User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
A Case of Leprosy in Central Florida
Case Report
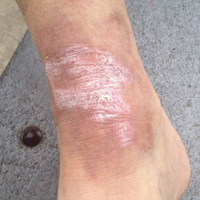
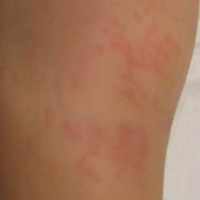
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
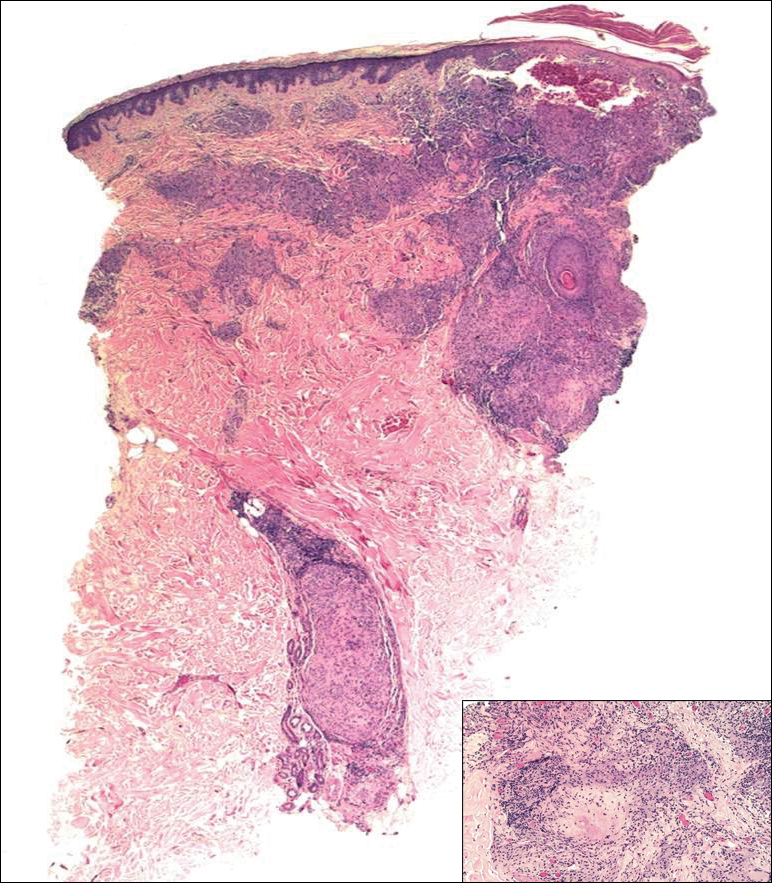
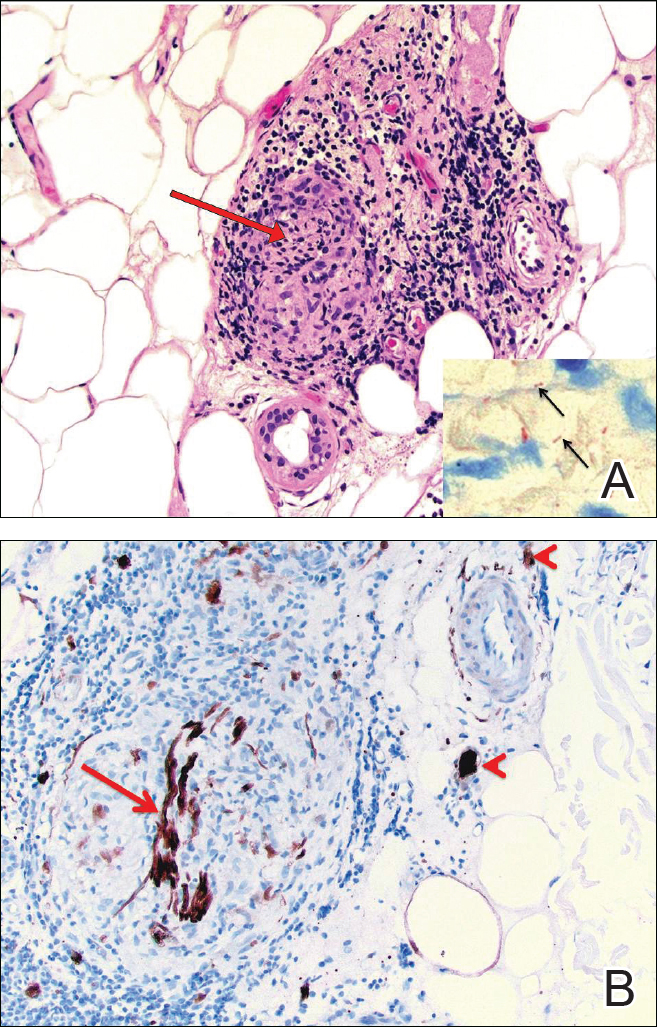
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.
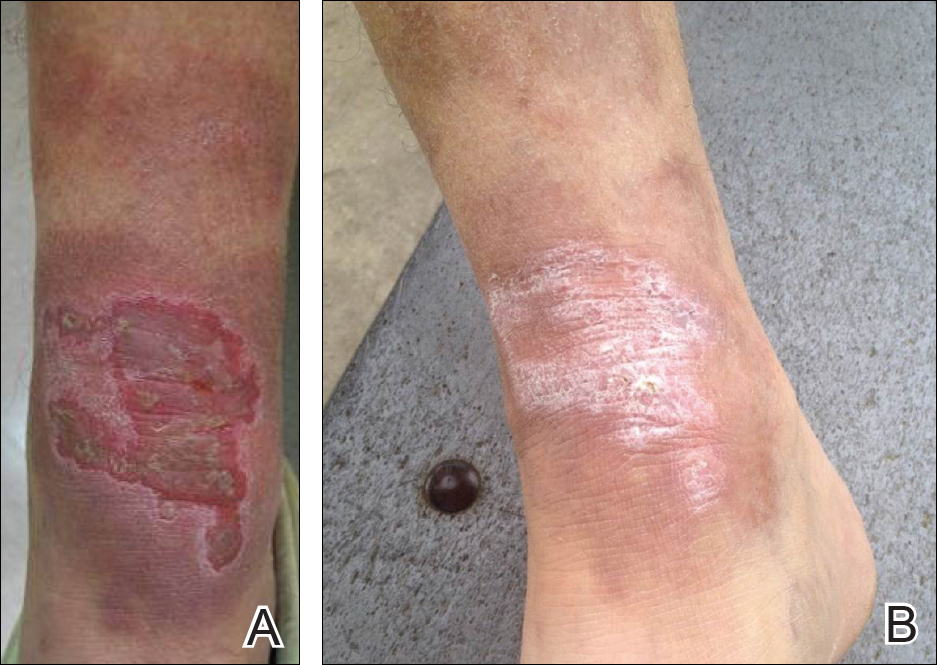
The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
Case Report
A 65-year-old man presented with multiple anesthetic, annular, erythematous, scaly plaques with a raised border of 6 weeks’ duration that were unresponsive to topical steroid therapy. Four plaques were noted on the lower back ranging from 2 to 4 cm in diameter as well as a fifth plaque on the anterior portion of the right ankle that was approximately 6×6 cm. He denied fever, malaise, muscle weakness, changes in vision, or sensory deficits outside of the lesions themselves. The patient also denied any recent travel to endemic areas or exposure to armadillos.
Biopsies were taken from lesions on the lumbar back and anterior aspect of the right ankle (Figure 1A). Hematoxylin and eosin staining revealed a granulomatous infiltrate spreading along neurovascular structures (Figure 2). Granulomas also were identified in the dermal interstitium exhibiting partial necrosis (Figure 2 inset). Conspicuous distension of lymphovascular and perineural areas also was noted. Immunohistochemical studies with S-100 and neurofilament stains allowed insight into the pathomechanism of the clinically observed anesthesia, as nerve fibers were identified showing different stages of damage elicited by the granulomatous inflammatory infiltrate (Figure 3). Fite staining was positive for occasional bacilli within histiocytes (Figure 3A inset). Despite the clinical, histologic, and immunohistochemical evidence, the patient had no known exposure to leprosy; consequently, a polymerase chain reaction (PCR) assay was ordered for confirmation of the diagnosis. Surprisingly, the PCR was positive for Mycobacterium leprae DNA. These findings were consistent with borderline tuberculoid leprosy.



