User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
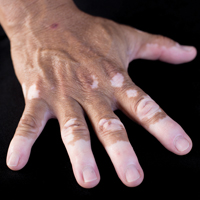
Vitiligo Patients Experience Barriers in Accessing Care
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
Vitiligo is a disorder typified by loss of pigmentation. Worldwide estimates of disease demonstrate 0.4% to 2% prevalence.1 Vitiligo generally is felt to be an autoimmune disorder with a complex multifactorial inheritance.2 Therapeutic options for vitiligo are largely off label and include topical corticosteroids, topical calcineurin inhibitors, narrowband UVB (NB-UVB) light phototherapy, and excimer (308 nm) laser therapy.3,4 Therapies for vitiligo are time consuming, as most topical therapies require twice-daily application. Additionally, many patients require 2 or more topical therapies due to involvement of both the head and neck as well as other body sites.3,4 Generalized disease often is treated with NB-UVB therapy 3 times weekly in-office visits, while excimer laser therapy is used for limited disease resistant to topical agents.3,4
Many barriers to good outcomes and care exist for patients with vitiligo.5 Patients may experience reduced quality of life and/or sexual dysfunction because of vitiligo lesions. The purpose of this pilot study was to identify barriers to access of care in vitiligo patients.
Methods
A survey was designed and then reviewed for unclear wording by members of the local vitiligo support group at Mount Sinai St. Luke’s-Roosevelt Hospital and Beth Israel Medical Centers (New York, New York). Linguistic revision and clarifications were added to the survey to correct identified communication problems. The survey was then posted using an Internet-based survey software. Links to the survey were sent via email to 107 individuals in a LISTSERV comprising Vitiligo Support International members who participated in a New York City support group (led by C.G. and N.B.S.). Only 1 email was used per household and only individuals 18 years or older could participate. These individuals were asked to complete a deidentified, 82-question, institutional review board–reviewed and exempted survey addressing issues affecting delivery and receipt of medical care for vitiligo.
Data were analyzed using the χ2 test, analysis of variance, or Student t test depending on the type of variable (categorical vs continuous). Fisher exact or Wilcoxon-Mann-Whitney tests were used when distributional assumptions were not met. A type I error rate (α=.05) was used to determine statistical significance. All analyses were performed using SAS 9.3 software.
Results
Respondents
The survey was completed by 81% (n=87) of individuals. The mean (SD) age of the treated patients about whom the respondents communicated was 33 (16) years and 71% (n=62) were women. The majority of respondents (64 [74%]) reported their race as white, followed by African American/black (12 [14%]), Hispanic (7 [8%]), and Asian (4 [5%]). Twenty-nine percent (22/76) of respondents reported a family income of less than $50,000 per year, 34% (26/76) reported an income of $50,000 to $100,000, and 37% (28/76) reported an income greater than $100,000, while 11 respondents did not report income.
Number of Physicians Seen
Respondents had reportedly seen an average (SD) number of 2 (1) physicians in the past/present before being offered any therapy for vitiligo and only 37% (32/87) of respondents reported being offered therapy by the first physician they saw. The number of physicians seen did not have a statistical relationship with years with vitiligo (ie, disease duration), sex, race, age of onset, income level, or number of sites affected.
Number of Sites Affected
The survey identified the following 23 sites affected by vitiligo: scalp, forehead, eyelids, lips, nose, cheeks, chin, neck, chest, stomach, back, upper arms, forearms, hands, wrists, fingers, genitalia, buttocks, thighs, calves/shins, ankles, feet, and toes. The average (SD) number of sites affected was 12 (6). The number of sites affected was correlated to the recommendation for phototherapy, while the recommendation for excimer laser therapy was inversely associated with the number of sites affected. The median number of sites affected for those who were not prescribed phototherapy was 10 (interquartile range [IQR]=9; P=.05); the median number of sites affected for those who were prescribed phototherapy was 15 (IQR=11). The association between the number of sites affected and whether the patient proceeded with phototherapy was not statistically significant. The need for phototherapy was not related to years with vitiligo (ie, disease duration), sex, or race.
Excimer laser therapy was prescribed more often to patients with fewer sites affected (median of 9 [IQR=3] vs median of 15 [IQR=9]; P=.04). Respondents who had fewer sites affected were on average more likely to proceed with excimer laser therapy (median of 8 [IQR=4] vs median of 11 [IQR=5]). The association between the number of sites affected and whether the patient proceeded with excimer laser therapy was not statistically significant.
Access to Topical Medications
Forty-one percent (36/87) of respondents reported difficulty accessing 1 or more topical therapies. Of 52 respondents who were prescribed a topical corticosteroid, 12 (23%) reported difficulty accessing therapy. Of 67 respondents who were prescribed a topical calcineurin inhibitor, 27 (40%) reported difficulty accessing medication (tacrolimus, n=17; pimecrolimus, n=10). Calcipotriene prescription coverage was not specifically addressed in this survey, as it usually is a second-line or adjunctive medication. Difficulty getting topical tacrolimus but not topical corticosteroids was associated with female sex (P=.03) but was not associated with race, income level, or level of education. Difficulty obtaining medication was not related to race, sex, level of education, or income level.
Consequences of Phototherapy
Twenty-three of 34 respondents (68%) who were told they required phototherapy actually received phototherapy and reported paying $38 weekly (IQR=$75). The majority of patients who proceeded with phototherapy lived (17/23 [74%]) or worked (16/23 [70%]) within 20 minutes of the therapy center. Self-reported response to phototherapy was good to very good in 65% (15/23) of respondents and no response in 30% (7/23); only 1 respondent reported worsening vitiligo. Sixty percent (15/25) of respondents said they were not satisfied with phototherapy. Respondents who were satisfied with the outcome of phototherapy had on average fewer sites affected by vitiligo (mean [SD], 10 [8]; P=.05). The association with other demographic and economic parameters (eg, sex, race, level of education, income level) was not statistically significant. Proceeding with phototherapy was not related to race, sex, level of education, or income level.
When questioned how many aspects of daily life (eg, work, home, school) were affected by phototherapy, 40% (35/87) of respondents reported that more than one life parameter was disturbed. Thirty-five percent (8/23) of respondents who received phototherapy reported that it affected their daily life “quite a bit” or “severely.” More respondents were likely to report that the therapy interfered with their life “somewhat,” “quite a bit,” or “severely” (76% [19/25]; 95% confidence interval, 55%-92%; P=.01) rather than “not at all” or “a little.”
Excimer Laser
Nine of 17 respondents (53%) who were recommended to undergo excimer laser therapy actually received therapy and reported paying $100 weekly (IQR=$60).
There was a trend toward significance of excimer usage being associated with lower age quartile (0–20 years)(P=.0553) and income more than $100,000 (P=.0788), neither of which reached statistical significance.
Insurance Coverage
Respondents were offered 7 answer options regarding the reason for noncoverage of topical calcineurin inhibitors. They were allowed to pick more than one reason where appropriate. For individuals who were prescribed topical tacrolimus but did not receive drug (n=17), the following reasons were cited: “no insurance coverage for the medication” (59% [10/17]), “your deductible was too high” (24% [4/17]), “prior authorization failed to produce coverage of the medication” (24% [4/17]), “your copay was prohibitively expensive” (24% [4/17]), “you were uncomfortable with the medication’s side effects” (18% [3/17]), “the tube was too small to cover your skin affected areas” (12% [2/17]), and “other” (29% [5/17]). Three patients selected 3 or more reasons, 8 patients selected 2 reasons, and 5 patients selected one reason.
