User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


The Emerging Physician-Scientist Crisis in America
Recent reporting has shown that That’s a problem, because physician-scientists are uniquely equipped to make scientific discoveries in the laboratory and translate them to the clinic. Indeed, many of the discoveries that have transformed medicine for the better were made by physician-scientists. For example, Jonas Salk developed the polio vaccine, Timothy Ley sequenced the first cancer genome, and Anthony Fauci coordinated public health responses to both the HIV/AIDS and COVID-19 pandemics. Indicative of their sheer impact, at least a third and as many as half of all Nobel Prizes and Lasker Awards in physiology/medicine have gone to physician-scientists.
So why is the supply of physician-scientists shrinking so precipitously at a time when medical discoveries are being made at a record-high rate? Immunotherapy and proton therapy are transforming cancer care; RNA technology led to COVID vaccines; CRISPR is facilitating gene editing and treatment of diseases like sickle cell anemia. Yet, as exciting as medical science has become, only 1.5% of American doctors work as physician-scientists, more than a threefold drop compared with 30 years ago when the figure was a more robust 4.7%. What’s going on?
Residency training programs at prestigious academic medical centers have standard infolded research years; for example, neurosurgery residents at academic medical centers will often get 2 years of protected research time. And the National Institutes of Health has training grants dedicated to physician-scientists, such as the K08 award program. Several foundations are also dedicated to supporting early-career physician-scientists. Yet, the number of physicians deciding to become physician-scientists remains low, and, more troubling, the attrition rate of those who do decide to go this route is quite high.
The underlying issue is multifold. First, funding rates from the federal government for grants have become competitive to the point of being unrealistic. For example, the current funding rate for the flagship R01 program from the National Cancer Institute is only 12%. Promotions are typically tied to these grant awards, which means physician-scientists who are unable to acquire substantial grant funding are unable to pay for their research or win promotion — and often exit the physician-scientist track altogether.
Compounding this issue is a lack of mentorship for early-career physician-scientists. With the rise of “careerism” in medicine, senior-level physician-scientists may have less incentive to mentor those who are earlier in their careers. Rather, there seems to be greater reward to “managing up” — that is, spending time to please hospital administrators and departmental leadership. Being involved in countless committees appears to carry more value in advancing an established investigator’s career than does mentorship.
Finally, physician-scientists typically earn less than their clinician colleagues, despite juggling both scientific and clinical responsibilities. While many are comfortable with this arrangement when embarking on this track, the disparity may become untenable after a while, especially as departmental leadership will often turn to physician-scientists to fill clinical coverage gaps when faculty leave the department, or as the medical center expands to satellite centers outside the primary hospital. Indeed, physician-scientists get pulled in several directions, which can lead to burnout and attrition, with many who are highly equipped for this track ultimately hanging up their cleats and seeking more clinical or private industry–oriented opportunities.
Every academic medical center operates differently. Some clearly have done a better job than others promoting and fostering physician-scientists. What we find in the centers that manage to retain physician-scientists is leadership plays a major role: If a medical center values the importance of physician-scientists, they will do things to foster the success of those people, such as assembling mentorship committees, establishing clear criteria for promotion and career advancement, protecting research time while maintaining some level of pay equity, advocating for team science approaches, and supporting investigators in cases of gaps in federal funding. Different countries also have different models for physician-scientist training, with Germany, for example, allowing medical residents to have 3 years of protected time to engage in research after their second year of residency.
The stakes here are high. If we can’t address the physician-scientist recruitment and retention crisis in America now, we risk falling behind other countries in our ability to innovate and deliver world-class care.
Dr Chaudhuri is a tenure-track physician-scientist at Washington University in St. Louis, a Paul and Daisy Soros Fellow, and a Public Voices Fellow of The OpEd Project.
Aadel Chaudhuri, MD, PhD, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recent reporting has shown that That’s a problem, because physician-scientists are uniquely equipped to make scientific discoveries in the laboratory and translate them to the clinic. Indeed, many of the discoveries that have transformed medicine for the better were made by physician-scientists. For example, Jonas Salk developed the polio vaccine, Timothy Ley sequenced the first cancer genome, and Anthony Fauci coordinated public health responses to both the HIV/AIDS and COVID-19 pandemics. Indicative of their sheer impact, at least a third and as many as half of all Nobel Prizes and Lasker Awards in physiology/medicine have gone to physician-scientists.
So why is the supply of physician-scientists shrinking so precipitously at a time when medical discoveries are being made at a record-high rate? Immunotherapy and proton therapy are transforming cancer care; RNA technology led to COVID vaccines; CRISPR is facilitating gene editing and treatment of diseases like sickle cell anemia. Yet, as exciting as medical science has become, only 1.5% of American doctors work as physician-scientists, more than a threefold drop compared with 30 years ago when the figure was a more robust 4.7%. What’s going on?
Residency training programs at prestigious academic medical centers have standard infolded research years; for example, neurosurgery residents at academic medical centers will often get 2 years of protected research time. And the National Institutes of Health has training grants dedicated to physician-scientists, such as the K08 award program. Several foundations are also dedicated to supporting early-career physician-scientists. Yet, the number of physicians deciding to become physician-scientists remains low, and, more troubling, the attrition rate of those who do decide to go this route is quite high.
The underlying issue is multifold. First, funding rates from the federal government for grants have become competitive to the point of being unrealistic. For example, the current funding rate for the flagship R01 program from the National Cancer Institute is only 12%. Promotions are typically tied to these grant awards, which means physician-scientists who are unable to acquire substantial grant funding are unable to pay for their research or win promotion — and often exit the physician-scientist track altogether.
Compounding this issue is a lack of mentorship for early-career physician-scientists. With the rise of “careerism” in medicine, senior-level physician-scientists may have less incentive to mentor those who are earlier in their careers. Rather, there seems to be greater reward to “managing up” — that is, spending time to please hospital administrators and departmental leadership. Being involved in countless committees appears to carry more value in advancing an established investigator’s career than does mentorship.
Finally, physician-scientists typically earn less than their clinician colleagues, despite juggling both scientific and clinical responsibilities. While many are comfortable with this arrangement when embarking on this track, the disparity may become untenable after a while, especially as departmental leadership will often turn to physician-scientists to fill clinical coverage gaps when faculty leave the department, or as the medical center expands to satellite centers outside the primary hospital. Indeed, physician-scientists get pulled in several directions, which can lead to burnout and attrition, with many who are highly equipped for this track ultimately hanging up their cleats and seeking more clinical or private industry–oriented opportunities.
Every academic medical center operates differently. Some clearly have done a better job than others promoting and fostering physician-scientists. What we find in the centers that manage to retain physician-scientists is leadership plays a major role: If a medical center values the importance of physician-scientists, they will do things to foster the success of those people, such as assembling mentorship committees, establishing clear criteria for promotion and career advancement, protecting research time while maintaining some level of pay equity, advocating for team science approaches, and supporting investigators in cases of gaps in federal funding. Different countries also have different models for physician-scientist training, with Germany, for example, allowing medical residents to have 3 years of protected time to engage in research after their second year of residency.
The stakes here are high. If we can’t address the physician-scientist recruitment and retention crisis in America now, we risk falling behind other countries in our ability to innovate and deliver world-class care.
Dr Chaudhuri is a tenure-track physician-scientist at Washington University in St. Louis, a Paul and Daisy Soros Fellow, and a Public Voices Fellow of The OpEd Project.
Aadel Chaudhuri, MD, PhD, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recent reporting has shown that That’s a problem, because physician-scientists are uniquely equipped to make scientific discoveries in the laboratory and translate them to the clinic. Indeed, many of the discoveries that have transformed medicine for the better were made by physician-scientists. For example, Jonas Salk developed the polio vaccine, Timothy Ley sequenced the first cancer genome, and Anthony Fauci coordinated public health responses to both the HIV/AIDS and COVID-19 pandemics. Indicative of their sheer impact, at least a third and as many as half of all Nobel Prizes and Lasker Awards in physiology/medicine have gone to physician-scientists.
So why is the supply of physician-scientists shrinking so precipitously at a time when medical discoveries are being made at a record-high rate? Immunotherapy and proton therapy are transforming cancer care; RNA technology led to COVID vaccines; CRISPR is facilitating gene editing and treatment of diseases like sickle cell anemia. Yet, as exciting as medical science has become, only 1.5% of American doctors work as physician-scientists, more than a threefold drop compared with 30 years ago when the figure was a more robust 4.7%. What’s going on?
Residency training programs at prestigious academic medical centers have standard infolded research years; for example, neurosurgery residents at academic medical centers will often get 2 years of protected research time. And the National Institutes of Health has training grants dedicated to physician-scientists, such as the K08 award program. Several foundations are also dedicated to supporting early-career physician-scientists. Yet, the number of physicians deciding to become physician-scientists remains low, and, more troubling, the attrition rate of those who do decide to go this route is quite high.
The underlying issue is multifold. First, funding rates from the federal government for grants have become competitive to the point of being unrealistic. For example, the current funding rate for the flagship R01 program from the National Cancer Institute is only 12%. Promotions are typically tied to these grant awards, which means physician-scientists who are unable to acquire substantial grant funding are unable to pay for their research or win promotion — and often exit the physician-scientist track altogether.
Compounding this issue is a lack of mentorship for early-career physician-scientists. With the rise of “careerism” in medicine, senior-level physician-scientists may have less incentive to mentor those who are earlier in their careers. Rather, there seems to be greater reward to “managing up” — that is, spending time to please hospital administrators and departmental leadership. Being involved in countless committees appears to carry more value in advancing an established investigator’s career than does mentorship.
Finally, physician-scientists typically earn less than their clinician colleagues, despite juggling both scientific and clinical responsibilities. While many are comfortable with this arrangement when embarking on this track, the disparity may become untenable after a while, especially as departmental leadership will often turn to physician-scientists to fill clinical coverage gaps when faculty leave the department, or as the medical center expands to satellite centers outside the primary hospital. Indeed, physician-scientists get pulled in several directions, which can lead to burnout and attrition, with many who are highly equipped for this track ultimately hanging up their cleats and seeking more clinical or private industry–oriented opportunities.
Every academic medical center operates differently. Some clearly have done a better job than others promoting and fostering physician-scientists. What we find in the centers that manage to retain physician-scientists is leadership plays a major role: If a medical center values the importance of physician-scientists, they will do things to foster the success of those people, such as assembling mentorship committees, establishing clear criteria for promotion and career advancement, protecting research time while maintaining some level of pay equity, advocating for team science approaches, and supporting investigators in cases of gaps in federal funding. Different countries also have different models for physician-scientist training, with Germany, for example, allowing medical residents to have 3 years of protected time to engage in research after their second year of residency.
The stakes here are high. If we can’t address the physician-scientist recruitment and retention crisis in America now, we risk falling behind other countries in our ability to innovate and deliver world-class care.
Dr Chaudhuri is a tenure-track physician-scientist at Washington University in St. Louis, a Paul and Daisy Soros Fellow, and a Public Voices Fellow of The OpEd Project.
Aadel Chaudhuri, MD, PhD, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Five Bold Predictions for Long COVID in 2024
With a number of large-scale clinical trials underway and researchers on the hunt for new therapies, long COVID scientists are hopeful that this is the year patients — and doctors who care for them — will finally see improvements in treating their symptoms.
Here are five bold predictions — all based on encouraging research — that could happen in 2024. At the very least, they are promising signs of progress against a debilitating and frustrating disease.
#1: We’ll gain a better understanding of each long COVID phenotype
This past year, a wide breadth of research began showing that long COVID can be defined by a number of different disease phenotypes that present a range of symptoms.
Researchers identified four clinical phenotypes: Chronic fatigue-like syndrome, headache, and memory loss; respiratory syndrome, which includes cough and difficulty breathing; chronic pain; and neurosensorial syndrome, which causes an altered sense of taste and smell.
Identifying specific diagnostic criteria for each phenotype would lead to better health outcomes for patients instead of treating them as if it were a “one-size-fits-all disease,” said Nisha Viswanathan, MD, director of the long COVID program at UCLA Health, Los Angeles, California.
Ultimately, she hopes that this year her patients will receive treatments based on the type of long COVID they’re personally experiencing, and the symptoms they have, leading to improved health outcomes and more rapid relief.
“Many new medications are focused on different pathways of long COVID, and the challenge becomes which drug is the right drug for each treatment,” said Dr. Viswanathan.
#2: Monoclonal antibodies may change the game
We’re starting to have a better understanding that what’s been called “viral persistence” as a main cause of long COVID may potentially be treated with monoclonal antibodies. These are antibodies produced by cloning unique white blood cells to target the circulating spike proteins in the blood that hang out in viral reservoirs and cause the immune system to react as if it’s still fighting acute COVID-19.
Smaller-scale studies have already shown promising results. A January 2024 study published in The American Journal of Emergency Medicine followed three patients who completely recovered from long COVID after taking monoclonal antibodies. “Remission occurred despite dissimilar past histories, sex, age, and illness duration,” wrote the study authors.
Larger clinical trials are underway at the University of California, San Francisco, California, to test targeted monoclonal antibodies. If the results of the larger study show that monoclonal antibodies are beneficial, then it could be a game changer for a large swath of patients around the world, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
“The idea is that the downstream damage caused by viral persistence will resolve itself once you wipe out the virus,” said Dr. Putrino.
#3: Paxlovid could prove effective for long COVID
The US Food and Drug Administration granted approval for Paxlovid last May for the treatment of mild to moderate COVID-19 in adults at a high risk for severe disease. The medication is made up of two drugs packaged together. The first, nirmatrelvir, works by blocking a key enzyme required for virus replication. The second, ritonavir, is an antiviral that’s been used in patients with HIV and helps boost levels of antivirals in the body.
In a large-scale trial headed up by Dr. Putrino and his team, the oral antiviral is being studied for use in the post-viral stage in patients who test negative for acute COVID-19 but have persisting symptoms of long COVID.
Similar to monoclonal antibodies, the idea is to quell viral persistence. If patients have long COVID because they can’t clear SAR-CoV-2 from their bodies, Paxlovid could help. But unlike monoclonal antibodies that quash the virus, Paxlovid stops the virus from replicating. It’s a different mechanism with the same end goal.
It’s been a controversial treatment because it’s life-changing for some patients and ineffective for others. In addition, it can cause a range of side effects such as diarrhea, nausea, vomiting, and an impaired sense of taste. The goal of the trial is to see which patients with long COVID are most likely to benefit from the treatment.
#4: Anti-inflammatories like metformin could prove useful
Many of the inflammatory markers persistent in patients with long COVID were similarly present in patients with autoimmune diseases like rheumatoid arthritis, according to a July 2023 study published in JAMA.
