moharami M, et al. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci. 2017;8:150-154.
- Bennardo F, Liborio F, Barone S, et al. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin Oral Investig. 2021;25:3747-3755.
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15.
- Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569-578.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529-535.
Lichen planus is a chronic inflammatory mucocutaneous disease that usually affects the skin and/or the genital and oral mucosae.1,2 This disease classically presents with clinical relapses or outbreaks that alternate with periods of remission or latency. Oral lichen planus (OLP) can present with or without extraoral manifestation. It sometimes is difficult to differentiate OLP from oral lichenoid reactions, which can be related to dental materials, some drugs, and systemic conditions or can be idiopathic.1,2
Oral lichen planus is one of the most common noninfectious diseases of the oral cavity, with a reported prevalence of 1% worldwide and marked geographical differences. In Europe, the prevalence of OLP ranges from 1% to 2%.3,4 It is more frequent in women (1.5:1 to 2:1) and usually appears in the fourth and fifth decades of life.1-4
The causes of OLP have not been entirely elucidated, but it is broadly accepted that there is a deregulation on different T lymphocytes that in turn causes effects on CD8 lymphocytes in response to an external noxa. This unknown “trigger” or starting factor also produces an impact on basal keratinocytes. Therefore, the pathogenesis of lichen planus is influenced by a series of cellular events mediated by different cytokines.2,5,6 Among these, tumor necrosis factor α and IL-1 are known to have important roles in the disease. More recently, other cytokines, such as IL-4, secreted by type 2 helper T cells, also have been related to the development and progression of the oral lesions.5,6 In addition to the factors that generate the onset of the disease, there are others that may precipitate clinical outbreaks. Different factors have been related to the progression of the disease, influencing the initiation, perpetuation, and/or worsening of OLP lesions.1,2 Exactly how these factors affect disease progression is another challenging question. The list of possible or potential factors related to disease progression is long; nonetheless, in the vast majority, a clear explanation at a molecular level has not been clearly demonstrated.2,5
Conventionally, 6 clinical presentations of OLP lesions divided into 2 main groups have been described in the oral cavity: white forms (reticular, papular, and plaquelike) and red forms (erythematous, atrophic-erosive, and bullous).1,7-9
Oral lichen planus mainly is treated with topically or systemically administered steroids based on the presence of symptoms such as pain and inability to perform daily activities (eg, eating, talking).5,10 The treatment of choice often is based on the professional’s experience, as there are no broadly accepted national or international clinical practice guidelines on steroid type, administration route, dose, vehicle for administration, or maintenance.11 Despite this lack of unified criteria, different topical and systemic steroid administration protocols allow a reduction in the symptoms or even the disappearance of the red lesions to be achieved in many cases. Unfortunately, there are many patients with lesions refractory to standard treatments for OLP.12 Several alternatives for these patients have been described in the literature, though on many occasions these alternatives present substantial side effects for the patient.13 The search for an effective treatment without side effects is still challenging. One of the treatments tested under this premise has been the application of plasma rich in growth factors (PRGF) by means of infiltration or topical application, in both cases obtaining good results without side effects.14
We sought to analyze the information from a case series of patients treated at the Eduardo Anitua Clinic (Vitoria-Gasteiz, Spain) and describe the results and follow-up of patients with erosive OLP refractory to standard therapy who have been successfully treated by local infiltration of PRGF as the only treatment.
Material and Methods
Patients—We included data from the database of the clinical center with de-identified information of patients with erosive OLP diagnosed clinically and histopathologically who did not respond to conventional treatment (ie, topical and/or systemic corticosteroids [depending on the case]) as well as patients who presented with extensive erosive OLP with systemic involvement and whose systemic treatment was not effective in resolving oral manifestations.
Therapies Administered and Evaluations—Lesions refractory to conventional corticosteroid protocols had been previously treated for 30 days with 0.5% triamcinolone acetonide mouth rinse followed by a cycle of 1% triamcinolone acetonide mouth rinse. Subsequently, a cycle of oral corticosteroids (prednisone for 30 days: 1 mg/kg/d in a single morning dose with staged reduction after the first week) had been administered. One dayafter the corticosteroid treatment was suspended, the patients were treated by PRGF-Endoret (BTI Biotechnology Institute) infiltration following the protocol described by Anitua et al.15,16
Before starting the infiltrations with PRGF, the patient had been asked to rate the pain level on a visual analog scale (VAS) of 1 to 10, with 10 being the most intense imaginable pain. Pain score was subsequently rated and registered during every visit. An initial photograph of the lesion also was obtained to establish a starting point for further comparisons of clinical evolution of the lesions.
Prior to each infiltration, the plasma was separated into 2 fractions. The second fraction was the one that corresponded to the highest number of platelets and included the 2 mL of plasma just above the white series (or buffy coat). This fraction of plasma was the one used to infiltrate the lesions.
Plasma rich in growth factors was activated just before infiltration. The activation was done by adding 10% calcium chloride. Once activated, it was infiltrated into the active lesion using a 31-G × 1/6-in hypodermic needle and a 2-mL Luer-lock syringe. Infiltrations were performed without anesthesia. Four punctures were made for each ulcerative lesion, dividing the lesion into 4 points: upper, lower, right, and left. Plasma rich in growth factors was infiltrated until a slight blanching was observed in the surrounding tissue. At that moment, the infiltration was stopped and was carried out in the next infiltration site.
One treatment session was performed per week, with follow-up 1 week after treatment. In the control visit, the state of the lesions was re-evaluated, and it was decided whether new infiltrations were needed. The treatment was finished when complete epithelialization of the lesion was visualized or the associated symptoms disappeared. At each visit, photographs were taken, and the patient assessed the severity of pain on the VAS.
Statistical Analysis—A Shapiro-Wilk test was carried out with the obtained data to check the normal distribution of the sample. The evolution of pain during the study was compared by paired t test. The qualitative variables were described by means of a frequency analysis. Quantitative variables were described by the mean and the SD. The data were analyzed with SPSS V15.0 for Windows (SPSS Inc). P<.05 showed statistical significance.
Results
A total of 15 patients were included in the study, all with atrophic-erosive lichen planus. Two patients were male, and 13 were female. The mean age (SD) of the patients included in the study was 55.27 (14.19) years. The mean number of outbreaks per year (SD) was 3.2 (1.7), with a range of 1 to 8 outbreaks.
Healing of OLP Lesions—The number of treatment sessions to achieve complete healing varied among the patients (Figures 1 and 2). Ten patients (66.7%) required a single session, 2 patients (13.3%) required 2 sessions, and 3 patients (20%) required 3 sessions. The mean time (SD) without lesions for the patients who required a single session was 10.9 (5.2) months (range, 6–24 months).

Pain Assessment—The mean (SD) score obtained on the VAS before treatment with PRGF was 8.27 (1.16); this score dropped to 1.27 (1.53) after the first treatment session and was a statistically significant difference (P=.006).

For those patients requiring more than 1 session, the mean (SD) pain scores decreased by 0.75 (0.97) points and 0 points after the first and second sessions of treatment, respectively. The mean (SD) amount of PRGF infiltrated in each patient in the first session was 2.60 (0.63) mL. In the second session, the mean (SD) amount was 1.2 (0.33) mL; these differences were statistically significant (P=.008). In the last session, the mean (SD) amount was 1.1 (0.22) mL.
