User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Adverse Effects of the COVID-19 Vaccine in Patients With Psoriasis
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
To the Editor:
Because the SARS-CoV-2 virus is constantly changing, routine vaccination to prevent COVID-19 infection is recommended. The messenger RNA (mRNA) vaccines from Pfizer-BioNTech and Moderna as well as the Ad26.COV2.S (Johnson & Johnson) and NVX-CoV2373 (Novavax) vaccines are the most commonly used COVID-19 vaccines in the United States. Adverse effects following vaccination against SARS-CoV-2 are well documented; recent studies report a small incidence of adverse effects in the general population, with most being minor (eg, headache, fever, muscle pain).1,2 Interestingly, reports of exacerbation of psoriasis and new-onset psoriasis following COVID-19 vaccination suggest a potential association.3,4 However, the literature investigating the vaccine adverse effect profile in this demographic is scarce. We examined the incidence of adverse effects from SARS-CoV-2 vaccines in patients with psoriasis.
This retrospective cohort study used the COVID-19 Research Database (https://covid19researchdatabase.org/) to examine the adverse effects following the first and second doses of the mRNA vaccines in patients with and without psoriasis. The sample size for the Ad26.COV2.S vaccine was too small to analyze.
Claims were evaluated from August to October 2021 for 2 diagnoses of psoriasis prior to January 1, 2020, using the International Classification of Diseases, Tenth Revision (ICD-10) code L40.9 to increase the positive predictive value and ensure that the diagnosis preceded the COVID-19 pandemic. Patients younger than 18 years and those who did not receive 2 doses of a SARS-CoV-2 vaccine were excluded. Controls who did not have a diagnosis of psoriasis were matched for age, sex, and hypertension at a 4:1 ratio. Hypertension represented the most common comorbidity that could feasibly be controlled for in this study population. Other comorbidities recorded included obesity, type 2 diabetes mellitus, congestive heart failure, asthma, chronic obstructive pulmonary disease, chronic ischemic heart disease, rhinitis, and chronic kidney disease.
Common adverse effects as long as 30 days after vaccination were identified using ICD-10 codes. Adverse effects of interest were anaphylactic reaction, initial encounter of adverse effect of viral vaccines, fever, allergic urticaria, weakness, altered mental status, malaise, allergic reaction, chest pain, symptoms involving circulatory or respiratory systems, localized rash, axillary lymphadenopathy, infection, and myocarditis.5 Poisson regression was performed using Stata 17 analytical software.
We identified 4273 patients with psoriasis and 17,092 controls who received mRNA COVID-19 vaccines (Table). Adjusted odds ratios (aORs) for doses 1 and 2 were calculated for each vaccine (eTable). Adverse effects with sufficient data to generate an aOR included weakness, altered mental status, malaise, chest pain, and symptoms involving the circulatory or respiratory system. The aORs for allergic urticaria and initial encounter of adverse effect of viral vaccines were only calculated for the Moderna mRNA vaccine due to low sample size.

This study demonstrated that patients with psoriasis do not appear to have a significantly increased risk of adverse effects from mRNA SARS-CoV-2 vaccines. Although the ORs in this study were not significant, most recorded adverse effects demonstrated an aOR less than 1, suggesting that there might be a lower risk of certain adverse effects in psoriasis patients. This could be explained by the immunomodulatory effects of certain systemic psoriasis treatments that might influence the adverse effect presentation.

The study is limited by the lack of treatment data, small sample size, and the fact that it did not assess flares or worsening of psoriasis with the vaccines. Underreporting of adverse effects by patients and underdiagnosis of adverse effects secondary to SARS-CoV-2 vaccines due to its novel nature, incompletely understood consequences, and limited ICD-10 codes associated with adverse effects all contributed to the small sample size.
Our findings suggest that the risk for immediate adverse effects from the mRNA SARS-CoV-2 vaccines is not increased among psoriasis patients. However, the impact of immunomodulatory agents on vaccine efficacy and expected adverse effects should be investigated. As more individuals receive the COVID-19 vaccine, the adverse effect profile in patients with psoriasis is an important area of investigation.
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
- Singh A, Khillan R, Mishra Y, et al. The safety profile of COVID-19 vaccinations in the United States. Am J Infect Control. 2022;50:15-19. doi: 10.1016/j.ajic.2021.10.015
- Beatty AL, Peyser ND, Butcher XE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4:e2140364. doi:10.1001/jamanetworkopen.2021.40364
- Bellinato F, Maurelli M, Gisondi P, et al. Cutaneous adverse reactions associated with SARS-CoV-2 vaccines. J Clin Med. 2021;10:5344. doi:10.3390/jcm10225344
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol. 2022;47:153-155. doi:10.1111/ced.14895
- Remer EE. Coding COVID-19 vaccination. ICD10monitor. Published March 2, 2021. Updated October 18, 2022. Accessed January 17, 2023. https://icd10monitor.medlearn.com/coding-covid-19-vaccination/
PRACTICE POINTS
- Patients who have psoriasis do not appear to have an increased incidence of adverse effects from messenger RNA COVID-19 vaccines.
- Clinicians can safely recommend COVID-19 vaccines to patients who have psoriasis.
How to Effectively Utilize Consultation Codes: 2023 Updates
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
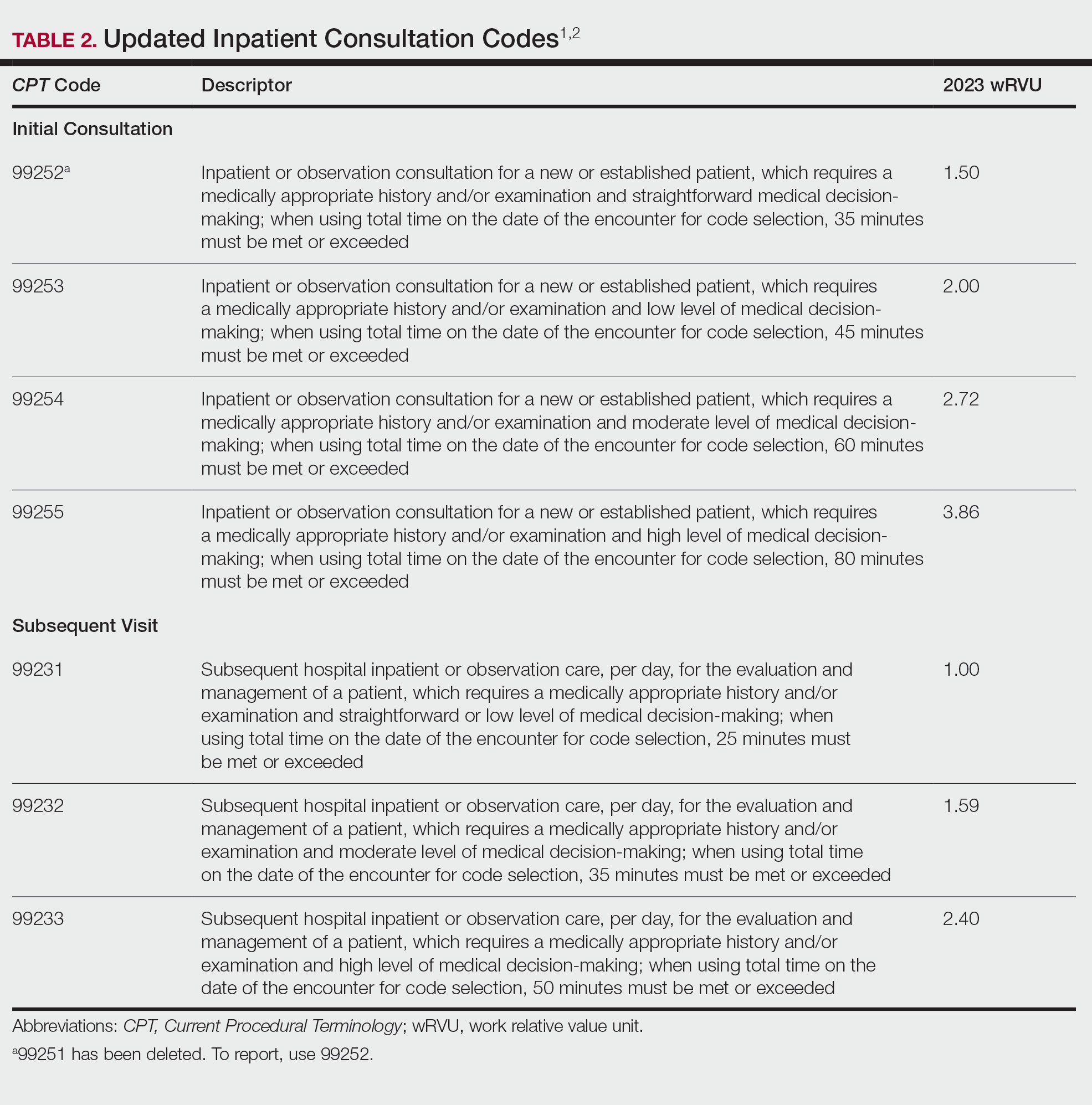
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.
Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.
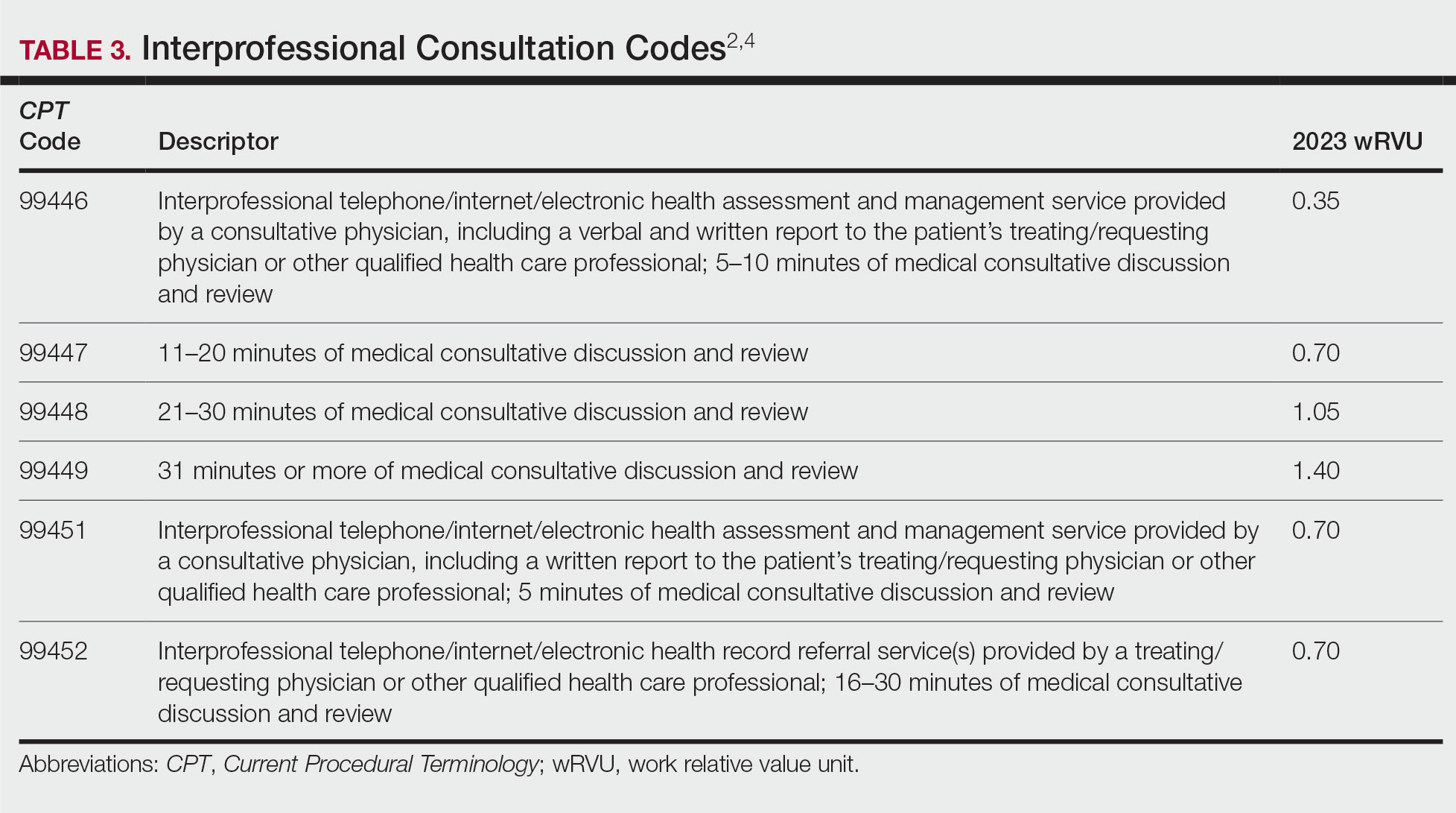
To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
Consultations and referrals are an important component of many dermatology practices. There are several families of consultation codes that can be utilized based on the setting and format of the patient encounter. In this article, I describe appropriate use of 3 families of consultation codes and recent updates in these areas.
Consultation Definitions
For all of these code sets, the same definition of consultationapplies—namely that the encounter is provided at the request of another physician, other qualified health care professional, or other appropriate source (eg, nonclinical social worker, educator, lawyer, insurance company) for a specific condition or problem. Importantly, a consultation initiated by a patient or family, or both, and not requested by one of the professionals listed above is not reported using a consultation code.1
The consultant’s opinion and any services that were ordered or performed also must be communicated to the requesting provider. The type of communication required varies based on the consultation code set in question.
Outpatient Consultation Codes
Outpatient consultation CPT (Current Procedural Terminology) codes (99241-99245) are a family of codes that can be utilized for evaluation of a new patient or an existing patient with a new problem in the outpatient setting. These codes are not reimbursed by the Centers for Medicare & Medicaid Services, but some private payers do recognize and reimburse for them.2
The consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1 Modifier -32 should not be used for a second request by a patient or a patient’s family.1
This family of codes has been revised in tandem with other evaluation and management (E/M) code sets; changes went into effect January 1, 2023. These updates are part of the ongoing effort to update code wording and structures to reflect guiding principles of the American Medical Association when redesigning E/M codes. These principles include decreasing administrative burden and the need for audits, decreasing unnecessary documentation that is not needed for patient care, and ensuring that payment for E/M is resource based.3 Updated code language and payment structure is found in Table 1.1,2 The main updates to these codes include:
• Code 99241 was deleted. This was in line with removal of 99201 from the outpatient E/M family set.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 99417 can be utilized.

