User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Clindamycin Phosphate–Tretinoin Combination Gel Revisited: Status Report on a Specific Formulation Used for Acne Treatment
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
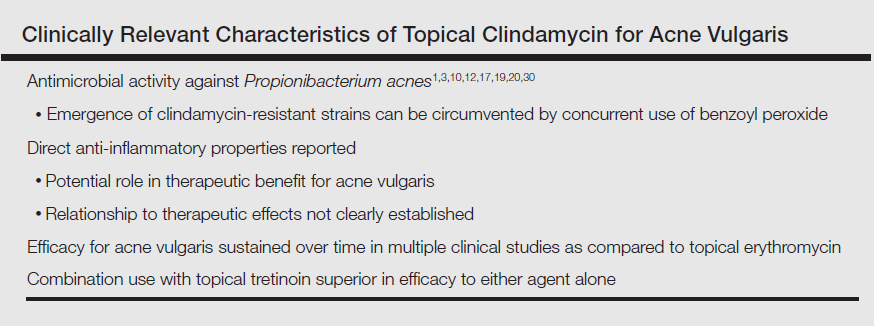
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.
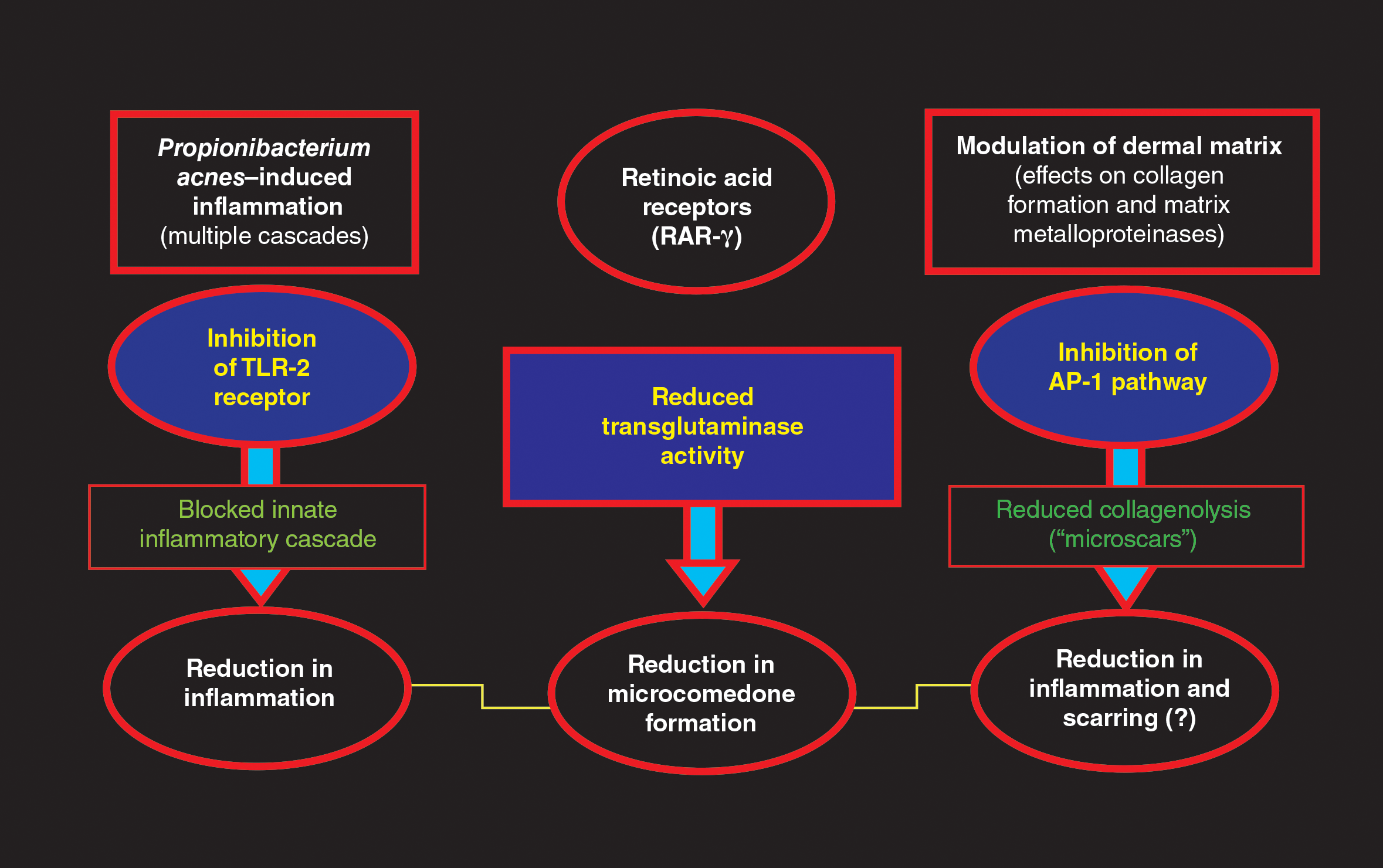
Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14
What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.

Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14

What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
Topical management of acne vulgaris (AV) incorporates a variety of agents with diverse modes of action (MOAs), including retinoids and antibiotics.1-3 The first topical retinoid developed for acne therapy was tretinoin, available in the United States since 1971.2,4 Topical retinoids, including tretinoin, exhibit multiple pharmacologic effects that are believed to correlate with efficacy for acne treatment,1,2,4,5 such as the reduction of inflammatory and comedonal lesions and contribution to dermal matrix remodeling.1,2,4-9 The predominant topical antibiotic used for acne treatment, often in combination with benzoyl peroxide (BP) and/or a topical retinoid, is clindamycin. Clindamycin is a lincosamide antibiotic that is closely related to erythromycin, a member of the macrolide antibiotic category.1,3,10 Available data support that over time topical clindamycin has sustained greater efficacy in reducing AV lesions than topical erythromycin; the latter also has been shown to exhibit a greater prevalence of Propionibacterium acnes resistance than clindamycin.1,3,10-12
Combination gel formulations of clindamycin phosphate 1.2%–tretinoin 0.025% (CP-Tret) are approved by the US Food and Drug Administration and available in the United States for once-daily treatment of AV in patients 12 years of age and older.13-15 Large-scale randomized controlled trials (RCTs) have demonstrated both efficacy and safety for these formulations.16,17 This article reviews important considerations related to both individual active ingredients (clindamycin phosphate [CP] and tretinoin [Tret]), formulation characteristics, and data from pivotal RCTs with a CP-Tret gel that has more recently been reintroduced into the US marketplace for acne therapy (Veltin, Aqua Pharmaceuticals).
What is the rationale behind combining CP and Tret in a single combination formulation?
Clindamycin is a lincosamide antibiotic that has been used for the treatment of AV for approximately 5 decades.1,3,10,17 The main MOA of clindamycin in the treatment of AV is believed to be reduction of P acnes; however, anti-inflammatory effects maypotentially play some role in AV lesion reduction.3,10,12,17-19 Multiple RCTs completed over approximately 3 decades and inclusive of more than 2000 participants treated topically with clindamycin as monotherapy have shown that the efficacy of this agent in reducing AV lesions has remained consistent overall,3,20-24 unlike topical erythromycin, which did not sustain its efficacy over a similar comparative time period.20 Importantly, these data are based on RCTs designed to evaluate the efficacy and safety of individual agents, including topical clindamycin; however, topical antibiotic therapy is not recommended as monotherapy for AV treatment due to emergence of antibiotic-resistant bacterial strains.1,3,11,12,25-28 Although the prevalence of resistant strains of P acnes is lower in the United States and many other countries for clindamycin versus erythromycin, the magnitude of clindamycin-resistant P acnes strains increases and response to clindamycin therapy may decrease when this agent is used alone.12,25-27,29,30 Therefore, it is recommended that a BP formulation that exhibits the ability to adequately reduce P acnes counts be used concurrently with antibiotic therapy for AV to reduce the emergence and proliferation of antibiotic-resistant P acnes organisms; short-contact BP therapy using a high-concentration (9.8%) emollient foam formulation and sufficient contact time (ie, 2 minutes) prior to washing off also has been shown to markedly reduce truncal P acnes organism counts.1,3,10-12,25-33 The Table depicts the major characteristics of clindamycin related to its use for treatment of AV.

Tretinoin has been used extensively for the treatment of AV since its introduction in the United States in 1971.1,2,4,5 The proposed MOAs of topical retinoids, including tretinoin, based on available data include a variety of pharmacologic effects such as inhibition of follicular hyperkeratosis (decreased microcomedone formation), modulation of keratinocyte differentiation, anti-inflammatory properties, and inhibition of dermal matrix degradation (Figure).1,2,4,5,14,34,35 Topical retinoids, including tretinoin, have been shown to reduce both inflammatory and comedonal acne lesions, likely due to multiple MOAs, and are devoid of antibiotic properties.2,4-8,16 Available data support that topical combination therapy for AV with a retinoid and a topical antimicrobial agent augments the therapeutic benefit as compared to use of either agent alone.1-4,12,15,16,28,31,32
The rationale for incorporating both clindamycin and tretinoin together into one topical formulation includes combining different MOAs that appear to correlate with suppression of AV lesion formation and to improve patient adherence through once-daily application of a single topical product.16,31,32,36 Importantly, formulation researchers were able to combine CP-Tret into a specific aqueous gel formulation that maintained the stability of both active ingredients and proved to be effective and safe in preliminary studies completed in participants with AV.16,23,37-39 This aqueous formulation incorporated a limited number of excipients with low to negligible potential for cutaneous irritation or allergenicity, including anhydrous citric acid (chelating agent, preservative, emulsifier, acidulent), butylated hydroxytoluene (antioxidant), carbomer homopolymer type C (thickening agent, dispersing agent, biocompatible gel matrix), edetate disodium (chelating agent), laureth 4 (emulsifier, dissolution agent), methylparaben (preservative), propylene glycol (low-concentration humectant), purified water (diluent), and tromethamine (buffer, permeability enhancer).14

