User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Late-Onset Bexarotene-Induced CD4 Lymphopenia in a Cutaneous T-cell Lymphoma Patient
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
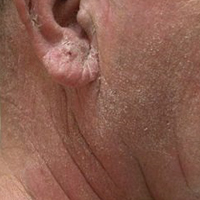
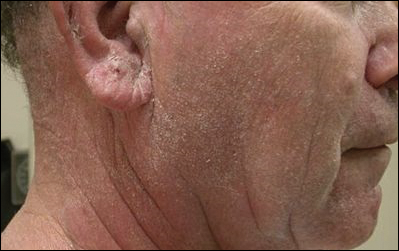
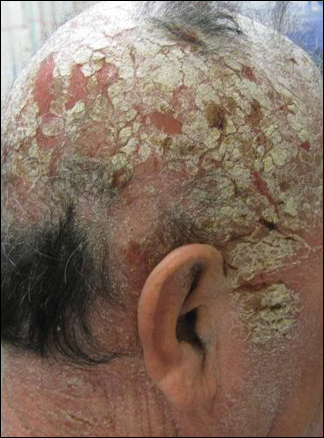
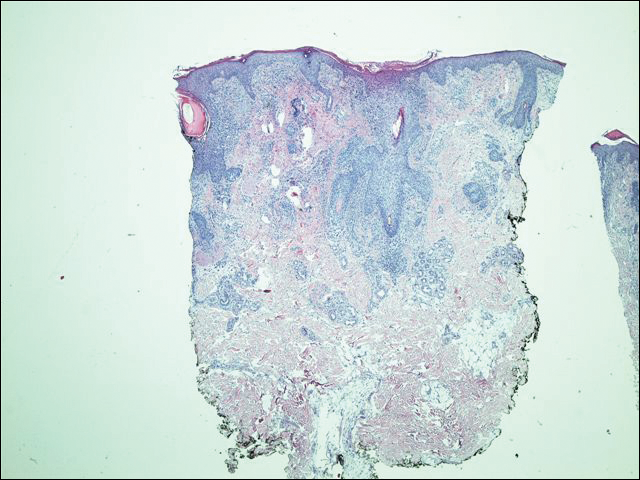
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.

The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
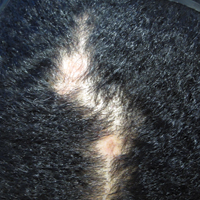
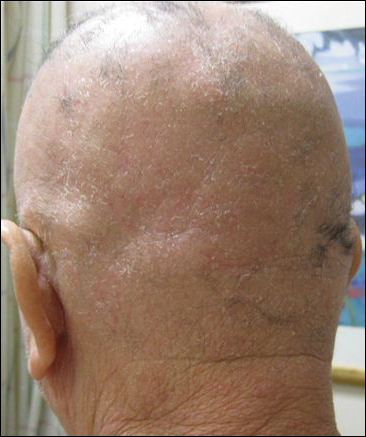
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.
Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.


The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.



Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.


The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.



Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
