User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Stroke may be the first symptom of COVID-19 in younger patients
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Investigators carried out a meta-analysis of data, including 160 patients with COVID-19 and stroke, and found that nearly half of patients under the age of 50 were asymptomatic at the time of stroke onset.
Although younger patients had the highest risk of stroke, the highest risk of death was in patients who were older, had other chronic conditions, and had more severe COVID-19–associated respiratory symptoms.
“One of the most eye-opening findings of this study is that, for patients under 50 years old, many were totally asymptomatic when they had a stroke related to COVID-19, [which] means that, for these patients, the stroke was their first symptom of the disease,” lead author Luciano Sposato, MD, MBA, associate professor and chair in stroke research at Western University, London, Ont.
The study was published online Sept. 15 in Neurology.
Anecdotal reports
“In early April of 2020, we realized that COVID-19 was a highly thrombogenic disease,” said Dr. Sposato. “Almost in parallel, I started to see anecdotal reports in social media of strokes occurring in patients with COVID-19, and there were also very few case reports.”
The investigators “thought it would be a good idea to put all the data together in one paper,” he said, and began by conducting a systematic review of 10 published studies of COVID-19 and stroke (n = 125 patients), which were then pooled with 35 unpublished cases from Canada, the United States, and Iran for a total of 160 cases.
The analysis examined in-hospital mortality rates of patients with stroke and COVID-19.
In addition, the researchers conducted a second review of 150 papers, encompassing a final cohort of 3,306 COVID-19 patients with stroke of any type and 5,322 with ischemic stroke.
“Some studies reported data for only ischemic stroke, and some reported data for all strokes considered together, which resulted in a different number of patients on each meta-analysis, with a lower number of ‘any stroke’ cases,” Dr. Sposato explained. “This review looked at the number of patients who developed a stroke during admission and included thousands of patients.”
Dr. Sposato noted that the first review was conducted on single case reports and small case series “to understand the clinical characteristics of strokes in patients with COVID-19 on an individual patient level,” since “large studies, including hundreds of thousands of patients, usually do not provide the level of detail for a descriptive analysis of the clinical characteristics of a disease.”
Cluster analyses were used to “identify specific clinical phenotypes and their relationship with death.” Patients were stratified into three age groups: <50, 50-70, and >70 years (“young,” “middle aged,” and “older,” respectively). The median age was 65 years and 43% were female.
Mortality ‘remarkably high’
The review showed that 1.8% (95% confidence interval, 0.9%-3.7%) of patients experienced a new stroke, while 1.5% (95% CI, 0.8%-2.8%) of these experienced an ischemic stroke. “These numbers are higher than historical data for other infectious diseases – for example, 0.75% in SARS-CoV-1, 0.78% in sepsis, and 0.2% in influenza,” Dr. Sposato commented.
Moreover, “this number may be an underestimate, given that many patients die without a confirmed diagnosis and that some patients did not come to the emergency department when experiencing mild symptoms during the first months of the pandemic,” he added.
Focusing on the review of 160 patients, the researchers described in-hospital mortality for strokes of all types and for ischemic strokes alone as “remarkably high” (34.4% [95% CI, 27.2%-42.4%] and 35.7% [95% CI, 27.5%-44.8%], respectively), with most deaths occurring among ischemic stroke patients.
“This high mortality rate is higher than the [roughly] 15% to 30% reported for stroke patients without COVID-19 admitted to intensive care units,” Dr. Sposato said.
High-risk phenotype
Many “young” COVID-19 patients (under age 50) who had a stroke (42.9%) had no previous risk factors or comorbidities. Moreover, in almost half of these patients (48.3%), stroke was more likely to occur before the onset of any COVID-19 respiratory symptoms.
Additionally, younger patients showed the highest frequency of elevated cardiac troponin compared with middle-aged and older patients (71.4% vs. 48.4% and 27.8%, respectively). On the other hand, mortality was 67% lower in younger versus older patients (odds ratio, 0.33; 95% CI, 0.12-0.94; P = .039).
Dr. Sposato noted that the proportion of ischemic stroke patients with large-vessel occlusion was “higher than previously reported” for patients with stroke without COVID-19 (47% compared with 29%, respectively).
“We should consider COVID-19 as a new cause or risk factor for stroke. At least, patients with stroke should probably be tested for SARS-CoV-2 infection if they are young and present with a large-vessel occlusion, even in the absence of typical COVID-19 respiratory symptoms,” he suggested.
The researchers identified a “high-risk phenotype” for death for all types of stroke considered together: older age, a higher burden of comorbidities, and severe COVID-19 respiratory symptoms. Patients with all three characteristics had the highest in-hospital mortality rate (58.6%) and a threefold risk of death, compared with the rest of the cohort (OR, 3.52; 95% CI, 1.53-8.09; P = .003).
“Several potential mechanisms can explain the increased risk of stroke among COVID-19 patients, but perhaps the most important one is increased thrombogenesis secondary to an exaggerated inflammatory response,” Dr. Sposato said.
Not just elders
Commenting on the study, Jodi Edwards, PhD, director of the Brain and Heart Nexus Research Program at the University of Ottawa Heart Institute, said the findings are “consistent with and underscore public health messaging emphasizing that COVID-19 does not only affect the elderly and those with underlying health conditions, but can have serious and even fatal consequences at any age.”
Dr. Edwards, who was not involved with the study, emphasized that “adherence to public health recommendations is critical to begin to reduce the rising incidence in younger adults.”
Dr. Sposato acknowledged that the study was small and that there “can be problems associated with a systematic review of case reports, such as publication bias, lack of completeness of data, etc, so more research is needed.”
Dr. Sposato is supported by the Kathleen & Dr. Henry Barnett Research Chair in Stroke Research at Western University, the Edward and Alma Saraydar Neurosciences Fund of the London Health Sciences Foundation, and the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario. Dr. Sposato reported speaker honoraria from Boehringer Ingelheim, Pfizer, Gore, and Bayer and research/quality improvement grants from Boehringer Ingelheim and Bayer. The other authors’ disclosures are listed on the original article. Dr. Edwards has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Neurology
CDC flips, acknowledges aerosol spread of COVID-19
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
The information reiterates, however, that “COVID-19 is thought to spread mainly through close contact from person to person, including between people who are physically near each other (within about 6 feet). People who are infected but do not show symptoms can also spread the virus to others.”
In a statement to the media, the CDC said, “Today’s update acknowledges the existence of some published reports showing limited, uncommon circumstances where people with COVID-19 infected others who were more than 6 feet away or shortly after the COVID-19–positive person left an area. In these instances, transmission occurred in poorly ventilated and enclosed spaces that often involved activities that caused heavier breathing, like singing or exercise. Such environments and activities may contribute to the buildup of virus-carrying particles.”
“This is HUGE and been long delayed. But glad it’s now CDC official,” tweeted Eric Feigl-Ding, MD, an epidemiologist and health economist at Harvard University, Boston on Oct. 5.
The CDC announcement follows an abrupt flip-flop on information last month surrounding the aerosol spread of the virus.
Information deleted from website last month
On September 18, the CDC had added to its existing guidance that the virus is spread “through respiratory droplets or small particles, such as those in aerosols, produced when an infected person coughs, sneezes, sings, talks, or breathes. These particles can be inhaled into the nose, mouth, airways, and lungs and cause infection.”
The CDC then deleted that guidance on Sept. 21, saying it was a draft update released in error.
A key element of the now-deleted guidance said, “this is thought to be the main way the virus spreads.”
The information updated today reverses the now-deleted guidance and says aerosol transmission is not the main way the virus spreads.
It states that people who are within 6 feet of a person with COVID-19 or have direct contact with that person have the greatest risk of infection.
