User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Atypical Herpes Zoster Presentation in a Healthy Vaccinated Pediatric Patient
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
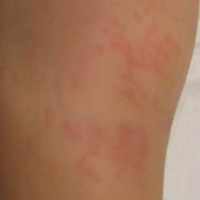
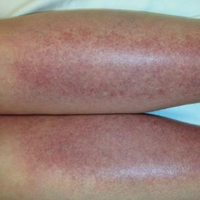
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.
The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Practice Points
- Both wild-type and vaccine-strain varicella-zoster virus (VZV) can establish latency in dorsal root ganglia and can cause herpes zoster (HZ) in vaccinated children.
- When HZ due to a vaccine strain of VZV occurs, the rash often presents near the site of initial vaccination.
- Although most cases of HZ in vaccinated children present with a characteristic HZ rash, physicians should be aware of the possibility for atypical presentations.
Nominate a Colleague or Patient for a NORD Rare Impact Award
Nominations are now open for the 2018 NORD Rare Impact Awards. These awards honor individuals who have made a positive impact on the rare disease community through research, patient care, advocacy, or other areas of involvement.
Awards are presented in May each year at NORD’s annual Rare Impact Celebration. Nominations may be submitted online. The deadline is January 12, 2018.
In recognition of the 35th anniversaries of NORD and the Orphan Drug Act in 2018, the awards will be presented in four categories representing the four pillars of NORD’s mission: Advocacy, Education, Research, and Patient Assistance.
Honorees from previous years have included members of Congress, staff and senior officials from NIH and FDA, medical researchers and clinicians, patient organization leaders, and individual patients and caregivers. The awards honor those who have helped to improve the lives of those affected by rare diseases.
Nominations are now open for the 2018 NORD Rare Impact Awards. These awards honor individuals who have made a positive impact on the rare disease community through research, patient care, advocacy, or other areas of involvement.
Awards are presented in May each year at NORD’s annual Rare Impact Celebration. Nominations may be submitted online. The deadline is January 12, 2018.
In recognition of the 35th anniversaries of NORD and the Orphan Drug Act in 2018, the awards will be presented in four categories representing the four pillars of NORD’s mission: Advocacy, Education, Research, and Patient Assistance.
Honorees from previous years have included members of Congress, staff and senior officials from NIH and FDA, medical researchers and clinicians, patient organization leaders, and individual patients and caregivers. The awards honor those who have helped to improve the lives of those affected by rare diseases.
Nominations are now open for the 2018 NORD Rare Impact Awards. These awards honor individuals who have made a positive impact on the rare disease community through research, patient care, advocacy, or other areas of involvement.
Awards are presented in May each year at NORD’s annual Rare Impact Celebration. Nominations may be submitted online. The deadline is January 12, 2018.
In recognition of the 35th anniversaries of NORD and the Orphan Drug Act in 2018, the awards will be presented in four categories representing the four pillars of NORD’s mission: Advocacy, Education, Research, and Patient Assistance.
Honorees from previous years have included members of Congress, staff and senior officials from NIH and FDA, medical researchers and clinicians, patient organization leaders, and individual patients and caregivers. The awards honor those who have helped to improve the lives of those affected by rare diseases.
Cosmetic Corner: Dermatologists Weigh in on Men’s Moisturizers
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s moisturizers. Consideration must be given to:
- Clinique For Men Oil Control Mattifying Moisturizer
Clinique Laboratories, LLC
“I recommend this product for men with oily or combination skin. It’s very lightweight and provides good hydration benefits without leaving the skin shiny.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Moisture Treatment for Men
Kiehl’s
“I commonly recommend this moisturizer. The Facial Fuel line is great for most skin types and the products are moderately priced.”—Gary Goldenberg, MD, New York, New York
- Neutrogena Men Triple Protect Face Lotion With Sunscreen
Johnson & Johnson Consumer Inc
“This is a light, daily moisturizer with broad-spectrum UV protection.”—Shari Lipner, MD, PhD, New York, New York
- Triple Lipid Restore 2:4:2
SkinCeuticals
“This moisturizer has the precise lipid content needed by the skin.”— Jerome Potozkin, MD, Danville, California
Cutis invites readers to send us their recommendations. Wet skin moisturizer, lip plumper, and pigment corrector will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s moisturizers. Consideration must be given to:
- Clinique For Men Oil Control Mattifying Moisturizer
Clinique Laboratories, LLC
“I recommend this product for men with oily or combination skin. It’s very lightweight and provides good hydration benefits without leaving the skin shiny.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Moisture Treatment for Men
Kiehl’s
“I commonly recommend this moisturizer. The Facial Fuel line is great for most skin types and the products are moderately priced.”—Gary Goldenberg, MD, New York, New York
- Neutrogena Men Triple Protect Face Lotion With Sunscreen
Johnson & Johnson Consumer Inc
“This is a light, daily moisturizer with broad-spectrum UV protection.”—Shari Lipner, MD, PhD, New York, New York
- Triple Lipid Restore 2:4:2
SkinCeuticals
“This moisturizer has the precise lipid content needed by the skin.”— Jerome Potozkin, MD, Danville, California
Cutis invites readers to send us their recommendations. Wet skin moisturizer, lip plumper, and pigment corrector will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s moisturizers. Consideration must be given to:
- Clinique For Men Oil Control Mattifying Moisturizer
Clinique Laboratories, LLC
“I recommend this product for men with oily or combination skin. It’s very lightweight and provides good hydration benefits without leaving the skin shiny.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Moisture Treatment for Men
Kiehl’s
“I commonly recommend this moisturizer. The Facial Fuel line is great for most skin types and the products are moderately priced.”—Gary Goldenberg, MD, New York, New York
- Neutrogena Men Triple Protect Face Lotion With Sunscreen
Johnson & Johnson Consumer Inc
“This is a light, daily moisturizer with broad-spectrum UV protection.”—Shari Lipner, MD, PhD, New York, New York
- Triple Lipid Restore 2:4:2
SkinCeuticals
“This moisturizer has the precise lipid content needed by the skin.”— Jerome Potozkin, MD, Danville, California
Cutis invites readers to send us their recommendations. Wet skin moisturizer, lip plumper, and pigment corrector will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Debunking Acne Myths: Does Back Acne Need to Be Treated?
Myth: Back acne will clear on its own
Patients with back acne may not seek treatment options because they assume it will clear on its own; however, deep painful lesions typically require treatment by a dermatologist. Mild to moderate cases may respond to less aggressive over-the-counter treatments but also may benefit from a combination of topical and systemic antibiotic therapies. According to James Q. Del Rosso, MD, a dermatologist in Las Vegas, Nevada, “Many dermatologists believe that truncal acne vulgaris warrants use of systemic antibiotic therapy, which may not necessarily be the case, especially in patients presenting with mild to moderate acne severity.” Cases of deep inflammatory acne on the back often warrant using systemic therapies such as oral isotretinoin. These severe cases may be less responsive to standard therapies and may require repeated treatment.
Although back acne is not as cosmetically visible as facial acne, it has been associated with sexual and bodily self-consciousness in both males and females. Preliminary data from one study showed that 78% of patients with truncal acne (n=141) on the back and/or chest indicated they were definitely interested in treatment, but truncal acne was not mentioned by these patients without direct inquiry from a physician. As a result, it may be beneficial for dermatologists to ask acne patients about lesions presenting on the back and inform them that treatment options are available.
Treatment application also is a consideration for back acne. Benzoyl peroxide cleanser/wash formulations are convenient, and the foam formulation of clindamycin phosphate allows for easy use due to its spreadability, rapid penetration, and lack of residue and fabric bleaching.
It is important to inform patients that back acne can flare even during active treatment. Patients should be instructed to wear loose-fitting clothes made of cotton or other sweat-wicking fabrics when working out and to shower and change clothes immediately after. Sheets and pillowcases should be changed regularly to avoid exposure to dead skin cells and bacteria, which can exacerbate acne on the back. Backpacks and handbags also can rub against the skin on the back, causing acne to flare. As an alternative, patients should be encouraged to carry handheld bags or bags with shoulder straps to avoid irritation of the skin on the back.
Back acne: how to see clearer skin. American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/back-acne-how-to-see-clearer-skin. Accessed October 30, 2017.
Del Rosso JQ. Management of truncal acne vulgaris: current perspectives on treatment. Cutis. 2006;77:285-289.
Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
Hassan J, Grogan S, Clark-Carter D, et al. The individual health burden of acne: appearance-related distress in male and female adolescents and adults with back, chest and facial acne. J Health Psychol. 2009;14:1105-11118.
Myth: Back acne will clear on its own
Patients with back acne may not seek treatment options because they assume it will clear on its own; however, deep painful lesions typically require treatment by a dermatologist. Mild to moderate cases may respond to less aggressive over-the-counter treatments but also may benefit from a combination of topical and systemic antibiotic therapies. According to James Q. Del Rosso, MD, a dermatologist in Las Vegas, Nevada, “Many dermatologists believe that truncal acne vulgaris warrants use of systemic antibiotic therapy, which may not necessarily be the case, especially in patients presenting with mild to moderate acne severity.” Cases of deep inflammatory acne on the back often warrant using systemic therapies such as oral isotretinoin. These severe cases may be less responsive to standard therapies and may require repeated treatment.
Although back acne is not as cosmetically visible as facial acne, it has been associated with sexual and bodily self-consciousness in both males and females. Preliminary data from one study showed that 78% of patients with truncal acne (n=141) on the back and/or chest indicated they were definitely interested in treatment, but truncal acne was not mentioned by these patients without direct inquiry from a physician. As a result, it may be beneficial for dermatologists to ask acne patients about lesions presenting on the back and inform them that treatment options are available.
Treatment application also is a consideration for back acne. Benzoyl peroxide cleanser/wash formulations are convenient, and the foam formulation of clindamycin phosphate allows for easy use due to its spreadability, rapid penetration, and lack of residue and fabric bleaching.
It is important to inform patients that back acne can flare even during active treatment. Patients should be instructed to wear loose-fitting clothes made of cotton or other sweat-wicking fabrics when working out and to shower and change clothes immediately after. Sheets and pillowcases should be changed regularly to avoid exposure to dead skin cells and bacteria, which can exacerbate acne on the back. Backpacks and handbags also can rub against the skin on the back, causing acne to flare. As an alternative, patients should be encouraged to carry handheld bags or bags with shoulder straps to avoid irritation of the skin on the back.
Myth: Back acne will clear on its own
Patients with back acne may not seek treatment options because they assume it will clear on its own; however, deep painful lesions typically require treatment by a dermatologist. Mild to moderate cases may respond to less aggressive over-the-counter treatments but also may benefit from a combination of topical and systemic antibiotic therapies. According to James Q. Del Rosso, MD, a dermatologist in Las Vegas, Nevada, “Many dermatologists believe that truncal acne vulgaris warrants use of systemic antibiotic therapy, which may not necessarily be the case, especially in patients presenting with mild to moderate acne severity.” Cases of deep inflammatory acne on the back often warrant using systemic therapies such as oral isotretinoin. These severe cases may be less responsive to standard therapies and may require repeated treatment.
Although back acne is not as cosmetically visible as facial acne, it has been associated with sexual and bodily self-consciousness in both males and females. Preliminary data from one study showed that 78% of patients with truncal acne (n=141) on the back and/or chest indicated they were definitely interested in treatment, but truncal acne was not mentioned by these patients without direct inquiry from a physician. As a result, it may be beneficial for dermatologists to ask acne patients about lesions presenting on the back and inform them that treatment options are available.
Treatment application also is a consideration for back acne. Benzoyl peroxide cleanser/wash formulations are convenient, and the foam formulation of clindamycin phosphate allows for easy use due to its spreadability, rapid penetration, and lack of residue and fabric bleaching.
It is important to inform patients that back acne can flare even during active treatment. Patients should be instructed to wear loose-fitting clothes made of cotton or other sweat-wicking fabrics when working out and to shower and change clothes immediately after. Sheets and pillowcases should be changed regularly to avoid exposure to dead skin cells and bacteria, which can exacerbate acne on the back. Backpacks and handbags also can rub against the skin on the back, causing acne to flare. As an alternative, patients should be encouraged to carry handheld bags or bags with shoulder straps to avoid irritation of the skin on the back.
Back acne: how to see clearer skin. American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/back-acne-how-to-see-clearer-skin. Accessed October 30, 2017.
Del Rosso JQ. Management of truncal acne vulgaris: current perspectives on treatment. Cutis. 2006;77:285-289.
Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
Hassan J, Grogan S, Clark-Carter D, et al. The individual health burden of acne: appearance-related distress in male and female adolescents and adults with back, chest and facial acne. J Health Psychol. 2009;14:1105-11118.
Back acne: how to see clearer skin. American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/back-acne-how-to-see-clearer-skin. Accessed October 30, 2017.
Del Rosso JQ. Management of truncal acne vulgaris: current perspectives on treatment. Cutis. 2006;77:285-289.
Dunn LK, O’Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatol Online J. 2011;17:1.
Hassan J, Grogan S, Clark-Carter D, et al. The individual health burden of acne: appearance-related distress in male and female adolescents and adults with back, chest and facial acne. J Health Psychol. 2009;14:1105-11118.
What’s Eating You? Scabies in the Developing World
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
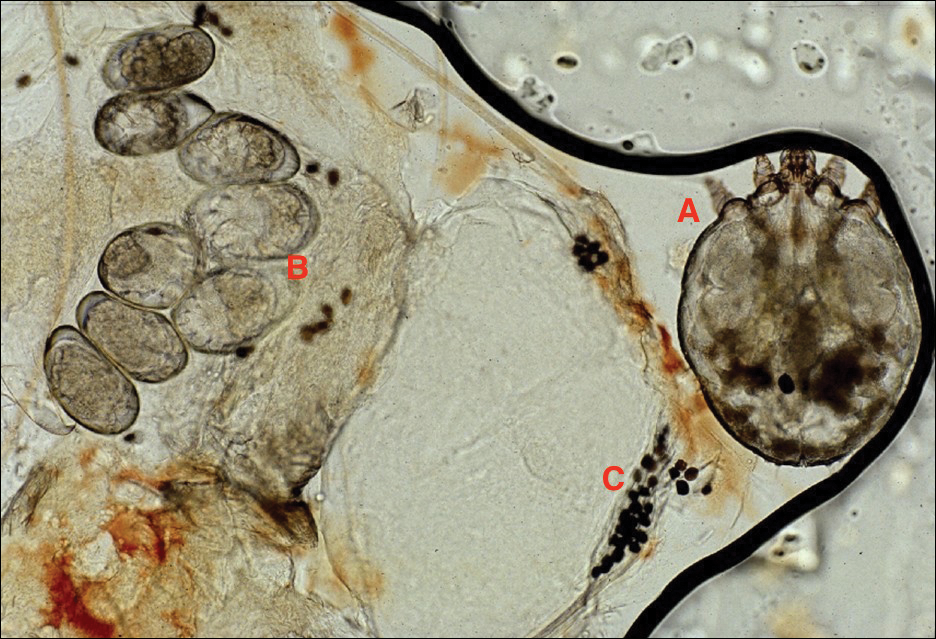
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).
Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
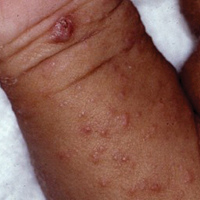
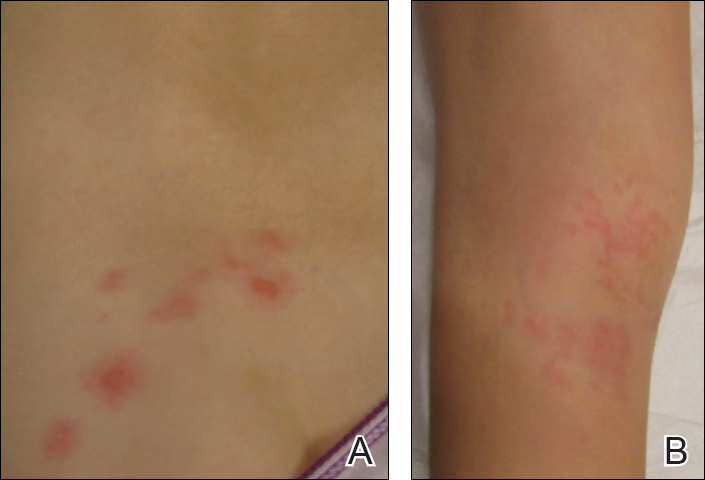
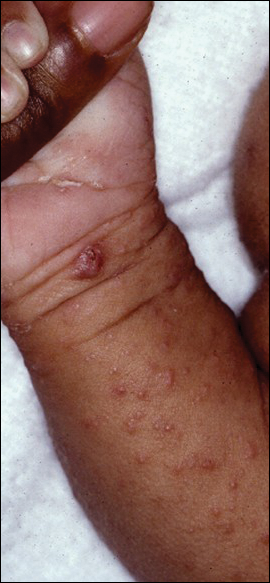
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.
Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
Scabies is caused by the mite Sarcoptes scabiei var hominis.1 It is in the arthropod class Arachnida, subclass Acari, and family Sarcoptidae.2 Historically, scabies was first described in the Old Testament and by Aristotle,2 but the causative organism was not identified until 1687 using a light microscope.3 Scabies affects all age groups, races, and social classes and is globally widespread. It is most prevalent in developing tropical countries.1 It is estimated that 300 million individuals worldwide are infested with scabies mites annually, with the highest burden in young children.4-7 In industrialized societies, infections often are seen in young adults and in institutional settings such as nursing homes.8 Scabies disproportionately impacts impoverished communities with crowded living conditions, poor hygiene and nutrition, and substandard housing.5,9 Controlling the spread of the disease in these communities presents challenges but is important because of the connection between scabies and chronic kidney disease.10 As such, scabies represents a major health problem in the developing world and has been the focus of major health initiatives.1,11
Identifying Characteristics
Adult females are 0.4-mm long and 0.3-mm wide, with males being smaller. Adult nymphs have 8 legs and larvae have 6 legs. Scabies mites are distinguishable from other arachnids by the position of a distinct gnathosoma and the lack of a division between the abdomen and cephalothorax.12 They are ovoid with a small anterior cephalic and caudal thoracoabdominal portion with hairlike projections coming off from the rudimentary legs. They can crawl as fast as 2.5 cm per minute on warm skin.2 The life cycle of the mite begins after mating: the male mite dies, and the female lays up to 3 eggs per day, which hatch in 3 to 4 days,2 in skin burrows within the stratum granulosum.12 Maturation from larva to adult takes 10 to 14 days.12 A female mite can live for 4 to 6 weeks and can produce up to 40 ova (Figure 1).

Disease Transmission
Without a host, mites are able to survive and remain capable of infestation for 24 to 36 hours at 21°C and 40% to 80% relative humidity. Lower temperatures and higher humidity prolong survival, but infectivity decreases the longer they are without a host.13
An adult human with ordinary scabies will have an average of 12 adult female mites on the body surface at a given time.14 However, hundreds of mites can be found in neglected children in underprivileged communities and millions in patients with crusted scabies.13 Transmission of typical scabies requires close direct skin-to-skin contact for 15 to 20 minutes.2,8 Transmission from clothing or fomites are an unlikely source of infestation with the exception of patients who are heavily infested such as in crusted scabies.12 In adults, sexual contact is an important method of transmission,12 and patients with scabies should be screened for other sexually transmitted diseases.8
Clinical Manifestations
Signs of scabies on the skin include burrows, erythematous papules, and generalized pruritus (Figure 2).12 The scalp, face, and neck frequently are involved in infants and children,2 and the hands, wrists, elbows, genitalia, axillae, umbilicus, belt line, nipples, and buttocks commonly are involved in adults.12 Itching is characteristically worse at night.8 In tropical climates, patients with scabies are predisposed to secondary bacterial skin infections, particularly Streptococcus pyogenes (group A streptococci). The association between scabies and pyoderma caused by group A streptococci has been well established.15,16 Mika et al10 suggested that local complement inhibition plays an important role in the development of pyoderma in scabies-infested skin.

Prevention and Control in the Developing World
Low-cost diagnostic equipment can play a key role in the definitive diagnosis and management of scabies outbreaks in the developing world. Micali et al28 found that a $30 videomicroscope was as effective in scabies diagnosis as a $20,000 videodermatoscope. Because of the low cost of benzyl benzoate, it is commonly used as a first-line drug in many parts of the world,13 whereas permethrin cream 5% is the standard treatment in the developed world.29 Recognition of the role of scabies in patients with pyoderma is key, and one study indicated clinically apparent scabies went unnoticed by physicians in 52% of patients presenting with skin lesions.30 Drug shortages also can contribute to a high prevalence of scabies infestation in the community.31 Mass treatment with ivermectin has proven to be an effective means of reducing the prevalence of many parasitic diseases,1,32,33 and it shows great promise for crusted scabies, institutional outbreaks, and mass administration in highly endemic communites.8 However, there is evidence of ivermectin tolerance among mites, which could undermine the success of mass drug administration.34 Another important consideration is population mobility and the risk for rapid reintroduction of scabies infection across regions.35
Complicating disease control are the socioeconomic factors associated with scabies in the developing world. Families with scabies infestation typically do not own their homes, are less likely to have constant electricity, have a lower monthly income, and live in substandard housing.20 Families can spend a substantial part of their household income on treatment, impacting what they can spend on food.8,11 In addition to medication, control of scabies requires community education and involvement, along with access to primary care and attention to living conditions and environmental factors.34,36
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015;373:2305-2313.
- Hicks MI, Elston DM. Scabies. Dermatol Ther. 2009;22:279-292.
- Ramos-e-Silva M. Giovan Cosimo Bonomo (1663-1696): discoverer of the etiology of scabies. Int J Dermatol. 1998;37:625-630.
- Chung SD, Wang KH, Huang CC, et al. Scabies increased the risk of chronic kidney disease: a 5-year follow-up study. J Eur Acad Dermatol Venereol. 2014;28:286-292.
- Wong SS, Poon RW, Chau S, et al. Development of conventional and real-time quantitative PCR assays for diagnosis and monitoring of scabies. J Clin Microbiol. 2015;53:2095-2102.
- Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis. 2015;9:e0004151.
- Gilmore SJ. Control strategies for endemic childhood scabies. PLoS One. 2011;6:e15990.
- Hay RJ, Steer AC, Engelman D, Walton S. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect. 2012;18:313-323.
- Hoy WE, White AV, Dowling A, et al. Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney Int. 2012;81:1026-1032.
- Mika A, Reynolds SL, Pickering D, et al. Complement inhibitors from scabies mites promote streptococcal growth—a novel mechanism in infected epidermis? PLoS Negl Trop Dis. 2012;6:e1563.
- McLean FE. The elimination of scabies: a task for our generation. Int J Dermatol. 2013;52:1215-1223.
- Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006;6:769-779.
- Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
- Johnston G, Sladden M. Scabies: diagnosis and treatment. BMJ. 2005;331:619-622.
- Yeoh DK, Bowen AC, Carapetis JR. Impetigo and scabies—disease burden and modern treatment strategies [published online May 11, 2016]. J Infect. 2016;(72 suppl):S61-S67.
- Bowen AC, Mahé A, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10:e0136789.
- Bowen AC, Tong SY, Chatfield MD, et al. The microbiology of impetigo in indigenous children: associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infect Dis. 2014;14:727.
- Sesso R, Pinto SW. Five-year follow-up of patients with epidemic glomerulonephritis due to Streptococcus zooepidemicus. Nephrol Dial Transplant. 2005;20:1808-1812.
- Singh GR. Glomerulonephritis and managing the risks of chronic renal disease. Pediatr Clin North Am. 2009;56:1363-1382.
- La Vincente S, Kearns T, Connors C, et al. Community management of endemic scabies in remote aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Negl Trop Dis. 2009;3:e444.
- Clucas DB, Carville KS, Connors C, et al. Disease burden and health-care clinic attendances for young children in remote aboriginal communities of northern Australia. Bull World Health Organ. 2008;86:275-281.
- Stanton B, Khanam S, Nazrul H, et al. Scabies in urban Bangladesh. J Trop Med Hyg. 1987;90:219-226.
- Heukelbach J, de Oliveira FA, Feldmeier H. Ecoparasitoses and public health in Brazil: challenges for control [in Portuguese]. Cad Saude Publica. 2003;19:1535-1540.
- Edison L, Beaudoin A, Goh L, et al. Scabies and bacterial superinfection among American Samoan children, 2011-2012. PLoS One. 2015;10:e0139336.
- Steer AC, Jenney AW, Kado J, et al. High burden of impetigo and scabies in a tropical country. PLoS Negl Trop Dis. 2009;3:e467.
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15:960-967.
- Romani L, Koroivueta J, Steer AC, et al. Scabies and impetigo prevalence and risk factors in Fiji: a national survey. PLoS Negl Trop Dis. 2015;9:e0003452.
- Micali G, Lacarrubba F, Verzì AE, et al. Low-cost equipment for diagnosis and management of endemic scabies outbreaks in underserved populations. Clin Infect Dis. 2015;60:327-329.
- Pasay C, Walton S, Fischer K, et al. PCR-based assay to survey for knockdown resistance to pyrethroid acaricides in human scabies mites (Sarcoptes scabiei var hominis). Am J Trop Med Hyg. 2006;74:649-657.
- Heukelbach J, van Haeff E, Rump B, et al. Parasitic skin diseases: health care-seeking in a slum in north-east Brazil. Trop Med Int Health. 2003;8:368-373.
- Potter EV, Mayon-White R, Poon-King T, et al. Acute glomerulonephritis as a complication of scabies. In: Orkin M, Maibach HI, eds. Cutaneous Infestations and Insect Bites. New York, NY: Marcel Dekker; 1985.
- Mahé A. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689.
- Steer AC, Romani L, Kaldor JM. Mass drug administration for scabies control. N Engl J Med. 2016;374:1690.
- Mounsey KE, Holt DC, McCarthy JS, et al. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840-841.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371-2372.
- O’Donnell V, Morris S, Ward J. Mass drug administration for scabies control. N Engl J Med. 2016;374:1689-1690.
Practice Points
- Scabies infestation is one of the world’s leading causes of chronic kidney disease.
- Ivermectin can be used to treat mass infestations, and older topical therapies also are commonly used.
Syphilis and the Dermatologist
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
Unsuspected Lymphomatoid Granulomatosis in a Patient With Antisynthetase Syndrome
Lymphomatoid granulomatosis (LYG) is a rare Epstein-Barr virus (EBV)–related extranodal angiocentric lymphoproliferative disorder. Most patients are adults in the fifth decade of life, and men are twice as likely as women to be affected.1 The most common site of involvement is the lungs, which has been observed in more than 90% of patients.2 The skin is the most common extrapulmonary site of involvement with variable manifestations including “rash,” subcutaneous nodules, and ulceration. Although a small subset of patients experience remission without treatment, most patients report a progressive course with median survival of less than 2 years.1,2 Clinical diagnosis often is challenging due to underrecognition of this rare condition by multidisciplinary physicians.
Case Report
A 60-year-old woman presented with fatigue, night sweats, poor appetite, unintentional weight loss, and dyspnea with minor exertion of 2 weeks’ duration. Her medical history was remarkable for antisynthetase syndrome manifested as polymyositis and interstitial lung disease, as well as recurrent breast cancer treated with wide excision, chemotherapy, and radiation therapy completed 2 months prior. Antisynthetase syndrome was controlled with azathioprine for 2 years, which was stopped during chemotherapy but restarted to treat worsened myalgia 4 months prior to presentation. Two weeks prior to hospital admission, she was treated with antibiotics at an outside hospital for presumed pneumonia without improvement. Upon admission to our hospital she was pancytopenic. Chest computed tomography showed interval development of extensive patchy ground-glass opacities in all lung lobes with areas of confluent consolidation. Broad infectious workup was negative. Given the time course of presentation and anterior accentuation of the lung infiltrates, the greatest clinical concern was radiation pneumonitis followed by drug toxicity. A bone marrow biopsy was hypocellular but without evidence of malignancy. Her pancytopenia was thought to be induced by azathioprine and/or antibiotics. Antibiotics were discontinued and prednisone was started for treatment of presumed radiation pneumonitis.