Inpatient Consultation Codes
Similar to the outpatient consultation codes, the inpatient consultation codes also have been revised as part of E/M updates; revisions went into effect January 1, 2023. Also, as with the outpatient consultation codes, the consultant’s opinion and any services that were ordered or performed must be communicated by written report to the requesting physician, other qualified health care professional, or other appropriate source. If a consultation is mandated (eg, by a third-party payer), then modifier -32 also should be reported.1
When inpatient consultations are performed, 2 code families generally are utilized. For initial consultation, initial inpatient consultation codes (99251-99255) are used; for any follow-up encounters performed while the patient is an inpatient, subsequent inpatient consultation codes (99231-99233) are used. The subsequent code family is the same that is utilized for all subsequent care within the inpatient or observation care setting, regardless of how the care was initiated.1
“Initial service” is when the patient has not received any professional services from either the physician or other qualified health care professional or from another physician or other qualified health care professional ofthe exact same specialty and subspecialty who belongs to the same group practice during the inpatient, observation, or nursing facility admission and stay. “Subsequent service” is when the patient has received professional service(s) from either the physician or other qualified health care professional or from another physician or other qualified health care professional.1 Updated code language and payment structure is found in Table 2.1,2 Major changes include:
• Code 99251 was deleted. This is in line with deletion of a new low-level patient encounter in the outpatient E/M family set and consultation code family set, as noted above.
• Level of service is now based solely on either time on the date of encounter or medical decision-making.
• Definitions regarding medical decision-making are in line with those utilized for outpatient E/M codes.
• If coding by time and the maximum amount of time has been exceeded by 15 or more minutes, prolonged services code 993X0 can be utilized.

Interprofessional Consultation Codes
An additional code family that can be utilized for consultations is the interprofessional consultation codes. These codes can be utilized when assisting in the diagnosis or management, or both, of a patient without face-to-face contact. These codes are listed in Table 3.2,4 For all of these codes, the consultation is performed by telephone, internet or electronic health record, or a combination of these means. The consultation can be for a new problem or a worsening existing problem. The patient can be a new or established patient to the consultant. Documentation should be performed in the patient’s medical record, including the reason for the request.

To bill for interprofessional consultation, the consultant should not have seen the patient in a face-to-face encounter within the prior 14 days or see them in the following 14 days. The codes should not be reported more than once in a 7-day period or more than once in a 14-day period in the case of code 99452.4 For codes 99446 to 99449, more than 50% of the time spent by the consulting physician must be devoted to verbal or internet discussion, or both, with the referring physician. For code 99451, service time is based on total review and interprofessional communication time.4 The correct code is chosen based on the following parameters:
• 99446-99449: Describes interprofessional consultation services, which include both a written and a verbal report to the patient’s treating or requesting physician or qualified health care professional. These codes can be utilized by a consulting physician. The correct code is chosen based on time spent by the consulting physician.
• 99451: Describes an interprofessional consultation service, which includes a written report to the patient’s treating or requesting physician or qualified health care professional. This code can be utilized by a consulting physician once 5 minutes of consultative discussion and review has been performed.
• 99452: Describes an interprofessional consultation service provided by the requesting physician. This code can be utilized when a requesting physician spends 16 to 30 minutes in medical consultative discussion and review.
Final Thoughts
Consultation codes can be an important part of a dermatologist’s practice. Differences exist between consultation code sets based on the encounter setting and whether the encounter was performed with or without face-to-face contact. In addition, updates to the E/M inpatient and outpatient consultation codes went into effect January 1, 2023. It is important to understand those changes to correctly bill for these encounters.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
- CPT® evaluation and management (E/M) code and guideline changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/system/files/2023-e-m-descriptors-guidelines.pdf
- RVU23A. US Centers for Medicare and Medicaid Services; January 2023. Accessed January 18, 2023. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu23a
- Understanding the landmark E/M office visit changes. American Medical Association. Accessed January 15, 2023. https://www.ama-assn.org/practice-management/cpt/understanding-landmark-em-office-visit-changes
- Synovec MS, Jagmin CL, Hochstetler Z, et al, eds. CPT 2022: Professional Edition. 4th ed. American Medical Association Press; 2021.
PRACTICE POINTS
- Updates to the inpatient and outpatient consultation codes went into effect January 1, 2023.
- For inpatient and outpatient consultation codes, level of service is now solely based on either time on the date of encounter or medical decision-making.
- Interprofessional consultation codes can be utilized when assisting in the diagnosis and/or management of a patient without face-to-face contact.
More New Therapeutics for Psoriasis
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
New Treatments for Psoriasis: An Update on a Therapeutic Frontier
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
PRACTICE POINTS
- Roflumilast, a phosphodiesterase 4 inhibitor, and tapinarof, an aryl hydrocarbon receptor–modulating agent, are 2 novel nonsteroidal topical treatments safe for regular long-term use on all affected areas of the skin in adult patients with plaque psoriasis.
- Deucravacitinib is an oral selective tyrosine kinase 2 allosteric inhibitor that has demonstrated a favorable safety profile and greater levels of efficacy than other available oral medications for plaque psoriasis.
- The dual inhibition of IL-17A and IL-17F with bimekizumab provides faster responses and greater clinical benefits for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone, achieving higher levels of efficacy than has been reported with any other biologic therapy.
- Spesolimab, an IL-36 receptor inhibitor, is an effective, US Food and Drug Administration–approved treatment for patients with generalized pustular psoriasis.
Annular Plaques Overlying Hyperpigmented Telangiectatic Patches on the Neck
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
A 58-year-old man with a history of type 2 diabetes mellitus, nephrolithiasis, hypovitaminosis D, and hypercholesterolemia presented to our dermatology clinic for a follow-up total-body skin examination after a prior diagnosis of basal cell carcinoma on the vertex of the scalp. Physical examination revealed extensive photodamage and annular plaques overlying hyperpigmented telangiectatic patches on the dorsal portion of the neck. The eruption persisted for 1 year and failed to improve with clotrimazole cream. His medications included simvastatin, metformin, chlorthalidone, vitamin D, and tamsulosin. Two shave biopsies from the posterior neck were performed.

