User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.

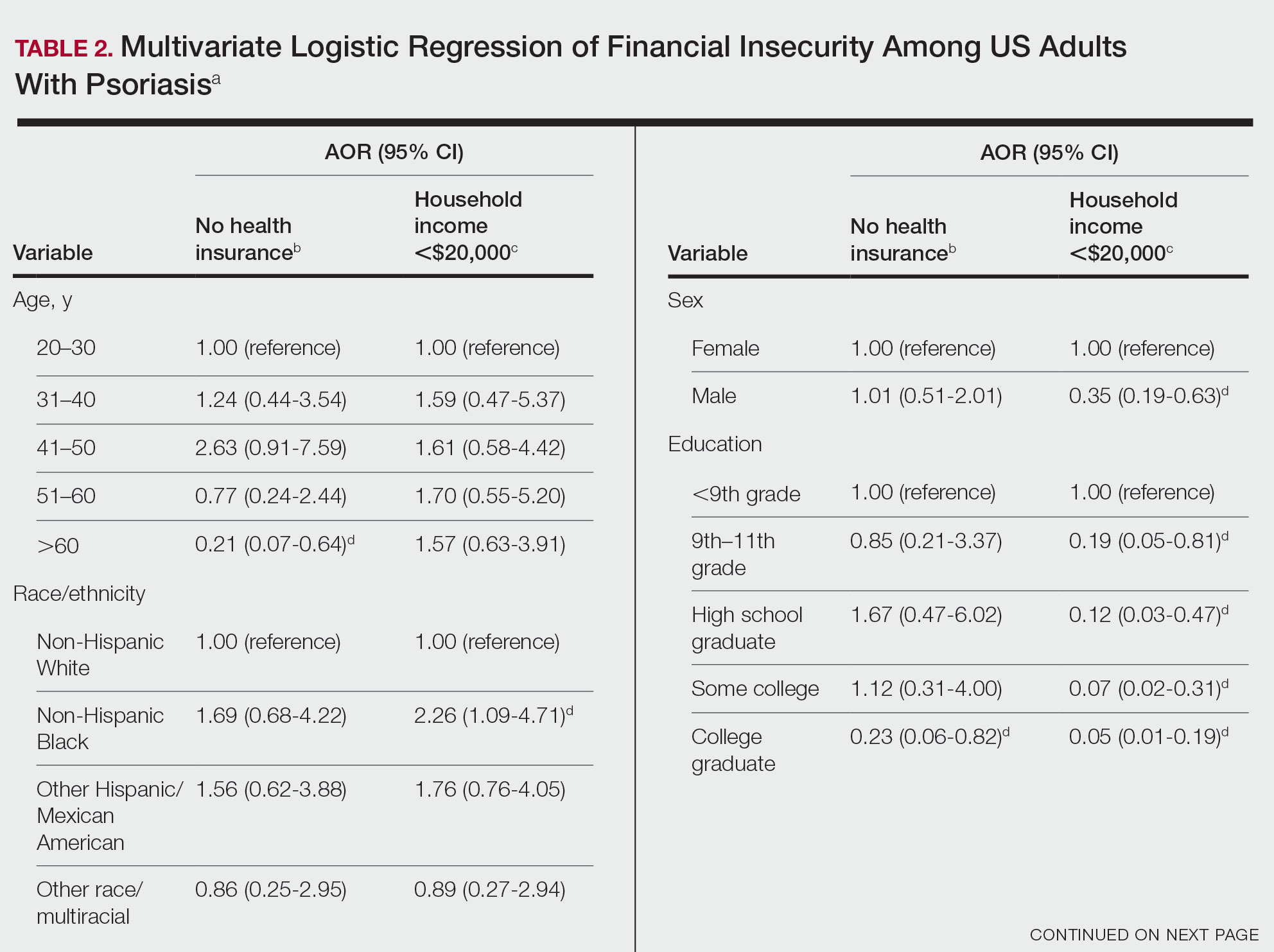
Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
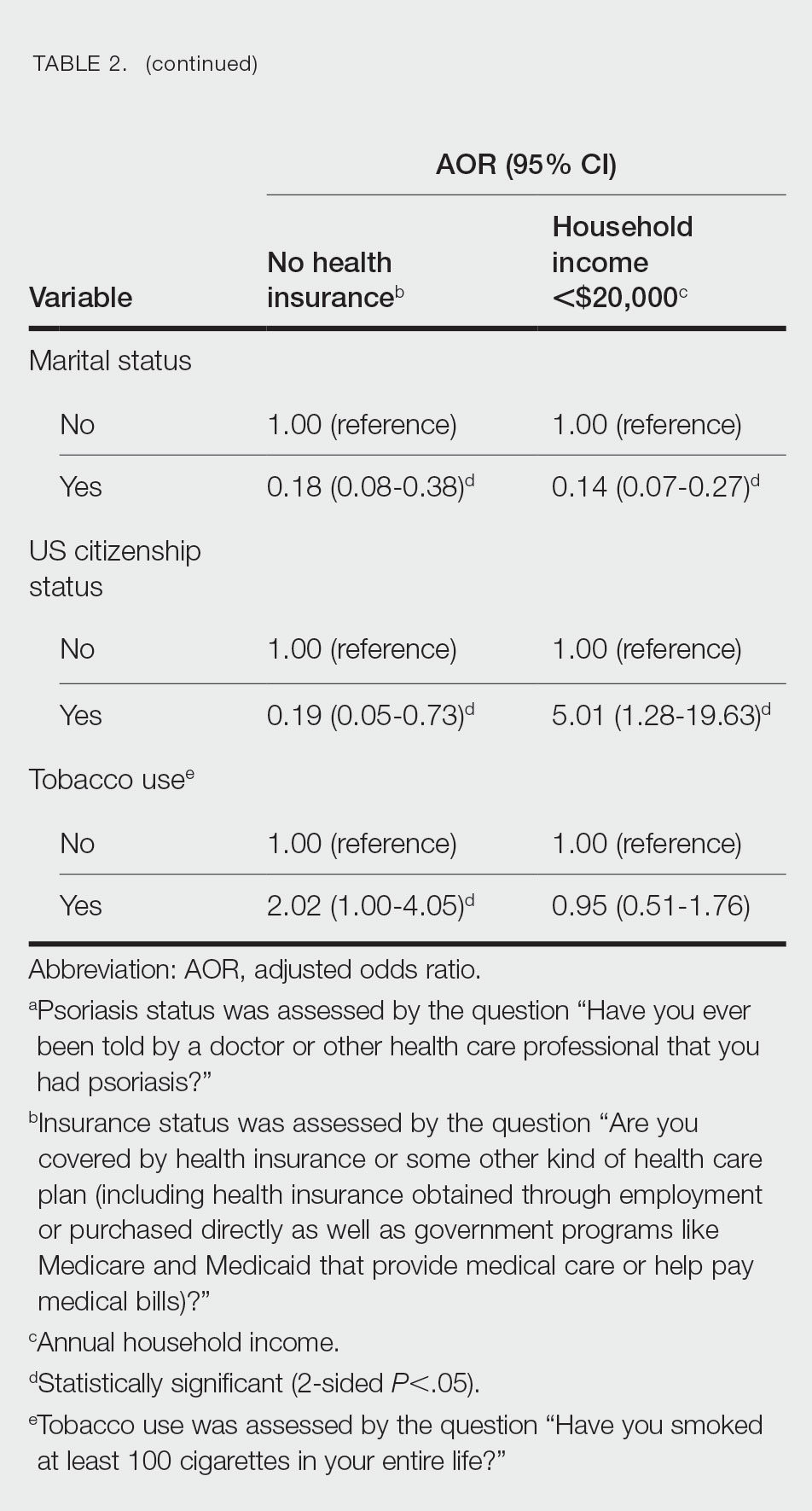
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
Cystic Presentation of High-Grade Ductal Carcinoma In Situ in an Inframammary Accessory Nipple
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.
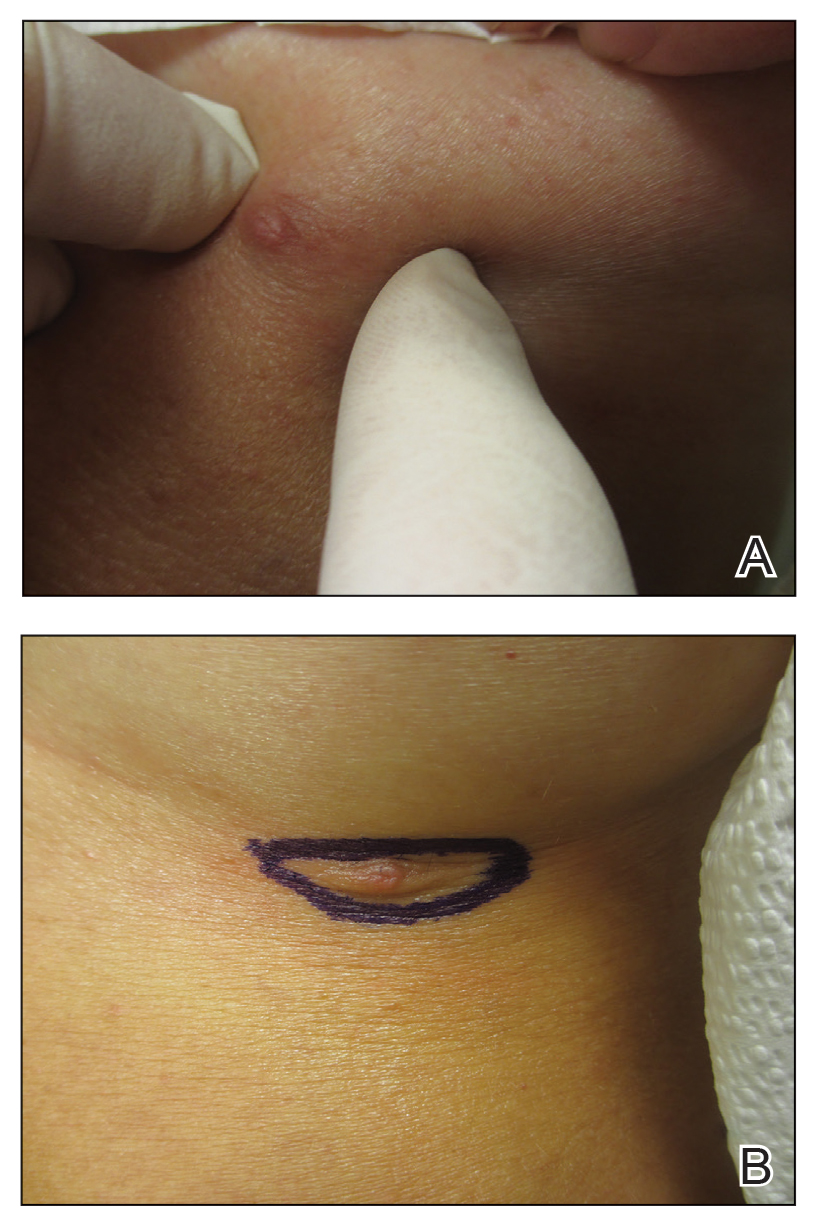
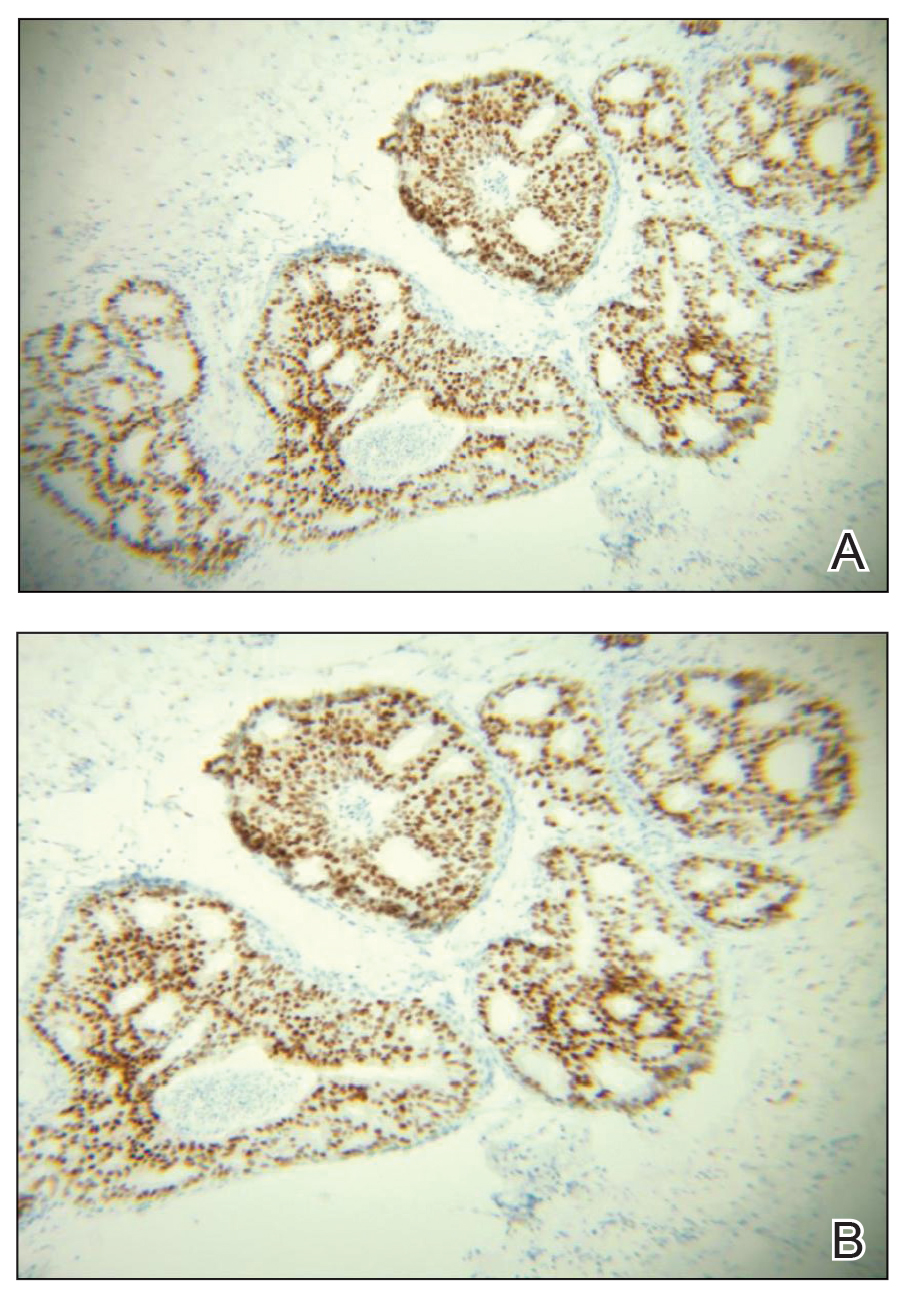
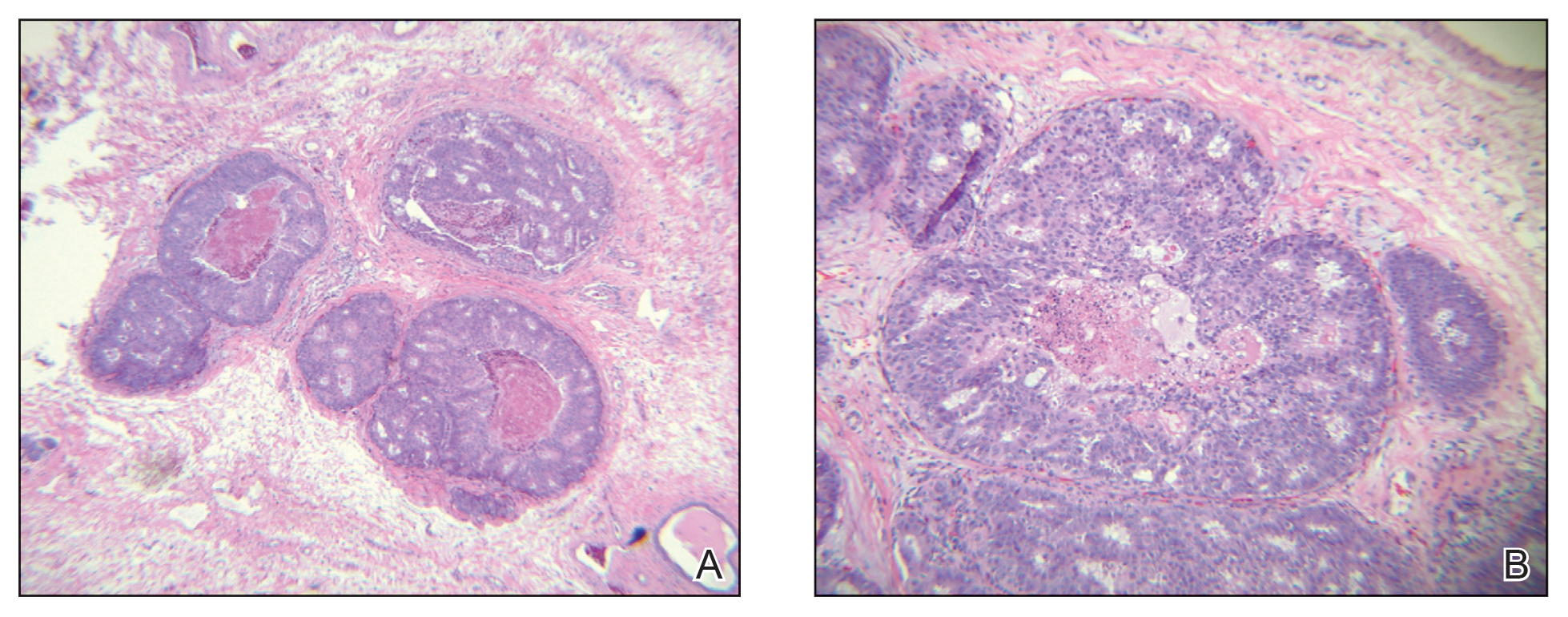
A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).
Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11
There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
Practice Points
- Accessory breasts (also referred to as ectopic breast tissue) develop when breast tissue is retained along the mammary ridge outside of the usual pectoral regions.
- Because accessory breasts may contain the same structures as anatomically correct breasts, they can be subject to the same benign or malignant changes.
- Clinical and pathologic correlation is prudent when interpreting ectopic mammary tissue, as various benign or malignant neoplasms may arise in this setting, especially if there are underlying genetic aberrancies or genodermatoses.
ChatGPT in Dermatology Clinical Practice: Potential Uses and Pitfalls
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
Artificial intelligence (AI) technology has increasingly been incorporated in medicine. In dermatology, AI has been used to detect and diagnose skin lesions, including skin cancer.1 ChatGPT (OpenAI) is a novel, highly popular development in generative AI technology. A large language model released in 2022, ChatGPT is a chatbot designed to mimic human conversation and generate specific detailed information when prompted. Free and publicly available, it has been used by millions of people. ChatGPT’s application in the medical field currently is being evaluated across several specialties, including plastic surgery, radiology, and urology.2-4 ChatGPT has the potential to assist health care professionals, including dermatologists, though its use raises important ethical considerations. Herein, we focus on the potential benefits as well as the pitfalls of using ChatGPT in dermatology clinical practice.
Potential Uses of ChatGPT in Practice
A major benefit of ChatGPT is its ability to improve clinical efficiency. First, ChatGPT can provide quick access to general medical information, similar to a search engine but with more natural language processing and contextual understanding to synthesize information.5 This function is useful for rapid concise answers to specific and directed questions. ChatGPT also can interact with its user by asking follow-up questions to produce more precise and relevant responses; this feature may help dermatologists form more accurate differential diagnoses. Additionally, ChatGPT can increase efficiency in clinical practice by drafting generic medical documents,2 including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. Another useful feature of ChatGPT is its ability to output information modeling human conversation. Because of this feature, ChatGPT also could be employed in clinical practice to serve as an interpreter for patients during clinic visits. Currently, the use of virtual translators can be cumbersome and subject to technical constraints. ChatGPT can provide accurate and conversational translations for patients and dermatologists, improving the patient-provider relationship.
ChatGPT also can contribute to major advancements in the field of dermatology beyond the clinical setting. Because of its ability to draw from extensive data that have already been uploaded, there are some uses of ChatGPT in a research context: to assist in finding resources for research and reviews, formulating hypotheses, drafting study protocols, and collecting large amounts of data within seconds.6