The hope is that anti-inflammatory medications may be used to reduce inflammation causing long COVID symptoms. But drugs used to treat rheumatoid arthritis like abatacept and infliximabcan also have serious side effects, including increased risk for infection, flu-like symptoms, and burning of the skin.
“Powerful anti-inflammatories can change a number of pathways in the immune system,” said Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. Anti-inflammatories hold promise but, Dr. McComsey said, “some are more toxic with many side effects, so even if they work, there’s still a question about who should take them.”
Still, other anti-inflammatories that could work don’t have as many side effects. For example, a study published in The Lancet Infectious Diseases found that the diabetes drug metformin reduced a patient’s risk for long COVID up to 40% when the drug was taken during the acute stage.
Metformin, compared to other anti-inflammatories (also known as immune modulators), is an inexpensive and widely available drug with relatively few side effects compared with other medications.
#5: Serotonin levels — and selective serotonin reuptake inhibitors (SSRIs) — may be keys to unlocking long COVID
One of the most groundbreaking studies of the year came last November. A study published in the journal Cell found lower circulating serotonin levels in patents with long COVID than in those who did not have the condition. The study also found that the SSRI fluoxetine improved cognitive function in rat models infected with the virus.
Researchers found that the reduction in serotonin levels was partially caused by the body’s inability to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactivated blood platelets may also have played a role.
Michael Peluso, MD, an assistant research professor of infectious medicine at the UCSF School of Medicine, San Francisco, California, hopes to take the finding a step further, investigating whether increased serotonin levels in patients with long COVID will lead to improvements in symptoms.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said last month in an interview with this news organization.
If patients show an improvement in symptoms, then the next step is looking into whether SSRIs boost serotonin levels in patients and, as a result, reduce their symptoms.
A version of this article appeared on Medscape.com.
With a number of large-scale clinical trials underway and researchers on the hunt for new therapies, long COVID scientists are hopeful that this is the year patients — and doctors who care for them — will finally see improvements in treating their symptoms.
Here are five bold predictions — all based on encouraging research — that could happen in 2024. At the very least, they are promising signs of progress against a debilitating and frustrating disease.
#1: We’ll gain a better understanding of each long COVID phenotype
This past year, a wide breadth of research began showing that long COVID can be defined by a number of different disease phenotypes that present a range of symptoms.
Researchers identified four clinical phenotypes: Chronic fatigue-like syndrome, headache, and memory loss; respiratory syndrome, which includes cough and difficulty breathing; chronic pain; and neurosensorial syndrome, which causes an altered sense of taste and smell.
Identifying specific diagnostic criteria for each phenotype would lead to better health outcomes for patients instead of treating them as if it were a “one-size-fits-all disease,” said Nisha Viswanathan, MD, director of the long COVID program at UCLA Health, Los Angeles, California.
Ultimately, she hopes that this year her patients will receive treatments based on the type of long COVID they’re personally experiencing, and the symptoms they have, leading to improved health outcomes and more rapid relief.
“Many new medications are focused on different pathways of long COVID, and the challenge becomes which drug is the right drug for each treatment,” said Dr. Viswanathan.
#2: Monoclonal antibodies may change the game
We’re starting to have a better understanding that what’s been called “viral persistence” as a main cause of long COVID may potentially be treated with monoclonal antibodies. These are antibodies produced by cloning unique white blood cells to target the circulating spike proteins in the blood that hang out in viral reservoirs and cause the immune system to react as if it’s still fighting acute COVID-19.
Smaller-scale studies have already shown promising results. A January 2024 study published in The American Journal of Emergency Medicine followed three patients who completely recovered from long COVID after taking monoclonal antibodies. “Remission occurred despite dissimilar past histories, sex, age, and illness duration,” wrote the study authors.
Larger clinical trials are underway at the University of California, San Francisco, California, to test targeted monoclonal antibodies. If the results of the larger study show that monoclonal antibodies are beneficial, then it could be a game changer for a large swath of patients around the world, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
“The idea is that the downstream damage caused by viral persistence will resolve itself once you wipe out the virus,” said Dr. Putrino.
#3: Paxlovid could prove effective for long COVID
The US Food and Drug Administration granted approval for Paxlovid last May for the treatment of mild to moderate COVID-19 in adults at a high risk for severe disease. The medication is made up of two drugs packaged together. The first, nirmatrelvir, works by blocking a key enzyme required for virus replication. The second, ritonavir, is an antiviral that’s been used in patients with HIV and helps boost levels of antivirals in the body.
In a large-scale trial headed up by Dr. Putrino and his team, the oral antiviral is being studied for use in the post-viral stage in patients who test negative for acute COVID-19 but have persisting symptoms of long COVID.
Similar to monoclonal antibodies, the idea is to quell viral persistence. If patients have long COVID because they can’t clear SAR-CoV-2 from their bodies, Paxlovid could help. But unlike monoclonal antibodies that quash the virus, Paxlovid stops the virus from replicating. It’s a different mechanism with the same end goal.
It’s been a controversial treatment because it’s life-changing for some patients and ineffective for others. In addition, it can cause a range of side effects such as diarrhea, nausea, vomiting, and an impaired sense of taste. The goal of the trial is to see which patients with long COVID are most likely to benefit from the treatment.
#4: Anti-inflammatories like metformin could prove useful
Many of the inflammatory markers persistent in patients with long COVID were similarly present in patients with autoimmune diseases like rheumatoid arthritis, according to a July 2023 study published in JAMA.
The hope is that anti-inflammatory medications may be used to reduce inflammation causing long COVID symptoms. But drugs used to treat rheumatoid arthritis like abatacept and infliximabcan also have serious side effects, including increased risk for infection, flu-like symptoms, and burning of the skin.
“Powerful anti-inflammatories can change a number of pathways in the immune system,” said Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. Anti-inflammatories hold promise but, Dr. McComsey said, “some are more toxic with many side effects, so even if they work, there’s still a question about who should take them.”
Still, other anti-inflammatories that could work don’t have as many side effects. For example, a study published in The Lancet Infectious Diseases found that the diabetes drug metformin reduced a patient’s risk for long COVID up to 40% when the drug was taken during the acute stage.
Metformin, compared to other anti-inflammatories (also known as immune modulators), is an inexpensive and widely available drug with relatively few side effects compared with other medications.
#5: Serotonin levels — and selective serotonin reuptake inhibitors (SSRIs) — may be keys to unlocking long COVID
One of the most groundbreaking studies of the year came last November. A study published in the journal Cell found lower circulating serotonin levels in patents with long COVID than in those who did not have the condition. The study also found that the SSRI fluoxetine improved cognitive function in rat models infected with the virus.
Researchers found that the reduction in serotonin levels was partially caused by the body’s inability to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactivated blood platelets may also have played a role.
Michael Peluso, MD, an assistant research professor of infectious medicine at the UCSF School of Medicine, San Francisco, California, hopes to take the finding a step further, investigating whether increased serotonin levels in patients with long COVID will lead to improvements in symptoms.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said last month in an interview with this news organization.
If patients show an improvement in symptoms, then the next step is looking into whether SSRIs boost serotonin levels in patients and, as a result, reduce their symptoms.
A version of this article appeared on Medscape.com.
With a number of large-scale clinical trials underway and researchers on the hunt for new therapies, long COVID scientists are hopeful that this is the year patients — and doctors who care for them — will finally see improvements in treating their symptoms.
Here are five bold predictions — all based on encouraging research — that could happen in 2024. At the very least, they are promising signs of progress against a debilitating and frustrating disease.
#1: We’ll gain a better understanding of each long COVID phenotype
This past year, a wide breadth of research began showing that long COVID can be defined by a number of different disease phenotypes that present a range of symptoms.
Researchers identified four clinical phenotypes: Chronic fatigue-like syndrome, headache, and memory loss; respiratory syndrome, which includes cough and difficulty breathing; chronic pain; and neurosensorial syndrome, which causes an altered sense of taste and smell.
Identifying specific diagnostic criteria for each phenotype would lead to better health outcomes for patients instead of treating them as if it were a “one-size-fits-all disease,” said Nisha Viswanathan, MD, director of the long COVID program at UCLA Health, Los Angeles, California.
Ultimately, she hopes that this year her patients will receive treatments based on the type of long COVID they’re personally experiencing, and the symptoms they have, leading to improved health outcomes and more rapid relief.
“Many new medications are focused on different pathways of long COVID, and the challenge becomes which drug is the right drug for each treatment,” said Dr. Viswanathan.
#2: Monoclonal antibodies may change the game
We’re starting to have a better understanding that what’s been called “viral persistence” as a main cause of long COVID may potentially be treated with monoclonal antibodies. These are antibodies produced by cloning unique white blood cells to target the circulating spike proteins in the blood that hang out in viral reservoirs and cause the immune system to react as if it’s still fighting acute COVID-19.
Smaller-scale studies have already shown promising results. A January 2024 study published in The American Journal of Emergency Medicine followed three patients who completely recovered from long COVID after taking monoclonal antibodies. “Remission occurred despite dissimilar past histories, sex, age, and illness duration,” wrote the study authors.
Larger clinical trials are underway at the University of California, San Francisco, California, to test targeted monoclonal antibodies. If the results of the larger study show that monoclonal antibodies are beneficial, then it could be a game changer for a large swath of patients around the world, said David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City.
“The idea is that the downstream damage caused by viral persistence will resolve itself once you wipe out the virus,” said Dr. Putrino.
#3: Paxlovid could prove effective for long COVID
The US Food and Drug Administration granted approval for Paxlovid last May for the treatment of mild to moderate COVID-19 in adults at a high risk for severe disease. The medication is made up of two drugs packaged together. The first, nirmatrelvir, works by blocking a key enzyme required for virus replication. The second, ritonavir, is an antiviral that’s been used in patients with HIV and helps boost levels of antivirals in the body.
In a large-scale trial headed up by Dr. Putrino and his team, the oral antiviral is being studied for use in the post-viral stage in patients who test negative for acute COVID-19 but have persisting symptoms of long COVID.
Similar to monoclonal antibodies, the idea is to quell viral persistence. If patients have long COVID because they can’t clear SAR-CoV-2 from their bodies, Paxlovid could help. But unlike monoclonal antibodies that quash the virus, Paxlovid stops the virus from replicating. It’s a different mechanism with the same end goal.
It’s been a controversial treatment because it’s life-changing for some patients and ineffective for others. In addition, it can cause a range of side effects such as diarrhea, nausea, vomiting, and an impaired sense of taste. The goal of the trial is to see which patients with long COVID are most likely to benefit from the treatment.
#4: Anti-inflammatories like metformin could prove useful
Many of the inflammatory markers persistent in patients with long COVID were similarly present in patients with autoimmune diseases like rheumatoid arthritis, according to a July 2023 study published in JAMA.
The hope is that anti-inflammatory medications may be used to reduce inflammation causing long COVID symptoms. But drugs used to treat rheumatoid arthritis like abatacept and infliximabcan also have serious side effects, including increased risk for infection, flu-like symptoms, and burning of the skin.
“Powerful anti-inflammatories can change a number of pathways in the immune system,” said Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, Ohio. Anti-inflammatories hold promise but, Dr. McComsey said, “some are more toxic with many side effects, so even if they work, there’s still a question about who should take them.”
Still, other anti-inflammatories that could work don’t have as many side effects. For example, a study published in The Lancet Infectious Diseases found that the diabetes drug metformin reduced a patient’s risk for long COVID up to 40% when the drug was taken during the acute stage.
Metformin, compared to other anti-inflammatories (also known as immune modulators), is an inexpensive and widely available drug with relatively few side effects compared with other medications.
#5: Serotonin levels — and selective serotonin reuptake inhibitors (SSRIs) — may be keys to unlocking long COVID
One of the most groundbreaking studies of the year came last November. A study published in the journal Cell found lower circulating serotonin levels in patents with long COVID than in those who did not have the condition. The study also found that the SSRI fluoxetine improved cognitive function in rat models infected with the virus.
Researchers found that the reduction in serotonin levels was partially caused by the body’s inability to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactivated blood platelets may also have played a role.
Michael Peluso, MD, an assistant research professor of infectious medicine at the UCSF School of Medicine, San Francisco, California, hopes to take the finding a step further, investigating whether increased serotonin levels in patients with long COVID will lead to improvements in symptoms.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said last month in an interview with this news organization.
If patients show an improvement in symptoms, then the next step is looking into whether SSRIs boost serotonin levels in patients and, as a result, reduce their symptoms.
A version of this article appeared on Medscape.com.
Low Vitamin D Levels May Signal CVD Risk in Young Adults
TOPLINE:
, small study finds.
METHODOLOGY:
- A secondary analysis of the Activating Brown Adipose Tissue Through Exercise (ACTIBATE) trial assessed the association between serum 25(OH)D levels and CVD risk factors.
- The cross-sectional study used baseline data of in 177 healthy sedentary adults ages 18-25 years (65% women; all White individuals), who were recruited between October 2015 and December 2016 from Granada, a region in the south of Spain.
- Study participants were nonsmokers, led a sedentary lifestyle, and did not have a prior history of CVD or chronic illnesses.
- The CVD risk factors included anthropometrical and body composition profiles, glucose and lipid metabolism, liver, and pro- and anti-inflammatory biomarkers.
- 25(OH)D serum concentrations were measured with a competitive chemiluminescence immunoassay and defined as deficient (< 20 ng/mL), insufficient (21-29 ng/mL), or normal (> 30 ng/mL).
TAKEAWAY:
- The levels correlated inversely with body mass index (BMI; standardized regression coefficient [beta], −0.177; P = .018), fat mass index (beta, −0.195; P = .011), and systolic blood pressure (beta, −0.137; P = .038), after adjusting for sex.
- Glucose metabolism markers (serum glucose and insulin concentrations, insulin/glucose ratio, and homeostatic model assessment of index) also correlated inversely with vitamin D levels.
- The trend was similar for liver markers serum γ-glutamyl transferase and alkaline phosphatase) and the anti-inflammatory marker interleukin-4.
- BMI, waist/hip ratio, fat mass index, blood pressure, and levels of glucose, insulin, , and liver markers were higher in the 44 participants with vitamin D deficiency vs 41 participants with normal vitamin D levels.
IN PRACTICE:
“Collectively, these findings support the idea that 25(OH)D concentrations may be used as a useful marker of CVD status, which can be easily monitored in young individuals,” the authors wrote.
SOURCE:
This study was led by first author Francisco J. AmaroGahete, MD, PhD, from the Department of Physiology, Faculty of Medicine, University of Granada, Spain, who also holds positions in other institutions. It was published online in the Journal of Endocrinological Investigation.
LIMITATIONS:
This study could not establish causal relationships due to its cross-sectional design. The results might not apply to younger or older people from different locations and ethnic backgrounds. The gold standard method for analyzing vitamin D levels, liquid chromatography–mass spectrometry, was not used in this study.
