User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Serum Ferritin Levels: A Clinical Guide in Patients With Hair Loss
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
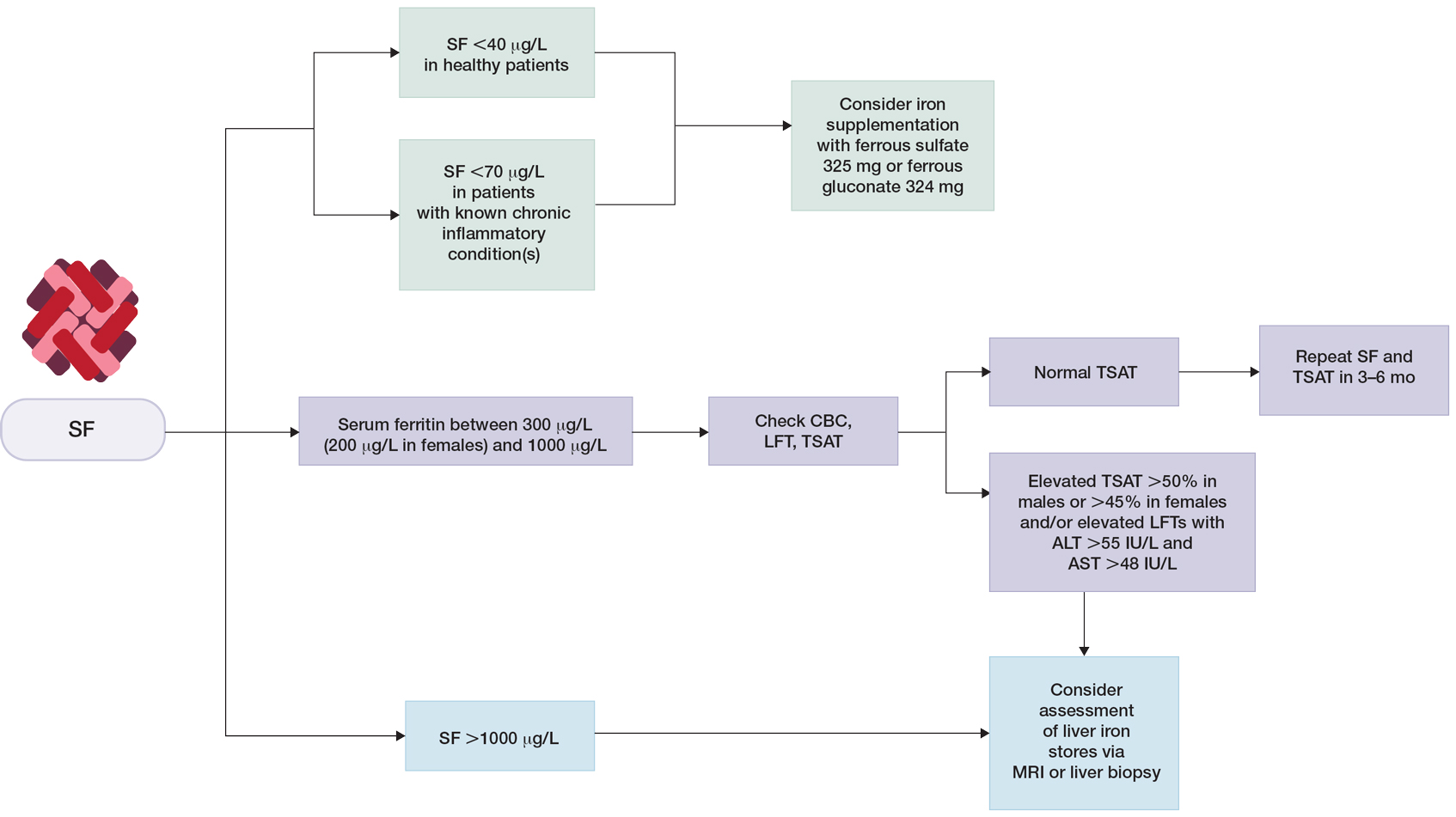
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.
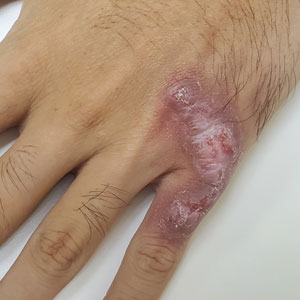
Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Practice Points
- In patients who are otherwise healthy without chronic systemic disease, hepatic disease, or inflammatory disorders, serum ferritin levels directly correlate with body iron status.
- Elevated serum ferritin should be interpreted in the context of other indicators of iron status, including transferrin saturation, complete blood cell count, and/or liver function panel.
- Low serum ferritin is a specific marker for iron deficiency, and iron supplementation should be initiated based on age-, sex-, and condition-specific thresholds.
Minimally Invasive Nail Surgery: Techniques to Improve the Patient Experience
Nail surgical procedures including biopsies, correction of onychocryptosis and other deformities, and excision of tumors are essential for diagnosing and treating nail disorders. Nail surgery often is perceived by dermatologists as a difficult-to-perform, high-risk procedure associated with patient anxiety, pain, and permanent scarring, which may limit implementation. Misconceptions about nail surgical techniques, aftercare, and patient outcomes are prevalent, and a paucity of nail surgery randomized clinical trials hinder formulation of standardized guidelines.1 In a survey-based study of 95 dermatology residency programs (240 total respondents), 58% of residents said they performed 10 or fewer nail procedures, 10% performed more than 10 procedures, 25% only observed nail procedures, 4% were exposed by lecture only, and 1% had no exposure; 30% said they felt incompetent performing nail biopsies.2 In a retrospective study of nail biopsies performed from 2012 to 2017 in the Medicare Provider Utilization and Payment Database, only 0.28% and 1.01% of all general dermatologists and Mohs surgeons, respectively, performed nail biopsies annually.3 A minimally invasive nail surgery technique is essential to alleviating dermatologist and patient apprehension, which may lead to greater adoption and improved outcomes.
Reduce Patient Anxiety During Nail Surgery
The prospect of undergoing nail surgery can be psychologically distressing to patients because the nail unit is highly sensitive, intraoperative and postoperative pain are common concerns, patient education materials generally are scarce and inaccurate,4 and procedures are performed under local anesthesia with the patient fully awake. In a prospective study of 48 patients undergoing nail surgery, the median preoperative Spielberger State-Trait Anxiety Inventory level was 42.00 (IQR, 6.50).5 Patient distress may be minimized by providing verbal and written educational materials, discussing expectations, and preoperatively using fast-acting benzodiazepines when necessary.6 Utilizing a sleep mask,7 stress ball,8 music,9 and/or virtual reality10 also may reduce patient anxiety during nail surgery.
Use Proper Anesthetic Techniques
Proper anesthetic technique is crucial to achieve the optimal patient experience during nail surgery. With a wing block, the anesthetic is injected into 3 points: (1) the proximal nail fold, (2) the medial/lateral fold, and (3) the hyponychium. The wing block is the preferred technique by many nail surgeons because the second and third injections are given in skin that is already anesthetized, reducing patient discomfort to a single pinprick11; additionally, there is lower postoperative paresthesia risk with the wing block compared with other digital nerve blocks.12 Ropivacaine, a fast-acting and long-acting anesthetic, is preferred over lidocaine to minimize immediate postoperative pain. Buffering the anesthetic solution to physiologic pH and slow infiltration can reduce pain during infiltration.12 Distraction12 provided by ethyl chloride refrigerant spray, an air-cooling device,13 or vibration also can reduce pain during anesthesia.
Punch Biopsy and Excision Tips
The punch biopsy is a minimally invasive method for diagnosing various neoplastic and inflammatory nail unit conditions, except for pigmented lesions.12 For polydactylous nail conditions requiring biopsy, a digit on the nondominant hand should be selected if possible. The punch is applied directly to the nail plate and twisted with downward pressure until the bone is reached, with the instrument withdrawn slowly to prevent surrounding nail plate detachment. Hemostasis is easily achieved with direct pressure and/or use of epinephrine or ropivacaine during anesthesia, and a digital tourniquet generally is not required. Applying microporous polysaccharide hemospheres powder14 or kaolin-impregnated gauze15 with direct pressure is helpful in managing continued bleeding following nail surgery. Punching through the proximal nail matrix should be avoided to prevent permanent onychodystrophy.
A tangential matrix shave biopsy requires a more practiced technique and is preferred for sampling longitudinal melanonychia. A partial proximal nail plate avulsion adequately exposes the origin of pigment and avoids complete avulsion, which may cause more onychodystrophy.16 For broad erythronychia, a total nail avulsion may be necessary. For narrow, well-defined erythronychia, a less-invasive approach such as trap-door avulsion, longitudinal nail strip, or lateral nail plate curl, depending on the etiology, often is sufficient. Tissue excision should be tailored to the specific etiology, with localized excision sufficient for glomus tumors; onychopapillomas require tangential excision of the distal matrix, entire nail bed, and hyperkeratotic papule at the hyponychium. Pushing the cuticle with an elevator/spatula instead of making 2 tangential incisions on the proximal nail fold has been suggested to decrease postoperative paronychia risk.12 A Teflon-coated blade is used to achieve a smooth cut with minimal drag, enabling collection of specimens less than 1 mm thick, which provides sufficient nail matrix epithelium and dermis for histologic examination.16 After obtaining the specimen, the avulsed nail plate may be sutured back to the nail bed using a rapidly absorbable suture such as polyglactin 910, serving as a temporary biological dressing and splint for the nail unit during healing.12 In a retrospective study of 30 patients with longitudinal melanonychia undergoing tangential matrix excision, 27% (8/30) developed postoperative onychodystrophy.17 Although this technique carries relatively lower risk of permanent onychodystrophy compared to other methods, it still is important to acknowledge during the preoperative consent process.12
The lateral longitudinal excision is a valuable technique for diagnosing nail unit inflammatory conditions. Classically, a longitudinal sample including the proximal nail fold, complete matrix, lateral plate, lateral nail fold, hyponychium, and distal tip skin is obtained, with a 10% narrowing of the nail plate expected. If the lateral horn of the nail matrix is missed, permanent lateral malalignment and spicule formation are potential risks. To minimize narrowing of the nail plate and postoperative paronychia, a longitudinal nail strip—where the proximal nail fold and matrix are left intact—is an alternative technique.18
Pain Management Approaches
Appropriate postoperative pain management is crucial for optimizing patient outcomes. In a prospective study of 20 patients undergoing nail biopsy, the mean pain score 6 to 12 hours postprocedure was 5.7 on a scale of 0 to 10. Patients with presurgery pain vs those without experienced significantly higher pain levels both during anesthesia and after surgery (both P<.05).19 Therefore, a personalized approach to pain management based on presence of presurgical pain is warranted. In a randomized clinical trial of 16 patients anesthetized with lidocaine 2% and intraoperative infiltration with a combination of ropivacaine 0.5 mL and triamcinolone (10 mg/mL [0.5 mL]) vs lidocaine 2% alone, the intraoperative mixture reduced postoperative pain (mean pain score, 2 of 10 at 48 hours postprocedure vs 7.88 of 10 in the control group [P<.001]).20
A Cochrane review of 4 unpublished dental and orthopedic surgery studies showed that gabapentin is superior to placebo in the treatment of acute postoperative pain. Therefore, a single dose of gabapentin (250 mg) may be considered in patients at risk for high postoperative pain.21 In a randomized double-blind trial of 210 Mohs micrographic surgery patients, those receiving acetaminophen and ibuprofen reported lower pain scores at 2, 4, 8, and 12 hours postprocedure compared with patients taking acetaminophen and codeine or acetaminophen alone.22 However, the role of opioids in pain management following nail surgery has not been adequately studied.
Wound Care
An efficient dressing protects the surgical wound, facilitates healing, and provides comfort. In our experience, an initial layer of petrolatum-impregnated gauze followed by a pressure-padded bandage consisting of folded dry gauze secured in place with longitudinally applied tape to avoid a tourniquet effect is effective for nail surgical wounds. As the last step, self-adherent elastic wrap is applied around the digit and extended proximally to prevent a tourniquet effect.23
Final Thoughts
Due to the intricate anatomy of the nail unit, nail surgeries are inherently more invasive than most dermatologic surgical procedures. It is crucial to adopt a minimally invasive approach to reduce tissue damage and potential complications in both the short-term and long-term. Adopting this approach may substantially improve patient outcomes and enhance diagnostic and treatment efficacy.
- Ricardo JW, Lipner SR. Nail surgery myths and truths. J Drugs Dermatol. 2020;19:230-234.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.E4835. doi:10.1016/j.jaad.2010.05.044
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:E14928. doi:10.1111/dth.14928
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Göktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39. doi:10.1177/1203475415588645
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient—a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097/DSS.0000000000001854
- Jellinek NJ, Vélez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271. doi:10.1016/j.det.2014.12.007
- Baltz JO, Jellinek NJ. Nail surgery: six essential techniques. Dermatol Clin. 2021;39:305-318. doi:10.1016/j.det.2020.12.015
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Ricardo JW, Lipner SR. Microporous polysaccharide hemospheres powder for hemostasis following nail surgery [published online March 26, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.069
- Ricardo JW, Lipner SR. Kaolin-impregnated gauze for hemostasis following nail surgery. J Am Acad Dermatol. 2021;85:E13-E14. doi:10.1016/j.jaad.2020.02.008
- Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol. 2007;56:803-810. doi:10.1016/j.jaad.2006.12.001
- Richert B, Theunis A, Norrenberg S, et al. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol. 2013;69:96-104. doi:10.1016/j.jaad.2013.01.029
- Godse R, Jariwala N, Rubin AI. How we do it: the longitudinal nail strip biopsy for nail unit inflammatory dermatoses. Dermatol Surg. 2023;49:311-313. doi:10.1097/DSS.0000000000003707
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Di Chiacchio N, Ocampo-Garza J, Villarreal-Villarreal CD, et al. Post-nail procedure analgesia: a randomized control pilot study. J Am Acad Dermatol. 2019;81:860-862. doi:10.1016/j.jaad.2019.05.015
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12, 2010]. Cochrane Database Syst Rev. 2010;2010:CD008183. doi:10.1002/14651858.CD008183.pub2
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013. doi:10.1111/j.1524-4725.2011.02022.x
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442-444. doi:10.1097/DSS.0000000000002371
Nail surgical procedures including biopsies, correction of onychocryptosis and other deformities, and excision of tumors are essential for diagnosing and treating nail disorders. Nail surgery often is perceived by dermatologists as a difficult-to-perform, high-risk procedure associated with patient anxiety, pain, and permanent scarring, which may limit implementation. Misconceptions about nail surgical techniques, aftercare, and patient outcomes are prevalent, and a paucity of nail surgery randomized clinical trials hinder formulation of standardized guidelines.1 In a survey-based study of 95 dermatology residency programs (240 total respondents), 58% of residents said they performed 10 or fewer nail procedures, 10% performed more than 10 procedures, 25% only observed nail procedures, 4% were exposed by lecture only, and 1% had no exposure; 30% said they felt incompetent performing nail biopsies.2 In a retrospective study of nail biopsies performed from 2012 to 2017 in the Medicare Provider Utilization and Payment Database, only 0.28% and 1.01% of all general dermatologists and Mohs surgeons, respectively, performed nail biopsies annually.3 A minimally invasive nail surgery technique is essential to alleviating dermatologist and patient apprehension, which may lead to greater adoption and improved outcomes.
Reduce Patient Anxiety During Nail Surgery
The prospect of undergoing nail surgery can be psychologically distressing to patients because the nail unit is highly sensitive, intraoperative and postoperative pain are common concerns, patient education materials generally are scarce and inaccurate,4 and procedures are performed under local anesthesia with the patient fully awake. In a prospective study of 48 patients undergoing nail surgery, the median preoperative Spielberger State-Trait Anxiety Inventory level was 42.00 (IQR, 6.50).5 Patient distress may be minimized by providing verbal and written educational materials, discussing expectations, and preoperatively using fast-acting benzodiazepines when necessary.6 Utilizing a sleep mask,7 stress ball,8 music,9 and/or virtual reality10 also may reduce patient anxiety during nail surgery.
Use Proper Anesthetic Techniques
Proper anesthetic technique is crucial to achieve the optimal patient experience during nail surgery. With a wing block, the anesthetic is injected into 3 points: (1) the proximal nail fold, (2) the medial/lateral fold, and (3) the hyponychium. The wing block is the preferred technique by many nail surgeons because the second and third injections are given in skin that is already anesthetized, reducing patient discomfort to a single pinprick11; additionally, there is lower postoperative paresthesia risk with the wing block compared with other digital nerve blocks.12 Ropivacaine, a fast-acting and long-acting anesthetic, is preferred over lidocaine to minimize immediate postoperative pain. Buffering the anesthetic solution to physiologic pH and slow infiltration can reduce pain during infiltration.12 Distraction12 provided by ethyl chloride refrigerant spray, an air-cooling device,13 or vibration also can reduce pain during anesthesia.
Punch Biopsy and Excision Tips
The punch biopsy is a minimally invasive method for diagnosing various neoplastic and inflammatory nail unit conditions, except for pigmented lesions.12 For polydactylous nail conditions requiring biopsy, a digit on the nondominant hand should be selected if possible. The punch is applied directly to the nail plate and twisted with downward pressure until the bone is reached, with the instrument withdrawn slowly to prevent surrounding nail plate detachment. Hemostasis is easily achieved with direct pressure and/or use of epinephrine or ropivacaine during anesthesia, and a digital tourniquet generally is not required. Applying microporous polysaccharide hemospheres powder14 or kaolin-impregnated gauze15 with direct pressure is helpful in managing continued bleeding following nail surgery. Punching through the proximal nail matrix should be avoided to prevent permanent onychodystrophy.
A tangential matrix shave biopsy requires a more practiced technique and is preferred for sampling longitudinal melanonychia. A partial proximal nail plate avulsion adequately exposes the origin of pigment and avoids complete avulsion, which may cause more onychodystrophy.16 For broad erythronychia, a total nail avulsion may be necessary. For narrow, well-defined erythronychia, a less-invasive approach such as trap-door avulsion, longitudinal nail strip, or lateral nail plate curl, depending on the etiology, often is sufficient. Tissue excision should be tailored to the specific etiology, with localized excision sufficient for glomus tumors; onychopapillomas require tangential excision of the distal matrix, entire nail bed, and hyperkeratotic papule at the hyponychium. Pushing the cuticle with an elevator/spatula instead of making 2 tangential incisions on the proximal nail fold has been suggested to decrease postoperative paronychia risk.12 A Teflon-coated blade is used to achieve a smooth cut with minimal drag, enabling collection of specimens less than 1 mm thick, which provides sufficient nail matrix epithelium and dermis for histologic examination.16 After obtaining the specimen, the avulsed nail plate may be sutured back to the nail bed using a rapidly absorbable suture such as polyglactin 910, serving as a temporary biological dressing and splint for the nail unit during healing.12 In a retrospective study of 30 patients with longitudinal melanonychia undergoing tangential matrix excision, 27% (8/30) developed postoperative onychodystrophy.17 Although this technique carries relatively lower risk of permanent onychodystrophy compared to other methods, it still is important to acknowledge during the preoperative consent process.12