A few days later, the patient developed new skin lesions and worsening bilateral leg edema. There were multiple small erythematous and hemorrhagic papules, macules, and blisters on the medial aspect of the right lower leg and ankle, each measuring less than 1 cm in diameter (Figure 1). The clinical differential diagnosis included vasculitis related to an underlying collagen vascular disease, atypical edema blisters, and drug hypersensitivity reaction. A punch biopsy of one of the lesions showed a moderately dense superficial and deep perivascular lymphoid infiltrate with marked papillary dermal edema and early subepidermal split (Figure 2). The infiltrate was comprised of small- to medium-sized lymphocytes admixed with large cells, histiocytes, and plasma cells (Figure 3). Immunohistochemistry revealed a predominance of CD3+ and CD4+ small- to medium-sized T cells. CD20 highlighted the large angiocentric B cells (Figure 4), which also were positive on EBV-encoded small RNA (EBER) in situ hybridization (Figure 5). A diagnosis of LYG was rendered. Approximately 40 to 50 EBV-positive large B cells were present per high-power field (HPF), consistent with grade 2 disease.
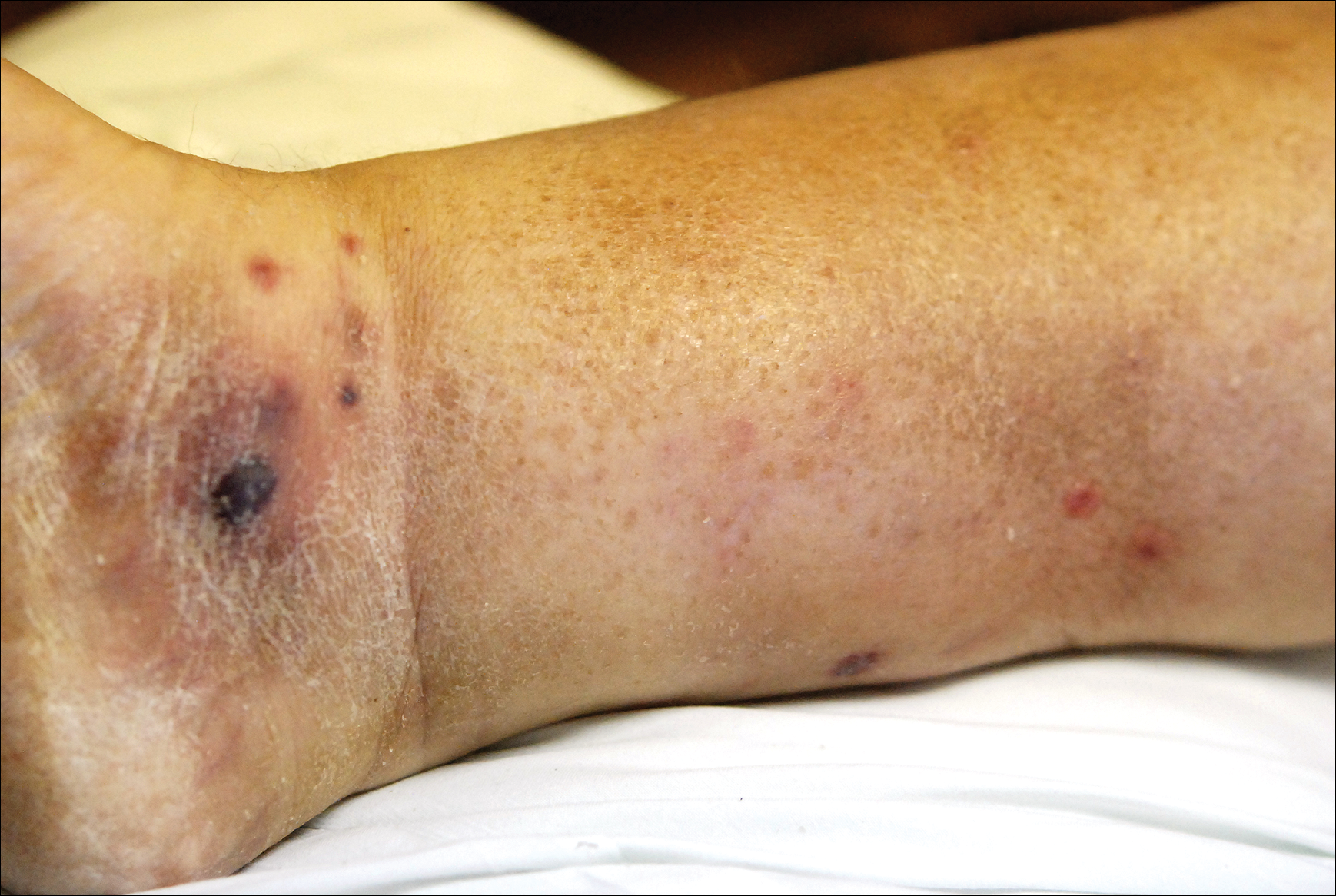
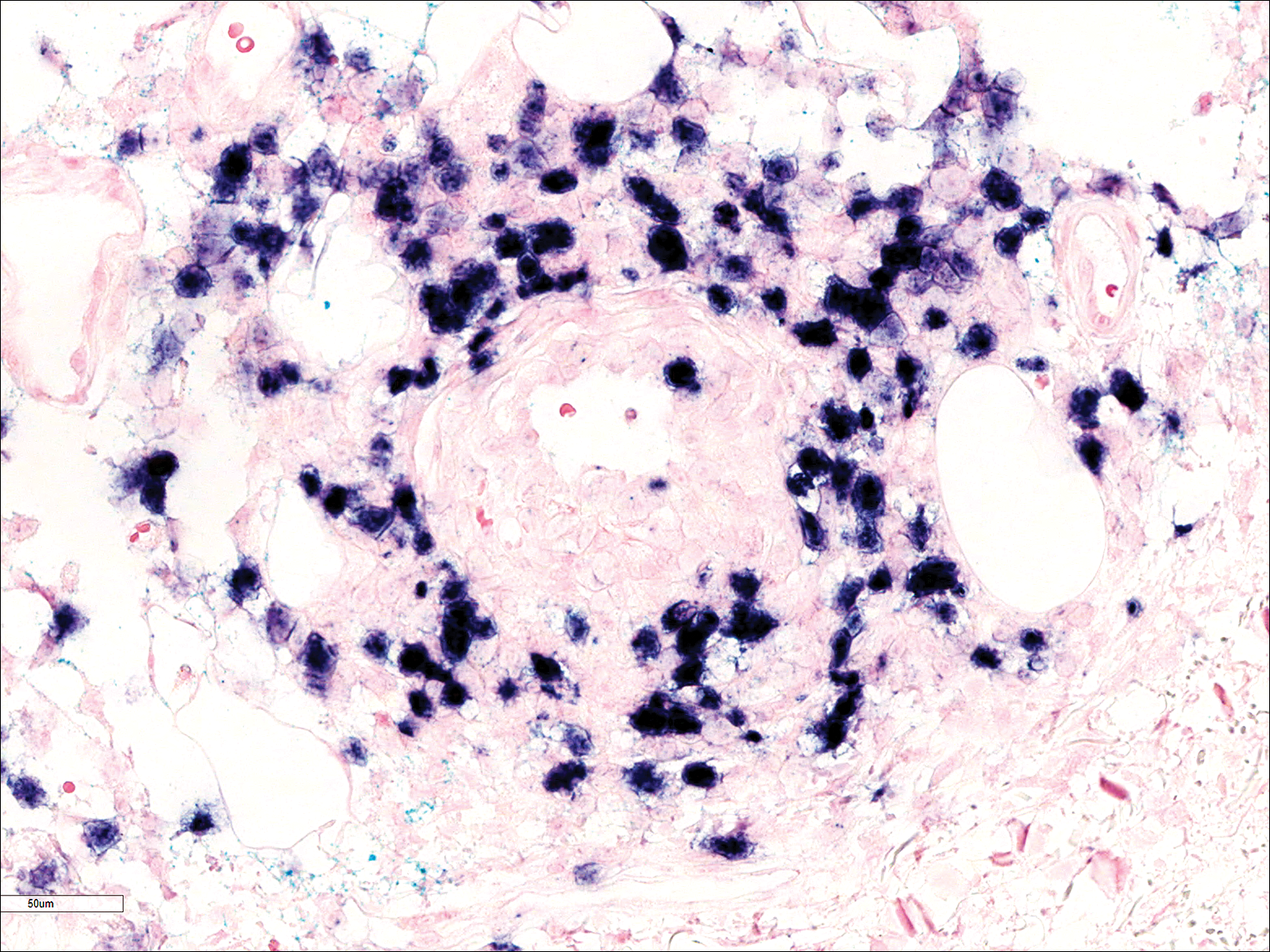
Soon after diagnosis, follow-up computed tomography of the chest, abdomen, and pelvis revealed suspicious lesions in the kidneys, liver, spleen, and inguinal and iliac lymph nodes. The ground-glass opacities in the lungs continued to progress, with 2 additional nodules noted in the right upper and lower lobes. Four days later, core needle biopsies of the right inguinal lymph node showed a large B-cell lymphoma with extensive necrosis (Figure 6). EBER in situ hybridization was suboptimal, probably due to extensive necrosis.
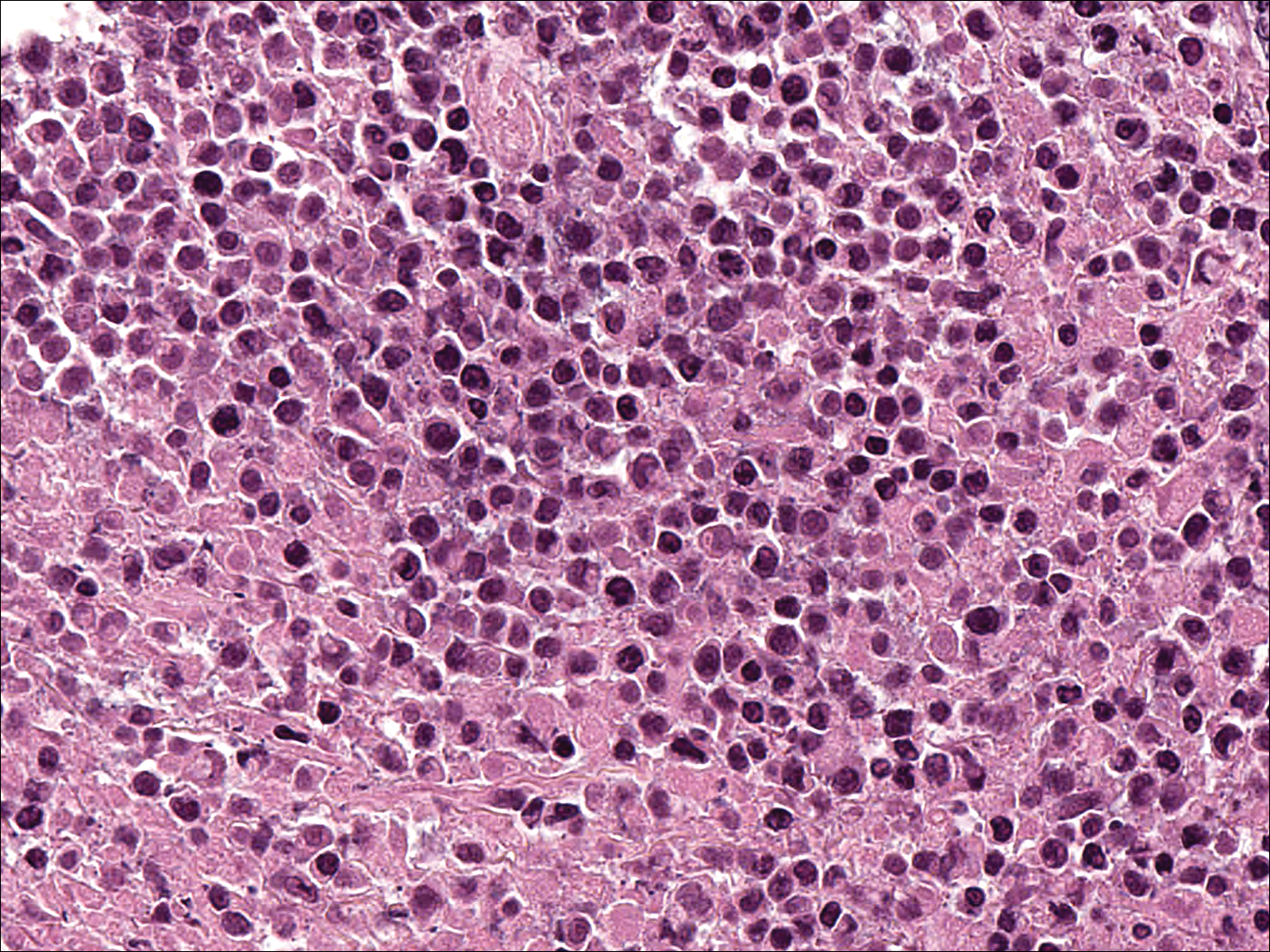
She was started on etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) for 5 days before developing Klebsiella pneumoniae sepsis and acute kidney injury. She was transferred to the critical care unit due to increasing oxygen requirement. Despite medical interventions, she continued to decompensate and elected to transition to palliative care. She died 6 weeks after the initial presentation. Her family did not request an autopsy.
Comment
Lymphomatoid granulomatosis is a rare lymphoproliferative disorder associated with various immunocompromised states including primary immunodeficiency disorders, human immunodeficiency virus infection, and immunosuppression for organ transplantation and autoimmune diseases. Our patient was receiving azathioprine for antisynthetase syndrome, which put her at risk for EBV infection and LYG. Azathioprine rarely has been reported as a possible culprit of LYG,3,4 but there are no known reported cases that were related to antisynthetase syndrome. There are multiple reports of development of LYG in patients receiving methotrexate for rheumatoid arthritis.5-10 Other iatrogenic causes reported in the literature include thiopurines11,12 and imatinib.13,14
The clinical diagnosis of our patient was particularly challenging given her complicated medical history including interstitial lung disease, predisposition to infection secondary to immunosuppression, and recent radiation therapy to the chest. This case illustrates the importance of maintaining a high index of suspicion for LYG in immunosuppressed patients presenting with lung infiltrates.
Presentation
Radiologically, LYG typically manifests as nodular densities accentuated in the lower lung lobes, which may become confluent.15 Because the nodular pattern in LYG is nonspecific and may mimic sarcoidosis, hypersensitivity pneumonitis, vasculitis, and infectious and neoplastic diseases,16 open lung biopsy often is required to establish the diagnosis in the absence of more accessible lesions.
Cutaneous lesions are seen in 40% to 50% of patients2 and may be the presenting sign of LYG. In a retrospective study, 16% (3/19) of LYG patients presented with cutaneous lesions months before diagnostic pulmonary lesions were identified.17 The skin is the most accessible site for biopsy, allowing definitive tissue diagnosis even when the condition is not clinically suspected. Therefore, dermatologists and dermatopathologists should be aware of this rare entity.