Practice Points
- Most adverse effects of bexarotene (eg, hypothyroidism, hyperlipidemia, leukopenia) occur within the first several months of therapy.
- Delayed-onset leukopenia, including CD4 lymphopenia, may occur several years after initiating bexarotene therapy, resulting in opportunistic infections.
- Long-term periodic monitoring of T lymphocyte counts at least twice yearly in addition to standard quarterly complete blood cell count with differential are recommended.
Online Patient-Reported Reviews of Mohs Micrographic Surgery: Qualitative Analysis of Positive and Negative Experiences
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
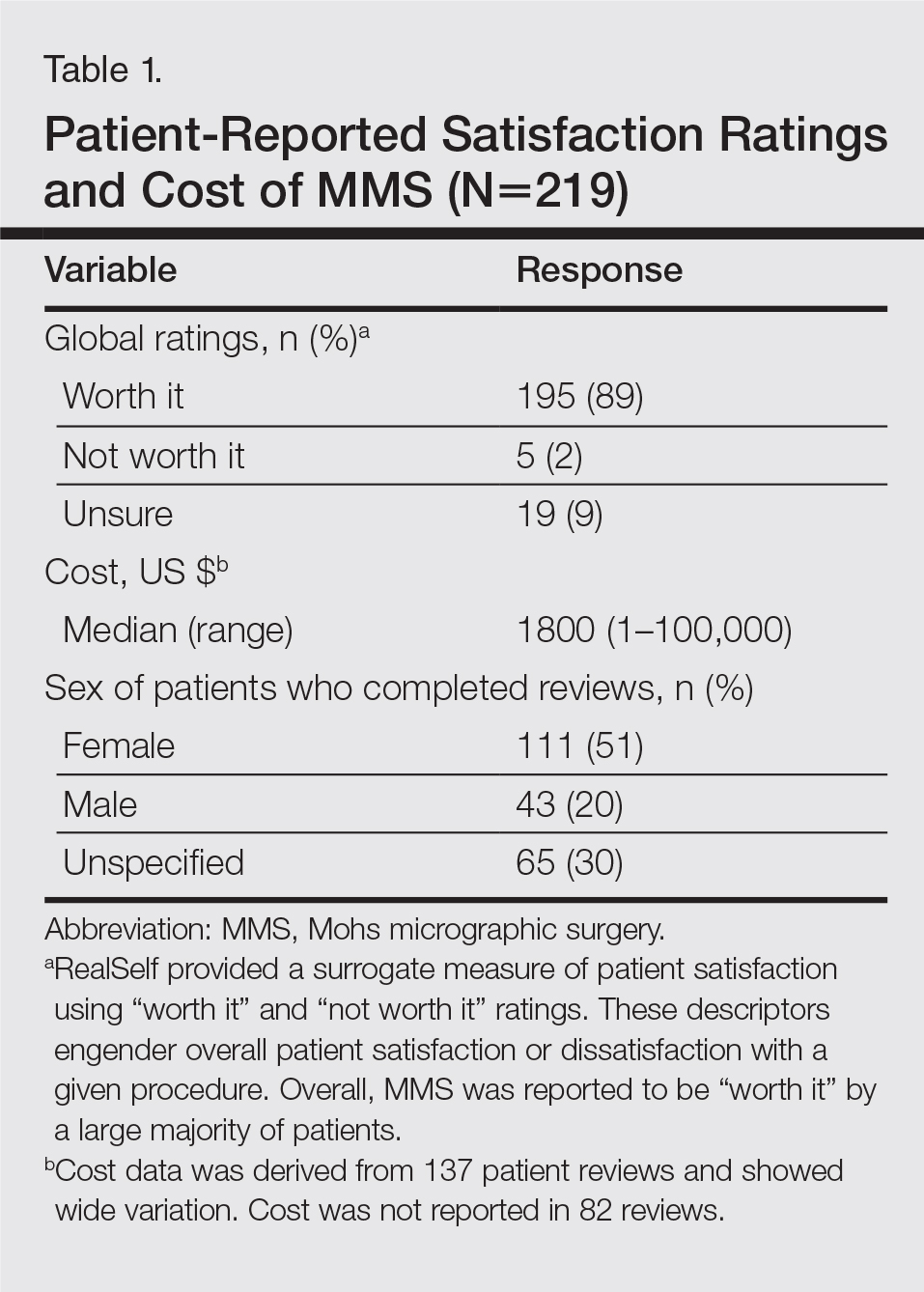
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).

Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
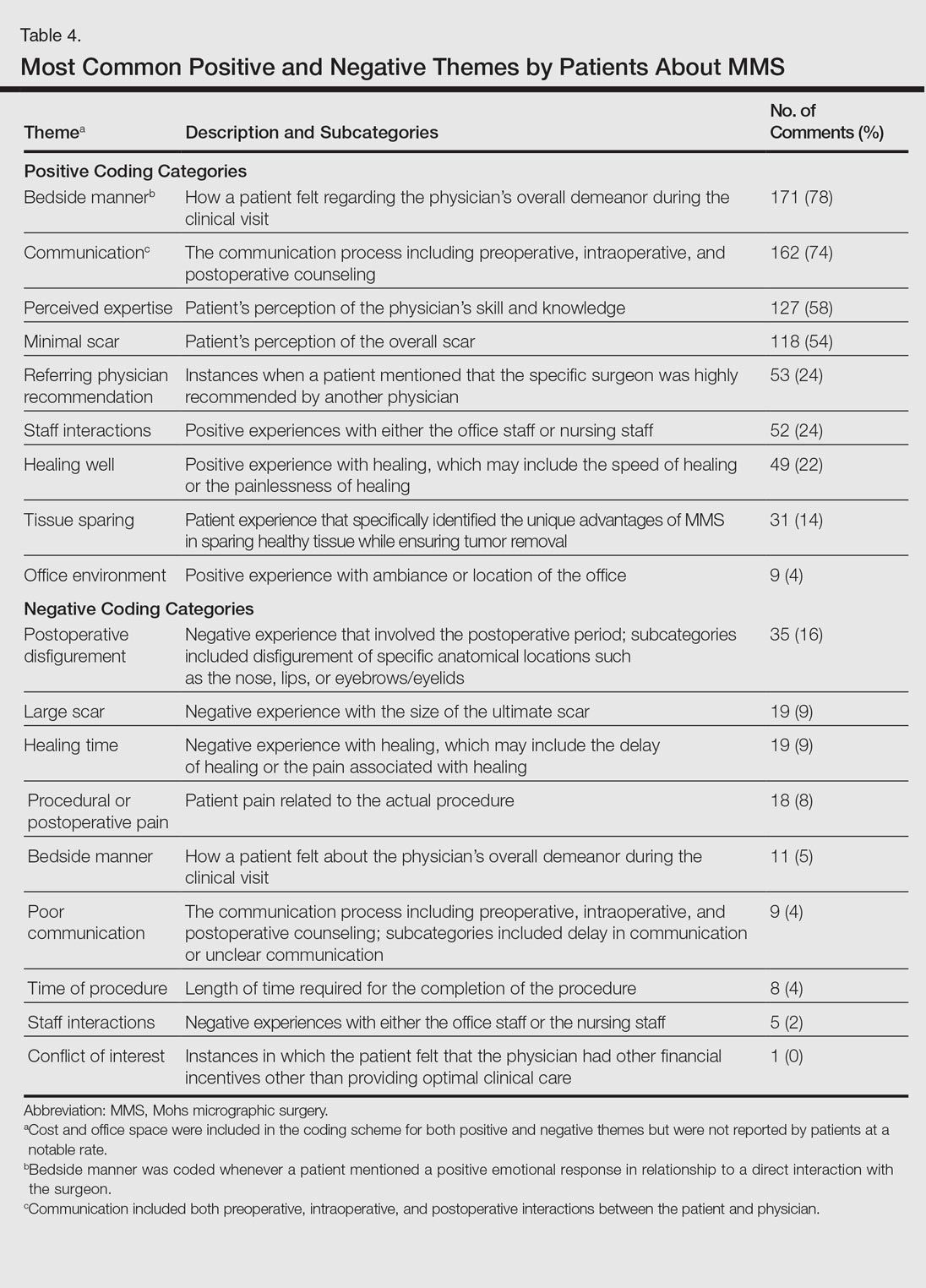
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).
Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).


Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).

Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).


Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).

Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
Resident Pearl
Patients are posting reviews online now more than ever regarding their experiences with dermatologic surgical procedures. Mohs micrographic surgery is rated highly by patients but suspect to missing information and a higher than expected attribution of the procedure to plastic surgeons.
Lupus Erythematosus Tumidus of the Scalp Masquerading as Alopecia Areata
Lupus erythematosus tumidus (LET) is a relatively rare condition but may simply be underdiagnosed in the literature. It presents as urticarialike papules and plaques in sun-exposed areas, characterized by induration and erythema. Lesions occur on the face, neck, upper extremities, and trunk and heal without scarring.1,2 Rarely, lesions can show fine scaling and associated pruritus, but most often the lesions are asymptomatic.3
Case Report
A 45-year-old woman presented with 2 asymptomatic self-described bald spots on the top of the head of 2 months’ duration. The patient denied prior treatment of the lesions and noted one patch was resolving. She reported no involvement of the eyebrows, eyelashes, and axillary and pubic hair. A review of systems was negative. The patient denied personal or family history of lupus, thyroid disease, or vitiligo.
Clinical examination revealed a 1.1-cm round patch of nonscarring alopecia on the right vertex scalp and a 0.9-cm round patch of nonscarring alopecia with moderate hair regrowth on the left vertex scalp. There was no erythema, scaling, or induration. The rest of the scalp was normal in appearance and the eyebrows and eyelashes were uninvolved. The patient was diagnosed with alopecia areata and was treated with 10 mg/mL of intralesional triamcinolone once monthly for 4 months.