DISCLOSURES:
This study was supported by the Spanish Ministry of Economy and Competitiveness, Spanish Ministry of Education, AstraZeneca HealthCare Foundation, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, small study finds.
METHODOLOGY:
- A secondary analysis of the Activating Brown Adipose Tissue Through Exercise (ACTIBATE) trial assessed the association between serum 25(OH)D levels and CVD risk factors.
- The cross-sectional study used baseline data of in 177 healthy sedentary adults ages 18-25 years (65% women; all White individuals), who were recruited between October 2015 and December 2016 from Granada, a region in the south of Spain.
- Study participants were nonsmokers, led a sedentary lifestyle, and did not have a prior history of CVD or chronic illnesses.
- The CVD risk factors included anthropometrical and body composition profiles, glucose and lipid metabolism, liver, and pro- and anti-inflammatory biomarkers.
- 25(OH)D serum concentrations were measured with a competitive chemiluminescence immunoassay and defined as deficient (< 20 ng/mL), insufficient (21-29 ng/mL), or normal (> 30 ng/mL).
TAKEAWAY:
- The levels correlated inversely with body mass index (BMI; standardized regression coefficient [beta], −0.177; P = .018), fat mass index (beta, −0.195; P = .011), and systolic blood pressure (beta, −0.137; P = .038), after adjusting for sex.
- Glucose metabolism markers (serum glucose and insulin concentrations, insulin/glucose ratio, and homeostatic model assessment of index) also correlated inversely with vitamin D levels.
- The trend was similar for liver markers serum γ-glutamyl transferase and alkaline phosphatase) and the anti-inflammatory marker interleukin-4.
- BMI, waist/hip ratio, fat mass index, blood pressure, and levels of glucose, insulin, , and liver markers were higher in the 44 participants with vitamin D deficiency vs 41 participants with normal vitamin D levels.
IN PRACTICE:
“Collectively, these findings support the idea that 25(OH)D concentrations may be used as a useful marker of CVD status, which can be easily monitored in young individuals,” the authors wrote.
SOURCE:
This study was led by first author Francisco J. AmaroGahete, MD, PhD, from the Department of Physiology, Faculty of Medicine, University of Granada, Spain, who also holds positions in other institutions. It was published online in the Journal of Endocrinological Investigation.
LIMITATIONS:
This study could not establish causal relationships due to its cross-sectional design. The results might not apply to younger or older people from different locations and ethnic backgrounds. The gold standard method for analyzing vitamin D levels, liquid chromatography–mass spectrometry, was not used in this study.
DISCLOSURES:
This study was supported by the Spanish Ministry of Economy and Competitiveness, Spanish Ministry of Education, AstraZeneca HealthCare Foundation, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
, small study finds.
METHODOLOGY:
- A secondary analysis of the Activating Brown Adipose Tissue Through Exercise (ACTIBATE) trial assessed the association between serum 25(OH)D levels and CVD risk factors.
- The cross-sectional study used baseline data of in 177 healthy sedentary adults ages 18-25 years (65% women; all White individuals), who were recruited between October 2015 and December 2016 from Granada, a region in the south of Spain.
- Study participants were nonsmokers, led a sedentary lifestyle, and did not have a prior history of CVD or chronic illnesses.
- The CVD risk factors included anthropometrical and body composition profiles, glucose and lipid metabolism, liver, and pro- and anti-inflammatory biomarkers.
- 25(OH)D serum concentrations were measured with a competitive chemiluminescence immunoassay and defined as deficient (< 20 ng/mL), insufficient (21-29 ng/mL), or normal (> 30 ng/mL).
TAKEAWAY:
- The levels correlated inversely with body mass index (BMI; standardized regression coefficient [beta], −0.177; P = .018), fat mass index (beta, −0.195; P = .011), and systolic blood pressure (beta, −0.137; P = .038), after adjusting for sex.
- Glucose metabolism markers (serum glucose and insulin concentrations, insulin/glucose ratio, and homeostatic model assessment of index) also correlated inversely with vitamin D levels.
- The trend was similar for liver markers serum γ-glutamyl transferase and alkaline phosphatase) and the anti-inflammatory marker interleukin-4.
- BMI, waist/hip ratio, fat mass index, blood pressure, and levels of glucose, insulin, , and liver markers were higher in the 44 participants with vitamin D deficiency vs 41 participants with normal vitamin D levels.
IN PRACTICE:
“Collectively, these findings support the idea that 25(OH)D concentrations may be used as a useful marker of CVD status, which can be easily monitored in young individuals,” the authors wrote.
SOURCE:
This study was led by first author Francisco J. AmaroGahete, MD, PhD, from the Department of Physiology, Faculty of Medicine, University of Granada, Spain, who also holds positions in other institutions. It was published online in the Journal of Endocrinological Investigation.
LIMITATIONS:
This study could not establish causal relationships due to its cross-sectional design. The results might not apply to younger or older people from different locations and ethnic backgrounds. The gold standard method for analyzing vitamin D levels, liquid chromatography–mass spectrometry, was not used in this study.
DISCLOSURES:
This study was supported by the Spanish Ministry of Economy and Competitiveness, Spanish Ministry of Education, AstraZeneca HealthCare Foundation, and other sources. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Dana-Farber Moves to Retract, Correct Dozens of Cancer Papers Amid Allegations
News of the investigation follows a blog post by British molecular biologist Sholto David, MD, who flagged almost 60 papers published between 1997 and 2017 that contained image manipulation and other errors. Some of the papers were published by Dana-Farber’s chief executive officer, Laurie Glimcher, MD, and chief operating officer, William Hahn, MD, on topics including multiple myeloma and immune cells.
Mr. David, who blogs about research integrity, highlighted numerous errors and irregularities, including copying and pasting images across multiple experiments to represent different days within the same experiment, sometimes rotating or stretching images.
In one case, Mr. David equated the manipulation with tactics used by “hapless Chinese papermills” and concluded that “a swathe of research coming out of [Dana-Farber] authored by the most senior researchers and managers appears to be hopelessly corrupt with errors that are obvious from just a cursory reading the papers.”
“Imagine what mistakes might be found in the raw data if anyone was allowed to look!” he wrote.
Barrett Rollins, MD, PhD, Dana-Farber Cancer Institute’s research integrity officer, declined to comment on whether the errors represent scientific misconduct, according to STAT. Rollins told ScienceInsider that the “presence of image discrepancies in a paper is not evidence of an author’s intent to deceive.”
Access to new artificial intelligence tools is making it easier for data sleuths, like Mr. David, to unearth data manipulation and errors.
The current investigation closely follows two other investigations into the published work of Harvard University’s former president, Claudine Gay, and Stanford University’s former president, Marc Tessier-Lavigne, which led both to resign their posts.
A version of this article appeared on Medscape.com.
News of the investigation follows a blog post by British molecular biologist Sholto David, MD, who flagged almost 60 papers published between 1997 and 2017 that contained image manipulation and other errors. Some of the papers were published by Dana-Farber’s chief executive officer, Laurie Glimcher, MD, and chief operating officer, William Hahn, MD, on topics including multiple myeloma and immune cells.
Mr. David, who blogs about research integrity, highlighted numerous errors and irregularities, including copying and pasting images across multiple experiments to represent different days within the same experiment, sometimes rotating or stretching images.
In one case, Mr. David equated the manipulation with tactics used by “hapless Chinese papermills” and concluded that “a swathe of research coming out of [Dana-Farber] authored by the most senior researchers and managers appears to be hopelessly corrupt with errors that are obvious from just a cursory reading the papers.”
“Imagine what mistakes might be found in the raw data if anyone was allowed to look!” he wrote.
Barrett Rollins, MD, PhD, Dana-Farber Cancer Institute’s research integrity officer, declined to comment on whether the errors represent scientific misconduct, according to STAT. Rollins told ScienceInsider that the “presence of image discrepancies in a paper is not evidence of an author’s intent to deceive.”
Access to new artificial intelligence tools is making it easier for data sleuths, like Mr. David, to unearth data manipulation and errors.
The current investigation closely follows two other investigations into the published work of Harvard University’s former president, Claudine Gay, and Stanford University’s former president, Marc Tessier-Lavigne, which led both to resign their posts.
A version of this article appeared on Medscape.com.
News of the investigation follows a blog post by British molecular biologist Sholto David, MD, who flagged almost 60 papers published between 1997 and 2017 that contained image manipulation and other errors. Some of the papers were published by Dana-Farber’s chief executive officer, Laurie Glimcher, MD, and chief operating officer, William Hahn, MD, on topics including multiple myeloma and immune cells.
Mr. David, who blogs about research integrity, highlighted numerous errors and irregularities, including copying and pasting images across multiple experiments to represent different days within the same experiment, sometimes rotating or stretching images.
In one case, Mr. David equated the manipulation with tactics used by “hapless Chinese papermills” and concluded that “a swathe of research coming out of [Dana-Farber] authored by the most senior researchers and managers appears to be hopelessly corrupt with errors that are obvious from just a cursory reading the papers.”
“Imagine what mistakes might be found in the raw data if anyone was allowed to look!” he wrote.
Barrett Rollins, MD, PhD, Dana-Farber Cancer Institute’s research integrity officer, declined to comment on whether the errors represent scientific misconduct, according to STAT. Rollins told ScienceInsider that the “presence of image discrepancies in a paper is not evidence of an author’s intent to deceive.”
Access to new artificial intelligence tools is making it easier for data sleuths, like Mr. David, to unearth data manipulation and errors.
The current investigation closely follows two other investigations into the published work of Harvard University’s former president, Claudine Gay, and Stanford University’s former president, Marc Tessier-Lavigne, which led both to resign their posts.
A version of this article appeared on Medscape.com.
Obstructive Sleep Apnea May Promote Early Bone Loss
TOPLINE:
Indicators of early bone loss were significantly higher in adults with severe obstructive sleep apnea (OSA) than in those with mild or moderate OSA and controls.
METHODOLOGY:
- The researchers enrolled 90 men aged 30-59 years who were patients at a single sleep and respiratory center between August 2017 and February 2019; the average age was 47.1 years, and the average body mass index was 25.7 kg/m2.
- The study population included 25 individuals with mild OSA, 21 with moderate OSA, 34 with severe OSA, and 10 controls without OSA.
- Bone loss was assessed using high-resolution peripheral quantitative computed tomography and blood samples. The researchers collected information on metabolic and inflammatory bone turnover indicators, as well as bone geometric parameters, bone microstructure parameters, and measures of bone mineral density (BMD).
TAKEAWAY:
- Total volumetric bone mineral density was significantly lower in patients with OSA than in controls and significantly different among OSA groups, as were the meta trabecular volumetric BMD, trabecular thickness (Tb.Th), and cortical thickness (Ct.Th).
- Differences in bone microstructure between patients with OSA and controls were most evident in measures of Tb.Th and Ct.Th.
- No significant differences appeared in blood bone turnover indicators or inflammation indicators among the groups.
IN PRACTICE:
“A study with a larger sample is necessary to further assess the relationship and mechanisms between OSA and osteoporosis,” the researchers wrote.
SOURCE:
The lead author on the study was Yixian Qiao, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China. The study was published online in BMC Pulmonary Medicine.
LIMITATIONS:
The cross-sectional design, small sample size, and inability to control for several key confounders such as nutritional status and amount of exercise, as well as the exclusion of women and elderly individuals, limited the findings.
DISCLOSURES:
The study was supported by the National Key Research and Development Projects of China. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Indicators of early bone loss were significantly higher in adults with severe obstructive sleep apnea (OSA) than in those with mild or moderate OSA and controls.
METHODOLOGY:
- The researchers enrolled 90 men aged 30-59 years who were patients at a single sleep and respiratory center between August 2017 and February 2019; the average age was 47.1 years, and the average body mass index was 25.7 kg/m2.
- The study population included 25 individuals with mild OSA, 21 with moderate OSA, 34 with severe OSA, and 10 controls without OSA.
- Bone loss was assessed using high-resolution peripheral quantitative computed tomography and blood samples. The researchers collected information on metabolic and inflammatory bone turnover indicators, as well as bone geometric parameters, bone microstructure parameters, and measures of bone mineral density (BMD).
TAKEAWAY:
- Total volumetric bone mineral density was significantly lower in patients with OSA than in controls and significantly different among OSA groups, as were the meta trabecular volumetric BMD, trabecular thickness (Tb.Th), and cortical thickness (Ct.Th).
- Differences in bone microstructure between patients with OSA and controls were most evident in measures of Tb.Th and Ct.Th.
- No significant differences appeared in blood bone turnover indicators or inflammation indicators among the groups.
IN PRACTICE:
“A study with a larger sample is necessary to further assess the relationship and mechanisms between OSA and osteoporosis,” the researchers wrote.
SOURCE:
The lead author on the study was Yixian Qiao, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China. The study was published online in BMC Pulmonary Medicine.
LIMITATIONS:
The cross-sectional design, small sample size, and inability to control for several key confounders such as nutritional status and amount of exercise, as well as the exclusion of women and elderly individuals, limited the findings.
DISCLOSURES:
The study was supported by the National Key Research and Development Projects of China. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Indicators of early bone loss were significantly higher in adults with severe obstructive sleep apnea (OSA) than in those with mild or moderate OSA and controls.
METHODOLOGY:
- The researchers enrolled 90 men aged 30-59 years who were patients at a single sleep and respiratory center between August 2017 and February 2019; the average age was 47.1 years, and the average body mass index was 25.7 kg/m2.
- The study population included 25 individuals with mild OSA, 21 with moderate OSA, 34 with severe OSA, and 10 controls without OSA.
- Bone loss was assessed using high-resolution peripheral quantitative computed tomography and blood samples. The researchers collected information on metabolic and inflammatory bone turnover indicators, as well as bone geometric parameters, bone microstructure parameters, and measures of bone mineral density (BMD).
TAKEAWAY:
- Total volumetric bone mineral density was significantly lower in patients with OSA than in controls and significantly different among OSA groups, as were the meta trabecular volumetric BMD, trabecular thickness (Tb.Th), and cortical thickness (Ct.Th).
- Differences in bone microstructure between patients with OSA and controls were most evident in measures of Tb.Th and Ct.Th.
- No significant differences appeared in blood bone turnover indicators or inflammation indicators among the groups.
IN PRACTICE:
“A study with a larger sample is necessary to further assess the relationship and mechanisms between OSA and osteoporosis,” the researchers wrote.
SOURCE:
The lead author on the study was Yixian Qiao, MD, of the Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China. The study was published online in BMC Pulmonary Medicine.
LIMITATIONS:
The cross-sectional design, small sample size, and inability to control for several key confounders such as nutritional status and amount of exercise, as well as the exclusion of women and elderly individuals, limited the findings.