The CDC reiterated in the statement to the media today, “People can protect themselves from the virus that causes COVID-19 by staying at least 6 feet away from others, wearing a mask that covers their nose and mouth, washing their hands frequently, cleaning touched surfaces often, and staying home when sick.”
Among the journals that have published evidence on aerosol spread is Clinical Infectious Diseases, which, on July 6, published the paper, “It Is Time to Address Airborne Transmission of Coronavirus Disease 2019,” which was supported by 239 scientists.
The authors wrote, “there is significant potential for inhalation exposure to viruses in microscopic respiratory droplets (microdroplets) at short to medium distances (up to several meters, or room scale).”
Aerosols and airborne transmission “are the only way to explain super-spreader events we are seeing,” said Kimberly Prather, PhD, an atmospheric chemist at the University of California at San Diego, in an interview Oct. 5 with the Washington Post.
Dr. Prather added that, once aerosolization is acknowledged, this becomes a “fixable” problem through proper ventilation.
“Wear masks at all times indoors when others are present,” Dr. Prather said. But when inside, she said, there’s no such thing as a completely safe social distance.
This article first appeared on Medscape.com.
Substance use tied to increased COVID-19 risk
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Substance use disorders (SUD), particularly opioid addiction and smoking, are tied to an increased risk for COVID-19 and serious adverse outcomes including hospitalization and death, new research suggests.
A study funded by the National Institutes of Health assessed electronic health records of more than 73 million patients in the United States. Although only 10.3% of the participants had an SUD, “they represented 15.6% of the COVID-19 cases,” the investigators reported.
In addition, those with a recent diagnosis of SUD were eight times more likely to develop COVID-19 versus those without such a diagnosis. For specific SUDs, the greatest risk was for those with an opioid addiction followed by those who were addicted to cigarettes.
coinvestigator Nora Volkow, MD, director of the National Institute on Drug Abuse, said in a press release.
It may also be harder for individuals with addiction to access health care services for a variety of reasons, including low socioeconomic status or stigma, she said in an interview.
Dr. Volkow said she has encountered patients with medical emergencies who refuse to seek treatment at the emergency department because of previous experiences where they have been mistreated and encountered discrimination, and “that’s really very tragic.”
The findings were published online Sept. 14 in Molecular Psychiatry.
Is nicotine protective?
Dr. Volkow, her fellow senior author Rong Xu, PhD, Case Western Reserve University, Cleveland, and their team conducted the study because data released before the pandemic showed a significant increase in opioid overdose in 2019. “We were in an opioid crisis where we again saw an increase in mortality associated with overdose – and then COVID comes along. So the question was how are people who are already struggling faring? And if they were getting infected [with the coronavirus], what happened to them?”
Patients with SUDs have multiple medical comorbidities that are known risk factors for COVID-19, Dr. Volkow noted.
However, the only specific SUD that has been previously studied in this context is tobacco use disorder, she said. A report from Chinese investigators released early in the pandemic showed that smokers were more likely to be infected by coronavirus and more likely to die from COVID-19.
Interestingly, a cross-sectional study published in April suggested that smoking may be protective against COVID, and Dr. Volkow noted that a clinical study currently being conducted in France is assessing whether wearing a nicotine patch has the potential to prevent the virus.
“That’s very different from looking at a chronic smoker,” she pointed out. “It’s a potential that nicotine as a chemical [could be] a preventive measure as opposed to saying smoking will prevent you from getting COVID.”
Patients with SUDs, said Dr. Volkow, “are likely to be at greater risk because of the effects of drugs in the metabolic system and the interfering with oxygenation in the pulmonary vessels.”
The retrospective case-control study included EHR data from 73.1 million patients. In the study population, 54% were women, 55% were White, 10% Black, 2% Asian, 1% Hispanic/Latino, and the others were classified as other or unknown.
EHRs were collected through June 15 at 360 hospitals in all 50 states and were deidentified to ensure privacy. SUDs included alcohol, tobacco, cannabis, opioid, and cocaine.
Racial disparities
Results showed that about 7.5 million participants had a previous SUD diagnosis; of these, 722,370 had been diagnosed within the past year.
Tobacco use disorder was the most common diagnosis (n = 6,414,580), followed by alcohol (1,264,990), cannabis (490,420), opioid (471,520), and cocaine (222,680).
In addition, 12,030 (60% women) were diagnosed with COVID-19 and 1,880 had both COVID-19 and an SUD.
Adjusted analyses revealed that those who had a recent diagnosis of SUD were at a significantly greater increased risk for COVID-19 than individuals without an SUD (adjusted odds ratio, 8.7; 95% confidence interval, 8.4-9.0; P < 10–30).
This increased risk was greatest in participants with opioid use disorder (aOR, 10.2; 95% CI, 9.1-11.5; P < 10–30), followed by those with tobacco use disorder (aOR, 8.2; 95% CI, 7.9 - 8.5; P < 10–30).
Alcohol, cocaine, and cannabis had aORs of 7.7, 6.5, and 5.3, respectively. The aOR for lifetime SUD and COVID-19 was 1.5.
Among all patients with COVID-19, hospitalization rates were significantly greater in those with an SUD (43.8%) versus those without (30.1%), as were death rates at 9.6% versus 6.6%, respectively.
Race was a significant risk factor. Black patients with a recent SUD diagnosis were twice as likely as White patients to develop COVID-19 (aOR, 2.2; P < 10–30), and those specifically with opioid use disorder were four times more likely to develop the disease (aOR, 4.2 P < 10–25).
Black patients with both COVID-19 and lifetime SUD also had greater hospitalization and death rates versus their White peers (50.7% vs. 35.2% and 13% vs. 8.6%, respectively).
“This surprised me,” Dr. Volkow noted. “You can see the emergence of the racial disparities even under these conditions of really negative outcomes.”
Vulnerable populations
Cancer; obesity; HIV; diabetes; cardiovascular disease; and chronic kidney, liver, and lung diseases, which are all risk factors for COVID-19, were more prevalent in the group of patients with a recent SUD diagnosis versus those without.
In addition, asthma, type 2 diabetes, hypertension, obesity, and chronic kidney disease were more prevalent in the Black patents with a recent SUD than in the White patients.
Overall, the findings “identify individuals with SUD as a vulnerable population, especially African Americans with SUDs, who are at significantly increased risk for COVID-19 and its adverse outcomes,” the investigators wrote.
The results also highlight “the need to screen and treat individuals with SUD as part of the strategy to control the pandemic while ensuring no disparities in access to healthcare support,” they added.
Dr. Volkow noted that “marginalization” often occurs for individuals with addiction, making it more difficult for them to access health care services.
“It is incumbent upon clinicians to meet the unique challenges of caring for this vulnerable population, just as they would any other high-risk group,” she said.
“Patients should not just be treated for COVID, but should also be provided with treatment for their substance use disorder,” Dr. Volkow added.
‘Pretty convincing’
Andrew J. Saxon, MD, professor in the department of psychiatry and behavioral sciences at the University of Washington, Seattle, called the findings interesting.
“I found it pretty convincing that people who have substance use disorders are probably at higher risk for getting COVID-19 infection and more complications once they are infected,” he said.
Dr. Saxon, who was not involved with the research, is also director of the Center of Excellence in Substance Addiction Treatment and Education and is a member of the American Psychiatric Association’s Council on Addiction Psychiatry.
He noted that an important point from the study was not just about a patient having an SUD being at increased risk for COVID-19 “and a more severe disease trajectory.” Other factors associated with having an SUD, such as increased comorbidities, also likely play a part.