The patient initially showed improvement with moderate hair regrowth. After 4 months of treatment, she developed 3 new 1- to 1.5-cm erythematous alopecic patches on the vertex scalp and had worsening in the initial patches (Figure 1). Given the resistance to standard therapy and the onset of multiple new areas with evidence of inflammatory involvement, a punch biopsy was performed. Histopathologic examination revealed a fairly unremarkable epidermis and a dense dermal inflammatory infiltrate that was present both in the superficial and deep dermis (Figure 2). The inflammatory cells, which appeared to be predominantly comprised of lymphocytes, had a predilection for the vasculature but also were observed within the interstitial dermis. Additionally, mucin appeared to be slightly increased in the deep dermis. The lymphocytic phenotype was confirmed by immunohistochemical studies for CD20 and CD3. The most likely possibilities for this reaction pattern were LET, Jessner lymphocytic infiltrate of the skin (JLIS), gyrate erythema, and lymphoma; however, the immunohistochemical studies effectively ruled out lymphoma. Additionally, there was pronounced dermal mucin noted in the specimen. The patient was diagnosed with LET of the scalp based on the constellation of findings.
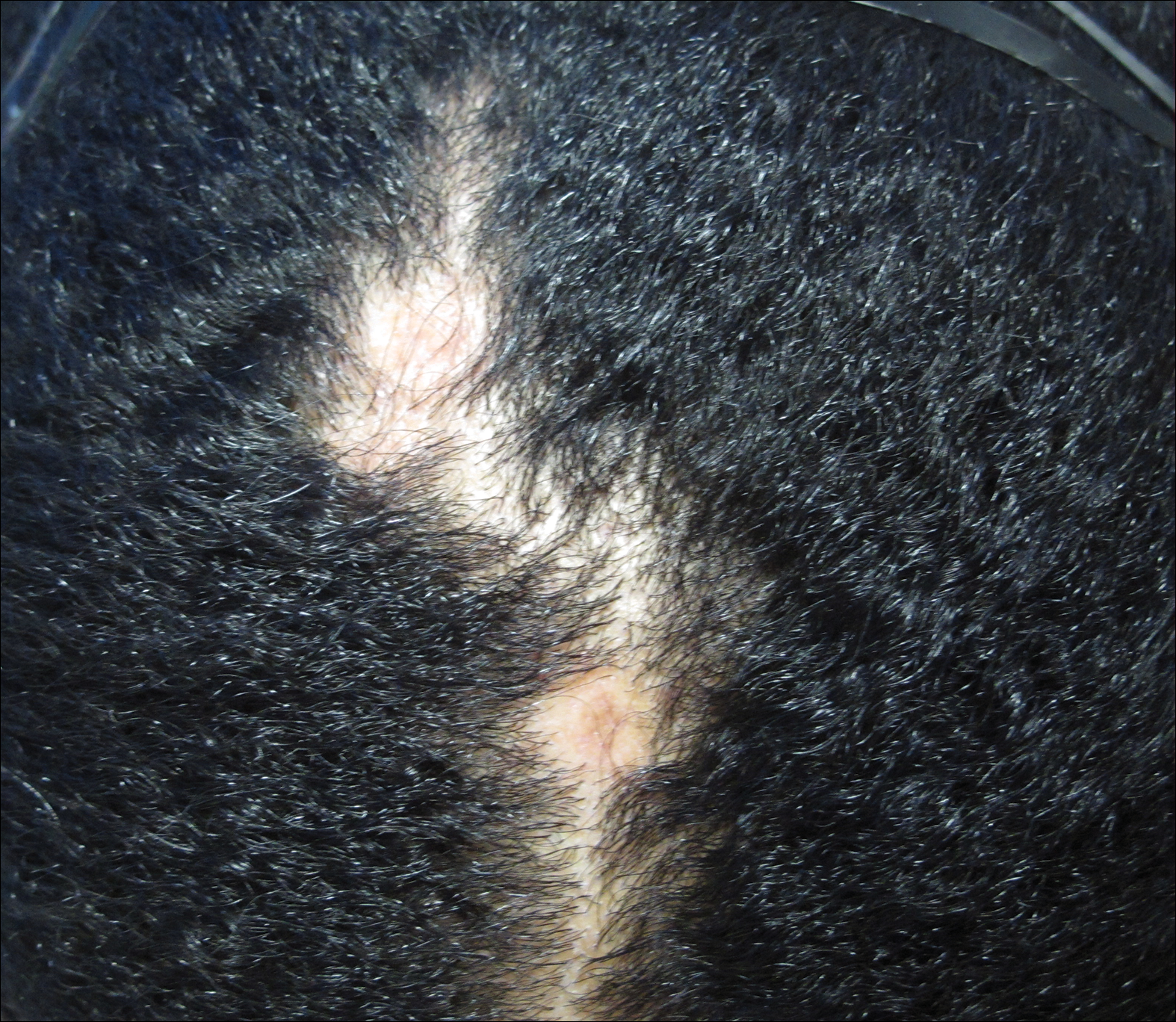
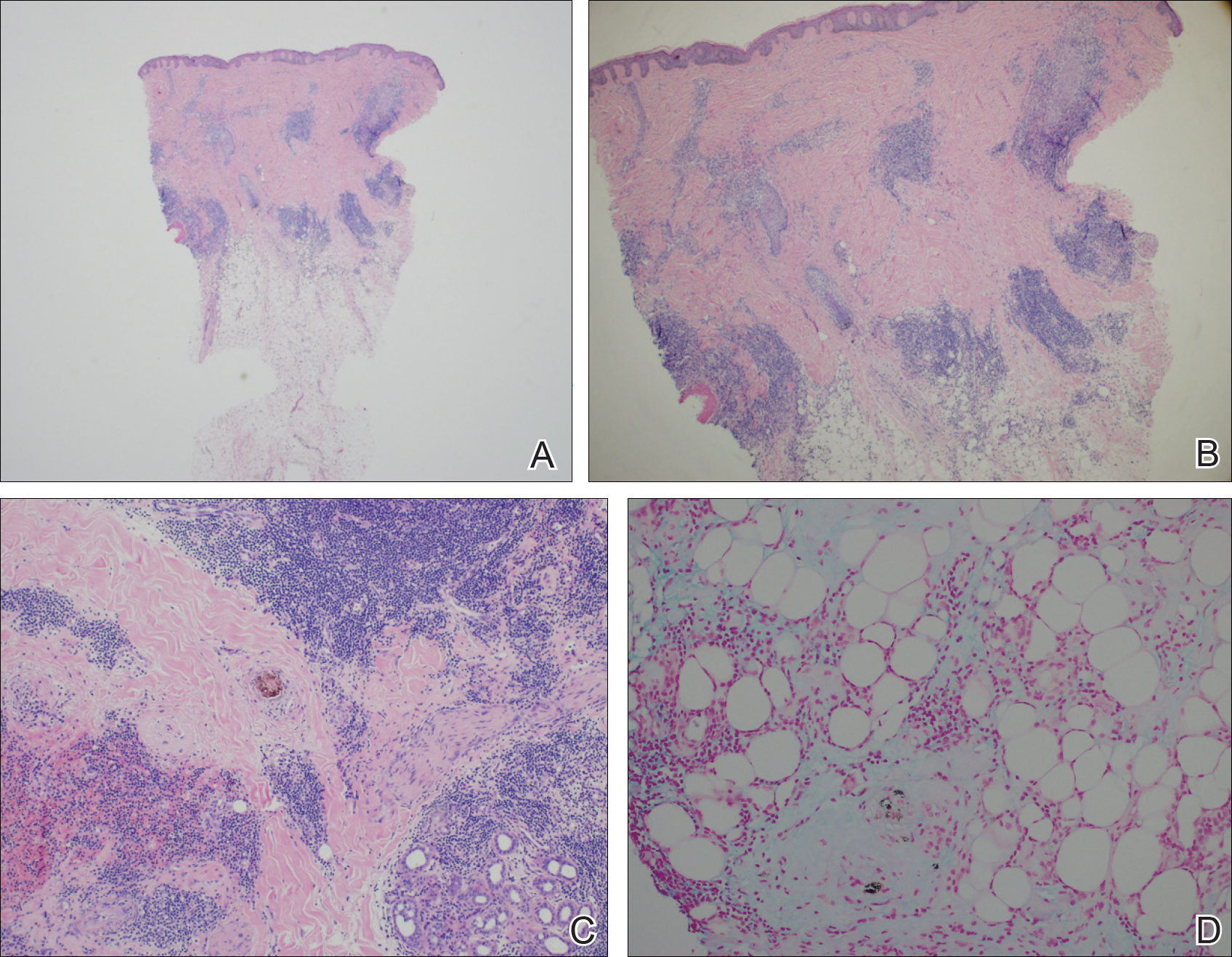
Comment
The classification of LET as a single unique entity or disease process sui generis has been in flux in the last decade. Its similarities to JLIS and other forms of chronic cutaneous lupus erythematosus (CCLE) have brought debate.4-6 In 1930, Gougerot and Burnier7 documented the first case of LET in the literature, describing smooth, infiltrated, erythematous lesions with no desquamation or other superficial changes seen in 5 patients.