What are the clinical data evaluating the efficacy and tolerability/safety of the specific aqueous-based gel formulation of CP-Tret?
An aqueous-based gel formulation (referred to in the literature as a hydrogel) of CP-Tret is devoid of alcohol and contains the excipients described above.14 This formulation was shown to be efficacious, well tolerated, and safe in smaller clinical studies of participants with AV.23,37-39 Two large-scale phase 3 studies were completed (N=2219), powered to compare the efficacy and tolerability/safety of CP-Tret hydrogel (n=634) versus CP hydrogel (n=635), Tret hydrogel (n=635), and vehicle hydrogel (n=315) in participants with facial AV. All 4 study drug formulations in both studies—CP-Tret, CP, Tret, vehicle—used the same hydrogel vehicle, hereafter referred to simply as gel.16
In both trials, participants 12 years of age and older with AV were randomized to active drug groups versus vehicle (2:2:2:1 randomization), each applied once daily at bedtime for 12 weeks.16 The baseline demographics among all 4 study groups were well matched, with approximately two-thirds of white participants and one-third Asian (2%–3%), black (19%–21%), or Hispanic (9%–10%). Approximately half of enrolled participants were 16 years of age or younger (mean age [range], 19.0–20.2 years). Enrolled participants in each study group presented at baseline predominantly with facial AV of mild (grade 2 [20%–23% of enrolled participants]) or moderate (grade 3 [60%–62% of enrolled participants]) severity based on a protocol-mandated, 6-point investigator static global assessment scale. Investigator static global assessment scores and acne lesion counts, including noninflammatory (comedonal), inflammatory (papules, pustules), and total AV lesions, were evaluated at baseline and weeks 2, 4, 8, and 12 (end of study [EOS]). Among the 4 study groups at baseline, the range of mean lesion counts was 27.7 to 29.3 for noninflammatory lesions, 26.0 to 26.4 for inflammatory lesions, and 76.4 to 78.3 for total lesions. All enrolled participants met protocol-mandated, standardized, inclusion, exclusion, and prestudy washout period criteria.16
The primary efficacy end points determined based on intention-to-treat analysis were the percentage reduction in all 3 lesion counts at EOS compared to baseline and the proportion of participants who achieved scores of clear (grade 0) or almost clear (grade 1) at EOS. The secondary end point parameter was time to 50% reduction in total lesion counts.16
The study efficacy outcomes were as follows: The mean percentage reduction in inflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 53.4%; CP, 47.5%; Tret, 43.3%; vehicle, 30.3%)(P<.005).16 The mean percentage reduction in noninflammatory lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 45.2%; CP, 31.6%; Tret, 37.9%; vehicle, 18.5%)(P≤.0004). The mean percentage reduction in total AV lesions at EOS versus baseline was significantly higher in the CP-Tret group than in each of the other 3 groups (CP-Tret, 48.7%; CP, 38.3%; Tret, 40.3%; vehicle, 23.2%)(P≤.0001). The median time to 50% reduction in total AV lesion counts was significantly faster with CP-Tret (8 weeks) compared to the other 3 groups (CP, 12 weeks [P<.0001]; Tret, 12 weeks [P<.001]; vehicle, not reached by EOS [P<.0001]). The consistency of results, methodologies, and overall study characteristics between the 2 phase 3 RCTs allowed for accurate pooling of data.16
Tolerability and safety assessments were completed at each visit for all enrolled participants. No adverse events (AEs) were noted in approximately 90% of enrolled participants.16 The most common AEs noted over the course of the study were mild to moderate application-site reactions (eg, dryness, erythema, burning, pruritus, desquamation), mostly correlated with the 2 groups containing tretinoin—CP-Tret and Tret—which is not unanticipated with topical retinoid use; 1.2% of these participants withdrew from the study due to such application-site AEs. No serious AEs or systemic safety signals emerged during the study.16
What summarizing statements can be made about CP-Tret gel from these study results that may be helpful to clinicians treating patients with AV?
The gel formulation of CP-Tret incorporates active ingredients that target different pathophysiologic cascades in AV, providing antimicrobial, anti-inflammatory, and anticomedonal effects.
Applied once daily, CP-Tret gel demonstrated the ability to achieve complete or near-complete clearance of comedonal and papulopustular facial AV lesions of mild to moderate severity in approximately 40% of participants within 12 weeks of use in 2 large-scale RCTs.16 The ability to achieve a median 50% reduction in total lesions by 8 weeks of use provides relevant information for patients regarding reasonable expectations with therapy.
The favorable cutaneous tolerability profile and low number of AEs demonstrated with CP-Tret gel are major considerations, especially as skin tolerability reactions can impede patient adherence with treatment. Any issues that interfere with achieving a favorable therapeutic outcome can lead to patients giving up with their therapy.
The large number of patients with skin of color treated with CP-Tret gel (n=209) in the 2 phase 3 RCTs is important, as the spectrum of racial origins, skin types, and skin colors seen in dermatology practices is diversifying across the United States. Both clinicians and patients with skin of color are often concerned about the sequelae of medication-induced skin irritation, which can lead to ensuing dyschromia.
Concerns related to potential development of clindamycin-resistant P acnes with CP-Tret gel may be addressed by concurrent use of BP, including with leave-on or short-contact therapy.
Although phase 3 RCTs evaluate therapeutic agents as monotherapy, in real world clinical practice, combination topical regimens using different individual products are common to optimize therapeutic outcomes. Advantages of the CP-Tret gel formulation, if a clinician desires to use it along with another topical product, are once-daily use and the low risk for cutaneous irritation.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
- Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from the Global Alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(suppl 1):S1-S37.
- Hui AM, Shalita AR. Topical retinoids. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:86-94.
- Del Rosso JQ. Topical antibiotics. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:95-104.
- Sami N. Topical retinoids. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:505-517.
- Baldwin HE, Nighland M, Kendall C, et al. 40 years of topical tretinoin use in review. J Drugs Dermatol. 2013;12:638-642.
- Retin-A Micro [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2015.
- Tazorac [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Differin [package insert]. Fort Worth, TX: Galderma Laboratories, LP; 2011.
- Kang S. The mechanism of action of topical retinoids. Cutis. 2005;75(suppl 2):10-13; discussion 13.
- Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013:445-459.
- Leyden JJ. The evolving role of Propionibacterium acnes in acne. Semin Cutan Med Surg. 2001;20:139-143.
- Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: focus on antibiotic resistance. Cutis. 2007;79(suppl 6):9-25.
- Ziana [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2016.
- Veltin [package insert]. Exton, PA: Aqua Pharmaceuticals; 2015.
- Ochsendorf F. Clindamycin phosphate 1.2%/tretinoin 0.025%: a novel fixed-dose combination treatment for acne vulgaris. J Eur Acad Dermatol Venereol. 2015;29(suppl 5):8-13.
- Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:73-81.
- Del Rosso JQ. Topical and oral antibiotics for acne vulgaris. Semin Cutan Med Surg. 2016;35:57-61.
- Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49(suppl 6A):8-11.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15-24.
- Simonart T, Dramaix M. Treatment of acne with topical antibiotics: lessons from clinical studies. Br J Dermatol. 2005;153:395-403.
- Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498-505.
- Del Rosso JQ. Topical therapy for acne in women: is there a role for clindamycin phosphate-benzoyl peroxide gel? Cutis. 2014;94:177-182.
- Del Rosso JQ, Zeichner JA. The clinical relevance of antibiotic resistance: thirteen principles that every dermatologist needs to consider when prescribing antibiotic therapy. Dermatol Clin. 2016;34:167-173.
- Leyden JJ. Antibiotic resistance in the topical treatment of acne vulgaris. Cutis. 2004;73(6 suppl):6-10.
- Del Rosso JQ, Webster GF, Rosen T, et al. Status report from the Scientific Panel on Antibiotic Use in Dermatology of the American Acne and Rosacea Society: part 1: antibiotic prescribing patterns, sources of antibiotic exposure, antibiotic consumption and emergence of antibiotic resistance, impact of alterations in antibiotic prescribing, and clinical sequelae of antibiotic use. J Clin Aesthet Dermatol. 2016;9:18-24.
- Layton AM. Top ten list of clinical pearls in the treatment of acne vulgaris. Dermatol Clin. 2016;34:147-157.
- Leyden JJ. In vivo antibacterial effects of tretinoin-clindamycin and clindamycin alone on Propionibacterium acnes with varying clindamycin minimum inhibitory. J Drugs Dermatol. 2012;11:1434-1438.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24:1117-1133.
- Villasenor J, Berson DS, Kroshinsky D. Combination therapy. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:105-112.
- Feneran A, Kaufman WS, Dabade TS, et al. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79-92.
- Leyden JJ, Del Rosso JQ. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830-833.
- Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol. 2005;4:41-47.
- Del Rosso JQ. Retinoic acid receptors and topical acne therapy: establishing the link between gene expression and drug efficacy. Cutis. 2002;70:127-129.
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015-1021.
- Richter JR, Bousema MT, DeBoulle KLVM, et al. Efficacy of fixed clindamycin 1.2%, tretinoin 0.025% gel formulation (Velac) in topical control of facial acne lesions. J Dermatol Treat. 1998;9:81-90.
- Richter JR, Fӧrstrӧm LR, Kiistala UO, et al. Efficacy of fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Dermatol Venereol. 1998;11:227-233.
- Cambazard F. Clinical efficacy of Velac, a new tretinoin and clindamycin gel in acne vulgaris. J Eur Acad Dermatol Venereol. 1998;11(suppl 1):S20-S27; discussion S28-S29.
Practice Points
- Clindamycin phosphate (CP)–tretinoin (Tret) formulated in an aqueous gel is effective based on clinical trials of the management of acne vulgaris (AV).
- The favorable tolerability of CP-Tret gel is advantageous, as topical agents often are used in combination with other therapies to treat AV, especially with a benzoyl peroxide–containing product.
- The availability of 2 active agents in 1 formulation is likely to optimize compliance.
Genital Wart Treatment
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
What does your patient need to know?
When a patient presents with a history of genital warts (GWs), find out when and where the lesions started; where the lesions are currently located; what new lesions have developed; what treatments have been administered (eg, physician applied, prescription) and which one(s) worked; what side effects to treatments have been experienced and at what dose; does a partner(s) have similar lesions; is there a history of other sexually transmitted diseases or genital cancer; is he/she immunocompromised (eg, human immunodeficiency virus, transplant, medications); and what is his/her sexual orientation.
Once all of the information has been gathered and the entire anogenital region has been examined, a treatment plan can be formulated. If the patient is immunocompromised or is a man who has sex with men, the risk for anogenital malignancy due to human papillomavirus (HPV) is higher, and GWs, which can be coinfected with oncogenic HPV types, should be treated more aggressively. If the patient is still getting new lesions, use of only a destructive method such as cryotherapy will likely lead to suboptimal results.
Any patients with GWs in the anal region but particularly those in high-risk groups such as men who have sex with men and human immunodeficiency virus–infected patients should have an anoscopy to evaluate for lesions on the anal mucosa and in the rectum.
What are your go-to treatments?
Prior treatments need to be taken into account; make sure to understand any side effects and how he/she applied the prior treatment before eliminating it as a viable option. Treatment usually depends on the number of lesions, surface area, anatomic locations involved, and size of the lesions. I start with a 2-pronged approach—a debulking therapy and a patient-applied topical therapy—which allows me to physically remove some of the lesions, typically the larger ones, and then have the patient apply a topical medication at home that will treat the smaller lesions as well as help to clear or decrease the burden of HPV virus on the skin. I use cryotherapy as a debulking agent, but curettage or podophyllin 25% also can be used in the office. I use imiquimod cream 5% as a first-line topical agent at the recommended dose of 3 times weekly; however, if after the first 2 weeks the patient has little response or too much irritation, I titrate the dose so that the patient has mild inflammation on the skin. The dose ultimately can range from daily to once weekly. Some patients who can only tolerate imiquimod once or twice weekly may require zinc oxide paste for the inguinal folds and scrotum to protect from irritation. Alternate topical medications for GWs include sinecatechins ointment 15% or cidofovir ointment 2%.
How do you keep patients compliant?
Start the visit with open communication about the disease, where it came from, what the risks are if it is not treated, and how we can best treat it to make sure we minimize those risks. I explain all of the treatment options as well as our role in treating these lesions and minimizing the risk for disease progression.
What do you do if they refuse treatment?
Most patients with GWs are motivated to be treated. If pain is a concern, such as with cryotherapy, I recommend topical treatments.
What patient resources do you recommend?
The American Academy of Dermatology (https://www.aad.org/public/diseases/contagious-skin-diseases/genital-warts), Harvard Medical School patient education center (Boston, Massachusetts)(http://www.patienteducationcenter.org/articles/genital-warts/), and American Family Physician (http://www.aafp.org/afp/2004/1215/p2345.html) provide patient materials that I recommend.
One Diagnosis and Modifier -25: Appropriate or Audit Target?
An established patient comes into your office with a painful new lesion on the hand. He thinks it may be a wart. You take a focused history of the lesion, do a physical examination, and confirm the diagnosis of verruca vulgaris. You discuss treatment options, risks, and the benefits of treatment, as well as the pathophysiology of warts. The decision is made to proceed that same day with cryosurgical destruction, which is performed. You feel that billing both an office visit with an appended modifier -25 and the benign destruction code 17110 is warranted, but your biller says only the procedure should be reported. Who is correct?
Modifier -25 use has come under increased scrutiny by insurers and regulators. There is a perception that this modifier is frequently used inappropriately or unnecessarily. In fact, the Office of Inspector General reported that 35% of claims using modifier -25 that Medicare allowed did not meet the requirements. The Office of Inspector General has recommended that the “[Centers for Medicare & Medicaid Services] should work with carriers to reduce the number of claims submitted using modifier -25” and “include modifier -25 reviews in their medical review strategies.”1 Translation: More chart reviews and audits! In my discussions with insurer medical directors, they point to the single diagnosis modifier -25 as likely abused and feel that its use in this context is almost never appropriate. Their audits have been focused on this aspect of dermatologists’ coding. In addition, some private insurers have started to discount reimbursement for office visits billed with modifier -25 by 50% to account for the level of perceived overuse.2
The Current Procedural Terminology description of modifier -25 is relatively clear: Modifier -25 is used to facilitate billing of evaluation and management (E/M) services on the day of a procedure for which separate payment may be made.3 This modifier indicates that a significant, separately identifiable E/M service was performed by the same physician on the day of a procedure. To appropriately bill both the E/M service and the procedure, the physician must indicate that the patient’s condition required an E/M service “above and beyond the usual pre- and post-operative work of a procedure.”4 However, it is largely left up to the physician to decide what constitutes the significant, separately identifiable E/M service.
As dermatologists, we all report modifier -25 appropriately as part of our daily practice. Performance of a medically necessary procedure on the same day as an E/M service generally is done to facilitate a prompt diagnosis or streamline treatment of a complex condition. Providing distinct medically necessary services on the same date allows physicians to provide effective and efficient high-quality care, in many cases saving patients a return visit. The most common scenario for using modifier -25 involves multiple concerns and multiple diagnoses, some of which are not associated with a procedure(s) that is performed on the same date of service. With multiple diagnoses, it is straightforward to demonstrate the separate E/M service associated with the nonprocedure-related diagnosis code(s); however, with one diagnosis for both the office visit and the procedure, clear documentation of the separate and identifiable E/M service is critical and is dependent on understanding what is included in the global surgical package.
Insurer payment for procedures includes local or topical anesthesia, the surgical service/procedure itself, immediate postoperative care including dictating the operative note, meeting/discussing the patient’s procedure with family and other physicians, evaluating the patient in postanesthesia/recovery area, and writing orders for the patient. This group of services is called the global surgical package. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M associated with the decision to perform surgery. An appropriate history and physical examination, as well as the discussion of differential diagnosis, treatment options, and risk and benefits of treatments, are all included in the payment of a minor procedure itself. Therefore, if an E/M service is performed on the same day as a minor procedure to decide whether to proceed with the minor surgical procedure, this E/M service cannot be separately reported. Moreover, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M service with such a minor procedure. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
Therefore, it is clear that the clinical scenario for verruca vulgaris treatment as described at the start of this article does not meet criteria for an office visit billed in addition to the destruction. The E/M services performed prior to the patient’s verruca vulgaris treatment are integral to and necessary for the decision to perform the procedure. Making and confirming the diagnosis of a condition or lesion prior to a procedure either by physical evaluation or by interpretation of a pathology report is part of the evaluation required to make the decision to proceed with a particular procedure.
There are clinical scenarios in which a physician can support additional E/M services beyond that of the procedure with just one diagnosis. If a patient presents with warts on the hand and face with resultant cryosurgical destruction done on the hand and a prescription for imiquimod to be used on the face to induce immunologic clearance of viral infection and decrease the risk of scarring, it is clear that a significant and separately identifiable E/M service exists. The evaluation of the facial warts and the prescription of medication and discussion of the risks, benefits, and therapeutic effects of that prescription is definitely distinct from the procedure. Similarly, if an evaluation of a patient with a rash results in only a diagnostic biopsy with no separate cognitive services other than the decision to perform the biopsy, an office visit charge in addition to the biopsy charge would not be warranted. However, if in addition to the biopsy the rash also is treated with topical or systemic steroids because of pruritus or a more extensive evaluation for systemic complications is required, an office visit charge is appropriate.
The frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of E/M services and minor procedures allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. However, modifier -25 use, particularly in the context of the same diagnosis for the office visit and the procedure, is under intense insurer scrutiny. Careful and complete documentation of the additional E/M service provided, including the additional history, physical examination results, and treatment considerations above and beyond those typically required by the minor procedure, will reduce the likelihood of redeterminations from reviews and audits. Understanding Medicare guidelines and National Correct Coding Initiative recommendations will help keep the dermatologist out of hot water.5
- Levinson DR. Use of modifier 25. Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Published November 2005. Accessed January 31, 2017.
- Modifier tables. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-tables-payment-policy. Revised April 2016. Accessed February 24, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Updated November 1, 2012. Accessed January 31, 2017.
- National Correct Coding Initiative Policy Manual for Medicare Services—Effective January 1, 2017. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/Downloads/2017-NCCI-Policy-Manual.zip. Accessed February 24, 2017.
An established patient comes into your office with a painful new lesion on the hand. He thinks it may be a wart. You take a focused history of the lesion, do a physical examination, and confirm the diagnosis of verruca vulgaris. You discuss treatment options, risks, and the benefits of treatment, as well as the pathophysiology of warts. The decision is made to proceed that same day with cryosurgical destruction, which is performed. You feel that billing both an office visit with an appended modifier -25 and the benign destruction code 17110 is warranted, but your biller says only the procedure should be reported. Who is correct?
Modifier -25 use has come under increased scrutiny by insurers and regulators. There is a perception that this modifier is frequently used inappropriately or unnecessarily. In fact, the Office of Inspector General reported that 35% of claims using modifier -25 that Medicare allowed did not meet the requirements. The Office of Inspector General has recommended that the “[Centers for Medicare & Medicaid Services] should work with carriers to reduce the number of claims submitted using modifier -25” and “include modifier -25 reviews in their medical review strategies.”1 Translation: More chart reviews and audits! In my discussions with insurer medical directors, they point to the single diagnosis modifier -25 as likely abused and feel that its use in this context is almost never appropriate. Their audits have been focused on this aspect of dermatologists’ coding. In addition, some private insurers have started to discount reimbursement for office visits billed with modifier -25 by 50% to account for the level of perceived overuse.2
The Current Procedural Terminology description of modifier -25 is relatively clear: Modifier -25 is used to facilitate billing of evaluation and management (E/M) services on the day of a procedure for which separate payment may be made.3 This modifier indicates that a significant, separately identifiable E/M service was performed by the same physician on the day of a procedure. To appropriately bill both the E/M service and the procedure, the physician must indicate that the patient’s condition required an E/M service “above and beyond the usual pre- and post-operative work of a procedure.”4 However, it is largely left up to the physician to decide what constitutes the significant, separately identifiable E/M service.
As dermatologists, we all report modifier -25 appropriately as part of our daily practice. Performance of a medically necessary procedure on the same day as an E/M service generally is done to facilitate a prompt diagnosis or streamline treatment of a complex condition. Providing distinct medically necessary services on the same date allows physicians to provide effective and efficient high-quality care, in many cases saving patients a return visit. The most common scenario for using modifier -25 involves multiple concerns and multiple diagnoses, some of which are not associated with a procedure(s) that is performed on the same date of service. With multiple diagnoses, it is straightforward to demonstrate the separate E/M service associated with the nonprocedure-related diagnosis code(s); however, with one diagnosis for both the office visit and the procedure, clear documentation of the separate and identifiable E/M service is critical and is dependent on understanding what is included in the global surgical package.
Insurer payment for procedures includes local or topical anesthesia, the surgical service/procedure itself, immediate postoperative care including dictating the operative note, meeting/discussing the patient’s procedure with family and other physicians, evaluating the patient in postanesthesia/recovery area, and writing orders for the patient. This group of services is called the global surgical package. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M associated with the decision to perform surgery. An appropriate history and physical examination, as well as the discussion of differential diagnosis, treatment options, and risk and benefits of treatments, are all included in the payment of a minor procedure itself. Therefore, if an E/M service is performed on the same day as a minor procedure to decide whether to proceed with the minor surgical procedure, this E/M service cannot be separately reported. Moreover, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M service with such a minor procedure. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
Therefore, it is clear that the clinical scenario for verruca vulgaris treatment as described at the start of this article does not meet criteria for an office visit billed in addition to the destruction. The E/M services performed prior to the patient’s verruca vulgaris treatment are integral to and necessary for the decision to perform the procedure. Making and confirming the diagnosis of a condition or lesion prior to a procedure either by physical evaluation or by interpretation of a pathology report is part of the evaluation required to make the decision to proceed with a particular procedure.
There are clinical scenarios in which a physician can support additional E/M services beyond that of the procedure with just one diagnosis. If a patient presents with warts on the hand and face with resultant cryosurgical destruction done on the hand and a prescription for imiquimod to be used on the face to induce immunologic clearance of viral infection and decrease the risk of scarring, it is clear that a significant and separately identifiable E/M service exists. The evaluation of the facial warts and the prescription of medication and discussion of the risks, benefits, and therapeutic effects of that prescription is definitely distinct from the procedure. Similarly, if an evaluation of a patient with a rash results in only a diagnostic biopsy with no separate cognitive services other than the decision to perform the biopsy, an office visit charge in addition to the biopsy charge would not be warranted. However, if in addition to the biopsy the rash also is treated with topical or systemic steroids because of pruritus or a more extensive evaluation for systemic complications is required, an office visit charge is appropriate.
The frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of E/M services and minor procedures allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. However, modifier -25 use, particularly in the context of the same diagnosis for the office visit and the procedure, is under intense insurer scrutiny. Careful and complete documentation of the additional E/M service provided, including the additional history, physical examination results, and treatment considerations above and beyond those typically required by the minor procedure, will reduce the likelihood of redeterminations from reviews and audits. Understanding Medicare guidelines and National Correct Coding Initiative recommendations will help keep the dermatologist out of hot water.5
An established patient comes into your office with a painful new lesion on the hand. He thinks it may be a wart. You take a focused history of the lesion, do a physical examination, and confirm the diagnosis of verruca vulgaris. You discuss treatment options, risks, and the benefits of treatment, as well as the pathophysiology of warts. The decision is made to proceed that same day with cryosurgical destruction, which is performed. You feel that billing both an office visit with an appended modifier -25 and the benign destruction code 17110 is warranted, but your biller says only the procedure should be reported. Who is correct?
Modifier -25 use has come under increased scrutiny by insurers and regulators. There is a perception that this modifier is frequently used inappropriately or unnecessarily. In fact, the Office of Inspector General reported that 35% of claims using modifier -25 that Medicare allowed did not meet the requirements. The Office of Inspector General has recommended that the “[Centers for Medicare & Medicaid Services] should work with carriers to reduce the number of claims submitted using modifier -25” and “include modifier -25 reviews in their medical review strategies.”1 Translation: More chart reviews and audits! In my discussions with insurer medical directors, they point to the single diagnosis modifier -25 as likely abused and feel that its use in this context is almost never appropriate. Their audits have been focused on this aspect of dermatologists’ coding. In addition, some private insurers have started to discount reimbursement for office visits billed with modifier -25 by 50% to account for the level of perceived overuse.2
The Current Procedural Terminology description of modifier -25 is relatively clear: Modifier -25 is used to facilitate billing of evaluation and management (E/M) services on the day of a procedure for which separate payment may be made.3 This modifier indicates that a significant, separately identifiable E/M service was performed by the same physician on the day of a procedure. To appropriately bill both the E/M service and the procedure, the physician must indicate that the patient’s condition required an E/M service “above and beyond the usual pre- and post-operative work of a procedure.”4 However, it is largely left up to the physician to decide what constitutes the significant, separately identifiable E/M service.
As dermatologists, we all report modifier -25 appropriately as part of our daily practice. Performance of a medically necessary procedure on the same day as an E/M service generally is done to facilitate a prompt diagnosis or streamline treatment of a complex condition. Providing distinct medically necessary services on the same date allows physicians to provide effective and efficient high-quality care, in many cases saving patients a return visit. The most common scenario for using modifier -25 involves multiple concerns and multiple diagnoses, some of which are not associated with a procedure(s) that is performed on the same date of service. With multiple diagnoses, it is straightforward to demonstrate the separate E/M service associated with the nonprocedure-related diagnosis code(s); however, with one diagnosis for both the office visit and the procedure, clear documentation of the separate and identifiable E/M service is critical and is dependent on understanding what is included in the global surgical package.
Insurer payment for procedures includes local or topical anesthesia, the surgical service/procedure itself, immediate postoperative care including dictating the operative note, meeting/discussing the patient’s procedure with family and other physicians, evaluating the patient in postanesthesia/recovery area, and writing orders for the patient. This group of services is called the global surgical package. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M associated with the decision to perform surgery. An appropriate history and physical examination, as well as the discussion of differential diagnosis, treatment options, and risk and benefits of treatments, are all included in the payment of a minor procedure itself. Therefore, if an E/M service is performed on the same day as a minor procedure to decide whether to proceed with the minor surgical procedure, this E/M service cannot be separately reported. Moreover, the fact that the patient is new to the physician is not sufficient to allow reporting of an E/M service with such a minor procedure. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
Therefore, it is clear that the clinical scenario for verruca vulgaris treatment as described at the start of this article does not meet criteria for an office visit billed in addition to the destruction. The E/M services performed prior to the patient’s verruca vulgaris treatment are integral to and necessary for the decision to perform the procedure. Making and confirming the diagnosis of a condition or lesion prior to a procedure either by physical evaluation or by interpretation of a pathology report is part of the evaluation required to make the decision to proceed with a particular procedure.
There are clinical scenarios in which a physician can support additional E/M services beyond that of the procedure with just one diagnosis. If a patient presents with warts on the hand and face with resultant cryosurgical destruction done on the hand and a prescription for imiquimod to be used on the face to induce immunologic clearance of viral infection and decrease the risk of scarring, it is clear that a significant and separately identifiable E/M service exists. The evaluation of the facial warts and the prescription of medication and discussion of the risks, benefits, and therapeutic effects of that prescription is definitely distinct from the procedure. Similarly, if an evaluation of a patient with a rash results in only a diagnostic biopsy with no separate cognitive services other than the decision to perform the biopsy, an office visit charge in addition to the biopsy charge would not be warranted. However, if in addition to the biopsy the rash also is treated with topical or systemic steroids because of pruritus or a more extensive evaluation for systemic complications is required, an office visit charge is appropriate.
The frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of E/M services and minor procedures allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. However, modifier -25 use, particularly in the context of the same diagnosis for the office visit and the procedure, is under intense insurer scrutiny. Careful and complete documentation of the additional E/M service provided, including the additional history, physical examination results, and treatment considerations above and beyond those typically required by the minor procedure, will reduce the likelihood of redeterminations from reviews and audits. Understanding Medicare guidelines and National Correct Coding Initiative recommendations will help keep the dermatologist out of hot water.5
- Levinson DR. Use of modifier 25. Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Published November 2005. Accessed January 31, 2017.
- Modifier tables. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-tables-payment-policy. Revised April 2016. Accessed February 24, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Updated November 1, 2012. Accessed January 31, 2017.
- National Correct Coding Initiative Policy Manual for Medicare Services—Effective January 1, 2017. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/Downloads/2017-NCCI-Policy-Manual.zip. Accessed February 24, 2017.
- Levinson DR. Use of modifier 25. Office of Inspector General website. https://oig.hhs.gov/oei/reports/oei-07-03-00470.pdf. Published November 2005. Accessed January 31, 2017.
- Modifier tables. Tufts Health Plan website. https://tuftshealthplan.com/documents/providers/payment-policies/modifier-tables-payment-policy. Revised April 2016. Accessed February 24, 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago, IL: American Medical Association; 2016.
- Centers for Medicare & Medicaid Services. Payment for evaluation and management services provided during global period of surgery. MLN Matters. May 19, 2006. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM5025.pdf. Updated November 1, 2012. Accessed January 31, 2017.
- National Correct Coding Initiative Policy Manual for Medicare Services—Effective January 1, 2017. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/Downloads/2017-NCCI-Policy-Manual.zip. Accessed February 24, 2017.
Practice Points
- Modifier -25 use is appropriate and critical for high-quality, efficient dermatology care.
- Correct use and documentation can help avoid loss of audits associated with modifier -25.
Erythematous Pearly Papule on the Chest
Primary Cutaneous B-cell Lymphoma
Cutaneous B-cell lymphomas (CBCLs) are a diverse but rare group of cutaneous lymphoproliferative neoplasms that make up approximately 20% of the total number of hematolymphoid neoplasms primary to the skin.1 These lymphomas are comprised of neoplastic B cells in various stages of differentiation. As a whole, they are rare neoplasms that primarily involve the head, neck, trunk, arms, or legs.1 Clinically, patients present with nontender, compressible, solitary, red to violaceous papules or nodules. Most CBCLs are considered low-grade malignancies with nonaggressive behavior and excellent prognosis; however, the diffuse large B-cell lymphomas, including but not limited to intravascular and leg type; lymphomatoid granulomatosis; and B-cell lymphoblastic lymphoma can act more aggressively.1
Histopathologic examination of primary CBCL generally reveals a relatively normal epidermis accompanied by a nodular to diffuse monomorphic lymphocytic cellular infiltrate in the dermis that can occasionally extend into the subcutaneous tissue (quiz image). Although not specific for CBCLs, oftentimes there is an acellular portion of the superficial papillary dermis known as a grenz zone that can serve as a histopathologic clue to the diagnosis of a cutaneous lymphoproliferative disorder. The list of malignant B-cell neoplasms is extensive (eg, cutaneous marginal zone B-cell lymphoma, primary cutaneous follicle center lymphoma, diffuse large B-cell lymphoma, intravascular large B-cell lymphoma), and few are seen in the skin.
The most common type of CBCL is marginal zone B-cell lymphoma, which is considered to be a tumor of mucosa-associated (or skin-associated) lymphoid tissue. It is characterized by a monomorphous population of small mature lymphocytes showing characteristics of the B cells of the marginal zone of the lymph node. Some cells have the features of centrocytes/centroblasts (Figure 1) demonstrated by slightly irregular or indented nuclei and generous amounts of cytoplasm. Larger and more pleomorphic cells such as immunoblasts are similarly noted (Figure 1). The quiz image and Figure 1 demonstrate a cutaneous marginal zone B-cell lymphoma. A histomorphologic clue supporting a diagnosis of marginal zone B-cell lymphoma over reactive lymphoid hyperplasia is a B-cell predominate (B- to T-cell ratio of at least 3 to 1) infiltrate that is comprised of marginal zone-type cells. Immunohistochemistry demonstrating fewer differentiated B cells with light chain restriction may provide additional evidence that supports a clonal and potentially malignant process.
Erythematous to violaceous nodules on the head and neck of older individuals are characteristic of both granuloma faciale and CBCL. Histologically, granuloma faciale is characterized by a dense cellular infiltrate, often with a nodular outline, occupying the mid dermis.2 Granuloma faciale typically spares the immediate subepidermis and hair follicles, forming a grenz zone. The cellular infiltrate is polymorphic and consists of eosinophils and neutrophils with scattered plasma cells, mast cells, and lymphocytes in a vasculocentric distribution, eventually with chronic concentric fibrosis (Figure 2).
Leukemia cutis demonstrates a dermal infiltrate that contains atypical mononuclear cells (myeloblasts and myelocytes)(Figure 3).3 These markedly atypical mononuclear cells can have kidney bean-shaped nuclei and percolate through the dermal collagen, resembling single-file cells. They have increased nuclear to cytoplasmic ratios and occasionally have prominent nucleoli. Correlation with immunophenotypic and cytochemical studies is required for specific typing of the leukemic infiltrate.
Similar to primary CBCL, lymphomatoid papulosis (LyP) consists of erythematous papules or nodules that can occur anywhere on the body. In contrast to CBCL, the lesions of LyP classically self-resolve. However, approximately 10% to 20% of patients develop a malignant lymphoma, with mycosis fungoides, Hodgkin disease, and anaplastic large cell lymphoma being the most commonly associated.
Histologic examination of lesions of LyP classically demonstrates a wedge-shaped dermal infiltrate with variable epidermal changes (Figure 4). The wedge-shaped infiltrate is composed of large atypical cells. Three main types of lesions have been delineated: types A, B, and C. Type A is characterized by an increased number of cells with large vesicular nuclei with clumped chromatin, prominent nucleoli, and pronounced cytoplasm. Reed-Sternberg-like cells with an admixture of inflammatory cells including small lymphocytes, macrophages, neutrophils, and eosinophils also are present. Type B neoplastic cells vary in size and feature hyperchromatic, convoluted, or cerebriform nuclei. The infiltrate can be dense and bandlike with fewer cells resembling mycosis fungoides; type B LyP has neoplastic cells, not inflammatory cells. Finally, type C demonstrates solid sheets of large atypical cells resembling anaplastic large cell lymphoma. Immunohistochemically, the atypical cells often are CD4+ and CD8- with variable loss of pan-T-cell antigens. The atypical cells of types A and C express CD30 reactivity.4
Merkel cell carcinoma (MCC) is a primary neuroendocrine carcinoma of the skin that usually arises on sun-exposed skin in elderly patients with lesions that histologically and clinically resemble cutaneous lymphoma.5 It classically is composed of small, round to oval, basophilic cells with a vesicular nucleus and multiple small nucleoli. Apoptotic cells and mitoses often are present.6 One key finding that helps to differentiate MCC from lymphoma is the presence of finely dispersed salt-and-pepper chromatin and molded nuclear contour in MCC (Figure 5).
Immunophenotyping is important in the differentiation of these diagnoses. The atypical cells of LyP are positive for CD3, CD4, and CD30 but are negative for CD8. However, in type B LyP, the large CD30+ cells seen in the other types are not commonly seen. In contrast, MCC expresses reactivity with cytokeratins, in particular cytokeratin 20 and CAM5.2, classically in a paranuclear dotlike pattern. In keeping with MCC's neuroendocrine differentiation, the tumor cells will demonstrate reactivity with synaptophysin, chromogranin, and CD56. The immunohistochemistry for leukemia cutis varies depending on the type of leukemia. Acute myelomonocytic leukemia is positive for myeloperoxidase, CD13, CD33, and CD68. The immunophenotype of these marginal zone lymphoma cells is as follows: positive for CD20, CD79a, and Bcl-2; negative for Bcl-6, CD5, CD10, CD23, and cyclin D1 (Bcl-1).7
- Olsen EA. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin. 2015;33:643-654.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Wieser I, Wohlmuth C, Nunez CA, et al. Lymphomatoid papulosis in children and adolescents: a systematic review. Am J Clin Dermatol. 2016;17:319-327.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin: I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Frigerio B, Capella C, Eusebi V, et al. Merkel cell carcinoma of the skin: the structure and origin of normal Merkel cells. Histopathology. 1983;7:229-249.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
Primary Cutaneous B-cell Lymphoma
Cutaneous B-cell lymphomas (CBCLs) are a diverse but rare group of cutaneous lymphoproliferative neoplasms that make up approximately 20% of the total number of hematolymphoid neoplasms primary to the skin.1 These lymphomas are comprised of neoplastic B cells in various stages of differentiation. As a whole, they are rare neoplasms that primarily involve the head, neck, trunk, arms, or legs.1 Clinically, patients present with nontender, compressible, solitary, red to violaceous papules or nodules. Most CBCLs are considered low-grade malignancies with nonaggressive behavior and excellent prognosis; however, the diffuse large B-cell lymphomas, including but not limited to intravascular and leg type; lymphomatoid granulomatosis; and B-cell lymphoblastic lymphoma can act more aggressively.1
Histopathologic examination of primary CBCL generally reveals a relatively normal epidermis accompanied by a nodular to diffuse monomorphic lymphocytic cellular infiltrate in the dermis that can occasionally extend into the subcutaneous tissue (quiz image). Although not specific for CBCLs, oftentimes there is an acellular portion of the superficial papillary dermis known as a grenz zone that can serve as a histopathologic clue to the diagnosis of a cutaneous lymphoproliferative disorder. The list of malignant B-cell neoplasms is extensive (eg, cutaneous marginal zone B-cell lymphoma, primary cutaneous follicle center lymphoma, diffuse large B-cell lymphoma, intravascular large B-cell lymphoma), and few are seen in the skin.
The most common type of CBCL is marginal zone B-cell lymphoma, which is considered to be a tumor of mucosa-associated (or skin-associated) lymphoid tissue. It is characterized by a monomorphous population of small mature lymphocytes showing characteristics of the B cells of the marginal zone of the lymph node. Some cells have the features of centrocytes/centroblasts (Figure 1) demonstrated by slightly irregular or indented nuclei and generous amounts of cytoplasm. Larger and more pleomorphic cells such as immunoblasts are similarly noted (Figure 1). The quiz image and Figure 1 demonstrate a cutaneous marginal zone B-cell lymphoma. A histomorphologic clue supporting a diagnosis of marginal zone B-cell lymphoma over reactive lymphoid hyperplasia is a B-cell predominate (B- to T-cell ratio of at least 3 to 1) infiltrate that is comprised of marginal zone-type cells. Immunohistochemistry demonstrating fewer differentiated B cells with light chain restriction may provide additional evidence that supports a clonal and potentially malignant process.

