User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Evidence builds linking anticoagulation to COVID-19 survival
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
Operation Quack Hack: FDA moves to stop fraudulent COVID-19 products
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.
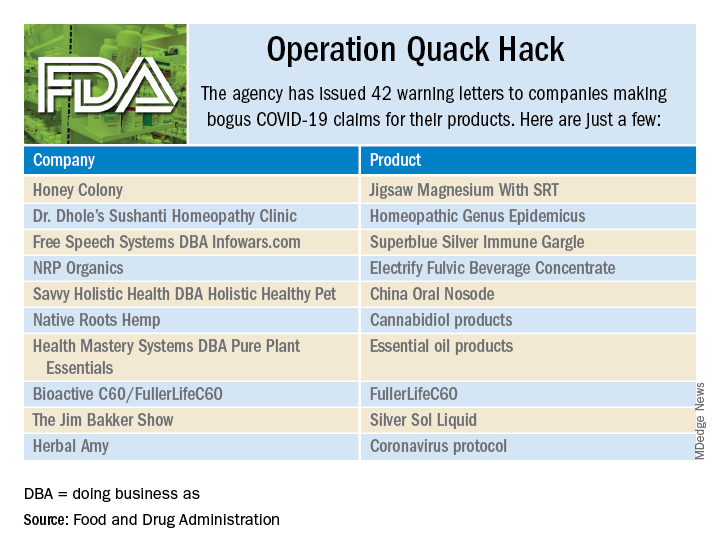
As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
Coronary CT angiography gives superior MI risk prediction
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
In patients with stable chest pain, the burden of low-attenuation noncalcified plaque on coronary CT angiography is a better predictor of future myocardial infarction risk than a cardiovascular risk score, an Agatson coronary artery calcium score, or angiographic severity of coronary stenoses, Michelle C. Williams, MBChB, PhD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
These findings from a post hoc analysis of the large multicenter SCOT-HEART trial challenge current concepts regarding the supposed superiority of the classic tools for MI risk prediction, noted Dr. Williams, a senior clinical research fellow at the University of Edinburgh.
Indeed, it’s likely that the current established predictors of risk – that is, coronary artery calcium, severity of stenosis, and cardiovascular risk score – are associated with clinical events only indirectly through their correlation with low-attenuated calcified plaque burden, which is the real driver of future MI, she continued.
Histologically, low-attenuated noncalcified plaque on coronary CT angiography (CCTA) is defined by a thin fibrous cap, a large, inflamed, lipid-rich necrotic core, and microcalcification. Previously, Dr. Williams and her coinvestigators demonstrated that visual identification of this unstable plaque subtype is of benefit in predicting future risk of MI (J Am Coll Cardiol. 2019 Jan 29;73[3]:291-301).
But visual identification of plaque subtypes is a crude and laborious process. In her current study, she and her coworkers have taken things a giant step further, using commercially available CCTA software to semiautomatically quantify the burden of this highest-risk plaque subtype as well as all the other subtypes.
This post hoc analysis of the previously reported main SCOT-HEART trial (N Engl J Med. 2018 Sep 6;379[10]:924-933) included 1,769 patients with stable chest pain randomized to standard care with or without CCTA guidance and followed for a median of 4.7 years, during which 41 patients had a fatal or nonfatal MI. At enrollment, 37% of participants had normal coronary arteries, 38% had nonobstructive coronary artery disease (CAD), and the remainder had obstructive CAD.
In a multivariate analysis, low-attenuation noncalcified plaque burden was the strongest predictor of future MI, with an adjusted hazard ratio of 1.6 per doubling. This metric was strongly correlated with coronary artery calcium score, underscoring the limited value of doing noncontrast CT in order to determine a coronary artery calcium score when CCTA is performed.
Low-attenuation plaque burden correlated very strongly with angiographic severity of stenosis, and only weakly with cardiovascular risk score, perhaps explaining the poor prognostic performance of cardiovascular risk scores in SCOT-HEART and other studies, according to Dr. Williams.
