User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Nearly 10% of hospitalized patients with COVID-19 later readmitted
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Should our patients really go home for the holidays?
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Patients with mental illness a priority for COVID vaccine, experts say
With this week’s announcement that Pfizer’s vaccine candidate against SARS-CoV-2 was 90% effective in preventing COVID-19, the world is one step closer to an effective vaccine.
Nevertheless, with a limited supply of initial doses, the question becomes, who should get it first? Individuals with severe mental illness should be a priority group to receive a COVID-19 vaccine, assert the authors of a perspective article published Nov. 1 in World Psychiatry.
Patients with underlying physical conditions, such as cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, obesity, immunodeficiency, and cancer, are particularly vulnerable to developing more severe illness and dying from COVID-19.
In these populations, the risk of a more severe course of infection or early death is significant enough for the U.S. National Academies of Sciences, Engineering, and Medicine to make these patients priority recipients of a vaccine against COVID-19.
Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors argued that those with severe mental illness also fit into this group.
Even without factoring COVID-19 into the calculation, those with severe mental illness have a two- to threefold higher mortality rate than the general population, resulting in reduction in life expectancy of 10-20 years, they noted. This is largely because of physical diseases including cardiovascular disease, type 2 diabetes, and respiratory ailments.
Individuals with severe mental illness also have higher rates of obesity than the general population and obesity is a risk factor for dying from COVID-19.
High-risk population
Like their peers with physical illnesses, recent studies suggest that those with severe mental illness are also at increased risk of morbidity and mortality from COVID-19.
For example, a recent U.S. case-control study with over 61 million adults showed that those recently diagnosed with a mental health disorder had a significantly increased risk for COVID-19 infection, an effect strongest for depression and schizophrenia.
Other recent studies have confirmed these data, including one linking a psychiatric diagnosis in patients hospitalized with COVID-19 to a significantly increased risk for death, as reported by Medscape Medical News.
Dr. De Hert and colleagues put these findings into perspective with this example: In 2017, there were an estimated 11.2 million adults in the United States with severe mental illness. Taking into account the 8.5% death rate in COVID-19 patients recently diagnosed with a severe mental illness, this means that about 1 million patients with severe mental illness in the United States would die if all were infected with the virus.
In light of this knowledge, and taking into account published ethical principles that should guide vaccine allocation, Dr. De Hert and colleagues said it is “paramount” that persons with severe mental illness be prioritized to guarantee that they receive a COVID-19 vaccine during the first phase of its distribution.
“It is our responsibility as psychiatrists in this global health crisis to advocate for the needs of our patients with governments and public health policy bodies,” they wrote.
The authors also encourage public health agencies to develop and implement targeted programs to ensure that patients with severe mental illness and their health care providers “are made aware of these increased risks as well as the benefits of vaccination.”
An argument for fairness
Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, also believes those with severe mental illness should be a priority group for a COVID vaccine.
“When we’re prioritizing groups for a COVID-19 vaccine, let’s not forget that people with serious mental illness have much lower life expectancies, more obesity, and more undiagnosed chronic conditions. They should be a priority group,” Dr. Appelbaum said in an interview.
“The argument for including people with severe mental illnesses among the vulnerable populations who should be prioritized for receipt of a COVID-19 vaccine is an argument for fairness in constructing that group,” he added.
“Like people with other chronic conditions associated with poor outcomes after SARS-CoV-2 infection, people with severe mental illnesses are more likely to be hospitalized and more likely to die. Although they are often systematically ignored when decisions are made about allocation of resources, there is some hope that, with enough public attention to this issue, they can be included this time,” Dr. Appelbaum said.
Dr. De Hert and Dr. Applebaum disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
With this week’s announcement that Pfizer’s vaccine candidate against SARS-CoV-2 was 90% effective in preventing COVID-19, the world is one step closer to an effective vaccine.
Nevertheless, with a limited supply of initial doses, the question becomes, who should get it first? Individuals with severe mental illness should be a priority group to receive a COVID-19 vaccine, assert the authors of a perspective article published Nov. 1 in World Psychiatry.
Patients with underlying physical conditions, such as cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, obesity, immunodeficiency, and cancer, are particularly vulnerable to developing more severe illness and dying from COVID-19.
In these populations, the risk of a more severe course of infection or early death is significant enough for the U.S. National Academies of Sciences, Engineering, and Medicine to make these patients priority recipients of a vaccine against COVID-19.
Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors argued that those with severe mental illness also fit into this group.
Even without factoring COVID-19 into the calculation, those with severe mental illness have a two- to threefold higher mortality rate than the general population, resulting in reduction in life expectancy of 10-20 years, they noted. This is largely because of physical diseases including cardiovascular disease, type 2 diabetes, and respiratory ailments.
Individuals with severe mental illness also have higher rates of obesity than the general population and obesity is a risk factor for dying from COVID-19.
High-risk population
Like their peers with physical illnesses, recent studies suggest that those with severe mental illness are also at increased risk of morbidity and mortality from COVID-19.
For example, a recent U.S. case-control study with over 61 million adults showed that those recently diagnosed with a mental health disorder had a significantly increased risk for COVID-19 infection, an effect strongest for depression and schizophrenia.
Other recent studies have confirmed these data, including one linking a psychiatric diagnosis in patients hospitalized with COVID-19 to a significantly increased risk for death, as reported by Medscape Medical News.
Dr. De Hert and colleagues put these findings into perspective with this example: In 2017, there were an estimated 11.2 million adults in the United States with severe mental illness. Taking into account the 8.5% death rate in COVID-19 patients recently diagnosed with a severe mental illness, this means that about 1 million patients with severe mental illness in the United States would die if all were infected with the virus.
In light of this knowledge, and taking into account published ethical principles that should guide vaccine allocation, Dr. De Hert and colleagues said it is “paramount” that persons with severe mental illness be prioritized to guarantee that they receive a COVID-19 vaccine during the first phase of its distribution.
“It is our responsibility as psychiatrists in this global health crisis to advocate for the needs of our patients with governments and public health policy bodies,” they wrote.
The authors also encourage public health agencies to develop and implement targeted programs to ensure that patients with severe mental illness and their health care providers “are made aware of these increased risks as well as the benefits of vaccination.”
An argument for fairness
Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, also believes those with severe mental illness should be a priority group for a COVID vaccine.
“When we’re prioritizing groups for a COVID-19 vaccine, let’s not forget that people with serious mental illness have much lower life expectancies, more obesity, and more undiagnosed chronic conditions. They should be a priority group,” Dr. Appelbaum said in an interview.
“The argument for including people with severe mental illnesses among the vulnerable populations who should be prioritized for receipt of a COVID-19 vaccine is an argument for fairness in constructing that group,” he added.
“Like people with other chronic conditions associated with poor outcomes after SARS-CoV-2 infection, people with severe mental illnesses are more likely to be hospitalized and more likely to die. Although they are often systematically ignored when decisions are made about allocation of resources, there is some hope that, with enough public attention to this issue, they can be included this time,” Dr. Appelbaum said.
Dr. De Hert and Dr. Applebaum disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
With this week’s announcement that Pfizer’s vaccine candidate against SARS-CoV-2 was 90% effective in preventing COVID-19, the world is one step closer to an effective vaccine.
Nevertheless, with a limited supply of initial doses, the question becomes, who should get it first? Individuals with severe mental illness should be a priority group to receive a COVID-19 vaccine, assert the authors of a perspective article published Nov. 1 in World Psychiatry.
Patients with underlying physical conditions, such as cardiovascular disease, chronic obstructive pulmonary disease, diabetes, chronic kidney disease, obesity, immunodeficiency, and cancer, are particularly vulnerable to developing more severe illness and dying from COVID-19.
In these populations, the risk of a more severe course of infection or early death is significant enough for the U.S. National Academies of Sciences, Engineering, and Medicine to make these patients priority recipients of a vaccine against COVID-19.
Marc De Hert, MD, PhD, professor of psychiatry at KU Leuven (Belgium), and coauthors argued that those with severe mental illness also fit into this group.
Even without factoring COVID-19 into the calculation, those with severe mental illness have a two- to threefold higher mortality rate than the general population, resulting in reduction in life expectancy of 10-20 years, they noted. This is largely because of physical diseases including cardiovascular disease, type 2 diabetes, and respiratory ailments.
Individuals with severe mental illness also have higher rates of obesity than the general population and obesity is a risk factor for dying from COVID-19.