The lateral longitudinal excision is a valuable technique for diagnosing nail unit inflammatory conditions. Classically, a longitudinal sample including the proximal nail fold, complete matrix, lateral plate, lateral nail fold, hyponychium, and distal tip skin is obtained, with a 10% narrowing of the nail plate expected. If the lateral horn of the nail matrix is missed, permanent lateral malalignment and spicule formation are potential risks. To minimize narrowing of the nail plate and postoperative paronychia, a longitudinal nail strip—where the proximal nail fold and matrix are left intact—is an alternative technique.18
Pain Management Approaches
Appropriate postoperative pain management is crucial for optimizing patient outcomes. In a prospective study of 20 patients undergoing nail biopsy, the mean pain score 6 to 12 hours postprocedure was 5.7 on a scale of 0 to 10. Patients with presurgery pain vs those without experienced significantly higher pain levels both during anesthesia and after surgery (both P<.05).19 Therefore, a personalized approach to pain management based on presence of presurgical pain is warranted. In a randomized clinical trial of 16 patients anesthetized with lidocaine 2% and intraoperative infiltration with a combination of ropivacaine 0.5 mL and triamcinolone (10 mg/mL [0.5 mL]) vs lidocaine 2% alone, the intraoperative mixture reduced postoperative pain (mean pain score, 2 of 10 at 48 hours postprocedure vs 7.88 of 10 in the control group [P<.001]).20
A Cochrane review of 4 unpublished dental and orthopedic surgery studies showed that gabapentin is superior to placebo in the treatment of acute postoperative pain. Therefore, a single dose of gabapentin (250 mg) may be considered in patients at risk for high postoperative pain.21 In a randomized double-blind trial of 210 Mohs micrographic surgery patients, those receiving acetaminophen and ibuprofen reported lower pain scores at 2, 4, 8, and 12 hours postprocedure compared with patients taking acetaminophen and codeine or acetaminophen alone.22 However, the role of opioids in pain management following nail surgery has not been adequately studied.
Wound Care
An efficient dressing protects the surgical wound, facilitates healing, and provides comfort. In our experience, an initial layer of petrolatum-impregnated gauze followed by a pressure-padded bandage consisting of folded dry gauze secured in place with longitudinally applied tape to avoid a tourniquet effect is effective for nail surgical wounds. As the last step, self-adherent elastic wrap is applied around the digit and extended proximally to prevent a tourniquet effect.23
Final Thoughts
Due to the intricate anatomy of the nail unit, nail surgeries are inherently more invasive than most dermatologic surgical procedures. It is crucial to adopt a minimally invasive approach to reduce tissue damage and potential complications in both the short-term and long-term. Adopting this approach may substantially improve patient outcomes and enhance diagnostic and treatment efficacy.
Nail surgical procedures including biopsies, correction of onychocryptosis and other deformities, and excision of tumors are essential for diagnosing and treating nail disorders. Nail surgery often is perceived by dermatologists as a difficult-to-perform, high-risk procedure associated with patient anxiety, pain, and permanent scarring, which may limit implementation. Misconceptions about nail surgical techniques, aftercare, and patient outcomes are prevalent, and a paucity of nail surgery randomized clinical trials hinder formulation of standardized guidelines.1 In a survey-based study of 95 dermatology residency programs (240 total respondents), 58% of residents said they performed 10 or fewer nail procedures, 10% performed more than 10 procedures, 25% only observed nail procedures, 4% were exposed by lecture only, and 1% had no exposure; 30% said they felt incompetent performing nail biopsies.2 In a retrospective study of nail biopsies performed from 2012 to 2017 in the Medicare Provider Utilization and Payment Database, only 0.28% and 1.01% of all general dermatologists and Mohs surgeons, respectively, performed nail biopsies annually.3 A minimally invasive nail surgery technique is essential to alleviating dermatologist and patient apprehension, which may lead to greater adoption and improved outcomes.
Reduce Patient Anxiety During Nail Surgery
The prospect of undergoing nail surgery can be psychologically distressing to patients because the nail unit is highly sensitive, intraoperative and postoperative pain are common concerns, patient education materials generally are scarce and inaccurate,4 and procedures are performed under local anesthesia with the patient fully awake. In a prospective study of 48 patients undergoing nail surgery, the median preoperative Spielberger State-Trait Anxiety Inventory level was 42.00 (IQR, 6.50).5 Patient distress may be minimized by providing verbal and written educational materials, discussing expectations, and preoperatively using fast-acting benzodiazepines when necessary.6 Utilizing a sleep mask,7 stress ball,8 music,9 and/or virtual reality10 also may reduce patient anxiety during nail surgery.
Use Proper Anesthetic Techniques
Proper anesthetic technique is crucial to achieve the optimal patient experience during nail surgery. With a wing block, the anesthetic is injected into 3 points: (1) the proximal nail fold, (2) the medial/lateral fold, and (3) the hyponychium. The wing block is the preferred technique by many nail surgeons because the second and third injections are given in skin that is already anesthetized, reducing patient discomfort to a single pinprick11; additionally, there is lower postoperative paresthesia risk with the wing block compared with other digital nerve blocks.12 Ropivacaine, a fast-acting and long-acting anesthetic, is preferred over lidocaine to minimize immediate postoperative pain. Buffering the anesthetic solution to physiologic pH and slow infiltration can reduce pain during infiltration.12 Distraction12 provided by ethyl chloride refrigerant spray, an air-cooling device,13 or vibration also can reduce pain during anesthesia.
Punch Biopsy and Excision Tips
The punch biopsy is a minimally invasive method for diagnosing various neoplastic and inflammatory nail unit conditions, except for pigmented lesions.12 For polydactylous nail conditions requiring biopsy, a digit on the nondominant hand should be selected if possible. The punch is applied directly to the nail plate and twisted with downward pressure until the bone is reached, with the instrument withdrawn slowly to prevent surrounding nail plate detachment. Hemostasis is easily achieved with direct pressure and/or use of epinephrine or ropivacaine during anesthesia, and a digital tourniquet generally is not required. Applying microporous polysaccharide hemospheres powder14 or kaolin-impregnated gauze15 with direct pressure is helpful in managing continued bleeding following nail surgery. Punching through the proximal nail matrix should be avoided to prevent permanent onychodystrophy.
A tangential matrix shave biopsy requires a more practiced technique and is preferred for sampling longitudinal melanonychia. A partial proximal nail plate avulsion adequately exposes the origin of pigment and avoids complete avulsion, which may cause more onychodystrophy.16 For broad erythronychia, a total nail avulsion may be necessary. For narrow, well-defined erythronychia, a less-invasive approach such as trap-door avulsion, longitudinal nail strip, or lateral nail plate curl, depending on the etiology, often is sufficient. Tissue excision should be tailored to the specific etiology, with localized excision sufficient for glomus tumors; onychopapillomas require tangential excision of the distal matrix, entire nail bed, and hyperkeratotic papule at the hyponychium. Pushing the cuticle with an elevator/spatula instead of making 2 tangential incisions on the proximal nail fold has been suggested to decrease postoperative paronychia risk.12 A Teflon-coated blade is used to achieve a smooth cut with minimal drag, enabling collection of specimens less than 1 mm thick, which provides sufficient nail matrix epithelium and dermis for histologic examination.16 After obtaining the specimen, the avulsed nail plate may be sutured back to the nail bed using a rapidly absorbable suture such as polyglactin 910, serving as a temporary biological dressing and splint for the nail unit during healing.12 In a retrospective study of 30 patients with longitudinal melanonychia undergoing tangential matrix excision, 27% (8/30) developed postoperative onychodystrophy.17 Although this technique carries relatively lower risk of permanent onychodystrophy compared to other methods, it still is important to acknowledge during the preoperative consent process.12
The lateral longitudinal excision is a valuable technique for diagnosing nail unit inflammatory conditions. Classically, a longitudinal sample including the proximal nail fold, complete matrix, lateral plate, lateral nail fold, hyponychium, and distal tip skin is obtained, with a 10% narrowing of the nail plate expected. If the lateral horn of the nail matrix is missed, permanent lateral malalignment and spicule formation are potential risks. To minimize narrowing of the nail plate and postoperative paronychia, a longitudinal nail strip—where the proximal nail fold and matrix are left intact—is an alternative technique.18
Pain Management Approaches
Appropriate postoperative pain management is crucial for optimizing patient outcomes. In a prospective study of 20 patients undergoing nail biopsy, the mean pain score 6 to 12 hours postprocedure was 5.7 on a scale of 0 to 10. Patients with presurgery pain vs those without experienced significantly higher pain levels both during anesthesia and after surgery (both P<.05).19 Therefore, a personalized approach to pain management based on presence of presurgical pain is warranted. In a randomized clinical trial of 16 patients anesthetized with lidocaine 2% and intraoperative infiltration with a combination of ropivacaine 0.5 mL and triamcinolone (10 mg/mL [0.5 mL]) vs lidocaine 2% alone, the intraoperative mixture reduced postoperative pain (mean pain score, 2 of 10 at 48 hours postprocedure vs 7.88 of 10 in the control group [P<.001]).20
A Cochrane review of 4 unpublished dental and orthopedic surgery studies showed that gabapentin is superior to placebo in the treatment of acute postoperative pain. Therefore, a single dose of gabapentin (250 mg) may be considered in patients at risk for high postoperative pain.21 In a randomized double-blind trial of 210 Mohs micrographic surgery patients, those receiving acetaminophen and ibuprofen reported lower pain scores at 2, 4, 8, and 12 hours postprocedure compared with patients taking acetaminophen and codeine or acetaminophen alone.22 However, the role of opioids in pain management following nail surgery has not been adequately studied.
Wound Care
An efficient dressing protects the surgical wound, facilitates healing, and provides comfort. In our experience, an initial layer of petrolatum-impregnated gauze followed by a pressure-padded bandage consisting of folded dry gauze secured in place with longitudinally applied tape to avoid a tourniquet effect is effective for nail surgical wounds. As the last step, self-adherent elastic wrap is applied around the digit and extended proximally to prevent a tourniquet effect.23
Final Thoughts
Due to the intricate anatomy of the nail unit, nail surgeries are inherently more invasive than most dermatologic surgical procedures. It is crucial to adopt a minimally invasive approach to reduce tissue damage and potential complications in both the short-term and long-term. Adopting this approach may substantially improve patient outcomes and enhance diagnostic and treatment efficacy.
- Ricardo JW, Lipner SR. Nail surgery myths and truths. J Drugs Dermatol. 2020;19:230-234.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.E4835. doi:10.1016/j.jaad.2010.05.044
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:E14928. doi:10.1111/dth.14928
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Göktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39. doi:10.1177/1203475415588645
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient—a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097/DSS.0000000000001854
- Jellinek NJ, Vélez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271. doi:10.1016/j.det.2014.12.007
- Baltz JO, Jellinek NJ. Nail surgery: six essential techniques. Dermatol Clin. 2021;39:305-318. doi:10.1016/j.det.2020.12.015
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Ricardo JW, Lipner SR. Microporous polysaccharide hemospheres powder for hemostasis following nail surgery [published online March 26, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.069
- Ricardo JW, Lipner SR. Kaolin-impregnated gauze for hemostasis following nail surgery. J Am Acad Dermatol. 2021;85:E13-E14. doi:10.1016/j.jaad.2020.02.008
- Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol. 2007;56:803-810. doi:10.1016/j.jaad.2006.12.001
- Richert B, Theunis A, Norrenberg S, et al. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol. 2013;69:96-104. doi:10.1016/j.jaad.2013.01.029
- Godse R, Jariwala N, Rubin AI. How we do it: the longitudinal nail strip biopsy for nail unit inflammatory dermatoses. Dermatol Surg. 2023;49:311-313. doi:10.1097/DSS.0000000000003707
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Di Chiacchio N, Ocampo-Garza J, Villarreal-Villarreal CD, et al. Post-nail procedure analgesia: a randomized control pilot study. J Am Acad Dermatol. 2019;81:860-862. doi:10.1016/j.jaad.2019.05.015
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12, 2010]. Cochrane Database Syst Rev. 2010;2010:CD008183. doi:10.1002/14651858.CD008183.pub2
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013. doi:10.1111/j.1524-4725.2011.02022.x
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442-444. doi:10.1097/DSS.0000000000002371
- Ricardo JW, Lipner SR. Nail surgery myths and truths. J Drugs Dermatol. 2020;19:230-234.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.E4835. doi:10.1016/j.jaad.2010.05.044
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:E14928. doi:10.1111/dth.14928
- Ishack S, Lipner SR. Evaluating the impact and educational value of YouTube videos on nail biopsy procedures. Cutis. 2020;105:148-149, E1.
- Göktay F, Altan ZM, Talas A, et al. Anxiety among patients undergoing nail surgery and skin punch biopsy: effects of age, gender, educational status, and previous experience. J Cutan Med Surg. 2016;20:35-39. doi:10.1177/1203475415588645
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Vachiramon V, Sobanko JF, Rattanaumpawan P, et al. Music reduces patient anxiety during Mohs surgery: an open-label randomized controlled trial. Dermatol Surg. 2013;39:298-305. doi:10.1111/dsu.12047
- Higgins S, Feinstein S, Hawkins M, et al. Virtual reality to improve the experience of the Mohs patient—a prospective interventional study. Dermatol Surg. 2019;45:1009-1018. doi:10.1097/DSS.0000000000001854
- Jellinek NJ, Vélez NF. Nail surgery: best way to obtain effective anesthesia. Dermatol Clin. 2015;33:265-271. doi:10.1016/j.det.2014.12.007
- Baltz JO, Jellinek NJ. Nail surgery: six essential techniques. Dermatol Clin. 2021;39:305-318. doi:10.1016/j.det.2020.12.015
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Ricardo JW, Lipner SR. Microporous polysaccharide hemospheres powder for hemostasis following nail surgery [published online March 26, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.069
- Ricardo JW, Lipner SR. Kaolin-impregnated gauze for hemostasis following nail surgery. J Am Acad Dermatol. 2021;85:E13-E14. doi:10.1016/j.jaad.2020.02.008
- Jellinek N. Nail matrix biopsy of longitudinal melanonychia: diagnostic algorithm including the matrix shave biopsy. J Am Acad Dermatol. 2007;56:803-810. doi:10.1016/j.jaad.2006.12.001
- Richert B, Theunis A, Norrenberg S, et al. Tangential excision of pigmented nail matrix lesions responsible for longitudinal melanonychia: evaluation of the technique on a series of 30 patients. J Am Acad Dermatol. 2013;69:96-104. doi:10.1016/j.jaad.2013.01.029
- Godse R, Jariwala N, Rubin AI. How we do it: the longitudinal nail strip biopsy for nail unit inflammatory dermatoses. Dermatol Surg. 2023;49:311-313. doi:10.1097/DSS.0000000000003707
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Di Chiacchio N, Ocampo-Garza J, Villarreal-Villarreal CD, et al. Post-nail procedure analgesia: a randomized control pilot study. J Am Acad Dermatol. 2019;81:860-862. doi:10.1016/j.jaad.2019.05.015
- Straube S, Derry S, Moore RA, et al. Single dose oral gabapentin for established acute postoperative pain in adults [published online May 12, 2010]. Cochrane Database Syst Rev. 2010;2010:CD008183. doi:10.1002/14651858.CD008183.pub2
- Sniezek PJ, Brodland DG, Zitelli JA. A randomized controlled trial comparing acetaminophen, acetaminophen and ibuprofen, and acetaminophen and codeine for postoperative pain relief after Mohs surgery and cutaneous reconstruction. Dermatol Surg. 2011;37:1007-1013. doi:10.1111/j.1524-4725.2011.02022.x
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442-444. doi:10.1097/DSS.0000000000002371
Evaluation of Micrographic Surgery and Dermatologic Oncology Fellowship Program Websites
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.
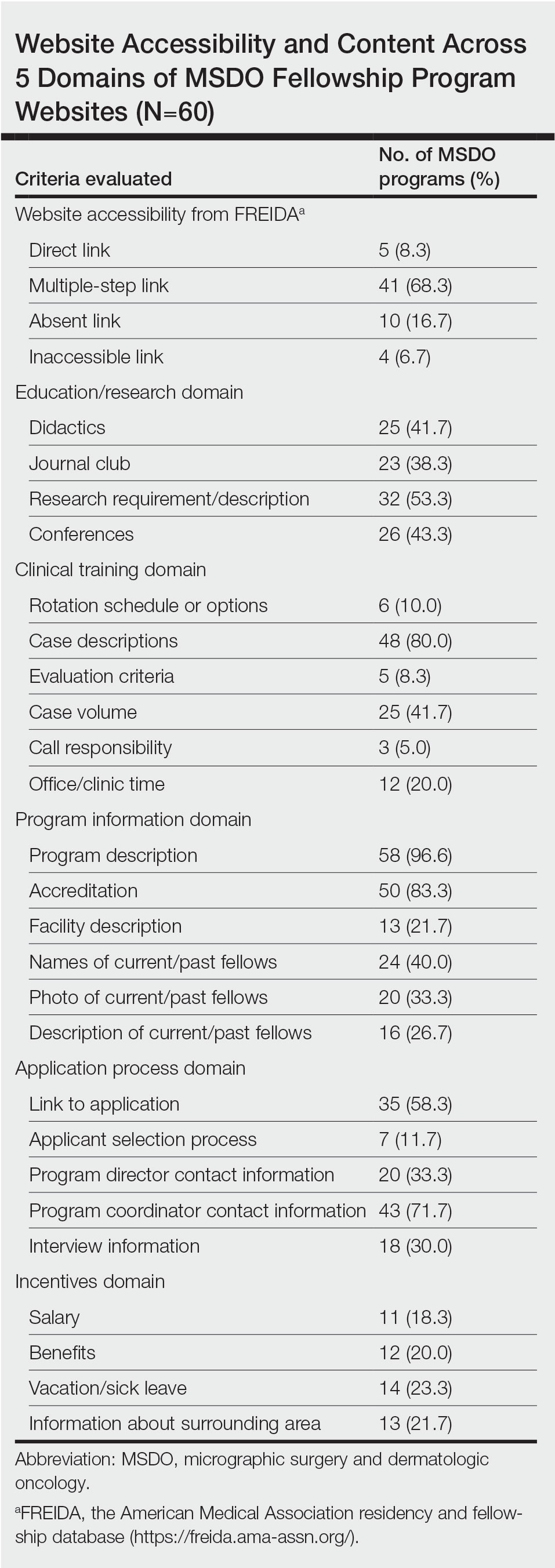
The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.