Patients with a low-attenuation noncalcified plaque burden greater than 4% in their coronary tree were 4.7 times more likely to have a subsequent MI than were those with a lesser burden. The predictive power was even greater in patients with nonobstructive CAD, where a low-attenuation noncalcified plaque burden in excess of 4% conferred a 6.6-fold greater likelihood of fatal or nonfatal MI, she observed.
Two things need to happen before measurement of low-attenuation noncalcified plaque via CCTA to predict MI risk is ready to be adopted in routine clinical practice, according to Dr. Williams. These SCOT-HEART results need to be validated in other cohorts, a process now underway in the SCOT-HEART 2 trial and other studies. Also, improved software incorporating machine learning is needed in order to speed up the semiautomated analysis of plaque subtypes, which now takes 20-30 minutes.
Dr. Williams reported having no financial conflicts regarding her study, funded by the National Health Service.
In conjunction with her virtual presentation at ACC 2020, the SCOT-HEART study results were published online (Circulation. 2020 Mar 16. doi: 10.1161/CIRCULATIONAHA.119.044720. [Epub ahead of print]).
SOURCE: Williams MC et al. ACC 2020, Abstract 909-06.
FROM ACC 2020
U.S. ‘deaths of despair’ from COVID-19 could top 75,000, experts warn
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
The number of “deaths of despair” could be even higher if the country fails to take bold action to address the mental health toll of unemployment, isolation, and uncertainty, according to the report from the Well Being Trust (WBT) and the Robert Graham Center for Policy Studies in Family Medicine and Primary Care.
“If nothing happens and nothing improves – ie, the worst-case scenario – we could be looking at an additional 150,000 people who died who didn’t have to,” Benjamin Miller, PsyD, WBT chief strategy officer, told Medscape Medical News.
“We can prevent these deaths. We know how and have a bevy of evidence-based solutions. We lack the resources to really stand this up in a way that can most positively impact communities,” Miller added.
Slow recovery, quick recovery scenarios
For the analysis, Miller and colleagues combined information on the number of deaths from suicide, alcohol, and drugs from 2018 as a baseline (n = 181,686). They projected levels of unemployment from 2020 to 2029 and then used economic modeling to estimate the additional annual number of deaths.
Across nine different scenarios, the number of additional deaths of despair range from 27,644 (quick recovery, smallest impact of unemployment on suicide, alcohol-, and drug-related deaths) to 154,037 (slow recovery, greatest impact of unemployment on these deaths), with 75,000 being the most likely.
The report offers several policy solutions to prevent a surge in “avoidable” deaths. They include finding ways to ameliorate the effects of unemployment and provide meaningful work to those who are out of work. Making access to care easier and fully integrating mental health and addiction care into primary and clinical care as well as community settings are also essential.
These solutions should also serve to prevent drug and alcohol misuse and suicide in normal times, the researchers say.
Miller believes it’s time for the federal government to fully support a framework of excellence in mental health and well-being and to invest in mental health now.
“In the short term, we need at least $48 billion to keep the lights on in the current system,” he said.
“This is because 92.6% of mental health organizations have had to reduce their operations in some capacity, 61.8% have had to completely close at least one program, and 31.0% have had to turn away patients. This scenario is not optimal for people who will need a system to help them right now during a crisis,” he added.
In the long term, $150 billion is needed for a “massive structural redesign” of the US mental health system, Miller said.
“This means bringing mental health fully into all facets of our healthcare system, of our community. It will take robust investment in creating new mechanisms for care ― those that are team-based, create a new type of workforce to deliver that care, and one that is seamless across clinical and community settings,” said Miller.
A version of this article first appeared on Medscape.com.
UNTOUCHED: Inappropriate shocks cut by subcutaneous ICD improvements
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
Patients with an indication for an implantable cardiac defibrillator for primary prevention of sudden cardiac death and a sharply reduced left ventricular ejection fraction of 35% or less safely received treatment from a refined, subcutaneous device that produced one of the lowest rates of inappropriate cardiac shocks ever seen in a reported ICD study, in a single-arm trial with 1,111 patients followed for 18 months.