ChatGPT also has potential in advancing medical education. It could be used by medical schools to model interactive patient encounters to help students practice taking a patient’s history and creating differential diagnoses.6 This application of ChatGPT may help medical students hone their clinical skills in a low-stress environment without the restrictions that can come with hiring and training standardized patients, especially when mimicking dermatologic clinical encounters.
Other possibilities for ChatGPT in dermatologic practice include survey administration, clinical trial recruitment, and even automatic high-risk medication monitoring. Despite the many potential applications of ChatGPT in clinical practice, the question raised in each scenario is the quality, accuracy, and safety of what it produces.
Potential Pitfalls of ChatGPT in Practice and Possible Mitigation Strategies
A main concern in using ChatGPT in clinical practice is its potential to produce inaccurate or biased information. When prompted to create a research abstract based on previously published research, ChatGPT drafted abstracts that were clear and digestible but supplemented with incorrect data.7 A group of medical researchers who reviewed these ChatGPT-generated abstracts mistook 32% of the abstracts as having been written by human researchers. The implications of this finding are worrisome. If inaccurate or false information is used by ChatGPT in documents sent to insurance companies or patients, the patient’s safety as well as the dermatologist’s license and credibility are at stake. Thus, dermatologists looking to use ChatGPT to draft generic medical documents should actively review the output to ensure that the information is accurate. Importantly, ChatGPT also is only currently programmed with information up to 2021, limiting its access to recently published research articles and updated International Classification of Diseases, Tenth Revision codes.5 The continued development of ChatGPT will lead to regular updates by OpenAI that resolve this shortcoming in the future. Further, AI models may encode and perpetuate harmful stereotypes and social biases that are present in training data.8
When considering its potential in clinical practice, ChatGPT itself states that it can aid in clinical decision-making by processing patient information, including history, current symptoms, and biopsy and test results. This is uncharted territory, and providers who use ChatGPT at this stage to aid in decision-making should regard it similarly to information retrieved from a search engine. The information produced by ChatGPT should be used to substantiate but not definitively confirm a diagnosis or dictate management. Clinical suspicion by the dermatologist should always trump ChatGPT output. If physicians recommend ChatGPT output over their own advice, it can become a liability, as the technology is not regulated to any degree. Patients also should be cautious when seeking and following medical advice from ChatGPT, as it could be misleading or incorrect and could undermine the patient-physician relationship.6
Additionally, the specific use of ChatGPT to evaluate patient health records raises concerns related to patient privacy and confidentiality. Providers should never enter patient information protected by the Health Insurance Portability and Accountability Act into ChatGPT or anywhere else on the internet. Mitigation of this risk is not currently possible; ChatGPT requires more development and further understanding to even consider its integration into current electronic health records without compromising patient confidentiality.