The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.

The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
Practice Points
- With the COVID-19 pandemic and the movement to a virtual fellowship application process, fellowship program websites that are comprehensive and accessible may help programs attract compatible candidates, improve transparency, and guide applicants through the application process.
- There is variation in the content of current micrographic surgery and dermatologic oncology fellowship program websites and areas upon which programs may seek to augment their website content to better reflect program strengths while attracting competitive candidates best suited for their program.
Economic Burden and Quality of Life of Patients With Moderate to Severe Atopic Dermatitis in a Tertiary Care Hospital in Helsinki, Finland: A Survey-Based Study
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.
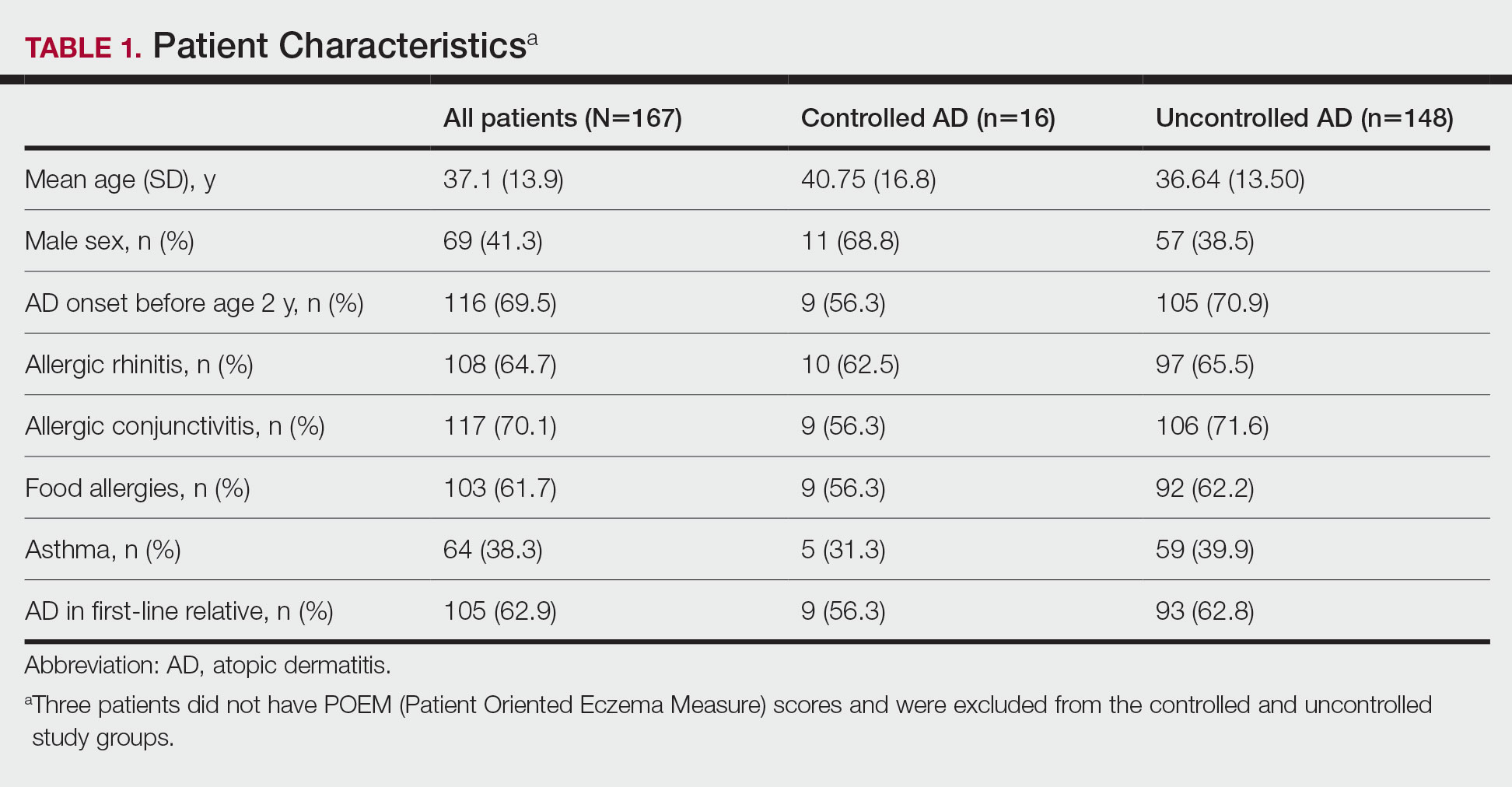
The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.
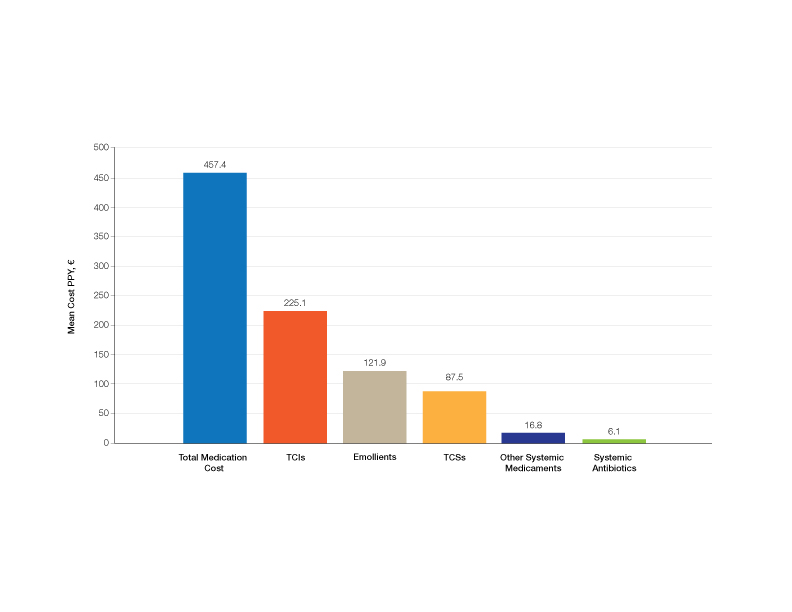
Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.
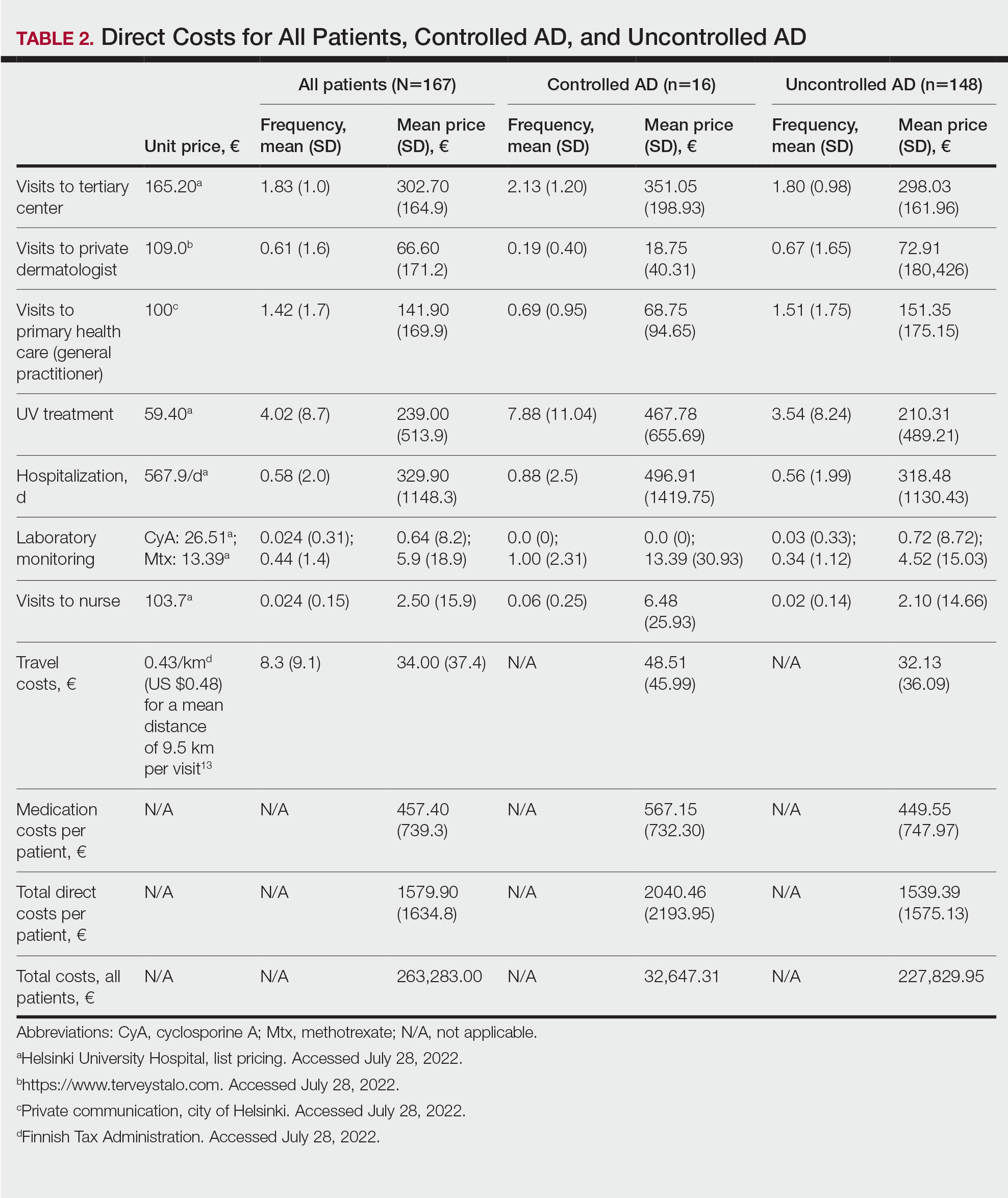
Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.
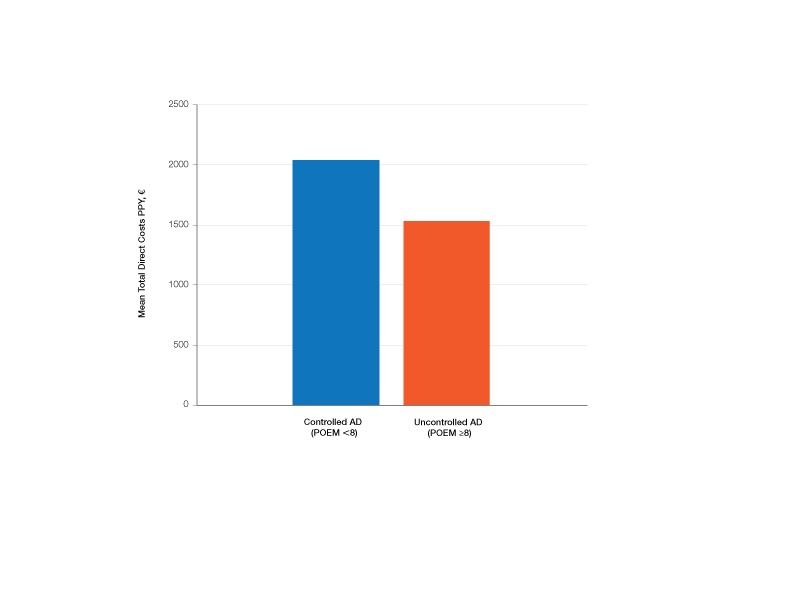
AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.

