User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
CDC shortens COVID-19 quarantine time to 10 or 7 days, with conditions
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Separating myth from reality: The role of cannabinoids in COVID-19
An intriguing pattern has emerged for cannabis enthusiasts as a result of lockdowns and statewide safety restrictions for COVID-19.
Consumers, as of late, have been shopping for larger marijuana baskets per trip to the dispensaries in various states, including California, Colorado, Nevada, and Washington, . However, they are also cutting down on the number of trips, perhaps, as a preventive measure to reduce the risk of exposure to coronavirus during this pandemic. Sales dipped considerably by the end of March only to experience a resurgence after the issuing of stimulus checks and unemployment benefits.
For the past few years, cannabis consumption remained steady while the industry continued to thrive with robust sales of the drug. It is a recession-proof phenomenon, therefore presenting a unique opportunity for clinicians with respect to patient education and individualized care.1
An unfortunate carryover of the governmental restrictions, self-isolation, and social estrangement is that consumers are now turning to the dark web as a source for continuous supply of cannabis. Prepandemic, according to the U.N. 2020 World Drug Report, there was already a 30% increase in sales of cannabis between 2009 and 2018. COVID-19 has fractured the drug’s supply chain and created an inescapable void that is being filled by drug traffickers.2 A clinical dilemma is posed when a user procures counterfeit cannabis or a drug batch with impurities.
Riding the cytokine storm
Cytokines are a host of proteins with designated regulatory and immune responses that play an instrumental role in cell signaling. The aptly named “cytokine storm” conjures up the image of an imperiled immune system spiraling out of control; it is, in fact, an extreme immune response that culminates into a massive influx of cytokines released into the bloodstream. Without the presence of an immunologic threat, cytokines are responsible for maintaining homeostasis and the functionality of immune cells. However, acute cytokine release (i.e., cytokine storm), as is the case with severe COVID-19, jeopardizes organ function (for example, interstitial lung disease) with clinical symptoms, such as fever, cough, dyspnea, and myalgia.
Benefits and drawbacks of immunosuppressive agents
To inhibit cytokine release (e.g., interleukin-6 cytokine levels), immunosuppressive agents such as tocilizumab have been leveraged to damper the body’s overactive inflammatory response to perceived immunologic stressors, in particular, COVID-19. While the aforementioned agent was remarkably effective with respect to lung consolidation clearance in most of the patients tested, a host of untoward effects prevent its general applicability and use. However, a team of researchers from the University of Nebraska, Omaha, with the Texas Biomedical Research Institute, San Antonio, might have stumbled upon a strategic workaround for mitigating the immune response.
They have proposed that cannabidiol (CBD) be used in lieu of other agents with potentially toxic effects. Animal and human trials have established that CBD confers a relatively high margin of safety coupled with favorable tolerance, providing a viable option for effectively targeting the inflammatory processes of SARS-CoV-2–based pulmonary disease. Furthermore, efficacy increased when CBD was combined with a terpene formulation, especially with respect to the more traditional steroid therapy.3
SARS-CoV-2 exhibits binding affinity for the ACE2 receptor, which is expressed in the lungs as well as other known predilection sites of infection. Ongoing studies attempt to modulate ACE2 expression, thereby eliminating its conspicuous role as “viral gateways,” perhaps even more so in patients with lung pathologies (e.g., people with chronic obstructive pulmonary disease [COPD] and smokers) as they already are prone to increased respiratory morbidity. CBD lacks tetrahydrocannabinol (THC), or the psychoactive component of cannabis sativa, rendering the agent to be particularly attractive from a therapeutic perspective. In addition to being devoid of abuse potential, CBD exhibits remarkable anti-inflammatory properties. It should be noted that considerable overlap exists between tobacco and cannabis users, and it is too early to determine the impact on COVID-19. As opposed to cannabis’s effect on ACE2 levels, smoking exhibits a proinflammatory role by up-regulating ACE2 expression.3 However, there are currently numerous conflicting reports in circulation about the positive effect of nicotine on COVID-19 outcome; confounding variables will need to be explored further in patients with a history of using nicotine and cannabis together.
From an immunologic perspective, the endocannabinoid system (ECS) plays an integral role in cell signaling by interacting with natural chemicals of the body, namely, cannabinoids with designated targets at the cannabinoid receptor 1 (CB1) and the CB2, respectively. The CB2 receptor is of particular interest as it is intimately involved in immune homeostasis; the primary goal of these COVID-19 studies is to modulate the endocannabinoid system via targeted CB2 therapies to produce an immunosuppressant effect.4 CB2 activation, be it by means of THC or CBD agonism, may prove to be beneficial by inhibiting the cytokine influx.
Unfortunately, there is a general dearth of data on COVID-19–exposed cannabis users, whether the drug is consumed for medication or recreational purposes. It has been suggested that cannabis intake might contribute toward the development of a cough, complicating the overall clinical outcome for those infected with the virus. The presence of a cough, even in an otherwise asymptomatic individual, facilitates viral spread. As for those cannabis users experiencing COVID-19 symptomatology, they can expect rapid clinical deterioration, including pronounced fatigue and a change in mental status.
According to pulmonary specialists and representatives of the American Lung Association, recreational cannabis use may be associated with a bronchitis-like inflammation (comparable with chronic bronchitis/COPD for chronic users) of the airways, along the lines of cigarette smoking.5 As far as cannabis smokers are concerned, the rationale for lung irritation is believed to stem from the relatively large portion of unburnt plant content that is inhaled in a given joint. If there is a superimposed infection, as is the case with COVID-19, the patient may experience further risk of adverse respiratory effects. This serves as a diagnostic dilemma for physicians, especially when they encounter patients who recently started dabbling with cannabis as a means of placating themselves or because they’ve heard rumors that it will somehow protect them from COVID-19. The entire assessment plan is slowed down as a result of the confounding variable (onset of a cough), which may arise independently of COVID-19 in cannabis users. Vulnerable populations include smokers and those with COPD or asthma, as they are more likely to require ventilator assistance during the course of COVID-19 therapy.5 Asthmatics and COPD patients are prone to bronchospasms because of sensitive airways.
COVID-19 safety protocols for cannabis users
Because of increased risk of respiratory morbidity, clinicians advise that consumption of recreational cannabinoids be scaled back during the course of the pandemic. In light of conflicting news from several media outlets regarding the efficacy of cannabis intake with respect to COVID-19, preexisting users might unwittingly increase their consumption as a preemptive measure against being exposed to the infection. To prevent transmission among users, clinicians should discourage patients from sharing joints. This recommendation is thematically consistent with general precautionary measures about the dangers of sharing utensils, drinking cups/glasses, and so on, amid the pandemic.
Despite promising preliminary research results, CBD cannot be wholeheartedly recommended at this time; patients already on medically administered cannabinoids are urged to discuss the risk-benefit ratio with their respective health care clinicians. Cannabinoid therapies present a massive opportunity from the perspective of immunomodulation, especially when considering the prevalence of drug use. However, to improve clinical guidelines with respect to COVID-19 outcomes, it would be prudent to increase the overall volume of preclinical knowledge by gathering retrospective data (from case-control designs) and randomized prospective trials.
A more comprehensive list of advice from physicians concerning casual or chronic cannabis users may also include: adopting a dedicated delivery or dispensing system for cannabis products, making considerations for decontamination (i.e., disinfecting mouthpieces), ensuring cleansing precautions are maintained (washing thoroughly before and after use or procurement), switching to inhalation alternates (e.g., tinctures, edibles, and/or oils) to decrease further irritation to the lungs. For bong users, it is recommended that they apply rubbing alcohol to clean their device followed with a minute of air-drying.6
Conclusion
The literature from preclinical studies appears to largely favor the use of CBD, but there remains an element of uncertainty with respect to implementing cannabinoids for the treatment of coronavirus.
COVID-19 cannabinoid intervention is a hot topic with renewed interest from the industry and the public at large, but viral-focused therapies remain a relatively underused area worth exploring with case-control designs and randomized prospective trials. As cannabis legalization is picking up momentum across five additional states, the time is ripe to systematically investigate the therapeutic applications of the drug beyond merely preclinical data. Aside from educational reform initiatives, clinicians might proactively launch a platform that integrates telemedicine as well as digital apps, directly linking the patient to the clinician and monitoring the efficacy of program initiatives in real time.
References
1. Long A. Consumers’ cannabis buying patterns change markedly in wake of COVID-19 pandemic. Marijuana Business Daily. 2020 Sep 22. https://mjbizdaily.com/consumers-cannabis-buying-patterns-change-markedly-in-wake-of-covid-pandemic/.
2. Bures B. How the coronavirus pandemic is increasing global demand for marijuana. Chicago Tribune. 2020 Jul 1. https://www.chicagotribune.com/marijuana/sns-tft-coronavirus-increases-global-marijuana-demand-20200701-oygaxryb7vhcjfeu44cgacicaa-story.html.
3. Walters J. Marijuana and COVID-19: Top studies. CannaMD. 2020 Aug 19. https://www.cannamd.com/marijuana-covid-19-top-studies/.
