User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
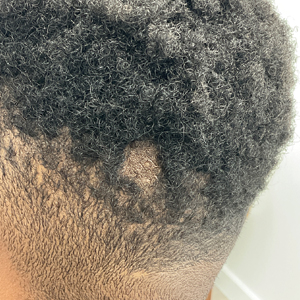
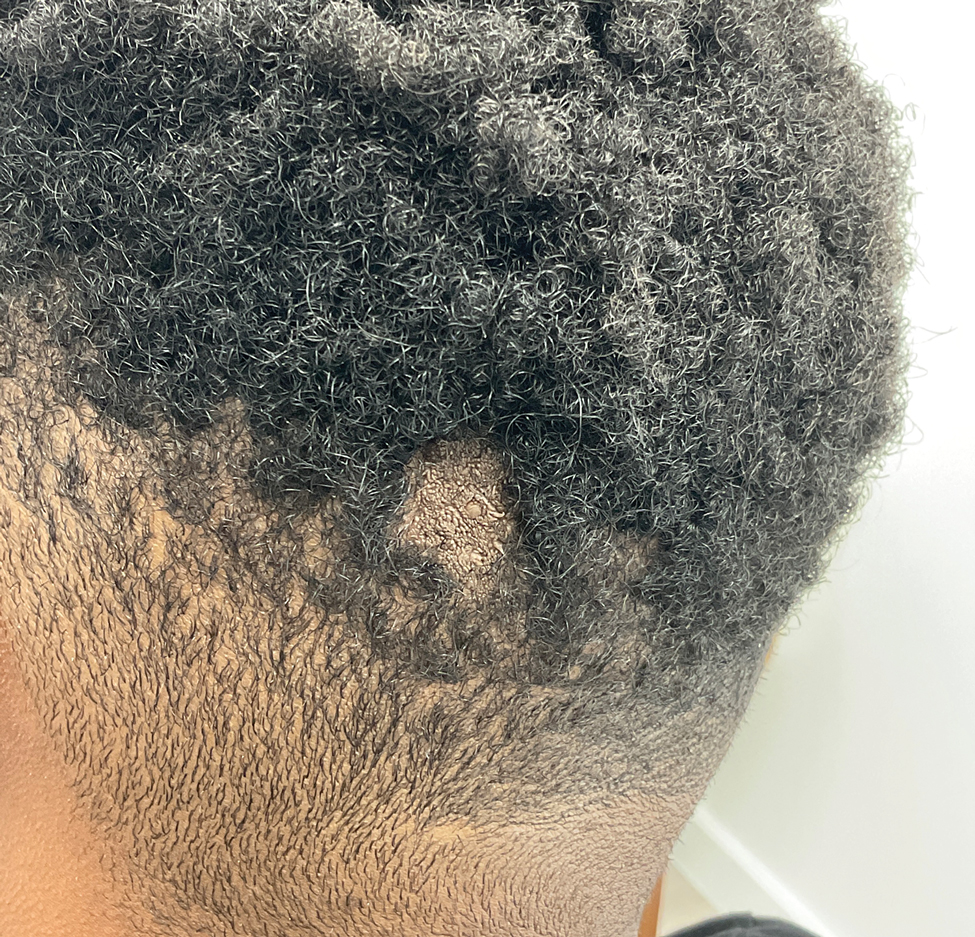
Hairless Scalp Lesion
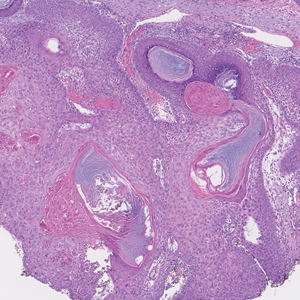
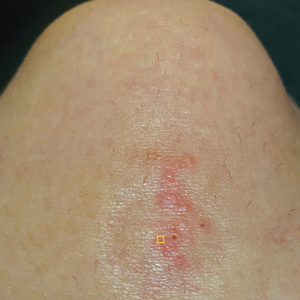
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
A 23-year-old man presented to the dermatology clinic with hair loss on the scalp of several years’ duration. The patient reported persistent pigmented bumps on the back of the scalp. He denied any pruritus or pain and had no systemic symptoms or comorbidities. Physical examination revealed a 1×1.5-cm, yellow-brown, hairless plaque on the left parietal scalp.
Western Pygmy Rattlesnake Envenomation and Bite Management
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
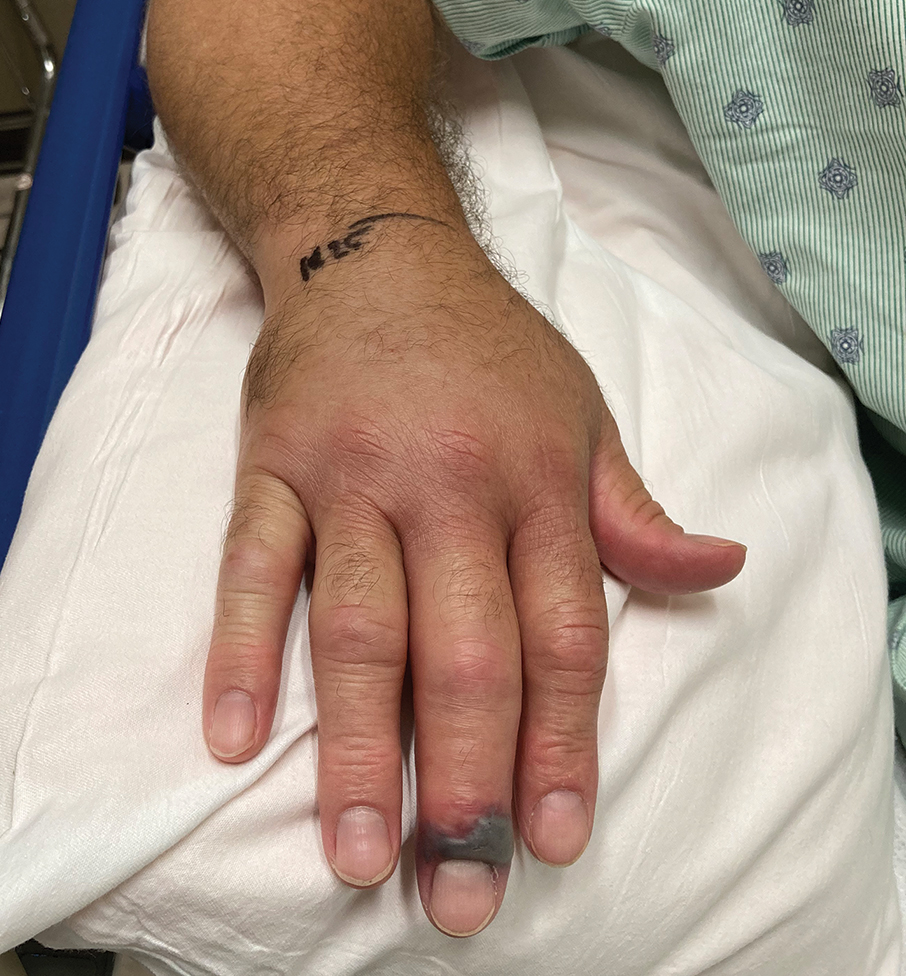
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.

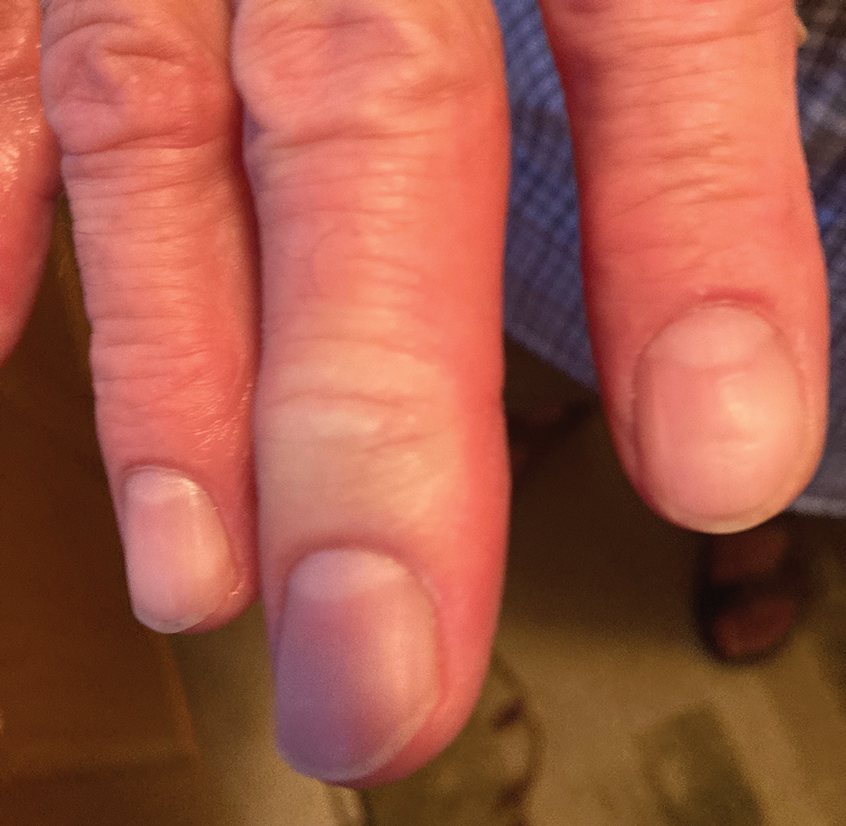
After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
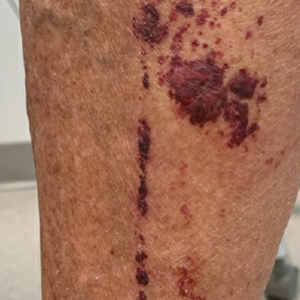
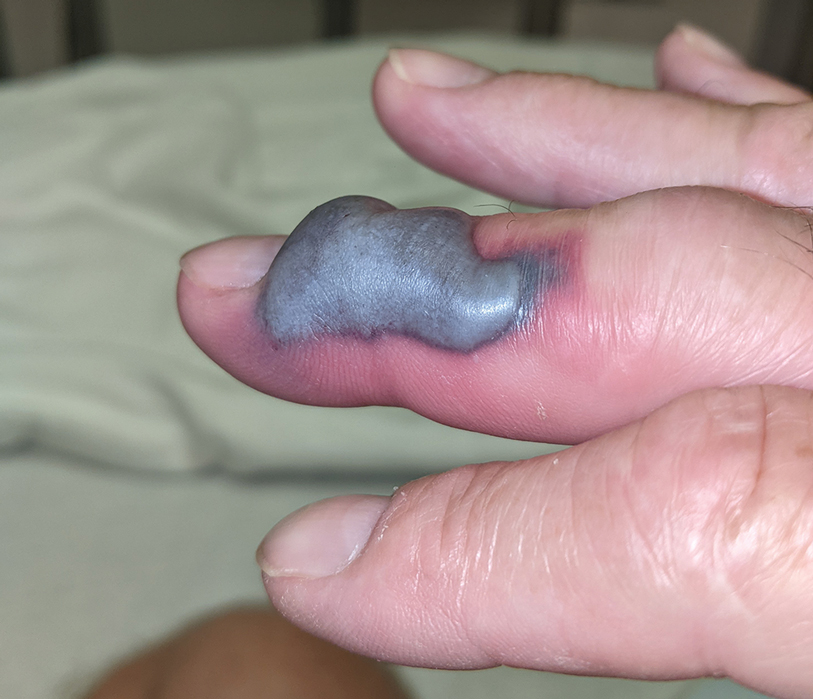
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.
Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.
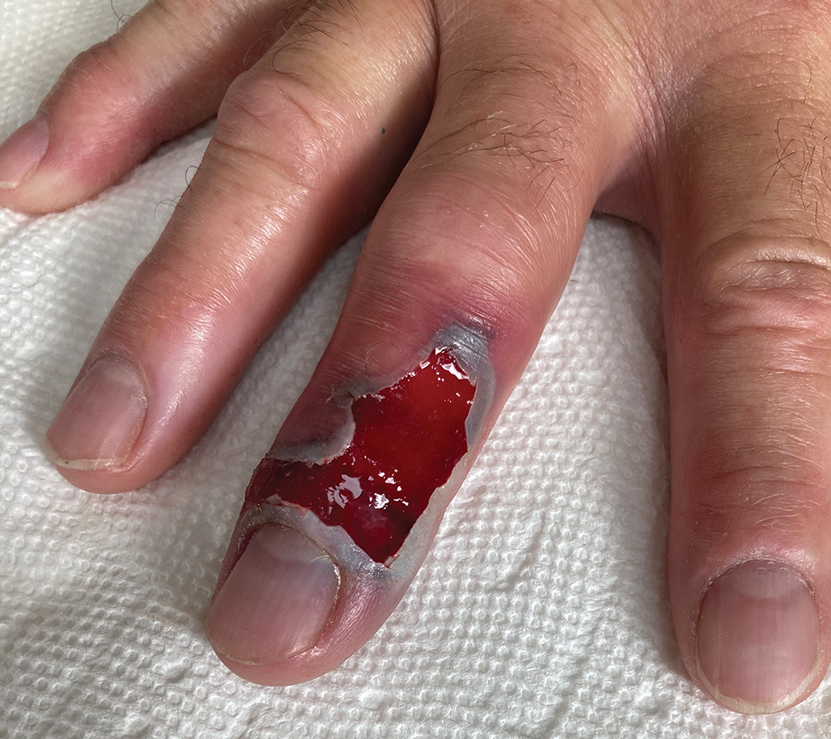
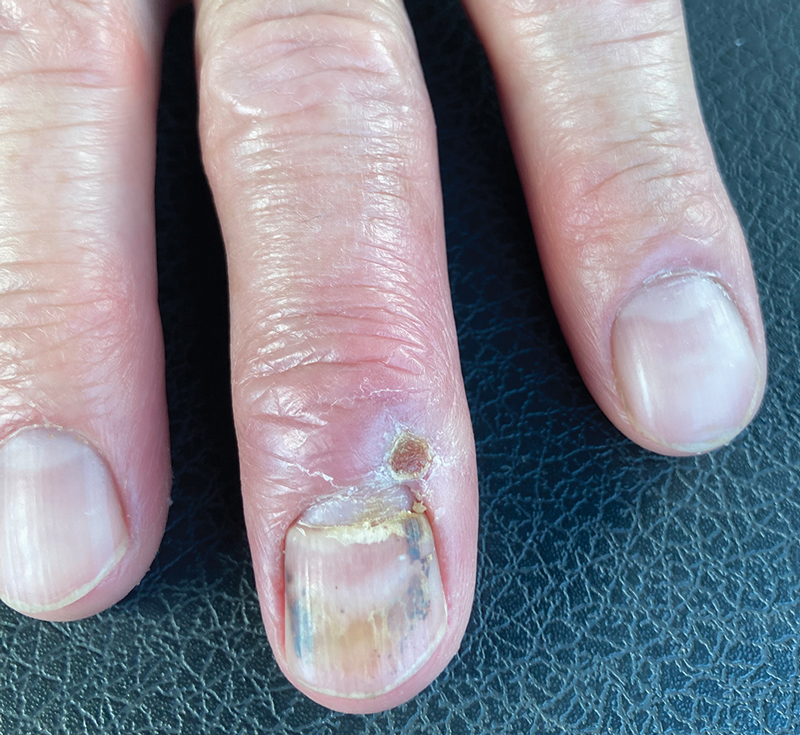
After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.
Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.



After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.

Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.

After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.

Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
There are 375 species of poisonous snakes, with approximately 20,000 deaths worldwide each year due to snakebites, mostly in Asia and Africa.1 The death rate in the United States is 14 to 20 cases per year. In the United States, a variety of rattlesnakes are poisonous. There are 2 genera of rattlesnakes: Sistrurus (3 species) and Crotalus (23 species). The pygmy rattlesnake belongs to the Sistrurus miliarius species that is divided into 3 subspecies: the Carolina pigmy rattlesnake (S miliarius miliarius), the western pygmy rattlesnake (S miliarius streckeri), and the dusky pygmy rattlesnake (S miliarius barbouri).2
The western pygmy rattlesnake belongs to the Crotalidae family. The rattlesnakes in this family also are known as pit vipers. All pit vipers have common characteristics for identification: triangular head, fangs, elliptical pupils, and a heat-sensing pit between the eyes. The western pygmy rattlesnake is found in Missouri, Arkansas, Oklahoma, Kentucky, and Tennessee.1 It is small bodied (15–20 inches)3 and grayish-brown, with a brown dorsal stripe with black blotches on its back. It is found in glades, second-growth forests near rock ledges, and areas where powerlines cut through dense forest.3 Its venom is hemorrhagic, causing tissue damage, but does not contain neurotoxins.4 Bites from the western pygmy rattlesnake often do not lead to death, but the venom, which contains numerous proteins and enzymes, does cause necrotic hemorrhagic ulceration at the site of envenomation and possible loss of digit.5,6
We present a case of a man who was bitten on the right third digit by a western pygmy rattlesnake. We describe the clinical course and treatment.
Case Report
A 56-year-old right-handed man presented to the emergency department with a rapidly swelling, painful hand following a snakebite to the dorsal aspect of the right third digit (Figure 1). He was able to capture a photograph of the snake at the time of injury, which helped identify it as a western pygmy rattlesnake (Figure 2). He also photographed the hand immediately after the bite occurred (Figure 3). Vitals on presentation included an elevated blood pressure of 161/100 mm Hg; no fever (temperature, 36.4 °C); and normal pulse oximetry of 98%, pulse of 86 beats per minute, and respiratory rate of 16 breaths per minute.



After the snakebite, the patient’s family called the Missouri Poison Center immediately. The family identified the snake species and shared this information with the poison center. Poison control recommended calling the nearest hospitals to determine if antivenom was available and make notification of arrival.
The patient’s tetanus toxoid immunization was updated immediately upon arrival. The hand was marked to monitor swelling. Initial laboratory test results revealed the following values: sodium, 133 mmol/L (reference range, 136–145 mmol/L); potassium, 3.4 mmol/L (3.6–5.2 mmol/L); lactic acid, 2.4 mmol/L (0.5–2.2 mmol/L); creatine kinase, 425 U/L (55–170 U/L); platelet count, 68/µL (150,000–450,000/µL); fibrinogen, 169 mg/dL (185–410 mg/dL); and glucose, 121 mg/dL (74–106 mg/dL). The remainder of the complete blood cell count and metabolic panel was unremarkable. Radiographs of the hand did not show any fractures, dislocations, or foreign bodies. Missouri Poison Center was consulted. Given the patient’s severe pain, edema beyond 40 cm, and developing ecchymosis on the inner arm, the bite was graded as a 3 on the traditional snakebite severity scale. Poison control recommended 4 to 6 vials of antivenom over 60 minutes. Six vials of Crotalidae polyvalent immune fab antivenom were given.
The patient’s complete blood cell count remained unremarkable throughout his admission. His metabolic panel returned to normal at 6 hours postadmission: sodium, 139 mmol/L; potassium, 4.0 mmol/L. His lactate and creatinine kinase were not rechecked. His fibrinogen was trending upward. Serial laboratory test results revealed fibrinogen levels of 153, 158, 161, 159, 173, and 216 mg/dL at 6, 12, 18, 24, 30, and 36 hours, respectively. Other laboratory test results including prothrombin time (11.0 s) and international normalized ratio (0.98) remained within reference range (11–13 s and 0.80–1.39, respectively) during serial monitoring.
The patient was hospitalized for 40 hours while waiting for his fibrinogen level to normalize. The local skin necrosis worsened acutely in this 40-hour window (Figure 4). Intravenous antibiotics were not administered during the hospital stay. Before discharge, the patient was evaluated by the surgery service, who did not recommend debridement.

Following discharge, the patient consulted a wound care expert. The area of necrosis was unroofed and debrided in the outpatient setting (Figure 5). The patient was started on oral cefalexin 500 mg twice daily for 10 days and instructed to perform twice-daily dressing changes with silver sulfadiazine cream 1%. A hand surgeon was consulted for consideration of a reverse cross-finger flap, which was not recommended. Twice-daily dressing changes for the wound—consisting of application of silver sulfadiazine cream 1% directly to the wound followed by gauze, self-adhesive soft-rolled gauze, and elastic bandages—were performed for 2 weeks.

After 2 weeks, the wound was left open to the air and cleaned with soap and water as needed. At 6 weeks, the wound was completely healed via secondary intention, except for some minor remaining ulceration at the location of the fang entry point (Figure 6). The patient had no loss of finger function or sensation.

Surgical Management of Snakebites
The surgeon’s role in managing snakebites is controversial. Snakebites were once perceived as a surgical emergency due to symptoms mimicking compartment syndrome; however, snakebites rarely cause a true compartment syndrome.7 Prophylactic bite excision and fasciotomies are not recommended. Incision and suction of the fang marks may be beneficial if performed within 15 to 30 minutes from the time of the bite.8 With access to a surgeon in this short time period being nearly impossible, incision and suctioning of fang marks generally is not recommended.9 Retained snake fangs are a possibility, and the infection could spread to a nearby joint, causing septic arthritis,10 which would be an indication for surgical intervention. Bites to the finger often cause major swelling, and the benefits of dermotomy are documented.11 Generally, early administration of antivenom will decrease local tissue reaction and prevent additional tissue loss.12 In our patient, the decision to perform dermotomy was made when the area of necrosis had declared itself and the skin reached its elastic limit. Bozkurt et al13 described the neurovascular bundles within the digit as functioning as small compartments. When the skin of the digit reaches its elastic limit, pressure within the compartment may exceed the capillary closing pressure, and the integrity of small vessels and nerves may be compromised. Our case highlights the benefit of dermotomy as well as the functional and cosmetic results that can be achieved.
Wound Care for Snakebites
There is little published on the treatment of snakebites after patients are stabilized medically for hospital discharge. Venomous snakes inject toxins that predominantly consist of enzymes (eg, phospholipase A2, phosphodiesterase, hyaluronidase, peptidase, metalloproteinase) that cause tissue destruction through diverse mechanisms.14 The venom of western pygmy rattlesnakes is hemotoxic and can cause necrotic hemorrhagic ulceration,4 as was the case in our patient.
Silver sulfadiazine commonly is used to prevent infection in burn patients. Given the large surface area of exposed dermis after debridement and concern for infection, silver sulfadiazine was chosen in our patient for local wound care treatment. Silver sulfadiazine is a widely available and low-cost drug.15 Its antibacterial effects are due to the silver ions, which only act superficially and therefore limit systemic absorption.16 Application should be performed in a clean manner with minimal trauma to the tissue. This technique is best achieved by using sterile gloves and applying the medication manually. A 0.0625-inch layer should be applied to entirely cover the cleaned debrided area.17 When performing application with tongue blades or cotton swabs, it is important to never “double dip.” Patient education on proper administration is imperative to a successful outcome.
Final Thoughts
Our case demonstrates the safe use of Crotalidae polyvalent immune fab antivenom for the treatment of western pygmy rattlesnake (S miliarius streckeri) envenomation. Early administration of antivenom following pit viper rattlesnake envenomations is important to mitigate systemic effects and the extent of soft tissue damage. There are few studies on local wound care treatment after rattlesnake envenomation. This case highlights the role of dermotomy and wound care with silver sulfadiazine cream 1%.
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
- Biggers B. Management of Missouri snake bites. Mo Med. 2017;114:254-257.
- Stamm R. Sistrurus miliarius pigmy rattlesnake. University of Michigan Museum of Zoology. Accessed September 23, 2024. https://animaldiversity.org/accounts/Sistrurus_miliarius/
- Missouri Department of Conservation. Western pygmy rattlesnake. Accessed September 18, 2024. https://mdc.mo.gov/discover-nature/field-guide/western-pygmy-rattlesnake
- AnimalSake. Facts about the pigmy rattlesnake that are sure to surprise you. Accessed September 18, 2024. https://animalsake.com/pygmy-rattlesnake
- King AM, Crim WS, Menke NB, et al. Pygmy rattlesnake envenomation treated with crotalidae polyvalent immune fab antivenom. Toxicon. 2012;60:1287-1289.
- Juckett G, Hancox JG. Venomous snakebites in the United States: management review and update. Am Fam Physician. 2002;65:1367-1375.
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217:726-735.
- Cribari C. Management of poisonous snakebite. American College of Surgeons Committee on Trauma; 2004. https://www.hartcountyga.gov/documents/PoisonousSnakebiteTreatment.pdf
- Walker JP, Morrison RL. Current management of copperhead snakebite. J Am Coll Surg. 2011;212:470-474.
- Gelman D, Bates T, Nuelle JAV. Septic arthritis of the proximal interphalangeal joint after rattlesnake bite. J Hand Surg Am. 2022;47:484.e1-484.e4.
- Watt CH Jr. Treatment of poisonous snakebite with emphasis on digit dermotomy. South Med J. 1985;78:694-699.
- Corneille MG, Larson S, Stewart RM, et al. A large single-center experience with treatment of patients with crotalid envenomations: outcomes with and evolution of antivenin therapy. Am J Surg. 2006;192:848-852.
- Bozkurt M, Kulahci Y, Zor F, et al. The management of pit viper envenomation of the hand. Hand (NY). 2008;3:324-331.
- Aziz H, Rhee P, Pandit V, et al. The current concepts in management of animal (dog, cat, snake, scorpion) and human bite wounds. J Trauma Acute Care Surg. 2015;78:641-648.
- Hummel RP, MacMillan BG, Altemeier WA. Topical and systemic antibacterial agents in the treatment of burns. Ann Surg. 1970;172:370-384.
- Modak SM, Sampath L, Fox CL. Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359-363.
- Oaks RJ, Cindass R. Silver sulfadiazine. StatPearls [Internet]. Updated January 22, 2023. Accessed September 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556054/
Practice Points
- Patients should seek medical attention immediately for western pygmy rattlesnake bites for early initiation of antivenom treatment.
- Contact the closest emergency department to confirm they are equipped to treat rattlesnake bites and notify them of a pending arrival.
- Consider dermotomy or local debridement of bites involving the digits.
- Monitor the wound in the days and weeks following the bite to ensure adequate healing.
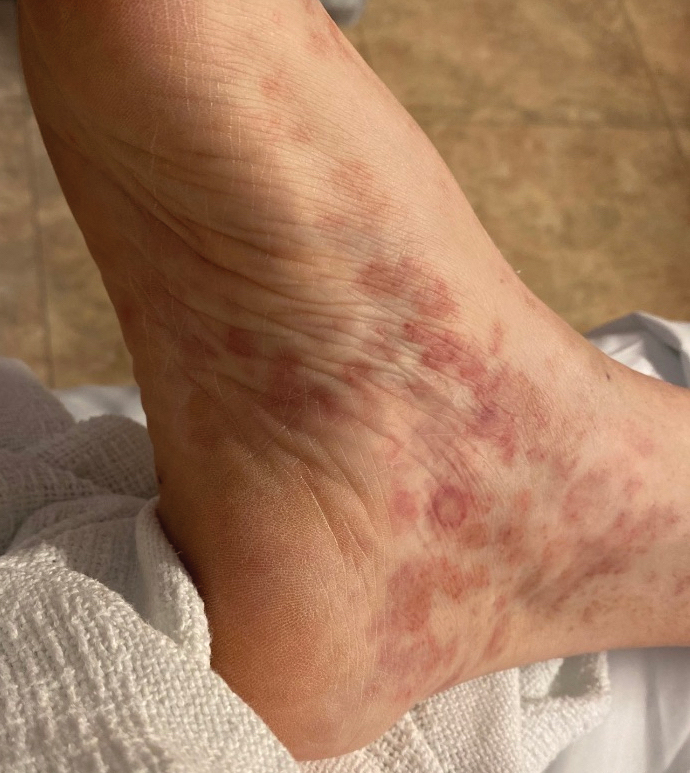
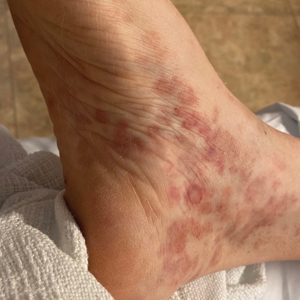
Multiple Painless Whitish Papules on the Vulva and Perianal Region
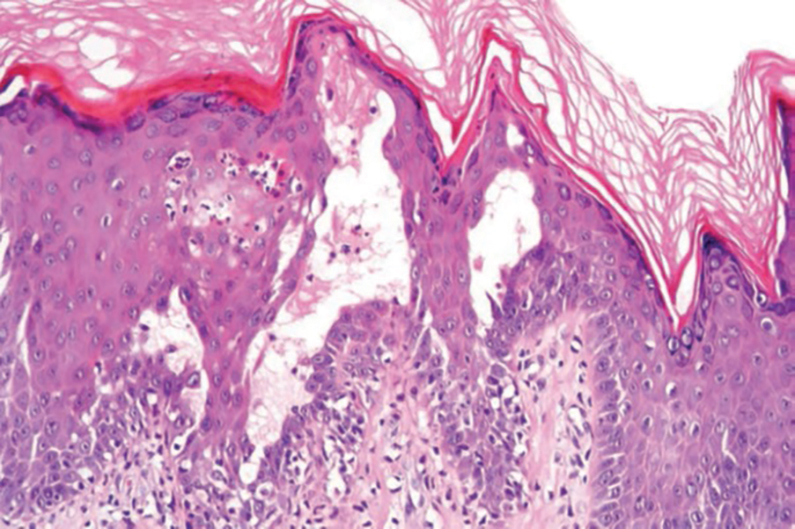
THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
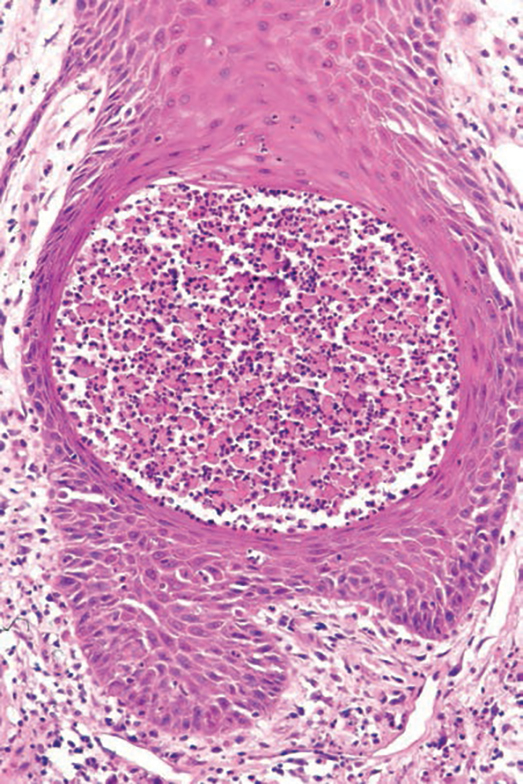
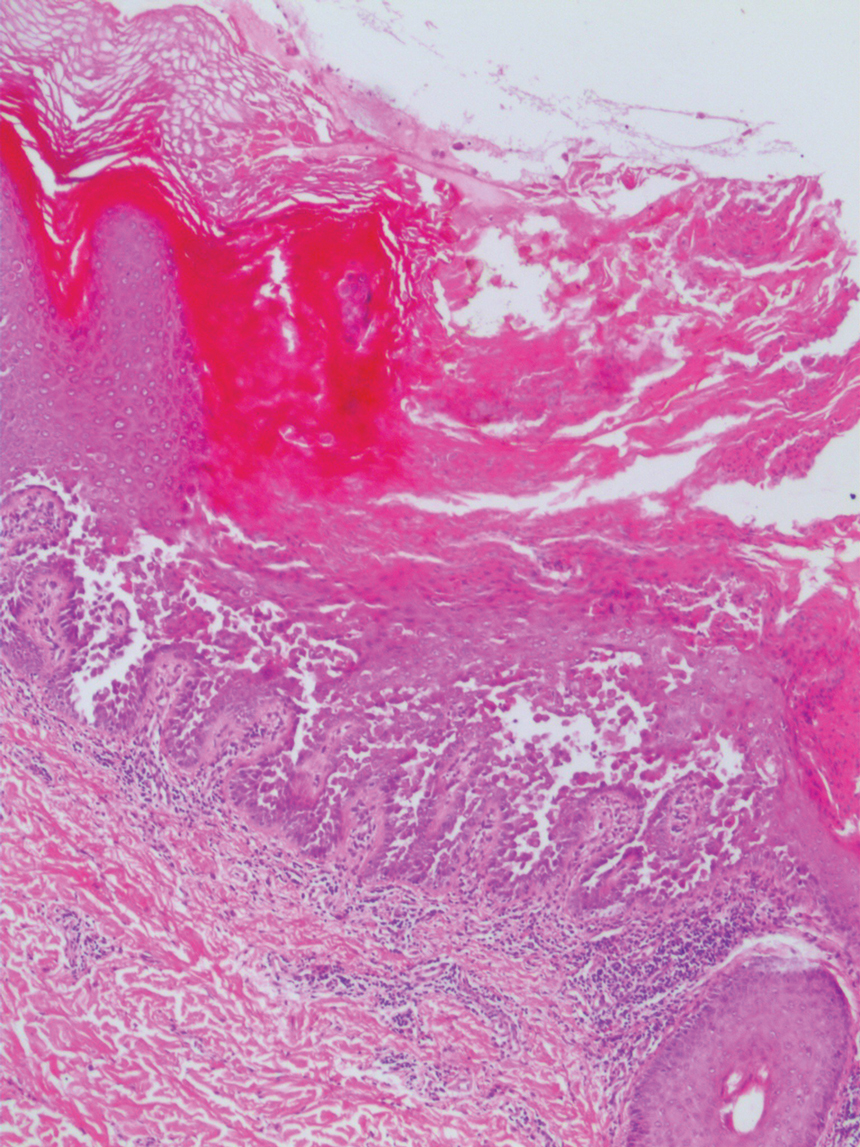
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5
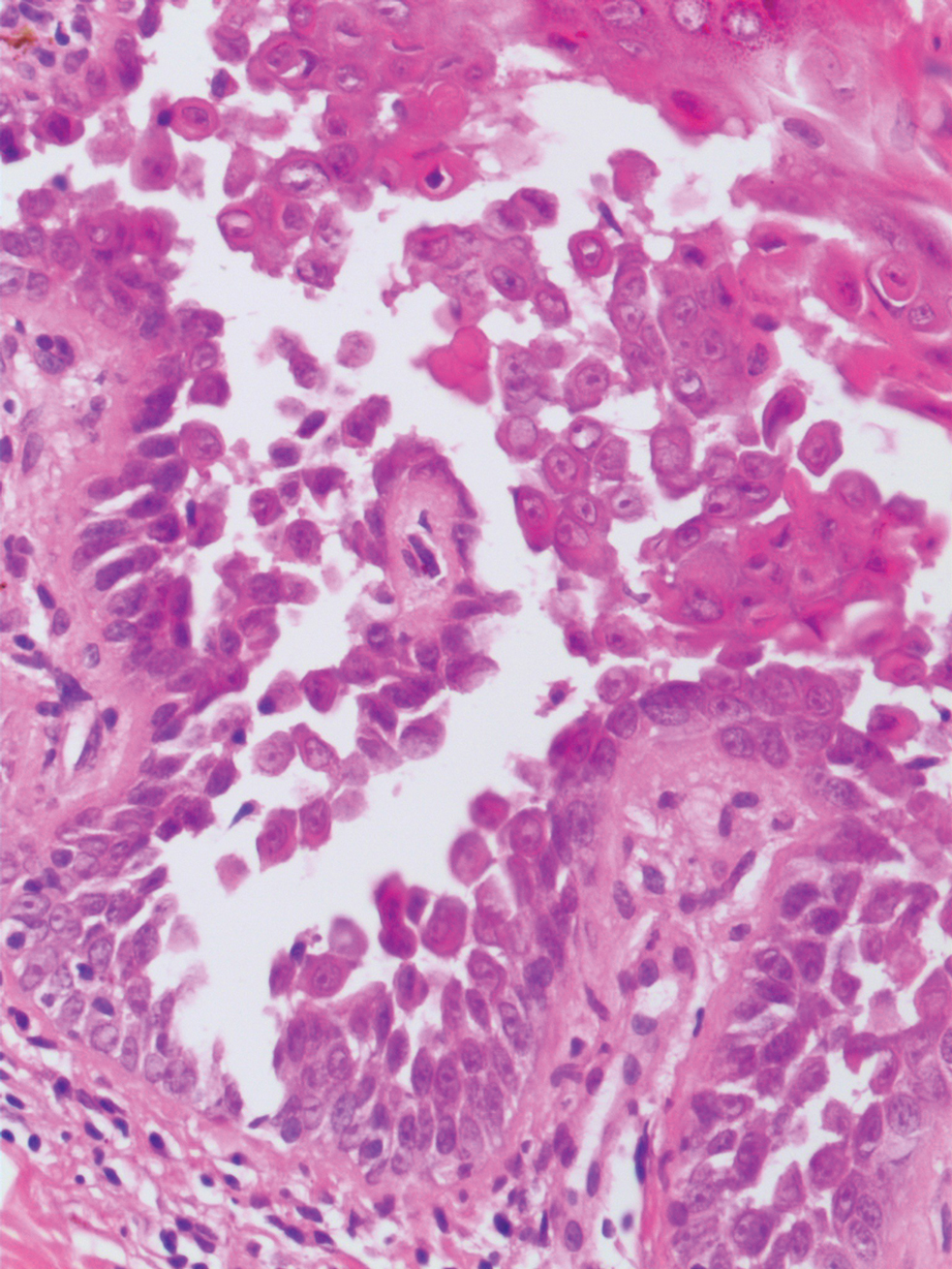
Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4
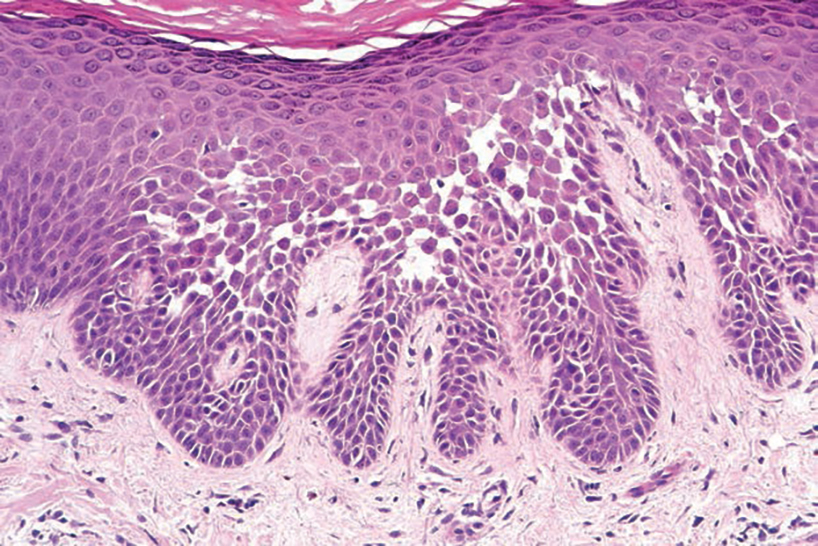
characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4
- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5

Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4

characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4

THE DIAGNOSIS: Papular Acantholytic Dyskeratosis
Histopathology of the lesion in our patient revealed hyperkeratosis, parakeratosis, dyskeratosis, and acantholysis of keratinocytes. The dermis showed variable chronic inflammatory cells. Corps ronds and grains in the acantholytic layer of the epidermis were identified. Hair follicles were not affected by acantholysis. Anti–desmoglein 1 and anti–desmoglein 3 serum antibodies were negative. Based on the combined clinical and histologic findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD) of the genitocrural area.
Although its typical histopathologic pattern mimics both Hailey-Hailey disease and Darier disease, PAD is a rare unique clinicopathologic entity recognized by dermatopathologists. It usually occurs in middle-aged women with no family history of similar conditions. The multiple localized, flesh-colored to whitish papules of PAD tend to coalesce into plaques in the anogenital and genitocrural regions. Plaques usually are asymptomatic but may be pruritic. Histopathologically, PAD will demonstrate hyperkeratosis, dyskeratosis, and acantholysis. Corps ronds and grains will be present in the acantholytic layer of the epidermis.1,2
The differential diagnosis for PAD includes pemphigus vegetans, Hailey-Hailey disease, Darier disease, and Grover disease. Patients usually develop pemphigus vegetans at an older age (typically 50–70 years).3 Histopathologically, it is characterized by pseudoepitheliomatous hyperplasia with an eosinophilic microabscess as well as acantholysis that involves the follicular epithelium (Figure 1),4 which were not seen in our patient. Direct immunofluorescence will show the intercellular pattern of the pemphigus group, and antidesmoglein antibodies can be detected by enzyme-linked immunosorbent assay.4,5

Hailey-Hailey disease (also known as benign familial pemphigus) typically manifests as itchy malodorous vesicles and erosions, especially in intertriginous areas. The most commonly affected sites are the groin, neck, under the breasts, and between the buttocks. In one study, two-thirds of affected patients reported a relevant family history.4 Histopathology will show minimal dyskeratosis and suprabasilar acantholysis with loss of intercellular bridges, classically described as resembling a dilapidated brick wall (Figure 2).4,5 There is no notable follicular involvement with acantholysis.4

characteristic dilapidated brick wall appearance (H&E, original
magnification ×40).
Darier disease (also known as keratosis follicularis) typically is inherited in an autosomal-dominant pattern.4 It is found on the seborrheic areas such as the scalp, forehead, nasolabial folds, and upper chest. Characteristic features include distal notching of the nails, mucosal lesions, and palmoplantar papules. Histopathology will reveal acantholysis, dyskeratosis, suprabasilar acantholysis, and corps ronds and grains.4 Acantholysis in Darier disease can be in discrete foci and/or widespread (Figure 3).4 Darier disease demonstrates more dyskeratosis than Hailey-Hailey disease.4,5

Grover disease (also referred to as transient acantholytic dermatosis) is observed predominantly in individuals who are middle-aged or older, though occurrence in children has been rarely reported.4 It affects the trunk, neck, and proximal limbs but spares the genital area. Histopathology may reveal acantholysis (similar to Hailey-Hailey disease or pemphigus vulgaris), dyskeratosis (resembling Darier disease), spongiosis, parakeratosis, and a superficial perivascular lymphocytic infiltrate with eosinophils.4 A histologic clue to the diagnosis is small lesion size (1–3 mm). Usually, only 1 or 2 small discrete lesions that span a few rete ridges are noted (Figure 4).4 Grover disease can cause follicular or acrosyringeal involvement.4

- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
- Al-Muriesh M, Abdul-Fattah B, Wang X, et al. Papular acantholytic dyskeratosis of the anogenital and genitocrural area: case series and review of the literature. J Cutan Pathol. 2016;43:749-758. doi:10.1111/cup.12736
- Harrell J, Nielson C, Beers P, et al. Eruption on the vulva and groin. JAAD Case Reports. 2019;6:6-8. doi:10.1016/j.jdcr.2019.11.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls [Internet]. Updated June 26, 2023. Accessed September 18, 2024. https://www.ncbi.nlm.nih.gov/books/NBK545229
- Acantholytic disorders. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin: With Clinical Correlations. Elsevier/ Saunders; 2012:171-200.
- Mohr MR, Erdag G, Shada AL, et al. Two patients with Hailey- Hailey disease, multiple primary melanomas, and other cancers. Arch Dermatol. 2011;147:211215. doi:10.1001/archdermatol.2010.445
A 21-year-old woman presented with a chronic eruption in the anogenital region of 4 years’ duration. Clinical examination revealed numerous painless, mildly itchy, malodorous, whitish papules on an erythematous base that were distributed on the vulva and perianal region. There were no erosions, and no other areas were involved. Routine laboratory tests were within reference range. The patient had no sexual partner and no family history of similar lesions. A skin biopsy was performed.
Pediatric Melanoma Outcomes by Race and Socioeconomic Factors
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
To the Editor:
Skin cancers are extremely common worldwide. Malignant melanomas comprise approximately 1 in 5 of these cancers. Exposure to UV radiation is postulated to be responsible for a global rise in melanoma cases over the past 50 years.1 Pediatric melanoma is a particularly rare condition that affects approximately 6 in every 1 million children.2 Melanoma incidence in children ranges by age, increasing by approximately 10-fold from age 1 to 4 years to age 15 to 19 years. Tumor ulceration is a feature more commonly seen among children younger than 10 years and is associated with worse outcomes. Tumor thickness and ulceration strongly predict sentinel lymph node metastases among children, which also is associated with a poor prognosis.3
A recent study evaluating stage IV melanoma survival rates in adolescents and young adults (AYAs) vs older adults found that survival is much worse among AYAs. Thicker tumors and public health insurance also were associated with worse survival rates for AYAs, while early detection was associated with better survival rates.4
Health disparities and their role in the prognosis of pediatric melanoma is another important factor. One study analyzed this relationship at the state level using Texas Cancer Registry data (1995-2009).5 Patients’ socioeconomic status (SES) and driving distance to the nearest pediatric cancer care center were included in the analysis. Hispanic children were found to be 3 times more likely to present with advanced disease than non-Hispanic White children. Although SES and distance to the nearest treatment center were not found to affect the melanoma stage at presentation, Hispanic ethnicity or being in the lowest SES quartile were correlated with a higher mortality risk.5
When considering specific subtypes of melanoma, acral lentiginous melanoma (ALM) is known to develop in patients with skin of color. A 2023 study by Holman et al6 reported that the percentage of melanomas that were ALMs ranged from 0.8% in non-Hispanic White individuals to 19.1% in Hispanic Black, American Indian/Alaska Native, and Asian/Pacific Islander individuals. However, ALM is rare in children. In a pooled cohort study with patient information retrieved from the nationwide Dutch Pathology Registry, only 1 child and 1 adolescent were found to have ALM across a total of 514 patients.7 We sought to analyze pediatric melanoma outcomes based on race and other barriers to appropriate care.
We conducted a search of the Surveillance, Epidemiology, and End Results (SEER) database from January 1995 to December 2016 for patients aged 21 years and younger with a primary melanoma diagnosis. The primary outcome was the 5-year survival rate. County-level SES variables were used to calculate a prosperity index. Kaplan-Meier analysis and Cox proportional hazards model were used to compare 5-year survival rates among the different racial/ethnic groups.
A sample of 2742 patients was identified during the study period and followed for 5 years. Eighty-two percent were White, 6% Hispanic, 2% Asian, 1% Black, and 5% classified as other/unknown race (data were missing for 4%). The cohort was predominantly female (61%). White patients were more likely to present with localized disease than any other race/ethnicity (83% vs 65% in Hispanic, 60% in Asian/Pacific Islander, and 45% in Black patients [P<.05]).
Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis. On multivariate analysis, this finding remained significant for Hispanic patients when compared with White patients (hazard ratio, 2.37 [P<.05]). Increasing age, male sex, advanced stage at diagnosis, and failure to receive surgery were associated with increased odds of mortality.
Patients with regionalized and disseminated disease had increased odds of mortality (6.16 and 64.45, respectively; P<.05) compared with patients with localized disease. Socioeconomic status and urbanization were not found to influence 5-year survival rates.
Pediatric melanoma often presents a clinical challenge with special considerations. Pediatric-specific predisposing risk factors for melanoma and an atypical clinical presentation are some of the major concerns that necessitate a tailored approach to this malignancy, especially among different age groups, skin types, and racial and socioeconomic groups.5
Standard ABCDE criteria often are inadequate for accurate detection of pediatric melanomas. Initial lesions often manifest as raised, red, amelanotic lesions mimicking pyogenic granulomas. Lesions tend to be very small (<6 mm in diameter) and can be uniform in color, thereby making the melanoma more difficult to detect compared to the characteristic findings in adults.5 Bleeding or ulceration often can be a warning sign during physical examination.
With regard to incidence, pediatric melanoma is relatively rare. Since the 1970s, the incidence of pediatric melanoma has been increasing; however, a recent analysis of the SEER database showed a decreasing trend from 2000 to 2010.4
Our analysis of the SEER data showed an increased risk for pediatric melanoma in older adolescents. In addition, the incidence of pediatric melanoma was higher in females of all racial groups except Asian/Pacific Islander individuals. However, SES was not found to significantly influence the 5-year survival rate in pediatric melanoma.
White pediatric patients were more likely to present with localized disease compared with other races. Pediatric melanoma patients with regional disease had a 6-fold increase in mortality rate vs those with localized disease; those with disseminated disease had a 65-fold higher risk. Consistent with this, Black and Hispanic patients had the worst 5-year survival rates on bivariate analysis.
These findings suggest a relationship between race, melanoma spread, and disease severity. Patient education programs need to be directed specifically to minority groups to improve their knowledge on evolving skin lesions and sun protection practices. Physicians also need to have heightened suspicion and better knowledge of the unique traits of pediatric melanoma.5
Given the considerable influence these disparities can have on melanoma outcomes, further research is needed to characterize outcomes based on race and determine obstacles to appropriate care. Improved public outreach initiatives that accommodate specific cultural barriers (eg, language, traditional patterns of behavior) also are required to improve current circumstances.
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
- Arnold M, Singh D, Laversanne M, et al. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 2022;158:495-503.
- McCormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153:707-715.
- Saiyed FK, Hamilton EC, Austin MT. Pediatric melanoma: incidence, treatment, and prognosis. Pediatric Health Med Ther. 2017;8:39-45.
- Wojcik KY, Hawkins M, Anderson-Mellies A, et al. Melanoma survival by age group: population-based disparities for adolescent and young adult patients by stage, tumor thickness, and insurance type. J Am Acad Dermatol. 2023;88:831-840.
- Hamilton EC, Nguyen HT, Chang YC, et al. Health disparities influence childhood melanoma stage at diagnosis and outcome. J Pediatr. 2016;175:182-187.
- Holman DM, King JB, White A, et al. Acral lentiginous melanoma incidence by sex, race, ethnicity, and stage in the United States, 2010-2019. Prev Med. 2023;175:107692. doi:10.1016/j.ypmed.2023.107692
- El Sharouni MA, Rawson RV, Potter AJ, et al. Melanomas in children and adolescents: clinicopathologic features and survival outcomes. J Am Acad Dermatol. 2023;88:609-616. doi:10.1016/j.jaad.2022.08.067
Practice Points
- Pediatric melanoma is a unique clinical entity with a different clinical presentation than in adults.
- Thicker tumors and disseminated disease are associated with a worse prognosis, and these factors are more commonly seen in Black and Hispanic patients.
Eyelid Dermatitis: Common Patterns and Contact Allergens
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.
Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46
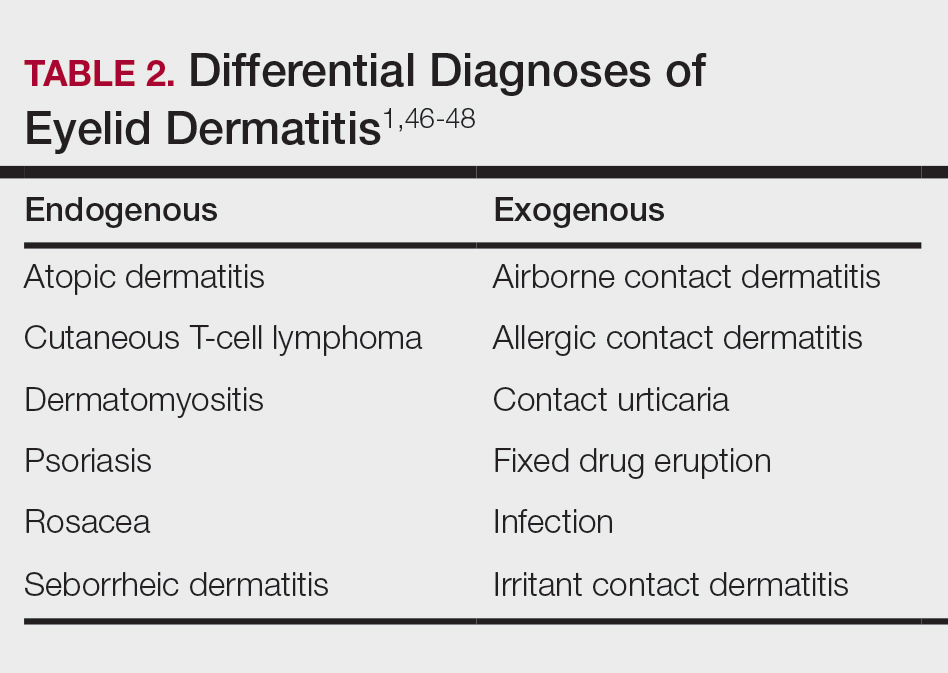
Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49 Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49 Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
Eyelid dermatitis is a common dermatologic concern representing a broad group of inflammatory dermatoses and typically presenting as eczematous lesions on the eyelids.1 One of the most common causes of eyelid dermatitis is thought to be allergic contact dermatitis (ACD), a type IV delayed hypersensitivity reaction caused by exposure to external allergens.2 Although ACD can occur anywhere on the body, dermatitis on the face and eyelids is quite common.1,2 This article aims to explore the clinical manifestation, evaluation, and management of eyelid ACD.
Pathophysiology of Eyelid ACD
Studies have shown that ACD is the most common cause of eyelid dermatitis, estimated to account for 46% to 72% of cases worldwide.3-6 Allergic contact dermatitis is a T cell–mediated type IV hypersensitivity reaction to external antigens that manifests as eczematous lesions at the site of contact with the allergen that may spread.7 Allergic contact dermatitis is a common condition, and it is estimated that at least 20% of the general worldwide population has a contact allergy.8,9 Histologically, ACD manifests as spongiotic dermatitis, though this is not unique and also may be seen in atopic dermatitis (AD) and irritant contact dermatitis.2 Allergic contact dermatitis is diagnosed via epicutaneous patch testing, and treatment involves allergen avoidance with or without adjuvant topical and/or systemic immunomodulatory treatments.7
The eyelids are uniquely prone to the development of ACD given their thinner epidermis and increased susceptibility to irritation. They frequently are exposed to allergens through the direct topical route as well as indirectly via airborne exposure, rinse-down products (eg, shampoos), and substances transferred from an individual’s own hands. The occluded skin folds of the eyelids facilitate increased exposure to trapped allergens.10,11 Additionally, the skin of the eyelids is thin, flexible, highly vascularized, and lacking in subcutaneous tissue, making this area more susceptible to antigen penetration than other locations on the body.1,2,10,12,13
Clinical Manifestations
Eyelid ACD is more common in females than males, which is thought to be related to increased use of cosmetics and fragrances.1,3,12,14-16 Clinical manifestations may resemble eczematous papules and plaques.1 Eyelid ACD commonly spreads beyond the eyelid margin, which helps to differentiate it from AD and irritant contact dermatitis. Symptoms of ACD on the eyelids typically include pruritus, redness, swelling, tearing, scaling, and pain.2 Persistent untreated eyelid dermatitis can lead to eyelash loss, damage to meibomian glands, and hyperpigmentation.2,17,18
Patterns of Eyelid ACD
Allergic contact dermatitis on the eyelids can occur due to direct application of allergens onto the skin of the eyelids, runoff of products from the hair/scalp (eg, shampoo), transfer of allergens from the hands, or contact with airborne allergens.1,2,11,12 Some reports have suggested that eyelid ACD more often is caused by products applied to the scalp or face rather than those applied directly to the eyelids.11 Because the scalp and face are less reactive to contact allergens, in some cases the eyelids may be the only affected site.10,12,13
The specific pattern of dermatitis on or around the eyelids can provide clues to the allergenic source. Dermatitis present around the eyelids and periorbital region with involvement of the bilateral upper and lower eyelids suggests direct exposure to a contact allergen, such as makeup or other cosmetic products.1 Unilateral involvement of only 1 eyelid can occur with ectopic transfer of allergens from the hands or nails.1,19 Involvement of the fingers or nails in addition to the eyelids may further suggest ectopic transfer, such as from allergens in nail polish.10 Unilateral eyelid dermatitis also could be caused by unique exposures such as a microscope or camera eyepiece.19 Distribution around the lower eyelids and upper cheeks is indicative of a drip or runoff pattern, which may result from an ophthalmic solution such as eye drops or contact lens solution.1,19 Finally, dermatitis affecting the upper eyelids along with the nasolabial folds and upper chest may suggest airborne contact dermatitis to fragrances or household cleaning products.1,11
Common Culprits of Eyelid ACD
Common causes of eyelid ACD include cosmetic products, ophthalmic medications, nail lacquers, and jewelry.10,13,20 Within the broader category of cosmetics, allergens may be found in makeup and makeup removers, cosmetic applicators and brushes, soaps and cleansers, creams and sunscreens, antiaging products, hair products, nail polish and files, and hair removal products, among many others.10,13,16,20 Additionally, ophthalmologic and topical medications are common sources of ACD, including eyedrops, contact lens solution, and topical antibiotics.10,13,21 Costume jewelry commonly contains allergenic metals, which also can be found in eyelash curlers, eyeglasses, toys, and other household items.22,23 Finally, contact allergens can be found in items such as goggles, gloves, textiles, and a variety of other occupational and household exposures.
Allergic contact dermatitis of the eyelids occurs predominantly—but not exclusively—in females.16,20,24 This finding has been attributed to the traditionally greater use of cosmetics and fragrances among women; however, the use of skin care products among men is increasing, and recent studies have shown the eyelids to be a common location of facial contact dermatitis among men.16,24 Although eyelid dermatitis has not been specifically analyzed by sex, a retrospective analysis of 1332 male patients with facial dermatitis found the most common sites to be the face (not otherwise specified)(48.9%), eyelids (23.5%), and lips (12.6%). In this cohort, the most common allergens were surfactants in shampoos and paraphenylenediamine in hair dyes.24
Common Allergens
Common contact allergens among patients with ACD of the eyelids include metals, fragrances, preservatives, acrylates, and topical medications.3,10,16,20,25-27 Sources of common contact allergens are reviewed in Table 1.

Metals—Metals are among the most common causes of ACD overall, and nickel frequently is reported as one of the top contact allergens in patients with eyelid dermatitis.16,27 A retrospective analysis of 2332 patients with eyelid dermatitis patch tested by the North American Contact Dermatitis Group from 1994 to 2016 found that 18.6% of patients with eyelid ACD had a clinically relevant nickel allergy. Sources of nickel exposure include jewelry, grooming devices, makeup and makeup applicators, and eyelash curlers, as well as direct transfer from the hands after contact with consumer products.16
Other metals that can cause ACD include cobalt (found in similar products to nickel) and gold. Gold often is associated with eyelid dermatitis, though its clinical relevance has been debated, as gold is a relatively inert metal that rarely is present in eye cosmetics and its ions are not displaced from objects and deposited on the skin via sweat in the same way as nickel.4,16,20,28-30 Despite this, studies have shown that gold is a common positive patch test reaction among patients with eyelid dermatitis, even in patients with no dermatitis at the site of contact with gold jewelry.20,29,31 Gold has been reported to be the most common allergen causing unilateral eyelid dermatitis via ectopic transfer.16,19,20,29 It has been proposed that titanium dioxide, present in many cosmetics and sunscreens, displaces gold allowing its release from jewelry, thereby liberating the fine gold ions and allowing them to desposit on the face and eyelids.30,31 Given the uncertain clinical relevance of positive patch test reactions to gold, Warshaw at al16 recommend a 2- to 3-month trial of gold jewelry avoidance to establish relevance, and Ehrlich and Gold29 noted that avoidance of gold leads to improvement.
Fragrances—Fragrances represent a broad category of naturally occurring and man-made components that often are combined to produce a desired scent in personal care products.32 Essential oils and botanicals are both examples of natural fragrances.33 Fragrances are found in numerous products including makeup, hair products, and household cleaning supplies and represent some of the most common contact allergens.32 Common fragrance allergens include fragrance mixes I and II, hydroperoxides of linalool, and balsam of Peru.12,32,34 Allergic contact dermatitis to fragrances typically manifests on the eyelids, face, or hands.33 Several studies have found fragrances to be among the top contact allergens in patients with eyelid dermatitis.3,12,20,25,34 Patch testing for fragrance allergy may include baseline series, supplemental fragrance series, and personal care products.32,35
Preservatives—Preservatives, including formaldehyde and formaldehyde releasers (eg, quaternium-15 and bronopol) and methylchloroisothiazolinone/methylisothiazolinone, may be found in personal care products such as makeup, makeup removers, emollients, shampoos, hair care products, and ophthalmologic solutions and are among the most common cosmetic sources of ACD.13,36-39 Preservatives are among the top allergens causing eyelid dermatitis.20 In particular, patch test positivity rates to methylchloroisothiazolinone/methylisothiazolinone have been increasing in North America.40 Sensitization to preservatives may occur through direct skin contact or transfer from the hands.41
Acrylates—Acrylates are compounds derived from acrylic acid that may be found in acrylic and gel nails, eyelash extensions, and other adhesives and are frequent causes of eyelid ACD.4,10,42 Acrylate exposure may be cosmetic among consumers or occupational (eg, aestheticians).42,43 Acrylates on the nails may cause eyelid dermatitis via ectopic transfer from the hands and also may cause periungual dermatitis manifesting as nail bed erythema.10 Hydroxyethyl methacrylate is one of the more common eyelid ACD allergens, and studies have shown increasing prevalence of positive reaction rates to hydroxyethylmethacrylate.10,44Topical Medications—Contact allergies to topical medications are quite common, estimated to occur in 10% to 17% of patients undergoing patch testing.45 Both active and inactive ingredients of topical medications may be culprits in eyelid ACD. The most common topical medication allergens include antibiotics, steroids, local anesthetics, and nonsteroidal anti-inflammatory drugs.45 Topical antibiotics such as neomycin and bacitracin represent some of the most common causes of eyelid dermatitis4,10 and may be found in a variety of products, including antibacterial ointments and eye drops.1 Many ophthalmologic medications also contain corticosteroids, with the most common allergenic steroids being tixocortol pivalate (a marker for hydrocortisone allergy) and budesonide.10,20 Topical steroids pose a particular dilemma, as they can be either the source of or a treatment for ACD.10 Eye drops also may contain anesthetics, β-blockers, and antihistamines, as well as the preservative benzalkonium chloride, all of which may be contact allergens.21,39
Differential Diagnosis of Eyelid Dermatitis
Although ACD is reported to be the most common cause of eyelid dermatitis, the differential diagnosis is broad, including endogenous inflammatory dermatoses and exogenous exposures (Table 2). Symptoms of eyelid ACD can be nonspecific (eg, erythema, pruritus), making diagnosis challenging.46