The case was reported to the National Hansen’s Disease Program (Baton Rouge, Louisiana). The patient was started on rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The lesions exhibited marked improvement after completion of therapy (Figure 1B).
Comment
Disease Transmission
Hansen disease, also known as leprosy, is a chronic granulomatous infectious disease that is caused by M leprae, an obligate intracellular bacillus aerobe.1 The mechanism of spread of M leprae is not clear. It is thought to be transmitted via respiratory droplets, though it may occur through injured skin.2 Studies have suggested that in addition to humans, nine-banded armadillos are a source of infection.2,3 Exposure to infected individuals, particularly multibacillary patients, increases the likelihood of contracting leprosy.2
According to the Centers for Disease Control and Prevention, 81 cases of Hansen disease were diagnosed in the United States in 2013,4 compared to 178 cases registered in 2015.5 Cases from Hawaii, Texas, California, Louisiana, New York, and Florida made up 72% (129/178) of the reported cases. There was an increase from 34 cases to 49 cases in Florida from 2014 to 2015.5 The spread of leprosy throughout Florida may be from the merge of 2 armadillo populations, an M leprae–infected population migrating east from Texas and one from south central Florida that historically had not been infected with M leprae until recently.3,6 Our patient did not have any known exposures to armadillos.
Classification and Presentation
The clinical presentation of Hansen disease is widely variable, as it can present at any point along a spectrum ranging from tuberculoid leprosy to lepromatous leprosy with borderline conditions in between, according to the Ridley-Jopling critera.7 The World Health Organization also classifies leprosy based on the number of acid-fast bacilli seen in a skin smear as either paucibacillary or multibacillary.2 The paucibacillary classification correlates with tuberculoid, borderline tuberculoid, and indeterminate leprosy, and multibacillary correlates with borderline lepromatous and lepromatous leprosy. Paucibacillary leprosy usually presents with a less dramatic clinical picture than multibacillary leprosy. The clinical presentation is dependent on the magnitude of immune response to M leprae.2
Paucibacillary infection occurs when the body generates a strong cell-mediated immune response against the bacteria,8 which causes the activation and proliferation of CD4 and CD8 T cells, limiting the spread of the mycobacterium. Subsequently, the patient typically presents with a mild clinical picture with few skin lesions and limited nerve involvement.8 The skin lesions are papules or plaques with raised borders that are usually hypopigmented on dark skin and erythematous on light skin. Nerve involvement in paucibacillary forms of leprosy include sensory impairment and anhidrosis within the lesions. Nerve enlargement usually affects superficial nerves, with the posterior tibial nerve being most commonly affected.
Multibacillary leprosy presents with systemic involvement due to a weak cell-mediated immune response. Patients generally present with diffuse, poorly defined nodules; greater nerve impairment; and other systemic symptoms such as blindness, swelling of the fingers and toes, and testicular atrophy (in men). Additionally, enlargement of the earlobes and widening of the nasal bridge may contribute to the appearance of leonine facies. Nerve impairment in multibacillary forms of leprosy may be more severe, including more diffuse sensory involvement (eg, stocking glove–pattern neuropathy, nerve-trunk palsies), which ultimately may lead to foot drop, claw toe, and lagophthalmos.8
In addition to the clinical presentation, the histology of the paucibacillary and multibacillary types differ. Multibacillary leprosy shows diffuse histiocytes without granulomas and multiple bacilli seen on Fite staining.8 In the paucibacillary form, there are well-formed granulomas with Langerhans giant cells and a perineural lymphocytic infiltrate seen on hematoxylin and eosin staining with rare acid-fast bacilli seen on Fite staining.
To diagnose leprosy, at least one of the following 3 clinical signs must be present: (1) a hypopigmented or erythematous lesion with loss of sensation, (2) thickened peripheral nerve, or (3) acid-fast bacilli on slit-skin smear.2
Management
The World Health Organization guidelines involve multidrug therapy over an extended period of time.2 For adults, the paucibacillary regimen includes rifampicin 600 mg once monthly and dapsone 100 mg once daily for 6 months. The adult regimen for multibacillary leprosy includes clofazimine 300 mg once monthly and 50 mg once daily, in addition to rifampicin 600 mg once monthly and dapsone 100 mg once daily for 12 months. If classification cannot be determined, it is recommended the patient be treated for multibacillary disease.2
Reversal Reactions
During the course of the disease, patients may upgrade (to a less severe form) or downgrade (to a more severe form) between the tuberculoid, borderline, and lepromatous forms.8 The patient’s clinical picture also may change with complications of leprosy, which include type 1 and type 2 reactions. Type 1 reaction is a reversal reaction seen in 15% to 30% of patients at risk, usually those with borderline forms of leprosy.9 Reversal reactions usually manifest as erythema and edema of current skin lesions, formation of new tumid lesions, and tenderness of peripheral nerves with loss of nerve function.8 Treatment of reversal reactions involves systemic corticosteroids.10 Type 2 reaction is classified as erythema nodosum leprosum. It presents within the first 2 years of treatment in approximately 20% of lepromatous patients and approximately 10% of borderline lepromatous patients but is rare in paucibacillary infections.11 It presents with fever and crops of pink nodules and may include iritis, neuritis, lymphadenitis, orchitis, dactylitis, arthritis, and proteinuria.8 Treatment options for erythema nodosum leprosum include corticosteroids, clofazimine, and thalidomide.12,13
Conclusion
Hansen disease is a rare condition in the United States. This case is unique because, to our knowledge, it is the first known PCR-confirmed case of Hansen disease in Okeechobee County, Florida. Additionally, the patient had no known exposure to M leprae. Exposure is increasing due to the increased geographical range of infected armadillos. Infection rates also may rise due to travel to endemic countries. Initially lesions may appear as innocuous erythematous plaques. When they do not respond to standard therapy, infectious agents such as M leprae should be part of the differential diagnosis. Because hematoxylin and eosin staining does not always yield results, if clinical suspicion is present, PCR should be performed. If a patient meets the clinical and histological diagnosis, the case should be reported to the National Hansen’s Disease Program.
After completion of treatment, our patient has shown excellent results. He has not yet demonstrated a reversal reaction; however, he may still be at risk, as it most commonly presents 2 months after starting treatment but can present years after treatment has been initiated.8 Cutaneous leprosy must be considered in the differential diagnosis for steroid-nonresponsive skin lesions, particularly in states such as Florida with a documented increase in incidence.
Acknowledgment
We thank Sharon Barrineau, ARNP (Okeechobee, Florida), for her acumen, contributions, and support on this case.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- World Health Organization. WHO Expert Committee on Leprosy, 8th Report. Geneva, Switzerland: World Health Organization; 2010.
- Truman RW, Singh P, Sharma R, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;364:1626-1633.
- Adams DA, Fullerton K, Jajosky R, et al; Division of Notifiable Diseases and Healthcare Information, Office of Surveillance, Epidemiology, and Laboratory Services, CDC. Summary of notifiable diseases—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62:1-122.
- A summary of Hansen’s disease in the United States—2015. Department of Health and Human Services, Health Resources and Services Administration, National Hansen’s Disease Program. https://www.hrsa.gov/sites/default/files/hansensdisease/pdfs/hansens2015report.pdf. Accessed October 23, 2017.
- Loughry WJ, Truman RW, McDonough CM, et al. Is leprosy spreading among nine-banded armadillos in the southeastern United States? J Wildl Dis. 2009;45:144-152.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity: a five group system. Int J Lepr. 1966;34:225-273.
- Lee DJ, Rea TH, Modlin RL. Leprosy. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Scollard DM, Adams LB, Gillis TP, et al. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338-381.
- Britton WJ. The management of leprosy reversal reactions. Lepr Rev. 1998;69:225-234.
- Manandhar R, LeMaster JW, Roche PW. Risk factors for erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1999;67:270-278.
- Lockwood DN. The management of erythema nodosum leprosum: current and future options. Lepr Rev. 1996;67:253-259.
- Jakeman P, Smith WC. Thalidomide in leprosy reaction. Lancet. 1994;343:432-433.
Practice Points
- A majority of leprosy cases in the United States have been reported in Florida, California, Texas, Louisiana, Hawaii, and New York.
- Leprosy should be included in the differential diagnosis for annular plaques, particularly those not responding to traditional treatment.
Atypical Disseminated Herpes Zoster: Management Guidelines in Immunocompromised Patients
Well-known for its typical presentation, classic herpes zoster (HZ) presents as a dermatomal eruption of painful erythematous papules that evolve into grouped vesicles or bullae.1,2 Thereafter, the lesions can become pustular or hemorrhagic.1 Although the diagnosis most often is made clinically, confirmatory techniques for diagnosis include viral culture, direct fluorescent antibody testing, or polymerase chain reaction (PCR) assay.1,3
The main risk factor for HZ is advanced age, most commonly affecting elderly patients.4 It is hypothesized that a physiological decline in varicella-zoster virus (VZV)–specific cell-mediated immunity among elderly individuals helps trigger reactivation of the virus within the dorsal root ganglion.1,5 Similarly affected are immunocompromised individuals, including those with human immunodeficiency virus (HIV) infection, due to suppression of T cells immune to VZV,1,5 as well as immunosuppressed transplant recipients who have diminished VZV-specific cellular responses and VZV IgG antibody avidity.6
Secondary complications of VZV infection (eg, postherpetic neuralgia, bacterial superinfection progressing to cellulitis) lead to increased morbidity.7,8 Disseminated cutaneous HZ is another grave complication of VZV infection and almost exclusively occurs with immunosuppression.1,8 It manifests as an eruption of at least 20 widespread vesiculobullous lesions outside the primary and adjacent dermatomes.6 Immunocompromised patients also are at increased risk for visceral involvement of VZV infection, which may affect vital organs such as the brain, liver, or lungs.7,8 Given the atypical presentation of VZV infection among some immunocompromised individuals, these patients are at increased risk for diagnostic delay and morbidity in the absence of high clinical suspicion for disseminated HZ.
Case Reports
Patient 1
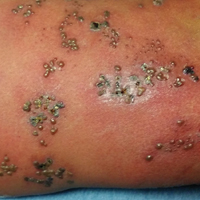
A 52-year-old man developed a painless nonpruritic rash on the left leg of 4 days’ duration. It initially appeared as an erythematous maculopapular rash on the medial aspect of the left knee without any prodromal symptoms. Over the next 4 days, erythematous vesicles developed that progressed to pustules, and the rash spread both proximally and distally along the left leg. Shortly following hospital admission, he developed a fever (temperature, 38.4°C). His medical history included alcoholic liver cirrhosis and AIDS, with a CD4 count of 174 cells/µL (reference range, 500–1500 cells/µL). He had been taking antiretroviral therapy (abacavir-lamivudine and dolutegravir) and prophylaxis against opportunistic infections (dapsone and itraconazole).
Physical examination was remarkable for an extensive rash consisting of multiple 1-cm clusters of approximately 40 pustules each scattered in a nondermatomal distribution along the left leg (Figure 1). Many of the vesicles were confluent with an erythematous base and were in different stages of evolution with some crusted and others emanating a thin liquid exudate. The lesions were nontender and without notable induration. The leg was warm and edematous.
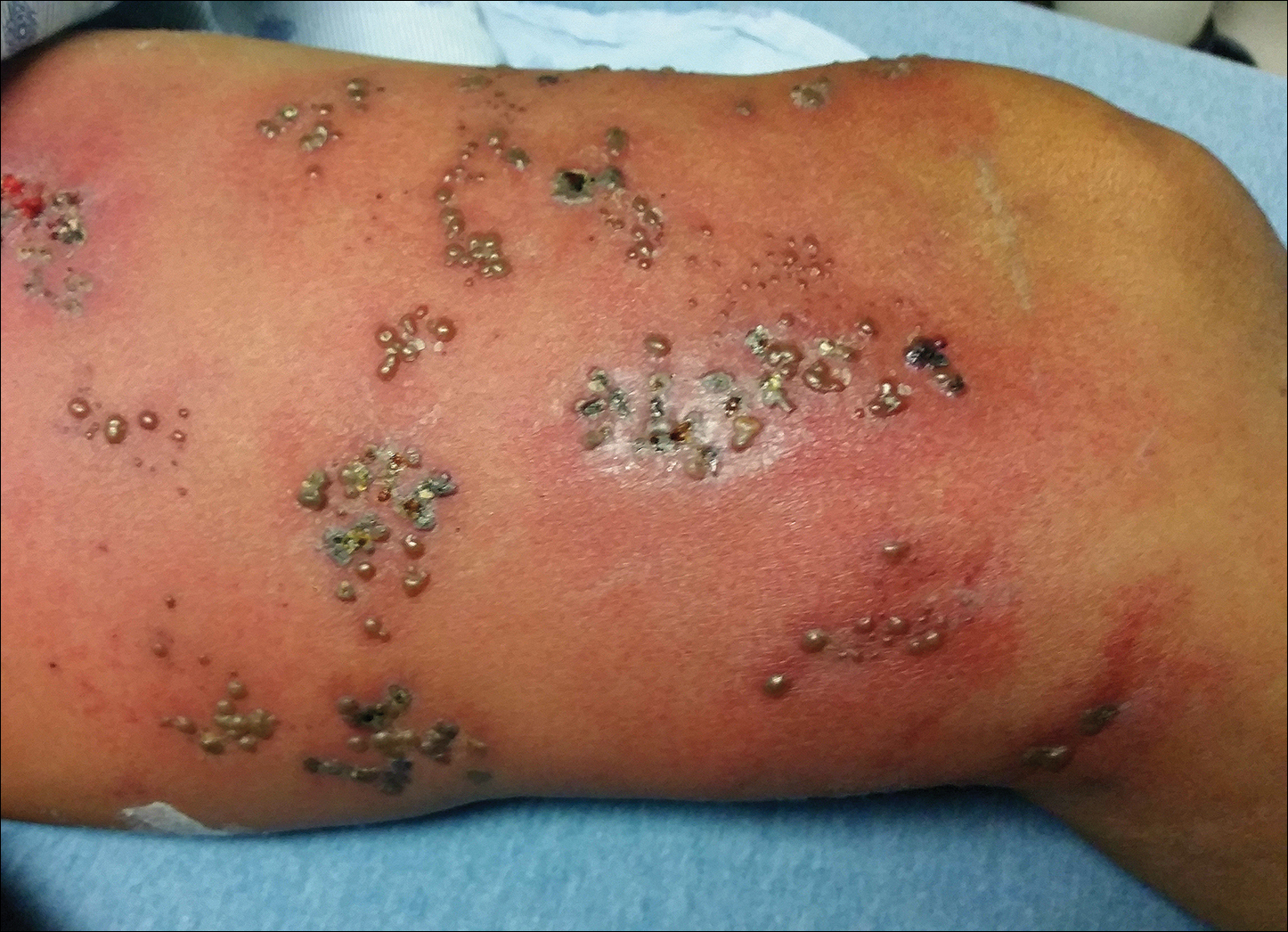
Clinically, the differential diagnosis included disseminated HZ with bacterial superinfection, Vibrio vulnificus infection, and herpes simplex virus (HSV) infection. The patient was treated with intravenous vancomycin, levofloxacin, and acyclovir, and no new lesions developed throughout the course of treatment. On this regimen, his fever resolved after 1 day, the active lesions began to crust, and the edema and erythema diminished. Results of bacterial cultures and plasma PCR and IgM for HSV types 1 and 2 were negative. Viral culture results were negative, but a PCR assay for VZV was positive, reflective of acute reactivation of VZV.
Patient 2
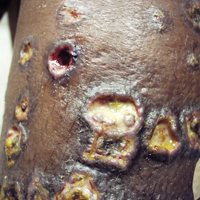
A 63-year-old man developed a pruritic burning rash involving the face, trunk, arms, and legs of 6 days’ duration. His medical history included a heart transplant 6 months prior to presentation, type 2 diabetes mellitus, and chronic kidney disease. He was taking antirejection therapy with mycophenolate mofetil (MMF), prednisone, and tacrolimus.
Physical examination was remarkable for an extensive rash consisting of clusters of 1- to 2-mm vesicles scattered in a nondermatomal pattern. Isolated vesicles involved the forehead, nose, and left ear, and diffuse vesicles with a relatively symmetric distribution were scattered across the back, chest, and proximal and distal arms and legs (Figure 2). Many of the vesicles had an associated overlying crust with hemorrhage. Some of the vesicles coalesced with central necrotic plaques.
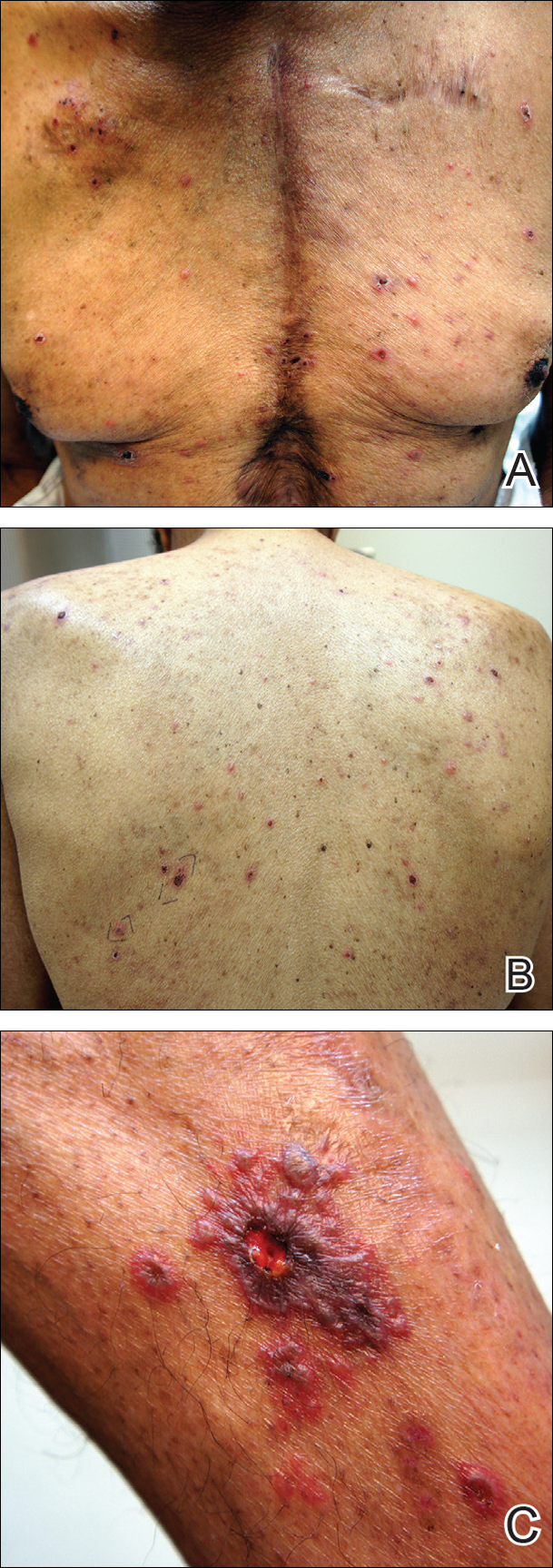
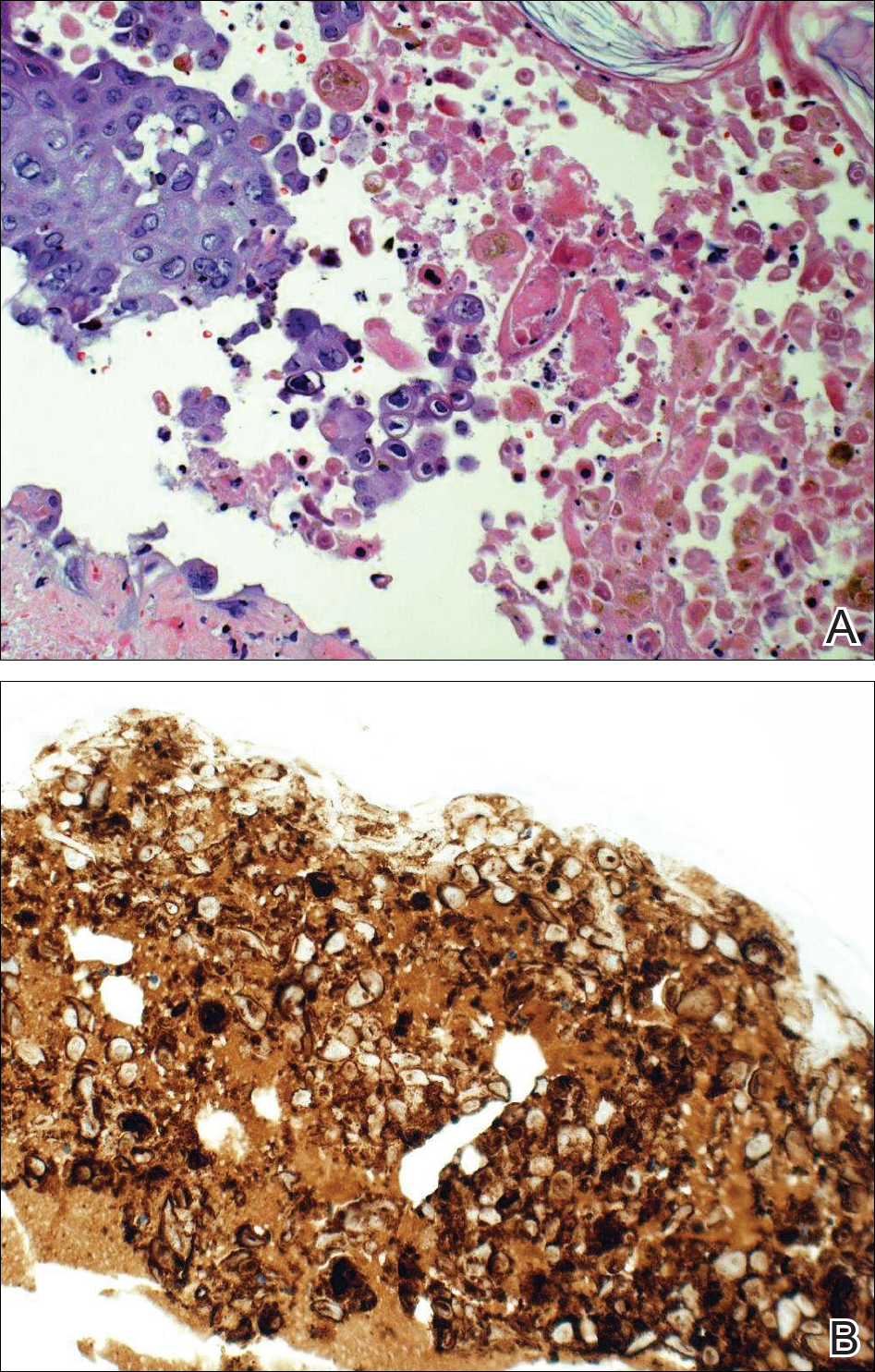
Given a clinical suspicion for disseminated HZ, therapy with oral valacyclovir was initiated. Two punch biopsies were consistent with herpesvirus cytopathic changes. Multiple sections demonstrated ulceration as well as acantholysis and necrosis of keratinocytes with multinucleation and margination of chromatin. There was an intense lichenoid and perivascular lymphocytic infiltrate in the dermis. Immunohistochemistry staining was positive for VZV and negative for HSV, indicating acute reactivation of VZV (Figure 3). Upon completion of an antiviral regimen, the patient returned to clinic with healed crusted lesions.
Comment
Frequently, the clinical features of HZ in immunocompromised patients mirror those in immunocompetent hosts.8 However, each of our 2 patients developed an unusual presentation of atypical generalized HZ.7 In this clinical variant, lesions develop along a single dermatome, then a diffuse vesicular eruption subsequently develops without dermatomal localization. These lesions can be chronic, persisting for months or years.7
The classic clinical presentation of HZ is distinct and often is readily diagnosed by visual inspection.7 However, atypical presentations and their associated complications can pose diagnostic and therapeutic challenges.7 Painless HZ lesions in a nondermatomal pattern were described in a patient who also had AIDS.9 Interestingly, multiple reports have found that patients with a severe but painless rash are less likely to have experienced a viral prodrome consisting of hyperesthesia, paresthesia, or pruritus.2,10 This observation suggests that lack of a prodrome, as in the case of patient 1 in our report, may aid in the recognition of painless HZ. Because of these atypical presentations, laboratory testing is even more important than in immunocompetent hosts, as diagnosis may be more difficult to establish on clinical presentation alone.
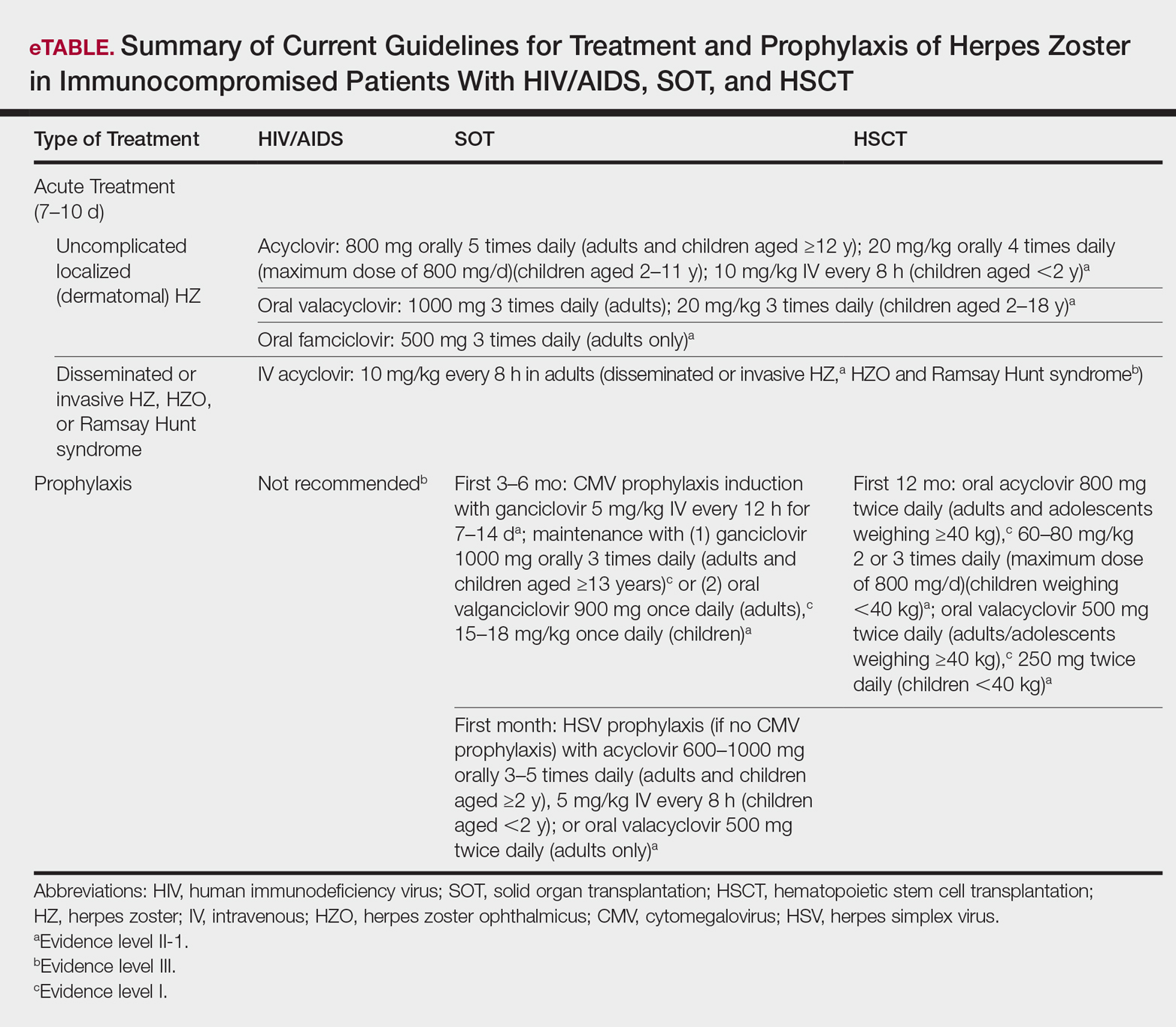
Several studies11-32 have evaluated modalities for treatment and prophylaxis for disseminated HZ in immunocompromised hosts, given its increased risk and potentially fatal complications in this population. The current guidelines in patients with HIV/AIDS, solid organ transplantation (SOT), and hematopoietic stem cell transplantation (HSCT) are summarized in the eTable.
HIV/AIDS Patients
Given their efficacy and low rate of toxicity, oral acyclovir, valacyclovir, and famciclovir are recommended treatment options for HIV patients with localized, mild, dermatomal HZ.11 Two exceptions include HZ ophthalmicus and Ramsay Hunt syndrome for which some experts recommend intravenous acyclovir given the risk for vision loss and facial palsy, respectively. Intravenous acyclovir often is the drug of choice for treating complicated, disseminated, or severe HZ in HIV-infected patients, though prospective efficacy data remain limited.11
With regard to prevention of infection, a large randomized trial in 2016 found that acyclovir prophylaxis resulted in a 68% reduction in HZ over 2 years among HIV patients.12 Despite data that acyclovir may be effective for this purpose, long-term antiviral prophylaxis is not routinely recommended for HZ,11,13 as it has been linked to rare cases of acyclovir-resistant HZ in HIV patients.14,15 However, antiviral prophylaxis against HSV type 2 reactivation in HIV patients also confers protection against VZV reactivation.11,12
Solid Organ Transplantation
Localized, mild to moderately severe dermatomal HZ can be treated with oral acyclovir, valacyclovir, or famciclovir. As in HIV patients, SOT patients with severe, disseminated, or complicated HZ should receive IV acyclovir.11 In the first 3 to 6 months following the procedure, SOT patients receive cytomegalovirus prophylaxis with ganciclovir or valgan-ciclovir, which also provides protection against HZ.13-18 For patients not receiving cytomegalovirus prophylaxis, HSV prophylaxis with oral acyclovir or valacyclovir is given for at least the first month after transplantation, which also confers protection against HZ.16,19 Antiviral therapy is critical during the early posttransplantation period when patients are most severely immunosuppressed and thus have the highest risk for VZV-associated complications.20 Although immunosuppression is lifelong in most SOT recipients, there is insufficient evidence for extending prophylaxis beyond 6 months.16,21
As a possible risk factor for HZ,22 MMF use is another consideration among SOT patients, similar to patient 2 in our report. A 2003 observational study supported withdrawal of MMF therapy during active VZV infection due to clinical observation of an association with HZ.23 However, a multicenter, randomized, controlled trial reported no cases of HZ in renal transplant recipients on MMF.24 Additionally, MMF has been observed to enhance the antiviral activity of acyclovir, at least in vitro.25 Given the lack of evidence of MMF as a risk factor for HZ, there is insufficient evidence for cessation of use during VZVreactivation in SOT patients.
Hematopoietic Stem Cell Transplantation
The preferred agents for treatment of localized mild dermatomal HZ are oral acyclovir or valacyclovir, as data on the safety and efficacy of famciclovir among HSCT recipients are limited.13,26 Patients should receive antiviral prophylaxis with one of these agents during the first year following allogeneic or autologous HSCT. This 1-year course has proven highly effective in reducing HZ in the first year following transplantation when most severe cases occur,21,26-29 and it has been associated with a persistently decreased risk for HZ even after discontinuation.21 Prophylaxis may be continued beyond 1 year in allogeneic HSCT recipients experiencing graft-versus-host disease who should receive acyclovir until 6 months after the end of immunosuppressive therapy.21,26
Vaccination remains a potential strategy to reduce the incidence of HZ in this patient population. A heat-inactivated vaccine administered within the first 3 months after the procedure has been shown to be safe among autologous and allogeneic HSCT patients.30,31 The vaccine notably reduced the incidence of HZ in patients who underwent autologous HSCT,32 but no known data are available on its clinical efficacy in allogeneic HSCT patients. Accordingly, there are no known official recommendations to date regarding vaccine use in these patient populations.26
Conclusion
It is incumbent upon clinicians to recognize the spectrum of atypical presentations of HZ and maintain a low threshold for performing appropriate diagnostic or confirmatory studies among at-risk patients with impaired immune function. Disseminated HZ can have potentially life-threatening visceral complications such as encephalitis, hepatitis, or pneumonitis.7,8 As such, an understanding of prevention and treatment modalities for VZV infection among immunocompromised patients is critical. Because the morbidity associated with complications of VZV infection is substantial and the risks associated with antiviral agents are minimal, antiviral prophylaxis is recommended for 6 months following SOT or 1 year following HSCT, and prompt treatment is warranted in cases of reasonable clinical suspicion for HZ.
Acknowledgment
The authors gratefully acknowledge the generosity of our patients in permitting photography of their skin findings for the furthering of medical education.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41:1-16.
- Nagasako EM, Johnson RW, Griffin DR, et al. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46:834-839.
- Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23-32.
- Herpes Zoster and Functional Decline Consortium. Functional decline and herpes zoster in older people: an interplay of multiple factors. Aging Clin Exp Res. 2015;27:757-765.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341-357.
- Prelog M, Schonlaub J, Jeller V, et al. Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine. 2013;31:2420-2426.
- Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
- Glesby MJ, Moore RD, Chaisson RE. Clinical spectrum of herpes zoster in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:370-375.
- Blankenship W, Herchline T, Hockley A. Asymptomatic vesicles in a patient with the acquired immunodeficiency syndrome. disseminated varicella-zoster virus (VZV) infection. Arch Dermatol. 1994;130:1193, 1196.
- Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342-348.
- Gnann JW. Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al, eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, United Kingdom: Cambridge University Press; 2007:1175-1191.
- Barnabas RV, Baeten JM, Lingappa JR, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: results of a randomized clinical trial. J Infect Dis. 2016;213:551-555.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
- Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990;112:187-191.
- Linnemann CC Jr, Biron KK, Hoppenjans WG, et al. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577-579.
- Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S108-S115.
- Preiksaitis JK, Brennan DC, Fishman J, et al. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218-227.
- Fishman JA, Doran MT, Volpicelli SA, et al. Dosing of intravenous ganciclovir for the prophylaxis and treatment of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2000;69:389-394.
- Zuckerman R, Wald A; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S104-S107.
- Arness T, Pedersen R, Dierkhising R, et al. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10:260-268.
- Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110:3071-3077.
- Rothwell WS, Gloor JM, Morgenstern BZ, et al. Disseminated varicella infection in pediatric renal transplant recipients treated with mycophenolate mofetil. Transplantation. 1999;68:158-161.
- Lauzurica R, Bayés B, Frías C, et al. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35:1758-1759.
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. TheTricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029-1037.
- Neyts J, De Clercq E. Mycophenolate mofetil strongly potentiates the anti-herpesvirus activity of acyclovir. Antiviral Res. 1998;40:53-56.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238.
- Boeckh M, Kim HW, Flowers ME, et al. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood. 2006;107:1800-1805.
- Kawamura K, Hayakawa J, Akahoshi Y, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol. 2015;102:230-237.
- Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Long-term follow-up after hematopoietic stem cell transplant general guidelines for referring physicians. Fred Hutchinson Cancer Research Center website. https://www.fredhutch.org/content/dam/public/Treatment-Suport/Long-Term-Follow-Up/physician.pdf. Published July 17, 2014. Accessed October 19, 2017.
- Kussmaul SC, Horn BN, Dvorak CC, et al. Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant. 2010;45:1602-1606.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26-34.
- Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20:285-287.
Well-known for its typical presentation, classic herpes zoster (HZ) presents as a dermatomal eruption of painful erythematous papules that evolve into grouped vesicles or bullae.1,2 Thereafter, the lesions can become pustular or hemorrhagic.1 Although the diagnosis most often is made clinically, confirmatory techniques for diagnosis include viral culture, direct fluorescent antibody testing, or polymerase chain reaction (PCR) assay.1,3
The main risk factor for HZ is advanced age, most commonly affecting elderly patients.4 It is hypothesized that a physiological decline in varicella-zoster virus (VZV)–specific cell-mediated immunity among elderly individuals helps trigger reactivation of the virus within the dorsal root ganglion.1,5 Similarly affected are immunocompromised individuals, including those with human immunodeficiency virus (HIV) infection, due to suppression of T cells immune to VZV,1,5 as well as immunosuppressed transplant recipients who have diminished VZV-specific cellular responses and VZV IgG antibody avidity.6
Secondary complications of VZV infection (eg, postherpetic neuralgia, bacterial superinfection progressing to cellulitis) lead to increased morbidity.7,8 Disseminated cutaneous HZ is another grave complication of VZV infection and almost exclusively occurs with immunosuppression.1,8 It manifests as an eruption of at least 20 widespread vesiculobullous lesions outside the primary and adjacent dermatomes.6 Immunocompromised patients also are at increased risk for visceral involvement of VZV infection, which may affect vital organs such as the brain, liver, or lungs.7,8 Given the atypical presentation of VZV infection among some immunocompromised individuals, these patients are at increased risk for diagnostic delay and morbidity in the absence of high clinical suspicion for disseminated HZ.
Case Reports
Patient 1
A 52-year-old man developed a painless nonpruritic rash on the left leg of 4 days’ duration. It initially appeared as an erythematous maculopapular rash on the medial aspect of the left knee without any prodromal symptoms. Over the next 4 days, erythematous vesicles developed that progressed to pustules, and the rash spread both proximally and distally along the left leg. Shortly following hospital admission, he developed a fever (temperature, 38.4°C). His medical history included alcoholic liver cirrhosis and AIDS, with a CD4 count of 174 cells/µL (reference range, 500–1500 cells/µL). He had been taking antiretroviral therapy (abacavir-lamivudine and dolutegravir) and prophylaxis against opportunistic infections (dapsone and itraconazole).
Physical examination was remarkable for an extensive rash consisting of multiple 1-cm clusters of approximately 40 pustules each scattered in a nondermatomal distribution along the left leg (Figure 1). Many of the vesicles were confluent with an erythematous base and were in different stages of evolution with some crusted and others emanating a thin liquid exudate. The lesions were nontender and without notable induration. The leg was warm and edematous.