4. El Biali M et al. Med Cannabis Cannabinoids. 2020 Aug 19. doi: 10.1159/000510799.
5. LaMotte S. “Smoking weed and coronavirus: Even occasional use raises risk of COVID-19 complications.” CNN Health. 2020 Apr 10. https://www.cnn.com/2020/04/10/health/smoking-weed-coronavirus-wellness/index.html
6. Yafai S and Etengoff S. The case for cannabis: Advising cannabis users about COVID-19. Emergency Medicine News. 2020 May 20;42(5B).
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Mr. Choudhry is a research assistant at the IMCHF. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. Dr. Islam, Mr. Choudhry, and Dr. Choudhry reported no relevant disclosures.
An intriguing pattern has emerged for cannabis enthusiasts as a result of lockdowns and statewide safety restrictions for COVID-19.
Consumers, as of late, have been shopping for larger marijuana baskets per trip to the dispensaries in various states, including California, Colorado, Nevada, and Washington, . However, they are also cutting down on the number of trips, perhaps, as a preventive measure to reduce the risk of exposure to coronavirus during this pandemic. Sales dipped considerably by the end of March only to experience a resurgence after the issuing of stimulus checks and unemployment benefits.
For the past few years, cannabis consumption remained steady while the industry continued to thrive with robust sales of the drug. It is a recession-proof phenomenon, therefore presenting a unique opportunity for clinicians with respect to patient education and individualized care.1
An unfortunate carryover of the governmental restrictions, self-isolation, and social estrangement is that consumers are now turning to the dark web as a source for continuous supply of cannabis. Prepandemic, according to the U.N. 2020 World Drug Report, there was already a 30% increase in sales of cannabis between 2009 and 2018. COVID-19 has fractured the drug’s supply chain and created an inescapable void that is being filled by drug traffickers.2 A clinical dilemma is posed when a user procures counterfeit cannabis or a drug batch with impurities.
Riding the cytokine storm
Cytokines are a host of proteins with designated regulatory and immune responses that play an instrumental role in cell signaling. The aptly named “cytokine storm” conjures up the image of an imperiled immune system spiraling out of control; it is, in fact, an extreme immune response that culminates into a massive influx of cytokines released into the bloodstream. Without the presence of an immunologic threat, cytokines are responsible for maintaining homeostasis and the functionality of immune cells. However, acute cytokine release (i.e., cytokine storm), as is the case with severe COVID-19, jeopardizes organ function (for example, interstitial lung disease) with clinical symptoms, such as fever, cough, dyspnea, and myalgia.
Benefits and drawbacks of immunosuppressive agents
To inhibit cytokine release (e.g., interleukin-6 cytokine levels), immunosuppressive agents such as tocilizumab have been leveraged to damper the body’s overactive inflammatory response to perceived immunologic stressors, in particular, COVID-19. While the aforementioned agent was remarkably effective with respect to lung consolidation clearance in most of the patients tested, a host of untoward effects prevent its general applicability and use. However, a team of researchers from the University of Nebraska, Omaha, with the Texas Biomedical Research Institute, San Antonio, might have stumbled upon a strategic workaround for mitigating the immune response.
They have proposed that cannabidiol (CBD) be used in lieu of other agents with potentially toxic effects. Animal and human trials have established that CBD confers a relatively high margin of safety coupled with favorable tolerance, providing a viable option for effectively targeting the inflammatory processes of SARS-CoV-2–based pulmonary disease. Furthermore, efficacy increased when CBD was combined with a terpene formulation, especially with respect to the more traditional steroid therapy.3
SARS-CoV-2 exhibits binding affinity for the ACE2 receptor, which is expressed in the lungs as well as other known predilection sites of infection. Ongoing studies attempt to modulate ACE2 expression, thereby eliminating its conspicuous role as “viral gateways,” perhaps even more so in patients with lung pathologies (e.g., people with chronic obstructive pulmonary disease [COPD] and smokers) as they already are prone to increased respiratory morbidity. CBD lacks tetrahydrocannabinol (THC), or the psychoactive component of cannabis sativa, rendering the agent to be particularly attractive from a therapeutic perspective. In addition to being devoid of abuse potential, CBD exhibits remarkable anti-inflammatory properties. It should be noted that considerable overlap exists between tobacco and cannabis users, and it is too early to determine the impact on COVID-19. As opposed to cannabis’s effect on ACE2 levels, smoking exhibits a proinflammatory role by up-regulating ACE2 expression.3 However, there are currently numerous conflicting reports in circulation about the positive effect of nicotine on COVID-19 outcome; confounding variables will need to be explored further in patients with a history of using nicotine and cannabis together.
From an immunologic perspective, the endocannabinoid system (ECS) plays an integral role in cell signaling by interacting with natural chemicals of the body, namely, cannabinoids with designated targets at the cannabinoid receptor 1 (CB1) and the CB2, respectively. The CB2 receptor is of particular interest as it is intimately involved in immune homeostasis; the primary goal of these COVID-19 studies is to modulate the endocannabinoid system via targeted CB2 therapies to produce an immunosuppressant effect.4 CB2 activation, be it by means of THC or CBD agonism, may prove to be beneficial by inhibiting the cytokine influx.
Unfortunately, there is a general dearth of data on COVID-19–exposed cannabis users, whether the drug is consumed for medication or recreational purposes. It has been suggested that cannabis intake might contribute toward the development of a cough, complicating the overall clinical outcome for those infected with the virus. The presence of a cough, even in an otherwise asymptomatic individual, facilitates viral spread. As for those cannabis users experiencing COVID-19 symptomatology, they can expect rapid clinical deterioration, including pronounced fatigue and a change in mental status.
According to pulmonary specialists and representatives of the American Lung Association, recreational cannabis use may be associated with a bronchitis-like inflammation (comparable with chronic bronchitis/COPD for chronic users) of the airways, along the lines of cigarette smoking.5 As far as cannabis smokers are concerned, the rationale for lung irritation is believed to stem from the relatively large portion of unburnt plant content that is inhaled in a given joint. If there is a superimposed infection, as is the case with COVID-19, the patient may experience further risk of adverse respiratory effects. This serves as a diagnostic dilemma for physicians, especially when they encounter patients who recently started dabbling with cannabis as a means of placating themselves or because they’ve heard rumors that it will somehow protect them from COVID-19. The entire assessment plan is slowed down as a result of the confounding variable (onset of a cough), which may arise independently of COVID-19 in cannabis users. Vulnerable populations include smokers and those with COPD or asthma, as they are more likely to require ventilator assistance during the course of COVID-19 therapy.5 Asthmatics and COPD patients are prone to bronchospasms because of sensitive airways.
COVID-19 safety protocols for cannabis users
Because of increased risk of respiratory morbidity, clinicians advise that consumption of recreational cannabinoids be scaled back during the course of the pandemic. In light of conflicting news from several media outlets regarding the efficacy of cannabis intake with respect to COVID-19, preexisting users might unwittingly increase their consumption as a preemptive measure against being exposed to the infection. To prevent transmission among users, clinicians should discourage patients from sharing joints. This recommendation is thematically consistent with general precautionary measures about the dangers of sharing utensils, drinking cups/glasses, and so on, amid the pandemic.
Despite promising preliminary research results, CBD cannot be wholeheartedly recommended at this time; patients already on medically administered cannabinoids are urged to discuss the risk-benefit ratio with their respective health care clinicians. Cannabinoid therapies present a massive opportunity from the perspective of immunomodulation, especially when considering the prevalence of drug use. However, to improve clinical guidelines with respect to COVID-19 outcomes, it would be prudent to increase the overall volume of preclinical knowledge by gathering retrospective data (from case-control designs) and randomized prospective trials.
A more comprehensive list of advice from physicians concerning casual or chronic cannabis users may also include: adopting a dedicated delivery or dispensing system for cannabis products, making considerations for decontamination (i.e., disinfecting mouthpieces), ensuring cleansing precautions are maintained (washing thoroughly before and after use or procurement), switching to inhalation alternates (e.g., tinctures, edibles, and/or oils) to decrease further irritation to the lungs. For bong users, it is recommended that they apply rubbing alcohol to clean their device followed with a minute of air-drying.6
Conclusion
The literature from preclinical studies appears to largely favor the use of CBD, but there remains an element of uncertainty with respect to implementing cannabinoids for the treatment of coronavirus.
COVID-19 cannabinoid intervention is a hot topic with renewed interest from the industry and the public at large, but viral-focused therapies remain a relatively underused area worth exploring with case-control designs and randomized prospective trials. As cannabis legalization is picking up momentum across five additional states, the time is ripe to systematically investigate the therapeutic applications of the drug beyond merely preclinical data. Aside from educational reform initiatives, clinicians might proactively launch a platform that integrates telemedicine as well as digital apps, directly linking the patient to the clinician and monitoring the efficacy of program initiatives in real time.
References
1. Long A. Consumers’ cannabis buying patterns change markedly in wake of COVID-19 pandemic. Marijuana Business Daily. 2020 Sep 22. https://mjbizdaily.com/consumers-cannabis-buying-patterns-change-markedly-in-wake-of-covid-pandemic/.