DISCLOSURES:
The study was supported by the National Key Research and Development Projects of China. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Cancer Identified as a New Cardiovascular Risk Factor
A history of cancer is an independent predictor of major cardiovascular events in patients undergoing coronary angioplasty. Cancer should be considered a new cardiovascular risk factor in primary and secondary prevention, according to a study presented at the 2023 American Heart Association Congress in Philadelphia.
, for example, equating it to the situation of a patient with diabetes or chronic renal failure,” said lead author Renzo Melchiori, MD, a cardiologist at the University Hospital Austral in Pilar, Argentina.
The researchers also advocate for intensifying cardiovascular control measures in secondary prevention for these patients, reconsidering goals, and ensuring compliance with prescribed pharmacological regimens and healthy lifestyle habits.
“Previously, when a patient had oncological pathology, thinking about associated cardiovascular risk seemed somewhat superfluous. But today, oncological diseases are treated so effectively, increasing survival and life expectancy, that we begin to focus on what happens with the arteries of these patients after treatment,” said Dr. Melchiori.
Higher Incidence Density
The retrospective analysis included 937 patients of both sexes aged 18 years and older who underwent coronary angioplasty for acute coronary syndrome between 2008 and 2022 at a university hospital. Of these participants, 89 (9.5%) had a history of cancer, with a median time since oncologic diagnosis of around 2 years for solid and hematologic tumors. Most participants had treated and resolved cancer.
Over a median follow-up of 45 months (range, 14-72 months), the cumulative incidence rates of a major cardiovascular event (nonfatal stroke, nonfatal acute myocardial infarction, cardiovascular death, or new angioplasty) were 22.2% (155/698) and 28.4% (25/88) in the groups without and with a history of cancer, respectively. The incidence density was significantly higher in the group with an oncologic history than in the group without such a history: 0.78 events/100 patients/month vs 0.48 events/100 patients/month (P = .01).
Kaplan-Meier analysis showed a higher probability of a major cardiovascular event in the group of patients with cancer or a history of cancer (P = .0086). In multivariate Cox regression analysis, cancer history was an independent predictor of major cardiovascular events adjusted for other risk factors such as age, hypertension, diabetes, smoking, sedentary lifestyle, and family history (hazard ratio, 1.66; P = .025).
Dr. Melchiori clarified that the increased incidence of cardiovascular events in patients with cancer or a history of cancer cannot be attributed to differences in percutaneous intervention or the indication or compliance of post-treatment pharmacological therapy.
In addition, the specialist acknowledged that due to the sample size, discrimination by cancer type, disease stage, or therapeutic strategies couldn’t be performed. A subanalysis, which has not been presented, indicated that the effect could not be explained solely by the application of radiotherapy or chemotherapy in the 90 days before angioplasty — two factors that cause arterial inflammation.
Intensifying Prevention Measures
Two independent experts told this news organization that the new study is "interesting" and reinforces the close connection between oncologic and cardiovascular pathology.
Andrés Daniele, MD, cardiologist and president of the Argentine Cardio-Oncology Association, a local chapter of the International Cardio-Oncology Society, emphasized that the study “reiterates an observation seen in other works: A higher rate of atherosclerotic pathology and cardiovascular events in patients with a history of cancer. And that has a reason to be: Both pathologies present common risk factors, and on the other hand, there is greater endothelial dysfunction secondary to the inflammatory syndrome and oncologic therapies.”
“There needs to be a continuum in the intensification of measures in primary and secondary cardiovascular prevention in cancer survivors, whether in remission or with chronic disease. We need to be very aggressive in managing risk factors and insist that patients who have had a cardiovascular event enter cardiovascular rehabilitation therapies,” said Dr. Daniele, who also heads the Cardio-Oncology Department at the centenary Roffo Institute of Oncology at the University of Buenos Aires, Argentina.
The study provides a valuable contribution because “we need to understand the epidemiology and natural history of patients with cancer at risk of developing cardiovascular complications to implement personalized cardiovascular prevention strategies,” said Teresa López Fernández, MD, cardiologist, coordinator of the Cardio-Oncology Program at La Paz University Hospital in Madrid, member of the Cardio-Oncology Working Group of the Spanish Society of Cardiology, member of the board of the International Cardio-Oncology Society, and cochair of the first clinical practice guidelines in cardio-oncology of the European Society of Cardiology.
“We have to be aware that perhaps we should not guide ourselves in these patients with the usual risk stratification scores as cancer or cardiotoxic treatment are not included as variables. However, they require our attention and effort to improve their quality and quantity of life, avoiding potentially preventable cardiovascular events that could negatively impact the survival achieved thanks to advances in cancer treatments,” said Dr. López Fernández.
Dr. Melchiori and Dr. Daniele declared no relevant economic conflicts of interest. Dr. López Fernández reported relationships with Daiichi Sankyo, Almirall España, Janssen-Cilag, Bayer, Roche, Philips, and Incyte.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
A history of cancer is an independent predictor of major cardiovascular events in patients undergoing coronary angioplasty. Cancer should be considered a new cardiovascular risk factor in primary and secondary prevention, according to a study presented at the 2023 American Heart Association Congress in Philadelphia.
, for example, equating it to the situation of a patient with diabetes or chronic renal failure,” said lead author Renzo Melchiori, MD, a cardiologist at the University Hospital Austral in Pilar, Argentina.
The researchers also advocate for intensifying cardiovascular control measures in secondary prevention for these patients, reconsidering goals, and ensuring compliance with prescribed pharmacological regimens and healthy lifestyle habits.
“Previously, when a patient had oncological pathology, thinking about associated cardiovascular risk seemed somewhat superfluous. But today, oncological diseases are treated so effectively, increasing survival and life expectancy, that we begin to focus on what happens with the arteries of these patients after treatment,” said Dr. Melchiori.
Higher Incidence Density
The retrospective analysis included 937 patients of both sexes aged 18 years and older who underwent coronary angioplasty for acute coronary syndrome between 2008 and 2022 at a university hospital. Of these participants, 89 (9.5%) had a history of cancer, with a median time since oncologic diagnosis of around 2 years for solid and hematologic tumors. Most participants had treated and resolved cancer.
Over a median follow-up of 45 months (range, 14-72 months), the cumulative incidence rates of a major cardiovascular event (nonfatal stroke, nonfatal acute myocardial infarction, cardiovascular death, or new angioplasty) were 22.2% (155/698) and 28.4% (25/88) in the groups without and with a history of cancer, respectively. The incidence density was significantly higher in the group with an oncologic history than in the group without such a history: 0.78 events/100 patients/month vs 0.48 events/100 patients/month (P = .01).
Kaplan-Meier analysis showed a higher probability of a major cardiovascular event in the group of patients with cancer or a history of cancer (P = .0086). In multivariate Cox regression analysis, cancer history was an independent predictor of major cardiovascular events adjusted for other risk factors such as age, hypertension, diabetes, smoking, sedentary lifestyle, and family history (hazard ratio, 1.66; P = .025).
Dr. Melchiori clarified that the increased incidence of cardiovascular events in patients with cancer or a history of cancer cannot be attributed to differences in percutaneous intervention or the indication or compliance of post-treatment pharmacological therapy.
In addition, the specialist acknowledged that due to the sample size, discrimination by cancer type, disease stage, or therapeutic strategies couldn’t be performed. A subanalysis, which has not been presented, indicated that the effect could not be explained solely by the application of radiotherapy or chemotherapy in the 90 days before angioplasty — two factors that cause arterial inflammation.
Intensifying Prevention Measures
Two independent experts told this news organization that the new study is "interesting" and reinforces the close connection between oncologic and cardiovascular pathology.
Andrés Daniele, MD, cardiologist and president of the Argentine Cardio-Oncology Association, a local chapter of the International Cardio-Oncology Society, emphasized that the study “reiterates an observation seen in other works: A higher rate of atherosclerotic pathology and cardiovascular events in patients with a history of cancer. And that has a reason to be: Both pathologies present common risk factors, and on the other hand, there is greater endothelial dysfunction secondary to the inflammatory syndrome and oncologic therapies.”
“There needs to be a continuum in the intensification of measures in primary and secondary cardiovascular prevention in cancer survivors, whether in remission or with chronic disease. We need to be very aggressive in managing risk factors and insist that patients who have had a cardiovascular event enter cardiovascular rehabilitation therapies,” said Dr. Daniele, who also heads the Cardio-Oncology Department at the centenary Roffo Institute of Oncology at the University of Buenos Aires, Argentina.
The study provides a valuable contribution because “we need to understand the epidemiology and natural history of patients with cancer at risk of developing cardiovascular complications to implement personalized cardiovascular prevention strategies,” said Teresa López Fernández, MD, cardiologist, coordinator of the Cardio-Oncology Program at La Paz University Hospital in Madrid, member of the Cardio-Oncology Working Group of the Spanish Society of Cardiology, member of the board of the International Cardio-Oncology Society, and cochair of the first clinical practice guidelines in cardio-oncology of the European Society of Cardiology.
“We have to be aware that perhaps we should not guide ourselves in these patients with the usual risk stratification scores as cancer or cardiotoxic treatment are not included as variables. However, they require our attention and effort to improve their quality and quantity of life, avoiding potentially preventable cardiovascular events that could negatively impact the survival achieved thanks to advances in cancer treatments,” said Dr. López Fernández.
Dr. Melchiori and Dr. Daniele declared no relevant economic conflicts of interest. Dr. López Fernández reported relationships with Daiichi Sankyo, Almirall España, Janssen-Cilag, Bayer, Roche, Philips, and Incyte.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
A history of cancer is an independent predictor of major cardiovascular events in patients undergoing coronary angioplasty. Cancer should be considered a new cardiovascular risk factor in primary and secondary prevention, according to a study presented at the 2023 American Heart Association Congress in Philadelphia.
, for example, equating it to the situation of a patient with diabetes or chronic renal failure,” said lead author Renzo Melchiori, MD, a cardiologist at the University Hospital Austral in Pilar, Argentina.
The researchers also advocate for intensifying cardiovascular control measures in secondary prevention for these patients, reconsidering goals, and ensuring compliance with prescribed pharmacological regimens and healthy lifestyle habits.
“Previously, when a patient had oncological pathology, thinking about associated cardiovascular risk seemed somewhat superfluous. But today, oncological diseases are treated so effectively, increasing survival and life expectancy, that we begin to focus on what happens with the arteries of these patients after treatment,” said Dr. Melchiori.
Higher Incidence Density
The retrospective analysis included 937 patients of both sexes aged 18 years and older who underwent coronary angioplasty for acute coronary syndrome between 2008 and 2022 at a university hospital. Of these participants, 89 (9.5%) had a history of cancer, with a median time since oncologic diagnosis of around 2 years for solid and hematologic tumors. Most participants had treated and resolved cancer.
Over a median follow-up of 45 months (range, 14-72 months), the cumulative incidence rates of a major cardiovascular event (nonfatal stroke, nonfatal acute myocardial infarction, cardiovascular death, or new angioplasty) were 22.2% (155/698) and 28.4% (25/88) in the groups without and with a history of cancer, respectively. The incidence density was significantly higher in the group with an oncologic history than in the group without such a history: 0.78 events/100 patients/month vs 0.48 events/100 patients/month (P = .01).
Kaplan-Meier analysis showed a higher probability of a major cardiovascular event in the group of patients with cancer or a history of cancer (P = .0086). In multivariate Cox regression analysis, cancer history was an independent predictor of major cardiovascular events adjusted for other risk factors such as age, hypertension, diabetes, smoking, sedentary lifestyle, and family history (hazard ratio, 1.66; P = .025).
Dr. Melchiori clarified that the increased incidence of cardiovascular events in patients with cancer or a history of cancer cannot be attributed to differences in percutaneous intervention or the indication or compliance of post-treatment pharmacological therapy.
In addition, the specialist acknowledged that due to the sample size, discrimination by cancer type, disease stage, or therapeutic strategies couldn’t be performed. A subanalysis, which has not been presented, indicated that the effect could not be explained solely by the application of radiotherapy or chemotherapy in the 90 days before angioplasty — two factors that cause arterial inflammation.
Intensifying Prevention Measures
Two independent experts told this news organization that the new study is "interesting" and reinforces the close connection between oncologic and cardiovascular pathology.
Andrés Daniele, MD, cardiologist and president of the Argentine Cardio-Oncology Association, a local chapter of the International Cardio-Oncology Society, emphasized that the study “reiterates an observation seen in other works: A higher rate of atherosclerotic pathology and cardiovascular events in patients with a history of cancer. And that has a reason to be: Both pathologies present common risk factors, and on the other hand, there is greater endothelial dysfunction secondary to the inflammatory syndrome and oncologic therapies.”
“There needs to be a continuum in the intensification of measures in primary and secondary cardiovascular prevention in cancer survivors, whether in remission or with chronic disease. We need to be very aggressive in managing risk factors and insist that patients who have had a cardiovascular event enter cardiovascular rehabilitation therapies,” said Dr. Daniele, who also heads the Cardio-Oncology Department at the centenary Roffo Institute of Oncology at the University of Buenos Aires, Argentina.
The study provides a valuable contribution because “we need to understand the epidemiology and natural history of patients with cancer at risk of developing cardiovascular complications to implement personalized cardiovascular prevention strategies,” said Teresa López Fernández, MD, cardiologist, coordinator of the Cardio-Oncology Program at La Paz University Hospital in Madrid, member of the Cardio-Oncology Working Group of the Spanish Society of Cardiology, member of the board of the International Cardio-Oncology Society, and cochair of the first clinical practice guidelines in cardio-oncology of the European Society of Cardiology.
“We have to be aware that perhaps we should not guide ourselves in these patients with the usual risk stratification scores as cancer or cardiotoxic treatment are not included as variables. However, they require our attention and effort to improve their quality and quantity of life, avoiding potentially preventable cardiovascular events that could negatively impact the survival achieved thanks to advances in cancer treatments,” said Dr. López Fernández.
Dr. Melchiori and Dr. Daniele declared no relevant economic conflicts of interest. Dr. López Fernández reported relationships with Daiichi Sankyo, Almirall España, Janssen-Cilag, Bayer, Roche, Philips, and Incyte.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
A Military Nurse Saves a Life After a Brutal Rollover Crash
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
Emergencies happen anywhere and anytime, and sometimes, medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
A week earlier I’d had a heart surgery and was heading out for a post-op appointment when I saw it: I had a flat tire. It didn’t make sense. The tire was brand new, and there was no puncture. But it was flat.
I swapped out the flat for the spare and went off base to a tire shop. While I was there, my surgeon’s office called and rescheduled my appointment for a couple of hours later. That was lucky because by the time the tire was fixed, I had just enough time to get there.
