User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


COVID-19 vaccine hesitancy ‘somewhat understandable,’ expert says
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 and the superspreaders: Teens
Although cases of COVID-19 in children is reported to be low, we are seeing a surge in Wisconsin with a 27.6% positivity rate reported on Sept. 27. Numerous other states across the country are reporting similar jumps of 10% or more.
According to the Wisconsin Department of Health Services as of Sept. 20, 2020, there were 10,644 cumulative cases in persons aged less than 18 years. This rise in cases is consistent with a return to school and sports. This cumulative case load amounts to 836.7/100, 000 cases. This population may not experience the level of illness seen in the older populations with hospitalization rates of only 3% under the age of 9 years and 13% of those age 10- 19-years, yet exposing older family and members of the community is driving the death rates. The combined influenza and COVID-19 season may greatly impact hospitalization rates of young and old. Additionally, we may see a surge in pediatric cancer rates and autoimmune diseases secondary to these trends.
I believe the overall number of adolescents with COVID-19 is underreported. Teens admit to a lack of understanding of symptoms. Many do not realize they have COVID-19 until someone points out the symptoms they describe such as a loss of taste or smell are COVID-19 symptoms. Others report they do not report symptoms to prevent quarantine. Additionally, others endorse ridicule from peers if they have tested positive and contract tracing identifies others potentially exposed and forced to sit out of sports because of quarantine. They have been bullied into amnesia when contract tracers call to prevent identifying others at school or in the community. All these behaviors proliferate the spread of disease within the community and will continue to drive both exposures and death rates.
Teens in high schools require increased education of the symptoms of COVID-19, promotion of the flu vaccine, and knowledge of the impact they can have on preventing the spread of viruses.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She said she had no relevant financial disclosures. Email her at [email protected].
Reference
COVID-19: Wisconsin Cases, Wisconsin Department of Health Services. Accessed 2020 Sep 27.
Although cases of COVID-19 in children is reported to be low, we are seeing a surge in Wisconsin with a 27.6% positivity rate reported on Sept. 27. Numerous other states across the country are reporting similar jumps of 10% or more.
According to the Wisconsin Department of Health Services as of Sept. 20, 2020, there were 10,644 cumulative cases in persons aged less than 18 years. This rise in cases is consistent with a return to school and sports. This cumulative case load amounts to 836.7/100, 000 cases. This population may not experience the level of illness seen in the older populations with hospitalization rates of only 3% under the age of 9 years and 13% of those age 10- 19-years, yet exposing older family and members of the community is driving the death rates. The combined influenza and COVID-19 season may greatly impact hospitalization rates of young and old. Additionally, we may see a surge in pediatric cancer rates and autoimmune diseases secondary to these trends.
I believe the overall number of adolescents with COVID-19 is underreported. Teens admit to a lack of understanding of symptoms. Many do not realize they have COVID-19 until someone points out the symptoms they describe such as a loss of taste or smell are COVID-19 symptoms. Others report they do not report symptoms to prevent quarantine. Additionally, others endorse ridicule from peers if they have tested positive and contract tracing identifies others potentially exposed and forced to sit out of sports because of quarantine. They have been bullied into amnesia when contract tracers call to prevent identifying others at school or in the community. All these behaviors proliferate the spread of disease within the community and will continue to drive both exposures and death rates.
Teens in high schools require increased education of the symptoms of COVID-19, promotion of the flu vaccine, and knowledge of the impact they can have on preventing the spread of viruses.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She said she had no relevant financial disclosures. Email her at [email protected].
Reference
COVID-19: Wisconsin Cases, Wisconsin Department of Health Services. Accessed 2020 Sep 27.
Although cases of COVID-19 in children is reported to be low, we are seeing a surge in Wisconsin with a 27.6% positivity rate reported on Sept. 27. Numerous other states across the country are reporting similar jumps of 10% or more.
According to the Wisconsin Department of Health Services as of Sept. 20, 2020, there were 10,644 cumulative cases in persons aged less than 18 years. This rise in cases is consistent with a return to school and sports. This cumulative case load amounts to 836.7/100, 000 cases. This population may not experience the level of illness seen in the older populations with hospitalization rates of only 3% under the age of 9 years and 13% of those age 10- 19-years, yet exposing older family and members of the community is driving the death rates. The combined influenza and COVID-19 season may greatly impact hospitalization rates of young and old. Additionally, we may see a surge in pediatric cancer rates and autoimmune diseases secondary to these trends.
I believe the overall number of adolescents with COVID-19 is underreported. Teens admit to a lack of understanding of symptoms. Many do not realize they have COVID-19 until someone points out the symptoms they describe such as a loss of taste or smell are COVID-19 symptoms. Others report they do not report symptoms to prevent quarantine. Additionally, others endorse ridicule from peers if they have tested positive and contract tracing identifies others potentially exposed and forced to sit out of sports because of quarantine. They have been bullied into amnesia when contract tracers call to prevent identifying others at school or in the community. All these behaviors proliferate the spread of disease within the community and will continue to drive both exposures and death rates.
Teens in high schools require increased education of the symptoms of COVID-19, promotion of the flu vaccine, and knowledge of the impact they can have on preventing the spread of viruses.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She said she had no relevant financial disclosures. Email her at [email protected].
Reference
COVID-19: Wisconsin Cases, Wisconsin Department of Health Services. Accessed 2020 Sep 27.
Pediatric fractures shift during pandemic
Pediatric fractures dropped by 2.5-fold during the early months of the COVID-19 pandemic, but more breaks happened at home and on bicycles, and younger kids were more affected, new research indicates.
The study of 1,745 patients also found that those with distal radius torus fractures were more likely to receive a Velcro splint during the pandemic. Experts said this key trend points toward widespread shifts to streamline treatment, which should persist after the pandemic.
“We expected to see a drop in fracture volume, but what was a bit unexpected was the proportional rise in at-home injuries, which we weren’t immediately aware of,” said senior author Apurva Shah, MD, MBA, of Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania in Philadelphia.
“As time went on, it became more apparent that trampoline and bicycle injuries were on the rise, but at the beginning of the pandemic, we didn’t intuitively expect that,” he added.
“Whenever there’s a major shift in how the world is working, we want to understand how that impacts child safety,” Dr. Shah said in an interview. “The message to get out to parents is that it’s obviously difficult to supervise kids while working from home” during the pandemic “and that supervision obviously is not always working as well as intended.”
Joshua T. Bram, a medical student, presented the study at the virtual American Academy of Pediatrics (AAP) 2020 National Conference.
Dr. Bram, Dr. Shah, and colleagues compared patients with acute fractures who presented at CHOP between March and April 2020 with those who presented during the same months in 2018 and 2019.
Overall, the number of patients with pediatric fractures who presented to CHOP fell to an average of just under 10 per day, compared with more than 22 per day in prior years (P < .001). In addition, the age of the patients fell from an average of 9.4 years to 7.5 years (P < .001), with fewer adolescents affected in 2020.
“I think when you cancel a 14-year-old’s baseball season” because of the pandemic, “unfortunately, that lost outdoor time might be substituted with time on a screen,” he explained. “But canceling a 6-year-old’s soccer season might mean substituting that with more time outside on bikes or on a trampoline.”
As noted, because of the pandemic, a higher proportion of pediatric fractures occurred at home (57.8% vs. 32.5%; P < .001) or on bicycles (18.3% vs. 8.2%; P < .001), but there were fewer organized sports–related (7.2% vs. 26.0%; P < .001) or playground-related injuries (5.2% vs. 9.0%; P < .001).
In the study period this year, the researchers saw no increase in the amount of time between injury and presentation. However, data suggest that, in more recent months, “kids are presenting with fractures late, with sometimes great consequences,” Dr. Shah said.
“What has changed is that a lot of adults have lost their jobs, and as a consequence, a lot of children have lost their access to private insurance,” he said. “But fracture is really a major injury, and this is a reminder for pediatricians and primary care physicians to recognize that families are going through these changes and that delays in care can really be detrimental to children.”
Velcro splints more common
A potential upside to shifts seen during the pandemic, Dr. Shah said, is the finding that distal radius torus fractures were more likely to be treated with a Velcro splint than in previous years (44.2% vs. 25.9%; P = .010).
“This is hitting on something important – that sometimes it’s crisis that forces us as physicians to evolve,” he said. “This is something I think is here to stay.
“Although research had already been there suggesting a close equivalent between splints and casting, culturally, a lot of surgeons hadn’t made that shift when historically the gold standard had been casting,” Dr. Shah added. “But with the pandemic, the shift to minimize contact with the health care system to keep families safe in their COVID bubble helped [usage of] splints take off.
“I suspect – and we’ll only know when we’re on the other side of this – when physicians see good results in splints in their own patients, they’re going to adopt those strategies more permanently,” he said.
Benjamin Shore, MD, MPH, of Boston Children’s Hospital, agreed with Dr. Shah’s prediction that fracture care will be more streamlined after the pandemic. Dr. Shore, who wasn’t involved in the study, said not only are more orthopedic providers treating patients with Velcro splints and bivalve casts, but they are also monitoring patients via telehealth.
“All of these are great examples of innovation, and one of the unique parts of the pandemic is it created a lot of rapid change across healthcare because it caused us to scrutinize the ways we practice and make a change,” Dr. Shore said in an interview.
“It wasn’t a very fancy study, but it’s very important in terms of demonstrating a change in practice,” Dr. Shore said. “The research here basically validated what many of us are seeing and hopefully will help us in future pandemics – which hopefully won’t happen – to tell families what to be proactive about.”
Dr. Shah and Dr. Shore agreed that, because fewer fractures are occurring in kids during the pandemic, there is an opportunity to redeploy orthopedic providers to other clinical areas on the basis of volume and need.
Dr. Shah and Dr. Shore have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pediatric fractures dropped by 2.5-fold during the early months of the COVID-19 pandemic, but more breaks happened at home and on bicycles, and younger kids were more affected, new research indicates.
The study of 1,745 patients also found that those with distal radius torus fractures were more likely to receive a Velcro splint during the pandemic. Experts said this key trend points toward widespread shifts to streamline treatment, which should persist after the pandemic.
“We expected to see a drop in fracture volume, but what was a bit unexpected was the proportional rise in at-home injuries, which we weren’t immediately aware of,” said senior author Apurva Shah, MD, MBA, of Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania in Philadelphia.
“As time went on, it became more apparent that trampoline and bicycle injuries were on the rise, but at the beginning of the pandemic, we didn’t intuitively expect that,” he added.
“Whenever there’s a major shift in how the world is working, we want to understand how that impacts child safety,” Dr. Shah said in an interview. “The message to get out to parents is that it’s obviously difficult to supervise kids while working from home” during the pandemic “and that supervision obviously is not always working as well as intended.”
Joshua T. Bram, a medical student, presented the study at the virtual American Academy of Pediatrics (AAP) 2020 National Conference.
Dr. Bram, Dr. Shah, and colleagues compared patients with acute fractures who presented at CHOP between March and April 2020 with those who presented during the same months in 2018 and 2019.
Overall, the number of patients with pediatric fractures who presented to CHOP fell to an average of just under 10 per day, compared with more than 22 per day in prior years (P < .001). In addition, the age of the patients fell from an average of 9.4 years to 7.5 years (P < .001), with fewer adolescents affected in 2020.
“I think when you cancel a 14-year-old’s baseball season” because of the pandemic, “unfortunately, that lost outdoor time might be substituted with time on a screen,” he explained. “But canceling a 6-year-old’s soccer season might mean substituting that with more time outside on bikes or on a trampoline.”
As noted, because of the pandemic, a higher proportion of pediatric fractures occurred at home (57.8% vs. 32.5%; P < .001) or on bicycles (18.3% vs. 8.2%; P < .001), but there were fewer organized sports–related (7.2% vs. 26.0%; P < .001) or playground-related injuries (5.2% vs. 9.0%; P < .001).
In the study period this year, the researchers saw no increase in the amount of time between injury and presentation. However, data suggest that, in more recent months, “kids are presenting with fractures late, with sometimes great consequences,” Dr. Shah said.
“What has changed is that a lot of adults have lost their jobs, and as a consequence, a lot of children have lost their access to private insurance,” he said. “But fracture is really a major injury, and this is a reminder for pediatricians and primary care physicians to recognize that families are going through these changes and that delays in care can really be detrimental to children.”
