User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Vitiligo
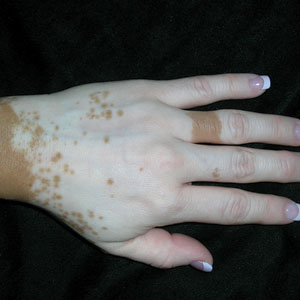
THE COMPARISON
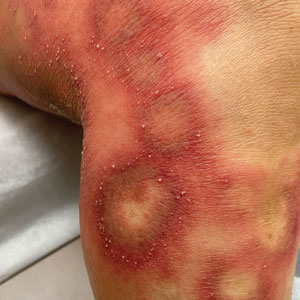
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
Janus Kinase Inhibitors: A Promising Therapeutic Option for Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29
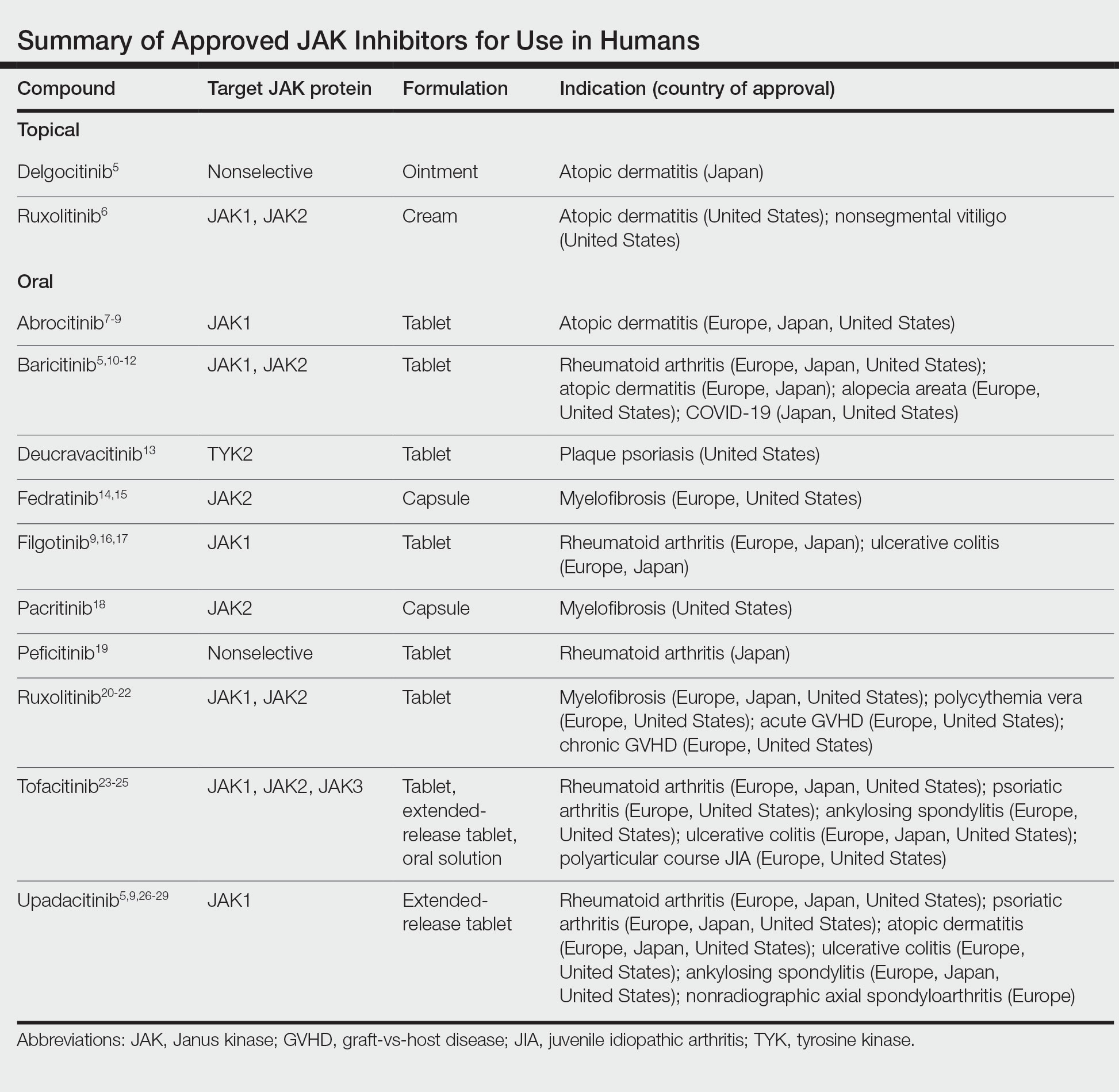
Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
PRACTICE POINTS
- Janus kinase (JAK) inhibitors are a novel class of small molecule inhibitors that modulate the JAK/signal transducer and activator of transcription signaling pathway.