High-risk population
Like their peers with physical illnesses, recent studies suggest that those with severe mental illness are also at increased risk of morbidity and mortality from COVID-19.
For example, a recent U.S. case-control study with over 61 million adults showed that those recently diagnosed with a mental health disorder had a significantly increased risk for COVID-19 infection, an effect strongest for depression and schizophrenia.
Other recent studies have confirmed these data, including one linking a psychiatric diagnosis in patients hospitalized with COVID-19 to a significantly increased risk for death, as reported by Medscape Medical News.
Dr. De Hert and colleagues put these findings into perspective with this example: In 2017, there were an estimated 11.2 million adults in the United States with severe mental illness. Taking into account the 8.5% death rate in COVID-19 patients recently diagnosed with a severe mental illness, this means that about 1 million patients with severe mental illness in the United States would die if all were infected with the virus.
In light of this knowledge, and taking into account published ethical principles that should guide vaccine allocation, Dr. De Hert and colleagues said it is “paramount” that persons with severe mental illness be prioritized to guarantee that they receive a COVID-19 vaccine during the first phase of its distribution.
“It is our responsibility as psychiatrists in this global health crisis to advocate for the needs of our patients with governments and public health policy bodies,” they wrote.
The authors also encourage public health agencies to develop and implement targeted programs to ensure that patients with severe mental illness and their health care providers “are made aware of these increased risks as well as the benefits of vaccination.”
An argument for fairness
Paul S. Appelbaum, MD, professor of psychiatry, medicine, and law at Columbia University, New York, also believes those with severe mental illness should be a priority group for a COVID vaccine.
“When we’re prioritizing groups for a COVID-19 vaccine, let’s not forget that people with serious mental illness have much lower life expectancies, more obesity, and more undiagnosed chronic conditions. They should be a priority group,” Dr. Appelbaum said in an interview.
“The argument for including people with severe mental illnesses among the vulnerable populations who should be prioritized for receipt of a COVID-19 vaccine is an argument for fairness in constructing that group,” he added.
“Like people with other chronic conditions associated with poor outcomes after SARS-CoV-2 infection, people with severe mental illnesses are more likely to be hospitalized and more likely to die. Although they are often systematically ignored when decisions are made about allocation of resources, there is some hope that, with enough public attention to this issue, they can be included this time,” Dr. Appelbaum said.
Dr. De Hert and Dr. Applebaum disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Poverty raises depression risk in patients with cystic fibrosis
Poor people with chronic illness have greater difficulty managing their disease than do their better-off counterparts, and a new study confirms this reality for patients with cystic fibrosis.
and anxiety symptoms, according to a new cross-sectional study. The data were drawn from the Cystic Fibrosis Foundation’s Success with Therapies Research Consortium.
“Assessing the special challenges that individuals with lower SES face, including financial barriers, is essential to understand how we can address the unique combinations of adherence barriers. In other chronic disorders, financial barriers or lower socioeconomic status is associated with nonadherence, but this relationship has not been well established in cystic fibrosis,” said Kimberly Dickinson, MD, MPH, of Johns Hopkins University, Baltimore, during her presentation of the results at the virtual North American Cystic Fibrosis Conference.
“I’ve always thought that my patients in the poorer population were doing worse, and I think this demonstrates that that’s true,” said Robert Giusti, MD, in an interview. Dr. Giusti is a clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center in New York. He was not involved in the study.
“These are very pertinent issues, especially if you think about the pandemic, and some of the issues related to mental health. It just highlights the importance of socioeconomic status and screening for some of the known risk factors so that we can develop interventions or programs to provide equitable care to all of our cystic fibrosis patients,” said Ryan Perkins, MD, who moderated the session where the study was presented. He is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, also in Boston.
The researchers looked retrospectively at 1 year’s worth of pharmacy refill receipts and number of times prescriptions were refilled versus the number of times prescribed, then calculated medicinal possession ratios. This was cross-referenced with annual household income and insurance status of patients with CF at 12 pediatric and 9 adult CF care centers, for a total of 376 patients (128 pediatric and 248 adult).
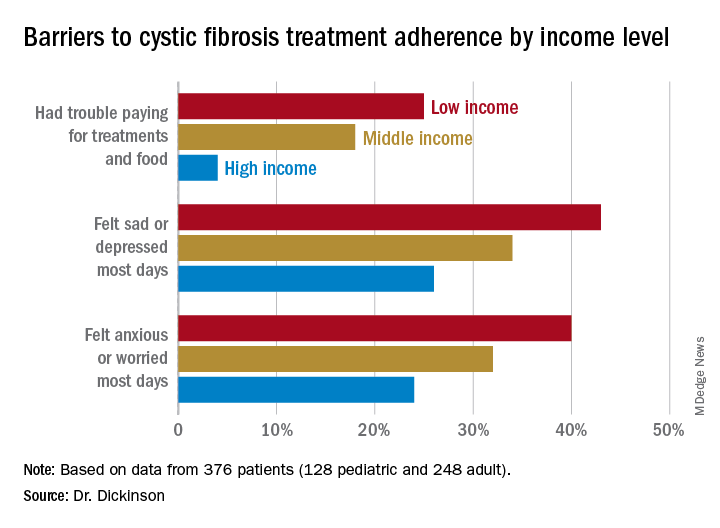
In this population, 32% of participants had public or no insurance, 68% had private or military insurance. The public/no insurance group was more likely than the private/military insurance group to report having trouble paying for treatments, food, or critical expenses related to CF care (23.3% vs. 12.1%, respectively); feeling symptoms on most days of depression (42.5% vs. 31.3%) or anxiety (40.0% vs. 28.5%); and experiencing conflict or stress with loved ones over treatments (30.0% vs. 20.3%) (P < .05 for all).
In all, 35% had a household income less than $40,000 per year, 33% between $44,000 and $100,000, and 32% higher than $100,000. The low-income group had a lower composite medication possession ratio (0.41) than the middle- (0.44) or high-income (0.52) groups, were more likely to have trouble paying for treatments, food, or treatment-related expenses (25%, 18%, 4%, respectively); were more likely most days to report symptoms of depression (43%, 34%, 26%) or anxiety (40%, 32%, 24%), and to have concerns about whether treatments were effective (42%, 27%, 29%). They were more likely to not be able to maintain a daily schedule or routine for treatments (28%, 22%, 14%).
The study showed that adherence barriers and suboptimal adherence are issues that cross all socioeconomic categories, though they were more problematic in the lowest bracket. Greater anxiety and depression among lower income individuals and those with private or no insurance was a key finding, according to Dr. Dickinson. “It highlights the importance of screening for mental health comorbidities that may impact non-adherence,” she said.
The study received funding from the Cystic Fibrosis Foundation. Dr. Dickinson, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
Poor people with chronic illness have greater difficulty managing their disease than do their better-off counterparts, and a new study confirms this reality for patients with cystic fibrosis.
and anxiety symptoms, according to a new cross-sectional study. The data were drawn from the Cystic Fibrosis Foundation’s Success with Therapies Research Consortium.
“Assessing the special challenges that individuals with lower SES face, including financial barriers, is essential to understand how we can address the unique combinations of adherence barriers. In other chronic disorders, financial barriers or lower socioeconomic status is associated with nonadherence, but this relationship has not been well established in cystic fibrosis,” said Kimberly Dickinson, MD, MPH, of Johns Hopkins University, Baltimore, during her presentation of the results at the virtual North American Cystic Fibrosis Conference.
“I’ve always thought that my patients in the poorer population were doing worse, and I think this demonstrates that that’s true,” said Robert Giusti, MD, in an interview. Dr. Giusti is a clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center in New York. He was not involved in the study.
“These are very pertinent issues, especially if you think about the pandemic, and some of the issues related to mental health. It just highlights the importance of socioeconomic status and screening for some of the known risk factors so that we can develop interventions or programs to provide equitable care to all of our cystic fibrosis patients,” said Ryan Perkins, MD, who moderated the session where the study was presented. He is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, also in Boston.
The researchers looked retrospectively at 1 year’s worth of pharmacy refill receipts and number of times prescriptions were refilled versus the number of times prescribed, then calculated medicinal possession ratios. This was cross-referenced with annual household income and insurance status of patients with CF at 12 pediatric and 9 adult CF care centers, for a total of 376 patients (128 pediatric and 248 adult).