The Bottom Line
Overall, ChatGPT has the potential to improve efficiency in clinical practice by assisting with informational searches and document drafting. Importantly, increased efficiency can reduce provider burnout and lead to improved patient care. However, dermatologists should carefully weigh the ethical issues of nonmaleficence and justice in evaluating ChatGPT for accuracy, biases, and the protection of patient confidentiality.
Finally, we asked ChatGPT on February 28, 2023, what it thinks:
User: Should dermatologists use ChatGPT in clinical practice?
ChatGPT: As an AI language model, ChatGPT can be a useful tool in dermatology clinical practice, but it should be used with caution in conjunction with other sources of information and clinical judgement.
Dermatologists should be aware of the potential benefits and risks of using ChatGPT and make informed decisions about how to integrate it into their clinical practice. It’s important to remember that ChatGPT is not a substitute for clinical expertise or human interaction with patients, and it should only be used as a supplement to enhance the quality of patient care.
Dermatologists who choose to use ChatGPT should also be aware of its limitations and potential risks, such as limited accuracy, lack of context, and liability issues if it provides incorrect information or recommendations that lead to patient harm. Dermatologists should use their professional judgement and consult with other health care professionals when making important decisions regarding patient care.
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
- Thomsen K, Iversen L, Titlestad TL, et al. Systematic review of machine learning for diagnosis and prognosis in dermatology. J Dermatol Treat. 2020;31:496-510. doi:10.1080/09546634.2019.1682500
- Shen Y, Heacock L, Elias J, et al. ChatGPT and other large language models are double-edged swords. Radiology. 2023;307:E230163. doi:10.1148/radiol.230163
- Gupta R, Pande P, Herzog I, et al. Application of ChatGPT in cosmetic plastic surgery: ally or antagonist? Aesthet Surg J. 2023;43:NP587-NP590. doi: 10.1093/asj/sjad042
- Gabrielson AT, Odisho AY, Canes D. Harnessing generative artificial intelligence to improve efficiency among urologists: welcome ChatGPT. J Urol. 2023;209:827-829. doi:10.1097/JU.0000000000003383
- What is ChatGPT? OpenAI. Accessed August 10, 2023. https://help.openai.com/en/articles/6783457-chatgpt-general-faq
- Haupt CE, Marks M. AI-generated medical advice—GPT and beyond. JAMA. 2023;329:1349-1350. doi:10.1001/jama.2023.5321
- Gao CA, Howard FM, Markov NS, et al. Comparing Scientific Abstracts Generated by ChatGPT to Original Abstracts Using an Artificial Intelligence Output Detector, Plagiarism Detector, and Blinded Human Reviewers. Scientific Communication and Education; 2022. doi:10.1101/2022.12.23.521610
- Weidinger L, Mellor J, Rauh M, et al. Ethical and social risks of harm from language models. arXiv. Preprint posted online December 8, 2021. https://doi.org/10.48550/arXiv.2112.04359
Practice Points
- ChatGPT potentially can play a beneficial role in dermatologic practice by quickly accessing and synthesizing information, drafting generic medical documents, interpreting visits, advancing medical education, and more.
- Dermatologists using ChatGPT should be extremely cautious, as it can produce false or biased information, perpetuate harmful stereotypes, and present information that is not up-to-date.
Applications for the CUTIS 2024 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2024 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2024.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
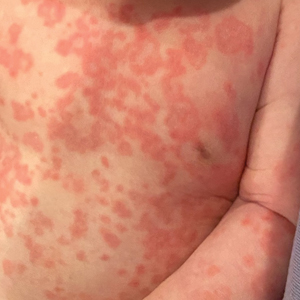
Diffuse Annular Plaques in an Infant
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
The Diagnosis: Neonatal Lupus Erythematosus
A review of the medical records of the patient’s mother from her first pregnancy revealed positive anti-Ro/SSA (Sjögren syndrome A) (>8.0 U [reference range <1.0 U]) and anti-La/SSB (Sjögren syndrome B) antibodies (>8.0 U [reference range <1.0 U]), which were reconfirmed during her pregnancy with our patient (the second child). The patient’s older brother was diagnosed with neonatal lupus erythematosus (NLE) 2 years prior at 1 month of age; therefore, the mother took hydroxychloroquine during the pregnancy with the second child to help prevent heart block if the child was diagnosed with NLE. Given the family history, positive antibodies in the mother, and clinical presentation, our patient was diagnosed with NLE. He was referred to a pediatric cardiologist and pediatrician to continue the workup of systemic manifestations of NLE and to rule out the presence of congenital heart block. The rash resolved 6 months after the initial presentation, and he did not develop any systemic manifestations of NLE.
Neonatal lupus erythematosus is a rare acquired autoimmune disorder caused by the placental transfer of anti-Ro/SSA and anti-La/SSB antibodies and less commonly anti-U1 ribonucleoprotein antinuclear autoantibodies.1,2 Approximately 1% to 2% of mothers with these positive antibodies will have infants affected with NLE.2 The annual prevalence of NLE in the United States is approximately 1 in 20,000 live births. Mothers of children with NLE most commonly have clinical Sjögren syndrome; however, anti-Ro/SSA and anti-LA/SSB antibodies may be present in 0.1% to 1.5% of healthy women, and 25% to 60% of women with autoimmune disease may be asymptomatic.1 As demonstrated in our case, when there is a family history of NLE in an infant from an earlier pregnancy, the risk for NLE increases to 17% to 20% in subsequent pregnancies1,3 and up to 25% in subsequent pregnancies if the initial child was diagnosed with a congenital heart block in the setting of NLE.1
Neonatal lupus erythematosus classically presents as annular erythematous macules and plaques with central scaling, telangictasia, atrophy, and pigmentary changes. It may start on the scalp and face and spread caudally.1,2 Patients may develop these lesions after UV exposure, and 80% of infants may not have dermatologic findings at birth. Importantly, 40% to 60% of mothers may be asymptomatic at the time of presentation of their child’s NLE.1 The diagnosis can be confirmed via antibody testing in the mother and/or infant. If performed, a punch biopsy shows interface dermatitis, vacuolar degeneration, and possible periadnexal lymphocytic infiltrates on histopathology.1,2
Management of cutaneous NLE includes sun protection (eg, application of sunscreen) and topical corticosteroids. Most dermatologic manifestations of NLE are transient, resolving after clearance of maternal IgG antibodies in 6 to 9 months; however, some telangiectasia, dyspigmentation, and atrophic scarring may persist.1-3
Neonatal lupus erythematosus also may have hepatobiliary, cardiac, hematologic, and less commonly neurologic manifestations. Hepatobiliary manifestations usually present as hepatomegaly or asymptomatic elevated transaminases or γ-glutamyl transferase.1,3 Approximately 10% to 20% of infants with NLE may present with transient anemia and thrombocytopenia.1 Cardiac manifestations are permanent and may require pacemaker implantation.1,3 The incidence of a congenital heart block in infants with NLE is 15% to 30%.3 Cardiac NLE most commonly injures the conductive tissue, leading to a congenital atrioventricular block. The development of a congenital heart block develops in the 18th to 24th week of gestation. Manifestations of a more advanced condition can include dilation of the ascending aorta and dilated cardiomyopathy.1 As such, patients need to be followed by a pediatric cardiologist for monitoring and treatment of any cardiac manifestations.
The overall prognosis of infants affected with NLE varies. Cardiac involvement is associated with a poor prognosis, while isolated cutaneous involvement requires little treatment and portends a favorable prognosis. It is critical for dermatologists to recognize NLE to refer patients to appropriate specialists to investigate and further monitor possible extracutaneous manifestations. With an understanding of the increased risk for a congenital heart block and NLE in subsequent pregnancies, mothers with positive anti-Ro/La antibodies should receive timely counseling and screening. In expectant mothers with suspected autoimmune disease, testing for antinuclear antibodies and SSA and SSB antibodies can be considered, as administration of hydroxychloroquine or prenatal systemic corticosteroids has proven to be effective in preventing a congenital heart block.1 Our patient was followed by pediatric cardiology and was not found to have a congenital heart block.
The differential diagnosis includes other causes of annular erythema in infants, as NLE can mimic several conditions. Tinea corporis may present as scaly annular plaques with central clearing; however, it rarely is encountered fulminantly in neonates.4 Erythema multiforme is a mucocutaneous hypersensitivy reaction distinguished by targetoid morphology.5 It is an exceedingly rare diagnosis in neonates; the average pediatric age of onset is 5.6 years.6 Erythema multiforme often is associated with an infection, most commonly herpes simplex virus,5 and mucosal involvement is common.6 Urticaria multiforme (also known as acute annular urticaria) is a benign disease that appears between 2 months to 3 years of age with blanchable urticarial plaques that likely are triggered by viral or bacterial infections, antibiotics, or vaccines.6 Specific lesions usually will resolve within 24 hours. Annular erythema of infancy is a benign and asymptomatic gyrate erythema that presents as annular plaques with palpable borders that spread centrifugally in patients younger than 1 year. Notably, lesions should periodically fade and may reappear cyclically for months to years. Evaluation for underlying disease usually is negative.6
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
- Derdulska JM, Rudnicka L, Szykut-Badaczewska A, et al. Neonatal lupus erythematosus—practical guidelines. J Perinat Med. 2021;49:529-538. doi:10.1515/jpm-2020-0543
- Wu J, Berk-Krauss J, Glick SA. Neonatal lupus erythematosus. JAMA Dermatol. 2021;157:590. doi:10.1001/jamadermatol.2021.0041
- Hon KL, Leung AK. Neonatal lupus erythematosus. Autoimmune Dis. 2012;2012:301274. doi:10.1155/2012/301274
- Khare AK, Gupta LK, Mittal A, et al. Neonatal tinea corporis. Indian J Dermatol. 2010;55:201. doi:10.4103/0019-5154.6274
- Ang-Tiu CU, Nicolas ME. Erythema multiforme in a 25-day old neonate. Pediatr Dermatol. 2013;30:E118-E120. doi:10.1111 /j.1525-1470.2012.01873.x
- Agnihotri G, Tsoukas MM. Annular skin lesions in infancy [published online February 3, 2022]. Clin Dermatol. 2022;40:505-512. doi:10.1016/j.clindermatol.2021.12.011
A 5-week-old infant boy presented with a rash at birth (left). The pregnancy was full term without complications, and he was otherwise healthy. A family history revealed that his older brother developed a similar rash 2 weeks after birth (right). Physical examination revealed polycyclic annular patches with an erythematous border and central clearing diffusely located on the trunk, extremities, scalp, and face with periorbital edema.