Clinically, the differential diagnosis included disseminated HZ with bacterial superinfection, Vibrio vulnificus infection, and herpes simplex virus (HSV) infection. The patient was treated with intravenous vancomycin, levofloxacin, and acyclovir, and no new lesions developed throughout the course of treatment. On this regimen, his fever resolved after 1 day, the active lesions began to crust, and the edema and erythema diminished. Results of bacterial cultures and plasma PCR and IgM for HSV types 1 and 2 were negative. Viral culture results were negative, but a PCR assay for VZV was positive, reflective of acute reactivation of VZV.
Patient 2
A 63-year-old man developed a pruritic burning rash involving the face, trunk, arms, and legs of 6 days’ duration. His medical history included a heart transplant 6 months prior to presentation, type 2 diabetes mellitus, and chronic kidney disease. He was taking antirejection therapy with mycophenolate mofetil (MMF), prednisone, and tacrolimus.
Physical examination was remarkable for an extensive rash consisting of clusters of 1- to 2-mm vesicles scattered in a nondermatomal pattern. Isolated vesicles involved the forehead, nose, and left ear, and diffuse vesicles with a relatively symmetric distribution were scattered across the back, chest, and proximal and distal arms and legs (Figure 2). Many of the vesicles had an associated overlying crust with hemorrhage. Some of the vesicles coalesced with central necrotic plaques.

Given a clinical suspicion for disseminated HZ, therapy with oral valacyclovir was initiated. Two punch biopsies were consistent with herpesvirus cytopathic changes. Multiple sections demonstrated ulceration as well as acantholysis and necrosis of keratinocytes with multinucleation and margination of chromatin. There was an intense lichenoid and perivascular lymphocytic infiltrate in the dermis. Immunohistochemistry staining was positive for VZV and negative for HSV, indicating acute reactivation of VZV (Figure 3). Upon completion of an antiviral regimen, the patient returned to clinic with healed crusted lesions.

Comment
Frequently, the clinical features of HZ in immunocompromised patients mirror those in immunocompetent hosts.8 However, each of our 2 patients developed an unusual presentation of atypical generalized HZ.7 In this clinical variant, lesions develop along a single dermatome, then a diffuse vesicular eruption subsequently develops without dermatomal localization. These lesions can be chronic, persisting for months or years.7
The classic clinical presentation of HZ is distinct and often is readily diagnosed by visual inspection.7 However, atypical presentations and their associated complications can pose diagnostic and therapeutic challenges.7 Painless HZ lesions in a nondermatomal pattern were described in a patient who also had AIDS.9 Interestingly, multiple reports have found that patients with a severe but painless rash are less likely to have experienced a viral prodrome consisting of hyperesthesia, paresthesia, or pruritus.2,10 This observation suggests that lack of a prodrome, as in the case of patient 1 in our report, may aid in the recognition of painless HZ. Because of these atypical presentations, laboratory testing is even more important than in immunocompetent hosts, as diagnosis may be more difficult to establish on clinical presentation alone.
Several studies11-32 have evaluated modalities for treatment and prophylaxis for disseminated HZ in immunocompromised hosts, given its increased risk and potentially fatal complications in this population. The current guidelines in patients with HIV/AIDS, solid organ transplantation (SOT), and hematopoietic stem cell transplantation (HSCT) are summarized in the eTable.

HIV/AIDS Patients
Given their efficacy and low rate of toxicity, oral acyclovir, valacyclovir, and famciclovir are recommended treatment options for HIV patients with localized, mild, dermatomal HZ.11 Two exceptions include HZ ophthalmicus and Ramsay Hunt syndrome for which some experts recommend intravenous acyclovir given the risk for vision loss and facial palsy, respectively. Intravenous acyclovir often is the drug of choice for treating complicated, disseminated, or severe HZ in HIV-infected patients, though prospective efficacy data remain limited.11
With regard to prevention of infection, a large randomized trial in 2016 found that acyclovir prophylaxis resulted in a 68% reduction in HZ over 2 years among HIV patients.12 Despite data that acyclovir may be effective for this purpose, long-term antiviral prophylaxis is not routinely recommended for HZ,11,13 as it has been linked to rare cases of acyclovir-resistant HZ in HIV patients.14,15 However, antiviral prophylaxis against HSV type 2 reactivation in HIV patients also confers protection against VZV reactivation.11,12
Solid Organ Transplantation
Localized, mild to moderately severe dermatomal HZ can be treated with oral acyclovir, valacyclovir, or famciclovir. As in HIV patients, SOT patients with severe, disseminated, or complicated HZ should receive IV acyclovir.11 In the first 3 to 6 months following the procedure, SOT patients receive cytomegalovirus prophylaxis with ganciclovir or valgan-ciclovir, which also provides protection against HZ.13-18 For patients not receiving cytomegalovirus prophylaxis, HSV prophylaxis with oral acyclovir or valacyclovir is given for at least the first month after transplantation, which also confers protection against HZ.16,19 Antiviral therapy is critical during the early posttransplantation period when patients are most severely immunosuppressed and thus have the highest risk for VZV-associated complications.20 Although immunosuppression is lifelong in most SOT recipients, there is insufficient evidence for extending prophylaxis beyond 6 months.16,21
As a possible risk factor for HZ,22 MMF use is another consideration among SOT patients, similar to patient 2 in our report. A 2003 observational study supported withdrawal of MMF therapy during active VZV infection due to clinical observation of an association with HZ.23 However, a multicenter, randomized, controlled trial reported no cases of HZ in renal transplant recipients on MMF.24 Additionally, MMF has been observed to enhance the antiviral activity of acyclovir, at least in vitro.25 Given the lack of evidence of MMF as a risk factor for HZ, there is insufficient evidence for cessation of use during VZVreactivation in SOT patients.
Hematopoietic Stem Cell Transplantation
The preferred agents for treatment of localized mild dermatomal HZ are oral acyclovir or valacyclovir, as data on the safety and efficacy of famciclovir among HSCT recipients are limited.13,26 Patients should receive antiviral prophylaxis with one of these agents during the first year following allogeneic or autologous HSCT. This 1-year course has proven highly effective in reducing HZ in the first year following transplantation when most severe cases occur,21,26-29 and it has been associated with a persistently decreased risk for HZ even after discontinuation.21 Prophylaxis may be continued beyond 1 year in allogeneic HSCT recipients experiencing graft-versus-host disease who should receive acyclovir until 6 months after the end of immunosuppressive therapy.21,26
Vaccination remains a potential strategy to reduce the incidence of HZ in this patient population. A heat-inactivated vaccine administered within the first 3 months after the procedure has been shown to be safe among autologous and allogeneic HSCT patients.30,31 The vaccine notably reduced the incidence of HZ in patients who underwent autologous HSCT,32 but no known data are available on its clinical efficacy in allogeneic HSCT patients. Accordingly, there are no known official recommendations to date regarding vaccine use in these patient populations.26
Conclusion
It is incumbent upon clinicians to recognize the spectrum of atypical presentations of HZ and maintain a low threshold for performing appropriate diagnostic or confirmatory studies among at-risk patients with impaired immune function. Disseminated HZ can have potentially life-threatening visceral complications such as encephalitis, hepatitis, or pneumonitis.7,8 As such, an understanding of prevention and treatment modalities for VZV infection among immunocompromised patients is critical. Because the morbidity associated with complications of VZV infection is substantial and the risks associated with antiviral agents are minimal, antiviral prophylaxis is recommended for 6 months following SOT or 1 year following HSCT, and prompt treatment is warranted in cases of reasonable clinical suspicion for HZ.
Acknowledgment
The authors gratefully acknowledge the generosity of our patients in permitting photography of their skin findings for the furthering of medical education.
Well-known for its typical presentation, classic herpes zoster (HZ) presents as a dermatomal eruption of painful erythematous papules that evolve into grouped vesicles or bullae.1,2 Thereafter, the lesions can become pustular or hemorrhagic.1 Although the diagnosis most often is made clinically, confirmatory techniques for diagnosis include viral culture, direct fluorescent antibody testing, or polymerase chain reaction (PCR) assay.1,3
The main risk factor for HZ is advanced age, most commonly affecting elderly patients.4 It is hypothesized that a physiological decline in varicella-zoster virus (VZV)–specific cell-mediated immunity among elderly individuals helps trigger reactivation of the virus within the dorsal root ganglion.1,5 Similarly affected are immunocompromised individuals, including those with human immunodeficiency virus (HIV) infection, due to suppression of T cells immune to VZV,1,5 as well as immunosuppressed transplant recipients who have diminished VZV-specific cellular responses and VZV IgG antibody avidity.6
Secondary complications of VZV infection (eg, postherpetic neuralgia, bacterial superinfection progressing to cellulitis) lead to increased morbidity.7,8 Disseminated cutaneous HZ is another grave complication of VZV infection and almost exclusively occurs with immunosuppression.1,8 It manifests as an eruption of at least 20 widespread vesiculobullous lesions outside the primary and adjacent dermatomes.6 Immunocompromised patients also are at increased risk for visceral involvement of VZV infection, which may affect vital organs such as the brain, liver, or lungs.7,8 Given the atypical presentation of VZV infection among some immunocompromised individuals, these patients are at increased risk for diagnostic delay and morbidity in the absence of high clinical suspicion for disseminated HZ.
Case Reports
Patient 1
A 52-year-old man developed a painless nonpruritic rash on the left leg of 4 days’ duration. It initially appeared as an erythematous maculopapular rash on the medial aspect of the left knee without any prodromal symptoms. Over the next 4 days, erythematous vesicles developed that progressed to pustules, and the rash spread both proximally and distally along the left leg. Shortly following hospital admission, he developed a fever (temperature, 38.4°C). His medical history included alcoholic liver cirrhosis and AIDS, with a CD4 count of 174 cells/µL (reference range, 500–1500 cells/µL). He had been taking antiretroviral therapy (abacavir-lamivudine and dolutegravir) and prophylaxis against opportunistic infections (dapsone and itraconazole).
Physical examination was remarkable for an extensive rash consisting of multiple 1-cm clusters of approximately 40 pustules each scattered in a nondermatomal distribution along the left leg (Figure 1). Many of the vesicles were confluent with an erythematous base and were in different stages of evolution with some crusted and others emanating a thin liquid exudate. The lesions were nontender and without notable induration. The leg was warm and edematous.