In this population, 32% of participants had public or no insurance, 68% had private or military insurance. The public/no insurance group was more likely than the private/military insurance group to report having trouble paying for treatments, food, or critical expenses related to CF care (23.3% vs. 12.1%, respectively); feeling symptoms on most days of depression (42.5% vs. 31.3%) or anxiety (40.0% vs. 28.5%); and experiencing conflict or stress with loved ones over treatments (30.0% vs. 20.3%) (P < .05 for all).
In all, 35% had a household income less than $40,000 per year, 33% between $44,000 and $100,000, and 32% higher than $100,000. The low-income group had a lower composite medication possession ratio (0.41) than the middle- (0.44) or high-income (0.52) groups, were more likely to have trouble paying for treatments, food, or treatment-related expenses (25%, 18%, 4%, respectively); were more likely most days to report symptoms of depression (43%, 34%, 26%) or anxiety (40%, 32%, 24%), and to have concerns about whether treatments were effective (42%, 27%, 29%). They were more likely to not be able to maintain a daily schedule or routine for treatments (28%, 22%, 14%).
The study showed that adherence barriers and suboptimal adherence are issues that cross all socioeconomic categories, though they were more problematic in the lowest bracket. Greater anxiety and depression among lower income individuals and those with private or no insurance was a key finding, according to Dr. Dickinson. “It highlights the importance of screening for mental health comorbidities that may impact non-adherence,” she said.
The study received funding from the Cystic Fibrosis Foundation. Dr. Dickinson, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
Poor people with chronic illness have greater difficulty managing their disease than do their better-off counterparts, and a new study confirms this reality for patients with cystic fibrosis.
and anxiety symptoms, according to a new cross-sectional study. The data were drawn from the Cystic Fibrosis Foundation’s Success with Therapies Research Consortium.
“Assessing the special challenges that individuals with lower SES face, including financial barriers, is essential to understand how we can address the unique combinations of adherence barriers. In other chronic disorders, financial barriers or lower socioeconomic status is associated with nonadherence, but this relationship has not been well established in cystic fibrosis,” said Kimberly Dickinson, MD, MPH, of Johns Hopkins University, Baltimore, during her presentation of the results at the virtual North American Cystic Fibrosis Conference.
“I’ve always thought that my patients in the poorer population were doing worse, and I think this demonstrates that that’s true,” said Robert Giusti, MD, in an interview. Dr. Giusti is a clinical professor of pediatrics at the New York University and director of the Pediatric Cystic Fibrosis Center in New York. He was not involved in the study.
“These are very pertinent issues, especially if you think about the pandemic, and some of the issues related to mental health. It just highlights the importance of socioeconomic status and screening for some of the known risk factors so that we can develop interventions or programs to provide equitable care to all of our cystic fibrosis patients,” said Ryan Perkins, MD, who moderated the session where the study was presented. He is a pediatric and adult pulmonary fellow at Boston Children’s Hospital and Brigham and Women’s Hospital, also in Boston.
The researchers looked retrospectively at 1 year’s worth of pharmacy refill receipts and number of times prescriptions were refilled versus the number of times prescribed, then calculated medicinal possession ratios. This was cross-referenced with annual household income and insurance status of patients with CF at 12 pediatric and 9 adult CF care centers, for a total of 376 patients (128 pediatric and 248 adult).

In this population, 32% of participants had public or no insurance, 68% had private or military insurance. The public/no insurance group was more likely than the private/military insurance group to report having trouble paying for treatments, food, or critical expenses related to CF care (23.3% vs. 12.1%, respectively); feeling symptoms on most days of depression (42.5% vs. 31.3%) or anxiety (40.0% vs. 28.5%); and experiencing conflict or stress with loved ones over treatments (30.0% vs. 20.3%) (P < .05 for all).
In all, 35% had a household income less than $40,000 per year, 33% between $44,000 and $100,000, and 32% higher than $100,000. The low-income group had a lower composite medication possession ratio (0.41) than the middle- (0.44) or high-income (0.52) groups, were more likely to have trouble paying for treatments, food, or treatment-related expenses (25%, 18%, 4%, respectively); were more likely most days to report symptoms of depression (43%, 34%, 26%) or anxiety (40%, 32%, 24%), and to have concerns about whether treatments were effective (42%, 27%, 29%). They were more likely to not be able to maintain a daily schedule or routine for treatments (28%, 22%, 14%).
The study showed that adherence barriers and suboptimal adherence are issues that cross all socioeconomic categories, though they were more problematic in the lowest bracket. Greater anxiety and depression among lower income individuals and those with private or no insurance was a key finding, according to Dr. Dickinson. “It highlights the importance of screening for mental health comorbidities that may impact non-adherence,” she said.
The study received funding from the Cystic Fibrosis Foundation. Dr. Dickinson, Dr. Giusti, and Dr. Perkins have no relevant financial disclosures.
FROM NACFC 2020
.
Biden plan to lower Medicare eligibility age to 60 faces hostility from hospitals
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Of his many plans to expand insurance coverage, President-elect Joe Biden’s simplest strategy is lowering the eligibility age for Medicare from 65 to 60.
But the plan is sure to face long odds, even if the Democrats can snag control of the Senate in January by winning two runoff elections in Georgia.
Republicans, who fought the creation of Medicare in the 1960s and typically oppose expanding government entitlement programs, are not the biggest obstacle. Instead, the nation’s hospitals, a powerful political force, are poised to derail any effort.
“Hospitals certainly are not going to be happy with it,” said Jonathan Oberlander, professor of health policy and management at the University of North Carolina at Chapel Hill.
Medicare reimbursement rates for patients admitted to hospitals average half what commercial or employer-sponsored insurance plans pay.
“It will be a huge lift [in Congress] as the realities of lower Medicare reimbursement rates will activate some powerful interests against this,” said Josh Archambault, a senior fellow with the conservative Foundation for Government Accountability.
Biden, who turns 78 this month, said his plan will help Americans who retire early and those who are unemployed or can’t find jobs with health benefits.
“It reflects the reality that, even after the current crisis ends, older Americans are likely to find it difficult to secure jobs,” Biden wrote in April.
Lowering the Medicare eligibility age is popular. About 85% of Democrats and 69% of Republicans favor allowing those as young as 50 to buy into Medicare, according to a KFF tracking poll from January 2019. (KHN is an editorially independent program of KFF.)
Although opposition from the hospital industry is expected to be fierce, that is not the only obstacle to Biden’s plan.
Critics, especially Republicans on Capitol Hill, will point to the nation’s $3 trillion budget deficit as well as the dim outlook for the Medicare Hospital Insurance Trust Fund. That fund is on track to reach insolvency in 2024. That means there won’t be enough money to fully pay hospitals and nursing homes for inpatient care for Medicare beneficiaries.
Moreover, it’s unclear whether expanding Medicare will fit on the Democrats’ crowded health agenda, which also includes dealing with the COVID-19 pandemic, possibly rescuing the Affordable Care Act if the Supreme Court strikes down part or all of the law in a current case, expanding Obamacare subsidies and lowering drug costs.
Biden’s proposal is a nod to the liberal wing of the Democratic Party, which has advocated for Sen. Bernie Sanders’ (I-Vt.) government-run “Medicare for All” health system that would provide universal coverage. Biden opposed that effort, saying the nation could not afford it. He wanted to retain the private health insurance system, which covers 180 million people.
To expand coverage, Biden has proposed two major initiatives. In addition to the Medicare eligibility change, he wants Congress to approve a government-run health plan that people could buy into instead of purchasing coverage from insurance companies on their own or through the Obamacare marketplaces. Insurers helped beat back this “public option” initiative in 2009 during the congressional debate over the ACA.
The appeal of lowering Medicare eligibility to help those without insurance lies with leveraging a popular government program that has low administrative costs.
“It is hard to find a reform idea that is more popular than opening up Medicare” to people as young as 60, Oberlander said. He said early retirees would like the concept, as would employers, who could save on their health costs as workers gravitate to Medicare.
The eligibility age has been set at 65 since Medicare was created in 1965 as part of President Lyndon Johnson’s Great Society reform package. It was designed to coincide with the age when people at that time qualified for Social Security. Today, people generally qualify for early, reduced Social Security benefits at age 62, though they have to wait until age 66 for full benefits.
While people can qualify on the basis of other criteria, such as having a disability or end-stage renal disease, 85% of the 57 million Medicare enrollees are in the program simply because they’re old enough.
Lowering the age to 60 could add as many as 23 million people to Medicare, according to an analysis by the consulting firm Avalere Health. It’s unclear, however, if everyone who would be eligible would sign up or if Biden would limit the expansion to the 1.7 million people in that age range who are uninsured and the 3.2 million who buy coverage on their own.