The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.

Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.

Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.

AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.

The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.

Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.

Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.

AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
Practice Points
- Atopic dermatitis (AD) causes a substantial economic burden.
- Atopic dermatitis profoundly affects quality of life and is associated with psychiatric comorbidities. With effective treatments, AD-associated comorbidities may be decreased and the economic burden for the patient and health care system reduced.
Imaging Tools for Noninvasive Hair Assessment
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.
Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).
VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.
Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
Practice Points
- Reflectance confocal microscopy (RCM) imaging can be taken at levels from the stratum corneum to the papillary dermis and can be used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, alopecia areata, and androgenetic alopecia.
- Because of its ability to distinguish different stages of disease, RCM can be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.
- Optical coherence tomography has the potential to monitor early subclinical responses to alopecia therapies while also improving hair transplantation outcomes by allowing for visualization of the subcutaneous angle of hair follicles.
- Software development paired with trichoscopy has the ability to quantify hair growth parameters such as hair count, density, and diameter.
Affixing a Scalp Dressing With Hairpins
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).
Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).

Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
Practice Gap
Wound dressings protect the skin and prevent contamination. The hair often makes it difficult to affix a dressing after a minor scalp trauma or local surgery on the head. Traditional approaches for fastening a dressing on the head include bandage winding or adhesive tape, but these methods often affect aesthetics or cause discomfort—bandage winding can make it inconvenient for the patient to move their head, and adhesive tape can cause pain by pulling the hair during removal.
To better position a scalp dressing, tie-over dressings, braid dressings, and paper clips have been used as fixators.1-3 These methods have benefits and disadvantages.
Tie-over Dressing—The dressing is clasped with long sutures that were reserved during wound closure. This method is sturdy, can slightly compress the wound, and is applicable to any part of the scalp. However, it requires more sutures, and more careful wound care may be required due to the edge of the dressing being close to the wound.
Braid Dressing—Tape, a rubber band, or braided hair is used to bind the gauze pad. This dressing is simple and inexpensive. However, it is limited to patients with long hair; even then, it often is difficult to anchor the dressing by braiding hair. Moreover, removal of the rubber band and tape can cause discomfort or pain.
Paper Clip—This is a simple scalp dressing fixator. However, due to the short and circular structure of the clip, it is not conducive to affixing a gauze dressing for patients with short hair, and it often hooks the gauze and hair, making it inconvenient for the physician and a source of discomfort for the patient when the paper clip is being removed.
The Technique
To address shortcomings of traditional methods, we encourage the use of hairpins to affix a dressing after a scalp wound is sutured. Two steps are required:
- Position the gauze to cover the wound and press the gauze down with your hand.
- Clamp the 4 corners of the dressing and adjacent hair with hairpins (Figure, A).