Erythematous to violaceous nodules on the head and neck of older individuals are characteristic of both granuloma faciale and CBCL. Histologically, granuloma faciale is characterized by a dense cellular infiltrate, often with a nodular outline, occupying the mid dermis.2 Granuloma faciale typically spares the immediate subepidermis and hair follicles, forming a grenz zone. The cellular infiltrate is polymorphic and consists of eosinophils and neutrophils with scattered plasma cells, mast cells, and lymphocytes in a vasculocentric distribution, eventually with chronic concentric fibrosis (Figure 2).

Leukemia cutis demonstrates a dermal infiltrate that contains atypical mononuclear cells (myeloblasts and myelocytes)(Figure 3).3 These markedly atypical mononuclear cells can have kidney bean-shaped nuclei and percolate through the dermal collagen, resembling single-file cells. They have increased nuclear to cytoplasmic ratios and occasionally have prominent nucleoli. Correlation with immunophenotypic and cytochemical studies is required for specific typing of the leukemic infiltrate.

Similar to primary CBCL, lymphomatoid papulosis (LyP) consists of erythematous papules or nodules that can occur anywhere on the body. In contrast to CBCL, the lesions of LyP classically self-resolve. However, approximately 10% to 20% of patients develop a malignant lymphoma, with mycosis fungoides, Hodgkin disease, and anaplastic large cell lymphoma being the most commonly associated.
Histologic examination of lesions of LyP classically demonstrates a wedge-shaped dermal infiltrate with variable epidermal changes (Figure 4). The wedge-shaped infiltrate is composed of large atypical cells. Three main types of lesions have been delineated: types A, B, and C. Type A is characterized by an increased number of cells with large vesicular nuclei with clumped chromatin, prominent nucleoli, and pronounced cytoplasm. Reed-Sternberg-like cells with an admixture of inflammatory cells including small lymphocytes, macrophages, neutrophils, and eosinophils also are present. Type B neoplastic cells vary in size and feature hyperchromatic, convoluted, or cerebriform nuclei. The infiltrate can be dense and bandlike with fewer cells resembling mycosis fungoides; type B LyP has neoplastic cells, not inflammatory cells. Finally, type C demonstrates solid sheets of large atypical cells resembling anaplastic large cell lymphoma. Immunohistochemically, the atypical cells often are CD4+ and CD8- with variable loss of pan-T-cell antigens. The atypical cells of types A and C express CD30 reactivity.4

Merkel cell carcinoma (MCC) is a primary neuroendocrine carcinoma of the skin that usually arises on sun-exposed skin in elderly patients with lesions that histologically and clinically resemble cutaneous lymphoma.5 It classically is composed of small, round to oval, basophilic cells with a vesicular nucleus and multiple small nucleoli. Apoptotic cells and mitoses often are present.6 One key finding that helps to differentiate MCC from lymphoma is the presence of finely dispersed salt-and-pepper chromatin and molded nuclear contour in MCC (Figure 5).

Immunophenotyping is important in the differentiation of these diagnoses. The atypical cells of LyP are positive for CD3, CD4, and CD30 but are negative for CD8. However, in type B LyP, the large CD30+ cells seen in the other types are not commonly seen. In contrast, MCC expresses reactivity with cytokeratins, in particular cytokeratin 20 and CAM5.2, classically in a paranuclear dotlike pattern. In keeping with MCC's neuroendocrine differentiation, the tumor cells will demonstrate reactivity with synaptophysin, chromogranin, and CD56. The immunohistochemistry for leukemia cutis varies depending on the type of leukemia. Acute myelomonocytic leukemia is positive for myeloperoxidase, CD13, CD33, and CD68. The immunophenotype of these marginal zone lymphoma cells is as follows: positive for CD20, CD79a, and Bcl-2; negative for Bcl-6, CD5, CD10, CD23, and cyclin D1 (Bcl-1).7
Primary Cutaneous B-cell Lymphoma
Cutaneous B-cell lymphomas (CBCLs) are a diverse but rare group of cutaneous lymphoproliferative neoplasms that make up approximately 20% of the total number of hematolymphoid neoplasms primary to the skin.1 These lymphomas are comprised of neoplastic B cells in various stages of differentiation. As a whole, they are rare neoplasms that primarily involve the head, neck, trunk, arms, or legs.1 Clinically, patients present with nontender, compressible, solitary, red to violaceous papules or nodules. Most CBCLs are considered low-grade malignancies with nonaggressive behavior and excellent prognosis; however, the diffuse large B-cell lymphomas, including but not limited to intravascular and leg type; lymphomatoid granulomatosis; and B-cell lymphoblastic lymphoma can act more aggressively.1
Histopathologic examination of primary CBCL generally reveals a relatively normal epidermis accompanied by a nodular to diffuse monomorphic lymphocytic cellular infiltrate in the dermis that can occasionally extend into the subcutaneous tissue (quiz image). Although not specific for CBCLs, oftentimes there is an acellular portion of the superficial papillary dermis known as a grenz zone that can serve as a histopathologic clue to the diagnosis of a cutaneous lymphoproliferative disorder. The list of malignant B-cell neoplasms is extensive (eg, cutaneous marginal zone B-cell lymphoma, primary cutaneous follicle center lymphoma, diffuse large B-cell lymphoma, intravascular large B-cell lymphoma), and few are seen in the skin.
The most common type of CBCL is marginal zone B-cell lymphoma, which is considered to be a tumor of mucosa-associated (or skin-associated) lymphoid tissue. It is characterized by a monomorphous population of small mature lymphocytes showing characteristics of the B cells of the marginal zone of the lymph node. Some cells have the features of centrocytes/centroblasts (Figure 1) demonstrated by slightly irregular or indented nuclei and generous amounts of cytoplasm. Larger and more pleomorphic cells such as immunoblasts are similarly noted (Figure 1). The quiz image and Figure 1 demonstrate a cutaneous marginal zone B-cell lymphoma. A histomorphologic clue supporting a diagnosis of marginal zone B-cell lymphoma over reactive lymphoid hyperplasia is a B-cell predominate (B- to T-cell ratio of at least 3 to 1) infiltrate that is comprised of marginal zone-type cells. Immunohistochemistry demonstrating fewer differentiated B cells with light chain restriction may provide additional evidence that supports a clonal and potentially malignant process.

Erythematous to violaceous nodules on the head and neck of older individuals are characteristic of both granuloma faciale and CBCL. Histologically, granuloma faciale is characterized by a dense cellular infiltrate, often with a nodular outline, occupying the mid dermis.2 Granuloma faciale typically spares the immediate subepidermis and hair follicles, forming a grenz zone. The cellular infiltrate is polymorphic and consists of eosinophils and neutrophils with scattered plasma cells, mast cells, and lymphocytes in a vasculocentric distribution, eventually with chronic concentric fibrosis (Figure 2).

Leukemia cutis demonstrates a dermal infiltrate that contains atypical mononuclear cells (myeloblasts and myelocytes)(Figure 3).3 These markedly atypical mononuclear cells can have kidney bean-shaped nuclei and percolate through the dermal collagen, resembling single-file cells. They have increased nuclear to cytoplasmic ratios and occasionally have prominent nucleoli. Correlation with immunophenotypic and cytochemical studies is required for specific typing of the leukemic infiltrate.

Similar to primary CBCL, lymphomatoid papulosis (LyP) consists of erythematous papules or nodules that can occur anywhere on the body. In contrast to CBCL, the lesions of LyP classically self-resolve. However, approximately 10% to 20% of patients develop a malignant lymphoma, with mycosis fungoides, Hodgkin disease, and anaplastic large cell lymphoma being the most commonly associated.
Histologic examination of lesions of LyP classically demonstrates a wedge-shaped dermal infiltrate with variable epidermal changes (Figure 4). The wedge-shaped infiltrate is composed of large atypical cells. Three main types of lesions have been delineated: types A, B, and C. Type A is characterized by an increased number of cells with large vesicular nuclei with clumped chromatin, prominent nucleoli, and pronounced cytoplasm. Reed-Sternberg-like cells with an admixture of inflammatory cells including small lymphocytes, macrophages, neutrophils, and eosinophils also are present. Type B neoplastic cells vary in size and feature hyperchromatic, convoluted, or cerebriform nuclei. The infiltrate can be dense and bandlike with fewer cells resembling mycosis fungoides; type B LyP has neoplastic cells, not inflammatory cells. Finally, type C demonstrates solid sheets of large atypical cells resembling anaplastic large cell lymphoma. Immunohistochemically, the atypical cells often are CD4+ and CD8- with variable loss of pan-T-cell antigens. The atypical cells of types A and C express CD30 reactivity.4

Merkel cell carcinoma (MCC) is a primary neuroendocrine carcinoma of the skin that usually arises on sun-exposed skin in elderly patients with lesions that histologically and clinically resemble cutaneous lymphoma.5 It classically is composed of small, round to oval, basophilic cells with a vesicular nucleus and multiple small nucleoli. Apoptotic cells and mitoses often are present.6 One key finding that helps to differentiate MCC from lymphoma is the presence of finely dispersed salt-and-pepper chromatin and molded nuclear contour in MCC (Figure 5).