2. Bures B. How the coronavirus pandemic is increasing global demand for marijuana. Chicago Tribune. 2020 Jul 1. https://www.chicagotribune.com/marijuana/sns-tft-coronavirus-increases-global-marijuana-demand-20200701-oygaxryb7vhcjfeu44cgacicaa-story.html.
3. Walters J. Marijuana and COVID-19: Top studies. CannaMD. 2020 Aug 19. https://www.cannamd.com/marijuana-covid-19-top-studies/.
4. El Biali M et al. Med Cannabis Cannabinoids. 2020 Aug 19. doi: 10.1159/000510799.
5. LaMotte S. “Smoking weed and coronavirus: Even occasional use raises risk of COVID-19 complications.” CNN Health. 2020 Apr 10. https://www.cnn.com/2020/04/10/health/smoking-weed-coronavirus-wellness/index.html
6. Yafai S and Etengoff S. The case for cannabis: Advising cannabis users about COVID-19. Emergency Medicine News. 2020 May 20;42(5B).
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Mr. Choudhry is a research assistant at the IMCHF. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. Dr. Islam, Mr. Choudhry, and Dr. Choudhry reported no relevant disclosures.
An intriguing pattern has emerged for cannabis enthusiasts as a result of lockdowns and statewide safety restrictions for COVID-19.
Consumers, as of late, have been shopping for larger marijuana baskets per trip to the dispensaries in various states, including California, Colorado, Nevada, and Washington, . However, they are also cutting down on the number of trips, perhaps, as a preventive measure to reduce the risk of exposure to coronavirus during this pandemic. Sales dipped considerably by the end of March only to experience a resurgence after the issuing of stimulus checks and unemployment benefits.
For the past few years, cannabis consumption remained steady while the industry continued to thrive with robust sales of the drug. It is a recession-proof phenomenon, therefore presenting a unique opportunity for clinicians with respect to patient education and individualized care.1
An unfortunate carryover of the governmental restrictions, self-isolation, and social estrangement is that consumers are now turning to the dark web as a source for continuous supply of cannabis. Prepandemic, according to the U.N. 2020 World Drug Report, there was already a 30% increase in sales of cannabis between 2009 and 2018. COVID-19 has fractured the drug’s supply chain and created an inescapable void that is being filled by drug traffickers.2 A clinical dilemma is posed when a user procures counterfeit cannabis or a drug batch with impurities.
Riding the cytokine storm
Cytokines are a host of proteins with designated regulatory and immune responses that play an instrumental role in cell signaling. The aptly named “cytokine storm” conjures up the image of an imperiled immune system spiraling out of control; it is, in fact, an extreme immune response that culminates into a massive influx of cytokines released into the bloodstream. Without the presence of an immunologic threat, cytokines are responsible for maintaining homeostasis and the functionality of immune cells. However, acute cytokine release (i.e., cytokine storm), as is the case with severe COVID-19, jeopardizes organ function (for example, interstitial lung disease) with clinical symptoms, such as fever, cough, dyspnea, and myalgia.
Benefits and drawbacks of immunosuppressive agents
To inhibit cytokine release (e.g., interleukin-6 cytokine levels), immunosuppressive agents such as tocilizumab have been leveraged to damper the body’s overactive inflammatory response to perceived immunologic stressors, in particular, COVID-19. While the aforementioned agent was remarkably effective with respect to lung consolidation clearance in most of the patients tested, a host of untoward effects prevent its general applicability and use. However, a team of researchers from the University of Nebraska, Omaha, with the Texas Biomedical Research Institute, San Antonio, might have stumbled upon a strategic workaround for mitigating the immune response.
They have proposed that cannabidiol (CBD) be used in lieu of other agents with potentially toxic effects. Animal and human trials have established that CBD confers a relatively high margin of safety coupled with favorable tolerance, providing a viable option for effectively targeting the inflammatory processes of SARS-CoV-2–based pulmonary disease. Furthermore, efficacy increased when CBD was combined with a terpene formulation, especially with respect to the more traditional steroid therapy.3
SARS-CoV-2 exhibits binding affinity for the ACE2 receptor, which is expressed in the lungs as well as other known predilection sites of infection. Ongoing studies attempt to modulate ACE2 expression, thereby eliminating its conspicuous role as “viral gateways,” perhaps even more so in patients with lung pathologies (e.g., people with chronic obstructive pulmonary disease [COPD] and smokers) as they already are prone to increased respiratory morbidity. CBD lacks tetrahydrocannabinol (THC), or the psychoactive component of cannabis sativa, rendering the agent to be particularly attractive from a therapeutic perspective. In addition to being devoid of abuse potential, CBD exhibits remarkable anti-inflammatory properties. It should be noted that considerable overlap exists between tobacco and cannabis users, and it is too early to determine the impact on COVID-19. As opposed to cannabis’s effect on ACE2 levels, smoking exhibits a proinflammatory role by up-regulating ACE2 expression.3 However, there are currently numerous conflicting reports in circulation about the positive effect of nicotine on COVID-19 outcome; confounding variables will need to be explored further in patients with a history of using nicotine and cannabis together.
From an immunologic perspective, the endocannabinoid system (ECS) plays an integral role in cell signaling by interacting with natural chemicals of the body, namely, cannabinoids with designated targets at the cannabinoid receptor 1 (CB1) and the CB2, respectively. The CB2 receptor is of particular interest as it is intimately involved in immune homeostasis; the primary goal of these COVID-19 studies is to modulate the endocannabinoid system via targeted CB2 therapies to produce an immunosuppressant effect.4 CB2 activation, be it by means of THC or CBD agonism, may prove to be beneficial by inhibiting the cytokine influx.
Unfortunately, there is a general dearth of data on COVID-19–exposed cannabis users, whether the drug is consumed for medication or recreational purposes. It has been suggested that cannabis intake might contribute toward the development of a cough, complicating the overall clinical outcome for those infected with the virus. The presence of a cough, even in an otherwise asymptomatic individual, facilitates viral spread. As for those cannabis users experiencing COVID-19 symptomatology, they can expect rapid clinical deterioration, including pronounced fatigue and a change in mental status.
According to pulmonary specialists and representatives of the American Lung Association, recreational cannabis use may be associated with a bronchitis-like inflammation (comparable with chronic bronchitis/COPD for chronic users) of the airways, along the lines of cigarette smoking.5 As far as cannabis smokers are concerned, the rationale for lung irritation is believed to stem from the relatively large portion of unburnt plant content that is inhaled in a given joint. If there is a superimposed infection, as is the case with COVID-19, the patient may experience further risk of adverse respiratory effects. This serves as a diagnostic dilemma for physicians, especially when they encounter patients who recently started dabbling with cannabis as a means of placating themselves or because they’ve heard rumors that it will somehow protect them from COVID-19. The entire assessment plan is slowed down as a result of the confounding variable (onset of a cough), which may arise independently of COVID-19 in cannabis users. Vulnerable populations include smokers and those with COPD or asthma, as they are more likely to require ventilator assistance during the course of COVID-19 therapy.5 Asthmatics and COPD patients are prone to bronchospasms because of sensitive airways.
COVID-19 safety protocols for cannabis users
Because of increased risk of respiratory morbidity, clinicians advise that consumption of recreational cannabinoids be scaled back during the course of the pandemic. In light of conflicting news from several media outlets regarding the efficacy of cannabis intake with respect to COVID-19, preexisting users might unwittingly increase their consumption as a preemptive measure against being exposed to the infection. To prevent transmission among users, clinicians should discourage patients from sharing joints. This recommendation is thematically consistent with general precautionary measures about the dangers of sharing utensils, drinking cups/glasses, and so on, amid the pandemic.
Despite promising preliminary research results, CBD cannot be wholeheartedly recommended at this time; patients already on medically administered cannabinoids are urged to discuss the risk-benefit ratio with their respective health care clinicians. Cannabinoid therapies present a massive opportunity from the perspective of immunomodulation, especially when considering the prevalence of drug use. However, to improve clinical guidelines with respect to COVID-19 outcomes, it would be prudent to increase the overall volume of preclinical knowledge by gathering retrospective data (from case-control designs) and randomized prospective trials.
A more comprehensive list of advice from physicians concerning casual or chronic cannabis users may also include: adopting a dedicated delivery or dispensing system for cannabis products, making considerations for decontamination (i.e., disinfecting mouthpieces), ensuring cleansing precautions are maintained (washing thoroughly before and after use or procurement), switching to inhalation alternates (e.g., tinctures, edibles, and/or oils) to decrease further irritation to the lungs. For bong users, it is recommended that they apply rubbing alcohol to clean their device followed with a minute of air-drying.6
Conclusion
The literature from preclinical studies appears to largely favor the use of CBD, but there remains an element of uncertainty with respect to implementing cannabinoids for the treatment of coronavirus.