Dissociating Fibroepithelioma of Pinkus From Internal Malignancy: A Single-Center Retrospective Study
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).
Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.
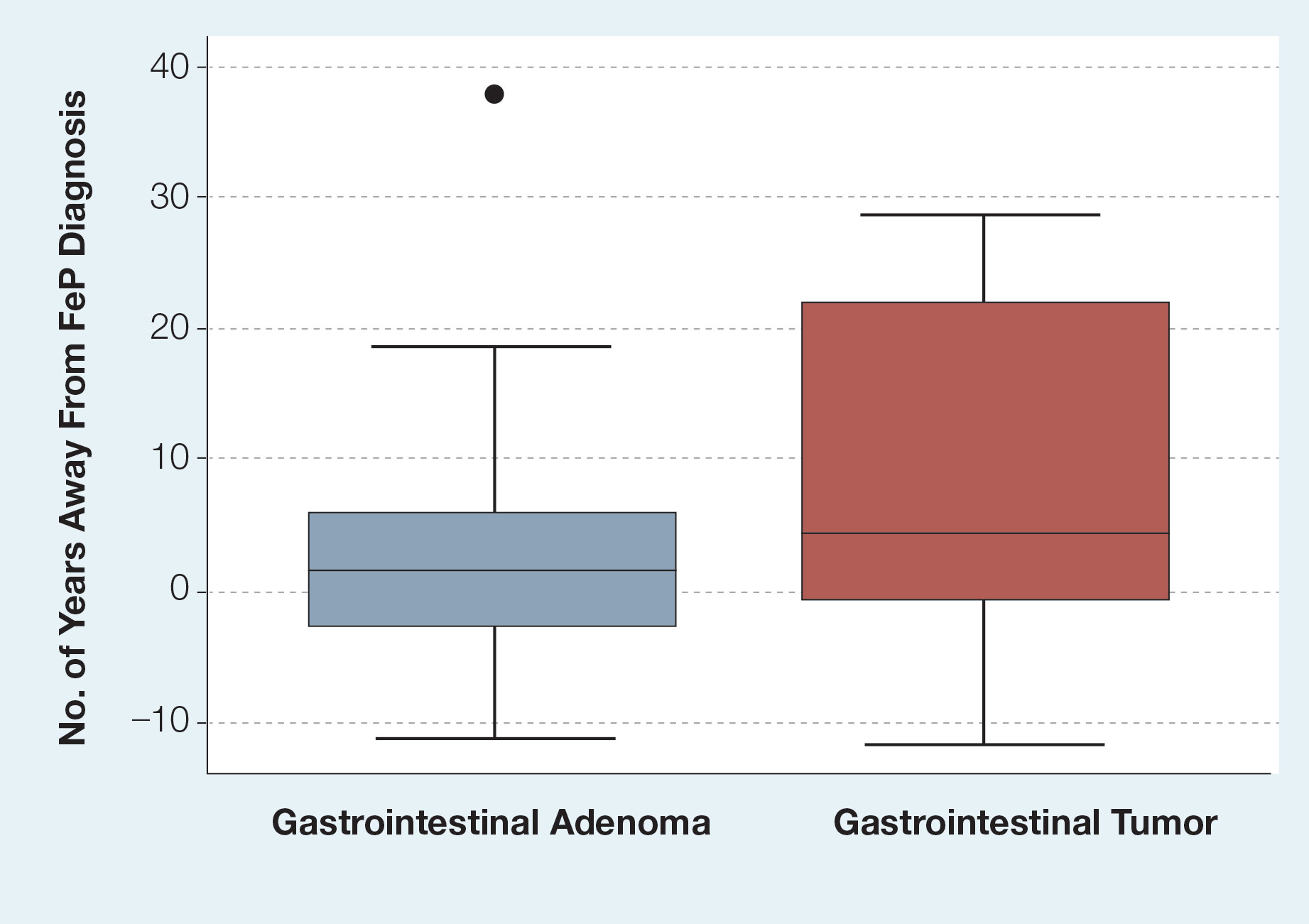
Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.
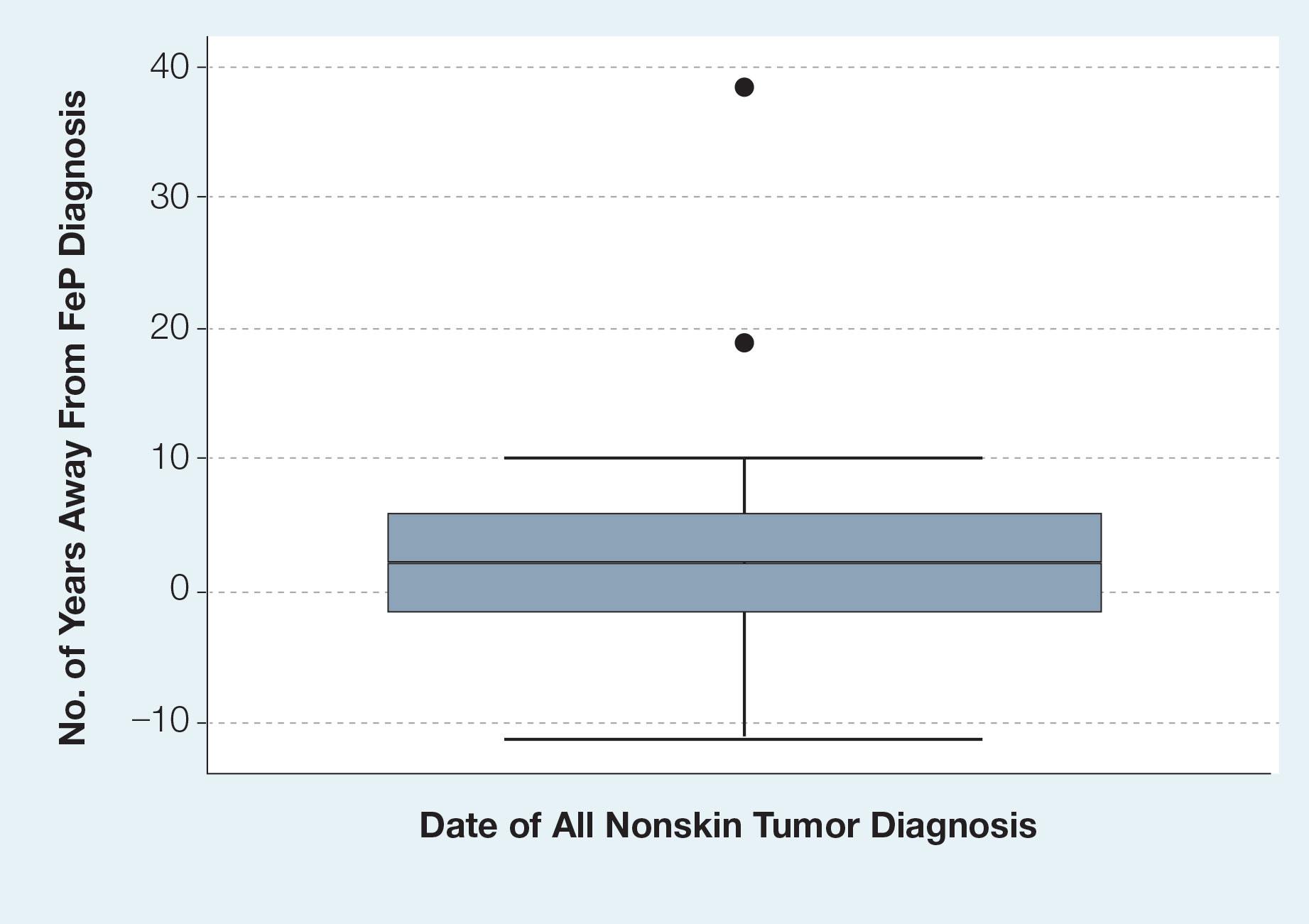
Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
Fibroepithelioma of Pinkus (FeP), or Pinkus tumor, is a rare tumor with a presentation similar to benign neoplasms such as acrochordons and seborrheic keratoses. Classically, FeP presents as a nontender, solitary, flesh-colored, firm, dome-shaped papule or plaque with a predilection for the lumbosacral region rather than sun-exposed areas. This tumor typically develops in fair-skinned older adults, more often in females.1
The association between cutaneous lesions and internal malignancies is well known to include dermatoses such as erythema repens in patients with lung cancer, or tripe palms and acanthosis nigricans in patients with gastrointestinal malignancy. Outside of paraneoplastic presentations, many syndromes have unique constellations of clinical findings that require the clinician to investigate for internal malignancy. Cancer-associated genodermatoses such as Birt-Hogg-Dubé, neurofibromatosis, and Cowden syndrome have key findings to alert the provider of potential internal malignancies.2 Given the rarity and relative novelty of FeP, few studies have been performed that evaluate for an association with internal malignancies.
There potentially is a common pathophysiologic mechanism between FeP and other benign and malignant tumors. Some have noted a possible common embryonic origin, such as Merkel cells, and even a common gene mutation involving tumor protein p53 or PTCH1 gene.3,4 Carcinoembryonic antigen is a glycoprotein often found in association with gastrointestinal tract tumors and also is elevated in some cases of FeP.5 A single-center retrospective study performed by Longo et al3 demonstrated an association between FeP and gastrointestinal malignancy by calculating a percentage of those with FeP who also had gastrointestinal tract tumors. Moreover, they noted that FeP preceded gastrointestinal tract tumors by up to 1 to 2 years. Using the results of this study, they suggested that a similar pathogenesis underlies the association between FeP and gastrointestinal malignancy, but a shared pathogenesis has not yet been elucidated.3
With a transition to preventive medicine and age-adjusted malignancy screening in the US medical community, the findings of FeP as a marker of gastrointestinal tract tumors could alter current recommendations of routine skin examinations and colorectal cancer screening. This study investigates the association between FeP and internal malignancy, especially gastrointestinal tract tumors.
Methods
Patient Selection—A single-center, retrospective, case-control study was designed to investigate an association between FeP and internal malignancy. The study protocol was approved by the institutional review board of the Naval Medical Center San Diego, California, in compliance with all applicable federal regulations governing the protection of human subjects. A medical record review was initiated using the Department of Defense (DoD) electronic health record to identify patients with a history of FeP. The query used a natural language search for patients who had received a histopathology report that included Fibroepithelioma of Pinkus, Pinkus, or Pinkus tumor within the diagnosis or comment section for pathology specimens processed at our institution (Naval Medical Center San Diego). A total of 45 patients evaluated at Naval Medical Center San Diego had biopsy specimens that met inclusion criteria. Only 42 electronic medical records were available to review between January 1, 2003, and March 1, 2020. Three patients were excluded from the study for absent or incomplete medical records.
Study Procedures—Data extracted by researchers were analyzed for statistical significance. All available data in current electronic health records prior to the FeP diagnosis until March 1, 2020, was reviewed for other documented malignancy or colonoscopy data. Data extracted included age, sex, date of diagnosis of FeP, location of FeP, social history, and medical and surgical history to identify prior malignancy. Colorectal cancer screening results were drawn from original reports, gastrointestinal clinic notes, biopsy results, and/or primary care provider documentation of colonoscopy results. If the exact date of internal tumor diagnosis could not be determined but the year was known, the value “July, year” was utilized as the diagnosis date.
Statistical Analysis—Data were reviewed for validity, and the Shapiro-Wilk test was used to test for normality. Graphical visualization assisted in reviewing the distribution of the data in relation to the internal tumors. The Fisher exact test was performed to test for associations, while continuous variables were assessed using the Student t test or the nonparametric Mann-Whitney U test. Analysis was conducted with StataCorp. 2017 Stata Statistical Software: Release 15 (StataCorp LLC). Significance was set at P<.05.
Results
Patient Demographics—Of the 42 patients with FeP included in this study, 28 (66.7%) were male and 14 (33.3%) were female. The overall mean age at FeP diagnosis was 56.83 years. The mean age (SD) at FeP diagnosis for males was 59.21 (19.00) years and 52.07 (21.61) for females (P=.2792)(Table 1). Other pertinent medical history, including alcohol and tobacco use, obesity, and diabetes mellitus, is included in Table 1.