Velcro splints more common
A potential upside to shifts seen during the pandemic, Dr. Shah said, is the finding that distal radius torus fractures were more likely to be treated with a Velcro splint than in previous years (44.2% vs. 25.9%; P = .010).
“This is hitting on something important – that sometimes it’s crisis that forces us as physicians to evolve,” he said. “This is something I think is here to stay.
“Although research had already been there suggesting a close equivalent between splints and casting, culturally, a lot of surgeons hadn’t made that shift when historically the gold standard had been casting,” Dr. Shah added. “But with the pandemic, the shift to minimize contact with the health care system to keep families safe in their COVID bubble helped [usage of] splints take off.
“I suspect – and we’ll only know when we’re on the other side of this – when physicians see good results in splints in their own patients, they’re going to adopt those strategies more permanently,” he said.
Benjamin Shore, MD, MPH, of Boston Children’s Hospital, agreed with Dr. Shah’s prediction that fracture care will be more streamlined after the pandemic. Dr. Shore, who wasn’t involved in the study, said not only are more orthopedic providers treating patients with Velcro splints and bivalve casts, but they are also monitoring patients via telehealth.
“All of these are great examples of innovation, and one of the unique parts of the pandemic is it created a lot of rapid change across healthcare because it caused us to scrutinize the ways we practice and make a change,” Dr. Shore said in an interview.
“It wasn’t a very fancy study, but it’s very important in terms of demonstrating a change in practice,” Dr. Shore said. “The research here basically validated what many of us are seeing and hopefully will help us in future pandemics – which hopefully won’t happen – to tell families what to be proactive about.”
Dr. Shah and Dr. Shore agreed that, because fewer fractures are occurring in kids during the pandemic, there is an opportunity to redeploy orthopedic providers to other clinical areas on the basis of volume and need.
Dr. Shah and Dr. Shore have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pediatric fractures dropped by 2.5-fold during the early months of the COVID-19 pandemic, but more breaks happened at home and on bicycles, and younger kids were more affected, new research indicates.
The study of 1,745 patients also found that those with distal radius torus fractures were more likely to receive a Velcro splint during the pandemic. Experts said this key trend points toward widespread shifts to streamline treatment, which should persist after the pandemic.
“We expected to see a drop in fracture volume, but what was a bit unexpected was the proportional rise in at-home injuries, which we weren’t immediately aware of,” said senior author Apurva Shah, MD, MBA, of Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania in Philadelphia.
“As time went on, it became more apparent that trampoline and bicycle injuries were on the rise, but at the beginning of the pandemic, we didn’t intuitively expect that,” he added.
“Whenever there’s a major shift in how the world is working, we want to understand how that impacts child safety,” Dr. Shah said in an interview. “The message to get out to parents is that it’s obviously difficult to supervise kids while working from home” during the pandemic “and that supervision obviously is not always working as well as intended.”
Joshua T. Bram, a medical student, presented the study at the virtual American Academy of Pediatrics (AAP) 2020 National Conference.
Dr. Bram, Dr. Shah, and colleagues compared patients with acute fractures who presented at CHOP between March and April 2020 with those who presented during the same months in 2018 and 2019.
Overall, the number of patients with pediatric fractures who presented to CHOP fell to an average of just under 10 per day, compared with more than 22 per day in prior years (P < .001). In addition, the age of the patients fell from an average of 9.4 years to 7.5 years (P < .001), with fewer adolescents affected in 2020.
“I think when you cancel a 14-year-old’s baseball season” because of the pandemic, “unfortunately, that lost outdoor time might be substituted with time on a screen,” he explained. “But canceling a 6-year-old’s soccer season might mean substituting that with more time outside on bikes or on a trampoline.”
As noted, because of the pandemic, a higher proportion of pediatric fractures occurred at home (57.8% vs. 32.5%; P < .001) or on bicycles (18.3% vs. 8.2%; P < .001), but there were fewer organized sports–related (7.2% vs. 26.0%; P < .001) or playground-related injuries (5.2% vs. 9.0%; P < .001).
In the study period this year, the researchers saw no increase in the amount of time between injury and presentation. However, data suggest that, in more recent months, “kids are presenting with fractures late, with sometimes great consequences,” Dr. Shah said.
“What has changed is that a lot of adults have lost their jobs, and as a consequence, a lot of children have lost their access to private insurance,” he said. “But fracture is really a major injury, and this is a reminder for pediatricians and primary care physicians to recognize that families are going through these changes and that delays in care can really be detrimental to children.”
Velcro splints more common
A potential upside to shifts seen during the pandemic, Dr. Shah said, is the finding that distal radius torus fractures were more likely to be treated with a Velcro splint than in previous years (44.2% vs. 25.9%; P = .010).
“This is hitting on something important – that sometimes it’s crisis that forces us as physicians to evolve,” he said. “This is something I think is here to stay.
“Although research had already been there suggesting a close equivalent between splints and casting, culturally, a lot of surgeons hadn’t made that shift when historically the gold standard had been casting,” Dr. Shah added. “But with the pandemic, the shift to minimize contact with the health care system to keep families safe in their COVID bubble helped [usage of] splints take off.
“I suspect – and we’ll only know when we’re on the other side of this – when physicians see good results in splints in their own patients, they’re going to adopt those strategies more permanently,” he said.
Benjamin Shore, MD, MPH, of Boston Children’s Hospital, agreed with Dr. Shah’s prediction that fracture care will be more streamlined after the pandemic. Dr. Shore, who wasn’t involved in the study, said not only are more orthopedic providers treating patients with Velcro splints and bivalve casts, but they are also monitoring patients via telehealth.
“All of these are great examples of innovation, and one of the unique parts of the pandemic is it created a lot of rapid change across healthcare because it caused us to scrutinize the ways we practice and make a change,” Dr. Shore said in an interview.
“It wasn’t a very fancy study, but it’s very important in terms of demonstrating a change in practice,” Dr. Shore said. “The research here basically validated what many of us are seeing and hopefully will help us in future pandemics – which hopefully won’t happen – to tell families what to be proactive about.”
Dr. Shah and Dr. Shore agreed that, because fewer fractures are occurring in kids during the pandemic, there is an opportunity to redeploy orthopedic providers to other clinical areas on the basis of volume and need.
Dr. Shah and Dr. Shore have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Burnout/depression: Half of pulmonology trainees report symptoms
results from a national survey demonstrated.
“Given the high prevalence of burnout and depressive symptoms among fellows training in pulmonary and critical care medicine, it is crucial for fellowship training programs and academic hospitals to consider policies and programs that can improve this public health crisis,” first author Michelle Sharp, MD, MHS, and colleagues wrote in a study published in CHEST.
Dr. Sharp, of the division of pulmonary and critical care medicine at Johns Hopkins University, Baltimore, and colleagues developed a cross-sectional electronic survey to assess burnout and depression symptoms in fellows enrolled in pulmonary and critical care medicine training programs in the United States. Between January and February 2019, a total of 976 fellows received the survey, which used the Maslach Burnout Index two-item measure to assess burnout and the two-item Primary Care Evaluation of Mental Disorders Procedure to screen for depressive symptoms. For both burnout and depression, the researchers constructed three multivariate logistic regression models to assess individual fellow characteristics, program structure, and institutional policies associated with the symptoms.
Of the 976 surveys sent, 502 completed both outcome measures, for a response rate of 51%. More than half (59%) were male, 57% described themselves as White/non-Hispanic, and 39% reported at least $200,000 in student loan debt. The researchers found that 50% of respondents screened positive for either burnout of depressive symptoms. Specifically, 41% met criteria for depressive symptoms, 32% were positive for burnout, and 23% were positive for both.
Factors significantly associated with a higher odds of burnout included working more than 70 hours in an average clinical week (adjusted odds ratio, 2.80) and reporting a somewhat negative or very negative impact of the EHR on joy in medicine (aOR, 1.91).
Factors significantly associated with a higher odds of depressive symptoms were financial concern (aOR, 1.13), being located in the Association of American Medical Colleges West region (aOR 3.96), working more than 70 hours in an average clinical week (aOR, 2.24), and spending a moderately high or excessive amount of time at home on the EHR (aOR, 1.71).
Of respondents who reported working in an institution with a coverage system for personal illness or emergency, 29% were uncomfortable accessing the system or felt comfortable only if unable to find their own coverage. In addition, among respondents who indicated that they had access to mental health resources through their place of employment, 15% said they were reluctant to access those resources if needed. Formal use of these programs was not measured by the survey.
“Our results suggest that further study of systemic solutions at the programmatic and institutional levels rather than at the individual level are needed,” Dr. Sharp and colleagues wrote. “Strategies such as providing an easily accessible coverage system, providing access to mental health resources, addressing work hour burden, reducing the EHR burden, and addressing financial concerns among trainees may help reduce burnout and/or depressive symptoms and should be further studied.”
In an interview, David Schulman, MD, FCCP, characterized the survey findings as “disheartening” but not surprising. “Burnout and depressive symptoms are a problem because almost everything we do to mitigate them works a little, but nothing works a lot,” said Dr. Schulman, professor of medicine in the division of pulmonary, allergy, critical care, and sleep medicine at Emory University, Atlanta, who was not affiliated with the study. “The limited availability of resources to fight this is a challenge. The thing that seems to correlate best with mitigating burnout and depression rates is just giving people time. In my experience, most people just want the space and time they need to mitigate burnout in their own way by having schedule flexibility or arranging time to spend with family or involved in other wellness activities.”
Dr. Schulman, who served as training program director of pulmonary and critical care medicine fellows at Emory for 14 years until stepping down from that role in September 2020, said that nurturing a culture where trainees and seasoned colleagues are comfortable talking about burnout and depressive symptoms is one way to foster change. “It’s weird to say that we should try to normalize burnout, but I don’t think the health care system is changing anytime soon. The health care system is a harsh mistress. It will continue to take and take from everyone involved in it until they have nothing left to give. It’s unfortunate, because people are sick, and hospitals can be relatively understaffed, particularly in the context of a major public health emergency. What we really need to do is try to normalize this by saying to trainees: ‘Hey. Everybody is under the gun. We’re going to share in this workload together because we can’t abandon our patients. We will do our best to make sure that the workload is shared amongst everybody.’ ”
He emphasized that most trainees recognize the importance of the work they do, “and they don’t shirk from it. But I think that drive sometimes gets in the way of self-care. I do think there needs to be a happy medium, where we definitely want you to work, because that’s how you learn and the system needs you, but we also recognize that there’s a need for you to take care of yourself.”
Dr. Schulman recommended that such discussions take place not remotely on Zoom calls and the like but rather in person with small groups of trainees and seasoned clinicians, “where people are more comfortable candidly discussing how they’re feeling. I don’t think grand rounds on burnout or depression are particularly effective. It needs to be interactive, and we need to listen as much as we’re talking.”
Although the survey by Dr. Sharp and colleagues was completed prior to the COVID-19 pandemic, Dr. Schulman has a hunch that the current driver of burnout and depression has more to do with trainees feeling a sense of physical isolation than with being overwhelmed by their workload. “I don’t think that’s unique to medicine,” he said. “When people get home from work, they can’t go out with friends or out to dinner, or travel, whatever they do to decompress. I think that’s a major driver for the current phenomenon, and I don’t think that’s unique to medicine. The psychological ramifications of isolation due to the coronavirus may eventually outpace the physical ramifications of all the illness that we have seen. Depression and burnout may not be as obviously damaging to people, but I think they’re affecting many more people than the virus itself.”
The survey was supported by the Association of Pulmonary and Critical Care Medicine Program Directors.
results from a national survey demonstrated.
“Given the high prevalence of burnout and depressive symptoms among fellows training in pulmonary and critical care medicine, it is crucial for fellowship training programs and academic hospitals to consider policies and programs that can improve this public health crisis,” first author Michelle Sharp, MD, MHS, and colleagues wrote in a study published in CHEST.
Dr. Sharp, of the division of pulmonary and critical care medicine at Johns Hopkins University, Baltimore, and colleagues developed a cross-sectional electronic survey to assess burnout and depression symptoms in fellows enrolled in pulmonary and critical care medicine training programs in the United States. Between January and February 2019, a total of 976 fellows received the survey, which used the Maslach Burnout Index two-item measure to assess burnout and the two-item Primary Care Evaluation of Mental Disorders Procedure to screen for depressive symptoms. For both burnout and depression, the researchers constructed three multivariate logistic regression models to assess individual fellow characteristics, program structure, and institutional policies associated with the symptoms.