Follow-up and Adverse Effects—The mean (SD) follow-up time was 47.16 (15.78) months. The patients were free of symptoms, and there were no adverse effects derived from the treatment during follow-up.
Comment
The primary goal of OLP treatment is to stop the outbreaks.1,9,13 The lack of potency of corticosteroids in some patients with OLP could be due in part to the inadequate selection of the vehicle (ointment/oral rinse) for the extension and characteristics of the lesion or because of an inappropriate prescription dose, time, and/or frequency, as described by González-Moles.17 However, even when using an appropriate protocol, some lesions are resistant to topical treatment and require other therapeutic modalities.1,9,13 Previously proposed topical treatments include different immunosuppressants, such as the mammalian target of rapamycin, tacrolimus ointment 0.1%, pimecrolimus cream 1%, or cyclosporine A (50–100 mg/mL) formulations.18 Nevertheless, these drugs seem to have a greater number of side effects than topical steroids, and tacrolimus has been associated with cases of oral malignancy after continuing treatment.15
Severe and/or recalcitrant lesions and extraoral involvement have been successfully treated with systemic prednisone (40–80 mg/d).1,9,13 Nevertheless, systemic corticosteroid toxicity requires that these treatments should be used only when necessary at the lowest possible dose and for the shortest possible duration.19 Other nonpharmacologic options for treatment are photodynamic, UV, and low-level laser therapy.20,21 They have been accepted as supplementary modalities in different inflammatory skin conditions but present important technical requirements. Their effectiveness in corticosteroid-resistant cases have not been definitively assessed. Interestingly, promising results recently have been reported by Bennardo et al22 when comparing the efficacy of autologous platelet concentrates with triamcinolone injection.
In our study, the use of PRGF stopped the lesions’ evolution since the first treatment session, reducing them by 6.5-fold. The positive effects observed may have been promoted by the activity of different proteins present in PRGF (eg, platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor, epidermal growth factor, fibroblast growth factor, fibronectin). These molecules contribute to collagen synthesis; angiogenesis; endothelial cell migration and proliferation; or keratinocyte cell migration, proliferation, differentiation, growth, and migration—phenomena that are essential for healing and re-epithelialization.23-25
Different studies also have supported an anti-inflammatory effect of PRGF mediated by an inhibition of the transcription of nuclear factor–κB and the expression of cyclooxygenase-2 and chemokine receptor type 4 produced by its high content of hepatocyte growth factor or the reduction of inflammatory marker expression, such as intercellular adhesion molecule 1. The development of an efficient 3-dimensional fibrin scaffold formation that occurs after PRGF administration also could facilitate healing, helping some cell populations to guide their position and function.23-25
Limitations of our study include the small number of patients and the absence of a control group. The higher number of female patients in the study did not seem to affect the results, as differences related to gender have not been reported when treating patients with OLP with autologous platelet concentrates or other modalities of treatment.
Conclusion
Results from our study indicate that the use of PRGF could be a new treatment option for OLP cases refractory to conventional therapy. No complications were observed during the treatment procedure or during the complete follow-up period. Nonetheless, new prospective studies with a greater number of patients and longer follow-up periods are needed to confirm these preliminary results.
Lichen planus is a chronic inflammatory mucocutaneous disease that usually affects the skin and/or the genital and oral mucosae.1,2 This disease classically presents with clinical relapses or outbreaks that alternate with periods of remission or latency. Oral lichen planus (OLP) can present with or without extraoral manifestation. It sometimes is difficult to differentiate OLP from oral lichenoid reactions, which can be related to dental materials, some drugs, and systemic conditions or can be idiopathic.1,2
Oral lichen planus is one of the most common noninfectious diseases of the oral cavity, with a reported prevalence of 1% worldwide and marked geographical differences. In Europe, the prevalence of OLP ranges from 1% to 2%.3,4 It is more frequent in women (1.5:1 to 2:1) and usually appears in the fourth and fifth decades of life.1-4
The causes of OLP have not been entirely elucidated, but it is broadly accepted that there is a deregulation on different T lymphocytes that in turn causes effects on CD8 lymphocytes in response to an external noxa. This unknown “trigger” or starting factor also produces an impact on basal keratinocytes. Therefore, the pathogenesis of lichen planus is influenced by a series of cellular events mediated by different cytokines.2,5,6 Among these, tumor necrosis factor α and IL-1 are known to have important roles in the disease. More recently, other cytokines, such as IL-4, secreted by type 2 helper T cells, also have been related to the development and progression of the oral lesions.5,6 In addition to the factors that generate the onset of the disease, there are others that may precipitate clinical outbreaks. Different factors have been related to the progression of the disease, influencing the initiation, perpetuation, and/or worsening of OLP lesions.1,2 Exactly how these factors affect disease progression is another challenging question. The list of possible or potential factors related to disease progression is long; nonetheless, in the vast majority, a clear explanation at a molecular level has not been clearly demonstrated.2,5
Conventionally, 6 clinical presentations of OLP lesions divided into 2 main groups have been described in the oral cavity: white forms (reticular, papular, and plaquelike) and red forms (erythematous, atrophic-erosive, and bullous).1,7-9
Oral lichen planus mainly is treated with topically or systemically administered steroids based on the presence of symptoms such as pain and inability to perform daily activities (eg, eating, talking).5,10 The treatment of choice often is based on the professional’s experience, as there are no broadly accepted national or international clinical practice guidelines on steroid type, administration route, dose, vehicle for administration, or maintenance.11 Despite this lack of unified criteria, different topical and systemic steroid administration protocols allow a reduction in the symptoms or even the disappearance of the red lesions to be achieved in many cases. Unfortunately, there are many patients with lesions refractory to standard treatments for OLP.12 Several alternatives for these patients have been described in the literature, though on many occasions these alternatives present substantial side effects for the patient.13 The search for an effective treatment without side effects is still challenging. One of the treatments tested under this premise has been the application of plasma rich in growth factors (PRGF) by means of infiltration or topical application, in both cases obtaining good results without side effects.14
We sought to analyze the information from a case series of patients treated at the Eduardo Anitua Clinic (Vitoria-Gasteiz, Spain) and describe the results and follow-up of patients with erosive OLP refractory to standard therapy who have been successfully treated by local infiltration of PRGF as the only treatment.
Material and Methods
Patients—We included data from the database of the clinical center with de-identified information of patients with erosive OLP diagnosed clinically and histopathologically who did not respond to conventional treatment (ie, topical and/or systemic corticosteroids [depending on the case]) as well as patients who presented with extensive erosive OLP with systemic involvement and whose systemic treatment was not effective in resolving oral manifestations.