Dr. Saxon agreed that the ongoing opioid epidemic combined with the pandemic led to a “perfect storm” of problems.
“We were making slow but some progress getting more people the medications they need [to treat opioid use disorder], but the pandemic coming along disrupted those efforts. A lot of health care entities had to shut down for a while, seeing patients only remotely,” which led to barriers as many clinicians needed to learn how to proceed using telehealth options, said Dr. Saxon.
Universal screening?
Asked whether physicians should screen all patients for SUDs, Dr. Saxon said it’s a complicated question.
“Screening for tobacco and alcohol has a really good evidence base and practices should be doing that. The stigma is there but it’s a lot less than with illegal substances,” he said.
Screening for illegal substances or misuse of prescription substances may not be a good idea in health care settings “when it’s something they can’t do anything about. If you’re going to screen, you would have to have either referral processes in place or treatment available in your facility,” Dr. Saxon said.
Opioid use disorder is “especially amenable to treatment in a primary care or health care setting with prescribers,” he noted.
However, stimulant or cannabis use disorders “require fairly intensive behavioral interventions that are not easy to deliver in many health care settings. And we don›t have the workforce trained up to provide those treatments as widely as they should be,” said Dr. Saxon.
“Unless there’s some way to treat the issue, what’s the point of screening for it? That just creates frustration for patients and clinicians, as well,” he said. “It’s something we’re moving toward but we’re not quite there yet.”
The report authors and Dr. Saxon have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Minorities bear brunt of pediatric COVID-19 cases
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
FROM PEDIATRICS
COVID-19 may discourage pediatric flu vaccination
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
Parents who did not vaccinate their children against influenza last year were significantly less likely to do so this year than parents whose children were vaccinated last year, based on survey data from more than 2,000 parents with babies and young children.
“Pediatric vaccination will be an important component to mitigating a dual influenza/COVID-19 epidemic,” Rebeccah L. Sokol, PhD, of Wayne State University, Detroit, and Anna H. Grummon, PhD, of Harvard School of Public Health, Boston, reported in Pediatrics.
Although the pandemic has increased acceptance of some healthy behaviors including handwashing and social distancing, the impact on influenza vaccination rates remains unknown, they said.
To assess parents’ current intentions for flu vaccination of young children this season, the researchers conducted an online survey of 2,164 parents or guardians of children aged between 6 months and 5 years in the United States. The 15-minute online survey was conducted in May 2020 and participants received gift cards. The primary outcome was the impact of the COVID-19 pandemic on parental intentions for having their child vaccinated against seasonal flu this year.
“We measured change categorically, with response options ranging from 1 (I became much less likely to get my child the flu shot next year) to 5 (I became much more likely to get my child the flu shot next year),” the researchers said.
Pandemic changes some parents’ plans
Overall, 60% of parents said that the ongoing pandemic had altered their flu vaccination intentions for their children. About 34% percent of parents whose children did not receive flu vaccine last year said they would not seek the vaccine this year because of the pandemic, compared with 25% of parents whose children received last year’s flu vaccine, a statistically significant difference (P < .001).
Approximately 21% of parents whose children received no flu vaccine last year said the pandemic made them more likely to seek vaccination for the 2020-2021 season, compared with 38% of parents whose children received last year’s flu vaccine.
“These results suggest that overall seasonal influenza vaccination rates may not increase simply because of an ongoing infectious disease pandemic. Instead, a significant predictor of future behavior remains past behavior,” Dr. Sokol and Dr. Grummon said.
The study findings were limited by several factors including the use of a convenience sample and the timing of the survey in May 2020, meaning that survey results might not be generalizable this fall as the pandemic persists, they noted. “Additionally, we assessed intentions to vaccinate; future research will clarify the COVID-19 pandemic’s influence on actual vaccination behaviors.”
The challenge of how to increase uptake of the influenza vaccine during the era of COVID-19 remains, and targeted efforts could include social norms messaging through social media, mass media, or health care providers to increase parents’ intentions to vaccinate, as well as vaccination reminders and presumptive announcements from health care providers that present vaccination as the default option, the researchers added.
Potential for ‘twindemic’ is real
The uptake of flu vaccination is especially important this year, Christopher J. Harrison, MD, director of the vaccine and treatment evaluation unit and professor of pediatrics at the University of Missouri–Kansas City, said in an interview.
“This year we are entering a flu season where the certainty of the timing as well as the potential severity of the season are not known. That said, social distancing and wearing masks – to the extent that enough people conform to COVID-19 precautions – could delay or even blunt the usual influenza season,” he noted.
Unfortunately, the Centers for Disease Control and Prevention and the Food and Drug Administration have had their credibility damaged by the challenges of creating a successful response on the fly to a uniquely multifaceted virus to which previous rules do not apply, Dr. Harrison said. In addition, public confidence was eroded when information about testing and reopening policies were released by non-CDC nonscientists and labeled “CDC recommended,” with no opportunity for the scientific community to correct inaccuracies.
“The current study reveals that public trust in influenza vaccine and indirectly in health authorities has been affected by the pandemic,” said Dr. Harrison. “Vaccine hesitancy has increased somewhat even among previous vaccine accepters. One wonders if promises of a quick COVID-19 vaccine increased mistrust of the FDA because of safety concerns, even among the most ardent provaccine population, and whether these concerns are bleeding over into influenza vaccine concerns.
“This only adds to the anxiety that families feel about visiting any medical facility for routine vaccines while the pandemic rages, and we now are in a fall SARS-CoV-2 resurgence,” he added.
Although the current study data are concerning, “there could still be a net gain of pediatric influenza vaccine uptake this season because the 34% less likely to immunize among previously nonimmunizing families would be counterbalanced by 21% of the same group being more likely to immunize their children [theoretical net loss of 13%],” Dr. Harrison explained. “But the pandemic seems to have motivated previously influenza-immunizing families, i.e. while 24% were less likely, 39% are more likely to immunize [theoretical net gain of 15%]. That said, we would still be way short of the number needed to get to herd immunity.”
Dr. Harrison said he found the findings somewhat surprising, but perhaps he should not have. “I had hoped for more acceptance rather than most people staying in their prior vaccine ‘opinion lanes,’ ending up with likely little overall net change in plans to immunize despite increased health awareness caused by a pandemic.”
However, “the U.S. population has been polarized on vaccines and particularly influenza vaccines for more than 50 years, so why would a pandemic make us less polarized, particularly when the pandemic itself has been a polarizing event?” he questioned.
The greatest barriers to flu vaccination for children this year include a lack of motivation among families to visit immunization sites, given the ongoing need for social distancing and masks, Dr. Harrison said.
“Another barrier is the waning public confidence in our medical/scientific national leaders and organizations,” he emphasized. “This makes it crucial that primary care providers step up and be extra strong vaccine advocates, despite the fact that pandemic economics and necessary safety processes have stressed providers and devastated practices. Indeed, in times of medical stress, no one gets more trust from families than their own personal provider.”
Ultimately, avenues for future research include asking diverse groups of families what they feel they need to hear to be more engaged in immunizing children against influenza. But for now, the current study findings identify that “the public is not uniformly responding to the pandemic’s influence on their likelihood of immunizing their children against influenza,” Dr. Harrison said.
“We now know the size of the problem and hopefully governments, public health organizations, pediatric advocates and clinical care givers can find ways to magnify the message that a pandemic year is not a year to avoid seasonal influenza vaccine unless one has a true contraindication,” Dr. Harrison said.
In addition, “one wonders if the poll were taken today – post the president’s COVID-19 illness – would the answers be different?” he noted.