The clinical morphologies of the skin lesions are nonspecific, ranging from erythematous papules and subcutaneous nodules to indurated plaques. Ulceration may be present. The lesions may be widely disseminated or limited to the arms and legs. Our patient presented with erythematous and hemorrhagic papules, macules, and blisters on the lower leg. The hemorrhagic and blistering nature of some of these lesions in our patient may be attributable to thrombocytopenia and lymphedema in addition to LYG.
Histopathology and Differential
The skin biopsy from our patient demonstrated typical features of LYG, namely EBV-positive neoplastic large B cells in a background of predominating reactive T cells.18 The neoplastic large cells frequently invade blood vessels, leading to luminal narrowing without necrosis of the vessel walls. Grading is based on the density of EBV-positive large B cells: grade 1 is defined as fewer than 5 cells per HPF; grade 2, 5 to 50 cells per HPF; and grade 3, more than 50 cells per HPF.18 Grade 2 or 3 disease predicts worse outcome,2 as observed in our case. It is important for pathologists and clinicians to be aware that the proportion of EBV-positive large B cells is variable even within a single lesion; therefore, more than 1 biopsy may be necessary for appropriate grading and management.1,17 Additionally, skin biopsy may have a lower sensitivity for detecting EBV-positive B cells compared to lung biopsy, possibly due to sampling error in small biopsies.17
The histopathologic features of LYG frequently overlap with other lymphomas. Due to the abundance of T cells, LYG may be misclassified as T-cell/histiocyte-rich large B-cell lymphoma.19 Because the latter is not associated with EBV, EBER in situ hybridization is helpful in distinguishing the 2 conditions. On the other hand, EBER in situ hybridization has no value in discriminating LYG and extranodal natural killer (NK)/T-cell lymphoma, as both are EBV driven. Unlike LYG, the neoplastic EBV-positive cells in extranodal NK/T-cell lymphoma make up the majority of the infiltrate and exhibit an NK-cell immunophenotype (positive CD56 and cytoplasmic CD3 epsilon).20 Pulmonary involvement also is uncommon in NK/T-cell lymphoma.
Aside from lymphomas, LYG also resembles granulomatosis with polyangiitis (GPA)(formerly known as Wegener granulomatosis). Clinically, both LYG and GPA can present with constitutional symptoms, as well as lung, kidney, and skin lesions. The 2 conditions differ microscopically, with leukocytoclastic vasculitis and necrotizing granulomatous inflammation being characteristic of GPA but absent in LYG.1,21 Neutrophils and eosinophils are much more likely to be present in GPA.22,23
Disease Progression
Although LYG is an extranodal disease, there is a 7% to 45% risk of progression to nodal lymphoma in patients with high-grade disease.2,22,24 Our patient progressed to nodal large B-cell lymphoma shortly after the diagnosis of high-grade LYG. She developed additional lesions in the liver, spleen, and kidneys, and ultimately succumbed to the disease. Prior studies have shown higher mortality in patients with bilateral lung involvement and neurologic abnormalities, whereas cutaneous involvement does not affect outcome.2
Treatment
A prospective study used an initial treatment regimen of cyclophosphamide and prednisone but mortality was high.24 More recently, chemotherapy regimens including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), CVP or CHOP combined with rituximab, C-MOPP (cyclophosphamide, vincristine, prednisone, and procarbazine), EPOCH, and rituximab with high-dose cytarabine have been used with variable success for grades 2 and 3 LYG.17,23,25,26 Antiviral and immunomodulatory (interferon alfa) therapy has been used to induce remission in a majority of patients with grades 1 or 2 LYG.3,17,27,28 There is a report of successful treatment of relapsed LYG with the retinoid agent bexarotene.29 Autologous or allogeneic stem cell transplantation was effective for some patients with refractory or relapsed LYG.30 Further studies are needed to clarify optimal treatment of LYG, especially high-grade disease.
Conclusion
We report a rare case of LYG in a patient with antisynthetase syndrome, which highlights the critical role of skin biopsy in establishing the diagnosis of LYG when the clinical and radiologic presentations are obscured by other comorbidities. Dermatologists should be familiar with this rare disease and maintain a low threshold for biopsy in immunocompromised patients presenting with nodular lung infiltrates and/or nonspecific skin lesions.
- Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010;34:E35-E48.
- Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43:360-373.
- Connors W, Griffiths C, Patel J, et al. Lymphomatoid granulomatosis associated with azathioprine therapy in Crohn disease. BMC Gastroenterol. 2014;14:127.
- Katherine Martin L, Porcu P, Baiocchi RA, et al. Primary central nervous system lymphomatoid granulomatosis in a patient receiving azathioprine therapy. Clin Adv Hematol Oncol. 2009;7:65-68.
- Barakat A, Grover K, Peshin R. Rituximab for pulmonary lymphomatoid granulomatosis which developed as a complication of methotrexate and azathioprine therapy for rheumatoid arthritis. Springerplus. 2014;3:751.
- Kobayashi S, Kikuchi Y, Sato K, et al. Reversible iatrogenic, MTX-associated EBV-driven lymphoproliferation with histopathological features of a lymphomatoid granulomatosis in a patient with rheumatoid arthritis. Ann Hematol. 2013;92:1561-1564.
- Kameda H, Okuyama A, Tamaru J, et al. Lymphomatoid granulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26:1585-1589.
- Oiwa H, Mihara K, Kan T, et al. Grade 3 lymphomatoid granulomatosis in a patient receiving methotrexate therapy for rheumatoid arthritis. Intern Med. 2014;53:1873-1875.
- Blanchart K, Paciencia M, Seguin A, et al. Fatal pulmonary lymphomatoid granulomatosis in a patient taking methotrexate for rheumatoid arthritis. Minerva Anestesiol. 2014;80:119-120.
- Schalk E, Krogel C, Scheinpflug K, et al. Lymphomatoid granulomatosis in a patient with rheumatoid arthritis receiving methotrexate: successful treatment with the anti-CD20 antibody mabthera. Onkologie. 2009;32:440-441.
- Subramaniam K, Cherian M, Jain S, et al. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J. 2013;43:1339-1342.
- Destombe S, Bouron-DalSoglio D, Rougemont AL, et al. Lymphomatoid granulomatosis: a unique complication of Crohn disease and its treatment in pediatrics. J Pediatr Gastroenterol Nutr. 2010;50:559-561.
- Yazdi AS, Metzler G, Weyrauch S, et al. Lymphomatoid granulomatosis induced by imatinib treatment. Arch Dermatol. 2007;143:1222-1223.
- Salmons N, Gregg RJ, Pallalau A, et al. Lymphomatoid granulomatosis in a patient previously diagnosed with a gastrointestinal stromal tumour and treated with imatinib. J Clin Pathol. 2007;60:199-201.
- Dee PM, Arora NS, Innes DJ Jr. The pulmonary manifestations of lymphomatoid granulomatosis. Radiology. 1982;143:613-618.
- Rezai P, Hart EM, Patel SK. Case 169: lymphomatoid granulomatosis. Radiology. 2011;259:604-609.
- Beaty MW, Toro J, Sorbara L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25:1111-1120.
- Pittaluga S, Wilson WH, Jaffe E. Lymphomatoid granulomatosis. In: Swerdlow S, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008:247-249.
- Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392.
- Jaffe E. Nasal and nasal-type T/NK cell lymphoma: a unique form of lymphoma associated with the Epstein-Barr virus. Histopathology. 1995;27:581-583.
- Barksdale SK, Hallahan CW, Kerr GS, et al. Cutaneous pathology in Wegener’s granulomatosis: a clinicopathologic study of 74 biopsies in 46 patients. Am J Surg Pathol. 1995;19:161-172.
- Koss MN, Hochholzer L, Langloss JM, et al. Lymphomatoid granulomatosis: a clinicopathologic study of 42 patients. Pathology. 1986;18:283-288.
- Aoki T, Harada Y, Matsubara E, et al. Long-term remission after multiple relapses in an elderly patient with lymphomatoid granulomatosis after rituximab and high-dose cytarabine chemotherapy without stem-cell transplantation. J Clin Oncol. 2013;31:E390-E393.
- Fauci AS, Haynes BF, Costa J, et al. Lymphomatoid granulomatosis: prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982;306:68-74.
- Jung KH, Sung HJ, Lee JH, et al. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009;55:386-390.
- Hernandez-Marques C, Lassaletta A, Torrelo A, et al. Rituximab in lymphomatoid granulomatosis. J Pediatr Hematol Oncol. 2014;36:E69-E74.
- Wilson WH, Gutierrez M, Raffeld M, et al. Lymphomatoid granulomatosis: phase 2 study of dose-adjusted interferon-alfa or EPOCH chemotherapy. Blood. 1999;94:599A.
- Wilson WH, Kingma DW, Raffeld M, et al. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996;87:4531-4537.
- Berg SE, Downs LH, Torigian DA, et al. Successful treatment of relapsed lymphomatoid granulomatosis with bexarotene. Cancer Biol Ther. 2008;7:1544-1546.
- Siegloch K, Schmitz N, Wu HS, et al. Hematopoietic stem cell transplantation in patients with lymphomatoid granulomatosis: a European group for blood and marrow transplantation report. Biol Blood Marrow Transplant. 2013;19:1522-1525.
Lymphomatoid granulomatosis (LYG) is a rare Epstein-Barr virus (EBV)–related extranodal angiocentric lymphoproliferative disorder. Most patients are adults in the fifth decade of life, and men are twice as likely as women to be affected.1 The most common site of involvement is the lungs, which has been observed in more than 90% of patients.2 The skin is the most common extrapulmonary site of involvement with variable manifestations including “rash,” subcutaneous nodules, and ulceration. Although a small subset of patients experience remission without treatment, most patients report a progressive course with median survival of less than 2 years.1,2 Clinical diagnosis often is challenging due to underrecognition of this rare condition by multidisciplinary physicians.
Case Report
A 60-year-old woman presented with fatigue, night sweats, poor appetite, unintentional weight loss, and dyspnea with minor exertion of 2 weeks’ duration. Her medical history was remarkable for antisynthetase syndrome manifested as polymyositis and interstitial lung disease, as well as recurrent breast cancer treated with wide excision, chemotherapy, and radiation therapy completed 2 months prior. Antisynthetase syndrome was controlled with azathioprine for 2 years, which was stopped during chemotherapy but restarted to treat worsened myalgia 4 months prior to presentation. Two weeks prior to hospital admission, she was treated with antibiotics at an outside hospital for presumed pneumonia without improvement. Upon admission to our hospital she was pancytopenic. Chest computed tomography showed interval development of extensive patchy ground-glass opacities in all lung lobes with areas of confluent consolidation. Broad infectious workup was negative. Given the time course of presentation and anterior accentuation of the lung infiltrates, the greatest clinical concern was radiation pneumonitis followed by drug toxicity. A bone marrow biopsy was hypocellular but without evidence of malignancy. Her pancytopenia was thought to be induced by azathioprine and/or antibiotics. Antibiotics were discontinued and prednisone was started for treatment of presumed radiation pneumonitis.
A few days later, the patient developed new skin lesions and worsening bilateral leg edema. There were multiple small erythematous and hemorrhagic papules, macules, and blisters on the medial aspect of the right lower leg and ankle, each measuring less than 1 cm in diameter (Figure 1). The clinical differential diagnosis included vasculitis related to an underlying collagen vascular disease, atypical edema blisters, and drug hypersensitivity reaction. A punch biopsy of one of the lesions showed a moderately dense superficial and deep perivascular lymphoid infiltrate with marked papillary dermal edema and early subepidermal split (Figure 2). The infiltrate was comprised of small- to medium-sized lymphocytes admixed with large cells, histiocytes, and plasma cells (Figure 3). Immunohistochemistry revealed a predominance of CD3+ and CD4+ small- to medium-sized T cells. CD20 highlighted the large angiocentric B cells (Figure 4), which also were positive on EBV-encoded small RNA (EBER) in situ hybridization (Figure 5). A diagnosis of LYG was rendered. Approximately 40 to 50 EBV-positive large B cells were present per high-power field (HPF), consistent with grade 2 disease.





Soon after diagnosis, follow-up computed tomography of the chest, abdomen, and pelvis revealed suspicious lesions in the kidneys, liver, spleen, and inguinal and iliac lymph nodes. The ground-glass opacities in the lungs continued to progress, with 2 additional nodules noted in the right upper and lower lobes. Four days later, core needle biopsies of the right inguinal lymph node showed a large B-cell lymphoma with extensive necrosis (Figure 6). EBER in situ hybridization was suboptimal, probably due to extensive necrosis.

She was started on etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) for 5 days before developing Klebsiella pneumoniae sepsis and acute kidney injury. She was transferred to the critical care unit due to increasing oxygen requirement. Despite medical interventions, she continued to decompensate and elected to transition to palliative care. She died 6 weeks after the initial presentation. Her family did not request an autopsy.
Comment
Lymphomatoid granulomatosis is a rare lymphoproliferative disorder associated with various immunocompromised states including primary immunodeficiency disorders, human immunodeficiency virus infection, and immunosuppression for organ transplantation and autoimmune diseases. Our patient was receiving azathioprine for antisynthetase syndrome, which put her at risk for EBV infection and LYG. Azathioprine rarely has been reported as a possible culprit of LYG,3,4 but there are no known reported cases that were related to antisynthetase syndrome. There are multiple reports of development of LYG in patients receiving methotrexate for rheumatoid arthritis.5-10 Other iatrogenic causes reported in the literature include thiopurines11,12 and imatinib.13,14
The clinical diagnosis of our patient was particularly challenging given her complicated medical history including interstitial lung disease, predisposition to infection secondary to immunosuppression, and recent radiation therapy to the chest. This case illustrates the importance of maintaining a high index of suspicion for LYG in immunosuppressed patients presenting with lung infiltrates.
Presentation
Radiologically, LYG typically manifests as nodular densities accentuated in the lower lung lobes, which may become confluent.15 Because the nodular pattern in LYG is nonspecific and may mimic sarcoidosis, hypersensitivity pneumonitis, vasculitis, and infectious and neoplastic diseases,16 open lung biopsy often is required to establish the diagnosis in the absence of more accessible lesions.