Therapies Administered and Evaluations—Lesions refractory to conventional corticosteroid protocols had been previously treated for 30 days with 0.5% triamcinolone acetonide mouth rinse followed by a cycle of 1% triamcinolone acetonide mouth rinse. Subsequently, a cycle of oral corticosteroids (prednisone for 30 days: 1 mg/kg/d in a single morning dose with staged reduction after the first week) had been administered. One dayafter the corticosteroid treatment was suspended, the patients were treated by PRGF-Endoret (BTI Biotechnology Institute) infiltration following the protocol described by Anitua et al.15,16
Before starting the infiltrations with PRGF, the patient had been asked to rate the pain level on a visual analog scale (VAS) of 1 to 10, with 10 being the most intense imaginable pain. Pain score was subsequently rated and registered during every visit. An initial photograph of the lesion also was obtained to establish a starting point for further comparisons of clinical evolution of the lesions.
Prior to each infiltration, the plasma was separated into 2 fractions. The second fraction was the one that corresponded to the highest number of platelets and included the 2 mL of plasma just above the white series (or buffy coat). This fraction of plasma was the one used to infiltrate the lesions.
Plasma rich in growth factors was activated just before infiltration. The activation was done by adding 10% calcium chloride. Once activated, it was infiltrated into the active lesion using a 31-G × 1/6-in hypodermic needle and a 2-mL Luer-lock syringe. Infiltrations were performed without anesthesia. Four punctures were made for each ulcerative lesion, dividing the lesion into 4 points: upper, lower, right, and left. Plasma rich in growth factors was infiltrated until a slight blanching was observed in the surrounding tissue. At that moment, the infiltration was stopped and was carried out in the next infiltration site.
One treatment session was performed per week, with follow-up 1 week after treatment. In the control visit, the state of the lesions was re-evaluated, and it was decided whether new infiltrations were needed. The treatment was finished when complete epithelialization of the lesion was visualized or the associated symptoms disappeared. At each visit, photographs were taken, and the patient assessed the severity of pain on the VAS.
Statistical Analysis—A Shapiro-Wilk test was carried out with the obtained data to check the normal distribution of the sample. The evolution of pain during the study was compared by paired t test. The qualitative variables were described by means of a frequency analysis. Quantitative variables were described by the mean and the SD. The data were analyzed with SPSS V15.0 for Windows (SPSS Inc). P<.05 showed statistical significance.
Results
A total of 15 patients were included in the study, all with atrophic-erosive lichen planus. Two patients were male, and 13 were female. The mean age (SD) of the patients included in the study was 55.27 (14.19) years. The mean number of outbreaks per year (SD) was 3.2 (1.7), with a range of 1 to 8 outbreaks.
Healing of OLP Lesions—The number of treatment sessions to achieve complete healing varied among the patients (Figures 1 and 2). Ten patients (66.7%) required a single session, 2 patients (13.3%) required 2 sessions, and 3 patients (20%) required 3 sessions. The mean time (SD) without lesions for the patients who required a single session was 10.9 (5.2) months (range, 6–24 months).

Pain Assessment—The mean (SD) score obtained on the VAS before treatment with PRGF was 8.27 (1.16); this score dropped to 1.27 (1.53) after the first treatment session and was a statistically significant difference (P=.006).

For those patients requiring more than 1 session, the mean (SD) pain scores decreased by 0.75 (0.97) points and 0 points after the first and second sessions of treatment, respectively. The mean (SD) amount of PRGF infiltrated in each patient in the first session was 2.60 (0.63) mL. In the second session, the mean (SD) amount was 1.2 (0.33) mL; these differences were statistically significant (P=.008). In the last session, the mean (SD) amount was 1.1 (0.22) mL.
Follow-up and Adverse Effects—The mean (SD) follow-up time was 47.16 (15.78) months. The patients were free of symptoms, and there were no adverse effects derived from the treatment during follow-up.
Comment
The primary goal of OLP treatment is to stop the outbreaks.1,9,13 The lack of potency of corticosteroids in some patients with OLP could be due in part to the inadequate selection of the vehicle (ointment/oral rinse) for the extension and characteristics of the lesion or because of an inappropriate prescription dose, time, and/or frequency, as described by González-Moles.17 However, even when using an appropriate protocol, some lesions are resistant to topical treatment and require other therapeutic modalities.1,9,13 Previously proposed topical treatments include different immunosuppressants, such as the mammalian target of rapamycin, tacrolimus ointment 0.1%, pimecrolimus cream 1%, or cyclosporine A (50–100 mg/mL) formulations.18 Nevertheless, these drugs seem to have a greater number of side effects than topical steroids, and tacrolimus has been associated with cases of oral malignancy after continuing treatment.15
Severe and/or recalcitrant lesions and extraoral involvement have been successfully treated with systemic prednisone (40–80 mg/d).1,9,13 Nevertheless, systemic corticosteroid toxicity requires that these treatments should be used only when necessary at the lowest possible dose and for the shortest possible duration.19 Other nonpharmacologic options for treatment are photodynamic, UV, and low-level laser therapy.20,21 They have been accepted as supplementary modalities in different inflammatory skin conditions but present important technical requirements. Their effectiveness in corticosteroid-resistant cases have not been definitively assessed. Interestingly, promising results recently have been reported by Bennardo et al22 when comparing the efficacy of autologous platelet concentrates with triamcinolone injection.
In our study, the use of PRGF stopped the lesions’ evolution since the first treatment session, reducing them by 6.5-fold. The positive effects observed may have been promoted by the activity of different proteins present in PRGF (eg, platelet-derived growth factor, vascular endothelial growth factor, transforming growth factor, epidermal growth factor, fibroblast growth factor, fibronectin). These molecules contribute to collagen synthesis; angiogenesis; endothelial cell migration and proliferation; or keratinocyte cell migration, proliferation, differentiation, growth, and migration—phenomena that are essential for healing and re-epithelialization.23-25
Different studies also have supported an anti-inflammatory effect of PRGF mediated by an inhibition of the transcription of nuclear factor–κB and the expression of cyclooxygenase-2 and chemokine receptor type 4 produced by its high content of hepatocyte growth factor or the reduction of inflammatory marker expression, such as intercellular adhesion molecule 1. The development of an efficient 3-dimensional fibrin scaffold formation that occurs after PRGF administration also could facilitate healing, helping some cell populations to guide their position and function.23-25
Limitations of our study include the small number of patients and the absence of a control group. The higher number of female patients in the study did not seem to affect the results, as differences related to gender have not been reported when treating patients with OLP with autologous platelet concentrates or other modalities of treatment.
Conclusion
Results from our study indicate that the use of PRGF could be a new treatment option for OLP cases refractory to conventional therapy. No complications were observed during the treatment procedure or during the complete follow-up period. Nonetheless, new prospective studies with a greater number of patients and longer follow-up periods are needed to confirm these preliminary results.
- Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:1-12.
- Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72-80.
- McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447-453.
- González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27:813-828.
- Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19:1161-1167.
- Mehrbani SP, Motahari P, Azar FP, et al. Role of interleukin-4 in pathogenesis of oral lichen planus: a systematic review. Med Oral Patol Oral Cir Bucal. 2020;25:E410-E415.
- Edwards PC, Kelsch R. Oral lichen planus: clinical presentation and management. J Can Dent Assoc. 2002;68:494-499.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826.
- Babu A, Chellaswamy S, Muthukumar S, et al. Bullous lichen planus: case report and review. J Pharm Bioallied Sci. 2019;11(suppl 2):S499-S506.
- Thongprasom K, Carrozzo M, Furness S, et al. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011;7:CD001168.