- Select JAK inhibitors have been approved by the US Food and Drug Administration for the management of atopic dermatitis. Their use in allergic contact dermatitis is under active investigation.
- Regular follow-up and laboratory monitoring for patients on oral JAK inhibitors is recommended, given the potential for treatment-related adverse effects.
Dome-Shaped Periorbital Papule
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
A 76-year-old woman presented with a slowly growing, asymptomatic, 5-mm, pink-brown, dome-shaped papule adjacent to the left lateral canthus of several years’ duration. Dermoscopic examination revealed fine linear peripheral blood vessels. The lesional cells were positive with cytokeratin 7, estrogen receptor, progesterone receptor, chromogranin, synaptophysin, and neuron-specific enolase. Cytokeratin 20 and p63 were negative, and the Ki-67 proliferative index was less than 5%.
Bone Wax as a Physical Hemostatic Agent
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.
Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
Dermatology Articles in Preprint Servers: A Cross-sectional Study
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).
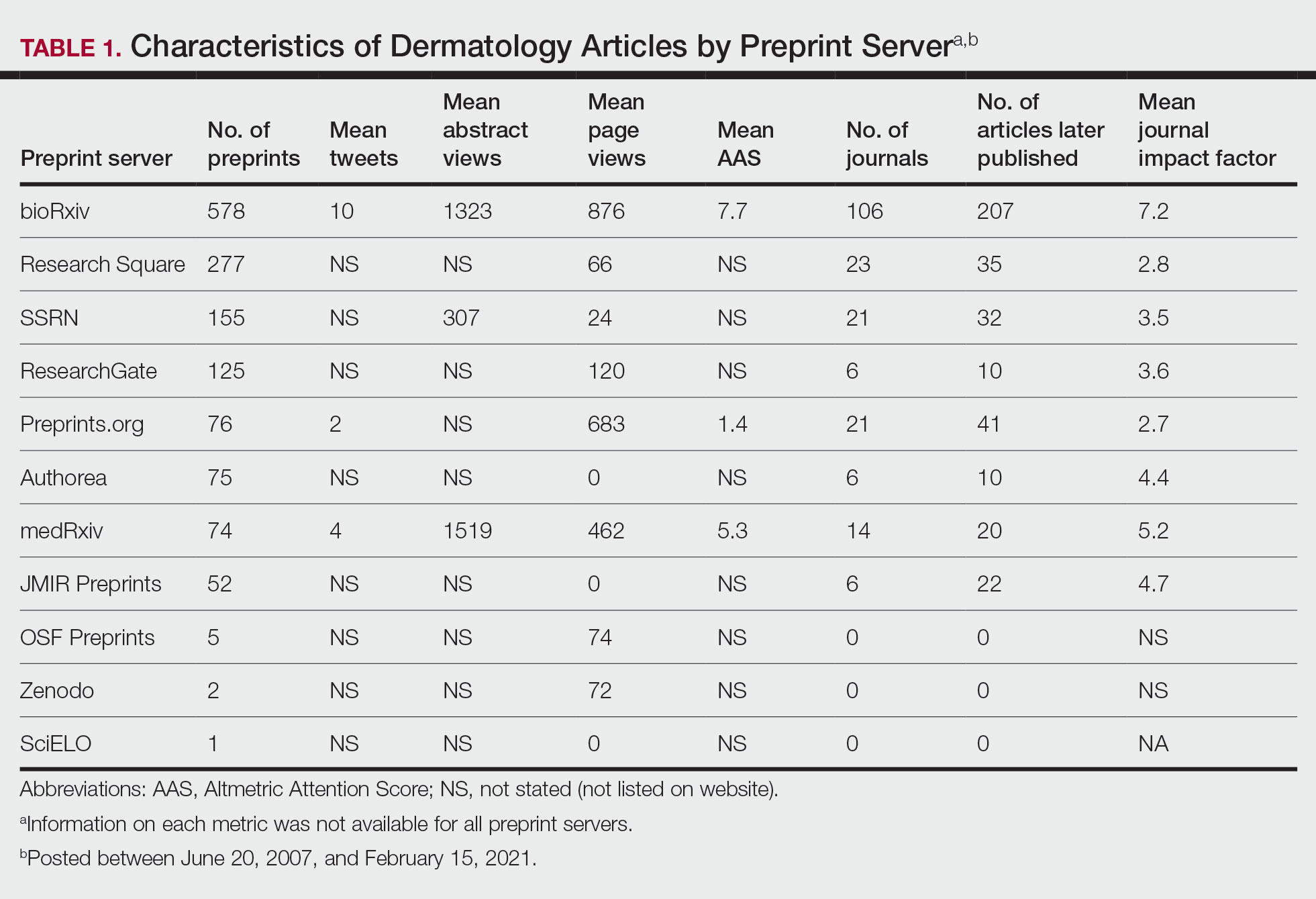
A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.
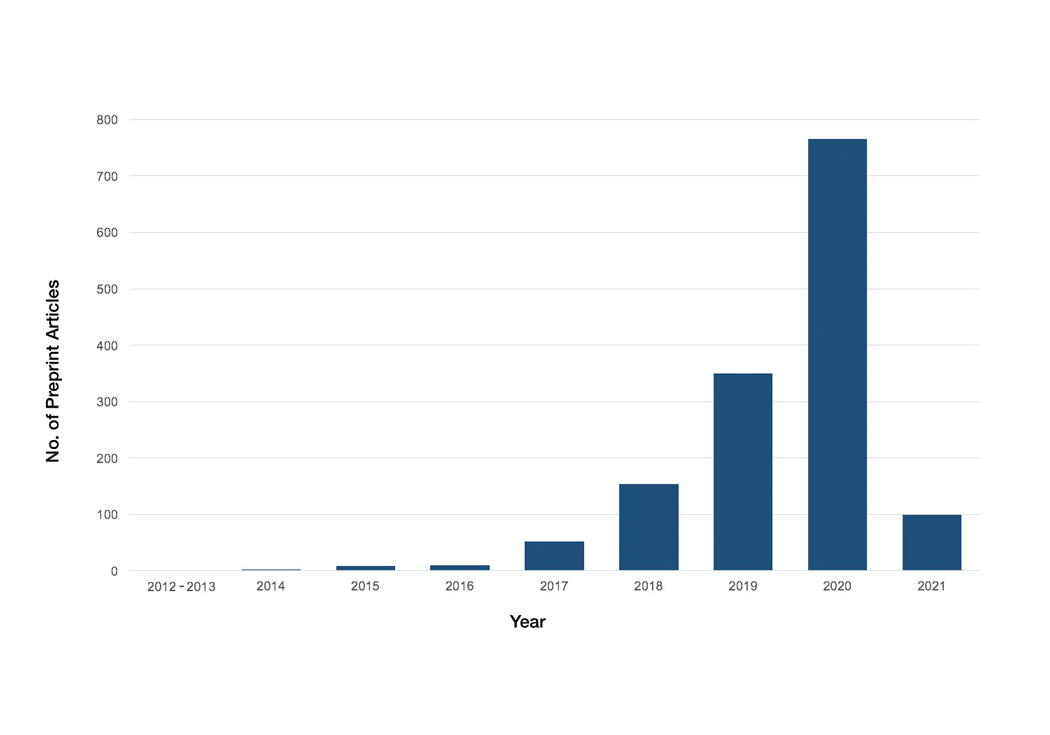
Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.