Atopic dermatitis represents another common cause of eyelid dermatitis, accounting for 14% to 39.5% of cases.3-5,49 Atopic dermatitis of the eyelids classically manifests with lichenification of the medial aspects of the eyelids.50 Atopic dermatitis and ACD may be difficult to distinguish, as the 2 conditions appear clinically similar and can develop concomitantly.51 Additionally, atopic patients are likely to have comorbid allergic rhinitis and sensitivity to environmental allergens, which may lead to chronic eye scratching and lichenification.1,51 Clinical features of eyelid dermatitis suggesting allergic rhinitis and likely comorbid AD include creases in the lower eyelids (Dennie-Morgan lines) and periorbital hyperpigmentation (known as the allergic shiner) due to venous congestion.1,52
Seborrheic dermatitis is an inflammatory reaction to Malassezia yeast that occurs in sebaceous areas such as the groin, scalp, eyebrows, eyelids, and nasolabial folds.1,53,54
Irritant contact dermatitis, a nonspecific inflammatory reaction caused by direct cell damage from external irritants, also may affect the eyelids and appear similar to ACD.1 It typically manifests with a burning or stinging sensation, as opposed to pruritus, and generally develops and resolves more rapidly than ACD.1 Personal care products are common causes of eyelid irritant contact dermatitis.16
Patch Testing for Eyelid ACD
The gold standard for diagnosis of ACD is patch testing, outlined by the International Contact Dermatitis Research Group.55-57 Patch testing generally is performed with standardized panels of allergens and can be customized either with supplemental panels based on unique exposures or with the patient’s own personal care products to increase the sensitivity of testing. Therefore, a thorough history is crucial to identifying potential allergens in a patient’s environment.
False negatives are possible, as the skin on the back may be thicker and less sensitive than the skin at the location of dermatitis.2,58 This is particularly relevant when using patch testing to diagnose ACD of the eyelids, where the skin is particularly thin and sensitive.2 Additionally, ingredients of ophthalmic medications are known to have an especially high false-negative rate with standard patch testing and may require repeated testing with higher drug concentrations or modified patch testing procedures (eg, open testing, scratch-patch testing).1,59
Treatment
Management of ACD involves allergen avoidance, typically dictated by patch test results.10 Allergen avoidance may be facilitated using online resources such as the Contact Allergen Management Program (https://www.acdscamp.org/) created by the American Contact Dermatitis Society.10,18 Patient counseling following patch testing is crucial to educating patients about sources of potential allergen exposures and strategies for avoidance. In the case of eyelid dermatitis, it is particularly important to consider exposure to airborne allergens such as fragrances.16 Fragrance avoidance is uniquely difficult, as labelling standards in the United States currently do not require disclosure of specific fragrance components.33 Additionally, products labelled as unscented may still contain fragrances. As such, some patients with fragrance allergy may need to carefully avoid all products containing fragrances.33
In addition to allergen avoidance, eyelid ACD may be treated with topical medications (eg, steroids, calcineurin inhibitors, Janus kinase inhibitors); however, these same topical medications also can cause ACD due to some ingredients such as propylene glycol.10 Topical steroids should be used with caution on the eyelids given the risk for atrophy, cataracts, and glaucoma.1
Final Interpretation
Eyelid dermatitis is a common dermatologic condition most frequently caused by ACD due to exposure to allergens in cosmetic products, ophthalmic medications, nail lacquers, and jewelry, among many other potential sources. The most common allergens causing eyelid dermatitis include metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications. Eyelid ACD is diagnosed via patch testing, and the mainstay of treatment is strict allergen avoidance. Patient counseling is vital for successful allergen avoidance and resolution of eyelid ACD.
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
- Hine AM, Waldman RA, Grzybowski A, et al. Allergic disorders of the eyelid. Clin Dermatol. 2023;41:476-480. doi:10.1016/j.clindermatol.2023.08.002
- Turkiewicz M, Shah A, Yang YW, et al. Allergic contact dermatitis of the eyelids: an interdisciplinary review. Ocul Surf. 2023;28:124-130. doi:10.1016/j.jtos.2023.03.001
- Valsecchi R, Imberti G, Martino D, et al. Eyelid dermatitis: an evaluation of 150 patients. Contact Dermatitis. 1992;27:143-147. doi:10.1111/j.1600-0536.1992.tb05242.x
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002;47:755-765. doi:10.1067/mjd.2002.122736
- Nethercott JR, Nield G, Holness DL. A review of 79 cases of eyelid dermatitis. J Am Acad Dermatol. 1989;21(2 pt 1):223-230. doi:10.1016/s0190-9622(89)70165-1
- Shah M, Lewis FM, Gawkrodger DJ. Facial dermatitis and eyelid dermatitis: a comparison of patch test results and final diagnoses. Contact Dermatitis. 1996;34:140-141. doi:10.1111/j.1600-0536.1996.tb02148.x
- Brites GS, Ferreira I, Sebastião AI, et al. Allergic contact dermatitis: from pathophysiology to development of new preventive strategies. Pharmacol Res. 2020;162:105282. doi:10.1016/j.phrs.2020.105282
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85. doi:10.1111/cod.13119
- Adler BL, DeLeo VA. Allergic contact dermatitis. JAMA Dermatol. 2021;157:364. doi:10.1001/jamadermatol.2020.5639
- Huang CX, Yiannias JA, Killian JM, et al. Seven common allergen groups causing eyelid dermatitis: education and avoidance strategies. Clin Ophthalmol Auckl NZ. 2021;15:1477-1490. doi:10.2147/OPTH.S297754
- Rozas-Muñoz E, Gamé D, Serra-Baldrich E. Allergic contact dermatitis by anatomical regions: diagnostic clues. Actas Dermo-Sifiliográficas Engl Ed. 2018;109:485-507. doi:10.1016/j.adengl.2018.05.016
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006;55:280-285. doi:10.1111/j.1600-0536.2006.00927.x
- Wolf R, Orion E, Tüzün Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014;32:131-140. doi:10.1016/j.clindermatol.2013.05.035
- Ockenfels HM, Seemann U, Goos M. Contact allergy in patients with periorbital eczema: an analysis of allergens. data recorded by the Information Network of the Departments of Dermatology. Dermatol Basel Switz. 1997;195:119-124. doi:10.1159/000245712
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients—patch test results during a 10-year period. Contact Dermatitis. 2014;70:205-212. doi:10.1111/cod.12157
- Warshaw EM, Voller LM, Maibach HI, et al. Eyelid dermatitis in patients referred for patch testing: retrospective analysis of North American Contact Dermatitis Group data, 1994-2016. J Am Acad Dermatol. 2021;84:953-964. doi:10.1016/j.jaad.2020.07.020
- McMonnies CW. Management of chronic habits of abnormal eye rubbing. Contact Lens Anterior Eye. 2008;31:95-102. doi:10.1016/j.clae.2007.07.008
- Chisholm SAM, Couch SM, Custer PL. Etiology and management of allergic eyelid dermatitis. Ophthal Plast Reconstr Surg. 2017;33:248-250. doi:10.1097/IOP.0000000000000723
- Lewallen R, Feldman S, eds. Regional atlas of contact dermatitis. The Dermatologist. Accessed April 22, 2024. https://s3.amazonaws.com/HMP/hmp_ln/imported/Regional%20Atlas%20of%20Contact%20Dermatitis%20Book_lr.pdf
- Rietschel RL, Warshaw EM, Sasseville D, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermat Contact Atopic Occup Drug. 2007;18:78-81. doi:10.2310/6620.2007.06041
- Mughal AA, Kalavala M. Contact dermatitis to ophthalmic solutions. Clin Exp Dermatol. 2012;37:593-597; quiz 597-598. doi:10.1111/j.1365-2230.2012.04398.x
- Goossens A. Contact allergic reactions on the eyes and eyelids. Bull Soc Belge Ophtalmol. 2004;292:11-17.
- Silverberg NB, Pelletier JL, Jacob SE, et al. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145:E20200628. doi:10.1542/peds.2020-0628
- Warshaw EM, Schlarbaum JP, Maibach HI, et al. Facial dermatitis in male patients referred for patch testing. JAMA Dermatol. 2020;156:79-84. doi:10.1001/jamadermatol.2019.3531
- Wenk KS, Ehrlich A. Fragrance series testing in eyelid dermatitis. Dermatitis. 2012;23:22-26. doi:10.1097/DER.0b013e31823d180f
- Crouse L, Ziemer C, Ziemer C, et al. Trends in eyelid dermatitis. Dermat Contact Atopic Occup Drug. 2018;29:96-97. doi:10.1097/DER.0000000000000338
- Yazdanparast T, Nassiri Kashani M, Shamsipour M, et al. Contact allergens responsible for eyelid dermatitis in adults. J Dermatol. 2024;51:691-695. doi:10.1111/1346-8138.17140
- Fowler J, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000;65:323-326.
- Danesh M, Murase JE. Titanium dioxide induces eyelid dermatitis in patients allergic to gold. J Am Acad Dermatol. 2015;73:E21. doi:10.1016/j.jaad.2015.03.046
- Katta R. Common misconceptions in contact dermatitis counseling. Dermatol Online J. 2008;14:2.
- De Groot AC. Fragrances: contact allergy and other adverse effects. Dermatitis. 2020;31:13-35. doi:10.1097/DER.0000000000000463
- Reeder MJ. Allergic contact dermatitis to fragrances. Dermatol Clin. 2020;38:371-377. doi:10.1016/j.det.2020.02.009
- Warshaw EM, Zhang AJ, DeKoven JG, et al. Epidemiology of nickel sensitivity: retrospective cross-sectional analysis of North American Contact Dermatitis Group data 1994-2014. J Am Acad Dermatol. 2019;80:701-713. doi:10.1016/j.jaad.2018.09.058
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society core allergen series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yim E, Baquerizo Nole KL, Tosti A. Contact dermatitis caused by preservatives. Dermatitis. 2014;25:215-231. doi:10.1097/DER.0000000000000061
- Alani JI, Davis MDP, Yiannias JA. Allergy to cosmetics. Dermatitis. 2013;24:283-290. doi:10.1097/DER.0b013e3182a5d8bc
- Hamilton T, de Gannes GC. Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Ther Lett. 2011;16:1-4.
- Ashton SJ, Mughal AA. Contact dermatitis to ophthalmic solutions: an update. Dermat Contact Atopic Occup Drug. 2023;34:480-483. doi:10.1089/derm.2023.0033
- Reeder MJ, Warshaw E, Aravamuthan S, et al. Trends in the prevalence of methylchloroisothiazolinone/methylisothiazolinone contact allergy in North America and Europe. JAMA Dermatol. 2023;159:267-274. doi:10.1001/jamadermatol.2022.5991
- Herro EM, Elsaie ML, Nijhawan RI, et al. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012;23:17-21. doi:10.1097/DER.0b013e31823d191f
- Kucharczyk M, Słowik-Rylska M, Cyran-Stemplewska S, et al. Acrylates as a significant cause of allergic contact dermatitis: new sources of exposure. Adv Dermatol Allergol Dermatol Alergol. 2021;38:555-560. doi:10.5114/ada.2020.95848
- Rodriguez I, George SE, Yu J, et al. Tackling acrylate allergy: the sticky truth. Cutis. 2023;112:282-286. doi:10.12788/cutis.0909
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019–2020. Dermatitis. 2023;34:90-104. doi:10.1089/derm.2022.29017.jdk
- de Groot A. Allergic contact dermatitis from topical drugs: an overview. Dermatitis. 2021;32:197-213. doi:10.1097/DER.0000000000000737
- Zug KA, Palay DA, Rock B. Dermatologic diagnosis and treatment of itchy red eyelids. Surv Ophthalmol. 1996;40:293-306. doi:10.1016/s0039-6257(96)82004-2
- Beltrani VS. Eyelid dermatitis. Curr Allergy Asthma Rep. 2001;1:380-388. doi:10.1007/s11882-001-0052-0
- Hirji SH, Maeng MM, Tran AQ, et al. Cutaneous T-cell lymphoma of the eyelid masquerading as dermatitis. Orbit Amst Neth. 2021;40:75-78. doi:10.1080/01676830.2020.1739080
- Svensson A, Möller H. Eyelid dermatitis: the role of atopy and contact allergy. Contact Dermatitis. 1986;15:178-182. doi:10.1111/j.1600-0536.1986.tb01321.x
- Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. Am Fam Physician. 2007;76:1815-1824.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142. doi:10.12788cutis.0599
- Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004;6:233-250. doi:10.2165/00148581-200406040-00003
- Singh A, Kansal NK, Kumawat D, et al. Ophthalmic manifestations of seborrheic dermatitis. Skinmed. 2023;21:397-401.
- Clark GW, Pope SM, Jaboori KA. Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician. 2015;91:185-190.
- Lachapelle JM, Maibach HI. Patch Testing and Prick Testing. Springer; 2012.
- Fregert S. Manual of Contact Dermatitis: On Behalf of the International Contact Dermatitis Research Group. Munksgaard; 1974.
- Reeder M, Reck Atwater A. Patch testing 101, part 1: performing the test. Cutis. 2020;106:165-167. doi:10.12788/cutis.0093
- Wolf R, Perluk H. Failure of routine patch test results to detect eyelid dermatitis. Cutis. 1992;49:133-134.
- Grey KR, Warshaw EM. Allergic contact dermatitis to ophthalmic medications: relevant allergens and alternative testing methods. Dermat Contact Atopic Occup Drug. 2016;27:333-347. doi:10.1097/DER.0000000000000224
Practice Points
- Eyelid dermatitis is a common dermatologic concern representing a broad range of inflammatory dermatoses, most often caused by allergic contact dermatitis (ACD).
- The most common contact allergens associated with eyelid dermatitis are metals (particularly nickel), fragrances, preservatives, acrylates, and topical medications, which may be found in a variety of sources, including cosmetics, ophthalmic medications, nail lacquers, and jewelry.
- Eyelid ACD is diagnosed via patch testing, and management involves strict allergen avoidance.
Purpuric Lesions on the Leg
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.
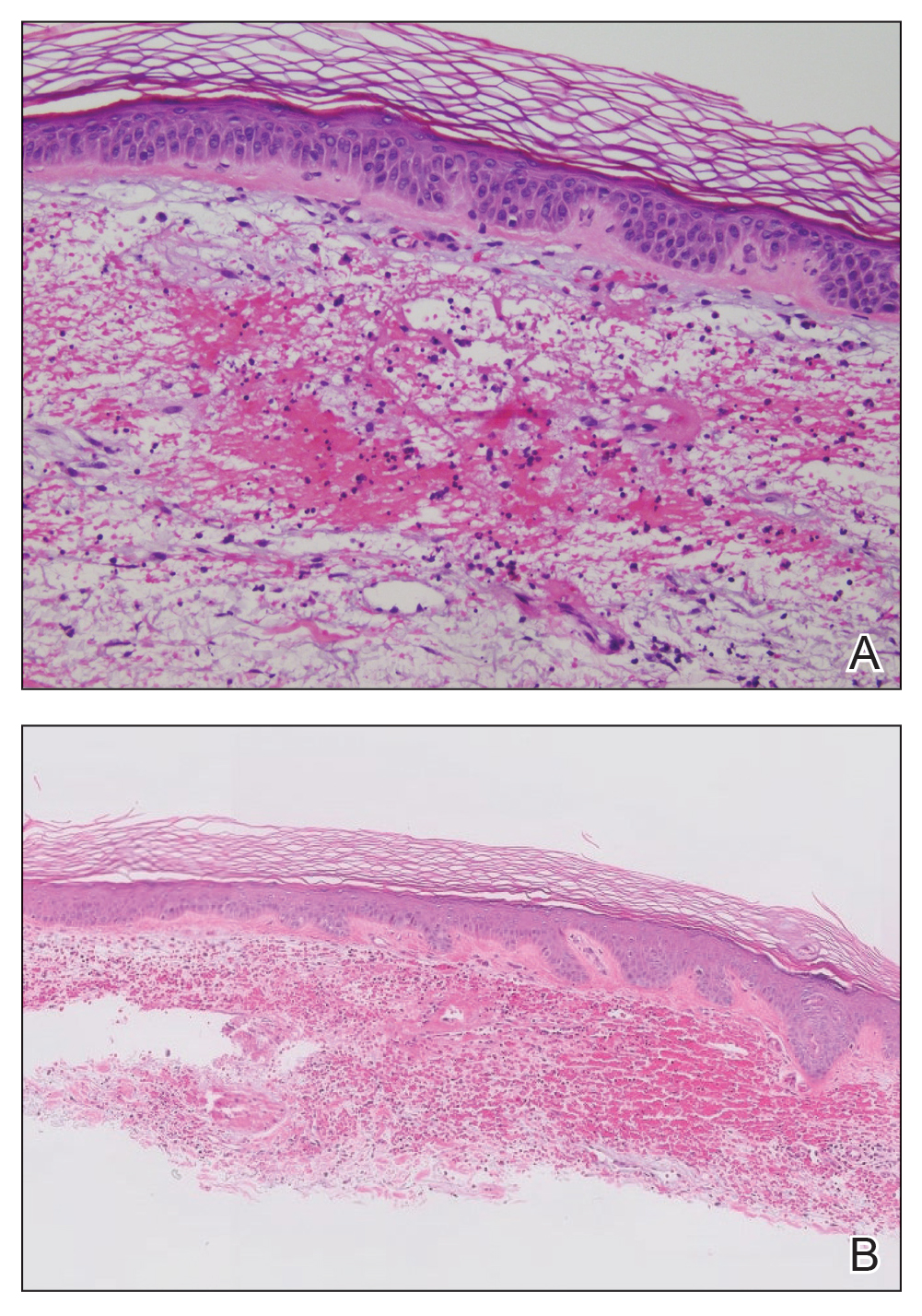
Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1
Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
THE DIAGNOSIS: Dengue Hemorrhagic Fever
The retiform purpura observed in our patient was suggestive of a vasculitic, thrombotic, or embolic etiology. Dengue IgM serologic testing performed based on her extensive travel history and recent return from a dengue-endemic area was positive, indicating acute infection. A clinical diagnosis of dengue hemorrhagic fever (DHF) was made based on the hemorrhagic appearance of the lesion. Histopathology revealed leukocytoclastic vasculitis (Figure). Anti–double-stranded DNA, antideoxyribonuclease, C3 and C4, CH50 (total hemolytic complement), antineutrophil cytoplasmic antibodies, HIV, and hepatitis B virus tests were normal. Direct immunofluorescence was negative.