- López-Jornet P, Martínez-Beneyto Y, Nicolás AV, et al. Professional attitudes toward oral lichen planus: need for national and international guidelines. J Eval Clin Pract. 2009;15:541-542.
- Yang H, Wu Y, Jiang L, et al. Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:496-509.
- Ribero S, Borradori L. Re: risk of malignancy and systemic absorption after application of topical tacrolimus in oral lichen planus. J Eur Acad Dermatol Venereol. 2017;31:E85-E86.
- Piñas L, Alkhraisat MH, Fernández RS, et al. Biological therapy of refractory ulcerative oral lichen planus with plasma rich in growth factors. Am J Clin Dermatol. 2017;18:429-433.
- Anitua E, Zalduendo MM, Prado R, et al. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103:1011-1020.
- Anitua E, Prado R, Sánchez M, et al. Platelet-rich plasma: preparation and formulation. Oper Tech Orthop. 2012;22:25-32.
- González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Pathol Oral Cir Bucal. 2010;15:E827-E831.
- Siponen M, Huuskonen L, Kallio-Pulkkinen S, et al. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: a pilot randomized controlled trial. Oral Dis. 2017;23:660-668.
- Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388-393.
- Lavaee F, Shadmanpour M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019;25:1954-1963.
- Derikvand N, Ghasemi SS, Moharami M, et al. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci. 2017;8:150-154.
- Bennardo F, Liborio F, Barone S, et al. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin Oral Investig. 2021;25:3747-3755.
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15.
- Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569-578.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529-535.
- Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:1-12.
- Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:72-80.
- McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37:447-453.
- González-Moles MÁ, Warnakulasuriya S, González-Ruiz I, et al. Worldwide prevalence of oral lichen planus: a systematic review and meta-analysis. Oral Dis. 2021;27:813-828.
- Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19:1161-1167.
- Mehrbani SP, Motahari P, Azar FP, et al. Role of interleukin-4 in pathogenesis of oral lichen planus: a systematic review. Med Oral Patol Oral Cir Bucal. 2020;25:E410-E415.
- Edwards PC, Kelsch R. Oral lichen planus: clinical presentation and management. J Can Dent Assoc. 2002;68:494-499.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826.
- Babu A, Chellaswamy S, Muthukumar S, et al. Bullous lichen planus: case report and review. J Pharm Bioallied Sci. 2019;11(suppl 2):S499-S506.
- Thongprasom K, Carrozzo M, Furness S, et al. Interventions for treating oral lichen planus. Cochrane Database Syst Rev. 2011;7:CD001168.
- López-Jornet P, Martínez-Beneyto Y, Nicolás AV, et al. Professional attitudes toward oral lichen planus: need for national and international guidelines. J Eval Clin Pract. 2009;15:541-542.
- Yang H, Wu Y, Jiang L, et al. Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:496-509.
- Ribero S, Borradori L. Re: risk of malignancy and systemic absorption after application of topical tacrolimus in oral lichen planus. J Eur Acad Dermatol Venereol. 2017;31:E85-E86.
- Piñas L, Alkhraisat MH, Fernández RS, et al. Biological therapy of refractory ulcerative oral lichen planus with plasma rich in growth factors. Am J Clin Dermatol. 2017;18:429-433.
- Anitua E, Zalduendo MM, Prado R, et al. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: evaluation of the effect of leukocyte inclusion. J Biomed Mater Res A. 2015;103:1011-1020.
- Anitua E, Prado R, Sánchez M, et al. Platelet-rich plasma: preparation and formulation. Oper Tech Orthop. 2012;22:25-32.
- González-Moles MA. The use of topical corticoids in oral pathology. Med Oral Pathol Oral Cir Bucal. 2010;15:E827-E831.
- Siponen M, Huuskonen L, Kallio-Pulkkinen S, et al. Topical tacrolimus, triamcinolone acetonide, and placebo in oral lichen planus: a pilot randomized controlled trial. Oral Dis. 2017;23:660-668.
- Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol. 2019;31:388-393.
- Lavaee F, Shadmanpour M. Comparison of the effect of photodynamic therapy and topical corticosteroid on oral lichen planus lesions. Oral Dis. 2019;25:1954-1963.
- Derikvand N, Ghasemi SS, Moharami M, et al. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci. 2017;8:150-154.
- Bennardo F, Liborio F, Barone S, et al. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin Oral Investig. 2021;25:3747-3755.
- Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4-15.
- Barrientos S, Brem H, Stojadinovic O, et al. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569-578.
- Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529-535.
Practice Points
- Treating erosive oral lichen planus lesions refractory to conventional steroid treatments can be challenging for clinicians.
- Complete re-epithelialization and total pain relief could be observed after 1 to 3 weekly perilesional infiltrations with plasma rich in growth factors.
- No relapse of the lesions in the same area or other complications could be observed during the follow-up time.
Interstitial Granulomatous Dermatitis as an Adverse Reaction to Vedolizumab
The number of monoclonal antibodies developed for therapeutic use has rapidly expanded over the last decade due to their generally favorable adverse effect (AE) profiles and efficacy.1 Tumor necrosis factor α inhibitors and general integrin antagonists are well-known examples of such monoclonal antibodies. Common conditions utilizing immunotherapy include inflammatory bowel diseases (IBDs), such as Crohn disease and ulcerative colitis (UC).2
The monoclonal antibody vedolizumab, approved in 2014 for moderate to severe UC and Crohn disease, selectively antagonizes α4β7 integrin to target a specific population of gastrointestinal T lymphocytes, preventing their mobilization to areas of inflammation.3 Adverse effects in patients treated with vedolizumab occur at a rate comparable to placebo and largely are considered nonserious4,5; the most commonly reported AE is disease exacerbation (13%–17% of patients).5,6 Published reports of cutaneous AEs at administration of vedolizumab include urticaria during infusion, appearance of cutaneous manifestations characteristic of IBD, psoriasis, Henoch-Schönlein purpura, and Sweet syndrome.7-10
We present the case of a 61-year-old woman with UC who developed reactive granulomatous dermatitis (RGD), interstitial granulomatous dermatitis (IGD) type secondary to vedolizumab. This adverse reaction has not, to our knowledge, been previously reported.
Case Report
A 61-year-old woman with a medical history of UC treated with vedolizumab and myelodysplastic syndrome treated with intravenous immunoglobulin (due to hypogammaglobulinemia following allogeneic stem cell transplantation 14 months prior) presented with a concern of a rash. The patient had been in a baseline state of health until 1 week after receiving her second dose of vedolizumab, at which time she developed a mildly pruritic maculopapular rash on the back and chest. Triamcinolone ointment and hydroxyzine were recommended during an initial telehealth consultation with an oncologist with minimal improvement. The rash continued to spread distally with worsening pruritus.
The patient returned to her oncologist for a routine follow-up appointment 5 days after initial teleconsultation. She reported poor oral intake due to oropharyngeal pain and a worsening rash; her husband added a report of recent onset of somnolence. She was admitted to the hospital, and intravenous fluids were administered.
At admission, the patient was hypotensive; vital signs were otherwise normal. Physical examination revealed the oropharynx was erythematous. Pink lichenoid papules coalescing into plaques were present diffusely across the trunk, arms, and legs; the hands, feet, and face were spared (Figure 1).