Dr. Sokol’s work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development but otherwise had no financial conflicts to disclose. Dr. Harrison disclosed that his institution receives grant funding from Merck, Pfizer, and GlaxoSmithKline for pediatric noninfluenza vaccine studies on which he is a subinvestigator, and support from the CDC for pediatric respiratory and gastrointestinal virus surveillance studies on which he is an investigator.
SOURCE: Sokol RL, Grummon AH. Pediatrics. 2020 Sep 30. doi: 10.1542/peds.2020-022871.
FROM PEDIATRICS
Inside the flawed White House testing scheme that did not protect Trump
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
The president has said others are tested before getting close to him, appearing to hold it as an iron shield of safety. He has largely eschewed mask-wearing and social distancing in meetings, travel and public events, while holding rallies for thousands of often maskless supporters.
The Trump administration has increasingly pinned its coronavirus testing strategy for the nation on antigen tests, which do not need a traditional lab for processing and quickly return results to patients. But the results are less accurate than those of the slower PCR tests.
An early antigen test used by the White House was woefully inaccurate. But the new antigen test the White House is using has not been independently evaluated for accuracy and reliability. Moreover, this is the kit the Trump administration is pushing out to thousands of nursing homes to test residents and staff.
Testing “isn’t a ‘get out of jail free card,’” said Dr. Alan Wells, medical director of clinical labs at the University of Pittsburgh Medical Center and creator of its test for the novel coronavirus. In general, antigen tests can miss up to half the cases that are detected by polymerase chain reaction tests, depending on the population of patients tested, he said.
The White House said the president’s diagnosis was confirmed with a PCR test but declined to say which test delivered his initial result. The White House has been using a new antigen test from Abbott Laboratories to screen its staff for COVID-19, according to two administration officials.
The test, known as BinaxNOW, received an emergency use authorization from the Food and Drug Administration in August. It produces results in 15 minutes. Yet little is independently known about how effective it is. According to the company, the test is 97% accurate in detecting positives and 98.5% accurate in identifying those without disease. Abbott’s stated performance of its antigen test was based on examining people within 7 days of COVID symptoms appearing.
The president and first lady have both had symptoms, according to White House chief of staff Mark Meadows and the first lady’s Twitter account. The president was admitted to Walter Reed National Military Medical Center on Friday evening “out of an abundance of caution,” White House press secretary Kayleigh McEnany said in a statement.
Vice President Mike Pence is also tested daily for the virus and tested negative, spokesperson Devin O’Malley said Friday, but he did not respond to a follow-up question about which test was used.
Trump heavily promoted another Abbott rapid testing device, the ID NOW, earlier this year. But that test relies on different technology than the newer Abbott antigen test.
“I have not seen any independent evaluation of the Binax assay in the literature or in the blogs,” Wells said. “It is an unknown.”
The Department of Health and Human Services announced in August that it had signed a $760 million contract with Abbott for 150 million BinaxNOW antigen tests, which are now being distributed to nursing homes and historically black colleges and universities, as well as to governors to help inform decisions about opening and closing schools. The Big Ten football conference has also pinned playing hopes on the deployment of antigen tests following Trump’s political pressure.
However, even senior federal officials concede that a test alone isn’t likely to stop the spread of a virus that has sickened more than 7 million Americans.
“Testing does not substitute for avoiding crowded indoor spaces, washing hands, or wearing a mask when you can’t physically distance; further, a negative test today does not mean that you won’t be positive tomorrow,” Adm. Brett Giroir, the senior HHS official helming the administration’s testing effort, said in a statement at the time.
Trump could be part of a “super-spreading event,” said Dr. Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota.
Given the timing of Trump’s positive test — which he announced on Twitter early Friday – his infection “likely happened 5 or more days ago,” Osterholm said. “If so, then he was widely infectious as early as Tuesday,” the day of the first presidential debate in Cleveland.
At least seven people who attended a Rose Garden announcement last Saturday, when Trump announced his nomination of Judge Amy Coney Barrett to the Supreme Court, have since tested positive for the coronavirus. They include Trump’s former adviser Kellyanne Conway, Republican Sens. Mike Lee and Thom Tillis, and the president of the University of Notre Dame, the Rev. John Jenkins.
“Having that many infected people there all at one time, we’re still going to see transmission coming off that event for a couple days,” Osterholm said.
Osterholm notes that about 20% of infected people lead to 80% of COVID-19 cases, because “super spreaders” can infect so many people at once.
He notes that participants and audience members at Tuesday’s debate were separated by at least 6 feet. But 6 feet isn’t always enough to prevent infection, he said.
While many COVID-19 infections appear to be spread by respiratory droplets, which usually fall to the ground within 6 feet, people who are singing or speaking loudly can project virus much further. Evidence also suggests that the novel coronavirus can spread through aerosols, floating in the air like a speck of dust.
“I wonder how much virus was floating in that room that night,” Osterholm said.
Other experts say it’s too soon to say whether Trump was infected in a super-spreader event. “The president and his wife have had many exposures to many people in enclosed venues without protection,” so they could have been infected at any number of places, said Dr. William Schaffner, an infectious disease specialist at the Vanderbilt University School of Medicine.
Although Democratic presidential candidate and former Vice President Joe Biden tested negative for the virus with a PCR test Friday, experts note that false-negative results are common in the first few days after infection. Test results over the next several days will yield more useful information.
It can take more than a week for the virus to reproduce enough to be detected, Wells said: “You are probably not detectable for 3, 5, 7, even 10 days after you’re exposed.”
In Minnesota, where Trump held an outdoor campaign rally in Duluth with hundreds of attendees Wednesday, health officials warned that a 14-day quarantine is necessary, regardless of test results.
“Anyone who was a direct contact of President Trump or known COVID-19 cases needs to quarantine and should get tested,” the Minnesota Department of Health said.
Ongoing lapses in test result reporting could hamper efforts to track and isolate sick people. As of Sept. 10, 21 states and the District of Columbia were not reporting all antigen test results, according to a KHN investigation, a lapse in reporting that officials say leaves them blind to disease spread. Since then, public health departments in Arizona, North Carolina and South Dakota all have announced plans to add antigen testing to their case reporting.
Requests for comment to the D.C. Department of Health were referred to Mayor Muriel Bowser’s office, which did not respond. District health officials told KHN in early September that the White House does not report antigen test results to them – a potential violation of federal law under the CARES Act, which says any institution performing tests to diagnose COVID-19 must report all results to local or state public health departments.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said it’s not surprising that Trump tested positive, given that so many of his close associates – including his national security adviser and Secret Service officers – have also been infected by the virus.
“When you look at the number of social contacts and travel schedules, it’s not surprising,” Adalja said.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Use of e-cigarettes may be linked to sleep deprivation
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.
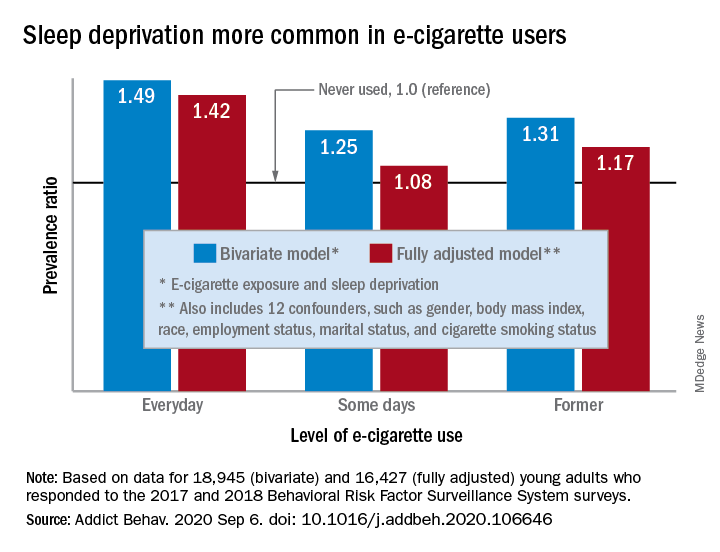
“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
FROM ADDICTIVE BEHAVIORS
FDA OKs combination immunotherapy for first-line mesothelioma treatment
This is the first drug regimen to receive regulatory approval for mesothelioma in 16 years and only the second systemic therapy to be approved for this indication.