In 2000, interest in LET and other forms of CCLE was increasing, and reports in the literature paralleled. That year, Kuhn et al4 reported 40 cases of LET, characterizing the clinical and histological features of each case to demonstrate that LET should be separate from other forms of CCLE. Until then, it is likely that many lesions that should have been classified as LET were instead classified as various forms of CCLE. The investigators maintained that LET also should be distinct from JLIS because it is associated with UV exposure.4 Kuhn et al8 reviewed phototesting in 60 patients with LET in 2001 and confirmed this subset was the most photosensitive type of lupus erythematosus.
In general, the histopathologic and immunohistochemical studies in LET and JLIS can be quite similar. Relatively distinguishing histopathologic findings in JLIS include no evidence of epidermal atrophy, basal vacuolar change, or follicular plugging, as well as negative immunofluorescence studies. Both entities show a predominantly T-cell population with a smaller component of B cells and thus a distinction cannot be made based on relative proportions of T and B cells in lesions.2
In 2003, Alexiades-Armenakas et al6 determined immunohistochemical criteria for LET, finding a predominance of T cells and more CD4 lymphocytes than CD8 lymphocytes with a mean ratio of roughly 3 to 1. Their study results maintained LET should be classified as a form of CCLE due to the chronicity of the lesions, the serologic profile with negative anti–double-stranded DNA, anticentromere, anti-Smith, anti-Ro/Sjögren syndrome antigen A, anti-La/Sjögren syndrome antigen B, and anti-nuclear ribonucleoprotein antibodies and the rare association with systemic disease.6 This conclusion was further solidified by a review published that same year citing unique histopathological features when compared to subacute cutaneous LE and discoid lupus erythematosus.5
This case illustrates the importance of histologic evaluation in determining the correct diagnosis in a patient with alopecia areata recalcitrant to treatment. Including LET in the differential of alopecic patches on the scalp could prove beneficial for patients, as LET responds well to antimalarial drugs and photoprotection.9 This patient had a normal antinuclear antibody panel and no signs or symptoms of systemic lupus. It was recommended that she avoid sun exposure and begin treatment with hydroxychloroquine but she declined. At a follow-up visit 6 months later she reported the lesions had improved, but a permanent wig had been sewn over the area, so it could not be examined.
- Lee L, Werth V. Rheumatologic disease. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 3rd ed. Mosby Elsevier; 2008:615-629.
- Weedon D. The lichenoid reaction pattern. In: Weedon D. Skin Pathology. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2002:35-70.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am Acad Dermatol. 1999;41:250-253.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Kuhn A, Sonntag M, Ruzicka T, et al. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003;48:901-908.
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003;49:494-500.
- Gougerot H, Burnier R. Lupuse rythe mateux “tumidus.” Bull Soc Fr Dermatol Syph. 1930;37:1291-1292.
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus tumidus—review of 60 patients. Photochem Photobiol. 2001;73:532-536.
- Cozzani E, Christana K, Rongioletti F, et al. Lupus erythematosus tumidus: clinical, histopathological and serological aspects and therapy response of 21 patients. Eur J Dermatol. 2010;20:797-801.
Lupus erythematosus tumidus (LET) is a relatively rare condition but may simply be underdiagnosed in the literature. It presents as urticarialike papules and plaques in sun-exposed areas, characterized by induration and erythema. Lesions occur on the face, neck, upper extremities, and trunk and heal without scarring.1,2 Rarely, lesions can show fine scaling and associated pruritus, but most often the lesions are asymptomatic.3
Case Report
A 45-year-old woman presented with 2 asymptomatic self-described bald spots on the top of the head of 2 months’ duration. The patient denied prior treatment of the lesions and noted one patch was resolving. She reported no involvement of the eyebrows, eyelashes, and axillary and pubic hair. A review of systems was negative. The patient denied personal or family history of lupus, thyroid disease, or vitiligo.
Clinical examination revealed a 1.1-cm round patch of nonscarring alopecia on the right vertex scalp and a 0.9-cm round patch of nonscarring alopecia with moderate hair regrowth on the left vertex scalp. There was no erythema, scaling, or induration. The rest of the scalp was normal in appearance and the eyebrows and eyelashes were uninvolved. The patient was diagnosed with alopecia areata and was treated with 10 mg/mL of intralesional triamcinolone once monthly for 4 months.
The patient initially showed improvement with moderate hair regrowth. After 4 months of treatment, she developed 3 new 1- to 1.5-cm erythematous alopecic patches on the vertex scalp and had worsening in the initial patches (Figure 1). Given the resistance to standard therapy and the onset of multiple new areas with evidence of inflammatory involvement, a punch biopsy was performed. Histopathologic examination revealed a fairly unremarkable epidermis and a dense dermal inflammatory infiltrate that was present both in the superficial and deep dermis (Figure 2). The inflammatory cells, which appeared to be predominantly comprised of lymphocytes, had a predilection for the vasculature but also were observed within the interstitial dermis. Additionally, mucin appeared to be slightly increased in the deep dermis. The lymphocytic phenotype was confirmed by immunohistochemical studies for CD20 and CD3. The most likely possibilities for this reaction pattern were LET, Jessner lymphocytic infiltrate of the skin (JLIS), gyrate erythema, and lymphoma; however, the immunohistochemical studies effectively ruled out lymphoma. Additionally, there was pronounced dermal mucin noted in the specimen. The patient was diagnosed with LET of the scalp based on the constellation of findings.


Comment
The classification of LET as a single unique entity or disease process sui generis has been in flux in the last decade. Its similarities to JLIS and other forms of chronic cutaneous lupus erythematosus (CCLE) have brought debate.4-6 In 1930, Gougerot and Burnier7 documented the first case of LET in the literature, describing smooth, infiltrated, erythematous lesions with no desquamation or other superficial changes seen in 5 patients.