A complete blood cell count and comprehensive metabolic panel were unremarkable. A lumbar puncture, chest radiograph, blood cultures, urinalysis, and urine cultures did not identify a clear infectious cause for the rash, though the workup for infection did raise concern about active cytomegalovirus (CMV) infection with colitis and pneumonitis. Computed tomography of the head showed no acute hemorrhage.
Dermatology was consulted and determined that the appearance of the rash was most consistent with a lichenoid drug eruption, likely secondary to vedolizumab that was administered 1 week before the rash onset. Analysis of a skin biopsy revealed a dense dermal histiocytic and lymphocytic infiltrate in close approximation to blood vessels, confirmed by immunohistochemical staining for CD45, CD43, CD68, CD34, c-KIT, and myeloperoxidase (Figures 2A and 2B). Colloidal iron staining of the specimen revealed no mucin (Figure 2C).
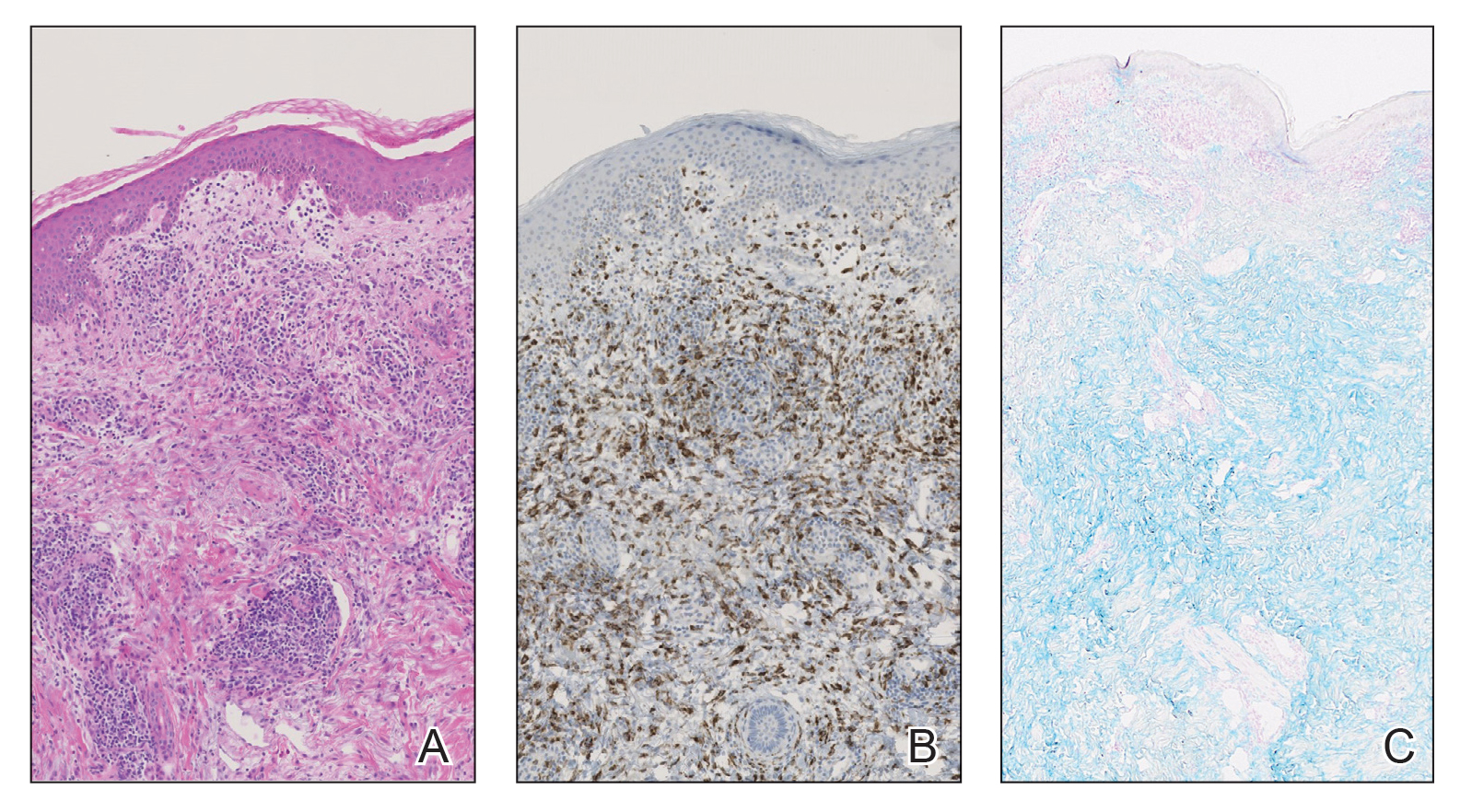
Taken together, the clinical presentation and histopathologic findings were determined to be most consistent with RGD, IGD type, with secondary vasculitis due to vedolizumab. The patient was treated with triamcinolone ointment and low-dose prednisone. Vedolizumab was discontinued. The rash resolved several weeks after cessation of vedolizumab.
Comment
This case describes the development of RGD, IGD type, as an AE of vedolizumab for the treatment of IBD. Reactive granulomatous dermatitis encompasses a spectrum of cutaneous reactions that includes the diagnosis formerly distinctly identified as IGD.11 This variety of RGD is characterized by histologic findings of heavy histiocytic inflammation in the reticular layer of the dermis with interstitial and perivascular neutrophils, lymphocytes, and histiocytes, as well as the absence of mucin. Interstitial granulomatous dermatitis–type reactions commonly are associated with autoimmune conditions and medications, with accumulating examples occurring in the setting of other biologic therapies, including the IL-6 receptor inhibitor tocilizumab; the programmed death receptor-1 inhibitor nivolumab; and the tumor necrosis factor α inhibitors infliximab, etanercept, and adalimumab.12-15
Although our patient represents CMV infection while being treated with vedolizumab, the relationship between the two is unclear. Development of CMV infection while receiving vedolizumab has been reported in the literature in a patient who was concurrently immunosuppressed with azathioprine.16 In contrast, vedolizumab administration has been utilized as a treatment of CMV infection in IBD patients, either alone or in combination with antiviral agents, with successful resolution of infection.17,18 Additional observations of the interaction between CMV infection and vedolizumab would be required to determine if the onset of CMV infection in this patient represents an additional risk of the medication.
Identifying a relationship between a monoclonal antibody therapy, such as vedolizumab, and RGD, IGD type, might be difficult in clinical practice, particularly if this type of reaction has not been previously associated with the culprit medication. In our patient, onset of cutaneous findings in relation to dosing of vedolizumab and exclusion of other possible causes of the rash supported the decision to stop vedolizumab. However, this decision often is challenging in patients with multiple concurrent medical conditions and those whose therapeutic options are limited.