Clinically, the differential diagnosis included disseminated HZ with bacterial superinfection, Vibrio vulnificus infection, and herpes simplex virus (HSV) infection. The patient was treated with intravenous vancomycin, levofloxacin, and acyclovir, and no new lesions developed throughout the course of treatment. On this regimen, his fever resolved after 1 day, the active lesions began to crust, and the edema and erythema diminished. Results of bacterial cultures and plasma PCR and IgM for HSV types 1 and 2 were negative. Viral culture results were negative, but a PCR assay for VZV was positive, reflective of acute reactivation of VZV.
Patient 2
A 63-year-old man developed a pruritic burning rash involving the face, trunk, arms, and legs of 6 days’ duration. His medical history included a heart transplant 6 months prior to presentation, type 2 diabetes mellitus, and chronic kidney disease. He was taking antirejection therapy with mycophenolate mofetil (MMF), prednisone, and tacrolimus.
Physical examination was remarkable for an extensive rash consisting of clusters of 1- to 2-mm vesicles scattered in a nondermatomal pattern. Isolated vesicles involved the forehead, nose, and left ear, and diffuse vesicles with a relatively symmetric distribution were scattered across the back, chest, and proximal and distal arms and legs (Figure 2). Many of the vesicles had an associated overlying crust with hemorrhage. Some of the vesicles coalesced with central necrotic plaques.

Given a clinical suspicion for disseminated HZ, therapy with oral valacyclovir was initiated. Two punch biopsies were consistent with herpesvirus cytopathic changes. Multiple sections demonstrated ulceration as well as acantholysis and necrosis of keratinocytes with multinucleation and margination of chromatin. There was an intense lichenoid and perivascular lymphocytic infiltrate in the dermis. Immunohistochemistry staining was positive for VZV and negative for HSV, indicating acute reactivation of VZV (Figure 3). Upon completion of an antiviral regimen, the patient returned to clinic with healed crusted lesions.

Comment
Frequently, the clinical features of HZ in immunocompromised patients mirror those in immunocompetent hosts.8 However, each of our 2 patients developed an unusual presentation of atypical generalized HZ.7 In this clinical variant, lesions develop along a single dermatome, then a diffuse vesicular eruption subsequently develops without dermatomal localization. These lesions can be chronic, persisting for months or years.7
The classic clinical presentation of HZ is distinct and often is readily diagnosed by visual inspection.7 However, atypical presentations and their associated complications can pose diagnostic and therapeutic challenges.7 Painless HZ lesions in a nondermatomal pattern were described in a patient who also had AIDS.9 Interestingly, multiple reports have found that patients with a severe but painless rash are less likely to have experienced a viral prodrome consisting of hyperesthesia, paresthesia, or pruritus.2,10 This observation suggests that lack of a prodrome, as in the case of patient 1 in our report, may aid in the recognition of painless HZ. Because of these atypical presentations, laboratory testing is even more important than in immunocompetent hosts, as diagnosis may be more difficult to establish on clinical presentation alone.
Several studies11-32 have evaluated modalities for treatment and prophylaxis for disseminated HZ in immunocompromised hosts, given its increased risk and potentially fatal complications in this population. The current guidelines in patients with HIV/AIDS, solid organ transplantation (SOT), and hematopoietic stem cell transplantation (HSCT) are summarized in the eTable.