Of the 976 surveys sent, 502 completed both outcome measures, for a response rate of 51%. More than half (59%) were male, 57% described themselves as White/non-Hispanic, and 39% reported at least $200,000 in student loan debt. The researchers found that 50% of respondents screened positive for either burnout of depressive symptoms. Specifically, 41% met criteria for depressive symptoms, 32% were positive for burnout, and 23% were positive for both.
Factors significantly associated with a higher odds of burnout included working more than 70 hours in an average clinical week (adjusted odds ratio, 2.80) and reporting a somewhat negative or very negative impact of the EHR on joy in medicine (aOR, 1.91).
Factors significantly associated with a higher odds of depressive symptoms were financial concern (aOR, 1.13), being located in the Association of American Medical Colleges West region (aOR 3.96), working more than 70 hours in an average clinical week (aOR, 2.24), and spending a moderately high or excessive amount of time at home on the EHR (aOR, 1.71).
Of respondents who reported working in an institution with a coverage system for personal illness or emergency, 29% were uncomfortable accessing the system or felt comfortable only if unable to find their own coverage. In addition, among respondents who indicated that they had access to mental health resources through their place of employment, 15% said they were reluctant to access those resources if needed. Formal use of these programs was not measured by the survey.
“Our results suggest that further study of systemic solutions at the programmatic and institutional levels rather than at the individual level are needed,” Dr. Sharp and colleagues wrote. “Strategies such as providing an easily accessible coverage system, providing access to mental health resources, addressing work hour burden, reducing the EHR burden, and addressing financial concerns among trainees may help reduce burnout and/or depressive symptoms and should be further studied.”
In an interview, David Schulman, MD, FCCP, characterized the survey findings as “disheartening” but not surprising. “Burnout and depressive symptoms are a problem because almost everything we do to mitigate them works a little, but nothing works a lot,” said Dr. Schulman, professor of medicine in the division of pulmonary, allergy, critical care, and sleep medicine at Emory University, Atlanta, who was not affiliated with the study. “The limited availability of resources to fight this is a challenge. The thing that seems to correlate best with mitigating burnout and depression rates is just giving people time. In my experience, most people just want the space and time they need to mitigate burnout in their own way by having schedule flexibility or arranging time to spend with family or involved in other wellness activities.”
Dr. Schulman, who served as training program director of pulmonary and critical care medicine fellows at Emory for 14 years until stepping down from that role in September 2020, said that nurturing a culture where trainees and seasoned colleagues are comfortable talking about burnout and depressive symptoms is one way to foster change. “It’s weird to say that we should try to normalize burnout, but I don’t think the health care system is changing anytime soon. The health care system is a harsh mistress. It will continue to take and take from everyone involved in it until they have nothing left to give. It’s unfortunate, because people are sick, and hospitals can be relatively understaffed, particularly in the context of a major public health emergency. What we really need to do is try to normalize this by saying to trainees: ‘Hey. Everybody is under the gun. We’re going to share in this workload together because we can’t abandon our patients. We will do our best to make sure that the workload is shared amongst everybody.’ ”
He emphasized that most trainees recognize the importance of the work they do, “and they don’t shirk from it. But I think that drive sometimes gets in the way of self-care. I do think there needs to be a happy medium, where we definitely want you to work, because that’s how you learn and the system needs you, but we also recognize that there’s a need for you to take care of yourself.”
Dr. Schulman recommended that such discussions take place not remotely on Zoom calls and the like but rather in person with small groups of trainees and seasoned clinicians, “where people are more comfortable candidly discussing how they’re feeling. I don’t think grand rounds on burnout or depression are particularly effective. It needs to be interactive, and we need to listen as much as we’re talking.”
Although the survey by Dr. Sharp and colleagues was completed prior to the COVID-19 pandemic, Dr. Schulman has a hunch that the current driver of burnout and depression has more to do with trainees feeling a sense of physical isolation than with being overwhelmed by their workload. “I don’t think that’s unique to medicine,” he said. “When people get home from work, they can’t go out with friends or out to dinner, or travel, whatever they do to decompress. I think that’s a major driver for the current phenomenon, and I don’t think that’s unique to medicine. The psychological ramifications of isolation due to the coronavirus may eventually outpace the physical ramifications of all the illness that we have seen. Depression and burnout may not be as obviously damaging to people, but I think they’re affecting many more people than the virus itself.”
The survey was supported by the Association of Pulmonary and Critical Care Medicine Program Directors.
results from a national survey demonstrated.
“Given the high prevalence of burnout and depressive symptoms among fellows training in pulmonary and critical care medicine, it is crucial for fellowship training programs and academic hospitals to consider policies and programs that can improve this public health crisis,” first author Michelle Sharp, MD, MHS, and colleagues wrote in a study published in CHEST.
Dr. Sharp, of the division of pulmonary and critical care medicine at Johns Hopkins University, Baltimore, and colleagues developed a cross-sectional electronic survey to assess burnout and depression symptoms in fellows enrolled in pulmonary and critical care medicine training programs in the United States. Between January and February 2019, a total of 976 fellows received the survey, which used the Maslach Burnout Index two-item measure to assess burnout and the two-item Primary Care Evaluation of Mental Disorders Procedure to screen for depressive symptoms. For both burnout and depression, the researchers constructed three multivariate logistic regression models to assess individual fellow characteristics, program structure, and institutional policies associated with the symptoms.
Of the 976 surveys sent, 502 completed both outcome measures, for a response rate of 51%. More than half (59%) were male, 57% described themselves as White/non-Hispanic, and 39% reported at least $200,000 in student loan debt. The researchers found that 50% of respondents screened positive for either burnout of depressive symptoms. Specifically, 41% met criteria for depressive symptoms, 32% were positive for burnout, and 23% were positive for both.
Factors significantly associated with a higher odds of burnout included working more than 70 hours in an average clinical week (adjusted odds ratio, 2.80) and reporting a somewhat negative or very negative impact of the EHR on joy in medicine (aOR, 1.91).
Factors significantly associated with a higher odds of depressive symptoms were financial concern (aOR, 1.13), being located in the Association of American Medical Colleges West region (aOR 3.96), working more than 70 hours in an average clinical week (aOR, 2.24), and spending a moderately high or excessive amount of time at home on the EHR (aOR, 1.71).
Of respondents who reported working in an institution with a coverage system for personal illness or emergency, 29% were uncomfortable accessing the system or felt comfortable only if unable to find their own coverage. In addition, among respondents who indicated that they had access to mental health resources through their place of employment, 15% said they were reluctant to access those resources if needed. Formal use of these programs was not measured by the survey.
“Our results suggest that further study of systemic solutions at the programmatic and institutional levels rather than at the individual level are needed,” Dr. Sharp and colleagues wrote. “Strategies such as providing an easily accessible coverage system, providing access to mental health resources, addressing work hour burden, reducing the EHR burden, and addressing financial concerns among trainees may help reduce burnout and/or depressive symptoms and should be further studied.”
In an interview, David Schulman, MD, FCCP, characterized the survey findings as “disheartening” but not surprising. “Burnout and depressive symptoms are a problem because almost everything we do to mitigate them works a little, but nothing works a lot,” said Dr. Schulman, professor of medicine in the division of pulmonary, allergy, critical care, and sleep medicine at Emory University, Atlanta, who was not affiliated with the study. “The limited availability of resources to fight this is a challenge. The thing that seems to correlate best with mitigating burnout and depression rates is just giving people time. In my experience, most people just want the space and time they need to mitigate burnout in their own way by having schedule flexibility or arranging time to spend with family or involved in other wellness activities.”
Dr. Schulman, who served as training program director of pulmonary and critical care medicine fellows at Emory for 14 years until stepping down from that role in September 2020, said that nurturing a culture where trainees and seasoned colleagues are comfortable talking about burnout and depressive symptoms is one way to foster change. “It’s weird to say that we should try to normalize burnout, but I don’t think the health care system is changing anytime soon. The health care system is a harsh mistress. It will continue to take and take from everyone involved in it until they have nothing left to give. It’s unfortunate, because people are sick, and hospitals can be relatively understaffed, particularly in the context of a major public health emergency. What we really need to do is try to normalize this by saying to trainees: ‘Hey. Everybody is under the gun. We’re going to share in this workload together because we can’t abandon our patients. We will do our best to make sure that the workload is shared amongst everybody.’ ”
He emphasized that most trainees recognize the importance of the work they do, “and they don’t shirk from it. But I think that drive sometimes gets in the way of self-care. I do think there needs to be a happy medium, where we definitely want you to work, because that’s how you learn and the system needs you, but we also recognize that there’s a need for you to take care of yourself.”
Dr. Schulman recommended that such discussions take place not remotely on Zoom calls and the like but rather in person with small groups of trainees and seasoned clinicians, “where people are more comfortable candidly discussing how they’re feeling. I don’t think grand rounds on burnout or depression are particularly effective. It needs to be interactive, and we need to listen as much as we’re talking.”
Although the survey by Dr. Sharp and colleagues was completed prior to the COVID-19 pandemic, Dr. Schulman has a hunch that the current driver of burnout and depression has more to do with trainees feeling a sense of physical isolation than with being overwhelmed by their workload. “I don’t think that’s unique to medicine,” he said. “When people get home from work, they can’t go out with friends or out to dinner, or travel, whatever they do to decompress. I think that’s a major driver for the current phenomenon, and I don’t think that’s unique to medicine. The psychological ramifications of isolation due to the coronavirus may eventually outpace the physical ramifications of all the illness that we have seen. Depression and burnout may not be as obviously damaging to people, but I think they’re affecting many more people than the virus itself.”
The survey was supported by the Association of Pulmonary and Critical Care Medicine Program Directors.
FROM CHEST
One measure of child COVID-19 may be trending downward
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
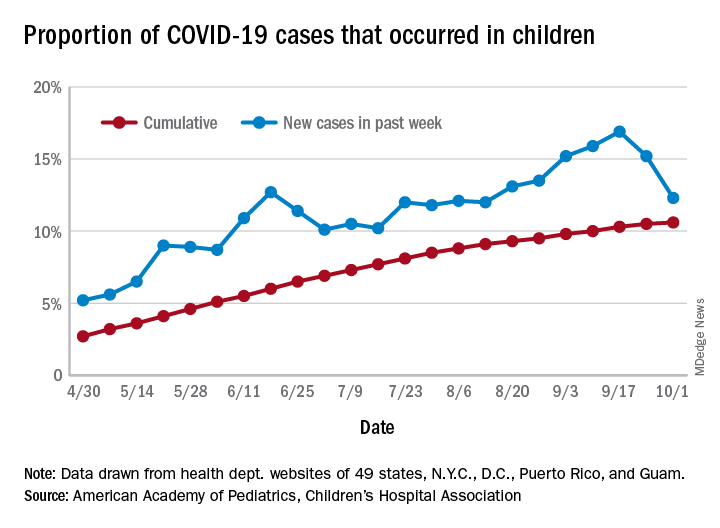
COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
CMS gives hospitals 14 weeks to start daily COVID, flu reports
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
Optimal sedation strategies for COVID-19 ICU patients: A work in progress
According to the best available evidence, analagosedation remains the focus for managing COVID-19 ICU patients, according to Steven B. Greenberg, MD, FCCP, FCCM.
“The choice of sedation and analgesia is important,” Dr. Greenberg, vice chair of education in the department of anesthesiology at Evanston Hospital, part of NorthShore University Health System, Chicago, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. “We know that the right choice of these two components may increase liberation from ventilators, earlier ICU discharge, and return to normal brain function and independent functional status.”
Analgesia first
Prior to the current pandemic, the approach to sedation of patients in the ICU was based on the PADIS Guidelines of 2018, which call for an assessment-driven, protocol-based stepwise approach to pain and sedation management in critically ill adults (Crit Care Med. 2018;46:e825-73). “” Dr. Greenberg said. “We know that pain management should be a priority of sedation, because pain may increase the risk of delirium, anxiety, and endocrine suppression, and may increase the risk of release of endogenous catecholamines, ischemia, and hypermetabolic states.”
Fentanyl appears to be the most common opioid analgesic used for patients in the ICU, “but fentanyl is a very lipophilic drug and has a long context-sensitive half-life,” he said. “There are components to fentanyl that allow it to become a very long-acting drug upon days and days of infusion. Another opioid used is remifentanil, which is typically short-acting because it is broken down in the blood by esterases, but may cause rigidity at higher doses. Dilaudid seems to be the least affected by organ dysfunction. In our very critically ill, prolonged mechanically ventilated COVID-19 patients, we’ve been using methadone for its NMDA [N-methyl-D-aspartate] antagonistic effect and its opioid-sparing effects.”