Comment
It has been reported that patients with vitiligo may have difficulty related to treatment compliance for a variety of reasons.5 We identified notable barriers that arise for some, if not all, patients with vitiligo in the United States at some point in their care, including interference with other aspects of daily life, lack of coverage by current health insurance provider, and high out-of-pocket expenses, in addition to the negative effects of vitiligo on quality of life that have already been reported.6,7 These barriers are not a function of race/ethnicity, income level, or age of onset, but they may be impacted, as in the case of tacrolimus, by female sex. It is clear that, based on this study’s numbers, many patients will be unable to receive and/or comply with recommended treatment plans.
A limitation of this analysis is the study population, a select group of patients who had not been prescribed all the therapies in question. The sample size may not be large enough to demonstrate differences between level of education, race, or income level; however, even with a sample size of 87 respondents, the barriers to access of care are prominent. Larger population-based surveys would potentially tease out patterns of barriers not apparent with a smaller sample. No data were generated specific to calcipotriene, and this medication was not specified as a write-in agent on open question by any respondents; therefore, access to topical calcipotriene cannot be projected from this study. Phototherapy was queried as a nonspecific term and the breakdown of NB-UVB versus psoralen plus UVA was not available for this survey. Data suggesting a burden of socioeconomic barriers have been reported for atopic dermatitis8 and psoriasis,9 which corroborate the need for greater research in the field of access to care in dermatology.
Despite some advancement in the care of vitiligo, patients often are unable to access preferred or recommended treatment modalities. Standard recommendations for care are initial usage of calcineurin inhibitors for facial involvement and topical high-potency corticosteroids for involvement of the body.3,4 Based on this survey, it would seem that many patients are not able to receive the standard of care. Similarly, NB-UVB phototherapy and excimer laser therapy are recommended for widespread vitiligo and lesions unresponsive to topical care. It would seem that almost half of our respondents did not have access to one or more of the recommended therapies. Barriers to care may have substantial clinical and psychological outcomes, which were not evaluated in this study but merit future research.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51:1206-1212.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44:676-680.
- Silverberg NB. Pediatric vitiligo. Pediatr Clin North Am. 2014;61:347-366.
- Taieb A, Alomar A, Böhm M, et al, Vitiligo European Task Force (VETF); European Academy of Dermatology and Venereology (EADV); Union Europénne des Médecins Spécialistes (UEMS). Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168:5-19.
- Abraham S, Raghavan P. Myths and facts about vitiligo: an epidemiological study. Indian J Pharm Sci. 2015;77:8-13.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
- Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