Characterization of Tumors—The classification of the number of patients with any other nonskin neoplasm is presented in Table 2. Fifteen (35.7%) patients had 1 or more gastrointestinal tubular adenomas. Three patients were found to have colorectal adenocarcinoma. Karsenti et al6 published a large study of colonic adenoma detection rates in the World Journal of Gastroenterology stratified by age and found that the incidence of adenoma for those aged 55 to 59 years was 28.3% vs 35.7% in our study (P=.2978 [Fisher exact test]).

Given the number of gastrointestinal tract tumors detected, most of which were found during routine surveillance, and a prior study6 suggesting a relationship between FeP and gastrointestinal tract tumors, we analyzed the temporal relationship between the date of gastrointestinal tract tumor diagnosis and the date of FeP diagnosis to assess if gastrointestinal tract tumor or FeP might predict the onset of the other (Figure 1). By assigning a temporal category to each gastrointestinal tract tumor as occurring either before or after the FeP diagnosis by 0 to 3 years, 3 to 10 years, 10 to 15 years, and 15 or more years, the box plot in Figure 1 shows that gastrointestinal adenoma development had no significant temporal relationship to the presence of FeP, excluding any outliers (shown as dots). Additionally, in Figure 1, the same concept was applied to assess the relationship between the dates of all gastrointestinal tract tumors—benign, precancerous, or malignant—and the date of FeP diagnosis, which again showed that FeP and gastrointestinal tract tumors did not predict the onset of the other. Figure 2 showed the same for all nonskin tumor diagnoses and again demonstrated that FeP and all other nondermatologic tumors did not predict the onset of the other.

Comment
Malignancy Potential—The malignant potential of FeP—characterized as a trichoblastoma (an adnexal tumor) or a basal cell carcinoma (BCC) variant—has been documented.1 Haddock and Cohen1 noted that FeP can be considered as an intermediate variant between BCC and trichoblastomas. Furthermore, they questioned the relevance of differentiating FeP as benign or malignant.1 There are additional elements of FeP that currently are unknown, which can be partially attributed to its rarity. If we can clarify a more accurate pathogenic model of FeP, then common mutational pathways with other malignancies may be identified.

Screening for Malignancy in FeP Patients—Until recently, FeP has not been demonstrated to be associated with other cancers or to have increased metastatic potential.1 In a 1985 case series of 2 patients, FeP was found to be specifically overlying infiltrating ductal carcinoma of the breast. After a unilateral mastectomy, examination of the overlying skin of the breast showed a solitary, lightly pigmented nodule, which was identified as an FeP after histopathologic evaluation.7 There have been limited investigations of whether FeP is simply a solitary tumor or a harbinger for other malignancies, despite a study by Longo et al3 that attempted to establish this temporal relationship. They recommended that patients with FeP be clinically evaluated and screened for gastrointestinal tract tumors.3 Based on these recommendations, textbooks for dermatopathology now highlight the possible correlation of FeP and gastrointestinal malignancy,8 which may lead to earlier and unwarranted screening.
Comparison to the General Population—Although our analysis showed a portion of patients with FeP have gastrointestinal tract tumors, we do not detect a significant difference from the general population. The average age at the time of FeP diagnosis in our study was 56.83 years compared with the average age of 64.0 years by Longo et al,3 where they found an association with gastrointestinal adenocarcinoma and neuroendocrine tumors. As the rate of gastrointestinal adenoma and malignancy increases with age, the older population in the study by Longo et al3 may have developed colorectal cancer independent of FeP development. However, the rate of gastrointestinal or other malignancies in their study was substantially higher than that of the general population. The Longo et al3 study found that 22 of 49 patients developed nondermatologic malignancies within 2 years of FeP diagnosis. Additionally, no data were provided in the study regarding precancerous lesions.
In our study population, benign gastrointestinal tract tumors, specifically tubular adenomas, were noted in 35.7% of patients with FeP compared with 28.3% of the general population in the same age group reported by Karsenti et al.6 Although limited by our sample size, our study demonstrated that patients with FeP diagnosis showed no significant difference in age-stratified incidence of tubular adenoma compared with the general population (P=.2978). Figures 1 and 2 showed no obvious temporal relationship between the development of FeP and the diagnosis of gastrointestinal tumor—either precancerous or malignant lesions—suggesting that diagnosis of one does not indicate the presence of the other.
Relationship With Colonoscopy Results—By analyzing those patients with FeP who specifically had documented colonoscopy results, we did not find a correlation between FeP and gastrointestinal tubular adenoma or carcinoma at any time during the patients’ available records. Although some patients may have had undocumented colonoscopies performed outside the DoD medical system, most had evidence that these procedures were being performed by transcription into primary care provider notes, uploaded gastroenterologist clinical notes, or colonoscopy reports. It is unlikely a true colorectal or other malignancy would remain undocumented over years within the electronic medical record.
Study Limitations—Because of the nature of electronic medical records at multiple institutions, the quality and/or the quantity of medical documentation is not standardized across all patients. Not all pathology reports may include FeP as the primary diagnosis or description, as FeP may simply be reported as BCC. Despite thorough data extraction by physicians, we were limited to the data available within our electronic medical records. Colonoscopies and other specialty care often were performed by civilian providers. Documentation regarding where patients were referred for such procedures outside the DoD was not available unless reports were transmitted to the DoD or transcribed by primary care providers. Incomplete records may make it more difficult to identify and document the number and characteristics of patients’ tubular adenomas. Therefore, a complete review of civilian records was not possible, causing some patients’ medical records to be documented for a longer period of their lives than for others.
Conclusion
Given the discrepancies in our findings with the previous study,3 future investigations on FeP and associated tumors should focus on integrated health care systems with longitudinal data sets for all age-appropriate cancer screenings in a larger sample size. Another related study is needed to evaluate the pathophysiologic mechanisms of FeP development relative to known cancer lines.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
- Haddock ES, Cohen PR. Fibroepithelioma of Pinkus revisited. Dermatol Ther (Heidelb). 2016;6:347-362.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Longo C, Pellacani G, Tomasi A, et al. Fibroepithelioma of Pinkus: solitary tumor or sign of a complex gastrointestinal syndrome. Mol Clin Oncol. 2016;4:797-800.
- Warner TF, Burgess H, Mohs FE. Extramammary Paget’s disease in fibroepithelioma of Pinkus. J Cutan Pathol. 1982;9:340-344.
- Stern JB, Haupt HM, Smith RR. Fibroepithelioma of Pinkus. eccrine duct spread of basal cell carcinoma. Am J Dermatopathol. 1994;16:585-587.
- Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019;25:447-456.
- Bryant J. Fibroepithelioma of Pinkus overlying breast cancer. Arch Dermatol. 1985;121:310.
- Calonje E, Brenn T, Lazar A, et al. McKee’s Pathology of the Skin: With Clinical Correlations. 5th ed. Elsevier; 2020.
PRACTICE POINTS
- Dermatologic reactions may be the initial presentation of an internal malignancy.
- Fibroepithelioma of Pinkus is considered on the spectrum between adnexal neoplasms and a nonaggressive variant of basal cell carcinoma (BCC).
- Fibroepithelioma of Pinkus should be managed similar to nonaggressive variants of BCC such as nodular BCC.
- Fibroepithelioma of Pinkus is not associated with internal malignancy.
Chronic Ulcerative Lesion
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.
Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
The Diagnosis: Marjolin Ulcer
A skin biopsy during his prior hospital admission demonstrated an ulcer with granulation tissue and mixed inflammation, and the patient was discharged with close outpatient follow-up. Two repeat skin biopsies from the peripheral margin at the time of the outpatient follow-up confirmed an invasive, well-differentiated squamous cell carcinoma (Figure), consistent with a Marjolin ulcer. Radiography demonstrated multiple left iliac chain and inguinal lymphadenopathies with extensive subcutaneous disease overlying the left medial tibia. After tumor board discussion, surgery was not recommended due to the size and likely penetration into the muscle. The patient began treatment with cemiplimab-rwlc, a PD-1 inhibitor. Within 4 cycles of treatment, he had improved pain and ambulation, and a 3-month follow-up positron emission tomography scan revealed decreased lymph node and cutaneous metabolic activity as well as clinical improvement.