As for nonopioid analgesics, Dr. Greenberg said that clinicians have shied away from using NSAIDs because of their side effects. “Tramadol indirectly inhibits reuptake of norepinephrine and serotonin, and ketamine is being used a lot more because of its NMDA antagonist effect,” he said. “Lidocaine and gabapentin have also been used.”
In a recent systematic review and meta-analysis, researchers assessed 34 trials that examined adjuvant analgesic use with an opioid in critically ill patients versus an opioid alone (Crit Care Expl. 2020;2:e0157). They found that when using an adjuvant such as acetaminophen, clonidine, dexmedetomidine, gabapentin, ketamine, magnesium, nefopam, NSAIDs, pregabalin, and tramadol, there was a reduction in pain scores as well as a reduction in opioid consumption. “So, clinicians should consider using adjuvant agents to limit opioid exposure and improve pain scores in the critically ill,” Dr. Greenberg said.
ICU delirium: Risk factors, prevention
Delirium in COVID-19 patients treated in the ICU of particular concern. According to a systematic review of 33 studies, 11 risk factors for delirium in the ICU were supported by strong or moderate levels of evidence (Crit Care Med. 2015;43:40-7). These include age, dementia, hypertension, emergency surgery, trauma, APACHE score of II, need for mechanical ventilation, metabolic acidosis, delirium on prior day, coma, and dexmedetomidine use. Risk factors for ICU delirium among COVID-19 patients, however, “are far different,” Dr. Greenberg said. “Why? First and foremost, we are restricting visitation of family,” he said. “That family connection largely can be lost. Second, there are limitations of nonpharmacologic interventions. There is less mobility and physical therapy employed because of the risk of health care workers’ exposure to the virus. There’s also uncertainty about the global pandemic. Anxiety and depression come with that, as well as disruptions to spiritual and religious services.”
Strategies for preventing delirium remain the same as before the pandemic and in accord with recent clinical practice guidelines: Reduce the use of certain drugs such as benzodiazepines and narcotics, reorient the patients, treat dehydration, use hearing aids and eyeglasses in patients who have them, use ear plugs to cancel noise, mobilize patients, maintain sleep/awake cycles, and encourage sedation holidays (Crit Care Med. 2018;46[9]:e825-73).
A recent study from France found that among 58 patients with COVID-19, 65% had positive Confusion Assessment Method (CAM)–ICU findings and 69% had agitation (N Engl J Med 2020;382:2268-70). Most of the patients (86%) received midazolam, 47% received propofol, and all received sufentanil. “In the pre-COVID days, we would use midazolam as a second-line agent for many of these patients,” Dr. Greenberg said. “So, times really have changed.”
The fate of COVID-19 patients following discharge from the ICU remains a concern, continued Dr. Greenberg, clinical professor of anesthesiology at the University of Chicago. A recent journal article by Michelle Biehl, MD, and Denise Sese, MD, noted that post–intensive care syndrome (PICS) or new or worsening impairment in any physical, cognitive, or mental domain is of significant concern among COVID-19 patients following their ICU stay (Cleveland Clin J Med 2020 Aug doi: 10.3949/ccjm.87a.ccc055). The authors stated that COVID-19 patients may face a higher risk of PICS because of restricted family visitation, prolonged mechanical ventilation, exposure to higher amounts of sedatives, and limited physical therapy during hospital stay.
No ideal sedative agent
The 2018 PADIS Guidelines on the use of ICU sedation suggested strong evidence for modifiable risk factors producing delirium in the context of benzodiazepines and blood transfusion. They recommend a light level of sedation and the use of propofol or dexmedetomidine over benzodiazepines. They also recommend routine delirium testing such as using the CAM-ICU or Intensive Care Delirium Screening Checklist (ICDSC) and nonpharmacologic therapies such as reorientation, cognitive stimulation, sleep improvement, and mobilization.
Several sedation-related factors may be related to an increased risk of delirium. “The type, dose, duration, and mode of delivery are very important,” Dr. Greenberg said. “The ideal sedative agent has a rapid, predictable onset; is short-acting; has anxiolytic, amnestic, and analgesic properties; is soluble; has a high therapeutic index; and no toxicity. The ideal sedative is also easy to administrate, contains no active metabolites, has minimal actions with other drugs, is reversible, and is cost effective. The problem is, there really is no ideal sedative agent. There is inadequate knowledge about the drugs [used to treat COVID-19 in the ICU] available to us, the dosage, and importantly, the pharmacokinetics and dynamics of these medications.”
The classic types of sedation being used in the ICU, he said, include the benzodiazepines midazolam, lorazepam, and diazepam, as well as propofol. Alternatives include dexmedetomidine, clonidine, ketamine, and the neuroleptics – haloperidol, quetiapine, olanzapine, ziprasidone, and risperidone. “The advantages of benzos are that they are anxiolytics, amnestics, and they are good sedatives with minimal hemodynamic effects,” Dr. Greenberg said.
Advantages of propofol include its sedative, hypnotic, and anxiolytic properties, he said. It reduces the cerebral metabolic rate and can relieve bronchospasm. “However, small studies have found that its use may be associated with an increased risk of delirium,” he said. “It is a respiratory depressant, and it can cause hypotension and decreased contractility. It has no analgesic properties, and two of the big concerns of its use in COVID-19 are the potential for hypertriglyceridemia and propofol infusion syndrome, particularly at doses of greater than 5 mg/kg per hour for greater than 48 hours. It is being given in high doses because patients are requiring higher doses to maintain ventilator synchrony.”
Choosing the right drug
The keys to success for sedation of ICU patients are choosing the right drug at the right dose for the right duration and the right mode of delivery, and applying them to the right population. However, as noted in a recent study, the pandemic poses unique challenges to clinicians in how they care for critically ill COVID-19 patients who require sedation (Anesth Analg. 2020 Apr 22. doi: 10.1213/ANE.0000000000004887). The use of provisional work areas “has escalated because of the amount of patients we’ve had to care for over the past nine months,” Dr. Greenberg said. “We’ve used alternate providers who are not necessarily familiar with the sedation and analgesic protocols and how to use these specific medications. Drug shortages have been on the rise, so there’s a need to understand alternative agents that can be used.”
COVID-19 patients face the potential risk for an increase in drug-drug interactions and side effects due to the polypharmacy that is often required to provide adequate sedation during mechanical ventilation. He noted that these patients may have “unusually high” analgesia and sedation requirements, particularly when they’re mechanically ventilated. A hypothesis as to why patients with COVID-19 require so much sedation and analgesia is that they often have a high respiratory drive and ventilator dyssynchrony, which requires increased neuromuscular blockade. “They also have an intense inflammatory response, which may be linked to tolerance of specific opioids and other medications,” Dr. Greenberg said. “Many ventilated COVID-19 patients are of younger age and previously in good health, and therefore, have an excellent metabolism. Health care providers are concerned about self-extubation. This prompts bedside providers to administer more sedatives to prevent this unwanted complication. There may also be a reduction of drip modifications by health care workers because of the potential risk of contracting COVID-19 when going into the room multiple times and for long periods of time” (Anesth Analg. 2020;131[1]:e34-e35).
According to a sedation resource on the SCCM website, about 5% of COVID-19 patients require mechanical ventilation. “There has been a massive shortage of the usual drugs that we use,” Dr. Greenberg said. “The demand for sedatives has increased by approximately 91%, while the demand for analgesics has increased by 79%, and neuromuscular blocker demand has increased by 105%.”
A retrospective study of 24 COVID-19 patients who required ventilation in the ICU found that the median daily dose of benzodiazepines was significantly higher, compared with the median daily dose used in the OSCILLATE trial (a median of 270 mg vs. 199 mg, respectively; Anesth Analg. 2020;131[4]e198-e200. doi: 10.1213/ane.0000000000005131). In addition, their median daily dose of opioid was approximately three times higher, compared with patients in the OSCILLATE trial (a median of 775 mg vs. 289 mg). Other agents used included propofol (84%), dexmedetomidine (53%), and ketamine (11%).
“A potential strategy for COVID-19 ICU patient sedation should be analgesia first, as indicated in the 2018 PADIS guidelines,” Dr. Greenberg advised. “We should also apply nonpharmacologic measures to reduce delirium. In nonintubated patients, we should use light to moderate sedation, targeting a RASS of –2 to +1, using hydromorphone or fentanyl boluses for analgesia and midazolam boluses or dexmedetomidine for sedation,.”
For intubated patients, he continued, target a RASS of –3 to –4, or –4 to –5 in those who require neuromuscular blockade. “Use propofol first then intermittent boluses of benzodiazepines,” said Dr. Greenberg, editor-in-chief of the Anesthesia Patient Safety Foundation newsletter. “For heavy sedation, use midazolam and supplement with ketamine and other analgesics and sedatives such as barbiturates, methadone, and even inhalation anesthetics in some cases.”
For analgesia in intubated patients, use fentanyl boluses then infusion. “Patients can easily become tachyphylactic to fentanyl, and it has a long context-sensitive half time,” he said. “Hydromorphone may be least affected by organ dysfunction.”
Dr. Greenberg concluded his presentation by stating that more studies are required “to delineate the best analgesia/sedation strategies and monitoring modalities for COVID-19 ICU patients.”
In commenting on the presentation, Mangala Narasimhan, DO, FCCP, senior vice president and director of critical care services at Northwell Health, said that the recommendations regarding sedation highlight a struggle that ICU providers have been dealing with during the COVID-19 epidemic.
“There have been unique challenges with COVID-19 and intubated patients. We have seen severe ventilator dyssynchrony and prolonged duration of mechanical ventilation. I think we can all agree that these patients have extremely high metabolic rates, have required high levels of sedation, have an increased need for neuromuscular blockade, and have high levels of delirium for extended periods of time. The recommendations provided here are reasonable. Strategies to prevent delirium should be employed, pain management should be prioritized, analgesics can help reduce the need for opioids. Alternatives to sedation are useful in this patient population and are well tolerated. Drug shortages have provided additional challenges to these strategies and have required us to think about the use of alternative agents. The recommendations echo the experience we have had with large numbers of intubated COVID-19 patients.”
Dr. Greenberg disclosed that he receives a stipend from the Anesthesia Patient Safety Foundation for serving as editor-in-chief of the foundation’s newsletter.
According to the best available evidence, analagosedation remains the focus for managing COVID-19 ICU patients, according to Steven B. Greenberg, MD, FCCP, FCCM.
“The choice of sedation and analgesia is important,” Dr. Greenberg, vice chair of education in the department of anesthesiology at Evanston Hospital, part of NorthShore University Health System, Chicago, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. “We know that the right choice of these two components may increase liberation from ventilators, earlier ICU discharge, and return to normal brain function and independent functional status.”
Analgesia first
Prior to the current pandemic, the approach to sedation of patients in the ICU was based on the PADIS Guidelines of 2018, which call for an assessment-driven, protocol-based stepwise approach to pain and sedation management in critically ill adults (Crit Care Med. 2018;46:e825-73). “” Dr. Greenberg said. “We know that pain management should be a priority of sedation, because pain may increase the risk of delirium, anxiety, and endocrine suppression, and may increase the risk of release of endogenous catecholamines, ischemia, and hypermetabolic states.”
Fentanyl appears to be the most common opioid analgesic used for patients in the ICU, “but fentanyl is a very lipophilic drug and has a long context-sensitive half-life,” he said. “There are components to fentanyl that allow it to become a very long-acting drug upon days and days of infusion. Another opioid used is remifentanil, which is typically short-acting because it is broken down in the blood by esterases, but may cause rigidity at higher doses. Dilaudid seems to be the least affected by organ dysfunction. In our very critically ill, prolonged mechanically ventilated COVID-19 patients, we’ve been using methadone for its NMDA [N-methyl-D-aspartate] antagonistic effect and its opioid-sparing effects.”
As for nonopioid analgesics, Dr. Greenberg said that clinicians have shied away from using NSAIDs because of their side effects. “Tramadol indirectly inhibits reuptake of norepinephrine and serotonin, and ketamine is being used a lot more because of its NMDA antagonist effect,” he said. “Lidocaine and gabapentin have also been used.”