- Hamilton MP, Ntais D, Griffiths CE, et al. Psoriasis treatment and management—a systematic review of full economic evaluations. Br J Dermatol. 2015;172:574-583.
Practice Points
- Patients with vitiligo may experience difficulty receiving the care prescribed to them.
- It is best to identify barriers such as work schedule or distance before recommending a treatment plan.
Tinea Capitis Caused by Trichophyton rubrum Mimicking Favus
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
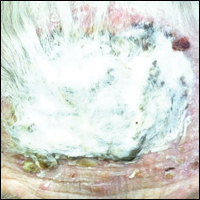
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.
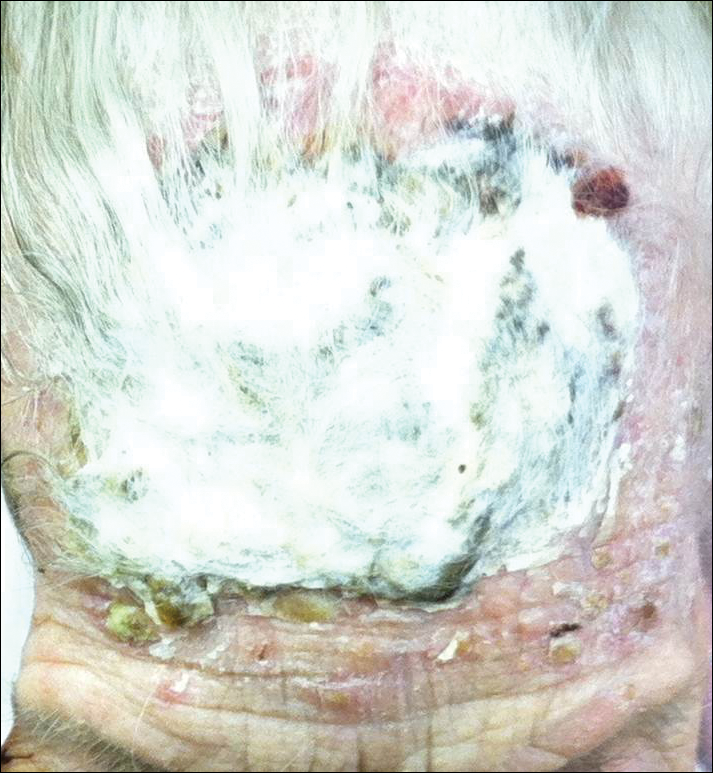
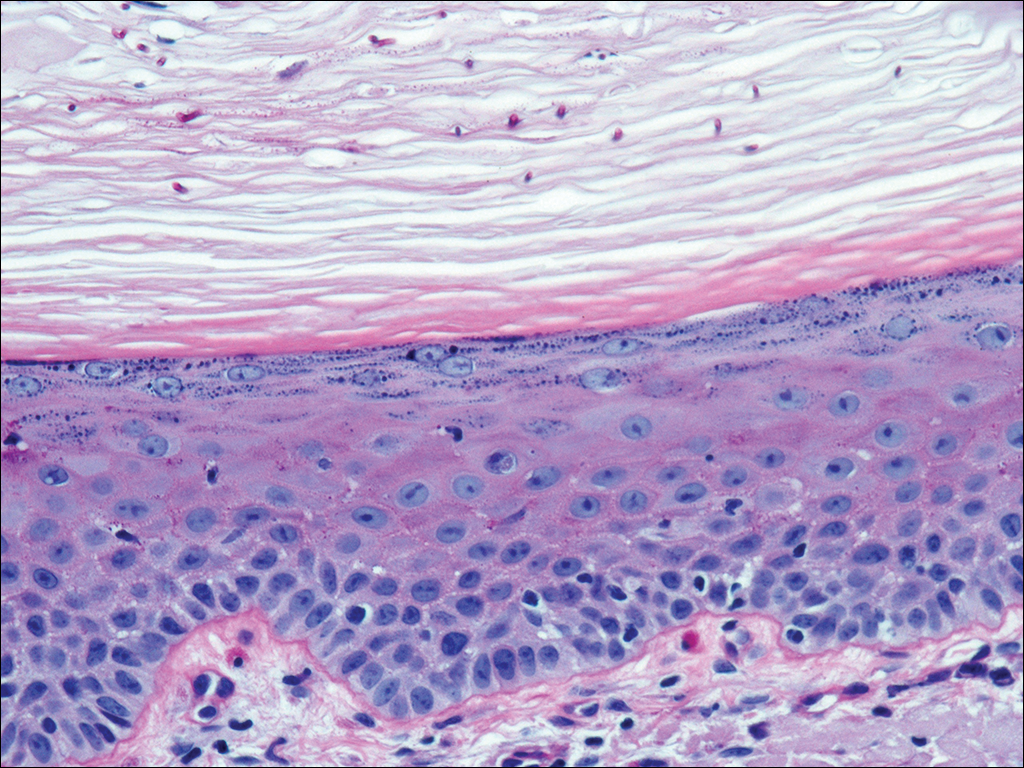
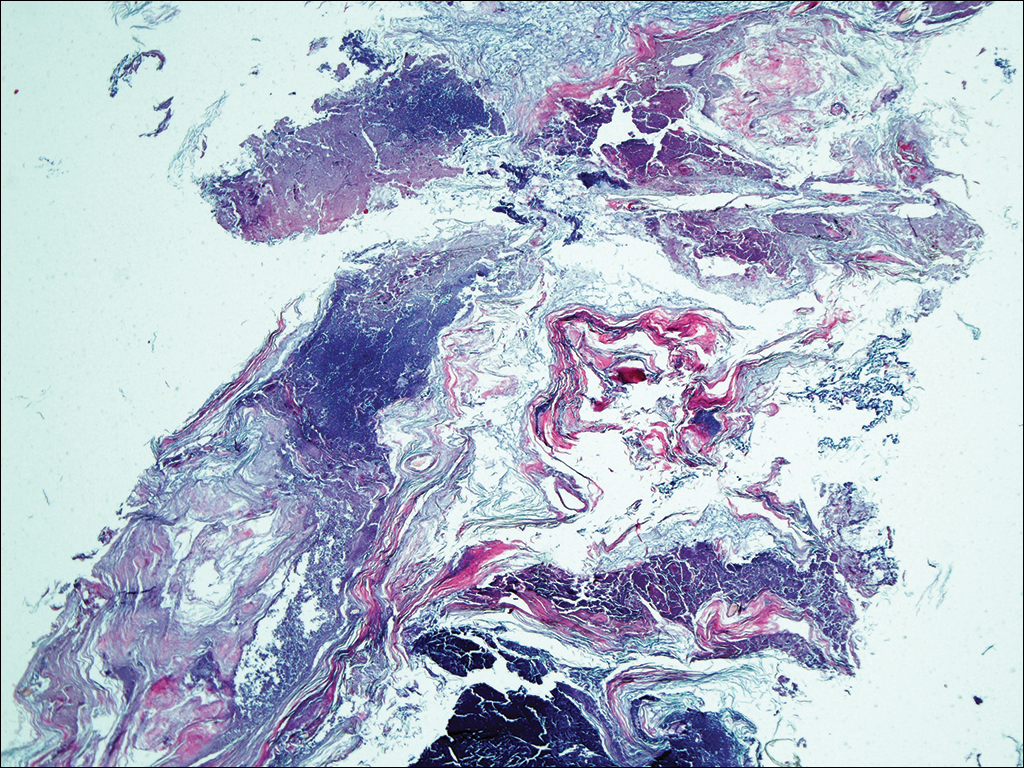
At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).

Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
Practice Points
- Although favus is uncommonly seen in developed countries, it still exists and can mimick other conditions, notably cutaneous malignancies.
- Favus may affect the skin and nails in addition to the hair.
- The lesions of favus may persist for many years.
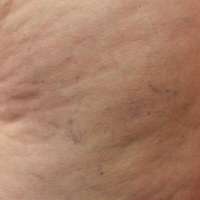
A Noninvasive Mechanical Treatment to Reduce the Visible Appearance of Cellulite
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).
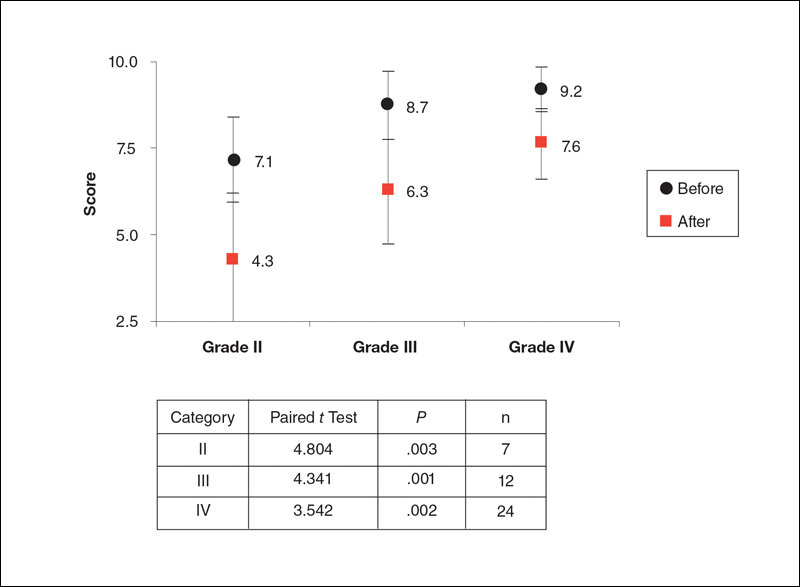
Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).
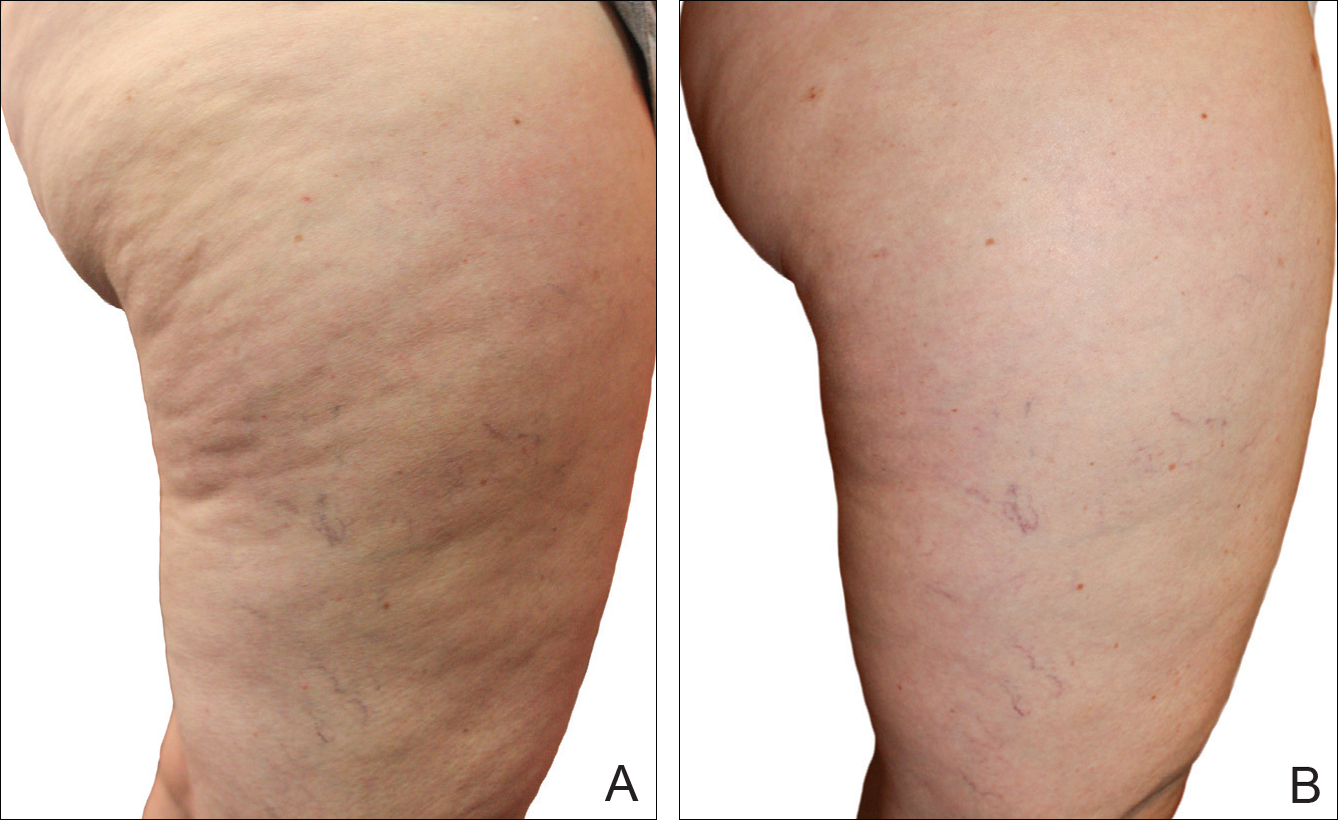
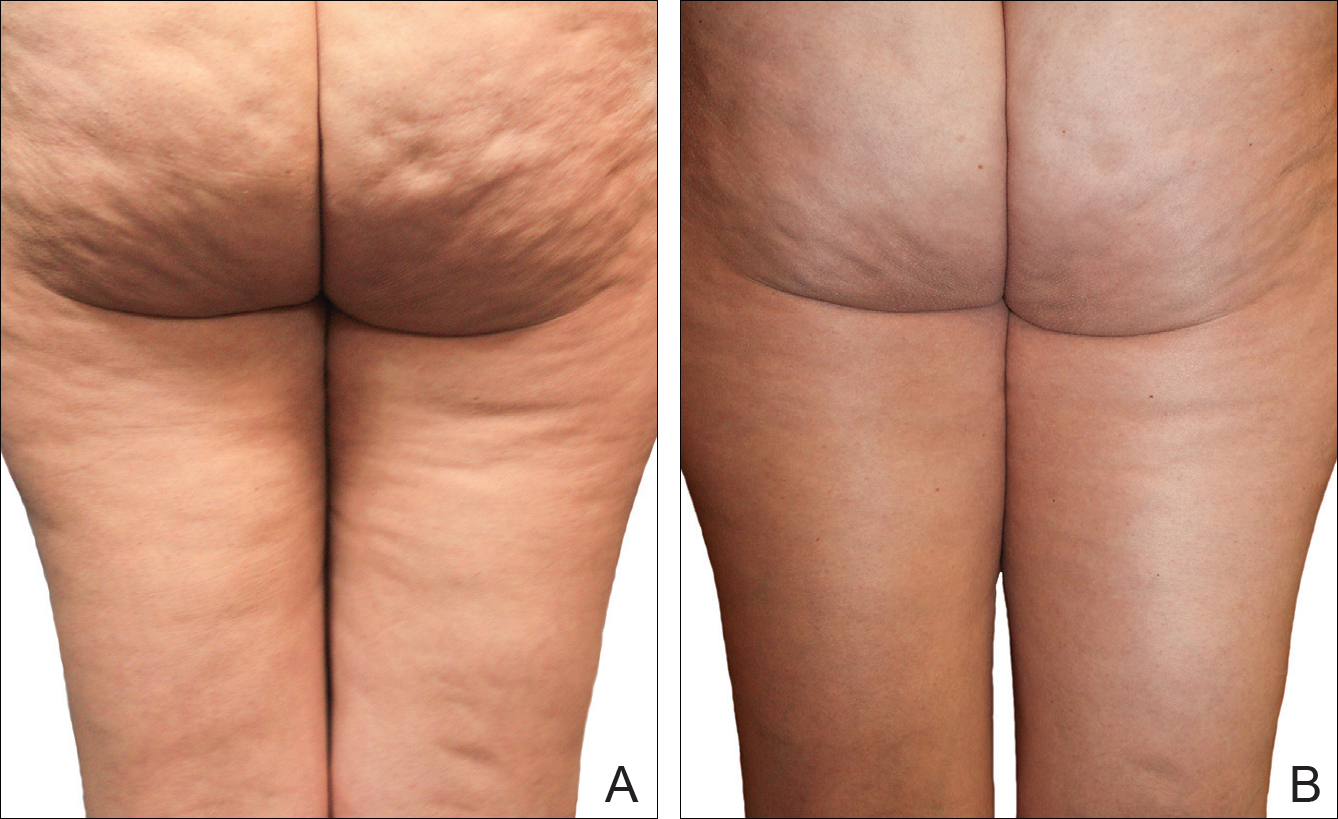
The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).

Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).


The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
Cellulite is a cosmetic problem, not a disease process. It affects 85% to 90% of all women worldwide and was described nearly 100 years ago.1 Causes may be genetic, hormonal, or vascular in nature and may be related to the septa configuration in the subdermal tissue. Fibrosis at the dermal-subcutaneous junction as well as decreased vascular and lymphatic circulation also may be causative factors.
Cellulite has a multifactorial etiology. Khan et al2 noted that there are specific classic patterns of cellulite that affect women exclusively. White women tend to have somewhat higher rates of cellulite than Asian women. The authors also stated that lifestyle factors such as high carbohydrate diets may lead to an increase in total body fat content, which enhances the appearance of cellulite.2
The subdermal anatomy affects the appearance of cellulite. Utilizing in vivo magnetic resonance imaging, Querleux et al3 showed that women with visible cellulite have dermal septa that are thinner and generally more perpendicular to the skin’s surface than women without cellulite. In women without cellulite, the orientation of the septa is more angled into a crisscross pattern. In women with a high percentage of perpendicular septa, the perpendicular septa allow for fat herniation with dimpling of the skin compared to the crisscross septa pattern.2 Other investigators have discussed the reduction of blood flow in specific areas of the body in women, particularly in cellulite-prone areas such as the buttocks and thighs, as another causative factor.2,4,5 Rossi and Vergnanini6 showed that the blood flow was 35% lower in affected cellulite regions than in nonaffected regions without cellulite, which can cause congestion of blood and lymphatic flow and increased subdermal pressure, thus increasing the appearance of cellulite.