The results showed “high efficacy and safety with contemporary devices and programming” despite being “the ‘sickest’ cohort studied to date” for use of a subcutaneous ICD (S-ICD), Michael R. Gold, MD, said at the annual scientific sessions of the Heart Rhythm Society, held online because of COVID-19. The 3.1% 1-year rate of patients who received at least one inappropriate shock was “the lowest reported for the S-ICD, and lower than in many transvenous ICD device studies,” and was also “the lowest 1-year rate reported to date for a multicenter ICD trial,” said Dr. Gold, a cardiac electrophysiologist and professor of medicine at the Medical University of South Carolina, Charleston. The upshot is that these data may help convince clinicians to be more liberal about offering a S-ICD device to patients with left ventricular function in this low range who need an ICD and do not need pacing.
The study’s primary endpoint was the rate of freedom from inappropriate shocks during 18 months of follow-up, which happened in 95.9% of patients and was highly statistically significant for meeting the prespecified performance goal of 91.6% that had been set using “standard Food and Drug Administration benchmarks,” with particular reliance on the performance shown in the MADIT-RIT trial (N Engl J Med. 2012 Dec 13;367[24]:2275-83).
S-ICDs maintain ‘niche’ status despite advantages
The S-ICD first received Food and Drug Administration clearance for U.S. use in 2012, but despite not requiring placement of a transvenous lead and thus eliminating the possibility for lead complications and deterioration, it so far has had very modest penetration into American practice. Recently, roughly 4% of U.S. patients who’ve received an ICD have had a subcutaneous model placed, relegating the S-ICD to “niche device” status, noted Andrea M. Russo, MD, director of electrophysiology and arrhythmia services at Cooper University Health Care in Camden, N.J. A major limitation of S-ICD devices is that they cannot provide chronic pacing and so aren’t an option for the many patients who also need this function in addition to protection from life-threatening ventricular arrhythmias.
“We have had a bias for whom we place an S-ICD,” explained Dr. Gold. “They have mostly been used in younger patients with less heart disease,” but when used in the current study cohort with markedly depressed heart function, the results showed that “we didn’t appear to harm patients in any way,” including no episodes of syncope because of an arrhythmia. Compared with other S-ICD studies, the patients in the new study, UNTOUCHED, had “lower ejection fractions, more heart failure diagnoses, and a higher rate of ischemic etiology.”
The tested S-ICD device appears to have safety and efficacy that is “just as good, and perhaps better” than many ICDs that use transvenous leads, “which was very surprising to us,” said Dr. Gold during a press briefing. “I think it will change practice” for ICD placement in patients who do not need pacing. “We found the device works even in the sickest patients.”
“This was a classic ICD population, with a low ejection fraction, and the results showed that the device performed well,” commented Dr. Russo, who served on the steering committee for the study. “I agree that the results will help” increase use of this device, but she added that other factors in addition to concerns about the inappropriate shock rate and the lack of most pacing functions have hobbled uptake since the device came on the market. These notably include a somewhat different placement approach than operators need to learn. The device is not always offered as an option to patients by their clinicians “in part because of their lack of familiarity, and concern about inappropriate shocks,” she said in an interview. That’s despite the clear attractions of a leaderless device, which obviates issues of lead deterioration, lead placement complications like perforations and pneumothorax, and sizing issues that can come up for women with narrower veins, as well as cutting the risk both for infections overall and for infections that progress to bacteremia, noted Dr. Russo, who is president of the Heart Rhythm Society.
Device improvements boost performance
The low 1-year and 18-month rates of inappropriate shocks likely occurred because of new filtering and programming incorporated into the tested device. “By changing the filter, we could make it more like a transvenous device” that is not fooled by T wave over sensing. The programing also included a high beat threshold, with a conditional zone above 200 beats per minute and an “aggressive shock zone” of 250 bpm, Dr. Gold said. This helped make the tested S-ICD more immune to inappropriately shocking a supraventricular arrhythmia; the study recorded no inappropriate shocks of this type, he reported.