Marjolin ulcers are rare and aggressive squamous cell carcinomas that arise from chronic wounds such as burn scars or pressure ulcers.1 Although an underlying well-differentiated squamous cell carcinoma is the most common etiology, patients also may present with underlying basal cell carcinomas, melanomas, or angiosarcomas.2 The exact pathogenesis underlying the malignant degeneration is unclear but appears to be driven by chronic inflammation. Patients classically present with a nonhealing ulcer associated with raised, friable, or crusty borders, as well as surrounding scar tissue. There is a median latency of 30 years after the trauma, though acute transformation within 12 months of an injury is possible.3 The diagnosis is confirmed with a peripheral wound biopsy. Surgical excision with wide margins remains the most common and effective intervention, especially for localized disease.1 The addition of lymph node dissection remains controversial, but treatment decisions can be guided by radiographic staging.4
The prognosis of Marjolin ulcers remains poor, with a predicted 5-year survival rate ranging from 43% to 58%.1 Dermatologists and trainees should be aware of Marjolin ulcers, especially as a mimicker of other chronic ulcerating conditions. Among the differential diagnosis, ulcerative lichen planus is a condition that commonly affects the oral and genital regions; however, patients with erosive lichen planus may develop an increased risk for the subsequent development of squamous cell carcinoma in the region.5 Furthermore, arterial ulcers typically develop on the distal lower extremities with other signs of chronic ischemia, including absent peripheral pulses, atrophic skin, hair loss, and ankle-brachial indices less than 0.5. Conversely, a venous ulcer classically affects the medial malleolus and will have evidence of venous insufficiency, including stasis dermatitis and peripheral edema.6
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
- Iqbal FM, Sinha Y, Jaffe W. Marjolin’s ulcer: a rare entity with a call for early diagnosis [published online July 15, 2015]. BMJ Case Rep. doi:10.1136/bcr-2014-208176
- Kanth AM, Heiman AJ, Nair L, et al. Current trends in management of Marjolin’s ulcer: a systematic review. J Burn Care Res. 2021;42:144-151. doi:10.1093/jbcr/iraa128
- Copcu E. Marjolin’s ulcer: a preventable complication of burns? Plast Reconstr Surg. 2009;124:E156-E164. doi:10.1097/PRS.0b013e3181a8082e
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Coll Certif Wound Spec. 2011; 3:60-64. doi:10.1016/j.jcws.2012.04.001
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Spentzouris G, Labropoulos N. The evaluation of lower-extremity ulcers. Semin Intervent Radiol. 2009;26:286-295. doi:10.1055/s-0029-1242204
A 46-year-old man with a history of a left leg burn during childhood that was unsuccessfully treated with multiple skin grafts presented as a hospital follow-up for outpatient management of an ulcer. The patient had an ulcer that gradually increased in size over 7 years. Over the course of 2 weeks prior to the hospital presentation, he noted increased pain and severe difficulty with ambulation but remained afebrile without other systemic symptoms. Prior to the outpatient follow-up, he had been admitted to the hospital where he underwent imaging, laboratory studies, and skin biopsy, as well as treatment with empiric vancomycin. Physical examination revealed a large undermined ulcer with an elevated peripheral margin and crusting on the left lower leg with surrounding chronic scarring.
Cutaneous T-Cell Lymphoma Treatment: Case Series of Combination Therapy With Intralesional Injections of 5-Fluorouracil and Topical Imiquimod
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.
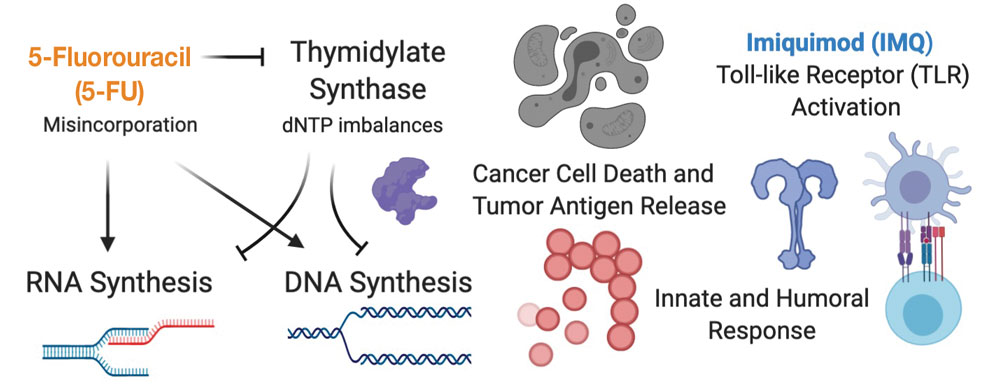
Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea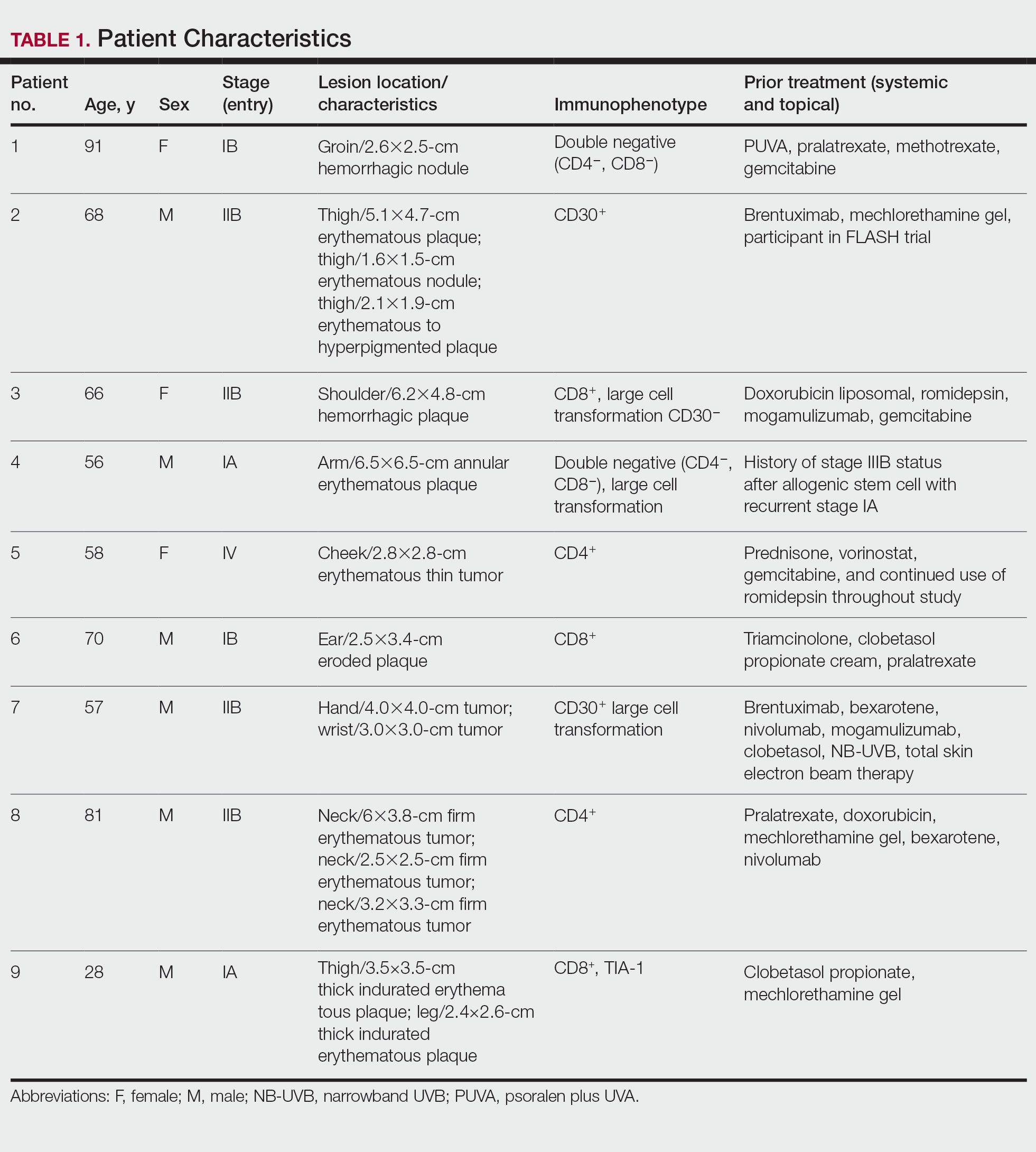
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.
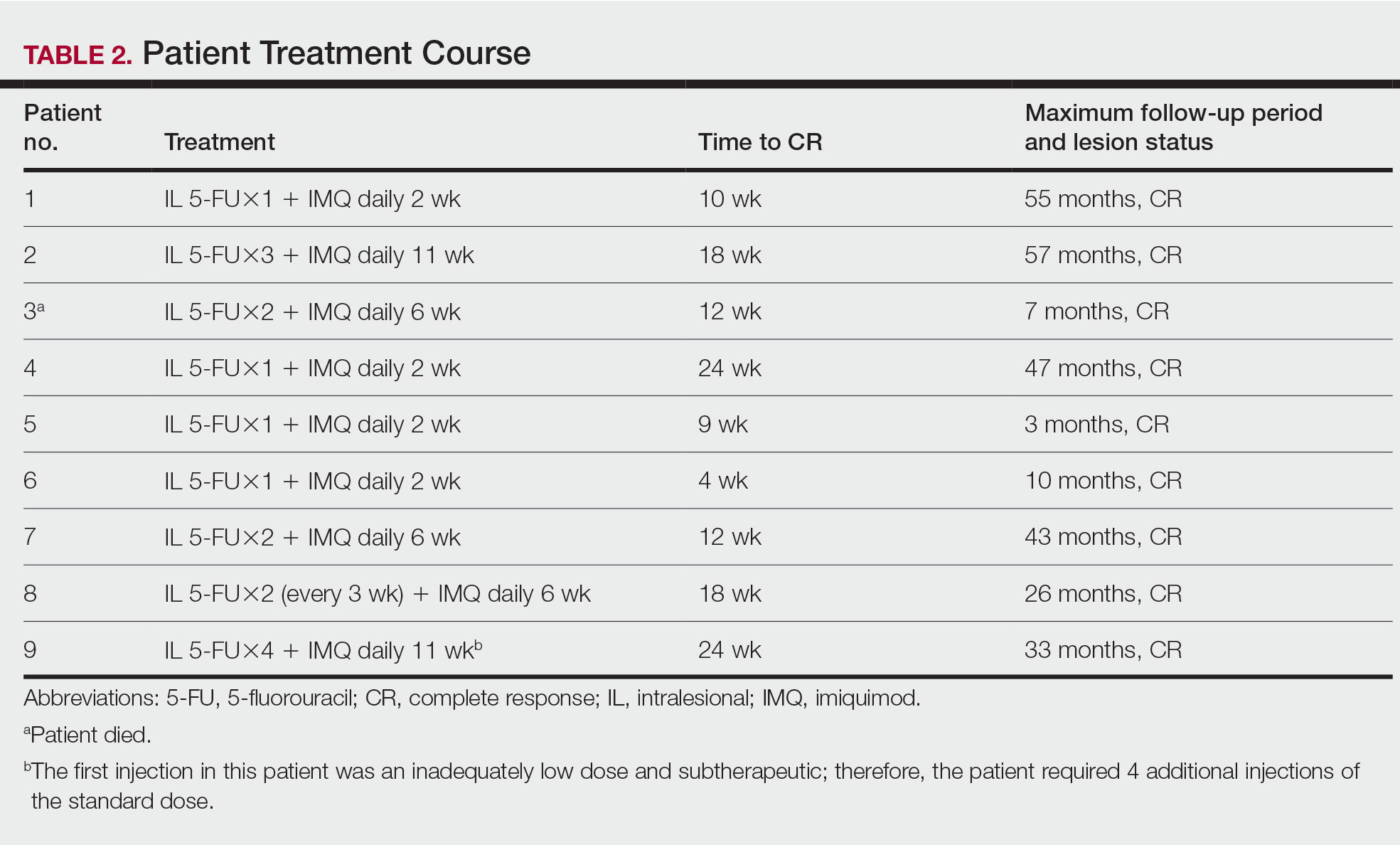
Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.
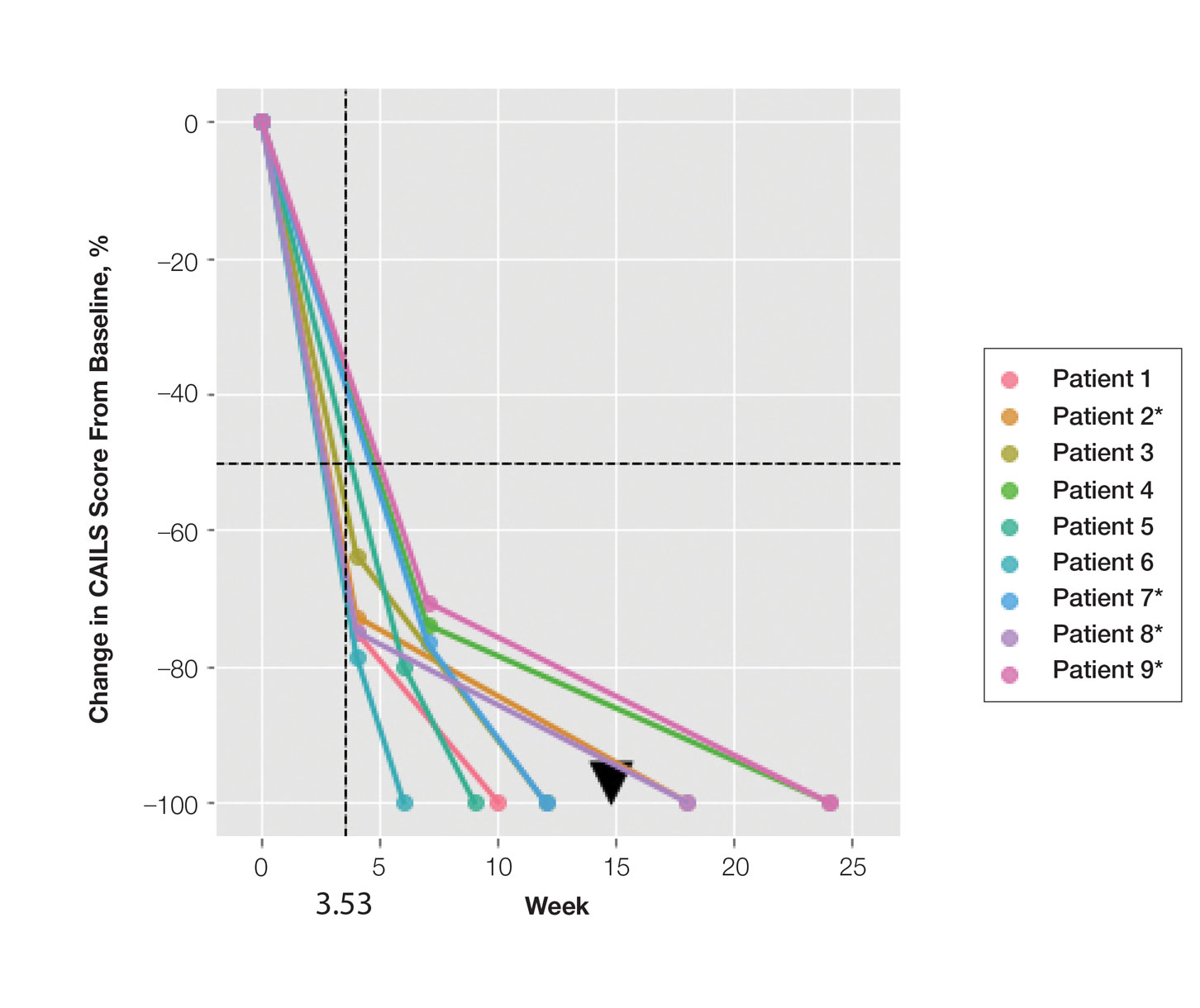
Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.
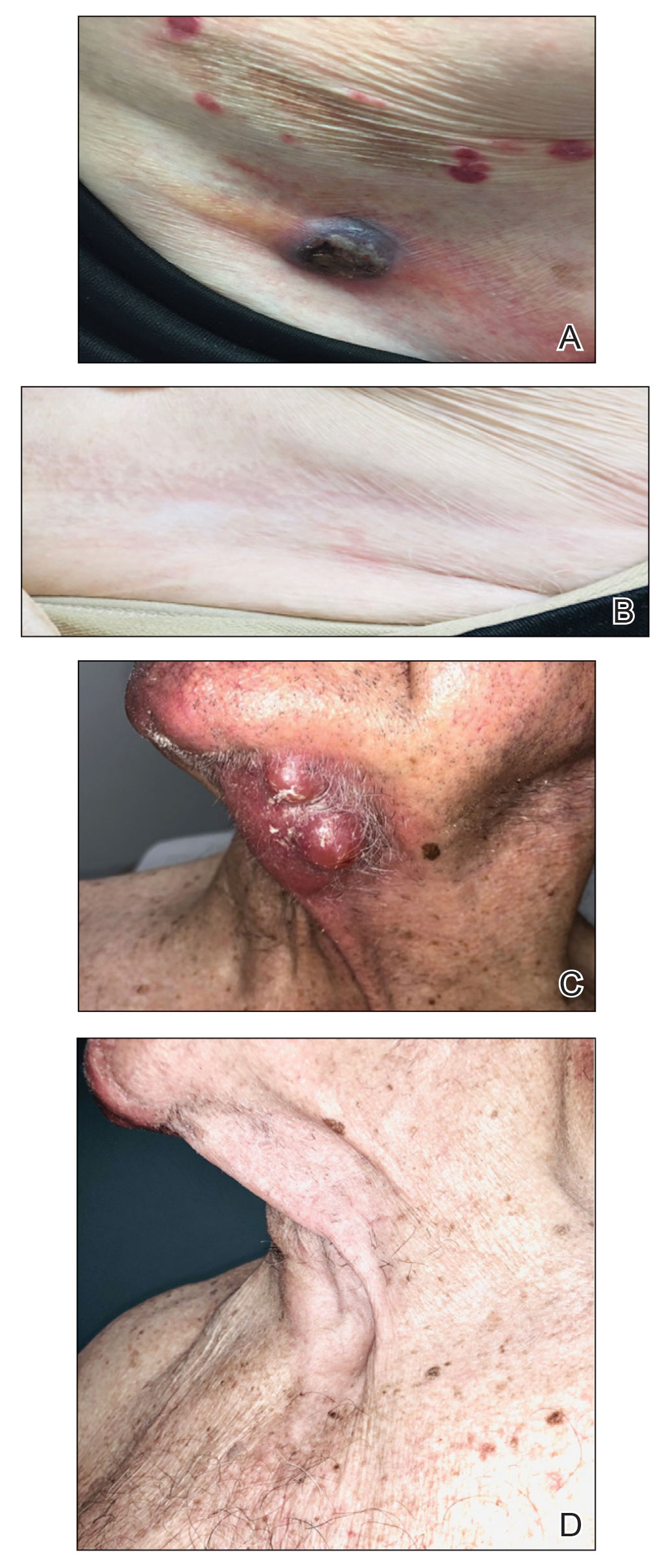
Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
Cutaneous T-cell lymphoma (CTCL) is a diverse group of skin-homing T-cell neoplasms with a wide array of clinical presentations, immunohistopathologic subtypes, and prognoses. The age-adjusted incidence of CTCL in the United States is 6.4 per million individuals.1 In the early stages of CTCL, the malignant lymphocytes are isolated to the skin, while more advanced disease involves metastatic spread to the lymphatic and peripheral blood compartments. Mycosis fungoides (MF) is the most common subtype of CTCL, comprising roughly 50% of all cases. The etiology of CTCL and MF remains poorly understood and no unifying driver mutation has been identified.2 However, recent sequencing efforts have revealed recurrent genomics alterations primarily in 3 pathways: constitutive T-cell activation, resistance to apoptosis/cell-cycle dysregulation, and DNA structural/gene expression dysregulation.3-8 These studies, among others, support the assertion that CTCL may be an epigenetic phenomenon.9-14
Most patients with MF will experience an indolent course of skin-limited disease with a favorable prognosis and a 5-year survival rate of 88%.15-17 A large study of patients with MF (N=525) followed for more than 40 years determined that approximately 20% of early-stage (IA-IIA) patients with MF progress to develop tumors, metastasis to the lymphatic tissue, and/or leukemic blood disease.18
Cutaneous T-cell lymphoma is a chronic disease, and most treatment responses are partial and short-lived. Allogenic hematopoietic transplantation is the only potentially curative option, and all other therapies are aimed at arresting progression and achieving remission.19 Skin-directed therapies include topical steroids, topical nitrogen mustard, phototherapy, and radiation. Systemic therapies such as oral retinoids, chemotherapy, and immunotherapy may be used alone or in combination with skin-directed therapies based on the overall disease stage and clinical presentation. Unfortunately, complete response (CR) to therapy is rare and fleeting, and most patients require multiple sequential treatments over their lifetimes.20
Across all stages of CTCL, there is a therapeutic push to combination and immune-based therapies to achieve more durable responses. The imidazoquinolines are a family of toll-like receptor (TLR) agonists including imiquimod (TLR7) and resiquimod (TLR7 and TLR8). Imiquimod (IMQ) is a topical immunomodulator, which increases the local cytotoxic helper T-cell profile (T
Skin-directed chemotherapy using 5-fluorouracil (5-FU) has shown activity against many cutaneous malignancies. 5-Fluorouracil is an antimetabolite drug that inhibits thymidylate synthase, resulting in interrupted DNA and RNA synthesis and leading to an apoptotic cell death (Figure 1). It has been administered via intravenous, oral (prodrug), intralesional (IL), and topical routes with well-documented success in treating cutaneous squamous cell carcinoma, keratoacanthoma, basal cell carcinoma, and precancerous actinic keratosis.35 As a topical, 5-FU has been shown to provide a good response in 6 patients with early MF.36 In late-stage MF, 5-FU has been used in combination with methotrexate as an infusion.37 We present a single-center case series of 9 patients with CTCL who received combination IL 5-FU and IMQ cream 5%.