Immunophenotyping is important in the differentiation of these diagnoses. The atypical cells of LyP are positive for CD3, CD4, and CD30 but are negative for CD8. However, in type B LyP, the large CD30+ cells seen in the other types are not commonly seen. In contrast, MCC expresses reactivity with cytokeratins, in particular cytokeratin 20 and CAM5.2, classically in a paranuclear dotlike pattern. In keeping with MCC's neuroendocrine differentiation, the tumor cells will demonstrate reactivity with synaptophysin, chromogranin, and CD56. The immunohistochemistry for leukemia cutis varies depending on the type of leukemia. Acute myelomonocytic leukemia is positive for myeloperoxidase, CD13, CD33, and CD68. The immunophenotype of these marginal zone lymphoma cells is as follows: positive for CD20, CD79a, and Bcl-2; negative for Bcl-6, CD5, CD10, CD23, and cyclin D1 (Bcl-1).7
- Olsen EA. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin. 2015;33:643-654.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Wieser I, Wohlmuth C, Nunez CA, et al. Lymphomatoid papulosis in children and adolescents: a systematic review. Am J Clin Dermatol. 2016;17:319-327.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin: I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Frigerio B, Capella C, Eusebi V, et al. Merkel cell carcinoma of the skin: the structure and origin of normal Merkel cells. Histopathology. 1983;7:229-249.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Olsen EA. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin. 2015;33:643-654.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Wieser I, Wohlmuth C, Nunez CA, et al. Lymphomatoid papulosis in children and adolescents: a systematic review. Am J Clin Dermatol. 2016;17:319-327.
- Sibley RK, Dehner LP, Rosai J. Primary neuroendocrine (Merkel cell?) carcinoma of the skin: I. a clinicopathologic and ultrastructural study of 43 cases. Am J Surg Pathol. 1985;9:95-108.
- Frigerio B, Capella C, Eusebi V, et al. Merkel cell carcinoma of the skin: the structure and origin of normal Merkel cells. Histopathology. 1983;7:229-249.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
An 81-year-old man with a history of hyperthyroidism, paroxysmal atrial fibrillation, hypertension, and nonmelanoma skin cancer presented with an erythematous pearly papule on the right lateral chest of 1 year's duration. The patient reported no symptoms of pruritus, bleeding, or burning. He was otherwise asymptomatic, and a review of systems revealed no abnormalities. His current medications included aspirin, benazepril, finasteride, levothyroxine, tamsulosin, warfarin, and alprazolam. He denied any new medications, recent travel, or preceding trauma. He had a history of Agent Orange exposure. Physical examination revealed a 0.4-cm erythematous pearly papule on the right lateral chest. A shave biopsy was obtained.
Editorial Note
With great pleasure we announce a collaboration between Cutis® and the Skin of Color Society (SOCS) to increase the knowledge available to dermatologists to help improve delivery of care to this underserved population.
Established in 2004 by Susan C. Taylor, MD (who also serves on the Cutis Editorial Board), SOCS (http://www.skinofcolorsociety.org) promotes, supports, and stimulates the development of information related to all aspects of skin of color within the specialty of dermatology, making this information readily available to the general population.
“Although a relatively new organization, SOCS has been essential in supporting and encouraging research and scholarly activity to increase our understanding of the ethnic differences that occur in problems related to hair, skin, and nails of the growing population of darker-skinned individuals in our country,” said Vincent A. DeLeo, MD, Editor-in-Chief of Cutis and a founding member of SOCS. “In addition, SOCS has been essential in mentoring young students and increasing minority participation in dermatology, and Cutis will strive to assist in those endeavors.”
The society also seeks to increase the body of dermatologic literature related to skin of color. To achieve this goal, SOCS will be collaborating with the editors of Cutis to publish quarterly Skin of Color columns to educate dermatologists and residents on basic science and clinical, surgical, and cosmetic research relevant to this patient population.
“SOCS is very excited to collaborate with Cutis in our mutual academic pursuits,” said Seemal R. Desai, MD, current secretary/treasurer of SOCS and president-elect. “It is vitally important to the mission of SOCS that dermatologists and patients be educated with the most up-to-date objective data, studies, and information that is available to most effectively help those suffering from skin disease in the skin of color population.”
Look for Skin of Color columns in upcoming issues of Cutis.
With great pleasure we announce a collaboration between Cutis® and the Skin of Color Society (SOCS) to increase the knowledge available to dermatologists to help improve delivery of care to this underserved population.
Established in 2004 by Susan C. Taylor, MD (who also serves on the Cutis Editorial Board), SOCS (http://www.skinofcolorsociety.org) promotes, supports, and stimulates the development of information related to all aspects of skin of color within the specialty of dermatology, making this information readily available to the general population.
“Although a relatively new organization, SOCS has been essential in supporting and encouraging research and scholarly activity to increase our understanding of the ethnic differences that occur in problems related to hair, skin, and nails of the growing population of darker-skinned individuals in our country,” said Vincent A. DeLeo, MD, Editor-in-Chief of Cutis and a founding member of SOCS. “In addition, SOCS has been essential in mentoring young students and increasing minority participation in dermatology, and Cutis will strive to assist in those endeavors.”
The society also seeks to increase the body of dermatologic literature related to skin of color. To achieve this goal, SOCS will be collaborating with the editors of Cutis to publish quarterly Skin of Color columns to educate dermatologists and residents on basic science and clinical, surgical, and cosmetic research relevant to this patient population.
“SOCS is very excited to collaborate with Cutis in our mutual academic pursuits,” said Seemal R. Desai, MD, current secretary/treasurer of SOCS and president-elect. “It is vitally important to the mission of SOCS that dermatologists and patients be educated with the most up-to-date objective data, studies, and information that is available to most effectively help those suffering from skin disease in the skin of color population.”
Look for Skin of Color columns in upcoming issues of Cutis.
With great pleasure we announce a collaboration between Cutis® and the Skin of Color Society (SOCS) to increase the knowledge available to dermatologists to help improve delivery of care to this underserved population.
Established in 2004 by Susan C. Taylor, MD (who also serves on the Cutis Editorial Board), SOCS (http://www.skinofcolorsociety.org) promotes, supports, and stimulates the development of information related to all aspects of skin of color within the specialty of dermatology, making this information readily available to the general population.
“Although a relatively new organization, SOCS has been essential in supporting and encouraging research and scholarly activity to increase our understanding of the ethnic differences that occur in problems related to hair, skin, and nails of the growing population of darker-skinned individuals in our country,” said Vincent A. DeLeo, MD, Editor-in-Chief of Cutis and a founding member of SOCS. “In addition, SOCS has been essential in mentoring young students and increasing minority participation in dermatology, and Cutis will strive to assist in those endeavors.”
The society also seeks to increase the body of dermatologic literature related to skin of color. To achieve this goal, SOCS will be collaborating with the editors of Cutis to publish quarterly Skin of Color columns to educate dermatologists and residents on basic science and clinical, surgical, and cosmetic research relevant to this patient population.
“SOCS is very excited to collaborate with Cutis in our mutual academic pursuits,” said Seemal R. Desai, MD, current secretary/treasurer of SOCS and president-elect. “It is vitally important to the mission of SOCS that dermatologists and patients be educated with the most up-to-date objective data, studies, and information that is available to most effectively help those suffering from skin disease in the skin of color population.”
Look for Skin of Color columns in upcoming issues of Cutis.
Oral Contraceptives for Acne Treatment: US Dermatologists’ Knowledge, Comfort, and Prescribing Practices
The incidence of acne in adult females is rising,1 and treatment with combined oral contraceptive pills (OCPs) is becoming an increasingly important therapy for women with acne. Prior reports have indicated that OCPs were as effective as systemic antibiotics in reducing inflammatory, noninflammatory, and total facial acne lesions after 6 months of treatment.2,3 The acne management guidelines of the American Academy of Dermatology confer OCPs a grade A recommendation based on consistent and good-quality patient-oriented evidence.4
The US Food and Drug Administration (FDA) has approved 3 OCPs for the treatment of acne in adult women: norgestimate–ethinyl estradiol in 1997, norethindrone acetate–ethinyl estradiol in 2001, and drospirenone–ethinyl estradiol in 2007.5 However, the use of these OCPs is poorly understood by many dermatologists. One study showed that dermatologists prescribed OCPs in only 2% of visits with female patients aged 12 to 55 years who presented for acne treatment, which is less often than obstetrician/gynecologists (36%) and internists (11%),6 perhaps due to perceived risks or unfamiliarity with OCP formulations and guidelines among dermatologists.7 Adverse effects of OCPs include venous thromboembolism (VTE), myocardial infarction, and hypertension,8 but they generally are well tolerated.9
Even less is known about dermatologists’ use of drospirenone-containing OCPs (DCOCPs), which contain the only FDA-approved progestin that blocks androgen receptors. In prior studies, treatment with DCOCPs was associated with greater reductions in total lesion count and investigator-graded acne severity compared to early-generation OCPs.10,11 However, DCOCPs have been associated with a greater risk for VTE (4.0–6.3 times higher than OCP nonuse; 1.0–3.3 times higher than levonorgestrel-containing OCPs),12 which may explain the decline in DCOCP prescriptions among gynecologists in Germany from 23.8% of OCP prescriptions in 2007 to 11.4% in 2011.13
In this study, we surveyed US dermatologists about their knowledge, comfort, and prescribing practices pertaining to the use of OCPs. We compare OCP-prescribing to nonprescribing dermatologists, and those frequently prescribing DCOCPs to those who infrequently prescribe DCOCPs.
Methods
Survey Design
We performed a cross-sectional survey study using convenience sampling. The instrument was designed based on primary literature on OCPs in acne treatment and questionnaires assessing the use of OCPs in other specialties. Topics included prescribing practices, contraindications for OCPs defined by the Centers for Disease Control and Prevention (CDC),14 VTE risk, patient selection for hormonal acne therapy, comfort with prescribing OCP therapy, and participant demographics.
Skip logic was employed (ie, subsequent questions depended on prior answers). A pilot study surveyed 9 board-certified dermatologists at our home institution (Weill Cornell Medical College, New York, New York).
Data Collection
Eligible participants were board-certified US dermatologists. Data were collected and managed using an electronic data capture tool through the Weill Cornell Medical College Clinical & Translational Science Center. Surveys were distributed electronically to dermatologic society members, university alumni networks, investigators’ professional contacts, and dermatologists whose contact information was purchased from an email marketing company. Chain-referral sampling (ie, participants’ recruitment among their colleagues) was used. Surveys were distributed at a regional dermatology meeting. Responses were collected from November 2014 to April 2015. This study was approved by the institutional review board.
Statistical Analysis
For the descriptive data, all responses including pilot study participants were analyzed regardless of survey completion and were summarized using frequency counts and percentages (N=130).
For the analysis of OCP prescription predictors, the sample included all respondents answering the demographic questions and indicating if they prescribe OCPs (N=116). One respondent was excluded for answering other for current practice setting. Demographic predictors of OCP prescription were physician characteristics, geographic region, practice location population density, practice attributes, time spent on medical versus pediatric dermatology, number of weekly acne patients, and percentage of total patients who are female. Medical school graduation year was a categorical variable and was categorized as prior to 1997 (when norgestimate–ethinyl estradiol was FDA approved for acne5) versus 1997 or later. Respondents’ practice states were analyzed according to US regions—Northeast, Midwest, South, West/Pacific—and population density (persons per square mile) using US Census Bureau data.15,16
Univariate logistic regressions modeling OCP prescribing probability were performed for each demographic variable; a multivariable logistic model was constructed including all variables significant at α=.20 from univariate modeling.
To compare frequent prescribers versus infrequent prescribers of DCOCPs, we included all respondents answering whether they frequently prescribe DCOCPs and whether they believed the risk for VTE associated with DCOCPs differed from other OCPs (n=68). A univariate logistic regression was performed to model the probability of responding “Yes, they pose a greater risk” versus any of the other 3 responses by whether or not the respondent frequently prescribed DCOCPs for acne, and an unadjusted odds ratio was obtained. All P values were 2-tailed with statistical significance evaluated at α=.05. Ninety-five percent confidence intervals were calculated to assess precision of obtained estimates. Analyses were performed using SAS software version 9.4.
Results
Demographics
Participant demographics as predictors of OCP prescription practices are described in Table 1.
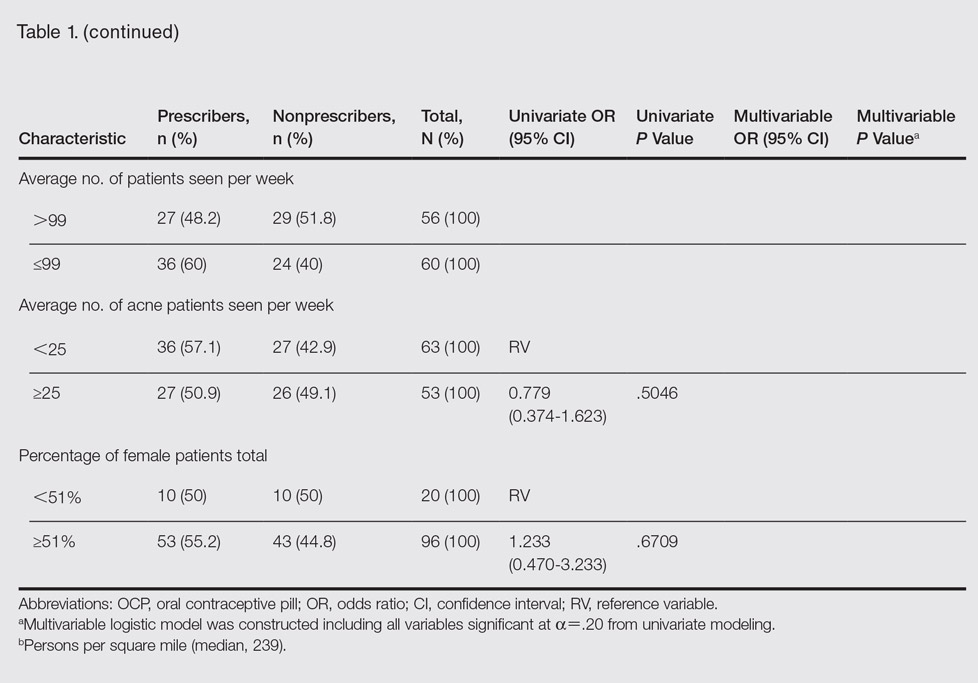
Knowledge
Oral contraceptive pills were endorsed as effective in the treatment of acne in women by 95.4% (124/130) of respondents. Among prescribers of OCPs for acne, 94.2% (65/69) believed OCPs were associated with an increased risk for VTE, no respondents thought OCPs were associated with a decreased VTE risk, 2.9% (2/69) believed OCPs did not affect VTE risk, and 2.9% (2/69) were unsure.
Among prescribers of OCPs for acne, 46.4% (32/69) believed DCOCPs posed a greater VTE risk than other OCPs. Odds of this response did not differ with frequent DCOCP prescribers versus infrequent prescribers (odds ratio, 0.731 [95% confidence interval, 0.272-1.964]; P=.5342). Participant responses on VTE risk and DCOCPs are provided in Table 2.

Dermatologists prescribing OCPs for acne endorsed greater likelihood of doing so in cases of cyclical flares with menstrual cycle (94.2% [65/69]), acne unresponsive to conventional therapy (87.0% [60/69]), acne on the lower half of the face (78.3% [54/69]), diagnosis of polycystic ovary syndrome (PCOS)(76.8% [53/69]), clinical suspicion of PCOS (71.0% [49/69]), concomitant hirsutism (71.0% [49/69]), late- or adult-onset acne (66.7% [46/69]), laboratory evidence of hyperandrogenism (60.9% [42/69]), and concomitant androgenetic alopecia (49.3% [34/69]).
Among dermatologists who prescribed OCPs for acne, CDC-defined absolute contraindications identified correctly were blood pressure of 160/100 mm Hg (59.4% [41/69]) and history of migraine with focal neurologic symptoms (49.3% [34/69]). The CDC-defined relative contraindications identified correctly were history of deep vein thrombosis or pulmonary embolism (1.4% [1/69]), breast cancer history with 5 years of no disease (15.9% [11/69]), hyperlipidemia (42.0% [29/69]), and 36 years or older smoking fewer than 15 cigarettes per day (21.7% [15/69]).
Comfort
Dermatologist self-reported comfort levels in prescribing OCPs for acne are shown in Table 3.

Prescribing Practices
Among all respondents, acne medications prescribed often included oral antibiotics (76.9% [100/130]), isotretinoin (41.5% [54/130]), and spironolactone (40.8% [53/130]).
Overall, 55.4% (72/130) of respondents prescribed OCPs for the following uses: acne (95.8% [69/72]), concomitant treatment with teratogenic medication (48.6% [35/72]), PCOS (34.7% [25/72]), hirsutism (26.4% [19/72]), androgenetic alopecia (19.4% [14/72]), SAHA (seborrhea, acne, hirsutism, alopecia) syndrome (12.5% [9/72]), and HAIR-AN (hyperandrogenism, insulin resistance, acanthosis nigricans) syndrome (11.1% [8/72]). For teratogenic medications, dermatologists prescribing OCPs did so with isotretinoin (77.8% [56/72]), spironolactone (73.6% [53/72]), tetracycline antibiotics (37.5% [27/72]), and other (34.7% [25/72]).
Of dermatologists prescribing OCPs for acne, frequency included often (19% [13/69]), sometimes (45% [31/69]), and rarely (36% [25/69]). The most frequently prescribed OCPs included Ortho Tri-Cyclen (Janssen Pharmaceuticals, Inc)(80% [55/69]), Yaz (Bayer)(64% [44/69]), and Estrostep (Warner Chilcott)(19% [13/69]). Fill-in responses included Desogen (Merck & Co, Inc)(3/69 [4%]), Alesse (Wyeth Pharmaceuticals, Inc)(3/69 [4%]), Lutera (Watson Pharma, Inc)(1/69 [1%]), Loestrin (Warner Chilcott)(1/69 [1%]), and Yasmin (Bayer)(1/69 [1%]).
In univariate regressions, graduation from medical school in 1997 or later (P=.0416), academic practice setting (P=.0130), and low-density practice setting (P=.0034) were significant predictors of prescribing OCPs. In multivariable regression, only academic practice setting (P=.0295) and low-density practice setting (P=.0050) remained significant predictors. Demographic predictors are summarized in Table 1.
Comment
Our results suggest that most dermatologists (95.4%) believe OCPs effectively treat acne; however, only 54% of respondents reported prescribing them. Academic dermatologists were more likely to prescribe OCPs than nonacademic dermatologists, possibly indicating that academic dermatologists are more familiar with the literature on the efficacy and use of OCPs. Nearly half of respondents seeing 25 or more acne patients weekly did not prescribe OCPs, suggesting a notable practice gap. Dermatologists in less dense US regions were more likely to prescribe OCPs, perhaps because dermatologists may be more likely to prescribe OCPs than refer patients in health care access–limited areas, just as primary care providers treat a broader range of conditions in low-density rural areas than urban ones.17 Exploring all dermatologists’ referral patterns for OCPs is warranted.
A strong knowledge area revealed from this study was hormonal treatment of acne in women, a vital area because appropriate patient selection is key to treatment success.8 Weaker knowledge areas included OCP contraindications and differences in VTE risk between formulations containing drospirenone and those not containing drospirenone. Only half the sample identified CDC-defined absolute contraindications, suggesting an education target for dermatologists to ensure patient safety. In contrast, respondents were conservative about relative contraindications, with most identifying deep vein thrombosis or pulmonary embolism, remote breast cancer history, and light smoking at 36 years or older as absolute contraindications. These results could reflect weighing the risk of relative contraindications against the benefit in acne, resulting in appropriately more conservative management than overall guidelines suggest. If so, it may suggest that dermatologists are adapting overall guidelines appropriately for use of OCPs in skin conditions.
Nearly all respondents knew that OCPs are associated with an increased risk for VTE. Approximately half understood that DCOCPs are associated with a greater VTE risk than other OCPs, with no difference between frequent and infrequent prescribers. Comparing these results to the findings on OCP prescribing overall, some dermatologists’ risk-benefit calculation for VTE differs from other specialties because DCOCPs have superior efficacy in acne, whereas DCOCPs have similar contraceptive efficacy to other OCPs.18 The fact that more dermatologists believed VTE to be an absolute contraindication than hypertension suggests dermatologists have a heightened awareness of VTE risk but prescribe DCOCPs for acne despite it.
Most OCP prescribers felt very comfortable selecting good candidates for OCPs (55.5%) and counseling on treatment initiation (45.8%) and side effects (48.6%). Only 22.2%, by contrast, were very comfortable managing side effects. This finding likely reflects the notion that VTEs are not most appropriately managed by a dermatologist. Exploring if a greater comfort level in managing side effects would make dermatologists more likely to prescribe OCPs is worthwhile. Additionally, exploring why many dermatologists do not prescribe OCPs despite believing they are effective for acne is warranted.
Study limitations included the use of convenience sampling. Additionally, our study did not investigate dermatologists’ reasons for not prescribing OCPs.
Conclusion
This study demonstrates that dermatologists believe OCPs effectively treat acne in women and that most dermatologists prescribing OCPs do so for acne treatment. Academic practice setting was associated with higher odds of prescribing OCPs than a nonacademic setting, but the number of weekly acne patients did not impact the likelihood of prescribing OCPs, which suggests a treatment gap warranting education efforts for dermatologists in nonacademic settings seeing many acne patients. Our study also suggests that awareness of the increased risk for VTE associated with DCOCPs is not associated with lower likelihood of prescribing DCOCPs, suggesting dermatologists may find greater treatment efficacy to be worth the higher risk.
Acknowledgments
We are grateful to the Department of Dermatology at the Weill Cornell College of Medicine (New York, New York) for providing funding to complete this study. We also acknowledge Paul Christos, DrPH, MS (New York, New York), and Xuming Sun, MS (New York, New York), for their assistance with the survey design. We also are indebted to numerous dermatologic professional societies for allowing the survey to be distributed to their membership.
- Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. July 11, 2012:CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459.
- Strauss JS, Krowchuk DP, Leyden JJ, et al; American Academy of Dermatology/American Academy of Dermatology Association. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651-663.
- Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatol Treat. 2012;23:272-277.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Katsambas AD, Dessinioti C. Hormonal therapy for acne: why not as first line therapy? facts and controversies. Clin Dermatol. 2010;28:17-23.
- Dragoman MV. The combined oral contraceptive pill—recent developments, risks and benefits. Best Pract Res Clin Obstet Gynaecol. 2014;28:825-834.
- Thorneycroft IH, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis. 2004;74:123-130.
- Mansour D, Verhoeven C, Sommer W, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. Eur J Contracept Reproduct Health Care. 2011;16:430-443.
- Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
- Ziller M, Rashed AN, Ziller V, et al. The prescribing of contraceptives for adolescents in German gynecologic practices in 2007 and 2011: a retrospective database analysis. J Pediatr Adolesc Gynecol. 2013;26:261-264.
- Centers for Disease Control and Prevention. US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- United States Census Bureau. Census Regions and Divisions of the United States. New York, NY: United States Department of Commerce; 2010.
- Resident Population Data—Population Density, 1910 to 2010. U.S. Census Bureau; 2012. http ://www.census.gov/2010census/data/apportionment-dens-text.php. Accessed January 9, 2017.
- Reschovsky A, Zahner SJ. Forecasting the revenues of local public health departments in the shadows of the “Great Recession.” J Public Health Manag Pract. 2016;22:120-128.
- Klipping C, Duijkers I, Fortier MP, et al. Contraceptive efficacy and tolerability of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38:73-83.
The incidence of acne in adult females is rising,1 and treatment with combined oral contraceptive pills (OCPs) is becoming an increasingly important therapy for women with acne. Prior reports have indicated that OCPs were as effective as systemic antibiotics in reducing inflammatory, noninflammatory, and total facial acne lesions after 6 months of treatment.2,3 The acne management guidelines of the American Academy of Dermatology confer OCPs a grade A recommendation based on consistent and good-quality patient-oriented evidence.4
The US Food and Drug Administration (FDA) has approved 3 OCPs for the treatment of acne in adult women: norgestimate–ethinyl estradiol in 1997, norethindrone acetate–ethinyl estradiol in 2001, and drospirenone–ethinyl estradiol in 2007.5 However, the use of these OCPs is poorly understood by many dermatologists. One study showed that dermatologists prescribed OCPs in only 2% of visits with female patients aged 12 to 55 years who presented for acne treatment, which is less often than obstetrician/gynecologists (36%) and internists (11%),6 perhaps due to perceived risks or unfamiliarity with OCP formulations and guidelines among dermatologists.7 Adverse effects of OCPs include venous thromboembolism (VTE), myocardial infarction, and hypertension,8 but they generally are well tolerated.9
Even less is known about dermatologists’ use of drospirenone-containing OCPs (DCOCPs), which contain the only FDA-approved progestin that blocks androgen receptors. In prior studies, treatment with DCOCPs was associated with greater reductions in total lesion count and investigator-graded acne severity compared to early-generation OCPs.10,11 However, DCOCPs have been associated with a greater risk for VTE (4.0–6.3 times higher than OCP nonuse; 1.0–3.3 times higher than levonorgestrel-containing OCPs),12 which may explain the decline in DCOCP prescriptions among gynecologists in Germany from 23.8% of OCP prescriptions in 2007 to 11.4% in 2011.13
In this study, we surveyed US dermatologists about their knowledge, comfort, and prescribing practices pertaining to the use of OCPs. We compare OCP-prescribing to nonprescribing dermatologists, and those frequently prescribing DCOCPs to those who infrequently prescribe DCOCPs.
Methods
Survey Design
We performed a cross-sectional survey study using convenience sampling. The instrument was designed based on primary literature on OCPs in acne treatment and questionnaires assessing the use of OCPs in other specialties. Topics included prescribing practices, contraindications for OCPs defined by the Centers for Disease Control and Prevention (CDC),14 VTE risk, patient selection for hormonal acne therapy, comfort with prescribing OCP therapy, and participant demographics.
Skip logic was employed (ie, subsequent questions depended on prior answers). A pilot study surveyed 9 board-certified dermatologists at our home institution (Weill Cornell Medical College, New York, New York).
Data Collection
Eligible participants were board-certified US dermatologists. Data were collected and managed using an electronic data capture tool through the Weill Cornell Medical College Clinical & Translational Science Center. Surveys were distributed electronically to dermatologic society members, university alumni networks, investigators’ professional contacts, and dermatologists whose contact information was purchased from an email marketing company. Chain-referral sampling (ie, participants’ recruitment among their colleagues) was used. Surveys were distributed at a regional dermatology meeting. Responses were collected from November 2014 to April 2015. This study was approved by the institutional review board.
Statistical Analysis
For the descriptive data, all responses including pilot study participants were analyzed regardless of survey completion and were summarized using frequency counts and percentages (N=130).
For the analysis of OCP prescription predictors, the sample included all respondents answering the demographic questions and indicating if they prescribe OCPs (N=116). One respondent was excluded for answering other for current practice setting. Demographic predictors of OCP prescription were physician characteristics, geographic region, practice location population density, practice attributes, time spent on medical versus pediatric dermatology, number of weekly acne patients, and percentage of total patients who are female. Medical school graduation year was a categorical variable and was categorized as prior to 1997 (when norgestimate–ethinyl estradiol was FDA approved for acne5) versus 1997 or later. Respondents’ practice states were analyzed according to US regions—Northeast, Midwest, South, West/Pacific—and population density (persons per square mile) using US Census Bureau data.15,16
Univariate logistic regressions modeling OCP prescribing probability were performed for each demographic variable; a multivariable logistic model was constructed including all variables significant at α=.20 from univariate modeling.
To compare frequent prescribers versus infrequent prescribers of DCOCPs, we included all respondents answering whether they frequently prescribe DCOCPs and whether they believed the risk for VTE associated with DCOCPs differed from other OCPs (n=68). A univariate logistic regression was performed to model the probability of responding “Yes, they pose a greater risk” versus any of the other 3 responses by whether or not the respondent frequently prescribed DCOCPs for acne, and an unadjusted odds ratio was obtained. All P values were 2-tailed with statistical significance evaluated at α=.05. Ninety-five percent confidence intervals were calculated to assess precision of obtained estimates. Analyses were performed using SAS software version 9.4.
Results
Demographics
Participant demographics as predictors of OCP prescription practices are described in Table 1.