HIV/AIDS Patients
Given their efficacy and low rate of toxicity, oral acyclovir, valacyclovir, and famciclovir are recommended treatment options for HIV patients with localized, mild, dermatomal HZ.11 Two exceptions include HZ ophthalmicus and Ramsay Hunt syndrome for which some experts recommend intravenous acyclovir given the risk for vision loss and facial palsy, respectively. Intravenous acyclovir often is the drug of choice for treating complicated, disseminated, or severe HZ in HIV-infected patients, though prospective efficacy data remain limited.11
With regard to prevention of infection, a large randomized trial in 2016 found that acyclovir prophylaxis resulted in a 68% reduction in HZ over 2 years among HIV patients.12 Despite data that acyclovir may be effective for this purpose, long-term antiviral prophylaxis is not routinely recommended for HZ,11,13 as it has been linked to rare cases of acyclovir-resistant HZ in HIV patients.14,15 However, antiviral prophylaxis against HSV type 2 reactivation in HIV patients also confers protection against VZV reactivation.11,12
Solid Organ Transplantation
Localized, mild to moderately severe dermatomal HZ can be treated with oral acyclovir, valacyclovir, or famciclovir. As in HIV patients, SOT patients with severe, disseminated, or complicated HZ should receive IV acyclovir.11 In the first 3 to 6 months following the procedure, SOT patients receive cytomegalovirus prophylaxis with ganciclovir or valgan-ciclovir, which also provides protection against HZ.13-18 For patients not receiving cytomegalovirus prophylaxis, HSV prophylaxis with oral acyclovir or valacyclovir is given for at least the first month after transplantation, which also confers protection against HZ.16,19 Antiviral therapy is critical during the early posttransplantation period when patients are most severely immunosuppressed and thus have the highest risk for VZV-associated complications.20 Although immunosuppression is lifelong in most SOT recipients, there is insufficient evidence for extending prophylaxis beyond 6 months.16,21
As a possible risk factor for HZ,22 MMF use is another consideration among SOT patients, similar to patient 2 in our report. A 2003 observational study supported withdrawal of MMF therapy during active VZV infection due to clinical observation of an association with HZ.23 However, a multicenter, randomized, controlled trial reported no cases of HZ in renal transplant recipients on MMF.24 Additionally, MMF has been observed to enhance the antiviral activity of acyclovir, at least in vitro.25 Given the lack of evidence of MMF as a risk factor for HZ, there is insufficient evidence for cessation of use during VZVreactivation in SOT patients.
Hematopoietic Stem Cell Transplantation
The preferred agents for treatment of localized mild dermatomal HZ are oral acyclovir or valacyclovir, as data on the safety and efficacy of famciclovir among HSCT recipients are limited.13,26 Patients should receive antiviral prophylaxis with one of these agents during the first year following allogeneic or autologous HSCT. This 1-year course has proven highly effective in reducing HZ in the first year following transplantation when most severe cases occur,21,26-29 and it has been associated with a persistently decreased risk for HZ even after discontinuation.21 Prophylaxis may be continued beyond 1 year in allogeneic HSCT recipients experiencing graft-versus-host disease who should receive acyclovir until 6 months after the end of immunosuppressive therapy.21,26
Vaccination remains a potential strategy to reduce the incidence of HZ in this patient population. A heat-inactivated vaccine administered within the first 3 months after the procedure has been shown to be safe among autologous and allogeneic HSCT patients.30,31 The vaccine notably reduced the incidence of HZ in patients who underwent autologous HSCT,32 but no known data are available on its clinical efficacy in allogeneic HSCT patients. Accordingly, there are no known official recommendations to date regarding vaccine use in these patient populations.26
Conclusion
It is incumbent upon clinicians to recognize the spectrum of atypical presentations of HZ and maintain a low threshold for performing appropriate diagnostic or confirmatory studies among at-risk patients with impaired immune function. Disseminated HZ can have potentially life-threatening visceral complications such as encephalitis, hepatitis, or pneumonitis.7,8 As such, an understanding of prevention and treatment modalities for VZV infection among immunocompromised patients is critical. Because the morbidity associated with complications of VZV infection is substantial and the risks associated with antiviral agents are minimal, antiviral prophylaxis is recommended for 6 months following SOT or 1 year following HSCT, and prompt treatment is warranted in cases of reasonable clinical suspicion for HZ.
Acknowledgment
The authors gratefully acknowledge the generosity of our patients in permitting photography of their skin findings for the furthering of medical education.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41:1-16.
- Nagasako EM, Johnson RW, Griffin DR, et al. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46:834-839.
- Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23-32.
- Herpes Zoster and Functional Decline Consortium. Functional decline and herpes zoster in older people: an interplay of multiple factors. Aging Clin Exp Res. 2015;27:757-765.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341-357.
- Prelog M, Schonlaub J, Jeller V, et al. Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine. 2013;31:2420-2426.
- Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
- Glesby MJ, Moore RD, Chaisson RE. Clinical spectrum of herpes zoster in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:370-375.
- Blankenship W, Herchline T, Hockley A. Asymptomatic vesicles in a patient with the acquired immunodeficiency syndrome. disseminated varicella-zoster virus (VZV) infection. Arch Dermatol. 1994;130:1193, 1196.
- Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342-348.
- Gnann JW. Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al, eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, United Kingdom: Cambridge University Press; 2007:1175-1191.
- Barnabas RV, Baeten JM, Lingappa JR, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: results of a randomized clinical trial. J Infect Dis. 2016;213:551-555.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
- Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990;112:187-191.
- Linnemann CC Jr, Biron KK, Hoppenjans WG, et al. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577-579.
- Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S108-S115.
- Preiksaitis JK, Brennan DC, Fishman J, et al. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218-227.
- Fishman JA, Doran MT, Volpicelli SA, et al. Dosing of intravenous ganciclovir for the prophylaxis and treatment of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2000;69:389-394.
- Zuckerman R, Wald A; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S104-S107.
- Arness T, Pedersen R, Dierkhising R, et al. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10:260-268.
- Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110:3071-3077.
- Rothwell WS, Gloor JM, Morgenstern BZ, et al. Disseminated varicella infection in pediatric renal transplant recipients treated with mycophenolate mofetil. Transplantation. 1999;68:158-161.
- Lauzurica R, Bayés B, Frías C, et al. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35:1758-1759.
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. TheTricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029-1037.
- Neyts J, De Clercq E. Mycophenolate mofetil strongly potentiates the anti-herpesvirus activity of acyclovir. Antiviral Res. 1998;40:53-56.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238.
- Boeckh M, Kim HW, Flowers ME, et al. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood. 2006;107:1800-1805.
- Kawamura K, Hayakawa J, Akahoshi Y, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol. 2015;102:230-237.
- Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Long-term follow-up after hematopoietic stem cell transplant general guidelines for referring physicians. Fred Hutchinson Cancer Research Center website. https://www.fredhutch.org/content/dam/public/Treatment-Suport/Long-Term-Follow-Up/physician.pdf. Published July 17, 2014. Accessed October 19, 2017.
- Kussmaul SC, Horn BN, Dvorak CC, et al. Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant. 2010;45:1602-1606.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26-34.
- Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20:285-287.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41:1-16.
- Nagasako EM, Johnson RW, Griffin DR, et al. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46:834-839.
- Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23-32.
- Herpes Zoster and Functional Decline Consortium. Functional decline and herpes zoster in older people: an interplay of multiple factors. Aging Clin Exp Res. 2015;27:757-765.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341-357.
- Prelog M, Schonlaub J, Jeller V, et al. Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine. 2013;31:2420-2426.
- Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
- Glesby MJ, Moore RD, Chaisson RE. Clinical spectrum of herpes zoster in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:370-375.
- Blankenship W, Herchline T, Hockley A. Asymptomatic vesicles in a patient with the acquired immunodeficiency syndrome. disseminated varicella-zoster virus (VZV) infection. Arch Dermatol. 1994;130:1193, 1196.
- Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342-348.
- Gnann JW. Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al, eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, United Kingdom: Cambridge University Press; 2007:1175-1191.
- Barnabas RV, Baeten JM, Lingappa JR, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: results of a randomized clinical trial. J Infect Dis. 2016;213:551-555.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
- Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990;112:187-191.
- Linnemann CC Jr, Biron KK, Hoppenjans WG, et al. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577-579.
- Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S108-S115.
- Preiksaitis JK, Brennan DC, Fishman J, et al. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218-227.
- Fishman JA, Doran MT, Volpicelli SA, et al. Dosing of intravenous ganciclovir for the prophylaxis and treatment of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2000;69:389-394.
- Zuckerman R, Wald A; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S104-S107.
- Arness T, Pedersen R, Dierkhising R, et al. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10:260-268.
- Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110:3071-3077.
- Rothwell WS, Gloor JM, Morgenstern BZ, et al. Disseminated varicella infection in pediatric renal transplant recipients treated with mycophenolate mofetil. Transplantation. 1999;68:158-161.
- Lauzurica R, Bayés B, Frías C, et al. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35:1758-1759.
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. TheTricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029-1037.
- Neyts J, De Clercq E. Mycophenolate mofetil strongly potentiates the anti-herpesvirus activity of acyclovir. Antiviral Res. 1998;40:53-56.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238.
- Boeckh M, Kim HW, Flowers ME, et al. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood. 2006;107:1800-1805.
- Kawamura K, Hayakawa J, Akahoshi Y, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol. 2015;102:230-237.
- Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Long-term follow-up after hematopoietic stem cell transplant general guidelines for referring physicians. Fred Hutchinson Cancer Research Center website. https://www.fredhutch.org/content/dam/public/Treatment-Suport/Long-Term-Follow-Up/physician.pdf. Published July 17, 2014. Accessed October 19, 2017.
- Kussmaul SC, Horn BN, Dvorak CC, et al. Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant. 2010;45:1602-1606.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26-34.
- Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20:285-287.
Practice Points
- Clinician awareness of management guidelines for the prevention and treatment of varicella-zoster virus infection in immunocompromised individuals is critical to minimize the risk for disease and associated morbidity.
- Antiviral prophylaxis is recommended for 6 months following solid organ transplantation or 1 year following hematopoietic stem cell transplantation, and prompt treatment is warranted in cases of reasonable clinical suspicion for herpes zoster.
Mycobacterium marinum Remains an Unrecognized Cause of Indolent Skin Infections
An environmental pathogen, Mycobacterium marinum can cause cutaneous infection when traumatized skin is exposed to fresh, brackish, or salt water. Fishing, aquarium cleaning, and aquatic recreational activities are risk factors for infection.1,2 Diagnosis often is delayed and is made several weeks or even months after initial symptoms appear.3 Due to the protracted clinical course, patients may not recall the initial exposure, contributing to the delay in diagnosis and initiation of appropriate treatment. It is not uncommon for patients with M marinum infection to be initially treated with antibiotics or antifungal drugs.
We present a review of 5 patients who were diagnosed with M marinum infection at our institution between January 2003 and March 2013.
Methods
This study was conducted at Henry Ford Hospital, a 900-bed tertiary care center in Detroit, Michigan. Patients who had cultures positive for M marinum between January 2003 and March 2013 were identified using the institution’s laboratory database. Medical records were reviewed, and relevant demographic, epidemiologic, and clinical data, including initial clinical presentation, alternative diagnoses, time between initial presentation and definitive diagnosis, and specific treatment, were recorded.
Results
We identified 5 patients who were diagnosed with culture-confirmed M marinum skin infections during the study period: 3 men and 2 women aged 43 to 72 years (Table 1). Two patients had diabetes mellitus and 1 had hepatitis C virus. None had classic immunosuppression. On repeated questioning after the diagnosis was established, all 5 patients reported that they kept a home aquarium, and all recalled mild trauma to the hand prior to the onset of symptoms; however, none of the patients initially linked the minor skin injury to the subsequent infection.
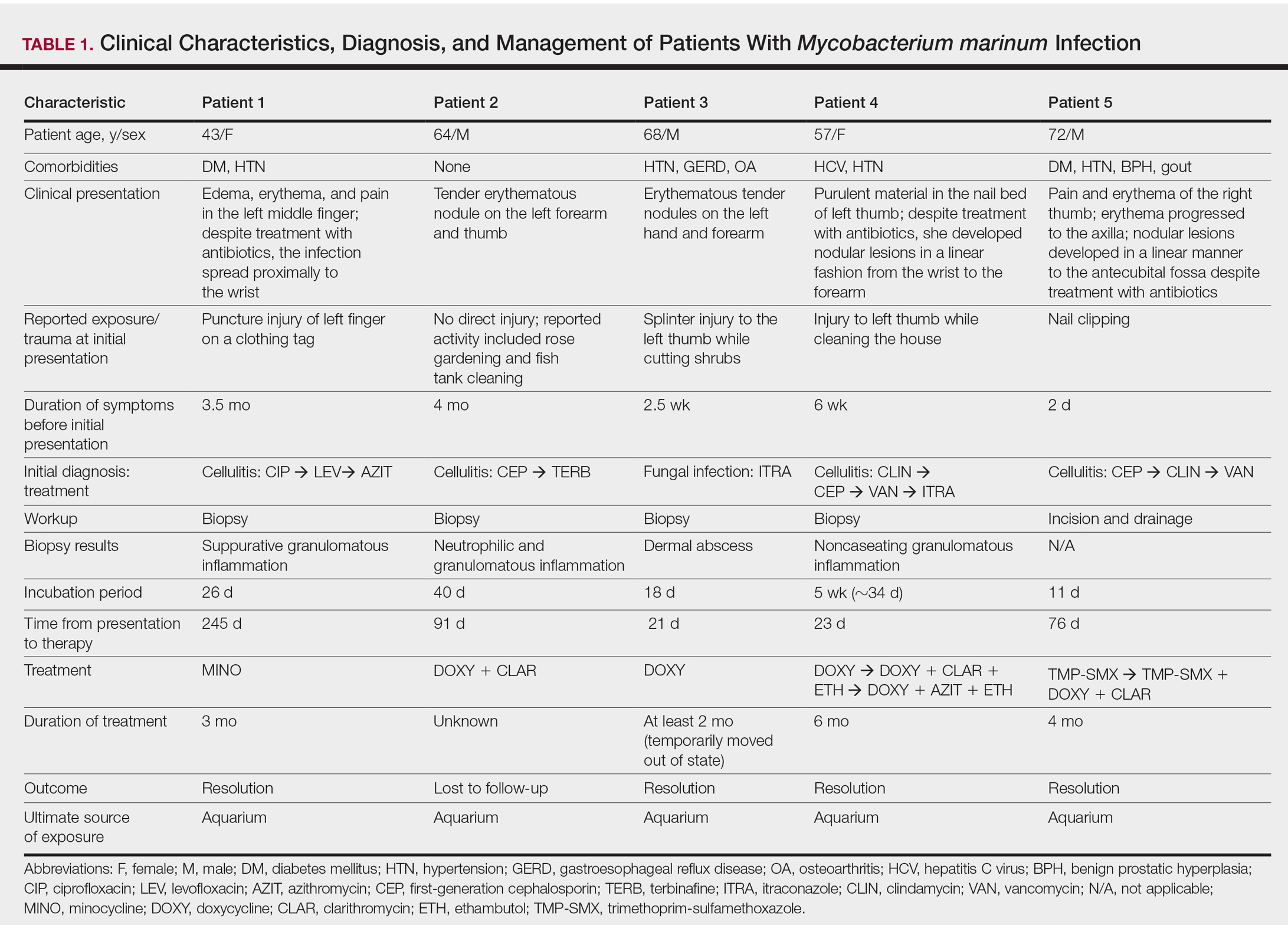
All 5 patients initially presented with erythema and swelling at the site of the injury, which evolved into inflammatory nodules that progressed proximally up to the arm despite empiric treatment with antibiotics active against streptococci and staphylococci (Figures 1 and 2). Three patients also received empiric antifungal therapy due to suspicion of sporotrichosis.
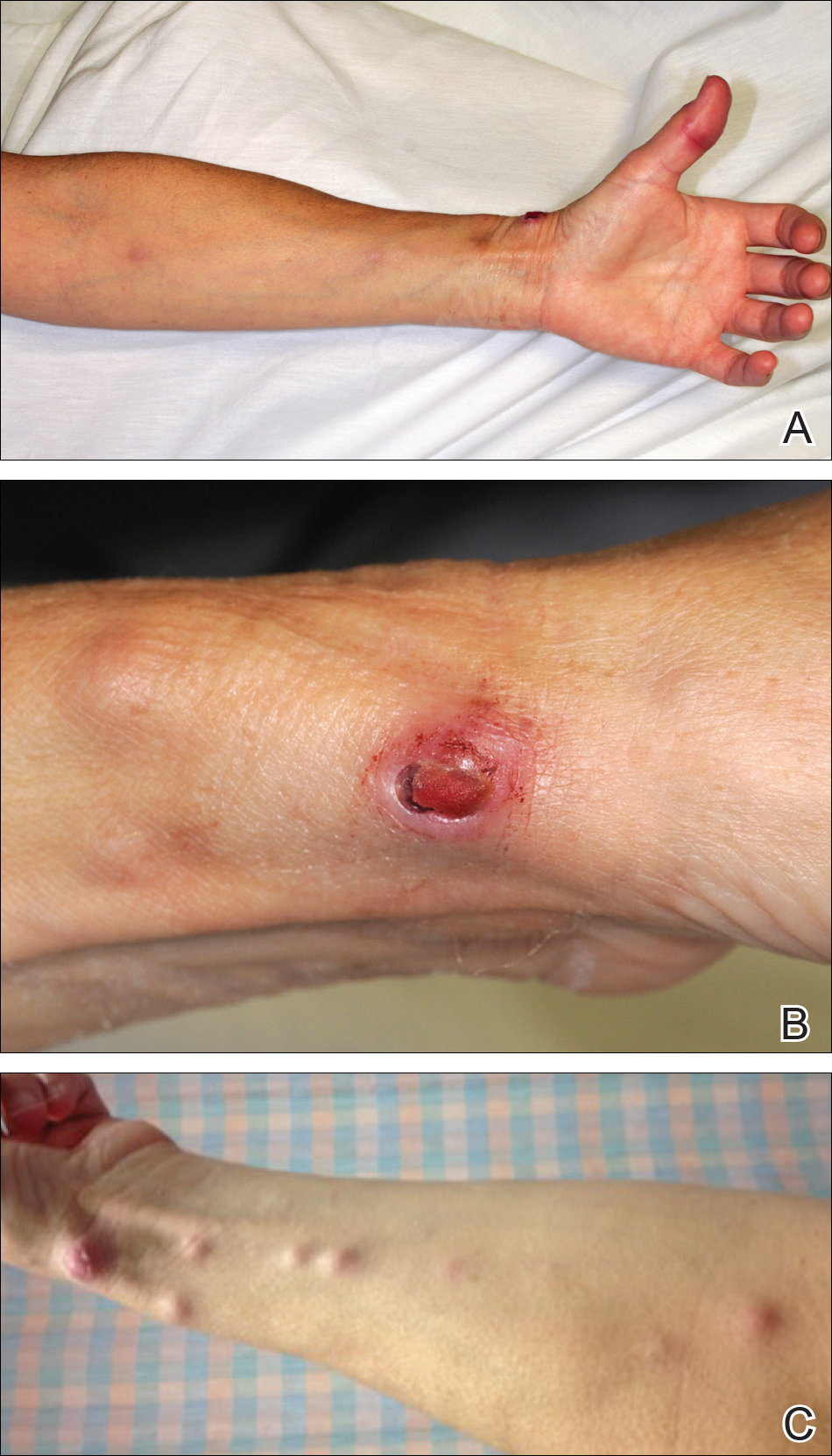
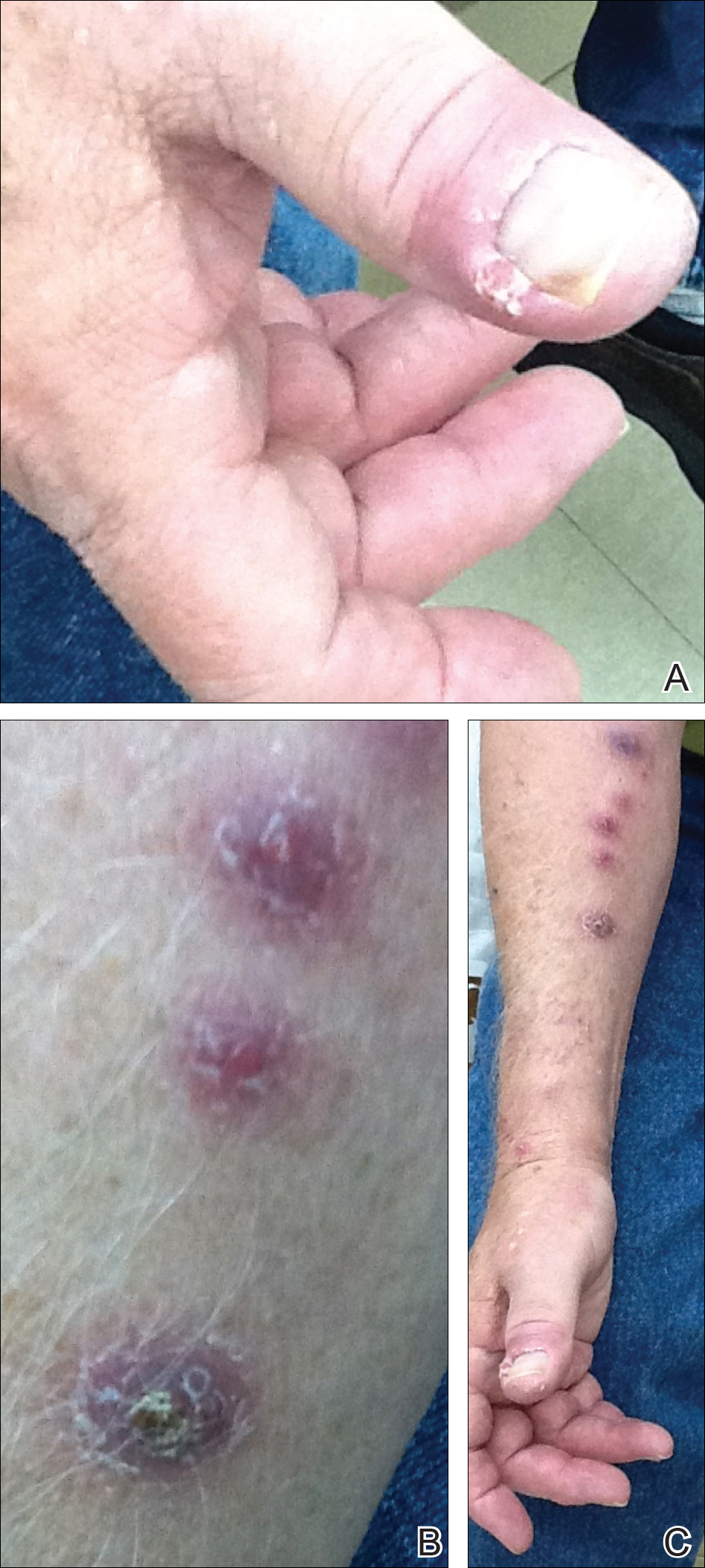
Skin biopsies were performed on 4 patients, and incision and drainage of purulent material was performed on the fifth patient. Histopathologic examination revealed granulomatous inflammation in 3 patients. Stains for acid-fast bacilli were positive in all 5 patients. Definitive diagnosis of the organism was confirmed by growth of M marinum within 11 to 40 days from the tissue in 4 patients and purulent material in the fifth patient. Susceptibility testing was performed on only 1 of the 5 isolates and showed that the organism was susceptible to amikacin, clarithromycin, doxycycline, ethambutol, rifampin, and trimethoprim-sulfamethoxazole (TMP-SMX).
The mean time from initial presentation to initiation of appropriate therapy for M marinum infection was 91 days (range, 21–245 days). Several different treatment regimens were used. All patients received either doxycycline or minocycline with or without a macrolide. Two also received other agents (TMP-SMX or ethambutol). Treatment duration varied from 2 to 6 months in 4 patients, and all 4 had complete resolution of the lesions; 1 patient was lost to follow-up.
Comment
Diagnosing the Infection
Diagnosis of M marinum infection remains problematic. In the 5 patients included in this study, the time between initial onset of symptoms and diagnosis of M marinum infection was delayed, as has been noted in other reports.4-7 Delays as long as 2 years before the diagnosis is made have been described.7 The clinical presentation of cutaneous infection with M marinum varies, which may delay diagnosis. Nodular lymphangitis is classic, but papules, pustules, ulcers, inflammatory plaques, and single nodules also can occur.1,2 Lymphadenopathy may or may not be present.4,8,9 The differential diagnosis is broad and includes infection by other nontuberculous mycobacteria such as Mycobacterium chelonae; Mycobacterium fortuitum; Nocardia species, especially Nocardia brasiliensis; Francisella tularensis; Sporothrix schenckii; and Leishmania species. It is not surprising that 4 patients in our study were initially treated for a gram-positive bacterial infection and 3 were treated for a fungal infection before the diagnosis of M marinum was made. Distinctive features that may help to differentiate these infections are summarized in Table 2.
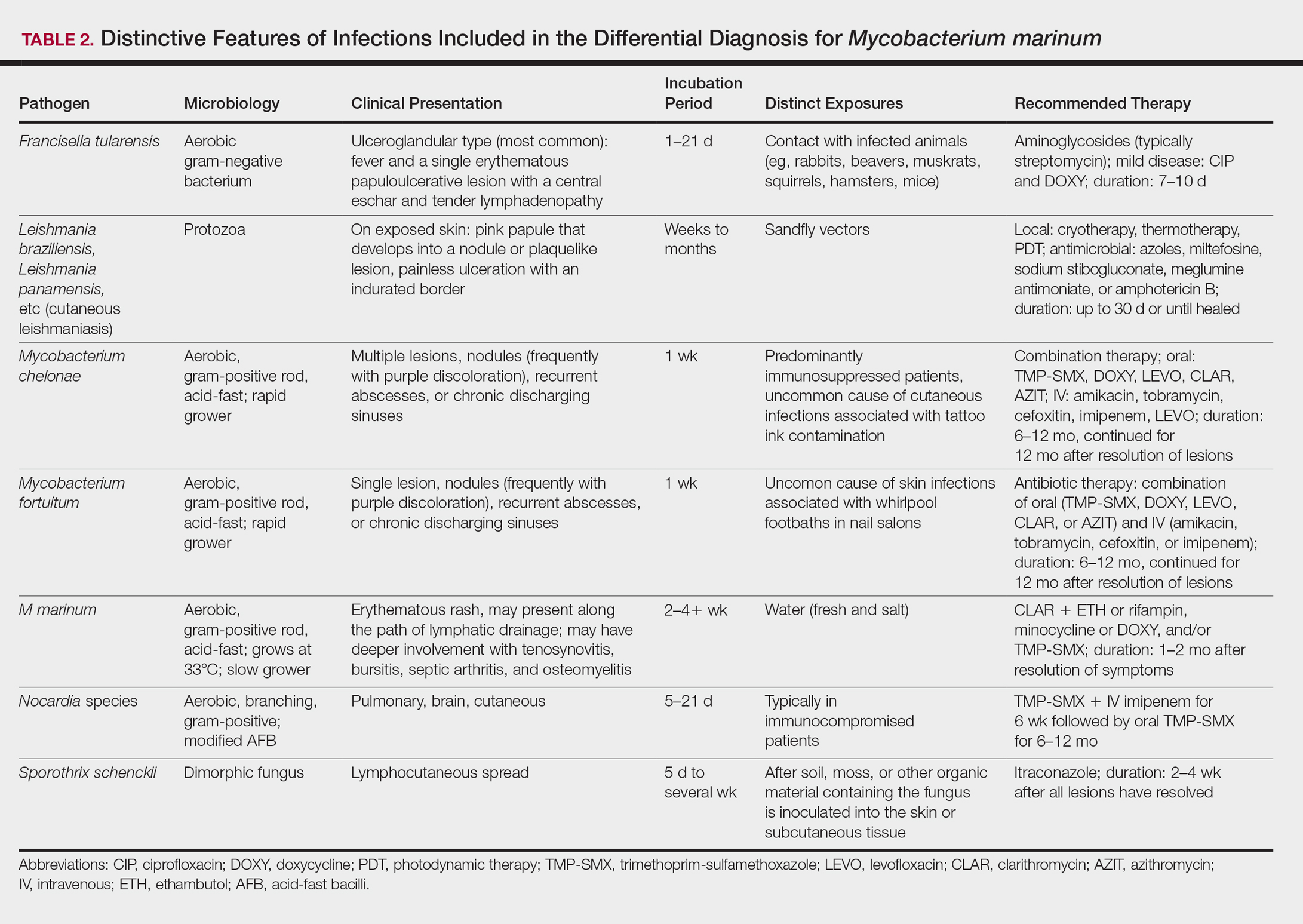
We found that the main cause of delayed diagnosis was the failure of physicians to obtain a thorough history regarding patients’ recreational activities and animal exposure. Patients often do not associate a remote aquatic exposure with their symptoms and will not volunteer this information unless directly asked.2,10 It was only after repeated questioning in all of these patients that they recounted prior trauma to the involved hand related to the aquarium.
Biopsy and Culture
Histopathologic examination of material from a biopsied lesion can give an early clue that a mycobacterial infection might be involved. Biopsy can reveal either noncaseating or necrotizing granulomas that have larger numbers of neutrophils in addition to lymphocytes and macrophages. Giant cells often are noted.5,9,11 Organisms can be seen with the use of a tissue acid-fast stain, but species cannot be differentiated by acid-fast staining.12 However, the sensitivity of acid-fast stains on biopsy material is low.3,13,14
Culture of the involved tissue is crucial for establishing the diagnosis of this infection. However, the rate of growth of M marinum is slow. Temperature requirements for incubation and delay in transporting specimens to the laboratory can lead to bacterial overgrowth, resulting in the inability to recover M marinum from the culture.13Mycobacterium marinum grows preferentially between 28°C and 32°C, and growth is limited at temperatures above 33°C.13,15,16 As illustrated in the cases presented, recovery of the organism may not be accomplished from the first culture performed, and additional biopsy material for culture may be needed. Liquid media generally is more sensitive and produces more rapid results than solid media (eg, Löwenstein-Jensen, Middlebrook 7H10/7H11 agar). However, solid media carry the advantage of allowing observation of morphology and estimation of the number of organisms.12,17
Rapid Detection
Advancements in molecular methods have allowed for more definitive and rapid identification of M marinum, substantially reducing the delay in diagnosis. Commercial molecular assays utilize in-solution hybridization or solid-format reverse-hybridization assays to allow mycobacterial detection as soon as growth appears.18 Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry can substantially shorten the time to species identification.19,20 Nonculture-based tests that have been developed for the rapid detection of M marinum infection include polymerase chain reaction-restriction fragment length polymorphism and polymerase chain reaction amplification of the 16S RNA gene.21 It should be noted, however, that M marinum and Mycobacterium ulcerans have a very homologous 16S ribosomal RNA gene sequence, differing by only 1 nucleotide; thus, distinguishing between M marinum and M ulcerans using this method may be challenging.22,23
Management
Treatment depends on the extent of the disease. Generally, localized cutaneous disease can be treated with monotherapy with agents such as doxycycline, clarithromycin, or TMP-SMX. Extensive disease typically requires a combination of 2 antimycobacterial agents, typically clarithromycin-rifampin, clarithromycin-ethambutol, or rifampin-ethambutol.12 Amikacin has been used in combination with other agents such as rifampin and clarithromycin in refractory cases.22,24 The use of ciprofloxacin is not encouraged because some isolates are resistant; however, other fluoroquinolones, such as moxifloxacin, may be options for combination therapy. Isoniazid, pyrazinamide, and streptomycin are not effective to treat M marinum.
Susceptibility testing of M marinum usually is performed to guide antimicrobial therapy in cases of poor clinical response or intolerance to first-line antimicrobials such as macrolides.25 The likelihood of M marinum developing resistance to the agents used for treatment appears to be low. Unfortunately, in vitro antimicrobial susceptibility tests do not correlate well with treatment efficiency.10
The duration of therapy is not standardized but usually is 5 to 6 months,7,10,26 with therapy often continuing 1 to 2 months after lesions appear to have resolved.12 However, in some cases (usually those who have more extensive disease), therapy has been extended to as long as 1 to 2 years.10 The ideal length of therapy in immunocompromised individuals has not been established27; however, a treatment duration of 6 to 9 months was reported in one study.28 Surgical debridement may be necessary in some patients who have involvement of deep structures of the hand or knee, those with persistent pain, or those who fail to respond to a prolonged period of medical therapy.29 Successful use of less conventional therapeutic approaches, including cryotherapy, radiation therapy, electrodesiccation, photodynamic therapy, curettage, and local hyperthermic therapy has been reported.30-32
Conclusion
Diagnosis and management of M marinum infection is difficult. Patients presenting with indolent nodular skin infections affecting the upper extremities should be asked about aquatic exposure. Tissue biopsy for histopathologic examination and culture is essential to establish an early diagnosis and promptly initiate appropriate therapy.
Acknowledgment
We would like to thank Carol A. Kauffman, MD (Ann Arbor, Michigan), for her thoughtful comments that greatly improved this manuscript.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390-397.
- Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis. 2000;31:439-443.
- Edelstein H. Mycobacterium marinum skin infections. report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359-1364.
- Janik JP, Bang RH, Palmer CH. Case reports: successful treatment of Mycobacterium marinum infection with minocycline after complication of disease by delayed diagnosis and systemic steroids. J Drugs Dermatol. 2005;4:621-624.
- Jolly HW Jr, Seabury JH. Infections with Myocbacterium marinum. Arch Dermatol. 1972;106:32-36.
- Sette CS, Wachholz PA, Masuda PY, et al. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Incl Trop Dis. 2015;21:7.
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662.
- Eberst E, Dereure O, Guillot B, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:E15-E16.
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma. a study of 290 cases. Arch Dermatol. 1963;88:158-162.
- Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-1752.
- Feng Y, Xu H, Wang H, et al. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71:267-272.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343-347.
- Wu TS, Chiu CH, Yang CH, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296.
- Ho WL, Chuang WY, Kuo AJ, et al. Nasal fish tank granuloma: an uncommon cause for epistaxis. Am J Trop Med Hyg. 2011;85:195-196.
- Dobos KM, Quinn FD, Ashford DA, et al. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg Infect Dis. 1999;5:367-378.
- van Ingen J. Diagnosis of non-tuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109.
- Piersimoni C, Scarparo C. Extrapulmonary infections associated with non-tuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351-1358; quiz 1544.
- Saleeb PG, Drake SK, Murray PR, et al. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790-1794.
- Adams LL, Salee P, Dionne K, et al. A novel protein extraction method for identification of mycobacteria using MALDI-ToF MS. J Microbiol Methods. 2015;119:1-3.
- Posteraro B, Sanguinetti M, Garcovich A, et al. Polymerase chain reaction-reverse cross-blot hybridization assay in the diagnosis of sporotrichoid Mycobacterium marinum infection. Br J Dermatol. 1998;139:872-876.
- Lau SK, Curreem SO, Ngan AH, et al. First report of disseminated Mycobacterium skin infections in two liver transplant recipients and rapid diagnosis by hsp65 gene sequencing. J Clin Microbiol. 2011;49:3733-3738.
- Hofer M, Hirschel B, Kirschner P, et al. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med. 1993;328:1007-1009.
- Huang Y, Xu X, Liu Y, et al. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clin Interv Aging. 2012;7:533-538.
- Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000;31:1209-1215.
- Balaqué N, Uçkay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main. 2015;34:18-23.
- Pandian TK, Deziel PJ, Otley CC, et al. Mycobacterium marinum infections in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2008;10:358-363.
- Jacobs S, George A, Papanicolaou GA, et al. Disseminated Mycobacterium marinum infection in a hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:410-414.
- Chow SP, Ip FK, Lau JH, et al. Mycobacterium marinum infection of the hand and wrist. results of conservative treatment in twenty-four cases. J Bone Joint Surg Am. 1987;69:1161-1168.
- Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978.
- Nenoff P, Klapper BM, Mayser P, et al. Infections due to Mycobacterium marinum: a review. Hautarzt. 2011;62:266-271.
- Prevost E, Walker EM Jr, Kreutner A Jr, et al. Mycobacterium marinum infections: diagnosis and treatment. South Med J. 1982;75:1349-1352.
An environmental pathogen, Mycobacterium marinum can cause cutaneous infection when traumatized skin is exposed to fresh, brackish, or salt water. Fishing, aquarium cleaning, and aquatic recreational activities are risk factors for infection.1,2 Diagnosis often is delayed and is made several weeks or even months after initial symptoms appear.3 Due to the protracted clinical course, patients may not recall the initial exposure, contributing to the delay in diagnosis and initiation of appropriate treatment. It is not uncommon for patients with M marinum infection to be initially treated with antibiotics or antifungal drugs.
We present a review of 5 patients who were diagnosed with M marinum infection at our institution between January 2003 and March 2013.
Methods
This study was conducted at Henry Ford Hospital, a 900-bed tertiary care center in Detroit, Michigan. Patients who had cultures positive for M marinum between January 2003 and March 2013 were identified using the institution’s laboratory database. Medical records were reviewed, and relevant demographic, epidemiologic, and clinical data, including initial clinical presentation, alternative diagnoses, time between initial presentation and definitive diagnosis, and specific treatment, were recorded.
Results
We identified 5 patients who were diagnosed with culture-confirmed M marinum skin infections during the study period: 3 men and 2 women aged 43 to 72 years (Table 1). Two patients had diabetes mellitus and 1 had hepatitis C virus. None had classic immunosuppression. On repeated questioning after the diagnosis was established, all 5 patients reported that they kept a home aquarium, and all recalled mild trauma to the hand prior to the onset of symptoms; however, none of the patients initially linked the minor skin injury to the subsequent infection.