In 2000, interest in LET and other forms of CCLE was increasing, and reports in the literature paralleled. That year, Kuhn et al4 reported 40 cases of LET, characterizing the clinical and histological features of each case to demonstrate that LET should be separate from other forms of CCLE. Until then, it is likely that many lesions that should have been classified as LET were instead classified as various forms of CCLE. The investigators maintained that LET also should be distinct from JLIS because it is associated with UV exposure.4 Kuhn et al8 reviewed phototesting in 60 patients with LET in 2001 and confirmed this subset was the most photosensitive type of lupus erythematosus.
In general, the histopathologic and immunohistochemical studies in LET and JLIS can be quite similar. Relatively distinguishing histopathologic findings in JLIS include no evidence of epidermal atrophy, basal vacuolar change, or follicular plugging, as well as negative immunofluorescence studies. Both entities show a predominantly T-cell population with a smaller component of B cells and thus a distinction cannot be made based on relative proportions of T and B cells in lesions.2
In 2003, Alexiades-Armenakas et al6 determined immunohistochemical criteria for LET, finding a predominance of T cells and more CD4 lymphocytes than CD8 lymphocytes with a mean ratio of roughly 3 to 1. Their study results maintained LET should be classified as a form of CCLE due to the chronicity of the lesions, the serologic profile with negative anti–double-stranded DNA, anticentromere, anti-Smith, anti-Ro/Sjögren syndrome antigen A, anti-La/Sjögren syndrome antigen B, and anti-nuclear ribonucleoprotein antibodies and the rare association with systemic disease.6 This conclusion was further solidified by a review published that same year citing unique histopathological features when compared to subacute cutaneous LE and discoid lupus erythematosus.5
This case illustrates the importance of histologic evaluation in determining the correct diagnosis in a patient with alopecia areata recalcitrant to treatment. Including LET in the differential of alopecic patches on the scalp could prove beneficial for patients, as LET responds well to antimalarial drugs and photoprotection.9 This patient had a normal antinuclear antibody panel and no signs or symptoms of systemic lupus. It was recommended that she avoid sun exposure and begin treatment with hydroxychloroquine but she declined. At a follow-up visit 6 months later she reported the lesions had improved, but a permanent wig had been sewn over the area, so it could not be examined.
Lupus erythematosus tumidus (LET) is a relatively rare condition but may simply be underdiagnosed in the literature. It presents as urticarialike papules and plaques in sun-exposed areas, characterized by induration and erythema. Lesions occur on the face, neck, upper extremities, and trunk and heal without scarring.1,2 Rarely, lesions can show fine scaling and associated pruritus, but most often the lesions are asymptomatic.3
Case Report
A 45-year-old woman presented with 2 asymptomatic self-described bald spots on the top of the head of 2 months’ duration. The patient denied prior treatment of the lesions and noted one patch was resolving. She reported no involvement of the eyebrows, eyelashes, and axillary and pubic hair. A review of systems was negative. The patient denied personal or family history of lupus, thyroid disease, or vitiligo.
Clinical examination revealed a 1.1-cm round patch of nonscarring alopecia on the right vertex scalp and a 0.9-cm round patch of nonscarring alopecia with moderate hair regrowth on the left vertex scalp. There was no erythema, scaling, or induration. The rest of the scalp was normal in appearance and the eyebrows and eyelashes were uninvolved. The patient was diagnosed with alopecia areata and was treated with 10 mg/mL of intralesional triamcinolone once monthly for 4 months.
The patient initially showed improvement with moderate hair regrowth. After 4 months of treatment, she developed 3 new 1- to 1.5-cm erythematous alopecic patches on the vertex scalp and had worsening in the initial patches (Figure 1). Given the resistance to standard therapy and the onset of multiple new areas with evidence of inflammatory involvement, a punch biopsy was performed. Histopathologic examination revealed a fairly unremarkable epidermis and a dense dermal inflammatory infiltrate that was present both in the superficial and deep dermis (Figure 2). The inflammatory cells, which appeared to be predominantly comprised of lymphocytes, had a predilection for the vasculature but also were observed within the interstitial dermis. Additionally, mucin appeared to be slightly increased in the deep dermis. The lymphocytic phenotype was confirmed by immunohistochemical studies for CD20 and CD3. The most likely possibilities for this reaction pattern were LET, Jessner lymphocytic infiltrate of the skin (JLIS), gyrate erythema, and lymphoma; however, the immunohistochemical studies effectively ruled out lymphoma. Additionally, there was pronounced dermal mucin noted in the specimen. The patient was diagnosed with LET of the scalp based on the constellation of findings.


Comment
The classification of LET as a single unique entity or disease process sui generis has been in flux in the last decade. Its similarities to JLIS and other forms of chronic cutaneous lupus erythematosus (CCLE) have brought debate.4-6 In 1930, Gougerot and Burnier7 documented the first case of LET in the literature, describing smooth, infiltrated, erythematous lesions with no desquamation or other superficial changes seen in 5 patients.
In 2000, interest in LET and other forms of CCLE was increasing, and reports in the literature paralleled. That year, Kuhn et al4 reported 40 cases of LET, characterizing the clinical and histological features of each case to demonstrate that LET should be separate from other forms of CCLE. Until then, it is likely that many lesions that should have been classified as LET were instead classified as various forms of CCLE. The investigators maintained that LET also should be distinct from JLIS because it is associated with UV exposure.4 Kuhn et al8 reviewed phototesting in 60 patients with LET in 2001 and confirmed this subset was the most photosensitive type of lupus erythematosus.