The hospital is right near I-35 in San Antonio, Texas. I got off the freeway and onto the access road and paused to turn into the parking lot. That’s when I heard an enormous crash.
I saw a big poof of white smoke, and a car barreled off the freeway and came rolling down the embankment.
When the car hit the access road, I saw a woman ejected through the windshield. She bounced and landed in the road about 25 feet in front of me.
I put my car in park, grabbed my face mask and gloves, and started running toward her. But another vehicle — a truck towing a trailer — came from behind to drive around me. The driver didn’t realize what had happened and couldn’t stop in time…
The trailer ran over her.
I didn’t know if anyone could’ve survived that, but I went to her. I saw several other bystanders, but they were frozen in shock. I was praying, dear God, if she’s alive, let me do whatever I need to do to save her life.
It was a horrible scene. This poor lady was in a bloody heap in the middle of the road. Her right arm was twisted up under her neck so tightly, she was choking herself. So, the first thing I did was straighten her arm out to protect her airway.
I started yelling at people, “Call 9-1-1! Run to the hospital! Let them know there’s an accident out here, and I need help!”
The woman had a pulse, but it was super rapid. On first glance, she clearly had multiple fractures and a bad head bleed. With the sheer number of times she’d been injured, I didn’t know what was going on internally, but it was bad. She was gargling on her own blood and spitting it up. She was drowning.
A couple of technicians from the hospital came and brought me a tiny emergency kit. It had a blood pressure cuff and an oral airway. All the vital signs indicated the lady was going into shock. She’d lost a lot of blood on the pavement.
I was able to get the oral airway in. A few minutes later, a fire chief showed up. By now, the traffic had backed up so badly, the emergency vehicles couldn’t get in. But he managed to get there another way and gave me a cervical collar (C collar) and an Ambu bag.
I was hyper-focused on what I could do at that moment and what I needed to do next. Her stats were going down, but she still had a pulse. If she lost the pulse or went into a lethal rhythm, I’d have to start cardiopulmonary resuscitation (CPR). I asked the other people, but nobody else knew CPR, so I wouldn’t have help.
I could tell the lady had a pelvic fracture, and we needed to stabilize her. I directed people how to hold her neck safely and log-roll her flat on the ground. I also needed to put pressure on the back of her head because of all the bleeding. I got people to give me their clothes and tried to do that as I was bagging her.
The windows of her vehicle had all been blown out. I asked somebody to go find her purse with her ID. Then I noticed something …
My heart jumped into my stomach.
A car seat. There was an empty child’s car seat in the back of the car.
I started yelling at everyone, “Look for a baby! Go up and down the embankment and across the road. There might have been a baby in the car!”
But there wasn’t. Thank God. She hadn’t been driving with her child.
At that point, a paramedic came running from behind all the traffic. We did life support together until the ambulance finally arrived.
Emergency medical services got an intravenous line in and used medical anti-shock trousers. Thankfully, I already had the C collar on, and we’d been bagging her, so they could load her very quickly.
I got rid of my bloody gloves. I told a police officer I would come back. And then I went to my doctor’s appointment.
The window at my doctor’s office faced the access road, so the people there had seen all the traffic. They asked me what happened, and I said, “It was me. I saw it happen. I tried to help.” I was a little frazzled.
When I got back to the scene, the police and the fire chief kept thanking me for stopping. Why wouldn’t I stop? It was astounding to realize that they imagined somebody wouldn’t stop in a situation like this.
They told me the lady was alive. She was in the intensive care unit in critical condition, but she had survived. At that moment, I had this overwhelming feeling: God had put me in this exact place at the exact time to save her life.
Looking back, I think about how God ordered my steps. Without the mysterious flat tire, I would’ve gone to the hospital earlier. If my appointment hadn’t been rescheduled, I wouldn’t have been on the access road. All those events brought me there.
Several months later, the woman’s family contacted me and asked if we could meet. I found out more about her injuries. She’d had multiple skull fractures, facial fractures, and a broken jaw. Her upper arm was broken in three places. Her clavicle was broken. She had internal bleeding, a pelvic fracture, and a broken leg. She was 28 years old.
She’d had multiple surgeries, spent 2 months in the ICU, and another 3 months in intensive rehab. But she survived. It was incredible.
We all met up at a McDonald’s. First, her little son — who was the baby I thought might have been in the car — ran up to me and said, “Thank you for saving my mommy’s life.”
Then I turned, and there she was — a beautiful lady looking at me with awe and crying, saying, “It’s me.”
She obviously had gone through a transformation from all the injuries and the medications. She had a little bit of a speech delay, but mentally, she was there. She could walk.
She said, “You’re my angel. God put you there to save my life.” Her family all came up and hugged me. It was so beautiful.
She told me about the accident. She’d been speeding that day, zigzagging through lanes to get around the traffic. And she didn’t have her seatbelt on. She’d driven onto the shoulder to try to pass everyone, but it started narrowing. She clipped somebody’s bumper, went into a tailspin, and collided with a second vehicle, which caused her to flip over and down the embankment.
“God’s given me a new lease on life,” she said, “a fresh start. I will forever wear my seatbelt. And I’m going to do whatever I can to give back to other people because I don’t even feel like I deserve this.”
I just cried.
I’ve been a nurse for 29 years, first on the civilian side and later in the military. I’ve led codes and responded to trauma in a hospital setting or a deployed environment. I was well prepared to do what I did. But doing it under such stress with adrenaline bombarding me ... I’m amazed. I just think God’s hand was on me.
At that time, I was personally going through some things. After my heart surgery, I was in an emotional place where I didn’t feel loved or valued. But when I had that realization — when I knew that I was meant to be there to save her life, I also got the very clear message that I was valued and loved so much.
I know I have a very strong purpose. That day changed my life.
US Air Force Lt. Col. Anne Staley is the officer in charge of the Military Training Network, a division of the Defense Health Agency Education and Training Directorate in San Antonio, Texas.
A version of this article appeared on Medscape.com.
Even Intentional Weight Loss Linked With Cancer
This transcript has been edited for clarity.
As anyone who has been through medical training will tell you, some little scenes just stick with you. I had been seeing a patient in our resident clinic in West Philly for a couple of years. She was in her mid-60s with diabetes and hypertension and a distant smoking history. She was overweight and had been trying to improve her diet and lose weight since I started seeing her. One day she came in and was delighted to report that she had finally started shedding some pounds — about 15 in the past 2 months.
I enthusiastically told my preceptor that my careful dietary counseling had finally done the job. She looked through the chart for a moment and asked, “Is she up to date on her cancer screening?” A workup revealed adenocarcinoma of the lung. The patient did well, actually, but the story stuck with me.
The textbooks call it “unintentional weight loss,” often in big, scary letters, and every doctor will go just a bit pale if a patient tells them that, despite efforts not to, they are losing weight. But true unintentional weight loss is not that common. After all, most of us are at least half-heartedly trying to lose weight all the time. Should doctors be worried when we are successful?
A new study suggests that perhaps they should. We’re talking about this study, appearing in JAMA, which combined participants from two long-running observational cohorts: 120,000 women from the Nurses’ Health Study, and 50,000 men from the Health Professionals Follow-Up Study. (These cohorts started in the 1970s and 1980s, so we’ll give them a pass on the gender-specific study designs.)
The rationale of enrolling healthcare providers in these cohort studies is that they would be reliable witnesses of their own health status. If a nurse or doctor says they have pancreatic cancer, it’s likely that they truly have pancreatic cancer. Detailed health surveys were distributed to the participants every other year, and the average follow-up was more than a decade.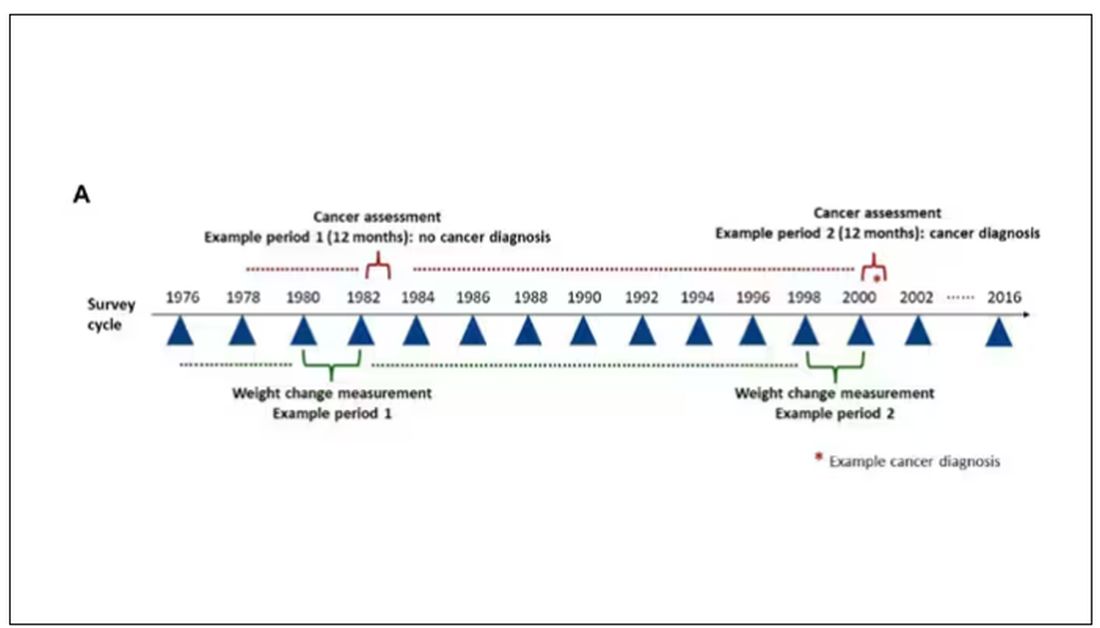
Participants recorded their weight — as an aside, a nested study found that self-reported rate was extremely well correlated with professionally measured weight — and whether they had received a cancer diagnosis since the last survey.
This allowed researchers to look at the phenomenon described above. Would weight loss precede a new diagnosis of cancer? And, more interestingly, would intentional weight loss precede a new diagnosis of cancer.
I don’t think it will surprise you to hear that individuals in the highest category of weight loss, those who lost more than 10% of their body weight over a 2-year period, had a larger risk of being diagnosed with cancer in the next year. That’s the yellow line in this graph. In fact, they had about a 40% higher risk than those who did not lose weight.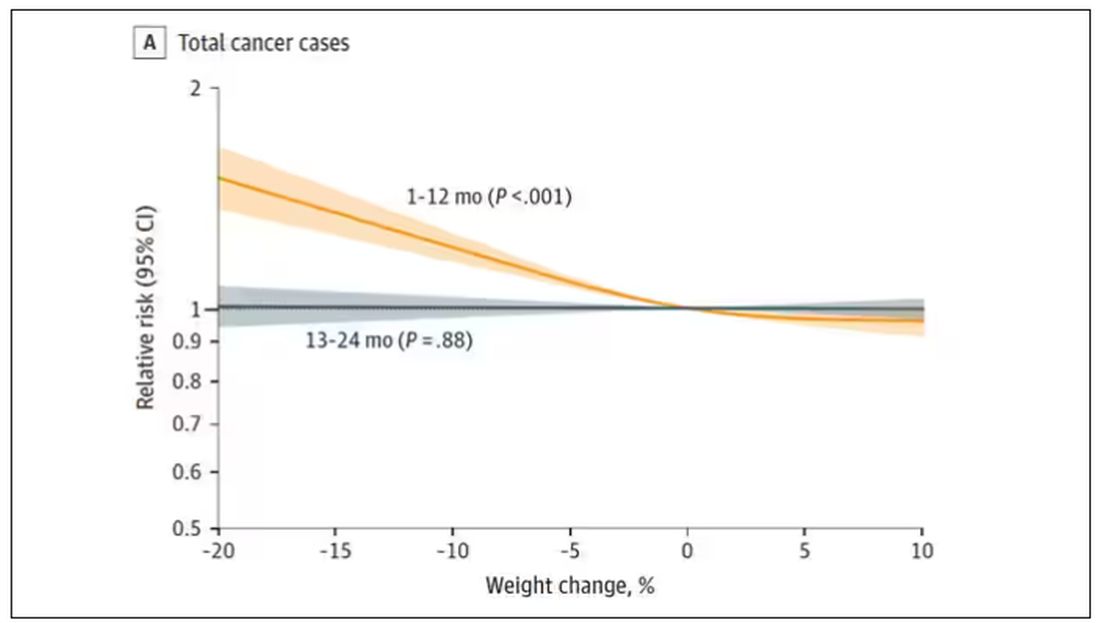
Increased risk was found across multiple cancer types, though cancers of the gastrointestinal tract, not surprisingly, were most strongly associated with antecedent weight loss.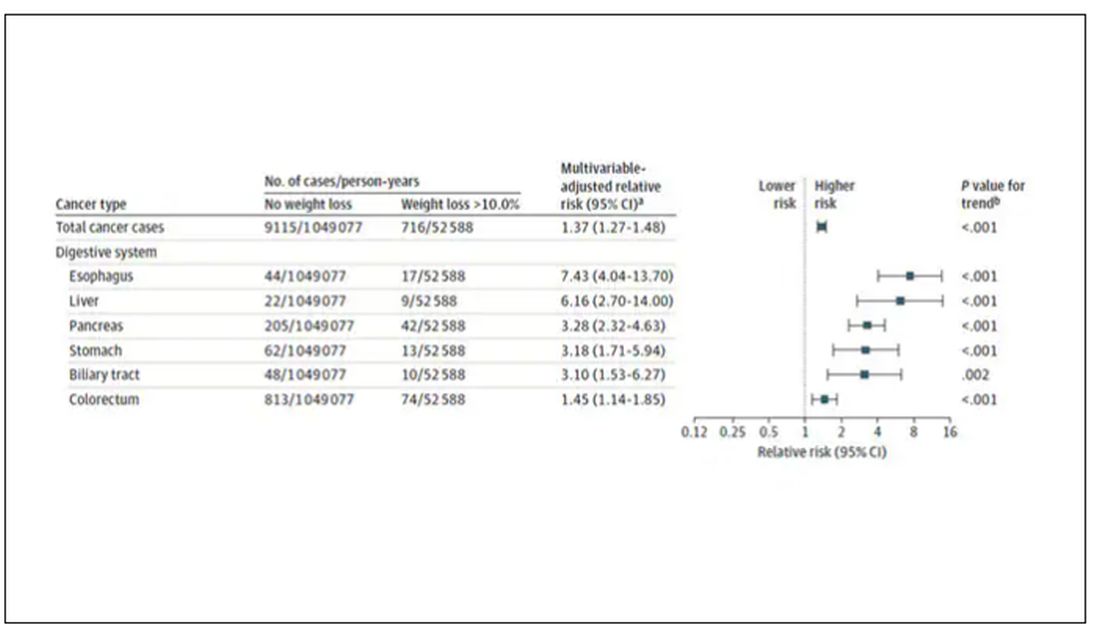
What about intentionality of weight loss? Unfortunately, the surveys did not ask participants whether they were trying to lose weight. Rather, the surveys asked about exercise and dietary habits. The researchers leveraged these responses to create three categories of participants: those who seemed to be trying to lose weight (defined as people who had increased their exercise and dietary quality); those who didn’t seem to be trying to lose weight (they changed neither exercise nor dietary behaviors); and a middle group, which changed one or the other of these behaviors but not both.