Avalere says 3.2 million people in that age group buy coverage on the individual market.
While the 60-to-65 group has the lowest uninsured rate (8%) among adults, it has the highest health costs and pays the highest rates for individual coverage, said Cristina Boccuti, director of health policy at West Health, a nonpartisan research group.
About 13 million of those between 60 and 65 have coverage through their employer, according to Avalere. While they would not have to drop coverage to join Medicare, they could possibly opt to also pay to join the federal program and use it as a wraparound for their existing coverage. Medicare might then pick up costs for some services that the consumers would have to shoulder out-of-pocket.
Some 4 million people between 60 and 65 are enrolled in Medicaid, the state-federal health insurance program for low-income people. Shifting them to Medicare would make that their primary health insurer, a move that would save states money since they split Medicaid costs with the federal government.
Chris Pope, a senior fellow with the conservative Manhattan Institute, said getting health industry support, particularly from hospitals, will be vital for any health coverage expansion. “Hospitals are very aware about generous commercial rates being replaced by lower Medicare rates,” he said.
“Members of Congress, a lot of them are close to their hospitals and do not want to see them with a revenue hole,” he said.
President Barack Obama made a deal with the industry on the way to passing the ACA. In exchange for gaining millions of paying customers and lowering their uncompensated care by billions of dollars, the hospital industry agreed to give up future Medicare funds designed to help them cope with the uninsured. Showing the industry’s prowess on Capitol Hill, Congress has delayed those funding cuts for more than six years.
Jacob Hacker, a Yale University political scientist, noted that expanding Medicare would reduce the number of Americans who rely on employer-sponsored coverage. The pitfalls of the employer system were highlighted in 2020 as millions lost their jobs and workplace health coverage.
Even if they can win the two Georgia seats and take control of the Senate with the vice president breaking any ties, Democrats would be unlikely to pass major legislation without GOP support — unless they are willing to jettison the long-standing filibuster rule so they can pass most legislation with a simple 51-vote majority instead of 60 votes.
Hacker said that slim margin would make it difficult for Democrats to deal with many health issues all at once.
“Congress is not good at parallel processing,” Hacker said, referring to handling multiple priorities at the same time. “And the window is relatively short.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Dripping, dabbing, and bongs: Can’t tell the players without a scorecard
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
E-cigarettes may be synonymous with vaping to most physicians, but there are other ways for patients to inhale nicotine or tetrahydrocannabinol-containing aerosols, according to investigators at the Cleveland Clinic. 
Humberto Choi, MD, and associates wrote in the Annals of the American Thoracic Society.
These “alternate aerosol inhalation methods” have been poorly described thus far, so little is known about their scope of use and potential health impact, they noted.
Dripping involves an e-cigarette modified to expose the heating coil. The e-cigarette liquid is dripped directly onto the hot coil, which produces immediate aerosolization and results in a thicker cloud.
Dripping “may expose users to higher levels of nicotine compared to e-cigarette inhalation” and lead to “increased release of volatile aldehydes as a result of the higher heating potential of direct atomizer exposure,” the investigators suggested.
Water pipes, or bongs, produce both smoke and vapor, although an electronic vaporizer can be attached to create a “vape bong.” About 21% of daily cannabis users report using a bong, but tobacco inhalation is less common. Cases of severe pulmonary infections have been associated with bong use, along with a couple of tuberculosis clusters, Dr. Choi and associates said.
Dabbing uses butane-extracted, concentrated cannabis oil inhaled through a modified water pipe or bong or a smaller device called a “dab pen.” A small amount, or “dab,” of the product is placed on the “nail,” which replaces the bowl of the water pipe, heated with a blowtorch, and inhaled through the pipe, the researchers explained.
The prevalence of dabbing is unknown, but “the most recent Monitoring the Future survey of high school seniors shows that 11.9% of students have used a marijuana vaporizer at some point in their life,” they said.
Besides the fire risks involved in creating the material needed for dabbing – use of heating plates, ovens, and devices for removing butane vapors – inhalation of residual butane vapors could lead to vomiting, cardiac arrhythmias, acute encephalopathy, and respiratory depression, Dr. Choi and associates said.
Nicotine dependence is also a concern, as is the possibility of withdrawal symptoms. “Patients presenting with prolonged and severe vomiting, psychotic symptoms, or other acute neuropsychiatric symptoms should raise the suspicion of [tetrahydrocannabinol]-containing products especially synthetic cannabinoids,” they wrote.
SOURCE: Choi H et al. Ann Am Thorac Soc. 2020 Oct 14. doi: 10.1513/AnnalsATS.202005-511CME.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Search for a snakebite drug might lead to a COVID treatment, too
Matthew Lewin, MD, PhD, founder of the Center for Exploration and Travel Health at the California Academy of Sciences, was researching snakebite treatments in rural locations in preparation for an expedition to the Philippines in 2011.
The story of a renowned herpetologist from the academy, Joseph Slowinski, who was bitten by a highly venomous krait in Myanmar and couldn’t get to a hospital in time to save his life a decade earlier, weighed on the emergency room doctor.
“I concluded that I needed something small and compact and that doesn’t care what kind of snake,” Dr. Lewin said.
It didn’t exist. That set Dr. Lewin in pursuit of a modern snakebite drug, a journey that finds his Corte Madera, Calif., company, Ophirex, nearing a promising oral treatment that fits in a pocket; is stable, easy to use, and affordable; and treats the venom from many species. “That’s the holy grail of snakebite treatment,” he said.
His work has gotten a boost with multimillion-dollar grants from a British charity and the U.S. Army. If it works – and it has been shown to work extremely well in mice and pigs – it could save tens of thousands of lives a year.
Dr. Lewin and Ophirex are not alone in their quest. Snakebites kill nearly 140,000 people a year, overwhelmingly in impoverished rural areas of Asia and Africa without adequate medical infrastructure and knowledge to administer antivenom. Though just a few people die each year in the United States from snakebites, the problem has risen to the top of the list of global health concerns in recent years. Funding has soared, and other research groups have also done promising work on new treatments. Herpetologists say deforestation and climate change are increasing human-snake encounters by forcing snakes to move to new habitats.
Dr. Lewin’s research is centered on a drug called varespladib. The enzyme inhibitor has proven itself in in-vitro lab studies and has effectively saved mice and pigs dosed with venom.
Along the way, Dr. Lewin and his team have come across another potential use for the drug. Varespladib has a positive effect on acute respiratory distress syndrome, associated with COVID-19. Next year, Ophirex will conduct human trials for the possible treatment of the condition funded with $9.9 million from the Army.
The link to a snakebite? The inflammation of the lungs caused by the coronavirus produces the sPLA2 enzyme. A more deadly version of the same enzyme is produced by snake venom.
The other companies that have come up with promising approaches to snakebite aren’t as far along as Ophirex. At the University of California-Irvine, chemist Ken Shea and his team created a nanogel – a kind of polymer used in medical applications – that blocks key proteins in the venom that cause cell destruction. At the Technical University of Denmark, Copenhagen, Andreas Laustsen is looking at engineering bacteria to manufacture anti-venom in fermentation tanks.
The days of incising a snakebite and sucking out the poison are long over, but the current treatment for venomous snakebites remains archaic.
Since the early 1900s, antivenom has been made by injecting horses or other animals with venom milked from snakes and diluted. The animals’ immune systems generate antibodies over several months, and blood plasma is taken from the animals and antibodies extracted from it.
It’s extremely expensive. Hospitals in the United States can charge as much as $15,000 a vial – and a single snakebite might require anywhere from 4 to 50 vials. Moreover, antivenom exists for little more than half the world’s species of venomous snakes.
A major problem is the roughly 2 hours it takes on average for a snakebite victim to reach a hospital and begin treatment. The chemical weapon that is venom starts immediately to destroy cells as it digests its next meal, making fast treatment essential to saving lives and preventing tissue loss.
“The two-hour window between fang and needle is where the most damage occurs,” said Leslie Boyer, director of the University of Arizona’s Venom Immunochemistry, Pharmacology and Emergency Response (VIPER) Institute. “We have a saying, ‘Time is tissue.’ ”
That’s why the search for a new snakebite drug has focused on an inexpensive treatment that can be taken into the field. Dr. Lewin’s drug wouldn’t replace antivenom. Instead, he thinks of it as the first line of defense until the victim can reach a hospital for antivenom treatment.