In a recent systematic review and meta-analysis, researchers assessed 34 trials that examined adjuvant analgesic use with an opioid in critically ill patients versus an opioid alone (Crit Care Expl. 2020;2:e0157). They found that when using an adjuvant such as acetaminophen, clonidine, dexmedetomidine, gabapentin, ketamine, magnesium, nefopam, NSAIDs, pregabalin, and tramadol, there was a reduction in pain scores as well as a reduction in opioid consumption. “So, clinicians should consider using adjuvant agents to limit opioid exposure and improve pain scores in the critically ill,” Dr. Greenberg said.
ICU delirium: Risk factors, prevention
Delirium in COVID-19 patients treated in the ICU of particular concern. According to a systematic review of 33 studies, 11 risk factors for delirium in the ICU were supported by strong or moderate levels of evidence (Crit Care Med. 2015;43:40-7). These include age, dementia, hypertension, emergency surgery, trauma, APACHE score of II, need for mechanical ventilation, metabolic acidosis, delirium on prior day, coma, and dexmedetomidine use. Risk factors for ICU delirium among COVID-19 patients, however, “are far different,” Dr. Greenberg said. “Why? First and foremost, we are restricting visitation of family,” he said. “That family connection largely can be lost. Second, there are limitations of nonpharmacologic interventions. There is less mobility and physical therapy employed because of the risk of health care workers’ exposure to the virus. There’s also uncertainty about the global pandemic. Anxiety and depression come with that, as well as disruptions to spiritual and religious services.”
Strategies for preventing delirium remain the same as before the pandemic and in accord with recent clinical practice guidelines: Reduce the use of certain drugs such as benzodiazepines and narcotics, reorient the patients, treat dehydration, use hearing aids and eyeglasses in patients who have them, use ear plugs to cancel noise, mobilize patients, maintain sleep/awake cycles, and encourage sedation holidays (Crit Care Med. 2018;46[9]:e825-73).
A recent study from France found that among 58 patients with COVID-19, 65% had positive Confusion Assessment Method (CAM)–ICU findings and 69% had agitation (N Engl J Med 2020;382:2268-70). Most of the patients (86%) received midazolam, 47% received propofol, and all received sufentanil. “In the pre-COVID days, we would use midazolam as a second-line agent for many of these patients,” Dr. Greenberg said. “So, times really have changed.”
The fate of COVID-19 patients following discharge from the ICU remains a concern, continued Dr. Greenberg, clinical professor of anesthesiology at the University of Chicago. A recent journal article by Michelle Biehl, MD, and Denise Sese, MD, noted that post–intensive care syndrome (PICS) or new or worsening impairment in any physical, cognitive, or mental domain is of significant concern among COVID-19 patients following their ICU stay (Cleveland Clin J Med 2020 Aug doi: 10.3949/ccjm.87a.ccc055). The authors stated that COVID-19 patients may face a higher risk of PICS because of restricted family visitation, prolonged mechanical ventilation, exposure to higher amounts of sedatives, and limited physical therapy during hospital stay.
No ideal sedative agent
The 2018 PADIS Guidelines on the use of ICU sedation suggested strong evidence for modifiable risk factors producing delirium in the context of benzodiazepines and blood transfusion. They recommend a light level of sedation and the use of propofol or dexmedetomidine over benzodiazepines. They also recommend routine delirium testing such as using the CAM-ICU or Intensive Care Delirium Screening Checklist (ICDSC) and nonpharmacologic therapies such as reorientation, cognitive stimulation, sleep improvement, and mobilization.
Several sedation-related factors may be related to an increased risk of delirium. “The type, dose, duration, and mode of delivery are very important,” Dr. Greenberg said. “The ideal sedative agent has a rapid, predictable onset; is short-acting; has anxiolytic, amnestic, and analgesic properties; is soluble; has a high therapeutic index; and no toxicity. The ideal sedative is also easy to administrate, contains no active metabolites, has minimal actions with other drugs, is reversible, and is cost effective. The problem is, there really is no ideal sedative agent. There is inadequate knowledge about the drugs [used to treat COVID-19 in the ICU] available to us, the dosage, and importantly, the pharmacokinetics and dynamics of these medications.”
The classic types of sedation being used in the ICU, he said, include the benzodiazepines midazolam, lorazepam, and diazepam, as well as propofol. Alternatives include dexmedetomidine, clonidine, ketamine, and the neuroleptics – haloperidol, quetiapine, olanzapine, ziprasidone, and risperidone. “The advantages of benzos are that they are anxiolytics, amnestics, and they are good sedatives with minimal hemodynamic effects,” Dr. Greenberg said.
Advantages of propofol include its sedative, hypnotic, and anxiolytic properties, he said. It reduces the cerebral metabolic rate and can relieve bronchospasm. “However, small studies have found that its use may be associated with an increased risk of delirium,” he said. “It is a respiratory depressant, and it can cause hypotension and decreased contractility. It has no analgesic properties, and two of the big concerns of its use in COVID-19 are the potential for hypertriglyceridemia and propofol infusion syndrome, particularly at doses of greater than 5 mg/kg per hour for greater than 48 hours. It is being given in high doses because patients are requiring higher doses to maintain ventilator synchrony.”
Choosing the right drug
The keys to success for sedation of ICU patients are choosing the right drug at the right dose for the right duration and the right mode of delivery, and applying them to the right population. However, as noted in a recent study, the pandemic poses unique challenges to clinicians in how they care for critically ill COVID-19 patients who require sedation (Anesth Analg. 2020 Apr 22. doi: 10.1213/ANE.0000000000004887). The use of provisional work areas “has escalated because of the amount of patients we’ve had to care for over the past nine months,” Dr. Greenberg said. “We’ve used alternate providers who are not necessarily familiar with the sedation and analgesic protocols and how to use these specific medications. Drug shortages have been on the rise, so there’s a need to understand alternative agents that can be used.”
COVID-19 patients face the potential risk for an increase in drug-drug interactions and side effects due to the polypharmacy that is often required to provide adequate sedation during mechanical ventilation. He noted that these patients may have “unusually high” analgesia and sedation requirements, particularly when they’re mechanically ventilated. A hypothesis as to why patients with COVID-19 require so much sedation and analgesia is that they often have a high respiratory drive and ventilator dyssynchrony, which requires increased neuromuscular blockade. “They also have an intense inflammatory response, which may be linked to tolerance of specific opioids and other medications,” Dr. Greenberg said. “Many ventilated COVID-19 patients are of younger age and previously in good health, and therefore, have an excellent metabolism. Health care providers are concerned about self-extubation. This prompts bedside providers to administer more sedatives to prevent this unwanted complication. There may also be a reduction of drip modifications by health care workers because of the potential risk of contracting COVID-19 when going into the room multiple times and for long periods of time” (Anesth Analg. 2020;131[1]:e34-e35).
According to a sedation resource on the SCCM website, about 5% of COVID-19 patients require mechanical ventilation. “There has been a massive shortage of the usual drugs that we use,” Dr. Greenberg said. “The demand for sedatives has increased by approximately 91%, while the demand for analgesics has increased by 79%, and neuromuscular blocker demand has increased by 105%.”
A retrospective study of 24 COVID-19 patients who required ventilation in the ICU found that the median daily dose of benzodiazepines was significantly higher, compared with the median daily dose used in the OSCILLATE trial (a median of 270 mg vs. 199 mg, respectively; Anesth Analg. 2020;131[4]e198-e200. doi: 10.1213/ane.0000000000005131). In addition, their median daily dose of opioid was approximately three times higher, compared with patients in the OSCILLATE trial (a median of 775 mg vs. 289 mg). Other agents used included propofol (84%), dexmedetomidine (53%), and ketamine (11%).
“A potential strategy for COVID-19 ICU patient sedation should be analgesia first, as indicated in the 2018 PADIS guidelines,” Dr. Greenberg advised. “We should also apply nonpharmacologic measures to reduce delirium. In nonintubated patients, we should use light to moderate sedation, targeting a RASS of –2 to +1, using hydromorphone or fentanyl boluses for analgesia and midazolam boluses or dexmedetomidine for sedation,.”
For intubated patients, he continued, target a RASS of –3 to –4, or –4 to –5 in those who require neuromuscular blockade. “Use propofol first then intermittent boluses of benzodiazepines,” said Dr. Greenberg, editor-in-chief of the Anesthesia Patient Safety Foundation newsletter. “For heavy sedation, use midazolam and supplement with ketamine and other analgesics and sedatives such as barbiturates, methadone, and even inhalation anesthetics in some cases.”
For analgesia in intubated patients, use fentanyl boluses then infusion. “Patients can easily become tachyphylactic to fentanyl, and it has a long context-sensitive half time,” he said. “Hydromorphone may be least affected by organ dysfunction.”
Dr. Greenberg concluded his presentation by stating that more studies are required “to delineate the best analgesia/sedation strategies and monitoring modalities for COVID-19 ICU patients.”
In commenting on the presentation, Mangala Narasimhan, DO, FCCP, senior vice president and director of critical care services at Northwell Health, said that the recommendations regarding sedation highlight a struggle that ICU providers have been dealing with during the COVID-19 epidemic.
“There have been unique challenges with COVID-19 and intubated patients. We have seen severe ventilator dyssynchrony and prolonged duration of mechanical ventilation. I think we can all agree that these patients have extremely high metabolic rates, have required high levels of sedation, have an increased need for neuromuscular blockade, and have high levels of delirium for extended periods of time. The recommendations provided here are reasonable. Strategies to prevent delirium should be employed, pain management should be prioritized, analgesics can help reduce the need for opioids. Alternatives to sedation are useful in this patient population and are well tolerated. Drug shortages have provided additional challenges to these strategies and have required us to think about the use of alternative agents. The recommendations echo the experience we have had with large numbers of intubated COVID-19 patients.”
Dr. Greenberg disclosed that he receives a stipend from the Anesthesia Patient Safety Foundation for serving as editor-in-chief of the foundation’s newsletter.
According to the best available evidence, analagosedation remains the focus for managing COVID-19 ICU patients, according to Steven B. Greenberg, MD, FCCP, FCCM.
“The choice of sedation and analgesia is important,” Dr. Greenberg, vice chair of education in the department of anesthesiology at Evanston Hospital, part of NorthShore University Health System, Chicago, said at a Society for Critical Care virtual meeting: COVID-19: What’s Next. “We know that the right choice of these two components may increase liberation from ventilators, earlier ICU discharge, and return to normal brain function and independent functional status.”
Analgesia first
Prior to the current pandemic, the approach to sedation of patients in the ICU was based on the PADIS Guidelines of 2018, which call for an assessment-driven, protocol-based stepwise approach to pain and sedation management in critically ill adults (Crit Care Med. 2018;46:e825-73). “” Dr. Greenberg said. “We know that pain management should be a priority of sedation, because pain may increase the risk of delirium, anxiety, and endocrine suppression, and may increase the risk of release of endogenous catecholamines, ischemia, and hypermetabolic states.”
Fentanyl appears to be the most common opioid analgesic used for patients in the ICU, “but fentanyl is a very lipophilic drug and has a long context-sensitive half-life,” he said. “There are components to fentanyl that allow it to become a very long-acting drug upon days and days of infusion. Another opioid used is remifentanil, which is typically short-acting because it is broken down in the blood by esterases, but may cause rigidity at higher doses. Dilaudid seems to be the least affected by organ dysfunction. In our very critically ill, prolonged mechanically ventilated COVID-19 patients, we’ve been using methadone for its NMDA [N-methyl-D-aspartate] antagonistic effect and its opioid-sparing effects.”
As for nonopioid analgesics, Dr. Greenberg said that clinicians have shied away from using NSAIDs because of their side effects. “Tramadol indirectly inhibits reuptake of norepinephrine and serotonin, and ketamine is being used a lot more because of its NMDA antagonist effect,” he said. “Lidocaine and gabapentin have also been used.”
In a recent systematic review and meta-analysis, researchers assessed 34 trials that examined adjuvant analgesic use with an opioid in critically ill patients versus an opioid alone (Crit Care Expl. 2020;2:e0157). They found that when using an adjuvant such as acetaminophen, clonidine, dexmedetomidine, gabapentin, ketamine, magnesium, nefopam, NSAIDs, pregabalin, and tramadol, there was a reduction in pain scores as well as a reduction in opioid consumption. “So, clinicians should consider using adjuvant agents to limit opioid exposure and improve pain scores in the critically ill,” Dr. Greenberg said.