Cutaneous lesions are seen in 40% to 50% of patients2 and may be the presenting sign of LYG. In a retrospective study, 16% (3/19) of LYG patients presented with cutaneous lesions months before diagnostic pulmonary lesions were identified.17 The skin is the most accessible site for biopsy, allowing definitive tissue diagnosis even when the condition is not clinically suspected. Therefore, dermatologists and dermatopathologists should be aware of this rare entity.
The clinical morphologies of the skin lesions are nonspecific, ranging from erythematous papules and subcutaneous nodules to indurated plaques. Ulceration may be present. The lesions may be widely disseminated or limited to the arms and legs. Our patient presented with erythematous and hemorrhagic papules, macules, and blisters on the lower leg. The hemorrhagic and blistering nature of some of these lesions in our patient may be attributable to thrombocytopenia and lymphedema in addition to LYG.
Histopathology and Differential
The skin biopsy from our patient demonstrated typical features of LYG, namely EBV-positive neoplastic large B cells in a background of predominating reactive T cells.18 The neoplastic large cells frequently invade blood vessels, leading to luminal narrowing without necrosis of the vessel walls. Grading is based on the density of EBV-positive large B cells: grade 1 is defined as fewer than 5 cells per HPF; grade 2, 5 to 50 cells per HPF; and grade 3, more than 50 cells per HPF.18 Grade 2 or 3 disease predicts worse outcome,2 as observed in our case. It is important for pathologists and clinicians to be aware that the proportion of EBV-positive large B cells is variable even within a single lesion; therefore, more than 1 biopsy may be necessary for appropriate grading and management.1,17 Additionally, skin biopsy may have a lower sensitivity for detecting EBV-positive B cells compared to lung biopsy, possibly due to sampling error in small biopsies.17
The histopathologic features of LYG frequently overlap with other lymphomas. Due to the abundance of T cells, LYG may be misclassified as T-cell/histiocyte-rich large B-cell lymphoma.19 Because the latter is not associated with EBV, EBER in situ hybridization is helpful in distinguishing the 2 conditions. On the other hand, EBER in situ hybridization has no value in discriminating LYG and extranodal natural killer (NK)/T-cell lymphoma, as both are EBV driven. Unlike LYG, the neoplastic EBV-positive cells in extranodal NK/T-cell lymphoma make up the majority of the infiltrate and exhibit an NK-cell immunophenotype (positive CD56 and cytoplasmic CD3 epsilon).20 Pulmonary involvement also is uncommon in NK/T-cell lymphoma.
Aside from lymphomas, LYG also resembles granulomatosis with polyangiitis (GPA)(formerly known as Wegener granulomatosis). Clinically, both LYG and GPA can present with constitutional symptoms, as well as lung, kidney, and skin lesions. The 2 conditions differ microscopically, with leukocytoclastic vasculitis and necrotizing granulomatous inflammation being characteristic of GPA but absent in LYG.1,21 Neutrophils and eosinophils are much more likely to be present in GPA.22,23
Disease Progression
Although LYG is an extranodal disease, there is a 7% to 45% risk of progression to nodal lymphoma in patients with high-grade disease.2,22,24 Our patient progressed to nodal large B-cell lymphoma shortly after the diagnosis of high-grade LYG. She developed additional lesions in the liver, spleen, and kidneys, and ultimately succumbed to the disease. Prior studies have shown higher mortality in patients with bilateral lung involvement and neurologic abnormalities, whereas cutaneous involvement does not affect outcome.2
Treatment
A prospective study used an initial treatment regimen of cyclophosphamide and prednisone but mortality was high.24 More recently, chemotherapy regimens including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), CVP or CHOP combined with rituximab, C-MOPP (cyclophosphamide, vincristine, prednisone, and procarbazine), EPOCH, and rituximab with high-dose cytarabine have been used with variable success for grades 2 and 3 LYG.17,23,25,26 Antiviral and immunomodulatory (interferon alfa) therapy has been used to induce remission in a majority of patients with grades 1 or 2 LYG.3,17,27,28 There is a report of successful treatment of relapsed LYG with the retinoid agent bexarotene.29 Autologous or allogeneic stem cell transplantation was effective for some patients with refractory or relapsed LYG.30 Further studies are needed to clarify optimal treatment of LYG, especially high-grade disease.
Conclusion
We report a rare case of LYG in a patient with antisynthetase syndrome, which highlights the critical role of skin biopsy in establishing the diagnosis of LYG when the clinical and radiologic presentations are obscured by other comorbidities. Dermatologists should be familiar with this rare disease and maintain a low threshold for biopsy in immunocompromised patients presenting with nodular lung infiltrates and/or nonspecific skin lesions.
Lymphomatoid granulomatosis (LYG) is a rare Epstein-Barr virus (EBV)–related extranodal angiocentric lymphoproliferative disorder. Most patients are adults in the fifth decade of life, and men are twice as likely as women to be affected.1 The most common site of involvement is the lungs, which has been observed in more than 90% of patients.2 The skin is the most common extrapulmonary site of involvement with variable manifestations including “rash,” subcutaneous nodules, and ulceration. Although a small subset of patients experience remission without treatment, most patients report a progressive course with median survival of less than 2 years.1,2 Clinical diagnosis often is challenging due to underrecognition of this rare condition by multidisciplinary physicians.
Case Report
A 60-year-old woman presented with fatigue, night sweats, poor appetite, unintentional weight loss, and dyspnea with minor exertion of 2 weeks’ duration. Her medical history was remarkable for antisynthetase syndrome manifested as polymyositis and interstitial lung disease, as well as recurrent breast cancer treated with wide excision, chemotherapy, and radiation therapy completed 2 months prior. Antisynthetase syndrome was controlled with azathioprine for 2 years, which was stopped during chemotherapy but restarted to treat worsened myalgia 4 months prior to presentation. Two weeks prior to hospital admission, she was treated with antibiotics at an outside hospital for presumed pneumonia without improvement. Upon admission to our hospital she was pancytopenic. Chest computed tomography showed interval development of extensive patchy ground-glass opacities in all lung lobes with areas of confluent consolidation. Broad infectious workup was negative. Given the time course of presentation and anterior accentuation of the lung infiltrates, the greatest clinical concern was radiation pneumonitis followed by drug toxicity. A bone marrow biopsy was hypocellular but without evidence of malignancy. Her pancytopenia was thought to be induced by azathioprine and/or antibiotics. Antibiotics were discontinued and prednisone was started for treatment of presumed radiation pneumonitis.
A few days later, the patient developed new skin lesions and worsening bilateral leg edema. There were multiple small erythematous and hemorrhagic papules, macules, and blisters on the medial aspect of the right lower leg and ankle, each measuring less than 1 cm in diameter (Figure 1). The clinical differential diagnosis included vasculitis related to an underlying collagen vascular disease, atypical edema blisters, and drug hypersensitivity reaction. A punch biopsy of one of the lesions showed a moderately dense superficial and deep perivascular lymphoid infiltrate with marked papillary dermal edema and early subepidermal split (Figure 2). The infiltrate was comprised of small- to medium-sized lymphocytes admixed with large cells, histiocytes, and plasma cells (Figure 3). Immunohistochemistry revealed a predominance of CD3+ and CD4+ small- to medium-sized T cells. CD20 highlighted the large angiocentric B cells (Figure 4), which also were positive on EBV-encoded small RNA (EBER) in situ hybridization (Figure 5). A diagnosis of LYG was rendered. Approximately 40 to 50 EBV-positive large B cells were present per high-power field (HPF), consistent with grade 2 disease.





Soon after diagnosis, follow-up computed tomography of the chest, abdomen, and pelvis revealed suspicious lesions in the kidneys, liver, spleen, and inguinal and iliac lymph nodes. The ground-glass opacities in the lungs continued to progress, with 2 additional nodules noted in the right upper and lower lobes. Four days later, core needle biopsies of the right inguinal lymph node showed a large B-cell lymphoma with extensive necrosis (Figure 6). EBER in situ hybridization was suboptimal, probably due to extensive necrosis.

She was started on etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH) for 5 days before developing Klebsiella pneumoniae sepsis and acute kidney injury. She was transferred to the critical care unit due to increasing oxygen requirement. Despite medical interventions, she continued to decompensate and elected to transition to palliative care. She died 6 weeks after the initial presentation. Her family did not request an autopsy.
Comment
Lymphomatoid granulomatosis is a rare lymphoproliferative disorder associated with various immunocompromised states including primary immunodeficiency disorders, human immunodeficiency virus infection, and immunosuppression for organ transplantation and autoimmune diseases. Our patient was receiving azathioprine for antisynthetase syndrome, which put her at risk for EBV infection and LYG. Azathioprine rarely has been reported as a possible culprit of LYG,3,4 but there are no known reported cases that were related to antisynthetase syndrome. There are multiple reports of development of LYG in patients receiving methotrexate for rheumatoid arthritis.5-10 Other iatrogenic causes reported in the literature include thiopurines11,12 and imatinib.13,14
The clinical diagnosis of our patient was particularly challenging given her complicated medical history including interstitial lung disease, predisposition to infection secondary to immunosuppression, and recent radiation therapy to the chest. This case illustrates the importance of maintaining a high index of suspicion for LYG in immunosuppressed patients presenting with lung infiltrates.
Presentation
Radiologically, LYG typically manifests as nodular densities accentuated in the lower lung lobes, which may become confluent.15 Because the nodular pattern in LYG is nonspecific and may mimic sarcoidosis, hypersensitivity pneumonitis, vasculitis, and infectious and neoplastic diseases,16 open lung biopsy often is required to establish the diagnosis in the absence of more accessible lesions.
Cutaneous lesions are seen in 40% to 50% of patients2 and may be the presenting sign of LYG. In a retrospective study, 16% (3/19) of LYG patients presented with cutaneous lesions months before diagnostic pulmonary lesions were identified.17 The skin is the most accessible site for biopsy, allowing definitive tissue diagnosis even when the condition is not clinically suspected. Therefore, dermatologists and dermatopathologists should be aware of this rare entity.
The clinical morphologies of the skin lesions are nonspecific, ranging from erythematous papules and subcutaneous nodules to indurated plaques. Ulceration may be present. The lesions may be widely disseminated or limited to the arms and legs. Our patient presented with erythematous and hemorrhagic papules, macules, and blisters on the lower leg. The hemorrhagic and blistering nature of some of these lesions in our patient may be attributable to thrombocytopenia and lymphedema in addition to LYG.
Histopathology and Differential
The skin biopsy from our patient demonstrated typical features of LYG, namely EBV-positive neoplastic large B cells in a background of predominating reactive T cells.18 The neoplastic large cells frequently invade blood vessels, leading to luminal narrowing without necrosis of the vessel walls. Grading is based on the density of EBV-positive large B cells: grade 1 is defined as fewer than 5 cells per HPF; grade 2, 5 to 50 cells per HPF; and grade 3, more than 50 cells per HPF.18 Grade 2 or 3 disease predicts worse outcome,2 as observed in our case. It is important for pathologists and clinicians to be aware that the proportion of EBV-positive large B cells is variable even within a single lesion; therefore, more than 1 biopsy may be necessary for appropriate grading and management.1,17 Additionally, skin biopsy may have a lower sensitivity for detecting EBV-positive B cells compared to lung biopsy, possibly due to sampling error in small biopsies.17
The histopathologic features of LYG frequently overlap with other lymphomas. Due to the abundance of T cells, LYG may be misclassified as T-cell/histiocyte-rich large B-cell lymphoma.19 Because the latter is not associated with EBV, EBER in situ hybridization is helpful in distinguishing the 2 conditions. On the other hand, EBER in situ hybridization has no value in discriminating LYG and extranodal natural killer (NK)/T-cell lymphoma, as both are EBV driven. Unlike LYG, the neoplastic EBV-positive cells in extranodal NK/T-cell lymphoma make up the majority of the infiltrate and exhibit an NK-cell immunophenotype (positive CD56 and cytoplasmic CD3 epsilon).20 Pulmonary involvement also is uncommon in NK/T-cell lymphoma.
Aside from lymphomas, LYG also resembles granulomatosis with polyangiitis (GPA)(formerly known as Wegener granulomatosis). Clinically, both LYG and GPA can present with constitutional symptoms, as well as lung, kidney, and skin lesions. The 2 conditions differ microscopically, with leukocytoclastic vasculitis and necrotizing granulomatous inflammation being characteristic of GPA but absent in LYG.1,21 Neutrophils and eosinophils are much more likely to be present in GPA.22,23
Disease Progression
Although LYG is an extranodal disease, there is a 7% to 45% risk of progression to nodal lymphoma in patients with high-grade disease.2,22,24 Our patient progressed to nodal large B-cell lymphoma shortly after the diagnosis of high-grade LYG. She developed additional lesions in the liver, spleen, and kidneys, and ultimately succumbed to the disease. Prior studies have shown higher mortality in patients with bilateral lung involvement and neurologic abnormalities, whereas cutaneous involvement does not affect outcome.2
Treatment
A prospective study used an initial treatment regimen of cyclophosphamide and prednisone but mortality was high.24 More recently, chemotherapy regimens including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CVP (cyclophosphamide, vincristine, and prednisone), CVP or CHOP combined with rituximab, C-MOPP (cyclophosphamide, vincristine, prednisone, and procarbazine), EPOCH, and rituximab with high-dose cytarabine have been used with variable success for grades 2 and 3 LYG.17,23,25,26 Antiviral and immunomodulatory (interferon alfa) therapy has been used to induce remission in a majority of patients with grades 1 or 2 LYG.3,17,27,28 There is a report of successful treatment of relapsed LYG with the retinoid agent bexarotene.29 Autologous or allogeneic stem cell transplantation was effective for some patients with refractory or relapsed LYG.30 Further studies are needed to clarify optimal treatment of LYG, especially high-grade disease.
Conclusion
We report a rare case of LYG in a patient with antisynthetase syndrome, which highlights the critical role of skin biopsy in establishing the diagnosis of LYG when the clinical and radiologic presentations are obscured by other comorbidities. Dermatologists should be familiar with this rare disease and maintain a low threshold for biopsy in immunocompromised patients presenting with nodular lung infiltrates and/or nonspecific skin lesions.
- Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010;34:E35-E48.
- Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43:360-373.
- Connors W, Griffiths C, Patel J, et al. Lymphomatoid granulomatosis associated with azathioprine therapy in Crohn disease. BMC Gastroenterol. 2014;14:127.
- Katherine Martin L, Porcu P, Baiocchi RA, et al. Primary central nervous system lymphomatoid granulomatosis in a patient receiving azathioprine therapy. Clin Adv Hematol Oncol. 2009;7:65-68.