Although there is some controversy regarding the effects of weight loss on the appearance of cellulite,2,7 it appears that the subdermal septa and morphology have more of an effect on the appearance of cellulite.2,3,8
Rossi and Vergnanini6 proposed a 4-grade system for evaluating the appearance of cellulite (grade I, no cellulite; grade II, skin that is smooth and without any pronounced dimpling upon standing or lying down but may show some dimpling upon pinching and strong muscle contraction; grade III, cellulite is present in upright positions but not when the patient is in a supine position; grade IV, cellulite can be seen when the patient is standing and in a supine position). Both grades III and IV can be exacerbated by maximal voluntary contraction and strong pinching of the skin because these actions cause the subcutaneous fat to move toward the surface of the skin between the septa. This grading system aligns with categories I through III described by Mirrashed et al.9
There are many cellulite treatments available but few actually create a reduction in the visible appearance of cellulite. A number of these treatments were reviewed by Khan et al,10 including massage; a noninvasive suction-assisted massage technique; and topical agents such as xanthine, retinols, and other botanicals.4,11-14 Liposuction has not been shown to be effective in the treatment of cellulite and in fact may increase the appearance of cellulite.9,15 Mesotherapy, a modality that entails injecting substances into the subcutaneous fat layer, is another treatment of cellulite. Two of the most common agents purported to dissolve fat include phosphatidylcholine and sodium deoxycholate. The efficacy and safety of mesotherapy remains controversial and unproven. A July 2008 position statement from the American Society of Plastic Surgeons stated that “low levels of validity and quality of the literature does not allow [American Society of Plastic Surgeons] to support a recommendation for the use of mesotherapy/injection lipolysis for fat reduction.”16 Other modalities such as noninvasive dual-wavelength laser/suction devices; low-energy diode laser, contact cooling, suction, and massage devices; and infrared, bipolar radiofrequency, and suction with mechanical massage devices are available and show some small improvements in the visible appearance of cellulite, but no rating scales were used in any of these studies.17,18 DiBernardo19 utilized a 1440-nm pulsed laser to treat cellulite. It is an invasive treatment that works by breaking down some of the connective tissue septa responsible for the majority and greater severity of the dermal dimpling seen in cellulite, increasing the thickness of the dermis as well as its elasticity, reducing subcutaneous fat, and improving circulation and reducing general lymphatic congestion.19 The system showed promise but was an invasive treatment, and one session could cost $5000 to $7000 for bilateral areas and another $2500 for each additional area.20 Burns21 expressed that the short-term results showed promise in reducing the appearance of cellulite. Noninvasive ultrasound22,23 as well as extracorporeal shock wave therapy24,25 also has shown some improvement in the firmness of collagen but generally not in the appearance of cellulite.