COVID-19 cannabinoid intervention is a hot topic with renewed interest from the industry and the public at large, but viral-focused therapies remain a relatively underused area worth exploring with case-control designs and randomized prospective trials. As cannabis legalization is picking up momentum across five additional states, the time is ripe to systematically investigate the therapeutic applications of the drug beyond merely preclinical data. Aside from educational reform initiatives, clinicians might proactively launch a platform that integrates telemedicine as well as digital apps, directly linking the patient to the clinician and monitoring the efficacy of program initiatives in real time.
References
1. Long A. Consumers’ cannabis buying patterns change markedly in wake of COVID-19 pandemic. Marijuana Business Daily. 2020 Sep 22. https://mjbizdaily.com/consumers-cannabis-buying-patterns-change-markedly-in-wake-of-covid-pandemic/.
2. Bures B. How the coronavirus pandemic is increasing global demand for marijuana. Chicago Tribune. 2020 Jul 1. https://www.chicagotribune.com/marijuana/sns-tft-coronavirus-increases-global-marijuana-demand-20200701-oygaxryb7vhcjfeu44cgacicaa-story.html.
3. Walters J. Marijuana and COVID-19: Top studies. CannaMD. 2020 Aug 19. https://www.cannamd.com/marijuana-covid-19-top-studies/.
4. El Biali M et al. Med Cannabis Cannabinoids. 2020 Aug 19. doi: 10.1159/000510799.
5. LaMotte S. “Smoking weed and coronavirus: Even occasional use raises risk of COVID-19 complications.” CNN Health. 2020 Apr 10. https://www.cnn.com/2020/04/10/health/smoking-weed-coronavirus-wellness/index.html
6. Yafai S and Etengoff S. The case for cannabis: Advising cannabis users about COVID-19. Emergency Medicine News. 2020 May 20;42(5B).
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Mr. Choudhry is a research assistant at the IMCHF. Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. Dr. Islam, Mr. Choudhry, and Dr. Choudhry reported no relevant disclosures.
ACIP: Health workers, long-term care residents first tier for COVID-19 vaccine
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted 13-1 that both groups be in the highest-priority group for vaccination. As such, ACIP recommends that both be included in phase 1a of the committee’s allocation plan.
The recommendation now goes to CDC director Robert Redfield, MD, for approval. State health departments are expected to rely on the recommendation, but ultimately can make their own decisions on how to allocate vaccine in their states.
“We hope that this vote gets us all one step closer to the day when we can all feel safe again and when this pandemic is over,” said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, at today’s meeting.
Health care workers are defined as paid and unpaid individuals serving in health care settings who have the potential for direct or indirect exposure to patients or infectious materials. Long-term care residents are defined as adults who reside in facilities that provide a variety of services, including medical and personal care. Phase 1a would not include children who live in such facilities.
“Our goal in phase 1a with regard to health care personnel is to preserve the workforce and health care capacity regardless of where exposure occurs,” said ACIP panelist Grace Lee, MD, MPH, professor of paediatrics at Stanford (Calif.) University. Thus vaccination would cover clinical support staff, such as nursing assistants, environmental services staff, and food support staff.
“It is crucial to maintain our health care capacity,” said ACIP member Sharon Frey, MD, clinical director at the Center for Vaccine Development at Saint Louis University. “But it’s also important to prevent severe disease and death in the group that is at highest risk of those complications and that includes those in long-term care facilities.”
CDC staff said that staff and residents in those facilities account for 6% of COVID-19 cases and 40% of deaths.
But Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., voted against putting long-term care residents into the 1a phase. “We have traditionally tried a vaccine in a young healthy population and then hope it works in our frail older adults. So we enter this realm of ‘we hope it works and that it’s safe,’ and that concerns me on many levels particularly for this vaccine,” she said, noting that the vaccines closest to FDA authorization have not been studied in elderly adults who live in nursing homes or assisted living facilities.
She added: “I have no reservations for health care workers taking this vaccine.”
Prioritization could change
The phase 1a allocation fits within the “four ethical principles” outlined by ACIP and CDC staff Nov. 23: to maximize benefits and minimize harms, promote justice, mitigate health inequities, and promote transparency.
“My vote reflects maximum benefit, minimum harm, promoting justice and mitigating the health inequalities that exist with regard to distribution of this vaccine,” said ACIP Chair Jose Romero, MD. Romero, chief medical officer of the Arkansas Department of Health, voted in favor of the phase 1a plan.
He and other panelists noted, however, that allocation priorities could change after the FDA reviews and authorizes a vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) will meet December 10 to review the Pfizer/BioNTech’s messenger RNA-based vaccine (BNT162b2). The companies filed for emergency use on November 20.
A second vaccine, made by Moderna, is not far behind. The company reported on Nov. 30 that its messenger RNA vaccine was 94.1% effective and filed for emergency use the same day. The FDA’s VRBPAC will review the safety and efficacy data for the Moderna vaccine on Dec. 17.
“If individual vaccines receive emergency use authorization, we will have more data to consider, and that could lead to revision of our prioritization,” said ACIP member Robert Atmar, MD, John S. Dunn Research Foundation Clinical Professor in Infectious Diseases at Baylor College of Medicine, Houston.
ACIP will meet again after the Dec. 10 FDA advisory panel. But it won’t recommend a product until after the FDA has authorized it, said Amanda Cohn, MD, senior advisor for vaccines at the CDC’s National Center for Immunization and Respiratory Diseases.
Staggered immunization subprioritization urged
The CDC staff said that given the potential that not enough vaccine will be available immediately, it was recommending that health care organizations plan on creating a hierarchy of prioritization within institutions. And, they also urged staggering vaccination for personnel in similar units or positions, citing potential systemic or other reactions among health care workers.
“Consider planning for personnel to have time away from clinical care if health care personnel experience systemic symptoms post vaccination,” said Sarah Oliver, MD, MSPH, from the CDC.
The CDC will soon be issuing guidance on how to handle systemic symptoms with health care workers, Dr. Oliver noted.
Some 40 million doses of the Pfizer/BioNTech and Moderna vaccines are expected to be available by the end of December, with 5 million to 10 million a week coming online after that, Dr. Cohn said. That means not all health care workers will be vaccinated immediately. That may require “subprioritization, but for a limited period of time,” she said.
Dr. Messonnier said that, even with limited supplies, most of the states have told the CDC that they think they can vaccinate all of their health care workers within 3 weeks – some in less time.
The ACIP allocation plan is similar to but not exactly the same as that issued by the National Academy of Sciences, Engineering, and Medicine, which issued recommendations in October. That organization said that health care workers, first responders, older Americans living in congregate settings, and people with underlying health conditions should be the first to receive a vaccine.
ACIP has said that phase 1b would include essential workers, including police officers and firefighters, and those in education, transportation, and food and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years or older.
This article first appeared on Medscape.com.
Treating insomnia, anxiety in a pandemic
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
What the Biden-Harris COVID-19 Advisory Board is missing
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
On Nov. 9, the Biden-Harris administration announced the members of its COVID-19 Advisory Board. Among them were many esteemed infectious disease and public health experts – encouraging, given that, for now, the COVID-19 pandemic shows no signs of slowing down. Not among them was a mental health professional.
As psychiatrists, we did not find this omission surprising, given the sidelined role our specialty too often plays among medical professionals. But we did find it disappointing. Not having a single behavioral health provider on the advisory board will prove to be a mistake that could affect millions of Americans.
Studies continue to roll in showing that patients with COVID-19 can present during and after infection with neuropsychiatric symptoms, including delirium, psychosis, and anxiety. In July, a meta-analysis published in The Lancet regarding the neuropsychological outcomes of earlier diseases caused by coronaviruses – severe acute respiratory syndrome and Middle East respiratory syndrome – suggested that, in the short term, close to one-quarter of patients experienced confusion representative of delirium. In the long term, following recovery, respondents frequently reported emotional lability, impaired concentration, and traumatic memories. Additionally, more recent research published in The Lancet suggests that rates of psychiatric disorders, dementia, and insomnia are significantly higher among survivors of COVID-19. This study echoes the findings of an article in JAMA from September that reported that, among patients who were hospitalized for COVID-19, mortality rates were higher for those who had previously been diagnosed with a psychiatric condition. And overall, the pandemic has been associated with significantly increased rates of anxiety and depression symptoms.
Although this research is preliminary,
This is especially true when you consider the following:
- It is very difficult to diagnose and treat mental health symptoms in a primary care setting that is already overburdened. Doing so results in delayed treatment and increased costs.
- In the long term, COVID-19 survivors will overburden the already underfunded mental healthcare system.
- Additional unforeseen psychological outcomes stem from the myriad traumas of events in 2020 (eg, racial unrest, children out of school, loss of jobs, the recent election).
Psychiatric disorders are notoriously difficult to diagnose and treat in the outpatient primary care setting, which is why mental health professionals will need to be a more integral part of the postpandemic treatment model and should be represented on the advisory board. Each year in the United States, there are more than 8 million doctors’ visits for depression, and more than half of these are in the primary care setting. Yet fewer than half of those patients leave with a diagnosis of depression or are treated for it.