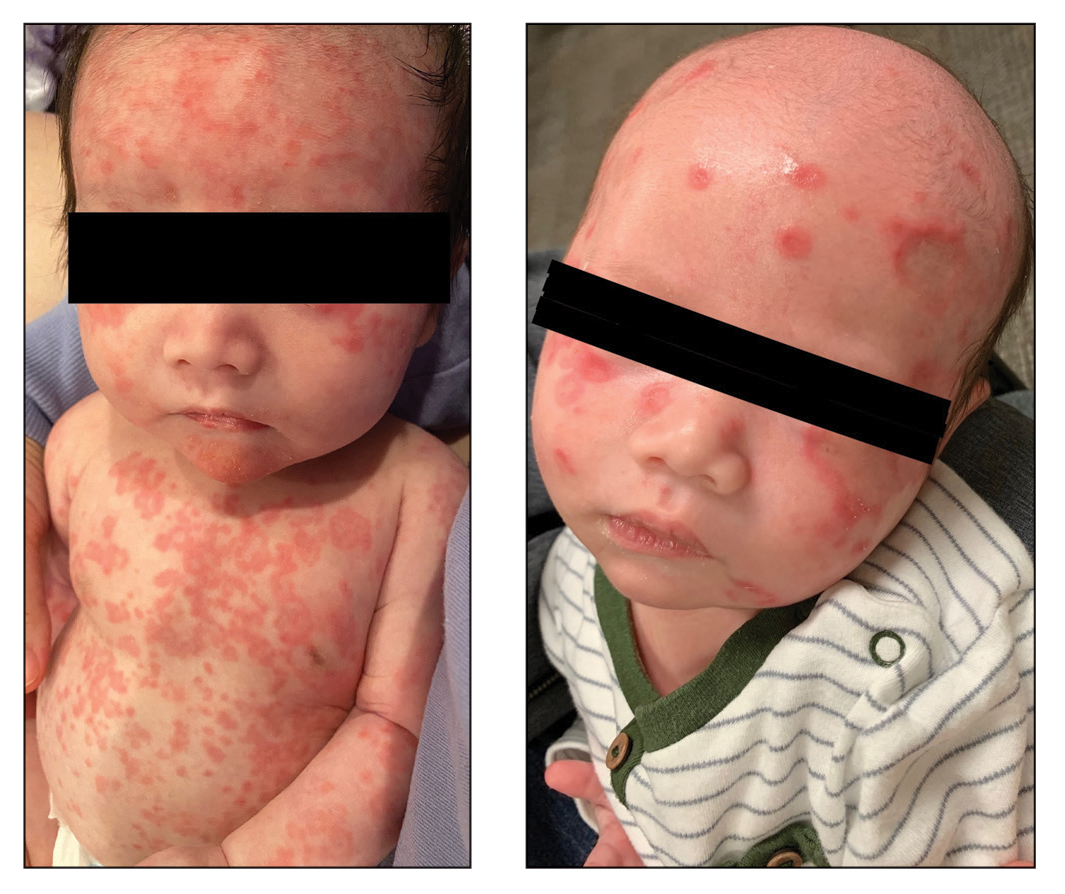
Shiny Indurated Plaques on the Legs
The Diagnosis: Pretibial Myxedema
Histopathology showed superficial and deep mucin deposition with proliferation of fibroblasts and thin wiry collagen bundles that were consistent with a diagnosis of pretibial myxedema. The patient was treated with clobetasol ointment 0.05% twice daily for 3 months, followed by a trial of pentoxifylline 400 mg 3 times daily for 3 months. After this treatment failed, she was started on rituximab infusions of 1 g biweekly for 1 month, followed by 500 mg at 6 months, with marked improvement after the first 2 doses of 1 g.
Pretibial myxedema is an uncommon cutaneous manifestation of autoimmune thyroid disease, occurring in 1% to 5% of patients with Graves disease. It usually occurs in older adult women on the pretibial regions and less commonly on the upper extremities, face, and areas of prior trauma.1-3 Although typically asymptomatic, it can be painful and ulcerate.3 The clinical presentation consists of bilateral nonpitting edema with overlying indurated skin as well as flesh-colored, yellow-brown, violaceous, or peau d’orange papules and plaques.2,3 Lesions develop over months and often have been associated with hyperhidrosis and hypertrichosis.2 Many variants have been identified including nodular, plaquelike, diffuse swelling (ie, nonpitting edema), tumor, mixture, polypoid, and elephantiasis; severe cases with acral involvement are termed thyroid acropachy.1-3 Pathogenesis likely involves the activation of thyrotropin receptors on fibroblasts by the circulating thyrotropin autoantibodies found in Graves disease. Activated fibroblasts upregulate glycosaminoglycan production, which osmotically drives the accumulation of dermal and subdermal fluid.1,3
This diagnosis should be considered in any patient with pretibial edema or edema in areas of trauma. Graves disease most commonly is diagnosed 1 to 2 years prior to the development of pretibial myxedema; other extrathyroidal manifestations, most commonly ophthalmopathies, almost always are found in patients with pretibial myxedema. If a diagnosis of Graves disease has not been established, thyroid studies, including thyrotropin receptor antibody serum levels, should be obtained. Histopathology showing increased mucin in the dermis and increased fibroblasts can aid in diagnosis.2,3
The differential diagnosis includes inflammatory dermatoses, such as stasis dermatitis and lipodermatosclerosis. Stasis dermatitis is characterized by lichenified yellowbrown plaques that present on the lower extremities; lipodermatosclerosis then can develop and present as atrophic sclerotic plaques with a champagne bottle–like appearance. Necrobiosis lipoidica demonstrates atrophic, shiny, yellow plaques with telangiectases and ulcerations. Hypertrophic lichen planus presents with hyperkeratotic hyperpigmented plaques on the shins.1,2 Other diseases of cutaneous mucin deposition, namely scleromyxedema, demonstrate similar physical findings but more commonly are located on the trunk, face, and dorsal hands rather than the lower extremities.1-3
Treatment of pretibial myxedema is difficult; normalization of thyroid function, weight reduction, and compression stockings can help reduce edema. Medical therapies aim to decrease glycosaminoglycan production by fibroblasts. First-line treatment includes topical steroids under occlusion, and second-line therapies include intralesional steroids, systemic corticosteroids, pentoxifylline, and octreotide.2,3 Therapies for refractory disease include plasmapheresis, surgical excision, radiotherapy, and intravenous immunoglobulin; more recent studies also endorse the use of isotretinoin, intralesional hyaluronidase, and rituximab.2,4 Success also has been observed with the insulin growth factor 1 receptor inhibitor teprotumumab in active thyroid eye disease, in which insulin growth factor 1 receptor is overexpressed by fibroblasts. Given the similar pathogenesis of thyroid ophthalmopathy with other extrathyroidal manifestations, teprotumumab is a promising option for refractory cases of pretibial myxedema and has led to disease resolution in several patients.4
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7. doi:10.1097/00005792-199401000-00001
- Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis, and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-662. doi:10.1067/mjd.2003.257
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446. doi:10.1210/jcem.87.2.8220
- Varma A, Rheeman C, Levitt J. Resolution of pretibial myxedema with teprotumumab in a patient with Graves disease. JAAD Case Reports. 2020;6:1281-1282. doi:10.1016/j.jdcr.2020.09.003
The Diagnosis: Pretibial Myxedema
Histopathology showed superficial and deep mucin deposition with proliferation of fibroblasts and thin wiry collagen bundles that were consistent with a diagnosis of pretibial myxedema. The patient was treated with clobetasol ointment 0.05% twice daily for 3 months, followed by a trial of pentoxifylline 400 mg 3 times daily for 3 months. After this treatment failed, she was started on rituximab infusions of 1 g biweekly for 1 month, followed by 500 mg at 6 months, with marked improvement after the first 2 doses of 1 g.
Pretibial myxedema is an uncommon cutaneous manifestation of autoimmune thyroid disease, occurring in 1% to 5% of patients with Graves disease. It usually occurs in older adult women on the pretibial regions and less commonly on the upper extremities, face, and areas of prior trauma.1-3 Although typically asymptomatic, it can be painful and ulcerate.3 The clinical presentation consists of bilateral nonpitting edema with overlying indurated skin as well as flesh-colored, yellow-brown, violaceous, or peau d’orange papules and plaques.2,3 Lesions develop over months and often have been associated with hyperhidrosis and hypertrichosis.2 Many variants have been identified including nodular, plaquelike, diffuse swelling (ie, nonpitting edema), tumor, mixture, polypoid, and elephantiasis; severe cases with acral involvement are termed thyroid acropachy.1-3 Pathogenesis likely involves the activation of thyrotropin receptors on fibroblasts by the circulating thyrotropin autoantibodies found in Graves disease. Activated fibroblasts upregulate glycosaminoglycan production, which osmotically drives the accumulation of dermal and subdermal fluid.1,3
This diagnosis should be considered in any patient with pretibial edema or edema in areas of trauma. Graves disease most commonly is diagnosed 1 to 2 years prior to the development of pretibial myxedema; other extrathyroidal manifestations, most commonly ophthalmopathies, almost always are found in patients with pretibial myxedema. If a diagnosis of Graves disease has not been established, thyroid studies, including thyrotropin receptor antibody serum levels, should be obtained. Histopathology showing increased mucin in the dermis and increased fibroblasts can aid in diagnosis.2,3
The differential diagnosis includes inflammatory dermatoses, such as stasis dermatitis and lipodermatosclerosis. Stasis dermatitis is characterized by lichenified yellowbrown plaques that present on the lower extremities; lipodermatosclerosis then can develop and present as atrophic sclerotic plaques with a champagne bottle–like appearance. Necrobiosis lipoidica demonstrates atrophic, shiny, yellow plaques with telangiectases and ulcerations. Hypertrophic lichen planus presents with hyperkeratotic hyperpigmented plaques on the shins.1,2 Other diseases of cutaneous mucin deposition, namely scleromyxedema, demonstrate similar physical findings but more commonly are located on the trunk, face, and dorsal hands rather than the lower extremities.1-3
Treatment of pretibial myxedema is difficult; normalization of thyroid function, weight reduction, and compression stockings can help reduce edema. Medical therapies aim to decrease glycosaminoglycan production by fibroblasts. First-line treatment includes topical steroids under occlusion, and second-line therapies include intralesional steroids, systemic corticosteroids, pentoxifylline, and octreotide.2,3 Therapies for refractory disease include plasmapheresis, surgical excision, radiotherapy, and intravenous immunoglobulin; more recent studies also endorse the use of isotretinoin, intralesional hyaluronidase, and rituximab.2,4 Success also has been observed with the insulin growth factor 1 receptor inhibitor teprotumumab in active thyroid eye disease, in which insulin growth factor 1 receptor is overexpressed by fibroblasts. Given the similar pathogenesis of thyroid ophthalmopathy with other extrathyroidal manifestations, teprotumumab is a promising option for refractory cases of pretibial myxedema and has led to disease resolution in several patients.4
The Diagnosis: Pretibial Myxedema
Histopathology showed superficial and deep mucin deposition with proliferation of fibroblasts and thin wiry collagen bundles that were consistent with a diagnosis of pretibial myxedema. The patient was treated with clobetasol ointment 0.05% twice daily for 3 months, followed by a trial of pentoxifylline 400 mg 3 times daily for 3 months. After this treatment failed, she was started on rituximab infusions of 1 g biweekly for 1 month, followed by 500 mg at 6 months, with marked improvement after the first 2 doses of 1 g.