Practical Implications
Hairpins are common for fixing hairstyles and decorating hair. They are inexpensive, easy to obtain, simple in structure, convenient to use without additional discomfort, and easy to remove (Figure, B). Because most hairpins have a powerful clamping force, they can affix dressings in short hair (Figure, A). All medical staff can use hairpins to anchor the scalp dressing. Even a patient’s family members can carry out simple dressing replacement and wound cleaning using this method. Patients also have many options for hairpin styles, which is especially useful in easing the apprehension of surgery in pediatric patients.
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
- Ginzburg A, Mutalik S. Another method of tie-over dressing for surgical wounds of hair-bearing areas. Dermatol Surg. 1999;25:893-894. doi:10.1046/j.1524-4725.1999.99155.x
- Yanaka K, Nose T. Braid dressing for hair-bearing scalp wound. Neurocrit Care. 2004;1:217-218. doi:10.1385/NCC:1:2:217
- Bu W, Zhang Q, Fang F, et al. Fixation of head dressing gauzes with paper clips is similar to and better than using tape. J Am Acad Dermatol. 2019;81:E95-E96. doi:10.1016/j.jaad.2018.10.046
Cancer Screening for Dermatomyositis: A Survey of Indirect Costs, Burden, and Patient Willingness to Pay
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18
The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).
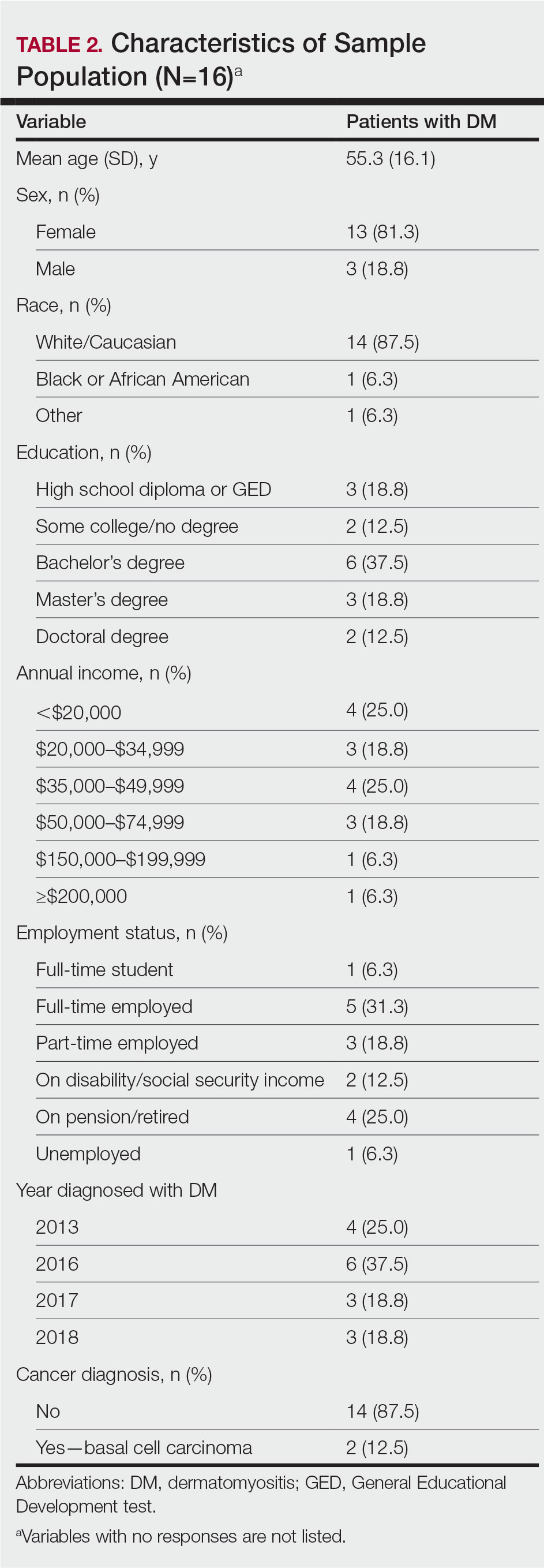
Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.
Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.
Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17
The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)
“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
Dermatomyositis (DM) is an uncommon idiopathic inflammatory myopathy (IIM) characterized by muscle inflammation; proximal muscle weakness; and dermatologic findings, such as the heliotrope eruption and Gottron papules.1-3 Dermatomyositis is associated with an increased malignancy risk compared to other IIMs, with a 13% to 42% lifetime risk for malignancy development.4,5 The incidence for malignancy peaks during the first year following diagnosis and falls gradually over 5 years but remains increased compared to the general population.6-11 Adenocarcinoma represents the majority of cancers associated with DM, particularly of the ovaries, lungs, breasts, gastrointestinal tract, pancreas, bladder, and prostate. The lymphatic system (non-Hodgkin lymphoma) also is overrepresented among cancers in DM.12
Because of the increased malignancy risk and cancer-related mortality in patients with DM, cancer screening generally is recommended following diagnosis.13,14 However, consensus guidelines for screening modalities and frequency currently do not exist, resulting in widely varying practice patterns.15 Some experts advocate for a conventional cancer screening panel (CSP), as summarized in Table 1.15-18 These tests may be repeated annually for 3 to 5 years following the diagnosis of DM. Although the use of myositis-specific antibodies (MSAs) recently has helped to risk-stratify DM patients, up to half of patients are MSA negative,19 and broad malignancy screening remains essential. Individualized discussions with patients about their risk factors, screening options, and risks and benefits of screening also are strongly encouraged.19-22 Studies of the direct costs and effectiveness of streamlined screening with positron emission tomography/computed tomography (PET/CT) compared with a CSP have shown similar efficacy and lower out-of-pocket costs for patients receiving PET/CT imaging.16-18

The goal of our study was to further characterize patients’ perspectives and experience of cancer screening in DM as well as indirect costs, both of which must be taken into consideration when developing consensus guidelines for DM malignancy screening. Inclusion of patient voice is essential given the similar efficacy of both screening methods. We assessed the indirect costs (eg, travel, lost work or wages, childcare) of a CSP in patients with DM. We theorized that the large quantity of tests involved in a CSP, which are performed at various locations on multiple days over the course of several years, may have substantial costs to patients beyond the co-pay and deductible. We also sought to measure patients’ perception of the burden associated with an annual CSP, which we defined to participants as the inconvenience or unpleasantness experienced by the patient, compared with an annual whole-body PET/CT. Finally, we examined the relative value of these screening methods to patients using a willingness-to-pay (WTP) analysis.
Materials and Methods
Patient Eligibility—Our study included Penn State Health (Hershey, Pennsylvania) patients 18 years or older with a recent diagnosis of DM—International Classification of Diseases, Ninth Revision code 710.3 or International Classification of Diseases, Tenth Revision codes M33.10 or M33.90—who were undergoing or had recently completed a CSP. Patients were excluded from the study if they had a concurrent or preceding diagnosis of malignancy (excluding nonmelanoma skin cancers) or had another IIM. The institutional review board at Penn State Health College of Medicine approved the study. Data for all patients were prospectively obtained.
Survey Design—A survey was generated to assess the burden and indirect costs associated with a CSP, which was modified from work done by Tchuenche et al23 and Teni et al.24 Focus groups were held in 2018 and 2019 with patients who met our inclusion criteria with the purpose of refining the survey instrument based on patient input. A summary explanation of research was provided to all participants, and informed consent was obtained. Patients were compensated for their time for focus groups. Audio of each focus group was then transcribed and analyzed for common themes. Following focus group feedback, a finalized survey was generated for assessing burden and indirect costs (survey instrument provided in the Supplementary Information). REDCap (Vanderbilt University), a secure web application, was used to construct the finalized survey and to collect and manage data.25
Patients who fit our inclusion criteria were identified and recruited in multiple ways. Patients with appointments at the Penn State Milton S. Hershey Medical Center Department of Dermatology were presented with the opportunity to participate, Penn State Health records with the appropriate billing codes were collected and patients were contacted, and an advertisement for the study was posted on StudyFinder. Surveys constructed on REDCap were then sent electronically to patients who agreed to participate in the study. A second summary explanation of research was included on the first page of the survey to describe the process.
The survey had 3 main sections. The first section collected demographic information. In the second section, we surveyed patients regarding the various aspects of a CSP that focus groups identified as burdensome. In addition, patients were asked to compare their feelings regarding an annual CSP vs whole-body PET/CT for a 3-year period utilizing a rating scale of strongly disagree, somewhat disagree, somewhat agree, and strongly agree. This section also included a willingness-to-pay (WTP) analysis for each modality. We defined WTP as the maximum out-of-pocket cost that the patient would be willing to pay to receive testing, which was measured in a hypothetical scenario where neither whole-body PET/CT nor CSP was covered by insurance.26 Although WTP may be influenced by external factors such as patient income, it can serve as a numerical measure of how much the patient values each service. Furthermore, these external factors become less relevant when comparing the relative value of 2 separate tests, as such factors apply equally in both scenarios. In the third section of the survey, patients were queried regarding various indirect costs associated with a CSP. Descriptions for a CSP and whole-body PET/CT, including risks and benefits, were provided to allow patients to make informed decisions.
Statistical Analysis—Because of the rarity of DM and the subsequently limited sample size, summary and descriptive statistics were utilized to characterize the sample and identify patterns in the results. Continuous variables are presented with means and standard deviations, and proportions are presented with frequencies and percentages. All analyses were done using SAS Version 9.4 (SAS Institute Inc).

Results
Patient Demographics—Fifty-four patients were identified using StudyFinder, physician referral, and search of the electronic health record. Nine patients agreed to take part in the focus groups, and 27 offered email addresses to be contacted for the survey. Of those 27 patients, 16 (59.3%) fit our inclusion criteria and completed the survey. Patient demographics are detailed in Table 2. The mean age was 55 years, and most patients were White (88% [14/16]), female (81% [13/16]), and had at least a bachelor’s degree (69% [11/16]). Most patients (69% [11/16]) had an annual income of less than $50,000, and half (50% [8/16]) were employed. All patients had been diagnosed with DM in or after 2013. Two patients were diagnosed with basal cell carcinoma during or after cancer screening.

Patient Preference for Screening and WTP—A majority (81% [13/16]) of patients desired some form of screening for occult malignancy following the diagnosis of DM, even in the hypothetical situation in which screening did not provide survival benefit (Figure 1). Twenty-five percent (4/16) of patients expressed that a CSP was burdensome, and 12.5% of patients (2/16) missed a CSP appointment; all of these patients rescheduled or were planning to reschedule. Assuming that both screening methods had similar predictive value in detecting malignancy, all 16 patients felt annual whole-body PET/CT for a 3-year period would be less burdensome than a CSP, and most (73% [11/15]) felt that it would decrease the likelihood of missed appointments. Overall, 93% (13/14) of patients preferred whole-body PET/CT over a CSP when given the choice between the 2 options (Figure 2). This preference was consistent with the patients’ WTP for these tests; patients reliably reported that they would pay more for annual whole-body PET/CT than for a CSP (Figure 3). Specifically, 75% (12/16) and 38% (6/16) of patients were willing to spend $250 or more and $1000 or more for annual whole-body PET/CT, respectively, compared with 56% (9/16) and 19% (3/16), respectively, for an annual CSP. Many patients (38% [6/16]) reported that they would not be willing to pay any out-of-pocket cost for a CSP compared with 13% (2/16) for PET/CT.Indirect Costs of Screening for Patients—Indirect costs incurred by patients undergoing a CSP are summarized in Table 3. Specifically, a large percentage of employed patients missed work (63% [5/8]) or had family miss work (38% [3/8]), necessitating the use of vacation and/or sick days to attend CSP appointments. A subset (25% [2/8]) lost income (average, $1500), and 1 patient reported that a family member lost income due to attending a CSP appointment. Most (75% [12/16]) patients also incurred substantial transportation costs (average, $243), with 1 patient spending $1000. No patients incurred child or elder care costs. One patient paid a small sum for lodging/meals while traveling to attend a CSP appointment.