Knowledge
Oral contraceptive pills were endorsed as effective in the treatment of acne in women by 95.4% (124/130) of respondents. Among prescribers of OCPs for acne, 94.2% (65/69) believed OCPs were associated with an increased risk for VTE, no respondents thought OCPs were associated with a decreased VTE risk, 2.9% (2/69) believed OCPs did not affect VTE risk, and 2.9% (2/69) were unsure.
Among prescribers of OCPs for acne, 46.4% (32/69) believed DCOCPs posed a greater VTE risk than other OCPs. Odds of this response did not differ with frequent DCOCP prescribers versus infrequent prescribers (odds ratio, 0.731 [95% confidence interval, 0.272-1.964]; P=.5342). Participant responses on VTE risk and DCOCPs are provided in Table 2.

Dermatologists prescribing OCPs for acne endorsed greater likelihood of doing so in cases of cyclical flares with menstrual cycle (94.2% [65/69]), acne unresponsive to conventional therapy (87.0% [60/69]), acne on the lower half of the face (78.3% [54/69]), diagnosis of polycystic ovary syndrome (PCOS)(76.8% [53/69]), clinical suspicion of PCOS (71.0% [49/69]), concomitant hirsutism (71.0% [49/69]), late- or adult-onset acne (66.7% [46/69]), laboratory evidence of hyperandrogenism (60.9% [42/69]), and concomitant androgenetic alopecia (49.3% [34/69]).
Among dermatologists who prescribed OCPs for acne, CDC-defined absolute contraindications identified correctly were blood pressure of 160/100 mm Hg (59.4% [41/69]) and history of migraine with focal neurologic symptoms (49.3% [34/69]). The CDC-defined relative contraindications identified correctly were history of deep vein thrombosis or pulmonary embolism (1.4% [1/69]), breast cancer history with 5 years of no disease (15.9% [11/69]), hyperlipidemia (42.0% [29/69]), and 36 years or older smoking fewer than 15 cigarettes per day (21.7% [15/69]).
Comfort
Dermatologist self-reported comfort levels in prescribing OCPs for acne are shown in Table 3.

Prescribing Practices
Among all respondents, acne medications prescribed often included oral antibiotics (76.9% [100/130]), isotretinoin (41.5% [54/130]), and spironolactone (40.8% [53/130]).
Overall, 55.4% (72/130) of respondents prescribed OCPs for the following uses: acne (95.8% [69/72]), concomitant treatment with teratogenic medication (48.6% [35/72]), PCOS (34.7% [25/72]), hirsutism (26.4% [19/72]), androgenetic alopecia (19.4% [14/72]), SAHA (seborrhea, acne, hirsutism, alopecia) syndrome (12.5% [9/72]), and HAIR-AN (hyperandrogenism, insulin resistance, acanthosis nigricans) syndrome (11.1% [8/72]). For teratogenic medications, dermatologists prescribing OCPs did so with isotretinoin (77.8% [56/72]), spironolactone (73.6% [53/72]), tetracycline antibiotics (37.5% [27/72]), and other (34.7% [25/72]).
Of dermatologists prescribing OCPs for acne, frequency included often (19% [13/69]), sometimes (45% [31/69]), and rarely (36% [25/69]). The most frequently prescribed OCPs included Ortho Tri-Cyclen (Janssen Pharmaceuticals, Inc)(80% [55/69]), Yaz (Bayer)(64% [44/69]), and Estrostep (Warner Chilcott)(19% [13/69]). Fill-in responses included Desogen (Merck & Co, Inc)(3/69 [4%]), Alesse (Wyeth Pharmaceuticals, Inc)(3/69 [4%]), Lutera (Watson Pharma, Inc)(1/69 [1%]), Loestrin (Warner Chilcott)(1/69 [1%]), and Yasmin (Bayer)(1/69 [1%]).
In univariate regressions, graduation from medical school in 1997 or later (P=.0416), academic practice setting (P=.0130), and low-density practice setting (P=.0034) were significant predictors of prescribing OCPs. In multivariable regression, only academic practice setting (P=.0295) and low-density practice setting (P=.0050) remained significant predictors. Demographic predictors are summarized in Table 1.
Comment
Our results suggest that most dermatologists (95.4%) believe OCPs effectively treat acne; however, only 54% of respondents reported prescribing them. Academic dermatologists were more likely to prescribe OCPs than nonacademic dermatologists, possibly indicating that academic dermatologists are more familiar with the literature on the efficacy and use of OCPs. Nearly half of respondents seeing 25 or more acne patients weekly did not prescribe OCPs, suggesting a notable practice gap. Dermatologists in less dense US regions were more likely to prescribe OCPs, perhaps because dermatologists may be more likely to prescribe OCPs than refer patients in health care access–limited areas, just as primary care providers treat a broader range of conditions in low-density rural areas than urban ones.17 Exploring all dermatologists’ referral patterns for OCPs is warranted.
A strong knowledge area revealed from this study was hormonal treatment of acne in women, a vital area because appropriate patient selection is key to treatment success.8 Weaker knowledge areas included OCP contraindications and differences in VTE risk between formulations containing drospirenone and those not containing drospirenone. Only half the sample identified CDC-defined absolute contraindications, suggesting an education target for dermatologists to ensure patient safety. In contrast, respondents were conservative about relative contraindications, with most identifying deep vein thrombosis or pulmonary embolism, remote breast cancer history, and light smoking at 36 years or older as absolute contraindications. These results could reflect weighing the risk of relative contraindications against the benefit in acne, resulting in appropriately more conservative management than overall guidelines suggest. If so, it may suggest that dermatologists are adapting overall guidelines appropriately for use of OCPs in skin conditions.
Nearly all respondents knew that OCPs are associated with an increased risk for VTE. Approximately half understood that DCOCPs are associated with a greater VTE risk than other OCPs, with no difference between frequent and infrequent prescribers. Comparing these results to the findings on OCP prescribing overall, some dermatologists’ risk-benefit calculation for VTE differs from other specialties because DCOCPs have superior efficacy in acne, whereas DCOCPs have similar contraceptive efficacy to other OCPs.18 The fact that more dermatologists believed VTE to be an absolute contraindication than hypertension suggests dermatologists have a heightened awareness of VTE risk but prescribe DCOCPs for acne despite it.
Most OCP prescribers felt very comfortable selecting good candidates for OCPs (55.5%) and counseling on treatment initiation (45.8%) and side effects (48.6%). Only 22.2%, by contrast, were very comfortable managing side effects. This finding likely reflects the notion that VTEs are not most appropriately managed by a dermatologist. Exploring if a greater comfort level in managing side effects would make dermatologists more likely to prescribe OCPs is worthwhile. Additionally, exploring why many dermatologists do not prescribe OCPs despite believing they are effective for acne is warranted.
Study limitations included the use of convenience sampling. Additionally, our study did not investigate dermatologists’ reasons for not prescribing OCPs.
Conclusion
This study demonstrates that dermatologists believe OCPs effectively treat acne in women and that most dermatologists prescribing OCPs do so for acne treatment. Academic practice setting was associated with higher odds of prescribing OCPs than a nonacademic setting, but the number of weekly acne patients did not impact the likelihood of prescribing OCPs, which suggests a treatment gap warranting education efforts for dermatologists in nonacademic settings seeing many acne patients. Our study also suggests that awareness of the increased risk for VTE associated with DCOCPs is not associated with lower likelihood of prescribing DCOCPs, suggesting dermatologists may find greater treatment efficacy to be worth the higher risk.
Acknowledgments
We are grateful to the Department of Dermatology at the Weill Cornell College of Medicine (New York, New York) for providing funding to complete this study. We also acknowledge Paul Christos, DrPH, MS (New York, New York), and Xuming Sun, MS (New York, New York), for their assistance with the survey design. We also are indebted to numerous dermatologic professional societies for allowing the survey to be distributed to their membership.
The incidence of acne in adult females is rising,1 and treatment with combined oral contraceptive pills (OCPs) is becoming an increasingly important therapy for women with acne. Prior reports have indicated that OCPs were as effective as systemic antibiotics in reducing inflammatory, noninflammatory, and total facial acne lesions after 6 months of treatment.2,3 The acne management guidelines of the American Academy of Dermatology confer OCPs a grade A recommendation based on consistent and good-quality patient-oriented evidence.4
The US Food and Drug Administration (FDA) has approved 3 OCPs for the treatment of acne in adult women: norgestimate–ethinyl estradiol in 1997, norethindrone acetate–ethinyl estradiol in 2001, and drospirenone–ethinyl estradiol in 2007.5 However, the use of these OCPs is poorly understood by many dermatologists. One study showed that dermatologists prescribed OCPs in only 2% of visits with female patients aged 12 to 55 years who presented for acne treatment, which is less often than obstetrician/gynecologists (36%) and internists (11%),6 perhaps due to perceived risks or unfamiliarity with OCP formulations and guidelines among dermatologists.7 Adverse effects of OCPs include venous thromboembolism (VTE), myocardial infarction, and hypertension,8 but they generally are well tolerated.9
Even less is known about dermatologists’ use of drospirenone-containing OCPs (DCOCPs), which contain the only FDA-approved progestin that blocks androgen receptors. In prior studies, treatment with DCOCPs was associated with greater reductions in total lesion count and investigator-graded acne severity compared to early-generation OCPs.10,11 However, DCOCPs have been associated with a greater risk for VTE (4.0–6.3 times higher than OCP nonuse; 1.0–3.3 times higher than levonorgestrel-containing OCPs),12 which may explain the decline in DCOCP prescriptions among gynecologists in Germany from 23.8% of OCP prescriptions in 2007 to 11.4% in 2011.13
In this study, we surveyed US dermatologists about their knowledge, comfort, and prescribing practices pertaining to the use of OCPs. We compare OCP-prescribing to nonprescribing dermatologists, and those frequently prescribing DCOCPs to those who infrequently prescribe DCOCPs.
Methods
Survey Design
We performed a cross-sectional survey study using convenience sampling. The instrument was designed based on primary literature on OCPs in acne treatment and questionnaires assessing the use of OCPs in other specialties. Topics included prescribing practices, contraindications for OCPs defined by the Centers for Disease Control and Prevention (CDC),14 VTE risk, patient selection for hormonal acne therapy, comfort with prescribing OCP therapy, and participant demographics.
Skip logic was employed (ie, subsequent questions depended on prior answers). A pilot study surveyed 9 board-certified dermatologists at our home institution (Weill Cornell Medical College, New York, New York).
Data Collection
Eligible participants were board-certified US dermatologists. Data were collected and managed using an electronic data capture tool through the Weill Cornell Medical College Clinical & Translational Science Center. Surveys were distributed electronically to dermatologic society members, university alumni networks, investigators’ professional contacts, and dermatologists whose contact information was purchased from an email marketing company. Chain-referral sampling (ie, participants’ recruitment among their colleagues) was used. Surveys were distributed at a regional dermatology meeting. Responses were collected from November 2014 to April 2015. This study was approved by the institutional review board.
Statistical Analysis
For the descriptive data, all responses including pilot study participants were analyzed regardless of survey completion and were summarized using frequency counts and percentages (N=130).
For the analysis of OCP prescription predictors, the sample included all respondents answering the demographic questions and indicating if they prescribe OCPs (N=116). One respondent was excluded for answering other for current practice setting. Demographic predictors of OCP prescription were physician characteristics, geographic region, practice location population density, practice attributes, time spent on medical versus pediatric dermatology, number of weekly acne patients, and percentage of total patients who are female. Medical school graduation year was a categorical variable and was categorized as prior to 1997 (when norgestimate–ethinyl estradiol was FDA approved for acne5) versus 1997 or later. Respondents’ practice states were analyzed according to US regions—Northeast, Midwest, South, West/Pacific—and population density (persons per square mile) using US Census Bureau data.15,16
Univariate logistic regressions modeling OCP prescribing probability were performed for each demographic variable; a multivariable logistic model was constructed including all variables significant at α=.20 from univariate modeling.
To compare frequent prescribers versus infrequent prescribers of DCOCPs, we included all respondents answering whether they frequently prescribe DCOCPs and whether they believed the risk for VTE associated with DCOCPs differed from other OCPs (n=68). A univariate logistic regression was performed to model the probability of responding “Yes, they pose a greater risk” versus any of the other 3 responses by whether or not the respondent frequently prescribed DCOCPs for acne, and an unadjusted odds ratio was obtained. All P values were 2-tailed with statistical significance evaluated at α=.05. Ninety-five percent confidence intervals were calculated to assess precision of obtained estimates. Analyses were performed using SAS software version 9.4.
Results
Demographics
Participant demographics as predictors of OCP prescription practices are described in Table 1.


Knowledge
Oral contraceptive pills were endorsed as effective in the treatment of acne in women by 95.4% (124/130) of respondents. Among prescribers of OCPs for acne, 94.2% (65/69) believed OCPs were associated with an increased risk for VTE, no respondents thought OCPs were associated with a decreased VTE risk, 2.9% (2/69) believed OCPs did not affect VTE risk, and 2.9% (2/69) were unsure.
Among prescribers of OCPs for acne, 46.4% (32/69) believed DCOCPs posed a greater VTE risk than other OCPs. Odds of this response did not differ with frequent DCOCP prescribers versus infrequent prescribers (odds ratio, 0.731 [95% confidence interval, 0.272-1.964]; P=.5342). Participant responses on VTE risk and DCOCPs are provided in Table 2.

Dermatologists prescribing OCPs for acne endorsed greater likelihood of doing so in cases of cyclical flares with menstrual cycle (94.2% [65/69]), acne unresponsive to conventional therapy (87.0% [60/69]), acne on the lower half of the face (78.3% [54/69]), diagnosis of polycystic ovary syndrome (PCOS)(76.8% [53/69]), clinical suspicion of PCOS (71.0% [49/69]), concomitant hirsutism (71.0% [49/69]), late- or adult-onset acne (66.7% [46/69]), laboratory evidence of hyperandrogenism (60.9% [42/69]), and concomitant androgenetic alopecia (49.3% [34/69]).
Among dermatologists who prescribed OCPs for acne, CDC-defined absolute contraindications identified correctly were blood pressure of 160/100 mm Hg (59.4% [41/69]) and history of migraine with focal neurologic symptoms (49.3% [34/69]). The CDC-defined relative contraindications identified correctly were history of deep vein thrombosis or pulmonary embolism (1.4% [1/69]), breast cancer history with 5 years of no disease (15.9% [11/69]), hyperlipidemia (42.0% [29/69]), and 36 years or older smoking fewer than 15 cigarettes per day (21.7% [15/69]).
Comfort
Dermatologist self-reported comfort levels in prescribing OCPs for acne are shown in Table 3.