Dengue virus is a single-stranded RNA virus transmitted by Aedes aegypti and Aedes albopictus mosquitoes and is one of the most prevalent arthropod-borne viruses affecting humans today.1,2 Infection with the dengue virus generally is seen in travelers visiting tropical regions of Africa, Mexico, South America, South and Central Asia, Southeast Asia, and the Caribbean.1 The Table shows the global distribution of dengue serotypes from 2000 to 2014.3,4 There are 4 serotypes of the dengue virus: DENV-1 to DENV-4. Infection with 1 strain elicits longlasting immunity to that strain, but subsequent infection with another strain can result in severe DHF due to antibody cross-reaction.1

Dengue virus infection ranges from mildly symptomatic to a spectrum of increasingly severe conditions that comprise dengue fever (DF) and DHF, as well as dengue shock syndrome and brain stem hemorrhage, which may be fatal.2,5 Dengue fever manifests as severe myalgia, fever, headache (usually retro-orbital), arthralgia, erythema, and rubelliform exanthema.6 The frequency of skin eruptions in patients with DF varies with the virus strain and outbreaks.7 The lesions initially develop with the onset of fever and manifest as flushing or erythematous mottling of the face, neck, and chest areas.1,7 The morbilliform eruption develops 2 to 6 days after the onset of the fever, beginning on the trunk and spreading to the face and extremities.1,7 The rash may become confluent with characteristic sparing of small round areas of normal skin described as white islands in a sea of red.2 Verrucous papules on the ears also have been described and may resemble those seen in Cowden syndrome. In patients with prior infection with a different strain of the virus, hemorrhagic lesions may develop, including characteristic retiform purpura, a positive tourniquet test, and the appearance of petechiae on the lower legs. Pruritus and desquamation, especially on the palms and soles, may follow the termination of the eruption.7
The differential diagnosis of DF includes measles, rubella, enteroviruses, and influenza. Chikungunya and West Nile viruses in Asia and Africa and the O’nyong-nyong virus in Africa are also arboviruses that cause a clinical picture similar to DF but not DHF. Other diagnostic considerations include phases of scarlet fever, typhoid, malaria, leptospirosis, hepatitis A, and trypanosomal and rickettsial diseases.7 The differential diagnosis of DHF includes antineutrophil cytoplasmic antibody–associated vasculitis, rheumatoid vasculitis, and bacterial septic vasculitis.
Acute clinical diagnosis of DF can be challenging because of the nonspecific symptoms that can be seen in almost every infectious disease. Clinical presentation assessment should be confirmed with laboratory testing.6 Dengue virus infection usually is confirmed by the identification of viral genomic RNA, antigens, or the antibodies it elicits. Enzyme-linked immunosorbent assay–based serologic tests are cost-effective and easy to perform.5 IgM antibodies usually show cross-reactivity with platelets, but the antibody levels are not positively correlated with the severity of DF.8 Primary infection with the dengue virus is characterized by the elevation of specific IgM levels that usually occurs 3 to 5 days after symptom onset and persists during the postfebrile stage (up to 30 to 60 days). In secondary infections, the IgM levels usually rise more slowly and reach a lower level than in primary infections.9 For both primary and secondary infections, testing IgM levels after the febrile stage may be helpful with the laboratory diagnosis.
Currently, there is no antiviral drug available for dengue. Treatment of dengue infection is symptomatic and supportive.2
Dengue hemorrhagic fever is indicated by a rising hematocrit (≥20%) and a falling platelet count (>100,000/mm3) accompanying clinical signs of hemorrhage. Treatment includes intravenous fluid replacement and careful clinical monitoring of hematocrit levels, platelet count, vitals, urine output, and other signs of shock.5 For patients with a history of dengue infection, travel to areas with other serotypes is not recommended.
If any travel to a high-risk area is planned, countryspecific travel recommendations and warnings should be reviewed from the Centers for Disease Control and Prevention’s website (https://wwwnc.cdc.gov/travel/notices/level1/dengue-global). Use of an Environmental Protection Agency–registered insect repellent to avoid mosquito bites and acetaminophen for managing symptoms is advised. During travel, staying in places with window and door screens and using a bed net during sleep are suggested. Long-sleeved shirts and long pants also are preferred. Travelers should see a health care provider if they have symptoms of dengue.10
African tick bite fever (ATBF) is caused by Rickettsia africae transmitted by Amblyomma ticks. Skin findings in ATBF include erythematous, firm, tender papules with central eschars consistent with the feeding patterns of ticks.11 Histopathology of ATBF usually includes fibrinoid necrosis of vessels in the dermis with a perivascular inflammatory infiltrate and coagulation necrosis of the surrounding dermis consistent with eschar formation.12 The lack of an eschar weighs against this diagnosis.
African trypanosomiasis (also known as sleeping sickness) is caused by protozoa transmitted by the tsetse fly. A chancrelike, circumscribed, rubbery, indurated red or violaceous nodule measuring 2 to 5 cm in diameter often develops as the earliest cutaneous sign of the disease.13 Nonspecific histopathologic findings, such as infiltration of lymphocytes and macrophages and proliferation of endothelial cells and fibroblasts, may be observed.14 Extravascular parasites have been noted in skin biopsies.15 In later stages, skin lesions called trypanids may be observed as macular, papular, annular, targetoid, purpuric, and erythematous lesions, and histopathologic findings consistent with vasculitis also may be seen.13
Chikungunya virus infection is an acute-onset, mosquito-borne viral disease. Skin manifestations may start with nonspecific, generalized, morbilliform, maculopapular rashes coinciding with fever, which also may be seen initially with DHF. Skin hyperpigmentation, mostly centrofacial and involving the nose (chik sign); purpuric and ecchymotic lesions over the trunk and flexors of limbs in adults, often surmounted by subepidermal bullae and lesions resembling toxic epidermal necrolysis; and nonhealing ulcers in the genital and groin areas are common skin manifestations of chikungunya infection.16 Intraepithelial splitting with acantholysis and perivascular lymphohistiocytic infiltration may be observed in the histopathology of blistering lesions, which are not consistent with DHF.17
Zika virus infection is caused by an arbovirus within the Flaviviridae family, which also includes the dengue virus. Initial mucocutaneous findings of the Zika virus include nonspecific diffuse maculopapular eruptions. The eruption generally spares the palms and soles; however, various manifestations including involvement of the palms and soles have been reported.18 The morbilliform eruption begins on the face and extends to the trunk and extremities. Mild hemorrhagic manifestations, including petechiae and bleeding gums, may be observed. Distinguishing between dengue and Zika virus infection relies on the severity of symptoms and laboratory tests, including polymerase chain reaction or IgM antibody testing.19 The other conditions listed do not produce hemorrhagic fever.
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
- Pincus LB, Grossman ME, Fox LP. The exanthem of dengue fever: clinical features of two US tourists traveling abroad. J Am Acad Dermatol. 2008;58:308-316. doi:10.1016/j.jaad.2007.08.042
- Radakovic-Fijan S, Graninger W, Müller C, et al. Dengue hemorrhagic fever in a British travel guide. J Am Acad Dermatol. 2002;46:430-433. doi:10.1067/mjd.2002.111904
- Yamashita A, Sakamoto T, Sekizuka T, et al. DGV: dengue genographic viewer. Front Microbiol. 2016;7:875. doi:10.3389/fmicb.2016.00875
- Centers for Disease and Prevention. Dengue in the US states and territories. Updated October 7, 2020. Accessed September 30, 2024. https://www.cdc.gov/dengue/data-research/facts-stats/?CDC_AAref_Val=https://www.cdc.gov/dengue/areaswithrisk/in-the-us.html
- Khetarpal N, Khanna I. Dengue fever: causes, complications, and vaccine strategies. J Immunol Res. 2016;2016:6803098. doi:10.1155/2016/6803098
- Muller DA, Depelsenaire AC, Young PR. Clinical and laboratory diagnosis of dengue virus infection. J Infect Dis. 2017;215(suppl 2):S89-S95. doi:10.1093/infdis/jiw649
- Waterman SH, Gubler DJ. Dengue fever. Clin Dermatol. 1989;7:117-122. doi:10.1016/0738-081x(89)90034-5
- Lin CF, Lei HY, Liu CC, et al. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143-149. doi:10.1002/1096- 9071(20000201)63:2<143::AID-JMV1009>3.0.CO;2-L
- Tripathi NK, Shrivastava A, Dash PK, et al. Detection of dengue virus. Methods Mol Biol. 2011;665:51-64. doi:10.1007/978-1-60761-817-1_4
- Centers for Disease Control and Prevention. Plan for travel. Accessed September 30, 2024. https://wwwnc.cdc.gov/travel
- Mack I, Ritz N. African tick-bite fever. N Engl J Med. 2019;380:960. doi:10.1056/NEJMicm1810093
- Lepidi H, Fournier PE, Raoult D. Histologic features and immunodetection of African tick-bite fever eschar. Emerg Infect Dis. 2006;12:1332- 1337. doi:10.3201/eid1209.051540
- McGovern TW, Williams W, Fitzpatrick JE, et al. Cutaneous manifestations of African trypanosomiasis. Arch Dermatol. 1995;131:1178-1182.
- Kristensson K, Bentivoglio M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, eds. Progress in Human African Trypanosomiasis, Sleeping Sickness. Springer; 1999:157-181.
- Capewell P, Cren-Travaillé C, Marchesi F, et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife. 2016;5:e17716. doi:10.7554/eLife.17716
- Singal A. Chikungunya and skin: current perspective. Indian Dermatol Online J. 2017;8:307-309. doi:10.4103/idoj.IDOJ_93_17
- Robin S, Ramful D, Zettor J, et al. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2009;169:67-72. doi:10.1007/s00431-009-0986-0
- Hussain A, Ali F, Latiwesh OB, et al. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus. 2018;10:E3290. doi:10.7759/cureus.3290
- Farahnik B, Beroukhim K, Blattner CM, et al. Cutaneous manifestations of the Zika virus. J Am Acad Dermatol. 2016;74:1286-1287. doi:10.1016/j.jaad.2016.02.1232
A 74-year-old woman who frequently traveled abroad presented to the dermatology department with retiform purpura of the lower leg along with gastrointestinal cramps, fatigue, and myalgia. The patient reported that the symptoms had started 10 days after returning from a recent trip to Africa.
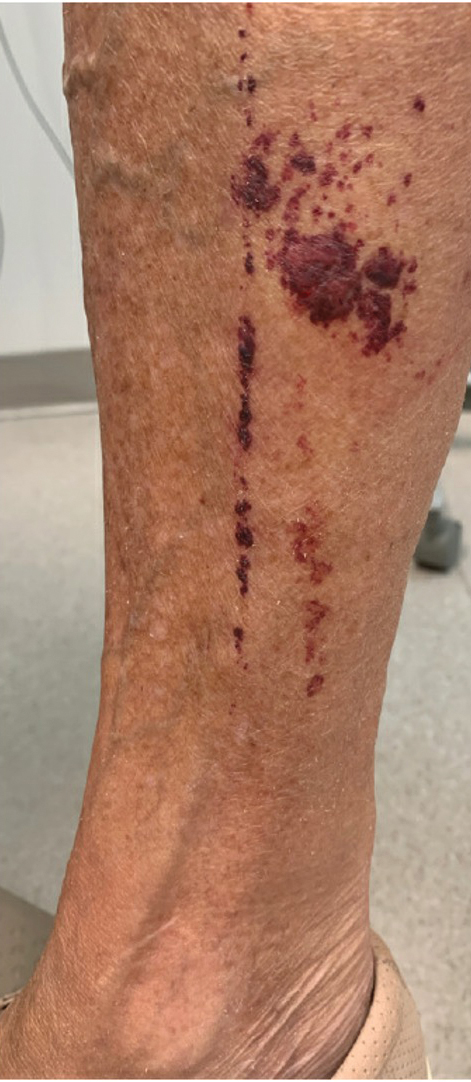
Inspection of Deep Tumor Margins for Accurate Cutaneous Squamous Cell Carcinoma Staging
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.
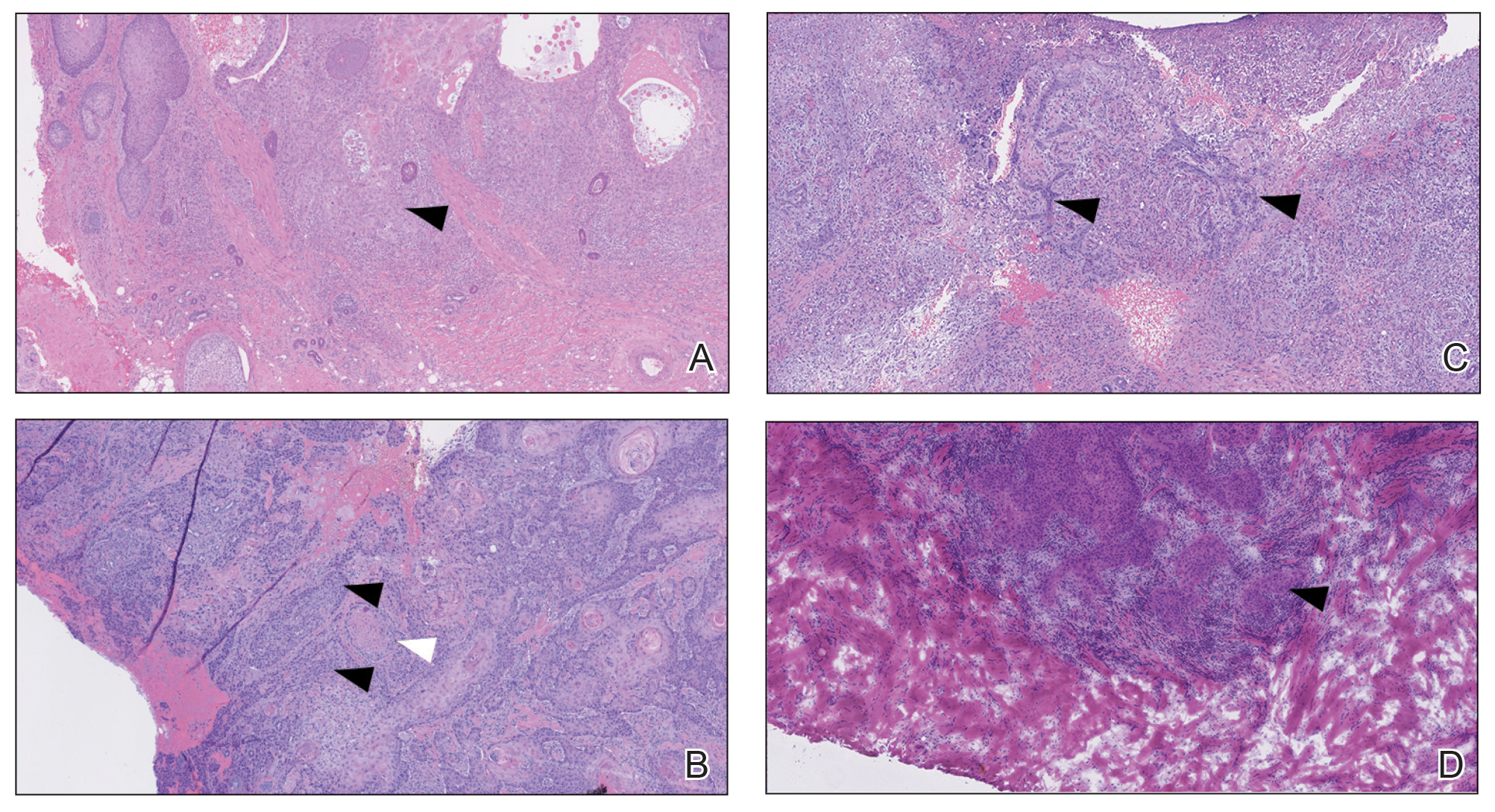
A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.
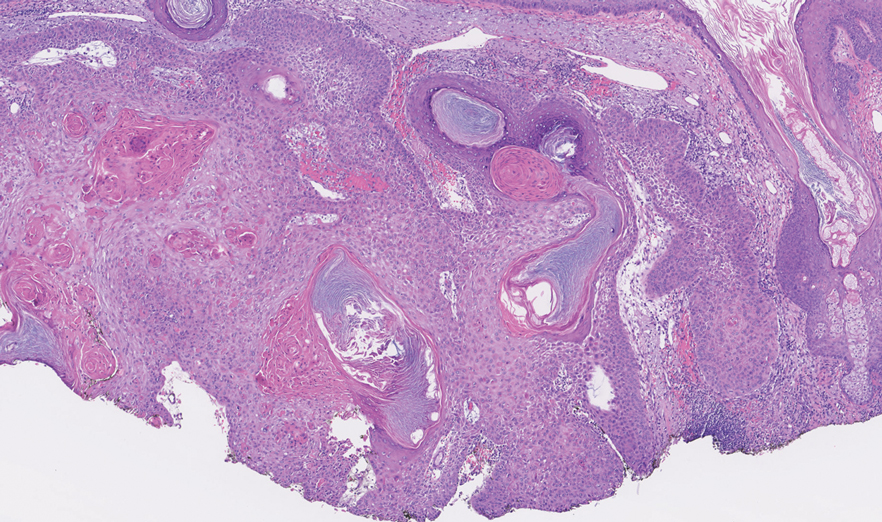
Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
To the Editor:
Histopathologic analysis of debulk specimens in Mohs micrographic surgery (MMS) may augment identification of high-risk factors in cutaneous squamous cell carcinoma (cSCC), which may warrant tumor upstaging.1 Intratumor location has not been studied when looking at these high-risk factors. Herein, we report 4 cSCCs initially categorized as well differentiated that were reclassified as moderate to poorly differentiated on analysis of debulk specimens obtained via shave removal.
An 80-year-old man (patient 1) presented with a tender 2-cm erythematous plaque with dried hemorrhagic crusting on the frontal scalp. He had a history of nonmelanoma skin cancers. A biopsy revealed a well-differentiated cSCC, which was upgraded from a T2a tumor to T2b during MMS due to galea involvement. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells at the deep margin (Figure 1A). Given T2b staging, baseline imaging and radiation therapy were recommended.