All 5 patients initially presented with erythema and swelling at the site of the injury, which evolved into inflammatory nodules that progressed proximally up to the arm despite empiric treatment with antibiotics active against streptococci and staphylococci (Figures 1 and 2). Three patients also received empiric antifungal therapy due to suspicion of sporotrichosis.


Skin biopsies were performed on 4 patients, and incision and drainage of purulent material was performed on the fifth patient. Histopathologic examination revealed granulomatous inflammation in 3 patients. Stains for acid-fast bacilli were positive in all 5 patients. Definitive diagnosis of the organism was confirmed by growth of M marinum within 11 to 40 days from the tissue in 4 patients and purulent material in the fifth patient. Susceptibility testing was performed on only 1 of the 5 isolates and showed that the organism was susceptible to amikacin, clarithromycin, doxycycline, ethambutol, rifampin, and trimethoprim-sulfamethoxazole (TMP-SMX).
The mean time from initial presentation to initiation of appropriate therapy for M marinum infection was 91 days (range, 21–245 days). Several different treatment regimens were used. All patients received either doxycycline or minocycline with or without a macrolide. Two also received other agents (TMP-SMX or ethambutol). Treatment duration varied from 2 to 6 months in 4 patients, and all 4 had complete resolution of the lesions; 1 patient was lost to follow-up.
Comment
Diagnosing the Infection
Diagnosis of M marinum infection remains problematic. In the 5 patients included in this study, the time between initial onset of symptoms and diagnosis of M marinum infection was delayed, as has been noted in other reports.4-7 Delays as long as 2 years before the diagnosis is made have been described.7 The clinical presentation of cutaneous infection with M marinum varies, which may delay diagnosis. Nodular lymphangitis is classic, but papules, pustules, ulcers, inflammatory plaques, and single nodules also can occur.1,2 Lymphadenopathy may or may not be present.4,8,9 The differential diagnosis is broad and includes infection by other nontuberculous mycobacteria such as Mycobacterium chelonae; Mycobacterium fortuitum; Nocardia species, especially Nocardia brasiliensis; Francisella tularensis; Sporothrix schenckii; and Leishmania species. It is not surprising that 4 patients in our study were initially treated for a gram-positive bacterial infection and 3 were treated for a fungal infection before the diagnosis of M marinum was made. Distinctive features that may help to differentiate these infections are summarized in Table 2.

We found that the main cause of delayed diagnosis was the failure of physicians to obtain a thorough history regarding patients’ recreational activities and animal exposure. Patients often do not associate a remote aquatic exposure with their symptoms and will not volunteer this information unless directly asked.2,10 It was only after repeated questioning in all of these patients that they recounted prior trauma to the involved hand related to the aquarium.
Biopsy and Culture
Histopathologic examination of material from a biopsied lesion can give an early clue that a mycobacterial infection might be involved. Biopsy can reveal either noncaseating or necrotizing granulomas that have larger numbers of neutrophils in addition to lymphocytes and macrophages. Giant cells often are noted.5,9,11 Organisms can be seen with the use of a tissue acid-fast stain, but species cannot be differentiated by acid-fast staining.12 However, the sensitivity of acid-fast stains on biopsy material is low.3,13,14
Culture of the involved tissue is crucial for establishing the diagnosis of this infection. However, the rate of growth of M marinum is slow. Temperature requirements for incubation and delay in transporting specimens to the laboratory can lead to bacterial overgrowth, resulting in the inability to recover M marinum from the culture.13Mycobacterium marinum grows preferentially between 28°C and 32°C, and growth is limited at temperatures above 33°C.13,15,16 As illustrated in the cases presented, recovery of the organism may not be accomplished from the first culture performed, and additional biopsy material for culture may be needed. Liquid media generally is more sensitive and produces more rapid results than solid media (eg, Löwenstein-Jensen, Middlebrook 7H10/7H11 agar). However, solid media carry the advantage of allowing observation of morphology and estimation of the number of organisms.12,17
Rapid Detection
Advancements in molecular methods have allowed for more definitive and rapid identification of M marinum, substantially reducing the delay in diagnosis. Commercial molecular assays utilize in-solution hybridization or solid-format reverse-hybridization assays to allow mycobacterial detection as soon as growth appears.18 Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry can substantially shorten the time to species identification.19,20 Nonculture-based tests that have been developed for the rapid detection of M marinum infection include polymerase chain reaction-restriction fragment length polymorphism and polymerase chain reaction amplification of the 16S RNA gene.21 It should be noted, however, that M marinum and Mycobacterium ulcerans have a very homologous 16S ribosomal RNA gene sequence, differing by only 1 nucleotide; thus, distinguishing between M marinum and M ulcerans using this method may be challenging.22,23
Management
Treatment depends on the extent of the disease. Generally, localized cutaneous disease can be treated with monotherapy with agents such as doxycycline, clarithromycin, or TMP-SMX. Extensive disease typically requires a combination of 2 antimycobacterial agents, typically clarithromycin-rifampin, clarithromycin-ethambutol, or rifampin-ethambutol.12 Amikacin has been used in combination with other agents such as rifampin and clarithromycin in refractory cases.22,24 The use of ciprofloxacin is not encouraged because some isolates are resistant; however, other fluoroquinolones, such as moxifloxacin, may be options for combination therapy. Isoniazid, pyrazinamide, and streptomycin are not effective to treat M marinum.
Susceptibility testing of M marinum usually is performed to guide antimicrobial therapy in cases of poor clinical response or intolerance to first-line antimicrobials such as macrolides.25 The likelihood of M marinum developing resistance to the agents used for treatment appears to be low. Unfortunately, in vitro antimicrobial susceptibility tests do not correlate well with treatment efficiency.10
The duration of therapy is not standardized but usually is 5 to 6 months,7,10,26 with therapy often continuing 1 to 2 months after lesions appear to have resolved.12 However, in some cases (usually those who have more extensive disease), therapy has been extended to as long as 1 to 2 years.10 The ideal length of therapy in immunocompromised individuals has not been established27; however, a treatment duration of 6 to 9 months was reported in one study.28 Surgical debridement may be necessary in some patients who have involvement of deep structures of the hand or knee, those with persistent pain, or those who fail to respond to a prolonged period of medical therapy.29 Successful use of less conventional therapeutic approaches, including cryotherapy, radiation therapy, electrodesiccation, photodynamic therapy, curettage, and local hyperthermic therapy has been reported.30-32
Conclusion
Diagnosis and management of M marinum infection is difficult. Patients presenting with indolent nodular skin infections affecting the upper extremities should be asked about aquatic exposure. Tissue biopsy for histopathologic examination and culture is essential to establish an early diagnosis and promptly initiate appropriate therapy.
Acknowledgment
We would like to thank Carol A. Kauffman, MD (Ann Arbor, Michigan), for her thoughtful comments that greatly improved this manuscript.
An environmental pathogen, Mycobacterium marinum can cause cutaneous infection when traumatized skin is exposed to fresh, brackish, or salt water. Fishing, aquarium cleaning, and aquatic recreational activities are risk factors for infection.1,2 Diagnosis often is delayed and is made several weeks or even months after initial symptoms appear.3 Due to the protracted clinical course, patients may not recall the initial exposure, contributing to the delay in diagnosis and initiation of appropriate treatment. It is not uncommon for patients with M marinum infection to be initially treated with antibiotics or antifungal drugs.
We present a review of 5 patients who were diagnosed with M marinum infection at our institution between January 2003 and March 2013.
Methods
This study was conducted at Henry Ford Hospital, a 900-bed tertiary care center in Detroit, Michigan. Patients who had cultures positive for M marinum between January 2003 and March 2013 were identified using the institution’s laboratory database. Medical records were reviewed, and relevant demographic, epidemiologic, and clinical data, including initial clinical presentation, alternative diagnoses, time between initial presentation and definitive diagnosis, and specific treatment, were recorded.
Results
We identified 5 patients who were diagnosed with culture-confirmed M marinum skin infections during the study period: 3 men and 2 women aged 43 to 72 years (Table 1). Two patients had diabetes mellitus and 1 had hepatitis C virus. None had classic immunosuppression. On repeated questioning after the diagnosis was established, all 5 patients reported that they kept a home aquarium, and all recalled mild trauma to the hand prior to the onset of symptoms; however, none of the patients initially linked the minor skin injury to the subsequent infection.

All 5 patients initially presented with erythema and swelling at the site of the injury, which evolved into inflammatory nodules that progressed proximally up to the arm despite empiric treatment with antibiotics active against streptococci and staphylococci (Figures 1 and 2). Three patients also received empiric antifungal therapy due to suspicion of sporotrichosis.


Skin biopsies were performed on 4 patients, and incision and drainage of purulent material was performed on the fifth patient. Histopathologic examination revealed granulomatous inflammation in 3 patients. Stains for acid-fast bacilli were positive in all 5 patients. Definitive diagnosis of the organism was confirmed by growth of M marinum within 11 to 40 days from the tissue in 4 patients and purulent material in the fifth patient. Susceptibility testing was performed on only 1 of the 5 isolates and showed that the organism was susceptible to amikacin, clarithromycin, doxycycline, ethambutol, rifampin, and trimethoprim-sulfamethoxazole (TMP-SMX).
The mean time from initial presentation to initiation of appropriate therapy for M marinum infection was 91 days (range, 21–245 days). Several different treatment regimens were used. All patients received either doxycycline or minocycline with or without a macrolide. Two also received other agents (TMP-SMX or ethambutol). Treatment duration varied from 2 to 6 months in 4 patients, and all 4 had complete resolution of the lesions; 1 patient was lost to follow-up.
Comment
Diagnosing the Infection
Diagnosis of M marinum infection remains problematic. In the 5 patients included in this study, the time between initial onset of symptoms and diagnosis of M marinum infection was delayed, as has been noted in other reports.4-7 Delays as long as 2 years before the diagnosis is made have been described.7 The clinical presentation of cutaneous infection with M marinum varies, which may delay diagnosis. Nodular lymphangitis is classic, but papules, pustules, ulcers, inflammatory plaques, and single nodules also can occur.1,2 Lymphadenopathy may or may not be present.4,8,9 The differential diagnosis is broad and includes infection by other nontuberculous mycobacteria such as Mycobacterium chelonae; Mycobacterium fortuitum; Nocardia species, especially Nocardia brasiliensis; Francisella tularensis; Sporothrix schenckii; and Leishmania species. It is not surprising that 4 patients in our study were initially treated for a gram-positive bacterial infection and 3 were treated for a fungal infection before the diagnosis of M marinum was made. Distinctive features that may help to differentiate these infections are summarized in Table 2.