Prescribing Practices
Among all respondents, acne medications prescribed often included oral antibiotics (76.9% [100/130]), isotretinoin (41.5% [54/130]), and spironolactone (40.8% [53/130]).
Overall, 55.4% (72/130) of respondents prescribed OCPs for the following uses: acne (95.8% [69/72]), concomitant treatment with teratogenic medication (48.6% [35/72]), PCOS (34.7% [25/72]), hirsutism (26.4% [19/72]), androgenetic alopecia (19.4% [14/72]), SAHA (seborrhea, acne, hirsutism, alopecia) syndrome (12.5% [9/72]), and HAIR-AN (hyperandrogenism, insulin resistance, acanthosis nigricans) syndrome (11.1% [8/72]). For teratogenic medications, dermatologists prescribing OCPs did so with isotretinoin (77.8% [56/72]), spironolactone (73.6% [53/72]), tetracycline antibiotics (37.5% [27/72]), and other (34.7% [25/72]).
Of dermatologists prescribing OCPs for acne, frequency included often (19% [13/69]), sometimes (45% [31/69]), and rarely (36% [25/69]). The most frequently prescribed OCPs included Ortho Tri-Cyclen (Janssen Pharmaceuticals, Inc)(80% [55/69]), Yaz (Bayer)(64% [44/69]), and Estrostep (Warner Chilcott)(19% [13/69]). Fill-in responses included Desogen (Merck & Co, Inc)(3/69 [4%]), Alesse (Wyeth Pharmaceuticals, Inc)(3/69 [4%]), Lutera (Watson Pharma, Inc)(1/69 [1%]), Loestrin (Warner Chilcott)(1/69 [1%]), and Yasmin (Bayer)(1/69 [1%]).
In univariate regressions, graduation from medical school in 1997 or later (P=.0416), academic practice setting (P=.0130), and low-density practice setting (P=.0034) were significant predictors of prescribing OCPs. In multivariable regression, only academic practice setting (P=.0295) and low-density practice setting (P=.0050) remained significant predictors. Demographic predictors are summarized in Table 1.
Comment
Our results suggest that most dermatologists (95.4%) believe OCPs effectively treat acne; however, only 54% of respondents reported prescribing them. Academic dermatologists were more likely to prescribe OCPs than nonacademic dermatologists, possibly indicating that academic dermatologists are more familiar with the literature on the efficacy and use of OCPs. Nearly half of respondents seeing 25 or more acne patients weekly did not prescribe OCPs, suggesting a notable practice gap. Dermatologists in less dense US regions were more likely to prescribe OCPs, perhaps because dermatologists may be more likely to prescribe OCPs than refer patients in health care access–limited areas, just as primary care providers treat a broader range of conditions in low-density rural areas than urban ones.17 Exploring all dermatologists’ referral patterns for OCPs is warranted.
A strong knowledge area revealed from this study was hormonal treatment of acne in women, a vital area because appropriate patient selection is key to treatment success.8 Weaker knowledge areas included OCP contraindications and differences in VTE risk between formulations containing drospirenone and those not containing drospirenone. Only half the sample identified CDC-defined absolute contraindications, suggesting an education target for dermatologists to ensure patient safety. In contrast, respondents were conservative about relative contraindications, with most identifying deep vein thrombosis or pulmonary embolism, remote breast cancer history, and light smoking at 36 years or older as absolute contraindications. These results could reflect weighing the risk of relative contraindications against the benefit in acne, resulting in appropriately more conservative management than overall guidelines suggest. If so, it may suggest that dermatologists are adapting overall guidelines appropriately for use of OCPs in skin conditions.
Nearly all respondents knew that OCPs are associated with an increased risk for VTE. Approximately half understood that DCOCPs are associated with a greater VTE risk than other OCPs, with no difference between frequent and infrequent prescribers. Comparing these results to the findings on OCP prescribing overall, some dermatologists’ risk-benefit calculation for VTE differs from other specialties because DCOCPs have superior efficacy in acne, whereas DCOCPs have similar contraceptive efficacy to other OCPs.18 The fact that more dermatologists believed VTE to be an absolute contraindication than hypertension suggests dermatologists have a heightened awareness of VTE risk but prescribe DCOCPs for acne despite it.
Most OCP prescribers felt very comfortable selecting good candidates for OCPs (55.5%) and counseling on treatment initiation (45.8%) and side effects (48.6%). Only 22.2%, by contrast, were very comfortable managing side effects. This finding likely reflects the notion that VTEs are not most appropriately managed by a dermatologist. Exploring if a greater comfort level in managing side effects would make dermatologists more likely to prescribe OCPs is worthwhile. Additionally, exploring why many dermatologists do not prescribe OCPs despite believing they are effective for acne is warranted.
Study limitations included the use of convenience sampling. Additionally, our study did not investigate dermatologists’ reasons for not prescribing OCPs.
Conclusion
This study demonstrates that dermatologists believe OCPs effectively treat acne in women and that most dermatologists prescribing OCPs do so for acne treatment. Academic practice setting was associated with higher odds of prescribing OCPs than a nonacademic setting, but the number of weekly acne patients did not impact the likelihood of prescribing OCPs, which suggests a treatment gap warranting education efforts for dermatologists in nonacademic settings seeing many acne patients. Our study also suggests that awareness of the increased risk for VTE associated with DCOCPs is not associated with lower likelihood of prescribing DCOCPs, suggesting dermatologists may find greater treatment efficacy to be worth the higher risk.
Acknowledgments
We are grateful to the Department of Dermatology at the Weill Cornell College of Medicine (New York, New York) for providing funding to complete this study. We also acknowledge Paul Christos, DrPH, MS (New York, New York), and Xuming Sun, MS (New York, New York), for their assistance with the survey design. We also are indebted to numerous dermatologic professional societies for allowing the survey to be distributed to their membership.
- Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. July 11, 2012:CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459.
- Strauss JS, Krowchuk DP, Leyden JJ, et al; American Academy of Dermatology/American Academy of Dermatology Association. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651-663.
- Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatol Treat. 2012;23:272-277.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Katsambas AD, Dessinioti C. Hormonal therapy for acne: why not as first line therapy? facts and controversies. Clin Dermatol. 2010;28:17-23.
- Dragoman MV. The combined oral contraceptive pill—recent developments, risks and benefits. Best Pract Res Clin Obstet Gynaecol. 2014;28:825-834.
- Thorneycroft IH, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis. 2004;74:123-130.
- Mansour D, Verhoeven C, Sommer W, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. Eur J Contracept Reproduct Health Care. 2011;16:430-443.
- Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
- Ziller M, Rashed AN, Ziller V, et al. The prescribing of contraceptives for adolescents in German gynecologic practices in 2007 and 2011: a retrospective database analysis. J Pediatr Adolesc Gynecol. 2013;26:261-264.
- Centers for Disease Control and Prevention. US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- United States Census Bureau. Census Regions and Divisions of the United States. New York, NY: United States Department of Commerce; 2010.
- Resident Population Data—Population Density, 1910 to 2010. U.S. Census Bureau; 2012. http ://www.census.gov/2010census/data/apportionment-dens-text.php. Accessed January 9, 2017.
- Reschovsky A, Zahner SJ. Forecasting the revenues of local public health departments in the shadows of the “Great Recession.” J Public Health Manag Pract. 2016;22:120-128.
- Klipping C, Duijkers I, Fortier MP, et al. Contraceptive efficacy and tolerability of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38:73-83.
- Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. July 11, 2012:CD004425.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459.
- Strauss JS, Krowchuk DP, Leyden JJ, et al; American Academy of Dermatology/American Academy of Dermatology Association. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56:651-663.
- Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatol Treat. 2012;23:272-277.
- Lam C, Zaenglein AL. Contraceptive use in acne. Clin Dermatol. 2014;32:502-515.
- Katsambas AD, Dessinioti C. Hormonal therapy for acne: why not as first line therapy? facts and controversies. Clin Dermatol. 2010;28:17-23.
- Dragoman MV. The combined oral contraceptive pill—recent developments, risks and benefits. Best Pract Res Clin Obstet Gynaecol. 2014;28:825-834.
- Thorneycroft IH, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis. 2004;74:123-130.
- Mansour D, Verhoeven C, Sommer W, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. Eur J Contracept Reproduct Health Care. 2011;16:430-443.
- Wu CQ, Grandi SM, Filion KB, et al. Drospirenone-containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. BJOG. 2013;120:801-810.
- Ziller M, Rashed AN, Ziller V, et al. The prescribing of contraceptives for adolescents in German gynecologic practices in 2007 and 2011: a retrospective database analysis. J Pediatr Adolesc Gynecol. 2013;26:261-264.
- Centers for Disease Control and Prevention. US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1-86.
- United States Census Bureau. Census Regions and Divisions of the United States. New York, NY: United States Department of Commerce; 2010.
- Resident Population Data—Population Density, 1910 to 2010. U.S. Census Bureau; 2012. http ://www.census.gov/2010census/data/apportionment-dens-text.php. Accessed January 9, 2017.
- Reschovsky A, Zahner SJ. Forecasting the revenues of local public health departments in the shadows of the “Great Recession.” J Public Health Manag Pract. 2016;22:120-128.
- Klipping C, Duijkers I, Fortier MP, et al. Contraceptive efficacy and tolerability of ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen: an open-label, multicentre, randomised, controlled study. J Fam Plann Reprod Health Care. 2012;38:73-83.
Practice Points
- In prior reports, oral contraceptive pills (OCPs) were found to be as effective as systemic antibiotics in reducing acne lesion counts at 6 months of treatment.
- Most dermatologists have prescribed OCPs and most believed they were an effective treatment for acne in women.
Misdiagnosed Crusted Scabies in an AIDS Patient Leads to Hyperinfestation
Case Report
A recently incarcerated 34-year-old man with an 11-year history of multidrug-resistant human immunodeficiency virus/AIDS (CD4 count, 121 cells/mm3; viral load, 49,625 particles/mm3 one week prior to presentation) was admitted to the hospital for an intensely pruritic, hyperkeratotic, scaly rash involving the entire body. The rash first appeared on the feet approximately 1 year prior to admission. At that time the patient was given oral fluconazole and a steroid cream with near resolution of the rash. He was then transferred multiple times to different units with subsequent discontinuation of the medications. The rash flared and progressed to involve the knees. He was restarted on the fluconazole and steroid cream and placed in isolation by medical personnel at the prison 6 months prior to presentation. The rash continued to spread, and he was given a working diagnosis of plaque-type psoriasis by several providers after several months of nonresponse to treatment. Additional attempts at treatment at outside facilities included oral fluconazole, trimethoprim-sulfamethoxazole, and other antibiotics. He was referred to dermatology at our institution but missed the appointment and was admitted to the hospital before the appointment could be rescheduled.
On admission to the hospital, he denied similar lesions in close contacts. On review of systems he had subjective fevers and chills, decreased appetite, nausea without vomiting, dysphagia to solids, epigastric pain, and 70-lb weight loss over the last 6 months. Facial involvement of the rash impaired the ability to open the mouth, speak, and eat. He had no known drug allergies. His only medications at the time of admission were nortriptyline, trimethoprim-sulfamethoxazole, and oral combination elvitegravir-cobicistat-emtricitabine-tenofovir for hu-man immunodeficiency virus treatment.
On physical examination he was cachectic, shivering, and foul smelling. He was afebrile, slightly tachycardic (112 beats per minute), and hypertensive (144/83 mm Hg) with a respiratory rate of 18 breaths per minute. His height was 1.83 m (6 ft) and weight was 48.5 kg (107 lb) with a body mass index of 14.5. Extensive erythematous, hyperkeratotic, crusted, and fissured plaques covered the entire body including the face, hands, and feet. The tongue was covered with bilateral white-colored plaques, and he had patches of alopecia, excoriations, and scales on the scalp. The elbows were fixed in a flexed position and he had decreased range of motion in the wrists and fingers due to the severe hyperkeratosis (Figure 1A). Hyperkeratosis also was prominent on the knees and feet with associated burrows (Figure 2A). He had foot drop on the left.
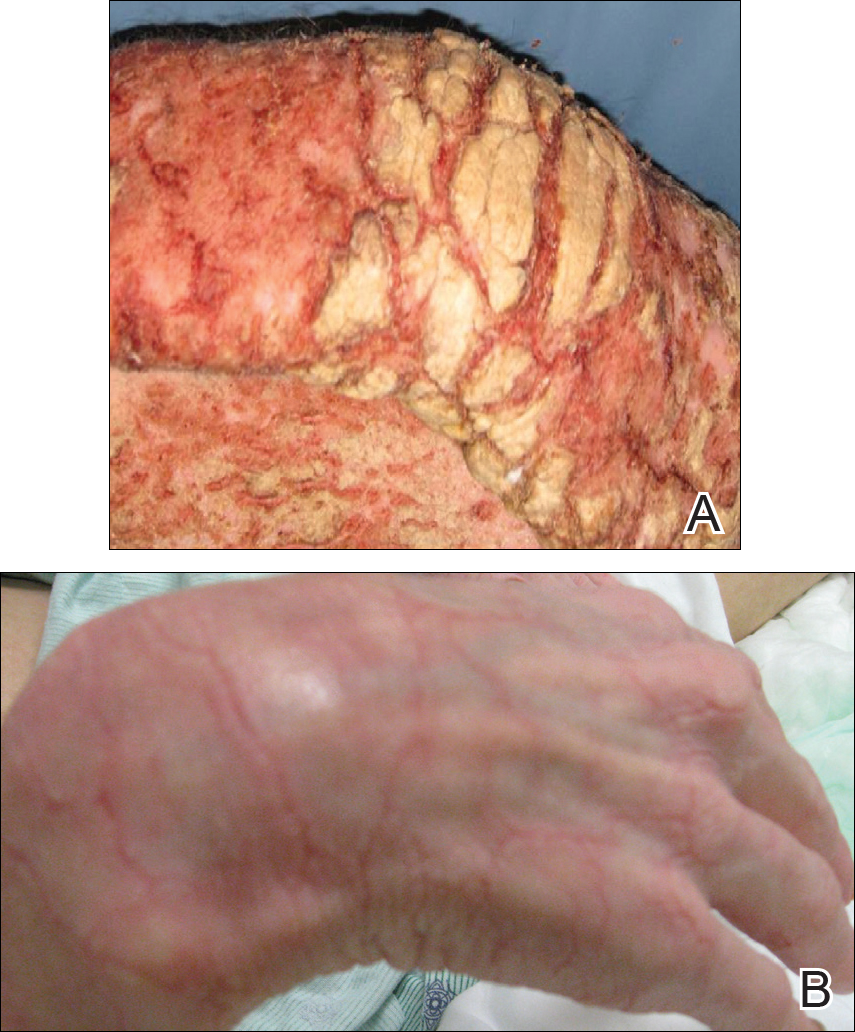
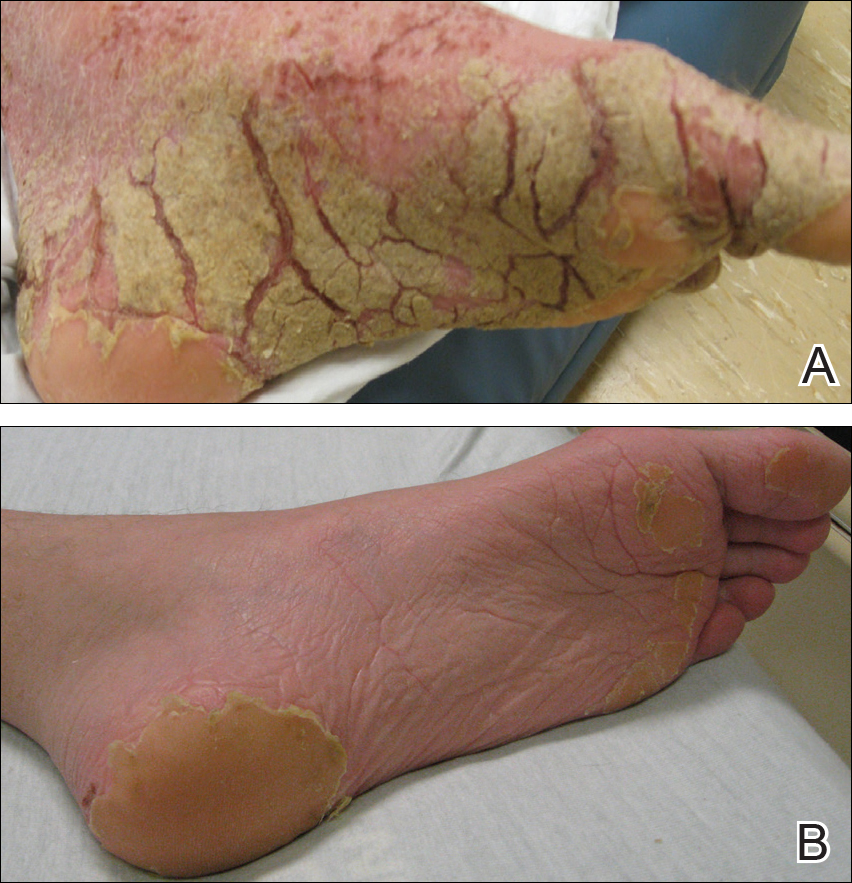
The differential diagnosis included a drug eruption; fungal or parasite infestation, such as crusted scabies; psoriasis; or cutaneous lymphoma. Laboratory studies were difficult to obtain, as there were limited areas suitable for vascular access. Blood work showed leukocytosis (18.9×109 cells/L [reference range, 4.8–10.8×109 cells/L) with 13.3% eosinophils (reference range, 1%–6%). This eosinophilia narrowed the likely diagnoses to a drug eruption or parasite infection.
The dermatology service was consulted. A mineral oil preparation was performed and showed numerous mites and feces consistent with a diagnosis of crusted scabies (Figure 3). The patient was started on a regimen of permethrin cream 5% applied to the entire body, except the face, which was left on overnight and washed off. This regimen was repeated daily for 1 week, then twice weekly until the rash resolved after a total of 3 weeks. Due to the severity of his condition, immunocompromised status, and concern for superinfection, oral ivermectin 200 μg/kg once daily was added on days 1, 2, 8, 9, 15, 22, and 29.1
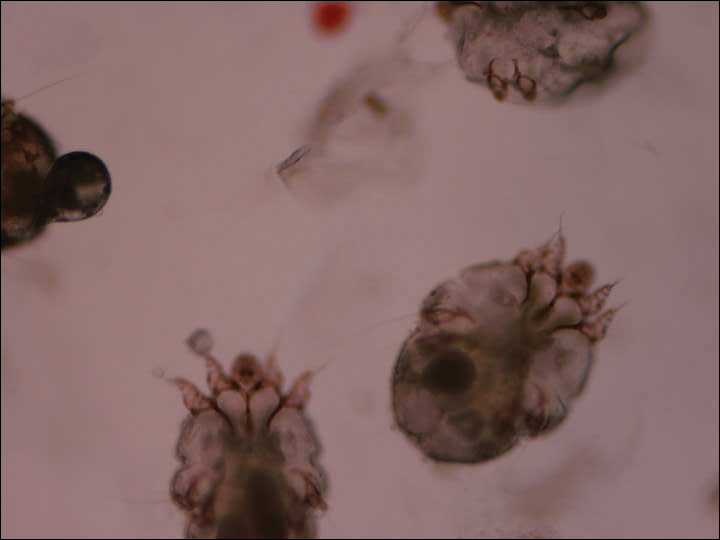
Our patient’s hospital course was further complicated by symptomatic hypoglycemia, altered mental status, and superimposed methicillin-resistant Staphylococcus aureus bacteremia, as well as Pseudomonas aeruginosa bacteremia, pneumonia, and coffee ground emesis. He was transferred to the intensive care unit but fortunately did not require intubation. His overall condition, mental status, and rash gradually improved. Three weeks after admission he only had a few residual lesions on the feet with clearing elsewhere (Figures 1B and 2B). He was discharged with a skin moisturizer and was referred for physical and occupational therapy. On follow-up clinic visits at 3 and 6 months, he had recovered well with general improvement in his condition.
Comment
Classic (noncrusted) scabies is common worldwide, with an estimated 300 million cases per year. It is caused by the mite Sarcoptes scabiei var hominis, and transmission occurs by direct skin-to-skin contact or less commonly by fomites (eg, linens, bedsheets) and therefore is common in overcrowded environments.2 Crusted scabies is a severe, highly contagious form of the disease in which the host’s immune system is overwhelmed and unable to defend against mites on the skin, resulting in hyperinfestation of the host. The mites use secretions to dissolve the epidermis and burrow through the skin, leaving feces in their tracks.3 Interestingly, the native aboriginal populations of Australia have a high incidence of crusted scabies even though they show no signs of immunosuppression. The reason remains unclear but may be due to a skewed T-cell response.4 Various mechanisms have been described for the symptoms of scabies, and it is believed that there is a hypersensitivity reaction to the mites and the feces. Increased IL-17 production by skin T cells may be responsible.5
Clinical Features
Crusted scabies is characterized by severe hyperkeratosis and plaques with desquamation and erythroderma that is worse in the acral regions and large joints, such as the elbows and the knees, as seen in our patient. Because of the deep burrows, patients are predisposed to secondary superinfections by bacteria. In our case, the patient had methicillin-resistant S aureus bacteremia, which persisted for some time despite treatment with intravenous antibiotics.
Diagnosis
Because scabies can imitate different conditions, it can be difficult to diagnose. Misdiagnosis of psoriasis in our patient led to ineffective treatment and subsequent worsening of his condition. Burrows are pathognomonic for scabies, though in severe cases, the burrows may be concealed by extreme hyperkeratosis. Diagnosis is confirmed by mineral oil preparation from the plaques showing numerous scabies mites and feces.
Treatment
It is important to control the spread of scabies, as it is highly contagious, and if the living environment is not properly cleaned, the patient can be reinfected. All clothing, bedsheets, and linens in the household must be washed in hot water and dried in a hot dryer, and nonwashable items should be placed in a closed plastic bag for 72 hours. All contacts also should be treated with 1 application of permethrin cream to the entire body including the head and neck, left on overnight, and washed off with warm water.1 The washing also helps remove some of the skin crusts. Patients should be educated that pruritus and burning may initially worsen with permethrin treatment due to the body’s reaction to the parasite.1,2 In addition, keratolytic agents such as topical urea or salicylic acid can be used as an adjuvant therapy to improve the efficacy of permethrin.
Permethrin is effective against both mites and eggs and works by inhibiting sodium channels, resulting in nerve signal conduction block and subsequent paralysis. Ivermectin is thought to act on glutamate-gated chloride channels, which are present in invertebrates but absent in vertebrates, causing hyperpolarization and paralysis of the adult mite.1,6
Conclusion
Crusted scabies is a highly contagious and intensely pruritic condition. Scabies can mimic other conditions, such as psoriasis or severe dermatitis, so it is important to keep this diagnosis in mind, especially in immunocompromised patients or populations in overcrowded areas (eg, those who are incarcerated or in nursing homes). Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts. A delay in diagnosis can lead to severe debilitating disease, as seen in the extreme case of our patient. However, our patient made a full recovery with appropriate treatment and care.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- World Health Organization. Water-related diseases: scabies. http://www.who.int/water_sanitation_health/diseases-risks/diseases/scabies/en/. Accessed February 23, 2017.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
- Liu X, Walton SF, Murray HC, et al. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 2014;36:594-604.
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug [published online August 26, 2005]. Trends Parasitol. 2005;21:530-532.
Case Report
A recently incarcerated 34-year-old man with an 11-year history of multidrug-resistant human immunodeficiency virus/AIDS (CD4 count, 121 cells/mm3; viral load, 49,625 particles/mm3 one week prior to presentation) was admitted to the hospital for an intensely pruritic, hyperkeratotic, scaly rash involving the entire body. The rash first appeared on the feet approximately 1 year prior to admission. At that time the patient was given oral fluconazole and a steroid cream with near resolution of the rash. He was then transferred multiple times to different units with subsequent discontinuation of the medications. The rash flared and progressed to involve the knees. He was restarted on the fluconazole and steroid cream and placed in isolation by medical personnel at the prison 6 months prior to presentation. The rash continued to spread, and he was given a working diagnosis of plaque-type psoriasis by several providers after several months of nonresponse to treatment. Additional attempts at treatment at outside facilities included oral fluconazole, trimethoprim-sulfamethoxazole, and other antibiotics. He was referred to dermatology at our institution but missed the appointment and was admitted to the hospital before the appointment could be rescheduled.
On admission to the hospital, he denied similar lesions in close contacts. On review of systems he had subjective fevers and chills, decreased appetite, nausea without vomiting, dysphagia to solids, epigastric pain, and 70-lb weight loss over the last 6 months. Facial involvement of the rash impaired the ability to open the mouth, speak, and eat. He had no known drug allergies. His only medications at the time of admission were nortriptyline, trimethoprim-sulfamethoxazole, and oral combination elvitegravir-cobicistat-emtricitabine-tenofovir for hu-man immunodeficiency virus treatment.
On physical examination he was cachectic, shivering, and foul smelling. He was afebrile, slightly tachycardic (112 beats per minute), and hypertensive (144/83 mm Hg) with a respiratory rate of 18 breaths per minute. His height was 1.83 m (6 ft) and weight was 48.5 kg (107 lb) with a body mass index of 14.5. Extensive erythematous, hyperkeratotic, crusted, and fissured plaques covered the entire body including the face, hands, and feet. The tongue was covered with bilateral white-colored plaques, and he had patches of alopecia, excoriations, and scales on the scalp. The elbows were fixed in a flexed position and he had decreased range of motion in the wrists and fingers due to the severe hyperkeratosis (Figure 1A). Hyperkeratosis also was prominent on the knees and feet with associated burrows (Figure 2A). He had foot drop on the left.