Dr. Lewin said he expects the drug to be inexpensive, so people in regions where snakebites are common can afford it.
Venom is extremely complicated chemically, and Dr. Lewin began his search by sussing out which of its myriad components to block. He zeroed in on the sPLA2 enzyme.
Surveying the literature about drugs that had been clinically tested for other conditions, he came across varespladib. It had been developed jointly by Eli Lilly and Shionogi, a Japanese pharmaceutical company, as a possible treatment for sepsis. They had never taken it to market.
If it worked, Dr. Lewin could license the right to produce the drug, which had already been thoroughly studied and was shown to be safe.
He placed venom in an array of test tubes. Varespladib and other drugs were added to the venom. He then added a reagent. If the venom was still active, the solution would turn yellow; if it was neutralized, it would remain clear.
The vials with varespladib “came up completely blank,” he said. “It was so stunning I said, ‘I must have made a mistake.’ ”
With a small grant, he sent the drug to the Yale Center for Molecular Discovery and found that varespladib effectively neutralized the venom of snakes found on six continents. The results were published in the journal Toxins and sent ripples through the small community of snakebite researchers.
Dr. Lewin then conducted tests on mice and pigs. Both were successful.
Human clinical trials are next, but they have been delayed by the pandemic. They are scheduled to get underway next spring.
Along the way, Dr. Lewin was fortunate enough to make some good connections that led to funding. In 2012, he attended a party at the Mill Valley, Calif., home of Jerry Harrison, the former guitarist and keyboardist for Talking Heads. Mr. Harrison had long been interested in business and start-ups – he said he was the most careful reader of the ’80s band’s contracts – and at the party he asked “if anyone had any ideas lying fallow,” Mr. Harrison said.
“And Matt pipes up and says, ‘I have this idea how to prevent people from dying from snakebites,’ ” Mr. Harrison said.
The musician said he was a bit taken aback by such an unusual and dire problem, but “I thought if it can save lives we have to do it,” he said. He became an investor and cofounder of Ophirex with Dr. Lewin.
Dr. Lewin met Lt. Col. Rebecca Carter, a biochemist who was assigned to lead the Medical Modernization Division of Air Force Special Operations Command, in 2016 when she attended a Venom Week conference in Greenville, N.C. He was presenting the results of his mouse studies. She told him about her first mission: to find a universal antivenom for medics on special operations teams in Africa. She persuaded the Special Operations Command Biomedical Research Advisory Group, which specializes in getting critical projects to production, to grant Ophirex $148,000 in 2017. She later retired from the Air Force and now works for Ophirex as vice president.
More multimillion-dollar grants followed, including the Army’s COVID grant. Clinical trials are scheduled to begin this winter.
Despite the progress and the sudden cash flow, Dr. Lewin tamps down talk of a universal snakebite cure. “There’s enough evidence to say the drug deserves to have its day in clinical trials,” he said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Matthew Lewin, MD, PhD, founder of the Center for Exploration and Travel Health at the California Academy of Sciences, was researching snakebite treatments in rural locations in preparation for an expedition to the Philippines in 2011.
The story of a renowned herpetologist from the academy, Joseph Slowinski, who was bitten by a highly venomous krait in Myanmar and couldn’t get to a hospital in time to save his life a decade earlier, weighed on the emergency room doctor.
“I concluded that I needed something small and compact and that doesn’t care what kind of snake,” Dr. Lewin said.
It didn’t exist. That set Dr. Lewin in pursuit of a modern snakebite drug, a journey that finds his Corte Madera, Calif., company, Ophirex, nearing a promising oral treatment that fits in a pocket; is stable, easy to use, and affordable; and treats the venom from many species. “That’s the holy grail of snakebite treatment,” he said.
His work has gotten a boost with multimillion-dollar grants from a British charity and the U.S. Army. If it works – and it has been shown to work extremely well in mice and pigs – it could save tens of thousands of lives a year.
Dr. Lewin and Ophirex are not alone in their quest. Snakebites kill nearly 140,000 people a year, overwhelmingly in impoverished rural areas of Asia and Africa without adequate medical infrastructure and knowledge to administer antivenom. Though just a few people die each year in the United States from snakebites, the problem has risen to the top of the list of global health concerns in recent years. Funding has soared, and other research groups have also done promising work on new treatments. Herpetologists say deforestation and climate change are increasing human-snake encounters by forcing snakes to move to new habitats.
Dr. Lewin’s research is centered on a drug called varespladib. The enzyme inhibitor has proven itself in in-vitro lab studies and has effectively saved mice and pigs dosed with venom.
Along the way, Dr. Lewin and his team have come across another potential use for the drug. Varespladib has a positive effect on acute respiratory distress syndrome, associated with COVID-19. Next year, Ophirex will conduct human trials for the possible treatment of the condition funded with $9.9 million from the Army.
The link to a snakebite? The inflammation of the lungs caused by the coronavirus produces the sPLA2 enzyme. A more deadly version of the same enzyme is produced by snake venom.
The other companies that have come up with promising approaches to snakebite aren’t as far along as Ophirex. At the University of California-Irvine, chemist Ken Shea and his team created a nanogel – a kind of polymer used in medical applications – that blocks key proteins in the venom that cause cell destruction. At the Technical University of Denmark, Copenhagen, Andreas Laustsen is looking at engineering bacteria to manufacture anti-venom in fermentation tanks.
The days of incising a snakebite and sucking out the poison are long over, but the current treatment for venomous snakebites remains archaic.
Since the early 1900s, antivenom has been made by injecting horses or other animals with venom milked from snakes and diluted. The animals’ immune systems generate antibodies over several months, and blood plasma is taken from the animals and antibodies extracted from it.
It’s extremely expensive. Hospitals in the United States can charge as much as $15,000 a vial – and a single snakebite might require anywhere from 4 to 50 vials. Moreover, antivenom exists for little more than half the world’s species of venomous snakes.
A major problem is the roughly 2 hours it takes on average for a snakebite victim to reach a hospital and begin treatment. The chemical weapon that is venom starts immediately to destroy cells as it digests its next meal, making fast treatment essential to saving lives and preventing tissue loss.
“The two-hour window between fang and needle is where the most damage occurs,” said Leslie Boyer, director of the University of Arizona’s Venom Immunochemistry, Pharmacology and Emergency Response (VIPER) Institute. “We have a saying, ‘Time is tissue.’ ”
That’s why the search for a new snakebite drug has focused on an inexpensive treatment that can be taken into the field. Dr. Lewin’s drug wouldn’t replace antivenom. Instead, he thinks of it as the first line of defense until the victim can reach a hospital for antivenom treatment.
Dr. Lewin said he expects the drug to be inexpensive, so people in regions where snakebites are common can afford it.
Venom is extremely complicated chemically, and Dr. Lewin began his search by sussing out which of its myriad components to block. He zeroed in on the sPLA2 enzyme.
Surveying the literature about drugs that had been clinically tested for other conditions, he came across varespladib. It had been developed jointly by Eli Lilly and Shionogi, a Japanese pharmaceutical company, as a possible treatment for sepsis. They had never taken it to market.
If it worked, Dr. Lewin could license the right to produce the drug, which had already been thoroughly studied and was shown to be safe.
He placed venom in an array of test tubes. Varespladib and other drugs were added to the venom. He then added a reagent. If the venom was still active, the solution would turn yellow; if it was neutralized, it would remain clear.
The vials with varespladib “came up completely blank,” he said. “It was so stunning I said, ‘I must have made a mistake.’ ”
With a small grant, he sent the drug to the Yale Center for Molecular Discovery and found that varespladib effectively neutralized the venom of snakes found on six continents. The results were published in the journal Toxins and sent ripples through the small community of snakebite researchers.
Dr. Lewin then conducted tests on mice and pigs. Both were successful.
Human clinical trials are next, but they have been delayed by the pandemic. They are scheduled to get underway next spring.
Along the way, Dr. Lewin was fortunate enough to make some good connections that led to funding. In 2012, he attended a party at the Mill Valley, Calif., home of Jerry Harrison, the former guitarist and keyboardist for Talking Heads. Mr. Harrison had long been interested in business and start-ups – he said he was the most careful reader of the ’80s band’s contracts – and at the party he asked “if anyone had any ideas lying fallow,” Mr. Harrison said.
“And Matt pipes up and says, ‘I have this idea how to prevent people from dying from snakebites,’ ” Mr. Harrison said.
The musician said he was a bit taken aback by such an unusual and dire problem, but “I thought if it can save lives we have to do it,” he said. He became an investor and cofounder of Ophirex with Dr. Lewin.