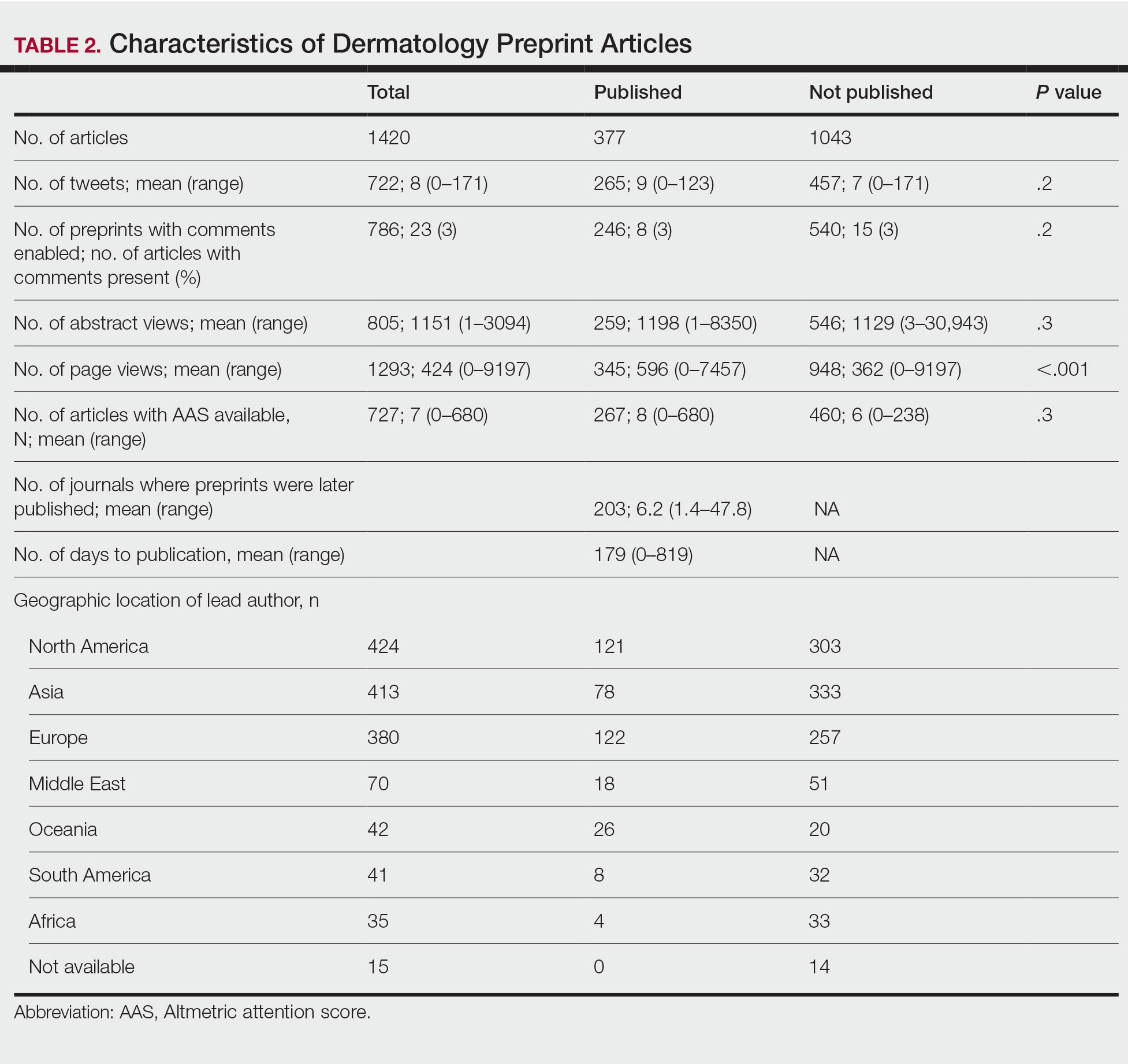
Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
PRACTICE POINTS
- Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals.
- The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission; therefore, dermatologists may use these servers to disseminate research quickly and freely but may not receive constructive criticism.
- Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice.
The Ins and Outs of Transferring Residency Programs
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
RESIDENT PEARL
- Transferring residency programs is difficult but possible. The decision to transfer residencies may be anxiety producing, but with substantial motives, the rewards of transferring can be worthwhile.