Methods
Patient Selection—Patients were selected from our multidisciplinary CTCL subspecialty clinic at the Inova Schar Cancer Institute (Fairfax, Virginia). Patients with single to few recalcitrant CTCL plaques or tumors that were symptomatic or otherwise bothersome were included. All patients had at least 2 prior skin-directed therapies that failed, and many had advanced-stage disease requiring systemic therapy. All patients provided verbal consent.
Study Materials and Evaluations—Patients received IL injections of 5-FU 50 mg/mL. The volume injected was approximately 0.2 cc per cubic centimeter of lesion tissue. Injections were repeated at 2- to 3-week intervals until the target lesions achieved an acute hemorrhagic phase characterized by erosion, flattening, and crust formation. The total number of serial injections administered ranged from 1 to 5. The patients concomitantly treated all lesions with IMQ cream 5% daily for a duration of 2 to 3 months.
Medical photography and physical examination were performed every 2 to 3 weeks until the hemorrhagic phase resolved and treated sites re-epithelialized. Index lesions were assessed using the Composite Assessment of Index Lesion Severity (CAILS) score by a single investigator for all patients.38 Scores were retrospectively assigned using the investigator’s detailed physical examination descriptions and extensive medical photography. Any hyperpigmentation was scored as residual disease, despite the fair interpretation of it as procedure-related postinflammatory dyspigmentation. Complete response was strictly defined as a CAILS score of 0. The patients were screened for possible systemic effects of IMQ, including the presence of fever, chills, fatigue, and myalgia. Patients were evaluated every 6 to 12 weeks as a standing follow-up.
Statistical Analysis—Reductions were calculated using local regression from baseline to the 4- to 7-week follow-up. Patients with multiple lesions had their CAILS score averaged at ea
Results
Nine patients aged 28 to 91 years (median age, 66 years) with CTCL stages IA to IVA2, who had lesions located throughout their body, achieved CR; 3 patients were female (Table 1). The most common phenotype was CD8+ (n=3). All patients had at least 2 prior skin-directed therapies at treatment sites that failed, and 1 patient had 7 prior treatments that failed. Prior treatments included a variety of modalities, including all standard-of-care options and enrollment in clinical trials. One patient died from pneumonia following CR (Table 2). Seven patients had previously received systemic therapy for CTCL, and 1 patient was stable on romidepsin during our study. In patients who received more than 1 injection of 5-FU—1 injection: 3 patients; 2 injections: 3 patients; 3 injections: 1 patient; 4 injections: 1 patient; 5 injections: 1 patient—injections were spaced by 2 to 3 weeks. There was 1 patient who initially had an inadequate dosing of IL 5-FU and was restarted 14 months later; this was the patient with 5 total injections. This occurred in one of the first patients in the study, who presented with a facial lesion. The investigator used approximately 0.02 cc per cubic centimeter (dose reduction of nearly 90%), which was inadequate and did not achieve the requisite hemorrhagic phase.