ICU delirium: Risk factors, prevention
Delirium in COVID-19 patients treated in the ICU of particular concern. According to a systematic review of 33 studies, 11 risk factors for delirium in the ICU were supported by strong or moderate levels of evidence (Crit Care Med. 2015;43:40-7). These include age, dementia, hypertension, emergency surgery, trauma, APACHE score of II, need for mechanical ventilation, metabolic acidosis, delirium on prior day, coma, and dexmedetomidine use. Risk factors for ICU delirium among COVID-19 patients, however, “are far different,” Dr. Greenberg said. “Why? First and foremost, we are restricting visitation of family,” he said. “That family connection largely can be lost. Second, there are limitations of nonpharmacologic interventions. There is less mobility and physical therapy employed because of the risk of health care workers’ exposure to the virus. There’s also uncertainty about the global pandemic. Anxiety and depression come with that, as well as disruptions to spiritual and religious services.”
Strategies for preventing delirium remain the same as before the pandemic and in accord with recent clinical practice guidelines: Reduce the use of certain drugs such as benzodiazepines and narcotics, reorient the patients, treat dehydration, use hearing aids and eyeglasses in patients who have them, use ear plugs to cancel noise, mobilize patients, maintain sleep/awake cycles, and encourage sedation holidays (Crit Care Med. 2018;46[9]:e825-73).
A recent study from France found that among 58 patients with COVID-19, 65% had positive Confusion Assessment Method (CAM)–ICU findings and 69% had agitation (N Engl J Med 2020;382:2268-70). Most of the patients (86%) received midazolam, 47% received propofol, and all received sufentanil. “In the pre-COVID days, we would use midazolam as a second-line agent for many of these patients,” Dr. Greenberg said. “So, times really have changed.”
The fate of COVID-19 patients following discharge from the ICU remains a concern, continued Dr. Greenberg, clinical professor of anesthesiology at the University of Chicago. A recent journal article by Michelle Biehl, MD, and Denise Sese, MD, noted that post–intensive care syndrome (PICS) or new or worsening impairment in any physical, cognitive, or mental domain is of significant concern among COVID-19 patients following their ICU stay (Cleveland Clin J Med 2020 Aug doi: 10.3949/ccjm.87a.ccc055). The authors stated that COVID-19 patients may face a higher risk of PICS because of restricted family visitation, prolonged mechanical ventilation, exposure to higher amounts of sedatives, and limited physical therapy during hospital stay.
No ideal sedative agent
The 2018 PADIS Guidelines on the use of ICU sedation suggested strong evidence for modifiable risk factors producing delirium in the context of benzodiazepines and blood transfusion. They recommend a light level of sedation and the use of propofol or dexmedetomidine over benzodiazepines. They also recommend routine delirium testing such as using the CAM-ICU or Intensive Care Delirium Screening Checklist (ICDSC) and nonpharmacologic therapies such as reorientation, cognitive stimulation, sleep improvement, and mobilization.
Several sedation-related factors may be related to an increased risk of delirium. “The type, dose, duration, and mode of delivery are very important,” Dr. Greenberg said. “The ideal sedative agent has a rapid, predictable onset; is short-acting; has anxiolytic, amnestic, and analgesic properties; is soluble; has a high therapeutic index; and no toxicity. The ideal sedative is also easy to administrate, contains no active metabolites, has minimal actions with other drugs, is reversible, and is cost effective. The problem is, there really is no ideal sedative agent. There is inadequate knowledge about the drugs [used to treat COVID-19 in the ICU] available to us, the dosage, and importantly, the pharmacokinetics and dynamics of these medications.”
The classic types of sedation being used in the ICU, he said, include the benzodiazepines midazolam, lorazepam, and diazepam, as well as propofol. Alternatives include dexmedetomidine, clonidine, ketamine, and the neuroleptics – haloperidol, quetiapine, olanzapine, ziprasidone, and risperidone. “The advantages of benzos are that they are anxiolytics, amnestics, and they are good sedatives with minimal hemodynamic effects,” Dr. Greenberg said.
Advantages of propofol include its sedative, hypnotic, and anxiolytic properties, he said. It reduces the cerebral metabolic rate and can relieve bronchospasm. “However, small studies have found that its use may be associated with an increased risk of delirium,” he said. “It is a respiratory depressant, and it can cause hypotension and decreased contractility. It has no analgesic properties, and two of the big concerns of its use in COVID-19 are the potential for hypertriglyceridemia and propofol infusion syndrome, particularly at doses of greater than 5 mg/kg per hour for greater than 48 hours. It is being given in high doses because patients are requiring higher doses to maintain ventilator synchrony.”
Choosing the right drug
The keys to success for sedation of ICU patients are choosing the right drug at the right dose for the right duration and the right mode of delivery, and applying them to the right population. However, as noted in a recent study, the pandemic poses unique challenges to clinicians in how they care for critically ill COVID-19 patients who require sedation (Anesth Analg. 2020 Apr 22. doi: 10.1213/ANE.0000000000004887). The use of provisional work areas “has escalated because of the amount of patients we’ve had to care for over the past nine months,” Dr. Greenberg said. “We’ve used alternate providers who are not necessarily familiar with the sedation and analgesic protocols and how to use these specific medications. Drug shortages have been on the rise, so there’s a need to understand alternative agents that can be used.”
COVID-19 patients face the potential risk for an increase in drug-drug interactions and side effects due to the polypharmacy that is often required to provide adequate sedation during mechanical ventilation. He noted that these patients may have “unusually high” analgesia and sedation requirements, particularly when they’re mechanically ventilated. A hypothesis as to why patients with COVID-19 require so much sedation and analgesia is that they often have a high respiratory drive and ventilator dyssynchrony, which requires increased neuromuscular blockade. “They also have an intense inflammatory response, which may be linked to tolerance of specific opioids and other medications,” Dr. Greenberg said. “Many ventilated COVID-19 patients are of younger age and previously in good health, and therefore, have an excellent metabolism. Health care providers are concerned about self-extubation. This prompts bedside providers to administer more sedatives to prevent this unwanted complication. There may also be a reduction of drip modifications by health care workers because of the potential risk of contracting COVID-19 when going into the room multiple times and for long periods of time” (Anesth Analg. 2020;131[1]:e34-e35).
According to a sedation resource on the SCCM website, about 5% of COVID-19 patients require mechanical ventilation. “There has been a massive shortage of the usual drugs that we use,” Dr. Greenberg said. “The demand for sedatives has increased by approximately 91%, while the demand for analgesics has increased by 79%, and neuromuscular blocker demand has increased by 105%.”
A retrospective study of 24 COVID-19 patients who required ventilation in the ICU found that the median daily dose of benzodiazepines was significantly higher, compared with the median daily dose used in the OSCILLATE trial (a median of 270 mg vs. 199 mg, respectively; Anesth Analg. 2020;131[4]e198-e200. doi: 10.1213/ane.0000000000005131). In addition, their median daily dose of opioid was approximately three times higher, compared with patients in the OSCILLATE trial (a median of 775 mg vs. 289 mg). Other agents used included propofol (84%), dexmedetomidine (53%), and ketamine (11%).
“A potential strategy for COVID-19 ICU patient sedation should be analgesia first, as indicated in the 2018 PADIS guidelines,” Dr. Greenberg advised. “We should also apply nonpharmacologic measures to reduce delirium. In nonintubated patients, we should use light to moderate sedation, targeting a RASS of –2 to +1, using hydromorphone or fentanyl boluses for analgesia and midazolam boluses or dexmedetomidine for sedation,.”
For intubated patients, he continued, target a RASS of –3 to –4, or –4 to –5 in those who require neuromuscular blockade. “Use propofol first then intermittent boluses of benzodiazepines,” said Dr. Greenberg, editor-in-chief of the Anesthesia Patient Safety Foundation newsletter. “For heavy sedation, use midazolam and supplement with ketamine and other analgesics and sedatives such as barbiturates, methadone, and even inhalation anesthetics in some cases.”
For analgesia in intubated patients, use fentanyl boluses then infusion. “Patients can easily become tachyphylactic to fentanyl, and it has a long context-sensitive half time,” he said. “Hydromorphone may be least affected by organ dysfunction.”
Dr. Greenberg concluded his presentation by stating that more studies are required “to delineate the best analgesia/sedation strategies and monitoring modalities for COVID-19 ICU patients.”
In commenting on the presentation, Mangala Narasimhan, DO, FCCP, senior vice president and director of critical care services at Northwell Health, said that the recommendations regarding sedation highlight a struggle that ICU providers have been dealing with during the COVID-19 epidemic.
“There have been unique challenges with COVID-19 and intubated patients. We have seen severe ventilator dyssynchrony and prolonged duration of mechanical ventilation. I think we can all agree that these patients have extremely high metabolic rates, have required high levels of sedation, have an increased need for neuromuscular blockade, and have high levels of delirium for extended periods of time. The recommendations provided here are reasonable. Strategies to prevent delirium should be employed, pain management should be prioritized, analgesics can help reduce the need for opioids. Alternatives to sedation are useful in this patient population and are well tolerated. Drug shortages have provided additional challenges to these strategies and have required us to think about the use of alternative agents. The recommendations echo the experience we have had with large numbers of intubated COVID-19 patients.”
Dr. Greenberg disclosed that he receives a stipend from the Anesthesia Patient Safety Foundation for serving as editor-in-chief of the foundation’s newsletter.
FROM AN SCCM VIRTUAL MEETING
FDA posts COVID vaccine guidance amid White House pushback
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
Chronic, preventive care fell as telemedicine soared during COVID-19
As the COVID-19 pandemic drove down the number of primary care visits and altered the method – moving many to telehealth appointments instead of in-person visits – the content of those appointments also changed, researchers reported in JAMA Network Open.
For the study, G. Caleb Alexander, MD, from the Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, and colleagues analyzed data from the IQVIA National Disease and Therapeutic Index, a nationally representative audit of outpatient care in the United States, from the first quarter of 2018 through the second quarter of 2020.
Most primary care visits in 2018 and 2019 were office based, the authors noted. In the second quarter (Q2, April-May) of 2020, as the COVID-19 pandemic spread across the country, the total number of primary care encounters decreased by 21.4%, and the number of office visits dropped by 50.2%, compared with the average of visits during Q2 in 2018 and 2019.
At the same time, telemedicine visits increased from just 1.1% of total visits in Q2 of 2018 and 2019 to 4.1% of visits in the first quarter (January through March) of 2020 and to 35.3% of visits in Q2 of 2020.
The authors also found that the use of telemedicine in the first half of 2020 varied by geographical region and was not associated with the regional COVID-19 burden. In the Pacific region (Washington, Oregon, and California), 26.8% of encounters were virtual. By contrast, the proportion of telemedicine encounters accounted for only 15.1% of visits in the East North Central states (Wisconsin, Michigan, Illinois, Indiana, and Ohio).
Adults between the ages of 19 and 55 years were more likely to attend telemedicine visits than were those younger or older. Additionally, adults who were commercially insured were more likely to adopt telemedicine versus those with public or no insurance. The study did not find substantial differences in telemedicine use by payer type, nor evidence of a racial disparity between Black and White people in their use of telemedicine.
Drop-off in preventive and chronic care
During the second quarter of this year, the authors reported, the number of visits that included blood pressure assessments dropped by 50.1% and the number of visits in which cholesterol levels were assessed fell by 36.9%, compared with the Q2 of 2018 and 2019.
Visits in which providers prescribed new antihypertensive or cholesterol-lowering medications decreased by 26% in Q2 of 2020 versus the same periods in the previous 2 years. The number of visits in which such prescriptions were renewed dropped by 8.9%.
New treatments also decreased significantly in Q2 of 2020 for patients with chronic conditions, including hypertension, diabetes, high cholesterol, asthma, depression, and insomnia.
When the authors compared the content of telemedicine versus in-person visits in Q2 of 2020, they found a substantial difference. Blood pressure was assessed in 69.7% of office visits, compared with 9.6% of telemedicine. Similarly, cholesterol levels were evaluated in 21.6% of office visits versus 13.5% of telemedicine encounters. New medications were ordered in similar proportions of office-based and telemedicine visits.
The authors concluded that “the COVID-19 pandemic has been associated with changes in the structure of primary care delivery, with the content of telemedicine visits differing from that of office-based encounters.”
While limited in scope, the authors noted, their study is one of the first to evaluate the changes in the content of primary care visits during the pandemic. They attributed the decline in evaluations of cardiovascular risk factors such as blood pressure and cholesterol to “fewer total visits and less frequent assessments during telemedicine encounters.”