Dr. Lewin met Lt. Col. Rebecca Carter, a biochemist who was assigned to lead the Medical Modernization Division of Air Force Special Operations Command, in 2016 when she attended a Venom Week conference in Greenville, N.C. He was presenting the results of his mouse studies. She told him about her first mission: to find a universal antivenom for medics on special operations teams in Africa. She persuaded the Special Operations Command Biomedical Research Advisory Group, which specializes in getting critical projects to production, to grant Ophirex $148,000 in 2017. She later retired from the Air Force and now works for Ophirex as vice president.
More multimillion-dollar grants followed, including the Army’s COVID grant. Clinical trials are scheduled to begin this winter.
Despite the progress and the sudden cash flow, Dr. Lewin tamps down talk of a universal snakebite cure. “There’s enough evidence to say the drug deserves to have its day in clinical trials,” he said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Matthew Lewin, MD, PhD, founder of the Center for Exploration and Travel Health at the California Academy of Sciences, was researching snakebite treatments in rural locations in preparation for an expedition to the Philippines in 2011.
The story of a renowned herpetologist from the academy, Joseph Slowinski, who was bitten by a highly venomous krait in Myanmar and couldn’t get to a hospital in time to save his life a decade earlier, weighed on the emergency room doctor.
“I concluded that I needed something small and compact and that doesn’t care what kind of snake,” Dr. Lewin said.
It didn’t exist. That set Dr. Lewin in pursuit of a modern snakebite drug, a journey that finds his Corte Madera, Calif., company, Ophirex, nearing a promising oral treatment that fits in a pocket; is stable, easy to use, and affordable; and treats the venom from many species. “That’s the holy grail of snakebite treatment,” he said.
His work has gotten a boost with multimillion-dollar grants from a British charity and the U.S. Army. If it works – and it has been shown to work extremely well in mice and pigs – it could save tens of thousands of lives a year.
Dr. Lewin and Ophirex are not alone in their quest. Snakebites kill nearly 140,000 people a year, overwhelmingly in impoverished rural areas of Asia and Africa without adequate medical infrastructure and knowledge to administer antivenom. Though just a few people die each year in the United States from snakebites, the problem has risen to the top of the list of global health concerns in recent years. Funding has soared, and other research groups have also done promising work on new treatments. Herpetologists say deforestation and climate change are increasing human-snake encounters by forcing snakes to move to new habitats.
Dr. Lewin’s research is centered on a drug called varespladib. The enzyme inhibitor has proven itself in in-vitro lab studies and has effectively saved mice and pigs dosed with venom.
Along the way, Dr. Lewin and his team have come across another potential use for the drug. Varespladib has a positive effect on acute respiratory distress syndrome, associated with COVID-19. Next year, Ophirex will conduct human trials for the possible treatment of the condition funded with $9.9 million from the Army.
The link to a snakebite? The inflammation of the lungs caused by the coronavirus produces the sPLA2 enzyme. A more deadly version of the same enzyme is produced by snake venom.
The other companies that have come up with promising approaches to snakebite aren’t as far along as Ophirex. At the University of California-Irvine, chemist Ken Shea and his team created a nanogel – a kind of polymer used in medical applications – that blocks key proteins in the venom that cause cell destruction. At the Technical University of Denmark, Copenhagen, Andreas Laustsen is looking at engineering bacteria to manufacture anti-venom in fermentation tanks.
The days of incising a snakebite and sucking out the poison are long over, but the current treatment for venomous snakebites remains archaic.
Since the early 1900s, antivenom has been made by injecting horses or other animals with venom milked from snakes and diluted. The animals’ immune systems generate antibodies over several months, and blood plasma is taken from the animals and antibodies extracted from it.
It’s extremely expensive. Hospitals in the United States can charge as much as $15,000 a vial – and a single snakebite might require anywhere from 4 to 50 vials. Moreover, antivenom exists for little more than half the world’s species of venomous snakes.
A major problem is the roughly 2 hours it takes on average for a snakebite victim to reach a hospital and begin treatment. The chemical weapon that is venom starts immediately to destroy cells as it digests its next meal, making fast treatment essential to saving lives and preventing tissue loss.
“The two-hour window between fang and needle is where the most damage occurs,” said Leslie Boyer, director of the University of Arizona’s Venom Immunochemistry, Pharmacology and Emergency Response (VIPER) Institute. “We have a saying, ‘Time is tissue.’ ”
That’s why the search for a new snakebite drug has focused on an inexpensive treatment that can be taken into the field. Dr. Lewin’s drug wouldn’t replace antivenom. Instead, he thinks of it as the first line of defense until the victim can reach a hospital for antivenom treatment.
Dr. Lewin said he expects the drug to be inexpensive, so people in regions where snakebites are common can afford it.
Venom is extremely complicated chemically, and Dr. Lewin began his search by sussing out which of its myriad components to block. He zeroed in on the sPLA2 enzyme.
Surveying the literature about drugs that had been clinically tested for other conditions, he came across varespladib. It had been developed jointly by Eli Lilly and Shionogi, a Japanese pharmaceutical company, as a possible treatment for sepsis. They had never taken it to market.
If it worked, Dr. Lewin could license the right to produce the drug, which had already been thoroughly studied and was shown to be safe.
He placed venom in an array of test tubes. Varespladib and other drugs were added to the venom. He then added a reagent. If the venom was still active, the solution would turn yellow; if it was neutralized, it would remain clear.
The vials with varespladib “came up completely blank,” he said. “It was so stunning I said, ‘I must have made a mistake.’ ”
With a small grant, he sent the drug to the Yale Center for Molecular Discovery and found that varespladib effectively neutralized the venom of snakes found on six continents. The results were published in the journal Toxins and sent ripples through the small community of snakebite researchers.
Dr. Lewin then conducted tests on mice and pigs. Both were successful.
Human clinical trials are next, but they have been delayed by the pandemic. They are scheduled to get underway next spring.
Along the way, Dr. Lewin was fortunate enough to make some good connections that led to funding. In 2012, he attended a party at the Mill Valley, Calif., home of Jerry Harrison, the former guitarist and keyboardist for Talking Heads. Mr. Harrison had long been interested in business and start-ups – he said he was the most careful reader of the ’80s band’s contracts – and at the party he asked “if anyone had any ideas lying fallow,” Mr. Harrison said.
“And Matt pipes up and says, ‘I have this idea how to prevent people from dying from snakebites,’ ” Mr. Harrison said.
The musician said he was a bit taken aback by such an unusual and dire problem, but “I thought if it can save lives we have to do it,” he said. He became an investor and cofounder of Ophirex with Dr. Lewin.
Dr. Lewin met Lt. Col. Rebecca Carter, a biochemist who was assigned to lead the Medical Modernization Division of Air Force Special Operations Command, in 2016 when she attended a Venom Week conference in Greenville, N.C. He was presenting the results of his mouse studies. She told him about her first mission: to find a universal antivenom for medics on special operations teams in Africa. She persuaded the Special Operations Command Biomedical Research Advisory Group, which specializes in getting critical projects to production, to grant Ophirex $148,000 in 2017. She later retired from the Air Force and now works for Ophirex as vice president.
More multimillion-dollar grants followed, including the Army’s COVID grant. Clinical trials are scheduled to begin this winter.
Despite the progress and the sudden cash flow, Dr. Lewin tamps down talk of a universal snakebite cure. “There’s enough evidence to say the drug deserves to have its day in clinical trials,” he said.
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Moral distress: COVID-19 shortages prompt tough decisions at bedside
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
Choosing which hospitalized COVID-19 patients receive potentially lifesaving care, making urgent calls for ventilators and other equipment, and triaging care based on patient age and comorbidities were among the challenges revealed in new feedback from health care leaders and frontline workers.
Even though many hospitals have contingency plans for how to allocate resources and triage patient care during crisis capacity, for many providers during the real-world COVID-19 trial of these protocols, they fell short.
Many hospital crisis capacity plans, for example, were too general to address all the specific challenges arising during the pandemic, investigators report in a study published online Nov. 6 in JAMA Network Open.
“Our research shows that the types of challenges and approach to resource limitation in real-world clinical settings during the pandemic differed in practice from how we had prepared in theory,” lead author Catherine Butler, MD, told Medscape Medical News. Insufficient dialysis treatment time, staff shortages, and routine supply scarcity are examples “for which there was not an established plan or approach for appropriate allocation.”