Treatment was well tolerated overall. In all cases, a hemorrhagic phase was achieved, characterized by erosion and crusting that was rated as mildly uncomfortable by 7 patients and moderately uncomfortable by 2 patients. In total, 15 lesions in all 9 patients achieved a CR within 24 weeks of the final injection. The longest treatment course required 12 weeks of therapy with IMQ and 5 IL injections of 5-FU. The fastest CR was achieved in patient 6 within 6 weeks following a single IL injection of 5-FU and 2 applications of IMQ. The average time to CR was 14.78 weeks (95% CI, 1.75-27.81)(Figure 2), and the time to CR ranged from 4 to 24 weeks. On average, patients achieved more than 50% reduction in CAILS score by 3.53 weeks (95% CI, 1.55-5.51) and nearly a 4-fold (74.7%) reduction at the time of initial follow-up (occurring at 4–7 weeks). By 7 weeks, patient 3 had the most modest improvement in CAILS score with a 2.75-fold reduction, while patient 5 had the largest decrease with a 5-fold reduction. Figure 3 shows representative clinical photographs of 2 patients before and after treatment, with all patients having similar results.

Comment
Cutaneous T-cell lymphoma is a chronic skin cancer with a pattern of limited response to therapy and frequent recurrence. Currently available skin-directed therapies function as temporizing measures rather than curative treatments. Immunotherapy offers the promise of lasting disease control even after cessation of treatment, as it may essentially awaken cutaneous immune surveillance to malignant lymphocytes.