We found that the main cause of delayed diagnosis was the failure of physicians to obtain a thorough history regarding patients’ recreational activities and animal exposure. Patients often do not associate a remote aquatic exposure with their symptoms and will not volunteer this information unless directly asked.2,10 It was only after repeated questioning in all of these patients that they recounted prior trauma to the involved hand related to the aquarium.
Biopsy and Culture
Histopathologic examination of material from a biopsied lesion can give an early clue that a mycobacterial infection might be involved. Biopsy can reveal either noncaseating or necrotizing granulomas that have larger numbers of neutrophils in addition to lymphocytes and macrophages. Giant cells often are noted.5,9,11 Organisms can be seen with the use of a tissue acid-fast stain, but species cannot be differentiated by acid-fast staining.12 However, the sensitivity of acid-fast stains on biopsy material is low.3,13,14
Culture of the involved tissue is crucial for establishing the diagnosis of this infection. However, the rate of growth of M marinum is slow. Temperature requirements for incubation and delay in transporting specimens to the laboratory can lead to bacterial overgrowth, resulting in the inability to recover M marinum from the culture.13Mycobacterium marinum grows preferentially between 28°C and 32°C, and growth is limited at temperatures above 33°C.13,15,16 As illustrated in the cases presented, recovery of the organism may not be accomplished from the first culture performed, and additional biopsy material for culture may be needed. Liquid media generally is more sensitive and produces more rapid results than solid media (eg, Löwenstein-Jensen, Middlebrook 7H10/7H11 agar). However, solid media carry the advantage of allowing observation of morphology and estimation of the number of organisms.12,17
Rapid Detection
Advancements in molecular methods have allowed for more definitive and rapid identification of M marinum, substantially reducing the delay in diagnosis. Commercial molecular assays utilize in-solution hybridization or solid-format reverse-hybridization assays to allow mycobacterial detection as soon as growth appears.18 Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry can substantially shorten the time to species identification.19,20 Nonculture-based tests that have been developed for the rapid detection of M marinum infection include polymerase chain reaction-restriction fragment length polymorphism and polymerase chain reaction amplification of the 16S RNA gene.21 It should be noted, however, that M marinum and Mycobacterium ulcerans have a very homologous 16S ribosomal RNA gene sequence, differing by only 1 nucleotide; thus, distinguishing between M marinum and M ulcerans using this method may be challenging.22,23
Management
Treatment depends on the extent of the disease. Generally, localized cutaneous disease can be treated with monotherapy with agents such as doxycycline, clarithromycin, or TMP-SMX. Extensive disease typically requires a combination of 2 antimycobacterial agents, typically clarithromycin-rifampin, clarithromycin-ethambutol, or rifampin-ethambutol.12 Amikacin has been used in combination with other agents such as rifampin and clarithromycin in refractory cases.22,24 The use of ciprofloxacin is not encouraged because some isolates are resistant; however, other fluoroquinolones, such as moxifloxacin, may be options for combination therapy. Isoniazid, pyrazinamide, and streptomycin are not effective to treat M marinum.
Susceptibility testing of M marinum usually is performed to guide antimicrobial therapy in cases of poor clinical response or intolerance to first-line antimicrobials such as macrolides.25 The likelihood of M marinum developing resistance to the agents used for treatment appears to be low. Unfortunately, in vitro antimicrobial susceptibility tests do not correlate well with treatment efficiency.10
The duration of therapy is not standardized but usually is 5 to 6 months,7,10,26 with therapy often continuing 1 to 2 months after lesions appear to have resolved.12 However, in some cases (usually those who have more extensive disease), therapy has been extended to as long as 1 to 2 years.10 The ideal length of therapy in immunocompromised individuals has not been established27; however, a treatment duration of 6 to 9 months was reported in one study.28 Surgical debridement may be necessary in some patients who have involvement of deep structures of the hand or knee, those with persistent pain, or those who fail to respond to a prolonged period of medical therapy.29 Successful use of less conventional therapeutic approaches, including cryotherapy, radiation therapy, electrodesiccation, photodynamic therapy, curettage, and local hyperthermic therapy has been reported.30-32
Conclusion
Diagnosis and management of M marinum infection is difficult. Patients presenting with indolent nodular skin infections affecting the upper extremities should be asked about aquatic exposure. Tissue biopsy for histopathologic examination and culture is essential to establish an early diagnosis and promptly initiate appropriate therapy.
Acknowledgment
We would like to thank Carol A. Kauffman, MD (Ann Arbor, Michigan), for her thoughtful comments that greatly improved this manuscript.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390-397.
- Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis. 2000;31:439-443.
- Edelstein H. Mycobacterium marinum skin infections. report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359-1364.
- Janik JP, Bang RH, Palmer CH. Case reports: successful treatment of Mycobacterium marinum infection with minocycline after complication of disease by delayed diagnosis and systemic steroids. J Drugs Dermatol. 2005;4:621-624.
- Jolly HW Jr, Seabury JH. Infections with Myocbacterium marinum. Arch Dermatol. 1972;106:32-36.
- Sette CS, Wachholz PA, Masuda PY, et al. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Incl Trop Dis. 2015;21:7.
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662.
- Eberst E, Dereure O, Guillot B, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:E15-E16.
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma. a study of 290 cases. Arch Dermatol. 1963;88:158-162.
- Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-1752.
- Feng Y, Xu H, Wang H, et al. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71:267-272.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343-347.
- Wu TS, Chiu CH, Yang CH, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296.
- Ho WL, Chuang WY, Kuo AJ, et al. Nasal fish tank granuloma: an uncommon cause for epistaxis. Am J Trop Med Hyg. 2011;85:195-196.
- Dobos KM, Quinn FD, Ashford DA, et al. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg Infect Dis. 1999;5:367-378.
- van Ingen J. Diagnosis of non-tuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109.
- Piersimoni C, Scarparo C. Extrapulmonary infections associated with non-tuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351-1358; quiz 1544.
- Saleeb PG, Drake SK, Murray PR, et al. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790-1794.
- Adams LL, Salee P, Dionne K, et al. A novel protein extraction method for identification of mycobacteria using MALDI-ToF MS. J Microbiol Methods. 2015;119:1-3.
- Posteraro B, Sanguinetti M, Garcovich A, et al. Polymerase chain reaction-reverse cross-blot hybridization assay in the diagnosis of sporotrichoid Mycobacterium marinum infection. Br J Dermatol. 1998;139:872-876.
- Lau SK, Curreem SO, Ngan AH, et al. First report of disseminated Mycobacterium skin infections in two liver transplant recipients and rapid diagnosis by hsp65 gene sequencing. J Clin Microbiol. 2011;49:3733-3738.
- Hofer M, Hirschel B, Kirschner P, et al. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med. 1993;328:1007-1009.
- Huang Y, Xu X, Liu Y, et al. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clin Interv Aging. 2012;7:533-538.
- Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000;31:1209-1215.
- Balaqué N, Uçkay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main. 2015;34:18-23.
- Pandian TK, Deziel PJ, Otley CC, et al. Mycobacterium marinum infections in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2008;10:358-363.
- Jacobs S, George A, Papanicolaou GA, et al. Disseminated Mycobacterium marinum infection in a hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:410-414.
- Chow SP, Ip FK, Lau JH, et al. Mycobacterium marinum infection of the hand and wrist. results of conservative treatment in twenty-four cases. J Bone Joint Surg Am. 1987;69:1161-1168.
- Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978.
- Nenoff P, Klapper BM, Mayser P, et al. Infections due to Mycobacterium marinum: a review. Hautarzt. 2011;62:266-271.
- Prevost E, Walker EM Jr, Kreutner A Jr, et al. Mycobacterium marinum infections: diagnosis and treatment. South Med J. 1982;75:1349-1352.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390-397.
- Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis. 2000;31:439-443.
- Edelstein H. Mycobacterium marinum skin infections. report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359-1364.
- Janik JP, Bang RH, Palmer CH. Case reports: successful treatment of Mycobacterium marinum infection with minocycline after complication of disease by delayed diagnosis and systemic steroids. J Drugs Dermatol. 2005;4:621-624.
- Jolly HW Jr, Seabury JH. Infections with Myocbacterium marinum. Arch Dermatol. 1972;106:32-36.
- Sette CS, Wachholz PA, Masuda PY, et al. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Incl Trop Dis. 2015;21:7.
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662.
- Eberst E, Dereure O, Guillot B, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:E15-E16.
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma. a study of 290 cases. Arch Dermatol. 1963;88:158-162.
- Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-1752.
- Feng Y, Xu H, Wang H, et al. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71:267-272.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343-347.
- Wu TS, Chiu CH, Yang CH, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296.
- Ho WL, Chuang WY, Kuo AJ, et al. Nasal fish tank granuloma: an uncommon cause for epistaxis. Am J Trop Med Hyg. 2011;85:195-196.
- Dobos KM, Quinn FD, Ashford DA, et al. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg Infect Dis. 1999;5:367-378.
- van Ingen J. Diagnosis of non-tuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109.
- Piersimoni C, Scarparo C. Extrapulmonary infections associated with non-tuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351-1358; quiz 1544.
- Saleeb PG, Drake SK, Murray PR, et al. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790-1794.
- Adams LL, Salee P, Dionne K, et al. A novel protein extraction method for identification of mycobacteria using MALDI-ToF MS. J Microbiol Methods. 2015;119:1-3.
- Posteraro B, Sanguinetti M, Garcovich A, et al. Polymerase chain reaction-reverse cross-blot hybridization assay in the diagnosis of sporotrichoid Mycobacterium marinum infection. Br J Dermatol. 1998;139:872-876.
- Lau SK, Curreem SO, Ngan AH, et al. First report of disseminated Mycobacterium skin infections in two liver transplant recipients and rapid diagnosis by hsp65 gene sequencing. J Clin Microbiol. 2011;49:3733-3738.
- Hofer M, Hirschel B, Kirschner P, et al. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med. 1993;328:1007-1009.
- Huang Y, Xu X, Liu Y, et al. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clin Interv Aging. 2012;7:533-538.
- Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000;31:1209-1215.
- Balaqué N, Uçkay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main. 2015;34:18-23.
- Pandian TK, Deziel PJ, Otley CC, et al. Mycobacterium marinum infections in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2008;10:358-363.
- Jacobs S, George A, Papanicolaou GA, et al. Disseminated Mycobacterium marinum infection in a hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:410-414.
- Chow SP, Ip FK, Lau JH, et al. Mycobacterium marinum infection of the hand and wrist. results of conservative treatment in twenty-four cases. J Bone Joint Surg Am. 1987;69:1161-1168.
- Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978.
- Nenoff P, Klapper BM, Mayser P, et al. Infections due to Mycobacterium marinum: a review. Hautarzt. 2011;62:266-271.
- Prevost E, Walker EM Jr, Kreutner A Jr, et al. Mycobacterium marinum infections: diagnosis and treatment. South Med J. 1982;75:1349-1352.
Practice Points
- Mycobacterium marinum infection should be suspected in patients with skin/soft tissue infections that fail to respond or progress despite treatment with antibiotics active against streptococci and staphylococci.
- Inquiring about environmental exposure prior to the onset of the symptoms is key to elaborate a differential diagnosis list.
- Biopsy for pathology evaluation and acid-fast bacilli smear and culture are key to establish the diagnosis of M marinum infection.
Small Kansas Town Turns Night Into Day for Boy With Rare Disease
A new documentary video developed by NORD shows how a small community in Kansas came together to support an 11-year-old boy who has a rare disease that makes it necessary for him to avoid sunlight.
Peyton Madden has xeroderma pigmentosum (XP), a rare condition resulting in extreme sensitivity to the sun’s ultraviolet rays. As a result, he is not able to join friends and classmates in typical activities such as swimming in the community pool.
As part of NORD’s “Do Your Share” campaign, community leaders in El Dorado, Kansas, organized a surprise event for Peyton on a recent evening. NORD was on hand with a videographer. Watch the 5-minute video.
A new documentary video developed by NORD shows how a small community in Kansas came together to support an 11-year-old boy who has a rare disease that makes it necessary for him to avoid sunlight.
Peyton Madden has xeroderma pigmentosum (XP), a rare condition resulting in extreme sensitivity to the sun’s ultraviolet rays. As a result, he is not able to join friends and classmates in typical activities such as swimming in the community pool.
As part of NORD’s “Do Your Share” campaign, community leaders in El Dorado, Kansas, organized a surprise event for Peyton on a recent evening. NORD was on hand with a videographer. Watch the 5-minute video.
A new documentary video developed by NORD shows how a small community in Kansas came together to support an 11-year-old boy who has a rare disease that makes it necessary for him to avoid sunlight.
Peyton Madden has xeroderma pigmentosum (XP), a rare condition resulting in extreme sensitivity to the sun’s ultraviolet rays. As a result, he is not able to join friends and classmates in typical activities such as swimming in the community pool.
As part of NORD’s “Do Your Share” campaign, community leaders in El Dorado, Kansas, organized a surprise event for Peyton on a recent evening. NORD was on hand with a videographer. Watch the 5-minute video.
Ulcerative Sarcoidosis: A Prototypical Presentation and Review
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).
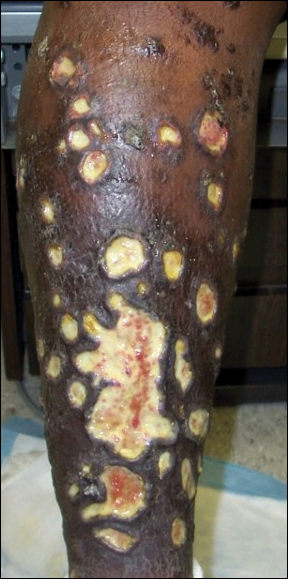
Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.
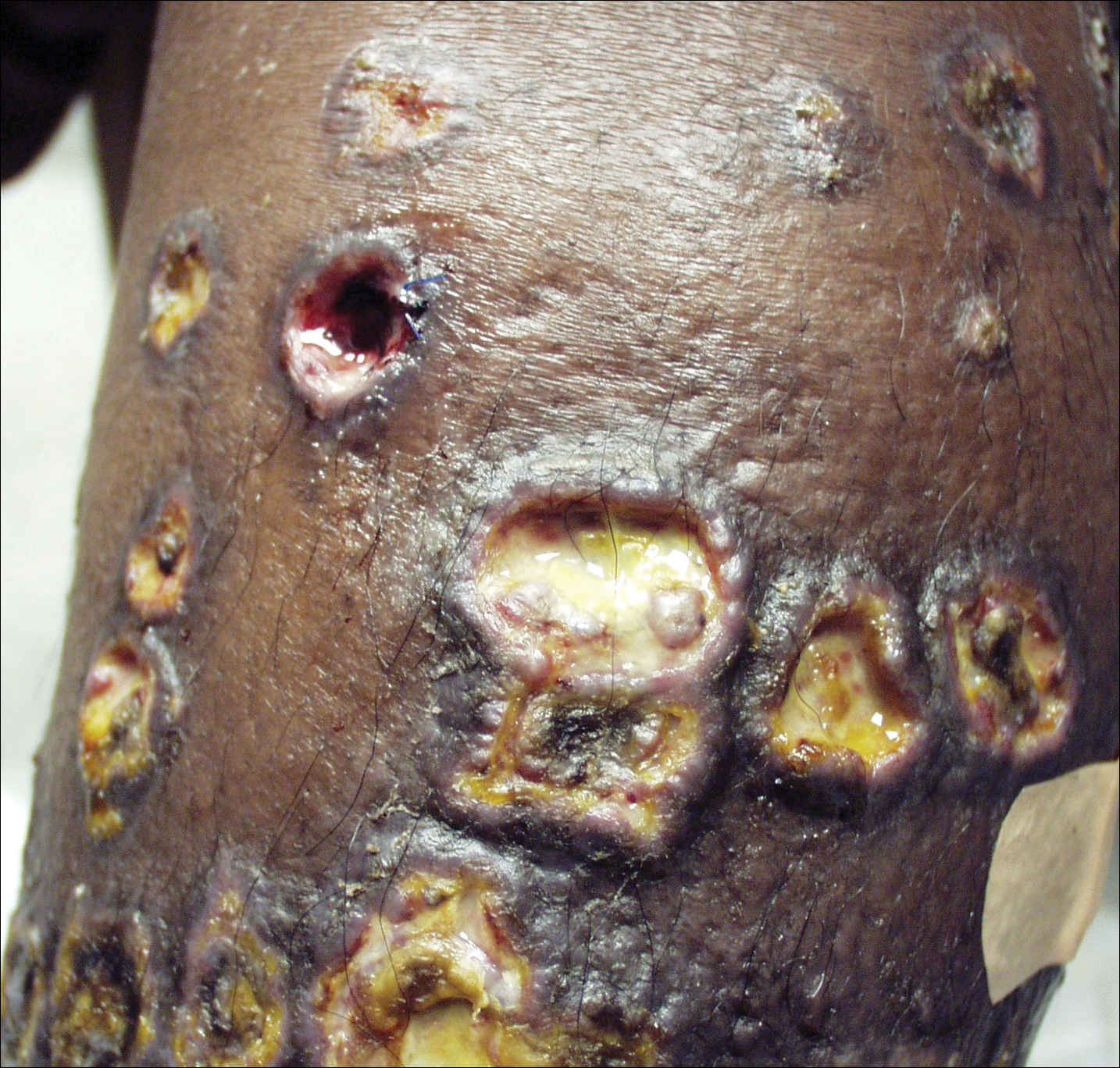
Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.
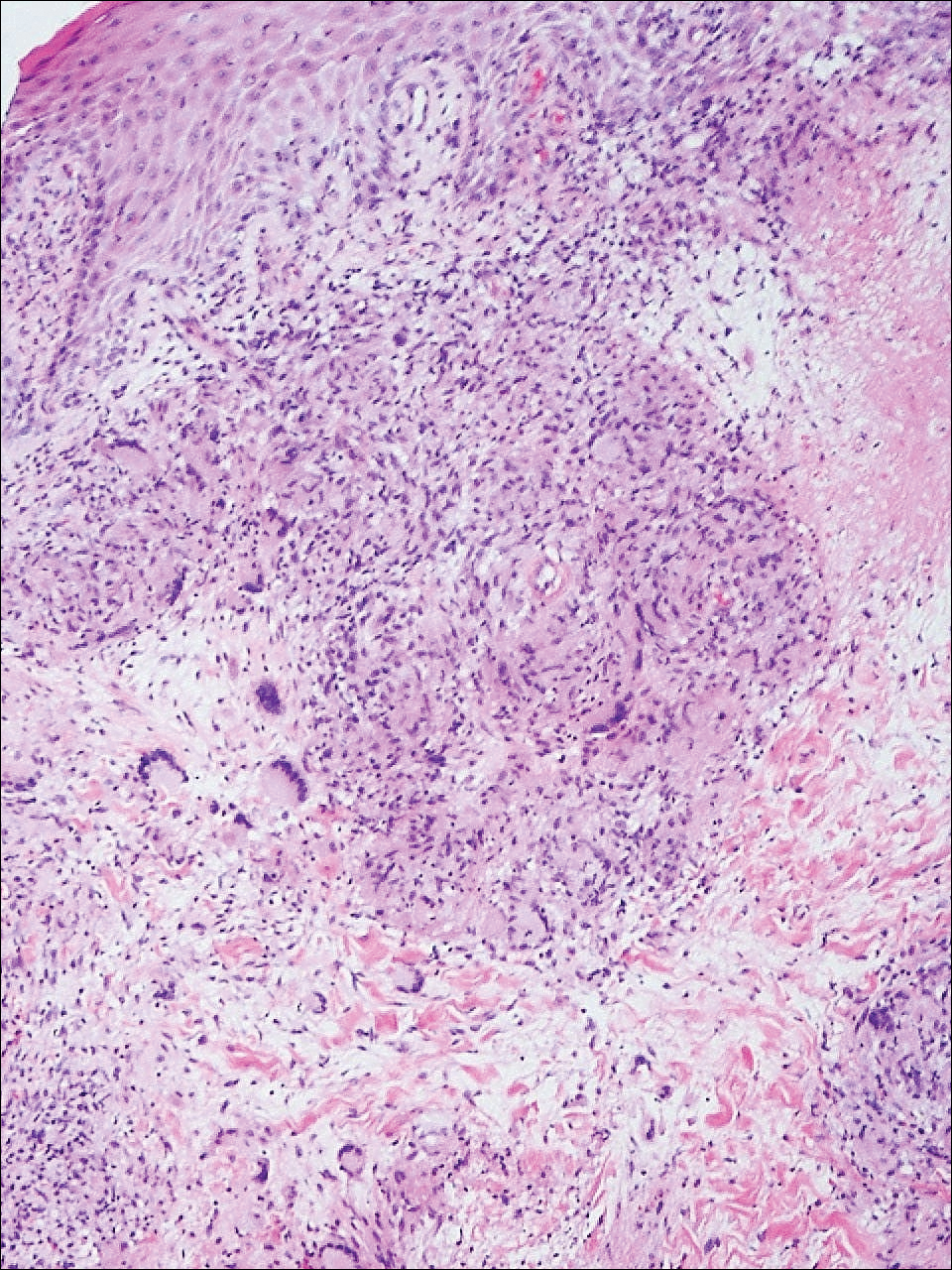
Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.
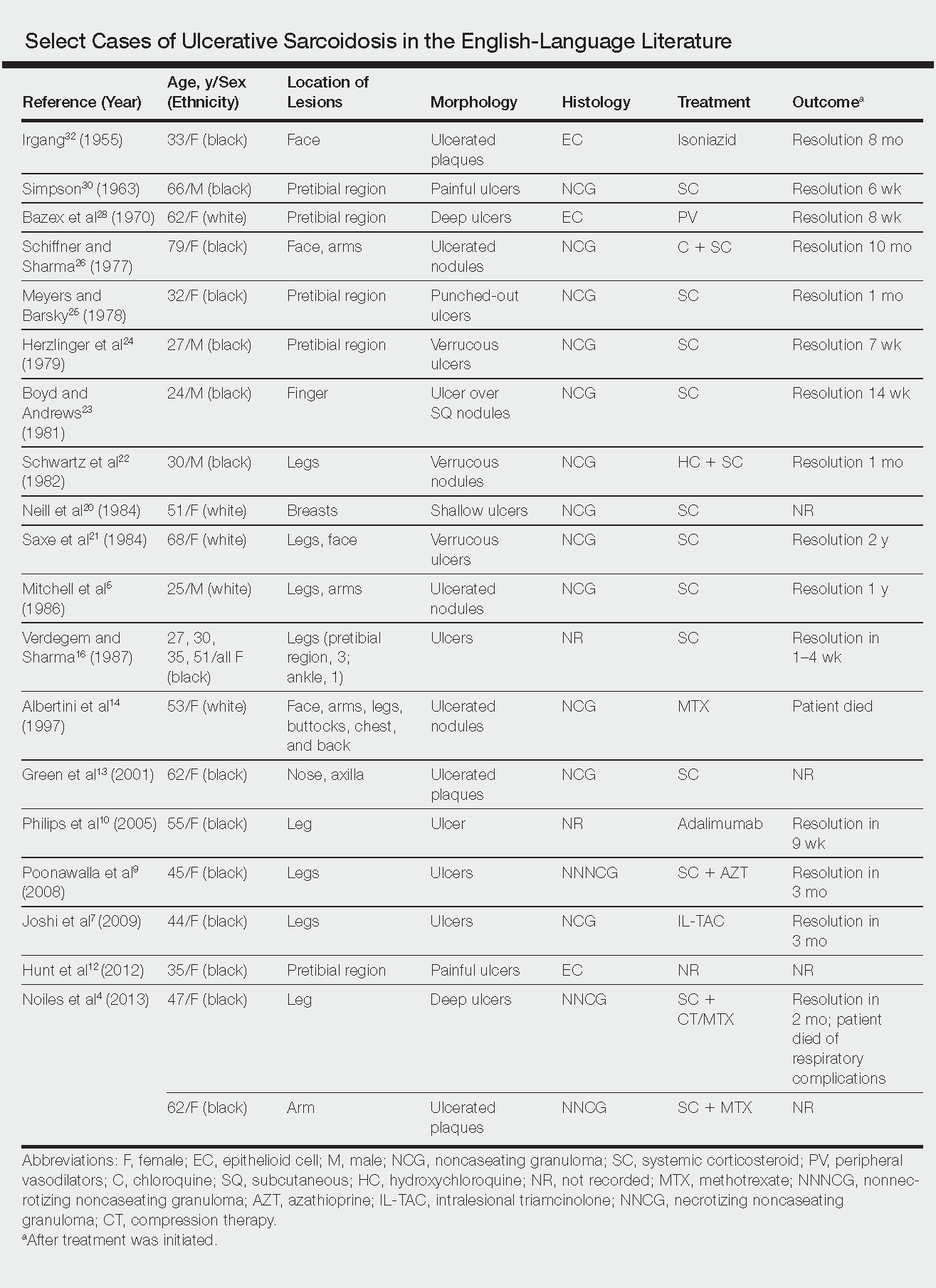
The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Practice Points
- Sarcoidosis can present as a primary ulcerative disease.
- Suspect ulcerative sarcoidosis when ulcerations are seen on the leg.
- Systemic corticosteroids may be the most effective treatment of ulcerative sarcoidosis.
NORD Supports Orphan Drug Tax Credit
NORD recently joined 140 other patient advocacy organizations in sending a letter to Congress urging that the Orphan Drug Tax Credit remain in place. The Orphan Drug Tax Credit is one of the incentives in the Orphan Drug Act that have promoted R & D on diagnostics and treatments for people with rare diseases since 1983.
As Congress discusses possible approaches to tax reform, some options would include doing away with the ODTC. However, NORD and its policy partners believe patients with rare diseases would suffer if that were to happen. A 2015 study showed that one-third fewer orphan drugs would be developed if the ODTC did not exist.
NORD recently joined 140 other patient advocacy organizations in sending a letter to Congress urging that the Orphan Drug Tax Credit remain in place. The Orphan Drug Tax Credit is one of the incentives in the Orphan Drug Act that have promoted R & D on diagnostics and treatments for people with rare diseases since 1983.
As Congress discusses possible approaches to tax reform, some options would include doing away with the ODTC. However, NORD and its policy partners believe patients with rare diseases would suffer if that were to happen. A 2015 study showed that one-third fewer orphan drugs would be developed if the ODTC did not exist.
NORD recently joined 140 other patient advocacy organizations in sending a letter to Congress urging that the Orphan Drug Tax Credit remain in place. The Orphan Drug Tax Credit is one of the incentives in the Orphan Drug Act that have promoted R & D on diagnostics and treatments for people with rare diseases since 1983.
As Congress discusses possible approaches to tax reform, some options would include doing away with the ODTC. However, NORD and its policy partners believe patients with rare diseases would suffer if that were to happen. A 2015 study showed that one-third fewer orphan drugs would be developed if the ODTC did not exist.
NORD Board Chair to Moderate Social Security Administration Forum
NORD Board Chair Marshall Summar, MD, will moderate a Social Security Administration (SSA) National Disability Forum on Compassionate Allowances and Rare Diseases on Nov. 7, 2017. Speakers will discuss the challenges of living with rare diseases and will recommend specific diseases for SSA’s Compassionate Allowances list.
The Compassionate Allowances list is a list of diseases or medical conditions that almost invariably meet SSA guidelines for disability assistance. Applications from individuals with those diagnoses qualify for expedited review.
Medical professionals and patient representatives are invited to participate in this event. Registration to attend the Washington, DC forum in person or to listen to the forum via teleconference may be done online.
SSA will also be accepting questions or comments about Compassionate Allowances on Twitter during the forum. Use the hashtag #SSANDForum and/or direct comments to @SSAOutreach. Questions or comments may also be sent by email to [email protected].
For two weeks after the event, SSA will be accepting suggestions online regarding diseases or conditions to be considered for the list. Read about the Compassionate Allowances Initiative and view the current list of conditions here.
NORD Board Chair Marshall Summar, MD, will moderate a Social Security Administration (SSA) National Disability Forum on Compassionate Allowances and Rare Diseases on Nov. 7, 2017. Speakers will discuss the challenges of living with rare diseases and will recommend specific diseases for SSA’s Compassionate Allowances list.
The Compassionate Allowances list is a list of diseases or medical conditions that almost invariably meet SSA guidelines for disability assistance. Applications from individuals with those diagnoses qualify for expedited review.
Medical professionals and patient representatives are invited to participate in this event. Registration to attend the Washington, DC forum in person or to listen to the forum via teleconference may be done online.
SSA will also be accepting questions or comments about Compassionate Allowances on Twitter during the forum. Use the hashtag #SSANDForum and/or direct comments to @SSAOutreach. Questions or comments may also be sent by email to [email protected].
For two weeks after the event, SSA will be accepting suggestions online regarding diseases or conditions to be considered for the list. Read about the Compassionate Allowances Initiative and view the current list of conditions here.
NORD Board Chair Marshall Summar, MD, will moderate a Social Security Administration (SSA) National Disability Forum on Compassionate Allowances and Rare Diseases on Nov. 7, 2017. Speakers will discuss the challenges of living with rare diseases and will recommend specific diseases for SSA’s Compassionate Allowances list.
The Compassionate Allowances list is a list of diseases or medical conditions that almost invariably meet SSA guidelines for disability assistance. Applications from individuals with those diagnoses qualify for expedited review.
Medical professionals and patient representatives are invited to participate in this event. Registration to attend the Washington, DC forum in person or to listen to the forum via teleconference may be done online.
SSA will also be accepting questions or comments about Compassionate Allowances on Twitter during the forum. Use the hashtag #SSANDForum and/or direct comments to @SSAOutreach. Questions or comments may also be sent by email to [email protected].
For two weeks after the event, SSA will be accepting suggestions online regarding diseases or conditions to be considered for the list. Read about the Compassionate Allowances Initiative and view the current list of conditions here.
NORD Call for Abstracts Reopened
NORD has reopened the call to submit abstracts and letters of intent for the following research grants.
- Cat Eye Syndrome
- Malonic Aciduria
- Post-Orgasmic Illness Syndrome
The deadline is ongoing and all US and international researchers interested in studying these diseases are encouraged to consider applying. For additional information and to view the RFPs, visit the NORD website.
NORD has reopened the call to submit abstracts and letters of intent for the following research grants.
- Cat Eye Syndrome
- Malonic Aciduria
- Post-Orgasmic Illness Syndrome
The deadline is ongoing and all US and international researchers interested in studying these diseases are encouraged to consider applying. For additional information and to view the RFPs, visit the NORD website.
NORD has reopened the call to submit abstracts and letters of intent for the following research grants.
- Cat Eye Syndrome
- Malonic Aciduria
- Post-Orgasmic Illness Syndrome
The deadline is ongoing and all US and international researchers interested in studying these diseases are encouraged to consider applying. For additional information and to view the RFPs, visit the NORD website.
Orphan Drugs Are Not Driving Up the Cost of Health Care, According to New Study
Orphan drugs accounted for only 7.9% of total drug sales in the United States in 2016, according to a new study conducted by the QuintilesIMS Institute. The study, commissioned by NORD, was released at the recent NORD Rare Diseases and Orphan Products Annual Summit in Washington DC.
The study analyzed the role of the Orphan Drug Act, orphan drug usage, and costs. Key findings included that:
- Of the $46 billion spent on pharmaceutical drugs in the US in 2016, only 7.9% was for orphan drugs.
- The orphan drug share of the total volume of pharmaceutical use in the US in 2016 was just 0.3%, down from a peak of 0.6% in 2003.
Findings also suggested that the Orphan Drug Act remains as important today as it was in 1983, when it was enacted. Read the QuintilesIMS report and a corresponding NORD document providing additional analysis.
Orphan drugs accounted for only 7.9% of total drug sales in the United States in 2016, according to a new study conducted by the QuintilesIMS Institute. The study, commissioned by NORD, was released at the recent NORD Rare Diseases and Orphan Products Annual Summit in Washington DC.
The study analyzed the role of the Orphan Drug Act, orphan drug usage, and costs. Key findings included that:
- Of the $46 billion spent on pharmaceutical drugs in the US in 2016, only 7.9% was for orphan drugs.
- The orphan drug share of the total volume of pharmaceutical use in the US in 2016 was just 0.3%, down from a peak of 0.6% in 2003.
Findings also suggested that the Orphan Drug Act remains as important today as it was in 1983, when it was enacted. Read the QuintilesIMS report and a corresponding NORD document providing additional analysis.
Orphan drugs accounted for only 7.9% of total drug sales in the United States in 2016, according to a new study conducted by the QuintilesIMS Institute. The study, commissioned by NORD, was released at the recent NORD Rare Diseases and Orphan Products Annual Summit in Washington DC.
The study analyzed the role of the Orphan Drug Act, orphan drug usage, and costs. Key findings included that:
- Of the $46 billion spent on pharmaceutical drugs in the US in 2016, only 7.9% was for orphan drugs.
- The orphan drug share of the total volume of pharmaceutical use in the US in 2016 was just 0.3%, down from a peak of 0.6% in 2003.
Findings also suggested that the Orphan Drug Act remains as important today as it was in 1983, when it was enacted. Read the QuintilesIMS report and a corresponding NORD document providing additional analysis.
Atypical Herpes Zoster Presentation in a Healthy Vaccinated Pediatric Patient
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.
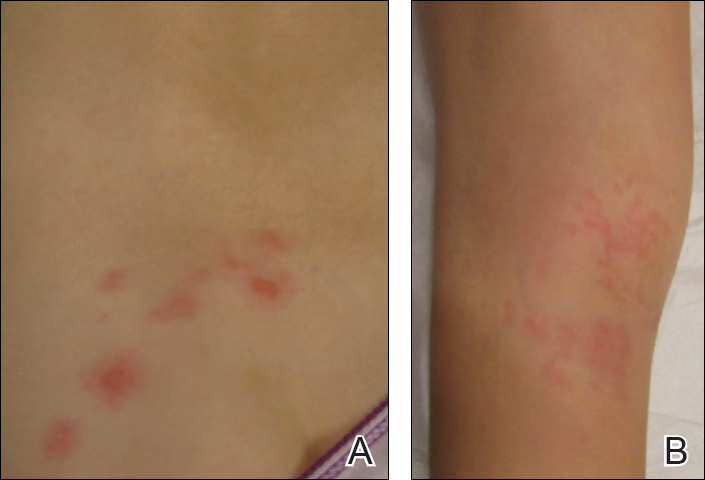
The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
Varicella-zoster virus (VZV) is a neurotropic human herpesvirus that causes varicella (chicken pox) and herpes zoster (shingles). During infection, the virus invades the dorsal root ganglia and establishes permanent latency. It can later reactivate and travel through sensory nerves to the skin where localized viral replication causes herpes zoster (HZ), which manifests with pain in a unilateral dermatomal distribution followed closely by an eruption of grouped macules and papules that evolve into vesicles on an erythematous base.1 These lesions form pustules and crusts over 7 to 10 days and heal completely within 4 weeks. Although postherpetic neuralgia is rare in children, the pain associated with HZ can last months or years.1,2
Universal childhood vaccination against VZV has existed in the United States since 1995, with a 2-dose vaccine regimen recommended by the CDC since 2007. Consequently, primary varicella infection in children is uncommon, and the majority of cases now occur in the vaccinated population.3 However, breakthrough varicella infection and postvaccination HZ are rare due to the long-lasting immunity and low virulence of the attenuated vaccine strain. We recount the case of a 6-year-old vaccinated girl with a unique presentation of HZ with no known primary varicella infection.
Case Report
A healthy 6-year-old girl presented with a stabbing burning pain in the left thigh extending down the calf of 4 days’ duration. The intense pain made walking difficult and responded minimally to ibuprofen and naproxen. Poor appetite, nausea, colicky abdominal pain, and fever (temperature, 38°C) accompanied the pain. Three days after the pain began she developed a pruritic rash on the same leg. Notably, she reported falling on a rosebush and sustaining a thorn prick in the left thigh 3 days prior to the onset of pain. Before presenting to our dermatology clinic, she was seen by a pediatrician, an emergency department physician, and an infectious disease specialist. The initial workup included a complete blood cell count, C-reactive protein test, erythrocyte sedimentation rate test, and hip and femur radiograph, which were all unremarkable. She was referred to dermatology with a differential diagnosis of sporotrichosis, contact dermatitis, reactive arthritis, viral myalgia, and Legg-Calvé-Perthes disease.
Physical examination revealed a well-appearing child with pink eczematous patches and plaques extending from the left side of the lower back to the mid shin in an L5 distribution (Figure). The left thigh was tender to palpation, and nontender left inguinal lymphadenopathy was present. A single isolated 2-mm vesicle was found on the anterior aspect of the left lower leg. Direct fluorescent antibody testing of vesicle fluid was positive for VZV antigen, confirming the diagnosis of HZ.