The UNTOUCHED study enrolled 1,116 patients at any of 110 sites in the United States and elsewhere who had a need for primary prevention of sudden cardiac death, a left ventricular ejection fraction of 35% or less, no need for pacing, and had successfully passed an S-ICD screening test. The investigators were able to include 1,111 of these patients in their endpoint analysis. Patients averaged 56 years of age, a quarter were women, and their average ejection fraction was 26%.
In addition to the primary endpoint and the 1-year inappropriate-shock rate, the results also showed an all-cause shock-free rate of 90.6% during 18-months’ follow-up, which significantly surpassed the prespecified performance goal for this metric of 85.8%. The tested device also appeared to successfully apply appropriate shocks when needed, delivering a total of 64 of these with just 1 shock failure, a case where the patient spontaneously reverted to normal rhythm. During the study period, 53 patients died (5%), including 3 arrhythmia-related deaths: 1 caused by asystole and 2 from pulseless electrical activity.
“The data show that in a standard ICD population, the device worked well, and was safe and effective,” Dr. Russo said. “These data say, at least consider this device along with a transvenous device” for appropriate patients. “It’s a great option for some patients. I’ve seen so may lead problems, and this avoids them.”
UNTOUCHED was sponsored by Boston Scientific, the company that markets the tested S-ICD. Dr. Gold has been a consultant to Boston Scientific and Medtronic and has been an investigator for trials sponsored by each of these companies. Dr. Russo served on the steering committee for UNTOUCHED but received no compensation and has no financial disclosures.
FROM HEART RHYTHM 2020
Novel inflammatory syndrome in children possibly linked to COVID-19
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
according to reports from National Health Service England, The Lancet, and the New York City health department.
Fifteen children in New York City hospitals have presented with the condition, provisionally called pediatric multisystem inflammatory syndrome, between April 17 and May 1, according to a health alert from New York City health department deputy commissioner Demetre C. Daskalakis, MD, MPH, on May 4. On May 5, the New York state department of health released a health advisory that 64 suspected cases had been reported in children in New York state hospitals, including New York City.
The New York City reports follow a case study published April 7 in Hospital Pediatrics about the presentation. There also was a statement from the U.K.’s Paediatric Intensive Care Society (PICS) on April 27 that noted “blood parameters consistent with severe COVID-19 in children” as well as abdominal pain, gastrointestinal symptoms, and cardiac inflammation.
“Whilst it is too early to say with confidence, features appear to include high CRP [C-reactive protein], high [erythrocyte sedimentation rate] and high ferritin,” the PICS release stated. The cardiac inflammation consists of “myocarditis with raised troponin and [prohormone brain natriuretic peptide],” according to the PICS statement. “Some have an appearance of their coronary arteries in keeping with Kawasaki disease.”
The initial 15 New York City patients reportedly all had “subjective or measured fever, and more than half reported rash, abdominal pain, vomiting, or diarrhea,” but fewer than half had respiratory symptoms.
The case study described a 6-month-old infant who was admitted and diagnosed with classic Kawasaki disease, who also tested positive for COVID-19 with fever and mild respiratory symptoms, reported Veena G. Jones, MD, a pediatric hospitalist in Palo Alto, Calif., and associates.
While many of the U.K. children presenting with the symptoms had a positive polymerase chain reaction tests for infection from SARS-CoV-2, some also had a negative test. Polymerase chain reaction testing in New York City was positive for 4 children and negative for 11 children, but 6 of the those who tested negative had positive serology tests, potentially pointing to postinfection sequelae.
At press time, more cases were reported from the United Kingdom in The Lancet. In London, eight children with hyperinflammatory shock, showing features similar to atypical Kawasaki disease, Kawasaki disease shock syndrome, or toxic shock syndrome, presented within 10 days to Evelina London Children’s Hospital Paediatric ICU, Shelley Riphagen, MBChB, and colleagues revealed.
Clinically, their presentations were similar, with persistent fever, rash, conjunctivitis, peripheral edema, extremity pain, and gastrointestinal symptoms. They all developed warm vasoplegic shock that did not respond to volume resuscitation; noradrenaline and milrinone were administered for hemodynamic support. Seven of the children needed mechanical ventilation for cardiovascular stabilization, although most of them had no significant respiratory involvement.