Comment
Patients with DM have an increased incidence of malignancy, thus cancer screening serves a crucial role in the detection of occult disease.13 Up to half of DM patients are MSA negative, and most cancers in these patients are found with blind screening. Whole-body PET/CT has emerged as an alternative to a CSP. Evidence suggests that it has similar efficacy in detecting malignancy and may be particularly useful for identifying malignancies not routinely screened for in a CSP. In a prospective study of patients diagnosed with DM and polymyositis (N=55), whole-body PET/CT had a positive predictive value of 85.7% and negative predictive value for detecting occult malignancy of 93.8% compared with 77.8% and 95.7%, respectively, for a CSP.17

The results of our study showed that cancer screening is important to patients diagnosed with DM and that most of these patients desire some form of cancer screening. This finding held true even when patients were presented with a hypothetical situation in which screening was proven to have no survival benefit. Based on focus group data, this desire was likely driven by the fear generated by not knowing whether cancer is present, as reported by the following DM patients:
“I mean [cancer screening] is peace of mind. It is ultimately worth it. You know, better than . . . not doing the screenings and finding 3 years down the road that you have, you know, a serious problem . . . you had the cancer, and you didn’t have the screenings.” (DM patient 1)

“I would rather know than not know, even if it is bad news, just tell me. The sooner the better, and give me the whole spiel . . . maybe all the screenings don’t need to be done, done so much, so often afterwards if the initial ones are ok, but I think too, for peace of mind, I would rather know it all up front.” (DM patient 2)
Further, when presented with the hypothetical situation that insurance would not cover screenings, a few patients remarked they would relocate to obtain them:
“I would find a place where the screenings were done. I’d move.” (DM patient 4)
“If it was just sky high and [insurance companies] weren’t willing to negotiate, I would consider moving.” (DM patient 3).
Sentiments such as these emphasize the importance and value that DM patients place on being screened for cancer and also may explain why only 25% of patients felt a CSP was burdensome and only 13% reported missing appointments, all of whom planned on making them up at a later time.
When presented with the choice of a CSP or annual whole-body PET/CT for a 3-year period following the diagnosis of DM, all patients expressed that whole-body PET/CT would be less burdensome. Most preferred annual whole-body PET/CT despite the slightly increased radiation exposure associated and thought that it would limit missed appointments. Accordingly, more patients responded that they would pay more money out-of-pocket for annual whole-body PET/CT. Given that WTP can function as a numerical measure of value, our results showed that patients placed a higher value on whole-body PET/CT compared with a CSP. The indirect costs associated with a CSP also were substantial, particularly regarding missed work, use of vacation and/or sick days, and travel expenses, which is particularly important because most patients reported an annual income less than $50,000.
The direct costs of a CSP and whole-body PET/CT have been studied. Specifically, Kundrick et al18 found that whole-body PET/CT was less expensive for patients (by approximately $111) out-of-pocket compared with a CSP, though cost to insurance companies was slightly greater. The present study adds to these findings by better illustrating the burden and indirect costs that patients experience while undergoing a CSP and by characterizing the patient’s perception and preference of these 2 screening methods.
Limitations of our study include a small sample size willing to complete the survey. There also was a predominance of White and female participants, partially attributed to the greater number of female patients who develop DM compared to male patients. However, this still may limit applicability of this study to males and patients of other races. Another limitation includes recall bias on survey responses, particularly regarding indirect costs incurred with a CSP. A final limitation was that only patients with a recent diagnosis of DM who were actively undergoing screening or had recently completed malignancy screening were included in the study. Given that these patients were receiving (or had completed) exclusively a CSP, patients were comparing their personal experience with a described experience. In addition, only 2 patients were diagnosed with cancer—both with basal cell carcinoma diagnosed on physical examination—which may have influenced their perception of a CSP, given that nothing was found on an extensive number of tests. However, these patients still greatly valued their screening, as evidenced in the survey.
Conclusion
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982. doi:10.1016/S0140-6736(03)14368-1
- Schmidt J. Current classification and management of inflammatory myopathies. J Neuromuscul Dis. 2018;5:109-129. doi:10.3233/JND-180308
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol. 201;40:550-564. doi:10.3899/jrheum.120682
- Wang J, Guo G, Chen G, et al. Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol. 2013;169:838-847. doi:10.1111/bjd.12564
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9:449-453. doi:10.1016/j.autrev.2009.12.005
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. a population-based study. N Engl J Med. 1992;326:363-367. doi:10.1056/nejm199202063260602
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. doi:10.1186/ar2987
- Chen YJ, Wu CY, Shen JL. Predicting factors of malignancy in dermatomyositis and polymyositis: a case-control study. Br J Dermatol. 2001;144:825-831. doi:10.1046/j.1365-2133.2001.04140.x
- Targoff IN, Mamyrova G, Trieu EP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006;54:3682-3689. doi:10.1002/art.22164
- Chow WH, Gridley G, Mellemkjær L, et al. Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control. 1995;6:9-13. doi:10.1007/BF00051675
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy: a population-based cohort study. Ann Intern Med. 2001;134:1087-1095. doi:10.7326/0003-4819-134-12-200106190-00008
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. doi:10.1016/S0140-6736(00)03540-6
- Leatham H, Schadt C, Chisolm S, et al. Evidence supports blind screening for internal malignancy in dermatomyositis: data from 2 large US dermatology cohorts. Medicine (Baltimore). 2018;97:E9639. doi:10.1097/MD.0000000000009639
- Sparsa A, Liozon E, Herrmann F, et al. Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol. 2002;138:885-890.
- Dutton K, Soden M. Malignancy screening in autoimmune myositis among Australian rheumatologists. Intern Med J. 2017;47:1367-1375. doi:10.1111/imj.13556
- Selva-O’Callaghan A, Martinez-Gómez X, Trallero-Araguás E, et al. The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol. 2018;30:630-636. doi:10.1097/BOR.0000000000000535
- Selva-O’Callaghan A, Grau JM, Gámez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123:558-562. doi:10.1016/j.amjmed.2009.11.012
- Kundrick A, Kirby J, Ba D, et al. Positron emission tomography costs less to patients than conventional screening for malignancy in dermatomyositis. Semin Arthritis Rheum. 2019;49:140-144. doi:10.1016/j.semarthrit.2018.10.021
- Satoh M, Tanaka S, Ceribelli A, et al. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol. 2017;52:1-19. doi:10.1007/s12016-015-8510-y
- Vaughan H, Rugo HS, Haemel A. Risk-based screening for cancer in patients with dermatomyositis: toward a more individualized approach. JAMA Dermatol. 2022;158:244-247. doi:10.1001/jamadermatol.2021.5841
- Khanna U, Galimberti F, Li Y, et al. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Transl Med. 2021;9:432. doi:10.21037/atm-20-5215
- Oldroyd AGS, Allard AB, Callen JP, et al. Corrigendum to: A systematic review and meta-analysis to inform cancer screening guidelines in idiopathic inflammatory myopathies. Rheumatology (Oxford). 2021;60:5483. doi:10.1093/rheumatology/keab616
- Tchuenche M, Haté V, McPherson D, et al. Estimating client out-of-pocket costs for accessing voluntary medical male circumcision in South Africa. PLoS One. 2016;11:E0164147. doi:10.1371/journal.pone.0164147
- Teni FS, Gebresillassie BM, Birru EM, et al. Costs incurred by outpatients at a university hospital in northwestern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2018;18:842. doi:10.1186/s12913-018-3628-2
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi:10.1016/j.jbi.2008.08.010
- Bala MV, Mauskopf JA, Wood LL. Willingness to pay as a measure of health benefits. Pharmacoeconomics. 1999;15:9-18. doi:10.2165/00019053-199915010-00002
Practice Points
- Dermatomyositis (DM) is associated with an increased risk for malignancy. Patient perspective needs to be considered in developing cancer screening guidelines for patients with DM, particularly given the similar efficacy of available screening modalities.
- Current modalities for cancer screening in DM include whole-body positron emission tomography/computed tomography (PET/CT) and a conventional cancer screening panel (CSP), which includes a battery of tests typically requiring multiple visits. Patients may find the simplicity of PET/CT more preferrable than the more complex CSP.
- Indirect costs of cancer screening include missed work, travel and childcare expenses, and lost wages. Conventional cancer screening has greater indirect costs than PET/CT.
Brachioradial Pruritus: An Etiologic Review and Treatment Summary
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).
Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
Brachioradial pruritus (BRP) is a neuropathic condition typically characterized by localized dysesthesia of the dorsolateral arms.1 This dysesthesia has been described as a persistent painful itching, burning, tingling, or stinging sensation2-4 and has a median duration of expression of 24 months.5,6 The condition may be unilateral or bilateral in nature but tends to have a predilection for a bilateral distribution along the C5 to C6 dermatomes.1,7,8 There are no primary skin lesions associated with BRP; however, excoriations, prurigo nodules, and lichenification may arise secondary to scratching of the irritated skin.1,4,5,9 Brachioradial pruritus tends to have a predilection for adult females (3:1 ratio) with lighter skin. The mean age at diagnosis is 59 years, but cases have been reported in patients aged 12 to 84 years.1,5 The diagnosis of BRP is based on clinical signs and symptoms, though the ice-pack sign tends to be pathognomonic for the diagnosis.10,11 Although there is no clear evidence on the exact cause of BRP, there are 2 prevalent theories: cervical radiculopathy secondary to cervical spine pathology and/or excessive exposure to UV radiation (UVR) in the summer months.3-5,12 Brachioradial pruritus remains poorly described in the literature, and even its origin is under debate. As such, the clinician may have difficulty deciding on the best course of management. The goal of this article is to identify and discuss known treatment options for BRP (Table).