Conclusion
Ulcerative colitis is not an uncommon condition; utilization of targeted monoclonal antibodies as a treatment strategy is expanding.2,19 As implementation of vedolizumab as a targeted biologic therapy for this disease increases, additional cases of IGD might emerge with greater frequency. Because IBD and autoimmune conditions have a tendency to coincide, awareness of the reaction presented here might be particularly important for dermatologists managing cutaneous manifestations of autoimmune conditions, as patients might present with a clinical picture complicated by preexisting skin findings.20 Furthermore, as reports of RGD, IGD type, in response to several monoclonal antibodies accumulate, it is prudent for all physicians to be aware of this potential complication of this class of medication so that they can make educated decisions about continuing monoclonal antibody therapy.
- Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019;37:9-16. doi:10.1016/j.tibtech.2018.05.014
- Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364-370. doi:10.1111/apt.14430
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437-1444. doi:10.1093/ecco-jcc/jjw092
- Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151-1159. doi:10.1097/MIB.0000000000000396
- Cohen RD, Bhayat F, Blake A, et al. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. 2020;14:192-204. doi:10.1093/ecco-jcc/jjz137
- Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. doi:10.1053/j.gastro.2014.05.008
- Tadbiri S, Peyrin-Biroulet L, Serrero M, et al; . Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485-493. doi:10.1111/apt.14419
- Pereira Guedes T, Pedroto I, Lago P. Vedolizumab-associated psoriasis: until where does gut selectivity go? Rev Esp Enferm Dig. 2020;112:580-581. doi:10.17235/reed.2020.6817/2019
- Gold SL, Magro C, Scherl E. A unique infusion reaction to vedolizumab in a patient with Crohn’s disease. Gastroenterology. 2018;155:981-982. doi:10.1053/j.gastro.2018.03.048
- Martínez Andrés B, Sastre Lozano V, Sánchez Melgarejo JF. Sweet syndrome after treatment with vedolizumab in a patient with Crohn’s disease. Rev Esp Enferm Dig. 2018;110:530. doi:10.17235/reed.2018.5603/2018
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Crowson AN, Magro C. Interstitial granulomatous dermatitis with arthritis. Hum Pathol. 2004;35:779-780. doi:10.1016/j.humpath.2004.05.001
- Altemir A, Iglesias-Sancho M, Sola-Casas MdeLA, et al. Interstitial granulomatous dermatitis following tocilizumab, a paradoxical reaction? Dermatol Ther. 2020;33:e14207. doi:10.1111/dth.14207
- Singh P, Wolfe SP, Alloo A, et al. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. 2020;47:65-69. doi:10.1111/cup.13562
- Deng A, Harvey V, Sina B, et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol. 2006;142:198-202. doi:10.1001/archderm.142.2.198
- Bonfanti E, Bracco C, Biancheri P, et al. Fever during anti-integrin therapy: new immunodeficiency. Eur J Case Rep Intern Med. 2020;7:001288. doi:10.12890/2020_001288
- A, Lenarcik M, E. Resolution of CMV infection in the bowel on vedolizumab therapy. J Crohns Colitis. 2019;13:1234-1235. doi:10.1093/ecco-jcc/jjz033
- Hommel C, Pillet S, Rahier J-F. Comment on: ‘Resolution of CMV infection in the bowel on vedolizumab therapy’. J Crohns Colitis. 2020;14:148-149. doi:10.1093/ecco-jcc/jjz108
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137-6146. doi:10.3748/wjg.v23.i33.6137
The number of monoclonal antibodies developed for therapeutic use has rapidly expanded over the last decade due to their generally favorable adverse effect (AE) profiles and efficacy.1 Tumor necrosis factor α inhibitors and general integrin antagonists are well-known examples of such monoclonal antibodies. Common conditions utilizing immunotherapy include inflammatory bowel diseases (IBDs), such as Crohn disease and ulcerative colitis (UC).2
The monoclonal antibody vedolizumab, approved in 2014 for moderate to severe UC and Crohn disease, selectively antagonizes α4β7 integrin to target a specific population of gastrointestinal T lymphocytes, preventing their mobilization to areas of inflammation.3 Adverse effects in patients treated with vedolizumab occur at a rate comparable to placebo and largely are considered nonserious4,5; the most commonly reported AE is disease exacerbation (13%–17% of patients).5,6 Published reports of cutaneous AEs at administration of vedolizumab include urticaria during infusion, appearance of cutaneous manifestations characteristic of IBD, psoriasis, Henoch-Schönlein purpura, and Sweet syndrome.7-10
We present the case of a 61-year-old woman with UC who developed reactive granulomatous dermatitis (RGD), interstitial granulomatous dermatitis (IGD) type secondary to vedolizumab. This adverse reaction has not, to our knowledge, been previously reported.
Case Report
A 61-year-old woman with a medical history of UC treated with vedolizumab and myelodysplastic syndrome treated with intravenous immunoglobulin (due to hypogammaglobulinemia following allogeneic stem cell transplantation 14 months prior) presented with a concern of a rash. The patient had been in a baseline state of health until 1 week after receiving her second dose of vedolizumab, at which time she developed a mildly pruritic maculopapular rash on the back and chest. Triamcinolone ointment and hydroxyzine were recommended during an initial telehealth consultation with an oncologist with minimal improvement. The rash continued to spread distally with worsening pruritus.
The patient returned to her oncologist for a routine follow-up appointment 5 days after initial teleconsultation. She reported poor oral intake due to oropharyngeal pain and a worsening rash; her husband added a report of recent onset of somnolence. She was admitted to the hospital, and intravenous fluids were administered.
At admission, the patient was hypotensive; vital signs were otherwise normal. Physical examination revealed the oropharynx was erythematous. Pink lichenoid papules coalescing into plaques were present diffusely across the trunk, arms, and legs; the hands, feet, and face were spared (Figure 1).

A complete blood cell count and comprehensive metabolic panel were unremarkable. A lumbar puncture, chest radiograph, blood cultures, urinalysis, and urine cultures did not identify a clear infectious cause for the rash, though the workup for infection did raise concern about active cytomegalovirus (CMV) infection with colitis and pneumonitis. Computed tomography of the head showed no acute hemorrhage.
Dermatology was consulted and determined that the appearance of the rash was most consistent with a lichenoid drug eruption, likely secondary to vedolizumab that was administered 1 week before the rash onset. Analysis of a skin biopsy revealed a dense dermal histiocytic and lymphocytic infiltrate in close approximation to blood vessels, confirmed by immunohistochemical staining for CD45, CD43, CD68, CD34, c-KIT, and myeloperoxidase (Figures 2A and 2B). Colloidal iron staining of the specimen revealed no mucin (Figure 2C).

Taken together, the clinical presentation and histopathologic findings were determined to be most consistent with RGD, IGD type, with secondary vasculitis due to vedolizumab. The patient was treated with triamcinolone ointment and low-dose prednisone. Vedolizumab was discontinued. The rash resolved several weeks after cessation of vedolizumab.