We sought to evaluate the efficacy and safety of a noninvasive mechanical treatment of cellulite.
Methods
This study was conducted in accordance with the guidelines set forth by the US Department of Health and Human Services’ Policy for Protection of Human Research Subjects and the World Medical Association’s Declaration of Helsinki. Participants were recruited through local area medical facilities in southeastern Michigan. Written informed consent was obtained from all participants prior to beginning the study.
Patients with grades II to IV cellulite, according to the Rossi and Vergnanini6 grading system, were allowed to participate. All participants in the study were asked not to make lifestyle changes (eg, exercise habits, diet) or use any other treatments for cellulite that might be available to them during the study period. Exclusion criteria included history of deep vein thrombosis, cancer diagnosed within the last year, pregnancy, hemophilia, severe lymphedema, presence of a pacemaker, epilepsy, seizure disorder, or current use of anticoagulants. History of partial or total joint replacements, acute hernia, nonunited fractures, advanced arthritis, or detached retina also excluded participation in the study.
Participants completed an 8-week, twice-weekly treatment protocol with a noninvasive mechanical device performed in clinic. The device consisted of a 10.16-cm belt with a layer of nonslip material wrapped around the belt. The belt was attached to a mechanical oscillator. We adjusted the stroke length to approximately 2 cm and moved the dermis at that length at approximately 1000 strokes per minute.
Each participant was treated for a total treatment time of 18 to 24 minutes. The total treatment area included the top of the iliac crest to just above the top of the popliteal space. The width of the belt (10.16 cm) was equal to 1 individual treatment area. Each individual treatment area was treated for 2 minutes. First the buttocks and bilateral thighs were treated, followed by the right lateral thigh and the left lateral thigh. The belt was moved progressively down the total treatment area until all individual treatment areas were addressed. The average participant had 3 to 4 bilateral thigh and buttocks treatment areas and 3 to 4 lateral treatment areas on both the left and right sides of the body.
Digital photographs were taken with standardized lighting for all participants. Photographs were taken before the first treatment on the lateral and posterior aspects of the participant and were taken again at the end of the treatment program immediately before the last treatment. Participants were asked to contract the gluteal musculature for all photographs.
Two board-certified plastic surgeons were asked to rate the before/after photographs in a blinded manner. They graded each photograph on a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite). These data were analyzed using a Wilcoxon signed rank test. These data were compared to the participants self-evaluation of the appearance of cellulite in the photographs from the initial and final treatments using a rating scale of 0 to 10 (0=no cellulite; 10=worst possible cellulite).
The circumference of the widest part of the gluteal area was measured before and after treatment (+/–0.5 cm). The data were analyzed using a paired t test.
Results
The study included 43 participants (age range, 21–67 years; mean age, 37.6 years; weight range, 51–97 kg; mean weight, 64.95 kg) who resided in the Midwestern United States, were interested in reducing their cellulite, and were willing to commit to treatment 2 times weekly for the duration of the 8-week study. Fourteen percent (6/43) of participants were smokers. Participant self-assessments were divided into 3 categories based on the Rossi and Vergnanini6 grading system: category II, n=7; category III, n=12; and category IV, n=24. Although all the categories in our analysis showed statistically significant improvements, we found that there was more improvement in category II participants versus category III, and then again more improvement in category III versus category IV. The data for each treatment were analyzed separately using a paired t test, as we were not interested in comparing categories, only the effect of the treatment. We were testing to see if the difference was greater than 0, and the paired t values were statistically significant in all cases (category II, P=.003; category III, P=.001; category IV, P=.002)(Figure 1).

Using a correlation analysis, we found that age, body weight, or body mass index were not significantly correlated with the difference between the before and after physician rating. The difference between before and after treatment also was independent of whether or not the participant exercised or had an adverse reaction to the belt. Adverse reactions to the belt were characterized by redness and/or minor raising of the skin immediately following the treatment. These reactions all dissipated within 12 hours. It also appeared that the rating scales correlated well with the participants self-perception of their cellulite and the improvements seen in the photographs (Figures 2 and 3).


The mean circumference of the widest part of the gluteal area before treatment was 100.2 cm and the standard deviation was 8.14 cm. The mean circumference after treatment was 98.3 cm and the standard deviation was 8.02 (t=–2.81; P<.05). Many of the women commented that they felt more “toned,” which probably accounted for the slight difference in circumference rather than weight loss.
Of the 2 blinded board-certified plastic surgeons, one physician rated all participants in category III as significantly improved (P<.05) and rated the other categories as marginally insignificantly improved; the second physician rated all categories as marginally insignificantly improved.
Comment
Although there are a large number of treatment protocols that have been introduced and studied for the reduction of the appearance of cellulite,4,9,11-18 many have not shown promising long-term results. Some treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.22-25 One of the only treatments that has shown some promise is an expensive invasive treatment.20
The system used in this study was shown to be safe in all study participants. No significant adverse reactions were noted, and each participant successfully completed the protocol. Figures 2 and 3 show the strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population, which was supported by statistical analysis, particularly the participant self-reported ratings. The participants and the blinded physicians were not in agreement on the improvement of cellulite. Although the participants knew the changes that occurred to their bodies, the physicians only had photographs from which to make their decisions. The participants clearly observed noticeable differences to their bodies, while the physicians either saw no change or some improvement.
The physicians were asked to evaluate only the cellulite, but the process we employed changed more than the cellulite. The first step in the process was a toning of the legs and buttocks, which was readily observable by the patients but was outside the scope of the physicians’ assessment. After the body toning, the cellulite began to improve. It is possible that the participants were responding to the entire process, which clearly was positive, while the physicians were responding only to the cellulite end point.
Our treatment regimen accomplished reduction of the visible appearance of cellulite by breaking down connective tissue septa as well as increasing the thickness of the dermis and its elasticity. It also helped reduce subcutaneous fat, improve circulation, and reduce general lymphatic congestion. The parallel motions of the unit could be adjusted, but we kept them at a mid-level range of motion. The motion at this frequency would have a tendency to not only heat the epidermis and dermal layer that we were attempting to affect but would also help accomplish breaking down the septa and improving the elasticity of the dermis. Also, the rapid motion over a period of time of pulling the dermis parallel to the subdermal tissue and fascia most likely helped improve the circulation and lymphatic flow in treated areas as well as possibly broke down the subcutaneous fat. All of these factors appear to have led to an improvement in the appearance of cellulite in our study participants.