Let’s look at those who really seemed to be trying to lose weight. Over 2 years, they got more exercise and improved their diet.
If they succeeded in losing 10% or more of their body weight, they still had a higher risk for cancer than those who had not lost weight — about 30% higher, which is not that different from the 40% increased risk when you include those folks who weren’t changing their lifestyle.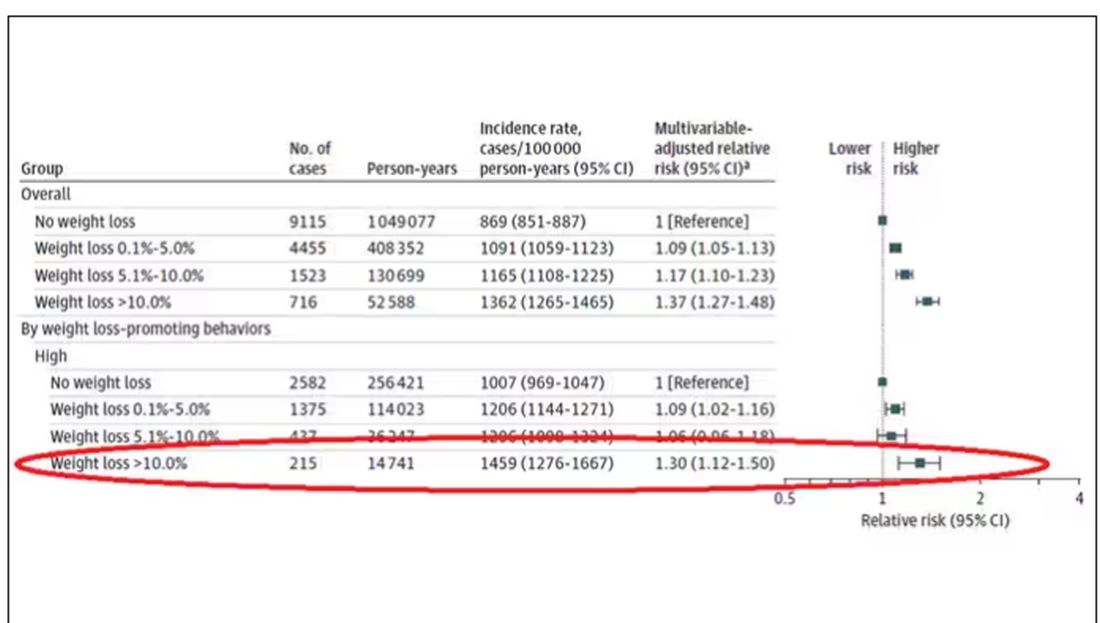
This is why this study is important. The classic teaching is that unintentional weight loss is a bad thing and needs a workup. That’s fine. But we live in a world where perhaps the majority of people are, at any given time, trying to lose weight.
We need to be careful here. I am not by any means trying to say that people who have successfully lost weight have cancer. Both of the following statements can be true:
Significant weight loss, whether intentional or not, is associated with a higher risk for cancer.
Most people with significant weight loss will not have cancer.
Both of these can be true because cancer is, fortunately, rare. Of people who lose weight, the vast majority will lose weight because they are engaging in healthier behaviors. A small number may lose weight because something else is wrong. It’s just hard to tell the two apart.
Out of the nearly 200,000 people in this study, only around 16,000 developed cancer during follow-up. Again, although the chance of having cancer is slightly higher if someone has experienced weight loss, the chance is still very low.
We also need to avoid suggesting that weight loss causes cancer. Some people lose weight because of an existing, as of yet undiagnosed cancer and its metabolic effects. This is borne out if you look at the risk of being diagnosed with cancer as you move further away from the interval of weight loss.
The further you get from the year of that 10% weight loss, the less likely you are to be diagnosed with cancer. Most of these cancers are diagnosed within a year of losing weight. In other words, if you’re reading this and getting worried that you lost weight 10 years ago, you’re probably out of the woods. That was, most likely, just you getting healthier.
Last thing: We have methods for weight loss now that are way more effective than diet or exercise. I’m looking at you, Ozempic. But aside from the weight loss wonder drugs, we have surgery and other interventions. This study did not capture any of that data. Ozempic wasn’t even on the market during this study, so we can’t say anything about the relationship between weight loss and cancer among people using nonlifestyle mechanisms to lose weight.
It’s a complicated system. But the clinically actionable point here is to notice if patients have lost weight. If they’ve lost it without trying, further workup is reasonable. If they’ve lost it but were trying to lose it, tell them “good job.” And consider a workup anyway.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
As anyone who has been through medical training will tell you, some little scenes just stick with you. I had been seeing a patient in our resident clinic in West Philly for a couple of years. She was in her mid-60s with diabetes and hypertension and a distant smoking history. She was overweight and had been trying to improve her diet and lose weight since I started seeing her. One day she came in and was delighted to report that she had finally started shedding some pounds — about 15 in the past 2 months.
I enthusiastically told my preceptor that my careful dietary counseling had finally done the job. She looked through the chart for a moment and asked, “Is she up to date on her cancer screening?” A workup revealed adenocarcinoma of the lung. The patient did well, actually, but the story stuck with me.
The textbooks call it “unintentional weight loss,” often in big, scary letters, and every doctor will go just a bit pale if a patient tells them that, despite efforts not to, they are losing weight. But true unintentional weight loss is not that common. After all, most of us are at least half-heartedly trying to lose weight all the time. Should doctors be worried when we are successful?
A new study suggests that perhaps they should. We’re talking about this study, appearing in JAMA, which combined participants from two long-running observational cohorts: 120,000 women from the Nurses’ Health Study, and 50,000 men from the Health Professionals Follow-Up Study. (These cohorts started in the 1970s and 1980s, so we’ll give them a pass on the gender-specific study designs.)
The rationale of enrolling healthcare providers in these cohort studies is that they would be reliable witnesses of their own health status. If a nurse or doctor says they have pancreatic cancer, it’s likely that they truly have pancreatic cancer. Detailed health surveys were distributed to the participants every other year, and the average follow-up was more than a decade.
Participants recorded their weight — as an aside, a nested study found that self-reported rate was extremely well correlated with professionally measured weight — and whether they had received a cancer diagnosis since the last survey.
This allowed researchers to look at the phenomenon described above. Would weight loss precede a new diagnosis of cancer? And, more interestingly, would intentional weight loss precede a new diagnosis of cancer.
I don’t think it will surprise you to hear that individuals in the highest category of weight loss, those who lost more than 10% of their body weight over a 2-year period, had a larger risk of being diagnosed with cancer in the next year. That’s the yellow line in this graph. In fact, they had about a 40% higher risk than those who did not lose weight.
Increased risk was found across multiple cancer types, though cancers of the gastrointestinal tract, not surprisingly, were most strongly associated with antecedent weight loss.
What about intentionality of weight loss? Unfortunately, the surveys did not ask participants whether they were trying to lose weight. Rather, the surveys asked about exercise and dietary habits. The researchers leveraged these responses to create three categories of participants: those who seemed to be trying to lose weight (defined as people who had increased their exercise and dietary quality); those who didn’t seem to be trying to lose weight (they changed neither exercise nor dietary behaviors); and a middle group, which changed one or the other of these behaviors but not both.
Let’s look at those who really seemed to be trying to lose weight. Over 2 years, they got more exercise and improved their diet.
If they succeeded in losing 10% or more of their body weight, they still had a higher risk for cancer than those who had not lost weight — about 30% higher, which is not that different from the 40% increased risk when you include those folks who weren’t changing their lifestyle.
This is why this study is important. The classic teaching is that unintentional weight loss is a bad thing and needs a workup. That’s fine. But we live in a world where perhaps the majority of people are, at any given time, trying to lose weight.
We need to be careful here. I am not by any means trying to say that people who have successfully lost weight have cancer. Both of the following statements can be true:
Significant weight loss, whether intentional or not, is associated with a higher risk for cancer.
Most people with significant weight loss will not have cancer.
Both of these can be true because cancer is, fortunately, rare. Of people who lose weight, the vast majority will lose weight because they are engaging in healthier behaviors. A small number may lose weight because something else is wrong. It’s just hard to tell the two apart.
Out of the nearly 200,000 people in this study, only around 16,000 developed cancer during follow-up. Again, although the chance of having cancer is slightly higher if someone has experienced weight loss, the chance is still very low.
We also need to avoid suggesting that weight loss causes cancer. Some people lose weight because of an existing, as of yet undiagnosed cancer and its metabolic effects. This is borne out if you look at the risk of being diagnosed with cancer as you move further away from the interval of weight loss.
The further you get from the year of that 10% weight loss, the less likely you are to be diagnosed with cancer. Most of these cancers are diagnosed within a year of losing weight. In other words, if you’re reading this and getting worried that you lost weight 10 years ago, you’re probably out of the woods. That was, most likely, just you getting healthier.
Last thing: We have methods for weight loss now that are way more effective than diet or exercise. I’m looking at you, Ozempic. But aside from the weight loss wonder drugs, we have surgery and other interventions. This study did not capture any of that data. Ozempic wasn’t even on the market during this study, so we can’t say anything about the relationship between weight loss and cancer among people using nonlifestyle mechanisms to lose weight.
It’s a complicated system. But the clinically actionable point here is to notice if patients have lost weight. If they’ve lost it without trying, further workup is reasonable. If they’ve lost it but were trying to lose it, tell them “good job.” And consider a workup anyway.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
As anyone who has been through medical training will tell you, some little scenes just stick with you. I had been seeing a patient in our resident clinic in West Philly for a couple of years. She was in her mid-60s with diabetes and hypertension and a distant smoking history. She was overweight and had been trying to improve her diet and lose weight since I started seeing her. One day she came in and was delighted to report that she had finally started shedding some pounds — about 15 in the past 2 months.
I enthusiastically told my preceptor that my careful dietary counseling had finally done the job. She looked through the chart for a moment and asked, “Is she up to date on her cancer screening?” A workup revealed adenocarcinoma of the lung. The patient did well, actually, but the story stuck with me.
The textbooks call it “unintentional weight loss,” often in big, scary letters, and every doctor will go just a bit pale if a patient tells them that, despite efforts not to, they are losing weight. But true unintentional weight loss is not that common. After all, most of us are at least half-heartedly trying to lose weight all the time. Should doctors be worried when we are successful?
A new study suggests that perhaps they should. We’re talking about this study, appearing in JAMA, which combined participants from two long-running observational cohorts: 120,000 women from the Nurses’ Health Study, and 50,000 men from the Health Professionals Follow-Up Study. (These cohorts started in the 1970s and 1980s, so we’ll give them a pass on the gender-specific study designs.)
The rationale of enrolling healthcare providers in these cohort studies is that they would be reliable witnesses of their own health status. If a nurse or doctor says they have pancreatic cancer, it’s likely that they truly have pancreatic cancer. Detailed health surveys were distributed to the participants every other year, and the average follow-up was more than a decade.
Participants recorded their weight — as an aside, a nested study found that self-reported rate was extremely well correlated with professionally measured weight — and whether they had received a cancer diagnosis since the last survey.
This allowed researchers to look at the phenomenon described above. Would weight loss precede a new diagnosis of cancer? And, more interestingly, would intentional weight loss precede a new diagnosis of cancer.
I don’t think it will surprise you to hear that individuals in the highest category of weight loss, those who lost more than 10% of their body weight over a 2-year period, had a larger risk of being diagnosed with cancer in the next year. That’s the yellow line in this graph. In fact, they had about a 40% higher risk than those who did not lose weight.
Increased risk was found across multiple cancer types, though cancers of the gastrointestinal tract, not surprisingly, were most strongly associated with antecedent weight loss.
What about intentionality of weight loss? Unfortunately, the surveys did not ask participants whether they were trying to lose weight. Rather, the surveys asked about exercise and dietary habits. The researchers leveraged these responses to create three categories of participants: those who seemed to be trying to lose weight (defined as people who had increased their exercise and dietary quality); those who didn’t seem to be trying to lose weight (they changed neither exercise nor dietary behaviors); and a middle group, which changed one or the other of these behaviors but not both.
Let’s look at those who really seemed to be trying to lose weight. Over 2 years, they got more exercise and improved their diet.
If they succeeded in losing 10% or more of their body weight, they still had a higher risk for cancer than those who had not lost weight — about 30% higher, which is not that different from the 40% increased risk when you include those folks who weren’t changing their lifestyle.
This is why this study is important. The classic teaching is that unintentional weight loss is a bad thing and needs a workup. That’s fine. But we live in a world where perhaps the majority of people are, at any given time, trying to lose weight.
We need to be careful here. I am not by any means trying to say that people who have successfully lost weight have cancer. Both of the following statements can be true:
Significant weight loss, whether intentional or not, is associated with a higher risk for cancer.
Most people with significant weight loss will not have cancer.
Both of these can be true because cancer is, fortunately, rare. Of people who lose weight, the vast majority will lose weight because they are engaging in healthier behaviors. A small number may lose weight because something else is wrong. It’s just hard to tell the two apart.
Out of the nearly 200,000 people in this study, only around 16,000 developed cancer during follow-up. Again, although the chance of having cancer is slightly higher if someone has experienced weight loss, the chance is still very low.
We also need to avoid suggesting that weight loss causes cancer. Some people lose weight because of an existing, as of yet undiagnosed cancer and its metabolic effects. This is borne out if you look at the risk of being diagnosed with cancer as you move further away from the interval of weight loss.
The further you get from the year of that 10% weight loss, the less likely you are to be diagnosed with cancer. Most of these cancers are diagnosed within a year of losing weight. In other words, if you’re reading this and getting worried that you lost weight 10 years ago, you’re probably out of the woods. That was, most likely, just you getting healthier.
Last thing: We have methods for weight loss now that are way more effective than diet or exercise. I’m looking at you, Ozempic. But aside from the weight loss wonder drugs, we have surgery and other interventions. This study did not capture any of that data. Ozempic wasn’t even on the market during this study, so we can’t say anything about the relationship between weight loss and cancer among people using nonlifestyle mechanisms to lose weight.