Comment
This case describes the development of RGD, IGD type, as an AE of vedolizumab for the treatment of IBD. Reactive granulomatous dermatitis encompasses a spectrum of cutaneous reactions that includes the diagnosis formerly distinctly identified as IGD.11 This variety of RGD is characterized by histologic findings of heavy histiocytic inflammation in the reticular layer of the dermis with interstitial and perivascular neutrophils, lymphocytes, and histiocytes, as well as the absence of mucin. Interstitial granulomatous dermatitis–type reactions commonly are associated with autoimmune conditions and medications, with accumulating examples occurring in the setting of other biologic therapies, including the IL-6 receptor inhibitor tocilizumab; the programmed death receptor-1 inhibitor nivolumab; and the tumor necrosis factor α inhibitors infliximab, etanercept, and adalimumab.12-15
Although our patient represents CMV infection while being treated with vedolizumab, the relationship between the two is unclear. Development of CMV infection while receiving vedolizumab has been reported in the literature in a patient who was concurrently immunosuppressed with azathioprine.16 In contrast, vedolizumab administration has been utilized as a treatment of CMV infection in IBD patients, either alone or in combination with antiviral agents, with successful resolution of infection.17,18 Additional observations of the interaction between CMV infection and vedolizumab would be required to determine if the onset of CMV infection in this patient represents an additional risk of the medication.
Identifying a relationship between a monoclonal antibody therapy, such as vedolizumab, and RGD, IGD type, might be difficult in clinical practice, particularly if this type of reaction has not been previously associated with the culprit medication. In our patient, onset of cutaneous findings in relation to dosing of vedolizumab and exclusion of other possible causes of the rash supported the decision to stop vedolizumab. However, this decision often is challenging in patients with multiple concurrent medical conditions and those whose therapeutic options are limited.
Conclusion
Ulcerative colitis is not an uncommon condition; utilization of targeted monoclonal antibodies as a treatment strategy is expanding.2,19 As implementation of vedolizumab as a targeted biologic therapy for this disease increases, additional cases of IGD might emerge with greater frequency. Because IBD and autoimmune conditions have a tendency to coincide, awareness of the reaction presented here might be particularly important for dermatologists managing cutaneous manifestations of autoimmune conditions, as patients might present with a clinical picture complicated by preexisting skin findings.20 Furthermore, as reports of RGD, IGD type, in response to several monoclonal antibodies accumulate, it is prudent for all physicians to be aware of this potential complication of this class of medication so that they can make educated decisions about continuing monoclonal antibody therapy.
The number of monoclonal antibodies developed for therapeutic use has rapidly expanded over the last decade due to their generally favorable adverse effect (AE) profiles and efficacy.1 Tumor necrosis factor α inhibitors and general integrin antagonists are well-known examples of such monoclonal antibodies. Common conditions utilizing immunotherapy include inflammatory bowel diseases (IBDs), such as Crohn disease and ulcerative colitis (UC).2
The monoclonal antibody vedolizumab, approved in 2014 for moderate to severe UC and Crohn disease, selectively antagonizes α4β7 integrin to target a specific population of gastrointestinal T lymphocytes, preventing their mobilization to areas of inflammation.3 Adverse effects in patients treated with vedolizumab occur at a rate comparable to placebo and largely are considered nonserious4,5; the most commonly reported AE is disease exacerbation (13%–17% of patients).5,6 Published reports of cutaneous AEs at administration of vedolizumab include urticaria during infusion, appearance of cutaneous manifestations characteristic of IBD, psoriasis, Henoch-Schönlein purpura, and Sweet syndrome.7-10
We present the case of a 61-year-old woman with UC who developed reactive granulomatous dermatitis (RGD), interstitial granulomatous dermatitis (IGD) type secondary to vedolizumab. This adverse reaction has not, to our knowledge, been previously reported.
Case Report
A 61-year-old woman with a medical history of UC treated with vedolizumab and myelodysplastic syndrome treated with intravenous immunoglobulin (due to hypogammaglobulinemia following allogeneic stem cell transplantation 14 months prior) presented with a concern of a rash. The patient had been in a baseline state of health until 1 week after receiving her second dose of vedolizumab, at which time she developed a mildly pruritic maculopapular rash on the back and chest. Triamcinolone ointment and hydroxyzine were recommended during an initial telehealth consultation with an oncologist with minimal improvement. The rash continued to spread distally with worsening pruritus.
The patient returned to her oncologist for a routine follow-up appointment 5 days after initial teleconsultation. She reported poor oral intake due to oropharyngeal pain and a worsening rash; her husband added a report of recent onset of somnolence. She was admitted to the hospital, and intravenous fluids were administered.
At admission, the patient was hypotensive; vital signs were otherwise normal. Physical examination revealed the oropharynx was erythematous. Pink lichenoid papules coalescing into plaques were present diffusely across the trunk, arms, and legs; the hands, feet, and face were spared (Figure 1).

A complete blood cell count and comprehensive metabolic panel were unremarkable. A lumbar puncture, chest radiograph, blood cultures, urinalysis, and urine cultures did not identify a clear infectious cause for the rash, though the workup for infection did raise concern about active cytomegalovirus (CMV) infection with colitis and pneumonitis. Computed tomography of the head showed no acute hemorrhage.
Dermatology was consulted and determined that the appearance of the rash was most consistent with a lichenoid drug eruption, likely secondary to vedolizumab that was administered 1 week before the rash onset. Analysis of a skin biopsy revealed a dense dermal histiocytic and lymphocytic infiltrate in close approximation to blood vessels, confirmed by immunohistochemical staining for CD45, CD43, CD68, CD34, c-KIT, and myeloperoxidase (Figures 2A and 2B). Colloidal iron staining of the specimen revealed no mucin (Figure 2C).

Taken together, the clinical presentation and histopathologic findings were determined to be most consistent with RGD, IGD type, with secondary vasculitis due to vedolizumab. The patient was treated with triamcinolone ointment and low-dose prednisone. Vedolizumab was discontinued. The rash resolved several weeks after cessation of vedolizumab.
Comment
This case describes the development of RGD, IGD type, as an AE of vedolizumab for the treatment of IBD. Reactive granulomatous dermatitis encompasses a spectrum of cutaneous reactions that includes the diagnosis formerly distinctly identified as IGD.11 This variety of RGD is characterized by histologic findings of heavy histiocytic inflammation in the reticular layer of the dermis with interstitial and perivascular neutrophils, lymphocytes, and histiocytes, as well as the absence of mucin. Interstitial granulomatous dermatitis–type reactions commonly are associated with autoimmune conditions and medications, with accumulating examples occurring in the setting of other biologic therapies, including the IL-6 receptor inhibitor tocilizumab; the programmed death receptor-1 inhibitor nivolumab; and the tumor necrosis factor α inhibitors infliximab, etanercept, and adalimumab.12-15
Although our patient represents CMV infection while being treated with vedolizumab, the relationship between the two is unclear. Development of CMV infection while receiving vedolizumab has been reported in the literature in a patient who was concurrently immunosuppressed with azathioprine.16 In contrast, vedolizumab administration has been utilized as a treatment of CMV infection in IBD patients, either alone or in combination with antiviral agents, with successful resolution of infection.17,18 Additional observations of the interaction between CMV infection and vedolizumab would be required to determine if the onset of CMV infection in this patient represents an additional risk of the medication.
Identifying a relationship between a monoclonal antibody therapy, such as vedolizumab, and RGD, IGD type, might be difficult in clinical practice, particularly if this type of reaction has not been previously associated with the culprit medication. In our patient, onset of cutaneous findings in relation to dosing of vedolizumab and exclusion of other possible causes of the rash supported the decision to stop vedolizumab. However, this decision often is challenging in patients with multiple concurrent medical conditions and those whose therapeutic options are limited.