A maintenance-type program, if continued, would likely demonstrate improved results by further breaking down the septa and improving the other factors that reduce the appearance of cellulite. We believe that the participants would eventually be able to discontinue the use of the unit or reduce its use substantially once the desired results were obtained.
When utilizing the device, the participants were in a standing posture and leaning into the belt with a moderate force, which seemed to secondarily improve the tone of the gluteal and thigh musculature that was being treated. It may be that the oscillatory motion and the standing posture caused the muscles to isometrically co-contract, adding a secondary exerciselike effect.26-29
Proving our suggested mechanisms of action would require tissue biopsies and/or magnetic resonance imaging studies that were beyond the scope of this study. However, regardless of the mechanism of action, we do believe that this treatment has been shown to be effective, convenient, and most importantly safe.
Conclusion
The unique device that was utilized in our study is a safe and cost-effective method of reducing the appearance of cellulite for home use and would allow for a noninvasive, low-risk procedure.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
- Scherwitz C, Braun-Falco O. So-called cellulite. J Dermatol Surg Oncol. 1978;4:230-234.
- Khan MH, Victor F, Rao B, et al. Treatment of cellulite: part I. pathophysiology. J Am Acad Dermatol. 2010;62:361-370, quiz 371-372.
- Querleux B, Cornillon C, Jolivet O, et al. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: relationships with sex and presence of cellulite. Skin Res Technol. 2002;8:118-124.
- Rawlings A. Cellulite and its treatment. Int J Cos Sci. 2006;28:175-190.
- Rosenbaum M, Prieto V, Hellmer J, et al. An exploratory investigation of the morphology and biochemistry of cellulite. Plast Reconstr Surg. 1998;101:1934-1939.
- Rossi AB, Vergnanini AL. Cellulite: a review. J Eur Acad Dermatol Venereol. 2000;14:251-262.
- Smalls LK, Hicks M, Passeretti D, et al. Effect of weight loss on cellulite: gynoid lypodystrophy. Plast Reconstr Surg. 2006;118:510-516.
- Nürnberger F, Müller G. So-called cellulite: an invented disease. J Dermatol Surg Oncol. 1978;4:221-229.
- Mirrashed F, Sharp JC, Krause V, et al. Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res Technol. 2004;10:161-168.
- Khan M, Victor F, Rao B, et al. Treatment of cellulite, part II. advances and controversies. J Am Acad Dermatol. 2010;62:373-384.
- Collis N, Elliot L, Sharp C, et al. Cellulite treatment: a myth or reality: a prospective randomized, controlled trial of two therapies, endermologie and aminophylline cream. Plast Reconstr Surg. 1999;104:1110-1114.
- Adcock D, Paulsen S, Jabour K, et al. Analysis of the effects of deep mechanical massage in the porcine model. Plast Reconstr Surg. 2000;108:233-240.
- Güleç AT. Treatment of cellulite with LPG endermologie. Int J Dermatol. 2009;48:265-270.
- Piérard-Franchimont C, Piérard GE, Henry F, et al. A randomized, placebo-controlled trial of tropical retinol in the treatment of cellulite. Am J Clin Dermatol. 2000;1:369-374.
- Coleman WP. Liposuction. In: Coleman WP, Hanke CW, Alt TH, eds. Cosmetic Surgery of the Skin: Principles and Practice. Philadelphia, PA: BC Decker; 1991:213-238.
- ASPS guiding principles for mesotherapy/injection lipolysis. American Society of Plastic Surgeons website. http://www.plasticsurgery.org/Documents/medical-professionals/health-policy/guiding-principles/ASPS-Guiding-Principles-for-Mesotherapy-Injection-Lipolysis-7-08.pdf. Published July 2008. Accessed February 17, 2016.
- Kulick MI. Evaluation of a noninvasive, dual-wavelength laser-suction and massage device for the regional treatment of cellulite. Plast Reconstr Surg. 2010;125:1788-1796.
- Nootheti PK, Magpantay A, Yosowitz G, et al. A single center, randomized, comparative, prospective clinical study to determine the efficacy of the VelaSmooth system versus the TriActive system for the treatment of cellulite. Lasers Surg Med. 2006;38:908-912.
- DiBernardo BE. Treatment of cellulite using a 1440-nm pulsed laser with one-year follow up. Aesthet Surg J. 2011;31:328-341.
- Johannes L. New laser aims to zap cellulite at the source. Wall Street Journal. July 3, 2012. http://www.wsj.com/articles/SB10001424052702303649504577496981754619546. Accessed November 21, 2016.
- Burns AJ. Commentary on: treatment of cellulite using a 1440-nm pulsed laser with one-year follow up: preliminary report. Aesthet Surg J. 2011;31:342-343.
- Teitelbaum SA, Burns JL, Kubota J, et al. Noninvasive body contouring by focused ultrasound: safety efficacy of the contour I device in a multicenter, controlled, clinical study. Plast Reconstr Surg. 2007;120:779-789.
- Brown SA, Greenbaum L, Shtukmaster S, et al. Characterization of nonthermal focused ultrasound for noninvasive selective fat cell disruption (lysis): technical and preclinical assessment. Plast Reconstr Surg. 2009;124:92-101.
- Angehrn F, Kuhn C, Voss A. Can cellulite be treated with low energy extracorporeal shock wave therapy? Clin Interv Aging. 2007;2:623-630.
- Christ C, Brenke R, Sattler G, et al. Improvement in skin elasticity in the treatment of cellulite and connective tissue weakness by means of extracorporeal pulse activation therapy. Aesthet Surg J. 2008;28:538-544.
- Bosco C, Colli R, Introini E, et al. Adaptive responses of human skeletal muscle to vibration exposure. Clin Physiol. 1999;19:183-187.
- Luo J, McNamara B, Moran K. The use of vibration training to enhance muscle strength and power. Sports Med. 2005;35:23-41.
- Annino G, Padua E, Castagna C, et al. Effect of whole body vibration training on lower limb performance in selected high-level ballet students. J Strength Cond Res. 2007;21:1072-1076.
- Verschueren SM, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study [published online December 22, 2003]. J Bone Miner Res. 2004;19:352-359.
Practice Points
- Several cellulite treatments have shown improvement in the firmness of collagen and the dermis but not in the appearance of cellulite.
- The noninvasive mechanical treatment for women with cellulite evaluated in this study showed a strong correlation between the treatment and the reduction in the visible appearance of cellulite in this study population.
Spot Psoriatic Arthritis Early in Psoriasis Patients
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
How does psoriatic arthritis present?
Psoriatic arthritis (PsA) can present in psoriasis patients with an average latency of approximately 10 years. In patients with a strong genetic predisposition, another more severe form of PsA can present earlier in life (<20 years of age). Although PsA generally is classified as a seronegative spondyloarthropathy, more than 10% of patients may in fact be rheumatoid factor-positive. Nail pitting is a feature that can suggest the possibility of PsA, present in almost 90% of patients with PsA.
Who should treat PsA?