Of note was development of small pleural, pericardial, and ascitic effusion – “suggestive of a diffuse inflammatory process,” Dr. Riphagen and associates wrote. None of the children initially was positive for SARS-CoV-2; laboratory evidence of infection or inflammation included “elevated concentrations of CRP, procalcitonin, ferritin, triglycerides or d-dimers.”
“A common echocardiographic finding was echobright coronary vessels,” they wrote. “One child developed arrhythmia with refractory shock, requiring extracorporeal life support, and died from a large cerebrovascular infarct.”
As the article went to press, the doctors in that same ICU had seen more than 20 children with similar clinical presentations, Dr. Riphagen and associates reported, and the first 10 tested positive for SARS-CoV-2 antibody, including the 8 described above.
“Most of the children appear to have antibodies to the novel coronavirus, even when they do not have virus detectable in their nose,” said Audrey John, MD, PhD, chief of the division of pediatric infectious diseases at Children’s Hospital of Philadelphia, where clinicians have seen several cases similar to those described by NHS England and the New York City health department. “This suggests that these symptoms are ‘postinfectious,’ likely due to an abnormal immune response that happens after viral infection.”
She noted at the time of her interview, however, that fewer than 100 U.S. pediatric cases appear to have been reported.
“While our understanding is evolving, given the scope of the COVID-19 pandemic, this suggests that this kind of severe disease in children is very rare indeed,” Dr. John said. “Because this syndrome is so newly described, we have to continue to be cautious in attributing this syndrome to COVID-19, as there are many other diseases that look quite similar.”
She advised clinicians to be “wary of attributing fever/rash/shock to this syndrome, as the differential is broad, and we do not want to fail to recognize and treat true toxic shock or tick-borne disease.”
Dawn Nolt, MD, MPH, an associate professor of pediatrics in infectious diseases at Oregon Health & Science University’s Doernbecher Children’s Hospital, Portland, also underscored the need to avoid drawing conclusions too quickly.
“At this time, there is no causality established between SARS-COV-2 and these inflammatory syndromes other than a temporal association,” said Dr. Nolt, whose hospital has not yet seen any of these cases. “If there is a link, then the symptoms may be from a ‘direct hit’ of the virus on tissues, or from an overly exuberant immune response.”
None of the initial 15 New York City children died, although 5 needed mechanical ventilation and over half needed blood pressure support. The one child in London died from a large cerebrovascular infarct.
If the cases are connected to COVID-19, one explanation for the presentation may be related to the leading hypothesis “that SARS-CoV-2 may stimulate the immune system in such a way to promote vasculitis,” Dr. Nolt said in an interview.
“It is unusual that this particular constellation was not reported from the known pediatric cases out of China, where the COVID-19 pandemic originated,” Dr. Nolt said. “If there is a link between SARS-CoV-2 and these inflammatory syndromes, this may have resulted from genetic/host differences, changes in the SARS-CoV-2 virus, or other factors yet to be determined.”
The New York City bulletin recommended that clinicians immediately refer children presenting with the described symptoms to a specialist in pediatric infectious disease, rheumatology, or critical care.
“Early diagnosis and treatment of patients meeting full or partial criteria for Kawasaki disease is critical to preventing end-organ damage and other long-term complications,” the bulletin stated. It recommended aspirin and intravenous immunoglobulin for those who met Kawasaki criteria.
Dr. John said that children with the presentation appear to be responding well to intravenous immunoglobulin and/or steroids. She further emphasized that virtually all pediatric patients recover from COVID-19.
“Physicians should advise families to bring their children and teens back in for evaluation if they develop new fever, rash, or abdominal pain and diarrhea,” Dr. John said. “Families should not be afraid to seek care when their kids are sick. Our pediatric hospitals and EDs are open for business and working hard to protect staff and patients.”
A Kawasaki syndrome diagnosis requires at least 5 days of a fever at 101-104° F or higher along with four of the following five symptoms: rash over the torso; redness and swelling on palms and soles of the feet with later skin peeling; bloodshot, light-sensitive eyes; swollen lymph glands in the neck; and irritation and inflammation of the mouth, lips and throat, sometimes with “strawberry” tongue, according to the American Heart Association.