Historically, screening for depression in the primary care setting is difficult given its broad presentation of symptoms, which include nonspecific physical complaints, such as digestive problems, headaches, insomnia, or general aches and pains. These shortcomings exist despite multiple changes in guidelines, such as regarding the use of self-screening tools and general screening for specific populations, such as postpartum women.
But screening alone has not been an effective strategy, especially when certain groups are less likely to be screened. These include older adults, Black persons, and men, all of whom are at higher risk for mortality after COVID-19. There is a failure to consistently apply standards of universal screening across all patient groups, and even if it occurs, there is a failure to establish reliable treatment and follow-up regimens. As clinicians, imagine how challenging diagnosis and treatment of more complicated psychiatric syndromes, such as somatoform disorder, will be in the primary care setting after the pandemic.
When almost two-thirds of symptoms in primary care are already “medically unexplained,” how do we expect primary care doctors to differentiate between those presenting with vague coronavirus-related “brain fog,” the run of the mill worrywart, and the 16%-34% with legitimate hypochondriasis of somatoform disorder who won’t improve without the involvement of a mental health provider?
A specialty in short supply
The mental health system we have now is inadequate for those who are currently diagnosed with mental disorders. Before the pandemic, emergency departments were boarding increasing numbers of patients with psychiatric illness because beds on inpatient units were unavailable. Individuals with insurance faced difficulty finding psychiatrists or psychotherapists who took insurance or who were availabile to accept new patients, given the growing shortage of providers in general. Community health centers continued to grapple with decreases in federal and state funding despite public political support for parity. Individuals with substance use faced few options for the outpatient, residential, or pharmacologic treatment that many needed to maintain sobriety.
Since the pandemic, we have seen rates of anxiety, depression, and suicidal thinking increase among adults and youth while many clinics have been forced to lay off employees, reduce services, or close their doors. As psychiatrists, we not only see the lack of treatment options for our patients but are forced to find creative solutions to meet their needs. How are we supposed to adapt (or feel confident) when individuals with or without previous mental illness face downstream consequences after COVID-19 when not one of our own is represented in the advisory board? How can we feel confident that downstream solutions acknowledge and address the intricacy of the behavioral health system that we, as mental health providers, know so intimately?
And what about the cumulative impact of everything else that has happened in 2020 in addition to the pandemic?! Although cataloging the various negative events that have happened this year is beyond the scope of this discussion, such lists have been compiled by the mainstream media and include the Australian brush fires, the crisis in Armenia, racial protests, economic uncertainties, and the run-up to and occurrence of the 2020 presidential election. Research is solid in its assertion that chronic stress can disturb our immune and cardiovascular systems, as well as mental health, leading to depression or anxiety. As a result of the pandemic itself, plus the events of this year, mental health providers are already warning not only of the current trauma underlying our day-to-day lives but also that of years to come.
More importantly, healthcare providers, both those represented by members of the advisory board and those who are not, are not immune to these issues. Before the pandemic, rates of suicide among doctors were already above average compared with other professions. After witnessing death repeatedly, self-isolation, the risk for infection to family, and dealing with the continued resistance to wearing masks, who knows what the eventual psychological toll our medical workforce will be?
Mental health providers have stepped up to the plate to provide care outside of traditional models to meet the needs that patients have now. One survey found that 81% of behavioral health providers began using telehealth for the first time in the past 6 months, owing to the COVID-19 pandemic. If not for the sake of the mental health of the Biden-Harris advisory board members themselves, who as doctors are likely to downplay the impact when struggling with mental health concerns in their own lives, a mental health provider deserves a seat at the table.
Plus, the outcomes speak for themselves when behavioral health providers collaborate with primary care providers to give treatment or when mental health experts are members of health crisis teams. Why wouldn’t the same be true for the Biden-Harris advisory board?
Kali Cyrus, MD, MPH, is an assistant professor of psychiatry and behavioral medicine at the Johns Hopkins School of Medicine, Baltimore, Maryland. She sees patients in private practice and offers consultation services in diversity strategy. Ranna Parekh, MD, MPH, is past deputy medical director and director of diversity and health equity for the American Psychiatric Association. She is currently a consultant psychiatrist at the Massachusetts General Hospital, Boston, and the chief diversity and inclusion officer at the American College of Cardiology.
A version of this article originally appeared on Medscape.com.
Moderna filing for FDA emergency COVID-19 vaccine approval, reports 94.1% efficacy
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
Approval of COVID-19 vaccines will change nature of clinical trials
While stressing the urgent need to vaccinate the whole U.S. population, infectious disease experts and medical ethicists are raising questions about the clinical trials needed to answer important questions about the new COVID-19 vaccines.
In a statement released on Nov. 20, Barbara Alexander, MD, president of the Infectious Diseases Society of America (IDSA) and a professor at Duke University, Durham, N.C., commented on Pfizer and BioNTech’s application to the Food and Drug Administration for an emergency use authorization (EUA) for its COVID-19 vaccine. Besides emphasizing the need for a transparent review of the companies’ trial data prior to the FDA’s granting an EUA, she said, “If emergency use authorization is granted, clinical trials and data collection must continue.”
In an interview, Dr. Alexander said she is convinced that both Pfizer and Moderna, which is also expected to seek an EUA soon, will continue their clinical trials to monitor the long-term safety and efficacy of their vaccines.
“The EUA guidance for COVID vaccine authorization is very clear that clinical trials will move forward,” she said. “Any EUA request would have to include a strategy to ensure that the long-term safety and efficacy of a vaccine could be monitored. I see no evidence that either Pfizer or Moderna is not prepared to follow those regulations.”
Eventually, she added, the drug makers will have to seek full FDA approval to replace an EUA, which as its name signifies, is designed for public health emergencies. “The EUA is a tool to help us get the vaccine into circulation and have it start working as quickly as possible in the current health crisis,” she said. “But once the crisis is over, if the sponsors want to continue to market their vaccines, they have to go forward and get full approval.”
Medical ethicists, however, point out there may be ethical and practical dilemmas involved in continuing or initiating clinical trials once a vaccine has been approved for use even on an emergency basis.
In a commentary in Annals of Internal Medicine, Rafael Dal-Re, MD, PhD, Arthur L. Caplan, PhD, and two other ethicists stipulated that the pandemic requires early licensing and deployment of COVID-19 vaccines. Nevertheless, they noted, additional months of data are required to establish the long-term efficacy and safety of the vaccines. “Moreover, early deployment could interfere with the acquisition of long-term data,” both on these vaccines and on others coming through the pipeline, they wrote.
In countries where an approved vaccine is deployed, the ethicists noted, investigators must inform participants in an ongoing trial about the approved vaccine’s status and ask if they want to continue in the study. If enough participants decline, the trial might have to be terminated early. At that point, researchers may not have sufficient long-term data to identify late-term safety issues, determine how long efficacy lasts, determine whether waning immunity is associated with reduced levels of antibodies, or identify the level of neutralizing antibodies that correlates with immunity.
Moreover, they observed, long-term trials are especially important for vaccines that use mRNA technology, because less is known about them than about traditional kinds of vaccines.
The authors also pointed out that early licensing of any vaccine might make it harder to evaluate vaccines that haven’t yet been approved. “Once a vaccine is licensed, new placebo-controlled RCTs [randomized controlled trials] of other vaccines will not be acceptable ethically, and noninferiority RCTs will be the most likely alternative.
“The goal of noninferiority trials will be to demonstrate that the immune response (that is, neutralizing antibody titers or levels) of the candidate vaccine is not inferior to that of the approved vaccine within a prespecified margin, which the FDA has established as less than 10% for COVID-19 vaccines,” the authors noted.
More data with more study designs
Dial Hewlett Jr., MD, medical director for disease control services, Westchester County Department of Health, White Plains, N.Y., said in an interview that the ethicists raise important issues that have been discussed in other forums, including a recent webinar of the National Academy of Medicine.
“As the authors point out, once you have a vaccine that has been shown to be effective and safe, it’s no longer ethical to enroll people in placebo trials,” he said.
Therefore, he said, Pfizer and Moderna will undoubtedly offer their vaccines to the people in their studies’ placebo groups after the vaccines receive an EUA. Then they will follow everyone who has been vaccinated for 2 years to determine long-term safety. Efficacy will also continue to be measured as an adjunct of safety, he said.
With regard to the difficulty of reconsenting individuals to enter a new clinical trial after a vaccine has been approved, he said, “I’d agree that trying to get all the same participants to come into another study would be a challenge. You can, however, design studies that will allow you to obtain the same information. You will have a large number of people out there who haven’t been vaccinated, and you can do single-arm longitudinal studies and measure a number of things in the individuals who are enrolled in those studies,” he said.
“You can look at the immunologic markers, both antibody and T-cell. You can follow these individuals longitudinally to see if they do develop disease over a period of time. If they do, you can determine what their levels of response were,” he added. “So there are opportunities to design studies that would give you some of the same information, although it would not be in the same population that was in the randomized trials.”