Although involving our colleagues in rheumatology is usually beneficial for our patients, in most cases dermatologists can and should effectively manage the care of PsA. The immunology of PsA is the same as psoriasis, which contrasts with rheumatoid arthritis (RA). Although active human immunodeficiency virus infection can trigger widespread psoriasis and PsA, RA conversely improves with the depletion of CD4+ cells. Methotrexate, which is used cavalierly by rheumatologists for RA, has a different effect in psoriasis; liver damage is 3 times as likely in psoriasis versus RA at the same doses, while cirrhosis without transaminitis is much more likely with psoriasis patients. Thus, a dermatologist's experience with using systemic medications to treat psoriasis is paramount in successful treatment of PsA.
What medications can we use to treat PsA?
Because halting the progression of PsA is the key to limiting long-term sequelae, systemic therapy is the mainstay of treatment. Treatment options range from methotrexate to most of the newer biologics. Acitretin tends to be ineffective. Apremilast is approved by the US Food and Drug Administration, and Janus kinase (JAK) inhibitors also have demonstrated efficacy in PsA trials. There are some biologics that are used for PsA but do not have an approval for psoriasis, such as certolizumab pegol.
What's new in PsA?
The literature is well established in the classic progression and presentation of PsA, but there is new evidence that the development of PsA in patients with psoriasis is preceded by a period of nonspecific musculoskeletal symptoms, such as joint pain, arthralgia, fatigue, heel pain, and stiffness (Eder et al). The presence of these symptoms may help guide focused questioning and examination.
Another recent study has shown that the incidence of Crohn disease and ulcerative colitis are more likely in patients with PsA (Zohar et al). It is another important consideration for our patients, especially with recent concerns regarding onset of inflammatory bowel disease with some of the newer biologics we may use to treat psoriasis.
As newer classes of biologic treatments emerge, it will be interesting to see how effective they are in treating PsA in addition to plaque psoriasis. We should be aggressive about treating our patients with psoriasis using systemic therapy if they develop joint pain.
Suggested Readings
Eder L, Polachek A, Rosen CF, et al. The development of PsA in patients with psoriasis is preceded by a period of non-specific musculoskeletal symptoms: a prospective cohort study [published online October 28, 2016]. Arthritis Rheumatol. doi:10.1002/art.39973.
Zohar A, Cohen AD, Bitterman H, et al. Gastrointestinal comorbidities in patients with psoriatic arthritis [published online August 17, 2016]. Clin Rheumatol. 2016;35:2679-2684.
A Potpourri of Things to Do Correctly
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
Practice Points
- A biopsy is not separately reportable when another procedure was done at the same site on the same day (eg, shave, excision, destruction).
- Use site-specific biopsy codes when their localization is more precise than the general skin biopsy.
- A simple nail clipping for culture or periodic acid-Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
Physicians Must Encourage HPV Vaccine
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
Despite overwhelming evidence indicating vaccines are safe and effective at preventing diseases,1 physicians are still faced with the dilemma of convincing patients to receive their recommended vaccinations. The topic comes up regularly on television talk shows; presidential debates; or in new documentary films, such as “Vaxxed: From Cover-up to Catastrophe,” which was pulled from the Tribeca Film Festival in March 2016.2 The central debate over vaccines traces back almost 20 years to the study published in The Lancet regarding the measles-mumps-rubella vaccine and the link to autism. Although the article was retracted in 2010 and no evidence has been found linking vaccines with autism,1,3 vaccination coverage gaps still exist. These gaps can leave communities vulnerable to vaccine-preventable diseases.4 This lack of protection is especially glaring for the human papillomavirus (HPV) vaccine, putting health care professionals including dermatologists in the position of educating parents and guardians to have their children immunized.
More than 10 years after the federal government approved the first vaccines to fight the cancer-causing HPV, less than half of adolescent girls and only a fifth of adolescent boys are getting immunized. The reasons for the low vaccination rates are particularly complicated because they play not only into fears over vaccines but also over a perceived risk the vaccine may encourage sexual activity in adolescents, which has not been proven.5 Another factor is reluctance on the part of physicians to discuss the vaccine with patients and to fully embrace its lifesaving potential. A recent study showed how physicians are contributing to the low rate.6 “The single biggest barrier to increasing HPV vaccination is not receiving a health care provider’s recommendation,” said Harvard University researcher Melissa Gilkey.7
According to the Centers for Disease Control and Prevention (CDC), as of 2014, only 40% of adolescent girls aged 13 to 17 years had completed the 3-dose course of the HPV vaccine and just 22% of adolescent boys,8 which is short of the 80% public health goal set in 2010 by the federal government.9 Vexingly, HPV vaccination rates lag behind the other 2 vaccines recommended in the same age group: the tetanus-diphtheria-acellular pertussis booster (88%) and the vaccine to prevent meningococcal disease (79%).8
Malo et al6 surveyed 776 primary care physicians and reported that more than a quarter of primary care respondents (27%) do not strongly endorse the HPV vaccine when talking with their patients’ families. Nearly 2 in 5 physicians (39%) did not recommend on-time HPV vaccination for their male patients compared to 26% for female patients.6
The starkest findings, however, related to how the physicians approached their discussions with parents and guardians. Only half recommended the vaccine the same day they discussed it, and 59% said they approached discussions by assessing the child’s risk for contracting the disease rather than consistently recommending it to all children as a routine immunization.6
Despite physician hesitancy, when looking at the facts there should be no debate. In December 2014, the US Food and Drug Administration approved the 9-valent HPV (9vHPV) vaccine for males and females aged 9 to 26 years. The vaccine covers HPV types 6, 11, 16, and 18, which are part of the quadrivalent HPV (qHPV) vaccine, along with HPV types 31, 33, 45, 52, and 58. The 9vHPV vaccine has the potential to offer protection against 30% to 35% more high-grade cervical lesions and to increase cervical cancer prevention from approximately 70% to 90%.10 It also will protect against 90% of the virus strains responsible for causing anogenital warts. According to CDC estimates, for every year that coverage does not increase, an additional 4400 women will develop cervical cancer. If providers can push the HPV vaccination rate up to the goal rate of 80%, the CDC estimates that 53,000 cases of cervical cancer could be prevented during the lifetime of patients younger than 12 years.11
In a clinical trial of 14,215 women, Joura et al12 reported that the 9vHPV vaccine had an efficacy of 96.7% to prevent high-grade cervical, vulvar, or vaginal dysplasia related to HPV types 31, 33, 45, 52, and 58 in women. Antibody responses to HPV-6, 11, 16, and 18 among participants who received the 9vHPV vaccine were noninferior to those who received the qHPV vaccine. The incidence of disease related to HPV-6, 11, 16, and 18 was similar in the 2 vaccine groups. The introduction of 9vHPV vaccination in both males and females was cost saving compared to the qHPV vaccine in cost-effectiveness analyses. Injection-site reactions were slightly more common with the 9vHPV vaccine compared to the qHPV vaccine but were generally mild with less than 0.1% of study participants discontinuing due to vaccine-related adverse events.12
Additionally, the vaccine has the potential to offer protection against penile, anal, vulvar, vaginal, and oropharyngeal cancers (OPCs). Data from Joura et al12 demonstrate that 55% of anal and penile cancers biopsied in the study carried the 5 HPV types that are included only in the 9vHPV vaccine.