The differential diagnosis included a drug eruption; fungal or parasite infestation, such as crusted scabies; psoriasis; or cutaneous lymphoma. Laboratory studies were difficult to obtain, as there were limited areas suitable for vascular access. Blood work showed leukocytosis (18.9×109 cells/L [reference range, 4.8–10.8×109 cells/L) with 13.3% eosinophils (reference range, 1%–6%). This eosinophilia narrowed the likely diagnoses to a drug eruption or parasite infection.
The dermatology service was consulted. A mineral oil preparation was performed and showed numerous mites and feces consistent with a diagnosis of crusted scabies (Figure 3). The patient was started on a regimen of permethrin cream 5% applied to the entire body, except the face, which was left on overnight and washed off. This regimen was repeated daily for 1 week, then twice weekly until the rash resolved after a total of 3 weeks. Due to the severity of his condition, immunocompromised status, and concern for superinfection, oral ivermectin 200 μg/kg once daily was added on days 1, 2, 8, 9, 15, 22, and 29.1

Our patient’s hospital course was further complicated by symptomatic hypoglycemia, altered mental status, and superimposed methicillin-resistant Staphylococcus aureus bacteremia, as well as Pseudomonas aeruginosa bacteremia, pneumonia, and coffee ground emesis. He was transferred to the intensive care unit but fortunately did not require intubation. His overall condition, mental status, and rash gradually improved. Three weeks after admission he only had a few residual lesions on the feet with clearing elsewhere (Figures 1B and 2B). He was discharged with a skin moisturizer and was referred for physical and occupational therapy. On follow-up clinic visits at 3 and 6 months, he had recovered well with general improvement in his condition.
Comment
Classic (noncrusted) scabies is common worldwide, with an estimated 300 million cases per year. It is caused by the mite Sarcoptes scabiei var hominis, and transmission occurs by direct skin-to-skin contact or less commonly by fomites (eg, linens, bedsheets) and therefore is common in overcrowded environments.2 Crusted scabies is a severe, highly contagious form of the disease in which the host’s immune system is overwhelmed and unable to defend against mites on the skin, resulting in hyperinfestation of the host. The mites use secretions to dissolve the epidermis and burrow through the skin, leaving feces in their tracks.3 Interestingly, the native aboriginal populations of Australia have a high incidence of crusted scabies even though they show no signs of immunosuppression. The reason remains unclear but may be due to a skewed T-cell response.4 Various mechanisms have been described for the symptoms of scabies, and it is believed that there is a hypersensitivity reaction to the mites and the feces. Increased IL-17 production by skin T cells may be responsible.5
Clinical Features
Crusted scabies is characterized by severe hyperkeratosis and plaques with desquamation and erythroderma that is worse in the acral regions and large joints, such as the elbows and the knees, as seen in our patient. Because of the deep burrows, patients are predisposed to secondary superinfections by bacteria. In our case, the patient had methicillin-resistant S aureus bacteremia, which persisted for some time despite treatment with intravenous antibiotics.
Diagnosis
Because scabies can imitate different conditions, it can be difficult to diagnose. Misdiagnosis of psoriasis in our patient led to ineffective treatment and subsequent worsening of his condition. Burrows are pathognomonic for scabies, though in severe cases, the burrows may be concealed by extreme hyperkeratosis. Diagnosis is confirmed by mineral oil preparation from the plaques showing numerous scabies mites and feces.
Treatment
It is important to control the spread of scabies, as it is highly contagious, and if the living environment is not properly cleaned, the patient can be reinfected. All clothing, bedsheets, and linens in the household must be washed in hot water and dried in a hot dryer, and nonwashable items should be placed in a closed plastic bag for 72 hours. All contacts also should be treated with 1 application of permethrin cream to the entire body including the head and neck, left on overnight, and washed off with warm water.1 The washing also helps remove some of the skin crusts. Patients should be educated that pruritus and burning may initially worsen with permethrin treatment due to the body’s reaction to the parasite.1,2 In addition, keratolytic agents such as topical urea or salicylic acid can be used as an adjuvant therapy to improve the efficacy of permethrin.
Permethrin is effective against both mites and eggs and works by inhibiting sodium channels, resulting in nerve signal conduction block and subsequent paralysis. Ivermectin is thought to act on glutamate-gated chloride channels, which are present in invertebrates but absent in vertebrates, causing hyperpolarization and paralysis of the adult mite.1,6
Conclusion
Crusted scabies is a highly contagious and intensely pruritic condition. Scabies can mimic other conditions, such as psoriasis or severe dermatitis, so it is important to keep this diagnosis in mind, especially in immunocompromised patients or populations in overcrowded areas (eg, those who are incarcerated or in nursing homes). Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts. A delay in diagnosis can lead to severe debilitating disease, as seen in the extreme case of our patient. However, our patient made a full recovery with appropriate treatment and care.
Case Report
A recently incarcerated 34-year-old man with an 11-year history of multidrug-resistant human immunodeficiency virus/AIDS (CD4 count, 121 cells/mm3; viral load, 49,625 particles/mm3 one week prior to presentation) was admitted to the hospital for an intensely pruritic, hyperkeratotic, scaly rash involving the entire body. The rash first appeared on the feet approximately 1 year prior to admission. At that time the patient was given oral fluconazole and a steroid cream with near resolution of the rash. He was then transferred multiple times to different units with subsequent discontinuation of the medications. The rash flared and progressed to involve the knees. He was restarted on the fluconazole and steroid cream and placed in isolation by medical personnel at the prison 6 months prior to presentation. The rash continued to spread, and he was given a working diagnosis of plaque-type psoriasis by several providers after several months of nonresponse to treatment. Additional attempts at treatment at outside facilities included oral fluconazole, trimethoprim-sulfamethoxazole, and other antibiotics. He was referred to dermatology at our institution but missed the appointment and was admitted to the hospital before the appointment could be rescheduled.
On admission to the hospital, he denied similar lesions in close contacts. On review of systems he had subjective fevers and chills, decreased appetite, nausea without vomiting, dysphagia to solids, epigastric pain, and 70-lb weight loss over the last 6 months. Facial involvement of the rash impaired the ability to open the mouth, speak, and eat. He had no known drug allergies. His only medications at the time of admission were nortriptyline, trimethoprim-sulfamethoxazole, and oral combination elvitegravir-cobicistat-emtricitabine-tenofovir for hu-man immunodeficiency virus treatment.
On physical examination he was cachectic, shivering, and foul smelling. He was afebrile, slightly tachycardic (112 beats per minute), and hypertensive (144/83 mm Hg) with a respiratory rate of 18 breaths per minute. His height was 1.83 m (6 ft) and weight was 48.5 kg (107 lb) with a body mass index of 14.5. Extensive erythematous, hyperkeratotic, crusted, and fissured plaques covered the entire body including the face, hands, and feet. The tongue was covered with bilateral white-colored plaques, and he had patches of alopecia, excoriations, and scales on the scalp. The elbows were fixed in a flexed position and he had decreased range of motion in the wrists and fingers due to the severe hyperkeratosis (Figure 1A). Hyperkeratosis also was prominent on the knees and feet with associated burrows (Figure 2A). He had foot drop on the left.


The differential diagnosis included a drug eruption; fungal or parasite infestation, such as crusted scabies; psoriasis; or cutaneous lymphoma. Laboratory studies were difficult to obtain, as there were limited areas suitable for vascular access. Blood work showed leukocytosis (18.9×109 cells/L [reference range, 4.8–10.8×109 cells/L) with 13.3% eosinophils (reference range, 1%–6%). This eosinophilia narrowed the likely diagnoses to a drug eruption or parasite infection.
The dermatology service was consulted. A mineral oil preparation was performed and showed numerous mites and feces consistent with a diagnosis of crusted scabies (Figure 3). The patient was started on a regimen of permethrin cream 5% applied to the entire body, except the face, which was left on overnight and washed off. This regimen was repeated daily for 1 week, then twice weekly until the rash resolved after a total of 3 weeks. Due to the severity of his condition, immunocompromised status, and concern for superinfection, oral ivermectin 200 μg/kg once daily was added on days 1, 2, 8, 9, 15, 22, and 29.1