The patient’s mother confirmed that she had no obvious history of VZV. She had received VZV vaccinations in the left leg and arm at 1 and 4 years of age, respectively. She was treated with acyclovir (80 mg/kg daily at 6-hour intervals for 5 days) with immediate improvement in symptoms and resolution of the rash by day 5 of treatment. She experienced intermittent burning pain in the leg throughout the course of treatment, which resolved shortly thereafter.
Comment
Herpes zoster is rare in young healthy children, and its incidence has decreased since the introduction of universal varicella vaccination.4 Reported incidence rates in vaccinated children vary from approximately 15 to 93 per 100,000 person-years,5,6 and the reported relative risk is 0.08 to 0.36 in vaccinated compared to unvaccinated children.6,7 No correlations with gender, race, or ethnicity and postvaccination HZ have been observed.5,8 Reported intervals between vaccination and HZ presentation are as short as 3 months and as long as 11 years.9 Although HZ is uncommon in immunocompetent children, the diagnosis of HZ itself is not an indication for formal workup for an underlying immunodeficiency or malignancy.10
Both wild-type and vaccine-strain VZV establish latent infection and can cause HZ in vaccinated children. Direct fluorescent antibody testing or polymerase chain reaction of HZ lesions can be used to identify VZV. Genotyping can distinguish the wild-type versus the vaccine strain but is not required for clinical management.3 In previously vaccinated children with HZ, approximately half present with wild-type and half with vaccine-strain VZV. In approximately half of wild-type cases, prior clinical varicella infection also occurred.8
Regardless of virus strain, vaccinated children typically present with the characteristic painful, vesicular, dermatomal HZ rash.8,9 This presentation can be milder with less pain and fewer vesicles than with unvaccinated cases.6 When vaccine-strain HZ occurs, the rash often presents at or near the site of initial vaccination, which typically is the arm or thigh.3,4,6,9 The vaccine strain has lower virulence than the wild-type virus. Eight cases of vaccine-strain zoster severe enough to cause neurological complications such as meningitis or encephalitis have been reported in children, with 6 cases reported in healthy children.9,11-17 Antiviral drugs hasten the healing of the HZ rash and shorten the duration of associated pain.1
Although pediatric HZ is uncommon, all physicians should be aware of possible atypical presentations in healthy vaccinated children to appropriately and quickly manage treatment.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274-280.
- Hillebrand K, Bricout H, Schulze-Rath R, et al. Incidence of herpes zoster and its complications in Germany, 2005-2009. J Infect. 2015;70:178-186.
- Lopez A, Schmid S, Bialek S. Varicella. In: Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th ed. 2011:1-16.
- Tanuseputroa P, Zagorskia B, Chanc KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine. 2011;29:8580- 8584.
- Wen SY, Liu WL. Epidemiology of pediatric herpes zoster after varicella infection: a population-based study. Pediatrics. 2015;135:565-571.
- Civen R, Chaves SS, Jumaan A, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
- Stein M, Cohen R, Bromberg M, et al. Herpes zoster in a partially vaccinated pediatric population in Central Israel. Pediatr Infect Dis J. 2012;31:906-909.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005-2009. J Infect Dis. 2013;208:1859-1868.
- Horien C, Grose C. Neurovirulence of varicella and the live attenuated varicella vaccine virus. Semin Pediatr Neurol. 2012;19:124-129.
- Petursson G, Helgason S, Gudmundsson S, et al. Herpes zoster in children and adolescents. Pediatr Infect Dis J. 1998;17:905-908.
- Levin MJ, Dahl KM, Weinberg A, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus in an immunosuppressed child. J Infect Dis. 2003;188:954-959.
- Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995-2005. J Infect Dis. 2008;197(suppl 2):S170-S177.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus and encephalitis in an immunocompetent child. Pediatrics. 2010;125:e969-e972.
- Pahud BA, Glaser CA, Dekker CL, et al. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316-323.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
Practice Points
- Both wild-type and vaccine-strain varicella-zoster virus (VZV) can establish latency in dorsal root ganglia and can cause herpes zoster (HZ) in vaccinated children.
- When HZ due to a vaccine strain of VZV occurs, the rash often presents near the site of initial vaccination.
- Although most cases of HZ in vaccinated children present with a characteristic HZ rash, physicians should be aware of the possibility for atypical presentations.