“Today’s approval of nivolumab plus ipilimumab provides a new treatment that has demonstrated an improvement in overall survival for patients with malignant pleural mesothelioma,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement.
“In 2004, FDA approved pemetrexed in combination with cisplatin for this indication, and now patients have an important, additional treatment option after more than a decade with only one FDA-approved drug regimen,” Dr. Pazdur added.
Improved overall survival
The approval is based on efficacy results from the CheckMate 743 trial, which compared immunotherapy with a chemotherapy regimen in a cohort of more than 600 treatment-naive patients (no systemic therapies) with unresectable mesothelioma.
Patients were randomized 1:1 to nivolumab and ipilimumab for up to 2 years (n = 303) or six cycles of combination chemotherapy with cisplatin or carboplatin plus pemetrexed (n = 302).
The study results were initially presented during the presidential symposium of the World Congress on Lung Cancer 2020.
The combined immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive versus 27% in the chemotherapy group.
Overall, the trial demonstrated a statistically significant improvement in overall survival for patients who received nivolumab plus ipilimumab versus those treated with chemotherapy. Median overall survival was 18.1 months versus 14.1 months (hazard ratio, 0.74; P = .002).
Median progression-free survival per blinded independent central review was 6.8 months in the nivolumab plus ipilimumab arm and 7.2 months in the chemotherapy arm (HR, 1.0). The confirmed overall response rate was 40% versus 43% in the immunotherapy and chemotherapy arms, respectively.
Median response duration was 11.0 months in the nivolumab plus ipilimumab arm and 6.7 months in the chemotherapy arm. At 24 months, 32% of the immunotherapy patients were still experiencing a response, compared with 8% of those in the chemotherapy arm.
The recommended doses for unresectable malignant pleural mesothelioma are nivolumab 360 mg every 3 weeks and ipilimumab 1 mg/kg every 6 weeks until disease progression or unacceptable toxicity, or up to 2 years in patients without disease progression.
The most common adverse reactions (incidence ≥20%) in patients receiving combination immunotherapy were fatigue, musculoskeletal pain, rash, diarrhea, dyspnea, nausea, decreased appetite, cough, and pruritus.
New standard of care?
The CheckMate 743 trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported study author Paul Baas, MD, PhD, of the Netherlands Cancer Institute, Amsterdam, at the time of its presentation.
He suggested that combination nivolumab and ipilimumab should therefore “be considered as a new standard of care.”
This article first appeared on Medscape.com.
This is the first drug regimen to receive regulatory approval for mesothelioma in 16 years and only the second systemic therapy to be approved for this indication.
“Today’s approval of nivolumab plus ipilimumab provides a new treatment that has demonstrated an improvement in overall survival for patients with malignant pleural mesothelioma,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement.
“In 2004, FDA approved pemetrexed in combination with cisplatin for this indication, and now patients have an important, additional treatment option after more than a decade with only one FDA-approved drug regimen,” Dr. Pazdur added.
Improved overall survival
The approval is based on efficacy results from the CheckMate 743 trial, which compared immunotherapy with a chemotherapy regimen in a cohort of more than 600 treatment-naive patients (no systemic therapies) with unresectable mesothelioma.
Patients were randomized 1:1 to nivolumab and ipilimumab for up to 2 years (n = 303) or six cycles of combination chemotherapy with cisplatin or carboplatin plus pemetrexed (n = 302).
The study results were initially presented during the presidential symposium of the World Congress on Lung Cancer 2020.
The combined immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive versus 27% in the chemotherapy group.
Overall, the trial demonstrated a statistically significant improvement in overall survival for patients who received nivolumab plus ipilimumab versus those treated with chemotherapy. Median overall survival was 18.1 months versus 14.1 months (hazard ratio, 0.74; P = .002).
Median progression-free survival per blinded independent central review was 6.8 months in the nivolumab plus ipilimumab arm and 7.2 months in the chemotherapy arm (HR, 1.0). The confirmed overall response rate was 40% versus 43% in the immunotherapy and chemotherapy arms, respectively.
Median response duration was 11.0 months in the nivolumab plus ipilimumab arm and 6.7 months in the chemotherapy arm. At 24 months, 32% of the immunotherapy patients were still experiencing a response, compared with 8% of those in the chemotherapy arm.
The recommended doses for unresectable malignant pleural mesothelioma are nivolumab 360 mg every 3 weeks and ipilimumab 1 mg/kg every 6 weeks until disease progression or unacceptable toxicity, or up to 2 years in patients without disease progression.
The most common adverse reactions (incidence ≥20%) in patients receiving combination immunotherapy were fatigue, musculoskeletal pain, rash, diarrhea, dyspnea, nausea, decreased appetite, cough, and pruritus.
New standard of care?
The CheckMate 743 trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported study author Paul Baas, MD, PhD, of the Netherlands Cancer Institute, Amsterdam, at the time of its presentation.
He suggested that combination nivolumab and ipilimumab should therefore “be considered as a new standard of care.”
This article first appeared on Medscape.com.
This is the first drug regimen to receive regulatory approval for mesothelioma in 16 years and only the second systemic therapy to be approved for this indication.
“Today’s approval of nivolumab plus ipilimumab provides a new treatment that has demonstrated an improvement in overall survival for patients with malignant pleural mesothelioma,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research, said in a statement.
“In 2004, FDA approved pemetrexed in combination with cisplatin for this indication, and now patients have an important, additional treatment option after more than a decade with only one FDA-approved drug regimen,” Dr. Pazdur added.
Improved overall survival
The approval is based on efficacy results from the CheckMate 743 trial, which compared immunotherapy with a chemotherapy regimen in a cohort of more than 600 treatment-naive patients (no systemic therapies) with unresectable mesothelioma.
Patients were randomized 1:1 to nivolumab and ipilimumab for up to 2 years (n = 303) or six cycles of combination chemotherapy with cisplatin or carboplatin plus pemetrexed (n = 302).
The study results were initially presented during the presidential symposium of the World Congress on Lung Cancer 2020.
The combined immunotherapy regimen was associated with a 26% improvement in overall survival. At 2 years, 41% of patients in the immunotherapy arm were still alive versus 27% in the chemotherapy group.
Overall, the trial demonstrated a statistically significant improvement in overall survival for patients who received nivolumab plus ipilimumab versus those treated with chemotherapy. Median overall survival was 18.1 months versus 14.1 months (hazard ratio, 0.74; P = .002).
Median progression-free survival per blinded independent central review was 6.8 months in the nivolumab plus ipilimumab arm and 7.2 months in the chemotherapy arm (HR, 1.0). The confirmed overall response rate was 40% versus 43% in the immunotherapy and chemotherapy arms, respectively.
Median response duration was 11.0 months in the nivolumab plus ipilimumab arm and 6.7 months in the chemotherapy arm. At 24 months, 32% of the immunotherapy patients were still experiencing a response, compared with 8% of those in the chemotherapy arm.
The recommended doses for unresectable malignant pleural mesothelioma are nivolumab 360 mg every 3 weeks and ipilimumab 1 mg/kg every 6 weeks until disease progression or unacceptable toxicity, or up to 2 years in patients without disease progression.