Hemorrhagic Lacrimation and Epistaxis: Rare Findings in Acute Hemorrhagic Edema of Infancy
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.
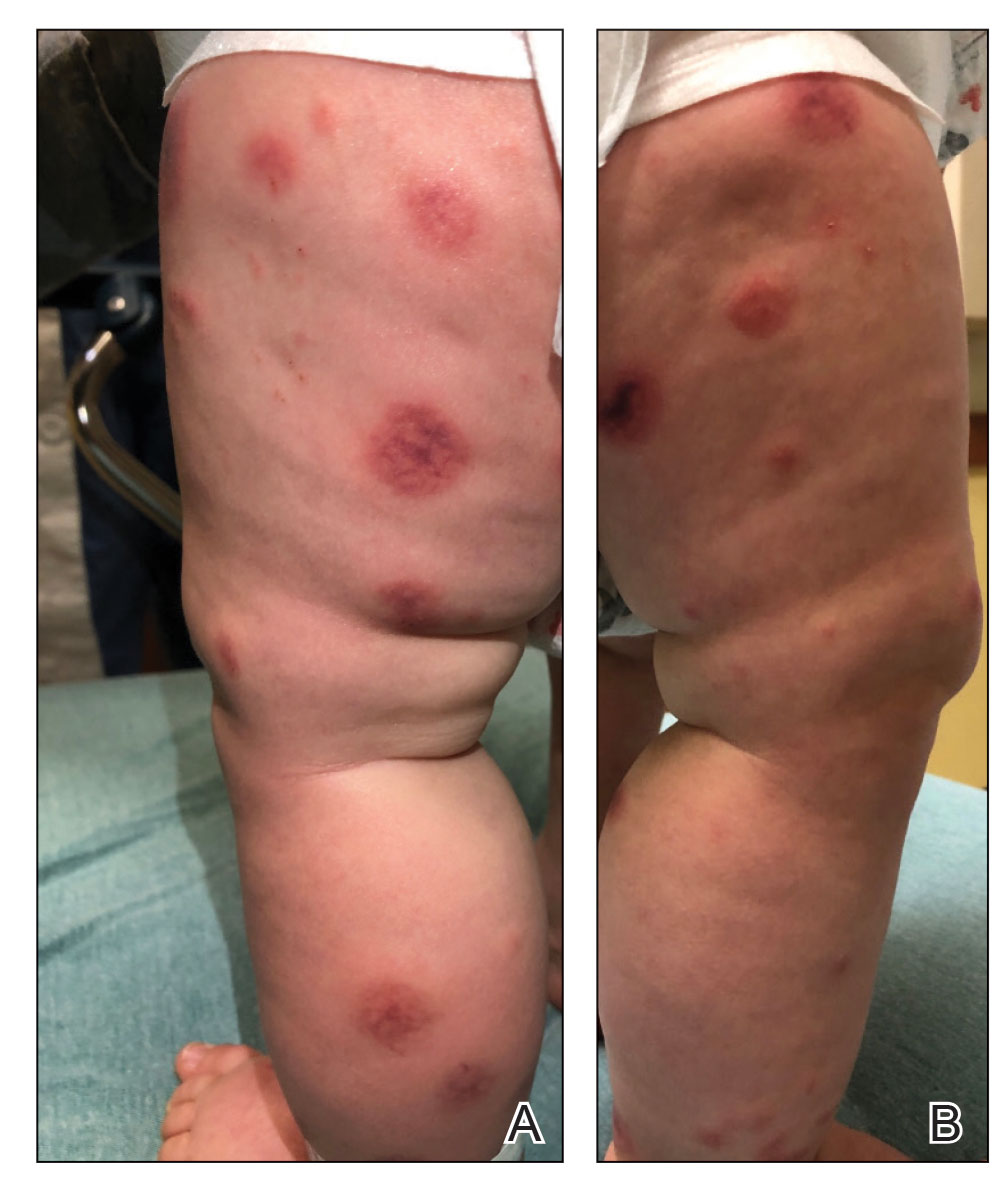
A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.
Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.

A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.

Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.

A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.

Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
PRACTICE POINTS
- Acute hemorrhagic edema of infancy (AHEI) is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.
- It is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.
- On rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis. Patients should be assured that the condition is self-limited and resolves without permanent sequalae.
Fungal Osler Nodes Indicate Candidal Infective Endocarditis
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.
Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).
Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.
Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.

Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).

Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.

Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
To the Editor:
A 44-year-old woman presented with a low-grade fever (temperature, 38.0 °C) and painful acral lesions of 1 week’s duration. She had a history of hepatitis C viral infection and intravenous (IV) drug use, as well as polymicrobial infective endocarditis that involved the tricuspid and aortic valves; pathogenic organisms were identified via blood culture as Enterococcus faecalis, Serratia species, Streptococcus viridans, and Candida albicans. The patient had received a mechanical aortic valve and bioprosthetic tricuspid valve replacement 5 months prior with warfarin therapy and had completed a postsurgical 6-week course of high-dose micafungin. She reported that she had developed painful, violaceous, thin papules on the plantar surface of the left foot 2 weeks prior to presentation. Her symptoms improved with a short systemic steroid taper; however, within a week she developed new tender, erythematous, thin papules on the plantar surface of the right foot and the palmar surface of the left hand with associated lower extremity swelling. She denied other symptoms, including fever, chills, neurologic symptoms, shortness of breath, chest pain, nausea, vomiting, hematuria, and hematochezia. Due to worsening cutaneous findings, the patient presented to the emergency department, prompting hospital admission for empiric antibacterial therapy with vancomycin and piperacillin-tazobactam for suspected infectious endocarditis. Dermatology was consulted after 1 day of antibacterial therapy without improvement to determine the etiology of the patient’s skin findings.
Physical examination revealed the patient was afebrile with partially blanching violaceous to purpuric, tender, edematous papules on the left fourth and fifth finger pads, as well as scattered, painful, purpuric patches with stellate borders on the right plantar foot (Figure 1). Laboratory test results revealed mild anemia (hemoglobin, 11.9 g/dL [reference range, 12.0–15.0 g/dL], mild neutrophilia (neutrophils, 8.4×109/L [reference range, 1.9–7.9×109/L], elevated acute-phase reactants (erythrocyte sedimentation rate, 71 mm/h [reference range, 0–20 mm/h]; C-reactive protein, 5.7 mg/dL [reference range, 0.0–0.5 mg/dL]), and positive hepatitis C virus antibody with an undetectable viral load. At the time of dermatologic evaluation, admission blood cultures and transthoracic echocardiogram were negative. Additionally, a transesophageal echocardiogram, limited by artifact from the mechanical aortic valve, was equivocal for valvular pathology. Subsequent ophthalmologic evaluation was negative for lesions associated with endocarditis, such as retinal hemorrhages.

Punch biopsies of the left fourth finger pad were submitted for histopathologic analysis and tissue cultures. Histopathology demonstrated deep dermal perivascular neutrophilic inflammation with multiple intravascular thrombi, perivascular fibrin, and karyorrhectic debris (Figure 2). Periodic acid–Schiff and Grocott-Gomori methenamine-silver stains revealed fungal spores with rare pseudohyphae within the thrombosed vascular spaces and the perivascular dermis, consistent with fungal septic emboli (Figure 3).

Empiric systemic antifungal coverage composed of IV liposomal amphotericin B and oral flucytosine was initiated, and the patient’s tender acral papules rapidly improved. Within 48 hours of biopsy, skin tissue culture confirmed the presence of C albicans. Four days after the preliminary dermatopathology report, confirmatory blood cultures resulted with pansensitive C albicans. Final tissue and blood cultures were negative for bacteria including mycobacteria. In addition to a 6-week course of IV amphotericin B and flucytosine, repeat surgical intervention was considered, and lifelong suppressive antifungal oral therapy was recommended. Unfortunately, the patient did not present for follow-up. Three months later, she presented to the emergency department with peritonitis; in the operating room, she was found to have ischemia of the entirety of the small and large intestines and died shortly thereafter.