Pretibial myxedema is an uncommon cutaneous manifestation of autoimmune thyroid disease, occurring in 1% to 5% of patients with Graves disease. It usually occurs in older adult women on the pretibial regions and less commonly on the upper extremities, face, and areas of prior trauma.1-3 Although typically asymptomatic, it can be painful and ulcerate.3 The clinical presentation consists of bilateral nonpitting edema with overlying indurated skin as well as flesh-colored, yellow-brown, violaceous, or peau d’orange papules and plaques.2,3 Lesions develop over months and often have been associated with hyperhidrosis and hypertrichosis.2 Many variants have been identified including nodular, plaquelike, diffuse swelling (ie, nonpitting edema), tumor, mixture, polypoid, and elephantiasis; severe cases with acral involvement are termed thyroid acropachy.1-3 Pathogenesis likely involves the activation of thyrotropin receptors on fibroblasts by the circulating thyrotropin autoantibodies found in Graves disease. Activated fibroblasts upregulate glycosaminoglycan production, which osmotically drives the accumulation of dermal and subdermal fluid.1,3
This diagnosis should be considered in any patient with pretibial edema or edema in areas of trauma. Graves disease most commonly is diagnosed 1 to 2 years prior to the development of pretibial myxedema; other extrathyroidal manifestations, most commonly ophthalmopathies, almost always are found in patients with pretibial myxedema. If a diagnosis of Graves disease has not been established, thyroid studies, including thyrotropin receptor antibody serum levels, should be obtained. Histopathology showing increased mucin in the dermis and increased fibroblasts can aid in diagnosis.2,3
The differential diagnosis includes inflammatory dermatoses, such as stasis dermatitis and lipodermatosclerosis. Stasis dermatitis is characterized by lichenified yellowbrown plaques that present on the lower extremities; lipodermatosclerosis then can develop and present as atrophic sclerotic plaques with a champagne bottle–like appearance. Necrobiosis lipoidica demonstrates atrophic, shiny, yellow plaques with telangiectases and ulcerations. Hypertrophic lichen planus presents with hyperkeratotic hyperpigmented plaques on the shins.1,2 Other diseases of cutaneous mucin deposition, namely scleromyxedema, demonstrate similar physical findings but more commonly are located on the trunk, face, and dorsal hands rather than the lower extremities.1-3
Treatment of pretibial myxedema is difficult; normalization of thyroid function, weight reduction, and compression stockings can help reduce edema. Medical therapies aim to decrease glycosaminoglycan production by fibroblasts. First-line treatment includes topical steroids under occlusion, and second-line therapies include intralesional steroids, systemic corticosteroids, pentoxifylline, and octreotide.2,3 Therapies for refractory disease include plasmapheresis, surgical excision, radiotherapy, and intravenous immunoglobulin; more recent studies also endorse the use of isotretinoin, intralesional hyaluronidase, and rituximab.2,4 Success also has been observed with the insulin growth factor 1 receptor inhibitor teprotumumab in active thyroid eye disease, in which insulin growth factor 1 receptor is overexpressed by fibroblasts. Given the similar pathogenesis of thyroid ophthalmopathy with other extrathyroidal manifestations, teprotumumab is a promising option for refractory cases of pretibial myxedema and has led to disease resolution in several patients.4
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7. doi:10.1097/00005792-199401000-00001
- Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis, and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-662. doi:10.1067/mjd.2003.257
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446. doi:10.1210/jcem.87.2.8220
- Varma A, Rheeman C, Levitt J. Resolution of pretibial myxedema with teprotumumab in a patient with Graves disease. JAAD Case Reports. 2020;6:1281-1282. doi:10.1016/j.jdcr.2020.09.003
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7. doi:10.1097/00005792-199401000-00001
- Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis, and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-662. doi:10.1067/mjd.2003.257
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446. doi:10.1210/jcem.87.2.8220
- Varma A, Rheeman C, Levitt J. Resolution of pretibial myxedema with teprotumumab in a patient with Graves disease. JAAD Case Reports. 2020;6:1281-1282. doi:10.1016/j.jdcr.2020.09.003
A 70-year-old woman presented with pain and swelling in both legs of many years’ duration. She had no history of skin disease. Physical examination revealed shiny indurated plaques on the legs, ankles, and toes with limited range of motion in the ankles (top). Marked thickening of the hands and index fingers also was noted (bottom). A punch biopsy of the distal pretibial region was performed.
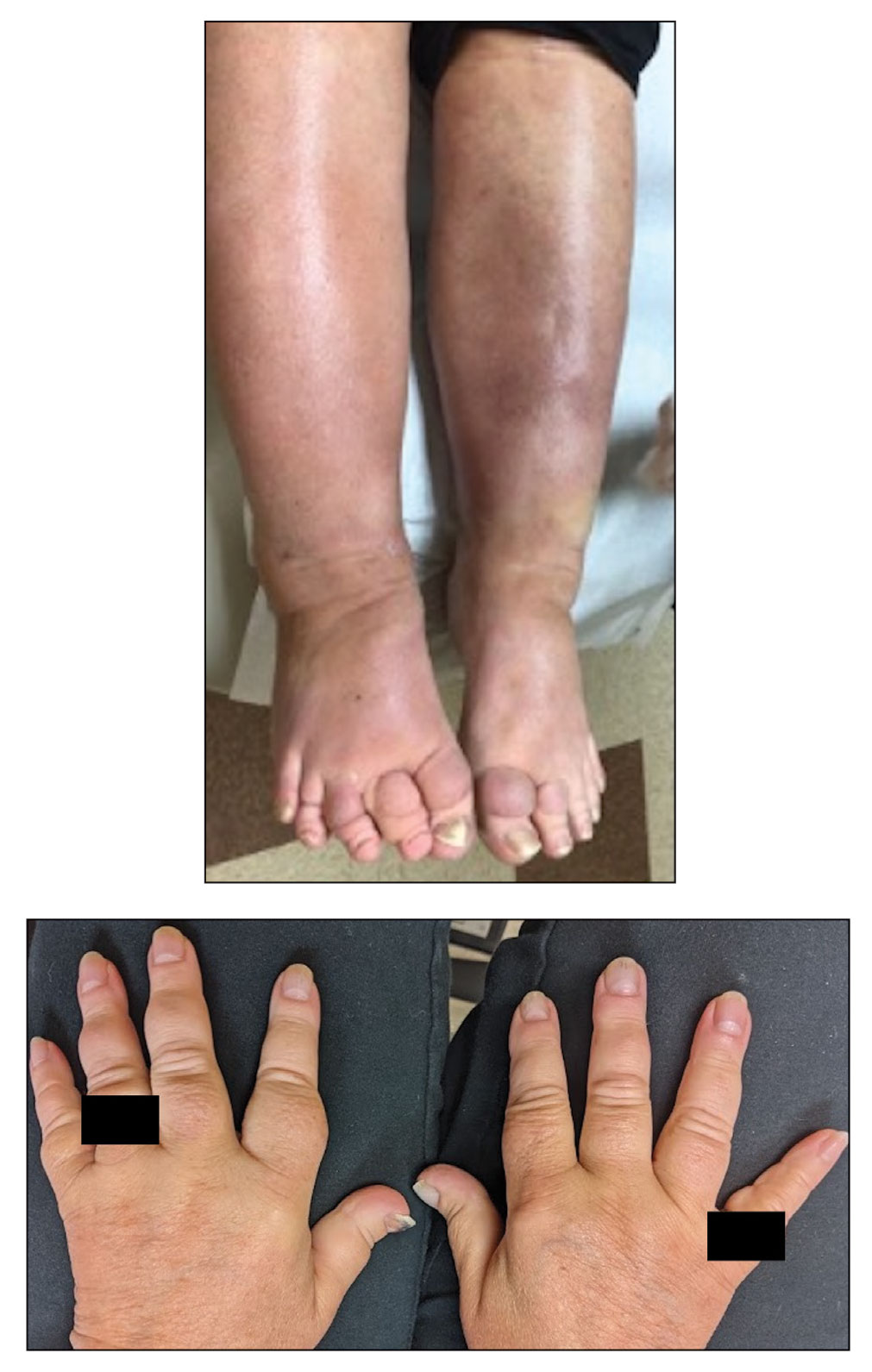
Squamous Cell Carcinoma
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3
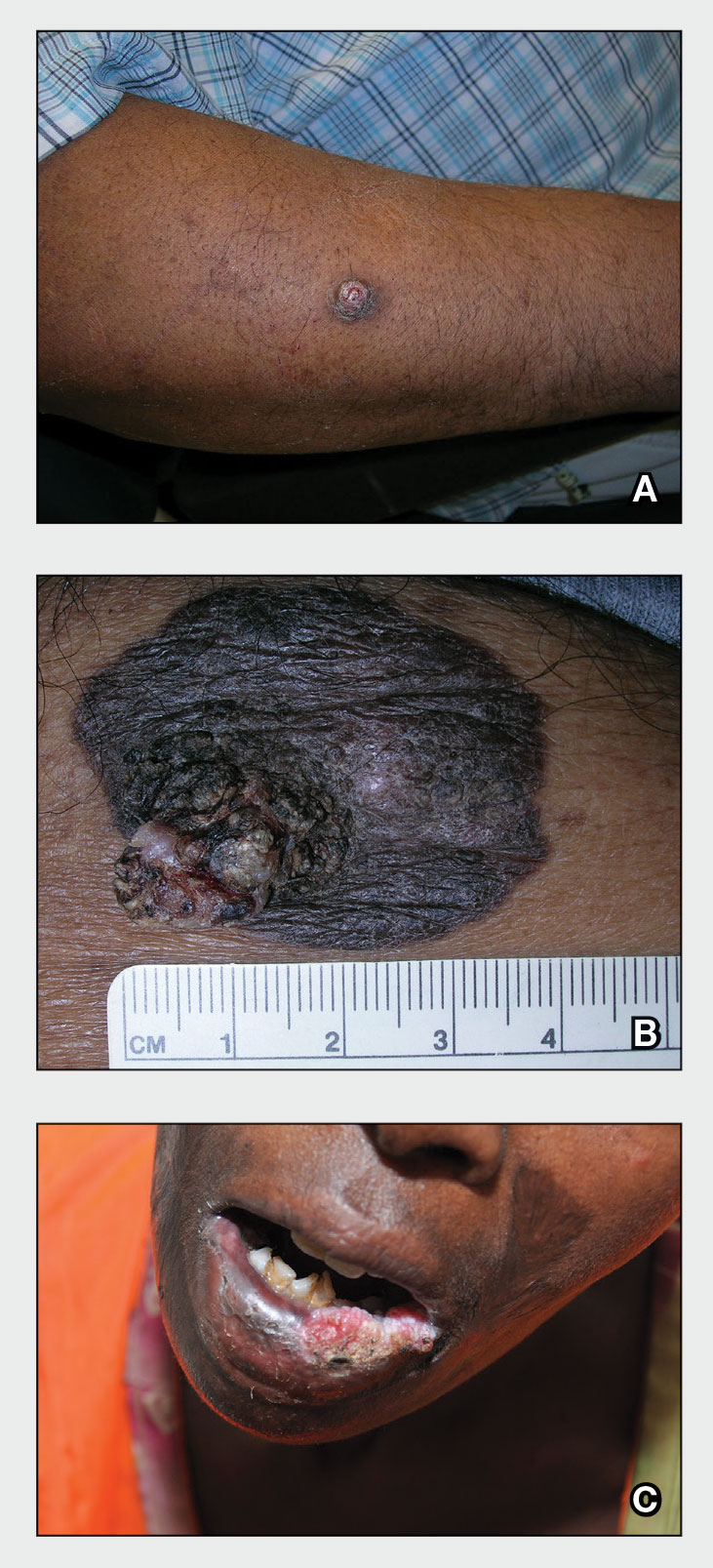
Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
THE COMPARISON
A A 51-year-old Hispanic man with a squamous cell carcinoma (SCC) of the keratoacanthoma type on the arm.
B A 75-year-old Black man with an SCC of the keratoacanthoma type on the abdomen.
C An African woman with an SCC on the lower lip decades after a large facial burn, which is known as a Marjolin ulcer.