The most common adverse reactions (incidence ≥20%) in patients receiving combination immunotherapy were fatigue, musculoskeletal pain, rash, diarrhea, dyspnea, nausea, decreased appetite, cough, and pruritus.
New standard of care?
The CheckMate 743 trial “met its primary endpoint of statistically improving overall survival for the experimental arm vs chemotherapy in a prespecified interim analysis,” reported study author Paul Baas, MD, PhD, of the Netherlands Cancer Institute, Amsterdam, at the time of its presentation.
He suggested that combination nivolumab and ipilimumab should therefore “be considered as a new standard of care.”
This article first appeared on Medscape.com.
High-dose TRT: A new standard of care for LS-SCLC?
In a phase 2 trial, the 2-year overall survival rate was 51.3% when twice-daily TRT was given at a dose of 45 Gy in 30 fractions and 75% when it was given at a dose of 60 Gy in 40 fractions in patients with LS-SCLC. The two treatment arms had similar safety and quality of life outcomes.
The higher dose “did not add toxicity,” a significant concern with higher radiation doses, said Bjorn Gronberg, MD, PhD, of the Norwegian University of Science and Technology in Trondheim, when presenting this study at the European Society for Medical Oncology Virtual Congress 2020.
However, the discussant for this study pointed out several limitations of the trial and concluded that the 45 Gy dose should remain the standard of care.
Dr. Gonberg explained that concurrent platinum/etoposide (PE) chemotherapy and TRT is the standard treatment for LS-SCLC, and the most recommended schedule for TRT is twice daily at 45 Gy in 30 fractions. He noted, however, that “there’s clearly a need for better treatment” because less than 30% of patients are cured.
“We hypothesized that increasing the dose of radiotherapy might improve survival,” he said.
Study details
Dr. Gonberg and colleagues conducted a phase 2 trial of patients with stage I-III SCLC confined to one hemithorax plus regional lymph nodes. The trial enrolled 176 patients and randomized 170 of them.
The patients received four courses of PE 3 weeks apart. For TRT, 81 patients were randomized to 45 Gy in 30 fractions, and 89 patients were randomized to 60 Gy in 40 fractions, with 10 fractions per week starting with the second PE course.
All patients who responded to chemoradiotherapy were offered prophylactic cranial irradiation at 25 Gy in 10 fractions or 30 Gy in 15 fractions.
Baseline characteristics were well balanced between the treatment arms. The median age was 65 years in both arms, and most patients were women (60.5% in the 45 Gy arm and 56% in the 60 Gy arm).
The mean number of chemotherapy courses was 3.8 in each arm, about 85% of patients received prophylactic cranial radiation, and roughly half received second-line chemotherapy. Overall, 73 patients completed TRT in the 45 Gy arm, and 81 completed TRT in the 60 Gy arm.
Efficacy
There was no significant difference in overall response rate between the treatment arms. It was 81.6% in the 45 Gy arm and 82.1% in the 60 Gy arm (P = .81).
Similarly, there was no significant difference in progression-free survival. The median progression-free survival was 11.1 months in the lower-dose arm and 18.7 months in the higher-dose arm (P = .22).
Still, there was a significant difference in overall survival between the arms. The 2-year overall survival rate was 51.3% in the lower-dose arm and 75% in the higher-dose arm (P = .002). The median overall survival was 24 months and 37.2 months, respectively (P = .034).
Discussant Corinne Faivre-Finn, MD, PhD, of the University of Manchester (England), cautioned that the lower-dose arm appeared to underperform, compared with prior research.
Additionally, “the survival curves separate at about 9 months, [with a] significant difference at 2 years, but the survival curves are coming back together at around 5 years, and that shows that there is a small difference in terms of long-term cure,” she said.
Safety
There were no significant differences in toxicity between the treatment arms.
Dr. Gronberg noted that esophagitis is considered the main dose-limiting toxicity with TRT, but there was no difference in incidence between the two arms (P = .916). Grade 3 esophagitis occurred in 18.4% of patients in the lower-dose arm and 19% of those in the higher-dose arm. There was no grade 4 esophagitis.
Rates of grade 3 and 4 neutropenic infections were higher in the lower-dose arm than in the higher-dose arm, but the difference was not statistically significant (P = .08).
There were also no significant differences in quality of life surveys that patients filled out periodically from baseline until week 52.
“Not so surprisingly,” Dr. Gronberg said, dysphagia was more common at the end of TRT. However, patients had recovered to baseline levels at week 22.
“There’s no difference in the maximum dysphagia reported between the treatment arms, but ... patients in the high-dose arm needed longer time to recover from dysphagia,” Dr. Gronberg said.
Scores for dyspnea, physical function, and global quality of life were similar between the treatment arms.
The similar toxicity between the arms “is quite puzzling,” Dr. Faivre-Finn said, given the 33% increase in radiation dose in the experimental arm. She said this “probably points out an imbalance in some of the factors” between the groups, including tumor volume and doses to organs at risk, which were not reported.
“There are some important missing data, in terms of interpretation of results,” she said.
Given the limitations, and the fact that the study population was relatively small, Dr. Faivre-Finn said “the results cannot be considered definitive and practice changing,” pending additional study.
“So my final conclusion is that twice-a-day radiotherapy at a dose of 45 Gy remains the standard of care, as recommended in the recently published ASTRO [American Society for Radiation Oncology] guidelines,” Dr. Faivre-Finn said.
The study was funded by the Norwegian Cancer Society, the Nordic Cancer Union, and the Norwegian University of Science and Technology. Dr. Gronberg disclosed relationships with Pfizer, Roche, Eli Lilly, and other companies. Dr. Faivre-Finn disclosed relationships with AstraZeneca, Merck, Pfizer, Elekta, and Boehringer Ingelheim.
SOURCE: Gronberg B et al. ESMO 2020, Abstract 1783O.
In a phase 2 trial, the 2-year overall survival rate was 51.3% when twice-daily TRT was given at a dose of 45 Gy in 30 fractions and 75% when it was given at a dose of 60 Gy in 40 fractions in patients with LS-SCLC. The two treatment arms had similar safety and quality of life outcomes.
The higher dose “did not add toxicity,” a significant concern with higher radiation doses, said Bjorn Gronberg, MD, PhD, of the Norwegian University of Science and Technology in Trondheim, when presenting this study at the European Society for Medical Oncology Virtual Congress 2020.
However, the discussant for this study pointed out several limitations of the trial and concluded that the 45 Gy dose should remain the standard of care.
Dr. Gonberg explained that concurrent platinum/etoposide (PE) chemotherapy and TRT is the standard treatment for LS-SCLC, and the most recommended schedule for TRT is twice daily at 45 Gy in 30 fractions. He noted, however, that “there’s clearly a need for better treatment” because less than 30% of patients are cured.
“We hypothesized that increasing the dose of radiotherapy might improve survival,” he said.
Study details
Dr. Gonberg and colleagues conducted a phase 2 trial of patients with stage I-III SCLC confined to one hemithorax plus regional lymph nodes. The trial enrolled 176 patients and randomized 170 of them.
The patients received four courses of PE 3 weeks apart. For TRT, 81 patients were randomized to 45 Gy in 30 fractions, and 89 patients were randomized to 60 Gy in 40 fractions, with 10 fractions per week starting with the second PE course.
All patients who responded to chemoradiotherapy were offered prophylactic cranial irradiation at 25 Gy in 10 fractions or 30 Gy in 15 fractions.
Baseline characteristics were well balanced between the treatment arms. The median age was 65 years in both arms, and most patients were women (60.5% in the 45 Gy arm and 56% in the 60 Gy arm).