While pointing to the inherent limitations of telemedicine, the study did not mention the availability of digital home blood pressure cuffs or home cholesterol test kits. Both kinds of devices are available at consumer-friendly price points and can help people track their indicators, but they’re not considered a substitute for sphygmomanometers used in offices or conventional lab tests. It’s not known how many consumers with cardiovascular risk factors have this kind of home monitoring equipment or how many doctors look at this kind of data.
Dr. Alexander reported serving as a paid adviser to IQVIA; that he is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and that he is a member of OptumRx’s National P&T Committee. One coauthor reported serving as an unpaid adviser to IQVIA and receiving personal fees from the states of California, Washington, and Alaska outside the submitted work. No other disclosures were reported.
A version of this article originally appeared on Medscape.com.
As the COVID-19 pandemic drove down the number of primary care visits and altered the method – moving many to telehealth appointments instead of in-person visits – the content of those appointments also changed, researchers reported in JAMA Network Open.
For the study, G. Caleb Alexander, MD, from the Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, and colleagues analyzed data from the IQVIA National Disease and Therapeutic Index, a nationally representative audit of outpatient care in the United States, from the first quarter of 2018 through the second quarter of 2020.
Most primary care visits in 2018 and 2019 were office based, the authors noted. In the second quarter (Q2, April-May) of 2020, as the COVID-19 pandemic spread across the country, the total number of primary care encounters decreased by 21.4%, and the number of office visits dropped by 50.2%, compared with the average of visits during Q2 in 2018 and 2019.
At the same time, telemedicine visits increased from just 1.1% of total visits in Q2 of 2018 and 2019 to 4.1% of visits in the first quarter (January through March) of 2020 and to 35.3% of visits in Q2 of 2020.
The authors also found that the use of telemedicine in the first half of 2020 varied by geographical region and was not associated with the regional COVID-19 burden. In the Pacific region (Washington, Oregon, and California), 26.8% of encounters were virtual. By contrast, the proportion of telemedicine encounters accounted for only 15.1% of visits in the East North Central states (Wisconsin, Michigan, Illinois, Indiana, and Ohio).
Adults between the ages of 19 and 55 years were more likely to attend telemedicine visits than were those younger or older. Additionally, adults who were commercially insured were more likely to adopt telemedicine versus those with public or no insurance. The study did not find substantial differences in telemedicine use by payer type, nor evidence of a racial disparity between Black and White people in their use of telemedicine.
Drop-off in preventive and chronic care
During the second quarter of this year, the authors reported, the number of visits that included blood pressure assessments dropped by 50.1% and the number of visits in which cholesterol levels were assessed fell by 36.9%, compared with the Q2 of 2018 and 2019.
Visits in which providers prescribed new antihypertensive or cholesterol-lowering medications decreased by 26% in Q2 of 2020 versus the same periods in the previous 2 years. The number of visits in which such prescriptions were renewed dropped by 8.9%.
New treatments also decreased significantly in Q2 of 2020 for patients with chronic conditions, including hypertension, diabetes, high cholesterol, asthma, depression, and insomnia.
When the authors compared the content of telemedicine versus in-person visits in Q2 of 2020, they found a substantial difference. Blood pressure was assessed in 69.7% of office visits, compared with 9.6% of telemedicine. Similarly, cholesterol levels were evaluated in 21.6% of office visits versus 13.5% of telemedicine encounters. New medications were ordered in similar proportions of office-based and telemedicine visits.
The authors concluded that “the COVID-19 pandemic has been associated with changes in the structure of primary care delivery, with the content of telemedicine visits differing from that of office-based encounters.”
While limited in scope, the authors noted, their study is one of the first to evaluate the changes in the content of primary care visits during the pandemic. They attributed the decline in evaluations of cardiovascular risk factors such as blood pressure and cholesterol to “fewer total visits and less frequent assessments during telemedicine encounters.”
While pointing to the inherent limitations of telemedicine, the study did not mention the availability of digital home blood pressure cuffs or home cholesterol test kits. Both kinds of devices are available at consumer-friendly price points and can help people track their indicators, but they’re not considered a substitute for sphygmomanometers used in offices or conventional lab tests. It’s not known how many consumers with cardiovascular risk factors have this kind of home monitoring equipment or how many doctors look at this kind of data.
Dr. Alexander reported serving as a paid adviser to IQVIA; that he is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and that he is a member of OptumRx’s National P&T Committee. One coauthor reported serving as an unpaid adviser to IQVIA and receiving personal fees from the states of California, Washington, and Alaska outside the submitted work. No other disclosures were reported.
A version of this article originally appeared on Medscape.com.
As the COVID-19 pandemic drove down the number of primary care visits and altered the method – moving many to telehealth appointments instead of in-person visits – the content of those appointments also changed, researchers reported in JAMA Network Open.
For the study, G. Caleb Alexander, MD, from the Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, and colleagues analyzed data from the IQVIA National Disease and Therapeutic Index, a nationally representative audit of outpatient care in the United States, from the first quarter of 2018 through the second quarter of 2020.
Most primary care visits in 2018 and 2019 were office based, the authors noted. In the second quarter (Q2, April-May) of 2020, as the COVID-19 pandemic spread across the country, the total number of primary care encounters decreased by 21.4%, and the number of office visits dropped by 50.2%, compared with the average of visits during Q2 in 2018 and 2019.
At the same time, telemedicine visits increased from just 1.1% of total visits in Q2 of 2018 and 2019 to 4.1% of visits in the first quarter (January through March) of 2020 and to 35.3% of visits in Q2 of 2020.
The authors also found that the use of telemedicine in the first half of 2020 varied by geographical region and was not associated with the regional COVID-19 burden. In the Pacific region (Washington, Oregon, and California), 26.8% of encounters were virtual. By contrast, the proportion of telemedicine encounters accounted for only 15.1% of visits in the East North Central states (Wisconsin, Michigan, Illinois, Indiana, and Ohio).
Adults between the ages of 19 and 55 years were more likely to attend telemedicine visits than were those younger or older. Additionally, adults who were commercially insured were more likely to adopt telemedicine versus those with public or no insurance. The study did not find substantial differences in telemedicine use by payer type, nor evidence of a racial disparity between Black and White people in their use of telemedicine.
Drop-off in preventive and chronic care
During the second quarter of this year, the authors reported, the number of visits that included blood pressure assessments dropped by 50.1% and the number of visits in which cholesterol levels were assessed fell by 36.9%, compared with the Q2 of 2018 and 2019.
Visits in which providers prescribed new antihypertensive or cholesterol-lowering medications decreased by 26% in Q2 of 2020 versus the same periods in the previous 2 years. The number of visits in which such prescriptions were renewed dropped by 8.9%.
New treatments also decreased significantly in Q2 of 2020 for patients with chronic conditions, including hypertension, diabetes, high cholesterol, asthma, depression, and insomnia.
When the authors compared the content of telemedicine versus in-person visits in Q2 of 2020, they found a substantial difference. Blood pressure was assessed in 69.7% of office visits, compared with 9.6% of telemedicine. Similarly, cholesterol levels were evaluated in 21.6% of office visits versus 13.5% of telemedicine encounters. New medications were ordered in similar proportions of office-based and telemedicine visits.
The authors concluded that “the COVID-19 pandemic has been associated with changes in the structure of primary care delivery, with the content of telemedicine visits differing from that of office-based encounters.”
While limited in scope, the authors noted, their study is one of the first to evaluate the changes in the content of primary care visits during the pandemic. They attributed the decline in evaluations of cardiovascular risk factors such as blood pressure and cholesterol to “fewer total visits and less frequent assessments during telemedicine encounters.”
While pointing to the inherent limitations of telemedicine, the study did not mention the availability of digital home blood pressure cuffs or home cholesterol test kits. Both kinds of devices are available at consumer-friendly price points and can help people track their indicators, but they’re not considered a substitute for sphygmomanometers used in offices or conventional lab tests. It’s not known how many consumers with cardiovascular risk factors have this kind of home monitoring equipment or how many doctors look at this kind of data.
Dr. Alexander reported serving as a paid adviser to IQVIA; that he is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and that he is a member of OptumRx’s National P&T Committee. One coauthor reported serving as an unpaid adviser to IQVIA and receiving personal fees from the states of California, Washington, and Alaska outside the submitted work. No other disclosures were reported.
A version of this article originally appeared on Medscape.com.
Primary care isn’t bouncing back
published by the Primary Care Collaborative (PCC) and Larry A. Green Center.
Since mid-March these organizations have issued short weekly and biweekly surveys to U.S. primary care physicians in an attempt to find the pulse of the county’s first line of care. “There is not a federal office for primary care, and it’s been anemically funded for decades,” Rebecca Etz, PhD, said in an interview. Yet these clinics represent the front lines of U.S. health care, and it’s where most Americans go for care and COVID-19 care, said Dr. Etz, director of the Virginia-based Larry A. Green Center, which is devoted to primary care research, development, and advocacy.
The latest survey responses, collected between Sept. 4 and 6, confirm what researchers had suspected: Primary care isn’t on solid footing. Eighty-one percent of respondents disagreed emphatically that primary care has bounced back, and another 13% said things were better than earlier this year but not normal.
Meanwhile, 35% of respondents said that revenue and pay are significantly lower than they were before the pandemic and net losses threaten their practices’ viability. Almost half (49%) said their mental exhaustion from work was at an all-time high.
“Because of how our system is set up – it’s a fee-for-service model – the more patients you see, the more money you get,” said Yalda Jabbarpour, MD, medical director at the Graham Center, a leading think tank on family medicine and health care policy. But the stay-at-home order, aversion to telehealth, and fear of in-person visits have been keeping patients away – and driving primary care revenue down. Even when practices transition to and expand their telehealth, payer reimbursement is not yet on parity with in-person visits.
Right now, primary care physicians are doing fewer procedures and spending more time on video visits. “So you may have the same overhead and time investment but you’re getting paid a fraction,” Dr. Etz said. In August, 50% of primary care physicians reported they were working the same or more hours per week as they did before the pandemic but for less money, according to an earlier survey from the Green Center and the PCC. That loss of revenue is compounded by the need for expensive personal protective equipment and preparation for the upcoming flu season, Dr. Etz said.
Ongoing surveys reveal stress
Over the last 20 weeks or so, the Green Center and PCC together have disseminated weekly (through June) or biweekly surveys to 100 professional organizations. Because there isn’t an entity that represents all of primary care and claims data take years to process, these surveys are intended to get real-time feedback from clinicians who are providing a lot of patient care during the pandemic.
The sample sizes are admittedly small, with the most recent survey including 489 clinicians. Dr. Jabbarpour noted, “489 – it’s a good number, but you would want more.” Generally, for a great survey response you’d want 20%-30% of the physician population because then you could assume you’re getting a good mix of geographies, practice sizes, and settings, she said.
Respondents to the most recent survey were from 49 different states; 70% identified their practice as family medicine. One-third had between one and three physicians in their practice and 40% had 10 or more clinicians. “It’s not perfect, but it sounds pretty representative of the primary care workforce,” Dr. Jabbarpour continued.
The latest Green Center-PCC survey, published last week, also found that one in five physicians surveyed said at least one clinician in their practice had opted for early retirement or left practice as a direct result of the pandemic. These declines in clinician staffing come as school reopenings threaten to cause a resurgence of COVID-19 cases and the 2021 flu season could complicate COVID-19 care and testing.
Shortfall could cascade to other specialties
News that primary care is both struggling and shrinking doesn’t come as a surprise to those who research this area, according to Dr. Jabbarpour. Yet it matters “because primary care is where the majority of Americans get their health care.” According to the Centers for Disease Control and Prevention, primary care accounts for 50% of all office visits. But the sector only encompasses 30% of the clinician workforce, according to a 2019 study of physician supply, and accounts for just 7% of national health expenditures, according to a separate 2019 study that measured primary care investment.
If primary care doesn’t bounce back, the shortfall could overwhelm the rest of the health care system, Dr. Jabbarpour said. “If primary care shortages increase, then urgent cares, ERs, and hospitals will become overwhelmed.”
Or public health could suffer as people don’t seek care at all. A study published earlier this summer found that up to 35% of excess deaths during the pandemic were not caused by COVID-19. Instead, they can be attributed to treatable causes, like heart disease, diabetes, and Alzheimers, the researchers concluded. In Dr. Etz’s estimation, this high cost is a glimpse of what happens when there isn’t adequate access to primary care.