“This left frontline clinicians to determine what constituted an acceptable standard of care and to make difficult allocation decisions at the bedside,” added Butler, acting instructor in the Division of Nephrology at the University of Washington in Seattle and a research fellow at the VA Health Services Research and Development Seattle-Denver Center of Innovation.
The investigators conducted semistructured interviews in April and May with 61 clinicians and health leaders. Mean age was 46 years, 63% were women, and participants practiced in 15 states. Most participants hailed from locations hard-hit by the pandemic at the time, including Seattle, New York City, and New Orleans.
Triage tribulations
The qualitative study included comments from respondents on three major themes that emerged: planning for crisis capacity, adapting to resource limitation, and the multiple unprecedented barriers to care delivery.
Overall, planning and support from institutional leaders varied. One provider said, “Talking to administration, and they just seemed really disengaged with the problem. We asked multiple times if there was a triage command center or a plan for what would occur if we got to the point where we had to triage resources. They said there was, but they wouldn’t provide it to us.”
Another had a more positive experience. “The biggest deal in the ethics world in the last 2 months has been preparing in case we need to triage. So, we have a very detailed, elaborate, well thought-out triage policy … that was done at the highest levels of the system.”
Clinicians said they participate on triage teams – despite the moral weight and likely emotional burden – out of a sense of duty.
Interestingly, some providers on these teams also reported a reluctance to reveal their participation to colleagues. “I didn’t feel like I should tell anybody … even some of my close friends who are physicians and nurses here … that I’ve been asked to be on this [triage team],” one respondent said. “I didn’t feel like I should make it known.”
Adapting to scarce resources
Multiple providers said they faced difficult care decisions because of limited dialysis or supply shortages. “They felt that this patient had the greatest likelihood of benefiting from most aggressive therapy. … I think there was probably like 5 or 6 patients in the ICU … and then you had this 35-year-old with no comorbidities,” one respondent said. “That’s who the ICU dialyzed, and I couldn’t really disagree.”
“I emailed all of [my colleagues], and I said ‘Help! We need X, we need CRRT [continuous renal replacement therapy] machines, we need dialysates,’ “ another responded.
“One of the attendings had a tweet when we were running out of CRRT. He had a tweet about, ‘Can anybody give us supplies for CRRT?’ So, it got to that. You do anything. You get really desperate,” the clinician said.
Other providers reported getting innovative under the circumstances. “My partner’s son, he actually borrowed a couple of 3D printers. He printed some of these face shields, and then they got the formula, or the specifics as to how to make this particular connection to connect to a dialysis machine to generate dialysate. So, he also printed some of those from the 3D printer.”
Dire situations with dialysis
Another respondent understood the focus on ventilators and ICU beds throughout the crisis, but said “no one has acknowledged that dialysis has been one of the most, if not the most, limited resources.”
Another clinician expressed surprise at a decision made in the face of limited availability of traditional dialysis. “A month ago, people said we were going to do acute peritoneal dialysis [PD]. And I said, ‘No, we’re not going to do acute PD. PD, it’s not that great for acute patients, sick people in the ICUs. I don’t think we’re going to do PD.’
“Three days later we were doing acute PD. I mean, that was unbelievable!”
Some institutions rationed dialysis therapy. “We went through the entire list at the beginning of the week and [said], this person has to dialyze these days, this person would probably benefit from a dialysis session, a third group person we could probably just string along and medically manage if we needed to,” one provider said.
Another respondent reported a different strategy. “No one was not getting dialysis, but there were a lot of people getting minimal dialysis. Even though people were getting treated, resources were very stretched.”
Changing family dynamics
COVID-19 has naturally changed how clinicians speak with families. One respondent recalled looking at the ICU physician and being like, ‘Have you talked to the son this week?’ And she’s like, ‘Oh my God, no. … Did you talk to the son?’ I’m like, ‘Oh my God, no.’ “
They realized, the respondent added, “that none of us had called the family because it’s just not in your workflow. You’re so used to the family being there.”
Multiple providers also feared a conversation with family regarding necessary changes to care given the limitation of resources during the pandemic.
“Most families have been actually very understanding. This is a crisis, and we’re in a pandemic, and we’re all doing things we wouldn’t normally do.”
Another respondent said, “We were pretty honest about how resources were limited and how we were doing with this COVID-19 surge. And I think we talked about how the usual ability to provide aggressive dialysis was not the case with COVID-19. There was a lot of understanding, sometimes to my surprise. I would think people would be more upset when hearing something like that.”
Many clinicians facing these challenges experience moral distress, the researchers noted.
“Early in the pandemic, it became quickly apparent that possible resource limitation, such as scarce ventilators, was a major ethical concern. There was robust debate and discussion published in medical journals and the popular press about how to appropriately allocate health care resources,” the University of Washington’s Butler said.
“Transparency, accountability, and standardized processes for rationing these resources in ‘crisis capacity’ settings were seen as key to avoiding the impact of implicit bias and moral distress for clinicians,” she added.
Lessons learned
In terms of potential solutions that could mitigate these challenges in the future, health care leaders “could develop standardized protocols or guidelines for allocating a broader range of potentially scarce health care resources even before ‘crisis capacity’ is declared,” Butler said.
Furthermore, no frontline worker should have to go it alone. “Medical ethicists and/or other clinicians familiar with ethical considerations in settings of scarce health care resources might provide bedside consultation and collaborate with frontline providers who must grapple with the impact of more subtle forms of resource limitation on clinical decision-making.”
The study was partially funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and a COVID-19 Research Award from the University of Washington Institute of Translational Health Sciences given to Butler.
This article first appeared on Medscape.com.
What happened to melanoma care during COVID-19 sequestration
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
Initial evidence suggests that the , Rebecca I. Hartman, MD, MPH, said at a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medication Education.
This is not what National Comprehensive Cancer Network officials expected when they issued short-term recommendations on how to manage cutaneous melanoma during the first wave of the COVID-19 pandemic. Those recommendations for restriction of care, which Dr. Hartman characterized as “pretty significant changes from how we typically practice melanoma care in the U.S.,” came at a time when there was justifiable concern that the first COVID-19 surge would strain the U.S. health care system beyond the breaking point.
The rationale given for the NCCN recommendations was that most time-to-treat studies have shown no adverse patient outcomes for 90-day delays in treatment, even for thicker melanomas. But those studies, all retrospective, have been called into question. And the first real-world data on the impact of care restrictions during the lockdown, reported by Italian dermatologists, highlights adverse effects with potentially far-reaching consequences, noted Dr. Hartman, director of melanoma epidemiology at Brigham and Women’s Hospital and a dermatologist, Harvard University, Boston.
Analysis of the impact of lockdown-induced delays in melanoma care is not merely an academic exercise, she added. While everyone hopes that the spring 2020 COVID-19 shelter-in-place was a once-in-a-lifetime event, there’s no guarantee that will be the case. Moreover, the lockdown provides a natural experiment addressing the possible consequences of melanoma care delays on patient outcomes, a topic that for ethical reasons could never be addressed in a randomized trial.
The short-term NCCN recommendations included the use of excisional biopsies for melanoma diagnosis whenever possible; and delay of up to 3 months for wide local excision of in situ melanoma, any invasive melanoma with negative margins, and even T1 melanomas with positive margins provided the bulk of the lesion had been excised. The guidance also suggested delaying sentinel lymph node biopsy (SLNB), along with increased use of neoadjuvant therapy in patients with clinically palpable regional lymph nodes in order to delay surgery for up to 8 weeks. Single-agent systemic therapy at the least-frequent dosing was advised in order to minimize toxicity and reduce the need for additional health care resources: for example, nivolumab (Opdivo) at 480 mg every 4 weeks instead of every 2 weeks, and pembrolizumab (Keytruda) at 400 mg every 6 weeks, rather than every 3 weeks.
So, that’s what the NCCN recommended. Here’s what actually happened during shelter-in-place as captured in Dr. Hartman’s survey of 18 U.S. members of the Melanoma Prevention Working Group, all practicing dermatology in centers particularly hard-hit in the first wave of the pandemic: In-person new melanoma patient visits plunged from an average of 4.83 per week per provider to 0.83 per week. Telemedicine visits with new melanoma patients went from zero prepandemic to 0.67 visits per week per provider, which doesn’t come close to making up for the drop in in-person visits. Interestingly, two respondents reported turning to gene-expression profile testing for patient prognostication because of delays in SLNB.