In general, the histopathologic and immunohistochemical studies in LET and JLIS can be quite similar. Relatively distinguishing histopathologic findings in JLIS include no evidence of epidermal atrophy, basal vacuolar change, or follicular plugging, as well as negative immunofluorescence studies. Both entities show a predominantly T-cell population with a smaller component of B cells and thus a distinction cannot be made based on relative proportions of T and B cells in lesions.2
In 2003, Alexiades-Armenakas et al6 determined immunohistochemical criteria for LET, finding a predominance of T cells and more CD4 lymphocytes than CD8 lymphocytes with a mean ratio of roughly 3 to 1. Their study results maintained LET should be classified as a form of CCLE due to the chronicity of the lesions, the serologic profile with negative anti–double-stranded DNA, anticentromere, anti-Smith, anti-Ro/Sjögren syndrome antigen A, anti-La/Sjögren syndrome antigen B, and anti-nuclear ribonucleoprotein antibodies and the rare association with systemic disease.6 This conclusion was further solidified by a review published that same year citing unique histopathological features when compared to subacute cutaneous LE and discoid lupus erythematosus.5
This case illustrates the importance of histologic evaluation in determining the correct diagnosis in a patient with alopecia areata recalcitrant to treatment. Including LET in the differential of alopecic patches on the scalp could prove beneficial for patients, as LET responds well to antimalarial drugs and photoprotection.9 This patient had a normal antinuclear antibody panel and no signs or symptoms of systemic lupus. It was recommended that she avoid sun exposure and begin treatment with hydroxychloroquine but she declined. At a follow-up visit 6 months later she reported the lesions had improved, but a permanent wig had been sewn over the area, so it could not be examined.
- Lee L, Werth V. Rheumatologic disease. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 3rd ed. Mosby Elsevier; 2008:615-629.
- Weedon D. The lichenoid reaction pattern. In: Weedon D. Skin Pathology. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2002:35-70.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am Acad Dermatol. 1999;41:250-253.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Kuhn A, Sonntag M, Ruzicka T, et al. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003;48:901-908.
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003;49:494-500.
- Gougerot H, Burnier R. Lupuse rythe mateux “tumidus.” Bull Soc Fr Dermatol Syph. 1930;37:1291-1292.
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus tumidus—review of 60 patients. Photochem Photobiol. 2001;73:532-536.
- Cozzani E, Christana K, Rongioletti F, et al. Lupus erythematosus tumidus: clinical, histopathological and serological aspects and therapy response of 21 patients. Eur J Dermatol. 2010;20:797-801.
- Lee L, Werth V. Rheumatologic disease. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 3rd ed. Mosby Elsevier; 2008:615-629.
- Weedon D. The lichenoid reaction pattern. In: Weedon D. Skin Pathology. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 2002:35-70.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am Acad Dermatol. 1999;41:250-253.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Kuhn A, Sonntag M, Ruzicka T, et al. Histopathologic findings in lupus erythematosus tumidus: review of 80 patients. J Am Acad Dermatol. 2003;48:901-908.
- Alexiades-Armenakas MR, Baldassano M, Bince B, et al. Tumid lupus erythematosus: criteria for classification with immunohistochemical analysis. Arthritis Rheum. 2003;49:494-500.
- Gougerot H, Burnier R. Lupuse rythe mateux “tumidus.” Bull Soc Fr Dermatol Syph. 1930;37:1291-1292.
- Kuhn A, Sonntag M, Richter-Hintz D, et al. Phototesting in lupus erythematosus tumidus—review of 60 patients. Photochem Photobiol. 2001;73:532-536.
- Cozzani E, Christana K, Rongioletti F, et al. Lupus erythematosus tumidus: clinical, histopathological and serological aspects and therapy response of 21 patients. Eur J Dermatol. 2010;20:797-801.
Practice Points
- Lupus erythematosus tumidus (LET) of the scalp can mimic alopecia areata on clinical presentation.
- A unique variant of chronic cutaneous lupus erythematosus, LET presents in sun-exposed areas without any corresponding systemic signs.
- Lupus erythematosus tumidus may respond well to antimalarial drugs.
Foundation for Prader-Willi Research Highlights Study of Genetic Therapy for Prader-Willi Syndrome
The Foundation for Prader-Willi Research has published a blog that discusses a new study which shows promising first steps toward genetic therapy for Prader-Willi syndrome.
The Foundation for Prader-Willi Research has published a blog that discusses a new study which shows promising first steps toward genetic therapy for Prader-Willi syndrome.
The Foundation for Prader-Willi Research has published a blog that discusses a new study which shows promising first steps toward genetic therapy for Prader-Willi syndrome.
APS Type 1 Symposium Is Planned
The second APS Type 1 Symposium for physicians and families will take place July 13 to15 at the State University of New York at Stony Brook. Additional information will be available from the APS Type 1 Foundation.
The second APS Type 1 Symposium for physicians and families will take place July 13 to15 at the State University of New York at Stony Brook. Additional information will be available from the APS Type 1 Foundation.
The second APS Type 1 Symposium for physicians and families will take place July 13 to15 at the State University of New York at Stony Brook. Additional information will be available from the APS Type 1 Foundation.
Aplastic Anemia & MDS International Foundation Research Portal Is Open
AAMDSIF has announced that its 2017 research grant portal is now open. Each year, the foundation’s medical advisors review research proposals from new and established investigators in the topic areas of aplastic anemia, myelodysplastic syndromes, and paroxysmal nocturnal hemoglobinuria. The deadline to submit an application is February 28, 2017.
AAMDSIF has announced that its 2017 research grant portal is now open. Each year, the foundation’s medical advisors review research proposals from new and established investigators in the topic areas of aplastic anemia, myelodysplastic syndromes, and paroxysmal nocturnal hemoglobinuria. The deadline to submit an application is February 28, 2017.