A 75-year-old man (patient 2) presented with a 2-cm erythematous plaque on the left vertex scalp with hemorrhagic crusting, yellow scale, and purulent drainage. He had a history of cSCCs. A biopsy revealed well-differentiated invasive cSCC, which was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Examination of the second Mohs stage revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, infiltration beyond the subcutaneous fat, and perineural invasion (Figure 1B). Given T2b staging, baseline imaging and radiation therapy were recommended.
An 86-year-old woman (patient 3) presented with a tender 2.4-cm plum-colored nodule on the right lower leg. She had a history of basal cell carcinoma. A biopsy revealed a well-differentiated invasive cSCC staged at T2a. Debulk analysis revealed moderately differentiated cSCC, with the least-differentiated cells at the deep margin, though the staging remained the same (Figure 1C).
An 82-year-old man (patient 4) presented with a 2.7-cm ulcerated nodule with adjacent scaling on the vertex scalp. He had no history of skin cancer. A biopsy revealed a well-differentiated cSCC (Figure 2) that was upgraded from a T2a tumor to T2b during MMS due to tumor extension beyond the subcutaneous fat. Debulk analysis revealed moderate to poorly differentiated cSCC, with the least-differentiated cells with single-cell extension at the deep margin in the galea (Figure 1D). Given T2b staging, baseline imaging and radiation therapy were recommended.