The mean number of chemotherapy courses was 3.8 in each arm, about 85% of patients received prophylactic cranial radiation, and roughly half received second-line chemotherapy. Overall, 73 patients completed TRT in the 45 Gy arm, and 81 completed TRT in the 60 Gy arm.
Efficacy
There was no significant difference in overall response rate between the treatment arms. It was 81.6% in the 45 Gy arm and 82.1% in the 60 Gy arm (P = .81).
Similarly, there was no significant difference in progression-free survival. The median progression-free survival was 11.1 months in the lower-dose arm and 18.7 months in the higher-dose arm (P = .22).
Still, there was a significant difference in overall survival between the arms. The 2-year overall survival rate was 51.3% in the lower-dose arm and 75% in the higher-dose arm (P = .002). The median overall survival was 24 months and 37.2 months, respectively (P = .034).
Discussant Corinne Faivre-Finn, MD, PhD, of the University of Manchester (England), cautioned that the lower-dose arm appeared to underperform, compared with prior research.
Additionally, “the survival curves separate at about 9 months, [with a] significant difference at 2 years, but the survival curves are coming back together at around 5 years, and that shows that there is a small difference in terms of long-term cure,” she said.
Safety
There were no significant differences in toxicity between the treatment arms.
Dr. Gronberg noted that esophagitis is considered the main dose-limiting toxicity with TRT, but there was no difference in incidence between the two arms (P = .916). Grade 3 esophagitis occurred in 18.4% of patients in the lower-dose arm and 19% of those in the higher-dose arm. There was no grade 4 esophagitis.
Rates of grade 3 and 4 neutropenic infections were higher in the lower-dose arm than in the higher-dose arm, but the difference was not statistically significant (P = .08).
There were also no significant differences in quality of life surveys that patients filled out periodically from baseline until week 52.
“Not so surprisingly,” Dr. Gronberg said, dysphagia was more common at the end of TRT. However, patients had recovered to baseline levels at week 22.
“There’s no difference in the maximum dysphagia reported between the treatment arms, but ... patients in the high-dose arm needed longer time to recover from dysphagia,” Dr. Gronberg said.
Scores for dyspnea, physical function, and global quality of life were similar between the treatment arms.
The similar toxicity between the arms “is quite puzzling,” Dr. Faivre-Finn said, given the 33% increase in radiation dose in the experimental arm. She said this “probably points out an imbalance in some of the factors” between the groups, including tumor volume and doses to organs at risk, which were not reported.
“There are some important missing data, in terms of interpretation of results,” she said.
Given the limitations, and the fact that the study population was relatively small, Dr. Faivre-Finn said “the results cannot be considered definitive and practice changing,” pending additional study.
“So my final conclusion is that twice-a-day radiotherapy at a dose of 45 Gy remains the standard of care, as recommended in the recently published ASTRO [American Society for Radiation Oncology] guidelines,” Dr. Faivre-Finn said.
The study was funded by the Norwegian Cancer Society, the Nordic Cancer Union, and the Norwegian University of Science and Technology. Dr. Gronberg disclosed relationships with Pfizer, Roche, Eli Lilly, and other companies. Dr. Faivre-Finn disclosed relationships with AstraZeneca, Merck, Pfizer, Elekta, and Boehringer Ingelheim.
SOURCE: Gronberg B et al. ESMO 2020, Abstract 1783O.
In a phase 2 trial, the 2-year overall survival rate was 51.3% when twice-daily TRT was given at a dose of 45 Gy in 30 fractions and 75% when it was given at a dose of 60 Gy in 40 fractions in patients with LS-SCLC. The two treatment arms had similar safety and quality of life outcomes.
The higher dose “did not add toxicity,” a significant concern with higher radiation doses, said Bjorn Gronberg, MD, PhD, of the Norwegian University of Science and Technology in Trondheim, when presenting this study at the European Society for Medical Oncology Virtual Congress 2020.
However, the discussant for this study pointed out several limitations of the trial and concluded that the 45 Gy dose should remain the standard of care.
Dr. Gonberg explained that concurrent platinum/etoposide (PE) chemotherapy and TRT is the standard treatment for LS-SCLC, and the most recommended schedule for TRT is twice daily at 45 Gy in 30 fractions. He noted, however, that “there’s clearly a need for better treatment” because less than 30% of patients are cured.
“We hypothesized that increasing the dose of radiotherapy might improve survival,” he said.
Study details
Dr. Gonberg and colleagues conducted a phase 2 trial of patients with stage I-III SCLC confined to one hemithorax plus regional lymph nodes. The trial enrolled 176 patients and randomized 170 of them.
The patients received four courses of PE 3 weeks apart. For TRT, 81 patients were randomized to 45 Gy in 30 fractions, and 89 patients were randomized to 60 Gy in 40 fractions, with 10 fractions per week starting with the second PE course.
All patients who responded to chemoradiotherapy were offered prophylactic cranial irradiation at 25 Gy in 10 fractions or 30 Gy in 15 fractions.
Baseline characteristics were well balanced between the treatment arms. The median age was 65 years in both arms, and most patients were women (60.5% in the 45 Gy arm and 56% in the 60 Gy arm).
The mean number of chemotherapy courses was 3.8 in each arm, about 85% of patients received prophylactic cranial radiation, and roughly half received second-line chemotherapy. Overall, 73 patients completed TRT in the 45 Gy arm, and 81 completed TRT in the 60 Gy arm.
Efficacy
There was no significant difference in overall response rate between the treatment arms. It was 81.6% in the 45 Gy arm and 82.1% in the 60 Gy arm (P = .81).
Similarly, there was no significant difference in progression-free survival. The median progression-free survival was 11.1 months in the lower-dose arm and 18.7 months in the higher-dose arm (P = .22).
Still, there was a significant difference in overall survival between the arms. The 2-year overall survival rate was 51.3% in the lower-dose arm and 75% in the higher-dose arm (P = .002). The median overall survival was 24 months and 37.2 months, respectively (P = .034).
Discussant Corinne Faivre-Finn, MD, PhD, of the University of Manchester (England), cautioned that the lower-dose arm appeared to underperform, compared with prior research.
Additionally, “the survival curves separate at about 9 months, [with a] significant difference at 2 years, but the survival curves are coming back together at around 5 years, and that shows that there is a small difference in terms of long-term cure,” she said.
Safety
There were no significant differences in toxicity between the treatment arms.
Dr. Gronberg noted that esophagitis is considered the main dose-limiting toxicity with TRT, but there was no difference in incidence between the two arms (P = .916). Grade 3 esophagitis occurred in 18.4% of patients in the lower-dose arm and 19% of those in the higher-dose arm. There was no grade 4 esophagitis.
Rates of grade 3 and 4 neutropenic infections were higher in the lower-dose arm than in the higher-dose arm, but the difference was not statistically significant (P = .08).
There were also no significant differences in quality of life surveys that patients filled out periodically from baseline until week 52.
“Not so surprisingly,” Dr. Gronberg said, dysphagia was more common at the end of TRT. However, patients had recovered to baseline levels at week 22.
“There’s no difference in the maximum dysphagia reported between the treatment arms, but ... patients in the high-dose arm needed longer time to recover from dysphagia,” Dr. Gronberg said.
Scores for dyspnea, physical function, and global quality of life were similar between the treatment arms.
The similar toxicity between the arms “is quite puzzling,” Dr. Faivre-Finn said, given the 33% increase in radiation dose in the experimental arm. She said this “probably points out an imbalance in some of the factors” between the groups, including tumor volume and doses to organs at risk, which were not reported.
“There are some important missing data, in terms of interpretation of results,” she said.