It’s a complicated system. But the clinically actionable point here is to notice if patients have lost weight. If they’ve lost it without trying, further workup is reasonable. If they’ve lost it but were trying to lose it, tell them “good job.” And consider a workup anyway.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Will New Lung Cancer Screening Guidelines Save More Lives?
When the American Cancer Society recently unveiled changes to its lung cancer screening guidance, the aim was to remove barriers to screening and catch more cancers in high-risk people earlier.
Although the lung cancer death rate has declined significantly over the past few decades, lung cancer remains the leading cause of cancer deaths worldwide.
Detecting lung cancer early is key to improving survival. Still, lung cancer screening rates are poor. In 2021, the American Lung Association estimated that 14 million US adults qualified for lung cancer screening, but only 5.8% received it.
Smokers or former smokers without symptoms may forgo regular screening and only receive their screening scan after symptoms emerge, explained Janani S. Reisenauer, MD, Division Chair of Thoracic Surgery at Mayo Clinic, Rochester, Minnesota. But by the time symptoms develop, the cancer is typically more advanced, and treatment options become more limited.
The goal of the new American Cancer Society guidelines, published in early November 2023 in CA: A Cancer Journal for Physicians, is to identify lung cancers at earlier stages when they are easier to treat.
Almost 5 million more high-risk people will now qualify for regular lung cancer screening, the guideline authors estimated.
But will expanding screening help reduce deaths from lung cancer? And perhaps just as important, will the guidelines move the needle on the “disappointingly low” lung cancer screening rates up to this point?
“I definitely think it’s a step in the right direction,” said Lecia V. Sequist, MD, MPH, clinical researcher and lung cancer medical oncologist, Massachusetts General Hospital Cancer Center, Boston, Massachusetts.
The new guidelines lowered the age for annual lung cancer screening among asymptomatic former or current smokers from 55-74 years to 50-80 years. The update also now considers a high-risk person anyone with a 20-pack-year history, down from a 30-pack-year history, and removes the requirement that former smokers must have quit within 15 years to be eligible for screening.
As people age, their risk for lung cancer increases, so it makes sense to screen all former smokers regardless of when they quit, explained Kim Lori Sandler, MD, from Vanderbilt University Medical Center, Nashville, Tennessee, and cochair of the American College of Radiology’s Lung Cancer Screening Steering Committee.
“There’s really nothing magical or drastic that happens at the 15-year mark,” Dr. Sequist agreed. For “someone who quit 14 years ago versus 16 years ago, it is essentially the same risk, and so scientifically it doesn’t really make sense to impose an artificial cut-off where no change in risk exists.”
The latest evidence reviewed in the new guidelines shows that expanding the guidelines would identify more early-stage cancers and potentially save lives. The authors modeled the benefits and harms of lung cancer screening using several scenarios.
Moving the start age from 55 to 50 years would lead to a 15% reduction in lung cancer mortality in men aged 50-54 years, the model suggested.
Removing the 15-year timeline for quitting smoking also would also improve outcomes. Compared with scenarios that included the 15-year quit timeline for former smokers, those that removed the limit would result in a 37.3% increase in screening exams, a 21% increase in would avert lung cancer deaths, and offer a 19% increase in life-years gained per 100,000 population.
Overall, the evidence indicates that, “if fully implemented, these recommendations have a high likelihood of significantly reducing death and suffering from lung cancer in the United States,” the guideline authors wrote.
But screening more people also comes with risks, such as more false-positive findings, which could lead to extra scans, invasive tests for tissue sampling, or even procedures for benign disease, Dr. Sandler explained. The latter “is what we really need to avoid.”
Even so, Dr. Sandler believes the current guidelines show that the benefit of screening “is great enough that it’s worth including these additional individuals.”
Guidelines Are Not Enough
But will expanding the screening criteria prompt more eligible individuals to receive their CT scans?
Simply expanding the eligibility criteria, by itself, likely won’t measurably improve screening uptake, said Paolo Boffetta, MD, MPH, of Stony Brook Cancer Center, Stony Brook, New York.
Healthcare and insurance access along with patient demand may present the most significant barriers to improving screening uptake.
The “issue is not the guideline as much as it’s the healthcare system,” said Otis W. Brawley, MD, professor of oncology at the Johns Hopkins University School of Medicine, Baltimore, Maryland.
Access to screening at hospitals with limited CT scanners and staff could present one major issue.
When Dr. Brawley worked at a large inner-city safety net hospital in Atlanta, patients with lung cancer frequently had to wait over a week to use one of the four CT scanners, he recalled. Adding to these delays, we didn’t have enough people to read the screens or enough people to do the diagnostics for those who had abnormalities, said Dr. Brawley.
To increase lung cancer screening in this context would increase the wait time for patients who do have cancer, he said.
Insurance coverage could present a roadblock for some as well. While the 2021 US Preventive Services Task Force (USPSTF) recommendations largely align with the new ones from the American Cancer Society, there’s one key difference: The USPSTF still requires former smokers to have quit within 15 years to be eligible for annual screening.
Because the USPSTF recommendations dictate insurance coverage, some former smokers — those who quit more than 15 years ago — may not qualify for coverage and would have to pay out-of-pocket for screening.
Dr. Sequist, however, had a more optimistic outlook about screening uptake.
The American Cancer Society guidelines should remove some of the stigma surrounding lung cancer screening. Most people, when asked a lot of questions about their tobacco use and history, tend to downplay it because there’s shame associated with smoking, Dr. Sequist said. The new guidelines limit the information needed to determine eligibility.
Dr. Sequist also noted that the updated American Cancer Society guideline would improve screening rates because it simplifies the eligibility criteria and makes it easier for physicians to determine who qualifies.
The issue, however, is that some of these individuals — those who quit over 15 years ago — may not have their scan covered by insurance, which could preclude lower-income individuals from getting screened.
The American Cancer Society guidelines” do not necessarily translate into a change in policy,” which is “dictated by the USPSTF and payors such as Medicare,” explained Peter Mazzone, MD, MPH, director of the Lung Cancer Program and Lung Cancer Screening Program for the Respiratory Institute, Cleveland Clinic, Cleveland, Ohio.
On the patient side, Dr. Brawley noted, “we don’t yet have a large demand” for screening.
Many current and former smokers may put off lung cancer screening or not seek it out. Some may be unaware of their eligibility, while others may fear the outcome of a scan. Even among eligible individuals who do receive an initial scan, most — more than 75% — do not return for their next scan a year later, research showed.
Enhancing patient education and launching strong marketing campaigns would be a key element to encourage more people to get their annual screening and reduce the stigma associated with lung cancer as a smoker’s disease.
“Primary care physicians are integral in ensuring all eligible patients receive appropriate screening for lung cancer,” said Steven P. Furr, MD, president of the American Academy of Family Physicians and a family physician in Jackson, Alabama. “It is imperative that family physicians encourage screening in at-risk patients and counsel them on the importance of continued screening, as well as smoking cessation, if needed.”
Two authors of the new guidelines reported financial relationships with Seno Medical Instruments, the Genentech Foundation, Crispr Therapeutics, BEAM Therapeutics, Intellia Therapeutics, Editas Medicine, Freenome, and Guardant Health.
A version of this article appeared on Medscape.com.
When the American Cancer Society recently unveiled changes to its lung cancer screening guidance, the aim was to remove barriers to screening and catch more cancers in high-risk people earlier.
Although the lung cancer death rate has declined significantly over the past few decades, lung cancer remains the leading cause of cancer deaths worldwide.
Detecting lung cancer early is key to improving survival. Still, lung cancer screening rates are poor. In 2021, the American Lung Association estimated that 14 million US adults qualified for lung cancer screening, but only 5.8% received it.
Smokers or former smokers without symptoms may forgo regular screening and only receive their screening scan after symptoms emerge, explained Janani S. Reisenauer, MD, Division Chair of Thoracic Surgery at Mayo Clinic, Rochester, Minnesota. But by the time symptoms develop, the cancer is typically more advanced, and treatment options become more limited.
The goal of the new American Cancer Society guidelines, published in early November 2023 in CA: A Cancer Journal for Physicians, is to identify lung cancers at earlier stages when they are easier to treat.
Almost 5 million more high-risk people will now qualify for regular lung cancer screening, the guideline authors estimated.
But will expanding screening help reduce deaths from lung cancer? And perhaps just as important, will the guidelines move the needle on the “disappointingly low” lung cancer screening rates up to this point?
“I definitely think it’s a step in the right direction,” said Lecia V. Sequist, MD, MPH, clinical researcher and lung cancer medical oncologist, Massachusetts General Hospital Cancer Center, Boston, Massachusetts.
The new guidelines lowered the age for annual lung cancer screening among asymptomatic former or current smokers from 55-74 years to 50-80 years. The update also now considers a high-risk person anyone with a 20-pack-year history, down from a 30-pack-year history, and removes the requirement that former smokers must have quit within 15 years to be eligible for screening.
As people age, their risk for lung cancer increases, so it makes sense to screen all former smokers regardless of when they quit, explained Kim Lori Sandler, MD, from Vanderbilt University Medical Center, Nashville, Tennessee, and cochair of the American College of Radiology’s Lung Cancer Screening Steering Committee.
“There’s really nothing magical or drastic that happens at the 15-year mark,” Dr. Sequist agreed. For “someone who quit 14 years ago versus 16 years ago, it is essentially the same risk, and so scientifically it doesn’t really make sense to impose an artificial cut-off where no change in risk exists.”
The latest evidence reviewed in the new guidelines shows that expanding the guidelines would identify more early-stage cancers and potentially save lives. The authors modeled the benefits and harms of lung cancer screening using several scenarios.
Moving the start age from 55 to 50 years would lead to a 15% reduction in lung cancer mortality in men aged 50-54 years, the model suggested.
Removing the 15-year timeline for quitting smoking also would also improve outcomes. Compared with scenarios that included the 15-year quit timeline for former smokers, those that removed the limit would result in a 37.3% increase in screening exams, a 21% increase in would avert lung cancer deaths, and offer a 19% increase in life-years gained per 100,000 population.
Overall, the evidence indicates that, “if fully implemented, these recommendations have a high likelihood of significantly reducing death and suffering from lung cancer in the United States,” the guideline authors wrote.
But screening more people also comes with risks, such as more false-positive findings, which could lead to extra scans, invasive tests for tissue sampling, or even procedures for benign disease, Dr. Sandler explained. The latter “is what we really need to avoid.”
Even so, Dr. Sandler believes the current guidelines show that the benefit of screening “is great enough that it’s worth including these additional individuals.”
Guidelines Are Not Enough
But will expanding the screening criteria prompt more eligible individuals to receive their CT scans?
Simply expanding the eligibility criteria, by itself, likely won’t measurably improve screening uptake, said Paolo Boffetta, MD, MPH, of Stony Brook Cancer Center, Stony Brook, New York.
Healthcare and insurance access along with patient demand may present the most significant barriers to improving screening uptake.
The “issue is not the guideline as much as it’s the healthcare system,” said Otis W. Brawley, MD, professor of oncology at the Johns Hopkins University School of Medicine, Baltimore, Maryland.
Access to screening at hospitals with limited CT scanners and staff could present one major issue.
When Dr. Brawley worked at a large inner-city safety net hospital in Atlanta, patients with lung cancer frequently had to wait over a week to use one of the four CT scanners, he recalled. Adding to these delays, we didn’t have enough people to read the screens or enough people to do the diagnostics for those who had abnormalities, said Dr. Brawley.
To increase lung cancer screening in this context would increase the wait time for patients who do have cancer, he said.
Insurance coverage could present a roadblock for some as well. While the 2021 US Preventive Services Task Force (USPSTF) recommendations largely align with the new ones from the American Cancer Society, there’s one key difference: The USPSTF still requires former smokers to have quit within 15 years to be eligible for annual screening.
Because the USPSTF recommendations dictate insurance coverage, some former smokers — those who quit more than 15 years ago — may not qualify for coverage and would have to pay out-of-pocket for screening.
Dr. Sequist, however, had a more optimistic outlook about screening uptake.
The American Cancer Society guidelines should remove some of the stigma surrounding lung cancer screening. Most people, when asked a lot of questions about their tobacco use and history, tend to downplay it because there’s shame associated with smoking, Dr. Sequist said. The new guidelines limit the information needed to determine eligibility.
Dr. Sequist also noted that the updated American Cancer Society guideline would improve screening rates because it simplifies the eligibility criteria and makes it easier for physicians to determine who qualifies.
The issue, however, is that some of these individuals — those who quit over 15 years ago — may not have their scan covered by insurance, which could preclude lower-income individuals from getting screened.
The American Cancer Society guidelines” do not necessarily translate into a change in policy,” which is “dictated by the USPSTF and payors such as Medicare,” explained Peter Mazzone, MD, MPH, director of the Lung Cancer Program and Lung Cancer Screening Program for the Respiratory Institute, Cleveland Clinic, Cleveland, Ohio.
On the patient side, Dr. Brawley noted, “we don’t yet have a large demand” for screening.
Many current and former smokers may put off lung cancer screening or not seek it out. Some may be unaware of their eligibility, while others may fear the outcome of a scan. Even among eligible individuals who do receive an initial scan, most — more than 75% — do not return for their next scan a year later, research showed.
Enhancing patient education and launching strong marketing campaigns would be a key element to encourage more people to get their annual screening and reduce the stigma associated with lung cancer as a smoker’s disease.
“Primary care physicians are integral in ensuring all eligible patients receive appropriate screening for lung cancer,” said Steven P. Furr, MD, president of the American Academy of Family Physicians and a family physician in Jackson, Alabama. “It is imperative that family physicians encourage screening in at-risk patients and counsel them on the importance of continued screening, as well as smoking cessation, if needed.”
Two authors of the new guidelines reported financial relationships with Seno Medical Instruments, the Genentech Foundation, Crispr Therapeutics, BEAM Therapeutics, Intellia Therapeutics, Editas Medicine, Freenome, and Guardant Health.
A version of this article appeared on Medscape.com.
When the American Cancer Society recently unveiled changes to its lung cancer screening guidance, the aim was to remove barriers to screening and catch more cancers in high-risk people earlier.
Although the lung cancer death rate has declined significantly over the past few decades, lung cancer remains the leading cause of cancer deaths worldwide.
Detecting lung cancer early is key to improving survival. Still, lung cancer screening rates are poor. In 2021, the American Lung Association estimated that 14 million US adults qualified for lung cancer screening, but only 5.8% received it.
Smokers or former smokers without symptoms may forgo regular screening and only receive their screening scan after symptoms emerge, explained Janani S. Reisenauer, MD, Division Chair of Thoracic Surgery at Mayo Clinic, Rochester, Minnesota. But by the time symptoms develop, the cancer is typically more advanced, and treatment options become more limited.