Fungal endocarditis is rare, tending to develop in patient populations with particular risk factors such as immune compromise, structural heart defects or prosthetic valves, and IV drug use. Candida infective endocarditis (CIE) represents less than 2% of infective endocarditis cases and carries a high mortality rate (30%–80%).1-3 Diagnosis may be challenging, as the clinical presentation varies widely. Although some patients may present with classic features of infective endocarditis, including fever, cardiac murmurs, and positive blood cultures, many cases of infective endocarditis present with nonspecific symptoms, raising a broad clinical differential diagnosis. Delay in diagnosis, which is seen in 82% of patients with fungal endocarditis, may be attributed to the slow progression of symptoms, inconclusive cardiac imaging, or negative blood cultures seen in almost one-third of cases.2,3 The feared complication of systemic embolization via infective endocarditis may occur in up to one-half of cases, with the highest rates associated with staphylococcal or fungal pathogens.2 The risk for embolization in fungal endocarditis is independent of the size of the cardiac valve vegetations; accordingly, sequelae of embolic complications may arise despite negative cardiac imaging.4 Embolic complications, which typically are seen within the first 2 to 4 weeks of treatment, may serve as the presenting feature of endocarditis and may even occur after completion of antimicrobial therapy.
Detection of cutaneous manifestations of infective endocarditis, including Janeway lesions, Osler nodes, and splinter hemorrhages, may allow for earlier diagnosis. Despite eponymous recognition, Janeway lesions and Osler nodes are relatively uncommon manifestations of infective endocarditis and may be found in only 5% to 15% of cases.5 Biopsies of suspected Janeway lesions and Osler nodes may allow for recognition of relevant vascular pathology, identification of the causative pathogen, and strong support for the diagnosis of infective endocarditis.4-7
The initial photomicrograph of corresponding Janeway lesion histopathology was published by Kerr in 1955 and revealed dermal microabscesses posited to be secondary to bacterial emboli.8,9 Additional cases through the years have reported overlapping histopathologic features of Janeway lesions and Osler nodes, with the latter often defined by the presence of vasculitis.4 Although there appears to be ongoing debate and overlap between the 2 integumentary findings, a general consensus on differentiation takes into account both the clinical signs and symptoms as well as the histopathologic findings.10,11
Osler nodes present as tender, violaceous, subcutaneous nodules on the acral surfaces, usually on the pads of the fingers and toes.5 The pathogenesis involves the deposition of immune complexes as a sequela of vascular occlusion by microthrombi classically seen in the late phase of subacute endocarditis. Janeway lesions present as nontender erythematous macules on the acral surfaces and are thought to represent microthrombi with dermal microabscesses, more common in acute endocarditis. Our patient demonstrated features of both Osler nodes and Janeway lesions. Despite the presence of fungal thrombi—a pathophysiology closer to that of Janeway lesions—the clinical presentation of painful acral nodules affecting finger pads and histologic features of vasculitis may be better characterized as Osler nodes. Regardless of pathogenesis, these cutaneous findings serve as a minor clinical criterion in the Duke criteria for the diagnosis of infective endocarditis when present.12
Candida infective endocarditis should be suspected in a patient with a history of valvular disease or prior infective endocarditis with fungemia, unexplained neurologic signs, or manifestations of peripheral embolization despite negative blood cultures.3 Particularly in the setting of negative cardiac imaging, recognition of CIE requires heightened diagnostic acumen and clinicopathologic correlation. Although culture and pathologic examination of valvular vegetations represents the gold standard for diagnosis of CIE, aspiration and culture of easily accessible septic emboli may provide rapid identification of the etiologic pathogen. In 1976, Alpert et al13 identified C albicans from an aspirated Osler node. Postmortem examination revealed extensive involvement of the homograft valve and aortic root with C albicans.13 Many other examples exist in the literature demonstrating matching pathogenic isolates from microbiologic cultures of skin and blood.4,9,14,15 Thadepalli and Francis7 investigated 26 cases of endocarditis in heroin users in which the admitting diagnosis was endocarditis in only 4 cases. The etiologic pathogen was aspirated from secondary sites of localized infections secondary to emboli, including cutaneous lesions in 10 of the cases. Gram stain and culture revealed the causative organism leading to the ultimate diagnosis and management in 17 of 26 patients with endocarditis.7
The incidence of fungal endocarditis is increasing, with a reported 67% of cases caused by nosocomial infection.1 Given the rising incidence of fungal endocarditis and its accompanying diagnostic difficulties, including frequently negative blood cultures and cardiac imaging, clinicians must perform careful skin examinations, employ judicious use of skin biopsy, and carefully correlate clinical and pathologic findings to improve recognition of this disease and guide patient care.