Etiology
Cervical Spine Pathology—A correlation appears to exist between BRP and cervical spine changes seen on plain film radiographs at the levels of C3 to C7, with increased incidence at the C5 to C6 levels. These plain film radiographs typically show degenerative joint disease and neural foraminal stenosis at levels that correlate to the dermatomal distribution of BRP.1,7,10,12-14 In addition to plain film radiography, some studies have utilized magnetic resonance imaging to view the cervical spine and have documented evidence of intervertebral disc protrusion/bulging, central canal stenosis, neuroforaminal stenosis, and spondylosis at the affected regions.5,15-17 Moreover, supporting the theory that the cervical spine is responsible for the emergence of BRP, Marziniak et al17 investigated 41 patients with BRP utilizing magnetic resonance tomography to find that 33 patients (80.5%) had changes in nerve compression, and 8 patients (19.5%) had degenerative changes. In addition to these findings, they found that there was a significant correlation (P<.01) between the dermatomal expression of BRP and the location of cervical anatomical changes.17 Further validating the relationship between cervical spine pathology and BRP is a case study of a patient who saw rapid and complete resolution of the pruritus following spinal decompression surgery.10 Another case study described an intramedullary tumor found in a patient with BRP that was diagnosed as an ependymoma after magnetic resonance imaging revealed an intramedullary lesion within the spinal cord between C4 and C7. The location of the tumor and dermatomal pattern of the neuropathic itch pointed to a possible association between nerve compression and BRP.14 Electromyography studies performed on individuals with BRP have shown an increase in polyphasic units, decreased motor units, and/or denervation changes along the C5/C6 or C6 nerve roots, which provides additional support for the theory of cervical spine pathology as a causative factor for BRP.16
UVR Exposure—Another etiologic theory for BRP is that UVR exposure may be responsible for the genesis of pruritus. Previously known as solar pruritus, BRP was deemed a clinical condition, as there was increased prevalence in patients living in warmer climates, such as Florida.9 Wallengren and Dahlbäck18 reported that sun exposure is a notable factor in the onset of BRP, as they saw an increase in symptoms during the late summer and a decrease in symptoms over the winter months. To further support the theory that UVR is linked to BRP, several studies have shown that the utilization of sun protection is linked to a reduction of symptoms, specifically in patients who showed seasonal variations of their symptoms.9,12,19 Additionally, a study by Mirzoyev and Davis5 retrospectively reviewed 111 patients diagnosed with BRP. Of these patients, 84 (75.7%) presented with bilateral symptoms, and 54 (48.6%) reported prolonged sun exposure. Both of these findings demonstrate correlation between UVR and BRP.5 Interestingly, UV light exposure is known to release β-endorphin in the skin and may theoretically provide an area of exploration between UVR and cervical spine theories.
Conservative Treatment
Chiropractic Manipulation—Because one etiologic theory includes disease of the cervical spine, there is evidence that targeting this region with treatment is beneficial.7 Two case reports found in the literature noted that cervical spine manipulation and cervical traction yielded positive results.20,21 It has been established that pain generated by disc lesions can be the result of local nociceptive fiber activation, direct mechanical compression of the nerve roots, or inflammatory mediators.22 There are several postulated models describing the hypoalgesic effects of spinal manipulation, which contains both biomechanical and neurophysiological mechanisms. Biomechanical changes theorized to elicit analgesia include restoration of faulty biomechanical movement patterns, breaking up of periarticular adhesions, and reflexogenic muscle inhibition of hypertonic musculature. Hypothesized neurophysiological effects of joint manipulation include an increase in afferent information overwhelming the nociceptive input, reduction of temporal summation, and autonomic activation leading to non–opioid-induced hypoalgesia.23 Cervical traction is another plausible treatment for BRP, wherein the physiological effects of traction allow for a separation of vertebral bodies and expansion of the intervertebral foramen circumference, thus decreasing compression of the nerve roots.24
Acupuncture—Neurogenic pruritus, including BRP, is a group of conditions that have been treated using acupuncture. Acupuncture treatment consists of intramuscular needle stimulation and has been found to alleviate itching in patients with neurogenic pruritus. In 1 retrospective case series, acupuncture was used to treat 16 patients who were identified as having segmental pruritus. Acupuncture targeted the spasmed paravertebral muscles of the affected dermatomal levels as well as other regions of the body, and it was found that 12 patients (75%) experienced full resolution of symptoms. However, relapse did occur in 6 patients (37%) within 1 to 12 months following treatment.25 Multiple theories exist as to why acupuncture may help. One is that it relieves muscle spasms, which in turn relieves neural irritation of the spinal nerves as they traverse the respective paraspinal musculature. Another is that acupuncture decreases nociception by stimulating release of opioid peptides in the dorsal horn.26 A third proposed theory is that acupuncture acts on the afferent nerve fibers responsible for transmitting pain—Aδ and C fibers—activating these afferent nerves to produce an analgesic effect.27
Physiotherapy—The literature suggests that possible first-line therapies for neurogenic pruritus, including BRP and notalgia paresthetica, consist of noninvasive nondermatologic treatments that target cervical spine disease. Notalgia paresthetica and BRP have similar proposed mechanisms of nerve impingement; therefore, they often are grouped together when discussing proposed manual treatment options. Physiotherapy treatment includes cervical muscle strengthening, increased range of motion, application of cervical soft collars, massage, transcutaneous electronic nerve stimulation, and cervical traction.7 A study of 12 patients by Raison-Peyron et al28 in 1999 discussed the use of spinal and paraspinal ultrasound or radiation physiotherapy. Six patients underwent this treatment, and the symptoms subsided in 4 cases.28 Another study by Fleischer et al29 in 2011 discussed improvement in 2 patients with notalgia paresthetica by exercise involving active range of motion and strengthening.
Photoprotection—Avoidance of UVR exposure has been beneficial to some patients to reduce symptoms. Use of sunscreen and long-sleeved UV-protective clothing during outdoor activities or the warmer summer months may be beneficial.1
Medical Treatment
Medication—Because of the nonspecific clinical presentation of BRP, initial treatment often involves prescription of first-line antipruritic agents, including steroid creams and systemic antihistamines, both of which generally fail to provide symptom relief.1,30 Medications with neurologic mechanisms of action appear to provide potentially superior outcomes.
Topical interventions for BRP and related neurogenic pruritus have shown limited success. A case series evaluating capsaicin for pruritus offered only transient relief, likely because of its temporary hyperstimulatory and desensitizing effect on neuropeptides.7,33 In small populations, the use of topical antidepressants has yielded cutaneous and pathological relief for BRP. A case study of a 70-year-old woman evaluated the efficacy of a combination cream of ketamine and amitriptyline (a tricyclic antidepressant) yielding moderate pruritus improvement and notable improvement of secondary brachial skin lesions.34 Oral steroids also have shown success in the treatment of chronic pruritus; however, limited research is available on the efficacy of such medications for BRP, and the long-term use of oral steroids is limited by many side effects.30
Interventional Pain Procedure—A 2018 case series investigated 3 patients with a clinical diagnosis of BRP who were treated between 2010 and 2016 with
Surgery—There are multiple case studies in the literature that discuss
Conclusion
The pathogenesis of BRP continues to be an area of debate—it may be secondary to cervical spine disease or UVR. This review found there is more research pointing to cervical spine disease. There is an abundance of literature discussing both conservative and invasive treatment strategies, both of which carry benefits. Further research is needed to better establish the etiology of BRP so that formal treatment guidelines may be established.
Neuropathic itch can be a frustrating condition for providers and patients, and many treatment modalities often are tried before arriving at a helpful treatment for a particular patient. Clinicians who may encounter BRP in practice benefit from up-to-date literature reviews that provide a summary of management strategies.
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
- Robbins BA, Schmieder GJ. Brachioradial pruritus. StatPearls Publishing; 2020. Updated September 12, 2022. Accessed July 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459321/
- Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803-805.
- Lane J, McKenzie J, Spiegel J. Brachioradial pruritus: a case report and review of the literature. Cutis. 2008;81:37-40.
- Wallengren J. Brachioradial pruritus: a recurrent solar dermopathy. J Am Acad Dermatol. 1998;39:803-806.
- Mirzoyev S, Davis M. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007-1015.
- Pinto AC, Wachholz PA, Masuda PY, et al. Clinical, epidemiological and therapeutic profile of patients with brachioradial pruritus in a reference service in dermatology. An Bras Dermatol. 2016;91:549-551. doi:10.1590/abd1806-4841.201644767
- Alai NN, Skinner HB. Concurrent notalgia paresthetica and brachioradial pruritus associated with cervical degenerative disc disease. Cutis. 2018;102:185, 186, 189, 190.
- Atis¸ G, Bilir Kaya B. Pregabalin treatment of three cases with brachioradial pruritus. Dermatol Ther. 2017;30:e12459.
- Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus). Arch Dermatol. 1968;98:481-485.
- Binder A, Fölster-Holst R, Sahan G, et al. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338-342.
- Bernhard JD, Bordeaux JS. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073.
- Veien N, Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183-185.
- Mataix J, Silvestre JF, Climent JM, et al. Brachioradial pruritus as a symptom of cervical radiculopathy. Article in Spanish. Actas Dermosifiliogr. 2008;99:719-722.
- Kavak A, Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437-440.
- Zeidler C, Pereira MP, Ständer S. Brachioradial pruritus successfully treated with intravenous naloxone. J Eur Acad Dermatol Venereol. 2023;37:e87-e89. doi:10.1111/jdv.18553
- Shields LB, Iyer VG, Zhang Y, et al. Brachioradial pruritus: clinical, electromyographic, and cervical MRI features in nine patients. Cureus. 2022;14:e21811. doi:10.7759/cureus.21811
- Marziniak M, Phan NQ, Raap U, et al. Brachioradial pruritus as a result of cervical spine pathology: the results of a magneticresonance tomography study. J Am Acad Dermatol. 2011;65:756-762. doi:10.1016/j.jaad.2010.07.036
- Wallengren J, Dahlbäck K. Familial brachioradial pruritus. Br J Dermatol. 2005;153:1016-1018.
- Salzmann SN, Okano I, Shue J, et al. Disabling pruritus in a patient with cervical stenosis. J Am Acad Orthop Surg Glob Res Rev. 2020;4:e19.00178. doi:10.5435/JAAOSGlobal-D-19-00178
- Golden KJ, Diana RM. A case of brachioradial pruritus treated with chiropractic and acupuncture. Case Rep Dermatol. 2022;14:93-97. doi:10.1159/000524054
- Tait CP, Grigg E, Quirk CJ. Brachioradial pruritus and cervical spine manipulation. Australas J Dermatol. 1998;39:168-170. doi:10.1111/j.1440-0960.1998.tb01274.x
- Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185-190. doi:10.1007/s11916-009-0032-y
- Gyer G, Michael J, Inklebarger J, et al. Spinal manipulation therapy: is it all about the brain? A current review of the neurophysiological effects of manipulation. J Integr Med. 2019;17:328-337. doi:10.1016/j.joim.2019.05.004
- Graham N, Gross A, Goldsmith CH, et al. Mechanical traction for neck pain with or without radiculopathy. Cochrane Database Syst Rev. 2008:CD006408. doi:10.1002/14651858.CD006408.pub2
- Stellon A. Neurogenic pruritus: an unrecognised problem? A retrospective case series of treatment by acupuncture. Acupunct Med. 2002;20:186-190. doi:10.1136/aim.20.4.186
- Bowsher D. Mechanisms of acupuncture. In: Filshie J, White A, eds. Medical Acupuncture: A Western Scientific Approach. Churchill Livingstone; 1998:69-82.
- Lim TK, Ma Y, Berger F, et al. Acupuncture and neural mechanism in the management of low back pain-an update. Medicines (Basel). 2018;5:63.
- Raison-Peyron N, Meunier L, Acevedo M, et al. Notalgia paresthetica: clinical, physiopathological and therapeutic aspects. a study of 12 cases. J Eur Acad Dermatol Venereol. 1999;12:215-221.
- Fleischer AB, Meade TJ, Fleischer AB. Notalgia paresthetica: successful treatment with exercises. Acta Derm Venereol. 2011;91:356-357. doi:10.2340/00015555-1039
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77:1068-1073.e7. doi:10.1016/j.jaad.2017.08.025
- Matsuda KM, Sharma D, Schonfeld AR, et al. Gabapentin and pregabalin for the treatment of chronic pruritus. J Am Acad Dermatol. 2016;75:619-625.e6. doi:10.1016/j.jaad.2016.02.1237
- Okuno S, Hashimoto T, Satoh T. Case of neuropathic itch-associated prurigo nodules on the bilateral upper arms after unilateral herpes zoster in a patient with cervical herniated discs: successful treatment with mirogabalin. J Dermatol. 2021;48:e585-e586.
- Papoiu AD, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi:10.1517/14656566.2010.481670
- Magazin M, Daze RP, Okeson N. Treatment refractory brachioradial pruritus treated with topical amitriptyline and ketamine. Cureus. 2019;11:e5117. doi:10.7759/cureus.5117
- Weinberg BD, Amans M, Deviren S, et al. Brachioradial pruritus treated with computed tomography-guided cervical nerve root block: a case series. JAAD Case Rep. 2018;4:640-644. doi:10.1016/j.jdcr.2018.03.025
- De Ridder D, Hans G, Pals P, et al. A C-fiber-mediated neuropathic brachioradial pruritus. J Neurosurg. 2010;113:118-121. doi:10.3171/2009.9.JNS09620
- Morosanu CO, Etim G, Alalade AF. Brachioradial pruritus secondary to cervical disc protrusion—a case report. J Surg Case Rep. 2022:rjac277. doi:10.1093/jscr/rjac277
Practice Points
- The etiology of brachioradial pruritus (BRP) has been associated with cervical spine pathology and/or UV radiation exposure.
- Treatment options for BRP range from conservative to invasive, and clinicians should consider the evidence for all options to decide what is best for each patient.
Violaceous Plaque on the Metacarpophalangeal Joints
The Diagnosis: Mycobacterial Infection
Mycobacterium marinum is a waterborne nontuberculous mycobacterium prevailing in salt water, brackish water, and still or streaming fresh water that infects fish and amphibians worldwide.1,2 Although first described in 1926 as the organism responsible for the demise of fish in an aquarium in Philadelphia, Pennsylvania, it was not until 1954 that the organism was linked to the cause of infection in humans after it was identified in 80 individuals who had utilized the same swimming pool.1 Due to its ability to secondarily contaminate aquariums, swimming pools, and rivers, this species can give rise to infection in humans, likely though an impaired skin barrier or points of trauma. It commonly is known as swimming pool or fish tank granuloma.3,4
Infection by M marinum commonly presents with lesions on the upper extremities, particularly the hands, that appear approximately 2 to 3 weeks following exposure to the organism.2 Lesions are categorized as superficial (type 1), granulomatous (type 2), or deep (type 3).1 Superficial lesions usually are solitary and painless; may exhibit purulent secretions; and consist of papulonodular, verrucose, or ulcerated granulomatous inflammation.1 These lesions may spread in a sporotrichoidlike pattern or in a linear fashion along lymphatic channels, similar to sporotrichosis. Granulomatous lesions present as solitary or numerous granulomas that typically are swollen, tender, and purulent. Deep lesions are the rarest form and primarily are seen in immunocompromised patients, particularly transplant recipients. Infection can lead to arthritis, tenosynovitis, or osteomyelitis.1