For newer vaccines that have yet to be tested, he said, developers can compare “historical controls” from the trials of approved vaccines, i.e., data from the unvaccinated participants in those studies, with the data from inoculating people with the novel agents. The historical data can be sex- and age-matched, among other things, to individuals in the new trials. Moreover, because the study protocols have been harmonized for all trials under Operation Warp Speed, it doesn’t matter what kind of vaccine they’re testing, he said.
It may be necessary to do additional studies to find out how long immunity lasts after people have been vaccinated, Dr. Hewlett pointed out.
“You may have a different trial design. You don’t need a control arm to determine how long immunity lasts. You’re just comparing the patients who were vaccinated to nothing,” he said. “So you could have a single-arm trial on a group of people who consent to be immunized and followed. You can see what their antibody levels are and other surrogate markers, and you can see when they might develop disease, if they do. You’d need a large sample, but you can do that.”
Dr. Hewlett noted that additional studies will be required to determine whether the new vaccines stop transmission of the coronavirus or just prevent symptoms of COVID-19. Until it’s established that a vaccine halts transmission or the country achieves herd immunity, he said, “we’ll still have to wear masks and take other precautions, because a significant portion of people will still be at risk.”
‘A lot of redundancy’
Dr. Alexander emphasized that any safety or efficacy issues with the first COVID-19 vaccines must be identified before the vaccine is offered to a large portion of the U.S. population.
“While the data from the Pfizer and Moderna trials are said to be favorable, we at IDSA want to make sure that whatever vaccine comes to market is safe,” she said. “Having an unsafe vaccine on the market would be worse than no vaccine, because you’re compromising the public confidence. We have to make sure the public trusts the process and that sufficient data have been evaluated to ensure the vaccine is safe and efficacious.
“I believe the FDA is being very careful and thoughtful in [its] response,” Dr. Alexander said. “They realize how important it is to get a vaccine and save lives. While they’re doing things differently and moving much faster than before, they’re still trying to be thoughtful and reasonable. They don’t seem to be putting people at risk or circumventing the regulatory standards.”
Moreover, she pointed out, the FDA’s Vaccines and Related Biological Products Advisory Committee, which is expected to meet on Dec. 10, will review the trial data before the agency grants an EUA to Pfizer or Moderna. Then the FDA will post the data publicly.
The next step is for the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention to look at the data and decide who in the United States should receive the vaccine first, she pointed out. And both Pfizer and Moderna have shown their data to advisory panels of outside experts.
“There’s a lot of redundancy, and a lot of people are looking at the data,” Dr. Alexander said. “So I don’t think we’re cutting corners to get it out there more quickly.”
A version of this article originally appeared on Medscape.com.
While stressing the urgent need to vaccinate the whole U.S. population, infectious disease experts and medical ethicists are raising questions about the clinical trials needed to answer important questions about the new COVID-19 vaccines.
In a statement released on Nov. 20, Barbara Alexander, MD, president of the Infectious Diseases Society of America (IDSA) and a professor at Duke University, Durham, N.C., commented on Pfizer and BioNTech’s application to the Food and Drug Administration for an emergency use authorization (EUA) for its COVID-19 vaccine. Besides emphasizing the need for a transparent review of the companies’ trial data prior to the FDA’s granting an EUA, she said, “If emergency use authorization is granted, clinical trials and data collection must continue.”
In an interview, Dr. Alexander said she is convinced that both Pfizer and Moderna, which is also expected to seek an EUA soon, will continue their clinical trials to monitor the long-term safety and efficacy of their vaccines.
“The EUA guidance for COVID vaccine authorization is very clear that clinical trials will move forward,” she said. “Any EUA request would have to include a strategy to ensure that the long-term safety and efficacy of a vaccine could be monitored. I see no evidence that either Pfizer or Moderna is not prepared to follow those regulations.”
Eventually, she added, the drug makers will have to seek full FDA approval to replace an EUA, which as its name signifies, is designed for public health emergencies. “The EUA is a tool to help us get the vaccine into circulation and have it start working as quickly as possible in the current health crisis,” she said. “But once the crisis is over, if the sponsors want to continue to market their vaccines, they have to go forward and get full approval.”
Medical ethicists, however, point out there may be ethical and practical dilemmas involved in continuing or initiating clinical trials once a vaccine has been approved for use even on an emergency basis.
In a commentary in Annals of Internal Medicine, Rafael Dal-Re, MD, PhD, Arthur L. Caplan, PhD, and two other ethicists stipulated that the pandemic requires early licensing and deployment of COVID-19 vaccines. Nevertheless, they noted, additional months of data are required to establish the long-term efficacy and safety of the vaccines. “Moreover, early deployment could interfere with the acquisition of long-term data,” both on these vaccines and on others coming through the pipeline, they wrote.
In countries where an approved vaccine is deployed, the ethicists noted, investigators must inform participants in an ongoing trial about the approved vaccine’s status and ask if they want to continue in the study. If enough participants decline, the trial might have to be terminated early. At that point, researchers may not have sufficient long-term data to identify late-term safety issues, determine how long efficacy lasts, determine whether waning immunity is associated with reduced levels of antibodies, or identify the level of neutralizing antibodies that correlates with immunity.
Moreover, they observed, long-term trials are especially important for vaccines that use mRNA technology, because less is known about them than about traditional kinds of vaccines.
The authors also pointed out that early licensing of any vaccine might make it harder to evaluate vaccines that haven’t yet been approved. “Once a vaccine is licensed, new placebo-controlled RCTs [randomized controlled trials] of other vaccines will not be acceptable ethically, and noninferiority RCTs will be the most likely alternative.
“The goal of noninferiority trials will be to demonstrate that the immune response (that is, neutralizing antibody titers or levels) of the candidate vaccine is not inferior to that of the approved vaccine within a prespecified margin, which the FDA has established as less than 10% for COVID-19 vaccines,” the authors noted.
More data with more study designs
Dial Hewlett Jr., MD, medical director for disease control services, Westchester County Department of Health, White Plains, N.Y., said in an interview that the ethicists raise important issues that have been discussed in other forums, including a recent webinar of the National Academy of Medicine.
“As the authors point out, once you have a vaccine that has been shown to be effective and safe, it’s no longer ethical to enroll people in placebo trials,” he said.
Therefore, he said, Pfizer and Moderna will undoubtedly offer their vaccines to the people in their studies’ placebo groups after the vaccines receive an EUA. Then they will follow everyone who has been vaccinated for 2 years to determine long-term safety. Efficacy will also continue to be measured as an adjunct of safety, he said.
With regard to the difficulty of reconsenting individuals to enter a new clinical trial after a vaccine has been approved, he said, “I’d agree that trying to get all the same participants to come into another study would be a challenge. You can, however, design studies that will allow you to obtain the same information. You will have a large number of people out there who haven’t been vaccinated, and you can do single-arm longitudinal studies and measure a number of things in the individuals who are enrolled in those studies,” he said.
“You can look at the immunologic markers, both antibody and T-cell. You can follow these individuals longitudinally to see if they do develop disease over a period of time. If they do, you can determine what their levels of response were,” he added. “So there are opportunities to design studies that would give you some of the same information, although it would not be in the same population that was in the randomized trials.”
For newer vaccines that have yet to be tested, he said, developers can compare “historical controls” from the trials of approved vaccines, i.e., data from the unvaccinated participants in those studies, with the data from inoculating people with the novel agents. The historical data can be sex- and age-matched, among other things, to individuals in the new trials. Moreover, because the study protocols have been harmonized for all trials under Operation Warp Speed, it doesn’t matter what kind of vaccine they’re testing, he said.
It may be necessary to do additional studies to find out how long immunity lasts after people have been vaccinated, Dr. Hewlett pointed out.
“You may have a different trial design. You don’t need a control arm to determine how long immunity lasts. You’re just comparing the patients who were vaccinated to nothing,” he said. “So you could have a single-arm trial on a group of people who consent to be immunized and followed. You can see what their antibody levels are and other surrogate markers, and you can see when they might develop disease, if they do. You’d need a large sample, but you can do that.”
Dr. Hewlett noted that additional studies will be required to determine whether the new vaccines stop transmission of the coronavirus or just prevent symptoms of COVID-19. Until it’s established that a vaccine halts transmission or the country achieves herd immunity, he said, “we’ll still have to wear masks and take other precautions, because a significant portion of people will still be at risk.”
‘A lot of redundancy’
Dr. Alexander emphasized that any safety or efficacy issues with the first COVID-19 vaccines must be identified before the vaccine is offered to a large portion of the U.S. population.
“While the data from the Pfizer and Moderna trials are said to be favorable, we at IDSA want to make sure that whatever vaccine comes to market is safe,” she said. “Having an unsafe vaccine on the market would be worse than no vaccine, because you’re compromising the public confidence. We have to make sure the public trusts the process and that sufficient data have been evaluated to ensure the vaccine is safe and efficacious.
“I believe the FDA is being very careful and thoughtful in [its] response,” Dr. Alexander said. “They realize how important it is to get a vaccine and save lives. While they’re doing things differently and moving much faster than before, they’re still trying to be thoughtful and reasonable. They don’t seem to be putting people at risk or circumventing the regulatory standards.”