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
- Arnold CJ, Johnson M, Bayer AS, et al. Infective endocarditis: an observational cohort study with a focus on therapy. Antimicrob Agents Chemother. 2015;59:2365. doi:10.1128/AAC.04867-14
- Chaudhary SC, Sawlani KK, Arora R, et al. Native aortic valve fungal endocarditis. BMJ Case Rep. 2013;2013:bcr2012007144. doi:10.1136/bcr-2012-007144
- Ellis ME, Al-Abdely H, Sandridge A, et al. Fungal endocarditis: evidence in the world literature, 1965–1995. Clin Infect Dis. 2001;32:50-62. doi:10.1086/317550
- Gil MP, Velasco M, Botella R, et al. Janeway lesions: differential diagnosis with Osler’s nodes. Int J Dermatol. 1993;32:673-674. doi:10.1111/j.1365-4362.1993.tb04025.x
- Gomes RT, Tiberto LR, Bello VNM, et al. Dermatologic manifestations of infective endocarditis. An Bras Dermatol. 2016;91:92-94.
- Yee JM. Osler’s nodes and the recognition of infective endocarditis: a lesion of diagnostic importance. South Med J. 1987;80:753-757.
- Thadepalli H, Francis C. Diagnostic clues in metastatic lesions of endocarditia in addicts. West J Med. 1978;128:1-5.
- Kerr A Jr. Subacute Bacterial Endocarditis. Charles C. Thomas; 1955.
- Kerr A Jr, Tan JS. Biopsies of the Janeway lesion of infective endocarditis. J Cutan Pathol. 1979;6:124-129. doi:10.1111/j.1600-0560.1979.tb01113.x
- Marrie TJ. Osler’s nodes and Janeway lesions. Am J Med. 2008;121:105-106. doi:10.1016/j.amjmed.2007.07.035
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. Australas J Dermatol. 2007;48:251-255. doi:10.1111/j.1440-0960.2007.00397.x
- Durack DT, Lukes AS, Bright DK, et al. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200-209.
- Alpert JS, Krous HF, Dalen JE, et al. Pathogenesis of Osler’s nodes. Ann Intern Med. 1976;85:471-473. doi:10.7326/0003-4819-85-4-471
- Cardullo AC, Silvers DN, Grossman ME. Janeway lesions and Osler’s nodes: a review of histopathologic findings. J Am Acad Dermatol. 1990;22:1088-1090. doi:10.1016/0190-9622(90)70157-D
- Vinson RP, Chung A, Elston DM, et al. Septic microemboli in a Janeway lesion of bacterial endocarditis. J Am Acad Dermatol. 1996;35:984-985. doi:10.1016/S0190-9622(96)90125-5
PRACTICE POINTS
- Fungal infective endocarditis is rare, and diagnostic tests such as blood cultures and echocardiography may not detect the disease.
- The mortality rate of fungal endocarditis is high, with improved clinical outcomes if diagnosed and treated early.
- Clinicopathologic correlation between integumentary examination and skin biopsy findings may provide timely diagnosis, thereby guiding appropriate therapy.
Generalized Pustular Psoriasis Treated With Risankizumab
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.
Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.
Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.

Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.

Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.

Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.

Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
PRACTICE POINTS
- Generalized pustular psoriasis (GPP) is a potentially life-threatening condition that can be precipitated by systemic steroids.
- Although more than 20 systemic medications have been tried with varying success, there has not been a single US Food and Drug Administration–approved medication for GPP until recently with the approval of spesolimab, an IL-36 receptor inhibitor.
- Risankizumab, a high-affinity humanized monoclonal antibody that targets the p19 subunit of the IL-23 cytokine, also has shown promise in a recent phase 3, open-label study for GPP.