Wide local excision was delayed by an average of 6 weeks in roughly one-third of melanoma patients with early tumor stage disease, regardless of margin status. For patients with stage T1b disease, wide local excision was typically performed on time during shelter-in-place; however, SLNB was delayed by an average of 5 weeks in 22% of patients with positive margins and 28% of those with negative margins. In contrast, 80% of patients with more advanced T2-T4 melanoma underwent on-schedule definitive management with wide local excision and SLNB, Dr. Hartman reported.
Critics have taken issue with the NCCN’s conclusion that most time-to-treatment studies show no harm arising from 90-day treatment delays. A review of the relevant published literature by Dr. Hartman’s Harvard colleagues, published in July, found that the evidence is mixed. “There is insufficient evidence to definitively conclude that delayed wide resection after gross removal of the primary melanoma is without harm,” they concluded in the review.
Spanish dermatologists performed a modeling study in order to estimate the potential impact of COVID-19 lockdowns on 5- and 10-year survival of melanoma patients. Using the growth rate of a random sample of 1,000 melanomas to model estimates of tumor thickness after various delays, coupled with American Joint Committee on Cancer survival data for different T stages, they estimated that 5-year survival would be reduced from 94.2% to 92.3% with a 90-day delay in diagnosis, and that 10-year survival would drop from 90.0% to 87.6%.
But that’s merely modeling. Francesco Ricci, MD, PhD, and colleagues from the melanoma unit at the Istituto Dermopatico dell’Immacolata, Rome, have provided a first look at the real-world impact of the lockdown. In the prelockdown period of January through March 9th, 2020, the referral center averaged 2.3 new melanoma diagnoses per day. During the Rome lockdown, from March 10th through May 3rd, this figure dropped to a mean of 0.6 melanoma diagnoses per day. Postlockdown, from May 4th to June 6th, the average climbed to 1.3 per day. The rate of newly diagnosed nodular melanoma was 5.5-fold greater postlockdown, compared with prelockdown; the rate of ulcerated melanoma was 4.9-fold greater.
“We can hypothesize that this may have been due to delays in diagnosis and care,” Dr. Hartman commented. “This is important because we know that nodular melanoma as well as ulceration tend to have a worse prognosis in terms of mortality.”
The mean Breslow thickness of newly diagnosed melanomas was 0.88 mm prelockdown, 0.66 mm during lockdown, and 1.96 mm postlockdown. The investigators speculated that the reduced Breslow thickness of melanomas diagnosed during lockdown might be explained by a greater willingness of more health-conscious people to defy the shelter-in-place instructions because of their concern about a suspicious skin lesion. “Though it is way too early to gauge the consequences of such diagnostic delay, should this issue be neglected, dermatologists and their patients may pay a higher price later with increased morbidity, mortality, and financial burden,” according to the investigators.
Dr. Hartman observed that it will be important to learn whether similar experiences occurred elsewhere during lockdown.
Another speaker, John M. Kirkwood, MD, said he has seen several melanoma patients referred from outside centers who had delays of up to 3 months in sentinel lymph node management of T2 and T3 tumors during lockdown who now have widespread metastatic disease.
“Now, is that anecdotal? I don’t know, it’s just worrisome to me,” commented Dr. Kirkwood, professor of medicine, dermatology, and translational science at the University of Pittsburgh.
Merrick Ross, MD, professor of surgical oncology at M.D. Anderson Cancer Center, Houston, recalled, “There was a period of time [during the lockdown] when we weren’t allowed to do certain elective procedures, if you want to call cancer surgery elective.”
“It’s too soon to talk about outcomes because a lot of patients are still in the process of being treated after what I would consider a significant delay in diagnosis,” the surgeon added.
An audience member asked if there will be an opportunity to see data on the damage done by delaying melanoma management as compared to lives saved through the lockdown for COVID-19. Dr. Ross replied that M.D. Anderson is in the midst of an institution-wide study analyzing the delay in diagnosis of a range of cancers.
“In our melanoma center it is absolutely clear, although we’re still collecting data, that the median tumor thickness is much higher since the lockdown,” Dr. Ross commented.
Dr. Hartman said she and her coinvestigators in the Melanoma Prevention Working Group are attempting to tally up the damage done via the lockdown by delaying melanoma diagnosis and treatment. But she agreed with the questioner that the most important thing is overall net lives saved through shelter-in-place.
“I’m sure that, separately, nondermatologists – perhaps infectious disease doctors and internists – are looking at how many lives were saved by the lockdown policy. So I do think all that data will come out,” Dr. Hartman predicted.
She reported having no financial conflicts regarding her presentation.
Global Academy for Medical Education and this news organization are owned by the same company.
SOURCE: Hartman, R. Cutaneous malignancies forum.
REPORTING FROM THE CUTANEOUS MALIGNANCIES FORUM
Trump could clean house at health agencies
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.
Others may soon depart voluntarily. Politico reported in late October that more than two dozen political appointees had already left the U.S. Department Health and Human Services (HHS) since the start of the COVID-19 pandemic in February and that potentially dozens of the more than 100 in the department would leave if Trump was not reelected.
Trump hasn’t conceded, he is challenging the election results, and he has already fired his Defense Secretary, Mark Esper.
Among those possibly in Trump’s sights: HHS Secretary Alex Azar, US Food and Drug Administration (FDA) Commissioner Stephen Hahn, MD, Centers for Disease Control and Prevention (CDC) Director Robert Redfield, MD, and White House Coronavirus Task Force member Anthony Fauci, MD, who is also the director of the National Institutes of Allergy and Infectious Diseases.
Seema Verma, the administrator of the Centers for Medicare & Medicaid Services (CMS), is likely safe. According to Politico, Verma is expected to leave on her own terms.
Azar has had a long run as a Trump appointee. He took office in January 2018 and has been a staunch loyalist. But he’s frequently been the butt of grousing by Trump for not doing enough to help lower drug prices and for his handling of the coronavirus pandemic. Azar was initially in charge of the Trump virus effort but was quickly replaced by Vice President Mike Pence.
It was widely reported in late April that Trump was considering firing Azar, but the president called that “fake news” in a tweet.
Azar has complained about Hahn, who was confirmed in December 2019. According to Politico, Azar was looking into how to remove Hahn as commissioner because of the FDA’s battle with the White House over standards for emergency use authorization of a coronavirus vaccine.
In addition, Trump was infuriated by the agency’s insistence that it stick to the highest bar for an emergency approval. “The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics. Obviously, they are hoping to delay the answer until after November 3rd,” Trump tweeted at Hahn.
Fauci on the firing line?
Most of the president’s ire has been directed at Fauci. As far back as April, Trump retweeted a call for Fauci’s firing. Twitter removed the original tweet but kept Trump’s comments on the original tweet.
The president has frequently questioned Fauci’s advice, sidelined him from task force meetings, and infrequently met with him. Trump called Fauci a “disaster” during a call with supporters in October, and then, at a campaign rally in November, intimated that he would fire the scientist after the election, according to The Washington Post.
But such a firing cannot be easily done. Some have speculated that Trump could pressure Fauci’s boss, Francis Collins, MD, PhD — the director of the National Institutes of Health (NIH), who is a political appointee — to get rid of him. But Collins would have to come up with a reason to fire Fauci. Because he is not a political appointee, Fauci is afforded a raft of protections given to civil service employees of the federal government.
To demote or fire Fauci, Collins would have to give him 30 days’ notice unless there’s a belief that he committed a crime. Fauci would have at least a week to offer evidence and affidavits in support of his service.
He’d also be entitled to legal representation, a written decision, and the specific reasons for the action being taken quickly. He could also request a hearing, and he’d be able to appeal any action to the Merit Systems Protection Board. The process could take months, if not years.
In late October, Trump issued an executive order that would reclassify certain federal employees so that they wouldn’t have such protections. But agencies have until mid-January to come up with lists of such workers, according to Government Executive.
Collins has been with NIH since 1993, when he headed the Human Genome Project and the National Human Genome Research Institute. Politico has speculated that Collins, 70, might retire if Trump was reelected. It’s unclear what he’ll do now.
Redfield, who has taken heat for his leadership from many in public health — and was asked in October to stand up to Trump by former CDC Director William H. Foege, MD — has been openly contradicted by the president on more than one occasion, according to The New York Times.
In September, The Hill reported that Trump told reporters that he’d chastised Redfield by phone soon after Redfield had told a Senate committee that a coronavirus vaccine would not be available until mid-2021.
This article first appeared on Medscape.com.