Cutaneous squamous cell carcinoma (SCC) develops from a malignant tumor of the keratinocytes, eccrine glands, or pilosebaceous units that invades the dermis. Risk factors include lighter skin tone, higher cumulative sun exposure, human papillomavirus (HPV) infection, hidradenitis suppurativa (HS), lichen sclerosus, family history of skin cancer,1 and immunosuppression.2 It typically affects sun-exposed areas of the body such as the face, scalp, neck, and extensor surfaces of the arms (Figure, A).3,4 However, in those with darker skin tones, the most common anatomic sites are those that are not exposed to the sun (Figure, B). Squamous cell carcinoma is diagnosed via skin biopsy. Treatment options include surgical excision, destructive methods such as electrodesiccation and curettage, and Mohs micrographic surgery. Cutaneous SCC has a cure rate of more than 95% and a mortality rate of 1.5% to 2% in the United States.3

Epidemiology
Squamous cell carcinoma is the most common skin cancer occurring in Black individuals, manifesting primarily in the fifth decade of life.5-7 It is the second most common skin cancer in White, Hispanic, and Asian individuals and is more common in males.8 In a study of organ transplant recipients (N=413), Pritchett et al9 reported that HPV infection was a major risk factor in Hispanic patients because 66.7% of those with SCC had a history of HPV. However, HPV is a risk factor for SCC in all ethnic groups.10
Key clinical features in people with darker skin tones
Anatomic location
- The lower legs and anogenital areas are the most common sites for SCC in patients with skin of color.4,11
- In Black women, SCC occurs more often on sun-exposed areas such as the arms and legs compared to Black men.7,12-14
- The genitalia, perianal area, ocular mucosa, and oral mucosa are the least likely areas to be routinely examined, even in skin cancer clinics that see high-risk patients, despite the SCC risk in the anogenital area.15,16
- Squamous cell carcinoma of the lips and scalp is more likely to occur in Black women vs Black men.4,7,17 Clinical appearance
- In those with darker skin tones, SCCs may appear hyperpigmented4 or hyperkeratotic with a lack of erythema and an inconsistent appearance.6,7,18
- A nonhealing ulceration of the skin should prompt a biopsy to rule out SCC.3,19
Worth noting
In patients with darker skin tones, the risk for SCC increases in areas with chronic inflammation and scarring of the skin.4,6,7,11,18,20-22 In Black patients, 20% to 40% of cases of SCC occur in the setting of chronic inflammation and scarring.6,7,18 Chronic inflammatory conditions include ulcers, lupus vulgaris, discoid lupus erythematosus, and HPV. In patients with discoid lupus erythematosus, there is an additive effect of sun exposure on the scars, which may play a role in the pathogenesis and metastasis risk for skin cancer in Black patients.4 Other scarring conditions include thermal or chemical burn scars, areas of physical trauma, and prior sites of radiation treatment.14,23 Squamous cell carcinoma arising in a burn scar is called a Marjolin ulcer or malignant degeneration of a scar (Figure, C). It is reported more often in lower-income, underresourced countries, which may suggest the need for early detection in populations with skin of color.24
Squamous cell carcinoma is more aggressive in sites that are not exposed to sun compared to sun-exposed areas.17,25
The risk for SCC is increased in immunocompromised patients,2 especially those with HPV.10
The prevalence of SCC in those with HS is approximately 4.6%. The chronic inflammation and irritation from HS in association with other risk factors such as tobacco use may contribute to the malignant transformation to SCC.26
Health disparity highlight
- The risk for metastasis from SCC is 20% to 40% in Black patients vs 1% to 4% in White patients.4,6,27
- Penile SCC was associated with a lower overall survival rate in patients of African descent.20,21
- The increased morbidity and mortality from SCC in patients with skin of color may be attributed to delays in diagnosis and treatment as well as an incomplete understanding of tumor genetics.4,6,18
Acknowledgment—The authors thank Elyse Gadra (Philadelphia, Pennsylvania) for assistance in the preparation of this manuscript.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
- Asgari MM, Warton EM, Whittemore AS. Family history of skin cancer is associated with increased risk of cutaneous squamous cell carcinoma. Dermatol Surg. 2015;41:481-486. doi:10.1097/DSS.0000000000000292
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297. doi:10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z
- Kallini JR, Nouran H, Khachemoune A. Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends. Int J Dermatol. 2015;54:130-140. https://doi.org/10.1111/ijd.12553.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public [published online January 28, 2014]. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Bradford PT. Skin cancer in skin of color. Dermatol Nurse. 2009;21:170-177.
- Gloster HM, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134. https://doi.org/10.1016/j.ijwd.2021.01.017
- Baum B, Duarte AM. Skin cancer epidemic in American Hispanic and Latino patients. In: Silverberg N, Duran-McKinster C, Tay Y-K, eds. Pediatric Skin of Color. Springer; 2015:453-460.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152: 1348-1353. doi:10.1001/jamadermatol.2016.3328
- Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389-395. doi:10.1093/jnci/djj092
- Gohara M. Skin cancer: an African perspective. Br J Dermatol. 2015;173: 17-21. https://doi.org/10.1111/bjd.13380
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. doi:10.1016/s1011-1344(01)00198-1
- Halder RM, Bang KM. Skin cancer in African Americans in the United States. Dermatol Clin. 1988;6:397-407.
- Mora RG, Perniciaro C. Cancer of the skin in blacks. I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543. doi:10.1016/s0190-9622(81)70113-0
- Bajaj S, Wolner ZJ, Dusza SW, et al. Total body skin examination practices: a survey study amongst dermatologists at high-risk skin cancer clinics. Dermatol Pract Concept. 2019;9:132-138. doi:10.5826/dpc.0902a09
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin. 2003;21:725-732, x. doi: 10.1016/s0733-8635(03)00085-8
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Sng J, Koh D, Siong WC, et al. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426-432.
- Shao K, Feng H. Racial and ethnic healthcare disparities in skin cancer in the United States: a review of existing inequities, contributing factors, and potential solutions. J Clin Aesthet Dermatol. 2022;15:16-22.
- Shao K, Hooper J, Feng H. Racial and ethnic health disparities in dermatology in the United States. part 2: disease-specific epidemiology, characteristics, management, and outcomes. J Am Acad Dermatol. 2022;87:733-744. https://doi.org/10.1016/j.jaad.2021.12.062
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. https://doi.org/10.1007/s40257-021-00662-z
- Copcu E, Aktas A, Sis¸man N, et al. Thirty-one cases of Marjolin’s ulcer. Clin Exp Dermatol. 2003;28:138-141. doi:10.1046/j.1365-2230.2003.01210.x
- Abdi MA, Yan M, Hanna TP. Systematic review of modern case series of squamous cell cancer arising in a chronic ulcer (Marjolin’s ulcer) of the skin. JCO Glob Oncol. 2020;6:809-818. doi:10.1200/GO.20.00094
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Chapman S, Delgadillo D, Barber C, et al. Cutanteous squamous cell complicating hidradenitis suppurativa: a review of the prevalence, pathogenesis, and treatment of this dreaded complication. Acta Dermatovenerol Al Pannocica Adriat. 2018;27:25-28.
- Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Lanolin: The 2023 American Contact Dermatitis Society Allergen of the Year
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
Lanolin was announced as the Allergen of the Year by the American Contact Dermatitis Society in March 2023.1 However, allergic contact dermatitis (ACD) to lanolin remains a matter of fierce debate among dermatologists. Herein, we discuss this important contact allergen, emphasizing the controversy behind its allergenicity and nuances to consider when patch testing.
What is Lanolin?
Lanolin is a greasy, yellow, fatlike substance derived from the sebaceous glands of sheep. It is extracted from wool using an intricate process of scouring with dilute alkali, centrifuging, and refining with hot alkali and bleach.2 It is comprised of a complex mixture of esters, alcohols, sterols, fatty acids, lactose, and hydrocarbons.3
The hydrophobic property of lanolin helps sheep shed water from their coats.3 In humans, this hydrophobicity benefits the skin by retaining moisture already present in the epidermis. Lanolin can hold as much as twice its weight in water and may reduce transepidermal water loss by 20% to 30%.4-6 In addition, lanolin maintains tissue breathability, which supports proper gas exchange, promoting wound healing and protecting against infection.3,7
Many personal care products (PCPs), cosmetics, and topical medicaments contain lanolin, particularly products marketed to help restore dry cracked skin. The range of permitted concentrations of lanolin in over-the-counter products in the United States is 12.5% to 50%.3 Lanolin also may be found in industrial goods. The Table provides a comprehensive list of common items that may contain lanolin.1,3,8,9
A Wolf in Sheep’s Clothing?
Despite its benefits, lanolin is a potential source of ACD. The first reported positive patch test (PPT) to lanolin worldwide was in the late 1920s.10 Subsequent cases of ACD to lanolin were described over the next 30 years, reaching a peak of recognition in the latter half of the 20th century with rates of PPT ranging from 0% to 7.4%, though the patient population and lanolin patch-test formulation used differed across studies.9 The North American Contact Dermatitis Group observed that 3.3% (1431/43,691) of patients tested from 2001 to 2018 had a PPT to either lanolin alcohol 30% in petrolatum (pet) or Amerchol L101 (10% lanolin alcohol dissolved in mineral oil) 50% pet.11 Compared to patients referred for patch testing, the prevalence of contact allergy to lanolin is lower in the general population; 0.4% of the general population in Europe (N=3119) tested positive to wool alcohols 1.0 mg/cm2 on the thin-layer rapid use Epicutaneous (TRUE) test.12
Allergic contact dermatitis to lanolin is unrelated to an allergy to wool itself, which probably does not exist, though wool is well known to cause irritant contact dermatitis, particularly in atopic individuals.13

Who Is at Risk for Lanolin Allergy?
In a recent comprehensive review of lanolin allergy, Jenkins and Belsito1 summarized 4 high-risk subgroups of patients for the development of lanolin contact allergy: stasis dermatitis, chronic leg ulcers, atopic dermatitis (AD), and perianal/genital dermatitis. These chronic inflammatory skin conditions may increase the risk for ACD to lanolin via increased exposure in topical therapies and/or increased allergen penetration through an impaired epidermal barrier.14-16 Demographically, older adults and children are at-risk groups, likely secondary to the higher prevalence of stasis dermatitis/leg ulcers in the former group and AD in the latter.1
Lanolin Controversies
The allergenicity of lanolin is far from straightforward. In 1996, Wolf17 first described the “lanolin paradox,” modeled after the earlier “paraben paradox” described by Fisher.18 There are 4 clinical phenomena of the lanolin paradox17:
- Lanolin generally does not cause contact allergy when found in PCPs but may cause ACD when found in topical medicaments.
- Some patients can use lanolin-containing PCPs on healthy skin without issue but will develop ACD when a lanolin-containing topical medicament is applied to inflamed skin. This is because inflamed skin is more easily sensitized.
- False-negative patch test reactions to pure lanolin may occur. Since Wolf’s17 initial description of the paradox, free alcohols of lanolin have been found to be its principal allergen, though it also is possible that oxidation of lanolin could generate additional allergenic substances.1
- Patch testing with wool alcohol 30% can generate both false-negative and false-positive results.
At one extreme, Kligman19 also was concerned about false-positive reactions to lanolin, describing lanolin allergy as a myth attributed to overzealous patch testing and a failure to appreciate the limitations of this diagnostic modality. Indeed, just having a PPT to lanolin (ie, contact allergy) does not automatically translate to a relevant ACD,1 and determining the clinical relevance of a PPT is of utmost importance. In 2001, Wakelin et al20 reported that the majority (71% [92/130]) of positive reactions to Amerchol L101 50% or 100% pet showed current clinical relevance. Data from the North American Contact Dermatitis Group in 2009 and in 2022 were similar, with 83.4% (529/634) of positive reactions to lanolin alcohol 30% pet and 86.5% (1238/1431) of positive reactions to Amerchol L101 50% pet classified as current clinical relevance.11,21 These findings demonstrate that although lanolin may be a weak sensitizer, a PPT usually represents a highly relevant cause of dermatitis.