Several small observational studies have evaluated topical IMQ and TLR agonist therapy in CTCL. The construct of prior reports varies widely, including many different pretreatments, dosing schemes, and follow-up periods.24-33 Dosing intervals with IMQ ranged from daily to 3 times per week and treatment duration from 2 weeks to 1 year. Complete response rates from 50% to 100% were reported, and partial responses were observed in all but 1 patient, with recurrence-free follow-up ranging from 6 months to 8 years. Comparatively, combining IL 5-FU and IMQ appears to be at least as effective as IMQ alone or in other sequential treatments and combinations.24-33
Resiquimod, an experimental TLR7/8 agonist, has shown promising results in CTCL. Rook et al34 conducted a phase 1 trial of topical resiquimod in 12 early-stage patients with CTCL, all of whom responded to therapy. Two patients achieved CR, and 9 achieved a partial response, including 5 patients with the folliculotropic subtype. Interestingly, an abscopal effect was observed in 92% (11/12) of patients. Molecular evidence of reduction of the malignant clone was observed in 90% of patients via high-throughput sequencing of lesional tissue.34 These exciting findings suggest that topical immune therapy with TLR agonists may achieve robust, sustained, and possibly global disease control in CTCL.
Topical therapies are limited by depth of absorption, which can present a barrier to using these treatments for thicker plaques and tumors. Combining IL and topical routes was critical in our study design. Having good clinical experience using IL 5-FU in nonmelanoma skin cancers, we hypothesized that IL 5-FU would achieve a cytotoxic response through the full depth of thicker lesions and erode the surface of these lesions to facilitate penetration of topical IMQ. We additionally hypothesized that the combination of mechanisms of action would lead to an additive or synergistic response (Figure 1). By first inducing apoptotic cell death via 5-FU, we hoped to spill malignant lymphocyte neoantigens. Coupling that antigen exposure with an enhanced T
In our case series, all 15 lesions in 9 patients completely cleared, and no recurrences were observed at 26-month follow-up. No patients encountered any major adverse events, and the procedure was well tolerated by all.
Study Limitations—Limitations of this small study certainly exist. It is impossible to prove that our mechanistic theory is accurate given our strictly clinical assessment tools. We speculate that if our results had been achieved with IL 5-FU alone, future investigation with a prospective study using multiple treatment arms including a control would be warranted. Kannangara et al36 reported the use of topical 5-FU for MF and the drug’s utility in either topical or IL routes for CTCL, which deserves further study. It is less likely that results were achieved exclusively by IMQ because of the rapid tissue breakdown observed in the acute hemorrhagic phase. This phenomenon is best explained by the sudden apoptosis caused by DNA intercalation from 5-FU. The follow-up period is not uniform because this was a rolling enrollment study. Follow-up will be ongoing, and we aim to assess all patients up to at least the 5-year point. A final limitation of this study is the purely clinical end point. In the future, pretreatment and posttreatment biopsies would be useful in assessing proof of histologic response, and high-throughput sequencing may be used to look for molecular clearance via liquid biopsy. Lastly, careful observation for possible abscopal effect using the Severity-Weighted Assessment Tool score would be interesting and potentially contributory to our understanding of the impact of topical immune therapy on cutaneous tumor surveillance.
Conclusion
Combination IL 5-FU and topical IMQ is a well-tolerated, effective, and durable therapy for recalcitrant thick plaques and tumors of CTCL. This treatment is convenient and cost-effective. The procedure is performed in less than 5 minutes in an outpatient dermatology clinic. All patients received full insurance coverage for both drug and procedure fees under Medicare and commercial carriers.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-859.
- DeSimone JA, Sodha P, Ignatova D, et al. Recent advances in primary cutaneous T-cell lymphoma. Curr Opin Oncol. 2015;27:128-133.
- Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011-1019.
- Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056-1060.
- McGirt LY, Jia P, Baerenwald DA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126:508-519.
- da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47:1465-1470.
- Litvinov IV, Netchiporouk E, Cordeiro B, et al. The use oftranscriptional profiling to improve personalized diagnosis and management of cutaneous T-cell lymphoma (CTCL). Clin Cancer Res. 2015;21:2820-2829.
- Cyrenne BM, Lewis JM, Weed JG, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130:2073-2083.
- Cancer Genome Atlas Research Network; Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113-1120.
- Kiel MJ, Sahasrabuddhe AA, Rolland DCM, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. 2015;6:8470.
- Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426-1434.
- van Doorn R, Slieker RC, Boonk SE, et al. Epigenomic analysis of Sézary syndrome defines patterns of aberrant DNA methylation and identifies diagnostic markers. J Invest Dermatol. 2016;136:1876-1884.
- Qiu L, Liu F, Yi S, et al. Loss of 5-hydroxymethylcytosine is an epigenetic biomarker in cutaneous T-cell lymphoma. J Invest Dermatol. 2018;138:2388-2397.
- Kim SR, Lewis JM, Cyrenne BM, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9:29193-29207.
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-16.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Lechowicz MJ, Lazarus HM, Carreras J, et al. Allogeneic hematopoietic cell transplantation for mycosis fungoides and Sezary syndrome. Bone Marrow Transplant. 2014;49:1360-1365.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome, part II: prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196-200.
- Gibson SJ, Lindh JM, Riter TR, et al. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74-86.
- Schön MP, Schön M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190-199.
- Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137-1139.
- Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116-118.
- Didona B, Benucci R, Amerio P, et al. Primary cutaneous CD30+ T-cell lymphoma responsive to topical imiquimod (Aldara). Br J Dermatol. 2004;150:1198-1201.
- Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275-280.
- Coors EA, Schuler G, Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391-393.
- Chiam LYT, Chan YC. Solitary plaque mycosis fungoides on the penis responding to topical imiquimod therapy. Br J Dermatol. 2007;156:560-562.
- Soler-Machín J, Gilaberte-Calzada Y, Vera-Alvarez J, et al. Imiquimod in treatment of palpebral mycosis fungoides. Article in Spanish. Arch Soc Esp Oftalmol. 2006;81:221-223.
- Martínez-González MC, Verea-Hernando MM, Yebra-Pimentel MT, et al. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148-152.
- Gordon MC, Sluzevich JC, Jambusaria-Pahlajani A. Clearance of folliculotropic and tumor mycosis fungoides with topical 5% imiquimod. JAAD Case Rep. 2015;1:348-350.
- Lewis DJ, Byekova YA, Emge DA, et al. Complete resolution of mycosis fungoides tumors with imiquimod 5% cream: a case series. J Dermatolog Treat. 2017;28:567-569.
- Rook AH, Gelfand JM, Wysocka M, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126:1452-1461.
- Morse LG, Kendrick C, Hooper D, et al. Treatment of squamous cell carcinoma with intralesional 5-fluorouracil. Dermatol Surg. 2003;29:1150-1153.
- Kannangara AP, Levitan D, Fleischer AB Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9:1017-1018.
- Schappell DL, Alper JC, McDonald CJ. Treatment of advanced mycosis fungoides and Sézary syndrome with continuous infusions of methotrexate followed by fluorouracil and leucovorin rescue. Arch Dermatol. 1995;131:307-313.
- Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598-2607.
PRACTICE POINTS
- Cutaneous T-cell lymphoma (CTCL) is a chronic lymphoma affecting the skin with limited durable effective skin-directed therapies.
- Combination intralesional 5-fluorouracil and topical imiquimod is a well-tolerated, fast, convenient, and durable therapy for recalcitrant thick plaques and tumors of CTCL.
- This regimen may be utilized as monotherapy or as the skin-directed component of combination therapy based on disease stage.
Microneedling With Bimatoprost to Treat Hypopigmented Skin Caused by Burn Scars
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.
Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).
We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

To the Editor:
Microneedling is a percutaneous collagen induction therapy frequently used in cosmetic dermatology to promote skin rejuvenation and hair growth and to treat scars by taking advantage of the body’s natural wound-healing cascade.1 The procedure works by generating thousands of microscopic wounds in the dermis with minimal damage to the epidermis, thus initiating the wound-healing cascade and subsequently promoting collagen production in a manner safe for all Fitzpatrick classification skin types.1-3 This therapy effectively treats scars by breaking down scarred collagen and replacing it with new healthy collagen. Microneedling also has application in drug delivery by increasing the permeability of the skin; the microwounds generated can serve as a portal for drug delivery.4
Bimatoprost is a prostaglandin analogue typically used to treat hypotrichosis and open-angle glaucoma.5-7 A known side effect of bimatoprost is hyperpigmentation of surrounding skin; the drug increases melanogenesis, melanocyte proliferation, and melanocyte dendricity, resulting in activation of the inflammatory response and subsequent prostaglandin release, which stimulates melanogenesis. This effect is similar to UV radiation–induced inflammation and hyperpigmentation.6,8
Capitalizing on this effect, a novel application of bimatoprost has been proposed—treating vitiligo, in which hypopigmentation results from destruction of melanocytes in certain areas of the skin. Bimatoprost ophthalmic solution 0.3% utilized as an off-label treatment for vitiligo has been shown to notably increase melanogenesis and return pigmentation to hypopigmented areas.8-10
A 32-year-old Black woman presented to our clinic with a 40×15-cm scar that was marked by postinflammatory hypopigmentation from a second-degree burn on the right proximal arm. The patient had been burned 5 months prior by boiling water that was spilled on the arm while cooking. She had immediately sought treatment at an emergency department and subsequently in a burn unit, where the burn was debrided twice; medication was not prescribed to continue treatment. The patient reported that the scarring and hypopigmentation had taken a psychologic toll; her hope was to have pigmentation restored to the affected area to boost her confidence.
Physical examination revealed that the burn wound had healed but visible scarring and severe hypopigmentation due to destroyed melanocytes remained (Figure 1). To inhibit inflammation and stimulate repigmentation, we prescribed the calcineurin inhibitor tacrolimus ointment 0.1% to be applied daily to the affected area. The patient returned to the clinic 1 month later. Perifollicular hyperpigmentation was noted at the site of the scar.

Monthly microneedling sessions with bimatoprost ophthalmic solution 0.3% were started. To avoid damaging any potentially remaining unhealed hypodermis and vasculature, the first microneedling session was performed with 9 needles set at minimal needle depth and frequency. The number of needles and their depth and frequency gradually were increased with each subsequent treatment. The patient continued tacrolimus ointment 0.1% throughout the course of treatment.
For each microneedling procedure, a handheld motorized microneedling device was applied to the skin at a depth of 0.25 mm, which was gradually increased until pinpoint petechiae were achieved. Bimatoprost ophthalmic solution 0.3% was then painted on the skin and allowed to absorb. Microneedling was performed again, ensuring that bimatoprost entered the skin in the area of the burn scar.
Microneedling procedures were performed monthly for 6 months, then once 3 months later, and once more 3 months later—8 treatments in total over the course of 1 year. Improvement in skin pigmentation was noted at each visit (Figure 2). Repigmentation was first noticed surrounding hair follicles; after later visits, it was observed that pigmentation began to spread from hair follicles to fill in remaining skin. The darkest areas of pigmentation were first noted around hair follicles; over time, melanocytes appeared to spontaneously regenerate and fill in surrounding areas as the scar continued to heal. The patient continued use of tacrolimus during the entire course of microneedling treatments and for the following 4 months. Sixteen months after initiation of treatment, the appearance of the skin was texturally smooth and returned to almost its original pigmentation (Figure 3).

We report a successful outcome in a patient with a hypopigmented burn scar who was treated with bimatoprost administered with traditional microneedling and alongside a tacrolimus regimen. Tacrolimus ointment inhibited the inflammatory response to allow melanocytes to heal and regenerate; bimatoprost and microneedling promoted hyperpigmentation of hair follicles in the affected area, eventually restoring pigmentation to the entire area. Our patient was extremely satisfied with the results of this combination treatment. She has reported feeling more confident going out and wearing short-sleeved clothing. Percutaneous drug delivery of bimatoprost ophthalmic solution 0.3% combined with topical tacrolimus may be an effective treatment for skin repigmentation. Further investigation of this regimen is needed to develop standardized treatment protocols.

- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
- Juhasz MLW, Cohen JL. Micro-needling for the treatment of scars: an update for clinicians. Clin Cosmet Investig Dermatol. 2020;13:997-1003. doi:10.2147/CCID.S267192
- Alster TS, Li MKY. Micro-needling of scars: a large prospective study with long-term follow-up. Plast Reconstr Surg. 2020;145:358-364. doi:10.1097/PRS.0000000000006462
- Aust MC, Knobloch K, Reimers K, et al. Percutaneous collagen induction therapy: an alternative treatment for burn scars. Burns. 2010;36:836-843. doi:10.1016/j.burns.2009.11.014
- Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547-1568. doi:10.1016/j.addr.2012.04.005
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. 2006;113:1961-1967. doi:10.1016/j.ophtha.2006.05.041
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005;123:1541-1546. doi:10.1001/archopht.123.11.1541
- Priluck JC, Fu S. Latisse-induced periocular skin hyperpigmentation. Arch Ophthalmol. 2010;128:792-793. doi:10.1001/archophthalmol.2010.89
- Grimes PE. Bimatoprost 0.03% solution for the treatment of nonfacial vitiligo. J Drugs Dermatol. 2016;15:703-710.
- Barbulescu C, Goldstein N, Roop D, et al. Harnessing the power of regenerative therapy for vitiligo and alopecia areata. J Invest Dermatol. 2020;140: 29-37. doi:10.1016/j.jid.2019.03.1142
- Kanokrungsee S, Pruettivorawongse D, Rajatanavin N. Clinicaloutcomes of topical bimatoprost for nonsegmental facial vitiligo: a preliminary study. J Cosmet Dermatol. 2021;20:812-818. doi.org/10.1111/jocd.13648
PRACTICE POINTS
- Microneedling is a percutaneous collagen induction therapy that also may be used in drug delivery.
- Hypopigmentation can cause considerable distress for patients with skin of color.
- Percutaneous drug delivery of bimatoprost may be helpful in skin repigmentation.