A press release from the AHA noted that Kawasaki disease is the most common cause of acquired heart disease in developed countries, but the condition remains rare.
Kawasaki disease’s etiology is unknown, but “some evidence suggests an infectious trigger, with winter-spring seasonality of the disease,” wrote the case study authors, noting that past research has linked Kawasaki disease with previous or concurrent infections of rhinovirus/enterovirus, parainfluenza, respiratory syncytial virus, influenza, adenovirus, and the four common human coronavirus strains.
“We have to remember that our experience with this pandemic is less than 12 months,” Dr. Nolt said. “We are still accumulating information, and any additional manifestations, particularly severe ones, adds to our ability to more quickly detect and treat children.”
Dr. Nolt and Dr. John had no disclosures.
SOURCES: Jones VG et al. Hosp Pediatr. 2020 Apr 7. doi: 10.1542/hpeds.2020-0123; Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
Volunteering during the pandemic: What doctors need to know
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
Androgens may explain male vulnerability to COVID-19
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
COVID-19 and impact on sleep medicine practices
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Join us for CHEST Annual Meeting 2020
Registration for CHEST Annual Meeting 2020 has opened! It is important now, more than ever, to stay up to date in clinical chest education. CHEST Annual Meeting is prepared to equip attendees with the latest education and original research in the field that can be taken back home and implemented into practices.
While CHEST is excited to bring the premier event in clinical chest medicine to their Second City Home of Chicago, Illinois, this October 17-21, it is understood that now may not be the best time to be planning for a conference that is 6 months down the road. Currently, your full attention is likely on your patients, your families, your health, and your safety, and it should be! Here at CHEST, the hope is to create a “light at the end of the tunnel” to give you and your colleagues something to look forward to – an opportunity to relax, learn, explore, and reconnect with your peers in the chest medicine field.
This year’s annual meeting will be filled with both new and returning educational opportunities, including CHEST Games; virtual patient tours; hands-on simulation courses; problem-based learning; and the return of FISH Bowl, an innovation competition. Along with the advanced education, there will be countless opportunities to network at after-hour events, such as the CHEST Challenge final competition, the Young Professionals Reception, and the CHEST Foundation Casino Night. Our hope is that you will be able to look ahead to October and be excited about the chance to experience everything that will be offered at CHEST 2020.
Before the meeting in October, don’t forget to submit your abstracts and case reports for consideration to be presented at CHEST 2020. CHEST is excited to give you and your colleagues the opportunity to present new and original research at this year’s meeting, which is why the deadline for submissions has been extended to June 1, 2020.
CHEST acknowledges that your workload is becoming increasingly heavier each day, and we are also making the safety of attendees the top priority.
That is why CHEST will be granting full refunds to any registrant who finds that they can no longer attend CHEST 2020 as the meeting approaches. Any hotel reservation that is made through CHEST’s official housing site, onPeak, will be able to be changed or canceled up to 24 hours in advance of the reservation date. Visit chestmeeting.chestnet.org/hotel-accommodations for more information.
CHEST 2020 meeting chair, Victor Test, MD, FCCP, hopes to leave CHEST learners with a beacon of hope, saying, “Signing up to come to the meeting and participating may seem impossible to think about right now. We are working hard to provide a high-quality experience and are encouraging everyone to look forward to the future, which will be a lot brighter.”
For all of the latest information on CHEST 2020, visit chestmeeting.chestnet.org.
Registration for CHEST Annual Meeting 2020 has opened! It is important now, more than ever, to stay up to date in clinical chest education. CHEST Annual Meeting is prepared to equip attendees with the latest education and original research in the field that can be taken back home and implemented into practices.
While CHEST is excited to bring the premier event in clinical chest medicine to their Second City Home of Chicago, Illinois, this October 17-21, it is understood that now may not be the best time to be planning for a conference that is 6 months down the road. Currently, your full attention is likely on your patients, your families, your health, and your safety, and it should be! Here at CHEST, the hope is to create a “light at the end of the tunnel” to give you and your colleagues something to look forward to – an opportunity to relax, learn, explore, and reconnect with your peers in the chest medicine field.