Mycobacterium marinum infection is diagnosed via tissue biopsy for concomitant histopathologic examination and culture from a nonulcerated area close to the lesion.1,2 If cultures do not grow, polymerase chain reaction (PCR) or PCR restriction fragment length polymorphism analysis can be conducted. These techniques can exclude other potential diagnoses; however, PCR is unable to provide information on antibiotic susceptibility.1 Biopsy of lesions reveals a nonspecific inflammatory type of reaction within the dermis consisting of lymphocytes, polymorphonuclear cells, and histiocytes.1,4 Additionally, a granulomatous inflammatory infiltrate resembling tuberculoid granuloma, sarcoidlike granuloma, or rheumatoidlike nodules also may be observed.1 With staining, the acid-fast organisms can be viewed within histiocytes, sometimes demonstrating transverse bands.4
The preferred treatment of M marinum infection is antibiotic therapy.2 It generally is not recommended to obtain in vitro drug sensitivity testing, as mutational resistance to the commonly utilized drugs is minimal. Microbiologic investigation may be warranted in cases of treatment failure or persistently positive cultures over a period of several months.1,2 Due to its rarity, no clinical trials exist to guide optimal management of M marinum infection, according to a search of ClinicalTrials.gov. Nonetheless, anecdotal evidence of prior cases can direct the selection of antibiotics. Mycobacterium marinum appears to respond to certain tetracyclines, including minocycline followed by doxycycline. Other options include clarithromycin, clarithromycin in combination with rifampin, rifampin in combination with ethambutol, trimethoprimsulfamethoxazole, and ciprofloxacin.1,2 Surgical debridement or excision may be indicated, especially in an infection involving deep structures, though recurrences have been reported in some individuals following surgery.2,4 Nonspecific treatment such as hyperthermic or liquid nitrogen local treatment have been used experimentally with positive outcomes; however, experience with this treatment modality is limited.2
Sarcoidosis is an immune-mediated systemic disorder that most commonly affects the lungs and skin. Histopathology shows sarcoidal granulomas with features similar to M marinum infection. The clinical presentation often is described as red-brown macules or papules affecting the face, rarely with overlying scale or ulceration.5 Majocchi granuloma is a dermatophyte fungal infection involving the hair follicles. Although application of topical steroids can worsen the involvement, it commonly displays perifollicular pustules,6 which were not seen in our patient. Granuloma annulare is a benign granulomatous disorder that will spontaneously resolve, typically within 2 years of onset. It presents as an annular or arcuate red-brown papule or plaque without overlying scale or ulceration,7 unlike the lesion seen in our patient. Cutaneous lymphoma is a malignant lymphoproliferative disease most commonly affecting middle-aged White men. The presentation is variable and may include an ulcerated plaque8; the lack of systemic symptoms and notable progression over several years in our patient made this a less likely diagnosis.
- Karim S, Devani A, Brassard A. Dermacase. can you identify this condition? Mycobacterium marinum infection. Can Fam Physician. 2013;59:53-54.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006; 25:609-613. doi:10.1007/s10096-006-0201-4
- Gray SF, Smith RS, Reynolds NJ, et al. Fish tank granuloma. BMJ. 1990;300:1069-1070. doi:10.1136/bmj.300.6731.1069
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma: a study of 290 cases. Arch Dermatol. 1963;88:158-162. doi:10.1001/archderm.1963.01590200046008
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives [published online May 22, 2018]. Infect Drug Resist. 2018;11:751-760. doi:10.2147/IDR.S145027
- Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23:37-50. doi:10.1007/s40257-021-00636-1
- Charli-Joseph YV, Gatica-Torres M, Pincus LB. Approach to cutaneous lymphoid infiltrates: when to consider lymphoma? Indian J Dermatol. 2016;61:351-374. doi:10.4103/0019-5154.185698
The Diagnosis: Mycobacterial Infection
Mycobacterium marinum is a waterborne nontuberculous mycobacterium prevailing in salt water, brackish water, and still or streaming fresh water that infects fish and amphibians worldwide.1,2 Although first described in 1926 as the organism responsible for the demise of fish in an aquarium in Philadelphia, Pennsylvania, it was not until 1954 that the organism was linked to the cause of infection in humans after it was identified in 80 individuals who had utilized the same swimming pool.1 Due to its ability to secondarily contaminate aquariums, swimming pools, and rivers, this species can give rise to infection in humans, likely though an impaired skin barrier or points of trauma. It commonly is known as swimming pool or fish tank granuloma.3,4
Infection by M marinum commonly presents with lesions on the upper extremities, particularly the hands, that appear approximately 2 to 3 weeks following exposure to the organism.2 Lesions are categorized as superficial (type 1), granulomatous (type 2), or deep (type 3).1 Superficial lesions usually are solitary and painless; may exhibit purulent secretions; and consist of papulonodular, verrucose, or ulcerated granulomatous inflammation.1 These lesions may spread in a sporotrichoidlike pattern or in a linear fashion along lymphatic channels, similar to sporotrichosis. Granulomatous lesions present as solitary or numerous granulomas that typically are swollen, tender, and purulent. Deep lesions are the rarest form and primarily are seen in immunocompromised patients, particularly transplant recipients. Infection can lead to arthritis, tenosynovitis, or osteomyelitis.1
Mycobacterium marinum infection is diagnosed via tissue biopsy for concomitant histopathologic examination and culture from a nonulcerated area close to the lesion.1,2 If cultures do not grow, polymerase chain reaction (PCR) or PCR restriction fragment length polymorphism analysis can be conducted. These techniques can exclude other potential diagnoses; however, PCR is unable to provide information on antibiotic susceptibility.1 Biopsy of lesions reveals a nonspecific inflammatory type of reaction within the dermis consisting of lymphocytes, polymorphonuclear cells, and histiocytes.1,4 Additionally, a granulomatous inflammatory infiltrate resembling tuberculoid granuloma, sarcoidlike granuloma, or rheumatoidlike nodules also may be observed.1 With staining, the acid-fast organisms can be viewed within histiocytes, sometimes demonstrating transverse bands.4
The preferred treatment of M marinum infection is antibiotic therapy.2 It generally is not recommended to obtain in vitro drug sensitivity testing, as mutational resistance to the commonly utilized drugs is minimal. Microbiologic investigation may be warranted in cases of treatment failure or persistently positive cultures over a period of several months.1,2 Due to its rarity, no clinical trials exist to guide optimal management of M marinum infection, according to a search of ClinicalTrials.gov. Nonetheless, anecdotal evidence of prior cases can direct the selection of antibiotics. Mycobacterium marinum appears to respond to certain tetracyclines, including minocycline followed by doxycycline. Other options include clarithromycin, clarithromycin in combination with rifampin, rifampin in combination with ethambutol, trimethoprimsulfamethoxazole, and ciprofloxacin.1,2 Surgical debridement or excision may be indicated, especially in an infection involving deep structures, though recurrences have been reported in some individuals following surgery.2,4 Nonspecific treatment such as hyperthermic or liquid nitrogen local treatment have been used experimentally with positive outcomes; however, experience with this treatment modality is limited.2
Sarcoidosis is an immune-mediated systemic disorder that most commonly affects the lungs and skin. Histopathology shows sarcoidal granulomas with features similar to M marinum infection. The clinical presentation often is described as red-brown macules or papules affecting the face, rarely with overlying scale or ulceration.5 Majocchi granuloma is a dermatophyte fungal infection involving the hair follicles. Although application of topical steroids can worsen the involvement, it commonly displays perifollicular pustules,6 which were not seen in our patient. Granuloma annulare is a benign granulomatous disorder that will spontaneously resolve, typically within 2 years of onset. It presents as an annular or arcuate red-brown papule or plaque without overlying scale or ulceration,7 unlike the lesion seen in our patient. Cutaneous lymphoma is a malignant lymphoproliferative disease most commonly affecting middle-aged White men. The presentation is variable and may include an ulcerated plaque8; the lack of systemic symptoms and notable progression over several years in our patient made this a less likely diagnosis.
The Diagnosis: Mycobacterial Infection
Mycobacterium marinum is a waterborne nontuberculous mycobacterium prevailing in salt water, brackish water, and still or streaming fresh water that infects fish and amphibians worldwide.1,2 Although first described in 1926 as the organism responsible for the demise of fish in an aquarium in Philadelphia, Pennsylvania, it was not until 1954 that the organism was linked to the cause of infection in humans after it was identified in 80 individuals who had utilized the same swimming pool.1 Due to its ability to secondarily contaminate aquariums, swimming pools, and rivers, this species can give rise to infection in humans, likely though an impaired skin barrier or points of trauma. It commonly is known as swimming pool or fish tank granuloma.3,4
Infection by M marinum commonly presents with lesions on the upper extremities, particularly the hands, that appear approximately 2 to 3 weeks following exposure to the organism.2 Lesions are categorized as superficial (type 1), granulomatous (type 2), or deep (type 3).1 Superficial lesions usually are solitary and painless; may exhibit purulent secretions; and consist of papulonodular, verrucose, or ulcerated granulomatous inflammation.1 These lesions may spread in a sporotrichoidlike pattern or in a linear fashion along lymphatic channels, similar to sporotrichosis. Granulomatous lesions present as solitary or numerous granulomas that typically are swollen, tender, and purulent. Deep lesions are the rarest form and primarily are seen in immunocompromised patients, particularly transplant recipients. Infection can lead to arthritis, tenosynovitis, or osteomyelitis.1
Mycobacterium marinum infection is diagnosed via tissue biopsy for concomitant histopathologic examination and culture from a nonulcerated area close to the lesion.1,2 If cultures do not grow, polymerase chain reaction (PCR) or PCR restriction fragment length polymorphism analysis can be conducted. These techniques can exclude other potential diagnoses; however, PCR is unable to provide information on antibiotic susceptibility.1 Biopsy of lesions reveals a nonspecific inflammatory type of reaction within the dermis consisting of lymphocytes, polymorphonuclear cells, and histiocytes.1,4 Additionally, a granulomatous inflammatory infiltrate resembling tuberculoid granuloma, sarcoidlike granuloma, or rheumatoidlike nodules also may be observed.1 With staining, the acid-fast organisms can be viewed within histiocytes, sometimes demonstrating transverse bands.4
The preferred treatment of M marinum infection is antibiotic therapy.2 It generally is not recommended to obtain in vitro drug sensitivity testing, as mutational resistance to the commonly utilized drugs is minimal. Microbiologic investigation may be warranted in cases of treatment failure or persistently positive cultures over a period of several months.1,2 Due to its rarity, no clinical trials exist to guide optimal management of M marinum infection, according to a search of ClinicalTrials.gov. Nonetheless, anecdotal evidence of prior cases can direct the selection of antibiotics. Mycobacterium marinum appears to respond to certain tetracyclines, including minocycline followed by doxycycline. Other options include clarithromycin, clarithromycin in combination with rifampin, rifampin in combination with ethambutol, trimethoprimsulfamethoxazole, and ciprofloxacin.1,2 Surgical debridement or excision may be indicated, especially in an infection involving deep structures, though recurrences have been reported in some individuals following surgery.2,4 Nonspecific treatment such as hyperthermic or liquid nitrogen local treatment have been used experimentally with positive outcomes; however, experience with this treatment modality is limited.2
Sarcoidosis is an immune-mediated systemic disorder that most commonly affects the lungs and skin. Histopathology shows sarcoidal granulomas with features similar to M marinum infection. The clinical presentation often is described as red-brown macules or papules affecting the face, rarely with overlying scale or ulceration.5 Majocchi granuloma is a dermatophyte fungal infection involving the hair follicles. Although application of topical steroids can worsen the involvement, it commonly displays perifollicular pustules,6 which were not seen in our patient. Granuloma annulare is a benign granulomatous disorder that will spontaneously resolve, typically within 2 years of onset. It presents as an annular or arcuate red-brown papule or plaque without overlying scale or ulceration,7 unlike the lesion seen in our patient. Cutaneous lymphoma is a malignant lymphoproliferative disease most commonly affecting middle-aged White men. The presentation is variable and may include an ulcerated plaque8; the lack of systemic symptoms and notable progression over several years in our patient made this a less likely diagnosis.
- Karim S, Devani A, Brassard A. Dermacase. can you identify this condition? Mycobacterium marinum infection. Can Fam Physician. 2013;59:53-54.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006; 25:609-613. doi:10.1007/s10096-006-0201-4
- Gray SF, Smith RS, Reynolds NJ, et al. Fish tank granuloma. BMJ. 1990;300:1069-1070. doi:10.1136/bmj.300.6731.1069
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma: a study of 290 cases. Arch Dermatol. 1963;88:158-162. doi:10.1001/archderm.1963.01590200046008
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives [published online May 22, 2018]. Infect Drug Resist. 2018;11:751-760. doi:10.2147/IDR.S145027
- Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23:37-50. doi:10.1007/s40257-021-00636-1
- Charli-Joseph YV, Gatica-Torres M, Pincus LB. Approach to cutaneous lymphoid infiltrates: when to consider lymphoma? Indian J Dermatol. 2016;61:351-374. doi:10.4103/0019-5154.185698
- Karim S, Devani A, Brassard A. Dermacase. can you identify this condition? Mycobacterium marinum infection. Can Fam Physician. 2013;59:53-54.
- Petrini B. Mycobacterium marinum: ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006; 25:609-613. doi:10.1007/s10096-006-0201-4
- Gray SF, Smith RS, Reynolds NJ, et al. Fish tank granuloma. BMJ. 1990;300:1069-1070. doi:10.1136/bmj.300.6731.1069
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma: a study of 290 cases. Arch Dermatol. 1963;88:158-162. doi:10.1001/archderm.1963.01590200046008
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702. doi:10.1016/j.ccm.2015.08.010
- Boral H, Durdu M, Ilkit M. Majocchi’s granuloma: current perspectives [published online May 22, 2018]. Infect Drug Resist. 2018;11:751-760. doi:10.2147/IDR.S145027
- Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23:37-50. doi:10.1007/s40257-021-00636-1
- Charli-Joseph YV, Gatica-Torres M, Pincus LB. Approach to cutaneous lymphoid infiltrates: when to consider lymphoma? Indian J Dermatol. 2016;61:351-374. doi:10.4103/0019-5154.185698
A 24-year-old man presented with a slowly growing, asymptomatic lesion on the left dorsal fourth and fifth metacarpophalangeal joints of 5 years’ duration that was recalcitrant to potent topical corticosteroids. Physical examination revealed an L-shaped, violaceous, firm plaque with focal areas of serous crust. There was no regional lymphadenopathy or lymphangitic spread. The patient had no history of recent travel, and he reported no associated pain or signs of systemic infection.