Moreover, she pointed out, the FDA’s Vaccines and Related Biological Products Advisory Committee, which is expected to meet on Dec. 10, will review the trial data before the agency grants an EUA to Pfizer or Moderna. Then the FDA will post the data publicly.
The next step is for the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention to look at the data and decide who in the United States should receive the vaccine first, she pointed out. And both Pfizer and Moderna have shown their data to advisory panels of outside experts.
“There’s a lot of redundancy, and a lot of people are looking at the data,” Dr. Alexander said. “So I don’t think we’re cutting corners to get it out there more quickly.”
A version of this article originally appeared on Medscape.com.
While stressing the urgent need to vaccinate the whole U.S. population, infectious disease experts and medical ethicists are raising questions about the clinical trials needed to answer important questions about the new COVID-19 vaccines.
In a statement released on Nov. 20, Barbara Alexander, MD, president of the Infectious Diseases Society of America (IDSA) and a professor at Duke University, Durham, N.C., commented on Pfizer and BioNTech’s application to the Food and Drug Administration for an emergency use authorization (EUA) for its COVID-19 vaccine. Besides emphasizing the need for a transparent review of the companies’ trial data prior to the FDA’s granting an EUA, she said, “If emergency use authorization is granted, clinical trials and data collection must continue.”
In an interview, Dr. Alexander said she is convinced that both Pfizer and Moderna, which is also expected to seek an EUA soon, will continue their clinical trials to monitor the long-term safety and efficacy of their vaccines.
“The EUA guidance for COVID vaccine authorization is very clear that clinical trials will move forward,” she said. “Any EUA request would have to include a strategy to ensure that the long-term safety and efficacy of a vaccine could be monitored. I see no evidence that either Pfizer or Moderna is not prepared to follow those regulations.”
Eventually, she added, the drug makers will have to seek full FDA approval to replace an EUA, which as its name signifies, is designed for public health emergencies. “The EUA is a tool to help us get the vaccine into circulation and have it start working as quickly as possible in the current health crisis,” she said. “But once the crisis is over, if the sponsors want to continue to market their vaccines, they have to go forward and get full approval.”
Medical ethicists, however, point out there may be ethical and practical dilemmas involved in continuing or initiating clinical trials once a vaccine has been approved for use even on an emergency basis.
In a commentary in Annals of Internal Medicine, Rafael Dal-Re, MD, PhD, Arthur L. Caplan, PhD, and two other ethicists stipulated that the pandemic requires early licensing and deployment of COVID-19 vaccines. Nevertheless, they noted, additional months of data are required to establish the long-term efficacy and safety of the vaccines. “Moreover, early deployment could interfere with the acquisition of long-term data,” both on these vaccines and on others coming through the pipeline, they wrote.
In countries where an approved vaccine is deployed, the ethicists noted, investigators must inform participants in an ongoing trial about the approved vaccine’s status and ask if they want to continue in the study. If enough participants decline, the trial might have to be terminated early. At that point, researchers may not have sufficient long-term data to identify late-term safety issues, determine how long efficacy lasts, determine whether waning immunity is associated with reduced levels of antibodies, or identify the level of neutralizing antibodies that correlates with immunity.
Moreover, they observed, long-term trials are especially important for vaccines that use mRNA technology, because less is known about them than about traditional kinds of vaccines.
The authors also pointed out that early licensing of any vaccine might make it harder to evaluate vaccines that haven’t yet been approved. “Once a vaccine is licensed, new placebo-controlled RCTs [randomized controlled trials] of other vaccines will not be acceptable ethically, and noninferiority RCTs will be the most likely alternative.
“The goal of noninferiority trials will be to demonstrate that the immune response (that is, neutralizing antibody titers or levels) of the candidate vaccine is not inferior to that of the approved vaccine within a prespecified margin, which the FDA has established as less than 10% for COVID-19 vaccines,” the authors noted.
More data with more study designs
Dial Hewlett Jr., MD, medical director for disease control services, Westchester County Department of Health, White Plains, N.Y., said in an interview that the ethicists raise important issues that have been discussed in other forums, including a recent webinar of the National Academy of Medicine.
“As the authors point out, once you have a vaccine that has been shown to be effective and safe, it’s no longer ethical to enroll people in placebo trials,” he said.
Therefore, he said, Pfizer and Moderna will undoubtedly offer their vaccines to the people in their studies’ placebo groups after the vaccines receive an EUA. Then they will follow everyone who has been vaccinated for 2 years to determine long-term safety. Efficacy will also continue to be measured as an adjunct of safety, he said.
With regard to the difficulty of reconsenting individuals to enter a new clinical trial after a vaccine has been approved, he said, “I’d agree that trying to get all the same participants to come into another study would be a challenge. You can, however, design studies that will allow you to obtain the same information. You will have a large number of people out there who haven’t been vaccinated, and you can do single-arm longitudinal studies and measure a number of things in the individuals who are enrolled in those studies,” he said.
“You can look at the immunologic markers, both antibody and T-cell. You can follow these individuals longitudinally to see if they do develop disease over a period of time. If they do, you can determine what their levels of response were,” he added. “So there are opportunities to design studies that would give you some of the same information, although it would not be in the same population that was in the randomized trials.”
For newer vaccines that have yet to be tested, he said, developers can compare “historical controls” from the trials of approved vaccines, i.e., data from the unvaccinated participants in those studies, with the data from inoculating people with the novel agents. The historical data can be sex- and age-matched, among other things, to individuals in the new trials. Moreover, because the study protocols have been harmonized for all trials under Operation Warp Speed, it doesn’t matter what kind of vaccine they’re testing, he said.
It may be necessary to do additional studies to find out how long immunity lasts after people have been vaccinated, Dr. Hewlett pointed out.
“You may have a different trial design. You don’t need a control arm to determine how long immunity lasts. You’re just comparing the patients who were vaccinated to nothing,” he said. “So you could have a single-arm trial on a group of people who consent to be immunized and followed. You can see what their antibody levels are and other surrogate markers, and you can see when they might develop disease, if they do. You’d need a large sample, but you can do that.”
Dr. Hewlett noted that additional studies will be required to determine whether the new vaccines stop transmission of the coronavirus or just prevent symptoms of COVID-19. Until it’s established that a vaccine halts transmission or the country achieves herd immunity, he said, “we’ll still have to wear masks and take other precautions, because a significant portion of people will still be at risk.”
‘A lot of redundancy’
Dr. Alexander emphasized that any safety or efficacy issues with the first COVID-19 vaccines must be identified before the vaccine is offered to a large portion of the U.S. population.
“While the data from the Pfizer and Moderna trials are said to be favorable, we at IDSA want to make sure that whatever vaccine comes to market is safe,” she said. “Having an unsafe vaccine on the market would be worse than no vaccine, because you’re compromising the public confidence. We have to make sure the public trusts the process and that sufficient data have been evaluated to ensure the vaccine is safe and efficacious.
“I believe the FDA is being very careful and thoughtful in [its] response,” Dr. Alexander said. “They realize how important it is to get a vaccine and save lives. While they’re doing things differently and moving much faster than before, they’re still trying to be thoughtful and reasonable. They don’t seem to be putting people at risk or circumventing the regulatory standards.”
Moreover, she pointed out, the FDA’s Vaccines and Related Biological Products Advisory Committee, which is expected to meet on Dec. 10, will review the trial data before the agency grants an EUA to Pfizer or Moderna. Then the FDA will post the data publicly.
The next step is for the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention to look at the data and decide who in the United States should receive the vaccine first, she pointed out. And both Pfizer and Moderna have shown their data to advisory panels of outside experts.
“There’s a lot of redundancy, and a lot of people are looking at the data,” Dr. Alexander said. “So I don’t think we’re cutting corners to get it out there more quickly.”
A version of this article originally appeared on Medscape.com.
CDC panel delves into priorities for COVID vaccine distribution
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
On Monday, members of an influential federal panel delved into the challenges ahead in deciding who will get the first doses of COVID-19 vaccines, including questions about which healthcare workers need those initial vaccinations the most.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) did not take any votes or seek to establish formal positions. Instead, the meeting served as a forum for experts to discuss the thorny issues ahead. The US Food and Drug Administration (FDA) could make a decision next month regarding clearance for the first COVID-19 vaccine.
An FDA advisory committee will meet December 10 to review the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, in partnership with BioNTech. Moderna Inc said on November 16 that it expects to soon ask the FDA for an EUA of its rival COVID vaccine.
ACIP will face a two-part task after the FDA clears COVID-19 vaccines, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. ACIP will need to first decide whether to recommend use of the vaccine and then address the “complicated and difficult” question of which groups should get the initial limited quantities.
“There aren’t any perfect decisions,” she told the ACIP members. “I know this is something that most of you didn’t anticipate doing, making these kinds of huge decisions in the midst of a pandemic.”