Tumor differentiation is a factor included in the Brigham and Women’s Hospital staging system, and intratumor variability can be clinically relevant for tumor staging.1 Specifically, cSCCs may exhibit intratumor heterogeneity in which predominantly well-differentiated tumors contain focal areas of poorer differentiation.2 This intratumor heterogeneity complicates estimation of tumor risk, as a well-differentiated tumor on biopsy may exhibit poor differentiation at a deeper margin. Our cases highlight that the cells at the deeper margin indeed can show poorer differentiation or other higher-risk tumor features. Thus, the most clinically relevant cells for tumor staging and prognostication may not be visible on initial biopsy, underscoring the utility of close examination of the deep layer of the debulk specimen and Mohs layer for comprehensive staging.
Genetic studies have attempted to identify gene expression patterns in cSCCs that predispose to invasion.3 Three of the top 6 genes in this “invasion signature gene set” were matrix metalloproteases; additionally, IL-24 messenger RNA was upregulated in both the cSCC invasion front and in situ cSCCs. IL-24 has been shown to upregulate the expression of matrix metalloprotease 7 in vitro, suggesting that it may influence tumor progression.3 Although gene expression was not included in this series, the identification of genetic variability in the most poorly differentiated cells residing in the deep margins is of great interest and may reveal mutations contributing to irregular cell morphology and cSCC invasiveness.
Prior studies have indicated that a proportion of cSCCs are histopathologically upgraded from the initial biopsy during MMS due to evidence of perineural invasion, bony invasion, or lesser differentiation noted during MMS stages or debulk analysis.1,4 However, the majority of Mohs surgeons report immediately discarding debulk specimens without further evaluation.5 Herein, we highlight 4 cSCC cases in which the deep margins of the debulk specimen contained the most dedifferentiated cells. Our findings emphasize the importance of thoroughly examining deep tumor margins for complete staging yet also highlight that identifying cells at these margins may not change patient management when high-risk criteria are already met.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
- McIlwee BE, Abidi NY, Ravi M, et al. Utility of debulk specimens during Mohs micrographic surgery for cutaneous squamous cell carcinoma. Dermatol Surg. 2021;47:599-604.
- Ramón y Cajal S, Sesé M, Capdevila C, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med. 2020;98:161-177.
- Mitsui H, Suárez-Fariñas M, Gulati N, et al. Gene expression profiling of the leading edge of cutaneous squamous cell carcinoma: IL-24-driven MMP-7. J Invest Dermatol. 2014;134:1418-1427.
- Chung E, Hoang S, McEvoy AM, et al. Histopathologic upgrading of cutaneous squamous cell carcinomas during Mohs micrographic surgery: a retrospective cohort study. J Am Acad Dermatol. 2021;85:923-930.
- Alniemi DT, Swanson AM, Lasarev M, et al. Tumor debulking trends for keratinocyte carcinomas among Mohs surgeons. Dermatol Surg. 2021;47:1660-1661.
Practice Points
- A proportion of cutaneous squamous cell carcinomas are upgraded from the initial biopsy during Mohs micrographic surgery due to evidence of perineural invasion, bony invasion, or lesser differentiation noted on Mohs stages or debulk analysis.
- Thorough inspection of the deep tumor margins may be required for accurate tumor staging and evaluation of metastatic risk. Cells at the deep margin of the tumor may demonstrate poorer differentiation and/or other higher-risk tumor features than those closer to the surface.
- Tumor staging may be incomplete until the deep margins are assessed to find the most dysplastic and likely clinically relevant cells, which may be missed without evaluation of the debulked tumor.
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
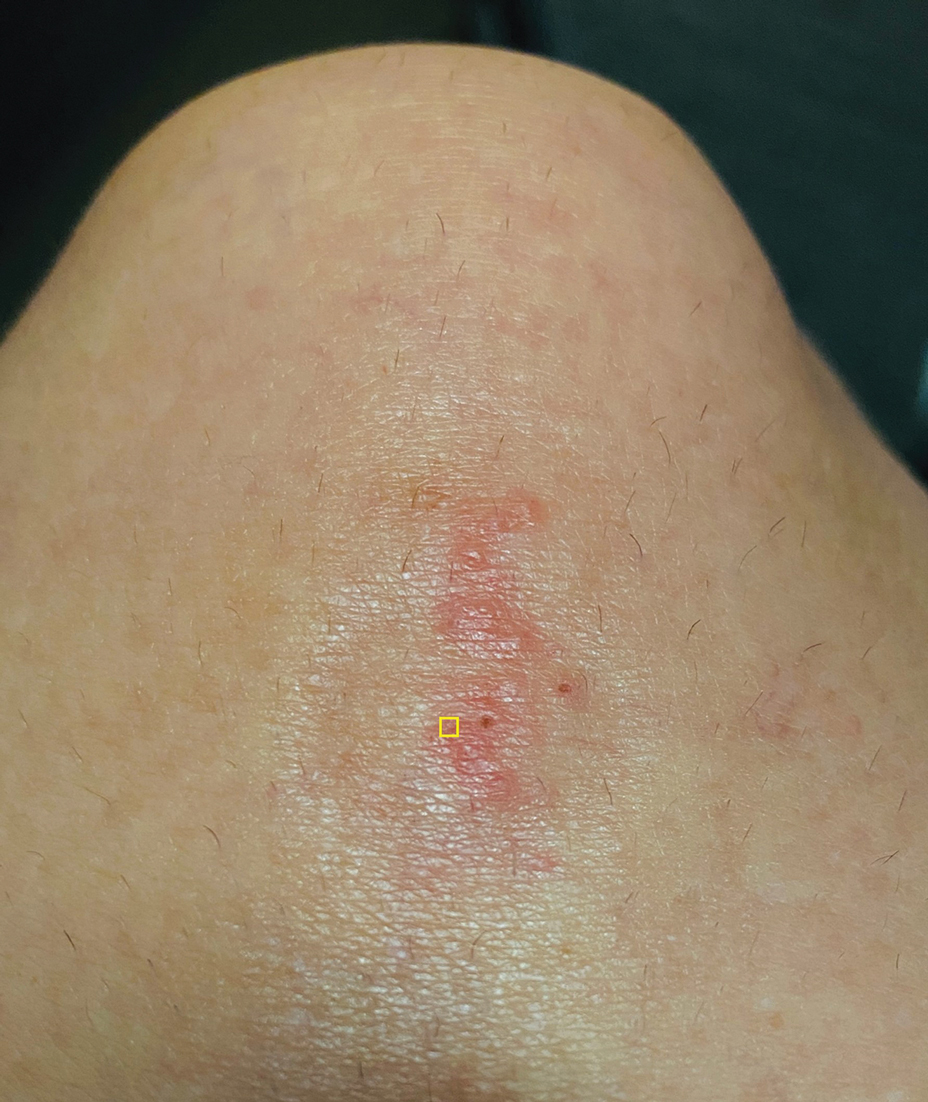
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
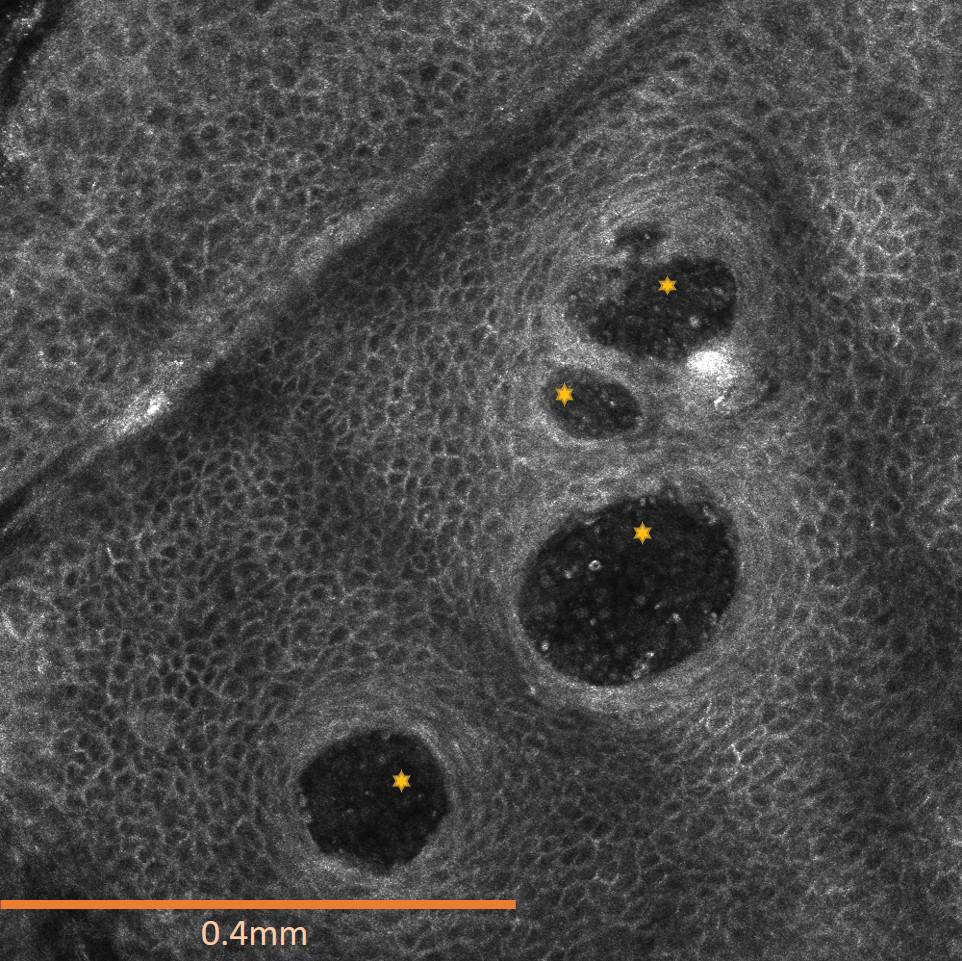
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.
Transient Eruption of Verrucous Keratoses During Encorafenib Therapy: Adverse Event or Paraneoplastic Phenomenon?
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.
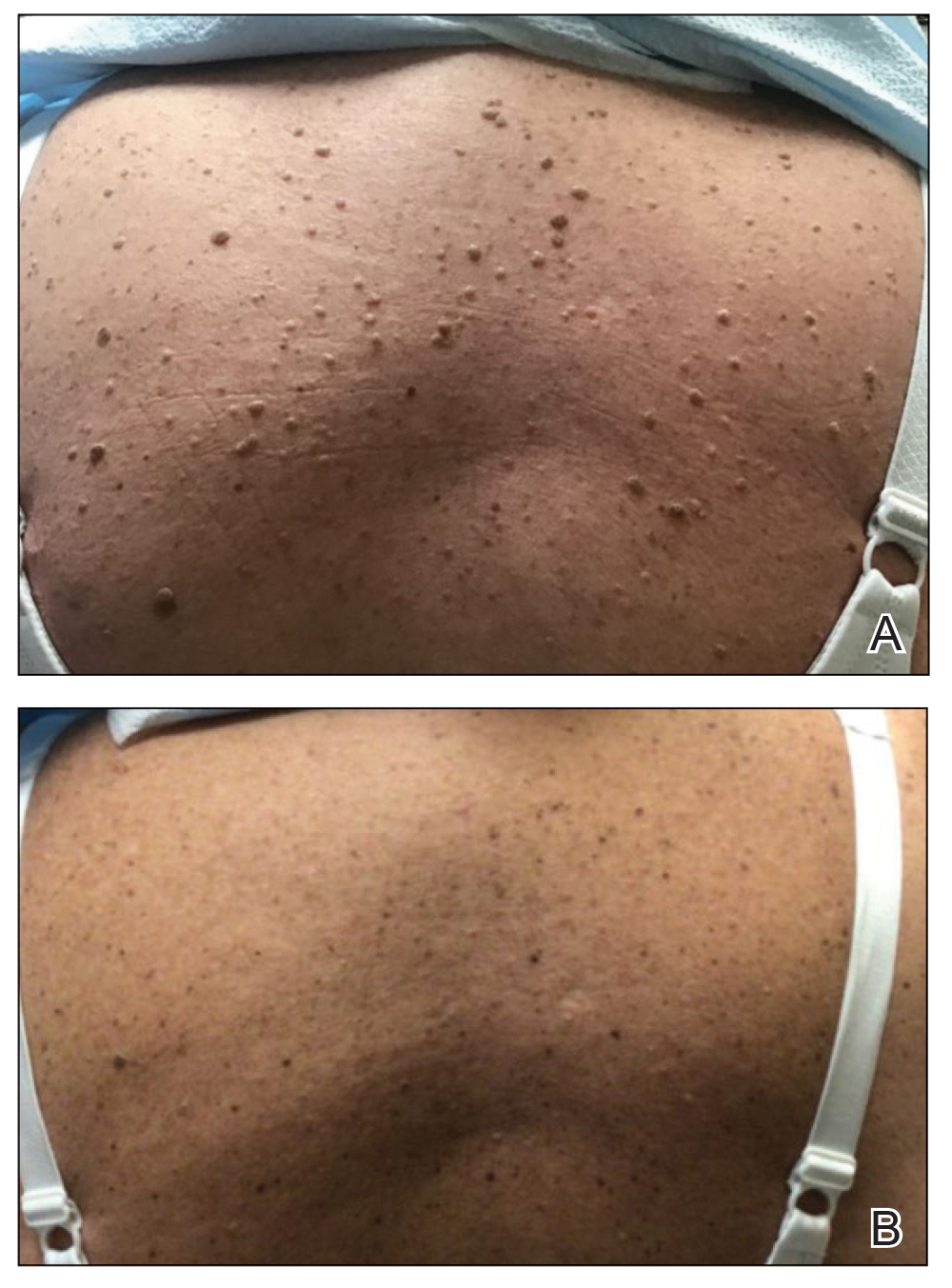
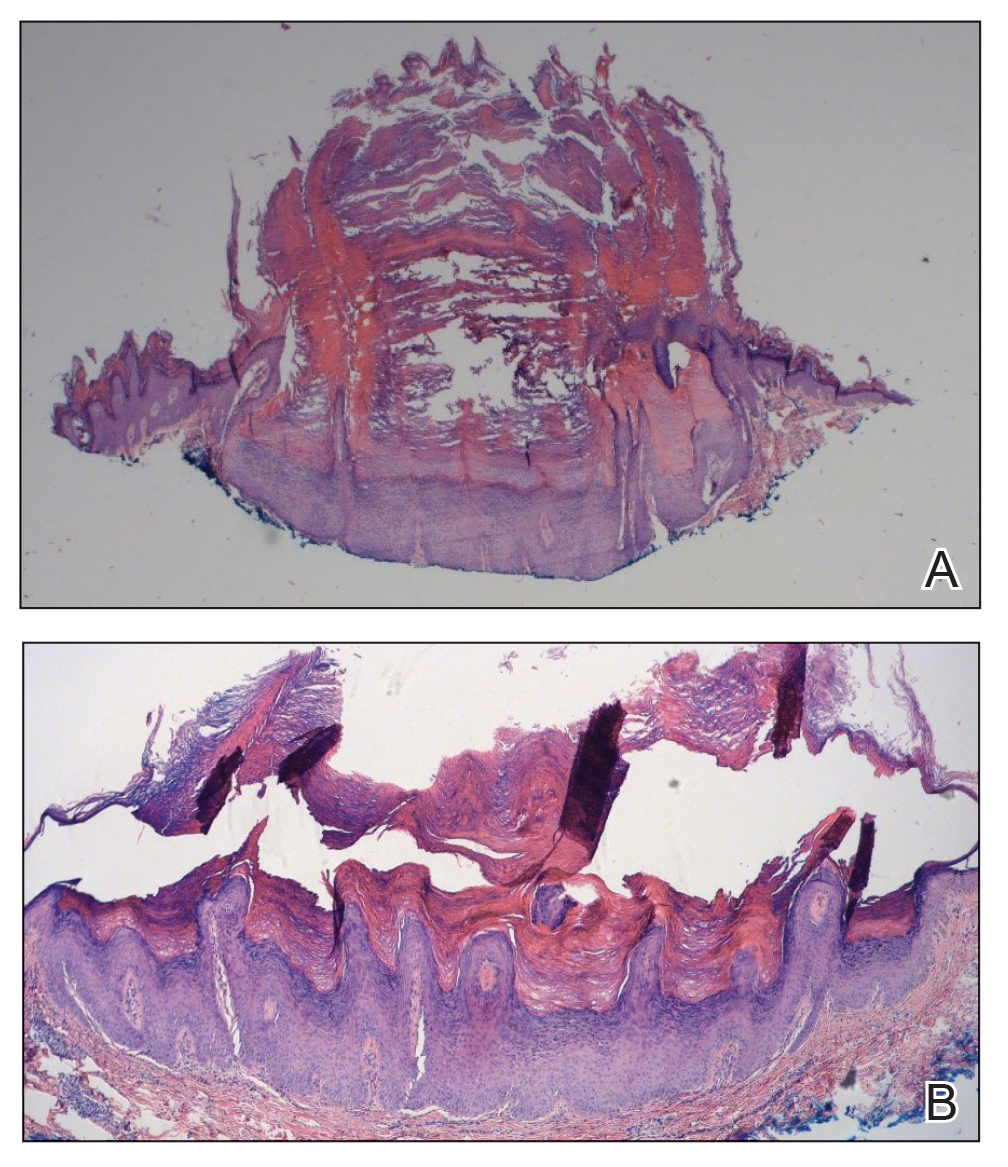
Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
Practice Points
- Verrucous keratoses are common cutaneous adverse events (AEs) associated with BRAF inhibitor therapy.
- Verrucous papules may be a paraneoplastic phenomenon and can be differentiated from a treatment-related AE based on the timing and progression in relation to tumor burden.
- Although treatment of particularly bothersome lesions with cryotherapy may be warranted, verrucous papules secondary to BRAF inhibitor therapy may resolve spontaneously.
Nonscaly Red-Brown Macules on the Feet and Ankles
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.
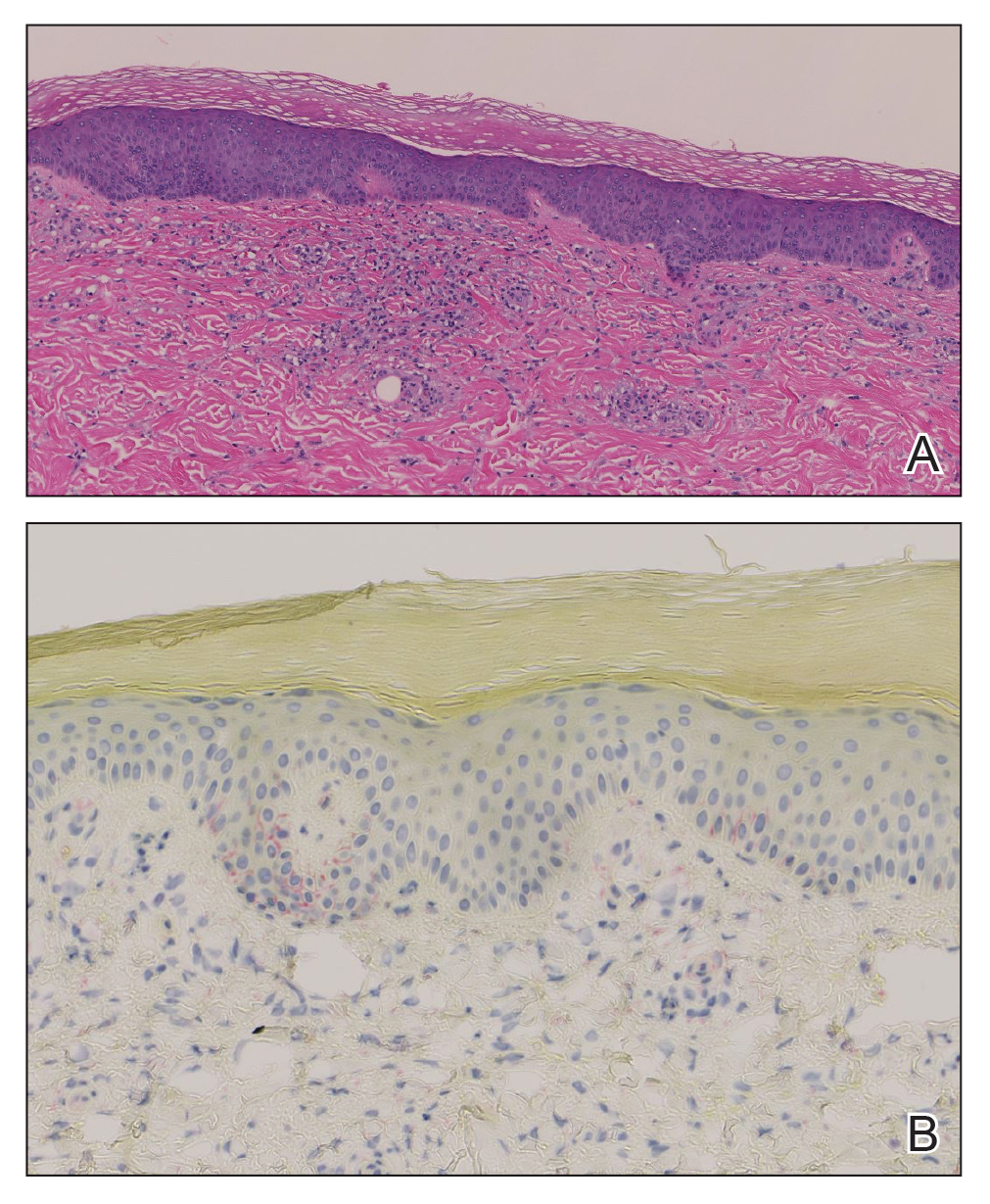
Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
A 59-year-old man presented with a nontender nonpruritic rash on the feet of 2 days’ duration. The patient had a several-year history of granulomatosis with polyangiitis (GPA) and was taking methotrexate and prednisone. The rash appeared suddenly—first on the right foot and then on the left foot—and was preceded by 1 week of worsening polyarthralgia, most notably in the ankles. He denied any fever, chills, sore throat, or weight loss. His typical GPA symptoms included inflammatory arthritis, scleritis, leukocytoclastic vasculitis, and sinonasal and renal involvement. He recently experienced exacerbation of inflammatory arthritis that required an increase in the prednisone dosage (from 40 mg to 60 mg daily), but there were no other GPA symptoms. He had a history of multiple female sexual partners but no known history of HIV and no recent testing for sexually transmitted infections. Hepatitis C antibody testing performed 5 years earlier was nonreactive. He denied any illicit drug use, recent travel, sick contacts, or new medications.
Dermatologic examination revealed nonscaly, clustered, red-brown macules, some with central clearing, on the medial and lateral aspects of the feet and ankles with a few faint copper-colored macules on the palms and soles. The ankles had full range of motion with no edema or effusion. There were no oral or genital lesions. The remainder of the skin examination was normal. Punch biopsies of skin on the left foot were obtained for histopathology and direct immunofluorescence.