Studies also show that the rate of OPC caused by HPV is rising rapidly and increasing more among men than women. Remarkably, OPC is projected to become more common than cervical cancer in 2020, with an estimated 70% of OPCs being caused by HPV in the United States.13 Theoretically, the 9vHPV vaccine has the potential to protect against even more cases of OPC because of its even broader coverage.14
Although optimal timing for the HPV vaccine would still be in preadolescence prior to sexual activity when exposure to HPV is less likely, CDC studies have shown benefit even in older patients who may have already been exposed to 1 or more HPV strains.15
Simply put, all the combined data highlight the overwhelming importance of HPV vaccination, with the 9vHPV vaccine representing a meaningful advantage over existing HPV vaccines. As physicians, we have a duty to our patients to emphasize the importance of this vaccine. It is a vaccine that has the potential to prevent multiple cancers, cancers for which we currently have no evidence-based prevention modalities, except in the case of cervical cancer. This responsibility falls on all providers, not just primary care providers. With a strong message from providers to vaccinate age-eligible males and females, we can move the United States from among the lowest rates of HPV vaccination to the highest, with subsequent reductions in the national cancer burden to follow.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
- Demicheli V, Rivetti A, Debalini MG, et al. Vaccines for measles, mumps, and rubella in children. Cochrane Database Syst Rev. 2012:CD004407.
- Cha EA. 7 Things about vaccines and autism that the movie ‘Vaxxed’ won’t tell you. Washington Post. May 25, 2016. https://www.washingtonpost.com/news/to-your-health/wp/2016/05/25/7-things-about-vaccines-and-autism-that-the-movie-vaxxed-wont-tell-you/. Accessed July 4, 2016.
- Carroll AE. Not up for debate: the science behind vaccination. New York Times. September 17, 2015. https://www.nytimes.com/2015/09/18/upshot/not-up-for-debate-the-science-behind-vaccination.html?_r=0. Accessed November 9, 2016.
- Steenhuysen J. U.S. vaccination rates high, but pockets of unvaccinated pose risk. Reuters. August 27, 2015. http://www.reuters.com/article/us-usa-vaccine-exemptions-idUSKCN0QW2JY20150827. Accessed November 9, 2016.
- HPV vaccine not linked to sexual promiscuity in girls, study finds. The Guardian. October 15, 2012. https://www.theguardian.com/society/2012/oct/15/hpv-vaccine-link-sexual-promiscuity. Accessed November 9, 2016.
- Malo TL, Gilkey MB, Hall ME, et al. Messages to motivate human papillomavirus vaccination: national studies of parents and physicians. Cancer Epidemiol Biomarkers Prev. 2016;25:1383-1391.
- Haelle T. Doctors, not parents, are the biggest obstacle to the HPV vaccine. NPR. October 22, 2015. http://www.npr.org/sections/health-shots/2015/10/22/450827102/doctors-not-parents-are-the-biggest-obstacle-to-the-hpv-vaccine. Accessed November 9, 2016.
- Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years- United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784-792.
- Healthy People 2020. Centers for Disease Control and Prevention website. http://www.cdc.gov/nchs/healthy_people/hp2020.htm. Updated October 14, 2011. Accessed November 9, 2016.
- Joura E, Clark L, Luxembourg A. Additional protection from 9-valent HPV vaccine if administered before HPV exposure. Am Fam Physician. 2016;93:254-256.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62:591-595.
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711-723.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294-4301.
- Barbieri RL. Advances in protection against oncogenic human papillomaviruses: the 9-valent vaccine. OBG Manag. 2015;27:6-8.
- Beachler DC, Kreimer AR, Schiffman M, et al. Multisite HPV16/18 vaccine efficacy against cervical, anal, and oral HPV [published online October 14, 2015]. J Natl Cancer Inst. doi:10.1093/jnci/djv302.
Debunking Melanoma Myths: Do Sunscreens Cause Cancer?
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Update on New Drugs in Dermatology
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
Debunking Psoriasis Myths: Do Psoriasis Therapies Cause Depression?
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Psoriasis treatments may cause depression
It has been well documented that patients with inflammatory diseases such as psoriasis have an increased risk for depression. One population-based cohort study in the United Kingdom reported the risk of depression was greater in patients with severe psoriasis versus mild psoriasis. Younger psoriasis patients also had a higher risk compared to older patients. A US population-based study also reported that psoriasis was associated with major depression, but the severity of psoriasis and patient's age were unrelated. Therefore, all psoriasis patients may be at risk.
But are some therapies associated with an increased risk of depression? Increased concentrations of proinflammatory cytokines such as tumor necrosis factor α have been associated with depression apart from psoriasis. Administering immunomodulating agents has been shown to increase the risk of depression.
Depression has been cited as an adverse effect of apremilast in the drug's package insert, which states, "Before using [apremilast] in patients with a history of depression and/or suicidal thoughts or behavior prescribers should carefully weigh the risks and benefits of treatment." In clinical trials, 1.3% (12/920) of participants treated with apremilast reported depression compared to 0.4% (2/506) treated with placebo. Dermatologists should remain vigilant about monitoring for symptoms of depression in patients treated with apremilast.
However, depression in the context of autoimmune disorders or any disorder with increased inflammation has responded to treatment with tumor necrosis factor α antagonists. The relationship between depression and inflammation suggests that there is an inflammatory subtype of depression and use of anti-inflammatory agents may treat both inflammation and depression.
Disease control has been shown to improve symptoms of depression in psoriasis patients. A study of 618 patients with moderate to severe psoriasis who were treated with etanercept or placebo for 12 weeks revealed that more patients receiving etanercept experienced 50% improvement in 2 rating scales of depression compared to placebo.
Excessive worrying, a form of psychological distress, can impact treatment outcomes in patients with psoriasis. A 2003 study found that patients with psoriasis who are classified as high-level worriers may benefit from adjunctive psychological intervention before and during treatment. In this cohort of psoriasis patients receiving psoralen plus UVA (PUVA) therapy, high-level worry was the only significant predictor of time taken for PUVA to clear psoriasis (P=.01). Patients in the high-level worry group cleared with PUVA treatment at a rate of 1.8 times slower than the low-level worry group.
In conclusion, psoriasis patients should follow the treatment plan outlined by dermatologists, as improving psoriasis symptoms may help alleviate depression or prevent it from occurring. Patients with a history of depression should be monitored carefully by dermatologists or referred to another health care professional, and patients as well as family and friends should be encouraged to report any depression symptoms.
Expert Commentary
The prescribing information for apremilast lists a warning (but not a black-box warning) for depression. Long-term registries will determine if there is truly an increased risk of depression when taking apremilast. When I counsel patients before prescribing apremilast, I mention this potential increased risk of depression as noted in the prescribing information, but I tell them that the risk is very low and that a true risk has not yet been determined in long-term registries. I mention to patients that if they really do feel depressed after starting apremilast, they should stop taking apremilast and contact me.
Long-term registries for etanercept, adalimumab, infliximab, and ustekinumab do not indicate an increased risk for depression. Intuitively, if a patient with severe psoriasis has depression worsened by their psoriasis, it stands to reason that improving their skin will likely improve their mood, which clinical trials have shown using patient-related outcomes.
—Jashin J. Wu, MD (Los Angeles, California)
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35
Almond M. Depression and inflammation: examining the link. Current Psychiatry. 2013;12:24-32.
Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009-2012. JAMA Dermatol. 2016;152:73-79.
Fortune DG, Richards HL, Kirby B, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Arch Dermatol. 2003;139:752-756.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Otezla [package insert]. Summit, NJ: Celgene Corporation; 2015.Research links psoriasis, depression [press release]. New York, NY: American Academy of Dermatology; August 20, 2015. https://www.aad.org/media/news-releases/research-links-psoriasis-depression. Accessed November 16, 2016.
Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35