AAMDSIF has announced that its 2017 research grant portal is now open. Each year, the foundation’s medical advisors review research proposals from new and established investigators in the topic areas of aplastic anemia, myelodysplastic syndromes, and paroxysmal nocturnal hemoglobinuria. The deadline to submit an application is February 28, 2017.
Alport Syndrome Foundation Announces Research Funding
The Alport Syndrome Foundation has announced the availability of funding for research to find novel treatments to prevent kidney failure and hearing loss in all patients with Alport syndrome. Two $100,000 projects are anticipated. Proposals are due March 20, 2017.
The Alport Syndrome Foundation has announced the availability of funding for research to find novel treatments to prevent kidney failure and hearing loss in all patients with Alport syndrome. Two $100,000 projects are anticipated. Proposals are due March 20, 2017.
The Alport Syndrome Foundation has announced the availability of funding for research to find novel treatments to prevent kidney failure and hearing loss in all patients with Alport syndrome. Two $100,000 projects are anticipated. Proposals are due March 20, 2017.
NORD Publishes Report on Spontaneous Intracranial Hypotension (SIH)
As part of its ongoing educational outreach, NORD has published a report on spontaneous intracranial hypotension (SIH) in its Rare Disease Database. The database covers approximately 1,300 rare diseases and is available to all on the NORD website. Reports may be read online or downloaded free of charge.
The report on SIH was developed in collaboration with Connie Deline, MD, co-founder of the Spinal CSF Leak Foundation, and Wouter I. Schievink, MD, Professor of Neurosurgery, Department of Neurosurgery, Cedars-Sinai Medical Center. The hope is that the new report will reduce diagnosis delay and also be helpful to newly diagnosed patients.
The new report may be accessed here. SIH is secondary to a cerebrospinal fluid leak at the level of the spine, and the resulting loss of CSF volume to support the brain and spinal cord. To promote awareness of the condition, February 26 to March 4 has been designated the first spinal CSF leak awareness week.
As part of its ongoing educational outreach, NORD has published a report on spontaneous intracranial hypotension (SIH) in its Rare Disease Database. The database covers approximately 1,300 rare diseases and is available to all on the NORD website. Reports may be read online or downloaded free of charge.
The report on SIH was developed in collaboration with Connie Deline, MD, co-founder of the Spinal CSF Leak Foundation, and Wouter I. Schievink, MD, Professor of Neurosurgery, Department of Neurosurgery, Cedars-Sinai Medical Center. The hope is that the new report will reduce diagnosis delay and also be helpful to newly diagnosed patients.
The new report may be accessed here. SIH is secondary to a cerebrospinal fluid leak at the level of the spine, and the resulting loss of CSF volume to support the brain and spinal cord. To promote awareness of the condition, February 26 to March 4 has been designated the first spinal CSF leak awareness week.
As part of its ongoing educational outreach, NORD has published a report on spontaneous intracranial hypotension (SIH) in its Rare Disease Database. The database covers approximately 1,300 rare diseases and is available to all on the NORD website. Reports may be read online or downloaded free of charge.
The report on SIH was developed in collaboration with Connie Deline, MD, co-founder of the Spinal CSF Leak Foundation, and Wouter I. Schievink, MD, Professor of Neurosurgery, Department of Neurosurgery, Cedars-Sinai Medical Center. The hope is that the new report will reduce diagnosis delay and also be helpful to newly diagnosed patients.
The new report may be accessed here. SIH is secondary to a cerebrospinal fluid leak at the level of the spine, and the resulting loss of CSF volume to support the brain and spinal cord. To promote awareness of the condition, February 26 to March 4 has been designated the first spinal CSF leak awareness week.
CORD Offers Consensus Framework for Ethical Collaboration
The Canadian Organization for Rare Disorders (CORD) has developed a Consensus Framework to encourage ethical collaboration among patient organizations, health care professionals, and the pharmaceutical industry.
The Canadian Organization for Rare Disorders (CORD) has developed a Consensus Framework to encourage ethical collaboration among patient organizations, health care professionals, and the pharmaceutical industry.
The Canadian Organization for Rare Disorders (CORD) has developed a Consensus Framework to encourage ethical collaboration among patient organizations, health care professionals, and the pharmaceutical industry.
Apply Now to Join NORD’s Charity Marathon Team
Running for Rare, NORD’s charity marathon team, will be running the Los Angeles Marathon on March 19 to raise funds for NORD’s patient assistance program for undiagnosed patients and to raise awareness of rare diseases. Each runner is paired with a community partner (a patient or caregiver) who provides inspiration to the runner.
Applications are open for runners and community partners. The LA Marathon is the 5th largest marathon in the US and the 11th largest worldwide. More than 25,000 people participate. Details.
Running for Rare, NORD’s charity marathon team, will be running the Los Angeles Marathon on March 19 to raise funds for NORD’s patient assistance program for undiagnosed patients and to raise awareness of rare diseases. Each runner is paired with a community partner (a patient or caregiver) who provides inspiration to the runner.
Applications are open for runners and community partners. The LA Marathon is the 5th largest marathon in the US and the 11th largest worldwide. More than 25,000 people participate. Details.
Running for Rare, NORD’s charity marathon team, will be running the Los Angeles Marathon on March 19 to raise funds for NORD’s patient assistance program for undiagnosed patients and to raise awareness of rare diseases. Each runner is paired with a community partner (a patient or caregiver) who provides inspiration to the runner.
Applications are open for runners and community partners. The LA Marathon is the 5th largest marathon in the US and the 11th largest worldwide. More than 25,000 people participate. Details.