The goal of the new American Cancer Society guidelines, published in early November 2023 in CA: A Cancer Journal for Physicians, is to identify lung cancers at earlier stages when they are easier to treat.
Almost 5 million more high-risk people will now qualify for regular lung cancer screening, the guideline authors estimated.
But will expanding screening help reduce deaths from lung cancer? And perhaps just as important, will the guidelines move the needle on the “disappointingly low” lung cancer screening rates up to this point?
“I definitely think it’s a step in the right direction,” said Lecia V. Sequist, MD, MPH, clinical researcher and lung cancer medical oncologist, Massachusetts General Hospital Cancer Center, Boston, Massachusetts.
The new guidelines lowered the age for annual lung cancer screening among asymptomatic former or current smokers from 55-74 years to 50-80 years. The update also now considers a high-risk person anyone with a 20-pack-year history, down from a 30-pack-year history, and removes the requirement that former smokers must have quit within 15 years to be eligible for screening.
As people age, their risk for lung cancer increases, so it makes sense to screen all former smokers regardless of when they quit, explained Kim Lori Sandler, MD, from Vanderbilt University Medical Center, Nashville, Tennessee, and cochair of the American College of Radiology’s Lung Cancer Screening Steering Committee.
“There’s really nothing magical or drastic that happens at the 15-year mark,” Dr. Sequist agreed. For “someone who quit 14 years ago versus 16 years ago, it is essentially the same risk, and so scientifically it doesn’t really make sense to impose an artificial cut-off where no change in risk exists.”
The latest evidence reviewed in the new guidelines shows that expanding the guidelines would identify more early-stage cancers and potentially save lives. The authors modeled the benefits and harms of lung cancer screening using several scenarios.
Moving the start age from 55 to 50 years would lead to a 15% reduction in lung cancer mortality in men aged 50-54 years, the model suggested.
Removing the 15-year timeline for quitting smoking also would also improve outcomes. Compared with scenarios that included the 15-year quit timeline for former smokers, those that removed the limit would result in a 37.3% increase in screening exams, a 21% increase in would avert lung cancer deaths, and offer a 19% increase in life-years gained per 100,000 population.
Overall, the evidence indicates that, “if fully implemented, these recommendations have a high likelihood of significantly reducing death and suffering from lung cancer in the United States,” the guideline authors wrote.
But screening more people also comes with risks, such as more false-positive findings, which could lead to extra scans, invasive tests for tissue sampling, or even procedures for benign disease, Dr. Sandler explained. The latter “is what we really need to avoid.”
Even so, Dr. Sandler believes the current guidelines show that the benefit of screening “is great enough that it’s worth including these additional individuals.”
Guidelines Are Not Enough
But will expanding the screening criteria prompt more eligible individuals to receive their CT scans?
Simply expanding the eligibility criteria, by itself, likely won’t measurably improve screening uptake, said Paolo Boffetta, MD, MPH, of Stony Brook Cancer Center, Stony Brook, New York.
Healthcare and insurance access along with patient demand may present the most significant barriers to improving screening uptake.
The “issue is not the guideline as much as it’s the healthcare system,” said Otis W. Brawley, MD, professor of oncology at the Johns Hopkins University School of Medicine, Baltimore, Maryland.
Access to screening at hospitals with limited CT scanners and staff could present one major issue.
When Dr. Brawley worked at a large inner-city safety net hospital in Atlanta, patients with lung cancer frequently had to wait over a week to use one of the four CT scanners, he recalled. Adding to these delays, we didn’t have enough people to read the screens or enough people to do the diagnostics for those who had abnormalities, said Dr. Brawley.
To increase lung cancer screening in this context would increase the wait time for patients who do have cancer, he said.
Insurance coverage could present a roadblock for some as well. While the 2021 US Preventive Services Task Force (USPSTF) recommendations largely align with the new ones from the American Cancer Society, there’s one key difference: The USPSTF still requires former smokers to have quit within 15 years to be eligible for annual screening.
Because the USPSTF recommendations dictate insurance coverage, some former smokers — those who quit more than 15 years ago — may not qualify for coverage and would have to pay out-of-pocket for screening.
Dr. Sequist, however, had a more optimistic outlook about screening uptake.
The American Cancer Society guidelines should remove some of the stigma surrounding lung cancer screening. Most people, when asked a lot of questions about their tobacco use and history, tend to downplay it because there’s shame associated with smoking, Dr. Sequist said. The new guidelines limit the information needed to determine eligibility.
Dr. Sequist also noted that the updated American Cancer Society guideline would improve screening rates because it simplifies the eligibility criteria and makes it easier for physicians to determine who qualifies.
The issue, however, is that some of these individuals — those who quit over 15 years ago — may not have their scan covered by insurance, which could preclude lower-income individuals from getting screened.
The American Cancer Society guidelines” do not necessarily translate into a change in policy,” which is “dictated by the USPSTF and payors such as Medicare,” explained Peter Mazzone, MD, MPH, director of the Lung Cancer Program and Lung Cancer Screening Program for the Respiratory Institute, Cleveland Clinic, Cleveland, Ohio.
On the patient side, Dr. Brawley noted, “we don’t yet have a large demand” for screening.
Many current and former smokers may put off lung cancer screening or not seek it out. Some may be unaware of their eligibility, while others may fear the outcome of a scan. Even among eligible individuals who do receive an initial scan, most — more than 75% — do not return for their next scan a year later, research showed.
Enhancing patient education and launching strong marketing campaigns would be a key element to encourage more people to get their annual screening and reduce the stigma associated with lung cancer as a smoker’s disease.
“Primary care physicians are integral in ensuring all eligible patients receive appropriate screening for lung cancer,” said Steven P. Furr, MD, president of the American Academy of Family Physicians and a family physician in Jackson, Alabama. “It is imperative that family physicians encourage screening in at-risk patients and counsel them on the importance of continued screening, as well as smoking cessation, if needed.”
Two authors of the new guidelines reported financial relationships with Seno Medical Instruments, the Genentech Foundation, Crispr Therapeutics, BEAM Therapeutics, Intellia Therapeutics, Editas Medicine, Freenome, and Guardant Health.
A version of this article appeared on Medscape.com.
Noninvasive AI-Driven Tool Speeds Idiopathic Pulmonary Fibrosis Diagnosis
When clinicians suspect lung fibrosis and particularly its most devastating form, idiopathic pulmonary fibrosis (IPF), a noninvasive artificial intelligence (AI)-driven digital diagnostic tool may identify subtype classifications facilitating proper treatment at earlier disease stages. On January 16, 2024, the tool, Simultaneously, the American Medical Association adopted relevant CPT [Current Procedural Terminology] billing codes, according to an IMVARIA Inc. press release.
Diagnosis and treatment of the lung inflammation and fibrosis that drive IPF lung function decline are often long delayed, Joshua Reicher, MD, CEO of IMVARIA Inc. and an adjunct clinical professor at Stanford (California) University said in an interview for CHEST Physician.
“There are multiple challenges with this somewhat uncommon condition. Part of the frequent delays in diagnosis is the lack of access to local experts. Another part is vague presenting symptoms like general fatigue, for example, which can have an overlap with a lot of other conditions. The published median average delay in diagnosis after first presenting symptoms is about 2.2 years. But it’s often longer.”
Determining Type of Lung Fibrosis
Conventional diagnosis based on lab tests for inflammatory biomarkers and extensive clinical history is “fairly straightforward,” Dr. Reicher continued, for determining that a patient has some form of lung fibrosis. “The critical element is to find out what type of lung fibrosis and then begin appropriate therapy. The literature lists about 200 different subtypes, but the top 5 make up the majority of cases. The focus with Fibresolve is on improving noninvasive sensitivity, especially for the cases that are less straightforward, but rather indeterminate and therefore particularly challenging,” Dr. Reicher stated.
Will adjunctive diagnostic use of Fibresolve obviate the need for invasive confirmatory tests? Dr. Reicher was cautious. “We like to be thoughtful about our positioning of artificial intelligence and prefer to say that it puts complementary information in the hands of the physician. It’s really up to the clinicians to decide if they have sufficient information to avoid that biopsy.” The uniqueness of Fibresolve, Dr. Reicher pointed out, is that it is widely accessible and does not require hyper-specialized providers. “You can use it at any center that has standard CT scans.”
Reducing Burden on Physicians
An essential feature of Fibresolve use is that its software analysis is conducted centrally. “Part of our goal is to reduce the burden on the clinicians as much as possible, and we try to offload as much of the technical work from them as we can.”
The clinicians send images to IMVARIA Inc. (typically electronically) where they are processed rapidly, and a report is generated with outputs identifying the specific classification, perhaps with one indicating that the findings are suggestive of IPF. Dr. Reicher observed that the Fibresolve’s deep learning algorithm was trained on thousands of cases. “We’re very confident in the results that it puts out,” he said.
“We’re very excited. This is the first FDA-authorized diagnostic tool of any type in lung fibrosis. We really think this supports doctors and patients in areas where there’s a high unmet need,” Dr. Reicher said.
IMVARIA is next developing, in collaboration with the Mayo Clinic, a Fibresolve application for use in lung cancer, he said.
When clinicians suspect lung fibrosis and particularly its most devastating form, idiopathic pulmonary fibrosis (IPF), a noninvasive artificial intelligence (AI)-driven digital diagnostic tool may identify subtype classifications facilitating proper treatment at earlier disease stages. On January 16, 2024, the tool, Simultaneously, the American Medical Association adopted relevant CPT [Current Procedural Terminology] billing codes, according to an IMVARIA Inc. press release.
Diagnosis and treatment of the lung inflammation and fibrosis that drive IPF lung function decline are often long delayed, Joshua Reicher, MD, CEO of IMVARIA Inc. and an adjunct clinical professor at Stanford (California) University said in an interview for CHEST Physician.
“There are multiple challenges with this somewhat uncommon condition. Part of the frequent delays in diagnosis is the lack of access to local experts. Another part is vague presenting symptoms like general fatigue, for example, which can have an overlap with a lot of other conditions. The published median average delay in diagnosis after first presenting symptoms is about 2.2 years. But it’s often longer.”
Determining Type of Lung Fibrosis
Conventional diagnosis based on lab tests for inflammatory biomarkers and extensive clinical history is “fairly straightforward,” Dr. Reicher continued, for determining that a patient has some form of lung fibrosis. “The critical element is to find out what type of lung fibrosis and then begin appropriate therapy. The literature lists about 200 different subtypes, but the top 5 make up the majority of cases. The focus with Fibresolve is on improving noninvasive sensitivity, especially for the cases that are less straightforward, but rather indeterminate and therefore particularly challenging,” Dr. Reicher stated.
Will adjunctive diagnostic use of Fibresolve obviate the need for invasive confirmatory tests? Dr. Reicher was cautious. “We like to be thoughtful about our positioning of artificial intelligence and prefer to say that it puts complementary information in the hands of the physician. It’s really up to the clinicians to decide if they have sufficient information to avoid that biopsy.” The uniqueness of Fibresolve, Dr. Reicher pointed out, is that it is widely accessible and does not require hyper-specialized providers. “You can use it at any center that has standard CT scans.”
Reducing Burden on Physicians
An essential feature of Fibresolve use is that its software analysis is conducted centrally. “Part of our goal is to reduce the burden on the clinicians as much as possible, and we try to offload as much of the technical work from them as we can.”
The clinicians send images to IMVARIA Inc. (typically electronically) where they are processed rapidly, and a report is generated with outputs identifying the specific classification, perhaps with one indicating that the findings are suggestive of IPF. Dr. Reicher observed that the Fibresolve’s deep learning algorithm was trained on thousands of cases. “We’re very confident in the results that it puts out,” he said.
“We’re very excited. This is the first FDA-authorized diagnostic tool of any type in lung fibrosis. We really think this supports doctors and patients in areas where there’s a high unmet need,” Dr. Reicher said.
IMVARIA is next developing, in collaboration with the Mayo Clinic, a Fibresolve application for use in lung cancer, he said.
When clinicians suspect lung fibrosis and particularly its most devastating form, idiopathic pulmonary fibrosis (IPF), a noninvasive artificial intelligence (AI)-driven digital diagnostic tool may identify subtype classifications facilitating proper treatment at earlier disease stages. On January 16, 2024, the tool, Simultaneously, the American Medical Association adopted relevant CPT [Current Procedural Terminology] billing codes, according to an IMVARIA Inc. press release.
Diagnosis and treatment of the lung inflammation and fibrosis that drive IPF lung function decline are often long delayed, Joshua Reicher, MD, CEO of IMVARIA Inc. and an adjunct clinical professor at Stanford (California) University said in an interview for CHEST Physician.
“There are multiple challenges with this somewhat uncommon condition. Part of the frequent delays in diagnosis is the lack of access to local experts. Another part is vague presenting symptoms like general fatigue, for example, which can have an overlap with a lot of other conditions. The published median average delay in diagnosis after first presenting symptoms is about 2.2 years. But it’s often longer.”
Determining Type of Lung Fibrosis
Conventional diagnosis based on lab tests for inflammatory biomarkers and extensive clinical history is “fairly straightforward,” Dr. Reicher continued, for determining that a patient has some form of lung fibrosis. “The critical element is to find out what type of lung fibrosis and then begin appropriate therapy. The literature lists about 200 different subtypes, but the top 5 make up the majority of cases. The focus with Fibresolve is on improving noninvasive sensitivity, especially for the cases that are less straightforward, but rather indeterminate and therefore particularly challenging,” Dr. Reicher stated.
Will adjunctive diagnostic use of Fibresolve obviate the need for invasive confirmatory tests? Dr. Reicher was cautious. “We like to be thoughtful about our positioning of artificial intelligence and prefer to say that it puts complementary information in the hands of the physician. It’s really up to the clinicians to decide if they have sufficient information to avoid that biopsy.” The uniqueness of Fibresolve, Dr. Reicher pointed out, is that it is widely accessible and does not require hyper-specialized providers. “You can use it at any center that has standard CT scans.”
Reducing Burden on Physicians
An essential feature of Fibresolve use is that its software analysis is conducted centrally. “Part of our goal is to reduce the burden on the clinicians as much as possible, and we try to offload as much of the technical work from them as we can.”
The clinicians send images to IMVARIA Inc. (typically electronically) where they are processed rapidly, and a report is generated with outputs identifying the specific classification, perhaps with one indicating that the findings are suggestive of IPF. Dr. Reicher observed that the Fibresolve’s deep learning algorithm was trained on thousands of cases. “We’re very confident in the results that it puts out,” he said.
“We’re very excited. This is the first FDA-authorized diagnostic tool of any type in lung fibrosis. We really think this supports doctors and patients in areas where there’s a high unmet need,” Dr. Reicher said.
IMVARIA is next developing, in collaboration with the Mayo Clinic, a Fibresolve application for use in lung cancer, he said.