Conclusion
Ulcerative colitis is not an uncommon condition; utilization of targeted monoclonal antibodies as a treatment strategy is expanding.2,19 As implementation of vedolizumab as a targeted biologic therapy for this disease increases, additional cases of IGD might emerge with greater frequency. Because IBD and autoimmune conditions have a tendency to coincide, awareness of the reaction presented here might be particularly important for dermatologists managing cutaneous manifestations of autoimmune conditions, as patients might present with a clinical picture complicated by preexisting skin findings.20 Furthermore, as reports of RGD, IGD type, in response to several monoclonal antibodies accumulate, it is prudent for all physicians to be aware of this potential complication of this class of medication so that they can make educated decisions about continuing monoclonal antibody therapy.
- Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019;37:9-16. doi:10.1016/j.tibtech.2018.05.014
- Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364-370. doi:10.1111/apt.14430
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437-1444. doi:10.1093/ecco-jcc/jjw092
- Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151-1159. doi:10.1097/MIB.0000000000000396
- Cohen RD, Bhayat F, Blake A, et al. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. 2020;14:192-204. doi:10.1093/ecco-jcc/jjz137
- Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. doi:10.1053/j.gastro.2014.05.008
- Tadbiri S, Peyrin-Biroulet L, Serrero M, et al; . Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485-493. doi:10.1111/apt.14419
- Pereira Guedes T, Pedroto I, Lago P. Vedolizumab-associated psoriasis: until where does gut selectivity go? Rev Esp Enferm Dig. 2020;112:580-581. doi:10.17235/reed.2020.6817/2019
- Gold SL, Magro C, Scherl E. A unique infusion reaction to vedolizumab in a patient with Crohn’s disease. Gastroenterology. 2018;155:981-982. doi:10.1053/j.gastro.2018.03.048
- Martínez Andrés B, Sastre Lozano V, Sánchez Melgarejo JF. Sweet syndrome after treatment with vedolizumab in a patient with Crohn’s disease. Rev Esp Enferm Dig. 2018;110:530. doi:10.17235/reed.2018.5603/2018
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Crowson AN, Magro C. Interstitial granulomatous dermatitis with arthritis. Hum Pathol. 2004;35:779-780. doi:10.1016/j.humpath.2004.05.001
- Altemir A, Iglesias-Sancho M, Sola-Casas MdeLA, et al. Interstitial granulomatous dermatitis following tocilizumab, a paradoxical reaction? Dermatol Ther. 2020;33:e14207. doi:10.1111/dth.14207
- Singh P, Wolfe SP, Alloo A, et al. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. 2020;47:65-69. doi:10.1111/cup.13562
- Deng A, Harvey V, Sina B, et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol. 2006;142:198-202. doi:10.1001/archderm.142.2.198
- Bonfanti E, Bracco C, Biancheri P, et al. Fever during anti-integrin therapy: new immunodeficiency. Eur J Case Rep Intern Med. 2020;7:001288. doi:10.12890/2020_001288
- A, Lenarcik M, E. Resolution of CMV infection in the bowel on vedolizumab therapy. J Crohns Colitis. 2019;13:1234-1235. doi:10.1093/ecco-jcc/jjz033
- Hommel C, Pillet S, Rahier J-F. Comment on: ‘Resolution of CMV infection in the bowel on vedolizumab therapy’. J Crohns Colitis. 2020;14:148-149. doi:10.1093/ecco-jcc/jjz108
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137-6146. doi:10.3748/wjg.v23.i33.6137
- Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. 2019;37:9-16. doi:10.1016/j.tibtech.2018.05.014
- Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364-370. doi:10.1111/apt.14430
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437-1444. doi:10.1093/ecco-jcc/jjw092
- Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151-1159. doi:10.1097/MIB.0000000000000396
- Cohen RD, Bhayat F, Blake A, et al. The safety profile of vedolizumab in ulcerative colitis and Crohn’s disease: 4 years of global post-marketing data. J Crohns Colitis. 2020;14:192-204. doi:10.1093/ecco-jcc/jjz137
- Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. doi:10.1053/j.gastro.2014.05.008
- Tadbiri S, Peyrin-Biroulet L, Serrero M, et al; . Impact of vedolizumab therapy on extra-intestinal manifestations in patients with inflammatory bowel disease: a multicentre cohort study nested in the OBSERV-IBD cohort. Aliment Pharmacol Ther. 2018;47:485-493. doi:10.1111/apt.14419
- Pereira Guedes T, Pedroto I, Lago P. Vedolizumab-associated psoriasis: until where does gut selectivity go? Rev Esp Enferm Dig. 2020;112:580-581. doi:10.17235/reed.2020.6817/2019
- Gold SL, Magro C, Scherl E. A unique infusion reaction to vedolizumab in a patient with Crohn’s disease. Gastroenterology. 2018;155:981-982. doi:10.1053/j.gastro.2018.03.048
- Martínez Andrés B, Sastre Lozano V, Sánchez Melgarejo JF. Sweet syndrome after treatment with vedolizumab in a patient with Crohn’s disease. Rev Esp Enferm Dig. 2018;110:530. doi:10.17235/reed.2018.5603/2018
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Crowson AN, Magro C. Interstitial granulomatous dermatitis with arthritis. Hum Pathol. 2004;35:779-780. doi:10.1016/j.humpath.2004.05.001
- Altemir A, Iglesias-Sancho M, Sola-Casas MdeLA, et al. Interstitial granulomatous dermatitis following tocilizumab, a paradoxical reaction? Dermatol Ther. 2020;33:e14207. doi:10.1111/dth.14207
- Singh P, Wolfe SP, Alloo A, et al. Interstitial granulomatous dermatitis and granulomatous arteritis in the setting of PD-1 inhibitor therapy for metastatic melanoma. J Cutan Pathol. 2020;47:65-69. doi:10.1111/cup.13562
- Deng A, Harvey V, Sina B, et al. Interstitial granulomatous dermatitis associated with the use of tumor necrosis factor alpha inhibitors. Arch Dermatol. 2006;142:198-202. doi:10.1001/archderm.142.2.198
- Bonfanti E, Bracco C, Biancheri P, et al. Fever during anti-integrin therapy: new immunodeficiency. Eur J Case Rep Intern Med. 2020;7:001288. doi:10.12890/2020_001288
- A, Lenarcik M, E. Resolution of CMV infection in the bowel on vedolizumab therapy. J Crohns Colitis. 2019;13:1234-1235. doi:10.1093/ecco-jcc/jjz033
- Hommel C, Pillet S, Rahier J-F. Comment on: ‘Resolution of CMV infection in the bowel on vedolizumab therapy’. J Crohns Colitis. 2020;14:148-149. doi:10.1093/ecco-jcc/jjz108
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137-6146. doi:10.3748/wjg.v23.i33.6137
Practice Points
- Reactive granulomatous dermatitis, interstitial granulomatous dermatitis (IGD) type, can occur as an adverse reaction to vedolizumab despite the minimal adverse effect profile of the medication.
- Evidence of IGD type reactions to monoclonal antibodies is accumulating; this disorder can be considered in the differential diagnosis for patients who develop a new rash when treated with an agent of this therapeutic class.