This year’s annual meeting will be filled with both new and returning educational opportunities, including CHEST Games; virtual patient tours; hands-on simulation courses; problem-based learning; and the return of FISH Bowl, an innovation competition. Along with the advanced education, there will be countless opportunities to network at after-hour events, such as the CHEST Challenge final competition, the Young Professionals Reception, and the CHEST Foundation Casino Night. Our hope is that you will be able to look ahead to October and be excited about the chance to experience everything that will be offered at CHEST 2020.
Before the meeting in October, don’t forget to submit your abstracts and case reports for consideration to be presented at CHEST 2020. CHEST is excited to give you and your colleagues the opportunity to present new and original research at this year’s meeting, which is why the deadline for submissions has been extended to June 1, 2020.
CHEST acknowledges that your workload is becoming increasingly heavier each day, and we are also making the safety of attendees the top priority.
That is why CHEST will be granting full refunds to any registrant who finds that they can no longer attend CHEST 2020 as the meeting approaches. Any hotel reservation that is made through CHEST’s official housing site, onPeak, will be able to be changed or canceled up to 24 hours in advance of the reservation date. Visit chestmeeting.chestnet.org/hotel-accommodations for more information.
CHEST 2020 meeting chair, Victor Test, MD, FCCP, hopes to leave CHEST learners with a beacon of hope, saying, “Signing up to come to the meeting and participating may seem impossible to think about right now. We are working hard to provide a high-quality experience and are encouraging everyone to look forward to the future, which will be a lot brighter.”
For all of the latest information on CHEST 2020, visit chestmeeting.chestnet.org.
Registration for CHEST Annual Meeting 2020 has opened! It is important now, more than ever, to stay up to date in clinical chest education. CHEST Annual Meeting is prepared to equip attendees with the latest education and original research in the field that can be taken back home and implemented into practices.
While CHEST is excited to bring the premier event in clinical chest medicine to their Second City Home of Chicago, Illinois, this October 17-21, it is understood that now may not be the best time to be planning for a conference that is 6 months down the road. Currently, your full attention is likely on your patients, your families, your health, and your safety, and it should be! Here at CHEST, the hope is to create a “light at the end of the tunnel” to give you and your colleagues something to look forward to – an opportunity to relax, learn, explore, and reconnect with your peers in the chest medicine field.
This year’s annual meeting will be filled with both new and returning educational opportunities, including CHEST Games; virtual patient tours; hands-on simulation courses; problem-based learning; and the return of FISH Bowl, an innovation competition. Along with the advanced education, there will be countless opportunities to network at after-hour events, such as the CHEST Challenge final competition, the Young Professionals Reception, and the CHEST Foundation Casino Night. Our hope is that you will be able to look ahead to October and be excited about the chance to experience everything that will be offered at CHEST 2020.
Before the meeting in October, don’t forget to submit your abstracts and case reports for consideration to be presented at CHEST 2020. CHEST is excited to give you and your colleagues the opportunity to present new and original research at this year’s meeting, which is why the deadline for submissions has been extended to June 1, 2020.
CHEST acknowledges that your workload is becoming increasingly heavier each day, and we are also making the safety of attendees the top priority.
That is why CHEST will be granting full refunds to any registrant who finds that they can no longer attend CHEST 2020 as the meeting approaches. Any hotel reservation that is made through CHEST’s official housing site, onPeak, will be able to be changed or canceled up to 24 hours in advance of the reservation date. Visit chestmeeting.chestnet.org/hotel-accommodations for more information.
CHEST 2020 meeting chair, Victor Test, MD, FCCP, hopes to leave CHEST learners with a beacon of hope, saying, “Signing up to come to the meeting and participating may seem impossible to think about right now. We are working hard to provide a high-quality experience and are encouraging everyone to look forward to the future, which will be a lot brighter.”
For all of the latest information on CHEST 2020, visit chestmeeting.chestnet.org.