- Barakat A, Grover K, Peshin R. Rituximab for pulmonary lymphomatoid granulomatosis which developed as a complication of methotrexate and azathioprine therapy for rheumatoid arthritis. Springerplus. 2014;3:751.
- Kobayashi S, Kikuchi Y, Sato K, et al. Reversible iatrogenic, MTX-associated EBV-driven lymphoproliferation with histopathological features of a lymphomatoid granulomatosis in a patient with rheumatoid arthritis. Ann Hematol. 2013;92:1561-1564.
- Kameda H, Okuyama A, Tamaru J, et al. Lymphomatoid granulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26:1585-1589.
- Oiwa H, Mihara K, Kan T, et al. Grade 3 lymphomatoid granulomatosis in a patient receiving methotrexate therapy for rheumatoid arthritis. Intern Med. 2014;53:1873-1875.
- Blanchart K, Paciencia M, Seguin A, et al. Fatal pulmonary lymphomatoid granulomatosis in a patient taking methotrexate for rheumatoid arthritis. Minerva Anestesiol. 2014;80:119-120.
- Schalk E, Krogel C, Scheinpflug K, et al. Lymphomatoid granulomatosis in a patient with rheumatoid arthritis receiving methotrexate: successful treatment with the anti-CD20 antibody mabthera. Onkologie. 2009;32:440-441.
- Subramaniam K, Cherian M, Jain S, et al. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J. 2013;43:1339-1342.
- Destombe S, Bouron-DalSoglio D, Rougemont AL, et al. Lymphomatoid granulomatosis: a unique complication of Crohn disease and its treatment in pediatrics. J Pediatr Gastroenterol Nutr. 2010;50:559-561.
- Yazdi AS, Metzler G, Weyrauch S, et al. Lymphomatoid granulomatosis induced by imatinib treatment. Arch Dermatol. 2007;143:1222-1223.
- Salmons N, Gregg RJ, Pallalau A, et al. Lymphomatoid granulomatosis in a patient previously diagnosed with a gastrointestinal stromal tumour and treated with imatinib. J Clin Pathol. 2007;60:199-201.
- Dee PM, Arora NS, Innes DJ Jr. The pulmonary manifestations of lymphomatoid granulomatosis. Radiology. 1982;143:613-618.
- Rezai P, Hart EM, Patel SK. Case 169: lymphomatoid granulomatosis. Radiology. 2011;259:604-609.
- Beaty MW, Toro J, Sorbara L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25:1111-1120.
- Pittaluga S, Wilson WH, Jaffe E. Lymphomatoid granulomatosis. In: Swerdlow S, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008:247-249.
- Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392.
- Jaffe E. Nasal and nasal-type T/NK cell lymphoma: a unique form of lymphoma associated with the Epstein-Barr virus. Histopathology. 1995;27:581-583.
- Barksdale SK, Hallahan CW, Kerr GS, et al. Cutaneous pathology in Wegener’s granulomatosis: a clinicopathologic study of 74 biopsies in 46 patients. Am J Surg Pathol. 1995;19:161-172.
- Koss MN, Hochholzer L, Langloss JM, et al. Lymphomatoid granulomatosis: a clinicopathologic study of 42 patients. Pathology. 1986;18:283-288.
- Aoki T, Harada Y, Matsubara E, et al. Long-term remission after multiple relapses in an elderly patient with lymphomatoid granulomatosis after rituximab and high-dose cytarabine chemotherapy without stem-cell transplantation. J Clin Oncol. 2013;31:E390-E393.
- Fauci AS, Haynes BF, Costa J, et al. Lymphomatoid granulomatosis: prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982;306:68-74.
- Jung KH, Sung HJ, Lee JH, et al. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009;55:386-390.
- Hernandez-Marques C, Lassaletta A, Torrelo A, et al. Rituximab in lymphomatoid granulomatosis. J Pediatr Hematol Oncol. 2014;36:E69-E74.
- Wilson WH, Gutierrez M, Raffeld M, et al. Lymphomatoid granulomatosis: phase 2 study of dose-adjusted interferon-alfa or EPOCH chemotherapy. Blood. 1999;94:599A.
- Wilson WH, Kingma DW, Raffeld M, et al. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996;87:4531-4537.
- Berg SE, Downs LH, Torigian DA, et al. Successful treatment of relapsed lymphomatoid granulomatosis with bexarotene. Cancer Biol Ther. 2008;7:1544-1546.
- Siegloch K, Schmitz N, Wu HS, et al. Hematopoietic stem cell transplantation in patients with lymphomatoid granulomatosis: a European group for blood and marrow transplantation report. Biol Blood Marrow Transplant. 2013;19:1522-1525.
- Katzenstein AL, Doxtader E, Narendra S. Lymphomatoid granulomatosis: insights gained over 4 decades. Am J Surg Pathol. 2010;34:E35-E48.
- Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43:360-373.
- Connors W, Griffiths C, Patel J, et al. Lymphomatoid granulomatosis associated with azathioprine therapy in Crohn disease. BMC Gastroenterol. 2014;14:127.
- Katherine Martin L, Porcu P, Baiocchi RA, et al. Primary central nervous system lymphomatoid granulomatosis in a patient receiving azathioprine therapy. Clin Adv Hematol Oncol. 2009;7:65-68.
- Barakat A, Grover K, Peshin R. Rituximab for pulmonary lymphomatoid granulomatosis which developed as a complication of methotrexate and azathioprine therapy for rheumatoid arthritis. Springerplus. 2014;3:751.
- Kobayashi S, Kikuchi Y, Sato K, et al. Reversible iatrogenic, MTX-associated EBV-driven lymphoproliferation with histopathological features of a lymphomatoid granulomatosis in a patient with rheumatoid arthritis. Ann Hematol. 2013;92:1561-1564.
- Kameda H, Okuyama A, Tamaru J, et al. Lymphomatoid granulomatosis and diffuse alveolar damage associated with methotrexate therapy in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26:1585-1589.
- Oiwa H, Mihara K, Kan T, et al. Grade 3 lymphomatoid granulomatosis in a patient receiving methotrexate therapy for rheumatoid arthritis. Intern Med. 2014;53:1873-1875.
- Blanchart K, Paciencia M, Seguin A, et al. Fatal pulmonary lymphomatoid granulomatosis in a patient taking methotrexate for rheumatoid arthritis. Minerva Anestesiol. 2014;80:119-120.
- Schalk E, Krogel C, Scheinpflug K, et al. Lymphomatoid granulomatosis in a patient with rheumatoid arthritis receiving methotrexate: successful treatment with the anti-CD20 antibody mabthera. Onkologie. 2009;32:440-441.
- Subramaniam K, Cherian M, Jain S, et al. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J. 2013;43:1339-1342.
- Destombe S, Bouron-DalSoglio D, Rougemont AL, et al. Lymphomatoid granulomatosis: a unique complication of Crohn disease and its treatment in pediatrics. J Pediatr Gastroenterol Nutr. 2010;50:559-561.
- Yazdi AS, Metzler G, Weyrauch S, et al. Lymphomatoid granulomatosis induced by imatinib treatment. Arch Dermatol. 2007;143:1222-1223.
- Salmons N, Gregg RJ, Pallalau A, et al. Lymphomatoid granulomatosis in a patient previously diagnosed with a gastrointestinal stromal tumour and treated with imatinib. J Clin Pathol. 2007;60:199-201.
- Dee PM, Arora NS, Innes DJ Jr. The pulmonary manifestations of lymphomatoid granulomatosis. Radiology. 1982;143:613-618.
- Rezai P, Hart EM, Patel SK. Case 169: lymphomatoid granulomatosis. Radiology. 2011;259:604-609.
- Beaty MW, Toro J, Sorbara L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25:1111-1120.
- Pittaluga S, Wilson WH, Jaffe E. Lymphomatoid granulomatosis. In: Swerdlow S, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008:247-249.
- Abramson JS. T-cell/histiocyte-rich B-cell lymphoma: biology, diagnosis, and management. Oncologist. 2006;11:384-392.
- Jaffe E. Nasal and nasal-type T/NK cell lymphoma: a unique form of lymphoma associated with the Epstein-Barr virus. Histopathology. 1995;27:581-583.
- Barksdale SK, Hallahan CW, Kerr GS, et al. Cutaneous pathology in Wegener’s granulomatosis: a clinicopathologic study of 74 biopsies in 46 patients. Am J Surg Pathol. 1995;19:161-172.
- Koss MN, Hochholzer L, Langloss JM, et al. Lymphomatoid granulomatosis: a clinicopathologic study of 42 patients. Pathology. 1986;18:283-288.
- Aoki T, Harada Y, Matsubara E, et al. Long-term remission after multiple relapses in an elderly patient with lymphomatoid granulomatosis after rituximab and high-dose cytarabine chemotherapy without stem-cell transplantation. J Clin Oncol. 2013;31:E390-E393.
- Fauci AS, Haynes BF, Costa J, et al. Lymphomatoid granulomatosis: prospective clinical and therapeutic experience over 10 years. N Engl J Med. 1982;306:68-74.
- Jung KH, Sung HJ, Lee JH, et al. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009;55:386-390.
- Hernandez-Marques C, Lassaletta A, Torrelo A, et al. Rituximab in lymphomatoid granulomatosis. J Pediatr Hematol Oncol. 2014;36:E69-E74.
- Wilson WH, Gutierrez M, Raffeld M, et al. Lymphomatoid granulomatosis: phase 2 study of dose-adjusted interferon-alfa or EPOCH chemotherapy. Blood. 1999;94:599A.
- Wilson WH, Kingma DW, Raffeld M, et al. Association of lymphomatoid granulomatosis with Epstein-Barr viral infection of B lymphocytes and response to interferon-alpha 2b. Blood. 1996;87:4531-4537.
- Berg SE, Downs LH, Torigian DA, et al. Successful treatment of relapsed lymphomatoid granulomatosis with bexarotene. Cancer Biol Ther. 2008;7:1544-1546.
- Siegloch K, Schmitz N, Wu HS, et al. Hematopoietic stem cell transplantation in patients with lymphomatoid granulomatosis: a European group for blood and marrow transplantation report. Biol Blood Marrow Transplant. 2013;19:1522-1525.
Practice Points
- Lymphomatoid granulomatosis (LYG) is a rare extranodal angiocentric large B-cell lymphoma driven by the Epstein-Barr virus.
- Lymphomatoid granulomatosis should be suspected when immunocompromised patients present with nodular lung infiltrates and/or nonspecific skin lesions.
- Skin biopsy serves a critical role in establishing the diagnosis of LYG, especially when clinical and radiologic findings are obscured by other comorbidities.
Vesiculobullous and Pustular Diseases in Newborns
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1
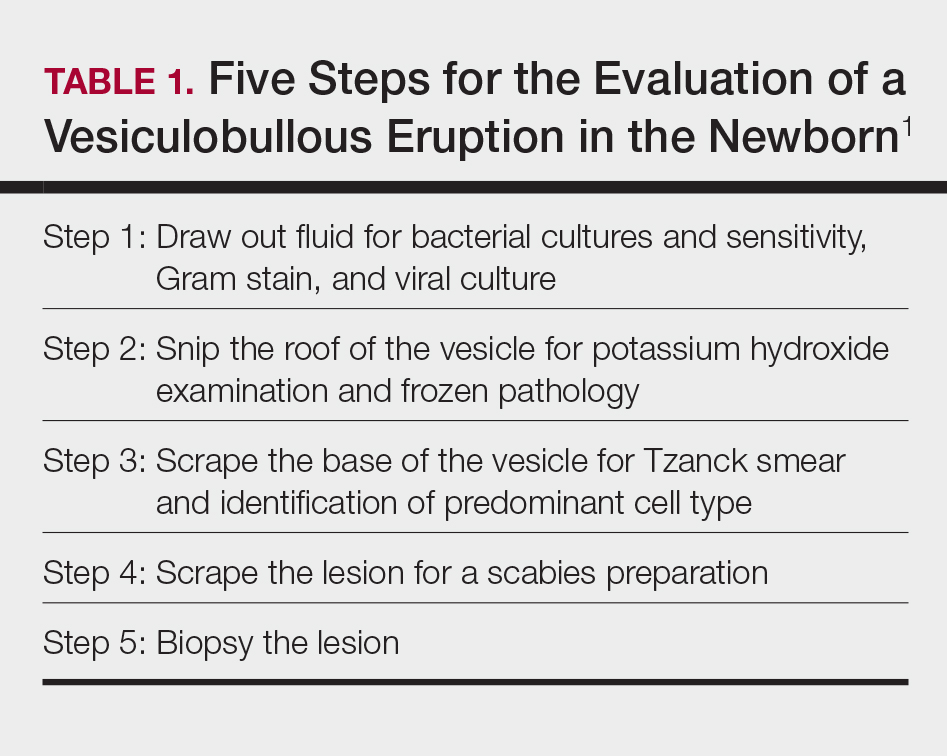
First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.