When asked about the frequency of the PCC/Green Center surveys, Bianca Frogner, PhD, a health economist and deputy director at the University of Washington Primary Care Innovation Lab in Seattle, said it’s unusual to have this regularity. Also, it’s unique in that it offers a constant mix of physicians. “It’s a small sample, but it still gives a voice where there isn’t one.”
Smaller weekly surveys and secondary analysis projects from the Graham Center, an American Academy of Family Physicians affiliated research center, reinforce findings of the Green Center. The Graham Center surveys also found primary care is taking a financial hit, staff is being furloughed, and patient volume is down, according to Dr. Jabbarpour, who has been involved in most of the Graham Center’s work on COVID.
Dr. Frogner, Dr. Etz, and Dr. Jabbarpour agree that, as a nation, the United States has chronically underinvested in primary care, and now the system is in crisis. The hope is that the survey data gives policymakers, state leaders, and the federal government a better idea of what’s happening on the ground.
It’s also important for researchers “keeping an eye out for the available supply of primary care for certain populations,” Dr. Frogner said. The current conditions are especially a threat to rural and underserved areas, she added.
If primary health care isn’t near recovery that’s a problem for the entire population, Dr. Etz said. And what happens if there’s another surge of COVID-19 or even a second pandemic in our lifetime? Her recommendation: Treat it like disaster recovery. Step one is “Stop the hemorrhaging – they need immediate cash flow.”
A version of this article originally appeared on Medscape.com.
published by the Primary Care Collaborative (PCC) and Larry A. Green Center.
Since mid-March these organizations have issued short weekly and biweekly surveys to U.S. primary care physicians in an attempt to find the pulse of the county’s first line of care. “There is not a federal office for primary care, and it’s been anemically funded for decades,” Rebecca Etz, PhD, said in an interview. Yet these clinics represent the front lines of U.S. health care, and it’s where most Americans go for care and COVID-19 care, said Dr. Etz, director of the Virginia-based Larry A. Green Center, which is devoted to primary care research, development, and advocacy.
The latest survey responses, collected between Sept. 4 and 6, confirm what researchers had suspected: Primary care isn’t on solid footing. Eighty-one percent of respondents disagreed emphatically that primary care has bounced back, and another 13% said things were better than earlier this year but not normal.
Meanwhile, 35% of respondents said that revenue and pay are significantly lower than they were before the pandemic and net losses threaten their practices’ viability. Almost half (49%) said their mental exhaustion from work was at an all-time high.
“Because of how our system is set up – it’s a fee-for-service model – the more patients you see, the more money you get,” said Yalda Jabbarpour, MD, medical director at the Graham Center, a leading think tank on family medicine and health care policy. But the stay-at-home order, aversion to telehealth, and fear of in-person visits have been keeping patients away – and driving primary care revenue down. Even when practices transition to and expand their telehealth, payer reimbursement is not yet on parity with in-person visits.
Right now, primary care physicians are doing fewer procedures and spending more time on video visits. “So you may have the same overhead and time investment but you’re getting paid a fraction,” Dr. Etz said. In August, 50% of primary care physicians reported they were working the same or more hours per week as they did before the pandemic but for less money, according to an earlier survey from the Green Center and the PCC. That loss of revenue is compounded by the need for expensive personal protective equipment and preparation for the upcoming flu season, Dr. Etz said.
Ongoing surveys reveal stress
Over the last 20 weeks or so, the Green Center and PCC together have disseminated weekly (through June) or biweekly surveys to 100 professional organizations. Because there isn’t an entity that represents all of primary care and claims data take years to process, these surveys are intended to get real-time feedback from clinicians who are providing a lot of patient care during the pandemic.
The sample sizes are admittedly small, with the most recent survey including 489 clinicians. Dr. Jabbarpour noted, “489 – it’s a good number, but you would want more.” Generally, for a great survey response you’d want 20%-30% of the physician population because then you could assume you’re getting a good mix of geographies, practice sizes, and settings, she said.
Respondents to the most recent survey were from 49 different states; 70% identified their practice as family medicine. One-third had between one and three physicians in their practice and 40% had 10 or more clinicians. “It’s not perfect, but it sounds pretty representative of the primary care workforce,” Dr. Jabbarpour continued.
The latest Green Center-PCC survey, published last week, also found that one in five physicians surveyed said at least one clinician in their practice had opted for early retirement or left practice as a direct result of the pandemic. These declines in clinician staffing come as school reopenings threaten to cause a resurgence of COVID-19 cases and the 2021 flu season could complicate COVID-19 care and testing.
Shortfall could cascade to other specialties
News that primary care is both struggling and shrinking doesn’t come as a surprise to those who research this area, according to Dr. Jabbarpour. Yet it matters “because primary care is where the majority of Americans get their health care.” According to the Centers for Disease Control and Prevention, primary care accounts for 50% of all office visits. But the sector only encompasses 30% of the clinician workforce, according to a 2019 study of physician supply, and accounts for just 7% of national health expenditures, according to a separate 2019 study that measured primary care investment.
If primary care doesn’t bounce back, the shortfall could overwhelm the rest of the health care system, Dr. Jabbarpour said. “If primary care shortages increase, then urgent cares, ERs, and hospitals will become overwhelmed.”
Or public health could suffer as people don’t seek care at all. A study published earlier this summer found that up to 35% of excess deaths during the pandemic were not caused by COVID-19. Instead, they can be attributed to treatable causes, like heart disease, diabetes, and Alzheimers, the researchers concluded. In Dr. Etz’s estimation, this high cost is a glimpse of what happens when there isn’t adequate access to primary care.
When asked about the frequency of the PCC/Green Center surveys, Bianca Frogner, PhD, a health economist and deputy director at the University of Washington Primary Care Innovation Lab in Seattle, said it’s unusual to have this regularity. Also, it’s unique in that it offers a constant mix of physicians. “It’s a small sample, but it still gives a voice where there isn’t one.”
Smaller weekly surveys and secondary analysis projects from the Graham Center, an American Academy of Family Physicians affiliated research center, reinforce findings of the Green Center. The Graham Center surveys also found primary care is taking a financial hit, staff is being furloughed, and patient volume is down, according to Dr. Jabbarpour, who has been involved in most of the Graham Center’s work on COVID.
Dr. Frogner, Dr. Etz, and Dr. Jabbarpour agree that, as a nation, the United States has chronically underinvested in primary care, and now the system is in crisis. The hope is that the survey data gives policymakers, state leaders, and the federal government a better idea of what’s happening on the ground.
It’s also important for researchers “keeping an eye out for the available supply of primary care for certain populations,” Dr. Frogner said. The current conditions are especially a threat to rural and underserved areas, she added.
If primary health care isn’t near recovery that’s a problem for the entire population, Dr. Etz said. And what happens if there’s another surge of COVID-19 or even a second pandemic in our lifetime? Her recommendation: Treat it like disaster recovery. Step one is “Stop the hemorrhaging – they need immediate cash flow.”
A version of this article originally appeared on Medscape.com.
published by the Primary Care Collaborative (PCC) and Larry A. Green Center.
Since mid-March these organizations have issued short weekly and biweekly surveys to U.S. primary care physicians in an attempt to find the pulse of the county’s first line of care. “There is not a federal office for primary care, and it’s been anemically funded for decades,” Rebecca Etz, PhD, said in an interview. Yet these clinics represent the front lines of U.S. health care, and it’s where most Americans go for care and COVID-19 care, said Dr. Etz, director of the Virginia-based Larry A. Green Center, which is devoted to primary care research, development, and advocacy.
The latest survey responses, collected between Sept. 4 and 6, confirm what researchers had suspected: Primary care isn’t on solid footing. Eighty-one percent of respondents disagreed emphatically that primary care has bounced back, and another 13% said things were better than earlier this year but not normal.
Meanwhile, 35% of respondents said that revenue and pay are significantly lower than they were before the pandemic and net losses threaten their practices’ viability. Almost half (49%) said their mental exhaustion from work was at an all-time high.
“Because of how our system is set up – it’s a fee-for-service model – the more patients you see, the more money you get,” said Yalda Jabbarpour, MD, medical director at the Graham Center, a leading think tank on family medicine and health care policy. But the stay-at-home order, aversion to telehealth, and fear of in-person visits have been keeping patients away – and driving primary care revenue down. Even when practices transition to and expand their telehealth, payer reimbursement is not yet on parity with in-person visits.
Right now, primary care physicians are doing fewer procedures and spending more time on video visits. “So you may have the same overhead and time investment but you’re getting paid a fraction,” Dr. Etz said. In August, 50% of primary care physicians reported they were working the same or more hours per week as they did before the pandemic but for less money, according to an earlier survey from the Green Center and the PCC. That loss of revenue is compounded by the need for expensive personal protective equipment and preparation for the upcoming flu season, Dr. Etz said.
Ongoing surveys reveal stress
Over the last 20 weeks or so, the Green Center and PCC together have disseminated weekly (through June) or biweekly surveys to 100 professional organizations. Because there isn’t an entity that represents all of primary care and claims data take years to process, these surveys are intended to get real-time feedback from clinicians who are providing a lot of patient care during the pandemic.
The sample sizes are admittedly small, with the most recent survey including 489 clinicians. Dr. Jabbarpour noted, “489 – it’s a good number, but you would want more.” Generally, for a great survey response you’d want 20%-30% of the physician population because then you could assume you’re getting a good mix of geographies, practice sizes, and settings, she said.
Respondents to the most recent survey were from 49 different states; 70% identified their practice as family medicine. One-third had between one and three physicians in their practice and 40% had 10 or more clinicians. “It’s not perfect, but it sounds pretty representative of the primary care workforce,” Dr. Jabbarpour continued.
The latest Green Center-PCC survey, published last week, also found that one in five physicians surveyed said at least one clinician in their practice had opted for early retirement or left practice as a direct result of the pandemic. These declines in clinician staffing come as school reopenings threaten to cause a resurgence of COVID-19 cases and the 2021 flu season could complicate COVID-19 care and testing.
Shortfall could cascade to other specialties
News that primary care is both struggling and shrinking doesn’t come as a surprise to those who research this area, according to Dr. Jabbarpour. Yet it matters “because primary care is where the majority of Americans get their health care.” According to the Centers for Disease Control and Prevention, primary care accounts for 50% of all office visits. But the sector only encompasses 30% of the clinician workforce, according to a 2019 study of physician supply, and accounts for just 7% of national health expenditures, according to a separate 2019 study that measured primary care investment.
If primary care doesn’t bounce back, the shortfall could overwhelm the rest of the health care system, Dr. Jabbarpour said. “If primary care shortages increase, then urgent cares, ERs, and hospitals will become overwhelmed.”
Or public health could suffer as people don’t seek care at all. A study published earlier this summer found that up to 35% of excess deaths during the pandemic were not caused by COVID-19. Instead, they can be attributed to treatable causes, like heart disease, diabetes, and Alzheimers, the researchers concluded. In Dr. Etz’s estimation, this high cost is a glimpse of what happens when there isn’t adequate access to primary care.
When asked about the frequency of the PCC/Green Center surveys, Bianca Frogner, PhD, a health economist and deputy director at the University of Washington Primary Care Innovation Lab in Seattle, said it’s unusual to have this regularity. Also, it’s unique in that it offers a constant mix of physicians. “It’s a small sample, but it still gives a voice where there isn’t one.”
Smaller weekly surveys and secondary analysis projects from the Graham Center, an American Academy of Family Physicians affiliated research center, reinforce findings of the Green Center. The Graham Center surveys also found primary care is taking a financial hit, staff is being furloughed, and patient volume is down, according to Dr. Jabbarpour, who has been involved in most of the Graham Center’s work on COVID.
Dr. Frogner, Dr. Etz, and Dr. Jabbarpour agree that, as a nation, the United States has chronically underinvested in primary care, and now the system is in crisis. The hope is that the survey data gives policymakers, state leaders, and the federal government a better idea of what’s happening on the ground.
It’s also important for researchers “keeping an eye out for the available supply of primary care for certain populations,” Dr. Frogner said. The current conditions are especially a threat to rural and underserved areas, she added.
If primary health care isn’t near recovery that’s a problem for the entire population, Dr. Etz said. And what happens if there’s another surge of COVID-19 or even a second pandemic in our lifetime? Her recommendation: Treat it like disaster recovery. Step one is “Stop the hemorrhaging – they need immediate cash flow.”
A version of this article originally appeared on Medscape.com.