There has been considerable public discussion of prioritization of COVID-19 vaccines, including a set of recommendations offered by a special committee created by the National Academies of Sciences, Engineering and Medicine. In addition, CDC staff and members of ACIP outlined what they termed the “four ethical principles” meant to guide these decisions in a November 23 report in the agency’s Morbidity and Mortality Weekly Report. These four principles are to maximize benefits and minimize harms; promote justice; mitigate health inequities; and promote transparency.
But as the issuing of the first EUA nears, it falls to ACIP to move beyond endorsing broad goals. The panel will need to make decisions as to which groups will have to wait for COVID-19 vaccines.
ACIP members on Monday delved into these kinds of more detailed questions, using a proposed three-stage model as a discussion point.
In phase 1a of this model, healthcare workers and residents of long-term care facilities would be the first people to be vaccinated. Phase 1b would include those deemed essential workers, including police officers, firefighters, and those in education, transportation, food, and agriculture sectors. Phase 1c would include adults with high-risk medical conditions and those aged 65 years and older.
ACIP member Grace M. Lee, MD, MPH, of Stanford University, Stanford, California, questioned whether healthcare workers who are not seeing patients in person should wait to get the vaccines. There has been a marked rise in the use of telehealth during the pandemic, which has spared some clinicians from in-person COVID-19 patient visits in their practices.
“Close partnership with our public health colleagues will be critically important to make sure that we are not trying to vaccinate 100% of our healthcare workforce, if some proportion of our workforce can work from home,” Lee said.
ACIP member Pablo Sánchez, MD, of the Research Institute at Nationwide Children’s Hospital in Columbus, Ohio, concurred. Some clinicians, he noted, may have better access to personal protective equipment than others, he said.
“Unfortunately, not all healthcare workers are equal in terms of risk,” Sánchez said. “Within institutions, we’re going to have to prioritize which ones will get” the vaccine.
Clinicians may also make judgments about their own risk and need for early access to COVID-19 vaccinations, Sánchez said.
“I’m 66, and I’d rather give it to somebody much older and sicker than me,” he said.
Broader access
Fairly large populations will essentially be competing for limited doses of the first vaccines to reach the market.
The overlap is significant in the four priority groups put forward by CDC. The CDC staff estimated that about 21 million people would fall into the healthcare personnel category, which includes hospital staff, pharmacists, and those working in long-term care facilities. There are about 87 million people in the essential workers groups. More than 100 million adults in the United States, such as those with diabetes and cancers, fall into the high-risk medical conditions group. Another 53 million people are aged 65 and older.
Department of Health and Human Services Secretary Alex Azar on November 18 said the federal government expects to have about 40 million doses of these two vaccines by the end of December, which is enough to provide the two-dose regimen for about 20 million. If all goes as expected, Pfizer and Moderna will ramp up production.
Moderna has said that it expects by the end of this year to have approximately 20 million doses of its vaccine ready to ship in the United States and that it is on track to manufacture 500 million to 1 billion doses globally in 2021. Pfizer and BioNTech have said they expect to produce globally up to 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021.
At the Monday meeting, several ACIP panelists stressed the need to ensure that essential workers get early doses of vaccines.
In many cases, these workers serve in jobs with significant public interaction and live in poor communities. They put themselves and their families at risk. Many of them lack the resources to take precautions available to those better able to isolate, said ACIP member Beth Bell, MD, MPH, of the University of Washington, Seattle, Washington.
“These essential workers are out there putting themselves at risk to allow the rest of us to socially distance,” she said. “Recognizing that not all of them may want to be vaccinated at this stage, we need to provide them with the opportunity early on in the process.”
In Bell’s view, the initial rollout of COVID-19 vaccines will send an important message about sharing this resource.
“If we’re serious about valuing equity, we need to have that baked in early on in the vaccination program,” she said.
Bell also said she was in favor of including people living in nursing homes in the initial wave of vaccinations. Concerns were raised about the frailty of this population.
“Given the mortality impact on the healthcare system from the number of nursing home residents that have been dying, I think on balance it makes sense to include them in phase 1a,” Bell said.
Other ACIP panelists said missteps with early vaccination of people in nursing homes could undermine faith in the treatments. Because of the ages and medical conditions of people in nursing homes, many of them may die after receiving the COVID-19 vaccine. Such deaths would not be associated with vaccine, but the medical community would not yet have evidence to disprove a connection.
There could be a backlash, with people falsely linking the death of a grandparent to the vaccine.
Fellow ACIP member Robert L. Atmar, MD, Baylor College of Medicine, Houston, Texas, was among those who had raised concerns about including people living in long-term care facilities in phase 1a. He said there are not yet enough data to judge the balance of benefits and harms of vaccination for this population.
The Pfizer and Moderna vaccines are “reactagenic,” meaning people may not feel well in the days after receiving the shots. The symptoms could lead to additional health evaluations of older people in nursing homes as clinicians try to figure out whether the patient’s reactions to the vaccine are caused by some condition or infection, Atmar said.
“Those of us who see these patients in the hospital recognize that there are often medical interventions that are done in the pursuit of a diagnosis, of a change in clinical status, that in and of themselves can lead to harm,” Atmar said.
Clinicians likely will have to encourage their patients of all ages to receive second doses of COVID-19 vaccines, despite the malaise they may provoke.
“We really need to make patients aware that this is not going to be a walk in the park. I mean, they’re going to know they had a vaccine, they’re probably not going to feel wonderful, but they’ve got to come back for that second dose,” said Sandra Adamson Fryhofer, MD, who represented the American Medical Association.
ACIP is expected to meet again to offer specific recommendations on the Pfizer and Moderna vaccines. ACIP’s recommendations trigger reimbursement processes, Azar said at a Tuesday press conference. ACIP’s work will inform decisions made by the federal government and governors about deploying shipments of COVID-19 vaccines, he said.
“At the end of the day, that is a decision, though, of the US government to make, which is where to recommend the prioritization,” Azar said. “It will be our nation’s governors in implementing the distribution plans to tell us” where to ship the vaccine.
This article first appeared on Medscape.com.
50.6 million tobacco users are not a homogeneous group
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.
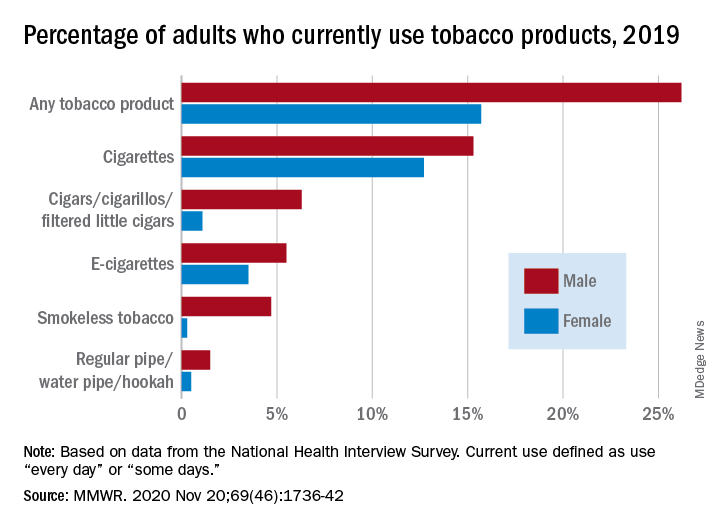
with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
Cigarettes are still the product of choice among U.S. adults who use tobacco, but the youngest adults are more likely to use e-cigarettes than any other product, according to data from the 2019 National Health Interview Survey.

with cigarette use reported by the largest share of respondents (14.0%) and e-cigarettes next at 4.5%, Monica E. Cornelius, PhD, and associates said in the Morbidity and Mortality Weekly Report.
Among adults aged 18-24 years, however, e-cigarettes were used by 9.3% of respondents in 2019, compared with 8.0% who used cigarettes every day or some days. Current e-cigarette use was 6.4% in 25- to 44-year-olds and continued to diminish with increasing age, said Dr. Cornelius and associates at the Centers for Disease Control and Prevention’s National Center for Chronic Disease Prevention and Health Promotion.
Men were more likely than women to use e-cigarettes (5.5% vs. 3.5%), and to use any tobacco product (26.2% vs. 15.7%). Use of other products, including cigarettes (15.3% for men vs. 12.7% for women), followed the same pattern to varying degrees, the national survey data show.
“Differences in prevalence of tobacco use also were also seen across population groups, with higher prevalence among those with a [high school equivalency degree], American Indian/Alaska Natives, uninsured adults and adults with Medicaid, and [lesbian, gay, or bisexual] adults,” the investigators said.
Among those groups, overall tobacco use and cigarette use were highest in those with an equivalency degree (43.8%, 37.1%), while lesbian/gay/bisexual individuals had the highest prevalence of e-cigarette use at 11.5%, they reported.
“As part of a comprehensive approach” to reduce tobacco-related disease and death, Dr. Cornelius and associates suggested, “targeted interventions are also warranted to reach subpopulations with the highest prevalence of use, which might vary by tobacco product type.”
SOURCE: Cornelius ME et al. MMWR. 2020 Nov 20;69(46);1736-42.
FROM MMWR