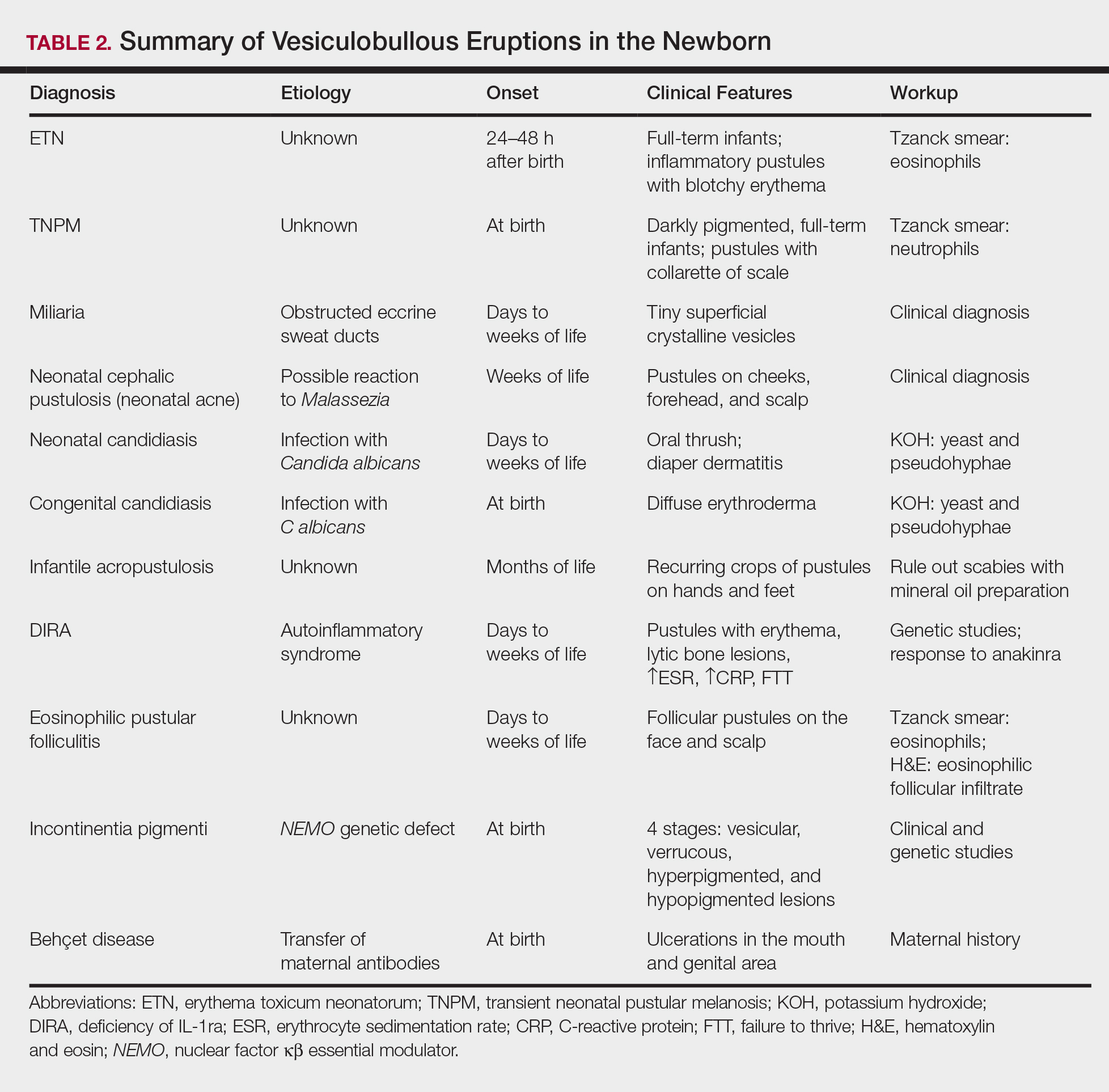
Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1

First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.

Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
Vesiculobullous eruptions in neonates can readily generate anxiety from parents/guardians and pediatricians over both infectious and noninfectious causes. The role of the dermatology resident is critical to help diminish fear over common vesicular presentations or to escalate care in rarer situations if a more obscure or ominous diagnosis is clouding the patient’s clinical presentation and well-being. This article summarizes both common and uncommon vesiculobullous neonatal diseases to augment precise and efficient diagnoses in this vulnerable patient population.
Steps for Evaluating a Vesiculopustular Eruption
Receiving a consultation for a newborn with widespread vesicles can be a daunting scenario for a dermatology resident. Fear of missing an ominous diagnosis or aggressively treating a newborn for an erroneous infection when the diagnosis is actually a benign presentation can lead to an anxiety-provoking situation. Additionally, performing a procedure on a newborn can cause personal uneasiness. Dr. Lawrence A. Schachner, an eminent pediatric dermatologist at the University of Miami Miller School of Medicine (Miami, Florida), recently lectured on 5 key steps (Table 1) for the evaluation of a vesiculobullous eruption in the newborn to maximize the accuracy of diagnosis and patient care.1

First, draw out the fluid from the vesicle to send for bacterial and viral culture as well as Gram stain. Second, snip the roof of the vesicle to perform potassium hydroxide examination for yeast or fungi and frozen pathology when indicated. Third, use the base of the vesicle to obtain cells for a Tzanck smear to identify the predominant cell infiltrate, such as multinucleated giant cells in herpes simplex virus or eosinophils in erythema toxicum neonatorum (ETN). Fourth, a mineral oil preparation can be performed on several lesions, especially if a burrow is observed, to rule out bullous scabies in the appropriate clinical presentation. Lastly, a perilesional or lesional punch biopsy can be performed if the above steps have not yet clinched the diagnosis.2 By utilizing these steps, the resident efficiently utilizes 1 lesion to narrow down a formidable differential list of bullous disorders in the newborn.
Specific Diagnoses
A number of common diagnoses can present during the newborn period and can usually be readily diagnosed by clinical manifestations alone; a summary of these eruptions is provided in Table 2. Erythema toxicum neonatorum is the most common pustular eruption in neonates and presents in up to 50% of full-term infants at days 1 to 2 of life. Inflammatory pustules surrounded by characteristic blotchy erythema are displayed on the face, trunk, arms, and legs, usually sparing the palms and soles.3 Erythema toxicum neonatorum typically is a clinical diagnosis; however, it can be confirmed by demonstrating the predominance of eosinophils on Tzanck smear.

Transient neonatal pustular melanosis (TNPM) also presents in full-term infants; usually favors darkly pigmented neonates; and exhibits either pustules with a collarette of scale that lack surrounding erythema or with residual brown macules on the face, genitals, and acral surfaces. Postinflammatory pigmentary alteration on lesion clearance is another clue to diagnosis. Similarly, it is a clinical diagnosis but can be confirmed with a Tzanck smear demonstrating neutrophils as the major cell infiltrate.
In a prospective 1-year multicenter study performed by Reginatto et al,4 2831 neonates born in southern Brazil underwent a skin examination by a dermatologist within 72 hours of birth to characterize the prevalence and demographics of ETN and TNPM. They found a 21.3% (602 cases) prevalence of ETN compared to a 3.4% (97 cases) prevalence of TNPM, but they noted that most patients were white, and thus the diagnosis of TNPM likely is less prevalent in this group, as it favors darkly pigmented individuals. Additional predisposing factors associated with ETN were male gender, an Apgar score of 8 to 10 at 1 minute, non–neonatal intensive care unit (NICU) patients, and lack of gestational risk factors. The TNPM population was much smaller, though the authors were able to conclude that the disease also was correlated with healthy, non-NICU patients. The authors hypothesized that there may be a role of immune system maturity in the pathogenesis of ETN and thus dermatology residents should be aware of the setting of their consultation.4 A NICU consultation for ETN should raise suspicion, as ETN and TNPM favor healthy infants who likely are not residing in the NICU; we are reminded of the target populations for these disease processes.
Additional common causes of vesicular eruptions in neonates can likewise be diagnosed chiefly with clinical inspection. Miliaria presents with tiny superficial crystalline vesicles on the neck and back of newborns due to elevated temperature and resultant obstruction of the eccrine sweat ducts. Reassurance can be provided, as spontaneous resolution occurs with cooling and limitation of occlusive clothing and swaddling.2
Infants at a few weeks of life may present with a noncomedonal pustular eruption on the cheeks, forehead, and scalp commonly known as neonatal acne or neonatal cephalic pustulosis. The driving factor is thought to be an abnormal response to Malassezia and can be treated with ketoconazole cream or expectant management.2
Cutaneous candidiasis is the most common infectious cause of vesicles in the neonate and can present in 2 fashions. Neonatal candidiasis is common, presenting a week after birth and manifesting as oral thrush and red plaques with satellite pustules in the diaper area. Congenital candidiasis is due to infection in utero, presents prior to 1 week of life, exhibits diffuse erythroderma, and requires timely parenteral antifungals.5 Newborns and preterm infants are at higher risk for systemic disease, while full-term infants may experience a mild course of skin-limited lesions.
It is imperative to rule out other infectious etiologies in ill-appearing neonates with vesicles such as herpes simplex virus, bacterial infections, syphilis, and vertically transmitted TORCH (toxoplasmosis, other infections rubella, cytomegalovirus infection, and herpes simplex) diagnoses.6 Herpes simplex virus classically presents with grouped vesicles on an erythematous base; however, such characteristic lesions may be subtle in the newborn. The site of skin involvement usually is the area that first comes into contact with maternal lesions, such as the face for a newborn delivered in a cephalic presentation.2 It is critical to be cognizant of this diagnosis, as a delay in antiviral therapy can result in neurologic consequences due to disseminated disease.
If the clinical picture of vesiculobullous disease in the newborn is not as clear, less common causes must be considered. Infantile acropustulosis presents with recurring crops of pustules on the hands and feet at several months of age. The most common differential diagnosis is scabies; therefore, a mineral oil preparation should be performed to rule out this common mimicker. Potent topical corticosteroids are first-line therapy, and episodes generally resolve with time.
Another mimicker of pustules in neonates includes deficiency of IL-1ra, a rare entity described in 2009.7 Deficiency of IL-1ra is an autoinflammatory syndrome of skin and bone due to unopposed action of IL-1 with life-threatening inflammation; infants present with pustules, lytic bone lesions, elevated erythrocyte sedimentation rate and C-reactive protein, and failure to thrive.8 The characteristic mutation was discovered when the infants dramatically responded to therapy with anakinra, an IL-1ra.
Eosinophilic pustular folliculitis is an additional pustular dermatosis that manifests with lesions predominately in the head and neck area, and unlike the adult population, it usually is self-resolving and not associated with other comorbidities in newborns.2
Incontinentia pigmenti is an X-linked dominant syndrome due to a genetic mutation in NEMO, nuclear factor κβ essential modulator, which protects against apoptosis.3 Incontinentia pigmenti presents in newborn girls shortly after birth with vesicles in a blaschkoid distribution before evolving through 4 unique stages of vesicular lesions, verrucous lesions, hyperpigmentation, and ultimately resolves with residual hypopigmentation in the affected area.
Lastly, neonatal Behçet disease can present with vesicles in the mouth and genital region due to transfer of maternal antibodies. It is self-limiting in nature and would be readily diagnosed with a known maternal history, though judicious screening for infections may be needed in specific settings.2
Conclusion
In summary, a vast array of benign and worrisome dermatoses present in the neonatal period. A thorough history and physical examination, including the temporality of the lesions, the health status of the newborn, and the maternal history, can help delineate the diagnosis. The 5-step method presented can further elucidate the underlying mechanism and reduce an overwhelming differential diagnosis list by reviewing each finding yielded from each step. Dermatology residents should feel comfortable addressing this unique patient population to ameliorate unclear cutaneous diagnoses for pediatricians.
Acknowledgment
A special thank you to Lawrence A. Schachner, MD (Miami, Florida), for his help providing resources and guidance for this topic.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
- Schachner L. Vesiculopustular dermatosis in neonates and infants. Lecture presented at: University of Miami Department of Dermatology & Cutaneous Surgery Grand Rounds; August 23, 2017; Miami, Florida.
- Eichenfield LF, Lee PW, Larraide M, et al. Neonatal skin and skin disorders. In: Schachner LA, Hansen RC, eds. Pediatric Dermatology. 4th ed. Philadelphia, PA: Elsevier Mosby; 2011:299-373.
- Goddard DS, Gilliam AE, Frieden IJ. Vesiculobullous and erosive diseases in the newborn. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:523-537.
- Reginatto FP, Muller FM, Peruzzo J, et al. Epidemiology and predisposing factors for erythema toxicum neonatorum and transient neonatal pustular melanosis: a multicenter study [published online May 25, 2017]. Pediatr Dermatol. 2017;34:422-426.
- Aruna C, Seetharam K. Congenital candidiasis. Indian Dermatol Online J. 2014;5(suppl 1):S44-S47.
- O’Connor NR, McLaughlin MR, Ham P. Newborn skin: part I. common rashes. Am Fam Physician. 2008;77:47-52.
- Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360:2438-2444.
- Minkis K, Aksentijevich I, Goldbach-Mansky R, et al. Interleukin 1 receptor antagonist deficiency presenting as infantile pustulosis mimicking infantile pustular psoriasis. Arch Dermatol. 2012;148:747-752.
The Exam of the Future: What Should Residents Expect?
Sunburn Purpura
To the Editor:
Chronic UV exposure has been linked to increased skin fragility and the development of purpuric lesions, a benign condition known as actinic purpura and commonly seen in elderly patients. Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
A 19-year-old woman presented with red and purple spots on the pretibial region of both legs extending to the thigh. One week prior to presentation she had a severe sunburn affecting most of the body, which resolved without blistering. Two days later, the spots appeared within the most severely sunburned areas of both legs. The patient reported that the lesions were mildly painful to palpation, but she was more concerned about the appearance. She denied any history of similar skin changes associated with sun exposure. The patient was otherwise healthy and denied any recent illnesses. She noted a history of mild bruising and bleeding with a resulting unremarkable workup by her primary care physician. The only medication taken was etonogestrel-ethinyl estradiol vaginal ring.
The scalp, face, arms, trunk, and legs were examined, and nonpalpable petechial changes were noted on the anterior aspect of the legs (Figure 1), with changes more prominent on the distal aspect of the legs. Mild superficial epidermal exfoliation was noted on both anterior thighs. The area of the lesions was not warm. The lesions were mildly tender to palpation. The remainder of the physical examination was unremarkable.
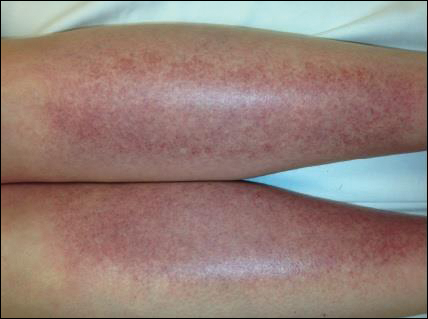
Given the timing of onset, preceding sun exposure, and the morphologic characteristics of the lesions, sunburn purpura was suspected. A punch biopsy of the anterior aspect of the left thigh was performed to rule out vasculitis. Microscopic examination revealed reactive epidermal changes with mild vascular ectasia and erythrocyte extravasation not associated with appreciable inflammation or evidence of vascular injury (Figure 2). Biopsy exposure to fluorescein-labeled antibodies directed against IgG, IgM, IgA, C3, and polyvalent immunoglobulins (IgG, IgM, and IgA) yielded no immunofluorescence. These biopsy results were consistent with sunburn purpura. Given the patient's normal platelet count, a diagnosis of idiopathic sunburn purpura was made. The patient was informed of the biopsy results and advised that the petechiae should resolve without treatment in 1 to 2 weeks, which occurred.