Considerations for Patch Testing
Considering Wolf’s17 claim that even pure lanolin is not an appropriate formulation to use for patch testing due to the risk for inaccurate results, you might now be wondering which preparation should be used. Mortensen22 popularized another compound, Amerchol L101, in 1979. In this small study of 60 patients with a PPT to lanolin and/or its derivatives, the highest proportion (37% [22/60]) were positive to Amerchol L101 but negative to wool alcohol 30%, suggesting the need to test to more than one preparation simultaneously.22 In a larger study by Miest et al,23 3.9% (11/268) of patients had a PPT to Amerchol L101 50% pet, whereas only 1.1% (3/268) had a PPT to lanolin alcohol 30% pet. This highlighted the importance of including Amerchol L101 when patch testing because it was thought to capture more positive results; however, some studies suggest that Amerchol L101 is not superior at predicting lanolin contact allergy vs lanolin alcohol 30% pet. The risk for an irritant reaction when patch testing with Amerchol L101 should be considered due to its mineral oil component.24
Although there is no universal consensus to date, some investigators suggest patch testing both lanolin alcohol 30% pet and Amerchol L101 50% pet simultaneously.1 The TRUE test utilizes 1000 µg/cm2 of wool alcohols, while the North American 80 Comprehensive Series and the American Contact Dermatitis Society Core 90 Series contain Amerchol L101 50% pet. Patch testing to the most allergenic component of lanolin—the free fatty alcohols (particularly alkane-α,β-diols and alkane-α,ω-diols)—has been suggested,1 though these formulations are not yet commercially available.
When available, the patient’s own lanolin-containing PCPs should be tested.1 Performing a repeat open application test (ROAT) to a lanolin-containing product also may be highly useful to distinguish weak-positive from irritant patch test reactions and to determine if sensitized patients can tolerate lanolin-containing products on intact skin. To complete a ROAT, a patient should apply the suspected leave-on product to a patch of unaffected skin (classically the volar forearm) twice daily for at least 10 days.25 If the application site is clear after 10 days, the patient is unlikely to have ACD to the product in question. Compared to patch testing, ROAT more accurately mimics a true use situation, which is particularly important for lanolin given its tendency to preferentially impact damaged or inflamed skin while sparing healthy skin.
Alternatives to Lanolin
Patients with confirmed ACD to lanolin may use plain petrolatum, a safe and inexpensive substitute with equivalent moisturizing efficacy. It can reduce transepidermal water loss by more than 98%,4 with essentially no risk for ACD. Humectants such as glycerin, sorbitol, and α-hydroxy acids also have moisturizing properties akin to those of lanolin. In addition, some oils may provide benefit to patients with chronic skin conditions. Sunflower seed oil and extra virgin coconut oil have anti-inflammatory, antibacterial, and barrier repair properties.26,27 Allergic contact dermatitis to these oils rarely, if ever, occurs.28
Final Interpretation
Lanolin is a well-known yet controversial contact allergen that is widely used in PCPs, cosmetics, topical medicaments, and industrial goods. Lanolin ACD preferentially impacts patients with stasis dermatitis, chronic leg ulcers, AD, and perianal/genital dermatitis. Patch testing with more than one lanolin formulation, including lanolin alcohol 30% pet and/or Amerchol L101 50% pet, as well as testing the patient’s own products may be necessary to confirm the diagnosis. In cases of ACD to lanolin, an alternative agent, such as plain petrolatum, may be used.
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089/derm.2022.0002
- National Center for Biotechnology Information (2023). PubChem Annotation Record for LANOLIN, Source: Hazardous Substances Data Bank (HSDB). Accessed July 21, 2023. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/1817
- National Center for Biotechnology Information. PubChem compound summary lanolin. Accessed July 17, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/Lanolin
- Purnamawati S, Indrastuti N, Danarti R, et al. the role of moisturizers in addressing various kinds of dermatitis: a review. Clin Med Res. 2017;15:75-87. doi:10.3121/cmr.2017.1363
- Sethi A, Kaur T, Malhotra SK, et al. Moisturizers: the slippery road. Indian J Dermatol. 2016;61:279-287. doi:10.4103/0019-5154.182427
- Souto EB, Yoshida CMP, Leonardi GR, et al. Lipid-polymeric films: composition, production and applications in wound healing and skin repair. Pharmaceutics. 2021;13:1199. doi:10.3390/pharmaceutics13081199
- Rüther L, Voss W. Hydrogel or ointment? comparison of five different galenics regarding tissue breathability and transepidermal water loss. Heliyon. 2021;7:E06071. doi:10.1016/j.heliyon.2021.e06071
- Zirwas MJ. Contact alternatives and the internet. Dermatitis. 2012;23:192-194. doi:10.1097/DER.0b013e31826ea0d2