Given the limitations, and the fact that the study population was relatively small, Dr. Faivre-Finn said “the results cannot be considered definitive and practice changing,” pending additional study.
“So my final conclusion is that twice-a-day radiotherapy at a dose of 45 Gy remains the standard of care, as recommended in the recently published ASTRO [American Society for Radiation Oncology] guidelines,” Dr. Faivre-Finn said.
The study was funded by the Norwegian Cancer Society, the Nordic Cancer Union, and the Norwegian University of Science and Technology. Dr. Gronberg disclosed relationships with Pfizer, Roche, Eli Lilly, and other companies. Dr. Faivre-Finn disclosed relationships with AstraZeneca, Merck, Pfizer, Elekta, and Boehringer Ingelheim.
SOURCE: Gronberg B et al. ESMO 2020, Abstract 1783O.
FROM ESMO 2020
‘Celebration’ will be ‘short-lived’ if COVID vaccine rushed: Experts
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.
on Wednesday.
The career staff of the Food and Drug Administration can be counted on to appropriately weigh whether a vaccine should be cleared for use in preventing COVID-19, witnesses, including Paul A. Offit, MD, of Children’s Hospital of Philadelphia, told the House Energy and Commerce Committee’s oversight and investigations panel.
FDA staffers would object to attempts by the Trump administration to rush a vaccine to the public without proper vetting, as would veteran federal researchers, including National Institutes of Health Director Francis S. Collins, MD, PhD, and Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, Offit said.
“If COVID-19 vaccines are released before they’re ready to be released, you will hear from these people, and you will also hear from people like Dr. Francis Collins and Tony Fauci, both of whom are trusted by the American public, as well as many other academicians and researchers who wouldn’t stand for this,” he said.
“The public is already nervous about these vaccines,” said Offit, who serves on key FDA and Centers for Disease Control and Prevention committees overseeing vaccine policy. “If trusted health officials stand up and decry a premature release, the celebration by the administration will be short-lived.”
Overly optimistic estimates about a potential approval can only serve to erode the public’s trust in these crucial vaccines, said another witness, Ashish K. Jha, MD, MPH, the dean of Brown University’s School of Public Health, in Providence, Rhode Island.
“All political leaders need to stop talking about things like time lines,” Jha told the lawmakers.
President Donald Trump has several times suggested that a COVID vaccine might be approved ahead of the November 3 election, where he faces a significant challenge from his Democratic rival, former Vice President Joe Biden.
In a Tuesday night debate with Biden, Trump again raised the idea of a quick approval. “Now we’re weeks away from a vaccine,” Trump said during the debate.
Trump’s estimates, though, are not in line with those offered by most firms involved with making vaccines. The most optimistic projections have come from Pfizer Inc. The drugmaker’s chief executive, Albert Bourla, has spoken about his company possibly having data to present to the FDA as early as late October about the safety and effectiveness of a vaccine.
In a September 8 interview with the Today show, Bourla said there was a 60% chance his company would meet that goal. In response to a question, he made it clear his comments applied to a potential Pfizer application, not an approval or release of a vaccine by that time.
In response to concerns about political pressures, the FDA in June issued guidance outlining what its staff would require for approval of a COVID-19 vaccine.
Pushback on politics
Another witness at the Wednesday hearing, Mark McClellan, MD, PhD, a former FDA commissioner (2002 – 2004), pushed back on objections to a potential release of further guidance from the agency.
“Some recent statements from the White House have implied that FDA’s plan to release additional written guidance on its expectations for emergency use authorization of a vaccine is unnecessarily raising the bar on regulatory standards for authorization,” said McClellan in his testimony for the House panel. “That is not the case.”
Instead, further FDA guidance would be a welcome form of feedback for the firms trying to develop COVID-19 vaccines, according to McClellan, who also serves on the board of directors for Johnson & Johnson. Johnson & Johnson is among the firms that have advanced a COVID-19 vaccine candidate to phase 3 testing. In his role as a director, he serves on the board’s regulatory compliance committee.
Along with politics, recent stumbles at FDA with emergency use authorizations (EUAs) of treatments for COVID-19 have eroded the public’s confidence in the agency, Jha told the House panel. The FDA approved hydroxychloroquine, a medicine promoted by Trump for use in COVID, under an EUA in March and then revoked this clearance in June.
Jha said the FDA’s most serious misstep was its handling of convalescent plasma, which was approved through an EUA on August 23 “in a highly advertised and widely televised announcement including the president.
“The announcement solidified in the public conversation the impression that, increasingly with this administration, politics are taking over trusted, nonpartisan scientific institutions,” he said in his testimony.
Approving a COVID-19 vaccine on the limited evidence through an EUA would mark a serious departure from FDA policy, according to Jha.
“While we sometimes accept a certain level of potential harm in experimental treatments for those who are severely ill, vaccines are given to healthy people and therefore need to have a substantially higher measure of safety and effectiveness,” he explained.
Jha said the FDA has only once before used this EUA approach for a vaccine. That was for a vaccine against inhaled anthrax and was mostly distributed to high-risk soldiers and civilians in war zones.
COVID-19, in contrast, is an infection that has changed lives around the world. The virus has contributed to more than 1 million deaths, including more than 200,000 in the United States, according to the World Health Organization.
Scientists are hoping vaccines will help curb this infection, although much of the future success of vaccines depends on how widely they are used, witnesses told the House panel.
Debate on approaches for vaccine effectiveness
In his testimony, Jha also noted concerns about COVID-19 vaccine trials. He included a reference to a Sept. 22 opinion article titled, “These Coronavirus Trials Don›t Answer the One Question We Need to Know,” which was written by Peter Doshi, PhD, of the University of Maryland School of Pharmacy, in Baltimore, and Eric Topol, MD, a professor of molecular medicine at Scripps Research in La Jolla, Calif. Topol is also editor in chief of Medscape.
Topol and Doshi questioned why the firms Moderna, Pfizer, and AstraZeneca structured their competing trials such that “a vaccine could meet the companies’ benchmark for success if it lowered the risk of mild Covid-19, but was never shown to reduce moderate or severe forms of the disease, or the risk of hospitalization, admissions to the intensive care unit or death.”
“To say a vaccine works should mean that most people no longer run the risk of getting seriously sick,” Topol and Doshi wrote. “That’s not what these trials will determine.”
There was disagreement about this point at the hearing. U.S. Representative Morgan Griffith (R-Va.) read the section of the Doshi-Topol article quoted above and asked one witness, Offit, to weigh in.
“Do you agree with those concerns? And either way, tell me why,” Griffith asked.
“I don’t agree,” Offit responded.
“I think it’s actually much harder to prevent asymptomatic infection or mildly symptomatic infection,” he said. “If you can prevent that, you are much more likely to prevent moderate to severe disease. So I think they have it backwards.”
But other researchers also question the approaches used with the current crop of COVID-19 vaccines.
“With the current protocols, it is conceivable that a vaccine might be considered effective – and eventually approved – based primarily on its ability to prevent mild cases alone,” wrote William Haseltine, PhD, president of the nonprofit ACCESS Health International, in a September 22 opinion article in the Washington Post titled: “Beware of COVID-19 Vaccine Trials Designed to Succeed From the Start.”
In an interview with Medscape Medical News on Wednesday, Haseltine said he maintains these concerns about the tests. Earlier in his career, he was a leader in HIV research through his lab at Harvard University in Cambridge, Massachusetts, and he subsequently led a biotech company, Human Genome Sciences.
He fears consumers will not get what they might expect from the vaccines being tested.
“What people care about is if this is going to keep them out of the hospital and will it keep them alive. And that’s not even part of this protocol,” Haseltine said.
This article first appeared on Medscape.com.