Sunburn purpura remains a rare phenomenon in which a petechial or purpuric rash develops acutely after intense sun exposure. We prefer the term sunburn purpura because it reflects the acuity of the phenomenon, as opposed to the previous labels solar purpura or photolocalized purpura, which also could suggest causality from chronic sun exposure. It has been proposed that sunburn purpura is a finding associated with a number of conditions rather than a unique entity.1 The following characteristics can be helpful in describing the development of sunburn purpura: delay following UV exposure, gross morphology, histologic findings, and possible associated medical conditions.1 Our case represents an important addition to the literature, as it differs from previously reported cases. Most importantly, the nonspecific biopsy findings and unremarkable laboratory findings associated with our case may represent primary or idiopathic sunburn purpura.
Previously reported cases of sunburn purpura have occurred in patients aged 10 to 66 years. It has been seen following UV exposure, vigorous exercise and high-dose aspirin, or concurrent fluoroquinolone therapy, or in the setting of erythropoietic protoporphyria, idiopathic thrombocytopenic purpura, or polymorphous light eruption.2-8 When performed, histology has revealed capillaritis, solar elastosis, perivascular infiltrate, lymphocytic perivascular infiltrate with dermal edema, or leukocytoclastic vasculitis.1,2,7-9 Our patient did not have a history of erythropoietic protoporphyria, polymorphous light eruption, or idiopathic thrombocytopenic purpura. She had not recently exercised, was not thrombocytopenic, and was not taking antiplatelet medications. She had no recent history of fluoroquinolone use. On histologic examination, our patient's biopsy demonstrated nonspecific petechial changes without signs of chronic UV exposure, dermal edema, vasculitis, lymphocytic infiltrate, or capillaritis.
Idiopathic sunburn purpura should only be diagnosed after other conditions are excluded. When evaluating a patient who presents with new-onset petechial rash following sun exposure, it is important to rule out vasculitis or thrombocytopenia as the cause, which is best achieved through skin biopsy and a platelet count, respectively. If there are no associated symptoms or thrombocytopenia and biopsy shows nonspecific vascular ectasia and erythrocyte extravasation, the physician should consider the diagnosis of idiopathic sunburn (solar or photolocalized) purpura. Along with regular UV protection, the physician should advise that the rash typically resolves without treatment in 1 to 2 weeks.
- Waters AJ, Sandhu DR, Green CM, et al. Solar capillaritis as a cause of solar purpura. Clin Exp Dermatol. 2009;34:E821-E824.
- Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
- Rubegni P, Feci L, Pellegrino M, et al. Photolocalized purpura during levofloxacin therapy. Photodermatol Photoimmunol Photomed. 2012;28:105-107.
- Urbina F, Barrios M, Sudy E. Photolocalized purpura during ciprofloxacin therapy. Photodermatol Photoimmunol Photomed. 2006;22:111-112.
- Torinuki W, Miura T. Erythropoietic protoporphyria showing solar purpura. Dermatologica. 1983;167:220-222.
- Leung AK. Purpura associated with exposure to sunlight. J R Soc Med. 1986;79:423-424.
- Kalivas J, Kalivas L. Solar purpura appearing in a patient with polymorphous light eruption. Photodermatol Photoimmunol Photomed. 1995;11:31-32.
- Ros AM. Solar purpura--an unusual manifestation of polymorphous light eruption. Photodermatol. 1988;5:47-48.
- Guarrera M, Parodi A, Rebora A. Solar purpura is not related to polymorphous light eruption. Photodermatol. 1989;6:293-294.
To the Editor:
Chronic UV exposure has been linked to increased skin fragility and the development of purpuric lesions, a benign condition known as actinic purpura and commonly seen in elderly patients. Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
A 19-year-old woman presented with red and purple spots on the pretibial region of both legs extending to the thigh. One week prior to presentation she had a severe sunburn affecting most of the body, which resolved without blistering. Two days later, the spots appeared within the most severely sunburned areas of both legs. The patient reported that the lesions were mildly painful to palpation, but she was more concerned about the appearance. She denied any history of similar skin changes associated with sun exposure. The patient was otherwise healthy and denied any recent illnesses. She noted a history of mild bruising and bleeding with a resulting unremarkable workup by her primary care physician. The only medication taken was etonogestrel-ethinyl estradiol vaginal ring.
The scalp, face, arms, trunk, and legs were examined, and nonpalpable petechial changes were noted on the anterior aspect of the legs (Figure 1), with changes more prominent on the distal aspect of the legs. Mild superficial epidermal exfoliation was noted on both anterior thighs. The area of the lesions was not warm. The lesions were mildly tender to palpation. The remainder of the physical examination was unremarkable.

Given the timing of onset, preceding sun exposure, and the morphologic characteristics of the lesions, sunburn purpura was suspected. A punch biopsy of the anterior aspect of the left thigh was performed to rule out vasculitis. Microscopic examination revealed reactive epidermal changes with mild vascular ectasia and erythrocyte extravasation not associated with appreciable inflammation or evidence of vascular injury (Figure 2). Biopsy exposure to fluorescein-labeled antibodies directed against IgG, IgM, IgA, C3, and polyvalent immunoglobulins (IgG, IgM, and IgA) yielded no immunofluorescence. These biopsy results were consistent with sunburn purpura. Given the patient's normal platelet count, a diagnosis of idiopathic sunburn purpura was made. The patient was informed of the biopsy results and advised that the petechiae should resolve without treatment in 1 to 2 weeks, which occurred.

Sunburn purpura remains a rare phenomenon in which a petechial or purpuric rash develops acutely after intense sun exposure. We prefer the term sunburn purpura because it reflects the acuity of the phenomenon, as opposed to the previous labels solar purpura or photolocalized purpura, which also could suggest causality from chronic sun exposure. It has been proposed that sunburn purpura is a finding associated with a number of conditions rather than a unique entity.1 The following characteristics can be helpful in describing the development of sunburn purpura: delay following UV exposure, gross morphology, histologic findings, and possible associated medical conditions.1 Our case represents an important addition to the literature, as it differs from previously reported cases. Most importantly, the nonspecific biopsy findings and unremarkable laboratory findings associated with our case may represent primary or idiopathic sunburn purpura.
Previously reported cases of sunburn purpura have occurred in patients aged 10 to 66 years. It has been seen following UV exposure, vigorous exercise and high-dose aspirin, or concurrent fluoroquinolone therapy, or in the setting of erythropoietic protoporphyria, idiopathic thrombocytopenic purpura, or polymorphous light eruption.2-8 When performed, histology has revealed capillaritis, solar elastosis, perivascular infiltrate, lymphocytic perivascular infiltrate with dermal edema, or leukocytoclastic vasculitis.1,2,7-9 Our patient did not have a history of erythropoietic protoporphyria, polymorphous light eruption, or idiopathic thrombocytopenic purpura. She had not recently exercised, was not thrombocytopenic, and was not taking antiplatelet medications. She had no recent history of fluoroquinolone use. On histologic examination, our patient's biopsy demonstrated nonspecific petechial changes without signs of chronic UV exposure, dermal edema, vasculitis, lymphocytic infiltrate, or capillaritis.
Idiopathic sunburn purpura should only be diagnosed after other conditions are excluded. When evaluating a patient who presents with new-onset petechial rash following sun exposure, it is important to rule out vasculitis or thrombocytopenia as the cause, which is best achieved through skin biopsy and a platelet count, respectively. If there are no associated symptoms or thrombocytopenia and biopsy shows nonspecific vascular ectasia and erythrocyte extravasation, the physician should consider the diagnosis of idiopathic sunburn (solar or photolocalized) purpura. Along with regular UV protection, the physician should advise that the rash typically resolves without treatment in 1 to 2 weeks.
To the Editor:
Chronic UV exposure has been linked to increased skin fragility and the development of purpuric lesions, a benign condition known as actinic purpura and commonly seen in elderly patients. Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
A 19-year-old woman presented with red and purple spots on the pretibial region of both legs extending to the thigh. One week prior to presentation she had a severe sunburn affecting most of the body, which resolved without blistering. Two days later, the spots appeared within the most severely sunburned areas of both legs. The patient reported that the lesions were mildly painful to palpation, but she was more concerned about the appearance. She denied any history of similar skin changes associated with sun exposure. The patient was otherwise healthy and denied any recent illnesses. She noted a history of mild bruising and bleeding with a resulting unremarkable workup by her primary care physician. The only medication taken was etonogestrel-ethinyl estradiol vaginal ring.
The scalp, face, arms, trunk, and legs were examined, and nonpalpable petechial changes were noted on the anterior aspect of the legs (Figure 1), with changes more prominent on the distal aspect of the legs. Mild superficial epidermal exfoliation was noted on both anterior thighs. The area of the lesions was not warm. The lesions were mildly tender to palpation. The remainder of the physical examination was unremarkable.

Given the timing of onset, preceding sun exposure, and the morphologic characteristics of the lesions, sunburn purpura was suspected. A punch biopsy of the anterior aspect of the left thigh was performed to rule out vasculitis. Microscopic examination revealed reactive epidermal changes with mild vascular ectasia and erythrocyte extravasation not associated with appreciable inflammation or evidence of vascular injury (Figure 2). Biopsy exposure to fluorescein-labeled antibodies directed against IgG, IgM, IgA, C3, and polyvalent immunoglobulins (IgG, IgM, and IgA) yielded no immunofluorescence. These biopsy results were consistent with sunburn purpura. Given the patient's normal platelet count, a diagnosis of idiopathic sunburn purpura was made. The patient was informed of the biopsy results and advised that the petechiae should resolve without treatment in 1 to 2 weeks, which occurred.

Sunburn purpura remains a rare phenomenon in which a petechial or purpuric rash develops acutely after intense sun exposure. We prefer the term sunburn purpura because it reflects the acuity of the phenomenon, as opposed to the previous labels solar purpura or photolocalized purpura, which also could suggest causality from chronic sun exposure. It has been proposed that sunburn purpura is a finding associated with a number of conditions rather than a unique entity.1 The following characteristics can be helpful in describing the development of sunburn purpura: delay following UV exposure, gross morphology, histologic findings, and possible associated medical conditions.1 Our case represents an important addition to the literature, as it differs from previously reported cases. Most importantly, the nonspecific biopsy findings and unremarkable laboratory findings associated with our case may represent primary or idiopathic sunburn purpura.
Previously reported cases of sunburn purpura have occurred in patients aged 10 to 66 years. It has been seen following UV exposure, vigorous exercise and high-dose aspirin, or concurrent fluoroquinolone therapy, or in the setting of erythropoietic protoporphyria, idiopathic thrombocytopenic purpura, or polymorphous light eruption.2-8 When performed, histology has revealed capillaritis, solar elastosis, perivascular infiltrate, lymphocytic perivascular infiltrate with dermal edema, or leukocytoclastic vasculitis.1,2,7-9 Our patient did not have a history of erythropoietic protoporphyria, polymorphous light eruption, or idiopathic thrombocytopenic purpura. She had not recently exercised, was not thrombocytopenic, and was not taking antiplatelet medications. She had no recent history of fluoroquinolone use. On histologic examination, our patient's biopsy demonstrated nonspecific petechial changes without signs of chronic UV exposure, dermal edema, vasculitis, lymphocytic infiltrate, or capillaritis.
Idiopathic sunburn purpura should only be diagnosed after other conditions are excluded. When evaluating a patient who presents with new-onset petechial rash following sun exposure, it is important to rule out vasculitis or thrombocytopenia as the cause, which is best achieved through skin biopsy and a platelet count, respectively. If there are no associated symptoms or thrombocytopenia and biopsy shows nonspecific vascular ectasia and erythrocyte extravasation, the physician should consider the diagnosis of idiopathic sunburn (solar or photolocalized) purpura. Along with regular UV protection, the physician should advise that the rash typically resolves without treatment in 1 to 2 weeks.
- Waters AJ, Sandhu DR, Green CM, et al. Solar capillaritis as a cause of solar purpura. Clin Exp Dermatol. 2009;34:E821-E824.
- Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
- Rubegni P, Feci L, Pellegrino M, et al. Photolocalized purpura during levofloxacin therapy. Photodermatol Photoimmunol Photomed. 2012;28:105-107.
- Urbina F, Barrios M, Sudy E. Photolocalized purpura during ciprofloxacin therapy. Photodermatol Photoimmunol Photomed. 2006;22:111-112.
- Torinuki W, Miura T. Erythropoietic protoporphyria showing solar purpura. Dermatologica. 1983;167:220-222.
- Leung AK. Purpura associated with exposure to sunlight. J R Soc Med. 1986;79:423-424.
- Kalivas J, Kalivas L. Solar purpura appearing in a patient with polymorphous light eruption. Photodermatol Photoimmunol Photomed. 1995;11:31-32.
- Ros AM. Solar purpura--an unusual manifestation of polymorphous light eruption. Photodermatol. 1988;5:47-48.
- Guarrera M, Parodi A, Rebora A. Solar purpura is not related to polymorphous light eruption. Photodermatol. 1989;6:293-294.
- Waters AJ, Sandhu DR, Green CM, et al. Solar capillaritis as a cause of solar purpura. Clin Exp Dermatol. 2009;34:E821-E824.
- Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
- Rubegni P, Feci L, Pellegrino M, et al. Photolocalized purpura during levofloxacin therapy. Photodermatol Photoimmunol Photomed. 2012;28:105-107.
- Urbina F, Barrios M, Sudy E. Photolocalized purpura during ciprofloxacin therapy. Photodermatol Photoimmunol Photomed. 2006;22:111-112.
- Torinuki W, Miura T. Erythropoietic protoporphyria showing solar purpura. Dermatologica. 1983;167:220-222.
- Leung AK. Purpura associated with exposure to sunlight. J R Soc Med. 1986;79:423-424.
- Kalivas J, Kalivas L. Solar purpura appearing in a patient with polymorphous light eruption. Photodermatol Photoimmunol Photomed. 1995;11:31-32.
- Ros AM. Solar purpura--an unusual manifestation of polymorphous light eruption. Photodermatol. 1988;5:47-48.
- Guarrera M, Parodi A, Rebora A. Solar purpura is not related to polymorphous light eruption. Photodermatol. 1989;6:293-294.
Practice Points
- Petechial skin changes acutely following intense sun exposure is a rare phenomenon referred to as sunburn purpura, photolocalized purpura, or solar purpura.
- Idiopathic sunburn purpura should only be diagnosed after vasculitis and/or thrombocytopenia is ruled out, which is best achieved through skin biopsy and a platelet count, respectively.
- The rash typically resolves without treatment in 1 to 2 weeks; however, a variety of UV protection modalities and education should be offered to the patient.