Our patient’s hospital course was further complicated by symptomatic hypoglycemia, altered mental status, and superimposed methicillin-resistant Staphylococcus aureus bacteremia, as well as Pseudomonas aeruginosa bacteremia, pneumonia, and coffee ground emesis. He was transferred to the intensive care unit but fortunately did not require intubation. His overall condition, mental status, and rash gradually improved. Three weeks after admission he only had a few residual lesions on the feet with clearing elsewhere (Figures 1B and 2B). He was discharged with a skin moisturizer and was referred for physical and occupational therapy. On follow-up clinic visits at 3 and 6 months, he had recovered well with general improvement in his condition.
Comment
Classic (noncrusted) scabies is common worldwide, with an estimated 300 million cases per year. It is caused by the mite Sarcoptes scabiei var hominis, and transmission occurs by direct skin-to-skin contact or less commonly by fomites (eg, linens, bedsheets) and therefore is common in overcrowded environments.2 Crusted scabies is a severe, highly contagious form of the disease in which the host’s immune system is overwhelmed and unable to defend against mites on the skin, resulting in hyperinfestation of the host. The mites use secretions to dissolve the epidermis and burrow through the skin, leaving feces in their tracks.3 Interestingly, the native aboriginal populations of Australia have a high incidence of crusted scabies even though they show no signs of immunosuppression. The reason remains unclear but may be due to a skewed T-cell response.4 Various mechanisms have been described for the symptoms of scabies, and it is believed that there is a hypersensitivity reaction to the mites and the feces. Increased IL-17 production by skin T cells may be responsible.5
Clinical Features
Crusted scabies is characterized by severe hyperkeratosis and plaques with desquamation and erythroderma that is worse in the acral regions and large joints, such as the elbows and the knees, as seen in our patient. Because of the deep burrows, patients are predisposed to secondary superinfections by bacteria. In our case, the patient had methicillin-resistant S aureus bacteremia, which persisted for some time despite treatment with intravenous antibiotics.
Diagnosis
Because scabies can imitate different conditions, it can be difficult to diagnose. Misdiagnosis of psoriasis in our patient led to ineffective treatment and subsequent worsening of his condition. Burrows are pathognomonic for scabies, though in severe cases, the burrows may be concealed by extreme hyperkeratosis. Diagnosis is confirmed by mineral oil preparation from the plaques showing numerous scabies mites and feces.
Treatment
It is important to control the spread of scabies, as it is highly contagious, and if the living environment is not properly cleaned, the patient can be reinfected. All clothing, bedsheets, and linens in the household must be washed in hot water and dried in a hot dryer, and nonwashable items should be placed in a closed plastic bag for 72 hours. All contacts also should be treated with 1 application of permethrin cream to the entire body including the head and neck, left on overnight, and washed off with warm water.1 The washing also helps remove some of the skin crusts. Patients should be educated that pruritus and burning may initially worsen with permethrin treatment due to the body’s reaction to the parasite.1,2 In addition, keratolytic agents such as topical urea or salicylic acid can be used as an adjuvant therapy to improve the efficacy of permethrin.
Permethrin is effective against both mites and eggs and works by inhibiting sodium channels, resulting in nerve signal conduction block and subsequent paralysis. Ivermectin is thought to act on glutamate-gated chloride channels, which are present in invertebrates but absent in vertebrates, causing hyperpolarization and paralysis of the adult mite.1,6
Conclusion
Crusted scabies is a highly contagious and intensely pruritic condition. Scabies can mimic other conditions, such as psoriasis or severe dermatitis, so it is important to keep this diagnosis in mind, especially in immunocompromised patients or populations in overcrowded areas (eg, those who are incarcerated or in nursing homes). Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts. A delay in diagnosis can lead to severe debilitating disease, as seen in the extreme case of our patient. However, our patient made a full recovery with appropriate treatment and care.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- World Health Organization. Water-related diseases: scabies. http://www.who.int/water_sanitation_health/diseases-risks/diseases/scabies/en/. Accessed February 23, 2017.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
- Liu X, Walton SF, Murray HC, et al. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 2014;36:594-604.
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug [published online August 26, 2005]. Trends Parasitol. 2005;21:530-532.
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725.
- World Health Organization. Water-related diseases: scabies. http://www.who.int/water_sanitation_health/diseases-risks/diseases/scabies/en/. Accessed February 23, 2017.
- Chosidow O. Scabies and pediculosis. Lancet. 2000;355:819-826.
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381.
- Liu X, Walton SF, Murray HC, et al. Crusted scabies is associated with increased IL-17 secretion by skin T cells. Parasite Immunol. 2014;36:594-604.
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug [published online August 26, 2005]. Trends Parasitol. 2005;21:530-532.
Practice Points
- Keep scabies in mind, especially in immunocompromised patients or populations in overcrowded areas.
- Treatment consists of isolating the patient, starting topical permethrin and oral ivermectin (in severe cases), washing all linens, and prophylactically treating contacts.
Hidradenitis Suppurativa Scoring Systems: Can We Choose Just One?
Interest in hidradenitis suppurativa (HS) has exploded in the last few years. A PubMed search of articles indexed for MEDLINE using the MeSH term hidradenitis suppurativa yielded more than 900 articles on HS since 1947, with a sharp increase in publications over the last few years and 119 articles published in 2015 alone. In addition to publications, we recently saw adalimumab become the first and only US Food and Drug Administration–approved treatment of moderate to severe HS.
With new treatment options and enthusiasm for HS, further attention needs to be paid to the scoring systems or outcome measures that clinicians use to grade HS severity and disease. Utilization of validated outcome measures allows for comparability between treatment effects, which is essential for clinical trials, meta-analyses, and monitoring of treatment response in daily clinical practice. Designing a scoring scale for any dermatologic disease is challenging; however, as we move forward with value-based reimbursement models, we likely will encounter quality reporting guidelines that mandate providers demonstrate the positive impact of treatment. Thus, scoring systems for HS, particularly ones that accurately assess this impact of treatment, are essential. For psoriasis, the physician global assessment (PGA) and psoriasis area and severity index are standard outcome measures of disease severity in clinical trials. The PGA also can be used in a clinical setting to longitudinally track patient treatment outcomes.1 Both the psoriasis area and severity index and PGA were cited as acceptable scoring tools for Medicare’s Physician Quality Reporting System quality metrics reporting (Measure #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications). Unfortunately, no such outcome measures consensus currently exists for scoring systems in HS.
Many scoring systems have been proposed for HS. The most well known is the Hurley staging system. Developed in 1989 for surgical approaches, it is a straightforward tool to categorize disease severity but does not emphasize the inflammatory component of HS. Recently, a refined Hurley stage classification system was proposed. This 3-step algorithm expanded the Hurley stage classification to incorporate disease extensiveness, degree of inflammation, and presence of sinus tracts.2 The modified Sartorius score (also known as the modified HS score) is a more detailed scoring system for assessing disease activity that requires measurements and precise counting of lesions.3 The HS-PGA is an ordinal scale specific to HS that categorizes patients into clear, minimal, mild, moderate, severe, or very severe disease, and it was used successfully in a phase 2 interventional clinical trial.4 The HS clinical response (HiSCR) score is an HS-specific, binary scoring system for patients with 3 or more abscesses or inflammatory nodules. It was engineered using raw data and outcomes from a large clinical trial, and subsequently was employed as the primary end point in 2 randomized controlled trials.5,6 It is the only HS scoring system to undergo an extensive validation process of both physician- and patient-reported measures for assessment of therapeutic response in controlling the inflammatory manifestations of HS.
Designing a scoring system for clinical trials can be complicated. Sample sizes are dependent on the delta, or change, in efficacy or variation in response, and the design of the score will affect how easy it is to detect a statistically meaningful difference. These choices are a critical part of the design of small studies, particularly if obtaining enough statistical power can be challenging. Additionally, it is easier to detect change in more homogenous populations where we expect a more consistent response. Hidradenitis suppurativa is not a particularly homogenous disease, which furthers the risk of designing a trial that cannot detect important differences. The PGA often is required by the US Food and Drug Administration and has the major advantage that it is easy to understand, but the categories can sometimes be too broad to detect change easily, and more granular data can provide the basis for more in-depth analyses. An ideal outcome measure is a simplified scoring system that assesses disease severity and responsiveness to treatment while accurately serving as a surrogate for patient-reported outcomes, such as the dermatology life quality index, visual analog scale for HS skin pain, the work productivity and activity impairment questionnaire (specific health problem), or the patient global assessment. Validation processes for outcome measures, such as the one that HiSCR underwent, are essential to ensure that the proposed scoring system has clinical meaningfulness to both the physician and patient.
A 2016 Cochrane review of interventions for HS included 12 randomized controlled trials that employed a total of 30 different outcome measures instruments. Because use of multiple scoring systems makes it difficult to compare analyses of treatment, the authors concluded that there was a need for improved validation of HS outcome measures for future clinical trials.7 Schmitt et al8 recognized that atopic dermatitis also was in a similar predicament; they noted that more than 20 outcome measures were employed to assess disease severity in clinical trials. The authors called this situation “a significant threat to evidence-based health care” and outlined the Harmonizing Outcome Measures for Eczema (HOME) research initiative’s methodology for creation of core outcome sets for any dermatologic disease. Their consensus process involved first identifying what to measure, termed outcome domains, followed by developing how to measure these domains through outcome measures instruments, which would be assessed for validity, reliability, sensitivity to change, and feasibility.8
Using the framework set forth by the HOME initiative and data from the 2016 Cochrane review,7 a recent review of all outcome measures instruments currently employed in HS found that 90% (27/30) were not validated.9 Even those that were validated still could not be fully recommended by the authors. The authors identified 10 potential outcome domains for measurement, including quality of life, pain, lesion count, PGA, patient global self-assessment, recurrence rate, overall satisfaction with treatment, impairment of function, cosmesis, and duration of recovery. They recommended a further consensus process to better define these outcomes.9
Measuring all of these variables seems daunting, but as the speed of HS research rapidly progresses, we would greatly benefit from employing a standard validated scoring system that captures both disease severity and activity. Several groups are working to improve our current tools, but we will need to move quickly to a common approach so we can better compare treatment effects and build an evidence base for treatment decisions. For now, the HiSCR is the most validated clinical trials instrument, but it may not be ideal for the clinical setting. In our practice, we grade all patients each visit with Hurley staging, the validated HS-PGA scoring system to track improvement in inflammatory lesions, and a 10-point pain scale to monitor disease activity and severity. We have found these tools to be quick and effective for measuring treatment response and would recommend employment of these scoring systems as a standard measure in clinical practice until further consensus is reached.
- Pascoe VL, Enamandram M, Corey KC, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375-381.
- Horváth B, Janse IC, Blok JL, et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group [published online August 18, 2016]. Acta Derm Venereol. doi:10.2340/00015555-2513.
- Revuz J. Modifications to the Sartorius score and instructions for evaluating the severity of suppurative hidradenitis [in French]. Ann Dermatol Venereol. 2007;134:173-174.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study [published online July 22, 2015]. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality [published online March 30, 2016]. Br J Dermatol. 2016;174:970-978.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology [published online September 4, 2014]. J Invest Dermatol. 2015;135:24-30.
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process [published online May 2, 2016]. Br J Dermatol. 2016;175:263-272.
Interest in hidradenitis suppurativa (HS) has exploded in the last few years. A PubMed search of articles indexed for MEDLINE using the MeSH term hidradenitis suppurativa yielded more than 900 articles on HS since 1947, with a sharp increase in publications over the last few years and 119 articles published in 2015 alone. In addition to publications, we recently saw adalimumab become the first and only US Food and Drug Administration–approved treatment of moderate to severe HS.
With new treatment options and enthusiasm for HS, further attention needs to be paid to the scoring systems or outcome measures that clinicians use to grade HS severity and disease. Utilization of validated outcome measures allows for comparability between treatment effects, which is essential for clinical trials, meta-analyses, and monitoring of treatment response in daily clinical practice. Designing a scoring scale for any dermatologic disease is challenging; however, as we move forward with value-based reimbursement models, we likely will encounter quality reporting guidelines that mandate providers demonstrate the positive impact of treatment. Thus, scoring systems for HS, particularly ones that accurately assess this impact of treatment, are essential. For psoriasis, the physician global assessment (PGA) and psoriasis area and severity index are standard outcome measures of disease severity in clinical trials. The PGA also can be used in a clinical setting to longitudinally track patient treatment outcomes.1 Both the psoriasis area and severity index and PGA were cited as acceptable scoring tools for Medicare’s Physician Quality Reporting System quality metrics reporting (Measure #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications). Unfortunately, no such outcome measures consensus currently exists for scoring systems in HS.
Many scoring systems have been proposed for HS. The most well known is the Hurley staging system. Developed in 1989 for surgical approaches, it is a straightforward tool to categorize disease severity but does not emphasize the inflammatory component of HS. Recently, a refined Hurley stage classification system was proposed. This 3-step algorithm expanded the Hurley stage classification to incorporate disease extensiveness, degree of inflammation, and presence of sinus tracts.2 The modified Sartorius score (also known as the modified HS score) is a more detailed scoring system for assessing disease activity that requires measurements and precise counting of lesions.3 The HS-PGA is an ordinal scale specific to HS that categorizes patients into clear, minimal, mild, moderate, severe, or very severe disease, and it was used successfully in a phase 2 interventional clinical trial.4 The HS clinical response (HiSCR) score is an HS-specific, binary scoring system for patients with 3 or more abscesses or inflammatory nodules. It was engineered using raw data and outcomes from a large clinical trial, and subsequently was employed as the primary end point in 2 randomized controlled trials.5,6 It is the only HS scoring system to undergo an extensive validation process of both physician- and patient-reported measures for assessment of therapeutic response in controlling the inflammatory manifestations of HS.
Designing a scoring system for clinical trials can be complicated. Sample sizes are dependent on the delta, or change, in efficacy or variation in response, and the design of the score will affect how easy it is to detect a statistically meaningful difference. These choices are a critical part of the design of small studies, particularly if obtaining enough statistical power can be challenging. Additionally, it is easier to detect change in more homogenous populations where we expect a more consistent response. Hidradenitis suppurativa is not a particularly homogenous disease, which furthers the risk of designing a trial that cannot detect important differences. The PGA often is required by the US Food and Drug Administration and has the major advantage that it is easy to understand, but the categories can sometimes be too broad to detect change easily, and more granular data can provide the basis for more in-depth analyses. An ideal outcome measure is a simplified scoring system that assesses disease severity and responsiveness to treatment while accurately serving as a surrogate for patient-reported outcomes, such as the dermatology life quality index, visual analog scale for HS skin pain, the work productivity and activity impairment questionnaire (specific health problem), or the patient global assessment. Validation processes for outcome measures, such as the one that HiSCR underwent, are essential to ensure that the proposed scoring system has clinical meaningfulness to both the physician and patient.
A 2016 Cochrane review of interventions for HS included 12 randomized controlled trials that employed a total of 30 different outcome measures instruments. Because use of multiple scoring systems makes it difficult to compare analyses of treatment, the authors concluded that there was a need for improved validation of HS outcome measures for future clinical trials.7 Schmitt et al8 recognized that atopic dermatitis also was in a similar predicament; they noted that more than 20 outcome measures were employed to assess disease severity in clinical trials. The authors called this situation “a significant threat to evidence-based health care” and outlined the Harmonizing Outcome Measures for Eczema (HOME) research initiative’s methodology for creation of core outcome sets for any dermatologic disease. Their consensus process involved first identifying what to measure, termed outcome domains, followed by developing how to measure these domains through outcome measures instruments, which would be assessed for validity, reliability, sensitivity to change, and feasibility.8
Using the framework set forth by the HOME initiative and data from the 2016 Cochrane review,7 a recent review of all outcome measures instruments currently employed in HS found that 90% (27/30) were not validated.9 Even those that were validated still could not be fully recommended by the authors. The authors identified 10 potential outcome domains for measurement, including quality of life, pain, lesion count, PGA, patient global self-assessment, recurrence rate, overall satisfaction with treatment, impairment of function, cosmesis, and duration of recovery. They recommended a further consensus process to better define these outcomes.9
Measuring all of these variables seems daunting, but as the speed of HS research rapidly progresses, we would greatly benefit from employing a standard validated scoring system that captures both disease severity and activity. Several groups are working to improve our current tools, but we will need to move quickly to a common approach so we can better compare treatment effects and build an evidence base for treatment decisions. For now, the HiSCR is the most validated clinical trials instrument, but it may not be ideal for the clinical setting. In our practice, we grade all patients each visit with Hurley staging, the validated HS-PGA scoring system to track improvement in inflammatory lesions, and a 10-point pain scale to monitor disease activity and severity. We have found these tools to be quick and effective for measuring treatment response and would recommend employment of these scoring systems as a standard measure in clinical practice until further consensus is reached.
Interest in hidradenitis suppurativa (HS) has exploded in the last few years. A PubMed search of articles indexed for MEDLINE using the MeSH term hidradenitis suppurativa yielded more than 900 articles on HS since 1947, with a sharp increase in publications over the last few years and 119 articles published in 2015 alone. In addition to publications, we recently saw adalimumab become the first and only US Food and Drug Administration–approved treatment of moderate to severe HS.
With new treatment options and enthusiasm for HS, further attention needs to be paid to the scoring systems or outcome measures that clinicians use to grade HS severity and disease. Utilization of validated outcome measures allows for comparability between treatment effects, which is essential for clinical trials, meta-analyses, and monitoring of treatment response in daily clinical practice. Designing a scoring scale for any dermatologic disease is challenging; however, as we move forward with value-based reimbursement models, we likely will encounter quality reporting guidelines that mandate providers demonstrate the positive impact of treatment. Thus, scoring systems for HS, particularly ones that accurately assess this impact of treatment, are essential. For psoriasis, the physician global assessment (PGA) and psoriasis area and severity index are standard outcome measures of disease severity in clinical trials. The PGA also can be used in a clinical setting to longitudinally track patient treatment outcomes.1 Both the psoriasis area and severity index and PGA were cited as acceptable scoring tools for Medicare’s Physician Quality Reporting System quality metrics reporting (Measure #410: Psoriasis: Clinical Response to Oral Systemic or Biologic Medications). Unfortunately, no such outcome measures consensus currently exists for scoring systems in HS.
Many scoring systems have been proposed for HS. The most well known is the Hurley staging system. Developed in 1989 for surgical approaches, it is a straightforward tool to categorize disease severity but does not emphasize the inflammatory component of HS. Recently, a refined Hurley stage classification system was proposed. This 3-step algorithm expanded the Hurley stage classification to incorporate disease extensiveness, degree of inflammation, and presence of sinus tracts.2 The modified Sartorius score (also known as the modified HS score) is a more detailed scoring system for assessing disease activity that requires measurements and precise counting of lesions.3 The HS-PGA is an ordinal scale specific to HS that categorizes patients into clear, minimal, mild, moderate, severe, or very severe disease, and it was used successfully in a phase 2 interventional clinical trial.4 The HS clinical response (HiSCR) score is an HS-specific, binary scoring system for patients with 3 or more abscesses or inflammatory nodules. It was engineered using raw data and outcomes from a large clinical trial, and subsequently was employed as the primary end point in 2 randomized controlled trials.5,6 It is the only HS scoring system to undergo an extensive validation process of both physician- and patient-reported measures for assessment of therapeutic response in controlling the inflammatory manifestations of HS.
Designing a scoring system for clinical trials can be complicated. Sample sizes are dependent on the delta, or change, in efficacy or variation in response, and the design of the score will affect how easy it is to detect a statistically meaningful difference. These choices are a critical part of the design of small studies, particularly if obtaining enough statistical power can be challenging. Additionally, it is easier to detect change in more homogenous populations where we expect a more consistent response. Hidradenitis suppurativa is not a particularly homogenous disease, which furthers the risk of designing a trial that cannot detect important differences. The PGA often is required by the US Food and Drug Administration and has the major advantage that it is easy to understand, but the categories can sometimes be too broad to detect change easily, and more granular data can provide the basis for more in-depth analyses. An ideal outcome measure is a simplified scoring system that assesses disease severity and responsiveness to treatment while accurately serving as a surrogate for patient-reported outcomes, such as the dermatology life quality index, visual analog scale for HS skin pain, the work productivity and activity impairment questionnaire (specific health problem), or the patient global assessment. Validation processes for outcome measures, such as the one that HiSCR underwent, are essential to ensure that the proposed scoring system has clinical meaningfulness to both the physician and patient.
A 2016 Cochrane review of interventions for HS included 12 randomized controlled trials that employed a total of 30 different outcome measures instruments. Because use of multiple scoring systems makes it difficult to compare analyses of treatment, the authors concluded that there was a need for improved validation of HS outcome measures for future clinical trials.7 Schmitt et al8 recognized that atopic dermatitis also was in a similar predicament; they noted that more than 20 outcome measures were employed to assess disease severity in clinical trials. The authors called this situation “a significant threat to evidence-based health care” and outlined the Harmonizing Outcome Measures for Eczema (HOME) research initiative’s methodology for creation of core outcome sets for any dermatologic disease. Their consensus process involved first identifying what to measure, termed outcome domains, followed by developing how to measure these domains through outcome measures instruments, which would be assessed for validity, reliability, sensitivity to change, and feasibility.8
Using the framework set forth by the HOME initiative and data from the 2016 Cochrane review,7 a recent review of all outcome measures instruments currently employed in HS found that 90% (27/30) were not validated.9 Even those that were validated still could not be fully recommended by the authors. The authors identified 10 potential outcome domains for measurement, including quality of life, pain, lesion count, PGA, patient global self-assessment, recurrence rate, overall satisfaction with treatment, impairment of function, cosmesis, and duration of recovery. They recommended a further consensus process to better define these outcomes.9
Measuring all of these variables seems daunting, but as the speed of HS research rapidly progresses, we would greatly benefit from employing a standard validated scoring system that captures both disease severity and activity. Several groups are working to improve our current tools, but we will need to move quickly to a common approach so we can better compare treatment effects and build an evidence base for treatment decisions. For now, the HiSCR is the most validated clinical trials instrument, but it may not be ideal for the clinical setting. In our practice, we grade all patients each visit with Hurley staging, the validated HS-PGA scoring system to track improvement in inflammatory lesions, and a 10-point pain scale to monitor disease activity and severity. We have found these tools to be quick and effective for measuring treatment response and would recommend employment of these scoring systems as a standard measure in clinical practice until further consensus is reached.
- Pascoe VL, Enamandram M, Corey KC, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375-381.
- Horváth B, Janse IC, Blok JL, et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group [published online August 18, 2016]. Acta Derm Venereol. doi:10.2340/00015555-2513.
- Revuz J. Modifications to the Sartorius score and instructions for evaluating the severity of suppurative hidradenitis [in French]. Ann Dermatol Venereol. 2007;134:173-174.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study [published online July 22, 2015]. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality [published online March 30, 2016]. Br J Dermatol. 2016;174:970-978.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology [published online September 4, 2014]. J Invest Dermatol. 2015;135:24-30.
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process [published online May 2, 2016]. Br J Dermatol. 2016;175:263-272.
- Pascoe VL, Enamandram M, Corey KC, et al. Using the Physician Global Assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 2015;151:375-381.
- Horváth B, Janse IC, Blok JL, et al. Hurley staging refined: a proposal by the Dutch Hidradenitis Suppurativa Expert Group [published online August 18, 2016]. Acta Derm Venereol. doi:10.2340/00015555-2513.
- Revuz J. Modifications to the Sartorius score and instructions for evaluating the severity of suppurative hidradenitis [in French]. Ann Dermatol Venereol. 2007;134:173-174.
- Kimball AB, Kerdel F, Adams D, et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med. 2012;157:846-855.
- Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52-59.
- Kimball AB, Sobell JM, Zouboulis CC, et al. HiSCR (hidradenitis suppurativa clinical response): a novel clinical endpoint to evaluate therapeutic outcomes in patients with hidradenitis suppurativa from the placebo-controlled portion of a phase 2 adalimumab study [published online July 22, 2015]. J Eur Acad Dermatol Venereol. 2016;30:989-994.
- Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality [published online March 30, 2016]. Br J Dermatol. 2016;174:970-978.
- Schmitt J, Apfelbacher C, Spuls PI, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology [published online September 4, 2014]. J Invest Dermatol. 2015;135:24-30.
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process [published online May 2, 2016]. Br J Dermatol. 2016;175:263-272.
Imatinib Mesylate–Induced Lichenoid Drug Eruption
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.
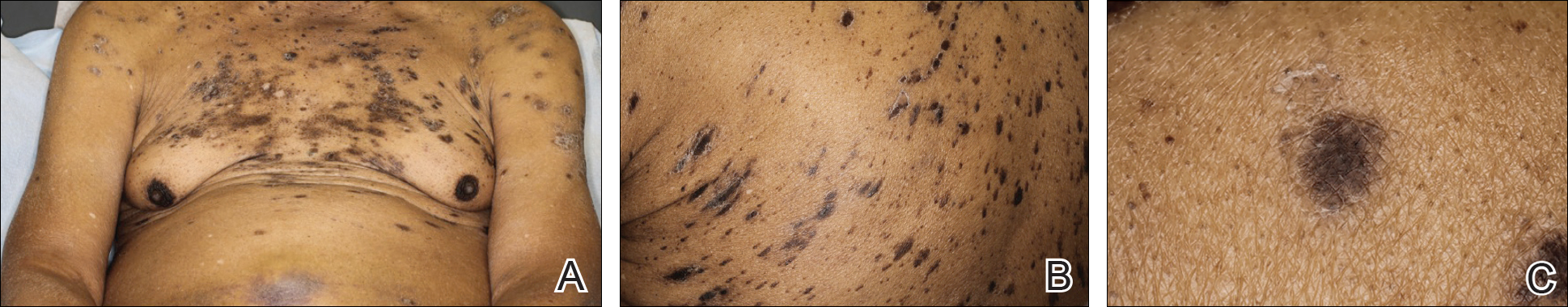
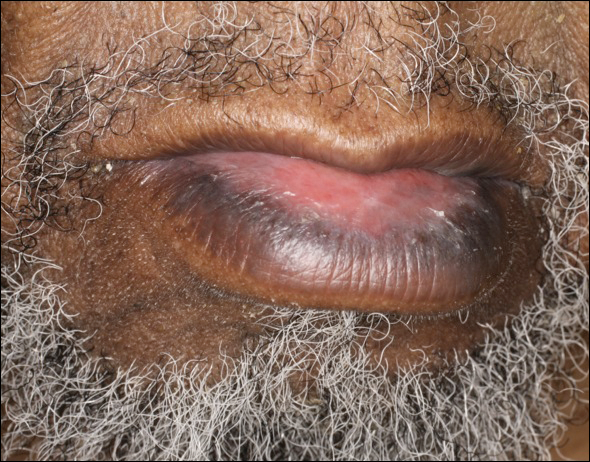
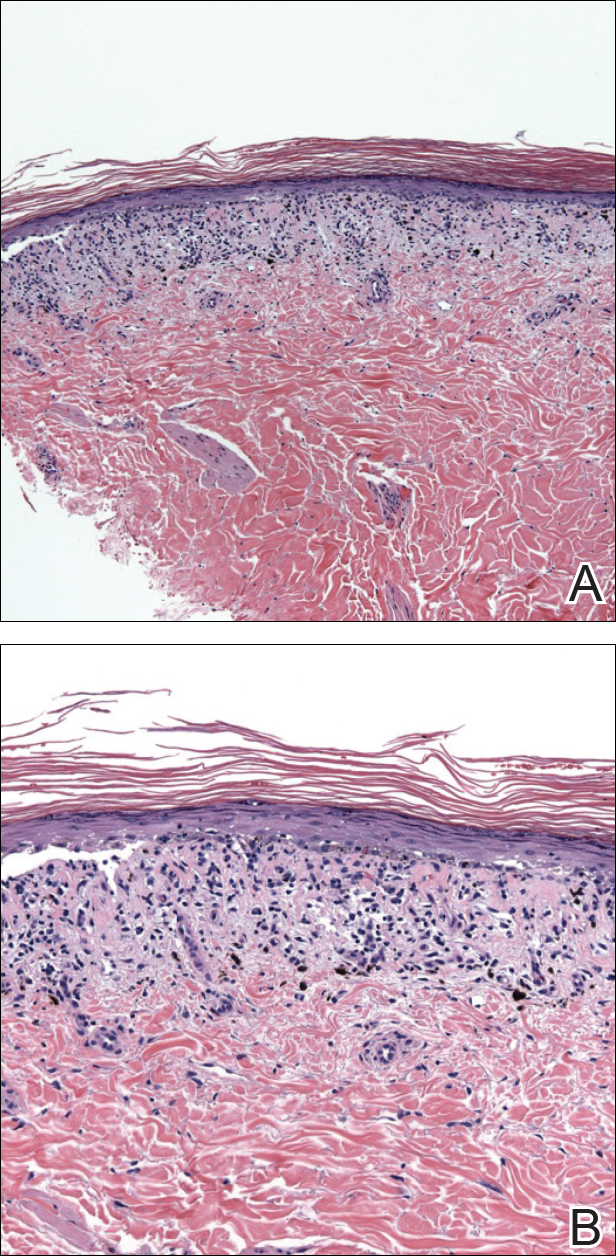
Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.



Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.



Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
Practice Points
- Imatinib mesylate can cause cutaneous adverse reactions including dry skin, alopecia, facial edema, photosensitivity rash, and lichenoid drug eruption (LDE).
- Topical corticosteroids, oral acitretin, and oral steroids may be reasonable treatment options for imatinib-induced LDE if discontinuing imatinib is not possible in a symptomatic patient.
Cosmetic Corner: Dermatologists Weigh in on OTC Adult Acne Products
To improve patient care and outcomes, leading dermatologists offered their recommendations on adult acne products. Consideration must be given to:
- Bioclear Face Lotion and Face Cream
Jan Marini Skin Research, Inc
“These products contain a powerful combination of glycolic, salicylic, and azelaic acids to smooth and brighten acne-prone skin.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- Neutrogena Clear Pore Cleanser/Mask
Johnson & Johnson Consumer Inc
“This is a good daily product for acne-prone skin. It is formulated with benzoyl peroxide and can be used as a daily wash or mask.”—Anthony M. Rossi, MD, New York, New York
- Offects Sulfur Masque Acne Treatment
ZO Skin Health Inc
“This nonirritating mask reduces inflammation and oiliness and is safe to use in pregnancy.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- PanOxyl Acne Foaming Wash and Acne Creamy Wash
Stiefel, a GSK company
Recommended by Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, as well as products for dry cuticles, hyperhidrosis, and sensitive skin will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
[polldaddy:9711250]
To improve patient care and outcomes, leading dermatologists offered their recommendations on adult acne products. Consideration must be given to:
- Bioclear Face Lotion and Face Cream
Jan Marini Skin Research, Inc
“These products contain a powerful combination of glycolic, salicylic, and azelaic acids to smooth and brighten acne-prone skin.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- Neutrogena Clear Pore Cleanser/Mask
Johnson & Johnson Consumer Inc
“This is a good daily product for acne-prone skin. It is formulated with benzoyl peroxide and can be used as a daily wash or mask.”—Anthony M. Rossi, MD, New York, New York
- Offects Sulfur Masque Acne Treatment
ZO Skin Health Inc
“This nonirritating mask reduces inflammation and oiliness and is safe to use in pregnancy.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- PanOxyl Acne Foaming Wash and Acne Creamy Wash
Stiefel, a GSK company
Recommended by Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, as well as products for dry cuticles, hyperhidrosis, and sensitive skin will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
[polldaddy:9711250]
To improve patient care and outcomes, leading dermatologists offered their recommendations on adult acne products. Consideration must be given to:
- Bioclear Face Lotion and Face Cream
Jan Marini Skin Research, Inc
“These products contain a powerful combination of glycolic, salicylic, and azelaic acids to smooth and brighten acne-prone skin.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- Neutrogena Clear Pore Cleanser/Mask
Johnson & Johnson Consumer Inc
“This is a good daily product for acne-prone skin. It is formulated with benzoyl peroxide and can be used as a daily wash or mask.”—Anthony M. Rossi, MD, New York, New York
- Offects Sulfur Masque Acne Treatment
ZO Skin Health Inc
“This nonirritating mask reduces inflammation and oiliness and is safe to use in pregnancy.”—Larisa Ravitskiy, MD, Gahanna, Ohio
- PanOxyl Acne Foaming Wash and Acne Creamy Wash
Stiefel, a GSK company
Recommended by Gary Goldenberg, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, as well as products for dry cuticles, hyperhidrosis, and sensitive skin will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
[polldaddy:9711250]