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Ramirez M, Eller JJ. The patch test in contact dermatitis. Allergy. 1929;1:489-493.
- Silverberg JI, Patel N, Warshaw EM, et al. Lanolin allergic reactions: North American Contact Dermatitis Group experience, 2001 to 2018. Dermatitis. 2022;33:193-199. doi:10.1097/DER.0000000000000871
- Diepgen TL, Ofenloch RF, Bruze M, et al. Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174:319-329. doi:10.1111/bjd.14167
- Zallmann M, Smith PK, Tang MLK, et al. Debunking the myth of wool allergy: reviewing the evidence for immune and non-immune cutaneous reactions. Acta Derm Venereol. 2017;97:906-915. doi:10.2340/00015555-2655
- Yosipovitch G, Nedorost ST, Silverberg JI, et al. Stasis dermatitis: an overview of its clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2023;24:275-286. doi:10.1007/s40257-022-00753-5
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788/cutis.0599
- Gilissen L, Schollaert I, Huygens S, et al. Iatrogenic allergic contact dermatitis in the (peri)anal and genital area. Contact Dermatitis. 2021;84:431-438. doi:10.1111/cod.13764
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Fisher AA. The paraben paradox. Cutis. 1973;12:830-832.
- Kligman AM. The myth of lanolin allergy. Contact Dermatitis. 1998;39:103-107. doi:10.1111/j.1600-0536.1998.tb05856.x
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046/j.1365-2133.2001.04277.x
- Warshaw EM, Nelsen DD, Maibach HI, et al. Positive patch test reactions to lanolin: cross-sectional data from the North American Contact Dermatitis group, 1994 to 2006. Dermatitis. 2009;20:79-88.
- Mortensen T. Allergy to lanolin. Contact Dermatitis. 1979;5:137-139. doi:10.1111/j.1600-0536.1979.tb04824.x
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097/DER.0b013e3182937aa4
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Amsler E, Assier H, Soria A, et al. What is the optimal duration for a ROAT? the experience of the French Dermatology and Allergology group (DAG). Contact Dermatitis. 2022;87:170-175. doi:10.1111/cod.14118
- Msika P, De Belilovsky C, Piccardi N, et al. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol. 2008;25:606-612. doi: 10.1111/j.1525-1470.2008.00783.x
- Lio PA. Alternative therapies in atopic dermatitis care: part 2. Pract Dermatol. July 2011:48-50.
- Karagounis TK, Gittler JK, Rotemberg V, et al. Use of “natural” oils for moisturization: review of olive, coconut, and sunflower seed oil. Pediatr Dermatol. 2019;36:9-15. doi:10.1111/pde.13621
Practice Points
- Lanolin is a common ingredient in personal care products (PCPs), cosmetics, topical medicaments, and industrial materials.
- Allergic contact dermatitis to lanolin appears to be most common in patients with stasis dermatitis, chronic leg ulcers, atopic dermatitis, and perianal/genital dermatitis.
- There is no single best lanolin patch test formulation. Patch testing and repeat open application testing to PCPs containing lanolin also may be of benefit.
Enlarging Pigmented Lesion on the Thigh
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6
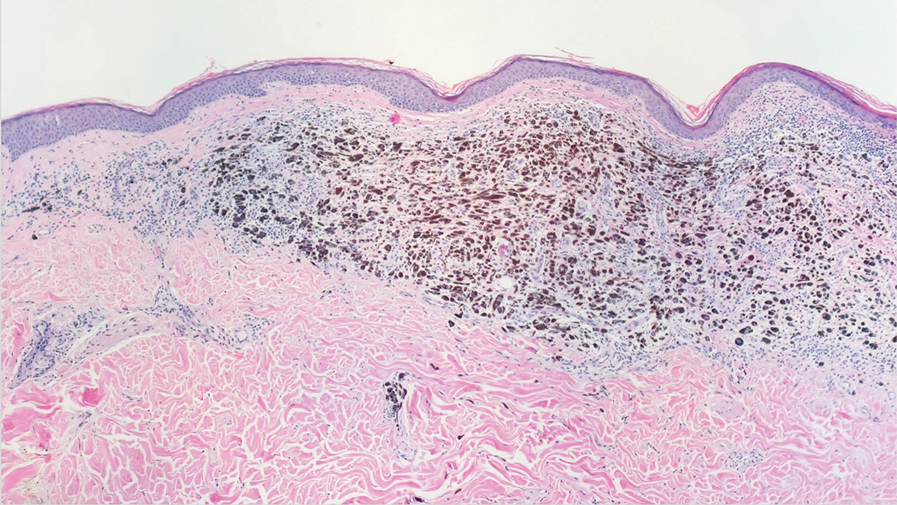
Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7
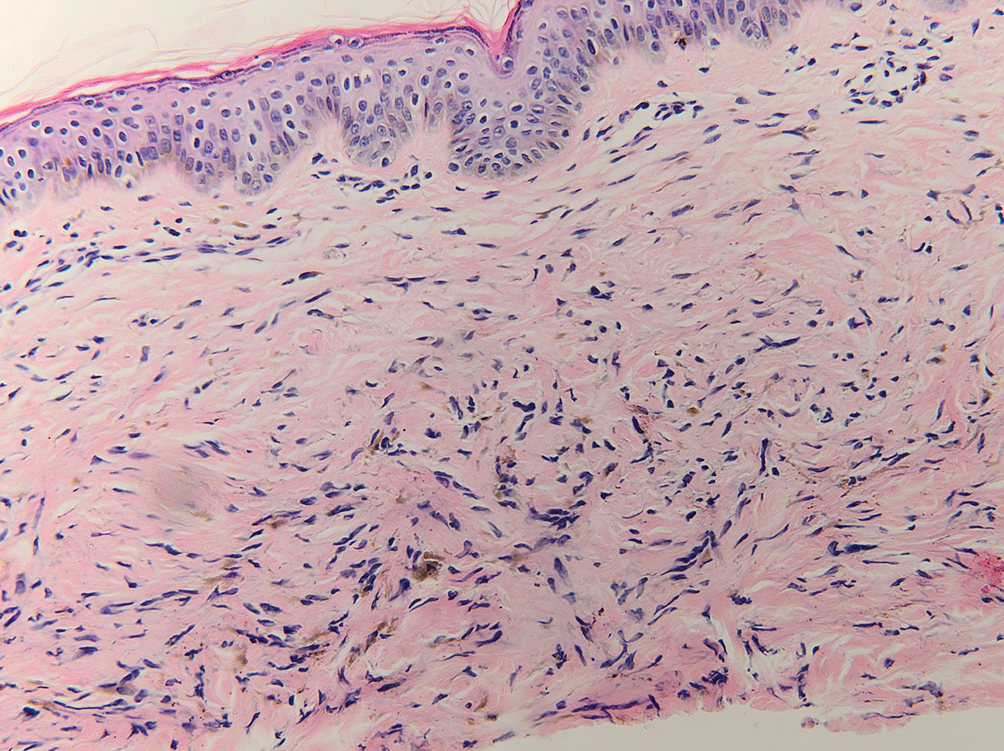
Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8
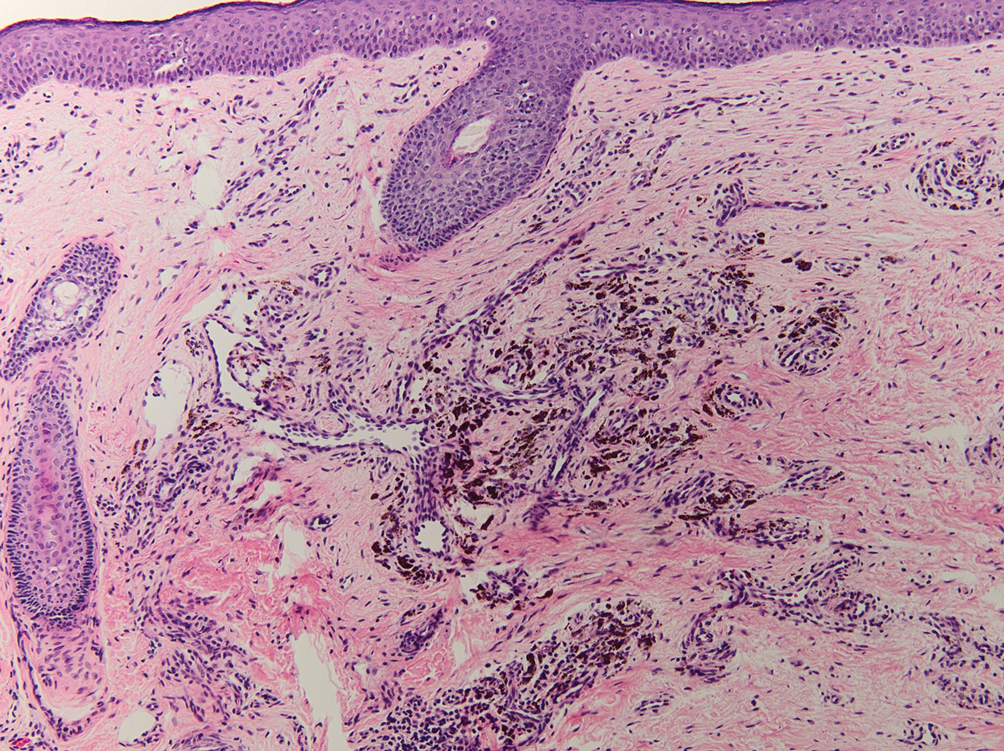
Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3
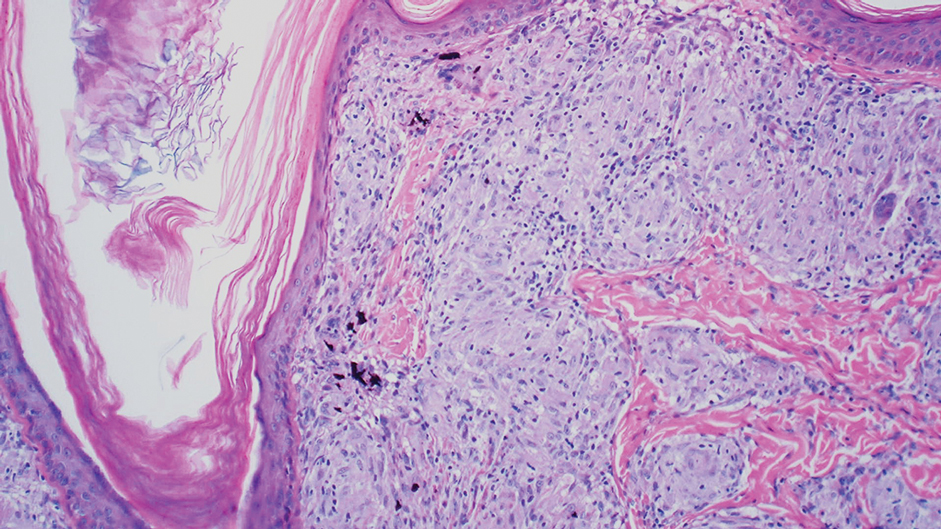
Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
An 80-year-old man presented with a pigmented lesion on the left lateral thigh near the knee that had been gradually enlarging over several weeks (top [inset]). He underwent a left knee replacement surgery for advanced osteoarthritis many months prior that was complicated by postoperative Staphylococcus aureus infection with sinus tract formation that was persistent for 6 months and treated with a topical medication. A pigmented lesion developed near the opening of the sinus tract. His medical history was remarkable for extensive actinic damage as well as multiple actinic keratoses treated with cryotherapy but no history of melanoma. An excisional biopsy was performed (top and bottom).
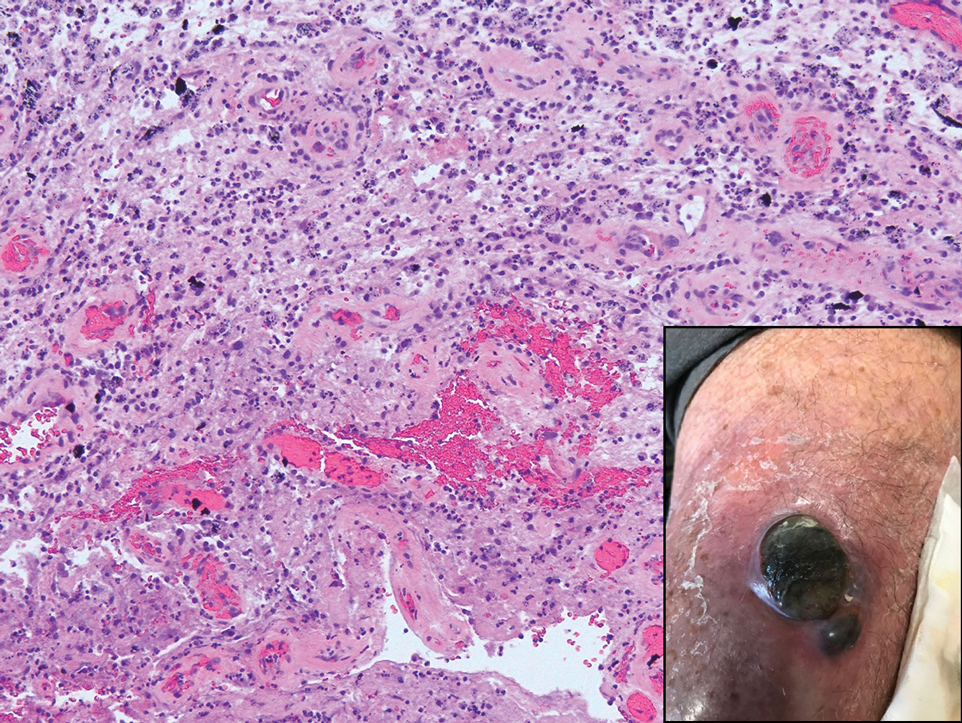
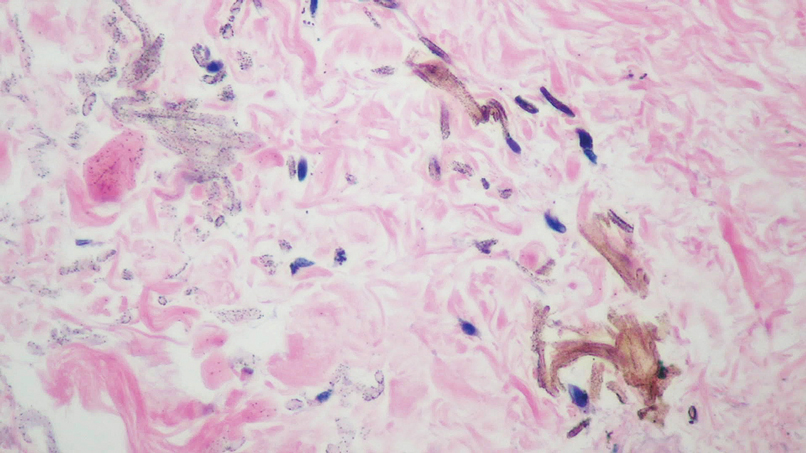
Serum Ferritin Levels: A Clinical Guide in Patients With Hair Loss
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.
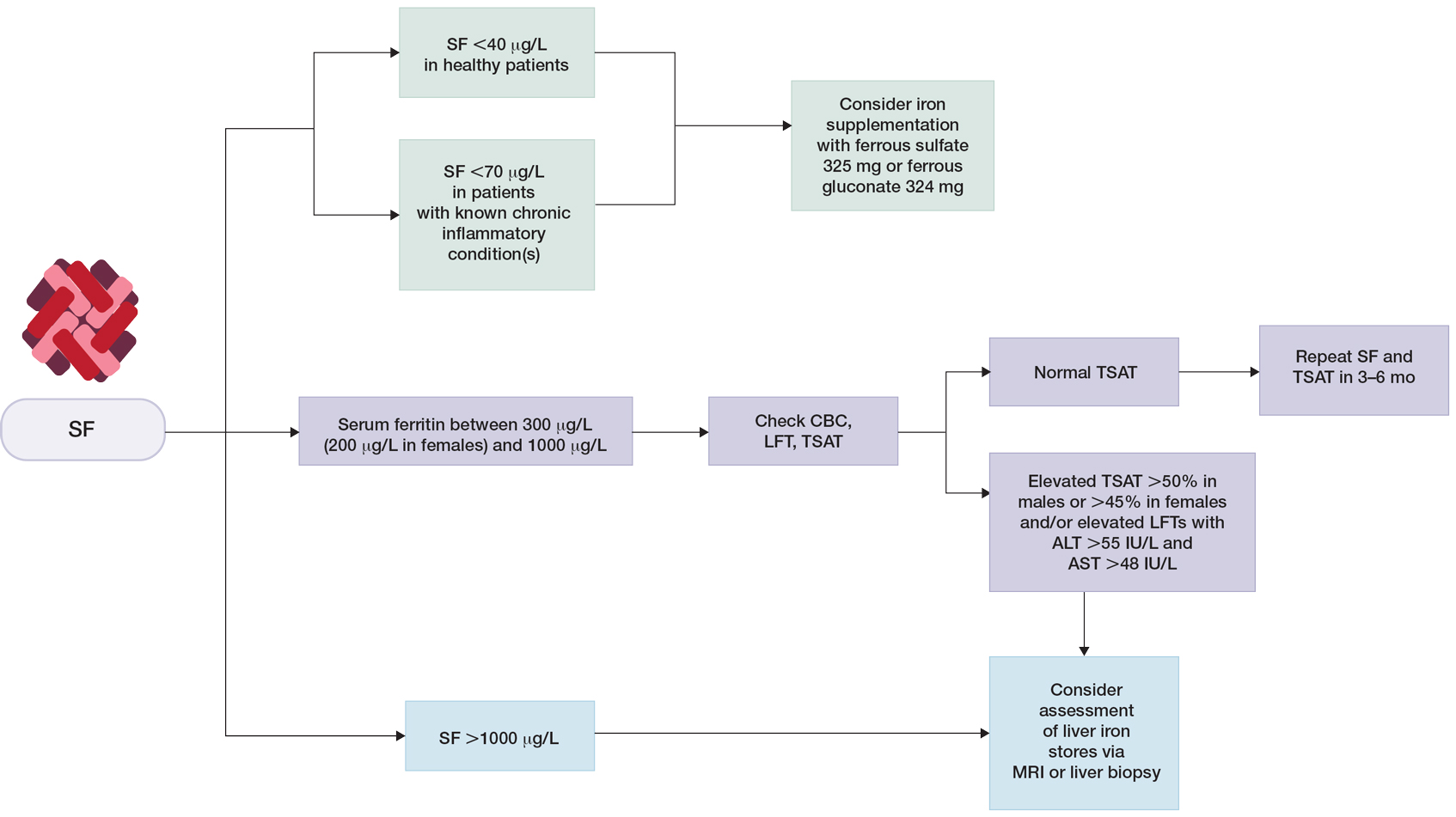
Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Practice Points
- In patients who are otherwise healthy without chronic systemic disease, hepatic disease, or inflammatory disorders, serum ferritin levels directly correlate with body iron status.
- Elevated serum ferritin should be interpreted in the context of other indicators of iron status, including transferrin saturation, complete blood cell count, and/or liver function panel.
- Low serum ferritin is a specific marker for iron deficiency, and iron supplementation should be initiated based on age-, sex-, and condition-specific thresholds.